WO2007080463A1 - An improved process for the preparation of dipyridamole - Google Patents
An improved process for the preparation of dipyridamole Download PDFInfo
- Publication number
- WO2007080463A1 WO2007080463A1 PCT/IB2007/000004 IB2007000004W WO2007080463A1 WO 2007080463 A1 WO2007080463 A1 WO 2007080463A1 IB 2007000004 W IB2007000004 W IB 2007000004W WO 2007080463 A1 WO2007080463 A1 WO 2007080463A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- dipyridamole
- solvent
- process according
- formula
- preparation
- Prior art date
Links
- IZEKFCXSFNUWAM-UHFFFAOYSA-N OCCN(CCO)c(nc12)nc(N3CCCCC3)c1nc(N(CCO)CCO)nc2N1CCCCC1 Chemical compound OCCN(CCO)c(nc12)nc(N3CCCCC3)c1nc(N(CCO)CCO)nc2N1CCCCC1 IZEKFCXSFNUWAM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
Definitions
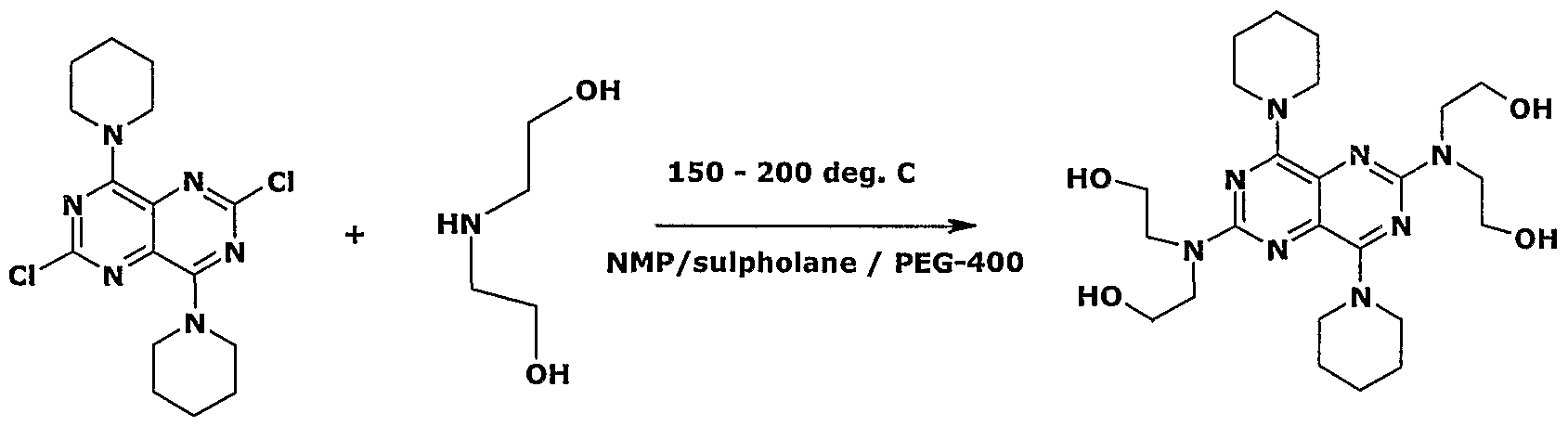
- the present invention' relates to an improved process for the preparation of
- Dipyridamole of formula (I) by reacting 2,6-Dichloro-4,8-dipiperidinopyrimido(5,4-d) pyrimidine (DDH) with Diethanolamine (DEA) using l-Methyl-2-pyrrolidinone (NMP) as a solvent.
- DDH 2,6-Dichloro-4,8-dipiperidinopyrimido(5,4-d) pyrimidine
- DEA Diethanolamine
- NMP l-Methyl-2-pyrrolidinone
- Dipyridamole which is chemically known as 4,8-Bis(piperidino)-N,N,N',N'- tetra(2-hydroxyethyl)pyrimido[5,4-d]pyrimidine-2,6-diamine is a platelet adhesion inhibitor. It is useful in anti-platelet therapy and it is marketed as Persantin ® by Boehringer Ingelheim.
- German patent 117456 describes the process for the production of Dipyridamole from 2,6-dichloro-4,8-dipiperidinopyrimido[5,4-d]pyrimidine and diethanolamine at 130 to 200° C under vacuum.
- German patent 1812918 describes the process for the preparation and purification of Dipyridamole. According to this patent 2,6-dichloro-4,8,- dipiperidinopyrimido[5,4-d]pyrimidine and diethanolamine are heated to 150 to 200 0 C to obtain Dipyridamole. This is characterized by the fact that after the completion of the reaction, the reaction mixture is dissolved in chloroform, which is further separated into the upper layer of diethanolamine and its hydrochloride and the chloroform solution. Thus obtained chloroform solution is reduced to dryness after stirring with water.
- RO 104718 Bl describes a process where Dipyridamole is manufactured and purified by reaction of diethanolamine with 2,6-dichloro-4,8-dipiperidinopyrimido[5,4- d]pyrimidine. In this process the yield is very low (58%) and purity is only 98%.
- Another patent DDl 15670 Z describes a process for the purification of Dipyridamole by refluxing it in AcOBu for 2 h in the presence of an equal amount of silica gel or by column chromatography on silica gel at 60-100 0 C.
- the purification by column chromatography is not economical and feasible at industrial scale.
- the main objective of the present invention is to provide an improved process for the preparation and purification of compound of formula (I), which gives better purity and high yield of the product.
- Another objective of the present invention is to provide a process for the preparation and purification of compound of formula (I), which would be easy to scale up and implement at industrial level.
- Yet another objective of the present invention is to provide a process for the preparation and purification of compound of formula (I), which avoids, use of hazardous gas (SO 2 ) and corrosive chemicals like HCl, H 2 SO 4 , acetic acid, NaOH, NH 3 etc.
- SO 2 hazardous gas
- corrosive chemicals like HCl, H 2 SO 4 , acetic acid, NaOH, NH 3 etc.
- the present invention provides a process for the preparation of
- Dipyridamole of formula (I) comprising reacting 2,6-Dichloro-4,8- dipiperidinopyrimido- (5,4-d)pyrimidine (DDH) of formula (II) with Diethanolamine (DEA) of formula (III) using a solvent.
- DDH 2,6-Dichloro-4,8- dipiperidinopyrimido- (5,4-d)pyrimidine
- DEA Diethanolamine
- the obtained wet or optionally dried crude Dipyridamole is purified by using ketonic solvent and aqueous alcoholic solvent or mixture thereof.
- the solvent is selected from the group consisting of l-Methyl-2-pyrrolidinone, Sulpholane and Polyethylene glycol, preferably l-Methyl-2-pyrrolidinone (NMP).
- the polyethylene glycol used is PEG-20Q or PEG-400, preferably PEG-400.
- the reaction is carried out at a temperature of about 25° C to reflux temperature, preferably at a temperature of about 150° C to 200 0 C.
- the starting material of this invention is prepared according to the literature available in the prior art.
- the ketonic solvent is acetone, methyl ethyl ketone, methyl vinyl ketone or methyl isobutyl ketone (MIBK), preferably MIBK.
- the alcoholic solvent is selected from the group having Ci to C 4 alkanol preferably isopropyl alcohol (IPA) or methanol.
- Methyl isobutyl ketone (750 ml) and 50 g of Dipyridamole (crude) were charged into a clean 2.0 L four-necked RBF at 25-3O 0 C and heated to 100-120 0 C. It was stirred to dissolve and cooled to 25-35 0 C and stirred for 30-60 min. The solid was Filtered and washed with 100 ml MIBK. The product was dried at 45-50 0 C under vacuum. The obtained material was further purified as follows:
- Polyethylene glycol-400 (15 mL), 5g of 2,6-Dichloro-4,8-dipiperidinopyrimido (5,4-d) pyrimidine (DDH) and 8.6 gm Diethanolamine (DEA) were charged into 10OmL four necked RBF at 25-35°C.
- the reaction mass was heated to 190-200 0 C and maintained for 2-3 hrs.
- the mixture was cooled to 25-35 0 C.
- Water 45 mL was added to the reaction mixture and stirred.
- the solid was filtered and washed with water.
- the solid was purified with MIBK,IPA- Water as given in example 1.
- Methyl isobutyl ketone (750 ml) and 65 g of Dipyridamole (wet crude) were charged into a clean 2.0 L four-necked RBF at 25-3O 0 C and heated to 100-120 0 C followed by azeotrophic separation of water. It was cooled to 25-35 0 C and stirred for
Abstract
The present invention relates to an improved process for the preparation of Dipyridamole of formula (I) by reacting 2,6-Dichloro-4, 8-dipiperidinopyrimido (5,4- d) pyrimidine (DDH) with Diethanolamine (DEA) using l-Methyl-2-pyrrolidinone (NMP) as a solvent.
Description
AN IMPROVED PROCESS FOR THE PREPARATION OF DIPYRIDAMOLE
Field of the Invention
The present invention' relates to an improved process for the preparation of
Dipyridamole of formula (I) by reacting 2,6-Dichloro-4,8-dipiperidinopyrimido(5,4-d) pyrimidine (DDH) with Diethanolamine (DEA) using l-Methyl-2-pyrrolidinone (NMP) as a solvent.
(I)
Background of the Invention
Dipyridamole which is chemically known as 4,8-Bis(piperidino)-N,N,N',N'- tetra(2-hydroxyethyl)pyrimido[5,4-d]pyrimidine-2,6-diamine is a platelet adhesion inhibitor. It is useful in anti-platelet therapy and it is marketed as Persantin ® by Boehringer Ingelheim.
The platelet aggregation inhibiting properties, anti-thrombotic and vasodilator properties of Dipyridamole is reported in US patent 3031450 which also describe a process for its preparation by reacting 2-chloro-6-diethanolamino-4, 8-dipiperidyl- pyrimido-pyrimidine with diethanolamine.
German patent 117456 describes the process for the production of Dipyridamole from 2,6-dichloro-4,8-dipiperidinopyrimido[5,4-d]pyrimidine and diethanolamine at 130 to 200° C under vacuum.
German patent 1812918 describes the process for the preparation and purification of Dipyridamole. According to this patent 2,6-dichloro-4,8,- dipiperidinopyrimido[5,4-d]pyrimidine and diethanolamine are heated to 150 to 2000C to obtain Dipyridamole. This is characterized by the fact that after the completion of the reaction, the reaction mixture is dissolved in chloroform, which is further separated into the upper layer of diethanolamine and its hydrochloride and the chloroform solution. Thus obtained chloroform solution is reduced to dryness after stirring with water.
RO 104718 Bl describes a process where Dipyridamole is manufactured and purified by reaction of diethanolamine with 2,6-dichloro-4,8-dipiperidinopyrimido[5,4- d]pyrimidine. In this process the yield is very low (58%) and purity is only 98%.
Another patent DDl 15670 Z describes a process for the purification of Dipyridamole by refluxing it in AcOBu for 2 h in the presence of an equal amount of silica gel or by column chromatography on silica gel at 60-1000C. The purification by column chromatography is not economical and feasible at industrial scale.
All the above mentioned prior art are neat reaction in which it is difficult to control the impurity and is not easy to scale up. In the prior art process the obtained product is pasty which needs decanting the mother liquor and further purification.
We focused our research to develop an improved and efficient process for the preparation and purification of Dipyridamole of formula (I) which will overcome the above mentioned prior art problems and will produce Dipyridamole in substantially good yield, high purity and with no mixture of solvents.
Objectives of the Invention
The main objective of the present invention is to provide an improved process for the preparation and purification of compound of formula (I), which gives better purity and high yield of the product.
Another objective of the present invention is to provide a process for the preparation and purification of compound of formula (I), which would be easy to scale up and implement at industrial level.
Yet another objective of the present invention is to provide a process for the preparation and purification of compound of formula (I), which avoids, use of hazardous gas (SO2) and corrosive chemicals like HCl, H2SO4, acetic acid, NaOH, NH3 etc.
Summary of the Invention
Accordingly, the present invention provides a process for the preparation of
Dipyridamole of formula (I) comprising reacting 2,6-Dichloro-4,8- dipiperidinopyrimido- (5,4-d)pyrimidine (DDH) of formula (II) with Diethanolamine (DEA) of formula (III) using a solvent. This process can be represented by the scheme given below:
(II) (III) (I)
The obtained wet or optionally dried crude Dipyridamole is purified by using ketonic solvent and aqueous alcoholic solvent or mixture thereof.
Description of the Invention
In an embodiment of the present invention the solvent is selected from the group consisting of l-Methyl-2-pyrrolidinone, Sulpholane and Polyethylene glycol, preferably l-Methyl-2-pyrrolidinone (NMP).
In another embodiment of the present invention, the polyethylene glycol used is PEG-20Q or PEG-400, preferably PEG-400.
In yet another embodiment of the present invention, the reaction is carried out at a temperature of about 25° C to reflux temperature, preferably at a temperature of about 150° C to 2000C.
In still another embodiment of the present invention the starting material of this invention is prepared according to the literature available in the prior art.
In yet another embodiment the ketonic solvent is acetone, methyl ethyl ketone, methyl vinyl ketone or methyl isobutyl ketone (MIBK), preferably MIBK.
In yet another embodiment the alcoholic solvent is selected from the group having Ci to C4 alkanol preferably isopropyl alcohol (IPA) or methanol.
The present invention is illustrated with the following examples, which should not be construed for limiting the scope of the invention.
Example 1
Preparation of Dipyridamole (Crude)
250 mL of l-Methyl-2-pyrrolidinone (NMP), 50g of 2,6-Dichloro-4,8 dipiperidinopyrimido(5,4-d)pyrimidine (DDH) and 136g of Diethanolamine (DEA) were charged into a 2.0 L four-necked RBF at 25-35 0C. The reaction mass was heated to 190 - 2000C and maintained for 1.5 to 2.5 hrs under stirring. The reaction mass was cooled to 25-350C and 450 mL of purified water was charged slowly into it and stirred for lhr. The solid reaction mass was filtered and washed with 500ml-purified water and dried the solid under vacuum at 50-550C for 8 to 10 hrs to get 55-60 g of crude Dipyridamole of 90-94% HPLC purity.
Purification Of Dipyridamole
Methyl isobutyl ketone (750 ml) and 50 g of Dipyridamole (crude) were charged into a clean 2.0 L four-necked RBF at 25-3O0C and heated to 100-1200C. It was stirred to dissolve and cooled to 25-350C and stirred for 30-60 min. The solid was
Filtered and washed with 100 ml MIBK. The product was dried at 45-50 0C under vacuum. The obtained material was further purified as follows:
Isopropyl alcohol (200 mL) and 40-45 g of Dipyridamole were charged into a clean 1.0 lit four-necked RBF at 25-350C. It was heated to 60-650C. Carbon (Ig) was added at 30-350C and filtered through celite and washed with 50 mL IPA. Water (500 mL) was charged slowly and stirred for 30 min. The solid was filtered and washed with a mixture of IPA : Water (1 :2) and dried the product at 45-50 0C under vacuum to obtain 43-5Og of Dipyridamole having HPLC purity 99.0 - 99.5%
Example 2
Sulpholane (15 mL), 5.0g of 2,6-Dichloro-4,8-dipiperidinopyrimido(5,4-d) pyrimidine (DDH) and 8.6g Diethanolamine (DEA) were charged into 100 mL four- necked RBF at 25-35°C. It was heated to 190-2000C and stirred for 2-3 hrs. The reaction mass was cooled to 25-35°C and 45 mL of water was added into it. The reaction mass was stirred. The solid was filtered and washed with water. The solid was purified with MIBK,IPA-Water as given in example 1.
Example 3
Polyethylene glycol-400 (15 mL), 5g of 2,6-Dichloro-4,8-dipiperidinopyrimido (5,4-d) pyrimidine (DDH) and 8.6 gm Diethanolamine (DEA) were charged into 10OmL four necked RBF at 25-35°C. The reaction mass was heated to 190-2000C and maintained for 2-3 hrs. The mixture was cooled to 25-350C. Water (45 mL) was added to the reaction mixture and stirred. The solid was filtered and washed with water. The solid was purified with MIBK,IPA- Water as given in example 1.
Example 4 (Azeotrophic removal of water in MIBK purification)
Preparation of Dipyridamole (Crude*) l-Methyl-2-pyrrolidinone (150 mL), 50g of 2,6-Dichloro-4,8-dipiperidinopyrimido
(5,4-d)pyrimidine (DDH) and 136g Diethanolamine (DEA) was charged into a 2.0 L four-necked RBF at 25-35 0C. The reaction mass was stirred & heated to 190 - 2000C
and stirring was continued for 1.5 to 2.5 hrs. The reaction mass was cooled to 25-350C and 450 mL of purified water was charged slowly into it and stirred for lhr. The solid reaction mass was filtered and washed with 500ml-purified water to obtain 110-13Og of wet crude Dipyridamole.
Purification Of Dipyridamole
Methyl isobutyl ketone (750 ml) and 65 g of Dipyridamole (wet crude) were charged into a clean 2.0 L four-necked RBF at 25-3O0C and heated to 100-1200C followed by azeotrophic separation of water. It was cooled to 25-350C and stirred for
30-60 min. The solid was filtered and washed with 100 ml MIBK. The product was dried at 45-50 0C under vacuum. The obtained material was further purified as follows:
Isopropyl alcohol (200 mL) and 45-48 g of Dipyridamole were charged into a clean 1.0 L four-necked RBF at 25-350C. It was heated to 60-650C and stirred to dissolve. Carbon (Ig) was added at 30-350C and filtered through celite and washed with 50 mL IPA. Water (500 mL) was charged slowly and stirred for 30 min. The solid was filtered and washed with a mixture of IPA : Water (1:2) and dried the product at 45-50 0C under vacuum to obtain 43-5Og of Dipyridamole having HPLC purity 99.0-99.5%
Purification with Methaanol-water
Methanol (200 mL) and 45-48 g of Dipyridamole were charged into a clean 1.0 L four-necked RBF at 25-350C. It was heated to 60-650C and stirred to dissolve. Carbon (Ig) was added at 30-350C and filtered through celite and washed with 50 mL methanol. Water (500 mL) was charged slowly and stirred for 30 min. The solid was filtered and washed with a mixture of methanol : Water (1 :2) and dried the product at 45-50 0C under vacuum to obtain 45g of Dipyridamole having HPLC purity 99%.
Claims
1. A process for preparing Dipyridamole of formula (I) comprising reacting 2,6- Dichloro-4,8-dipiperidinopyrimido- (5,4-d)pyrimidine (DDH) of formula (II) with Diethanolamine (DEA) of formula (III) using a solvent selecting from the group consisting of l-Methyl-2-pyrrolidinone, Sulpholane and Polyethylene glycol.
(D CII) (πI>
2. A process according to claim 1, wherein the solvent used is l-Methyl-2- pyrrolidinone.
3. A process according to claim 1, wherein the solvent polyethylene glycol used is Polyethylene glycol-400 or Polyethylene glycol-200.
4. A process according to claim 1, wherein the reaction is carried out at a temperature of about 25° C to reflux temperature.
5. A process according to claim 1, wherein the reaction is carried out at a temperature of about 150° C to 2000C.
6. A process according to claim 1, wherein the obtained Dipyridamole of formula (I) is further purified using ketonic solvent and aqueous alcoholic solvent or mixture thereof.
7. A process according to claim 6, wherein the ketonic solvent is methyl isobutyl ketone.
8. A process according to claim 6, wherein the alcoholic solvent is isopropyl alcohol or methanol.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN50/CHE/2006 | 2006-01-12 | ||
| IN50CH2006 | 2006-01-12 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2007080463A1 true WO2007080463A1 (en) | 2007-07-19 |
Family
ID=38256028
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2007/000004 WO2007080463A1 (en) | 2006-01-12 | 2007-01-03 | An improved process for the preparation of dipyridamole |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007080463A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011151640A1 (en) | 2010-05-31 | 2011-12-08 | Generics [Uk] Limited | Processes for the preparation of dipyridamole |
| CN108069972A (en) * | 2016-11-16 | 2018-05-25 | 湖南尔康制药股份有限公司 | A kind of production method of Dipyridamole bulk pharmaceutical chemicals |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5191295A (en) * | 1975-02-05 | 1976-08-10 | Jipiridamooruno kairyoseizoho | |
| DE2927539A1 (en) * | 1979-07-07 | 1981-01-08 | Margineanu Dan Axente Dipl Ing | Bis:di:ethanol-amino-di:piperidino-pyrimido-pyrimidine prepn. - from methyl acetoacetate and urea via amino-orotic acid |
-
2007
- 2007-01-03 WO PCT/IB2007/000004 patent/WO2007080463A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5191295A (en) * | 1975-02-05 | 1976-08-10 | Jipiridamooruno kairyoseizoho | |
| DE2927539A1 (en) * | 1979-07-07 | 1981-01-08 | Margineanu Dan Axente Dipl Ing | Bis:di:ethanol-amino-di:piperidino-pyrimido-pyrimidine prepn. - from methyl acetoacetate and urea via amino-orotic acid |
Non-Patent Citations (1)
| Title |
|---|
| DATABASE WPI Week 197639, Derwent World Patents Index; AN 1976-72913X, XP003016033 * |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011151640A1 (en) | 2010-05-31 | 2011-12-08 | Generics [Uk] Limited | Processes for the preparation of dipyridamole |
| JP2013527221A (en) * | 2010-05-31 | 2013-06-27 | ジェネリクス・[ユーケー]・リミテッド | Process for the preparation of dipyridamole |
| US8946414B2 (en) | 2010-05-31 | 2015-02-03 | Generics [Uk] Limited | Processes for the preparation of dipyridamole |
| US9381197B2 (en) | 2010-05-31 | 2016-07-05 | Generics [Uk] Limited | Processes for the preparation of dipyridamole |
| JP2016155810A (en) * | 2010-05-31 | 2016-09-01 | ジェネリクス・[ユーケー]・リミテッド | Process for preparation of dipyridamole |
| CN108069972A (en) * | 2016-11-16 | 2018-05-25 | 湖南尔康制药股份有限公司 | A kind of production method of Dipyridamole bulk pharmaceutical chemicals |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DK162647B (en) | PROCEDURE FOR PREPARING PYRIDO-IMIDAZORIFAMYCINES | |
| WO2008047388A2 (en) | Improved process for the preparation of ranolazine | |
| US10214532B2 (en) | Process for preparing ibrutinib | |
| CN101426795B (en) | Process for preparing dorzolamide | |
| CN101316820B (en) | Process for preparation of chiral amlodipine gentisate | |
| WO2009121791A1 (en) | CONVERSION OF TRYPTOPHAN INTO ß-CARBOLINE DERIVATIVES | |
| WO2007080463A1 (en) | An improved process for the preparation of dipyridamole | |
| CN114105984A (en) | Preparation method of indolizine corrosion inhibitor | |
| EP2114856A1 (en) | Process for the preparation of ethyl-n-(2, 3-dichloro-6-nitrobenzyl) glycine hydrochloride | |
| JP2014524929A (en) | How to prepare Prasugrel | |
| WO2012017441A1 (en) | Improved process to prepare s-2-hydroxy-3-methoxy-3,3-diphenyl propionic acid | |
| CN108947800B (en) | Synthesis method of (1S) -4, 5-dimethoxy-1- (carbonylaminomethyl) benzocyclobutane | |
| CN103562207B (en) | For preparing the preparation method of 2-amino-9-((2-phenyl-1,3-dioxane-5-base epoxide) methyl)-1H-purine-6 (9H) the-one compound of valganciclovir | |
| CN101522680A (en) | Process for the preparation of abacavir | |
| CN112939814B (en) | Preparation method of deuterated dacarbazine intermediate | |
| WO2009101634A2 (en) | A novel process for the preparation of eszopiclone | |
| CN111116593B (en) | Continuous preparation method of imatinib | |
| CN108299173B (en) | Asymmetric synthesis method of dezocine key intermediate | |
| EP1397335A1 (en) | Resolution process for (r)-(-)-2-hydroxy-2-(2-chlorophenyl)acetic acid | |
| CN111440173B (en) | Preparation method of PI3K inhibitor | |
| US20060030730A1 (en) | Purification and production methods of 1-aminocyclopropanecarboxylic acid | |
| CN108503583B (en) | Alkylation method of nitrogen-hydrogen-containing compound and application thereof | |
| FI64596B (en) | PROCEDURE FOR FRAMSTAELLNING AV 9- (2,6-DIHALOGENBENYL) ADENIN VAESENTLIGEN FRI FRAON 3-ISOMEREN | |
| KR101396686B1 (en) | Process for the preparation of abacavir | |
| WO2004099208A1 (en) | Process for the preparation of famciclovir |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07700439 Country of ref document: EP Kind code of ref document: A1 |