SYSTEMATIC MODULAR PRODUCTION OF AMINIMIDE- AND OXAZOLONE- BASED MOLECULES HAVING AT LEAST TWO STRUCTURAL DIVERSITY ELEMENTS FIELD OF THE INVENTION
The present invention relates to the modular development of aminimide-based and oxazolone-derived synthetic organic molecules, possessing selected properties for a particular application. This invention involves: a) the synthesis of an array of different molecules generated from base modules of aminimide-forming, oxazolone, oxazolone-forming and/or oxazolone-derived molecules containing a chosen set of substituent groups which confer structural diversity; and/or the reaction of these modules with other appropriate reactive groups to produce an array of molecules possessing a chosen set of diverse structural moieties; and, b) the screening of some or all of the molecules in the array for the desired set of properties in a target application. The iterative application of this invention enables molecules to be produced, having an optimum balance of properties for the particular application.
BACKGROUND OF THE INVENTION
The discovery of new molecules has traditionally focused in two broad areas, biologically active molecules, which are used as drugs for the treatment of life-threatening diseases, and new materials, which are used in commercial, and especially, in high technological applications. In both areas, the strategy used to discover new molecules has involved two basic operations: (i) a more or less random choice of a molecular candidate, prepared either via chemical synthesis or isolated from natural sources; and, (ii) the testing of the molecular candidate for the property or properties of interest. This discovery cycle is repeated indefinitely until a molecule possessing the desirable property, i.e., "lead molecule", is located. This "lead molecule" discovery process has been inherently ad hoc in nature and is time-consuming, laborious, unpredictable and costly.
Once a candidate lead molecule has been determined, the synthetic chemist must subsequently find ways to synthesize structural variants of this molecule to optimize its properties in the desired application. In the case where the lead molecule is a synthesized organic species or a natural product, the chemist is usually limited to certain structural and synthetic reaction schemes. These schemes are dictated largely by the structural composition of the lead molecule and by the requirements of the specific application. For example, in cases where the lead molecule possesses a functionally important aromatic ring, various electrophilic and nucleophilic substitutions may be carried out on the ring to produce variants. Each such case must be approached as a specific independent design and synthesis problem, starting each time from the beginning, because of the lack of availability of an appropriate chemistry to simply alter the structure of the lead compound to produce the variant.
Recently, some attempts have been made to modularize certain synthetic organic reaction schemes to facilitate modification and transformation of a lead or base compound (see, for example, 1993 Proc. Natl. Acad. Sci. USA, 90, 6909). However, the molecules which can be produced by such attempts are extremely limited in their achievable diversity and are still bounded by factors dictated by the choice of specific structural themes. In the case where the "lead molecule" is a naturally occurring biological molecule, such as a peptide, a protein, an oligonucleotide or a carbohydrate, simple synthetic point-modifications to the lead molecule to produce variants are quite difficult to achieve.
A brief account of the strategies and tactics used in the discovery of new molecules is described below. The emphasis is on biologically interesting molecules; however, the technical problems encountered in the discovery of biologically active molecules as outlined here are also illustrative of the problems encountered in the discovery of molecules which can serve as building blocks for the development of new tools and materials for a variety of high technological applications. Furthermore,
as discussed below, these problems are also illustrative of the problems encountered in the development of fabricated structures and materials for high technological applications.
Drug Design
Modern theories of biological activity state that biological activities, and therefore physiological states, are the result of molecular recognition events. For example, nucleotides can form complementary base pairs so that complementary single-stranded molecules hybridize resulting in double- or triple-helical structures that appear to be involved in regulation of gene expression. In another example, a biologically active molecule, referred to as a ligand, binds with another molecule, usually a macromolecule referred to as ligand-acceptor (e.g., a receptor, an enzyme, etc.), and this binding elicits a chain of molecular events which ultimately gives rise to a physiological state, e.g., normal cell growth and differentiation, abnormal cell growth leading to carcinogenesis, blood-pressure regulation, nerve-impulse- generation and -propagation, etc. The binding between ligand and ligand-acceptor is geometrically characteristic and extraordinarily specific, involving appropriate three- dimensional structural arrangements and chemical interactions.
A currently favored strategy for the development of agents which can be used to treat diseases involves the discovery of forms of ligands of biological receptors, enzymes, or related macromolecules, which mimic such ligands and either boost, i.e., agonize, or suppress, i.e., antagonize, the activity of the ligand. The discovery of such desirable ligand forms has traditionally been carried out either by random screening of molecules (produced through chemical synthesis or isolated from natural sources), or by using a so-called "rational" approach involving identification of a lead-structure, usually the structure of the native ligand, and optimization of its properties through numerous cycles of structural redesign and
biological testing. Since most useful drugs have been discovered not through the "rational" approach but through the screening of randomly chosen compounds, a hybrid approach to drug discovery has recently emerged which is based on the use of combinatorial chemistry to construct huge libraries of randomly-built chemical structures which are screened for specific biological activities. (S. Brenner and R. A. Lerner, 1992, Proc. Natl. Acad. Sci. USA 89:53, 81)
Most lead-structures which have been used in the "rational" drug design approach are native polypeptide ligands of receptors or enzymes. The majority of polypeptide ligands, especially the small ones, are relatively unstable in physiological fluids, due to the tendency of the peptide bond to undergo facile hydrolysis in acidic media or in the presence of peptidases. Thus, such ligands are decisively inferior in a pharmacokinetic sense to non-peptidic compounds, and are not favored as drugs. An additional limitation of small peptides as drugs is their low affinity for ligand acceptors. This phenomenon is in sharp contrast to the affinity demonstrated by large, folded polypeptides, e.g., proteins, for specific acceptors, e.g., receptors or enzymes, which can be in the sub-nanomolar range. For peptides to become effective drugs, they must be transformed into non-peptidic organic structures, i.e., peptide mimetics, which bind tightly, preferably in the nanomolar range, and can withstand the chemical and biochemical rigors of coexistence with biological tissues and fluids.
Despite numerous incremental advances in the art of peptidomimetic design, no general solution to the problem of converting a polypeptide-ligand structure to a peptidomimetic has been defined. At present, "rational" peptidomimetic design is done on an ad hoc basis. Using numerous redesign-synthesis- screening cycles, peptidic ligands belonging to a certain biochemical class have been converted by groups of organic chemists and pharmacologists to specific peptidomimetics; however, in the majority of cases, results in one biochemical area, e.g., peptidase inhibitor design using the enzyme substrate as a lead, cannot be transferred for use in another
area, e.g., tyrosine-kinase inhibitor design using the kinase substrate as a lead.
In many cases, the peptidomimetics that result from a peptide structural lead using the "rational" approach comprise unnatural alpha-amino acids. Many of these mimetics exhibit several of the troublesome features of native peptides (which also comprise alpha-amino acids) and are, thus, not favored for use as drugs. Recently, fundamental research on the use of nonpeptidic scaffolds, such as steroidal or sugar structures, to anchor specific receptor-binding groups in fixed geometric relationships have been described (see for example Hirschmann, R. et al., 1992 J. Am. Chem. Soc., 114:9699-9701; Hirschmann, R. et al., 1992 J. Am. Chem. Soc., 114:9217-9218); however, the success of this approach remains to be seen.
In an attempt to accelerate the identification of lead- structures, and also the identification of useful drug candidates through screening of randomly chosen compounds, researchers have developed automated methods for the generation of large combinatorial libraries of peptides and certain types of peptide mimetics, e.g., "peptoids", which are screened for a desirable biological activity. For example, the method of H. M. Geysen, (1984 Proc. Natl. Acad. Sci. USA 81:3998) employs a modification of the Merrifield peptide synthesis, wherein the C-terminal amino acid residues of the peptides to be synthesized are linked to solid-support particles shaped as polyethylene pins; these pins are treated individually or collectively in sequence to introduce additional amino-acid residues forming the desired peptides. The peptides are then screened for activity without removing them from the pins. Houghton, (1985, Proc. Natl. Acad. Sci. USA 82:5131; and U. S. Patent No. 4,631,211) utilizes individual polyethylene bags ("tea bags") containing C-terminal amino acids bound to a solid support. These are mixed and coupled with the requisite amino acids using solid phase synthesis techniques. The peptides produced are then recovered and tested individually. Fodor et al., (1991, Science 251:767) described light-directed, spatially addressable parallel-peptide synthesis on a silicon wafer to generate large
arrays of addressable peptides that can be directly tested for binding to biological targets. These workers have also developed recombinant DNA/genetic engineering methods for expressing huge peptide libraries on the surface of phages (Cwirla et al., 1990, Proc. Natl. Acad. Sci. USA 87:6378).
In another combinatorial approach, V. D. Huebner and D. V. Santi (U. S. Patent No. 5,182,366) utilized functionalized polystyrene beads divided into portions each of which was acylated with a desired amino acid; the bead portions were mixed together, then divided into portions each of which was re- subjected to acylation with a second desirable amino acid producing dipeptides, using the techniques of solid phase peptide synthesis. By using this synthetic scheme, exponentially increasing numbers of peptides were produced in uniform amounts which were then separately screened for a biological activity of interest. Another method of producing libraries of organic compounds based on dipeptides, hydantioins and benzodiazepines using a polystyrene based solid support is described by DeWitt et al. (1993, Proc. Natl. Acad. Sci. USA, 90:6909). Bunin et al. (1992, J. Am. Chem. Soc. 114:10997) describe a method for the combinatorial synthesis of large libraries of peptides. According to Bunin, 2-amino benzophenones are attached to a polystyrene solid support and converted into various 1,4 benzodiazepine derivatives, which can then be screened for specific receptor or enzyme activity.
Zuckerman et al., (1992, Int. J. Peptide Protein Res. 91:1 and 1993, Structural Biology, 3:580) also have developed similar methods for the synthesis of peptide libraries and applied these methods to the automation of a modular synthetic chemistry for the production of libraries of, for example, N-alkyl glycine peptide derivatives, called "peptoids"., which are screened for activity against a variety of biochemical targets. (See also, Symon et al., 1992, Proc. Natl. Acad. Sci. USA 89:9367). Encoded combinatorial chemical syntheses have been described recently (S. Brenner and R. A. Lerner, 1992, Proc. Natl. Acad. Sci. USA 89:5381).
The focus of these structural diversity activities on peptide synthesis chemistry is a direct result of the fact that the ability to generate structural diversity requires, as its starting point, the access to practical stepwise sequential synthesis chemistries which allow the incorporation of varied structural elements with orthogonal reactivities. To date, these have only been worked out for the Merrifield synthesis of peptides and the Carruthers synthesis of oligonucleotides. Thus, there remains a need for an improved method for the structure-directed generation and screening of organic compounds to determine which may be suitable in a particular application.
SUMMARY OF THE INVENTION
The present invention relates to compounds having selected properties for a particular application which are made by forming base modules having at least two structural diversity elements. Such base modules are formed by reaction of a first reactive group, with a compound having at least one structural diversity element and a second reactive group. The first and second reactive groups can be combined by an addition reaction to produce a first array of molecules when at least one of the structural diversity elements of the compounds is varied when producing the base modules. This array can be screened to determine a first suitable compound for a particular application.
If desired, this method can be repeated by producing a second array of molecules through the formation of base modules having structural diversity elements that are different from those of the first array of molecules, and screening the second array of molecules to determine a second suitable compound for the particular application. The second array can be produced by forming base modules having at least two structural diversity elements in the same manner as the first array, except that the structural diversity elements are modified from those of the first suitable compound. The steps of producing and screening an array of molecules can be repeated as often as necessary to achieve an optimum compound for the particular application.
Preferably, the first compound is produced by forming an oxazole compound having at least one structural diversity element attached thereto and reacting it with a nucleophile or carbonyl compound which contains at least one structural diversity element to form a base module having one of the following structures:
wherein at least two of the unconnected lines can be connected to structural diversity elements.
Alternatively, it is also preferred to provide the first compound as an aminimide-forming compound having at least one structural diversity element attached thereto and to react it with an oxazolone or other compound which contains at least one structural diversity element to form a base module having one of the following structures:
e.g.,
wherein at least two of the unconnected lines are connected to structural diversity elements.
Advantageously, the first and second structural diversity elements can be any of the following; (a) an amino acid derivative of the form (AA)n; (b) a nucleotide derivative of the
form (NUCL)n; (c) a carbohydrate derivative of the form (CH)n; (d) an organic moiety of an alkyl, carboxycyclic, aryl, alkylaryl, aralkyl, alkaryl group or a substituted or heterocyclic derivative thereof; (e) of a naturally occurring or synthetic organic structural motif, optionally containing a reporter element, an electrophilic group, a nucleophilic group or a polymerizable group; or, (f) a macromolecular component.
If desired, at least one of the first and second compounds can be provided with two or more structural diversity elements, two of which can form a ring structure. Thus, a wide variety of compounds can be made. Various combinations of these compounds can be placed into arrays which represent another embodiment of the invention.
These arrays are useful in a method for obtaining compounds having selected properties for a particular application by producing a first structurally diverse array of molecules having at least two orthogonal reactivity elements wherein a first orthogonal reactivity element is held constant for each molecule and a second orthogonal reactivity element is varied. The array is screened to determine a first suitable compound for the intended application. Further modifying the first suitable compound can form a second structurally diverse array of molecules. Preferably, the first suitable compound has at least two orthogonal reactivity elements, so that the first suitable compound can be modified by holding a first orthogonal reactivity element constant while varying the second orthogonal reactivity element to produce a second structurally diverse array which can be screened to determine a second suitable compound for the intended application. The modifying and screening steps can be repeated as often as necessary to achieve the optimum compound for the intended application.
The various base compounds represent another aspect of the present invention. These compounds include those which have any of the following structures:
wherein A, B, C, D, E, F, G, H, I, J, K, L and, M are structured diversity elements of the types mentioned above;
Y is an oxygen, sulfur or nitrogen atom;
Z is
and; n can be any integer from 1 to four, inclusive.
A wide variety of molecular arrays can thus be generated for use in screening to determine which compounds would be suitable for a particular application.
The first structurally diverse array of molecules is advantageously produced by reacting either an oxazolone or an aminimide compound, or combinations thereof, with the first and second components which provide the orthogonal reactivity elements. It is useful for the first structurally diverse array of molecules to have one of the specific structures disclosed herein. These structures may include components such as an amino acid derivative, a nucleotide derivative, a carbohydrate derivative, an organic structural motif, a reporter element, a polymerizable moiety, or a macromolecular component.
This method is useful for a wide variety of applications, including the development of new biopharmaceutical agents, new monomeric species for the modular construction of separations tools, including chiral selectors, industrial detergents and additives, and for the development of modular chemical intermediates for the production of new materials and polymers. Specifically, the method relates to the selection of molecular modules containing appropriate structural diversity and reactivity elements, the connecting of these modules together via facile high-yield addition reactions which produce discrete, highly pure molecules in microscopic (less than or equal to 1 milligram) to macroscopic quantities (greater than 1 milligram) in a manner such that the properties of these molecules are determined by the contributions of the individual building modules. The molecular modules of the invention may be chiral, and can be used to synthesize new compounds, structures and materials which are able to recognize biological receptors,
enzymes, genetic materials, and other chiral molecules, and are thus of great interest in the fields of biopharmaceuticals, separation industrial and materials science. DETAILED DESCRIPTION OF THE INVENTION
For the purposes of this invention the following terms are defined to clearly delineate the scope of the present invention;
The term "addition reaction" is considered to be any reaction in which the number of original atoms or bonds in a compound is increased after such reaction has occurred.
"Compartments" is defined as any structure in, or on which a discrete amount of a compound is situated. This term is considered to encompass structures which have classically been considered to be compartments such as sample vials and test tubes, as well as non-traditional compartments, such as, for example, silicon wafers, gelatin, polystyrene or other macromolecular media.
A base module is a set of molecules which is common to a group of larger molecules in an array of said larger molecules, where said larger molecules have one or more structural diversity elements. The term "base module" is equivalent to the term "molecular scaffolding" for the present invention.
Structural diversity elements are any organic or inorganic atom(s), molecule(s), or bond(s) which adds to or changes the structure of a base module.
A reactive group is a molecule (s) capable of forming a structural diversity element.
When a numerical variable is specified as a part of any structure or formula, such numerical variable is intended to represent each embodiment of the subject structure or formula that would correspond to each numerical value that said variable could be.
The present invention is able to generate a number of different molecules for screening purposes by first forming a base module that contains at least two structural diversity elements attached thereto. These modules are formed by reacting first and second compounds, each of which has at least one
structural diversity element and a reactive group. The reactive groups of the first and second compounds are such that they react with each other to form the base module by an additional reaction. By fixing one of the positions and structures of the structural diversity elements and by varying at least one of the others, an array of different molecules is easily generated. These molecules can then be screened to determine which are suitable for a particular application or target use. Once a suitable compound is identified, it can be selected for generating a further array of molecules. This is done by modifying the particular structural diversity elements that are found to be suitable, or by combining the chosen structural diversity element with an expanded or different set of second compounds or elements. This process can be repeated as often as necessary to develop the optimum compound for the particular use.
The particular base module chosen for use in accordance with the present invention is not critical and can be any one of a wide variety of structures. It has been found, however, that two particular structures which are known in the art are highly useful as such base modules, these known compounds being the oxazolones and aminimides. Thus, it is preferred to utilize compounds which are aminimide forming, oxazolone forming, oxazolone or oxazolone-derived molecules for use as the base module. Depending upon the specific structure and feature selected, these base modules can have between two and nine structural diversity elements. The specific chemistry of these molecules, as well as an identification of the structural diversity elements and reactivity groups, follows.
Oxazolones
Oxazolones, or azlactones, are structures of the general formula:
where A, R, and R' are functional groups, and n is an integer between 0 and 3.
Oxazolones may possess up to two substituents at the 5- position, represented by R and R' . When these substituents are not equivalent, that is when R ≠ R', the carbon atom at the 5- position is asymmetric and two non-superimposable oxazolone structures (azlactones) result as shown below:
Chiral oxazolones possessing a single, non-hydrogen substituent at the 5-position (also known as 5(4H)-oxazolones), derived from chiral natural amino acid derivatives, including activated acylamino acyl structures, have been prepared and isolated in the pure, crystalline state (Bodansky, M.; Klausner, Y. S.; Ondetti, M. A. in "Peptide Synthesis", Second Edition, John Wiley & Sons, New York, 1976, p. 14 and references cited therein). The facile, base-catalyzed racemization of several of these oxazolones has been studied in connection with investigations of the serious racemization problem confronting peptide synthesis (see Kemp, D. S. in "The Peptides, Analysis,
Synthesis, and Biology", Vol. 1, Gross, E. & Meienhofer, J. editors, 1979, p. 315).
Racemization during peptide synthesis becomes very extensive when the desired peptide is produced by aminolysis of activated peptidyl carboxyl, as in the case of peptide chain extension from the amino terminus, e.g., I - VI shown below (see Atherton, E.; Sheppard, R. C. "Solid Phase Peptide Synthesis, A Practical Approach," IRL Press at Oxford University Press, 1989, pages 11 and 12). An extensively studied mechanism describing this racemization involves conversion of the activated acyl derivative (II) to an oxazolone (III) followed by facile base-catalyzed racemization of the oxazolone via a resonance-stabilized intermediate (IV) and aminolysis of the racemic oxazolone (V) producing racemic peptide products (VI).
Extensive research on the trapping of oxazolones III (or of their activated acyl precursors II) to give acylating agents which undergo little or no racemization upon aminolysis has been carried out. Successes in this area (such as the use of N- hydroxybenzotriazole) have greatly advanced the art of peptide synthesis (Kemp, D. S. in "The Peptides, Analysis, Synthesis, and Biology," Vol. 1, Gross, E. & Meienhofer, J. editors, 1979, p. 315). However, the control of such racemization is difficult, and at times unpredictable. Thus, attempts to deal with the racemization problem in peptide synthesis have involved suppressing or avoiding the formation of oxazolone intermediates altogether.
Oxazolones having at least one hydrogen substituent at the 5-position can also undergo a variety of rearrangements and side-reactions (cf., 1967 Tetrahedron 23, 3363), which may interfere with other desired transformations. This is illustrated for the case of the oxazolone formed from the cyclization of N-acryloyl glycine through a 1,5-hydrogen shift, (a [5,1] sigmatropic rearrangement) from the corresponding mono- substituted vinyl azlactone:
Oxazolones containing no hydrogen substituents at the five position (e.g., where R and R' are alkyl substituents, or where an exo-olefin protrudes from the oxazolone ring at this position) are structurally precluded from undergoing these racemizations and side-reactions. These di-substituted oxazolones may be obtained chirally pure and may be subjected to the transformations, which are the subject of this invention, with retention of the chirality at this position.
The formation of these substituted vinyl azlactones containing no hydrogen substituents at the 5-position can be produced through the cyclization of N-acryloyl glycine (where
R is a hydrogen atom) or an equivalent reagent (e.g., where R an alkyl group) in the presence of a carbonyl-containing reagent
(e.g., an aldehyde or ketone compound) as shown below:
The substituent at the 2-position can be capable of undergoing addition reactions, as in the case of the substituted vinyl group (where R can be a hydrogen or other substituent). Chemical modifications may be carried out with retention of the chirality at the 5-position to produce new oxazolones. This is shown for the Michael-type addition of the reagent A'XH to the alkenyl oxazolone as follows:
Functional groups that are capable of this Michael-type transformation without opening of the azlactone ring under appropriate conditions are, for example, mercaptans (where X = S) or secondary amines (where X = NR, and R can be a structural diversity element that does not adversely affect the outcome of the desired reaction). In both cases, A' can be a structural diversity group that does not adversely affect the formation of the molecular scaffold, as shown below:
where Y is a hetero-atom capable of opening the azlactone ring to form the molecular scaffold unit, and B' is a structural diversity element.
Synthesis of Oxazolones The compounds of the present invention can be synthesized by many routes. It is well known in the art of organic synthesis that many different synthetic protocols can be used to prepare a given compound. Different routes can involve more or less expensive reagents, easier or more difficult separation or purification procedures, straightforward or cumbersome scale- up, and higher or lower yield. The skilled synthetic organic chemist knows well how to balance the competing characteristics of synthetic strategies. Thus the compounds of the present invention are not limited by the choice of synthetic strategy, and any synthetic strategy that yields the compounds described above can be used.
Oxazolones may be prepared from the appropriate amino acid using any of a number of standard acylation and cyclization techniques well-known to those skilled in the art, e.g.:
The size of the azlactone ring, as well as the geometric configuration of the structural diversity elements Q, T, U, V, W and Z will be dictated by the choice of the amino-carboxylic acid containing reagent, e.g.:
The features of the structural diversity elements, R1 and
R2, as well as Q, T, U, V, W, and Z will be dictated by the amino-carboxylic acid containing reagent. The diversity elements R1 and R2 could also be added onto the azlactone module
(e.g., alpha-alkylation of the carbonyl in the presence of a non-nucleophilic base like DBU (1,8-diazobicyclo[5.4.0]undec-7- ene) and an alkylated agent, similar to the ones described below).
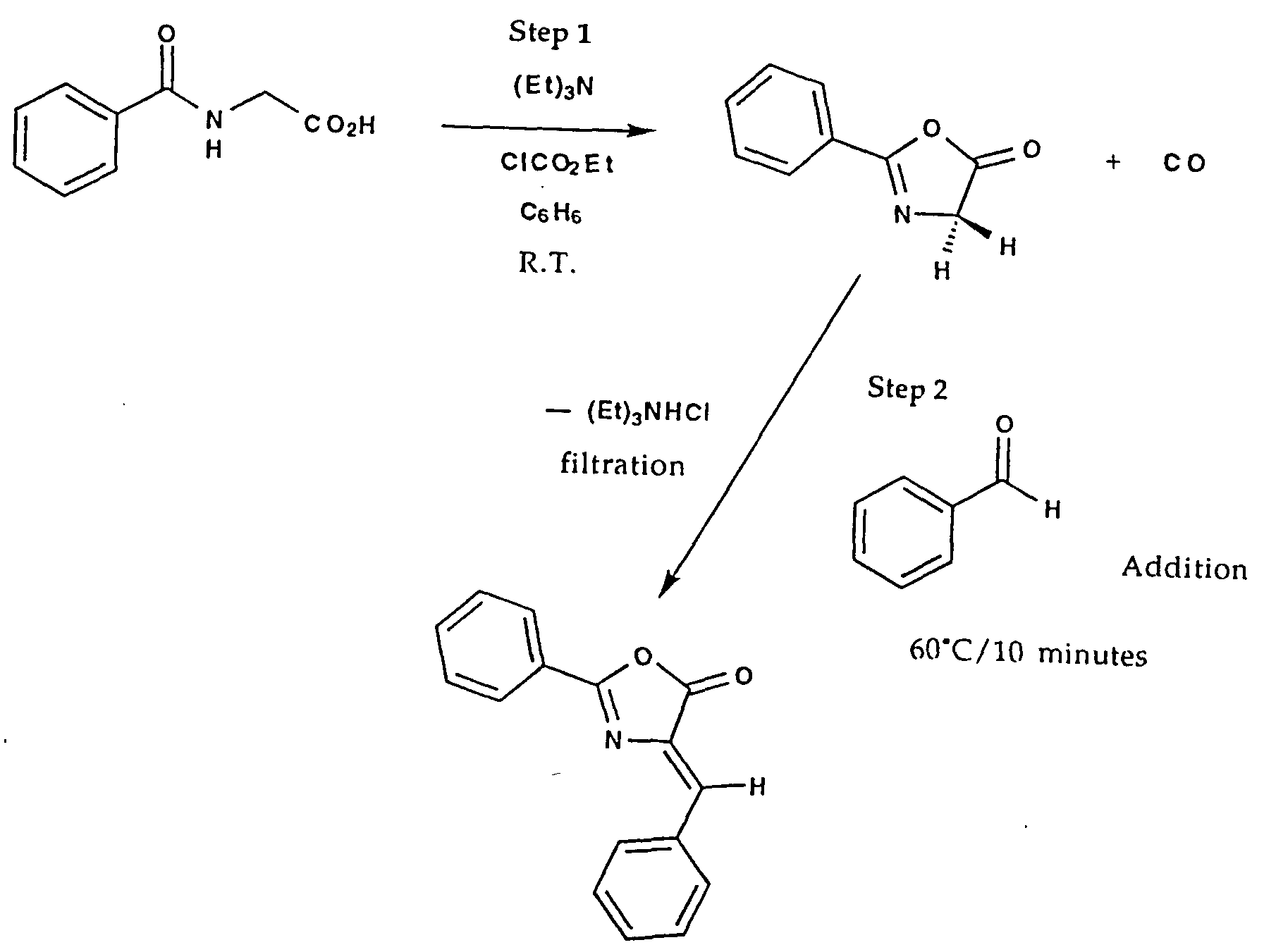
These oxazolones may be isolated in the pure state or may be generated in-situ from the acyl amino acid by treatment, for example, with equivalent amounts of triethyl amine and ethyl chloroformate in benzene. Following the evolution of carbon monoxide and the removal of the triethyl ammonium chloride formed by filtration, the solution of the oxazolone may be utilized directly for subsequent transformations.
For the purposes of this invention a structural diversity element can be any organic or inorganic atom, molecule or bond which adds to or changes the structure of a base module. Examples of structural diversity elements, are for example, any linear or branched chain alkyl group that is substituted or unsubstituted, any substituted or unsubstituted carbocyclic compound and any substituted or unsubstituted aryl group.
Further, the structural diversity elements preferably do not interfere or adversely affect the formation of the azlactone module. While the structural diversity element is broadly defined above, diversity element A can be, for example, straight or branched chain alkyl groups such as methyl, ethyl, propyl, butyl including n-butyl, sec-butyl, iso-butyl, tert-butyl, pentyl, hexyl, heptyl, octyl, etc., and their variants, straight and branch chain alkenyl chains such as ethenyl, propenyl,
butenyl, pentenyl, hexenyl, heptenyl, octenyl, etc., and their variants, straight and branch chain alkynyl chains such as ethynyl, propynyl, butynyl, pentynyl, hexynyl, heptynyl, octynyl, etc., and their variants, for example, aryl, aralkyl, alkaryl, cycloalkyl, cycloalkylalkyl and heterocycles. Functionalized diversity elements (e.g., 2-bromoethyl) and their variants, functionalized surfaces such as films, membranes, wafers, resins and beads may also be used.
Preferred reagents for the synthesis of structural diversity element A are compounds such as straight and branch chain alkyl carboxylic acids such as formic acid, acetic acid, propanoic acid, butanoic acid including n-butanoic acid, sec- butanoic acid, iso-butanoic acid, tert-butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, etc. , aryl carboxylic acids, aralkyl carboxylic acids, alkaryl carboxylic acids, cycloalkyl carboxylic acids, cycloalkylalkyl carboxylic acids and their variants, heterocyclic acids, N-protected amino acids, peptides and proteins such as N,S-Di-CBZ-L-Cysteine, N,N '-bis(t-BOC)-L-Cystine, N-t-Butoxycarbonyl-S-phenylalanine, N-t-Butoxycarbonyl-R-phenylalanine, etc., other carboxylic acids such as 3-Aminobenzoic acid, 4-Aminobenzoic acid, 2- Aminoisobutyric acid, cis-4-(Aminomethyl)cyclohexanecarboxylic acid, trans-4-(Aminomethyl)cyclohexanecarboxylic acid, 5- Aminovaleric acid, Bromoacetic acid, 3-Bromopropionic acid, Cyclohexanecarboxylic acid, Diphenylacetic acid, Ethylenediaminetetraacetic acid, 2-Formylphenoxyacetic acid, 4- Formylphenoxyacetic acid, Hippuric acid, Isonipecotic acid, (R)- (-)-Mandelic acid, (S)-(+)-Mandelic acid, (±)-2-Methylbutyric acid, D-Tartaric acid, L-Tartaric acid, Thiosalicylic acid, Trifluoroacetic acid, esters and acid halides listed below.
Reactions of Oxazolones
Ring-opening Addition
Oxazolones may be subjected to ring opening reactions with a variety of nucleophiles, as shown below:
In the structure above, Y represents a hetero-atom, such as an oxygen, sulfur, or nitrogen atom. R1 and R2 differ from one another, and taken alone, each signifies one of the following: alkyl including carbocyclic and substituted forms thereof; aryl, aralkyl, alkaryl, and substituted or heterocyclic versions thereof.
The above ring-opening reaction can be carried out either in an organic solvent such as methylene chloride, ethyl acetate, dimethyl formamide (DMF) or in water at room or higher temperatures, in the presence or absence of acids, such as carboxylic acids, other proton or Lewis-acids, or bases, such as tertiary amines or hydroxides, serving as catalysts.
This reaction may be used to generate an array of adducts, possessing combinations of the structural diversity elements A and C, as shown:
The reagents for the synthesis of diversity element B may be, for instance, straight or branched chain alkyl amines such as methyl amine, ethyl amine, propyl amine, butyl amine including n-butyl amine, sec-butyl amine, iso-butyl amine, tert- butyl amine, pentyl amine, hexyl amine, heptyl amine, octyl amine, etc., aryl amines, aralkyl amines, alkaryl amines, cycloalkyl amines, cycloalkylalkyl amines, heterocyclic amines and their variants, other amines such as 1- Adamantanemethylamine, 4'-Aminoacetophenone, 3-Aminobenzoic
acid, 4-Aminobenzoic acid, 4-Amino-1-benzylpiperidine, 4-Amino- 1-butanol, 4-Aminobutyraldehyde diethyl acetal, DL-α-Amino-ε- caprolactam, 1-Amino-2,6-dimethylpiperidine,
Aminodiphenylmethane, 4-(2-Aminoethyl)morpholine, 2-(2- Aminoethyl)-1-methylpyrrole, 2-(2-Aminoethyl)-1- methylpyrrolidine, 2-(2-Aminoethyl) pyridine, 1-(2-
Aminoethyl)pyrrolide, 1-Aminohomopiperidine, 1-Amino-4-(2- hydroxyethyl)piperazine, 2-Aminoisobutyric acid, 1-Aminoindan, (R)-(+)-1-Amino-2-(methoxymethyl)pyrrolidine, (S)-(-)-1-Amino-2- (methoxymethyl ) pyrrolidine , trans-4 -
(Aminomethyl) cyclohexanecarboxylie acid, 2-(2-
Aminomethylphenylthio) benzyl alcohol, 1-Amino-4- methylpiperazine, 3-(Aminomethyl) pyridine, 4-Aminomorpholine,
2-Amino-1-phenylethanol, 2-(4-Aminophenyl) ethylamine, 1- Ammopiperidine, (R)-(-)-1-Amino-2-propanol, (S)-(+)-1-Amino-2- propanol, (R)-(-)-2-Amino-1-propanol, (S)-(+)-2-Amino-1- propanol, 3-Amino-1-propanol, 3-Aminorhodanine, N-Amino-1,2,3,4- tetrahydroisoquinoline, 4-Amino-1,2,4-triazole, 5-Aminovaleric acid, Benzylamine, Cyclohexylamine, Dehydroabiethylamine, Diacetone acrylamine, Diethylamine, N,N-Diethylethylenediamine, N,N'-Diethylethylenediamine, N,N-Diethylethylenetriamine, 2,4- Difluorobenzylamine, Diisopropylamine, N, N-
Diisopropylethylamine, 2,2-Dimethoxypropane, 3-
Dimethylaminopropylamine, N,N-Dimethylethylenediamine, 1,1- Dimethylhydrazine, 2,2-Diphenylethylamine, Ethanolamine, 2- Ethoxybenzylamine, Furfurylamine, Histamine, Hydrazine, 2- Methoxybenzylamine, 3-Methoxybenzylamine, 4-Methoxybenzylamine, 2-Methoxyphenethylamine, 3-Methoxyphenethylamine, 4-
Methoxyphenethylamine, Methyl 3-aminobenzoate, Methyl 4- aminobenzoate, (R)-(+)-α-Methylbezylamine, 1-Methyl-3- phenylpropylamine, 1-Naphthalenemethylamine, (S)-(-)-α-
Methylbezylamine, Phenethylamine, 4-Phenylbutylamine, 3-Phenyl- 1-propylamine, Tetrahydrofurfurylamine, 1,2,3,4-Tetrahydro-1- naphthylamine, 2- (p-Tolyl) ethylamine, Tris (Hydroxymethyl)aminomethane, Tryptamine, Tyramine,
Vincamine; straight and branch chain alkyl mercaptans such as methyl mercaptan, Ethanethiol, propyl mercaptan, butyl mercaptan
including n-butyl mercaptan, sec-butyl mercaptan, isobutyl mercaptan, tert-butyl mercaptan, pentyl mercaptan, hexyl mercaptan, heptyl mercaptan, octyl mercaptan, etc., aryl mercaptans, alkaryl mercaptans, aralkyl mercaptans, cycloalkyl mercaptans, cycloalkylalkyl mercaptans, heterocyclic mercaptans and their variants, other thiols, such as 4-Acetamidothiophenol, 2-(2-Aminomethylphenylthio) benzyl alcohol, 3-Chloro-1- propanethiol, DL-Dithiothreitol, Methyl (methylthio) acetate, Methyl 3-(methylthio) propionate, 5-Methyl-1,3,4-thiadiazole-2- thiol, Methyl thioglycolate, Methyl thiosalicylate, 4- (Methylthio) benzaldehyde, 2-(Methylthio) ethanol, 3- (Methylthio)propionaldehyde, 1-Phenyl-1H-tetrazole-5-thiol, 1- Thio-β-D-glucose, 2-Thionaphthol, 2-Thiophenecarboxaldehyde, Thiosalicylic acid, Benzyl mercaptan, 2-Mercaptobenzothiazole, 2-Mercaptoethanol, 2-Mercaptopyridine; straight and branch chain alkyl alcohols such as methyl alcohol, ethyl alcohol, propyl alcohol, butyl alcohol including n-butyl alcohol, sec-butyl alcohol, iso-butyl alcohol, tert-butyl alcohol, pentyl alcohol, hexyl alcohol, heptyl alcohol, octyl alcohol, etc., aryl alcohols, alkaryl alcohols, aralkyl alcohols, cycloalkyl alcohols, cycloalkylalkyl alcohols, heterocyclic alcohols and their variants, other alcohols such as 4-Acetamidothiophenol, 4-Amino-1-butanol, 2-(2-Aminomethylphenylthio) benzyl alcohol, 2-Amino-1-phenylethanol, (R)-(-)-1-Amino-2-propanol, (S)-(+)-1- Amino-2-propanol, (R)-(-)-2-Amino-1-propanol, (S)-(+)-2-Amino-1- propanol, 3-Amino-1-propanol, 2-Bromoethanol, N-t- Butoxycarbonyl-(S)-phenylalaninol, N-t-Butoxycarbonyl- (R)- phenylalaninol, (R)-(-)-Epinephrine, (S)-(+)-Epinephrine, Ethanolamine, Glycerol, Glycidol, 2-Mercaptoethanol, (R)-2- Methylglycidol, (S)-2-Methylglycidol, 2-(Methylthio) ethanol. Phenol, (R)-(-)-2-Phenylglycinol, (S)-(+)-2-Phenylglycinol, 2- Thionaphthol, 4- (Trifluoromethyl) benzyl alcohol, and 2- (Trifluoromethyl)phenethyl alcohol.
In a preferred embodiment of the present invention the above described structural diversity elements are substituents on the following general structure:
wherein where A and B.are structured diversity elements of the types mentioned above;
Y is an oxygen, sulfur or nitrogen atom;
Z is selected from
n can be any integer from 1 to four, inclusive with the proviso that when n=1, A is a nitrogen group, Z is a compound according to structure III and Y is an oxygen atom, B is not a resin bead. In another preferred embodiment of the present invention the substituents on formula I are such that when n=1, Z is a compound according to structure III, C and D are hydrogen atoms, A is a carbon atom bonded to: (a) a secondary amine; (b) a hydrogen atom; and, (c) another carbon atom which is bonded to a substituted or unsubstituted aminal.
Still another preferred embodiment is when formula I is substituted such that when n=1, Z is a compound according to structure III, C and D are hydrogen atoms, and A is a carbon atom bonded to 2 hydrogen atoms and a primary or secondary amine.
In addition, by appropriate selection of the R1 and R2 groups, two additional diversity elements can be provided in those positions. Thus, the compound shown can have from two to four structural diversity elements attached to the base module as desired.
Carbonyl Addition
When both substituents in the 5-position are hydrogen, i.e., the oxazolone is formed from cyclization of an acyl glycine, the ring may undergo a high yield condensation addition reaction with aldehydes or ketone-containing structural groups through an Aldol-type condensation (e.g., the Erlenmeyer azlactone synthesis). This reaction may be used to generate an array of adducts, possessing combinations of the structural diversity elements A, B and E, as shown:
Again, as noted above, the C and D groups can be selected to be diversity elements to provide an additional structural diversity group on the oxazolone molecule.
When D is a hydrogen, diversity element C can be, for example, straight or branched chain alkyl aldehydes such as Formaldehyde, ethanal, propanal, butanal including n-butyl aldehyde, sec-butyl aldehyde, iso-butyl aldehyde, pentanal, hexanal, heptanal, octanal, etc., aryl aldehydes, alkaryl aldehydes, aralkyl aldehydes, cycloalkyl aldehydes, cycloalkylalkyl aldehydes, heterocyclic aldehydes and their variants, other aldehydes such as o-Anisaldehyde, m- Anisaldehyde, p-Anisaldehyde, Benzaldehyde, 1,4-Benzodioxan-6-
carboxaldehyde, 3-Benzyloxybenzaldehyde, 4-
Benzyloxybenzaldehyde, 4-Biphenylcarboxaldehyde, 3,5-
Bis(trifluoromethyl) benzaldehyde, 4-Bromobenzaldehyde, 3-(4- tert-Butylphenoxy)benzaldehyde, 4-Carboxybenzaldehyde, 2- Chlorobenzaldehyde, 3-Chlorobenzaldehyde, 4-Chlorobenzaldehyde, trans-Cinnamaldehyde, (S)-(-)-Citronellal,
Cyclohexanecarboxaldehyde, Cyclopropanecarboxaldehyde, 3-(3,4- Dichlorophenoxy) benzaldehyde, 2,3-Difluorobenzaldehyde, 2,4- Difluorobenzaldehyde, 2,5-Difluorobenzaldehyde, 2,6- Difluorobenzaldehyde, 3,4-Difluorobenzaldehyde, 3,5-
Difluorobenzaldehyde, 2,3-Dimethoxybenzaldehyde, 2,4-
Dimethoxybenzaldehyde, 2,5-Dimethoxybenzaldehyde, 4-
(Dimethylamino) benzaldeyde, Diphenylacetaldehyde, 2-
Ethoxybenzaldehyde, 4-Ethoxybenzaldehyde, 4-Ethylbenzaldehyde, 3-Fluoro-p-anisaldehyde, 2-Fluorobenzaldehyde, 3-
Fluorobenzaldehyde, 4-Fluorobenzaldehyde, 3-Fluoro-2- methylbenzaldehyde, 2-Fluoro-3-trifluoromethylbenzaldehyde,
Formaldehyde, 4-Formyl-1,3-benzenedisulfonic acid, 4-
Formylbenzenesulfonic acid, 5-Formyl-2-furansulfonic acid, 2- Formylphenoxyacetic acid, 4-Formylphenoxyacetic acid, trans- trans-2,4-Hexadienal, 4-Hydrocinnamaldehyde, Hydroxybezaldehyde, Indole-3-carboxaldehyde, 4-Isopropylbenzaldehyde,
Isovaleraldehyde, 2-Methoxy-1-pyrrolidinecarboxaldehyde, 3- Methyl-p-anisaldehyde, 3-(4-Methylphenoxy)benzaldehyde, 4- (Methylthio) benzaldehyde, 3- (Methylthio)propionaldehyde, 1- Naphthaldehyde, 2-Naphthaldehyde, 2-Nitrobenzaldehyde, 5- Norbornene-2-carboxaldehyde, 3-Phenoxybenzaldehyde, 4-
Phenoxybenzaldehyde, Phenylacetaldehyde, 3-Phenylbutyraldehyde, Phenylpropionaldehyde, 4-Propoxybenzaldehyde, Piperonal, 2- Pyridinecarboxaldehyde, 3-Pyridinecarboxaldehyde, 4-
Pyridinecarboxaldehyde, Pyrrole-2-carboxaldehyde,
Stilbenecarboxaldehyde, 2-Thiophenecarboxaldehyde, o-
Tolualdehyde, m-Tolualdehyde, p-Tolualdehyde, 3-
( Tri f luoromethoxy ) benz a ldehyde , 4 - (Trifluoromethoxy) benzaldehyde, 3- [ 3-
(Trifluoromethyl)phenoxy]benzaldehyde, α,α,α-Trifluoro-o-
tolualdehyde, α,α,α-Trifluoro-m-tolualdehyde, α,α,α-Trifluoro-p- tolualdehyde, and Valeraldehyde.
Where D is not Hydrogen, either of the diversity elements C and D can be, for example, generated from reagents such as straight or branched chain alkyl ketones such as propanone, 2- butanone, 3-butanone, pentanone, hexanone, heptanone, octanone, etc., aryl aryl ketones, alkyl aryl ketones, aryl alkyl ketones, cycloalkyl ketones, cycloalkylalkyl ketones, heterocyclic ketones and their variants, other ketones such as 5-(2- Adamantylidene)-2,2-dimethyl-1,3-dioxane-4,6-dione, 4'- Aminoacetophenone, Benzophenone, Cyclopropyl phenyl ketone, Diacetone aerylamine, 2,2-Dimethyl-1,3-dioxane-4,6-dione, 10- Methyl-9 (10H)-acridone, 1-Methyl-2-pyrazolidinone, and 3-Methyl- 3-pyrazolin-5-one.
Combination of the Two Reactions
The resulting adduct may subsequently undergo a high yield ring-opening addition reaction with a wide variety of nucleophiles, such as reagents mercaptans, amino groups and alcohols. This reaction sequence may, thus, be used to generate an array of adducts, possessing combinations of the structural diversity elements A, B, C and D, as shown:
Again, as noted above, the A, C and D groups can be selected to be diversity elements to provide additional structural diversity groups on the oxazolone molecule. And the diversity element B can be selected to provide additional
structural diversity on the oxazolone-based molecule that is formed after ring opening in the presence of a nucleophile.
This is illustrated for the case of the in-situ generation of the oxazolone from hippuric acid, followed by removal of the triethylammonium chloride by filtration, the addition of benzaldehyde to form the unsaturated adduct and the ring opening addition of benzylamine to give the tri-phenyl substituted adduct shown. In this specific case, the reagents have been chosen such that the diversity element A is a phenyl ring, as generated from the azlactone module, diversity element C is a phenyl ring and diversity element D is a hydrogen atom, as generated by benzaldehyde, and the diversity element B is a benzyl group, as generated by the nucleophilic opening of the azlactone by benzylamine:
The ability of these various reactions to be carried out in a stepwise sequential process using modules chosen in a structure-directed manner allows the production of structurally directed thematic diversity libraries, having structural elements systematically varied around a basic motif.
Aminimides
Aminimides are Zwitterionic structures described by the resonance hybrid of the two energetically comparable Lewis structures shown below:
The tetrasubstituted nitrogen of the aminimide group can be asymmetric , rendering aminimides chiral as shown by the two enantiomers below:
As a result of the polarity of their structures but lack of net charge, simple aminimides are freely soluble in both water and organic solvents.
Dilute aqueous solutions of aminimides are neutral and of very low conductivity; the conjugate acids of simple aminimides are weakly acidic, with a pKa of about 4.5. A striking property of aminimides is their hydrolytic stability, under acidic, basic, or enzymatic conditions. For example, boiling trimethyl amine benzamide in 6 N NaOH for 24 hrs leaves the aminimide unchanged. Upon thermolytic treatment, at temperatures exceeding 180 °C, aminimides decompose to give isocyanates as follows:
Synthetic Routes to Aminimides
Aminimides can be synthesized in a variety of different ways. It is well known in the art of organic synthesis that
many different synthetic protocols can be used to prepare a given compound. Different routes can involve more or less expensive reagents, easier or more difficult separation or purification procedures, straight forward or cumbersome scale- up, and higher or lower yields. The skilled synthetic organic chemist knows well how to balance the competing characteristics of different strategies. Thus, the compounds of the present invention are not limited by the choice of synthetic strategy. Any synthetic strategy that yields the compounds described can be used.
Aminimides via Alkylation of N,N-Di-substituted Hydrazides
Alkylation of a hydrazide followed by neutralization with a base produces an aminimide.
This alkylation is carried out in a suitable solvent, such as a protic solvent, e.g., water, ethanol, isopropyl alcohol or a dipolar aprotic solvent, e.g., DMF, DMSO, acetonitrile, usually with heating. An example of this reaction is the synthesis of the trifluoroacyl-analide dipeptide elastase inhibitor mimetics shown in the examples below.
The synthesis of hydrazides is well known. For example, hydrazides can be generated from the reaction of hydrazines with acid chlorides. The diversity elements E and F may be, for example, derived from reagents containing di-substituted hydrazines. The structural diversity element H may be, for example, derived from reagents such as acid halides and reagents that are capable of being converted to acid halides, such as carboxylic acids and esters as described below.
Diversity element G may be, for example, straight or branched chain alkyl bromides such as bromomethane, bromoethane,
1-bromopropane, 2-Bromopropane, bromobutane including 1- bromobutane, 2-Bromobutane, 1-Bromo-2-methylpropane, sec-butyl bromide, iso-butyl bromide, bromopentane, bromohexane, bromoheptane, bromooctane, etc., aryl bromides, alkaryl bromides, aralkyl bromides, cycloalkyl bromides, cycloalkylalkyl bromides and their variants, other bromides such as Benzyl bromide, 2-Bromoacetamide, Bromoacetic acid, 4- Bromobenzaldehyde, 1-Bromo-2,2-dimethoxypropane, 2-Bromoethanol,
2-(2-Bromoethyl)-1,3-dioxane, (2-Bromoethyl) benzene, 3-
(Bromomethyl)-2,4,10-trioxaadamantane, 3-Bromopropionic acid, tert-Butyl bromoacetate, Carbon tetrabromide, Cinnamyl bromide,
Methyl bromoacetate, Methyl 3-bromopropionate, straight and branch chain alkyl chlorides such as chloromethane, chloroethane, 1-chloropropane, 2-chloropropane, chlorobutane including 1-chlorobutane, 2-chlorobutane, sec-butyl chloride, iso-butyl chloride, chloropentane, chlorohexane, chloroheptane, chlorooctane, etc., aryl chlorides, alkaryl chlorides, aralkyl chlorides, cycloalkyl chlorides, cycloalkylalkyl chlorides and their variants, other chlorides such as Benzyl chloride, 2-
Chloroethyl methyl sulfide, 3-Chloro-1-propanethiol, 1,2-
Dichloroethane, straight and branch chain alkyl iodides such as iodomethane, iodoethane, 1-iodopropane, 2-iodopropane, iodobutane including 1-iodobutane, 2-iodobutane, sec-butyl iodide, iso-butyl iodide, iodopentane, iodohexane, iodoheptane, iodooctane, etc., aryl iodides, alkaryl iodides, aralkyl iodides, cycloalkyl iodides, cycloalkylalkyl iodides and their variants, other iodides such as Benzyl iodide, substituted alcohols (e.g., mesitylated or tosylated derivatives) for the alcohols such as those previously listed.
Aminimides via Acylation of 1,1,1-Trialkyl Hydrazinium Salts
Acylation of a suitable trialkyl hydrazinium salt by an acyl derivative or isocyanate in the presence of a strong base in a suitable organic solvent, e.g., dioxane, ether, acetonitrile, etc., produces good yields of aminimides.
The formation of the hydrazinium salt is well known. For example, alkylation of a di-substituted hydrazine with an alkyl halide will generally alkylate the hydrazine on the more substituted nitrogen, thus forming the hydrazinium salt. The structural diversity elements E and F may be generated from reagents that contain a di-substituted hydrazine, as those described above. The structural diversity element G may be generated from reagents capable of alkylation, also described as above for the alkylation of hydrazides.
Diversity element H may be any diversity element such as those defined above. In particular, H can be derived from reagents such as straight or branched chain alkyl esters, such as alkyl formate, alkyl acetate, alkyl propionate, alkyl butanoate including alkyl n-butanoate, alkyl sec-butanoate, alkyl iso-butanoate, alkyl pentanoate, alkyl hexanoate, alkyl heptanoate, alkyl octanoate, etc., alkaryl esters, aralkyl esters, cycloalkylalkyl esters, heterocyclic esters and their variants, other esters such as Diethyloxalate, Dimethyl L- Tartrate, Ethyl 3,4-dihydroxyhydrocinnamate, Ethyl 2,3- Epoxybutyrate, Ethyl hydrocinnamate, Ethyl N-hydroxyacetimidate, Ethyl isonipecotate, Ethyl 2-methyl-4-pentenoate, Ethyl 4- methyl-5-imidazolecarboxylate, Ethyl (±) -nipecotate, Ethyl (±)- 3-phenylglycidate, Ethyl 1-piperazinecarboxylate, Ethyl 1- piperidineacetate, Ethyl o-tolylacetate, Methyl acetate, Methyl 3-aminobenzoate, Methyl 4-aminobenzoate, Methyl benzoate, Methyl 1-benzyl-5-oxo-3-pyrrolidinecarboxylate. Methyl bromoacetate, Methyl 3-bromopropionate, Methyl butyrate, Methyl caproate, Methyl trans-cinnamate. Methyl cyclohexanecarboxylate, Methyl cyclohexanepropionate, Methyl cyclohexylacetate, Methyl cyclopropanecarboxylate, Methyl 2,5-dichlorobenzoate, Methyl 2,4-dihydroxybenzoate, Methyl 3,5-dimethoxybenzoate, Methyl 2,2-
dimethyl-3-hydroxypropionate, Methyl 3 ,3-dimethyl-4-pentenoate,
Methyl diphenylacetate, Methyl 10,11-Epoxyundecanoate, Methyl
4-fluorobenzoylacetate, Methyl 4-formylbenzoate, Methyl 2- furoate, Methyl 3-hydroxybenzoate, Methyl 4-hydroxybenzoate, Methyl 2-hydroxyisobutyrate, Methyl 4-hydroxymethylbenzoate,
Methyl 3-(4-hydroxyphenyl) propionate. Methyl 4- hydroxyphenylacetate. Methyl isobutyrate. Methyl isonicotinate,
Methyl (S)-(-)-lactate, Methyl (±)-mandelate, Methyl methanesulfonate, Methyl methoxyacetate, Methyl 2- methoxybenzoate. Methyl 4-methoxybenzoate, Methyl trans-(±)-3-
(4-methoxyphenyl) glycidate, Methyl 4-methoxyphenylacetate,
Methyl 2-methylbenzoate, Methyl 3-methylbenzoate, Methyl 4- methylbenzoate, (±)-Methyl 2-methylbutyrate, Methyl 2-methyl-3- furancarboxylate, Methyl 6-methylnicotinate, Methyl o- Methylpodacarpate, Methyl 1-methyl-2-pyrroleacetate, Methyl
(methylthio) acetate, Methyl 3-(methylthio) propionate, Methyl 1- naphthaleneacetate, Methyl nicotinate, Methyl 2- oxocyclopentanecarboxylate, Methyl phenoxyacetate, Methyl 2- phenyl-4-quinolinecarboxylate, Methyl propionate, Methyl 3- pyridylcarbamate. Methyl salicylate, Methyl thioglycolate.
Methyl thiosalicylate, Methyl trifluoroacetate, Methyl trimethylacetate, Methyl valerate, Methyl vanillate,
Methylphenylacetate, straight and branch chain acid halides such as formoyl halide, Acetyl halide, Propionyl halide, butyryl halide including n-butyryl halide, sec-butyryl halide,
Isobutyryl halide, pentionyl halide, Isovaleryl halide, hexionyl halide, heptionyl halide, octionyl halide, Palmitoyl chloride, etc., aryl acid halides such as Benzoyl chloride, alkaryl acid halides, aralkyl acid halides such as 4-Biphenylcarbonyl chloride, cycloalkylalkyl acid halides such as
Cyclohexanecarbonyl chloride, Cyclopentanecarbonyl chloride,
Cyclopropanecarbonyl chloride and their variants, other acid halides such as, Acryloyl chloride, 1-Adamantanecarbonyl chloride, Bromoacetyl bromide, 3-Bromopropionyl chloride, Diphenylacetyl chloride, 2-Furoyl chloride, Hydrocinnamoyl chloride, lminodibenzyl-5-carbonyl chloride, 2-
Mesitylenesulfonyl chloride, Methacryloyl chloride,
Methanesulfonyl chloride, 4-Morpholinecarbonyl chloride, Nicotinoyl chloride, 3-Nitrobenzoyl chloride, 4-Nitrobenzoyl chloride, Oxalyl chloride, Phenylacetyl chloride, Piperonyloyl chloride, Terephthaloyl chloride, Valeryl chloride, straight and branch chain alkyl haloformates, a such as Ethyl chloroformate, and Isobutyl chloroformate, aryl haloformates, alkaryl haloformates, aralkyl haloformates, cycloalkyl haloformates, cycloalkylalkyl haloformates and their variants, the carboxylic acids, previously described, that can be converted to esters (e.g., propionic acid in the presence of boron trifluoride etherate in methanol will form methyl propionate) or acid halides (e.g., propionic acid in the presence of thionyl chloride will yield propionyl chloride). Aminimides via the Hydrazine-Epoxide-Ester Reaction
A very useful and versatile synthesis of aminimides involves the one-pot reaction of an epoxide, an asymmetrically di-substituted hydrazine, and an ester in a protic solvent, usually water or an alcohol, which is allowed to proceed usually at room temperature over several hours to several days.
The structural diversity elements E, F and H may be any structural diversity element. In particular, E, F and H may be
derived from reagents containing substituents such as alkyl, carbocyclic, cycloalkyl, aryl or alkaryl, and those carboxylates as described above. The structural diversity element J may be selected from reagents containing a terminal epoxide, for example ethylene oxide, propylene oxide and styrene oxide. Other oxiranes are listed in preferred examples set forth for structural diversity elements J, K and L.
The rates for the above reaction increase with increasing electrophilicity of the ester component. Generally, a mixture of 0.1 mole of each of the reactants in 50-100 mL of an appropriate solvent is stirred for the required period at room temperature (the reaction may be monitored by thin layer chromatography). At the end of this period, the solvent is removed in vacuo to give the crude product.
Any of the various structural diversity elements illustrated in all of these aminimide and aminimide-forming structures may be selected to be a structural diversity element.
The ability of these various reactions to be carried out using modules chosen in a structure-directed manner allows the production of structurally directed thematic diversity libraries, having structural elements systematically varied around a basic motif.
Other methods of producing aminimides are detailed in an article entitled "Chemistry of Aminimides", Stanley Wawzonek, Ind. Eng. Chem. Prod. Res. Dev., Volume 19, pages 338-349, 1980, herein specifically incorporated by reference. Further details on the reaction possibilities for the subject oxazolone and aminimide compounds can be found in PCT applications, PCT/US93/12591 and PCT/US93/12612, each filed on December 28, 1993, and entitled Modular Design And Synthesis Of Oxazolone- Derived Molecules and Modular Design And synthesis Of Aminimide- Derived Molecules, respectively. The content of each of those applications is expressly incorporated herein by reference thereto to the extent necessary to understand the metes and bounds of this invention.
Mixed Aminimide-Oxazolones
A particularly useful embodiment of the invention is the synthesis of mixed aminimide-oxazolone molecules, as shown below. This scenario allows the incorporation of multiple structural diversity elements as indicated:
The diversity element A represents that diversity element from the module azlactone, the diversity elements C and D represent those for the carbonyl-derived diversity element of the azlactone module, the diversity elements E and F represent diversity elements derived from an unsymmetric, 1,1- disubstituted hydrazine, and the diversity element J represents diversity elements derived from a functionalized oxirane, in this example, a terminal oxirane.
The oxirane used in the formation of the hydrazinium ion in the example shown above can be di-substituted or tri- substituted. In the case where a tri-substituted oxirane is
used, an additional two structural diversity elements, K and L, can be introduced.
Some preferred reagents for the synthesis of the diversity element J, K and L may be epoxides such as straight and branch chain oxiranes such as ethylene oxide, Propylene oxide, 1,2- Epoxybutane, cis-2,3-Epoxybutane, trans-2,3-Epoxybutane, 1,2- Epoxypentane, 2,3-Epoxypentane, 1,2-Epoxyhexane, 2,3- Epoxyhexane, 3,4-Epoxyhexane, Epoxyheptane, Epoxyoctane, etc., aryl epoxides, alkaryl epoxides, aralkyl epoxides, cycloalkyl epoxides, cycloalkylalkyl epoxides, heterocyclic epoxides and their variants, other oxiranes such as (±)-1,3-Butadiene diepoxide, Butyl glycidyl ether, 4-Chlorophenyl glycidyl ether,
Cyclohexene oxide, Cyclooctene oxide, Cyclopentene oxide, Ethyl
(±)-3-phenylglycidate, 2-Ethylhexyl glycidyl ether, Glycidol, (±)-Glycidyl 2-methylphenyl ether, (±)-Glycidyl isopropyl ether,
(+)-Limonene oxide, Methyl trans -(±)-3-(4- methoxyphenyl)glycidate, (R)-2-Methylglycidol, (S)-2-
Methylglycidol, a-Pinene oxide, Styrene oxide, 4-tert-
Butylphenyl 2,3-epoxypropyl ether, Epichlorohydrin, (±)-1,2- Epoxy-3-phenoxypropane, 1,2-Epoxy-5-hexene, 1,2-Epoxyhexane, exo-2,3-Epoxynorbornane, (±)-(2,3-Epoxypropyl) benzene, 2,3- Epoxypropyl 4-methoxyphenyl ether, Ethyl 2,3-Epoxybutyrate, Methyl 10,11-Epoxyundecanoate.
Furthermore, the hydroxyl group can be modified to accommodate yet another structural diversity element, represented by M. The structural diversity element M may be derived from those reagents described for the structural diversity element G. Thus, a total of nine diversity elements can be provided on the mixed aminimide-oxazolone base module as shown below.
Structural Diversity Elements
Any of a wide variety of structural diversity elements can be used. These elements would include:
1.) Amino acid derivatives of the form (AA)n, which would include, for example, natural and synthetic amino acid residues (n=1) including all of the naturally occurring alpha amino acids, such as alanine, arginine, asparagnine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, etc.; the naturally occurring di-substituted amino acids, such as amino isobutyric acid, and isovaline, etc.; a variety of synthetic amino acid residues, including alpha-disubstituted variants, species with olefinic substitution at the alpha position, species having derivatives, variants or mimetics of the naturally occurring side chains; N-Substituted glycine residues; natural and synthetic species known to functionally mimic amino acid residues, such as statine, bestatin, etc. Peptides (n = 2-30) constructed from the amino acids listed above, such as angiotensinogen and its family of physiologically important angiotensin hydrolysis products, as well as derivatives, variants and mimetics made from various combinations and permutations of all the natural and synthetic residues listed above. Polypeptides (n = 31-70), such as big endothelin, pancreastatin, human growth hormone releasing factor and human pancreatic polypeptide. Proteins (n > 70) including structural proteins such as collagen, functional proteins such as hemoglobin, regulatory proteins such as the dopamine and thrombin receptors.
2.) Nucleotide derivatives of the form (NUCL)n, which includes natural and synthetic nucleotides (n=1) such as adenosine, thymine, guanidine, uridine, cystosine, derivatives of these and a variety of variants and mimetics of the purine ring, the sugar ring, the phosphate linkage and combinations of some or all of these. Nucleotide probes (n=2-25) and oligonucleotides (n>25) including all of the various possible
homo and heterosynthetic combinations and permutations of the naturally occurring nucleotides, derivatives and variants containing synthetic purine or pyrimidine species or mimics of these, various sugar ring mimetics, and a wide variety of alternate backbone analogs including but not limited to phosphodiester, phosphorothionate, phosphorodithionate, phosphoramidate, alkyl phosphotriester, sulfamate, 3'- thioformacetal, methylene (methylimino), 3-N-carbamate, morpholino carbamate and peptide nucleic acid analogs.
3.) Carbohydrate derivatives of the form (CH)n, including natural physiologically active carbohydrates such as glucose, galactose, sialic acids, beta-D-glucosylamine and nojorimycin which are both inhibitors of glucosidase; pseudo sugars, such as 5a-carba-2-D-galactopyranose, which is known to inhibit the growth of Klebsiella pneumonia (n=1), synthetic carbohydrate residues and derivatives of these (n=1) and all of the complex oligomeric permutations of these as found in nature, including high mannose oligosaccharides, the known antibiotic streptomycin (n>1).
4.) A naturally occurring or synthetic organic structural motif. This term is defined as meaning an organic molecule having a specific structure that has biological activity, such as having a complementary structure to an enzyme, for instance. This term includes any of the well known base structures of pharmaceutical compounds including pharmacophores or metabolites thereof. These motifs include beta-lactams, such as penicillin, known to inhibit bacterial cell wall biosynthesis; dibenzazepines, known to bind to CNS receptors, used as antidepressants; polyketide macrolides, known to bind to bacterial ribosymes, etc. These structural motifs are generally known to have specific desirable binding properties to ligand acceptors.
5.) A reporter element, such as a natural or synthetic dye or a residue capable of photographic amplification which possesses reactive groups which may be synthetically incorporated into the oxazolone structure or reaction scheme, and may be attached through the groups without adversely
interfering with the reporting functionality of the group. Preferred reactive groups are amino, thio, hydroxy, carboxylic acid, carboxylic acid ester, particularly methyl ester, acid chloride, isocyanate alkyl halides, aryl halides and oxirane groups.
6.) An organic moiety containing a polymerizable group such as a double bond or other functionalities capable of undergoing condensation polymerization or co-polymerization. Suitable groups include vinyl groups, oxirane groups, carboxylic acids, acid chlorides, esters, amides, lactones and lactams. Other organic moiety such as those defined for R and R' may also be used.
7.) A macromolecular component, such as a macromolecular surface or structures which may be attached to the oxazolone modules via the various reactive groups outlined above in a manner where the binding of the attached species to a ligand- receptor molecule is not adversely affected, and the interactive activity of the attached functionality is determined or limited by the macromolecule. This includes porous and non-porous inorganic macromolecular components, such as, but not limited to silica, alumina, zirconia, titania and the like, as commonly used for various applications, such as normal and reverse phase chromatographic separations, water purification, pigments for paints, etc.; porous and non-porous organic macromolecular components, including synthetic components such as styrene- divinyl benzene beads, various methacrylate beads, PVA beads, and the like, commonly used for protein purification, water softening and a variety of other applications, natural components such as native and functionalized celluloses, such as, for example, agarose and chitin, sheet and hollow fiber membranes made from nylon, polyether sulfone or any of the materials mentioned above. The molecular weight of these macromolecules may range from about 1000 Daltons to as high as possible. They may take the form of nanoparticles (dp = 100- 1000 Angstroms), latex particles (dp = 1000-5000 Angstroms), porous or non-porous beads (dp = 0.5-1000 microns), membranes,
gels, macroscopic surfaces or functionalized or coated versions or composites of these.
8) A structural moiety selected from the group including cyano, nitro, halogen, oxygen, hydroxy, alkoxy, thio, straight or branched chain alkyl, carbocyclic aryl and substituted or heterocyclic derivatives thereof.
As used herein, the phrase linear chain or branched chained alkyl groups means any substituted or unsubstituted acyclic carbon-containing compounds, including alkanes, alkenes and alkynes. Alkyl groups having up to 30 carbon atoms are preferred. Examples of alkyl groups include lower alkyl, for example, methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl or tert-butyl; upper alkyl, for example, octyl, nonyl, decyl, and the like; lower alkylene, for example, ethylene, propylene, propyldiene, butylene, butyldiene; upper alkenyl such as 1- decene, 1-nonene, 2,6-dimethyl-5-octenyl, 6-ethyl-5-octenyl or heptenyl, and the like; alkynyl such as 1-ethynyl, 2-butynyl, 1-pentynyl and the like. The ordinary skilled artisan is familiar with numerous linear and branched alkyl groups, which are within the scope of the present invention.
In addition, such alkyl group may also contain various substituents in which one or more hydrogen atoms has been replaced by a functional group. Functional groups include but are not limited to hydroxyl, amino, carboxyl, amide, ester, ether, and halogen (fluorine, chlorine, bromine and iodine), to mention but a few. Specific substituted alkyl groups can be, for example, alkoxy such as methoxy, ethoxy, butoxy, pentoxy and the like, polyhydroxy such as 1,2-dihydroxypropyl, 1,4- dihydroxy-1-butyl, and the like; methylamino, ethylamino, dimethylamino, diethylamino, triethylamino, cyclopentylamino, benzylamino, dibenzylamino, and the like; propanoic, butanoic or pentanoic acid groups, and the like; formamido, acetamido, butanamido, and the like, methoxycarbonyl, ethoxycarbonyl or the like, chloroformyl, bromoformyl, 1,1-chloroethyl, bromo ethyl, and the like, or dimethyl or diethyl ether groups or the like.
As used herein, substituted and unsubstituted carbocyclic groups of up to about 20 carbon atoms means cyclic carbon-
containing compounds, including but not limited to cyclopentyl, cyclohexyl, cycloheptyl, admantyl, and the like. such cyclic groups may also contain various substituents in which one or more hydrogen atoms has been replaced by a functional group. Such functional groups include those described above, and lower alkyl groups as described above. The cyclic groups of the invention may further comprise a heteroatom. For example, in a specific embodiment, R2 is cycohexanol.
As used herein, substituted and unsubstituted aryl groups means a hydrocarbon ring bearing a system of conjugated double bonds, usually comprising an even number of 6 or more pi-bond electrons. Examples of aryl groups include, but are not limited to, phenyl, naphthyl, anisyl, toluyl, xylenyl and the like. According to the present invention, aryl also includes aryloxy, aralkyl, aralkyloxy and heteroaryl groups, e.g., pyrimidine, morpholine, piperazine, piperidine, benzoic acid, toluene or thiophene and the like. These aryl groups may also be substituted with any number of a variety of functional groups. In addition to the functional groups described above in connection with substituted alkyl groups and carbocyclic groups, functional groups on the aryl groups can be nitro groups.
As mentioned above, these structural moieties can also be any combination of alkyl, carbocyclic or aryl groups, for example, 1-cyclohexylpropyl, benzylcyclohexylmethyl, 2- cyclohexyl-propyl, 2,2-methylcyclohexylpropyl, 2,2- methylphenylpropyl, 2,2-methylphenylbutyl, and the like.
In one preferred embodiment of the present invention one or more of the structural diversity elements; A, B, C, D, E, F, G, H, J, K, L and M are reactive groups that are capable of further reactions to produce a base module or an orthogonal reactive group. For example, the present invention is directed to structural diversity groups that may themselves be capable of further reaction to form base modules as described herein. For example, a structural diversity element that is an oxazolone based reactive group that upon further reaction can form an aminimide base module. Such ring opening reactions are described in PCT applications, PCT/US93/12591 and PCT/US93/12612, each
filed on December 28, 1993, and entitled Modular Design And Synthesis Of Oxazolone-Derived Molecules and Modular Design And synthesis Of Aminimide-Derived Molecules, respectively. The content of each of those applications is expressly incorporated herein by reference thereto to the extent necessary to understand the metes and bounds of this invention.
Orthogonal Reactivities
A key element of the present method is the presence of at least two compounds, each having a reactive group capable of forming an addition compound with the other and carrying at least one of the structural diversity groups. These compounds are used to form the aminimide and the oxazolone base modules. These compounds may take the form of either a) multiple reactive groups which are capable of being "turned on" independently of each other, or b) groups with multiple states with differing reactivities which may be addressed or brought into being at different times or under different conditions in a reaction sequence. It is highly desirable, although not absolutely necessary, that each individual reaction be a high-yielding addition reaction without possible interfering side-reactions, so that isolation and purification steps are not necessary, or, at least, are held to a minimum.
Specifically preferred reactive groups to generate the aminimide and oxazolone structures and the resulting base modules are listed below in tables 1, 2 and 3. The bonds in the structures in these figures represent potential points of attachment for the attachment of the structural diversity elements to the first and second compounds and to the base modules.
EXAMPLES
In order to exemplify the results achieved using the methods and compounds of the present invention, the following examples are provided without any intent to limit the scope of the instant invention to the discussion therein, all parts and percentages are by weight unless otherwise indicated.
EXAMPLE 1.
This example describes the generation of a matrix of 16 molecules around the following aryl-heterocycle-alicyclic amine structural theme.
Theme:
The 2-phenyl and 2-(2-naphthyl)-5-oxazolones (produced by reacting the lithium salt of glycine with the aryl acid chlorides, followed by cyclization with ethyl chloroformate at 0 °C) were reacted with 2-furfural, 3-furfural, 2-thiophenal and 3-thiophenyl to produce the oxazolones functionalized at the 5- position. This reaction was followed by subsequent ring-opening addition of 4-(3-aminopropylmorpholine and 1-(3-aminopropyl)-2- pipicoline to form the addύcts shown. The reactions were carried out in individual vials such that each vial contained one pure final compound as follows:
1) equimolar quantities of the oxazolone and the aldehyde dissolved in dry benzene (25 mL/gm reactants) were heated to 75 35 °C for 15 minutes; 2) the reaction mixture was cooled to 10 °C, and the amine was added dropwise with stirring; 3) the mixture
was re-heated to 75 °C for 20 minutes and 4) the solvent was removed in vacuo to give the crude solid product.
The following example outlines the generation of a matrix of 16 molecules around the basic structural theme of a hydroxy- proline transition state mimetic inhibitor for proteases:
Structural Theme:
This mimetic was synthesized by reacting styrene oxide or propylene oxide, ethyl acetate or methyl benzoate with four commercially available cyclic hydrazines (as mimetics of proline) in isopropanol in 16 individual sample vials, as shown in figure 1.
These 16 materials were isolated in essentially quantitative yield on removal of the reaction solvent by evaporation and purified samples were obtained as crystalline solids after recrystallization from ethyl acetate and characterized by
1H-NMR, FTIR and other analytical techniques. The set of molecules where X = CH
2 was tested as competitive inhibitors of the enzyme chymotrypsin in a standard assay using a BTEE substrate. The results found for K
i were 200 uM for R
1 = Ph, R
2= Me; 130 uM for R
1 = Me, R
2 = Ph; 500 uM for R
1 = Ph, R
2 = Ph; and R
1 = Me, R
2 = Me was found to not be an inhibitor. These results indicate a preference of the enzyme in this assay for one phenyl and one methyl, with the phenyl being preferred in the R
1 position. Based on these results, a second array was synthesized using phenyl groups in this position having a variety of different substituent groups for further testing against the enzyme.
From the foregoing, it is seen that various arrays of molecules can be prepared. These arrays can be generated in the desired size to facilitate the screening of a large number of molecules at one time. In Example 2, 4 x 4 arrays of molecules were prepared, but the invention is not to be limited to that specific embodiment. For example, standard trays having 96 compartments in an 8 x 12 array can be used where any number of compartments contain different molecules, while the other can contain controls or duplicate samples. It is possible, and preferred, to include 16 controls and 80 different samples in the array. After an initial screening identifies molecules having certain beneficial or desirable properties, a second tray containing, e.g., 20 samples of each of 4 different molecules, again with 16 controls, samples, can be used, to confirm the original results. The samples can be placed in columns of the same material, or a completely random array can be generated to have a completely blind analysis.
In view of these variations, one of ordinary skill in the act understands that any m x p array of molecules can be generated, where n and p are integers, m being greater than o
and p being greater than 1. There is no upper limit to m and p other than the capabilities of the testing or screening equipment. As noted above, an 8 x 12 array would be typical, but of compounds can be tested from arrays where m or p is as high as 25 or more; of being the total of m times p. At this time, it is specifically preferred that m and p be integers of between 3 and 15, and that a few control molecules be included so that q is less than the product of m and p. However, this invention contemplates that use of any integer for m or n, with each integer or combination of m x p integers relied upon as representing a useful embodiment.
As noted above, the molecules used in the array would be generated from one or more of the base molecules described herein. In this manner, combinatorial libraries of r different compounds, where r is any integer, can be made. Typically, r will be greater than 5, other 25 or greater. As noted, r can be as high as 80 or 96 using available trays, or can even be higher using specifically designed trays. Although for convenience, linear arrays are described, the specific arrangement of the molecules and tray compartments can be circular, staggered or in any other configuration which can have a completely blind analysis.
In view of these variations, one of ordinary skill in the art understands that any m x p array of molecules can be generated, where n and p are integers, m being greater than o and p are integers, me being greater than o and p being greater than 1. There is no upper limit to m and p other than the capabilities of the testing or screening equipment. As noted above, an 8 x 12 array would be typical, but of compounds can be tested from arrays where m or p is as high as 25 or more; of being the total of m times p. At this time, it is specifically preferred that m and p be integers of between 3 and 15, and that a few control molecules be included so that q is less than the product of m and p. However, this invention contemplates the use of any integer for m or n, with each integer or combination of m x p integers relied upon as representing a useful embodiment.
As noted above, the molecules used in the array would be generated from one or more of the base molecules described herein. In this manner, combinatorial libraries of r different compounds, where r is an integer, can be made. Typically, r will be greater than 5, often 25 or greater. As noted, r can be as high as 80 or 96 using available trays. Although for convenience, linear arrays are described, the specific arrangement of the molecules and tray compartments can be circular, rectangular, staggered or in any configuration which can be analyzed by the testing or screening device used.
The relevant portions of all cited patents, patent applications and other publications are specifically incorporated herein by reference.
The scope of the following claims is intended to encompass all obvious changes in the details, materials and arrangement of parts that will occur to one of ordinary skill in the art .