WO2007069986A1 - New oxabispidine compounds for the treatment of cardiac arrhythmias - Google Patents
New oxabispidine compounds for the treatment of cardiac arrhythmias Download PDFInfo
- Publication number
- WO2007069986A1 WO2007069986A1 PCT/SE2006/001426 SE2006001426W WO2007069986A1 WO 2007069986 A1 WO2007069986 A1 WO 2007069986A1 SE 2006001426 W SE2006001426 W SE 2006001426W WO 2007069986 A1 WO2007069986 A1 WO 2007069986A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- oxa
- ethyl
- diazabicyclo
- acetamide
- cyano
- Prior art date
Links
- 0 *N(CC(CN(*)C1)O)CC1O Chemical compound *N(CC(CN(*)C1)O)CC1O 0.000 description 5
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D498/00—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms
- C07D498/02—Heterocyclic compounds containing in the condensed system at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms in which the condensed system contains two hetero rings
- C07D498/08—Bridged systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/06—Antiarrhythmics
Definitions
- This invention relates to novel pharmaceutically useful compounds, in particular compounds which are useful in the treatment of cardiac arrhythmias.
- Cardiac arrhythmias may be defined as abnormalities in the rate, regularity, or site of origin of the cardiac impulse or as disturbances in conduction which causes an abnormal sequence of activation.
- Arrhythmias may be classified clinically by means of the presumed site of origin (i.e. as supraventricular, including atrial and atrioventricular, arrhythmias and ventricular arrhythmias) and/or by means of rate (i.e. bradyarrhythmias (slow) and tachyarrhythmias (fast)).
- Class III antiarrhythmic drugs may be defined as drugs which prolong the trans-membrane action potential duration (which can be caused by a block of outward K + currents or from an increase of inward ion currents) and refractoriness, without affecting cardiac conduction.
- Antiarrhythmic drugs based on bispidines are known from inter alia international patent applications WO 91/07405 and WO 99/31100, European patent applications 306 871, 308 843 and 655 228 and US patents 3,962,449, 4,556,662, 4,550,112, 4,459,301 and 5,468,858, as well as journal articles including, inter alia, J. Med. Chem. 39, 2559, (1996), Pharmacol Res., 24, 149 (1991), Circulation, 90, 2032 (1994) m ⁇ Anal. ScI 9, 429 (1993).
- Certain oxabispidine compounds are disclosed as chemical curiosities in Chem. Ber., 96, 2872 (1963).
- the use of certain other oxabispidine compounds in the treatment of cardiac arrhythmias is disclosed in WO 01/28992.
- Methods for the preparation of such oxabispidine compounds are disclosed in WO 02/28863, WO 02/28864, WO 02/83690 and WO 02/83691.
- acid addition salts that are useful in such methods of preparation are disclosed in WO 04/035592.
- oxabispidine-based compounds comprising an amide-substituted alkyl group exhibit unexpectedly beneficial properties that render them particularly suitable for use in the treatment of cardiac arrhythmias.
- R 1 represents C 1-12 alkyl (which alkyl group is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het 1 , -N(R 5a )R 6 , -C(O)R 5b , -OR 5c , -C(O)-E-R 7 , -C(O)N(R 8a )R 5d , -OC(O)N(R 8b )R 5e , -S(O) 2 R 9a , -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ) or R 1 represents -C(O)-E-R 7 , -C(O)N(R 8a )R 5d or -S(O) 2 R 9a ;
- R >5a a represents H or C 1-6 alkyl (which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d );
- R 5b to R 5e independently represent, at each occurrence when used herein, H, C 1-6 alkyl (which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, Het 2 , -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ), aryl or Het 3 , or R 5d or R 5e , together with, respectively, R 8a or R 8b , may represent C 3-6 alkylene (which alkylene group is optionally interrupted by an O atom and/or is optionally substituted by one or more C 1-3 alkyl groups);
- R 6 represents H, C 1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ), aryl,
- R IOa , R 1Ob , R 1Oc and R 1Od independently represent C 1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl), aryl, or R 1Oa represents H;
- R represents, at each occurrence when used herein, C 1-12 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -6 alkoxy, Het 4 , -S(O) 2 N(R 9 y b D ⁇ )Ro 9c >9ck
- R and R independently represent H, C 1-12 alkyl, C 1-6 alkoxy (which latter two groups are optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, C 1-4 alkyl, C 1-4 alkoxy, -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ), -D-aryl, -D-aryloxy, -D-Het 5 , -D-N(H)C(O)R Ua , -D-S(O) 2 R 12a , -D-C(O)R llb , -D-C(O)OR 12b , -D-C(O)N(R llc )R lld , or R 8a or R 8b , together with, respectively,
- R 12a and R 12b independently represent C 1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl) or aryl;
- D represents, at each occurrence when used herein, a direct bond or C 1-6 alkylene;
- E represents O or S;
- R 9a represents, at each occurrence when used herein, C 1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ) or aryl;
- R 9b represents, at each occurrence when used herein, H or C 1-6 alkyl;
- R 9c and R 9d independently represent, at each occurrence when used herein, C 1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl and Het 6 ), aryl or Het 7 , or R 9c represents H;
- Het 1 to Het 7 independently represent five- to twelve-membered heterocyclic groups containing one or more heteroatoms selected from oxygen, nitrogen and/or sulfur, which groups are optionally substituted by one or more groups selected from -OH, oxo, halo, cyano, nitro, C 1-6 alkyl, C 1-6 alkoxy, aryl, aryloxy, -N(R 13a )R 13b , -C(O)R 13c , -C(O)OR 13d , -C(O)N(R 13e )R 13f , -N(R 13g )C(O)R 13h , -S(O) 2 N(R 13i )R 13j and -N(R 13k )S(O) 2 R 13m ; R 13a to R 13m independently represent C 1-6 alkyl, aryl or R 13a to R 13k independently represent H; R 2 and R 3 independently represent H, F or C 1-3 alkyl;
- A represents a direct bond or C 1-3 alkylene optionally substituted by one or more groups selected from F and C 1-3 alkyl;
- C 1-3 alkylene optionally substituted by one or more groups selected from F and C 1-3 alkyl or when X represents a direct bond
- B may alternatively, together with R 18 , represent a structural fragment of formula Ia
- J represents a direct bond or Ci -3 alkylene
- R 15a to R 15d indepe mnedent ly represent H or C 1-3 alkyl or, when the dashed line represents a bond, R 15a is absent;
- R 14a represents H or C 1-6 alkyl
- R 14b represents H, Ci -6 alkyl or, together with R 18 , R 14b may alternatively represent a direct bond or -C(O)-;
- R 4 represents Ci -6 alkyl or a structural fragment of formula Ib, in which the wavy line indicates the position of attachment of the fragment;
- Cu alkylene optionally substituted by one or more groups selected from F and C 1-3 alkyl, or
- n 2 or 3 or, when Z represents -C(0)-N(R 14 )-B-, then n may alternatively represent 1 ;
- X I to X 4 independently represent N or C(R 17 ) and X 5 represents N or C(R 18 ), provided that at least one of X 1 to X 5 is other than N;
- R 17 and R 18 independently represent H, -OH, cyano, halo, nitro, C 1-6 alkyl (optionally terminated by -N(H)C(0)0R 19a ), C 1-6 alkoxy, -N(R 20a )R 20b , -C(O)R 20c , -C(O)OR 20d , -C(O)N(R 20e )R 20f , -N(R 20g )C(O)R 20h , -N(R 20i )C(O)N(R 20j )R 20k , -N(R 20m )S(O) 2 R 19b , -S(O) 2 N(R 20n )R 20p , -S(O) 2 R 19c , -OS(O) 2 R 19d , -Si(R 19e ) 3 and aryl or R 18
- (b) together with R 14b may alternatively represent a direct bond or -C(O)-;
- R 19a to R 19e represent, independently at each occurrence, Ci -6 alkyl or phenyl, which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, C t-4 alkyl and C 1-4 alkoxy;
- R 2Oa and R 20b independently represent H, C 1-6 alkyl or together represent C 3-6 alkylene, resulting in a four- to seven-membered nitrogen-containing ring;
- R 2Oc to R 20p independently represent H or Ci -6 alkyl;
- R 41 to R 46 independently represent H or C 1 ⁇ alkyl
- alkyl groups and alkoxy groups as defined herein may be straight-chain or, when there is a sufficient number (i.e. a minimum of three) of carbon atoms be branched-chain, and/or cyclic. Further, when there is a sufficient number (i.e. a minimum of four) of carbon atoms, such alkyl and alkoxy groups may also be part cyclic/acyclic. Such alkyl and alkoxy groups may also be saturated or, when there is a sufficient number (i.e. a minimum of two) of carbon atoms, be unsaturated and/or interrupted by one or more oxygen and/or sulfur atoms. Unless otherwise specified, alkyl and alkoxy groups may also be substituted by one or more halo, and especially fluoro, atoms.
- alkylene groups as defined herein may be straight- chain or, when there is a sufficient number (i.e. a minimum of two) of carbon atoms, be branched-chain. Such alkylene chains may also be saturated or, when there is a sufficient number (i.e. a minimum of two) of carbon atoms, be unsaturated and/or interrupted by one or more oxygen and/or sulfur atoms. Unless otherwise specified, alkylene groups may also be substituted by one or more halo atoms.
- aryl when used herein, includes C 6- io aryl groups such as phenyl, naphthyl and the like.
- aryloxy when used herein includes C 6-1O aryloxy groups such as phenoxy, naphthoxy and the like. For the avoidance of doubt, aryloxy groups referred to herein are attached to the rest of the molecule via the O-atom of the oxy-group.
- aryl and aryloxy groups may be substituted by one or more groups including -OH, halo, cyano, nitro, C 1-6 alkyl, C 1-6 alkoxy, -N(R 20a )R 20b , -C(O)R 20c , -C(O)OR 20d , -C(O)N(R 20e )R 20f , -N(R 20g )C(O)R 20h , -N(R 20m )S(O) 2 R 19b , -S(O) 2 N(R 20a )R 20p , -S(O) 2 R 190 , -OS(O) 2 R 19d and/or -Si(R 19e ) 3 (wherein R 19b to R 19e and R 20a to R 20P are as hereinbefore defined).
- aryl groups are preferably substituted by between one and three substituents.
- halo when used herein, includes fluoro, chloro, bromo and iodo.
- Heterocyclic (Het 1 to Het 7 ) groups that may be mentioned include those containing 1 to 4 heteroatoms (selected from the group oxygen, nitrogen and/or sulfur) and in which the total number of atoms in the ring system are between five and twelve. Heterocyclic (Het 1 to Het 7 ) groups may be fully saturated, partly unsaturated, wholly aromatic or partly aromatic in character.
- Het 1 , Het 2 and Het a are examples of heterocyclic (Het 1 , Het 2 and Het a ) groups that may be mentioned include l-azabicyclo[2.2.2]octanyl, benzimidazolyl, benzo[c]isoxazolidinyl, benzisoxazolyl, benzodioxanyl, benzodioxepanyl, benzodioxolyl, benzofuranyl, benzofurazanyl, benzomorpholinyl, 2,1,3-benzoxadiazolyl, benzoxazolidinyl, benzoxazolyl, benzopyrazolyl, benzo[e]pyrimidine, 2,1,3-benzothiadiazolyl, benzothiazolyl, benzothienyl, benzotriazolyl, chromanyl, chromenyl, cinnolinyl, 2,3-dihydrobenzimidazolyl, 2,3-d
- Het 1 Values of Het 1 that may be mentioned include benzodioxanyl (e.g. benzo-1,3- dioxanyl or benzo-l,4-dioxanyl), benzodioxolyl, benzisoxazolyl, benzofuranyl, 2,3-dihydrobenzo[&]furanyl, imidazo[l,2- ⁇ 2]pyridinyl, indolyl, isoxazolyl, pyrazolyl, pyridinyl and quinolinyl.
- benzodioxanyl e.g. benzo-1,3- dioxanyl or benzo-l,4-dioxanyl
- benzodioxolyl e.g. benzo-1,3- dioxanyl or benzo-l,4-dioxanyl
- benzodioxolyl e.g. benzo-1,3- dioxanyl or benzo-
- heterocyclic (Het 1 to Het 7 ) groups may, where appropriate, be located on any atom in the ring system including a heteroatom.
- the point of attachment of heterocyclic (Het 1 to Het 7 ) groups may be via any atom in the ring system including (where appropriate) a heteroatom, or an atom on any fused carbocyclic ring that may be present as part of the ring system.
- Heterocyclic (Het 1 to Het 7 ) groups may also be in the N- or S-oxidised form.
- Pharmaceutically acceptable derivatives include salts and solvates. Salts which may be mentioned include acid addition salts. Pharmaceutically acceptable derivatives also include, at the oxabispidine or other heterocyclic nitrogen atoms, C 1-4 alkyl quaternary ammonium salts and iV-oxides.
- the compounds of the invention may exhibit tautomerism. All tautomeric forms and mixtures thereof are included within the scope of the invention.
- the compounds of the invention may also contain one or more asymmetric carbon atoms and may therefore exist as enantiomers or diastereoisomers, and may exhibit optical activity.
- Diastereoisomers may be separated using conventional techniques, e.g. chromatography or fractional crystallisation.
- the various stereoisomers may be isolated by separation of a racemic or other mixture of the compounds using conventional, e.g. fractional crystallisation or HPLC, techniques.
- the desired optical isomers may be made by reaction of the appropriate optically active starting materials under conditions which will not cause racemisation or epimerisation, or by derivatisation, for example with a homochiral acid followed by separation of the diastereomeric esters by conventional means (e.g. HPLC, chromatography over silica). All stereoisomers are included within the scope of the invention. Abbreviations are listed at the end of this specification.
- R 1 does not represent C 1-12 alkyl, which alkyl group is optionally substituted by one or more groups including one -N(R 5a )C(O)OR 10b group.
- compounds of the invention that may be mentioned include those in which:
- R 1 represents (a) C 1-5 alkyl terminated by a group selected from aryl, Het 1 , -N(R 5a )R 6 ,
- R 5a and R 5d independently represent H or methyl;
- R 5b represents C 1-5 alkyl;
- R 5c represents aryl or Q. 3 alkyl substituted by aryl
- R 6 represents -C(O)OR 10b ;
- R 1Ob represents C 1-5 alkyl
- R 7 represents C 1-5 alkyl
- R 8a represents C 1-5 alkyl or -D-phenyl (the phenyl part of which latter group is optionally substituted by one or more (e.g. one to three) groups selected from F and cyano);
- D represents C 1-3 alkylene
- Het 1 represents a heterocyclic group that is (a) five- or six-membered and aromatic, or
- heterocyclic group contains one or more (e.g. one to three) heteroatoms selected from oxygen, nitrogen and/or sulfur (e.g. selected from oxygen and nitrogen), which group is are optionally substituted by one or more groups selected from halo (e.g. F or Cl), cyano, C 1-3 alkyl (e.g. methyl) and C 1-3 alkoxy
- R and R both represent H; Z represents
- B represents C 1-S n-alkylene
- R 14a and R 14b independently represent H or Ci -3 alkyl (e.g. methyl);
- R 4 represents C 1- S alkyl (e.g. tert-butyl) or, particularly, a structural fragment of formula Ib, as defined above;
- X represents a direct bond (e.g. when Z represents -C(O)-N(R 14b )-B-), Ci- 3 /z-alkylene optionally substituted by one or more (e.g. one or two) methyl groups, or
- R 16a and R 16b both represent H; n represents 3 or, particularly, 2; X 1 to X 4 all represent C(R 17 ); X 5 represents C(R 18 );
- R and R independently represent H, cyano or halo (e.g. Cl or, particularly, F), provided that no more than three of X 1 to X 5 represent other than C(H);
- R 41 to R 46 all represent H;
- aryl represents naphthyl or, particularly, phenyl, which aryl group is optionally substituted by one or more (e.g. one to three) groups selected from halo (e.g. F or Cl), cyano, C 1-3 alkyl, C 1-3 alkoxy (which latter two groups are optionally substituted by one or more F atoms), -OS(O) 2 R 19d and -Si(CH 3 ) 3
- R 19d represents C 1-3 alkyl (e.g. methyl) or, particularly, phenyl, which latter group is optionally substituted by one or more (e.g. one to three) groups selected from methyl and halo (e.g. Cl).
- compounds of the invention that may be mentioned include those that either (a) are or (b) are not of the formula Ic, wherein R 1 , R 14a and R 41 to R 46 are as hereinbefore defined and:
- R 17a represents one or more substituents on the phenyl ring, each substituent taking the same definition as R 17 above.
- R 14a represents methyl or, particularly, H
- X a represents O(CH 2 ) 2 (in which group the O-atom is attached to the phenyl ring),
- R a represents H or one to three substituents (e.g. three substituents at the 2-, 4- and 6-positions, two substituents at the 2- and 4-positions or one substituent at the 2-, 3- or, particularly, 4-position) selected from halo (e.g. F) and CN.
- substituents e.g. three substituents at the 2-, 4- and 6-positions, two substituents at the 2- and 4-positions or one substituent at the 2-, 3- or, particularly, 4-position
- halo e.g. F
- X a represents O(CH 2 ) 2 (in which group the O-atom is attached to the phenyl ring), C(CH 3 ) 2 , or, particularly, CH 2 ; the substitution pattern on the phenyl ring is as follows
- R 17al represents H, F or, particularly, cyano
- R 17a2 and R 17a3 independently represent F or, particularly, H;
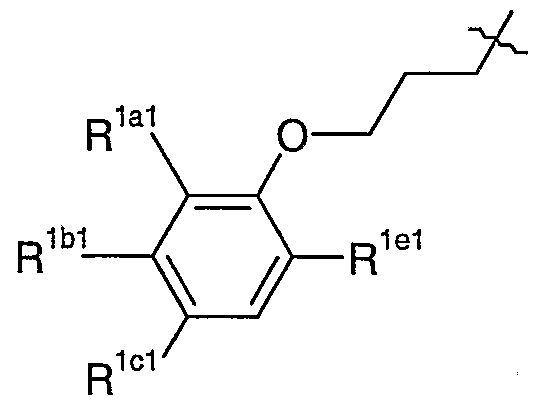
- R 1 represents a structural fragment of the formula
- Q 3 represents a direct bond, CH 2 or OCH 2 (in which latter group the O- atom is attached to the phenyl ring) and
- R la to R le independently represent H, halo (e.g. F or Cl), cyano or C 1-2 alkoxy (which latter group is optionally substituted by one or more halo (e.g. F) atoms), provided that at least two of R la to R le represent H.
- halo e.g. F or Cl
- cyano or C 1-2 alkoxy which latter group is optionally substituted by one or more halo (e.g. F) atoms
- Still further compounds of formula Ic include those in which: X a represents C(CH 3 ) 2 (e.g. when R lcl represents cyano), O(CH 2 ) 2 (in which group the O-atom is attached to the phenyl ring) or, particularly, CH 2 ; R 17al represents cyano or, when X a represents C(CHs) 2 , R 17al may alternatively represent H; R 17a2 represent F or, particularly, H; R 17a3 represents H; R 1 represents a structural fragment of the formula wherein R lal to R lel and R la2 to R Ic2 take the same definitions as R la to R le above or, in particular
- R lal represents H or, particularly, F,
- R , lbl and R > lel independently represent F or, particularly, H,
- R , lc c l represents Cl, cyano or, particularly, F,
- R , la2 represents F or, particularly, H,
- R , 162 represents methoxy or, particularly, H,
- R , lc c l represents Cl, F, methoxy or, particularly, H and
- Q 1 represents a direct bond or, particularly, CH 2 .
- compounds of the invention that may be mentioned include those that either (a) are or (b) are not of the formula Id,
- R 1 , R 41 to R 46 and X are as hereinbefore defined and:
- R 14bl represents H or C 1-6 alkyl
- R 17b represents one or more substituents on the phenyl ring, each substituent taking the same definition as R 17 above.
- Compounds of formula Id are hereinafter also referred to as "the compounds of the invention”. Further, for the avoidance of doubt, the specific definitions of groups mentioned herein in relation to compounds of formula I are also, where relevant, specific definitions of the equivalent groups in compounds of formulae Id (and vice versa).
- B represents (CHb) 2 or, particularly, CH 2 ;
- R 14bl represents H or, particularly, methyl;
- X represents (CH 2 ) 2 , CEfe or a direct bond
- R 17b represents H or one to three substituents (e.g. two substituents at the 2- and 4-or
- X represents (CH 2 ) 2 or, particularly, a direct bond; the substitution pattern on the phenyl ring is as follows
- R represents H, F or, particularly (e.g. when R represents H), cyano, and
- R 17b2 represents F (e.g. when R 17bl represents F) or, particularly, H; R 1 represents a structural fragment of the formula
- Q 2 represents Ci -3 alkylene (e.g. CH 2 ) and R lf represents one to three (e.g. one or two) substituents selected from C 1-2 alkoxy (e.g. methoxy) optionally substituted by one or more (e.g. two) F atoms.
- R represents methyl
- X represents a direct bond
- R I7bI represents cyano
- R 17b2 represents H
- Q 2 represents CH 2
- R lf represents one substituent at the 2- or, particularly, 3 -position (relative to the point of attachment of Q 2 ) or two substituents at the 3- and 5-positions (relative to the point of attachment
- each R lf substituent represents methoxy or (e.g. when only one R lf substituent is present) fiuoro-substituted methoxy (such as OCHF 2 ).
- compounds of the invention that may be mentioned include those that either (a) are or (b) are not of the formula Ie,
- R 1 , R 14a and R 41 to R are as hereinbefore defined.
- R 1 represents a structural fragment of the formula
- Q represents O or OCH 2 (in which latter group the 0-atom is attached to the phenyl ring),
- R Ig and R Ih indepi ndently represent H or F; R 14a represents H.
- R 1 and R 41 to R 46 are as hereinbefore defined and in which the left hand side-chain (LHS) represents
- Particular compounds of the invention include: (a) compounds (i) to (cclvi) above, other than compounds (ccxxv) to (ccl) above; (b) compounds (xxiv), (xxix), (xli), (xliv), (lxix), (lxxiii), (lxxxvii) to (lxxxix), (xcviii), (cii), (cviii), (ex), (cxxxi), (cxlv), (clii), (cc) and (ccli) to (cclvi) above; or
- L 1 represents a leaving group such as halo, alkanesulfonate, perfluoroalkanesulfonate, arenesulfonate, -OC(O)-E-R 7 , imidazole or R 21 O- (wherein R 21 represents, for example, C 1-1 Q alkyl or aryl, which groups are optionally substituted by one or more halo or nitro groups) and X, R 1 and R 7 are as hereinbefore defined, for example at between ambient temperature (e.g. 25°C) and reflux temperature in the presence of a suitable base (e.g.
- an appropriate solvent e.g. dichloromethane, chloroform, acetonitrile, iVjV-dimethylformamide, THF, toluene, water, a lower alkyl alcohol (e.g. ethanol) or mixtures thereof;
- L represents a leaving group such as halo, alkanesulfonate (e.g. mesylate), perfluoroalkanesulfonate or arenesulfonate (e.g. 2- or 4-nitrobenzenesvufonate, toluenesulfonate or benzenesulfonate) and R 2 , R 3 , R 4 and Z are as hereinbefore defined, for example at elevated temperature (e.g. between 35°C and reflux temperature) in the presence of a suitable base (e.g. triethylamine or potassium carbonate) and an appropriate organic solvent (e.g.
- a suitable base e.g. triethylamine or potassium carbonate
- an appropriate organic solvent e.g.
- R 4 -C(O)-L 3 Vn wherein L 3 represents a leaving group, such as halo, OH, imidazole or R 21 O-, and R 4 and R 21 are as hereinbefore defined, for example under conditions known to those skilled in the art (such as in the presence of an appropriate base (e.g. pyridine, DMAP, TEA, 2,4,6-collidine or DIPEA) and a suitable organic solvent (e.g. dichloromethane, acetonitrile, EtOAc or DMF) and optionally in the presence of a coupling agent (e.g. oxalyl chloride in DMF, EDC, DCC, HBTU, HATU, PyBOP or TBTU, optionally in combination with an alcohol such as HOBT));
- an appropriate base e.g. pyridine, DMAP, TEA, 2,4,6-collidine or DIPEA
- a suitable organic solvent e.g. dichloromethane, aceton
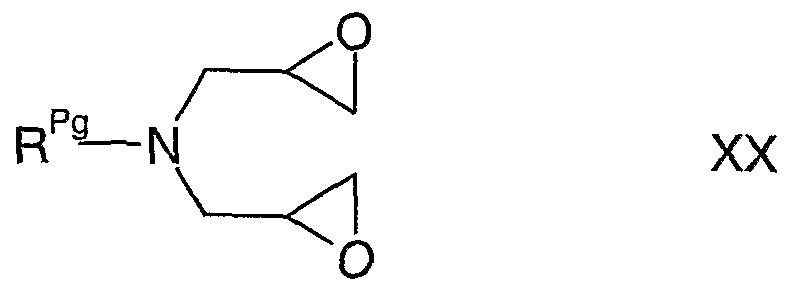
- R 1 represents C 1-12 alkyl (which alkyl group is attached to the oxabispidine iV-atom via a CH 2 group and is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het 1 , -0R 5c , -C(O)-E-R 7 , -C(O)N(R 8a )R 5d , -OC(O)N(R 8b )R 5e , -S(O) 2 R 9a , -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ), reaction of a compound of formula II, as hereinbefore defined, with a compound of formula VHI,
- R 1X -CHO vm wherein R lx represents aryl, Het 1 or C 1-U alkyl (which alkyl group is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het 1 , -0R 5c , -C(O)-E-R 7 , -C(O)N(R 8a )R 5d , -OC(O)N(R 8b )R 5e , -S(O) 2 R 9a , -S(O) 2 N(R 9b )R 9c and -N(R 9b )S(O) 2 R 9d ), and R 5c to R 5e , R 7 , R 8a , R 8b , R 9a to R 9d , E and Het 1 are as hereinbefore defined, for example under conditions known to those skilled in the art (such as at from ambient temperature (e.g.
- a suitable solvent e.g. THF, dichloromethane or a C 1-3 alkyl alcohol
- a catalyst such as a base (e.g. a tertiary amine such as triethylamine) or a Lewis acid or proton source (e.g. a carboxylic acid such as acetic acid)
- a reducing agent e.g. NaB(OAc) 3 H or NaBH 3 CN
- a suitable solvent e.g. THF, dichloromethane or a C 1-3 alkyl alcohol
- Z a represents -N(R 14a )-C(O)-A a - or -C(O)-N(R l4b )-B-
- a a represents C 1-3 alkylene (optionally substituted by one or more groups selected from F and C 1-3 alkyl)
- R 4 , R 14a , R 14b and B are as hereinbefore defined, for example under conditions known to those skilled in the art (such as those described in respect of process (d) above), followed by reduction in the presence of a reducing agent (e.g.
- a suitable strong base such as an alkali metal hydride (e.g. NaH), an alkali metal alkoxide (e.g. potassium te/t-butoxide) or an alkali metal amide (e.g. IJDA or, particularly, LiHMDS)
- an appropriate solvent e.g. THF
- R g represents an amino protective group (e;g. benzyl or benzenesulfonyl) and R 1 and R 41 to R 46 are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. when R Pg represents benzyl, catalytic hydrogenation, or, when R ? ⁇ represents benzenesulfonyl, hydrolysis in the presence of a strong acid such as hydrobromic or concentrated sulfuric acid (e.g. about 80% sulfuric acid at about 13O 0 C)).
- a strong acid such as hydrobromic or concentrated sulfuric acid (e.g. about 80% sulfuric acid at about 13O 0 C)).
- R 4 -N(H)R 14a XIII wherein R 4 and R l4a are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. those described in relation to process (c) above).
- B a represents a direct bond or C 1-2 alkylene optionally substituted by one or more groups selected from F and C 1-3 alkyl
- R 1 to R 3 and R 41 to R 46 are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. catalytic hydrogentation, i.e. reaction with hydrogen gas in the presence of a suitable catalyst (e.g. a platinum group metal optionally supported on a substrate such as a carbon black, particularly palladium on carbon or Raney- Nickel ® ) and an appropriate solvent (e.g. a C 1-4 alcohol, such as ethanol or, particularly, methanol), and optionally in the presence of ammonia).
- a suitable catalyst e.g. a platinum group metal optionally supported on a substrate such as a carbon black, particularly palladium on carbon or Raney- Nickel ®
- an appropriate solvent e.g. a C 1-4 alcohol, such as ethanol or, particularly, methanol
- Compounds of formula IX may be prepared by oxidation of a corresponding compound of formula XV, wherein R 4 and Z a are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. Swern oxidation conditions, such as reaction, at from -80 to -60 0 C, in the presence of a mixture of oxalyl chloride and DMSO, a suitable base (e.g. a tertiary amine such as TEA) and an appropriate solvent (e.g. DCM)).
- Swern oxidation conditions such as reaction, at from -80 to -60 0 C
- a suitable base e.g. a tertiary amine such as TEA
- an appropriate solvent e.g. DCM
- R 1 and R Pg are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. reaction in the presence of a suitable dehydrating reagent, such as sulfuric acid (e.g. concentrated sulfuric acid) or a sulfonic acid (e.g. an alkane or perfluoroalkanesulfonic acid, such as methanesulfonic acid, including anhydrous methanesulfonic acid)).
- a suitable dehydrating reagent such as sulfuric acid (e.g. concentrated sulfuric acid) or a sulfonic acid (e.g. an alkane or perfluoroalkanesulfonic acid, such as methanesulfonic acid, including anhydrous methanesulfonic acid)).
- compounds of formula XI in which R Pg represents an acid-labile protective group may be converted in situ (i.e. in a "one- pot" process starting with a compound of formula XVI), by use of concentrated (e.g. about 80%) sulfuric acid at elevated temperature (e.g. about 130°C), to a corresponding compound of formula IV.
- the compounds of formulae XI and XVI When reacted with sulfuric acid, the compounds of formulae XI and XVI may either be added to sulfuric acid or vice versa.
- Compounds of formula XIV may be prepared by reaction of a corresponding compound of formula IV, as hereinbefore defined, with a compound of formula XVII, NC-B a -C(R 2 )(R 3 )-L 2 XVII wherein R 2 , R 3 , B a and L 2 are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. those described in respect of process (b) above).
- R 1 is as hereinbefore defined, with a compound of formula XXIII, R Pg -NH 2 XXIII wherein R Pg is as hereinbefore defined, in either case under conditions known to those skilled in the art (e.g. by reaction at between ambient (e.g. 25°C) and reflux temperature in the presence of a C 1-4 alkyl alcohol (e.g. ethanol, IMS or methanol)).
- a C 1-4 alkyl alcohol e.g. ethanol, IMS or methanol
- Reaction may, in one embodiment, be conducted such that the compounds of formula XX and XXI (or, alternatively, the compounds of formulae XXII and XXIII) are added, separately, simultaneously and at a substantially equivalent rate of moles per minute, to a reaction vessel containing solvent.
- L 4 represents a leaving group (e.g. arenesulfonate, perfluoroalkanesulfonate or alkanesulfonate (such as ⁇ -toluenesulfonate, 2- or 4-nitrobenzenesulfonate, methanesulfonate, benzenesulfonate or trifluoromethanesulfonate) or, particularly, halo) with:
- compounds of formulae XX and XXII are prepared by reaction of either a compound of formula XXIII or a compound of formula XXI with at least two equivalents of a compound of formula XXIV and at least two equivalents of base under conditions where the reaction is performed by addition of base to an aqueous mixture of the compounds of formulae XXIII and XXIV (or, alternatively, compounds of formulae XXI and XXIV) and the rate of base addition is controlled so as to maintain, during the addition of base, the reaction pH within a certain range (e.g. between pH 10 and pH 13) for as long as possible (e.g. at least 5 times longer within said range than outside of it).
- a certain range e.g. between pH 10 and pH 13
- aryl e.g. phenyl
- heterocyclic, group(s) in compounds defined herein may be converted to other claimed substituents using techniques well known to those skilled in the art. For example, hydroxy may be converted to alkoxy, phenyl may be halogenated to give halophenyl, nitro may be reduced to give amino, halo may be displaced by cyano, etc.
- hydroxy may be either reduced to alkylene or converted to halo.
- the compounds of the invention may be isolated from their reaction mixtures using conventional techniques.
- the functional groups of intermediate compounds may be, or may need to be, protected by protecting groups.
- Functional groups which it is desirable to protect include hydroxy, amino and carboxylic acid.
- Suitable protecting groups for hydroxy include trialkylsilyl and diarylalkylsilyl groups (e.g. fert-butyldimethylsilyl, t ⁇ t-butyldiphenylsilyl or trimethylsilyl), tetrahydropyranyl and alkylcarbonyl groups (e.g. methyl- and ethylcarbonyl groups).
- Suitable protecting groups for phenoxy include alkyl (i.e. ether) groups, such as methyl.
- Suitable protecting groups for amino include benzyl, sulfonamido (e.g. benzenesulfonarnido), tert-butyloxycarbonyl, 9-fluorenyl- methoxycarbonyl or benzyloxycarbonyl.
- Suitable protecting groups for amidino and guanidino include benzyloxycarbonyl.
- Suitable protecting groups for carboxylic acid include C 1-6 alkyl or benzyl esters.
- protection and deprotection of functional groups may take place before or after any of the reaction steps described hereinbefore.
- Protecting groups may be removed in accordance with techniques which are well known to those skilled in the art and as described hereinafter.
- R lal to R lel are as hereinbefore defined, or a protected derivative thereof;
- R lal represents F
- R lbl and R lel both represent H
- R lcl represents Cl, cyano or, particularly, F.
- the compounds defined at (C) above can alternatively be represented as compounds of formulae XXV, XXVI, XXVH and XXVDI,
- R 41 to R 46 , R Pg , R la to R le and Q 4 are as hereinbefore defined, or protected derivatives thereof.
- Q 4 represents unsubstituted C 2-4 alkylene, such as (CH 2 ) 3 or, particularly, (CH 2 ) 2 ;
- R la represents F or, particularly, H;
- R lb represents methoxy or, particularly, H;
- R ld and R le both represent H.
- R 41 to R 46 that may be mentioned in relation to compounds of formulae XXV and XXVI include H.
- R Pg that may be mentioned in relation to compounds of formulae XXVI and XXV ⁇ include benzyl and benzenesulfonyl.
- the compounds of the invention exhibit myocardial electrophysiological activity, for example as demonstrated in the test described below.
- the compounds of the invention are thus expected to be useful in both the prophylaxis and the treatment of arrhythmias, and in particular atrial and ventricular arrhythmias.
- the compounds of the invention are thus indicated in the treatment or prophylaxis of cardiac diseases, or in indications related to cardiac diseases, in which arrhythmias are believed to play a major role, including ischaemic heart disease, sudden heart attack, myocardial infarction, heart failure, cardiac surgery and thromboembolic events.
- a method of treatment of an arrhythmia which method comprises administration of a therapeutically effective amount of a compound of the invention to a person suffering from, or susceptible to, such a condition.
- the compounds of the invention will normally be administered orally, subcutaneously, intravenously, intraarterially, transdermally, intranasally, by inhalation, or by any other parenteral route, in the form of pharmaceutical preparations comprising the active ingredient either as a free base or a non-toxic organic or inorganic acid addition salt, in a pharmaceutically acceptable dosage form.
- the compositions may be administered at varying doses.
- a pharmaceutical formulation including a compound of the invention in admixture with a pharmaceutically acceptable adjuvant, diluent or carrier.
- Suitable daily doses of the compounds of the invention in therapeutic treatment of humans are about 0.005 to 50.0 mg/kg body weight at oral administration and about 0.005 to 15.0 mg/kg body weight at parenteral administration.
- Preferable ranges of daily doses of the compounds of the invention in therapeutic treatment of humans are about 0.005 to 20.0 mg/kg body weight at oral administration and about 0.005 to 10.0 mg/kg body weight at parenteral administration.
- the compounds of the invention may also be combined with any other drugs useful in the treatment of arrhythmias and/or other cardiovascular disorders.
- a combination product comprising: (A) a compound of the invention, as hereinbefore defined, or a pharmaceutically acceptable derivative thereof; and
- each of components (A) and (B) is formulated in admixture with a pharmaceutically-acceptable adjuvant, diluent or carrier.
- Such combination products provide for the administration of compounds of the invention in conjunction with the other therapeutic agent, and may thus be presented either as separate formulations, wherein at least one of those formulations comprises a compound of the invention and at least one comprises the other therapeutic agent, or may be presented (i.e. formulated) as a combined preparation (i.e. presented as a single formulation including a compound of the invention and the other therapeutic agent).
- a pharmaceutical formulation including a compound of the invention, as hereinbefore defined, or a pharmaceutically acceptable derivative thereof, an anticoagulant, and a pharmaceutically-acceptable adjuvant, diluent or carrier;
- a pharmaceutical formulation including an anticoagulant with a pharmaceutically-acceptable adjuvant, diluent or carrier, which components (a) and (b) are each provided in a form that is suitable for administration in conjunction with the other.
- an anticoagulant includes references to one a substance selected from the group consisting of aspirin, warfarin, enoxaparin, heparin, low molecular weight heparin, cilostazol, clopidogrel, ticlopidine, tirofiban, abciximab, dipyridamole, plasma protein fraction, human albumin, low molecular weight dextran, hetastarch, reteplase, alteplase, streptokinase, urokinase, dalteparin, filgrastin, immunoglogulin, ginkolide B, hirudins, foropafant, rocepafant, bivalirudin, dermatan sulfate mediolanum, eptilibatide, tirofiban, thrombomodulin, abcxmab, low molecular weight dermatan sulfate- opocrin
- anticoagulants include aspirin and warfarin.
- an anticoagulant' also includes references to thrombin inhibitors.
- thrombin inhibitors that may be mentioned include low molecular weight thrombin inhibitors.
- the term ''low molecular weight thrombin inhibitors will be understood by those skilled in the art, and includes' references to any composition of matter (e.g. chemical compound) that inhibits thrombin to an experimentally determinable degree (as determined by in vivo and/or in vitro tests), and which possesses a molecular weight of below about 2,000, preferably below about 1,000.
- Preferred low molecular weight thrombin inhibitors include low molecular weight peptide-based, amino acid-based, and/or peptide analogue-based, thrombin inhibitors, as well as derivatives thereof.
- the term "low molecular weight peptide-based, amino acid-based, and/or peptide analogue-based, thrombin inhibitors" will be well understood by one skilled in the art to include references to low molecular weight thrombin inhibitors with one to four peptide linkages, and includes those described in the review paper by Claesson in Blood Coagul. Fibrin.
- derivatives of thrombin inhibitors include chemical modifications, such as esters, prodrugs and metabolites, whether active or inactive, and pharmaceutically acceptable salts and solvates, such as hydrates, of any of these, and solvates of any such salt.
- Preferred low molecular weight peptide-based thrombin inhibitors include those known collectively as the "gatrans". Particular gatrans which may be mentioned include HOOC-CH 2 -(R)Cha-Pic-Nag-H (known as inogatran) and HOOC-CH 2 - (R)Cgl-Aze-Pab-H (known as melagatran) (see International Patent Application WO 93/11152 and WO 94/29336, respectively, and the lists of abbreviations contained therein). International Patent Application WO 97/23499 discloses a number of compounds which have been found to be useful as prodrugs of thrombin inhibitors. Said prodrugs have the general formula
- R a OOC-CH 2 -(R)Cgl-Aze-Pab-R b wherein R a represents H, benzyl or C 1-1O alkyl, R b (which replaces one of the hydrogen atoms in the amidino unit of Pab-H) represents OH, OC(O)R 0 or C(O)OR d , R c represents C 1-17 alkyl, phenyl or 2-naphthyl and R d represents C 1-12 alkyl, phenyl, C 1-3 alkylphenyl, or 2-naphthyl.
- Preferred compounds include R a OOC-CH 2 -(R)Cgl-Aze-Pab-OH, wherein R a represents benzyl or C 1-10 alkyl, e.g. ethyl or isopropyl, especially EtOOC-CH 2 -(R)Cgl-Aze-Pab-OH.
- R a represents benzyl or C 1-10 alkyl, e.g. ethyl or isopropyl, especially EtOOC-CH 2 -(R)Cgl-Aze-Pab-OH.
- the active thrombin inhibitors themselves are disclosed in WO 94/29336.
- thrombin inhibitors include those disclosed in WO 02/44145, such as compounds of the following general formula,
- R° represents -OH or -CH 2 OH;
- R 1 represents at least one optional halo substituent;
- R 2 represents one or two C 1-3 alkoxy substituents, the alkyl parts of which substituents are themselves substituted with one or more fluoro substituents (i.e. R 2 represents one or two fluoroalkoxy(C 1-3 ) groups);
- Y represents -CH 2 - or -(CH 2 ) 2 -; and
- R 3 represents a structural fragment of formula I(i) or I(ii):
- R j4 represents H or one or more fluoro substituents
- R 5 represents H, OR 6 or C(O)OR 7
- R 6 represents H, C 1-1 O alkyl, C 1-3 alkylaryl or C 1-3 alkyloxyaryl (the alkyl parts of which latter two groups are optionally interrupted by one or more oxygen atoms, and the aryl parts of which latter two groups are optionally substituted by one or more substituents selected from halo, phenyl, methyl or methoxy, which latter three groups are also optionally substituted by one or more halo substituents);
- R 7 represents Ci -1O alkyl (which latter group is optionally interrupted by one or more oxygen atoms), or C 1-3 alkylaryl or C 1 - 3 alkyloxyaryl (the alkyl parts of which latter two groups are optionally interrupted by one or more oxygen atoms, and the aryl parts of which latter two groups are optionally substituted by one or more substituents selected from halo, phen
- R >2 ⁇ r. e presents -OCHF 2 , -OCF 3 , -OCH 2 CH 2 F or -OCH 2 CHF 2 ;
- R 5 represents H or OR 6 ; and
- R 6 represents methyl, ethyl, rc-propyl, /-propyl or cyclobutyl.
- the compounds of the invention have the advantage that they are effective against cardiac arrhythmias.
- Compounds of the invention have advantageous properties compared to compounds of the prior art, in particular enhanced potency, enhanced selectivity, and/or reduction of total clearance. These advantages may provide for corresponding useful properties in practice.
- compounds of the present invention when used as pharmaceutical agents, may have a lower daily clinical dose, longer duration of action, and/or an improved side effect profile.
- Compounds of the invention may also have the advantage that they may be more efficacious than, be less toxic than, have a broader range of activity (including exhibiting any combination of class I, class II, class III and/or class IV activity (especially class I and/or class IV activity in addition to class III activity)) than, be more potent than, be longer acting than, produce fewer side effects (including a lower incidence of proarrhythmias such as torsades de pointes) than, be more easily absorbed than, or that they may have other useful pharmacological properties over, compounds known in the prior art.
- Anaesthesia was induced by an intraperitoneal injection of pentobarbital (50 to 60 mg/kg) and catheters were introduced into one carotid artery (for blood pressure recording and blood sampling) and into one jugular vein (for drug infusions). Needle electrodes were placed on the limbs for recording, of ECGs (lead II). A thermistor was placed in the rectum and the animal was placed on a heating pad, set to a rectal temperature of between 37.5 and 38.5°C.
- a tracheotomy was performed and the animal was artificially ventilated with room air by use of a small animal ventilator, set to keep blood gases within the normal range for the species.
- a small animal ventilator set to keep blood gases within the normal range for the species.
- both vagi were cut in the neck, and 0.5 mg/kg of propranolol was given intravenously, 15 minutes before the start of the experiment.
- the left ventricular epicardium was exposed by a left-sided thoracotomy, and a custom-designed suction electrode for recording of the rnonophasic action potential (MAP) was applied to the left ventricular free wall.
- the electrode was kept in position as long as an acceptable signal could be recorded, otherwise it was moved to a new position.
- a bipolar electrode for pacing was clipped to the left atrium. Pacing (1 ms duration, twice the diastolic threshold) was performed with a custom- made constant current stimulator. The heart was paced at a frequency just above the spontaneous sinus rate during 30 s every fifth minute throughout the study.
- the MAP signal, the blood pressure signal and the lead II ECG were collected (the sampling frequency was 1000 Hz and each sampling period 10 s) on a personal computer during the last 10 s of each 30 s pacing sequence and the last 10 s of the following min of sinus rhythm.
- the signals were processed using a custom- designed computer program (PharmLab v 4.0).
- test procedure consisted of two basal control recordings, 3 minutes apart, during both pacing and sinus rhythm. After the second control recording, the first dose of the test substance was infused in a volume of 0.2 mL/kg into the jugular vein catheter for 30 seconds. Three minutes later, pacing was started and a new recording was made. Five minutes after the previous dose, the next dose of test substance was administered. Six to ten consecutive doses were given during each experiment.
- the three variables selected were the MAP duration at 75 percent repolarization during pacing, the atrio-ventricular (AV) conduction time (defined as the interval between the atrial pace pulse and the start of the ventricular MAP) during pacing, and the heart rate (defined as the RR interval during sinus rhythm).
- AV atrio-ventricular
- AV atrio-ventricular
- AV atrio-ventricular
- RR interval the heart rate
- Systolic and diastolic blood pressure were measured in order to judge the haemodynamic status of the anaesthetised animal. Further, the ECG was checked for arrhythmias and/or morphological changes.
- the mean of the two control recordings was set to zero and the effects recorded after consecutive doses of test substance were expressed as percentage changes from this value. By plotting these percentage values- against the cumulative dose administered before each recording, it was possible to construct dose-response curves. In this way, each experiment generated three dose-response curves, one for MAP duration, one for AV-conduction time and one for the sinus frequency (RR interval). A mean curve of all experiments performed with a test substance was calculated, and potency values were derived from the mean curve. All dose- response curves in these experiments were constructed by linear connection of the data points obtained. The cumulative dose prolonging the MAP duration by 10% from the baseline was used as an index to assess the class III electrophysiological potency of the agent under investigation (D 1O ).
- the human ether-a-go-go related gene encodes the voltage-gated K + channel underlying the cardiac rapid delayed rectifier current I ⁇ r -
- the IC50 value for HERG channel blockade was determined using a high throughput functional assay based on depolarisation-induced Rb + -efflux from Chinese hamster ovary cells stably expressing the HERG-channel.
- Cells were grown in Ham F12 (Life Technologies 31765-027) supplemented with 10% FBS and 0.6 mg/mL hygromycin B and were routinely passaged twice- weekly. For experimental studies, cells were plated at a density of 15,000 cells/well in Falcon, 384-well tissue culture-treated black- walled clear-bottomed plates and were thereafter incubated overnight at 37°C in a cell culture incubator.
- Rb + -Load buffer a physiological buffer containing Rb +
- test compounds were added.
- the cell plates were then incubated for another 10 minutes and, following this incubation period, external K + concentration was increased in order to depolarize the cells and activate HERG channels.
- supernatants were transferred to new microplates for subsequent determination of Rb + content, using Atomic Absorption Spectrometry analysis.
- the basal Rb + efflux (content of Rb + (mg/L) in supernatants of wells receiving only wash buffer) was defined as 100% inhibition and the stimulated Rb + efflux (content of Rb + (mg/L) in supernatants of wells exposed only to increased external potassium concentration) was defined as 0% inhibition.
- A Rb + content in wells receiving test compound +increased external K + .
- B Basal Rb + efflux.
- C Stimulated Rb + efflux.
- Buffers containing 10 mM ammonium acetate or 5 mM ammonium formate / 5 mM formic acid were used.
- the mass spectra were recorded using a Waters ZQ2000 equipped with an electrospray or ESCI interface, switching positive and negative ionization mode.
- UV spectra were collected by a Agilent 1100 PDA and the evaporative light scattering (ELS) signal by a Sedere Sedex 55 or 75.
- 1 H NMR and 13 C NMR measurements were performed on a BRUKER ACP 300 and Varian 300, 400, 500 and 600 Mercury, Unity plus and Unity Inova spectrometers, operating at 1 H frequencies of 300, 400, 500 and 600 MHz respectively and at 13 C frequencies of 75.4, 100.6, 125.7 and 150.9 MHz respectively.
- Rotamers may or may not be denoted in spectra depending upon ease of interpretation of spectra. Unless otherwise stated, chemical shifts are given in ppm with the solvent as internal standard.
- 1,3-Propane dithiol (16.59 g, 0.1533 mol) was added to a solution of 4-(azido- methyl)-3-fluorobenzonitrile (9 g, 0.0511 mol; see step (iii) above) and triethylamine (15.48 g, 0.1533 mol) in dry methanol (90 mL). The resulting mixture was stirred for 4 h under a nitrogen atmosphere. The reaction mixture was then filtered and the solvent was concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 3% methanol in DCM as eluent, to give 4.6 g of the sub-title compound as a yellow liquid.
- reaction mixture was stirred at room temperature overnight before being diluted with water and extracted with DCM.
- the combined organic layers were washed with brine and dried over sodium sulfate and then concentrated.
- the resulting residue was then purified by column chromatography over silica gel, using 1% methanol in chloroform as eluent, to yield 18 g of the sub-title product as a pale yellow solid.
- nnon-3-vnethyll- benzamide tert ⁇ Buty ⁇ 7- ⁇ 2-[(4-cyanobenzoyl)(methyl)amino]eihyl ⁇ -9-oxa-3,7-diazabicyclo- [3.3.1]nonane-3-carboxylate (11.5 g; see step (iii) above) was dissolved in dry dioxane (50 mL). Dioxane (100 mL, saturated with HCl gas) was added to the resulting solution. The reaction mixture was stirred at RT for 2 h before being diluted with dry diethyl ether.
- the Boc-protected compound was taken in (25 mL) of dioxane (saturated with HCl gas) and stirred for 30 minutes. Dioxane was decanted and the precipitated solid was washed with diethyl ether and dried under vacuum to yield 3.8 g of the sub-title compound as a white powder.
- Oxalyl chloride (6.5 g, 0.0517 mol) was added, dropwise, to a stirred solution of
- Trifluoroacetic acid 50 mL was added and the resulting mixture was stirred at room temperature for 2.5 hours before being concentrated in vacuo.
- the crude product was dissolved in 1 M HCl (50 mL) to provide an aqueous layer that was extracted with ethyl acetate (2 x 50 mL).
- the aqueous phase was concentrated in vacuo and to the remainder was added aqueous Na 2 CO 3 (50 mL).
- the resulting aqueous phase was extracted with DCM (3 x 50 mL), after which the combined DCM layers were dried over Na 2 SO 4 and concentrated in vacuo. This afforded 2.45 g (66.2% over two steps, i.e.
- step (iv) l-( " 2-Bromoethyl)-4-trifluoromethylbenzene
- HBr in acetic acid
- sulfuric acid 10 mL
- the reaction mixture was then poured onto ice and extracted with diethyl ether.
- the organic layer was washed with NaHCO 3 and water and then dried over sodium sulfate.
- Triphenylphosphine 14.97 g, 0.0571 mol was added to a solution of 3-(2- hydroxyethyl)benzonitrile (4.2 g. 0.0285 mol; see ste ⁇ (ii) above) in DCM (50 mL).
- the reaction mixture was cooled to 0 0 C and solution of CBr 4 (18.93 g, 0.0571 mol) in DCM was added, dropwise. After stirring at room temperature overnight, the reaction mixture was filtered and the solids washed with petroleum ether. The filtrate was concentrated and the resulting crude product was purified by column chromatography (using 3% ethyl acetate / petroleum ether as eluent) to give 4.2 g of the title compound as yellow liquid.
- reaction mixture was diluted with DCM (40 mL) and the organic phase was washed with water (2 x 30 mL) and brine (30 mL), dried over Na 2 SO 4 , and then concentrated in vacuo to give 0.78 g (95%) of the title compound as a colourless oil.
- Methyl iodide (4.4 niL, 0.071 mol) was added, at O 0 C, to a suspension of (4- bromo-3-fluorophenyl)acetic acid (8.2 g, 0.035 mol; see step (iii) above) and potassium carbonate (12.2 g, 0.088 mol) in dry acetonitrile (100 mL).
- the resulting mixture was stirred at 60°C overnight under a nitrogen atmosphere.
- the reaction mixture was filtered and solvent concentrated under reduced pressure to provide 6.4 g of the sub-title compound as pale yellow oil. This was employed directly in the next step without further purification.
- Methyl iodide (6.3 g, 0.0446 mol, 2 eq.) was added, drop by drop at O 0 C, to a mixture of (4-bromo-2-fluoro ⁇ henyl)acetic acid (5.5 g, 0.0223 mol; see step (vi) above) and dry K 2 CO 3 (8.46 g, 0.6125 mol) in dry acetonitrile (100 mL) under a nitrogen atmosphere.
- the reaction mixture was stirred at 60°C overnight under a nitrogen atmosphere and then filtered through Celite ® . Solvent evaporation under reduced pressure afforded 5.5 g of the sub-title compound as a brownish liquid. This was employed directly in the next step without further purification.
- Methanesulfonyl chloride (10.76 g, 0.094 mol) was added, at 0°C, to a solution of 2-(2,6-difluorophenyl)ethanol (13.5 g, 0.085 mol; see step (ii) above) and triethylamine (12.93 g, 0.128 mol) in dry DCM (135 mL). The resulting mixture was stirred for 3 h under a nitrogen atmosphere before the reaction was quenched with water and extracted with DCM. The organic (DCM) layer was dried over sodium sulfate and then concentrated under reduced pressure to give 18 g of the title compound as a pale yellow oil.
- Benzenesulfonamide 120 g, 0.763 moles
- (7?)-epichlorohydri ⁇ 282.6 g, 3.054 moles
- water 960 g
- the mixture was heated to 40 0 C and then sufficient sodium hydroxide solution (31%) was added over approximately 5 mins such that the pH was raised to 11.5-12.0 (in an alternative procedure, 25% sodium hydroxide solution can be employed).
- the remainder of the sodium hydroxide (201 g, 1.557 moles in total) was then added at such a rate as to maintain the pH at 11.5-12.0 and the temperature at 40-50°C (usually requires addition over 3-4 hours).
- reaction mixture was then stirred for 2 hours at 40-45 0 C and distilled to remove 3 volumes (360 mL) of water/epichlorohydrin at 50 mbar (5 kPa) with a maximum contents (source vessel) temperature of 43°C. Chlorobenzene was then added (221.4 g, 1.67 volumes) and the mixture was stirred for 0.5 hours before being allowed to settle. The lower product (chlorobenzene) layer was separated and the extraction process repeated using a further portion of chlorobenzene (44.3 g, 0.33 vols.). The two product layers were combined for use in the next step (see step (ii), Alternative 1 below).
- Chlorobenzene (266 g, 240 mL) was then added and the distillation continued until a further portion of methanol (4 volumes, 240 mL) had been collected from the reaction vessel.
- a second portion of chlorobenzene (133 g, 120 mL) was added and a mixture of solvent (4 volumes, 240 mL of a mixture of chlorobenzene/methanol) was distilled from the reaction mixture at 50 mbar (5 kPa).
- the remaining mixture (after the distillation) comprised the sub-title compound and chlorobenzene with a methanol content of ⁇ 0.1% w/w. This solution was employed in the next step (see step (iii), Alternative 1 below).
- Chlorobenzene (598 g, 9 volumes) and water (7.2 g, 0.4 moles) were added to a solution of S-benzyl-SJ-dihydroxy-l-phenylsulfonyl-l ⁇ -diazacyclooctane in chlorobenzene (0.382 moles; see step (ii), Alternative 1 above) and heated to 75°C.
- Sulfuric acid (98%, 134 g, 1.337 moles) was then added over 1 hour, whilst maintaining the temperature in the range 75-90°C.
- S-benzyl-SJ-dihydroxy-l-phenylsulfonyl-l j S-diazacyclooctane may be added to sulfuric acid.
- the biphasic reaction mixture was heated to 95 0 C and stirred for 3 hours. The temperature was adjusted to 50 0 C and methanol (57 g, 1.2 volumes) was added at such a rate as to maintain the temperature at between 50 and 60 0 C.
- the reaction mixture was basified by adding aqueous ammonia (17.5%, 346 g, 372 mL) over 2 hours at between 60 and 70 0 C, and then allowed to settle after 15 min of stirring (the mixture is kept at 60 0 C during the period in which it is allowed to settle).
- the lower aqueous layer was separated and the upper organic layer transferred to the crystallising vessel.
- the aqueous layer was returned to the reaction vessel and the temperature was adjusted to 45°C before chlorobenzene (133 g, 120 mL) was added.
- the separation process was repeated (i.e. the aqueous layer extracted and the phases separated) and the second organic phase combined with the first organic phase in the crystallising vessel.
- Chlorobenzene was then distilled (660 mL, 11 volumes) from the product layer at 50 mbar (5 kPa) and then methanol (470 g, 594 mL) was added over the course of 1 hour.
- the aqueous layer was returned to the reaction vessel and sodium hydroxide (31%, 181 g, 141 mL) was added over 45 mins, allowing the temperature to rise to a maximum of .60 0 C.
- Toluene (156 g, 180 mL) was added and the temperature adjusted to 6O 0 C before the layers were separated and the lower aqueous layer discarded.
- the toluene layer, containing the product, was washed with water (120 g) at 60 0 C before being cooled to 40 0 C, after which iso-propanol (345 g, 440 mL) was added.
- 3-benzyl-7- (phenylsulfonyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane 100.06 g, 279 mmol; see step (iii) above).
- 3-benzyl-7-(phenylsulfonyl)-9- oxa-3,7-diazabicyclo[3.3.1]nonane may be added to sulfuric acid.
- the reaction mixture was heated for 9 hours at 130 0 C, then left to cool to room temperature overnight.
- the acidic solution was poured into a clean vessel containing water (300 mL), and concentrated aqueous ammonia (35%) added dropwise over 2 hours (550 mL). After ammonia addition was complete, the pH of the reaction mixture was checked and found to be 10. Toluene (450 mL) was then added, and the temperature adjusted to 6O 0 C. The lower (aqueous) layer was separated and discarded. To the remaining upper layers (organic layer and interfacial layer), 5 M sodium hydroxide solution (300 mL) was added. The mixture was re-heated to 60 0 C, and stirred for 15 minutes. The layers were separated and the lower aqueous phase removed.
- the product is isolated by filtration, washed with isopropanol (126 g, 160 mL) and then dried by suction (on the filter) for 30 mins, before being transferred to a vacuum oven.
- the title compound is then dried to constant weight at 4O 0 C (30.1 g, 92.5%).
- the reaction was re-heated to 70 0 C and the phases were separated, keeping the small amount of interfacial material in the toluene phase.
- the aqueous phase was discarded.
- the remaining organic phase was extracted with 10% w/w aqueous citric acid (312.30 mmoles; 576.81 mL; 600.00 g), at 70°C, keeping the small amount of interfacial material with the toluene phase.
- the organic phase was then discarded.
- 2-Butanol (8.15 moles; 750.00 mL; 604.12 g) and aqueous 15% w/w sodium hydroxide (1.13 moles; 257.73 mL; 300.00 g) were added to the citric acid phase.
- the phases were separated at 70 0 C and the aqueous phase discarded.
- the 2-butanol phase was distilled at atmospheric pressure (88-90 0 C); 375 mL of solvent was distilled off.
- the remaining solution (524 g) was decanted to a clean flask and was then allowed to cool to room temperature overnight whilst stirring.
- the product slurry was cooled to 5 0 C and, after 2 hours at that temperature, was filtered.
- the filter cake was washed with cold (5°C) 2-butanol (1.89 moles; 173.81 mL; 140.00 g) and was then sucked as dry as possible on the filter.
- the damp filter cake (146 g) was dried in vacuo at 30 0 C for 3.5 hours, at 35 0 C for 15.5 hours and finally at 40 0 C for 5.5 hours to provide 114.1 g (69%) of the title compound as a white solid.
- the crystallisation mother liquors were concentrated by distillation at atmospheric pressure (and 90 to 100°C); 400 mL of solvent was distilled off. The solution was cooled to 26°C over the course of 2 hours, after which crystallisation seed (ca. 200 mg, taken from the first crop of title compound) was added. After 1 hour, the mixture was cooled to 18 0 C and then filtered.
- the filter cake was washed with cold (5°C) 2-butanol (543.36 mmoles; 50.00 mL; 40.28 g) and then sucked as dry as possible on the filter.
- the damp filter cake was dried in vacuo (at 4O 0 C) to provide a second crop of the title compound as a white solid (22.04 g, 13%). Total yield: 135.94 g (82%).
- the reaction was heated to 70 0 C (stirring at 300 rpm) and was held at this temperature for 8 hours before being stirred at room temperature overnight (for convenience).
- the reaction mixture was re-heated to 70 0 C and the phases were separated, leaving interfacial material with the toluene phase.
- the lower (aqueous) phase (pH 14) was discarded and a solution of 10% w/w aqueous citric acid (260.25 mmoles; 480.68 mL; 500.00 g) was added.
- the mixture was re-heated to 70 0 C and the phases separated, leaving interfacial material with the toluene phase, and the upper (organic) phase was discarded.
- the reaction was heated to 7O 0 C (stirring at 250 rpm) arid was held at this temperature for 8 hours before being left to stand at room temperature overnight (for convenience).
- the reaction was reheated to 70 0 C and the phases were separated.
- the lower (aqueous) phase was discarded and a solution of 10% w/w citric acid (130.12 mmoles; 240.34 mL; 250.00 g) was added to the retained (organic) phase.
- the mixture was re-heated to 50 0 C and the phases were separated again and the upper (organic) phase was discarded.
- Toluene (1.09 moles; 114.78 mL; 100.00 g) was added to the retained (aqueous) phase and the mixture re-heated to 50 0 C. The phases were separated and the upper (organic) phase was discarded. Toluene (1.09 moles; 114.78 mL; 100.00 g) and an aqueous solution of 20% w/w sodium hydroxide (500.04 mmoles; 82.02 mL; 100.00 g) were added to the retained (aqueous) phase and the temperature of the mixture was adjusted to 50 0 C. The phases were separated and the lower (aqueous) phase was discarded.
- This solid was partitioned between toluene (3.78 moles; 400.00 mL; 348.48 g) and a solution of sodium hydroxide (2.00 moles; 80.00 g) in water (22.20 moles; 400.00 mL; 400.00 g).
- the organic phase was dried over magnesium sulfate, filtered and concentrated in vacuo to give a white solid, 75 g (94% yield of free base).
- This solid was dissolved in isopropanol (9.80 moles; 750.00 mL; 588.75 g) and the solution heated to 47°C.
- Concentrated hydrochloric acid (708.76 mmoles; 60.00 mL; 70.80 g) was added within one minute, which caused the temperature to rise to 57°C and a precipitate to form.
- the mixture was allowed to cool to 30 0 C over the course of 1 hour, and was then cooled to 5 0 C over the course of 20 minutes. After 45 minutes at this temperature, the product was collected by filtration.
- the filter cake was washed with isopropanol (2.61 moles; 200.00 mL; 157.00 g) and sucked dry on the filter to give 116 g of a white solid.
- the combined filtrate and washings were heated to 40 0 C and concentrated hydrochloric acid (378.01 mmoles; 32.00 mL; 37.76 g) was added over the course of 12 minutes, such that the temperature of the reaction mixture was kept between 40 and 45 0 C. After stirring for 5 minutes, a precipitate started to form (at this point the temperature of the mixture was 42°C). The mixture was allowed to cool to 28 0 C over the course of 65 minutes, and was then cooled to 5 0 C over the course of 15 minutes. The mixture was stirred at 5°C for 90 minutes before the product was collected by filtration on a 7 cm diameter Buchner funnel (this took 2 minutes).
- the filter cake was washed with cold (5 0 C) isopropanol (1.31 moles; 100.00 mL; 78.50 g), which took 1 minute.
- the filter cake was sucked as dry as possible on the filter (10 minutes) to provide a white solid (52 g).
- This solid was dried in vacuo, at 4O 0 C for 3.5 hours, to provide the title compound as a white solid (42.45 g, 85% for two steps (i.e. from 3-benzyl-9- oxa-3,7-diazabicyclo[3.3.1]nonane dihydrochloride)).
- Bromofluoromethane (3 ml) was condensed down into a measuring cylinder cooled with dry ice.
- the liquid bromofluoromethane was added to a mixture of 3,5-dihydroxybenzaldehyde (1.00 g, 7.24 mrnol), potassium carbonate (3.00 g, 21.7 mmol) and acetonitrile (15 ml) in a 20 ml vial designed for microwave reactions (BiotageTM) equipped with a magnetic stirring bar.
- the vial was capped with a cap, containing a septum, belonging to the microwave vial.
- the vial was heated using an oil bath so that the reaction mixture was stirred at 70 0 C over night.
- the reaction mixture was filtered and evaporated.
- the product was purified by chromatography on silica gel (HorizonTM, flash system from BiotageTM; column: Flash 40+M, 40 x 150 mm), using methanol saturated with ammonia in DCM as eluent, to yield 0.187 g (16%) of the title compound.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Cardiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Heart & Thoracic Surgery (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Abstract
There is provided compounds of formula I, [Chemical formula should be inserted here. Please see paper copy] wherein R1 to R4, R41 to R46 and Z have meanings given in the description, which are useful in the prophylaxis and in the treatment of arrhythmias, in particular atrial and ventricular arrhythmias.
Description
NEW OXABISPIDINE COMPOUNDS FOR THE TREATMENT OF CARDIAC ARRHYTHMIAS.
Field of the Invention
This invention relates to novel pharmaceutically useful compounds, in particular compounds which are useful in the treatment of cardiac arrhythmias.
Background and Prior Art
Cardiac arrhythmias may be defined as abnormalities in the rate, regularity, or site of origin of the cardiac impulse or as disturbances in conduction which causes an abnormal sequence of activation. Arrhythmias may be classified clinically by means of the presumed site of origin (i.e. as supraventricular, including atrial and atrioventricular, arrhythmias and ventricular arrhythmias) and/or by means of rate (i.e. bradyarrhythmias (slow) and tachyarrhythmias (fast)).
hi the treatment of cardiac arrhythmias, the negative outcome in clinical trials (see, for example, the outcome of the Cardiac Arrhythmia Suppression Trial (CAST) reported in New England Journal of Medicine, 321, 406 (1989)) with "traditional" antiarrhythmic drugs, which act primarily by slowing the conduction velocity (class I antiarrhythmic drugs), has prompted drug development towards compounds which selectively delay cardiac repolarization, thus prolonging the QT interval. Class III antiarrhythmic drugs may be defined as drugs which prolong the trans-membrane action potential duration (which can be caused by a block of outward K+ currents or from an increase of inward ion currents) and refractoriness, without affecting cardiac conduction.
One of the key disadvantages of hitherto known drugs which act by delaying repolarization (class III or otherwise) is that they all are known to exhibit a unique form of proarrhythmia known as torsades de pointes (turning of points), which may, on occasion be fatal. From the point of view of safety, the minimisation of this
phenomenon (which has also been shown to be exhibited as a result of administration of non-cardiac drugs such as phenothiazines, tricyclic antidepressants, antihistamines and antibiotics) is a key problem to be solved in the provision of effective antiarrhythmic drugs.
Antiarrhythmic drugs based on bispidines (3,7-diazabicyclo[3.3.1]nonanes), are known from inter alia international patent applications WO 91/07405 and WO 99/31100, European patent applications 306 871, 308 843 and 655 228 and US patents 3,962,449, 4,556,662, 4,550,112, 4,459,301 and 5,468,858, as well as journal articles including, inter alia, J. Med. Chem. 39, 2559, (1996), Pharmacol Res., 24, 149 (1991), Circulation, 90, 2032 (1994) mάAnal. ScI 9, 429 (1993).
Certain oxabispidine compounds are disclosed as chemical curiosities in Chem. Ber., 96, 2872 (1963). The use of certain other oxabispidine compounds in the treatment of cardiac arrhythmias is disclosed in WO 01/28992. Methods for the preparation of such oxabispidine compounds are disclosed in WO 02/28863, WO 02/28864, WO 02/83690 and WO 02/83691. Further, acid addition salts that are useful in such methods of preparation are disclosed in WO 04/035592.
Further groups of oxabispidine compounds that are useful in the treatment of cardiac arrhythmias are disclosed in unpublished international patent application numbers PCT/SE2005/000890 and PCT/SE2005/000891.
We have surprisingly found that a sub-group of oxabispidine-based compounds comprising an amide-substituted alkyl group exhibit unexpectedly beneficial properties that render them particularly suitable for use in the treatment of cardiac arrhythmias.
Disclosure of the Invention
R1 represents C1-12 alkyl (which alkyl group is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het1, -N(R5a)R6, -C(O)R5b, -OR5c, -C(O)-E-R7, -C(O)N(R8a)R5d, -OC(O)N(R8b)R5e, -S(O)2R9a, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d) or R1 represents -C(O)-E-R7, -C(O)N(R8a)R5d or -S(O)2R9a;
R >5aa represents H or C1-6 alkyl (which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d);
R5b to R5e independently represent, at each occurrence when used herein, H, C1-6 alkyl (which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, Het2, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), aryl or Het3, or R5d or R5e, together with, respectively, R8a or R8b, may represent C3-6 alkylene (which alkylene group is optionally interrupted by an O atom and/or is optionally substituted by one or more C1-3 alkyl groups);
R6 represents H, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), aryl,
-C(O)R10a, -C(O)OR10b or -C(O)N(H)R10c or -S(O)2R10d; RIOa, R1Ob, R1Oc and R1Od independently represent C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl), aryl, or R1Oa represents H;
R represents, at each occurrence when used herein, C1-12 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -6 alkoxy, Het4, -S(O)2N(R 9ybDγ)Ro 9c >9ck
C1 yc and -N(R ,9ybDx)S(O)2Ryα);
R and R independently represent H, C1-12 alkyl, C1-6 alkoxy (which latter two groups are optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, C1-4 alkyl, C1-4 alkoxy, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), -D-aryl, -D-aryloxy, -D-Het5, -D-N(H)C(O)RUa, -D-S(O)2R12a, -D-C(O)Rllb, -D-C(O)OR12b, -D-C(O)N(Rllc)Rlld, or R8a or R8b, together with, respectively, R5d or R5e, may represent C3-6 alkylene (which alkylene group is optionally interrupted by an O atom and/or is optionally substituted by one or more C1-3 alkyl groups); Rlla to Rlld independently represent H, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl), aryl, or Rllc and Rlld together represent C3-6 alkylene;
R12a and R12b independently represent C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl) or aryl; D represents, at each occurrence when used herein, a direct bond or C1-6 alkylene; E represents O or S;
R9a represents, at each occurrence when used herein, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d) or aryl; R9b represents, at each occurrence when used herein, H or C1-6 alkyl; R9c and R9d independently represent, at each occurrence when used herein, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl and Het6), aryl or Het7, or R9c represents H;
Het1 to Het7 independently represent five- to twelve-membered heterocyclic groups containing one or more heteroatoms selected from oxygen, nitrogen and/or sulfur, which groups are optionally substituted by one or more groups selected from -OH, oxo, halo, cyano, nitro, C1-6 alkyl, C1-6 alkoxy, aryl, aryloxy, -N(R13a)R13b, -C(O)R13c, -C(O)OR13d, -C(O)N(R13e)R13f, -N(R13g)C(O)R13h, -S(O)2N(R13i)R13j and -N(R13k)S(O)2R13m; R13a to R13m independently represent C1-6 alkyl, aryl or R13a to R13k independently represent H;
R2 and R3 independently represent H, F or C1-3 alkyl;
Z represents
-N(R14a)-C(O)-A- (in which group N(R14a) is attached to R4) or -C(O)-N(R14b)-B- (in which group the C(O) moiety is attached to R4);
A represents a direct bond or C1-3 alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl;
B represents
C1-3 alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl or when X represents a direct bond, B may alternatively, together with R18, represent a structural fragment of formula Ia,
in which the wavy lines indicate the positions of attachment of the fragment, wherein G is attached to N(R14b) and J is attached to C(R2)(R3), and the dashed line represents a bond or, when G represents C(R15c)(R15d), is absent; G repre sseennttss NN,, CC((RR1155bb)) oorr CC((RR1155cc))((RR1155dd));;
J represents a direct bond or Ci-3 alkylene;
R15a to R15d indepe mnedently represent H or C1-3 alkyl or, when the dashed line represents a bond, R15a is absent;
R14a represents H or C1-6 alkyl;
R14b represents H, Ci-6 alkyl or, together with R18, R14b may alternatively represent a direct bond or -C(O)-;
R4 represents Ci-6 alkyl or a structural fragment of formula Ib,
in which the wavy line indicates the position of attachment of the fragment;
X represents a direct bond,
Cu alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl, or
-Y-[C(R16a)(R16b)]π-, in which latter group C(R16a)(R16b) is attached to Z; Y represents O, -N(R16c)- or S(O)0-2; RI6a to RWc independently represent H or C1-3 alkyl; n represents 2 or 3 or, when Z represents -C(0)-N(R14)-B-, then n may alternatively represent 1 ;
XI to X4 independently represent N or C(R17) and X5 represents N or C(R18), provided that at least one of X1 to X5 is other than N;
R17 and R18 independently represent H, -OH, cyano, halo, nitro, C1-6 alkyl (optionally terminated by -N(H)C(0)0R19a), C1-6 alkoxy, -N(R20a)R20b, -C(O)R20c, -C(O)OR20d, -C(O)N(R20e)R20f, -N(R20g)C(O)R20h, -N(R20i)C(O)N(R20j)R20k, -N(R20m)S(O)2R19b, -S(O)2N(R20n)R20p, -S(O)2R19c, -OS(O)2R19d, -Si(R19e)3 and aryl or R18
(a) together with B and when X represents a direct bond, may alternatively represent a structural fragment of formula Ia, as defined above, or
(b) together with R14b, may alternatively represent a direct bond or -C(O)-;
R19a to R19e represent, independently at each occurrence, Ci-6 alkyl or phenyl, which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, Ct-4 alkyl and C1-4 alkoxy;
R2Oa and R20b independently represent H, C1-6 alkyl or together represent C3-6 alkylene, resulting in a four- to seven-membered nitrogen-containing ring; R2Oc to R20p independently represent H or Ci-6 alkyl; and
R41 to R46 independently represent H or C1^ alkyl;
wherein each aryl group, unless otherwise specified, is optionally substituted;
or a pharmaceutically acceptable derivative thereof,
provided that the compound is not:
(a) 2-[7-(2- { (aminocarbonyl)[2-(4-cyanophenoxy)ethyl] amino } ethyl)-9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl]-iV-(fert-butyl)acetaπiide;
(b) 2-[7-(2-{(aminocarbonyl)[2-(4-cyanophenoxy)ethyl]amino}ethyl)-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl]-JV-benzylacetamide;
(c) 2-[7-(2-{(aminocarbonyl)[2-(4-cyanophenoxy)ethylJamino}ethyl)-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl]-iV-(l-methyl-l-ρhenylethyl)acetamide;
(d) N-(^rt-butyl)-2-(7-{2-[t2-(4rcyanophenoxy)ethyl](methylsulfonyl)- ammojethylJ-θ-oxa-SJ-diazabicyclotS.S.ljnon-S-yOacetamide; (e) iV-benzyl-2-(7- { 2- [[2-(4-cyanophenoxy)ethyl] (methylsulfonyl)amino] -ethyl } -
9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)acetamide;
(f) 2-(7-{2-[[2-(4-cyanophenoxy)ethyl](methylsulfonyl)amino]ethyl}-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl)-iV-(l-methyl-l-phenylethyl)acetamide;
(g) N-(tert-butyl)-2-(7-{2-[[3-(4-cyanophenyl)propyl](methylsulfonyl)- arnino]ethyl } -9-oxa-3 ,7-diazabicyclo[3.3.1 ]non-3-yl)acetamide;
(h) 2-{7-[3-(4-cyanoρhenoxy)-2-hydroxyρropyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}-iV-isopropylacetamide;
(i) 2- {7-[3-(4-cyanoanilino)proρyl]-9-oxa-3,7-diazabicyclo[3.3. l]non-3-yl }-N- isopropylacetamide; (j) 2-{7-[2-(4-cyanoρhenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
JV-isopropylacetamide;
(k) 2-(7-{ 3-[(4-cyanophenyl)sulfonyl]propyl } -9-oxa-3 ,7-diazabicyclo[3.3.1]-
non-3 -yl)-iV-isoproρylacetamide; or
(1) 2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } - JV,Λf~diethylacetainide,
which compounds are referred to hereinafter as "the compounds of the invention".
Unless otherwise specified, alkyl groups and alkoxy groups as defined herein may be straight-chain or, when there is a sufficient number (i.e. a minimum of three) of carbon atoms be branched-chain, and/or cyclic. Further, when there is a sufficient number (i.e. a minimum of four) of carbon atoms, such alkyl and alkoxy groups may also be part cyclic/acyclic. Such alkyl and alkoxy groups may also be saturated or, when there is a sufficient number (i.e. a minimum of two) of carbon atoms, be unsaturated and/or interrupted by one or more oxygen and/or sulfur atoms. Unless otherwise specified, alkyl and alkoxy groups may also be substituted by one or more halo, and especially fluoro, atoms.
Unless otherwise specified, alkylene groups as defined herein may be straight- chain or, when there is a sufficient number (i.e. a minimum of two) of carbon atoms, be branched-chain. Such alkylene chains may also be saturated or, when there is a sufficient number (i.e. a minimum of two) of carbon atoms, be unsaturated and/or interrupted by one or more oxygen and/or sulfur atoms. Unless otherwise specified, alkylene groups may also be substituted by one or more halo atoms.
The term "aryl", when used herein, includes C6-io aryl groups such as phenyl, naphthyl and the like. The term "aryloxy", when used herein includes C6-1O aryloxy groups such as phenoxy, naphthoxy and the like. For the avoidance of doubt, aryloxy groups referred to herein are attached to the rest of the molecule via the O-atom of the oxy-group. Unless otherwise specified, aryl and aryloxy groups may be substituted by one or more groups including -OH, halo, cyano, nitro, C1-6 alkyl, C1-6 alkoxy, -N(R20a)R20b, -C(O)R20c, -C(O)OR20d, -C(O)N(R20e)R20f, -N(R20g)C(O)R20h, -N(R20m)S(O)2R19b, -S(O)2N(R20a)R20p,
-S(O)2R190, -OS(O)2R19d and/or -Si(R19e)3 (wherein R19b to R19e and R20a to R20P are as hereinbefore defined). When substituted, aryl groups are preferably substituted by between one and three substituents.
The term "halo", when used herein, includes fluoro, chloro, bromo and iodo.
Heterocyclic (Het1 to Het7) groups that may be mentioned include those containing 1 to 4 heteroatoms (selected from the group oxygen, nitrogen and/or sulfur) and in which the total number of atoms in the ring system are between five and twelve. Heterocyclic (Het1 to Het7) groups may be fully saturated, partly unsaturated, wholly aromatic or partly aromatic in character. Values of heterocyclic (Het1, Het2 and Heta) groups that may be mentioned include l-azabicyclo[2.2.2]octanyl, benzimidazolyl, benzo[c]isoxazolidinyl, benzisoxazolyl, benzodioxanyl, benzodioxepanyl, benzodioxolyl, benzofuranyl, benzofurazanyl, benzomorpholinyl, 2,1,3-benzoxadiazolyl, benzoxazolidinyl, benzoxazolyl, benzopyrazolyl, benzo[e]pyrimidine, 2,1,3-benzothiadiazolyl, benzothiazolyl, benzothienyl, benzotriazolyl, chromanyl, chromenyl, cinnolinyl, 2,3-dihydrobenzimidazolyl, 2,3-dihydrobenzo[&]furanyl, 1 ,3-dihydrobenzo[c]- furanyl, l,3-dihydro-2,l-benzisoxazolyl 2,3-dihydropyrrolo[2,3-&]pyridinyl, dioxanyl, furanyl, hexahydropyrimidinyl, hydantoinyl, imidazolyl, imidazo[l,2- α]pyridinyl, imidazo[2,3-ό]thiazolyl, indolyl, isoquinolinyl, isoxazolidinyl, isoxazolyl, maleimido, morpholinyl, naphtho[l,2-&]furanyl, oxadiazolyl, 1,2- or 1,3-oxazinanyl, oxazolyl, phthalazinyl, piperazinyl, piperidinyl, purinyl, pyranyl, pyrazinyl, pyrazolyl, pyridinyl, pyrimidinyl, pyrrolidinonyl, pyrrolidinyl, pyrrolinyl, pyrrolo[2,3-&]pyridinyl, pyrrolo[5,l-b]pyridinyl, pyrrolo[2,3- c]pyridinyl, pyrrolyl, quinazolinyl, quinolinyl, sulfolanyl, 3-sulfolenyl, 4,5,6,7-tetrahydrobenzimidazolyl, 4,5,6,7-tetrahydrobenzopyrazolyl, 5,6,7, 8-tetra- hydrobenzotøpyrimidine, tetrahydrofuranyl, tetrahydropyranyl, 3,4,5,6-tetra- hydropyridinyl, 1 ,2,3 ,4-tetrahydropyrimidinyl, 3 ,4,5,6-tetrahydropyrimidinyl, thiadiazolyl, thiazolidinyl, thiazolyl, thienyl, thieno[5,l-c]pyridinyl, thiochromanyl, triazolyl, l,3,4-triazolo[2,3-^]pyrimidinyl, xanthenyl and the like.
Values of Het1 that may be mentioned include benzodioxanyl (e.g. benzo-1,3- dioxanyl or benzo-l,4-dioxanyl), benzodioxolyl, benzisoxazolyl, benzofuranyl, 2,3-dihydrobenzo[&]furanyl, imidazo[l,2-<2]pyridinyl, indolyl, isoxazolyl, pyrazolyl, pyridinyl and quinolinyl.
Substituents on heterocyclic (Het1 to Het7) groups may, where appropriate, be located on any atom in the ring system including a heteroatom. The point of attachment of heterocyclic (Het1 to Het7) groups may be via any atom in the ring system including (where appropriate) a heteroatom, or an atom on any fused carbocyclic ring that may be present as part of the ring system. Heterocyclic (Het1 to Het7) groups may also be in the N- or S-oxidised form.
Pharmaceutically acceptable derivatives include salts and solvates. Salts which may be mentioned include acid addition salts. Pharmaceutically acceptable derivatives also include, at the oxabispidine or other heterocyclic nitrogen atoms, C1-4 alkyl quaternary ammonium salts and iV-oxides.
The compounds of the invention may exhibit tautomerism. All tautomeric forms and mixtures thereof are included within the scope of the invention.
The compounds of the invention may also contain one or more asymmetric carbon atoms and may therefore exist as enantiomers or diastereoisomers, and may exhibit optical activity. Diastereoisomers may be separated using conventional techniques, e.g. chromatography or fractional crystallisation. The various stereoisomers may be isolated by separation of a racemic or other mixture of the compounds using conventional, e.g. fractional crystallisation or HPLC, techniques. Alternatively the desired optical isomers may be made by reaction of the appropriate optically active starting materials under conditions which will not cause racemisation or epimerisation, or by derivatisation, for example with a homochiral acid followed by separation of the diastereomeric esters by conventional means (e.g. HPLC, chromatography over silica). All stereoisomers are included within the scope of the invention.
Abbreviations are listed at the end of this specification.
Compounds of the invention that may be mentioned include those in which R1 does not represent C1-12 alkyl, which alkyl group is optionally substituted by one or more groups including one -N(R5a)C(O)OR10b group.
In one embodiment of the invention, compounds of the invention that may be mentioned include those in which:
R1 represents (a) C1-5 alkyl terminated by a group selected from aryl, Het1, -N(R5a)R6,
-C(O)R5b, -OR5c, -C(O)OR7 and -C(O)N(R8a)R5d, and optionally further substituted by OH and/or one or more halo groups, or (b) -C(O)OR7;
R5a and R5d independently represent H or methyl; R5b represents C1-5 alkyl;
R5c represents aryl or Q.3 alkyl substituted by aryl;
R6 represents -C(O)OR10b;
R1Ob represents C1-5 alkyl;
R7 represents C1-5 alkyl; R8a represents C1-5 alkyl or -D-phenyl (the phenyl part of which latter group is optionally substituted by one or more (e.g. one to three) groups selected from F and cyano);
D represents C1-3 alkylene;
Het1 represents a heterocyclic group that is (a) five- or six-membered and aromatic, or
(b) nine- or ten-membered, bicyclic and aromatic or part-aromatic, which heterocyclic group contains one or more (e.g. one to three) heteroatoms selected from oxygen, nitrogen and/or sulfur (e.g. selected from oxygen and nitrogen), which group is are optionally substituted by one or more groups selected from halo (e.g. F or Cl), cyano, C1-3 alkyl (e.g. methyl) and C1-3 alkoxy
(e.g. methoxy);
R and R both represent H;
Z represents
-N(R14a)-C(O)- or -C(O)-N(R14b)-B-; B represents C1-S n-alkylene;
R14a and R14b independently represent H or Ci-3 alkyl (e.g. methyl); R4 represents C1-S alkyl (e.g. tert-butyl) or, particularly, a structural fragment of formula Ib, as defined above; X represents a direct bond (e.g. when Z represents -C(O)-N(R14b)-B-), Ci-3 /z-alkylene optionally substituted by one or more (e.g. one or two) methyl groups, or
-Y-[C(R16a)(R16b)]n- (e.g. when Z represents -N(R14a)-C(O)-A-); Y represents O;
R16a and R16b both represent H; n represents 3 or, particularly, 2; X1 to X4 all represent C(R17); X5 represents C(R18);
1 *7 IS
R and R independently represent H, cyano or halo (e.g. Cl or, particularly, F), provided that no more than three of X1 to X5 represent other than C(H); R41 to R46 all represent H; aryl represents naphthyl or, particularly, phenyl, which aryl group is optionally substituted by one or more (e.g. one to three) groups selected from halo (e.g. F or Cl), cyano, C1-3 alkyl, C1-3 alkoxy (which latter two groups are optionally substituted by one or more F atoms), -OS(O)2R19d and -Si(CH3)3; R19d represents C1-3 alkyl (e.g. methyl) or, particularly, phenyl, which latter group is optionally substituted by one or more (e.g. one to three) groups selected from methyl and halo (e.g. Cl).
In two further embodiments of the invention, compounds of the invention that may be mentioned include those that either (a) are or (b) are not of the formula Ic,
wherein R1, R14a and R41 to R46 are as hereinbefore defined and:
(a) Xa takes the same definition as X above, except that it does not represent a direct bond; and
(b) R17a represents one or more substituents on the phenyl ring, each substituent taking the same definition as R17 above.
Compounds of formula Ic are hereinafter also referred to as "the compounds of the invention". Further, for the avoidance of doubt, the specific definitions of groups mentioned herein in relation to compounds of formula I are also, where relevant, specific definitions of the equivalent groups in compounds of formulae Ic (and vice versa).
Particular compounds of formula Ic that may be mentioned include those in which:
R14a represents methyl or, particularly, H;
Xa represents O(CH2)2 (in which group the O-atom is attached to the phenyl ring),
(CHa)2, C(CHg)2 or CH2;
R a represents H or one to three substituents (e.g. three substituents at the 2-, 4- and 6-positions, two substituents at the 2- and 4-positions or one substituent at the 2-, 3- or, particularly, 4-position) selected from halo (e.g. F) and CN.
Further compounds of formula Ic that may be mentioned include those in which: Xa represents O(CH2)2 (in which group the O-atom is attached to the phenyl ring), C(CH3)2, or, particularly, CH2;
the substitution pattern on the phenyl ring is as follows
wherein the wavy line indicates the position of attachment to the group Xa and R17al represents H, F or, particularly, cyano, and R17a2 and R17a3 independently represent F or, particularly, H;
R1 represents a structural fragment of the formula
Q3 represents a direct bond, CH2 or OCH2 (in which latter group the O- atom is attached to the phenyl ring) and
Rla to Rle independently represent H, halo (e.g. F or Cl), cyano or C1-2 alkoxy (which latter group is optionally substituted by one or more halo (e.g. F) atoms), provided that at least two of Rla to Rle represent H.
Still further compounds of formula Ic that may be mentioned include those in which: Xa represents C(CH3)2 (e.g. when Rlcl represents cyano), O(CH2)2 (in which group the O-atom is attached to the phenyl ring) or, particularly, CH2; R17al represents cyano or, when Xa represents C(CHs)2, R17al may alternatively represent H; R17a2 represent F or, particularly, H; R17a3 represents H; R1 represents a structural fragment of the formula
wherein Rlal to Rlel and Rla2 to RIc2 take the same definitions as Rla to Rle above or, in particular
Rlal represents H or, particularly, F,
R , lbl and R > lel independently represent F or, particularly, H,
R , lccl represents Cl, cyano or, particularly, F,
R , la2 represents F or, particularly, H,
R , 162 represents methoxy or, particularly, H,
R , lccl represents Cl, F, methoxy or, particularly, H and
Q1 represents a direct bond or, particularly, CH2.
In two still further embodiments of the invention, compounds of the invention that may be mentioned include those that either (a) are or (b) are not of the formula Id,
(a) R14bl represents H or C1-6 alkyl;
(b) R17b represents one or more substituents on the phenyl ring, each substituent taking the same definition as R 17 above.
Compounds of formula Id are hereinafter also referred to as "the compounds of the invention". Further, for the avoidance of doubt, the specific definitions of groups mentioned herein in relation to compounds of formula I are also, where relevant, specific definitions of the equivalent groups in compounds of formulae Id (and vice versa).
Particular compounds of formula Id that may be mentioned include those in which:
B represents (CHb)2 or, particularly, CH2; R14bl represents H or, particularly, methyl;
X represents (CH2)2, CEfe or a direct bond;
R17b represents H or one to three substituents (e.g. two substituents at the 2- and 4-or
3- and 4-positions, or one substituent at the 4-position) selected from halo (e.g. F) and CN.
Further compounds of formula Id that may be mentioned include those in which:
X represents (CH2)2 or, particularly, a direct bond; the substitution pattern on the phenyl ring is as follows
R represents H, F or, particularly (e.g. when R represents H), cyano, and
R17b2 represents F (e.g. when R17bl represents F) or, particularly, H; R1 represents a structural fragment of the formula
Q2 represents Ci-3 alkylene (e.g. CH2) and
Rlf represents one to three (e.g. one or two) substituents selected from C1-2 alkoxy (e.g. methoxy) optionally substituted by one or more (e.g. two) F atoms.
Still further compounds of formula Id that may be mentioned include those in which:
R represents methyl;
X represents a direct bond; RI7bI represents cyano; R17b2 represents H; Q2 represents CH2; Rlf represents one substituent at the 2- or, particularly, 3 -position (relative to the point of attachment of Q2) or two substituents at the 3- and 5-positions (relative to the point of attachment
Of Q2), wherein each Rlf substituent represents methoxy or (e.g. when only one Rlf substituent is present) fiuoro-substituted methoxy (such as OCHF2).
In two further embodiments of the invention, compounds of the invention that may be mentioned include those that either (a) are or (b) are not of the formula Ie,
Compounds of formula Ie are hereinafter also referred to as "the compounds of the invention". Further, for the avoidance of doubt, the specific definitions of groups mentioned herein in relation to compounds of formula I are also, where relevant,
specific definitions of the equivalent groups in compounds of formulae Ie (and vice versa).
Particular compounds of formula Ie that may be mentioned include those in which:
R1 represents a structural fragment of the formula
Q represents O or OCH2 (in which latter group the 0-atom is attached to the phenyl ring),
RIg and RIh indepi ndently represent H or F; R14a represents H.
hi one embodiment of the invention, compounds of the invention that may be mentioned include those in which R1 represents
[3,5-bis(fluoromethoxy)phenyl]methyl,
(2,3-dimethoxyphenyl)methyl,
(2,4-difluorophenyl)methyl,
(2,6-difluorophenyl)methyl, (2,6-dimethoxyphenyl)methyl,
(2,6-dimethylphenyl)methyl,
(2-cyanophenyl)methyl,
(2-methoxyphenyl)methyl,
(2-methylpropan-2-yl)oxycarbonyl, (2-methylpropan-2-yl)oxycarbonylmethyl,
(3 ,3-dimethyl-2-oxo-butyl) ,
(3 ,5-diethoxyphenyl)methyl,
(3 ,5-drmethoxyphenyl)methyl,
(3,5-dimethyll,2-oxazol-4-yl)methyl, .
(3-cyanophenyl)methyl,
(3-ethoxyphenyl)methyl,
(3-methoxyphenyl)methyl,
(3-propan-2-yloxyphenyl)methyl, (4-chlorophenyl)methyl,
(4-cyanophenyl)methylcarbamoylmethyl,
(4-fluorophenyl)methyl,
(4-methylsulfonyloxyphenyl)methyl,
(5-fluoro-2-methyl-phenyl)rαethyl, (8-fluoro-2,4-dioxabicyclo[4.4.0]deca-7,9,H-trien-10-yl)methyl,
[(2R)-3-(4-cyanophenoxy)-2-hydroxy-propyl],
[2-(4-methoxyphenyl)-2-oxo-ethyl] ,
[2-(difluoromethoxy)phenyl]methyl,
[2-(triJfluoromethyl)phenyl]raethyl, [2,4-bis(trifluoromethyl)phenyl]methyl,
[3-(4-methylphenyl)sulfonyloxyplienyl]rDethyl,
[3-(difluorotnethoxy)phenyl]methyl,
[3-(fluoromethoxy)phenyl]methyl,
[3-(trifluoromethoxy)phenyl]methyl, [4-(difluoromethoxy)phenyl]methyl,
[4-(trifluoromethyl)phenyl]raeth.yl,
[4-fluoro-2-(trifluoromethyl)phenyl]methyl, l,7-diazabicyclo[4.3.0]nona-2,4,6,8-tetraen-8-ylmethyl,
2-(lH-indol-3-yl)ethyl, 2-(2,3-dihydrobenzofuran-5~yl)ethyl,
2-(2,4-difluorophenoxy)ethyl,
2-(2,4-difluorophenyl)ethyl,
2-(2,6-difluorophenoxy)ethyl,
2-(2,6-difluorophenyl)eihyl, 2-(2,6-dimethylphenoxy)ethyl,
2-(2-chloroρhenyl)ethyl,
2-(2-fluorophenoxy)ethyl,
2-(2-fluorophenyl)ethyl,
2-(2-methoxyphenyl)ethyl,
2-(2-methylphenyl)ethyl,
2-(3 ,4-difluorophenyl)ethyl, 2-(3,4-dimetiioxyphenyl)ethyl,
2-(3 ,5-dimethyll ,2-oxazol-4-yl)ethyl,
2-(3 ,5-dimethylpyrazol- l-yl)ethyl,
2-(3-chlorophenyl)ethyl,
2-(3 -cyanophenyl)ethyl, 2-(3~fluorophenyl)ethyl,
2-(3 -methoxyphenyl)ethyl,
2-(4-acetylphenyl)ethyl,
2-(4-chlorophenyl)ethyl,
2-(4-cyano-2-fluoro-phenoxy)ethyl, 2-(4-cyano-2-fluoro-phenyl)ethyl,
3-(4-cyano-3-fluoro-phenoxy)propyl,
2-(4-cyano-3 -fluoro-phenyl)eihyl,
2-(4-cyanophenoxy)ethyl,
2-(4-cyanophenyl)eihyl, 2-(4-fluorophenoxy)ethyl,
2-(4-fluorophenyl)ethyl,
2-(4-methoxyphenyl)ethyl,
2-(4-trimethylsilylphenyl)ethyl,
2-(6-methylpyridin-2-yl)ethyl, 2- [(2-methylpropan-2-yl)oxycarbonyl]propan-2-yl,
2-[(2-methylpropan-2-yl)oxycarbonylamino]ethyl,
2-[(3-chlorophenyl)methoxy]ethyl,
2-[(3-cyanophenyl)methoxy]ethyl,
2-[(3-fluorophenyl)methoxy]ethyl, 2-[(4-chlorophenyl)methoxy]ethyl,
2-[(4-cyanobenzoyl)amino]ethyl,
2-[(4-cyanophenyl)methoxy]ethyl,
2-[(4-fluorophenyl)methoxy]ethyl,
2-[[3-(trifluoromethyl)phenyl]methoxy]ethyl,
2-[[4-(trifluorome1hyl)phenyl]methoxy]ethyl,
2-[2-(difluoromethoxy)phenyl]ethyl, 2-[2-(trifluoromethyl)phenyl]ethyl,
2-[3-(4-cyanophenyl)propanoyl-methyl-amino]ethyl,
2-[3,4-bis(difluoromethoxy)phenyl]ethyl,
2-[4-(difluoromethoxy)phenyl]ethyl,
2-[4-(trϋluoromethyl)phenyl]ethyl, 2-benzo[d]isoxazol-3-ylethyl,
2-benzofuran-5-ylethyl,
2-phenylmethoxyethyl,
2-pyridin-2-ylethyl,
2-pyridin-4-ylethyl, 3-(2,4-difluorophenoxy)propyl,
3-(2,5-difluorophenoxy)propyl,
3-(2,6-difluorophenoxy)propyl,
3 -(2-cyanoρhenoxy)propyl,
3 -(2-fluorophenoxy)propyl, 3-(2-methoxyphenoxy)propyl,
3 -(3 ,4-difluorophenoxy)propyl,
3 -(3 ,5-difluorophenoxy)propyl,
3-(3-chlorophenoxy)propyl,
3-(3-cyanophenoxy)propyl, 3-(3-fluorophenoxy)propyl,
3 -(3 -methoxyphenyl)propyl,
3-(4-chloro-2-fluoro-phenoxy)propyl, • 3-(4-chloro-3-fluoro-phenoxy)propyl,
3 -(4-chlorophenoxy)propyl, 3-(4-cyano-2,6-difluoro-phenoxy)propyl,
3-(4-cyano-2-fluoro-phenoxy)propyl,-
3-(4-cyanophenoxy)propyl,
3-(4-cyanophenyl)propyl,
3-(4-cyanophenyl)sulfonylpropyl,
3-(4-fluoroρhenoxy)propyl,
3 - [(2-methylpropan-2-yl)oxy]propyl, 3-[3-(trifluorometfayl)phenoxy]propyl,
3-[4-(trifluoromethyl)phenoxy]propyl,
3-phenoxypropyl,
3-phenylpropyl,
4-(4-cyanophenyϊ)butyl, (4-cyanophenyl)methyl, benzo[ 1 ,3]dioxol-4-ylmethyl, benzo[ 1 ,3] dioxol-5-ylmethyl, benzyl, naphthalen- 1 -ylmethyl, phenethyl, quinolin-8-ylmethyl, or tert-butylcarbamoylmethyl.
In one embodiment of the invention, compounds of the invention that may be mentioned include those of the formula If,
wherein R1 and R41 to R46 are as hereinbefore defined and in which the left hand side-chain (LHS) represents
2-[(4-cyanobenzoyl)-methyl-amino] ethyl, (4-oxo-3H-phthalazin-l-yl)methyl, 2-(l ,3-dioxoisoindol-2-yl)ethyl, 2-(4-methylsulfonyloxyphenyl)ethyl,
2-[(3,4-difluorobenzoyl)amino]ethyl,
2-[(4-cyanobenzoyl)amino]ethyl,
2-[(4-cyanoberLZoyl)-methyl-amiQθ]ethyl,
2-benzamidoethyl, 3-benzamidopropyl,
(2,4-difluorophenyl)methylcarbamoylmethyl,
(2-fluorophenyl)methylcarbainoylmeth.yl,
(3-fluorophenyl)methylcarbamoylmethyl,
(4-cyano-2,6-difluoro-phenyl)methylcarbamoylmethyl, (4-cyano-2-fluoro-phenyl)methylcarbamoylmethyl,
(4-cyanophenyl)methylcarbamoylmethyl,
(4-fluorophenyl)methylcarbamoylmethyl,
(benzyl-methyl-carbamoyl)methyl,
(methyl-tert-butyl-carbamoyl)methyl, [(4-cyanophenyl)methyl-methyl-carbamoyl]methyl,
2-(4-cyanophenoxy)ethylcarbamoylmethyl,
2-(4-fluorophenyl)ethylcarbamoylmethyl,
2-phenylpropan-2-ylcarbamoylmethyl, benzylcarbamoylmethyl, dipropan-2-ylcarbamoylmethyl, phenethylcarbamoylmethyl, tert-butylcarbamoylmethyl,
2-(2,2-dimethylpropanoylamino)ethyl,
2-(3,3-dimethyl-2-oxo-indol-l-yl)ethyl, 2-(3,3-dimethylbutanoylamino)ethyl,
2-(tert-butylcarbamoyl)ethyl,
2-[3-(4-cyanophenyl)propanoylamino]ethyl, or
2-[3-(4-cyanophenyl)propanoyl-methyl-amino]ethyl.
Particular compounds of the invention that may be mentioned include the compounds of the Examples disclosed hereinafter, such as:
(i) N-(4-cyanobenzyl)-N-methyl-2-{7-[2-(2-methylρhenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl}acetamide;
(ii) 2-{7-[2-(2-chlorophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-(4- cyanobenzyl)-iV-methylacetamide; (iii) iV-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenyl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } -iV-methylacetamide;
(iv) iV-(4-cyanobenzyl)-N-me1iιyl-2-(7-{2-[2-(trifluoromethyl)phenyl]ethyl}-9- oxa-3,7-diazabicyclo[3.3. l]non-3-yl)acetamide;
(v) 2-(7-{2-[3,4-bis(difluoromethoxy)phenyl]ethyl}-9-oxa-3,7-diazabicycb- [3.3. l]non-3-yl)-N-(4-cyanobenzyl)-iV-nietb.ylacetamide;
(vi) iV--(4-cyanobenzyl)-iV-methyl-2-(7-{2-[4-(trimethylsilyl)phenyl]ethyl}-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl)acetamide;
(vii) iV-(4-cyanobenzyl)-2-(7- { 2~[4-(tximethylsilyl)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl)acetamide; (viii) N-(4-cyanobenzyl)-2-{7-[2-(3,4-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicydo[3.3.1]non-3-yl}-N-methylacetamide;
(ix) N-(4-cyanobenzyl)- N-methyl-2-(7- { 2-[4-(trifluoromethyl)phenyl]ethyl } -9- oxa-3 ,7-dia2abicyclo[3.3. l]non-3-yl)acetamide;
(x) iV-benzyl-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yljacetamide;
(xi) iV-(4-cyanobenzyl)-2- { 7-[2-(3-cyanophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl} acetamide;
(xii) iV-(4-cyanobenzyl)-2-{7-[2-(2,4-difluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (xiii) N-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(xiv) 2- {7-[2-(l ,2-benzisoxazol-3-yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl } -iV-(4-cyanobenzyl)acetamide
(xv) N-(4-cyanobenzyl)-2-(7-{2-[4-(difluoromethoxy)phenyl]ethyl}-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl)acetamide;
(xvi) N-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-cyano-3-fluorophenyl)ethyl]-9-oxa-
3,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xvii) iV-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-cyano-2-fluoroplienyl)ethyl]-9-oxa-
3,7-diazabicyclo[3.3.1]non~3-yl}acetamide;
(xviii) JV-(4-cyano-2-fluorobenzyl)-2- { 7-[2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7- diazabicyclo [3.3.1 ]non-3 -yl } acetamide; (xix) N-(4-cyanobenzyl)-2-{7-[2-(4-cyano-3-fluoroplienyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl }acetamide;
(xx) N-(4-cyanobenzyl)-2-{7-[2-(4-cyano-2-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xxi) JV-(4-cyanobenzyl)-2-(7-{2-[2-(difluoromethoxy)phenyl]ethyl}-9-oxa-3,7- diazabicyclo[3.3.l]non-3-yl)acetamide;
(xxii) iV:-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9- oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxiii) iV-(4-cyano-2-fl.uorobenzyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl } acetamide; (xxiv) N-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1 Jnon-3-yl} acetamide;
(xxv) N-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl}acetamide;
(xxvi) N-(4-cyano-2-fluorobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl]acetamide;
(xxvii) N-(fert-butyl)-2-{7-[4-(4-cyanophenyl)butyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl} acetamide;
(xxviii) N-(ferϊ-butyl)-2-{7-[3-(4-cyano-3-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl} acetamide; (xxix) N-(tert-butyl)-2- { 7-[2-(4-cyano-2-fluorophenoxy)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xxx) Λr-(4-cyano-2,6-difluorobenzyl)-2-{7-[3-(4-cyanophenoxy)proρyl]-9-oxa-
3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } acetamide;
(xxxi) iV-(4-cyano-2,6-difluorobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa- 3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxxii) iV-(4-cyano-2,6-difluorobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxxiii) N-(4-cyano-2,6-difluorobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3,7-diaza- bicyclo[3.3.1]non~3-yl]acetamide;
(xxxiv) iV-(4-cyanoben2yl)-2-{7~[3-(3-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (xxxv) iV-(4~cyanobenzyl)-2-{7-[3-(2-cyanophenoxy)proρyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3 -yl } acetamide;
(xxxvi) iV-(4-cyanobenzyl)-2-(7-{2-[2-(trifluoromethyl)phenyl]ethyl}-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(xxxvii) iV-(4-cyanobenzyl)-2-{7-[2-(2-methylplieπyl)ethyl]-9-oxa-3,7- diazabicyclo [3.3.1 ]non-3-yl } acetamide;
(xxxviii) 2- {7-[2-(2-chlorophenyl)ethyl]-9-oxa-3 ?7-diazabicyclo[3.3. l]non-3-yl} - iV-(4-cyanobenzyl)acetamide;
(xxxix) 2-{7-[2-(3-chlorophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-cyanobenzyl)acetamide; (xl) iV-(4-cyanobenzyl)-2-{ 7-[2-(2-methoxyphenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl}acetamiάe;
(xli) N-(4-cyanobenzyl)-2-{7-[2-(4-methoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1 ]non-3-yI } acetamide;
(xlii) N-(4-cyanobenzyl)-2-{7-[3-(4-cyano-2,6-difluorophenoxy)propyl]-9-oxa- SJ-diazabicyclotS.S.ljnon-S-ylJacetamide;
(xliii) JV-(4-cyanobenzyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl } acetamide;
(xliv) iV-(4-cyanobenzyl)-2-{7-[3-(4-cyano-3-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide; (xlv) iV-(4-cyanobenzyl)-2- {7-[3-(2-methoxyρhenoxy)ρropyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(xlvi) /V-(4-cyanobenzyl)-2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(xlvii) N-(4-cyanobenzyl)-2-{7-[2-(6-methylpyridin-2-yl)ethylj-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xlviii) N-(4-cyanobenzyl)-2-[7-(2-pyridin-2-ylethyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl] acetamide;
(xlix) N-(4-cyanobenzyl)-2-[7-(2-pyridin-4-ylethyl)-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl]acetamide;
(1) 2- { 7- [2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -N-(2- phenylethyl)acetamide; (li) 2-{7-[2-(4-cyanoρhenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-[2-
(4-fluorophenyl)ethyl]acetamide;
(Hi) N-(4-cyanobenzyl)-2- { 7-[2-(3 ,5-dimethylisoxazol-4-yl)ethyl]-9-oxa-3 J- diazabicyclo[3.3. l]non-3 -yl } acetamide;
(liii) N-(4-cyanobenzyl)-2-{7-[2-(lH-indol-3-yl)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}acetamide;
(liv) N-(4-cyanobenzyl)-2-[7-(imidazo[l,2-a]pyridin-2-ylmethyl)-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl]acetamide;
(lv) N-(4-cyanobenzyl)-2-{7-[2-(3,5-dimethyl-lH-ρyrazol-l-yl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3 -yl } acetamide; (lvi) N-(4-cyanobenzyl)-2-{7-[2-(3,4-dimethoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lvii) iV-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo [3.3.1 ]non-3 -yl } acetamide;
(lviii) 2-{7-t2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N- (4-fluorobenzyl)acetamide;
(lix) N-benzyl-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}acetamide;
(Ix) 2- { 7-[2~(4-cyanophenoxy)ethylj -9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl} -N-
[2-(4-fluorophenyl)ethyl]acetamide; (lxi) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-fluorobenzyl)acetamide;
(lxii) 2- { 7-[3 -(4-cyanophenoxy)proρyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } - iV-[2-(4-fluorophenyl)ethyl]acetamide;
(lxiii) N-(4-cyanobenzyl)-2-{7-[(3,5-dimethylisoxazol-4-yl)methyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(lxiv) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(2,4-difluorobenzyl)acetamide;
(lxv) 2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1 ]non-3-yl }- iV-(3-fluorobenzyl)acetamide;
(lxvi) N-(4-cyanobenzyl)-2-{7-[2-(2~fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (lxvii) 2- { 7-[3-(4-cyanophenoxy)propyl] -9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } - iV-(2-phenylethyl)acetamide;
(lxviii) 2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } - iV-(2-phenylethyl)acetamide;
(lxix)iV-(?-?^-butyl)-2-{7-[3-(4-cyano-2,6-difluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(lxx) N-(re/t-butyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxi) N-(4-cyanobenzyl)-2-{7-[3-(3,5-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide; (lxxii) iV-(4-cyanobenzyl)-2-{7-[3-(2,5-difluprophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lxxiii) iV-(4--cyanobenzyl)-2-{7-[3-(2,6-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(lxxiv) N-(4-cyanobenzyl)-2~{7-[3-(2-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(Ixxv) 2- { 7- t3-(4-cyanophenoxy)propyl] -9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } -
N-(2-fluorobenzyl)acetamide;
(lxxvi) 2-{7~[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV-(3-fluorobenzyl)acetamide; (lxxvii) iV-(4-cyanobenzyl)-2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(lxxviii) N-(4-cyanobenzyl)-2-{7-[4-(trifluoromethyl)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lxxix) 2-[7-(4-chlorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]-iV-(4-cyano- benzyl)acetamide;
(lxxx) iV-(4-cyanobenzyl)-2-[7-(4-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(lxxxi) N-(4-cyanobenzyl)-2-[7~(4-fluorobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yI]acetamide;
(lxxxii) iV-(4-cyanobenzyl)-2- { 7-[2-(3 ,4-difluorophenyl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide; (lxxxiii) iV-benzyl-2-{7-[3-(4-cyanoplienoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }-N-methylacetamide;
(lxxxiv) iV-benzyl-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } -N-methylacetamide;
(lxxxv) Λr-(4-cyanobenzyl)-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lxxxvi) iV-(4-cyanobenzyl)-2-(7-{2-[4-(trifluoromethyl)phenyl]ethyl}-9-oxa-3,7- diazabicycl,o[3.3.1]non-3-yl)acetamide;
(lxxxvii) 2- { 7-[2-(4-chlorophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -
JV-(4-cyanobenzyl)acetaπiide; (lxxxviii) iV-(4-cyanobenzyl)-2-{7-[2-(2,4-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1 ]non-3 -yl } acetamide;
(lxxxix) N-(4-cyanobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xc) iV-(4-cyanobenzyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xci) N-(4-cyanobenzyl)-2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}-iV-methylacetaniide;
(xcii) iV-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } -iV-methylacetamide; (xciii) N-(4~cyanobenzyl)-AT-methyl-2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl] acetamide;
(xciv) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xcv) iV-[2-(4-cyanophenoxy)ethyl]-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(xcvi) N- [2-(4-cy anophenoxy)ethyl] -2- [7-(3 -phenoxypropyl)-9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl]acetamide;
(xcvii) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-cyanoρhenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl } acetamide;
(xcviii) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl} acetamide; (xcix) iV-[2-(4~cyanophenoxy)ethyl]~2- [7-(2-phenylethyl)-9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl]acetamide;
(c) 2-[7-(4-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]-iV-[2-(4-cyano- phenoxy)ethyl] acetamide ;
(ci) iV-[2-(4-cyanophenoxy)ethyl]-2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl]acetamide;
(cii) 2-(7-benzyl-9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 ~yl)-N- [2-(4-cyanoρhenoxy)- ethyl] acetamide;
(ciii) iV-(4-cyanobenzyl)-2-(7- { 3 - [3 -(trifluoromethyl)phenoxy]propyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide; (civ) 2- {7-[3-(3-chlorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo [3.3. l]non-3-yl }- iV-(4-cyanobenzyl)acetamide;
(cv) iV-(4-cyanobenzyl)-2-{7-[3-(3-fluorophenoxy)ρroρyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cvi) 2-{7-[3-(4-chloro-2-fluoropb.enoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}-iV-(4-cyanobenzyl)acetamide;
(cvii) 2- { 7-[3-(4-chloro-3-fluorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl}-N-(4-cyanobenzyl)acetamide;
(cviii) N-(4-cyanobenzyl)-2- { 7-[3-(3,4-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (cvix) N-(4-cyanobenzyl)-2-(7- { 3 -[4-(trifluoromethyl)phenoxy]propyl } -9-oxa-3 ,7- diazabicyclo [3.3. l]non-3-yl)acetamide;
(cx) 2-{7-[3-(4-chlorophenoxy)ρropyl]-9-oχa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-cyanobenzyl)acetamide;
(cxi) iV-(4-cyanobenzyl)-2-[7-(3-phenoxypropyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(cxii) iV-(4-cyanobenzyl)-2-{7-[2-(3-fluoropb.enyl)etb.yl]-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3-yl } acetamide;
(cxiii) N-(4-cyanobenzyl)-2-(7-{2-[(3-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxiv) 2-(7- { 2-[(3-chlorobenzyl)oxy] ethyl } -9-oxa-3 ,7-diazabicyclo[3.3.1 ]non-3- yl)-ΛT-(4-cyanobenzyl)acetamide; (cxv) Λf-(4-cyanobenzyl)-2-[7-(2-{ [3-(trifluoromethyl)benzyl]oxy}eihyl)-9-oxa-
3,7-diazabicyclo[3.3.1]non-3-yl]acetamide;
(cxvi) N-(4-cyanobenzyl)-2-(7-{2-[(3-fluorobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxvii) 2-(7-{2-[(4-chlorobeiizyl)oxy]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl)-iV-(4-cyanobenzyl)acetamide;
(cxviii) N-(4-cyanobenzyl)-2-[7-(2-{[4-(trifluoromethyl)benzyl]oxy}ethyl)-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl]acetamide;
(cxix) iV-(4-cyanobenzyl)-2-(7- { 2-[(4-fluorobenzyl)oxy] ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide; (cxx) 2-{7-[2-(benzyloxy)ethyl]-9-oxa-3,7~diazabicyclo[3.3.1]non-3-yl}-N-(4- . cyanobenzyl)acetamide;
(cxxi) iV-(4-cyanobenzyl)-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)acetamide;
(cxxii) 2-{7-[2-(l-benzofuran-5-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- JV-(4-cyanobenzyl)acetamide;
(cxxiii) iV-benzyl-2-(7- { 2- [(4-cy anobenzyl)oxy] ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxxiv) N-(tert-butyϊ)-2- { 7-[2-(4-cyanophenoxy)ethylJ-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3-yl } -iV-methylacetamide; (cxxv) N-(tert-butyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } -iV-methylacetamide;
(cxxvi) N-(fert-butyl)-2-(7- { 2- [(4-cyanobenzyl)oxy] ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl)-iV-methylacetamide;
(cxxvii) N-(tert-butyϊ)-2-(7- { 2-[(4-cyanobenzyl)oxy]ethyl } -9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)acetamide;
(cxχviii) 2-{7-[2-(benzylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } -iV-(4-cyanobenzyl)acetamide;
(cxxix) N-(4-cyanobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cxxx) N-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide; (cxxxi) JV-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(cxxxii) Λ/'-(4-cyanobenzyl)-2-{7-[2-(2,3-dihydro-l-benzofuran-5-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(cxxxiii) iV-(4-cyanobenzyl)-2-[7-(2-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3 -yl] acetamide;
(cxxxiv) 2,2'-(9-oxa-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis[iV-(4-cyano- benzyl)acetamide] ;
(cxxxv) 2-(7-benzyl~9-oxa~3,7-diazabicydo[3.3.1]non-3-yl)-N-(4-cyanobenzyl)- acetamide; (cxxxvi) tert-butyl [2-(7-{2~[(4-cyanobenzyl)amino]-2-oxoethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)ethyl]carbamate;
(cxxxvii) 2- { 7-[2-(tert-butylamino)-2-oxoethyl] -9-oxa-3 ,7-diazabicyclo[3.3.1] - non-3-yl}-N-(4-cyanobenzyl)acetamide;
(cxxxviii) tert-butyl 7- { 2-[(4-cyanobenzyl)amino]-2-oxoethyl } -9-oxa-3 ,7-diaza- bicycloP-Sllnonane-S-carboxylate;
(cxxxix) _V-(2-{ 7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-4-cyanobenzaniide;
(cxl) iV-(tert-butyl)-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide; (cxli) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -N-
(l-metb.yl-l-phenylethyl)acetamide;
(cxlii) N-benzyl-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl} acetamide;
(cxliii) N-(ter?-butyl)-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}acetamide;
(cxliv) N-(2-{7-[2-(ter?-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)-3-(4-cyanophenyl)-N-methylpropanamide;
(cxlv) 2- { 7- [3 -(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1 ]non-3 -yl } -
N-( 1 -methyl- 1 -phenylethyl)acetamide;
(cxlvi) iV-benzyl-2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl } acetamide; (cxlvii) 2-{7-[(2S)-3 -(4-cyanophenoxy)-2-hydroxypropyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} -iV-(l-methyl-l-phenylethyl)acetamide;
(cxlviii) iV-benzyl-2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(cxlix) N-(fe^-butyl)-2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa- 3 ,7-diazabicyclo[3.3.1 ]non-3-yl } acetamide;
(cl) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV,JV-diisoρropylacetamide;
(cli) 2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}-N,N-diisopropylacetamide; (clii) N~(tert~butyl)-2- { 7- [3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl}acetamide;
(cliii) 3-[(7-{2-[(4-cyanobenzoyl)(methyl)amino3ethyl}-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3~yl)methyl]phenyl 4-methylbenzenesulfonate;
(cliv) 4-cyano-N-(2-{7-[3-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(civ) 4-cyano-iV- {2-[7-(3,5-diethoxybenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-iV-inethylbenzamide;
(clvi) 4-cyano-iV-methyl-N-(2-{7-[3-(trifluorometb.oxy)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide; (clvii) 4-cy ano-N- { 2- [7-(3 -isopropoxybenzyl)-9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-
3-yl]etb.yl}-iV-methylbenzamide;
(clviii) 4-cyano-iV-{2-[7-(3-ethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl } -N-methylbenzamide;
(clix) N-(2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl}ethyl)-3,4-difluorobenzamide;
(clx) iV-(2-{7-[2-(4-cyanophenoxy)eth.yl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- ethyl)-3 ,4-difluorobenzamide;
(clxi) 4-cyano-iV-{2-[7-(2,6-(iimethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl]ethyl}-N-methylbenzamide;
(clxii) 4-cyano-N- {2-[7-(2,3-dimethoxybenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-
3-yl] ethyl } -iV-methylbenzamide; (clxiii) 4-cyano-iV-{2-[7-(2-methoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl}-iV~methylbenzamide;
(clxiv) 4-cyano-iV-{2-[7-(3-methoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl] ethyl } -JV-methylbenzamide;
(clxv) 4-cyano-7V-(2- { 7-[2-(2,6-difluorophenoxy)ethyl]-9-oxa-3,7~diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(clxvi) 4-cyano-JV-(2- { 7- [2-(3 ,5-dimethylisoxazol-4-yl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide;
(clxvii) 4-cyano-N-(2-{7-[(3,5-dimethylisoxazol-4-yl)methyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide; (clxviii) 4-cyano-iV-(2-{7-[2-(3,4-difluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(clxix) 4-cyano-iV-(2-{7-[2-(2,4-difluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide;
(clxx) N-(2~ { 7-[3-(4-chlorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl}ethyl)-4-cyanobenzamide;
(clxxi) 4-cyano-iV-(2-{7-[3-(2,6-difluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(clxxii) 4-cyano-iV-(2-{7-[3-(2,4-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)benzamide; (clxxiii) 4- [(7- { 2- [(4-cyanobenzoyl)amino]ethyl } -9-oxa-3 ,7-diazabicycIo[3.3.1]- non-3-yl)methyl]ρhenyl methanesulfonate;
(clxxiv) 4-cyano-N-(2-{7-[(6-fluoro-4H-l,3-benzodioxin-8-yl)methyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}ethyl)benzamide;
(clxxv) 4-cyano-N-(2-{7-[2-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo- [3.3. l]non-3-yl}ethyl)-N-methylbenzamide;
(clxxvi) 4-cyano-iV-(2-{7-[4-(difluoroniethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3~yl} ethyl)-N-methylbenzamide;
(clxxvii) 4-[2-(7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl)ethyl]phenyl meihanesulfonate;
(clxxviii) 4-cyano-iV-(2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo-
[3.3.1 ]non-3-yl } ethyl)benzamide; (clxxix) 4-cyano-iV-(2-{7-[2-(4-cyanophenyl)eth.yl]-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3 -yl } ethyl)benzamide;
(clxxx) iV-(2-{7-[2-(4-cyanophenoxy)etb.yl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } ethyl)benzamide;
(clxxxi) 4-cyano-iV-(2- { 7-[2-(4-fluorophenyl)ethyl]-9~oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(clxxxii) 4-cyano-iV-(2- {7- [2-(4-fluorophenoxy)ethyl] -9-oχa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide;
(clxxxiii) 4-cyano-iV-(2-{7-[3-(4-fluoroρhenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3-yl } ethyl)benzamide; (clxxxix) iV-(3-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl }propyl)benzamide;
(cxc) 4-cyano-N- { 2- [7-(2,6-difluorobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3 - yl]ethyl}benzamide;
(cxci) 4-cyano-iV- { 2-[7-(2,6-dimethylbenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}benzamide;
(cxcii) N- { 2-[7-(l ,3-benzodioxol-4-ylmethyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-4-cyanobenzamide;
(cxciii) 4-cyano-iV-metb.yl-iV-{2-[7-(3-plienylpropyl)-9-oxa-3J7-diazabicyclo-
[3.3.1]non-3-yl]ethyl}benzamide; (cxciv) 4-cyano-iV-methyl-iV- { 2- [7-(2~phenylethyl)~9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl]ethyl}benzamide;
(cxcv) iV-[2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]-4-cyano-N- methylbenzamide;
(cxcvi) 4-cyano-N-(2-{7-[4-fluoro-2-(trifluoromethyl)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)benzamide;
(cxcvii) 4-cyano-N-(2-{7-[2-(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3~yl } ethyl)benzamide;
(cxcviii) 4-cyano-iV- { 2-[7-(5-fluoro-2-methylbenzyl)-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl]ethyl }benzamide;
(cxcix) iV-(2-{7-[2,4-bis(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3 -yl } ethyl)-4-cyanobenzamide; (cc) 4-cyano-iV-(2- { 7-[2-(diflu.oromethoxy)benzyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)benzamide;
(cci) 4-cyano-N-{2-[7-(3-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl }benzamide;
(ccii) 4-cyano-iV-(2-{7-[3-(3-methoxyphenyl)propyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(cciii) 4-cyano-N-{2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl jbenzamide;
(cciv) 4-cyano-N-(2-{7-[3-(4-cyanophenyl)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3 -yl } ethyl)benzamide; (ccv) N-{2-[7-(l,3-benzodioxol-5-ylmethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yljethyl }-4-cyanoben2amide;
(ccvi) 4-cyano-iV- { 2-[7-( l-naphthylmethyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl }benzamide;
(ccvii) 4-cyano-Λir-{2-[7-(quinolin-8-ylmetiiyl)-9-oxa-3,7-diazabicyclo[3.3.1]non- 3-yl]ethyl}benzaxnide;
(ccviii) 4-cyano-iV-(2-{7-[3-(trifluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(ccix) N-{ 2-[7-(4-chlorobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]ethyl } -4- cyanobenzamide; (ccx) 4-cyano-N-(2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl}ethyl)benzamide;
(ccxi) 4-cyano-N-{2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl }benzamide;
(ccxii) 4-cyano-N-(2-{7-[4-(trifluororQethyl)benzyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(ccxiii) 4-cyano-iV-{ 2-[7-(2-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl}benzamide;
(ccxiv) 4-cyano-iV-{2-[7-(2,4-difluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl}benzamide;
(ccxv) 4-cyano-iV- { 2-[7-(4-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl } benzamide;- (ccxvi) 4-(3 - { 7- [(4-oxo-3 ,4-dihydrophthalazin- 1 -yl)methyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl }propoxy)benzonitrile;
(ccxvii) 4-cyano-N-{2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl }benzamide;
(ccxviii) tert-butyl 2-(7- { 2-[(4-cyanobenzoyl)amino]ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl)-2-methylpropanoate;
(ccxix) tert-butyl [2-(7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)ethyl]carbamate;
(ccxx) N-(2- { 7-[2-(tert-butylamino)~2-oxoethyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3 -yl } ethyl)-4-cyanobenzamide; (ccxxi) tert-butyl (7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3-yl)acetate;
(ccxxii) 4-cyano-iV- { 2-[7-(3 ,3-dimethyl-2-oxobutyl)-9-oxa-3 ,7-diazabicyclo-
[3.3.1]non-3-yl]ethyl}benzamide;
(ccxxiii) 7Y-[2-(7-benzyl-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)ethyl]-4-cyano~ benzamide;
(ccxxiv) 4-[((2S)-3-{7-[2-(l,3-dioxo-l,3-dihydro-2H-isoindol-2-yl)ethyl]-9-oxa-
3,7-diazabicyclo[3.3. l]non-3-yl } -2-hydroxypropyl)oxy]benzonitrile;
(ccxxv) iV-(fer?-butyl)-3-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.l]non-3-yl}propanamide; (ccxxvi) 4-(2-{7-[2-(2,2-dimethyl-4-oxo-3,4-dihydro-l,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl} ethoxy)benzonitrile;
(ccxxvii) 4-(3- { 7- [2-(2,2-dimethyl-4-oxo-3 ,4-dihydro- 1 ,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl }propoxy)benzonitrile;
(ccxxviii) 4-[((2S)-3-{7-[2-(3,3'dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]- 9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } -2-hydroxypropyl)oxy]benzojQitri]e;
(ccxxix) 4-(3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dϋiydro-lH-indol-l-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl }propoxy)benzonitrile;
(ccxxx) 4-[(3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3.1 Jnon-3 -yl }propyl)sulf onyljbenzonitrile;
(ccxxxi) 4-(2- { 7-[2-(3 ,3-dimethyl-2-oxo-2,3-dihydro- lH-indol- l-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3.1 ]non-3-yl } ethoxy)benzonitrile; (ccxxxii) iV-(2-{7-[2-(4-acetylphenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } ethyl)-3 -(4-cy anophenyl)propanamide;
(ccxxxiii) 3-(4-cyanophenyl)-iV-(2- {7-[2-(4-methoxyphenyl)ethyl]-9-oxa-3 ,7- diazabicyclo [3.3.1 ]non-3 -yl } ethyl)propanamide;
(ccxxxiv) 3~(4-cyanophenyϊ)-N-(2- { 7-[2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)propanamide;
(ccxxxv) 3-(4-cyanophenyl)-iV-(2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)propanamide;
(ccxxxvi) 3~(4:cyanophenyl)-N-(2-{7-[2-(2,6-dimethylphenoxy)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3 -yl } ethyl)pr opanamide; (ccxxxvii) N- { 2-[7-(3-tert-butoxypropyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl } -3 -(4-cyanophenyl)propaπamide;
(ccxxxviii) 3-(4-cyanophenyl)-Λ/r- { 2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3-yl] ethyl }propanamide;
(ccxxxix) 4-[((2S)-3- { 7-[2-(2,2-dimethyl- l-oxido-4-oxo-3 ,4-dihydro- 1 ,5-benzo- thiazepin-5(2H)-yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -2-hydroxy- propyl)oxy]benzonitrile;
(ccxl) 4-[((2S)-3-{7-[2-(2,2~dimethyl-4-oxo-3,4-dihydro-l,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3 -yl } -2-hydroxypropyl)oxy]- benzonitrile; (ccxli) N-(2- { 7- [2-(ter?-butylamino)-2-oxoethyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)-3-(4-cyanophenyl)-N-methylpropanamide;
(ccxlii) 4-[((2S)-3-{7-[2-(2,2-dimethyl-l,l-dioxido~4-oxo-3,4-dihydro-l,5-benzo- thiazepin-5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxy- propyl)oxy]benzonitrile; (ccxliii) 3-(4-cyanoρhenyl)-iV-(2- { 7-[2-(4-methoxyphenyl)-2-oxoethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl}ethyl)-iV-methylρropanainide;
(ccxliv) N-[2-(7-benzyl-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)etitiyl]-3-(4-cyano- phenyl)-N~methylpropanamide;
(ccxlv) tert-butyl [2-(7-{2-[[3-(4-cyanoρhenyl)propanoyl](methyl)amino]ethyl}-9- oxa-3,7-diazabicyclo[3.3. l]non-3-yl)ethyl]carbamate; (ccxlvi) 3-(4-cyanophenyl)-ΛT-{2-[7-(3,3-dimethyl-2-oxobutyl)-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl]ethyl }propanamide;
(ccxlvii) 4-[((2S)-3-{7-[2-(2,2-dimethyl-4-oxo-3;4-dihydro-l,5-benzothiazepin-
5(2H)-yl)ethyl]-9-oxa~3 ,7-diazabicyclo[3.3. l]non-3-yl } -2-hydroxyρropyl)oxy]- benzonitrile; (ccxlviii) tert-butyl { 2-[7-(2- { [3-(4-cyanophenyl)propanoyl] amino } ethyl)-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl] ethyl } carbamate;
(ccxlix) Λ/r-(2-{7-[(2S)-3-(4~cyanoρhenoxy)-2-hydroxypropyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)-3 ,3-dimethylbutanamide;
(ccl) iV-(2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)-2,2-dimethylpropanamide;
(ccli) 4-cyano-iV-{2-[7-(3,5-dimethoxybeiizyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl]-ethyl}-N-methylbenzamide;
(cclii) iV-(4-cyano-2-fluorobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7- diaza-bicyclo[3.3. l]non-3-yl } acetamide; (ccliii) N-(4-cyanobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl] acetamide;
(ccliv) iV-(4-cyanobenzyl)-2-{7-[3-(2,4-difluorophenoxy)proρyl]-9-oxa-3,7-diaza- bicyclo[3.3.1 Jnon-3-yl } acetamide;
(cclv) iV-(4-cyanobenzyl)-2-{7-[2-(3-methoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cclvi) 4-cyano-2V-(2- { 7-[3-(difluoromethoxy)benzyl]-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3 -yl } ethyl)-iV-methylbenzamide;
(cclvii) 4-cvano-Ν-(2-{7-r3-ffluoromethoxy)benzyn-9-oxa-3J-diazabicyclo-
F3.3. nnon-3-yl )ethyl)-N-methylbenzamide; (cclvm) N-(2-{7-r3,5-bis(fluoromethoxy)benzyl1-9-oxa-3,7-diazabicyclo-
[3.3. llnon-3-yl I ethyl)-4-cvano-N-methylbenzamide,
and salts and/or solvates thereof.
Particular compounds of the invention that may be mentioned include: (a) compounds (i) to (cclvi) above, other than compounds (ccxxv) to (ccl) above; (b) compounds (xxiv), (xxix), (xli), (xliv), (lxix), (lxxiii), (lxxxvii) to (lxxxix), (xcviii), (cii), (cviii), (ex), (cxxxi), (cxlv), (clii), (cc) and (ccli) to (cclvi) above; or
(c) compounds (ccli) to (cclviii) above, and salts and/or solvates thereof.
Preparation
Compounds of formulae I, Ic, Id and Ie may be prepared by analogy with procedures known to those skilled in the art (e.g. the procedures outlined in WO 01/28992, WO 02/83690, WO 02/83691, WO 02/28863, WO 02/28864 and WO 2004/035592, the disclosures of which documents are hereby incorporated by reference). Thus, according to the invention there is also provided a process for the preparation of compounds of formulae I, Ic, Id and Ie which comprises:
(a) reaction of a compound of formula II,
R!-L! in wherein L1 represents a leaving group such as halo, alkanesulfonate, perfluoroalkanesulfonate, arenesulfonate, -OC(O)-E-R7, imidazole or R21O- (wherein R21 represents, for example, C1-1Q alkyl or aryl, which groups are optionally
substituted by one or more halo or nitro groups) and X, R1 and R7 are as hereinbefore defined, for example at between ambient temperature (e.g. 25°C) and reflux temperature in the presence of a suitable base (e.g. triethylamine, potassium carbonate or a bicarbonate, such as sodium bicarbonate) and an appropriate solvent (e.g. dichloromethane, chloroform, acetonitrile, iVjV-dimethylformamide, THF, toluene, water, a lower alkyl alcohol (e.g. ethanol) or mixtures thereof);
(b) reaction of a compound of formula IV,
wherein L represents a leaving group such as halo, alkanesulfonate (e.g. mesylate), perfluoroalkanesulfonate or arenesulfonate (e.g. 2- or 4-nitrobenzenesvufonate, toluenesulfonate or benzenesulfonate) and R2, R3, R4 and Z are as hereinbefore defined, for example at elevated temperature (e.g. between 35°C and reflux temperature) in the presence of a suitable base (e.g. triethylamine or potassium carbonate) and an appropriate organic solvent (e.g. acetonitrile, dichloromethane, chloroform, dimethylsulfoxide, MN-dimemylformamide, a lower alkyl alcohol (e.g. ethanol), isopropyl acetate, water or mixtures thereof);
(c) for compounds of formula I in which Z represents -C(O)-N(R14b)-B-, coupling of a corresponding compound of formula VI,
wherein R1 to R3, R14b, R41 to R46 and B are as hereinbefore defined, with a compound of formula VII,
R4-C(O)-L3 Vn wherein L3 represents a leaving group, such as halo, OH, imidazole or R21O-, and R4 and R21 are as hereinbefore defined, for example under conditions known to those skilled in the art (such as in the presence of an appropriate base (e.g. pyridine, DMAP, TEA, 2,4,6-collidine or DIPEA) and a suitable organic solvent (e.g. dichloromethane, acetonitrile, EtOAc or DMF) and optionally in the presence of a coupling agent (e.g. oxalyl chloride in DMF, EDC, DCC, HBTU, HATU, PyBOP or TBTU, optionally in combination with an alcohol such as HOBT));
(d) for compounds of formula I in which R1 represents C1-12 alkyl (which alkyl group is attached to the oxabispidine iV-atom via a CH2 group and is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het1, -0R5c, -C(O)-E-R7, -C(O)N(R8a)R5d, -OC(O)N(R8b)R5e, -S(O)2R9a, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), reaction of a compound of formula II, as hereinbefore defined, with a compound of formula VHI,
R1X-CHO vm wherein Rlx represents aryl, Het1 or C1-U alkyl (which alkyl group is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het1, -0R5c, -C(O)-E-R7, -C(O)N(R8a)R5d, -OC(O)N(R8b)R5e, -S(O)2R9a, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), and R5c to R5e, R7, R8a, R8b, R9a to R9d, E and Het1 are as hereinbefore defined, for example under conditions known to those skilled in the art (such as at from ambient temperature (e.g. 15 to 250C) to elevated temperature (e.g. 50 to 700C) in the presence of a suitable solvent (e.g. THF, dichloromethane or a C1-3 alkyl alcohol) and optionally in the presence of a
catalyst, such as a base (e.g. a tertiary amine such as triethylamine) or a Lewis acid or proton source (e.g. a carboxylic acid such as acetic acid)), followed by reduction in the presence of a reducing agent (e.g. NaB(OAc)3H or NaBH3CN), for example under conditions known to those skilled in the art (e.g. at from ambient temperature (e.g. 15 to 25°C) to elevated temperature (e.g. 50 to 700C) in the presence of a suitable solvent (e.g. THF, dichloromethane or a C1-3 alkyl alcohol));
(e) for compounds of formula I in which R2 and R3 both represent H and A (if present) represents C1-3 alkylene (optionally substituted by one or more groups selected from F and C1^ alkyl), reaction of a compound of formula IV, as hereinbefore defined, with a compound of formula IX,
O wherein Za represents -N(R14a)-C(O)-Aa- or -C(O)-N(Rl4b)-B-, Aa represents C1-3 alkylene (optionally substituted by one or more groups selected from F and C1-3 alkyl) and R4, R14a, R14b and B are as hereinbefore defined, for example under conditions known to those skilled in the art (such as those described in respect of process (d) above), followed by reduction in the presence of a reducing agent (e.g.
NaB(OAc)3H or NaBH3CN), for example under conditions known to those skilled in the art (such as those described in respect of process (d) above);
17 I S 17 I R
(f) conversion of one R or R group to another R or R group using techniques well known to those skilled in the art; or
(g) for acid addition salts of compounds of formula I, reaction of a corresponding compound of formula I with an acid.
Compounds of formulae II, III, IV, V, VI, VII, VIII and IX may be prepared according to or by analogy with procedures known to those skilled in the art (e.g. procedures described or referred to in WO 01/28992, WO 02/28863, WO 02/28864, WO 02/83690, WO 02/83691 and WO 2004/035592, the disclosures of which
documents are hereby incorporated by reference). However, particular routes to specific intermediates are described below.
Compounds of formula II in which R14a or R14b represents C1-6 alkyl may be prepared by reaction of a corresponding compound of formula II in which R14a or R14b, respectively, represents H, or a (oxabispidine N-) protected derivative thereof, with a compound of formula X,
Cx-6 alkyl-L2 X wherein L2 is as hereinbefore defined, for example under conditions known to those skilled in the art, such as reaction, at sub-ambient temperature (e.g. from -80 to
-60°C or from 0 to 15°C), in the presence of a suitable strong base (such as an alkali metal hydride (e.g. NaH), an alkali metal alkoxide (e.g. potassium te/t-butoxide) or an alkali metal amide (e.g. IJDA or, particularly, LiHMDS)) and an appropriate solvent (e.g. THF).
Compounds of formula IV may be prepared by deprotection of a corresponding compound of formula XI,
or an acid addition salt thereof, wherein R g represents an amino protective group (e;g. benzyl or benzenesulfonyl) and R1 and R41 to R46 are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. when RPg represents benzyl, catalytic hydrogenation, or, when R?ε represents benzenesulfonyl, hydrolysis in the presence of a strong acid such as hydrobromic or concentrated sulfuric acid (e.g. about 80% sulfuric acid at about 13O0C)).
Compounds of formula V in which Z represents -N(R14a)-C(O)-A- may be prepared by reaction of a corresponding compound of formula XH,
wherein R2, R3, L2 and L3 are as hereinbefore defined, with a compound of formula XIII,
R4-N(H)R14a XIII wherein R4 and Rl4a are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. those described in relation to process (c) above).
Compounds of formula VI in which R 4b represents H and B represents C1-3 alkylene that is unsubstituted α- to the NH2 group may be prepared by reduction of a corresponding compound of formula XIV,
wherein Ba represents a direct bond or C1-2 alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl, and R1 to R3 and R41 to R46 are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. catalytic hydrogentation, i.e. reaction with hydrogen gas in the presence of a suitable catalyst (e.g. a platinum group metal optionally supported on a substrate such as a carbon black, particularly palladium on carbon or Raney- Nickel®) and an appropriate solvent (e.g. a C1-4 alcohol, such as ethanol or, particularly, methanol), and optionally in the presence of ammonia).
Compounds of formula IX may be prepared by oxidation of a corresponding compound of formula XV,
wherein R4 and Za are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. Swern oxidation conditions, such as reaction, at from -80 to -600C, in the presence of a mixture of oxalyl chloride and DMSO, a suitable base (e.g. a tertiary amine such as TEA) and an appropriate solvent (e.g. DCM)).
Compounds of formula XI in which R41 to R46 all represent H may be prepared by dehydrative cyclisation of a corresponding compound of formula XVI,
wherein R1 and RPg are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. reaction in the presence of a suitable dehydrating reagent, such as sulfuric acid (e.g. concentrated sulfuric acid) or a sulfonic acid (e.g. an alkane or perfluoroalkanesulfonic acid, such as methanesulfonic acid, including anhydrous methanesulfonic acid)).
When prepared via sulfuric acid-catalysed dehydrative cyclisation of compounds of formula XVI, compounds of formula XI in which RPg represents an acid-labile protective group (e.g. benzenesulfonyl) may be converted in situ (i.e. in a "one- pot" process starting with a compound of formula XVI), by use of concentrated (e.g. about 80%) sulfuric acid at elevated temperature (e.g. about 130°C), to a corresponding compound of formula IV.
When reacted with sulfuric acid, the compounds of formulae XI and XVI may either be added to sulfuric acid or vice versa.
Compounds of formula XIV may be prepared by reaction of a corresponding compound of formula IV, as hereinbefore defined, with a compound of formula XVII,
NC-Ba-C(R2)(R3)-L2 XVII wherein R2, R3, Ba and L2 are as hereinbefore defined, for example under conditions known to those skilled in the art (e.g. those described in respect of process (b) above).
Compounds of formula XV in which Za represents -C(O)-N(R14b)-B- may be prepared by reaction of a corresponding compound of formula XVIII,
XVIII
wherein R14b and B are as hereinbefore defined, with a compound of formula XIX, R4-C(O)-L3 XIX for example under conditions known to those skilled in the art (e.g. those described in relation to process (c) above).
Compounds of formula XVI may be prepared: (a) by reaction of a compound of formula XX,
in either racemic or diastereomerically enriched form, wherein RPg is as hereinbefore defined, with a compound of formula XXI, R!-NH2 XXI wherein R1 is as hereinbefore defined; or
(b) by reaction of a compound of formula XXII,
in either racemic or diastereomerically enriched form, wherein R1 is as hereinbefore defined, with a compound of formula XXIII, RPg-NH2 XXIII wherein RPg is as hereinbefore defined,
in either case under conditions known to those skilled in the art (e.g. by reaction at between ambient (e.g. 25°C) and reflux temperature in the presence of a C1-4 alkyl alcohol (e.g. ethanol, IMS or methanol)). Reaction may, in one embodiment, be conducted such that the compounds of formula XX and XXI (or, alternatively, the compounds of formulae XXII and XXIII) are added, separately, simultaneously and at a substantially equivalent rate of moles per minute, to a reaction vessel containing solvent.
Compounds of formulae XX and XXII may be prepared by reaction of a corresponding compound of formula XXIV,
in either racemic, enantiomeric or enantiomerically enriched form, wherein L4 represents a leaving group (e.g. arenesulfonate, perfluoroalkanesulfonate or alkanesulfonate (such as ^-toluenesulfonate, 2- or 4-nitrobenzenesulfonate, methanesulfonate, benzenesulfonate or trifluoromethanesulfonate) or, particularly, halo) with:
(a) for the preparation of a compound of formula XX, a compound of formula XXIII, as hereinbefore defined; or
(b) for the preparation of a compound of formula XXII, a compound of formula XXI, as hereinbefore defined, in both instances under conditions known to those skilled in the art (e.g. reaction at from ambient temperature (e.g. 25°C) to elevated temperature (e.g. 35 to 600C) in the presence of a suitable base (e.g. an alkali metal carbonates, an alkali metal hydrogencarbonates or, particularly, an alkali metal hydroxide such as sodium hydroxide) and an appropriate solvent (e.g. water, a C1-4 alkyl alcohol (e.g. ethanol, IMS or methanol), or a mixture thereof)).
In one embodiment, compounds of formulae XX and XXII are prepared by reaction of either a compound of formula XXIII or a compound of formula XXI with at least two equivalents of a compound of formula XXIV and at least two equivalents of base under conditions where the reaction is performed by addition
of base to an aqueous mixture of the compounds of formulae XXIII and XXIV (or, alternatively, compounds of formulae XXI and XXIV) and the rate of base addition is controlled so as to maintain, during the addition of base, the reaction pH within a certain range (e.g. between pH 10 and pH 13) for as long as possible (e.g. at least 5 times longer within said range than outside of it).
Compounds of formulae X, XII, XIII, XVII, XVIII, XEK, XXI, XXIII and XXW, and derivatives thereof, are either commercially available, are known in the literature, or may be obtained either by analogy with the processes described herein, or by conventional synthetic procedures, in accordance with standard techniques, from readily available starting materials using appropriate reagents and reaction conditions.
Substituents on the aryl (e.g. phenyl), and (if appropriate) heterocyclic, group(s) in compounds defined herein may be converted to other claimed substituents using techniques well known to those skilled in the art. For example, hydroxy may be converted to alkoxy, phenyl may be halogenated to give halophenyl, nitro may be reduced to give amino, halo may be displaced by cyano, etc.
The skilled person will also appreciate that various standard substituent or functional group interconversions and transformations within certain compounds of formula I will provide other compounds of formulae I. For example, hydroxy may be either reduced to alkylene or converted to halo.
The compounds of the invention may be isolated from their reaction mixtures using conventional techniques.
It will be appreciated by those skilled in the art that, in the process described above, the functional groups of intermediate compounds may be, or may need to be, protected by protecting groups.
Functional groups which it is desirable to protect include hydroxy, amino and carboxylic acid. Suitable protecting groups for hydroxy include trialkylsilyl and diarylalkylsilyl groups (e.g. fert-butyldimethylsilyl, tøt-butyldiphenylsilyl or trimethylsilyl), tetrahydropyranyl and alkylcarbonyl groups (e.g. methyl- and ethylcarbonyl groups). Suitable protecting groups for phenoxy include alkyl (i.e. ether) groups, such as methyl. Suitable protecting groups for amino include benzyl, sulfonamido (e.g. benzenesulfonarnido), tert-butyloxycarbonyl, 9-fluorenyl- methoxycarbonyl or benzyloxycarbonyl. Suitable protecting groups for amidino and guanidino include benzyloxycarbonyl. Suitable protecting groups for carboxylic acid include C1-6 alkyl or benzyl esters.
The protection and deprotection of functional groups may take place before or after any of the reaction steps described hereinbefore. Protecting groups may be removed in accordance with techniques which are well known to those skilled in the art and as described hereinafter.
The use of protecting groups is described in "Protective Groups in Organic Chemistry", edited by J.W.F. McOmie, Plenum Press (1973), and "Protective Groups in Organic Synthesis", 3rd edition, T.W. Greene & P.G.M. Wutz, Wiley- Interscience (1999).
Persons skilled in the art will appreciate that, in order to obtain compounds of the invention in an alternative, and, on some occasions, more convenient, manner, the individual process steps mentioned herein may be performed in a different order, and/or the individual reactions may be performed at a different stage in the overall route (i.e. substituents may be added to and/or chemical transformations performed upon, different intermediates to those associated hereinbefore with a particular reaction). This will depend inter alia on factors such as the nature of other functional groups present in a particular substrate, the availability of key intermediates and the protecting group strategy (if any) to be adopted. Clearly, the type of chemistry involved will influence the choice of reagent that is used in the
said synthetic steps, the need, and type, of protecting groups that are employed, and the sequence for accomplishing the synthesis.
It will also be appreciated by those skilled in the art that, although certain protected derivatives of compounds of formula I, which may be made prior to a final deprotection stage, may not possess pharmacological activity as such, they may be administered parenterally or orally and thereafter metabolised in the body to form compounds of the invention which are pharmacologically active. Such derivatives may therefore be described as "prodrugs". Moreover, certain compounds of formula I may act as prodrugs of other compounds of formula I.
All prodrugs of compounds of formula I are included within the scope of the invention.
Some of the intermediates referred to hereinbefore are novel. According to a further aspect of the invention there is thus provided:
(A) a compound of formula II, as hereinbefore defined, or a protected derivative thereof;
(B) a compound of formula IV, as hereinbefore defined, wherein R1 represents the following structural fragment
(C) a compound of formula IV, XI, XVI or XXII, as hereinbefore defined, in which the group R1 represents a structural fragment of the formula
wherein Q4 represents C2-4 n-alkylene optionally substituted by one or more substituents selected from halo, methyl and hydroxy, and Rla to Rle are as hereinbefore defined, or a protected derivative thereof; and
(D) a compound of formula XFV, as hereinbefore defined, or a protected derivative thereof.
Compounds of formula IV described at (B) above that may be mentioned include those in which: Rlal represents F; Rlbl and Rlel both represent H; Rlcl represents Cl, cyano or, particularly, F.
The compounds defined at (C) above can alternatively be represented as compounds of formulae XXV, XXVI, XXVH and XXVDI,
XXV
XXVlI
XXVIII
in which compounds, R41 to R46, RPg, Rla to Rle and Q4 are as hereinbefore defined, or protected derivatives thereof.
Compounds of formulae XXV to XXVIII that may be mentioned include those in which:
Q4 represents unsubstituted C2-4 alkylene, such as (CH2)3 or, particularly, (CH2)2; Rla represents F or, particularly, H;
Rlb represents methoxy or, particularly, H; R Rllcc rreepprreesseennttss CCll,, FF,, mmeetthhooxxyy or, particularly, H; Rld and Rle both represent H.
Particular compounds of formulae XXV to XXVIII that may be mentioned include those in which:
Q4 represents (CH2)2; and Rla to Rle all represent H.
Values of R41 to R46 that may be mentioned in relation to compounds of formulae XXV and XXVI include H.
Values of RPg that may be mentioned in relation to compounds of formulae XXVI and XXVπ include benzyl and benzenesulfonyl.
Medical and pharmaceutical use
Compounds of the invention are useful because they possess pharmacological activity. They are therefore indicated as pharmaceuticals.
Thus, according to a further aspect of the invention there is provided the compounds of the invention for use as pharmaceuticals.
In particular, the compounds of the invention exhibit myocardial electrophysiological activity, for example as demonstrated in the test described below.
The compounds of the invention are thus expected to be useful in both the prophylaxis and the treatment of arrhythmias, and in particular atrial and ventricular arrhythmias.
The compounds of the invention are thus indicated in the treatment or prophylaxis of cardiac diseases, or in indications related to cardiac diseases, in which arrhythmias are believed to play a major role, including ischaemic heart disease, sudden heart attack, myocardial infarction, heart failure, cardiac surgery and thromboembolic events.
In the treatment of arrhythmias, compounds of the invention have been found to selectively delay cardiac repolarization and increase refractoriness.
According to a further aspect of the invention, there is provided a method of treatment of an arrhythmia which method comprises administration of a therapeutically effective amount of a compound of the invention to a person suffering from, or susceptible to, such a condition.
Pharmaceutical preparations
The compounds of the invention will normally be administered orally, subcutaneously, intravenously, intraarterially, transdermally, intranasally, by inhalation, or by any other parenteral route, in the form of pharmaceutical preparations comprising the active ingredient either as a free base or a non-toxic organic or inorganic acid addition salt, in a pharmaceutically acceptable dosage form. Depending upon the disorder and patient to be treated, as well as the route of administration, the compositions may be administered at varying doses.
According to a further aspect of the invention there is thus provided a pharmaceutical formulation including a compound of the invention in admixture with a pharmaceutically acceptable adjuvant, diluent or carrier.
Suitable daily doses of the compounds of the invention in therapeutic treatment of humans are about 0.005 to 50.0 mg/kg body weight at oral administration and about 0.005 to 15.0 mg/kg body weight at parenteral administration. Preferable ranges of daily doses of the compounds of the invention in therapeutic treatment of humans are about 0.005 to 20.0 mg/kg body weight at oral administration and about 0.005 to 10.0 mg/kg body weight at parenteral administration.
The compounds of the invention may also be combined with any other drugs useful in the treatment of arrhythmias and/or other cardiovascular disorders.
Thus, according to a further aspect of the invention, there is provided a combination product comprising:
(A) a compound of the invention, as hereinbefore defined, or a pharmaceutically acceptable derivative thereof; and
(B) an anticoagulant,
wherein each of components (A) and (B) is formulated in admixture with a pharmaceutically-acceptable adjuvant, diluent or carrier.
Such combination products provide for the administration of compounds of the invention in conjunction with the other therapeutic agent, and may thus be presented either as separate formulations, wherein at least one of those formulations comprises a compound of the invention and at least one comprises the other therapeutic agent, or may be presented (i.e. formulated) as a combined preparation (i.e. presented as a single formulation including a compound of the invention and the other therapeutic agent).
Thus, there is further provided:
(1) a pharmaceutical formulation including a compound of the invention, as hereinbefore defined, or a pharmaceutically acceptable derivative thereof, an anticoagulant, and a pharmaceutically-acceptable adjuvant, diluent or carrier; and
(2) a kit of parts comprising components:
(a) a pharmaceutical formulation including a compound of the invention, as hereinbefore defined, or a pharmaceutically acceptable derivative thereof, in admixture with a pharmaceutically-acceptable adjuvant, diluent or carrier; and
(b) a pharmaceutical formulation including an anticoagulant with a pharmaceutically-acceptable adjuvant, diluent or carrier,
which components (a) and (b) are each provided in a form that is suitable for administration in conjunction with the other.
When used herein, the term "an anticoagulant" includes references to one a substance selected from the group consisting of aspirin, warfarin, enoxaparin, heparin, low molecular weight heparin, cilostazol, clopidogrel, ticlopidine, tirofiban, abciximab, dipyridamole, plasma protein fraction, human albumin, low molecular weight dextran, hetastarch, reteplase, alteplase, streptokinase, urokinase, dalteparin, filgrastin, immunoglogulin, ginkolide B, hirudins, foropafant, rocepafant, bivalirudin, dermatan sulfate mediolanum, eptilibatide, tirofiban, thrombomodulin, abcxmab, low molecular weight dermatan sulfate- opocrin, eptacog alfa, argatroban, fondaparinux sodium, tifacogin, lepirudin, desirudin, OP2000, roxifiban, parnaparin sodium, human hemoglobin (Hemosol), bovine hemoglobin (Biopure), human hemoglobin (Northfield), antithrombin III, RSR 13, heparin-oral (Emisphere) transgenic antithrombin III, H37695, enoxaparin sodium, mesoglycan, CTC 111, bivalirudin, and any derivatives and/or combinations thereof.
Particular anticoagulants that may be mentioned include aspirin and warfarin.
The term "an anticoagulant' also includes references to thrombin inhibitors. Thrombin inhibitors that may be mentioned include low molecular weight thrombin inhibitors. The term ''low molecular weight thrombin inhibitors" will be understood by those skilled in the art, and includes' references to any composition of matter (e.g. chemical compound) that inhibits thrombin to an experimentally determinable degree (as determined by in vivo and/or in vitro tests), and which possesses a molecular weight of below about 2,000, preferably below about 1,000.
Preferred low molecular weight thrombin inhibitors include low molecular weight peptide-based, amino acid-based, and/or peptide analogue-based, thrombin inhibitors, as well as derivatives thereof.
The term "low molecular weight peptide-based, amino acid-based, and/or peptide analogue-based, thrombin inhibitors" will be well understood by one skilled in the art to include references to low molecular weight thrombin inhibitors with one to four peptide linkages, and includes those described in the review paper by Claesson in Blood Coagul. Fibrin. 5, 411 (1994), as well as those disclosed in US Patent No 4,346,078, International Patent Applications WO 93/11152, WO 93/18060, WO 93/05069, WO 94/20467, WO 94/29336, WO 95/35309, WO 95/23609, WO 96/03374, WO 96/06832, WO 96/06849, WO 96/25426, WO 96/32110, WO 97/01338, WO 97/02284, WO 97/15190, WO 97/30708, WO 97/40024, WO 97/46577, WO 98/06740, WO 97/49404, WO 97/11693, WO 97/24135, WO 97/47299, WO 98/01422, WO 98/57932, WO 99/29664, WO 98/06741, WO 99/37668, WO 99/37611, WO 98/37075, WO 99/00371, WO 99/28297, WO 99/29670, WO 99/40072, WO 99/54313, WO 96/31504, WO 00/01704 and WO 00/08014; and European Patent Applications 648 780, 468 231, 559 046, 641 779, 185 390, 526 877, 542 525, 195 212, 362 002, 364 344, 530 167, 293 881, 686 642, 669 317, 601 459 and 623 596, the disclosures in all of which documents are hereby incorporated by reference.
In the present application, derivatives of thrombin inhibitors include chemical modifications, such as esters, prodrugs and metabolites, whether active or inactive, and pharmaceutically acceptable salts and solvates, such as hydrates, of any of these, and solvates of any such salt.
Preferred low molecular weight peptide-based thrombin inhibitors include those known collectively as the "gatrans". Particular gatrans which may be mentioned include HOOC-CH2-(R)Cha-Pic-Nag-H (known as inogatran) and HOOC-CH2- (R)Cgl-Aze-Pab-H (known as melagatran) (see International Patent Application WO 93/11152 and WO 94/29336, respectively, and the lists of abbreviations contained therein).
International Patent Application WO 97/23499 discloses a number of compounds which have been found to be useful as prodrugs of thrombin inhibitors. Said prodrugs have the general formula
RaOOC-CH2-(R)Cgl-Aze-Pab-Rb wherein Ra represents H, benzyl or C1-1O alkyl, Rb (which replaces one of the hydrogen atoms in the amidino unit of Pab-H) represents OH, OC(O)R0 or C(O)ORd, Rc represents C1-17 alkyl, phenyl or 2-naphthyl and Rd represents C1-12 alkyl, phenyl, C1-3 alkylphenyl, or 2-naphthyl. Preferred compounds include RaOOC-CH2-(R)Cgl-Aze-Pab-OH, wherein Ra represents benzyl or C1-10 alkyl, e.g. ethyl or isopropyl, especially EtOOC-CH2-(R)Cgl-Aze-Pab-OH. The active thrombin inhibitors themselves are disclosed in WO 94/29336.
Further low molecular weight thrombin inhibitors that may be mentioned include those disclosed in WO 02/44145, such as compounds of the following general formula,
R° represents -OH or -CH2OH; R1 represents at least one optional halo substituent; R2 represents one or two C1-3 alkoxy substituents, the alkyl parts of which substituents are themselves substituted with one or more fluoro substituents (i.e. R2 represents one or two fluoroalkoxy(C1-3) groups); Y represents -CH2- or -(CH2)2-; and R3 represents a structural fragment of formula I(i) or I(ii):
1(0 1(10 wherein
R j4 . represents H or one or more fluoro substituents; R5 represents H, OR6 or C(O)OR7; R6 represents H, C1-1O alkyl, C1-3 alkylaryl or C1-3 alkyloxyaryl (the alkyl parts of which latter two groups are optionally interrupted by one or more oxygen atoms, and the aryl parts of which latter two groups are optionally substituted by one or more substituents selected from halo, phenyl, methyl or methoxy, which latter three groups are also optionally substituted by one or more halo substituents); R7 represents Ci-1O alkyl (which latter group is optionally interrupted by one or more oxygen atoms), or C1-3 alkylaryl or C1-3 alkyloxyaryl (the alkyl parts of which latter two groups are optionally interrupted by one or more oxygen atoms, and the aryl parts of which latter two groups are optionally substituted by one or more substituents selected from halo, phenyl, methyl or methoxy, which latter three groups are also optionally substituted by one or more halo substituents); and one or two of X1, X2, X3 and X4 represent -N- and the others represent -CH-; or a pharmaceutically-acceptable derivative thereof.
Compounds of the above general formula in which R is other than H have been found to be useful as prodrugs of thrombin inhibitors (which thrombin inhibitors include the corresponding compounds of the above general formula in which R5 is H).
Particular compounds disclosed in WO 02/44145 that may be mentioned include those of the following general formula:
wherein
R >2^ r. epresents -OCHF2, -OCF3, -OCH2CH2F or -OCH2CHF2; R5 represents H or OR6; and R6 represents methyl, ethyl, rc-propyl, /-propyl or cyclobutyl.
In this respect, more particular compounds disclosed in WO 02/44145 that may be mentioned include the thrombin inhibitor
Ph(3-Cl)(5-OCHF2)-(i?)CH(OH)C(O)-Aze-Pab and its methoxyamidino prodrug
Ph(3-Cl)(5-OCHF2)~(i?)CH(OH)C(O)-Aze-Pab(OMe).
The compounds of the invention have the advantage that they are effective against cardiac arrhythmias.
Compounds of the invention have advantageous properties compared to compounds of the prior art, in particular enhanced potency, enhanced selectivity, and/or reduction of total clearance. These advantages may provide for corresponding useful properties in practice. For example, when used as pharmaceutical agents, compounds of the present invention may have a lower daily clinical dose, longer duration of action, and/or an improved side effect profile.
Compounds of the invention may also have the advantage that they may be more efficacious than, be less toxic than, have a broader range of activity (including exhibiting any combination of class I, class II, class III and/or class IV activity (especially class I and/or class IV activity in addition to class III activity)) than, be more potent than, be longer acting than, produce fewer side effects (including a lower incidence of proarrhythmias such as torsades de pointes) than, be more
easily absorbed than, or that they may have other useful pharmacological properties over, compounds known in the prior art.
Biological Tests
Test A
Primary Electrophysiological Effects In Anaesthetised Guinea Pigs Guinea pigs weighing between 500 and 1000 g were used. The animals were housed for at least one week before the experiment and had free access to food and tap water during that period.
Anaesthesia was induced by an intraperitoneal injection of pentobarbital (50 to 60 mg/kg) and catheters were introduced into one carotid artery (for blood pressure recording and blood sampling) and into one jugular vein (for drug infusions). Needle electrodes were placed on the limbs for recording, of ECGs (lead II). A thermistor was placed in the rectum and the animal was placed on a heating pad, set to a rectal temperature of between 37.5 and 38.5°C.
A tracheotomy was performed and the animal was artificially ventilated with room air by use of a small animal ventilator, set to keep blood gases within the normal range for the species. In order to reduce autonomic influences both vagi were cut in the neck, and 0.5 mg/kg of propranolol was given intravenously, 15 minutes before the start of the experiment.
The left ventricular epicardium was exposed by a left-sided thoracotomy, and a custom-designed suction electrode for recording of the rnonophasic action potential (MAP) was applied to the left ventricular free wall. The electrode was kept in position as long as an acceptable signal could be recorded, otherwise it was moved to a new position. A bipolar electrode for pacing was clipped to the left atrium. Pacing (1 ms duration, twice the diastolic threshold) was performed with a custom- made constant current stimulator. The heart was paced at a frequency just above the spontaneous sinus rate during 30 s every fifth minute throughout the study.
The MAP signal, the blood pressure signal and the lead II ECG were collected (the sampling frequency was 1000 Hz and each sampling period 10 s) on a personal computer during the last 10 s of each 30 s pacing sequence and the last 10 s of the following min of sinus rhythm. The signals were processed using a custom- designed computer program (PharmLab v 4.0).
The test procedure consisted of two basal control recordings, 3 minutes apart, during both pacing and sinus rhythm. After the second control recording, the first dose of the test substance was infused in a volume of 0.2 mL/kg into the jugular vein catheter for 30 seconds. Three minutes later, pacing was started and a new recording was made. Five minutes after the previous dose, the next dose of test substance was administered. Six to ten consecutive doses were given during each experiment.
Data analysis
Of the numerous variables measured in this analysis, three were selected as the most important for comparison and selection of active compounds. The three variables selected were the MAP duration at 75 percent repolarization during pacing, the atrio-ventricular (AV) conduction time (defined as the interval between the atrial pace pulse and the start of the ventricular MAP) during pacing, and the heart rate (defined as the RR interval during sinus rhythm). Systolic and diastolic blood pressure were measured in order to judge the haemodynamic status of the anaesthetised animal. Further, the ECG was checked for arrhythmias and/or morphological changes.
The mean of the two control recordings was set to zero and the effects recorded after consecutive doses of test substance were expressed as percentage changes from this value. By plotting these percentage values- against the cumulative dose administered before each recording, it was possible to construct dose-response curves. In this way, each experiment generated three dose-response curves, one for MAP duration, one for AV-conduction time and one for the sinus frequency (RR interval). A mean curve of all experiments performed with a test substance was
calculated, and potency values were derived from the mean curve. All dose- response curves in these experiments were constructed by linear connection of the data points obtained. The cumulative dose prolonging the MAP duration by 10% from the baseline was used as an index to assess the class III electrophysiological potency of the agent under investigation (D1O).
Test B
Rb+-efflux assay for detection of HERG channel blockers
The human ether-a-go-go related gene (HERG) encodes the voltage-gated K+ channel underlying the cardiac rapid delayed rectifier current Iκr- The IC50 value for HERG channel blockade was determined using a high throughput functional assay based on depolarisation-induced Rb+-efflux from Chinese hamster ovary cells stably expressing the HERG-channel.
Cells were grown in Ham F12 (Life Technologies 31765-027) supplemented with 10% FBS and 0.6 mg/mL hygromycin B and were routinely passaged twice- weekly. For experimental studies, cells were plated at a density of 15,000 cells/well in Falcon, 384-well tissue culture-treated black- walled clear-bottomed plates and were thereafter incubated overnight at 37°C in a cell culture incubator.
Following incubating overnight, cell plates were washed and a Rb+-Load buffer (a physiological buffer containing Rb+) was added. Cell plates were then incubated for 3 hours and were thereafter washed. Following this wash, the test compounds were added. The cell plates were then incubated for another 10 minutes and, following this incubation period, external K+ concentration was increased in order to depolarize the cells and activate HERG channels. After a ten minute exposure period to the increased K+ concentration, supernatants were transferred to new microplates for subsequent determination of Rb+ content, using Atomic Absorption Spectrometry analysis.
The basal Rb+ efflux (content of Rb+ (mg/L) in supernatants of wells receiving only wash buffer) was defined as 100% inhibition and the stimulated Rb+efflux (content of Rb+ (mg/L) in supernatants of wells exposed only to increased external potassium concentration) was defined as 0% inhibition.
Compound activity was expressed as:
A- B
100 x 1 —
C-B_
A: Rb+ content in wells receiving test compound +increased external K+. B: Basal Rb+ efflux. C: Stimulated Rb+ efflux.
The invention is illustrated by way of the following examples.
Examples
General Experimental Procedures
Mass spectra were recorded on one of the following instruments: MUX(8)-LCT,
ZQ Masspectrometer and Quattro micro, all from Waters Micromass.
LC-MS:
Separation was performed using Agilent 1100 Series Modules on a Synergi MAX
RP (3 x 50 mm, C12, 4 μm particles) with gradient elution. Samples were injected using a Waters 2700 Sample Manager.
Mobile phases: Generic gradients were applied from 5% to 95% acetonitrile.
Buffers containing 10 mM ammonium acetate or 5 mM ammonium formate / 5 mM formic acid were used.
The mass spectra were recorded using a Waters ZQ2000 equipped with an electrospray or ESCI interface, switching positive and negative ionization mode. UV spectra were collected by a Agilent 1100 PDA and the evaporative light scattering (ELS) signal by a Sedere Sedex 55 or 75.
Data collection and evaluation were performed using the MassLynx software.
Accurate mass was determined using a LCT or Q-TOF micro mass spectrometer.
1H NMR and 13C NMR measurements were performed on a BRUKER ACP 300 and Varian 300, 400, 500 and 600 Mercury, Unity plus and Unity Inova spectrometers, operating at 1H frequencies of 300, 400, 500 and 600 MHz respectively and at 13C frequencies of 75.4, 100.6, 125.7 and 150.9 MHz respectively.
Rotamers may or may not be denoted in spectra depending upon ease of interpretation of spectra. Unless otherwise stated, chemical shifts are given in ppm with the solvent as internal standard.
Synthesis of Intermediates The following intermediates were not commercially available, and were therefore prepared by the methods described below.
Preparation A
N-(4-Cyanobenzyl)-2-(9-oxa-3,7-diazabicyclor3.3.nnon-3-yl)acetamide, hydrochloric acid salt
Ci) 2-Chloro-Ar-(4-cvanobenzyl)acetamide
ALTERNATIVE 1 Triethylamine (16.7 mL, 0.119 mol), followed by chloroacetyl chloride (5.76 g, 0.0523 mol) were added, drop by drop, to a vigorously stirred solution of 4-cyano benzylamine, methanesulfonic acid salt (10 g, 0.0476 mol; see WO 99/64391) in
dry DCM (100 mL) at 00C. The resulting mixture was then stirred overnight at room temperature under a nitrogen atmosphere. The reaction mixture was diluted with water and extracted with DCM. The organic layer was washed with water, brine and dried over sodium sulfate. Solvent evaporation, followed by purification by column chromatography (over silica gel, using 3% methanol in DCM as eluent) yielded 13 g of the sub-title compound as pale yellow solid.
ALTERNATIVE 2
To 4-cyanobenzylamine hydrochloride (10.00 g, 59.30 mmol) and tetrahydrofuran (50 mL) was added chloroacetic anhydride (10.36 g, 59.38 mmol). The resulting mixture was cooled to 0°C - 5°C. Triethylamine (19 mL, 136.32 mmol) was then added, drop wise, over a period of 10 minutes. Once the addition had been completed, the reaction was allowed to warm to room temperature, at which temperature it was stirred overnight. Water (50 mL) and isopropyl acetate (100 mL) were added to precipitate the product and the resulting biphasic mixture was heated to 5O0C. The phases were separated and the lower (aqueous) phase discarded. Then, a 20% w/w aqueous sodium chloride solution (30 mL) was added and the mixture, which was subsequently re-heated to 5O0C and stirred for 10 minutes. The phases were separated and the lower (aqueous) phase discarded. The remaining mixture was filtered, whilst hot, into a clean vessel in order to remove all insoluble material, and washed through with isopropyl acetate (10 mL). The resulting filtrate and washings were re-heated to 65°C. The reaction mixture was then cooled to room temperature, causing the product to crystallize from solution. The temperature of the mixture was reduced to 5°C and the product was isolated by filtration. The filter cake was washed with isopropyl acetate (30 mL) and sucked as dry as possible on the filter. Oven drying in vacuo (500C, 16 h) gave the sub-title compound as an off-white solid (8.24 g, 39.49 mmol, 67%). 1H NMR (400 MHz, DMSO-d6): δ 8.85 (t, / = 5.6 Hz, IH), 7.81 (dd, / = 6.4, 1.8 Hz, 2H), 7.46 (d, J = 8.5 Hz, 2H), 4.39 (d, J = 5.9 Hz, 2H), 4.16 (s, 2H). 13C NMR (75 MHz, DMSO-d6): δ 166.36 (amide C=O), 144.84 (aromatic ipso-C), 132.28 (aromatic C-H), 128.02 (aromatic C-H), 118.86 (aromatic ipso-C), 109.68 (CN), 42.58 (CH2), 42.18 (CH2).
ALTERNATIVE 3
Toluene (38 mL) was added to 4-cyanobenzylamine hydrochloride (7.52 g, 44.60 mmol) and chloroacetic anhydride (7.79 g, 44.65 mmol). The resulting mixture was cooled to 0 to 5°C. Triethylamine (14 mL, 100.44 mmol) was then added, dropwise over a period of 20 minutes. Once the addition was complete the reaction was allowed to warm to room temperature, before being stirred for 3 hours at that temperature. Water (70 mL) was added (in order to precipitate the product) and the resulting heterogeneous mixture was stirred at room temperature for 30 minutes. The temperature of the mixture was reduced to 5°C and the product was isolated by filtration. The filter cake was washed three times with water (20 mL) and sucked as dry as possible on the filter. Oven drying in vacuo (50°C, 18h) gave the title compound as a pale brown solid (7.52 g, 36.04 mmol, 81%). mp = 137.5°C 1H NMR (300 MHz, DMSO-d6): δ 8.85 (t, / = 5.5 Hz, IH); 7.81 (dd, J = 6.6, 1.8 Hz, 2H); 7.45 (d, J = 8.4 Hz, 2H); 4.39 (d, J = 6.1 Hz, 2H); 4.16 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 166.36 (amide C=O); 144.84 (aromatic ipso-C); 132.28 (aromatic C-H); 128.02 (aromatic C-H); 118.86 (aromatic ipso-C); 109.68 (CN); 42.58 (CH2); 42.18 (CH2).
ALTERNATIVE 4
4-Cyanobenzylamine hydrochloride (87.7 g) was suspended in dichloromethane (0.6 L) under nitrogen atmosphere. The suspension was cooled using an ice bath before triethylamine (152 mL) was added. Chloroacetyl chloride (70.5 g) was then continuously added to the resulting suspension over the course of 2 hours. After addition was complete, the reaction was left stirring for another hour. The reaction mixture was diluted with dichloromethane (3 L) and washed with HCI solution (2.5 M, 0.2 L). The organic layer was washed with saturated NaHCO3 solution (0.3 L) before being dried (MgSO4), filtered and concentrated under reduced pressure to provide the crude title compound (119.5 g). LC-MS: 207 (M-H)+ 13C-NMR (CDCl3): δ 166.4, 143, 132.9, 128.5, 118.8, 112, 43.5, 42.8.
1H-NMR (CDCl3): δ 7.66 (d, 2H), 7.42 (d, 2H), 4.59 (s, IH), 4.57 (s, IH), 4.15 (S5 2H).
ALTERNATIVE 5 Chloroacetic anhydride (31.17 g, 178.66 mmol) was added to 4- cyanobenzylamine hydrochloride (30.07 g, 178.32 mmol) and 2-butanone (150 mL). The resulting heterogeneous mixture was cooled to 5 0C. Triethylamine (62 mL, 444.82 mmol) was then added to the reaction mixture at a constant dropwise rate over approximately 45 minutes. Once the addition was complete, the mixture was warmed to 25 0C and stirred for 16 hours. 12.5 % w/w sodium chloride solution (150 mL) and butan-1-ol (240 mL) were added and the resulting biphasic mixture heated to 50 °C. The phases were separated and to the retained organic phase was added 12.5 % w/w sodium chloride solution (90 mL). Re-heated to 50 0C and stirred for 10 minutes. The phases were separated and the organic phase retained. Solvent (90 mL) was removed by distillation under reduced pressure to azeo-dry the reaction solution. Heated to 75 °C and filtered hot to remove all insoluble material, washing through with butan-1-ol (30 mL). The reaction solution was then cooled to room temperature, causing the crystallisation of the product from solution. Cooled to 5 0C. The product was collected by filtration and washed with butan-1-ol (90 mL). The filter cake was sucked as dry as possible and then oven dried in vacuo (50 °C, 16 h) to give the sub-title compound as a pale brown crystalline solid (27.42 g, 131.42 mmol, 74 %). mp = 140.6 °C
1U NMR (400 MHz, DMSO-d6) δ 8.86 (s, IH), 7.78 (d, / = 8.3 Hz, 2H), 7.44 (d, 7 = 7.9 Hz, 2H), 4.37 (d, / = 6.2 Hz, 2H), 4.14 (s, 2H). 13C NMR (100.MHz, DMSO-d6) δ 166.32 (amide C=O), 144.77 (ipso- aromatic C), 132.24 (aromatic C-H), 127.98 (aromatic C-H), 118.81 (ipso- aromatic C), 109.65 (nitrile CN), 42.54 (CH2), 42.16 (CH2).
ALTERNATIVE 6
Chloroacetic anhydride (20.72 g, 118.76 mmol) was added to 4- cyanobenzylamine hydrochloride (20.06 g, 118.96 mmol) and 2-butanone (100 mL). The resulting heterogeneous mixture was cooled to 5 0C. Triethylamine (42 mL, 301.33 mmol) was then added to the reaction mixture at a constant dropwise rate over approximately 30 minutes. Once the addition was complete, the mixture was warmed to 25 0C. 12.5 % w/w sodium chloride solution (100 mL) was added and the resulting biphasic mixture heated to 50 °C. The phases were separated and both phases retained. To the retained aqueous phase was added 2-butanone (60 mL). Heated to 50 °C and stirred for 15 minutes. The phases were separated and the organic phase retained. The organic layers were combined and butan-1- ol (160 mL) and 12.5 % w/w sodium chloride solution (60 mL) added. Re- heated to 50 0C and stirred for 15 minutes. The phases were separated and the organic phase retained. Solvent (158 mL) was removed by distillation under reduced pressure to azeo-dry the reaction solution. Heated to 75 0C and filtered hot to remove all insoluble material, washing through with butan-1-ol (20 mL). Further solvent (30 mL) was removed by distillation under reduced pressure, causing the precipitation of the product from solution. The reaction mixture was then cooled to room temperature. Cooled to 5 0C. The product was collected by filtration and washed with butan-1-ol (60 mL). The filter cake was sucked as dry as possible and then oven dried in vacuo (45 0C, 90 h) to give the sub-title compound as a pale brown crystalline solid (20.42 g, 97.87 mmol, 82 %).
1H NMR (400 MHz, DMSO-d6) δ 8.86 (s, IH), 7.81 (dd, / = 6.5, 1.7 Hz, 2H), 7.45 (d, J= 8.3 Hz, 2H), 4.39 (d, J = 6.2 Hz, 2H), 4.16 (s, 2H). 13C NMR (100.MHz, DMSO-d6) δ 166.31 (amide C=O), 144.78 (ipso- aromatic C), 132.24 (aromatic C-H), 127.98 (aromatic C-H), 118.80 (ipso- aromatic C), 109.65 (nitrile CN), 42.54 (CH2), 42.15 (CH2).
(ii) tert-Butyl 7-{2-F(4-cyanobenzyl)aminol-2-oxoethyl )-9-oxa-3,7-diazabicyclo- \3.3. llnonane-3-carboxylate
A mixture of 2-chloro-N-(4-cyanobenzyl)acetamide (13 g, 0.0625 mol; see step (i) above), 9-oxa-3,7-diaza-bicyclo[3.3.1]nonane-3-carboxylic acid tert-butyl ester
(13.6 g, 0.0625 mol; see WO 01/28992) and potassium carbonate (12.95 g,
0.0937 mol) in dry acetonitrile (200 mL) was stirred at 6O0C overnight under a nitrogen atmosphere. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 2.5% methanol in DCM as eluent, to yield
18 g of the sub-title compound as pale yellow solid.
(iii) iV-(4-Cvanobenzyl)-2-(9-oxa-3 J-diazabicvclo[3.3.11non-3-yl)acetamide, hydrochloric acid salt tert-Butyl 7- { 2-[(4-cyanobenzyl)amino] -2-oxoethyl } -9-oxa-3 ,7-diazabicyclo- [3.3.1]nonane-3-carboxylate (16 g, 40 mmol; see step (ii) above) was taken in dry ether (saturated with HCl, 50 mL). The resulting mixture was stirred overnight under a nitrogen atmosphere. The reaction mixture was filtered; the solid was washed with diethyl ether and dried under vacuum to yield 15 g (40 mmol) of the title compound as pale yellow solid.
Preparation B
N-(4-CvanobenzylV N-methyl-2-(9~oxa-3 J-diazabicvcloD .3. llnon-3-ylV acetamide, hydrochloric acid salt
(i) fert-Butyl 7- {2-r(4-cyanobenzyl)(methyl')aminol-2-oxoethyl } -9-oxa-3 ,7-diaza- bicyclor3.3.11nonane-3-carboxylate
To a solution of tert-butyl 7-{2-[(4-cyanobenzyl)amino]-2-oxoethyl}-9-oxa-3,7- diazabicyclo[3.3.1]nonane-3-carboχylate (18 g, 0.045 mol; see Preparation A, step (ii) above) in dry THF (200 mL) was added LiHMDS (67 mL, 1 M solution in
THF), dropwise. The reaction mixture was stirred at -78°C for 30 minutes, after which methyl iodide (9.65 g, 0.0675 mol) was added, dropwise over a period of
15 minutes. The reaction mixture was stirred at RT overnight, cooled to O0C, quenched with 10% NaHCO3 solution and concentrated partially to remove THF. The resulting aqueous layer was extracted with DCM (2 x 200 mL). The combined organic layers were washed with water and brine, dried over Na2SO4 and concentrated. The resulting residue was purified by column chromatography over silica gel, using 4% methanol in DCM as eluent, to yield the sub-title compound (16 g, 86%) as a yellow, gummy liquid.
αi)iV-(4-Cvanobenzyl)-iy-methyl-2-('9-oxa-3.7-diazabicvclor3.3.nnon-3-yl)- acetamide, hydrochloric acid salt tert-Butyl 7-{2-[(4-cyanobenzyl)(methyl)amino]-2-oxoethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]nonane-3-carboxylate (15 g, 36.1 mmol; see step (i) above) was taken in dry dioxane (saturated with HCl, 100 mL) and stirred for 1 h under a nitrogen atmosphere. During this time, the hydrochloride salt product precipitated as white solid. Dioxane was decanted from the reaction mixture and the solid was washed with diethyl ether and dried under vacuum to yield the title compound in quantitative yield as a pale yellow solid.
Preparation C N-(4-Cvano-2,6-difluorobenzylV2-(9-oxa-3 J-diazabicvcloB .3. Hnon-3-vD- acetamide, hydrochloric acid salt
(i) 2-Chloro-iV-(4-cvano-2,6-difluorobenzyl)acetamide
Chloroacetyl chloride (1.0 g, 8.8 mmol) was added, drop by drop at 0°C, to a solution of 4-(aminomethyl)-3,5-difluorobenzonitrile hydrochloride (1.5 g, 7.3 mmol; see WO 2003/101956) and triethylamine (1.49 g, 14.7 mmol) in 20 mL of DCM (dry). The reaction mixture was stirred at room temperature for 2 h under a nitrogen atmosphere and was then partitioned between water and DCM. The organic layer was washed with water then brine, and was then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by column chromatography over silica gel (using 2% methanol in DCM as eluent) afforded 2.0 g of the sub-title compound as light brown solid.
(ii) tert-Butyl 7-{2-r(4-cyano-216-difluorobenzyI)amino1-2-oxoethyl }-9-oxa-3,7- diazabicvclof3.3. llnonane-3-caiboxylate
A suspension of 2-chloro-Λ/L(4-cyano-2,6-difluorobenzyl)acetainide (2.0 g, 8.2 mmol; see step (i) above), 9-oxa-3,7-diaza-bicyclo[3.3.1]nonane-3-carboxylic acid tert-butyl ester (2.47 g, 8.26 mmol; see WO 01/28992) and dry K2CO3 (2.2 g,
16.5 mmol) in 30 mL of dry acetonitrile was stirred at 600C overnight under a nitrogen atmosphere. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 2.5% methanol in DCM as eluent, to yield
2.2 g of the sub-title compound as pale yellow, gummy liquid.
(iii) JV-(4-Cvano-2,6-difluorobenzylV2-(9-oxa-3J-diazabicvclor3.3.nnon-3-vD- acetamide, hydrochloric acid salt tert-Butyl 7- { 2-[(4-cyano-2,6-difluorobenzyl)amino]-2-oxoethyl }-9-oxa-3 ,7- diazabicyclo[3.3.1Jnonane-3-carboxylate (2.2 g; see step (ii) above) was taken in 25 mL of dry dioxane (saturated with HCl gas) and stirred at room temperature for 30 min. The precipitated solid was filtered, washed with dry diethyl ether (twice) and finally dried under vacuum to give 1.85 g of the title compound as pale yellow solid.
Preparation D iV-(4-Cvano-2-fluorobenzyl)-2-(9-oxa-3 ,7-diazabicvclor3.3.11non-3- ypacetamide, hydrochloric acid salt
(i) (4-Bromo-2-fluorophenyl)methanol
Sodium borohydride (4.63 g, 0.1219 mol) was added to a solution of 4-bromo-2- fluorobenzaldehyde (16.5 g, 0.0813 mol) in methanol (200 mL) at 00C. The resulting mixture was stirred for 1 h at RT before being quenched with water. Solvent (methanol) was concentrated under reduced pressure before the aqueous phase was extracted with DCM (3 times). The combined organic layers were washed with water and brine and then dried over sodium sulfate. Solvent
evaporation under reduced pressure afforded the sub-title compound (15.3 g) as a pale yellow liquid.
(iϊ) 3-Fluoro-4-(hydroxymethyl)benzonitrile A solution of (4-bromo-2-fluorophenyl)methanol (15.3 g, 0.0746 mol; see step (i) above) in DMF (80 mL) was purged with argon for 15 min. At this point, Zn(CN)2 (5.97 g, 0.0522 mol) and Pd(PPh3)4 (4.27 g, 0.0037 mol) were added, successively. The resulting suspension was stirred at 95°C for 17 h under an argon atmosphere, before being cooled to RT, quenched with water and extracted with ethyl acetate. The organic (ethyl acetate) layer was then washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by column chromatography over silica gel (using 25% ethyl acetate in petroleum ether as eluent) afforded 8.9 g of the sub-title compound as a white solid.
(iii) 4-(Azidomethyl)-3 -fluorobenzonitrile
Diphenylphosphonic azide (16.83 g, 13.2 mL, 0.061 mol), followed by DBU (9.31 g, 9.1 mL, 0.061 mol) were added at 00C to a solution of 3-fϊuoro-4- (hydroxymethyl)benzonitrile (8.4 g, 0.0556 mol; see step (ii) above) in THF (dry, 85 mL) under a nitrogen atmosphere. The reaction mixture was stirred at room temperature overnight and then concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 5 to 6% ethyl acetate in petroleum ether as eluent, to give 8.8 g of the sub-title compound as a yellow liquid.
(iv) 4-(Aminomethyl)-3-fluorobenzonitrile
1,3-Propane dithiol (16.59 g, 0.1533 mol) was added to a solution of 4-(azido- methyl)-3-fluorobenzonitrile (9 g, 0.0511 mol; see step (iii) above) and triethylamine (15.48 g, 0.1533 mol) in dry methanol (90 mL). The resulting mixture was stirred for 4 h under a nitrogen atmosphere. The reaction mixture was then filtered and the solvent was concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 3%
methanol in DCM as eluent, to give 4.6 g of the sub-title compound as a yellow liquid.
(v) 2-Chloro-iV-('4-cvano-2-fluorobeiizyl)acetamide Chloroacetyl chloride (4.15 g, 0.0368 mol) was added at 0°C to a solution of 4- (aminomethyl)-3-fluorobenzonitrile (4.6 g, 0.0307 mol; see step (iv) above) and triethylamine (7.75 g, 0.0768 mol) in dry DCM (100 mL). The resulting mixture was stirred for 30 minutes under a nitrogen atmosphere befoer the reaction was quenched with ice water and extracted with DCM. The organic layer was washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by column chromatography over silica gel using 23% ethyl acetate in petroleum ether as eluent, afforded 4.1 g of the sub-title compound as a yellow solid.
(vi) fert-Butyl 7-12-r(4-cvano-2-fluorobenzyl)amino]-2-oxoethyl ) -9-oxa-3 J- diazabicvclo P3.3.11nonane-3 -carbox ylate
A suspension of 2-chloro-N-(4-cyano-2-fluorobenzyl)acetamide (4.1 g, 0.0181 mol; see step (v) above), 9-oxa-3,7-diaza-bicyclo[3.3.1]nonane-3- carboxylic acid tert-butyl ester (4.79 g, 0.0181 mol; see WO 01/28992) and K2CO3 (10 g, 0.0724 mol) in dry acetonitrile (70 mL) was stirred at RT for 26 h under a nitrogen atmosphere. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The resulting residue was purified by column chromatography over silica gel, using 50% ethyl acetate in petroleum ether as eluent, to give 4.7 g of the sub-title compound as pale yellow solid.
(vii) /Y-(4-Cvano-2-fluorobenzyl)-2-('9-oxa-3 J-diazabicvcloH .3. llnon-3-ylV acetamide, hydrochloric acid salt tert-Butyl 7- { 2-[(4-cyano-2-fluorobenzyl)amino]-2-oxoethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1]nonane-3-carboxylate (4.7 g, 0.0112, from step (vi) above) in dry dioxane (20 mL) was added to diethyl ether saturated with HCl gas (65 mL). The reaction mixture was stirred at room temperature for 1.5 h and the solvent was
decanted. The precipitated solid was washed with dry diethyl ether (3 times) and dried under vacuum to give 5.2 g of the title compound as pale yellow solid.
Preparation E 4-Cyano-N-r2-(9-oxa-3 J-diazabicvcloP .3.11non-3-yl)ethyllbenzamide, hydrochloric acid salt
(i) fert-Butyl 7-(cvanomethyl)-9-oxa-3,7-diazabicyclor3.3.nnonane-3-carboxylate To a stirred suspension of 9-oxa-3,7-diaza-bicyclo[3.3.1]nonane-3-carboxylic acid tert-bntyl ester hydrochloride (19 g, 0.0718 mol; see WO 01/28992) and dry K2CO3 (25 g, 0.1798 mol) in dry acetonitrile at O0C was added chloroacetonitrile (10.9 g, 0.439 mol), dropwise. The reaction mixture was stirred at room temperature overnight before being diluted with water and extracted with DCM. The combined organic layers were washed with brine and dried over sodium sulfate and then concentrated. The resulting residue was then purified by column chromatography over silica gel, using 1% methanol in chloroform as eluent, to yield 18 g of the sub-title product as a pale yellow solid.
(ii) tgr?-Butyl 7-(2-aminoethyl)-9-oxa-3,7-diazabicyclor3.3.nnonane-3- carboxylate
Ammonia gas was purged through a solution of tert-butyl 7-(cyanomethyl)-9-oxa- 3,7-diazabicyclo[3.3.1]nonane-3-carboxylate (12 g, 0.0447 mol) in dry methanol (200 mL) for 5 min. Raney-Nickel (9 g) was added and the mixture was hydrogenated under 3 kg pressure for 2 days. The reaction mixture was filtered and filtrate was concentrated under reduced pressure. The resulting residue was purified by column chromatography, using 10% methanol in chloroform as eluent, to yield 9 g of the sub-title compound as a brownish liquid.
(iii) fert-Butyl 7-{2-rC4-cyanobenzoyl')amino1ethyl}-9-oxa-3,7-diazabicvclo- F3.3.1 "lnonane-3 -carboxylate
EDC (7.6 g, 0.039 mol), followed by HOBT (0.45 g, 0.033 mol) were added to a solution of tert-butyl 7-(2-aminoethyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane-3- carboxylate (9 g, 0.033 mol; see step (ii) above), 4-cyanobenzoic acid (4.87 g,
0.033 mol) and triethylamine (4 g, 0.039 mol) in dry DCM (100 mL) at 0°C. The reaction mixture was stirred at RT overnight under a nitrogen atmosphere before being diluted with water and extracted with DCM. The combined organic layers were washed with brine, dried over sodium sulfate and then concentrated. The resulting residue was purified by column chromatography, using 2% methanol in
DCM as eluent, to yield 10.5 g of the sub-title compound as a pale brown solid.
(iv) 4-Cyano-N-r2-(9-oxa-3,7-diazabicyclor3.3.1]non-3-yl)ethyllbenzamide, hydrochloric acid salt ter?-Butyl 7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]- nonane-3-carboxylate (10.5 g, 0.0262 mol; see step (iii) above) was taken in dioxane (50 mL, saturated with HCl gas) and stirred at room' temperature for 1 h.
Dioxane was decanted and the precipitated solid was washed with diethyl ether and dried under vacuum. This yielded the title compound 10 g as off-white solid.
Preparation F
4-Cvano-A/'-methyl-N-r2-(9-oxa-3,7-diazabicvclor3.3.11non-3-yl)ethvnbenzamide
(i) 4-Cvano-N-(2-hvdroxyethyl)-N-methylbenzamide 4-Cyanobenzoyl chloride (25 g) in dry DCM (50 mL) was added, at 0°C, to a solution of iV-methyl ethanolamine (12.75 g, 0.17 mol) and triethylamine (36 mL, 0.255 mol) in dry DCM (300 mL). The resulting mixture was stirred at RT overnight under a nitrogen atmosphere before being quenched with water and extracted with DCM. The organic layer (DCM) was then washed with water and brine and dried over sodium sulfate. Solvent was evaporated under reduced pressure and the resulting residue was purified by column chromatography over
silica gel, using 1% methanol in DCM as eluent, to give sub-title compound 18 g as white solid.
(ii) 4-Cvano-JV-methyl-N-(2-oxoethyl)benz amide Oxalyl chloride (10.9 mL, 0.125 mol) was added to a solution of DMSO (17.7 mL, 0.25 mol) in dry DCM (200 mL) at -78°C. The resulting mixture was stirred at -78°C for 15 minutes before 4-cyano~N-(2-hydroxyethyl)-N-methyl- benzamide (17 g, 0.083 mol; see step (i) above) in dry DCM (50 mL) was added. Stirring was continued at -78°C for 3 h before triethylamine (58 mL) was added and the reaction mixture was slowly warmed to RT. Citric acid (10% aqueous) was added and the resulting mixture was diluted with water and DCM. The aqueous layer was extracted with DCM. The combined organic layers were then washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure afforded the sub-title compound (18 g) as a brownish oil.
(iii) fe/t-Butyl 7-{2-ff4-Cvanobenzoyl)fmethyl)amino1ethyl}-9-oxa-37-diaza- bicvclo [3.3.1 lnonane-3 -carboxylate
A mixture of crude 4-cyano-N-methyl-iV-(2-oxoethyl)benzamide (14 g, 0.069 mol; see step (ii) above), 9-oxa-3,7-diaza-bicyclo[3.3.1]nonane-3-carboxylic acid tert- butyl ester (11 g, 0.048 mol; see WO 01/28992) and acetic acid (6.4 mL, 0.104 mol) in dry DCM (200 mL) was stirred at RT for 8 h. Sodium cyanoborohydride (6.55 g, 0.104 mol) was added and the reaction mixture was stirred at RT for 2 h. The reaction mixture was diluted with water. The aqueous layer was then extracted with DCM and the combined organic layers washed with water and brine and dried over sodium sulfate. Solvent was evaporated under reduced pressure and the resulting residue was purified by column chromatography over silica gel, using 1.5% methanol in DCM as eluent, to give the sub-title compound (11.5 g) as yellow oil.
(iv) 4-Cvano-N-methyl-N-r2-(9-oxa-3 J-diazabicvcloH .3. nnon-3-vnethyll- benzamide tert~Buty\ 7-{2-[(4-cyanobenzoyl)(methyl)amino]eihyl}-9-oxa-3,7-diazabicyclo- [3.3.1]nonane-3-carboxylate (11.5 g; see step (iii) above) was dissolved in dry dioxane (50 mL). Dioxane (100 mL, saturated with HCl gas) was added to the resulting solution. The reaction mixture was stirred at RT for 2 h before being diluted with dry diethyl ether. The precipitated solid was filtered and dried under vacuum to give the hydrochloride salt of the title compound (9.5 g) as pale yellow powder. HPLC analysis of this salt indicated a purity of <90%. The hydrochloride salt was partitioned between 10% NaHCO3 and DCM. The organic layer was separated and then washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 6 g of crude product, for which HPLC analysis again indicated a purity of <90%. Purification by column chromatography (three times), using 2% methanol in DCM as eluent, gave the title compound (2.45 g with 97% purity and 630 mg with 96% purity) as an off-white solid.
Preparation G
JV-r2-r4-Cvanoρhenoxy)ethvn-2-(9-oxa-3J-diazabicvclor3.3.11non-3-yl)- acetamide, hydrochloric acid salt
(i) 4-(2-Aminoethoxy)benzonitrile hydrochloride
A suspension of 4-hydroxybenzonitrile (4 g, 0.0336 mol), tert-butyl (2-bromo- ethyl)carbamate (7.5 g, 0.0336 mol; see WO 01/28992) and dry K2CO3 (4.6 g, 0.0336 mol) in dry acetonitrile (50 mL) was stirred at 6O0C overnight under a nitrogen atmosphere. The reaction mixture was filtered and solvent was concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 2% MeOH in DCM as eluent, to yield 4 g of Boc-protected compound. The Boc-protected compound was taken in (25 mL) of dioxane (saturated with HCl gas) and stirred for 30 minutes. Dioxane was decanted and the precipitated
solid was washed with diethyl ether and dried under vacuum to yield 3.8 g of the sub-title compound as a white powder.
(ii) 2-Chloro-iV- F2-(4-c vanophenoxy)eth yli acetamide Chloroacetyl chloride (1.96 g, 0.0174 mol) was added, drop by drop at 0°C, to a solution of 4-(2-aminoethoxy)benzonitrile hydrochloride (3.8 g, 0.0145 mol; see step (i) above) and triethylamine (2.92 g, 0.029 mol) in 50 mL of DCM (dry). The reaction mixture was stirred at room temperature for 5 h under a nitrogen atmosphere and partitioned between water and DCM. The organic layer was washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by column chromatography over silica gel (using 2.5% methanol in DCM as eluent) yielded 3 g of the sub-title compound as a brownish solid.
(iii) fer?-Butyl 7-(2-{r2-(4-cvanophenoxy)etfayl1amino)-2-oxoethyl)-9-oxa-3.7- diazabicvclorS.S.linonane-S-carboxylate
A suspension of 2-chloro-iV-[2-(4-cyanophenoxy)ethyl]acetamide (3 g, 0.0126 mol; see step (ii) above), 9-oxa-3,7-diaza-bicyclo [3.3.1 ]nonane-3- carboxylic acid tert-butyl ester (2.88 g, 0.0126 mol; see WO 01/28992) and dry K2CO3 (5.22 g, 0.0378 mol) in dry acetonitrile (100 mL) was stirred at 6O0C overnight under a nitrogen atmosphere. The reaction mixture was filtered and the filtrate was concentrated. The resulting residue was purified by column chromatography over silica gel, using 3% methanol in DCM as eluent, to yield 5.2 g of the sub-title compound as pale yellow solid.
(iv) N-r2-(4-Cvanophenoxy)ethyl]-2-(9-oxa-3,7-diazabicvclor3.3.11non-3-. vPacetamide. hydrochloric acid salt tert-Butyl 7-(2-{[2-(4-cyanophenoxy)ethyl]amino}-2-oxoethyl)-9-oχa-3,7-diaza- bicyclo[3.3.1]nonane-3-carboxylate (5.2 g, 0.012 mol; see step (iii) above) was taken in 25 mL of dioxane (saturated with HCl gas) and stirred at room temperature for 30 minutes. Dioxane was decanted from the reaction mixture and
the precipitated solid was washed with diethyl ether and dried under vacuum to yield 5.8 g of the title compound as white solid (hygroscopic).
Preparation H 3-(4-Cvanophenyl)-N-methyl-iV-r2-(9-oxa-3J-diazabicvclor3.3.nnon-3-vnethyll- propanamide, hydrochloric acid salt
(i) 3-(4-Cvanophenyl)-Λr-methyl-N-(2-hvdroxyethvI)propanamide iV-Methyl ethanolamine (8.5 mL, 0.1108 mol) and ethyl 3-(4-cyanophenyl)- propanoate (15 g, 0.0738 mol; see Syn. Lett. 8, 1133-1136 (2003)) were heated together at 1600C for 4 h. The reaction mixture was cooled to room temperature, diluted with water and then extracted with DCM. The organic layer was washed with brine and dried over sodium sulfate before being concentrated. The resulting residue was purified by column chromatography, using methanol in DCM as the eluent, to yield the sub-title compound (11 g) as a pale yellow solid.
(ii) 3-(4-Cyanophenyl)-iVr-methyl-N-(2-oxoethyl)propanamide
Oxalyl chloride (6.5 g, 0.0517 mol) was added, dropwise, to a stirred solution of
DMSO (8.06 g, 0.1034 mol) in DCM at -78°C. 3-(4-Cyanophenyl)-N-methyl-N- (2-hydroxyethyl)ρropanamide (8 g, 0.0344 mol; see step (i) above) in DCM (50 mL) was added, dropwise, and stirring was continued for 2 h at the same temperature under a nitrogen atmosphere. Triethylamine (approximately 45 mL) was added and stirring was continued for 15 min. The reaction mixture was quenched with 10% (aqueous) citric acid and extracted with DCM. The organic layer was washed with water and brine, dried over sodium sulfate. Solvent was evaporated under reduced pressure to yield the sub-title compound (7.8 g) as a pale yellow liquid.
(iii) fert-Butyl 7-(2-{r3-(4-cvanophenyl)propanoyn(methyl)amino)ethyl)-9-oxa- SJ-diazabicvcloP.S.πnonane-S-carboxylate
3-(4-Cyanophenyl)-N-methyl-N-(2-oxoethyl)propanamide (7.5 g, 0.0326 mol; see step (ii) above) and 9-oxa-3,7-diazabicycIo[3.3.1]nonane-3-carboxylic acid ten-
butyl ester (6.3 g, 0.0277 mol, see WO 01/28992) were taken in dry DCM (100 mL) and cooled to 00C. Acetic acid (glacial) was added and the resulting mixture was stirred for 1 h at room temperature. Sodium cyanoborohydride (3.07 g, 0.0489 mol) was added at O0C and the reaction mixture was stirred at room temperature overnight under a nitrogen atmosphere. The reaction was quenched with water and extracted with DCM. The organic layer was washed with water and brine, dried over sodium sulfate and then concentrated. The resulting residue was purified by column chromatography, using methanol in chloroform as eluent, to yield 9.5 g of the sub-title compound as a yellow liquid.
(iv) 3-(4-Cvanophenyl)-N-methyl-iV- r2-(9-oxa-3 J-diazabicvcloD .3.1 ]non-3- yDethyllpropanamide, hydrochloric acid salt tert-Butyl 7-(2~ { [3-(4-cyanophenyl)propanoyl] (methyl)amino } ethyl)-9-oxa-3 ,7- diazabicyclo[3.3.1]nonane-3-carboxylate (9.5 g, 0.0214 mol; see step (iii) above) was taken in 50 mL of dioxane (saturated with HCl gas). The resulting mixture was stirred for 2 h. Solvent was decanted and the precipitated solid was washed with diethyl ether and dried under vacuum to yield the title compound (8 g) as a highly hygroscopic, off-white solid.
Preparation I
3-(4-Cvanoρhenyl)-N-r2-(9-oxa-3 J-diazabicvclor3.3. llnon-3- yPethyllpropanamide, hydrochloric acid salt
(i) fert-Butyl 7-(2-{ [3-(4-cvanophenyl)propanoyllaminoiethyl)-9-oxa-3.7- diazabicyclor3.3.1~[nonane-3-carboxylate
To a solution of iert-butyl 7-(2-aminoethyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane- 3-carboxylate (1 g, 0.00367 mol; see Preparation E(ii) above) and 3-(4-cyano- ρhenyl)ρropionic acid (0.64 g, 0.00367 mol) in DCM (25 mL) at 0°C was added EDC (0.845 g, 0.0044 mol), HOBT (0.050 g, 0.3 mmol) and triethylamine (0.445 g, 0.0044 mol). The reaction mixture was stirred at RT overnight, diluted with water and extracted with DCM. The combined organic layers were washed with water and brine, dried over Na2SO4 and then concentrated. The resulting
residue was purified by column chromatography over silica gel (1% methanol in chloroform as the eluent) and further recrystallised from hexane/diethyl ether to yield the sub-title compound (0.8 g) as a white solid.
(ii) 3-(4-Cvanophenyl>N-r2~(9-oxa-3 J-diazabicvcloβ -3.11nαn-3-yl)ett.yri- propanamide
To dioxane (150 mL, saturated with HCl gas), tert-bntyl 7-(2-{[3-(4- cyanopheny^propanoylJaminoJethy^-P-oxa-SJ-diazabicyclotS.S.^nonane-S- carboxylate (5g, 0.0116 mol; see step (i) above) was added. The resulting mixture was stirred for 1.5h. On completion of reaction (as determined by TLC), the reaction mixture was filtered and the product was washed with ether to give 4.2 g (99%) of the title compound.
Preparation J iV-fferg-ButvD^-rg-oxa-SJ-diazabicvclorS.a.llnon-S-vDacetamide
(i) fert-Butyl 7-r2-(fert-butylamino)-2-oxoethyll-9-oxa-3,7--diazabicvclor3.3.11- nonane-3-carboxylate
To a mixture of fert-butyl 9-oxa-3,7-diazabicyclo[3.3.1]nonane-3-carboxylate (3.50 g, 15.33 mmol; see WO 01/28992), N-(fert-butyl)-2-chloroacetamide (3.44 g, 23.00 mmol) and anhydrous potassium carbonate (3.18 g, 23.00 mmol) was added dry acetonitrile (40 mL). The resulting mixture was heated to 60°C and stirred overnight. The mixture was then filtered and the filtrate was concentrated in vacuo. The crude product was purified by chromatography on silica gel (Horizon™, flash system from Biotage™; column: Flash 40+M, 100 g), using DCM / MeOH (9:1) as eluent, to afford 5.1 g of a 2:1 mixture of the title compound and N-(tert-butyl)-2-chloroacetamide.
(ii) N-(tgrt-Butyl)-2-(9-oxa-3J-diazabicyclor3.3.nnon-3-yl)acetamide To the mixture from step (i) above (of tert-bntyl 7-[2-(terϊ-butylamino)-2- oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]nonane-3-carboxylate and JV-(fe?t-butyl)- 2-chloroacetamide) was dissolved in DCM (200 mL). Trifluoroacetic acid
(50 mL) was added and the resulting mixture was stirred at room temperature for 2.5 hours before being concentrated in vacuo. The crude product was dissolved in 1 M HCl (50 mL) to provide an aqueous layer that was extracted with ethyl acetate (2 x 50 mL). The aqueous phase was concentrated in vacuo and to the remainder was added aqueous Na2CO3 (50 mL). The resulting aqueous phase was extracted with DCM (3 x 50 mL), after which the combined DCM layers were dried over Na2SO4 and concentrated in vacuo. This afforded 2.45 g (66.2% over two steps, i.e. from tert-bntyl 9-oxa-3,7-diazabicyclo[3.3.1]nonane-3-carboxylate) of the title compound. 13C NMR (100.6 MHz, CDCl3): δ 169.24, 67.88, 63.58, 57.27, 50.91, 49.90, 28.99
Preparation K(a) 2-r(4-Cyanobenzyl)oxylethyl methanesulfonate
(i) 4-r(2-Hydroxyethoxy)methvnbenzonitrile
NaH (2.4 g of a 60% suspension in mineral oil, 60 mmol) was washed twice with heptane (2 x 20 mL). Dry THF (20 mL) was added, followed, in portions during 15 minutes (gas evolution and heat generation), by ethylene glycol (24.8 g, 400 mmol). The resulting mixture was stirred for 2 h at RT, after which the mixture was warmed to 8O0C. After 20 minutes at this temperature, 4-cyano- benzyl bromide (7.84 g, 40 mmol) was added in portions over the course of 60 minutes. The reaction mixture was then stirred at 800C for 2 h. The mixture was cooled to RT and saturated NH4Cl solution (150 mL) was added slowly. The mixture was extracted with diethyl ether (3 x 100 mL). The organic (diethyl ether) layer was washed with NH4Cl solution and brine, dried over Na2SO4 and then evaporated to provide a residue that was purified by chromatography on silica (using heptane / ethyl acetate (60:40) as eluent). This gave 5.5 g (76.5%) of the sub-title compound.
(ii) 2-F(4-Cvanobenzyl)oxy1 ethyl methanesulfonate
4-[(2-Hydroxyethoxy)methyl]benzonitrile (5.5g, 30.5 mmol; see step (i) above) was dissolved in DCM (61 mL) and cooled to -1O0C. Triethylamine (5 mL,
36.5 mmol) and methanesulfonyl chloride (3.84 g, 33.5 mmol) were added and the resulting mixture was stirred at below -50C for 1.5 h, and then at RT overnight. DCM (200 mL) was added and the mixture was washed with water (2 x 100 mL) and then dried over Na2SO4. Evaporation gave 7.3 g (94%) of the title compound, which was used without further purification.
Preparation KCo)
The following compounds were prepared according to or by analogy with the procedure outlined in Preparation K(a) above, replacing 4-cyanobenzyl bromide with the appropriate (substituted) benzyl halide:
2-[(3-cyanobenzyl)oxy]ethyl methanesulfonate;
2- [(3 ~chlorobenzyl)oxy] ethyl methanesulf onate;
2-{[3-(trifluoromethyl)benzyl]oxy}ethyl methanesulfonate;
2-[(3-fluorobenzyl)oxy]ethyl methanesulfonate; 2-[(4-fluorobenzyl)oxy]ethyl methanesulfonate;
2-[(4-chlorobenzyl)oxy]ethyl methanesuLfonate;
2-{[4-(trifluoromethyl)benzyl]oxy}ethyl methanesulfonate; and
2-(benzyloxy)ethyl methanesulfonate.
Preparation L
2-(2-Fluorophenyl)ethyl methanesulfonate
To a solution of 2-(2-fluorophenyl)ethanol (4.00 g, 28.5 mmol) and triethylamine (6.36 mL, 45.7 mmol) in DCM (15 mL) was added methanesulfonyl chloride (2.88 mL, 37.1 mmol) at -5°C. The resulting mixture was stirred for 4 hours and was allowed to slowly warm to room temperature. The mixture was diluted with DCM and extracted with 1 M HCl and water. The organic layer was dried over Na2SO4 and concentrated in vacuo. The resulting crude product was purified by column chromatography, using DCM as eluent, to afford 5.88 g (94.4%) of the title compound. 1H NMR (400 MHz, CDCl3): δ 7.28-7.22 (2H, m), 7.12-7.02 (2H, m), 4.42 (2H, t), 3.10 (2H, t), 2.88 (3H, s)
Preparation M
2-(3-Memoxyphenyl)ethyl methanesulfonate
The title compound was prepared by a procedure analogous to that described in Preparation L above, but using 2-(3~methoxyphenyl)ethanol in place of 2-(2- fluorophenyl)ethanol.
Preparation N l-(2-Brom.oethyl)-4-trifluoromethylbenzene
(i) l-(2-Methoxwinyl)-4-trifluorometh.ylbenzene
Potassium tert-butoxide (13.46 g, 0.120 mol) was added to methoxymethyl triphenyl phosphonium chloride (38.30 g, 0.112 mol) in 150 mL dry THF at -30°C under an argon atmosphere. The resulting mixture was stirred for 1 h before 4- trifluoromethylbenzaldehyde (15.0 g, 0.086 mol), dissolved in 60 mL THF, was added, dropwise at the same temperature. Stirring was continued for 3 h at room temperature before the reaction mixture was treated with 100 mL water. Petroleum ether (150 mL) was added and the resulting mixture was and filtered through Celite®. The organic layer was separated and the aqueous layer was extracted with petroleum ether (2 x 100 mL). The combined organic layers were washed with brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by purification over silica gel using petroleum ether as eluent, afforded 10.5 g of the sub-title compound as a colorless liquid. This was employed directly in the next step without further purification.
(ii) (4-Trifluoromethylphenyl)acetaldehyde
To a solution of l-(2-methoxyvinyl)-4-trifluoromethylbenzene (10.5 g, 0.052 mol; see step (i) above) in 158 mL acetone was added 107.3 mL of 3 M HCl. The resulting mixture was stirred at 450C for 4 h, after which acetone was evaporated under reduced pressure and the residue was partitioned between water and diethyl ether. The organic layer was washed with NaHCθ3 and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure afforded the sub-title
compound (9.4 g)as pale yellow liquid. This was employed directly in the next step without further purification.
(iii) 2-f4-Trifluoromethylphenyl)ethanol NaBH4 (2 g, 0.055 mol) was added, at 0°C, to a solution of 4-trifluoromethyl- phenyl)acetaldehyde (9.4 g, 0.05 mol; see step (ii) above) in 154 mL ethanol. The resulting mixture was stirred at RT overnight before the solvent was evaporated under reduced pressure and the residue was partitioned between 50 mL of 1 N HCl and 50 mL of diethyl ether. The organic layer was washed with water, dried over sodium sulfate and the solvent evaporated under reduced pressure to give 10 g of the sub-title compound as a liquid. This was employed directly in the next step without further purification.
(iv) l-("2-Bromoethyl)-4-trifluoromethylbenzene A mixture of step 2-(4-trifluoromethylphenyl)ethanol (10 g, 0.053 mol; see step (iiϊ) above), HBr in acetic acid (86 mL of 1.06 M) and 10 mL of sulfuric acid was stirred at 100°C for 4 h. The reaction mixture was then poured onto ice and extracted with diethyl ether. The organic layer was washed with NaHCO3 and water and then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by purification over silica gel (using petroleum ether as eluent). afforded 5 g of the title compound as a liquid.
Preparation O 4-(2-Bromoethyl)-l,2-difluorobenzene
(i) 1 ,2-Difluoro-4-(2-methoxyvinyl)benzene
Potassium tert-butoxide (11 g, 0.098 mol) was added to methoxymethyl triphenyl phosphonium chloride (31.12 g, 0.091 mol) in 150 mL of dry THF at -300C under ' an argon atmosphere and stirred for 1 h. 3,4-Difluorobenzaldehyde (10 g, 0.07 mol) in 60 mL of THF was added, dropwise at the same temperature, and then stirring was continued for 3 h at room temperature. The reaction mixture was treated with 100 mL of water and 150 mL of petroleum ether and was then filtered
through Celite®. The organic layer was separated and the aqueous layer was extracted with petroleum ether (2 x 100 mL). The combined organic layers were washed with brine and dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by purification over silica gel using petroleum ether as ■ eluent, afforded 5.5 g of the sub-title compound as a colorless liquid.
(ip (3 ,4-Difluorophenyl') acetaldehyde
To a solution of l,2-difluoro-4-(2-methoxyvinyl)benzene (5.5 g, 0.032 mol; see step (i) above) in 80 mL acetone was added 66 mL of 3 M HCl. The resulting mixture was stirred at 450C for 4 h before acetone was evaporated under reduced pressure and the residue was partitioned between water and diethyl ether. The organic layer was washed with NaHCO3 and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 5.5 g of the sub-title compound as a liquid. This was employed directly in the next step without further purification.
(iii) 2-(3 ,4-Difluorophenyl)ethanol
NaBH4 (1.46 g, 0.038 mol) was added, at 0°C, to a solution of (3,4-difluoro- phenyl)acetaldehyde (5.5 g 0.35 mol; see step (ii) above) in 90 mL ethanol. The resulting mixture was stirred at RT overnight before the solvent was evaporated under reduced pressure and the residue was partitioned between 50 mL of 1 N HCl and 50 mL of diethyl ether. The organic layer was washed with water, dried over sodium sulfate and solvent evaporated under reduced pressure to give 5.5 g of the sub-title compound as a liquid. This was employed directly in the next step without further purification.
(iv) 4-(2-BromoethvD- 1 ,2-difluorobenzene
A mixture of 2-(3,4-difluorophenyl)ethanol (5 g, 0.032 mol; see step (iii) above), HBr in acetic acid (51.84 mL of 49%, 0.64 mol) and 5 mL of sulfuric acid was stirred at 1000C for 4 h. The reaction mixture was poured onto ice and extracted with diethylether. The organic layer was washed with NaHCO3 and water and then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed
by purification (three times) over silica gel (using petroleum ether as eluent), afforded 1.8 g of the title compound as a liquid.
Preparation P 3-(2-Bromoemyl)benzonitrile
(i) 2-(3-Bromophenyl)ethanol
3-Bromophenyl acetic acid (10 g, 0.0465 mol), dissolved in THF (50 mJL), was added dropwise added to a LAH (3.5 g, 0.93 mol) suspension in THF (100 mL), at 00C under a nitrogen atmosphere. The reaction mixture was stirred for 2 h at room temperature before being quenched with water (9.5 mL), 10% NaOH (9.5 mL) and then water (9.5 mL) again. Precipitated solid was filtered and the residue was washed with DCM. The filtrate was concentrated to afford 8.3 g of the sub-title compound as pale yellow liquid.
(ii) 3-f2-Hydroxyethyl)benzonitrile
Zn(CN)2 (5.03 g, 0.028 mol) and Pd(PPh3)4 (2.3 g, 0.002 mol) were added to a solution of 2-(3-bromophenyl)ethanol (8.3 g, 0.412 mol; see step (i) above) in dry DMF (75 mL). The reaction mixture was stirred overnight at 100°C before being cooled to room temperature and quenched with water. The resulting mixture was extracted with ethyl acetate and the organic layer was separated, dried over anhydrous Na2SO4 and then concentrated. The resulting crude product was purified by column chromatography, using 15% ethyl acetate / petroleum ether as eluent, to give 4.2 g of the sub-title compound as yellow liquid.
(iii) 3-(2-Bromoethyl)benzonitrile
Triphenylphosphine (14.97 g, 0.0571 mol) was added to a solution of 3-(2- hydroxyethyl)benzonitrile (4.2 g. 0.0285 mol; see steρ(ii) above) in DCM (50 mL). The reaction mixture was cooled to 00C and solution of CBr4 (18.93 g, 0.0571 mol) in DCM was added, dropwise. After stirring at room temperature overnight, the reaction mixture was filtered and the solids washed with petroleum ether. The filtrate was concentrated and the resulting crude product was purified
by column chromatography (using 3% ethyl acetate / petroleum ether as eluent) to give 4.2 g of the title compound as yellow liquid.
Preparation O Methanesulfonic acid 2-(4-difluoromethoxyphenyl)ethyl ester
(i) l-Difluoromethoxy-4-((E,Z)-2-methoxwinγl)benzene (E/Z ratio -3:2)
A suspension of methoxymethyl triphenylphosphonium chloride (6.69 g,
19.5 mmol) in THF (200 mL) was cooled to ca. -2O0C. n-BuLi (2.5 M in hexanes, 7.2 mL, 18 mmol) was added in portions over 5 minutes and the orange-red reaction mixture was then stirred for an additional 15 minutes. 4-Difluoro- methoxybenzaldehyde (2.58 g, 15.0 mmol) in THF (50 mL) was added, at -100C, and the reaction mixture was stirred at this temperature for 30 minutes, and then at room temperature for 19 h. Water (100 mL) was added and the mixture was concentrated under reduced pressure. The remaining aqueous phase was extracted with ethyl acetate (2 x 100 mL) and the combined organic phase was washed with water (2 x 100 mL) and brine (100 mL), dried over Na2SO4, and then concentrated in vacuo. The crude product was purified three times by chromatography on silica gel (Horizon™, flash system from Biotage™; column: Flash 40+M, 40 x 150 mm), using 3% ethyl acetate in heptane as eluent. Fractions containing pure product were combined and concentrated in vacuo. This afforded 1.60 g (53%) of the sub-title compound as a colourless oil.
1H-NMR (500 MHz, CDCl3): δ 7.56 (2H, minor isomer), 7.20 (2H, major isomer), 7.03 (d, 2H, minor isomer), 7.02 (d, 2H, major isomer), 7.00 (d, IH, major isomer), 6.47 (t, IH, minor isomer), 6.47 (t, IH, major isomer), 6.14 (d, IH, minor isomer), 5.78 (d, IH, major isomer), 5.20 (d, IH, minor isomer), 3.78 (s, 3H, minor isomer), 3.69 (s, 3H, major isomer)
(ii) (4-Difluoromethoxyphenyl)acetaldehyde To a solution of l-difluoromethoxy-4-((E,Z)-2-methoxyvinyl)benzene (1.58 g, 7.9 mmol; see step (i) above) in acetone (20 mL) was added 3 M HCl (3.7 mL, 11.1 mmol). The resulting mixture was stirred at 450C for 4 h before being
concentrated under reduced pressure. Water (25 mL) was added to the resulting residue and the aqueous phase was then extracted with diethyl ether (2 x 50 mL). The combined organic phase was washed with saturated aqueous NaHCθ3 (50 mL) and brine (50 mL), dried over Na2SO4, and then concentrated in vacuo to give 1.32 g of crude sub-title compound as a yellow oil (with a purity of ca. 80%). This was employed directly in the next step without further purification. 1H-KMR (500 MHz, CDCl3): δ 9.76 (t, IH), 7.21 (d, 2H), 7.13 (d, 2H), 6.51 (t, IH), 3.70 (d, 2H)
(iii) 2-(4-Difluorom.ethoxyphenyl)-ethanol
A solution of (4-difluoromethoxypbenyl)acetaldehyde (1.22 g, 6 mmol; see step (ii) above) in ethanol (20 mL) was cooled to 0°C. Sodium borohydride (0.27 g, 7.2 mmol) was added and the resulting reaction mixture was stirred at 0°C for 2 h and then at room temperature for 16 h. After concentration under reduced pressure, the residue was diluted with diethyl ether (50 mL) and 1 M HCl (50 mL). The phases were separated and the organic phase was washed with water (50 mL) and brine (50 mL), dried over Na2SO4, and then concentrated in vacuo. This gave a crude product that was purified (twice) by chromatography on silica gel (Horizon™, flash system from Biotage™; column: Flash 40+M, 40 x 150 mm), using eluents of ethyl acetate in heptane (25 to 40% ethyl acetate). Concentration in vacuo of the combined fractions containing pure product afforded 0.62 g (50%) of the sub-title compound as a light yellow oil. 1H-NMR (500 MHz, CDCl3): δ 7.22 (d, 2H), 7.07 (d, 2H), 6.48 (t, IH), 3.88-3.80 (m, 2H), 2.85 (t, 2H), 1.55 (m, br, IH)
(iv) Methanesulfonic acid 2-(4-difluoromethoxyphenyl)ethyl ester A solution of 2-(4-difluoromethoxyphenyl)ethanol (0.582 g, 3.09 mmol; see step (iii) above) in DCM (8 mL) was cooled to 00C. Triethylamine (0.52 mL, 3.7 mmol) was added, followed by a solution of methanesulfonyl chloride (0.26 mL, 3.4 mmol) in DCM (2 mL), and the reaction mixture was stirred at 00C for 90 minutes. The reaction mixture was diluted with DCM (40 mL) and the organic phase was washed with water (2 x 30 mL) and brine (30 mL), dried over
Na2SO4, and then concentrated in vacuo to give 0.78 g (95%) of the title compound as a colourless oil.
1H-NMR (500 MHz, CDCl3): δ 7.23 (d, 2H), 7.09 (d, 2H), 6.49 (t, IH), 4.40 (t, 2H), 3.05 (t, 2H), 2.89 (s, 3H)
Preparation R 4-(2-Bromoethyl)-2-fluoroberizonitrile
(i) 1 -Bromo-4-(bromorDethyl)-2-fluorobenzene To a solution of 4-bromo-3-fluorotoluene (20 g, 0.1058 mol) in CCl4 (50 mL) were added NBS (21.6 g, 0.121 mol) and AtBN (1.7 g, 10.5 mmol). The resulting suspension was stirred at 900C overnight under a nitrogen atmosphere. The reaction mixture was filtered and solvent concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using petroleum ether as eluent, to give 26 g of the sub-title compound as a colorless liquid.
(ii) (4-Bromo-3-fluorophenyl)acetonitrile
NaCN (10.6 g, 0.21 mol) was added to a solution of l-bromo-4-(bromomethyl)-2- fluorobenzene (26 g, 0.097 mol; see step( i) above) in dry DMF (169 mL). The resulting mixture was stirred at 500C overnight under a nitrogen atmosphere before the reaction was quenched with water. DMF was evaporated under reduced pressure and the product was extracted with ethyl acetate. The organic layer was washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 20 g of the sub-title compound as red solid. This was employed directly in the next step without further purification.
(iii) (4-Bromo-3-fluorophenyl)acetic acid A suspension of (4-bromo-3-fluorophenyl)acetonitrile (20 g; see step (ii) above) in cone. HCl (100 mL) was stirred at 8O0C overnight. The reaction mixture was basified with saturated NaHCO3 (aq.) and then extracted with ethyl acetate. The
aqueous layer was acidified with cone. HCl and filtered to provide 8.2 g of the sub-title compound as a pink solid. This was employed directly in the next step without further purification.
(iv) Methyl (4-bromo-3-fluorophenv0acetate
Methyl iodide (4.4 niL, 0.071 mol) was added, at O0C, to a suspension of (4- bromo-3-fluorophenyl)acetic acid (8.2 g, 0.035 mol; see step (iii) above) and potassium carbonate (12.2 g, 0.088 mol) in dry acetonitrile (100 mL). The resulting mixture was stirred at 60°C overnight under a nitrogen atmosphere. The reaction mixture was filtered and solvent concentrated under reduced pressure to provide 6.4 g of the sub-title compound as pale yellow oil. This was employed directly in the next step without further purification.
(v) Methyl (4-cyano-3-fluorophenyl)acetate A suspension of methyl (4-bromo-3 -fluorophenyl) acetate (6.4 g, 0.026 mol; see step (iv) above) and Zn(CN)2 (2.09 g, 0.018 mol) in dry DMF (32 mL) was evacuated and purged with argon for 15 minutes. Pd(PPli3)4 (1.5 g, 0.0013 mol) was added and the resulting suspension was stirred at 8O0C overnight under an argon atmosphere. The reaction was quenched with water, DMF was evaporated under reduced pressure and the resulting aqueous layer was extracted with ethyl acetate. The organic (ethyl acetate) layer was washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by purification over silica gel using 15% ethyl acetate in petroleum ether as eluent, afforded 4.3 g of the sub-title compound as pale yellow liquid.
(vi) 2-Fluoro-4-(2-hydroxyethyl)benzonitrile
Methanol (3 mL) was added to a suspension of lithium borohydride (4.3 g, 0.022 mol) in dry ether (30 mL) at 00C. Methyl (4-cyano-3-fluorophenyl)acetate (4.3 g, 0.022 mol; see step (v) above) in dry ether (30 mL) was added, dropwise, and stirring was continued overnight under a nitrogen atmosphere. The reaction was quenched with water and extracted with ethyl acetate. The organic layer was washed with water and brine and then dried over sodium sulfate. Solvent
evaporation under reduced pressure, followed by column chromatography over silica gel using 20% ethyl acetate in petroleum ether as eluent, afforded 2.23 g of the sub-title compound as white solid.
(vii) 4-(2-Bromoethyl)-2-fluorobenzonitrile
Carbon tetrabromide (8.96 g, 0.027 mol) in dry DCM (20 mL) was added, drop by drop at O0C, to a solution of 2-fluoro-4-(2-hydroxyethyl)benzonitrile (2.23 g, 0.013 mol) and triphenylphosphine (7.07 g, 0.027 mol) in DCM (100 mL) under a nitrogen atmosphere. The reaction mixture was stirred at RT overnight under a nitrogen atmosphere. Solvent evaporation under reduced pressure, followed by purification of the residue by column chromatography over silica gel (using 6% ethyl acetate in petroleum ether as eluent) gave 2.23 g of the title compound as a violet oil.
Preparation S
2-(4-Cvano-2-fluorophenyl)ethyl methanesulfo∑tate
(i) (4-Brorno~2-fluorophenyl)methanol
BH3 (1 M, in THF, 205 mL) was added to 4-bromo-2-fluoro benzoic acid (15 g, 0.0684 mol) in dry THF (150 mL) under a nitrogen atmosphere. The resulting mixture was stirred at RT overnight, after which the reaction mixture was cooled to 0°C and quenched with aqueous citric acid. Solvents (THF) were evaporated from the reaction mixture and the resulting aqueous mixture was extracted with ethyl acetate. The combined organic layers were washed with 10% NaHCO3, water and brine, dried over sodium sulfate and then concentrated under reduced pressure to afforded 14.7 g of the sub-title compound as pale yellow liquid. This was employed directly in the next step without further purification.
(ii) 4-Bromo-2-fluorobenzyl methanesulfonate Methanesulfonyl chloride (5.63 g, 0.0492 mol) was added, drop by drop at 00C, to a solution of (4-bromo-2-fluorophenyl)methanol (10.1 g, 0.0492 mol; see step (i) above) and triethylamine (5.47 g, 0.0541 mol) in dry DCM (25 mL). The
resulting mixture was stirred at RT overnight under a nitrogen atmosphere. The reaction was quenched with water, extracted with DCM, washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 8.4 g of the sub-title compound as a red oil. This was employed directly in the next step without further purification.
(iii) (4-Bromo-2-fluorophenyl)acetonitrile
A suspension of 4-bromo-2-fluorobenzyl methanesulfonate (8.4 g, 0.0376 mol; see step (ii) above) and sodium cyanide (3.6 g, 0.0752 mL) in dry DMSO (80 mL) was stirred at RT overnight under a nitrogen atmosphere. The reaction was quenched with water and then extracted with ethyl acetate. The organic (ethyl acetate) layer was washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 6.5 g of the sub-title compound as a dark red liquid. This was employed directly in the next step without further purification.
(iv) (4-Bromo-2-fluorophenyl)acetic acid
(4-Bromo-2-fluorophenyl)acetonitrile (6.5 g, 0.0303 mol; see step (iii) above) was taken in cone. HCl (35 mL) and stirred at 90°C overnight under a nitrogen atmosphere. The reaction mixture was poured onto ice-water and extracted with DCM. The organic layer was washed with water and brine and then dried over sodium sulfate. Solvent evaporation under reduced pressure afforded a crude product that was washed with petroleum ether and dried under vacuum to give 5.7 g of the sub-title compound as a brown solid.
(v) Methyl (4-bromo-2-fluorophenyl)acetate
Methyl iodide (6.3 g, 0.0446 mol, 2 eq.) was added, drop by drop at O0C, to a mixture of (4-bromo-2-fluoroρhenyl)acetic acid (5.5 g, 0.0223 mol; see step (vi) above) and dry K2CO3 (8.46 g, 0.6125 mol) in dry acetonitrile (100 mL) under a nitrogen atmosphere. The reaction mixture was stirred at 60°C overnight under a nitrogen atmosphere and then filtered through Celite®. Solvent evaporation under
reduced pressure afforded 5.5 g of the sub-title compound as a brownish liquid. This was employed directly in the next step without further purification.
Qi) Methyl (4-cyano-2-fluorophenyl)acetate A suspension of methyl (4-bromo-2-fluorophenyl)acetate (5.5 g, 0.0223 mol; see step (v) above), Zn(CN)2 (1.79 g, 0.0156 mol) in dry DMF (30 mL) was purged with argon for 15 minutes before Pd(PPh3)4 (1.27 g, 0.0011 mol) was added. The resulting suspension was stirred at 800C overnight under a nitrogen atmosphere before the reaction was quenched with water and extracted with ethyl acetate. The organic layer was washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure, followed by purification over silica gel (using 18% ethyl acetate in petroleum ether as eluent) gave the sub-title compound (3.6 g) as a pale yellow solid.
(vii) 3-Fluoro-4-(2-hvdroxyethyl)benzonitrile
Methanol (3 mL), followed by methyl (4-cyano-2-fluorophenyl)acetate (3.6 g, 0.018 mol; see step (vi) above) was added, at O0C, to a suspension of LiBH4 (1.11 g, 0.0572 mol) in dry diethyl ether (15 mL). The resulting mixture was stirred at RT overnight under a nitrogen atmosphere. The reaction was quenched with water and extracted with diethyl ether. The organic layer was washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure provided a residue that was purified by column chromatography over silica gel, using 23% ethyl acetate in petroleum ether as eluent. This gave 0.6 g of the sub-title compound as a yellow liquid.
(viii) 2-(4-Cvano-2-fluorophenγl)ethyl methanesulfonate
Methanesulfonyl chloride (0.3 mL, 0.0036 mol) was added, at 00C, to a solution of 3-fluoro-4-(2-hydroxyethyl)benzonitrile (0.6 g, 0.0036 mol; see step (vii) above) and triethylamine (1.55 mL, 11.2 mmol) in dry DCM (10 mL) under a nitrogen atmosphere. The reaction mixture was stirred at RT for 2 h and then quenched with water. The organic layer was washed with water and brine and then dried
over sodium sulfate. Solvent evaporation under reduced pressure afforded 0.9 g of the title compound as an off-white solid.
Preparation T 2-(2,6-Difluorophenyl)ethyl methanesulfonate
(i) Methyl (2,6-difluorophenyl)acetate
H2SO4 (cone, 0.75 mL) was added to a solution of (2,6-difluorophenyl)acetic acid
(14 g, 0.0814 mol) in dry methanol (160 mL). The resulting mixture was stirred at room temperature overnight. Solvent was evaporated under reduced pressure and the residue was partitioned between water and diethyl ether. The organic layer was washed water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure yielded 16 g of the sub-title compound as a pale yellow liquid. This was employed directly in the next step without further purification.
(ii) 2-(2,6-Difluorophenyl')ethanol
LAH (4.7 g, 0.129 mol) in dry diethyl ether (400 mL) was added, at 0°C, to a stirred suspension of methyl (2",6-difluorophenyl)acetate (16 g, 0.086 mol; see step (i) above) in dry diethyl ether (80 mL). The resulting mixture was stirred at room temperature for 2 h under a nitrogen atmosphere before the reaction mixture was quenched with water and NaOH and then filtered. The aqueous layer was acidified with 1.5 N HCl and extracted with diethyl ether. The organic layer was washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 13.5 g of the sub-title compound as yellow liquid. This was employed directly in the next step without further purification.
(iii) 2-(2,6-Difluorophenyl)ethyl methanesuIfonate
Methanesulfonyl chloride (10.76 g, 0.094 mol) was added, at 0°C, to a solution of 2-(2,6-difluorophenyl)ethanol (13.5 g, 0.085 mol; see step (ii) above) and triethylamine (12.93 g, 0.128 mol) in dry DCM (135 mL). The resulting mixture was stirred for 3 h under a nitrogen atmosphere before the reaction was quenched with water and extracted with DCM. The organic (DCM) layer was dried over
sodium sulfate and then concentrated under reduced pressure to give 18 g of the title compound as a pale yellow oil.
Preparation U l-(2-Bromoethoxy')-2,4-difluorobenzene
A suspension of 2,4-difluorophenol (8 g, 0.0615 mol), 1,2-dibromoethane (37.5 mL, 81.75 g, 0.435 mol) and K2CO3 (27.49 g, 0.198 mol) in acetonitrile (80 mL) was stirred at 60°C overnight under a nitrogen atmosphere. The reaction mixture was filtered and solvent concentrated under reduced pressure. The residue was purified by column chromatography over silica gel, using 5% ethyl acetate in petroleum ether as eluent, to give the title compound (9.93 g) as a colorless liquid.
Preparation VCa) l-(3-Bromopropoxy)-2,4-difluorobenzene
2,4-Difluorophenol (100 g, 0.769 mol), 1,3-dibromoproρane (931 g, 4.615 mol) and anhydrous potassium carbonate (212.2 g, 1.538 mol) were refluxed for 2 h in acetonitrile (1.3 L). The reaction mixture was then cooled to room temperature and filtered. The filtrate was concentrated to provide a crude product that was purified by reduced pressure distillation to give 140 g (73%)' of the title compound.
1H-NMR (400 MHz, CDCl3): δ 6.96 (m, IH), 6.87 (m, IH), 6.81 (m, IH), 4.16 (t, 2H), 3.65 (t, 2H), 2.38-2.32 (m, 2H)
Preparation VCb)
The following compounds were prepared according to or by analogy with the procedure outlined in either Preparation U or Preparation V(a) above (as appropriate), replacing 2,4-difluorophenol with the appropriate substituted phenol: 4-(3 -bromopropoxy)benzonitrile; 4-(3-bromopropoxy)~3 ,5-difluorobenzonitrile; 4-(3-bromopropoxy)-3-fluorobenzonitrile; l-(3-bromopropoxy)-4-(trifluoromethyl)benzene;
4-(3-bromopropoxy)- 1 ,2-difluorobenzene;
4-(3-bromopropoxy)-l-chloro-2-fluorobenzene; l-(3-bromopropoxy)-4-chloro-2-fluorobenzene; l-(3-bromopropoxy)-3-fluorobenzene l-(3-bromopropoxy)-3-chlorobenzene;
1 -(3 -bromopropoxy)-3 -(txifluoromethyl)benzene;
1 -(3 -bromopropoxy)-4-chlorobenzene; l-(3-bromopropoxy)-2-fluorobenzene;
2-(3-bromopropoxy)-l,3-difluorobenzene; 4-(3-bromopropoxy)isophthalonitrile;
4-(3-bromopropoxy)-2-fluorobenzonitrile;
2-(3 -bromopropoxy)benzonitxile;
3-(3-bromopropoxy)benzonitrile;
4-(2-bromoethoxy)benzonitrile; 4-(2-bromoethoxy)isophthalonitrile;
2-(2-bromoethoxy)-l ,3 -dimethylbenzene;
4-(2-bromoethoxy)-3-fluorobenzonitrile;
4-(2-bromoethoxy)-3,5-difluorobenzonitrile; l-(2-bromoemoxy)-2-fluorobenzene; 2-(2-bromoethoxy)- 1 ,3-difluorobenzene;
4-(2-bromoethoxy)- 1 ,2-difluorobenzene; and l-(2-bromoethoxy)-3-fluorobenzene.
Preparation X Methanesulfonic acid C2-benzor^πisoxazol-3-yl')ethyl ester
(i) (2-Benzorάπisoxazol-3-yl)ethanol
To a solution of (benzo[<]isoxazol-3-yl)acetic acid (0.86 g, 4.85 mmol) in anhydrous THF (40 mL) was added, dropwise at 0°C, borane-dimethylsulfide complex (0.58 mL, 10 M, 5.8 mmol). The resulting mixture was allowed to warm to room temperature and was then stirred overnight. MeOH (10 mL) was carefully added and, when gas evolution had ceased, the mixture was heated under
gentle reflux for 4 hours. The reaction mixture was concentrated in vacuo and the resulting residue was dissolved in DCM (20 mL). An aqueous solution of Na2CC>3 (20 mL) was added and the aqueous layer was extracted with DCM (3 x 20 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuo. The crude product was then purified by chromatography on silica gel to provide 537 mg (67.8%) of the title compound.
13C NMR (125.7 MHz, CDCl3): δ 163.06, 156.92, 130.27, 123.65, 121.98, 121.65, 110.10, 60.34, 29.01
(ii) Methanesulfonic acid (2-benzor<i1isoxazol-3-yl)ethyl ester
To a solution of (2-benzo[<fjisoxazol-3-yl)ethanol (0.537 g, 3.29 mmol; see step (i) above) and triethylamine (0.55 mL, 3.95 mmol) in DCM (20 mL) was added methanesulfonyl chloride (0.31 mL, 3.95 mmol), at 00C. The reaction mixture was stirred for 1 hour and was then extracted with water (2 x 20 mL) and brine (20 mL). The organic layer was dried over Na2SO4 and concentrated in vacuo, which afforded 818 mg (98%) of the title compound.
1H NMR (500 MHz, CDCl3): δ 7.73 (IH, d), 7.61 (2H, m), 7.37 (IH, m), 4.73 (2H, t), 3.49 (2H, t), 3.01 (3H, s)
Preparation Y
2-ChIQrQ-N- r2-(4-fluorophenyl)ethvn acetamide
Chloroacetyl chloride (1.94 g, 9 mmol) was added, drop by drop at 00C, to a solution of 2-(4-fluorophenyl)ethylamine (2 g, 17.2 mmol) and triethylamine (3.61 g, 35.7 mmol) in 30 mL of DCM (dry). The reaction mixture was stirred at room temperature for 1 h under a nitrogen atmosphere before being partitioned between water and DCM. The organic layer was washed with water and brine and dried over sodium sulfate. Solvent evaporation under reduced pressure afforded 3.4 g of the title compound as a brown solid. This was employed directly without further purification.
Preparation Z(a)
2-Chloro-JV-d-methyl-l-phenylethyl)acetamide
To 2-phenylpropan-2-amine (541 rag, 4.00 mmol) in THF (20 xnL) was added three equivalents of polymer-bound diisopropylethylamine. The resulting mixture was cooled to 00C and chloroacetyl chloride (904 mg, 8.00 mmol) in THF (20 mL) was added stepwise over the course of 5 to 10 minutes. The resulting mixture was stirred at 0°C for 20 minutes and then at room temperature for 2 hours. The resulting mixture was passed through a column of polymer-bound amine (NH2, Isolute™) with DCM/MeCN/MeOH (2:1:1) as eluent. The eluate was concentrated in vacuo and the resulting residue was dissolved in DCM and loaded onto a cation exchange column (SCX-2, Isolute™, 5 g). Elution of the column with DCM/MeCN/MeOH (2:1:1) afforded 735 mg (87%) of the title compound as a white solid.
Preparation Z(b)
The following compounds were prepared according to or by analogy with the procedure outlined in Preparation Z(a) above, replacing 2-phenylpropan-2-amine with the appropriate amine:
2-chloro-ΛT-(2-phenylethyl)acetamide; 2-chloro-iV-f2-(4-fluorophenyl)ethyl]acetamide;
2-chloro-N-(4-fluorobenzyl)acetamide; iV-benzyl-2-chloroacetamide;
2-chloro-N-(2,4-difluorobenzyl)acetamide;
2-chloro-N-(3-fluorobenzyl)acetamide; 2-chloro-iV-(2-fluorobenzyl)acetamide;
N-benzyl-2-chloro-N-methylacetamide; iV-(ter?-butyl)-2-chloro-iV-methylacetamide; and
N-(tert-butyl)-3-chloropropanamide.
Preparation AA 3-Benzyl-9-oxa-3J-diazabicyclor3.3J1nonane dihydrocnloride
(i)iV,iV-Bis(2-oxiranylmethyl)benzenesulfonamide
ALTERNATIVE 1
Benzenesulfonamide (120 g, 0.763 moles), (7?)-epichlorohydriα (282.6 g, 3.054 moles) and water (960 g) were added to a 2 L reaction flask. The mixture was heated to 400C and then sufficient sodium hydroxide solution (31%) was added over approximately 5 mins such that the pH was raised to 11.5-12.0 (in an alternative procedure, 25% sodium hydroxide solution can be employed). The remainder of the sodium hydroxide (201 g, 1.557 moles in total) was then added at such a rate as to maintain the pH at 11.5-12.0 and the temperature at 40-50°C (usually requires addition over 3-4 hours). The reaction mixture was then stirred for 2 hours at 40-450C and distilled to remove 3 volumes (360 mL) of water/epichlorohydrin at 50 mbar (5 kPa) with a maximum contents (source vessel) temperature of 43°C. Chlorobenzene was then added (221.4 g, 1.67 volumes) and the mixture was stirred for 0.5 hours before being allowed to settle. The lower product (chlorobenzene) layer was separated and the extraction process repeated using a further portion of chlorobenzene (44.3 g, 0.33 vols.). The two product layers were combined for use in the next step (see step (ii), Alternative 1 below).
ALTERNATIVE 2 Benzenesulfonamide (175 kg, 1 eq.), water (1365 kg, 8 rel. vol.) and (ft)-epichlorohydrin (412 kg, 4 eq.) were charged to a reaction vessel. The reactants were heated to 400C. Sufficient aqueous sodium hydroxide was added, over the course of approximately 20 minutes, to adjust the pH to 11.5 - 12.0. The remainder was then charged in a controlled manner over approximately 150 minutes, such that the temperature of the reaction was maintained between 400C and 500C, and the pH remained in the range 11.5 to 12.0 (total charge: 90.8 kg in 202 kg of water). After the addition, of sodium hydroxide was complete, the
reaction was stirred between 400C and 45°C for 2 hours. The excess (R)- epichlorohydrin was removed as a water azeotrope by vacuum distillation (ca. 60 mbar (6 kPa), internal temperature 43°C maximum, 525 litres of distillate, 3 rel. vol.). Chlorobenzene (total of 387 kg, 2 rel. vol.) was then charged to the reaction in two portions. Following each addition, the mixture was stirred and then allowed to settle before the chlorobezene layer was separated. The two chlorobenzene layers were then combined and used without further treatment in the next step (see step (ii), Alternative 2 below).
(ii) 5-B enzyl-3 J-dihydroxy-l-phenylsulf onyl-1 ,5-diazacyclooctane
ALTERNATIVE 1
Methanol (854 g, 18 volumes) was heated to reflux. ΛζN-bis(2-oxiranylmethyl)- benzenesulfonamide (0.382 mol; see step (i), Alternative 1 above) and benzylamine (37.3 g, 0.347 moles) were concurrently added via syringe pumps over 6 hours into the reaction vessel at opposite sides of the reaction vessel. The reaction was maintained at reflux throughout the addition of the reagents. After addition was complete, the reaction solution was maintained at reflux for a further 3 hours before methanol (14 volumes, 840 mL) was distilled from the reaction vessel at atmospheric pressure. Chlorobenzene (266 g, 240 mL) was then added and the distillation continued until a further portion of methanol (4 volumes, 240 mL) had been collected from the reaction vessel. A second portion of chlorobenzene (133 g, 120 mL) was added and a mixture of solvent (4 volumes, 240 mL of a mixture of chlorobenzene/methanol) was distilled from the reaction mixture at 50 mbar (5 kPa). The remaining mixture (after the distillation) comprised the sub-title compound and chlorobenzene with a methanol content of <0.1% w/w. This solution was employed in the next step (see step (iii), Alternative 1 below).
ALTERNATIVE 2
Methanol (2494 kg, 18 rel. vol. - either fresh or recycled) was charged to a reaction vessel and heated to reflux temperature (approx. 650C). Simultaneously,
and over approximately 6 hours were charged the chlorobenzene solution (containing N,iV-bis(2-oxiranylmethyl)benzenesulfonamide) from step (i), Alternative 2 above and benzylamine (109 kg, 0.91 eq.). The batch was maintained at reflux throughout the addition. The reaction was stirred at approximately 65°C (reflux temperature) for a further 3 hours. Methanol (1938 kg, 14 rel. vol.) was then removed by distillation at atmospheric pressure before chlorobenzene (775 kg, 4 rel. vol.) was added. The resulting solution was used without further treatment in the next step (see step (iii), Alternative 2 below).
(iii) 3-Benzyl-7-(phenylsulfonyl)-9-oxa-3,7-diazabicyclor3.3.1]nonane
ALTERNATIVE 1
Chlorobenzene (598 g, 9 volumes) and water (7.2 g, 0.4 moles) were added to a solution of S-benzyl-SJ-dihydroxy-l-phenylsulfonyl-l^-diazacyclooctane in chlorobenzene (0.382 moles; see step (ii), Alternative 1 above) and heated to 75°C. Sulfuric acid (98%, 134 g, 1.337 moles) was then added over 1 hour, whilst maintaining the temperature in the range 75-90°C. (In an alternative embodiment, S-benzyl-SJ-dihydroxy-l-phenylsulfonyl-ljS-diazacyclooctane may be added to sulfuric acid.) The biphasic reaction mixture was heated to 950C and stirred for 3 hours. The temperature was adjusted to 500C and methanol (57 g, 1.2 volumes) was added at such a rate as to maintain the temperature at between 50 and 600C. The reaction mixture was basified by adding aqueous ammonia (17.5%, 346 g, 372 mL) over 2 hours at between 60 and 700C, and then allowed to settle after 15 min of stirring (the mixture is kept at 600C during the period in which it is allowed to settle). The lower aqueous layer was separated and the upper organic layer transferred to the crystallising vessel. The aqueous layer was returned to the reaction vessel and the temperature was adjusted to 45°C before chlorobenzene (133 g, 120 mL) was added. The separation process was repeated (i.e. the aqueous layer extracted and the phases separated) and the second organic phase combined with the first organic phase in the crystallising vessel. Chlorobenzene was then distilled (660 mL, 11 volumes) from the product layer at 50 mbar (5 kPa) and then methanol (470 g, 594 mL) was added over the course of 1 hour. The temperature
was allowed to fall during this addition, after which the resulting slurry was cooled to 50C and held at that temperature for 1 hour before being filtered. The filter cake was then washed with two portions of methanol (2 x 47.4 g, (2 x 60 rnL)), at either 5°C or ambient temperature, and then suction dried for 30 mins. The product was transferred to a vacuum oven and dried to constant weight at 4O0C to provide the sub-title compound (yield 31% (42.5 g) over Alternative 1 of steps (i), (ii) and (iii)).
ALTERNATIVE 2 The chlorobenzene / methanol solution from step (ii), Alternative 2 above was distilled further at atmospheric pressure (removing a total of 700 litres (4 rel. vol.) of solvent). Fresh and/or recycled chlorobenzene (350 litres, 2 vols.) was charged, then distillation was continued under vacuum (ca. 50 mbar (5 kPa)) to complete solvent exchange to chlorobenzene (a total of a further 700 litres (4 rel. vol.) being removed through distillation). Further chlorobenzene (fresh or recovered, 1575 litres, 9 rel. vol.) and water (21 kg, 1.05 eq.) were charged and the batch heated to 750C. Sulfuric acid (382 kg, 3.5 eq. of 98%) was charged over approximately 1 hour, whilst allowing the temperature to rise up to 900C. (In an alternative embodiment, the chlorobenzene / water mixture may be added to sulfuric acid.) The biphasic reaction mixture was maintained for a further 3 hours at 95°C. After cooling to 50 - 55°C, methanol (160 kg, 1.2 rel. vol.) was charged, whilst maintaining the temperature at 50 - 550C. Aqueous ammonia (176 kg in 830 kg of water) was charged in a controlled manner whilst maintaining the contents at between 60 - 7O0C. After stirring for 15 minutes, the batch was settled for 30 minutes and the layers separated. After back extracting the aqueous layer with chlorobenzene (388 kg, 2 vol.) the organic layers were combined and a total of 1925 litres (11 rel. vol.) of chlorobenzene were distilled under vacuum (50 mbar (5 kPa), 450C). Methanol (1330 kg, 9.6 rel. vol) was charged to the residue. The resultant slurry was cooled to 5°C, stirred for 1 hour, then the solids were isolated by filtration. The wet filter cake was dried under vacuum (-50 mbar (5 kPa), 400C maximum temperature) to afford 130.5 kg of the sub-title compound (32.7% yield over Alternative 2 of steps (i), (ii) and (iii)). "
(iv) 3-Benzyl-9-oxa-3,7-diazabicvclo[3.3.1"lnonane dihydrochloride
ALTERNATIVE 1
3-Benzyl-7-(phenylsulfonyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane (40 g, 0.112 mol; see step (iii), Alternative 1 above) and hydrobromic acid (48%, 179 g, 120 niL) were heated to 1220C and stirred for 9 hours. The solution was cooled to 200C before toluene (173 g, 200 mL) was added and the resulting biphasic mixture stirred for 30 mins. After being allowed to settle, the lower aqueous layer of the biphasic mixture was separated and the upper toluene layer discarded. The aqueous layer was returned to the reaction vessel and sodium hydroxide (31%, 181 g, 141 mL) was added over 45 mins, allowing the temperature to rise to a maximum of .600C. Toluene (156 g, 180 mL) was added and the temperature adjusted to 6O0C before the layers were separated and the lower aqueous layer discarded. The toluene layer, containing the product, was washed with water (120 g) at 600C before being cooled to 400C, after which iso-propanol (345 g, 440 mL) was added. Hydrochloric acid (36%, 25.9 g, 0.256 mol) was then added over 1 hour at 40-450C, after which the mixture was cooled to 5°C and stirred for 1 hour. The product was filtered, washed with wø-propanol (141 g, 180 mL) and then suction dried for 30 mins before being transferred to the vacuum oven and dried to constant weight at 400C (yield: 88%, 28.6 g).
ALTERNATIVE 2
3-Benzyl-7-(phenylsulfonyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane (107 kg, 1 eq.; see step (iii), Alternative 2 above) and hydrobromic acid (229 kg, 9.5 eq. in 248 kg water) were charged to a vessel, heated initially to 110 to 115°C with the scrubber vent open, the vent was sealed then heating was continued under a slight positive pressure (4psi (0.27 atmospheres)) to 122°C then stirred for 9 hours at 1220C. After cooling to 15 - 20°C, toluene (463 kg, 5 rel. vol) was charged and the resulting biphasic mixture was stirred before being allowed to settle for 30 minutes. The layers were separated and the lower aqueous layer was returned to the original vessel. To this vessel was then charged aqueous sodium hydroxide (149 kg, 12.5 eq. in 332 kg water) whilst maintaining the contents temperature
below 800C. The reaction mixture was cooled to a temperature in the range of 15 to 2O0C before toluene (416 kg, 4.5 rel. vol) was charged. The resulting biphasic mixture was heated to 6O0C and then stirred and settled for 30 minutes at 600C. After separation, the toluene layer was washed with water (214 kg, 2 rel. vol.) at 6O0C, then cooled to 15 - 200C. Isopropyl alcohol (925 kg, 11 rel. vols.) was charged, and the contents adjusted to 4O0C. Hydrochloric acid (25 kg, 2.3 eq. in 44.5 kg water) was charged in a controlled manner, keeping the contents in the range 40 - 45°C. After stirring for 1 hour at 40°C, the resultant slurry was cooled to 5°C and stirring continued for a further 2 hours. The solids were isolated by filtration and dried (400C maximum temperature) to afford 78 kg (89.7%) of the title compound.
ALTERNATIVE 3
Water (72 mL) and concentrated sulfuric acid (228 mL) were added to 3-benzyl-7- (phenylsulfonyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane (100.06 g, 279 mmol; see step (iii) above). (In an alternative embodiment, 3-benzyl-7-(phenylsulfonyl)-9- oxa-3,7-diazabicyclo[3.3.1]nonane may be added to sulfuric acid.) The reaction mixture was heated for 9 hours at 1300C, then left to cool to room temperature overnight. The acidic solution was poured into a clean vessel containing water (300 mL), and concentrated aqueous ammonia (35%) added dropwise over 2 hours (550 mL). After ammonia addition was complete, the pH of the reaction mixture was checked and found to be 10. Toluene (450 mL) was then added, and the temperature adjusted to 6O0C. The lower (aqueous) layer was separated and discarded. To the remaining upper layers (organic layer and interfacial layer), 5 M sodium hydroxide solution (300 mL) was added. The mixture was re-heated to 600C, and stirred for 15 minutes. The layers were separated and the lower aqueous phase removed. Isopropanol (1100 mL) was added to the organic phase and the resulting solution warmed to 43°C. Concentrated hydrochloric acid (54 mL) was then added over 1 hour, maintaining the temperature at between 40 and 45°C, which precipitated the product. The mixture was then cooled to 50C, and stirred for 1 hour. The product was collected by filtration and the filter cake was washed by displacement with isopropanol (400 mL) before being dried as
much as possible by suction (on the filter) and then in vacuo (for 64 hours at 400C). This gave the title compound as a crystalline, white solid (77.16 g, 95%).
ALTERNATIVE 4 Water (11.2 mL) and concentrated sulfuric acid (24.5 mL) were added to 5- benzyWJ-dmydroxy-l-phenylsulfonyl-l.S-diazacyclooctane (2.92 g, 7.76 mmol; see step (ii) above). The reaction mixture was heated for 24 hours at 950C. The temperature was adjusted to 600C, and toluene (40 mL) was added. Sodium hydroxide solution was then added (150 mL of 5 M), causing the internal temperature to rise to 85°C. The pH of the reaction mixture was then checked, and was found to be 2. A few pellets of solid sodium hydroxide were then added. The pH was measured again, and was found to be 13. The layers were separated, and the aqueous phase extracted with toluene (50 mL). The organic phases were combined, dried (MgSO4), filtered and concentrated in vacuo to provide 3-benzyl- 9-oxa-3,7-diazabicyclo[3.3.1]nonane as an orange-brown oil. This can be converted to the title compound (dihydrochloride salt) by reaction with hydrochloric acid under standard conditions.
1H NMR (300 MHz, DMSOd6) δ 7.25 (m, 5H), 4.38 (s, IH), 3.69 (s, IH), 3.49 (m, 2H), 3.34 (m, 2H), 2.99 (d, J = 13.8 Hz, IH), 2.86 (m, 2H), 2.74 (m, IH), 2.64 (m, 2H).
ALTERNATIVE 5
77% sulfuric acid (126.3 g, 0.99 moles) and 98% sulfuric acid (68.4 g,
0.683 moles) are mixed carefully to afford 195 g of 85% sulfuric acid (1.675 moles, 15 eq.). (Alternatively, water and 98% sulfuric acid are mixed carefully to prepare the same quantity of 85% sulfuric acid.) The reaction mixture is heated to 1000C, before 3-benzyl-7-(phenylsulfonyl)-9-oxa-3,7-diazabicyclo- [3.3.1]nonane (40 g, 0.112moles; see step (iii) above) is added, portion-wise, over the course of approximately 45 to 60 minutes. The reaction mixture is heated to 13O0C, and stirred at this temperature for 9 hours. After the reaction mixture is cooled to 20 to 25°C, water (120 g) is added over the course of approximately 30 minutes, during which addition the reaction mixture is maintained at 20 to
5O0C. At this point, 35% ammonia solution (193.6 g, 3.96 moles) is added over the course of approximately 2 hours, during which addition the reaction mixture is maintained at below 7O0C. After verifying that the pH of the batch is 10 or above, toluene is added and the reaction mixture is stirred rapidly, at 70 to 750C, for 15 minutes. The layers are allowed to settle for approximately 30 minutes, then the lower (aqueous) layer is discarded. To the remaining upper layers (organic layer and interfacial layer),- is added 5M sodium hydroxide solution (139 g, 0.60 moles), and the reaction mixture is stirred for approximately 15 minutes at 60 to 65°C. After settling for 30 minutes, the layers are separated, keeping any interfacial material with the aqueous layer. The product (toluene) layer is cooled to 40 to 450C before isopropanol (345 g, 440 mL) is added, followed by, over the course of 1 hour and at 40 to 45°C, 36% hydrochloric acid (26.0 g, 0.257 mols). The resulting mixture is cooled to 50C and stirred at this temperature for 1 hour. The product is isolated by filtration, washed with isopropanol (126 g, 160 mL) and then dried by suction (on the filter) for 30 mins, before being transferred to a vacuum oven. The title compound is then dried to constant weight at 4O0C (30.1 g, 92.5%).
Preparation AB 3-Benzyl-7-(2-phenethyl)-9-oxa-3 J-diazabicycloP .3. linonane
ALTERNATIVE 1
Sodium carbonate (830.28 mmoles; 88.00 g), was added to a 2L flask. Water
(11.10 moles; 200.00 mL; 200.00 g) was added and the mixture stirred to give a solution. A solution of l-bromo-2-phenylethane (1.18 eq.; 607.90 mmoles; 83.03 mL; 112.50 g) in toluene (3.26 moles; 344.35 mL; 300.00 g) was added, at which point the temperature of the resulting mixture was 3O0C. A solution of 3- benzyl-9-oxa-3,7-diazabicyclo[3.3.1]nonane dihydrochloride (1.00 equiv; 515.07 mmoles; 150.00 g; see Preparation AA above) in water (24.98 moles; 450.00 mL) was added over 15 minutes. The residues were washed into the reaction flask with water (3.33 moles; 60.00 mL; 60.00 g). The resulting mixture was heated to 7O0C over 15 minutes and then held at this temperature for 8.5 hours
before being stirred at room temperature overnight (for convenience). The reaction was re-heated to 700C and the phases were separated, keeping the small amount of interfacial material in the toluene phase. The aqueous phase was discarded. The remaining organic phase was extracted with 10% w/w aqueous citric acid (312.30 mmoles; 576.81 mL; 600.00 g), at 70°C, keeping the small amount of interfacial material with the toluene phase. The organic phase was then discarded. 2-Butanol (8.15 moles; 750.00 mL; 604.12 g) and aqueous 15% w/w sodium hydroxide (1.13 moles; 257.73 mL; 300.00 g) were added to the citric acid phase. The phases were separated at 700C and the aqueous phase discarded. The 2-butanol phase was distilled at atmospheric pressure (88-900C); 375 mL of solvent was distilled off. The remaining solution (524 g) was decanted to a clean flask and was then allowed to cool to room temperature overnight whilst stirring. The product slurry was cooled to 50C and, after 2 hours at that temperature, was filtered. The filter cake was washed with cold (5°C) 2-butanol (1.89 moles; 173.81 mL; 140.00 g) and was then sucked as dry as possible on the filter. The damp filter cake (146 g) was dried in vacuo at 300C for 3.5 hours, at 350C for 15.5 hours and finally at 400C for 5.5 hours to provide 114.1 g (69%) of the title compound as a white solid. The crystallisation mother liquors were concentrated by distillation at atmospheric pressure (and 90 to 100°C); 400 mL of solvent was distilled off. The solution was cooled to 26°C over the course of 2 hours, after which crystallisation seed (ca. 200 mg, taken from the first crop of title compound) was added. After 1 hour, the mixture was cooled to 180C and then filtered. The filter cake was washed with cold (5°C) 2-butanol (543.36 mmoles; 50.00 mL; 40.28 g) and then sucked as dry as possible on the filter. The damp filter cake was dried in vacuo (at 4O0C) to provide a second crop of the title compound as a white solid (22.04 g, 13%). Total yield: 135.94 g (82%).
1H NMR (300 MHz, CDCl3): δ 2.5-2.65 (m, 6H); 2.78-2.95 (m, 6H); 3.49 (s, 2H); 3.89 (t, 2H); 7.15-7.35 (m, 8H); 7.4-7.43 (m, 2H).
ALTERNATIVE 2
A flask was charged with 3-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]nonane dihydrochloride (1.00 equiv; 343.38 mmoles; 100.00 g; see Preparation AA above). A solution of l-bromo-2-ρhenylethane (1.20 equiv; 410.67 mmoles; 56.09 mL; 76.00 g) in toluene (2.17 moles; 229.57 mL; 200.00 g) was added. The mixture was stirred and a solution of sodium hydroxide (1.10 moles; 44.00 g) in water (22.20 moles; 400.00 mL; 400.00 g) was added. The reaction was heated to 700C (stirring at 300 rpm) and was held at this temperature for 8 hours before being stirred at room temperature overnight (for convenience). The reaction mixture was re-heated to 700C and the phases were separated, leaving interfacial material with the toluene phase. The lower (aqueous) phase (pH 14) was discarded and a solution of 10% w/w aqueous citric acid (260.25 mmoles; 480.68 mL; 500.00 g) was added. The mixture was re-heated to 700C and the phases separated, leaving interfacial material with the toluene phase, and the upper (organic) phase was discarded. Toluene (1.09 moles; 114.78 mL; 100.00 g) was added and the mixture re-heated to 70°C. The phases were separated, leaving interfacial material with the toluene phase, and the upper organic phase was discarded. 2-Butanol (5.40 moles; 496.59 mL; 400.00 g) and 20% w/w aqueous sodium hydroxide (1.00 moles; 164.04 mL; 200.00 g) were added and the mixture re-heated to 700C. The phases were separated, leaving interfacial material with the aqueous phase, and the lower (aqueous) phase was discarded. The 2-butanol solution of the title compound was assumed to contain 100% yield of product and was used in the next step (see Preparation AC, Alternative 2 below) directly.
ALTERNATIVE 3
A flask was charged with 3-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]nonane dihydrochloride (1.00 equiv; 171.69 mmoles; 50.00 g; see Preparation AA above). A solution of l-bromo-2-phenylethane (1.20 equiv; 205.33 mmoles; 28.04 mL; 38.00 g) in toluene (1.09 moles; 114.78 mL; 100.00 g) was added. The mixture was stirred and a solution of sodium hydroxide (550.04 mmoles; 22.00 g) in water (11.10 moles; 200.00 mL; 200.00 g) was added. The reaction was heated to 7O0C (stirring at 250 rpm) arid was held at this temperature for 8 hours before being left
to stand at room temperature overnight (for convenience). The reaction was reheated to 700C and the phases were separated. The lower (aqueous) phase was discarded and a solution of 10% w/w citric acid (130.12 mmoles; 240.34 mL; 250.00 g) was added to the retained (organic) phase. The mixture was re-heated to 500C and the phases were separated again and the upper (organic) phase was discarded. Toluene (1.09 moles; 114.78 mL; 100.00 g) was added to the retained (aqueous) phase and the mixture re-heated to 500C. The phases were separated and the upper (organic) phase was discarded. Toluene (1.09 moles; 114.78 mL; 100.00 g) and an aqueous solution of 20% w/w sodium hydroxide (500.04 mmoles; 82.02 mL; 100.00 g) were added to the retained (aqueous) phase and the temperature of the mixture was adjusted to 500C. The phases were separated and the lower (aqueous) phase was discarded. Water (2.78 moles; 50.00 mL; 50.00 g) was added and the temperature adjusted to 600C. The phases were separated and the lower (aqueous) phase was discarded. The retained toluene solution of the title compound (151.26 g) was assumed to contain 100% yield of product and was used in the next step (see Preparation AC, Alternative 3 below) directly.
ALTERNATIVE 4 3-Benzyl-9-oxa-3,7-diaza-bicyclo[3.3.1]nonane dihydrochloride (145.6 g; see Preparation AA above) was dissolved in water (0.5 L) and a solution of NaOH (62.8 g) in water (0.5L) was added. 2-Phenylethyl bromide (105.4 g), dissolved in toluene (0.5 L), was added. The resulting mixture was stirred at 6O0C fox 16 hours. The aqueous layer was separated and discarded and the toluene layer was then extracted with an aqueous solution of citric acid (10%, 1 L). The toluene layer was separated and discarded before the aqueous (citric acid) layer was basified with 50% NaOH solution until pH was above 12. The aqueous layer was then extracted with ethyl acetate (0.5 L + 0.3 L). The combined organic layers were dried (MgSO4), filtered and then concentrated under reduced pressure to provide the title compound (151.5 g, 94% yield). LC-MS: 323 (M+H)+
1H-NMR (CDCl3): δ 7.43 (d, 2H), 7.35-7.29 (m, 6H), 7.27-7.20 (m, 2H), 3.91 (t, 2H), 3.52 (s, 2H), 2.97-2.81 (6H), 2.64-2.56 (m, 6H).
Preparation AC 3~(2-Phenethyl)-9-oxa-3 ,7-diazabicyclor3.3. llnonane dihydrochloride
ALTERNATIVE 1
3-Benzyl-7-(2-phenethyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane (1.00 equiv; 93.04 mmoles; 30.00 g; see Preparation AB above) was charged to a hydrogenation vessel. Isopropanol (2.00 moles; 152.87 niL; 120.00 g), citric acid (28.11 mmoles; 5.40 g) and water (166.53 mmoles; 3.00 mL; 3.00 g) were added, followed by 5% palladium on charcoal (1.50 g; ca. 60% water wet; Johnson Matthey Type 440). The resulting mixture was warmed to 60°C under a hydrogen atmosphere (3 bar) until hydrogen uptake ceased (ca. 2.5 hours). Heating and stirring was stopped and the reaction left to stand overnight (for convenience). The catalyst was removed by filtration and washed with isopropanol (783.75 mmoles; 60.00 mL; 47.10 g). The combined filtrate and washings were heated to between 40 and 45°C. Concentrated hydrochloric acid (212.63 mmoles; 18.00 mL; 21.24 g) was added over the course of 2 minutes, which caused the temperature of the mixture to rise from 45 to 520C. The mixture was allowed to cool and, after 5 minutes, precipitate started to form. The mixture was then allowed to cool to room temperature over the course of 75 minutes. It was then cooled to 50C over 15 minutes and held at that temperature for 70 minutes. The product was collected by filtration and was washed with cold (5°C) isopropanol (783.75 mmoles; 60.00 mL; 47.10 g). The filter cake was sucked as dry as possible on the filter then the damp product (32 g) was dried in vacuo (at 400C) to provide the title compound as a white solid (25.82 g, 91%). 1H NMR (400 MHz, CD3OD + 4 drops of D2O): δ 2.7-3.0 (m, 6H); 3.3-3.5 (m, 6H); 4.15 (br s, 2H); 7.20-7.36 (m, 5H). Chloride = 23.8% w/w (di-HCl requires 23.9% w/w) Water = 3.20% w/w
ALTERNATIVE 2
The hot (60°C) solution of 3-benzyl-7-(2-phenethyl)-9-oxa-3,7-diazabicyclo- [3.3.1]nonane in 2-butanol generated in Preparation AB, Alternative 2 above was added to a hydrogenation vessel. Isopropanol (1.66 moles; 127.39 mL; 100.00 g) and citric acid (103.06 mmoles; 19.80 g) were added, which provided a slightly yellow solution. To this solution was added 5% palladium on charcoal (5.50 g; ca. 60% water wet; Johnson Matthey Type 440) and the resulting reaction mixture was warmed to 500C under a hydrogen atmosphere (3 bar), with stirring at 500 rpm. Hydrogen gas uptake was complete after 14 hours. The reaction was cooled to 25°C, then the catalyst removed by filtration, washing through with isopropanol (3.33 moles; 254.78 mL; 200.00 g). The combined filtrate and washings were heated to 47°C. Concentrated hydrochloric acid (767.83 mmoles; 65.00 mL; 76.70 g) was added over the course of 7 minutes, which resulted in a reaction mixture having a temperature of 52°C. The mixture was allowed to cool but no precipitate formed. The solution was concentrated in vacuo, at <50°C, to provide a pink solid. This solid was partitioned between toluene (3.78 moles; 400.00 mL; 348.48 g) and a solution of sodium hydroxide (2.00 moles; 80.00 g) in water (22.20 moles; 400.00 mL; 400.00 g). The organic phase was dried over magnesium sulfate, filtered and concentrated in vacuo to give a white solid, 75 g (94% yield of free base). This solid was dissolved in isopropanol (9.80 moles; 750.00 mL; 588.75 g) and the solution heated to 47°C. Concentrated hydrochloric acid (708.76 mmoles; 60.00 mL; 70.80 g) was added within one minute, which caused the temperature to rise to 57°C and a precipitate to form. The mixture was allowed to cool to 300C over the course of 1 hour, and was then cooled to 50C over the course of 20 minutes. After 45 minutes at this temperature, the product was collected by filtration. The filter cake was washed with isopropanol (2.61 moles; 200.00 mL; 157.00 g) and sucked dry on the filter to give 116 g of a white solid. This solid was further dried in vacuo overnight, at 400C, to provide the title compound (89.18 g, 85% for two steps (i.e. from 3-benzyl-9-oxa~3,7- diazabicyclo[3.3. l]nonane dihydrochloride)).
1H NMR (400 MHz, CD3OD + 4 drops Of D2O): δ 2.7-2.9 (m, 4H); 2.93 (t, 2H); 3.21 (d, 2H); 3.3-3.5 (m, 6H); 4.10 (br s, 2H); 7.20-7.36 (m, 5H).
Chloride = 23.4% w/w (di-HCl requires 23.9% w/w). Water = 0.16% w/w.
ALTERNATIVE 3 Isopropanol (3.33 moles; 254.78 rxiL; 200.00 g) was added to the solution of 3-benzyl-7-(2-phenethyI)-9-oxa-3,7-diazabicyclo[3.3. l]nonane in toluene (1.00 equiv; 164.18 mmoles; 151.26 g) generated in Preparation AB, Alternative 3 above and the combined solution was then added to a hydrogenation vessel. Solid citric acid (49.97 mmoles; 9.60 g) and water (222.03 mmoles; 4.00 mL; 4.00 g) were added to provide a solution having a temperature of 25°C. To this solution was added 5% palladium on charcoal (2.60 g; ca. 60% water wet; Johnson Matthey Type 440), and the resulting mixture was warmed to 40°C under a hydrogen atmosphere (3 bar) with stirring at 500 rpm. Hydrogen uptake was complete after 7 hours. The reaction was left to stand overnight (for convenience). The catalyst was removed by filtration using a glass microfibre filter paper on a 7 cm diameter Buchner funnel and was washed with isopropanol (1.66 moles; 127.39 mL; 100.00 g). The combined filtrate and washings were heated to 400C and concentrated hydrochloric acid (378.01 mmoles; 32.00 mL; 37.76 g) was added over the course of 12 minutes, such that the temperature of the reaction mixture was kept between 40 and 450C. After stirring for 5 minutes, a precipitate started to form (at this point the temperature of the mixture was 42°C). The mixture was allowed to cool to 280C over the course of 65 minutes, and was then cooled to 50C over the course of 15 minutes. The mixture was stirred at 5°C for 90 minutes before the product was collected by filtration on a 7 cm diameter Buchner funnel (this took 2 minutes). The filter cake was washed with cold (50C) isopropanol (1.31 moles; 100.00 mL; 78.50 g), which took 1 minute. The filter cake was sucked as dry as possible on the filter (10 minutes) to provide a white solid (52 g). This solid was dried in vacuo, at 4O0C for 3.5 hours, to provide the title compound as a white solid (42.45 g, 85% for two steps (i.e. from 3-benzyl-9- oxa-3,7-diazabicyclo[3.3.1]nonane dihydrochloride)).
1H NMR (400 MHz, CD3OD + 4 drops of D2O): δ 2.7-3.0 (m, 6H); 3.2-3.5 (m, 6H); 4.13 (br s, 2H); 7.20-7.36 (m, 5H).
Chloride = 24.1% w/w (di-HCl requires 23.9% w/w). Water = 3.8% w/w.
Preparation AD 3 -(2-Phenethyl)-9-oxa-3 ,7-diazabicvcloF3.3. llnonane
3-Benzyl-7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane (151.5 g; see Preparation AB, Alternative 4 above) was dissolved in methanol (0.7 L) and acetic acid (0.25 L). Pd(OH)2 on carbon (10 g) was added and the mixture was stirred under a hydrogen atmosphere (at 3.5 bar pressure) for 20 hours. The catalyst was removed by filtration and the filtrate was concentrated under reduced pressure. The residue was diluted with water and then basified until the pH was above 11. The resulting aqueous mixture was extracted with DCM- (6 x 100 mL) and the combined organic layers were dried (MgSO4), filtered and combined with ethyl acetate washes of the drying agent. The filtrate was concentrated under reduced pressure to provide the title compound in crude form (115.9 g). LC-MS: 233 (M+H)+
13C-NMR (CDCl3): δ 140, 128.9, 128.7, 126.8, 66.9, 60.2, 57.4, 48.4, 33.2. 1H-NMR (CDCl3): δ 7.35-7.31 (m, 2H), 7.27-7.19 (m, 3H), 3.82 (m, 2H), 3.34- 3.28 (m, 2H), 3.09-2.98 (m, 4H), 2.87-2.82 (m, 2H), 2.73-2.67 (m, 2H), 2.60-2.55 (m, 2H).
Preparation AE 3-(fluoromethoxy)benzaldehyde
3-Hydroxybenzaldehyde (202 mg, 1.65 mmol) and acetonitrile (3 ml) were mixed in a 5 ml vial designed for microwave reactions (Biotage™) equipped with a magnetic stirring bar. Bromofluoromethane was bubbled through the mixture at -78 0C until the volume of the mixture had increased by 0.5 ml.
Potassium carbonate (343 mg, 2.48 mmol) was added and the vial was capped with a cap, containing a septum, belonging to the microwave vial. The vial was allowed to reach room temperature and the reaction mixture was stirred at room temperature over night, filtered, and concentrated under reduced pressure. The residue was dissolved in ether (5 ml), washed with 2
M NaOH (2 x 5 ml) and water, dried (Na2SO4), and filtered. Removal of the solvent gave 227 mg (89 %) of a colourless oil.
1H-NMR (500 MHz, CDCl3): δ 9.99 (s, 1 H), 7.62 (m, 1 H), 7.58 (m, 1 H), 7.51 (m, 1 H), 7.34 (m, 1 H), 5.76 (d, 2 H).
Preparation AF
3,5-bis(fluoromethoxy)benzaldehyde
Bromofluoromethane (3 ml) was condensed down into a measuring cylinder cooled with dry ice. The liquid bromofluoromethane was added to a mixture of 3,5-dihydroxybenzaldehyde (1.00 g, 7.24 mrnol), potassium carbonate (3.00 g, 21.7 mmol) and acetonitrile (15 ml) in a 20 ml vial designed for microwave reactions (Biotage™) equipped with a magnetic stirring bar. The vial was capped with a cap, containing a septum, belonging to the microwave vial. The vial was heated using an oil bath so that the reaction mixture was stirred at 70 0C over night. The reaction mixture was filtered and evaporated. The residue was dissolved in diethyl ether (50 ml), washed with 2 M NaOH (2 x 20 ml) and water, dried over MgSO4, and filtered. Evaporation of the solvent gave 1.21 g (83 %) of an oil, which crystallised after evaporation to dryness from a dichloromethane solution. 1H-NMR (500 MHz, CDCl3): δ 9.95 (s, 1 H), 7.34 (m, 2 H), 7.05 (m, 1 H), 5.76 (d, 4 H).
Examples
Examplel
4-Cvano-iV-{2-r7-(3.5-dimethoxybenzylV9-oxa-3J-diazabicvclor3.3.nnon-3-vn- ethyl )-N-methylbenzamide
4-Cyano-iV-methyl-N-[2-(9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)ethyl]benzamide
(0.81 g, 2.57 mmol; see Preparation F above) and 3,5-dimethoxybenzaldehyde (0.86 g, 5.19 mol) were mixed in dry tetrahydrofuran (80 mL). Triethylamine
(0.72 mL, 5.14 mmol) and sodium triacetoxyborohydride (0.71 g, 3.36 mmol)
were added and the reaction mixture was stirred at 600C under a nitrogen atmosphere for 46 h. The solids were filtered off and washed with acetonitrile, after which the solvent was evaporated from the filtrate. A saturated solution of NaHCO3 (55 mL) was added to the crude product and was extracted three times with DCM (120 mL). The combined organic phases were dried (Na2SO4), filtered and concentrated in vacuo. The product was purified by chromatography on silica gel (Horizon™, flash system from Biotage™; column: Flash 40+M, 40 x 150 mm), using methanol saturated with ammonia in DCM as eluent, to yield 0.187 g (16%) of the title compound. 13C-NMR (500 MHz, CDCl3): δ 170.12, 169.64, 161.04, 141.25, 140.97, 132.56, 132.48, 128.05, 127.76, 118.48, 113.4, 107.16, 98.67, 98.46, 68.73, 68.5, 63.54, 58.00, 56.95, 56.64, 56.49, 55.52, 48.69, 44.15, 38.06, 33.74.
Example 2 iV-(4-Cvano-2-fluorobenzyl)-2- ( 7-F2-(2-fluorophenyl)ethyll-9-oxa-3 ,7-diaza- bicyclor3.3. nnon-3-yll acetamide
AT-(4-Cyano-2-fluorobenzyl)-2-(9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)acetamide dihydrochloride (2.14 g, 5.47 mmol; see Preparation D above), 2-(2- fluorophenyl)ethyl methanesulfonate (1.52 g, 6.94 mmol; see Preparation L above) and K2CO3 (2.95 g, 21.4 mmol) were mixed in acetonitrile (65 mL) and water (2 mL). The reaction mixture was stirred at 600C for 72 h, after which the solids were filtered off and washed with acetonitrile. The solvent was evaporated from the combined filtrates and the crude product was purified by chromatography on silica gel (SPl flash system from Biotage™; column: Flash 40+M, 40 x 150 mm), using methanol saturated with ammonia in DCM as eluent. This yielded 1.07 g (44%) of the title compound.
13C-NMR (500 MHz, CDCl3): δ 172.87, 162.24, 161.31, 160.3, 159.32, 132.54, 132.42, 131.06, 131.02, 130.67, 130.63, 128.47,128.41, 126.55, 126.42, 124.45, 124.42, 119.27, 119.07, 117.7, 115.72, 115.54, 112.72, 112.65,68.69, 60.32, 60.29, 56.96, 55.88, 31.11, 26.53.
Example 3
N-^-CvanobenzvD-a-ry-α-phenylethvD-g-oxa-S.y-diazabicvclorS.a.linon-S- yllacetamide
ALTERNATIVE I
N-(4-Cyanobenzyl)-2-(9-oxa-3,7~diazabicyclo[33.1]non-3-yl)acetamide dihydro- chloride (6.08 g, 16.3 mmol; see Preparation A above), 2-phenylethyl bromide (2.55 mL, 18.7 mmol) and K2CO3 (7.89 g, 57.1 mmol) were mixed in acetonitrile (150 mL) and water (4 mL). The reaction mixture was stirred at 6O0C for 66 h, after which the solids were filtered off and washed with acetonitrile. The solvent was evaporated from the combined filtrates before toluene (100 mL) and a 10% solution of citric acid (75 mL) were added to the resulting crude product. The resulting mixture was stirred for 1.5 h before being transferred to a separating funnel. The phases were separated and the upper (organic) phase was discarded. The aqueous phase was basified with 2 M sodium hydroxide (70 mL), extracted twice with ethyl acetate (150 mL) and then stirred together with ethyl acetate at room temperature for 30 minutes. This was transferred to a separating funnel and the lower (aqueous) phase was then removed and discarded. The organic phase was dried (MgSO4), filtered and concentrated in vacuo to give the title compound as a yellow-brown oil (which later solidified to give a yellow solid). The crude product was then purified by recrystallisation according to the following procedure.
Isopropanol (53 mL) was added to 5.83 g of the crude product and the resulting mixture was heated to 70°C by using a water bath. At 700C, all material had dissolved. The resulting solution was allowed to cool to room temperature, at which point crystallisation was noted to have occurred. The mixture was left at room temperature over a weekend (approximately 2.5 days). The mixture was filtered and the solid was then washed with cold (70C) isopropanol. The damp solid was then dried (using a rotary evaporator) in vacuo to provide the title compound as a pale yellow solid. Yield: (5.02 g, 76.2%)
13C-NMR (500 MHz, CDCl3): δ 174.04, 144.88, 139.91, 132.44, 128.32, 127-83, 126.37, 118.37,.110.94, 68.9, 61.63, 59.79, 56.66, 55.38, 42.01, 32.49.
ALTERNATIVE II 3-(2-Phenylethyl)-9-oxa-3,7-diazabicyclo[3.3.1]nonane (115.9 g of crude material; see Preparation AD above) was suspended in acetonitrile (0.7 L) and 2-chloro-iV-(4-cyanobenzyl)acetamide (102.8 g of crude material; see Preparation A(i), Alternative 4 above), suspended in acetonitrile (0.5 L), was added. Water (0.1 L) and K2CO3 (68 g) were added and the resulting mixture was stirred overnight at room temperature. Solid K2CO3 was removed by filtration and washed with acetonitrile. The filtrate was concentrated under reduced pressure and the residue was diluted with ethyl acetate (0.7 L). The mixture in ethyl acetate was extracted with aqueous citric acid solution (10%, 1.2 L), and the organic (ethyl acetate) layer was separated and discarded. The aqueous (citric acid) layer was then washed with ethyl acetate (2 x 200 mL) before being basified with 50% NaOH (until the pH of the mixture was above 12) and extracted with ethyl acetate (1 L). The organic layer was stored at 4 degrees for one week and the resulting precipitate (title compound, 85.2 g, 45% yield) was collected by filtration. LC-MS: 405 (M+H)
1H-NMR (MeOH-d4): δ 7.64 (d, 2H), 7.41 (d, 2H), 7.25 (t, 2H), 7.20-7.16 (m, IH), 7.00-6.95 (d, 2H), 4.54 (s, 2H), 3.89-3.84 (2H), 3.12 (s, 2H), 3.02-2.87 (m, 6H), 2.61-2.54 (m, 2H), 2.52-2.45 (m, 2H), 2.29-2.21 (m, 2H). 13C-NMR (MeOH-d4): δ 174.0, 144.9, 139.9, 132.4, 128.3, 127.8, 126.0, 118.4, 110.9, 68.9, 61.6, 59.8, 56.7, 55.4, 42.0, 32.5.
As a means for purification the title compound can be crystallized or slurried in solvents selected from acetonitrile, ethanol, acetone, water, isopropanol, THF, 2- butanone, methyl isobutyl ketone, methanol, n-heptane, iso-octane, 1-butanol and ethyl acetate; or a mixture thereof.
ALTERNATIVE III
2-chloro-N-(4-cyanobenzyl)acetamide (20.97 g, 98.49 mmol) was added to 3-phenethyl-9-oxa-3,7-diazabicyclo[3.3.1]nonane (30.00 g, 98.28 mmol), propan-2-ol (150 mL), and 1 M sodium carbonate solution (160 mL) and thereafter heated at reflux (78 0C) for 3 hours. The temperature was adjusted to 45 0C and solvent (180 mL) removed by distillation under reduced pressure, maintaining the temperature in the range 45-50 °C. Toluene (210 mL) and 1 M sodium hydroxide solution (90 mL) were added and the resulting biphasic mixture heated to 60 0C. The phases were separated and to the retained organic phase was added 10 % w/v citric acid (150 mL). Stirred for 15 minutes at room temperature. The phases were separated and to the retained aqueous phase was added butan-1-ol (240 mL) and 2 M sodium hydroxide solution (120 mL). Heated to 60 °C and stirred for 15 minutes. The phases were separated and the retained organic phase washed with 20 % w/w sodium chloride solution (60 mL) at 60 °C. Solvent (60 mL) was removed by distillation under reduced pressure, maintaining the temperature at approximately 60 0C. The reaction mixture was filtered whilst hot to remove any insoluble material, and washed through with butan-1-ol (30 mL). Cooled to room temperature, causing the product to crystallise from solution. Cooled to 5 °C. The product was collected by filtration and washed with butan-1-ol (90 mL). The filter cake was sucked as dry as possible, and then oven dried in vacuo (50 °C, 18 h) to give the sub-title compound as a white crystalline solid (31.46 g, 77.77 mmol, 79 %). mp = 129.7 0C 1H NMR (300 MHz, DMSO-d6) δ 9.48 (t, J = 6.2 Hz, IH), 7.76 (dt, J = 8.2, 1.7 Hz, 2H), 7.43 (d, / = 8.4 Hz, 2H), 7.26 - 7.12 (m, 3H), 6.91 (d, / = 6.6 Hz, 2H), 4.50 (d, J = 6.3 Hz, 2H), 3.82 (s, 2H), 3.02 (s, 2H), 2.91 (d, J = 11.5 Hz, 2H), 2.82 (s, 3H), 2.52 - 2.41 (m, 3H), 2.33 (dd, J = 11.2, 3.5 Hz, 2H), 2.17 - 2.08 (m, 2H).
13C NMR (100 MHz, DMSO-d6) δ 171.49 (amide C=O), 145.44 (C6H4 ipso-C), 139.62 (C6H5 ipso-Q, 132.34 (C6H4 C-H), 128.25 (C6H5 σ/m-C-H), 128.19 (C6H5 o/m-C-B), 127.51 (C6H4 C-H), 125.91 (C6H5 p-C-H), 118.73 (nitrile CN), 109.64 (C6H4 ipso-C(CN)), 67.65 (oxabispidine CH-O), 61.29 (CH2), 59.52 (CH2), 56.48 (oxabispidine CH2-N), 55.18 (oxabispidine CH2- N), 41.49 (C6H4-CH2-NH-), 32.27 (C6H5-CH2-)
Example 4 N-(4-CvanobenzylV2-|7-r3-('2,4-difluorophenoxy)propyn-9-oxa-3,7-diaza- bicycloP .3. linon-3 - yl I acetamide
N-(4-Cyanobenzyl)-2-(9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl) acetamide dihydro- chloride (3.72 g, 10 mmol; see Preparation A above), l-(3-bromopropoxy)-2,4- difluorobenzene (3.01 g, 12 mmol; see Preparation V(a) above) and K2CO3 (5.18 g, 37.5 mmol) were mixed in acetonitrile (50 mL) and water (1.3 mL). The reaction mixture was stirred at 90°C for 3 h, after which the solids were filtered off and the solvent was evaporated from the filtrate. The resulting crude product was purified by chromatography on silica gel (Horizon™ flash system from Biotage™; column: Flash 40+M, 40 x 150 mm), using methanol saturated with ammonia in DCM as eluent. This yielded 2.57 g (54%) of the title compound. 13C-NMR (500 MHz, CDCl3): δ 172.58, 157.78, 157.7, 155.85, 155.77, 153.66, 153.57, 151.68, 151.59, 145.2, 143.36, 143.34, 143.28, 143.25, 132.44, 128.15, 118.83, 115.85, 115.83, 115.77, 115. 75, 111.11, 110.75, 110.72, 110.57, 110.54, 105.34, 105.17, 105.13, 104.95, 68.49, 68.34, 60.08, 56.94, 56.88, 55.64, 42.5, 27.03
Example 5
N-(4-cvanobenzylV2- 17-r2-(3-methoxyphenyl)ethyl1-9-oxa-3.7- diazabicyclor3.3. llnon-3-yl } acetamide
Potassium carbonate (1.41 g, 10.2 mmol) was added to a mixture of the dihydrochloride salt of N-(4-cyanobenzyl)-2-(9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl)acetamide (1.00 g, 2.68 mmol; see Preparation A above) and 2-(3- methoxyphenyl)ethyl methanesulfonate (0.617 g, 2.68 mmol; see Preparation M
above) in acetonitrile (20 mL) and water (0.5 mL). The reaction mixture was heated to reflux for seven hours, after which it was stirred at room temperature overnight and then filtered. Solvent was and evaporated from the filtrate and the resulting crude product was purified by preparative HPLC on a Gilson preparative HPLC system equipped with a Kromasil column (C8, 10 μm, 50.8 x 250 mm), using a MeCN/NHUOAc eluent system. The product fractions were pooled and the acetonitrile was removed on a rotary evaporator. The aqueous phase was extracted with DCM and the combined organic phase was dried (MgSO4) and evaporated, giving 0.41 g (35%) of the title compound as a colourless oil, which solidified on standing.
1H-NMR (500 MHz, CDCl3): δ 9.51 (m, 1 H), 7.53 (d, 2 H), 7.27 (d, 2 H), 7.18 (t, 1 H), 6.74 m (1 H), 6.53 (m, 1 H), 6,49 (m, 1 H), 4.48 (m, 2 H), 3.86 (m, 2 H), 3.77 (s, 3 H), 3.10 (s, 2 H), 2.97 (m, 2 H), 2.80 (m, 4 H), 2.53 (m, 4 H), 2.25 (m, 2 H).
Example 6
4-Cvano-iy-(2- 17-r3-(difluoromethoxy)benzyl'l-9-oxa-3 J-diazabicvcloβ .3. Hnon-
3-yl }ethyl)-N-methylbenzamide
Potassium carbonate (0.67 g, 4.8 mmol) was added to a mixture of 4-cyano-N- methyl-N-[2-(9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]benzamide (0.61 g, 1.9 mmol; see Preparation F above) and l-(bromomethyl)-3-(difluoromethoxy)- benzene (0.46, 1.9 mmol) in acetonitrile (35 mL). The reaction mixture was stirred at 6O0C under nitrogen for 24 hours, before being filtered. Solvent was evaporated from the filtrate and the resulting crude product was purified by flash chromatography on silica gel (Horizon™ flash system from Biotage™; column: Si 25 + M, 2.5 x 15 cm), using methanol saturated with ammonia in DCM as eluent. This gave 0.48 g (53%) of the title compound.
1H-NMR (500 MHz, CD3OD): 7.79 and 7.73 (rotamers, d, 2H), 7.59 and 7.56 (rotamers, d, 2H), 7.32-7.16 (m, 3 H), 6.98 (m, 1 H), 6.80 and 6.76 (rotamers, t, 1 H), 3.90-3.38 (m, 6 H), 3.19 and 3.04 (rotamers, s, 3 H), 3.03-2.69 (m, 4 H), 2.63- 2.35 (m, 6 H).
Example 7
N-(247-r3,5-bis(fluoromethoxy)beiizyl1-9-oxa-3,7-diazabicvclor3.3.11non- 3-yl}ethyl)-4-cvano-N-methylbenzamide, tartrate salt Sodium triacetoxyborohydride (1.98 g, 9.34 mmol) was added to a mixture of 4-cyano-N-methyl-N-[2-(9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]- benzamide (see Preparation F above)(2.26 g, 7.19 mmol), 3,5-bis- (fluoromethoxy)benzaldehyde (2.13 g, 10.5 mmol) (see Prepareation AE above), and triethylamine (2.0 ml, 14 mmol) in THF (120 ml). The reaction mixture was stirred at room temperature over night, filtered, and evaporated. The residue was dissolved in ethyl acetate (100 ml) and washed with saturated NaHCO3 (2 X 50 ml). The organic phase was extracted with 10 % citric acid (3 X 40 ml); some toluene was added in order to get better phase separation. The citric acid phase was washed with ethyl acetate (2 X 30 ml) and was then basified with 50 % NaOH to pH 12. The aqueous phase was then extracted with ethyl acetate (3 X 50 ml). The combined organic layers were dried over MgSO4, filtered, and evaporated. The crude product was purified by flash chromatography on silica gel (SP4™ flash system from Biotage™, column: Si 40 + M, 4.0 x 15 cm) using methanol saturated with ammonia in dichloromethane as eluent giving 1.29 g (36 %) of a sticky oil. The oil was dissolved in methanol (50 ml), tartaric acid (387 mg, 2.56 mmol) was added, and the resulting solution was stirred for 30 minutes at room temperature. Removal of the solvent and drying in vacuo gave 1.68 g of the title compound as a white powder. 1H-NMR (500 MHz, CD3OD): 7.83 (dm, 2 H), 7.66 (dm, 2 H), 6.96 (m, 2 H), 6.84 (m, 1 H), 5.75 (d, 4 H), 4.38 (s, 2 H), 4.16 (m, 2 H), 3.93 (s, 2 H), 3.83 (t, 2 H), 3.56 (d, 2 H), 3.33 (d, 2 H), 3.16 (m, 2 H), 3.10 (t, 2 H), 3.05 (s, 3 H), 3.01 (m, 2 H).
Example 8
The following compounds were prepared, from appropriate intermediates (such as those described hereinbefore), according to or by analogy with methods described herein:
(i) N-(4-cyanobenzyl)-N-methyl-2-{7-[2-(2-methylphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(ii) 2-{7-[2-(2-chlorophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-(4- cyanobenzyl)-N-methylacetamide;
(iii) N-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} -iV-methylacetamide; (iv) N-(4-cyanobenzyl)-Λ/r-methyl-2-(7-{2-[2-(trifluoromethyl)phenyl]ethyl}-9- oxa-3,7-diazabicyclo[3.3. l]non-3-yl)acetamide;
(v) 2-(7-{ 2-[3 ,4-bis(difluoromethoxy)phenyl]ethyl } -9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl)-N-(4-cyanobenzyl)-N-methylacetamide;
(vi) N-(4-cyanobenzyl)-N-methyl-2-(7-{2-[4-(trimethylsilyl)phenyl]ethyl}-9-oxa- 3 ,7-diazabicyclo[3.3. l]npn-3-yl)acetamide;
(vii) N-(4-cyanobenzyl)-2-(7- { 2-[4-(trimethylsilyl)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(viii) iV-(4-cy anobenzyl)-2- { 7- [2-(3 ,4-difluorophenyl)ethyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } -iV-methylacetamide; (ix) N-(4-cyanobenzyl)-N-methyl-2-(7-{2-[4-(trifluorometibιyl)phenyl]ethyl}-9- oxa-3,7-diazabicyclo[3.3.1]non-3-yl)acetamide;
(x) N-benzyl-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl} acetamide;
(xi) N-(4-cyanobenzyl)-2-{7-[2-(3-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}acetamide;
(xii) N-(4-cyanobenzyl)-2-{7-[2-(2,4-difluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(xiii) N-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide; (xiv) 2- {7-[2-(l ,2-benzisoxazol-3-yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl } -iV-(4-cyanobenzyl) acetamide
(xv) N-(4-cyanobenzyl)-2-(7-{2-[4-(difluoromethoxy)ρhenyl]ethyl}-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl)acetamide;
(xvi) iV-(4-cyano-2-fluorobenzyl)-2- { 7- [2-(4-cyano-3 -fluorophenyl)ethyl] -9-oxa-
3,7-diazabicyclo[3.3.1]non-3-yl}acetamide; (xvii) N-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-cyano-2-fluorophenyl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3 -yl } acetamide;
(xviii) iV-(4-cyano~2-fluorobenzyl)-2- { 7-[2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(xix) N-(4-cyanobenzyl)-2-{7-[2-(4-cyano-3-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xx) iV-(4-cyanobenzyl)-2-{7-[2-(4-cyano-2-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xxi) N-(4-cyanobenzyl)-2-(7- { 2-[2-(difluoromethoxy)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide; (xxii) N-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9- oxa-3,7-diazabicyclo[3.3.1]non-3-yl}acetamide;
(xxiii) N-(4-cyano-2-fluorobenzyl)-2- {7- [3 -(4-cyanophenoxy)propyl] -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxiv) N-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-fluoropb.enoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3 -yl } acetamide;
(xxv) N-(4-cyano-2-fluorobenzyl)-2- { 7-[2-(4-fluorophenyl)ethyl]-9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxvi) iV-(4-cyano-2-fluorobenzyl)-2- [7-(2-phenylethyl)-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl] acetamide; (xxvii) JV-(tert-butyl)-2- { 7-[4-(4-cyanophenyl)butyl]-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide;
(xxviii) N-(tert-butyl)-2-{7-[3-(4-cyano-3-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl } acetamide;
(xxix) N-(tert-butyϊ)-2- { 7-[2-(4-cyano-2-fluorophenoxy)ethyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(xxx) N-(4-cyano-2,6-difluorobenzyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxxi) N-(4-cyano-2,6-difluorobenzyl)-2- { 7-[3-(4-fluorophenoxy)propyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxxii) N-(4-cyano-2,6-difluorobenzyl)-2- { 7-[2-(2-fluorophenyl)ethyl] -9-oxa-3 ,7- diazabicyclo [3.3.1 ]non-3 -yl } acetamide; (xxxiii) N-(4-cyano-2,6-difluorobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3 ,7-diaza- bicyclo [3.3.1 ]non-3 -yl] acetamide ;
(xxxiv) N-(4-cyanobenzyl)-2-{7-[3-(3-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(xxxv) N-(4-cyanobenzyl)-2-{7-[3-(2-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(xxxvi) N-(4-cyanobenzyl)-2-(7-{2-[2-(trifluoromethyl)phenyl]ethyl}-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(xxxvii) N-(4-cyanobenzyl)-2-{7-[2-(2-methylphenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3 -yl } acetamide; (xxxviii) 2-{7-[2-(2-chlorophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV-(4-cyanobenzyl) acetamide;
(xxxix) 2-{7-[2-(3-chlorophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-cyanobenzyl)acetamide;
(xl) N-(4-cyanobenzyl)-2- { 7-[2-(2-methoxyphenyl)ethyl]-9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl}acetamide;
(xli) N-(4-cyanobenzyl)-2- { 7-[2-(4-methoxyphenyl)ethyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xlii) iV-(4-cyanobenzyl)-2- { 7-[3-(4-cyano-2,6-difluoropnenoxy)propyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide; (xliii) N-(4-cyanobenzyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xliv) N-(4-cyanobenzyl)-2-{7-[3-(4-cyano-3-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3 -yl } acetamide;
(xlv) N-(4-cyanobenzyl)-2-{7-[3-(2-methoxyphenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xlvi) N-(4-cyanobenzyl)-2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(xlvii) JV-(4-cyanobenzyl)-2- { 7-[2-(6-methylpyridin-2-yl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xlviii) N-(4-cyanobenzyl)-2-[7-(2-pyridin-2-ylethyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl]acetaniide; (xlix) iV-(4-cyanobenzyl)-2-[7-(2-pyridin-4-ylethyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl]acetamide;
(l) 2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-(2- phenylethyl)acetamide;
(li) 2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-iV-[2- (4-fluorophenyl)ethyl] acetamide;
(lii) N-(4-cyanobenzyl)-2-{7-[2-(3,5-dimethylisoxazol-4-yl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(liii) iV-(4-cyanobenzyl)-2-{7-[2-(lH-indol-3-yl)ethyl]-9-oxa-3,7-diazabicyclo- .
[3.3. l]non-3-yl } acetamide; (liv) N-(4-cyanobenzyl)-2-[7-(imidazo[l,2-a]pyridin-2-ylmethyl)-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl]acetamide;
(lv) N-(4-cyanobenzyl)-2-{7-[2-(3,5-dimethyl-lH-pyrazol-l-yl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(lvi) N-(4-cyanobenzyl)-2-{7-[2-(3,4-dimethoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lvii) N-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lviii) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-fluorobenzyl)acetamide; (lix) N-benzyl-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide;
(lx) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}.-N-
[2-(4-fluorophenyl)ethyl]acetamide;
(lxi) 2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl }-N- (4-fluorobenzyl)acetamide;
(lxii) 2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3. l]non-3-yl }-
N-[2-(4-fluorophenyl)ethyl]acetamide;
(lxiii) N-(4-cyanobenzyl)-2-{7-[(3,5-dimethylisoxazol-4-yl)methyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(lxiv) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV-(2,4-difluorobenzyl)acetamide; (lxv) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(3-fluorobenzyl)acetamide;
(lxvi) N-(4-cyanobenzyl)-2-{7-[2-(2-fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lxvii) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV-(2-phenylethyl)acetamide;
(lxviii) 2- {7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } - iV-(2-phenylethyl)acetamide;
(lxix) N-(tert-butyϊ)-2- { 7-[3-(4-cyano-2,6-difluorophenoxy)propyl]-9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl } acetamide; (lxx) N-(tert-butyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxi) N-(4-cyanobenzyl)-2-{7-[3-(3,5-difluorophenoxy)propyl]-9::oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(lxxii) N-(4-cyanobenzyl)-2-{7-[3-(2,5-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxiii) N-(4-cyanobenzyl)-2-{7-[3-(2,6-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(lxxiv) N-(4-cyanobenzyl)-2-{7-[3-(2-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide; (lxxv) 2- { 7- [3 -(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -
N-(2-fluorobenzyl)acetamide;
(lxxvi) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(3-fluorobenzyl)acetamide;
(lxxyii) A''-(4-cyanobenzyl)-2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(lxxviii) N-(4-cyanobenzyl)-2-{7-[4-(trifluoromethyl)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxix) 2-[7-(4-chlorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]-N-(4-cyano- benzyl)acetamide;
(lxxx) N-(4-cyanobenzyl)-2-[7-(4-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide; (lxxxi) N-(4-cyanobenzyl)-2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(lxxxii) N-(4-cyanobenzyl)-2-{7-[2-(3,4-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1 Jnon~3-yl } acetamide;
(lxxxiii) N-benzyl-2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl}-N-methylacetamide;
(lxxxiv) N-benzyl-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }-N-methylacetamide;
(lxxxv) N-(4-cyanobenzyl)-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (lxxxvi) N-(4-cyanobenzyl)-2-(7- { 2-[4-(trifluoromeihyl)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(lxxxvii) 2-{7-[2-(4-chlorophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(4-cyanobenzyl)acetamide;
(lxxxviii) N-(4-cyanobenzyl)-2-{7-[2-(2,4-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(lxxxix) N-(4-cyanobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xc) N-(4-cyanobenzyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (xci) N-(4-cyanobenzyl)-2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } -iV-methylacetamide;
(xcii) iV-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenyl)etb.yl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }-N-methylacetamide;
(xciii) N-(4-cyanobenzyl)-N-methyl-2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl]acetamide;
(xciv) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xcv) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(xcvi) N-[2-(4-cyanophenoxy)ethyl]-2-[7-(3-phenoxypropyl)-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl] acetamide; (xcvii) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xcviii) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo [3.3. l]non-3 -yl } acetamide;
(xcix) N-[2-(4-cyanophenoxy)ethyl]-2-[7-(2-phenylethyl)-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl]acetamide;
(c) 2-[7-(4-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]-N-[2-(4-cyano- phenoxy)ethyl] acetamide ;
(ci) N-[2-(4-cyanophenoxy)ethyl]-2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl]acetamide; (cii) 2-(7-benzyl-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)-iV-[2-(4-cyanophenoxy)- ethyl]acetamide;
(ciii) N-(4-cyanobenzyl)-2-(7- { 3- [3 -(trifluoromethyl)phenoxy]propyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(civ) 2- { 7-[3-(3-chlorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } - JV-(4-cyanobenzyl) acetamide;
(cv) iV-(4-cyanobenzyl)-2-{7-[3-(3-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(cvi) 2-{7-[3-(4-chloro-2-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } -iV-(4-cyanobenzyl)acetamide; (cvii) 2-{7-[3-(4-chloro-3-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}-iV-(4-cyanobenzyl)acetamide;
(cviii) N-(4-cyanobenzyl)-2-{7-[3-(3,4-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(cvix) iV-(4-cyanobenzyl)-2~(7- { 3 -[4-(trifluoromethyl)phenoxy]propyl } -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl)acetamide;
(cx) 2-{7-[3-(4-chlorophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-cyanobenzyl)acetamide;
(cxi) N-(4-cyanobenzyl)-2-[7-(3-phenoxypropyl)-9-oxa-3,7-diazabicyclo[3.3.1]~ non-3 -yl] acetamide;
(cxii) N-(4-cyanobenzyl)-2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide; (cxiii) N-(4-cyanobenzyl)-2-(7- { 2-[(3-cyanobenzyl)oxy]ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl)acetamide;
(cxiv) 2-(7-{2-[(3-chlorobenzyl)oxy]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl)-N-(4-cyanobenzyl)acetamide;
(cxv) iV-(4-cyanobenzyl)-2-[7-(2- { [3-(trifluoromethyl)benzyl] oxy } ethyl)-9-oxa- 3 ,7-diazabicyclo[3.3.1 ]non-3-yl] acetamide;
(cxvi) iV-(4-cyanobenzyl)r2-(7-{2-[(3-fluorobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxvii) 2-(7-{2-[(4-chlorobenzyl)oxy]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl)-N-(4-cyanobenzyl)acetamide; (cxviii) 2V-(4-cyanobenzyl)-2-[7-(2-{ [4-(trifluoromethyl)benzyl]oxy } ethyl)-9-oxa-
3 ,7-diazabicyclo[3.3.1 ]non-3-yl] acetamide;
(cxix) iV-(4-cyanobenzyl)-2-(7- { 2-[(4-fluorobenzyl)oxy]ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxx) 2-{7-[2-(benzyloxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-(4- cyanobenzyl)acetamide;
(cxxi) iV-(4-cyanobenzyl)-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxxii) 2-{7-[2-(l-benzofuran-5-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(4-cyanobenzyl) acetamide ; (cxxiii) Ν-benzyl-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxxiv) iV-(tert-butyl)-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]nόn-3-yl}-N-methylacetamide;
(cxxv) N-(?ert-butyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo- [3.3. l]non-3-yl } -JV-methylacetamide;
(cxxvi) N-(te?t-butyl)-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)-N-methylacetamide;
(cxxvii) N-(tert-butyl)-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)acetamide;
(cxxviii) 2-{7-[2-(benzylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } -N-(4-cyanobenzyl)acetamide; (cxxix) N-(4-cyanobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(cxxx) Λ/'-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cxxxi) N-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cxxxii) N-(4-cyanobenzyl)-2-{7-[2-(2,3-dihydro-l-benzofuxan-5-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(cxxxiii) iV-(4-cyanobenzyl)-2-[7-(2-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3 -yl] acetamide; (cxxxiv) 2,2'-(9-oxa-3 ,7-diazabicyclo[3.3.1 ]nonane-3 ,7-diyl)bis [iV-(4-cyano- benzyl)acetamide] ;
(cxxxv) 2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3. l]non-3-yl)-iV-(4-cyanobenzyl)- acetamide;
(cxxxvi) tert-butyl [2-(7- { 2-[(4-cyanobenzyl)amino] -2-oxoethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl)ethyl]carbamate;
(cxxxvii) 2-{7-[2-(fert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3 -yl } -N-(4-cyanobenzyl)acetamide;
(cxxxviii) tert-butyl 7-{2-[(4-cyanobenzyl)amino]-2-oxoethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]nonane-3-carboxylate; (cxxxix) N-(2-{7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)-4-cyanobenzamide;
(cxl) Λ/-(tert-butyl)-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl} acetamide;
(cxli) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-iV- (1 -methyl- 1 -phenylethyl)acetamide;
(cxlii) N-benzyl-2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3 -yl } acetamide;
(cxliii) N-(tert-butyl)-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide;
(cxliv) N-(2-{7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-3-(4-cyanophenyl)-iV-methylpropanamide; (cxlv) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(l-methyl-l-phenylethyl)acetamide;
(cxlvi) iV-benzyl-2- { 7-[3 ~(4-cyanophenoxy)propyl] -9-oxa-3 ,7-diazabicyclo [3.3.1]- non-3-yl } acetamide;
(cxlvii) 2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}-N-(l-methyl-l-phenylethyl)acetamide;
(cxlviii) iV-benzyl-2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl }acetamide;
(cxlix) iV-(tert-butyl)-2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide; (cl) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
JV,iV-diisopropylacetamide;
(cli) 2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }-N,iV-diisopropylacetamide;
(clii) iV-(fert-butyl)-2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl}acetamide;
(cliii) 3-[(7-{2-[(4-cyanobenzoyl)(methyl)amino]ethyl}-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl)methyl]phenyl 4-methylbenzenesulfonate;
(cliv) 4-cyano-iV-(2- { 7-[3-(difluoromethoxy)benzyl]-9-oxa-3 ,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide; (civ) 4-cyano-ΛT- { 2-[7-(3 ,5-diethoxybenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-N-methylbenzamide;
(clvi) 4-cyano-N-methyl-N-(2-{7-[3-(trifluoromethoxy)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)benzamide;
(clvii) 4-cyano-N- { 2-[7-(3-isopropoxybenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non- 3-yl]ethyl}-N-methylbenzamide;
(clviii) 4-cyano-N-{2-[7-(3-ethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl}-N-methylbenzamide;
(clix) _V-(2-{ 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl } ethyl)-3 ,4-difluorobenzamide;
(clx) ΛT-(2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- ethyl)-3 ,4-difluorobenzamide; (clxi) 4-cyano-N-{2-[7-(2,6-dimeihoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3 -yl] ethyl } -N-methylbenz amide;
(clxii) 4-cyano-N- { 2-[7-(2,3-dimethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-
3-yl]ethyl } -JV-methylbenzamide;
(clxiii) 4-cyano-N- { 2-[7-(2-methoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-N-methylbenzamide;
(clxiv) 4-cyano-iV- { 2-[7-(3-methoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yl] ethyl } -iV-methylbenzamide;
(clxv) 4-cyano-N-(2-{7-[2-(2,6-difluorophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide; (clxvi) 4-cyano-iV-(2- { 7-[2-(3 ,5-dimethylisoxazol-4-yl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide;
(clxvii) 4-cyano-iV-(2-{7-[(3,5-dimethylisoxazol-4-yl)methyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}ethyl)benzamide;
(clxviii) 4-cyario-N-(2- { 7- [2-(3 ,4-difluorophenyl)ethyl] -9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(clxix) 4-cyano-N-(2-{7-[2-(2,4-difluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(clxx) JV-(2-{ 7-[3-(4-chlorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl } ethyl)-4-cyanobenzamide; (clxxi) 4-cyano-N-(2-{7-[3-(2,6-difluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(clxxii) 4-cyano-N-(2-{7-[3-(2,4-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide;
(clxxiii) 4-[(7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl)methyl]phenyl methanesulfonate;
(clxxiv) 4-cyano-N-(2-{7-[(6-fluoro-4H-l,3-benzodioxin-8-yl)methyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}ethyl)benzamide;
(clxxv) 4-cyano-N-.(2-{7-[2-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)-N-methylbenzamide;
(clxxvi) 4-cyano-N-(2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)-N-methylbenzamide; (clxxvii) A-[I-(J- { 2-[(4-cyanobenzoyl)amino]ethyl } -9-oxa-3 ,7-diazabicyclo-
[3.3.1]non-3-yl)ethyl]phenyl methanesulfonate;
(clxxviii) 4-cyano-N-(2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(clxxix) 4-cyano-N-(2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(clxxx) N-(2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } ethyl)benzamide;
(clxxxi) 4-cyano-iV-(2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide; (clxxxii) 4-cyano-N-(2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide;
(clxxxiii) 4-cyano-N-(2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(clxxxix) N-(3-{ 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non- 3-yl}propyl)benzamide;
(cxc) 4-cyano-N- { 2-[7-(2,6-difluorobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}benzamide;
(cxci) 4-cyano-iV-{2-[7-(2,6-dimethylbenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl }benzamide; (cxcii) N-{2-[7-(l,3-benzodioxol-4-ylmethyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-4-cyanobenzamide;
(cxciii) 4-cyano-iV-methyl-N-{2-[7-(3-plienylpropyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl]ethyl }benzamide;
(cxciv) 4-cyano-N-methyl-iV-{2-[7-(2-phenyletliyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]ethyl}benzamide;
(cxcv) N-[2-(7-benzyl-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)ethyl]-4-cyano-iV- methylbenzamide;
(cxcvi) 4-cyano-iV-(2- { 7- [4-fluoro-2-(trifluoromethyl)benzyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide;
(cxcvii) 4-cyano-N-(2-{7-[2-(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide; (cxcviii) 4-cyano-N-{2-[7-(5-fluoro-2-methylbenzyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl]ethyl}benzamide;
(cxcix) N-(2-{7-[2,4-bis(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3 -yl } ethyl)-4-cyanobenzamide;
(cc) 4-cyano-N-(2-{7-[2-(difluorometiioxy)benzyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)benzamide;
(cci) 4-cyano-iV- { 2-[7-(3-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl }benzamide;
(ccii) 4-cyano-JV-(2- { 7-[3-(3-methoxyphenyl)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide; (cciii) 4-cyano-iV-{2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl } benzamide;
(cciv) 4-cyano-N-(2- { 7-[3-(4-cyanophenyl)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)benzamide;
(ccv) iV-{2-[7-(l ,3-benzodioxol-5-ylmethyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-4-cyanobenzamide;
(ccvi) 4-cyano-N-{2-[7-(l-naphthylmethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl] ethyl }benzamide;
(ccvii) 4-cyano-N- { 2-[7-(quinolin-8-ylmethyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-
3 -yl] ethyl } benzamide; (ccviii) 4-cyano-N-(2-{7-[3-(trifluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide;
(ccix) N- { 2-[7-(4-chlorobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3 -yl] ethyl } -A- cyanobenzamide;
(ccx) 4-cyano-N-(2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}ethyl)benzamide;
(ccxi) 4-cyano-N- { 2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yl]ethyl }benzamide;
(ccxii) 4-cyano-N-(2-{7-[4-(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide;
(ccxiii) 4-cyano-iV-{2-[7-(2-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl }benzamide; (ccxiv) 4-cyano-iV- {2-[7-(2,4-difluorobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl }benzamide;
(ccxv) 4-cyano-N-{2-[7-(4-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl }benzamide;-
(ccxvi) 4-(3 - { 7- [(4-oxo-3 ,4-dihydrophthalazin- 1 -yl)methyl] -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl}propoxy)benzonitrile;
(ccxvii) 4-cyano-iV- { 2-[7-(2-phenylethyl)-9-oxa~3 ,7-diazabicyclo[3.3. l]non-3-yl]- eihyl }benzamide;
(ccxviii) tert-butyl 2-(7- { 2- [(4-cyanobenzoyl)amino] ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1Jnon-3-yl)-2-methylpropanoate; (ccxix) tert-bntyl [2-(7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)ethyl]carbamate;
(ccxx) N-(2-{7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-4-cyanobenzamide;
(ccxxi) tert-butyl (J- { 2-[(4-cyanobenzoyl)amino]ethyl } -9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl)acetate;
(ccxxii) 4-cyano-N-{2-[7-(3,3-dimethyl-2-oxobutyl)-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl]ethyl}benzamide;
(ccxxiii) iV-[2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]-4-cyano- benzamide; (ccxxiv) 4-[((2S)-3-{7-[2-(l,3-dioxo-l,3-dihydro-2H-isoindol-2-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } -2-hydroxypropyl)oxy]benzonitrile;
(ccxxv) N-(tert-butyl)-3-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }propanamide;
(ccxxvi) 4-(2-{7-[2-(2,2-dimethyl-4-oxo-3,4-dihydro-l,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl} ethoxy)benzonitrile;
(ccxxvii) 4-(3-{7-[2-(2,2-dimethyl-4-oxo-3,4-dihydro-l,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}propoxy)benzonitrile;
(ccxxviii) 4-[((2S)-3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-
9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxypropyl)oxy]benzonitrile;
(ccxxix) 4-(3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl }propoxy)benzonitrile; (ccxxx) 4-[(3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } propyl)sulf onyl]benzonitrile; ς
(ccxxxi) 4-(2-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-9-oxa-
3,7-diazabicyclo[3.3.1]non-3-yl}ethoxy)benzonitrile;
(ccxxxii) iV-(2-{7-[2-(4-acetylphenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl}ethyl)-3-(4-cyanophenyl)propanamide;
(ccxxxiii) 3-(4-cyanophenyl)-N-(2-{7-[2-(4-methoxyphenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } ethyl)propanamide;
(ccxxxiv) 3-(4-cyanophenyl)-N-(2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } ethyl)propanamide; (ccxxxv) 3-(4-cyanophenyl)-N-(2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)propanamide;
(ccxxxvi) 3-(4-cyanophenyl)-N-(2-{7-[2-(2,6-dimethylphenoxy)ethyl]-9-oxa-3,7- diazabicyclo [3.3.1 ]non-3 -yl } ethyl)propanamide;
(ccxxxvii) N-{2-[7-(3-tert-butoxypropyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl}-3-(4-cyanophenyl)propanamide;
(ccxxxviii) 3-(4-cyanophenyl)-N-{2-[7-(2-phenyleihyl)-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl]ethyl}propanamide;
(ccxxxix) 4-[((2S)-3-{7-[2-(2,2-dimethyl-l-oxido-4-oxo-3,4-dihydro-l,5-benzo- thiazepin-5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxy- propyl)oxy]benzonitrile;
(ccxl) 4-[((2S)-3- { 7-[2-(2,2-dimethyl-4-oxo-3 ,4-dihydro-l ,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxypropyl)oxy]- benzonitrile;
(ccxli) N-(2- { 7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)-3-(4-cyanophenyl)-N-methylpropanamide;
(ccxlii) 4-[((2S)-3- { 7-[2-(2,2-dimethyl- 1 , 1 -dioxido-4-oxo-3 ,4-dihydro- 1 ,5-benzo- thiazepin-5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxy- propyl)oxy]benzonitrile;
(ccxliii) 3-(4-cyanophenyl)-N-(2-{7-[2-(4-methoxyphenyl)-2-oxoethyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}ethyl)-N-methylpropanamide;
(ccxliv) N-[2-(7-benzyl-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)ethyl]-3-(4-cyano- phenyl)-N-methylpropanamide;
(ccxlv) tert-butyl [2-(7-{2-[[3-(4-cyanophenyl)propanoyl](methyl)amino]ethyl}-9- oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)ethyl]carbamate; (ccxlvi) 3-(4-cyanophenyl)-N-{2-[7-(3,3-dimethyl-2-oxobutyl)-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl]ethyl}propanamide;
(ccxlvii) 4-[((2S)-3-{7-[2-(2,2-dimethyl-4-oxo-3,4-dihydro-l,5-benzotliiazepin-
5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxypropyl)oxy]- benzonitrile; (ccxlviii) tert-butyl {2-[7-(2-{[3-(4-cyanophenyl)propanoyl]amino}ethyl)-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl] ethyl } carbamate;
(ccxlix) N-(2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)-3,3-dimethylbutanamide; and
(ccl) N-(2- { 7-[(2S)-3 -(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3 ,7-diaza- bicyGlo[3.3.1]non-3-yl}ethyl)-2,2-dimethylpropanamide.
(ccli) 4-cyano-Ν-(2- j 7-F3-ffluoromethoxy)benzyl"|-9-oxa-3 ,7-diazabicvclor3.3.11- non-3-yl } ethyl)-N-methylbenzamide
Example 9 Title compounds of the above Examples were tested in Test A above and were found to exhibit D10 values greater than 5.5. Indeed the compounds of Examples 1, 3 and 8(xxix) were found to have D1O values of 6.5, 7.1 and 7.7, respectively.
Example 10 Title compounds of the above Examples were tested in Test B above and were found to exhibit pICso values of greater than 4.5. Indeed the compounds of Examples 1, 3 and 8(xxix) were found to have pICso values of 4.9, 5.37 and 4.77, respectively.
Abbreviations
Ac acetyl
AIBN 2,2'-azobis(2-methylpropionitrile)
API atmospheric pressure ionisation (in relation to MS) aq. aqueous br broad (in relation to NMR)
Bt benzotriazole t-BuOH tø/t-butanol
CI chemical ionisation (in relation to MS) mCPBA meta-chloroperoxybenzoic acid d doublet (in relation to NMR)
DBU diazabicyclo [5.4.0] undec-7-ene
DCM dichloromethane dd doublet of doublets (in relation to NMR)
DEPEA diisopropylethylamine
DMAP 4-dimethylaminopyridine
DMF N^V-dimethylformamide
DMSO dimethylsulfoxide
EDC 1 - [3 -(dimethylamino)propyl] -3 -ethylcarbodiimi
Et ethyl
EtOAc ethyl acetate eq. equivalents
ES electrospray (in relation to MS)
FAB fast atom bombardment (in relation to MS)
FBS foetal bovine serum h hour(s)
HCl hydrochloric acid
HEPES 4-(2-hydroxyethyl)- 1 -piperazineethanesulf onic acid
HOBT 1 -hydroxybenzotriazole
HPLC high performance liquid chromatography
IMS industrial methylated spirit (denatured ethanol)
IPA wo-propyl alcohol (propan-2-ol)
LAH lithium aluminium hydride
LDA lithium diisopropylamide
LiHMDS = lithium hexamethyldisilazide m = multiplet (in relation to NMR)
Me methyl
MeCN acetonitrile
MeOH methanol min. = minute(s) m.p. = melting point
MS mass spectroscopy
NADPH nicotinamide adenine dinucleotide phosphate, reduced form
NBS N-bromosuccinimide
OAc acetate
Pd/C palladium on carbon q quartet (in relation to NMR)
RT room temperature s = singlet (in relation to NMR) t = triplet (in relation to NMR)
TEA triethylamine
THF tetrahydrofuran tic thin layer chromatography
Prefixes n-, s-, i-, t- and tert- have their usual meanings: normal, secondary, iso, and tertiary.
Claims
1. A compound of formula I,
R1 represents C1-12 alkyl (which alkyl group is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl, Het1, -N(R5a)R6, -C(O)R5b, -OR5c, -C(O)-E-R7, -C(O)N(R8a)R5d, -OC(O)N(R8b)R5e, -S(O)2R9a, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d) or R1 represents -C(O)-E-R7, -C(O)N(R8a)R5d or -S(O)2R9a;
R5a represents H or C1-6 alkyl (which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d); R b to R e independently represent, at each occurrence when used herein, H, C1-6 alkyl (which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, Het2, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), aryl or Het3, or R5d or R5e, together with, respectively, R8a or R8b, may represent C3-6 alkylene (which alkylene group is optionally interrupted by an O atom and/or is optionally substituted by one or more C1-3 alkyl groups);
R6 represents H, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), aryl, -C(O)R10a, -C(O)OR10b or -C(O)N(H)R10c or -S(O)2R10d; R1Oa, R1Ob, R1Oc and R1Od independently represent C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl), aryl, or R1Oa represents H; R7 represents, at each occurrence when used herein, C1-12 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl, C1-6 alkoxy, Het4, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d);
R and R independently represent H, C1-12 alkyl, C1-6 alkoxy (which latter two groups are optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, C1-4 alkyl, C1-4 alkoxy, -S(O)2N(R9b)R9c and -N(R9b)S(O)2R9d), -D-aryl, -D-aryloxy, -D-Het5, -D-N(H)C(O)R1 la, -D-S(O)2R12a, -D-C(0)Rllb, -D-C(O)OR12b, -D-C(O)N(Rllc)Rlld, or R8a or R8b, together with, respectively, R5d or R5e, may represent C3-6 alkylene (which alkylene group is optionally interrupted by an O atom and/or is optionally substituted by one or more C1-3 alkyl groups); Rlla to Rlld independently represent H, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl), aryl, or Rllc and Rlld together represent C3-6 alkylene; R12a and R12b independently represent C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro and aryl) or aryl;
D represents, at each occurrence when used herein, a direct bond or C1-6 alkylene; E represents O or S;
R9a represents, at each occurrence when used herein, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl,
-S(O)2N(R9b)R9c and ~N(R9b)S(O)2R9d) or aryl;
R9b represents, at each occurrence when used herein, H or C1-6 alkyl;
R9c and R9d independently represent, at each occurrence when used herein, C1-6 alkyl (optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, aryl and Het ), aryl or Het7, or R9c represents H;
Het1 to Het7 independently represent five- to twelve-membered heterocyclic groups containing one or more heteroatoms selected from oxygen, nitrogen and/or sulfur, which groups are optionally substituted by one or more groups selected from -OH, oxo, halo, cyano, nitro, C1-6 alkyl, C1-6 alkoxy, aryl, aryloxy, -N(R13a)R13b, -C(O)R13c, -C(O)OR13d, -C(O)N(R13e)R13f, -N(R13g)C(O)R13h, -S(O)2N(R13i)R13j and -N(R13k)S(O)2R13m; R13a to R13m independently represent C1-6 alkyl, aryl or R13a to R13k independently represent H;
R2 and R3 independently represent H, F or C1-3 alkyl;
Z represents
-N(R14a)-C(O)-A- (in which group N(R14a) is attached to R4) or -C(O)-N(R14b)-B- (in which group the C(O) moiety is attached to R4);
A represents a direct bond or C1-3 alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl;
B represents
C1-3 alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl or when X represents a direct bond, B may alternatively, together with R18, represent a structural fragment of formula Ia,
in which the wavy lines indicate the positions of attachment of the fragment, wherein G is attached to N(R14b) and J is attached to C(R2)(R3), and the dashed line represents a bond or, when G represents C(R15c)(R15d), is absent; G represents N, C(R15b) or C(R15c)(R15d);
J represents a direct bond or C1-3 alkylene; RR1155aa ttoo RR1155dd iinnddeeppeennddeennttllyy rreeppiresent H or C1-3 alkyl or, when the dashed line represents a bond, R15a is absent;
R , 14a represents H or C1-6 alkyl; R14b represents H, C1-6 alkyl or, together with R18, R14b may alternatively represent a direct bond or -C(O)-; R4 represents C1-6 alkyl or a structural fragment of formula Ib,
X represents a direct bond,
C1-3 alkylene optionally substituted by one or more groups selected from F and C1-3 alkyl, or
-Y-[C(R16a)(R16b)]n-, in which latter group C(R16a)(R16b) is attached to Z; Y represents O, -N(R16c)- or S(O)0-2;
R i6a to R i6c independently represent H or C1-3 alkyl; nn rreepprreesseennttss 2 or 3 or, when Z represents -C(O)-N(R14)-B-, then n may alternatively represent 1;
X1 to X4 independently represent N or C(R17) and X5 represents N or C(R18), provided that at least one of X1 to X5 is other than N;
17 I S
R and R independently represent H, -OH, cyano, halo, nitro, C1-6 alkyl (optionally terminated by -N(H)C(O)OR19a), C1-6 alkoxy, -N(R20a)R20b, -C(O)R20c, -C(O)OR20d, -C(O)N(R20e)R20f, -N(R20s)C(O)R20h, -N(R20i)C(O)N(R20j)R20k, -N(R20m)S(O)2R19b, -S(O)2N(R20n)R20p, -S(O)2R19c, -OS(O)2R19d, -Si(R19e)3 and aryl or R18
(a) together with B and when X represents a direct bond, may alternatively represent a structural fragment of formula Ia, as defined above, or (b) together with R14b, may alternatively represent a direct bond or -C(O)-;
R19a to R19e represent, independently at each occurrence, C1-6 alkyl or phenyl, which latter group is optionally substituted by one or more groups selected from -OH, halo, cyano, C1-4 alkyl and C1-4 alkoxy; R2Oa and R20b independently represent H, C1-6 alkyl or together represent C3-6 alkylene, resulting in a four- to seven-membered nitrogen-containing ring; R20c to R20p independently represent H or C1-6 alkyl; and
R41 to R46 independently represent H or C1-3 alkyl;
wherein each aryl group, unless otherwise specified, is optionally substituted;
or a pharmaceutically acceptable salt and/or derivative thereof,
provided that the compound is not:
(a) 2-[7-(2-{(arninocarbonyl)[2-(4-cyanophenoxy)ethyl]amino}ethyl)-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl]-N-(te?t-butyl)acetamide;
(b) 2-[7-(2-{(aminocarbonyl)[2-(4-cyanophenoxy)ethyl]amino}ethyl)-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl]-N-benzylacetamide;
(c) 2- [7-(2- { (aminocarbonyl) [2-(4-cyanophenoxy) ethyl] amino } ethyl)-9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl]-iV-(l-methyl-l-phenylethyl)acetamide;
(d) /y-(?er?-butyl)-2-(7-{2-[[2-(4-cyanophenoxy)ethyl](methylsulfonyl)- amino]ethyl } -9-oxa-3,7-diazabicyclo[3.3. l]non-3-yl)acetamide; (e) ΛT-benzyl-2-(7-{2-[[2-(4-cyanophenoxy)ethyl](methylsulfoήyl)amino]-ethyl}-
9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)acetamide;
(f) 2-(7-{2-[[2-(4-cyanophenoxy)ethyl](methylsulfonyl)amino]ethyl}-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl)-N-(l-methyl-l-phenylethyl)acetamide;
(g) N-(tert-butyl)-2-(7-{2-[[3-(4-cyanophenyl)propyl](methylsulfonyl)- amino] ethyl } -9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)acetamide;
(h) 2-{7-[3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }-N-isopropylacetamide;
(i) 2-{7-[3-(4-cyanoanilino)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N- isopropylacetamide; (j) 2- {7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl }-
N-isopropylacetamide;
(k) 2-(7-{3-[(4-cyanophenyl)sulfonyl]propyl}-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl)-iV-isopropylacetamide; or
(l) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV,iV~diethylacetamide.
2. A compound as claimed in Claim 1, wherein each aryl or aryloxy group is, unless otherwise specified, optionally substituted by one or more groups selected from -OH, halo, cyano, nitro, C1-6 alkyl, C1-6 alkoxy, -N(R20a)R20b, -C(O)R20c, -C(O)OR20d, -C(O)N(R20e)R20f, -N(R20g)C(O)R20h, -N(R20m)S(O)2R19b, -S(O)2N(R20n)R20p, -S(O)2R19c, -OS(O)2R19d and/or -Si(R19β)3, wherein R19b to R19e and R2Oa to R20p are as defined in Claim 1.
3. A compound as claimed in Claim 1 or Claim 2, wherein R1 represents:
(a) C1-5 alkyl terminated by a group selected from aryl, Het1, -N(R5a)R6, -C(O)R5b, -OR5c, -C(O)OR7 and -C(O)N(R8a)R5d, and optionally further substituted by OH and/or one or more halo groups, or
(b) -C(O)OR7;
4. A compound as claimed in any one of the preceding claims, wherein: R5a and R5d independently represent H or methyl; R b represents C1-5 alkyl; R5c represents aryl or C1-3 alkyl substituted by aryl; R6 represents -C(O)OR10b; R1Ob represents C1-5 alkyl; R7 represents C1-5 alkyl; R a represents C1-5 alkyl or -D- phenyl (the phenyl part of which latter group is optionally substituted by one or more (e.g. one to three) groups selected from F and cyano); and/or D represents C1-3 alkylene.
5. A compound as claimed in any one of the preceding claims, wherein Het1 represents a heterocyclic group that is
(a) five- or six-membered and aromatic, or
(b) nine- or ten-membered, bicyclic and aromatic or part-aromatic, which heterocyclic group contains one or more heteroatoms selected from oxygen, nitrogen and/or sulfur, which group is are optionally substituted by one or more groups selected from halo, cyano, C1-3 alkyl and C1-3 alkoxy.
6. A compound as claimed in any one of the preceding claims, wherein R2 and R both represent H.
7. A compound as claimed in any one of the preceding claims, wherein Z represents -N(R14^-C(O)- or -C(O)-N(R14b)-B-.
8. A compound as claimed in any one of the preceding claims, wherein B represents C1-3 n-alkylene.
9. A compound as claimed in any one of the preceding claims, wherein R14a and R14b independently represent H or C1-3 alkyl.
10. A compound as claimed in any one of the preceding claims, wherein R4 represents C1-5 alkyl or a structural fragment of formula Ib, as defined in Claim 1.
11. A compound as claimed in any one of the preceding claims, wherein X represents:
(a) when Z represents -C(O)-N(R14b)-B~, a direct bond;
(b) C1-3 n-alkylene optionally substituted by one or more methyl groups; or (c) when Z represents -N(R14a)-C(O)-A-, -Y-[C(R16a)(R16b)]n-.
12. A compound as claimed in any one of the preceding claims, wherein: Y represents O; R16a and R16b both represent H; and/or n represents 2 or 3.
13. A compound as claimed in any one of the preceding claims, wherein X1 to X4 all represent C(R17) and X5 represents C(R18).
14. A compound as claimed in any one of the preceding claims, wherein R17 aanndd RR iinnddeeppeennddeennttllyy rreepprreesseenntt HH,, ccyyaannoo or halo, provided that no more than three of X1 to X5 represent other than C(H).
15. A compound as claimed in any one of the preceding claims, wherein R 41 to R all represent H.
16. A compound as claimed in any one of the preceding claims, wherein: aryl represents phenyl, which aryl group is optionally substituted by one to three groups selected from halo, cyano, C1-3 alkyl, C1-3 alkoxy (which latter two groups are optionally substituted by one or more F atoms), -OS(O)2R19d and -Si(CHs)3; and/or R19d represents C1-3 alkyl or phenyl, which latter group is optionally substituted by one to three groups selected from methyl and halo.
17. A compound as claimed in any one of the preceding claims, wherein R1 represents
[3,5-bis(fluoromethoxy)phenyl]methyl,
(2,3 -dimethoxyphenyl)methyl, (2,4-difluorophenyl)methyl,
(2,6-difluorophenyl)methyl,
(2,6-dimethoxyphenyl)methyl,
(2,6-dimethylphenyl)methyl,
(2-cyanophenyl)methyl, (2-methoxyphenyl)methyl,
(2-methylpropan-2-yl)oxycarbonyl,
(2-methylpropan-2-yl)oxycarbonyhnethyl,
(3 ,3-dimethyl-2-oxo-butyl),
(3 ,5-diethoxyphenyl)methyl, (3,5-dimethoxyphenyl)methyl,
(3,5-dimethyll,2-oxazol-4-yl)methyl,
(3 -cyanophenyl)methyl,
(3-ethoxyphenyl)methyl,
(3-methoxyphenyl)mefhyl, (3-propan-2-yloxyphenyl)methyl,
(4-chlorophenyl)methyl,
(4-cyanophenyl)methylcarbamoylmethyl, (4-fluorophenyl)methyl,
(4-methylsulfonyloxyphenyl)methyl,
(5-fluoro-2-methyl-ρhenyl)methyl,
(8-fluoro-2,4-dioxabicyclo[4.4.0]deca-7,9,ll-trien-10-yl)methyl, [(2R)-3-(4-cyanophenoxy)-2-hydroxy-propyl] ,
[2-(4-methoxyphenyl)-2-oxo-ethyl],
[2-(difluoromethoxy)phenyl]methyl,
[2-(trifluoromethyl)phenyl]methyl,
[2,4-bis(trifluoromethyl)phenyl]methyl, [3-(4-methylphenyl)sulfonyloxyphenyl]methyl,
[3-(difluoromethoxy)phenyl]methyl,
[3-(fluoromethoxy)phenyl]methyl,
[3 -(trifluoromethoxy)phenyl]methyl,
[4-(difluoromethoxy)phenyl]methyl, [4-(trifluorometh.yl)phenyl]methyl,
[4-fluoro-2-(trifluoromethyl)plienyl]methyl,
1 ,7-diazabicyclo[4.3.0]nona-2,4,6,8-tetraen-8-ylmethyl,
2-(lH-indol-3-yl)ethyl,
2-(2,3-dihydrobenzofuran-5-yl)ethyl, 2-(2,4-difluorophenoxy)ethyl,
2-(2,4-difluorophenyl)eihyl,
2-(2,6-difluorophenoxy)ethyl,
2-(2,6-difluorophenyl)ethyl,
2-(2,6-dimethylphenoxy)ethyl, 2-(2-chlorophenyl)ethyl,
2-(2-fluorophenoxy)ethyl,
2-(2-fluorophenyl)ethyl,
2-(2-methoxyphenyl)ethyl,
2-(2-methylphenyl)ethyl, 2-(3,4-difluorophenyl)ethyl,
2-(3 ,4-dimethoxyphenyl)ethyl,
2-(3,5-dimethyll,2-oxazol-4-yl)ethyl, 2-(3 ,5-dimethylpyrazol- 1 -yl)ethyl,
2-(3-chlorophenyl)ethyl,
2-(3-cyanophenyl)ethyl,
2-(3-fluorophenyl)ethyl, 2-(3-methoxyphenyl)eihyl,
2-(4-acetylphenyl)ethyl, '
2-(4-chlorophenyl)ethyl,
2-(4-cyano-2-fluoro-phenoxy)ethyl,
2-(4-cyano-2-fluoro-phenyl)ethyl, 3-(4-cyano-3-fluoro-phenoxy)propyl,
2-(4-cyano-3 -fluoro-phenyl)ethyl,
2-(4-cyanophenoxy)ethyl,
2-(4-cyanophenyl)ethyl,
2-(4-fluorophenoxy)ethyl, 2-(4-fluorophenyl)ethyl,
2-(4-methoxyphenyl)ethyl,
2-(4-trimethylsilylphenyl)ethyl,
2-(6-methylpyridin-2-yl)ethyl,
2-[(2-methylpropan-2-yl)oxycarbonyl]propan-2-yl, 2-[(2-methylpropan-2-yl)oxycarbonylamino]ethyl,
2-[(3-chlorophenyl)methoxy]ethyl,
2- [(3-cyanophenyl)methoxy] ethyl,
2-[(3-fluorophenyl)methoxy]ethyl,
2-[(4-chlorophenyl)methoxy]ethyl, 2-[(4-cyanobenzoyl)amino]ethyl,
2-[(4-cyanophenyl)methoxy]ethyl,
2-[(4-fluorophenyl)methoxy]ethyl,
2-[[3-(trJfluoronietliyl)phenyl]methoxy]efhyl,
2-[[4-(trifluoromethyl)phenyl]methoxy]ethyl, 2-[2-(difluoromethoxy)phenyl]ethyl,
2-[2-(trifluoromethyl)phenyl]ethyl,
2-[3-(4-cyanophenyl)propanoyl-methyl-amino]ethyl, 2-[3,4-bis(difluoromethoxy)phenyl]ethyl,
2-[4-(difluoromethoxy)phenyl]ethyl,
2-[4-(trifluoromethyl)plienyl]ethyl,
2-benzo[d]isoxazol-3-ylethyl, 2-benzofuran-5-ylethyl,
2-phenylmethoxyethyl,
2-pyridin-2-ylethyl,
2-pyridin-4-ylethyl,
3-(2,4-difluorophenoxy)propyl, 3-(2,5-difluorophenoxy)propyl,
3-(2,6-difluorophenoxy)propyl,
3-(2-cyanophenoxy)propyl,
3-(2-fluorophenoxy)propyl,
3-(2-methoxyphenoxy)propyl, 3-(3,4-difluorophenoxy)propyl,
3-(3 ,5-difluorophenoxy)propyl,
3-(3-chlorophenoxy)propyl,
3-(3-cyanophenoxy)propyl,
3 -(3 -fluorophenoxy)propyl, 3-(3-methoxyphenyl)propyl,
3-(4-chloro-2-fluoro-phenoxy)propyl,
3-(4-chloro-3-fluoro-phenoxy)propyl,
3-(4-chlorophenoxy)propyl,
3-(4-cyano-2,6-difluoro-phenoxy)propyl, 3 -(4-cyano-2-fluoro-phenoxy)propyl,
3-(4-cyanophenoxy)ρropyl,
3-(4-cyanophenyl)propyl,
3 -(4-cyanophenyl)sulf onylpropyl,
3-(4-fluorophenoxy)propyl, 3-[(2-methylpropan-2-yl)oxy]propyl,
3-[3-(trifluoromethyl)phenoxy]propyl,
3-[4-(trifluoromethyl)phenoxy]propyl, 3-phenoxypropyl, 3-phenylpropyl, 4-(4-cyanophenyl)butyl, (4-cyanophenyl)meihyl, benzo[l,3]dioxol-4-ylmethyl, benzo[l,3]dioxol-5-ylmethyl, benzyl, naphthalen-1-ylmethyl, phenethyl, quinolin-8-ylmethyl, or tert-butylcarbamoylmethyl.
4 18. A compound as claimed in any one of the preceding claims, wherein R - Z-
CR R - represents 2-[(4-cyanobenzoyl)-methyl-amino]ethyl,
(4-oxo-3H-phthalazin-l-yl)methyl,
2-( 1 ,3 -dioxoisoindol-2-yl)ethyl,
2-(4-methylsulfonyloxyphenyl)ethyl,
2- [(3 ,4-difluorobenzoyl)amino] ethyl, 2-[(4-cyanobenzoyl)amino]ethyl,
2- [(4-cyanobenzoyl)-methyl-amino] ethyl,
2-benzamidoethyl,
3-benzamidopropyl,
(2,4-difluorophenyl)methylcarbamoylmethyl, (2-fluorophenyl)methylcarbamoyhnethyl,
(3-fluorophenyl)methylcarbamoylmethyl,
(4-cyano-2,6-difluoro-phenyl)methylcarbamoylmethyl,
(4-cyano-2-fluoro-phenyl)methylcarbamoylmethyl,
(4-cyanophenyl)methylcarbamoylmethyl, (4-fluorophenyl)methylcarbamoylmethyl,
(benzyl-methyl-carbamoyl)methyl,
(methyl-tert-butyl-carbamoyl)methyl, [(4-cyanophenyl)methyl-methyl-carbamoyl]methyl,
2-(4-cyanophenoxy)ethylcarbamoylniethyl,
2~(4-fluorophenyl)ethylcarbamoylmethyl,
2-phenylpropan-2-ylcarbamoylmethyl, benzylcarbamoylmethyl, dipropan-2-ylcarbamoylmethyl, phenethylcarbamoylniethyl, tert-butylcarbamoylmethyl,
2-(2,2-dimethylpropanoylamino)ethyl, 2-(3,3-dimethyl-2-oxo-indol-l-yl)ethyl,
2-(3 ,3 -dimethylbutanoylamino)ethyl,
2-(tert-butylcarbamoyl)ethyl,
2-[3-(4-cyanophenyl)propanoylamino]ethyl, or
2-[3-(4-cyanophenyl)propanoyl-methyl-amino]ethyl.
19. A compound as claimed in any one of the preceding claims, wherein the compound is selected from:
(i) iV-(4-cyanobenzyl)-N-methyl-2-{7-[2-(2-methylphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide; (ii) 2- { 7-[2-(2-chlorophenyl)ethyl] -9-oxa-3 ,7-diazabicyclo[3.3. l]non-3 -yl } -N-(4- cyanobenzyl)-N-methylacetamide;
(iii) N-(4-cyanobenzyl)-2- { 7- [2-(2,6-difluorophenyl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl}-N-methylacetamide;
(iv) N-(4-cyanobenzyl)-N-methyl-2-(7- { 2-[2-(trifluoromethyl)phenyl] ethyl } -9- oxa-3,7-diazabicyclo[3.3.1]non-3-yl)acetamide;
(v) 2-(7- { 2-[3 ,4-bis(difluoromethoxy)phenyl] ethyl } -9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl)-N-(4-cyanobenzyl)-N-methylacetamide;
(vi) iV-(4-cyanobenzyl)-N-methyl-2-(7- { 2- [4-(trimethylsilyl)phenyl] ethyl } -9-oxa-
3,7-diazabicyclo[3.3.1]non-3-yl)acetamide; (vii) iV-(4-cyanobenzyl)-2-(7- { 2-[4-(trimethylsilyl)phenyl]ethyl } -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl)acetamide; (viii) N-(4-cyanobenzyl)-2-{7-[2-(3,4-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } -iV-methylacetamide;
(ix) N-(4-cyanobenzyl)-N-methyl-2-(7-{2-[4-(trifluoromethyl)phenyl]ethyl}-9- oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 -yl)acetamide; (x) N-benzyl-2-{ 7-[2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo [3.3. l]non-3- yl}acetamide;
(xi) N-(4-cyanobenzyl)-2-{7-[2-(3-cyanophenyl)emyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}acetamide;
(xii) N-(4-cyanobenzyl)-2-{7-[2-(2,4-difluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xiii) N-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xiv) 2-{7-[2-(l,2-benzisoxazol-3-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } -iV-(4-cyanobenzyl)acetamide (xv) N-(4-cyanobenzyl)-2-(7-{2-[4-(difluoromethoxy)phenyl]ethyl}-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl)acetamide;
(xvi) N-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-cyano-3-fluorophenyl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xvii) iV-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-cyano-2-fluorophenyl)ethyl]-9-oxa- 3,7-diazabicyclo[3.3.1]non-3-yl}acetamide;
(xviii) iV-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-cyanoph.enyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xix) N~(4-cyanobenzyl)-2- { 7-[2-(4-cyano-3-fluorophenyl)ethyl]-9-oxa-3 ,7- diazabicyclo [3.3. l]non-3-yl } acetamide; (xx) N-(4-cyanobenzyl)-2-{7-[2-(4-cyano-2-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xxi) N-(4-cyanobenzyl)-2-(7- { 2-[2-(difluoromethoxy)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(xxii) N-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9- oxa-3 ,7-diazabicyclo [3.3. l]non-3-yl } acetamide;
(xxiii) N-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide; (xxiv) N-(4-cyano-2-fluorobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl} acetamide;
(xxv) N-(4-cyano-2-fluorobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide; (xxvi) N-(4-cyano-2-fluorobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl]acetamide;
(xxvii) A''-(tert-butyl)-2-{7-[4-(4-cyanophenyl)butyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl} acetamide;
(xxviii) iV-(te?t-butyl)-2-{7-[3-(4-cyano-3-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxix) N-(ter?-butyl)-2-{7-[2-(4-cyano-2-fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xxx) N-(4-cyano-2,6-difluorobenzyl)-2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-
3,7-diazabicyclo[3.3. l]non-3-yl } acetamide; (xxxi) iV-(4-cyano-2,6-difluorobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xxxii) N-(4-cyano-2,6-difluorobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl} acetamide;
(xxxiii) iV-(4-cyano-2,6-difluorobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl]acetamide;
(xxxiv) iV-(4-cyanobenzyl)-2-{7-[3-(3-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(xxxv) N-(4-cyanobenzyl)-2-{7-[3-(2-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo [3.3.1 ]non-3 -yl } acetamide; (xxxvi) _V-(4-cyanobenzyl)-2-(7- { 2-[2-(trifluoromethyl)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl)acetamide;
(xxxvii) N-(4-cyanobenzyl)-2-{7-[2-(2-methylphenyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(xxxviii) 2-{7-[2-(2-chlorophenyl)etiiyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- N-(4-cyanobenzyl)acetamide;
(xxxix) 2-{7-[2-(3-chloroρhenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-cyanobenzyl)acetamide; (xl) N-(4-cyanobenzyl)-2-{7-[2-(2-methoxyphenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}acetamide;
(xli) N-(4-cyanobenzyl)-2-{7-[2-(4-methoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (xlii) iV-(4-cyanobenzyl)-2-{7-[3-(4-cyano-2,6-difluoroplienoxy)propyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(xliii) N-(4-cyanobenzyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1 ]non-3-yl } acetamide;
(xliv) iV-(4-cyanobenzyl)-2- { 7- [3 -(4-cyano-3 -fluorophenoxy)propyl] -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(xlv) N-(4-cyanobenzyl)-2-{7-[3-(2-methoxyphenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1 ]non-3 -yl } acetamide ;
(xlvi) N-(4-cyanobenzyl)-2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide; (xlvii) N-(4-cyanobenzyl)-2-{7-[2-(6-methylpyridin-2-yl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide;
(xlviii) N-(4-cyanobenzyl)-2-[7-(2-pyridin-2-ylethyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl]acetamide;
(xlix) iV-(4-cyanobenzyl)-2- [7-(2-pyridin-4-ylethyl)-9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl]acetamide;
(1) 2-{ 7-[2-(4-cyanophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl} -_V-(2- phenylethyl)acetamide;
(li) 2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-[2-
(4-fluorophenyl)ethyl] acetamide; (lii) N-(4-cyanobenzyl)-2- { 7-[2-(3 ,5-dimethylisoxazol-4-yl)ethyl]-9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl} acetamide;
(liii) N-(4-cyanobenzyl)-2-{7-[2-(lH-indol-3-yl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl} acetamide;
(liv) N-(4-cyanobenzyl)-2-[7-(imidazo[l,2-a]pyridin-2-ylmethyl)-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl] acetamide;
(lv) N-(4-cyanobenzyl)-2-{7-[2-(3,5-dimethyl-lH-pyrazol-l-yl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide; (lvi) N-(4-cyanobenzyl)-2-{7-[2-(3,4-dimethoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lvii) N-(4-cyanobenzyl)-2-{7-[2-(2,6-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo [3.3.1 ]non-3 -yl } acetamide; (lviii) 2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3. l]non-3-yl } -N-
(4-fluorobenzyl)acetamide;
(lix) N-benzyl-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide;
(lx) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N- [2-(4-fluorophenyl)ethyl] acetamide;
(lxi) 2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl}-N-
(4-fluorobenzyl)acetamide ;
(lxii) 2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl}-
N-[2-(4-fluorophenyl)ethyl]acetamide; (lxiii) N-(4-cyanobenzyl)-2-{7-[(3,5-dimethylisoxazol-4-yl)methyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(lxiv) 2- {7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -
N-(2,4-difluorobenzyl)acetamide;
(lxv) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- N-(3-fluorobenzyl)acetamide;
(lxvi) N-(4-cyanobenzyl)-2-{7-[2-(2-fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl} acetamide;
(lxvii) 2-{ 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -
N-(2-phenylethyl)acetamide; (lxviii) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-
N-(2-phenylethyl)acetamide;
(lxix) N-(tert-butyl)-2-{7-[3-(4-cyano-2,6-difluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(lxx) N-(?ert-butyl)-2-{7-[3-(4-cyano-2-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxi) N-(4-cyanobenzyl)-2-{7-[3-(3,5-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (lxxii) N-(4-cyanobenzyl)-2-{7-[3-(2,5-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxiii) N-(4-cyanobenzyl)-2-{7-[3-(2,6-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl} acetamide; (lxxiv) N-(4-cyanobenzyl)-2-{7-[3-(2-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxv) 2-{ 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl }- iV-(2-fluorobenzyl)acetamide;
(lxxvi) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- JV-(3-fluorobenzyl)acetamide;
(lxxvii) 7V-(4-cyanobenzyl)-2-{ 7-[4-(difluoromethoxy)benzyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxviii) iV-(4-cyanobenzyl)-2- { 7-[4-(trifluoromethyl)benzyl]-9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl} acetamide; (lxxix) 2-[7-(4-chlorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]-N-(4-cyano- benzyl)acetamide;
(lxxx) N-(4-cyanobenzyl)-2-[7-(4-cyanobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3 -yl] acetamide;
(lxxxi) N-(4-cyanobenzyl)-2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl] acetamide;
(lxxxii) iV-(4-cyanobenzyl)-2- { 7- [2-(3 ,4-difluorophenyl)ethyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxxiii) N-benzyl-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}-N-methylacetamide; (lxxxiv) N-benzyl-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }-iV-methylacetamide;
(lxxxv) N-(4-cyanobenzyl)-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxxvi) N-(4-cyanobenzyl)-2-(7- { 2-[4-(trifluoromethyl)phenyl] ethyl } -9-oxa-3 ,7- diazabicyclo[3.3.1]non-3-yl)acetamide;
(lxxxvii) 2-{ 7-[2-(4-chlorophenyl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl} -
N-(4-cyanobenzyl)acetamide; (lxxxviii) N-(4-cyanobenzyl)-2-{7-[2-(2,4-difluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(lxxxix) N-(4-cyanobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (xc) iV-(4-cyanobenzyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1 ]non-3-yl } acetamide;
(xci) N-(4-cyanobenzyl)-2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } -iV-methylacetamide;
(xcii) N-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}-iV-methylacetamide;
(xciii) N-(4-cyanobenzyl)-N-methyl-2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl]acetamide;
(xciv) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl }acetamide; (xcv) N-[2-(4-cyanophenoxy)ethyl]-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide;
(xcvi) iV-[2-(4-cyanophenoxy)ethyl]-2-[7-(3-phenoxypropyl)-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3 -yl] acetamide;
(xcvii) N- [2-(4-cyanophenoxy)ethyl] -2- { 7- [2-(4-cyanophenyl)ethyl] -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xcviii) iV-[2-(4-cyanophenoxy)ethyl]-2-{7-[2-(4-fluorop]ienyl)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(xcix) N-[2-(4-cyanophenoxy)ethyl]-2-[7-(2-phenylethyl)-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl]acetamide; (c) 2-[7-(4-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]-iV-[2-(4-cyano- phenoxy)ethyl]acetamide;
(ci) N-[2-(4-cyanophenoxy)ethyl]-2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl] acetamide;
(cii) 2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)-N-[2-(4-cyanophenoxy)- ethyl] acetamide;
(ciii) N-(4-cyanobenzyl)-2-(7-{3-[3-(trifluoromethyl)phenoxy]ρropyl}-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl)acetamide; (civ) 2- { 7-[3-(3-chlorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1 ]non-3-yl } - iV-(4-cyanobenzyl)acetamide;
(cv) N-(4-cyanobenzyl)-2-{7-[3-(3-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide; (cvi) 2-{7-[3-(4-chloro-2-fluoroρhenoxy)proρyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}-N-(4-cyanobenzyl)acetamide;
(cvii) 2-{7-[3-(4-chloro-3-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}-N-(4-cyanobenzyl)acetamide;
(cviii) iV-(4-cyanobenzyl)-2- { 7- [3 -(3 ,4-difluorophenoxy)propyl] -9-oxa-3 ,7-diaza- bicycloP^JJnon-S-ylJacetamide;
(cvix) N-(4-cyanobenzyl)-2-(7- { 3- [4-(trifluoromethyl)phenoxy]propyl } -9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl)acetamide;
(cx)-2-{7-[3-(4-chlorophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
(4-cyanobenzyl)acetamide; (cxi) N-(4-cyanobenzyl)-2-[7-(3-phenoxypropyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(cxii) iV-(4-cyanobenzyl)-2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl} acetamide;
(cxiii) iV-(4-cyanobenzyl)-2-(7-{2-[(3-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)acetamide;
(cxiv) 2-(7- { 2-[(3-chlorobenzyl)oxy] ethyl }-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yl)-iV-(4-cyanobenzyl)acetamide;
(cxv) iV-(4-cyanobenzyl)-2-[7-(2-{[3-(txifluόromethyl)ben2;yl]oxy}ethyl)-9-oxa-
3,7-diazabicyclo[3.3.1]non-3-yl]acetamide; (cxvi) N-(4-cyanobenzyl)-2-(7-{2-[(3-fluorobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxvii) 2-(7-{2-[(4-chlorobenzyl)oxy]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl)-N-(4-cyanobenzyl)acetaniide;
(cxviii) N-(4-cyanobenzyl)-2-[7-(2- { [4-(trifluoromethyl)benzyl]oxy } efhyl)-9-oxa- 3 ,7-diazabicyclo[3.3. l]non-3-yl]acetamide;
(cxix) N-(4-cyanobenzyl)-2-(7- { 2- [(4-fluorobenzyl)oxy] ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3.1 ]non-3~yl)acetamide; (cxx) 2-{7-[2-(benzyloxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-(4- cyanobenzyl)acetamide;
(cxxi) N-(4-cyanobenzyl)-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide; (cxxii) 2- { 7-[2-(l-benzofuran-5-yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } -
JV-(4-cyanobenzyl)acetamide;
(cxxiii) N-benzyl-2-(7-{2-[(4-cyanobenzyl)oxy]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)acetamide;
(cxxiv) iV-(terf-butyl)-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}-N-methylacetamide;
(cxxv) N-(tert-butyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3.1 ]non-3 -yl } -iV-methylacetamide;
(cxxvi) N-(tert-butyl)-2-(7- { 2-[(4-cyanobenzyl)oxy] ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl)-iV-methylacetamide; (cxxvii) iV-(tert-butyl)-2-(7~ { 2-[(4-cyanobenzyl)oxy] ethyl} -9-oxa-3 ,7-diaza- bicyclo[3.3.1]non-3-yl)acetamide;
(cxxviii) 2-{7-[2-(benzylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } -N-(4-cyanobenzyl)acetamide;
(cxxix) N-(4-cyanobenzyl)-2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cxxx) N-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide;
(cxxxi) iV-(4-cyanobenzyl)-2-{7-[2-(4-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } acetamide; (cxxxii) iV-(4-cyanobenzyl)-2-{7-[2-(2,3-dihydro-l-benzofuran-5-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } acetamide;
(cxxxiii) iV-(4-cyanobenzyl)-2-[7-(2-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl]acetamide;
(cxxxiv) 2,2'-(9-oxa-3,7-diazabicyclo[3.3.1]nonane-3,7-diyl)bis[N-(4-cyano- benzyl)acetamide];
(cxxxv) 2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)-N-(4-cyanobenzyl)- acetamide; (cxxxvi) tert-bntyl [2-(7- { 2-[(4-cyanobenzyl)amino]-2-oxoethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl)ethyl]carbamate;
(cxxxvii) 2-{7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } -JV-(4-cyanobenzyl)acetamide; (cxxxviii) tert-butyl 7-{2-[(4-cyanobenzyl)amino]-2-oxoethyl}-9-oxa-3,7-diaza- bicyclo[3.3.1]nonane-3-carboxylate;
(cxxxix) N-(2-{7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-4-cyanobenzamide;
(cxl) N-(tert-buty\)-2- {7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}acetamide;
(cxli) 2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-N-
( 1-methyl- 1 -phenylethyl)acetamide;
(cxlii) N-benzyl-2-{7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } acetamide; (cxliii) N-(tert-butyl)-2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }acetamide;
(cxliv) N-(2-{7-[2-(tert-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)-3-(4-cyanophenyl)-N-methylpropanamide;
(cxlv) 2- { 7- [3-(4-cyanophenoxy)propyl] -9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } - N-(l-methyl-l-phenylethyl)acetamide;
(cxlvi) N-benzyl-2- { 7-[3-(4-cyanophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo[3.3.1]- non-3-yl } acetamide;
(cxlvii) 2- { 7- [(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl] -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} -N-(l-methyl- l-phenylethyl)acetamide; (cxlviii) N-benzyl-2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl } acetamide;
(cxlix) N-(tert-butyϊ)-2- { 7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-
3 ,7-diazabicyclo [3.3.1 ]non-3-yl } acetamide;
(cl) 2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}- iV,iV-diisopropylacetamide;
(cli) 2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } -iV^/V-diisopropylacetamide; (clii) iV-(tert-butyl)-2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } acetamide;
(cliii) 3-[(7-{2-[(4-cyanobenzoyl)(methyl)amino]ethyl}-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl)methyl]phenyl 4-methylbenzenesulfonate; (cliv) 4-cyano-iV-(2-{7-[3-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(civ) 4-cyano-iV- { 2-[7-(3 ,5-diethoxybenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}-N-methylbenzamide;
(clvi) 4-cyano-N-methyl-iV-(2- { 7-[3-(trifluoromethoxy)benzyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)benzamide;
(clvii) 4-cyano-iV-{2-[7-(3-isopropoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3 -yl] ethyl } -JV-methylbenzamide;
(clviii) 4-cyano-N-{2-[7-(3-ethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl] ethyl } -iV-methylbenzamide; (clix) N-(2-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } ethyl)-3 ,4-difluorobenzamide;
(clx) N-(2- {7- [2-(4-cyanophenoxy)ethyl] -9-oxa-3 ,7-diazabicyclo [3.3.1 ]non-3 -yl } - ethyl)-3 ,4-difluorobenzamide;
(clxi) 4-cyano-iV- {2-[7-(2,6-dimethoxybenzyl)-9-oxa-3 ,7-diazabicyclo [3.3. l]non- 3-yl]ethyl)-N-methylbenzamide;'
(clxii) 4-cyano-iV-{2-[7-(2,3-dimethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl]ethyl}-N-methylbenzamide;
(clxiii) 4-cyano-N- { 2-[7-(2-methoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yljethyl }-iV-methylbenzamide; (clxiv) 4-cyano-N-{2-[7-(3-methoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- . yl] ethyl } -N-methylbenzamide;
(clxv) 4-cyano-iV-(2-{7-[2-(2,6-difluorophenoxy)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide;
(clxvi) 4-cyano-N-(2- { 7-[2-(3 ,5-dimethylisoxazol-4-yl)ethyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide;
(clxvii) 4-cyano-N-(2- { 7-[(3 ,5-dimethylisoxazol-4-yl)methyl]-9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl} ethyl)benzamide; (clxviii) 4-cyano-N-(2-{7-[2-(3,4-difluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(clxix) 4-cyano-N-(2-{7-[2-(2,4-difluorophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)benzamide; (clxx)N-(2-{7-[3-(4-chlorophenoxy)ρropyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl } ethyl)-4-cyanobenzamide;
(clxxi) 4-cyano-iV-(2-{ 7-[3-(2,6-difluorophenoxy)propyl]-9-oxa-3 ,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(clxxϊi) 4-cyano-N-(2-{7-[3-(2,4-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl }ethyl)benzamide;
(clxxiii) 4-[(7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl)methyl]phenyl methanesulfonate;
(clxxiv) 4-cyano-N-(2-{7-[(6-fluoro-4H-l,3-benzodioxin-8-yl)methyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}ethyl)benzamide; (clxxv) 4-cyano-iV-(2- { 7-[2-(difluoromethoxy)benzyl] -9-oxa-3 ,7-diazabicyclo-
[3.3. l]non-3-yl} ethyl)-N-methylbenzamide;
(clxxvi) 4-cyano-Λ''-(2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)-iV-meihylbenzamide;
(clxxvii) 4-[2-(7- {2-[(4-cyanobenzoyl)amino]ethyl } -9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl)ethyl]phenyl methanesulfonate;
(clxxviii) 4-cyano-iV-(2- { 7- [2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo-
[3.3.1 ]non-3-yl } ethyl)benzamide;
(clxxix) 4-cyano-N-(2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide; (clxxx) N-(2- { 7-[2-(4-cyanophenoxy)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl}ethyl)benzamide;
(clxxxi) 4-cyano-N-(2-{7-[2-(4-fluoroρhenyl)ethyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(clxxxii) 4-cyano-N-(2-{7-[2-(4-fluorophenoxy)ethyl]-9-oxa-3,7-diazabicyclo- [3.3. l]non-3-yl} ethyl)benzamide;
(clxxxiii) 4-cyano-N-(2-{7-[3-(4-fluorophenoxy)propyl]-9-oxa-3,7-diazabicyclo- [3.3. l]non-3-yl } ethyl)benzamide; (clxxxix) N-(3-{7-[3-(4-cyanophenoxy)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl }propyl)benzamide;
(cxc) 4-cyano-N-{2-[7-(2,6-difluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl }benzamide; (cxci) 4-cyano-yV-{2-[7-(2,6-dimethylbenzyl)-9-oxa-3,7-diazabicyclo[3.3. l]non-3- yl] ethyl }benzamide;
(cxcii) N-{2-[7-(l,3-benzodioxol-4-ylmethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl } -4-cyanobenzamide;
(cxciii) 4-cyano-Λ/r;-methyl-N-{2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl]ethyl}benzamide;
(cxciv) 4-cyano-N-methyl-N-{2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl]ethyl}benzamide;
(cxcv) iV-[2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]-4-cyano-N- methylbenzamide; (cxcvi) 4-cyano-N-(2-{7-[4-fluoro-2-(trifluoromethyl)benzyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)benzamide;
(cxcvii) 4-cyano-N-(2-{7-[2-(trifluoromethyl)benz;yl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(cxcviii) 4-cyano-7V- { 2- [7-(5-fluoro-2-methylbenzyl)-9-oxa-3 ,7-diazabicyclo- [3.3.1]non-3-yl]ethyl}benzamide;
(cxcix) iV-(2-{7-[2,4-bis(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-4-cyanobenzamide;
(cc) 4-cyano-iV-(2- { 7- [2-(difluoromethoxy)benzyl]-9-oxa-3 ,7-diazabicyclo [3.3.1]- non-3 -yl } ethyl)benzamide ; (cci) 4-cyano-iV- { 2-[7-(3-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl jbenzamide;
(ccii) 4-cyano-N-(2-{7-[3-(3-methoxyphenyl)propyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(cciii) 4-cyano-N-{2-[7-(3-phenylpropyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl }benzamide;
(cciv) 4-cyano-Λ/r-(2-{7-[3-(4-cyanophenyl)propyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)benzamide; (ccv) N-{2-[7-(l,3-benzodioxol-5-ylmethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl] ethyl } -4-cyanobenzamide;
(ccvi) 4-cyano-iV-{2-[7-(l-naphthylmethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl}benzamide; (ccvii) 4-cyano-iV-{ 2-[7-(quinolin-8-ylmethyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-
3 -yl] ethyl }benzaniide;
(ccviii) 4-cyano-N-(2-{7-[3-(trifluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)benzamide;
(ccix) iV-{2-[7-(4-chlorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]ethyl}-4- cyanobenzamide;
(ccx) 4-cyano-N-(2-{7-[4-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)benzamide;
(ccxi) 4-cyano-iV-{2-[7-(4-fluorobenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3- yl]ethyl}benzamide; (ccxii) 4-cyano-N-(2-{7-[4-(trifluoromethyl)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl} ethyl)benzamide;
(ccxiii) 4-cyano-JV- { 2-[7-(2-cyanobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl}benzamide;
(ccxiv) 4-cyano-iV-{ 2-[7-(2,4~difluorobenzyl)~9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl]ethyl}benzamide;
(ccxv) 4-cyano-iV- { 2-[7-(4-cyaήobenzyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl] - ethyl }benzamide;-
(ccxvi) 4-(3-{7-[(4-oxo-3,4-dihydrophthalazin-l-yl)methyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } propoxy)benzonitrile; (ccxvii) 4-cyano-N- { 2-[7-(2-phenylethyl)-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl]- ethyl}benzamide;
(ccxviii) tert-butyl 2-(7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl)-2-methylpropanoate;
(ccxix) tert-butyl [2-(7- { 2-[(4-cyanobenzoyl)amino]ethyl } -9-oxa-3 ,7-diaza- bicyclo[3.3. l]non-3-yl)ethyl]carbaniate;
(ccxx) N-(2-{7-[2-(ter?-butylatniαo)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-4-cyanobenzamide; (ccxxi) terf-butyl (7-{2-[(4-cyanobenzoyl)amino]ethyl}-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl)acetate;
(ccxxii) 4-cyano-iV- { 2- [7-(3 ,3 -dimethyl-2-oxobutyl)-9-oxa-3 ,7-diazabicyclo-
[3.3.1 ]non-3 -yl] ethyl }benzamide; (ccxxiii) iV-[2~(7-benzyl-9-oxa-3 ,7-diazabicyclo[3.3. l]non~3-yl)ethyl]-4-cyano- benzamide;
(ccxxiv) 4-[((2S)-3-{7-[2-(l,3-dioxo-l,3-dihydro-2H-isoindol-2-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } -2-hydroxypropyl)oxy]benzonitrile;
(ccxxv) N-(ϊerϊ-butyl)-3-{7-[2-(4-cyanopb.enoxy)ethyl]-9-oxa-3,7-diazabicyclo- [3.3.1]non-3-yl}propanamide;
(ccxxvi) 4-(2- { 7-[2-(2,2-dimethyl-4-oxo-3 ,4-dihydro- 1 ,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl } ethoxy)benzonitrile;
(ccxxvii) 4-(3- { 7-[2-(2,2-dimethyl-4-oxo-3 ,4-dihydro- 1 ,5-benzoxazepin-5(2H)- yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}propoxy)benzonitrile; (ccxxviii) 4-[((2S)-3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-
9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxypropyl)oxy]benzonitrile;
(ccxxix) 4-(3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indoM-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl }propoxy)benzonitrile;
(ccxxx) 4-[(3-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-9-oxa- 3,7-diazabicyclo[3.3.1]non-3-yl}propyl)sulfonyl]benzonitrile;
(ccxxxi) 4-(2-{7-[2-(3,3-dimethyl-2-oxo-2,3-dihydro-lH-indol-l-yl)ethyl]-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl } ethoxy)benzonitrile;
(ccxxxii) N-(2- {7-[2-(4-acetylphenyl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl } ethyl)-3-(4-cyanophenyl)propanamide; (ccxxxiii) 3-(4-cyanophenyl)-N-(2- { 7-[2-(4-methoxyphenyl)ethyl]-9-oxa-3 ,7- diazabicyclo[3.3. l]non-3-yl } ethyl)propanamide;
(ccxxxiv) 3-(4-cyanophenyl)-N-(2-{7-[2-(4-cyanophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. ljnon-3-yl } ethyl)propanamide;
(ccxxxv) 3-(4-cyanophenyl)-iV-(2-{7-[2-(3-fluorophenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)propanamide;
(ccxxxvi) 3-(4-cyanophenyl)-iV-(2-{7-[2-(2,6-dimethylphenoxy)ethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl} ethyl)propanamide; (ccxxxvii) N-{2-[7-(3-tert-butoxypropyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl]- ethyl } -3-(4-cyanophenyl)propanamide;
(ccxxxviii) 3-(4-cyanophenyl)-N-{2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl]ethyl}propanamide; (ccxxxix) 4-[((2S)-3-{7-[2-(2,2-dimethyl-l-oxido-4-oxo-3,4-dihydro-l,5-benzo- thiazepin-5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxy- propyl)oxy]benzonitrile;
(ccxl) 4-[((2S)-3-{7-[2-(2,2-dimethyl-4-oxo-3,4-dihydro-l,5-benzoxazeρin-5(2H)- yl)ethyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl} -2-hydroxypropyl)oxy]- benzonitrile;
(ccxli) N-(2-{7-[2-(rer?-butylamino)-2-oxoethyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl } ethyl)-3-(4-cyanophenyl)-N-methylpropanamide;
(ccxlii) 4-[((2S)-3-{7-[2-(2,2-dimethyl-l,l-dioxido-4-oxo-3,4-dihydro-l,5-benzo- thiazepin-5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxy- propyl)oxy]benzonitrile;
(ccxliii) 3-(4-cyanophenyl)-N-(2-{7-[2-(4-methoxyphenyl)-2-oxoethyl]-9-oxa-3,7- diazabicyclo[3.3. l]non-3-yl }ethyl)-N-methylpropanamide;
(ccxliv) N-[2-(7-benzyl-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]-3-(4-cyano- phenyl)-N-methylpropanamide; (ccxlv) tert-butyl [2-(7-{2-[[3-(4-cyanophenyl)propanoyl](methyl)amino]ethyl}-9- oxa-3 ,7-diazabicyclo[3.3. l]non-3-yl)ethyl]carbamate;
(ccxlvi) 3-(4-cyanophenyl)-N-{2-[7-(3,3-dimethyl-2-oxobutyl)-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl]ethyl}propanamide;
(ccxlvii) 4-[((2S)-3-{7-[2-(2,2-dimethyl-4-oxo-3,4-dihydro-l,5-benzothiazepin- 5(2H)-yl)ethyl]-9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl}-2-hydroxypropyl)oxy]- benzonitrile;
(ccxlviii) fert-butyl {2-[7-(2-{ [3-(4-cyanophenyl)propanoyl]amino}ethyl)-9-oxa-
3 ,7-diazabicyclo[3.3. l]non-3-yl] ethyl } carbamate;
(ccxlix) iV-(2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)-3 ,3-dimethylbutanamide;
(ccl) iV-(2-{7-[(2S)-3-(4-cyanophenoxy)-2-hydroxypropyl]-9-oxa-3,7-diaza- bicyclo[3.3. l]non-3-yl } ethyl)-2,2-dimethylpropanamide; (ccli) 4-cyano-iV-{2-[7-(3,5-dimethoxybenzyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3 -yl] -ethyl } -iV-methylbenzamide;
(cclii) N'-(4-cyano-2-fluorobenzyl)-2-{7-[2-(2-fluorophenyl)ethyl]-9-oxa-3,7- diaza-bicyclo[3.3. l]non-3-yl } acetamide; (ccliii) iV-(4-cyanobenzyl)-2-[7-(2-phenylethyl)-9-oxa-3,7-diazabicyclo[3.3.1]non-
3-yl]acetamide;
(ccliv) N-(4-cyanobenzyl)-2-{7-[3-(2,4-difluorophenoxy)propyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide;
(cclv) N-(4-cyanobenzyl)-2-{7-[2-(3-methoxyphenyl)ethyl]-9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl} acetamide;
(cclvi) 4-cyano-iV-(2-{7-[3-(difluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl }ethyl)-N-methylbenzamide;
(cclvii) 4-cyano-Ν-(2-{7-[3-(fluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3. l]non-3-yl } ethyl)-N-methylbenzamide; (cclviii) N-(2-{7-[3,5-bis(fluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo-
[3.3.1]non-3-yl}ethyl)-4-cyano-N-methylbenzamide, and salts and/or solvates thereof.
20. A compound as claimed in any one of the preceding claims, wherein the compound is of the formula Ic,
wherein R1, Rl4a and R41 to R46 are as defined in Claim 1 and: (a) Xa takes the same definition as X in Claim 1, except that it does not represent a direct bond; and (b) R17a represents one or more substituents on the phenyl ring, each substituent
1*7 taking the same definition as R in Claim 1.
21. A compound as claimed in Claim 20, wherein: R1 represents a structural fragment of the formula
Rlal , Rlbl and Rlel independently represent H or F;
Rlcl represents Cl, cyano or F;
Rla2 represents F or H; Rlb2 represents methoxy or H;
Rlcl represents Cl, F, methoxy or H; Q1 represents a direct bond or CH2; R41 to R46 all represent H; R14a represents methyl or H; Xa represents
O(CH2)2 (in which group the O-atom is attached to the phenyl ring),
CH2 or, when Rlcl represents cyano, C(CHz)^, the substitution pattern on the phenyl ring to which Xa is attached is as follows
R17al represents cyano or, when Xa represents C(CH3)2, R17al may alternatively represent H; and
R17a2 represent F or H.
22. A compound as claimed in any one of Claims 1 to 19, wherein the compound is of the formula Id,
(a) R14bl represents H or C1-6 alkyl;
(b) R17b represents one or more substituents on the phenyl ring, each substituent taking the same definition as R 17 in Claim 1.
23. A compound as claimed in Claim 22, wherein: R1 represents a structural fragment of the formula
R • if represents one substituent at the 2- or 3-position (relative to the point of attachment of
Q2), or two substituents at the 3- and 5-positions (relative to the point of attachment of Q2), and each Rlf substituent represents methoxy or fluoro-substituted methoxy; R41 to R46 all represent H; B represents (CEb)2 or CH2; R14bl represents H or methyl; X represents a direct bond; the substitution pattern on the phenyl ring to which X is attached is as follows wherein the wavy line indicates the position of attachment to the group X;
R , 17bl represents cyano; and R17b2 represents H.
24. A compound as claimed in any one of Claims 1 to 19, wherein the compound is of the formula Ie,
25. A compound as claimed in Claim 24, wherein: R1 represents a structural fragment of the formula
Rlg and Rlh independently represent H or F; and R14a represents H.
26. 4-Cyano-iV- { 2-[7-(3 ,5-dimethoxybenzyl)-9-oχa-3 ,7-diazabicyclo[3.3.1]- non-3-yl]-ethyl}-N-methylbenzamide or a salt and/or solvate thereof.
27. N-(4-Cyanobenzyl)-2-[7-(2-phenylethyl)-9-oχa-3 ,7-diazabicyclo[3.3.1]- non-3-yl]acetamide or a salt and/or solvate thereof.
28. N-(4-Cyanobenzyl)-2-{7-[3-(2,4-difluorophenoxy)propyl]-9-oxa-3,7- diazabicyclo[3.3.1]non-3-yl}acetamide or a salt and/or solvate thereof.
29. iV-(4-Cyanobenzyl)-2-{7-[2-(3-methoxyphenyl)ethyl]~9-oxa-3,7-diaza- bicyclo[3.3.1]non-3-yl}acetamide or a salt and/or solvate thereof.
30. 4-cyano-N-(2-{7-[3-(fluoromethoxy)benzyl]-9-oxa-3,7-diazabicyclo[3.3.1]- non-3-yl}ethyl)-N-methylbenzamide or a salt and/or solvate thereof .
31. N-(2- { 7-[3 ,5-bis(fluoromethoxy)benzyl]-9-oxa-3 ,7-diazabicyclo[3.3. l]non-3- yl}ethyl)-4-cyano-N-methylbenzamide or a salt and/or solvate thereof
32. A pharmaceutical formulation including a compound as defined in any one of Claims 1 to 31 in admixture with a pharmaceutically-acceptable adjuvant, diluent or carrier.
33. A pharmaceutical formulation for use in the prophylaxis or the treatment of an arrhythmia, comprising a compound as defined in any one of Claims 1 to 31.
34. A compound as defined in any one of Claims 1 to 31 for use as a pharmaceutical.
35. A compound as defined in any one of Claims 1 to 31 for use in the prophylaxis or the treatment of an arrhythmia.
36. The use of a compound as defined in any one of Claims 1 to 31 for the manufacture of a medicament for the prophylaxis or the treatment of an arrhythmia.
37. The use as claimed in Claim 36, wherein the arrhythmia is an atrial or a ventricular arrhythmia.
38. A method of prophylaxis or treatment of an arrhythmia which method comprises administration of a therapeutically effective amount of a compound as defined in any one of Claims 1 to 31 to a person suffering from, or susceptible to, such a condition.
39. A process for the preparation of a compound as defined in Claim 1, which process comprises:
(a) reaction of a compound of formula II,
R^L1 III wherein L1 represents a leaving group;
(b) reaction of a compound of formula IV,
wherein R1 and R41 to R46 are as defined in Claim 1, with a compound of formula V, wherein L2 represents a leaving group and R2, R3, R4 and Z are as defined in Claim 1; (c) for compounds of formula I in which Z represents -C(O)-N(R14b)-B-, coupling of a corresponding compound of formula VI,
R4-C(O)-L3 VH wherein L3 represents a leaving group and R4 is as defined in Claim 1; (d) for compounds of formula I in which R1 represents C1-12 alkyl (which alkyl group is attached to the oxabispidine iV-atom via a CH2 group and is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl and Het1), reaction of a compound of formula π, as hereinbefore defined, with a compound of formula VIII, Rlx-CHO VHI wherein Rlx represents aryl, Het1 or C1-11 alkyl (which alkyl group is optionally substituted by one or more groups selected from halo, cyano, nitro, aryl and Het1), and Het1 is as defined in Claim 1, followed by reduction in the presence of a reducing agent; (e) for compounds of formula I in which R2 and R3 both represent H and A (if present) represents C1-3 alkylene (optionally substituted by one or more groups selected from F and C1-3 alkyl), reaction of a compound of formula IV, as defined above, with a compound of formula IX,
wherein Za represents -N(R14a)-C(O)-Aa- or -C(O)-N(R14b)-B-, Aa represents C1-3 alkylene (optionally substituted by one or more groups selected from F and C1-3 alkyl) and R4, R14a, R14b and B are as defined in Claim 1, followed by reduction in the presence of a reducing agent;
1*7 1 K T7 I S (f) conversion of one R or R group to another R or R group using techniques well known to those skilled in the art;
(g) for acid addition salts of compounds of formula I, reaction of a corresponding compound of formula I with an acid; or (h) deprotection of a protected derivative of formula I.
40. A compound of formula II, as defined in Claim 39, or a protected derivative thereof.
41. A compound of formula IV, as defined in Claim 39, wherein R1 represents the following structural fragment
42. A compound as claimed in Claim 41, wherein: Rlal represents F;
Rlbl and Rlel both represent H; R c represents Cl, cyano or F.
XXVII
XXVIII
Q ^4 represents C2-4 n-alkylene optionally substituted by one or more substituents selected from halo, methyl and hydroxy; Rla to Rle independently represent H, halo, cyano or C1-2 alkoxy (which latter group is optionally substituted by one or more halo atoms), provided that at least two of Rla to Rle represent H; RPg represents an amino protective group; and R41 to R46 are as defined in Claim 1 , or a protected derivative thereof.
44. A compound as claimed in Claim 43, wherein: R41 to R46 all represent H; Q4 represents (CH2)2;
Rla to Rle all represent H; and if present, RPg represents benzyl or benzenesulfonyl.
45. N-(4-cyanobenzyl)-2-(9-oxa-3,7-diazabicyclo[3.3. l]non-3-yl)acetamide or a salt thereof.
46. 3-(2-Phenylethyl)-9~oxa-3,7-diazabicyclo[3.3.1]nonane or a salt thereof.
47. 4-Cyano-N-methyl-N-[2-(9-oxa-3,7-diazabicyclo[3.3.1]non-3-yl)ethyl]- benzamide or a salt thereof.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE0502774-3 | 2005-12-15 | ||
| SE0502774 | 2005-12-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2007069986A1 true WO2007069986A1 (en) | 2007-06-21 |
Family
ID=38163196
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/SE2006/001426 WO2007069986A1 (en) | 2005-12-15 | 2006-12-14 | New oxabispidine compounds for the treatment of cardiac arrhythmias |
Country Status (4)
| Country | Link |
|---|---|
| AR (1) | AR058382A1 (en) |
| TW (1) | TW200734342A (en) |
| UY (1) | UY30014A1 (en) |
| WO (1) | WO2007069986A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011012896A2 (en) | 2009-07-31 | 2011-02-03 | Astrazeneca Ab | Compounds - 801 |
| US8148373B2 (en) | 2008-02-06 | 2012-04-03 | Astrazeneca Ab | Compounds |
| US8952034B2 (en) | 2009-07-27 | 2015-02-10 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US8962610B2 (en) | 2011-07-01 | 2015-02-24 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9079901B2 (en) | 2010-07-02 | 2015-07-14 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9115096B2 (en) | 2011-05-10 | 2015-08-25 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9193694B2 (en) | 2011-07-01 | 2015-11-24 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001028992A2 (en) * | 1999-10-18 | 2001-04-26 | Astrazeneca Ab | New oxabispidine compounds useful in the treatment of cardiac arrhythmias |
| WO2002083688A1 (en) * | 2001-04-12 | 2002-10-24 | Astrazeneca Ab | 3,7-diazabicyclo [3.3.1] formulations as antiarhythmic compounds |
| WO2004035592A1 (en) * | 2002-10-14 | 2004-04-29 | Astrazeneca Ab | Chemical intermediate |
| WO2005123748A1 (en) * | 2004-06-15 | 2005-12-29 | Astrazeneca Ab | Novel oxabispidine compounds and their use in the treatment of cardiac arrhythmias |
-
2006
- 2006-12-05 TW TW095145210A patent/TW200734342A/en unknown
- 2006-12-14 AR ARP060105514A patent/AR058382A1/en not_active Application Discontinuation
- 2006-12-14 WO PCT/SE2006/001426 patent/WO2007069986A1/en active Application Filing
- 2006-12-15 UY UY30014A patent/UY30014A1/en not_active Application Discontinuation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001028992A2 (en) * | 1999-10-18 | 2001-04-26 | Astrazeneca Ab | New oxabispidine compounds useful in the treatment of cardiac arrhythmias |
| WO2002083688A1 (en) * | 2001-04-12 | 2002-10-24 | Astrazeneca Ab | 3,7-diazabicyclo [3.3.1] formulations as antiarhythmic compounds |
| WO2004035592A1 (en) * | 2002-10-14 | 2004-04-29 | Astrazeneca Ab | Chemical intermediate |
| WO2005123748A1 (en) * | 2004-06-15 | 2005-12-29 | Astrazeneca Ab | Novel oxabispidine compounds and their use in the treatment of cardiac arrhythmias |
Cited By (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8148373B2 (en) | 2008-02-06 | 2012-04-03 | Astrazeneca Ab | Compounds |
| US8629271B2 (en) | 2008-02-06 | 2014-01-14 | Astrazeneca Ab | Compounds |
| US8952034B2 (en) | 2009-07-27 | 2015-02-10 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9371329B2 (en) | 2009-07-27 | 2016-06-21 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| WO2011012896A2 (en) | 2009-07-31 | 2011-02-03 | Astrazeneca Ab | Compounds - 801 |
| US8455483B2 (en) | 2009-07-31 | 2013-06-04 | Astrazeneca Ab | Compounds—801 |
| US8476265B2 (en) | 2009-07-31 | 2013-07-02 | Astrazeneca Ab | Compounds-801 |
| US9079901B2 (en) | 2010-07-02 | 2015-07-14 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9115096B2 (en) | 2011-05-10 | 2015-08-25 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9403782B2 (en) | 2011-05-10 | 2016-08-02 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9682998B2 (en) | 2011-05-10 | 2017-06-20 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9193694B2 (en) | 2011-07-01 | 2015-11-24 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US8962610B2 (en) | 2011-07-01 | 2015-02-24 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9598435B2 (en) | 2011-07-01 | 2017-03-21 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9676760B2 (en) | 2011-07-01 | 2017-06-13 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
| US9695192B2 (en) | 2011-07-01 | 2017-07-04 | Gilead Sciences, Inc. | Fused heterocyclic compounds as ion channel modulators |
Also Published As
| Publication number | Publication date |
|---|---|
| UY30014A1 (en) | 2007-07-31 |
| TW200734342A (en) | 2007-09-16 |
| AR058382A1 (en) | 2008-01-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007069986A1 (en) | New oxabispidine compounds for the treatment of cardiac arrhythmias | |
| US20090270383A1 (en) | Novel Oxabispidine Compounds And Their Use In The Treatment Of Cardiac Arrhythmias | |
| NO343185B1 (en) | Merged heterocyclic derivative, medical composition containing the same as well as medical use thereof | |
| SK5062002A3 (en) | New oxabispidine compounds useful in the treatment of cardiac arrhythmias | |
| ZA200502579B (en) | Chemical intermediate. | |
| KR20200101330A (en) | Amine-substituted heterocyclic compounds as EHMT2 inhibitors, salts thereof, and methods for their synthesis | |
| NZ519497A (en) | New azabicyclooctane derivatives useful in the treatment of cardiac arrhythmias | |
| US20080319198A2 (en) | Novel Oxabispidine Compounds And Their Use In The Treatment Of Cardiac Arrhythmias | |
| US7928225B2 (en) | Oxabispidine compounds for the treatment of cardiac arrhythmias | |
| CA2378603C (en) | Benzofuran derivatives | |
| SK18282001A3 (en) | New bispidine compounds useful in the treatment of cardiac arrhythmias | |
| ZA200503509B (en) | Benzoxazocines and their use as monoamine-reuptake inhibitors | |
| KR20070032330A (en) | Novel oxabispidine compounds and their use in the treatment of cardiac arrhythmias |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 06824525 Country of ref document: EP Kind code of ref document: A1 |