US20030225152A1 - 3-(Arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors - Google Patents
3-(Arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors Download PDFInfo
- Publication number
- US20030225152A1 US20030225152A1 US10/259,703 US25970302A US2003225152A1 US 20030225152 A1 US20030225152 A1 US 20030225152A1 US 25970302 A US25970302 A US 25970302A US 2003225152 A1 US2003225152 A1 US 2003225152A1
- Authority
- US
- United States
- Prior art keywords
- dihydro
- indol
- methylene
- phenylamino
- fluoro
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 *C.C[RaH].[2*]N1C(=O)C(=CCC2=CC=CC=C2)C2=C1C=CC=C2 Chemical compound *C.C[RaH].[2*]N1C(=O)C(=CCC2=CC=CC=C2)C2=C1C=CC=C2 0.000 description 20
- QDVWJMUHZAMGNO-UHFFFAOYSA-N CCN(CC)CCCOC(C)C Chemical compound CCN(CC)CCCOC(C)C QDVWJMUHZAMGNO-UHFFFAOYSA-N 0.000 description 9
- PWPUPXJJCPIOGY-UHFFFAOYSA-N CC(C)CCN1CCOCC1 Chemical compound CC(C)CCN1CCOCC1 PWPUPXJJCPIOGY-UHFFFAOYSA-N 0.000 description 7
- XLZMWNWNBXSZKF-UHFFFAOYSA-N CC(C)N1CCOCC1 Chemical compound CC(C)N1CCOCC1 XLZMWNWNBXSZKF-UHFFFAOYSA-N 0.000 description 7
- XVUVQAGOLUXWCB-UHFFFAOYSA-N CC(C)OCC1CCCN(C)C1 Chemical compound CC(C)OCC1CCCN(C)C1 XVUVQAGOLUXWCB-UHFFFAOYSA-N 0.000 description 7
- QGKOHSUFRXIKPO-UHFFFAOYSA-N CC(C)OCCCN1CCCCC1 Chemical compound CC(C)OCCCN1CCCCC1 QGKOHSUFRXIKPO-UHFFFAOYSA-N 0.000 description 7
- PARDALBXSPCGRP-UHFFFAOYSA-N CC(C)CCCCN1CCCCC1 Chemical compound CC(C)CCCCN1CCCCC1 PARDALBXSPCGRP-UHFFFAOYSA-N 0.000 description 6
- HDEUOOBSAKUIRJ-UHFFFAOYSA-N CC(C)CCCCN1CCOCC1 Chemical compound CC(C)CCCCN1CCOCC1 HDEUOOBSAKUIRJ-UHFFFAOYSA-N 0.000 description 6
- PWMYYGYCTJRBBX-UHFFFAOYSA-N CC(C)CCN1CCCCC1 Chemical compound CC(C)CCN1CCCCC1 PWMYYGYCTJRBBX-UHFFFAOYSA-N 0.000 description 6
- BNBLWPUWMNURAX-UHFFFAOYSA-N CC(C)CN1CCCCC1 Chemical compound CC(C)CN1CCCCC1 BNBLWPUWMNURAX-UHFFFAOYSA-N 0.000 description 6
- QKVSMSABRNCNRS-UHFFFAOYSA-N CC(C)CN1CCOCC1 Chemical compound CC(C)CN1CCOCC1 QKVSMSABRNCNRS-UHFFFAOYSA-N 0.000 description 6
- PRKXEYYPWVQBLM-UHFFFAOYSA-N CC(C)NCCCN1CCOCC1 Chemical compound CC(C)NCCCN1CCOCC1 PRKXEYYPWVQBLM-UHFFFAOYSA-N 0.000 description 6
- XTWYARJGACRMGR-UHFFFAOYSA-N CC(C)OCCN1CCCC1 Chemical compound CC(C)OCCN1CCCC1 XTWYARJGACRMGR-UHFFFAOYSA-N 0.000 description 6
- DQTFXWXEDYSIRO-UHFFFAOYSA-N CC(C)OCCN1CCCCC1 Chemical compound CC(C)OCCN1CCCCC1 DQTFXWXEDYSIRO-UHFFFAOYSA-N 0.000 description 6
- NVFSQXGPNCPGOR-UHFFFAOYSA-N CC(C)NCCN1CCOCC1 Chemical compound CC(C)NCCN1CCOCC1 NVFSQXGPNCPGOR-UHFFFAOYSA-N 0.000 description 5
- IJUOMCMYAFYRGH-UHFFFAOYSA-N CC1CN(C(C)C)CC(C)O1 Chemical compound CC1CN(C(C)C)CC(C)O1 IJUOMCMYAFYRGH-UHFFFAOYSA-N 0.000 description 5
- ODIQTOYGORNLPE-UHFFFAOYSA-N CC(C)N1CCN(C)CC1 Chemical compound CC(C)N1CCN(C)CC1 ODIQTOYGORNLPE-UHFFFAOYSA-N 0.000 description 4
- YJAJULNXPNZYQX-UHFFFAOYSA-N CC(C)NCCCN1CCN(C)CC1 Chemical compound CC(C)NCCCN1CCN(C)CC1 YJAJULNXPNZYQX-UHFFFAOYSA-N 0.000 description 3
- FAWYUMGHXFVCFW-UHFFFAOYSA-N CC(C)OCCCCN1CCCCC1 Chemical compound CC(C)OCCCCN1CCCCC1 FAWYUMGHXFVCFW-UHFFFAOYSA-N 0.000 description 3
- FGGUOSGGXBUWRK-UHFFFAOYSA-N CCN(CC)CCOC(C)C Chemical compound CCN(CC)CCOC(C)C FGGUOSGGXBUWRK-UHFFFAOYSA-N 0.000 description 3
- KXIXHISTUVHOCY-UHFFFAOYSA-N CC(C)N1CCCCC1 Chemical compound CC(C)N1CCCCC1 KXIXHISTUVHOCY-UHFFFAOYSA-N 0.000 description 2
- UJQOOKDZIXZAJG-UHFFFAOYSA-N CC(C)OCCCCN1CCSCC1 Chemical compound CC(C)OCCCCN1CCSCC1 UJQOOKDZIXZAJG-UHFFFAOYSA-N 0.000 description 2
- DPUCWQUWEJBKPK-UHFFFAOYSA-N N=C(c1ccccc1N1)C1=O Chemical compound N=C(c1ccccc1N1)C1=O DPUCWQUWEJBKPK-UHFFFAOYSA-N 0.000 description 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Nc1ccccc1 Chemical compound Nc1ccccc1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- JTNXQVCPQMQLHK-UHFFFAOYSA-N CC(C)=S Chemical compound CC(C)=S JTNXQVCPQMQLHK-UHFFFAOYSA-N 0.000 description 1
- KJUXBAKIRGSUAC-UHFFFAOYSA-N CC(C)C(=S)N1CCOCC1 Chemical compound CC(C)C(=S)N1CCOCC1 KJUXBAKIRGSUAC-UHFFFAOYSA-N 0.000 description 1
- ATDQIPIFGWOSJZ-UHFFFAOYSA-N CC(C)OCCCCN1CCCC1 Chemical compound CC(C)OCCCCN1CCCC1 ATDQIPIFGWOSJZ-UHFFFAOYSA-N 0.000 description 1
- QDWOAXATZDTVBO-UHFFFAOYSA-N CC(C)OCCCCN1CCN(C)CC1 Chemical compound CC(C)OCCCCN1CCN(C)CC1 QDWOAXATZDTVBO-UHFFFAOYSA-N 0.000 description 1
- MUSSLOCHKDAEIK-UHFFFAOYSA-N CC(C)OCCCCN1CCOCC1 Chemical compound CC(C)OCCCCN1CCOCC1 MUSSLOCHKDAEIK-UHFFFAOYSA-N 0.000 description 1
- DQDMFVPHODNTIZ-UHFFFAOYSA-N CC(C)OCCCN1CCC(F)C1 Chemical compound CC(C)OCCCN1CCC(F)C1 DQDMFVPHODNTIZ-UHFFFAOYSA-N 0.000 description 1
- IXUBECFKQFSDRM-UHFFFAOYSA-N CC(C)OCCCN1CCCC1 Chemical compound CC(C)OCCCN1CCCC1 IXUBECFKQFSDRM-UHFFFAOYSA-N 0.000 description 1
- BHTCSYCYFDJJIZ-UHFFFAOYSA-N CC(C)OCCCN1CCN(C)CC1 Chemical compound CC(C)OCCCN1CCN(C)CC1 BHTCSYCYFDJJIZ-UHFFFAOYSA-N 0.000 description 1
- IZEQCHGSCUYCJE-UHFFFAOYSA-N CC(C)OCCCN1CCOCC1 Chemical compound CC(C)OCCCN1CCOCC1 IZEQCHGSCUYCJE-UHFFFAOYSA-N 0.000 description 1
- KMVUFFGTJAHXDV-UHFFFAOYSA-N CC(C)OCCCN1CCSCC1 Chemical compound CC(C)OCCCN1CCSCC1 KMVUFFGTJAHXDV-UHFFFAOYSA-N 0.000 description 1
- ZKLQIVPPHFQZOK-UHFFFAOYSA-N COCCN1CCCC1 Chemical compound COCCN1CCCC1 ZKLQIVPPHFQZOK-UHFFFAOYSA-N 0.000 description 1
- SGSJRUJTZDJOEF-UHFFFAOYSA-N CSOCCCN1CCOCC1 Chemical compound CSOCCCN1CCOCC1 SGSJRUJTZDJOEF-UHFFFAOYSA-N 0.000 description 1
- XLMKBZYPBUIFFI-UHFFFAOYSA-N SOCCCN1CCSCC1 Chemical compound SOCCCN1CCSCC1 XLMKBZYPBUIFFI-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/08—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms
- C07D295/084—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms with the ring nitrogen atoms and the oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings
- C07D295/088—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly bound oxygen or sulfur atoms with the ring nitrogen atoms and the oxygen or sulfur atoms attached to the same carbon chain, which is not interrupted by carbocyclic rings to an acyclic saturated chain
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P41/00—Drugs used in surgical methods, e.g. surgery adjuvants for preventing adhesion or for vitreum substitution
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D209/00—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D209/02—Heterocyclic compounds containing five-membered rings, condensed with other rings, with one nitrogen atom as the only ring hetero atom condensed with one carbocyclic ring
- C07D209/04—Indoles; Hydrogenated indoles
- C07D209/30—Indoles; Hydrogenated indoles with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, directly attached to carbon atoms of the hetero ring
- C07D209/32—Oxygen atoms
- C07D209/34—Oxygen atoms in position 2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D295/00—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms
- C07D295/04—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms
- C07D295/12—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms
- C07D295/135—Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms with substituted hydrocarbon radicals attached to ring nitrogen atoms substituted by singly or doubly bound nitrogen atoms with the ring nitrogen atoms and the substituent nitrogen atoms separated by carbocyclic rings or by carbon chains interrupted by carbocyclic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings
- C07D401/10—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing two hetero rings linked by a carbon chain containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D403/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00
- C07D403/02—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings
- C07D403/12—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, not provided for by group C07D401/00 containing two hetero rings linked by a chain containing hetero atoms as chain links
Definitions
- Tyrosine kinases can be of the receptor-type (having extracellular, transmembrane and intracellular domains) or the non-receptor type (being wholly intracellular).
- tBu refers to t-butyl
- Alkoxyl refers to an “O-alkyl” group.
- the substituent on the aniline moiety is referred to as an o, m or p substituent or a 2, 3 or 4 substituent, respectively.
- the 5 substituent is also a m substituent and the 6 substituent is an o substituent.
- the present invention relates to compounds capable of regulating and/or modulating tyrosine kinase signal transduction and more particularly receptor and non-receptor tyrosine kinase signal transduction.
- Tyrosine kinase signal transduction results in, among other responses, cell proliferation, differentiation and metabolism.
- Abnormal cell proliferation may result in a wide array of disorders and diseases, including the development of neoplasia such as carcinoma, sarcoma, leukemia, glioblastoma, hemangioma, psoriasis, arteriosclerosis, arthritis and diabetic retinopathy (or other disorders related to uncontrolled angiogenesis and/or vasculogenesis, e.g. macular degeneration).
- Examples 242, 243, 244, and 245 are prepared by substituting the appropriate 4′-methyl or 6′-fluoro or 5′-fluoro or 5′-chloro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-(2-morpholin-4-yl-ethyl)-benzene-1,4-diamine, used for the preparation of Example 235, for aniline in the reaction of Example 1.
- a room temperature solution of 5.04 gms. 4-nitro-benzoic acid in tetrahydrofuran (10 mL) is treated with 5.14 gms. 1′,1′-carbonyl-diimidizole and immediately immersed in an ice bath.
- the reaction mixture is stirred in the ice bath for 30 minutes, then it is allowed to warm to room temperature.
- Once at room temperature the reaction mixture is treated with 3 mL piperidine.
- the reaction mixture is allowed to stir at room temperature ovenight.
- the reaction is then made basic with the addition of saturated aqueous sodium bicarbonate solution, and the resulting mixture is extracted with ethyl acetate.
- the quenched reaction mixture is then extracted with ethyl acetate and water.
- the organic layer is separated, and concentrated in vacuo, while the aqueous layer is separated and made basic with the addition of aqueous 1 M NaOH.
- the basic aqueous layer is then extracted with ethyl acetate and the ethyl acetate layer from this extraction is concentrated in vacuo.
- the solids isolated from both concentrated ethyl acetate layers are combined to yield 92 mg of 1-(4-nitro-benzyl)-piperidine as a white-yellow solid.
- 6-(3-Methoxy-phenyl)-1,3-dihydro-indol-2-one (0.2282 gms.) is combined with 0.23 mL of ethylformate in 0.67 mL anhydrous ethanol, and is treated with a solution of 21%, by weight, sodium formate in ethanol (0.40 mL). The resulting solution is allowed to stand at room temperature for 30 minutes, and then is refluxed for 2 h to yield a suspension. Once at room temperature the suspension is acidified to pH 1.0 with 10% HCl(aq), then diluted with 5 mL of H 2 O. The resulting precipitate.
- the named compound is prepared by refluxing 0.020 gms E & Z 3-Hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one (see example 268) with 0.0327 gms 4-(3-diethylamino-propoxy)-phenylamine (used in the preparation of Example 214) in tetrahydrofuran (0.33 mL) for 36 h. Following cooling to room temperature, solvent evaporation in vacuo, trituration with ethyl acetate/(min) hexanes and filtration the reaction yields the named compound as a solid in the amount of 9.0 mg.
- the named compound is prepared by refluxing 0.0505 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.085 gms. 4-(2-piperidin-1-yl-ethyl)-phenylamine in tetrahydrofuran (1.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound in the amount of 66 mg.
- 4-(2-Piperidin-1-yl-ethyl)-phenylamine is prepared from (4-Nitro-phenyl)-acetic acid by the following method:
- a room temperature solution of 2.53 gms. (4-Nitro-phenyl)-acetic acid in tetrahydrofuran (10 mL) is treated with 2.44 gms. 1′,1′-carbonyl-diimidizole and immediately immersed in an ice bath.
- the reaction mixture is stirred in the ice bath for 30 minutes then it is allowed to warm to room temperature.
- Once at room temperature the reaction mixture is treated with 1.21 mL piperidine, and then is stirred overnight at room temperature.
- the reaction is then made basic with the addition of saturated aqueous sodium bicarbonate, and the resulting mixture is extracted with ethyl acetate.
- the named compound is prepared by refluxing 0.051 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.085 gms. 4-morpholin-4-ylmethyl-phenylamine in tetrahydrofuran (2.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 0.070 gms.
- N-(3-Morpholin-4-yl-propyl)-benzene-1,4-diamine (4.39 gms.) is isolated upon evaporation of the filtrate, and is subsequently used without purification in the reaction of Example 297.
- a suspension of 0.1385 gms. of [3-(4-methyl-piperazin-1-yl)-propyl]-(4-nitro-phenyl)-amine in 3 mL of ethanol is heated to 50° C. Once dissolution is achieved 0.144 mL hydrazine monohydrate is added to the solution.
- a Raney nickel slurry in water is added to the 50° C. solution dropwise, waiting after each addition for the gas evolution to cease. Sufficient quantities of Raney nickel have been added when continued addition of Raney nickel causes no further gas evolution. The reaction is then maintained at 50° C. for an additional hour, and subsequently is cooled to room temperature.
- the named compound is prepared by substituting 4-methyl-1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-[3-(4-methyl-piperazin-1-yl)-propyl]-benzene-1,4-diamine (used for the preparation of Example 302) for aniline in the reaction of Example 1.
- 4-(2-Morpholin-4-yl-ethyl)-phenylamine is prepared from (4-nitro-phenyl)-acetic acid by the following method:
- reaction mixture is maintained at refluxing temperature overnight, then cooled to 0° C., and quenched with the addition of concentrated HCl (added until fizzing stops).
- the quenched reaction mixture is then warmed to room temperature, and extracted with ethyl acetate and water.
- the organic layer is then separated, dried over sodium sulfate and concentrated in vacuo.
- the solid isolated is then chromatographed by flash silica gel chromatography using 30% ethyl acetate in hexanes as the eluant to yield 0.7993 gms. of 4-[2-(4-nitro-phenyl)-ethyl]-morpholine as a white solid.
- the named compound is prepared by refluxing 0.0707 gms. E & Z-3-Hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, as prepared in the reaction of Example 1, with 0.1088 gms. 4-(2-morpholin-4-yl-ethyl)-phenylamine (used in the preparation of Example 306) in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 77 mg.
- 4-(4-Morpholin-4-yl-butyl)-phenylamine is prepared from 4-(4-nitro-phenyl)-butyric acid by the following method:
- a room temperature solution of 1.1180 gms. 4-(4-nitro-phenyl)-butyric acid in tetrahydrofuran (3 mL) is treated with 0.9052 gms. 1′,1′-carbonyl-diimidizole using an ice bath to attenuate the intensity of the reaction.
- the reaction mixture is stirred for 1 h.
- the reaction mixture is then treated with 0.5 mL morpholine.
- the reaction is heated overnight at 35° C.
- the reaction is then allowed to cool to room temperature, and is made basic with the addition of saturated aqueous sodium bicarbonate.
- the resulting mixture is extracted with ethyl acetate.
- the named compound is prepared by refluxing 0.131 gms. E & Z-3-hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, as prepared in the reaction of Example 1, with 0.0759 gms. 4-(4-morpholin-4-yl-butyl)-phenylamine (used in the preparation of Example 312) in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 98.0 mg.
- a room temperature solution of 1.1220 gms. 4-(4-nitro-phenyl)-butyric acid in tetrahydrofuran (3 mL) is treated with 0.8978 gms. 1′,1′-carbonyl-diimidizole using an ice bath to attenuate the intensity of the reaction.
- the reaction mixture is stirred for 30 minutes in the ice bath and 30 minutes at room temperature.
- the reaction mixture is then treated with 0.5 mL morpholine.
- the reaction was heated overnight at 35° C.
- the reaction is then allowed to cool to room temperature, and is made basic with the addition of saturated aqueous sodium bicarbonate.
- the resulting mixture is extracted with ethyl acetate.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Diabetes (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Neurosurgery (AREA)
- Rheumatology (AREA)
- Epidemiology (AREA)
- Dermatology (AREA)
- Urology & Nephrology (AREA)
- Obesity (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Hospice & Palliative Care (AREA)
- Emergency Medicine (AREA)
- Surgery (AREA)
- Ophthalmology & Optometry (AREA)
- Transplantation (AREA)
- Cardiology (AREA)
- Vascular Medicine (AREA)
- Endocrinology (AREA)
- Psychiatry (AREA)
- Pain & Pain Management (AREA)
- Heart & Thoracic Surgery (AREA)
- Pulmonology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
Abstract
The present invention relates to organic molecules capable of modulating tyrosine kinase signal transduction in order to regulate, modulate and/or inhibit abnormal cell proliferation.
Description
- This application claims priority under 35 U.S.C. § 119(e)(1) to provisional application Nos. 60/325,819 and 60/325,815, filed Sep. 27, 2001.
- 1. Field of the Invention
- The present invention relates to novel compounds capable of modulating, regulating and/or inhibiting tyrosine kinase signal transduction. The present invention is also directed to methods of regulating, modulating or inhibiting tyrosine kinases, whether of the receptor or non-receptor class, for the prevention and/or treatment of disorders related to unregulated tyrosine kinase signal transduction, including cell growth, metabolic, and blood vessel proliferative disorders.
- 2. Description of the Related Art
- Protein tyrosine kinases (PTKs) comprise a large and diverse class of proteins having enzymatic activity. The PTKs play an important role in the control of cell growth and differentiation.
- For example, receptor tyrosine kinase mediated signal transduction is initiated by extracellular interaction with a specific growth factor (ligand), followed by receptor dimerization, transient stimulation of the intrinsic protein tyrosine kinase activity and phosphorylation. Binding sites are thereby created for intracellular signal transduction molecules and lead to the formation of complexes with a spectrum of cytoplasmic signaling molecules that facilitate the appropriate cellular response (e.g., cell division, metabolic homeostasis, and responses to the extracellular microenvironment).
- With respect to receptor tyrosine kinases, it has been shown also that tyrosine phosphorylation sites function as high-affinity binding sites for SH2 (src homology) domains of signaling molecules. Several intracellular substrate proteins that associate with receptor tyrosine kinases (RTKs) have been identified. They may be divided into two principal groups: (1) substrates which have a catalytic domain; and (2) substrates which lack such domain but serve as adapters and associate with catalytically active molecules. The specificity of the interactions between receptors or proteins and SH2 domains of their substrates is determined by the amino acid residues immediately surrounding the phosphorylated tyrosine residue. Differences in the binding affinities between SH2 domains and the amino acid sequences surrounding the phosphotyrosine residues on particular receptors are consistent with the observed differences in their substrate phosphorylation profiles. These observations suggest that the function of each receptor tyrosine kinase is determined not only by its pattern of expression and ligand availability but also by the array of downstream signal transduction pathways that are activated by a particular receptor. Thus, phosphorylation provides an important regulatory step which determines the selectivity of signaling pathways recruited by specific growth factor receptors, as well as differentiation factor receptors.
- Aberrant expression or mutations in the PTKs have been shown to lead to either uncontrolled cell proliferation (e.g. malignant tumor growth) or to defects in key developmental processes. Consequently, the biomedical community has expended significant resources to discover the specific biological role of members of the PTK family, their function in differentiation processes, their involvement in tumorigenesis and in other diseases, the biochemical mechanisms underlying their signal transduction pathways activated upon ligand stimulation and the development of novel drugs.
- Tyrosine kinases can be of the receptor-type (having extracellular, transmembrane and intracellular domains) or the non-receptor type (being wholly intracellular).
- The RTKs comprise a large family of transmembrane receptors with diverse biological activities. The intrinsic function of RTKs is activated upon ligand binding, which results in phophorylation of the receptor and multiple cellular substrates, and subsequently in a variety of cellular responses.
- At present, at least nineteen (19) distinct RTK subfamilies have been identified. One RTK subfamily, designated the HER subfamily, is believed to be comprised of EGFR, HER2, HER3 and HER4. Ligands to the Her subfamily of receptors include epithelial growth factor (EGF), TGF-α, amphiregulin, HB-EGF, betacellulin and heregulin.
- A second family of RTKs, designated the insulin subfamily, is comprised of the INS-R, the IGF-1R and the IR-R. A third family, the “PDGF” subfamily includes the PDGF α and β receptors, CSFIR, c-kit and FLK-II. Another subfamily of RTKs, identified as the FLK family, is believed to be comprised of the Kinase insert Domain-Receptor fetal liver kinase-1 (KDR/FLK-1), the fetal liver kinase 4 (FLK-4) and the fins-like tyrosine kinase 1 (flt-1). Each of these receptors was initially believed to be receptors for hematopoictic growth factors. Two other subfamilies of RTKs have been designated as the FGF receptor family (FGFR1, FGFR2, FGFR3 and FGFR4) and the Met subfamily (c-met and Ron).
- Because of the similarities between the PDGF and FLK subfamilies, the two subfamilies are often considered together. The known RTK subfamilies are identified in Plowman et al, 1994, DN&P 7(6): 334-339, which is incorporated herein by reference.
- The non-receptor tyrosine kinases represent a collection of cellular enzymes which lack extracellular and transmembrane sequences. At present, over twenty-four individual non-receptor tyrosine kinases, comprising eleven (11) subfamilies (Src, Frk, Btk, Csk, Abl, Zap70, Fes/Fps, Fak, Jak, Ack and LIMK) have been identified. At present, the Src subfamily of non-receptor tyrosine kinases is comprised of the largest number of PTKs and include Src, Yes, Fyn, Lyn, Lck, Blk, Hck, Fgr and Yrk. The Src subfamily of enzymes has been linked to oncogenesis. A more detailed discussion of non-receptor tyrosine kinases is provided in Bolen, 1993, Oncogen 8: 2025-2031, which is incorporated herein by reference.
- Many of the tyrosine kinases, whether an RTK or non-receptor tyrosine kinase, have been found to be involved in cellular signaling pathways leading to cellular signal cascades leading to pathogenic conditions, including cancer, psoriasis and hyper immune response.
- In view of the surmised importance of PTKs to the control, regulation and modulation of cell proliferation the diseases and disorders associated with abnormal cell proliferation, many attempts have been made to identify receptor and non-receptor tyrosine kinase “inhibitors” using a variety of approaches, including the use of mutant ligands (U.S. Pat. No. 4,966,849), soluble receptors and antibodies (PCT Application No. WO 94/10202; Kendall & Thomas, 1994, Proc. Nat'l Acad. Sci 90: 10705-09; Kim, et al, 1993, Nature 362: 841-844), RNA ligands (Jellinek, et al, Biochemistry 33: 10450-56); Takano, et al, 1993, Mol. Bio. Cell 4:358A; Kinsella, et al, 1992, Exp. Cell Res. 199: 56-62; Wright, et al, 1992, J. Cellular Phys. 152: 448-57) and tyrosine kinase inhibitors (PCT Application Nos. WO 94/03427; WO 92/21660; WO 91/15495; WO 94/14808; U.S. Pat. No. 5,330,992; Mariani, et al, 1994, Proc. Am. Assoc. Cancer Res. 35: 2268).
- More recently, attempts have been made to identify small molecules which act as tyrosine kinase inhibitors. For example, bis monocyclic, bicyclic or heterocyclic aryl compounds (PCT Application No. WO 92/20642), vinylene-azaindole derivatives (PCT Application No. WO 94/14808) and 1-cyclopropyl-4-pyridyl-quinolones (U.S. Pat. No. 5,330,992) have been described generally as tyrosine kinase inhibitors. Styryl compounds (U.S. Pat. No. 5,217,999), styryl-substituted pyridyl compounds (U.S. Pat. No. 5,302,606), certain quinazoline derivatives (EP Application No. 0 566 266 A1), selcoindoles and selenides (PCT Application No. WO 94/03427), tricyclic polyhydroxylic compounds (PCT Application No. WO 92/21660) and benzylphosphonic acid compounds (PCT Application No. WO 91/15495) have been described as compounds for use as tyrosine kinase inhibitors for use in the treatment of cancer.
- The identification of effective small compounds which specifically inhibit signal transduction by modulating the activity of receptor and non-receptor tyrosine kinases to regulate and modulate abnormal or inappropriate cell proliferation is therefore desirable and one object of this invention.
- Finally, certain small compounds are disclosed in U.S. Pat. Nos. 5,792,783; 5,834,504; 5,883,113; 5,883,116 and 5,886,020 as useful for the treatment of diseases related to unregulated TKS transduction. These patents are hereby incorporated by reference in its entirety for the purpose of disclosing starting materials and methods for the preparation thereof, screens and assays to determine a claimed compound's ability to modulate, regulate and/or inhibit cell proliferation, indications which are treatable with said compounds, formulations and routes of administration, effective dosages, etc.
- The present invention relates to organic molecules capable of modulating, regulating and/or inhibiting tyrosine kinase signal transduction. Such compounds are useful for the treatment of diseases related to unregulated TKS transduction, including cell proliferative diseases such as cancer, atherosclerosis, restenosis, metabolic diseases such as diabetes, inflammatory diseases such as psoriasis and chronic obstructive pulmonary disease, vascular proliferative disorders such as diabetic retinopathy, age-related macular degeneration and retinopathy of prematurity, autoimmune diseases and transplant rejection.
-
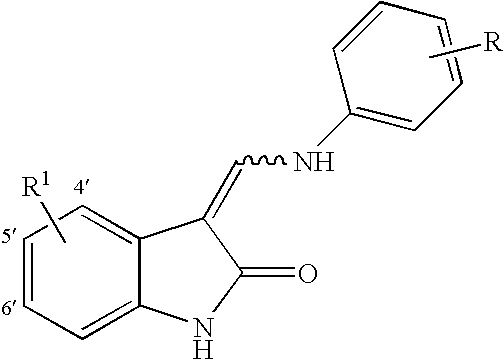
- wherein R′ is selected from the group consisting of halogen, NO 2, CN, C1 to C4 alkyl and aryl, e.g. phenyl; R2 is selected from the group consisting of hydrogen, C1 to C8 alkyl, COCH3, CH2CH2OH,CH2CH2CH2OH and phenyl; R is selected from the group consisting of D, halogen, C1 to C8 alkyl, CF3, OCF3, OCF2H, CH2CN, CN, SR2, (CR7R8)cC(O)OR2, C(O)N(R2)2, (CR7R8)cOR2,HNC(O)R2, HN—C(O)OR2,(CR7R8)cN(R2)2, SO2 (CR7R8)cN(R2)2,OP(O)(OR2)2, OC(O)OR2, OCH2O, HN—CH═CH, —N(COR2)CH2CH2, HC═N—NH, N═CH—S, O(CR7R8)d—R6 and (CR7R8)c—R6, —NR2(CR7R8)dR6 wherein R6 is selected from the group consisting of halogen, 3-fluoropyrrolidinyl, 3-fluoropiperidinyl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 3-pyrrolinyl, pyrrolidinyl, methyl isonipecotate, N-(2-methoxyethyl)-N-methylamyl, 1,2,3,6-tetrahydropyridinyl, morpholinyl, hexamethyleneiminyl, piperazinyl-2-one, piperazinyl, N-(2-methoxyethyl)ethylaminyl, thiomorpholinyl, heptamethyleneiminyl, 1-piperazinylcarboxaldehyde, 2,3 ,6,7-tetrahydro-(1H)-1,4-diazepinyl-5(4H)-one, N-methylhomopiperazinyl, (3-dimethylamino)pyrrolidinyl, N-(2-methoxyethyl)-N-propylaminyl, isoindolinyl, nipecotamidinyl, isonipecotamidinyl, 1-acetylpiperazinyl, 3-acetamidopyrrolidinyl, trans- decahydroisoquinolinyl, cis-decahydroisoquinolinyl, N-acetylhomopiperazinyl, 3-(diethylamino)pyrrolidinyl, 1,4-dioxa-8-azaspiro[4.5]decaninyl, 1-(2-methoxyethyl)-piperazinyl, 2-pyrrolidin-3-ylpyridinyl, 4-pyrrolidin-3-ylpyridinyl, 3-(methylsulfonyl)pyrrolidinyl, 3-picolylmethylaminyl, 2-(2-methylaminoethyl)pyridinyl, 1-(2-pyrimidyl)-piperazinyl, 1-(2-pyrazinyl)-piperazinyl, 2-methylaminomethyl-1,3-dioxolane, 2-(N-methyl-2-aminoethyl)-1,3-dioxolane, 3-(N-acetyl-N-methylamino)pyrrolidinyl, 2-methoxyethylaminyl, tetrahydrofurfurylaminyl, 4-aminotetrahydropyran, 2-amino-1-methoxybutane, 2-methoxyisopropylaminyl, 1-(3-aminopropyl)imidazole, histamyl , N,N-diisopropylethylenediaminyl, 1-benzyl-3-aminopyrrolidyl 2-(aminomethyl)-5-methylpyrazinyl, 2,2-dimethyl-1,3-dioxolane-4-methanaminyl, (R)-3-amino-1-N-BOC-pyrrolidinyl, 4-amino-1,2,2,6,6-pentamethylpiperidinyl, 4-aminomethyltetrahydropyran, ethanolamine and alkyl-substituted derivatives thereof and wherein when c is 1 said CH2 may be
- and CH 2CH2CH2; provided said alkyl or phenyl radicals may be substituted with one or two halo, hydroxy or lower alkyl amino radicals wherein R7 and R8 may be selected from the group consisting of H, F and C1-C4 alkyl or CR7R8 may represent a carbocyclic ring of from 3 to 6 carbons, preferably R7 and R8 are H or CH3;
- b is 0 or an integer of from 1 to 3;
- a is 0 or an integer of from 1 to 5, preferably 1 to 3;
- c is 0 or an integer of from 1 to 4,
- d is an integer of from 2 to 5;
- the wavy line represents a E or Z bond and pharmaceutically acceptable salts thereof.
- In one embodiment of the present invention R 1 is selected from the group consisting of H, i.e. b is 0; CH3, F, Cl and phenyl.
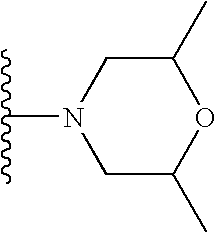
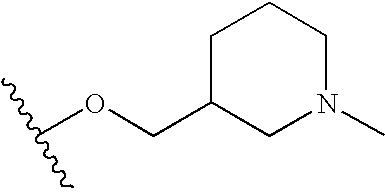
- Preferably, R is selected from the group consisting of CH 3, CH2CH3, OCH3, OH, t-butyl, F, CN, C(O)NH2, HN C(O)CH3, CH2C(O)OH, SO2NH2, C(O)OH, OCF2H, isopropyl, C2H5OH, C(O)OCH3, CH2OH, NH—CH═CH, HC═N—N—H, N═CH—S, O(CR7R8)dR6, (CR7R8)cR6 and —NR2(CR7R8)dR6, wherein R6 is selected from the group consisting of 3-fluoropyrrolidinyl, 3-fluoropiperidinyl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 3-pyrrolinyl, pyrrolidinyl, methyl isonipecotate, N-(2-methoxyethyl)-N-methylamyl, 1,2,3,6-tetrahydropyridinyl, morpholinyl, hexamethyleneiminyl, piperazinyl-2-one, piperazinyl, N-(2-methoxyethyl)ethylaminyl, thiomorpholinyl, heptamethyleneiminyl, 1-piperazinylcarboxaldehyde, 2,3,6,7-tetrahydro-(1H)-1,4-diazcpinyl-5(4H)-one, N-methylhomopiperazinyl, (3-dimethylamino)pyrrolidinyl, N-(2-methoxyethyl)-N-propylaminyl, isoindolinyl, nipecotamidinyl, isonipecotamidinyl, 1-acetylpiperazinyl, 3-acetamidopyrrolidinyl, trans-decahydroisoquinolinyl, cis-decahydroisoquinolinyl, N-acetylhomopiperazinyl, 3-(diethylamino)pyrrolidinyl, 1,4-dioxa-8-azaspiro[4.5]decaninyl, 1-(2-methoxyethyl)-piperazinyl, 2-pyrrolidin-3-ylpyridinyl, 4-pyrrolidin-3-ylpyridinyl, 3-(methylsulfonyl)pyrrolidinyl, 3-picolylmethylaminyl, 2-(2-methylaminoethyl)pyridinyl, 1-(2-pyrimidyl)-piperazinyl, 1-(2-pyrazinyl)-piperazinyl, 2-methylaminomethyl-1,3-dioxolane, 2-(N-methyl-2-aminoethyl)-1,3-dioxolane, 3-(N-acetyl-N-methylamino)pyrrolidinyl, 2-methoxyethylaminyl, tetrahydrofurfurylaminyl, 4-aminotetrahydropyran, 2-amino-1-methoxybutane, 2-methoxyisopropylaminyl, 1-(3-aminopropyl)imidazole, histamyl , N,N-diisopropylethylenediaminyl, 1-benzyl-3-aminopyrrolidyl 2-(aminomethyl)-5-methylpyrazinyl, 2,2-dimethyl-1,3-dioxolane-4-methanaminyl, (R)-3-amino-1-N-BOC-pyrrolidinyl, 4-amino-1,2,2,6,6-pentamethylpiperidinyl, 4-aminomethyltetrahydropyranyl, ethanolamine and alkyl-substituted derivatives thereof, e.g. R6 is morpholinyl or CH2N(CH3)2.
- More preferably, R is selected from the group consisting of m-ethyl, p-methoxy, p-hydroxy, m-hydroxy, p-cyano, m-C(O)NH 2, p-HNC(O)CH3, p-CH2C(O)OH, p-SO2NH2, p-CH2OH, m-methoxy, p-CH2CH2OH, HNCH═CH, HC═N—NH, p-morpholinyl, N═CH—S, p-OCHF2, p-COOH, p-CH3, p-OCH3, m-F, m-CH2N(C2H3)2, (CR7R8)cR6, O(CR7R8)dR6 and NR2(CR7R8)dR6.
- It is noted that R may represent a condensed ring that is attached to the above phenyl ring at two positions. For example, as shown in Example 23, below, CH 2CH2CH2 may be attached at the 3 and 4 (or m and p) positions of the phenyl ring.
- Still more preferably, R is selected from the group consisting of fluoro, methyl, (CR 7R8)cR6, O(CR7R8)dR6 and NR2(CR7R8)dR6 wherein R6 is selected from dimethylamino, diethylamino, 3-fluoropyrrolidinyl, 3-fluoropiperidinyl, 3-pyridinyl, 4-pyridinyl, pyrrolidinyl, morpholinyl, piperazinyl, heptamethyleneiminyl, tetrahydrofurfurylaminyl, 4-aminotetrahydropyranyl, N,N-diisopropylethylenediaminyl and 4-aminomethyltetrahydropyran.
- In particular, the compounds of the present invention are selected from the compounds of Table 1, below.
TABLE 1 Unsubstituted 4-Methyl & 5-Chloro 3-[(Substituted Phenylamino)-methylene]- 1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 1 H H H H H H 2 H H Br H H H 3 H H H Br H H 4 H Br H H H H 6 H H Et H H H 7 H H H OMe H H 8 H H H CO2Et H H 9 H Et H H H H 10 H H F Me H H 11 H Me F H H H 12 H H H OH H H 13 H H Cl OH H H 14 H Me H F H H 15 H H OH H H H 16 H H OMe H OMe H 17 H H H tBu H H 18 H H H Me H H 19 H H Me H Me H 20 H H Me Me H H 21 H H F OMe H H 22 H H CF3 H H H 23 H H —CH2CH2CH2— H H 24 H F H Cl H H 25 H H H CF3 H H 26 H F H Me OCO2Et H 27 H F H Me OCO2CH2C(CH3)3 H 28 H F H Cl OH H 29 H H H CN H H 30 H H H CH2CN H H 31 H H —CH═CH—NH— H H 32 H H —NH—N═CH— H H 33 H H H CONH2 H H 34 H H H NHCOCH3 H H 35 H H CH2CO2H H H H 36 H H H Cl H H Unsubstituted, 4-methyl & 5-Chloro 3-[(Substituted Phenylamino)- methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 37 H H CO2H Cl H H 38 H H H SO2NH2 H H 39 H H H SO2NHCOCH3 H H 40 H H H N-morpholino H H 41 H H H OPh H H 42 H H OMe OMe H H 43 H H —S—CH═N— H H 44 H H OH CO2H H H 45 H H CF3 Cl H H 46 H H CF3 H CF3 H 47 H H CF3 F H H 48 H H OH Me H H 49 H H OH OMe H H 50 H H H OCHF2 H H 51 H H H OCF3 H H 52 H H H iPr H H 53 H F H Me H H 54 H H Me Cl H H 55 H H CF3 OMe H H 56 H H CF3 Me H H 57 5′-Cl H OMe H H H 58 4′-Me H H H H H 59 4′-Me H H OMe H H 60 4′-Me H OH H H H 61 4′-Me H OMe H OMe H 62 4′-Me H H Me H H 63 4′-Me H Me H Me H 64 5′-Cl H H OCHF2 H H 65 5′-Cl H OH OMe H H 66 5′-Cl H H OCF3 H H 67 5′-Cl H Me OH H H 68 5′-Cl H —OCH2O— H H 69 5′-Cl H Me Me H H 70 5′-Cl H H iPr H H 71 5′-Cl H OH Me H H 72 5′-Cl H H (CH2)2OH H H Unsubstituted, 4-methyl & 5-Chloro 3-[(Substituted Phenylamino)- methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 73 5′-Cl H H OMe H H 74 5′-Cl H H H H H 75 5′-Cl H OMe H OMe H 76 5′-Cl H OH H H H 77 5′-Cl H H OH H H 78 5′-Cl H Me H Me H 79 5′-Cl H H Me H H 80 H H —OCH2O— H H 81 H H CO2H OH H H 82 H H H OEt H H 83 H H —N(COMe)—CH2—CH2— H H 84 H H H OPO(OH)2 H H 85 H H CO2H CO2H H H 86 H H H CO2H H H 87 H H H (CH2)2OH H H 88 H H H CH2OH H H 89 H H OMe CO2CH3 H H 90 4′-Me H —NH—N═CH— H H 91 4′-Me H F OMe H H 92 4′-Me H —S—CH═N— H H 93 4′-Me H OMe CO2CH3 H H 94 H H OMe H H H 95 4′-Me H Me Me H H 96 4′-Me H H OH H H 97 4′-Me H —CH═CH—NH— H H 98 4′-Me H H t-Bu H H 99 4′-Me H H CH2OH H H 100 5′-Cl H H t-Bu H H 101 5′-Cl H —S—CH═N— H H 102 5′-Cl H OMe OMe H H 103 5′-Cl H —NH—N═CH— H H 104 5′-Cl OMe H Cl OMe H 105 5′-Cl H F OMe H H 106 5′-Cl H H N-morpholino H H 107 5′-Cl H H OEt H H 108 5′-Cl H CO2H OH H H Unsubstituted, 4-methyl & 5-Chloro 3-[(Substituted Phenylamino)- methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 109 5′-Cl H CH2NEt2 OH H H 110 5′-Cl H —CH═CH—NH— H H 111 5′-Cl H H CH2OH H H 112 5′-Cl H Me iPr H H 113 4′-Me H H CH2CH2OH H H 114 5′-Cl H H NHCOMe H H 115 5′-Cl H H CH2CO2H H H 116 5′-Cl H H SO2NH2 H H 117 4′-Me H OH OMe H H 118 4′-Me H CO2H OH H H 119 4′-Me H H OCHF2 H H 120 4′-Me H H OCF3 H H 121 4′-Me H CF3 OMe H H 122 4′-Me H H OEt H H 123 4′-Me H H iPr H H 124 4′-Me H —O—CH2—O— H H 125 4′-Me H OH Me H H 126 4′-Me H OMe OMe H H 127 4′-Me Et H H H H 128 4′-Me H H CN H H 129 4′-Me H H CONH2 H H 130 4′-Me H H NHCOCH3 H H 131 4′-Me H H CH2CO2H H H 132 4′-Me H Me OH H H 133 H H Me OH H H 134 H H OH NHCO2Et H H 135 4′-Me F H OMe H H 136 H H H SMe H H 137 4′-Me H H SMe H H 138 5′-Cl H H SMe H H 139 H H H —CH2CH2CH2CO2H H H 140 4′-Me H H —CH2CH2CH2CO2H H H 141 H H —CH2CH2CO2H H H H 142 4′-Me H —CH2CH2CO2H H H H 143 5′-Cl H —CH2CH2CO2H H H H 144 H H H —CH2CH2CO2H H H 145 4′-Me H H —CH2CH2CO2H H H 146 5′-Cl H H —CH2CH2CO2H H H Unsubstituted, 4-methyl, 5-Chloro & 5-Fluoro 3-[(Substituted Phenylamino)- methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 147 4′-Me H Et H H H 148 5′-Cl H Et H H H 149 5′-Cl H H Et H H 150 5′-Cl H H —CH2CH2CH2CO2H H H 151 4′-Me H H Et H H 152 5′-Cl H H —CN H H 155 4′-Me H OH CO2H H H 156 H H H N(Me)2 H H 157 H H H H H 158 H H H H H 159 H H H H H 160 H H CH2N(Et)2 OH H H 161 4′-Me H CH2N(Et)2 OH H H 162 5′-F H —CH═CH—NH— H H 163 5′-F H —NH—N═CH— H H 164 5′-F H OH OMe H H 165 5′-F H H CH2CH2CO2H H H 166 5′-F H H SO2NH2 H H 167 5′-F H H H H 168 5′-F H H H H 169 5′-F H H H H H 170 5′-F H H CONH2 H H 171 5′-F H H SMe H H 172 5′-F H F OMe H H 173 5′-F H —S—CH═N— H H 174 5′-F H CH2CO2H H H 175 5′-F H CH2CH2CO2H H H H 176 5′-F H Et H H H Unsubstituted, 4-methyl, 5-Chloro & 5-Fluoro 3-[(Substituted Phenylamino)- methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 177 5′-F H OH H H H 178 5′-F H H CH2OH H H 179 H H H H H 180 H H H NH2 H H 181 4′-Me H H NH2 H H 182 H H CH(OH)CH3 H H H 183 4′-Me H CH(OH)CH3 H H H 184 H H CH2OH H H H 185 4′-Me H CH2OH H H H 186 H H NHCO2t-Bu H H H 187 4′-Me H NHCO2t-Bu H H H 188 H H H N(Et)2 H H 189 4′-Me H H N(Et)2 H H 190 H H SO2N(CH2CH2OH)2 H H H 191 4′-Me H SO2N(CH2CH2OH)2 H H H 192 H H H SO2NCH2CH2OH H H 193 H H SO2NCH2CH2CH2OH H H H 194 4′-Me H SO2NCH2CH2CH2OH H H H 195 H H CO2H H H 196 4′-Me H H H H 197 4′-Me H H SO2NCH2CH2OH H H 198 H H H OCH2CH2CH2Cl H H 199 H H H OCH2CH2CH2CH2Cl H H 200 H H H OCH2CH2CH2I H H 201 H H H OCH2CH2CH2CH2I H H 202 4′-Me D D D D D 203 H D D CO2H D D 204 H D D NH2 D D 205 4′-Me D D NH2 D D Unsubstituted, 4-methyl, 5-Chloro & 5-Fluoro 3-[(Substituted Phenylamino)- methylene]-1,3-dibydro-indol-2-Ones. R Substitution Example # R1 2 3 4 5 6 206 H H H H H 207 H H H OCH2CH2CH2CH2N(Et)2 H H 208 H H H H H 209 H H H H H 210 4′-Me H NH2 H H H 211 H H NH2 H H H 212 H H Me H H 213 4′-Me H NH2 Me H H 214 H H H OCH2CH2CH2N(Et)2 H H 215 H H H H H 216 H H H H H 217 H H H H H 218 H H H H H 219 5′-F H H H H 220 4′-Me H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Cyano, 5-Fluoro, 5-Nitro, 6- Fluoro & 6-Aryl 3-[(Substituted Phenylamino)-methylene]-1,3-dihydro-indol- 2-ones. R Substitution Example # R1 2 3 4 5 6 221 5′-F H H H H 222 5′-F H H OMe H H 223 H D D D D D 224 H H H CH2CO2H H H 225 H H H H H 226 H H H H H 227 4′-Me H H H H 228 6′-F H H H H 229 6′-F H H H H 230 6′-F H H H H 231 4′-Me H H H H 232 5′-Cl H H H H 233 5′-F H H H H 234 6′-F H H H H 235 H H H H H 236 5′-NO2 H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Cyano, 5-Fluoro, 5-Nitro, 6- Fluoro & 6-Aryl 3-[(Substituted Phenylamino)-methylene]-1,3-dihydro-indol- 2-ones. R Substitution Example # R1 2 3 4 5 6 237 5′-CN H H H H 238 4′-Me H H H H 239 6′-F H H H H 240 5′-F H H H H 241 5′-Cl H H H H 242 4′-Me H H H H 243 6′-F H H H H 244 5′-F H H H H 245 5′-Cl H H H H 246 4′-Me H H H H 247 6′-F H H H H 248 H H F H H 249 4′-Me H F H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Cyano, 5-Fluoro, 5-Nitro, 6- Fluoro & 6-Aryl 3-[(Substituted Phenylamino)-methylene]-1,3-dihydro-indol- 2-ones. R Substitution Example # R1 2 3 4 5 6 250 6′-F H F H H 251 H H H H H 252 4′-Me H H H H 253 6′-F H H H H 254 H H H H H 255 4′-F H H H H 256 4′-Me H H H H 257 4′-F H H H H 258 5′-F H H H H 259 6′-F H H H H 260 5′-Cl H H H H 261 4′-F H H H H 262 5′-Cl H H H H 263 5′-F H H H H 264 4′-Me H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Cyano, 5-Fluoro, 5-Nitro, 6- Fluoro & 6-Aryl 3-[(Substituted Phenylamino)-methylene]-1,3-dihydro-indol- 2-ones. R Substitution Example # R1 2 3 4 5 6 265 H H H H H 266 6′-F H H H H 267 4′-F H H H H 268 6′-(3- Methoxyphenyl) H H H H 269 6′-(3- Methoxyphenyl) H H H H 270 4′-Me H H H H 271 6′-F H H H H 272 H H H H H 273 4′-F H H H H 274 5′-F H H H H 275 5′-Cl H H H H 276 6′-(3- Methoxyphenyl) H H H H 277 6′-(3- Methoxyphenyl) H H H H 278 4′-Me H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Cyano, 5-Fluoro, 5-Nitro, 6- Fluoro & 6-Aryl 3-[(Substituted Phenylamino)-methylene]-1,3-dihydro-indol- 2-ones. R Substitution Example # R1 2 3 4 5 6 279 6′-F H H H H 280 H H H H H 281 4′-F H H H H 282 5′-F H H H H 283 5′-Cl H H H H 284 H H H H H 285 5′-Cl H H H H 286 4′-Me H H H H 287 4′-F H H H H 288 5′-F H H H H 289 6′-F H H H H 290 H H H H H 291 5′Cl H H H H 292 4′-Me H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Cyano, 5-Fluoro, 5-Nitro, 6- Fluoro & 6-Aryl 3-[(Substituted Phenylamino)-methylene]-1,3-dihydro-indol- 2-ones. R Substitution Example # R1 2 3 4 5 6 293 4′-F H H H H 294 5′-F H H H H 295 6′-F H H H H 296 4′-Me H H H H 297 H H H H H 298 6′-F H H H H 299 5′-Cl H H H H 300 5′-F H H H H 301 4′-F H H H H 302 H H H H H 303 4′-Me H H H H 304 6′-F H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Fluoro & 6-Fluoro 3- [(Substituted Phenylamino)-methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 305 H H H H H 306 H H H H H 307 5′-Cl H H H H 308 4′-Me H H H H 309 4′-F H H H H 310 5′-F H H H H 311 6′-F H H H H 312 H H H H H 313 5′-Cl H H H H 314 4′-Me H H H H 315 4′-F H H H H 316 5′-F H H H H 317 6′-F H H H H Unsubstituted, 4-Fluoro, 4-methyl, 5-Chloro, 5-Fluoro & 6-Fluoro 3- [(Substituted Phenylamino)-methylene]-1,3-dihydro-indol-2-ones. R Substitution Example # R1 2 3 4 5 6 318 H H H H H 319 5′-Cl H H H H 320 4′-Me H H H H 321 4′-F H H H H 322 5′-F H H H H 323 6′-F H H H H - and a pharmaceutically acceptable carrier or excipient. Such a composition is believed to modulate signal transduction by a tyrosine kinase, either by inhibition of catalytic activity, affinity to ATP or ability to interact with a substrate.
- More particularly, the compositions of the present invention may be included in methods for treating diseases comprising proliferation, fibrotic or metabolic disorders, for example cancer, fibrosis, psoriasis, atherosclerosis, arthritis, and other disorders related to abnormal vasculogenesis and/or angiogenesis, such as diabetic retinopathy.
- The following defined terms are used throughout this specification:
- “Me” refers to methyl.
- “Et” refers to ethyl.
- “tBu” refers to t-butyl.
- “iPr” refers to i-propyl.
- “Ph” refers to phenyl.
- “Pharmaceutically acceptable salt” refers to those salts which retain the biological effectiveness and properties of the free bases and which are obtained by reaction with inorganic acids such as hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid and the like.
- “Alkyl” refers to a straight-chain, branched or cyclic saturated aliphatic hydrocarbon. Preferably, the alkyl group has 1 to 12 carbons. More preferably, it is a lower alkyl of from 1 to 7 carbons, most preferably 1 to 4 carbons. Typical alkyl groups include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tertiary butyl, pentyl, hexyl and the like. The alkyl group may be optionally substituted with one or more substituents are selected from the group consisting of hydroxyl, cyano, alkoxy, ═O, ═S, NO 2, halogen, dimethyl amino, and SH.
- “Alkenyl” refers to a straight-chain, branched or cyclic unsaturated hydrocarbon group containing at least one carbon-carbon double bond. Preferably, the alkenyl group has 1 to 12 carbons. More preferably it is a lower alkenyl of from 1 to 7 carbons, most preferably 1 to 4 carbons. The alkenyl group may be optionally substituted with one or more substituents selected from the group consisting of hydroxyl, cyano, alkoxy, ═O, ═S, NO 2, halogen, dimethyl amino, and SH.
- “Alkynyl” refers to a straight-chain, branched or cyclic unsaturated hydrocarbon containing at least one carbon-carbon triple bond. Preferably, the alkynyl group has 1 to 12 carbons. More preferably it is a lower alkynyl of from 1 to 7 carbons, most preferably 1 to 4 carbons. The alkynyl group may be optionally substituted with one or more substituents selected from the group consisting of hydroxyl, cyano, alkoxy, ═O, ═S, NO 2, halogen, dimethyl amino, and SH.
- “Alkoxyl” refers to an “O-alkyl” group.
- “Aryl” refers to an aromatic group which has at least one ring having a conjugated pi electron system and includes carbocyclic aryl, heterocyclic aryl and biaryl groups. The aryl group may be optionally substituted with one or more substituents selected from the group consisting of halogen, trihalomethyl, hydroxyl, SH, OH, NO 2, amine, thioether, cyano, alkoxy, alkyl, and amino.
- “Alkaryl” refers to an alkyl that is covalently joined to an aryl group. Preferably, the alkyl is a lower alkyl.
- “Carbocyclic aryl” refers to an aryl group wherein the ring atoms are carbon.
- “Heterocyclic aryl” refers to an aryl group having from 1 to 3 heteroatoms as ring atoms, the remainder of the ring atoms being carbon. Heteroatoms include oxygen, sulfur, and nitrogen. Thus, heterocyclic aryl groups include furanyl, thienyl, pyridyl, pyrrolyl, N-lower alkyl pyrrolo, pyrimidyl, pyrazinyl, imidazolyl and the like.
- “Hydrocarbyl” refers to a hydrocarbon radical having only carbon and hydrogen atoms. Preferably, the hydrocarbyl radical has from 1 to 20 carbon atoms, more preferably from 1 to 12 carbon atoms and most preferably from 1 to 7 carbon atoms.
- “Substituted hydrocarbyl” refers to a hydrocarbyl radical wherein one or more, but not all, of the hydrogen and/or the carbon atoms are replaced by a halogen, nitrogen, oxygen, sulfur or phosphorus atom or a radical including a halogen, nitrogen, oxygen, sulfur or phosphorus atom, e.g. fluoro, chloro, cyano, nitro, hydroxyl, phosphate, thiol, etc.
- “Amide” refers to —C(O)—NH—R′, wherein R′ is alkyl, aryl, alkylaryl or hydrogen.
- “Thioamide” refers to —C(S)—NH—R′, wherein R′ is alkyl, aryl, alkylaryl or hydrogen.
- “Amine” refers to a —N(R″)R′″ group, wherein R″ and R″′ are independently selected from the group consisting of alkyl, aryl, and alkylaryl.
- “Thioether” refers to —S—R″, wherein R″ is alkyl, aryl, or alkylaryl.
- “Sulfonyl” refers to —S(O) 2—R″″, where R″″ is aryl, C(CN)═C-aryl, CH2CN, alkyaryl, sulfonamide, NH-alkyl, NH-alkylaryl, or NH-aryl.
- Also, alternatively the substituent on the aniline moiety is referred to as an o, m or p substituent or a 2, 3 or 4 substituent, respectively. (Obviously, the 5 substituent is also a m substituent and the 6 substituent is an o substituent.
- The present invention relates to compounds capable of regulating and/or modulating tyrosine kinase signal transduction and more particularly receptor and non-receptor tyrosine kinase signal transduction.
- Receptor tyrosine kinase mediated signal transduction is initiated by extracellular interaction with a specific growth factor (ligand), followed by receptor dimerization, transient stimulation of the intrinsic protein tyrosine kinase activity and phosphorylation. Binding sites are thereby created for intracellular signal transduction molecules and lead to the formation of complexes with a spectrum of cytoplasmic signaling molecules that facilitate the appropriate cellular response (e.g., cell division, metabolic effects and responses to the extracellular microenvironment).
- It has been shown that tyrosine phosphorylation sites in growth factor receptors function as high-affinity binding sites for SH2 (src homology) domains of signaling molecules. Several intracellular substrate proteins that associate with receptor tyrosine kinases have been identified. They may be divided into two principal groups: (1) substrates which have a catalytic domain; and (2) substrates which lack such domain but serve as adapters and associate with catalytically active molecules. The specificity of the interactions between receptors and SH2 domains of their substrates is determined by the amino acid residues immediately surrounding the phosphorylated tyrosine residue. Differences in the binding affinities between SH2 domains and the amino acid sequences surrounding the phosphotyrosine residues on particular receptors are consistent with the observed differences in their substrate phosphorylation profiles. These observations suggest that the function of each receptor tyrosine kinase is determined not only by its pattern of expression and ligand availability but also by the array of downstream signal transduction pathways that are activated by a particular receptor. Thus, phosphorylation provides an important regulatory step which determines the selectivity of signaling pathways recruited by specific growth factor receptors, as well as differentiation factor receptors.
- Tyrosine kinase signal transduction results in, among other responses, cell proliferation, differentiation and metabolism. Abnormal cell proliferation may result in a wide array of disorders and diseases, including the development of neoplasia such as carcinoma, sarcoma, leukemia, glioblastoma, hemangioma, psoriasis, arteriosclerosis, arthritis and diabetic retinopathy (or other disorders related to uncontrolled angiogenesis and/or vasculogenesis, e.g. macular degeneration).
- This invention is therefore directed to compounds which regulate, modulate and/or inhibit tyrosine kinase signal transduction by affecting the enzymatic activity of the RTKs and/or the non-receptor tyrosine kinases and interfering with the signal transduced by such proteins. More particularly, the present invention is directed to compounds which regulate, modulate and/or inhibit the RTK and/or non-receptor tyrosine kinase mediated signal transduction pathways as a therapeutic approach to cure many kinds of solid tumors, including but not limited to carcinoma, sarcoma, leukemia, erythroblastoma, glioblastoma, meningioma, astrocytoma, melanoma and myoblastoma. Indications may include, but are not limited to brain cancers, bladder cancers, ovarian cancers, gastric cancers, pancreas cancers, colon cancers, blood cancers, lung cancers and bone cancers.
- Biological data for the compounds of the present invention was generated by use of the following assays.
- VEGF Stimulated Ca ++ Signal in vitro
- Automated FLIPR (Fluorometric Imaging Plate Reader) technology was used to screen for inhibitors of VEGF induced increases in intracellular calcium levels in fluorescent dye loaded endothelial cells. HUVEC (human umbilical vein endothelial cells) (Clonetics) were seeded in 96-well fibronectin coated black-walled plates overnight at 37° C./5%CO 2. Cells were loaded with calcium indicator Fluo-4 for 45 minutes at 37° C. Cells were washed 4 times (Original Cell Wash, Labsystems) to remove extracellular dye. Test compounds were reconstituted in 100% DMSO and added to the cells to give a final DMSO concentration of 0.1%. For screening, cells were pre-incubated with test agents for 30 minutes, at a single concentration (10 μM) or at concentrations ranging from 0.01 to 10.0 μM followed by VEGF stimulation (5 ng/mL). Changes in fluorescence at 516 nm were measured simultaneously in all 96 wells using a cooled CCD camera. Data were generated by determining max-min fluorescence levels for unstimulated, stimulated, and drug treated samples. IC50 values for test compounds were calculated from % inhibition of VEGF stimulated responses in the absence of inhibitor.
- Protocol for KDR Assay:
- KDR Assay:
- The cytoplasmic domain of the human VEGF receptor (VEGFR-2) was expressed as a Histidine-tagged fusion protein following infection of insect cells using an engineered baculovirus. His-VEGFR-2 was purified to homogeneity, as determined by SDS-PAGE, using nickel resin chromatography. Kinase assays were performed in 96 well microtiter plates that were coated overnight with 30 μg of poly-Glu-Tyr (4:1) in 10 mM Phosphate Buffered Saline (PBS), pH 7.2-7.4. The plates were incubated with 1% BSA and then washed four times with PBS prior to starting the reaction. Reactions were carried out in 120 μL reaction volumes containing 3.6 μM ATP in kinase buffer (50 mM Hepes buffer pH 7.4, 20 mM MgCl 2, 0.1 mM MnCl2 and 0.2 mM Na3VO4). Test compounds were reconstituted in 100% DMSO and added to the reaction to give a final DMSO concentration of 5%. Reactions were initiated by the addition 0.5 ng of purified protein. Following a ten minute incubation at 25° C., the reactions were washed four times with PBS containing 0.05% Tween-20. 100 μl of a monoclonal anti-phosphotyrosine antibody-peroxidase conjugate was diluted 1:10000 in PBS-Tween-20 and added to the wells for 30 minutes. Following four washes with PBS-Tween-20, 100 μl of O-phenylenediamine Dihydrochloride in Phosphate-citrate buffer, containing urea hydrogen peroxide, was added to the wells for 7 minutes as a colorimetric substrate for the peroxidase. The reaction was terminated by the addition of 100 μl of 2.5N H2SO4 to each well and read using a microplate ELISA reader set at 492 nm. IC50 values for compound inhibition were calculated directly from graphs of optical density (arbitrary units) versus compound concentration following subtraction of blank values.
- VEGF-induced Dermal Extravasation in Guinea Pig (Miles Assay). Male Hartley guinea pigs (300-600 g) were anesthetized with isofluorane, sheared, and given a single dose of drug or the respective vehicle. The guinea pigs were dosed orally unless indicated otherwise in Table 3. Ten minutes prior to the end of drug treatment, guinea pigs were anesthetized with isofluorane, and 0.5% Evans blue dye (EBD) in PBS (13-15 mg/kg dose of EBD) was injected intravenously. After 5 minutes, triplicate intradermal injections of 100 ng rhVEGF 165 in 100 μl PBS and of 100 μl PBS alone were administered on the flank. After 20 minutes, each animal was euthanized with Pentosol, and the skin containing the intradermal injection sites was removed for image analysis.
- Using an analog video camera coupled to a PC, an image of each trans-illuminated skin sample was captured, and the integrated optical density of each injection site was measured using ImagePro 4. For each skin sample, the difference between the mean optical density of the VEGF sites and mean optical density of the PBS sites is the measure of VEGF-induced EBD extravasation in that animal. These measured values were averaged per study group to determine the mean VEGF-induced EBD extravasation for each experimental condition, and the group means were then compared to assess inhibition of VEGF-induced EBD extravasation in the drug-treated groups relative to the vehicle-treated controls.
- To determine the dose required for 50% inhibition (ID 50), the percent inhibition data was plotted as a function of oral dose, using the ‘best-fit’ analysis within MicroSoft Excel software. The ID50 value was verified visually by using the plotted data (horizontal line from 50% y value, at intersection with best-fit line drop vertical line to x axis (dose)).
- Laser-induced Choroidal Neovascularization (CNV) in Rat (CNV Assay). CNV was induced and quantified in this model as previously described (Edelman and Castro. Exp. Eye Res. 2000; 71:523-533). On day 0, male Brown Norway rats (200-300 g) were anesthetized with 100 mg/kg Ketamine and 10 mg/kg Xylazine, and pupils were dilated with 1% Tropicamide. Using the blue-green setting of a Coherent Novus Argon Laser, 3 laser bums (90 mW for 0.1 s; 100 μm diameter) were given to each eye between the retinal vessels around the optic nerve head. Rats were dosed with test compounds in their indicated vehicles orally once daily.
- On day 10, rats were sacrificed with 100% CO 2, and blood vessels were labeled by vascular perfusion with 10 mg/ml FITC-dextran (MW 2×106). Using an epifluorescence microscope (20×) coupled to a spot digital camera and a PC, images were obtained from the flat mounts of the RPE-choroid-sclera from each eye, and the area occupied by hyperfluorescent neovessels within each laser lesion was measured using ImagePro 4 software.
- To determine the dose required for 50% inhibition (ID50), the percent inhibition data was plotted as a function of oral dose, using the ‘best-fit’ analysis within MicroSoft Excel software. The ID 50 value was verified visually by using the plotted data (horizontal line from 50% y value, at intersection with best-fit line drop vertical line to x axis (dose)).
- The results of said assays are set forth in Tables 2, 3 and 4 below, wherein NT means not tested.
TABLE 2 Kinase Inhibition Data VEGF Stimulated VEGF Stimulated Ca++ signal assay Ca++ signal assay KDR Assay Example # % inhibition @ 10 μM mean IC50 (μM) mean IC50 (μM) 1 92 4.05 NT 2 −0.5 NT NT 3 3.50 NT NT 4 16.5 NT NT 5 77.280 1.82 0.48 6 90.06 1.84 0.52 7 94.50 0.95 0.69 8 9 NT NT 9 13.50 NT NT 10 46.00 10 NT 11 −14 NT NT 12 94.25 1.07 NT 13 −12.50 NT NT 14 −14.00 NT NT 15 93.70 0.19 0.91 16 50 10 1.27 17 76.5 3.00 0.79 18 35.5 NT NT 19 14 NT NT 20 63 1.82 1.21 21 88 1.50 0.72 22 22.5 NT NT 23 42 NT NT 24 −13.5 NT NT 25 −1.5 NT NT 26 37.5 NT NT 27 −9 NT NT 28 32.5 NT NT 29 81.50 7.29 0.68 30 39.50 NT NT 31 98.56 0.72 0.57 32 72.20 2.66 0.60 33 95.86 1.35 0.88 34 94.50 2.25 0.47 35 95.10 3.10 0.19 36 30.00 NT NT 37 −4.00 NT NT 38 92.08 1.45 0.50 39 −14.50 NT NT 40 96.71 0.25 0.25 41 16.00 NT NT 42 59.50 NT NT 43 84.00 1.74 0.23 44 96.00 2.02 0.66 45 50.00 NT NT 46 −4.00 NT NT 47 −2.50 NT NT 48 95.44 0.42 0.74 49 97.11 0.10 0.51 50 93.50 1.65 0.55 51 45.50 NT NT 52 69.00 2.28 0.58 53 2.50 NT NT 54 40.50 NT NT 55 14.50 NT NT 56 21.00 NT NT 57 60.00 5.85 1.88 58 86.61 0.81 0.36 59 86.00 1.65 0.18 60 94.00 1.33 0.28 61 −4.50 NT NT 62 33.00 NT NT 63 −14.50 NT NT 64 37.00 NT NT 65 95.50 1.10 0.53 66 18.50 NT NT 67 19.00 NT NT 68 −2.50 NT NT 69 −10.68 NT NT 70 25.00 NT NT 71 38.50 NT NT 72 81.50 1.56 0.43 73 56.50 3.65 0.26 74 44.00 NT NT 75 −18.00 NT NT 76 55.50 NT NT 77 35.00 NT NT 78 8.00 NT NT 79 −4.00 NT NT 80 3.50 NT NT 81 NT NT NT 82 NT 2.5 0.38 83 NT NT NT 84 11.50 NT NT 85 NT NT NT 86 48.50 NT NT 87 79.50 2.25 0.46 88 97.51 1.00 0.41 89 39.50 NT NT 90 97.81 0.42 0.22 91 93.50 3.02 0.24 92 69.00 1.77 0.15 93 51.50 6.62 0.63 94 92.50 3.88 0.64 95 14.00 NT NT 96 97.00 1.68 0.20 97 96.50 1.86 0.20 98 47.50 NT NT 99 97.80 0.48 0.21 100 6.00 NT NT 101 22.00 NT NT 102 82.50 5.91 0.65 103 96.59 1.04 0.48 104 −14.50 NT NT 105 17.50 NT NT 106 96.59 0.60 0.23 107 41.50 NT NT 108 −14.50 NT NT 109 96.50 1.70 0.60 110 95.00 1.55 0.57 111 98.50 1.17 0.43 112 10.50 NT NT 113 97.52 0.35 0.20 114 88.96 1.93 0.92 115 98.23 3.08 0.47 116 99.37 1.64 0.98 117 97.72 0.29 0.31 118 −26.96 NT NT 119 89.28 1.34 0.53 120 12.67 NT NT 121 4.86 NT NT 122 71.71 1.54 0.37 123 49.94 4.43 0.59 124 71.93 1.96 0.52 125 96.55 1.86 0.67 126 95.15 1.22 0.34 127 −11.79 NT NT 128 42.88 8.26 0.46 129 99.00 1.27 NT 130 97.31 0.57 0.38 131 96.71 1.55 0.77 132 73 7.38 NT 133 73 4.91 NT 134 83.09 7.39 1.12 135 27.98 NT 7.77 136 63.74 2.07 0.49 137 65.36 1.67 0.27 138 80.90 7.07 0.73 139 99.26 1.62 0.53 140 96.89 0.46 0.40 141 92.56 2.96 0.51 142 99.27 4.16 0.21 143 66.92 7.61 0.52 144 96.51 2.70 0.46 145 98.73 0.59 0.19 146 98.38 2.07 0.51 147 71.57 5.49 0.15 148 42.22 NT NT 149 17.67 NT 0.62 150 90.86 1.85 0.30 151 50.83 NT 0.46 152 18.73 NT 10 155 97.15 2.60 0.40 156 95.36 0.83 0.51 157 97.89 0.23 0.25 158 97.55 1.14 0.39 159 97.42 0.58 0.35 160 91.44 1.29 0.70 161 95.23 0.46 0.16 162 89.94 1.05 0.30 163 95.34 0.85 0.32 164 98.82 0.16 0.38 165 99.33 1.34 0.36 166 49.17 NT 0.66 167 95.67 0.36 0.19 168 94.33 0.15 0.14 169 93.44 1.16 0.61 170 96.67 0.59 0.41 171 38.07 NT 0.43 172 93.33 1.40 0.69 173 94.54 1.48 0.52 174 76.40 6.02 0.84 175 38.03 NT 0.71 176 67.88 2.54 0.80 177 98.17 0.74 0.75 178 98.78 1.06 0.53 179 97.35 0.64 0.31 180 98.33 1.03 0.44 181 97.73 0.49 0.35 182 99.29 0.95 1.65 183 98.88 0.75 0.52 184 98.21 1.56 0.61 185 98.44 0.87 0.33 186 99.03 1.92 0.89 187 96.84 1.67 0.27 188 98.04 0.53 0.18 189 98.16 0.34 0.07 190 54.11 9.99 6.96 191 98.65 2.41 0.70 192 98.66 1.53 0.59 193 81.51 4.77 2.47 194 96.91 2.67 0.66 195 91.12 4.67 0.96 196 73.10 0.77 0.05 197 98.08 0.32 0.09 198 56.28 7.92 0.47 199 32.43 NT 0.29 200 75.19 3.48 0.74 201 44.15 NT 0.91 202 NT NT NT 203 NT NT NT 204 NT NT NT 205 NT NT NT 206 96.28 0.67 0.24 207 98.64 0.51 0.24 208 99.04 0.53 0.26 209 98.61 0.48 0.24 210 97.40 0.49 0.17 211 96.69 0.66 0.46 212 96.51 0.82 0.50 213 94.97 0.65 0.15 214 97.35 0.35 0.33 215 96.66 0.67 0.34 216 95.31 0.57 0.34 217 97.68 0.86 0.28 218 98.12 0.44 0.17 219 97.38 0.62 0.22 220 94.93 0.30 0.06 221 97.59 0.93 0.38 222 48.76 NT 0.47 223 NT NT NT 224 56.18 6.31 2.27 225 95.70 0.56 0.29 226 97.97 0.70 0.28 227 85.90 0.17 0.05 228 97.25 0.15 0.08 229 97.19 0.23 0.11 230 96.39 0.05 0.08 231 93.29 0.19 0.07 232 97.09 0.86 0.16 233 96.89 0.37 0.19 234 96.09 0.17 0.11 235 98.20 0.28 0.24 236 7.20 NT 0.97 237 36.44 NT 0.71 238 98.39 0.78 0.06 239 97.65 0.19 0.15 240 98.32 0.41 0.23 241 95.81 1.01 0.13 242 95.82 0.13 0.07 243 95.63 0.10 0.11 244 95.98 0.20 0.19 245 95.54 0.47 0.14 246 NT 0.36 0.09 247 NT 0.48 0.20 248 NT 0.71 0.63 249 NT 0.38 0.07 250 NT 0.30 0.15 251 NT 0.46 0.14 252 NT 0.29 0.15 253 NT 0.19 0.17 254 NT 0.47 0.50 255 NT 1.30 0.44 256 NT 0.67 0.19 257 NT 1.63 0.51 258 NT 0.72 0.56 259 NT 0.22 0.30 260 NT 0.88 0.30 261 NT 1.06 0.39 262 NT 0.87 0.21 263 NT 0.47 0.36 264 NT 0.26 0.07 265 NT 0.53 0.61 266 NT 0.12 0.17 267 NT 1.42 0.23 268 NT 10 0.08 269 NT 10 1.68 270 NT 0.28 0.11 271 NT 0.10 0.13 272 NT 0.43 0.46 273 NT 1.07 0.33 274 NT 0.45 0.21 275 NT 0.62 0.13 276 NT 8.81 0.22 277 NT 10 0.25 278 NT 0.18 0.08 279 NT 0.17 0.16 280 NT 0.42 0.46 281 NT 0.73 0.32 282 NT 0.34 0.33 283 NT 0.94 0.15 284 NT 0.25 0.25 285 NT 0.56 0.11 286 NT 0.13 0.04 287 NT 0.62 0.11 288 NT 0.30 0.33 289 NT 0.42 0.26 290 NT 0.44 0.58 291 NT 0.66 0.21 292 NT 0.14 0.09 293 NT 0.88 0.47 294 NT 0.40 0.38 295 NT 0.13 0.18 296 NT 0.16 0.14 297 NT 0.42 0.39 298 NT 0.10 0.34 299 NT 0.49 0.14 300 NT 0.29 0.42 301 NT 0.77 0.27 302 NT 0.71 NT 303 NT 0.52 NT 304 NT 0.12 NT 305 NT 0.55 0.31 306 NT 0.28 0.62 307 NT 0.65 0.27 308 NT 0.16 0.12 309 NT 0.68 0.23 310 NT 0.43 0.23 311 NT 0.12 0.10 312 NT 0.54 NT 313 NT 0.99 NT 314 NT 0.32 NT 315 NT 1.54 NT 316 NT 0.59 NT 317 NT 0.17 NT 318 NT NT NT 319 NT 0.95 NT 320 NT 0.33 NT 321 NT 1.18 NT 322 NT NT NT 323 NT NT NT -
TABLE 3 Miles Assay Results Miles Assay Miles Assay Miles Assay Miles Assay Preincubation Miles Assay Example # dose (mg/kg) vehicle % inhibition period (h) ID50 (mg/kg) 40 75 PEG400 100 18 75 corn oil 47 75 micronized corn 52 oil 75 homogenized corn 40 oil 1 I.V. bolus in 0 0.5 20% methyl cyclodextrin 1 I.V. bolus in 0 1 20% methyl cyclodextrin 1 I.V. bolus in 0 2 20% methyl cyclodextrin 10 43% methyl 6 1 cyclodextrin 156 75 PEG400 (po) 100 157 75 PEG400 (po) 38 159 75 PEG400 91 167 75 PEG400 31 179 75 PEG400 65 181 75 PEG400 38 206 75 corn oil 74 207 75 corn oil 80 208 75 corn oil 32 209 75 PEG400 79 75 corn oil 84 211 75 PEG400 38 214 75 PEG400 61 75 corn oil 78 215 75 PEG400 53 216 75 PEG400 91 218 75 PEG400 99 75 corn oil 97 219 75 PEG400 28 220 75 PEG400 39 229 75 PEG400 97 231 75 corn oil 93 234 75 corn oil 95 235 75 corn oil 99 238 75 corn oil 60 239 75 corn oil 100 243 75 corn oil 100 247 75 corn oil 45 249 75 corn oil 29 250 75 corn oil 62 266 40 corn oil 82 271 40 corn oil 98 -
TABLE 4 CNV Assay results CNV Assay CNV Assay CNV Assay CNV Assay Example # dose (mg/kg) vehicle % inhibition ID50 (mg/kg) 40 50 (bid) corn oil 92 100 (sid) corn oil 74 40 (sid) PEG400 96 ˜20 100 (sid) PEG400 toxic 20 (sid) PEG400 54 20 (bid) PEG400 95 159 100 (sid) 10% DMAC, 41 10% NMP, 80% PEG400 209 80 (sid) corn oil 95 214 80 (sid) corn oil 85 218 80 (sid) corn oil 99 17 38.5 (sid) corn oil 68 9.3 (sid) corn oil 33 231 80 (sid) corn oil 85 234 80 (sid) corn oil 97 19 35 (sid) corn oil 76 8.6 (sid) corn oil 18 235 40 (sid) corn oil 63 239 40 (sid) corn oil 56 - As shown in Table 2, above, the compounds of Examples 1, 5, 6, 7, 12, 15, 17, 21, 29, 31-35, 38, 40, 43, 44, 48-50, 52, 58-60, 65, 72, 73, 82, 87, 88, 90-94, 96, 97, 99, 102, 103, 106, 109-111, 113-117, 119, 122-126, 128-131, 134, 136, 138-147, 149-151, 155-159, 160-189, 191-201, 206-222, 225-268, 270-314, 316, 317, 319 and 320 are preferred as they show either % inhibition of VEGF>79% or VEGIF IC 50<1.0 μM in either the VEGF stimulated Ca++signal assay or KDR assay.
- As also can be seen in Table 2, above, the Compounds of Examples 7, 15, 31, 40, 48, 49, 58, 88, 90, 99, 106, 113, 117, 130, 140, 145, 156, 157, 159, 161, 163, 164, 167, 168, 170, 177, 179, 181, 183, 185, 188, 189, 196, 197 and 206-221, 225-235, 238-240, 242-254, 256, 258-260, 262-266, 270-272, 274, 275, 278-301 and 305-311 are more preferred as they show VEGF IC 50≦1.0 μM in both VEGF stimulated Ca++signal assay and KDR assays.
- Finally, as shown in Tables 2, 3 and 4, the compounds of Examples 40, 156, 157, 159, 167, 179, 181, 206-209, 211, 214-216, 218-220, 229, 231, 234, 235, 238, 239, 243, 247, 249, 250, 266 and 271 are most preferred in that they show significant in-vivo activity and therefore would be effective in oral administration.
- The invention is further illustrated by the following non-limiting examples.
- Phenylaminomethylene-1,3-dihydro-indol-2-one
- 2.42 mL of ethylformate are combined with 1.33 gms of 1,3 dihydro-indol-2-one in a solution of 21%, by weight, sodium formate in ethanol. The resulting solution is allowed to stand at room temperature for 30 minutes and then refluxed for 30 minutes to yield a suspension. Once at room temperature the suspension was acidified to pH 1.0 with 10% HCl(aq), then 5 mL of H 2O was added. The resulting precipitate is filtered and washed with H2O (4×20 mL) to provide a mixture of E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as a solid.
- E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one is reacted with 0.022 gms. of aniline by refluxing in tetrahydrofuran (1.2 mL) for 12 hours to yield a quantitative amount (39 mg) of the named compound as a solid following concentration in vacuo, dilution with isopropanol and filtration.
- (3-Bromophenylamino)-methylene]-1,3-dihydro-indol-2-one
- The named compound is prepared by substituting 3-bromoaniline for aniline in the reaction of Example 1.
- The compounds of Example 3 through 198, 202-205, 210-213, 219-224, 227-230 and 236-237 are prepared by substituting the appropriate substituted aniline for aniline, or the appropriate 4′-methyl or 5′-fluoro or 5′-chloro or 6′-fluoro or 5′-nitro or 5′-cyano substituted 1,3 dihydro-indol-2-one for 1,3 dihydro-indol-2-one in the reaction of Example 1.
- 3-{[4-(4-Pyrrolidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 207, 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and pyrrolidine are converted to the named compound
- 3-{[4-(3-Chloro-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- A solution of 3-[(4-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one (0.945 g, 3.75 mmol) in 20 mL DMF is treated with potassium carbonate (777 mg, 1.5 equiv.) and 1-bromo-3-chloropropane (1.1 equiv.). The reaction mixture is heated to 40° C. for 3 h. The reaction mixture is partitioned between EtOAc and water. The organic layer is collected and washed sequentially with H 2O (1 X), saturated aqueous NaHCO3 solution (1 X), and brine (1 X). The organic phase was dried and concentrated to give an oil. The oil was purified by preparative chromatography (silica gel; 1:1 EtOAc:hexane) to give the named compound as a yellow solid (711 mg, 58%).
- 3-{[4-(4-Chloro-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- A solution of 3-[(4-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one (0.945 g, 3.75 mmol) in 20 mL DMF is treated with potassium carbonate (777 mg, 1.5 equiv.) and 1-bromo-4-chlorobutane (1.1 equiv.). The reaction mixture is heated to 40° C. for 3 h. The reaction mixture is partitioned between EtOAc and water. The organic layer is collected and washed sequentially with H 2O (1 X), saturated aqueous NaHCO3 solution (1 X), and brine (1 X). The organic phase is dried and concentrated to give a solid. The solid is triturated with MeOH and collected by filtration to give the named compound as yellow solid (600 mg, 47%).
- 3-{[4-(3-Iodo-propoxy)-phenylaminol-methylene}-1,3-dihydro-indol-2-one
- A solution of 3-{[4-(3-Chloro-propoxy)-phenyla-nino]-methylene}-1,3-dihydro-indol-2-one (45 mg, 0.137 mmol) and Nal (103 mg, 5 equiv.) in 1 mL acetone is heated at 80° C. overnight. The reaction mixture is cooled to room temperature and evaporated to dryess. The residue is dissolved in EtOAc and H 2O. The organic layer is collected and dried over anhydrous Na2SO4, filtered, and concentrated to give the named compound as yellow solid (100%).
- 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described for Example 200, 3-{[4-(4-Chloro-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one was converted to the named compound.
- 3-{[4-(4-Morpholin-4-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 207, 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and morpholine are converted to the named compound.
- 3-{[4-(4-Diethylamino-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- A 25 mL pressure tube is charged with 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one (75 mg, 0.173 mmol), diethylamine (2 mL) and the reaction mixture is heated at 50° C. for 3 h. The reaction mixture is cooled to room temperature and partitioned between EtOAc and H 2O. The organic layer is washed with saturated aqueous NaHCO3 solution, brine, and dried over anhydrous Na2SO4. The organic layer is collected by filtration and concentrated to give a yellow solid. The solid is broken up in MeOH and collected by filtration to give the named compound as a yellow solid.
- 3-({4-14-(4-Methyl-piperazin-1-yl)-butoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 207, 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dilhydro-indol-2-one and N-methylpiperazine are converted to the named compound.
- 3-{[4-(4-Piperidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 207, 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and piperidine are converted to the named compound
- 3-{[14-(3-Diethylamino-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a mamnner similar to that described in Example 217, 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and diethylamine are converted to the named compound.
- 3-{[4-(3-Morpholin-4-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 217, 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and morpholine are converted to the named compound.
- 3-{[4-(3-Pyrrolidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 217, 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and pyrrolidine are converted to the named compound.
- 3-({4-[3-(4-Methyl-piperazin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- A solution of 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one (66 mg, 0.157 mmol) in 1 mL THF is treated with 1-methylpiperazine (37.2 μL, 2.2 equiv.) and the resulting reaction mixture is heated at 50° C. for 4 h. The reaction mixture is cooled to room temperature and concentrated under reduced pressure. The residue is partitioned between EtOAc and H 2O. The organic layer is collected and washed with saturated aqueous NaHCO3, brine, and dried over anhydrous Na2SO4. The yellow solid residue obtained upon evaporation is broken up in 1:9 EtOAc:hexane and collected by filtration to give the named compound as a yellow solid.
- 3-{[4-(3-Piperidin-1-yl-propoxy)-phenylamino}-methylenel-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 217, 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and piperidine are converted to the named compound.
- 3-{[4-(3-Thiomorpholin-4-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 217, 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and thiomorpholine are converted to the named compound.
- 3-{[4-(4-Thiomorpholin-4-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 207, 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one and thiomo-lpholine are converted to the named compound.
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- To a solution of 4-(3-diethylamino-propoxy)-phenylamine (913 mg, 1.3 equiv.) in THF (14 mL) is added 4-methyl-3-hydroxymethylene-1,3-dihydro-indol-2-one (554 mg, 3.17 mmol) in one portion. The resulting reaction mixture is stirred and heated at 72° C. overnight. The reaction solution is cooled to room temperature and partitioned between EtOAc:H 2O. The organic layer is collected and washed with saturated aqueous NaHCO3 and dried over anhydrous Na2SO4. The organic phase is filtered and concentrated. The residue obtained is triturated with 1:4 EtOAc:hexane to give the named compound as a yellow solid (625.8 mg, 52%).
- Diethyl-[3-(4-nitro-phenoxy)-propyl]-amine
- A solution of 3-diethylamino-1-propanol (2.97 mL, 20 mmol) in THF (40 mL) at 0° C. is treated potassium tert-butoxide (2.36 g, 1.05 equiv.) in one portion. The reaction mixture is stirred at 0° C. for 5 min during which time it becomes reddish brown. Neat 1-fluoro-4-nitrobenzene (2.82 g, 1 equiv.) is added dropwise and the ice-bath is removed. The reaction mixture is stirred at room temperature for 15 min. The dark green reaction mixture is quenched with ice-water (300 mL), and extracted with EtOAc (2×200 mL). The combined organic extracts are washed with water (2×300 mL) and brine (1×300 mL) and dried over anhydrous Na 2SO4. The oily brown residue obtained after evaporation, is purified by chromatography, (silica gel, 1:4 MeOH:CHCl3) to give the named compound as a brown oil (3.59 g, 71%).
- 4-(3-Diethylamino-propoxy)-phenylamine
- A solution of diethyl-[3-(4-nitro-phenoxy)-propyl]-amine (15.2 g, 60.4 mmol) is dissolved in absolute ethanol (350 mL) and to this solution is added hydrazine monohydrate (18 mL, 6 equiv.) followed by the addition of a small portion of Raney nickel. The reaction mixture is heated to 50° C. with stirring for 3 h until all gas evolution has ceased. The reaction mixture is filtered through celite to remove the Raney nickel. The filtrate is concentrated under reduced pressure to give the named compound as a greenish brown oil (quantitative). This material is carried on in subsequent steps without purification.
- 5-Chloro-3-{[4-(3-diethylamino-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-diethylamino-propoxy)-phenylamine (911 mg, 1.3 equiv.) and 5-chloro-3-hydroxyiethylene-1,3-dihydro-indol-2-one (617 mg, 3.16 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (577 mg, 46%).
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-diethylamino- propoxy)-phenylamine (161 mg, 1.3 equiv.) and 5-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (100 mg, 0.56 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (88 mg, 41%).
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-diethylamino-propoxy)-phenylamine (5 g, 1.3 equiv.) and 6-fluoro-3-hydroxyn-ethylene-1,3-dihydro-indol-2-one (3.12 g, 17.4 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (4.1 g, 61%).
- 3-[{4-(2-Morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one.
- The named compound is prepared by refluxing 1.26 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 3.45 gms. of N-(2-morpholin-4-yl-ethyl)-benzene-1,4-diamine in tetrahydrofuran (34.02 mL) for 12 hours. Following concentration in vacuo, dilution with hot isopropanol and filtration provides the named compound is isolated as a yellow solid in the amount of 1.731 gms.
- N-(2-Morpholin-4-yl-ethyl)-benzene-1,4-diamine is prepared from p-fluoronitrobenzene by the following method:
- A mixture of 7.5 mL of p-fluoronitrobenzene, 10.25 mL 2-morpholin-4-yl-ethylamine and 15 mL N,N-diisopropylamine in 36 mL dioxane is heated at 105° C. for 2 days. The reaction mixture is cooled to room temperature, diluted with dichloromethane (360 mL) and washed with water (3×290 mL). Upon evaporation to dryness, the dichloromethane layer yields the (2-morpholin-4-yl-ethyl)-(4-nitro-phenyl)-amine product, which is recrystallized from methanol.
- A suspension of 4.05 gms. of (2-morpholin-4-yl-ethyl)-(4-nitro-phenyl)-amine in 93 mL of ethanol is heated to 50° C. Once dissolution is achieved 4.8 mL hydrazine monohydrate is added to the solution. A Raney nickel 2800 slurry in water (approximately 3.5 mL) is added to the 50° C. solution dropwise, waiting after each addition for the bubbling to cease. Sufficient quantities of Raney nickel have been added when continued addition of Raney nickel causes no further gas evolution. The reaction is then maintained at 50° C. for an additional hour, and subsequently is cooled to room temperature. The reaction mixture is filtered through a pad of celite (rinsing the pad with methanol). The N-(2-morpholin-4-yl-ethyl)-benzene-1,4-diamine (3.45 gms.) is isolated upon evaporation of the filtrate, and is subsequently used without purification in the reaction of Example 235.
- Examples 242, 243, 244, and 245 are prepared by substituting the appropriate 4′-methyl or 6′-fluoro or 5′-fluoro or 5′-chloro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-(2-morpholin-4-yl-ethyl)-benzene-1,4-diamine, used for the preparation of Example 235, for aniline in the reaction of Example 1.
- 4-Methyl-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}- 1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-Piperidin-1-yl-propoxy)-phenylamine (0.61 g, 1.3 equiv.) and 4-methyl-3-hydroxymethylene-1,3-dihydro-indol-2-one (0.35 g, 2 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (388 mg, 49%).
- 1-[3-(4-Nitro-phenoxy)-propyl]-piperidine
- In a manner similar to that described in Example 231a, 3-piperidino-propan-1-ol (1 g) is converted to the named compound as a light brown oil (1.85 g).
- 4-(3-Piperidin-1-yl-propoxy)-phenylamine
- In a manner similar to that described in Example 231b, 1-[3-(4-nitro-phenoxy)-propyl]-piperidine (6.99 mmol) is converted to the named compound as a brown oil (1.64 g).
- 6-Fluoro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-Piperidin-1-yl-propoxy)-phenylamine (0.61 g, 1.3 equiv.) and 6-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (0.358 g, 2.00 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (465 mg, 59%).
- 5-Fluoro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-piperidin-1-yl-propoxy)-phenylamine (0.57 g, 1.3 equiv.) and 5-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (0.363 g, 2 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (379 mg, 47%).
- 5-Chloro-3-{[4-(3-piperidin-1-yl-propox)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-piperidin-1-yl-propoxy)-phenylamine (550 mg, 1.3 equiv.) and 5-chloro-3-hydroxymethylene-1,3-dihydro-indol-2-one (380 mg, 2.00 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (464 mg, 58%).
- 4-Methyl-3-{[4-(4-piperidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(4-Piperidin-1-yl-butoxy)-phenylamine (1.19 g, 1.1 equiv.) and 4-metbyl-3-hydroxymethylene-1,3-dihydro-indol-2-one (0.79 g, 4.5 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (0.97 g, 53%).
- 1-[4-(4-Nitro-phenoxy)-butyl]-piperidine
- In a manner similar to that described in Example 231a, 4-piperldino-butan-1-ol (36 mmol), is converted to the named compound as a light brown oil (8.61 g, 89%).
- 4-(4-Piperidin-1-yl-butoxy)-phenylamine
- In a manner similar to that described in Example 231b, 1-[4-(4-nitro-phenoxy)-butyl]-piperidine (8.12 g, 29 mmol) is converted to the named compound as a light greenish soft solid (6.46 g, 89%).
- 6-Fluoro-3-{[4-(4-piperidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(4-piperidin-1-yl-butoxy)-phenylamine (400 mg, 1.1 equiv.) and 6-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (263 mg, 1.47 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (435 mg, 73%).
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-Diethylamino-propoxy)-3-fluoro-phenylamine (400 mg, 1.1 equiv.) and 3-hydroxynethylene-1,3-dihydro-indol-2-one (242 mg, 1.5 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (135 mg, 24%).
- Diethyl-[3-(2-fluoro-4-nitro-phenoxy)-propyl]-amine
-
- In a manner similar to that described in Example 231a, 3,4-difluoronitrobenzene (6.7 mL, 60.6 mmol, 1 equiv.) and 3-diethylamino-1-propanol (9 mL, 1 equiv.) are converted to the named compound as a brown oil (16.3 g).
- 4-(3-Diethylamino-propoxy)-3-fluoro-phenylamine
- In a manner similar to that described in Example 231b, diethyl-[3-(2-fluoro-4-nitro-phenoxy)-propyl]-amine (16.3 g, 60.3 mmol) is converted to the named compound as a brown oil (12.4 g, 86%).
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-diethylamino-propoxy)-3-fluoro-phenylamine (400 mg, 1.1 equiv.) and 4-methyl-3-hydroxyrethylene-1,3-dihydro-indol-2-one (263 mg, 1.5 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (294 mg, 49%).
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-diethylamino-propoxy)-3-fluoro-phenylamine (400 mg, 1.1 equiv.) and 6-fluoro-3-hydroxyrethylene-1,3-dihydro-indol-2-one (269 mg, 1.5 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (195 mg, 32%).
- 3-[(4-Piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one.
- The named compound is prepared by refluxing 0.025 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.036 gms. 4-piperidin-1-ylmethyl-phenylamine in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperture, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 0.038 gms.
- Piperidin-1-ylmethyl-phenylamine is prepared from 4-nitro-benzoic acid by the following method:
- A room temperature solution of 5.04 gms. 4-nitro-benzoic acid in tetrahydrofuran (10 mL) is treated with 5.14 gms. 1′,1′-carbonyl-diimidizole and immediately immersed in an ice bath. The reaction mixture is stirred in the ice bath for 30 minutes, then it is allowed to warm to room temperature. Once at room temperature the reaction mixture is treated with 3 mL piperidine. The reaction mixture is allowed to stir at room temperature ovenight. The reaction is then made basic with the addition of saturated aqueous sodium bicarbonate solution, and the resulting mixture is extracted with ethyl acetate. The organics are separated, dried over anhydrous sodium sulfate, and subsequently evaporated to dryness in vacuo. The crude product residue is then chromatographed by flash silica gel chromatography using 50% ethyl acetate in hexanes as the eluant. Following evaporation of solvent, 1-(4-nitrobenzoyl)piperidine is isolated as a white solid in the amount of 5.87 gms.
- 1-(4-Nitrobenzoyl)piperidine (0.1125 gms.) is then dissolved in tetrahydrofuran (1 mL) and slowly added dropwise to a 0° C., 1.0 M solution (4 mL) of Borane in tetrahydrofuran. The reaction mixture is maintained at 0° C. for 20 minutes following the completion of the addition to the Borane/tetrahydrofuran solution. The reaction mixture is then allowed to warm to room temperature, and is subsequently heated to 60° C. using an oil bath. The reaction mixture is maintained at 60° C. overnight, then is cooled to room temperature, and quenched with the addition of concentrated HCl (added until gas evolution stops). The quenched reaction mixture is then extracted with ethyl acetate and water. The organic layer is separated, and concentrated in vacuo, while the aqueous layer is separated and made basic with the addition of aqueous 1 M NaOH. The basic aqueous layer is then extracted with ethyl acetate and the ethyl acetate layer from this extraction is concentrated in vacuo. The solids isolated from both concentrated ethyl acetate layers are combined to yield 92 mg of 1-(4-nitro-benzyl)-piperidine as a white-yellow solid.
- 1-(4-Nitro-benzyl)-piperidine (0.5052 gms.) is suspended in 25 mL of a 1:1 (v:v) solution of acetic acid and water. The suspension is then treated with 45 mL of an ˜10 wt. % solution of TiCl 3 in 20-30 wt. % HCl, dropwise. Over the course of the addition a color change from colorless to purple to black ensues. The reaction mixture is immersed in a 60° C. oil bath overnight following the addition of TiCl3. The reaction mixture is then cooled to room temperature and made basic with the addition of a 10% aqueous solution of NaOH. The basic reaction mixture is extracted with chloroformn, and the organic layer is dried over anhydrous sodium sulfate. Following evaporation of the solvent the organic layer yields 0.333 gms. of piperidin-1-ylmethyl-phenylamine.
- 4-Methyl-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Exanple 231, 4-(2-piperidin-1-yl-ethoxy)-phenylamine (754 mg, 1.2 equiv.) and 4-methyl-3-hydroxymethylene-1,3- dilhydro-indol-2-one (500 mg, 2.86 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (530 mg, 49%).
- 1-[2-(4-Nitro-phenoxy)-ethyl]-piperidine
- In a manner similar to that described in Example 231a, 1-piperidine-ethanol (8.8 mL, 66 mmnol, 1.1 equiv.) is converted to the named compound as a light brown solid (14.6 g, 97%).
- 4-(2-Piperidin-1-yl-ethoxy)-phenylamine
- In a manner similar to that described in Example 231b, 1-[2-(4-nitro-phenoxy)-ethyl]-piperidine (5.0 g, 20 mmol) is converted to the named compound as a light brown oil (4.4 g).
- 6-Fluoro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-piperidin-1-yl-ethoxy)-phenylamine (737 mg, 1.2 equiv.) and 6-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.79 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (745 mg, 70%).
- 3-{[4-(2-Piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-piperidin-1-yl-ethoxy)-phenylamine (820mg, 1.2 equiv.) and 3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 3.11 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (899 mg, 80%).
- 4-Fluoro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-piperidin-1-yl-propoxy)-phenylamine (610 mg, 1.3 equiv.) and 4-fluoro-3-hydroxynethylene-1,3-dihydro-indol-2-one (360 mg, 2 mmol, 1 equiv.) are converted to the named compound as a yellow solid (625 mg, 79%).
- 4-Methyl-3-1(4-piperidin-1-ylmethyl-phenylamino)-methylene-1,3-dihydro-indol-2-one.
- The named compound is prepared by refluxing 0.045 gms E & Z-3-hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, prepared by substituting 4′-methyl-1,3 dihydro-indol-2-one for 1,3 dihydro-indol-2-one in the reaction of Example 1, with 0.064 gms. 4-piperidin-1-ylmethyl-phenylamine, prepared as in the reaction of Example 251, in tetrahydrofuran (1 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 0.0463 gms.
- Examples 257-260 are prepared by substituting the appropriate 4′-fluoro or 5′-fluoro or 6′-fluoro or 5′-chloro substituted 1,3-dihydro-indol-2-one for the 1, 3-dihydro-indol-2-one and 4-piperidin-1-ylmethyl-phenylamine (used in the preparation of Example 251) for aniline in the reaction of Example 1.
- 4-Fluoro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-piperidin-1-yl-ethoxy)-phenylamine (600 mg, 1.2 equiv.) and 4-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (407 mg, 2.27 mmol, 1 equiv.) (4-Fluorooxindole may be prepared as described in Synthesis 1991, 10, 871) are reacted to give the named compound as a yellow solid (542 mg, 63%).
- 5-Chloro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-piperidin-1-yl-ethoxy)-phenylamine (600 mg, 1.2 equiv.) and 5-chloro-3-hydroxymethylene-1,3-dihydro-indol-2-one (444 mg, 2.27 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (513 mg, 57%).
- 5-Fluoro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-piperidin-1-yl-ethoxy)-phenylamine (600 mg, 1.2 equiv.) and 5-fluoro-3-hydroxynethylene-1,3-dihydro-indol-2-one (407 mg, 2.27 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (644 mg, 74%).
- 3-{[4-(2-Diethylamino-ethoxy)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- In a manner similar to that describe in Example 231, 4-(2-diethylamino-ethoxy)-phenylamine (713 mg, 1.2 equiv.) and 4-methyl-3-hydroxynmethylene-1,3-dihydro-indol-2-one (500 mg, 2.86 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (237 mg, 23%).
- Diethyl-12-(4-nitro-phenoxy)-ethyl]-amine
- In a manner similar to that described in Example 231a, N,N-diethylethanolamine (8.75 mL, 66 mmol, 1.1 equiv.) is converted to the named compound as a brown oil (14.3 g).
- 4-(2-Diethylamino-ethoxy)-phenylamine
- In a manner similar to that described in Example 231b, diethyl-[2-(4-nitro-phenoxy)-ethyl]-amine (4.77 g, 20 mmol) is converted to the named compound as a brown oil (4.16 g).
- 3-{[4-(2-Diethylamino-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a maimner similar to that described in Example 231, 4-(2-diethylamino-ethoxy)-phenylamine (775 mg, 1.2 equiv.) and 3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 3.11 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (557 mg, 51%).
- 3-{[4-(2-Diethylamino-ethoxy)-phenylamino]-methylenel-6-fluoro-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-Diethylamino-ethoxy)-phenylamine (697 mg, 1.2 equiv.) and 6-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.79 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (399 mg, 39%).
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(3-Diethylamino-propoxy)-3-fluoro-phenylamine (720 mg, 1.5 equiv.) and 4-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (358 mg, 2.00 mmol) are reacted to give the named compound as a yellow solid (541 mg, 67%).
- 6-(3-Methoxy-phenyl)-3-{[4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.020 gms E & Z 3-hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one with 0.021 gms 4-(4-methyl-piperazin-1-yl)-phenylamine in tetrahydrofuran (0.33 mL) for 2 days. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol (plus a little chloroform) and filtration the reaction yields the named compound as a solid in the amount of 16.2 mg.
- E & Z 3-Hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one is prepared in 5 steps from 4-Bromo-1-fluoro-2-nitro-benzene by the following method:
- 4-Bromo-1-fluoro-2-nitro-benzene (5 gms.), 3.85 gms. 3-methoxyphenylboronic acid and 22 mL of a 2M aqueous solution of sodium carbonate are suspended in 100 mL of a 1:1 (v:v) mix of toluene and ethanol. The resulting suspension is then treated with 0.8 gms. tetrakis(triphenylphosphine)palladium (0) at room temperature. The reaction mixture is then heated at reflux for 12 h. The reaction mixture is subsequently concentrated in vacuo, and the resulting residue is taken up in ethyl acetate (200 mL). The ethyl acetate solution is then washed successively with water (2×200 mL), and brine (2×200 mL). The organic layer is then dried by filtering through phase separator paper and concentrated in vacuo to yield 7.19 gms. of crude product. The crude product is then recrystallized from hot ethanol yielding 4-fluoro-3′-methoxy-3-nitro-biphenyl as a yellow-white solid in the amount of 4.767 gms.
- A room temperature DMSO (50 mL) suspension of a 60% dispersion of sodium hydride in mineral oil (3.94 gms.) is treated dropwise with 10.41 mL of dimethylmalonate. The suspension is then heated to 100° C. for 35 minutes and subsequently treated with a solution of 4-fluoro-3′-methoxy-3-nitro-biphenyl (4.6138 gms.) in DMSO (55 mL). The reaction mixture is heated at 100° C. for an additional 1 h after which it is cooled to room temperature, and quenched with the addition of a saturated aqueous solution of ammonium chloride (300 mL). The quenched reaction mixture is then extracted with ethyl acetate (3×300 mL). The combined ethyl acetate washes are then washed with brine (1×500 mL), filtered through phase separator paper to dry the solution, and then concentrated in vacuo. The crude residue isolated is recrystallized from isopropanol/ethyl acetate yielding 2-(3′-methoxy-3-nitro-biphenyl-4-yl)-malonic acid dimethyl ester in the amount of 4.4023 gms.
- 2-(3′-Methoxy-3-nitro-biphenyl-4-yl)-malonic acid dimethyl ester (4.4023 gms.) is suspended in 45 mL of 6N HCl and heated at 110° C. for 4 days. The reaction mixture is cooled to room temperature and the precipitate is collected by filtration. As the solid material is in rather large chunks, the solid purification is simplified by first dissolving the solid chunks in an excess of hot methanol, and subsequently concentrating the solution in vacuo to get a more manageable powder. This powder is then triturated with approximately 10 mL of methanol and filtered yielding (3′-methoxy-3-nitro-biphenyl-4-yl)-acetic acid in the amount of 2.0120 gms.
- A solution of (3′-Methoxy-3-nitro-biphenyl-4-yl)-acetic acid (2.0120 gms.) in 35 mL methanol is treated with 0.3123 gms. of 10% palladium on carbon. The resulting suspension is then stirred vigorously under an atmosphere of hydrogen at room temperature and atmospheric pressure for 3 h. The reaction mixture is then filtered through a pad of celite, the filtrate is treated with decolorizing charcoal and filtered a second time through celite. The filtrate is then concentrated in vacuo and purified by trituration with isopropanol yielding 0.5736 gms. of 6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one.
- 6-(3-Methoxy-phenyl)-1,3-dihydro-indol-2-one (0.2282 gms.) is combined with 0.23 mL of ethylformate in 0.67 mL anhydrous ethanol, and is treated with a solution of 21%, by weight, sodium formate in ethanol (0.40 mL). The resulting solution is allowed to stand at room temperature for 30 minutes, and then is refluxed for 2 h to yield a suspension. Once at room temperature the suspension is acidified to pH 1.0 with 10% HCl(aq), then diluted with 5 mL of H 2O. The resulting precipitate. was filtered and washed with H2O (4×20 mL) to provide a mixture of E & Z 3-hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one, as a solid in the amount of 0.2064 gms.
- 6-(3-Methoxy-phenyl)-3-{[4-morpholin-4-yl-phenylamino)-methylene]- 1,3-dihydro-indol-2-one.
- The named compound is prepared by refluxing 0.020 gms E & Z 3-hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one with 0.020 gms. 4-morpholin-4-yl-phenylamine in tetrahydrofuran (0.33 mL) for 2 days. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 15.7 mg.
- 4-Methyl-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, (706 mg, 1.2 equiv.) and 4-methyl-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.86 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (478 mg, 46%).
- 1-[2-(4-Nitro-phenoxy)-ethyl]-pyrrolidine
- In a manner similar to that described in Example 231a, 1-(2-hydroxyethyl)-pyrroline (7.72 mL, 66 mmol, 1.1 equiv.) is converted to the named compound as a brown oil (14.2 g).
- 4-(2-Pyrrolidin-1-yl-ethoxy)-phenylamine
- In a manner similar to that described in Example 231b, 1-[2-(4-nitro-phenoxy)-ethyl]-pyrrolidine (7.5 g, 30 mmol) is converted to the named compound as a reddish oil (5.52 g, 89%).
- 6-Fluoro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-pyrrolidin-1-yl-ethoxy)-phenylamine (690 mg, 1.2 equiv.) and 6-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.79 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (500 mg, 48%).
- 3-{[4-(2-Pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2one
- In a manner similar to that described in Example 231, 4-(2-pyrrolidin-1-yl-ethoxy)-phenylamine (767 mg, 1.2 equiv.) and 3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 3.11 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (690 mg, 64%).
- 4-Fluoro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-pyrrolidin-1-yl-ethoxy)-phenylamine (690 mg, 1.2 equiv.) and 4-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.79 mmol, 1 equiv.) to give the named compound as a yellow solid (790 mg, 77.0%).
- 5-Fluoro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-Pyrrolidin-1-yl-ethoxy)-phenylamine (690 mg, 1.2 equiv.) and 5-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.79 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (705 mg, 69%).
- 5-Chloro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(2-Pyrrolidin-1-yl-ethoxy)-phenylamine (632 mg, 1.2 equiv.) and 5-chloro-3-hydroxymethylene-1,3-dihydro-indol-2-one (500 mg, 2.56 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (775 mg, 79%).
- 6-(3-Methoxy-phenyl)-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one.
- The named compound is prepared by refluxing 0.020 gms E & Z 3-hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one (see example 268) with 0.0375 gms 4-(3-piperidin-1-yl-propoxy)-phenylamine (used in the preparation of Example 218) in tetrahydrofuran (0.33 mL) for 36 h. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 16.8 mg.
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-6-(3-Methoxy-phenyl)-1,3-dihydro-indol-2-one.
- The named compound is prepared by refluxing 0.020 gms E & Z 3-Hydroxymethylene-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one (see example 268) with 0.0327 gms 4-(3-diethylamino-propoxy)-phenylamine (used in the preparation of Example 214) in tetrahydrofuran (0.33 mL) for 36 h. Following cooling to room temperature, solvent evaporation in vacuo, trituration with ethyl acetate/(min) hexanes and filtration the reaction yields the named compound as a solid in the amount of 9.0 mg.
- 4-Methyl-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(1-methyl-piperidin-3-ylmethoxy)-phenylamine (603 mg, 1.2 equiv.) and 4-methyl-3-hydroxymethylene-1,3-dihydro-indol-2-one (400 mg, 2.28 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (439 mg, 51%).
- 1-Methyl-3-(4-nitro-phenoxymethyl)-piperidine
- In a manner similar to that described in Example 231a, 1-methyl-3- piperidinemethanol (8.53 g, 66 mmol, 1.1 equiv.) is converted to the named compound as a brown oil (11.5 g, 77%).
- 4-(1-Methyl-piperidin-3-ylmethoxy)-phenylamine
- In a manner similar to that described in Example 231b, 1-Methyl-3-(4-nitro-phenoxymethyl)-piperidine (7.5 g, 30 mmol) is converted to the named compound as a light brown solid (4.82 g, 73%).
- 6-Fluoro-3-{[4-(1-methyl-piperidin-3-ylethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(1-methyl-piperidin-3-ylmethoxy)-phenylamine (590 mg, 1.2 equiv.) and 6-fluoro-3- hydroxymethylene-1,3-dihydro-indol-2-one (400 mg, 2.23 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (466 mg, 55%).
- 3-{[4-(1-Methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(1-methyl-piperidin-3-ylmethoxy)-phenylamine (656 mg, 1.2 equiv.) and 3-hydroxymethylene-1,3-dihydro-indol-2-one (400 mg, 2.48 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (732 mg, 81%).
- 4-Fluoro-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(1-methyl-piperidin-3-ylmethoxy)-phenylamine (590 mg, 1.2 equiv.) and 4-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (400 mg, 2.23 mmol, 1 equiv.) are reacted to give the named compound as a yellow solid (576 mg, 68%).
- 5-Fluoro-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, 4-(1-methyl- piperidin-3-ylmethoxy)-phenylamine hydrochloride (688 mg, 1.2 equiv.) and 5-fluoro-3-hydroxymethylene-1,3-dihydro-indol-2-one (400 mg, 2.23 mmol, 1 equiv.) with the addition of anhydrous Et 3N (2.25 equiv.) are reacted to give the named compound as a yellow solid (657 mg, 77%).
- 5-Chloro-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 231, , 4-(1-methyl-piperidin-3-ylmethoxy)-phenylamine hydrochloride (158 mg, 1.2 equiv.) and 5-chloro-3-hydroxymethylene-1,3-dihydro-indol-2-one (100 mg, 0.51 mmol, 1 equiv.) with the addition of anhydrous Et 3N (2.11 equiv.) are reacted to give the named compound as a yellow solid (100 mg, 49%).
- 3-{[4-(2-Piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.0505 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.085 gms. 4-(2-piperidin-1-yl-ethyl)-phenylamine in tetrahydrofuran (1.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound in the amount of 66 mg.
- 4-(2-Piperidin-1-yl-ethyl)-phenylamine is prepared from (4-Nitro-phenyl)-acetic acid by the following method:
- A room temperature solution of 2.53 gms. (4-Nitro-phenyl)-acetic acid in tetrahydrofuran (10 mL) is treated with 2.44 gms. 1′,1′-carbonyl-diimidizole and immediately immersed in an ice bath. The reaction mixture is stirred in the ice bath for 30 minutes then it is allowed to warm to room temperature. Once at room temperature the reaction mixture is treated with 1.21 mL piperidine, and then is stirred overnight at room temperature. The reaction is then made basic with the addition of saturated aqueous sodium bicarbonate, and the resulting mixture is extracted with ethyl acetate. The organics are separated, dried over anhydrous sodium sulfate, filtered, and subsequently evaporated to dryness in vacuo. The crude product residue is then chromatographed by flash silica gel chromatography using 50% ethyl acetate in hexanes as the eluant. Following evaporation of solvent, 2-(4-nitro-phenyl)-1-piperidin-1-yl-ethanone is isolated as a white solid in the amount of 3.18 gms.
- 2-(4-Nitro-phenyl)-1-piperidin-1-yl-ethanone (1.24 gms.) is then dissolved in tetrahydrofuran (5 mL), and slowly added dropwise to a 0° C., 1.0M solution (35 mL) of Borane in tetrahydrofuran. The reaction mixture is maintained at 0° C. for 20 minutes following the completion of the addition to the Borane/tetrahydrofuran solution. The reaction mixture is then allowed to warm to room temperature, and is subsequently refluxed using an oil bath. The reaction mixture is maintained at refluxing temperature overnight, then cooled to 0° C. and quenched with the addition of concentrated HCl (added until fizzing stops). The quenched reaction mixture is then extracted with ethyl acetate and water. The organic layer is then separated, dried over sodium sulfate and concentrated in vacuo. The solid isolated is then triturated with hexanes and a little ethyl acetate to yield 0.7563 gms. of 1-[2-(4-nitro-phenyl)-ethyl]-piperidine as a white solid following filtration.
- 1-[2-(4-Nitro-phenyl)-ethyl]-piperidine (0.1178 gms.) is suspended in 4.5 mL of a 1:1 (v:v) solution of acetic acid and water. The suspension is then immersed in a 65° C. oil bath and treated in dropwise fashion with 9 mL of an ˜10 wt. % solution of TiCl 3 in 20-30 wt. % HCl. Over the course of the addition a color change from colorless to purple to black ensues. The reaction mixture is maintained in the 65° C. oil bath overnight following the addition of TiCl3. The reaction mixture is then cooled to 0° C., and made basic with the addition of a 10% aqueous solution of NaOH. The basic reaction mixture is then extracted with chloroform. The emmulsion that forms is filtered through glass wool, re-extracted with chloroform, and then dried over anhydrous sodium sulfate. Following evaporation of the solvent the residue is chromatographed by flash silica gel chromatography (10% methanol in chloroform) to yield 60 mg of 4-(2-piperidin-1-yl-ethyl)-phenylamine.
- 4-Methyl-3-{[4-(2-piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.055 gms. E & Z-3-Hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, as prepared in the reaction of Example 1, with 0.085 gms. 4-(2-piperidin-1-yl-ethyl)-phenylamine (used in the preparation of Example 284) in tetrahydrofuran (1 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 38 mg.
- Examples 285 and 287-289 are prepared by substituting the appropriate 5′-chloro or 4′-fluoro or 5′-fluoro or 6′-fluoro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and 4-(2-piperidin-1-yl-ethyl)-phenylamine (used in the preparation of Example 284) for aniline in the reaction of Example 1.
- 3-[(4-Morpholin-4-ylmethyl-phenylamino)-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.051 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.085 gms. 4-morpholin-4-ylmethyl-phenylamine in tetrahydrofuran (2.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 0.070 gms.
- 4-Morpholin-4-ylmethyl-phenylamine is prepared from 4-Nitro-benzoic acid by the following method:
- A room temperature solution of 2.49 gms. 4-nitro-benzoic acid in tetrahydrofuran (15 mL) is treated with 2.44 gms. 1′,1′-carbonyl-diimidizole, and immediately immersed in an ice bath. The reaction mixture is stirred in the ice bath for 30 minutes then it is allowed to warm to room temperature. Once at room temperature the reaction mixture is treated with 1.5 mL piperidine. The reaction mixture is allowed to stir at room temperature overnight. The reaction is then made basic with the addition of saturated aqueous sodium bicarbonate solution, and the resulting mixture is extracted with ethyl acetate. The organics are separated, dried over anhydrous sodium sulfate and subsequently evaporated to dryness in vacuo. The crude product residue is then chromatographed by flash silica gel chromatography using 50% ethyl acetate in hexanes as the eluant. Following evaporation of solvent, 1-(4-nitrobenzoyl)morpholine is isolated as a white solid in the amount of 1.679 gms.
- 1-(4-Nitrobenzoyl)morpholine (1.1219 gms.) is then dissolved in tetrahydrofuran (6 mL) and slowly added dropwise to a 0° C., 1.0M solution (30 mL) of Borane in tetrahydrofuran. The reaction mixture is maintained at 0° C. for 20 minutes following the completion of the addition to the Borane/tetrahydrofuran solution. The reaction mixture is then allowed to warm to room temperature, and is subsequently heated to reflux using an oil bath. The reaction mixture is maintained at refluxing temperature overnight, then cooled to room temperature and quenched with the addition of concentrated HCl (added until gas evolution stops). The quenched reaction mixture is then extracted with ethyl acetate and water. The organic layer is then separated and concentrated in vacuo to yield 0.6650 gms. of 1-(4-nitro-benzyl)-morpholine as a yellow solid.
- 1-(4-Nitro-benzyl)-morpholine (0.6446 gms.) is suspended in 15 mL of a 1:1 (v:v) solution of acetic acid and water. The suspension is then heated with a heat gun until a homogeneous solution forms. The solution is then treated with 35 mL of an ˜10 wt. % solution of TiCl 3 in 20-30 wt. % HCl, dropwise. Over the course of the addition a color change from colorless to purple to black ensues. The reaction mixture is immersed in a 60° C. oil bath overnight following the addition of TiCl3. The reaction mixture is then cooled to room temperature and made basic with the addition of a 10% aqueous solution of NaOH. The basic reaction mixture is then extracted with chloroform four times and the combined organic layers are dried over anhydrous sodium sulfate. Following filtration and evaporation of the solvent in vacuo, the organic layer yields 0.5112 gms. 4-morpholin-4-ylmethyl-phenylamine.
- 4-Methyl-3-[(4-morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.056 gms E & Z-3-hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, prepared by substituting 4′-methyl-1,3 dihydro-indol-2-one for 1,3 dihydro-indol-2-one in the reaction of Example 1, with 0.085 gms. 4-morpholin-4-ylmethyl-phenylamine, prepared as in the reaction of Example 290, in tetrahydrofuran (2.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol, and filtration the reaction yields the named compound as a solid in the amount of 12 mg.
- Examples 291, and 293-295 are prepared by substituting the appropriate 5′-chloro or 4′-fluoro or 5′-fluoro or 6′-fluoro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and 4-morpholin-4-ylmethyl-phenylamine, used in the preparation of Example 290, for aniline in the reaction of Example 1.
- 4-Methyl-3-{[4-(3-morpholin-4-yl-propylamino)-phenylamino]- methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by substituting 4-methyl-1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-(3-morpholin-4-yl-propyl)-benzene-1,4-diamine (used for the preparation of Example 290) for aniline in the reaction of Example 1.
- 3-{[4-(3-Morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydroindol-2-one
- The named compound is prepared by refluxing 0.336 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.730 gms. N-(3-morpholin-4-yl-propyl)-benzene-1,4-diamine in tetrahydrofuran (12.5 mL) for 24 hours to yield the named compound as a solid in the amount of 0.513 gms. following concentration in vacuo, recrystallization with hot isopropanol and filtration.
- N-(3-Morpholin-4-yl-propyl)-benzene-1,4-diamine is prepared from p-fluoronitrobenzene by the following method:
- A mixture of 4.85 mL of p-fluoronitrobenzene, 6.0 gms. 2-morpholin-4-yl-ethylamine and 8.69 mL N,N-diisopropylamine in 20.8 mL dioxane is heated at 105° C. for 2 days. The reaction mixture is cooled to room temperature, evaporated to dryness, and recrystallized from hot isopropanol yielding the (3-morpholin-4-yl-propyl)-(4-nitro-phenyl)-amine product in the amount of 5.30 gms.
- A suspension of 5.00 gms. of (3-morpholin-4-yl-propyl)-(4-nitro-phenyl)-amine in 110 mL of ethanol is heated to 50° C. Once dissolution is achieved 5.49 mL hydrazine monohydrate is added to the solution. A Raney nickel slurry in water is added to the 50° C. solution dropwise, waiting after each addition for the bubbling to cease. Sufficient quantities of Raney nickel have been added when continued addition of Raney nickel causes no further gas evolution. The reaction is then maintained at 50° C. for an additional hour, and subsequently is cooled to room temperature. The reaction mixture is filtered through a pad of celite (rinsing the pad with methanol). N-(3-Morpholin-4-yl-propyl)-benzene-1,4-diamine (4.39 gms.) is isolated upon evaporation of the filtrate, and is subsequently used without purification in the reaction of Example 297.
- Example 298-301 are prepared by substituting the appropriate 6′-fluoro or 5′-chloro or 5′-fluoro or 4′-fluoro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-(3-morpholin-4-yl-propyl)-benzene-1,4-diamine (used in the preparation of Example 297) for aniline in the reaction of Example 1.
- 3-({4-[3-(4-Methyl-piperazin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.135 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.035 gms. N-[3-(4-Methyl-piperazin-1-yl)-propyl]-benzene-1,4-diamine in tetrahydrofuran (1 mL) for 24 hours. Following concentration in vacuo and recrystallization from hot isopropanol and filtration the named compound is isolated as a yellow solid in the amount of 0.0151 gms.
- N-[3-(4-Methyl-piperazin-1-yl)-propyl]-benzene-1,4-diamine was prepared from p-fluoronitrobenzene by the following method:
- A mixture of 4.44 mL of p-fluoronitrobenzene, 6 gms. 3-(4-methyl- piperazin-1-yl)-propylamine and 6.65 mL N,N-diisopropylamine in 19 mL dioxane is heated at 105° C. for 2 days. The reaction mixture is cooled to room temperature, evaporated to dryness, and recrystallized from hot isopropanol yielding [3-(4-methyl-piperazin-1-yl)-propyl]-(4-nitro-phenyl)-amine in the amount of 0.1385 gins.
- A suspension of 0.1385 gms. of [3-(4-methyl-piperazin-1-yl)-propyl]-(4-nitro-phenyl)-amine in 3 mL of ethanol is heated to 50° C. Once dissolution is achieved 0.144 mL hydrazine monohydrate is added to the solution. A Raney nickel slurry in water is added to the 50° C. solution dropwise, waiting after each addition for the gas evolution to cease. Sufficient quantities of Raney nickel have been added when continued addition of Raney nickel causes no further gas evolution. The reaction is then maintained at 50° C. for an additional hour, and subsequently is cooled to room temperature. The reaction mixture is filtered through a pad of celite (rinsing the pad with methanol). N-[3-(4-Methyl-piperazin-1-yl)-propyl]-benzene-1,4-diamine (0.1 16 gms.) is isolated upon evaporation of the filtrate, and is subsequently used without purification in the reaction of Example 302.
- 4-Methyl-3-({4-[3-(4-methyl-piperazin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- The named compound is prepared by substituting 4-methyl-1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-[3-(4-methyl-piperazin-1-yl)-propyl]-benzene-1,4-diamine (used for the preparation of Example 302) for aniline in the reaction of Example 1.
- 6-Fluoro-3-({4-[3-(4-methyl-piperazin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- The named compound is prepared by substituting 6-fluoro-1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and N-[3-(4-methyl-piperazin-1-yl)-propyl]-benzene-1,4-diamine (used for the preparation of Example 302) for aniline in the reaction of Example 1.
- 3-({4-[3-(3-Fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- In a manner similar to that described in Example 217, 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene)-1,3-dihydro-indol-2-one and 3-fluoro-pyrrolidine hydrochloride (226 mg, 1.2 equiv.) (prepared by the method of Giardina, G et al, Synlett (1995), (1), 55-7) are converted to the named compound as a slightly brownish yellow solid (258 mg, 45%).
- 3-{[4-(2-Morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.0644 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.1088 gms. 4-(2-morpholin-4-yl-ethyl)-phenylamine in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound in the amount of 91.1 mg.
- 4-(2-Morpholin-4-yl-ethyl)-phenylamine is prepared from (4-nitro-phenyl)-acetic acid by the following method:
- A room temperature solution of 1.8839 gms. (4-Nitro-phenyl)-acetic acid in tetrahydrofuran (5 mL) is treated with 1.8069 gms. 1′,1′-carbonyl-diimidizole. The reaction mixture is stirred for 1 h. The reaction mixture is then treated with 1.0 mL morpholine. The reaction is heated overnight at 35° C. The reaction is then allowed to cool to room temperature, and is made basic with the addition of saturated aqueous sodium bicarbonate. The resulting mixture is extracted with ethyl acetate. The aqueous layer is re-extracted four more times with ethyl acetate. The combined organics are evaporated to dryness in vacuo. The crude product residue is then chromatographed by flash silica gel chromatography using 60% ethyl acetate in hexanes as the eluant. Following evaporation of solvent, 1-morpholin-4-yl-2-(4-nitro-phenyl)-ethanone is isolated as a white solid in the amount of 1.4009 gms.
- 1-Morpholin-4-yl-2-(4-nitro-phenyl)-ethanone (1.4009 gms.) is then dissolved in tetrahydrofuran (7.5 mL) and slowly added dropwise to a 0° C., 1.0M solution (40 mL) of Borane in tetrahydrofuran. The reaction mixture is maintained at 0° C. for 30 minutes following the completion of the addition to the Borane/tetrahydrofuran solution. The reaction mixture is then allowed to warm to room temperature, and is subsequently refluxed using an oil bath. The reaction mixture is maintained at refluxing temperature overnight, then cooled to 0° C., and quenched with the addition of concentrated HCl (added until fizzing stops). The quenched reaction mixture is then warmed to room temperature, and extracted with ethyl acetate and water. The organic layer is then separated, dried over sodium sulfate and concentrated in vacuo. The solid isolated is then chromatographed by flash silica gel chromatography using 30% ethyl acetate in hexanes as the eluant to yield 0.7993 gms. of 4-[2-(4-nitro-phenyl)-ethyl]-morpholine as a white solid.
- 4-[2-(4-Nitro-phenyl)-ethyl]-morpholine (0.7993 gms.) is suspended in 30 mL of a 1:1 (v:v) solution of acetic acid and water. The suspension is then immersed in a 60° C. oil bath and treated in dropwise fashion with 40 mL of an ˜10 wt. % solution of TiCl 3 in 20-30 wt. % HCl. The reaction mixture is maintained in the 60° C. oil bath overnight following the addition of TiCl3. The reaction mixture is then cooled to 0° C., and is made basic with the addition of a 10% aqueous solution of NaOH. The basic reaction mixture is then extracted with chloroform. The emmulsion that forms is filtered through glass wool, re-extracted with chloroform and then dried over anhydrous sodium sulfate. Following evaporation of the solvent the residue is chromatographed by flash silica gel chromatography (10% methanol in chloroform) which then yielding 0.6528 gms. of 4-(2-morpholin-4-yl-ethyl)-phenylamine.
- 4-Methyl-3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.0707 gms. E & Z-3-Hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, as prepared in the reaction of Example 1, with 0.1088 gms. 4-(2-morpholin-4-yl-ethyl)-phenylamine (used in the preparation of Example 306) in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 77 mg.
- Examples 307 and 309-311 are prepared by substituting the appropriate 5′-chloro or 4′-fluoro or 5′-fluoro or 6′-fluoro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and 4-(2-morpholin-4-yl-ethyl)-phenylamine (used in the preparation of Example 306) for aniline in the reaction of Example 1.
- 3-{[4-(4-Morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.0687 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.131 gms. 4-(4-morpholin-4-yl-butyl)-phenylamine in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound in the amount of 0.1165 gms.
- 4-(4-Morpholin-4-yl-butyl)-phenylamine is prepared from 4-(4-nitro-phenyl)-butyric acid by the following method:
- A room temperature solution of 1.1180 gms. 4-(4-nitro-phenyl)-butyric acid in tetrahydrofuran (3 mL) is treated with 0.9052 gms. 1′,1′-carbonyl-diimidizole using an ice bath to attenuate the intensity of the reaction. The reaction mixture is stirred for 1 h. The reaction mixture is then treated with 0.5 mL morpholine. The reaction is heated overnight at 35° C. The reaction is then allowed to cool to room temperature, and is made basic with the addition of saturated aqueous sodium bicarbonate. The resulting mixture is extracted with ethyl acetate. The organics are dried over anhydrous sodium sulfate, filtered and evaporated to dryness in vacuo. The crude product residue is then chromatographed by flash silica gel chromatography using 60% ethyl acetate in hexanes as the eluant. Following evaporation of solvent, 1-morpholin-4-yl-4-(4-nitro-phenyl)-butan-1-one is isolated as a white solid in the amount of 1.3925 gms.
- 1-Morpholin-4-yl-4-(4-nitro-phenyl)-butan-1-one (1.3925 gms.) is then dissolved in tetrahydrofuran (7.0 mL) and added dropwise to a 0° C., 1.0M solution (40 mL) of Borane in tetrahydrofuran. The reaction mixture is maintained at 0° C. for 1 hour following the completion of the addition to the Borane/tetrahydrofuran solution. The reaction mixture is then allowed to warm to room temperature, and is subsequently refluxed using an oil bath. The reaction mixture is maintained at refluxing temperature overnight, then is cooled to 0° C. and quenched with the addition of concentrated HCl (added until fizzing stops). The quenched reaction mixture is then warmed to room temperature, and extracted with ethyl acetate and water. The aqueous layer is then extracted twice more with ethyl acetate. The combined organic layers are concentrated in vacuo. The solid isolated is then chromatographed by flash silica gel chromatography using 50% ethyl acetate in hexanes as the eluant to yield 0.9559 gms. of 4-[4-(4-nitro-phenyl)-butyl]-morpholine as a white solid.
- 4-[4-(4-Nitro-phenyl)-butyl]-morpholine (0.9337 gms.) is suspended in 30 mL of a 1:1 (v:v) solution of acetic acid and water. The suspension is then immersed in a 60° C. oil bath and treated in dropwise fashion with 40 mL of an ˜10 wt. % solution of TiCl 3 in 20-30 wt. % HCl. The reaction mixture is maintained in the 60° C. oil bath overnight following the addition of TiCl3. The reaction mixture is then cooled to 0° C., and is made basic with the addition of a 10% aqueous solution of NaOH. The basic reaction mixture is then extracted with chloroform. The emulsion that forms is filtered through glass wool, and then the layers are separated. The organic layer is dried over anhydrous sodium sulfate. Following filtration and evaporation of the solvent the residue is chromatographed by flash silica gel chromatography (10% methanol in chloroform) which then yielding 0.905 gms. of 4-(4-morpholin-4-yl-butyl)-phenylamine.
- 4-Methyl-3-{[4-(4-morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.131 gms. E & Z-3-hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, as prepared in the reaction of Example 1, with 0.0759 gms. 4-(4-morpholin-4-yl-butyl)-phenylamine (used in the preparation of Example 312) in tetrahydrofuran (1.5 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 98.0 mg.
- Examples 313 and 315-317 are prepared by substituting the appropriate 5′-chloro or 4′-fluoro or 5′-fluoro or 6′-fluoro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and 4-(4-morpholin-4-yl-butyl)-phenylamine (used in the preparation of Example 312) for aniline in the reaction of Example 1.
- 3-{[4-(4-Piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.0355 gms. E & Z 3-[(hydroxy)-methylene]-1,3-dihydro-indol-2-one, as prepared in Example 1, with 0.070 gms. 4-(4-piperidin-1-yl-butyl)-phenylamine in tetrahydrofuran (1.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound in the amount of 52.6 mg.
- 4-(4-Piperidin-1-yl-butyl)-phenylamine is prepared from 4-(4-nitro-phenyl)-butyric acid by the following method:
- A room temperature solution of 1.1220 gms. 4-(4-nitro-phenyl)-butyric acid in tetrahydrofuran (3 mL) is treated with 0.8978 gms. 1′,1′-carbonyl-diimidizole using an ice bath to attenuate the intensity of the reaction. The reaction mixture is stirred for 30 minutes in the ice bath and 30 minutes at room temperature. The reaction mixture is then treated with 0.5 mL morpholine. The reaction was heated overnight at 35° C. The reaction is then allowed to cool to room temperature, and is made basic with the addition of saturated aqueous sodium bicarbonate. The resulting mixture is extracted with ethyl acetate. The organics are dried over anhydrous sodium sulfate, filtered and evaporated to dryness in vacuo. The crude product residue is then purified by filtering it through a plug of silica gel using 50% ethyl acetate in hexanes as the eluant. Following evaporation of solvent, 4-(4-nitro-phenyl)-1-piperidin-1-yl-butan-1-one is isolated as a yellow oil in the amount of 1.47 gms.
- 4-(4-Nitro-phenyl)-1-piperidin-1-yl-butan-1-one (1.465 gms.) is then dissolved in tetrahydrofuran (7.0 mL) and added dropwise to a 0° C., 1.5M solution (25 mL) of Borane in tetrahydrofuran. The reaction mixture is maintained at 0° C. for 30 minutes following the completion of the addition to the Borane/tetrahydrofuran solution. The reaction mixture is then allowed to warm to room temperature for an hour, and is subsequently refluxed using an oil bath. The reaction mixture is maintained at refluxing temperature overnight, then cooled to 0° C. and quenched with the addition of concentrated HCl (added until gas evolution stops). The quenched reaction mixture is then warmed to room temperature, and extracted with ethyl acetate and water. The emulsion that forms is filtered through glass wool and the layers are separated. The aqueous layer is then extracted three additional times with ethyl acetate. The combined organic layers are concentrated in vacuo. The solid isolated is then chromatographed by flash silica gel chromatography using 30% ethyl acetate in hexanes as the eluant yielding 0.719 gms. of 1-[4-(4-nitro-phenyl)-butyl]-piperidine.
- 1-[4-(4-Nitro-phenyl)-butyl]-piperidine (0.719 gms.) is suspended in 30 mL of a 1:1 (v:v) solution of acetic acid and water. The suspension is then immersed in a 60° C. oil bath and treated in dropwise fashion with 40 mL of an ˜10 wt. % solution of TiCl 3 in 20-30 wt. % HCl. The reaction mixture is maintained in the 60° C. oil bath overnight following the addition of TiCl3. The reaction mixture is then cooled to 0° C., and is made basic with the addition of a 10% aqueous solution of NaOH. The basic reaction mixture is then extracted with chloroform. The emulsion that forms is filtered through glass wool, and then the layers are separated. The aqueous layer is re-extracted with ethyl acetate three additional times. The combined organic layers are concentrated in vacuo and the residue is chromatographed by flash silica gel chromatography (10% methanol in chloroform) yielding 0.428 gms. of 4-(4-piperidin-1-yl-butyl)-phenylamine.
- 4-Methyl-3-{[4-(4-piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- The named compound is prepared by refluxing 0.0410 gms. E & Z-3-Hydroxymethylene-4-methyl-1,3-dihydro-indol-2-one, as prepared in the reaction of Example 1, with 0.070 gms. 4-(4-piperidin-1-yl-butyl)-phenylamine (used in the preparation of Example 318) in tetrahydrofuran (1.0 mL) overnight. Following cooling to room temperature, solvent evaporation in vacuo, trituration with isopropanol and filtration the reaction yields the named compound as a solid in the amount of 38.3 mg.
- Examples 319 and 321-323 are prepared by substituting the appropriate 5′-chloro or 4′-fluoro or 5′-fluoro or 6′-fluoro substituted 1,3-dihydro-indol-2-one for the 1,3-dihydro-indol-2-one and 4-(4-piperidin-1-yl-butyl)-phenylamine, used in the preparation of Example 318, for aniline in the reaction of Example 1.
- Thus, in accordance with the above examples, the following compounds are synthesized:
- 3-Phenylaminomethylene-1,3-dihydro-indol-2-one
- 3-[(3-Bromo-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Bromo-phenylamino)-methyl]-1,3-dihydro-indol-2-one
- 3-[(3-Bromo-phenylamino)-methyl]-1,3-dihydro-indol-2-one
- 3-[(4-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-[(2-Oxo-1,3-dihydro-indol-3-ylididenemethyl)-amino]-benzoic acid ethyl ester
- 3-[(2-Ethyl-phenylamino)-methylene}-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-2-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Chloro-4-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Fluoro-2-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3,5-Dimethoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-tert-Butyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-(p-Tolylamino-methylene)-1,3-dihydro-indol-2-one
- 3-[(3,5-Dimethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3,4-Dimethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-(Indan-5-ylaminomethylene)-1,3-dihydro-indol-2-one
- 3-[(4-Chloro-2-fluoro-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- Carbonic acid ethyl ester 4-fluoro-2-methyl-5-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl ester
- Carbonic acid 2,2-dimethyl-propyl ester 4-fluoro-2-methyl-5-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl ester
- 3-[(4-Chloro-2-fluoro-5-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Cyanophenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Cyanomethylphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(5-Indolylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(5-Indazolylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Benzamidylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Acetanilidylamino)-methylene]-1,3-dihydro-indol-2-one
- (3-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl-)acetic acid
- 3-[(4-Chlorophenylamino)-methylene]-1,3-dihydro-indol-2-one
- 2-Chloro-5-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 2-Oxo-1,2-dihydro-indol-3-ylidenemethyl-sulfanilamide
- 2-Oxo-1,2-dihydro-indol-3-ylidenemethyl-sulfaacetamide
- 3-[(4-Morpholinoamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Phenoxyamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3,4-Dimethoxyamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(6-Benzthioazolylamino)-methylene]-1,3-dihydro-indol-2-one
- 2-Hydroxy-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 3-[(Chloro-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(Bis-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(Fluoro-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-4-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-4-methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(1,1-Difluoro-methoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Trifluoromethoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Isopropyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(2-Fluoro-4-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Chloro-3-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Methoxy-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(Methyl-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(3-methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Methyl-3-phenylaminomethylene-1,3-dihydro-indol-2-one
- 3-[(4-Methoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-phenylarmino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3,5-Dimethoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-(p-tolylamino-methylene)-1,3-dihydro-indol-2-one
- 3-[(3,5-Dimethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Difluoromethoxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-4-methoxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(4-Trifluoromethoxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol -2-one
- 3-[(3-Methyl-4-hydroxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3,4-Metylenedioxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3,4-Dimethylphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(4-i-Propylphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-4-methylphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(1-hydroxy-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Methoxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(Phenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3,5-Dimethoxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(4-Hydroxyphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3,5-Dimethylphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(4-Methylphenylamino)-methylene]-5-chloro-1,3-dihydro-indol-2-one
- 3-[(3,4-Methylenedioxyphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 2-Hydoxy-5-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 3-[(4-Ethoxyphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(1-Acetyl-2,3-dihydro-1-indol-6-ylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phospate
- 4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phthalic acid
- 4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 3-{[4-(2-Hydroxyethyl)phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Hydroxymethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 2-Methoxy-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid methyl ester
- 3-[(1H-Indazol-6-ylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-methoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-(Benzothiazol-6-ylaminomethylene)-4-methyl-1,3-dihydro-indol-2-one
- 2-Methoxy-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid methyl ester
- 3-[(3-Methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3,4-Dimethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Hydroxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(1H-Indol-6-ylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-tert-Butyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Hydroxymethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-t-butyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(phenylamino)-methylene]-1,3-dihydro-indol-2-one benzothiazol-6-yl-amine
- 5-Chloro-3-[(3,4-dimethoxyphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-6-indazolylamine
- 5-Chloro-3-[(4-chloro-2,5-dimethoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(3-flouro-4-methoxyphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-morpholinophenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-ethoxyphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethylamino)-2-hydroxybenzoic acid
- 5-Chloro-3-[(4-hydoxy-3-(diethylaminomethyl)phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethylamino)indole
- 5-Chloro-3-[(4-(hydoxymethyl)phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-i-propyl-3-methylphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(2-Hydroxy-ethyl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- N-{4-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-acetamide
- {4-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-acetic acid
- 4-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- 3-[(3-Hydroxy-4-methoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 2-Hydroxy-5-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 3-{[4-(1,1-Difluoro-methoxy)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-trifluoromethoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Methoxy-3-trifluoromethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Ethoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Isopropyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-(Benzo[1,3]dioxol-5-ylaminomethylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxy-4-methyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3,4-Dimethoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(2-Ethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzonitrile
- 4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzamide
- N-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-acetamide
- {4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-acetic acid
- 3-[(4-Hydroxy-3-methyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Hydroxy-3-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- {2-Hydroxy-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-carbamic acid ethyl ester
- 3-[(2-Fluoro-4-methoxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Methylsulfanyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-methylsulfanyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-methylsulfanyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-butyric acid
- 4-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-butyric acid
- 3-{3-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-{3-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-{3-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-{4-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-[(3-Ethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(3-ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-{4-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-butyric acid
- 3-[(4-Ethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 4-[(5-Chloro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzonitrile
- 2-Hydroxy-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 3-[(4-Dimethylamino-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(4-Methyl-piperazin-1-yl)-phenylamino]-methylenel}-1,3-dihydro-indol-2-one
- 3-[(4-Piperidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(3-Diethylaminomethyl-4-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Diethylaminomethyl-4-hydroxy-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(1 h-indol-5-ylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(1 h-indazol-6-ylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(3-hydroxy-4-methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- 5-Fluoro-3-[(4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-phenylaminomethylene-1,3-dihydro-indol-2-one
- 4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzamide
- 5-Fluoro-3-[(4-methylsulfanyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(3-fluoro-4-methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-(Benzothiazol-6-ylaminomethylene)-5-fluoro-1,3-dihydro-indol-2-one
- {4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl }-acetic acid
- 3-{3-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-propionic acid
- 3-[(3-Ethyl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(3-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-hydroxymethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(4-Pyrrolidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Amino-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Amino-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-(1-Hydroxy-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-(1-Hydroxy-ethyl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxymethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Hydroxymethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- {4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-carbamic acid tert-butyl ester
- {4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-carbamic acid tert-butyl ester
- 3-[(4-Diethylamino-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- N,N-Bis-(2-hydroxy-ethyl)-3-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- N,N-Bis-(2-hydroxy-ethyl)-3-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- N-(2-Hydroxy-ethyl)-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- N-(3-Hydroxy-propyl)-3-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- N-(3-Hydroxy-propyl)-3-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- 2-Morpholin-4-yl-5-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic acid
- 4-Methyl-3-{[4-(1-morpholin-4-yl-methanethiol)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- N-(2-Hydroxy-ethyl)-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzenesulfonamide
- 3-{[4-(3-Chloro-propoxy)-phenylamino]-methylene }-1,3-dihydro-indol-2-one
- 3-{[4-(4-Chloro-butoxy)-phenylamino]-methylenel}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Iodo-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Iodo-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-phenylaminomethylene-2,3,4,5,6-d4-1,3-dihydro-indol-2-one
- 4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-benzoic-2,3,5,6-d4 acid
- 3-[(4-Amino-phenylamino)-methylene]-2,3,5,6-d4-1,3-dihydro-indol-2-one
- 3-[(4-Amino-phenylamino)-methylene]-2,3,5,6-d4-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(4-Morpholin-4-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Diethylamino-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[4-(4-Methyl-piperazin-1-yl)-butoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(4-Piperidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(3-Amino-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3-Amino-phenylamino)-methylene]-1,3-dihydro-indo1-2-one
- 3-[(3-Amino-4-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(3-Amino-4-methyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylenel}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Morpholin-4-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Pyrrolidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-( {4-[3-(4-Methyl-piperazin-1-yl)-propoxy]-phenylamino}methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3-Piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-Phenylaminomethylene-2,3,4,5,6-d4-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-piperidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Carboxymethylphenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(3-thiomorpholin-4-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Thiomorpholin-4-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(3-diethylamino-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Morpholin-4-yl-phenylamino)-methylene]-5-nitro-1,3-dihydro-indol-2-one
- 3-[(4-Morpholin-4-yl-phenylamino)-methylene]-2-oxo-2 ,3-dihydro-1 h-indole-5-carbonitrile
- 4-Methyl-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-methyl-3-{[4-(4-piperidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-piperidin-1-yl-butoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene)}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(4-piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-piperidin-1-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-piperidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethoxy)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethoxy)-phenylamino]-methylenel}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethoxy)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 6-(3-Methoxy-phenyl)-3-{[4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-(3-Methoxy-phenyl)-3-{[4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene }-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylenel}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-Dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(2-pyrrolidin-1-yl-ethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-(3-Methoxy-phenyl)-3-{[4-(3-piperidin-1-yl-propoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propoxy)-phenylamino]-methylene}-6-(3-methoxy-phenyl)-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene }-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene)-}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-( 1-methyl-piperidin-3-ylmethoxy)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(2-piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-piperidin-1-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Chloro-3-[(4-morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(4-morpholin-4-ylmethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-( {4-[3-(4-Methyl-piperazin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Methyl-3-({4-[3-(4-methyl-piperazin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[3-(4-methyl-piperazin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[3-(3-Fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-morpholin-4-yl-ethyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Chloro-3-{[4-(4-morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(4-morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-morpholin-4-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indo1-2-one
- 5-Chloro-3-{[4-(4-piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(4-piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-piperidin-1-yl-butyl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- Furthermore, the compounds listed below may be synthesized in accordance with the working examples, using the appropriate substitutions and/or other methods known in the art and similarly will have utility in the method of the present invention.
- 3-[(4-Pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-({4-[(2-Methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Methyl-piperid in-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Methyl-piperid in-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(3-Oxo-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl )-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-[(4-Thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(5-Oxo-[1,4]diazepan-1-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-Methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 1-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carboxylic acid amide
- 1-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid amide
- 3-{[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- N-(1-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-acetamide
- 3-{[4-(4-Isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Octahydro-isoquinolin-2-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-phenylamino]-methylene}-1,3-dihydro-indol
- 3-({4-[4-(2-Methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3-Pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Pyridin-4-yl-pyrrolidin-1-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-on
- 3-{[4-(3-Methanesulfonyl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[Methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2,3,5,6-Tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-aminol-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Methoxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Methoxymethyl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(Pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(5-Methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,2,2,6,6-Pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(Tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Hydroxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 1-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 3-({4-[(2-Methoxy-ethyl)-methyl-amino-phenylamino}-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl )-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 4-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl )-amino]-phenyl}-piperazine-1-ca
- 3-{[4-(4-Ethyl-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[(2-Methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 1-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carbaldehyde acid amide
- N-(1-{4-[(4-Methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)acetamide
- 3-{[4-(4-Isopropyl-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene-}1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[4-(2-Methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2one
- 3-{[4-(3-Methanesulfonyl-pyrrolidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-methyl-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 1-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carboxylic acid amide
- 1-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid amide
- N-(1-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-acetamide
- 4-Fluoro-3-{[4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(methyl-pyridin-3-yl methyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indo-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-methoxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 1-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 5-Fluoro-3-({4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-oxo-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-phenylamino}-methylene)-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 4-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-methyl-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2 ,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- N-(1-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-acetamide
- 5-Fluoro-3-{[4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(octahydro-isoquinolin-2-y)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4. 5]dec-8-yl)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-phenylamino}-methylene)-5-fluoro-1,3-dihydro-indol-2-one
- N-(1-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-N-methyl-acetamide
- 5-Fluoro-3-{[4-(2-methoxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-phenylamino]-methylene-}5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(pyridin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-phenylamino}-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(4-thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 4-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl )-amino]-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl )-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 1-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid amide
- N-(1-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-acetamide
- 6-Fluoro-3-{[4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-methanesulfonyl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one 6-Fluoro-3-{[4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-phenylamino}-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-morpholin-4-yi-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[3-(2-methyl-piperidin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 6-Fluoro-3-({4-[(tetrahydro-pyran-4-ylmethyl )-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-3-fluoro-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 3-({3-Fluoro-4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-fluoro-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-oxo-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-fluoro-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-3-fluoro-phenylamino-methylene}-1,3-dihydro-indol-2-one
- 4-{2-Fluoro-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 3-{[3-Fluoro-4-(5-oxo-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carboxylic acid amide
- 3-{[4-(4-Acetyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- N-(1-{2-Fluoro-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-acetamide
- 3-{[3-Fluoro-4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yi)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([ ,3]Dioxolan-2-ylmethyl-methyl-amino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-3-fluoro-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- N-(1-{2-Fluoro-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-N-methyl-acetamide
- 3-{[3-Fluoro-4-(2-methoxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(1-methoxymethyl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(pyridin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-([4-(3-Diethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-imidazol-1-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(5-methyl-pyrazin-2-yl methyl)-amino]-phenylamino-methylene}-1,3-dihydro-indol-2-one 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-fluoro-phenylamino}-methylene)-1,3- dihydro-indol-2-one
- 3-{2-Fluoro-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 3-{[3-Fluoro-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-pyrrolidin-1-yl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-3-fluoro-phenylamino)-m ethylene]-4-m ethyl-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 3-({3-Fluoro-4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-[(3-Fluoro-4-morpholin-4-yl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-fluoro-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-oxo-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-fluoro-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-3-fluoro-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-{2-Fluoro-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 3-{[3-Fluoro-4-(5-oxo-[1,4]diazepan-1-yi)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-methyl-[1,4]diazepan-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(4-Acetyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(octahydro-isoquinolin-2-y3)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-3-fluoro-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- N-(1-{2-Fluoro-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-N-methyl-acetamide
- 3-{[3-Fluoro-4-(2-methoxy-ethylamino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(1-methoxymethyl-propylamino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-pyridin-2-y1-ethylamino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-([3-Fluoro-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(pyridin-2-ylmethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(pyridin-4-ylmethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-([3-Fluoro-4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({3-Fluoro-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-fluoro-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-{2-Fluoro-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 3-({3-Fluoro-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 3-{[3-Fluoro-4-(2-hydroxy-ethylamino)-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(3-fluoro-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-3-fluoro-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(4-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 4-Fluoro-3-({3-fluoro-4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(3-fluoro-4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-fluoro-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(3-oxo-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-fluoro-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl )-amino]-3-fluoro-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(3-fluoro-4-thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-{2-Fluoro-4-[(4-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 4-Fluoro-3-{[3-fluoro-4-(5-oxo-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(4-methyl-[1,4]diazepan-1-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2 ,6-Dimethyl-morpholin-4-y)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(4-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carboxylic acid amide
- 1-{2-Fluoro-4-[(4-fluoro-2-oxo-,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid amide
- 3-{[4-(4-Acetyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2one
- 4-Fluoro-3-{[3-fluoro-4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-3-fluoro-phenylamino]-methylene)-4-fuoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[4-(2-methoxy-ethyl )-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one 4-Fluoro-3-{[3-fluoro-4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-fluoro-phenylamino]-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-3-fluoro-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-methoxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-([3-fluoro-4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(l-methoxymethyl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[(pyridin-4-ylmethyl)-amino]-phenylamino)-methylene)-,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[3-(2-methyl-piperidin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[4-(2-Dimethylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(3-imidazol-1-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-fluoro-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 3-{2-Fluoro-4-[(4-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 4-Fluoro-3-([3-fluoro-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-fluoro-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-fluoro-4-(2-hydroxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,5-Dihydro-pyrrol-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(3-fluoro-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-3-fluoro-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 5-Fluoro-3-({3-fluoro-4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(3-fluoro-4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 4-{2-Fluoro-4-[(5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(4-methyl-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carboxylic acid amide
- 1-{2-Fluoro-4-[(5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid amide
- 5-Fluoro-3-{[3-fluoro-4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Acetyl-[1,4]diazepan-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl )-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(3-pyridin-4-yl-pyrrolidin-1-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,31 Dioxolan-2-yl-ethyl)-methyl-amino]-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- N-(1-{2-Fluoro-4-[(5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-N-methyl-acetamide
- 5-Fluoro-3-{[3-fluoro-4-(2-methoxy-ethylamino)-phenylamino]-methylene-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(1-methoxymethyl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[3-(2-methyl-piperidin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-5-fluoro-,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-fluoro-4-(3-imidazol-1-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-fluoro-phenylamino}-methylene)-5-fluoro-1,3-dihydro-indol-2-one
- 3-{2-Fluoro-4-[(5-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 5-Fluoro-3-{[3-fluoro-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-fluoro-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one 5-Fluoro-3-{[3-fluoro-4-(2-hydroxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,5-Dihydro-pyrrol-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(3-fluoro-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Diethylamino-3-fluoro-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(6-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid methyl ester
- 6-Fluoro-3-({3-fluoro-4-[(2-methoxy-ethyl)-methyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(3-fluoro-4-morpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(3-oxo-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2- 2
- 6-Fluoro-3-{[3-fluoro-4-(4-methyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-fluoro-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-3-fluoro-phenylamino}-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(3-fluoro-4-thiomorpholin-4-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 4-{2-Fluoro-4-[(6-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperazine-1-carbaldehyde
- 6-Fluoro-3-{[3-fluoro-4-(5-oxo-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-propyl-amino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-fluoro-4-[(2-methoxy-ethyl)-propyl-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 1-{2-Fluoro-4-[(6-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-3-carboxylic acid amide
- 1-{2-Fluoro-4-[(6-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-piperidine-4-carboxylic acid amide
- 6-Fluoro-3-{[3-fluoro-4-(4-isopropyl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-([4-(4-sec-Butyl-piperazin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-fluoro-4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(methyl-pyridin-3-ylmethyl-amino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(4-pyrimidin-2-yl-piperazin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2 ,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-aminol-3-fluoro-phenylamino]-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- N-(1-{2-Fluoro-4-[(6-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-N-methyl-acetamide
- 6-Fluoro-3-{[3-fluoro-4-(2-methoxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-fluoro-4-[(tetrahydro-furan-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-([3-fluoro-4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-([4-(3-Dimethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(l-methoxymethyl-propylamino)-phenylamino]-methylene}-,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-fluoro-4-[(pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-methoxy-1-methyl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-fluoro-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-fluoro-phenylamino}-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- 3-{2-Fluoro-4-[(6-fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 6-Fluoro-3-{[3-fluoro-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-fluoro-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-fluoro-4-(2-hydroxy-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-fluoro-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-[(3-Methyl-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-({4-[(2-Methoxy-ethyl )-methyl-amino]-3-methyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[3-Methyl-4-(5-oxo-[1,4]diazepan-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 1-{2-Methyl-4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl )-amino]-phenyl}-piperidine-4-carboxylic acid amide
- 3-{[4-(4-Isopropyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Methyl-4-(octahydro-isoquinolin-2-y)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Methoxymethyl-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Methyl-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[3-Methyl-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Methyl-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[3-Methyl-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({3-Methyl-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-methyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-({3-methyl-4-[methyl-(2-pyridin-2-yl-ethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-3-methyl-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- N-Methyl-N-(1-{2-methyl-4-[(4-methyl-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-phenyl}-pyrrolidin-3-yl)-acetamide
- 3-{[4-(3-Dimethylamino-propylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(1-Methoxymethyl-propylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[3-methyl-4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Methoxy-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(3-Imidazol-1-yl-propylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-methyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-({3-methyl-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(3-methyl-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 1-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-4-carboxylic acid methyl ester
- 4-Fluoro-3-{[3-methyl-4-(3-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-methyl-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-methyl-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-3-methyl-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-methyl-phenylamino]-methylene-}4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(2-methoxy-ethyl)-propyl-amino]-3-methyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 1-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-3-carboxylic acid amide
- 1-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-4-carboxylic acid amide
- 4-Fluoro-3-{[4-(4-isopropyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-y)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-3-methyl-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-methoxy-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(tetrahydro-pyran-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-methyl-4-[(pyridin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-methyl-4-[(pyridin-3-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[3-methyl-4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-methyl-4-[3-(2-methyl-piperidin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[2-(1H-imidazol-4-yl)-ethylamino]-3-methyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-methyl-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-methyl-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 3-{4-[(4-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 4-Fluoro-3-{[3-methyl-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({3-methyl-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-hydroxy-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(3-methyl-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 1-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-4-carboxylic acid methyl ester
- 5-Fluoro-3-{[3-methyl-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-methyl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 4-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 1-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-3-carboxylic acid amide
- 1-{4-[(5-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-4-carboxylic acid amide
- 5-Fluoro-3-{[3-methyl-4-(octahydro-isoquinolin-2-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(3-pyridin-2-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(3-pyridin-4-yl-pyrrolidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(2-pyridin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(2-pyridin-3-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-methyl-4-[(pyridin-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-methyl-4-[3-(2-methyl-piperidin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-imidazol-1-yl-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[2-(1H-imidazol-4-yl)-ethylamino]-3-methyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-methyl-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-methyl-phenylamino}-methylene)-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[3-methyl-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({3-methyl-4-[(tetrahydro-pyran-4-ylmethyl )-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(3-methyl-4-pyrrolidin-1-yl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 1-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-4-carboxylic acid methyl ester
- 6-Fluoro-3-{[3-methyl-4-(4-methyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 4-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 1-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-3-carboxylic acid amide
- 1-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-methyl-phenyl}-piperidine-4-carboxylic acid amide
- 3-{[4-(4-Butyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-pyrrolidin-1-yl)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-3-methyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(4-propyl-piperidin-1-yl)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(2,3,5,6-tetrahydro-[1,2′]bipyrazinyl-4-yl )-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-[1,3]Dioxolan-2-yl-ethyl)-methyl-amino]-3-methyl-phenylamino}-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(2-pyridin-2-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(2-morpholin-4-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(3-morpholin-4-yl-propylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-methyl-4-[(pyridin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-methyl-4-[(pyridin-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(2-piperidin-1-yl-ethylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-methyl-4-[3-(2-methyl-piperidin-1-yl)-propylamino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-methyl-4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-methyl-phenylamino}-methylene)-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[3-methyl-4-(1,2,2,6,6-pentamethyl-piperidin-4-ylamino)-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({3-methyl-4-[(tetrahydro-pyran-4-ylmethyl)-amino]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-methyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Pyrrolidin-1-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 1-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-trifluoromethyl-phenyl}-piperidine-4-carboxylic acid methyl ester
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Morpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(3-Methyl-piperidin-1-yl )-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Methyl-piperidin-1-yl )-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(4-Methyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-trifluoromethyl-phenyl}-piperazine-1-carbaldehyde
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(2-Methoxy-ethyl)-propyl-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 1-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-trifluoromethyl-phenyl}-piperidine-4-carboxylic acid amide
- 3-{[4-(4-Acetyl-piperazin-1-yl )-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(Octahydro-isoquinolin-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-sec-Butyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- N-Methyl-N-(1-{4-[(2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-trifluoromethyl-phenyl}-pyrrolidin-3-yl)-acetamide
- 3-{[4-(2-Methoxy-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(Tetrahydro-furan-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(Tetrahydro-pyran-4-ylamino)-3-trifluoromethyl-phenylamino]-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Methoxymethyl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Pyridin-2-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Pyridin-4-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Pyridin-3-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Morpholin-4-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Morpholin-4-yl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(Pyridin-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(Pyridin-3-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Piperidin-1-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[3-(2-Methyl-piperidin-1-yl)-propylamino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Methoxy-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-({4-[(5-Methyl-pyrazin-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{4-[(2-Oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-trifluoromethyl-phenylamino}-pyrrolidine-1-carboxylic acid tert-butyl ester
- 3-({4-[(Tetrahydro-pyran-4-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Hydroxy-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-pyrrolidin-1-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-({4-[(2-Methoxy-ethyl)-methyl-amino]-3-trifluoromethyl-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-morpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-trifluoromethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-[(4-thiomorpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene]-3,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-{[4-(4-Acetyl-piperazin-1-yl )-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(octahydro-isoquinolin-2-yl )-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(octahydro-isoquinolin-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl )-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(3-pyridin-2-yl-pyrrolidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Methoxy-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(tetrahydro-pyran-4-ylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(1-Methoxymethyl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-pyridin-2-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Methyl-3-{[4-(2-pyridin-4-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Methoxy-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-methyl-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-morpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-methyl-piperidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-methyl-piperidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-methyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-trifluoromethyl-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[(4-thiomorpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Acetyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-isopropyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(octahydro-isoquinolin-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-[4-(octahydro-isoquinolin-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[4-(2-methoxy-ethyl)-piperazin-1-yl]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(3-pyridin-2-yl-pyrrolidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(4-propyl-piperidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-([1,3]Dioxolan-2-ylmethyl-methyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-methoxy-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(tetrahydro-furan-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(tetrahydro-pyran-4-ylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-pyridin-4-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-piperidin-1-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[3-(2-methyl-piperidin-1-yl)-propylamino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 4-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[(2,2-Dimethyl-[1,3]dioxolan-4-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-4-fluoro-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[(tetrahydro-pyran-4-yl methyl )-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-4-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,6-Dihydro-2H-pyridin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-[(4-morpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol 2-one
- 3-[(4-Azepan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-methyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-[(4-Dipropylamino-3-trifluoromethyl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Butyl-ethyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1 3-dihydro-indol-2-one
- 3-({4-[Ethyl-(2-methoxy-ethyl )-amino]-3-trifluoromethyl-phenylamino}-methylene)-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(Cyclopropylmethyl-propyl-amino)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-[(4-Azocan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3,5-Dimethyl-piperidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(5-oxo-[1,4]diazepan-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(4-methyl-[1,4]diazepan-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(4-Ethyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Dimethylamino-pyrrolidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Acetyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Butyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,4-Dioxa-8-aza-spiro[4.5]dec-8-yl)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-methoxy-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(tetrahydro-pyran-4-ylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(3-morpholin-4-yl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 5-Fluoro-3-{[4-(2-piperidin-1-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-trifluoromethyl-phenylamino]-methylene)-5-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1-Benzyl-pyrrolidin-3-ylamino)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(5-methyl-pyrazin-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[(tetrahydro-pyran-4-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-5-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-[(4-morpholin-4-yl-3-trifluoromethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
- 3-[(4-Azepan-1-yl-3-trifluoromethyl-phenylamino)-methylene]-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(4-methyl-piperazin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(2,6-Dimethyl-morpholin-4-yl)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(1,3-Dihydro-isoindol-2-yl)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(4-Acetyl-piperazin-1-yl )-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- N-(1-{4-[(6-Fluoro-2-oxo-1,2-dihydro-indol-3-ylidenemethyl)-amino]-2-trifluoromethyl-phenyl}-pyrrolidin-3-yl)-acetamide
- 6-Fluoro-3-{[4-(3-methanesulfonyl-pyrrolidin-1-yl)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-methoxy-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(tetrahydro-pyran-4-ylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(1-methoxymethyl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-pyridin-2-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-pyridin-3-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(pyridin-2-ylmethyl)-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-piperidin-1-yl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[3-(2-methyl-piperidin-1-yl )-propylamino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(2-methoxy-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Dimethylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 3-{[4-(3-Diethylamino-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-{[4-(3-imidazol-1-yl-propylamino)-3-trifluoromethyl-phenylamino]-methylene}-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diisopropylamino-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[(tetrahydro-pyran-4-ylmethyl )-amino]-3-trifluoromethyl-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-{[4-(2-Diethylamino-1-methyl-ethylamino)-3-trifluoromethyl-phenylamino]-methylene}-6-fluoro-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[2-(3-fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[2-(3-fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[2-(3-Fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Chloro-3-({4-[2-(3-fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[2-(3-Fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2-one
- 4-Fluoro-3-({4-[2-(3-fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[2-(3-Fluoro-pyrrolidin-1-yl)-ethoxy]-phenylamino}-methylene)-1-piperidin-4-yl-1,3-dihydro-indol-2-one
- 6-Fluoro-3-({4-[3-(3-fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 5-Fluoro-3-({4-[3-(3-fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihyro-indol-2-one
- 5-Chloro-3-({4-[3-(3-fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[3-(3-Fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-4-methyl-1,3-dihydro-indol-2one
- 4-Fluoro-3-({4-[3-(3-fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1,3-dihydro-indol-2-one
- 3-({4-[3-(3-Fluoro-pyrrolidin-1-yl)-propoxy]-phenylamino}-methylene)-1-piperidin-4-yl-1,3-dihydroindol-2-one
- The present invention is not to be limited in scope by the exemplified embodiments which are intended as illustrations of single aspects of the invention only. Indeed, various modifications of the invention in addition to those described herein will become apparent to those skilled in the art from the foregoing description. For example novel compounds of formula II, below may be utilized in the method of treating diseases described above.
- wherein R 5 is selected from the group consisting of halogen, nitro, hydroxy, hydrocarbyl, substituted hydrocarbyl, amide, thioamide, amine, thioether and sulfonyl; R3 is selected from the group consisting of D, halogen, nitro, hydroxy, hydrocarbyl, substituted hydrocarbyl, amide, thioamide, amine, thioether and sulfonyl and phosphonic acid; R4 is selected from the group consisting of hydrogen, hydrocarbyl and substituted hydrocarbyl; b is 0 or an integer from 1 to 3; a is 0 or an integer of from I to 5; the wavy line represents a E or Z bond and pharmaceutically acceptable salts thereof. Said hydrocarbyl and/or substituted hydrocarbyl may be alkyl, alkenyl, alkynyl, aryl (including carbocylic aryl and heterocyclic aryl) and alkaryl.
- Such modifications are intended to fall within the scope of the appended claims:
- All references cited herein are hereby incorporated by reference in their entirety.
- The foregoing description details specific methods and compositions that can be employed to practice the present invention, and represents the best mode contemplated. However, it is apparent for one of ordinary skill in the art that further compounds with the desired pharmacological properties can be prepared in an analogous manner, and that the disclosed compounds can also be obtained from different starting compounds via different chemical reactions. Similarly, different pharmaceutical compositions may be prepared and used with substantially the same result. Thus, however detailed the foregoing may appear in text, it should not be construed as limiting the overall scope hereof, rather, the ambit of the present invention is to be governed only by the lawful construction of the appended claims.
Claims (11)
1. A compound selected from the group consisting of
3-Phenylaminomethylene-1,3-dihydro-indol-2-one,
3-[(3-Bromo-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-[(4-Bromo-phenylamino)-methyl]-1,3-dihydro-indol-2-one,
3-[(3-Bromo-phenylamino)-methyl]-1,3-dihydro-indol-2-one,
3-[(4-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-[(4-Methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
4-[(2-Oxo-1,3-dihydro-indol-3-ylididenemethyl)-amino]-benzoic acid ethyl ester,
3-[(2-Ethyl-phenylamino)-methylene}-1,3-dihydro-indol-2-one,
3-[(3-Fluoro-4-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-[(3-Fluoro-2-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-[(4-Hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-[(3-Chloro-4-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one,
3-[(4-Fluoro-2-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one and pharmaceutically acceptable salts thereof.
2. A pharmaceutical composition comprising a pharmaceutically acceptable carrier or excipient and a compound according to claim 1 .
3. A method for treating diseases related to unregulated tyrosine kinase signal transduction, the method comprising the step of administering to a subject in need thereof a therapeutically effective amount of a compound selected from the group consisting of
3-Phenylaminomethylene-1,3-dihydro-indol-2-one
3-[(3-Bromo-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(4-Bromo-phenylamino)-methyl]-1,3-dihydro-indol-2-one
3-[(3-Bromo-phenylamino)-methyl]-1,3-dihydro-indol-2-one
3-[(4-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(4-Methoxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
4-[(2-Oxo-1,3-dihydro-indol-3-ylididenemethyl)-amino]-benzoic acid ethyl ester
3-[(2-Ethyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(3-Fluoro-4-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(3-Fluoro-2-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(4-Hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(3-Chloro-4-hydroxy-phenylamino)-methylene]-1,3-dihydro-indol-2-one
3-[(4-Fluoro-2-methyl-phenylamino)-methylene]-1,3-dihydro-indol-2-one and pharmaceutically acceptable salts thereof.
4. The method of claim 3 wherein said disease is selected from the group consisting of cancer, blood vessel proliferative disorders, fibrotic disorders, mesangial cell proliferative disorders and metabolic diseases.
5. The method of claim 3 wherein said disease is a blood vessel proliferative disorder and the blood vessel proliferative disorder is selected from the group consisting of diabetic retinopathy, age-related macular degeneration, retinopathy of prematurity, arthritis and restenosis.
6. The method of claim 3 wherein said disease is a blood vessel proliferative disorder and the fibrotic disorder is selected from the group consisting of hepatic cirrhosis, atherosclerosis and surgical adhesions.
7. The method of claim 3 wherein said disease is a mesangial cell proliferative disorder and the mesangial cell proliferative disorder is selected from the group consisting of glomerulonephritis, diabetic nephropathy, malignant nephrosclerosis, thrombotic microangiopathy syndromes, transplant rejection and glomerulopathies.
8. The method of claim 3 wherein said disease is a metabolic disorder and the metabolic disorder is selected from the group consisting of psoriasis, diabetes mellitus, wound healing, inflammation and neurodegenerative diseases.
9. The method of claim 5 wherein said blood vessel proliferative disorder is diabetic retinopathy.
10. The method of claim 5 wherein said blood vessel proliferative disorder is age-related macular degeneration.
11. The method of claim 8 wherein said metabolic disorder is psoriasis.
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/259,703 US20030225152A1 (en) | 2001-09-27 | 2002-09-27 | 3-(Arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors |
| US10/886,213 US7098236B2 (en) | 2001-09-27 | 2004-07-06 | 3-(arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/396,036 US20060194863A1 (en) | 2001-09-27 | 2006-03-31 | 3-(Arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US32581501P | 2001-09-27 | 2001-09-27 | |
| US32581901P | 2001-09-27 | 2001-09-27 | |
| US10/259,703 US20030225152A1 (en) | 2001-09-27 | 2002-09-27 | 3-(Arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10824925 Continuation | |||
| US82492504A Continuation | 2001-09-27 | 2004-04-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030225152A1 true US20030225152A1 (en) | 2003-12-04 |
Family
ID=26985125
Family Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/256,879 Expired - Fee Related US6765012B2 (en) | 2001-09-27 | 2002-09-27 | 3-(Arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US10/259,703 Abandoned US20030225152A1 (en) | 2001-09-27 | 2002-09-27 | 3-(Arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors |
| US10/783,325 Expired - Lifetime US7015220B2 (en) | 2001-09-27 | 2004-02-20 | 3-(arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US10/886,213 Expired - Lifetime US7098236B2 (en) | 2001-09-27 | 2004-07-06 | 3-(arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/228,780 Abandoned US20060025413A1 (en) | 2001-09-27 | 2005-09-16 | 3-(arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/274,681 Expired - Lifetime US7414054B2 (en) | 2001-09-27 | 2005-11-15 | 3-(arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/396,036 Abandoned US20060194863A1 (en) | 2001-09-27 | 2006-03-31 | 3-(Arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/256,879 Expired - Fee Related US6765012B2 (en) | 2001-09-27 | 2002-09-27 | 3-(Arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
Family Applications After (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/783,325 Expired - Lifetime US7015220B2 (en) | 2001-09-27 | 2004-02-20 | 3-(arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US10/886,213 Expired - Lifetime US7098236B2 (en) | 2001-09-27 | 2004-07-06 | 3-(arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/228,780 Abandoned US20060025413A1 (en) | 2001-09-27 | 2005-09-16 | 3-(arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/274,681 Expired - Lifetime US7414054B2 (en) | 2001-09-27 | 2005-11-15 | 3-(arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
| US11/396,036 Abandoned US20060194863A1 (en) | 2001-09-27 | 2006-03-31 | 3-(Arylamino)methylene-1, 3-dihydro-2H-indol-2-ones as kinase inhibitors |
Country Status (6)
| Country | Link |
|---|---|
| US (7) | US6765012B2 (en) |
| EP (1) | EP1430048A1 (en) |
| JP (2) | JP2005508336A (en) |
| AU (1) | AU2002341881B2 (en) |
| CA (1) | CA2461812C (en) |
| WO (1) | WO2003027102A1 (en) |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050244477A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Tyrosine kinase microsphers |
| US20050244475A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Biodegradable intravitreal tyrosine kinase implants |
| US7799336B2 (en) | 2004-04-30 | 2010-09-21 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US20110091520A1 (en) * | 2004-04-30 | 2011-04-21 | Allergan, Inc. | Sustained Release Intraocular Implants and Methods for Treating Ocular Neuropathies |
| US7993634B2 (en) | 2004-04-30 | 2011-08-09 | Allergan, Inc. | Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and related methods |
| US8119154B2 (en) | 2004-04-30 | 2012-02-21 | Allergan, Inc. | Sustained release intraocular implants and related methods |
| US8147865B2 (en) | 2004-04-30 | 2012-04-03 | Allergan, Inc. | Steroid-containing sustained release intraocular implants and related methods |
| US8293210B2 (en) | 2004-04-30 | 2012-10-23 | Allergan, Inc. | Sustained release intraocular implants and methods for preventing retinal dysfunction |
| EP2583679A1 (en) | 2007-04-02 | 2013-04-24 | Allergan, Inc. | Methods and compositions for intraocular administration to treat ocular conditions |
| US8455656B2 (en) | 2004-04-30 | 2013-06-04 | Allergan, Inc. | Kinase inhibitors |
| US8506986B2 (en) | 2004-04-30 | 2013-08-13 | Allergan, Inc. | Sustained release intraocular implants and methods for treating ocular vasculopathies |
| US8673341B2 (en) | 2004-04-30 | 2014-03-18 | Allergan, Inc. | Intraocular pressure reduction with intracameral bimatoprost implants |
| US8846094B2 (en) | 2003-11-12 | 2014-09-30 | Allergan, Inc. | Peripherally administered viscous formulations |
| US8969415B2 (en) | 2006-12-01 | 2015-03-03 | Allergan, Inc. | Intraocular drug delivery systems |
| US9101583B2 (en) | 2004-04-30 | 2015-08-11 | Allergan, Inc. | Microparticles manufactured in an oil-in-water process comprising a prostamide |
| US9138480B2 (en) | 2009-11-09 | 2015-09-22 | Allergan, Inc. | Compositions and methods for stimulating hair growth |
| US9265775B2 (en) | 2003-11-12 | 2016-02-23 | Allergan, Inc. | Pharmaceutical compositions |
| US9572859B2 (en) | 2004-01-20 | 2017-02-21 | Allergan, Inc. | Compositions and methods for localized therapy of the eye |
| US9610246B2 (en) | 2013-02-15 | 2017-04-04 | Allergan, Inc. | Sustained drug delivery implant |
| US9775846B2 (en) | 2004-04-30 | 2017-10-03 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related implants |
Families Citing this family (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1998046588A2 (en) * | 1997-04-11 | 1998-10-22 | Neorx Corporation | Compounds and therapies for the prevention of vascular and non-vascular pathologies |
| US7164001B2 (en) * | 2000-01-20 | 2007-01-16 | Genentech, Inc. | Compositions and methods for the treatment of immune related diseases |
| KR100600550B1 (en) | 2000-10-20 | 2006-07-13 | 에자이 가부시키가이샤 | Nitrogenous aromatic ring compounds |
| AU2002341881B2 (en) * | 2001-09-27 | 2008-05-08 | Allergan, Inc. | 3-(arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors |
| DE10233366A1 (en) * | 2002-07-23 | 2004-02-12 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Indolinone derivatives substituted in the 6-position, their preparation and their use as medicaments |
| US7169936B2 (en) | 2002-07-23 | 2007-01-30 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Indolinone derivatives substituted in the 6-position, their preparation and their use as medicaments |
| PE20040701A1 (en) * | 2002-07-23 | 2004-11-30 | Boehringer Ingelheim Pharma | INDOLINONE DERIVATIVES SUBSTITUTED IN POSITION 6 AND THEIR PREPARATION AS MEDICINES |
| US7514468B2 (en) | 2002-07-23 | 2009-04-07 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Indolinone derivatives substituted in the 6 position, the preparation thereof and their use as pharmaceutical compositions |
| CN101337930B (en) | 2003-11-11 | 2010-09-08 | 卫材R&D管理有限公司 | Urea derivative preparation process |
| FR2866879A1 (en) * | 2004-02-27 | 2005-09-02 | Oreal | PARA-PHENYLENEDIAMINE SECONDARY N-ALKYLAMINEE, DYEING COMPOSITION OF KERATIN FIBERS CONTAINING SUCH A PARA-PHENYLENEDIAMINE, METHODS USING THE SAME AND USES THEREOF |
| US7338536B2 (en) | 2004-02-27 | 2008-03-04 | L'oreal S. A. | N-alkylamino secondary para-phenylenediamine, composition for dyeing keratin fibers comprising such a para-phenylenediamine, processes using this composition and uses thereof |
| KR100629713B1 (en) * | 2004-04-10 | 2006-09-29 | (주)아모레퍼시픽 | Pentaerythritol derivatives and a method for preparation thereof, and liquid crystal base containing the same |
| US20050244472A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Intraocular drug delivery systems containing excipients with reduced toxicity and related methods |
| US8591885B2 (en) * | 2004-04-30 | 2013-11-26 | Allergan, Inc. | Carbonic anhydrase inhibitor sustained release intraocular drug delivery systems |
| US20050244461A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Controlled release drug delivery systems and methods for treatment of an eye |
| US20050244471A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Estradiol derivative and estratopone containing sustained release intraocular implants and related methods |
| US20050244462A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Devices and methods for treating a mammalian eye |
| US20050244478A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Anti-excititoxic sustained release intraocular implants and related methods |
| US20050244466A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Photodynamic therapy in conjunction with intraocular implants |
| US20070059336A1 (en) * | 2004-04-30 | 2007-03-15 | Allergan, Inc. | Anti-angiogenic sustained release intraocular implants and related methods |
| ATE428421T1 (en) | 2004-09-17 | 2009-05-15 | Eisai R&D Man Co Ltd | MEDICAL COMPOSITION WITH IMPROVED STABILITY AND REDUCED GELING PROPERTIES |
| TW200640443A (en) * | 2005-02-23 | 2006-12-01 | Alcon Inc | Methods for treating ocular angiogenesis, retinal edema, retinal ischemia, and diabetic retinopathy using selective RTK inhibitors |
| US7309787B2 (en) * | 2005-07-13 | 2007-12-18 | Allergan, Inc. | Kinase inhibitors |
| CA2602389A1 (en) | 2005-07-13 | 2007-01-18 | Allergan, Inc. | Kinase inhibitors |
| US7749530B2 (en) * | 2005-07-13 | 2010-07-06 | Allergan, Inc. | Kinase inhibitors |
| EP1902024B1 (en) * | 2005-07-13 | 2013-04-17 | Allergan, Inc. | Kinase inhibitors |
| WO2007015578A1 (en) | 2005-08-02 | 2007-02-08 | Eisai R & D Management Co., Ltd. | Method for assay on the effect of vascularization inhibitor |
| US8338415B2 (en) | 2006-01-24 | 2012-12-25 | Allergan, Inc. | Substituted 3-(5-membered unsaturated heterocyclyl-1, 3-dihydro-indol-2-one's and derivatives thereof as kinase inhibitors |
| US7977351B2 (en) * | 2006-03-22 | 2011-07-12 | Allergan, Inc. | Heteroaryl dihydroindolones as kinase inhibitors |
| CN101443009A (en) | 2006-05-18 | 2009-05-27 | 卫材R&D管理有限公司 | Antitumor agent for thyroid cancer |
| US8034957B2 (en) * | 2006-08-11 | 2011-10-11 | Allergan, Inc. | Kinase inhibitors |
| KR101472600B1 (en) | 2006-08-28 | 2014-12-15 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | Antitumor agent for undifferentiated gastric cancer |
| US8143410B2 (en) | 2006-11-16 | 2012-03-27 | Allergan, Inc. | Kinase inhibitors |
| US8558002B2 (en) | 2006-11-16 | 2013-10-15 | Allergan, Inc. | Sulfoximines as kinase inhibitors |
| WO2008093855A1 (en) | 2007-01-29 | 2008-08-07 | Eisai R & D Management Co., Ltd. | Composition for treatment of undifferentiated-type of gastric cancer |
| US7911053B2 (en) * | 2007-04-19 | 2011-03-22 | Marvell World Trade Ltd. | Semiconductor packaging with internal wiring bus |
| AU2008265104B2 (en) * | 2007-06-21 | 2013-09-12 | Janssen Pharmaceutica Nv | Indolin-2-ones and aza-indolin-2-ones |
| JP5209254B2 (en) * | 2007-08-30 | 2013-06-12 | 日本曹達株式会社 | Process for producing substituted phenoxyazabicyclooctane derivatives |
| CA2704000C (en) | 2007-11-09 | 2016-12-13 | Eisai R&D Management Co., Ltd. | Combination of anti-angiogenic substance and anti-tumor platinum complex |
| BR112012004843A2 (en) | 2009-09-03 | 2019-09-24 | Allergan Inc | compounds as tyrosine kinase modulators |
| US9340555B2 (en) | 2009-09-03 | 2016-05-17 | Allergan, Inc. | Compounds as tyrosine kinase modulators |
| KR101677790B1 (en) | 2010-06-25 | 2016-11-18 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | Antitumor agent using compounds having kinase inhibitory effect in combination |
| RU2580609C2 (en) | 2011-04-18 | 2016-04-10 | Эйсай Ар Энд Ди Менеджмент Ко., Лтд. | Anticancer therapeutic agent |
| EP3444363B1 (en) | 2011-06-03 | 2020-11-25 | Eisai R&D Management Co., Ltd. | Biomarkers for prediciting and assessing responsiveness of thyroid and kidney cancer subjects to lenvatinib compounds |
| CN103304468A (en) * | 2012-03-13 | 2013-09-18 | 华东师范大学 | One-pot series synthetic method of oxoindole |
| US9642851B2 (en) | 2012-12-06 | 2017-05-09 | Kbp Biosciences Co., Ltd. | Indolinone derivative as tyrosine kinase inhibitor |
| KR20150098605A (en) | 2012-12-21 | 2015-08-28 | 에자이 알앤드디 매니지먼트 가부시키가이샤 | Amorphous form of quinoline derivative, and method for producing same |
| SG11201509278XA (en) | 2013-05-14 | 2015-12-30 | Eisai R&D Man Co Ltd | Biomarkers for predicting and assessing responsiveness of endometrial cancer subjects to lenvatinib compounds |
| US9642839B2 (en) | 2013-06-20 | 2017-05-09 | Institute Of Radiation Medicine, China Academy Of Military Medical Sciences Pla | Substance having tyrosine kinase inhibitory activity and preparation method and use thereof |
| SI3524595T1 (en) | 2014-08-28 | 2022-10-28 | Eisai R&D Management Co., Ltd. | High-purity quinoline derivative and method for manufacturing same |
| WO2016136745A1 (en) | 2015-02-25 | 2016-09-01 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | Method for suppressing bitterness of quinoline derivative |
| KR102662228B1 (en) | 2015-03-04 | 2024-05-02 | 머크 샤프 앤드 돔 코포레이션 | Combination of PD-1 antagonists and VEGFR/FGFR/RET tyrosine kinase inhibitors to treat cancer |
| CA2988707C (en) | 2015-06-16 | 2023-10-10 | Eisai R&D Management Co., Ltd. | Combination of cbp/catenin inhibitor and immune checkpoint inhibitor for treating cancer |
| CN108822017A (en) * | 2017-04-11 | 2018-11-16 | 中国医学科学院药物研究所 | New indole ketone compound and its preparation method and medicinal usage |
| SG11202103459WA (en) * | 2018-10-05 | 2021-05-28 | Ichnos Sciences S A | Indolinone compounds for use as map4k1 inhibitors |
| CN111285872B (en) * | 2018-12-06 | 2022-05-17 | 北京志健金瑞生物医药科技有限公司 | Indole-2-ketone derivative and preparation method and application thereof |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4956849A (en) * | 1988-08-04 | 1990-09-11 | Voest-Alpine Maschinenbau Gesellschaft M.B.H. | Process and apparatus for graphitizing carbon bodies |
| US4966849A (en) * | 1985-09-20 | 1990-10-30 | President And Fellows Of Harvard College | CDNA and genes for human angiogenin (angiogenesis factor) and methods of expression |
| US5217999A (en) * | 1987-12-24 | 1993-06-08 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Styryl compounds which inhibit EGF receptor protein tyrosine kinase |
| US5302606A (en) * | 1990-04-16 | 1994-04-12 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Styryl-substituted pyridyl compounds which inhibit EGF receptor tyrosine kinase |
| US5330992A (en) * | 1992-10-23 | 1994-07-19 | Sterling Winthrop Inc. | 1-cyclopropyl-4-pyridyl-quinolinones |
| US5792783A (en) * | 1995-06-07 | 1998-08-11 | Sugen, Inc. | 3-heteroaryl-2-indolinone compounds for the treatment of disease |
| US6316635B1 (en) * | 1995-06-07 | 2001-11-13 | Sugen, Inc. | 2-indolinone derivatives as modulators of protein kinase activity |
| US6369086B1 (en) * | 1997-09-05 | 2002-04-09 | Smithkline Beecham Corporation | Substituted oxidole derivatives as protein tyrosine and as protein serine/threonine kinase inhibitors |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4476307A (en) * | 1982-09-20 | 1984-10-09 | Pfizer Inc. | Heteroylidene indolone compounds |
| US5326905A (en) | 1990-04-02 | 1994-07-05 | Pfizer Inc. | Benzylphosphonic acid tyrosine kinase inhibitors |
| US5409930A (en) | 1991-05-10 | 1995-04-25 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Bis mono- and bicyclic aryl and heteroaryl compounds which inhibit EGF and/or PDGF receptor tyrosine kinase |
| US6194439B1 (en) | 1991-05-29 | 2001-02-27 | Pfizer Inc. | Tricyclic polyhydroxylic tyrosine kinase inhibitors |
| RU2155187C2 (en) | 1992-08-06 | 2000-08-27 | Варнер-Ламберт Компани | Derivatives of indole, their tautomers, mixtures of their isomers or separate isomers and pharmaceutically acceptable salts, pharmaceutical composition showing antitumor or inhibiting protein tyrosine kinase activity and method of inhibition of protein tyrosine kinase-depending disease or control of aberrant growth of mammalian or human cells |
| DK0666868T4 (en) | 1992-10-28 | 2006-09-18 | Genentech Inc | Use of anti-VEGF antibodies to treat cancer |
| GB9226855D0 (en) | 1992-12-23 | 1993-02-17 | Erba Carlo Spa | Vinylene-azaindole derivatives and process for their preparation |
| DE19824922A1 (en) * | 1998-06-04 | 1999-12-09 | Boehringer Ingelheim Pharma | Novel substituted indolinones, their preparation and their use as pharmaceuticals |
| US6525072B1 (en) | 1998-08-31 | 2003-02-25 | Sugen, Inc. | Geometrically restricted 2-indolinone derivatives as modulators of protein kinase activity |
| PT1115704E (en) * | 1998-09-25 | 2003-11-28 | Boehringer Ingelheim Pharma | NEW INDOLINONS REPLACED WITH INHIBITOR ACTION ON SEVERAL KINDS AND CYCLINE / CDK COMPLEXES |
| GB9904933D0 (en) * | 1999-03-04 | 1999-04-28 | Glaxo Group Ltd | Compounds |
| WO2001016130A1 (en) * | 1999-08-27 | 2001-03-08 | Boehringer Ingelheim Pharma Kg | Substituted indolinones as tyrosine kinase inhibitors |
| UA75054C2 (en) * | 1999-10-13 | 2006-03-15 | Бьорінгер Інгельхайм Фарма Гмбх & Ко. Кг | Substituted in position 6 indolinones, producing and use thereof as medicament |
| US6762180B1 (en) * | 1999-10-13 | 2004-07-13 | Boehringer Ingelheim Pharma Kg | Substituted indolines which inhibit receptor tyrosine kinases |
| EP1339680A1 (en) * | 2000-09-01 | 2003-09-03 | Glaxo Group Limited | Substituted oxindole derivatives as tyrosine kinase inhibitors |
| AU2002341881B2 (en) * | 2001-09-27 | 2008-05-08 | Allergan, Inc. | 3-(arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors |
-
2002
- 2002-09-27 AU AU2002341881A patent/AU2002341881B2/en not_active Ceased
- 2002-09-27 US US10/256,879 patent/US6765012B2/en not_active Expired - Fee Related
- 2002-09-27 WO PCT/US2002/030882 patent/WO2003027102A1/en active Application Filing
- 2002-09-27 CA CA2461812A patent/CA2461812C/en not_active Expired - Fee Related
- 2002-09-27 US US10/259,703 patent/US20030225152A1/en not_active Abandoned
- 2002-09-27 EP EP02776036A patent/EP1430048A1/en not_active Withdrawn
- 2002-09-27 JP JP2003530690A patent/JP2005508336A/en active Pending
-
2004
- 2004-02-20 US US10/783,325 patent/US7015220B2/en not_active Expired - Lifetime
- 2004-07-06 US US10/886,213 patent/US7098236B2/en not_active Expired - Lifetime
-
2005
- 2005-09-16 US US11/228,780 patent/US20060025413A1/en not_active Abandoned
- 2005-11-15 US US11/274,681 patent/US7414054B2/en not_active Expired - Lifetime
-
2006
- 2006-03-31 US US11/396,036 patent/US20060194863A1/en not_active Abandoned
-
2010
- 2010-01-25 JP JP2010013326A patent/JP2010116411A/en active Pending
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4966849A (en) * | 1985-09-20 | 1990-10-30 | President And Fellows Of Harvard College | CDNA and genes for human angiogenin (angiogenesis factor) and methods of expression |
| US5217999A (en) * | 1987-12-24 | 1993-06-08 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Styryl compounds which inhibit EGF receptor protein tyrosine kinase |
| US4956849A (en) * | 1988-08-04 | 1990-09-11 | Voest-Alpine Maschinenbau Gesellschaft M.B.H. | Process and apparatus for graphitizing carbon bodies |
| US5302606A (en) * | 1990-04-16 | 1994-04-12 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Styryl-substituted pyridyl compounds which inhibit EGF receptor tyrosine kinase |
| US5330992A (en) * | 1992-10-23 | 1994-07-19 | Sterling Winthrop Inc. | 1-cyclopropyl-4-pyridyl-quinolinones |
| US5792783A (en) * | 1995-06-07 | 1998-08-11 | Sugen, Inc. | 3-heteroaryl-2-indolinone compounds for the treatment of disease |
| US5834504A (en) * | 1995-06-07 | 1998-11-10 | Sugen, Inc. | 3-(2'-halobenzylidenyl)-2-indolinone compounds for the treatment of disease |
| US5883116A (en) * | 1995-06-07 | 1999-03-16 | Sugen, Inc. | 3-(2'-alkoxybenzylidenyl)-2-indolinone and analogues thereof for the treatment of disease |
| US5883113A (en) * | 1995-06-07 | 1999-03-16 | Sugen, Inc. | 3-(4'-Bromobenzylindenyl)-2-indolinone and analogues thereof for the treatment of disease |
| US5886020A (en) * | 1995-06-07 | 1999-03-23 | Sugen, Inc. | 3-(4'-dimethylaminobenzylidenyl)-2-indolinone and analogues thereof for the treatment of disease |
| US6316635B1 (en) * | 1995-06-07 | 2001-11-13 | Sugen, Inc. | 2-indolinone derivatives as modulators of protein kinase activity |
| US6369086B1 (en) * | 1997-09-05 | 2002-04-09 | Smithkline Beecham Corporation | Substituted oxidole derivatives as protein tyrosine and as protein serine/threonine kinase inhibitors |
Cited By (64)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8846094B2 (en) | 2003-11-12 | 2014-09-30 | Allergan, Inc. | Peripherally administered viscous formulations |
| US9265775B2 (en) | 2003-11-12 | 2016-02-23 | Allergan, Inc. | Pharmaceutical compositions |
| US9089478B2 (en) | 2003-11-12 | 2015-07-28 | Allergen, Inc. | Peripherally administered viscous formulations |
| US9572859B2 (en) | 2004-01-20 | 2017-02-21 | Allergan, Inc. | Compositions and methods for localized therapy of the eye |
| US8771722B2 (en) | 2004-04-30 | 2014-07-08 | Allergan, Inc. | Methods of treating ocular disease using steroid-containing sustained release intraocular implants |
| US8206736B2 (en) | 2004-04-30 | 2012-06-26 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US8119154B2 (en) | 2004-04-30 | 2012-02-21 | Allergan, Inc. | Sustained release intraocular implants and related methods |
| US8147865B2 (en) | 2004-04-30 | 2012-04-03 | Allergan, Inc. | Steroid-containing sustained release intraocular implants and related methods |
| US8900622B1 (en) | 2004-04-30 | 2014-12-02 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US8206737B2 (en) | 2004-04-30 | 2012-06-26 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US8257730B2 (en) | 2004-04-30 | 2012-09-04 | Allergan, Inc. | Steroid-containing sustained release intraocular implants and related methods |
| US8263110B2 (en) | 2004-04-30 | 2012-09-11 | Allergan, Inc. | Sustained release intraocular implants and related methods |
| US8293210B2 (en) | 2004-04-30 | 2012-10-23 | Allergan, Inc. | Sustained release intraocular implants and methods for preventing retinal dysfunction |
| US8298570B2 (en) | 2004-04-30 | 2012-10-30 | Allergan, Inc. | Sustained release intraocular implants and related methods |
| US8404267B2 (en) | 2004-04-30 | 2013-03-26 | Allergan, Inc. | Sustained release intraocular implants containing tyrosine kinase inhibitors and related methods |
| US8409607B2 (en) | 2004-04-30 | 2013-04-02 | Allergan, Inc. | Sustained release intraocular implants containing tyrosine kinase inhibitors and related methods |
| US8425929B2 (en) | 2004-04-30 | 2013-04-23 | Allergan, Inc. | Sustained release intraocular implants and methods for preventing retinal dysfunction |
| US10881608B2 (en) | 2004-04-30 | 2021-01-05 | Allergan, Inc. | Biodegradable intravitreal tyrosine kinase implants |
| US8440216B2 (en) | 2004-04-30 | 2013-05-14 | Allergan, Inc. | Sustained release intraocular implants and related methods |
| US8445027B2 (en) | 2004-04-30 | 2013-05-21 | Allergan, Inc. | Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and prostamide |
| US8455656B2 (en) | 2004-04-30 | 2013-06-04 | Allergan, Inc. | Kinase inhibitors |
| US8465778B2 (en) | 2004-04-30 | 2013-06-18 | Allergan, Inc. | Method of making tyrosine kinase microspheres |
| US8481069B2 (en) | 2004-04-30 | 2013-07-09 | Allergan, Inc. | Tyrosine kinase microspheres |
| US8506986B2 (en) | 2004-04-30 | 2013-08-13 | Allergan, Inc. | Sustained release intraocular implants and methods for treating ocular vasculopathies |
| US8512738B2 (en) | 2004-04-30 | 2013-08-20 | Allergan, Inc. | Biodegradable intravitreal tyrosine kinase implants |
| US8580292B2 (en) | 2004-04-30 | 2013-11-12 | Allergan, Inc. | Sustained release intraocular implants and methods for treating ocular vasculopathies |
| US8609144B2 (en) | 2004-04-30 | 2013-12-17 | Allergan, Inc. | Sustained release intraocular implants and methods for preventing retinal dysfunction |
| US8637068B2 (en) | 2004-04-30 | 2014-01-28 | Allergan, Inc. | Hypotensive prostamide-containing biodegradable intraocular implants and related methods |
| US10864218B2 (en) | 2004-04-30 | 2020-12-15 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US8673341B2 (en) | 2004-04-30 | 2014-03-18 | Allergan, Inc. | Intraocular pressure reduction with intracameral bimatoprost implants |
| US8715709B2 (en) | 2004-04-30 | 2014-05-06 | Allergan, Inc. | Sustained release intraocular implants and methods for treating ocular neuropathies |
| US20050244477A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Tyrosine kinase microsphers |
| US10406168B2 (en) | 2004-04-30 | 2019-09-10 | Allergan, Inc. | Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and related methods |
| US7993634B2 (en) | 2004-04-30 | 2011-08-09 | Allergan, Inc. | Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and related methods |
| US8911767B2 (en) | 2004-04-30 | 2014-12-16 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US8962009B2 (en) | 2004-04-30 | 2015-02-24 | Allergan, Inc. | Sustained release intraocular implants and related methods |
| US20110091520A1 (en) * | 2004-04-30 | 2011-04-21 | Allergan, Inc. | Sustained Release Intraocular Implants and Methods for Treating Ocular Neuropathies |
| US8968766B2 (en) | 2004-04-30 | 2015-03-03 | Allergan, Inc. | Sustained release intraocular implants containing tyrosine kinase inhibitors and related methods |
| US8999397B2 (en) | 2004-04-30 | 2015-04-07 | Allergan, Inc. | Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and related methods |
| US9056045B2 (en) | 2004-04-30 | 2015-06-16 | Allergan, Inc. | Intraocular biodegradable microspheres |
| US7799336B2 (en) | 2004-04-30 | 2010-09-21 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US9101583B2 (en) | 2004-04-30 | 2015-08-11 | Allergan, Inc. | Microparticles manufactured in an oil-in-water process comprising a prostamide |
| US10398707B2 (en) | 2004-04-30 | 2019-09-03 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related implants |
| US9144543B2 (en) | 2004-04-30 | 2015-09-29 | Allergan, Inc. | Sustained release intraocular implants and methods for preventing retinal dysfunction |
| US9161938B2 (en) | 2004-04-30 | 2015-10-20 | Allergan, Inc. | Sustained release intraocular implants and methods for treating ocular vasculopathies |
| US9233070B2 (en) | 2004-04-30 | 2016-01-12 | Allergan, Inc. | Biodegradable intravitreal tyrosine kinase implants |
| US7771742B2 (en) | 2004-04-30 | 2010-08-10 | Allergan, Inc. | Sustained release intraocular implants containing tyrosine kinase inhibitors and related methods |
| US9326949B2 (en) | 2004-04-30 | 2016-05-03 | Allergan, Inc. | Method of making oil-in-oil emulsified polymeric implants containing a hypotensive lipid |
| US9393223B2 (en) | 2004-04-30 | 2016-07-19 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US20050244475A1 (en) * | 2004-04-30 | 2005-11-03 | Allergan, Inc. | Biodegradable intravitreal tyrosine kinase implants |
| US10328086B2 (en) | 2004-04-30 | 2019-06-25 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US9669039B2 (en) | 2004-04-30 | 2017-06-06 | Allergan, Inc. | Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and related methods |
| US9707238B2 (en) | 2004-04-30 | 2017-07-18 | Allergan, Inc. | Oil-in-water method for making polymeric implants containing a hypotensive lipid |
| US9750751B2 (en) | 2004-04-30 | 2017-09-05 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related methods |
| US9775846B2 (en) | 2004-04-30 | 2017-10-03 | Allergan, Inc. | Hypotensive lipid-containing biodegradable intraocular implants and related implants |
| US10064872B2 (en) | 2004-04-30 | 2018-09-04 | Allergan, Inc. | Oil-in-water method for making polymeric implants containing a hypotensive lipid |
| US10076492B2 (en) | 2004-04-30 | 2018-09-18 | Allergan, Inc. | Biodegradable intravitreal tyrosine kinase implants |
| US10201641B2 (en) | 2004-04-30 | 2019-02-12 | Allergan, Inc. | Sustained release intraocular implants and methods for treating ocular vasculopathies |
| US8969415B2 (en) | 2006-12-01 | 2015-03-03 | Allergan, Inc. | Intraocular drug delivery systems |
| US8642067B2 (en) | 2007-04-02 | 2014-02-04 | Allergen, Inc. | Methods and compositions for intraocular administration to treat ocular conditions |
| EP2583679A1 (en) | 2007-04-02 | 2013-04-24 | Allergan, Inc. | Methods and compositions for intraocular administration to treat ocular conditions |
| US9138480B2 (en) | 2009-11-09 | 2015-09-22 | Allergan, Inc. | Compositions and methods for stimulating hair growth |
| US10231926B2 (en) | 2013-02-15 | 2019-03-19 | Allergan, Inc. | Sustained drug delivery implant |
| US9610246B2 (en) | 2013-02-15 | 2017-04-04 | Allergan, Inc. | Sustained drug delivery implant |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2005508336A (en) | 2005-03-31 |
| US20060025413A1 (en) | 2006-02-02 |
| CA2461812A1 (en) | 2003-04-03 |
| US7414054B2 (en) | 2008-08-19 |
| JP2010116411A (en) | 2010-05-27 |
| WO2003027102A1 (en) | 2003-04-03 |
| US20060063940A1 (en) | 2006-03-23 |
| US7015220B2 (en) | 2006-03-21 |
| US20040198720A1 (en) | 2004-10-07 |
| US20060194863A1 (en) | 2006-08-31 |
| AU2002341881B2 (en) | 2008-05-08 |
| US6765012B2 (en) | 2004-07-20 |
| US20030199478A1 (en) | 2003-10-23 |
| EP1430048A1 (en) | 2004-06-23 |
| US20050228036A1 (en) | 2005-10-13 |
| CA2461812C (en) | 2011-09-20 |
| US7098236B2 (en) | 2006-08-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6765012B2 (en) | 3-(Arylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors | |
| AU2002341881A1 (en) | 3-(arylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors | |
| US6747025B1 (en) | Kinase inhibitors for the treatment of disease | |
| US7060844B2 (en) | (3Z)-3-(2,3-dihydro-1H-inden-1-ylidene)-1,3-dihydro-2H-indol-2-ones as kinase inhibitors | |
| US8809534B2 (en) | Compounds as tyrosine kinase modulators | |
| US6699863B1 (en) | Kinase inhibitors for the treatment of disease | |
| US6559173B1 (en) | 3-(heteroarylamino)methylene-1,3-dihydro-2H-indol-2-ones as kinase inhibitors | |
| US6858641B2 (en) | Substituted indolinones | |
| EP1902025A1 (en) | Kinase inhibitors | |
| US7439371B2 (en) | Indol kinase inhibitors | |
| WO2004050621A2 (en) | Indol derivatives and their use as kinase inhibitors | |
| CN106565682B (en) | Substituted indole expires ketone derivatives and application thereof | |
| JP4879492B2 (en) | Kinase inhibitors for the treatment of diseases | |
| WO2003027109A1 (en) | 3-(heteroarylamino)methylene-1, 3-dihydro-2h-indol-2-ones as kinase inhibitors | |
| DE19940829A1 (en) | New 3-(anilino-methylidene)-2-indolinone derivatives, are kinase inhibitors and cell proliferation inhibitors, useful e.g. for treating tumors, metastasis, rheumatoid arthritis or psoriasis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: ALLERGAN, INC., CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:ANDREWS, STEVEN W.;WURSTER, JULIE A.;HULL III., CLARENCE E.;REEL/FRAME:013440/0596;SIGNING DATES FROM 20021010 TO 20021023 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |