WO1995003306A1 - Arthropodicidal azacyclic heterocycles - Google Patents
Arthropodicidal azacyclic heterocycles Download PDFInfo
- Publication number
- WO1995003306A1 WO1995003306A1 PCT/US1994/008404 US9408404W WO9503306A1 WO 1995003306 A1 WO1995003306 A1 WO 1995003306A1 US 9408404 W US9408404 W US 9408404W WO 9503306 A1 WO9503306 A1 WO 9503306A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- alkyl
- independently selected
- haloalkyl
- halogen
- Prior art date
Links
- 0 CC(CC1)N(*)C(*)C1*=C Chemical compound CC(CC1)N(*)C(*)C1*=C 0.000 description 3
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/04—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings directly linked by a ring-member-to-ring-member bond
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/34—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom
- A01N43/40—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings
- A01N43/42—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with one nitrogen atom as the only ring hetero atom six-membered rings condensed with carbocyclic rings
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/72—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms
- A01N43/82—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having rings with nitrogen atoms and oxygen or sulfur atoms as ring hetero atoms five-membered rings with three ring hetero atoms
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N43/00—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds
- A01N43/90—Biocides, pest repellants or attractants, or plant growth regulators containing heterocyclic compounds having two or more relevant hetero rings, condensed among themselves or with a common carbocyclic ring system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D453/00—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids
- C07D453/02—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids containing not further condensed quinuclidine ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D453/00—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids
- C07D453/06—Heterocyclic compounds containing quinuclidine or iso-quinuclidine ring systems, e.g. quinine alkaloids containing isoquinuclidine ring systems
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/08—Bridged systems
Definitions
- EP 412,798 A2 discloses pyridinyl-substituted azabicyclic compounds for use in dementia provided the pyridinyl ring is not attached to the 2 -carbon position of the azabicyclic moiety.
- J. Gen. Chem. U.S.S.R., (1963), 33, 3345 discloses
- WO 93/14636 discloses azacyclic rings substituted with an optionally substituted oxadiazolyl or thiadiazolyl ring as insecticides. None of these references specifically teaches the compounds of the present invention.
- This invention pertains to compounds of Formula I, including all geometric and stereoisomers and agriculturally suitable salts thereof.
- the compounds are:
- R 1 and R 2 are independently selected from the group H, halogen, C 1 -C 6 alkyl,
- C 1 -C 6 haloalkyl C 2 -C 6 alkenyl, C 2 -C 6 haloalkenyl, C 2 -C 6 alkynyl, C 2 -C 6 haloalkynyl, C 3 -C 6 cycloalkyl, C 3 -C 6 halocycloalkyl, CN, SCN, NO 2 , N(R5)R 6 , OR 5 , C(O)R 5 , C(O)OR 5 , C(O)N(R 5 )R 6 , SR 5 , S(O)R 5 , S(O) 2 R 5 , S(O) 2 N(R 5 )R 6 and C 1 -C 6 alkyl substituted with 1 or 2 groups independently selected from NO 2 , CN, C 1 -C 3 alkylthio, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 2 -C 4 alkylcarbonyl and
- R 3 which is attached to any carbon of the azabicyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 haloalkenyl, C 2 -C 6 alkynyl, C 2 -C 6 haloalkynyl, C 3 -C 6 cycloalkyl, C 3 -C 6 halocycloalkyl, CN, SCN, NO 2 , N(R 7 )R 8 , OR 7 , C(O)R 7 , C(O)OR 7 , C(O)N(R 7 )R 8 , SR 7 , S(O)R 7 , S(O) 2 R 7 , S(O) 2 N(R 7 )R 8 and C,-C 6 alkyl substituted with 1 or 2 groups independently selected
- R 4 is selected from the group H, C 1 -C 6 alkyl, C 3 -C 6 cycloalkyl, C 1 -C 6 haloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, N(R 9 )R 10 , C(O)R 9 , C(O)OR 9 ,
- R 5 , R 6 , R 7 ,R 8 , R 9 and R 10 are independently selected the group H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
- W is selected from the group halogen, NO 2 , CN, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkylthio, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 2 -C 4 alkylcarbonyl and C 2 -C 4 alkoxycarbonyl;
- n and n are independently 0, 1 or 2;
- p 1 or 2;
- Preferred Compounds A are compounds of Formula I wherein Q is Q- 1.
- Preferred Compounds B are compounds of Preferred A wherein:
- R 1 is selected from the group H, halogen and C 1 -C 2 alkyl
- R 2 is selected from the group H and Cl
- R 3 is selected from the group H, halogen, C 1 -C 6 alkyl and OR 7 ;
- R 5 is selected from the group H and C 1 -C 4 alkyl ;
- n 0 or 1.
- Compound C of Preferred B which is:
- This invention also includes arthropodicidal compositions and method of use in both agronomic and nonagronomic environments wherein the arthropodicidal compounds are those of the formula:
- Q 1 is selected from the group
- R 3 which is attached to any carbon of the azacyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 haloalkenyl, C 2 -C 6 alkynyl, C 2 -C 6 haloalkynyl, C 3 -C 6 cycloalkyl, C 3 -C 6 halocycloalkyl, CN, SCN, NO 2 , N(R 7 )R 8 , OR 7 , C(O)R 7 , C(O)
- R 4 and R 11 are independently selected from the group H, C 1 -C 6 alkyl, C 3 -C 6 cycloalkyl, C 1 -C 6 haloalkyl, C 2 -C 6 alkenyl, C 2 -C 6 alkynyl, N(R 9 )R 10 , C(O)R 9 , C(O)OR 9 , C(O)N(R 9 )R 10 , SR 9 , S(O)R 9 , S(O) 2 R 9,
- R 1 , R 2 , R 3 or R 4 is S(O)R 5 , S(O) 2 R 5 , S(O)R 7 , S(O) 2 R 7 , S(O)R 9 , or S(O) 2 R 9 then R 5 , R 7 and R 9 are other than H;
- R 5 , R 6 , R 7 ,R 8 , R 9 and R 10 are independently selected the group H, C 1 -C 6 alkyl, C 1 -C 6 haloalkyl, C 3 -C 6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
- W is selected from the group halogen, NO 2 , CN, C 1 -C 3 alkyl, C 1 -C 3 haloalkyl, C 1 -C 3 alkylthio, C 1 -C 3 alkoxy, C 1 -C 3 haloalkoxy, C 2 -C 4 alkylcarbonyl and C 2 -C 4 alkoxycarbonyl;
- n and n are independently 0, 1 or 2;
- p 1 or 2;
- q 1, 2 or 3.
- Preferred Method A is the method wherein:
- R 1 is selected from the group H, halogen and C 1 -C 2 alkyl
- R 2 is selected from the group H and Cl
- R 3 is selected from the group H, halogen, C 1 -C 6 alkyl and OR 7 ;
- R 5 is selected from the group H and C 1 -C 4 alkyl
- n 0 or 1.
- Preferred Method B is the method of Preferred A wherein:
- V is N
- Y and Z are -C(R 1 )-;
- Q 1 is Q-6.
- Preferred Method C is the method of Preferred A wherein:
- V is N
- X is S
- Y and Z are -C(R 1 )-;
- Q 1 is Q-6.
- Preferred Method D is the method of Preferred A wherein:
- the ring contains two N and one O or S;
- Q 1 is Q-6.
- Compounds of this invention can exist as one or more stereoisomers.
- the various stereoisomers include enantiomers, diastereomers and geometric isomers.
- one stereoisomer may be more active than the others and how to separate stereoisomers.
- the present invention comprises racemic and optically active compound(s) of Formulae I and II as well as agriculturally suitable salts thereof.
- optically active compound(s) includes individual stereoisomers, mixtures of stereoisomers enriched in one stereoisomer, and optically active mixtures of compounds.
- alkyl used either alone or in compound words such as “alkylthio” or “haloalkyl” denotes straight-chain or branched alkyl, such as, methyl, ethyl, n-propyl, i-propyl, or the different butyl, pentyl or hexyl isomers.
- Alkenyl denotes straight-chain or branched alkenes such as ethenyl, 1-propenyl, 2-propenyl, and the different butenyl, pentenyl and hexenyl isomers.
- Alkenyl also denotes polyenes such as 1,3-hexadiene.
- Alkynyl denotes straight-chain or branched alkynes such as ethynyl, 1-propynyl, 3-propynyl and the different butynyl, pentynyl and hexynyl isomers.
- Alkynyl can also denote moieties comprised of multiple triple bonds such as 2,4-hexadiyne.
- Alkoxy denotes methoxy, ethoxy, n-propyloxy and isopropyloxy isomers.
- Cycloalkyl denotes cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl.
- halogen either alone or in compound words such as
- C i -C j The total number of carbon atoms in a substituent group is indicated by the "C i -C j " prefix where i and j are numbers from 1 to 6.
- C 1 -C 3 alkyl designates methyl through propyl
- C 2 alkoxy designates CH 3 CH 2 O
- C 3 alkoxy designates CH 3 CH 2 CH 2 O or (CH 3 ) 2 CHO.
- Examples of Formula II heterocyclic rings, exclusive of Q 1 include pyrrolyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl, tetrazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, oxazinyl, thiazinyl, oxadiazinyl, thiadiazinyl, triazinyl and tetrazinyl.
- the 2-position of the azabicyclic ring is defined as any carbon atom directly attached to the nitrogen atom of the azabicyclic ring.
- the 3-position is defined as any carbon atom directly attached to any carbon atom which is directly attached to the nitrogen atom of the azabicyclic ring.
- Compounds of Formula I (Q-1) and Formula II (Q-6) can be prepared by intramolecular alkylation of amino compounds of Formula III where G is a suitable leaving group such as a halogen or sulfonate.
- the reaction can be performed in the absence or presence of a base in a suitable solvent. Suitable solvents include tetrahydrofuran, diethyl ether and dimethoxyethane. Examples of typical bases include sodium methoxide, potassium t-butoxide and sodium hydride.
- the reactions can be run at temperatures in the range of 25°C to reflux. This method is similar to procedures described in the art (Chimia (1976) 30, 60). Scheme I illustrates this reaction.
- Compounds of Formula III can be prepared by reaction of the hydroxy compounds of Formula IV with a halogenating agent such as thionyl chloride, phosphorous tribromide or a sulfonylating agent such as methanesulfonyl chloride or /7-toluenesulfonyl chloride using procedures that are known to one skilled in the art (March, J. Advanced Organic Chemistry, John Wiley & Sons, New York, 3rd ed.
- a halogenating agent such as thionyl chloride, phosphorous tribromide or a sulfonylating agent such as methanesulfonyl chloride or /7-toluenesulfonyl chloride
- Formula IV compounds can be prepared by reduction of ketones with a reducing agent such as sodium borohydride, borane, and lithium aluminum hydride using procedures that are known to one skilled in the art (March, J. Advanced Organic Chemistry, John Wiley & Sons, New York, 3rd ed. (1985) 1147). Scheme III illustrates this transformation.
- a reducing agent such as sodium borohydride, borane, and lithium aluminum hydride
- a suitable solvent such as diethyl ether, tetrahydrofuran and dimethoxyethane.
- the reaction can be run at temperatures from -78°C to 35°C.
- Compounds of Formula VIII can be prepared by de-alkylation of Formula IX compounds with a chloroformate derivative such as 1-chloroethyl chloroformate, benzyl chloroformate, and 2,2,2-trichloroethyl chloroformate in a suitable solvent such as methylene chloride, chloroform, and 1,2-dichloroethane.
- a chloroformate derivative such as 1-chloroethyl chloroformate, benzyl chloroformate, and 2,2,2-trichloroethyl chloroformate in a suitable solvent such as methylene chloride, chloroform, and 1,2-dichloroethane.
- a suitable solvent such as methylene chloride, chloroform, and 1,2-dichloroethane.
- Compounds of Formula IX can be prepared by addition of an organometallic compound of Formula VI to carbonyl compounds of Formula VII in an analogous procedure described for Formula IV compounds.
- Scheme VII illustrates this
- Carbonyl compounds of Formula VII where R z is -N(OMe)Me can be prepared from carboxylic acid derivatives using procedures known in the art (Org. Prep. Proc. (1993) 25, 15).
- Organometallic compounds of Formula VI can be prepared by reaction of Formula X with a reactive metal species, such as magnesium, using procedures similar to those described in the art (J. Med. Chem. (1965) 8, 829). One skilled in the art will recognize this as a Grignard reagent.
- compounds of Formula I (Q-1) and Formula II (Q-6) can be prepared by deprotection of salts of Formula XI by hydrogenation in the presence of a suitable catalyst and solvent.
- Suitable catalysts include palladium on carbon and platinum oxide.
- Appropriate solvents are methanol, ethanol and ethyl acetate. Similar procedures are described in the art (J. Chem. Soc, Perkin Trans. I, (1991) 1091). Scheme IX illustrates this transformation.
- Salts of Formula XI can be prepared by cyclization of Formula III compounds in a suitable solvent.
- suitable solvents are ethanol, 1,2-dichloroethane and toluene.
- the reaction can be run at temperatures in the range of 0°C to reflux. Scheme X illustrates this reaction.
- Compounds of Formula I (Q-2) and Formula II (Q-7) can be prepared by cycloaddition of compounds of Formula XII with imines of Formula XIII.
- the reaction can be performed in the absence or presence of an acid such as zinc chloride, boron trifluoride, and hydrogen chloride.
- Suitable solvents are dichloromethane, toluene, tetrahydrofuran, and water.
- the reactions can be run at temperatures ranging from -78°C to reflux temperature of the solvent. It will be recognized by those skilled in the art that fully saturated azabicyclic analogs can be prepared by simple reduction of the olefin. Scheme XII illustrates this transformation.
- compounds of Formula I (Q-2) and Formula II (Q-7) can be prepared by intramolecular alkylation of Formula XIV compounds in a similar fashion to methods described for Formula I (Q-1) compounds.
- Scheme XIII illustrates this transformation.
- Imines of Formula XIII can be prepared by condensation of Formula VII carbonyl compounds with a primary amine (R 4 NH 2 ) using procedures known to one skilled in the art (March, J. Advanced Organic Chemistry; John Wiley & Sons, New York, 3rd ed. (1985) 465). Scheme XIV illustrates this transformation.
- Formula I (Q-3) and Formula II (Q-7) compounds can be prepared by
- Formula XV compounds can be prepared by reacting heterocycles of
- compounds of Formula I (Q-3) and Formula II (Q-7) can be prepared by intramolecular alkylation of Formula XVIII compounds by procedures described for Formula I (Q-1) compounds.
- Scheme XVII illustrates this
- Formula XVIII compounds can be prepared by addition of organometallic compounds of Formula XIX to imines XX.
- Typical solvents can include
- Imines of Formula XX can be prepared by condensation of a primary amine R 4 NH 2 with ketones XXI under conditions known to one skilled in the art (March, J. Advanced Organic Chemistry; John Wiley & Sons, New York, 3rd ed. (1985) 465). Scheme XIX illustrates this transformation.
- Organometallic compounds of Formula XIX can be prepared by methods known in the art see, for example, EP 492,902-A1.
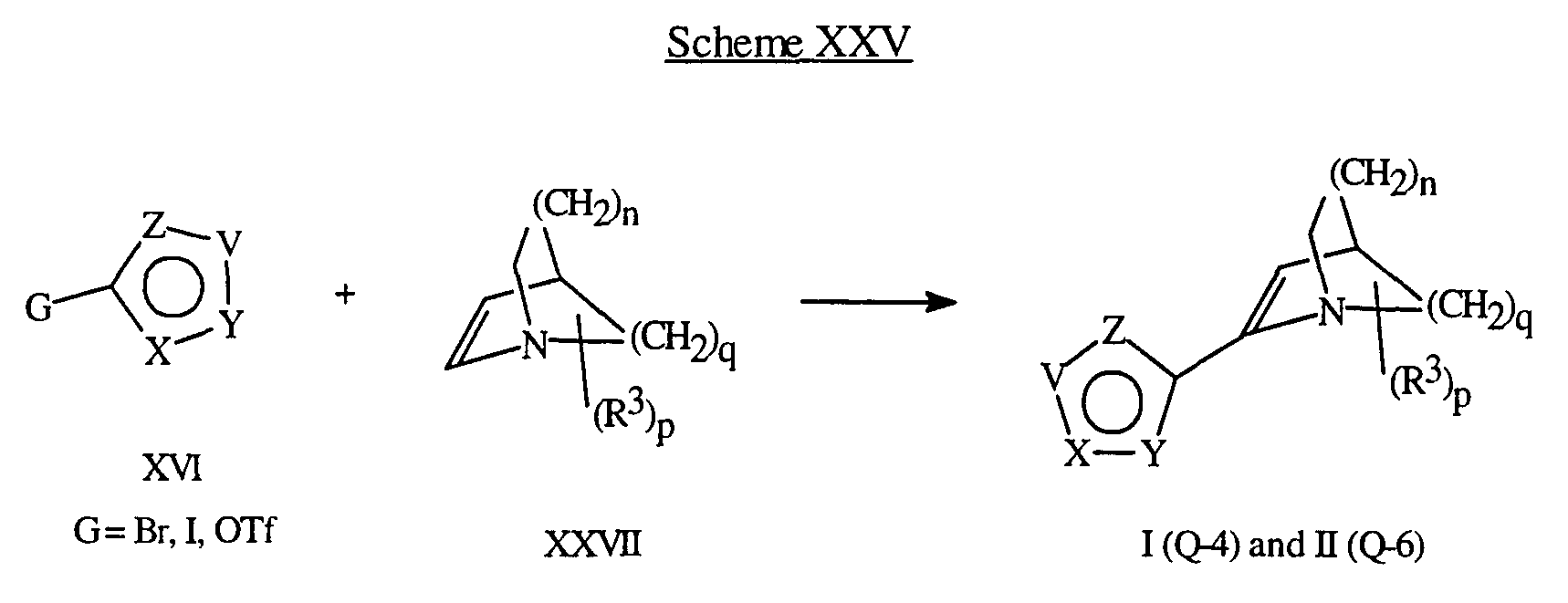
- Compounds of Formula I (Q-4) and Formula II (Q-6) can be synthesized by reacting a heterocycle of Formula XVI with an olefin XXVII under palladium catalysis in the presence of base.
- Typical solvents include acetonitrile, dimethylformamide, tetrahydrofuran, and dimethylsulfoxide. Reaction temperatures range from room temperature to the reflux temperature of the particular solvent. Scheme XXV illustrates this transformation.
- Olefins of the Formula XXVII can be prepared by using procedures known in the art (Helv. Chim. Acta. (1957) 40, 2170).
- N,O-dimethylhydroxylamine hydrochloride (14.0 g, 0.144 mol) in 200 mL of dichloromethane was added triethylamine (36.3 g, 0.358 mol) at 0°C. After complete addition, the reaction was warmed to room temperature and stirred for 2 h. Water was added and the mixture was extracted with dichloromethane . The organic layers were dried over MgSO 4 , filtered and concentrated. Ether was added and the triethylamine hydrochloride salts were removed by filtration. Concentration of the ether solution gave 27.0 g of a yellow oil, sufficiently pure for the next step.
- Step C (6-Chloro-3-pyridinyl )(4-piperidinyl) methanone hydrochloride
- 1,2-dichloroethane was added 1-chloroethyl chloroformate (9.0 g, 0.063 mol). The mixture was heated at reflux for 3-4 h. After cooling to room temperature, water was added and the mixture was extracted with dichloromethane. The combined extracts were dried over MgSO 4 , filtered, and concentrated to give 8.0 g of a yellow solid. The crude solid was dissolved in methanol and heated at reflux for 30 min. After cooling to room temperature, the solvent was removed and the solids were triturated with ether to give 5.5 g (6-chloro-3-pyridinyl) (4-piperidinyl) methanone hydrochloride.
- Step D 2-Chloro-5-[chloro(4-piperidinyl)methyl] pyridine
- Step E 7-(6-Chloro-3-pyridinyl)-1-azabicyclo[2.2.1] heptane
- Step B 7-(5.6-Dichloro-3-pyridinyl)-1-azabicyclo[2.2.1]heptane
- Step A The product of Step A was manipulated according to Steps B-E of Example 1 to give 0.95 g of a waxy solid, m.p. 30-32°C. 1 H ⁇ MR (CDCl 3 ) ⁇ 8.31 (s,1H), 7.88 (s,1H), 3.73 (s,1H), 3.05 (t,1H), 2.95-2.90 (m,1H), 2.7-2.6 (m,2H), 2.5-2.4 (m,1H), 1.9-1.8 (m,1H), 1.45-1.30 (m,2H), 1.3-1.2 (m,1H).
- Compounds of this invention will generally be used in formulation with an agriculturally suitable carrier comprising a liquid or solid diluent.
- Useful formulations include dusts, granules, baits, pellets, solutions, suspensions, emulsions, wettable powders, emulsifiable concentrates, dry flowables and the like, consistent with the physical properties of the active ingredient, mode of application and environmental factors such as soil type, moisture and temperature.
- Sprayable formulations can be extended in suitable media and used at spray volumes from about one to several hundred liters per hectare. High strength compositions are primarily used as intermediates for further formulation.
- the formulations will typically contain effective amounts of active ingredient, diluent and surfactant within the following approximate ranges which add up to 100 weight percent.
- Typical solid diluents are described in Watkins, et al., Handbook of Insecticide Dust Diluents and Carriers, 2nd Ed., Dorland Books, Caldwell, New Jersey. Typical liquid diluents and solvents are described in Marsden, Solvents Guide, 2nd Ed., Interscience, New York, 1950. McCutcheon's Detergents and Emulsifiers Annual, Allured Publ. Corp., Ridgewood, New Jersey, as well as Sisely and Wood,
- Fine solid compositions are made by blending and, usually, grinding as in a hammer mill or fluid energy mill.
- Water-dispersible granules can be produced by agglomerating a fine powder composition; see for example, Cross et al., Pesticide Formulations, Washington, D.C., 1988, pp 251-259.
- Suspensions are prepared by wet-milling; see, for example, U.S. 3,060,084.
- Granules and pellets can be made by spraying the active material upon preformed granular carriers or by agglomeration techniques. See Browning,
- the compounds of this invention exhibit activity against a wide spectrum of foliar-feeding, fruit-feeding, stem or root feeding, seed-feeding, aquatic and
- arthropods includes insects, mites and nematodes which are pests of growing and stored agronomic crops, forestry, greenhouse crops, ornamentals, nursery crops, stored food and fiber products, livestock, household, and public and animal health. Those skilled in the art will appreciate that not all compounds are equally effective against all growth stages of all pests.
- all of the compounds of this invention display activity against pests that include: eggs, larvae and adults of the Order Lepidoptera; eggs, foliar-feeding, fruit-feeding, root-feeding, seed-feeding larvae and adults of the Order Coleoptera; eggs, immatures and adults of the Orders Hemiptera and Homoptera; eggs, larvae, nymphs and adults of the Order Acari; eggs, immatures and adults of the Orders Thysanoptera, Orthoptera and Dermaptera; eggs, immatures and adults of the Order Diptera; and eggs, junveniles and adults of the Phylum Nematoda.
- the compounds of this invention are also active against pests of the Orders Hymenoptera, Isoptera, Siphonaptera, Blattaria, Thysanura and Psocoptera; pests belonging to the Class Arachnida and Phylum Platyhelminthes.
- the compounds are active against southern corn rootworm (Diabrotica undecimpunctata howardi), aster leafhopper (Mascrosteles fascifrons), boll weevil (Anthonomus grandis), two-spotted spider mite (Tetranychus urticae), fall armyworm (Spodoptera frugiperda), black bean aphid (Aphis fabae), green peach aphid (Myzus persica), cotton aphid (Aphis gossypii), Russian wheat aphid (Diuraphis noxia), English grain aphid (Sitobion avenae), tobacco budworm (Heliothis virescens), rice water weevil (Lissorhoptrus oryzophilus), rice leaf beetle (Oulema oryzae), whitebacked planthopper (Sogatella furcifera), green leafhopper (Nephotettix cincticeps), brown planthopper (
- Tetranychidae including Tetranychus urticae, Tetranychus cinnabarinus, Tetranychus mcdanieli, Tetranychus pacificus, Tetranychus turkestani, Byrobia rubrioculus, Panonychus ulmi, Panonychus citri, Eotetranychus carpini borealis, Eotetranychus, hicoriae, Eotetranychus sexmaculatus, Eotetranychus yumensis, Eotetranychus banksi and Oligonychus pratensis; Tenuipalpidae including Brevipalpus lew
- Compounds of this invention can also be mixed with one or more other insecticides, fungicides, nematocides, bactericides, acaricides, growth regulators, chemosterilants, semiochemicals, repellants, attractants, pheromones, feeding stimulants or other biologically active compounds to form a multi-component pesticide giving an even broader spectrum of agricultural protection.
- insecticides such as avermectin B, monocrotophos, carbofuran, tetrachlorvinphos, malathion, parathion-methyl, methomyl, chlordimeform, diazinon, deltamethrin, oxamyl, fenvalerate, esfenvalerate, permethrin, profenofos, sulprofos, triflumuron,
- fungicides such as carbendazim, thiuram, dodine, maneb, chloroneb, benomyl, cymoxanil, fenpropidine, fenpropimorph, triadimefon, captan, thiophanate-methyl, thiabendazole, phosethyl-Al, chlorothalonil, dichloran, metalaxyl, captafol, iprodione, oxadixyl, vinclozolin, kasugamycin, myclobutanil, tebuconazole, difenoconazole, diniconazole, fluquinconazole, ipconazole, metconazole, penconazole, propiconazole, uniconzole, flutriafol, prochloraz, pyrifenox, fenarimol, triadimenol, diclobutrazol, copper oxychloride, furala
- Arthropod pests are controlled and protection of agronomic, horticultural and specialty crops, animal and human health is achieved by applying one or more of the compounds of this invention, in an effective amount, to the environment of the pests including the agronomic and/or nonagronomic locus of infestation, to the area to be protected, or directly on the pests to be controlled.
- the present invention further comprises a method for the control of foliar and soil inhabiting arthropods and nematode pests and protection of agronomic and/or nonagronomic crops, comprising applying one or more of the compounds of Formula I or Formula II, or compositions containing at least one such compound, in an effective amount, to the environment of the pests including the agronomic and/or nonagronomic locus of infestation, to the area to be protected, or directly on the pests to be controlled.
- a preferred method of application is by spraying.
- granular formulations of these compounds can be applied to the plant foliage or the soil.
- Other methods of application include direct and residual sprays, aerial sprays, seed coats, microencapsulations, systemic uptake, baits, eartags, boluses, foggers, fumigants, aerosols, dusts and many others.
- the compounds can be incorporated into baits that are consumed by the arthropods or in devices such as traps and the like.
- the compounds of this invention can be applied in their pure state, but most often application will be of a formulation comprising one or more compounds with suitable carriers, diluents, and surfactants and possibly in combination with a food depending on the contemplated end use.
- a preferred method of application involves spraying a water dispersion or refined oil solution of the compounds. Combinations with spray oils, spray oil concentrations, spreader stickers, adjuvants, and synergists and other solvents such as piperonyl butoxide often enhance compound efficacy.
- the rate of application required for effective control will depend on such factors as the species of arthropod to be controlled, the pest's life cycle, life stage, its size, location, time of year, host crop or animal, feeding behavior, mating behavior, ambient moisture, temperature, and the like. Under normal circumstances, application rates of about 0.01 to 2 kg of active ingredient per hectare are sufficient to control pests in agronomic ecosystems, but as little as 0.001 kg/hectare may be sufficient or as much as 8 kg hectare may be required. For nonagronomic applications, effective use rates will range from about 1.0 to 50 mg/square meter but as little as 0.1 mg/square meter may be sufficient or as much as 150 mg/square meter may be required.
- Control efficacy represents inhibition of arthropod development (including mortality) that causes significantly reduced feeding.
- the pest control protection afforded by the compounds is not limited, however, to these species. See Index Tables A-D for compound descriptions.
- Test units each consisting of a H.I.S. (high impact styrene) tray with 16 cells were prepared.
- Wet filter paper and approximately 8 cm 2 of lima bean leaf was placed into twelve of the cells.
- a 0.5 cm layer of wheat germ diet was placed into the four remaining cells.
- Fifteen to twenty third-instar larvae of fall armyworm (Spodoptera frugiperda) were placed into an 8 ounce (230 mL) plastic cup. Solutions of each of the test compounds in 75/25 acetone/distilled water solvent were sprayed into the tray and cup. Spraying was accomplished by passing the tray and cup, on a conveyer belt, directly beneath a flat fan hydraulic nozzle which discharged the spray at a rate of
- Test units each consisting of an 8 ounce (230 mL) plastic cup containing a one-inch square (2.54 cm 2 ) of a wheatgerm diet, were prepared. Solutions of each of the test compounds in 75/25 acetone/distilled water solvent were sprayed into the tray and cup. Spraying was accomplished by passing the tray and cup, on a conveyer belt, directly beneath a flat fan hydraulic nozzle which discharged the spray at a rate of 0.5 pounds of active ingredient per acre (about 0.55 kg/ha) at 30 p.s.i. (207 kPa). After the spray on the cups had dried, five second-instar larvae of the southern corn rootworm (Diabrotica undecimpunctata howardi) were placed into each cup. The cups were then held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. The same units were read again at 8 days. Of the compounds tested, the following gave control efficacy levels of 80% or higher: 1, 3* and 6.
- Test units were prepared from a series of 12 ounce (350 mL) cups, each containing oat (Avena sativa) seedlings in a 1 inch (2.54 cm) layer of sterilized soil.
- test units were sprayed as described in TEST A with individual solutions of the test compounds. After the oats had dried from the spraying, between 10 and 15 adult aster leafhoppers (Mascrosteles fascifrons) were aspirated into each of the cups. The cups were covered with vented lids and held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1*, 3*.
- kidney bean leaves that had been infested on the undersides with 25 to 30 adult mites (Tetranychus urticae) were sprayed with their undersides facing up on a hydraulic sprayer with a solution of the test compound in 75/25 acetone/distilled water solvent. Spraying was accomplished by passing the leaves, on a conveyor belt, directly beneath a flat fan hydraulic nozzle which discharged the spray at a rate of 0.55 pounds of active ingredient per acre (about 0.5 kg/ha) at 30 p.s.i. (207 kPa).
- the leaf squares were then placed underside-up on square of wet cotton in a petri dish and the perimeter of the leaf square was tamped down onto the cotton with forceps so that the mites cannot escape onto the untreated leaf surface.
- the test units were held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1, 5 and 6.
- Test units consisting of 9 ounce (260 mL) cups containing five adult boll weevils (Anthonomus grandis grandis) were prepared. The test units were sprayed as described in TEST A with individual solutions of the test compounds. Each cup was covered with a vented lid and held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1, 2*.
Abstract
Arthropodicidal compounds having formula (I) and compositions comprising compounds of formula (II) and use of said compounds to control arthropods, wherein Q is selected from the group Q-1, Q-2, Q-3 and Q-4; and Q1 is selected from the group Q-5, Q-6 and Q-7.
Description
ARTHROPODICIDAL AZACYCLIC HETEROCYCLES
The present invention comprises compounds useful for the control of arthropods. EP 412,798 A2 discloses pyridinyl-substituted azabicyclic compounds for use in dementia provided the pyridinyl ring is not attached to the 2 -carbon position of the azabicyclic moiety. J. Gen. Chem. U.S.S.R., (1963), 33, 3345 discloses
2-(3-pyridinyl)-1-azabicyclo[2.2.2]octane, however, no utility is taught. WO 93/14636 discloses azacyclic rings substituted with an optionally substituted oxadiazolyl or thiadiazolyl ring as insecticides. None of these references specifically teaches the compounds of the present invention.
SUMMARY OF THE INVENTION
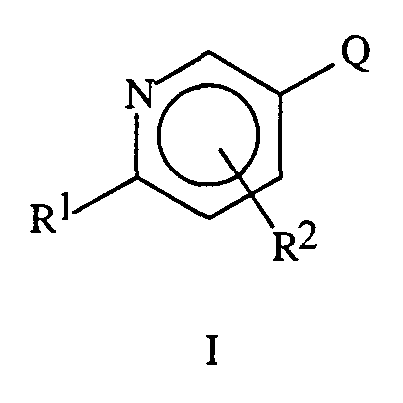
This invention pertains to compounds of Formula I, including all geometric and stereoisomers and agriculturally suitable salts thereof. The compounds are:
wherein:
Q is selected from the group
and
R1 and R2 are independently selected from the group H, halogen, C1-C6 alkyl,
C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R5)R6, OR5, C(O)R5, C(O)OR5, C(O)N(R5)R6, SR5, S(O)R5, S(O)2R5, S(O)2N(R5)R6 and C1-C6 alkyl substituted with 1 or 2 groups
independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl; R2 being attached to any unsubstituted aromatic ring carbon; and R1 and R2 are not both hydrogen when Q is Q-1 or Q-4, n is 1, R3 is H and q is 2;
R3, which is attached to any carbon of the azabicyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R7)R8, OR7, C(O)R7, C(O)OR7, C(O)N(R7)R8, SR7, S(O)R7, S(O)2R7, S(O)2N(R7)R8 and C,-C6 alkyl substituted with 1 or 2 groups independently selected from the group NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R4 is selected from the group H, C1-C6 alkyl, C3-C6 cycloalkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, N(R9)R10, C(O)R9, C(O)OR9,
C(O)N(R9)R10, SR9, S(O)R9, S(O)2R9, S(O)2N(R9)R10, benzyl and CH(CH3)Ph; provided when any of R1, R2, R3 or R4 is S(O)R5, S(O)2R5, S(O)R7, S(O)2R7, S(O)R9, or S(O)2R9 then R5, R7 and R9 are other than H;
R5, R6, R7,R8, R9 and R10 are independently selected the group H, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
W is selected from the group halogen, NO2, CN, C1-C3 alkyl, C1-C3 haloalkyl, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
m and n are independently 0, 1 or 2;
p is 1 or 2; and
q is 1, 2 or 3. Preferred Compounds A are compounds of Formula I wherein Q is Q- 1.
Preferred Compounds B are compounds of Preferred A wherein:
R1 is selected from the group H, halogen and C1-C2 alkyl;
R2 is selected from the group H and Cl;
R3 is selected from the group H, halogen, C1-C6 alkyl and OR7;
R5 is selected from the group H and C1-C4 alkyl ; and
n is 0 or 1.
Specifically preferred for biological activity is Compound C of Preferred B which is:
7-(6-chloro-3-pyridinyl)-1-azabicyclo[2.2.1]heptane. This invention also includes arthropodicidal compositions and method of use in both agronomic and nonagronomic environments wherein the arthropodicidal compounds are those of the formula:
wherein:
Q1 is selected from the group
where the broken line represents an optional chemical bond;
V, X, Y and Z of the ring are each independently selected from the group O, S, N, -C(R1)-, -C(R1)=C(R2)-, -C(R1)=N- and -N(R11)-; provided that (i) no more than one of V, X, Y or Z is -C(R1)=C(R2)-, -C(R1)=N-, -N(R11)-, O or S, (ii) at least one of V, X, Y or Z is N, (iii) when the ring is a 5- membered ring containing two N and one O or S, and Q is Q-6, then the ring is attached to the 2-position of Q1 and (iv) when the ring is a 5- membered ring containing two N and one O or S, then Q is other than Q-5; R1 and R2 are independently selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R5)R6, OR5, C(O)R5, C(O)OR5, C(O)N(R5)R6, SR5, S(O)R5, S(O)2R5, S(O)2N(R5)R6 and C1-C6 alkyl substituted with 1 or 2 groups
independently selected from the group NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R3, which is attached to any carbon of the azacyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R7)R8, OR7, C(O)R7, C(O)OR7,
C(O)N(R7)R8, SR7, S(O)R7, S(O)2R7, S(O)2N(R7)R8 and C1-C6 alkyl substituted with 1 or 2 groups independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R4 and R11 are independently selected from the group H, C1-C6 alkyl, C3-C6 cycloalkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, N(R9)R10, C(O)R9, C(O)OR9, C(O)N(R9)R10, SR9, S(O)R9, S(O)2R9,
S(O)2N(R9)R10, benzyl and CH(CH3)Ph; provided when any of R1, R2, R3 or R4 is S(O)R5, S(O)2R5, S(O)R7, S(O)2R7, S(O)R9, or S(O)2R9 then R5, R7 and R9 are other than H;
R5, R6, R7,R8, R9 and R10 are independently selected the group H, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
W is selected from the group halogen, NO2, CN, C1-C3 alkyl, C1-C3 haloalkyl, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
m and n are independently 0, 1 or 2;
p is 1 or 2; and
q is 1, 2 or 3.
Preferred Method A is the method wherein:
R1 is selected from the group H, halogen and C1-C2 alkyl;
R2 is selected from the group H and Cl;
R3 is selected from the group H, halogen, C1-C6 alkyl and OR7;
R5 is selected from the group H and C1-C4 alkyl; and
m and n are 0 or 1.
Preferred Method B is the method of Preferred A wherein:
V is N;
X is -C(R1)=C(R2)-;
Y and Z are -C(R1)-; and
Q1 is Q-6.
Preferred Method C is the method of Preferred A wherein:
V is N;
X is S;
Y and Z are -C(R1)-; and
Q1 is Q-6.
Preferred Method D is the method of Preferred A wherein:
the ring contains two N and one O or S; and
Q1 is Q-6. Compounds of this invention can exist as one or more stereoisomers. The various stereoisomers include enantiomers, diastereomers and geometric isomers. One skilled in the art will appreciate that one stereoisomer may be more active than the others and how to separate stereoisomers. Accordingly, the present invention comprises racemic and optically active compound(s) of Formulae I and II as well as agriculturally suitable salts thereof. The term optically active compound(s) includes individual stereoisomers, mixtures of stereoisomers enriched in one stereoisomer, and optically active mixtures of compounds.
In the above recitations, the term "alkyl", used either alone or in compound words such as "alkylthio" or "haloalkyl" denotes straight-chain or branched alkyl, such as, methyl, ethyl, n-propyl, i-propyl, or the different butyl, pentyl or hexyl isomers.
"Alkenyl" denotes straight-chain or branched alkenes such as ethenyl, 1-propenyl, 2-propenyl, and the different butenyl, pentenyl and hexenyl isomers. "Alkenyl" also denotes polyenes such as 1,3-hexadiene. "Alkynyl" denotes straight-chain or branched alkynes such as ethynyl, 1-propynyl, 3-propynyl and the different butynyl, pentynyl and hexynyl isomers. "Alkynyl" can also denote moieties comprised of multiple triple bonds such as 2,4-hexadiyne. "Alkoxy" denotes methoxy, ethoxy, n-propyloxy and isopropyloxy isomers. "Cycloalkyl" denotes cyclopropyl, cyclobutyl, cyclopentyl and cyclohexyl. The term "halogen", either alone or in compound words such as
"haloalkyl", denotes fluorine, chlorine, bromine or iodine. Further, when used in compound words such as "haloalkyl", said alkyl may be partially or fully substituted with halogen atoms which may be the same or different. Examples of "haloalkyl" include F3C, ClCH2, CF3CH2 and CF3CCl2. Examples of "haloalkenyl" include (Cl)2C=CHCH2 and CF3CH2CH=CHCH2. Examples of "haloalkynyl" include
HC≡CCHCl, CF3C≡C, CCl3C=C and FCH2C≡CCH2. The total number of carbon atoms in a substituent group is indicated by the "Ci-Cj" prefix where i and j are numbers from 1 to 6. For example, C1-C3 alkyl designates methyl through propyl; C2 alkoxy designates CH3CH2O; C3 alkoxy designates CH3CH2CH2O or (CH3)2CHO.
Examples of "alkoxycarbonyl" include CH3OC(=O), CH3CH2OC(=O),
CH3CH2CH2OC(=O) and (CH3)2CHOC(=O).
Examples of Formula II heterocyclic rings, exclusive of Q1, include pyrrolyl, imidazolyl, pyrazolyl, oxazolyl, thiazolyl, isoxazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, triazolyl, tetrazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, oxazinyl, thiazinyl, oxadiazinyl, thiadiazinyl, triazinyl and tetrazinyl.
The 2-position of the azabicyclic ring is defined as any carbon atom directly attached to the nitrogen atom of the azabicyclic ring. The 3-position is defined as any carbon atom directly attached to any carbon atom which is directly attached to the nitrogen atom of the azabicyclic ring.
When a compound is substituted with a substituent bearing a subscript that indicates the number of said substituents can exceed 1, said substituents (when they exceed 1) are independently selected from the group of defined substituents. Similarly, when a compound is substituted with a substituent which occurs more than once, said substituent is independently selected from the group defined for said substituent.
DETAILS OF THE INVENTION
Compounds of Formula I (Q-1) and Formula II (Q-6) can be prepared by intramolecular alkylation of amino compounds of Formula III where G is a suitable leaving group such as a halogen or sulfonate. The reaction can be performed in the absence or presence of a base in a suitable solvent. Suitable solvents include tetrahydrofuran, diethyl ether and dimethoxyethane. Examples of typical bases include sodium methoxide, potassium t-butoxide and sodium hydride. The reactions can be run at temperatures in the range of 25°C to reflux. This method is similar to procedures described in the art (Chimia (1976) 30, 60). Scheme I illustrates this reaction.
Compounds of Formula III can be prepared by reaction of the hydroxy compounds of Formula IV with a halogenating agent such as thionyl chloride, phosphorous tribromide or a sulfonylating agent such as methanesulfonyl chloride or /7-toluenesulfonyl chloride using procedures that are known to one skilled in the art
(March, J. Advanced Organic Chemistry, John Wiley & Sons, New York, 3rd ed.
(1985) 1 151). Scheme II illustrates this transformation.
Formula IV compounds can be prepared by reduction of ketones with a reducing agent such as sodium borohydride, borane, and lithium aluminum hydride using procedures that are known to one skilled in the art (March, J. Advanced Organic Chemistry, John Wiley & Sons, New York, 3rd ed. (1985) 1147). Scheme III illustrates this transformation.
Alternatively, Formula IV compounds can be prepared by addition of an appropriate organometallic compound VI (where M = Li, Mg, Cu, Zn) to a carbonyl compound VII in a suitable solvent such as diethyl ether, tetrahydrofuran and dimethoxyethane. The reaction can be run at temperatures from -78°C to 35°C.
Compounds of Formula V can be prepared by deprotection of compounds of Formula VIII using procedures known to one skilled in the art (Greene, Protective Groups in Organic Synthesis, 2nd ed. (1991) 315-348). Scheme V illustrates this transformation.
Compounds of Formula VIII can be prepared by de-alkylation of Formula IX compounds with a chloroformate derivative such as 1-chloroethyl chloroformate, benzyl chloroformate, and 2,2,2-trichloroethyl chloroformate in a suitable solvent such as methylene chloride, chloroform, and 1,2-dichloroethane. The reactions are usually run at temperatures in the range of 25°C to the reflux temperature of the particular solvent. Scheme VI illustrates this transformation.
Compounds of Formula IX can be prepared by addition of an organometallic compound of Formula VI to carbonyl compounds of Formula VII in an analogous procedure described for Formula IV compounds. Scheme VII illustrates this
transformation.
Carbonyl compounds of Formula VII where Rz is -N(OMe)Me can be prepared from carboxylic acid derivatives using procedures known in the art (Org. Prep. Proc. (1993) 25, 15).
Organometallic compounds of Formula VI can be prepared by reaction of Formula X with a reactive metal species, such as magnesium, using procedures similar to those described in the art (J. Med. Chem. (1965) 8, 829). One skilled in the art will recognize this as a Grignard reagent. Alternatively, Formula VI compounds (M=Li) can be obtained by lithium/halogen exchange using procedures known to one skilled in the art (March, J. Advanced Organic Chemistry, John Wiley & Sons, New York, 3rd ed. (1985) 1169).
Alternatively, compounds of Formula I (Q-1) and Formula II (Q-6) can be prepared by deprotection of salts of Formula XI by hydrogenation in the presence of a suitable catalyst and solvent. Suitable catalysts include palladium on carbon and platinum oxide. Appropriate solvents are methanol, ethanol and ethyl acetate. Similar procedures are described in the art (J. Chem. Soc, Perkin Trans. I, (1991) 1091). Scheme IX illustrates this transformation.
Salts of Formula XI can be prepared by cyclization of Formula III compounds in a suitable solvent. Suitable solvents are ethanol, 1,2-dichloroethane and toluene. The reaction can be run at temperatures in the range of 0°C to reflux. Scheme X illustrates this reaction.
One skilled in the art will recognize that one substituent can be converted into another. For example, compound I (V=CR1, R1=Cl) can be converted into
I (V=CR1, R1=OMe) by displacement with methoxide, or converted into
I (V=CR1, R1=alkyl) by reaction with an organostannane under palladium catalysis. Scheme XI illustrates this transformation.
Compounds of Formula I (Q-2) and Formula II (Q-7) can be prepared by cycloaddition of compounds of Formula XII with imines of Formula XIII. The reaction can be performed in the absence or presence of an acid such as zinc chloride, boron trifluoride, and hydrogen chloride. Suitable solvents are dichloromethane, toluene, tetrahydrofuran, and water. The reactions can be run at temperatures ranging from -78°C to reflux temperature of the solvent. It will be recognized by those skilled in the art that fully saturated azabicyclic analogs can be prepared by simple reduction of the olefin. Scheme XII illustrates this transformation.
Alternatively, compounds of Formula I (Q-2) and Formula II (Q-7) can be prepared by intramolecular alkylation of Formula XIV compounds in a similar fashion to methods described for Formula I (Q-1) compounds. Scheme XIII illustrates this transformation.
Imines of Formula XIII can be prepared by condensation of Formula VII carbonyl compounds with a primary amine (R4NH2) using procedures known to one skilled in the art (March, J. Advanced Organic Chemistry; John Wiley & Sons, New York, 3rd ed. (1985) 465). Scheme XIV illustrates this transformation.
Formula I (Q-3) and Formula II (Q-7) compounds can be prepared by
cycloaddition of compound XV using procedures known to one skilled in the art (J. Am. Chem. Soc, (1985) 107, 1768). It will be recognized by one skilled in the art that fully saturated azabicyclic analogs can be prepared by simple reduction of the olefin by known methods. Scheme XV illustrates this transformation.
Formula XV compounds can be prepared by reacting heterocycles of
Formula XVI with olefins XVII under palladium catalysis in the presence of base. Typical solvents include acetonitrile, dimethylformamide, and tetrahydrofuran. Typical bases include triethylamine, diisopropylethylamine and sodium bicarbonate. Reaction temperatures range from room temperature to the reflux temperature of the particular solvent. Scheme XVI illustrates this transformation.
Alternatively, compounds of Formula I (Q-3) and Formula II (Q-7) can be prepared by intramolecular alkylation of Formula XVIII compounds by procedures
described for Formula I (Q-1) compounds. Scheme XVII illustrates this
transformation.
Formula XVIII compounds can be prepared by addition of organometallic compounds of Formula XIX to imines XX. Typical solvents can include
tetrahydrofuran and diethylether. Typical reaction temperatures range from -78°C to room temperature. Scheme XVIII illustrates this transformation.
Imines of Formula XX can be prepared by condensation of a primary amine R4NH2 with ketones XXI under conditions known to one skilled in the art (March, J. Advanced Organic Chemistry; John Wiley & Sons, New York, 3rd ed. (1985) 465). Scheme XIX illustrates this transformation.
Organometallic compounds of Formula XIX can be prepared by methods known in the art see, for example, EP 492,902-A1.
Compounds of Formula II (Q-6) can be synthesized by addition of organometallic compounds XIX to azabicyclic ketones XXII followed by chlorination, elimination, and hydrogenation as described in the art. See, for example, EP 412,798. Scheme XX depicts this transformation.
Compounds of Formula II (Q-6) can be synthesized from azabicyclic esters XXIII and compounds XXIV, in a manner analogous to procedures described in the art. See, for example, EP 323,864. Scheme XXI depicts this transformation.
Formula XXV by procedures described for Formula II (Q-6) compounds. Scheme XXII depicts this transformation.
Compounds of Formula XXV can be prepared by hydrogenation of
Formula XXVI compounds, which are described in the art (J. Chem. Soc, Perkin Trans. I ( 1991), 1337). Scheme XXIII depicts this transformation.
Compounds of Formula II (Q-5) can be synthesized by addition of organometallic compounds XIX to azacyclic ketones XXVI, followed by chlorination and elimination. See, for example, J. Med. Chem. ( 1992) 35, 4011. Scheme XXIV depicts this transformation.
Compounds of Formula I (Q-4) and Formula II (Q-6) can be synthesized by reacting a heterocycle of Formula XVI with an olefin XXVII under palladium catalysis in the presence of base. Typical solvents include acetonitrile, dimethylformamide, tetrahydrofuran, and dimethylsulfoxide. Reaction temperatures range from room temperature to the reflux temperature of the particular solvent. Scheme XXV illustrates this transformation.
Olefins of the Formula XXVII can be prepared by using procedures known in the art (Helv. Chim. Acta. (1957) 40, 2170).
It is recognized that some reagents and reaction conditions described above for preparing compounds of Formulae I and II may not be compatible with certain functionalities present in the intermediates. In these instances, the incorporation of protection/deprotection sequences into the synthesis will aid in obtaining the desired products. The use and choice of appropriate protecting groups will be apparent to one skilled in chemical synthesis.
EXAMPLE 1
Preparation of 7-(6-chloro-3-pyridinyl)-1-azabicyclo[2.2.1]heptane Step A: 6-Chloro-N-methoxy-N-methyl-3-pyridine carboxamide
To a suspension of 6-chloronicotinyl chloride (24.0 g, 0.136 mol) and
N,O-dimethylhydroxylamine hydrochloride (14.0 g, 0.144 mol) in 200 mL of dichloromethane was added triethylamine (36.3 g, 0.358 mol) at 0°C. After complete addition, the reaction was warmed to room temperature and stirred for 2 h. Water was added and the mixture was extracted with dichloromethane . The organic layers were dried over MgSO4, filtered and concentrated. Ether was added and the triethylamine hydrochloride salts were removed by filtration. Concentration of the ether solution gave 27.0 g of a yellow oil, sufficiently pure for the next step. 1H ΝMR (CDCl3) δ 8.78 (s,1H), 8.04 (d,1H), 7.40 (d,1H), 3.56 (s,3H), 3.40 (s,3H).
Step B: (6-Chloro-3-pyridinyl)(1-methyl-4-piperidinyl) methanone
Chloro(1-methyl-4-piperidinyl) magnesium (prepared from 4-chloro-1-methyl piperidine (17.75 g, 0.133 mol) in 300 mL of tetrahydrofuran and magnesium (3.4 g, 0.140 mol) according to the procedure in J. Med. Chem. (1965) 8, 829), was added to a solution of the product of Step A (13.3 g, 0.066 mol) in 50 mL of tetrahydrofuran at 0°C. After addition was complete, the reaction was warmed to room temperature. After 1 h, dilute HCl solution was added and the mixture was extracted with ethyl acetate. The combined extracts were dried over MgSO4, filtered and concentrated. The crude material was triturated with hexane to give 9.8 g of an off-white solid, m.p.
104.5-106°C. 1H NMR (CDCl3) δ 8.90 (s,1H), 8.20 (d,1H), 7.45 (d,1H), 3.20-3.05 (m,1H), 2.95 (d,2H), 2.31 (s,3H), 2.20-2.00 (m,2H), 2.00-1.80 (m,4H).
Step C: (6-Chloro-3-pyridinyl )(4-piperidinyl) methanone hydrochloride
To a solution of the product of Step B (6.28 g, 0.026 mol) in 100 mL of
1,2-dichloroethane was added 1-chloroethyl chloroformate (9.0 g, 0.063 mol). The mixture was heated at reflux for 3-4 h. After cooling to room temperature, water was added and the mixture was extracted with dichloromethane. The combined extracts were dried over MgSO4, filtered, and concentrated to give 8.0 g of a yellow solid. The crude solid was dissolved in methanol and heated at reflux for 30 min. After cooling to room temperature, the solvent was removed and the solids were triturated with ether to give 5.5 g (6-chloro-3-pyridinyl) (4-piperidinyl) methanone hydrochloride.
Step D: 2-Chloro-5-[chloro(4-piperidinyl)methyl] pyridine
Sodium borohydride (0.81 g, 0.021 mol) was added portionwise to a suspension of the product of Step C (5.5 g, 0.021 mol), in 50 mL of methanol. After complete addition, the reaction was stirred for 10 min and concentrated. The residue was suspended in 60 mL of dichloromethane/pyridine (5: 1). Excess thionyl chloride (ca. 4 mL) was added. After 1 h, the mixture was concentrated. Water was added and the mixture was neutralized with potassium carbonate and extracted with ethyl acetate. The combined organic layers were dried over MgSO4, filtered, and concentrated to give 2.6 g of an off-white solid.
Step E: 7-(6-Chloro-3-pyridinyl)-1-azabicyclo[2.2.1] heptane
Potassium t-butoxide (2.5 g, 0.022 mol) was added to a suspension of the product of Step D (2.6 g, 0.009 mol) in 50 mL of tetrahydrofuran. The mixture was heated at reflux for 48 h. After cooling to room temperature, water was added and the mixture was extracted with ethyl acetate. The combined extracts were dried over MgSO4, filtered, and concentrated. Chromatography on silica gel (9: 1 chloroform:methanol) afforded 1.3 g of a yellow solid, m.p. 77-78°C. 1H NMR (CDCl3) δ 8.42 (s,1H), 7.75 (d,1H), 7.27 (s,1H), 3.75 (s,1H), 3.05 (dt,1H); 2.90 (d,1H), 2.70-2.60 (m,2H),
2.50-2.40 (m,1H), 1.9-1.7 (m,1H), 1.45-1.30 (m,2H), 1.20-1.10 (m,1H).
EXAMPLE 2
Preparation of 7-(3-pyridinyl)-1-azabicyclo[2.2.1]heptane To a solution of 7-(6-chloro-3-pyridinyl)-1-azabicyclo [2.2.1]heptane (100.0 mg, 0.48 mmol) in methanol was added a catalytic amount of 5% palladium on carbon and a balloon containing hydrogen gas. After stirring overnight, the mixture was filtered through a pad of Celite® and concentrated. The residue was taken up in
dichloromethane, washed with saturated sodium bicarbonate solution, dried over MgSO4, filtered, and concentrated to give 55 mg of a brown oil. 1H NMR (CDCI3) δ 8.65 (s,1H), 8.50 (d,1H), 7.80 (d,1H), 7.25 (d,1H), 3.81 (s,1H), 3.10-3.00 (m,1H), 3.00-2.95 (m,1H), 2.80-2.60 (m,2H), 2.50-2.40 (m,1H), 1.90-1.80 (m,1H), 1.45-1.10 (m,3H).
EXAMPLE 3
Preparation of 7-(5.6-dichloro-3-pyridinyl)-1-azabicyclo[2.2.1]heptane
Step A: 5,6-Dichloro-N-methoxy-N-methyl-3-pyridine carboxamide
To a solution of 5,6-dichloronicotinic acid (10.0 g, 0.052 mmol) and
triethylamine (15.2 mL, 0.109 mmol) in dichloromethane was added oxalyl chloride (6.94 g, 0.05 mmol). After the gas evolution ceased, NO-dimethylhydroxylamine hydrochloride (5.34 g, 0.055 mmol) was added and the mixture was stirred overnight. Saturated sodium bicarbonate solution was added and the mixture was extracted with dichloromethane. The combined organic layers were dried over MgSO4, filtered, and concentrated to give 9.1 g of a brown solid. 1H ΝMR (CDCl3) δ 8.68 (s,1H), 8.16 (s,1H), 3.59 (s,3H), 3.40 (s,3H)
Step B: 7-(5.6-Dichloro-3-pyridinyl)-1-azabicyclo[2.2.1]heptane
The product of Step A was manipulated according to Steps B-E of Example 1 to give 0.95 g of a waxy solid, m.p. 30-32°C. 1H ΝMR (CDCl3) δ 8.31 (s,1H), 7.88 (s,1H), 3.73 (s,1H), 3.05 (t,1H), 2.95-2.90 (m,1H), 2.7-2.6 (m,2H), 2.5-2.4 (m,1H), 1.9-1.8 (m,1H), 1.45-1.30 (m,2H), 1.3-1.2 (m,1H).
By the procedures described herein, the following compounds of Tables 1 to 23 can be prepared. The compounds in Table 1, line 1 can be referred to as 1-1, 1-2, 1-3, 1-4 and 1-5 (as designated by line and column). All the other specific compounds covered in these Tables can be designated in an analogous fashion. The following abbreviations have been used in Tables 1-23: Me = methyl, Et = ethyl, iPr = isopropyl, nPr = n-propyl and Bn = benzyl.
Formulation/Utility
Compounds of this invention will generally be used in formulation with an agriculturally suitable carrier comprising a liquid or solid diluent. Useful formulations
include dusts, granules, baits, pellets, solutions, suspensions, emulsions, wettable powders, emulsifiable concentrates, dry flowables and the like, consistent with the physical properties of the active ingredient, mode of application and environmental factors such as soil type, moisture and temperature. Sprayable formulations can be extended in suitable media and used at spray volumes from about one to several hundred liters per hectare. High strength compositions are primarily used as intermediates for further formulation. The formulations will typically contain effective amounts of active ingredient, diluent and surfactant within the following approximate ranges which add up to 100 weight percent.
Weight Percent
Active
Ingredient Diluent Surfactant
Wettable Powders 5-90 0-74 1-10
Oil Suspensions, Emulsions, 5-50 40-95 0-15
Solutions, (including Emulsifiable
Concentrates)
Dusts 1-25 70-99 0-5
Granules, Baits and Pellets 0.01-99 5-99.99 0-15
High Strength Compositions 90-99 0-10 0-2
Typical solid diluents are described in Watkins, et al., Handbook of Insecticide Dust Diluents and Carriers, 2nd Ed., Dorland Books, Caldwell, New Jersey. Typical liquid diluents and solvents are described in Marsden, Solvents Guide, 2nd Ed., Interscience, New York, 1950. McCutcheon's Detergents and Emulsifiers Annual, Allured Publ. Corp., Ridgewood, New Jersey, as well as Sisely and Wood,
Encyclopedia of Surface Active Agents, Chemical Publ. Co., Inc., New York, 1964, list surfactants and recommended uses. All formulations can contain minor amounts of additives to reduce foam, caking, corrosion, microbiological growth, and the like.
Solutions are prepared by simply mixing the ingredients. Fine solid compositions are made by blending and, usually, grinding as in a hammer mill or fluid energy mill. Water-dispersible granules can be produced by agglomerating a fine powder composition; see for example, Cross et al., Pesticide Formulations, Washington, D.C., 1988, pp 251-259. Suspensions are prepared by wet-milling; see, for example, U.S. 3,060,084. Granules and pellets can be made by spraying the active material upon preformed granular carriers or by agglomeration techniques. See Browning,
"Agglomeration", Chemical Engineering, December 4, 1967, pp 147-148, Perry's
Chemical Engineer's Handbook, 4th ed., McGraw-Hill, New York, (1963), pp 8-57 and following, and WO 91/13546.
For further information regarding the art of formulation, see U.S. 3,235,361, Col. 6, line 16 through Col. 7, line 19 and Examples 10-41; U.S. 3,309,192, Col. 5, line 43 through Col. 7, line 62 and Examples 8, 12, 15, 39, 41, 52, 53, 58, 132, 138 -140, 162-164, 166, 167 and 169-182; U.S. 2,891,855, Col. 3, line 66 through Col. 5, line 17 and Examples 1-4; Klingman, Weed Control as a Science, John Wiley and Sons, Inc., New York, (1961), pp 81-96; and Hance et al., Weed Control
Handbook, 8th ed., Blackwell Scientific Publications, Oxford, (1989).
In the following Examples, all percentages are by weight and all formulations are prepared in conventional ways. Compound numbers refer to compounds in Index Table A.
Example A
Wettable Powder
Compound 1 65.0%
dodecylphenol polyethylene glycol ether 2.0%
sodium ligninsulfonate 4.0%
sodium silicoaluminate 6.0%
montmorillonite (calcined) 23.0%.
Example B
Granule
Compound 1 10.0%
attapulgite granules (low volatile
matter, 0.71/0.30 mm; U.S.S. No.
25-50 sieves) 90.0%.
Example C
Extruded Pellet
Compound 1 25.0%
anhydrous sodium sulfate 10.0%
crude calcium ligninsulfonate 5.0%
sodium alkylnaphthalenesulfonate 1.0%
calcium/magnesium bentonite 59.0%.
Example D
Emulsifiable Concentrate
Compound 1 20.0%
blend of oil soluble sulfonates
and polyoxyethylene ethers 10.0%
isophorone 70.0%.
The compounds of this invention exhibit activity against a wide spectrum of foliar-feeding, fruit-feeding, stem or root feeding, seed-feeding, aquatic and
soil-inhabiting arthropods (term "arthropods" includes insects, mites and nematodes) which are pests of growing and stored agronomic crops, forestry, greenhouse crops, ornamentals, nursery crops, stored food and fiber products, livestock, household, and public and animal health. Those skilled in the art will appreciate that not all compounds are equally effective against all growth stages of all pests. Nevertheless, all of the compounds of this invention display activity against pests that include: eggs, larvae and adults of the Order Lepidoptera; eggs, foliar-feeding, fruit-feeding, root-feeding, seed-feeding larvae and adults of the Order Coleoptera; eggs, immatures and adults of the Orders Hemiptera and Homoptera; eggs, larvae, nymphs and adults of the Order Acari; eggs, immatures and adults of the Orders Thysanoptera, Orthoptera and Dermaptera; eggs, immatures and adults of the Order Diptera; and eggs, junveniles and adults of the Phylum Nematoda. The compounds of this invention are also active against pests of the Orders Hymenoptera, Isoptera, Siphonaptera, Blattaria, Thysanura and Psocoptera; pests belonging to the Class Arachnida and Phylum Platyhelminthes. Specifically, the compounds are active against southern corn rootworm (Diabrotica undecimpunctata howardi), aster leafhopper (Mascrosteles fascifrons), boll weevil (Anthonomus grandis), two-spotted spider mite (Tetranychus urticae), fall armyworm (Spodoptera frugiperda), black bean aphid (Aphis fabae), green peach aphid (Myzus persica), cotton aphid (Aphis gossypii), Russian wheat aphid (Diuraphis noxia), English grain aphid (Sitobion avenae), tobacco budworm (Heliothis virescens), rice water weevil (Lissorhoptrus oryzophilus), rice leaf beetle (Oulema oryzae), whitebacked planthopper (Sogatella furcifera), green leafhopper (Nephotettix cincticeps), brown planthopper (Nilaparvata lugens), small brown planthopper (Laodelphax striatellus), rice stem borer (Chilo suppressalis), rice leafroller (Cnaphalocrocis medinalis), black rice stink bug (Scotinophara lurida), rice stink bug (Oebalus pugnax), rice bug
(Leptocorisa chinensis), slender rice bug (Cletus puntiger), and southern green stink bug (Nezara viridula). The compounds are active on mites, demonstrating ovicidal, larvicidal and chemosterilant activity against such families as Tetranychidae including Tetranychus urticae, Tetranychus cinnabarinus, Tetranychus mcdanieli, Tetranychus pacificus, Tetranychus turkestani, Byrobia rubrioculus, Panonychus ulmi, Panonychus citri, Eotetranychus carpini borealis, Eotetranychus, hicoriae, Eotetranychus sexmaculatus, Eotetranychus yumensis, Eotetranychus banksi and Oligonychus pratensis; Tenuipalpidae including Brevipalpus lewisi, Brevipalpus phoenicis,
Brevipalpus californicus and Brevipalpus obovatus; Eriophyidae including
Phyllocoptruta oleivora, Eriophyes sheldoni, Aculus cornutus, Epitrimerus pyri and
Eriophyes mangiferae. See WO 90/10623 and WO 92/00673 for more detailed pest descriptions.
Compounds of this invention can also be mixed with one or more other insecticides, fungicides, nematocides, bactericides, acaricides, growth regulators, chemosterilants, semiochemicals, repellants, attractants, pheromones, feeding stimulants or other biologically active compounds to form a multi-component pesticide giving an even broader spectrum of agricultural protection. Examples of other agricultural protectants with which compounds of this invention can be formulated are: insecticides such as avermectin B, monocrotophos, carbofuran, tetrachlorvinphos, malathion, parathion-methyl, methomyl, chlordimeform, diazinon, deltamethrin, oxamyl, fenvalerate, esfenvalerate, permethrin, profenofos, sulprofos, triflumuron,
diflubenzuron, methoprene, buprofezin, thiodicarb, acephate, azinphosmethyl, chlorpyrifos, dimethoate, fipronil, flufenprox, fonophos, isofenphos, methidathion, metha-midophos, phosmet, phosphamidon, phosalone, pirimicarb, phorate, terbufos, trichlorfon, methoxychlor, bifenthrin, biphenate, cyfluthrin, tefluthrin, fenpropathrin, fluvalinate, flucythrinate, tralomethrin, imidacloprid, metaldehyde and rotenone;
fungicides such as carbendazim, thiuram, dodine, maneb, chloroneb, benomyl, cymoxanil, fenpropidine, fenpropimorph, triadimefon, captan, thiophanate-methyl, thiabendazole, phosethyl-Al, chlorothalonil, dichloran, metalaxyl, captafol, iprodione, oxadixyl, vinclozolin, kasugamycin, myclobutanil, tebuconazole, difenoconazole, diniconazole, fluquinconazole, ipconazole, metconazole, penconazole, propiconazole, uniconzole, flutriafol, prochloraz, pyrifenox, fenarimol, triadimenol, diclobutrazol, copper oxychloride, furalaxyl, folpet, flusilazol, blasticidin S, diclomezine, edifenphos, isoprothiolane, iprobenfos, mepronil, neo-asozin, pencycuron, probenazole, pyroquilon, tricyclazole, validamycin, and flutolanil; nematocides such as aldoxycarb, fenamiphos and fosthietan; bactericides such as oxytetracyline, streptomycin and tribasic copper sulfate; acaricides such as binapacryl, oxythioquinox, chlorobenzilate, dicofol, dienochlor, cyhexatin, hexythiazox, amitraz, propargite, tebufenpyrad and fenbutatin oxide; and biological agents such as entomopathogenic bacteria, virus and fungi.
In certain instances, combinations with other arthropodicides having a similiar spectrum of control but a different mode of action will be particularly advantageous for resistance management.
Arthropod pests are controlled and protection of agronomic, horticultural and specialty crops, animal and human health is achieved by applying one or more of the compounds of this invention, in an effective amount, to the environment of the pests including the agronomic and/or nonagronomic locus of infestation, to the area to be protected, or directly on the pests to be controlled. Thus, the present invention further comprises a method for the control of foliar and soil inhabiting arthropods and
nematode pests and protection of agronomic and/or nonagronomic crops, comprising applying one or more of the compounds of Formula I or Formula II, or compositions containing at least one such compound, in an effective amount, to the environment of the pests including the agronomic and/or nonagronomic locus of infestation, to the area to be protected, or directly on the pests to be controlled. A preferred method of application is by spraying. Alternatively, granular formulations of these compounds can be applied to the plant foliage or the soil. Other methods of application include direct and residual sprays, aerial sprays, seed coats, microencapsulations, systemic uptake, baits, eartags, boluses, foggers, fumigants, aerosols, dusts and many others. The compounds can be incorporated into baits that are consumed by the arthropods or in devices such as traps and the like.
The compounds of this invention can be applied in their pure state, but most often application will be of a formulation comprising one or more compounds with suitable carriers, diluents, and surfactants and possibly in combination with a food depending on the contemplated end use. A preferred method of application involves spraying a water dispersion or refined oil solution of the compounds. Combinations with spray oils, spray oil concentrations, spreader stickers, adjuvants, and synergists and other solvents such as piperonyl butoxide often enhance compound efficacy.
The rate of application required for effective control will depend on such factors as the species of arthropod to be controlled, the pest's life cycle, life stage, its size, location, time of year, host crop or animal, feeding behavior, mating behavior, ambient moisture, temperature, and the like. Under normal circumstances, application rates of about 0.01 to 2 kg of active ingredient per hectare are sufficient to control pests in agronomic ecosystems, but as little as 0.001 kg/hectare may be sufficient or as much as 8 kg hectare may be required. For nonagronomic applications, effective use rates will range from about 1.0 to 50 mg/square meter but as little as 0.1 mg/square meter may be sufficient or as much as 150 mg/square meter may be required.
The following TESTS demonstrate the control efficacy of compounds of this invention on specific pests. "Control efficacy" represents inhibition of arthropod development (including mortality) that causes significantly reduced feeding. The pest control protection afforded by the compounds is not limited, however, to these species. See Index Tables A-D for compound descriptions.
TEST A
Fall Armyworm
Test units, each consisting of a H.I.S. (high impact styrene) tray with 16 cells were prepared. Wet filter paper and approximately 8 cm2 of lima bean leaf was placed into twelve of the cells. A 0.5 cm layer of wheat germ diet was placed into the four remaining cells. Fifteen to twenty third-instar larvae of fall armyworm (Spodoptera frugiperda) were placed into an 8 ounce (230 mL) plastic cup. Solutions of each of the test compounds in 75/25 acetone/distilled water solvent were sprayed into the tray and cup. Spraying was accomplished by passing the tray and cup, on a conveyer belt, directly beneath a flat fan hydraulic nozzle which discharged the spray at a rate of
0.5 pounds of active ingredient per acre (about 0.55 kg/ha) at 30 p.s.i. (207 kPa). The insects were transferred from the 8 ounce cup into the cells of the H.I.S. tray (one insect per cell). The trays were covered and held at 27°C and 50% relative humidity for 48 h
after which time readings were taken on the twelve cells with lima bean leaves. The four remaining cells were read 7 days later for delayed toxicity readings. Of the compounds tested, the following gave control efficacy levels of 80% or higher: 3*. * - tested at 250 ppm.
TEST B
Southern Corn Rootworm
Test units, each consisting of an 8 ounce (230 mL) plastic cup containing a one-inch square (2.54 cm2) of a wheatgerm diet, were prepared. Solutions of each of the test compounds in 75/25 acetone/distilled water solvent were sprayed into the tray and cup. Spraying was accomplished by passing the tray and cup, on a conveyer belt, directly beneath a flat fan hydraulic nozzle which discharged the spray at a rate of 0.5 pounds of active ingredient per acre (about 0.55 kg/ha) at 30 p.s.i. (207 kPa). After the spray on the cups had dried, five second-instar larvae of the southern corn rootworm (Diabrotica undecimpunctata howardi) were placed into each cup. The cups were then held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. The same units were read again at 8 days. Of the compounds tested, the following gave control efficacy levels of 80% or higher: 1, 3* and 6.
* Test conducted at 250 ppm. TEST C
Aster Leafhopper
Test units were prepared from a series of 12 ounce (350 mL) cups, each containing oat (Avena sativa) seedlings in a 1 inch (2.54 cm) layer of sterilized soil.
The test units were sprayed as described in TEST A with individual solutions of the test compounds. After the oats had dried from the spraying, between 10 and 15 adult aster leafhoppers (Mascrosteles fascifrons) were aspirated into each of the cups. The cups were covered with vented lids and held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1*, 3*.
* Test conducted at 250 ppm.
TEST D
Two-Spotted Spider Mite
One inch squares (2.54 cm) of kidney bean leaves that had been infested on the undersides with 25 to 30 adult mites (Tetranychus urticae) were sprayed with their undersides facing up on a hydraulic sprayer with a solution of the test compound in 75/25 acetone/distilled water solvent. Spraying was accomplished by passing the leaves, on a conveyor belt, directly beneath a flat fan hydraulic nozzle which discharged the spray at a rate of 0.55 pounds of active ingredient per acre (about 0.5 kg/ha) at 30 p.s.i. (207 kPa). The leaf squares were then placed underside-up on square of wet cotton in a petri dish and the perimeter of the leaf square was tamped down onto the cotton with forceps so that the mites cannot escape onto the untreated leaf surface. The test units were held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1, 5 and 6.
TEST E
Boll Weevil
Test units consisting of 9 ounce (260 mL) cups containing five adult boll weevils (Anthonomus grandis grandis) were prepared. The test units were sprayed as described in TEST A with individual solutions of the test compounds. Each cup was covered with a vented lid and held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1, 2*.
* Test conducted at 250 ppm
TEST F
Contact Test Against Black Bean Aphid
Individual nasturtium leaves were infested with 10 to 15 aphids (all morphs and growth stages of Aphis fabae) and sprayed with their undersides facing up as described in TEST A. The leaves were then set in 3/8 inch (0.94 cm) diameter vials containing 4 mL of sugar solution (approximately 1.4 g per liter) and covered with a clear plastic 1 ounce (29 mL) cup to prevent escape of the aphids that drop from the leaves. The test units were held at 27°C and 50% relative humidity for 48 h, after which time mortality readings were taken. Of the compounds tested, the following gave mortality levels of 80% or higher: 1, 2*, 3*.
Claims
1. A compound of the formula
Q is selected from the group
, ,
R1 and R2 are independently selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R5)R6, OR5, C(O)R5, C(O)OR5, C(O)N(R5)R6, SR5, S(O)R5, S(O)2R5, S(O)2N(R5)R6 and C1-C6 alkyl substituted with 1 or 2 groups independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl; R2 being attached to any unsubstituted aromatic ring carbon; and R1 and R2 are not both hydrogen when Q is Q-1 or Q-4, n is 1, R3 is H and q is 2; R3, which is attached to any carbon of the azabicyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R7)R8, OR7, C(O)R7, C(O)OR7,
C(O)N(R7)R8, SR7, S(O)R7, S(O)2R7, S(O)2N(R7)R8 and C1-C6 alkyl substituted with 1 or 2 groups independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R4 is selected from the group H, C1-C6 alkyl, C3-C6 cycloalkyl, C1-C6 haloalkyl,
C2-C6 alkenyl, C2-C6 alkynyl, N(R9)R10, C(O)R9, C(O)OR9,
C(O)N(R9)R10, SR9, S(O)R9, S(O)2R9, S(O)2N(R9)R10, benzyl and CH(CH3)Ph; provided when any of R1, R2, R3 or R4 is S(O)R5, S(O)2R5, S(O)R7, S(O)2R7, S(O)R9, or S(O)2R9 then R5, R7 and R9 are other than H; R5, R6, R7,R8, R9 and R10 are independently selected from the group H, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
W is selected from the group halogen, NO2, CN, C1-C3 alkyl, C1-C3 haloalkyl, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and
C2-C4 alkoxycarbonyl;
m and n are independently 0, 1 or 2;
p is 1 or 2; and
q is 1, 2 or 3.
2. A compound according to Claim 1 wherein Q is Q-1.
3. A compound according to Claim 2 wherein
R1 is selected from the group H, halogen and C1-C2 alkyl;
R2 is selected from the group H and Cl;
R3 is selected from the group H, halogen, C1-C6 alkyl and OR7;
R5 is selected from the group H and C1-C4 alkyl; and
n is 0 or 1.
4. A compound according to Claim 3 which is
7-(6-chloro-3-pyridinyl)-1-azabicyclo[2.2.1]heptane.
5. A method for controlling arthropods comprising contacting the arthropods or their environment with an arthropodicidally effective amount of a compound of the formula:
Q1 is selected from the group
where the broken line represents an optional chemical bond;
V, X, Y and Z of the ring are each independently selected from the group O, S, N, -C(R1)-, -C(R1)=C(R2)-, -C(R1)=N- and -N(R11)-; provided that (i) no more than one of V, X, Y or Z is -C(R1)=C(R2)-, -C(R1)=N-, -N(R11)-, O or S, (ii) at least one of V, X, Y or Z is N, (iii) when the ring is a 5- membered ring containing two N and one O or S, and Q is Q-6, then the ring is attached to the 2-position of Q1 and (iv) when the ring is a 5- membered ring containing two N and one O or S, then Q is other than Q-5; R1 and R2 are independently selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R5)R6, OR5, C(O)R5, C(O)OR5, C(O)N(R5)R6, SR5, S(O)R5, S(O)2R5, S(O)2N(R5)R6 and C1-C6 alkyl substituted with 1 or 2 groups
independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy,
C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R3, which is attached to any carbon of the azacyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R7)R8, OR7, C(O)R7, C(O)OR7, C(O)N(R7)R8, SR7, S(O)R7, S(O)2R7, S(O)2N(R7)R8 and C1-C6 alkyl substituted with 1 or 2 groups independently selected from the group NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R4 and R11 are independently selected from the group H, C1-C6 alkyl, C3-C6 cycloalkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, N(R9)R10, C(O)R9, C(O)OR9, C(O)N(R9)R10, SR9, S(O)R9, S(O)2R9,
S(O)2N(R9)R10, benzyl and CH(CH3)Ph; provided when any of R1, R2, R3 or R4 is S(O)R5, S(O)2R5, S(O)R7, S(O)2R7, S(O)R9, or S(O)2R9 then R5, R7 and R9 are other than H;
R5, R6, R7,R8, R9 and R10 are independently selected the group H, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
W is selected from the group halogen, NO2, CN, C1-C3 alkyl, C1-C3 haloalkyl, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
m and n are independently 0, 1 or 2;
p is 1 or 2; and
q is 1, 2 or 3.
6. A method according to Claim 5 wherein:
R1 is selected from the group H, halogen and C1-C2 alkyl;
R2 is selected from the group H and Cl;
R3 is selected from the group H, halogen, C1-C6 alkyl and OR7;
R5 is selected from the group H and C1-C4 alkyl; and
m and n are independently 0 or 1.
7. A method according to Claim 6 wherein:
V is N;
X is -C(R1)=C(R2)-;
Y and Z are -C(R1)-; and
Q1 is Q-6.
8. A method according to Claim 6 wherein:
V is N; X is S;
Y and Z are -C(R1)-; and
Q1 is Q-6.
9. A method according to Claim 6 wherein:
the ring contains two N and one O or S; and
Q1 is Q-6.
10. An arthropodicidal composition comprising a carrier and an
arthropodicidally effective amount of a compound of the formula:
Q1 is selected from the group
V, X, Y and Z of the ring are each independently selected from the group O, S, N,
-C(R1)-, -C(R1)=C(R2)-, -C(R1)=N- and -N(R11)-; provided that (i) no more than one of V, X, Y or Z is -C(R1)=C(R2)-, -C(R1)=N-, -N(R11)-, O or S, (ii) at least one of V, X, Y or Z is N, (iii) when the ring is a 5- membered ring containing two N and one O or S, and Q is Q-6, then the ring is attached to the 2-position of Q- and (iv) when the ring is a 5- membered ring containing two N and one O or S, then Q is other than Q-5; R1 and R2 are independently selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R5)R6, OR5, C(O)R5, C(O)OR5, C(O)N(R5)R6, SR5, S(O)R5, S(O)2R5, S(O)2N(R5)R6 and C1-C6 alkyl substituted with 1 or 2 groups
independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R3, which is attached to any carbon of the azacyclic ring including the carbon directly attached to the heterocyclic aromatic ring, is selected from the group H, halogen, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, C3-C6 halocycloalkyl, CN, SCN, NO2, N(R7)R8, OR7, C(O)R7, C(O)OR7, C(O)N(R7)R8, SR7, S(O)R7, S(O)2R7, S(O)2N(R7)R8 and C1-C6 alkyl substituted with 1 or 2 groups independently selected from NO2, CN, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and C2-C4 alkoxycarbonyl;
R4 and R11 are independently selected from the group H, C1-C6 alkyl, C3-C6 cycloalkyl, C,-C6 haloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, N(R9)R10,
C(O)R9, C(O)OR9, C(O)N(R9)R10, SR9, S(O)R9, S(O)2R9,
S(O)2N(R9)R10, benzyl and CH(CH3)Ph; provided when any of R1, R2, R3 or R4 is S(O)R5, S(O)2R5, S(O)R7, S(O)2R7, S(O)R9, or S(O)2R9 then R5, R7 and R9 are other than H;
R5, R6, R7,R8, R9 and R10 are independently selected the group H, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, phenyl optionally substituted with 1 or 2 substituents independently selected from W, and benzyl optionally substituted with 1 or 2 substitutents independently selected from W;
W is selected from the group halogen, NO2, CN, C1-C3 alkyl, C1-C3 haloalkyl, C1-C3 alkylthio, C1-C3 alkoxy, C1-C3 haloalkoxy, C2-C4 alkylcarbonyl and
C2-C4 alkoxycarbonyl;
m and n are independently 0, 1 or 2;
p is 1 or 2; and
q is 1, 2 or 3.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU74747/94A AU7474794A (en) | 1993-07-22 | 1994-07-21 | Arthropodicidal azacyclic heterocycles |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US9587693A | 1993-07-22 | 1993-07-22 | |
| US08/095,876 | 1993-07-22 | ||
| US15619793A | 1993-11-22 | 1993-11-22 | |
| US08/156,197 | 1993-11-22 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1995003306A1 true WO1995003306A1 (en) | 1995-02-02 |
Family
ID=26790711
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1994/008404 WO1995003306A1 (en) | 1993-07-22 | 1994-07-21 | Arthropodicidal azacyclic heterocycles |
Country Status (2)
| Country | Link |
|---|---|
| AU (1) | AU7474794A (en) |
| WO (1) | WO1995003306A1 (en) |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996008968A1 (en) * | 1993-08-26 | 1996-03-28 | Dowelanco | Insect control with substituted triazole and tetrazole compounds |
| WO1996036637A1 (en) * | 1995-05-17 | 1996-11-21 | R.J. Reynolds Tobacco Company | Pharmaceutical compositions for prevention and treatment of central nervous system disorders |
| WO1998022462A1 (en) * | 1996-11-15 | 1998-05-28 | Zeneca Limited | 8-azabicyclo[3.2.1]octane-, 8-azabicyclo[3.2.1]oct-6-ene-, 9-azabicyclo[3.3.1]nonane-, 9-aza-3-oxabicyclo[3.3.1]nonane-, 9-aza-3-thiabicyclo[3.3.1]nonane derivatives, their preparation and their use as insecticides |
| WO1998025924A1 (en) * | 1996-11-26 | 1998-06-18 | Zeneca Limited | 8-azabicyclo[3.2.1]octane-, 8-azabicyclo[3.2.1]oct-6-ene-, 9-azabicyclo[3.3.1]nonane-, 9-aza-3-oxabicyclo[3.3.1]nonane- and 9-aza-3-thiabicyclo[3.3.1]nonane derivatives, their preparation and their use as insecticides |
| WO1998025923A1 (en) * | 1996-11-26 | 1998-06-18 | Zeneca Limited | 8-azabicyclo[3.2.1]octane derivatives, their preparation and their use as insecticides |
| US5849754A (en) * | 1996-11-20 | 1998-12-15 | Zeneca Limited | Bicyclic amine derivatives |
| WO1999000385A1 (en) * | 1997-06-30 | 1999-01-07 | R.J. Reynolds Tobacco Company | 3-pyridyl-1-aza-bicyclo-alkane derivatives for prevention and treatment of cns disorders |
| US5859024A (en) * | 1996-05-13 | 1999-01-12 | Zeneca Limited | Insecticidal, acaricidal or nematicidal 3-cyano-8-azabicyclo 3.2.1!octane derivatives |
| US5922732A (en) * | 1995-05-24 | 1999-07-13 | Zeneca Limited | Bicyclic amines |
| WO1999038865A1 (en) * | 1998-01-29 | 1999-08-05 | Zeneca Limited | 8-azabicyclo(3.2.1)oct-2-ene derivatives as pesticides |
| US5952339A (en) * | 1998-04-02 | 1999-09-14 | Bencherif; Merouane | Pharmaceutical compositions and methods of using nicotinic antagonists for treating a condition or disorder characterized by alteration in normal neurotransmitter release |
| EP0955301A2 (en) * | 1998-04-27 | 1999-11-10 | Pfizer Products Inc. | 7-aza-bicyclo[2.2.1]-heptane derivatives, their preparation and use according to their affinity for neuronal nicotinic acetylchloline receptors |
| US5986100A (en) * | 1998-04-02 | 1999-11-16 | Crooks; Peter Anthony | Pharmaceutical compositions and methods for use |
| US6057446A (en) * | 1998-04-02 | 2000-05-02 | Crooks; Peter Anthony | Certain 1-aza-tricyclo [3.3.1-13,7 ] decane compounds |
| US6432975B1 (en) | 1998-12-11 | 2002-08-13 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| US6525065B1 (en) | 1997-06-30 | 2003-02-25 | Targacept, Inc. | Pharmaceutical compositions and methods for effecting dopamine release |
| US6624173B1 (en) | 1997-06-30 | 2003-09-23 | Targacept, Inc. | Pharmaceutical compositions for treating and/or preventing CNS disorders |
| US6953855B2 (en) | 1998-12-11 | 2005-10-11 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US7101896B2 (en) | 1999-11-01 | 2006-09-05 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| US7223750B2 (en) | 2002-01-07 | 2007-05-29 | Sanofi-Aventis | Derivatives of 5-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane, the preparation thereof and the application of same in therapeutics |
| US20100035916A1 (en) * | 2007-02-09 | 2010-02-11 | Sanofi-Aventis | Azabicycloalkane derivatives, preparation thereof and use thereof in therapy |
| JP2011042671A (en) * | 2003-08-18 | 2011-03-03 | Fujifilm Finechemicals Co Ltd | Pyridyltetrahydropyridines and pyridylpiperidines, and methods for producing the same |
| US7981906B2 (en) | 2007-08-02 | 2011-07-19 | Targacept, Inc. | (2S,3R)-N-(2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl-benzofuran-2-carboxamide, novel salt forms, and methods of use thereof |
| US8476296B2 (en) | 2009-01-26 | 2013-07-02 | Targacept, Inc. | Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide |
| US9724340B2 (en) | 2015-07-31 | 2017-08-08 | Attenua, Inc. | Antitussive compositions and methods |
| JPWO2016175017A1 (en) * | 2015-04-28 | 2018-02-22 | アグロカネショウ株式会社 | Novel 4-pyridinecarboxamide derivative and agricultural and horticultural agent containing this as an active ingredient |
| CN109593088A (en) * | 2018-12-27 | 2019-04-09 | 华东理工大学 | Azabicyclic derivatives and its preparation and application |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993014636A1 (en) * | 1992-01-23 | 1993-08-05 | Dowelanco | Insect control with substituted oxadiazole and thiadiazole compounds |
-
1994
- 1994-07-21 AU AU74747/94A patent/AU7474794A/en not_active Abandoned
- 1994-07-21 WO PCT/US1994/008404 patent/WO1995003306A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1993014636A1 (en) * | 1992-01-23 | 1993-08-05 | Dowelanco | Insect control with substituted oxadiazole and thiadiazole compounds |
Non-Patent Citations (2)
| Title |
|---|
| CHEMICAL ABSTRACTS, vol. 62, no. 8, 1965, Columbus, Ohio, US; abstract no. 9101c, A.S. SADYKOV ET AL.: "Syntheses based on anabasine. XIX. Synthesis of 7-methylquinuclidine and alpha-(7-methylquinuclidyl)-beta-pyridine" * |
| ZH. OBSHCH. KHIM. 34(12), 4104-7 (1964) * |
Cited By (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996008968A1 (en) * | 1993-08-26 | 1996-03-28 | Dowelanco | Insect control with substituted triazole and tetrazole compounds |
| WO1996036637A1 (en) * | 1995-05-17 | 1996-11-21 | R.J. Reynolds Tobacco Company | Pharmaceutical compositions for prevention and treatment of central nervous system disorders |
| US6573275B1 (en) | 1995-05-24 | 2003-06-03 | Syngenta Limited | Bicyclic amines |
| US6391883B1 (en) | 1995-05-24 | 2002-05-21 | Syngenta Limited | Bicyclic amines |
| US6207676B1 (en) | 1995-05-24 | 2001-03-27 | Zeneca Limited | Bicyclic amines |
| US5922732A (en) * | 1995-05-24 | 1999-07-13 | Zeneca Limited | Bicyclic amines |
| US5859024A (en) * | 1996-05-13 | 1999-01-12 | Zeneca Limited | Insecticidal, acaricidal or nematicidal 3-cyano-8-azabicyclo 3.2.1!octane derivatives |
| WO1998022462A1 (en) * | 1996-11-15 | 1998-05-28 | Zeneca Limited | 8-azabicyclo[3.2.1]octane-, 8-azabicyclo[3.2.1]oct-6-ene-, 9-azabicyclo[3.3.1]nonane-, 9-aza-3-oxabicyclo[3.3.1]nonane-, 9-aza-3-thiabicyclo[3.3.1]nonane derivatives, their preparation and their use as insecticides |
| US6066646A (en) * | 1996-11-15 | 2000-05-23 | Zeneca Limited | Bicyclic amine derivatives |
| US5912254A (en) * | 1996-11-15 | 1999-06-15 | Zeneca Limited | Bicycle amine derivatives |
| US5849754A (en) * | 1996-11-20 | 1998-12-15 | Zeneca Limited | Bicyclic amine derivatives |
| US6291474B1 (en) | 1996-11-26 | 2001-09-18 | Zeneca Limited | Bicyclic amine derivatives |
| US6093726A (en) * | 1996-11-26 | 2000-07-25 | Zeneca Limited | Bicyclic amine derivatives |
| US6174894B1 (en) | 1996-11-26 | 2001-01-16 | Zeneca Limited | Bicyclic amine derivatives |
| US6177442B1 (en) | 1996-11-26 | 2001-01-23 | Zeneca Limited | Bicyclic amine derivatives |
| WO1998025923A1 (en) * | 1996-11-26 | 1998-06-18 | Zeneca Limited | 8-azabicyclo[3.2.1]octane derivatives, their preparation and their use as insecticides |
| WO1998025924A1 (en) * | 1996-11-26 | 1998-06-18 | Zeneca Limited | 8-azabicyclo[3.2.1]octane-, 8-azabicyclo[3.2.1]oct-6-ene-, 9-azabicyclo[3.3.1]nonane-, 9-aza-3-oxabicyclo[3.3.1]nonane- and 9-aza-3-thiabicyclo[3.3.1]nonane derivatives, their preparation and their use as insecticides |
| US6624173B1 (en) | 1997-06-30 | 2003-09-23 | Targacept, Inc. | Pharmaceutical compositions for treating and/or preventing CNS disorders |
| WO1999000385A1 (en) * | 1997-06-30 | 1999-01-07 | R.J. Reynolds Tobacco Company | 3-pyridyl-1-aza-bicyclo-alkane derivatives for prevention and treatment of cns disorders |
| US6525065B1 (en) | 1997-06-30 | 2003-02-25 | Targacept, Inc. | Pharmaceutical compositions and methods for effecting dopamine release |
| WO1999038865A1 (en) * | 1998-01-29 | 1999-08-05 | Zeneca Limited | 8-azabicyclo(3.2.1)oct-2-ene derivatives as pesticides |
| US5986100A (en) * | 1998-04-02 | 1999-11-16 | Crooks; Peter Anthony | Pharmaceutical compositions and methods for use |
| US5952339A (en) * | 1998-04-02 | 1999-09-14 | Bencherif; Merouane | Pharmaceutical compositions and methods of using nicotinic antagonists for treating a condition or disorder characterized by alteration in normal neurotransmitter release |
| US6211372B1 (en) | 1998-04-02 | 2001-04-03 | Peter Anthony Crooks | Pharmaceutical compositions and methods for use |
| US6417359B1 (en) | 1998-04-02 | 2002-07-09 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| US6057446A (en) * | 1998-04-02 | 2000-05-02 | Crooks; Peter Anthony | Certain 1-aza-tricyclo [3.3.1-13,7 ] decane compounds |
| EP0955301A3 (en) * | 1998-04-27 | 2001-04-18 | Pfizer Products Inc. | 7-aza-bicyclo[2.2.1]-heptane derivatives, their preparation and use according to their affinity for neuronal nicotinic acetylcholine receptors |
| EP0955301A2 (en) * | 1998-04-27 | 1999-11-10 | Pfizer Products Inc. | 7-aza-bicyclo[2.2.1]-heptane derivatives, their preparation and use according to their affinity for neuronal nicotinic acetylchloline receptors |
| US8124619B2 (en) | 1998-12-11 | 2012-02-28 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US6953855B2 (en) | 1998-12-11 | 2005-10-11 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US6432975B1 (en) | 1998-12-11 | 2002-08-13 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| US7425561B2 (en) | 1998-12-11 | 2008-09-16 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US8124620B2 (en) | 1998-12-11 | 2012-02-28 | Targacept, Inc. | 3-Substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US8084462B2 (en) | 1998-12-11 | 2011-12-27 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US8124618B2 (en) | 1998-12-11 | 2012-02-28 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US7754189B2 (en) | 1998-12-11 | 2010-07-13 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US7767193B2 (en) | 1998-12-11 | 2010-08-03 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US7101896B2 (en) | 1999-11-01 | 2006-09-05 | Targacept, Inc. | Pharmaceutical compositions and methods for use |
| US7223750B2 (en) | 2002-01-07 | 2007-05-29 | Sanofi-Aventis | Derivatives of 5-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane, the preparation thereof and the application of same in therapeutics |
| US7585974B2 (en) | 2002-01-07 | 2009-09-08 | Sanofi-Aventis | Derivatives of 5-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane, their preparation and their therapeutic application |
| US8541447B2 (en) | 2003-02-21 | 2013-09-24 | Targacept, Inc. | 3-substituted-2-(arlyalkyl)-1-azabicycloalkanes and methods of use thereof |
| US8143272B2 (en) | 2003-02-21 | 2012-03-27 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| US8158649B2 (en) | 2003-02-21 | 2012-04-17 | Targacept, Inc. | 3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof |
| JP2011042671A (en) * | 2003-08-18 | 2011-03-03 | Fujifilm Finechemicals Co Ltd | Pyridyltetrahydropyridines and pyridylpiperidines, and methods for producing the same |
| US8759343B2 (en) | 2007-02-09 | 2014-06-24 | Sanofi | Azabicycloalkane derivatives, preparation thereof and use thereof in therapy |
| JP2010518053A (en) * | 2007-02-09 | 2010-05-27 | サノフイ−アベンテイス | Azabicycloalkane derivatives, their preparation and their use in therapy |
| US20100035916A1 (en) * | 2007-02-09 | 2010-02-11 | Sanofi-Aventis | Azabicycloalkane derivatives, preparation thereof and use thereof in therapy |
| US8173669B2 (en) | 2007-02-09 | 2012-05-08 | Sanofi-Aventis | Azabicycloalkane derivatives, preparation thereof and use thereof in therapy |
| US8541446B2 (en) | 2007-08-02 | 2013-09-24 | Targacept, Inc. | (2S,3R)-N-(2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofurn-2-carboxamide, novel salt forms, and methods of use thereof |
| US7981906B2 (en) | 2007-08-02 | 2011-07-19 | Targacept, Inc. | (2S,3R)-N-(2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl-benzofuran-2-carboxamide, novel salt forms, and methods of use thereof |
| US8119659B2 (en) | 2007-08-02 | 2012-02-21 | Targacept, Inc. | (2S,3R)-N-(2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofuran-2-carboxamide, novel salt forms, and methods of use thereof |
| US8846715B2 (en) | 2007-08-02 | 2014-09-30 | Targacept, Inc. | (2S,3R)-N-(2((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofuran-2-carboxamide novel salt forms, and methods of use thereof |
| US8476296B2 (en) | 2009-01-26 | 2013-07-02 | Targacept, Inc. | Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide |
| US8901151B2 (en) | 2009-01-26 | 2014-12-02 | Targacept, Inc. | Preparation and therapeutic applications of (2S, 3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]OCT-3-yl)-3,5-difluorobenzamide |
| US9173876B2 (en) | 2009-01-26 | 2015-11-03 | Targacept, Inc. | Preparation and therapeutic applications of (2S,3R)-N-2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)-3,5-difluorobenzamide |
| JPWO2016175017A1 (en) * | 2015-04-28 | 2018-02-22 | アグロカネショウ株式会社 | Novel 4-pyridinecarboxamide derivative and agricultural and horticultural agent containing this as an active ingredient |
| US9724340B2 (en) | 2015-07-31 | 2017-08-08 | Attenua, Inc. | Antitussive compositions and methods |
| CN109593088A (en) * | 2018-12-27 | 2019-04-09 | 华东理工大学 | Azabicyclic derivatives and its preparation and application |
| CN109593088B (en) * | 2018-12-27 | 2022-03-25 | 华东理工大学 | Azabicyclo derivatives, their preparation and use |
Also Published As
| Publication number | Publication date |
|---|---|
| AU7474794A (en) | 1995-02-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO1995003306A1 (en) | Arthropodicidal azacyclic heterocycles | |
| EP0712394B1 (en) | Arthropodicidal oxazolines and thiazolines | |
| WO1995007278A1 (en) | Fungicidal, miticidal and arthropodicidal aminopyrimidines | |
| EP0737188B1 (en) | Arthropodicidal oxadiazine carboxanilides | |
| EP0646111B1 (en) | Arthropodicidal oxazolines and thiazolines | |
| WO1996017851A1 (en) | Arthropodicidal and fungicidal organosilanes and organogermanes | |
| WO1997011057A1 (en) | Arthropodicidal 1,4-dihydropyridines and 1,4-dihydropyrimidines | |
| WO1995019972A1 (en) | Arthropodicidal 2-oxa and thia-zolines | |
| WO1993021160A1 (en) | Arthropodicidal pyrazole sulfonates | |
| US5444079A (en) | Arthropodicidal oxazolines | |
| US5538967A (en) | Arthropodicidal oxazines and thiazines | |
| AU679350B2 (en) | Arthropodicidal tetrahydropyrimidines | |
| WO1995016676A1 (en) | Arthropodicidal pentafluorothio substituted anilides | |
| WO1994008954A1 (en) | Arthropodicidal semicarbazones | |
| US5767281A (en) | Arthropodicidal oxazolines and thiazolines | |
| WO1997013773A1 (en) | Arthropodicidal oxazolines and thiazolines | |
| WO1995000491A1 (en) | Arthropodicidal sulfonates | |
| WO1993021150A2 (en) | Arthropodicidal aryl sulfonates | |
| WO1993010096A1 (en) | Arthropodicidal and nematicidal sulfonates | |
| WO1996033180A1 (en) | Oxazoline and thiazoline arthropodicides | |
| EP0658163A1 (en) | Arthropodicidal tetrahydropyrimidines | |
| WO1997005145A1 (en) | Arthropodicidal tetrahydropyrimidines | |
| WO1994012491A1 (en) | Arthropodicidal nitroethylene diamines | |
| WO1995006631A1 (en) | Polycyclic arthropodicides | |
| WO1997005146A1 (en) | Arthropodicidal nitromethylenes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AM AU BB BG BR BY CA CN CZ FI GE HU JP KG KP KR KZ LK LT LV MD MG MN NO NZ PL RO RU SI SK TJ TT UA US US UZ VN |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): KE MW SD AT BE CH DE DK ES FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: CA |