KR810000552B1 - Method of manufacturing anti-malignant tumor agent - Google Patents
Method of manufacturing anti-malignant tumor agent Download PDFInfo
- Publication number
- KR810000552B1 KR810000552B1 KR770002155A KR770002155A KR810000552B1 KR 810000552 B1 KR810000552 B1 KR 810000552B1 KR 770002155 A KR770002155 A KR 770002155A KR 770002155 A KR770002155 A KR 770002155A KR 810000552 B1 KR810000552 B1 KR 810000552B1
- Authority
- KR
- South Korea
- Prior art keywords
- culture
- molecular weight
- malignant tumor
- leukemia
- solution
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired
Links
- 201000011510 cancer Diseases 0.000 title claims description 8
- 230000009876 antimalignant effect Effects 0.000 title claims description 7
- 238000004519 manufacturing process Methods 0.000 title claims description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 10
- 239000002244 precipitate Substances 0.000 claims description 8
- 241000191963 Staphylococcus epidermidis Species 0.000 claims description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 claims description 4
- 239000001963 growth medium Substances 0.000 claims description 4
- 239000007787 solid Substances 0.000 claims description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 2
- 230000000259 anti-tumor effect Effects 0.000 claims description 2
- 229910052799 carbon Inorganic materials 0.000 claims description 2
- 229910052757 nitrogen Inorganic materials 0.000 claims description 2
- 229910017053 inorganic salt Inorganic materials 0.000 claims 1
- 238000009630 liquid culture Methods 0.000 claims 1
- 239000000243 solution Substances 0.000 description 18
- 208000032839 leukemia Diseases 0.000 description 16
- 239000004480 active ingredient Substances 0.000 description 13
- 210000004027 cell Anatomy 0.000 description 12
- 241000699670 Mus sp. Species 0.000 description 11
- 238000000034 method Methods 0.000 description 10
- 206010028980 Neoplasm Diseases 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 238000001228 spectrum Methods 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 6
- 238000001704 evaporation Methods 0.000 description 6
- 230000008020 evaporation Effects 0.000 description 6
- 239000002609 medium Substances 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 5
- 210000004881 tumor cell Anatomy 0.000 description 5
- 241000700605 Viruses Species 0.000 description 4
- 239000012153 distilled water Substances 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 230000036210 malignancy Effects 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- 239000003826 tablet Substances 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 3
- 239000012141 concentrate Substances 0.000 description 3
- -1 etc.) Substances 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000011081 inoculation Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 244000005700 microbiome Species 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 239000011343 solid material Substances 0.000 description 3
- 206010003445 Ascites Diseases 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 208000006268 Sarcoma 180 Diseases 0.000 description 2
- 241000191940 Staphylococcus Species 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 210000001015 abdomen Anatomy 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000000706 filtrate Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 230000001717 pathogenic effect Effects 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 241000193798 Aerococcus Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- 241000699666 Mus <mouse, genus> Species 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 229920005654 Sephadex Polymers 0.000 description 1
- 239000012507 Sephadex™ Substances 0.000 description 1
- 241000191967 Staphylococcus aureus Species 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000012136 culture method Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 230000002440 hepatic effect Effects 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000001226 reprecipitation Methods 0.000 description 1
- 238000009938 salting Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 230000003393 splenic effect Effects 0.000 description 1
- 239000006190 sub-lingual tablet Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000005760 tumorsuppression Effects 0.000 description 1
Images
Landscapes
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Abstract
내용 없음.No content.
Description
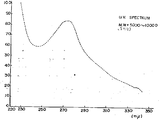
제1도-제4도는 실시예 2에 표시된 공정에 의하여 얻어진 분획의 U.V. 스펙트럼 및 I.R 스펙트럼을 나타낸다.1 through 4 show U.V. fractions of fractions obtained by the process shown in Example 2. Spectrum and I.R spectrum are shown.
제1도는 분자량 5,000~10,000을 가진 활성성분의 U.V. 스펙트럼을 나타낸다(원배양액과 물의 희석비는 1 : 10).1 shows U.V. of active ingredients having a molecular weight of 5,000 to 10,000. It shows the spectrum (dilution ratio of the original culture liquid and water is 1:10).
제2도는 분자량 1,000~5,000을 가진 활성성분의 U.V. 스펙트럼을 나타낸다(원배양액).2 shows U.V. The spectrum is shown (original culture solution).
제3도는 분자량 5,000~10,000을 가진 활성성분의 I.R. 스펙트럼을 표시하며.3 shows I.R. of active ingredients having a molecular weight of 5,000 to 10,000. Display the spectrum.
제4도는 분자량 1,000~5,000을 가진 활성성분의 I.R. 스펙트럼을 표시한다.4 shows I.R. of active ingredients having a molecular weight of 1,000 to 5,000. Display the spectrum.
본 발명은 신규한 항악성종양제의 제조방법에 관한 것이다. 좀더 상세히 설명하면 본 발명은 액체배지(液體培地)에 스타필로코쿠스 에피데르미디스 STE(staph ylococcus epidermidis STF)(微工硏寄託 No. 3706; ATCC 31310)를 배양하여 얻어지는 신규한 활성물질을 제조하는 방법에 관한 것이다.The present invention relates to a method for producing a novel anti-malignant tumor agent. In more detail, the present invention provides a novel active substance obtained by culturing Staphylococcus epidermidis ST (STC) No. 3706 (ATCC 31310) in a liquid medium. It relates to a manufacturing method.
상기물질은 백혈병 및 악성종양을 방지하는데 효과를 가지고 있다. 본 발명은 백혈병 환자에 있어 부스럼이 생겼을 때, 예상외로 백혈병세포의 수가 현저히 감소되고 마침내 환자의 백혈구수가 정상으로 된 것 발견하여 이루어진 발명인 것이다.The substance has an effect on preventing leukemia and malignancies. When the swelling occurs in a leukemia patient, the present invention unexpectedly reduced the number of leukemia cells and finally found that the leukocyte count of the patient was normal.
본 발명자들은 백혈병환자의 종기에서 미생물균주를 분리시키고 이것을 배양하여 백혈병을 감염시킨 동물에게 배양액의 여액을 투여한 결과, 이 동물들의 백혈병 바이러스 양이 줄어들고 백혈병 세포의 수가 줄어들며, 백혈병이 걸린 기관의 회복, 수명의 연장 및 살아남은 동물의 수를 증가시킨 효과를 나타냈다.The present inventors isolated microbial strains from the boil of leukemia patients and cultured them to the animal infected with leukemia. Administration of these animals resulted in a reduction in the amount of leukemia virus in these animals, a reduction in the number of leukemia cells, recovery of organs with leukemia, prolongation of lifespan, and an increase in the number of surviving animals.
연구를 계속하여, 본 발명자들은 상기 여액이 백혈병에 뿐만 아니라 일반적으로 다른 악성종양에 유효하다는 것도 발견하였다.Continuing the study, the inventors found that the filtrate is effective not only for leukemia, but generally for other malignancies.
본 발명자들은, 배양액의 여액을 농축시키고, 활성물로 생각되는 수용성물질을 몇 개의 성분으로 분리시키며, 이 물질이 상기 항생활성을 가지고 있다는 것을 입증하기 위하여 단리하고 정제하였다.The present inventors concentrated and purified the filtrate of the culture, separated the water-soluble substance considered to be the active ingredient into several components, and isolated and purified to demonstrate that this substance has the anti-bioactivity.
다음에 본 발명에 대하여 상세히 설명한다.Next, the present invention will be described in detail.
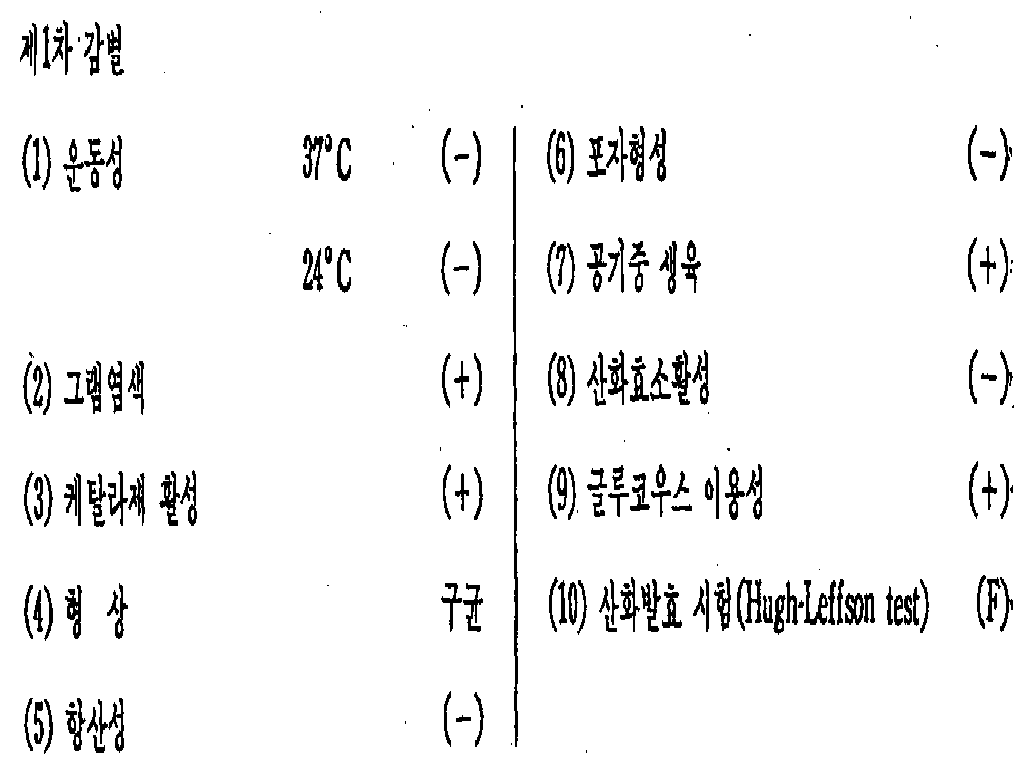
[1] 균주(菌株)의 식별[1] identification of strains
본 발명에 사용되는 미생물의 균주는 신규한 것이다. 이것은 미공연 기탁 번호 No. 3706 및 ATCC 31310로 미공연 및 "ATCC" 각각에 기탁되어 있다.Strains of microorganisms used in the present invention are novel. This is the no. Deposited as 3706 and ATCC 31310 in unperforming and “ATCC” respectively.
이 균주는 이들 연구소에서 누구나 자유로 사용할 수 있다.This strain is freely available to anyone in these laboratories.
이 균주는 다음과 같이 스타필로코쿠스 에피데르미디스의 일균으로 판명되었다.This strain was found to be a strain of Staphylococcus epidermides as follows.
상기 시험결과로부터 병원균은 스타필로코쿠스 또는 에어로코커스에 속한다는 것이 판명되었다.From the test results, it was found that the pathogen belongs to Staphylococcus or Aerococcus.
이리하여, 병원균은 마침내 스타필로코쿠스 에피데르미디스로 판단하였다. 본원 발명자들은 본 균주를 "STF"라고 명명하였다.Thus, the pathogen finally determined to be Staphylococcus epidermidis. The present inventors named this strain "STF".
[2] 활성성분의 제법 :[2] preparation of active ingredients:
병원균은 일반배지, 예컨대 탄소, 질소, 무기염류를 포함하는 영양배지에 잘 증식된다.Pathogens multiply well in general media, such as nutrient media containing carbon, nitrogen and inorganic salts.
세균에 의하여 만들어지는 유용한 활성성분을 이용하기 위하여 정치상태 또는 진탕상태하의 액체배지에 있어서의 호기배양이 바람직하다. 배지의 적당한 예는 다음과 같다.In order to utilize useful active ingredients made by bacteria, aerobic culture in a liquid medium in a stationary state or shaking state is preferable. Suitable examples of media are as follows.
위에는 액체배지만을 예시하였지만, 일반고체배지도 사용할 수 있다. 균류는 24시간-120시간동안 배양온도 -20℃~+40℃에서 상기 배지에 배양된다. 최적온도는 약 37℃였다. 집락표면(colony surface)은 희고 원형을 이루고 있었다.Although only liquid medium is illustrated above, a general solid medium may also be used. The fungus is incubated in the medium at -20 ° C to + 40 ° C for 24 hours-120 hours. The optimum temperature was about 37 ° C. The colony surface was white and round.
얻어진 배양액은 원심분리되고 고체물질은 분리되고 용액을 얻었다(이후는 "배양액"이라고 한다). 이 배양액에 여러 가지 실험을 가하였다.The obtained culture was centrifuged, the solid material was separated, and a solution was obtained (hereinafter referred to as "culture medium"). Various experiments were added to this culture.
[3] 활성성분의 호용[3] favorable active ingredients
얻어진 배양액은 다음의 결과를 얻기 위하여 여러 가지의 질병을 감염시킨 동물들에게 투여하였다 :The resulting culture was administered to animals infected with various diseases to achieve the following results:
(1) 백혈병 바이러스의 접종에 의하여 발생된 악성종양의 억제 : ICR 생쥐 303마리를 3군으로 나누었다.(1) Inhibition of malignancy caused by inoculation of leukemia virus: 303 ICR mice were divided into three groups.
그중 2개군에 대하여 프랜드(Friend)의 백혈병 바이러스를 접종한 후 1개군에 배양액을 0.ml/10g/day의 비율로 8일간 복강내에 투여하여 다음과 같은 비장 이상 비대 억제효과를 보았다.Two of the two groups were inoculated with Freind's leukemia virus, and one group of the culture solution was intraperitoneally administered at a rate of 0.ml/10g/day for 8 days, and the splenic abnormality was inhibited as follows.
(2) 백혈병 세포의 접종에 의하여 발생한 악성종양 억제 :(2) Malignant tumor suppression caused by inoculation of leukemia cells:
유발된 백혈병 상태를 발생시키기 위하여 BDF1생쥐들에게 생쥐백혈병세포 L-1210이 접종되었다. 생쥐들은 제한된 시간이 지나면 죽는다. 1개군 생쥐가 6마리로 이루어진 2개군의 생쥐들에게 L-1210 세포가 접종되고, 그후 상기 배양액이 아래의 결과를 얻기 위하여 5일간 계속해서 상기 1개군에 투약되었다. 주어진 배양액의 투여양은 (1)에 있어서와 동일 하였다.BDF 1 mice were inoculated with mouse leukemia cell L-1210 to induce induced leukemia. Mice die after a limited time. L-1210 cells were inoculated into two groups of mice consisting of six mice in one group, and then the culture solution was continuously administered to the group for 5 days to obtain the following results. The dose of the given culture was the same as in (1).
시험군/대조군(수명연장비)는 112% 이상으로 높았다.The test group / control group (lifetime equipment) was higher than 112%.
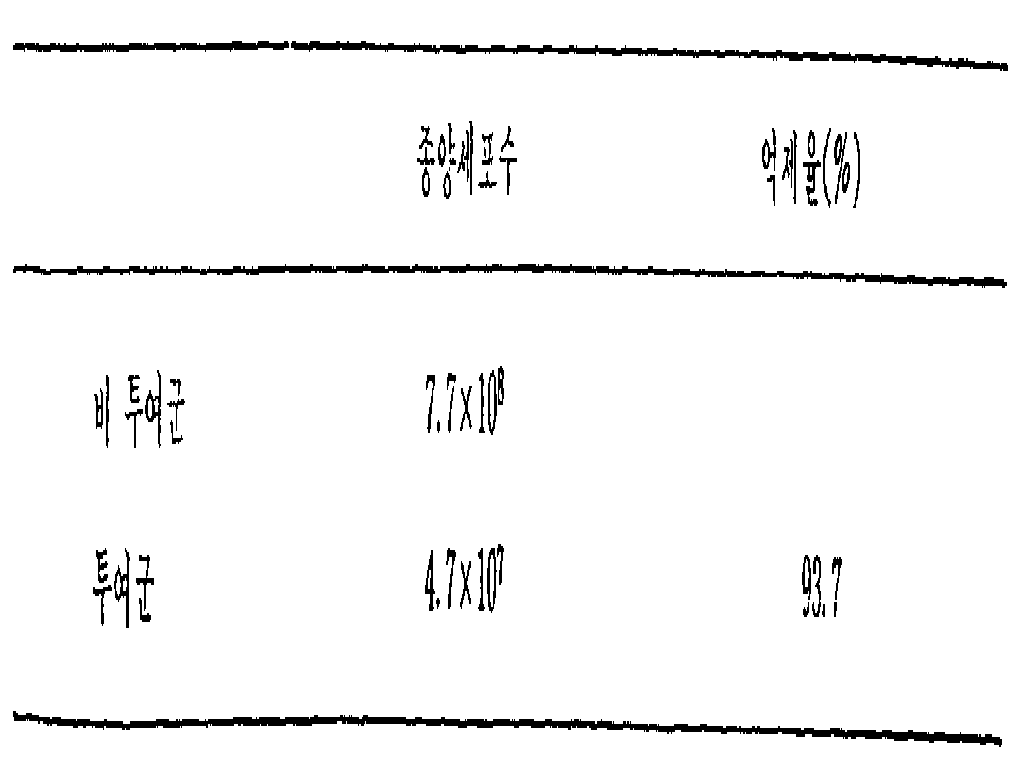
(3) 에어리히(Ehrlich) 암세포의 억제효과 :(3) Inhibitory effect of Ehrlich cancer cells:
1개군이 10마리의 생쥐로 이루어진 2개군에 에어리히 복수 종양세포를 접종 하였다. 5일간을 계속하여 1개군의 생쥐의 복부내에 배양액이 투여되었다. 그리하여 아래와 같이 10일째에 억제효과가 종양세포수에 의하여 판명되었다. 배양액의 투여양은 (1)에 있어서와 동일하였다.Two groups of 10 mice were inoculated in one group and inoculated with Errich's ascites tumor cells. The culture solution was administered to the abdomen of one group of mice continuously for 5 days. Thus, the inhibitory effect was confirmed by the tumor cell number on
(4) 사르코마(Sarcoma) 180세포의 억제효과 :(4) Inhibitory effect of Sarcoma 180 cells:
상기한 바와 같은 방법으로 1개군이 10마리의 생쥐로 이루어지는 ICR생쥐 2개군에 대하여 복부내에 사르코마 180세포를 접종하였다. 배양액은 5일간 계속하여 1개군에 투여되었다. 10일째의 세포수를 조사하여 다음결과를 얻었다. 배양액의 투여양은 (1)에 있어서와 동일하였다.As described above, two groups of ICR mice, in which one group consists of 10 mice, were inoculated with Sarcoma 180 cells in the abdomen. The culture solution was administered to one group for 5 days. The number of cells at
비교를 위하여 동일한 배양법에 의하여 얻어진 다른 공지의 미생물의 배양액의 효과가 조사되었다. 아래의 공지외 미생물의 배양액은 프랜드 백혈병 바이러스로 접종된 생쥐들에게 투여되었다. 각 경우에 있어서 아무런 효과가 관찰되지 않았다 :For comparison, the effect of the culture solution of other known microorganisms obtained by the same culture method was investigated. Cultures of the following unknown microorganisms were administered to mice inoculated with Friend Leukemia virus. In each case no effect was observed:
슈우도모나스(Pseudomonas)Pseudomonas
클렙시엘라(Klebsiella)Klebsiella
스타필로코커스 아우레우스(staphylococcus aureus)Staphylococcus aureus
(4) 활성성분의 단리 및 정제 :(4) Isolation and Purification of Active Ingredients:
스타필로코쿠스 에피데르미디스의 배양액에 함유된 활성성분은 예를 들면, 다음 방법으로 분리되고 정제될 수가 있다 :The active ingredient contained in the culture of Staphylococcus epidermidis can be isolated and purified, for example, by the following method:
(1) 배양액을 30분~10분간, 3,000~12,000rpm에서 원심분리시켜 용액과 침전물로 분리시켰다. 다음에 침전물은 증류수로 세척하고 다시 원심 분리시켰다. 세척액은 상기 용액과 병합시켰다. 이 방법을 1~5회(통상 3회) 반복했다.(1) The culture was centrifuged at 3,000 to 12,000 rpm for 30 minutes to 10 minutes to separate the solution and precipitate. The precipitate was then washed with distilled water and centrifuged again. Wash solution was combined with the solution. This method was repeated 1 to 5 times (usually 3 times).
(2) 이렇게 해서 수집된 용액은 1~6시간 5~30mmHg의 감압하에 온도 10~30℃에서 증발에 의하여 농축되었으며, 이리하여 침전된 고체물질은 증류수로 세척한 후 용해시켰다. 이 용액은 증발에 의하여 다시 농축시켰다. 이 공정은 백색, 황색 또는 갈색을 띤, 물에 용해가능한 백색 결정체 분말을 얻기 위하여 1회~5회(통상 3회) 반복하였다.(2) The solution thus collected was concentrated by evaporation at a temperature of 10-30 ° C. under a reduced pressure of 5-30 mmHg for 1-6 hours. Thus, the precipitated solid material was washed with distilled water and dissolved. This solution was concentrated again by evaporation. This process was repeated once to five times (typically three times) to obtain a white, yellow or brownish, white crystalline powder soluble in water.
(3) 이 결정체분말은 예컨대 세파덱스(Sphadex)(스웨덴, 바이오케미칼사에 의하여 제조된 흡착여과 겔) G-25와 같은 물레클라사이브 조작에 의하여 분류한다.(3) This crystalline powder is classified by water reclamation operation such as, for example, Sphadex (Sweden, adsorption filtration gel manufactured by Biochemical Co., Ltd.) G-25.
각 분획에 대한 활성을 조사하여 활성분획을 반복하여 선택하였다. 이러한 공정에 의하여 단일활성 피이크를 가진 물질이 얻어졌다.The active fractions were selected repeatedly by investigating the activity of each fraction. This process yielded a material with a single active peak.
이 활성물은 다시 물에 용해시켜서 이온교환 크로마토그래피 또는 투석(透析)과 같은 정제 방법에 의하여 정제하였다.This active material was again dissolved in water and purified by purification method such as ion exchange chromatography or dialysis.
(4) 이렇게 해서 정제된 활성성분은 수용액에 여러 가지의 유기 용제를 가하고 산성 영역에서 알칼리영역으로 pH를 변화시키고 염석후 재침전 및 여과 또는 증발에 의한 농축으로 분리시킬 수가 있다.(4) The active ingredient thus purified can be separated by adding various organic solvents to the aqueous solution, changing the pH from the acidic zone to the alkaline zone, followed by reprecipitation after salting and concentration by filtration or evaporation.
본 물질은 다음과 같은 특성을 갖는다.This material has the following properties.
이렇게해서 유리되고 정제된 활성성분의 항종양성 활성은 L-1210세포의 배양액에 이의 희석된 수용액을 가하고 48시간 35℃에서 이것을 배양하여 세포증식 억제율을 결정함으로써 다음과 같이 확인되었다.Thus, the antitumor activity of the purified and purified active ingredient was confirmed as follows by adding its diluted aqueous solution to the culture solution of L-1210 cells and culturing it at 35 ° C. for 48 hours to determine the cell proliferation inhibition rate.
이리하여 스타필로코쿠스 에피데르미디스에 의해 생산되는 물질은 액상암 및 고체상암과 같은 여러 가지의 악성종양을 제어하는 효과를 가지고 있다. 본 물질을 급성독성을 나타내지 않는다.Thus, the material produced by Staphylococcus epidermidis has the effect of controlling various malignancies such as liquid and solid cancers. The substance is not acutely toxic.
(5) 활성성분의 투여 :(5) Administration of the active ingredient:
투약량은 투약경로에 따라서 다르지만 약 3-300mg/kg이다. 고체물질이 제거된 배양액은 직접사용할 수가 있다. 그러나, 물론 투약량을 정확히 조절하기 위하여 분리되고 정제된 활성성분을 사용하는 것은 더 좋다. 정맥주사, 피하주사, 근육주사, 경구투약, 좌약, 정제 및 유제와 같은 여러 가지의 투약방법이 가능하다. 따라서 제제의 형태는 적당히 선택할 수 있고 예컨대 분말, 정제(단층정제, 중층정제, 장용제등) 캡슈울, 앰플, 또는 바이알에든 주사약, 설하정, 트로치제, 좌약 또는 지방유제등을 들 수 있다.Dosage depends on the route of administration but is about 3-300 mg / kg. The culture medium from which the solid material has been removed can be used directly. However, it is of course better to use the isolated and purified active ingredient in order to precisely control the dosage. Various dosage methods are possible, such as intravenous, subcutaneous, intramuscular, oral, suppositories, tablets and emulsions. Therefore, the form of the formulation may be appropriately selected, and examples thereof include powders, tablets (monolayer tablets, multilayer tablets, enteric agents, etc.), capsules, ampoules, or vials, injections, sublingual tablets, troches, suppositories, or fat emulsions.
아래 실시예는 본 발명을 예시한다. 아래 실시예에는 결코 본 발명을 한정하는 것은 아니다.The following examples illustrate the invention. The following examples in no way limit the invention.
[실시예 1]Example 1
스타필로코쿠스 에피데르미디스 STF는 아래배합의 배지에서 48시간 37℃에서 배양되었다 :Staphylococcus epidermidis STF was incubated at 37 ° C. for 48 hours in the media below:
상징액과 침전물로 나누기 위하여 30분간 10,000rpm에서 배양액을 원심 분리시켰다.The culture solution was centrifuged at 10,000 rpm for 30 minutes to divide the supernatant and the precipitate.
이 침전물은 1리터의 증류수로 재분산시켰으며 이 분산은 18시간 37℃에서 방치시키고 10,000rpm으로 다시 원심 분리시켜 상징액과 침전물로 나누었다. 이 공정은 5회에 걸쳐 반복되었다. 얻어진 상징액은 모아져 약 2시간 상온에서 10mmHg의 감압하에 증발에 의하여 농축되었다. 약 1/30 농축이 달성된 후, 액체는 세파덱스 D-25층을 통과하고 OD(광학밀도) 265의 인디케이터를 사용하여 U.V. 분광분석을 하였다.The precipitate was redispersed with 1 liter of distilled water and the dispersion was left at 18 ° C for 18 hours and centrifuged again at 10,000 rpm to separate the supernatant and precipitate. This process was repeated five times. The obtained supernatant was collected and concentrated by evaporation under reduced pressure of 10 mmHg at room temperature for about 2 hours. After about 1/30 enrichment was achieved, the liquid passed through the Sephadex D-25 layer and an indicator of OD (optical density) 265 was used to determine U.V. Spectroscopic analysis was performed.
분획의 분리에는 몰리쉬(Molisch) 반응과 같은 다당류 검출법이 사용된다. L-1210 세포 증식 억제활성의 크로마토그램을 조사하여 활성분획을 분리하였다. 이 공정은 최고 활성분획을 얻기 위하여 3회 반복되었다. 이 활성분획의 수용액은 백색분말을 얻기 위하여 증발에 의하여 농축되었다.The separation of fractions uses polysaccharide detection methods such as the Molisch reaction. Chromatogram of L-1210 cell proliferation inhibitory activity was investigated to isolate the active fraction. This process was repeated three times to obtain the highest active fraction. The aqueous solution of this active fraction was concentrated by evaporation to obtain a white powder.
이 분말은 물로 세척하고 다시 용해되어 증발에 의하여 농축되었다. 이 공정은 백색결정 분말을 얻기 위하여 5회 반복되었다. 배양액 1리터로부터의 산출량은 0.3g이었다.This powder was washed with water, dissolved again and concentrated by evaporation. This process was repeated five times to obtain a white crystalline powder. The yield from 1 liter of culture was 0.3 g.
[실시예 2]Example 2
실시예 1에 있어서와 마찬가지로 구성된 25리터의 액체배지가 만들어졌다. 배양은 2일간 37℃로 실시되었다. 배양액은 세균을 제거하기 위하여 원심분리( 6,000rpm 20분간)시켰다. 얻어진 상징액(약 23미터)은 감압하에 40℃에서 약 500c.c. 용적으로 농축되었다.A 25 liter liquid medium constructed as in Example 1 was made. The culture was carried out at 37 ° C. for 2 days. The culture was centrifuged (6,000 rpm for 20 minutes) to remove bacteria. The obtained supernatant (about 23 meters) was prepared at about 500c.c. Concentrated to volume.
이렇게 해서 형성된 침전물은 3회에 걸쳐 100c.c.의 증류수로 세척되었다. 이 세척물은 농축물과 결합되어, 이 전부가 감압하에 40℃에서 용적 500c.c.로 농축되었다.The precipitate thus formed was washed three times with 100 c.c. of distilled water. This wash was combined with the concentrate, all of which was concentrated to a volume of 500 c.c. at 40 ° C. under reduced pressure.
농축물은 아미콘 파아 이이스트 다이아플론 #10,000(Amicon Far East Diaf lon #10,000) 몰레클라 시이브막으로 2개분획(그중 1개분별 성분은 분자량이 10,000 이상이고 다른 것은 분자량 10,000 이하를 가지고 있다)으로 나뉘었다.The concentrate is an Amicon Far East Diaflon # 10,000 moleclave membrane, with two fractions (one fraction having a molecular weight of 10,000 or more and the other having a molecular weight of 10,000 or less). Divided into
10,000 이하의 분자량을 가진 용액은 산성물질, 중성물질 및 염기물질로 나누기 위하여 디메틸 아미노에틸 셀루로오스탑(OH-형)을 통과시켰다.A solution with a molecular weight of 10,000 or less was passed through a dimethyl aminoethyl cellulose tower (OH-type) to divide into acidic, neutral and basic substances.
그후, 이 중성물질과 염기물질은 다이아플론 #5,000으로 다시 2개분획으로(그 하나는 분자량 5,000~10,000(원용액)을 가진 것으로, 또 다른 것은 분자량이 5,000 이하를 가진 것으로) 나누었다. 분자량이 5,000 이하의 것은 다시 다이아플론 #1,000으로 2개 분획으로(분자량 1,000~5,000을 가진 것과 분자량 1,000 이하를 가진 것으로) 나누었다. 이리하여 농축물은 각개 분획으로 나눌 수가 있었다.The neutrals and bases were then divided into two separate fractions, diaphragm # 5,000, one with a molecular weight of 5,000 to 10,000 (original solution) and another with a molecular weight of 5,000 or less. The molecular weight less than 5,000 was again divided into two fractions (with molecular weight 1,000-5,000 and those with molecular weight 1,000 or less). Thus, the concentrate could be divided into individual fractions.
상기 각 분획의 U.V. 스펙트럼 및 IR 스펙트럼이 결정되었다(제1~4도 참조).U.V. of each fraction. Spectrum and IR spectrum were determined (see FIGS. 1-4).
다음에 상기 각개분획을 여러 가지의 유발된 질환에 감염된 동물들에게 투약하여 다음의 결과를 얻었다.The individual fractions were then administered to animals infected with various induced diseases to obtain the following results.
(A) 시험관내에 배양된 백혈병세포 L-1210에 대한 5,000<분자량<10,000 및 1,000<분자량<5,000을 가진 분획의 항악성종양효과 :(A) Anti-malignancy effect of fractions with 5,000 <molecular weight <10,000 and 1,000 <molecular weight <5,000 on in vitro cultured leukemia cells L-1210:
(B) 시험관내에 배양된 종양세포에 대한 5,000<분자량<10,000 및 1,000<분자량<5,000을 가진 분획의 항악성종양효과 :(B) Anti-malignancy effect of fractions with 5,000 <molecular weight <10,000 and 1,000 <molecular weight <5,000 on tumor cells cultured in vitro:
(C) 시험관내에 배양된 복수간장종양세포 AH41C에 대한 5,000<분자량<10,000 및 10,000<분자량<5,000을 가진 스티엔(styeen) 분획의 항악성종양효과 :(C) Anti-malignant tumor effect of styeen fraction with 5,000 <molecular weight <10,000 and 10,000 <molecular weight <5,000 on in vitro cultured hepatic hepatocellular tumor AH41C:
(D) 에어리히 복수 종양세포를 접종시킨 생쥐에 대한 5,000<분자량<10,000 및 10,000<분자량<5,000을 가진 스티엔 분획의 항악성종양효과(세포 접종후 제11일에 총 종양 세포수에 미치는 영향).(D) Anti-malignant tumor effect of styrene fractions containing 5,000 <molecular weight <10,000 and 10,000 <molecular weight <5,000 in mice inoculated with Aerrich ascites tumor cells (influence on total tumor cell number on day 11 after cell inoculation) ).
Claims (1)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR770002155A KR810000552B1 (en) | 1977-09-13 | 1977-09-13 | Method of manufacturing anti-malignant tumor agent |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR770002155A KR810000552B1 (en) | 1977-09-13 | 1977-09-13 | Method of manufacturing anti-malignant tumor agent |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR810000552B1 true KR810000552B1 (en) | 1981-06-01 |
Family
ID=19204921
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR770002155A Expired KR810000552B1 (en) | 1977-09-13 | 1977-09-13 | Method of manufacturing anti-malignant tumor agent |
Country Status (1)
| Country | Link |
|---|---|
| KR (1) | KR810000552B1 (en) |
-
1977
- 1977-09-13 KR KR770002155A patent/KR810000552B1/en not_active Expired
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| NO149239B (en) | OFFSHORE CONSTRUCTION. | |
| Sepčić et al. | Biological activities of aqueous extracts from marine sponges and cytotoxic effects of 3-alkylpyridinium polymers from Reniera sarai | |
| Lampis et al. | Sattabacins and sattazolins: new biologically active compounds with antiviral properties extracted from a Bacillus sp. | |
| US3923977A (en) | Antibiotic FR-1923 substance | |
| CN101628931B (en) | Antitumor antibiotics, pharmaceutically acceptable salts thereof, preparation method thereof and use thereof | |
| Okami | Potential use of marine microorganisms for antibiotics and enzyme production | |
| KR810000552B1 (en) | Method of manufacturing anti-malignant tumor agent | |
| US3674774A (en) | Pyrazomycin and process for production thereof | |
| EP0116690A1 (en) | Antiviral composition containing an indole-N-glycoside | |
| US3681491A (en) | Bleomycin and processes for the preparation thereof | |
| Martin et al. | GLYCOCINNAMOYLSPERMIDINES, A NEW CLASS OF ANTIBIOTICS II. ISOLATION, PHYSICOCHEMICAL AND BIOLOGICAL PROPERTIES OF LL-BM123β, γ 1, AND γ 2 | |
| US3592925A (en) | Antibiotics ah272alpha2 and ah272beta2 and process for producing same | |
| DE2040141C3 (en) | Dipeptides and processes for their preparation | |
| CN110627699A (en) | A kind of polyketide compound pyoluteorin and its preparation method and application | |
| Fleck et al. | Leukaemomycin, an antibiotic with antitumor activity. I. Screening, fermentation, and biological activity | |
| DE3586469T2 (en) | CRISAMICIN ANTIBIOTIC, ITS PRODUCTION AND USE AND A MICROORGANISM THAT CAN PRODUCE THIS. | |
| CN116283727A (en) | A kind of chaetoglobosin compound and its preparation method and application | |
| US4159321A (en) | Agent for inhibiting tumor induced by leukemia virus | |
| CN110407797B (en) | Secalonic acid K derived from Penicillium oxalicum and its preparation method | |
| EP0026485B1 (en) | Herbicoline, process for its preparation and compositions containing it | |
| CN110407794A (en) | Ryeonic acid K derived from Penicillium oxalicum and its application in inhibiting the proliferation of cancer cells | |
| JPS5946597B2 (en) | New antibiotic SF-1130-X1 substance, its manufacturing method and immunostimulant | |
| JPS6020000B2 (en) | New antibiotic Y-16482 substance and its manufacturing method | |
| US3592926A (en) | Antifungals bk217beta and bk217upsilon and process for producing same | |
| Ballio et al. | A nitrosophenol cobalt chelate produced by a streptomycete |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 19770913 |
|
| PG1605 | Publication of application before grant of patent |
Comment text: Decision on Publication of Application Patent event code: PG16051S01I Patent event date: 19810430 |
|
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 19810811 |