WO2007037134A1 - Inkjet recording medium and method for manufacturing the same - Google Patents
Inkjet recording medium and method for manufacturing the same Download PDFInfo
- Publication number
- WO2007037134A1 WO2007037134A1 PCT/JP2006/318319 JP2006318319W WO2007037134A1 WO 2007037134 A1 WO2007037134 A1 WO 2007037134A1 JP 2006318319 W JP2006318319 W JP 2006318319W WO 2007037134 A1 WO2007037134 A1 WO 2007037134A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- recording medium
- receiving layer
- inkjet recording
- ink receiving
- liquid
- Prior art date
Links
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/50—Recording sheets characterised by the coating used to improve ink, dye or pigment receptivity, e.g. for ink-jet or thermal dye transfer recording
- B41M5/52—Macromolecular coatings
- B41M5/5227—Macromolecular coatings characterised by organic non-macromolecular additives, e.g. UV-absorbers, plasticisers, surfactants
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/50—Recording sheets characterised by the coating used to improve ink, dye or pigment receptivity, e.g. for ink-jet or thermal dye transfer recording
- B41M5/52—Macromolecular coatings
- B41M5/5218—Macromolecular coatings characterised by inorganic additives, e.g. pigments, clays
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/50—Recording sheets characterised by the coating used to improve ink, dye or pigment receptivity, e.g. for ink-jet or thermal dye transfer recording
- B41M5/52—Macromolecular coatings
- B41M5/5254—Macromolecular coatings characterised by the use of polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. vinyl polymers
Definitions
- the present invention relates to an inkjet recording medium, which is medium suitably used for inkjet recording method, and a method for manufact BACKGROUND ART
- the inkjet recording method has been used widely m h addition to office use as a matter of course, in view of such advantages as capa recording on a vanety of recording matenals, relatively inexpensive and comp (apparatus), and excellent quietness
- Charactenstics required for a recording medium for inkjet recording i commonly (1) quick-drying (high ink absorption rate), (2) adequate and unifor ink dots (no bleeding), (3) good graininess, (4) high circulanty of dots, (5) hig concentration, (6) high color saturation (dullness-free), (7) excellent water resi resistance, and ozone resistance of a pnnted area, (8) high whiteness of a recor feeling similar to silver salt photography, and the like, in addition to the above various characteristics
- the recording medium is excellent in ink receiving property ( and has high glossiness
- an inkjet recording medium which h a recording layer having high void fraction and containing fine inorganic pigm and a water-soluble resm (see Japanese Patent Application Laid-Open Nos (JP and 10-217601)
- inkjet recording sheets particularly the inkjet recording maxim recording layer of a porous structure containing silica as the inorganic fine part excellent ink absorbing property owing to the structure thereof, and have high performance that enables formation of an image of high resolution and high gl
- a trace gas m the air, particularly ozone can be a cause for fading of image with age Since the recording materials having a recording layer of the above-mentioned porous structure have a lot of voids, the recorded image is ea ozone in the air Therefore, resistance to ozone gas (ozone resistance) is a very characteristic for a recording mate ⁇ al having a recording layer of the above-de structure
- inkjet recording media to which a liquid prepared by dis compound and a water-soluble polyvalent metal salt is disclosed
- an inkjet recording sheet which has a colorant recei containing cationic polymer-modified inorganic pigment fine particles prepare cationic polymer having a group capable of combining with the inorganic pig particle at the terminal thereof, and the inorganic pigment fine particles
- the present invention has been made in view of the above-described provides an inkjet recording medium and a method for manufacturing the sam
- a first aspect of the invention provides an inkjet recording medium h receiving layer on a support
- the ink receiving layer contains a compound con atom, and the center plane average roughness (SRa value) on the surface of the layer is less than 6 nm when measured under the condition of a cutoff of 2 to 2
- a second aspect of the invention provides an inkjet recording medium receiving layer on a support
- the ink receiving layer contains a sulfoxide-cont compound, and the center plane average roughness (SRa value) on the surface receiving layer is 11 nm or less when measured under the condition of a cutoff
- a third aspect of the invention provides a method for manufacturing t recording medium according to either of the first or second aspect
- a fourth aspect of the invention provides a method for manufacturin recording medium according to either of the first or second aspect
- the metho dispersing at least some of the components in a coating liquid for forming the layer by a head-on collision high-pressure disperser or an orifice-passing high disperser during preparation of the coating liquid
- a fifth aspect of the invention provides a method for manufacturing a recording medium having an ink receiving layer including a compound contai atom
- the center plane average roughness (SRa value) on the surface of the layer is 11 nm or less when measured under the condition of a cutoff of 2 to 2 method includes at least
- first and second liquids contains a compound containing sulfur
- the viscosity liquid before exhibiting the decreasing drying is 6000 Pa or more at a shear rat
- the inkjet recording medium according to the presen be desc ⁇ bed
- An inkjet recording medium has an i layer on a support, the ink receiving layer includes a compound containing a s the center plane average roughness (SRa value) on the surface of the ink recei which an image is to be formed is less than 6 nm when measured under the co cutoff of 2 to 2 5 ⁇ m to 2 5 ⁇ m
- SRa value center plane average roughness
- the first embodiment of the invention differs from the second embod invention in the compound contained in the ink receiving layer (either a comp a sulfur atom or a sulfoxide-containing compound), and the center plane avera (SRa value) (either less than 6 nm, or 11 run or less)
- SRa value center plane avera
- the ink receiving layer according to the invention includes a compou sulfur atom m addition to the major components constituting the ink receiving inorganic fine particles, a water-soluble resin, and a mordant
- containing a sulfur atom is a compound that can be oxidized Accordingly, wh compound is added to the ink receiving layer, it functions to improve the ozon the ink receiving layer
- the compo sulfur atom is added to the ink receiving layer, the surface thereof becomes co image density decreases due to diffused reflection of light
- pr decrease in the image density and improvement in ozone resistance are attaine simultaneously by defining the center plane average roughness (SRa value) on the ink receiving layer [Center plane average roughness (SRa)]
- SRa value center plane average roughness on the 10 nm or less, and more preferable is 8 nm or less in the second embodiment
- the center plane average roughness (SRa) means the average roughn three-dimensionally scanning the roughness on a certain flat plane, and is a di from a center line roughness (Ra value) obtained by scanning a linear roughne plane Concavities and convexities on the surface of a base material are not u are wavy concavities and convexities having a variety of wavelengths
- the ex "measurement under 2 to 2 5 ⁇ m cutoff condition” means a measurement of t and convexities having a wavelength of 2 to 2 5 ⁇ m
- the viscosity of the coating forming the ink receiving layer may be adjusted.
- SRa value center plane average roughness
- the head-on collision high-pressure disperser is a high-pressure dispe disperses the ink receiving layer coating liquid by head-on collision
- the orific Typical examples of high-pressure homogemzers include NANOMI (trade name), manufactured by Nanomizer Corporation, MICROFLUIDIZER manufactured by Microfluidics Corporation, and ULTIMAIZER (trade name) by Sugino Machine Corporation
- the orifice described above refers to a mechanism which has a thin p plate) having minute openings with a shape of a circle or the like inserted in a and which rapidly narrowing the flow path of the straight pipe
- the above-described high-pressure homogemzer is basically compos high-pressure generation section that pressurizes raw mate ⁇ al slurry or the lik collision section or an orifice section
- high-pressure pump which is commonly called a plunger pump
- high-pressure pumps of a variety of types such as a single pump, a d t ⁇ ple pump, pumps of any type can be used in the invention without particular [Compound containing sulfur atom]
- the compound containing a sulfur atom is a compound that can be ox described hereinbefore
- the compound containing a sulfur atom is preferably a compound ha group, or a sulfoxide-containing compound, and particularly preferably a sulfo compound
- the respective compounds are desc ⁇ bed in detail -Compound contaimng a thioether group-
- the compound contaimng a thioether group includes compounds containing a sul aromatic groups bonded to the both sides of the sulfur atom (the following ⁇ 1> compounds containing a sulfur atom and alkyl groups (having preferably four atoms) sandwiching the sulfur atom (the following ⁇ 4> to ⁇ 5>), DL-methionin 2-(ethylthio)ethanol ⁇ 1>
- the content of any of the thioether compound in the ink receiving lay from O 1 to 50 mmol/m , and more preferably from O 2 to 20 mmol/m O
- the sulfoxide-containmg compound having a structure represented b (1) may be substituted by a hydrophilic group
- a hydrophilic group examples of the hydrophilic gr substituted or unsubstituted amino groups, substituted or unsubstituted carba substituted or unsubstituted sulfamoyl groups, substituted or unsubstituted am hydroxyl group, sulfonic acid, carboxyhc acid, phosphoric acid, ethyleneoxy substituted or unsubstituted nitrogen-containing heterocycles
- the sulfoxide-containmg compound is preferably a compo by the following formula (2)
- R 1 and R 3 each independently represent a substituted alkyl group, a substituted or unsubstituted aryl group, a substituted or unsubsti heterocyclic group, or a polymer residue composed of such groups R 1 and R 3 same as or different from each other R 1 and R 3 may combine with each other R represents a substituted or unsubstituted bi- to hexa-valent linking group R with R or R to form a ⁇ ng, or combine with R or R to form a ring m is 0 o or greater n is 0 or 1
- At least one of R , R , and R represents an alkyl group, heterocyclic group, or a polymer residue each of which is substituted by a hyd selected from a substituted or unsubstituted ammo group, a substituted or unsu example, alkyl groups having 1 to 22 carbon atoms are preferable Specificall group is preferably a methyl group, an ethyl group, an ally
- the unsubstituted aryl group represented by R 1 or R 3 is preferably, fo aryl group having 6 to 22 carbon atoms Specific examples thereof include a p 1 -naphthyl group, and a 2-naphthyl group, and a phenyl group is particularly p
- Examples of the unsubstituted heterocyclic group represented by R 1 o thienyl group, a thiazolyl group, an oxazolyl group, a py ⁇ dyl group, a pyrazyl thiadiazoyl group, a t ⁇ azoyl group, a morphoryl group, a piperazyl group, a p a t ⁇ azyl group, an indolyl group, a benzothiazoyl group, and a benzoxazoyl gr others, a thiazolyl group, an oxazolyl group, a py ⁇ dyl group, a thiadiazoyl gro group, a morphoryl group, a pyrimidyl group, a t ⁇ azyl group, a benzothiazoyl benzoxazoyl group are particularly preferred
- R or R represents a polymer residue composed of groups sele substituted or unsubstituted alkyl groups, aryl groups, and heterocyclic residue of the polymer residue is a polymer having any of the following units
- R 4 represents a hydrogen atom, or an alkyl group having 1 to atoms
- R represents an alkylene group
- Q represents a linking group
- L represents 1 or 2
- P represents 1 and n have the same definitions as R , R , m, and n in the formula (2), respecti
- R 6 represents a hydrogen atom, an alkyl group, or an aryl gr
- R 1 or R 3 represents a substituted alkyl, aryl, or heterocyclic gro of the substituent(s) include substituted or unsubstituted amino groups (e g a having 30 or less carbon atoms, an amino group, alkylamino groups, dialkyla arylamino groups, and acylamino groups), substituted or unsubstituted carbam carbamoyl groups having 30 or less carbon atoms, a carbamoyl group, a meth group, a dimethylcarbamoyl group, a morpholinocarbamoyl group, and a pipe group), substituted or unsubstituted ammoniums (e g ammoniums having 30 atoms, ammonium, trimethylammonium, t ⁇ ethylammomum, dimethylbenzyla hydroxyethyldimethylammonium), substituted or unsub
- R 1 and R 3 may be the same as or different from each other, and may each other to form a ⁇ ng
- R represents a substituted or unsubstituted divalent to hexavalent lin may be bonded to R or R , or R or R to form a ⁇ ng
- Examples of the sulfur heterocycle formed by such a bonding include a thienyl group, a thiazoyl gro group, a dithiolan-2-yl group, a t ⁇ thian-2-yl group, and a dithian-2-yl group
- the sulfoxide-contaming compound according to preferably water-soluble
- Such a sulfoxide-contaming compound is a Lewis base, which has hi in water than a thioether compound Therefore, the sulfoxide-contaming co added in a larger amount than a thioether compound
- the sulfoxide-containing compound according to the invention it is preferred to add the sulfoxide-contaming compound to a coating liquid or containing the after-mentioned fine particles and water-soluble resin
- the sulfoxide-contaming compound according to the invention is preferred to add the sulfoxide-contaming compound to the coating liquid or containing fine particles and a water-soluble resin after the sulfoxide-contami emulsified or after the sulfoxide-contaming compound is added to an organic solvent
- the conten sulfoxide-containing compound is preferably 0 01 to 20 g/m , and more prefer g/m in view of further improvement in ozone resistance, resistance to bleed (i with age, and glossiness
- the sulfox compound which generally has a higher oxidation potential than conventional sulfur-containing compounds (thioethers, thioureas), can achieve higher ozone higher light resistance when combined with a supe ⁇ or colorant having a high potential for the sake of improving the ozone resistance and the light resistanc
- the fine partic as primary particles, or after forming secondary particles The average prim diameter of these fine particles is preferably 2 ⁇ m or less, more preferably 20
- silica fine particles with an average primary particle dia or less colloidal silica with an average primary particle diameter of 30 nm or l fine particles with an average primary particle diameter of 20 nm or less, and pseudo-boehmite with an average fine pore diameter of 2 to 15 nm are more p the silica fine particles, alumina fine particles and pseudo-boehmite are partic preferable
- the silica fine particles are roughly classified into wet method particl method (gas phase method) particles depending on their production method example of the wet method, active silica is formed by acidolysis of a silicate s silica is polymerized to an adequate degree, and then is coagulated and precipi hydrated silica
- active silica is formed by acidolysis of a silicate s silica is polymerized to an adequate degree, and then is coagulated and precipi hydrated silica
- an is obtained by hydrolysis of silicon hahde in gas phase at high temperature (fla method), or silica sand and coke are vaporized by reduction by heating with ar furnace, and the product thereof is oxidized with air (arc method)
- the "gas means anhydrous silica fine particles obtained by the gas phase method
- the silica fine particles are particularly preferable as the silica fine particles used
- the gas phase silica exhibits different properties from hydra the difference in the density of the silanol groups on the surface and in the pro voids, the gas phase silica is suitable for forming a three-dimensional structure void ratio While the reason thereof is not clear, the density of the silanol gro surface of the fine particles is as large as 5 to 8 groups/nm in the case of hydr thus the silica particles easily aggregate In contrast, the density of the silano surface of the fine particles is as small as 2 to 3 groups/nm m the case of gas p density and good glossiness of colors, not only m the uses requiring high tran an OHP film, but also in an application as a recording sheet such as a photogr paper
- the average primary particle diameter of the inorganic fine particles silica is preferably 50 nm or less, more preferably from 3 to 50 run, still mor from 3 to 30 nm, particularly preferably 3 to 20 nm, and most preferably 3 to of the quick drying property (ink absorption rate) Since the gas phase silica p liable to be coagulated with each other due to hydrogen bonds between the sil structure having a large void ratio can be formed when the average primary p is 50 nm or less, and ink absorbing charactenstics can be effectively improved
- gas phase silica may be used together with other inorganic fine p those described above
- the content of gas phase silica is preferably 30 mass more preferably 50 mass % or more, when the gas phase silica is used togethe particles
- Alumina fine particles, alumina hydrate, and a mixture or composite preferable as the inorganic fine particles used in the invention The alumina preferable among them since it absorbs ink well and fixes the ink, and pseudo (AI 2 O 3 11H 2 O) is particularly preferable While various forms of the alumina used, boehmite sol is preferably used as the raw material since a smooth layer obtained
- the fine void structure of pseudo-boehmite preferably has an average diameter of 1 to 30 nm, more preferably 2 to 15 nm
- the fine void volume is to 2 0 cc/g, more preferably 0 5 to 1 5 cc/g
- the fine void diameter and fine measured by a nitrogen absorption-desorption method using, for example, a g absorption-desorption analyzer (for example, OMNISORP 369 manufactured Coulter, Inc )
- the gas phase alumina fine particles are preferable among the alumn [Water-soluble resm]
- water-soluble resin used in the ink receiving layer i alcohol resins (e g , polyvinyl alcohol (PVA), acetoacetyl-modif ⁇ ed polyvinyl cation-modified polyvinyl alcohol, anion-modified polyvinyl alcohol, silanol- polyvinyl alcohol and polyvinyl acetal), which are resins having hydroxyl gro hydrophilic structural units, cellulose resins (methyl cellulose (MC), ethyl cell hydroxyethyl cellulose (HEC), carboxymethyl cellulose (CMC), hydroxyprop (HPC), hydroxyethylmethyl cellulose and hydroxypropylmethyl cellulose), ch starches, resins having ether bonds (polyethylene oxide (PEO), polypropylene polyethyleneglycol (PEG) and polyvinyl ether (PVE)), and resins having carb (polyacrylamide (PAAM), polyvinyl pyrrohdone (PVP) and polyacrylic acid
- PVA poly
- polyacrylic acid salts include polyacrylic acid salts, maleic acid resins, algi and gelatins, having carboxylic groups as dissociation groups
- the polyvinyl alcohol resins are particularly preferable among the re Examples of the polyvinyl alcohol resins are desc ⁇ bed in Japanese Patent App Publication (JP-B) Nos 4-52786, 5-67432 and 7-29479, Japanese Patent No No 7-57553, Japanese Patent Nos 2502998 and 3053231, JP-ANo 63-17617 Patent No 2604367, JP-A Nos 7-276787, 9-207425, 11-58941, 2000-135858, 2001-287444, 62-278080 and 9-39373, Japanese Patent No 2750433, JP-A N 2000-158801, 2001-213045, 2001-328345 and 8-324105, 11-348417
- the content of the water-soluble resm of the invention is preferably 9 more preferably 12 to 33 mass %, relative to the mass of the total solid content receiving layer more preferably 80 to 99 5%
- the polyvinyl alcohol resin has hydroxyl groups in its structur dimensional network structure with secondary particles of the silica fine parti chain units is readily formed since hydrogen bonds are formed between the h and the silanol groups on the surface of the silica fine particles It is conside receiving layer having a porous structure with a high void ratio and sufficient formed owing to the formation of the three dimensional network structure
- porous ink receiving layer obtained as described above rapidly a capillary action during inkjet recording, and thus dots of good circularity can without ink bleed
- the polyvinyl alcohol resin may be used together with other water-s
- the content of polyvinyl alcohol resin in the total water-soluble resins is pref mass % or more, more preferably 70 mass % or more, when the polyvinyl alc used together with other water-soluble resins ⁇ Composition ratio between fine particles and water-soluble resin>
- PB ratio mass composition ratio of inorganic fine particle water-soluble resin (y) largely affects the structure and strength of the ink rece While the void ratio, fine void volume and surface area (per unit mass) increa composition ratio (PB ratio) increases, the density and strength tend to be low
- the mass composition ratio (PB ratio, (x/y)) m the ink receiving laye invention is preferably in the range of 1 5 to 10, so as to prevent decrease in t and generation of cracks at drying resulting from an excessively large PB rati prevent decrease in ink absorbing property accompanying reduction of void ra easily occurring filling of voids with the resin resulting from an excessively s
- the ink receiving layer should have sufficient film strength strength of the ink receiving layer is required also for preventing cracks and p the coating liquid onto a support, and then drying the coated layer, whereby a light-transmitting porous layer with an average fine void diameter of 30 run or ratio of 50 to 80%, a specific void volume of 0 5 ml/g or more, and a specific 100 m /g or more can be readily formed (Crosshnking agent)
- the ink rec preferably contains a water-soluble resin
- the mk receiving layer is preferab layer obtained by forming the coated layer containing the sulfoxide-containin cation polymer, the inorganic fine particles, the water-soluble resin and a cross capable of crosshnking the water-soluble resin, and curing the coated layer thr crosshnking reaction between the crosshnking agent and the water-soluble res
- Boron compounds are preferably used for crosshnking the water-solu particularly polyvinyl alcohol resin
- the boron compound include acid, borate (for example orthoborate, InBC> 3 , ScBO 3 , YBO 3 , LaBC> 3 , Mg 3 (BO C ⁇ 3 (B ⁇ 3 )2), diborate (for example Mg2B2 ⁇ 5, CO2B2O5), methaborate (for exa Ca(BO 2 ) 2 NaBO 2 and KBO 2 ), tetraborate (for example Na 2 B 4 O 7 10H 2 O), and (for example KBsOg 4H 2 O, Ca 2 BoOn 7H 2 O, and CsB 5 Os) Borax, boric acid preferable since they can cause crosshnking reaction quickly, and boric acid is preferable
- crosshnking agents examples include aldehyde compou formaldehyde, glyoxal and glutaraldehyde, ketone compounds such as diacetyl cyclopentanedione, active halogen compounds such as bis(2-chloroethylurea), 2-hydroxy-4,6-dichloro-l,3,5-t ⁇ azine, 2,4-dichloro-6- t ⁇ azine sodium salt, act compounds such as di vinyl sulfonic acid, l,3-divinylsulfonyl-2-propanol, N,N'-ethylenebis(vinylsulfonylacetamide), and 1 ,3,5-triaclyroyl-hexahydro-S- such as 2,3-dihydroxydioxane, metal-containmg compounds such as titanium aluminum sulfate, chromium alum, potassium alum, zirconium acetate and ch poly
- crosslinking agent selected from the above may be use more crosslinking agents selected from the above may be used in combination
- crosslink curing is preferably carried out in manner a crosslinking agent is added to a coating liquid containing inorganic water-soluble resin and the like (hereinafter occasionally referred to as "first li a basic solution having a pH of 7 1 or higher (hereinafter occasionally referre liquid”), onto the coated layer formed by application of the first liquid, the sec applied (1) simultaneously with the application of the first liquid for forming t or (2) before the coated layer exhibits decreasing drying during drying of the c formed by application of the first liquid
- the compound containin is contained in either of the first or second liquid
- Application of the crosslinki preferably conducted as follows when a boron compound is used as an exampl the ink receiving layer is a layer obtained by crosslink-curing of a coated layer application of the coating liquid (first liquid) containing fine particles and a w resin containing polyvinyl alcohol, the crosslink cu ⁇ ng is earned out by apply solution having a pH of 7 1 or higher (the second liquid) onto the coated layer simultaneously with the application
- the amount of crosslinking agent to be used is preferably from 1 to 5 more preferably from 5 to 40 mass% with respect to the water-soluble resin
- Specific examples thereof include calcium acetate, calcium chloride, calcium sulfate, calcium butyrate, barium acetate, barium sulfate, barium pho oxalate, barium naphthoresorcin carboxylate, barium butyrate, manganese chl manganese acetate, manganese formate dihydrate, ammonium manganese sul cupnc chloride, ammonium copper (II) chloride dihydrate, copper sulfate, co copper oxalate, copper phthalate, copper citrate, copper gluconate, copper nap chlo ⁇ de, cobalt thiocyanate, cobalt sulfate, cobalt (II) acetate, cobalt naphthe sulfate hexahydrate, nickel chloride hexahydrate, mckel acetate
- water-soluble compounds contaimng titanium or zirconium are more pr
- a water-soluble compound contaimng titanium include titanium titanium sulfate, titanium tetrachloride, tetraisopropyl titanate, titanium acetyl titanium lactate
- the water-soluble polyvalent metal compound is ad amount of 0 1 to 10 mass %, and more preferably 0 5 to 8 mass % with respec inorganic fine particles [Mordant] mordants and inorganic mordants each may be used alone In an embodime organic mordants and one or more inorganic mordants are used in combinatio
- the catiomc mordant a polymer mordant having, as a cationic gro secondary, or tertiary amino group or a quaternary ammonium base is used in
- the cationic mordant may be a cationic non-polymer mordant in the
- non-mordant mo polymer mordants examples include homopolymers of a mono monomer) having a primary, secondary, or tertiary amino group, or a salt ther quaternary ammonium base, copolymers or condensation polymers of the mor and one or more other monomers (hereinafter referred to as "non-mordant mo polymer mordants may be used m the form of a water-soluble polymer or wat latex particles
- Examples of the monomer include t ⁇ methyl-p-vmylbenzylammomum chlo ⁇ de, trimethyl-m-vinylbenzylammom t ⁇ ethyl-p-vinylbenzylammonium chlo ⁇ de, t ⁇ ethyl-m-vinylbenzylammonium N,N-dimethyl-N-ethyl-N-p-vinylbenzylammonium chloride, N,N-diethyl-N-methyl-N-p-vmylbenzylammomum chloride, N,N-dimethyl-N-n-propyl-N-p-vinylbenzylammonium chlo ⁇ de, N ⁇ -dimethyl-N-n-octyl-N-p-vinylbenzylammonium chloride, N,N-dimethyl-N-benzyl-N-p-vmylbenzylammonium chlo ⁇ de, N
- mordant monomer examples include N-vinylimidazole,
- N-vinyl-2-methylimidazole 2-vinylpy ⁇ dine, 4-vinylpyndine, 4-vmyl-N-meth chloride, 4-vinyl-N-ethylpyndmium bromide, dimethyldiallylammonium chlo monomethyldiallylammonium chloride
- mordant monomers Only one of such mordant monomers may be used, or two or more co mordant monomers may be used in combination
- the non-mordant monomer refers to a monomer that does contain a b (meth)acrylates (such as cyclohexyl (meth)acrylate), aryl methacrylates (such (meth)acrylate)), aralkyl (meth)acrylates (such as benzyl(meth)acrylate), subs (meth)acrylates (such as 2-hydroxyethyl (meth)acrylate, methoxymethyl (met allyl (meth)acrylate), (meth)acrylamides (such as (meth)acrylamide, dimethyl (meth)acrylamide, N-ethyl (meth)acrylamide, and N-isopropyl (meth)acrylam vmyls (styrene, vinyltoluene and ⁇ -methylstyrene), vinyl esters (such as vinyl propionate and vinyl versatate), allyl esters (such as allyl acetate) halogen-co monomers (such as vmyh
- non-mordant monomer Only one non-mordant monomer may be used, or two or more non-m monomers may be used in combination
- polymer mordant examples include polyethyleneimine (and de thereof), polyvinylamine (and denvatives thereof), polyallyamme (and derivat polyamidme, cationic polysaccharides (such as cationic starch and chitosan), d resins (such as dicyan diamide-formahn polycondensate), polyamine cationic dicyan diamide-diethylenet ⁇ amine polycondensate), epichlorohydrm-dimethy polymers, and dimethyldiallylammonium chloride-sulfur dioxide copolymer
- Polymers having a quaternary ammonium base are preferable, and (m polymers, vinylbenzylammonium polymers and diallylammonium polymers h average molecular weight of 1,000 to 100,000 and having a quaternary ammo particularly preferable as the organic mordant in the invention
- the content of the mordant in the ink receiving layer from 0 01 to 10 g/m , more preferably from 0 1 to 5 g/m
- the ink receiving layer coating liquid (first liquid) preferably contain as the surfactant, cationic surfactants, anionic surfactants, noniomc surfactant surfactants, fluorosurfactants, and silicone surfactants are all usable
- noniomc surfactants examples include polyoxyalkylene glycerin fatty acid esters (such as glycerol monooleate), polyoxyethylene glyc esters (such as monostearic acid polyoxyethylene glycerin and monooleic aci polyoxyethylene glycerin), polyoxyethylene fatty acid esters (such as polyeth monolaurate, and polyethyleneglycol monooleate), polyoxyethylene alkylami acetylene glycols (such as 2,4,7,9-tetramethyl-5-decyn-4,7-diol, and ethylene and propylene oxide adducts of the diol) Polyoxyalkylene alkylethers are p them
- the nonionic surfactant may be used in the first solution and the seco Only one noniomc surfactant may be used, or two or more nonionic surfactan in combination
- amphoteric surfactants include those of amino acid type carboxyamonium betaine type, sulfonammonium betame type, ammonium sul betaine type and lmidazohum betame type, and those desc ⁇ bed in USP No 3, Nos 59-49535, 63-236546, 5-303205, 8-262742 and 10-282619 may be favor
- the amphoteric surfactant may be an amphoteric surfactant of amino acid typ de ⁇ ved from an ammo acid (such as glycme, glutamic acid or histidme) as de No 5-303205
- the amphoteric surfactant may be an N-aminoac long chain acyl group introduced thereto, or a salt thereof Only a single am surfactant may be used, or two or more amphoteric surfactants may be used in
- anionic surfactants include fatty acid salts (for example, and potassium oleate), salts of alkylsulfuric acid esters (for example, sodium l and t ⁇ ethanolamine lauryl sulfate), sulfonic acid slats (for example, sodium d sulfonate), alkylsulfosuccinic acid salts (for example, sodium dioctylsulfosucc alkyldiphenylether disulfonic acid salts, and alkylphospho ⁇ c acid salts
- cationic surfactants include alkylamine salts, quaternary salts, py ⁇ dinium salts and lmidazohum salts
- fluorosurfactants include a compound derived from an i having a perfluoroalkyl group using a method such as electrolytic fluo ⁇ nation, organic group, a structure in which the both terminals of a siloxane structure a with the organic group, or a structure in which one of the terminals of a siloxa modified with the organic group
- the content of surfactant is preferably from 0 01 to 2 preferably from 0 01 to 1 0%, relative to the ink receiving layer coating liquid
- the content of surfactant is preferably from 0 01 to 2 preferably from 0 01 to 1 0%, relative to the ink receiving layer coating liquid
- the ink receiving layer preferably contains a high bo organic solvent for preventing curling
- the high boiling point organic solve compound having a boiling point of 150 0 C or higher at atmospheric pressure, water-soluble or hydrophobic compound
- the high boiling point organic sol solid or liquid at room temperature and may be a low molecular weight comp molecular weight compound
- organic solvent examples include aromatic carboxylic acid est dibutyl phthalate, diphenyl phthalate and phenyl benzoate), aliphatic carboxyli (such as dioctyl adipate, dibutyl sebacate, methyl stearate, dibutyl maleate, dib and t ⁇ ethyl acetylcitrate), phosphoric acid esters (such as trioctyl phosphate a phosphate), epoxy compounds (such as epoxylated soy bean oil and epoxy late methyl esters), alcohols (such as stearyl alcohol, ethyleneglycol, propylenegly diethyleneglycol, triethyleneglycol, glyce ⁇ n, diethyl enegly col monobutylethe triethyleneglycol monobutylether, glycerin monomethylether, 1,2,3-butanet ⁇ o 1,2,4-butanetnol, 1 ,2,4-pentanet ⁇ ol, 1,2,6
- the material used for the transparent support is preferably transparen to radiant heat generated when used with an OHP or a backlight display
- Ex material include polyesters such as polyethylene terephthalate (PET), polysul polyphenylene oxide, polyimide, polycarbonate and polyamide Polyesters and polyethylene terephthalate is particularly preferable among them
- the thickness of the support is not particularly restricted, the th preferably 50 to 200 ⁇ m from the viewpoint of ease of the handling
- the opaque support having high glossiness preferably has a surface of 40% or more on which the ink receiving layer is to be provided
- the glos measured according to the method (a 75 degree specular glossiness test metho sheets and paper board) defined in Japanese Industrial Standards (JIS) P-8142, incorporated herein by reference Specific examples include the following s
- Examples include highly glossy paper supports such as art paper, coa cast-coat paper, and baryta paper used for silver salt photographic support, hig (which may have been subjected to a surface calendering treatment) comprisi that has been made opaque by adding a white pigment or the like, wherein the may be a polyester such as polyethylene terephthalate (PET), a cellulose ester nitrocellulose, cellulose acetate or cellulose acetate butylate, polysulfone, poly oxide, polyimide, polycarbonate or polyamide, and supports in which a coated polyolefin, which contains or does not contain a white pigment, on the surface various paper supports as described above, a transparent support as descnbed highly glossy film containing a white pigment or the like
- Foamed polyester films contaimng a white pigment for example, foa contains polyolefin fine particles and voids formed by stretching
- a white pigment for example, foa contains polyolefin fine particles and voids formed by stretching
- the thickness of the opaque support is not particularly rest ⁇ cte polyester fibers, as necessary While any one of LBKP, LBSP, NBKP, NBSP, LUKP and NUKP may be used as the wood pulp, it is preferable to use a great LBKP, NBSP, LBSP, NDP and LDP, which contain a high proportion of short other wood pulps
- the proportion of LBS and/or LDP is preferably 10 mass % mass % or less
- Chemical pulps (such as sulfate pulp and sulfite pulp) contaimng littl be preferably used, and a pulp whose b ⁇ ghtness has been improved by a bleac is also useful
- the following agents may be added to the raw paper sheet as necessa agent such as a higher fatty acid and alkylketene dimer, white pigment such as carbonate, talc and titanium oxide, a paper strength enhancer such as starch, p and polyvinyl alcohol, a fluorescent b ⁇ ghtener, a humectant such as polyethyl dispersing agent, a softening agent such as quaternary ammonium, and the like
- necessa agent such as a higher fatty acid and alkylketene dimer, white pigment such as carbonate, talc and titanium oxide, a paper strength enhancer such as starch, p and polyvinyl alcohol, a fluorescent b ⁇ ghtener, a humectant such as polyethyl dispersing agent, a softening agent such as quaternary ammonium, and the like
- the freeness of the pulp to be used for paper-making is preferably fro ml as defined in CSF Regarding the fiber length after beating, the sum of th mass of the 24 mesh filtration residue and the percentage by mass of the 42 me residue is preferably 30 to 70 mass % Such mesh filtration residues are define which is incorporated herein by reference The percentage by mass of the 4 residue is preferably 20mass % or less
- the basis weight of the raw paper is preferably from 30 to 250 g/m 2 , preferably from 50 to 200 g/m
- the thickness of the raw paper is preferably ⁇ m
- High smoothness can be rendered to the raw paper by applying a calend during paper making or after paper making
- the density of the raw paper is u to 1 2 g/m 3 (JIS P-8118, which is incorporated herein by reference)
- the rigidity of the raw paper is preferably from 2 to 20 mN m under t according to JIS P-8125, which is incorporated herein by reference polyethylene (HDPE)
- HDPE polyethylene
- LLDPE, polypropylene, and the like may als component
- the polyethylene layer on the side to be provided with the ink receivi preferably obtained by adding titanium oxide of rutile or anatase type, fluores and ultramarine blue to polyethylene such that opaqueness, whiteness, and hu as widely adopted in photographic paper
- the content of titanium oxide relat polyethylene is preferably from 3 to 20 mass %, more preferably from 4 to 13 While the thickness of the polyethylene layer is not particularly rest ⁇ cted, a th 50 ⁇ m is favorable for both the layers on the front and back sides
- the thickness of the undercoat layer is preferably fro
- the polyethylene coated paper may be used as glossy paper, or may b having such a matte surface or silky surface as realized in usual photographic so-called embossing treatment is conducted when polyethylene is coated on th melt-extrusion
- a back coat layer may be provided on the support, and components th to the back coat layer may be, for example, a white pigment, an aqueous binde
- white pigments such as light calcium carbonate, heavy calcium carbonate, kaol calcium sulfate, barium sulfate, titanium dioxide, zinc oxide, zinc sulfide, zmc satm white, aluminum silicate, diatomaceous earth, calcium silicate, magnesiu synthetic amorphous silica, colloidal silica, colloidal alumina, pseudo-boehmit hydroxide, alumina, hthopone, zeolite, hydrated halloysite, magnesium carbon magnesium hydroxide, and organic pigments such as styrene-based plastic pig plastic pigments, polyethylene, microcapsules, urea resin and melamine resin
- aqueous binder usable in the back coat layer examples include ⁇ Method for manufacturing inkjet recording medium>
- a method according to an embodiment of the invention for manufact recording medium is a method for manufacturing the inkjet recording maxim invention
- the method includes forming an ink receiving layer by a process in the following (A) and (B), and at least one of the following first liquid and the contains a compound containing sulfur
- a sulfoxide-containing compound is used as the containing a sulfur atom
- a method for manufacturing an in medium of the invention is a method for manufacturing an inkjet recording m ink receiving layer including a compound containing a sulfur atom, wherein th average roughness (SRa value) on the surface of the ink receiving layer is 11 r measured under a condition of a cutoff of 2 to 2 5 ⁇ m
- the method includes fo receiving layer by a process including at least the following (A) and (B), at lea following first and second liquids contains a compound containing a sulfur ato viscosity of the first liquid before exhibiting decreasing drying is 6000 Pa or rate of 1 s l
- the compound containing a sulfur atom may be contained i and second liquids, it is more preferably contained in the first liquid in view o condition of the ink receiving layer
- the first liquid preferably contains the inorganic fine the coating liquid for forming an ink receiving layer
- the first liquid contains inorganic fine particles (e g gas phase silica) and a water-soluble resin (e g p alcohol)
- the coating liquid can be prepared, for example, in the following ma inorganic fine particles such as gas phase silica and a dispersing agent are add 10 to 20 mass % of silica fine particles in the water)
- the mixture is dispersed of high-speed rotation of, for example, 10000 rpm (preferably 5000 to 20000 r example, 20 minutes (preferably 10 to 30 minutes) by using a beads mill (for ' KD-P" manufactured by Shima Enterprise Co , Ltd )
- a sulfoxide- compound compound contaimng a sulfur atom
- PV solution polyvinyl alcohol
- the amount of PVA is, for example amount that the mass of PVA is one-third of the mass of the gas phase silic
- a previously-prepared water dispersion of the gas phase silica may b
- SRa value center plane average roughness
- the solvent used in each process may be water, an organic solvent or liquids selected from water and organic solvents
- Organic solvents usable fo include alcohols such as methanol, ethanol, n-propanol, l-propanol and metho ketones such as acetone and methylethyl ketone, tetrahydrofuran, acetonitnle, and toluene
- a dispersing agent may be added for improving dispersibility of the c
- the dispersing agent is not particularly limited, and may be a known cationic agent
- the amount of the dispersing agent to be added is preferably from 0 1 preferably from 1 to 10%, relative to the amount of the inorganic fine particles
- the pH of the first liquid is not particularly restricted, and is preferab 6 0, more preferably from 3 to 5 Bleeding of image with time can be suppre ink receiving layer is formed from the coating liquid having a pH of 2 to 6
- the ink receiving layer coating liquid (first liquid) can be applied by coating method using an extrusion die coater, an air doctor coater, a blade coat a knife coater, a squeeze coater, a reverse roll coater or a bar coater
- the second liquid is applied onto the coated layer (i) simultaneously application of the coating liquid for forming an ink receiving layer (first liquid the coated layer exhibits decreasing drying during drying of the coated layer f application of the first liquid
- the ink receiving layer is suita manufactured by introducing the second liquid during constant drying of the c liquid caused by the crosslinking agent does not proceed sufficiently, so that t where bronzing occurs or defects such as cracking occurs in the ink receiving
- the second liquid may be prepared, for example by adding a metal c to 5%), a basic compound (e g 1 to 5%), and optionally paratoluenesulfonic a 3%) to ion-exchange water, and agitating the resultant mixture liquid sufficien values for the respective components each mean a mass % of the solid content
- the expression "before the coated layer exhibits decreasing drying ra refers to a process within a few minutes from immediately after the applicatio liquid for forming an ink receiving layer, during which a phenomenon, "const is observed
- the constant drying rate refers to a proportional decrease in conte solvent (dispersion medium) in the coated layer to time
- the time during whic drying rate is observed is desc ⁇ bed, for example, in 'Kagaku Kogaku Binran Chemical Enginee ⁇ ng) (pages 707 to 712, published from Maruzen Co , Ltd 1980)
- the coated layer is d ⁇ ed after the first liquid is a coated layer exhibits a constant rate of drying
- the drying is earned 18O 0 C for 0 5 to 10 minutes (preferably 0 5 to 5 minutes)
- the drying time nat depending on the amount of the coating liquid to be applied, but the above ran preferable
- the viscosity at a shear rate of 1 s * of the first liquid before exhibitin drying is preferably 6000 Pa or more, more preferably 7000 Pa or more, and p preferably from 8000 to 10000 Pa
- the center plane a roughness (SRa) on the surface of the ink receiving layer measured under a co cutoff of 2 to 2 5 ⁇ m can be 11 nm or less
- the viscosity may be measured by sampling the coating liquid from t when the coating liquid is dried until immediately before the coated layer exhi of immersing the support having the coated layer provided thereon in the seco the like
- the method usable for applying the second liquid in the method (1) coating method such as methods using a curtain flow coater, an extrusion die doctor coater, a blade coater, a rod coater, a knife coater, a squeeze coater, a re or a bar coater
- methods in which the coater does not directly cont formed first coated layer are preferable, such as an extrusion die coater, a curt and a bar coater
- the ink receiving layer is usually he 180 0 C for 0 5 to 30 minutes so as to be dried and cured Heating to 40 to 15 minutes is particularly preferable
- the simultaneous application can be performed b method using, for example, an extrusion die coater or a curtain flow coater coated layer is d ⁇ ed after the simultaneous application
- the drying is conduct heating the coated layer to 40 to 150 0 C for 0 5 to 10 minutes, preferably to 40 0 5 to 5 minutes
- water-soluble resin is added as a thickener in consideration of coatability
- water-soluble resin may be a polymer, whose examples include cellulose resi hydroxylpropylmethyl cellulose, methyl cellulose and hydroxyethyl cellulose pyrrohdone and gelatin
- the barrier layer liquid may contain a mordant suc described above
- the roll temperature at the calender treatment is preferably from 30 t preferably from 40 to 100 0 C
- the linear pressure between the rolls at the calender treatment is pref to 400 kg/cm, more preferably from 100 to 200 kg/cm
- the thickness of the ink receiving layer sh determined in relation to the void ratio m the layer For example, the thickne about 15 ⁇ m or more when the amount of ink is 8 nL/mm and the void ratio i
- the thickness of the ink receiving layer is pr 10 to 50 ⁇ m in the case of ink-jet recording
- the diameter of the void m the ink receiving layer is preferably from ⁇ m, more preferably from 0 01 to 0 025 ⁇ m, m terms of a median diameter
- the void ratio and median diameter can be measured with a mercury (trade name PORESIZER 9320-PC2, manufactured by Shimadzu Corporation
- the pH of the surface of the ink receiving layer of the invention is pr to 6, more preferably from 3 5 to 4 5
- the pH on the surface is measured in 3
- the ink receiving layer preferably has high transparency
- the haze value is p less, more preferably 15 or less
- the haze value can be measured with a haze meter (trade name HG manufactured by Suga Test Instrument Co , Ltd )
- a dispersion of polymer fine particles may be added to the layers con mkjet recording medium according to the invention (for example, the ink rece back layer)
- This polymer fine particle dispersion is used for improving film as dimensional stability, prevention of curl, prevention of adhesion and preven
- the polymer fine particle dispersion is desc ⁇ bed, for example m JP-A Nos 62 62- 1316648 and 62- 110066 Cracking and curling of the layer can be preven polymer fine particle dispersion having a low glass transition temperature (40 0 layer contaimng the mordant Curling may be also prevented by adding a po particle dispersion having a high glass transition temperature to the back layer of Japanese Patent Application No 2005-282489 is incorporated herein by ref entirety
- An inkjet recording medium comprising an ink receiving layer p support, wherein the ink receiving layer includes a compound containing a sul the center plane average roughness (SRa value) on the surface of the ink recei less than 6 nm when measured under a condition of a cutoff of 2 to 2 5 ⁇ m
- SRa value center plane average roughness
- An inkjet recording medium composing an ink receiving layer pr pH of the surface of the ink receiving layer is from 3 to 6
- Pulp slurry was prepared by beating 50 parts of LBKP made of acaci LBKP made of aspen with a disk free refiner to a Canadian Freeness of 300 m
- the pulp slurry prepared as desc ⁇ bed above was used for papermak fourd ⁇ nier
- the felt face of the resulting web was pushed forcibly to a drum d a dryer canvas so as to be d ⁇ ed
- the tensile force of the dry set at 1 6 kg/cm
- lg/m 2 of polyvinyl alcohol (trade name KL-118, manu Kuraray Co , Ltd ) was applied onto both sides of the raw paper with a size pre
- Gas phase silica fine particles (inorganic fine particles) 8 9 par (trade name AEROSIL 300SF75, manufactured by Nippon Aerosil C
- the front surface of the support was subjected to corona discharge tre 170 ml/m 2 of the first liquid together with 10 8 ml/m 2 of five-fold diluted aque poly aluminum chlo ⁇ de (trade name "ALUFESfE 83", manufactured by Taim Co , Ltd ) were in-line coated on the front surface of the support (coating proc thickness was 32 ⁇ m was obtained ⁇ Composition of second hquid>
- An mkjet recording medium was produced in the same manner as in except that the compound A contained in the coating liquid A (first liquid) for receiving layer was replaced by the following compound B (a compound cont groups), and 0 8 part of ethanol was also contained in the coating liquid A
- Compound B An lnkjet recording medium was produced in the same manner as in except that the compound A was not contained in the coating liquid A (first li an ink receiving layer, and 2 3 parts of ethanol was also contained m the coati [Comparative example 2]
- An inkjet recording medium was produced in the same manner as m except that 2 3 parts of ethanol was also contained in the coating liquid A (firs forming an ink receiving layer [Comparative example 3]
- An inkjet recording medium was produced m the same manner as in Example 2 except that the compound A contained in the coating liquid A (first forming an ink receiving layer was replaced with the same amount of the com [Evaluation]
- the center plane average roughness (SRa value) was measured with manufactured by Zygo Corporation at a cutoff of 2 to 2 5 ⁇ m based on the foll measurement and analysis conditions ⁇ Measurement and analysis conditions>
- the coating liquid which was drie coated layer exhibited a decreasing drying rate was sampled from the support, was measured with RHEOSTRESS 600 according to the following evaluation criteria Evaluation criteria
- a black solid image was printed on each of the recording media with (trade name "PMG-800C", manufactured by Seiko-Epson Corporation) loade genuine ink set
- the concentration in the black area was measured with a refle densitometer (trade name XRITE 938, manufactured by Xrite Corporation)
- an inkjet recording medium can be provi excellent ozone resistance and capable of suppressing decrease in image densi manufacturing such an mkjet recording medium is also provided
Landscapes
- Ink Jet Recording Methods And Recording Media Thereof (AREA)
- Ink Jet (AREA)
Abstract
An inkjet recording medium comprising an ink receiving layer provided on a support. The ink receiving layer includes a compound containing a sulfur atom, and a center plane average roughness (SRa value) on a surface of the ink receiving layer is less than 6 nm when measured under a condition of a cutoff of 2 to 2.5 m.
Description
DESCRIPTION
INKJET RECORDING MEDIUM AND METHOD FOR MANUFACTURI
TECHNICAL FIELD
The present invention relates to an inkjet recording medium, which is medium suitably used for inkjet recording method, and a method for manufact BACKGROUND ART
In recent years a vanety of information processing systems such as a recording method, a thermal recording method, a pressure sensitive recording photosensitive recording method, and a transfer-type recording method have b with rapid advancements in information-technology industry, and recording m recording instruments suitable for these information processing systems have developed and put into practical use
Among others, the inkjet recording method has been used widely m h addition to office use as a matter of course, in view of such advantages as capa recording on a vanety of recording matenals, relatively inexpensive and comp (apparatus), and excellent quietness
With an increase m resolution of inkjet pπnters in recent years, it has possible to obtain a high-quality recorded material, a so-called photo-like reco vanety of recording sheets for inkjet recording have been developed also with of hardware (apparatuses) as mentioned above
Charactenstics required for a recording medium for inkjet recording i commonly (1) quick-drying (high ink absorption rate), (2) adequate and unifor ink dots (no bleeding), (3) good graininess, (4) high circulanty of dots, (5) hig concentration, (6) high color saturation (dullness-free), (7) excellent water resi resistance, and ozone resistance of a pnnted area, (8) high whiteness of a recor
feeling similar to silver salt photography, and the like, in addition to the above various characteristics
For the purpose of improving the above-described various characteris recording medium m which the recording layer has a porous structure has bee put into practical use in recent years Since such an mkjet recording medium i porous structure, the recording medium is excellent in ink receiving property ( and has high glossiness
For instance, an inkjet recording medium has been proposed which h a recording layer having high void fraction and containing fine inorganic pigm and a water-soluble resm (see Japanese Patent Application Laid-Open Nos (JP and 10-217601)
These inkjet recording sheets, particularly the inkjet recording mediu recording layer of a porous structure containing silica as the inorganic fine part excellent ink absorbing property owing to the structure thereof, and have high performance that enables formation of an image of high resolution and high gl
However, such recording sheets have a problem in that the gas perme due to the porous film, which may accelerate the deterioration of the compone the recording layer
A trace gas m the air, particularly ozone, can be a cause for fading of image with age Since the recording materials having a recording layer of the above-mentioned porous structure have a lot of voids, the recorded image is ea ozone in the air Therefore, resistance to ozone gas (ozone resistance) is a very characteristic for a recording mateπal having a recording layer of the above-de structure
A variety of inkjet recording media aiming at satisfying the above-des characteristics have been reported
For instance, inkjet recording media to which a liquid prepared by dis
compound and a water-soluble polyvalent metal salt is disclosed Further, in J 2003-200657, an inkjet recording sheet is disclosed which has a colorant recei containing cationic polymer-modified inorganic pigment fine particles prepare cationic polymer having a group capable of combining with the inorganic pig particle at the terminal thereof, and the inorganic pigment fine particles
These inkjet recording sheets descπbed in JP-A Nos 2005-7849 and however, also fails to satisfy all of the ozone resistance, bleed, and image dens density of black)
When an additive for rendeπng ozone resistance is added, the ozone r be realized, however, there arises a problem in that the image density decrease it has been difficult to provide the ozone resistance and prevention the decreas density simultaneously
DISCLOSURE OF THE INVENTION
The present invention has been made in view of the above-described provides an inkjet recording medium and a method for manufacturing the sam
A first aspect of the invention provides an inkjet recording medium h receiving layer on a support The ink receiving layer contains a compound con atom, and the center plane average roughness (SRa value) on the surface of the layer is less than 6 nm when measured under the condition of a cutoff of 2 to 2
A second aspect of the invention provides an inkjet recording medium receiving layer on a support The ink receiving layer contains a sulfoxide-cont compound, and the center plane average roughness (SRa value) on the surface receiving layer is 11 nm or less when measured under the condition of a cutoff
A third aspect of the invention provides a method for manufacturing t recording medium according to either of the first or second aspect The metho least
A fourth aspect of the invention provides a method for manufacturin recording medium according to either of the first or second aspect The metho dispersing at least some of the components in a coating liquid for forming the layer by a head-on collision high-pressure disperser or an orifice-passing high disperser during preparation of the coating liquid
A fifth aspect of the invention provides a method for manufacturing a recording medium having an ink receiving layer including a compound contai atom The center plane average roughness (SRa value) on the surface of the layer is 11 nm or less when measured under the condition of a cutoff of 2 to 2 method includes at least
(A) applying a first liquid contaimng at least a water-soluble resin an agent onto a support to form a coated layer, and
(B) applying a second liquid containing a basic compound onto the c simultaneously with the application of the first liquid, or (2) before the coated decreasing drying during the drying of the coated layer formed by the applicat liquid, to crosslink and cure the coated layer to form an ink receiving layer At first and second liquids contains a compound containing sulfur The viscosity liquid before exhibiting the decreasing drying is 6000 Pa or more at a shear rat
BEST MODE FOR CARRYING OUT THE INVENTION
In the following, the inkjet recording medium according to the presen be descπbed
An inkjet recording medium according to a first embodiment has an i layer on a support, the ink receiving layer includes a compound containing a s the center plane average roughness (SRa value) on the surface of the ink recei which an image is to be formed is less than 6 nm when measured under the co cutoff of 2 to 2 5 μm
to 2 5 μm
Hereinafter, the description of the condition "when measured under t a cutoff of 2 to 2 5 μm" is sometimes omitted when referring to a center plane roughness (SRa value), in which case the center plane average roughness (SR to the value masured under the condition described above
The first embodiment of the invention differs from the second embod invention in the compound contained in the ink receiving layer (either a comp a sulfur atom or a sulfoxide-containing compound), and the center plane avera (SRa value) (either less than 6 nm, or 11 run or less) In this respect, since a sulfoxide-containing compound is an example of a compound contaimng a sul second embodiment can be considered to be a practical mode of the first embo Accordingly, the respective embodiments are explained simultaneously while on the first embodiment <Ink receiving layer>
The ink receiving layer according to the invention includes a compou sulfur atom m addition to the major components constituting the ink receiving inorganic fine particles, a water-soluble resin, and a mordant In the invention, containing a sulfur atom is a compound that can be oxidized Accordingly, wh compound is added to the ink receiving layer, it functions to improve the ozon the ink receiving layer However, the inventor has found that when the compo sulfur atom is added to the ink receiving layer, the surface thereof becomes co image density decreases due to diffused reflection of light In the invention, pr decrease in the image density and improvement in ozone resistance are attaine simultaneously by defining the center plane average roughness (SRa value) on the ink receiving layer [Center plane average roughness (SRa)]
In the invention, a center plane average roughness (SRa value) on the
10 nm or less, and more preferable is 8 nm or less in the second embodiment
The center plane average roughness (SRa) means the average roughn three-dimensionally scanning the roughness on a certain flat plane, and is a di from a center line roughness (Ra value) obtained by scanning a linear roughne plane Concavities and convexities on the surface of a base material are not u are wavy concavities and convexities having a variety of wavelengths The ex "measurement under 2 to 2 5 μm cutoff condition" means a measurement of t and convexities having a wavelength of 2 to 2 5 μm
A method for measuring the center plane average roughness (SRa) in will be described
The measurement of the center plane average roughness (SRa) under a cutoff of 2 to 2 5 μm is conducted by using NEW VIEW 5022 manufactured Corporation based on the following measurement and analysis conditions [Measurement and analysis conditions]
Measurement length 5 mm in X direction, 5 mm in Y direction
Objective lens 50 magnifications
Bandpass filter 2 to 2 5 μm
In the invention, in order to make the center plane average roughness the surface of an ink receiving layer 11 nm or less, the viscosity of the coating forming the ink receiving layer may be adjusted The embodiment therefor wil later in the method for manufacturing an mkjet recording medium of the inven
When the center plane average roughness (SRa value) on the surface receiving layer is set at less than 6 nm, it is preferred to use a head-on collisio disperser or an orifice-passing high-pressure disperser for dispersing the ingre the preparation of the coating liquid
The head-on collision high-pressure disperser is a high-pressure dispe disperses the ink receiving layer coating liquid by head-on collision The orific
Typical examples of high-pressure homogemzers include NANOMI (trade name), manufactured by Nanomizer Corporation, MICROFLUIDIZER manufactured by Microfluidics Corporation, and ULTIMAIZER (trade name) by Sugino Machine Corporation
The orifice described above refers to a mechanism which has a thin p plate) having minute openings with a shape of a circle or the like inserted in a and which rapidly narrowing the flow path of the straight pipe
The above-described high-pressure homogemzer is basically compos high-pressure generation section that pressurizes raw mateπal slurry or the lik collision section or an orifice section For the high-pressure generation section high-pressure pump, which is commonly called a plunger pump, can be suitabl There are high-pressure pumps of a variety of types such as a single pump, a d tπple pump, pumps of any type can be used in the invention without particular [Compound containing sulfur atom]
Next, a compound containing a sulfur atom will be descπbed
The compound containing a sulfur atom is a compound that can be ox described hereinbefore
The compound containing a sulfur atom is preferably a compound ha group, or a sulfoxide-containing compound, and particularly preferably a sulfo compound In the following, the respective compounds are descπbed in detail -Compound contaimng a thioether group-
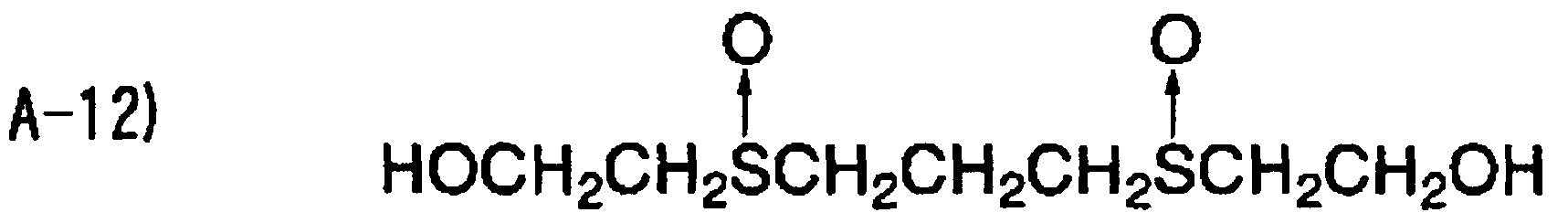
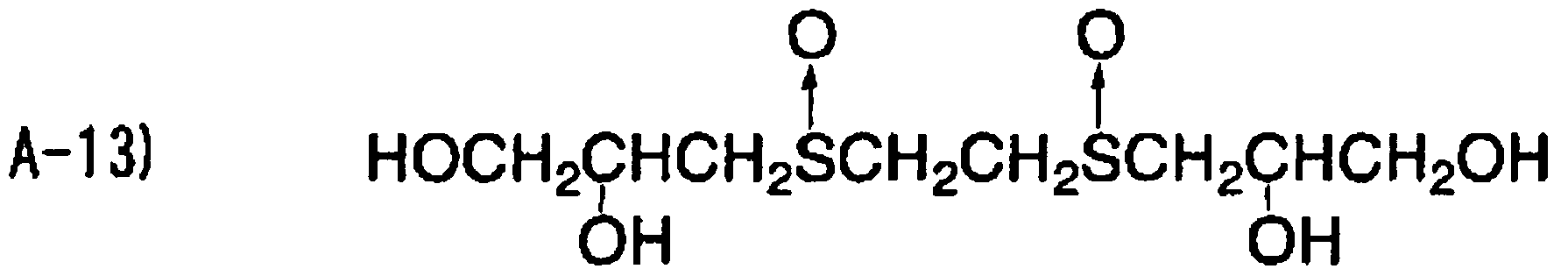
The compound contaimng a thioether group according to the inventio particularly limited, and examples thereof include compounds containing a sul aromatic groups bonded to the both sides of the sulfur atom (the following <1> compounds containing a sulfur atom and alkyl groups (having preferably four atoms) sandwiching the sulfur atom (the following <4> to <5>), DL-methionin 2-(ethylthio)ethanol
<1>
<2>
<3>
<4>
<5>
The content of any of the thioether compound in the ink receiving lay from O 1 to 50 mmol/m , and more preferably from O 2 to 20 mmol/m
O
— G-S-G- Formu l a (1)
The sulfoxide-containmg compound having a structure represented b (1) may be substituted by a hydrophilic group Examples of the hydrophilic gr substituted or unsubstituted amino groups, substituted or unsubstituted carba substituted or unsubstituted sulfamoyl groups, substituted or unsubstituted am hydroxyl group, sulfonic acid, carboxyhc acid, phosphoric acid, ethyleneoxy substituted or unsubstituted nitrogen-containing heterocycles
Moreover, the sulfoxide-containmg compound is preferably a compo by the following formula (2)
O
R1-(S — R2)m— S-R3 Formu l a (2)
(O)n
In formula (2), R1 and R3 each independently represent a substituted alkyl group, a substituted or unsubstituted aryl group, a substituted or unsubsti heterocyclic group, or a polymer residue composed of such groups R1 and R3 same as or different from each other R1 and R3 may combine with each other R represents a substituted or unsubstituted bi- to hexa-valent linking group R with R or R to form a πng, or combine with R or R to form a ring m is 0 o or greater n is 0 or 1 At least one of R , R , and R represents an alkyl group, heterocyclic group, or a polymer residue each of which is substituted by a hyd selected from a substituted or unsubstituted ammo group, a substituted or unsu
example, alkyl groups having 1 to 22 carbon atoms are preferable Specificall group is preferably a methyl group, an ethyl group, an allyl group, a n-butyl g group, a n-octyl group, a benzyl group, an iso-propyl group, an iso-butyl grou group, a cyclohexyl group, or a 2-ethylhexyl group, more preferably an alkyl to 10 carbon atoms, and particularly preferably a methyl group, an ethyl group a n-propyl group, an iso-butyl group, a cyclohexyl group, or a 2-ethylhexyl gr
The unsubstituted aryl group represented by R1 or R3 is preferably, fo aryl group having 6 to 22 carbon atoms Specific examples thereof include a p 1 -naphthyl group, and a 2-naphthyl group, and a phenyl group is particularly p
Examples of the unsubstituted heterocyclic group represented by R1 o thienyl group, a thiazolyl group, an oxazolyl group, a pyπdyl group, a pyrazyl thiadiazoyl group, a tπazoyl group, a morphoryl group, a piperazyl group, a p a tπazyl group, an indolyl group, a benzothiazoyl group, and a benzoxazoyl gr others, a thiazolyl group, an oxazolyl group, a pyπdyl group, a thiadiazoyl gro group, a morphoryl group, a pyrimidyl group, a tπazyl group, a benzothiazoyl benzoxazoyl group are particularly preferred
When R or R represents a polymer residue composed of groups sele substituted or unsubstituted alkyl groups, aryl groups, and heterocyclic residue of the polymer residue is a polymer having any of the following units
wherein R4 represents a hydrogen atom, or an alkyl group having 1 to atoms, R represents an alkylene group, Q represents a linking group, R and independently represent an alkylene group, L represents 1 or 2, P represents 1 and n have the same definitions as R , R , m, and n in the formula (2), respecti
wherein R6 represents a hydrogen atom, an alkyl group, or an aryl gr When R1 or R3 represents a substituted alkyl, aryl, or heterocyclic gro of the substituent(s) include substituted or unsubstituted amino groups (e g a having 30 or less carbon atoms, an amino group, alkylamino groups, dialkyla arylamino groups, and acylamino groups), substituted or unsubstituted carbam carbamoyl groups having 30 or less carbon atoms, a carbamoyl group, a meth group, a dimethylcarbamoyl group, a morpholinocarbamoyl group, and a pipe group), substituted or unsubstituted ammoniums (e g ammoniums having 30 atoms, ammonium, trimethylammonium, tπethylammomum, dimethylbenzyla hydroxyethyldimethylammonium), substituted or unsubstituted sulfamoyl gro sulfamoyl groups having 30 or less carbon atoms, a sulfamoyl group, a methyl group, a dimethylsulfamoyl group, a morpholinosulfamoyl group, and a pipen group), substituted or unsubstituted nitrogen-containing heterocycles (e g a py pyπmidyl group, a morphohno group, a pyrohdino group, a pipeπdmo group, group), hydrophilic groups represented by a hydroxyl group, a sulfomc acid, a a phosphoric acid, an ethyleneoxy group and the like, a cyano group, halogen fluorine atom, a chlorine atom, and a bromine atom), substituted or unsubstitut alkoxycarbonyl groups (e g alkoxycarbonyl groups having 30 or less carbon a methoxycarbonyl group, an ethoxycarbonyl group, a dimethylarninoethoxyeth
or less carbon atoms, an acetyloxy group, and a propionyloxy group), and sub unsubstituted acyl groups (e g acyl groups having 30 or less carbon atoms, a and a propionyl group)
R1 and R3 may be the same as or different from each other, and may each other to form a πng
R represents a substituted or unsubstituted divalent to hexavalent lin may be bonded to R or R , or R or R to form a πng Examples of the sulfur heterocycle formed by such a bonding include a thienyl group, a thiazoyl gro group, a dithiolan-2-yl group, a tπthian-2-yl group, and a dithian-2-yl group
Examples of the divalent to hexavalent linking group represented by containing carbon, mtrogen, oxygen, or phosphor, and a specific examples the following linking groups
-CH2CH2- -CH2CH2CH2- -CH2CH2-O-CH2CH2-
by a substituted or unsubstituted amino group, a substituted or unsubstituted c a substituted or unsubstituted sulfamoyl group, a substituted or unsubstituted hydroxyl group, a sulfonic acid, a carboxylic acid, a phosphoric acid, an ethyl or a substituted or unsubstituted nitrogen-containing heterocycle The hydrop be selected from the substituents mentioned in the descπption of R1 and R3
Since the preparation of the lnkjet recording medium of the inventio practically aqueous coating, the sulfoxide-contaming compound according to preferably water-soluble
Such a sulfoxide-contaming compound is a Lewis base, which has hi in water than a thioether compound Therefore, the sulfoxide-contaming co added in a larger amount than a thioether compound
When the sulfoxide-containing compound according to the invention it is preferred to add the sulfoxide-contaming compound to a coating liquid or containing the after-mentioned fine particles and water-soluble resin
When the sulfoxide-contaming compound according to the invention is preferred to add the sulfoxide-contaming compound to the coating liquid or containing fine particles and a water-soluble resin after the sulfoxide-contami emulsified or after the sulfoxide-contaming compound is added to an organic
In the inkjet recording medium according to the invention, the conten sulfoxide-containing compound is preferably 0 01 to 20 g/m , and more prefer g/m in view of further improvement in ozone resistance, resistance to bleed (i with age, and glossiness
In the inkjet recording medium according to the invention, the sulfox compound, which generally has a higher oxidation potential than conventional sulfur-containing compounds (thioethers, thioureas), can achieve higher ozone higher light resistance when combined with a supeπor colorant having a high potential for the sake of improving the ozone resistance and the light resistanc
O
A-3) CH3SCH2CH2OH
O
A-ID f
HOCH2CH2CH2SCH2CH2CH2OH
O
O
A-18) I
CH3SCH2COOH
O 0
A"25) HOOCCH2CH-SCH2CH2SCHCH2COOH
:OOH COOH
0
A-28) CH3SCH2CH2CHCOOH
0 0
A"33) C2H5SCHCHSC2H5
O O
A-36) t t
NaO3SCH2CH2CH2SCH2CH2SCH2CH2CH2SO3
A-43) C2H5SCH2CHCOOH
NH2
A-45)
O
A-51) HOOCCH2SCH2CHCOOH IsJH2
0
O
A-62)
A-66)
The content of the compound containing a sulfur atom in the inkjet re
particles, colloidal silica, alumina fine particles and pseudo-boehmite are pref them from the viewpoint of forming a good porous structure The fine partic as primary particles, or after forming secondary particles The average prim diameter of these fine particles is preferably 2 μm or less, more preferably 20
Furthermore, silica fine particles with an average primary particle dia or less, colloidal silica with an average primary particle diameter of 30 nm or l fine particles with an average primary particle diameter of 20 nm or less, and pseudo-boehmite with an average fine pore diameter of 2 to 15 nm are more p the silica fine particles, alumina fine particles and pseudo-boehmite are partic preferable
The silica fine particles are roughly classified into wet method particl method (gas phase method) particles depending on their production method example of the wet method, active silica is formed by acidolysis of a silicate s silica is polymerized to an adequate degree, and then is coagulated and precipi hydrated silica In contrast, in a typical example of the gas phase method, an is obtained by hydrolysis of silicon hahde in gas phase at high temperature (fla method), or silica sand and coke are vaporized by reduction by heating with ar furnace, and the product thereof is oxidized with air (arc method) The "gas means anhydrous silica fine particles obtained by the gas phase method The silica fine particles are particularly preferable as the silica fine particles used
Although the gas phase silica exhibits different properties from hydra the difference in the density of the silanol groups on the surface and in the pro voids, the gas phase silica is suitable for forming a three-dimensional structure void ratio While the reason thereof is not clear, the density of the silanol gro surface of the fine particles is as large as 5 to 8 groups/nm in the case of hydr thus the silica particles easily aggregate In contrast, the density of the silano surface of the fine particles is as small as 2 to 3 groups/nm m the case of gas p
density and good glossiness of colors, not only m the uses requiring high tran an OHP film, but also in an application as a recording sheet such as a photogr paper
The average primary particle diameter of the inorganic fine particles silica) is preferably 50 nm or less, more preferably from 3 to 50 run, still mor from 3 to 30 nm, particularly preferably 3 to 20 nm, and most preferably 3 to of the quick drying property (ink absorption rate) Since the gas phase silica p liable to be coagulated with each other due to hydrogen bonds between the sil structure having a large void ratio can be formed when the average primary p is 50 nm or less, and ink absorbing charactenstics can be effectively improved
The gas phase silica may be used together with other inorganic fine p those described above The content of gas phase silica is preferably 30 mass more preferably 50 mass % or more, when the gas phase silica is used togethe particles
Alumina fine particles, alumina hydrate, and a mixture or composite preferable as the inorganic fine particles used in the invention The alumina preferable among them since it absorbs ink well and fixes the ink, and pseudo (AI2O3 11H2O) is particularly preferable While various forms of the alumina used, boehmite sol is preferably used as the raw material since a smooth layer obtained
The fine void structure of pseudo-boehmite preferably has an average diameter of 1 to 30 nm, more preferably 2 to 15 nm The fine void volume is to 2 0 cc/g, more preferably 0 5 to 1 5 cc/g The fine void diameter and fine measured by a nitrogen absorption-desorption method using, for example, a g absorption-desorption analyzer (for example, OMNISORP 369 manufactured Coulter, Inc )
The gas phase alumina fine particles are preferable among the alumn
[Water-soluble resm]
Examples of the water-soluble resin used in the ink receiving layer i alcohol resins (e g , polyvinyl alcohol (PVA), acetoacetyl-modifϊed polyvinyl cation-modified polyvinyl alcohol, anion-modified polyvinyl alcohol, silanol- polyvinyl alcohol and polyvinyl acetal), which are resins having hydroxyl gro hydrophilic structural units, cellulose resins (methyl cellulose (MC), ethyl cell hydroxyethyl cellulose (HEC), carboxymethyl cellulose (CMC), hydroxyprop (HPC), hydroxyethylmethyl cellulose and hydroxypropylmethyl cellulose), ch starches, resins having ether bonds (polyethylene oxide (PEO), polypropylene polyethyleneglycol (PEG) and polyvinyl ether (PVE)), and resins having carb (polyacrylamide (PAAM), polyvinyl pyrrohdone (PVP) and polyacrylic acid
Other examples include polyacrylic acid salts, maleic acid resins, algi and gelatins, having carboxylic groups as dissociation groups
The polyvinyl alcohol resins are particularly preferable among the re Examples of the polyvinyl alcohol resins are descπbed in Japanese Patent App Publication (JP-B) Nos 4-52786, 5-67432 and 7-29479, Japanese Patent No No 7-57553, Japanese Patent Nos 2502998 and 3053231, JP-ANo 63-17617 Patent No 2604367, JP-A Nos 7-276787, 9-207425, 11-58941, 2000-135858, 2001-287444, 62-278080 and 9-39373, Japanese Patent No 2750433, JP-A N 2000-158801, 2001-213045, 2001-328345 and 8-324105, 11-348417
Examples of water-soluble resins other than polyvinyl alcohol resins i compounds described in paragraph [0011] to [0014] m JP-ANo 11-165461
Only one water-soluble resin may be used, or a combination of two o water-soluble resms may be used
The content of the water-soluble resm of the invention is preferably 9 more preferably 12 to 33 mass %, relative to the mass of the total solid content receiving layer
more preferably 80 to 99 5%
While the polyvinyl alcohol resin has hydroxyl groups in its structur dimensional network structure with secondary particles of the silica fine parti chain units is readily formed since hydrogen bonds are formed between the h and the silanol groups on the surface of the silica fine particles It is conside receiving layer having a porous structure with a high void ratio and sufficient formed owing to the formation of the three dimensional network structure
The porous ink receiving layer obtained as described above rapidly a capillary action during inkjet recording, and thus dots of good circularity can without ink bleed
The polyvinyl alcohol resin may be used together with other water-s The content of polyvinyl alcohol resin in the total water-soluble resins is pref mass % or more, more preferably 70 mass % or more, when the polyvinyl alc used together with other water-soluble resins <Composition ratio between fine particles and water-soluble resin>
The mass composition ratio (PB ratio (x/y)) of inorganic fine particle water-soluble resin (y) largely affects the structure and strength of the ink rece While the void ratio, fine void volume and surface area (per unit mass) increa composition ratio (PB ratio) increases, the density and strength tend to be low
The mass composition ratio (PB ratio, (x/y)) m the ink receiving laye invention is preferably in the range of 1 5 to 10, so as to prevent decrease in t and generation of cracks at drying resulting from an excessively large PB rati prevent decrease in ink absorbing property accompanying reduction of void ra easily occurring filling of voids with the resin resulting from an excessively s
Since the recording sheet may suffer stress when conveyed m a conv an inkjet printer, the ink receiving layer should have sufficient film strength strength of the ink receiving layer is required also for preventing cracks and p
the coating liquid onto a support, and then drying the coated layer, whereby a light-transmitting porous layer with an average fine void diameter of 30 run or ratio of 50 to 80%, a specific void volume of 0 5 ml/g or more, and a specific 100 m /g or more can be readily formed (Crosshnking agent)
In the lnkjet recording medium according to the invention, the ink rec preferably contains a water-soluble resin The mk receiving layer is preferab layer obtained by forming the coated layer containing the sulfoxide-containin cation polymer, the inorganic fine particles, the water-soluble resin and a cross capable of crosshnking the water-soluble resin, and curing the coated layer thr crosshnking reaction between the crosshnking agent and the water-soluble res
Boron compounds are preferably used for crosshnking the water-solu particularly polyvinyl alcohol resin Examples of the boron compound inclu acid, borate (for example orthoborate, InBC>3, ScBO3, YBO3, LaBC>3, Mg3(BO Cθ3(Bθ3)2), diborate (for example Mg2B2θ5, CO2B2O5), methaborate (for exa Ca(BO2)2 NaBO2 and KBO2), tetraborate (for example Na2B4O7 10H2O), and (for example KBsOg 4H2O, Ca2BoOn 7H2O, and CsB5Os) Borax, boric acid preferable since they can cause crosshnking reaction quickly, and boric acid is preferable
The following compounds other than boron compounds may be used crosshnking agent for the water-soluble resin
Examples of such other crosshnking agents include aldehyde compou formaldehyde, glyoxal and glutaraldehyde, ketone compounds such as diacetyl cyclopentanedione, active halogen compounds such as bis(2-chloroethylurea), 2-hydroxy-4,6-dichloro-l,3,5-tπazine, 2,4-dichloro-6- tπazine sodium salt, act compounds such as di vinyl sulfonic acid, l,3-divinylsulfonyl-2-propanol, N,N'-ethylenebis(vinylsulfonylacetamide), and 1 ,3,5-triaclyroyl-hexahydro-S-
such as 2,3-dihydroxydioxane, metal-containmg compounds such as titanium aluminum sulfate, chromium alum, potassium alum, zirconium acetate and ch poly amine compounds such as tetraethylenepentamine, hydrazide compounds dihydrazine adipate, and low molecular weight compounds or polymers conta two oxazoline groups
Only a single crosslinking agent selected from the above may be use more crosslinking agents selected from the above may be used in combination
As mentioned hereunder, crosslink curing is preferably carried out in manner a crosslinking agent is added to a coating liquid containing inorganic water-soluble resin and the like (hereinafter occasionally referred to as "first li a basic solution having a pH of 7 1 or higher (hereinafter occasionally referre liquid"), onto the coated layer formed by application of the first liquid, the sec applied (1) simultaneously with the application of the first liquid for forming t or (2) before the coated layer exhibits decreasing drying during drying of the c formed by application of the first liquid In this case, the compound containin is contained in either of the first or second liquid Application of the crosslinki preferably conducted as follows when a boron compound is used as an exampl the ink receiving layer is a layer obtained by crosslink-curing of a coated layer application of the coating liquid (first liquid) containing fine particles and a w resin containing polyvinyl alcohol, the crosslink cuπng is earned out by apply solution having a pH of 7 1 or higher (the second liquid) onto the coated layer simultaneously with the application of the first liquid, or (2) before the coated decreasing drying during drying of the coated layer formed by application of t The boron compound as a crosslinking agent may be contained at least one of second liquids, and may be contained in the both of the liquids
The amount of crosslinking agent to be used is preferably from 1 to 5 more preferably from 5 to 40 mass% with respect to the water-soluble resin
Specific examples thereof include calcium acetate, calcium chloride, calcium sulfate, calcium butyrate, barium acetate, barium sulfate, barium pho oxalate, barium naphthoresorcin carboxylate, barium butyrate, manganese chl manganese acetate, manganese formate dihydrate, ammonium manganese sul cupnc chloride, ammonium copper (II) chloride dihydrate, copper sulfate, co copper oxalate, copper phthalate, copper citrate, copper gluconate, copper nap chloπde, cobalt thiocyanate, cobalt sulfate, cobalt (II) acetate, cobalt naphthe sulfate hexahydrate, nickel chloride hexahydrate, mckel acetate tetrahydrate, nickel sulfate hexahydrate, amide nickel sulfate tetrahydrate, nickel sulfamina 2-ethylhexanoate, aluminum sulfate, aluminum sulfite, aluminum thiosulfate, polychloπde, aluminum nitrate nonahydrate, aluminum chloride hexahydrate, acetate, aluminum lactate, basic aluminum thioglycolate, ferrous bromide, ferr ferric chloπde, ferric sulfate, ferrous sulfate, iron (III) citrate, iron (III) lactate tπammomum iron (III) tπoxalate tπhydrate, zinc bromide, zinc chloride, zinc hexahydrate, zinc sulfate, zinc acetate, zinc lactate, zirconium acetate, zirconi zirconium chloπde, zirconium oxychloπde octahydrate, zirconium hydroxychl chromium acetate, chromium sulfate, magnesium acetate, magnesium oxalate, sulfate, magnesium chloride hexahydrate, magnesium citrate nonahydrate, sod tungstophosphate, tungsten sodium citrate, dodecatungstophosphate n-hydrate, dodecatungstosilicate hexacosahydrate, molybdenum chloπde, dodecamolybd n-hydrate and the like, aluminum alum, basic aluminum polyhydroxide, zinc p ammonium zinc acetate, and ammonium zinc carbonate Two or more of these polyvalent metal compounds may be used together In the invention, the term regarding water-soluble polyvalent metal compounds means that the polyvalen compounds are dissolves, at a concentration of 1 mass% or more, in water of 2
Among the above-descπbed water-soluble polyvalent metal compoun aluminum compound or a compound containing a metal belonging to Group 4
polyhydroxide the major component of which is represented by the following 3, and contains stably a basic and high-molecular polynuclear condensation i [Al6(OH)1S]3+, [Al8(OH)2Q]4+, [Al13 (OH)34] 5+, and [Al2 KOH)6O]3+
[Al2(OH)n Cl6 Jm formula 1 [Al(OH)3]nAlCl3 formula 2 Aln(0H)mCl(3n m) O < m < 3n formula 3
These compounds are supplied from Tagi Chemical Co , Ltd under t aluminum polychloπde (PAC) as a chemical for water treatment, from Asada Ltd under the name of aluminum polyhydroxide (Paho), from Riken Green C the name of HAP-25, from Taimei Chemicals Co , Ltd under the name of AL from other manufacturers for the same purpose Products of various grades ar available
As the water-soluble compound containing an element of Group 4 A Table, water-soluble compounds contaimng titanium or zirconium are more pr Examples of a water-soluble compound contaimng titanium include titanium titanium sulfate, titanium tetrachloride, tetraisopropyl titanate, titanium acetyl titanium lactate Examples of a water-soluble compound containing zirconium zirconium acetate, zirconium chloπde, zirconium hydroxychloπde, zirconium zirconium carbonate, zirconium hydroxide, zircomum lactate, ammonium zirc carbonate, potassium zirconium carbonate, zirconium sulfate, and zirconium f compounds
It is preferred that the water-soluble polyvalent metal compound is ad amount of 0 1 to 10 mass %, and more preferably 0 5 to 8 mass % with respec inorganic fine particles [Mordant]
mordants and inorganic mordants each may be used alone In an embodime organic mordants and one or more inorganic mordants are used in combinatio
As the catiomc mordant, a polymer mordant having, as a cationic gro secondary, or tertiary amino group or a quaternary ammonium base is used in However, the cationic mordant may be a cationic non-polymer mordant in the
Examples of the polymer mordant include homopolymers of a mono monomer) having a primary, secondary, or tertiary amino group, or a salt ther quaternary ammonium base, copolymers or condensation polymers of the mor and one or more other monomers (hereinafter referred to as "non-mordant mo polymer mordants may be used m the form of a water-soluble polymer or wat latex particles
Examples of the monomer (mordant monomer) include tπmethyl-p-vmylbenzylammomum chloπde, trimethyl-m-vinylbenzylammom tπethyl-p-vinylbenzylammonium chloπde, tπethyl-m-vinylbenzylammonium N,N-dimethyl-N-ethyl-N-p-vinylbenzylammonium chloride, N,N-diethyl-N-methyl-N-p-vmylbenzylammomum chloride, N,N-dimethyl-N-n-propyl-N-p-vinylbenzylammonium chloπde, N^-dimethyl-N-n-octyl-N-p-vinylbenzylammonium chloride, N,N-dimethyl-N-benzyl-N-p-vmylbenzylammonium chloπde, N,N-diethyl-N-benzyl-N-p-vinylbenzylammonium chloride, N,N-dimethyl-N-(4-methyl)benzyl-N-p-vinylbenzylammonium chloπde, and N,N-dimethyl-N-phenyl-N-p-vinylbenzylammonium chloride, tπmethyl-p-vinylbenzylammonium bromide, tπmethyl-m-vinylbenzylammoni tπmethyl-p-vinylbenzylammonium sulfonate, tπmethyl-m-vmylbenzylammon tπmethyl-p-vinylbenzylammonium acetate, tπmethyl-m-vinylbenzylammomu N,N,N-tnethyl-N-2-(4-vmylphenyl)ethylammonmm chloπde, N,N,N-tπethyl-N-2-(3 -vinylphenyl)ethylammonium chloride,
p-toluenesulfonates and the like), trimethyl-2-(methacryloyloxy)ethylammoni tπethyl-2-(methacryloyloxy)ethylammonium chloride, tπmethyl-2-(acryloyloxy)ethylammonium chloride, triethyl-2-(acryloyloxy)et chloride, tnmethyl-3-(methacryloyloxy)propylammonium chloride, tnethyl-3 -(methacryloyloxy)propylammonium chloride, tπmethyl-2-(methacryloylamino)ethylamrnonium chloride, tπethyl-2-(methacryloylamino)ethylammonium chloride, tπmethyl-2-(acryloylamino)ethylammonium chloride, tπethyl-2-(acryloylamino)ethylamrnonium chloride, tπmethyl-3 -(methacryloylamino)propylamrnonium chloride, tnethyl-3 -(methacryloylamino)propylammonmm chlonde, tπmethyl-3 -(acryloylarnino)propylammonium chloride, tπethyl-3 -(acryloylamino)propylamrnonium chlonde, N,N-dimethyl-N-ethyl-2-(methacryloyloxy)ethylamrnonium chloride, N,N-diethyl-N-methyl-2-(methacryloyloxy)ethylammonium chlonde, N,N-dimethyl-N-ethyl-3 -(acryloylamino)propylammonium chloride, tnmethyl-2-(methaciyloyloxy)ethylammonium bromide, tnmethyl-3 -(acryloylamino)propylammonium bromide, tnmethyl-2-(methacryloyloxy)ethylammonium sulfonate, and tπmethyl-3 -(acryloylamino)propylammonium acetate
Examples of other mordant monomer include N-vinylimidazole,
N-vinyl-2-methylimidazole, 2-vinylpyπdine, 4-vinylpyndine, 4-vmyl-N-meth chloride, 4-vinyl-N-ethylpyndmium bromide, dimethyldiallylammonium chlo monomethyldiallylammonium chloride
Only one of such mordant monomers may be used, or two or more co mordant monomers may be used in combination
The non-mordant monomer refers to a monomer that does contain a b
(meth)acrylates (such as cyclohexyl (meth)acrylate), aryl methacrylates (such (meth)acrylate)), aralkyl (meth)acrylates (such as benzyl(meth)acrylate), subs (meth)acrylates (such as 2-hydroxyethyl (meth)acrylate, methoxymethyl (met allyl (meth)acrylate), (meth)acrylamides (such as (meth)acrylamide, dimethyl (meth)acrylamide, N-ethyl (meth)acrylamide, and N-isopropyl (meth)acrylam vmyls (styrene, vinyltoluene and α-methylstyrene), vinyl esters (such as vinyl propionate and vinyl versatate), allyl esters (such as allyl acetate) halogen-co monomers (such as vmyhdene chlonde and vinyl chloride), vinyl cyanates (su (meth)acrylonitπle), and olefins (such as ethylene and propylene)
Only one non-mordant monomer may be used, or two or more non-m monomers may be used in combination
Examples of the polymer mordant include polyethyleneimine (and de thereof), polyvinylamine (and denvatives thereof), polyallyamme (and derivat polyamidme, cationic polysaccharides (such as cationic starch and chitosan), d resins (such as dicyan diamide-formahn polycondensate), polyamine cationic dicyan diamide-diethylenetπamine polycondensate), epichlorohydrm-dimethy polymers, and dimethyldiallylammonium chloride-sulfur dioxide copolymer
Polymers having a quaternary ammonium base are preferable, and (m polymers, vinylbenzylammonium polymers and diallylammonium polymers h average molecular weight of 1,000 to 100,000 and having a quaternary ammo particularly preferable as the organic mordant in the invention
In the invention, the content of the mordant in the ink receiving layer from 0 01 to 10 g/m , more preferably from 0 1 to 5 g/m
The ink receiving layer coating liquid (first liquid) preferably contain As the surfactant, cationic surfactants, anionic surfactants, noniomc surfactant surfactants, fluorosurfactants, and silicone surfactants are all usable
Examples of preferable noniomc surfactants include polyoxyalkylene
glycerin fatty acid esters (such as glycerol monooleate), polyoxyethylene glyc esters (such as monostearic acid polyoxyethylene glycerin and monooleic aci polyoxyethylene glycerin), polyoxyethylene fatty acid esters (such as polyeth monolaurate, and polyethyleneglycol monooleate), polyoxyethylene alkylami acetylene glycols (such as 2,4,7,9-tetramethyl-5-decyn-4,7-diol, and ethylene and propylene oxide adducts of the diol) Polyoxyalkylene alkylethers are p them The nonionic surfactant may be used in the first solution and the seco Only one noniomc surfactant may be used, or two or more nonionic surfactan in combination
Examples of amphoteric surfactants include those of amino acid type carboxyamonium betaine type, sulfonammonium betame type, ammonium sul betaine type and lmidazohum betame type, and those descπbed in USP No 3, Nos 59-49535, 63-236546, 5-303205, 8-262742 and 10-282619 may be favor The amphoteric surfactant may be an amphoteric surfactant of amino acid typ deπved from an ammo acid (such as glycme, glutamic acid or histidme) as de No 5-303205 Specifically, the amphoteric surfactant may be an N-aminoac long chain acyl group introduced thereto, or a salt thereof Only a single am surfactant may be used, or two or more amphoteric surfactants may be used in
Examples of anionic surfactants include fatty acid salts (for example, and potassium oleate), salts of alkylsulfuric acid esters (for example, sodium l and tπethanolamine lauryl sulfate), sulfonic acid slats (for example, sodium d sulfonate), alkylsulfosuccinic acid salts (for example, sodium dioctylsulfosucc alkyldiphenylether disulfonic acid salts, and alkylphosphoπc acid salts
Examples of cationic surfactants include alkylamine salts, quaternary salts, pyπdinium salts and lmidazohum salts
Examples of fluorosurfactants include a compound derived from an i having a perfluoroalkyl group using a method such as electrolytic fluoπnation,
organic group, a structure in which the both terminals of a siloxane structure a with the organic group, or a structure in which one of the terminals of a siloxa modified with the organic group Examples of modification with the organi amino modification, polyether modification, epoxy modification, carboxyl m carbinol modification, alkyl modification, aralkyl modification, phenol modifi fluorine modification
In the invention, the content of surfactant is preferably from 0 01 to 2 preferably from 0 01 to 1 0%, relative to the ink receiving layer coating liquid When two or more coating liquids for forming the ink receiving layer are use is preferable to add the surfactant to each coating liquid
In the invention, the ink receiving layer preferably contains a high bo organic solvent for preventing curling The high boiling point organic solve compound having a boiling point of 1500C or higher at atmospheric pressure, water-soluble or hydrophobic compound The high boiling point organic sol solid or liquid at room temperature, and may be a low molecular weight comp molecular weight compound
Examples of the organic solvent include aromatic carboxylic acid est dibutyl phthalate, diphenyl phthalate and phenyl benzoate), aliphatic carboxyli (such as dioctyl adipate, dibutyl sebacate, methyl stearate, dibutyl maleate, dib and tπethyl acetylcitrate), phosphoric acid esters (such as trioctyl phosphate a phosphate), epoxy compounds (such as epoxylated soy bean oil and epoxy late methyl esters), alcohols (such as stearyl alcohol, ethyleneglycol, propylenegly diethyleneglycol, triethyleneglycol, glyceπn, diethyl enegly col monobutylethe triethyleneglycol monobutylether, glycerin monomethylether, 1,2,3-butanetπo 1,2,4-butanetnol, 1 ,2,4-pentanetπol, 1,2,6-hexanetnol, thiodiglycol, tπethanol poly ethyleneglycol), vegetable oils (such as soy bean oil and sunflower oil), a aliphatic carboxylic acid (such as linoleic acid and oleic acid)
labeling face side
The material used for the transparent support is preferably transparen to radiant heat generated when used with an OHP or a backlight display Ex material include polyesters such as polyethylene terephthalate (PET), polysul polyphenylene oxide, polyimide, polycarbonate and polyamide Polyesters and polyethylene terephthalate is particularly preferable among them
While the thickness of the support is not particularly restricted, the th preferably 50 to 200 μm from the viewpoint of ease of the handling
The opaque support having high glossiness preferably has a surface of 40% or more on which the ink receiving layer is to be provided The glos measured according to the method (a 75 degree specular glossiness test metho sheets and paper board) defined in Japanese Industrial Standards (JIS) P-8142, incorporated herein by reference Specific examples include the following s
Examples include highly glossy paper supports such as art paper, coa cast-coat paper, and baryta paper used for silver salt photographic support, hig (which may have been subjected to a surface calendering treatment) comprisi that has been made opaque by adding a white pigment or the like, wherein the may be a polyester such as polyethylene terephthalate (PET), a cellulose ester nitrocellulose, cellulose acetate or cellulose acetate butylate, polysulfone, poly oxide, polyimide, polycarbonate or polyamide, and supports in which a coated polyolefin, which contains or does not contain a white pigment, on the surface various paper supports as described above, a transparent support as descnbed highly glossy film containing a white pigment or the like
Foamed polyester films contaimng a white pigment (for example, foa contains polyolefin fine particles and voids formed by stretching) are also favo Resin coat paper used for the silver salt photographic paper is also preferable
While the thickness of the opaque support is not particularly restπcte
polyester fibers, as necessary While any one of LBKP, LBSP, NBKP, NBSP, LUKP and NUKP may be used as the wood pulp, it is preferable to use a great LBKP, NBSP, LBSP, NDP and LDP, which contain a high proportion of short other wood pulps
However, the proportion of LBS and/or LDP is preferably 10 mass % mass % or less
Chemical pulps (such as sulfate pulp and sulfite pulp) contaimng littl be preferably used, and a pulp whose bπghtness has been improved by a bleac is also useful
The following agents may be added to the raw paper sheet as necessa agent such as a higher fatty acid and alkylketene dimer, white pigment such as carbonate, talc and titanium oxide, a paper strength enhancer such as starch, p and polyvinyl alcohol, a fluorescent bπghtener, a humectant such as polyethyl dispersing agent, a softening agent such as quaternary ammonium, and the like
The freeness of the pulp to be used for paper-making is preferably fro ml as defined in CSF Regarding the fiber length after beating, the sum of th mass of the 24 mesh filtration residue and the percentage by mass of the 42 me residue is preferably 30 to 70 mass % Such mesh filtration residues are define which is incorporated herein by reference The percentage by mass of the 4 residue is preferably 20mass % or less
The basis weight of the raw paper is preferably from 30 to 250 g/m2, preferably from 50 to 200 g/m The thickness of the raw paper is preferably μm High smoothness can be rendered to the raw paper by applying a calend during paper making or after paper making The density of the raw paper is u to 1 2 g/m3 (JIS P-8118, which is incorporated herein by reference)
The rigidity of the raw paper is preferably from 2 to 20 mN m under t according to JIS P-8125, which is incorporated herein by reference
polyethylene (HDPE) However, LLDPE, polypropylene, and the like may als component
The polyethylene layer on the side to be provided with the ink receivi preferably obtained by adding titanium oxide of rutile or anatase type, fluores and ultramarine blue to polyethylene such that opaqueness, whiteness, and hu as widely adopted in photographic paper The content of titanium oxide relat polyethylene is preferably from 3 to 20 mass %, more preferably from 4 to 13 While the thickness of the polyethylene layer is not particularly restπcted, a th 50 μm is favorable for both the layers on the front and back sides An under be provided on the polyethylene layer so as to provide the polyethylene layer adhesiveness to the ink receiving layer Aqueous polyester, gelatin and PVA as the undercoat layer The thickness of the undercoat layer is preferably fro
The polyethylene coated paper may be used as glossy paper, or may b having such a matte surface or silky surface as realized in usual photographic so-called embossing treatment is conducted when polyethylene is coated on th melt-extrusion
A back coat layer may be provided on the support, and components th to the back coat layer may be, for example, a white pigment, an aqueous binde
Examples of the white pigment contained in the back coat layer inclu white pigments such as light calcium carbonate, heavy calcium carbonate, kaol calcium sulfate, barium sulfate, titanium dioxide, zinc oxide, zinc sulfide, zmc satm white, aluminum silicate, diatomaceous earth, calcium silicate, magnesiu synthetic amorphous silica, colloidal silica, colloidal alumina, pseudo-boehmit hydroxide, alumina, hthopone, zeolite, hydrated halloysite, magnesium carbon magnesium hydroxide, and organic pigments such as styrene-based plastic pig plastic pigments, polyethylene, microcapsules, urea resin and melamine resin
Examples of the aqueous binder usable in the back coat layer include
<Method for manufacturing inkjet recording medium>
A method according to an embodiment of the invention for manufact recording medium is a method for manufacturing the inkjet recording mediu invention The method includes forming an ink receiving layer by a process in the following (A) and (B), and at least one of the following first liquid and the contains a compound containing sulfur
(A) applying a first liquid containing at least a water-soluble resin crosslinking agent onto a support to form a coated layer on the support
(B) applying a second liquid containing a basic compound onto the simultaneously with the application of the first liquid, or (2) before the coated decreasing drying during drying of the coated layer formed by the application liquid, to crosslink and cure the coated layer
In a method for manufacturing the inkjet recording medium accordin embodiment described above, a sulfoxide-containing compound is used as the containing a sulfur atom
According to another embodiment, a method for manufacturing an in medium of the invention is a method for manufacturing an inkjet recording m ink receiving layer including a compound containing a sulfur atom, wherein th average roughness (SRa value) on the surface of the ink receiving layer is 11 r measured under a condition of a cutoff of 2 to 2 5 μm The method includes fo receiving layer by a process including at least the following (A) and (B), at lea following first and second liquids contains a compound containing a sulfur ato viscosity of the first liquid before exhibiting decreasing drying is 6000 Pa or rate of 1 s l
(A) applying a first liquid contaimng at least a water-soluble resin an agent onto a support to form a coated layer on the support
(B) applying a second liquid containing a basic compound onto the c
roughness (SRa value) on the surface of the ink receiving layer is 11 nm or le measured under a condition of a cutoff of 2 to 2 5 μm
It is preferred to provide the ink receiving layer thus crosslink-cured absorbing property and prevention of film crack
Although the compound containing a sulfur atom may be contained i and second liquids, it is more preferably contained in the first liquid in view o condition of the ink receiving layer
In the invention, the first liquid preferably contains the inorganic fine the coating liquid for forming an ink receiving layer (the first liquid) contains inorganic fine particles (e g gas phase silica) and a water-soluble resin (e g p alcohol), the coating liquid can be prepared, for example, in the following ma inorganic fine particles such as gas phase silica and a dispersing agent are add 10 to 20 mass % of silica fine particles in the water) The mixture is dispersed of high-speed rotation of, for example, 10000 rpm (preferably 5000 to 20000 r example, 20 minutes (preferably 10 to 30 minutes) by using a beads mill (for ' KD-P" manufactured by Shima Enterprise Co , Ltd ) Thereafter, a sulfoxide- compound (compound contaimng a sulfur atom) and a polyvinyl alcohol (PV solution are added to the dispersion liquid The amount of PVA is, for example amount that the mass of PVA is one-third of the mass of the gas phase silica T mixture is dispersed in the same rotation condition as descπbed above, whereb liquid is obtained It is preferred to adjust the pH of the coating liquid to aroun aqueous ammonia or the like, or to use a dispersing agent in order to render st coating liquid The resulting coating liquid is in a homogeneous sol state Whe liquid is applied onto a support by the following coating method, and is dried, receiving layer having a three-dimensional network structure can be formed
When the water dispersion containing the gas phase silica and the dis prepared, a previously-prepared water dispersion of the gas phase silica may b
In order to set the center plane average roughness (SRa value) on the ink receiving layer at less than 6 nm when measured under a condition of a cu μm, it is preferable to use a head-on collision high pressure disperser or an ori high pressure disperser, as descπbed above Descπptions for the head-on co pressure disperser and the onfice-passing high pressure disperser are omitted since they are already descπbed in the above sections
The solvent used in each process may be water, an organic solvent or liquids selected from water and organic solvents Organic solvents usable fo include alcohols such as methanol, ethanol, n-propanol, l-propanol and metho ketones such as acetone and methylethyl ketone, tetrahydrofuran, acetonitnle, and toluene
A dispersing agent may be added for improving dispersibility of the c The dispersing agent is not particularly limited, and may be a known cationic agent
The amount of the dispersing agent to be added is preferably from 0 1 preferably from 1 to 10%, relative to the amount of the inorganic fine particles
The pH of the first liquid is not particularly restricted, and is preferab 6 0, more preferably from 3 to 5 Bleeding of image with time can be suppre ink receiving layer is formed from the coating liquid having a pH of 2 to 6
The ink receiving layer coating liquid (first liquid) can be applied by coating method using an extrusion die coater, an air doctor coater, a blade coat a knife coater, a squeeze coater, a reverse roll coater or a bar coater
The second liquid is applied onto the coated layer (i) simultaneously application of the coating liquid for forming an ink receiving layer (first liquid the coated layer exhibits decreasing drying during drying of the coated layer f application of the first liquid More specifically, the ink receiving layer is suita manufactured by introducing the second liquid during constant drying of the c
liquid caused by the crosslinking agent does not proceed sufficiently, so that t where bronzing occurs or defects such as cracking occurs in the ink receiving
The second liquid may be prepared, for example by adding a metal c to 5%), a basic compound (e g 1 to 5%), and optionally paratoluenesulfonic a 3%) to ion-exchange water, and agitating the resultant mixture liquid sufficien values for the respective components each mean a mass % of the solid content
The expression "before the coated layer exhibits decreasing drying ra refers to a process within a few minutes from immediately after the applicatio liquid for forming an ink receiving layer, during which a phenomenon, "const is observed The constant drying rate refers to a proportional decrease in conte solvent (dispersion medium) in the coated layer to time The time during whic drying rate" is observed is descπbed, for example, in 'Kagaku Kogaku Binran Chemical Engineeπng) (pages 707 to 712, published from Maruzen Co , Ltd 1980)
As described above, the coated layer is dπed after the first liquid is a coated layer exhibits a constant rate of drying In general, the drying is earned 18O0C for 0 5 to 10 minutes (preferably 0 5 to 5 minutes) The drying time nat depending on the amount of the coating liquid to be applied, but the above ran preferable
The viscosity at a shear rate of 1 s * of the first liquid before exhibitin drying is preferably 6000 Pa or more, more preferably 7000 Pa or more, and p preferably from 8000 to 10000 Pa When the viscosity of the first liquid befor decreasing drying is 6000 Pa or more at a shear rate of 1 s 1, the center plane a roughness (SRa) on the surface of the ink receiving layer measured under a co cutoff of 2 to 2 5 μm can be 11 nm or less
The viscosity may be measured by sampling the coating liquid from t when the coating liquid is dried until immediately before the coated layer exhi
of immersing the support having the coated layer provided thereon in the seco the like
The method usable for applying the second liquid in the method (1) coating method such as methods using a curtain flow coater, an extrusion die doctor coater, a blade coater, a rod coater, a knife coater, a squeeze coater, a re or a bar coater However, methods in which the coater does not directly cont formed first coated layer are preferable, such as an extrusion die coater, a curt and a bar coater
After applying the second liquid, the ink receiving layer is usually he 1800C for 0 5 to 30 minutes so as to be dried and cured Heating to 40 to 15 minutes is particularly preferable
When the second liquid is applied simultaneously with the applicatio liquid for forming the ink receiving layer (first liquid), the first liquid and sec simultaneously applied (dual layer coating) onto the support such that the first the support, and then are dried and cured to form the ink receiving layer
The simultaneous application (dual layer coating) can be performed b method using, for example, an extrusion die coater or a curtain flow coater coated layer is dπed after the simultaneous application The drying is conduct heating the coated layer to 40 to 1500C for 0 5 to 10 minutes, preferably to 40 0 5 to 5 minutes
When the simultaneous application (dual layer coating) is conducted, with an extrusion die coater, the simultaneously extruded two coating liquids f layer in the vicinity of the discharge port of the extrusion die coater before bei onto the support, and the dual layer is applied onto the support as it is Since liquids in the dual layer before application already tend to cause crosslinking r interface between the two coating liquids during transfer onto the support, the liquids are likely to mix and become viscous in the vicinity of the discharge po
The water-soluble resin is added as a thickener in consideration of coatability water-soluble resin may be a polymer, whose examples include cellulose resi hydroxylpropylmethyl cellulose, methyl cellulose and hydroxyethyl cellulose pyrrohdone and gelatin The barrier layer liquid may contain a mordant suc described above
The surface smoothness, glossiness, transparency and coated layer st improved by applying a calender treatment by passing through a roll nip unde pressure using a super calender or a gloss calender after forming an ink receiv support However, since the calender treatment may cause a decrease of the which may result in decrease in ink absorbing property), a condition giving s in the void ratio should be employed
The roll temperature at the calender treatment is preferably from 30 t preferably from 40 to 1000C
The linear pressure between the rolls at the calender treatment is pref to 400 kg/cm, more preferably from 100 to 200 kg/cm
Since the ink receiving layer should have an enough absorption capac the droplets in the ink-jet recording, the thickness of the ink receiving layer sh determined in relation to the void ratio m the layer For example, the thickne about 15 μm or more when the amount of ink is 8 nL/mm and the void ratio i
Consideπng these points, the thickness of the ink receiving layer is pr 10 to 50 μm in the case of ink-jet recording
The diameter of the void m the ink receiving layer is preferably from μm, more preferably from 0 01 to 0 025 μm, m terms of a median diameter
The void ratio and median diameter can be measured with a mercury (trade name PORESIZER 9320-PC2, manufactured by Shimadzu Corporation
The pH of the surface of the ink receiving layer of the invention is pr to 6, more preferably from 3 5 to 4 5 The pH on the surface is measured in 3
The ink receiving layer preferably has high transparency As a rough ink receiving layer is formed on a transparent film support, the haze value is p less, more preferably 15 or less
The haze value can be measured with a haze meter (trade name HG manufactured by Suga Test Instrument Co , Ltd )
A dispersion of polymer fine particles may be added to the layers con mkjet recording medium according to the invention (for example, the ink rece back layer) This polymer fine particle dispersion is used for improving film as dimensional stability, prevention of curl, prevention of adhesion and preven The polymer fine particle dispersion is descπbed, for example m JP-A Nos 62 62- 1316648 and 62- 110066 Cracking and curling of the layer can be preven polymer fine particle dispersion having a low glass transition temperature (400 layer contaimng the mordant Curling may be also prevented by adding a po particle dispersion having a high glass transition temperature to the back layer of Japanese Patent Application No 2005-282489 is incorporated herein by ref entirety
In the following, exemplary embodiments of the invention will be de
<1> An inkjet recording medium comprising an ink receiving layer p support, wherein the ink receiving layer includes a compound containing a sul the center plane average roughness (SRa value) on the surface of the ink recei less than 6 nm when measured under a condition of a cutoff of 2 to 2 5 μm
<2> The inkjet recording medium as descπbed in <1>, wherein the co containing a sulfur atom is a compound containing a thioether group, or a sulfoxide-containing compound
<3> The inkjet recording medium as described in <1> wherein the co containing a sulfur atom is a sulfoxide-containing compound
<4> An inkjet recording medium composing an ink receiving layer pr
pH of the surface of the ink receiving layer is from 3 to 6
<7> A method for manufacturing the mkjet recording medium as des one of <1> to <6>, the method comprising at least
(A) applying at least a first liquid containing a water-soluble resin an agent onto a support to form a coated layer on the support, and
(B) applying a second liquid containing a basic compound onto the c simultaneously with the application of the first liquid, or (2) before the coated decreasing drying during drying of the coated layer formed by the application liquid, to crosslink and cure the coated layer to form an ink receiving layer, wherein at least one of the first and second liquids includes a compo sulfur
<8> The method for manufacturing an inkjet recording medium as de wherein the viscosity of the first liquid before exhibiting decreasing drying is more at a shear rate of 1 s l
<9> The method for manufacturing an inkjet recording medium as de or <8>, wherein the first liquid further includes inorganic fine particles
<10> The method for manufacturing an inkjet recording medium as d one of <7> to <9>, wherein the water-soluble resin is polyvinyl alcohol
<11> The method for manufacturing an inkjet recording medium as d one of <7> to <10>, wherein the crosslinking agent is bone acid
<12> The method for manufacturing an mkjet recording medium as d one of <7> to <11>, wherein the pH of the first liquid is 6 0 or less, and the p liquid is 7 1 or more
<13> A method for manufacturing the mkjet recording medium as de one of <1> to <4>, the method comprising dispersing at least some of the com coating liquid for forming the ink receiving layer with a head-on collision hig disperser or an orifice-passing high-pressure disperser during a preparation pr
(B) applying a second liquid containing a basic compound onto the c simultaneously with the application of the first liquid, or (2) before the coated decreasing drying during drying of the coated layer formed by the application liquid, to crosslink and cure the coated layer to form an ink receiving layer, wherein at least one of the first and second liquids includes a compo sulfur, and the viscosity of the first liquid before exhibiting decreasing drying more at a shear rate of 1 s l
EXAMPLES
In the following, the present invention is specifically described by re Examples However, the Examples should not be construed as limiting the inv Examples, "part" and "%" mean "part by mass" and "mass %", respectively, u mentioned
Pulp slurry was prepared by beating 50 parts of LBKP made of acaci LBKP made of aspen with a disk free refiner to a Canadian Freeness of 300 m
Then, 1 3% of a catiomc starch (trade name CATO 304L, manufactu NSC Corporation), 0 15% of anionic polyacrylamide (trade name DA4104, m Seiko PMC Corporation), 0 29% of an alkylketen dimer (trade name SIZEPI manufactured by Arakawa Chemical Industπes, Ltd ), 0 29% of epoxylated be amide, and 0 32% of polyamide polyamine epichlorohydπn (trade name ARA manufactured by Arakawa Chemical Industries, Ltd ) per pulp were added to t pulp slurry, and then 0 12% of antifoaming agent was added thereto
The pulp slurry prepared as descπbed above was used for papermak fourdπnier The felt face of the resulting web was pushed forcibly to a drum d a dryer canvas so as to be dπed During the drying, the tensile force of the dry set at 1 6 kg/cm Then, lg/m2 of polyvinyl alcohol (trade name KL-118, manu Kuraray Co , Ltd ) was applied onto both sides of the raw paper with a size pre
discharge treatment, and then a dispersion liquid containing aluminum oxide ( ALUMINASOL 100, manufactured by Nissan Chemical Industries, Co , Ltd ) dioxide (trade name SNOWTEX O, manufactured by Nissan Chemical Indus as antistatic agents m a mass ratio of 1 2 dispersed m water was applied to gi of 0 2 g/m , whereby a support was obtained -Preparation of liquid A (first liquid) for forming ink receiving layer-
(1) Gas phase silica fine particles, (2) ion-exchange water, (3) ' Sharo and (4) "ZA-30" in the following composition were mixed, and the mixture w with a beads mill (for example, KD-P manufactured by Shmmaru Enterprises Then, the dispersion liquid was heated to 450C and kept at the temperature for Thereafter, the following (5) boric acid, (6) polyvinyl alcohol solution, (7) "S 600", (8) polyoxyethylene laurylether, and (9) compound A (the above-describ compound "A-41" as an example of the sulfoxide-containing compound) were dispersion liquid at 3O0C, so that a coating liquid A (first liquid) for forming a layer was prepared The mass ratio of the silica fine particles to the water-solu ratio = (1) (6)) was 4 5 1, and the pH of the coating liquid A for forming an layer was 3 8, which was acidic <Composition of the coating liquid A (first liquid) for forming an ink receivin
(1) Gas phase silica fine particles (inorganic fine particles) 8 9 par (trade name AEROSIL 300SF75, manufactured by Nippon Aerosil C
(2) Ion-exchange water 51 8 pa
(3) "SHAROL DC-902P" (51 5% aqueous solution) 0 78 pa (Dispersing agent, manufactured by Dai-ichi Kogyo Seiyaku Co , Ltd
(4) "ZA-30" 0 48 pa (manufactured by Daiichi Kigenso Kagakukogyo Co , Ltd )
(5) Boric acid (crosslmking agent) 0 33 pa
(6) Polyvinyl alcohol (water-soluble resin) solution 26 0 par
Ltd )
Ion-exchange water 22 7 p
(7) "SUPERFLEX 600" 1 11 p (manufactured by Dai-ichi Kogyo Seiyaku Co , Ltd )
(8) Polyoxyethylene laurylether (surfactant) 0 44 p (trade name "EMULGEN 109P" (10 % aqueous solution), HLB val manufactured by Kao Corporation)
(9) Compound A (the exemplary compound ' A-41" as an example o sulfoxide-containing compound) 0 43 p
Compound 1
-Production of inkjet recording medium-
The front surface of the support was subjected to corona discharge tre 170 ml/m2 of the first liquid together with 10 8 ml/m2 of five-fold diluted aque poly aluminum chloπde (trade name "ALUFESfE 83", manufactured by Taim Co , Ltd ) were in-line coated on the front surface of the support (coating proc
thickness was 32 μm was obtained <Composition of second hquid>
(1) Boric acid 0 65 pa
(2) Ammonium zirconyl carbonate 2 5 par
(trade name ZIRCOZOL AC-7 (28% aqueous solution), manufactur Kigenso Kagaku Kogyo Co , Ltd )
(3) Ammonium carbonate 4 0 par (Primary Kanto Chemical co , Inc )
(4) Ion-exchange water 89 4 pa
(5) Polyoxyethylene laurylether (surfactant) 6 0 par
(trade name "EMULGEN 109P", manufactured by Kao Corporation, solution), HLB value 13 6) [Example 2]
An mkjet recording medium was produced in the same manner as in except that the compound A contained in the coating liquid A (first liquid) for receiving layer was replaced by the following compound B (a compound cont groups), and 0 8 part of ethanol was also contained in the coating liquid A
Compound B
An lnkjet recording medium was produced in the same manner as in except that the compound A was not contained in the coating liquid A (first li an ink receiving layer, and 2 3 parts of ethanol was also contained m the coati [Comparative example 2]
An inkjet recording medium was produced in the same manner as m except that 2 3 parts of ethanol was also contained in the coating liquid A (firs forming an ink receiving layer [Comparative example 3]
An inkjet recording medium was produced m the same manner as in Example 2 except that the compound A contained in the coating liquid A (first forming an ink receiving layer was replaced with the same amount of the com [Evaluation]
The following evaluations were conducted on each of the inkjet reco obtained in Examples and Comparative Examples The evaluation results are 1
(1) Center plane average roughness (SRa value)
The center plane average roughness (SRa value) was measured with manufactured by Zygo Corporation at a cutoff of 2 to 2 5 μm based on the foll measurement and analysis conditions <Measurement and analysis conditions>
Measurement length 5 mm in X-direction, 5 mm in Y-direction
Objective lens 10 magnification
Bandpass filter 2 to 2 5 μm
(2) Viscosity of coating liquid
After application of the first liquid, the coating liquid which was drie coated layer exhibited a decreasing drying rate was sampled from the support, was measured with RHEOSTRESS 600
according to the following evaluation criteria Evaluation criteria
A Color fading was scarcely observed
B Slight color fading was observed
C Considerable color fading was observed
D Degree of color fading was severe
(4) Evaluation of image density
A black solid image was printed on each of the recording media with (trade name "PMG-800C", manufactured by Seiko-Epson Corporation) loade genuine ink set The concentration in the black area was measured with a refle densitometer (trade name XRITE 938, manufactured by Xrite Corporation)
(5) Cracking
Cracks in the produced inkjet recording media were evaluated with n
A No crack was observed
B Fine cracks were observed
C Remarkable cracks were observed
Table 1
As is clear from Table 1, the image density was high and the ozone r excellent in the case of the inkjet recording media of Examples 1 to 3 On the ozone resistance was insufficient in the case of the inkjet recording medium o Example 1 containing no sulfur compound, and the image density was decrea of the inkjet recording media of Comparative Examples 2 and 3 whose center roughness (SRa value) was outside the range defined in the invention
According to the invention, an inkjet recording medium can be provi excellent ozone resistance and capable of suppressing decrease in image densi manufacturing such an mkjet recording medium is also provided
All publications, patent applications, and technical standards mention specification are herein incorporated by reference to the same extent as if eac publication, patent application, or technical standard was specifically and indi indicated to be incorporated by reference
Claims
1 An mkjet recording medium comprising an ink receiving layer support wherein the ink receiving layer includes a compound containing a s center plane average roughness (SRa value) on a surface of the ink receivin 6 nm when measured under a condition of a cutoff of 2 to 2 5 μm
2 The inkjet recording medium according to claim 1 wherein the containing a sulfur atom is a compound containing a thioether group or a sulfoxide-containing compound
3 The inkjet recording medium according to claim 1 wherein the containing a sulfur atom is a sulfoxide-containing compound
4 An inkjet recording medium comprising an ink receiving layer support wherein the ink receiving layer includes a sulfoxide-containing com center plane average roughness (SRa value) on a surface of the ink receiving less when measured under a condition of a cutoff of 2 to 2 5 μm
5 The inkjet recording medium according to any one of claim 1 t wherein a haze value of the ink receiving layer is 20 or less
6 The inkjet recording medium according to any one of claim 1 t wherein a pH of a surface of the ink receiving layer is from 3 to 6
7 A method for manufacturing the inkjet recording medium accor of claim 1 to claim 4 the method comprising
sulfur
8 The method for manufacturing an lnkjet recording medium acco wherein a viscosity of the first liquid before exhibiting decreasing drying is 6 at a shear rate of 1 s l
9 The method for manufacturing an inkjet recording medium acco wherein the first liquid further includes inorganic fine particles
10 The method for manufacturing an inkjet recording medium acc 7, wherein the water-soluble resin is polyvinyl alcohol
11 The method for manufacturing an inkjet recording medium acc 7, wherein the crosslinking agent is boric acid
12 The method for manufacturing an inkjet recording medium acc 7, wherein a pH of the first liquid is 6 0 or less, and a pH of the second liquid i
13 A method for manufacturing the inkjet recording medium accor of claim 1 to claim 3, the method composing dispersing at least some of the c coating liquid for forming the ink receiving layer with a head-on collision hig disperser or an oπfice-passing high-pressure disperser during a preparation pr coating liquid
14 A method for manufacturing an mkjet recording medium comp receiving layer that includes a compound containing a sulfur atom and has a s center plane average roughness (SRa value) is 11 nm or less when measured u
wherein at least one of the first and second liquids includes a compo sulfur, and a viscosity of the first liquid before exhibiting decreasing drying is more at a shear rate of 1 s ]
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP06810163A EP1937484A4 (en) | 2005-09-28 | 2006-09-08 | Inkjet recording medium and method for manufacturing the same |
| US12/088,392 US20090246422A1 (en) | 2005-09-28 | 2006-09-08 | Inkjet recording medium and method for manufacturing the same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2005-282489 | 2005-09-28 | ||
| JP2005282489A JP2007090645A (en) | 2005-09-28 | 2005-09-28 | Inkjet recording medium and its manufacturing method |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007037134A1 true WO2007037134A1 (en) | 2007-04-05 |
| WO2007037134A9 WO2007037134A9 (en) | 2007-05-24 |
Family
ID=37899564
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2006/318319 WO2007037134A1 (en) | 2005-09-28 | 2006-09-08 | Inkjet recording medium and method for manufacturing the same |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20090246422A1 (en) |
| EP (1) | EP1937484A4 (en) |
| JP (1) | JP2007090645A (en) |
| CN (1) | CN101272917A (en) |
| WO (1) | WO2007037134A1 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100233374A1 (en) * | 2009-03-13 | 2010-09-16 | Fujifilm Corporation | Method of producing inkjet recording medium |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104822870B (en) * | 2013-01-31 | 2017-10-27 | 格拉特菲尔特盖恩斯巴赫股份有限公司 | Crosslinking/functionalization system for paper or nonwoven web |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000263928A (en) * | 1999-03-19 | 2000-09-26 | Konica Corp | Ink jet recording sheet and method for ink jet recording using it |
| JP2001341412A (en) * | 2000-03-27 | 2001-12-11 | Oji Paper Co Ltd | Ink jet recording medium |
| JP2002192830A (en) * | 2000-12-25 | 2002-07-10 | Konica Corp | Ink jet recording paper |
| JP2003165269A (en) * | 2001-11-30 | 2003-06-10 | Mitsubishi Paper Mills Ltd | Ink jet recording material and its manufacturing method |
| JP2003285535A (en) * | 2002-03-27 | 2003-10-07 | Mitsubishi Paper Mills Ltd | Ink jet recording material |
| JP2003291493A (en) * | 2002-03-29 | 2003-10-14 | Mitsubishi Paper Mills Ltd | Method for manufacturing inkjet recording material |
| JP2004299373A (en) * | 2003-03-19 | 2004-10-28 | Fuji Photo Film Co Ltd | Ink jet recording method |
| JP2005081802A (en) * | 2003-09-11 | 2005-03-31 | Konica Minolta Holdings Inc | Inkjet recording sheet and its manufacturing method |
| JP2005212172A (en) * | 2004-01-28 | 2005-08-11 | Fuji Photo Film Co Ltd | Inkjet recording medium and inkjet recording method |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6492005B1 (en) * | 1999-03-09 | 2002-12-10 | Konica Corporation | Ink jet recording sheet |
| US6551695B2 (en) * | 2000-01-14 | 2003-04-22 | Mitsubishi Paper Mills, Limited | Ink-jet recording material |
| DE60119799T2 (en) * | 2000-01-28 | 2007-04-26 | Oji Paper Co., Ltd. | Ink jet recording material |
| JP2003127533A (en) * | 2001-10-26 | 2003-05-08 | Fuji Photo Film Co Ltd | Ink jet recording sheet |
-
2005
- 2005-09-28 JP JP2005282489A patent/JP2007090645A/en not_active Abandoned
-
2006
- 2006-09-08 CN CNA2006800353435A patent/CN101272917A/en active Pending
- 2006-09-08 EP EP06810163A patent/EP1937484A4/en not_active Withdrawn
- 2006-09-08 WO PCT/JP2006/318319 patent/WO2007037134A1/en active Application Filing
- 2006-09-08 US US12/088,392 patent/US20090246422A1/en not_active Abandoned
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2000263928A (en) * | 1999-03-19 | 2000-09-26 | Konica Corp | Ink jet recording sheet and method for ink jet recording using it |
| JP2001341412A (en) * | 2000-03-27 | 2001-12-11 | Oji Paper Co Ltd | Ink jet recording medium |
| JP2002192830A (en) * | 2000-12-25 | 2002-07-10 | Konica Corp | Ink jet recording paper |
| JP2003165269A (en) * | 2001-11-30 | 2003-06-10 | Mitsubishi Paper Mills Ltd | Ink jet recording material and its manufacturing method |
| JP2003285535A (en) * | 2002-03-27 | 2003-10-07 | Mitsubishi Paper Mills Ltd | Ink jet recording material |
| JP2003291493A (en) * | 2002-03-29 | 2003-10-14 | Mitsubishi Paper Mills Ltd | Method for manufacturing inkjet recording material |
| JP2004299373A (en) * | 2003-03-19 | 2004-10-28 | Fuji Photo Film Co Ltd | Ink jet recording method |
| JP2005081802A (en) * | 2003-09-11 | 2005-03-31 | Konica Minolta Holdings Inc | Inkjet recording sheet and its manufacturing method |
| JP2005212172A (en) * | 2004-01-28 | 2005-08-11 | Fuji Photo Film Co Ltd | Inkjet recording medium and inkjet recording method |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP1937484A4 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100233374A1 (en) * | 2009-03-13 | 2010-09-16 | Fujifilm Corporation | Method of producing inkjet recording medium |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1937484A4 (en) | 2009-11-11 |
| WO2007037134A9 (en) | 2007-05-24 |
| CN101272917A (en) | 2008-09-24 |
| US20090246422A1 (en) | 2009-10-01 |
| JP2007090645A (en) | 2007-04-12 |
| EP1937484A1 (en) | 2008-07-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE60304525T2 (en) | Inkjet recording sheet, method, and ink | |
| US7785679B2 (en) | Support for image recording material and image recording material | |
| US20070237911A1 (en) | Coating liquid for ink receiving layer, production method thereof, ink jet recording medium and production method thereof | |
| JP2002052810A (en) | Sheet for ink jet recording | |
| EP1621359B1 (en) | Recording medium and its method of manufacture | |
| KR20070101320A (en) | Support for image recording material and image recording material | |
| JP4533321B2 (en) | Ink jet recording medium and manufacturing method thereof | |
| DE60310832T2 (en) | An ink jet recording medium and a method of forming an image | |
| JP2003011498A (en) | Sheet for ink-jet recording | |
| JP2010110944A (en) | Inkjet recording medium | |
| EP1937484A1 (en) | Inkjet recording medium and method for manufacturing the same | |
| US7736709B2 (en) | Recording medium and method for manufacturing recording medium | |
| US20070087936A1 (en) | Recording medium and method of manufacturing inkjet recording medium | |
| US8236395B2 (en) | Inkjet recording medium | |
| JP4291737B2 (en) | recoding media | |
| JP2006321176A (en) | Recording medium | |
| JP2004345309A (en) | Ink jet recording medium | |
| JP2003320747A (en) | Inkjet recording sheet | |
| JP4319470B2 (en) | Ink jet recording medium and manufacturing method thereof | |
| JP2003305944A (en) | Ink jet recording sheet | |
| JP2004358774A (en) | Inkjet record medium and method for manufacturing it | |
| JP2003326843A (en) | Ink jet recording sheet | |
| JP2007185885A (en) | Inkjet recording medium and its manufacturing method | |
| JP2006305939A (en) | Recording medium and its manufacturing method | |
| JP2006181877A (en) | Inkjet recording medium, its manufacturing method and coating liquid for recording layer of inkjet recording medium |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 200680035343.5 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 12088392 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2006810163 Country of ref document: EP |