WO1992010992A1 - Oral compositions effective against plaque and gingivitis - Google Patents
Oral compositions effective against plaque and gingivitis Download PDFInfo
- Publication number
- WO1992010992A1 WO1992010992A1 PCT/US1991/009377 US9109377W WO9210992A1 WO 1992010992 A1 WO1992010992 A1 WO 1992010992A1 US 9109377 W US9109377 W US 9109377W WO 9210992 A1 WO9210992 A1 WO 9210992A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- composition according
- chlorophenol
- antibacterial
- methyl
- water
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/81—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
- A61K8/8164—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by a carboxyl radical, and containing at least one other carboxyl radical in the molecule, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers, e.g. poly (methyl vinyl ether-co-maleic anhydride)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/19—Cosmetics or similar toiletry preparations characterised by the composition containing inorganic ingredients
- A61K8/24—Phosphorous; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/34—Alcohols
- A61K8/347—Phenols
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q11/00—Preparations for care of the teeth, of the oral cavity or of dentures; Dentifrices, e.g. toothpastes; Mouth rinses
Definitions
- the present invention relates " to oral compositions which provide antiplaque and antigingivitis benefits as well as being effective against other anaerobic infections of the mouth.
- Periodontal diseases include gingivitis and gingivitis, are believed to involve anaerobic bacterial infections.
- Periodontal disease affects the periodontiu , which is the investing and supporting tissue surrounding a tooth (i.e., the periodontal ligament, the gingiva, and the alveolar bone).
- Gingivitis and periodontitis are inflammatory disorders of the gingiva and the periodontal ligament, respectively.
- Gingivosis and periodontosis are more severe conditions involving degenerative disorders of the tissue. Combinations of inflammatory and degenerative conditions are termed periodontitis complex.
- Periodontal disease is a major cause of tooth loss in adults.
- Tooth loss from periodontal disease is a significant problem beginning at age 35, but even by age 15 it is estimated that about
- noncationic, water-insoluble antibacterial agents in oral products is disclosed in a number of references.
- One such reference is U.S. Patent 4.022.889 to Vinson et al .
- Vinson describes compositions containing zinc salts and antibacterial agents such as halogenated salicylanilides and halogenated hydroxydiphenyl ethers.
- Another reference disclosing noncationic water-insoluble antibacterial agents is U.K. Patent Application GB 2.200.551. published August 10, 1988.
- the compositions contain a molecularly dehydrated polyphosphate salt. The salt is stated to improve the effectiveness of the antibac ⁇ terial.
- Another reference disclosing noncationic water-insoluble antibacterials in oral compositions is U.S.
- the present invention in certain aspects, embraces oral care product containing water-insoluble, noncationic antibacterial agents, a polyethylene glycol solvent and an anionic linear, polycarboxylate.
- the present invention also encompasses a method for treating diseases of the oral cavity using noncationic water insoluble antibacterial agents.
- oral compositions as used herein means a product which in the ordinary course of usage is not intentionally swallowed for ⁇ SHEET purposes of systemic administration of particular therapeutic agents, but is rather retained in the oral cavity for a time sufficient to contact substantially all of the dental surfaces and/or oral tissues for purposes of oral activity.
- safe and effective amount as used herein means suffi ⁇ cient amount of material to provide the desired benefit while being safe to the hard and soft tissues of the oral cavity.
- carrier a suitable vehicle which is pharmaceutically acceptable and can be used to apply the present compositions in the oral cavity.
- the present invention in certain aspects involves the use of water-insoluble, noncationic antibacterials with a polyethylene glycol solvent and a carboxyvinyl polymer having molecular weight of 3,000,000 or greater.
- the essential and optional components of the compositions are made using the process described in detail below.
- Antibacterial Agents Given below are examples of antibacterial agents useful in the compositions of the present invention which are water insoluble and noncationic.
- Phenolic Compounds including phenol and its homologs, ono- and poly-alkyl and aromatic halophenols, resorcinol and its derivatives, bisphenolic compounds and halogenated salicylani- lides).
- the antibacterial agent is present in the oral compositions of the present invention in an effective antiplaque amount, typically about 0.01-5% by weight, preferably about 0.03-1%.
- the antibacterial agent is substantially water-insoluble, meaning that its solubility is less than about 1% by weight in water at 25 * C and may be even less than about 0.1%. If an ionizable group is present solubility is determined at a pH at which ionization does not occur.
- polyethylene glycols useful in this invention can be any of a wide range of molecular weights such as from about 100 to about 1,000, preferably from about 300 to about 600.
- the glycol is present in an amount of from about 1% to about 10%, preferably from about 3% to about 6%.
- the anionic polymeric polycarboxylates employed herein are well known, being employed in the form of their free acids or partially or preferably fully neutralized water soluble alkali metal (e.g. potassium and preferably sodium) or ammonium salts.
- Preferred are 1:4 to 4:1 copolymers of maleic anhydride or acid with another polymerizable ethylenically unsaturated monomer, preferably methyl vinyl ether (methoxyethylene) having a molecular weight (M.W.) of about 30,000 to about 1,000,000.
- M.W. molecular weight
- These copolymers are available for example as Gantrez (AN 139(M.W. 500,000), A.N. 119 (M.W. 250,000) and preferably S-97 Pharmaceutical Grade (M.W. 70,000), of GAF Corporation.
- operative polymeric polycarboxylates include those such as the 1:1 copolymers of maleic anhydride with ethyl acrylate, hydroxyethyl methacrylate, N-vinyl-2-pyrollidone, or ethylene, the latter being available for example as Monsanto EMA No. 1103, M.W. 10,000 and EMA Grade 61, and 1:1 copolymers of acrylic acid with methyl or hydroxyethyl methacrylate, methyl or ethyl acrylate, isobutyl vinyl ether or N-vinyl-2-pyrrolidone.
- Additional operative polymeric polycarboxylates disclosed in above referred to U.S. Patent Nos. 4.138.477 and 4.183.914. incorporated herein by reference, include copolymers of maleic anhydride with styrene, isobutylene or ethyl vinyl ether, poly- acrylic, polyitaconic and polymaleic acids, and sulfoacrylic oligomers of M.W. as low as 1,000 available as Uniroyal ND-2.
- Suitable generally are polymerized olefinically or ethylen- ically unsaturated carboxylic acids containing an activated carbon-to-carbon olefinic double bond and at least one carboxyl group, that is, an acid containing an olefinic double bond which readily functions in polymerization because of its presence in the monomer molecule either in the alpha-beta position with respect to a carboxyl group or as part of a terminal methylene grouping.
- Such acids are acrylic, ethacrylic, ethacrylic, alpha-chloroacrylic, crotonic, beta-acryloxy propionic, sorbic, alpha-chlorsorbic, cinnamic, beta-styrylacrylic, muconic, ita- conic, citraconic, mesaconic, glutaconic, aconitic, alpha-phenyl- acrylic, 2-benzyl acrylic, 2-cyclohexylacrylic, angelic, umbel!ic, fumaric, maleic acids and anhydrides.
- Other different olefinic monomers copolymerizable with such carboxylic monomers include vinylacetate, vinyl chloride, dimethyl aleate and the like. Copolymers contain sufficient carboxylic salt groups for water- solubility.
- the linear anionic polymeric polycarboxylate component is mainly a hydrocarbon with optional halogen and O-containing substituents and linkages as present in for example ester, ether and OH groups, and when present is generally employed in the instant compositions in approximate weight amounts of 0.05 to 3%, preferably 0.05 to 2%, more preferably 0.1 to 2%.
- Water Water is another essential component of this invention. Water employed in the preparation of commercially suitable compositions should preferably be deionized and free of organic impurities. Water generally comprises from about 10% to 50%, preferably from about 20% to 40%, by weight of the toothpaste compositions herein while mouthwashes contain from about 40% to about 95%. These amounts of water include the free water which is added plus that which is introduced with other materials as with sorbitol.
- compositions of the present invention may contain in addition to the above-listed components many others which will be somewhat dependent on the type of composition (mouthwashes, toothpastes, topical gels, prophylaxis pastes and the like). Toothpastes and mouthwashes are the preferred systems with toothpastes being the most preferred.
- the abrasive polishing material contemplated for use in the present invention can be any material which does not excessively abrade dentin. These include, for example, silicas including gels and precipitates, calcium carbonate, dicalcium orthophosphate dihy- drate, calcium pyrophosphate, tricalcium phosphate, calcium polymetaphosphate, insoluble sodium polymetaphosphate, hydrated alumina, and resinous abrasive materials such as particulate condensation products of urea and formaldehyde, and others such as disclosed by Cooley et al. in U.S. Patent 3.070.510. December 25, 1962, incorporated herein by reference. Mixtures of abrasives may also be used.
- Silica dental abrasives of various types, can provide the unique benefits of exceptional dental cleaning and polishing performance without unduly abrading tooth enamel or dentin.
- Silica abrasive materials are also exceptionally compatible with sources of soluble fluoride and other ion sources. For these reasons they are preferred for use herein.
- the silica abrasive polishing materials useful herein, as well as the other abrasives generally have an average particle size ranging between about 0.1 and 30 microns, preferably 5 and 15 microns.
- the silica abrasive can be precipitated silica or silica gels such as the silica xerogels described in Pader et al., U.S. Patent 3.538.230.
- silica xerogels marketed under the tradename "Syloid" by the W.R. Grace & Company, Davison Chemical Division.
- Preferred precipitated silica materials include those marketed by the J.M. Huber Corporation under the tradename, "Zeodent", parti ⁇ cularly the silica carrying the designation "Zeodent 119". These silica abrasive are described in U.S. Patent 4.340.583. July 29, 1982, incorporated herein by reference.
- the abrasive in the toothpaste compositions described herein is present at a level of from about 6% to about 70%, preferably from about 15% to about 30%.
- Flavoring agents can also be added to the dentifrice and other compositions of the present invention. Suitable flavoring agents include oil of wintergreen, oil of
- Sweetening agents are also useful and include aspartame, acesulfame, saccharin, dextrose, levulose and sodium cyclamate. Flavoring and sweetening agents are. generally used in the compo- sitions herein at levels of from about 0.005% to about 2% by weight and may be used as a solvent for the antibacterials hereinbefore indicated.
- thickening agents are carboxyvinyl polymers, carrageenan, hydroxy ⁇ ethyl cellulose and water soluble salts of cellulose ethers such as sodium carboxymethyl cellulose and sodium carboxymethyl hydroxyethyl cellulose.
- Natural gums such as gum karaya, gum arabic, and gum tragacanth and polysaccharide gums such as xanthan gum can also be used.
- Colloidal magnesium aluminum silicate or finely divided silica can be used as part of the thickening agent to further improve texture.
- Thickening agents in an amount from 0.05% to 1.5% by weight of the total composition may be used.
- a humectant in a toothpaste to keep it from hardening.
- Suitable hu ectants include glycerin, sorbitol, and other edible polyhydric alcohols at a combined level of from about 10% to about 70%.
- Mouthwashes generally comprise from about 20:1 to about 2:1 of a water/ethyl alcohol solution and preferably other ingredients such as flavor, sweeteners, humectants and sudsing agents such as those described above.
- the humectants, such as glycerin and sorbitol give a moist feel to the mouth.
- the mouthwashes of the invention comprise 5% to 60% (preferably 10% to 25%) ethyl alcohol, 0% to
- a humectant 20% (preferably 5% to 20%) of a humectant, 0% to 2% (preferably
- emulsifying agent 0% to 0.5% (preferably 0.005% to
- a fluoride ion source is a fluoride ion source.
- the sources of fluoride ions, or fluoride-providing compounds, are well known in the art as anticaries agents and also act as such agents in the practice of this invention as well as to inhibit pyrophosphatase. These compounds may be slightly soluble in water or may, preferably, be fully water-soluble. They are charac ⁇ terized by their ability to release fluoride ions in water and by freedom from undesired reaction with other compounds of the oral preparation.
- inorganic fluoride salts such as soluble alkali metal, alkaline earth metal salts, for example, sodium fluoride, barium fluoride, sodium fluorsilicate, ammonium fluorosilicate, sodium fluorozirconate, sodium mono- fluorophosphate, aluminum mono- and di-fluorophosphate, and fluorinated sodium calcium pyrophosphate.
- Alkali metal and tin fluorides such as sodium and stannous fluorides, sodium mono- fluorophosphate (MFP) and mixtures thereof, are preferred.
- the amount of fluoride-providing compound is dependent to some extent upon the type of compound, its solubility, and the type of oral preparation, but it must be a nontoxic amount, generally about 0.005 to about 3.0% in the preparation.
- a dentifrice preparation e.g. dental gel, toothpaste (including cream)
- an amount of such compound which releases up to about 5,000 ppm of F ion by weight of the preparation is considered satisfactory.
- Any suitable minimum amount of such compound may be used, but it is preferable to employ sufficient compound to release about 300 to 2,000 ppm, more preferably about 800 to about 1,500 ppm of fluoride ion.
- this component is present in an amount up to about 2% by weight, based on the weight of the preparation, and preferably in the range of about 0.05% to 1%.
- the compound may be present in an amount of about 0.1-3%, more typically about 0.76%.
- Still another optional component for use in the compositions of the present invention is an anticalculus agent.
- agents include any which are effective against calculus such as pyro ⁇ phosphate salts as disclosed in U.S. Patent 4.515.772. May 7, 1985 incorporated herein by reference.
- the preferred agents are mono, di, tri and tetra alkali metal and ammonium pyrophosphate. Such agents are used in amounts sufficient to reduce calculus. These amounts are preferably in an amount of at least about 1% P2O7, most preferably at least about 1.3%, most preferably at least about 1.5%.
- anticalculus agents are metal ions such as zinc disclosed in U.S. Patent 4.022.880. May 10, 1977 to Vinson incorporated herein by reference. Still others are polymers such as those described in U.S. Patent 4.661.341. April 28, 1987 to Benedict and U.S. Patent 3.429.963. February 25, 1969 to Shed- lovsky, both of which are incorporated herein by reference. Such metals are used in an amount of from about 0.05% to about 5%, preferably about 0.5% to about 2%, while such polymers are used in amounts of from about 0.01% to about 10%, preferably from about 0.1% to about 5%.
- Surfactants are also useful in the composition of this invention include many different materials. Suitable surfactants include any which are reasonably stable and function over a wide pH range. Included are non-soap anionic, nonionic, cationic, zwitterionic and amphoteric organic synthetic surfactants. Many of these are disclosed by Gieske et al. in U.S. Patent 4.051.234. September 27, 1988 incorporated herein in total by reference.
- Preferred surfactants include alkyl sulfates. Any surfactant used is at a level of from about 0.2% to about 6%, preferably from about 0.6% to about 2% in a toothpaste and from about 0.01% to about 5%, preferably from about 0.1% to about 0.5% in a mouthwash.
- the pH of the present compositions and/or its pH in the mouth can be any pH which is safe for the mouth's hard and soft tissues. Such pH's are generally from about 3 to about 10, preferably from about 4 to about 9.
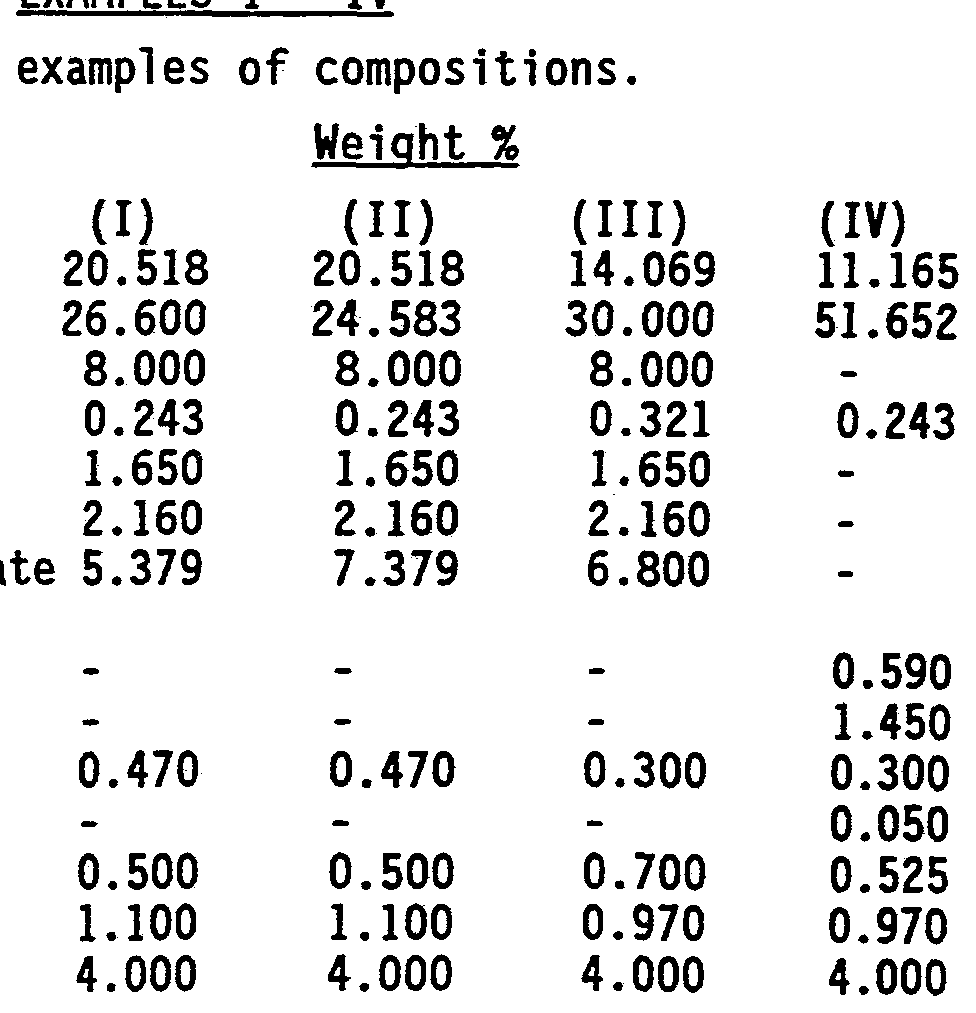
- Sorbitol (70%) Glycerin Sodium fluoride Sodium acid pyrophosphate Tetrasod um pyrophosphate Tetrapotassium pyrophospha (60-65%) Monosodium phosphate Trisodium phosphate Saccharin FD&C Blue No. 1 Titanium dioxide Flavor PEG-300
- compositions are made in a conventional manner.
- compositions of this present invention are superior due to the improved bioavailability of the triclosan antibacterial. 0
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Birds (AREA)
- Epidemiology (AREA)
- Inorganic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Emergency Medicine (AREA)
- Cosmetics (AREA)
Abstract
Disclosed are oral compositions which are effective against plaque and gingivitis and contain a noncationic water insoluble antibacterial agent.
Description
ORAL COMPOSITIONS EFFECTIVE AGAINST PLAQUE AND GINGIVITIS
Douglas Charles Mohl TECHNICAL FIELD The present invention relates" to oral compositions which provide antiplaque and antigingivitis benefits as well as being effective against other anaerobic infections of the mouth.
Plaque induced diseases, including periodontitis and gingivitis, are believed to involve anaerobic bacterial infections. Periodontal disease affects the periodontiu , which is the investing and supporting tissue surrounding a tooth (i.e., the periodontal ligament, the gingiva, and the alveolar bone). Gingivitis and periodontitis are inflammatory disorders of the gingiva and the periodontal ligament, respectively. Gingivosis and periodontosis are more severe conditions involving degenerative disorders of the tissue. Combinations of inflammatory and degenerative conditions are termed periodontitis complex.
Periodontal disease is a major cause of tooth loss in adults.
Tooth loss from periodontal disease is a significant problem beginning at age 35, but even by age 15 it is estimated that about
4 out of 5 persons already have gingivitis and 4 out of 10 have periodontitis.
While good oral hygiene, as achieved by brushing the teeth with a cleansing dentifrice, may help reduce the incidence of periodontal disease, it does not necessarily prevent or eliminate its occurrence. This is because microorganisms contribute to both the initiation and progress of periodontal disease. Thus, in order to prevent or treat periodontal disease, these micro¬ organisms must be suppressed by some means other than simple mechanical scrubbing. Towards this end, there has been a great deal of research aimed at developing therapeutic dentifrices, outhwashes, and methods of treating periodontal disease which are effective in suppressing these microorganisms.
The use of noncationic, water-insoluble antibacterial agents in oral products is disclosed in a number of references. One such reference is U.S. Patent 4.022.889 to Vinson et al . Vinson describes compositions containing zinc salts and antibacterial agents such as halogenated salicylanilides and halogenated hydroxydiphenyl ethers.
Another reference disclosing noncationic water-insoluble antibacterial agents is U.K. Patent Application GB 2.200.551. published August 10, 1988. In addition to the antibacterial, the compositions contain a molecularly dehydrated polyphosphate salt. The salt is stated to improve the effectiveness of the antibac¬ terial. Another reference disclosing noncationic water-insoluble antibacterials in oral compositions is U.S. 4.894.220. January 16, 1990 to Nabi et al. This reference teaches the use of solvents and polymers to enhance the antibacterial's effect. Still another reference disclosing such antibacterials combined with polyethylene glycols in oral compositions is European Patent Application 02.220.890. May 6, 1987. All prior art references are incorporated herein by reference in total.
It has now been found that the bioavailability and effective- ness of the antibacterial can be improved by incorporating the antibacterial, a linear anionic polycarboxylate and a polyethylene glycol into the composition.
It is therefore an object of the present invention to provide improved oral care products containing specific antibacterial agents.
It is a further object of the present invention to provide more effective products for treating diseases of the oral cavity.
It is still a further objective to provide methods for treating diseases of the oral cavity. These and other objects will become readily apparent from the detailed disclosure which follows.
All percentages and ratios used herein are by weight unless otherwise specified. Also, all measurements referred to herein are made at 25'C in the composition unless otherwise specified. SUMMARY OF THE INVENTION
The present invention, in certain aspects, embraces oral care product containing water-insoluble, noncationic antibacterial agents, a polyethylene glycol solvent and an anionic linear, polycarboxylate. The present invention also encompasses a method for treating diseases of the oral cavity using noncationic water insoluble antibacterial agents.
By "oral compositions" as used herein means a product which in the ordinary course of usage is not intentionally swallowed for ε SHEET
purposes of systemic administration of particular therapeutic agents, but is rather retained in the oral cavity for a time sufficient to contact substantially all of the dental surfaces and/or oral tissues for purposes of oral activity. By "safe and effective amount" as used herein means suffi¬ cient amount of material to provide the desired benefit while being safe to the hard and soft tissues of the oral cavity.
By the term "comprising", as used herein, is meant that various additional components can be conjointly employed in the compositions of this invention as long as the listed materials perform their intended functions.
By the term "carrier", as used herein, is meant a suitable vehicle which is pharmaceutically acceptable and can be used to apply the present compositions in the oral cavity. DETAILED DESCRIPTION OF THE INVENTION
The present invention in certain aspects involves the use of water-insoluble, noncationic antibacterials with a polyethylene glycol solvent and a carboxyvinyl polymer having molecular weight of 3,000,000 or greater. The essential and optional components of the compositions are made using the process described in detail below.
Antibacterial Agents Given below are examples of antibacterial agents useful in the compositions of the present invention which are water insoluble and noncationic.
Halogenated Diohenyl Ethers 2/,4,4'-trichloro-2-hydroxy-diphenyl ether (Triclosan) 2,2'-dihydroxy-5,5'-dibromo-diphenyl ether.
Phenolic Compounds (including phenol and its homologs, ono- and poly-alkyl and aromatic halophenols, resorcinol and its derivatives, bisphenolic compounds and halogenated salicylani- lides).
Phenol and its Homologs Phenol 2 Methyl - Phenol
3 Methyl - Phenol
4 Methyl - Phenol 4 Ethyl - Phenol 2,4-Dimethyl - Phenol
2,5-Dimethyl - Phenol
3,4-Dimethyl - Phenol
2,6-Dimethyl - Phenol
4-n-Propyl - Phenol
4-n-Butyl - Phenol
4-n-Amyl - Phenol
4-tert-Amyl - Phenol
4-n-Hexyl - Phenol
4-n-Heptyl - Phenol
Mono- and Polv-Alkyl and Aromatic Halophenols p-Chlorophenol
Methyl p-Chlorophenol
Ethyl p-Chlorophenol n-Propyl p-Chlorophenol n-Butyl p-Chlorophenol n-Amyl p-Chlorophenol sec-Amy1 p-Chlorophenol n-Hexyl p-Chlorophenol
Cyclohexyl p-Chlorophenol n-Heptyl p-Chlorophenol n-Octyl p-Chlorophenol o-Chlorophenol
Methyl o-Chlorophenol
Ethyl o-Chlorophenol n-Propyl o-Chlorophenol n-Butyl o-Chlorophenol n-Amyl o-Chlorophenol tert-A yl o-Chlorophenol n-Hexyl o-Chlorophenol n-Heptyl o-Chlorophenol o-Benzyl - p-Chlorophenol o-Benxyl-m-methyl - p-Chlorophenol o-Benzyl- , -dimethyl - p-Chlorophenol o-Phenylethyl - p-Chlorophenol o-Phenylethyl-m-methyl - p-Chlorophenol
3-Methyl - p-Chlorophenol
3,5-Dimethyl - p-Chlorophenol
6-Ethyl-3-methyl - p-Chlorophenol
6-n-Propyl -3-methyl - p-Chlorophenol
6- i so-Propyl -3-methyl •Chlorophenol
2-Ethyl -3,5-dimethyl ■Chlorophenol
6- sec- Butyl -3-methyl -Chlorophenol
2-iso-Propyl-3,5-dimethyl ■Chlorophenol
6-Diethyl ethyl-3-methyl ■Chlorophenol
6-iso-Propyl-2-ethyl-3-methyl •Chlorophenol
2-sec-Amyl-3,5-dimethyl ■Chlorophenol
2-Diethylmethyl-3,5-dimethyl ■Chlorophenol
6-sec-0ctyl-3-methyl -Chlorophenol p-Bromophenol
Methyl p-Bromophenol
Ethyl p-Bromophenol n-Propyl p-Bromophenol n-Butyl p-Bromophenol n-Amyl p-Bromophenol sec-Amyl p-Bromophenol n-Hexyl p-Bromophenol cyclohexyl p-Bromophenol o-Bromophenol tert-Am l o-Bromophenol n-Hexyl o-Bromophenol n-Propyl-m.mDimethyl o-Bromophenol
2-Phenyl Phenol
4-Chloro-2-methyl phenol
4-Chloro-3-methyl phenol
4-Chloro-3,5-dimethyl phenol
2,4-dichloro-3,5-dimethylphenol
3,4,5,6-terabromo-2-methylphenol
5-methyl-2-pentylphenol
4-isopropyl-3-methylphenol
5-Chloro-2-hydroxydiphenylmethane
Resorcinol and its Derivatives Resorcinol
Methyl - Resorcinol
Ethyl - Resorcinol n-Propyl - Resorcinol n-Butyl - Resorcinol n-Amyl - Resorcinol n-Hexyl - Resorcinol
n-Heptyl - Resorcinol n-Octyl - Resorcinol n-Nonyl - Resorcinol
Phenyl - Resorcinol
Benzyl - Resorcinol
Phenyl ethyl - Resorcinol
Phenyl propyl - Resorcinol p-Chlorobenzyl - Resorcinol
5-Chloro -2,4-Dihydroxydiphenyl Methane
44''--CChhlloorroo -2,4-Dihydroxydiphenyl Methane
5-Bromo -2,4-Dihydroxydiphenyl Methane
4' -Bromo -2,4-Dihydroxydiphenyl Methane Bisohenolic Compounds 2,2'-methylene bis (4-chlorophenol) 2,2'-methylene bis (3,4,6-trichlorophenol) 2,2'-methylene bis (4-chloro-6-bron.oph.enol) bis (2-hydroxy-3,5-dichlorophenyl) sulphide bis (2-hydroxy-5-chlorobenzyl) sulphide Halogenated SalicylaniTides 4',5-dibromosalicylanilide
3,4',5-trichlorosalcylanilide 3,4',5-tribromosalicylanilide 2,3,3',5-tetrachlorosalicylanilide 3,3',5-trichlorosalicylanilide 3,5-dibromo-3'-trifluoromethyl salicylanilide 5-n-octanoyl-3'-trif1uoromethyl salicylani1ide 3,5-dibromo-4'-trif1uoromethyl salicylani1ide 3,5-dibromo-3'-trifluoromethyl salicylanilide (Fluorophene) Benzoic Esters p-Hydroxybenzoic Acid Methyl - p-Hydroxybenzoic Acid Ethyl - p-Hydroxybenzoic Acid Propyl - p-Hydroxybenzoic Acid Butyl - p-Hydroxybenzoic Acid
Halogenated Carban 1ides 3,4,4'-trichlorocarbanilide -trif1uoromethyl-4,4'-dichlorocarbaniTide ,3',4-trichlorocarbanil de
The antibacterial agent is present in the oral compositions of the present invention in an effective antiplaque amount, typically about 0.01-5% by weight, preferably about 0.03-1%. The antibacterial agent is substantially water-insoluble, meaning that its solubility is less than about 1% by weight in water at 25*C and may be even less than about 0.1%. If an ionizable group is present solubility is determined at a pH at which ionization does not occur. Polyethylene Glvcols The polyethylene glycols useful in this invention can be any of a wide range of molecular weights such as from about 100 to about 1,000, preferably from about 300 to about 600. The glycol is present in an amount of from about 1% to about 10%, preferably from about 3% to about 6%. Anionic Linear Polycarboxylate
The anionic polymeric polycarboxylates employed herein are well known, being employed in the form of their free acids or partially or preferably fully neutralized water soluble alkali metal (e.g. potassium and preferably sodium) or ammonium salts. Preferred are 1:4 to 4:1 copolymers of maleic anhydride or acid with another polymerizable ethylenically unsaturated monomer, preferably methyl vinyl ether (methoxyethylene) having a molecular weight (M.W.) of about 30,000 to about 1,000,000. These copolymers are available for example as Gantrez (AN 139(M.W. 500,000), A.N. 119 (M.W. 250,000) and preferably S-97 Pharmaceutical Grade (M.W. 70,000), of GAF Corporation.
Other operative polymeric polycarboxylates include those such as the 1:1 copolymers of maleic anhydride with ethyl acrylate, hydroxyethyl methacrylate, N-vinyl-2-pyrollidone, or ethylene, the latter being available for example as Monsanto EMA No. 1103, M.W. 10,000 and EMA Grade 61, and 1:1 copolymers of acrylic acid with methyl or hydroxyethyl methacrylate, methyl or ethyl acrylate, isobutyl vinyl ether or N-vinyl-2-pyrrolidone.
Additional operative polymeric polycarboxylates disclosed in above referred to U.S. Patent Nos. 4.138.477 and 4.183.914. incorporated herein by reference, include copolymers of maleic anhydride with styrene, isobutylene or ethyl vinyl ether, poly- acrylic, polyitaconic and polymaleic acids, and sulfoacrylic oligomers of M.W. as low as 1,000 available as Uniroyal ND-2.
Suitable generally are polymerized olefinically or ethylen- ically unsaturated carboxylic acids containing an activated carbon-to-carbon olefinic double bond and at least one carboxyl group, that is, an acid containing an olefinic double bond which readily functions in polymerization because of its presence in the monomer molecule either in the alpha-beta position with respect to a carboxyl group or as part of a terminal methylene grouping. Illustrative of such acids are acrylic, ethacrylic, ethacrylic, alpha-chloroacrylic, crotonic, beta-acryloxy propionic, sorbic, alpha-chlorsorbic, cinnamic, beta-styrylacrylic, muconic, ita- conic, citraconic, mesaconic, glutaconic, aconitic, alpha-phenyl- acrylic, 2-benzyl acrylic, 2-cyclohexylacrylic, angelic, umbel!ic, fumaric, maleic acids and anhydrides. Other different olefinic monomers copolymerizable with such carboxylic monomers include vinylacetate, vinyl chloride, dimethyl aleate and the like. Copolymers contain sufficient carboxylic salt groups for water- solubility.
The linear anionic polymeric polycarboxylate component is mainly a hydrocarbon with optional halogen and O-containing substituents and linkages as present in for example ester, ether and OH groups, and when present is generally employed in the instant compositions in approximate weight amounts of 0.05 to 3%, preferably 0.05 to 2%, more preferably 0.1 to 2%. Water Water is another essential component of this invention. Water employed in the preparation of commercially suitable compositions should preferably be deionized and free of organic impurities. Water generally comprises from about 10% to 50%, preferably from about 20% to 40%, by weight of the toothpaste compositions herein while mouthwashes contain from about 40% to about 95%. These amounts of water include the free water which is added plus that which is introduced with other materials as with sorbitol.
Optional Components The compositions of the present invention may contain in addition to the above-listed components many others which will be somewhat dependent on the type of composition (mouthwashes, toothpastes, topical gels, prophylaxis pastes and the like). Toothpastes and mouthwashes are the preferred systems with
toothpastes being the most preferred.
Toothpastes contain as a major component an abrasive. The abrasive polishing material contemplated for use in the present invention can be any material which does not excessively abrade dentin. These include, for example, silicas including gels and precipitates, calcium carbonate, dicalcium orthophosphate dihy- drate, calcium pyrophosphate, tricalcium phosphate, calcium polymetaphosphate, insoluble sodium polymetaphosphate, hydrated alumina, and resinous abrasive materials such as particulate condensation products of urea and formaldehyde, and others such as disclosed by Cooley et al. in U.S. Patent 3.070.510. December 25, 1962, incorporated herein by reference. Mixtures of abrasives may also be used.
Silica dental abrasives, of various types, can provide the unique benefits of exceptional dental cleaning and polishing performance without unduly abrading tooth enamel or dentin. Silica abrasive materials are also exceptionally compatible with sources of soluble fluoride and other ion sources. For these reasons they are preferred for use herein. The silica abrasive polishing materials useful herein, as well as the other abrasives, generally have an average particle size ranging between about 0.1 and 30 microns, preferably 5 and 15 microns. The silica abrasive can be precipitated silica or silica gels such as the silica xerogels described in Pader et al., U.S. Patent 3.538.230. issued March 2, 1970 and DiGiulio, U.S. Patent 3.862.307. June 21, 1975, both incorporated herein by reference. Preferred are the silica xerogels marketed under the tradename "Syloid" by the W.R. Grace & Company, Davison Chemical Division. Preferred precipitated silica materials include those marketed by the J.M. Huber Corporation under the tradename, "Zeodent", parti¬ cularly the silica carrying the designation "Zeodent 119". These silica abrasive are described in U.S. Patent 4.340.583. July 29, 1982, incorporated herein by reference.
The abrasive in the toothpaste compositions described herein is present at a level of from about 6% to about 70%, preferably from about 15% to about 30%.
Flavoring agents, as was noted earlier, can also be added to the dentifrice and other compositions of the present invention. Suitable flavoring agents include oil of wintergreen, oil of
E SHEET
peppermint, oil of spearmint,, oil of sassafras, and oil of clove. Sweetening agents are also useful and include aspartame, acesulfame, saccharin, dextrose, levulose and sodium cyclamate. Flavoring and sweetening agents are. generally used in the compo- sitions herein at levels of from about 0.005% to about 2% by weight and may be used as a solvent for the antibacterials hereinbefore indicated.
In preparing toothpastes, it is necessary to add some thickening material to provide a desirable consistency. Preferred thickening agents are carboxyvinyl polymers, carrageenan, hydroxy¬ ethyl cellulose and water soluble salts of cellulose ethers such as sodium carboxymethyl cellulose and sodium carboxymethyl hydroxyethyl cellulose. Natural gums such as gum karaya, gum arabic, and gum tragacanth and polysaccharide gums such as xanthan gum can also be used. Colloidal magnesium aluminum silicate or finely divided silica can be used as part of the thickening agent to further improve texture. Thickening agents in an amount from 0.05% to 1.5% by weight of the total composition may be used.
It is also desirable to include a humectant in a toothpaste to keep it from hardening. Suitable hu ectants include glycerin, sorbitol, and other edible polyhydric alcohols at a combined level of from about 10% to about 70%.
Another preferred embodiment of the present invention is a mouthwash composition. Mouthwashes generally comprise from about 20:1 to about 2:1 of a water/ethyl alcohol solution and preferably other ingredients such as flavor, sweeteners, humectants and sudsing agents such as those described above. The humectants, such as glycerin and sorbitol give a moist feel to the mouth.
Generally, on a weight basis the mouthwashes of the invention comprise 5% to 60% (preferably 10% to 25%) ethyl alcohol, 0% to
20% (preferably 5% to 20%) of a humectant, 0% to 2% (preferably
0.01% to 0.5%) emulsifying agent, 0% to 0.5% (preferably 0.005% to
0.06%) sweetening agent such as saccharin, 0% to 0.3% (preferably from 0.03% to 0.3%) flavoring agent, and the balance water. Another optional component is a fluoride ion source. The sources of fluoride ions, or fluoride-providing compounds, are well known in the art as anticaries agents and also act as such agents in the practice of this invention as well as to inhibit pyrophosphatase. These compounds may be slightly soluble in water
or may, preferably, be fully water-soluble. They are charac¬ terized by their ability to release fluoride ions in water and by freedom from undesired reaction with other compounds of the oral preparation. Among these materials-are inorganic fluoride salts, such as soluble alkali metal, alkaline earth metal salts, for example, sodium fluoride, barium fluoride, sodium fluorsilicate, ammonium fluorosilicate, sodium fluorozirconate, sodium mono- fluorophosphate, aluminum mono- and di-fluorophosphate, and fluorinated sodium calcium pyrophosphate. Alkali metal and tin fluorides, such as sodium and stannous fluorides, sodium mono- fluorophosphate (MFP) and mixtures thereof, are preferred.
The amount of fluoride-providing compound is dependent to some extent upon the type of compound, its solubility, and the type of oral preparation, but it must be a nontoxic amount, generally about 0.005 to about 3.0% in the preparation. In a dentifrice preparation, e.g. dental gel, toothpaste (including cream), an amount of such compound which releases up to about 5,000 ppm of F ion by weight of the preparation is considered satisfactory. Any suitable minimum amount of such compound may be used, but it is preferable to employ sufficient compound to release about 300 to 2,000 ppm, more preferably about 800 to about 1,500 ppm of fluoride ion. Typically, in the cases of alkali metal fluorides and stannous fluoride, this component is present in an amount up to about 2% by weight, based on the weight of the preparation, and preferably in the range of about 0.05% to 1%. In the case of sodium monofluorophosphate, the compound may be present in an amount of about 0.1-3%, more typically about 0.76%.
Still another optional component for use in the compositions of the present invention is an anticalculus agent. These agents include any which are effective against calculus such as pyro¬ phosphate salts as disclosed in U.S. Patent 4.515.772. May 7, 1985 incorporated herein by reference. The preferred agents are mono, di, tri and tetra alkali metal and ammonium pyrophosphate. Such agents are used in amounts sufficient to reduce calculus. These amounts are preferably in an amount of at least about 1% P2O7, most preferably at least about 1.3%, most preferably at least about 1.5%.
Other anticalculus agents are metal ions such as zinc disclosed in U.S. Patent 4.022.880. May 10, 1977 to Vinson
incorporated herein by reference. Still others are polymers such as those described in U.S. Patent 4.661.341. April 28, 1987 to Benedict and U.S. Patent 3.429.963. February 25, 1969 to Shed- lovsky, both of which are incorporated herein by reference. Such metals are used in an amount of from about 0.05% to about 5%, preferably about 0.5% to about 2%, while such polymers are used in amounts of from about 0.01% to about 10%, preferably from about 0.1% to about 5%.
Surfactants are also useful in the composition of this invention include many different materials. Suitable surfactants include any which are reasonably stable and function over a wide pH range. Included are non-soap anionic, nonionic, cationic, zwitterionic and amphoteric organic synthetic surfactants. Many of these are disclosed by Gieske et al. in U.S. Patent 4.051.234. September 27, 1988 incorporated herein in total by reference.
Preferred surfactants include alkyl sulfates. Any surfactant used is at a level of from about 0.2% to about 6%, preferably from about 0.6% to about 2% in a toothpaste and from about 0.01% to about 5%, preferably from about 0.1% to about 0.5% in a mouthwash. The pH of the present compositions and/or its pH in the mouth can be any pH which is safe for the mouth's hard and soft tissues. Such pH's are generally from about 3 to about 10, preferably from about 4 to about 9.
Given below are non-limiting examples which illustrate the compositions of the present invention.
EXAMPLES I - IV
Component
Sorbitol (70%) Glycerin Sodium fluoride Sodium acid pyrophosphate Tetrasod um pyrophosphate Tetrapotassium pyrophospha (60-65%) Monosodium phosphate Trisodium phosphate Saccharin FD&C Blue No. 1 Titanium dioxide Flavor PEG-300
15
The above compositions are made in a conventional manner.
The performance of the above compositions of this present invention are superior due to the improved bioavailability of the triclosan antibacterial. 0
WHAT IS CLAIMED:
5
0
5
SUBSTITUTESHEET
Claims
1. An oral composition comprising a noncationic water insoluble antibacterial, a polyethylene glycol solvent, a linear anionic polycar¬ boxylate and water.
2. A composition according to Claim 1 wherein said antibacterial is a phenolic compound.
3. A composition according to either of Claims 1 or 2 wherein said polyethylene glycol has a molecular weight of from about 100 to about 1,000.
4. A composition according to any of Claims 1-3 wherein said polycar- boxylate is an acrylic acid polymer.
5. A composition according to any of Claims 1-4 wherein said polymer is a copolymer of vinyl methyl ether and maleic anhydride.
6. A composition according to any of Claims 1-5 wherein said composi¬ tion is a toothpaste or a mouthwash.
7. A composition according to any of Claims 1-6 wherein said composi¬ tion is a toothpaste which in addition contains an abrasive and a binder.
8. A composition according to any of Claim 1-7 wherein said tooth¬ paste also contains triclosan as the antibacterial agent.
9. A composition according to any of Claims 1-8 wherein said tooth¬ paste contains a soluble fluoride ion source.
10. A composition according to any of Claims 1-9 wherein said tooth¬ paste contains an anticalculus agent.
11. A composition according to any of Claims 1-10 wherein said anti¬ calculus agent is a soluble pyrophosphate salt.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US63009490A | 1990-12-19 | 1990-12-19 | |
| US630,094 | 1990-12-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1992010992A1 true WO1992010992A1 (en) | 1992-07-09 |
Family
ID=24525730
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1991/009377 WO1992010992A1 (en) | 1990-12-19 | 1991-12-13 | Oral compositions effective against plaque and gingivitis |
Country Status (2)
| Country | Link |
|---|---|
| AU (1) | AU9179791A (en) |
| WO (1) | WO1992010992A1 (en) |
Cited By (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0579333A2 (en) * | 1992-07-16 | 1994-01-19 | Colgate-Palmolive Company | Antibacterial antiplaque oral composition |
| WO1994022417A1 (en) * | 1993-04-02 | 1994-10-13 | Smithkline Beecham Plc | An oral hygiene pretreatment composition |
| WO1994023691A2 (en) * | 1993-04-08 | 1994-10-27 | The Procter & Gamble Company | Oral compositions for treating plaque and gingivitis containing a noncationic antibacterial agent and a bicarbonate salt |
| EP0691124A1 (en) * | 1994-07-07 | 1996-01-10 | Sara Lee/DE N.V. | Mouth care products |
| EP0743059A2 (en) * | 1995-05-18 | 1996-11-20 | Colgate-Palmolive Company | Process for applying antibacterial oral composition to dental implant areas |
| WO1997010800A2 (en) * | 1995-09-22 | 1997-03-27 | Colgate-Palmolive Company | Antiplaque oral composition |
| WO1998034587A1 (en) * | 1997-02-10 | 1998-08-13 | Colgate-Palmolive Company | Oral composition exhibiting enhance uptake |
| US5854194A (en) * | 1996-12-12 | 1998-12-29 | Colgate-Palmolive Co. | Chemical linker compositions |
| US5912274A (en) * | 1995-09-22 | 1999-06-15 | Colgate-Palmolive Company | Antiplaque oral composition and method |
| US5955407A (en) * | 1996-12-12 | 1999-09-21 | Colgate-Palmolive Co. | Chemical linker compositions |
| US6020301A (en) * | 1996-12-12 | 2000-02-01 | Colgate Palmolive Company | Chemical linker compositions |
| US6197741B1 (en) * | 1996-12-12 | 2001-03-06 | Colgate-Palmolive Company | Chemical linker compositions |
| GB2358584A (en) * | 1999-12-23 | 2001-08-01 | Smithkline Beecham Plc | Oral care composition |
| US6303555B1 (en) * | 1996-12-12 | 2001-10-16 | Colgate-Palmolive Company | Chemical linker compositions |
| US6306809B1 (en) * | 1996-12-12 | 2001-10-23 | Colgate-Palmolive Co. | Chemical linker compositions |
| US6420325B2 (en) * | 1996-12-12 | 2002-07-16 | Colgate-Palmolive Company | Chemical linker compositions |
| WO2003053897A1 (en) * | 2001-12-20 | 2003-07-03 | Warner-Lambert Company Llc | Non-halogenated hydroxyalkyl-substituted phenol compounds, antimicrobial compositions containing the same, and methods of using the same |
| US6790868B2 (en) | 2000-12-20 | 2004-09-14 | Warner-Lambert Company | Non-halogenated phenoxy and/or benzyloxy substituted phenols, antimicrobial compositions containing the same, and methods of using the same |
| US6838583B2 (en) | 2000-12-20 | 2005-01-04 | Warner-Lambert Company Llc | Non-halogenated naphthol compounds, antimicrobial compositions containing the same, and the methods of using the same |
| US7211700B2 (en) | 2000-12-20 | 2007-05-01 | Mcneil-Ppc, Inc. | Non-halogenated phenyl substituted phenols, antimicrobial compositions containing the same, and methods of using the same |
| EP2437596A1 (en) * | 2009-06-03 | 2012-04-11 | Ex-Tek, LLC | Skin treatment compositions |
| EP2996777B1 (en) | 2013-05-15 | 2017-03-01 | Unilever PLC | Oral care compositions |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2200551A (en) * | 1987-01-30 | 1988-08-10 | Colgate Palmolive Co | Antibacterial antiplaque, anticalculus oral composition |
| FR2641186A1 (en) * | 1988-12-29 | 1990-07-06 | Colgate Palmolive Co | ANTIBACIAL ANTIBACTERIAL BUCCAL COMPOSITION AND METHOD OF USE |
-
1991
- 1991-12-13 AU AU91797/91A patent/AU9179791A/en not_active Abandoned
- 1991-12-13 WO PCT/US1991/009377 patent/WO1992010992A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2200551A (en) * | 1987-01-30 | 1988-08-10 | Colgate Palmolive Co | Antibacterial antiplaque, anticalculus oral composition |
| FR2641186A1 (en) * | 1988-12-29 | 1990-07-06 | Colgate Palmolive Co | ANTIBACIAL ANTIBACTERIAL BUCCAL COMPOSITION AND METHOD OF USE |
Cited By (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0579333A3 (en) * | 1992-07-16 | 1994-05-25 | Colgate Palmolive Co | Antibacterial antiplaque oral composition |
| EP0579333A2 (en) * | 1992-07-16 | 1994-01-19 | Colgate-Palmolive Company | Antibacterial antiplaque oral composition |
| WO1994022417A1 (en) * | 1993-04-02 | 1994-10-13 | Smithkline Beecham Plc | An oral hygiene pretreatment composition |
| WO1994023691A2 (en) * | 1993-04-08 | 1994-10-27 | The Procter & Gamble Company | Oral compositions for treating plaque and gingivitis containing a noncationic antibacterial agent and a bicarbonate salt |
| WO1994023691A3 (en) * | 1993-04-08 | 1995-01-12 | Procter & Gamble | Oral compositions for treating plaque and gingivitis containing a noncationic antibacterial agent and a bicarbonate salt |
| US5670138A (en) * | 1994-07-07 | 1997-09-23 | Sara Lee/De N.V. | Mouth-care products |
| EP0691124A1 (en) * | 1994-07-07 | 1996-01-10 | Sara Lee/DE N.V. | Mouth care products |
| EP0743059A2 (en) * | 1995-05-18 | 1996-11-20 | Colgate-Palmolive Company | Process for applying antibacterial oral composition to dental implant areas |
| EP0743059A3 (en) * | 1995-05-18 | 1998-04-22 | Colgate-Palmolive Company | Process for applying antibacterial oral composition to dental implant areas |
| US5912274A (en) * | 1995-09-22 | 1999-06-15 | Colgate-Palmolive Company | Antiplaque oral composition and method |
| WO1997010800A2 (en) * | 1995-09-22 | 1997-03-27 | Colgate-Palmolive Company | Antiplaque oral composition |
| WO1997010800A3 (en) * | 1995-09-22 | 1997-05-22 | Colgate Palmolive Co | Antiplaque oral composition |
| US5723500A (en) * | 1995-09-22 | 1998-03-03 | Colgate-Palmolive Company | Antiplaque oral composition and method |
| AU708974B2 (en) * | 1995-09-22 | 1999-08-19 | Colgate-Palmolive Company, The | Antiplaque oral composition |
| US6303555B1 (en) * | 1996-12-12 | 2001-10-16 | Colgate-Palmolive Company | Chemical linker compositions |
| US5955407A (en) * | 1996-12-12 | 1999-09-21 | Colgate-Palmolive Co. | Chemical linker compositions |
| US6020301A (en) * | 1996-12-12 | 2000-02-01 | Colgate Palmolive Company | Chemical linker compositions |
| US6197741B1 (en) * | 1996-12-12 | 2001-03-06 | Colgate-Palmolive Company | Chemical linker compositions |
| US6306809B1 (en) * | 1996-12-12 | 2001-10-23 | Colgate-Palmolive Co. | Chemical linker compositions |
| US6420325B2 (en) * | 1996-12-12 | 2002-07-16 | Colgate-Palmolive Company | Chemical linker compositions |
| US5854194A (en) * | 1996-12-12 | 1998-12-29 | Colgate-Palmolive Co. | Chemical linker compositions |
| WO1998034587A1 (en) * | 1997-02-10 | 1998-08-13 | Colgate-Palmolive Company | Oral composition exhibiting enhance uptake |
| GB2358584A (en) * | 1999-12-23 | 2001-08-01 | Smithkline Beecham Plc | Oral care composition |
| US7211700B2 (en) | 2000-12-20 | 2007-05-01 | Mcneil-Ppc, Inc. | Non-halogenated phenyl substituted phenols, antimicrobial compositions containing the same, and methods of using the same |
| US6790868B2 (en) | 2000-12-20 | 2004-09-14 | Warner-Lambert Company | Non-halogenated phenoxy and/or benzyloxy substituted phenols, antimicrobial compositions containing the same, and methods of using the same |
| US6838583B2 (en) | 2000-12-20 | 2005-01-04 | Warner-Lambert Company Llc | Non-halogenated naphthol compounds, antimicrobial compositions containing the same, and the methods of using the same |
| WO2003053897A1 (en) * | 2001-12-20 | 2003-07-03 | Warner-Lambert Company Llc | Non-halogenated hydroxyalkyl-substituted phenol compounds, antimicrobial compositions containing the same, and methods of using the same |
| EP2437596A1 (en) * | 2009-06-03 | 2012-04-11 | Ex-Tek, LLC | Skin treatment compositions |
| CN102480945A (en) * | 2009-06-03 | 2012-05-30 | 艾克斯特克有限责任公司 | Skin treatment compositions |
| EP2437596A4 (en) * | 2009-06-03 | 2013-01-16 | Ex Tek Llc | Skin treatment compositions |
| EP2996777B1 (en) | 2013-05-15 | 2017-03-01 | Unilever PLC | Oral care compositions |
Also Published As
| Publication number | Publication date |
|---|---|
| AU9179791A (en) | 1992-07-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0696449B1 (en) | Antimicrobial oral composition | |
| US4894220A (en) | Antibacterial antiplaque oral composition | |
| AU629743B2 (en) | Antibacterial antiplaque anticalculus oral composition | |
| AU625379B2 (en) | Antiplaque antibacterial oral composition | |
| US5288480A (en) | Antiplaque antibacterial oral composition | |
| US5188821A (en) | Antibacterial antiplaque oral composition mouthwash or liquid dentifrice | |
| US5728756A (en) | Antiplaque antibacterial oral composition | |
| US5032386A (en) | Antiplaque antibacterial oral composition | |
| US5156835A (en) | Antibacterial antiplaque oral composition | |
| US5037637A (en) | Antibacterial antiplaque, anticalculus oral composition | |
| WO1992010992A1 (en) | Oral compositions effective against plaque and gingivitis | |
| US5474761A (en) | Oral compositions for treating plaque and gingivitis | |
| IL92694A (en) | Antibacterial antiplaque anticalculus oral composition | |
| US5290541A (en) | Methods for making oral compositions | |
| US5178851A (en) | Antiplaque antibacterial oral composition | |
| US5290542A (en) | Oral compositions for treating plaque and gingivitis | |
| WO1992010994A1 (en) | Oral compositions effective against plaque and gingivitis | |
| US5453265A (en) | Antibacterial antiplaque oral composition | |
| US5312618A (en) | Antibacterial antiplaque oral composition | |
| IE914419A1 (en) | Methods for making oral compositions | |
| EP1052967A2 (en) | Antiplaque oral composition and method | |
| CA2006717C (en) | Antibacterial anti-plaque oral composition mouthwash or liquid dentifrice | |
| CA2006716C (en) | Antibacterial antiplaque oral composition | |
| WO1993002658A1 (en) | Oral compositions effective against plaque and gingivitis | |
| NZ236644A (en) | Oral cleaning composition containing polymeric carboxylate phosphatase inhibitor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AT AU BB BG BR CA CH CS DE DK ES FI GB HU JP KP KR LK LU MG MN MW NL NO PL RO SD SE SU |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE BF BJ CF CG CH CI CM DE DK ES FR GA GB GN GR IT LU MC ML MR NL SE SN TD TG |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| NENP | Non-entry into the national phase |
Ref country code: CA |
|
| 122 | Ep: pct application non-entry in european phase |