WO1992004302A1 - Guanidine as a nitrogen fertilizer - Google Patents
Guanidine as a nitrogen fertilizer Download PDFInfo
- Publication number
- WO1992004302A1 WO1992004302A1 PCT/CA1991/000308 CA9100308W WO9204302A1 WO 1992004302 A1 WO1992004302 A1 WO 1992004302A1 CA 9100308 W CA9100308 W CA 9100308W WO 9204302 A1 WO9204302 A1 WO 9204302A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- guanidine
- fertilizer
- ammonium sulphate
- nitrogen
- ammonium
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C05—FERTILISERS; MANUFACTURE THEREOF
- C05C—NITROGENOUS FERTILISERS
- C05C11/00—Other nitrogenous fertilisers
-
- C—CHEMISTRY; METALLURGY
- C05—FERTILISERS; MANUFACTURE THEREOF
- C05C—NITROGENOUS FERTILISERS
- C05C3/00—Fertilisers containing other salts of ammonia or ammonia itself, e.g. gas liquor
Definitions
- This invention relates to slow nitrogen release fertilizer and a process for making the slow release nitrogen component by use of sulphur dioxide emissions.
- Nitrogen fertilizer is widely used to maintain and increase the growth in a variety of plants and in particular to increase yield and protein content of non- leguminous crops.
- One of the significant difficulties with nitrogen fertilizers is to maintain release of the nitrogen over extended periods of time and preferably during initial application of the nitrogen fertilizer to provide for a quick release of a certain portion thereof.
- nitrogen fertilizers which tend to suit different regions of application, the most common of which are urea, anhydrous ammonia, various ammonium salts such as ammonium nitrate, ammonium sulphate and calcium nitrate. It has been found, however, that the uptake by the crop of the fertilizer is normally in the range of
- the fast release nitrogens are the various salts of the above type which include ammonia, ammonia salts and nitrates.
- the slow release nitrogens may be compounds such as sulphur coated urea. It also generally understood that the slow release forms for nitrogen must be relatively inexpensive to manufacture.
- sulphur dioxide is normally converted to elemental sulphur, sulphuric acid or calcium sulphate.
- sulphur dioxide may be used in the manufacture of guanidine sulphate.
- guanidine sulfamate is prepared.
- sulfmates act as growth inhibitors or to some extent are poisonous to agricultural crops.
- sulphur dioxide emissions such as obtained from various types of industrial processes to manufacture guanidine sulphate for use as a fertilizer. Applicants have, however, discovered quite
- the invention provides a process for converting sulphur dioxide from waste gas streams to a non-toxic beneficial component to be returned to the soil.
- the invention provides a nitrogen based fertilizer having a slow nitrogen release component of guanidine ammonium sulphate.
- the invention provides an economical process for the conversion of sulfamates prepared by reacting waste sulphur dioxide with urea in the presence of ammonia to give guanidine ammonium sulphate in a form which is readily used as a fertilizer component and with the additional advantages that the final composition also includes fast release ammonium sulphate fertilizer as well as elemental sulphur which is an important nutrient for plant growth.
- a nitrogen fertilizer which has slow nitrogen release properties.
- the fertilizer comprises guanidine ammonium sulphate.
- a plant fertilizer has slow and fast nitrogen releasing properties, the fertilizer comprising an ammonium salt admixed with a guanidine based nitrogen source.
- the improvement comprises the guanidine based nitrogen source consisting of guanidine ammonium sulphate.
- the delayed release component is guanidine ammonium sulphate.
- the quick release component is ammonium sulphate or one of its other ammonium salts as well as various nitrates.
- composition may also optionally include elemental
- the resultant product has a combination of all three; that is, a slow release component in the form of guanidine ammonium sulphate, a quick release component in the form of ammonium sulphate and the plant nutrient element of sulphur.
- This invention provides for the very important recycle of hazardous sulphur dioxide emissions to the soil through an environmentally beneficial component; i.e., guanidine ammonium sulphate, ammonium sulphate and elemental sulphur.
- the process for making these components is cost competitive with existing costs for the commercial production of the widely used ammonium sulphate
- guanidine ammonium sulphate functions very effectively as a slow nitrogen release component in a fertilizer.
- quick release components may be added, such as anhydrous ammonia, aqua-ammonia, NH 4 NO 3 , Ca(NO 3 ) 2 , or (NH 4 ) 2 SO 4 .
- the preferred quick release component is ammonium sulphate since as will be discussed is a result of the process of this invention.
- the combination of the guanidine ammonium sulphate with ammonium sulphate provides both slow and fast release properties and is as significant if not more significant than existing slow and fast release fertilizers.
- a variety of suitable diluents, excipients, carriers, insecticides, herbicides, mixtures thereof and the like may be incorporated with the fertilizer composition to enhance the properties thereof.
- the amount of guanidine ammonium sulphate in the fertilizer is
- the guanidine ammonium sulphate would make up approximately 50 kg of nitrogen per hectare and the fast release component would make up the remaining 50 kg of the nitrogen per hectare, resulting in a molar ratio of 1 mole of guanidine ammonium sulphate to 1 mole of, for example, ammonium sulphate in the fertilizer composition.
- sulphur is also included as a plant nutrient, it may be present as 1 mole of elemental sulphur. This mixture in the fertilizer contains approximately 29.5% nitrogen and 25.2% sulphur by weight.
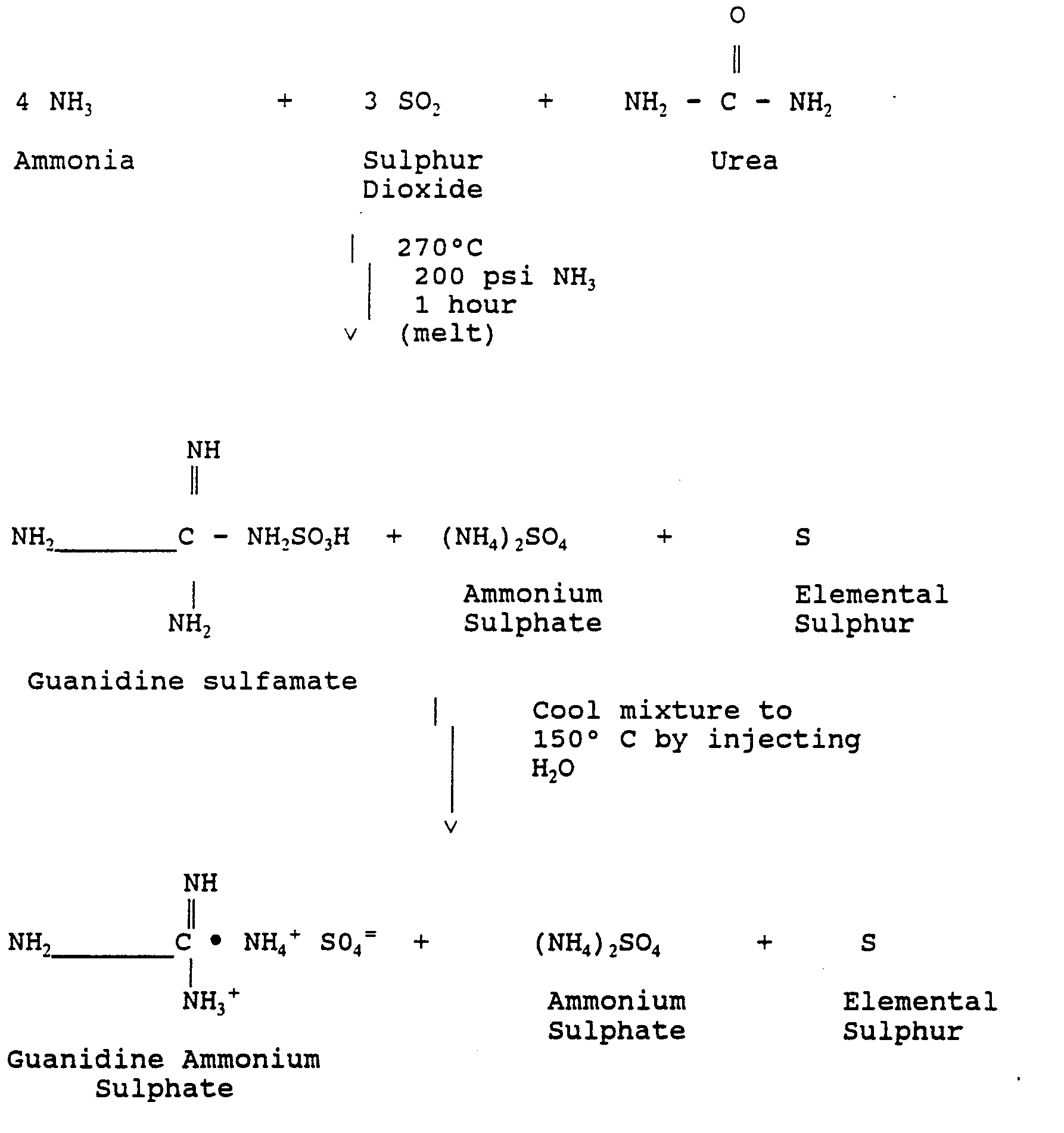
- the preferred process for preparing the slow and the fast nitrogen release components involves the use of sulphur dioxide emissions.
- the schematic for the process is as follows:
- the process of this invention provides in combination and in the correct molar ratio, the slow release component of guanidine ammonium sulphate, the fast release component of ammonium sulphate and the plant nutrient elemental sulphur.
- the less desirable guanidine sulfamate as a source of nitrogen can be converted to guanidine ammonium sulphate by the hydrolysing step. It has been found that hydrolysis of guanidine sulfamate to guanidine ammonium sulphate proceeds rapidly at temperatures in the range of 150° to 200°C by simply injecting into the reaction mixture a hydrolysing agent, such as water or a weak acid. Such hydrolysing step does not affect the ammonium sulphate or elemental sulphur, so the resultant product after hydrolysis is in a form usable as a fertilizer.
- guanidine sulfamate to guanidine ammonium sulphate include dilute sulphuric acid.
- the cost of preparing the slow and fast nitrogen release components as well as the elemental sulphur is in the range of $0.05 to $0.10 per pound which is cost competitive with other forms of fertilizer components and considerably less than the normal cost of manufacturing guanidine ammonium sulphate.
- the process of this invention has a particular benefit in the global environment because it is made from sulphur dioxide emissions as well as ammonia and urea which are also byproducts of industrial processes.
- the sulphur dioxide emissions are normally removed from waste gases of coal burning power plants, sour natural gas processing installations and smelters.
- guanidine ammonium sulphate is a useful fertilizer component, the driving force now exits to manufacture large quantities of fertilizer involving the use of sulphur dioxide.
- Such use diverts sulphur dioxide from being emitted to the atmosphere to instead being returned to the soil in a beneficial component. It also provides for a cost effective elimination of sulphur dioxide from emissions without any waste materials, such as the previously produced calcium sulphates. It has been found that the rates of nitrogen release from the guanidine ammonium sulphate and the ammonium sulphate, as established by the following Examples, is well within the specifications for both components and to some extent provides enhanced plant growth.
- the above schematic is not intended to be exclusive with respect to the process of making guanidine ammonium sulphate. It is appreciated that there are several variations in preparing a sulfamate. Sulphur dioxide and ammonium may be reacted in a gaseous phase at 150° to 200°C to prepare the solid ammonium sulfamate. The solid ammonium sulfamate may be reacted with urea as a melt at a temperature in the range of 280°C to produce the guanidine sulfamate in the presence of ammonia at a pressure of approximately 200 psi. Such process not only yields the sulfamate, but as well the ammonium sulphate and elemental sulphur.
- this reaction is carried out in a high pressure reactor which is capable of withstanding pressures in the range of 200 to 300 psi. Normally the reaction takes about one hour to complete conversion of urea to guanidine sulfamate with the byproducts of ammonium sulphate and elemental sulphur.
- the melt mixture is then cooled to a temperature in the range of 150° to 200°C and a hydrolysing agent is injected to the reactor to hydrolyse guanidine sulfamate to prepare guanidine ammonium sulphate salt.
- a hydrolysing agent is injected to the reactor to hydrolyse guanidine sulfamate to prepare guanidine ammonium sulphate salt.
- hydrolysis of the sulfamate provides a reaction product which includes the desired fast release fertilizer component of ammonium sulphate and the plant nutrient of elemental sulphur.
- the melt is then further cooled to room temperature. During such cooling process, the melt begins to solidify. At this time, the melt may be either granulated by use of
- the material may then be admixed with desired diluents, excipients, carriers, insecticides, herbicides, mixtures thereof and the like.
- desired diluents excipients, carriers, insecticides, herbicides, mixtures thereof and the like.
- the amount of nitrogen fertilizer components can be used at rates up to 100 kg of nitrogen per hectare.
- composition as used in the final fertilizer composition is not necessary that there be 100% conversion by the process of this invention of the sulfamates to the ammonium sulphate salts of the guanidine.
- the fertilizer compositions of this invention may be applied to the soils in a normal format. Usually they are applied in the granular or prill form; however, the compositions could be dissolved and applied in solution by a variety of spraying techniques.
- This example provides enabling disclosure with respect to the process and provides analysis data regarding identification of the reaction products.
- the resultant solid contained guanidine ammonium sulphate, ammonium sulphate and sulphur. It is appreciated that in the production of the guanidine sulfamate, all three reactants of ammonia, sulphur dioxide and urea may be combined in the reaction vessel to give that product.
- the other soil was high in soil organic matter (10.2%) and was finely textured (Clay loam).
- This example sets forth experimental results showing yield and nitrogen uptake of barley grown in soil containing no fertilizer, A.S. alone, G.A.S. alone and a mixture of A.S. and G.A.S. obtained from the process of this invention.
- barley Hadeum vulgare, cv. Empress
- soil moisture was maintained at 70 to 90% of field capacity.
- the G.A.S. produced little yield increase after 28 days on the Luvisolic soil (Table 2) but after 42 days yields were similar to those produced by the A.S.
- the uptake of N by the barley followed the same pattern. The patterns were not as clear on the Chernozemic soil (Table 3), except that at 42 days the yield and N-uptake was approximately alike for A.S. and G.A.S.
- This example sets forth experimental results showing yield and nitrogen uptake of barley grown in soil
- G.A.S. -A.S. mixture for both soils.
- Urea one of the most common commercial fertilizers, tended to yield less than A.S. or the G.A.S. -A.S.
- the rate of application in the experiment was 45 or 60 micrograms of N per gram of soil. If these rates were expressed as kilograms of N per hectare on an area basis, the values would be somewhat higher (up to 67.5 to 90 kg N/ha). Consequently, the rates used in the experiment were slightly greater than the rates used in the field in the Prairie provinces.
- This example compares sources of guanidine salts by yield and N-uptake of barley in the greenhouse.
- the experiment was conducted in a chamber (22 to 23°C) with 30 seeds place between moistened filter paper in a series of Petri dishes. There were 3
- the purpose was to find if G.A.S. decreases yield when sufficient A.S. nitrogen is added for optimum crop yield.
- the A.S. addition was 180 ug N/g, and the
- G.A.S. were 30, 60 and 90 ug N/g (see Table 7). There was a tendency for slightly less yield with 90 compared to 60 or 30 ug N/g of G.A.S. at 21 days (not shown). The yields at 56 days showed small but
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pest Control & Pesticides (AREA)
- Fertilizers (AREA)
Abstract
Description
Claims
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB9018893.9 | 1990-08-30 | ||
| GB909018893A GB9018893D0 (en) | 1990-08-30 | 1990-08-30 | Guanidine as a nitrogen fertilizer |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1992004302A1 true WO1992004302A1 (en) | 1992-03-19 |
Family
ID=10681362
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/CA1991/000308 WO1992004302A1 (en) | 1990-08-30 | 1991-08-30 | Guanidine as a nitrogen fertilizer |
Country Status (3)
| Country | Link |
|---|---|
| AU (1) | AU8405591A (en) |
| GB (1) | GB9018893D0 (en) |
| WO (1) | WO1992004302A1 (en) |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4083712A (en) * | 1971-02-19 | 1978-04-11 | Bayer Aktiengesellschaft | Nitrogenous fertilizer compositions |
| US4711659A (en) * | 1986-08-18 | 1987-12-08 | Moore William P | Attrition resistant controlled release fertilizers |
-
1990
- 1990-08-30 GB GB909018893A patent/GB9018893D0/en active Pending
-
1991
- 1991-08-30 AU AU84055/91A patent/AU8405591A/en not_active Abandoned
- 1991-08-30 WO PCT/CA1991/000308 patent/WO1992004302A1/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4083712A (en) * | 1971-02-19 | 1978-04-11 | Bayer Aktiengesellschaft | Nitrogenous fertilizer compositions |
| US4711659A (en) * | 1986-08-18 | 1987-12-08 | Moore William P | Attrition resistant controlled release fertilizers |
Non-Patent Citations (1)
| Title |
|---|
| CHEMICAL ABSTRACTS, vol. 90, no. 25, 18 June 1979, Columbus, Ohio, US; abstract no. 214508D, TANIHARA, KOICHI: 'Studies on the preparation of guanidinium salts. 1. Formation of guanidinium salts by the reaction of urea with ammonium amidosulfate upon fusion under atmospheric pressure.' page 718 ; SA 50631 030see abstract * |
Also Published As
| Publication number | Publication date |
|---|---|
| AU8405591A (en) | 1992-03-30 |
| GB9018893D0 (en) | 1990-10-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5352265A (en) | Granular urea-based fertilizer | |
| ES2805999T3 (en) | Preparations with enhanced urease inhibitory effect and urea-containing fertilizers containing them | |
| Byrnes et al. | Recent developments on the use of urease inhibitors in the tropics | |
| US4334905A (en) | Agrochemical agents and their use | |
| NO166942B (en) | PROCEDURE FOR THE PREPARATION OF HETRAZEPINS. | |
| CA2506093A1 (en) | A process for the manufacture of sulphur-containing ammonium phosphate fertilizers | |
| MXPA03006029A (en) | Fertilizing agricultural or horticultural substrates, especially for growth of fruit or vegetable crops, by applying water containing nitrogen fertilizer and nitrification inhibitor in the absence of excess water. | |
| AU2016334226B2 (en) | Managing ethylene in plants using a synergistic agricultural formula comprising diacyl or diary urea and at least one metal complex | |
| EP0949221B1 (en) | Composition suitable as liquid fertilizer containing sulphur as potassium tetrathionate | |
| CN1099373A (en) | Plant growth promoter | |
| JPH0515672B2 (en) | ||
| WO1992004302A1 (en) | Guanidine as a nitrogen fertilizer | |
| CN1041713C (en) | Chelate multicomponent mixed microfertilizer and its preparing method | |
| EP0463075B1 (en) | Stabilized urea based fertilizers for foliar application | |
| CN100513361C (en) | fertilizer combination | |
| US20020134124A1 (en) | Diureides and their use | |
| EP0013307B1 (en) | Liquid foliar fertilizer compositions containing water-soluble urea-formaldehyde products | |
| CN110357710A (en) | A kind of Resistance of Wheat To Adversity physiology slow release fertilizer synergist and the preparation method and application thereof | |
| CN101723723A (en) | Preparation method of vermiculite compound fertilizer | |
| AU2021100558A4 (en) | Carbon-coupled stabilized compound fertilizer and preparation method | |
| US4883530A (en) | Use of ammonium syngenite as a slow-acting nitrogen fertilizer | |
| US3453098A (en) | Fertilizer containing biuret free of cyanate ions | |
| US4113462A (en) | Promotion of plant growth with compositions containing a dithiocarbamic acid derivative | |
| JP2000302583A (en) | Slow-release liquid fertilizer | |
| CN114031447A (en) | Liquid fertilizer with strong leaf surface wetting and spreading capability and preparation method thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AT AU BB BG BR CA CH CS DE DK ES FI GB HU JP KP KR LK LU MC MG MN MW NL NO PL RO SD SE SU US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE BF BJ CF CG CH CI CM DE DK ES FR GA GB GN GR IT LU ML MR NL SE SN TD TG |
|
| ENP | Entry into the national phase |
Ref document number: 2090402 Country of ref document: CA Kind code of ref document: A Ref document number: 2090402 Country of ref document: CA |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| 122 | Ep: pct application non-entry in european phase | ||
| NENP | Non-entry into the national phase |
Ref country code: CA |