KR20180098325A - 친수성 의료 장치 - Google Patents
친수성 의료 장치 Download PDFInfo
- Publication number
- KR20180098325A KR20180098325A KR1020187021078A KR20187021078A KR20180098325A KR 20180098325 A KR20180098325 A KR 20180098325A KR 1020187021078 A KR1020187021078 A KR 1020187021078A KR 20187021078 A KR20187021078 A KR 20187021078A KR 20180098325 A KR20180098325 A KR 20180098325A
- Authority
- KR
- South Korea
- Prior art keywords
- polymer
- hydrophilic
- substrate
- medical device
- layer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
- 239000010410 layer Substances 0.000 claims abstract description 84
- 239000000758 substrate Substances 0.000 claims abstract description 74
- 238000009736 wetting Methods 0.000 claims abstract description 63
- 230000005660 hydrophilic surface Effects 0.000 claims abstract description 60
- 229920001477 hydrophilic polymer Polymers 0.000 claims abstract description 58
- 239000002344 surface layer Substances 0.000 claims abstract description 56
- 229920005601 base polymer Polymers 0.000 claims abstract description 35
- 239000012530 fluid Substances 0.000 claims abstract description 29
- 238000000034 method Methods 0.000 claims abstract description 25
- 229920002959 polymer blend Polymers 0.000 claims abstract description 23
- 238000004519 manufacturing process Methods 0.000 claims abstract description 12
- 239000000463 material Substances 0.000 claims description 59
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 51
- 239000000203 mixture Substances 0.000 claims description 28
- 239000004814 polyurethane Substances 0.000 claims description 28
- 229920000098 polyolefin Polymers 0.000 claims description 21
- 239000007788 liquid Substances 0.000 claims description 17
- 230000002485 urinary effect Effects 0.000 claims description 17
- 229920000642 polymer Polymers 0.000 claims description 16
- 229920001577 copolymer Polymers 0.000 claims description 13
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 13
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 13
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 claims description 12
- 239000000945 filler Substances 0.000 claims description 12
- 229920002635 polyurethane Polymers 0.000 claims description 11
- -1 vinyl compound Chemical class 0.000 claims description 11
- 150000001875 compounds Chemical class 0.000 claims description 8
- 239000004698 Polyethylene Substances 0.000 claims description 7
- 230000001954 sterilising effect Effects 0.000 claims description 7
- 238000004659 sterilization and disinfection Methods 0.000 claims description 7
- 230000007704 transition Effects 0.000 claims description 7
- 239000004433 Thermoplastic polyurethane Substances 0.000 claims description 6
- 229910000019 calcium carbonate Inorganic materials 0.000 claims description 6
- 150000004676 glycans Chemical class 0.000 claims description 6
- 229920001282 polysaccharide Polymers 0.000 claims description 6
- 239000005017 polysaccharide Substances 0.000 claims description 6
- 229920002803 thermoplastic polyurethane Polymers 0.000 claims description 6
- VLCAYQIMSMPEBW-UHFFFAOYSA-N methyl 3-hydroxy-2-methylidenebutanoate Chemical compound COC(=O)C(=C)C(C)O VLCAYQIMSMPEBW-UHFFFAOYSA-N 0.000 claims description 4
- 229920002554 vinyl polymer Polymers 0.000 claims description 4
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 claims description 3
- 229920002134 Carboxymethyl cellulose Polymers 0.000 claims description 3
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 claims description 3
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 3
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 claims description 3
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 3
- 229920002125 Sokalan® Polymers 0.000 claims description 3
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 claims description 3
- 150000005215 alkyl ethers Chemical class 0.000 claims description 3
- 150000008064 anhydrides Chemical class 0.000 claims description 3
- 239000001768 carboxy methyl cellulose Substances 0.000 claims description 3
- 235000010948 carboxy methyl cellulose Nutrition 0.000 claims description 3
- 239000008112 carboxymethyl-cellulose Substances 0.000 claims description 3
- 229920000669 heparin Polymers 0.000 claims description 3
- 229960002897 heparin Drugs 0.000 claims description 3
- 229920001600 hydrophobic polymer Polymers 0.000 claims description 3
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 3
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 3
- 229940071676 hydroxypropylcellulose Drugs 0.000 claims description 3
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 claims description 3
- 229920000609 methyl cellulose Polymers 0.000 claims description 3
- 239000001923 methylcellulose Substances 0.000 claims description 3
- 235000010981 methylcellulose Nutrition 0.000 claims description 3
- 229960002900 methylcellulose Drugs 0.000 claims description 3
- 229920000058 polyacrylate Polymers 0.000 claims description 3
- 229920000573 polyethylene Polymers 0.000 claims description 3
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 3
- 229940068984 polyvinyl alcohol Drugs 0.000 claims description 3
- 235000019422 polyvinyl alcohol Nutrition 0.000 claims description 3
- 229920002307 Dextran Polymers 0.000 claims description 2
- 229960002086 dextran Drugs 0.000 claims description 2
- 230000002401 inhibitory effect Effects 0.000 claims description 2
- 230000001678 irradiating effect Effects 0.000 claims description 2
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 claims description 2
- 229920003229 poly(methyl methacrylate) Polymers 0.000 claims description 2
- 239000000230 xanthan gum Substances 0.000 claims description 2
- 229920001285 xanthan gum Polymers 0.000 claims description 2
- 235000010493 xanthan gum Nutrition 0.000 claims description 2
- 229940082509 xanthan gum Drugs 0.000 claims description 2
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims 2
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims 1
- 239000004926 polymethyl methacrylate Substances 0.000 claims 1
- 238000000576 coating method Methods 0.000 description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 19
- 238000002156 mixing Methods 0.000 description 13
- 150000002431 hydrogen Chemical class 0.000 description 12
- 230000005855 radiation Effects 0.000 description 12
- 238000002474 experimental method Methods 0.000 description 10
- 239000011248 coating agent Substances 0.000 description 9
- 239000001257 hydrogen Substances 0.000 description 8
- 229910052739 hydrogen Inorganic materials 0.000 description 8
- 239000007789 gas Substances 0.000 description 7
- 238000002386 leaching Methods 0.000 description 7
- 238000001125 extrusion Methods 0.000 description 6
- 238000000465 moulding Methods 0.000 description 6
- 230000008961 swelling Effects 0.000 description 6
- 238000004132 cross linking Methods 0.000 description 5
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 150000001408 amides Chemical class 0.000 description 4
- 150000001412 amines Chemical class 0.000 description 4
- 239000010408 film Substances 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 229920006132 styrene block copolymer Polymers 0.000 description 4
- 150000003673 urethanes Chemical class 0.000 description 4
- 210000003708 urethra Anatomy 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 238000001000 micrograph Methods 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- 238000003860 storage Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 229920003317 Fusabond® Polymers 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- 150000001298 alcohols Chemical class 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 239000004295 calcium sulphite Substances 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 238000001746 injection moulding Methods 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 238000005498 polishing Methods 0.000 description 2
- 229920001707 polybutylene terephthalate Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 239000002861 polymer material Substances 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- RYECOJGRJDOGPP-UHFFFAOYSA-N Ethylurea Chemical compound CCNC(N)=O RYECOJGRJDOGPP-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 229920011250 Polypropylene Block Copolymer Polymers 0.000 description 1
- 229920001328 Polyvinylidene chloride Polymers 0.000 description 1
- 229920003082 Povidone K 90 Polymers 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 231100000987 absorbed dose Toxicity 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000002399 angioplasty Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 239000012267 brine Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 239000000819 hypertonic solution Substances 0.000 description 1
- 229940021223 hypertonic solution Drugs 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 230000005865 ionizing radiation Effects 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000011104 metalized film Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- TWNQGVIAIRXVLR-UHFFFAOYSA-N oxo(oxoalumanyloxy)alumane Chemical compound O=[Al]O[Al]=O TWNQGVIAIRXVLR-UHFFFAOYSA-N 0.000 description 1
- YWAKXRMUMFPDSH-UHFFFAOYSA-N pentene Chemical compound CCCC=C YWAKXRMUMFPDSH-UHFFFAOYSA-N 0.000 description 1
- 229940021222 peritoneal dialysis isotonic solution Drugs 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 238000007781 pre-processing Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000009877 rendering Methods 0.000 description 1
- 229910052814 silicon oxide Inorganic materials 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 229920002397 thermoplastic olefin Polymers 0.000 description 1
- 238000001269 time-of-flight mass spectrometry Methods 0.000 description 1
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/08—Materials for coatings
- A61L29/085—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/02—Inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/14—Materials characterised by their function or physical properties, e.g. lubricating compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0009—Making of catheters or other medical or surgical tubes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0017—Catheters; Hollow probes specially adapted for long-term hygiene care, e.g. urethral or indwelling catheters to prevent infections
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2400/00—Materials characterised by their function or physical properties
- A61L2400/10—Materials for lubricating medical devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2420/00—Materials or methods for coatings medical devices
- A61L2420/02—Methods for coating medical devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2420/00—Materials or methods for coatings medical devices
- A61L2420/06—Coatings containing a mixture of two or more compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M2025/0062—Catheters; Hollow probes characterised by structural features having features to improve the sliding of one part within another by using lubricants or surfaces with low friction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0222—Materials for reducing friction
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B05—SPRAYING OR ATOMISING IN GENERAL; APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D—PROCESSES FOR APPLYING FLUENT MATERIALS TO SURFACES, IN GENERAL
- B05D5/00—Processes for applying liquids or other fluent materials to surfaces to obtain special surface effects, finishes or structures
- B05D5/08—Processes for applying liquids or other fluent materials to surfaces to obtain special surface effects, finishes or structures to obtain an anti-friction or anti-adhesive surface
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Hematology (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Anesthesiology (AREA)
- Pulmonology (AREA)
- Biophysics (AREA)
- Urology & Nephrology (AREA)
- Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Materials For Medical Uses (AREA)
Abstract
Description
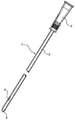
도 1은 본 발명에 따른 카테터의 실시예를 도시한다.
도 2는 본 발명의 실시예에 따른 예시적인 카테터 어셈블리의 단면도이다.
도 3은 본 발명의 다른 실시예에 따른 예시적인 카테터 어셈블리의 단면도이다.
| 샘플 | 베이스 폴리머 | 베이스 폴리머 양(g) | PEO 양(g) | T(℃) | 혼합 시간 (mts) |
혼합 속도(rpm) |
| A1 | T93 | 5 | 10 | 140 | 2 | 20 |
| A2 | T93 | 8 | 8 | 140 | 1.5 | 30 |
| A3 | PU | 5 | 10 | 140 | 2 | 30 |
| A4 | PU | 8 | 8 | 140 | 4 | 40 |
| A5 | T60 | 8 | 8 | 140 | 2 | 40 |
| A6 | PB | 10 | 5 | 170 | 4 | 50 |
| A7 | PB | 12 | 4 | 160 | 4 | 50 |
| A8 | PU | 10 | 5 | 140 | 1.5 | 50 |
| A9 | PU | 8 | 8 | 120 | 1.5 | 50 |
| 비 습윤시 치수(mm) | 비 습윤시 중량(g) | 침출 시간(mts) | 샘플 내의 PEO 함량(wt%) | 베이스 폴리머 | 물 내의 PEO 농도(g/l) |
| 5x10x1.7 | 0.097 | 60 | 40 | T93 | 0.74 |
| 5x10x0.8 | 0.054 | 3 | 75 | PU | 0 |
| 6x10x1 | 0.061 | 3 | 50 | T93 | 0 |
| 5x10x1.5 | 0.11 | 3 | 75 | PU | 0 |
| 5x10x1.5 | 0.11 | 60 | 75 | PU | 2.51 |
| 5x10x1.5 | 0.09 | 30 | 75 | PU | 1.10 |
| 5x10x1 | 0.064 | 30 | 50 | T93 | 0.40 |
| 5x10x1.2 | 0.074 | 3 | 50 | PU | 0.10 |
| 5x10x1.2 | 0.077 | 30 | 50 | PU | 0.47 |
| 5x10x1.2 | 0.072 | 60 | 50 | PU | 1.05 |
| 5x10x1.2 | 0.061 | 60 | 50 | PU | 0.82 |
| 5x10x1 | 0.03 | 3 | 50 | PU | 0.04 |
| 5x10x1 | 0.049 | 30 | 50 | PU | 0.38 |
| 샘플 | PU 양(wt%) |
T93 양(wt%) |
PEO 양(wt%) | T(℃) | 혼합 시간 (mts) |
혼합 속도(rpm) |
| B1 | 13 | 87 | 120 | 2 | 70 | |
| B2 | 33 | 66 | 120 | 2 | 70 | |
| B3 | 50 | 50 | 120 | 2 | 70 | |
| B4 | 66 | 33 | 120 | 2 | 70 | |
| B5 | 25 | 75 | 120 | 2 | 70 | |
| B6 | 33 | 66 | 120 | 2 | 70 |
| 샘플 | 팽윤(%) | 습윤(%) |
| B1 | 158 | 100 |
| B2 | 144 | 76 |
| B3 | 147 | 63 |
| B4 | 129 | 60 |
| B5 | 243 | 82 |
| B6 | N/A(경계가 확산되어 측정을 가능하게 함) | 63 |
| 샘플 | 마찰 (1회) |
마찰 (5회) |
마찰 (10회) |
| B3 | 0.083 | 0.133 | 0.166 |
| B4 | 0.093 | N/A | N/A |
| B5 | 0.098 | 0.096 | 0.100 |
| B3-단단한 | 0.091 | 0.090 | 0.091 |
| 샘플 | 베이스 폴리머 PE(wt%) | PEO 양(wt%) | 상용시약 양(wt%) | 탄산칼슘 양 (wt%) |
T(℃) | 혼합 시간 (mts) |
혼합 속도(rpm) |
| C1 | 84 | 10 | 6 | 190 | 2 | 20 | |
| C2 | 78 | 10 | 6 | 6 | 190 | 2 | 20 |
| C3 | 40 | 48 | 12 | 180 | 2 | 20 |
| 샘플 | 베이스 폴리머 PU 양(wt%) | PEO 양(wt%) | PVP 양 (wt%) |
탄산칼슘 양 (wt%) |
T(℃) | 혼합 시간 (mts) |
혼합 속도(rpm) |
| D1 | 60 | 20 | 20 | 140 | 1 | 30 | |
| D2 | 80 | 20 | 150 | 5 | 50 | ||
| D3 | 60 | 20 | 20 | 150 | 5 | 50 |
Claims (18)
- 표면을 구비하되, 습윤 유체에 의해 습윤 시 상기 표면의 적어도 일 부분에 의료 장치의 저-마찰 표면 특성을 제공하는 친수성 표면층을 갖는 기판을 포함하는 의료 장치로,
상기 친수성 표면층을 포함하는 기판의 적어도 하나의 베이스층은 적어도 하나의 베이스 폴리머 및 적어도 하나의 친수성 폴리머를 포함하는 폴리머 블렌드로 제조되고, 상기 적어도 하나의 친수성 폴리머의 농도는 상기 베이스층의 나머지 부분보다 상기 친수성 표면층에서 더 높은 것을 특징으로 하는 의료 장치. - 제1항에 있어서,
상기 친수성 표면과 베이스층의 나머지 부분 사이에 전이층이 있으며, 상기 전이층은 베이스층의 나머지 부분으로부터 친수성 표면 쪽으로 친수성 폴리머의 농도의 점진적인 증가를 제공하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 친수성 표면층의 재료 조성은 건조 상태에서 적어도 70wt%, 바람직하게는 적어도 80wt%, 더욱 바람직하게는 적어도 90wt%, 더욱 바람직하게는 적어도 95wt%, 가장 바람직하게는 적어도 99wt%의 적어도 하나의 폴리머를 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 베이스층의 내부의 폴리머 블렌드는 20-90wt%, 바람직하게는 25-75wt%, 가장 바람직하게는 25-50wt%의 적어도 하나의 친수성 폴리머를 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 베이스층의 내부의 폴리머 블렌드는 20-80wt%, 바람직하게는 25-75wt%, 가장 바람직하게는 50-75wt%의 적어도 하나의 베이스 폴리머를 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
적어도 하나의 친수성 폴리머는, 폴리비닐 화합물, 다당류, 폴리우레탄, 폴리아크릴레이트 또는 비닐 화합물과 아크릴레이트 또는 무수물의 공중합체, 폴리(알킬 에테르), 즉, 폴리옥시에틸렌, 특히, 폴리에틸렌 옥사이드와 같은 폴리에틸렌 옥사이드 또는 폴리프로필렌 옥사이드, 폴리비닐-피롤리돈, 헤파린, 덱스트란, 잔탄검, 폴리비닐 알코올, 하이드록시 프로필 셀룰로오스, 메틸 셀룰로오스, 비닐피롤리돈과 하이드록시 에틸메틸 아크릴레이트의 공중합체 또는 폴리메틸비닐 에테르와 말레 산 무수물의 공중합체, 및 이들의 공중합체로부터 선택되는 적어도 하나의 재료를 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 친수성 폴리머는 폴리에틸렌 옥사이드, 폴리(아크릴 산), 폴리비닐-피롤리돈 및 카르복시 메틸 셀룰로오스와 같은 폴리사카라이드 중 적어도 하나를 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 친수성 폴리머(들)는 적어도 50kDa, 바람직하게는 적어도 100kDa, 가장 바람직하게는 적어도 200kDa의 분자량을 갖는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 적어도 하나의 베이스 폴리머는 우세한 소수성 폴리머인 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 적어도 하나의 베이스 폴리머는 폴리올레핀 및 열가소성 폴리우레탄과 같은 폴리우레탄 중 적어도 하나를 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 의료 장치는 카테터이며, 바람직하게는 요로 카테터이고, 상기 친수성 표면층은 적어도 삽입 가능한 부분에 제공되는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 기판은 탄산 칼슘 필러와 같은 필러 재료를 또한 포함하는 것을 특징으로 하는 의료 장치. - 선행하는 청구항들 중 어느 한 항에 있어서,
상기 베이스층의 내부는 적어도 2개의 상이한 서브-층을 포함하며, 상기 친수성 표면층에 근접한 외부 서브-층은 상기 친수성 표면층에서 멀리 있는 내부 서브-층보다 높은 농도의 친수성 폴리머(들)를 갖는 것을 특징으로 하는 의료 장치. - 제13항에 있어서,
상기 적어도 2개의 상이한 서브-층들은 함께 공압출되는 것을 특징으로 하는 의료 장치. - 의료 장치, 바람직하게는 요로 카테터를 제조하는 방법으로,
적어도 하나의 베이스 폴리머 및 적어도 하나의 친수성 폴리머를 포함하는 폴리머 블렌드를 준비하는 단계;
상기 폴리머 블렌드로 제조된 적어도 하나의 층을 포함하는 기판을 형성하는 단계;
상기 기판을 습윤 액체에 배치하여, 적어도 하나의 친수성 폴리머가 상기 기판의 표면을 향해 이동하는 단계; 및
상기 기판을 조사하여 상기 폴리머들 사이에 가교 결합을 형성하고, 친수성 폴리머(들)의 추가 이동을 금지시킴으로써, 상기 폴리머 블렌드보다 높은 농도의 친수성 폴리머(들)를 포함하는 안정한 친수성 표면층을 상기 기판의 표면 상에 형성하는 단계를 포함하는 것을 특징으로 하는 제조 방법. - 제15항에 있어서,
습윤 액체에 상기 기판을 배치하는 단계는 습윤 액체와 함께 용기 내에 의료 장치를 배치하는 단계 및 용기를 폐쇄하는 단계를 포함하고, 후속하는 조사 단계는 의료 장치의 살균에도 영향을 미치는 것을 특징으로 하는 제조 방법. - 제15항 또는 제16항에 있어서,
상기 친수성 표면층의 특성을 제어하기 위해 습윤 유체 내에 상기 의료 장치의 배치 및 상기 조사 사이의 대기 시간이 제어되며, 상기 대기 시간은 바람직하게는 1분 내지 24시간의 범위, 더욱 바람직하게는 2분 내지 4시간의 범위, 가장 바람직하게는 3-180분의 범위 내에 있도록 제어되는 것을 특징으로 하는 제조 방법. - 제1항 내지 제14항 중 어느 한 항에 따른 의료 장치, 용기 및 습윤 유체를 포함하는 의료 장치 어셈블리로,
상기 용기는 습윤 유체와 함께 의료 장치를 적어도 일부, 바람직하게는 전체를 수용하여서, 적어도 일부 및 바람직하게는 전체 친수성 표면층이 습윤 상태로 유지되는 것을 특징으로 하는 의료 장치 어셈블리.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15202776.9A EP3187199B1 (en) | 2015-12-28 | 2015-12-28 | Hydrophilic medical device |
| EP15202776.9 | 2015-12-28 | ||
| PCT/EP2016/082410 WO2017114753A1 (en) | 2015-12-28 | 2016-12-22 | Hydrophilic medical device |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20180098325A true KR20180098325A (ko) | 2018-09-03 |
| KR102758594B1 KR102758594B1 (ko) | 2025-01-22 |
Family
ID=55066410
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187021078A Active KR102758594B1 (ko) | 2015-12-28 | 2016-12-22 | 친수성 의료 장치 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US11724008B2 (ko) |
| EP (2) | EP3693032B1 (ko) |
| JP (1) | JP6938508B2 (ko) |
| KR (1) | KR102758594B1 (ko) |
| CN (1) | CN108430528B (ko) |
| AU (1) | AU2016382838A1 (ko) |
| BR (1) | BR112018012806B1 (ko) |
| DK (2) | DK3693032T3 (ko) |
| HU (1) | HUE049479T2 (ko) |
| WO (1) | WO2017114753A1 (ko) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA3084050C (en) | 2017-12-05 | 2025-05-13 | Hollister Incorporated | Urinary catheters with stable mechanical properties over a range of temperatures |
| LT4176908T (lt) | 2018-05-17 | 2024-08-26 | Hollister Incorporated | Būdai gaminti hidrofilinių kateterių su rankovėmis rinkinius |
| US12351773B2 (en) | 2019-01-15 | 2025-07-08 | Avient Corporation | Lubricious thermoplastic compounds and thermoplastic articles made therefrom |
| KR102880227B1 (ko) * | 2019-02-08 | 2025-11-03 | 컬러플라스트 에이/에스 | 도뇨관 |
| EP4628107A3 (en) * | 2019-06-25 | 2025-12-17 | Hollister Incorporated | Reusable urinary catheter products |
| CN110124185A (zh) * | 2019-06-25 | 2019-08-16 | 华中科技大学同济医学院附属协和医院 | 一种带有可塑性支架的导尿管及用于该支架的消毒装置 |
| US12053593B2 (en) | 2020-04-24 | 2024-08-06 | Convatec Limited | Wetting mechanism for a catheter |
| GB202006055D0 (en) | 2020-04-24 | 2020-06-10 | Convatec Ltd | A wetting mechanism for a catheter |
| GB202006059D0 (en) | 2020-04-24 | 2020-06-10 | Convatec Ltd | A wetting mechanism for a catheter |
| AU2021265834B2 (en) | 2020-05-01 | 2025-07-17 | Hollister Incorporated | Medical device products including liquid-drawing members |
| AU2021373614A1 (en) * | 2020-11-09 | 2023-06-22 | Carefusion 303, Inc. | Multilayer medical tubing with low sorbability |
| US20220160970A1 (en) * | 2020-11-24 | 2022-05-26 | Carefusion 303, Inc. | Medical devices for anti-bubble medication delivery |
| DK181278B1 (en) * | 2021-05-03 | 2023-06-20 | Jonsman Innovation Aps | Moldable, hydrophilic polymer blend for molding devices which are hydrophilic and lubricious after molding |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5041100A (en) | 1989-04-28 | 1991-08-20 | Cordis Corporation | Catheter and hydrophilic, friction-reducing coating thereon |
| US20070184275A1 (en) * | 2006-02-01 | 2007-08-09 | Hollister Incorporated | Methods of applying a hydrophilic coating to a substrate, and substrates having a hydrophilic coating |
| JP2009501608A (ja) * | 2005-07-18 | 2009-01-22 | アストラ・テック・アクチエボラーグ | 尿カテーテル |
| US20100159116A1 (en) * | 2008-12-19 | 2010-06-24 | Rindlav-Westling Aasa | Method for producing a medical device with a cross-linked hydrophilic coating |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4642267A (en) * | 1985-05-06 | 1987-02-10 | Hydromer, Inc. | Hydrophilic polymer blend |
| US4781978A (en) * | 1987-03-02 | 1988-11-01 | Minnesota Mining And Manufacturing Company | Articles having a coating formed from a polymeric blend |
| US5061424A (en) * | 1991-01-22 | 1991-10-29 | Becton, Dickinson And Company | Method for applying a lubricious coating to an article |
| US5443907A (en) * | 1991-06-18 | 1995-08-22 | Scimed Life Systems, Inc. | Coating for medical insertion guides |
| US5688855A (en) * | 1995-05-01 | 1997-11-18 | S.K.Y. Polymers, Inc. | Thin film hydrophilic coatings |
| SE9900465D0 (sv) | 1999-02-12 | 1999-02-12 | Astra Ab | Storage package |
| SE0201330D0 (sv) | 2002-04-30 | 2002-04-30 | Astra Tech Ab | Catheter assembly |
| US7628784B2 (en) * | 2003-01-06 | 2009-12-08 | C. R. Bard, Inc. | Balloon catheter with improved resistance to non-deflation |
| US20060240060A1 (en) | 2005-04-22 | 2006-10-26 | Cardiac Pacemakers, Inc. | Lubricious compound and medical device made of the same |
| US20120077049A1 (en) * | 2007-08-06 | 2012-03-29 | Abbott Cardiovascular Systems, Inc. | Medical devices having a lubricious coating with a hydrophilic compound in an interlocking network |
| US20090155519A1 (en) * | 2007-12-12 | 2009-06-18 | Emembrane Inc. | Hydrophilic Coating Of polymeric Substrates |
| DK2301595T3 (da) * | 2009-09-23 | 2014-04-07 | Dentsply Ih Ab | Gennemskylleligt kateter og fremgangsmåde til fremstilling af sådan et kateter |
| EP2941279B1 (en) * | 2013-01-04 | 2023-05-17 | SurModics, Inc. | Low particulate lubricious coating with vinyl pyrrolidone and acidic polymer-containing layers |
| LT3283136T (lt) * | 2015-04-16 | 2021-06-25 | Hollister Incorporated | Hidrofilinės dangos ir jų sudarymo būdai |
-
2015
- 2015-12-28 DK DK20166707.8T patent/DK3693032T3/da active
- 2015-12-28 EP EP20166707.8A patent/EP3693032B1/en active Active
- 2015-12-28 HU HUE15202776A patent/HUE049479T2/hu unknown
- 2015-12-28 EP EP15202776.9A patent/EP3187199B1/en not_active Not-in-force
- 2015-12-28 DK DK15202776.9T patent/DK3187199T3/da active
-
2016
- 2016-12-22 US US16/336,061 patent/US11724008B2/en active Active
- 2016-12-22 BR BR112018012806-6A patent/BR112018012806B1/pt not_active IP Right Cessation
- 2016-12-22 KR KR1020187021078A patent/KR102758594B1/ko active Active
- 2016-12-22 JP JP2018533063A patent/JP6938508B2/ja active Active
- 2016-12-22 CN CN201680075227.XA patent/CN108430528B/zh not_active Expired - Fee Related
- 2016-12-22 AU AU2016382838A patent/AU2016382838A1/en not_active Abandoned
- 2016-12-22 WO PCT/EP2016/082410 patent/WO2017114753A1/en not_active Ceased
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5041100A (en) | 1989-04-28 | 1991-08-20 | Cordis Corporation | Catheter and hydrophilic, friction-reducing coating thereon |
| JP2009501608A (ja) * | 2005-07-18 | 2009-01-22 | アストラ・テック・アクチエボラーグ | 尿カテーテル |
| US20070184275A1 (en) * | 2006-02-01 | 2007-08-09 | Hollister Incorporated | Methods of applying a hydrophilic coating to a substrate, and substrates having a hydrophilic coating |
| JP2009525176A (ja) * | 2006-02-01 | 2009-07-09 | ホリスター・インコーポレイテッド | 親水性コーティングを基材へ塗布する方法、及び親水性コーティングを有する基材 |
| US20100159116A1 (en) * | 2008-12-19 | 2010-06-24 | Rindlav-Westling Aasa | Method for producing a medical device with a cross-linked hydrophilic coating |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102758594B1 (ko) | 2025-01-22 |
| EP3693032A1 (en) | 2020-08-12 |
| DK3693032T3 (da) | 2022-05-16 |
| EP3187199B1 (en) | 2020-04-01 |
| WO2017114753A1 (en) | 2017-07-06 |
| DK3187199T3 (da) | 2020-06-02 |
| BR112018012806B1 (pt) | 2021-07-20 |
| HUE049479T2 (hu) | 2020-09-28 |
| AU2016382838A1 (en) | 2018-06-21 |
| JP2019505277A (ja) | 2019-02-28 |
| CN108430528A (zh) | 2018-08-21 |
| US20190224384A1 (en) | 2019-07-25 |
| JP6938508B2 (ja) | 2021-09-22 |
| BR112018012806A2 (pt) | 2018-12-04 |
| EP3693032B1 (en) | 2022-02-23 |
| CN108430528B (zh) | 2021-06-01 |
| US11724008B2 (en) | 2023-08-15 |
| EP3187199A1 (en) | 2017-07-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102758594B1 (ko) | 친수성 의료 장치 | |
| EP2279767B1 (en) | Urinary catheter | |
| EP3744381B1 (en) | Urinary catheter having a soft tip | |
| EP1131112B2 (en) | A method for sterilising a medical device having a hydrophilic coating | |
| JP5799023B2 (ja) | 迅速放出可能な抗菌剤を有する短時間使用のための医療デバイス | |
| JP2009515604A (ja) | 潤滑化合物及びそれから作られる医療装置 | |
| EP4520358A1 (en) | Hydrophilic urinary catheter | |
| JP2007144062A (ja) | 医療用器具及びその製造方法 | |
| CN117838941A (zh) | 用于医疗器械应用的离子化合物 | |
| HK1132940A (en) | A method for sterilising a medical device having a hydrophilic coating | |
| HK1123510A (en) | A method for sterilising a medical device having a hydrophilic coating |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180720 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20211217 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20240522 Patent event code: PE09021S01D |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20250108 |
|
| PG1601 | Publication of registration |