WO2003064393A1 - Thio-carbostyril derivative, its n-oxides and the n-oxides of aripiprazole - Google Patents
Thio-carbostyril derivative, its n-oxides and the n-oxides of aripiprazole Download PDFInfo
- Publication number
- WO2003064393A1 WO2003064393A1 PCT/SE2003/000164 SE0300164W WO03064393A1 WO 2003064393 A1 WO2003064393 A1 WO 2003064393A1 SE 0300164 W SE0300164 W SE 0300164W WO 03064393 A1 WO03064393 A1 WO 03064393A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- oxides
- aripiprazole
- thio
- carbostyril derivative
- pharmaceutical composition
- Prior art date
Links
- CEUORZQYGODEFX-UHFFFAOYSA-N Aripirazole Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NC(=O)CCC4=CC=3)CC2)=C1Cl CEUORZQYGODEFX-UHFFFAOYSA-N 0.000 title claims abstract description 66
- 229960004372 aripiprazole Drugs 0.000 title claims abstract description 65
- KXZSVYHFYHTNBI-UHFFFAOYSA-N 1h-quinoline-2-thione Chemical class C1=CC=CC2=NC(S)=CC=C21 KXZSVYHFYHTNBI-UHFFFAOYSA-N 0.000 title claims abstract description 37
- 150000001204 N-oxides Chemical class 0.000 claims abstract description 51
- XSCIEQZAASEUST-UHFFFAOYSA-N 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1h-quinoline-2-thione Chemical compound ClC1=CC=CC(N2CCN(CCCCOC=3C=C4NC(=S)CCC4=CC=3)CC2)=C1Cl XSCIEQZAASEUST-UHFFFAOYSA-N 0.000 claims abstract description 29
- 150000003839 salts Chemical class 0.000 claims abstract description 24
- 201000000980 schizophrenia Diseases 0.000 claims abstract description 19
- 208000012661 Dyskinesia Diseases 0.000 claims abstract description 6
- 208000023105 Huntington disease Diseases 0.000 claims abstract description 4
- 208000018737 Parkinson disease Diseases 0.000 claims abstract description 4
- 239000008194 pharmaceutical composition Substances 0.000 claims description 31
- 238000000034 method Methods 0.000 claims description 20
- 238000002360 preparation method Methods 0.000 claims description 16
- 230000008569 process Effects 0.000 claims description 13
- NHQDETIJWKXCTC-UHFFFAOYSA-N 3-chloroperbenzoic acid Chemical compound OOC(=O)C1=CC=CC(Cl)=C1 NHQDETIJWKXCTC-UHFFFAOYSA-N 0.000 claims description 8
- 102000015554 Dopamine receptor Human genes 0.000 claims description 8
- 108050004812 Dopamine receptor Proteins 0.000 claims description 8
- 239000000969 carrier Substances 0.000 claims description 8
- 239000000126 substance Substances 0.000 claims description 8
- 210000003169 central nervous system Anatomy 0.000 claims description 7
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 7
- 239000004480 active ingredient Substances 0.000 claims description 6
- 239000003085 diluting agent Substances 0.000 claims description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 5
- CFHGBZLNZZVTAY-UHFFFAOYSA-N lawesson's reagent Chemical compound C1=CC(OC)=CC=C1P1(=S)SP(=S)(C=2C=CC(OC)=CC=2)S1 CFHGBZLNZZVTAY-UHFFFAOYSA-N 0.000 claims description 5
- -1 2, 3-dichloro- phenyl Chemical group 0.000 claims description 4
- 239000002253 acid Substances 0.000 claims description 4
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Natural products C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 4
- 206010013654 Drug abuse Diseases 0.000 claims description 3
- 208000010228 Erectile Dysfunction Diseases 0.000 claims description 3
- 241000124008 Mammalia Species 0.000 claims description 3
- 201000001881 impotence Diseases 0.000 claims description 3
- 208000020016 psychiatric disease Diseases 0.000 claims description 3
- BHEPBYXIRTUNPN-UHFFFAOYSA-N hydridophosphorus(.) (triplet) Chemical compound [PH] BHEPBYXIRTUNPN-UHFFFAOYSA-N 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 150000007522 mineralic acids Chemical class 0.000 claims description 2
- 150000007524 organic acids Chemical class 0.000 claims description 2
- 235000005985 organic acids Nutrition 0.000 claims description 2
- 208000011117 substance-related disease Diseases 0.000 claims description 2
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 39
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 24
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 24
- 241000700159 Rattus Species 0.000 description 20
- 235000002639 sodium chloride Nutrition 0.000 description 20
- 239000000243 solution Substances 0.000 description 19
- 229940079593 drug Drugs 0.000 description 18
- 239000003814 drug Substances 0.000 description 18
- 230000000694 effects Effects 0.000 description 16
- 239000007924 injection Substances 0.000 description 14
- 238000002347 injection Methods 0.000 description 14
- 210000004556 brain Anatomy 0.000 description 13
- 229940073584 methylene chloride Drugs 0.000 description 13
- 239000000047 product Substances 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 10
- 235000019441 ethanol Nutrition 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 239000002904 solvent Substances 0.000 description 9
- 238000004885 tandem mass spectrometry Methods 0.000 description 9
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 8
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 8
- 229920002472 Starch Polymers 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008107 starch Substances 0.000 description 8
- 235000019698 starch Nutrition 0.000 description 8
- 239000003826 tablet Substances 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 7
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 7
- 239000008280 blood Substances 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 230000005062 synaptic transmission Effects 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 208000009132 Catalepsy Diseases 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 206010047853 Waxy flexibility Diseases 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 239000013078 crystal Substances 0.000 description 6
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 6
- 238000004128 high performance liquid chromatography Methods 0.000 description 6
- 230000014759 maintenance of location Effects 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- LISFMEBWQUVKPJ-UHFFFAOYSA-N quinolin-2-ol Chemical class C1=CC=C2NC(=O)C=CC2=C1 LISFMEBWQUVKPJ-UHFFFAOYSA-N 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 208000024891 symptom Diseases 0.000 description 6
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 5
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 5
- 125000001309 chloro group Chemical group Cl* 0.000 description 5
- 238000000502 dialysis Methods 0.000 description 5
- 239000008101 lactose Substances 0.000 description 5
- 238000001690 micro-dialysis Methods 0.000 description 5
- 230000000144 pharmacologic effect Effects 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- CFFZDZCDUFSOFZ-UHFFFAOYSA-N 3,4-Dihydroxy-phenylacetic acid Chemical compound OC(=O)CC1=CC=C(O)C(O)=C1 CFFZDZCDUFSOFZ-UHFFFAOYSA-N 0.000 description 4
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 4
- 229960004046 apomorphine Drugs 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000003480 eluent Substances 0.000 description 4
- 235000019439 ethyl acetate Nutrition 0.000 description 4
- 239000008103 glucose Substances 0.000 description 4
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 4
- 210000001577 neostriatum Anatomy 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 238000007493 shaping process Methods 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 3
- 239000005995 Aluminium silicate Substances 0.000 description 3
- 235000013162 Cocos nucifera Nutrition 0.000 description 3
- 244000060011 Cocos nucifera Species 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- 229920001214 Polysorbate 60 Polymers 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 229960000583 acetic acid Drugs 0.000 description 3
- 235000012211 aluminium silicate Nutrition 0.000 description 3
- 229910021529 ammonia Inorganic materials 0.000 description 3
- VMWNQDUVQKEIOC-CYBMUJFWSA-N apomorphine Chemical compound C([C@H]1N(C)CC2)C3=CC=C(O)C(O)=C3C3=C1C2=CC=C3 VMWNQDUVQKEIOC-CYBMUJFWSA-N 0.000 description 3
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 230000037396 body weight Effects 0.000 description 3
- 235000014121 butter Nutrition 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 239000012153 distilled water Substances 0.000 description 3
- 229960003638 dopamine Drugs 0.000 description 3
- 230000003291 dopaminomimetic effect Effects 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 238000010992 reflux Methods 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 239000007858 starting material Substances 0.000 description 3
- 239000000829 suppository Substances 0.000 description 3
- 229910021642 ultra pure water Inorganic materials 0.000 description 3
- 239000012498 ultrapure water Substances 0.000 description 3
- 238000005303 weighing Methods 0.000 description 3
- DUUGKQCEGZLZNO-UHFFFAOYSA-N 5-hydroxyindoleacetic acid Chemical compound C1=C(O)C=C2C(CC(=O)O)=CNC2=C1 DUUGKQCEGZLZNO-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- 206010001540 Akathisia Diseases 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 208000014094 Dystonic disease Diseases 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical group C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 208000001431 Psychomotor Agitation Diseases 0.000 description 2
- 239000012891 Ringer solution Substances 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 239000000556 agonist Substances 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000003693 atypical antipsychotic agent Substances 0.000 description 2
- 150000007514 bases Chemical class 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 230000003542 behavioural effect Effects 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 239000004621 biodegradable polymer Substances 0.000 description 2
- 229910000019 calcium carbonate Inorganic materials 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 208000010118 dystonia Diseases 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 235000011187 glycerol Nutrition 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 230000003370 grooming effect Effects 0.000 description 2
- 229960003878 haloperidol Drugs 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 239000007942 layered tablet Substances 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 2
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- 229910000069 nitrogen hydride Inorganic materials 0.000 description 2
- 239000012044 organic layer Substances 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 2
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 2
- 235000012239 silicon dioxide Nutrition 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- 229940001584 sodium metabisulfite Drugs 0.000 description 2
- 235000010262 sodium metabisulphite Nutrition 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- BGRJTUBHPOOWDU-NSHDSACASA-N (S)-(-)-sulpiride Chemical compound CCN1CCC[C@H]1CNC(=O)C1=CC(S(N)(=O)=O)=CC=C1OC BGRJTUBHPOOWDU-NSHDSACASA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- LDVVTQMJQSCDMK-UHFFFAOYSA-N 1,3-dihydroxypropan-2-yl formate Chemical compound OCC(CO)OC=O LDVVTQMJQSCDMK-UHFFFAOYSA-N 0.000 description 1
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 1
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- 208000007848 Alcoholism Diseases 0.000 description 1
- 206010002942 Apathy Diseases 0.000 description 1
- 241000490494 Arabis Species 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 208000022497 Cocaine-Related disease Diseases 0.000 description 1
- 206010012239 Delusion Diseases 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 101100476962 Drosophila melanogaster Sirup gene Proteins 0.000 description 1
- 241001269524 Dura Species 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-N Formic acid Chemical compound OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 1
- DCXXMTOCNZCJGO-UHFFFAOYSA-N Glycerol trioctadecanoate Natural products CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 description 1
- 208000004547 Hallucinations Diseases 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- WTDRDQBEARUVNC-LURJTMIESA-N L-DOPA Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-LURJTMIESA-N 0.000 description 1
- WTDRDQBEARUVNC-UHFFFAOYSA-N L-Dopa Natural products OC(=O)C(N)CC1=CC=C(O)C(O)=C1 WTDRDQBEARUVNC-UHFFFAOYSA-N 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 101710138657 Neurotoxin Proteins 0.000 description 1
- ZPLJXLYKWWBBQC-UHFFFAOYSA-N O[N]1(CCN(CCCCOc2ccc(CCC(N3)S)c3c2)CC1)c(cccc1Cl)c1Cl Chemical compound O[N]1(CCN(CCCCOc2ccc(CCC(N3)S)c3c2)CC1)c(cccc1Cl)c1Cl ZPLJXLYKWWBBQC-UHFFFAOYSA-N 0.000 description 1
- 229930040373 Paraformaldehyde Natural products 0.000 description 1
- 208000027089 Parkinsonian disease Diseases 0.000 description 1
- 206010034010 Parkinsonism Diseases 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 208000028017 Psychotic disease Diseases 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 229910021607 Silver chloride Inorganic materials 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 206010001584 alcohol abuse Diseases 0.000 description 1
- 208000025746 alcohol use disease Diseases 0.000 description 1
- GZCGUPFRVQAUEE-SLPGGIOYSA-N aldehydo-D-glucose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O GZCGUPFRVQAUEE-SLPGGIOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 239000000538 analytical sample Substances 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000003354 anti-apomorphinic effect Effects 0.000 description 1
- 230000000561 anti-psychotic effect Effects 0.000 description 1
- 239000000305 astragalus gummifer gum Substances 0.000 description 1
- 229940127236 atypical antipsychotics Drugs 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000004067 bulking agent Substances 0.000 description 1
- 244000309464 bull Species 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 238000011088 calibration curve Methods 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 208000015114 central nervous system disease Diseases 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- ZPEIMTDSQAKGNT-UHFFFAOYSA-N chlorpromazine Chemical compound C1=C(Cl)C=C2N(CCCN(C)C)C3=CC=CC=C3SC2=C1 ZPEIMTDSQAKGNT-UHFFFAOYSA-N 0.000 description 1
- 229960001076 chlorpromazine Drugs 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 201000001272 cocaine abuse Diseases 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 231100000868 delusion Toxicity 0.000 description 1
- 239000003479 dental cement Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229910001873 dinitrogen Inorganic materials 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000000835 electrochemical detection Methods 0.000 description 1
- 230000002996 emotional effect Effects 0.000 description 1
- 239000002702 enteric coating Substances 0.000 description 1
- 238000009505 enteric coating Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000000763 evoking effect Effects 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000007941 film coated tablet Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 235000011194 food seasoning agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 1
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 1
- 108010025899 gelatin film Proteins 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 125000005456 glyceride group Chemical group 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 208000031424 hyperprolactinemia Diseases 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 210000004283 incisor Anatomy 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229940057952 methanol Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- KWKAKUADMBZCLK-UHFFFAOYSA-N methyl heptene Natural products CCCCCCC=C KWKAKUADMBZCLK-UHFFFAOYSA-N 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N n-Octanol Natural products CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 239000002581 neurotoxin Substances 0.000 description 1
- 231100000618 neurotoxin Toxicity 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- WLGDAKIJYPIYLR-UHFFFAOYSA-N octane-1-sulfonic acid Chemical compound CCCCCCCCS(O)(=O)=O WLGDAKIJYPIYLR-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- DIVDFFZHCJEHGG-UHFFFAOYSA-N oxidopamine Chemical compound NCCC1=CC(O)=C(O)C=C1O DIVDFFZHCJEHGG-UHFFFAOYSA-N 0.000 description 1
- 239000004031 partial agonist Substances 0.000 description 1
- 210000003899 penis Anatomy 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 125000004193 piperazinyl group Chemical group 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 238000010149 post-hoc-test Methods 0.000 description 1
- 230000001242 postsynaptic effect Effects 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 235000010413 sodium alginate Nutrition 0.000 description 1
- 239000000661 sodium alginate Substances 0.000 description 1
- 229940005550 sodium alginate Drugs 0.000 description 1
- ODZPKZBBUMBTMG-UHFFFAOYSA-N sodium amide Chemical compound [NH2-].[Na+] ODZPKZBBUMBTMG-UHFFFAOYSA-N 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000008259 solid foam Substances 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000007940 sugar coated tablet Substances 0.000 description 1
- 229960004940 sulpiride Drugs 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000028838 turning behavior Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
- XOOUIPVCVHRTMJ-UHFFFAOYSA-L zinc stearate Chemical class [Zn+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O XOOUIPVCVHRTMJ-UHFFFAOYSA-L 0.000 description 1
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/20—Oxygen atoms
- C07D215/22—Oxygen atoms attached in position 2 or 4
- C07D215/227—Oxygen atoms attached in position 2 or 4 only one oxygen atom which is attached in position 2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/18—Antipsychotics, i.e. neuroleptics; Drugs for mania or schizophrenia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/30—Drugs for disorders of the nervous system for treating abuse or dependence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/16—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D215/36—Sulfur atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07F—ACYCLIC, CARBOCYCLIC OR HETEROCYCLIC COMPOUNDS CONTAINING ELEMENTS OTHER THAN CARBON, HYDROGEN, HALOGEN, OXYGEN, NITROGEN, SULFUR, SELENIUM OR TELLURIUM
- C07F9/00—Compounds containing elements of Groups 5 or 15 of the Periodic Table
- C07F9/02—Phosphorus compounds
- C07F9/547—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom
- C07F9/6564—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having phosphorus atoms, with or without nitrogen, oxygen, sulfur, selenium or tellurium atoms, as ring hetero atoms
- C07F9/6581—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having phosphorus atoms, with or without nitrogen, oxygen, sulfur, selenium or tellurium atoms, as ring hetero atoms having phosphorus and nitrogen atoms with or without oxygen or sulfur atoms, as ring hetero atoms
- C07F9/6584—Heterocyclic compounds, e.g. containing phosphorus as a ring hetero atom having phosphorus atoms, with or without nitrogen, oxygen, sulfur, selenium or tellurium atoms, as ring hetero atoms having phosphorus and nitrogen atoms with or without oxygen or sulfur atoms, as ring hetero atoms having one phosphorus atom as ring hetero atom
- C07F9/65842—Cyclic amide derivatives of acids of phosphorus, in which one nitrogen atom belongs to the ring
Definitions
- the present invention relates to a novel thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole. More particularly, the invention relates to a novel thio- carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole and salts thereof, processes for preparing said thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole and salts thereof, as well as pharmaceutical compositions for treating e.g. schizophrenia containing, as the active ingredient, said thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole or salts thereof.
- Schizophrenia is the most common type of psychosis caused by an excessive neurotransmission activity of the dopaminergic nervous system in the central nervous system. [Cf. "Hypothesis of Excessive Dopamine” by Michio Tohru : TAISHA (Metabo- lism) , Vol. 22, pp. 49, (1985); and Pharmacia Review, No. 10, "KOKORO-TO-KUSURI (Mind and Drugs) " edited by Pharmaceutical Society of Japan] .
- a number of drugs having the ability to block the neuro- transmission of dopamine receptors in the central nervous system, have been developed.
- examples of said drugs are phe- nothiazine-type compounds such as Chlorpromazine; butyrophe- none-type compounds such as Haloperidol ; and benzamide-type compounds such as Sulpiride.
- phe- nothiazine-type compounds such as Chlorpromazine
- butyrophe- none-type compounds such as Haloperidol
- benzamide-type compounds such as Sulpiride.
- Drugs known in the prior art for treating schizophrenia induce a number of side-effects.
- side-effects are those induced by phenothiazine-type drugs, i.e. the or- thostatic hypotension and hypersedation on the basis of strong alpha-blocking activity; and in the case of drugs hav- ing strong activity for blocking neurotransmission of dopa- minergic receptor, the side-effects are so-called extrapyra- midal side-effects such as catalepsy, akathisia, dystonia and the like caused by blocking the neurotransmission of dopamine receptors in the striatum.
- carbostyril derivatives for therapeutical use has also been disclosed.
- carbostyril derivatives known in prior art are those disclosed in: EP-A-0367141 , Publication date: 1990-05-09, Applicant (s) : OTSUKA PHARMA CO LTD (JP) , Priority Number (s) : JP19880276953 19881031.
- JP Applicant
- Priority Number s
- JP19880276953 JP19880276953 19881031.
- the "3 rd generation anti-psychotic" Aripiprazole emanates from that patent application.
- carbostyril derivatives disclosed in US-A- 4,234,585 and EP-A-226,441 have chemical structural formula similar to that of Aripiprazole.
- carbostyril derivatives disclosed in US-A-4 , 234 , 584 have chemical structural formula similar to that of Aripiprazole and also have pharmacological activities similar to those shown by Aripiprazole.
- Carbostyril derivatives are also disclosed in Australian Patent No. 50252/85, Japanese Patent Kokai (Laid-open) Nos. 58- 43952 (1983), 56-49359 (1981), 56-49360 (1981) and 56-49361 (1981) .
- a further object of the present invention is to provide proc- esses for preparing said thio-carbostyril derivative, its N- oxides and the N-oxides of Aripiprazole and salts thereof.
- a still further object of the present invention is to provide a pharmaceutical composition for treating schizophrenia, Huntington's disease and dyskinesias in Parkinson's disease.
- a still further object of the present invention is to provide a pharmaceutical composition for treating erectile dysfunction.
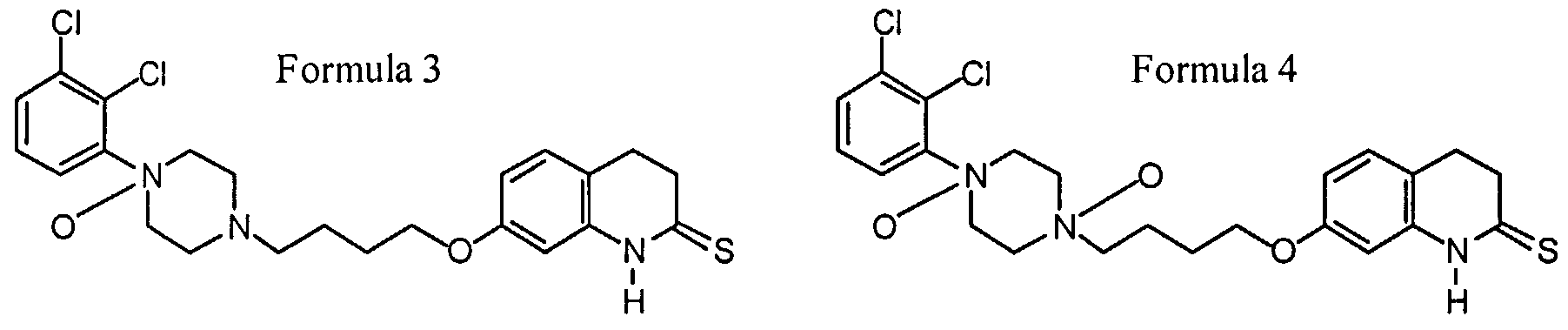
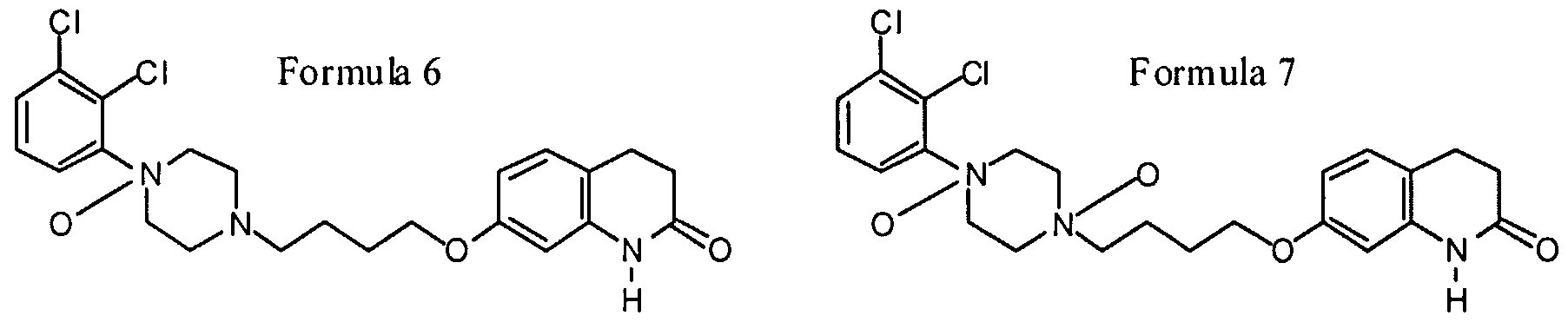
- a novel thio-carbostyril derivative which were surprisingly found to exhibit strong activity for influencing neurotransmission of dopamine receptors and being devoid of the side-effects induced by known drugs for treating schizophrenia, said derivative being 7- ⁇ 4- [4- (2 , 3-dichloro-phenyl) -piperazin-1-yl] - butoxy ⁇ -3 , 4-dihydro-lH-quinoline-2-thione, its N-oxides and the N-oxides of Aripiprazole, having the Formulas 1-7:
- Formula 1 thio-ari Formula 2 thio-ari n-ox (basic N) Formula 3 thio-ari n-ox (anilinic N) Formula 4 thio-ari di-n-ox Formula 5 ari n-ox (basic N) Formula 6 ari n-ox (anilinic N) Formula 7 ari di-n-ox CHEMICAL STRUCTURES OF ARIPIPRAZOLE AND THE NEW COMPOUNDS OF THE PRESENT INVENTION
- the present invention also provides a pharmaceutical composition
- a pharmaceutical composition comprising said thio-carbostyril derivative, its N- oxides and the N-oxides of Aripiprazole or a physiologically acceptable salt thereof as an active ingredient optionally together with at least one member selected from the group consisting of pharmaceutically acceptable carriers, diluents and excipients.
- the present invention further provides processes for the preparation of the thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole of the present invention and salts thereof.
- the present invention provides the use of the thio- carbo-styril derivative, its N-oxides and the N-oxides of Aripiprazole of the present invention for the preparation of a pharmaceutical formulation for the treatment of central nervous system (CNS) disorders in mammals including man.
- CNS central nervous system
- the thio-carbostyril derivative, its N-oxides and the N- oxides of Aripiprazole of Formulas 1-7 display a favorable atypical anti-psychotic pharmacological profile.
- the thio- carbostyril derivative of Formula 1 surprisingly displays a receptor binding selectivity profile, which is more favorable than that of Aripiprazole.
- the thio-carbostyril derivative of Formula 1 (thio-Aripiprazole) was, surprisingly, shown to form Aripiprazole in vivo after the administration of 100 ⁇ mol/kg i.p. In the brain sample there was more Aripiprazole than thio-Aripi- prazole. This was also true, at lower absolute concentrations, in the blood sample. This means that thio-Aripiprazole has both pharmacological effects in its own and it also forms Aripiprazole via bio-activation. Thus, thio-Aripiprazole works both as a drug and as a pro-drug.
- the thio-carbostyril derivative, its N-oxides and the N- oxides of Aripiprazole represented by the Formulas 1-7 of the present invention can easily be converted into their acid- addition salts by reacting them with a pharmaceutically acceptable acid.
- examples of such acids include inorganic acids such as hydrochloric acid, sulfuric acid, phosphoric acid, hydrobromic acid and the like; organic acids such as oxalic acid, maleic acid, fumaric acid, malic acid, tartaric acid, citric acid, benzoic acid and the like.
- a thio-carbostyril derivative, represented by the Formula 1 of the present invention, which has an acidic -NH- thio-amide group, can eas- ily be converted into its salts by reacting with basic compounds.
- basic compounds include sodium-amide and LDA.
- the present invention provides a pharmaceutical composition
- a pharmaceutical composition comprising said thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole or a physiologically acceptable salt thereof as an active ingredient optionally together with at least one member selected from the group consisting of carriers, diluents and excipi- ents.
- the thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole represented by the Formulas 1-7 can be used in the form of usual pharmaceutical compositions, which are prepared by using at least one member selected from the group consisting of carriers, diluents and excipients such as fillers, bulking agents, binders, wetting agents, disintegrating agents, surface active agents, lubricants and the like.
- compositions various types of administration unit forms can be selected depending on the therapeutic purposes, and the examples of pharmaceutical compositions are tablets, pills, powders, liquids, suspensions, emulsions, granules, capsules, suppositories, injection preparations (solutions and suspensions) , bio-degradable polymers and the like.
- any excipients which are known and used widely in this field can also be used, for example carriers such as lactose, white sugar, sodium chloride, glucose, urea, starch, calcium carbonate, kaolin, crystalline cellulose, silicic acid and the like; binders such as water, ethanol, propanol , simple sirup, glucose solutions, starch solutions, gelatin solutions, carboxymethyl cellulose, she- lac, methyl cellulose, potassium phosphate, polyvinylpyrroli- done and the like; disintegrating agents such as dried starch, sodium alginate, agar powder, laminalia powder, sodium hydrogen carbonate, calcium carbonate, fatty acid esters of polyoxyethylene sorbitan, sodium laurylsulfate, monoglyc- eride of stearic acid, starch, lactose and the like; disintegration inhibitors such as white sugar, stearin, coconut butter, hydrogenated oils; absorption accelerators such as
- any excipients which are known and widely used in this field can also be used, for example, carriers such as lactose, starch, coconut butter, hardened vegetable oils, kaolin, talc and the like; binders such as gum arabi powder, tragacanth gum powder, gelatin, ethanol and the like; disintegrating agents such as agar, laminalia and the like.
- carriers such as lactose, starch, coconut butter, hardened vegetable oils, kaolin, talc and the like
- binders such as gum arabi powder, tragacanth gum powder, gelatin, ethanol and the like
- disintegrating agents such as agar, laminalia and the like.
- any excipients which are known and widely used in this field can also be used, for example polyethylene glycols, coconut butter, higher alcohols, esters of higher alcohols, gelatin, semi-synthesized glycerides and the like.
- solutions and suspensions are sterilized and are preferably made isotonic to blood.
- any carriers which are usually used in this field can also be used, for example, water, ethyl alcohol, propylene glycol, ethoxylated isostearyl alcohol, polyoxylated isostearyl alcohol, fatty acid esters of polyoxyethylene sorbitan.
- adequate amounts of sodium chloride, glucose or glycerin can be added to the desired injection preparations to make them isotonic.
- usual dissolving agents, buffer agents, analgesic agents may be added.
- coloring agents, preservatives, perfumes, seasoning agents, sweetening agents and other medicines may also be added to the desired preparations during the treatment of schizophrenia.
- the amount of the thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole of Formulas 1-7 or salt thereof to be contained in a pharmaceutical composition for treating schizophrenia according to the present invention is not specifically restricted and can suitably be selected from a wide range, usually it is contained 1 to 70%, preferably 1 to 30% by weight of the whole composition.
- Administration methods of a pharmaceutical composition for treating schizophrenia of the present invention are not specifically restricted, and the thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole of the present invention can be administered in various forms of preparations depending on the age of the patient, distinction of sex, other conditions, as well as conditions of the symptoms.
- tablets, pills, solutions, suspensions, emulsions, granules and capsules are orally administered; and injection preparations are administered singly or mixed with injection transfusions such as glucose solutions and amino acid solutions intravenously; and if necessary, the injection preparations are administered singly intramuscularly, in- tracutaneously, subcutaneously or intraperitoneally.
- Suppositories are administered into the rectum.
- Bio-degradable polymers are implanted under the skin or used orally.
- the dosage of a pharmaceutical composition for treating schizophrenia according to the present invention is suitably selected according to the method of use, the age of the patient, distinction of sex, other conditions, as well as con- ditions of the symptoms, usually about 0.1 to 10 mg/kg of the body weight/day of the thio-carbostyril derivative, its N- oxides and the N-oxides of Aripiprazole of Formulas 1-7 as the active ingredient may be administered. Usually, 1 to 200 mg of the active ingredient may be contained in an admini- stration unit form.
- a process for the preparation of the thio-carbostyril derivative of the present invention which process comprises reacting aripiprazole with Lawesson's reagent (2,4-bis(4- methoxyphenyl) -1,3,2, 4-dithiadiphosphetane 2, 4-di-sulfide) or phosphorous pentasulphide at appropriate temperatures.
- a process for the preparation of N-oxides of the thio-carbostyril deriva- tive of Formula 1 or of aripiprazole which process comprises reacting the thio-carbostyril derivative of Formula 1 and aripiprazole, respectively, with m-chloroperbenzoic acid.
- the use of the thio-carbostyril derivative, its N-oxides and the N-oxides of Aripiprazole of the present invention for the manufacture of a pharmaceutical composition for the treatment of central nervous system CNS disorders in mammals, including man.
- said pharmaceutical composition is suited for treating dopa- mine receptor related central nervous neuro-psychiatric diseases and/or for treating circulatory disorders.
- dopamine receptor related central nervous neuro-psychiatric diseases and circulatory disorders are schizophrenia; dyskinesias by Parkinson's disease, e.g. dy- skinesias caused by long-term L-dopa; and Huntington's disease .
- said pharmaceutical composition is suited for treating drug abuse, in particular alcohol and/or cocaine abuse.
- said pharmaceutical composition is suited for the treatment of erectile dysfunction.
- a process for the preparation of a pharmaceutical composition characterized in that the compounds of Formulas 1 according to the invention or a physiologically acceptable salt thereof is incorporated in one or more inert carriers and/or diluents by a non-chemical method.
- Example 1 Aripiprazole (OPC-14597, CAS RN 129722-12-9; 1.00 g, 2.23 mmol) was dissolved in toluene (25 mL) . To this solution was added Lawesson's reagent (1.08 g, 2.68 mmol) and the reaction mixture was refluxed for one hour. TLC was best run on silica pretreated with ammonia (NH 3 (g) ) and with EtOAc as eluent. The solvent was evaporated and the orange residue was dissolved in methylene chloride (50 mL) and washed with 10 % Na 2 C0 3 (2 x 15 mL) .
- Aripiprazole (Mw 447, 25 mg, 56 ⁇ mol) was dissolved in about 1 mL methylene chloride. To this solution was added (dropwise at room temperature) m- chloroperbenzoic acid (MCPBA, Mw 173, 19 mg, 112 ⁇ mol).
- MCPBA m- chloroperbenzoic acid
- a TLC alumina eluting with methylene chloride/methanol 20/1
- Aripiprazole (Mw 447, 100 mg, 224 ⁇ mol) was dissolved in about 5 mL methylene chloride. To this solution was added (at room temperature) m-chloroperbenzoic acid (MCPBA, Mw 173, 200 mg, 1160 ⁇ mol).
- MCPBA m-chloroperbenzoic acid
- a TLC alumina eluting with methyl- ene chloride/methanol 20/1
- the same eluent was used when chromatographing in a Pasteur pipette (alumina).
- TLC was checked after night in room temperature but showed no further reaction, which indicates that the oxidation had stopped halfway due to the addition of too little MCPBA to convert all the starting material to N-oxidized products.
- Two rats were treated with either Aripiprazole (100 ⁇ mol/kg i.p.) or thio-Aripiprazole (100 ⁇ mol/kg i.p.). After 2 hours, the animals were killed and blood was collected via heart puntation. The brains were removed and homogenized. The biological sample were centrifuged at 10,000 r.p.m. and the supernatant was transferred to test tubes with a pipett and were stored until further workup and analysis. Samples were spiked with the standard mono-pivaloyl-apomorphine .
- Rat with aripiperazol injected Brain: 4.7 ⁇ M Aripiprazole Blood: 660 nM Aripiprazole (no thio-aripiperazole was detected in these samples)
- Aripiprazole-mono-N-oxide (100 ⁇ mol/kg) was difficult to dissolve (10 microliters acetic acid, water, PEG and DMSO, totally about 1 mL) and was administered orally to a rat weighing about 300 g. No dramatic behavioural the effects were seen, but the rat showed no signs of catalepsy behavior. After two hours, the rat was anesthetized (isoflurane) and was killed by heart puncture. Blood was col- lected and the brain was taken out to be homogenized in 60 percent
- Aripiprazole is generated from both Aripiprazole-mono-N-oxide and from thio-Aripiprazole.
- the compound of the present invention may be evaluated in rats unilaterally lesioned with 6-hydroxydopamine (6-OH-DA) (Ungerstedt and Arbuthnott, Brain Res. 1970, 24, 485-493) .
- 6-hydroxydopamine 6-hydroxydopamine
- the DA neurons of one side (left or right) of the nigrostriatal DA system are selectively and completely degen- erated by intracebral injection of the neurotoxin 6-OH-DA.
- Haloperidol was administered subcutaneously (s.c.) to the rats at a dose of 1 mg/kg body weight.
- Apomorphine was administered subcutaneously to the rats in doses of 0.05, 0.1 and 0.25 mg/kg body weight ("Apomorphine 0.05", etc. in the Ta- bles below).
- Aripriprazol and 7- (4- [4- (2 , 3-dichloro-phenyl) - piperazin-1-yl] -butoxy ⁇ -3 , 4 -dihydro-lH-quinoline-2 -thione ( "thio-aripriprazole” ) were each administered intraperito- neally (i.p.) at a dose of 10 mg/kg.
- Catalepsy was observed by placing the animals on an inclined grid 60 degrees for a maximum of 2.25 min, in a lit room. The animals were allowed 30 s of adaptation on the grid, at every measuring occasion, before the observation (stop watch) was started. The catalepsy was expressed as a score from 0 to 5, according to the time, square root transformation the rat re- mained immobile, min : 0 s 0.00-0.08; 1 s 0.09-0.35; 2 s
- mice Male Wistar rats (from Harlan, Zeist, The Netherlands) weighing 280-320 g were used, and housed as described for the lo- comotor activity experiments. On line brain microdialysis in freely moving animals was essentially performed as described previously (Westerink, Trends in Anal. Chem. 1992, 11, 176- 182) . Briefly, rats were anesthetized with choral hydrate (400 mg/kg ip) and 10% lidocaine locally applied. The rats were then mounted into a stereotaxic frame (Kopf) . The incisor bar was placed in position so that the scull was held in a horizontal position. The skull was exposed and burr holes were drilled.
- Kopf stereotaxic frame
- the dialysis tube (ID: 0.22 mm; OD : 0.31 mm) was prepared from polyacryloni- trile methalys sulfonate copolymer (AN 69, Hospal, Bologna, Italy) .
- the dialysis membrane was implanted in the Striatum with coordinates which were calculated relative to bregma: A + 1 , L 3, D 6 according to the brain atlas of Paxinos and Watson (1982) .
- the dura was removed with a sharp needle. Two anchor screws were positioned in different bone plates nearby.
- the dialysis probes were perfused with successively ultra pure water, methanol, ultra pure water and Ringer solution (1.2 mM Ca 2+ ) .
- the dialysis probe was positioned in the burr hole under stereotaxic guidance.
- the probe was cemented in this position with phosphatine dental cement (Associated dental products LTD, Kemdent Works, Purdon, Swinden, Wiltshire SN 5 9 HT) .
- the experiments were performed in conscious rats 17-56 h after implantation of the cannula.
- the striatum was perfused with a Ringer solution (147 mM NaCl , 4 mM KCl , 1.2 mM CaCl 2 , 1.1 M MgCl 2 ) at 2 1/min (CMA/102 microdialysis pump).
- a Ringer solution 147 mM NaCl , 4 mM KCl , 1.2 mM CaCl 2 , 1.1 M MgCl 2
- CMA/102 microdialysis pump 2 1/min
- Dopamine, dihydroxyphenylacetic acid (DOPAC) and 5-HIAA were quantitated by HPLC with electrochemical detection.
- An HPLC pump (LKB, Pharmacia) was used in conjugation with an EC- detector (Antec, Leiden) working at 625 mV versus Ag/AgCl reference electrode.
- the analytical column was a Supelco Su- pelcosil LC-18 Column (15 cm, 4.6 mm, 3 ⁇ m) .
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Psychiatry (AREA)
- Cardiology (AREA)
- Addiction (AREA)
- Heart & Thoracic Surgery (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Psychology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
Claims
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE0200301A SE0200301D0 (en) | 2002-02-01 | 2002-02-01 | Thio-carbostyril derivative |
| SE0200301-0 | 2002-02-01 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2003064393A1 true WO2003064393A1 (en) | 2003-08-07 |
Family
ID=20286844
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/SE2003/000164 WO2003064393A1 (en) | 2002-02-01 | 2003-01-31 | Thio-carbostyril derivative, its n-oxides and the n-oxides of aripiprazole |
Country Status (2)
| Country | Link |
|---|---|
| SE (1) | SE0200301D0 (en) |
| WO (1) | WO2003064393A1 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004017897A3 (en) * | 2002-08-20 | 2004-12-02 | Bristol Myers Squibb Co | Aripiprazole complex formulation and method |
| WO2008127188A1 (en) * | 2007-04-12 | 2008-10-23 | Allbay Ab | N-oxide and/or di-n-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| WO2008142461A1 (en) * | 2007-05-18 | 2008-11-27 | Richter Gedeon Nyrt. | Metabolites of (thio)carbamoyl-cyclohexane derivatives |
| WO2010099502A1 (en) | 2009-02-26 | 2010-09-02 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of utilizing arylpiperazine derivatives |
| US8207163B2 (en) | 2008-05-27 | 2012-06-26 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of using piperazine based antipsychotic agents |
| US8247420B2 (en) | 2007-05-21 | 2012-08-21 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of using quinolinone based atypical antipsychotic agents |
| US8569498B2 (en) | 2008-12-18 | 2013-10-29 | Richter Gedeon Nyrt. | Process for the preparation of piperazine compounds and hydrochloride salts thereof |
| US8569496B2 (en) | 2008-12-17 | 2013-10-29 | Richter Gedeon Nyrt. | Piperazine salt and a process for the preparation thereof |
| US8569497B2 (en) | 2008-12-18 | 2013-10-29 | Richter Gedeon Nyrt. | Process for the preparation of piperazine derivatives |
| US8802672B2 (en) | 2007-12-03 | 2014-08-12 | Richter Gedeon Nyrt. | Pyrimidinyl-piperazines useful as D3/D2 receptor ligands |
| US9012476B2 (en) | 2011-12-08 | 2015-04-21 | IVAX International GmbH | Hydrobromide salt of pridopidine |
| US11207308B2 (en) | 2012-04-04 | 2021-12-28 | Prilenia Neurotherapeutics Ltd. | Pharmaceutical compositions for combination therapy |
| US11274087B2 (en) | 2016-07-08 | 2022-03-15 | Richter Gedeon Nyrt. | Industrial process for the preparation of cariprazine |
| USRE49110E1 (en) | 2008-07-16 | 2022-06-21 | Richter Gedeon Nyrt. | Pharmaceutical formulations containing dopamine receptor ligands |
| US11547707B2 (en) | 2019-04-10 | 2023-01-10 | Richter Gedeon Nyrt. | Carbamoyl cyclohexane derivatives for treating autism spectrum disorder |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0367141A2 (en) * | 1988-10-31 | 1990-05-09 | Otsuka Pharmaceutical Co., Ltd. | Carbostyril derivatives |
-
2002
- 2002-02-01 SE SE0200301A patent/SE0200301D0/en unknown
-
2003
- 2003-01-31 WO PCT/SE2003/000164 patent/WO2003064393A1/en not_active Application Discontinuation
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0367141A2 (en) * | 1988-10-31 | 1990-05-09 | Otsuka Pharmaceutical Co., Ltd. | Carbostyril derivatives |
Non-Patent Citations (4)
| Title |
|---|
| BOURLAND JAMES A. AND FRENCH EDWARD D.: "Effects of remoxipride, an atyptical antipsychotic, on cocaine self-administration in the rat using fixed- and progressive-ratio schedules of reinforcement", DRUG AND ALCOHOL DEPENDENCE, vol. 40, no. 2, 1995, pages 111 - 114, XP002964965 * |
| INOUE ATSUKO ET AL.: "Aripiprazole, a novel antipsychotic drug, inhibits quinpirole-evoked GTPase activity but does not up-regulated dopamine D2 receptor following repeated treatment in the rat striatum", EUROPEAN JOURNAL OF PHARMACOLOGY, vol. 321, 1997, pages 105 - 111, XP002964963 * |
| SEEMAN P. AND VAN TOL H.H.: "Dopamine receptor pharmacology", TRENDS IN PHARMACOLOGICAL SCIENCES, vol. 15, no. 7, July 1994 (1994-07-01), pages 264 - 270, XP000992721 * |
| WISE ROY A.: "D-1- and D2-type contributions to psychomoter sensitization and reward: implications for pharmacological treatment strategies", CLINICAL NEUROPHARMACOLOGY, vol. 18, no. SUPPL. 1, 1995, pages 74 - 83, XP002964964 * |
Cited By (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7115587B2 (en) | 2002-08-20 | 2006-10-03 | Bristol-Myers Squibb Company | Aripiprazole complex formulation and method |
| CN100335047C (en) * | 2002-08-20 | 2007-09-05 | 布里斯托尔-迈尔斯斯奎布公司 | Aripiprazole complex formulation and method |
| US8999952B2 (en) | 2002-08-20 | 2015-04-07 | Otsuka Pharmaceutical Co., Ltd. | Aripiprazole complex formulation and method |
| US7550445B2 (en) | 2002-08-20 | 2009-06-23 | Bristol-Myers Squibb Company | Aripiprazole complex formulation and method |
| WO2004017897A3 (en) * | 2002-08-20 | 2004-12-02 | Bristol Myers Squibb Co | Aripiprazole complex formulation and method |
| KR101043866B1 (en) | 2002-08-20 | 2011-06-22 | 브리스톨-마이어스 스큅 컴퍼니 | Aripiprazole complex formulation and method |
| CN101711236B (en) * | 2007-04-12 | 2012-10-31 | Nsab神经研究瑞典公司分公司 | N-oxide and/or di-N-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| WO2008127188A1 (en) * | 2007-04-12 | 2008-10-23 | Allbay Ab | N-oxide and/or di-n-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| AU2008239841B2 (en) * | 2007-04-12 | 2013-07-18 | Teva Pharmaceuticals International Gmbh | N-oxide and/or di-N-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| JP2010523651A (en) * | 2007-04-12 | 2010-07-15 | エヌエスエイビー、フィリアル アヴ ノイロサーチ スウェーデン エービー、スヴェーリエ | N-oxide and / or di-N-oxide derivatives of dopamine receptor stabilizers / modulators exhibiting an improved cardiovascular side effect profile |
| US9139525B2 (en) | 2007-04-12 | 2015-09-22 | Teva Pharmaceuticals International Gmbh | N-oxide and/or di-N-oxide derivatives of dopamine receptor stabilizers/modulators displaying improved cardiovascular side-effects profiles |
| RU2470013C2 (en) * | 2007-04-12 | 2012-12-20 | НСАБ, Филиаль ау НьюроСёрч Свиден АБ, Сверийе | N-oxide and/or di-n-oxide derivatives of stabilisers/modulators of dopamine receptors, demonstrating improved profiles of cardio-vascular side effects |
| US20100137335A1 (en) * | 2007-05-18 | 2010-06-03 | Eva Againe Csongor | Metabolites of (thio) carbamoyl-cyclohexane derivatives |
| JP2010527983A (en) * | 2007-05-18 | 2010-08-19 | リチュテル・ゲデオン・ヴェジェーセティ・ジャール・ニュイルヴァーノシャン・ミューコェデー・レースヴェーニュタールシャシャーグ | Metabolites of (thio) carbamoyl-cyclohexane derivatives |
| WO2008142461A1 (en) * | 2007-05-18 | 2008-11-27 | Richter Gedeon Nyrt. | Metabolites of (thio)carbamoyl-cyclohexane derivatives |
| US8765765B2 (en) * | 2007-05-18 | 2014-07-01 | Richter Gedeon Nyrt. | Metabolites of (thio) carbamoyl-cyclohexane derivatives |
| US8247420B2 (en) | 2007-05-21 | 2012-08-21 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of using quinolinone based atypical antipsychotic agents |
| US8802672B2 (en) | 2007-12-03 | 2014-08-12 | Richter Gedeon Nyrt. | Pyrimidinyl-piperazines useful as D3/D2 receptor ligands |
| US8207163B2 (en) | 2008-05-27 | 2012-06-26 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of using piperazine based antipsychotic agents |
| USRE49302E1 (en) | 2008-07-16 | 2022-11-15 | Richter Gedeon Nyrt. | Pharmaceutical formulations containing dopamine receptor ligands |
| USRE49110E1 (en) | 2008-07-16 | 2022-06-21 | Richter Gedeon Nyrt. | Pharmaceutical formulations containing dopamine receptor ligands |
| US8569496B2 (en) | 2008-12-17 | 2013-10-29 | Richter Gedeon Nyrt. | Piperazine salt and a process for the preparation thereof |
| US8569498B2 (en) | 2008-12-18 | 2013-10-29 | Richter Gedeon Nyrt. | Process for the preparation of piperazine compounds and hydrochloride salts thereof |
| US8569497B2 (en) | 2008-12-18 | 2013-10-29 | Richter Gedeon Nyrt. | Process for the preparation of piperazine derivatives |
| WO2010099502A1 (en) | 2009-02-26 | 2010-09-02 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of utilizing arylpiperazine derivatives |
| US9604944B2 (en) | 2009-02-26 | 2017-03-28 | Reviva Pharmaceuticals, Inc. | Arylpiperazine derivatives and methods of utilizing same |
| US8575185B2 (en) | 2009-02-26 | 2013-11-05 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of utilizing quinazolinedione derivatives |
| US8188076B2 (en) | 2009-02-26 | 2012-05-29 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of utilizing arylpiperazine derivatives |
| US8461154B2 (en) | 2009-02-26 | 2013-06-11 | Reviva Pharmaceuticals, Inc. | Methods of utilizing arylpiperazine derivatives |
| US9255076B2 (en) | 2009-02-26 | 2016-02-09 | Reviva Pharmaceuticals, Inc. | Arylpiperazine derivatives and methods of utilizing same |
| EP2400968B1 (en) * | 2009-02-26 | 2016-09-28 | Reviva Pharmaceuticals, Inc. | Compositions, synthesis, and methods of utilizing arylpiperazine derivatives |
| US8859552B2 (en) | 2009-02-26 | 2014-10-14 | Reviva Pharmaceuticals, Inc. | Methods of utilizing arylpiperazine derivatives |
| US8431570B2 (en) | 2009-02-26 | 2013-04-30 | Reviva Pharmaceuticals, Inc. | Methods of utilizing arylpiperazine derivatives |
| US9975862B2 (en) | 2009-02-26 | 2018-05-22 | Reviva Pharmaceuticals, Inc. | Arylpiperazine derivatives and methods of utilizing same |
| US9814706B2 (en) | 2011-12-08 | 2017-11-14 | Teva Pharmaceuticals International Gmbh | Hydrobromide salt of pridopidine |
| US9012476B2 (en) | 2011-12-08 | 2015-04-21 | IVAX International GmbH | Hydrobromide salt of pridopidine |
| US11207308B2 (en) | 2012-04-04 | 2021-12-28 | Prilenia Neurotherapeutics Ltd. | Pharmaceutical compositions for combination therapy |
| US11274087B2 (en) | 2016-07-08 | 2022-03-15 | Richter Gedeon Nyrt. | Industrial process for the preparation of cariprazine |
| US11547707B2 (en) | 2019-04-10 | 2023-01-10 | Richter Gedeon Nyrt. | Carbamoyl cyclohexane derivatives for treating autism spectrum disorder |
Also Published As
| Publication number | Publication date |
|---|---|
| SE0200301D0 (en) | 2002-02-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2608788B2 (en) | Schizophrenia remedy | |
| US6683090B1 (en) | N-azabicyclo-amide derivatives | |
| WO2003064393A1 (en) | Thio-carbostyril derivative, its n-oxides and the n-oxides of aripiprazole | |
| KR0138529B1 (en) | Carbostyril derivatives | |
| PT725779E (en) | NEW (R) -5-CARBAMOYL-8-FLUORO-3-N, N-DISSUBSTITUTED-AMINO-3,4-DIHYDRO-2H-1-BENZOPYRANES | |
| JP2011136906A (en) | Heterocyclic compound | |
| WO2003097636A1 (en) | Novel compounds and their use | |
| CN114787156A (en) | Novel methyl quinazolinone derivatives | |
| KR19990028757A (en) | Benzo [g] quinoline derivatives | |
| ZA200405024B (en) | Tetrahydroquinoline analoques as muscarinic agonists | |
| JP2017019768A (en) | Hexahydrodibenzo[a,g]quinolizine compound, manufacturing method therefor, pharmaceutical composition and application thereof | |
| EP1701951B1 (en) | Modulators of peripheral 5-ht receptors | |
| KR870001072B1 (en) | Process for preparation of octahydrothiazolo(4,5-g)quinolines | |
| CN101970427A (en) | Method for treating pain syndrome and other disorders | |
| US6169094B1 (en) | Compositions of (S) (-)-amisulpride | |
| EP3986567B1 (en) | New egfr inhibitors | |
| AU2010340745B2 (en) | Sulfone compounds as 5-HT6 receptor ligands | |
| US20050096347A1 (en) | Beta3-Adrenoreceptor agonists, agonist compositions and methods of using | |
| JPH08502057A (en) | Heterocyclic amines with calmodulin-antagonistic properties | |
| US11773063B1 (en) | Pharmaceutically acceptable salts and compositions thereof | |
| JP4557112B2 (en) | Benzoxazole derivative and pharmaceutical comprising the derivative as an active ingredient | |
| CA2905950A1 (en) | Novel breathing control modulating compounds, and methods of using same | |
| US11738008B2 (en) | N-methyl-D-aspartic acid receptor modulators | |
| WO2005000305A1 (en) | 3-aminopiperidines and 3-aminoquinuclidines as inhibitors of monoamine uptake | |
| CZ51094A3 (en) | 3-(n-isopropyl-n-propylamino)-5-(n-isopropyl)carbamoylchroman |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ OM PH PL PT RO RU SC SD SE SG SK SL TJ TM TN TR TT TZ UA UG US UZ VC VN YU ZA ZM ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZM ZW AM AZ BY KG KZ MD RU TJ TM AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LU MC NL PT SE SI SK TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: JP |
|
| WWW | Wipo information: withdrawn in national office |
Country of ref document: JP |