WO2006007286A2 - Polymerizable compositions comprising nanoparticles - Google Patents
Polymerizable compositions comprising nanoparticles Download PDFInfo
- Publication number
- WO2006007286A2 WO2006007286A2 PCT/US2005/019774 US2005019774W WO2006007286A2 WO 2006007286 A2 WO2006007286 A2 WO 2006007286A2 US 2005019774 W US2005019774 W US 2005019774W WO 2006007286 A2 WO2006007286 A2 WO 2006007286A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- brightness enhancing
- meth
- film
- enhancing film
- acrylate
- Prior art date
Links
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B6/00—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings
- G02B6/0001—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings specially adapted for lighting devices or systems
- G02B6/0011—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings specially adapted for lighting devices or systems the light guides being planar or of plate-like form
- G02B6/0033—Means for improving the coupling-out of light from the light guide
- G02B6/005—Means for improving the coupling-out of light from the light guide provided by one optical element, or plurality thereof, placed on the light output side of the light guide
- G02B6/0053—Prismatic sheet or layer; Brightness enhancement element, sheet or layer
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G8/00—Condensation polymers of aldehydes or ketones with phenols only
- C08G8/04—Condensation polymers of aldehydes or ketones with phenols only of aldehydes
- C08G8/08—Condensation polymers of aldehydes or ketones with phenols only of aldehydes of formaldehyde, e.g. of formaldehyde formed in situ
- C08G8/10—Condensation polymers of aldehydes or ketones with phenols only of aldehydes of formaldehyde, e.g. of formaldehyde formed in situ with phenol
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F2/00—Processes of polymerisation
- C08F2/44—Polymerisation in the presence of compounding ingredients, e.g. plasticisers, dyestuffs, fillers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/102—Esters of polyhydric alcohols or polyhydric phenols of dialcohols, e.g. ethylene glycol di(meth)acrylate or 1,4-butanediol dimethacrylate
- C08F222/1025—Esters of polyhydric alcohols or polyhydric phenols of dialcohols, e.g. ethylene glycol di(meth)acrylate or 1,4-butanediol dimethacrylate of aromatic dialcohols
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/04—Optical elements characterised by the material of which they are made; Optical coatings for optical elements made of organic materials, e.g. plastics
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/04—Prisms
- G02B5/045—Prism arrays
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/12—Reflex reflectors
- G02B5/122—Reflex reflectors cube corner, trihedral or triple reflector type
- G02B5/124—Reflex reflectors cube corner, trihedral or triple reflector type plural reflecting elements forming part of a unitary plate or sheet
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B6/00—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings
- G02B6/0001—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings specially adapted for lighting devices or systems
- G02B6/0011—Light guides; Structural details of arrangements comprising light guides and other optical elements, e.g. couplings specially adapted for lighting devices or systems the light guides being planar or of plate-like form
- G02B6/0065—Manufacturing aspects; Material aspects
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y20/00—Nanooptics, e.g. quantum optics or photonic crystals
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F222/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical and containing at least one other carboxyl radical in the molecule; Salts, anhydrides, esters, amides, imides, or nitriles thereof
- C08F222/10—Esters
- C08F222/1006—Esters of polyhydric alcohols or polyhydric phenols
- C08F222/103—Esters of polyhydric alcohols or polyhydric phenols of trialcohols, e.g. trimethylolpropane tri(meth)acrylate
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2323/00—Functional layers of liquid crystal optical display excluding electroactive liquid crystal layer characterised by chemical composition
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2323/00—Functional layers of liquid crystal optical display excluding electroactive liquid crystal layer characterised by chemical composition
- C09K2323/03—Viewing layer characterised by chemical composition
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2323/00—Functional layers of liquid crystal optical display excluding electroactive liquid crystal layer characterised by chemical composition
- C09K2323/03—Viewing layer characterised by chemical composition
- C09K2323/031—Polarizer or dye
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/02—Diffusing elements; Afocal elements
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/913—Material designed to be responsive to temperature, light, moisture
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/773—Nanoparticle, i.e. structure having three dimensions of 100 nm or less
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/70—Nanostructure
- Y10S977/778—Nanostructure within specified host or matrix material, e.g. nanocomposite films
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/84—Manufacture, treatment, or detection of nanostructure
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/902—Specified use of nanostructure
- Y10S977/932—Specified use of nanostructure for electronic or optoelectronic application
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/902—Specified use of nanostructure
- Y10S977/932—Specified use of nanostructure for electronic or optoelectronic application
- Y10S977/939—Electron emitter, e.g. spindt emitter tip coated with nanoparticles
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24355—Continuous and nonuniform or irregular surface on layer or component [e.g., roofing, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24355—Continuous and nonuniform or irregular surface on layer or component [e.g., roofing, etc.]
- Y10T428/24372—Particulate matter
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31855—Of addition polymer from unsaturated monomers
Definitions
- Brightness enhancing films are utilized in many electronic products to increase the brightness of a backlit flat panel display such as a liquid crystal display (LCD) including those used in electroluminescent panels, laptop computer displays, word processors, desktop monitors, televisions, video cameras, as well as automotive and aviation displays.
- LCD liquid crystal display
- Brightness enhancing films desirably exhibit specific optical and physical properties including the index of refraction of a brightness enhancing film that is related to the brightness gain (i.e. "gain") produced.
- gain the brightness gain
- Improved brightness can allow the electronic product to operate more efficiently by using less power to light the display, thereby reducing the power consumption, placing a lower heat load on its components, and extending the lifetime of the product.
- Brightness enhancing films have been prepared from high index of refraction monomers that are cured or polymerized, as described for example in U.S. Pat. Nos. 5,908,874; 5,932,626; 6,107,364; 6,280,063; 6,355,754; as well as EP 1 014113 and WO 03/076528.
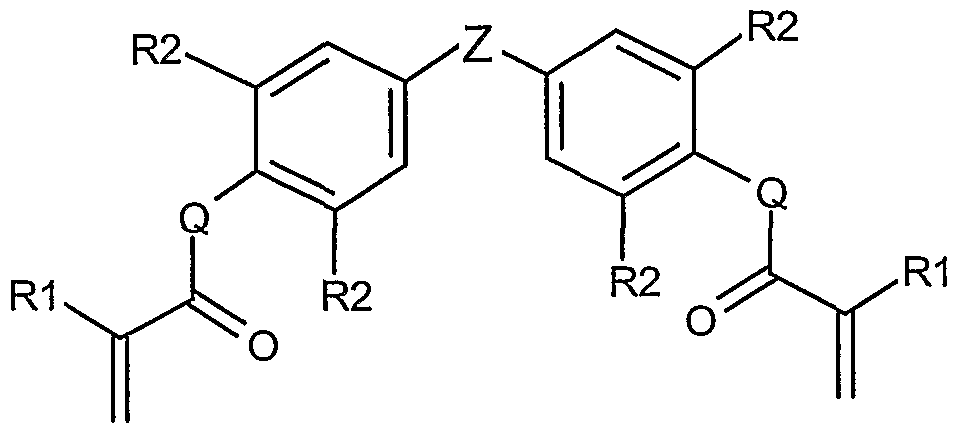
- a brightness enhancing film having a brightness enhancing polymerized structure comprises the reaction product of a polymerizable composition comprising at least about 15 wt-% of one or more first monomers selected from
- R2 is independently H or Br
- Z is independently -C(CH 3 ) 2 - 5 -CH 2 -, -C(O)-,. -S-, -S(O)-, or -S(O) 2 -, and
- Q is independently O or S
- R2 is independently H or Br
- Z is independently -C(CH 3 ) 2 -, -CH 2 -, -C(O)-, -S-, -S(O)-, or -S(O) 2 -, and
- L is a linking group independently selected from linear and branched C 2 - C 12 alkyl groups wherein the carbon chain is optionally substituted with one or more oxygen group and/or the carbon atoms are optionally substituted with one or more hydroxyl groups; and mixtures of i) and ii);
- a brightness enhancing film having a brightness enhancing polymerized structure comprises the reaction product of a polymerizable composition comprising a) at least about 15 wt-% of one or more (meth)acrylated aromatic epoxy oligomers; b) at least about 10 wt-% inorganic nanoparticles; and c) optionally a crosslinking agent comprising at least two (meth)acrylate functional groups.
- a brightness enhancing film having a brightness enhancing polymerized structure comprises the reaction product of a substantially solvent free polymerizable composition comprising an organic component, comprising one or more ethylenically unsaturated monomers, and at least 10 wt-% inorganic nanoparticles.
- the organic component has a viscosity of less than 1000 cps at 180°F.
- the organic component may comprise at least one oligomeric ethylenically unsaturated monomer having a number average molecular weight of greater than 450 g/mole.
- a brightness enhancing film having a brightness enhancing polymerized structure comprises the reaction product of a substantially solvent free polymerizable composition comprising an organic component comprising one or more ethylenically unsaturated monomers wherein the organic component has a refractive index of at least 1.54; and at least 10 wt-% inorganic nanoparticles.
- the amount of inorganic particles is typically less than about 60 wt-%.
- the inorganic nanoparticles are preferably surface modified.
- the inorganic nanoparticles typically comprise silica, zirconia, titania, antimony oxides, alumina, tin oxides, mixed metal oxides thereof, and mixtures thereof.
- the nanoparticles may range in primary particle size from 5 nm to 75 nm, from 10 run to 30 nm, from 5 nm to 15 nm.
- the first monomer preferably consists of the reaction product of Tetrabromobisphenol A diglycidyl ether and (meth) acrylic acid.
- the polymerizable compositions may further comprise at least one second high index monomer (i.e. different than the first monomer).
- the polymerizable composition is preferably free of methacrylate functional monomer.
- the invention relates to an article comprising the brightness enhancing film in contact with a second optical film or light guide.
- the second optical film may include a turning film, a diffuser, an absorbing polarizer, a reflective polarizer, or a protective cover film.
- compositions described herein may also be advantageous for other optical or microstructured articles.
- FIG. 1 is a perspective view of an illustrative microstructure-bearing optical product of the present invention.
- FIG. 2 is a schematic view of an illustrative backlit liquid crystal display including the brightness enhancing film of the invention.
- Index of refraction refers to the absolute refractive index of a material (e.g., a monomer) that is understood to be the ratio of the speed of electromagnetic radiation in free space to the speed of the radiation in that material.
- the refractive index can be measured using known methods and is generally measured using an Abbe refractometer in the visible light region (available commercially, for example, from Fisher Instruments of Pittsburgh, PA). It is generally appreciated that the measured index of refraction can vary to some extent depending on the instrument.
- (Meth)acrylate refers to both acrylate and methacrylate compounds.
- Polymerizable composition refers to the total composition including the organic component that comprises at least one polymerizable monomer and the optional inorganic nanoparticles.
- Organic component refers to all of the components of the composition except for the inorganic nanoparticles.
- the organic component and polymerizable composition are one in the same.
- nanoparticles is defined herein to mean particles (primary particles or associated primary particles) with a diameter less than about 100 nm.
- “Surface modified colloidal nanoparticle” refers to nanoparticles each with a modified surface such that the nanoparticles provide a stable dispersion.
- Aggregation refers to a strong association between primary particles that may be chemically bound to one another. The breakdown of aggregates into smaller particles is difficult to achieve.
- Agglomeration refers to a weak association between primary particles which may be held together by charge or polarity and can be broken down into smaller entities.
- Primary particle size refers to the mean diameter of a single (non-aggregate, non- agglomerate) particle.
- Brightness enhancing films generally enhance on-axis luminance (referred herein as "brightness") of a lighting device.
- Brightness enhancing films can be light transmissible, microstructured films.
- the microstructured topography can be a plurality of prisms on the film surface such that the films can be used to redirect light through reflection and refraction.
- the height of the prisms typically ranges from about 1 to about 75 microns.
- the microstructured optical film can increase brightness of an optical display by limiting light escaping from the display to within a pair of planes disposed at desired angles from a normal axis running through the optical display.
- Brightness enhancing films include microstructure-bearing articles having a regular repeating pattern of symmetrical tips and grooves.
- Other examples of groove patterns include patterns in which the tips and grooves are not symmetrical and in which the size, orientation, or distance between the tips and grooves is not uniform. Examples of brightness enhancing films are described in Lu et al., U.S. Pat. No. 5,175,030, and Lu, U.S. Pat. No. 5,183,597.
- a brightness enhancing film 30 may comprise a base layer 2 and optical layer 4.
- Optical layer 4 comprises a linear array of regular right prisms, identified as prisms 6, 8, 12, and 14.
- Each prism, for example, prism 6, has a first facet 10 and a second facet 11.
- the prisms 6, 8, 12, and 14 are formed on base 2 that has a first surface 18 on which the prisms are formed and a second surface 20 that is substantially flat or planar and opposite first surface 18.
- right prisms it is meant that the apex angle ⁇ is typically about 90°. However, this angle can range from 70° to 120° and may range from 80° to 100°. Further the apexes can be sharp, rounded, flattened or truncated.
- the prism facets need not be identical, and the prisms may be tilted with respect to each other.
- the relationship between the total thickness 24 of the optical article, and the height 22 of the prisms, may vary. However, it is typically desirable to use relatively thinner optical layers with well-defined prism facets.
- a typical ratio of prism height 22 to total thickness 24 is generally between 25/125 and 2/125.
- the base layer of the brightness enhancing film can be of a nature and composition suitable for use in an optical product, i.e. a product designed to control the flow of light.
- an optical product i.e. a product designed to control the flow of light.
- Many materials can be used as a base material provided the material is sufficiently optically clear and is structurally strong enough to be assembled into or used within a particular optical product.
- the base material is chosen that has sufficient resistance to temperature and aging that performance of the optical product is not compromised over time.
- the particular chemical composition and thickness of the base material for any optical product can depend on the requirements of the particular optical product that is being constructed. That is, balancing the needs for strength, clarity, temperature resistance, surface energy, adherence to the optical layer, among others.
- the thickness of the base layer is typically at least about 0.025 millimeters (mm) and more typically at least about 0.25 mm. Further, the base layer generally has a thickness of no more than about 1 mm.
- Useful base layer materials include cellulose acetate butyrate, cellulose acetate propionate, cellulose triacetate, polyether sulfone, polymethyl methacrylate, polyurethane, polyester, polycarbonate, polyvinyl chloride, syndiotactic polystyrene, polyethylene naphthalate, copolymers or blends based on naphthalene dicarboxylic acids, and glass.
- the base material can contain mixtures or combinations of these materials.
- the base may be multi-layered or may contain a dispersed phase suspended or dispersed in a continuous phase.
- Exemplary base layer materials include polyethylene terephthalate (PET) and polycarbonate. Examples of useful PET films include photograde polyethylene terephthalate (PET) and PET commercially available from DuPont Films of Wilmington, Delaware, under the trade designation "Melinex".
- the base layer material can be optically active, and can act as a polarizing material.
- a number of base layer materials are known to be useful as polarizing materials.
- Polarization of light through a film can be accomplished, for example, by the inclusion of dichroic polarizers in a film material that selectively absorbs passing light.
- Light polarization can also be achieved by including inorganic materials such as aligned mica chips or by a discontinuous phase dispersed within a continuous film, such as droplets of light modulating liquid crystals dispersed within a continuous film.
- a film can be prepared from microfine layers of different materials.
- the polarizing materials within the film can be aligned into a polarizing orientation, for example, by employing methods such as stretching the film, applying electric or magnetic fields, and coating techniques.
- polarizing films include those described in U.S. Pat. Nos. 5,825,543 and 5,783,120.
- the use of these polarizer films in combination with a brightness enhancement film has been described in U.S. Pat. No. 6,111,696.
- Another example of a polarizing film is described in U.S. Pat. No. 5,882,774.
- Multilayer polarizing films are sold by 3M Company, St. Paul, MN under the trade designation DBEF (Dual Brightness Enhancement Firm). The use of such multilayer polarizing optical film in a brightness enhancement film has been described in U.S. Pat. No. 5,828,488.
- polarizing and non-polarizing films can also be useful as the base layer for brightness enhancing films of the invention such as described in U.S. Pat. Nos. 5,612,820 and 5,486,949, among others.
- the present invention relates to polymerizable resin compositions useful for optical articles and in particular the optical layer of a brightness enhancing film.
- the brightness enhancing or other microstructured articles comprise a polymerized structure comprising the reaction product of an organic component optionally comprising a plurality of nanoparticles.
- the polymerized structure can be an optical element or optical product constructed of a base layer and an optical layer.
- the base layer and optical layer can be formed from the same or different polymer material.
- the polymerizable resin composition comprises a first monomer having a refractive index of at least 1.47, for most product applications; whereas the polymerizable resin composition of a turning film may have a refractive index as low as 1.44. High transmittance in the visible light spectrum is also typically preferred.
- the composition of the invention is preferably polymerizable by irradiation with ultraviolet or visible light in the presence of a photoinitiator. hi one embodiment, the invention relates to a polymerizable composition comprising a first monomer that comprises a major portion having the following general structures I or II:
- each Rl is independently hydrogen or methyl.
- Each R2 is independently hydrogen or bromine.
- Each Z is independently -C(CH 3 ) 2 -, -CH 2 -, -C(O)-, -S-, -S(O)-, or -S(O) 2 -, and each Q is independently O or S.
- the Rl groups are the same.
- the R2 groups are the same as each other well.
- L is a linking group. L may independently comprise a branched or linear C 2 -C 12 alkyl group. The carbon chain of the alkyl group may optionally be substituted with one or more oxygen groups.
- the carbon atoms of the alkyl group may optionally be substituted with one or more hydroxyl groups.
- L may be -CH 2 CH(OH)CH 2 -.
- the linking groups are the same.
- the alkyl group comprises no more than 8 carbon atoms and more preferably no more than 6 carbon atoms.
- the first monomer may be synthesized or purchased. As used herein, major portion refers to at least 60-70 wt-% of the monomer containing the specific structure(s) just described. It is commonly appreciated that other reaction products are also typically present as a byproduct of the synthesis of such monomers.
- the first monomer is preferably the reaction product of Tetrabromobisphenol A diglycidyl ether and acrylic acid.
- the first monomer may be obtained from UCB Corporation, Smyrna, GA under the trade designation "RDX-51027". This material comprises a major portion of 2-propenoic acid, (l-methylethylidene)bis[(2,6-dibromo-4,l- phenylene)oxy(2-hydroxy-3 , 1 -propanediyl)] ester.
- the brightness enhancing film is comprised of the reaction product of only one of these first monomers and in particular the reaction product of Tetrabromobisphenol A diglycidyl ether and acrylic acid.
- the polymerizable composition comprises at least one (meth)acrylated aromatic epoxy oligomer.
- Various (meth)acrylated aromatic epoxy oligomers are available commercially available.
- (meth)acrylated aromatic epoxy (described as a modified epoxy acrylates), are available from Sartomer, Exton , PA under the trade designation "CNl 18", “CNl 15” and "CNl 12C60”.
- a (meth)acrylated aromatic epoxy oligomer (described as an epoxy novolak acrylate blended with 40% trimethylolpropane triacrylate), is available from Sartomer under the trade designation "CN112C60".
- the aromatic epoxy acrylate is derived from bisphenol A, such as those of II. In other embodiments, however, the aromatic epoxy acrylate may be derived from a monomer different than bisphenol A.
- the organic component may comprise aromatic epoxy acrylate, at least one crosslinking agent, at least one reactive diluent, and at least one other ethylenically unsaturated monomer.
- the organic component of the polymerizable composition may only include the aromatic epoxy acrylate and crosslinking agent or the aromatic epoxy acrylate and reactive diluent, each of such including photoinitiator.
- the aromatic epoxy acrylate may be monofunctional provided that the polymerizable composition includes at least one ingredient that comprises at least two ethylenically unsaturated polymerizable groups.
- the aromatic epoxy acrylate may have three or more (meth)acrylate groups.
- the aromatic epoxy (meth)acrylate may be halogenated, typically having a refractive index of greater than 1.56. hi other aspects, the aromatic epoxy (meth)acrylate may have a refractive index of less than 1.56.
- the aromatic epoxy (meth)acrylate may have a viscosity of greater than 2150 cps at 65°C. Less than 30 wt-% of the aromatic epoxy (meth) acrylate may be employed, for example in combination with a reactive diluent, hi other embodiments, the aromatic epoxy (meth)acrylate may have a viscosity of less than 2150 cps at 65°C, and diluent may not be employed. Greater than 30 wt-% of the aromatic epoxy (meth)acrylate may be employed in organic component.
- the first monomer and/or aromatic epoxy (meth)acrylate is preferably present in the polymerizable composition in an amount of at least about 15 wt-% (e.g. 20 wt-%, 30 wt-%, 35 wt-%, 40 wt-%, 45 wt-% and 50 wt-% and any amount there between). Typically, the amount of the first monomer and/or aromatic epoxy (meth)acrylate does not exceed about 60 wt-%.
- the polymerizable composition of the invention can optionally include at least one and preferably only one crosslinking agent.
- Multi-functional monomers can be used as crosslinking agents to increase the glass transition temperature of the polymer that results from the polymerizing of the polymerizable composition.
- the glass transition temperature can be measured by methods known in the art, such as Differential Scanning Calorimetry (DSC), modulated DSC, or Dynamic Mechanical Analysis.
- DSC Differential Scanning Calorimetry
- modulated DSC modulated DSC
- Dynamic Mechanical Analysis Dynamic Mechanical Analysis
- the polymeric composition is sufficiently crosslinked to provide a glass transition temperature that is greater than 45°C.
- the crosslinking agent comprises at least two (meth)acrylate functional groups. Since methacrylate groups tend to be less reactive than acrylate groups, it is preferred that the crosslinking agent comprises three or more acrylate groups.
- Suitable crosslinking agents include for example hexanediol acrylate (HDDA), pentaerythritol tri(meth)acrylate, pentaerythritol tetra(meth)acrylate, trimethylolpropane tri(methacrylate), dipentaerythritol penta(meth) acrylate, dipentaerythritol hexa(meth)acrylate, trimethylolpropane ethoxylate tri(meth)acrylate, glyceryl tri(meth)acrylate, pentaerythritol propoxylate tri(meth)acrylate, and ditrimethylolpropane tetra(meth)acrylate. Any one or combination of crosslinking agents may
- the crosslinking agent is preferably present in the polymerizable composition in an amount of at least about 2 wt-%. Typically, the amount of crosslinking agent is not greater than about 25 wt-%. The crosslinking agent may be present in any amount ranging from about 5 wt-% and about 15 wt-%.
- Preferred crosslinking agents include hexanediol diacrylate (HDDA), pentaerythritol tri(meth)acrylate, pentaerythritol tetra(meth)acrylate, dipentaerythritol penta(meth)acrylate, trimethylolpropane tri(meth)acrylate, ditrimethylolpropane tetra(meth)acrylate, and mixtures thereof. More preferably the crosslinking agent(s) is free of methacrylate functionality.
- Pentaerythritol triacrylate PETA
- dipentaerythritol pentaacrylate are commercially available from Sartomer Company, Exton, PA under the trade designations "SR444" and “SR399LV” respectively; from Osaka Organic Chemical Industry, Ltd. Osaka, Japan under the trade designation “Viscoat #300”; from Toagosei Co. Ltd., Tokyo, Japan under the trade designation "Aronix M- 305"; and from Eternal Chemical Co., Ltd., Kaohsiung, Taiwan under the trade designation "Etermer 235".
- Trimethylol propane triacrylate (TMPTA) and ditrimethylol propane tetraacrylate (di-TMPTA) are commercially available from Sartomer Company under the trade designations "SR351" and "SR355".
- TMPTA is also available from Toagosei Co. Ltd. under the trade designation "Aronix M-309”.
- ethoxylated trimethylolpropane triacrylate and ethoxylated pentaerythritol triacrylate are commercially available from Sartomer under the trade designations "SR454" and "SR494" respectively.
- a crosslinking agent need not be employed.
- 10 wt-% phenoxy ethyl acrylate can be combined with the first monomer and at least 10 wt-% of the surface modified nanoparticles (e.g. of Example 1).
- the (e.g. first, aromatic epoxy (meth)acrylate) monomer(s) as well as optional crosslinking agent and optional reactive diluent typically comprise (meth)acrylate functional groups, hi preferred embodiments the polymerizable composition comprises solely acrylate functionality and thus is substantially free of methacrylate functional groups.
- the polymerizable composition described herein contains (e.g. surface modified) inorganic oxide particles.
- the size of such particles is chosen to avoid significant visible light scattering. It may be desirable employ a mixture of inorganic oxide particle types to optimize an optical or material property and to lower total composition cost.
- Hybrid polymers formed from inorganic nanoparticles and organic resin is amenable to achieving durability unobtainable with conventional organic resins alone. The inclusion of the inorganic nanoparticles can improve the durability of the articles (e.g. brightness enhancing film) thus formed.
- compositions just described are preferred compositions for providing a substantially solvent free polymerizable composition comprising inorganic nanoparticles and an organic component, wherein the organic component has a low viscosity, such as less than 1000 cps at 180°F.
- Substantially solvent free refer to the polymerizable composition having less than 5 wt-%, 4 wt-%, 3 wt-%, 2 wt-%, 1 wt-%, and 0.5 wt-% of (e.g. organic) solvent.
- concentration of solvent can be determined by known methods, such as gas chromatography. Solvent concentrations of less than 0.5 wt-% are preferred.
- the organic component can be a solid or comprise a solid component provided that the melting point in the polymerizable composition is less than the coating temperature.
- the organic component can be a liquid at ambient temperature.
- the components of the organic component are preferably chosen such that the organic component has a low viscosity.
- the viscosity of the organic component is substantially lower than the organic component of compositions previously employed.
- the viscosity of the organic component is less than 1000 cps and typically less than 900 cps.
- the viscosity of the organic component may be less than 800 cps, less than 450 cps, less than 600 cps, or less than 500 cps at the coating temperature.
- viscosity is measured with 25 mm parallel plates using a Dynamic Stress Rheometer (at a shear rate up to 1000 sec-1). Further, the viscosity of the organic component is typically at least 10 cps, more typically at least 50 cps, even more typically at least 100 cps, and most typically at least 200 cps at the coating temperature.
- the coating temperature typically ranges from ambient temperature, (i.e. 25°C) to 180°F (82°C).
- the coating temperature may be less than 170°F (77°C), less than 160°F (71 0 C), less than 150 0 F (66°C), less than 140 0 F (6O 0 C), less than 130 0 F (54 0 C), or less than 12O 0 F (49°C).
- the organic component can be a solid or comprise a solid component provided that the melting point is less than the coating temperature.
- the organic component can be a liquid at ambient temperature.
- the organic component as well as the polymerizable composition has a refractive index of at least 1.47, for most product applications; whereas the polymerizable resin composition of a turning film may have a refractive index as low as 1.44.
- the refractive index of the organic component or the polymerizable composition may be at least 1.48, 1.49, 1.50, 1.51, 1.52, 1.53, 1.54, 1.55, 1.56, 1.57, 1.58, 1.59, or 1.60.
- the polymerizable composition including the nanoparticles can have a refractive index as high as 1.70. (e.g. at least 1.61, 1.62, 1.63, 164, 1.65, 1.66, 1.67, 1.68, or 1.69) High transmittance in the visible light spectrum is also typically preferred.
- the polymerizable compositions just described are also preferred compositions for providing a substantially solvent free polymerizable composition comprising nanoparticles and an organic component comprising one or more ethylenically unsaturated monomers wherein the organic component has a high refractive index, i.e. of at least 1.54.
- the polymerizable composition having the low viscosity and/or high refractive index organic component may be prepared from other ethylenically unsaturated monomers as well.
- the organic component may comprise a (meth)acrylated urethane oligomer, (meth)acrylated polyester oligomer, a (meth)acrylated phenolic oligomer, a (meth)acrylated acrylic oligomer, and mixtures thereof. In some embodiments, however, the organic component is free of urethane linkages.
- the organic component may comprise at least one oligomeric ethylenically unsaturated monomer having a number average molecular weight of greater than 450 g/mole, typically in combination with a reactive diluent and/or crossliker.
- Suitable oligomeric (meth)acrylated aromatic epoxy oligomers are commercially available from Sartomer under the trade designations "CN104", “CNl 16", “CN120”, CN121” and “CN136”; from Cognis under the trade designation “Photomer 3016”; and from UCB under the trade designations "3200”, “3201”, “3211” and”3212".
- Suitable urethane (meth)acrylates are commercially available from Sartomer under the trade designations "CN965", “CN968”, “CN981”, “CN 983”, “CN 984", “CN972”, and “CN978”; from Cognis under the trade designation “Photomer 6210”, “Photomer 6217”, “Photomer 6230”, “Photomer 6623”, “Photomer 6891”, and “Photomer 6892”; and from UCB under the trade designations "Ebecryl 1290", “Ebecryl 2001”, and “Ebecryl 4842".
- Suitable polyester (meth)acrylates are commercially available from Sartomer under the trade designation "CN292"; from Cognis under the trade designation "Photomer 5010", “Photomer 5429”, “Photomer 5430”, “Photomer 5432”, “Photomer 5662”, “Photomer 5806”, and “Photomer 5920”; and from UCB under the trade designations "Ebecryl 80", “Ebecryl 81”, “Ebecryl 83", “Ebecryl 450”, “Ebecryl 524", “Ebecryl 525", “Ebecryl 585", “Ebecryl 588", “Ebecryl 810", and “Ebecryl 2047".
- Suitable phenolic (meth)acrylates are commercially available from Sartomer under the trade designation “SR601” and “SR602”; from Cognis under the trade designations "Photomer 4025” and "Photomer 4028”.
- Suitable (meth)acrylated acrylic oligomers are also commercially available or can be prepared by methods know in the art.
- the polymerizable resin composition optionally, yet preferably comprises up to about 35 wt-% (e.g. integers ranging from 1 to 35) reactive diluents to reduce the viscosity of the polymerizable resin composition and to improve the processability.
- Reactive diluents are mono- ethylenically unsaturated monomers such as (meth)acrylates or monomeric N-substituted or N,N-disubstituted (meth)acrylamides, especially an acrylamide. These include N-alkylacrylamides and N 5 N- dialkylacrylamides, especially those containing C 1-4 alkyl groups. Examples are N- isopropylacrylamide, N-t-butylacrylamide, N,N-dimethylacrylamide, N 5 N- diethylacrylamide, N- vinyl pyrrolidone and N- vinyl caprolactam.
- Preferred diluents can have a refractive index greater than 1.50 (e.g. greater than 1.55. Such reactive diluents can be halogenated or non-halogenated (e.g. non- brominated). Suitable monomers typically have a number average molecular weight no greater than 450 g/mole include
- Suitable reactive diluents include for example phenoxy ethyl (meth)acrylate; phenoxy-2-methylethyl (meth)acrylate; phenoxyethoxyethyl (meth)acrylate, 3-hydroxy-2- hydroxypropyl (meth)acrylate; benzyl (meth)acrylate, 4-(l-methyl-l- phenethyl)phenoxyethyl (meth)acrylate; phenylthio ethyl acrylate; 2-naphthylthio ethyl acrylate; 1-naphthylthio ethyl acrylate; 2,4,6-tribromophenoxy ethyl acrylate; 2,4- dibromophenoxy ethyl acrylate; 2-bromophenoxy ethyl acrylate; 1-naphthyloxy ethyl acrylate; 2-naphthyloxy ethyl
- a preferred diluent is phenoxyethyl (meth)acrylate, and in particular phenoxyethyl acrylate (PEA).
- PDA phenoxyethyl acrylate
- Phenoxyethyl acrylate is commercially available from more than one source including from Sartomer under the trade designation "SR339”; from Eternal Chemical Co. Ltd. under the trade designation "Etermer 210"; and from Toagosei Co. Ltd under the trade designation "TO-1166”.
- Benzyl acrylate is commercially available from AlfaAeser Corp, Ward Hill, MA.
- Such optional monomer(s) may be present in the polymerizable composition in amount of at least about 5 wt-%.
- the optional monomer(s) typically total no more than about 50 wt-% of the polymerizable composition.
- the some embodiments the total amount of optional high index monomer ranges from about 30 wt-% to about 45 wt-% (including integers between 30 and 45).
- the optional high index monomer may be halogenated (i.e. brominated).
- One exemplary high index optional monomer is 2,4,6-tribromophenoxyethyl (meth)acrylate commercially available from Daiichi Kogyo Seiyaku Co. Ltd (Kyoto, Japan) under the trade designation "BR-31".
- Suitable methods of polymerization include solution polymerization, suspension polymerization, emulsion polymerization, and bulk polymerization, as are known in the art. Suitable methods include heating in the presence of a free-radical initiator as well as irradiation with electromagnetic radiation such as ultraviolet or visible light in the presence of a photoinitiator. Inhibitors are frequently used in the synthesis of the polymerizable composition to prevent premature polymerization of the resin during synthesis, transportation and storage. Suitable inhibitors include hydroquinone, 4- methoxy phenol, and hindered amine nitroxide inhibitors at levels of 50-1000 ppm. Other kinds and/or amounts of inhibitors may be employed as known to those skilled in the art.
- the composition of the present invention optionally comprises a least one photoinitiator.
- a single photoinitiator or blends thereof may be employed in the brightness enhancement film of the invention.
- the photoinitiator(s) are at least partially soluble (e.g. at the processing temperature of the resin) and substantially colorless after being polymerized.
- the photoinitiator may be (e.g. yellow) colored, provided that the photoinitiator is rendered substantially colorless after exposure to the XJV light source.
- Suitable photoinitiators include monoacylphosphine oxide and bisacylphosphme oxide.
- Commercially available mono or bisacylphosphine oxide photoinitiators include 2,4,6-trimethylbenzoydiphenylphosphine oxide, commercially available from BASF (Charlotte, NC) under the trade designation "Lucirin TPO"; ethyl-2,4,6- trimethylbenzoylphenyl phosphinate, also commercially available from BASF under the trade designation "Lucirin TPO-L”; and bis (2,4,6-trimethylbenzoyl)-phenylphosphine oxide commercially available from Ciba Specialty Chemicals under the trade designation "Irgacure 819".
- photoinitiators include 2-hydroxy-2-methyl-l-phenyl- propan-1-one, commercially available from Ciba Specialty Chemicals under the trade designation “Darocur 1173” as well as other photoinitiators commercially available from Ciba Specialty Chemicals under the trade designations "Darocur 4265”, “Irgacure 651”, “Irgacure 1800”, “Irgacure 369”, “Irgacure 1700”, and "Irgacure 907".
- the photoinitiator can be used at a concentration of about 0.1 to about 10 weight percent. More preferably, the photoinitiator is used at a concentration of about 0.5 to about 5 wt-%. Greater than 5 wt-% is generally disadvantageous in view of the tendency to cause yellow discoloration of the brightness enhancing film.
- Other photoinitiators and photoinitiator may. also suitably be employed as may be determined by one of ordinary skill in the art.
- Surfactants such as fluorosurfactants and silicone based surfactants can optionally be included in the polymerizable composition to reduce surface tension, improve wetting, allow smoother coating and fewer defects of the coating, etc.
- the polymerizable compositions are energy curable in time scales preferably less than five minutes such as for a brightness enhancing film having a 75 micron thickness.
- the polymerizable composition is preferably sufficiently crosslinked to provide a glass transition temperature that is typically greater than 45 0 C.
- the glass transition temperature can be measured by methods known in the art, such as Differential Scanning Calorimetry (DSC), modulated DSC, or Dynamic Mechanical Analysis.
- DSC Differential Scanning Calorimetry
- modulated DSC modulated DSC
- Dynamic Mechanical Analysis Dynamic Mechanical Analysis.
- the polymerizable composition can be polymerized by conventional free radical polymerization methods.
- the inorganic nanoparticles are preferably surface modified such that the nanoparticles are polymerizable with the organic component.
- Surface modified (e.g. colloidal) nanoparticles can be present in the polymerized structure in an amount effective to enhance the durability and/or refractive index of the article or optical element.
- the surface modified colloidal nanoparticles described herein can have a variety of desirable attributes, including for example; nanoparticle compatibility with resin systems such that the nanoparticles form stable dispersions within the resin systems, surface modification can provide reactivity of the nanoparticle with the resin system making the composite more durable, properly surface modified nanoparticles added to resin systems provide a low impact on uncured composition viscosity.
- a combination of surface modifiers can be used to manipulate the uncured and cured properties of the composition.
- Appropriately surface modified nanoparticles can improve the optical and physical properties of the optical element such as, for example, improve resin mechanical strength, minimize viscosity changes while increasing solid volume loading in the resin system and maintain optical clarity while increasing solid volume loading in the resin system.
- the surface modified colloidal nanoparticles can be oxide particles having a primary particle size or associated particle size of greater than 1 nm and less than 100 nm. It is preferred that the nanoparticles are unassociated. Their measurements can be based on transmission electron miscroscopy (TEM).
- the nanoparticles can include metal oxides such as, for example, alumina, tin oxides, antimony oxides, silica, zirconia, titania, mixtures thereof, or mixed oxides thereof.
- Surface modified colloidal nanoparticles can be substantially fully condensed.
- Non-silica containing fully condensed nanoparticles typically have a degree of crystallinity (measured as isolated metal oxide particles) greater than 55%, preferably greater than 60%, and more preferably greater than 70%.
- the degree of crystallinity can range up to about 86% or greater.
- the degree of crystallinity can be determined by X-ray detraction techniques. Condensed crystalline (e.g. zirconia) nanoparticles have a high refractive index whereas amorphous nanoparticles typically have a lower refractive index.
- Silica nanoparticles can have a particle size from 5 to 75 nm or 10 to 30 nm or 20 nm. Silica nanoparticles can be present in the durable article or optical element in an amount from 10 to 60 wt-%, or 10 to 40 wt-%. Silicas for use in the materials of the invention are commercially available from Nalco Chemical Co., Naperville, IL under the trade designation "Nalco Collodial Silicas" such as products 1040, 1042, 1050, 1060, 2327 and 2329.
- Suitable fumed silicas include for example, products commercially available from DeGussa AG, (Hanau, Germany) under the trade designation, " Aerosil series OX- 50", as well as product numbers -130, -150, and -200. Fumed silicas are also commercially available from Cabot Corp., Tuscola, I, under the trade designations CAB- O-SPERSE 2095", “CAB-O-SPERSE A105", and "CAB-O-SIL M5".
- Zirconia nanoparticles can have a particle size from 5 to 50 nm, or 5 to 15 nm, or 10 nm. Zirconia nanoparticles can be present in the durable article or optical element in an amount from 10 to 70 wt-%, or 30 to 60 wt-%. Zirconias for use in composition and articles of the invention are available from Nalco Chemical Co. under the trade designation "Nalco 00SS008" and from Buhler AG Uzwil, Switzerland under the trade designation "Buhler zirconia Z-WO sol". Zirconia nanoparticle can also be prepared such as described in U.S. Patent Application serial No. 11/027426 filed Dec. 30, 2004 and U.S. Patent No. 6,376,590.
- Titania, antimony oxides, alumina, tin oxides, and/or mixed metal oxide nanoparticles can have a particle size or associated particle size from 5 to 50 nm, or 5 to 15 nm, or 10 nm. Titania, antimony oxides, alumina, tin oxides, and/or mixed metal oxide nanoparticles can be present in the durable article or optical element in an amount from 10 to 70 wt-%, or 30 to 60 wt-%.
- Mixed metal oxide for use in materials of the invention are commercially available from Catalysts & Chemical Industries Corp., Kawasaki, Japan, under the trade designation "Optolake 3".
- Surface-treating the nano-sized particles can provide a stable dispersion in the polymeric resin.
- the surface-treatment stabilizes the nanoparticles so that the particles will be well dispersed in the polymerizable resin and results in a substantially homogeneous composition.
- the nanoparticles can be modified over at least a portion of its surface with a surface treatment agent so that the stabilized particle can copolymerize or react with the polymerizable resin during curing.
- the nanoparticles of the present invention are preferably treated with a surface treatment agent.
- a surface treatment agent has a first end that will attach to the particle surface (covalently, ionically or through strong physisorption) and a second end that imparts compatibility of the particle with the resin and/or reacts with resin during curing.
- surface treatment agents include alcohols, amines, carboxylic acids, sulfonic acids, phosphonic acids, silanes and titanates.
- the preferred type of treatment agent is determined, in part, by the chemical nature of the metal oxide surface. Silanes are preferred for silica and other for siliceous fillers. Silanes and carboxylic acids are preferred for metal oxides such as zirconia.
- the surface modification can be done either subsequent to mixing with the monomers or after mixing. It is preferred in the case of silanes to react the silanes with the particle or nanoparticle surface before incorporation into the resin.
- the required amount of surface modifier is dependant upon several factors such particle size, particle type, modifier molecular wt, and modifier type. In general it is preferred that approximately a monolayer of modifier is attached to the surface of the particle. The attachment procedure or reaction conditions required also depend on the surface modifier used. For silanes it is preferred to surface treat at elevated temperatures under acidic or basic conditions for from 1-24 hr approximately. Surface treatment agents such as carboxylic acids may not require elevated temperatures or extended time.
- surface treatment agents suitable for the compositions include compounds such as, for example, isooctyl trimethoxy-silane, N-(3- triethoxysilylpropyl) methoxyethoxyethoxyethyl carbamate, N-(3- triethoxysilylpropyl) methoxyethoxyethyl carbamate, 3- (methacryloyloxy)propyltrimethoxysilane, 3- acryloxypropyltrimethoxysilane, 3 -(methacryloyloxy)propyltriethoxysilane, 3 - (methacryloyloxy) propyhnethyldimethoxysilane, 3-(acryloyloxypropyl)methyldimethoxysilane,
- the surface modification of the particles in the colloidal dispersion can be accomplished in a variety of ways.
- the process involves the mixture of an inorganic dispersion with surface modifying agents.
- a co-solvent can be added at this point, such as for example, l-niethoxy-2-propanol, ethanol, isopropanol, ethylene glycol, N,N-dimethylacetamide and l-methyl-2-pyrrolidinone.
- the co-solvent can enhance the solubility of the surface modifying agents as well as the surface modified particles.
- the mixture comprising the inorganic sol and surface modifying agents is subsequently reacted at room or an elevated temperature, with or without mixing.
- the mixture can be reacted at about 85°C for about 24 hours, resulting in the surface modified sol.
- the surface treatment of the metal oxide can preferably involve the adsorption of acidic molecules to the particle surface.
- the surface modification of the heavy metal oxide may take place at room temperature.
- the surface modification of ZrO 2 with silanes can be accomplished under acidic conditions or basic conditions, hi one preferred case the silanes are preferably heated under acid conditions for a suitable period of time. At which time the dispersion is combined with aqueous ammonia (or other base). This method allows removal of the acid counter ion from the ZrO 2 surface as well as reaction with the silane. In a preferred method the particles are precipitated from the dispersion and separated from the liquid phase.
- the surface modified particles can then be incorporated into the curable resin in various methods.
- a solvent exchange procedure is utilized whereby the resin is added to the surface modified sol, followed by removal of the water and co-solvent (if used) via evaporation, thus leaving the particles dispersed in the polymerizable resin.
- the evaporation step can be accomplished for example, via distillation, rotary evaporation or oven drying.
- the surface modified particles can be extracted into a water immiscible solvent followed by solvent exchange, if so desired.
- another method for incorporating the surface modified nanoparticles in the polymerizable resin involves the drying of the modified particles into a powder, followed by the addition of the resin material into which the particles are dispersed.
- the drying step in this method can be accomplished by conventional means suitable for the system, such as, for example, oven drying or spray drying.
- a combination of surface modifying agents can be useful, wherein at least one of the agents has a functional group co-polymerizable with a hardenable resin. Combinations of surface modifying agent can result in lower viscosity.
- the polymerizing group can be ethylenically unsaturated or a cyclic function subject to ring opening polymerization.
- An ethylenically unsaturated polymerizing group can be, for example, an acrylate or methacrylate, or vinyl group.
- a cyclic functional group subject to ring opening polymerization generally contains a heteroatom such as oxygen, sulfur or nitrogen, and preferably a 3-membered ring containing oxygen such as an epoxide.
- a preferred combination of surface modifying agent includes at least one surface modifying agent having a functional group that is co-polymerizable with the (organic component of the) hardenable resin and a second modifying agent different than the first modifying agent.
- the second modifying agent is optionally co-polymerizable with the organic component of the polymerizable composition.
- the second modifying agent may have a low refractive index (i.e. less than 1.52 or less than 1.50).
- the second modifying agent is preferably a polyalkyleneoxide containing modifying agent that is optionally co- polymerizable with the organic component of the polymerizable composition.
- a microstructure-bearing article e.g. brightness enhancing film
- a method including the steps of (a) preparing a polymerizable composition (i.e. the polymerizable composition of the invention); (b) depositing the polymerizable composition onto a master negative microstructured molding surface in an amount barely sufficient to fill the cavities of the master; (c) filling the cavities by moving a bead of the polymerizable composition between a preformed base and the master, at least one of which is flexible; and (d) curing the composition.
- a polymerizable composition i.e. the polymerizable composition of the invention
- the master can be metallic, such as nickel, nickel-plated copper or brass, or can be a thermoplastic material that is stable under the polymerization conditions, and that preferably has a surface energy that allows clean removal of the polymerized material from the master.
- One or more the surfaces of the base film can be optionally be primed or otherwise be treated to promote adhesion of the optical layer to the base.
- the brightness enhancing film of the invention is usefully employed in a display for the purpose of improving the gain.
- a schematic view of an illustrative backlit liquid crystal display generally indicated at 110 in FIG. 2.
- the various components depicted are often in contact with the brightness enhancing film.
- the brightness enhancing film 111 of the present invention is generally positioned between a light guide 118 and a liquid crystal display panel 114.
- the liquid crystal display panel typically includes an absorbing polarizer on both surfaces. Thus, such absorbing polarizer is positioned adjacent to the brightness enhancing film of the invention.
- the backlit liquid crystal display can also include a light source 116 such as a fluorescent lamp and a white reflector 120 also for reflecting light also toward the liquid crystal display panel.
- the brightness enhancing film 111 collimates light emitted from the light guide 118 thereby increasing the brightness of the liquid crystal display panel 114.
- the increased brightness enables a sharper image to be produced by the liquid crystal display panel and allows the power of the light source 116 to be reduced to produce a selected brightness.
- the backlit liquid crystal display is useful in equipment such as computer displays (laptop displays and computer monitors), televisions, video recorders, mobile communication devices, handheld devices (i.e. cell phone, personal digital assistant (PDA)), automobile and avionic instrument displays, and the like, represented by reference character 121.
- the display may further include another optical film 112 positioned between the brightness enhancing film and the liquid crystal display panel 114.
- the other optical film may include for example a diffuser, a reflective polarizer, or a second brightness enhancing film.
- Other optical films may be positioned between optical film 112 and the liquid crystal display panel 114 or between the brightness enhancing film 111 and the light guide 118, as are known in the art.
- a turning film may be located between lightguide and optical film.
- the brightness enhancing film may be a turning film.
- a turning film typically includes prism structures formed on an input surface, and the input surface is disposed adjacent the lightguide. The light rays exiting the lightguide at the glancing angle, usually less than 30 degrees to the output surface, encounter the prism structures.
- the light rays are refracted by a first surface of the prism structures and are reflected by a second surface of the prism structures such that they are directed by the turning lens or film in the desired direction, e.g., substantially parallel to a viewing axis of the display.
- Examples of polarizing firms include those described in U.S. Pat. Nos. 5,825,543 and 5,783,120. The use of these polarizer films in combination with a brightness enhancing film has been described in U.S. Pat. No. 6,111,696. Another example of a polarizing film is described in U.S. Pat. No. 5,882,774.
- One example of such films that are available commercially are the multilayer films sold under the trade designation DBEF (Dual Brightness Enhancement Film) from 3M Company. Multilayer polarizing optical films have been described, for example in U.S. Pat. No. 5,828,488.
- a turning film typically includes prism structures formed on an input surface and the input surface is disposed adjacent to a lightguide.
- the light rays exiting the lightguide at the glancing angles encounter the prism structures.
- the light rays are refracted by a first surface of the prism structures and are reflected by a second surface of the prism structures such that the rays are directed by the turning film in the desired direction, e.g. substantially parallel to a viewing axis of the display. If these additional optical films are included as the base layer of the brightness enhancing films, than the thickness of the base layer may be considerably greater than previously described.
- the polymerizable composition described herein may be advantageous for other optical materials such as microstructure-bearing optical articles (e.g. films).
- exemplary optical materials include optical lenses such as Fresnel lenses, optical films, such as high index of refraction films e.g., microreplicated films such as totally internal reflecting films, or brightness enhancing films, flat films, multilayer films, retroreflective sheeting, optical light fibers or tubes, flexible molds (e.g. suitable for making barrier ribs for plasma display panels) and others.
- optical lenses such as Fresnel lenses
- optical films such as high index of refraction films e.g., microreplicated films such as totally internal reflecting films, or brightness enhancing films, flat films, multilayer films, retroreflective sheeting, optical light fibers or tubes, flexible molds (e.g. suitable for making barrier ribs for plasma display panels) and others.
- the production of optical products from high index of refraction polymerizable compositions is described, for example, in U.S
- XS a second sheet of the same material is placed underneath the first sheet and orientated such that the grooves of the second sheet are normal to the front face of the Teflon light cube.
- polymerizable resin compositions were prepared into brightness enhancing films using a master tool that had a 90° apex angles as defined by the slope of the sides of the prisms, hi the first set of experiments, the mean distance between adjacent apices was about 50 micrometers and the apex of the prism vertices was round.
- brightness enhancing films were prepared from polymerizable resin compositions 1-3 along with a control (i.e. Control 1 of Table I), hi a second set of experiments brightness enhancing films were prepared from polymerizable resin composition 4 along with a control (i.e. Control 2 of Table I), hi a third set of experiments, brightness enhancing films were prepared from polymerizable resin composition 5 along with a control (i.e. Control 3 of Table I).
- control consisted of a mixture of 12.5 wt-% PEA, 37.5 wt-% BR-31, 30 wt-% RDX- 51027, 20 wt-% of a crosslinking agent obtained from UCB Corporation under the trade designation "EB-9220", 1 pph Darocur 1173, and 0.3 wt-% surfactant, commercially available from 3M Company under the trade designation "FC-430"'.
- Table I as follows sets forth the amount of first monomer, kind and amount of monofunctional diluent (i.e. phenoxyethyl acrylate (PEA)), crosslinking agent (i.e. PETA), inorganic nanoparticles, photoinitiator (Lucirin TPO-L) employed in the examples.
- the first monomer employed in the examples comprised at least about 60-70 wt-% of 2- propenoic acid, (l-methylethylidene)bis[(2,6-dibromo-4,l-phenylene)oxy(2-hydroxy-3,l- propanediyl)] ester.
- the SiO 2 containing resin from Example 1 (10 g) was mixed with 20/60/20 SR444/First Monomer/PEA (2.12 g) to give a 38 wt-% SiO 2 containing resin. 1 wt-% TPO-L was added.
- the SiO 2 containing resin from Example 1 (10 g) was mixed with 20/60/20 SR444/First Monomer/PEA (4.38 g) to give a 32 wt-% SiO 2 containing resin. 1 wt% TPO- L was added.
- silane-modified zirconia nanoparticle dispersion Nalco 00SS008 zirconia sol (372.56 g) and 2-[2-(2-methoxyethoxy)ethoxy]acetic acid (MEEAA) commercially available from Sigma- Aldrich (23.16g) were charged to a 1 L round bottom flask. The water and acetic acid were removed via rotary evaporation.
- MEEAA 2-[2-(2-methoxyethoxy)ethoxy]acetic acid
- the powder thus obtained was redispersed in 127.58 g D.I water and charged to a 2 L beaker to which was added with stirring 400 g l-methoxy-2-propanol, 36.62 g A-174, 24.61 g Silquest A-1230 and 0.4 g of a 5% solution of Prostab 5198 in water. This mixture was stirred 30 min at room temperature then poured into IL (quart) jars, sealed and heated to 9O 0 C for 3.0 h. The contents of the jars were removed and concentrated to 40% ZrO 2 via rotary evaporation.
- Buhler zirconia Z- WO sol (401.5 g) (available from Buhler AG Uzwil, Switzerland) was charged to a 1 qt jar to which was added with stirring, 450 g l-methoxy-2-propanol, 28.5 g Silane A174, 19.16 g Silquest A-1230 and 0.5 g of a 5% solution of Prostab 5198 in water. This mixture was stirred 30 min at room temperature then sealed and heated to 9O 0 C for 3.0 h. The contents of the jars were removed and concentrated to approximately 40% ZrO 2 via rotary evaporation.
- Deionized water (707.8 g) and 24.2 g concentrated aqueous ammonia (29 % NH 3 ) were charged to a 4L beaker.
- the concentrated dispersion (346.8 g) was added slowly to the beaker with stirring.
- the white precipitate thus obtained was isolated via vacuum filtration and washed with additional D.I. water.
- the damp solids were dispersed in l-methoxy-2-propanol.
- the resultant silane modified zirconia dispersion contained 20.58 % solids.
- the above modified ZrO 2 dispersion (225.2 g), 20/60/20 PEA/First Monomer/SR444 (30.9 g) and a 5% solution of Prostab 5198 in water (0.24 g) were charged to a IL round bottom flask. Water and alcohol were removed via rotary evaporation.
- the resultant formulation contained 47.85% ZrO2 by TGA and had a refractive index of 1.615. 1 wt% TPO-L was added.
- a ZrO 2 sol was prepared according to U.S. Patent Application Serial No. 11/027426 filed Dec. 30, 2004 yielding a sol with 45.78% solids.
- the ZrO 2 was tested according to the following ZrO2 Test Methods:
- PCS Photon Correlation Spectroscopy
- the volume-average particle size was determined by Photon Correlation Spectroscopy (PCS) using a Malvern Series 4700 particle size analyzer (available from Malvern Instruments Inc., Southborough, MA). Dilute zirconia sol samples were filtered through a 0.2 ⁇ m filter using syringe-applied pressure into a glass cuvette that was then covered. Prior to starting data acquisition the temperature of the sample chamber was allowed to equilibrate at 25 °C. The supplied software was used to do a CONTIN analysis with an angle of 90 degrees. CONTIN is a widely used mathematical method for analyzing general inverse transformation problems that is further described in S. W. Provencher, Comput. Phys. Commun., 27, 229 (1982).
- refractive index of water equal to 1.333
- viscosity of water equal to 0.890 centipoise
- refractive index of the zirconia particles equal to 1.9.
- the intensity-average particle size was equal to the size of a particle corresponding to the mean value of the scattered light intensity distribution.
- the scattered light intensity was proportional to the sixth power of the particle diameter.
- the volume- average particle size also reported in nanometers, was derived from a volume distribution that was calculated from the scattered light intensity distribution taking into account both the refractive index of the zirconia particles and the refractive index of the dispersing medium (i.e., water).
- the volume-average particle size was equal to the particle size corresponding to the mean of the volume distribution.
- the intensity-average particle size was divided by the volume-average particle size to provide a ratio that is indicative of the particle size distribution.
- the particle size of a dried zirconia sample was reduced by hand grinding using an agate mortar and pestle. A liberal amount of the sample was applied by spatula to a glass microscope slide on which a section of double coated tape had been adhered. The sample was pressed into the adhesive on the tape by forcing the sample against the tape with the spatula blade. Excess sample was removed by scraping the sample area with the edge of the spatula blade, leaving a thin layer of particles adhered to the adhesive. Loosely adhered materials remaining after the scraping were remove by forcefully tapping the microscope slide against a hard surface. In a similar manner, corundum (Linde 1.0 ⁇ m alumina polishing powder, Lot Number C062, Union Carbide, Indianapolis, IN) was prepared and used to calibrate the diffractometer for instrumental broadening.
- X-ray diffraction scans were obtained using a Philips vertical diffractometer having a reflection geometry, copper K a radiation, and proportional detector registry of the scattered radiation.
- the diffractometer was fitted with variable incident beam slits, fixed diffracted beam slits, and graphite diffracted beam monochromator.
- the survey scan was conducted from 25 to 55 degrees two theta (2 ⁇ ) using a 0.04 degree step size and 8 second dwell time.
- X-ray generator settings of 45 kV and 35 rnA were employed.
- Data collections for the corundum standard were conducted on three separate areas of several individual corundum mounts. Data was collected on three separate areas of the thin layer sample mount.
- the observed diffraction peaks were identified by comparison to the reference diffraction patterns contained within the International Center for Diffraction Data (ICDD) powder diffraction database (sets 1-47, ICDD, Newton Square, PA) and attributed to either cubic/tetragonal (C/T) or monoclinic (M) forms of zirconia.
- ICDD International Center for Diffraction Data
- C/T cubic/tetragonal
- M monoclinic
- Peak widths for the observed diffraction maxima due to corundum were measured by profile fitting. The relationship between mean corundum peak widths and corundum peak position (2 ⁇ ) was determined by fitting a polynomial to these data to produce a continuous function used to evaluate the instrumental breadth at any peak position within the corundum testing range. Peak widths for the observed diffraction maxima due to zirconia were measured by profile fitting observed diffraction peaks. The following peak widths were evaluated depending on the zirconia phase found to be present:
- C/T Cubic/Tetragonal (C/T): (1 1 1) Monoclinic (M): (-1 1 1), and (1 1 1)
- the cubic/tetragonal crystallite size was measured as the average of three measurements using (1 1 l) peak.
- the monoclinic crystallite size was measured as the average of three measurement using the (-1 1 1) peak and three measurements using the (1 1 1) peak.
- Monoclinic Mean Crystallite Size is the average of three measurement using the (-1 1 1) peak and three measurements using the (1 1 1) peak.
- Weighted average [(% C/T)(C/T size) + (% M)(M size)]/100
- %C/T the percent crystallinity contributed by the cubic and tetragonal crystallite content of the ZrO 2 particles
- C/T size the size of the cubic and tetragonal crystallites
- % M the percent crystallinity contributed by the monoclinic crystallite content of the ZrO 2 particles.
- M size the size of the monoclinic crystallites. Dispersion Index
- the Dispersion Index is equal to the volume-average size measured by PCS divided by the weighted average crystallite size measured by XRD.
- the weight percent solids were determined by drying a sample weighing 3 to 6 grams at 120 0 C for 30 minutes.
- the percent solids can be calculated from the weight of the wet sample (i.e., weight before drying, weight wet ) and the weight of the dry sample (i.e., weight after drying, weight dr y) using the following equation.
- wt-% solids 100 (weightdry) / weight wet
- the ZrO 2 Sol (50.00 g), MEEAA (2.22 g), BCEA (1.06 g), l-methoxy-2-propanol (75 g), and a 50/50 mix of PEA/RDX (17.60 g) were charged to a round bottom flask and the alcohol and water were removed via rotary evaporation.
- the ZrO 2 containing resin was 49.59 % ZrO 2 and had a refractive index of 1.639. 0.5 pph of TPO-L was added to the above mixture.
Landscapes
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Nanotechnology (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biophysics (AREA)
- Composite Materials (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Optical Elements Other Than Lenses (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Macromonomer-Based Addition Polymer (AREA)
- Polymerisation Methods In General (AREA)
- Manufacture Of Macromolecular Shaped Articles (AREA)
- Polarising Elements (AREA)
- Liquid Crystal (AREA)
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP20050789350 EP1756626B1 (en) | 2004-06-17 | 2005-06-06 | Polymerizable compositions comprising nanoparticles |
| KR1020067026535A KR101255417B1 (en) | 2004-06-17 | 2005-06-06 | Polymerizable compositions comprising nanoparticles |
| CN2005800197288A CN1969201B (en) | 2004-06-17 | 2005-06-06 | Polymerizable compositions comprising nanoparticles |
| JP2007516536A JP5038132B2 (en) | 2004-06-17 | 2005-06-06 | Polymerizable composition comprising nanoparticles |
Applications Claiming Priority (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US87036604A | 2004-06-17 | 2004-06-17 | |
| US10/870,366 | 2004-06-17 | ||
| US10/939,184 US7179513B2 (en) | 2003-09-12 | 2004-09-10 | Durable optical element |
| US10/939,184 | 2004-09-10 | ||
| US10/938,006 | 2004-09-10 | ||
| US10/938,006 US7289202B2 (en) | 2004-09-10 | 2004-09-10 | Methods for testing durable optical elements |
| US11/078,145 | 2005-03-11 | ||
| US11/078,145 US7282272B2 (en) | 2003-09-12 | 2005-03-11 | Polymerizable compositions comprising nanoparticles |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2006007286A2 true WO2006007286A2 (en) | 2006-01-19 |
| WO2006007286A3 WO2006007286A3 (en) | 2006-02-23 |
Family
ID=35169650
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2005/019774 WO2006007286A2 (en) | 2004-06-17 | 2005-06-06 | Polymerizable compositions comprising nanoparticles |
| PCT/US2005/021351 WO2006028543A2 (en) | 2004-06-17 | 2005-06-16 | Optical film, assembly and display device |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2005/021351 WO2006028543A2 (en) | 2004-06-17 | 2005-06-16 | Optical film, assembly and display device |
Country Status (6)
| Country | Link |
|---|---|
| US (6) | US7282272B2 (en) |
| EP (3) | EP1756626B1 (en) |
| JP (2) | JP5038132B2 (en) |
| KR (2) | KR101255417B1 (en) |
| CN (3) | CN101872028B (en) |
| WO (2) | WO2006007286A2 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006073856A2 (en) * | 2004-12-30 | 2006-07-13 | 3M Innovative Properties Company | Durable high index nanocomposites for ar coatings |
| WO2007001811A1 (en) * | 2005-06-28 | 2007-01-04 | General Electric Company | Compositions for brightness enhancing films |
| US7297810B2 (en) | 2004-12-30 | 2007-11-20 | 3M Innovative Properties Company | High refractive index monomers for optical applications |
| WO2007082919A3 (en) * | 2006-01-18 | 2008-04-17 | Lemnis Lighting Ip Gmbh | Novel monomeric and polymeric materials |
| WO2008082414A1 (en) * | 2007-01-04 | 2008-07-10 | General Electric Company | Methods for improving mold quality for use in the manufacture of light management film, manufactured film and corresponding mold |
| WO2008121465A1 (en) * | 2007-02-27 | 2008-10-09 | 3M Innovative Properties Company | Brightness enhancing film comprising nanocomposite structure having improved crack resistance |
| US7491441B2 (en) | 2004-12-30 | 2009-02-17 | 3M Innovative Properties Company | High refractive index, durable hard coats |
| WO2009103651A2 (en) | 2008-02-21 | 2009-08-27 | Basf Se | Preparation of cationic nanoparticles and personal care compositions comprising said nanoparticles |
| JP2010520939A (en) * | 2007-03-09 | 2010-06-17 | スリーエム イノベイティブ プロパティズ カンパニー | Triphenyl monomer suitable for microstructured optical film |
| JP2010520938A (en) * | 2007-03-09 | 2010-06-17 | スリーエム イノベイティブ プロパティズ カンパニー | Microstructured optical film containing biphenyl bifunctional monomer |
| US7957621B2 (en) * | 2008-12-17 | 2011-06-07 | 3M Innovative Properties Company | Light extraction film with nanoparticle coatings |
| US7981986B2 (en) | 2008-04-29 | 2011-07-19 | 3M Innovative Properties Company | Optical films comprising fluorenol (meth)acrylate monomer |
| EP2507161A2 (en) * | 2009-12-02 | 2012-10-10 | 3M Innovative Properties Company | Functionalized zirconia nanoparticles and high index films made therefrom |
| US8389599B2 (en) | 2008-10-22 | 2013-03-05 | 3M Innovative Properties Company | Dental composition comprising biphenyl di(meth)acrylate monomer comprising urethane moieties |
| US9164195B2 (en) | 2007-03-07 | 2015-10-20 | 3M Innovative Properties Company | Methods of making microstructured optical films comprising biphenyl difunctional monomers |
Families Citing this family (133)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7683098B2 (en) * | 1996-09-03 | 2010-03-23 | Ppg Industries Ohio, Inc. | Manufacturing methods for nanomaterial dispersions and products thereof |
| US7094461B2 (en) * | 2002-12-31 | 2006-08-22 | 3M Innovative Properties Company | P-polarizer with large z-axis refractive index difference |
| US7282272B2 (en) * | 2003-09-12 | 2007-10-16 | 3M Innovative Properties Company | Polymerizable compositions comprising nanoparticles |
| US7074463B2 (en) * | 2003-09-12 | 2006-07-11 | 3M Innovative Properties Company | Durable optical element |
| US20060128853A1 (en) * | 2004-12-13 | 2006-06-15 | General Electric Company | Compositions for articles comprising replicated microstructures |
| US7241437B2 (en) * | 2004-12-30 | 2007-07-10 | 3M Innovative Properties Company | Zirconia particles |
| JP2008530346A (en) * | 2005-02-17 | 2008-08-07 | スリーエム イノベイティブ プロパティズ カンパニー | Brightness enhancing film comprising a polymerized organic phase having a low glass transition temperature |
| US20060204676A1 (en) * | 2005-03-11 | 2006-09-14 | Jones Clinton L | Polymerizable composition comprising low molecular weight organic component |
| KR20060112451A (en) * | 2005-04-27 | 2006-11-01 | 삼성전자주식회사 | Plastic substrate for display panel and manufacturing process thereof |
| US8718437B2 (en) | 2006-03-07 | 2014-05-06 | Qd Vision, Inc. | Compositions, optical component, system including an optical component, devices, and other products |
| WO2007103310A2 (en) | 2006-03-07 | 2007-09-13 | Qd Vision, Inc. | An article including semiconductor nanocrystals |
| KR100615338B1 (en) * | 2005-06-15 | 2006-08-25 | 삼성에스디아이 주식회사 | Plasma display apparatus |
| US7838138B2 (en) | 2005-09-19 | 2010-11-23 | 3M Innovative Properties Company | Fuel cell electrolyte membrane with basic polymer |
| JP5268240B2 (en) * | 2005-10-03 | 2013-08-21 | キヤノン株式会社 | Optical composite material and optical element |
| US20070082988A1 (en) * | 2005-10-06 | 2007-04-12 | General Electric Company | Compositions for articles comprising replicated microstructures |
| EP1950239B1 (en) * | 2005-10-28 | 2017-01-04 | Sumitomo Osaka Cement Co., Ltd. | Transparent inorganic-oxide dispersion, resin composition containing inorganic oxide particles, composition for encapsulating luminescent element, luminescent element, hard coat, optical functional film, optical part, and process for producing resin composition containing inorganic oxide particles |
| US7547467B2 (en) | 2005-11-15 | 2009-06-16 | 3M Innovative Properties Company | Brightness enhancing film and methods of surface treating inorganic nanoparticles |
| US9951438B2 (en) | 2006-03-07 | 2018-04-24 | Samsung Electronics Co., Ltd. | Compositions, optical component, system including an optical component, devices, and other products |
| US9874674B2 (en) | 2006-03-07 | 2018-01-23 | Samsung Electronics Co., Ltd. | Compositions, optical component, system including an optical component, devices, and other products |
| US8031296B2 (en) * | 2006-04-05 | 2011-10-04 | Nitto Denko Corporation | Liquid crystal panel and liquid crystal display apparatus |
| US7981949B2 (en) * | 2006-05-23 | 2011-07-19 | 3M Innovative Properties Company | Curable hydrophilic compositions |
| WO2007140347A2 (en) * | 2006-05-25 | 2007-12-06 | I2Ic Corporation | Extraction of light from a light conducting medium in a preferred emanation pattern |
| WO2007146868A1 (en) * | 2006-06-12 | 2007-12-21 | 3M Innovative Properties Company | Led device with re-emitting semiconductor construction and converging optical element |
| US20070284565A1 (en) * | 2006-06-12 | 2007-12-13 | 3M Innovative Properties Company | Led device with re-emitting semiconductor construction and optical element |
| US7952110B2 (en) * | 2006-06-12 | 2011-05-31 | 3M Innovative Properties Company | LED device with re-emitting semiconductor construction and converging optical element |
| US7902542B2 (en) * | 2006-06-14 | 2011-03-08 | 3M Innovative Properties Company | Adapted LED device with re-emitting semiconductor construction |
| TWI317025B (en) * | 2006-08-28 | 2009-11-11 | Eternal Chemical Co Ltd | Optical film |
| US8836212B2 (en) | 2007-01-11 | 2014-09-16 | Qd Vision, Inc. | Light emissive printed article printed with quantum dot ink |
| KR100912260B1 (en) * | 2007-03-28 | 2009-08-17 | 제일모직주식회사 | Optical prism sheet having a certain roughness thereon |
| US8597959B2 (en) * | 2007-04-19 | 2013-12-03 | 3M Innovative Properties Company | Methods of use of solid support material for binding biomolecules |
| EP2137533A2 (en) * | 2007-04-19 | 2009-12-30 | 3M Innovative Properties Company | Uses of water-dispersible silica nanoparticles for attaching biomolecules |
| TWI348561B (en) * | 2007-05-02 | 2011-09-11 | Ind Tech Res Inst | Diffusing polarizer and backlight module using the same |
| CN101681057B (en) | 2007-05-20 | 2012-07-04 | 3M创新有限公司 | Thin hollow backlights with beneficial design characteristics |
| EP2160645A2 (en) | 2007-05-20 | 2010-03-10 | 3M Innovative Properties Company | Light recycling hollow cavity type display backlight |
| US9028108B2 (en) | 2007-05-20 | 2015-05-12 | 3M Innovative Properties Company | Collimating light injectors for edge-lit backlights |
| EP2160644B1 (en) | 2007-05-20 | 2019-05-01 | 3M Innovative Properties Company | Semi-specular components in hollow cavity light recycling backlights |
| JP5645237B2 (en) * | 2007-11-26 | 2014-12-24 | パナソニックIpマネジメント株式会社 | Infrared detector |
| JP2010531045A (en) | 2007-06-22 | 2010-09-16 | スリーエム イノベイティブ プロパティズ カンパニー | System and method for controlling backlight output characteristics |
| JP5773646B2 (en) | 2007-06-25 | 2015-09-02 | キユーデイー・ビジヨン・インコーポレーテツド | Compositions and methods comprising depositing nanomaterials |
| US8986812B2 (en) | 2007-07-09 | 2015-03-24 | 3M Innovative Properties Company | Thin microstructured optical films |
| WO2009014707A2 (en) | 2007-07-23 | 2009-01-29 | Qd Vision, Inc. | Quantum dot light enhancement substrate and lighting device including same |
| CN101363990B (en) * | 2007-08-08 | 2013-02-06 | 奇美电子股份有限公司 | Polarizing diaphragm and display apparatus using the same |
| US8128249B2 (en) | 2007-08-28 | 2012-03-06 | Qd Vision, Inc. | Apparatus for selectively backlighting a material |
| JP2011501219A (en) | 2007-10-16 | 2011-01-06 | スリーエム イノベイティブ プロパティズ カンパニー | Higher transmittance light control film |
| US7716999B2 (en) * | 2007-10-31 | 2010-05-18 | 3M Innovative Properties Company | Test method for determining microstructure deformation resistance of a microstructured film |
| CN101469250B (en) | 2007-12-26 | 2012-09-19 | 3M创新有限公司 | Removable antifogging coating, product, coating composition and method |
| KR101460155B1 (en) * | 2008-01-15 | 2014-11-10 | 삼성전자주식회사 | Backlight unit and liquid crystal display having the same |
| WO2009100307A1 (en) | 2008-02-07 | 2009-08-13 | 3M Innovative Properties Company | Hollow backlight with structured films |
| WO2009105450A1 (en) | 2008-02-22 | 2009-08-27 | 3M Innovative Properties Company | Backlights having selected output light flux distributions and display systems using same |
| JP2009276726A (en) * | 2008-04-17 | 2009-11-26 | Olympus Corp | Optical material composition and optical element using the same |
| US20090275720A1 (en) * | 2008-04-30 | 2009-11-05 | 3M Innovative Properties Company | Ortho-benzylphenol mono(meth)acrylate monomers suitable for microstructured optical films |
| WO2009151515A1 (en) | 2008-05-06 | 2009-12-17 | Qd Vision, Inc. | Solid state lighting devices including quantum confined semiconductor nanoparticles |
| WO2009137053A1 (en) | 2008-05-06 | 2009-11-12 | Qd Vision, Inc. | Optical components, systems including an optical component, and devices |
| US9207385B2 (en) | 2008-05-06 | 2015-12-08 | Qd Vision, Inc. | Lighting systems and devices including same |
| EP2288946A2 (en) * | 2008-06-03 | 2011-03-02 | 3M Innovative Properties Company | Microstructures comprising polyalkyl nitrogen or phosphorus onium fluoroalkyl sulfonyl salts |
| US8757858B2 (en) | 2008-06-04 | 2014-06-24 | 3M Innovative Properties Company | Hollow backlight with tilted light source |
| US8343622B2 (en) | 2008-07-01 | 2013-01-01 | 3M Innovative Properties Company | Flexible high refractive index hardcoat |
| TWM352035U (en) * | 2008-10-21 | 2009-03-01 | Optivision Technology Inc | Optical film with light diverging and converging functions |
| WO2010074862A1 (en) | 2008-12-15 | 2010-07-01 | 3M Innovative Properties Company | High refractive index inorganic oxide nanoparticles comprising surface treatment, polymerizable resin, and articles |
| CN102307917B (en) * | 2008-12-22 | 2019-07-12 | 3M创新有限公司 | Microstructured optical films comprising reactive UV-stabilizer |
| US9063264B2 (en) | 2009-02-09 | 2015-06-23 | 3M Innovative Properties Company | Simplified edge-lit backlight system |
| JP5823958B2 (en) * | 2009-06-02 | 2015-11-25 | スリーエム イノベイティブ プロパティズ カンパニー | Light re-deflecting film and display using the film |
| CN101941001B (en) | 2009-07-03 | 2014-04-02 | 3M创新有限公司 | Hydrophilic coating, product, coating composition and method |
| EP2470930A1 (en) | 2009-08-25 | 2012-07-04 | 3M Innovative Properties Company | Light redirecting film and display system incorporating same |
| US9110235B2 (en) | 2009-10-16 | 2015-08-18 | 3M Innovative Properties Company | Retroreflective sheeting and license plate with reduced retroreflectivity at high entrance angles |
| TW201115231A (en) | 2009-10-28 | 2011-05-01 | Coretronic Corp | Backlight module |
| US8917447B2 (en) * | 2010-01-13 | 2014-12-23 | 3M Innovative Properties Company | Microreplicated film for attachment to autostereoscopic display components |
| CN101839441B (en) * | 2010-04-23 | 2011-07-27 | 上海凯鑫森产业投资控股有限公司 | Optical prism sheet for backlight module |
| KR101842728B1 (en) | 2010-05-07 | 2018-03-27 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | Antireflective films comprising microstructured surface |
| CN102241899B (en) | 2010-05-11 | 2014-05-14 | 3M创新有限公司 | Coating composition, method for modifying matrix surface, and product |
| WO2011162971A2 (en) * | 2010-06-24 | 2011-12-29 | 3M Innovative Properties Company | Polymerizable compositions free of organic emulsifier and polymers and methods of making thereof |
| KR101710168B1 (en) * | 2010-09-16 | 2017-02-27 | 코오롱인더스트리 주식회사 | Spherical polymer powder having high refractive index and preparation method thereof |
| CA2755151C (en) * | 2010-10-18 | 2014-06-17 | Valspar Sourcing, Inc. | Anti-graffiti coatings |
| US8469551B2 (en) | 2010-10-20 | 2013-06-25 | 3M Innovative Properties Company | Light extraction films for increasing pixelated OLED output with reduced blur |
| US8547015B2 (en) | 2010-10-20 | 2013-10-01 | 3M Innovative Properties Company | Light extraction films for organic light emitting devices (OLEDs) |
| JP5646280B2 (en) * | 2010-10-28 | 2014-12-24 | スタンレー電気株式会社 | Surface light source device |
| CN104808270B (en) | 2010-12-04 | 2017-08-15 | 3M创新有限公司 | Light fixture and forming method thereof |
| EP2646860A1 (en) | 2010-12-04 | 2013-10-09 | 3M Innovative Properties Company | Illumination assembly and method of forming same |
| EP2655257A4 (en) | 2010-12-22 | 2016-10-19 | 3M Innovative Properties Co | Surface-modified zirconia nanoparticles |
| EP2500629B1 (en) | 2011-03-15 | 2017-09-06 | SMR Patents S.à.r.l. | Rearview mirror for a vehicle with lighting units with micro-optics |
| SG194729A1 (en) * | 2011-05-13 | 2013-12-30 | 3M Innovative Properties Co | Benzyl (meth)acrylate monomers suitable for microstructured optical films |
| JP5453346B2 (en) * | 2011-05-31 | 2014-03-26 | 京セラドキュメントソリューションズ株式会社 | Image reading apparatus and image forming apparatus having the same |
| US8708543B2 (en) * | 2011-08-10 | 2014-04-29 | Osram Sylvania Inc. | Light engine having distributed remote phosphors |
| JP5752522B2 (en) * | 2011-08-19 | 2015-07-22 | 富士フイルム株式会社 | Laminated film for supporting optical functional member and method for producing the same, sheet and method for producing the same, prism sheet |
| CN102952071B (en) * | 2011-08-30 | 2015-11-25 | 比亚迪股份有限公司 | UV-curable monomer and preparation method thereof, polymerisable compound and backlight module |
| WO2013109612A1 (en) * | 2012-01-16 | 2013-07-25 | Filmetrics, Inc. | High-lifetime broadband light source for low-power applications |
| JP6062922B2 (en) | 2012-03-30 | 2017-01-18 | 株式会社きもと | Edge light type backlight device and light diffusing member |
| KR102056505B1 (en) | 2012-03-30 | 2019-12-16 | 키모토 컴파니 리미티드 | Edge light-type backlight device and light diffusion member |
| US11133118B2 (en) | 2012-05-22 | 2021-09-28 | University Of Massachusetts | Patterned nanoparticle structures |
| US9929325B2 (en) | 2012-06-05 | 2018-03-27 | Samsung Electronics Co., Ltd. | Lighting device including quantum dots |
| WO2014004228A1 (en) * | 2012-06-27 | 2014-01-03 | 3M Innovative Properties Company | Optical component array |
| US9360591B2 (en) * | 2012-09-20 | 2016-06-07 | 3M Innovative Properties Company | Microstructured film comprising nanoparticles and monomer comprising alkylene oxide repeat units |
| GB201219171D0 (en) * | 2012-10-25 | 2012-12-12 | Epipole Ltd | Image acquisition apparatus |
| US9308616B2 (en) | 2013-01-21 | 2016-04-12 | Innovative Finishes LLC | Refurbished component, electronic device including the same, and method of refurbishing a component of an electronic device |
| US10144842B2 (en) | 2013-03-15 | 2018-12-04 | Pixelligent Technologies Llc | High refractive index nanocomposite layer |
| US10273365B2 (en) | 2013-03-15 | 2019-04-30 | Pixelligent Technologies Llc | High refractive index nanocomposite |
| US10033014B2 (en) | 2013-03-15 | 2018-07-24 | Pixelligent Technologies Llc. | Advanced light extraction structure |
| WO2014186096A1 (en) * | 2013-05-17 | 2014-11-20 | Innovative Finishes LLC | Refurbishing a component of an electronic device |
| US10050236B2 (en) | 2013-07-08 | 2018-08-14 | Pixelligent Technologies Llc | Advanced light extraction structure |
| BR112016006775A2 (en) | 2013-10-02 | 2017-08-01 | 3M Innovative Properties Co | optical film stack and article |
| JP6453864B2 (en) | 2013-10-02 | 2019-01-16 | スリーエム イノベイティブ プロパティズ カンパニー | An article comprising a polyacrylate pressure sensitive primer and an adhesive comprising a polyacrylate component |
| WO2015050750A1 (en) | 2013-10-02 | 2015-04-09 | 3M Innovative Properties Company | Microstuctured diffuser comprising first microstructured layer and coating, optical stacks, and method |
| WO2015086850A1 (en) | 2013-12-15 | 2015-06-18 | Vkr Holding A/S | Skylight with sunlight pivot |
| EP3084485A4 (en) * | 2013-12-16 | 2017-10-18 | GE Lighting Solutions, LLC | Optical components with surface micro/nano structure based on fluoro-acrylate material |
| WO2015116459A1 (en) | 2014-01-29 | 2015-08-06 | 3M Innovative Properties Company | Aqueous surface coating composition and modified particles |
| US9046637B1 (en) | 2014-02-25 | 2015-06-02 | 3M Innovative Properties Company | Tubular lighting systems with inner and outer structured surfaces |
| US10161593B2 (en) | 2014-02-25 | 2018-12-25 | 3M Innovative Properties Company | Solid state lighting device with virtual filament(s) |
| CN104125451B (en) * | 2014-07-08 | 2015-12-30 | 四川大学 | A kind of integration imaging 3D display unit of high brightness |
| WO2016099514A1 (en) * | 2014-12-18 | 2016-06-23 | GE Lighting Solutions, LLC | A micro-lens base resin for led lightguide/waveguide applications |
| WO2017023642A1 (en) * | 2015-07-31 | 2017-02-09 | Pixelligent Technologies Llc | Nanocomposite formulations for optical applications |
| US10571618B2 (en) * | 2015-08-18 | 2020-02-25 | Apple Inc. | Display backlight with an optical film |
| US11034845B2 (en) | 2016-02-04 | 2021-06-15 | Pixelligent Technologies, Llc | Nanocomposite formulations for optical applications |
| US10222510B2 (en) * | 2016-03-09 | 2019-03-05 | Lg Chem, Ltd | Anti-reflective film |
| US10377913B2 (en) | 2016-09-16 | 2019-08-13 | Corning Incorporated | High refractive index nanocomposites |
| DE102016225153B4 (en) | 2016-12-15 | 2018-07-12 | Magna Mirrors Holding Gmbh | lighting unit |
| US10919770B2 (en) * | 2017-06-23 | 2021-02-16 | Nanosys, Inc. | Homogeneous anaerobically stable quantum dot concentrates |
| CN107179574B (en) * | 2017-07-19 | 2020-07-31 | 宁波长阳科技股份有限公司 | High-luminance wide-viewing-angle brightness enhancement film |
| KR102113237B1 (en) * | 2017-09-26 | 2020-05-20 | 주식회사 엘지화학 | Optical film and liquid crystal display |
| CN109749017A (en) * | 2017-11-03 | 2019-05-14 | 宁波激智科技股份有限公司 | A kind of composition and a kind of brightness enhancement film |
| US10670964B2 (en) | 2017-11-21 | 2020-06-02 | International Business Machines Corporation | Ruggedized solder mask material |
| CN107957605A (en) * | 2018-01-12 | 2018-04-24 | 上海午井光电科技有限公司 | Speculum |
| CN207851852U (en) * | 2018-01-23 | 2018-09-11 | 金佶科技股份有限公司 | Electronic device and its taken module |
| US11906759B2 (en) | 2018-05-22 | 2024-02-20 | 3M Innovative Properties Company | Optical film with light control edge |
| CN110941039B (en) * | 2018-09-25 | 2021-04-30 | 深圳光峰科技股份有限公司 | Light reflecting material, reflecting layer and preparation method thereof |
| CN109796562A (en) * | 2018-12-21 | 2019-05-24 | 宁波激智科技股份有限公司 | A kind of composition and a kind of brightness enhancement film and its application |
| CN109581742A (en) * | 2018-12-21 | 2019-04-05 | 宁波激智科技股份有限公司 | A kind of brightness enhancement film and its application |
| KR20200091741A (en) * | 2019-01-23 | 2020-07-31 | 동우 화인켐 주식회사 | Polarizer and method of manufacturing the same |
| CN114207391A (en) | 2019-04-16 | 2022-03-18 | 华为技术有限公司 | Signal acquisition spectrometer |
| KR20220010484A (en) * | 2019-05-20 | 2022-01-25 | 크레인 앤 코, 인크 | Use of nanoparticles to tune the refractive index of a layer of a polymer matrix to optimize micro-optical (MO) focus |
| CN113050328B (en) * | 2021-03-15 | 2023-02-28 | 合肥京东方光电科技有限公司 | Front module and display device |
| KR102716656B1 (en) * | 2021-08-23 | 2024-10-15 | 주식회사 케이씨텍 | Metal oxide dispersion, film composiotion for display and optical member for display |
| US20240159953A1 (en) * | 2022-04-01 | 2024-05-16 | Radiant Opto-Electronics Corporation | Display device and backlight module thereof |
| CN116931160A (en) * | 2022-04-01 | 2023-10-24 | 瑞仪光电(南京)有限公司 | Backlight module with multiple cone structures designed on optical film and display device |
Family Cites Families (116)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2742604A (en) | 1951-04-25 | 1956-04-17 | Bosch Arma Corp | Electromechanical resolvers |
| EP0089041B1 (en) | 1982-03-16 | 1987-11-25 | E.I. Du Pont De Nemours And Company | Use of a negative acting photopolymerizable element as a solder mask |
| US4542449A (en) * | 1983-08-29 | 1985-09-17 | Canadian Patents & Development Limited | Lighting panel with opposed 45° corrugations |
| US4487904A (en) * | 1983-09-21 | 1984-12-11 | Toray Industries, Inc. | Urethanized acrylic resin material for plastic lens and lens composed thereof |
| EP0176874A3 (en) * | 1984-09-19 | 1988-02-10 | Toray Industries, Inc. | A highly-refractive plastic lens |
| US4812032A (en) * | 1984-09-19 | 1989-03-14 | Toray Industries, Inc. | Highly-refractive plastic lens |
| US4568445A (en) * | 1984-12-21 | 1986-02-04 | Honeywell Inc. | Electrode system for an electro-chemical sensor for measuring vapor concentrations |
| US4771774A (en) * | 1986-02-28 | 1988-09-20 | Devices For Vascular Intervention, Inc. | Motor drive unit |
| US4937172A (en) * | 1986-12-02 | 1990-06-26 | E. I. Du Pont De Nemours And Company | Photopolymerizable composition having superior adhesion, articles and processes |
| US5073462A (en) * | 1986-12-02 | 1991-12-17 | E. I. Du Pont De Nemours And Company | Photopolymerizable composition having superior adhesion, articles and processes |
| JPS63279201A (en) * | 1987-05-12 | 1988-11-16 | Kureha Chem Ind Co Ltd | Resin lens |
| US5354821A (en) * | 1988-08-31 | 1994-10-11 | Henkel Kommanditgesellschaft Auf Aktien | Radically polymerizable multicomponent mixtures and their use (III) |
| JPH0288615A (en) * | 1988-09-27 | 1990-03-28 | Mitsubishi Rayon Co Ltd | Flame retardant liquid photosensitive resin composition |
| US5164464A (en) * | 1989-01-17 | 1992-11-17 | The Dow Chemical Company | Vinyl ester resins containing mesogenic/rigid rodlike moieties |
| US4962163A (en) * | 1989-01-17 | 1990-10-09 | The Dow Chemical Company | Vinyl ester resins containing mesogenic/rigid rodlike moieties |
| US5066750A (en) * | 1989-01-17 | 1991-11-19 | The Dow Chemical Company | Vinyl ester resins containing mesogenic/rigid rodlike moieties |
| US5175030A (en) * | 1989-02-10 | 1992-12-29 | Minnesota Mining And Manufacturing Company | Microstructure-bearing composite plastic articles and method of making |
| US5183597A (en) * | 1989-02-10 | 1993-02-02 | Minnesota Mining And Manufacturing Company | Method of molding microstructure bearing composite plastic articles |
| US5486949A (en) * | 1989-06-20 | 1996-01-23 | The Dow Chemical Company | Birefringent interference polarizer |
| JPH03160058A (en) * | 1989-11-17 | 1991-07-10 | Mitsubishi Rayon Co Ltd | Crosslinkage-curable resin composition |
| US5247038A (en) * | 1989-11-29 | 1993-09-21 | Mitsubishi Rayon Co., Ltd. | Polybutylene glycol dimethacrylate and resin composition for cast polymerization |
| AU634338B2 (en) * | 1990-02-08 | 1993-02-18 | Mitsubishi Rayon Company Limited | Composition for plastic lenses |
| JP2873126B2 (en) * | 1991-04-17 | 1999-03-24 | 日本ペイント株式会社 | Photosensitive composition for volume hologram recording |
| JP3155327B2 (en) * | 1992-03-27 | 2001-04-09 | 三菱化学株式会社 | High refractive index optical material and method for producing the same |
| JP2958191B2 (en) * | 1992-08-11 | 1999-10-06 | 株式会社きもと | Light diffusion sheet |
| US5691846A (en) * | 1993-10-20 | 1997-11-25 | Minnesota Mining And Manufacturing Company | Ultra-flexible retroreflective cube corner composite sheetings and methods of manufacture |
| US5828488A (en) * | 1993-12-21 | 1998-10-27 | Minnesota Mining And Manufacturing Co. | Reflective polarizer display |
| US5882774A (en) * | 1993-12-21 | 1999-03-16 | Minnesota Mining And Manufacturing Company | Optical film |
| DE69525236T2 (en) * | 1994-10-18 | 2002-10-24 | Mitsubishi Rayon Co., Ltd. | Actinic radiation-curable composition and leaf-shaped lens |
| US5855983A (en) * | 1995-02-03 | 1999-01-05 | Minnesota Mining And Manufacturing Company | Flame retardant ultraviolet cured multi-layered film |
| US5635278A (en) * | 1995-02-03 | 1997-06-03 | Minnesota Mining And Manufacturing Company | Scratch resistant optical films and method for producing same |
| US5626800A (en) * | 1995-02-03 | 1997-05-06 | Minnesota Mining And Manufacturing Company | Prevention of groove tip deformation in brightness enhancement film |
| JP4168179B2 (en) * | 1995-03-03 | 2008-10-22 | スリーエム カンパニー | Light directional films having structured surfaces of various heights and light directional products made from the films |
| JP3298891B2 (en) | 1995-04-28 | 2002-07-08 | 日本油脂ビーエーエスエフコーティングス株式会社 | Paint composition, method for producing paint composition, and method for producing dispersion of inorganic oxide sol |
| JP3190251B2 (en) * | 1995-06-06 | 2001-07-23 | 太陽インキ製造株式会社 | Photocurable and thermosetting resin composition for alkali-developed flexible printed wiring boards |
| US5714218A (en) * | 1995-08-21 | 1998-02-03 | Dainippon Printing Co., Ltd. | Ionizing radiation-curable resin composition for optical article, optical article, and surface light source |
| US5917664A (en) * | 1996-02-05 | 1999-06-29 | 3M Innovative Properties Company | Brightness enhancement film with soft cutoff |
| US5825543A (en) * | 1996-02-29 | 1998-10-20 | Minnesota Mining And Manufacturing Company | Diffusely reflecting polarizing element including a first birefringent phase and a second phase |
| US5783120A (en) * | 1996-02-29 | 1998-07-21 | Minnesota Mining And Manufacturing Company | Method for making an optical film |
| US5919551A (en) * | 1996-04-12 | 1999-07-06 | 3M Innovative Properties Company | Variable pitch structured optical film |
| US5908874A (en) * | 1996-06-18 | 1999-06-01 | 3M Innovative Properties Company | Polymerizable compositions containing fluorochemicals to reduce melting temperature |
| US5760126A (en) * | 1996-12-20 | 1998-06-02 | Minnesota Mining And Manufacturing Company | Aqueous fluorochemical compositions and abrasion-resistant coatings therefrom |
| EP0860742B1 (en) * | 1997-02-25 | 2001-04-04 | E.I. Du Pont De Nemours And Company | Flexible, flame-retardant, photoimageable composition for coating printing circuits |
| WO1998050340A1 (en) | 1997-05-09 | 1998-11-12 | Minnesota Mining And Manufacturing Company | High index of refraction monomers |
| US6107364A (en) | 1997-05-09 | 2000-08-22 | 3M Innovative Properties Company | Methyl styrene as a high index of refraction monomer |
| US6355754B1 (en) | 1997-05-09 | 2002-03-12 | 3M Innovative Properties Company | High refractive index chemical composition and polymers and polymeric material derived therefrom |
| US5932626A (en) * | 1997-05-09 | 1999-08-03 | Minnesota Mining And Manufacturing Company | Optical product prepared from high index of refraction brominated monomers |
| US6280063B1 (en) * | 1997-05-09 | 2001-08-28 | 3M Innovative Properties Company | Brightness enhancement article |
| MY122234A (en) * | 1997-05-13 | 2006-04-29 | Inst Neue Mat Gemein Gmbh | Nanostructured moulded bodies and layers and method for producing same |
| US5898523A (en) * | 1997-07-02 | 1999-04-27 | Minnesota Mining & Manufacturing Company | Tiled retroreflective sheeting composed of highly canted cube corner elements |
| US6599631B2 (en) * | 2001-01-26 | 2003-07-29 | Nanogram Corporation | Polymer-inorganic particle composites |
| JPH11209144A (en) * | 1998-01-21 | 1999-08-03 | Hoya Corp | Glass for near infrared ray absorbing filter and near infrared ray absorbing filter using the same |
| JP3901822B2 (en) | 1998-02-06 | 2007-04-04 | 三菱化学株式会社 | Low birefringence optical member, resin composition for molding the same, and method for producing optical member |
| US5915551A (en) * | 1998-07-23 | 1999-06-29 | Vavro; David J. | Golf bag with integrated beverage cooler |
| US6329058B1 (en) * | 1998-07-30 | 2001-12-11 | 3M Innovative Properties Company | Nanosize metal oxide particles for producing transparent metal oxide colloids and ceramers |
| AU8761498A (en) | 1998-07-30 | 2000-02-21 | Minnesota Mining And Manufacturing Company | Nanosize metal oxide particles for producing transparent metal oxide colloids and ceramers |
| US6359170B1 (en) * | 1998-09-02 | 2002-03-19 | 3M Innovative Properties Company | Brominated materials |
| CA2340671A1 (en) | 1998-09-02 | 2000-03-16 | Minnesota Mining And Manufacturing Company | Brominated materials |
| JP2000147208A (en) | 1998-11-12 | 2000-05-26 | Tomoegawa Paper Co Ltd | Antireflection material and polarizing film using same |
| JP3900506B2 (en) * | 1998-11-06 | 2007-04-04 | Jsr株式会社 | Liquid curable resin composition, cured product thereof and antireflection film |
| EP1014113A3 (en) | 1998-12-21 | 2001-05-09 | Dsm N.V. | Photo curable resin composition and optical parts |
| US6261700B1 (en) * | 1998-12-30 | 2001-07-17 | 3M Innovative Properties Co | Ceramer containing a brominated polymer and inorganic oxide particles |
| US6146910A (en) * | 1999-02-02 | 2000-11-14 | The United States Of America, As Represented By The Secretary Of Commerce | Target configuration and method for extraction of overlay vectors from targets having concealed features |
| CA2414702C (en) * | 1999-06-30 | 2008-02-05 | Silverbrook Research Pty Ltd | Printhead support structure and assembly |
| US6288419B1 (en) * | 1999-07-09 | 2001-09-11 | Micron Technology, Inc. | Low resistance gate flash memory |
| KR20010050120A (en) | 1999-08-25 | 2001-06-15 | 고사이 아끼오 | Light scattering resin layer, color filter and liquid crystal display using the same |
| US6723096B1 (en) * | 1999-08-26 | 2004-04-20 | Sdgi Holdings, Inc. | Devices and methods for implanting fusion cages |
| US6356391B1 (en) * | 1999-10-08 | 2002-03-12 | 3M Innovative Properties Company | Optical film with variable angle prisms |
| JP4122661B2 (en) | 1999-10-22 | 2008-07-23 | Jsr株式会社 | Photocurable resin composition and plastic sheet |
| US6368682B1 (en) * | 1999-10-22 | 2002-04-09 | 3M Innovative Properties Company | Composition and structures made therefrom |
| US6376590B2 (en) * | 1999-10-28 | 2002-04-23 | 3M Innovative Properties Company | Zirconia sol, process of making and composite material |
| JP4011811B2 (en) | 2000-01-14 | 2007-11-21 | Jsr株式会社 | Photocurable resin composition and optical member |
| US6304365B1 (en) * | 2000-06-02 | 2001-10-16 | The University Of British Columbia | Enhanced effective refractive index total internal reflection image display |
| US6376704B1 (en) | 2000-06-28 | 2002-04-23 | 3M Innovative Properties Company | Naphthyoxyalkyl(meth)acrylates with high refractive indices and low glass transition temperatures |
| US6896958B1 (en) | 2000-11-29 | 2005-05-24 | Nanophase Technologies Corporation | Substantially transparent, abrasion-resistant films containing surface-treated nanocrystalline particles |
| US6541591B2 (en) * | 2000-12-21 | 2003-04-01 | 3M Innovative Properties Company | High refractive index microreplication resin from naphthyloxyalkylmethacrylates or naphthyloxyacrylates polymers |
| AR032424A1 (en) * | 2001-01-30 | 2003-11-05 | Procter & Gamble | COATING COMPOSITIONS TO MODIFY SURFACES. |
| US6593392B2 (en) * | 2001-06-22 | 2003-07-15 | Corning Incorporated | Curable halogenated compositions |
| US7420005B2 (en) * | 2001-06-28 | 2008-09-02 | Dai Nippon Printing Co., Ltd. | Photocurable resin composition, finely embossed pattern-forming sheet, finely embossed transfer sheet, optical article, stamper and method of forming finely embossed pattern |
| US6656990B2 (en) * | 2001-07-11 | 2003-12-02 | Corning Incorporated | Curable high refractive index compositions |
| JP4532898B2 (en) * | 2001-08-02 | 2010-08-25 | スリーエム イノベイティブ プロパティズ カンパニー | Abrasive particles and method for producing and using the same |
| JP4124991B2 (en) | 2001-10-23 | 2008-07-23 | Dic株式会社 | Active energy ray-curable resin composition for Fresnel lens and Fresnel lens sheet |
| GB0127252D0 (en) * | 2001-11-13 | 2002-01-02 | Vantico Ag | Production of composite articles composed of thin layers |
| US6962946B2 (en) * | 2001-11-21 | 2005-11-08 | 3M Innovative Properties Company | Nanoparticles having a rutile-like crystalline phase and method of preparing same |
| US6641577B2 (en) * | 2001-11-28 | 2003-11-04 | 20/10 Perfect Vision Optische Geraete Gmbh | Apparatus and method for creating a corneal flap |
| EP1478689A1 (en) * | 2002-02-19 | 2004-11-24 | Photon-X, Inc. | Polymer nanocomposites for optical applications |
| US20050256219A1 (en) | 2002-03-11 | 2005-11-17 | Hideaki Takase | Photocurable resin composition and optical component |
| AU2003218212A1 (en) * | 2002-03-15 | 2003-09-29 | Photon-X, Inc. | Optical polymer nanocomposite substrates with surface relief structures |
| JP4229731B2 (en) | 2002-03-18 | 2009-02-25 | 大日本印刷株式会社 | Resin composition and optical element |
| DE10212523A1 (en) | 2002-03-21 | 2003-10-02 | Degussa | Air-drying, silane-containing coating agents |
| ATE411407T1 (en) | 2002-04-15 | 2008-10-15 | Schott Ag | METHOD FOR COATING METAL SURFACES |
| TWI290328B (en) * | 2002-05-23 | 2007-11-21 | Nof Corp | Transparent conductive laminated film and touch panel |
| US20040021133A1 (en) * | 2002-07-31 | 2004-02-05 | Nagpal Vidhu J. | High refractive index polymerizable composition |
| DE60220518T2 (en) | 2002-08-08 | 2008-02-07 | Nissan Motor Co., Ltd., Yokohama | Acrylic urethane coating composition |
| US6844047B2 (en) * | 2002-10-07 | 2005-01-18 | Eastman Kodak Company | Optical element containing nanocomposite materials |
| US6727309B1 (en) * | 2002-10-08 | 2004-04-27 | 3M Innovative Properties Company | Floor finish composition |
| EP1418448A1 (en) * | 2002-11-06 | 2004-05-12 | Koninklijke DSM N.V. | Preparation of a mechanically durable single layer coating with anti-reflective properties |
| AU2003300010A1 (en) * | 2002-12-20 | 2004-07-22 | Dow Global Technologies Inc. | Polymerized macrocyclic oligomer nanocomposite compositions |
| US6833176B2 (en) | 2003-01-06 | 2004-12-21 | General Electric Company | Radiation curable microstructure-bearing articles |
| US6844950B2 (en) * | 2003-01-07 | 2005-01-18 | General Electric Company | Microstructure-bearing articles of high refractive index |
| US7041365B2 (en) | 2003-05-12 | 2006-05-09 | 3M Innovative Properties Company | Static dissipative optical construction |
| US6846089B2 (en) * | 2003-05-16 | 2005-01-25 | 3M Innovative Properties Company | Method for stacking surface structured optical films |
| US7046439B2 (en) * | 2003-05-22 | 2006-05-16 | Eastman Kodak Company | Optical element with nanoparticles |
| US7169375B2 (en) | 2003-08-29 | 2007-01-30 | General Electric Company | Metal oxide nanoparticles, methods of making, and methods of use |
| US7045558B2 (en) | 2003-08-29 | 2006-05-16 | General Electric Company | Method of making a high refractive index optical management coating and the coating |
| US7282272B2 (en) | 2003-09-12 | 2007-10-16 | 3M Innovative Properties Company | Polymerizable compositions comprising nanoparticles |
| US7074463B2 (en) * | 2003-09-12 | 2006-07-11 | 3M Innovative Properties Company | Durable optical element |
| US6998425B2 (en) * | 2003-12-23 | 2006-02-14 | General Electric Company | UV curable coating compositions and uses thereof |
| US20050147838A1 (en) * | 2003-12-30 | 2005-07-07 | 3M Innovative Properties Company | Polymerizable compositions for optical articles |
| JP2005314661A (en) | 2004-03-30 | 2005-11-10 | Mitsubishi Chemicals Corp | Resin molded product |
| JP2005316219A (en) | 2004-04-30 | 2005-11-10 | Olympus Corp | Optical material |
| TWI317444B (en) | 2004-09-03 | 2009-11-21 | Eternal Chemical Co Ltd | Optical film having high hardness and use thereof |
| US7326448B2 (en) | 2005-02-17 | 2008-02-05 | 3M Innovative Properties Company | Polymerizable oligomeric urethane compositions comprising nanoparticles |
| US20060226583A1 (en) * | 2005-04-04 | 2006-10-12 | Marushin Patrick H | Light directing film |
| TWI287115B (en) | 2005-10-07 | 2007-09-21 | Eternal Chemical Co Ltd | Brightness enhancement film |
| US7547467B2 (en) | 2005-11-15 | 2009-06-16 | 3M Innovative Properties Company | Brightness enhancing film and methods of surface treating inorganic nanoparticles |
-
2005
- 2005-03-11 US US11/078,145 patent/US7282272B2/en not_active Expired - Lifetime
- 2005-06-06 WO PCT/US2005/019774 patent/WO2006007286A2/en not_active Application Discontinuation
- 2005-06-06 JP JP2007516536A patent/JP5038132B2/en active Active
- 2005-06-06 CN CN2010101322911A patent/CN101872028B/en active Active
- 2005-06-06 EP EP20050789350 patent/EP1756626B1/en not_active Not-in-force
- 2005-06-06 KR KR1020067026535A patent/KR101255417B1/en active IP Right Grant
- 2005-06-06 CN CN2005800197288A patent/CN1969201B/en active Active
- 2005-06-06 EP EP20100185201 patent/EP2278363A3/en not_active Withdrawn
- 2005-06-16 EP EP05815069A patent/EP1756631A2/en not_active Withdrawn
- 2005-06-16 KR KR1020107014833A patent/KR101245880B1/en active IP Right Grant
- 2005-06-16 WO PCT/US2005/021351 patent/WO2006028543A2/en active Application Filing
- 2005-06-16 CN CNA2005800201353A patent/CN101288004A/en active Pending
- 2005-06-16 JP JP2007516750A patent/JP2008503774A/en not_active Withdrawn
-
2006
- 2006-06-08 US US11/422,900 patent/US7622164B2/en active Active
-
2007
- 2007-10-12 US US11/871,330 patent/US7547476B2/en not_active Expired - Lifetime
-
2009
- 2009-06-10 US US12/481,991 patent/US7763331B2/en not_active Expired - Fee Related
-
2010
- 2010-06-18 US US12/818,224 patent/US8168271B2/en not_active Expired - Fee Related
-
2012
- 2012-03-28 US US13/432,272 patent/US8389074B2/en not_active Expired - Lifetime
Non-Patent Citations (1)
| Title |
|---|
| None |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7491441B2 (en) | 2004-12-30 | 2009-02-17 | 3M Innovative Properties Company | High refractive index, durable hard coats |
| US7264872B2 (en) | 2004-12-30 | 2007-09-04 | 3M Innovative Properties Company | Durable high index nanocomposites for AR coatings |
| WO2006073856A3 (en) * | 2004-12-30 | 2007-10-04 | 3M Innovative Properties Co | Durable high index nanocomposites for ar coatings |
| US7297810B2 (en) | 2004-12-30 | 2007-11-20 | 3M Innovative Properties Company | High refractive index monomers for optical applications |
| WO2006073856A2 (en) * | 2004-12-30 | 2006-07-13 | 3M Innovative Properties Company | Durable high index nanocomposites for ar coatings |
| WO2007001811A1 (en) * | 2005-06-28 | 2007-01-04 | General Electric Company | Compositions for brightness enhancing films |
| WO2007082919A3 (en) * | 2006-01-18 | 2008-04-17 | Lemnis Lighting Ip Gmbh | Novel monomeric and polymeric materials |
| US8323594B2 (en) | 2006-01-18 | 2012-12-04 | Sparkxis B.V. | Method of making hybrid organic-inorganic monomeric materials |
| WO2008082414A1 (en) * | 2007-01-04 | 2008-07-10 | General Electric Company | Methods for improving mold quality for use in the manufacture of light management film, manufactured film and corresponding mold |
| JP2010519599A (en) * | 2007-02-27 | 2010-06-03 | スリーエム イノベイティブ プロパティズ カンパニー | Brightness enhancement film with nanocomposite structure with improved crack resistance |
| CN102746448B (en) * | 2007-02-27 | 2015-05-06 | 3M创新有限公司 | Brightness enhancing film comprising nanocomposite structure having improved crack resistance |
| WO2008121465A1 (en) * | 2007-02-27 | 2008-10-09 | 3M Innovative Properties Company | Brightness enhancing film comprising nanocomposite structure having improved crack resistance |
| US8530572B2 (en) | 2007-02-27 | 2013-09-10 | 3M Innovative Properties Company | Brightness enhancing film comprising nanocomposite structure having improved crack resistance |
| US9164195B2 (en) | 2007-03-07 | 2015-10-20 | 3M Innovative Properties Company | Methods of making microstructured optical films comprising biphenyl difunctional monomers |
| JP2010520938A (en) * | 2007-03-09 | 2010-06-17 | スリーエム イノベイティブ プロパティズ カンパニー | Microstructured optical film containing biphenyl bifunctional monomer |
| US8586154B2 (en) | 2007-03-09 | 2013-11-19 | 3M Innovative Properties Company | Triphenyl monomers suitable for microstructured optical films |
| US8871315B2 (en) | 2007-03-09 | 2014-10-28 | 3M Innovative Properties Company | Triphenyl monomers suitable for microstructured optical films |
| JP2010520939A (en) * | 2007-03-09 | 2010-06-17 | スリーエム イノベイティブ プロパティズ カンパニー | Triphenyl monomer suitable for microstructured optical film |
| US9221743B2 (en) | 2007-03-09 | 2015-12-29 | 3M Innovative Properties Company | Triphenyl monomers suitable for microstructured optical films |
| US9885807B2 (en) | 2007-03-09 | 2018-02-06 | 3M Innovative Properties Company | Triphenyl monomers suitable for microstructured optical films |
| US8449868B2 (en) | 2008-02-21 | 2013-05-28 | Basf Se | Preparation of cationic nanoparticles and personal care compositions comprising said nanoparticles |
| WO2009103651A2 (en) | 2008-02-21 | 2009-08-27 | Basf Se | Preparation of cationic nanoparticles and personal care compositions comprising said nanoparticles |
| US7981986B2 (en) | 2008-04-29 | 2011-07-19 | 3M Innovative Properties Company | Optical films comprising fluorenol (meth)acrylate monomer |
| US8389599B2 (en) | 2008-10-22 | 2013-03-05 | 3M Innovative Properties Company | Dental composition comprising biphenyl di(meth)acrylate monomer comprising urethane moieties |
| US7957621B2 (en) * | 2008-12-17 | 2011-06-07 | 3M Innovative Properties Company | Light extraction film with nanoparticle coatings |
| EP2507161A2 (en) * | 2009-12-02 | 2012-10-10 | 3M Innovative Properties Company | Functionalized zirconia nanoparticles and high index films made therefrom |
| EP2507161A4 (en) * | 2009-12-02 | 2014-01-01 | 3M Innovative Properties Co | Functionalized zirconia nanoparticles and high index films made therefrom |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1756626B1 (en) | 2014-04-23 |
| KR101255417B1 (en) | 2013-04-17 |
| US20090246417A1 (en) | 2009-10-01 |
| WO2006028543A2 (en) | 2006-03-16 |
| JP5038132B2 (en) | 2012-10-03 |
| KR101245880B1 (en) | 2013-03-20 |
| JP2008503768A (en) | 2008-02-07 |
| US7763331B2 (en) | 2010-07-27 |
| EP1756631A2 (en) | 2007-02-28 |
| JP2008503774A (en) | 2008-02-07 |
| US20050200278A1 (en) | 2005-09-15 |
| US8168271B2 (en) | 2012-05-01 |
| CN101872028A (en) | 2010-10-27 |
| CN101288004A (en) | 2008-10-15 |
| WO2006007286A3 (en) | 2006-02-23 |
| CN1969201B (en) | 2010-05-05 |
| WO2006028543A8 (en) | 2006-04-27 |
| US20100253885A1 (en) | 2010-10-07 |
| US20060210726A1 (en) | 2006-09-21 |
| US20080030829A1 (en) | 2008-02-07 |
| US7622164B2 (en) | 2009-11-24 |
| EP2278363A2 (en) | 2011-01-26 |
| US8389074B2 (en) | 2013-03-05 |
| KR20070022098A (en) | 2007-02-23 |
| KR20100084583A (en) | 2010-07-26 |
| US20120189784A1 (en) | 2012-07-26 |
| CN101872028B (en) | 2011-12-07 |
| EP2278363A3 (en) | 2011-05-25 |
| CN1969201A (en) | 2007-05-23 |
| US7282272B2 (en) | 2007-10-16 |
| EP1756626A2 (en) | 2007-02-28 |
| US7547476B2 (en) | 2009-06-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1756626B1 (en) | Polymerizable compositions comprising nanoparticles | |
| JP7138491B2 (en) | Polymerizable composition containing low molecular weight organic component | |
| US20080119583A1 (en) | Brightness Enhancement Film Comprising Polymerized Organic Phase Having Low Glass Transition Temperature | |
| TWI363062B (en) | Polymerizable compositions comprising nanoparticles | |
| WO2007059228A1 (en) | Brightness enhancing film and method of surface treating inorganic nanoparticles | |
| WO2006099168A2 (en) | Polymerizable compositions comprising nanoparticles | |
| EP1869115A1 (en) | Polymerizable oligomeric urethane compositions comprising nanoparticles | |
| US20060187366A1 (en) | Microstructured article comprising a polymerized composition having low glass transition temperature |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BW BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE EG ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KM KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NA NG NI NO NZ OM PG PH PL PT RO RU SC SD SE SG SK SL SM SY TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): BW GH GM KE LS MW MZ NA SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LT LU MC NL PL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2005789350 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020067026535 Country of ref document: KR Ref document number: 200580019728.8 Country of ref document: CN Ref document number: 2007516536 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 4635/CHENP/2006 Country of ref document: IN |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020067026535 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 2005789350 Country of ref document: EP |