NOVEL 3,4-DIALKOXYPHENYL DERIVATIVES AND THE USE THEREOF
BACKGROUND OF THE INVENTION
FIELD OF THE INVENTION
This invention is directed to the novel compounds of formula [1], their preparation, pharmaceutical compositions containing these compounds, and their pharmaceutical use in the treatment of disease states associated with cytokine that mediates cellular activity.
Disease states associated with abnormally high physiological levels of cytokines such as TNF are treatable according to the invention. TNF is an important pro-inflammatory cytokine which causes hemorrhagic necrosis of tumors and possesses other important biological activities.
It has been implicated as a mediator of endotoxic shock, inflammation of joints and of the airways, immune deficiency states, allograft rejection, and in the cachexia associated with malignant disease and some parasitic infections. In view of the association of the high serum levels of TNF with poor prognosis in sepsis, graft versus host disease and acute respiratory distress syndrome, and its role in many other immunological processes, this factor is regarded as an important mediator of general inflammation.
DESCRIPTION OF THE PRIOR ART
Tumor necrosis factor- a (TNF- ) is a primary cytokine secreted by mononuclear phagocytes stimulated by several immune activators. These factors are known to induce acute infection, shock, inflammation, heat, hemolysis, coagulation and acute reaction in human or animal.
If TNF- α is excessed or not regulated well, many diseases like endotoxemia, toxic shock syndromelNature 330. 662-664(1987)] or cachexiafLancet 335(1990), 662 (1990)]occur. It is predicted that inhibition of TNF- α production and action is a good theraphy for inflammatory, infectious, and immune diseases, inhibition of production and action of TNF- α can be deduced to treat the diseases like septic shock, sepsis, endotoxemia, septic syndrome, malaria mycobacterium infection, encephalomyelitis, psoriasis, cachexia, graft versus host reaction, asthma, cancer, autoimmune disease, rheumatoid arthritis, osteoarthritis, Crohn's disease, multiple sclerosis, systemic Lupus Erythematosus, etc.
Therefore, TNF- α inhibitor is now being studied extensively for therapeutics against the above diseases. Currently, steroids like dexamethasone and prednisolone which have the side effects by systemically inducing immunosuppression and poly-or monoclonal antibodies of TNF- α receptor are known to act as therapeutic agent in autoimmune diseaseslScience 234 470-474(1985), W09211383, Clinical and Experimental Rheumatoid 5173-5175(1993), PNAS 9784-9788(1992), Annals of Rheumatoid Disease 480-486(1990)]. Xanthine compounds like pentoxifyiline are known to inhibit TNF- α , but are weak in their potencytCirculatory Shock 44. 188-195(1994)].
Although rolipram has CNS side effects like nausea and vomitting.Drugs of the Future 28. 793-803(1995)] which provides an active pharmacophore currently being studied as novel derivatives for inhibition of TNF- α [W09212961 , WO9503794, WO9402465, WO9505386, WO9509624, WO9620926].
SUMMARY OF THE INVENTION
The inventors have studied extensively to settle the problems that the previous TNF- inhibitors possess. As a result, the novel 3,4-dialkoxyphenyl derivatives depicted in formula [1] have shown selective and potent TNF- α biosynthesis inhibition. We elucidated that they had no side effect like nausea, or vomitting. And hence we have achieved this invention.
The first aim of this invention presents the novel compounds with potent TNF-
a inhibition activity represented in formula [1].
The second aim of this invention presents the methods of synthesis of the novel 3,4-dialkoxyphenyl derivatives and their salts in formula [1].
The third aim of this invention presents pharmaceutical compositions of TNF- α inhibitor containing the novel 3,4-dialkoxyphenyl derivatives and their salts in formula [1] as use of joint inflammation, rheumatoid arthritis, osteoarthritis, sepsis, septic shock, asthma, graft versus host reaction, psoriasis, allergic inflammation, and autoimmune diseases.
DETAILED DESCRIPTION OF THE INVENTION
This invention provides the compound formula [1] of the novel 3,4-dialkoxyphenyl derivatives, and a pharmaceutically acceptable salt thereof.
wherein X is oxygen or sulfur ;
A, B, C, and D are independently carbon, nitrogen or N-oxide ; Ri is lower alkyl group ; R2 is lower alkyl, cycloalkyl, hydroxy cycloalkyl, arylalkyl, cycloalkylalkyl, bicycloalkyl group ; R3 is hydrogen, hydroxy ; R4 is hydrogen, halogen, azido, lower alkyl, lower alkenyl, lower alkoxy, phenyl, amino, cycloalkyl, cycloalkylalkyl group ; R5 is hydrogen, halogen, hydroxy, methylhydroxy, lower alkyl, lower alkoxy,
amino, lower alkylamino, lower diaikylamino, cyano, aldehyde, aldehydeoxime, -COORs, -N(R7)(RB), -CH N(R9)(Rιo) ; R6 is hydrogen, -NHNH2, lower alkyl group ; R7 is hydrogen ; R8 is -COCO-R11, -CH2CO-R11 ; R9 is hydroxy ; R10 is hydrogen, -CO-Rn ; R11 is lower alkoxy, hydroxy, and amino group ; or a pharmaceutically acceptable salt thereof.
As used above, and throughout the description of the invention, the following terms, unless otherwise indicated, shall be understood to have the following meanings :
Definition
"Halogen" means fluoro, chloro, or bromo. Preferred are fluoro and chioro.
"Lower alkyl" means aliphatic hydrocarbon group having 1 to 6 carbon atoms in the chain such as methyl, ethyl, propyl, butyl, pentyl and hexyl. Preferably, it means straight or branched hydrocarbon having 1 to 4 carbon atoms.
"Lower alkenyl" means an aliphatic hydrocarbon group containing a carbon-carbon double bond which may be straight or branched having 2 to 6 carbon atoms in the chain such as ethenyl, propenyl, butenyl and pentenyl. Preferably, it means hydrocarbon having 2 to 4 carbon atoms.
"Lower alkoxy" means alkyl oxy group which may be straight or branched having 1 to 4 carbon atoms in the chain such as methoxy, ethoxy, propoxy and butoxy.
"Cycloalkyl" means non-aromatic mono- or multicyclic ring system having 3 to 8 carbon atoms in the chain such as cyclopropyi, cyclobutyl, cyclopentyl, cyclohexyl, cyclooctyl and adamantyl. Preferably, it means hydrocarbon having 3 to 6 carbon atoms.
"Arylalkyl" means phenyl alkyl group having 3 to 10 carbon atoms in the chain such as phenylethyl, phenylpropyl, phenylbutyl and phenylpentyl. Preferably, it means hydrocarbon having 3 to 8 carbon atoms.
"Cycloalkylalkyl" means cycloalkylalkyl group in which cycloalkyl is as previously defined, alkyl is methyl and ethyl. Preferably, cycloalkylalkyl is cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl, and cyclopentylethyl.
"Bicycloalkyl" means indanyl group such as 1-indanyl and 2-indanyl.
"N-oxide" means a moiety of the following structure
According to a compound aspect of the invention, preferred compounds described formula [1] are following :
wherein X is oxygen or sulfur ;
A, B, C, and D are independently carbon, nitrogen or N-oxide ; Ri is C1-C4 lower alkyl group ; R2 is C1-C4 lower alkyl, C3-C6 cycloalkyl, hydroxy-C3-Ce cycloalkyl, aryl-Cj-Cβ
-alkyl, CJ-CS cycloalkyl-Cι-C alkyl, indanyl group ; R3 is hydrogen, hydroxy ; R4 is hydrogen, halogen, azido, Cι-C4 lower alkyl, C2-C4 lower alkenyl, C1-C4 lower alkoxy, phenyl, amino, C3-C6 cycloalkyl, C3-C6 cycloalkyl-Cι-C4 alkyl group ; R5 is hydrogen, halogen, hydroxy, methylhydroxy, C1-C4 lower alkyl, C1-C4 lower alkoxy, amino, C1-C4 lower alkylamino, di-Cι-C4 lower alkylamino, cyano, aldehyde, aidehydeoxime, -COORe, -N(R7)(Rβ), -CH2N(R9)(Rιo) ; Re is hydrogen, -NHNH2, C1-C4 lower alkyl group ; R7 is hydrogen ; Re is -COCO-R11, -CH2CO-R11 ; R9 is hydroxy ;
R10 is hydrogen, -CO-Rn ;
R11 is C1-C4 lower alkoxy, hydroxy, and amino group ; or a pharmaceutically acceptable salt thereof.
According to a compound aspect of the invention, more preferred compounds described formula [1] are following :
wherein X is oxygen or sulfur ;
A, B, C, and D are independently carbon, nitrogen or N-oxide ; R1 is C1-C2 lower alkyl group ; R2 is C1-C2 lower alkyl, C3-C6 cycloalkyl, hydroxy-C3-Ce cycloalkyl, aryl-C3-Ce alkyl, C3-C5 cycloalkyl-Ci-C∑ alkyl, 1 -indanyl or 2-indanyl ; R3 is hydrogen, hydroxy ; R4 is hydrogen, halogen, azido, C1-C2 lower alkyl, C2-C3 lower alkenyl, C1-C2 lower alkoxy, phenyl, amino, CJ-CB cycloalkyl, C3-C5 cycloalkyl-Ci-C∑ alkyl! Rs is hydrogen, halogen, hydroxy, methylhydroxy, methyl, methoxy, amino, methylamino, dimethylamino, cyano, aldehyde, aldehydeoxime, -COORβ, -N(R7)(Rβ), -CH2N(R9)(Rιo) ; R6 is hydrogen, -NHNH2, C1-C2 lower alkyl group ; R7 is hydrogen ; Re is -COCO-R11, -CH2CO-R11 ; Rg is hydroxy ; R10 is hydrogen, -CO-Rn ; R11 is methoxy, hydroxy, and amino group ; or a pharmaceutically acceptable salt thereof.
Preferred compounds for use according to the invention are selected from the following: 3-methyl-2-(3,4-dimethoxyphenyl)isoindolin-1-one
-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one -methyl-2-(3-cyclopentylmethyioxy-4-methoxyphenyl)isoindolin-1 -one -methyl-2-{3-(1 -indanyloxy)-4-methoxyphenyl}isoindolin-1 -one -methyl-2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1-one -methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione -methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one -methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-hydroxy-isoindolin-1-one -methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-methoxy-isoindolin-1-one
3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-4,5,6,7-tetrafluoro-isoindolin-1-one
3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one
3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-dimethylamino-isoindolin-1-one
3-methyl-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one
3-methyl-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
3-methyl-3-hydroxy-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-methyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one
3-methyl-3-hydroxy-2-{3-(1-indanyloxy)-4-methoxyphenyl}isoindolin-1-one
3-methyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione
3-methyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1
-one
3-methyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-methoxy-isoindolin-1- one
3-methyl-3-hydroxy-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1- one
3-methyl-3-hydroxy-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1- one
3-methylene-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-methylene-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one
3-methylene-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one
3-methylene-2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1-one
3-methylene-2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1-one
3-methyiene-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one
3-methylene-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-methoxy-isoindolin-1-one
3-methylene-2-(3-cyclopentyloxy-4-methoxyphenyl)-4,5,6,7-tetrafluoro-isoindolin-1- one
3-methylene-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one
3-methylene-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-dimethylamino-isoindolin-1- one
3-methylene-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one
3-methylene-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
3-hydroxy-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindoIin-1-one
3-hydroxy-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one
3-hydroxy-2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1-one
3-hydroxy-2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1-one
3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one
3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-methoxy-isoindolin-1-one
3-hydroxy-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one
3-hydroxy-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
3-methoxy-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-methoxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one
3-methoxy-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one
3-methoxy-2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1-one
3-methoxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione
3-methoxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one
3-methoxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-hydroxy-isoindolin-1-one
3-methoxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-4,5,6,7-tetrafluoro-isoindolin-1- one
3-methoxy-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one
3-methoxy-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one
3-methoxy-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
-fluoro-2-(3,4-dimethoxyphenyl)isoindolin-1-one -fluoro-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one -fluoro-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one -f luoro-2-{3-(1 -indanyloxy)-4-methoxyphenyl}isoindolin-1 -one -fluoro-2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1-one -fluoro-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione -fluoro-2-(3-cyclopentyloxy-4-methoxyphenyl)-4,5,6,7-tetrafluoro-isoindolin-1-one -fluoro-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one -fluoro-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one -fluoro-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
-azido-2-(3,4-dimethoxyphenyl)isoindolin-1-one -azido-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one -azido-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one -azido-2-{3-(1 -indanyloxy)-4-methoxyphenyl}isoindolin-1 -one -azido-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione -azido-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one -azido-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one -azido-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one -azido-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
-amino-2-(3,4-dimethoxyphenyl)isoindolin-1-one -amino-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one -amino-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one -amino-2-{3-(1 -indanyloxy)-4-methoxyphenyl}isoindolin-1 -one -amino-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione -amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one -amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one -amino-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one -amino-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one -phenyl-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-phenyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one
3-phenyl-2-{3-(1 -indanyloxy)-4-methoxyphenyl}isoindolin-1 -one
3-phenyl-2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1-one
3-phenyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione
3-phenyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one
3-phenyl-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one
3-phenyl-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
3-cyclopropyl-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-cyclopropyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one
3-cyclopropyl-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one
3-cyclopropyl-2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1-one
3-cyclopropyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-thione
3-cyclopropyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin-1-one
3-cyclopropyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-4,5,6,7-tetrafluoro-isoindolin-1- one
3-cyclopropyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1-one
3-cyclopropyl-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1-one
3-cyclopropyl-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1-one
3-cyclopropylmethyl-2-(3,4-dimethoxyphenyl)isoindolin-1-one
3-cyclopropylmethyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one
3-cyclopropylmethyl-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1-one
3-cyclopropylmethyl-2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1-one
3-cyclopropylmethyl-2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1-one
3-cyclopropylmethyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin-1- one
3-cyclopropylmethyl-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}isoindolin-1
-one
3-cyclopropylmethyl-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]isoindolin-1- one
6-(3,4-dimethoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6-{3-(1-indanyloxy)-4-methoxyphenyl}-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6-(3-cyclohexyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-thione
6-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-6,7-dihydro-5H-pyrrolot3,4-b]pyr idin-5-one
6-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-6,7-dihydro-5H-pyrrolot3,4-b]pyr idin-5-one
6-(3,4-dimethoxyphenyl)-7-methylene-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-7-methylene-6,7-dihydro-5H-pyrrolo[3,4-b]pyr idin-5-one
6-(3-cyclopentylmethyloxy-4-methoxyphenyl)-7-methylene-6,7-dihydro-5H-pyrrolo[3,
4-b]pyridin-5-one
6-{3-(2-indanyloxy)-4-methoxyphenyl}-7-methylene-6,7-dihydro-5H-pyrrolo[3,4-b]pyr idin-5-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-7-methylene-6,7-dihydro-5H-pyrrolo[3,4-b]pyr idin-5-thione
6-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-7-methylene-6,7-dihydro-5H-pyr rolo[3,4-b]pyridin-5-one
6-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-7-methylene-6,7-dihydro-5H-pyr rolo[3,4-b]pyridin-5-one
6-(3,4-dimethoxyphenyl)-7-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-7-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin
-5-one
6-{3-(1-indanyloxy)-4-methoxyphenyl}-7-methyl-6,7-dihydro-5H-pyrrolot3,4-b]pyridin
-5-one
6-{3-(2-indanyloxy)-4-methoxyphenyl}-7-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin
-5-one
6-(3-cyclohexyloxy-4-methoxyphenyl)-7-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin
-5-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-7-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin
-5-thione
6-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-7-methyl-6,7-dihydro-5H-pyrrolo
[3,4-b]pyridin-5-one
6-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-7-methyl-6,7-dihydro-5H-pyrrolo
[3,4-b]pyridin-5-one
2-(3,4-dimethoxyphenyl)-2,3-dihydro-1H-pyrrolo[3,4-c]pyridin-1-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one
2-(3-cyclopentymethyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1- one
2-{3-(1 -indanyloxy)-4-methoxyphenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1 -one
2-(3-cyclohexyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrroIo[3,4-c]pyridin-1-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-thione
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-1-one
2-t3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-1-one
2-(3,4-dimethoxyphenyl)-3-methylene-2,3-dihydro-1 H-pyrrolot3,4-c]pyridin-1-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methylene-2,3-dihydro-1 H-pyrrolot3,4-c] pyridin-1-one
2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-methylene-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-1-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methylene-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-1-thione
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-methylene-2,3-dihydro-1 H- pyrrolo[3,4-c]pyridin-1 -one
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-methylene-2,3-dihydro-1 H- pyrrolo[3,4-c]pyridin-1 -one
2-(3,4-dimethoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1-one
2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-1-one
2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1-one
2-{3-(2-indanyloxy)-4-methoxyphenyl}-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1-on
2-(3-cyclohexyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1-thione
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-methyl-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-1 -one
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-methyl-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-1 -one
6-(3,4-dimethoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-7-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolot3,4-b]pyridin-7-one
6-{3-(1-indanyloxy)-4-methoxyphenyl}-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-7-one
6-(3-cyclohexyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-7-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-7-thione
6-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-6,7-dihydro-5H-pyrrolo[3,4-b] pyridin-7-one
6-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyi]-6,7-dihydro-5H-pyrrolo[3,4-b]pyr idin-7-one
6-(3,4-dimethoxyphenyl)-5-methylene-6,7-dihydro-5H-pyrrolot3,4-b]pyridin-7-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-5-methylene-6,7-dihydro-5H-pyrrolo[3,4-b]pyr idin-7-one
6-{3-(2-indanyloxy)-4-methoxyphenyl}-5-methylene-6,7-dihydro-5H-pyrrolo[3,4-b]pyr idin-7-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-5-methylene-6,7-dihydro-5H-pyrrolot3,4-b]pyr
idin-7-thione
6-{4-methoxy-3-(1-methyl-3-phenyipropoxy)phenyl}-5-methylene-6,7-dihydro-5H-pyr rolo[3,4-b]pyridin-7-one
6-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-5-methylene-6,7-dihydro-5H-pyr rolo[3,4-b]pyridin-7-one
6-(3,4-dimethoxyphenyl)-5-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-7-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-5-methyl-6,7-dihydro-5H-pyrrolot3,4-b]pyridin
-7-one
6-(3-cyclopentylmethyloxy-4-methoxyphenyl)-5-methyl-6,7-dihydro-5H-pyrrolo[3,4-b] pyridin-7-one
6-{3-(2-indanyloxy)-4-methoxyphenyl}-5-methyl-6,7-dihydro-5H-pyrrolot3,4-b]pyridin
-7-one
6-(3-cyclohexyloxy-4-methoxyphenyl)-5-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin
-7-one
6-(3-cyclopentyloxy-4-methoxyphenyl)-5-methyl-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin
-7-thione
6-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-5-methyl-6,7-dihydro-5H-pyrrolo
[3,4-b]pyridin-7-one
6-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-5-methyl-6,7-dihydro-5H-pyrrolo
[3,4-b]pyridin-7-one
2-(3,4-dimethoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-3-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolot3,4-c]pyridin-3-one
2-(3-cyclopentymethyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-3- one
2-{3-(2-indanyloxy)-4-methoxyphenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-3-one
2-(3-cyclohexyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolot3,4-c]pyridin-3-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-3-thione
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyr idin-3-one
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[3,4-c]pyri
din-3-one
2-(3,4-dimethoxyphenyl)-1-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-3-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-1-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-3-one
2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-1-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-3-one
2-{3-(2-indanyloxy)-4-methoxyphenyl}-1-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-3-one
2-(3-cyclohexyloxy-4-methoxyphenyl)-1-methyl-2,3-dihydro-1H-pyrrolot3,4-c]pyridin
-3-one
2-(3-cyclopentyloxy-4-methoxyphenyl)-1-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-3-th ione
2-{4-methoxy-3-(1 -methyl-3-phenylpropoxy)phenyl}-1 -methyl-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-3-one
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-1-methyl-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-3-one
6-amino-2-(3,4-dimethoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one
6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1 -one
6-amino-2-(3-cyclopentymethyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]p yridin-1-one
6-amino-2-{3-(1-indanyloxy)-4-methoxyphenyl}-2,3-dihydro-1 H-pyrrolot3,4-c]pyridin
-1-one
6-amino-2-(3-cyclohexyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-
1-one
6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-1-th ione
6-amino-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-1 -one
6-amino-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[
3,4-c]pyridin-1 -one
6-amino-2-(3,4-dimethoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-3-one
6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-3-one
6-amino-2-(3-cyclopentymethyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]p yridin-3-one
6-amino-2-{3-(1-indanyloxy)-4-methoxyphenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-3-one
6-amino-2-(3-cyclohexyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-
3-one
6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-3]-thione
6-amino-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-3-one
6-amino-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[
3,4-c]pyridin-3-one
6-amino-2-(3,4-dimethoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-o ne
6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4
-c]pyridin-1-one
6-amino-2-(3-cyclopentymethyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrro lo[3,4-c]pyridin-1 -one
6-amino-2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4
-c]pyridin-1-one
6-amino-2-(3-(2-indanyloxy)-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4
-c]pyridin-1-one
6-amino-2-(3-cyclohexyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4- c]pyridin-1-one
6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4
-c]pyridin-1 -thione
6-amino-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1 -one
6-amino-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[ 3,4-c]pyridin-1 -one
ethyl 2-(3,4-dimethoxyphenyl)-3-oxo-5-isoindoline carboxylate ethyl 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylate ethyl 2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylate ethyl 2-{3-(1 -indanyloxy)-4-methoxyphenyl}-3-oxo-5-isoindoline carboxylate ethyl 2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylate ethyl 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-5-isoindoline carboxylate ethyl 2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-5-isoindoline carboxylate ethyl 2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-5-isoindoline carboxylate
2-(3,4-dimethoxyphenyl)-3-oxo-5-isoindoline carboxylic acid 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylic acid 2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylic acid 2-{3-(1 -indanyloxy)-4-methoxyphenyl}-3-oxo-5-isoindoline carboxylic acid 2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylic acid 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-5-isoindoline carboxylic acid 2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-5-isoindoline carboxylic acid
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-5-isoindoline carboxylic acid
2-(3,4-dimethoxyphenyl)-3-oxo-5-isoindoline carboaldehyde 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboaldehyde 2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboaldehyde 2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-5-isoindoline carboaldehyde 2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboaldehyde
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-5-isoindoline carboaldehyde
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-5-isoindoline carboaldehy de
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-5-isoindoline carboaldehy de
2-(3,4-dimethoxyphenyl)-3-oxo-5-isoindoline carboaldehydeoxime
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboaldehydeoxime
2-(3-cyclopentylmethyloxy-4-methoxypheny!)-3-oxo-5-isoindoline carboaldehydeoxim e
2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-5-isoindoline carboaldehydeoxime
2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboaldehydeoxime
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-5-isoindoline carboaldehydeoxime
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-5-isoindoline carboaldeh ydeoxime
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-5-isoindoline carboaldehy deoxime
2-(3,4-dimethoxyphenyl)-3-oxo-5-isoindoline carbonitrile 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carbonitrile 2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carbonitrile 2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-5-isoindoline carbonitrile 2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carbonitrile 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-5-isoindoline carbonitrile 2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-5-isoindoline carbonitrile 2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-5-isoindoline carbonitrile
2-(3,4-dimethoxyphenyl)-3-oxo-5-isoindoline carbohydrazide 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carbohydrazide 2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carbohydrazide 2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-5-isoindoline carbohydrazide 2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carbohydrazide
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-5-isoindoline carbohydrazide
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-5-isoindoline carbohydraz ide
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-5-isoindoline carbohydraz ide
2-{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}acetonitrile
2-{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}acetonit rile
2-{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}a cetonitrile
2-[2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl]acetonit rile
2-{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}acetonitr ile
2-{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}aceto nitrile
2-[2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-isoin dolyllacetonitrile
2-{2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-isoin dolyllacetonitrile
N-[2-{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}methyl]hydroxyure a
N-[2-{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}met hyl]hydroxyurea
N-[2-{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindol yl}methyl]hydroxyurea
N-(2-[2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl]meth yDhydroxyurea
N-[2-{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}meth yl]hydroxyurea
N-[2-{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}m ethyl]hydroxyurea
N-(2-[2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-i soindolyl]methyl)hydroxyurea
N-(2-{2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-is oindolyl}methyl)hydroxyurea
6-(aminomethyl)-2-(3,4-dimethoxyphenyl)-1-isoindolinone
6-(aminomethyl)-2-(3-cyclopentyloxy-4-methoxyphenyl)-1-isoindolinone
6-(aminomethyl)-2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-1-isoindolinone
6-(aminomethyl)-2-{3-(1-indanyloxy)-4-methoxyphenyl}-1-isoindolinone
6-(aminomethyl)-2-(3-cyclohexyloxy-4-methoxyphenyl)-1-isoindolinone
6-(aminomethyl)-2-(3-cyclopentyloxy-4-methoxyphenyl)-1-isoindolinthione
6-(aminomethyl)-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-1-isoindolinone
6-(aminomethyl)-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-1-isoindolinone
2-(3,4-dimethoxyphenyl)-6-hydroxymethyl-1-isoindolinone
2-(3-cyclopentyloxy-4-methoxyphenyl)-6-hydroxymethyl-1-isoindolinone
2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-6-hydroxymethyl-1-isoindolinone
2-{3-(1-indanyloxy)-4-methoxyphenyl}-6-hydroxymethyl-1-isoindolinone
2-(3-cyclohexyloxy-4-methoxyphenyl)-6-hydroxymethyl-1-isoindolinone
2-(3-cyclopentyloxy-4-methoxyphenyl)-6-hydroxymethyl-1-isoindolinthione
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-6-hydroxymethyl-1-isoindolinon e
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-6-hydroxymethyl-1-isoindolinone
2-(3,4-dimethoxyphenyl)-6-(hydroxyamino)methyl-1-isoindolinone
2-(3-cyclopentyloxy-4-methoxyphenyl)-6-(hydroxyamino)methyl-1-isoindolinone
2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-6-(hydroxyamino)methyl-1-isoindolinon e
2-{3-(1-indanyloxy)-4-methoxyphenyl}-6-(hydroxyamino)methyl-1-isoindolinone
2-(3-cyclohexyloxy-4-methoxyphenyl)-6-(hydroxyamino)methyl-1-isoindolinone
2-(3-cyclopentyloxy-4-methoxyphenyl)-6-(hydroxyamino)methyl-1-isoindolinthione
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-6-(hydroxyamino)methyl-1-isoin dolinone
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-6-(hydroxyamino)methyl-1-isoind olinone
{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}methylmethanesulfonate
(2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}methylme thanesulfonate
{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}me thylmethanesulfonate
[2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl]methylmet hanesulfonate
{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}methylmet hanesulfonate
{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}methyl methanesulfonate
[2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-isoind olyl]methylmethanesulfonate
{2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-isoindo lyl}methylmethanesulfonate
methyl 2-[{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]-2-oxo acetate methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl
}amino]-2-oxoacetate methyl 2-[{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-iso indoiyl}amino]-2-oxoacetate methyl 2-([2-{3-(1 -indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl] amino)-2-oxoacetate methyl 2-[{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl} amino]-2-oxoacetate
methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoind olyl}amino]-2-oxoacetate methyl 2-([2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1
H-5-isoindolyl]amino)-2-oxoacetate methyl 2-{(2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H
-5-isoindolyl)amino}-2-oxoacetate
N1 -{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}ethanamide
N1 -{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}ethan amide
N1-{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl lethanamide
N1 -[2-{3-(1 -indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl]ethana mide
N1-{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}ethana mide
N1 -{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}eth anamide
N1 -[2-{4-methoxy-3-(1 -methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-iso indolyllethanamide
N1-(2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-isoi ndoiyDethanamide
2-[{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]-2-oxoacetic acid
2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]
-2-oxoacetic acid
2-[{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl} amino]-2-oxoacetic acid
2-[{2-(3-(1-indanyloxy)-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]
-2-oxoacetic acid
2-[{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]-
2-oxoacetic acid
2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}amin o]-2-oxoacetic acid
2-([2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-isoi ndolyl]amino)-2-oxoacetic acid
2-{(2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-isoi ndolyl)amino}-2-oxoacetic acid
methyl 2-[{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]acetate methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl
}amino]acetate methyl 2-[{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-iso indolyllaminolacetate methyl 2-([2-{3-(1 -indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl
]amino)acetate methyl 2-[{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl} aminolacetate methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindo lyl}amino]acetate methyl 2-[{2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1
H-5-isoindolyl}amino]acetate methyl 2-[{2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H
-5-isoindolyl}amino]acetate
2-{[2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl]amino}acetic acid 2-{[2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl]amino) acetic acid
2-{[2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl] aminolacetic acid
2-([2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl]amino) acetic acid
2-[{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]a cetic acid
2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}amin olacetic acid
2-([2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-isoi ndolyl]amino)acetic acid
2-{(2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-isoi ndolyl)amino}acetic acid
2-[{2-(3,4-dimethoxyphenyl)-3-oxo-2,3-dihydro-1H-5-isoindolyl}amino]acetamide
2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino] acetamide
2-[{2-(3-cyclopentylmethyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl} aminolacetamide
2-([2-{3-(1-indanyloxy)-4-methoxyphenyl}-3-oxo-2,3-dihydro-1 H-5-isoindolyl]amino) acetamide
2-[{2-(3-cyclohexyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolyl}amino]a cetamide
2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-thioxo-2,3-dihydro-1 H-5-isoindolyl}amin olacetamide
2-([2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-oxo-2,3-dihydro-1 H-5-isoi ndolyl]amino)acetamide
2-({2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-oxo-2,3-dihydro-1 H-5-isoi ndolyl}amino)acetamide
2-(3,4-dimethoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one • hydrogen chl oride
2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one
• hydrogen chloride
2-(3-cyclopentymethyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1- one ■ hydrogen chloride 2-{3-(1 -indanyloxy)-4-methoxyphenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1 -one
■ hydrogen chloride 2-(3-cyclohexyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one
■ hydrogen chloride 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1 -thione
■ hydrogen chloride
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyr idin-1-one • hydrogen chloride
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[3,4-c]pyri din-1-one • hydrogen chloride
1-oxo-2-(3,4-dimethoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-5-ium-5-olate
1-oxo-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-5
-ium-5-olate
1 -oxo-2-{3-(1 -indanyloxy)-4-methoxyphenyl}-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-5- ium-5-olate
1-oxo-2-(3-cyclohexyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-5-i um-5-olate
1-oxo-2-(3-cyclopentymethyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyri din-5-ium-5-olate
1-thioxo-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin
-5-ium-5-olate
1 -oxo-2-{4-methoxy-3-(1 -methyl-3-phenylpropoxy)phenyl}-2,3-dihydro-1 H-pyrrolo[3,
4-c]pyridin-5-ium-5-olate
1-oxo-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-2,3-dihydro-1 H-pyrrolo[3,
4-c]pyridin-5-ium-5-olate
2-(3,4-dimethoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-1 -one • hydro gen chloride
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin -1-one • hydrogen chloride
2-(3-cyclopentymethyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-1-one • hydrogen chloride
2-(3-(2-indanyloxy)-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin -1-one • hydrogen chloride
2-(3-cyclohexyioxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin -1-one • hydrogen chloride
2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin -1-thione • hydrogen chloride
2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-methyl-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1-one • hydrogen chloride
2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-methyl-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1-one • hydrogen chloride
1 -oxo-2-(3,4-dimethoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c]pyridin-5-ium
-5-olate
1-oxo-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-5-ium-5-olate
1-oxo-2-(3-cyclopentymethyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-5-ium-5-olate
1-oxo-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-5-ium-5-thiolate
1-oxo-2-{4-methoxy-3-(1-methyl-3-phenylpropoxy)phenyl}-3-methyl-2,3-dihydro-1 H
-pyrrolo[3,4-c]pyridin-5-ium-5-olate
1-oxo-2-[3-{(3-hydroxycyclopentyl)oxy}-4-methoxyphenyl]-3-methyl-2,3-dihydro-1 H
-pyrrolo[3,4-c]pyridin-5-ium-5-olate
The compounds of the present invention are useful in the form of the acid, or N-oxide thereof or in the form of a pharmaceutically acceptable salt thereof. All forms are within the scope of the invention. Pharmaceutically acceptable salts within the scope of the invention are those derived from the following acids; mineral acids such as hydrochloric acid, sulfuric acid, phosphoric acid and sulfamic acid; organic acids such as acetic acid, citric acid, lactic acid, tartaric acid, malonic acid, methanesulfonic acid, ethanesuifonic acid, benzenesulfonic acid and
p-toluenesulfonic acid, cyclohexylsulfamic acid, quinic acid, and the like.
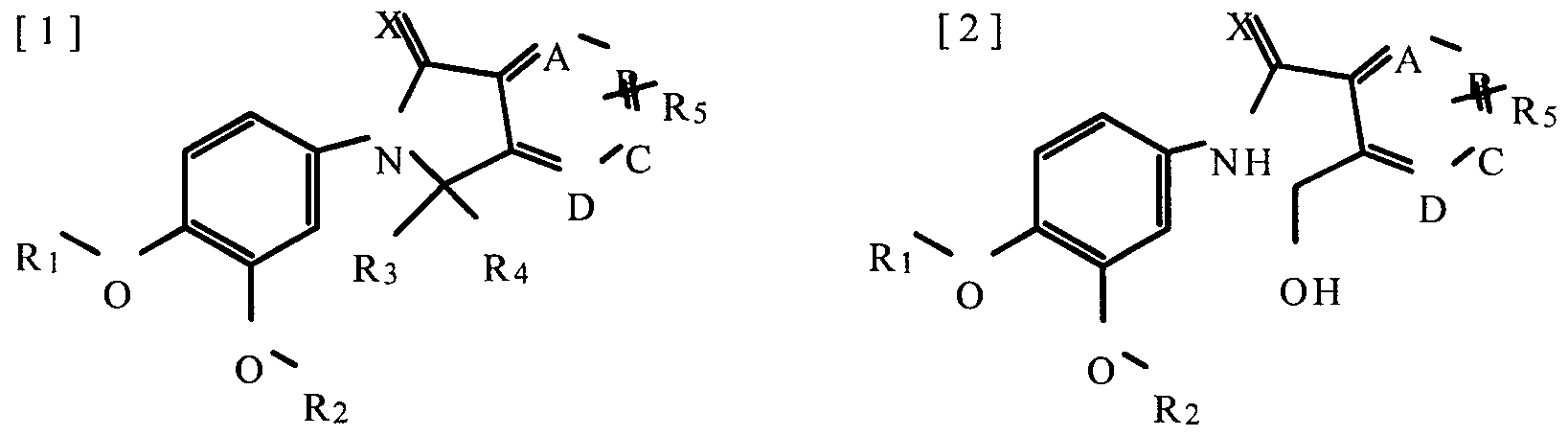
This invention relates to the synthesis and preparation method of the novel 3,4-dialkoxyphenyl derivatives. According to this invention, the compound of formula [4] is prepared by reaction of formula [5] with [6] or [7]. Then the compound of formula [4] (a) is reacted with Grignard reagents or (b) is reduced to afford the compound of formula [2] or [3]. The compound of formula [2] is cyclized to produce the compound of formula [1], in which R3 and R4 is hydrogen, and one of A, B, C and D is nitrogen. By this way, the 3,4-dialkoxyphenyl derivatives can be prepared.
Wherein,
A, B, C, D, X, Ri, R2, R3, R , and Rs are as defined above.
The preparation for the compound of formula [1] in this invention can be described in the following reaction scheme 1.
[ Scheme 1 ]
[5] [ ; 7]
Wherein, A, B, C, D, X, Ri, R2, R3, R, and Rs are as defined above.
In the following, the preparation of 3,4-dialkoxyphenyl derivatives of formula [1] is described in detail.
In the reaction A, the compound of formula [4] is prepared by reaction of amine compound of formula [5] with formula [6] or [7]. The reaction of formula [5] with [6] is accomplished by refluxing with the acidic catalyst in unreactive solvent. For this purpose either inorganic catalyst or organic catalyst is used. The inorganic catalyst is hydrochloric acid, sulfuric acid, or nitric acid, and organic catalyst is acetic acid or trifluoroacetic acid. Acetic acid is most preferably used among these catalysts. The reaction of formula [5] with [7] is accomplished by refluxing with the basic catalyst in unreactive solvent. For this purpose it is used basic catalyst such as triethylamine, pyridine, diisopropylethylamine, 2,6-lutidine and 1 ,8-diazabicyclo[5,4,0]undec-7-ene. Triethylamine is most preferably used among these catalysts. Unreactive solvent such as chloroform, dichloromethane, acetonitrile, tetrahydrofuran, benzene and toluene can desirably be used and chloroform is most preferable among these solvents. The reaction is performed at the temperature ranging 60-70 C for 4-48 hours.
The compound of formula [4] produced in the reaction A is reacted with Grignard reagent to produce the compound of formula [1] according to the reaction B. Grignard reagents used preferably in reaction B include the compound of chemical formula-R MgX(R4 is defined as above and X represents halogen) such as methylmagnesium bromide, vinyimagnesium bromide, cyclopropylmagnesium bromide, cyclopropylmethylmagnesium bromide, and phenylmagnesium bromide. From one to five equivalents of Grignard reagent in this reaction is preferably used with one equivalent of compound of formula [4]. Anhydrous diethylether, anhydrous tetrahydrofuran can be used in this reaction and anhydrous tetrahydrofuran is most preferable solvent among these solvents. The reaction is performed at 0-10
C for 0.5-1 h.
The compound of formula [4] is reduced to give compounds of formula [2] or [3] by using reaction C in different way. The reducing agents used preferably in reaction C is sodium borohydride, lithium borohydride, or lithium aluminum hydride, and sodium borohydride is most preferable among these. Either compound of formula [2] or [3] is obtained depending on the amount of reducing reagents in reaction C. Compound of formula [2] is obtained on using three to five equivalents of reducing reagent with one equivalent of compound of formula [4], but compound of formula [3] is obtained on using one equivalent of reagents. The reaction C is performed preferably in the alcohol solvent. The alcohol solvent such as methanol, ethanol, propanol, butanol, isopropyl alcohol can be desirable and ethanol, methanol is most preferable. In the reaction C, compound of formula [2] is prepared at ranging 0-100 C , preferably at room temperature for 1-2h, and the compound of formula [3] is prepared at between -10-0°C for 1-2h.
The Compound of formula [2] obtained from reaction C is cyclized by the following reaction D to produce compound of formula [1], in which R3 and R4 are hydrogen and one of A, B, C and D is nitrogen. Cyclization of reaction D can be done by performing the reactions such as halogen substitution reaction, and intramolecular Mitzunobu reaction, oxidation-dehydroxylation, and Mitzunobu reaction is most preferable in cyclization. In more detail, compound of formula
[2] is reacted with triphenylphosphine and diethylazodicarboxylate in anhydrous tetrahydrofuran solvent. This reaction is generally performed at the temperature between 0 and 100 C , preferably at room temperature for 1-2h.
The compound of formula [1] prepared by following the procedure described above in this invention can be transformed to variety of other compounds.
The 3,4-dialkoxyphenyl compounds of formula [1] prepared from the above method can be separated and purified by general method such as column chromatography, or recrystallization.
The novel 3,4-dialkoxyphenyl compounds of formula [1] in this invention can inhibit the action of detrimental excess of TNF- α and hence can prevent and treat various diseases such as joint inflammation, rheumatoid arthritis, osteoarthritis, sepsis, septic shock, asthma, graft versus host reaction, psoriasis, allergic inflammation and autoimmune disease. The representative compounds of this invention such as compound 12, 37, 43, 63 and 64 in Table 1 showed the oral median lethal dose of more than 3.5g per Kg of body weight in Table 3 which suggests that these compounds are acceptably non-toxic for pharmaceutical use. Therefore, in order to use compounds of formula [1] or pharmaceutically acceptable salt thereof for therapeutic purposes, it will normally be formulated into a pharmaceutical composition in accordance with standard pharmaceutical practice. This invention, therefore, also relates to a pharmaceutical composition comprising an effective, non-toxic amount of a compound of formula [1] and a pharmaceutically acceptable carrier or diluent.
The composition in this invention can be mixed in general pharmaceutical method with pharmaceutically acceptable carrier to give variety of useful pharmaceutical formulations for oral administration such as tablet, capsule, granules, powders, aqueous solutions or suspensions; for injection such as injectable solutions, suspended solutions; for local administration such as suppositories, ointments, creams, gel, spray and patches.
The products according to the invention may be presented in forms permitting administration by the most suitable route and the invention also relates to pharmaceutical compositions containing at least one product according to the invention which are suitable for use in human or
veterinary medicine. These compositions may be prepared according to the customary methods, using one or more pharmaceutically acceptable adjuvants or excipients. The adjuvants comprise, inter alia, diluents, sterile aqueous media and the various non-toxic organic solvents. The compositions may be presented in the form of tablets, pills, granules, powders, aqueous solutions or suspensions, injectable solutions, elixirs or syrups, and can contain one or more agents chosen from the group comprising sweeteners, flavorings, colorings, or stabilizers in order to obtain pharmaceutically acceptable preparations. The choice of vehicle and the content of active substance in the vehicle are generally determined in accordance with the solubility and chemical properties of the product, the particular mode of administration and the provisions to be observed in pharmaceutical practice. For example, excipients such as lactose, sodium citrate, calcium carbonate, dicalcium phosphate and disintegrating agents such as starch, alginic acids and certain complex silicates combined with lubricants such as magnesium stearate, sodium lauryl sulfate and talc may be used for preparing tablets. To prepare a capsule, it is advantageous to use lactose and high molecular weight polyethylene glycols. When aqueous suspensions are used they can contain emulsifying agents or agents which facilitate suspension. Diluents such as sucrose, ethanol, polyethylene glycol, propyiene glycol, glycerol and chloroform or mixtures thereof may also be used. For parenteral administration, emulsions, suspensions or solutions of the products according to the invention in vegetable oil, for example sesame oil, groundnut oil or olive oil, or aqueous-organic solutions such as water and propyiene glycol, injectable organic esters such as ethyl oleate, as well as steril aqueous solutions of the pharmaceutically acceptable salts, are used. The solutions of the salts of the products according to the invention are especially useful for administration by intramuscular or subcutaneous injection. The aqueous
solution, also comprising solutions of the salts in pure distilled water, may be used for intravenous administration with the proviso that their pH is suitably adjusted, that they are judiciously buffered and rendered isotonic with a sufficient quantity of glucose or sodium chloride and that they are sterilized by heating, irradiation or microfiltration. Suitable compositions containing the compounds of the invention may be prepared by conventional means. For example, compounds of the invention may be dissolved or suspended in a suitable carrier for use in a nebulizer or a suspension or solution aerosol, or may be absorbed or adsorbed onto a suitable solid carrier for use in a dry powder inhaler. Solid compositions for rectal administration include suppositories formulated in accordance with known methods and containing at least one compound of formula [1]. The dose employed will be determined by the physician, and depends upon the desired therapeutic effect, the route of administration and the duration of the treatment, and the condition of the patient. In the adult, the doses are generally from 0.001 to 50, preferably 0.001 to 5mg/kg body weight per day by inhalation, from 0.01 to 100, preferably 0.1 to 70, more especially 0.5 to 10mg/kg body weight per day by oral administration, and from 0.001 to 10, preferably 0.01 to 1 mg/kg body weight per day by intravenous administration. In each particular case, the doses will be determined in accordance with the factors distinctive to the subjects to be treated, such as age, weight, general state of health and other characteristics which can influence the efficacy of the medicinal product. The products according to the invention may be administered as frequency as necessary in order to obtain the desired therapeutic effect. It may be necessary to have long-term treatments at the rate of 1 to 4 doses per day, in accordance with the physiological requirements of each particular patient. Generally, the active product may be administered orally 1 to 4 times per day.
The following examples represent this invention and this invention is not
limited in only these examples.
Example 1 3-Cyclopentyloxy-4-methoxynitrobenzene [81
To a solution of 2-methoxy-5-nitrophenol(3g, 17.74mmol) in DMF(30ml) were added cyciopentyl bromide(4g, 26.61 mmol) and potassium carbonate(5g, 35.48mmol). The reaction mixture was stirred at 60 °C for 15h, cooled to room temperature, treated with distilled water(20ml), and extracted twice with ether. The ether layer was dried over gS04, filtered, and concentrated in vacuo to give the title compound(4g, 95%) as a pale yellow solid.
1H-N R(CDCI3, ppm) : 8 1.65-1.68(m, 2H), 1.86-2.02(m, 6H), 3.95(s, 3H),
4.87(m, 1 H), 6.9(d, J=8.9Hz, 1 H), 7.74(d, J=2.6Hz, 1 H), 7.87-7.91 (dd, J=2.6, 8.9 Hz, 1 H).
Example 2 3-Cyclopentyloxy-4-methoxyaniline [91
To a solution of 3-cyclopentyloxy-4-methoxynitrobenzene(4.2g, 17.7mmol) in methanol(30ml) were added ammonium formate(3.5g, 53.2 mmol) and 10% Pd-C(0.3g). The reaction mixture was refluxed for 2h, cooled to room temperature, filtered through Celite, and evaporated in vacuo to remove solvent. The residue was dissolved in ether, washed twice with distilled water, dried over MgS04, filtered, and concentrated in vacuo to give the title compound(3g, 81%) as pale brown liquid.
1H-N R(CDCI3, ppm) : δ 1.60-1.63(m, 2H), 1.85-1.95(m, 6H), 3.05(bs, 2H),
3.78(s, 3H), 4.73(m, 1 H), 6.24(dd, J=2.6, 8.4Hz, 1 H), 6.33(d, J=2.6Hz, 1 H), 6.73(d, J=8.4Hz, 1 H).
Example 3 N-(3-Cyclopentyloxy-4-methoxyphenyl)isoindolin-1.3-dione [101
To a solution of 3-cyclopentyloxy-4-methoxyaniline(0.52g, 2.42mmol) in chloroformdOml) was added phthaiic anhydride(0.36g, 2.43mmol). The reaction
mixture was stirred for 0.5h at room temperature, treated with acetic acidOOml), refluxed for 4h, cooled to room temperature, and then concentrated in vacuo to remove chloroform and acetic acid. The residue was crystallized from methanol to afford the title compound (0.75g, 91%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.60-1.64(m, 2H), 1.82-1.96(m, 6H), 3.90(s, 3H),
4.78(m, 1 H), 6.96-7.00(m, 3H), 7.77-7.80(m, 2H),
7.93-7.96(m, 2H).
Example 4 3-methyl-3-hvdroxy-2-(3-cvclopentyloxy-4-methoxyphenyl)isoind olin-1-one [111
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1 ,3-dione(0.5g, 1.48mmol) in anhydrous THF(IOml) was slowly added 3.0M methylmagnesium bromide(0.2ml, 4.44mmol) at 0°C . The reaction mixture was warmed to room temperature, stirred for 20 minutes, quanched with saturated ammonium chloride solution, and diluted with ethyl acetate. The organic layer was washed with distilled water, dried over MgS04, filtered, and concentrated in vacuo. To the residue was added ether, stirred at room temperature, and filtered to give the title compound(0.35g, 67%) as a white solid.
1H-NMR(CDC13, ppm) : δ 1.15-1.91 (m, 8H), 1.56(s, 3H), 3.86(s, 3H), 4.43(s, 1 H),
4.58(m, 1 H), 6.75(d, J=8.7Hz, 1 H), 6.90(dd, J=8.7, 2.4Hz, 1 H), 6.99(d, J=2.4Hz, 1 H), 7.28-7.56(m, 4H).
Example 5 3-Methyl-2-(3-cyclopentyloxy-4-methoχyplιenyl)isoindolin-1-one [121
To a solution of 3-methyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoi- ndolin -1-one(0.52g, 1.48mmol) in dichioromethane(IOml) were added triethylsilane (1.29ml, 1.78mmol) and trifluoroacetic acid(0.14ml, 1.78mmol). The reaction mixture was stirred for 4h at room temperature, evaporated in vacuo, and then diluted with ethyl acetate. The organic layer was washed with distilled water, dried over MgS04,
filtered, and concentrated in vacuo to give the title compound(0.46g, 92%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.49(d, J=6.3Hz, 3H), 1.64-2.05(m, 8H), 3.92(s, 3H),
4.87(m, 1 H), 5.15(q, J=6.3Hz, 1 H), 6.97(s, 2H), 7.34(s, 1 H), 7.56-7.62(m, 3H), 7.96(d, J=7.5Hz, 1 H).
Example 6 3-Methyleπe-2-(3-cvclopentyloxy-4-methoxyphenyl)isoindolin-1-o ne M31
To a solution of 3-methyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoi- ndolin-1-one(0.52g, 1.48mmol) in benzene(IOml) was added p-toluenesulfonic acid(0.28g, 1.48mmol). The reaction mixture was stirred for 4h at room temperature, diluted with ethyl acetate. The organic layer was washed with distilled water, dried over MgSθ4, filtered, and evaporated in vacuo to remove solvent. The residue was purified by flash chromatography on silica to afford the title compound(0.52g, 91 %) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.64-2.05(m, 8H), 3.29(dd, J=16.8, 3.9Hz, 2H),
3.42(dd, J=16.8, 6.3Hz, 2H), 3.89(s, 3H), 4.85(d, J=1.8Hz, 1 H), 5.20(m, 1 H), 5.26(d, J=1.8Hz, 1 H), 6.95-7.00(m, 3H), 7.16-7.28(m, 2H), 7.55-7.97(m, 2H).
Example 7 3-Cyclopropyl-3-hydroxy-2-(3-cvclopentyloxy-4-methoχyphenyl) isoindolin-1-one [14]
The title compound was prepared following the procedures described in example 4 with 2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1 ,3-dione(0.5g, 1.48mmol) and cyclopropyl magnesium bromide which obtained in situ with Mg(0.1g, 4.44mmol) and cyclopropyl bromide(0.54g, 4.44mmol), as a white solid(0.47g, 84%).
1H-NMR(CDCI3, ppm) : δ 0.50-0.70(m, 4H), 1.60-2.05(m, 9H), 3.45(s, 1H),
3.85(s, 3H), 4.55(m, 1 H), 6.80(d, J=8.7Hz, 1 H), 6.91 (dd, J=8.7, 2.1 Hz, 1 H), 6.98(d, J=2.1 Hz, 1 H),
7.20-7.75(m, 4H).
Example 8 3-Cyclopropyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1- one [151
The title compound was prepared following the procedures described in example 5 with 3-cyclopropyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)iso- indolin-1-one(0.56g, 1.48mmol) as a white solid(0.41g, 76%).
1H-NMR(CDCI3, ppm) : δ 0.50-0.70(m, 4H), 1.60-2.05(m, 9H), 3.90(s, 3H),
4.85(m, 1 H), 5.25(d, J=6.3Hz, 1 H), 6.90-7.00(m, 3H), 7.35-7.95(m, 4H). Example 9 3-Cyclopropylmethyl-3-hydroxy-2-(3-cvclopeπtyloxy-4-methoxyp- henvOisoindolin- 1-one [161
The title compound was prepared following the procedures described in example 4 with 2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1 ,3-dione(0.5g, 1.48mmol) and cyclopropyl methylmagnesium bromide which obtained in situ with Mg(0.1 g, 4.44mmol) and cyclopropyl methyl bromide(0.6g, 4.44mmol), as a white solid(0.46g, 79%).
1H-NMR(CDCI3, ppm) : δ 0.35-0.60(m, 4H), 1.15-1.90(m, 11 H), 3.45(s, 1 H),
3.85(s, 3H), 4.55(m, 1 H), 6.70(d, J=2.4Hz, 1 H), 6.78(d, J=8.7Hz, 1 H), 6.90(dd, J=8.7, 2.4Hz, 1 H), 7.25-7.60(m, 4H).
Example 10 3-Cyclopropylmethyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoi- ndolin-1-one [171
The title compound was prepared following the procedures described in example 5 with 3-cyclopropylmethyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphe- nyl)isoindolin-1-one(0.58g, 1.48mmol) as a white solid(0.43g, 76%).
1H-NMR(CDCI3, ppm) : δ 0.35-0.70(m, 4H), 1.50-2.20(m, 11 H), 3.84(s, 3H),
4.83(m, 1 H), 5.20(m, 1 H), 6.93(m, 2H), 7.28(m, 1 H),
7.46-7.95(m, 4H).
Example 11 3-Phenyl-3-hvdroxy-2-(3-cyclopentyloχy-4-methoxyphenyl)iso- indolin-1-one [181
The title compound was prepared following the procedures described in example 4 with 2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1 ,3-dione(0.5g, 1.48mmol) and phenyimagnesium bromide which obtained in situ with Mg(0.1g, 4.44mmol) and bromobenzene(0.7g, 4.44mmol), as a white solid(0.57g, 93%).
1H-NMR(CDCI3, ppm) : δ 1.30-1.76(m, 8H), 3.78(s, 3H), 3.89(s, 1 H), 4.46(m,
1 H), 6.67(d, J=8.5Hz, 1 H), 6.87(d, J=2.1 Hz, 1 H), 6.90(dd, J=8.5, 2.1 Hz, 1 H), 7.20-7.76(m, 9H).
Example 12 3-Phenyl-2-(3-cvclopentyloxy-4-methoχyphenyl)isoindoHn-1-one £191
The title compound was prepared following the procedures described in example 5 with 3-phenyl-3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin -1-one(0.61g, 1.48mmol) as a white solid(0.51g, 86%).
1H-NMR(CDCI3, ppm) : δ 1.58-2.00(m, 8H), 3.81 (s, 3H), 4.68(m, 1 H), 6.00(s,
1 H), 6.79(d, J=8.5Hz, 1 H), 6.97(dd, J=8.5, 2.1 Hz, 1 H), 7.20-7.32(m, 7H), 7.52(m, 2H), 7.98(d, J=2.1 Hz, 1 H).
Example 13 3-Methyl-2-(3-cvclopentyloxy-4-methoxyphenv0isoindolin-1-thi- one[201
To a solution of 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin -1-one(0.1g, 0.3mmol)in benzene(5ml) and toluene(5ml) was added Lawson's reagent(0.7g, 0.15mmol). The reaction mixture was refluxed for 5h, cooled to room temperature, added distilled water(IOml), and extracted with ethyl acetate. The organic layer was dried over MgS0 , filtered, evaporated in vacuo, and
recrystallized from ether to afford the title compound(0.04g, 40%) as a yellow solid. 1H-NMR(CDCI3, ppm) : δ 1.50(d, J=6.3Hz, 3H), 1.64-2.00(m, 8H), 3.90(s, 3H),
4.85(m, 1 H), 5.10(q, J=6.3Hz, 1 H), 7.34(s, 1 H), 7.50-7.95(m, 4H).
Example 14 3-Hvdroxy-2-(3-cvclopentyloxy-4-methoxypheπyl)isoindolin-1- one[211
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1 ,3-dione(0.5g, 1.48mmol) in methanol(IOml) was added sodium borohydride(0.06g, 1.48mmol) at 0 °C . The reaction mixture was treated with ice-water, extracted with ethyl acetate, dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.48g, 96%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.60-2.00(m, 8H), 3.84(s, 3H), 4.76(m, 1 H), 6.30(s,
1 H), 6.87(d, J=8.5Hz, 1 H), 7.43(d, J=2.1 Hz, 1 H), 7.50-7.80(m, 4H).
Example 15 3-Methoxy-2-(3-cvclopeπtyloxy-4-methoχyphenyl)isoindolin-1- one[22l
To a solution of 3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1- one(0.5g,1.48mmol) in DMF(IOml) were added potassium carbonate(0.4g, 2.96mmol) and iodomethane(0.42g, 2.96mmol). The reaction mixture was stirred for 12h at 60 "C , cooled to room temperature, added distilled waterOOml), and extracted twice with ether. The extract was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.48g, 92%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 2.97(s, 3H), 3.89(s, 3H), 4.86(m,
1 H), 6.39(s, 1 H), 6.94(d, J=8.5Hz, 1 H), 7.24(dd, J=8.5, 2.1 Hz, 1 H), 7.54(d, J=2.1 Hz, 1 H), 7.55-7.95(m, 4H).
Example 16 3-Fluoro-2-(3-cvclopentyloxy-4-methoxyphenyl)isoindolin-1-one
To a solution of 3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1- one(0.5g, 1.48mmol) in dichloromethaneOOml) was added diethylamino sulfurtrifluoride(0.29g, 1.78mmol). The reaction mixture was stirred for 6h at room temperature, washed twice with distilled water, dried over MgS04, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica to afford the title compound(0.24g, 40%) as a white solid.
1H-N R(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.95(s, 3H), 4.85(m, 1 H), 6.75(d,
J=5.7Hz, 1 H), 6.95(d, J=8.5Hz, 1 H), 7.10(dd, J=8.5, 2.1 Hz, 1 H), 7.40(d, J=2.1 Hz, 1 H), 7.65-8.00(m, 4H).
Example 17 3-Azido-2-(3-cvclopentyloxy-4-methoxyphenyl)isoindolin-1-one £241
To a solution of 3-hydroxy-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1- one(0.5g, 1.48mmol) in anhydrous tolueneOOml) were added diphenylphosphory- lazide(0.49g, 1.78mmol) and 1 ,8-diazabicyclo[5,4,0]undec-7-ene(0.27g, 1.78mmol). The reaction mixture was stirred for 4h at room temperature, added ethyl acetate, washed twice with distilled water, dried over MgS04, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica to afford the title compound(0.5g, 94%) as a white solid. H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.90(s, 3H), 4.80(m, 1 H), 6.00(s,
1 H), 6.96(d, J=8.5Hz, 1 H), 7.08(dd, J=8.5, 2.1 Hz, 1 H), 7.38(d, J=2.1 Hz, 1 H), 7.60-7.97(m, 4H).
Example 18 3-Amino-2-(3-cvclopentyloxy-4-methoχyphenyl)isoindolin-1-one [251
The title compound was prepared following the procedures described in example 2 with 3-azido-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one (0.54g, 1.48mmol) as a white solid(0.47g, 94%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.89(s, 3H), 4.84(m, 1 H), 5.87(s,
1 H), 6.88(d, J=8.5Hz, 1 H), 6.96(dd, J=8.5, 2.1 Hz, 1 H), 7.31 (d, J=2.1 Hz, 1 H), 7.55-7.92(m, 4H).
Example 19 6-(3-Cvclopentyloxy-4-metlιoxyphenyl)-6?7-dihvdro-5H-pyrrolo [3.4-b1pyridin-5.6-dioπe [261
To a solution of pyridine-2,3-dicarboxylic acid(1.62g, 9.65mmol) in toluenedOml) was added thionyl chioride(3.45g, 28.95mmol). The reaction mixture was refluxed for 4h, evaporated in vacuo to remove thionyl chloride. To the residue were added dichloromethanedOml), 3-cyclopentyloxy-4-methoxyaniline(2g, 9.65mmol) and triethylamine(2.44g, 25.13mmol), stirred for 6h at room temperature, and then concentrated in vacuo. The resultant mixture was treated with chioroformdOml) and acetic acid(2ml), refluxed for 48h, cooled to room temperature, concentrated in vacuo to remove chloroform and acetic acid, added ethanol, and stirred at room temperature. The resultant solids were filtered to give the title compound(1.5g, 46%) as a yellow solid.
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.91 (s, 3H), 4.77(m, 1 H),
6.95-7.00(m, 3H), 7.75(m, 1 H), 8.30(m, 1 H), 9. 05(m, 1 H).
Example 20 (A) N2-(3-Cvclopentyloxy-4-methoχyphenyl)-3-(hvdroxymethyl) -2-pyridine carboxamide [27]
To a solution of 6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo [3,4-b]pyridin-5,7-dione(0.5g, 1.48mmol) in methanol(IOml) was slowly added sodium boro hydride(0.28g, 7.41 mmol) at room temperature. The reaction mixture was stirred for 1 h at room temperature, evaporated in vacuo, added distilled waterdOml), and extracted twice with ethyl acetate. The organic layer was dried over MgS04, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica to afford the title compound(0.23g, 46%) as a white solid.
1H-N R(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.86(s, 3H), 4.83(m, 1 H), 4.91 (s,
2H), 5.09(bs, 1 H), 6.88(d, J=8.5Hz, 1 H), 7.18(dd, J=8.5, 2.2Hz, 1 H), 7.40-7.50(m, 2H), 7.81 (dd, J=8.1 , 1.2Hz, 1 H), 8.57(m, 1 H), 10.23(bs, 1 H).
(B) N3-(3-Cvclopentyloxy-4-methoxyphenyl)-2-(hvdroxymethyl)nicotinamide I28J
To a solution of 6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo [3,4-b]pyridin-5,7-dione(0.5g, 1.48mmol) in methanol(IOml) was slowly added sodium boro hydride(0.28g, 7.41 mmol) at room temperature. The reaction mixture was stirred for 1h at room temperature, evaporated in vacuo, added distilled waterdOml), and extracted twice with ethyl acetate. The organic layer was dried over MgS04, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica to afford of the title compound(0.24g, 48%) as a white solid.
1H-N R(CDCI3, ppm) : δ 1.50-2.05(m, 8H), 3.85(s, 3H), 4.79(m, 1 H), 4.94(s,
2H), 5.56(bs, 1 H), 6.85(d, J=8.7Hz, 1 H), 7.08(dd, J=8.7, 2.2Hz, 1 H), 7.33(dd, J=5.1 ,Hz, 1 H), 8.12(m, 1 H), 8.55(m, 1 H), 9.33(bs, 1 H).
Example 21 (A) 6-(3-Cvclopentyloxy-4-methoχyphenyl)-6.7-dihvdro-5H- pyrrolo[3.4-blpyridin-7-one [291
To a solution of N2-(3-cyclopentyloxy-4-methoxyphenyl)-3-(hydroxymethyl)-2- pyridine carboxamide(0.4g, 1.17mmol) in anhydrous THFdOml) were added triphenylphosphine(0.37g, 1.41 mmol) and diethylazodicarboxylate(0.25g, 1.41 mmol) at room temperature. The reaction mixture was stirred for 1 h at room temperature, evaporated in vacuo, treated with 6N HCl solutiondOml), and extracted with ethyl acetate. The aqueous layer was basified to pH 8-9 with 6N NaOH solution, and extracted with ethyl acetate. Then, the resultant organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.34g, 89%) as a white solid.
H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.85(s, 3H), 4.82(s, 2H), 4.85(m,
1 H), 6.87(d, J=8.5Hz, 1 H), 7.09(dd, J=8.5, 2.1 Hz, 1 H), 7.78(d, J=4.8Hz, 1 H), 7.87(dd, J=7.8, 1.5Hz, 1 H), 8.77(dd, J=4.8, 1.5Hz, 1 H).
(B) 6-(3-Cvclopentyloxy-4-methoxyphenyl)-6.7-dihydro-5H-pyrrolo[3.4-b1 pyridin-5-one [301
The title compound was prepared following the procedures described in example 21 (A) with N3-(3-cyclopentyloxy-4-methoxyphenyl)-2-(hydroxymethyl)nicot- inamide(0.4g, 1.17mmol) as a white solid(0.35g, 92%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.86(s, 3H), 4.84(m, 1 H), 4.88(s,
2H), 6.90(d, J=8.5Hz, 1 H), 7.04(dd, J=8.5, 2.1 Hz, 1 H), 7.45(dd, J=7.8, 4.8Hz, 1 H), 7.81 (d, J=2.1 Hz, 1 H), 8.16(d, J=7.8Hz, 1 H), 8.77(d, J=4.8, 1 H).
Example 22 β-(3-Cvclopentyloxy-4-methoxyphenyl)-7-hydroxy-7-methyl-6.7 -dihydro-5H-pyrrolo[3.4-b1pyridin-5-one [311
The title compound was prepared following the procedures described in example 4 with 6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo[3,4-b] pyridin-5,6-dione(0.5g, 1.48mmol) as a white solid(0.5g, 96%).
1H-N R(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 1.75(s, 3H), 3.90(s, 3H), 4.55(s,
1 H), 4.75(m, 1 H), 6.92(d, J=8.5Hz, 1 H), 7.05-7.10(m, 2H), 7.44(dd, J=7.8, 1.8Hz, 1 H), 8.10(dd, J=7.8, 1.5Hz, 1 H), 8.73(dd, J=4.8, 1.5Hz, 1 H).
Example 23 β-O-Cvclopentyloxy^-methoxyphenvD^-methylene-β,?- dihvdro-5H-pyrrolo[3.4-b]pyridin-5-one [321
The title compound was prepared following the procedures described in example 6 with 6-(3-cyclopentyloxy-4-methoxyphenyl)-7-hydroxy-7-methyl-6,7-
dihydro-5H-pyrrolo[3,4-b]pyridin-5-one(0.52g, 1.48mmol) as a white solid(0.45g,
91 %).
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.86(s, 3H), 4.85(d, J=1.8Hz, 1 H),
4.95(m, 1 H), 5.75(d, J=1.8Hz, 1 H), 7.05-7.10(m, 2H), 7.50(dd, J=7.8, 4.8Hz, 1 H), 8.22(dd, J=7.8, 1.5Hz, 1 H), 8.85(dd, J=4.8, 1.5Hz, 1 H).
Example 24 6-(3-Cvclopentyloxy-4-methoxyphenyl)-7-methyl-6?7-dihvdro-5H -pyrrolo[3T4-b1pyridin-5-one [331
To a solution of 6-(3-cyclopentyloxy-4-methoxyphenyl)-7-methylene-6,7-dihydro -5H-pyrrolo[3,4-b]pyridin-5-one(0.5g, 1.48mmol) in methanol(30ml) were added ammonium formate(0.3g, 4.44mmol) and 10% Pd-C(0.1g). The reaction mixture was stirred for 1 h at room temperature, filtered through Celite, and evaporated in vacuo to remove methanol. The residue was diluted with ethyl acetate, washed twice with distilled water, dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.48g, 96%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.55(d, J=6.7Hz, 3H), 1.60-2.05(m, 8H), 3.89(s, 3H),
4.83(m, 1 H), 5.12(q, J=6.7Hz, 1 H), 6.96(m, 2H), 7.46(dd, J=7.8, 4.8Hz, 1H), 8.19(dd, J=7.8, 1.5Hz, 1 H), 8.80(dd, J=4.8, 1.5Hz, 1 H).
Example 25 2-(3-Cvclopentyloxy-4-methoxyphenyl)-2.3-dihydro-1 H-pyrrolo [3.4-c]pyridin-1 ,3-dione [341
The title compound was prepared following the procedures described in example 19 with pyridine-3,4-dicarboxylic acid(1.62g, 9.65mmol) as a yellow solid(2.8g, 86%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.90(s, 3H), 4.75(m, 1 H), 6.92(d,
J=8.5Hz, 1H), 7.07(m, 2H), 7.44(dd, J=7.8, 4.8Hz, 1 H), 8.10(dd, J=7.8, 1.5Hz, 1 H), 8.72(dd, J=4.8, 1.5Hz, 1 H).
Example 26 (A) N4-(3-Cvclopentyloxy-4-methoxyphenyl)-3-(hvdroxymethyl) isonicotinamide [35]
The title compound was prepared following the procedures described in example 20(A) with 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1 ,3-dione(0.5 g, 1.48mmol) as a white solid(0.25g, 50%) purified by flash chromatography on silica.
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.85(s, 3H), 4.52(s, 1H), 4.74(d,
J=4.5Hz, 1 H), 4.79(m, 1 H), 6.84(d, J=8.5Hz, 1 H), 7.07(dd, J=8.5, 2.1Hz, 1 H), 7.38(m, 2H), 8.61 (d, J=4,8Hz, 1 H), 8.86(s, 2H).
(B) N3-(3-Cvclopentyloxy-4-methoχyphenyl)-4-(hvdroxymethyl)nicotinamide [361
The title compound was prepared following the procedures described in example 20(B) with 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1 ,3-dione(0.5g, 1.48mmoI) as a white solid(0.21 g, 42%) purified by flash chromatography on silica.
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.87(s, 3H), 4.08(s, 1 H), 4.80(s,
2H), 4.82(m, 1 H), 6.84(d, J=8.5Hz, 1 H), 7.07(dd, J=8.5, 2.1 Hz, 1 H), 7.46(d, J=2.4Hz, 1 H), 7.67(d, J=5.1 Hz, 1 H), 8.62(s, 1H), 8.71 (d, J=5.1 Hz, 1 H), 9.07(bs, 1 H).
Example 27 (A) 2-(3-Cvclopeπtyloxy-4-methoχyphenyl)-2.3-dihvdro-1 H- pyrrolo[3.4-c]pyridin-1-one [371
The title compound was prepared following the procedures described in example 21 (A) with N4-(3-cyclopentyloxy-4-methoxyphenyl)-3-(hydroxymethyl) isonicotinamide(0.4g, 1.17mmol) as a white solid(0.36g, 94%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.88(s, 3H), 4.85(m, 1 H), 4.87(s,
2H), 6.91 (d, J=8.5Hz, 1 H), 7.02(dd, J=8.5, 2.1 Hz, 1 H),
7.51 (d, J=5.1 Hz, 1 H), 7.82(d, J=2.1 Hz, 1 H), 8.82(d, J=5.1 Hz, 1 H), 9.18(s, 1 H).
(B) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-2.3-dihvdro-1 H-pyrrolo[3.4-c1 pyridin-1-one [381
The title compound was prepared following the procedures described in example 21 (A) with N3-(3-cyclopentyloxy-4-methoxyphenyl)-4-(hydroxymethyl) nicotinamide(0.4g, 1.17mmol) as a white solid(0.35g, 92%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.07(m, 8H), 3.90(s, 3H), 4.85(m, 1 H), 4.96(s,
2H), 6.92(d, J=8.5Hz, 1 H), 7.06(dd, J=8.5, 2.1 Hz, 1 H), 7.81 (d, J=5.1 Hz, 1 H), 7.82(d, J=2.1 Hz, 1 H), 8.84(d, J=5.1 Hz, 1 H), 8.93(s, 1 H).
Example 28 (A) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-hvdroxy-3- met yl-2-3-dihydro-1 H-pyrrolo[3T4-c]pyridin-1-one[391
The title compound was prepared following the procedures described in example 4 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-1 ,3-dione(0.5g, 1.48mmol) as a white solid(0.27g, 51%) purified by flash chromatography on silica.
1H-NMR(CDCI3, ppm) : δ 1.15-1.90(m, 8H), 1.81 (s, 3H), 3.86(s, 3H), 4.43(s,
1 H), 4.58(m, 1 H), 6.70(d, J=8.7Hz, 1 H), 6.95(dd, J=8.7, 2.4Hz, 1 H), 6.99(d, J=2.4Hz, 1 H), 7.45(dd, J=5.1 , 1.5Hz, 1 H), 8.80(d, J=5.1 Hz, 1 H), 9.15(s, 1 H).
(B) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-1-hydroxy-1-methyl-2T3 -dihydro-pyrrolo[3.4-c]pyridin-3-one [401
The title compound was prepared following the procedures described in example 4 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo
[3,4-c]pyridin-1 ,3-dione(0.5g, 1.48mmol) as a white solid(0.23g, 43%) purified by
flash chromatography on silica.
1H-N R(CDCI3, ppm) :δ 1.15-1.95(m, 8H), 1.81(s, 3H), 3.89(s, 3H), 4.50(s, 1H),
4.57(m, 1H), 6.72(d, J=8.7Hz, 1H), 7.06(dd, J=8.7, 2.4Hz, 1H), 7.08(d, J=2.4Hz, 1H), 7.70(dd, J=5.1, 1.5Hz, 1H), 8.82(d, J=5.1Hz, 1H), 9.17(s, 1H).
Example 29 (A) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-methylene-2.3 -dihvdro-1H-pyrrolo[3.4-c1pyridin-1-one [411
The title compound was prepared following the procedures described in example 6 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-hydroxy-3-methyl-2,3- dihydro-1H-pyrrolo[3,4-c]pyridin-1-one(0.52g, 1.48mmol) as a white solid(0.45g, 91%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.05(m, 8H), 3.91 (s, 3H), 4.85(m, 1H), 4.90(d,
J=1.8Hz, 1H), 6.95(m, 2H), 7.25(s, 1H), 7.45(d, J=5.1Hz, 1H), 8.90(d, J=5.1Hz, 1H), 9.20(s, 1H).
(B) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-1-methylene-2.3-dihvdro-1H -pyrrolo[3.4-c1pyridin-3-one [421
The title compound was prepared following the procedures described in example 6 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-1-hydroxy-1-methyl-2,3- dihydro-1H-pyrrolo[3,4-c]pyridin-3-one(0.52g, 1.48mmol) as a white solid(0.43g, 87%).
1H-NMR(CDCI3, ppm) : 8 1.50-2.10(m, 8H), 3.95(s, 3H), 4.83(m, 1H), 4.85(d,
J=2.1Hz, 1H), 5.24(d, J=2.1Hz, 1H), 6.93(m, 2H), 7.01 (dd, J=8.5, 2.4Hz, 1H), 7.79-7.83(m, 2H), 8.80-8.95(m, 2H).
Example 30 (A) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-methyl-2.3- dihvdro-1 H-pyrrolo[3.4-c1pyridin-1-one [431
The title compound was prepared following the procedures described in example 24 with _2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methylene-2,3-dihydro -1 H-pyrrolo[3,4-c]pyridin-1-one(0.5g, 1.48mmol) as a white solid(0.49g, 94%).
1H-NMR(CDCI3, ppm) : δ 1.24(d, J=6.8Hz, 3H), 1.60-2.05(m, 8H), 3.90(s, 3H),
4.82(m, 1 H), 5.14(q, J=6.8Hz, 1 H), 6.91 (m, 2H), 7.24(d, J=2.1 Hz, 1 H), 7.47(d, J=5.1 Hz, 1 H), 8.84(d, J=5.1 Hz, 1 H), 9.19(s, 1 H).
(B) 2-(3-Cvclopentyloxy-4-methoχyphenyl)-1 -methyl-2,3-dihvdro-1 H -pyrrolo[3.4-c1pyridin-3-one [441
The title compound was prepared following the procedures described in example 24 with _2-(3-cyclopentyloxy-4-methoxyphenyl)-1-methylene-2,3-dihydro -1 H-pyrroIo[3,4-c]pyridin-3-one(0.5g, 1.48mmol) as a white solid(0.49g, 94%).
1H-NMR(CDCI3, ppm) : δ 1.50(d, J=6.9Hz, 3H), 1.60-2.05(m, 8H), 3.88(s, 3H),
4.83(m, 1 H), 5.25(q, J=6.9Hz, 1 H), 6.98(m, 2H), 7.26(d, J=2.4Hz, 1 H), 7.77(d, J=5.1 Hz, 1 H), 8.83(d, J=5.1 Hz, 1 H), 8.91 (s, 1 H).
Example 31 2-(3-Cvclopentyloxy-4-methoxyphenyl)-3.5.6-trimethyl-isoindolin -1-one [451
The title compound was prepared following the procedures described in example 4 and 5 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-5,6-dimethyl-isoindolin -1 ,3-dione(0.54g, 1.48mmol) as a white solid(0.47g, 87%).
1H-N R(CDCI3, ppm) : δ 1.47(d, J=6.3Hz, 3H), 1.65-2.00(m, 8H), 2.30(s, 3H),
2.32(s, 3H), 3.93(s, 3H), 4.84(m, 1 H), 5.05(q, J=6.3Hz, 1 H), 6.90(m, 2H), 7.01 (s, 1 H), 7.02(s, 1 H), 7.20(d, J=8.4Hz, 1 H).
Example 32 3-Methyl-2-(3-cvclopeπtyloxy-4-methoxyphenyl)-7-hvdroxy -isoindolin-1-one [461
The title compound was prepared following the procedures described in example 4 and 5 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-4-hydroxy-isoindolin -1 ,3-dione(0.52g, 1.48mmol) as a white solid(0.39g, 75%).
1H-NMR(CDCI3, ppm) : δ 1.48(d, J=6.3Hz, 3H), 1.65-2.00(m, 8H), 3.92(s, 3H),
4.85(m, 1 H), 5.10(q, J=6.3Hz, 1 H), 6.96(m, 2H), 7.24(d, J=8.4Hz, 1 H), 7.49(d, J=6.6Hz, 1 H), 7.67(m, 1 H), 7.82(bs, 1 H).
Example 33 3-Methyl-2-(3-cvclopentyloxy-4-methoxyphenyl)-7-methoxy -isoindolin-1-one [471
To a solution of 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-7-hydroxy -isoindolin-1-one(0.2g, 0.59mmol) in dichloromethane(I Oml) was added methyltrifluoromethanesuifonate(0.2g, 1.18mmol). The reaction mixture was stirred for 2h at room temperature, and washed twice with distilled water. The organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.19g, 90%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.45(d, J=6.3Hz, 3H), 1.65-2.00(m, 8H), 3.93(s, 3H),
4.84(m, 1 H), 5.05(q, J=6.3Hz, 1 H), 6.90-7.30(m, 6H).
Example 34 3-Methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-4.5.6T7- tetrafluoro-isoindolin-1-one [481
The title compound was prepared following the procedures described in example 4 and 5 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-4,5,6,7-tetrafluoro- isoindolin-1 ,3-dione(0.6g, 1.48mmol) as a white solid(0.49g, 75%). H-NMR(CDC13, ppm) : δ 1.44(d, J=6.3Hz, 3H), 1.65-2.05(m, 8H), 3.94(s, 3H),
4.85(m, 1 H), 5.04(q, J=6.3Hz, 1 H), 6.85-6,98(m, 3H).
Example 35 3-Methyl-2-(3-cvclopentyloxy-4-methoxyphenyl)-6- benzylamino-isoindolin-1-one [491
The title compound was prepared following the procedures described in example 4 and 5 with 6-benzylamino-2-(3-cyclopentyloxy-4-methoxyphenyl) isoindolin-1 ,3-dione(0.65g, 1.48mmol ) as a white solid(0.51g, 78%).
1H-N R(CDCI3, ppm) : δ 1.44(d, J=6.3Hz, 3H), 1.65-2.00(m, 8H), 3.94(s, 3H),
4.78(d, J=5.4Hz, 1 H), 4.84(m, 1 H), 5.04(q, J=6.3Hz, 1 H), 6.92-7.80(m, 1 H)
Example 36 3-Methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino- isoindolin-1-one [501
The title compound was prepared following the procedures described in example 2 with 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-benzylamino- isoindolin-1-one(0.65g,1.48mmol) as a white solid(0.42g, 80%).
1H-N R(CDCI3, ppm) : δ 1.30-2.10(m, 8H), 1.40(d, J=6.3Hz, 3H), 3.88(s, 3H),
4.83(m, 1H), 5.03(q, J=6.3Hz, 1H), 6.85-7.05(m, 3H), 7.20-7.90(m, 3H).
Example 37 (A) 3-Methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6 -methylamino-isoindolin-1-one [511
The title compound was prepared following the procedures described in example 33 with 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin -1-one(0.52g, 1.48mmol) as a white solid(0.23g, 42%) purified by flash chromatography on silica.
1H-N R(CDCI3, ppm) : δ 1.30-2.10(m, 8H), 1.43(d, J=6.3Hz, 3H), 2.98(d,
J=4.7Hz, 3H), 3.87(s, 3H), 4.84(m, 1 H), 5.02(q, J=6.3Hz, 1 H), 6.85-7.04(m, 3H), 7.10-7.90(m, 3H).
(B) 3-Methyl-2-(3-cvclopentyloxy-4-methoxyphenyl)-6-dimethylamino -isoindolin-1-one [521
The title compound was prepared following the procedures described in example 33 with 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino-isoindolin -1-one(0.52g, 1.48mmol) as a white solid(0.44g, 78%) purified by flash chromatography on silica. H-N R(CDCI3, ppm) : δ 1.30-2.10(m, 8H), 1.42(d, J=6.3Hz, 3H), 3.15(s, 6H),
3.85(s, 3H), 4.82(m, 1 H), 5.01 (q, J=6.3Hz, 1 H), 6.80-7.00(m, 3H), 7.10-7.90(m, 3H).
Example 38 3-Methyl-2-(3.4-dimethoχyphenyl)isoindolin-1-one [531
The title compound was prepared following the procedures described in example 4 and 5 with 2-(3,4-dimethoxyphenyl)isoindolin-1 ,3-dione(0.42g,1.48mmol) as a white soiid(0.29g, 69%).
1H-NMR(CDCI3, ppm) : δ 1.48(d, J=6.3Hz, 3H), 1.50-2.00(m, 8H), 3.90(s, 3H),
3.94(s, 3H), 4.87(m, 1 H), 5.10(q, J=6.3Hz, 1 H), 6.98(s, 1H), 7.33(s, 1H), 7.50-7.95(m, 4H).
Example 39 3-Methyl-2-(3-cvclopentylmethyloxy-4-methoxyphenyl)isoindolin -1-one [541
The title compound was prepared following the procedures described in example 4 and 5 with 2-(3-cyclopentylmethyloxy-4-methoxyphenyl)isoindolin-1 ,3- dione(0.52g, 1.48mmol) as a white solid(0.43g, 82%).
1H-NMR(CDCI3, ppm) : δ 1.40-1.90(m, 8H), 1.45(d, J=6.3Hz, 3H), 2.49(m, 1 H),
3.90(s, 1 H), 3.95(d, J=7.2Hz, 2H), 5.12(q, J=6.3Hz, 1 H), 6.95(d, J=8.5Hz, 1 H), 7.19(dd, J=8.5, 2.1 Hz, 1 H), 7.52(d, J=2.1 Hz, 1 H), 7.55-7.92(m, 3H), 7.93(d, J=7.5Hz, 1 H).
Example 40 3-Methyl-2-(3-cvclohexyloxy-4-methoχyphenyl)isoindolin-1-one £551
The title compound was prepared following the procedures described in
example 4 and 5 with 2-(3-cyclohexyloxy-4-methoxyphenyl)isoindolin-1 ,3-dione (0.52g,1.48mmol)as a white solid(0.45g, 82%).
1H-NMR(CDCI3, ppm) : δ 1.20-2.10(m, 10H), 1.37(d, J=6.7Hz, 3H), 3.90(s, 3H),
4.25(m, 1 H), 5.11 (q, J=6.7Hz, 1 H), 6.93-6.99(m, 2H),
7.28(s, 1 H), 7.48-7.97(m, 4H).
Example 41 3-Methyl-2-{3-(1-indanyloxy)-4-methoxyphenyl}isoindolin-1-one [561
The title compound was prepared following the procedures described in example 4 and 5 with 2-{3-(1-indanyloxy)-4-methoxyphenyl}isoindolin-1 ,3-dione (0.57g,1.48mmol) as a white solid(0.48g, 84%).
1H-NMR(CDCI3, ppm) : δ 1.49(d, J=6.6Hz, 3H), 3.30-3.47(m, 4H), 3.89(s, 3H),
5.11 (q, J=6.7Hz, 1H), 5.27(m, 1 H), 6.99(m, 2H), 6.99-7.28(m, 4H), 7.41 (d, J=1.8Hz, 1 H), 7.52-7.97(m, 4H).
Example 42 3-Methyl-2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1-one £571
The title compound was prepared following the procedures described in example 4 and 5 with 2-{3-(2-indanyloxy)-4-methoxyphenyl}isoindolin-1 ,3-dione (0.57g, 1.48mmol) as a white solid(0.46g, 84%).
1H-NMR(CDCI3, ppm) : δ 1.45(d, J=6.6Hz, 3H), 1.80-3.10(m, 4H), 3.90(s, 3H),
5.10(q, J=6.7Hz, 1H), 5.25(m, 1 H), 6.98(m, 2H), 7.10-8.00(m, 9H).
Example 43 6-(3-Cyclopentyloxy-4-methoxyphenyl)-6.7-dihvdro-5H-pyrrolo [3T4-b1pyridin-7-one • hydrogen chloride [581
To a solution of 6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H- pyrrolo[3,4-b]pyridin-5-one(0.48g, 1.48mmol) in ethyl acetate was added cone.
HCI(0.5ml). The reaction mixture was stirred at room temperature. The resultant solid was filtered to give the title compound(0.5g, 94%) as a white solid.
1H-NMR(DMSO-d6, ppm) : δ 1.60-2.05(m, 8H), 3.75(s, 3H), 4.75(m, 1 H), 5.01 (s,
2H), 6.95(d, J=8.5Hz, 1 H), 6.96(m, 2H), 7.23(dd, J=8.5, 2,1 Hz, 1 H), 7.53(dd, J=7.5, 1.5Hz, 1 H), 7.68(d, J=2.1 Hz, 1 H), 7.80(d, J=7.5, 1 H), 8.86(m, 1 H).
Example 44 6-(3-Cvclopentyloxy-4-methoxyphenyl)-6.7-dihvdro-5H-pyrrolo [3.4-b1pyridin-5-one • hydrogen chloride [591
The title compound was prepared following the procedures described in example 43 with 6-(3-cyclopentyloxy-4-methoxyphenyl)-6,7-dihydro-5H-pyrrolo [3,4-b]pyridin-5-one(0.48g,1.48mmo0 as a yellow solid(0.48g, 90%).
1H-NMR(DMSO-d6, ppm) : δ 1.60-2.05(m, 8H), 3.80(s, 3H), 4.78(m, 1 H), 4.96(s,
2H), 6.92(d, J=8.5Hz, 1 H), 7.22(dd, J=8.5, 2,1 Hz, 1 H), 7.54(dd, J=7.5, 1.5Hz, 1 H), 7.62(d, J=2.1 Hz, 1 H), 7.80(d, J=7.5, 1 H), 8.86(m, 1 H).
Example 45 6-(3-Cvclopentyloxy-4-methoχyphenyl)-7-methyl-6.7-dihvdro -5H-pyrrolof3.4-b1pyridin-5-one - hydrogen chloride [601
The title compound was prepared following the procedures described in example 43 with 6-(3-cyclopentyloxy-4-methoxyphenyl)-7-methyl-6,7-dihydro-5H- pyrrolo[3,4-b]pyridin-5-one(0.5g, 1.48mmol) as a yellow solid(0.52g, 94%).
1H-NMR(DMSO-d6, ppm) : δ 1.55(d, J=6.7Hz, 3H), 1.60-2.05(m, 8H), 3.73(s,
3H), 4.78(m, 1 H), 5.27(q, J=6.7Hz, 1 H), 6.99(m, 2H), 7.20(s, 1 H), 7.36(m, 2H), 8.19(dd, J=7.5, 1.5Hz, 1 H), 8.85(m, 1 H).
Example 46 2-(3-Cvclopentyloxy-4-methoχyphenyl)-2.3-dihvdro-1 H-pyrrolo [3.4-c]pyridin-1-one • hydrogen chloride [611
The title compound was prepared following the procedures described in example 43 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1-one(0.48g,1.48mmol) as a yellow solid(0.47g, 88%).
1H-NMR(DMSO-d6, ppm) : δ 1.60-2.10(m, 8H), 3.75(s, 3H), 4.82(m, 1 H), 5.03(s,
2H), 6.97(d, J=8.5Hz, 1 H), 7.17(dd, J=8.5, 2,1 Hz, 1 H), 7.55(m, 1 H), 7.68(d, J=2.1 Hz, 1 H), 8.81 (d, J=5.5, 1 H), 9.15(s, 1 H).
Example 47 2-(3-Cvclopentyloxy-4-methoχyphenyl)-3-methyl-2.3-dihvdro- 1 H-pyrrolo[3.4-c1pyridin-1-one • hydrogen chloride [621
The title compound was prepared following the procedures described in example 43 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H- pyrrolo[3,4-c]pyridin-1-one(0.5g, 1.48mmol) as a yellow solid(0.52g, 94%).
1H-NMR(DMSO-d6, ppm) : δ 1.55(d, J=6.7Hz, 3H), 1.60-2.05(m, 8H), 3.73(s,
3H), 4.78(m, 1 H), 5.27(q, J=6.7Hz, 1 H), 6.99(m, 2H), 7.20(s, 1 H), 7.36(m, 2H), 8.19(dd, J=7.5, 1.5Hz, 1 H), 8.85(m, 1 H).
Example 48 1-Oxo-2-(3-cvclopentyloxy-4-methoxyphenyl)-2.3-dihydro-1 H -pyrrolo[3.4-c1Pyridin-5-ium-5-olate [631
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1-one(0.48g, 1.48mmol) in THFdOml) were added hydrogen peroxide(5ml) and acetic acid(5ml) at room temperature. The reaction mixture was refluxed for 12h, evaporated in vacuo, diluted with ethyl acetate, and washed twice with distilled water. The organic layer was dried over MgS04, filtered, and concentrated in vacuo. The residue was purified by flash chromatography on silica to afford the title compound(0.21g, 42%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.60-2.06(m, 8H), 3.85(s, 3H), 4.75(s, 2H), 4.87(m,
1 H), 6.85-7.03(m, 2H), 7.82(d, J=2.1Hz, 1H), 7.90-7.93(m, 2H), 9.05(s, 1 H).
Example 49 1-Oxo-2-(3-Cyclopentyloxy-4-methoxyphenyl)-3-methyl-2.3- dihvdro-1 H-pyrrolo[3.4-c]pyridin-5-ium-5-olate [641
The title compound was prepared following the procedures described in example 48 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl-2,3-dihydro-1 H- pyrrolo[3,4-c]pyridin-1-one(0.5g, 1.48mmol) as a white solid(0.24g, 46%).
1H-N R(CDCI3, ppm) : δ 1.50(d, J=6.9Hz, 3H), 1.60-2.05(m, 8H), 3.85(s, 3H),
4.84(m, 1 H), 5.10(q, J=6.9Hz, 1 H), 6.90(m, 2H), 7.23(d, J=2.1 Hz, 1 H), 8.10(m, 2H), 9.10(s, 1 H).
Example 50 2-(3-Cvclopentyloxy-4-methoχyphenyl)-6-nitro-2.3-dihvdro-1 H -pyrrolo[3.4-c]pyridin-1 ,3-dione [651
The title compound was prepared following the procedures described in example 19 with 6-nitro-3,4-pyridinedicarboxyiic acid(2g, 9.65mmol) as a yellow solid(2.5g, 67%). H-NMR(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.91 (s, 3H), 4.79(m, 1 H),
6.93-7.00(m, 3H), 9.15(s, 1 H), 9.20(s, 1 H).
Example 51 (A) N4-(3-Cvclopentyloxy-4-methoxyphenyl)-5-hvdroxymethyl -2-nitro-isonicotinamide [661
The title compound was prepared following the procedure described in example 20(A) with 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-nitro-2,3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1 ,3-dione(0.57g, 1.48mmol) as a white soiid(0.23g, 40%) purified by flash chromatography on silica.
1H-NMR(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.87(s, 3H), 4.50(bs, 1 H), 4.82(m,
1 H), 4.92(s, 2H), 6.84(d, J=8.7Hz, 1 H), 6.99(dd, J=8.7, 2.4Hz, 1 H), 7.40(d, J=2.4Hz, 1 H), 8.90(s, 1 H), 9.05(s, 1 H).
(B) N3-(3-Cyclopentyloxy-4-methoxyphenyl)-4-hvdroxymethyl-6-nitro-nicotin amide [671
The title compound was prepared following the procedures described in example 20(B) with 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-nitro-2,3-dihydro-1 H- pyrrolo[3,4-c]-pyridin-1 ,3- dione(0.57g, 1.48mmol) as a white solid(0.25g, 43%) purified by flash chromatography on silica.
1H-N R(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.85(s, 3H), 4.50(bs, 1H), 4.84(m,
1 H), 4.90(s, 2H), 6.85(d, J=8.7Hz, 1 H), 6.98(dd, J=8.7, 2.4Hz, 1 H), 7.41 (d, J=2.4Hz, 1 H), 8.60(s, 1 H), 9.11 (s, 1 H).
Example 52 (A) 2-(3-Cvclopentyloxy-4-methoχyphenyl)-6-πitro-2.3- dihvdro-1H-pyrrolo[3.4-clpyridin-1-one [681
The title compound was prepared following the procedures described in example 21 (A) with N4-(3-cyclopentyloxy-4-methoxyphenyl)-5-hydroxymethyl-2- nitro- isonicotin-amide(0.45g, 1.17mmol) as a white solid(0.38g, 88%).
1H-N R(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.88(s, 3H), 4.87(m, 1 H), 4.95(s, 2H),
6.92(d, J=8.7Hz, 1 H), 7.05(dd, J=8.7, 2.4Hz, 1 H), 7.83(d, J=2.4Hz, 1 H), 8.89(s, 1 H), 9.05(s, 1 H).
(B) 2-(3-Cvclopentyloxy-4-methoxyphenyl)-6-nitro-2.3-dihvdro-1 H- pyrrolo[3.4-c]pyridin-3-one [691
The title compound was prepared following the procedures described in example 21 (A) with N3-(3-cyclopentyloxy-4-methoxyphenyl)-4-hydroxymethyl-6-nitro -nicotinamide(0.45g, 1.17mmol) as a white solid(0.4g, 93%).
1H-N R(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.89(s, 3H), 4.89(m, 1 H), 4.97(s, 2H),
6.93(d, J=8.7Hz, 1 H), 7.06(dd, J=8.7, 2.4Hz, 1 H), 7.81 (d, J=2.4Hz, 1 H), 8.30(s, 1H), 8.90(s, 1 H).
Example 53 (A) 6-Amino-2-(3-cyclopentyloxy-4-methoχypheπyl)-2.3- dihvdro-1 H-pyrrolo[3.4-c1pyridin-1-one [701
The title compound was prepared following the procedures described in example 2 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-nitro-2,3-dihydro-1H- pyrrolo[3,4-c]pyridin-1-one(0.43g, 1.17mmol) as a white solid(0.37g, 92%).
1H-NMR(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.86(s, 3H), 4.76(s, 2H), 4.87(m, 1 H),
6.80-7.45(m, 3H), 7.90(d, J=2.4Hz, 1 H), 8.20(s, 1 H).
(B) 6-Amino-2-(3-Cvclopentyloxy-4-methoχyphenyl)-2.3-dihvdro-1 H- pyrrolo[3.4-c1pyridin-3-one [711
The title compound was prepared following the procedures described in example 2 with 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-nitro-2,3-dihydro-1 H-pyrrolo[3,4-c] pyridin-3-one(0.43g, 1.17mmol) as a white solid(0.38g, 95%).
1H-N R(CDCI3) ppm) : δ 1.60-2.10(m, 8H), 3.88(s, 3H), 4.75(s, 2H), 4.89(m, 1 H),
6.80-7.45(m, 3H), 7.89(d, J=2.4Hz, 1 H), 8.80(m, 1 H).
Example 54 (A) 6-Benzylamino-2-(3-cvclopentyloxy-4-methoxyphenyl)-1
-methylene-2.3-dihvdro-1 H-pyrrolo[3.4-c1pyridin-3-one [721
The title compound was prepared following the procedures described in example 4 and 6 with 6-benzylamino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro- 1 H-pyrrolo[3,4-c]pyridin-1 ,3-dione(0.66g, 1.48mmol) as a white solid(0.22g, 34%) purified by flash chromatography on silica.
1H-NMR(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.87(s, 3H), 4.40(d, J=5.0Hz, 1 H),
4.57(d, J=5.0Hz, 1 H), 4.78-4.84(m, 1 H), 4.86(s, 2H), 6.70-7.40(m, 8H), 8.87(s, 1 H), 9.15(s, 1 H).
(B) 6-Benzylamino-2-(3-cvclopentyloxy-4-methoxyphenyl)-3-methylene -2.3-dihvdro-1 H-pyrrolo[3.4-c1pyridin-1-one [731
The title compound was prepared following the procedures described in example
4 and 6 with 6-benzylamino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro-
1 H-pyrrolo[3,4-c]ρyridin-1 ,3-dione(0.66g, 1.48mmol) as a white solid(0.26g, 40%) purified by flash chromatography on silica.
1H-N R(CDCI3, ppm) : δ 1.60-2.10(m, 8H), 3.92(s, 3H), 4.84(m, 1 H), 4.86(s, 2H),
5.03(d, J=2.2Hz, 1 H), 5.40(d, J=2.2Hz, 1 H), 6.70-7.40(m, 7H), 7.85(d, J=2.5Hz, 1 H), 8.87(s, 1 H), 9.16(s, 1 H).
Example 55 6-Amino-2-(3-cvclopentyloxy-4-methoxyphenyl)-3-methyl-2T3 -dihvdro-1 H-pyrrolo[3.4-c1pyridin-1-one [741
The title compound was prepared following the procedures described in example
2 with 6-benzylamino-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methylene-2,3- dihydro-1 H-pyrrolo[3,4-c]pyridin-1-one(0.65g, 1.48mmol) as a white solid(0.46g, 88%,).
1H-N R(CDCI3, ppm) : δ 1.56(d, J=6.9Hz, 3H), 1.60-2.10(m, 8H), 3.86(s, 3H),
4.83(m,12H), 5.14(q, J=6.9Hz, 1 H), 6.90-6.98(m, 2H), 7.30(s, 1H), 7.55(s, 1 H), 8.45(s, 1H).
Example 56 2-(3-Cvclopentyloxy-4-methoxyphenyl)-1.3-dioxo-5-isoindoline carboxylic acid [751
The title compound was prepared following the procedures described in example
3 with3-cyclopentyloxy-4-methoxyaniline(0.52g, 2.42mmol) and 1 ,2,4- benzentricarboxylic-anhydride(0.46g, 2.42mmol) as a brown solid(0.84g, 91%).
1H-N R(CDCI3, ppm) : δ 1.63-1.68(m, 2H), 1.83-1.98(m, 6H), 3.92(s, 3H),
4.77-4.83(m, 1 H), 6.93-7.00(m, 3H), 8.11 (d, J=7.8Hz, 1 H), 8.57(d, J=7.8Hz, 1 H), 8.98(s, 1 H), 13.20(bs, 1 H).
Example 57 Ethyl 2-(3-cyclopentyloxy-4-methoχyphenyl)-1.3-dioxo-5- isoindoline carboxylate [761
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-1 ,3-dioxo-5-isoindoline
carboxylic acid(0.92g, 2.42mmol) in anhydrous acetonedOml) was added slowly triethylaminedml, 7.26mmol). The reaction mixture was stirred at room temperature for 10min, treated with iodoethane(1.13g, 7.26mmol), refluxed for 10h, cooled to room temperature, evaporated in vacuo, and added methanol. The resultant solid was filtered to afford the title compound(0.94g, 95%) as a pale yellow solid. H-N R(CDCI3, ppm) : δ 1.49(t, J=7.1 Hz, 3H), 1.61-1.65(m, 2H), 1.84-1.98(m,
6H), 3.91 (s, 3H), 4.48(q, J=7.1 Hz, 2H), 4.76-4.80(m, 1 H), 6.95-6.99(m, 3H), 8.03(d, J=7.8Hz, 1H), 8.50(dd, J=7.8, 1.3Hz, 1 H), 8.62(d, J=1.3Hz, 1 H).
Example 58 (A) Ethyl 4-(3-cvclopentyloxy-4-methoxyarylino)carbonyl-3- hvdroxy-methyl benzoate [771
The title compound was prepared following the procedures described in example 20(A) with ethyl 2-(3-cyclopentyloxy-4-methoxyphenyl)-1 ,3-dioxo-5-isoindoline -carboxylate(0.99g, 2.42mmol) in anhydrous methanol and sodiumborohydride(0.46g, 12.1 mmol) as a white solid(0.45g, 45%).
1H-NMR(CDCI3, ppm) : δ 1.44(t, J=7.1 Hz, 3H), 1.58-1.71 (m, 2H), 1.81 -2.01 (m,
6H), 3.87(s, 3H), 4.22(t, J=6.6Hz, 1 H), 4.42(q, J=7.1 Hz, 2H), 4.73(d, J=6.6Hz, 2H), 4.74-4.93(m, 1 H), 6.87(d, J=8.6Hz, 1 H), 7.06(dd, J=8.6, 2.4Hz, 1 H), 7.40(d, J=2.4Hz, 1H), 7.53(d, J=7.9Hz, 1 H), 8.14(dd, J=7.9, 1.6Hz, 1 H), 8.30(bs, 1 H), 8.36(d, J=1.6Hz, 1 H).
(B) Ethyl 3-(3-cvclopentyloxy-4-methoxyarylino)carbonyl-4- hydroxymethylbenzoate [781
To a solution of ethyl 2-(3-cyclopentyloxy-4-methoxyphenyl)-1 ,3-dioxo-5- isoindoline carboxylate(0.99g, 2.42mmol) in anhydrous methanol was added sodium borohydride(0.46g, 12.1 mmol). The reaction mixture was followed the procedures described in example 20(A). Then, the residue was recrystallized from ether, and solid was filtered off. The resultant solution was concentrated in vacuo
to give the title compound(0.48g, 48%) as a pale yellow solid.
1H-NMR(CDCI3, ppm) : δ 1.42(t, J=7.1 Hz, 3H), 1.62-1.67(m, 2H), 1.86-2.01 (m,
6H), 3.87(s, 3H), 4.22(t, J=6.6Hz, 1 H), 4.42(q, J=7.1 Hz, 2H), 4.73(d, J=6.6Hz, 2H), 4.74-4.93(m, 1 H), 6.87(d, J=8.6Hz, 1 H), 7.06(dd, J=8.6, 2.4Hz, 1 H), 7.40(d, J=2.4Hz, 1 H), 7.53(d, J=7.9Hz, 1 H), 8.14(dd, J=7.9, 1.6Hz, 1 H), 8.30(bs, 1 H), 8.36(d, J=1.6Hz, 1 H).
Example 59 (A) Ethyl 2-(3-cyclopentyloxy-4-methoχyphenyl)-1 -oxo-5- isoindoline carboxylate [791
The title compound was prepared following the procedures described in example 21 (A) with ethyl 4-(3-cyclopentyloxy-4-methoxyarylino)carbonyl-3-hydroxymethyl benzoatedg, 2.42mmol) as a white solid(0.86g, 89%).
1H-NMR(CDCI3, ppm) : δ 1.45(t, J=7.1Hz, 3H), 1.62-1.66(m, 2H), 1.83-2.07(m,
6H), 3.89(s, 3H), 4.43(q, J=7.1 Hz, 2H), 4.86-4.89(m, 1 H), 4.90(s, 2H), 6.92(d, J=8.7Hz, 1 H), 7.05(dd, J=8.7, 2.5Hz, 1 H), 7.88(d, J=2.5Hz, 1 H), 7.97(d, J=8.3Hz, 1 H), 8.21 (m, 2H).
(B) Ethyl 3-(3-cvclopentyloxy-4-methoxyphenyl)-3-oxo-5-isoindoline carboxylate[801
The title compound was prepared following the procedures described in example 21 (A) with ethyl 3-(3-cyclopentyloxy-4-methoxyarylino)carbonyl-4- hydroxymethyl benzoate (1 g, 2.42mmol) as a white solid(0.87g, 90%).
1H-NMR(CDCI3, ppm) : δ 1.44(t, J=7.1 Hz, 3H), 1.56-1.67(m, 2H), 1.83-2.08(m,
6H), 3.89(s, 3H), 4.43(q, J=7.1 Hz, 2H), 4.86-4.89(m, 1 H), 4.90(s, 2H), 6.92(d, J=8.7Hz, 1 H), 7.04(dd, J=8.7, 2.5Hz, 1 H), 7.61 (d, J=7.9Hz, 1 H), 7.87(d, J=2.5Hz, 1 H), 8.31 (dd, J=7.9, 1.5Hz, 1 H), 8.60(s, 1 H).
Example 60 2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindoliπecarbo hvdrazide [811
To a solution of ethyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindoiine carboxylate(0.96g, 2.42mmol) in ethanol(IOml) was added excess hydrazine solution. The reaction mixture was stirred for 24h at room temperature, evaporated in vacuo, and added distilled water. The resultant solid was filtered to afford the title compound(0.48g, 48%) as a white solid. H-NMR(CDC13, ppm) : δ 1.60-1.67(m, 2H), 1.84-2.06(m, 6H), 3.89(s, 3H),
4.43(bs, 2H), 4.87-4.89(m, 1 H), 4.90(s, 2H), 6.90(d, J=8.8Hz, 1 H), 7.06(dd, J=8.8, 2.5Hz, 1 H), 7.56(bs, 1 H), 7.64(d, J=7.7Hz, 1 H), 7.80(d, J=2.5Hz, 1 H), 8.13(dd, J=7.7, 1.5Hz, 1 H), 8.20(d, J=1.5Hz, 1 H).
Example 61 2-(3-Cvclopentyloxy-4-methoχyphenyl)-3-oxo-5- isoindolinecarboxylic acid [821
To a solution of ethyl 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindoiine carboxylate(0.96g, 2.42mmol) in ethanol(IOml) was added the solution of lithium hydroxide(0.25g, 6.05mmol) in waterdOml). The reaction mixture was stirred for 1 h at 80 °C , cooled to room temperature, and acidified to pH 1 with concentrated HCl solution. The resultant solid was filtered to afford the title compound(0.88g, 98%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.63-1.68(m, 2H), 1.81-2.08(m, 6H), 3.89(s, 3H),
4.86-4.90(m, 1 H), 4.93(s, 2H), 6.92(d, J=8.8Hz, 1 H), 7.05(dd, J=8.8, 2.5Hz, 1 H), 7.65(d, J=7.9Hz, 1 H), 7.86(d, J=2.5Hz, 1 H), 8.37(dd, J=7.9, 1.5Hz, 1 H), 8.69(d, J=1.5Hz, 1 H).
Example 62 2-(3-Cvclopentyloxy-4-methoxyphenyl)-6-hydroxymethyl-1 -isoindolinone [831
To a solution of ethyl 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindoline carboxylate(0.96g, 2.42mmol) in anhydrous THFdOml) was added lithium borohydride(0.08g, 3.63mmol). The reaction mixture was stirred for 1 h at room temperature, evaporated in vacuo, diluted with ethyl acetate, and washed twice with distilled water. The organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.83g, 97%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.61-1.68(m, 2H), 1.84-2.08(m, 6H), 3.89(s, 3H),
4.83-4.91 (m, 5H), 5.10(bs, 1 H), 6.90(d, J=8.8Hz, 1 H), 7.06(dd, J=8.9, 2.6Hz, 1 H), 7.52(d, J=7.8Hz, 1 H), 7.65(d, J=7.8Hz, 1 H), 7.88-7.91 (m, 2H).
Example 63 2-(3-Cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindolinecarbo aldehyde [841
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-hydroxymethyl-1- isoindolinone(0.85g, 2.42mmol) in anhydrous dichloromethane(IOml) was added manganase oxide(0.42g, 4.84mmol). The reaction mixture was stirred for 24h at room temperature, and then filtered through Celite to remove Mn02. The residue was concentrated in vacuo to give the title compound(0.82g, 96%) as a white solid. 1H-NMR(CDCI3, ppm) : δ 1.63-1.70(m, 2H), 1.84-2.07(m, 6H), 3.89(s, 3H),
4.86-4.91 (m, 1 H), 4.94(s, 2H), 6.92(d, J=8.7Hz, 1 H), 7.05(dd, J=8.7, 2.5Hz, 1 H), 7.70(d, J=7.8Hz, 1 H), 7.84(d, J=2.5Hz, 1 H), 8.17(dd, J=7.8, 1.4Hz, 1 H), 8.40(s, 1 H), 10.15(s, 1H).
Example 64 2-(3-Cyclopentyloxy-4-methoχyphenyl)-3-oxo-5- isoindolinecarbo aldehvdeoxime [851
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindolinecarbo-aldehyde(0.85g, 2.42mmol) in methanol(IOml) and waterdOml) was added hydroxyamine hydrochloride (0.17g, 2.42mmol). The reaction mixture was controlled to pH 4-5 with 1 N NaOH solution, stirred for 2h at room temperature,
extracted with ethyl acetate, and washed twice with distilled water. The organic layer was dried over MgSθ4, filtered, and concentrated in vacuo to give the title compound(0.83g, 93%) as a white solid.
1H-N R(CDCI3, ppm) : δ 1.60-1.67(m, 2H), 1.84-2.07(m, 6H), 3.89(s, 3H),
4.85-4.90(m, 3H), 6.91 (d, J=8.7Hz, 1 H), 7.05(dd, J=8.7, 2.5Hz, 1 H), 7.54(d, J=7.9Hz, 1 H), 7.70(s, 1 H), 7.86-7.89(m, 2H), 8.14(s, 1 H), 8.26(s, 1 H).
Example 65 2-(3-Cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindolinecarbonitrile[86l
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-5- isoindolinecarbo a!dehydeoxime(0.89g, 2.42mmol) in acetonitriiedOml) were added triphenylphosphine(2.54g, 9.68mmol) and carbon tetrachloride(0.75g, 4.84mmol). The reaction mixture was stirred for 24h at room temperature, evaporated in vacuo, diluted with ethyl acetate, and washed twice with distilled water. The organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.82g, 97%) as a white solid.
1H-N R(CDCI3, ppm) : δ 1.60-1.65(m, 2H), 1.83-2.06(m, 6H), 3.89(s, 3H),
4.85-4.90(m, 1 H), 4.92(s, 2H), 6.90(d, J=8.7Hz, 1 H), 7.02(dd, J=8.7, 2.5Hz, 1 H), 7.66(d, J=7.8Hz, 1 H), 7.81 (dd, J=2.5Hz, 1 H), 7.88(dd, J=7.8, 1.3Hz, 1 H), 8.20(s, 1 H).
Example 66 [2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-oxo-2.3-dihvdro- 1 H-5-isoindolyllmethyl methanesulfonate [871
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-hydroxymethyl-1-iso -indolinone(0.86g, 2.42mmol) in dichloromethane(IOml) were slowly added methane sulfonylchloride(0.33g, 2.90mmol) and triethylamine(0.37g, 3.63mmol) at O C . The reaction mixture was stirred for 0.5h at room temperature, washed twice with distilled water, dried over MgS04, filtered, and concentrated in vacuo to give the
title compound(0.96g, 96%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.61-1.66(m, 2H), 1.83-2.06(m, 6H), 3.02(s, 3H), 3.88(s,
3H), 4.87(s, 2H), 4.86-4.89(m, 1 H), 5.36(s, 2H), 6.91 (d, J=8.7Hz, 1 H), 7.04(dd, J=8.7, 2.5Hz, 1 H), 7.58(d, J=7.8Hz, 1 H), 7.68(dd, J=7.8, 1.5Hz, 1 H), 7.85(d, J=2.5Hz, 1 H), 7.96(s, 1 H).
Example 67 6-(Azidomethyl)-2-(3-cvclopentyloxy-4-methoχyphenyl)-1-iso indolinone [881
To a solution of [2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H -5-isoindolinyl]methyl methanesuifonatedg, 2.42mmol) in DMF(IOml) was added sodium azide(0.47g, 7.26mmol). The reaction mixture was stirred for 2h at 60°C , cooled to room temperature, added ethyl acetate, washed three times with distilled water, dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.9g, 97%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.61-1.66(m, 2H), 1.86-2.07(m, 6H), 3.89(s, 3H), 4.48(s,
2H), 4.85(s, 2H), 4.86-4.90(m, 1 H), 6.91 (d, J=8.7Hz, 1 H), 7.04(dd, J=8.7, 2.5Hz, 1H), 7.56(m, 2H), 7.86(m, 2H).
Example 68 6-(Aminomethyl)-2-(3-cvclopentyloxy-4-methoxyphenyl)-1-iso indolinone [891
To a solution of 6-(azidomethyl)-2-(3-cyclopentyloxy-4-methoxyphenyl)-1-iso -indolinone(0.92g, 2.42mmol) in THFdOml) was added triphenylphosphine(0.7g, 2.66mmol). The reaction mixture was stirred for 20min., added distilled waterd ml), stirred for 8h at room temperature, added 1 N HCl solution, and extracted with ethyl acetate. The aqueous layer was basified to pH 8-9 with 2N NaOH solution, and extracted with ethyl acetate. The organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.79g, 92%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.56-1.66(m, 2H), 1.85-2.07(m, 6H), 3.88(s, 3H), 4.01 (s,
2H), 4.83(s, 2H), 4.82-4.89(m, 1 H), 6.90(d, J=8.7Hz, 1 H),
7.05(dd, J=8.7, 2.5Hz, 1 H), 7.48(m, 1 H), 7.58(d, J=7.7Hz, 1 H), 7.87(m, 2H).
Example 69 2-[2-(3-Cvclopentyloxy-4-methoχyphenyl)-3-oxo-2.3-dihvdro- 1 H-5-isoindolinyl]acetonitrile [901
To a solution of [2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro- 1 H-5-isoindolyl]methyl methanesulfonatedg, 2.42mmol) in DMFd Oml) was added sodium cyanide(0.36g, 7.26mmol). The reaction mixture was stirred for 2h at 60 °C , added ethyl acetate, and washed three times with distilled water. The organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.85g, 96%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.60-1.67(m, 2H), 1.85-2.06(m, 6H), 3.89(s, 3H), 4.88(s,
2H), 4.86-4.90(m, 1 H), 6.92(d, J=8.7Hz, 1 H), 7.04(dd, J=8.7, 2.5Hz, 1 H), 7.55(d, J=7.8Hz, 1 H), 7.61 (d, J=7.8Hz, 1 H), 7.83-7.85(m, 2H).
Example 70 2-(3-Cvclopentyloxy-4-methoxyphenyl)-6-(hvdroxyamino)methyl -1-isoindolinone [911
A solution of hydroxyamine hydrogen chioiride(1.68g, 24.2mmol) in water(IOml) was basified to pH 8 with 1 N NaOH solution, added methanol(20ml), treated with [2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-dihydro-1 H-5-isoindolinyl]methyl methanesulfonatedg, 2.42mmol), and refluxed for 3h. The reaction mixture was evapoprated in vacuo, added water, and extracted three times with ethyl acetate. The organic layer was dried over MgS04, filtered, and concentrateed, and concentrated in vacuo to give the title compound(0.47g, 52%) as a white solid. 1H-NMR(CDC13, ppm) : δ 1.61-1.66(m, 2H), 1.83-2.06(m, 6H), 3.87(s, 3H), 4.26(s,
2H), 4.81 (s, 2H), 4.84-4.90(m, 1 H), 6.88(d, J=9.0Hz, 1 H), 7.03(dd, J=9.0, 2.4Hz, 1 H), 7.30(d, J=8.1 Hz, 1 H), 7.58(d, J=8.1 Hz, 1 H), 7.84(d, J=2.4Hz, 1 H), 7.91 (m, 1 H).
Example 71 N-[{2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-oxo-2.3-dihvdro- 1 H-5-isoindolinyl}methyl]hvdroxyurea [921
To a solution of 2-(3-cyclopentyloxy-4-methoxyphenyl)-6-(hydroxyamino) methyl-1-isoindolinone(0.89g, 2.42mmol) in anhydrous THF(IOml) was added trimethylsilylisocyanate(0.35g, 3.03mmol). The reaction mixture was refluxed for 1 h and evaporated in vacuo. The residue was crystallized from methanol to afford the title compound(0.42g, 42%) as a white solid.
1H-NMR(DMSO-d6, ppm) : δ 1.61-1.66(m, 2H), 1.70-1.78(m, 4H), 1.81-1.89(m,
2H), 3.58(s, 3H), 4.61 (s, 2H), 4.76-4.80(m, 1 H), 4.94(s, 2H), 6.42(s, 2H), 6.98(d, J=8.7Hz, 1 H), 7.24(d, J=7.9Hz, 1 H), 7.51-7.57(m, 2H), 7.61-7.65(m, 2H).
Example 72 Methyl 2-[{2-(3-cvclopentyloxy-4-methoxyphenyl)-3-oxo-2.3- dihvdro-1 H-5-isoindolinyl}amino1-2-oxoacetate [931
To a solution of 6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-1-isoindolinone (0.25g, 0.74mmoi) in dichloromethane(IOml) were slowly added methyloxalylchloride(0.1g, 0.89mmol) and triethylamine(0.16ml, 1.11 mmol) at 0°C . The reaction mixture was stirred for 1h, and washed twice with distilled water. The organic layer was dried over MgS04, filtered, and concentrated in vacuo to give the title compound(0.29g, 92%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.62-1.67(m, 2H), 1.83-2.07(m, 6H), 3.89(s, 3H), 4.02(s,
2H), 4.85(s, 2H), 4.87-4.89(m, 1 H), 6.90(d, J=8.7Hz, 1 H), 7.30(dd, J=8.7, 2.4Hz, 1 H), 7.54(d, J=8.2Hz, 1 H), 7.84(d, J=2.4Hz, 1 H), 8.02(d, J=1.9Hz, 1 H), 8.07(dd, J=8.2, 1.9Hz, 1 H), 9.03(bs, 1 H).
Example 73 N1-[2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-oxo-2.3-dihvdro- 1 H-5-isoindolinyl]ethaπe diamide [941
To a solution of methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2,3-
dihydro-1 H-5-isoindolinyl}amino]-2-oxoacetate(0.31g, 0.74mmol) in methanol(5ml) and dichloromethane(5ml) was added excess ammonia water. The reaction mixture was stirred for 1 h at room temperature, evaporated in vacuo, and added distilled water. The resultant solid was filtered to afford the title compound(0.26g, 86%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.60-1.65(m, 2H), 1.84-2.07(m, 6H), 3.89(s, 3H), 4.84(s,
2H), 4.87-4.90(m, 1 H), 5.73(bs, 1 H), 6.92(d, J=8.7Hz, 1 H), 7.04(dd, J=8.7, 2.4Hz, 1 H), 7.44(bs, 1 H), 7.53(d, J=8.2Hz, 1 H), 7.87(d, J=2.4Hz, 1 H), 7.94(dd, J=8.2, 2.0Hz, 1 H), 8.14(d, J=2.0Hz, 1 H), 9.38(bs, 1 H).
Example 74 Methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo-2.3- dihydro-1 H-5-isoindolinyl}amiπo1acetate [951
To a solution of 6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-1-isoindolinone (0.82g, 2.42mmol) in DMF(IOml) were added potassium carbonate(0.67g, 4.84mmol) and methylbromo acetate(0.45g, 2.91 mmol). The reaction mixture was stirred for 4h at 60 C , and added water. The resultant solid was filtered to afford the title compound(0.93g, 93%) as a white solid.
1H-NMR(CDCI3, ppm) : δ 1.61-1.66(m, 2H), 1.86-2.06(m, 6H), 3.88(s, 3H), 3.90(s,
2H), 4.02(s, 2H), 4.75(s, 2H), 4.84-4.86(m, 1 H), 6.90(d, J=8.7Hz, 1 H), 7.31 (dd, J=8.7, 2.4Hz, 1 H), 7.53(d, J=8.2Hz, 1 H), 7.84(d, J=2.4Hz, 1H), 8.03(d, J=1.9Hz, 1 H), 8.08(dd, J=8.2, 1.9Hz, 1 H).
Example 75 2-H"2-(3-Cvclopentyloxy-4-methoxyphenyl)-3-oxo-2.3-dihvdro- 1 H-5-isoindolinyl}amino1acetamide [961
The title compound was prepared following the procedures described in example 73 with methyl 2-[{2-(3-cyclopentyloxy-4-methoxyphenyl)-3-oxo- 2,3-dihydro-1 h-5-iso-indolinyl}amino]acetate(0.99g, 2.42mmol) as a white solid (0.62g, 64%).
H-NMR(CDCI3, ppm) : δ 1.61-1.66(m, 2H), 1.85-2.06(m, 6H), 3.88(s, 3H),
3.90(s, 2H), 4.75(s, 2H), 4.85-4.88(m, 1 H), 5.77(bs, 1 H), 6.47(bs, 1 H), 6.88-6.92(m, 2H), 7.02(m, 2H), 7.33-7.35(m, 1 H), 7.84-7.86(m, 1 H).
Example 76 N1-[2-(3-Cvclopentyloxy-4-methoxyphenyl)-1-oxo-2.3-dihvdro- 1 H-pyrrolo[3.4-c]pyridin-6-yl1ethane diamide [971
The title compound was prepared following the procedures described in example 72 and 73 with 6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3- dihydro-1 H-pyrrolo[3,4-c]pyridin -1-one(0.82g, 2.42mmol) as a white solid(0.96g, 96%).
1H-NMR(CDCI3, ppm) : δ 1.60-1.68(m, 2H), 1.82-2.01 (m, 6H), 3.87(s, 3H),
4.75(s, 2H), 4.87-4.90(m, 1 H), 5.73(bs, 1 H), 6.92(d, J=8.7Hz, 1 H), 7.01 (dd, J=8.7, 2.5Hz, 1 H), 7.47(bs, 1 H), 7.82(d, J=2.5Hz, 1 H), 8.82(s, 1 H), 9.18(s, 1 H), 9.37(bs, 1 H).
Example 77 N1-[2-(3-Cvclopeπtyloxy-4-methoxyphenyl)-3-methyl-1-oxo-2.3- dihvdro-1 H-pyrrolo[3.4-c]pyridin-6-yl1ethane diamide [981
The title compound was prepared following the procedures described in example 72 and 73 with 6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl- 2, 3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1-one(0.86g, 2.42mmol) as a white solid (0.98g, 98%).
1H-NMR(CDCI3, ppm) : δ 1.49(d, J=6.9Hz, 3H), 1.60-1.68(m, 2H), 1.82-2.01 (m,
6H), 3.90(s, 3H), 4.80-4.87(m, 1 H), 5.14(q, J=6.9Hz, 1H), 5.74(bs, 1 H), 6.90-6.98(m, 3H), 7.24(s, 1 H), 7.50(bs, 1 H), 8.84(s, 1 H), 9.19(s, 1 H), 9.36(bs, 1 H).
Example 78 N1-[2-(3-Cyclopentyloxy-4-methoxyphenyl>-1-methyl-3-oxo-2r3 -dihvdro-1 H-5-isoindolinyl1ethane diamide [991
The title compound was prepared following the procedures described in example 72 and 73 with 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino- isoindolin-1-one(0.85g, 2.42 mmol) as a white soiid(0.97g, 97%). H-N R(CDCI3, ppm) : δ 1.30-2.10(m, 8H), 1.40(d, J=6.3Hz, 3H), 3.88(s, 3H),
4.83(m, 1 H), 5.03(q, J=6.3Hz, 1 H), 5.75(bs, 1 H), 6.85-7.07(m, 3H), 7.22-7.95(m, 3H), 750(bs, 1 H), 9.40(bs, 1 H).
Example 79 2-ff2-(3-Cvclopentyloxy-4-methoχyphenyl)-1-oxo-2τ3-dihvdro- 1 H-pyrrolo[3.4-c]pyridin-6-yl}amino1acetamide [1001
The title compound was prepared following the procedures described in example 73 and 74 with 6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-2,3-dihydro -1 H-pyrrolo[3,4-c] pyridin-1-one(0.82g, 2.42mmol) as a white solid(0.92g, 95%).
1H-NMR(CDCI3, ppm) : δ 1.62-1.66(m, 2H), 1.82-2.01 (m, 6H), 3.89(s, 3H),
3.94(s, 2H), 4.85-4.88(m, 1 H), 4.89(s, 2H), 5.77(bs, 1H), 6.46(bs, 1H), 6.92(d, J=8.7Hz, 1 H), 7.01 (dd, J=8.7, 2.5Hz, 1 H), 7.84(d, J=2.5Hz, 1 H), 8.83(s, 1 H), 9.20(s. 1 H).
Example 80 2-[f2-(3-Cyclopentyloxy-4-methoχyphenyl)-3-methyl-1-oxo-2.3 -dihvdro-1 H-pyrrolo[3.4-c]pyridin-6-yl}amino]acetamide [1011
The title compound was prepared following the procedures described in example 73 and 74 with 6-amino-2-(3-cyclopentyloxy-4-methoxyphenyl)-3-methyl- 2, 3-dihydro-1 H-pyrrolo [3,4-c]pyridin-1-one(0.86g, 2.42mmol) as a white solid (0.93g, 93%).
1H-N R(CDCI3, ppm) : δ 1.54(d, J=6.9Hz, 3H), 1.60-1.67(m, 2H), 1.85-2.05(m,
6H), 3.87(s, 3H), 4.81-4.86(m, 1H), 5.15(q, J=6.9Hz, 1 H), 5.76(bs, 1 H), 6.49(bs, 1 H), 6.92-6.99(m, 2H), 7.30(s, 1 H), 8.84(s, 1 H), 9.19(s, 1 H).
Example 81 2-[{2-(3-Cyclopentyloxy-4-methoxyphenyl)-1-methyl-3-oxo-2.3 -dihydro-1 H-5-isoindolyl}amino1acetamide [102]
The title compound was prepared following the procedures described in example 73 and 74 with 3-methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)-6-amino- isoindolin-1-one(0.85g, 2.42mmol) as a white solid(0.95g, 95%).
1H-NMR(CDCI3, ppm) : δ 1.35-2.10(m, 8H), 1.41(d, J=6.3Hz, 3H), 3.87(s, 3H),
3.96(s, 2H), 4.81-4.86(m, 1 H), 5.04(q, J=6.3Hz, 1 H), 5.77(bs, 1 H), 6.48(bs, 1 H), 6.87-7.05(m, 3H), 7.25-7.97(m, 3H).
Experimental Example 1
TNF- α in vitro assay(reference; Taffet S.M. et al., Cellular Immunology (1989) 120, 291 -300);
After cancer cell line of mouse macrophage(RAW264.7) is diluted with RPMI1640 medium(containing 5% FCS), then plated out in 24 well plate at 1x106 cells/ml. Then, the culture is incubated for 18 hours at 5% CO2 and 37°C . 1 μM of compound and 1 μg/ml of lipopolysaccharide(LPS) are added to the plate and the culture is incubated for 6 hours at 37 °C . After incubated, the culture is centrifuged and supernatants are collected. The supernatants are stored at -20 C till measurement. The measurement of TNF- α in the media is performed with a mouse TNF- α kit(Amersham, UK). And the procedure is in accordance with the guidance provided by Amersham. Inhibition percentage of each compound is calculated by comparison of amount of TNF- α , released in the well treated with compound, with that in the well without any treatment. Inhibitory activities of compounds on in vitro TNF- α synthesis are listed in Table 1.
Table 1
Experimental Example 2
TNF- α in vivo assay(reference; Novogrodsky A. et al., Science (1994) 264, 319-322)
After compound is suspended in 5% sodium carboxymethyl celiulose(CMC), starved mouse(C57BL/6, 6-week old, male) is administered orally at the volume of 0.1 ml per 10g of body weight. Lipopolysaccharide (LPS) is injected intraperitoneally at the concentration of 1.5mg/mouse for 2 hours after compound administration. The control is administered orally with 5% Na CMC at the volume of 0.1 ml per 10g of body weight. After one and half hours, mice are anaesthetized with ether, blood is collected from vena cava, serum is collected from blood after 5 minute centrifugation at 12,000 rpm. The serum collected is stored at -20°C till TNF- α
ELISA assay. The amount of TNF- α in serum is measured with a mouse TNF- α kit (Amersham, UK). And the procedure is in accordance with the guidance provided by Amersham Inhibitory activities of compounds on in vivo TNF- α synthesis are listed in Table 2.
Table 2
According to Table 1 and Table 2, compounds invented by us, show high inhibitory effect on in vitro and in vivo TNF- α synthesis.
Experimental Example 3
Acute Toxicity Test(LDso)
The compounds in Table 3 are administered orally at various dose with SPF ICR mice(body weight 20± 1g). The animal number of each group is 5. The number of the dead is checked for 24 hours after administration. And animal condition and the number of the dead have been observed for 7 days. The lethal dose of 50%(LD5o) is calculated in accordance with Litchfield-Wilcoxon) method. The result is listed in Table 3. Table 3
* indicates post oral.
The following Composition Examples illustrate pharmaceutical compositions according to the present invention.
Composition Example 1
3-Methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1 -one(1 gMmean particle size 3.5 microns) and lactose(99g)(mean particle size 72 microns) are blended together for 30 minures in a mechanical shaker/mixer. The resultant blend is filled, to a fill weight of 25mg, into No. 3 hard gelatine capsules, to give a product suitable for use, for example, with a dry powder inhaler.
Composition Example 2
No. 2 size gelatine capsules each containing:
3-Methyl-2-(3-cyclopentyloxy-4-methoxyphenyl)isoindolin-1-one 20mg lactose 10Omg starch 60mg dextrin 40mg magnesium stearate 1 mg
are prepared in accordance with the usual procedure.
Compositions similar to those above are prepared from other compounds of formula [1].