METHOD AND DEVICE FOR COUNTING AND RECORDING FETAL MOVEMENT
Description
1. Cross Reference to Related Applications This application claims priority under 35 U.S.C. §119(e) to commonly-owned, co-pending U.S. Provisional Patent Application Serial No. 60/080,713, entitled DEVICE FOR COUNTING AND RECORDING FETAL MOVEMENT, filed April 3, 1998 , by Peter L. Rosenblatt, which is incorporated herein by reference in its entirety.
2. Technical Field The invention relates generally to a device and method for counting and recording fetal movement, and more particularly to a device which automatically prompts a pregnant woman to count fetal movement, registers each movement perceived by the mother over a predetermined amount of time and notifies the pregnant woman if an adequate number of movements have been registered during the predetermined amount of time.
3. Background of Related Art It is well established that maternal assessment of fetal activity, or so called "kick-count" protocols, are a simple, effective and reliable method of monitoring fetal well-being in high-risk pregnancies. (As used herein the term "kick-count" protocol refers to any protocol for maternal assessment of fetal activity or movement). More recently, it has been demonstrated that these kick-count protocols can lower antepartum stillbirth rates in low-risk pregnancies as well. Studies using real-time ultrasonography have shown that in the third trimester the human fetus spends approximately ten percent of its time making gross body
-2-
movements, and that the average fetus moves thirty times each hour. However, most women are able to perceive only 70 to 80 percent of such gross body movements. Factors that may reduce maternal perception of gross fetal movements include maternal obesity, anterior placentation, and both polyhudramnios as well as oligohydramnios. In addition, several medications, including narcotics and barbiturates, may reduce fetal activity. Contrary to popular belief, fetal activity does not increase following meals, but rather is enhanced by maternal hypoglycemia. Fetal activity tends to peak in the late evening, between 9:00 p.m. and 1 :00 a.m., which correlates with declining maternal glucose levels. Fetal movement appears to be strongly correlated with fetal oxygenation as well. Using a sheep model, Natale et al. demonstrated a significant decline in fetal activity associated with minor reductions in fetal Po2. The ability of kick counting protocols to predict fetal compromise is based upon this phenomenon of decreased fetal activity resulting from hypoxemia. Maternal monitoring of fetal movements in the third trimester of pregnancy has been advocated for all pregnancies. Although there is no single accepted protocol, several methods for monitoring fetal movements have been described in literature. One of the most popular methods, described by Pearson and Weaver, involves the use of the Cardiff Count to Ten chart. The patient is asked to count fetal movements each day and record how much time it takes to perceive ten movements. In this study, patients were asked to contact their obstetricians if 12 hours had passed before ten movements were perceived, or if the time to reach the tenth movement increased each day. Sadovsky et al. described an alternate method whereby patients count fetal movement for 30 to 60 minutes, two or three times each day. If less than ten movements are counted in 12 hours (the "movement alarm signal"), the patient is instructed to contact her physician for further fetal evaluation. In another method, Rayburn et al. recommended counting fetal movements for 60 minutes each day and suggested that less than three movements an hour for two consecutive days may indicate fetal distress. A study conducted by Sadovsky et al. which evaluated the
-3-
various techniques used for kick counts found the "movement alarm signal" and the "Count to Ten" methods to be the most valuable. More recently, an alternative protocol for kick counting was described by Moore and Piacquadio to accommodate the "busy life-style of the contemporary pregnant woman." Starting at 28 weeks gestation, women were asked to record the time required to appreciate ten fetal movements (defined as "any discrete kick, flutter, swish, or roll"), while lying on their left side. Women were instructed to perform this kick counting on a daily basis, but exclusively in the evening hours, between aboui 7 p.m. - 11 p.m., when fetal activity is most pronounced. If two hours elapsed before noting the ten movements, women were instructed to go to labor and delivery for an evaluation. During their prospective study, a total of 4,383 deliveries occurred (2,519 during a control period without formal kick counting and 1,864 during the study period with the new protocol). They found that this simplified protocol resulted in a significant drop in fetal mortality from 8.7 to 2.1 per 1 ,000 deliveries. Among those women who presented with decreased fetal movement, interventions for fetal compromise resulted in a reduction in fetal mortality from 44 to 10 per 1,000. In this study, the mean time to perceive ten fetal movements was only 20.9 + 18.1 min (mean + Standard Deviation). Furthermore, after 90 minutes, 99.5% of women perceived ten movements. Therefore, using a cutoff time of 2 hours, only 1 in 500 women were found to ever exceed this upper time limit and require additional testing. As the drop in fetal mortality in this study confirms, this 2 hour cutoff time appropriately selects those patients at significant risk. Instructing women in fetal kick counting did result in an overall increase in antenatal testing (e.g. nonstress test, contraction stress test) of 13%, although the drastic reduction in fetal mortality certainly justifies this modest increase in antenatal testing. The efficacy of "kick count" protocols in decreasing antenatal stillbirth has been well documented in a number of large clinical trials. In addition to Moore and Piacquadio, Nedlam conducted a prospective, randomized study in which 1,562 women at 32 weeks gestation were asked to count fetal activity 3 times a week for 2
hours after a meal. Patients who noted less than 3 movements each hour were further evaluated with ultrasound and nonstress testing. Only one stillbirth occurred in the monitored group, compared with ten stillbirths in the control group of 1 ,549 women. This study clearly demonstrates the sensitivity of this kick counting technique in selecting those patients at risk for fetal demise. Of the 4% of women in the monitored group who reported decreased movement, nearly 25% were confirmed to have fetal distress based on further testing. These investigators attributed the prevention of 14 fetal deaths in the monitored group to the use of this simple technique. Rayburn reported that when using his method (described above) of the 5% of the women who reported decreased fetal activity, the incidence of stillbirth was 60 times higher, and the incidence of severe intrauterine growth retardation and low Apgar scores were 10 times higher than for women who reported normal fetal activity. In view of the foregoing, it is apparent that accurately and consistently recording fetal activity in the third trimester can forewarn of fetal distress and help prevent antenatal still births. Of course, for any "kick count" protocol to be effective, the pregnant woman must accurately follow the prescribed protocol, report any deviation to her obstetrician, and follow-up with further testing, such as a nonstress test. However, compliance rates for fetal kick counting have been reported to range from 50% to 83%, depending upon several factors, such as the study population and the complexity and length of the kick counting protocol. Clark and Britton described factors which contribute to patient noncompliance with the Cardiff Count to Ten protocol. They reported that 27% of the patients filled out their charts less than 50% of the time and that 65% of the subjects who experienced movement alarm signals did not notify their care providers. In suggesting possible explanations for this poor compliance, they noted that more than a third of patients reported that their care providers never asked about their charts at subsequent prenatal visits. In addition, 82% of patients were unclear on how to record their interval times when not starting the counting at 9 a.m. Another 23% of patients did not know how to contact their care provider if they had a movement alarm signal. Additionally, difficulty in marking the
chart was reported in 16% of the subjects. These authors conclude that strategies for improving patient compliance need to be developed in order to make fetal kick counting more beneficial. It is therefore an object of the present invention to provide a device for simplifying administration and compliance with any of the various kick-count protocols. It is another object of the present invention to provide a device which automatically prompts a pregnant woman to count fetal movement and notifies the pregnant woman if an adequate number of movements have, or have not been counted during a predetermined amount of time. It is a further object of the present invention to provide a method of fetal monitoring which records fetal movement data during a predetermined time interval, compares the data to a target index of fetal movement and notifies the patient and/or care provider of the results .
Summary In accordance with the present invention, there is provided an electronic device that registers or records each perceived fetal movement input by a pregnant woman over a predetermined amount of time (depending upon the kick-count protocol utilized). The device is programmed to compare the number of movements input to an index for the particular protocol, in order to determine if an adequate number of movements have been input, and informs the user by a visual and/or auditory cue if an adequate number of movements have not been input. The device may then signal to the patient to contact her obstetrical provider for an evaluation of fetal well-being. In one embodiment, the device is preferably programmable to be utilized with any of the aforementioned kick-count protocols, depending upon the obstetrical providers preference. The device may also be programmed to inform the user if an adequate number of movements have been input, if the fetal movement session has been
-6-
interrupted, if no fetal movements have been input for a predetermined number of days, and to display the results of current and previous sessions. In one embodiment, the device may be programmable so that the patient can input her due date, and may then also function as a counter to indicate how many days remaining until the due date, or how many days have elapsed since the beginning of the pregnancy. If the due date is programmed into the device, then the device may also include information or prompts to the patient based upon the gestational week or date. For example, the device may provide information concerning the fetus, such as the development of sexual organs, and/or the device may provide reminders to the patient such as to practice breathing techniques or Kegel exercises. The device may also take any number of forms, including but not limited to a watch, necklace, electromc personal organizer and may be carried in a woman's pocket, clipped on to clothing (like a beeper) or worn on the woman's clothing or body. In a preferred embodiment, when not in use as a kick counter, the unit may be used as a conventional watch or clock. In use, the care provider chooses which of the programmed protocols he or she wishes the patient to utilize during the third trimester of pregnancy , Once the protocol is selected, the device automatically prompts the patient to begin counting fetal movement at a certain time of day, depending upon the protocol. The user then inputs perceived movements into the device during the predetermined time period for the session, which is again dependent upon the protocol. The device is programmed to count the inputted movements and compare the number of movements to an index for the particular protocol. The device then automatically notifies the user if the session has not been successfully completed (i.e. the number of movements registered compared to the index is not correct). The device may additionally notify the user if the session has been successfully completed (i.e. the number of movements registered compared to the index is correct). The patient may also interrupt the session, if necessary, in which case the device may record the interruption. Upon completion of
-7-
a session the device may revert back to a conventional watch or clock, and/or may display the results of the completed session until the next session is begun.
Brief Description of the Drawings It should be understood that the drawings are provided for the purpose of illustration only and are not intended to define the limits of the invention. The foregoing and other objects and advantages of the embodiments described herein will become apparent with reference to the following detailed description when taken in conjunction with the accompanying drawings in which: Fig. 1 is a front view of a device in accordance with one embodiment of the present invention in a "ready" mode prepared to receive input from the patient for counting fetal movement and configured to be worn as a wrist watch; Fig. 2 is a front view showing the device of Fig. 1 in an "in-use" mode during counting of fetal movement; Fig. 3 is a front view showing the device of Fig. 1 in an "alert" mode instructing the user to contact a physician; Fig. 4 is a front view showing the device of Fig. 1 in a "result" mode displaying the results to the user after receiving input and comparing it to the appropriate index for a given kick-count protocol; Fig. 5 is a front view showing the device of Fig. 1 also in a "result" mode and displaying to the user the number of days past the patient's due date; Fig. 6 is a front view showing the device of Fig. 1 in an "alarm" mode notifying the user that the number of kicks recorded did not meet the protocol standard and to notify her physician; Fig. 7 is a front view showing the device of Fig. 1 in a "pass" mode notifying the user that the number of kicks recorded did meet the protocol standard; Fig. 8 is a front view showing the device of Fig. 1 in an "non-use" mode in which the device displays no results as the patient has not entered any results for the day;
Fig. 9 is a front view showing the device of Fig. 1 in a "cancel" mode in which the input is interrupted by the user; and Fig. 10 is a front view showing the device of Fig. 1 in a "run" or "set" mode in which the device operates as a conventional watch and in which certain variables can be set by the user; Fig. 11 is a schematic block diagram of the device of Fig. 1 ; Fig. 12A is a perspective view of a second embodiment of the device in an off position and configured to be worn as a necklace; Fig. 12B is a front view of the device of Fig. 12A in the "in-use" mode; and Fig. 13 is a front view of a third embodiment showing the device in the "in- use" mode and configured as a personal organizer.
Detailed Description of the Illustrative Embodiments A device 10 for automatically prompting a pregnant woman to input or count perceived fetal movements, and which registers each movement perceived by the woman over a predetermined amount of time is illustrated in Fig. 1. The device preferably includes an input member 12 for actuation by the user to input fetal movements as they occur during a given session, a timer 14 for timing the elapsed time since the beginning of the session, a processor for comparing the number of ietui movements input relative to an index for a given "kick-count" protocol and an indicator or display 16 for conveying information to the user. The input member 12 may be in the form of a button 12 which is used to start the session and which, when depressed by the user, inputs the fetal movements which may then be registered by a counter. The counter may be any conventional counting mechanism which is capable of registering the number of fetal movements input by the user. The counter may be separate from the processor, or may be part of the processor itself. The device is preferably programmable so that the patient's doctor or clinician can chose which of several available "kick-count" protocols to utilize, as described hereinabove, depending upon the doctor's preference. For the illustrated embodiment, the device
-9-
is shown as utilizing the Moore and Piacquadio protocol, in which case the index utilized is a simple value of ten movements within a two hour period. Thus, the processor compares the number of movements input to the index and, for the present embodiment, once ten movements are input within a two hour time period the processor sends a signal that the session has been successfully completed. If, however, ten movements are not input within a two hour time period the processor sends a signal that the session has not been successfully completed and the indicator notifies the user to this effect. The processor which is utilized in the device may be any conventional processor, as would be known to one of skill in the art. The device may further include a second and third input member 20, 22 in order to set or change (increase or decrease) various parameters, such as the time, date and/or estimated due date, or other parameters described in further detail hereinbelow. In the present embodiment, input member 12 is preferably used to select the variable which is being adjusted, such as the due date, while the second and third input member 20, 22 are used to increase and decrease the value of each variable (Fig. 10). The third input member 20 may also function to turn on a back light for visualizing the LCD during the run mode and in low light environments. A toggle member 24 may also be provided to toggle between the available modes, for example the "run" mode which the patient uses for counting fetal movement, and the ' set" mode which is used to program the various parameters such as time, date, due date, etc. All of the input members may be in the form of button, or may take other forms as would be known to one of skill in the art. The device may be initially set by either the clinician or the patient, for example by inputting the correct time and date. In either case, the user may also be required to enter the estimated due date (EDD) once the current date and time are set. The kick counter may then be programmed to begin functioning during the 28th week of gestation until the 45th week of gestation is completed, after which time the device can be programmed to disable itself so that the device will no longer function as a kick counter. The kick counting device may also be turned off (for example in the "set"
-10-
mode) by either the patient or the clinician after the delivery of the baby. In addition, the device may also be programmed to allow the patient/clinician to change the due date only a certain amount of times, for example three, so that the user cannot extend the use of the device beyond the 45th week of gestation. Once disabled for use in counting fetal movement, the device may preferably still function as a conventional watch. Referring now to Fig. 2, the device also includes indicator or display 16 which is preferably utilized to convey various forms of information to the user. In the present embodiment, when utilized to count fetal movements the display preferably includes the current number of fetal movements registered 26 and the elapsed time 28 since the beginning of the session. The display may also display the number of sessions completed during the day, if an alternate protocol other than the Moore Piacquadio protocol is utilized. The display can also display other information of interest, such as the number of days left until the patient's due date 30 (or the days past the due date), information concerning the fetus, for example when sexual organs develop, and/or reminders to the patient, for example to practice breathing techniques or Kegel exercises, if desired. The information of interest can be programmed into the device to be displayed at appropriate times during the pregnancy, depending upon the estimated due date (i.e. a pregnancy calender). The display 16 may also provide various other prompts or signals to the user. For example, the display may prompt the user to begin a session by displaying indicia, such as the words "READY" or "BEGIN COUNT" (FIG. 1), may indicate the successful completion of a session by display indicia, such as the word "OK" (Fig. 7), may indicate the interruption of a session by displaying indicia, such as the word "CANCEL" (Fig. 9), may display a warning to the user if the session is not successfully completed (i.e. the 2 hour time limit is reached before the tenth movement is counted, a.k.a. "movement alarm signal"), such as "ALARM"(Fig. 6) or "CALL YOUR OB/GYN", and may also display a warning if the user has failed to track fetal movements for a predetermined amount of time (for example 5 days), such
-11-
as "CALL YOUR OB/GYN" (Fig. 3) or "CALL YOUR DOC". The non-use warning signal may additionally include some indication which differentiates non-use from the movement alarm signal, such as a predetermined letter, e.g. "F", or a number. In the case of either non-use or an unsuccessful session, the device may be programmed to disable itself until reset either manually by the user or by the doctor or clinician. The device may be reset, for example, by holding down a reset button, or by inputting a code provided by the patient's doctor or clinician. Any of the above visual displays may additionally be accompanied by an audible indication, such as a beep or buzz, or may alternately be replaced by the audible indication. For example, the "ALARM" indicia may be accompanied by an audible alarm in the form of two double beeps or may be replaced by the audible alarm. The display may be a liquid crystal display (LCD), LED, or similar display, as would be known to one of skill in the art. In a preferred embodiment, the device 10 stores the previous day's results, which can be easily accessed by the clinician or the patient. For example, the previous day's results may be accessed by holding down the input member 12 between 12 midnight and 6 p.m., i.e. when the device is not being used as a fetal movement counting device under the Moore and Piacquadio protocol (Fig. 5). Alternately, other methods of accessing the previous day's or other previous results may be accessed by known methods, such as by downloading the results to either a stand-alone base unit or through a peripheral device connected to a personal computer. In either case, a printout of the patient's results would be available for placement in the patient's prenatal record. The fetal movement device can store up to about sixteen weeks of patient's results (or as many weeks as appropriate). For the exemplary protocol, the previously recorded results preferably include the date and the elapsed time to reach the tenth movement or, if ten movements were not recorded, then the number of movements recorded and the elapsed time. When not functioning as a fetal movement counting device, the present device may function as a standard watch. For example, as depicted in Fig. 10 the device may display the date, time and any other pertinent information. The device may also take
-12-
any number of forms, including but not limited to a watch, necklace, electronic personal organizer and may be carried in a woman's pocket, clipped on to clothing (like a beeper) or worn on the woman's clothing or body. When utilized as part of a personal organizer, the device may include any number of other option, such as an address book, appointment calender, etc. as would be conventional with an electronic personal organizer. The device may also be programmed, as described above, to display other information of interest and/or reminders to the patient. The present invention offers several advantages, which may make it a valuable tool for both pregnant woman and their obstetricians. First, having a device specifically designed for recording fetal movement acts as a reminder and provides positive reinforcement for pregnant women who have been asked by their physicians to record fetal activity. Second, by automating this technique, the pregnant woman is automatically prompted to begin kick counting and receives automatic feedback, such as notification of the successful or unsuccessful completion of a session. These automated features are particularly useful to a pregnant woman who is often preoccupied with other tasks and who may, therefore forget to initiate kick-counting. In addition, there is no need for women to keep track of 1) the number of kicks perceived. 2) the starting time, or 3) the ending time in confusing and complicated charts. Tne electronic kick count recorder can also be used to provide a record of both kick count results and alarm signals, which can be documented in the patient's chart. Finally, women will most definitely appreciate the interest their obstetricians are taking in their prenatal care by prescribing the kick count recorder. Use of the fetal movement recording device will now be described with respect to the Moore and Piacquadio protocol. In the program based on the Moore and Piacquadio protocol, the device may only function as a kick count recorder between 6:00 PM and 12:00 MN. At all other times it may, if desired, display the time, date, day of week and days until the estimated due date. At 6:00 PM, each night from the 28th week of gestation on, the device will display "READY" and await input by the user (Figure 1). If no input is
-13-
entered by 7:00 PM, the device may double beep once every 30 minutes until 11 :30 PM, or until input member 12 is pressed, which initiates the session. At any time between 6:00 PM and 12:00 MN, the patient may begin her two hour session by pressing input member 12, and the message "BEGIN COUNT" may appear. At this point, the "ELAPSED TIME" is displayed in hours and minutes and the number of recorded kicks is displayed on the display, for example in box 32 (Figure 2). The present time and date may also be displayed as well in the upper portion 34 of the display. The patient then presses the input member 12 each time she perceives a fetal movement, i.e. any discrete kick, flutter, swish, or roll. If the tenth kick is recorded within the upper time limit of two hours for the instant protocol, the results will be displayed (the result mode); and the device will acknowledge the successful completion of the session, for example by a visual indication such as "OK" (Fig. 7) appearing on the display. After 10 seconds, the display may then revert to the current time, date, and day of week, and the device will not be available for kick count recording until the following day at 6:00 PM. If the two hour time limit expires prior to recording the tenth kick, otherwise defined as the movement alarm, the display may read "ALARM" (Fig. 6), and an audible alarm may also sound (for example, a double beep for 10 seconds, then two double beeps every 15 minutes) until the device has been reset, or the device may disable itself until reset by the patient's care giver. Alternately, the patient may reset the device by holding the input member 12 down for 4 seconds (the device may double beep after each second). The device may also be programmed to signal the user if other criteria which may indicate fetal compromise are met (e.g., a pattern of increasing amount of elapsed time to reach ten movements). If the patient interrupts her session (by pressing input member 12 for four seconds - which may be accompanied by double beeps every second), the display will read CANCEL (Fig. 9) for that day, unless the patient subsequently completes a valid session later in the same day, between 6 p.m. and 12 midnight, in which case the latter results will be displayed.
-14-
After 12:00 midnight, the device may again display the time, date, and day of week, but will not record kick counts. After five continuous days of non-use, the device may display a message such as "CALL YOUR OBGYN'V'CALL YOUR DOC" (Fig. 3), or other such phrase, and an audible alarm may sound, as described hereinabove. A predetermined letter (e.g., "F") or number may also appear in the box to differentiate this warning from a movement alarm signal. The patient may then reset the device as described herein above.
The steps of the present invention are further depicted in part in the following Tables 1 through 3. The present invention preferably includes three modes as described hereinabove, i.e. the run mode (Table 1) in which fetal movement is registered by the user, the results mode (Table 2) in which results from current/and or past sessions are displayed, and the set mode (Table 3) in which various parameters such as date and time are set and/or changed. As described above, toggle member 24 may be provided in order to shift between the various modes of the device.
In the run mode, the device may complete the steps enumerated in Table 1 below. The column labeled "function" indicates a particular function accomplished by the device, and the column labeled "display" indicates the reading on the display 16 at the time of the corresponding function. Table 1 is provided for illustrative purposes only, and is not intended to limit the scope of the invention as the steps enumerated in Table 1 may vary, as would be apparent to one of skill in the art.
Table 1 : Run mode (Moore/Piacquadio protocol)
STEP FUNCTION DISPLAY
1 During the day, the device functions as a 11-29-97 watch, displaying the time, date and day on the WED
LCD. A
At 6:00 PM, the device becomes available for 6:00 PM kick counting. READY
At 7:00 PM, if patient has not initiated 7:00 PM session, device may double-beep. With READY continued non-use, the device may double- beep (once) every 30 minutes until 11 :30 PM.
If no entry by 12 MN, device goes back to 11-30-97 watch function, and is unavailable for kick WED counting until the following evening at 6:00 A
PM. 12:01
If 5 days of continuous non-use, display may CALL YOUR read... OBGYN
If during step 2 or 3, session initiated ... ("6:06 6:06 PM
PM" may alternate with "START COUNT"). 0:00 o
Buttons 20, 22 and 24 are preferably inactive
ELAPSED TIME during kick counting sessions.
Here, four kicks have been recorded in ?0 6:26 PM minutes. 0:20 4
ELAPSED TIME
If 10 kicks recorded within 2:00 HR...device 6:48 PM may double-beep four times to let patient OK know she does not need to record any more kicks. After 10 seconds, "OK" may be displayed and the device may then revert back
to watch, until following day at 6:00 PM.
-16-
After successful completion of kick counting 11-29-97 WED session, display reverts back to watch P functions. 6:48
If the patient decides to interrupt her session 11:07 PM before counting 10 kicks (and before 2 hr), she READY may hold button 12 down for 4 seconds (accompanied by double-beeps every second). If the current time is still before 12:00 MN, the patient may initiate a new session for that same day. She may not initiate a session after 12:00 MN. This interruption may be recorded in the results (see Table 2 below).
If, however, 2 hours pass before 10 kicks are 8:06 PM recorded... (in this case, only 7 kicks were 2:00 7 recorded during the 2 hour session).
ELAPSED TIME
...a message appears (and the device may CALL YOUR double-beep ten times - cannot be silenced) OBGYN 7 instructing the patient to call her clinician. Device may not function until it is reset. The number of kicks recorded during the session may remain in the display.
-17-
13 The patient may reset the device herself by 11-29-87 WED holding button 12 down for 4 seconds P
(accompanied by double-beeps every second) 8:06 or it may be reset by her care provider. The
"alarm" may be recorded, however, in the previous day's result. The device may function
as a watch until the following day at 6:00 PM.
It should be noted that in step 2 of Table 1 a "Research Version" may be available that will be available for kick counting at any time of day. In step 12 of table 1, a reminder double-beep every 30 minutes to 1 hour could follow the initial beeps.
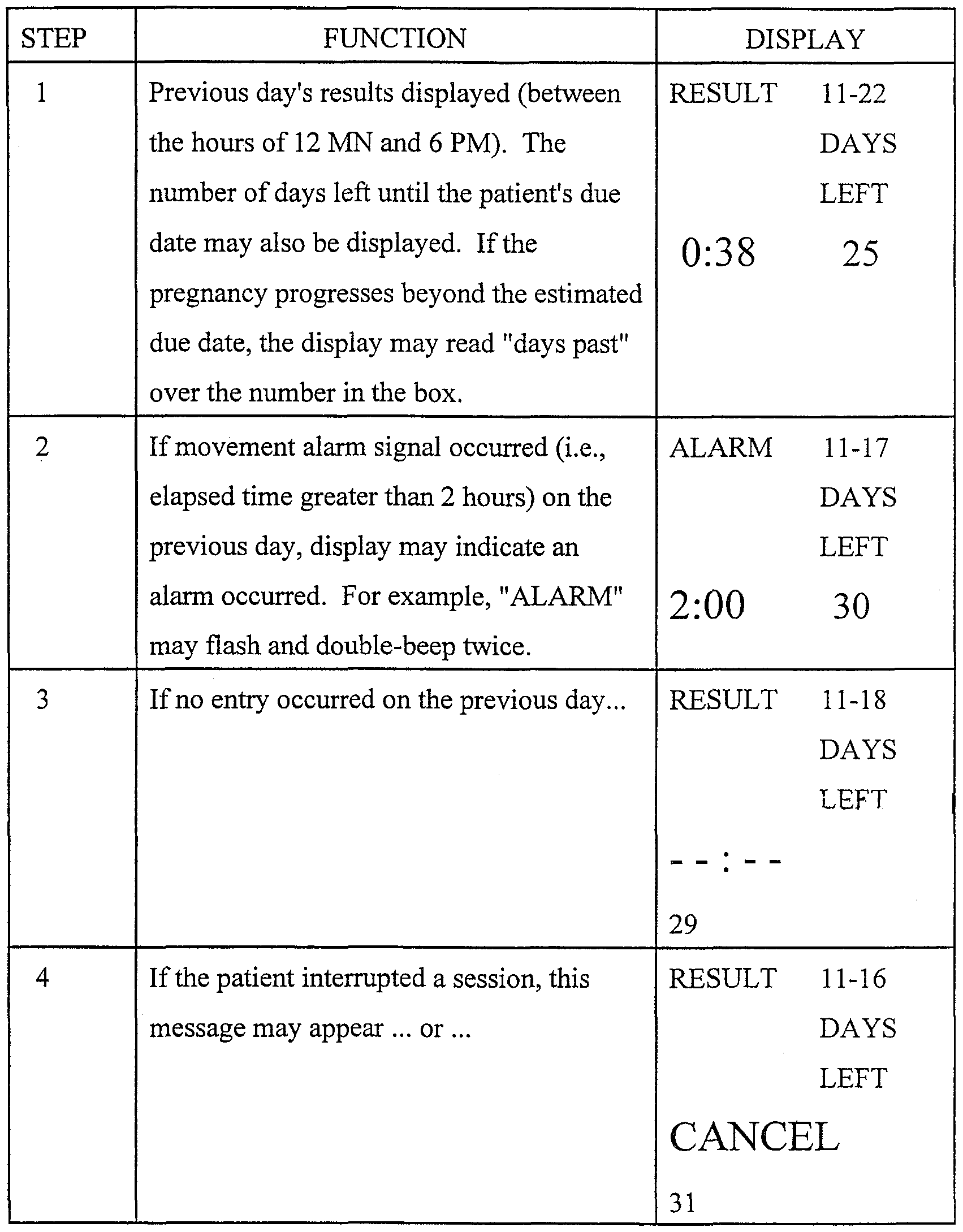
Table 2 depicts the result mode of the present invention, which may, for example, be accessed from run mode by pressing and holding the input button 12 depicted in Figure 1. The column labeled "function" indicates a particular function accomplished by the device, and the column labeled "display" indicates the display at the time of the corresponding function. Again, Table 2 is provided for illustrative purposes only, and is not intended to limit the scope of the invention as the steps enumerated in Table 2 may vary, as would be apparent to one of skill in the art
-18-
Table 2 Result mode
STEP FUNCTION DISPLAY
1 Previous day's results displayed (between RESULT 11-22 the hours of 12 MN and 6 PM). The DAYS number of days left until the patient's due LEFT date may also be displayed. If the 0:38 25 pregnancy progresses beyond the estimated due date, the display may read "days past" over the number in the box.
2 If movement alarm signal occurred (i.e., ALARM 11-17 elapsed time greater than 2 hours) on the DAYS previous day, display may indicate an LEFT alarm occurred. For example, "ALARM" 2:00 30 may flash and double-beep twice.
3 If no entry occurred on the previous day... RESULT 11-18 DAYS
LEFT
29
4 If the patient interrupted a session, this RESULT 11-16 message may appear ... or ... DAYS LEFT
CANCEL
5 If the patient then completed a session on RESULT 11-16 the same day... DAYS LEFT
0:52 31
6 Releasing input button 12 will return the 11-29-98 display to the current time, day and date. WED
A
11 :22
Table 3 depicts the set mode of the present invention, which may, for example, be accessed from the run mode by pressing toggle button 24. In the set mode, all time and date values can be adjusted by the patient. The column labeled "function" indicates a particular function accomplished by the device, and the column labeled "display" indicates the display at the time of the corresponding function. Table 3 is also provided for illustrative purposes only, and is not intended to limit the scope of the invention as the steps enumerated in Table 3 may vary, as would be apparent to one of skill in the art.
-20-
Table 3 Set mode
STEP FUNCTION DISPLAY
1 Input button 12 may be used to select the 11-30-97 variable (i.e., 11 → 30 → 97 → P → 12 → P 27 → Y) which will blink, indicating that 12:27 Y the value of the variable can be changed. Button 20 preferably increases and button 22 decreases the value of the variable. The letter in the box can toggle between Y (for
COUNT yes) or N (for no), depending on whether the patient would like the daily kick counting function (and the number of days left) activated. This allows patients to turn the kick counting function off after delivery. If she does not turn off the kick counting function, kick counting will automatically shut off five weeks after her entered due date (after 45th week of
gestation).
-21-
Upon completion of setting the above MAR- 17-98 variables (for the first time only), pressing button 24 may then toggle to a new Set EDD submode - the EDD (or estimated Date of Delivery). This date is determined by the clinician. Button 12 will again change the variable (i.e., MAR U 17 U 98) which will blink, indicating that the value of the variable can be changed. Button 20 again is used to increase and button 22 to decrease the value of the variable. Once set, this due date can preferably not be altered and will automatically begin 12 weeks before this due date (28th week of pregnancy) and will terminate automatically 5 weeks after the due date (45th week of pregnancy) if not shut off before this time, as described above.
Alternatively, the due date can be altered (to make allowances for clinical changes made in the due date) by not more than 30 days - in either direction, earlier or later preferably not more than 2-3 times. This will be reflected in the months available to the patient in the set mode.
It will be understood that various modifications may be made to the embodiment disclosed herein. For example, although described for use with the Moore/Piacquadio protocol, the device may be programmed for use with any kick-
-22-
count protocol and the device may include visual displays an/or audible displays, for example an audible beep to begin counting and a visual display to display the results of the session. Also, although the device may function in a separate mode as a personal organizer, watch, etc., it does not have to have a separate mode and may simply function only as a device for monitoring fetal movement. Likewise, although the device is preferably disabled after the 45th week or delivery of the baby, it does not have to disable itself and may continue to function in its capacity as a kick counter. In addition, although several different modes for providing information to the user are illustrated, only the alarm mode need be included in the device. Therefore, the above description should not be construed as limiting, but merely as exemplifications of a preferred embodiment. Those skilled in the art will envision other modifications within the scope spirit of the invention.