WO2004087154A1 - Use of cuminum cyminum extract and piperine for potentiation of bioefficacy of anti infectives - Google Patents
Use of cuminum cyminum extract and piperine for potentiation of bioefficacy of anti infectives Download PDFInfo
- Publication number
- WO2004087154A1 WO2004087154A1 PCT/IN2003/000110 IN0300110W WO2004087154A1 WO 2004087154 A1 WO2004087154 A1 WO 2004087154A1 IN 0300110 W IN0300110 W IN 0300110W WO 2004087154 A1 WO2004087154 A1 WO 2004087154A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition
- infective
- bioenhancer
- formula
- group
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/23—Apiaceae or Umbelliferae (Carrot family), e.g. dill, chervil, coriander or cumin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
- A61P31/06—Antibacterial agents for tuberculosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
Definitions
- the present invention relates to the field of chemotherapeutics, particularly to their formulation as of oral pharmaceutical compositions containing bioenhancers for increasing bioefficacy of anti-infectives and thereby requiring lower doses and/or decreased frequency of dosing of such anti-infectives while mamtaining the therapeutic efficacy of standard doses of such drugs. Background of the invention.
- pathogenic microorgnisms which include bacteria, virus and fungi.
- pathogenic microorgnisms lead to septicaemia, serious infections of upper and lower respiratory tract, CNS, meningitis, intra- abdominal including peritoneum, genito-urinary tract, skin, and soft tissue, and variety of other infections like systemic mycosis, candidiasis including infections caused by dermatophytes.
- antimicrobials include aminoglycosides, penicillins, cephalosporins, macrolides, glycopeptides, fluoroquinolones, tetracyclins, first and second line anti-TB drags, anti- leprosy, antivirals, polyene, triazole, and imidazole anti-fungals, combinations like pyrimidine derivatives and trimethoprim and sulphamethoxizole.
- Trimethoprim-sulfamethoxazole also known as co-trimoxazole or TMP-SMX, which was introduced in 1968 as a broad-spectrum antimicrobial agent.
- Tri ethoprim was specially developed as a potentiator of sulphonamide to act synergistically against bacteria and delay the development of bacterial resistance.
- the 1:5 ratio of trimethoprim to sulfamethoxazole achieves an approximate 1:20 ratio of peak serum concentrations which is the optimal synergistic ratio of serum concentrations against most susceptible bacteria (Gutman LT, Pediatr Infect Dis 1984;3 :349-57, Olin BR, Facts and Comparisons, Inc. 1998; 408b-409d, Cockerill FR, Edson RS, Mayo Clin Proc 1991;66:1260-9)
- the combination can also be between one antiinfective agent and another chemical agent which by itself is not antiinfective in nature but when combined with the antiinfective, enhances the effectiveness of this antiinfective.
- the example of such combination is Amoxicillin + Clavulanic acid, more commonly known as Augmentin.
- Amoxicillin is an antibiotic of the penicillin type. It is effective against different bacteria such as H. influenzae, N. gonorrhea, E. coli, Pneumococci, Streptococci, and certain strains of Staphylococci. Chemically, it is closely related to penicillin and ampicillin.
- Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus. It is a ⁇ -lactam structurally related to the penicillins and possesses the ability to inactivate a wide variety of ⁇ -lactamases by blocking the active sites of these enzymes. Clavulanic acid is particularly active against the clinically important plasimd mediated ⁇ -lactamases frequently responsible for transferred drug resistance to penicillins and cephalosporins.
- Ayurveda One of the most notable features that has been associated with the traditional Indian medicine and amply described in Ayurveda is the use of compositions which offer additive, synergistic and potentiating effect of one medicament when used in combination with the other.
- Ayurveda there are several natural products, which have been used as an essential ingredient of many formulations used against wide range of diseases. The most prominent of these being 'Trikatu' comprising black pepper, long pepper and dry ginger.
- 'Trikatu' comprising black pepper, long pepper and dry ginger.
- Detailed and systematic studies have shown that one of the active ingredients of peppers i.e., piperine is a potent bioavailability and/ or bioeffacicay enhancer of several drugs and nutrients.
- the main object of the invention is to provide a oral pharmaceutical compositions containing bioenhancers for increasing bioefficacy of anti-infectives and thereby requiring lower doses and/or decreased frequency of dosing of such anti-infectives while maintaining the therapeutic efficacy of standard doses of such drugs.
- the present invention deals with one such combinations, where piperine and other bioenhancers are used as potentiators when combined with various anti-infective agents in vitro using bacteria, viruses and yeast and in vivo using mice and guinea pig infection models.
- the present invention is aimed to overcome or avoid problems faced in the prior art.
- the use of products of the present invention offer a low dose regimen that produces enhanced therapeutic action comparable to that of standard dose alone.
- the present invention provides a composition useful for enhanced therapeutic effect at reduced doses of the anti infectives against infection caused bya microorganism comprising a mixture of an anti-infective agent and a bioenhancer selected from piperine of formula 1 and 3',5-Dihydroxy flavone 7-O- ⁇ -D-galacturonide-4'-O- ⁇ -D- glucopyranoside of formula 2 or a mixture thereof.
- the anti infective agent is selected from the group consisting of penicillins including semi synthetic, cephalosporins, aminoglycosides, glycopeptides, fluroquinolones, macrolides, tetracyclines, first and second line anti-TB drugs, antileprosy drugs, oxazolidelones, antifungal agents, antiviral agents and pyrimidine derivatives - sulphonamides combination.
- the anti-fungal agent is selected from the group consisting of polyenes, imidazoles and triazoles.
- the antiviral agent is selected from the group consisting of Zidovudines, idouridine, acyclovir and ribavarine.
- the 3',5-Dihydroxy flavone 7-O- ⁇ -D- galacturonide-4'-0- ⁇ -D-glucopyranoside is used in pure form or in the form of a HPLC fingerprinted fraction of 3',5-Dihydroxy flavone 7-O- ⁇ -D-galacturonide-4'-O- ⁇ -D- glucopyranoside from Cuminum cyminum or a sub fraction.
- the concentration of the anti infective is two to eight times lesser than when such anti infective is used without the bioenhancer.
- the composition includes one or more pharmaceutically acceptable additives and excipients.
- the additives/excipients are selected from the group consisting of nutrients comprising proteins, carbohydrates, sugar, talc, magnesium stearate, cellulose, calcium carbonate, starch-gelatin paste, and/ or pharmaceutically acceptable carriers, diluents and solvents.
- the composition is in oral administration form.
- the ratio of the anti-infective to the bioenhancer is in the range of 1 : 1 to 1:5.
- the additives have no effect on the anti- infective property of the said composition.
- the present invention also provides a process for the preparation of a composition useful for enhanced therapeutic effect at reduced doses of the anti infectives against infection caused bya microorganism comprising a mixture of an anti-infective agent and a bioenhancer selected from piperine of formula 1 and 3',5-Dihydroxy flavone 7-O- ⁇ -D- galacturonide-4'-O- ⁇ -D-glucopyranoside of formula 2 or a mixture thereof, said process comprising a physical admixing technique.
- a bioenhancer selected from piperine of formula 1 and 3',5-Dihydroxy flavone 7-O- ⁇ -D- galacturonide-4'-O- ⁇ -D-glucopyranoside of formula 2 or a mixture thereof, said process comprising a physical admixing technique.
- the physical admixing technique is selected from dialysis, molecular sieving and by membranes.
- the process of preparation of the bioenhancer comprises use of water, alcohol, combinations of water and alcohol, hydrocarbons, ketones and ethers.
- the anti infective agent is selected from the group consisting of penicillins including semi synthetic, cephalosporins, aminoglycosides, glycopeptides, fluroquinolones, macrolides, tetracyclines, first and second line anti-TB drugs, antileprosy drugs, oxazolidelones, antifungal agents, antiviral agents and pyrimidine derivatives - sulphonamides combination.
- the anti-fungal agent is selected from the group consisting of polyenes, imidazoles and triazoles.
- the antiviral agent is selected from the group consisting of Zidovudines, idouridine, acyclovir and ribavarine.
- the 3',5-Dihydroxy flavone 7-O- ⁇ -D- galacturonide-4'-O- ⁇ -D-glucopyranoside is used in pure form or in the form of a HPLC fingerprinted fraction of 3',5-Dihydroxy flavone 7-O- ⁇ -D-galacturonide-4'-O- ⁇ -D- glucopyranoside from Cuminum cyminum or a sub fraction.
- the concentration of the anti infective is two to eight times lesser than when such anti infective is used without the bioenhancer.
- the composition includes one or more pharmaceutically acceptable additives and excipients.
- the additives/excipients are selected from the group consisting of nutrients comprising proteins, carbohydrates, sugar, talc, magnesium stearate, cellulose, calcium carbonate, starch-gelatin paste, and/ or pharmaceutically acceptable carriers, diluents and solvents.
- the composition is in oral administration form.
- the ratio of the anti-infective to the bioenhancer is in the range of 1 : 1 to 1:5.
- Figure 2 is a graph showing the effect of rifampicin alone and in combination with piperine in an in vivo mice infection model.
- Figure 3 is a graph showing the effect of rifampicin alone and in combination with 3',5-Dihydroxy flavone 7-O- ⁇ -D-galacturonide-4'-O- ⁇ -D-glucopyranoside of formula 2 in in vivo mice infection model.
- Bioefficacy/bioavailability
- Chemoresistance is a major problem in drag therapy.
- the mechanisms underlying the clinical phenomena of de novo and acquired drag resistance may arise from alterations at any step in the cell-killing pathway. These include drug transport, drag metabolism, drag targets, cellular repair mechanisms and the ability of cells to recognize a harmful toxin or pathogen.
- a common mechanism of reduced cellular drag accumulation is the increased expression of P-glycoprotein, a membrane transporter that efficiently removes drugs from these cells.
- Another limiting factor is the high activity of cytochrome P 450 dependent proteins. Both these proteins P-gp and CYP 450 have been shown to regulate the oral bioavailability of a majority of drags.
- P-gp is considered to be associated with MDR caused by the levels of its expression in tumors and after drug therapy.
- the overall permeability changes may affect (i) specific ion transporter channels, and (ii) also lead to bulk movement of lipophilic solutes along the paracellular pathway.
- Such membrane changes have also been evidenced in the action of several polyene antibiotics (Milhaud J et al, Biochimica et Biophysica Acta, 1988; 943:315-325).
- the changes caused by piperine in membrane fluidity are, as already stated, short living, completely reversible but more than any thing is selective.
- the products of the present invention are novel mechanism based pharmaceutical entities acting through synergism and or additive effect so that drags contained in the formulation are more bioefficaceous as a result of one or more of the mechanism as revealed above and thereby increasing the sensitivity of the target cell to an anti-infective.
- the 'drag' in the present invention refers to a chemical entity capable of affecting organism's patho-physiology and used for the treatment or prevention of disease.
- Drags include a number of classes of compounds, but not limited to aminoglycoside, penicillins, cephalosporins and other ⁇ -lactam agents, macrolides, glycopeptides, fluoroquinolones, tetracyclines, first and second line anti-TB drags, anti-leprosy, antivirals, polyene, triazole, and imidazoles and combinations like pyrimidines, sulphamethoxazole.
- Drags may be a pro- drag, activated or metabolised form, consisting of charged, uncharged, hydrophilic, hydrophobic or zwitter-ion species which make their entry by simple diffusion, carrier mediated transport dependent and not dependent on energy requirements, through ion and/ or voltage gated channels.
- the 'bioenhancer' refers to piperine (formula 1) or other such molecules, characterised fractions and / or extracts as a chemical entity.
- the process of obtaining piperine as more than 98 % pure chemicaly characterized form has been disclosed in LP 172689, IP 172690, IP 176433, US 5439891 and a co-pending US patent application No 60/306917/2001.
- the processes for preparation of a characterised fraction (HPLC profile enclosed) and a pure chemically characterised molecule (Fig.2) from Cuminum cyminum have been disclosed in co-pending patent application No.
- the ratio of those two bioenhancers to drugs may vary from 1 to 50% for the fraction and from 0.1 to 30% for the pure molecule to obtain desired reduction in MIC values anti infectives.
- the ratios of the drag and the bioenhancers and / or in composite bioenhancers are governed by amounts sufficient to produce enhanced therapeutic efficacy as measured by MIC of the formulation being lesser than the drug alone.
- a pharmaceutical carrier is generally an inert bulk agent added to make the ingredients achieve superior admixing and can be solid or liquid.
- the inert parts of standard pharmaceutical compositions used in this process are also part of the present invention. Study design The checkerboard method:
- checkerboard refers to the pattern (of tubes or microtiter plate wells) formed by multiple dilutions of two drags being tested (Eliopoulos GM, Moellering RC. Antimicrobila Combinations. In: Antibiotics in Laboratory Medicine: USA: Williams & Wilkins).
- the checkerboard consisted of columns in which each tube (or well) contains the same amount of the standard drug (antibacterial/antifbngal/anti- TB/antiviral) being diluted along the x-axis and rows in which each tube (or well) contains the same amount of the bioenhancer being diluted on the y-axis (Fig.3).
- each square in the checkerboard (which represents one tube/ well or plate) contained a unique combination of the standard drag and bioenhancer.
- the concentration range of standard drag in the present study was 64 ⁇ g/ml to 0.03 ⁇ g/ml, whereas the bioenhancer was tested in the range of 500 ⁇ g/ml to 0.2 ⁇ g/ml.
- This checkerboard technique can be performed with liquid or semisolid (agar) media.
- the agar (Mueller Hinton agar, Middlebrook 7H10 agar) was autoclaved and allowed to cool to 50°C to 55°C.
- the combination of the standard drag and the bioenhancer was added to the agar.
- Serial two fold dilutions of each of standard drag and the bioenhancer were prepared in appropriate solvents.
- the volume of solvent (containing standard drag or bioenhancer) added to agar was kept small (i.e ⁇ 5% of the total volume).
- Methicillin Resistant Staphylococcus aureus ++++ No inhibition, +++ 20% inhibition, ++ 50% inhibition
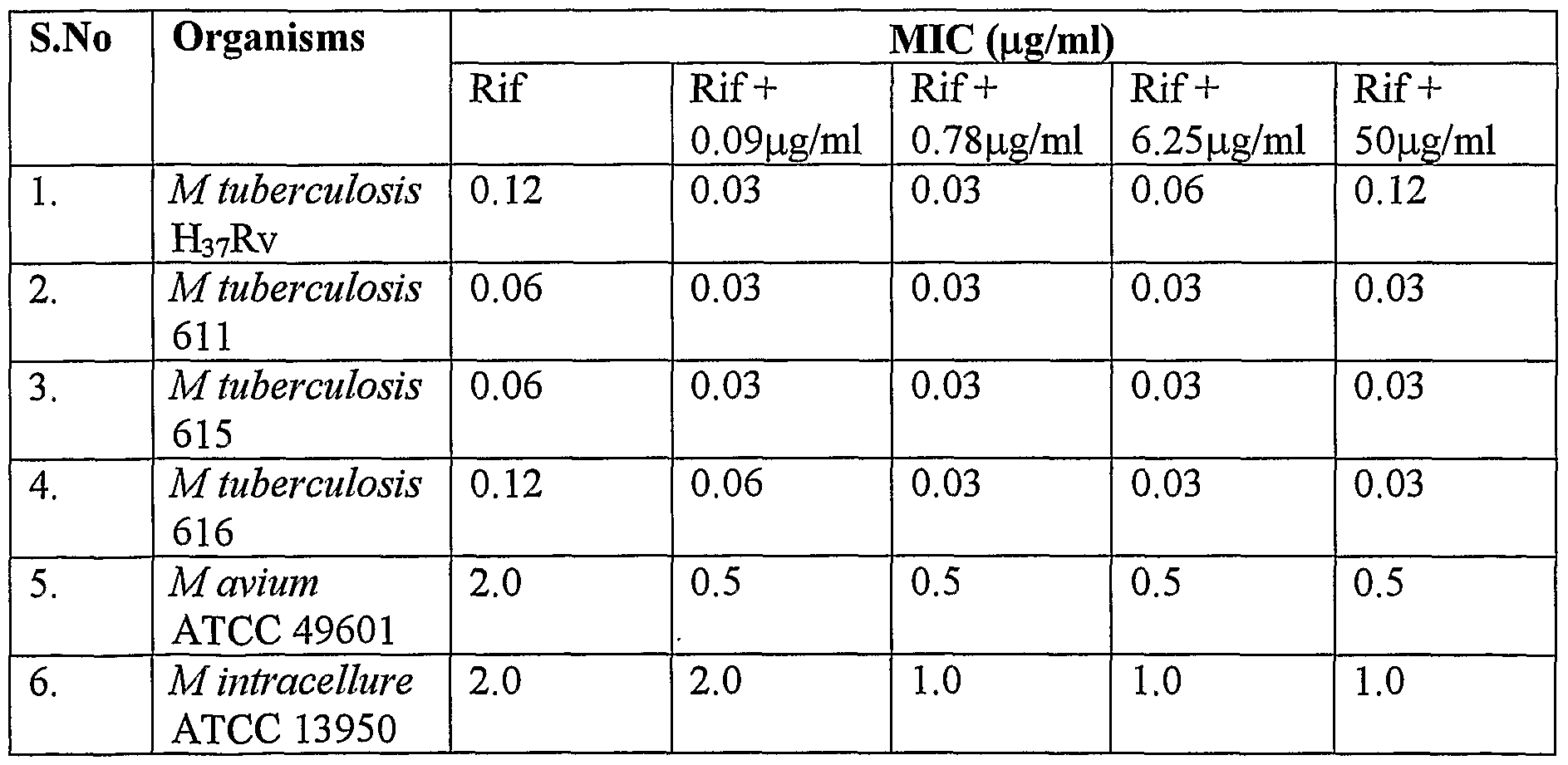
- Example 2 Decrease in the MICs of rifampicin against M.tuberculosis, M. avium and M. intracellure when used in combination with piperine and fraction of Cuminum cyminum.
- MIC Minimum Inhibitory Concentration
- Example 3 Reduction in the dose requirement of rifampicin when used in combination with piperine and fraction of Cuminum cyminum in systemic infection model of mice.
- the study was conducted to see the in vivo response of rifampicin in combination with piperine.
- the Swiss albino mice were infected intravenously with M.tuberculosis H 3 Rv (10 6 CFU/mouse). The infected mice were divided in groups and each group consisted of 6 mice.
- Example 4 Decrease in the MICs of Ciprofloxacin against Staphylococcus aureus, MRSA and Staphylococcus hemolyticus when used in combination with piperine.
- MIC Minimum Inhibitory Concentration
- Example 5 Decrease in the MICs of fluconazole against Candida albicans, Candida parapsilosis and Candida glabrata when used in combination with piperine.
- Minimum Inhibitory Concentration (MIC) of fluconazole alone and in combination with piperine was performed against fungal species, using method described in the study design Two to eight fold reductions in MIC of fluconazole was observed in combination wffli piperine. (Table -6)
- Example 6 List of drugs cited in accompanying Table 7 as some of the examples for the purpose of the present invention.
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP03719082A EP1608370A1 (en) | 2003-03-31 | 2003-03-31 | Use of cuminum cyminum extract and piperine for potention of bioeffiacy of anti infectives |
| PCT/IN2003/000110 WO2004087154A1 (en) | 2003-03-31 | 2003-03-31 | Use of cuminum cyminum extract and piperine for potentiation of bioefficacy of anti infectives |
| AU2003223111A AU2003223111A1 (en) | 2003-03-31 | 2003-03-31 | Use of cuminum cyminum extract and piperine for potentiation of bioefficacy of anti infectives |
| JP2004570079A JP4589126B2 (en) | 2003-03-31 | 2003-03-31 | Use of cumin extract and piperine to influence the biological effectiveness of anti-infectives |
| CNB038264668A CN100425236C (en) | 2003-03-31 | 2003-03-31 | Use of cuminum cyminum extract and piperine for potentiaion of bioeffectacy of anti infectives |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/IN2003/000110 WO2004087154A1 (en) | 2003-03-31 | 2003-03-31 | Use of cuminum cyminum extract and piperine for potentiation of bioefficacy of anti infectives |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2004087154A1 true WO2004087154A1 (en) | 2004-10-14 |
Family
ID=33104959
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IN2003/000110 WO2004087154A1 (en) | 2003-03-31 | 2003-03-31 | Use of cuminum cyminum extract and piperine for potentiation of bioefficacy of anti infectives |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP1608370A1 (en) |
| JP (1) | JP4589126B2 (en) |
| CN (1) | CN100425236C (en) |
| AU (1) | AU2003223111A1 (en) |
| WO (1) | WO2004087154A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008019292A2 (en) * | 2006-08-04 | 2008-02-14 | Trustees Of Boston University | Compositions and methods for potentiating antibiotic activity |
| WO2017220982A1 (en) * | 2016-06-22 | 2017-12-28 | Helperby Therapeutics Limited | Combination comprising piperine and polymyxins for treating microbial infections |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20190111067A1 (en) * | 2014-12-18 | 2019-04-18 | Helperby Therapeutics Limited | Antimicrobial combinations and their use in the treatment of microbial infection |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5439891A (en) | 1993-10-29 | 1995-08-08 | Kapil; Randhir S. | Process for preparation of pharmaceutical composition with enhanced activity for treatment of tuberculosis and leprosy |
| WO1997014319A1 (en) * | 1995-10-20 | 1997-04-24 | Hauser Chemical Research, Inc. | Foods and beverages containing anthocyanins stabilized by plant extracts |
| US5744161A (en) | 1995-02-24 | 1998-04-28 | Sabinsa Corporation | Use of piperine as a bioavailability enhancer |
| WO2004009061A2 (en) * | 2002-07-18 | 2004-01-29 | Council Of Scientific And Industrial Research | Bioavailability/bioefficacy enhaning activity of cuminum cyminum and extracts and fractions thereof |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IN176897B (en) * | 1993-10-29 | 1996-09-28 | Cadila Lab Ltd | |
| RU2002107449A (en) * | 2000-06-26 | 2003-11-20 | Бакулиш Мафатлал ХАМАР (IN) | CHEMOSENSIBILIZER |
-
2003
- 2003-03-31 AU AU2003223111A patent/AU2003223111A1/en not_active Abandoned
- 2003-03-31 WO PCT/IN2003/000110 patent/WO2004087154A1/en active Application Filing
- 2003-03-31 JP JP2004570079A patent/JP4589126B2/en not_active Expired - Fee Related
- 2003-03-31 CN CNB038264668A patent/CN100425236C/en not_active Expired - Lifetime
- 2003-03-31 EP EP03719082A patent/EP1608370A1/en not_active Withdrawn
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5439891A (en) | 1993-10-29 | 1995-08-08 | Kapil; Randhir S. | Process for preparation of pharmaceutical composition with enhanced activity for treatment of tuberculosis and leprosy |
| US5744161A (en) | 1995-02-24 | 1998-04-28 | Sabinsa Corporation | Use of piperine as a bioavailability enhancer |
| WO1997014319A1 (en) * | 1995-10-20 | 1997-04-24 | Hauser Chemical Research, Inc. | Foods and beverages containing anthocyanins stabilized by plant extracts |
| WO2004009061A2 (en) * | 2002-07-18 | 2004-01-29 | Council Of Scientific And Industrial Research | Bioavailability/bioefficacy enhaning activity of cuminum cyminum and extracts and fractions thereof |
Non-Patent Citations (3)
| Title |
|---|
| BIOCHIMICA ET BIOPHYSICA ACTA, vol. 943, 1988, pages 315 - 325 |
| DATABASE BIOSIS [online] BIOSCIENCES INFORMATION SERVICE, PHILADELPHIA, PA, US; 2002, HIWALE ET AL.: "Effect of co-administration of piperine on pharmacokinetics of eta-lactam antibiotics in rats", XP002275578, Database accession no. PREV200200231303 * |
| INDIAN JOURNAL OF EXPERIMENTAL BIOLOGY, vol. 40, no. 3, 2002, pages 277 - 281 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008019292A2 (en) * | 2006-08-04 | 2008-02-14 | Trustees Of Boston University | Compositions and methods for potentiating antibiotic activity |
| WO2008019292A3 (en) * | 2006-08-04 | 2008-08-14 | Univ Boston | Compositions and methods for potentiating antibiotic activity |
| WO2017220982A1 (en) * | 2016-06-22 | 2017-12-28 | Helperby Therapeutics Limited | Combination comprising piperine and polymyxins for treating microbial infections |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2006514972A (en) | 2006-05-18 |
| EP1608370A1 (en) | 2005-12-28 |
| CN1798560A (en) | 2006-07-05 |
| AU2003223111A1 (en) | 2004-10-25 |
| JP4589126B2 (en) | 2010-12-01 |
| CN100425236C (en) | 2008-10-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Zacchino et al. | Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs | |
| AU2009234283B2 (en) | Diterpene glycosides as natural solubilizers | |
| Mishra et al. | Natural products: an evolving role in future drug discovery | |
| JP2011517686A5 (en) | ||
| JP2009263371A (en) | Anti-cancer combination | |
| CN103391774B (en) | Comprise the pharmaceutical composition of trans-cinnamaldehyde and the purposes in treatment is infected thereof | |
| US10864188B2 (en) | Anti-microbial composition | |
| US20180207216A1 (en) | Compositions and Methods for Treating Multi-Drug Resistant Malaria | |
| US20110159124A1 (en) | Compositions and methods useful for treatment of respiratory illness | |
| US7119075B2 (en) | Use of herbal agents for potentiation of bioefficacy of anti infectives | |
| Zhai et al. | Success stories of natural product-derived compounds from plants as multidrug resistance modulators in microorganisms | |
| EP1608370A1 (en) | Use of cuminum cyminum extract and piperine for potention of bioeffiacy of anti infectives | |
| Zuo et al. | Potentiation effects by usnic acid in combination with antibiotics on clinical multi-drug resistant isolates of methicillin-resistant Staphylococcus aureus (MRSA) | |
| CN107812011B (en) | Antifungal pharmaceutical composition | |
| US20210060049A1 (en) | Phytochemical-antibiotic combination for the treatment of a bacterial infection | |
| CN111991390B (en) | Amphotericin B synergist and application thereof | |
| US20050214390A1 (en) | Bioavailability enhancing activity of Carum carvi extracts and fractions thereof | |
| CN110801455A (en) | Pharmaceutical composition for treating MRSA and application thereof | |
| AU2017235711B2 (en) | Enhanced tulathromycin | |
| EP3294317B1 (en) | Enhanced antibiotic composition | |
| JPH01180825A (en) | Agent for infectious disease | |
| Ayogu et al. | Effect of Garcinia Kola (Heckel) on Pharmacokinetic Parameters of Rifampicin | |
| CN116687929A (en) | Application of sanguinarine in preparing medicament for preventing and treating clinical rare fungus related infectious diseases | |
| CN116236496A (en) | Method for resisting candida glabrata by combining ambroxol and amphotericin B | |
| EP1370263B1 (en) | Antibiotic pharmaceutical composition with lysergol as bio-enhancer and method of treatment |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2975/DELNP/2004 Country of ref document: IN |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ OM PH PL PT RO RU SD SE SG SK SL TJ TM TN TR TT TZ UA UG UZ VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT RO SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2004570079 Country of ref document: JP |
|
| REEP | Request for entry into the european phase |
Ref document number: 2003719082 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2003719082 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 20038264668 Country of ref document: CN |
|
| WWP | Wipo information: published in national office |
Ref document number: 2003719082 Country of ref document: EP |