WO2002012185A1 - Process for preparing 2,3-diaminopropanols and synthesis of other compounds using 2,3-diaminopropanols - Google Patents
Process for preparing 2,3-diaminopropanols and synthesis of other compounds using 2,3-diaminopropanols Download PDFInfo
- Publication number
- WO2002012185A1 WO2002012185A1 PCT/KR2001/001362 KR0101362W WO0212185A1 WO 2002012185 A1 WO2002012185 A1 WO 2002012185A1 KR 0101362 W KR0101362 W KR 0101362W WO 0212185 A1 WO0212185 A1 WO 0212185A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- formula
- group
- compound
- process according
- aziridine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *C(C1N(*)C1)O Chemical compound *C(C1N(*)C1)O 0.000 description 2
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C247/00—Compounds containing azido groups
- C07C247/02—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton
- C07C247/08—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton being unsaturated
- C07C247/10—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton being unsaturated and containing rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/12—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms
- C07D295/125—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms with the ring nitrogen atoms and the substituent nitrogen atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings
- C07D295/13—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms with the ring nitrogen atoms and the substituent nitrogen atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings to an acyclic saturated chain
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/02—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton by reactions involving the formation of amino groups from compounds containing hydroxy groups or etherified or esterified hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C213/00—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton
- C07C213/08—Preparation of compounds containing amino and hydroxy, amino and etherified hydroxy or amino and esterified hydroxy groups bound to the same carbon skeleton by reactions not involving the formation of amino groups, hydroxy groups or etherified or esterified hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C247/00—Compounds containing azido groups
- C07C247/02—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton
- C07C247/04—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton being saturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C247/00—Compounds containing azido groups
- C07C247/02—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton
- C07C247/08—Compounds containing azido groups with azido groups bound to acyclic carbon atoms of a carbon skeleton being unsaturated
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D241/00—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings
- C07D241/02—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings
- C07D241/04—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D333/00—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom
- C07D333/02—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings
- C07D333/04—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom
- C07D333/06—Heterocyclic compounds containing five-membered rings having one sulfur atom as the only ring hetero atom not condensed with other rings not substituted on the ring sulphur atom with only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, directly attached to the ring carbon atoms
- C07D333/14—Radicals substituted by singly bound hetero atoms other than halogen
- C07D333/16—Radicals substituted by singly bound hetero atoms other than halogen by oxygen atoms
Definitions
- the obtained optically pure 2,3-diaminopropanol is used to produce, for example, hydroxymethylpiperazine which is useful as an intermediate of medicament.
- the processes for producing an optically active piperazine was proposed via a catalytic hydrogenation (U.S. Patent No. 5,977,364), or resolution of racemic mixture (U.S. Patent No. 5,945,534) were known.
- the present invention is characterized by a process for preparing 2,3- diaminopropanol from a chiral diamine.
- morpholine is used as amine in the present process, D-threo-l-phenyl-2-decanoylamino-3-morpholino-l-propanol etc. can be produced, which is biologically active. Disclosure of the Invention
- the present invention provides a process for producing 2,3-diaminopropanol in high yields of more than 90% by ring opening an inactivated optically pure aziridine and reacting it with various amines.
- the obtained 2,3-diaminopropanol is used in the synthesis of high value-added compounds, such as hydroxymethylpiperazine, D-threo-l-phenyl-2- decanoylamino-3 -morpholino- 1 -propanol, etc.
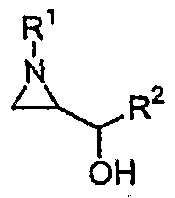
- the object of the present invention is to provide a process for producing 2,3- diaminopropanol derivatives of formula (2) which act as intermediates in the synthesis of medicaments from aziridine alcohol of formula (1) and a process for producing the other useful compounds therefrom. Accordingly, the processes of the present invention can have four preparations as follows.
- a compound of formula (1) was reacted with halotrialkylsilane [Si(R) 3 X] to carry out a regiospecific ring opening reaction of the aziridine, and then the resulting intermediate was reacted with an amine (NHR 3 R 4 ) to produce a compound of formula (2).
- This can be schematically illustrated in the following Scheme 1
- R 1 is selected from the group consisting of hydrogen; alkyl; cycloalkyl; 3- triazinyl or pyrimidyl acyl; aryl; hydrocarbon residues; aralkyl; and those substituted with suitable substituent(s), and includes 4-chlorophenyl, 4-methoxyphenyl, benzyl, 2,4- dimethoxyphenyl, (lR)-phenylethyl and (lS)-phenylethyl, preferences are given to (1R)- phenylethyl and (lS)-phenylethyl;
- R 2 is selected from the group consisting of hydrogen; alkyl including, but not limiting to methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butyl, n-pentyl, isopentyl, neopentyl, n-hexyl, n-
- Preparation 2 may be applied.
- aryl includes, but is not limited to, C 5 -C 14 aromatic hydrocarbon group such as phenyl, indenyl, naphthyl, phenanthrenyl, anthracenyl etc.
- heteroaryl includes, but is not limited to, 1 to 4 unsaturated 3- to 8- membered heterocyclic moieties containing 1 to 4 nitrogen atoms (e.g., pyrrolyl, pyrrolinyl, imidazolyl, pyrazolyl, pyridyl, dihydropyridyl, pyrimidyl, pyrazinyl, pyridazinyl, triazolyl, tetrazolyl etc.); saturated 3- to 8-membered heterocyclic moieties containing 1 to 4 nitrogen atom(s) (e.g., pyrrolidinyl, imidazolidinyl, piperidyl, piperazinyl etc.); unsaturated condensed heterocyclic moieties containing 1 to 4 nitrogen atom(s) (e.g., indolyl, isoindolyl, indolinyl, indolizinyl, benzimidazolyl,
- pyrrolyl pyrazolyl
- imidazolyl oxazolyl
- isoxazolyl isoxazolyl
- thiazolyl isothiazolyl
- 1,2,3 -oxadiazolyl triazolyl, tetrazolyl, thiadiazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, morpholinyl.
- suitable substituent(s) includes, but is not limited to, lower alkyl (e.g., methyl, ethyl, propyl, isopropyl, butyl, isobutyl, t-butyl, pentyl, neopentyl, t-pentyl, hexyl etc.), lower alkoxy (e.g., methoxy, ethoxy, propoxy, isopropoxy, isobutoxy, tert-butoxy, pentyloxy, neopentyloxy, tert-pentyloxy, hexyloxy etc.), lower alkenyl (e.g., vinyl, 1-propenyl, allyl, 1-methylallyl, 1-, 2- or 3-butenyI, 1-, 2-, 3- or 4-pentenyl etc.), lower alkynyl (e.g., ethynyl, 1-propynyl, propargyl, 1-methylpropargon
- haIotrialkylsilane[Si(R) 3 X] includes, but is not limited to, chlorotrimethylsilane, bromotrimethylsilane, iodotriethylsilane.
- lower means that the number of carbon is 1 to 6.
- lower alkyl indicates, but not limited to C,-C 6 linear or branched moiety(ies) such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, t-butyl, pentyl, neopentyl, t-pentyl, hexyl, 1,1-dimethylbutyl, 2,2- dimethylbutyl, and the term "alkyl” includes, but is not limited to, linear or branched C,-C 20 alkyl such as methyl, ethyl, propyl, 2-ethylhexyl, octyl, dodecyl, hexadecyl, and octadecyl.
- all the preparation processes of the present invention are usually carried out in a solvent such as acetonitrile, benzene, N,N-dimethylformamide, tetrahydrofuran, methylene chloride, ethylene chloride, chloroform, diethyl ether or other solvents which do not adversely influence the reaction.
- a solvent such as acetonitrile, benzene, N,N-dimethylformamide, tetrahydrofuran, methylene chloride, ethylene chloride, chloroform, diethyl ether or other solvents which do not adversely influence the reaction.
- a conventional organic solvent can be used. More preferably halogenated organic solvent, and most preferably methylene chloride can be used.
- the reaction temperature is not critical, and the reaction is usually carried out under cooling to warming.
- An azidoaminoalcohol of formula (3) was produced by reacting a compound of formula (1), the same starting material as in Preparation 1 by a known process (Y. Lim, Tetrahedron Letters, 1995, 36, 8431, and B. C. Kim, Tetrahedron, 1996, 52, 12117), reacting the compound with azidotrialkylsilane[Si(R') 3 N 3 ] to carry out a regiospecific ring opening reaction of the aziridine.
- 2,3-diaminopropanol of formula (4) was produced by reducing the obtained compound of formula (3) using a conventional azido reduction process. According to this procedure, a product wherein R 3 and R 4 are both hydrogen [i.e., a compound of formula (4)] can be produced.
- Aldotrialkylsilane[Si(R') 3 N 3 ] M as used herein includes, but is not limited to, azidotrimethylsilane or azidotriethylsilane. Preference is given to azidotrimethylsilane.
- the "reduction” according to this invention can be commonly carried out by the conventional procedures in the art such as chemical reduction and catalytic reduction. The following is a brief introduction of the reduction procedure, which can be used in the present invention.
- Suitable reducing agents to be used in the chemical reduction are hydride (e.g., lithium aluminum hydride, sodium borohydride, sodium cyanoborohydride etc.); mixture of borane and tetrahydrofuran or di(lower)alkyl sulfide (e.g., dimethyl sulfide etc.); or mixture of metal (e.g., tin, zinc, iron etc.) or acid compound (e.g., formic acid, acetic acid, propionic acid, trifluoroacetic acid, p-toluenesulfonic acid, hydrochloric acid, hydrobromic acid etc.).
- hydride e.g., lithium aluminum hydride, sodium borohydride, sodium cyanoborohydride etc.
- borane and tetrahydrofuran or di(lower)alkyl sulfide e.g., dimethyl sulfide etc.
- metal e.g., tin,
- Suitable catalysts to be used in catalytic reduction are conventional ones such as platinum catalyst (e.g., platinum plate, spongy platinum, platinum black, colloidal platinum, platinum oxide, platinum wire etc.), palladium catalyst (e.g., spongy palladium, palladium black, palladium oxide, palladium on carbon, colloidal palladium, palladium on barium sulfate, palladium on barium carbonate etc.), nickel catalyst (e.g., reduced nickel, nickel oxide, Raney nickel etc.), cobalt catalyst (e.g., reduced cobalt, Raney cobalt etc.), iron catalyst (e.g., reduced iron, Raney iron, Ullmann iron etc.) and the like.
- platinum catalyst e.g., platinum plate, spongy platinum, platinum black, colloidal platinum, platinum oxide, platinum wire etc.
- palladium catalyst e.g., spongy palladium, palladium black, palladium oxide, palladium on carbon, colloidal palla
- the known reduction procedures can be utilized in reducing an azido group to an amino group in Preparation 2, and the typical examples are reduction by LiAlH 4 , reduction by Ph 3 P and catalytic reduction (catalytic hydrogenation), etc.
- the reduction is usually carried out in a solvent such as water, alcohol, tetrahydrofuran, dioxane, N,N-dimethylformamide, or a mixture thereof; or other solvents which do not adversely influence the reaction.
- a solvent such as water, alcohol, tetrahydrofuran, dioxane, N,N-dimethylformamide, or a mixture thereof; or other solvents which do not adversely influence the reaction.
- the acid used in chemical reduction is liquid, it can also act as a solvent.
- the reaction temperature is not critical and the reaction is usually carried out under cooling to warming.
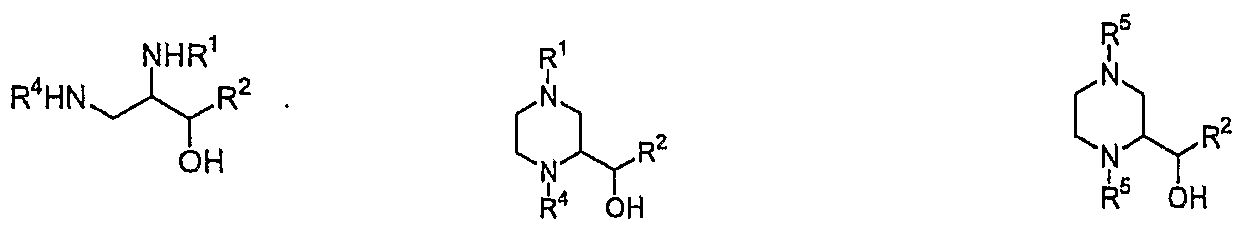
- a compound of formula (6) can be produced by subjecting 2,3-diaminopropanol of formula (5) (i.e., the compound of formula (2) obtained from Preparation 1, wherein R 3 is hydrogen) to reduction as shown in Scheme 3, preferably reduction using a compound having a dialdehyde group and a hydride reducing agent.
- the compound of formula (6) was subjected to a conventional reduction in the presence of a compound capable of producing an amino protecting group (i.e., amino protector), preferably reduction by catalytic hydrogenation (catalytic reduction) as described above to produce a compound of formula (7).
- Typical compound of formula (7) includes l,4-di-t-Boc-2(R)-hydroxymethylpiperazine (wherein "Boc” stands for tert-butyloxycarbonyl).
- R 1 , R 2 and R 4 are as defined hereinbefore, and R 5 is an amino protecting group, which is suitable for protecting (or blocking) an amino group from chemical reaction, and is easily removable after completing the desired reaction on the other site.
- Typical examples of this type include substituted or unsubstituted acyl, aryl, aralkoxymethyl or aralkyl group etc.
- the name or size of amino protecting groups is not critical because they are removed after completing the desired reaction. However, those having 1 to 20 carbon atom(s), particularly 1 to 8 carbon atom(s) can be preferably used.
- acyl group as used herein can have the broadest meaning in regard to this process. It includes an acyl group derived from aliphatic, aromatic aliphatic, aromatic or heterocyclic carboxylic acid or sulfonic acid as well as alkoxycarbonyl, aryloxycarbonyl, particularly aralkoxycarbonyl group.
- acyl group include, but are not limited to, alkanoyl such as acetyl, propionyl, butyryl, decanoyl etc.; aralkanoyl such as phenylacetyl etc.; aroyl such as benzoyl or toluyl etc.; aryloxyalkanoyl; alkoxycarbonyl such as methoxycarbonyl, ethoxycarbonyl, 2,2,2-trichloroethoxycarbonyl, Boc, 2-iodoethoxycarbonyl etc.; aralkylcarbonyl such as Cbz(carbobenzoxy), 4-methoxybenzyloxycarbonyl, Fmoc (9- fluorenylmethoxycarbonyl) etc.; arylsulfonyl.
- alkanoyl such as acetyl, propionyl, butyryl, decanoyl etc.
- aralkanoyl such as phenylacety
- amino protecting group the preference is given to Boc, and also to Cbz, Fmoc, benzyl or acetyl.
- amino protector t-butyl carbonate, methylcarbamate, ethylcarbamate or 9- fluorenylmethylcarbamate can be preferably used.
- the “hydride” includes, but is not limited to, lithium aluminum hydride, sodium borohydride or sodium cyanoborohydride.
- a compound having a dialdehyde group is well known in the art, and preferably glyoxal can be used.
- the compound of formula (8) can be prepared by subjecting R 1 group of the compound of formula (2) to a conventional reduction procedure, preferably to a reduction using catalytic hydrogenation as described hereinbefore, and then reacting it with alkanoyl halide (R 6 X).
- alkanoyl halide R 6 X
- Typical examples of the compound of formula (8) include D-threo-l-phenyl-2-decanoylamino-3-morpholino-l-propanol.
- R 1 , R 2 , R 3 and R 4 and the redction are as defined hereinbefore,
- R 6 includes, but is not limited to, alkanoyl group such as acetyl, propionyl, butyryl, valeryl, pivaloyl, hexanoyl, isobutyryl, 2-ethylbutyryl, 3,3-dimethylbutyryl, decanoyl, preference is given to decanoyl, and X is fluoro, chloro, bromo or iodo.
- alkanoyl group such as acetyl, propionyl, butyryl, valeryl, pivaloyl, hexanoyl, isobutyryl, 2-ethylbutyryl, 3,3-dimethylbutyryl, decanoyl, preference is given to decanoyl
- X is fluoro, chloro, bromo or iodo.
- N-[(S)-l-phenylethyl]aziridine-2(R)-[l(R)-methyl]methanol (201 mg) was dissolved in methylene chloride, to which azidotrimethylsilane (0.25 ml) was added.
- the reaction mixture was stirred at ambient temperature for 12 hours, was treated with IN of aqueous hydrochloric acid solution and then was stirred for 1 more hour.
- the solution was neutralized with sodium hydrogen carbonate, and the reaction product was extracted twice each with 5.0 mi of methylene chloride.
- the combined organic extracts were dried under anhydrous magnesium sulfate, and the solution was filtered, concentrated in vacuo and purified to give 154 mg of product as an oil.
- N-[(S)-l-phenylethyl]aziridine-2(R)-methanol 60 mg was dissolved in 1.0 ml of acetonitrile, and then 107 mg of sodium iodide and 86 mg of chlorotnmethylsilane were added to this solution. After stirring the reaction mixture at ambient temperature for 1 hour and 50 minutes, 16.8 mg of pyrrolidine was added, and then the reaction mixture was heated for 2 hours with stirring. After the reaction was completed, the reaction mixture was treated with 1.2N aqueous hydrochloric acid solution, neutralized with sodium hydrogen carbonate and extracted twice each with 5 mi of methylene chloride. The combined organic extracts were rinsed with brine, and dried under anhydrous magnesium sulfate. The solution was filtered, concentrated in vacuo, and purified to give 73 mg of titled product as an oil.
- the present invention produces the known 2,3-diaminopropanol in high yields of more than 90% by ring opening an inactivated optically pure aziridine and reacting with various amines.
- the obtained 2,3-diaminopropanol can be used in synthesis of high value-added fine chemical compounds, such as hydroxymethylpiperazine, D-threo-l-phenyl-2-decanoylamino- 3 -morpholino-1 -propanol etc.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
Claims
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2001277801A AU2001277801A1 (en) | 2000-08-10 | 2001-08-10 | Process for preparing 2,3-diaminopropanols and synthesis of other compounds using 2,3-diaminopropanols |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020000046394A KR20000063913A (en) | 2000-08-10 | 2000-08-10 | Process for preparing 1,2-diaminopropane alcohols from aziridines |
| KR2000/46394 | 2000-08-10 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2002012185A1 true WO2002012185A1 (en) | 2002-02-14 |
Family
ID=19682665
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/KR2001/001362 Ceased WO2002012185A1 (en) | 2000-08-10 | 2001-08-10 | Process for preparing 2,3-diaminopropanols and synthesis of other compounds using 2,3-diaminopropanols |
Country Status (3)
| Country | Link |
|---|---|
| KR (2) | KR20000063913A (en) |
| AU (1) | AU2001277801A1 (en) |
| WO (1) | WO2002012185A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20000063913A (en) * | 2000-08-10 | 2000-11-06 | 하현준 | Process for preparing 1,2-diaminopropane alcohols from aziridines |
| EP2198864A1 (en) | 2005-01-26 | 2010-06-23 | Allergan, Inc. | Pharmaceutical compositions having an analgesic effect and containing 1-benzyl-1-hydroxy-2,3-diamino-propyl amines, 3-benzyl-3-hydroxy-2-amino propionic acid amides or related compounds |
| CN103910655A (en) * | 2014-04-11 | 2014-07-09 | 太原理工大学 | Ring opening method of aziridine compounds |
| US9314466B2 (en) | 2007-03-06 | 2016-04-19 | Allergan, Inc. | Methods for treating cognitive disorders using 1-benzyl-1-hydroxy-2,3-diamino-propyl amines, 3-benzyl-3-hydroxy-2-amino-propionic acid amides and related compounds |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100420263B1 (en) * | 2001-04-16 | 2004-03-02 | 한솔케미언스 주식회사 | Process for preparing iso-serine and its derivatives from aziridine-2-carboxylates |
| KR101631481B1 (en) | 2015-04-07 | 2016-06-17 | 고려대학교 산학협력단 | Polymer using aziridine and method for preparing the same |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3957823A (en) * | 1972-04-15 | 1976-05-18 | Badische Anilin- & Soda-Fabrik Aktiengesellschaft | Electrophilic substitution of nitrosamines |
| US4040838A (en) * | 1975-03-05 | 1977-08-09 | Fuji Photo Film Co., Ltd. | Processing color photographic materials |
| JPH01228946A (en) * | 1988-03-09 | 1989-09-12 | Suntory Ltd | Synthesis of beta-hydroxyphenetylamines |
| EP0736509A2 (en) * | 1995-04-07 | 1996-10-09 | Sumitomo Chemical Company, Limited | Processes for preparing optically active alcohols and optically active amines |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS56164129A (en) * | 1980-05-20 | 1981-12-17 | Takasago Corp | Preparation of allyl alcohol |
| JPH0791239B2 (en) * | 1988-11-22 | 1995-10-04 | 株式会社日本触媒 | Method for producing ethylenediamine |
| US6063963A (en) * | 1995-07-05 | 2000-05-16 | Pharm-Eco Laboratories, Inc. | Amino acid-derived diaminopropanols |
| KR20000063913A (en) * | 2000-08-10 | 2000-11-06 | 하현준 | Process for preparing 1,2-diaminopropane alcohols from aziridines |
-
2000
- 2000-08-10 KR KR1020000046394A patent/KR20000063913A/en active Pending
-
2001
- 2001-08-10 WO PCT/KR2001/001362 patent/WO2002012185A1/en not_active Ceased
- 2001-08-10 AU AU2001277801A patent/AU2001277801A1/en not_active Abandoned
- 2001-08-10 KR KR1020037001904A patent/KR20030029816A/en not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3957823A (en) * | 1972-04-15 | 1976-05-18 | Badische Anilin- & Soda-Fabrik Aktiengesellschaft | Electrophilic substitution of nitrosamines |
| US4040838A (en) * | 1975-03-05 | 1977-08-09 | Fuji Photo Film Co., Ltd. | Processing color photographic materials |
| JPH01228946A (en) * | 1988-03-09 | 1989-09-12 | Suntory Ltd | Synthesis of beta-hydroxyphenetylamines |
| EP0736509A2 (en) * | 1995-04-07 | 1996-10-09 | Sumitomo Chemical Company, Limited | Processes for preparing optically active alcohols and optically active amines |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20000063913A (en) * | 2000-08-10 | 2000-11-06 | 하현준 | Process for preparing 1,2-diaminopropane alcohols from aziridines |
| US8513288B2 (en) | 2005-01-26 | 2013-08-20 | Allergan, Inc. | 1-aryl-1-hydroxy-2,3-diamino-propyl amines, 1-heteroaryl-1-hydroxy-2,3-diamino-propyl amines and related compounds having analgesic and/or immuno stimulant activity |
| US8013000B2 (en) | 2005-01-26 | 2011-09-06 | Allergan, Inc. | 3-heteroaryl-3-hydroxy-2-amino-propyl amines and related compounds having analgesic and/or immuno stimlant activity |
| US8153666B2 (en) | 2005-01-26 | 2012-04-10 | Allergan, Inc. | Compounds having analgesic and/or immunostimulant activity |
| EP2489658A1 (en) | 2005-01-26 | 2012-08-22 | Allergan, Inc. | 3-Heteroaryl-3-hydroxy-2-amino-propyl amines and related compounds having analgesic and/or immuno stimulant activity |
| US8288556B2 (en) | 2005-01-26 | 2012-10-16 | Allergan, Inc. | 3-aryl-3-hydroxy-2-amino-propionic acid amides, 3-heteroaryl-3-hydroxy-2-amino-propionic acid amides and related compounds having analgesic and/or immuno stimulant activity |
| EP2198864A1 (en) | 2005-01-26 | 2010-06-23 | Allergan, Inc. | Pharmaceutical compositions having an analgesic effect and containing 1-benzyl-1-hydroxy-2,3-diamino-propyl amines, 3-benzyl-3-hydroxy-2-amino propionic acid amides or related compounds |
| US8835463B2 (en) | 2005-01-26 | 2014-09-16 | Allergan, Inc. | Compounds having analgesic and/or immunostimulant activity |
| US8927589B2 (en) | 2005-01-26 | 2015-01-06 | Allergan, Inc. | 3-aryl-3-hydroxy-2-amino-propionic acid amides, 3-heteroaryl-3-hydroxy-2-aminopropionic acid amides and related compounds having analgesic and/or immuno stimulant activity |
| US9278943B2 (en) | 2005-01-26 | 2016-03-08 | Exonhit Therapeutics Sa | Methods of using as analgesics 1-benzyl-1-hydroxy-2, 3-diamino-propyl amines, 3-benzyl-3-hydroxy-2-amino-propionic acid amides and related compounds |
| US9399628B2 (en) | 2005-01-26 | 2016-07-26 | Allergan, Inc. | 1-aryl-1-hydroxy-2,3-diamino-propyl amines, 1-heteroaryl-1-hydroxy-2,3-diamino-propyl amines and related compounds having analgesic and/or immuno stimulant activity |
| US9828349B2 (en) | 2005-01-26 | 2017-11-28 | Exonhit Therapeutics Sa | 1-aryl-1-hydroxy-2,3-diamino-propyl amines, 1-heteroaryl-1-hydroxy-2,3-diamino-propyl amines and related compounds having analgesic and/or immuno stimulant activity |
| US9314466B2 (en) | 2007-03-06 | 2016-04-19 | Allergan, Inc. | Methods for treating cognitive disorders using 1-benzyl-1-hydroxy-2,3-diamino-propyl amines, 3-benzyl-3-hydroxy-2-amino-propionic acid amides and related compounds |
| CN103910655A (en) * | 2014-04-11 | 2014-07-09 | 太原理工大学 | Ring opening method of aziridine compounds |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20000063913A (en) | 2000-11-06 |
| KR20030029816A (en) | 2003-04-16 |
| AU2001277801A1 (en) | 2002-02-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8962839B2 (en) | Chiral spiro-pyridylamidophosphine ligand compound, synthesis method therefor and application thereof | |
| US20240246991A1 (en) | Manufacture of compounds and compositions for inhibiting the activity of shp2 | |
| US9718795B2 (en) | 1,4-cyclohexylamine derivatives and processes for the preparation thereof | |
| US7307091B2 (en) | Deuterated 3-piperidinopropiophenone and medicaments containing said compounds | |
| JP4915019B2 (en) | Binaphthol derivative and optical resolution and conversion method | |
| EP3372597A1 (en) | Method for preparing oxazolidinone intermediate | |
| EP3725765A1 (en) | A process for the preparation of enantiomerically pure norepinephrine | |
| JPWO2003097632A1 (en) | Propanolamine derivative, method for producing 3-N-methylamino-1- (2-thienyl) -1-propanol, and method for producing propanolamine derivative | |
| WO2002012185A1 (en) | Process for preparing 2,3-diaminopropanols and synthesis of other compounds using 2,3-diaminopropanols | |
| US20040242887A1 (en) | Deuterated n-substituted and alpha-substituted diphenylalkoxy acetic acid amino alkyl esters and medicaments containing these compounds | |
| CN104910158B (en) | 5,6,7,8-tetrahydropyrido[3,4-d] pyrimidine compound with bioactivity as well as preparation method and application thereof | |
| CA2702605C (en) | Unprotected amino aldehydes and applications for same | |
| Liu et al. | Ternary Aldehyde–Copper–Iridium Catalysis Enables Stereodivergent Allylation via α‐C‐H Functionalization of Primary Amines | |
| CN110885292A (en) | Synthetic method of β-amino alcohol compounds | |
| CN110283103B (en) | Method for synthesizing α amino acid ester/amide by base-catalyzed decarboxylation amination | |
| US20190345163A1 (en) | Processes for the Preparation of Ribociclib and Intermediates Thereof | |
| CN110963959B (en) | Preparation method for synthesizing N-protected and unprotected 3-hydroxy-4, 4-dimethylpiperidine | |
| CN111943945A (en) | A kind of Suwo Lexan intermediate and preparation method thereof | |
| CN111269147A (en) | A kind of chiral phosphine nitrogen phosphine ligand and chiral metal organic coordination complex and its application | |
| CN107459524B (en) | A kind of diazoxide ring spiro diketopiperazine skeleton class compound and construction method thereof | |
| CN112939849B (en) | (S, S) -2, 8-diazabicyclo [4.3.0] nonane intermediate and preparation method and application thereof | |
| US4045560A (en) | 2-Morpholine containing methano or ethano anthracene compounds | |
| JP5384353B2 (en) | Method for synthesizing aryloxypropylamine and heteroaryloxypropylamine | |
| CN112679363A (en) | Method for preparing pentazocine intermediate | |
| EP3411355B1 (en) | Process for the preparation of trans-4-amino-1-cyclohexanecarboxylic acid |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CO CR CU CZ DE DK DM DZ EC EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG US UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GQ GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 1020037001904 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020037001904 Country of ref document: KR |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| 32PN | Ep: public notification in the ep bulletin as address of the adressee cannot be established |
Free format text: COMMUNICATION PURSANT TO RULE 69 (EPO FORM 1205A) OF 20-06-03 |
|
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: JP |
|
| WWR | Wipo information: refused in national office |
Ref document number: 1020037001904 Country of ref document: KR |