US7803359B1 - UV-absorbers for ophthalmic lens materials - Google Patents
UV-absorbers for ophthalmic lens materials Download PDFInfo
- Publication number
- US7803359B1 US7803359B1 US12/435,975 US43597509A US7803359B1 US 7803359 B1 US7803359 B1 US 7803359B1 US 43597509 A US43597509 A US 43597509A US 7803359 B1 US7803359 B1 US 7803359B1
- Authority
- US
- United States
- Prior art keywords
- ophthalmic device
- absorber
- device material
- alkyl
- nothing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000463 material Substances 0.000 title claims description 51
- 239000006096 absorbing agent Substances 0.000 title claims description 46
- 150000001875 compounds Chemical class 0.000 claims abstract description 13
- 239000000178 monomer Substances 0.000 claims description 22
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 15
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 14
- 229910052760 oxygen Inorganic materials 0.000 claims description 9
- ILZXXGLGJZQLTR-UHFFFAOYSA-N 2-phenylethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCC1=CC=CC=C1 ILZXXGLGJZQLTR-UHFFFAOYSA-N 0.000 claims description 7
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 claims description 7
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 claims description 6
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 claims description 6
- 125000003161 (C1-C6) alkylene group Chemical group 0.000 claims description 6
- 125000000217 alkyl group Chemical group 0.000 claims description 6
- 239000003431 cross linking reagent Substances 0.000 claims description 5
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 4
- 229910007161 Si(CH3)3 Inorganic materials 0.000 claims description 4
- 125000005037 alkyl phenyl group Chemical group 0.000 claims description 4
- 229910052799 carbon Inorganic materials 0.000 claims description 4
- 229910052736 halogen Inorganic materials 0.000 claims description 4
- 150000002367 halogens Chemical class 0.000 claims description 4
- 229910052717 sulfur Inorganic materials 0.000 claims description 4
- 150000001252 acrylic acid derivatives Chemical class 0.000 claims description 3
- 125000002947 alkylene group Chemical group 0.000 claims description 3
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 claims description 3
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims description 2
- 125000004191 (C1-C6) alkoxy group Chemical group 0.000 claims description 2
- 125000001622 2-naphthyl group Chemical group [H]C1=C([H])C([H])=C2C([H])=C(*)C([H])=C([H])C2=C1[H] 0.000 claims description 2
- CWVFILUMTNJKBN-UHFFFAOYSA-N 2-phenylmethoxyethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCC1=CC=CC=C1 CWVFILUMTNJKBN-UHFFFAOYSA-N 0.000 claims description 2
- DJENTNKTVLSRNS-UHFFFAOYSA-N 3-phenylmethoxypropyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCOCC1=CC=CC=C1 DJENTNKTVLSRNS-UHFFFAOYSA-N 0.000 claims description 2
- IGVCHZAHFGFESB-UHFFFAOYSA-N 4-phenylbutyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCCC1=CC=CC=C1 IGVCHZAHFGFESB-UHFFFAOYSA-N 0.000 claims description 2
- RXPPWNDYCIQFQB-UHFFFAOYSA-N 5-phenylpentyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCCCC1=CC=CC=C1 RXPPWNDYCIQFQB-UHFFFAOYSA-N 0.000 claims description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 claims description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 2
- CBOIHMRHGLHBPB-UHFFFAOYSA-N hydroxymethyl Chemical compound O[CH2] CBOIHMRHGLHBPB-UHFFFAOYSA-N 0.000 claims description 2
- 125000005647 linker group Chemical group 0.000 claims description 2
- 125000001624 naphthyl group Chemical group 0.000 claims description 2
- 229920001296 polysiloxane Polymers 0.000 claims description 2
- 125000001424 substituent group Chemical group 0.000 claims description 2
- 125000003258 trimethylene group Chemical group [H]C([H])([*:2])C([H])([H])C([H])([H])[*:1] 0.000 claims description 2
- 239000000203 mixture Substances 0.000 description 22
- 239000000243 solution Substances 0.000 description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 18
- 238000006243 chemical reaction Methods 0.000 description 17
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 15
- 0 [8*]C1=CC(O[9*])=CC(N2N=C3C=C4O[1*][2*][3*]OC4=CC3=N2)=C1O Chemical compound [8*]C1=CC(O[9*])=CC(N2N=C3C=C4O[1*][2*][3*]OC4=CC3=N2)=C1O 0.000 description 12
- 229940125904 compound 1 Drugs 0.000 description 11
- 238000009472 formulation Methods 0.000 description 11
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 239000003999 initiator Substances 0.000 description 9
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 239000004615 ingredient Substances 0.000 description 7
- -1 2-nitroaniline compound Chemical class 0.000 description 6
- LDAJFLKWQVYIFG-UHFFFAOYSA-N 4,5-dimethoxy-2-nitroaniline Chemical compound COC1=CC(N)=C([N+]([O-])=O)C=C1OC LDAJFLKWQVYIFG-UHFFFAOYSA-N 0.000 description 6
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- 230000005540 biological transmission Effects 0.000 description 6
- 239000011541 reaction mixture Substances 0.000 description 6
- HPSGLFKWHYAKSF-UHFFFAOYSA-N 2-phenylethyl prop-2-enoate Chemical compound C=CC(=O)OCCC1=CC=CC=C1 HPSGLFKWHYAKSF-UHFFFAOYSA-N 0.000 description 5
- LGDHZCLREKIGKJ-UHFFFAOYSA-N 3,4-dimethoxyaniline Chemical compound COC1=CC=C(N)C=C1OC LGDHZCLREKIGKJ-UHFFFAOYSA-N 0.000 description 5
- 239000012964 benzotriazole Substances 0.000 description 5
- 230000000903 blocking effect Effects 0.000 description 5
- 238000000605 extraction Methods 0.000 description 5
- 238000001914 filtration Methods 0.000 description 5
- 238000000034 method Methods 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 4
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 4
- 150000001565 benzotriazoles Chemical class 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 239000012954 diazonium Substances 0.000 description 4
- 150000001989 diazonium salts Chemical class 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- NOBYOEQUFMGXBP-UHFFFAOYSA-N (4-tert-butylcyclohexyl) (4-tert-butylcyclohexyl)oxycarbonyloxy carbonate Chemical compound C1CC(C(C)(C)C)CCC1OC(=O)OOC(=O)OC1CCC(C(C)(C)C)CC1 NOBYOEQUFMGXBP-UHFFFAOYSA-N 0.000 description 3
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 3
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 239000012632 extractable Substances 0.000 description 3
- 125000000555 isopropenyl group Chemical group [H]\C([H])=C(\*)C([H])([H])[H] 0.000 description 3
- VHRYZQNGTZXDNX-UHFFFAOYSA-N methacryloyl chloride Chemical compound CC(=C)C(Cl)=O VHRYZQNGTZXDNX-UHFFFAOYSA-N 0.000 description 3
- SNVLJLYUUXKWOJ-UHFFFAOYSA-N methylidenecarbene Chemical compound C=[C] SNVLJLYUUXKWOJ-UHFFFAOYSA-N 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- XFCMNSHQOZQILR-UHFFFAOYSA-N 2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOC(=O)C(C)=C XFCMNSHQOZQILR-UHFFFAOYSA-N 0.000 description 2
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 2
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 238000006149 azo coupling reaction Methods 0.000 description 2
- 238000009835 boiling Methods 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 238000001723 curing Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- IQPQWNKOIGAROB-UHFFFAOYSA-N isocyanate group Chemical group [N-]=C=O IQPQWNKOIGAROB-UHFFFAOYSA-N 0.000 description 2
- 238000002386 leaching Methods 0.000 description 2
- 238000006396 nitration reaction Methods 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 2
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 238000002235 transmission spectroscopy Methods 0.000 description 2
- 238000000411 transmission spectrum Methods 0.000 description 2
- 238000002371 ultraviolet--visible spectrum Methods 0.000 description 2
- PVCVRLMCLUQGBT-UHFFFAOYSA-N (1-tert-butylcyclohexyl) (1-tert-butylcyclohexyl)oxycarbonyloxy carbonate Chemical compound C1CCCCC1(C(C)(C)C)OC(=O)OOC(=O)OC1(C(C)(C)C)CCCCC1 PVCVRLMCLUQGBT-UHFFFAOYSA-N 0.000 description 1
- XYLWRVUSFZZXFU-UHFFFAOYSA-N (4-tert-butylcyclohexyl)oxyperoxycarbonyl (4-tert-butylcyclohexyl)peroxy carbonate Chemical compound C1CC(C(C)(C)C)CCC1OOOC(=O)OC(=O)OOOC1CCC(C(C)(C)C)CC1 XYLWRVUSFZZXFU-UHFFFAOYSA-N 0.000 description 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 1
- JJBFVQSGPLGDNX-UHFFFAOYSA-N 2-(2-methylprop-2-enoyloxy)propyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OC(C)COC(=O)C(C)=C JJBFVQSGPLGDNX-UHFFFAOYSA-N 0.000 description 1
- DPJCXCZTLWNFOH-UHFFFAOYSA-N 2-nitroaniline Chemical class NC1=CC=CC=C1[N+]([O-])=O DPJCXCZTLWNFOH-UHFFFAOYSA-N 0.000 description 1
- NBOGODDTOPKOHH-UHFFFAOYSA-N 2-tert-butyl-4-(3-hydroxypropoxy)phenol Chemical compound CC(C)(C)C1=CC(OCCCO)=CC=C1O NBOGODDTOPKOHH-UHFFFAOYSA-N 0.000 description 1
- HTWRFCRQSLVESJ-UHFFFAOYSA-N 3-(2-methylprop-2-enoyloxy)propyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCOC(=O)C(C)=C HTWRFCRQSLVESJ-UHFFFAOYSA-N 0.000 description 1
- XOJWAAUYNWGQAU-UHFFFAOYSA-N 4-(2-methylprop-2-enoyloxy)butyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCCOC(=O)C(C)=C XOJWAAUYNWGQAU-UHFFFAOYSA-N 0.000 description 1
- DBCAQXHNJOFNGC-UHFFFAOYSA-N 4-bromo-1,1,1-trifluorobutane Chemical compound FC(F)(F)CCCBr DBCAQXHNJOFNGC-UHFFFAOYSA-N 0.000 description 1
- JHWGFJBTMHEZME-UHFFFAOYSA-N 4-prop-2-enoyloxybutyl prop-2-enoate Chemical compound C=CC(=O)OCCCCOC(=O)C=C JHWGFJBTMHEZME-UHFFFAOYSA-N 0.000 description 1
- SAPGBCWOQLHKKZ-UHFFFAOYSA-N 6-(2-methylprop-2-enoyloxy)hexyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCCCCCOC(=O)C(C)=C SAPGBCWOQLHKKZ-UHFFFAOYSA-N 0.000 description 1
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 description 1
- PASPUADQPHFXBX-UHFFFAOYSA-N C=C(C)C(=O)OCCCC1=CC(N2N=C3C=CC(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 Chemical compound C=C(C)C(=O)OCCCC1=CC(N2N=C3C=CC(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 PASPUADQPHFXBX-UHFFFAOYSA-N 0.000 description 1
- DZIPOJCPKZLMRG-UHFFFAOYSA-N C=C(C)C(=O)OCCCOC1=CC(N2N=C3C=C(CO)C(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 Chemical compound C=C(C)C(=O)OCCCOC1=CC(N2N=C3C=C(CO)C(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 DZIPOJCPKZLMRG-UHFFFAOYSA-N 0.000 description 1
- VDEYGKYGGKVVDS-UHFFFAOYSA-N C=C(C)C(=O)OCCCOC1=CC(N2N=C3C=CC(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 Chemical compound C=C(C)C(=O)OCCCOC1=CC(N2N=C3C=CC(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 VDEYGKYGGKVVDS-UHFFFAOYSA-N 0.000 description 1
- HLTVXJZFIFRXDB-UHFFFAOYSA-N C=C(C)CC1=CC(C)=CC(N2N=C3C=CC=CC3=N2)=C1O Chemical compound C=C(C)CC1=CC(C)=CC(N2N=C3C=CC=CC3=N2)=C1O HLTVXJZFIFRXDB-UHFFFAOYSA-N 0.000 description 1
- NAMSZFOHELZSOX-UHFFFAOYSA-N C=C[Si](C)(C)CCCOC1=CC(N2N=C3C=CC(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 Chemical compound C=C[Si](C)(C)CCCOC1=CC(N2N=C3C=CC(OC)=CC3=N2)=C(O)C(C(C)(C)C)=C1 NAMSZFOHELZSOX-UHFFFAOYSA-N 0.000 description 1
- LAQRRCSARQMIKB-UHFFFAOYSA-I COC1=C(OC)C=C(N)C=C1.COC1=C(OC)C=C(NC(C)=O)C=C1.COC1=C(OC)C=C([N+](=O)[O-])C(N)=C1.COC1=C(OC)C=C([N+](=O)[O-])C(NC(C)=O)=C1.I[V](I)I.I[V]I Chemical compound COC1=C(OC)C=C(N)C=C1.COC1=C(OC)C=C(NC(C)=O)C=C1.COC1=C(OC)C=C([N+](=O)[O-])C(N)=C1.COC1=C(OC)C=C([N+](=O)[O-])C(NC(C)=O)=C1.I[V](I)I.I[V]I LAQRRCSARQMIKB-UHFFFAOYSA-I 0.000 description 1
- GCHAZXGZNMOFSI-UHFFFAOYSA-N COC1=CC(N2N=C3C=C(CO)C(OC)=CC3=N2)=C(O)C=C1 Chemical compound COC1=CC(N2N=C3C=C(CO)C(OC)=CC3=N2)=C(O)C=C1 GCHAZXGZNMOFSI-UHFFFAOYSA-N 0.000 description 1
- PJCFJDWSOCOERX-UHFFFAOYSA-N COC1=CC2=NN(C3=C(O)C(C(C)(C)C)=CC(C)=C3)N=C2C=C1CO Chemical compound COC1=CC2=NN(C3=C(O)C(C(C)(C)C)=CC(C)=C3)N=C2C=C1CO PJCFJDWSOCOERX-UHFFFAOYSA-N 0.000 description 1
- GBJAUSOGMGJPTF-UHFFFAOYSA-N COC1=CC2=NN(C3=C(O)C(C(C)(C)C)=CC(OCCCO)=C3)N=C2C=C1CO Chemical compound COC1=CC2=NN(C3=C(O)C(C(C)(C)C)=CC(OCCCO)=C3)N=C2C=C1CO GBJAUSOGMGJPTF-UHFFFAOYSA-N 0.000 description 1
- MOIKUNVJUHMULY-UHFFFAOYSA-N COC1=CC2=NN(C3=C(O)C=CC(C)=C3)N=C2C=C1CO Chemical compound COC1=CC2=NN(C3=C(O)C=CC(C)=C3)N=C2C=C1CO MOIKUNVJUHMULY-UHFFFAOYSA-N 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- IOVCWXUNBOPUCH-UHFFFAOYSA-N Nitrous acid Chemical compound ON=O IOVCWXUNBOPUCH-UHFFFAOYSA-N 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- HFBMWMNUJJDEQZ-UHFFFAOYSA-N acryloyl chloride Chemical group ClC(=O)C=C HFBMWMNUJJDEQZ-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 239000012965 benzophenone Substances 0.000 description 1
- YHWCPXVTRSHPNY-UHFFFAOYSA-N butan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCC[O-].CCCC[O-].CCCC[O-].CCCC[O-] YHWCPXVTRSHPNY-UHFFFAOYSA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000007334 copolymerization reaction Methods 0.000 description 1
- 239000012043 crude product Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006704 dehydrohalogenation reaction Methods 0.000 description 1
- 238000006193 diazotization reaction Methods 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- STVZJERGLQHEKB-UHFFFAOYSA-N ethylene glycol dimethacrylate Substances CC(=C)C(=O)OCCOC(=O)C(C)=C STVZJERGLQHEKB-UHFFFAOYSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000012065 filter cake Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 239000005457 ice water Substances 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 239000002198 insoluble material Substances 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- FQPSGWSUVKBHSU-UHFFFAOYSA-N methacrylamide Chemical compound CC(=C)C(N)=O FQPSGWSUVKBHSU-UHFFFAOYSA-N 0.000 description 1
- 125000005395 methacrylic acid group Chemical group 0.000 description 1
- VYQNWZOUAUKGHI-UHFFFAOYSA-N monobenzone Chemical compound C1=CC(O)=CC=C1OCC1=CC=CC=C1 VYQNWZOUAUKGHI-UHFFFAOYSA-N 0.000 description 1
- LNOPIUAQISRISI-UHFFFAOYSA-N n'-hydroxy-2-propan-2-ylsulfonylethanimidamide Chemical compound CC(C)S(=O)(=O)CC(N)=NO LNOPIUAQISRISI-UHFFFAOYSA-N 0.000 description 1
- DZPYVOWPTBVRJR-UHFFFAOYSA-N n-(3,4-dimethoxyphenyl)acetamide Chemical compound COC1=CC=C(NC(C)=O)C=C1OC DZPYVOWPTBVRJR-UHFFFAOYSA-N 0.000 description 1
- AJDUTMFFZHIJEM-UHFFFAOYSA-N n-(9,10-dioxoanthracen-1-yl)-4-[4-[[4-[4-[(9,10-dioxoanthracen-1-yl)carbamoyl]phenyl]phenyl]diazenyl]phenyl]benzamide Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C=CC=C2NC(=O)C(C=C1)=CC=C1C(C=C1)=CC=C1N=NC(C=C1)=CC=C1C(C=C1)=CC=C1C(=O)NC1=CC=CC2=C1C(=O)C1=CC=CC=C1C2=O AJDUTMFFZHIJEM-UHFFFAOYSA-N 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 239000012044 organic layer Substances 0.000 description 1
- 230000020477 pH reduction Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 125000000864 peroxy group Chemical group O(O*)* 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000003505 polymerization initiator Substances 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- FBCQUCJYYPMKRO-UHFFFAOYSA-N prop-2-enyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCC=C FBCQUCJYYPMKRO-UHFFFAOYSA-N 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- AOJFQRQNPXYVLM-UHFFFAOYSA-N pyridin-1-ium;chloride Chemical compound [Cl-].C1=CC=[NH+]C=C1 AOJFQRQNPXYVLM-UHFFFAOYSA-N 0.000 description 1
- 239000010453 quartz Substances 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000010288 sodium nitrite Nutrition 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 125000003011 styrenyl group Chemical group [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000001029 thermal curing Methods 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 125000001425 triazolyl group Chemical group 0.000 description 1
- 229940124543 ultraviolet light absorber Drugs 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 239000001043 yellow dye Substances 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D249/00—Heterocyclic compounds containing five-membered rings having three nitrogen atoms as the only ring hetero atoms
- C07D249/16—Heterocyclic compounds containing five-membered rings having three nitrogen atoms as the only ring hetero atoms condensed with carbocyclic rings or ring systems
- C07D249/18—Benzotriazoles
- C07D249/20—Benzotriazoles with aryl radicals directly attached in position 2
Definitions
- This invention is directed to ophthalmic lens materials.
- this invention relates to ultraviolet light absorbers that are suitable for use in ophthalmic lens materials.
- UV light absorbers are known as ingredients for polymeric materials used to make ophthalmic lenses and, in particular, intraocular lenses. UV absorbers are preferably covalently bound to the polymeric network of the lens material instead of simply physically entrapped in the material to prevent the absorber from migrating, phase separating or leaching out of the lens material. Such stability is particularly important for implantable ophthalmic lenses where the leaching of the UV absorber may present both toxicological issues and lead to the loss of UV blocking activity in the implant.
- UV absorbers Numerous copolymerizable benzotriazole, benzophenone and triazine UV absorbers are known. Many of these UV absorbers contain conventional olefinic polymerizable groups, such as methacrylate, acrylate, methacrylamide, acrylamide or styrene groups. Copolymerization with other ingredients in the lens materials, typically with a radical initiator, incorporates the UV absorbers into the resulting polymer chain. Incorporation of additional functional groups on a UV absorber may influence one or more of the UV absorber's UV absorbing properties, solubility or reactivity. If the UV absorber does not have sufficient solubility in the remainder of the ophthalmic lens material ingredients or polymeric lens material, the UV absorber may coalesce into domains that could interact with light and result in decreased optical clarity of the lens.
- polymeric ophthalmic lens materials that incorporate UV absorbers can be found in U.S. Pat. Nos. 5,290,892; 5,331,073 and 5,693,095.
- some ophthalmic lenses In addition to blocking UV light, some ophthalmic lenses also block blue light. See, for example, U.S. Pat. Nos. 5,470,932 and 5,543,504. These lenses block both types of light by using two chromophores: a UV absorber and a yellow dye.
- UV absorbers that are suitable for use in implantable ophthalmic lenses and are capable of blocking not only UV light (400 nm and below) but also blocking at least some light between 400-450 nm.
- the present invention provides UV absorbers that block not only UV light but also light in the 400-450 nm range. These UV absorbers are suitable for use in ophthalmic devices, including contact lenses, and are particularly useful in implantable lenses, such as intraocular lenses (IOLs).
- the UV absorbers of the present invention are copolymerizable with other ingredients in ophthalmic device formulations.
- FIG. 1 shows the UV/VIS spectra of various UV absorbers.
- FIG. 2 shows the UV/VIS spectra of an acrylic IOL material containing the UV absorber of Example 1 (Compound 1) before and after extraction.
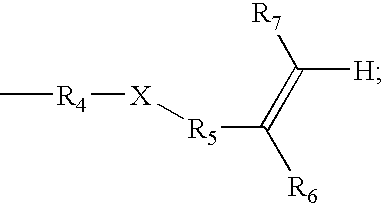
- UV absorbers of the present invention have the structure shown in formula I.
- R 4 is C 1 -C 6 alkylene
- X is O or NR 7 ;
- R 5 is C( ⁇ O) or C 1 -C 6 alkylphenyl
- R 6 is H or methyl
- R 7 is H
- R 8 is C 4 -C 12 t-alkyl.
- R 1 and R 3 methyl
- R 4 is C 2 -C 3 alkylene
- X is O
- R 5 is C( ⁇ O);
- R 6 is H or methyl
- R 7 is H
- R 8 is t-butyl
- reaction scheme 1 works best when R 9 does not contain an ester, carbonate, carbamate, or isocyanate group.
- the polymerizable group containing an ester, carbonate, carbamate, or isocyanate group
- the polymerizable group should be added after the scheme 1 reaction sequence.
- the phenolic reactant (IV) may have an omega-chain hydroxyl alkylene group (e.g., —CH 2 CH 2 OH) in place of the desired R 9 group during the reaction sequence of scheme 1.
- the desired R 9 group could then be added via a dehydrohalogenation reaction using acryloyl- or methacryloyl-chloride, for example.
- the specialty dialkoxy/diaryloxy-substituted 2-nitroanilines denoted as (II) in Scheme 1 above, as well as, the specialty target phenolic compound (IV), may be synthesized by methods and techniques known to those skilled in the art.
- the synthesis of a dimethoxy-substituted 2-nitroaniline is shown in Scheme 2.
- the 4,5-dimethoxy-2-nitroaniline can be prepared by the nitration of 4-amino veratrole (VII, Aldrich Chemical Co., a.k.a. 3,4-dimethoxyaniline) to provide the dimethoxy-substituted ortho-nitroaniline (VIII) starting material needed for the benzotriazole synthesis (Scheme 1).
- the UV absorbers of the present invention are particularly suitable for use in IOLs.
- IOL materials will generally contain from 0.1 to 5% (w/w) of a UV absorber of formula I.
- IOL materials will contain from 0.1 to 2% (w/w) of a UV absorber of the present invention.
- Ophthalmic device materials are prepared by copolymerizing the UV absorbers of the present invention with other ingredients, such as device-forming materials, cross-linking agents, and blue-light blocking chromophores.
- the ophthalmic device materials comprise an acrylic or methacrylic device-forming monomer. More preferably, the device-forming monomers comprise a monomer of formula II:
- Preferred monomers of formula II are those wherein A is H or CH 3 , B is (CH 2 ) m , m is 2-5, Y is nothing or O, w is 0-1, and D is H. Most preferred are 2-phenylethyl methacrylate; 4-phenylbutyl methacrylate; 5-phenylpentyl methacrylate; 2-benzyloxyethyl methacrylate; and 3-benzyloxypropyl methacrylate; and their corresponding acrylates.
- Monomers of formula II are known and can be made by known methods.
- the conjugate alcohol of the desired monomer can be combined in a reaction vessel with methyl methacrylate, tetrabutyl titanate (catalyst), and a polymerization inhibitor such as 4-benzyloxy phenol.
- the vessel can then be heated to facilitate the reaction and distill off the reaction by-products to drive the reaction to completion.
- Alternative synthesis schemes involve adding methacrylic acid to the conjugate alcohol and catalyzing with a carbodiimide or mixing the conjugate alcohol with methacryloyl chloride and a base such as pyridine or triethylamine.
- Device materials generally comprise a total of at least about 75%, preferably at least about 80%, of device-forming monomers.
- the device materials of the present invention generally comprise a cross-linking agent.
- the cross-linking agent used in the device materials of this invention may be any terminally ethylenically unsaturated compound having more than one unsaturated group.
- a preferred cross-linking monomer is CH 2 ⁇ C(CH 3 )C( ⁇ O)O—(CH 2 CH 2 O) p —C( ⁇ O)C(CH 3 ) ⁇ CH 2 where p is such that the number-average molecular weight is about 400, about 600, or about 1000.
- the total amount of the cross-linking component is at least 0.1% by weight and, depending on the identity and concentration of the remaining components and the desired physical properties, can range to about 20% by weight.
- the preferred concentration range for the cross-linking component is 0.1-17% (w/w).
- Suitable polymerization initiators for device materials containing a UV absorber of the present invention include thermal initiators and photoinitiators.
- Preferred thermal initiators include peroxy free-radical initiators, such as t-butyl (peroxy-2-ethyl)hexanoate and di-(tert-butylcyclohexyl) peroxydicarbonate (commercially available as Perkadox® 16 from Akzo Chemicals Inc., Chicago, Ill.). Initiators are typically present in an amount of about 5% (w/w) or less. Because free-radical initiators do not become chemically a part of the polymers formed, the total amount of initiator is customarily not included when determining the amounts of other ingredients.
- the device materials containing a UV absorber of the present invention may also contain a reactive colorant.
- Suitable reactive blue-light absorbing compounds include those described in U.S. Pat. No. 5,470,932. Blue-light absorbers are typically present in an amount from about 0.01-0.5% (weight).
- the IOLs constructed of the materials of the present invention can be of any design capable of being rolled or folded into a small cross section that can fit through a relatively smaller incision.
- the IOLs can be of what is known as a one piece or multipiece design, and comprise optic and haptic components.
- the optic is that portion which serves as the lens.
- the haptics are attached to the optic and hold the optic in its proper place in the eye.
- the optic and haptic(s) can be of the same or different material.
- a multipiece lens is so called because the optic and the haptic(s) are made separately and then the haptics are attached to the optic.
- the optic and the haptics are formed out of one piece of material. Depending on the material, the haptics are then cut, or lathed, out of the material to produce the IOL.
- the materials of the present invention are also suitable for use in other ophthalmic devices, such as contact lenses, keratoprostheses, and corneal inlays or rings.
- reaction schemes 1 and 2 shown above may be applied to this specific synthetic sequence.
- Scheme 2 10 grams (65.28 mmoles) of 4-amino veratrole (Scheme 2, structure VII, a.k.a. 3,4-dimethoxyaniline, Aldrich Chemical Co.) was dissolved in 20 grams of glacial acetic acid in a 100 mL round bottomed flask. To the flask was added 22 grams of ice followed by the dropwise addition of 1.1 equivalents of acetic anhydride (7.3 grams, Aldrich Chemical Co.) with stirring via an addition funnel over a 30 minute time interval.
- Scheme 2 10 grams (65.28 mmoles) of 4-amino veratrole (Scheme 2, structure VII, a.k.a. 3,4-dimethoxyaniline, Aldrich Chemical Co.) was dissolved in 20 grams of glacial acetic acid in a 100 mL round bottomed flask. To the flask was added 22 grams of ice followed by the
- the reaction to make the acetamide intermediate of 4-aminoveratrole occurred with the evolution of heat after which the reaction flask was placed into a water bath and heated for an hour at 55° C. The stirring was continued overnight.
- the 4-acetamidoveratrole product was not isolated, but the next reaction step was continued by the addition of 5.9 grams of nitric acid in 8 mL of water and the reaction was cooled to 15° C. in an ice-water bath. As the nitration reaction preceded the purple colored reaction solution turned to a red-orange color.
- the reaction flask was cooled in a refrigerator overnight and a red-orange powder precipitated from the solution.
- the solid was filtered off and washed with 100 mL of cold water and air dried on the filter.
- the solid from the previous step was placed into a 100 mL round bottomed flask and 50 mL of water and a solution of 5.9 grams of potassium hydroxide (1.5 equivalents) in 14 mL of water was added to the flask.
- a reflux condenser was attached to the flask and the mixture was heated to boiling and refluxed overnight. From the reaction flask was cooled in ice and an orange solid precipitated from solution.
- the solid was filtered off, washed with 200 mL of cold water, and dried in air and overnight in a vacuum oven at 50° C. to obtain 10.0 grams (50.5 mmoles) of 4,5-dimethoxy-2-nitroaniline (VIII), 77% yield, and mp 197-199° C.
- reaction mixture was kept below 0° C.
- the reaction mixture was further stirred below 0° C. for 2 hours, then allowed to warm up to room temperature.
- the nitro azo intermediate was isolated by acidification with hydrochloric acid, filtration, and washing with water, then used without further purification.
- the nitro azo intermediate above from Section b was dissolved in 150 mL of reagent ethanol in a 500 mL round bottomed flask equipped with a magnetic stirbar.
- a glucose solution (18.0 g, (100 mmoles) in 150 mL of aqueous 2N NaOH) was added dropwise to the nitro azo intermediate solution under nitrogen, at room temperature.
- the reaction temperature was kept below 30° C. by means of a water bath.
- the mixture was stirred overnight.
- Freshly activated (acid washed) zinc dust (16.5 grams) was added to the homogeneous reaction mixture.
- the mixture was stirred at room temperature for 2 hours and heated with a water bath at 50° C. for 1 hour.
- the resulting solution was filtered to remove a small amount of insoluble material and diluted with 40 mL of methanol.
- the solution was cooled slowly to room temperature, then in a refrigerator and finally placed in a freezer where the temperature was lowered to about ⁇ 5° C.
- the resulting crystals were separated by filtration, washed with 35 mL of cold methanol/methylene chloride (90/10 v/v) and dried in under vacuum to give 6.2 grams of >98% pure product (HPLC).
- Solutions containing from 1.70 to as much as 1.85% by weight of the benzotriazole compounds listed in Table 1 below were prepared in either chloroform (CHCl 3 ) or dichloromethane (CH 2 Cl 2 ). The solutions were prepared by dissolving about 0.018 grams of UV absorber into about 0.982 grams of solvent by weighing to an accuracy of ⁇ 0.01 mg. The UV-visible transmission spectrum of each solution was measured. The measurement was performed from 850 to 250 nm in 1-mm quartz cuvettes using a Perkin-Elmer Lambda 35 UV-Visible Spectrophotometer. The results are shown in FIG. 1 . From each spectrum, the wavelengths for the 1% T and 10% T cutoff were determined and those values are listed in Table 2.
- a monomer diluent formulation consisting of 2-phenylethyl acrylate (PEA), 2-phenylethyl methacrylate (PEMA), and 1,4-butanediol diacrylate (BDDA) was prepared by mixing the three monomers together in the proportions of 65:30:3.2 parts by weight.
- Formulations containing 1.8, 2.4 and 3.0% of Compound 1 was prepared by dissolving 0.036, 0.048, 0.060 grams of Compound 1, weighed to an accuracy of ⁇ 0.01 mg, into the PEA/PEMA/BDDA monomer diluent to make 2 grams of each formulation.
- a comparison formulation containing 1.8% of ortho-methallyl Tinuvin P (Compound D) was prepared by dissolving 0.036 grams of Compound D into 1.968 grams of the PEA/PEMA/BDDA monomer diluent. Just prior to curing, to each formulation was added 1.8% bis-(4-tert-butylcyclohexylperoxy) dicarbonate (Perkadox-16, Akzo Corp.) initiator, by dissolving approximately 0.020 grams into each formulation (1.0%) and mixing on a vortex mixer.
- a control formulation that did not contain any UV absorber was prepared by dissolving 1.0% of the Perkadox 16 initiator into the PEA/PEMA/BDDA monomer diluent.
- each formulation was passed through a 0.45 ⁇ m membrane syringe filter and purged with nitrogen. Finally, each formulation was cast into polypropylene molds to form 1 ⁇ 2-cm ⁇ ⁇ 1-mm rectangular films by thermal curing at 70° C. for 7 hours, followed by 7 hours at 100° C. in a programmable temperature oven (1000 Halfo Series, VWR Scientific Corp.). The films were demolded and 3 representative samples from each group were weighted and 2 were analyzed by UV-Visible transmission spectroscopy. The films were placed into polypropylene tissue capsules, Soxhlet extracted with acetone, and slowly dried in air and at 60° C. in a vacuum oven to remove residual acetone.
- a programmable temperature oven 1000 Halfo Series, VWR Scientific Corp.
- UV-Visible analysis was performed from 300-800 nm using a Perkin-Elmer Lambda 35 instrument equipped with a Lab Sphere RSA-PE-20 integrating sphere.
- the UV absorber Compound 1 exhibited inhibitory character with the 3.0% formulation not curing to a solid material and increased extractables in going from 1.8 to 2.4% Compound 1 concentration (Table 3). Nonetheless, this experiment verified the presence of the polymerizable methacrylate group in the Compound 1 structure given the minimal change between the before and after extraction for the 1 and 10% transmission cutoff wavelengths in the 1.8 and 2.4% Compound 1 materials. In comparison to Compound D, Compound 1 shows very good incorporation into the acrylic lens material on free radical polymerization (Table 3). As well, the transmission spectra before and after extraction for these materials are nearly identical ( FIG. 2 ).
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
Abstract
Description
-
- R1 and R3 independently=H; C1-C12 alkyl, optionally substituted; C1-C12 cycloalkyl, optionally substituted; phenyl, optionally substituted; or naphthyl; or R1 and R3 are linked through the optional (as designated by the dashed bonds) linking group R2 where R2 is a C1-C6 alkylene, optionally substituted; or R1, R2, and R3 taken together form an optionally substituted 1,2-phenyl, 1,2-naphthyl, or 2,3-naphthyl, wherein, in each case, the optional substituents are independently C1-C6 alkyl, C1-C6 alkoxy, OH, —Si(CH3)3, halogen, —(CH2CH2O)n—R10, or —(CH2CH(CH3)O)nR10;
- R10=H, —Si(CH3)3, or C1-C6 alkyl;
- R8=H or C1-C12 alkyl; and
- R9=H; C1-C12 alkyl, optionally substituted with OH; or
-
- R4=C1-C12 alkylene, (CH2CH2O)n, (CH2CH(CH3)O)n, or CH2CH2CH2(Si(CH3)2O)mSi(CH3)2CH2CH2CH2;
- X=nothing, O, NR7, or S, provided that if R4 is (CH2CH2O)n, or (CH2CH(CH3)O)n then X is nothing;
- R5=nothing, C(═O), C(═O)CjH2j, C1-C6 alkylene, phenyl, or C1-C6 alkylphenyl; C(═O)O, C(═O)OCjH2j, C(═O)NR7, C(═O)NR7CjH2j, C(═S)O, C(═S)OCjH2j, C(═S)NR7, C(═S)NR7CjH2j,
- n=2-10;
- j=1-6;
- m=1-9;
- R6=H or methyl; and
- R7=H, C1-C6 alkyl, or phenyl.
Preferred compounds of formula (I) are those wherein:
-
- where in formula [II]:
- A is H, CH3, CH2CH3, or CH2OH;
- B is (CH2)m or [O(CH2)2]Z;
- C is (CH2)w;
- m is 2-6;
- z is 1-10;
- Y is nothing, O, S, or NR′, provided that if Y is O, S, or NR′, then B is (CH2)m;
- R′ is H, CH3, Cn′H2n′+1 (n′=1-10), iso-OC3H7, C6H5, or CH2C6H5;
- w is 0-6, provided that m+w≦8; and
- D is H, C1-C4 alkyl, C1-C4 alkoxy, C6H5, CH2C6H5 or halogen.
| TABLE 2 |
| UV-Visible transmission data for solutions of benzotriazole |
| compounds |
| UV | ||||
| Absorber | Molecular | Estimated | Transmission cutoff | |
| Com- | Wt. (MW), | Conc. | Molar conc | Wavelength in nm |
| pound | mg/mmole | (wt %) | nmole/mL, |
1% T | 10% T |
| A | 285.3 | 1.78 | 0.0926 | 391.0 | 395.0 |
| B | 341.4 | 1.73 | 0.0672 | 394.5 | 398.0 |
| C | 423.5 | 1.77 | 0.0620 | 397.0 | 400.5 |
| D | 279.3 | 1.74 | 0.0925 | 398.0 | 401.5 |
| 2 | 301.3 | 1.82 | 0.0896 | 410.0 | 414.5 |
| 1 | 453.5 | 1.73 | 0.0566 | 416.0 | 420.5 |
| 3 | 401.5 | 1.77 | 0.0654 | 417.5 | 421.5 |
| E | 439.5 | 1.73 | 0.0584 | 421.5 | 426.0 |
| E | 439.5 | 1.85 | 0.0625 | 422.0 | 426.5 |
| F | 411.5 | 1.81 | 0.0653 | 424.5 | 429.5 |
| TABLE 3 |
| UV-Visible Transmission Cutoffs and Extractables for |
| Acrylic Lens Material Slabs Cured with 1.0% Perkadox 16 |
| Wavelength (nm) for | % Total | |||
| UV Absorber | B/A | Transmission Cutoff | Extractables |
| Compound | Conc. | Extraction | 1% T | 10% | AVE | SD | ||
| 1 | 1.80 | Before | 412.0 | 416.5 | 1.18 | 0.431 |
| After | 411.5 | 416.5 | ||||
| 1 | 2.40 | Before | 413.5 | 418.5 | 4.21 | 2.068 |
| After | 413.5 | 418.0 |
| 1 | 3.00 | Material did not cure | N/A | N/A |
| D | 1.80 | Before | 396.5 | 401.0 | 2.05 | 1.369 |
| After | 395.5 | 400.0 | ||||
| None | 0 | Before | 277.5 | 284.5 | 0.22 | 0.058 |
| After | 278.0 | 285.5 | ||||
Claims (18)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/435,975 US7803359B1 (en) | 2008-05-06 | 2009-05-05 | UV-absorbers for ophthalmic lens materials |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US5081108P | 2008-05-06 | 2008-05-06 | |
| US12/435,975 US7803359B1 (en) | 2008-05-06 | 2009-05-05 | UV-absorbers for ophthalmic lens materials |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US7803359B1 true US7803359B1 (en) | 2010-09-28 |
Family
ID=42753112
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/435,975 Active US7803359B1 (en) | 2008-05-06 | 2009-05-05 | UV-absorbers for ophthalmic lens materials |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US7803359B1 (en) |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8323631B2 (en) | 2008-07-15 | 2012-12-04 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| US8475691B2 (en) | 2011-08-15 | 2013-07-02 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| US8585938B1 (en) | 2012-03-30 | 2013-11-19 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| WO2015072991A1 (en) * | 2013-11-14 | 2015-05-21 | Novartis Ag | Uv-absorbers for ophthalmic lens materials |
| WO2015170278A1 (en) | 2014-05-07 | 2015-11-12 | Tubitak | A formulation and lens manufacturing process for the production of intraocular lens (iol) |
| US9594188B2 (en) | 2011-12-06 | 2017-03-14 | University Of Florida Research Foundation, Inc. | UV blocker loaded contact lenses |
| WO2017145024A1 (en) | 2016-02-22 | 2017-08-31 | Novartis Ag | Uv-absorbing vinylic monomers and uses thereof |
| EP3236296A1 (en) | 2016-04-21 | 2017-10-25 | ESSILOR INTERNATIONAL (Compagnie Générale d'Optique) | Optical material comprising a red-shifted benzotriazole uv absorber |
| CN107955007A (en) * | 2017-11-17 | 2018-04-24 | 南京工业大学连云港工业技术研究院 | The synthetic method of ultraviolet absorber UV-326 |
| CN109535092A (en) * | 2018-12-24 | 2019-03-29 | 温州大学 | A kind of preparation method of benzotriazole analog derivative |
| US10322993B2 (en) | 2015-12-02 | 2019-06-18 | Novartis Ag | Water-soluble UV-absorbing compounds and uses thereof |
| JP2019210289A (en) * | 2018-06-05 | 2019-12-12 | エバーライト ケミカル インダストリアル コーポレイション | Novel benzotriazole uv absorber with red shift and use thereof |
| US10526296B2 (en) | 2017-06-30 | 2020-01-07 | Johnson & Johnson Vision Care, Inc. | Hydroxyphenyl naphthotriazoles as polymerizable blockers of high energy light |
| US10723732B2 (en) | 2017-06-30 | 2020-07-28 | Johnson & Johnson Vision Care, Inc. | Hydroxyphenyl phenanthrolines as polymerizable blockers of high energy light |
| US10752720B2 (en) | 2017-06-26 | 2020-08-25 | Johnson & Johnson Vision Care, Inc. | Polymerizable blockers of high energy light |
| US10935695B2 (en) | 2018-03-02 | 2021-03-02 | Johnson & Johnson Vision Care, Inc. | Polymerizable absorbers of UV and high energy visible light |
| US11543683B2 (en) | 2019-08-30 | 2023-01-03 | Johnson & Johnson Vision Care, Inc. | Multifocal contact lens displaying improved vision attributes |
| WO2023228055A1 (en) | 2022-05-23 | 2023-11-30 | Alcon Inc. | Uv/hevl-filtering contact lenses |
| WO2023228054A1 (en) | 2022-05-23 | 2023-11-30 | Alcon Inc. | Method for making hevl-filtering contact lenses |
| WO2024074814A1 (en) | 2022-10-04 | 2024-04-11 | Sublino Limited | Composition comprising a functionalised dye and a diallylamine comonomer and use |
| US11958824B2 (en) | 2019-06-28 | 2024-04-16 | Johnson & Johnson Vision Care, Inc. | Photostable mimics of macular pigment |
| US11993037B1 (en) | 2018-03-02 | 2024-05-28 | Johnson & Johnson Vision Care, Inc. | Contact lens displaying improved vision attributes |
| WO2024236526A2 (en) | 2023-05-18 | 2024-11-21 | Alcon Inc. | High-energy-violet-absorbing vinylic monomers |
| WO2024236524A1 (en) | 2023-05-18 | 2024-11-21 | Alcon Inc. | Uv/hevl-filtering silicone hydrogel contact lenses |
| US12178938B2 (en) | 2018-12-10 | 2024-12-31 | Seed Co., Ltd. | UV absorbing ocular lens |
| WO2025032504A2 (en) | 2023-08-07 | 2025-02-13 | Alcon Inc. | Uv/hevl-filtering silicone hydrogel contact lenses |
Citations (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4716234A (en) | 1986-12-01 | 1987-12-29 | Iolab Corporation | Ultraviolet absorbing polymers comprising 2-(2'-hydroxy-5'-acryloyloxyalkoxyphenyl)-2H-benzotriazole |
| US4803254A (en) | 1987-03-11 | 1989-02-07 | Iolab Corporation | Vinylsilylalkoxy arylbenzotriazole compounds and UV absorbing compositions made therefrom |
| US5290892A (en) | 1990-11-07 | 1994-03-01 | Nestle S.A. | Flexible intraocular lenses made from high refractive index polymers |
| US5331073A (en) | 1992-11-09 | 1994-07-19 | Allergan, Inc. | Polymeric compositions and intraocular lenses made from same |
| US5455152A (en) * | 1994-09-27 | 1995-10-03 | Eastman Kodak Company | Benzotriazole based UV absorbing monomers and photographic elements containing polymers formed from them |
| US5470932A (en) | 1993-10-18 | 1995-11-28 | Alcon Laboratories, Inc. | Polymerizable yellow dyes and their use in opthalmic lenses |
| US5637726A (en) | 1993-04-22 | 1997-06-10 | Wesley-Jessen Corporation | UV-absorbing benzotriazoles having a styrene group |
| US5693095A (en) | 1995-06-07 | 1997-12-02 | Alcon Laboratories, Inc. | High refractive index ophthalmic lens materials |
| US6166218A (en) | 1996-11-07 | 2000-12-26 | Ciba Specialty Chemicals Corporation | Benzotriazole UV absorbers having enhanced durability |
| US6528602B1 (en) | 1999-09-07 | 2003-03-04 | Alcon Universal Ltd. | Foldable ophthalmic and otorhinolaryngological device materials |
| US6806337B2 (en) | 2002-07-16 | 2004-10-19 | Alcon | Ophthalmic and otorhinolaryngological device materials |
| US6846897B2 (en) | 2001-11-02 | 2005-01-25 | Bausch And Lomb, Inc. | High refractive index aromatic-based silyl monomers |
| US6852793B2 (en) | 2002-06-19 | 2005-02-08 | Bausch & Lomb Incorporated | Low water content, high refractive index, flexible, polymeric compositions |
| JP2005053058A (en) | 2003-08-04 | 2005-03-03 | Fuji Photo Film Co Ltd | Optical information recording medium and information recording method |
| US6872793B1 (en) | 2003-08-07 | 2005-03-29 | Alcon, Inc. | Ophthalmic and otorhinolaryngological device materials |
| CN1727338A (en) | 2004-12-27 | 2006-02-01 | 常州华钛化学有限公司 | Benzotriazole compound with alkenyl ester structure and preparation method thereof |
| US7037954B2 (en) | 2000-03-22 | 2006-05-02 | Menicon Co., Ltd. | Ocular lens material |
| US7067602B2 (en) | 2003-11-05 | 2006-06-27 | Benz Research And Development Corporation | Materials for making hydrophobic intraocular lens |
| US7101949B2 (en) | 2001-11-02 | 2006-09-05 | Bausch & Lomb Incorporated | High refractive index polymeric siloxysilane compositions |
| US20060252850A1 (en) | 2005-05-04 | 2006-11-09 | Bausch & Lomb Incorporated | Radiation-absorbing polymeric materials and ophthalmic devices comprising same |
| US20070092830A1 (en) | 2005-10-24 | 2007-04-26 | Bausch & Lomb Incorporated | Polymeric radiation-absorbing materials and ophthalmic devices comprising same |
| US20070092831A1 (en) | 2005-10-24 | 2007-04-26 | Bausch & Lomb Incorporated | Radiation-absorbing polymeric materials and ophthalmic devices comprising same |
| US7326423B2 (en) | 2004-11-22 | 2008-02-05 | Advanced Medical Optics, Inc. | Copolymerizable azo compounds and articles containing them |
| EP1033590B1 (en) | 1999-03-02 | 2008-05-07 | Menicon Co., Ltd. | Ocular lens material |
| WO2008109624A2 (en) | 2007-03-05 | 2008-09-12 | Benz Research And Development Corp. | Light filters comprising a naturally occurring chromophore and derivatives thereof |
| US20080266519A1 (en) | 2007-04-30 | 2008-10-30 | Alcon Research, Ltd. | Uv-absorbers for ophthalmic lens materials |
| JP2009013148A (en) | 2007-07-03 | 2009-01-22 | Shipro Kasei Kaisha Ltd | Chemical modification to bis type structure by adipoyl dichloride, isophthaloyl dichloride or terephthaloyl dichloride of benzotriazole-based ultraviolet light absorbent having hydroxymethyl group |
| US20090043007A1 (en) | 2007-08-09 | 2009-02-12 | Alcon, Inc. | Ophthalmic lens materials containing chromophores that absorb both uv and short wavelength visible light |
| US20090043105A1 (en) | 2007-08-09 | 2009-02-12 | Alcon, Inc. | Ophthalmic lens materials containing chromophores that absorb both uv and short wavelength visible light |
-
2009
- 2009-05-05 US US12/435,975 patent/US7803359B1/en active Active
Patent Citations (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4716234A (en) | 1986-12-01 | 1987-12-29 | Iolab Corporation | Ultraviolet absorbing polymers comprising 2-(2'-hydroxy-5'-acryloyloxyalkoxyphenyl)-2H-benzotriazole |
| US4803254A (en) | 1987-03-11 | 1989-02-07 | Iolab Corporation | Vinylsilylalkoxy arylbenzotriazole compounds and UV absorbing compositions made therefrom |
| US5290892A (en) | 1990-11-07 | 1994-03-01 | Nestle S.A. | Flexible intraocular lenses made from high refractive index polymers |
| US5331073A (en) | 1992-11-09 | 1994-07-19 | Allergan, Inc. | Polymeric compositions and intraocular lenses made from same |
| US5637726A (en) | 1993-04-22 | 1997-06-10 | Wesley-Jessen Corporation | UV-absorbing benzotriazoles having a styrene group |
| US5470932A (en) | 1993-10-18 | 1995-11-28 | Alcon Laboratories, Inc. | Polymerizable yellow dyes and their use in opthalmic lenses |
| US5543504A (en) | 1993-10-18 | 1996-08-06 | Alcon Laboratories, Inc. | Polymerizable yellow dyes and their use in ophthalmic lenses |
| US5455152A (en) * | 1994-09-27 | 1995-10-03 | Eastman Kodak Company | Benzotriazole based UV absorbing monomers and photographic elements containing polymers formed from them |
| US5693095A (en) | 1995-06-07 | 1997-12-02 | Alcon Laboratories, Inc. | High refractive index ophthalmic lens materials |
| US6166218A (en) | 1996-11-07 | 2000-12-26 | Ciba Specialty Chemicals Corporation | Benzotriazole UV absorbers having enhanced durability |
| EP1033590B1 (en) | 1999-03-02 | 2008-05-07 | Menicon Co., Ltd. | Ocular lens material |
| US6528602B1 (en) | 1999-09-07 | 2003-03-04 | Alcon Universal Ltd. | Foldable ophthalmic and otorhinolaryngological device materials |
| US7037954B2 (en) | 2000-03-22 | 2006-05-02 | Menicon Co., Ltd. | Ocular lens material |
| US6846897B2 (en) | 2001-11-02 | 2005-01-25 | Bausch And Lomb, Inc. | High refractive index aromatic-based silyl monomers |
| US7101949B2 (en) | 2001-11-02 | 2006-09-05 | Bausch & Lomb Incorporated | High refractive index polymeric siloxysilane compositions |
| US6852793B2 (en) | 2002-06-19 | 2005-02-08 | Bausch & Lomb Incorporated | Low water content, high refractive index, flexible, polymeric compositions |
| US6806337B2 (en) | 2002-07-16 | 2004-10-19 | Alcon | Ophthalmic and otorhinolaryngological device materials |
| JP2005053058A (en) | 2003-08-04 | 2005-03-03 | Fuji Photo Film Co Ltd | Optical information recording medium and information recording method |
| US6872793B1 (en) | 2003-08-07 | 2005-03-29 | Alcon, Inc. | Ophthalmic and otorhinolaryngological device materials |
| US7067602B2 (en) | 2003-11-05 | 2006-06-27 | Benz Research And Development Corporation | Materials for making hydrophobic intraocular lens |
| US7326423B2 (en) | 2004-11-22 | 2008-02-05 | Advanced Medical Optics, Inc. | Copolymerizable azo compounds and articles containing them |
| CN1727338A (en) | 2004-12-27 | 2006-02-01 | 常州华钛化学有限公司 | Benzotriazole compound with alkenyl ester structure and preparation method thereof |
| US20060252850A1 (en) | 2005-05-04 | 2006-11-09 | Bausch & Lomb Incorporated | Radiation-absorbing polymeric materials and ophthalmic devices comprising same |
| US20070092830A1 (en) | 2005-10-24 | 2007-04-26 | Bausch & Lomb Incorporated | Polymeric radiation-absorbing materials and ophthalmic devices comprising same |
| US20070092831A1 (en) | 2005-10-24 | 2007-04-26 | Bausch & Lomb Incorporated | Radiation-absorbing polymeric materials and ophthalmic devices comprising same |
| WO2008109624A2 (en) | 2007-03-05 | 2008-09-12 | Benz Research And Development Corp. | Light filters comprising a naturally occurring chromophore and derivatives thereof |
| US20080242818A1 (en) | 2007-03-05 | 2008-10-02 | Benz Research And Development Corp. | Light filters comprising a naturally occurring chromophore and derivatives thereof |
| US20080266519A1 (en) | 2007-04-30 | 2008-10-30 | Alcon Research, Ltd. | Uv-absorbers for ophthalmic lens materials |
| JP2009013148A (en) | 2007-07-03 | 2009-01-22 | Shipro Kasei Kaisha Ltd | Chemical modification to bis type structure by adipoyl dichloride, isophthaloyl dichloride or terephthaloyl dichloride of benzotriazole-based ultraviolet light absorbent having hydroxymethyl group |
| US20090043007A1 (en) | 2007-08-09 | 2009-02-12 | Alcon, Inc. | Ophthalmic lens materials containing chromophores that absorb both uv and short wavelength visible light |
| US20090043105A1 (en) | 2007-08-09 | 2009-02-12 | Alcon, Inc. | Ophthalmic lens materials containing chromophores that absorb both uv and short wavelength visible light |
Non-Patent Citations (1)
| Title |
|---|
| Takakis, et al., "Preparation of Benzofuroxans and Benzofurazans of 2,3,4,5-Tetrahydrobenzo[b][1.4]dioxocin and Related Compounds," J. Heterocyclic Chem., 1990, pp. 177-181, vol. 27. |
Cited By (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8425891B2 (en) | 2008-07-15 | 2013-04-23 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| US8323631B2 (en) | 2008-07-15 | 2012-12-04 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| US8475691B2 (en) | 2011-08-15 | 2013-07-02 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| US9885886B2 (en) | 2011-12-06 | 2018-02-06 | University Of Florida Research Foundation, Inc. | UV blocker loaded contact lenses |
| US9594188B2 (en) | 2011-12-06 | 2017-03-14 | University Of Florida Research Foundation, Inc. | UV blocker loaded contact lenses |
| US8585938B1 (en) | 2012-03-30 | 2013-11-19 | Novartis Ag | UV-absorbers for ophthalmic lens materials |
| WO2015072991A1 (en) * | 2013-11-14 | 2015-05-21 | Novartis Ag | Uv-absorbers for ophthalmic lens materials |
| WO2015170278A1 (en) | 2014-05-07 | 2015-11-12 | Tubitak | A formulation and lens manufacturing process for the production of intraocular lens (iol) |
| US10351637B2 (en) | 2014-05-07 | 2019-07-16 | Tubitak | Formulation and lens manufacturing process for the production of intraocular lens (IOL) |
| US10472319B2 (en) * | 2015-12-02 | 2019-11-12 | Novartis Ag | Water-soluble UV-absorbing compounds and uses thereof |
| US10322993B2 (en) | 2015-12-02 | 2019-06-18 | Novartis Ag | Water-soluble UV-absorbing compounds and uses thereof |
| US10254567B2 (en) | 2016-02-22 | 2019-04-09 | Novartis Ag | UV-absorbing vinylic monomers and uses thereof |
| WO2017145024A1 (en) | 2016-02-22 | 2017-08-31 | Novartis Ag | Uv-absorbing vinylic monomers and uses thereof |
| JP2019515337A (en) * | 2016-04-21 | 2019-06-06 | エシロール アテルナジオナール | Optical material comprising a redshifted benzotriazole UV absorber |
| EP3236296A1 (en) | 2016-04-21 | 2017-10-25 | ESSILOR INTERNATIONAL (Compagnie Générale d'Optique) | Optical material comprising a red-shifted benzotriazole uv absorber |
| US10838111B2 (en) | 2016-04-21 | 2020-11-17 | Essilor International | Optical material comprising a red-shifted benzotriazole UV absorber |
| WO2017182639A1 (en) | 2016-04-21 | 2017-10-26 | Essilor International (Compagnie Générale d'Optique) | Optical material comprising a red-shifted benzotriazole uv absorber |
| US10752720B2 (en) | 2017-06-26 | 2020-08-25 | Johnson & Johnson Vision Care, Inc. | Polymerizable blockers of high energy light |
| US10975040B2 (en) | 2017-06-30 | 2021-04-13 | Johnson & Johnson Vision Care, Inc. | Hydroxyphenyl naphthotriazoles as polymerizable blockers of high energy light |
| US10526296B2 (en) | 2017-06-30 | 2020-01-07 | Johnson & Johnson Vision Care, Inc. | Hydroxyphenyl naphthotriazoles as polymerizable blockers of high energy light |
| US10723732B2 (en) | 2017-06-30 | 2020-07-28 | Johnson & Johnson Vision Care, Inc. | Hydroxyphenyl phenanthrolines as polymerizable blockers of high energy light |
| CN107955007A (en) * | 2017-11-17 | 2018-04-24 | 南京工业大学连云港工业技术研究院 | The synthetic method of ultraviolet absorber UV-326 |
| US11820899B2 (en) | 2018-03-02 | 2023-11-21 | Johnson & Johnson Vision Care, Inc. | Polymerizable absorbers of UV and high energy visible light |
| US10935695B2 (en) | 2018-03-02 | 2021-03-02 | Johnson & Johnson Vision Care, Inc. | Polymerizable absorbers of UV and high energy visible light |
| US11993037B1 (en) | 2018-03-02 | 2024-05-28 | Johnson & Johnson Vision Care, Inc. | Contact lens displaying improved vision attributes |
| JP2019210289A (en) * | 2018-06-05 | 2019-12-12 | エバーライト ケミカル インダストリアル コーポレイション | Novel benzotriazole uv absorber with red shift and use thereof |
| US12178938B2 (en) | 2018-12-10 | 2024-12-31 | Seed Co., Ltd. | UV absorbing ocular lens |
| CN109535092B (en) * | 2018-12-24 | 2021-05-11 | 温州大学 | Preparation method of benzotriazole derivatives |
| CN109535092A (en) * | 2018-12-24 | 2019-03-29 | 温州大学 | A kind of preparation method of benzotriazole analog derivative |
| US11958824B2 (en) | 2019-06-28 | 2024-04-16 | Johnson & Johnson Vision Care, Inc. | Photostable mimics of macular pigment |
| US11543683B2 (en) | 2019-08-30 | 2023-01-03 | Johnson & Johnson Vision Care, Inc. | Multifocal contact lens displaying improved vision attributes |
| WO2023228054A1 (en) | 2022-05-23 | 2023-11-30 | Alcon Inc. | Method for making hevl-filtering contact lenses |
| WO2023228055A1 (en) | 2022-05-23 | 2023-11-30 | Alcon Inc. | Uv/hevl-filtering contact lenses |
| WO2024074814A1 (en) | 2022-10-04 | 2024-04-11 | Sublino Limited | Composition comprising a functionalised dye and a diallylamine comonomer and use |
| WO2024236526A2 (en) | 2023-05-18 | 2024-11-21 | Alcon Inc. | High-energy-violet-absorbing vinylic monomers |
| WO2024236524A1 (en) | 2023-05-18 | 2024-11-21 | Alcon Inc. | Uv/hevl-filtering silicone hydrogel contact lenses |
| WO2025032504A2 (en) | 2023-08-07 | 2025-02-13 | Alcon Inc. | Uv/hevl-filtering silicone hydrogel contact lenses |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7803359B1 (en) | UV-absorbers for ophthalmic lens materials | |
| US8425891B2 (en) | UV-absorbers for ophthalmic lens materials | |
| US8262947B2 (en) | UV/visible light absorbers for ophthalmic lens materials | |
| KR101592448B1 (en) | Uv/visible light absorbers for ophthalmic lens materials | |
| US8207244B2 (en) | Visible light absorbers for ophthalmic lens materials | |
| US8585938B1 (en) | UV-absorbers for ophthalmic lens materials | |
| EP2176243B1 (en) | Ophthalmic lens materials containing chromophores that absorb both uv and short wavelength visible light | |
| AU2011205622B2 (en) | Visible light absorbers for ophthalmic lens materials | |
| CA2694909A1 (en) | Ophthalmic lens materials containing chromophores that absorb both uv and short wavelength visible light | |
| CA2901817A1 (en) | Uv-absorbers for ophthalmic lens materials | |
| US8475691B2 (en) | UV-absorbers for ophthalmic lens materials | |
| TW201518282A (en) | UV-absorbers for ophthalmic lens materials |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ALCON, INC., SWITZERLAND Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:JINKERSON, DAVID L.;WEINSCHENK, JOSEPH I., III;DEAN, W. DENNIS;REEL/FRAME:022641/0660 Effective date: 20090504 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| AS | Assignment |
Owner name: NOVARTIS AG, SWITZERLAND Free format text: MERGER;ASSIGNOR:ALCON, INC.;REEL/FRAME:026376/0076 Effective date: 20110408 |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 8TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1552) Year of fee payment: 8 |
|
| AS | Assignment |
Owner name: ALCON INC., SWITZERLAND Free format text: CONFIRMATORY DEED OF ASSIGNMENT EFFECTIVE APRIL 8, 2019;ASSIGNOR:NOVARTIS AG;REEL/FRAME:051454/0788 Effective date: 20191111 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 12TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1553); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment: 12 |