US3649879A - Electrolytic color-indicating timer - Google Patents
Electrolytic color-indicating timer Download PDFInfo
- Publication number
- US3649879A US3649879A US64411A US3649879DA US3649879A US 3649879 A US3649879 A US 3649879A US 64411 A US64411 A US 64411A US 3649879D A US3649879D A US 3649879DA US 3649879 A US3649879 A US 3649879A
- Authority
- US
- United States
- Prior art keywords
- solution
- salt
- set forth
- combination set
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01R—MEASURING ELECTRIC VARIABLES; MEASURING MAGNETIC VARIABLES
- G01R29/00—Arrangements for measuring or indicating electric quantities not covered by groups G01R19/00 - G01R27/00
- G01R29/24—Arrangements for measuring quantities of charge
Definitions
- ABSTRACT An electrolytic coulometer useful as a timer which incorporates two electrolytes in solution and an indicator wherein the passage of electric current eliminates one electrolyte from solution before the other and thereafter acts on the second electrolyte to cause the indicator to change color.
- This invention relates to a coulometer or a colorimetric timer which indicates the lapse of a particular time period or the passage therethrough of a predetermined quantity of electric current by a relatively abrupt change in the color of the timer.
- Electrolytic timers presently exist in the art, but they are expensive and imprecise. Their general operation depends on the erosion of an electrode either to a measured degree or a totality. The difficulties of providing a precisely erodible amount of electrode material in order to obtain any sort of accuracy in the timing is a difficult and expensive process.
- This invention is directed to an electrolytic color-indicating timer or coulometer which depends, in principle, on the presence of two electrolytic salts in the electrolyte, one of which is acted upon first by a passage of electric current to disappear effectively from the solution and the other of which is thereafter acted upon the change the pH of the solution to effect an indicator change.
- an electrolytic color-indicating timer or coulometer which depends, in principle, on the presence of two electrolytic salts in the electrolyte, one of which is acted upon first by a passage of electric current to disappear effectively from the solution and the other of which is thereafter acted upon the change the pH of the solution to effect an indicator change.
- the device of this invention is one which lends itself well, for instance, to marking end points of guarantee periods or service intervals as, for instance, in household appliances. It provides a highly visible signal that the end point of the period has been reached, the transition point is sharp, and it is adaptable to the measurement of time periods up to as much as a year or more.
- the device has a further advantage in that it is self-stopping; i.e., after the lapse of the predetermined time period and the signalled termination thereof, the device contains integral provision effectively shutting off the flow of electric current therethrough.
- the device in question has an easily attained accuracy of plus or minus percent for timing intervals of up to a year.
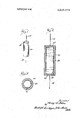
- HG. 3 is a horizontal section taken about centrally through the timer o'f'FlG. l.
- the illustrated embodiment of'the timer consists of an outer cylindrical transparent or translucent jacket 10, desirably made of a resilient plastic, a metal cathode 12 closing the top end of 'the cylinder and having a terminal wire '14 connected thereto and a metal anode 16 closing the bottom end of the cylinder and having a terminal wire 18 connected to it.
- a sleeve of indicator-impregnated paper 20 is contained within the cylinder, and the cylinder is filled full with an electrolyte solution 22.
- the reaction on which the instant color change timer or coulometer is based is one wherein a solution of a metallic salt is electrolyzed over a period of time to a point of exhaustion.
- a suitable way to obtain this exhaustion is by choosing a metal ion which will plate out on the cell cathode and anelectrolyte anion which will combine with the cell anode material to form an insoluble salt.
- the timing capability of the cell will be determined by the, gross quantity of the metallic salt consisting of this metal and this anion.
- a second salt of a strong base is also incorporated into the solution.
- This salt should include as its anion one which likewise will form an insoluble salt with the anode metal.
- the anion of the strong base be the same asthat of the exhaustible salt.
- the strong base salt Upon passage of current through the cell, the strong base is sufficiently greatly electropositive with respect to the metal to be deposited that the salt of the strong base will not be affected until the exhaustible salt is exhausted from the solution. The action of the cell, therefore, is sequential.
- the strong base salt will preserve conductivity of the cell through the point of exhaustion of the exhaustible salt so as to obtain removal of the exhaustible salt which is essentially linear with respect to time under constant voltage circumstances.
- the anode and cathode are silver and the electrolyte solution has the following composition:
- the proportion of cadmium chloride is selected such that the liter of solution has a total current capacity of IOuAH or IOuAI-I per ml. If the current flow through the cell is 10 uA, as will be later developed, the timing capability per ml. will be 1,000 hours.
- the cadmium chloride Upon the passage of electrical current, the cadmium chloride will be first affected, cadmium being lower on the electromotive scale. The cadmium will plate out on the cathode and the chloride ion will react with the silver of the anode to form silver chloride.
- the sodium sulphate has the effect of decreasing the impedance of the cell and aiding in the formation of a porous but adherent silver chloride deposit on the anode. This decomposition of the cadmium chloride will continue until the cadmium chloride is substantially exhausted.
- the sodium chloride enters into the electrolytic reaction, the chloride ion adding to the chloride deposit on the anode of the cadmium chloride and the sodium ion forming srfi ium hydroxide to increase sharply the pH of the electrolyte solution beyond the transition point of the indicator impregnated into the paper 20.
- Any strong base chloride may be substituted for the sodium chloride. Specific substitutes might be the chlorides of potassium, calcium, magnesium, barium, etc.
- an appropriate indicator is phenolphthalein, although other indicators or mixtures of indicators may be employed as is well known in the art.
- Other indicators found to be particularly suitable are litmus and o-cresolphthalein. It is desireable that the indicator be stable and insoluble in the electrolyte solution under the slightly acid conditions which prevail during the timed interval.
- the orientation of the cell as illustrated in the drawings is deliberate.
- the cell should be vertical.
- hydrogen is evolved from the cathode.
- the pressure within the cell would increase to the extent where the cell wall would rupture or the anode or cathode be displaced.
- a hydrogen bubble forms on top of the electrolyte solution and thus eventually isolates an electrode from the solution and renders the cell nonconductive.
- the anode should be uppermost to prevent movement of non-adherent cadmium from the cathode to the anode, where it would recombine to form cadmium chloride. This cadmium chloride would render the cell inaccurate by increasing its capacity beyond the intended timing value.
- the sodium sulphate present serves to cure a circumstance which sometimes arises in a cell lacking this compound. Occasionally, such latter cells develop an unaccountably higher resistance which augments the voltage drop across the cell with a concurrent tendency to increase the acidity of the solution. It is believed that this effect may arise from the deposit of a relatively impervious nonconducting silver chloride film on the anode. If this be the case, it is believed that the sodium sulphate has the effect of maintaining the AgCl film in pervious condition. The proportion of sodium sulphate in the solution was arrived at by adding the maximum possible without raising the freezing or solidifying temperature of the solution. Sulphates of other strong bases may be substituted for the sodium sulphate.
- strong base is meant a base having a dissociated constant in excess of 10 as defined on page 1623 of the Handbook of Chemistry and Physics, 38th Edition, 1956-1957 in a table entitled Formulas for Calculating Titration Data.”
- the cell electrodes may be equally well silver, as described, or silver plated.
- the plating should be of such quality and thickness that it will remain unbroken throughout the timed period.
- a copper cathode has been employed with some success, but a cell with two silver electrodes has been found to be more reliable.
- the electrodes must be clean for optimum reactivity. It has been found that cleaning by etching is unsatisfactory; silver chloride bonds strongly to an etched surface, generating an insulating barrier. An abrasive cleaning functions satisfactorily.
- an indicator dissolved in the electrolyte does not yield as good results as an indicator, insoluble in its acid phase, impregnated in a paper sheet. Dissolved indicators seemingly migrate to the anode by electrophoresis and are subject to oxidation during the running time of the cell.
- orthocresolphthalein gives the sharpest color change. Should impregnated papers not be commercially available, they may be prepared by dipping filter paper into an alcohol solution of the indicator and letting it dry.
- the range of solution components is not particularly critical.
- the cadmium chloride proportion will be dictated by the desired time interval and the cell size up to a content on the order of 100 g. per liter.
- the sodium chloride provides a good reaction within the range of l to 100 g. per liter.
- the sodium sulphate functions within the same range of proportion, but the cell loses conductivity at the low end and the solution solidifies at too high a temperature at the high end of the range.
- timer in an extremely low cost and simple application would be in determining the warranty period of a major appliance.
- the timer will be connected through a simple rectifier or diode to line current, and a it is possible to vary the timing capacity of the cell by varying the concentration of the cadmium chloride in the solution, it is preferred that variations in timing be accommodated by altering the cell capacity.
- a colorimetric coulometer comprising an electrolytic cell having a container, an anode and a cathode, and an electrolyte solution therein, said solution including a first, dissolved salt of a base material forming a cation productive of a soluble base material at the cathode and the anion of which forms an insoluble salt with said anode, a tlme-penod-exhaustible quantity of a second salt having a cation less electropositive than saidbase material cation of said first salt and being electrolytically platable on said cathode, and the anion of said second salt forming an insoluble salt with said anode, and means for indicating the transformation of said solution into a base solution.
Landscapes
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
Abstract
An electrolytic coulometer useful as a timer which incorporates two electrolytes in solution and an indicator wherein the passage of electric current eliminates one electrolyte from solution before the other and thereafter acts on the second electrolyte to cause the indicator to change color.
Description
United States Patent Gates 1451 Mar. 14, 1972 ELECTROLYTIC COLOR-INDICATING TIMER Inventor: Henry S. Gates, Salt Lake City, Utah Gibbs Manufacturing 81 Research Corporation, Janesville, Wis.
Aug. 17, 1970 Assignee:
Filed:
Appl. No.:
u.s. CI ..317/230, 324/94 Int. Cl. .1101; 9/02 Field of Search .324/94; 252/622; 204/95 R;
References Cited UNITED STATES PATENTS 1/1961 Eriksen ..324/94 3,017,612 1/1962 Singer ..340/173 Analytical Chemistry, Volume 27, No. 7, July 1955, pages 1 197- 1199.
Primary Examiner.lames D. Kallam Attorney-Gradolph, Love, Rogers and Van Sciver [5 7] ABSTRACT An electrolytic coulometer useful as a timer which incorporates two electrolytes in solution and an indicator wherein the passage of electric current eliminates one electrolyte from solution before the other and thereafter acts on the second electrolyte to cause the indicator to change color.
10 Claims, 3 Drawing Figures ELECTROLYTIC COLOR-INDICATING TIMER BACKGROUND OF THE INVENTION This invention relates to a coulometer or a colorimetric timer which indicates the lapse of a particular time period or the passage therethrough of a predetermined quantity of electric current by a relatively abrupt change in the color of the timer.
Electrolytic timers presently exist in the art, but they are expensive and imprecise. Their general operation depends on the erosion of an electrode either to a measured degree or a totality. The difficulties of providing a precisely erodible amount of electrode material in order to obtain any sort of accuracy in the timing is a difficult and expensive process.
SUMMARY OF THE INVENTION This invention is directed to an electrolytic color-indicating timer or coulometer which depends, in principle, on the presence of two electrolytic salts in the electrolyte, one of which is acted upon first by a passage of electric current to disappear effectively from the solution and the other of which is thereafter acted upon the change the pH of the solution to effect an indicator change. By virtue of this invention there is only one element which is present in critical quantity, the first electrolytic salt, and since solutions are easily made with high accuracy of content and containers can be easily made to precise volume, the cost of the timer is extraordinarily low as compared with devices presently known.
The device of this invention is one which lends itself well, for instance, to marking end points of guarantee periods or service intervals as, for instance, in household appliances. It provides a highly visible signal that the end point of the period has been reached, the transition point is sharp, and it is adaptable to the measurement of time periods up to as much as a year or more. The device has a further advantage in that it is self-stopping; i.e., after the lapse of the predetermined time period and the signalled termination thereof, the device contains integral provision effectively shutting off the flow of electric current therethrough. The device in question has an easily attained accuracy of plus or minus percent for timing intervals of up to a year.
DESCRIPTION OF THE DRAWINGS of FIG. 1; and
HG. 3 is a horizontal section taken about centrally through the timer o'f'FlG. l.
DESCRIPTIONOF A PREFERRED EMBODIMENT The illustrated embodiment of'the timer consists of an outer cylindrical transparent or translucent jacket 10, desirably made of a resilient plastic, a metal cathode 12 closing the top end of 'the cylinder and having a terminal wire '14 connected thereto and a metal anode 16 closing the bottom end of the cylinder and having a terminal wire 18 connected to it.
As illustrated, a sleeve of indicator-impregnated paper 20 is contained within the cylinder, and the cylinder is filled full with an electrolyte solution 22.
The reaction on which the instant color change timer or coulometer is based is one wherein a solution of a metallic salt is electrolyzed over a period of time to a point of exhaustion. A suitable way to obtain this exhaustion is by choosing a metal ion which will plate out on the cell cathode and anelectrolyte anion which will combine with the cell anode material to form an insoluble salt. The timing capability of the cell will be determined by the, gross quantity of the metallic salt consisting of this metal and this anion.
Use of this salt alone, however, gives no useful indication since, as the concentration approaches the vanishing point, the resistance of the cell would increase such that the end point would be indefinitely projected. Accordingly, a second salt of a strong base is also incorporated into the solution. This salt should include as its anion one which likewise will form an insoluble salt with the anode metal. Thus, it is suggested that the anion of the strong base be the same asthat of the exhaustible salt. Upon passage of current through the cell, the strong base is sufficiently greatly electropositive with respect to the metal to be deposited that the salt of the strong base will not be affected until the exhaustible salt is exhausted from the solution. The action of the cell, therefore, is sequential. At the same time the strong base salt will preserve conductivity of the cell through the point of exhaustion of the exhaustible salt so as to obtain removal of the exhaustible salt which is essentially linear with respect to time under constant voltage circumstances.
After exhaustion of the exhaustible salt, further electrolysis of the electrolyte solution will result in the formation of the strong base at the cathode together with the evolution of hydrogen and a further elimination of the common anion at the cell anode by virtue of combination with the oxidized anode metal. The formation of the strong base at the cathode generates quickly a sharp increase in the pH of the cell electrolyte which is readily detectable by an acid-base indicator. The starting solution is slightly acidic, in the region of pH 5.
To deal with a specific embodiment, the anode and cathode are silver and the electrolyte solution has the following composition:
Constituent g./liter CdCI Z'AI-QO 42.6 NaCl l0.0 M1 50, 40.0
Distilled H 0 to make 1 liter.
The proportion of cadmium chloride is selected such that the liter of solution has a total current capacity of IOuAH or IOuAI-I per ml. If the current flow through the cell is 10 uA, as will be later developed, the timing capability per ml. will be 1,000 hours.
Upon the passage of electrical current, the cadmium chloride will be first affected, cadmium being lower on the electromotive scale. The cadmium will plate out on the cathode and the chloride ion will react with the silver of the anode to form silver chloride. The sodium sulphate has the effect of decreasing the impedance of the cell and aiding in the formation of a porous but adherent silver chloride deposit on the anode. This decomposition of the cadmium chloride will continue until the cadmium chloride is substantially exhausted. At this point, the sodium chloride enters into the electrolytic reaction, the chloride ion adding to the chloride deposit on the anode of the cadmium chloride and the sodium ion forming srfi ium hydroxide to increase sharply the pH of the electrolyte solution beyond the transition point of the indicator impregnated into the paper 20. Any strong base chloride may be substituted for the sodium chloride. Specific substitutes might be the chlorides of potassium, calcium, magnesium, barium, etc.
An appropriate indicator is phenolphthalein, although other indicators or mixtures of indicators may be employed as is well known in the art. Other indicators found to be particularly suitable are litmus and o-cresolphthalein. It is desireable that the indicator be stable and insoluble in the electrolyte solution under the slightly acid conditions which prevail during the timed interval.
The orientation of the cell as illustrated in the drawings is deliberate. The cell should be vertical. As the current electrolyzes the sodium chloride solution hydrogen is evolved from the cathode. Were such evolution permitted to continue indefinitely the pressure within the cell would increase to the extent where the cell wall would rupture or the anode or cathode be displaced. With the orientation illustrated, a hydrogen bubble forms on top of the electrolyte solution and thus eventually isolates an electrode from the solution and renders the cell nonconductive.
The anode should be uppermost to prevent movement of non-adherent cadmium from the cathode to the anode, where it would recombine to form cadmium chloride. This cadmium chloride would render the cell inaccurate by increasing its capacity beyond the intended timing value.
The sodium sulphate present serves to cure a circumstance which sometimes arises in a cell lacking this compound. Occasionally, such latter cells develop an unaccountably higher resistance which augments the voltage drop across the cell with a concurrent tendency to increase the acidity of the solution. It is believed that this effect may arise from the deposit of a relatively impervious nonconducting silver chloride film on the anode. If this be the case, it is believed that the sodium sulphate has the effect of maintaining the AgCl film in pervious condition. The proportion of sodium sulphate in the solution was arrived at by adding the maximum possible without raising the freezing or solidifying temperature of the solution. Sulphates of other strong bases may be substituted for the sodium sulphate.
By strong base" is meant a base having a dissociated constant in excess of 10 as defined on page 1623 of the Handbook of Chemistry and Physics, 38th Edition, 1956-1957 in a table entitled Formulas for Calculating Titration Data."
The cell electrodes may be equally well silver, as described, or silver plated. The plating should be of such quality and thickness that it will remain unbroken throughout the timed period. A copper cathode has been employed with some success, but a cell with two silver electrodes has been found to be more reliable.
The electrodes must be clean for optimum reactivity. It has been found that cleaning by etching is unsatisfactory; silver chloride bonds strongly to an etched surface, generating an insulating barrier. An abrasive cleaning functions satisfactorily.
Generally, an indicator dissolved in the electrolyte does not yield as good results as an indicator, insoluble in its acid phase, impregnated in a paper sheet. Dissolved indicators seemingly migrate to the anode by electrophoresis and are subject to oxidation during the running time of the cell.
Of the indicators mentioned, orthocresolphthalein gives the sharpest color change. Should impregnated papers not be commercially available, they may be prepared by dipping filter paper into an alcohol solution of the indicator and letting it dry.
The range of solution components is not particularly critical. The cadmium chloride proportion will be dictated by the desired time interval and the cell size up to a content on the order of 100 g. per liter. The sodium chloride provides a good reaction within the range of l to 100 g. per liter. The sodium sulphate functions within the same range of proportion, but the cell loses conductivity at the low end and the solution solidifies at too high a temperature at the high end of the range.
A representative utilization of the timer in an extremely low cost and simple application would be in determining the warranty period of a major appliance. The timer will be connected through a simple rectifier or diode to line current, and a it is possible to vary the timing capacity of the cell by varying the concentration of the cadmium chloride in the solution, it is preferred that variations in timing be accommodated by altering the cell capacity.
I claim:
1. A colorimetric coulometer comprising an electrolytic cell having a container, an anode and a cathode, and an electrolyte solution therein, said solution including a first, dissolved salt of a base material forming a cation productive of a soluble base material at the cathode and the anion of which forms an insoluble salt with said anode, a tlme-penod-exhaustible quantity of a second salt having a cation less electropositive than saidbase material cation of said first salt and being electrolytically platable on said cathode, and the anion of said second salt forming an insoluble salt with said anode, and means for indicating the transformation of said solution into a base solution.
2. The combination set forth in claim 1 wherein said anode has a silver surface and wherein said salt is cadmium chloride.
3. The combination set forth in claim 1 wherein the contents of said cell are visible and said indicating means is litmus, phenolphthalein, or ortho-cresolphthalein.
4. The combination set forth in claim 1 wherein said indicating means is in said solution and is formed of an impregnated fabric.
5. The combination set forth in claim 3 wherein the container of said cell is light transmitting with some elasticity and said cell is mountable with one of said electrodes uppermost, said electrode being formed to escape said solution, said solution being removable from contact with said one electrode upon evolution of a gas from either of said electrodes.
6. The combination set forth in claim 2 wherein said solution further contains a substantial proportion of a sulphate of a strong base.
7. The combination set forth in claim 1 wherein said electrodes have silver surfaces, said first salt is a chloride of an electropositive element and said second salt is cadmium chloride.
8. The combination set forth in claim 7 wherein said indicating means is phenolphthalein, litmus, or orthocresolphthalein.
9. The combination set forth in claim 7 further including a sulphate of an electropositive element.
10. The combination set forth in claim 9 wherein said electrolyte solution has an approximate composition of 10 g. N aCl 42.6 g. CdCl;, 2% H 0, and 40 g. Na SO per liter of aqueous solution.
Claims (10)
1. A colorimetric coulometer comprising an electrolytic cell having a container, an anode and a cathode, and an electrolyte solution therein, said solution including a first, dissolved salt of a base material forming a cation productive of a soluble base material at the cathode and the anion of which forms an insoluble salt with said anode, a time-period-exhaustible quantity of a second salt having a cation less electropositive than said base material cation of said first salt and being electrolytically platable on said cathode, and the anion of said second salt forming an insoluble salt with said anode, and means for indicating the transformation of said solution into a base solution.
2. The combination set forth in claim 1 wherein said anode has a silver surface and wherein said second salt is cadmium chloride.
3. The combination set forth in claim 1 wherein the contents of said cell are visible and said indicating means is litmus, phenolphthalein, or ortho-cresolphthalein.
4. The combination set forth in claim 1 wherein said indicating means is in said solution and is formed of an impregnated fabric.
5. The combination set forth in claim 3 wherein the container of said cell is light transmitting with some elasticity and said cell is mountable with one of said electrodes uppermost, said electrode being formed to escape said solution, said solution being removable from contact with said one electrode upon evolution of a gas from either of said electrodes.
6. The combination set forth in claim 2 wherein said solution further contains a substantial proportion of a sulphate of a strong base.
7. The combination set forth in claim 1 wherein said electrodes have silver surfaces, said first salt is a chloride of an electropositive element and said second salt is cadmium chloride.
8. The combination set forth in claim 7 wherein said indicating means is phenolphthalein, litmus, or ortho-cresolphthalein.
9. The combination set forth in claim 7 further including a sulphate of an electropositive element.
10. The combination set forth in claim 9 wherein said electrolyte solution has an approximate composition of 10 g. NaCl, 42.6 g. CdC12 .2 1/2 H2O, and 40 g. Na2SO4 per liter of aqueous solution.
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US6441170A | 1970-08-17 | 1970-08-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US3649879A true US3649879A (en) | 1972-03-14 |
Family
ID=22055802
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US64411A Expired - Lifetime US3649879A (en) | 1970-08-17 | 1970-08-17 | Electrolytic color-indicating timer |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US3649879A (en) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2970264A (en) * | 1957-05-31 | 1961-01-31 | Raytheon Co | Elapsed time indicators |
| US3017612A (en) * | 1956-11-23 | 1962-01-16 | Nat Scient Lab Inc | Method and apparatus for storing information |
| US3119754A (en) * | 1961-02-10 | 1964-01-28 | Sperry Rand Corp | Elapsed time indicator |
| US3302091A (en) * | 1963-04-22 | 1967-01-31 | Ian H S Henderson | Coulometric device |
| US3463673A (en) * | 1967-09-12 | 1969-08-26 | Nasa | Electrochemical coulometer and method of forming same |
| US3564349A (en) * | 1969-05-02 | 1971-02-16 | Gen Electric | Coulometer with electrode containing excess of at least 200 percent cadmium hydroxide |
-
1970
- 1970-08-17 US US64411A patent/US3649879A/en not_active Expired - Lifetime
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3017612A (en) * | 1956-11-23 | 1962-01-16 | Nat Scient Lab Inc | Method and apparatus for storing information |
| US2970264A (en) * | 1957-05-31 | 1961-01-31 | Raytheon Co | Elapsed time indicators |
| US3119754A (en) * | 1961-02-10 | 1964-01-28 | Sperry Rand Corp | Elapsed time indicator |
| US3302091A (en) * | 1963-04-22 | 1967-01-31 | Ian H S Henderson | Coulometric device |
| US3463673A (en) * | 1967-09-12 | 1969-08-26 | Nasa | Electrochemical coulometer and method of forming same |
| US3564349A (en) * | 1969-05-02 | 1971-02-16 | Gen Electric | Coulometer with electrode containing excess of at least 200 percent cadmium hydroxide |
Non-Patent Citations (1)
| Title |
|---|
| Analytical Chemistry, Volume 27, No. 7, July 1955, pages 1197 1199. * |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Elder et al. | Anodic behaviour of the borohydride ion | |
| Burke et al. | Cyclic voltammetry as a technique for determining the surface area of RuO2 electrodes | |
| Bockris | Electrolytic polarisation—I. The overpotential of hydrogen on some less common metals at high current densities. Influence of current density and time | |
| Micka et al. | Theory of porous electrodes—XVI. The nickel hydroxide electrode | |
| Gritzner | Solvent effects on redox potentials: Studies in N-methylformamide | |
| ES326101A1 (en) | A procedure to electrolytically eliminate the metal shrinkle. (Machine-translation by Google Translate, not legally binding) | |
| GB1433962A (en) | Formation of adherent precipitates on surfaces immersed in liquids | |
| Sykut et al. | The influence of thiourea on the electroreduction of Zn2+ ions in various supporting electrolytes in respect to the cap-pair effect | |
| US3649879A (en) | Electrolytic color-indicating timer | |
| US3658593A (en) | Electrochemical cells with lithium negative electrodes | |
| Burrows et al. | The mechanism of oxidation of Cl− on platinum and RuO2/TiO2 electrodes, and the reduction of Cl2 on platinum | |
| Mer et al. | The activity coefficients and heats of transfer of cadmium sulfate from electromotive force measurements at 25 and 0. Application of the extended theory of Debye and Hückel | |
| Taraszewska et al. | Medium effects in the systems: Cd2+ or Zn2+ in some water+ organic solvent mixtures deduced from equilibrium potentials at amalgam electrodes | |
| Ceccattini et al. | Thermodynamics of NaCl in aqueous ethylene glycol, acetonitrile, and 1, 4-dioxane mixtures from emf measurements at 25° C | |
| Maget et al. | The electrochemical reduction of oxygen on platinum electrodes partially immersed in sulfuric acid | |
| Cady | The Electrolysis and Electrolytic Conductivity of Certain Substances dissolved in LiquidAmmonia | |
| Tanaka et al. | Electrode Processes of Nickel (II) Ions at the Mercury Electrode in Concentrated Calcium Chloride Solutions | |
| GB1501893A (en) | Continuous electrolytic colouring of a preanodised aluminium foil or strip | |
| Gillet | Electrolysis in Anhydrous Ether | |
| Fogg | A review of the electrochemistry of gallium | |
| Azzam et al. | Anodic behaviour and passivation of tin in alkaline—stannate plating solutions | |
| Santhanam et al. | Coulometric Oxidation of Thio-Urea | |
| US1346057A (en) | Apparatus for measuring ohmic resistance of liquids and solutions | |
| Amis et al. | The Equivalent Conductance of Electrolytes in Mixed Solvents: XII. Magnesium Sulfate in Water‐Ethanol Solvents | |
| Yoneda | Fundamental Research on Anodic Reaction. II. Activation Energy of Anodic Oxygen-producing Reaction on Some Electrode Materials in Aqueous Solution of n/10 Sodium Hydroxide |