SUPPLEMENTARY DRINKS USED IN THE DIAGNOSIS OF DIABETES
FIELD OF THE INVENTION
The present invention relates to supplementary solution used in the diagnosis of diabetes and more particularly, the composition of said solution used in the diagnosis by means of oral medication which provides a varietv of flavors and tastes sufficient to appeal to most subjects, minimizes the usual unwillingness for the test, prevents false diagnoses, and leads more accurate and precise diagnostic results by maintaining a standardized glucose concentration during the oral glucose tolerance tests.
BACKGROUND OF THE INVENTION
In general, diabetes mellitus is a chronic disorder in which glucose is excreted in urine when renal tubules are not able to reabsorb glucose as the plasma glucose level becomes higher than the normal due to none or insufficient insulin secretion or abnormal insulin receptors. If this condition is neglected to proceed, in short-term, it could develop into a diabetic coma, a so-called diabetic acidosis. In long-term, it could also lead to complications such as diabetic micro and macrovascular disorders such as diabetic neuropathy, retinopathy, and nephropathy. Therefore, diabetes mellitus requires an accurate and effective diagnostic method and treatment plan.
The fasting blood glucose test or oral glucose tolerance test is being used for diagnosis of diabetes. In particular, the oral glucose tolerance test is used for confirmation of diabetes or pregnancv-induced diabetes since it has advantages of measuring the metabolic change of glucose during the given time, and these values are used for an accurate diagnosis of diabetes mellitus. For the oral glucose tolerance test, the following cπteπas are used to diagnosis of
dιabetes_melhtus: a) WHO or NDDG of USA criteria are being used, aftei collecting the venous blood at fasting and 30 minute intervals for 2 hours aftei taking a 75 g dextrose diagnostic solution within 5 minutes after 8-14 hour fasting; and b) diagnosis based on the fasting and 2 hours blood sugar levels after glucose intake.
Conventionally, dextrose ringer solution or sugar water were used to be mixed arbitrarily in hospitals and were supplied to subjects. Since the solution thus obtained had a uniform taste and inaccurate glucose concentration, it was difficult to be manufactured into a single product. However, most of solutions described above were carbonated water with a relatively high concentration and strong taste, leading to a serious rejection from the subject due to side effects such as nausea, vomiting, and headaches.
Therefore, subjects suffered seriously from the test, and in some cases, the subjects did not even drink the solution at all or selectively drank only a small amount, which resulted an inaccurate diagnosis within and among individuals. Also, a certain amount of glucose tolerance measures required for the test was not sufficient to reflect the accuracy of diagnosis.
The above result was caused by the use of diabetes diagnostic solutions that were not suitable for the physical state and taste. Thus, diabetes diagnostics supplementary solution could be readily used upon selection of subjects without any rejection should be developed.
Among the requirements described above, the present invention particularly relates to manufacturing reliable supplementary drinks and their use to minimize the unwillingness from the subject for the test. Said soluhon assists in the diagnostic process as a suitable solution is selected for the oral glucose tolerance test from a variety of solutions (e.g., different concentrations
and quantities), provided with a considerahon that the d iagnostic method depends on the age or characteristic of the subject.
In other words, the soluhon is used to produce the optimal test results in that it gets to satisfy the standardized condition and suitable for the test as the subjects to be diagnosed.
SUMMARY OF THE INVENTION
An object of the present invention is to provide supplementary soluhon for the oral glucose tolerance test with an improved function, which vs ould prevent the unw illingness from the subject and misdiagnoses b\ adding various flavors and tastes and maintaining the sugar concentration and a unit cost of manufacture based on the international standard diagnostic method.
To accomplish the objectives described above, the present invention is characterized by a composition comprising 20 to 80% dextrose concentration, 0.1 to g monohydrate form citric acid, 0.1 to lg sodium benzoate, 0.01 to 1 °o flavoring agent, O.OOlg or more of food dye, and an adequate amount of water in a 150ml standard solution, to be utilized as a diagnostic soluhon for the oral glucose tolerance test.
Brief Description of the Drawings
Fig. 1, which describes the results of the animal test, is a graph representing the change of the blood sugar levels measured for 2 hours after oral administration of the diagnostic soluhon.
Fig. 2, which illustrates the results of the animal test, is a bar graph representing the area under the curve on dextrose measured for 2 hours aftei oral administration of the diagnoshc soluhon.
Fig. 3, which indicates the results of the clinical test, is a graph
\ represenhng the change of the blood sugar levels measured for 2 hours after oral administration of the diagnostic soluhon.
Fig. 4, which shows the results of the clinical test, is a bar graph represenhng the area under the curve on dextrose measured for 2 hours aftei oral administration of the diagnostic soluhon
Preferred Embodiments of the Invention
The present invenhon is described in more detail hereunder.
The chemical ingredients of each composition used in manufacturing supplementary drinks in the present invenhon are as follows.
Dextrose, which is D-glucose having chemical formula of ChHi2On has the molecular weight of 180.16 and is composed ot 40.00% of C, 6.72% of H, 53.29% of O.
A sour tashng agent (monohydrate form citric acid), which has chemical names of 2-hydroxy-l,2,3,-propanetrιcarboxylιc acid or β -hydroxyrricarballyic acid having the chemical formula of C6-H-O7, has molecular weight of 192.12 and is composed of 37.51% of C, 4.20% of H, 58.29% of O. Conventionally, the biggest problem upon administering the drinks for the oral glucose tolerance test was a nauseous feeling due to high dextrose concentration. In the present invenhon a sour flavor of monohydrate form citric acid is used to prevent such nauseous feeling by adding a monohydrate form citric acid to a soluhon with the range of 0.1 to 0.3% of the total soluhon with optimal effect on the subjects, the most preferably 0.2% .
Sodium benzoate, which has the chemical formula of CzHsNaO with molecular weight of 144.11, is composed of 58.34% of C, 3.50% of H, 15 96% ot Na, 22.21% of O. Sodium benzoate, with the range of 0.02-0.04% of the total soluhon, is also added to be functioned as a preservative for long-term storage
,) and stability.
It is preferred that water provided as carbonated water or d istilled water is provided particularly in the form of CH2O.
A food dye is selected from the group consisting of Red no. 40, Blue no- .1 I and Yellow no. 4, and a quantity added is variable depending on the preference of the subjects. Also, dyes may not be added separately but may be substituted by one of flavoring agents containing a food dye.
For flavoring agents, samples that are suitable for the taste of the subjects and that can rjroduce both tastes and flavors to give a variety to the 10 product should be used.
The tastes and flavors of the supplementary solution manufactured by the present invention can be made in a tremendous variety considering tastes.
The present invention is illustrating citron, lemon/lime, DoonggeuUe
(Pohjgonntuiii odorπtum), coffee, scorched rice tea, green tea, and strawberry
1.1 flavor for convenience; however, it is not necessarily limited to said drinks.
In the present invention, an amount of the flavoring agents is in the range of 0.01 to 1 %, and kinds and amount of flavoring agents are: α) citron 0.814 ± 0.01 citron oil 1.0%
20 ethyl alcohol 99.0%
(2) lemon/ lime 0.823±0.01 lemon oil 1.0% lime distilled oil 0.5% ethyl alcohol 98.5%
(3) DoonggeuUe (Polygoπatiiiii odorπtnm) 0.887±0.01 ethyl acetate 0.05 '•>^% acetic acid 0.03'
furfural o.o.
5-methyl furfural 0.03%
Fenugreek solid extract 0.2% vanilla extract 0.2% propylene glycol 31.54% ethyl alcohol 67.9%
(4) coffee 0.816 ± 0.01 furfural 0.05% furfuryl alcohol 0.02%
10 altol 0.03% ethyl maltol 0.01% vanillin 0.01% propylene glycol 4.88% ethyl alcohol 95.00% i .l (5 5)) scorched rice tea 1.025 ± 0.01 furfural 0.05% methyl cvclopentenolone 0.02% benzyl alcohol 0.04% acetone 0.01%
20 sulfurol 0.03% ethyl alcohol 4.53% propylene glycol 95.32%
6) green tea 0.857% ±0.01 cis-3-hexenol 0.12% linalool 0.33% linalool oxide 0.06% alpha-terpineol 0.27%
geraniol 0.36% propylene glycol 20.00% ethyl alcohol 78.86%
(7) strawberry 0.895 ± 0.01 ethyl acetate 0.17% ethyl butvrate 0.26% ethyl caproate 0.17% hexyl acetate 0.54% tians-2-hexenol 0.03% ethyl isovalerate 0.07% ethyl maltol 0.02% gamma-decalactone 0.01% propylene glycol 34% ethyl alcohol 64.73% The supplementary drinks in the present invenhon needs to be stored in a dry, dark place and requires refπgerahon before use.
The precauhon before use is to fast for 10 to 14 hours without having any food, beverages, and smoking before the test.
The present invenhon is illustrated in details in the following examples, but is not limited by these examples.
Example 1: Dextrose content and dissolving temperature, and minimum amount of solvent for complete dissolution
Each example to reproduce the present invenhon is performed identically, regardless of wt. % of dextrose solute, and a content of dextrose is in the range of 10 to 90%, depending on an amount of the solvent or need upon diagnosis of diabetes.
Also, contents to dissolve dextrose in the solvent when manufacturing the present invention are from ranges of 10% to 90% . However, at example of the mixture only 10%, 25%, 40%, 50%, 75%, and 90% methods are presented.
Table 1 shows a minimum amount of solvent (water) required to completely dissolve dextrose at different contents at each temperature
Table 1 : Dextrose content and dissolving temperature, and minimum amount of solvent (water) for complete dissolution
Example 2: Manufacturing supplementary drinks containing 50% dextrose
The manufacturing method based on a 50% dextrose supplementary drink (75g dextrose in 150ml solution) is as follows:
When 50 °C distilled water was used to make 25 °C-90°C distilled water,
75g of dextrose, for example, was added into 80ml distilled water to be dissolved completely.
Then the powder forms of 0.3g of monohydrate form citric acid (the range of the content when manufacturing is 0.1 to lg), O.Olg of sodium benzoate
(the range of the content when manufacturing is 0.001 to 0.06g) and O.Olg of food dye (the range of the content when manufacturing is 0.001 to O.lg) were added and dissolved completely.
After the solution was cooled to room temperature, 0.185ml(0.1 %) (the 1 range of content when manufacturing is 0.01 to 5%) of the flavoring agent was added, and distilled water at room temperature, carbonated water or purified water was added to make up the solution to the total volume of 150ml.
Example 3: Manufacturing method for 50% dextrose supplementary drinks 10 containing 50g, 75g, lOOg dextrose
The optimal amount of dextrose to be used to manufacture supplementary drinks of 50g, 75g, lOOg contents depending on the differences of the subjects was 225g, and that of distilled water was 450ml.
An amount of distilled water to dissolve 225g of dextrose at 50 °C was 1.1 92.25ml, and in some cases, more than 92.25ml at 50 °C was used; however, the total volume of the solution should not exceed 450ml after 225g of dextrose was dissolved.
After 200ml of distilled water is sufficiently heated to 50 °C, followed by addition of 225g dextrose, the solution was stirred to be dissolved completely. 20 After complete dissolution, 0.9g of monohydrate form citric acid (the adding range when manufacturing is 0.05 to 0.5%), O.Olg of sodium benzoate (the adding range when manufacturing is 0.001 to 0.06%) and 0.03g of food dye (the adding range when manufacturing is 0.001 to O.lg) were added and dissolved completely.
'>{ Then, said solution was cooled to room temperature, followed by addition of 0.554ml(0.1%) (the adding range when manufacturing is 0.01 to 5%) of a flavoring agent, and distilled, carbonated or purified water were also
added to make the solution up to 450ml. The total 450ml solution was then put and sealed in bottles of 100ml(50g), 150ml(75g) and 200ml(100g) to produce three kinds of testing solutions.
An amount and a range of ingredients depending on dextrose concentrations are shown in Table 2 below.
Table 2 : The amount of the ingredients depending on dextrose concentrations
10 Example 4: Food dyes
The purpose of adding a food dye was to distinguish the colors corresponding to flavors of each drink and to choose freely with visible appreciation and classification. citron Blue no. 1 + Yellow no. 4
1.1 lemon/ lime Blue no. 1 + Yellow no.4
DoonggeuUe (Po jgonatuni odoratuυή Yellow no. 4
coffee Red no. 40 + Yellow no. 4 scorched rice tea Yellow no. 4 + Red no. 40 green tea Blue no. 1 + Yellow no. 4 strawberry Red no. 40 + Yellow no. 4
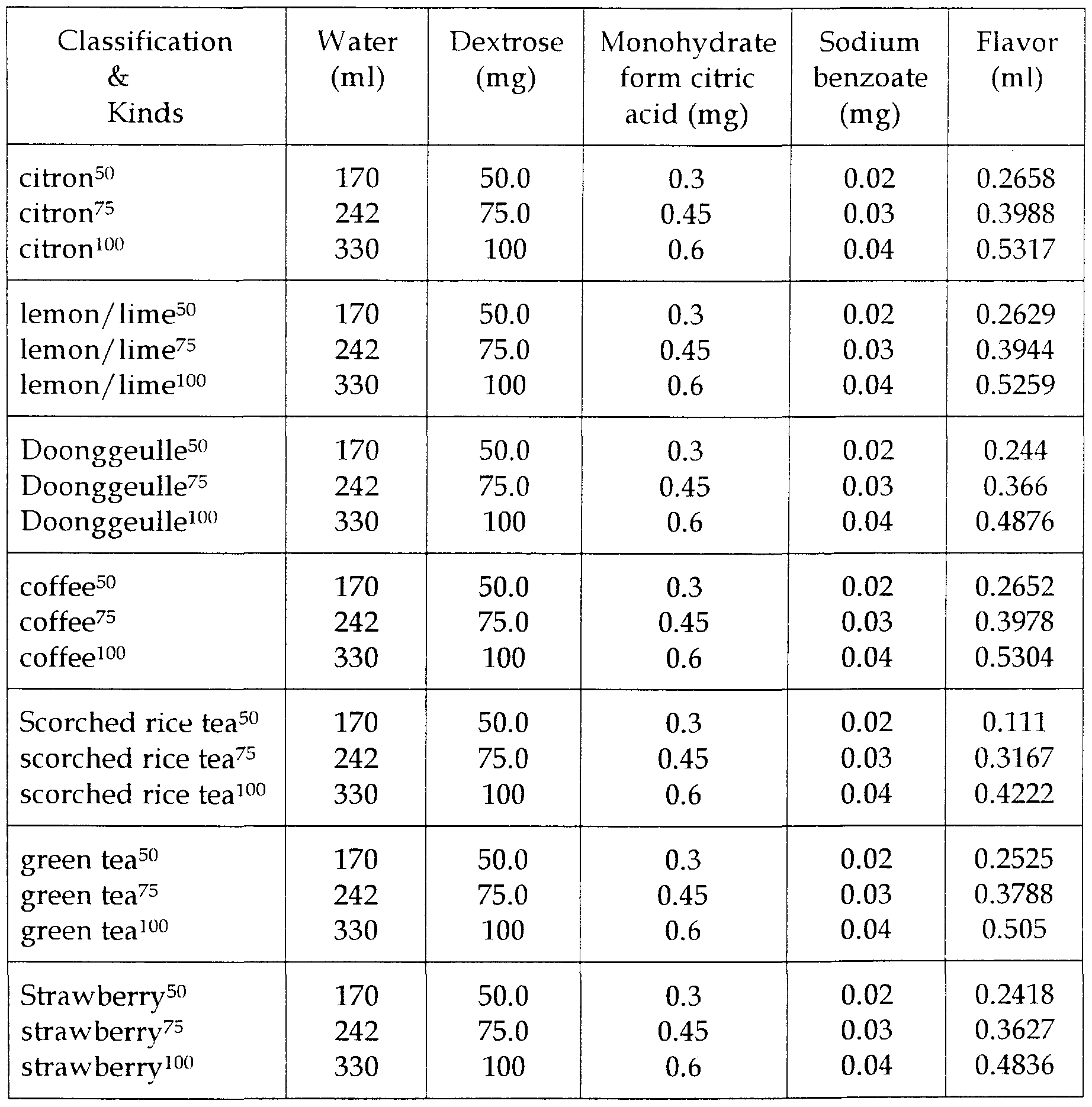
Tables 3-8 describes the optimal amounts of the ingredients that can be added in each solution when manufacturing the supplementary drinks with dextrose concentrations varied from 10% to 90% in the present invention.
The superscripts attached to the added flavoring agents represent the amount of dextrose required for the oral glucose tolerance test. For example, "strawberry100" refers to the one selected by the subject who likes the strawberry flavor and needs to take lOOg of dextrose for the glucose tolerance test.
Table 3 : 10% dextrose solution composition
Table 4 : 25% dextrose solution composition
Table 5 40' /o dextrose solution composition
1.1
Table 6 : 50% dextrose solution composition
lb
Table 7 : 75% dextrose solution composition
Table 8 : 90% Dextrose Solution Composition
Example 5: The amount of dextrose intake for diagnosis by the oral glucose tolerance test
Screening test for pregnancy-induced diabetes: 50g intake (see Table 9)
Test for diabetes (adults): 75g intake (see Table 9)
Test for pregnancy-induced diabetes: lOOg intake (see Table 9)
Test for diabetes (pediatric): 1.75g dextrose/ kg bodv weight (no more
than 75g)
55.5ml to 500ml for 50g test, 83.33ml to 750ml for 75g test; and 111.1ml to 1000ml for lOOg test was taken.
Table 9. Dextrose content and the optimal volume of intake for the glucose tolerance test
Example 6: Animal test and Clinical test To find out the diagnostic accuracy of the supplementary drinks in the present invenhon and the convenhonal dextrose diagnostic soluhon (Scientific, NJ, USA) for the glucose tolerance test, animal and clinical tests were performed to be compared and analyzed using the GI (gastrointestinal) glucose kinetic-Curve on blood sugar level change after taking each diagnostic soluhon for the oral glucose tolerance test Also, convenience, preference or side effects such as nausea and vomiting of the subjects were compared and evaluated in a pre-chnical study.
(1) Animal test
The convenhonal 25% dextrose diagnostic solution (dextrose 75g in the volume of 296ml; "Criterion", Scientific, NJ, USA) and the 50% dextrose solution (dextrose 75g in the volume of 150ml; "Diasol-S") in the present invention were administered orally into 15 male mice (Sprague-Dawely, about 250g), and the blood sugar levels were compared.
The 15 mice were allowed to adapt to the environment of the animal room for 3 days after the purchase From the fourth day, 5 mice were fasted for 12-14 hours daily and approximately 0.2ml of blood was collected from the tail vein around 8 a.m. next morning. Right after the fasting blood collection, the solution (50% Diasol-S) of 2g dextrose/kg body w t. was administered oralh using syringes into the mice. The blood was collected from the tail vein at 30, 60, 90 and 120 minutes after oral administration. During the blood collection, the blood vessels in the tail were catheteπzed, and the collected blood was centrifuged at 2500 rpm for 15 minutes to separate the plasma. The blood sugar levels were measured from the plasma by the glucose oxidase method using YSI 2300-STAT (Yellow Springs Instrument Co., Ohio, USA). The tested mice were allowed to rest for 3 days, and then the second test was performed. The second test was done identically as the first test, using the convenhonal diabetes diagnoshc solution (25% Criterion) on the mice used in the first test. The weight change of the mice during the first and second tests was about an average 15g. The blood sugar levels of the mice that took the 50% dextrose solution (Diasol-S) in the present invenhon at fashng were relatively lower than the levels of the mice that took 25% soluhon (Criterion), while the blood sugar levels at 30, 60 and 120 minutes appeared higher in 50% solution, but there was no statistical difference (Fig. 1). Also, the area under the curve on dextrose was higher in the 50% diagnoshc soluhon than the 25% solution, but there was no stahshcal difference (Fig. 2).
The highest blood sugar level in both 25% and 50% diagnoshc solutions among the altered levels after the 2-hours oral glucose tolerance test appeared 1 hour after administration of the soluhon. The blood sugar levels obtained from the two groups were evaluated from the graph on blood sugar level using the "repeated measures analysis", which produced no statistically significant difference (Fig. 1).
(2) Clinical test
In the clinical study, the conventional 25% dextrose diagnostic soluhon (75g dextrose in 296ml soluhon, "Criterion") and the 50% dextrose diagnostic solution (75g dextrose in 150ml, "Diasol-S") were administered into 15 health) volunteers (3 males and 12 females) without any chronic disease. And then the side effects due to the sugar metabolism and the use of the solution with a high concentration were evaluated. The two kinds of solutions were used for consecutive oral glucose tolerance tests for 2 days. 50% dextrose diagnostic solution (Diasol-S) was used on the first day while 25% diagnostic solution was used on the second day, and the results from the tests were compared and analyzed. The blood from the subjects who participated on the first day was collected during 8-14 hours of fashng. Within 5 minutes after the collection, the concentrated 50% dextrose solution was slowly administered into the subjects, and 1ml of the blood was collected from a vein of each subject at 30, 60, 90 and 120 minutes. The collected blood was transferred into a 5ml NaF tube and centrifuged at 3000rpm for 3 minutes. The blood sugar was measured by the glucose oxidase method using YSI 2300-STAT (Yellow Springs Instrument Co., Ohio, USA). On the second day, the same subjects were allowed to fast for 8-14 hours, and their blood was collected at the same time as the first day. The convenhonal 25% dextrose diagnoshc soluhon (Criterion) was administered
into the subjects within 5 minutes after the collection, and then the blood was collected from the vein at 30, 60, 90 and 120 minutes. The amount of the collected blood and the method of analysis used were identical as the ones performed on the first day.
(3) Statistical analysis
SPSS for Windows version 7.5 was used for the statistical verification, and paired t-test, ANONA and chi-square test were used to evaluate the statistical significance between two groups. The significance level was p<0.05. The blood sugar levels at different times (fashng, 30, 60, 90 and 120 min.) were divided into 25% and 50% dextrose solutions to be compared and analyzed using paired t-test. Also in the present invention, the graph on blood sugar level change with time after the administration of 25% and 50% solutions was analyzed. Since the first and second tests were performed for 2 consecuhve days, the second measurements after the first one were done in 24 hours. The fashng state of the subjects and the hme spent for the oral glucose tolerance test were to be performed at the same time as the previous day; however, there was 1-hour difference for the mean fashng hours. The mean fasting hours (average 13 hours) of the subjects on the first day was 1 hour longer than the second day (average 12 hours), but there was no statistical significance (p=0.254). The blood sugar levels measured at fashng, 60, 90 and 120 minutes except for the one measured at 30 minutes after administration of the solution appeared higher in 50% diagnoshc soluhon than 25% soluhon, but there was no statistical difference (Fig. 3). The area under the curve on dextrose also was higher on administration of 50% soluhon, but there was no statistical significance (Fig. 4).
The graphs on blood sugar level change after the 2-hour oral glucose
tolerance test were somewhat different in animal and clinical tests. Whereas the highest blood sugar level appeared 1 hour after the glucose tolerance test in the animal study, it appeared 30 minutes after the test in the clinical study. However, there was no statistically significant difference between two groups.
Also, the preference of the subjects on 25% and 50% diagnostic solutions were evaluated in the clinical study, and the results are listed in Table 10 hereunder. Table 10.
The unwillingness of the subjects due to the amount of the solution intake was 33.3% for 50% solution (150ml) and 90% for 25% solution (296ml), indicating 3 times rejection rates in the 25% solution when compared to the 50% solution (p<0.05). The degrees of dextrose concentration, thirsty, nausea, stomach pain and headache between two diagnostic solutions were very similar.
2Λ Furthermore, the vomihng caused by nauseous feeling was 6.7% (1 person) in the 25% solution but no such episode occurred in the 50% soluhon The subject who could not tolerate the test indicated that the excess intake of the soluhon was a heavy burden.
Thus, the present invenhon, which contains tastes and flavors, allows subjects to use it in the diagnosis of diabetes without unwillingness for the test, thereby enabling to maintain the ophmal physical condition upon testing and at the same time to provide the supplementary drinks that can increase the reliability of the diabehc test.
Although the present invenhon presented its best embodiments, it was not necessarily limited to them, and thus considering the possibility of alterations of the embodiments described above if the present invention could be performed withm the scope of the attached claims.