US20040204456A1 - Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate - Google Patents
Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate Download PDFInfo
- Publication number
- US20040204456A1 US20040204456A1 US10/820,397 US82039704A US2004204456A1 US 20040204456 A1 US20040204456 A1 US 20040204456A1 US 82039704 A US82039704 A US 82039704A US 2004204456 A1 US2004204456 A1 US 2004204456A1
- Authority
- US
- United States
- Prior art keywords
- cancer
- threo
- methylphenidate
- side effect
- cognitive
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [2*][C@@H](C(=O)OC)[C@@]1([H])CCCCN1.[2*][C@@H](C(=O)OC)[C@]1([H])CCCCN1.[2*][C@H](C(=O)OC)[C@@]1([H])CCCCN1.[2*][C@H](C(=O)OC)[C@]1([H])CCCCN1 Chemical compound [2*][C@@H](C(=O)OC)[C@@]1([H])CCCCN1.[2*][C@@H](C(=O)OC)[C@]1([H])CCCCN1.[2*][C@H](C(=O)OC)[C@@]1([H])CCCCN1.[2*][C@H](C(=O)OC)[C@]1([H])CCCCN1 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/08—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms
- C07D211/18—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D211/34—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with substituted hydrocarbon radicals attached to ring carbon atoms with hydrocarbon radicals, substituted by carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/445—Non condensed piperidines, e.g. piperocaine
- A61K31/4458—Non condensed piperidines, e.g. piperocaine only substituted in position 2, e.g. methylphenidate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/12—Drugs for genital or sexual disorders; Contraceptives for climacteric disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/26—Psychostimulants, e.g. nicotine, cocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P17/00—Preparation of heterocyclic carbon compounds with only O, N, S, Se or Te as ring hetero atoms
- C12P17/10—Nitrogen as only ring hetero atom
- C12P17/12—Nitrogen as only ring hetero atom containing a six-membered hetero ring
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P41/00—Processes using enzymes or microorganisms to separate optical isomers from a racemic mixture
- C12P41/006—Processes using enzymes or microorganisms to separate optical isomers from a racemic mixture by reactions involving C-N bonds, e.g. nitriles, amides, hydantoins, carbamates, lactames, transamination reactions, or keto group formation from racemic mixtures
Definitions
- the present invention is directed to methods for treating fatigue, neurobehavioral slowing and other cognitive disorders and defects due to cancers and treatments associated with cancers, and similar conditions.
- the present invention is directed to methods for treating disorders related to menopause, including executive function defects. The methods involve the administration of a composition comprising D -threo methylphenidate that is substantially free of L -threo methylphenidate and of erythro forms of methylphenidate.
- Advanced cancer typically produces severe pain in patients. This pain is often controlled by the administration of large doses of analgesics, including opioid analgesics. However, the pain relief is often accompanied by undesirable side-effects such as unacceptable sedation and/or a decrease in cognitive function. These side effects have a significant negative impact on the quality of life of the patient. In addition, cancer patients often display one or more of a decrease in cognitive function, fatigue, and neurobehavioral slowing that is unrelated to the administration of analgesics, but may be related to the underlying cancer, the treatment of the cancer, or both.
- Menopause is accompanied by several side effects, including an executive function defect.
- many menopausal women report impairment in short term memory, inability to screen distractions and sustain attention in organization of thoughts and tasks.
- women diagnosed with ADD prior to menopause report exacerbation of ADD symptoms during the protracted perimenopausal period and thereafter. See Brown, T. E., Attention-Deficit Disorders and Comorbidities in Children, Adolescents and Aduts, American Psychiatric Press, Washington, D.C., 2000, at p. 40-41.
- Methylphenidate has been used to treat nervous system disorders including Attention Deficit Disorder (ADD), a commonly diagnosed nervous system illness in children, Attention Deficit Hyperactivity Disorder (ADHD), and cognitive decline in patients with Acquired Immunodeficiency Syndrome (AIDS) or AIDS related conditions.
- ADD Attention Deficit Disorder
- ADHD Attention Deficit Hyperactivity Disorder
- AIDS Acquired Immunodeficiency Syndrome
- the racemic form of methylphenidate also has been proposed to improve cognitive function in patients receiving large doses of medication.
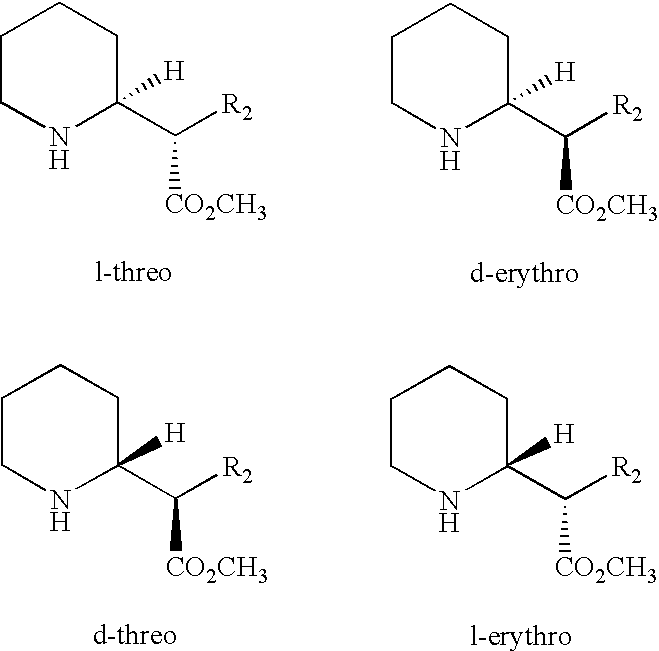
- Methylphenidate exists as four separate optical isomers as follows:
- R 2 is phenyl.
- Pharmaceutically acceptable salts are generally administered clinically.
- Other phenidate drugs which also can be administered according to the invention, include those in which the methyl group in the above structures is replaced by C 2 -C 4 alkyl and those in which R 2 is optionally substituted with C 1 -C 4 alkyl.
- methylphenidate hydrochloride salt is commonly referred to simply as “methylphenidate”.
- methylphenidate is used broadly herein to include methylphenidate and pharmaceutically acceptable salts thereof, including methylphenidate hydrochloride.
- the threo racemate (pair of enantiomers) of methylphenidate is a mild central nervous system stimulant with pharmacological activity qualitatively similar to that of amphetamines.
- Undesirable side effects associated with the use of the DL -threo racemate of methylphenidate include anorexia, weight loss, insomnia, dizziness and dysphoria.
- the racemate which is a Schedule II controlled substance, produces a euphoric effect when administered intravenously or through inhalation or ingestion, and thus carries a high potential for abuse.
- D -threo isomer (2R:2′R) of methylphenidate substantially free of the ‘ 1 -threo isomer, produces a methylphenidate medication which retains high activity levels and simultaneously may possess reduced euphoric effect and reduced potential for abuse among patients. See U.S. patent Ser. No. 5,908,850, incorporated herein by reference in its entirety.
- D -threo-methylphenidate (2R:2′R) may possesses enhanced therapeutic activity with reduced side effects, and 1-threo-methylphenidate may produce undesirable side effects, euphoria and drug abuse potential in patients.
- the present invention provides methods for treating fatigue, neurobehavioral slowing and cognitive side effects arising from cancer, or from a treatment therefor, such as chemotherapy, radiation therapy and administration of medication to control pain.
- the invention provides methods for alleviation of depression caused by cognitive dysfunction (a “cognitive side effect”) and fatigue associated with cancer, and treatments therefor.
- the methods of the invention involve the administration of D -threo-methylphenidate or a pharmaceutically acceptable salt thereof, substantially free of both L -threo-methylphenidate and erythro methylphenidates.
- methods for alleviating fatigue and/or neurobehavioral slowing arising from an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- the present invention provides methods for alleviating fatigue or neurobehavioral slowing arising from the administration of a treatment for an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- Also provided in accordance with the present invention are methods for alleviating a cognitive side effect of a treatment for an oncological condition, comprising the steps of identifying a patient suffering from a cognitive side-effect of a treatment for an oncological condition; and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- the treatment for the oncological condition is the administration of pain management and biological therapies, including pain relief medication, chemotherapy, radiation therapy, and surgery.
- the treatment for the oncological condition is chemotherapy or the administration of pain relief medication.
- the pain relief medication is one or more opioid analgesics, nerve blocks or other psychotropic agents.
- the oncological condition is a cancer selected from the group consisting of all malginant conditions, inclduing both solid tumors and nonsolid tumors.
- the oncological condition is a solid tumor.
- the cognitive side effect is sedation, decreased cognitive function, major depressive disorder, or neurobehavioral slowing. In some preferred embodiments, the cognitive side effect is sedation or decreased cognitive function.
- the present invention provides methods for treating a symptom of menopause comprising the steps of identifying a patient suffering from a symptom of menpoause; and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- the symptom of menopause is impairment in short term memory, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, and/or apathy.
- the administration of the D -threo-methylphenidate (2R:2′R), or the pharmaceutically acceptable salt thereof gives rise to efficacious treatment without interfering with patient sleep patterns or engendering anoretic behavior.
- the methods of the invention involve the administration of D -threo-methylphenidate or a pharmaceutically acceptable salt thereof, substantially free of both L -threo-methylphenidate and erythro methylphenidates. It is now believed that the L isomer may contribute to the side effects associated with the commercial drug, and that it is thus desirable to administer only the active D -threo form of the drug.
- methods for alleviating fatigue or neurobehavioral slowing arising from an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- the present invention provides methods for alleviating fatigue or neurobehavioral slowing arising from the administration of a treatment for an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- Also provided in accordance with the present invention are methods for alleviating a cognitive side effect (e.g., neurobehavioral slowing) of a treatment for an oncological condition, comprising the steps of identifying a patient suffering from a cognitive side-effect of a treatment for an oncological condition; and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- a cognitive side effect e.g., neurobehavioral slowing
- the treatment for the oncological condition is the administration of pain management and biological therapies, including pain relief medication, chemotherapy, radiation therapy, and surgery.
- the treatment for the oncological condition is chemotherapy or the administration of pain relief medication.
- the pain relief medication is one or more opioid analgesics, nerve blocks or other psychotropic agents.
- the oncological condition is a cancer selected from the group consisting of all malginant conditions, inclduing both solid tumors and nonsolid tumors.
- the oncological condition is a solid tumor.
- the cognitive side effect is sedation, decreased cognitive function, major depressive disorder, or neurobehavioral slowing. In some preferred embodiments, the cognitive side effect is sedation or decreased cognitive function.
- the present invention provides methods for treating a symptom of menopause comprising the steps of identifying a patient suffering from a symptom of menpoause; and administering to said patient a therapeutically effective amount of D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer.
- the symptom of menopause is impairment in short term memory, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing and/or apathy.
- the administration of the D -threo-methylphenidate (2R:2′R), or the pharmaceutically acceptable salt thereof gives rise to efficacious treatment without interfering with patient sleep patterns or engendering anoretic behavior.
- the administration of the pharmacodynamically active D -threo form of methylphenidate may provide efficacious treatment for an entire day with minimal undesirable side effects such as interference with patient sleep patterns or anoretic behavior. It has been surprisingly and unexpectedly discovered that the beneficial effects of the D -threo isomer persist for a longer period time when the D -threo isomer is administered alone than when it is administered in combination with the L -threo isomer.
- L isomer functions as an antagonist to the D isomer.
- another aspect of the present invention provides methods for ameliorating or counteracting the effects of methylphenidate drugs, comprising administering L -threo methylphenidate to a patient who has a serum level of D -threo methylphenidate.
- D -threo methylphenidate has a longer duration of action than DL -methylphenidate of at least six hours.
- Patients who were given the D -threo isomer free of the L isomer performed better in objective tests than patients who received the DL -threo racemate or a placebo, at the 6 hour time point.
- the patients who received DL -threo racemate did not perform better after that time period than those who received a placebo.
- subjective observations of the same patients indicated that those who received only the D -threo isomer experienced beneficial effects of the drug for longer times than did those who received the DL -threo racemate.
- D -threo methylphenidate will be particularly useful in treating patients affected by fatigue, neurobehavioral slowing, and other cognitive defects (‘cognitive side effects”) that are due to cancer, and that are exacerbated by the administration of treatments for cancer such as chemotherapy, radiation therapy, bone marrow transplants, stem cell transplants and administration of medication to control pain.
- cognitive defects include but are not limited to neurobehavioral slowing, sedation, diminished executive function, decreased cognitive function, major depressive disorder and impaired quality of life.
- oncological condition is intended to mean all malignant conditions, including all cancers, for example solid tumors and nonsolid tumors.
- oncological conditions include cancers of the skin, mouth, brain and other nervous tissue, bone, lung, colon and rectum, pancreas, prostate, urinary tract, leukemias and lymphomas.
- the term “arising from the administration of a treatment for an oncological condition” is intended to mean that the indicated symptom or condition is in whole or in part caused by (i.e., is a side-effect of) the administration of a therapeutic agent used for the treatment of cancer, or for the management of a symptom of the cancer.
- agents used for the treatment of cancer include chemotherapeutic agents, including both chemical and radiotherapeutics, and radiation.
- agents used for the management of a symptom of the cancer include pain relief medications such as opioid or opoid-like analgesics and non-steroidal anti-inflammatory agents.
- the term “alleviating a cognitive side effect of a treatment for an oncological condition” means the lessening of the severity of a cognitive side effect caused in whole or in part by the administration of a treatment for an oncological condition.
- the term “cognitive side effect” as used herein denotes an impairment of one or more cognitive functions that results in whole or in part from the administration of an agent used for the treatment of cancer. Examples of cognitive side effects include sedation, neurobehavioral slowing, decreased cognitive function, depression, apathy, decreased libido and derpersonalization.
- the term “decreased cognitive function” is intended to mean a decrease in any or all aspects of thought, attention, perception, and/or memory.
- menopause is given its normal meaning of the period during which marks the permanent cessation of menstrual activity.
- symptom of menopause in intended to include those symptoms associated with menopause, including vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, apathy, mental depression and impairment of short term memory.
- execution function defect is intended to include but is not limited to one or more defects in the cognitive mechanisms responsible for focusing attention, goal-related behavior, strategic planning and problem solving.
- the methods of the invention will find use with patients, including outpatients, with all types of cancer, either primary or metastatic.
- dosage forms are administered of D -threo methylphenidate substantially free of L -threo methylphenidate and of erythro methylphenidates.
- “Substantially free”, as used herein, means that the dosage forms comprise at least about 95 percent, preferably at least about 97 percent, and more preferably at least about 99 percent of the D -threo isomer, to the exclusion of the L -threo and erythro forms.
- the D -threo form can be isolated by methods known to those skilled in the art.
- the D -threo methylphenidate can be administered in any of a variety of dosage regimes.
- Such regimes include chronic single, bolus dosages, i.e., where one dose being administered in a predetermined time period, for example twenty four hours.
- Further dosage regimes include those where multiple dosages are manually administered, and dosage forms where a single dosage form is administered that effectively mimics multiple dosages, such as pulsatile release dosage forms.
- Further dosage forms useful with the present invention include delay release and extended release (i.e., “time release”) dosage forms. The selection of appropriate dosage forms for an individual patient will depend upon the individual circumstances, and will be apparent to those of skill in the art.
- “Chronic”, as used herein, refers to continuous, regular, long-term therapeutic administration, i.e. periodic administration without substantial interruption, such as, for example, daily, for a time period of at least several weeks or months to several years, for the purpose of treating a patient needing treatment.
- “Bolus”, as used herein, refers to administration of a drug as a single event.
- the term “bolus” is intended to exclude dosage forms such as sustained release, pulsed release, and time release, and includes any dosage form which can be used to deliver a single dose.
- a bolus is preferably administered to a patient in need of treatment once daily, more preferably in the morning.
- the bolus dosages of the present invention may be administered in any conventional form known to those skilled in the art. Suitable methods for administration include oral dosage forms, injection, and infusion.
- the D -threo methylphenidate substantially free of L -threo methylphenidate and of erythro methylphenidates as described herein can be taken up in pharmaceutically acceptable carriers, such as, for example, solutions, suspensions, tablets, capsules, ointments, elixirs and injectable compositions.
- Pharmaceutical preparations generally can contain from about 1% to about 90% by weight of active ingredient. Preparations which are in single dose form, “unit dosage form”, preferably contain from about 20% to about 90% active ingredient.
- active ingredient refers to D -threo methylphenidate substantially free of L -threo methylphenidate and of erythro methylphenidates as described herein, salts thereof, and mixtures of D -threo methylphenidate as described herein with other pharmaceutically active compounds.
- Dosage unit forms such as, for example, tablets or capsules, typically contain from about 0.001 to about 1.0 g of active ingredient.
- Pharmaceutical preparations may be administered orally, parenterally, or topically.
- Pharmaceutical preparations containing compounds described herein may be prepared by methods known to those skilled in the art, such as, for example, conventional mixing, granulating, dissolving, or lyophilizing.
- Oral dosage forms include capsules, pills, tablets, troches, lozenges, melts, powders, solutions, suspensions and emulsions.
- the oral dosage forms provided by the invention can be in the form of tablets, caplets, and the like and can be of any shape suitable for oral administration of a drug, such as spheroidal, cube-shaped, oval, bean shaped, or ellipsoidal.
- the compounds may be combined with one or more solid pharmaceutically acceptable carriers, optionally granulating the resulting mixture.
- Pharmaceutically acceptable adjuvants may optionally be included, such as, for example, flow-regulating agents and lubricants.
- Suitable carriers include, for example, fillers such as sugars, cellulose preparations, calcium phosphates; and binders such as methylcellulose, hydroxymethylcellulose, and starches, such as, for example, maize starch, potato starch, rice starch, and wheat starch.
- the dosage form may be in the form of granules, which may be irregularly shaped.
- the dosage form can comprise a capsule containing particles.
- orally administrable pharmaceutical preparations are dry-filled capsules consisting of gelatin, and soft sealed capsules consisting of gelatin and a plasticizer such as glycerol or sorbitol.
- the dry-filled capsules may contain the active ingredient in the form of a granulate, for example in admixture with fillers, binders, glidants, and stabilizers.
- the active ingredient is preferably dissolved or suspended in a suitable liquid adjuvant, such as, for example, a fatty oil, paraffin oil, or liquid polyethylene glycol, optionally in the presence of stabilizers.
- suitable liquid adjuvant such as, for example, a fatty oil, paraffin oil, or liquid polyethylene glycol
- Other oral administrable forms include syrups containing active ingredient, for example, in suspended form at a concentration of from about 0.01% to 20%, or in a similar concentration that provides a suitable single dose when administered, for example, in measures of from about 2 to about 5 milliliters.
- Suitable excipients for use in oral liquid dosage forms include diluents such as water and alcohols, for example ethanol, benzyl alcohol and polyethylene alcohols, either with or without the addition of a pharmaceutically acceptable surfactant, suspending agent, or emulsifying agent. Also suitable are powdered or liquid concentrates for combining with liquids such as milk. Such concentrates may also be packed in single dose quantities.
- D -threo methylphenidate as described herein may be administered parenterally, that is, subcutaneously, intravenously, intramuscularly, or interperitoneally, as injectable dosages of the compound in a physiologically acceptable diluent with a pharmaceutical carrier.
- Solutions for parenteral administration may be in the form of infusion solutions.
- a pharmaceutical carrier may be, for example, a sterile liquid or mixture of liquids such as water, saline, aqueous dextrose and related sugar solutions, an alcohol such as ethanol, glycols such as propylene glycol or polyethylene glycol, glycerol ketals such as 2,2-dimethyl-1,3-dioxolane-4-methanol, ethers such as poly(ethyleneglycol)400, oils, fatty acids, fatty acid esters or glycerides, with or without the addition of a pharmaceutically acceptable surfactant such as a soap or detergent, suspending agent such as pectin, carbomers, methylcellulose, hydroxypropylmethylcellulose, or carboxymethylcellulose, or emulsifying agent or other pharmaceutically acceptable adjuvants.
- a sterile liquid or mixture of liquids such as water, saline, aqueous dextrose and related sugar solutions, an alcohol such as ethanol, glycols such as propylene glycol or polyethylene glyco
- oils which may be used in parenteral formulations include petroleum, animal, vegetable, or synthetic oils such as, for example, peanut oil, soybean oil, sesame oil, cottonseed oil, corn oil, olive oil, petrolatum, and mineral oil.
- Suitable fatty acids include, for example, oleic acid, stearic acid, and isostearic acid.
- Suitable fatty acid esters include ethyl oleate and isopropyl myristate.
- Suitable soaps include alkaline metal, ammonium and triethanolamine salts of fatty acids.
- Suitable detergents include cationic detergents such as dimethyl dialkyl ammonium halides and alkyl pyridinium halides; anionic detergents such as alkyl, aryl and olefin sulfonates, monoglyceride sulfates and sulfosuccinates; nonionic detergents such as fatty amino oxides, fatty acid alkanolamides and polyoxyethylenepropylene copolymers; and amphoteric detergents such as alkyl-(-aminopropionates and 2-alkylimidazoline quaternary ammonium salts; as well as mixtures of detergents.
- Parenteral preparations will typically contain at least about 0.01% by weight of active ingredient in solution.
- injectable compositions may contain a non-ionic surfactant having a hydrophile-lipophile balance (HLB) of from about 12 to about 17.
- HLB hydrophile-lipophile balance
- the quantity of surfactant in such formulations ranges from about 5% to about 15% by weight.
- the surfactant may be a single component having the above HLB or a mixture of two or more components having the desired HLB.
- Particular examples of useful surfactants include polyethylene sorbitan fatty acid esters, such as, for example, sorbitan monooleate.
- D -threo methylphenidate as described herein can be formulated for nasal administration, particularly in the form of powders, nasal drops, or aerosols.
- the compounds of the invention also can be administered dermally, via, for example, trans-dermal patches.
- the preferred quantity of D -threo methylphenidate to be used in a dosage for treating a particular patient can be readily determined by one skilled in the art. Factors determining the appropriate dosage include the weight and age of the patient, the type and extent of the disorder being treated, and other conditions of the patient including other disorders and other medications, if any, that the patient is taking. Generally, the dosage of D -threo methylphenidate will be from about 0.01 mg/kg of patient body weight to about 1 mg/kg of patient body weight. Appropriate quantities can be determined by one skilled in the art. For example, a relatively small child will generally require a dose of from about 0.03 to about 0.3 mg/kg, while a larger child or an adult may require a dose of from about 0.1 mg/kg to about 0.4 or 0.5 mg/kg.
- a physician treating a patient with cancer will generally titrate the dose of methylphenidate until the desired therapeutic effects is achieved.
- a patient with cancer receiving an opioid analgesic for pain management will initially be administered a minimum dose of 2.5 mg of d-MPH b.i.d. at the time of the opioid analgesic, with dose increasing a clinically warranted.
- Response by patients with cognitive deficiencies described herein is generally determined by two types of measurements: objective measures of a patient's ability to concentrate and remain focused on a task such as performing a math test; and subjective scores of a patient's performance.
- HSCS High Sensitivity Cognitive Screen
- Six cognitive domains memory, language, visual-motor, spatial, attention/concentration, and self-regulation and planning (see, for example, Faust D. and Fogel B. S.: The development and initial validation of a sensitive bedside cognitive screening test. J. Nerv. Ment. Dis.; 177:25-31, 1989).
- MMSE Mini-Mental State Exam
- Attention and concentration can be evaluated via the Trial Making Test-Part A (See, for example, Reitan, R. M.: Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills 8:271-276, 1958), and the Digit Span, Forward and Backward test (see, for example, Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. New York; Psychological Corporation, 1981)).
- Visuospatial skills can be evaluated by, for example, the Revised-Rey Osterrieth Complex Figure test, (see, for example, Osterrieth R. A. Le test de copie d'une fugure complexe. Archives de Psychologie, 1944, 30, 206-356). Impairment to language functions can be evaluated by, for example, the Verbal Fluency (F-A-S) test (see, for example Kaplan, E. F., Goodglass, H., Weintraub, S. The Boston Naming Test. Boston: E. Kaplan & H. Goodglass, 1978).
- F-A-S Verbal Fluency
- Learning impairment can be evaluated by, for example, the California Verbal Learning Test (see, for example, Delis, D. C., Kramer, J. H., Kaplan, E., Ober, B. A. California Verbal Learning Test-Research Edition. The Psychological Corp., New York, 1987).
- California Verbal Learning Test see, for example, Delis, D. C., Kramer, J. H., Kaplan, E., Ober, B. A. California Verbal Learning Test-Research Edition. The Psychological Corp., New York, 1987).
- Memory impairment can be evaluated by, for example, the Revised-Rey Osterrieth Complex Figure, Immediate Recall test and/or the Revised-Rey Osterrieth Complex Figure, Delayed Recall test, (see, for example, Osterrieth R A, above); the California Verbal Learning Test, Immediate Recall, and/or the California Verbal Learning Test, Delayed Recall, the California Verbal Learning Test, Recognition (See, for example, Delis et al., above).
- Executive function impairment can be evaluated by, for example, the Trail Making Test-Part B (See, for example, Reitan et al., above).
- Depression can be evaluated by, for example, the Center for Epidemiologic Studies Depression (CES-D) Scale (see, for example, Radloff, L. S.: The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1:385-401, 1977), or by Beck Depression Inventory (BDI) (see, for example, Beck, A. T. and Beamesderfer, A: Assessment of depression: the Depression Inventory. Mod. Probl. Pharmacopsychiatry; 7:155-169, 1974).
- CES-D Center for Epidemiologic Studies Depression
- BDI Beck Depression Inventory
- Patients that have received at least one cycle of cytotoxic chemotherapy, preferably within 2 months prior to treatment, and who display one or more symptoms of cognitive dysfunction are evaluated as candidates for d-MPH treatment.
- patients Prior to commencement of treatment, patients are evaluated for the following: medical history/concomitant illnesses, physical examination, 12-lead electrocardiogram, routine laboratory tests and assessments of cognitive function. Tests for cognitive function can include those know to those of skill in the art, for example those described above.
- Patients having no medical contraindication to the use of methylphenidate are then initially administered d-MPH 5 mg/day (2.5 mg b.i.d given 4 to 6 hours apart).
- the dose may be increased as clinically warranted if there are no adverse effects that preclude dose-escalation and there is no significant therapeutic response.
- Daily doses can be administered two or three times per day.
- the maximum dose will be 50 mg/day, given two to three times per day.
- Patients are evaluated periodically for one or more of fatigue, neurobehavioral slowing, sedation, decreased cognitive function, and major depressive disorder. Patients receiving the foregoing treatment will display an alleviation of one or more of the foregoing symptoms.
- Menopausal women who display one or more symptoms including an executive function defect, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, apathy, or impairment of short term memory are evaluated as candidates for d-MPH treatment. Prior to commencement of treatment, patients are evaluated for the following: medical history/concomitant illnesses, physical examination, 12-lead electrocardiogram, routine laboratory tests and assessments of the severity of the symptom.
- Patients having no medical contraindication to the use of methylphenidate are then initially administered d-MPH 5 mg/day (2.5 mg b.i.d given 4 to 6 hours apart).
- the dose may be increased as clinically warranted if there are no adverse effects that preclude dose-escalation and there is no significant therapeutic response.
- Daily doses can be administered two or three times per day. The maximum dose will be 50 mg/day, given two to three times per day. Once a patient's optimal dose has been determined, the patient will remain on this dose for at least 2 weeks.
- Patients are evaluated for one or more of executive function defect, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, apathy, or impairment of short term memory. Patients receiving the foregoing treatment will display an alleviation of one or more of the foregoing symptoms.
- d-MPH D -Threo-Methylphenidate Hydrochloride
- Patients having no medical contraindication to the use of methylphenidate are then initially administered d-MPH 5 mg/day (2.5 mg b.i.d given 4 to 6 hours apart).
- the dose may be increased as clinically warranted if there are no adverse effects that preclude dose-escalation and there is no significant therapeutic response.
- Daily doses can be administered two or three times per day. The maximum dose will generally be approximately 50 mg/day, given two to three times per day.
- Patients are periodically evaluated for efficacy of treatment. Patients receiving the foregoing treatment will display an alleviation of one or more of the foregoing symptoms.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Psychiatry (AREA)
- Endocrinology (AREA)
- Pain & Pain Management (AREA)
- Reproductive Health (AREA)
- Analytical Chemistry (AREA)
- Anesthesiology (AREA)
- Diabetes (AREA)
- Hospice & Palliative Care (AREA)
- Cardiology (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Heart & Thoracic Surgery (AREA)
- Obesity (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
In one aspect, the present invention is directed to methods for treating fatigue, neurobehavioral slowing and other cognitive disorders and defects due to cancers and treatments associated with cancers, and similar conditions. In a further aspect, the present invention is directed to methods for treating disorders related to menopause, including executive function defects. The methods involve the administration of a composition comprising D -threo methylphenidate that is substantially free of L -threo methylphenidate and of erythro forms of methylphenidate.
Description
- This application is a continuation in part of U.S. Ser. No. 09/318,151, filed May 25, 1999, which is a continuation in part of U.S. Ser No. 08/583,317 filed Jan. 5, 1996, now U.S. Pat. No. 5,773,756, and a continuation in part of U.S. Ser. No. 08/827,230, filed Apr. 2, 1997, now U.S. Pat. No. 5,908,850, which is continuation of U.S. Ser. No. 08/567,131, filed Dec. 4, 1995. The contents of each of the foregoing applications are incorporated herein by reference in their entirety.
- In one aspect, the present invention is directed to methods for treating fatigue, neurobehavioral slowing and other cognitive disorders and defects due to cancers and treatments associated with cancers, and similar conditions. In a further aspect, the present invention is directed to methods for treating disorders related to menopause, including executive function defects. The methods involve the administration of a composition comprising
D -threo methylphenidate that is substantially free ofL -threo methylphenidate and of erythro forms of methylphenidate. - Advanced cancer typically produces severe pain in patients. This pain is often controlled by the administration of large doses of analgesics, including opioid analgesics. However, the pain relief is often accompanied by undesirable side-effects such as unacceptable sedation and/or a decrease in cognitive function. These side effects have a significant negative impact on the quality of life of the patient. In addition, cancer patients often display one or more of a decrease in cognitive function, fatigue, and neurobehavioral slowing that is unrelated to the administration of analgesics, but may be related to the underlying cancer, the treatment of the cancer, or both.
- Menopause is accompanied by several side effects, including an executive function defect. For example, many menopausal women report impairment in short term memory, inability to screen distractions and sustain attention in organization of thoughts and tasks. In addition, women diagnosed with ADD prior to menopause report exacerbation of ADD symptoms during the protracted perimenopausal period and thereafter. See Brown, T. E., Attention-Deficit Disorders and Comorbidities in Children, Adolescents and Aduts, American Psychiatric Press, Washington, D.C., 2000, at p. 40-41.
- Methylphenidate has been used to treat nervous system disorders including Attention Deficit Disorder (ADD), a commonly diagnosed nervous system illness in children, Attention Deficit Hyperactivity Disorder (ADHD), and cognitive decline in patients with Acquired Immunodeficiency Syndrome (AIDS) or AIDS related conditions. See, e.g., Brown, G., Intl. J. Psych. Med. 25(1): 21-37 (1995); Holmes et al., J. Clin. Psychiatry 50: 5-8 (1989). The racemic form of methylphenidate also has been proposed to improve cognitive function in patients receiving large doses of medication. See, for example, Bruera et al., Pain (1992) 163-166, Yee et al., Journal of Pain and Symptom Management (1994), Vol. 9, No.2, 122-125, and Meyers et al., Journal of Clinical Oncology (1998) Vol. 16, No. 7, 2522-2527.
-
- wherein R 2 is phenyl. Pharmaceutically acceptable salts are generally administered clinically. Other phenidate drugs, which also can be administered according to the invention, include those in which the methyl group in the above structures is replaced by C2-C4 alkyl and those in which R2 is optionally substituted with C1-C4 alkyl.
- Clinically, the threo pair of enantiomers of methylphenidate hydrochloride is generally administered for the treatment of ADD and ADHD. The hydrochloride salt is commonly referred to simply as “methylphenidate”. Unless indicated otherwise, the term “methylphenidate” is used broadly herein to include methylphenidate and pharmaceutically acceptable salts thereof, including methylphenidate hydrochloride.
- The threo racemate (pair of enantiomers) of methylphenidate is a mild central nervous system stimulant with pharmacological activity qualitatively similar to that of amphetamines. Undesirable side effects associated with the use of the
DL -threo racemate of methylphenidate include anorexia, weight loss, insomnia, dizziness and dysphoria. Furthermore, the racemate, which is a Schedule II controlled substance, produces a euphoric effect when administered intravenously or through inhalation or ingestion, and thus carries a high potential for abuse. - Srinivas et al. studied the administration of
DL -threo-,D -threo, andL -threo-methylphenidate to children suffering from ADHD, and reported that the pharmacodynamic activity ofDL -threo-methylphenidate resides in theD -threo isomer (Clin. Pharmacol. Ther., 52: 561-568 (1992)). WhileDL -threo-methylphenidate is generally used therapeutically, this racemate includes theL isomer which apparently makes no significant contribution to the pharmacological effectiveness of the drug. The removal of theL isomer is expensive, however, and there has been no reason to do so. - It has been discovered that the use of the
D -threo isomer (2R:2′R) of methylphenidate, substantially free of the ‘1-threo isomer, produces a methylphenidate medication which retains high activity levels and simultaneously may possess reduced euphoric effect and reduced potential for abuse among patients. See U.S. patent Ser. No. 5,908,850, incorporated herein by reference in its entirety. Thus,D -threo-methylphenidate (2R:2′R) may possesses enhanced therapeutic activity with reduced side effects, and 1-threo-methylphenidate may produce undesirable side effects, euphoria and drug abuse potential in patients. - There remains a need for improved methods for alleviating the undesirable symptoms and side-effects described above. This invention is directed to these, as well as other, important ends.
- In one aspect, the present invention provides methods for treating fatigue, neurobehavioral slowing and cognitive side effects arising from cancer, or from a treatment therefor, such as chemotherapy, radiation therapy and administration of medication to control pain. In further aspects, the invention provides methods for alleviation of depression caused by cognitive dysfunction (a “cognitive side effect”) and fatigue associated with cancer, and treatments therefor. The methods of the invention involve the administration of
D -threo-methylphenidate or a pharmaceutically acceptable salt thereof, substantially free of bothL -threo-methylphenidate and erythro methylphenidates. - In some embodiments of the invention, methods are provided for alleviating fatigue and/or neurobehavioral slowing arising from an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - In further aspects, the present invention provides methods for alleviating fatigue or neurobehavioral slowing arising from the administration of a treatment for an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - Also provided in accordance with the present invention are methods for alleviating a cognitive side effect of a treatment for an oncological condition, comprising the steps of identifying a patient suffering from a cognitive side-effect of a treatment for an oncological condition; and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - In some embodiments of the methods of the invention, the treatment for the oncological condition is the administration of pain management and biological therapies, including pain relief medication, chemotherapy, radiation therapy, and surgery. In some particularly preferred embodiments, the treatment for the oncological condition is chemotherapy or the administration of pain relief medication. In further embodiments of the foregoing methods, the pain relief medication is one or more opioid analgesics, nerve blocks or other psychotropic agents.
- In further embodiments of the foregoing methods, the oncological condition is a cancer selected from the group consisting of all malginant conditions, inclduing both solid tumors and nonsolid tumors. In some preferred embodiments, the oncological condition is a solid tumor.
- In some embodiments of the foregoing methods, the cognitive side effect is sedation, decreased cognitive function, major depressive disorder, or neurobehavioral slowing. In some preferred embodiments, the cognitive side effect is sedation or decreased cognitive function.
- In further aspects, the present invention provides methods for treating a symptom of menopause comprising the steps of identifying a patient suffering from a symptom of menpoause; and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - In some embodiments of the foregoing methods, the symptom of menopause is impairment in short term memory, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, and/or apathy.
- In some preferred embodiments of the foregoing methods, the administration of the
D -threo-methylphenidate (2R:2′R), or the pharmaceutically acceptable salt thereof, gives rise to efficacious treatment without interfering with patient sleep patterns or engendering anoretic behavior. - The methods of the invention involve the administration of
D -threo-methylphenidate or a pharmaceutically acceptable salt thereof, substantially free of bothL -threo-methylphenidate and erythro methylphenidates. It is now believed that theL isomer may contribute to the side effects associated with the commercial drug, and that it is thus desirable to administer only the activeD -threo form of the drug. - Thus, in some embodiments of the invention, methods are provided for alleviating fatigue or neurobehavioral slowing arising from an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - In further aspects, the present invention provides methods for alleviating fatigue or neurobehavioral slowing arising from the administration of a treatment for an oncological condition, said method comprising the steps of identifying a patient suffering from said fatigue or neurobehavioral slowing, and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - Also provided in accordance with the present invention are methods for alleviating a cognitive side effect (e.g., neurobehavioral slowing) of a treatment for an oncological condition, comprising the steps of identifying a patient suffering from a cognitive side-effect of a treatment for an oncological condition; and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - In some embodiments of the methods of the invention, the treatment for the oncological condition is the administration of pain management and biological therapies, including pain relief medication, chemotherapy, radiation therapy, and surgery. In some particularly preferred embodiments, the treatment for the oncological condition is chemotherapy or the administration of pain relief medication. In further embodiments of the foregoing methods, the pain relief medication is one or more opioid analgesics, nerve blocks or other psychotropic agents.
- In further embodiments of the foregoing methods, the oncological condition is a cancer selected from the group consisting of all malginant conditions, inclduing both solid tumors and nonsolid tumors. In some preferred embodiments, the oncological condition is a solid tumor.
- In some embodiments of the foregoing methods, the cognitive side effect is sedation, decreased cognitive function, major depressive disorder, or neurobehavioral slowing. In some preferred embodiments, the cognitive side effect is sedation or decreased cognitive function.
- In further aspects, the present invention provides methods for treating a symptom of menopause comprising the steps of identifying a patient suffering from a symptom of menpoause; and administering to said patient a therapeutically effective amount of
D -threo-methylphenidate (2R:2′R) or a pharmaceutically acceptable salt thereof, substantially free of the 1-threo isomer. - In some embodiments of the foregoing methods, the symptom of menopause is impairment in short term memory, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing and/or apathy.
- In some preferred embodiments of the foregoing methods, the administration of the
D -threo-methylphenidate (2R:2′R), or the pharmaceutically acceptable salt thereof, gives rise to efficacious treatment without interfering with patient sleep patterns or engendering anoretic behavior. - The administration of the pharmacodynamically active
D -threo form of methylphenidate may provide efficacious treatment for an entire day with minimal undesirable side effects such as interference with patient sleep patterns or anoretic behavior. It has been surprisingly and unexpectedly discovered that the beneficial effects of theD -threo isomer persist for a longer period time when theD -threo isomer is administered alone than when it is administered in combination with theL -threo isomer. - While it is not intended that the present invention be bound by any particular theory, it is believed that the
L isomer functions as an antagonist to theD isomer. Thus, another aspect of the present invention provides methods for ameliorating or counteracting the effects of methylphenidate drugs, comprising administeringL -threo methylphenidate to a patient who has a serum level ofD -threo methylphenidate. - The present inventors have observed that in the context of ADD,
D -threo methylphenidate has a longer duration of action thanDL -methylphenidate of at least six hours. Patients who were given theD -threo isomer free of theL isomer performed better in objective tests than patients who received theDL -threo racemate or a placebo, at the 6 hour time point. In contrast, the patients who receivedDL -threo racemate did not perform better after that time period than those who received a placebo. Furthermore, subjective observations of the same patients indicated that those who received only theD -threo isomer experienced beneficial effects of the drug for longer times than did those who received theDL -threo racemate. - It is expected that
D -threo methylphenidate will be particularly useful in treating patients affected by fatigue, neurobehavioral slowing, and other cognitive defects (‘cognitive side effects”) that are due to cancer, and that are exacerbated by the administration of treatments for cancer such as chemotherapy, radiation therapy, bone marrow transplants, stem cell transplants and administration of medication to control pain. Examples of such cognitive defects include but are not limited to neurobehavioral slowing, sedation, diminished executive function, decreased cognitive function, major depressive disorder and impaired quality of life. - As used herein, the term “oncological condition” is intended to mean all malignant conditions, including all cancers, for example solid tumors and nonsolid tumors. Examples of “oncological conditions” include cancers of the skin, mouth, brain and other nervous tissue, bone, lung, colon and rectum, pancreas, prostate, urinary tract, leukemias and lymphomas.
- As used herein, the term “arising from the administration of a treatment for an oncological condition” is intended to mean that the indicated symptom or condition is in whole or in part caused by (i.e., is a side-effect of) the administration of a therapeutic agent used for the treatment of cancer, or for the management of a symptom of the cancer. Examples of agents used for the treatment of cancer include chemotherapeutic agents, including both chemical and radiotherapeutics, and radiation. Examples of agents used for the management of a symptom of the cancer include pain relief medications such as opioid or opoid-like analgesics and non-steroidal anti-inflammatory agents.
- As used herein, the term “alleviating a cognitive side effect of a treatment for an oncological condition” means the lessening of the severity of a cognitive side effect caused in whole or in part by the administration of a treatment for an oncological condition. The term “cognitive side effect” as used herein denotes an impairment of one or more cognitive functions that results in whole or in part from the administration of an agent used for the treatment of cancer. Examples of cognitive side effects include sedation, neurobehavioral slowing, decreased cognitive function, depression, apathy, decreased libido and derpersonalization. The term “decreased cognitive function” is intended to mean a decrease in any or all aspects of thought, attention, perception, and/or memory.
- As used herein, the term “menopause” is given its normal meaning of the period during which marks the permanent cessation of menstrual activity. The term “symptom of menopause” in intended to include those symptoms associated with menopause, including vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, apathy, mental depression and impairment of short term memory. As used herein, the term “executive function defect” is intended to include but is not limited to one or more defects in the cognitive mechanisms responsible for focusing attention, goal-related behavior, strategic planning and problem solving.
- The methods of the invention will find use with patients, including outpatients, with all types of cancer, either primary or metastatic.
- According to one method of the present invention, dosage forms are administered of
D -threo methylphenidate substantially free ofL -threo methylphenidate and of erythro methylphenidates. “Substantially free”, as used herein, means that the dosage forms comprise at least about 95 percent, preferably at least about 97 percent, and more preferably at least about 99 percent of theD -threo isomer, to the exclusion of theL -threo and erythro forms. TheD -threo form can be isolated by methods known to those skilled in the art. - In accordance with the present invention, the
D -threo methylphenidate can be administered in any of a variety of dosage regimes. Such regimes include chronic single, bolus dosages, i.e., where one dose being administered in a predetermined time period, for example twenty four hours. Further dosage regimes include those where multiple dosages are manually administered, and dosage forms where a single dosage form is administered that effectively mimics multiple dosages, such as pulsatile release dosage forms. Further dosage forms useful with the present invention include delay release and extended release (i.e., “time release”) dosage forms. The selection of appropriate dosage forms for an individual patient will depend upon the individual circumstances, and will be apparent to those of skill in the art. - “Chronic”, as used herein, refers to continuous, regular, long-term therapeutic administration, i.e. periodic administration without substantial interruption, such as, for example, daily, for a time period of at least several weeks or months to several years, for the purpose of treating a patient needing treatment.
- “Bolus”, as used herein, refers to administration of a drug as a single event. The term “bolus” is intended to exclude dosage forms such as sustained release, pulsed release, and time release, and includes any dosage form which can be used to deliver a single dose. According to the present invention, a bolus is preferably administered to a patient in need of treatment once daily, more preferably in the morning. The bolus dosages of the present invention may be administered in any conventional form known to those skilled in the art. Suitable methods for administration include oral dosage forms, injection, and infusion.
- For pharmaceutical use, the
D -threo methylphenidate substantially free ofL -threo methylphenidate and of erythro methylphenidates as described herein can be taken up in pharmaceutically acceptable carriers, such as, for example, solutions, suspensions, tablets, capsules, ointments, elixirs and injectable compositions. Pharmaceutical preparations generally can contain from about 1% to about 90% by weight of active ingredient. Preparations which are in single dose form, “unit dosage form”, preferably contain from about 20% to about 90% active ingredient. As used herein, the term “active ingredient” refers toD -threo methylphenidate substantially free ofL -threo methylphenidate and of erythro methylphenidates as described herein, salts thereof, and mixtures ofD -threo methylphenidate as described herein with other pharmaceutically active compounds. Dosage unit forms such as, for example, tablets or capsules, typically contain from about 0.001 to about 1.0 g of active ingredient. Pharmaceutical preparations may be administered orally, parenterally, or topically. Pharmaceutical preparations containing compounds described herein may be prepared by methods known to those skilled in the art, such as, for example, conventional mixing, granulating, dissolving, or lyophilizing. Oral dosage forms include capsules, pills, tablets, troches, lozenges, melts, powders, solutions, suspensions and emulsions. The oral dosage forms provided by the invention can be in the form of tablets, caplets, and the like and can be of any shape suitable for oral administration of a drug, such as spheroidal, cube-shaped, oval, bean shaped, or ellipsoidal. For oral dosage forms, for example, the compounds may be combined with one or more solid pharmaceutically acceptable carriers, optionally granulating the resulting mixture. Pharmaceutically acceptable adjuvants may optionally be included, such as, for example, flow-regulating agents and lubricants. Suitable carriers include, for example, fillers such as sugars, cellulose preparations, calcium phosphates; and binders such as methylcellulose, hydroxymethylcellulose, and starches, such as, for example, maize starch, potato starch, rice starch, and wheat starch. The dosage form may be in the form of granules, which may be irregularly shaped. The dosage form can comprise a capsule containing particles. Examples of orally administrable pharmaceutical preparations are dry-filled capsules consisting of gelatin, and soft sealed capsules consisting of gelatin and a plasticizer such as glycerol or sorbitol. The dry-filled capsules may contain the active ingredient in the form of a granulate, for example in admixture with fillers, binders, glidants, and stabilizers. In soft capsules, the active ingredient is preferably dissolved or suspended in a suitable liquid adjuvant, such as, for example, a fatty oil, paraffin oil, or liquid polyethylene glycol, optionally in the presence of stabilizers. Other oral administrable forms include syrups containing active ingredient, for example, in suspended form at a concentration of from about 0.01% to 20%, or in a similar concentration that provides a suitable single dose when administered, for example, in measures of from about 2 to about 5 milliliters. Suitable excipients for use in oral liquid dosage forms include diluents such as water and alcohols, for example ethanol, benzyl alcohol and polyethylene alcohols, either with or without the addition of a pharmaceutically acceptable surfactant, suspending agent, or emulsifying agent. Also suitable are powdered or liquid concentrates for combining with liquids such as milk. Such concentrates may also be packed in single dose quantities. - In accordance with the present invention,
D -threo methylphenidate as described herein may be administered parenterally, that is, subcutaneously, intravenously, intramuscularly, or interperitoneally, as injectable dosages of the compound in a physiologically acceptable diluent with a pharmaceutical carrier. Solutions for parenteral administration may be in the form of infusion solutions. A pharmaceutical carrier may be, for example, a sterile liquid or mixture of liquids such as water, saline, aqueous dextrose and related sugar solutions, an alcohol such as ethanol, glycols such as propylene glycol or polyethylene glycol, glycerol ketals such as 2,2-dimethyl-1,3-dioxolane-4-methanol, ethers such as poly(ethyleneglycol)400, oils, fatty acids, fatty acid esters or glycerides, with or without the addition of a pharmaceutically acceptable surfactant such as a soap or detergent, suspending agent such as pectin, carbomers, methylcellulose, hydroxypropylmethylcellulose, or carboxymethylcellulose, or emulsifying agent or other pharmaceutically acceptable adjuvants. Examples of oils which may be used in parenteral formulations include petroleum, animal, vegetable, or synthetic oils such as, for example, peanut oil, soybean oil, sesame oil, cottonseed oil, corn oil, olive oil, petrolatum, and mineral oil. Suitable fatty acids include, for example, oleic acid, stearic acid, and isostearic acid. Suitable fatty acid esters include ethyl oleate and isopropyl myristate. Suitable soaps include alkaline metal, ammonium and triethanolamine salts of fatty acids. Suitable detergents include cationic detergents such as dimethyl dialkyl ammonium halides and alkyl pyridinium halides; anionic detergents such as alkyl, aryl and olefin sulfonates, monoglyceride sulfates and sulfosuccinates; nonionic detergents such as fatty amino oxides, fatty acid alkanolamides and polyoxyethylenepropylene copolymers; and amphoteric detergents such as alkyl-(-aminopropionates and 2-alkylimidazoline quaternary ammonium salts; as well as mixtures of detergents. Parenteral preparations will typically contain at least about 0.01% by weight of active ingredient in solution. Preservatives and buffers may also be used advantageously. Injection suspensions may include viscosity-increasing substances such as, for example, sodium carboxymethylcellulose, sorbitol or dextran, and may also include stabilizers. In order to minimize irritation at the site of injection, injectable compositions may contain a non-ionic surfactant having a hydrophile-lipophile balance (HLB) of from about 12 to about 17. The quantity of surfactant in such formulations ranges from about 5% to about 15% by weight. The surfactant may be a single component having the above HLB or a mixture of two or more components having the desired HLB. Particular examples of useful surfactants include polyethylene sorbitan fatty acid esters, such as, for example, sorbitan monooleate. - In addition to parenteral administration,
D -threo methylphenidate as described herein can be formulated for nasal administration, particularly in the form of powders, nasal drops, or aerosols. The compounds of the invention also can be administered dermally, via, for example, trans-dermal patches. - The preferred quantity of
D -threo methylphenidate to be used in a dosage for treating a particular patient can be readily determined by one skilled in the art. Factors determining the appropriate dosage include the weight and age of the patient, the type and extent of the disorder being treated, and other conditions of the patient including other disorders and other medications, if any, that the patient is taking. Generally, the dosage ofD -threo methylphenidate will be from about 0.01 mg/kg of patient body weight to about 1 mg/kg of patient body weight. Appropriate quantities can be determined by one skilled in the art. For example, a relatively small child will generally require a dose of from about 0.03 to about 0.3 mg/kg, while a larger child or an adult may require a dose of from about 0.1 mg/kg to about 0.4 or 0.5 mg/kg. - A physician treating a patient with cancer will generally titrate the dose of methylphenidate until the desired therapeutic effects is achieved. For example, a patient with cancer receiving an opioid analgesic for pain management will initially be administered a minimum dose of 2.5 mg of d-MPH b.i.d. at the time of the opioid analgesic, with dose increasing a clinically warranted.
- Response by patients with cognitive deficiencies described herein is generally determined by two types of measurements: objective measures of a patient's ability to concentrate and remain focused on a task such as performing a math test; and subjective scores of a patient's performance.
- The following examples are merely illustrative of the present invention and should not be considered limiting of the scope of the invention in any way. These examples and equivalents thereof will become more apparent to those skilled in the art in light of the present disclosure and the accompanying claims.
- Determination of Symptoms
- Patients can be evaluated for cognitive impairment by any of the tests known in the art. For example, High Sensitivity Cognitive Screen (HSCS) can test six cognitive domains: memory, language, visual-motor, spatial, attention/concentration, and self-regulation and planning (see, for example, Faust D. and Fogel B. S.: The development and initial validation of a sensitive bedside cognitive screening test. J. Nerv. Ment. Dis.; 177:25-31, 1989).
- Global cognitive function can be evaluated by the Mini-Mental State Exam (MMSE; see, for example, Folstein M. F., Folstin S. E., McHugh P. R.: “Minimental State”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatry Res. 12:189-198, 1975) Attention and concentration can be evaluated via the Trial Making Test-Part A (See, for example, Reitan, R. M.: Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Motor Skills 8:271-276, 1958), and the Digit Span, Forward and Backward test (see, for example, Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. New York; Psychological Corporation, 1981)).
- Visuospatial skills can be evaluated by, for example, the Revised-Rey Osterrieth Complex Figure test, (see, for example, Osterrieth R. A. Le test de copie d'une fugure complexe. Archives de Psychologie, 1944, 30, 206-356). Impairment to language functions can be evaluated by, for example, the Verbal Fluency (F-A-S) test (see, for example Kaplan, E. F., Goodglass, H., Weintraub, S. The Boston Naming Test. Boston: E. Kaplan & H. Goodglass, 1978).
- Learning impairment can be evaluated by, for example, the California Verbal Learning Test (see, for example, Delis, D. C., Kramer, J. H., Kaplan, E., Ober, B. A. California Verbal Learning Test-Research Edition. The Psychological Corp., New York, 1987).
- Memory impairment can be evaluated by, for example, the Revised-Rey Osterrieth Complex Figure, Immediate Recall test and/or the Revised-Rey Osterrieth Complex Figure, Delayed Recall test, (see, for example, Osterrieth R A, above); the California Verbal Learning Test, Immediate Recall, and/or the California Verbal Learning Test, Delayed Recall, the California Verbal Learning Test, Recognition (See, for example, Delis et al., above).
- Executive function impairment can be evaluated by, for example, the Trail Making Test-Part B (See, for example, Reitan et al., above).
- Quality of Life can be evaluated by the FACIT-F tests (Fatigue Scale), the FACT-F test (Functional Assessment of Cancer Therapy—Fatigue Scale), the FACT-G test (Functional Assessment of Cancer Therapy—General Scale), and the FACT-BR test (Functional Assessment of Cancer Therapy—Brain Subscale), (see, for example Cella, D. F., Tulsky, D. S., Gray, G., Sarafian, B., Linn E. Bonomi, A., et al.: The functional assessment of cancer therapy scale: development and validation of the general measure. J. Clin. Oncol. 11:570-579, 1993; Weitzner, M. A., Meyers, C. A., Gelke, C. K., Byrne, K. S., Cella, D. F., Levin, V. A.: The Functional Assessment of Cancer Therapy (FACT) scale: Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 75:1151-1161, 1995; and Yellin, S. B., Cella, D. F., Webster, K, Blendowsky, C., Kaplan, E.,: Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) Measurement System., J. Pain Symptom Manage. 13:63-74, 1997). Each of the foregoing publications are incorporated herein by reference in their entirety.
- Depression can be evaluated by, for example, the Center for Epidemiologic Studies Depression (CES-D) Scale (see, for example, Radloff, L. S.: The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1:385-401, 1977), or by Beck Depression Inventory (BDI) (see, for example, Beck, A. T. and Beamesderfer, A: Assessment of depression: the Depression Inventory. Mod. Probl. Pharmacopsychiatry; 7:155-169, 1974).
- Patients that have received at least one cycle of cytotoxic chemotherapy, preferably within 2 months prior to treatment, and who display one or more symptoms of cognitive dysfunction are evaluated as candidates for d-MPH treatment. Prior to commencement of treatment, patients are evaluated for the following: medical history/concomitant illnesses, physical examination, 12-lead electrocardiogram, routine laboratory tests and assessments of cognitive function. Tests for cognitive function can include those know to those of skill in the art, for example those described above. Patients having no medical contraindication to the use of methylphenidate are then initially administered d-MPH 5 mg/day (2.5 mg b.i.d given 4 to 6 hours apart). The dose may be increased as clinically warranted if there are no adverse effects that preclude dose-escalation and there is no significant therapeutic response. Daily doses can be administered two or three times per day. The maximum dose will be 50 mg/day, given two to three times per day.
- Patients are evaluated periodically for one or more of fatigue, neurobehavioral slowing, sedation, decreased cognitive function, and major depressive disorder. Patients receiving the foregoing treatment will display an alleviation of one or more of the foregoing symptoms.
- Menopausal women who display one or more symptoms including an executive function defect, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, apathy, or impairment of short term memory are evaluated as candidates for d-MPH treatment. Prior to commencement of treatment, patients are evaluated for the following: medical history/concomitant illnesses, physical examination, 12-lead electrocardiogram, routine laboratory tests and assessments of the severity of the symptom.
- Patients having no medical contraindication to the use of methylphenidate are then initially administered d-MPH 5 mg/day (2.5 mg b.i.d given 4 to 6 hours apart). The dose may be increased as clinically warranted if there are no adverse effects that preclude dose-escalation and there is no significant therapeutic response. Daily doses can be administered two or three times per day. The maximum dose will be 50 mg/day, given two to three times per day. Once a patient's optimal dose has been determined, the patient will remain on this dose for at least 2 weeks.
- Patients are evaluated for one or more of executive function defect, decreased cognitive function, mental depression, vasomotor instability, nervousness, excitability, fatigue, neurobehavioral slowing, apathy, or impairment of short term memory. Patients receiving the foregoing treatment will display an alleviation of one or more of the foregoing symptoms.
- Menopausal women who have been previously been diagnosed with Attention Deficit Disorder (“ADD”) and who are suspected having exacerbated ADD symptoms are evaluated for one or more symptoms of ADD according to previously published methods (for example, see American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington, D.C., 1994, pp 78-85).
- Patients having no medical contraindication to the use of methylphenidate are then initially administered d-MPH 5 mg/day (2.5 mg b.i.d given 4 to 6 hours apart). The dose may be increased as clinically warranted if there are no adverse effects that preclude dose-escalation and there is no significant therapeutic response. Daily doses can be administered two or three times per day. The maximum dose will generally be approximately 50 mg/day, given two to three times per day.
- Patients are periodically evaluated for efficacy of treatment. Patients receiving the foregoing treatment will display an alleviation of one or more of the foregoing symptoms.
- It is intended that each of the patents, applications, and printed publications mentioned or referred to in this specification be hereby incorporated by reference in their entirety.
- As those skilled in the art will appreciate, numerous changes and modifications may be made to the preferred embodiments of the invention without departing from the spirit of the invention. It is intended that all such variations fall within the scope of the invention.
Claims (33)
1-46. (canceled)
47. A method of alleviating a cognitive side effect due to cancer or a treatment for cancer comprising:
identifying a patient suffering from said side effect, and
administrating a therapeutically effective amount of D-threo methylphenidate to said patient in a pulsatile release dosage form.
48. The method of claim 47 wherein said D-threo methylphenidate is in the form of a pharmaceutically acceptable salt.
49. The method of claim 47 wherein said cancer is a solid tumor or nonsolid tumor.
50. The method of claim 49 wherein said cancer is a solid tumor.
51. The method of claim 47 wherein said treatment comprises chemotherapy or radiation therapy.
52. The method of claim 47 wherein said dosage form comprises a pharmaceutically acceptable carrier.
53. The method of claim 52 wherein said carrier is a tablet or a capsule.
54. The method of claim 47 wherein said administration is by oral dosage form.
55. The method of claim 47 wherein said side effect comprises sedation, neurobehavioral slowing, decreased cognitive function, depression, apathy, decreased libido, or depersonalization.
56. A method of alleviating a cognitive side effect due to cancer or a treatment for cancer comprising:
identifying a patient suffering from said side effect, and
administrating a therapeutically effective amount of D-threo methylphenidate to said patient in a chronic, bolus dosage form.
57. The method of claim 56 wherein said D-threo methylphenidate is in the form of a pharmaceutically acceptable salt.
58. The method of claim 56 wherein said cancer is a solid tumor or nonsolid tumor.
59. The method of claim 58 wherein said cancer is a solid tumor.
60. The method of claim 56 wherein said treatment comprises chemotherapy or radiation therapy.
61. The method of claim 56 wherein said dosage form comprises a pharmaceutically acceptable carrier.
62. The method of claim 61 wherein said carrier is a solution, suspension, tablet, capsule, ointment, elixir, or injectable composition.
63. The method of claim 56 wherein said administration is by oral dosage form, injection, or infusion.
64. The method of claim 56 wherein said side effect comprises sedation, neurobehavioral slowing, decreased cognitive function, depression, apathy, decreased libido, or depersonalization.
65. A method of alleviating a cognitive side effect due to cancer or a treatment for cancer comprising:
identifying a patient suffering from said side effect, and
administrating a therapeutically effective amount of D-threo methylphenidate to said patient in a time release dosage form.
66. The method of claim 65 wherein said D-threo methylphenidate is in the form of a pharmaceutically acceptable salt.
67. The method of claim 65 wherein said cancer is a solid tumor or nonsolid tumor.
68. The method of claim 67 wherein said cancer is a solid tumor.
69. The method of claim 65 wherein said treatment comprises chemotherapy or radiation therapy.
70. The method of claim 65 wherein said dosage form comprises a pharmaceutically acceptable carrier.
71. The method of claim 70 wherein said carrier is a solution, suspension, tablet, or a capsule.
72. The method of claim 65 wherein said administration is by oral dosage form, injection, or infusion.
73. The method of claim 65 wherein said side effect comprises sedation, neurobehavioral slowing, decreased cognitive function, depression, apathy, decreased libido, or depersonalization.
74. A method of alleviating a cognitive side effect of chemotherapy comprising:
identifying a patient suffering from said side effect, and
administrating a therapeutically effective amount of D-threo methylphenidate following the end of at least one cycle of chemotherapy.
75. The method of claim 74 wherein said D-threo methylphenidate is in the form of a pharmaceutically acceptable salt.
76. The method of claim 74 wherein said administration is by oral dosage form, injection, infusion, or pharmaceutically acceptable carrier.
77. The method of claim 76 wherein said carrier is a solution, suspension, tablet, capsule, ointment, elixir, or injectable composition.
78. The method of claim 74 wherein said side effect comprises sedation, neurobehavioral slowing, decreased cognitive function, depression, apathy, decreased libido or depersonalization.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/820,397 US20040204456A1 (en) | 1995-12-04 | 2004-04-08 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US56713195A | 1995-12-04 | 1995-12-04 | |
| US08/827,230 US5908850A (en) | 1995-12-04 | 1997-04-02 | Method of treating attention deficit disorders with d-threo methylphenidate |
| US09/318,151 US6355656B1 (en) | 1995-12-04 | 1999-05-25 | Phenidate drug formulations having diminished abuse potential |
| US09/903,803 US6486177B2 (en) | 1995-12-04 | 2001-07-12 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US10/195,974 US7115631B2 (en) | 1995-12-04 | 2002-07-16 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US10/820,397 US20040204456A1 (en) | 1995-12-04 | 2004-04-08 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/195,974 Continuation US7115631B2 (en) | 1995-12-04 | 2002-07-16 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040204456A1 true US20040204456A1 (en) | 2004-10-14 |
Family
ID=25418099
Family Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/903,803 Expired - Fee Related US6486177B2 (en) | 1995-12-04 | 2001-07-12 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US10/195,974 Expired - Fee Related US7115631B2 (en) | 1995-12-04 | 2002-07-16 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US10/820,397 Abandoned US20040204456A1 (en) | 1995-12-04 | 2004-04-08 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US11/402,385 Abandoned US20060183774A1 (en) | 1995-12-04 | 2006-04-11 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US12/640,734 Abandoned US20100093797A1 (en) | 1995-12-04 | 2009-12-17 | Methods For Treatment Of Cognitive And Menopausal Disorders With D-Threo Methylphenidate |
| US13/172,977 Abandoned US20110257227A1 (en) | 1995-12-04 | 2011-06-30 | Methods For Treatment Of Cognitive And Menopausal Disorders With D-Threo Methylphenidate |
| US13/661,472 Abandoned US20130053414A1 (en) | 1995-12-04 | 2012-10-26 | Methods for treatment of cognitive and menopausal disorders with d-threo methylphenidate |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/903,803 Expired - Fee Related US6486177B2 (en) | 1995-12-04 | 2001-07-12 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US10/195,974 Expired - Fee Related US7115631B2 (en) | 1995-12-04 | 2002-07-16 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/402,385 Abandoned US20060183774A1 (en) | 1995-12-04 | 2006-04-11 | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US12/640,734 Abandoned US20100093797A1 (en) | 1995-12-04 | 2009-12-17 | Methods For Treatment Of Cognitive And Menopausal Disorders With D-Threo Methylphenidate |
| US13/172,977 Abandoned US20110257227A1 (en) | 1995-12-04 | 2011-06-30 | Methods For Treatment Of Cognitive And Menopausal Disorders With D-Threo Methylphenidate |
| US13/661,472 Abandoned US20130053414A1 (en) | 1995-12-04 | 2012-10-26 | Methods for treatment of cognitive and menopausal disorders with d-threo methylphenidate |
Country Status (10)
| Country | Link |
|---|---|
| US (7) | US6486177B2 (en) |
| EP (1) | EP1411943A4 (en) |
| JP (1) | JP2005520780A (en) |
| KR (1) | KR20040029360A (en) |
| AU (1) | AU2002318302B2 (en) |
| CA (1) | CA2453510C (en) |
| IL (1) | IL159313A0 (en) |
| MX (1) | MXPA04000249A (en) |
| NZ (2) | NZ530211A (en) |
| WO (1) | WO2003005962A2 (en) |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU708873B2 (en) | 1995-07-14 | 1999-08-12 | Medeva Europe Limited | Composition comprising d-threo-methylphenidate and another drug |
| US5837284A (en) * | 1995-12-04 | 1998-11-17 | Mehta; Atul M. | Delivery of multiple doses of medications |
| US6486177B2 (en) * | 1995-12-04 | 2002-11-26 | Celgene Corporation | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US5922736A (en) * | 1995-12-04 | 1999-07-13 | Celegene Corporation | Chronic, bolus administration of D-threo methylphenidate |
| US6962997B1 (en) * | 1997-05-22 | 2005-11-08 | Celgene Corporation | Process and intermediates for resolving piperidyl acetamide steroisomers |
| WO2001097806A1 (en) * | 2000-06-20 | 2001-12-27 | Herbst Arthur L | Cox-2 inhibitors and the prevention of the side effects of radiation therapy |
| FR2823975B1 (en) * | 2001-04-27 | 2003-05-30 | Sanofi Synthelabo | NEW USE OF PYRIDOINDOLONE |
| JP4959335B2 (en) * | 2003-10-08 | 2012-06-20 | マリンクロッド エルエルシー | Methylphenidate solution and related administration and manufacturing methods |
| US20050239830A1 (en) * | 2004-04-26 | 2005-10-27 | Vikram Khetani | Methods of diminishing co-abuse potential |
| KR20070045278A (en) * | 2004-07-22 | 2007-05-02 | 와이어쓰 | Method for treating nervous system disorders and conditions |
| KR20070034126A (en) * | 2004-07-22 | 2007-03-27 | 와이어쓰 | Methods of treatment of diseases and disorders of the nervous system |
| BRPI0512182A (en) * | 2004-07-22 | 2008-02-19 | Wyeth Corp | Method for treating nervous system conditions and disorders |
| WO2006047330A2 (en) * | 2004-10-22 | 2006-05-04 | Mark Froimowitz | Methylphenidate analogs and methods of use thereof |
| JP2008523097A (en) * | 2004-12-09 | 2008-07-03 | セルジーン・コーポレーション | Treatment with D-threomethylphenidate |
| KR101318806B1 (en) | 2005-01-20 | 2013-10-16 | 인스티튜트 포 몰리큘러 메디신, 인코포레이티드 | Methylphenidate derivatives and uses of them |
| WO2006078984A2 (en) * | 2005-01-20 | 2006-07-27 | Institute For Molecular Medicine, Inc. | Uses of methylphenidate derivatives |
| WO2007013936A2 (en) * | 2005-07-21 | 2007-02-01 | Wyeth | Method for treating nervous system disorders and conditions |
| WO2007109104A2 (en) | 2006-03-16 | 2007-09-27 | Tris Pharma, Inc. | Modified release formulations containing drug-ion exchange resin complexes |
| JP5856843B2 (en) | 2008-05-27 | 2016-02-10 | アンピオ ファーマシューティカルズ,インコーポレイテッド | Pharmaceutical composition using diketopiperazine |
| US9345558B2 (en) | 2010-09-03 | 2016-05-24 | Ormco Corporation | Self-ligating orthodontic bracket and method of making same |
| EP2616055A1 (en) * | 2010-09-13 | 2013-07-24 | Tenera Therapeutics, LLC | Compositions for treating cancer treatment - related fatigue |
| US8287903B2 (en) | 2011-02-15 | 2012-10-16 | Tris Pharma Inc | Orally effective methylphenidate extended release powder and aqueous suspension product |
| BR112014005909B1 (en) * | 2011-09-15 | 2021-05-18 | Friulchem Spa | compositions for oral administration to animals, methods of production thereof and uses thereof |
| KR101312286B1 (en) * | 2012-11-26 | 2013-09-27 | (주)비씨월드제약 | Pharmaceutical composition having immediate release and controlled release properties and comprising d-threo-methylphenidate |
| US11590228B1 (en) | 2015-09-08 | 2023-02-28 | Tris Pharma, Inc | Extended release amphetamine compositions |
| US11590081B1 (en) | 2017-09-24 | 2023-02-28 | Tris Pharma, Inc | Extended release amphetamine tablets |
Citations (69)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US19535A (en) * | 1858-03-02 | Improvement in sewing-machines | ||
| US32335A (en) * | 1861-05-14 | Island | ||
| US49205A (en) * | 1865-08-01 | Improvement in machines for hulling grain | ||
| US105134A (en) * | 1870-07-05 | Thomas sheehan | ||
| US2507631A (en) * | 1944-01-19 | 1950-05-16 | Ciba Pharm Prod Inc | Pyridine and piperidine compounds and process of making same |
| US2838519A (en) * | 1953-12-23 | 1958-06-10 | Ciba Pharm Prod Inc | Process for the conversion of stereoisomers |
| US2957880A (en) * | 1953-12-23 | 1960-10-25 | Ciba Pharm Prod Inc | Process for the conversion of stereoisomers |
| US4137300A (en) * | 1976-08-20 | 1979-01-30 | Ciba-Geigy Corporation | Sustained action dosage forms |
| US4410700A (en) * | 1980-07-03 | 1983-10-18 | The United States Of America As Represented By The Department Of Health And Human Services | Preparation of chiral 1-benzyl-1,2,3,4-tetrahydroisoquinolines by optical resolution |
| US4794001A (en) * | 1986-03-04 | 1988-12-27 | American Home Products Corporation | Formulations providing three distinct releases |
| US4882166A (en) * | 1981-09-30 | 1989-11-21 | National Research Development Corporation | Compositions comprising encapsulated particles and their preparation |
| US4904476A (en) * | 1986-03-04 | 1990-02-27 | American Home Products Corporation | Formulations providing three distinct releases |
| US4968505A (en) * | 1988-08-16 | 1990-11-06 | Ss Pharmaceutical Company, Ltd. | Long-acting diclofenac sodium preparation |
| US4986987A (en) * | 1986-05-09 | 1991-01-22 | Alza Corporation | Pulsed drug delivery |
| US4992445A (en) * | 1987-06-12 | 1991-02-12 | American Cyanamid Co. | Transdermal delivery of pharmaceuticals |
| US5104899A (en) * | 1990-08-13 | 1992-04-14 | Sepracor, Inc. | Methods and compositions for treating depression using optically pure fluoxetine |
| US5114946A (en) * | 1987-06-12 | 1992-05-19 | American Cyanamid Company | Transdermal delivery of pharmaceuticals |
| US5133974A (en) * | 1989-05-05 | 1992-07-28 | Kv Pharmaceutical Company | Extended release pharmaceutical formulations |
| US5137733A (en) * | 1990-06-28 | 1992-08-11 | Tanabe Seiyaku Co., Ltd. | Controlled release pharmaceutical preparation |
| US5156850A (en) * | 1990-08-31 | 1992-10-20 | Alza Corporation | Dosage form for time-varying patterns of drug delivery |
| US5158777A (en) * | 1990-02-16 | 1992-10-27 | E. R. Squibb & Sons, Inc. | Captopril formulation providing increased duration of activity |
| US5160744A (en) * | 1991-06-27 | 1992-11-03 | Alza Corporation | Verapmil therapy |
| US5202128A (en) * | 1989-01-06 | 1993-04-13 | F. H. Faulding & Co. Limited | Sustained release pharmaceutical composition |
| US5217718A (en) * | 1989-08-18 | 1993-06-08 | Cygnus Therapeutic Systems | Method and device for administering dexmedetomidine transdermally |
| US5223265A (en) * | 1992-01-10 | 1993-06-29 | Alza Corporation | Osmotic device with delayed activation of drug delivery |
| US5229131A (en) * | 1990-02-05 | 1993-07-20 | University Of Michigan | Pulsatile drug delivery system |
| US5232705A (en) * | 1990-08-31 | 1993-08-03 | Alza Corporation | Dosage form for time-varying patterns of drug delivery |
| US5236689A (en) * | 1987-06-25 | 1993-08-17 | Alza Corporation | Multi-unit delivery system |
| US5283193A (en) * | 1988-06-27 | 1994-02-01 | Asahi Kasei Kogyo K.K. | Process for producing optically active α-substituted organic acid and microorganism and enzyme used therefor |
| US5284769A (en) * | 1989-10-16 | 1994-02-08 | Chiros Ltd. | Process for preparing a single enantiomer of a lactam using lactamase |
| US5299121A (en) * | 1992-06-04 | 1994-03-29 | Medscreen, Inc. | Non-prescription drug medication screening system |
| US5308348A (en) * | 1992-02-18 | 1994-05-03 | Alza Corporation | Delivery devices with pulsatile effect |
| US5326570A (en) * | 1991-07-23 | 1994-07-05 | Pharmavene, Inc. | Advanced drug delivery system and method of treating psychiatric, neurological and other disorders with carbamazepine |
| US5331000A (en) * | 1992-03-09 | 1994-07-19 | Sepracor Inc. | Antipyretic and analgesic methods and compositions containing optically pure R(-) ketoprofen |
| US5362755A (en) * | 1990-01-05 | 1994-11-08 | Sepracor, Inc. | Method for treating asthma using optically pure (R)-albuterol |
| US5375693A (en) * | 1992-08-03 | 1994-12-27 | Sepracor, Inc. | Methods and compositions for treating allergic disorders and other disorders metabolic derivatives of terfenadine |
| US5391381A (en) * | 1987-06-25 | 1995-02-21 | Alza Corporation | Dispenser capable of delivering plurality of drug units |
| US5425950A (en) * | 1991-10-30 | 1995-06-20 | Glaxo Group Limited | Controlled release pharmaceutical compositions |
| US5449743A (en) * | 1993-01-29 | 1995-09-12 | Shiro Kobayashi | Method for ring opening polymerization using a hydrolase catalyst |
| US5478573A (en) * | 1992-12-23 | 1995-12-26 | Kinaform Technology, Inc. | Delayed, sustained-release propranolol pharmaceutical preparation |
| US5496561A (en) * | 1993-08-25 | 1996-03-05 | Ss Pharmaceutical Co., Ltd. | Controlled release-initiation and controlled release-rate pharmaceutical composition |
| US5500227A (en) * | 1993-11-23 | 1996-03-19 | Euro-Celtique, S.A. | Immediate release tablet cores of insoluble drugs having sustained-release coating |
| US5512293A (en) * | 1992-07-23 | 1996-04-30 | Alza Corporation | Oral sustained release drug delivery device |
| US5567441A (en) * | 1995-03-24 | 1996-10-22 | Andrx Pharmaceuticals Inc. | Diltiazem controlled release formulation |
| US5580578A (en) * | 1992-01-27 | 1996-12-03 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| US5593694A (en) * | 1991-10-04 | 1997-01-14 | Yoshitomi Pharmaceutical Industries, Ltd. | Sustained release tablet |
| US5672360A (en) * | 1993-11-23 | 1997-09-30 | Purdue Pharma, L.P. | Method of treating pain by administering 24 hour oral opioid formulations |
| US5733756A (en) * | 1996-01-05 | 1998-03-31 | Celgene Corporation | Lactams and processes for stereoselective enrichment of lactams, amides, and esters |
| US5773478A (en) * | 1995-07-14 | 1998-06-30 | Medeva Europe Limited | Composition comprising methylphenidate and another drug |
| US5837284A (en) * | 1995-12-04 | 1998-11-17 | Mehta; Atul M. | Delivery of multiple doses of medications |
| US5874090A (en) * | 1995-07-14 | 1999-02-23 | Medeva Europe Limited | Sustained-release formulation of methylphenidate |
| US5908850A (en) * | 1995-12-04 | 1999-06-01 | Celgene Corporation | Method of treating attention deficit disorders with d-threo methylphenidate |
| US5922736A (en) * | 1995-12-04 | 1999-07-13 | Celegene Corporation | Chronic, bolus administration of D-threo methylphenidate |
| US5936091A (en) * | 1997-05-22 | 1999-08-10 | Celgene Corporation | Processes and intermediates for resolving piperidyl acetamide stereoisomers |
| US5965734A (en) * | 1997-01-31 | 1999-10-12 | Celgene Corporation | Processes and intermediates for preparing 2-substituted piperidine stereoisomers |
| US6031124A (en) * | 1996-03-27 | 2000-02-29 | Medeva Europe Limited | 7-amino-2-heptenoates and their use in the preparation of methylphenidate |
| US6121453A (en) * | 1996-03-08 | 2000-09-19 | Medeva Europe Limited | Resolution of threo-methylphenidate |
| US6127385A (en) * | 1999-03-04 | 2000-10-03 | Pharmaquest Limited | Method of treating depression using l-threo-methylphenidate |
| US6221883B1 (en) * | 2000-04-12 | 2001-04-24 | Ross Baldessarini | Method of dopamine inhibition using l-threo-methylphenidate |
| US6228398B1 (en) * | 1998-11-02 | 2001-05-08 | Elan Corporation, Plc | Multiparticulate modified release composition |
| US6242464B1 (en) * | 1996-01-22 | 2001-06-05 | Chiroscience Limited | Single isomer methylphenidate and resolution process |
| US6344215B1 (en) * | 2000-10-27 | 2002-02-05 | Eurand America, Inc. | Methylphenidate modified release formulations |
| US6355656B1 (en) * | 1995-12-04 | 2002-03-12 | Celgene Corporation | Phenidate drug formulations having diminished abuse potential |
| US6359139B1 (en) * | 2000-11-07 | 2002-03-19 | Celgene Corporation | Methods for production of piperidyl acetamide stereoisomers |
| US6395752B1 (en) * | 1999-03-04 | 2002-05-28 | Pharmaquest Limited | Method of treating depression using 1-threo-methylphenidate |
| US6441178B2 (en) * | 1997-01-17 | 2002-08-27 | Medeva Europe Limited | Resolution of ritalinic acid salt |
| US20020132793A1 (en) * | 2000-08-28 | 2002-09-19 | Mel Epstein | Use of methylphenidate compounds to enhance memory |
| US6486177B2 (en) * | 1995-12-04 | 2002-11-26 | Celgene Corporation | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US6962997B1 (en) * | 1997-05-22 | 2005-11-08 | Celgene Corporation | Process and intermediates for resolving piperidyl acetamide steroisomers |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB589625A (en) | 1944-01-19 | 1947-06-25 | Chem Ind Basel | Manufacture of new pyridine and piperidine compounds |
| GB788226A (en) | 1953-12-23 | 1957-12-23 | Ciba Ltd | Stereoisomers of ª‡-phenyl-ª‡-piperidyl-(2)-acetic acid and process of making same |
| GB878167A (en) | 1958-11-17 | 1961-09-27 | Ciba Ltd | New acetic acid esters |
| SU466229A1 (en) | 1973-01-23 | 1975-04-05 | Всесоюзный научно-исследовательский химико-фармацевтический институт им. С.Орджоникидзе | Method for preparing methyl trio-phenyl- (piperidyl-2) -acetic acid hydrochloride |
| IT1276689B1 (en) | 1995-06-09 | 1997-11-03 | Applied Pharma Res | SOLID PHARMACEUTICAL FORM FOR ORAL USE |
| EP0839038A1 (en) | 1995-07-14 | 1998-05-06 | Chiroscience Limited | THERAPEUTIC USE OF d-threo-METHYLPHENIDATE |
| MX9805870A (en) | 1996-01-22 | 1999-01-31 | ||
| PT879228E (en) | 1996-02-02 | 2003-01-31 | Medeva Europ Ltd | PROCESS FOR THE PREPARATION OF D-TREO- (R, R) -METHYLPHENIDATE AND RECYCLING OF UNSAFE BY EPIMERIZATION |
| DE69710051T2 (en) | 1996-03-08 | 2002-08-14 | Medeva Europe Ltd., Slough | RESOLUTION METHOD OF THREO-METHYLPHENIDATE |
| JP5173089B2 (en) | 1996-11-25 | 2013-03-27 | アルザ コーポレイション | Increasing dose drug dosage form |
| EP0948484A1 (en) | 1996-12-13 | 1999-10-13 | Medeva Europe Limited | The preparation of enantiomerically-enriched threo-methylphenidate |
| GB9913458D0 (en) | 1999-06-09 | 1999-08-11 | Medeva Europ Ltd | The therapeutic use of d-threo-methylphenidate |
| EP1239858B1 (en) | 1999-12-17 | 2003-10-29 | Celltech Pharma Europe Limited | Pharmaceutical for the treatment of convulsive states |
-
2001
- 2001-07-12 US US09/903,803 patent/US6486177B2/en not_active Expired - Fee Related
-
2002
- 2002-07-11 WO PCT/US2002/022039 patent/WO2003005962A2/en active IP Right Grant
- 2002-07-11 KR KR10-2004-7000312A patent/KR20040029360A/en not_active Application Discontinuation
- 2002-07-11 JP JP2003511771A patent/JP2005520780A/en active Pending
- 2002-07-11 AU AU2002318302A patent/AU2002318302B2/en not_active Ceased
- 2002-07-11 CA CA2453510A patent/CA2453510C/en not_active Expired - Fee Related
- 2002-07-11 NZ NZ530211A patent/NZ530211A/en not_active IP Right Cessation
- 2002-07-11 EP EP02748129A patent/EP1411943A4/en not_active Withdrawn
- 2002-07-11 MX MXPA04000249A patent/MXPA04000249A/en active IP Right Grant
- 2002-07-11 IL IL15931302A patent/IL159313A0/en unknown
- 2002-07-11 NZ NZ565754A patent/NZ565754A/en not_active IP Right Cessation
- 2002-07-16 US US10/195,974 patent/US7115631B2/en not_active Expired - Fee Related
-
2004
- 2004-04-08 US US10/820,397 patent/US20040204456A1/en not_active Abandoned
-
2006
- 2006-04-11 US US11/402,385 patent/US20060183774A1/en not_active Abandoned
-
2009
- 2009-12-17 US US12/640,734 patent/US20100093797A1/en not_active Abandoned
-
2011
- 2011-06-30 US US13/172,977 patent/US20110257227A1/en not_active Abandoned
-
2012
- 2012-10-26 US US13/661,472 patent/US20130053414A1/en not_active Abandoned
Patent Citations (79)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US19535A (en) * | 1858-03-02 | Improvement in sewing-machines | ||
| US32335A (en) * | 1861-05-14 | Island | ||
| US49205A (en) * | 1865-08-01 | Improvement in machines for hulling grain | ||
| US105134A (en) * | 1870-07-05 | Thomas sheehan | ||
| US2507631A (en) * | 1944-01-19 | 1950-05-16 | Ciba Pharm Prod Inc | Pyridine and piperidine compounds and process of making same |
| US2957880A (en) * | 1953-12-23 | 1960-10-25 | Ciba Pharm Prod Inc | Process for the conversion of stereoisomers |
| US2838519A (en) * | 1953-12-23 | 1958-06-10 | Ciba Pharm Prod Inc | Process for the conversion of stereoisomers |
| US4137300A (en) * | 1976-08-20 | 1979-01-30 | Ciba-Geigy Corporation | Sustained action dosage forms |
| US4410700A (en) * | 1980-07-03 | 1983-10-18 | The United States Of America As Represented By The Department Of Health And Human Services | Preparation of chiral 1-benzyl-1,2,3,4-tetrahydroisoquinolines by optical resolution |
| US4882166A (en) * | 1981-09-30 | 1989-11-21 | National Research Development Corporation | Compositions comprising encapsulated particles and their preparation |
| US4794001A (en) * | 1986-03-04 | 1988-12-27 | American Home Products Corporation | Formulations providing three distinct releases |
| US4904476A (en) * | 1986-03-04 | 1990-02-27 | American Home Products Corporation | Formulations providing three distinct releases |
| US4986987A (en) * | 1986-05-09 | 1991-01-22 | Alza Corporation | Pulsed drug delivery |
| US5114946A (en) * | 1987-06-12 | 1992-05-19 | American Cyanamid Company | Transdermal delivery of pharmaceuticals |
| US4992445A (en) * | 1987-06-12 | 1991-02-12 | American Cyanamid Co. | Transdermal delivery of pharmaceuticals |
| US5391381A (en) * | 1987-06-25 | 1995-02-21 | Alza Corporation | Dispenser capable of delivering plurality of drug units |
| US5236689A (en) * | 1987-06-25 | 1993-08-17 | Alza Corporation | Multi-unit delivery system |
| US5283193A (en) * | 1988-06-27 | 1994-02-01 | Asahi Kasei Kogyo K.K. | Process for producing optically active α-substituted organic acid and microorganism and enzyme used therefor |
| US4968505A (en) * | 1988-08-16 | 1990-11-06 | Ss Pharmaceutical Company, Ltd. | Long-acting diclofenac sodium preparation |
| US5202128A (en) * | 1989-01-06 | 1993-04-13 | F. H. Faulding & Co. Limited | Sustained release pharmaceutical composition |
| US5133974A (en) * | 1989-05-05 | 1992-07-28 | Kv Pharmaceutical Company | Extended release pharmaceutical formulations |
| US5217718A (en) * | 1989-08-18 | 1993-06-08 | Cygnus Therapeutic Systems | Method and device for administering dexmedetomidine transdermally |
| US5284769A (en) * | 1989-10-16 | 1994-02-08 | Chiros Ltd. | Process for preparing a single enantiomer of a lactam using lactamase |
| US5362755A (en) * | 1990-01-05 | 1994-11-08 | Sepracor, Inc. | Method for treating asthma using optically pure (R)-albuterol |
| US5229131A (en) * | 1990-02-05 | 1993-07-20 | University Of Michigan | Pulsatile drug delivery system |
| US5158777A (en) * | 1990-02-16 | 1992-10-27 | E. R. Squibb & Sons, Inc. | Captopril formulation providing increased duration of activity |
| US5137733A (en) * | 1990-06-28 | 1992-08-11 | Tanabe Seiyaku Co., Ltd. | Controlled release pharmaceutical preparation |
| US5104899A (en) * | 1990-08-13 | 1992-04-14 | Sepracor, Inc. | Methods and compositions for treating depression using optically pure fluoxetine |
| US5232705A (en) * | 1990-08-31 | 1993-08-03 | Alza Corporation | Dosage form for time-varying patterns of drug delivery |
| US5156850A (en) * | 1990-08-31 | 1992-10-20 | Alza Corporation | Dosage form for time-varying patterns of drug delivery |
| US5160744A (en) * | 1991-06-27 | 1992-11-03 | Alza Corporation | Verapmil therapy |
| US5326570A (en) * | 1991-07-23 | 1994-07-05 | Pharmavene, Inc. | Advanced drug delivery system and method of treating psychiatric, neurological and other disorders with carbamazepine |
| US5593694A (en) * | 1991-10-04 | 1997-01-14 | Yoshitomi Pharmaceutical Industries, Ltd. | Sustained release tablet |
| US5425950A (en) * | 1991-10-30 | 1995-06-20 | Glaxo Group Limited | Controlled release pharmaceutical compositions |
| US5223265A (en) * | 1992-01-10 | 1993-06-29 | Alza Corporation | Osmotic device with delayed activation of drug delivery |
| US5639476A (en) * | 1992-01-27 | 1997-06-17 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| US5580578A (en) * | 1992-01-27 | 1996-12-03 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| US5308348A (en) * | 1992-02-18 | 1994-05-03 | Alza Corporation | Delivery devices with pulsatile effect |
| US5331000A (en) * | 1992-03-09 | 1994-07-19 | Sepracor Inc. | Antipyretic and analgesic methods and compositions containing optically pure R(-) ketoprofen |
| US5299121A (en) * | 1992-06-04 | 1994-03-29 | Medscreen, Inc. | Non-prescription drug medication screening system |
| US5512293A (en) * | 1992-07-23 | 1996-04-30 | Alza Corporation | Oral sustained release drug delivery device |
| US5375693A (en) * | 1992-08-03 | 1994-12-27 | Sepracor, Inc. | Methods and compositions for treating allergic disorders and other disorders metabolic derivatives of terfenadine |
| US5478573A (en) * | 1992-12-23 | 1995-12-26 | Kinaform Technology, Inc. | Delayed, sustained-release propranolol pharmaceutical preparation |
| US5449743A (en) * | 1993-01-29 | 1995-09-12 | Shiro Kobayashi | Method for ring opening polymerization using a hydrolase catalyst |
| US5496561A (en) * | 1993-08-25 | 1996-03-05 | Ss Pharmaceutical Co., Ltd. | Controlled release-initiation and controlled release-rate pharmaceutical composition |
| US5500227A (en) * | 1993-11-23 | 1996-03-19 | Euro-Celtique, S.A. | Immediate release tablet cores of insoluble drugs having sustained-release coating |
| US5672360A (en) * | 1993-11-23 | 1997-09-30 | Purdue Pharma, L.P. | Method of treating pain by administering 24 hour oral opioid formulations |
| US5567441A (en) * | 1995-03-24 | 1996-10-22 | Andrx Pharmaceuticals Inc. | Diltiazem controlled release formulation |
| US6468504B1 (en) * | 1995-07-14 | 2002-10-22 | Medeva Europe, Ltd. | Composition comprising methylphenidate and another drug |
| US5773478A (en) * | 1995-07-14 | 1998-06-30 | Medeva Europe Limited | Composition comprising methylphenidate and another drug |
| US6113879A (en) * | 1995-07-14 | 2000-09-05 | Medeva Europe Limited | Composition comprising methylphenidate and another drug |
| US5874090A (en) * | 1995-07-14 | 1999-02-23 | Medeva Europe Limited | Sustained-release formulation of methylphenidate |
| US5908850A (en) * | 1995-12-04 | 1999-06-01 | Celgene Corporation | Method of treating attention deficit disorders with d-threo methylphenidate |
| US6486177B2 (en) * | 1995-12-04 | 2002-11-26 | Celgene Corporation | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US7115631B2 (en) * | 1995-12-04 | 2006-10-03 | Celgene Corporation | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate |
| US6635284B2 (en) * | 1995-12-04 | 2003-10-21 | Celegene Corporation | Delivery of multiple doses of medications |
| US6602887B2 (en) * | 1995-12-04 | 2003-08-05 | Celegene Corporation | Chronic, bolus administration of D-threo methylphenidate |
| US5837284A (en) * | 1995-12-04 | 1998-11-17 | Mehta; Atul M. | Delivery of multiple doses of medications |
| US6528530B2 (en) * | 1995-12-04 | 2003-03-04 | Celgene Corporation | Phenidate drug formulations having diminished abuse potential |
| US5922736A (en) * | 1995-12-04 | 1999-07-13 | Celegene Corporation | Chronic, bolus administration of D-threo methylphenidate |
| US6355656B1 (en) * | 1995-12-04 | 2002-03-12 | Celgene Corporation | Phenidate drug formulations having diminished abuse potential |
| US6255325B1 (en) * | 1995-12-04 | 2001-07-03 | Celgene Corporation | Chronic, bolus administration of D-threo methylphenidate |
| US5733756A (en) * | 1996-01-05 | 1998-03-31 | Celgene Corporation | Lactams and processes for stereoselective enrichment of lactams, amides, and esters |
| US6242464B1 (en) * | 1996-01-22 | 2001-06-05 | Chiroscience Limited | Single isomer methylphenidate and resolution process |
| US6531489B2 (en) * | 1996-01-22 | 2003-03-11 | Chiroscience, Ltd. | Single isomer methylphenidate and resolution process |
| US6121453A (en) * | 1996-03-08 | 2000-09-19 | Medeva Europe Limited | Resolution of threo-methylphenidate |
| US6031124A (en) * | 1996-03-27 | 2000-02-29 | Medeva Europe Limited | 7-amino-2-heptenoates and their use in the preparation of methylphenidate |
| US6441178B2 (en) * | 1997-01-17 | 2002-08-27 | Medeva Europe Limited | Resolution of ritalinic acid salt |
| US5965734A (en) * | 1997-01-31 | 1999-10-12 | Celgene Corporation | Processes and intermediates for preparing 2-substituted piperidine stereoisomers |
| US6962997B1 (en) * | 1997-05-22 | 2005-11-08 | Celgene Corporation | Process and intermediates for resolving piperidyl acetamide steroisomers |
| US5936091A (en) * | 1997-05-22 | 1999-08-10 | Celgene Corporation | Processes and intermediates for resolving piperidyl acetamide stereoisomers |
| US6228398B1 (en) * | 1998-11-02 | 2001-05-08 | Elan Corporation, Plc | Multiparticulate modified release composition |
| US6730325B2 (en) * | 1998-11-02 | 2004-05-04 | Elan Corporation, Plc | Multiparticulate modified release composition |
| US6395752B1 (en) * | 1999-03-04 | 2002-05-28 | Pharmaquest Limited | Method of treating depression using 1-threo-methylphenidate |
| US6127385A (en) * | 1999-03-04 | 2000-10-03 | Pharmaquest Limited | Method of treating depression using l-threo-methylphenidate |
| US6221883B1 (en) * | 2000-04-12 | 2001-04-24 | Ross Baldessarini | Method of dopamine inhibition using l-threo-methylphenidate |
| US20020132793A1 (en) * | 2000-08-28 | 2002-09-19 | Mel Epstein | Use of methylphenidate compounds to enhance memory |
| US6344215B1 (en) * | 2000-10-27 | 2002-02-05 | Eurand America, Inc. | Methylphenidate modified release formulations |
| US6359139B1 (en) * | 2000-11-07 | 2002-03-19 | Celgene Corporation | Methods for production of piperidyl acetamide stereoisomers |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2002318302B2 (en) | 2007-03-15 |
| US20130053414A1 (en) | 2013-02-28 |
| US7115631B2 (en) | 2006-10-03 |
| MXPA04000249A (en) | 2004-05-04 |
| US20100093797A1 (en) | 2010-04-15 |
| US20060183774A1 (en) | 2006-08-17 |
| JP2005520780A (en) | 2005-07-14 |
| WO2003005962A2 (en) | 2003-01-23 |
| US20110257227A1 (en) | 2011-10-20 |
| CA2453510C (en) | 2012-03-20 |
| IL159313A0 (en) | 2004-06-01 |
| US20020198234A1 (en) | 2002-12-26 |
| WO2003005962A3 (en) | 2003-06-12 |
| US6486177B2 (en) | 2002-11-26 |
| AU2002318302C1 (en) | 2003-01-29 |
| EP1411943A2 (en) | 2004-04-28 |
| NZ530211A (en) | 2008-04-30 |
| US20020022640A1 (en) | 2002-02-21 |
| KR20040029360A (en) | 2004-04-06 |
| EP1411943A4 (en) | 2005-09-28 |
| CA2453510A1 (en) | 2003-01-23 |
| NZ565754A (en) | 2009-05-31 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20130053414A1 (en) | Methods for treatment of cognitive and menopausal disorders with d-threo methylphenidate | |
| AU2002318302A1 (en) | Methods for treatment of cognitive and menopausal disorders with D-threo methylphenidate | |
| US6255325B1 (en) | Chronic, bolus administration of D-threo methylphenidate | |
| US20140194466A1 (en) | Perhexiline for use in the treatment of hypertrophic cardiomyopathy (hcm) | |
| US20100104621A1 (en) | Treating adhd and other diseases involving inflammation | |
| CN110167562A (en) | DMPC, DMPG, DMPC/DMPG, LYSOPG and LYSOPC are directed to the protective effect for causing the drug of ion channel disease | |
| US20050277626A1 (en) | Methods and compositions for treatment of nicotine dependence and dementias | |
| US6774131B1 (en) | Remedies for endothelin-induced diseases | |
| US20040024043A1 (en) | Method for treating cognitive disorders | |
| CN1338944A (en) | Cholesteryl ester transfer protein inhibitors and fibric acid derivatives for cardiovascular indications | |
| JP2008523097A (en) | Treatment with D-threomethylphenidate | |
| US20080261955A1 (en) | Use of Pharmaceutical Compositions of Lofepramine for the Treatment of Adhd, Cfs, Fm and Depression | |
| JPS63145231A (en) | Cerebral function activation agent containing steroid compound |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |