JP7547040B2 - Fine cellulose fiber and resin composite - Google Patents
Fine cellulose fiber and resin composite Download PDFInfo
- Publication number
- JP7547040B2 JP7547040B2 JP2019191926A JP2019191926A JP7547040B2 JP 7547040 B2 JP7547040 B2 JP 7547040B2 JP 2019191926 A JP2019191926 A JP 2019191926A JP 2019191926 A JP2019191926 A JP 2019191926A JP 7547040 B2 JP7547040 B2 JP 7547040B2
- Authority
- JP
- Japan
- Prior art keywords
- fine cellulose
- cellulose fibers
- resin
- mass
- acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 229920003043 Cellulose fiber Polymers 0.000 title claims description 261
- 239000000805 composite resin Substances 0.000 title claims description 59
- 229920005989 resin Polymers 0.000 claims description 119
- 239000011347 resin Substances 0.000 claims description 119
- 229920002678 cellulose Polymers 0.000 claims description 107
- 239000001913 cellulose Substances 0.000 claims description 107
- 238000000034 method Methods 0.000 claims description 95
- 239000006185 dispersion Substances 0.000 claims description 66
- 239000002994 raw material Substances 0.000 claims description 47
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 46
- 239000002002 slurry Substances 0.000 claims description 43
- 239000000835 fiber Substances 0.000 claims description 37
- 238000004519 manufacturing process Methods 0.000 claims description 28
- 238000012360 testing method Methods 0.000 claims description 25
- 229920001282 polysaccharide Polymers 0.000 claims description 21
- 239000005017 polysaccharide Substances 0.000 claims description 21
- 238000003756 stirring Methods 0.000 claims description 17
- 230000008569 process Effects 0.000 claims description 14
- 238000001035 drying Methods 0.000 claims description 13
- 238000002156 mixing Methods 0.000 claims description 13
- 239000007788 liquid Substances 0.000 claims description 10
- 238000007385 chemical modification Methods 0.000 claims description 6
- 230000010933 acylation Effects 0.000 claims description 3
- 238000005917 acylation reaction Methods 0.000 claims description 3
- 150000004676 glycans Chemical class 0.000 claims 2
- -1 cupra Polymers 0.000 description 63
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 40
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 30
- 239000002904 solvent Substances 0.000 description 29
- 229920005992 thermoplastic resin Polymers 0.000 description 24
- 239000007787 solid Substances 0.000 description 20
- 150000004804 polysaccharides Chemical class 0.000 description 19
- 239000003822 epoxy resin Substances 0.000 description 18
- 229920000647 polyepoxide Polymers 0.000 description 18
- 239000000047 product Substances 0.000 description 18
- 229920001971 elastomer Polymers 0.000 description 17
- 238000005259 measurement Methods 0.000 description 16
- 239000000203 mixture Substances 0.000 description 16
- 238000006116 polymerization reaction Methods 0.000 description 16
- 239000002253 acid Substances 0.000 description 15
- 229920002647 polyamide Polymers 0.000 description 15
- 239000004952 Polyamide Substances 0.000 description 14
- 125000004432 carbon atom Chemical group C* 0.000 description 14
- 239000004743 Polypropylene Substances 0.000 description 13
- 150000001875 compounds Chemical class 0.000 description 13
- 229920001577 copolymer Polymers 0.000 description 13
- DKGAVHZHDRPRBM-UHFFFAOYSA-N Tert-Butanol Chemical compound CC(C)(C)O DKGAVHZHDRPRBM-UHFFFAOYSA-N 0.000 description 12
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 12
- 239000003795 chemical substances by application Substances 0.000 description 12
- 238000000465 moulding Methods 0.000 description 12
- 229920001155 polypropylene Polymers 0.000 description 12
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 11
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 11
- 239000008188 pellet Substances 0.000 description 11
- 230000000704 physical effect Effects 0.000 description 11
- 238000012545 processing Methods 0.000 description 11
- 239000005060 rubber Substances 0.000 description 11
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Chemical compound CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 10
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 10
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 10
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 10
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 10
- 229920003049 isoprene rubber Polymers 0.000 description 10
- 239000000178 monomer Substances 0.000 description 10
- 230000035699 permeability Effects 0.000 description 10
- 229920006122 polyamide resin Polymers 0.000 description 10
- 229920005672 polyolefin resin Polymers 0.000 description 10
- 239000002023 wood Substances 0.000 description 10
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- 229920002292 Nylon 6 Polymers 0.000 description 9
- 239000000010 aprotic solvent Substances 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 239000013078 crystal Substances 0.000 description 9
- 239000002270 dispersing agent Substances 0.000 description 9
- 239000000463 material Substances 0.000 description 9
- 238000000691 measurement method Methods 0.000 description 9
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 8
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 8
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 8
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 8
- 238000005520 cutting process Methods 0.000 description 8
- 230000000694 effects Effects 0.000 description 8
- 238000011156 evaluation Methods 0.000 description 8
- 238000004062 sedimentation Methods 0.000 description 8
- 238000000926 separation method Methods 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 229920000742 Cotton Polymers 0.000 description 7
- 229920002488 Hemicellulose Polymers 0.000 description 7
- 238000010521 absorption reaction Methods 0.000 description 7
- 125000003118 aryl group Chemical group 0.000 description 7
- 238000001125 extrusion Methods 0.000 description 7
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 7
- 230000007062 hydrolysis Effects 0.000 description 7
- 238000006460 hydrolysis reaction Methods 0.000 description 7
- 238000002844 melting Methods 0.000 description 7
- 230000008018 melting Effects 0.000 description 7
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 239000004094 surface-active agent Substances 0.000 description 7
- 229920001187 thermosetting polymer Polymers 0.000 description 7
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 6
- 244000043261 Hevea brasiliensis Species 0.000 description 6
- OFOBLEOULBTSOW-UHFFFAOYSA-N Malonic acid Chemical compound OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 6
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 6
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 6
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 6
- 238000004061 bleaching Methods 0.000 description 6
- 239000004359 castor oil Substances 0.000 description 6
- 235000019438 castor oil Nutrition 0.000 description 6
- 239000003054 catalyst Substances 0.000 description 6
- 230000000052 comparative effect Effects 0.000 description 6
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 description 6
- 239000000806 elastomer Substances 0.000 description 6
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 6
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 6
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 6
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 6
- 229920005610 lignin Polymers 0.000 description 6
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 229920003052 natural elastomer Polymers 0.000 description 6
- 229920001194 natural rubber Polymers 0.000 description 6
- 229920003986 novolac Polymers 0.000 description 6
- 239000004627 regenerated cellulose Substances 0.000 description 6
- 238000002834 transmittance Methods 0.000 description 6
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 6
- 238000005406 washing Methods 0.000 description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical class OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 5
- 239000002841 Lewis acid Substances 0.000 description 5
- 238000006640 acetylation reaction Methods 0.000 description 5
- 150000008065 acid anhydrides Chemical class 0.000 description 5
- 125000002252 acyl group Chemical group 0.000 description 5
- 239000011324 bead Substances 0.000 description 5
- 238000005119 centrifugation Methods 0.000 description 5
- 238000009837 dry grinding Methods 0.000 description 5
- 150000004820 halides Chemical class 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 230000003287 optical effect Effects 0.000 description 5
- 239000003960 organic solvent Substances 0.000 description 5
- 239000000843 powder Substances 0.000 description 5
- 235000019260 propionic acid Nutrition 0.000 description 5
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 5
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 4
- 239000005711 Benzoic acid Substances 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 239000004593 Epoxy Substances 0.000 description 4
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 4
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical class C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 description 4
- 230000021736 acetylation Effects 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 4
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical compound OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 4
- 125000000217 alkyl group Chemical group 0.000 description 4
- 235000010233 benzoic acid Nutrition 0.000 description 4
- HQABUPZFAYXKJW-UHFFFAOYSA-N butan-1-amine Chemical compound CCCCN HQABUPZFAYXKJW-UHFFFAOYSA-N 0.000 description 4
- 238000001723 curing Methods 0.000 description 4
- NZNMSOFKMUBTKW-UHFFFAOYSA-N cyclohexanecarboxylic acid Chemical compound OC(=O)C1CCCCC1 NZNMSOFKMUBTKW-UHFFFAOYSA-N 0.000 description 4
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 4
- 239000012153 distilled water Substances 0.000 description 4
- 125000001165 hydrophobic group Chemical group 0.000 description 4
- 238000001746 injection moulding Methods 0.000 description 4
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical compound OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 4
- 229920002961 polybutylene succinate Polymers 0.000 description 4
- 239000004631 polybutylene succinate Substances 0.000 description 4
- 229920009537 polybutylene succinate adipate Polymers 0.000 description 4
- 239000004630 polybutylene succinate adipate Substances 0.000 description 4
- 229920001707 polybutylene terephthalate Polymers 0.000 description 4
- 229920001225 polyester resin Polymers 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 238000011160 research Methods 0.000 description 4
- 239000013049 sediment Substances 0.000 description 4
- 239000006228 supernatant Substances 0.000 description 4
- SZHOJFHSIKHZHA-UHFFFAOYSA-N tridecanoic acid Chemical compound CCCCCCCCCCCCC(O)=O SZHOJFHSIKHZHA-UHFFFAOYSA-N 0.000 description 4
- 125000002827 triflate group Chemical group FC(S(=O)(=O)O*)(F)F 0.000 description 4
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 4
- RSWGJHLUYNHPMX-UHFFFAOYSA-N Abietic-Saeure Natural products C12CCC(C(C)C)=CC2=CCC2C1(C)CCCC2(C)C(O)=O RSWGJHLUYNHPMX-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 239000005635 Caprylic acid (CAS 124-07-2) Substances 0.000 description 3
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 239000005639 Lauric acid Substances 0.000 description 3
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 240000000907 Musa textilis Species 0.000 description 3
- 229920002302 Nylon 6,6 Polymers 0.000 description 3
- 235000021314 Palmitic acid Nutrition 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 229920001131 Pulp (paper) Polymers 0.000 description 3
- KHPCPRHQVVSZAH-HUOMCSJISA-N Rosin Natural products O(C/C=C/c1ccccc1)[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 KHPCPRHQVVSZAH-HUOMCSJISA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 235000021355 Stearic acid Nutrition 0.000 description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 125000002723 alicyclic group Chemical group 0.000 description 3
- 125000001931 aliphatic group Chemical group 0.000 description 3
- 125000003342 alkenyl group Chemical group 0.000 description 3
- 229910052782 aluminium Inorganic materials 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 150000008064 anhydrides Chemical class 0.000 description 3
- 238000010009 beating Methods 0.000 description 3
- 238000009835 boiling Methods 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 3
- 150000001735 carboxylic acids Chemical class 0.000 description 3
- 125000002091 cationic group Chemical group 0.000 description 3
- 238000006757 chemical reactions by type Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000010411 cooking Methods 0.000 description 3
- 125000000753 cycloalkyl group Chemical group 0.000 description 3
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 3
- 238000002845 discoloration Methods 0.000 description 3
- 239000002612 dispersion medium Substances 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000002657 fibrous material Substances 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 125000005843 halogen group Chemical group 0.000 description 3
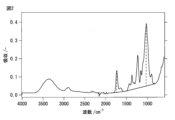
- 238000002329 infrared spectrum Methods 0.000 description 3
- 238000004898 kneading Methods 0.000 description 3
- 150000007517 lewis acids Chemical class 0.000 description 3
- 239000010445 mica Substances 0.000 description 3
- 229910052618 mica group Inorganic materials 0.000 description 3
- 210000001724 microfibril Anatomy 0.000 description 3
- 125000000896 monocarboxylic acid group Chemical group 0.000 description 3
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 3
- 239000002121 nanofiber Substances 0.000 description 3
- 239000002736 nonionic surfactant Substances 0.000 description 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 3
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 3
- 229960002446 octanoic acid Drugs 0.000 description 3
- 229920001568 phenolic resin Polymers 0.000 description 3
- 239000005011 phenolic resin Substances 0.000 description 3
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 3
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 3
- 229920002857 polybutadiene Polymers 0.000 description 3
- 239000004645 polyester resin Substances 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 229920000139 polyethylene terephthalate Polymers 0.000 description 3
- 239000005020 polyethylene terephthalate Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 239000003505 polymerization initiator Substances 0.000 description 3
- 230000000379 polymerizing effect Effects 0.000 description 3
- 229920001451 polypropylene glycol Polymers 0.000 description 3
- 238000010298 pulverizing process Methods 0.000 description 3
- 150000004040 pyrrolidinones Chemical class 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- 239000011342 resin composition Substances 0.000 description 3
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- 239000008117 stearic acid Substances 0.000 description 3
- 229920003048 styrene butadiene rubber Polymers 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- TUNFSRHWOTWDNC-HKGQFRNVSA-N tetradecanoic acid Chemical compound CCCCCCCCCCCCC[14C](O)=O TUNFSRHWOTWDNC-HKGQFRNVSA-N 0.000 description 3
- 238000005979 thermal decomposition reaction Methods 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- KHPCPRHQVVSZAH-UHFFFAOYSA-N trans-cinnamyl beta-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OCC=CC1=CC=CC=C1 KHPCPRHQVVSZAH-UHFFFAOYSA-N 0.000 description 3
- 229940005605 valeric acid Drugs 0.000 description 3
- 229920002554 vinyl polymer Polymers 0.000 description 3
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical group C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 2
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 2
- XQUPVDVFXZDTLT-UHFFFAOYSA-N 1-[4-[[4-(2,5-dioxopyrrol-1-yl)phenyl]methyl]phenyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1C(C=C1)=CC=C1CC1=CC=C(N2C(C=CC2=O)=O)C=C1 XQUPVDVFXZDTLT-UHFFFAOYSA-N 0.000 description 2
- BMVXCPBXGZKUPN-UHFFFAOYSA-N 1-hexanamine Chemical compound CCCCCCN BMVXCPBXGZKUPN-UHFFFAOYSA-N 0.000 description 2
- 238000004922 13C solid-state nuclear magnetic resonance spectroscopy Methods 0.000 description 2
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 2
- UJTRCPVECIHPBG-UHFFFAOYSA-N 3-cyclohexylpyrrole-2,5-dione Chemical class O=C1NC(=O)C(C2CCCCC2)=C1 UJTRCPVECIHPBG-UHFFFAOYSA-N 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- MHZGKXUYDGKKIU-UHFFFAOYSA-N Decylamine Chemical compound CCCCCCCCCCN MHZGKXUYDGKKIU-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 241000219146 Gossypium Species 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- 229920000459 Nitrile rubber Polymers 0.000 description 2
- 229920000571 Nylon 11 Polymers 0.000 description 2
- 229920000299 Nylon 12 Polymers 0.000 description 2
- 229920000572 Nylon 6/12 Polymers 0.000 description 2
- REYJJPSVUYRZGE-UHFFFAOYSA-N Octadecylamine Chemical compound CCCCCCCCCCCCCCCCCCN REYJJPSVUYRZGE-UHFFFAOYSA-N 0.000 description 2
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 2
- 229930182556 Polyacetal Natural products 0.000 description 2
- 239000005062 Polybutadiene Substances 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- WQDUMFSSJAZKTM-UHFFFAOYSA-N Sodium methoxide Chemical compound [Na+].[O-]C WQDUMFSSJAZKTM-UHFFFAOYSA-N 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- KKEYFWRCBNTPAC-UHFFFAOYSA-N Terephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 238000002441 X-ray diffraction Methods 0.000 description 2
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 2
- 238000005903 acid hydrolysis reaction Methods 0.000 description 2
- 235000011037 adipic acid Nutrition 0.000 description 2
- 229960000250 adipic acid Drugs 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000007605 air drying Methods 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 2
- 150000008041 alkali metal carbonates Chemical class 0.000 description 2
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 229910001860 alkaline earth metal hydroxide Inorganic materials 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 150000004703 alkoxides Chemical class 0.000 description 2
- 125000005037 alkyl phenyl group Chemical group 0.000 description 2
- 125000005360 alkyl sulfoxide group Chemical group 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 239000003945 anionic surfactant Substances 0.000 description 2
- 229910052785 arsenic Inorganic materials 0.000 description 2
- 239000010953 base metal Substances 0.000 description 2
- 239000004305 biphenyl Substances 0.000 description 2
- 235000010290 biphenyl Nutrition 0.000 description 2
- 229910052797 bismuth Inorganic materials 0.000 description 2
- PXKLMJQFEQBVLD-UHFFFAOYSA-N bisphenol F Chemical compound C1=CC(O)=CC=C1CC1=CC=C(O)C=C1 PXKLMJQFEQBVLD-UHFFFAOYSA-N 0.000 description 2
- 229920005549 butyl rubber Polymers 0.000 description 2
- 239000006227 byproduct Substances 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 150000001244 carboxylic acid anhydrides Chemical class 0.000 description 2
- 238000005266 casting Methods 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 229910001914 chlorine tetroxide Inorganic materials 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 229930003836 cresol Natural products 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- VZFUCHSFHOYXIS-UHFFFAOYSA-N cycloheptane carboxylic acid Natural products OC(=O)C1CCCCCC1 VZFUCHSFHOYXIS-UHFFFAOYSA-N 0.000 description 2
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- JQVDAXLFBXTEQA-UHFFFAOYSA-N dibutylamine Chemical compound CCCCNCCCC JQVDAXLFBXTEQA-UHFFFAOYSA-N 0.000 description 2
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 2
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical compound C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 125000003700 epoxy group Chemical group 0.000 description 2
- 238000005886 esterification reaction Methods 0.000 description 2
- FJKIXWOMBXYWOQ-UHFFFAOYSA-N ethenoxyethane Chemical compound CCOC=C FJKIXWOMBXYWOQ-UHFFFAOYSA-N 0.000 description 2
- YCUBDDIKWLELPD-UHFFFAOYSA-N ethenyl 2,2-dimethylpropanoate Chemical compound CC(C)(C)C(=O)OC=C YCUBDDIKWLELPD-UHFFFAOYSA-N 0.000 description 2
- MEGHWIAOTJPCHQ-UHFFFAOYSA-N ethenyl butanoate Chemical compound CCCC(=O)OC=C MEGHWIAOTJPCHQ-UHFFFAOYSA-N 0.000 description 2
- UIWXSTHGICQLQT-UHFFFAOYSA-N ethenyl propanoate Chemical compound CCC(=O)OC=C UIWXSTHGICQLQT-UHFFFAOYSA-N 0.000 description 2
- UHESRSKEBRADOO-UHFFFAOYSA-N ethyl carbamate;prop-2-enoic acid Chemical class OC(=O)C=C.CCOC(N)=O UHESRSKEBRADOO-UHFFFAOYSA-N 0.000 description 2
- 238000010097 foam moulding Methods 0.000 description 2
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 2
- 238000005227 gel permeation chromatography Methods 0.000 description 2
- JFCQEDHGNNZCLN-UHFFFAOYSA-N glutaric acid Chemical compound OC(=O)CCCC(O)=O JFCQEDHGNNZCLN-UHFFFAOYSA-N 0.000 description 2
- VOZRXNHHFUQHIL-UHFFFAOYSA-N glycidyl methacrylate Chemical class CC(=C)C(=O)OCC1CO1 VOZRXNHHFUQHIL-UHFFFAOYSA-N 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 229910052738 indium Inorganic materials 0.000 description 2
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 2
- 229910052747 lanthanoid Inorganic materials 0.000 description 2
- 150000002602 lanthanoids Chemical class 0.000 description 2
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 2
- 239000011976 maleic acid Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 239000000155 melt Substances 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- BDJRBEYXGGNYIS-UHFFFAOYSA-N nonanedioic acid Chemical compound OC(=O)CCCCCCCC(O)=O BDJRBEYXGGNYIS-UHFFFAOYSA-N 0.000 description 2
- IOQPZZOEVPZRBK-UHFFFAOYSA-N octan-1-amine Chemical compound CCCCCCCCN IOQPZZOEVPZRBK-UHFFFAOYSA-N 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 150000002894 organic compounds Chemical class 0.000 description 2
- LPNBBFKOUUSUDB-UHFFFAOYSA-N p-toluic acid Chemical compound CC1=CC=C(C(O)=O)C=C1 LPNBBFKOUUSUDB-UHFFFAOYSA-N 0.000 description 2
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Chemical compound [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- WLJVXDMOQOGPHL-UHFFFAOYSA-N phenylacetic acid Chemical compound OC(=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-UHFFFAOYSA-N 0.000 description 2
- WLJVNTCWHIRURA-UHFFFAOYSA-N pimelic acid Chemical compound OC(=O)CCCCCC(O)=O WLJVNTCWHIRURA-UHFFFAOYSA-N 0.000 description 2
- 229920003192 poly(bis maleimide) Polymers 0.000 description 2
- 229920001230 polyarylate Polymers 0.000 description 2
- 239000004629 polybutylene adipate terephthalate Substances 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 239000011112 polyethylene naphthalate Substances 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 229920006324 polyoxymethylene Polymers 0.000 description 2
- 238000012805 post-processing Methods 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 150000003141 primary amines Chemical class 0.000 description 2
- WGYKZJWCGVVSQN-UHFFFAOYSA-N propylamine Chemical compound CCCN WGYKZJWCGVVSQN-UHFFFAOYSA-N 0.000 description 2
- 238000007348 radical reaction Methods 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- 229920003987 resole Polymers 0.000 description 2
- CXMXRPHRNRROMY-UHFFFAOYSA-N sebacic acid Chemical compound OC(=O)CCCCCCCCC(O)=O CXMXRPHRNRROMY-UHFFFAOYSA-N 0.000 description 2
- 238000000371 solid-state nuclear magnetic resonance spectroscopy Methods 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 150000003440 styrenes Chemical class 0.000 description 2
- TYFQFVWCELRYAO-UHFFFAOYSA-N suberic acid Chemical compound OC(=O)CCCCCCC(O)=O TYFQFVWCELRYAO-UHFFFAOYSA-N 0.000 description 2
- 238000010998 test method Methods 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 2
- 239000003021 water soluble solvent Substances 0.000 description 2
- 238000001238 wet grinding Methods 0.000 description 2
- 238000004736 wide-angle X-ray diffraction Methods 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000004711 α-olefin Substances 0.000 description 2
- MUTGBJKUEZFXGO-OLQVQODUSA-N (3as,7ar)-3a,4,5,6,7,7a-hexahydro-2-benzofuran-1,3-dione Chemical compound C1CCC[C@@H]2C(=O)OC(=O)[C@@H]21 MUTGBJKUEZFXGO-OLQVQODUSA-N 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- HCNHNBLSNVSJTJ-UHFFFAOYSA-N 1,1-Bis(4-hydroxyphenyl)ethane Chemical compound C=1C=C(O)C=CC=1C(C)C1=CC=C(O)C=C1 HCNHNBLSNVSJTJ-UHFFFAOYSA-N 0.000 description 1
- ZXHDVRATSGZISC-UHFFFAOYSA-N 1,2-bis(ethenoxy)ethane Chemical compound C=COCCOC=C ZXHDVRATSGZISC-UHFFFAOYSA-N 0.000 description 1
- BPXVHIRIPLPOPT-UHFFFAOYSA-N 1,3,5-tris(2-hydroxyethyl)-1,3,5-triazinane-2,4,6-trione Chemical compound OCCN1C(=O)N(CCO)C(=O)N(CCO)C1=O BPXVHIRIPLPOPT-UHFFFAOYSA-N 0.000 description 1
- MPJPKEMZYOAIRN-UHFFFAOYSA-N 1,3,5-tris(2-methylprop-2-enyl)-1,3,5-triazinane-2,4,6-trione Chemical compound CC(=C)CN1C(=O)N(CC(C)=C)C(=O)N(CC(C)=C)C1=O MPJPKEMZYOAIRN-UHFFFAOYSA-N 0.000 description 1
- OUPZKGBUJRBPGC-UHFFFAOYSA-N 1,3,5-tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione Chemical compound O=C1N(CC2OC2)C(=O)N(CC2OC2)C(=O)N1CC1CO1 OUPZKGBUJRBPGC-UHFFFAOYSA-N 0.000 description 1
- KOMNUTZXSVSERR-UHFFFAOYSA-N 1,3,5-tris(prop-2-enyl)-1,3,5-triazinane-2,4,6-trione Chemical compound C=CCN1C(=O)N(CC=C)C(=O)N(CC=C)C1=O KOMNUTZXSVSERR-UHFFFAOYSA-N 0.000 description 1
- PXGZQGDTEZPERC-UHFFFAOYSA-N 1,4-cyclohexanedicarboxylic acid Chemical compound OC(=O)C1CCC(C(O)=O)CC1 PXGZQGDTEZPERC-UHFFFAOYSA-N 0.000 description 1
- PWGJDPKCLMLPJW-UHFFFAOYSA-N 1,8-diaminooctane Chemical compound NCCCCCCCCN PWGJDPKCLMLPJW-UHFFFAOYSA-N 0.000 description 1
- OVGRCEFMXPHEBL-UHFFFAOYSA-N 1-ethenoxypropane Chemical compound CCCOC=C OVGRCEFMXPHEBL-UHFFFAOYSA-N 0.000 description 1
- MCTWTZJPVLRJOU-UHFFFAOYSA-N 1-methyl-1H-imidazole Chemical compound CN1C=CN=C1 MCTWTZJPVLRJOU-UHFFFAOYSA-N 0.000 description 1
- VTRRCXRVEQTTOE-UHFFFAOYSA-N 1-methylsulfinylethane Chemical compound CCS(C)=O VTRRCXRVEQTTOE-UHFFFAOYSA-N 0.000 description 1
- LNETULKMXZVUST-UHFFFAOYSA-N 1-naphthoic acid Chemical compound C1=CC=C2C(C(=O)O)=CC=CC2=C1 LNETULKMXZVUST-UHFFFAOYSA-N 0.000 description 1
- RUFPHBVGCFYCNW-UHFFFAOYSA-N 1-naphthylamine Chemical compound C1=CC=C2C(N)=CC=CC2=C1 RUFPHBVGCFYCNW-UHFFFAOYSA-N 0.000 description 1
- 238000005160 1H NMR spectroscopy Methods 0.000 description 1
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 1
- WTYYGFLRBWMFRY-UHFFFAOYSA-N 2-[6-(oxiran-2-ylmethoxy)hexoxymethyl]oxirane Chemical compound C1OC1COCCCCCCOCC1CO1 WTYYGFLRBWMFRY-UHFFFAOYSA-N 0.000 description 1
- VUIWJRYTWUGOOF-UHFFFAOYSA-N 2-ethenoxyethanol Chemical compound OCCOC=C VUIWJRYTWUGOOF-UHFFFAOYSA-N 0.000 description 1
- KXGFMDJXCMQABM-UHFFFAOYSA-N 2-methoxy-6-methylphenol Chemical compound [CH]OC1=CC=CC([CH])=C1O KXGFMDJXCMQABM-UHFFFAOYSA-N 0.000 description 1
- OGJZJWVBRMAQBR-UHFFFAOYSA-N 2-methylheptane-1,7-diamine Chemical compound NCC(C)CCCCCN OGJZJWVBRMAQBR-UHFFFAOYSA-N 0.000 description 1
- ZSPDYGICHBLYSD-UHFFFAOYSA-N 2-methylnaphthalene-1-carboxylic acid Chemical compound C1=CC=CC2=C(C(O)=O)C(C)=CC=C21 ZSPDYGICHBLYSD-UHFFFAOYSA-N 0.000 description 1
- GAGWMWLBYJPFDD-UHFFFAOYSA-N 2-methyloctane-1,8-diamine Chemical compound NCC(C)CCCCCCN GAGWMWLBYJPFDD-UHFFFAOYSA-N 0.000 description 1
- JZUHIOJYCPIVLQ-UHFFFAOYSA-N 2-methylpentane-1,5-diamine Chemical compound NCC(C)CCCN JZUHIOJYCPIVLQ-UHFFFAOYSA-N 0.000 description 1
- BSKHPKMHTQYZBB-UHFFFAOYSA-N 2-methylpyridine Chemical compound CC1=CC=CC=N1 BSKHPKMHTQYZBB-UHFFFAOYSA-N 0.000 description 1
- UOBYKYZJUGYBDK-UHFFFAOYSA-N 2-naphthoic acid Chemical compound C1=CC=CC2=CC(C(=O)O)=CC=C21 UOBYKYZJUGYBDK-UHFFFAOYSA-N 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- CMLFRMDBDNHMRA-UHFFFAOYSA-N 2h-1,2-benzoxazine Chemical group C1=CC=C2C=CNOC2=C1 CMLFRMDBDNHMRA-UHFFFAOYSA-N 0.000 description 1
- HQNOODJDSFSURF-UHFFFAOYSA-N 3-(1h-imidazol-2-yl)propan-1-amine Chemical compound NCCCC1=NC=CN1 HQNOODJDSFSURF-UHFFFAOYSA-N 0.000 description 1
- OFNISBHGPNMTMS-UHFFFAOYSA-N 3-methylideneoxolane-2,5-dione Chemical compound C=C1CC(=O)OC1=O OFNISBHGPNMTMS-UHFFFAOYSA-N 0.000 description 1
- VPWNQTHUCYMVMZ-UHFFFAOYSA-N 4,4'-sulfonyldiphenol Chemical compound C1=CC(O)=CC=C1S(=O)(=O)C1=CC=C(O)C=C1 VPWNQTHUCYMVMZ-UHFFFAOYSA-N 0.000 description 1
- HMMBJOWWRLZEMI-UHFFFAOYSA-N 4,5,6,7-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1CCCC2=C1C(=O)OC2=O HMMBJOWWRLZEMI-UHFFFAOYSA-N 0.000 description 1
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 1
- JLBJTVDPSNHSKJ-UHFFFAOYSA-N 4-Methylstyrene Chemical compound CC1=CC=C(C=C)C=C1 JLBJTVDPSNHSKJ-UHFFFAOYSA-N 0.000 description 1
- PVFQHGDIOXNKIC-UHFFFAOYSA-N 4-[2-[3-[2-(4-hydroxyphenyl)propan-2-yl]phenyl]propan-2-yl]phenol Chemical compound C=1C=CC(C(C)(C)C=2C=CC(O)=CC=2)=CC=1C(C)(C)C1=CC=C(O)C=C1 PVFQHGDIOXNKIC-UHFFFAOYSA-N 0.000 description 1
- MWSKJDNQKGCKPA-UHFFFAOYSA-N 6-methyl-3a,4,5,7a-tetrahydro-2-benzofuran-1,3-dione Chemical compound C1CC(C)=CC2C(=O)OC(=O)C12 MWSKJDNQKGCKPA-UHFFFAOYSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 244000198134 Agave sisalana Species 0.000 description 1
- 239000004953 Aliphatic polyamide Substances 0.000 description 1
- 241000609240 Ambelania acida Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 235000017166 Bambusa arundinacea Nutrition 0.000 description 1
- 235000017491 Bambusa tulda Nutrition 0.000 description 1
- 229930185605 Bisphenol Natural products 0.000 description 1
- GIXXQTYGFOHYPT-UHFFFAOYSA-N Bisphenol P Chemical compound C=1C=C(C(C)(C)C=2C=CC(O)=CC=2)C=CC=1C(C)(C)C1=CC=C(O)C=C1 GIXXQTYGFOHYPT-UHFFFAOYSA-N 0.000 description 1
- SDDLEVPIDBLVHC-UHFFFAOYSA-N Bisphenol Z Chemical compound C1=CC(O)=CC=C1C1(C=2C=CC(O)=CC=2)CCCCC1 SDDLEVPIDBLVHC-UHFFFAOYSA-N 0.000 description 1
- 244000025254 Cannabis sativa Species 0.000 description 1
- 235000012766 Cannabis sativa ssp. sativa var. sativa Nutrition 0.000 description 1
- 235000012765 Cannabis sativa ssp. sativa var. spontanea Nutrition 0.000 description 1
- 239000005632 Capric acid (CAS 334-48-5) Substances 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 239000004641 Diallyl-phthalate Substances 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- 229920000875 Dissolving pulp Polymers 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 229920002430 Fibre-reinforced plastic Polymers 0.000 description 1
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 240000000797 Hibiscus cannabinus Species 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- 229920000433 Lyocell Polymers 0.000 description 1
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 1
- 229920000057 Mannan Polymers 0.000 description 1
- 229920000877 Melamine resin Polymers 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- KWYHDKDOAIKMQN-UHFFFAOYSA-N N,N,N',N'-tetramethylethylenediamine Chemical compound CN(C)CCN(C)C KWYHDKDOAIKMQN-UHFFFAOYSA-N 0.000 description 1
- SVYKKECYCPFKGB-UHFFFAOYSA-N N,N-dimethylcyclohexylamine Chemical compound CN(C)C1CCCCC1 SVYKKECYCPFKGB-UHFFFAOYSA-N 0.000 description 1
- SUAKHGWARZSWIH-UHFFFAOYSA-N N,N‐diethylformamide Chemical compound CCN(CC)C=O SUAKHGWARZSWIH-UHFFFAOYSA-N 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- GRSMWKLPSNHDHA-UHFFFAOYSA-N Naphthalic anhydride Chemical compound C1=CC(C(=O)OC2=O)=C3C2=CC=CC3=C1 GRSMWKLPSNHDHA-UHFFFAOYSA-N 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 229920000305 Nylon 6,10 Polymers 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 240000002834 Paulownia tomentosa Species 0.000 description 1
- LGRFSURHDFAFJT-UHFFFAOYSA-N Phthalic anhydride Natural products C1=CC=C2C(=O)OC(=O)C2=C1 LGRFSURHDFAFJT-UHFFFAOYSA-N 0.000 description 1
- 244000082204 Phyllostachys viridis Species 0.000 description 1
- 235000015334 Phyllostachys viridis Nutrition 0.000 description 1
- 239000004642 Polyimide Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 239000004373 Pullulan Substances 0.000 description 1
- 229920001218 Pullulan Polymers 0.000 description 1
- 229920000297 Rayon Polymers 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical compound [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 229910000831 Steel Inorganic materials 0.000 description 1
- 239000002174 Styrene-butadiene Substances 0.000 description 1
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 description 1
- 241000251555 Tunicata Species 0.000 description 1
- 239000004699 Ultra-high molecular weight polyethylene Substances 0.000 description 1
- 229920001807 Urea-formaldehyde Polymers 0.000 description 1
- 229920006311 Urethane elastomer Polymers 0.000 description 1
- 235000019498 Walnut oil Nutrition 0.000 description 1
- FDLQZKYLHJJBHD-UHFFFAOYSA-N [3-(aminomethyl)phenyl]methanamine Chemical compound NCC1=CC=CC(CN)=C1 FDLQZKYLHJJBHD-UHFFFAOYSA-N 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 238000000862 absorption spectrum Methods 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- FXXACINHVKSMDR-UHFFFAOYSA-N acetyl bromide Chemical compound CC(Br)=O FXXACINHVKSMDR-UHFFFAOYSA-N 0.000 description 1
- WETWJCDKMRHUPV-UHFFFAOYSA-N acetyl chloride Chemical compound CC(Cl)=O WETWJCDKMRHUPV-UHFFFAOYSA-N 0.000 description 1
- 239000012346 acetyl chloride Substances 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- LEKJTGQWLAUGQA-UHFFFAOYSA-N acetyl iodide Chemical compound CC(I)=O LEKJTGQWLAUGQA-UHFFFAOYSA-N 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 229920000800 acrylic rubber Polymers 0.000 description 1
- XECAHXYUAAWDEL-UHFFFAOYSA-N acrylonitrile butadiene styrene Chemical compound C=CC=C.C=CC#N.C=CC1=CC=CC=C1 XECAHXYUAAWDEL-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000001361 adipic acid Substances 0.000 description 1
- 229920003231 aliphatic polyamide Polymers 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000005215 alkyl ethers Chemical class 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000002280 amphoteric surfactant Substances 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 239000002216 antistatic agent Substances 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 229920003235 aromatic polyamide Polymers 0.000 description 1
- 125000003710 aryl alkyl group Chemical group 0.000 description 1
- 229960002255 azelaic acid Drugs 0.000 description 1
- 239000010905 bagasse Substances 0.000 description 1
- 239000011425 bamboo Substances 0.000 description 1
- RQPZNWPYLFFXCP-UHFFFAOYSA-L barium dihydroxide Chemical compound [OH-].[OH-].[Ba+2] RQPZNWPYLFFXCP-UHFFFAOYSA-L 0.000 description 1
- 229910001863 barium hydroxide Inorganic materials 0.000 description 1
- AYJRCSIUFZENHW-DEQYMQKBSA-L barium(2+);oxomethanediolate Chemical compound [Ba+2].[O-][14C]([O-])=O AYJRCSIUFZENHW-DEQYMQKBSA-L 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- UMIVXZPTRXBADB-UHFFFAOYSA-N benzocyclobutene Chemical compound C1=CC=C2CCC2=C1 UMIVXZPTRXBADB-UHFFFAOYSA-N 0.000 description 1
- AQIHMSVIAGNIDM-UHFFFAOYSA-N benzoyl bromide Chemical compound BrC(=O)C1=CC=CC=C1 AQIHMSVIAGNIDM-UHFFFAOYSA-N 0.000 description 1
- PASDCCFISLVPSO-UHFFFAOYSA-N benzoyl chloride Chemical compound ClC(=O)C1=CC=CC=C1 PASDCCFISLVPSO-UHFFFAOYSA-N 0.000 description 1
- WPCXDBCEDWUSOU-UHFFFAOYSA-N benzoyl iodide Chemical compound IC(=O)C1=CC=CC=C1 WPCXDBCEDWUSOU-UHFFFAOYSA-N 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- JZQAAQZDDMEFGZ-UHFFFAOYSA-N bis(ethenyl) hexanedioate Chemical compound C=COC(=O)CCCCC(=O)OC=C JZQAAQZDDMEFGZ-UHFFFAOYSA-N 0.000 description 1
- QUDWYFHPNIMBFC-UHFFFAOYSA-N bis(prop-2-enyl) benzene-1,2-dicarboxylate Chemical compound C=CCOC(=O)C1=CC=CC=C1C(=O)OCC=C QUDWYFHPNIMBFC-UHFFFAOYSA-N 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- MPMBRWOOISTHJV-UHFFFAOYSA-N but-1-enylbenzene Chemical compound CCC=CC1=CC=CC=C1 MPMBRWOOISTHJV-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- QAWBXZYPFCFQLA-UHFFFAOYSA-N butanoyl bromide Chemical compound CCCC(Br)=O QAWBXZYPFCFQLA-UHFFFAOYSA-N 0.000 description 1
- YHASWHZGWUONAO-UHFFFAOYSA-N butanoyl butanoate Chemical compound CCCC(=O)OC(=O)CCC YHASWHZGWUONAO-UHFFFAOYSA-N 0.000 description 1
- ZEEDSWFDNIDARI-UHFFFAOYSA-N butanoyl iodide Chemical compound CCCC(I)=O ZEEDSWFDNIDARI-UHFFFAOYSA-N 0.000 description 1
- JHIWVOJDXOSYLW-UHFFFAOYSA-N butyl 2,2-difluorocyclopropane-1-carboxylate Chemical compound CCCCOC(=O)C1CC1(F)F JHIWVOJDXOSYLW-UHFFFAOYSA-N 0.000 description 1
- DVECBJCOGJRVPX-UHFFFAOYSA-N butyryl chloride Chemical compound CCCC(Cl)=O DVECBJCOGJRVPX-UHFFFAOYSA-N 0.000 description 1
- ZMCUDHNSHCRDBT-UHFFFAOYSA-M caesium bicarbonate Chemical compound [Cs+].OC([O-])=O ZMCUDHNSHCRDBT-UHFFFAOYSA-M 0.000 description 1
- FJDQFPXHSGXQBY-UHFFFAOYSA-L caesium carbonate Chemical compound [Cs+].[Cs+].[O-]C([O-])=O FJDQFPXHSGXQBY-UHFFFAOYSA-L 0.000 description 1
- 229910000024 caesium carbonate Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 235000009120 camo Nutrition 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 239000004918 carbon fiber reinforced polymer Substances 0.000 description 1
- PFKFTWBEEFSNDU-UHFFFAOYSA-N carbonyldiimidazole Chemical compound C1=CN=CN1C(=O)N1C=CN=C1 PFKFTWBEEFSNDU-UHFFFAOYSA-N 0.000 description 1
- 238000010538 cationic polymerization reaction Methods 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 235000005607 chanvre indien Nutrition 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000006258 conductive agent Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- AQEDFGUKQJUMBV-UHFFFAOYSA-N copper;ethane-1,2-diamine Chemical compound [Cu].NCCN AQEDFGUKQJUMBV-UHFFFAOYSA-N 0.000 description 1
- 238000003851 corona treatment Methods 0.000 description 1
- LDHQCZJRKDOVOX-NSCUHMNNSA-N crotonic acid Chemical compound C\C=C\C(O)=O LDHQCZJRKDOVOX-NSCUHMNNSA-N 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- XLJMAIOERFSOGZ-UHFFFAOYSA-M cyanate Chemical compound [O-]C#N XLJMAIOERFSOGZ-UHFFFAOYSA-M 0.000 description 1
- YVWBQGFBSVLPIK-UHFFFAOYSA-N cyclohex-2-ene-1-carboxylic acid Chemical compound OC(=O)C1CCCC=C1 YVWBQGFBSVLPIK-UHFFFAOYSA-N 0.000 description 1
- XBZSBBLNHFMTEB-UHFFFAOYSA-N cyclohexane-1,3-dicarboxylic acid Chemical compound OC(=O)C1CCCC(C(O)=O)C1 XBZSBBLNHFMTEB-UHFFFAOYSA-N 0.000 description 1
- YQLZOAVZWJBZSY-UHFFFAOYSA-N decane-1,10-diamine Chemical compound NCCCCCCCCCCN YQLZOAVZWJBZSY-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000002781 deodorant agent Substances 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 150000001991 dicarboxylic acids Chemical class 0.000 description 1
- CCAFPWNGIUBUSD-UHFFFAOYSA-N diethyl sulfoxide Chemical compound CCS(=O)CC CCAFPWNGIUBUSD-UHFFFAOYSA-N 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- GYZLOYUZLJXAJU-UHFFFAOYSA-N diglycidyl ether Chemical compound C1OC1COCC1CO1 GYZLOYUZLJXAJU-UHFFFAOYSA-N 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- WEHWNAOGRSTTBQ-UHFFFAOYSA-N dipropylamine Chemical compound CCCNCCC WEHWNAOGRSTTBQ-UHFFFAOYSA-N 0.000 description 1
- QFTYSVGGYOXFRQ-UHFFFAOYSA-N dodecane-1,12-diamine Chemical compound NCCCCCCCCCCCCN QFTYSVGGYOXFRQ-UHFFFAOYSA-N 0.000 description 1
- 238000001523 electrospinning Methods 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- 238000004049 embossing Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 229920005558 epichlorohydrin rubber Polymers 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- WGXGKXTZIQFQFO-CMDGGOBGSA-N ethenyl (e)-3-phenylprop-2-enoate Chemical compound C=COC(=O)\C=C\C1=CC=CC=C1 WGXGKXTZIQFQFO-CMDGGOBGSA-N 0.000 description 1
- IYNRVIKPUTZSOR-HWKANZROSA-N ethenyl (e)-but-2-enoate Chemical compound C\C=C\C(=O)OC=C IYNRVIKPUTZSOR-HWKANZROSA-N 0.000 description 1
- FFYWKOUKJFCBAM-UHFFFAOYSA-N ethenyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OC=C FFYWKOUKJFCBAM-UHFFFAOYSA-N 0.000 description 1
- JZRGFKQYQJKGAK-UHFFFAOYSA-N ethenyl cyclohexanecarboxylate Chemical compound C=COC(=O)C1CCCCC1 JZRGFKQYQJKGAK-UHFFFAOYSA-N 0.000 description 1
- CMDXMIHZUJPRHG-UHFFFAOYSA-N ethenyl decanoate Chemical compound CCCCCCCCCC(=O)OC=C CMDXMIHZUJPRHG-UHFFFAOYSA-N 0.000 description 1
- GLVVKKSPKXTQRB-UHFFFAOYSA-N ethenyl dodecanoate Chemical compound CCCCCCCCCCCC(=O)OC=C GLVVKKSPKXTQRB-UHFFFAOYSA-N 0.000 description 1
- UJRIYYLGNDXVTA-UHFFFAOYSA-N ethenyl hexadecanoate Chemical compound CCCCCCCCCCCCCCCC(=O)OC=C UJRIYYLGNDXVTA-UHFFFAOYSA-N 0.000 description 1
- LZWYWAIOTBEZFN-UHFFFAOYSA-N ethenyl hexanoate Chemical compound CCCCCC(=O)OC=C LZWYWAIOTBEZFN-UHFFFAOYSA-N 0.000 description 1
- AFSIMBWBBOJPJG-UHFFFAOYSA-N ethenyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC=C AFSIMBWBBOJPJG-UHFFFAOYSA-N 0.000 description 1
- QBDADGJLZNIRFQ-UHFFFAOYSA-N ethenyl octanoate Chemical compound CCCCCCCC(=O)OC=C QBDADGJLZNIRFQ-UHFFFAOYSA-N 0.000 description 1
- ZQZUENMXBZVXIZ-UHFFFAOYSA-N ethenyl tetradecanoate Chemical compound CCCCCCCCCCCCCC(=O)OC=C ZQZUENMXBZVXIZ-UHFFFAOYSA-N 0.000 description 1
- 125000001033 ether group Chemical group 0.000 description 1
- FPIQZBQZKBKLEI-UHFFFAOYSA-N ethyl 1-[[2-chloroethyl(nitroso)carbamoyl]amino]cyclohexane-1-carboxylate Chemical compound ClCCN(N=O)C(=O)NC1(C(=O)OCC)CCCCC1 FPIQZBQZKBKLEI-UHFFFAOYSA-N 0.000 description 1
- 229920006242 ethylene acrylic acid copolymer Polymers 0.000 description 1
- 229920005680 ethylene-methyl methacrylate copolymer Polymers 0.000 description 1
- 238000004880 explosion Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 238000007380 fibre production Methods 0.000 description 1
- 239000011151 fibre-reinforced plastic Substances 0.000 description 1
- 239000011152 fibreglass Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 229920001973 fluoroelastomer Polymers 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 239000002783 friction material Substances 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- ANSXAPJVJOKRDJ-UHFFFAOYSA-N furo[3,4-f][2]benzofuran-1,3,5,7-tetrone Chemical compound C1=C2C(=O)OC(=O)C2=CC2=C1C(=O)OC2=O ANSXAPJVJOKRDJ-UHFFFAOYSA-N 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- 239000011487 hemp Substances 0.000 description 1
- PWSKHLMYTZNYKO-UHFFFAOYSA-N heptane-1,7-diamine Chemical compound NCCCCCCCN PWSKHLMYTZNYKO-UHFFFAOYSA-N 0.000 description 1
- 239000011964 heteropoly acid Substances 0.000 description 1
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 229920002681 hypalon Polymers 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910003475 inorganic filler Inorganic materials 0.000 description 1
- 239000011256 inorganic filler Substances 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 229920000592 inorganic polymer Polymers 0.000 description 1
- 229910001867 inorganic solvent Inorganic materials 0.000 description 1
- 239000003049 inorganic solvent Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- QQVIHTHCMHWDBS-UHFFFAOYSA-N isophthalic acid Chemical compound OC(=O)C1=CC=CC(C(O)=O)=C1 QQVIHTHCMHWDBS-UHFFFAOYSA-N 0.000 description 1
- 150000003951 lactams Chemical class 0.000 description 1
- 229920000092 linear low density polyethylene Polymers 0.000 description 1
- 239000004707 linear low-density polyethylene Substances 0.000 description 1
- 239000000944 linseed oil Substances 0.000 description 1
- 235000021388 linseed oil Nutrition 0.000 description 1
- XGZVUEUWXADBQD-UHFFFAOYSA-L lithium carbonate Chemical compound [Li+].[Li+].[O-]C([O-])=O XGZVUEUWXADBQD-UHFFFAOYSA-L 0.000 description 1
- 229910052808 lithium carbonate Inorganic materials 0.000 description 1
- 229910000032 lithium hydrogen carbonate Inorganic materials 0.000 description 1
- HQRPHMAXFVUBJX-UHFFFAOYSA-M lithium;hydrogen carbonate Chemical compound [Li+].OC([O-])=O HQRPHMAXFVUBJX-UHFFFAOYSA-M 0.000 description 1
- 229920001684 low density polyethylene Polymers 0.000 description 1
- 239000004702 low-density polyethylene Substances 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000010327 methods by industry Methods 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000002715 modification method Methods 0.000 description 1
- DTKANQSCBACEPK-UHFFFAOYSA-N n',n'-bis[3-(dimethylamino)propyl]-n,n-dimethylpropane-1,3-diamine Chemical compound CN(C)CCCN(CCCN(C)C)CCCN(C)C DTKANQSCBACEPK-UHFFFAOYSA-N 0.000 description 1
- TXXWBTOATXBWDR-UHFFFAOYSA-N n,n,n',n'-tetramethylhexane-1,6-diamine Chemical compound CN(C)CCCCCCN(C)C TXXWBTOATXBWDR-UHFFFAOYSA-N 0.000 description 1
- DMQSHEKGGUOYJS-UHFFFAOYSA-N n,n,n',n'-tetramethylpropane-1,3-diamine Chemical compound CN(C)CCCN(C)C DMQSHEKGGUOYJS-UHFFFAOYSA-N 0.000 description 1
- AJFDBNQQDYLMJN-UHFFFAOYSA-N n,n-diethylacetamide Chemical compound CCN(CC)C(C)=O AJFDBNQQDYLMJN-UHFFFAOYSA-N 0.000 description 1
- PSHKMPUSSFXUIA-UHFFFAOYSA-N n,n-dimethylpyridin-2-amine Chemical compound CN(C)C1=CC=CC=N1 PSHKMPUSSFXUIA-UHFFFAOYSA-N 0.000 description 1
- SXJVFQLYZSNZBT-UHFFFAOYSA-N nonane-1,9-diamine Chemical compound NCCCCCCCCCN SXJVFQLYZSNZBT-UHFFFAOYSA-N 0.000 description 1
- JFNLZVQOOSMTJK-KNVOCYPGSA-N norbornene Chemical compound C1[C@@H]2CC[C@H]1C=C2 JFNLZVQOOSMTJK-KNVOCYPGSA-N 0.000 description 1
- 239000004843 novolac epoxy resin Substances 0.000 description 1
- 239000010680 novolac-type phenolic resin Substances 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- ZWLPBLYKEWSWPD-UHFFFAOYSA-N o-toluic acid Chemical compound CC1=CC=CC=C1C(O)=O ZWLPBLYKEWSWPD-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 150000002978 peroxides Chemical class 0.000 description 1
- 239000013034 phenoxy resin Substances 0.000 description 1
- 229920006287 phenoxy resin Polymers 0.000 description 1
- 239000003279 phenylacetic acid Substances 0.000 description 1
- 229960003424 phenylacetic acid Drugs 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 238000009832 plasma treatment Methods 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229920001084 poly(chloroprene) Polymers 0.000 description 1
- 229920003207 poly(ethylene-2,6-naphthalate) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920000343 polyazomethine Polymers 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 238000006068 polycondensation reaction Methods 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001721 polyimide Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 229920001955 polyphenylene ether Polymers 0.000 description 1
- 239000005077 polysulfide Substances 0.000 description 1
- 229920001021 polysulfide Polymers 0.000 description 1
- 150000008117 polysulfides Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229910000028 potassium bicarbonate Inorganic materials 0.000 description 1
- 235000015497 potassium bicarbonate Nutrition 0.000 description 1
- 239000011736 potassium bicarbonate Substances 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 235000011181 potassium carbonates Nutrition 0.000 description 1
- TYJJADVDDVDEDZ-UHFFFAOYSA-M potassium hydrogencarbonate Chemical compound [K+].OC([O-])=O TYJJADVDDVDEDZ-UHFFFAOYSA-M 0.000 description 1
- 229940086066 potassium hydrogencarbonate Drugs 0.000 description 1
- LPNYRYFBWFDTMA-UHFFFAOYSA-N potassium tert-butoxide Chemical compound [K+].CC(C)(C)[O-] LPNYRYFBWFDTMA-UHFFFAOYSA-N 0.000 description 1
- 238000010248 power generation Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- RIBFXMJCUYXJDZ-UHFFFAOYSA-N propanoyl bromide Chemical compound CCC(Br)=O RIBFXMJCUYXJDZ-UHFFFAOYSA-N 0.000 description 1
- RZWZRACFZGVKFM-UHFFFAOYSA-N propanoyl chloride Chemical compound CCC(Cl)=O RZWZRACFZGVKFM-UHFFFAOYSA-N 0.000 description 1
- GLCSNYFRXVGJJF-UHFFFAOYSA-N propanoyl iodide Chemical compound CCC(I)=O GLCSNYFRXVGJJF-UHFFFAOYSA-N 0.000 description 1
- WYVAMUWZEOHJOQ-UHFFFAOYSA-N propionic anhydride Chemical compound CCC(=O)OC(=O)CC WYVAMUWZEOHJOQ-UHFFFAOYSA-N 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 235000019423 pullulan Nutrition 0.000 description 1
- 150000003215 pyranoses Chemical group 0.000 description 1
- HNJBEVLQSNELDL-UHFFFAOYSA-N pyrrolidin-2-one Chemical compound O=C1CCCN1 HNJBEVLQSNELDL-UHFFFAOYSA-N 0.000 description 1
- QGKLPGKXAVVPOJ-UHFFFAOYSA-N pyrrolidin-3-one Chemical compound O=C1CCNC1 QGKLPGKXAVVPOJ-UHFFFAOYSA-N 0.000 description 1
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 239000011134 resol-type phenolic resin Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000007790 scraping Methods 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- QDRKDTQENPPHOJ-UHFFFAOYSA-N sodium ethoxide Chemical compound [Na+].CC[O-] QDRKDTQENPPHOJ-UHFFFAOYSA-N 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000010959 steel Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000010902 straw Substances 0.000 description 1
- 229960005137 succinic acid Drugs 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- 238000003878 thermal aging Methods 0.000 description 1
- 229920002803 thermoplastic polyurethane Polymers 0.000 description 1
- 150000004992 toluidines Chemical class 0.000 description 1
- LDHQCZJRKDOVOX-UHFFFAOYSA-N trans-crotonic acid Natural products CC=CC(O)=O LDHQCZJRKDOVOX-UHFFFAOYSA-N 0.000 description 1
- DTQVDTLACAAQTR-DYCDLGHISA-N trifluoroacetic acid-d1 Chemical compound [2H]OC(=O)C(F)(F)F DTQVDTLACAAQTR-DYCDLGHISA-N 0.000 description 1
- SRPWOOOHEPICQU-UHFFFAOYSA-N trimellitic anhydride Chemical compound OC(=O)C1=CC=C2C(=O)OC(=O)C2=C1 SRPWOOOHEPICQU-UHFFFAOYSA-N 0.000 description 1
- KQBSGRWMSNFIPG-UHFFFAOYSA-N trioxane Chemical compound C1COOOC1 KQBSGRWMSNFIPG-UHFFFAOYSA-N 0.000 description 1
- 229920000785 ultra high molecular weight polyethylene Polymers 0.000 description 1
- 229920001862 ultra low molecular weight polyethylene Polymers 0.000 description 1
- KLNPWTHGTVSSEU-UHFFFAOYSA-N undecane-1,11-diamine Chemical compound NCCCCCCCCCCCN KLNPWTHGTVSSEU-UHFFFAOYSA-N 0.000 description 1
- 229920006337 unsaturated polyester resin Polymers 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- KOZCZZVUFDCZGG-UHFFFAOYSA-N vinyl benzoate Chemical compound C=COC(=O)C1=CC=CC=C1 KOZCZZVUFDCZGG-UHFFFAOYSA-N 0.000 description 1
- 229960000834 vinyl ether Drugs 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 239000008170 walnut oil Substances 0.000 description 1
- 229920001221 xylan Polymers 0.000 description 1
- 150000004823 xylans Chemical class 0.000 description 1
- 238000004383 yellowing Methods 0.000 description 1
Images
Landscapes
- Polysaccharides And Polysaccharide Derivatives (AREA)
- Paper (AREA)
Description
本発明は、微細セルロース繊維及び樹脂複合体に関する。 The present invention relates to fine cellulose fibers and a resin composite.
熱可塑性樹脂は、軽く、加工特性に優れるため、自動車部材、電気・電子部材、事務機器ハウジング、精密部品等の多方面に広く使用されている。しかしながら、樹脂単体では、機械特性、寸法安定性等が不十分である場合が多く、樹脂と各種繊維状物質をコンポジットしたものが一般的に用いられている。 Thermoplastic resins are lightweight and have excellent processability, so they are widely used in a wide range of applications, including automotive parts, electrical and electronic parts, office equipment housings, and precision parts. However, resin alone often lacks sufficient mechanical properties and dimensional stability, so composites of resin and various fibrous materials are commonly used.
近年、繊維状物質として、微細セルロース繊維(セルロースナノファイバー(CNF)ともいう。)をはじめとしたナノ繊維が用いられるようになってきている。CNFは、乾燥状態では凝集し易い性質があるため、安定分散が可能な水分散液として製造される。 In recent years, nanofibers, including fine cellulose fibers (also called cellulose nanofibers (CNF)), have come to be used as fibrous materials. CNF has a tendency to aggregate in a dry state, so it is manufactured as an aqueous dispersion that allows for stable dispersion.
セルロース繊維を他の材料中に分散させる技術は従来種々提案されている。例えば、特許文献1には、樹脂に対するセルロースの分散性向上を目的として、セルロースナノファイバーとブロック共重合体を含む組成物が、特許文献2には、アスペクト比が高いセルロース繊維を樹脂組成物中に良好に分散することを目的として、ポリビニルアルコール、ポリビニルピロリドン、およびこれらの共重合体からなる水溶性樹脂を含む樹脂組成物が、記載されている。 Various techniques for dispersing cellulose fibers in other materials have been proposed in the past. For example, Patent Document 1 describes a composition containing cellulose nanofibers and a block copolymer with the aim of improving the dispersibility of cellulose in resin, and Patent Document 2 describes a resin composition containing polyvinyl alcohol, polyvinylpyrrolidone, and a water-soluble resin consisting of a copolymer of these with the aim of dispersing cellulose fibers with a high aspect ratio well in a resin composition.
しかし上記の従来技術によってもなお、樹脂中での微細セルロース繊維の十分良好な分散は困難である。特に、微細セルロース繊維においては、凝集塊が一旦発生すると当該凝集塊を解消することが困難であり、この凝集塊は、微細セルロース繊維を樹脂中に分散させてなる樹脂複合体において物性を著しく低下又は不均一化させる要因となっていた。 However, even with the above-mentioned conventional techniques, it is still difficult to disperse fine cellulose fibers sufficiently well in the resin. In particular, once agglomerates form in fine cellulose fibers, it is difficult to eliminate the agglomerates, and these agglomerates cause a significant decrease or non-uniformity in the physical properties of the resin composite in which fine cellulose fibers are dispersed in the resin.
本発明は、上記の課題を解決し、凝集塊を形成しにくく樹脂複合体に対する物性向上効果が良好である、微細セルロース繊維の提供を目的とする。 The present invention aims to solve the above problems and provide fine cellulose fibers that are less likely to form agglomerates and have a good effect of improving the physical properties of resin composites.
本発明者は、前記課題を解決するため、鋭意検討を進めた結果、微細セルロース繊維の特定濃度の希薄分散液中での沈降挙動を制御することで表記の課題を解決できることを見出し、本発明をなすに至った。
すなわち、本発明は以下の態様を包含する。
[1] 微細セルロース繊維であって、
前記微細セルロース繊維を水中に濃度0.05質量%で含有させてなる試験用分散液を23℃、常圧にて24時間静置したときの、試験用分散液の全液高100%に対する微細セルロース繊維の沈降高さの比率が、6%以上である、微細セルロース繊維。
[2] 平均繊維径が2~1000nmである、上記態様1に記載の微細セルロース繊維。
[3] 化学修飾されている、上記態様1又は2に記載の微細セルロース繊維。
[4] 前記化学修飾がアシル化である、上記態様3に記載の微細セルロース繊維。
[5] 重量平均分子量(Mw)が100000以上であり、重量平均分子量(Mw)と数平均分子量(Mn)との比(Mw/Mn)が6以下である、上記態様1~4のいずれかに記載の微細セルロース繊維。
[6] アルカリ可溶多糖類平均含有率が12質量%以下であり、結晶化度が60%以上である、上記態様1~5のいずれかに記載の微細セルロース繊維。
[7] 上記態様1~6のいずれかに記載の微細セルロース繊維を含む、乾燥体。
[8] 含水率が50質量%以下である、上記態様7に記載の乾燥体。
[9] 上記態様1~6のいずれかに記載の微細セルロース繊維と、樹脂とを含む、樹脂複合体。
[10] 上記態様1~6のいずれかに記載の微細セルロース繊維の製造方法であって、
セルロース原料を叩解し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、及び
任意に、前記微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、
を含む、方法。
[11] 上記態様7又は8に記載の乾燥体の製造方法であって、
セルロース原料を叩解し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、
前記微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、及び
任意に、前記微細セルロース繊維スラリー及び/又は微細セルロース繊維と追加の成分とを混合すること、
を含む、方法。
[12] 上記態様9に記載の樹脂複合体の製造方法であって、
セルロース原料を叩解し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、及び
任意に、前記微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、
を含む方法で微細セルロース繊維を得る微細セルロース繊維製造工程、並びに、
前記微細セルロース繊維と前記樹脂とを混合する混合工程、
を含む、方法。
[13] 前記解繊の前、前記解繊と同時、及び/又は前記解繊の後にセルロースを化学修飾することを更に含む、上記態様10~12のいずれかに記載の方法。
As a result of intensive research into solving the above-mentioned problems, the inventors discovered that the above-mentioned problems can be solved by controlling the sedimentation behavior of fine cellulose fibers in a dilute dispersion of a specific concentration, and thus completed the present invention.
That is, the present invention includes the following aspects.
[1] A fine cellulose fiber,
The fine cellulose fibers have a ratio of the sedimentation height of the fine cellulose fibers to the total liquid height of the test dispersion (100%) of 6% or more when the test dispersion containing the fine cellulose fibers in water at a concentration of 0.05% by mass is allowed to stand at 23°C and normal pressure for 24 hours.
[2] The fine cellulose fibers according to the above aspect 1, having an average fiber diameter of 2 to 1000 nm.
[3] The fine cellulose fibers according to the above aspect 1 or 2, which are chemically modified.
[4] The fine cellulose fibers according to the above aspect 3, wherein the chemical modification is acylation.
[5] The fine cellulose fiber according to any one of the above aspects 1 to 4, having a weight average molecular weight (Mw) of 100,000 or more and a ratio (Mw/Mn) of the weight average molecular weight (Mw) to the number average molecular weight (Mn) of 6 or less.
[6] The fine cellulose fibers according to any one of the above aspects 1 to 5, having an average alkali-soluble polysaccharide content of 12% by mass or less and a crystallinity of 60% or more.
[7] A dried body comprising the fine cellulose fibers according to any one of aspects 1 to 6.
[8] The dried body according to aspect 7, having a moisture content of 50% by mass or less.
[9] A resin composite comprising the fine cellulose fiber according to any one of aspects 1 to 6 above and a resin.
[10] A method for producing the fine cellulose fiber according to any one of the above aspects 1 to 6,
beating a cellulose raw material and then defibrating the cellulose raw material with a homomixer to obtain a fine cellulose fiber slurry; and optionally drying the fine cellulose fiber slurry under reduced pressure while stirring.
A method comprising:
[11] A method for producing the dried body according to aspect 7 or 8, comprising the steps of:
A cellulose raw material is beaten and then defibrated with a homomixer to obtain a fine cellulose fiber slurry;
drying the fine cellulose fiber slurry under reduced pressure while stirring; and optionally mixing the fine cellulose fiber slurry and/or the fine cellulose fibers with additional components.
A method comprising:
[12] A method for producing the resin composite according to aspect 9, comprising the steps of:
beating a cellulose raw material and then defibrating the cellulose raw material with a homomixer to obtain a fine cellulose fiber slurry; and optionally drying the fine cellulose fiber slurry under reduced pressure while stirring.
A process for producing fine cellulose fibers by a method comprising the steps of:
A mixing step of mixing the fine cellulose fibers and the resin;
A method comprising:
[13] The method according to any one of aspects 10 to 12, further comprising chemically modifying cellulose before, simultaneously with, and/or after the defibration.
本発明の一態様によれば、凝集塊を形成し難く樹脂複合体に対する物性向上効果が良好である、微細セルロース繊維が提供され得る。 According to one aspect of the present invention, fine cellulose fibers can be provided that are less likely to form agglomerates and have a good effect of improving the physical properties of resin composites.
本発明の例示の態様について以下具体的に説明するが、本発明はこれらの態様に限定されるものではない。 The following describes exemplary embodiments of the present invention in detail, but the present invention is not limited to these embodiments.
≪微細セルロース繊維≫
本発明の一態様は、微細セルロース繊維を水中に濃度0.05質量%で含有させてなる試験用分散液を23℃、常圧にて24時間静置したときの、試験用分散液の全液高100%に対する微細セルロース繊維の沈降高さの比率が、6%以上である、微細セルロース繊維を提供する。微細セルロース繊維とは、セルロース原料をリファイナー、高圧ホモジナイザー、ボールミル、ホモミキサー等によって機械的に微細化する方法により解繊したセルロースを指す。微細セルロース繊維は、通常、極めて凝集しやすく繊維間に強固な相互作用を有するために樹脂中で良好に分散させることが困難である。本発明者は、予想外にも、0.05質量%という特定の希薄濃度の水分散液中で高い沈降高さを示すような微細セルロース繊維であれば、繊維間で適度な空間を保持して凝集体を形成し難く樹脂中でも良好に分散し得る一方で、繊維間の適度な絡み合いによって樹脂に対して優れた物性向上効果を付与できることを発見した。このような微細セルロース繊維は、比較的少量の使用であっても、微細セルロース繊維と樹脂とを含む複合体(本開示で、樹脂複合体ともいう。)の物性向上効果に優れる。本開示の微細セルロース繊維は、分散液(すなわちスラリー)中に、又は乾燥体として、存在してよい。微細セルロース繊維は、後述の化学修飾がされたものであってもよい。上記分散液としては、微細セルロース繊維を例えば0.001~2質量%、又は0.01~1.5質量%、又は0.1~1質量%含み、残部が分散媒である分散液を例示できる。分散媒は例えば水及び/又は有機溶媒であってよい。有機溶媒としては、水混和性有機溶媒、例えば、ポリオール(例えばポリプロピレングリコール、ポリエチレングリコールのようなポリエーテルポリオール等)、エタノール、メタノール、ジメチルホルムアミド、ジメチルスルホキシド、アセトニトリル、アセトン、酢酸、t-ブタノール等を例示できる。
<Fine cellulose fiber>
One aspect of the present invention provides fine cellulose fibers, in which the ratio of the settling height of the fine cellulose fibers to the total liquid height of the test dispersion (100%) when the test dispersion containing the fine cellulose fibers in water at a concentration of 0.05% by mass is left to stand at 23° C. and normal pressure for 24 hours is 6% or more. The fine cellulose fibers refer to cellulose defibrated by a method of mechanically finely pulverizing a cellulose raw material using a refiner, a high-pressure homogenizer, a ball mill, a homomixer, or the like. Fine cellulose fibers are usually very prone to aggregation and have strong interactions between the fibers, making it difficult to disperse them well in a resin. The present inventor unexpectedly discovered that fine cellulose fibers that exhibit a high settling height in a specific dilute aqueous dispersion of 0.05% by mass can maintain a suitable space between the fibers, making it difficult to form aggregates and can be dispersed well in a resin, while being able to impart an excellent physical property improvement effect to the resin due to the suitable entanglement between the fibers. Even when such fine cellulose fibers are used in a relatively small amount, they have an excellent effect of improving the physical properties of a composite containing fine cellulose fibers and a resin (also referred to as a resin composite in the present disclosure). The fine cellulose fibers of the present disclosure may be present in a dispersion (i.e., a slurry) or as a dried body. The fine cellulose fibers may be chemically modified as described below. Examples of the dispersion include dispersions containing, for example, 0.001 to 2 mass%, or 0.01 to 1.5 mass%, or 0.1 to 1 mass% of fine cellulose fibers, with the remainder being a dispersion medium. The dispersion medium may be, for example, water and/or an organic solvent. Examples of the organic solvent include water-miscible organic solvents, such as polyols (e.g., polyether polyols such as polypropylene glycol and polyethylene glycol), ethanol, methanol, dimethylformamide, dimethyl sulfoxide, acetonitrile, acetone, acetic acid, and t-butanol.
試験用分散液は以下の方法で調製される。微細セルロース繊維が分散液の形態で存在している場合には、当該分散液を溶媒置換、及び/又は希釈若しくは濃縮によって微細セルロース繊維濃度0.05質量%の水分散液に変換して試験用分散液とする。濃縮は、分散液を濾布、メンブレンフィルター、遠心分離等に供することで行う。微細セルロース繊維が乾燥体である場合には、水を添加してホモジナイザー等で再分散することで微細セルロース繊維濃度0.05質量%の試験用分散液を調製する。 The test dispersion is prepared by the following method. When the fine cellulose fibers are present in the form of a dispersion, the dispersion is converted into an aqueous dispersion with a fine cellulose fiber concentration of 0.05% by mass by solvent replacement and/or dilution or concentration to prepare the test dispersion. Concentration is performed by subjecting the dispersion to a filter cloth, membrane filter, centrifugation, etc. When the fine cellulose fibers are in a dry form, water is added and the fibers are redispersed using a homogenizer, etc. to prepare a test dispersion with a fine cellulose fiber concentration of 0.05% by mass.
沈降高さの上記比率は、一態様において6%以上であり、好ましくは、10%以上、又は15%以上、又は20%以上、又は25%以上、又は30%以上である。沈降高さの比率が高いことは、微細セルロース繊維が、良好に解繊されているとともに、繊維同士が適度な空間を保ち凝集塊を形成しにくい一方で適度な絡み合いも有していることの指標となる。一方、上記比率が高すぎると(例えば、100%の場合は微細セルロース繊維が沈降していない)、微細セルロース繊維のネットワークが過度に形成しやすく、樹脂中への分散が困難になり、結果として凝集塊を形成しやすく、樹脂複合体の物性低下を招く場合がある。このような観点から、上記比率の上限は、80%以下、又は70%以下、又は60%以下、50%以下、又は40%以下であってもよい。 In one embodiment, the ratio of the settling height is 6% or more, and preferably 10% or more, 15% or more, 20% or more, 25% or more, or 30% or more. A high ratio of the settling height is an indicator that the fine cellulose fibers are well defibrated, and that the fibers maintain a moderate space between each other and are unlikely to form agglomerates, while also having a moderate amount of entanglement. On the other hand, if the ratio is too high (for example, when the fine cellulose fibers do not settle in the case of 100%), the fine cellulose fibers are likely to form an excessive network, making it difficult to disperse them in the resin, and as a result, agglomerates are likely to form, which may lead to a decrease in the physical properties of the resin composite. From this perspective, the upper limit of the ratio may be 80% or less, 70% or less, 60% or less, 50% or less, or 40% or less.
沈降高さ比は分散液の波長850nmにおける透過度(以下、初期透過度ともいう。)を、例えば液中分散安定性評価装置 Turbiscan(三洋貿易(株)製)を用いて測定する。また、この試験管を23℃で24時間静置した後、分散液の底面から分離界面までの高さと、分散液底面から分散液上面までの高さとを、上記液中分散安定性評価装置を用い、上記波長で透過度50%になる高さを分離界面と定義して測定し、下記式(1):
沈降高さ比(%)=([A]/[B])×100・・・(1)
(式(1)中、[A]は分散液底面から分離界面までの高さであり、[B]は分散液底面から分散液上面までの高さである。)
に従って、沈降高さ比を求める。なお、上記の透過度50%になる高さが画定されない場合には、上記初期透過度よりも透過度が高い領域を上澄部、低い領域を堆積部とし、上澄部と堆積部との境界を分離界面と定義し、上記(1)に従って沈降高さ比を求める。
The sedimentation height ratio is measured by measuring the transmittance of the dispersion at a wavelength of 850 nm (hereinafter also referred to as initial transmittance) using, for example, a liquid dispersion stability evaluation device Turbiscan (manufactured by Sanyo Trading Co., Ltd.). After leaving the test tube at rest for 24 hours at 23° C., the height from the bottom of the dispersion to the separation interface and the height from the bottom of the dispersion to the top of the dispersion are measured using the liquid dispersion stability evaluation device, with the height at which the transmittance becomes 50% at the above wavelength being defined as the separation interface, and is calculated using the following formula (1):
Settling height ratio (%) = ([A] / [B]) × 100 ... (1)
(In formula (1), [A] is the height from the bottom surface of the dispersion to the separation interface, and [B] is the height from the bottom surface of the dispersion to the top surface of the dispersion.)
In addition, when the height at which the permeability becomes 50% is not defined, the region with a higher permeability than the initial permeability is defined as the supernatant portion, the region with a lower permeability than the initial permeability is defined as the sediment portion, and the boundary between the supernatant portion and the sediment portion is defined as the separation interface, and the sedimentation height ratio is calculated according to (1) above.
一態様において、本開示の微細セルロース繊維は、例えば、セルロース原料をジメチルスルホキシド(DMSOともいう)中でホモミキサーを用いて解繊することによって微細セルロース繊維スラリーを得ること、及び任意に微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、を含む方法で製造できる。減圧乾燥を経る場合には本開示の微細セルロース繊維は乾燥体として得られる。沈降高さを制御する手段としては、上記セルロース原料を乾式粉砕又は湿式粉砕処理し、一態様では長さ加重平均繊維長が5mm以下、好ましくは4mm以下、又は3mm以下、又は2mm以下、又は1mm以下となるセルロース原料を製造し、該セルロース原料を湿式で微細化することを例示できる。セルロース原料が5mm以下であると、続く微細化工程において均一な微細セルロース繊維が得られ、水中での凝集が抑えられる。この作用機序は明らかではないが、セルロース繊維が長すぎると解繊における剪断等のエネルギーが均一に掛かりにくくなる。このようにして得られる不均一な微細セルロース繊維は、太い繊維の存在により繊維径が太くなる他、撚糸しやすく、水中での分散性に劣ると考えられる。上記、セルロース原料の長さ加重平均繊維長の下限としては、過度の粉砕処理による結晶化度の低下を防ぐため、好ましくは0.1mm以上、又は0.2mm以上、又は0.3mm以上、又は0.4mm以上、又は0.5mm以上である。セルロース原料の長さ加重平均繊維長は、例えば、光学的計測方法によって測定することができる(JAPAN TAPPI 紙パルプ試験方法 No.52(パルプ及び紙-繊維長試験方法-光学的自動計測法)またはJIS P 8226(パルプ-光学的自動分析法による繊維長測定方法-第1部:偏光法)、JIS P 8226‐2(パルプ-光学的自動分析法による繊維長測定方法-第2部:非偏光法)を参照。) In one embodiment, the fine cellulose fiber of the present disclosure can be produced by a method including, for example, obtaining a fine cellulose fiber slurry by defibrating a cellulose raw material in dimethyl sulfoxide (also referred to as DMSO) using a homomixer, and optionally drying the fine cellulose fiber slurry under reduced pressure while stirring. When the cellulose raw material is dried under reduced pressure, the fine cellulose fiber of the present disclosure is obtained as a dry body. As a means for controlling the settling height, the above-mentioned cellulose raw material is subjected to a dry or wet grinding treatment, and in one embodiment, a cellulose raw material having a length-weighted average fiber length of 5 mm or less, preferably 4 mm or less, or 3 mm or less, or 2 mm or less, or 1 mm or less is produced, and the cellulose raw material is refined in a wet manner. If the cellulose raw material is 5 mm or less, uniform fine cellulose fibers are obtained in the subsequent refinement process, and aggregation in water is suppressed. Although the mechanism of this action is unclear, if the cellulose fibers are too long, it becomes difficult to apply shear energy, etc., uniformly during defibration. The non-uniform fine cellulose fibers obtained in this manner have a large fiber diameter due to the presence of thick fibers, and are prone to twisting and poorly dispersible in water. The lower limit of the length-weighted average fiber length of the cellulose raw material is preferably 0.1 mm or more, or 0.2 mm or more, or 0.3 mm or more, or 0.4 mm or more, or 0.5 mm or more to prevent a decrease in crystallinity due to excessive crushing treatment. The length-weighted average fiber length of the cellulose raw material can be measured, for example, by an optical measurement method (see JAPAN TAPPI Paper and Pulp Test Method No. 52 (Pulp and Paper - Fiber Length Test Method - Optical Automatic Measurement Method) or JIS P 8226 (Pulp - Fiber Length Measurement Method by Optical Automatic Analysis Method - Part 1: Polarized Method), JIS P 8226-2 (Pulp - Fiber Length Measurement Method by Optical Automatic Analysis Method - Part 2: Non-Polarized Method)).
セルロース原料としては、天然セルロース及び再生セルロースを用いることができる。天然セルロースとしては、木材種(広葉樹又は針葉樹)から得られる木材パルプ、非木材種(綿、竹、麻、バガス、ケナフ、コットンリンター、サイザル、ワラ等)から得られる非木材パルプ、動物(例えばホヤ類)や藻類、微生物(例えば酢酸菌)、が産生するセルロース繊維集合体を使用できる。再生セルロースとしては、再生セルロース繊維(ビスコース、キュプラ、テンセル等)、セルロース誘導体繊維、エレクトロスピニング法により得られた再生セルロース又はセルロース誘導体の極細糸等を使用できる。 As the cellulose raw material, natural cellulose and regenerated cellulose can be used. As the natural cellulose, wood pulp obtained from wood species (broadleaf or coniferous trees), non-wood pulp obtained from non-wood species (cotton, bamboo, hemp, bagasse, kenaf, cotton linters, sisal, straw, etc.), and cellulose fiber aggregates produced by animals (e.g., sea squirts), algae, and microorganisms (e.g., acetic acid bacteria) can be used. As the regenerated cellulose, regenerated cellulose fibers (viscose, cupra, tencel, etc.), cellulose derivative fibers, and ultra-fine threads of regenerated cellulose or cellulose derivatives obtained by the electrospinning method can be used.
前記セルロース原料は、アルカリ可溶分、及び硫酸不溶成分(リグニン等)を含有するため、蒸解処理による脱リグニン等の精製工程及び漂白工程を経て、アルカリ可溶分及び硫酸不溶成分を減らしても良い。他方、蒸解処理による脱リグニン等の精製工程及び漂白工程はセルロースの分子鎖を切断し、重量平均分子量、及び数平均分子量を変化させてしまうため、セルロース原料の精製工程及び漂白工程は、セルロースの重量平均分子量、及び重量平均分子量と数平均分子量との比が、適切な範囲から逸脱しない程度にコントロールされていることが重要である。 Since the cellulose raw material contains alkali-soluble and sulfuric acid-insoluble components (such as lignin), the alkali-soluble and sulfuric acid-insoluble components may be reduced by a purification process such as delignification by cooking and a bleaching process. On the other hand, the purification process such as delignification by cooking and the bleaching process cut the molecular chains of cellulose, changing the weight-average molecular weight and number-average molecular weight, so it is important that the purification process and bleaching process of the cellulose raw material control the weight-average molecular weight of cellulose and the ratio of the weight-average molecular weight to the number-average molecular weight so that they do not deviate from the appropriate range.
また、蒸解処理による脱リグニン等の精製工程及び漂白工程はセルロース分子の分子量を低下させるため、これらの工程によって、セルロースが低分子量化すること、及びセルロース原料が変質してアルカリ可溶分の存在比率が増加することが懸念される。アルカリ可溶分は耐熱性に劣るため、セルロース原料の精製工程及び漂白工程は、セルロース原料に含有されるアルカリ可溶分の量が一定の値以下の範囲となるようにコントロールされていることが重要である。 In addition, refining processes such as delignification by cooking and bleaching processes reduce the molecular weight of cellulose molecules, and there is concern that these processes will result in low molecular weight cellulose and alter the cellulose raw material, increasing the proportion of alkali-soluble matter present. Since alkali-soluble matter has poor heat resistance, it is important that the refining and bleaching processes of cellulose raw materials are controlled so that the amount of alkali-soluble matter contained in the cellulose raw material is kept below a certain value.
前記セルロース原料は乾式粉砕処理によってセルロース原料を得る。乾式粉砕において用いられる粉砕機はどのような形式のものでも用いることができるが、その中でも高速回転衝撃式粉砕機が良好な形状のセルロース原料が得られるため、好ましい。高速回転衝撃式粉砕機とは粉砕室内で回転するピンや特殊な構造を有するローターがセルロース原料に衝撃、あるいは剪断等を与え、これを粉砕する方式の粉砕機である。また、乾式粉砕時の繊維ダメージを抑制するために、セルロース原料重量のある程度水分を含有させても構わない。 The cellulose raw material is obtained by a dry grinding process. Any type of grinder can be used in the dry grinding process, but a high-speed rotary impact grinder is preferable because it can produce cellulose raw material with a good shape. A high-speed rotary impact grinder is a grinder that uses pins that rotate in a grinding chamber or a rotor with a special structure to impact or shear the cellulose raw material and grind it. In addition, to suppress fiber damage during dry grinding, the cellulose raw material may contain moisture to a certain extent based on its weight.
この形式を有する粉砕機としては自由粉砕機、ニューコスモマイザー((株)奈良機械製作所製)、ヴィクトリーミル、ファーインヴィクトリーミル(ホソカワミクロン(株)製)、ターボミル(ターボ工業(株)製)、ウルトラローター((株)W.I.R製)、マキノ式粉砕機、ウルトラプレックス(槙野産業(株)製)、ファインミル(日本ニューマチック工業(株)製)、インペラーミル((株)セイシン企業製)、ディスクリファイナー(相川鉄工(株)製)等が挙げられる。 Examples of mills with this type include the Jiyu mill, New Cosmomizer (manufactured by Nara Machinery Works, Ltd.), Victory Mill, Far In Victory Mill (manufactured by Hosokawa Micron, Ltd.), Turbo Mill (manufactured by Turbo Kogyo, Ltd.), Ultra Rotor (manufactured by W.I.R. Co., Ltd.), Makino type mill, Ultraplex (manufactured by Makino Sangyo, Ltd.), Fine Mill (manufactured by Nippon Pneumatic Mfg. Co., Ltd.), Impeller Mill (manufactured by Seishin Enterprise Co., Ltd.), and Disc Refiner (manufactured by Aikawa Iron Works, Ltd.).
前記セルロース原料を微細セルロース繊維とするために繊維径を小さくする方法としては、特に制限はないが、解繊の処理条件(剪断場を与える方法、剪断場の大きさ等)をより高効率にすることが好ましい。又、剪断による結晶化度の低下を抑制するために溶媒を用いた湿式での解繊が好ましい。解繊溶媒としては特に制限されないが、例えば、水、非プロトン性溶媒が挙げられる。水は溶媒として安価であり、製造設備の防爆化等が不要であるため、好ましい。非プロトン性溶媒は、セルロース原料を浸漬させると、セルロースの膨潤が短時間で起こり、わずかな攪拌と剪断エネルギーを与えるだけで微細化していく。又、微細化前又は微細化時又は微細化後にセルロース修飾化剤を加えることにより、化学修飾された微細セルロース繊維(化学修飾微細セルロース繊維ともいう)を溶媒置換せずに直接得ることができる。したがって、非プロトン性溶媒は製造効率の観点から好ましい。 There is no particular restriction on the method of reducing the fiber diameter in order to convert the cellulose raw material into fine cellulose fibers, but it is preferable to make the defibration processing conditions (method of applying a shear field, size of the shear field, etc.) more efficient. In addition, in order to suppress the decrease in crystallinity due to shear, wet defibration using a solvent is preferable. There is no particular restriction on the defibration solvent, but examples include water and aprotic solvents. Water is preferable because it is an inexpensive solvent and does not require explosion-proofing of the production equipment. When the cellulose raw material is immersed in an aprotic solvent, cellulose swells in a short time, and the cellulose is finely pulverized by only applying slight stirring and shear energy. In addition, by adding a cellulose modifying agent before, during, or after fine pulverization, chemically modified fine cellulose fibers (also called chemically modified fine cellulose fibers) can be obtained directly without solvent replacement. Therefore, aprotic solvents are preferable from the viewpoint of production efficiency.
非プロトン性溶媒としては、例えば、アルキルスルホキシド類、アルキルアミド類、ピロリドン類等が挙げられる。これらの溶媒は、単独で又は二種以上組み合わせて使用できる。 Examples of aprotic solvents include alkyl sulfoxides, alkyl amides, pyrrolidones, etc. These solvents can be used alone or in combination of two or more.
アルキルスルホキシド類としては、例えば、ジメチルスルホキシド(DMSO)、メチルエチルスルホキシド、ジエチルスルホキシド等のジC1-4アルキルスルホキシド等が挙げられる。 Examples of alkyl sulfoxides include di-C1-4 alkyl sulfoxides such as dimethyl sulfoxide (DMSO), methyl ethyl sulfoxide, and diethyl sulfoxide.
アルキルアミド類としては、例えば、N,N-ジメチルホルムアミド(DMF)、N,N-ジエチルホルムアミド等のN,N-ジC1-4アルキルホルムアミド;N,N-ジメチルアセトアミド(DMAc)、N,N-ジエチルアセトアミド等のN,N-ジC1-4アルキルアセトアミド等が挙げられる。 Examples of alkylamides include N,N-diC1-4 alkylformamides such as N,N-dimethylformamide (DMF) and N,N-diethylformamide; and N,N-diC1-4 alkylacetamides such as N,N-dimethylacetamide (DMAc) and N,N-diethylacetamide.
ピロリドン類としては、例えば、2-ピロリドン、3-ピロリドン等のピロリドン;N-メチル-2-ピロリドン(NMP)等のN-C1-4アルキルピロリドン等が挙げられる。 Examples of pyrrolidones include pyrrolidones such as 2-pyrrolidone and 3-pyrrolidone; N-C1-4 alkylpyrrolidone such as N-methyl-2-pyrrolidone (NMP); and the like.
これらの非プロトン性溶媒は、単独で又は二種以上組み合わせて使用できる。これらの非プロトン性溶媒のうち、DMSO、DMF、DMAc、NMP等、特に、DMSOを用いれば、化学修飾微細セルロース繊維をより効率的に製造することができる。この作用機序は必ずしも明らかではないが、非プロトン性溶媒中でのセルロース原料の均質なミクロ膨潤に起因するものと推察される。又、微細セルロース繊維製造に一般的に使用される水と非プロトン性溶媒では上述の様にセルロース原料の膨潤状態が異なるため、得られる微細セルロース繊維の繊維径及び形状が異なる場合がある。繊維径及び形状は水中や樹脂中での分散状態やネットワークの形成性と関係があり、沈降高さ比にも影響を与えると考えられる。 These aprotic solvents can be used alone or in combination of two or more. Among these aprotic solvents, DMSO, DMF, DMAc, NMP, etc., especially DMSO, can be used to more efficiently produce chemically modified fine cellulose fibers. The mechanism of action is not entirely clear, but it is presumed to be due to uniform micro-swelling of the cellulose raw material in the aprotic solvent. In addition, since the swelling state of the cellulose raw material differs between water and aprotic solvents, which are generally used in the production of fine cellulose fibers, as described above, the fiber diameter and shape of the obtained fine cellulose fibers may differ. The fiber diameter and shape are related to the dispersion state and network formation in water or resin, and are also thought to affect the sedimentation height ratio.
セルロース原料の微細化は、セルロース原料に剪断が効果的に掛かる装置であって、例えば、離解機、叩解機、リファイナー、低圧ホモジナイザー、高圧ホモジナイザー、超高圧ホモジナイザー、乳化機、ホモミキサー、グラインダー、マスコロイダー、カッターミル、ボールミル、ビーズミル、ジェットミル、単軸押出機、2軸押出機、超音波攪拌機、家庭用ジューサーミキサー等を用いることができる。中でも、非プロトン性溶媒を用いたホモミキサーでの微細化は低エネルギーで解繊できるとともに、非水系での微細セルロース繊維の化学修飾が可能となるため、好ましい。 The cellulose raw material can be refined using a device that effectively applies shear to the cellulose raw material, such as a disintegrator, beater, refiner, low-pressure homogenizer, high-pressure homogenizer, ultra-high-pressure homogenizer, emulsifier, homomixer, grinder, mass colloider, cutter mill, ball mill, bead mill, jet mill, single-screw extruder, twin-screw extruder, ultrasonic agitator, or household juicer mixer. Among these, the homomixer using an aprotic solvent is preferred because it can defibrate the cellulose raw material with low energy and allows chemical modification of the fine cellulose fibers in a non-aqueous system.
ホモミキサーでの微細化の手順としては、これに限定されないが以下を例示できる。・一態様において、ホモミキサーは、内容物を供給するための第一手段、該第一手段からの内容物に剪断力と突出圧を生じさせることができる回転要素を有する第二手段、及び任意に、該第二手段からの内容物を該第一手段に戻すことができる第三手段を備える。一態様においては、第一手段の下部に第二手段を配置し、内容物を、第一手段により第二手段に送り込み、第二手段の吐出圧により第三手段を介して第一手段に戻すことで、剪断力によるセルロースの解繊を極めて効率的に実施できる。第一手段は、撹拌プロペラ等のアジテーターであってよく、当該アジテーターは、タンク内壁面に沿って内容物を削ぎ落すことができるスクレーパーを有してよい。第二手段は、タンク底部に取り付けられた超高速ホモジナイザー(ローター/ステーター方式)等であってよい。第二手段は、剪断力、固形分同士の衝突等のエネルギーにより内容物をサブマイクロレベルへ粉砕、分散できる。第三手段は、第二手段からの内容物(例えばホモジナイザーからの吐出物)をタンク上部又は中段に送り出して第一手段に戻す再循環パイプ等であってよい。 The procedure for pulverization in the homomixer includes, but is not limited to, the following. In one embodiment, the homomixer includes a first means for supplying the contents, a second means having a rotating element capable of generating a shear force and a protruding pressure on the contents from the first means, and optionally a third means capable of returning the contents from the second means to the first means. In one embodiment, the second means is disposed below the first means, the contents are fed to the second means by the first means, and are returned to the first means via the third means by the discharge pressure of the second means, thereby enabling extremely efficient defibration of cellulose by shear force. The first means may be an agitator such as a stirring propeller, and the agitator may have a scraper capable of scraping off the contents along the inner wall surface of the tank. The second means may be an ultra-high speed homogenizer (rotor/stator type) attached to the bottom of the tank. The second means can crush and disperse the contents to a sub-micron level by energy such as shear force and collision between solids. The third means may be a recirculation pipe that sends the contents from the second means (e.g., the discharge from the homogenizer) to the top or middle of the tank and returns it to the first means.
一態様において、タンク容量35L以上のホモミキサーの場合、セルロース乾燥固形分1kgあたり定格消費電力量0.5~80kWhという極めて低いエネルギーをセルロース原料に付与できる。このような低エネルギー解繊は、微細セルロース繊維の沈降高さを容易に制御できる点で好適である。一態様において、第二手段の回転要素を、20m/s~80m/sの周速度で回転させ、好ましくは20℃~90℃で0.5時間~8時間、剪断力により低エネルギー解繊することは、微細セルロース繊維の沈降高さを容易に制御できる点で好適である。 In one embodiment, in the case of a homomixer with a tank capacity of 35 L or more, extremely low energy of 0.5 to 80 kWh of rated power consumption per kg of cellulose dry solids can be imparted to the cellulose raw material. Such low-energy defibration is preferable in that the settling height of the fine cellulose fibers can be easily controlled. In one embodiment, low-energy defibration using shear force by rotating the rotating element of the second means at a peripheral speed of 20 m/s to 80 m/s and preferably at 20°C to 90°C for 0.5 hours to 8 hours is preferable in that the settling height of the fine cellulose fibers can be easily controlled.
一態様において、微細セルロース繊維の数平均繊維径は、微細セルロース繊維による物性向上効果を良好に得る観点から、好ましくは2~1000nmである。微細セルロース繊維数平均繊維径は、より好ましくは4nm以上、又は5nm以上、又は10nm以上、又は15nm以上、又は20nm以上であり、より好ましくは500nm以下、又は450nm以下、又は400nm以下、又は350nm以下、又は300nm以下、又は250nm以下である。 In one aspect, the number average fiber diameter of the microfine cellulose fibers is preferably 2 to 1000 nm from the viewpoint of obtaining a good effect of improving physical properties by the microfine cellulose fibers. The number average fiber diameter of the microfine cellulose fibers is more preferably 4 nm or more, or 5 nm or more, or 10 nm or more, or 15 nm or more, or 20 nm or more, and more preferably 500 nm or less, or 450 nm or less, or 400 nm or less, or 350 nm or less, or 300 nm or less, or 250 nm or less.
微細セルロース繊維の平均L/Dは、微細セルロース繊維を含む樹脂複合体の機械的特性を少量の微細セルロース繊維で良好に向上させる観点から、好ましくは、50以上、又は80以上、又は100以上、又は120以上、又は150以上である。上限は特に限定されないが、取扱い性の観点から好ましくは5000以下である。 The average L/D of the fine cellulose fibers is preferably 50 or more, or 80 or more, or 100 or more, or 120 or more, or 150 or more, from the viewpoint of effectively improving the mechanical properties of the resin composite containing the fine cellulose fibers with a small amount of the fine cellulose fibers. The upper limit is not particularly limited, but is preferably 5,000 or less from the viewpoint of handleability.
本開示で、微細セルロース繊維の各々の長さ、径、及びL/D比は、微細セルロース繊維の水分散液を水溶性溶媒(例えば、水、エタノール、tert-ブタノール等)で0.01~0.1質量%まで希釈し、高剪断ホモジナイザー(例えばIKA製、商品名「ウルトラタラックスT18」)を用い、処理条件:回転数25,000rpm×5分間で分散させ、マイカ上にキャストし、風乾したものを測定サンプルとし、高分解能走査型顕微鏡(SEM)又は原子間力顕微鏡(AFM)で計測して求める。具体的には、少なくとも100本の繊維状物質が観測されるように倍率が調整された観察視野にて、無作為に選んだ100本の繊維状物質の長さ(L)及び径(D)を計測し、比(L/D)を算出する。微細セルロース繊維について、長さ(L)の数平均値、径(D)の数平均値、及び比(L/D)の数平均値を算出する。 In the present disclosure, the length, diameter, and L/D ratio of each of the fine cellulose fibers are determined by diluting an aqueous dispersion of the fine cellulose fibers with a water-soluble solvent (e.g., water, ethanol, tert-butanol, etc.) to 0.01 to 0.1% by mass, dispersing the fibers using a high-shear homogenizer (e.g., IKA, product name "Ultra Turrax T18") under processing conditions: rotation speed of 25,000 rpm for 5 minutes, casting the fibers on mica, and air-drying the fibers to obtain a measurement sample, which is then measured using a high-resolution scanning electron microscope (SEM) or atomic force microscope (AFM). Specifically, the length (L) and diameter (D) of 100 randomly selected fibrous substances are measured in an observation field where the magnification is adjusted so that at least 100 fibrous substances are observed, and the ratio (L/D) is calculated. The number average value of the length (L), the number average value of the diameter (D), and the number average value of the ratio (L/D) of the fine cellulose fibers are calculated.
なお、後述の樹脂複合体中の微細セルロース繊維の長さ、径、及びL/D比は、固体である樹脂複合体を測定サンプルとして、上述の測定方法により測定することで確認することができる。又は、樹脂複合体中の微細セルロース繊維の長さ、径、及びL/D比は、樹脂複合体の樹脂成分を溶解できる有機又は無機の溶媒に樹脂複合体中の樹脂成分を溶解させ、微細セルロース繊維を分離し、前記溶媒で充分に洗浄した後、水溶性溶媒(例えば、水、エタノール、tert-ブタノール等)で置換し、0.01~0.1質量%分散液を調製し、高剪断ホモジナイザー(例えばIKA製、商品名「ウルトラタラックスT18」)で再分散する。再分散液をマイカ上にキャストし、風乾したものを測定サンプルとして上述の測定方法により測定することで確認することができる。この際、測定する微細セルロース繊維は無作為に選んだ100本以上での測定を行う。 The length, diameter, and L/D ratio of the fine cellulose fibers in the resin composite described below can be confirmed by measuring the solid resin composite as a measurement sample using the above-mentioned measurement method. Alternatively, the length, diameter, and L/D ratio of the fine cellulose fibers in the resin composite can be confirmed by dissolving the resin components in the resin composite in an organic or inorganic solvent that can dissolve the resin components of the resin composite, separating the fine cellulose fibers, thoroughly washing with the solvent, and then replacing with a water-soluble solvent (e.g., water, ethanol, tert-butanol, etc.), preparing a 0.01 to 0.1 mass% dispersion, and redispersing with a high-shear homogenizer (e.g., IKA, product name "Ultra Turrax T18"). The redispersion can be confirmed by casting the redispersion on mica, air-drying it, and measuring it as a measurement sample using the above-mentioned measurement method. At this time, the measurement is performed on 100 or more randomly selected fine cellulose fibers.
微細セルロース繊維の結晶化度は、好ましくは55%以上である。結晶化度がこの範囲にあると、セルロース繊維自体の力学物性(強度、寸法安定性)が高いため、セルロース繊維を樹脂に分散した際に、樹脂複合体の強度、寸法安定性が高い傾向にある。より好ましい結晶化度の下限は、60%であり、さらにより好ましくは70%であり、最も好ましくは80%である。微細セルロース繊維の結晶化度についても上限は特に限定されず、高い方が好ましいが、生産上の観点から好ましい上限は99%である。 The crystallinity of the fine cellulose fibers is preferably 55% or more. When the crystallinity is in this range, the mechanical properties (strength, dimensional stability) of the cellulose fibers themselves are high, so that when the cellulose fibers are dispersed in a resin, the strength and dimensional stability of the resin composite tend to be high. A more preferable lower limit of the crystallinity is 60%, even more preferably 70%, and most preferably 80%. There is no particular upper limit for the crystallinity of the fine cellulose fibers, and the higher the better, but from a production standpoint, a preferable upper limit is 99%.
植物由来のセルロースのミクロフィブリル同士の間、及びミクロフィブリル束同士の間には、ヘミセルロース等のアルカリ可溶多糖類、及びリグニン等の酸不溶成分が存在する。ヘミセルロースはマンナン、キシラン等の糖で構成される多糖類であり、セルロースと水素結合して、ミクロフィブリル間を結びつける役割を果たしている。またリグニンは芳香環を有する化合物であり、植物の細胞壁中ではヘミセルロースと共有結合していることが知られている。微細セルロース繊維中のリグニン等の不純物の残存量が多いと、加工時の熱により変色をきたすことがあるため、押出加工時及び成形加工時の樹脂複合体の変色を抑制する観点からも、微細セルロース繊維の結晶化度は上述の範囲内にすることが望ましい。 Between the microfibrils of plant-derived cellulose and between the microfibril bundles, there are alkali-soluble polysaccharides such as hemicellulose, and acid-insoluble components such as lignin. Hemicellulose is a polysaccharide composed of sugars such as mannan and xylan, and forms hydrogen bonds with cellulose to bind the microfibrils together. Lignin is also a compound with an aromatic ring, and is known to be covalently bonded to hemicellulose in the cell walls of plants. If there is a large amount of impurities such as lignin remaining in the fine cellulose fibers, discoloration may occur due to heat during processing. Therefore, from the viewpoint of suppressing discoloration of the resin composite during extrusion processing and molding processing, it is desirable to set the crystallinity of the fine cellulose fibers within the above-mentioned range.
ここでいう結晶化度は、微細セルロース繊維がセルロースI型結晶(天然セルロース由来)である場合には、サンプルを広角X線回折により測定した際の回折パターン(2θ/deg.が10~30)からSegal法により、以下の式で求められる。
結晶化度(%)=([2θ/deg.=22.5の(200)面に起因する回折強度]-[2θ/deg.=18の非晶質に起因する回折強度])/[2θ/deg.=22.5の(200)面に起因する回折強度]×100
When the fine cellulose fibers are cellulose type I crystals (derived from natural cellulose), the degree of crystallinity can be calculated from the diffraction pattern (2θ/deg. is 10 to 30) obtained by measuring a sample by wide-angle X-ray diffraction using the Segal method, as shown in the following formula.
Crystallinity (%)=([diffraction intensity attributable to the (200) plane at 2θ/deg.=22.5]−[diffraction intensity attributable to amorphous at 2θ/deg.=18])/[diffraction intensity attributable to the (200) plane at 2θ/deg.=22.5]×100
また結晶化度は、微細セルロース繊維がセルロースII型結晶(再生セルロース由来)である場合には、広角X線回折において、セルロースII型結晶の(110)面ピークに帰属される2θ=12.6°における絶対ピーク強度h0 とこの面間隔におけるベースラインからのピーク強度h1 とから、下記式によって求められる。
結晶化度(%) =h1 /h0 ×100
When the fine cellulose fibers are cellulose II type crystals (derived from regenerated cellulose), the degree of crystallinity can be calculated from the absolute peak intensity h0 at 2θ=12.6° assigned to the (110) plane peak of the cellulose II type crystals in wide-angle X-ray diffraction and the peak intensity h1 from the baseline at this interplanar spacing, according to the following formula:
Crystallinity (%) = h1 /h0 ×100
セルロースの結晶形としては、I型、II型、III型、IV型などが知られており、その中でも特にI型及びII型は汎用されており、III型、IV型は実験室スケールでは得られているものの工業スケールでは汎用されていない。本開示の微細セルロース繊維としては、構造上の可動性が比較的高く、当該微細セルロース繊維を樹脂に分散させることにより、線膨張係数がより低く、引っ張り、曲げ変形時の強度及び伸びがより優れた樹脂複合体が得られることから、セルロースI型結晶又はセルロースII型結晶を含有するセルロース繊維が好ましく、セルロースI型結晶を含有し、かつ結晶化度が55%以上の微細セルロース繊維がより好ましい。 Known crystalline forms of cellulose include types I, II, III, and IV, of which types I and II are particularly widely used, and types III and IV have been obtained on a laboratory scale but are not widely used on an industrial scale. The fine cellulose fibers disclosed herein have relatively high structural mobility, and by dispersing the fine cellulose fibers in a resin, a resin composite having a lower linear expansion coefficient and superior strength and elongation during tensile and bending deformation can be obtained. Therefore, cellulose fibers containing cellulose type I crystals or cellulose type II crystals are preferred, and fine cellulose fibers containing cellulose type I crystals and having a crystallinity of 55% or more are more preferred.
また、微細セルロース繊維の重合度は、好ましくは100以上、より好ましくは150以上であり、より好ましくは200以上、より好ましくは300以上、より好ましくは400以上であり、好ましくは3500以下、より好ましく3300以下、より好ましくは3200以下、より好ましくは3100以下、より好ましくは3000以下である。 The degree of polymerization of the fine cellulose fibers is preferably 100 or more, more preferably 150 or more, more preferably 200 or more, more preferably 300 or more, more preferably 400 or more, and preferably 3500 or less, more preferably 3300 or less, more preferably 3200 or less, more preferably 3100 or less, more preferably 3000 or less.
加工性と機械的特性発現との観点から、微細セルロース繊維の重合度を上述の範囲内とすることが望ましい。加工性の観点から、重合度は高すぎない方が好ましく、機械的特性発現の観点からは低すぎないことが望まれる。 From the viewpoint of processability and mechanical property expression, it is desirable that the degree of polymerization of the fine cellulose fiber is within the above-mentioned range. From the viewpoint of processability, it is preferable that the degree of polymerization is not too high, and from the viewpoint of mechanical property expression, it is desirable that the degree of polymerization is not too low.
微細セルロース繊維の重合度は、「第十五改正日本薬局方解説書(廣川書店発行)」の確認試験(3)に記載の銅エチレンジアミン溶液による還元比粘度法に従って測定される平均重合度を意味する。 The degree of polymerization of fine cellulose fibers refers to the average degree of polymerization measured according to the reduced specific viscosity method using a copper ethylenediamine solution as described in the Verification Test (3) of the "15th Revised Japanese Pharmacopoeia Commentary (published by Hirokawa Shoten)."
一態様において、微細セルロース繊維の重量平均分子量(Mw)は100000以上であり、より好ましくは200000以上、さらに好ましくは250000以上である。重量平均分子量と数平均分子量(Mn)との比(Mw/Mn)は10以下、又は9以下、又は8以下、又は7以下、又は6以下、好ましくは5.4以下である。重量平均分子量が大きいほどセルロース分子の末端基の数は少ないことを意味する。また、重量平均分子量と数平均分子量との比(Mw/Mn)は分子量分布の幅を表すものであることから、Mw/Mnが小さいほどセルロース分子の末端の数は少ないことを意味する。セルロース分子の末端は熱分解の起点となるため、微細セルロース繊維のセルロース分子の重量平均分子量が大きいだけでなく、重量平均分子量が大きいと同時に分子量分布の幅が狭い場合に、特に高耐熱性の微細セルロース繊維、及び微細セルロース繊維と樹脂とを含む樹脂複合体が得られる。微細セルロース繊維の重量平均分子量(Mw)は、セルロース原料の入手容易性の観点から、例えば1000000以下、又は900000以下、又は800000以下、又は700000以下、又は600000以下、又は500000以下、であってよい。重量平均分子量と数平均分子量(Mn)との比(Mw/Mn)は微細セルロース繊維の製造容易性の観点から、例えば1.5以上、又は2以上であってよい。Mwは、目的に応じたMwを有するセルロース原料を選択すること、セルロース原料に対して物理的処理及び/又は化学的処理を適度な範囲で適切に行うこと、等によって上記範囲に制御できる。Mw/Mnもまた、目的に応じたMw/Mnを有するセルロース原料を選択すること、セルロース原料に対して物理的処理及び/又は化学的処理を適度な範囲で適切に行うこと、等によって上記範囲に制御できる。Mwの制御、及びMw/Mnの制御の両者において、上記物理的処理としては、マイクロフリュイダイザー、ボールミル、ディスクミル等の乾式粉砕若しくは湿式粉砕、擂潰機、ホモミキサー、高圧ホモジナイザー、超音波装置等による衝撃、剪断、ずり、摩擦等の機械的な力を加える物理的処理を例示でき、上記化学的処理としては、蒸解、漂白、酸処理、再生セルロース化等を例示できる。 In one embodiment, the weight average molecular weight (Mw) of the fine cellulose fiber is 100,000 or more, more preferably 200,000 or more, and even more preferably 250,000 or more. The ratio (Mw/Mn) of the weight average molecular weight to the number average molecular weight (Mn) is 10 or less, or 9 or less, or 8 or less, or 7 or less, or 6 or less, and preferably 5.4 or less. The larger the weight average molecular weight, the fewer the number of terminal groups of the cellulose molecule. In addition, since the ratio (Mw/Mn) of the weight average molecular weight to the number average molecular weight represents the width of the molecular weight distribution, the smaller the Mw/Mn, the fewer the number of terminals of the cellulose molecule. Since the terminals of the cellulose molecules are the starting points of thermal decomposition, particularly when the weight average molecular weight of the cellulose molecules of the fine cellulose fibers is large and the weight average molecular weight is large and the width of the molecular weight distribution is narrow, a particularly high heat resistant fine cellulose fiber and a resin composite containing the fine cellulose fibers and the resin can be obtained. The weight average molecular weight (Mw) of the fine cellulose fiber may be, for example, 1,000,000 or less, or 900,000 or less, or 800,000 or less, or 700,000 or less, or 600,000 or less, or 500,000 or less, from the viewpoint of the availability of the cellulose raw material. The ratio (Mw/Mn) of the weight average molecular weight to the number average molecular weight (Mn) may be, for example, 1.5 or more, or 2 or more, from the viewpoint of the ease of production of the fine cellulose fiber. Mw can be controlled to the above range by selecting a cellulose raw material having an Mw according to the purpose, appropriately performing physical treatment and/or chemical treatment on the cellulose raw material in an appropriate range, etc. Mw/Mn can also be controlled to the above range by selecting a cellulose raw material having an Mw/Mn according to the purpose, appropriately performing physical treatment and/or chemical treatment on the cellulose raw material in an appropriate range, etc. In both the control of Mw and the control of Mw/Mn, examples of the physical treatment include dry or wet grinding using a microfluidizer, ball mill, or disk mill, or physical treatment that applies mechanical forces such as impact, shear, shear, or friction using a crusher, homomixer, high-pressure homogenizer, or ultrasonic device, and examples of the chemical treatment include digestion, bleaching, acid treatment, and conversion to regenerated cellulose.
ここでいうセルロースの重量平均分子量及び数平均分子量とは、セルロースを塩化リチウムが添加されたN,N-ジメチルアセトアミドに溶解させたうえで、N,N-ジメチルアセトアミドを溶媒としてゲルパーミエーションクロマトグラフィによって求めた値である。 The weight average molecular weight and number average molecular weight of cellulose referred to here are values obtained by dissolving cellulose in N,N-dimethylacetamide containing added lithium chloride, and then performing gel permeation chromatography using N,N-dimethylacetamide as a solvent.
微細セルロース繊維の重合度(すなわち平均重合度)又は分子量を制御する方法としては、加水分解処理等が挙げられる。加水分解処理によって、微細セルロース繊維内部の非晶質セルロースの解重合が進み、平均重合度が小さくなる。また同時に、加水分解処理により、上述の非晶質セルロースに加え、ヘミセルロースやリグニン等の不純物も取り除かれるため、繊維質内部が多孔質化する。 Methods for controlling the degree of polymerization (i.e., average degree of polymerization) or molecular weight of fine cellulose fibers include hydrolysis. Hydrolysis promotes depolymerization of the amorphous cellulose inside the fine cellulose fibers, decreasing the average degree of polymerization. At the same time, hydrolysis removes impurities such as hemicellulose and lignin in addition to the above-mentioned amorphous cellulose, making the inside of the fibers more porous.
加水分解の方法は、特に制限されないが、酸加水分解、アルカリ加水分解、熱水分解、スチームエクスプロージョン、マイクロ波分解等が挙げられる。これらの方法は、単独で使用してもよく、2種以上を併用してもよい。酸加水分解の方法では、例えば、繊維性植物からパルプとして得たα-セルロースをセルロース原料とし、これを水系媒体に分散させた状態で、プロトン酸、カルボン酸、ルイス酸、ヘテロポリ酸等を適量加え、攪拌しながら加温することにより、容易に平均重合度を制御できる。この際の温度、圧力、時間等の反応条件は、セルロース種、セルロース濃度、酸種、酸濃度等により異なるが、目的とする平均重合度が達成されるよう適宜調製されるものである。例えば、2質量%以下の鉱酸水溶液を使用し、100℃以上、加圧下で、10分間以上セルロースを処理するという条件が挙げられる。この条件のとき、酸等の触媒成分が微細セルロース繊維内部まで浸透し、加水分解が促進され、使用する触媒成分量が少なくなり、その後の精製も容易になる。なお、加水分解時のセルロース原料の分散液は、水の他、本発明の効果を損なわない範囲において有機溶媒を少量含んでいてもよい。 The hydrolysis method is not particularly limited, and examples thereof include acid hydrolysis, alkali hydrolysis, hydrothermal decomposition, steam explosion, microwave decomposition, and the like. These methods may be used alone or in combination of two or more. In the acid hydrolysis method, for example, α-cellulose obtained as pulp from a fibrous plant is used as the cellulose raw material, and this is dispersed in an aqueous medium, and an appropriate amount of protonic acid, carboxylic acid, Lewis acid, heteropolyacid, etc. is added and heated while stirring, so that the average degree of polymerization can be easily controlled. The reaction conditions such as temperature, pressure, and time at this time vary depending on the cellulose type, cellulose concentration, acid type, acid concentration, etc., but are appropriately adjusted so that the desired average degree of polymerization is achieved. For example, a condition is that cellulose is treated for 10 minutes or more at 100°C or higher under pressure using an aqueous mineral acid solution of 2% by mass or less. Under these conditions, the catalyst components such as acid penetrate into the inside of the fine cellulose fibers, promoting hydrolysis, reducing the amount of catalyst components used, and making subsequent purification easier. In addition, the dispersion of the cellulose raw material during hydrolysis may contain a small amount of an organic solvent in addition to water, as long as the effects of the present invention are not impaired.
微細セルロース繊維が含み得るアルカリ可溶多糖類は、ヘミセルロースのほか、β-セルロース及びγ-セルロースも包含する。アルカリ可溶多糖類とは、植物(例えば木材)を溶媒抽出及び塩素処理して得られるホロセルロースのうちのアルカリ可溶部として得られる成分(すなわちホロセルロースからα-セルロースを除いた成分)として当業者に理解される。アルカリ可溶多糖類は、水酸基を含む多糖であり耐熱性が悪く、熱がかかった場合に分解すること、熱エージング時に黄変を引き起こすこと、セルロース繊維の強度低下の原因になること等の不都合を招来し得ることから、微細セルロース繊維中のアルカリ可溶多糖類含有量は少ない方が好ましい。 The alkali-soluble polysaccharides that may be contained in the fine cellulose fibers include hemicellulose, as well as β-cellulose and γ-cellulose. Those skilled in the art will understand alkali-soluble polysaccharides as the components obtained as the alkali-soluble portion of holocellulose obtained by solvent extraction and chlorine treatment of plants (e.g., wood) (i.e., the components obtained by removing α-cellulose from holocellulose). Alkali-soluble polysaccharides are polysaccharides that contain hydroxyl groups and have poor heat resistance, and may decompose when exposed to heat, cause yellowing during thermal aging, and cause a decrease in the strength of the cellulose fibers. Therefore, it is preferable that the content of alkali-soluble polysaccharides in the fine cellulose fibers is low.
一態様において、微細セルロース繊維中のアルカリ可溶多糖類平均含有率は、微細セルロース繊維の良好な分散性を得る観点から、微細セルロース繊維100質量%に対して、好ましくは、20質量%以下、又は18質量%以下、又は15質量%以下、又は12質量%以下である。上記含有率は、微細セルロース繊維の製造容易性の観点から、1質量%以上、又は2質量%以上、又は3質量%以上であってもよい。 In one embodiment, the average content of alkali-soluble polysaccharides in the fine cellulose fibers is preferably 20% by mass or less, 18% by mass or less, 15% by mass or less, or 12% by mass or less, based on 100% by mass of the fine cellulose fibers, from the viewpoint of obtaining good dispersibility of the fine cellulose fibers. The above content may be 1% by mass or more, 2% by mass or more, or 3% by mass or more, from the viewpoint of ease of production of the fine cellulose fibers.
アルカリ可溶多糖類平均含有率は、非特許文献(木質科学実験マニュアル、日本木材学会編、92~97頁、2000年)に記載の手法より求めることができ、ホロセルロース含有率(Wise法)からαセルロース含有率を差し引くことで求められる。なおこの方法は当業界においてヘミセルロース量の測定方法として理解されている。1つのサンプルにつき3回アルカリ可溶多糖類含有率を算出し、算出したアルカリ可溶多糖類含有率の数平均をアルカリ可溶多糖類平均含有率とする。 The average alkali-soluble polysaccharide content can be determined by the method described in the non-patent literature (Wood Science Experiment Manual, edited by the Japan Wood Research Society, pp. 92-97, 2000), by subtracting the α-cellulose content from the holocellulose content (Wise method). This method is understood in the industry as a method for measuring the amount of hemicellulose. The alkali-soluble polysaccharide content is calculated three times for each sample, and the number average of the calculated alkali-soluble polysaccharide contents is taken as the average alkali-soluble polysaccharide content.
一態様において、微細セルロース繊維中の酸不溶成分平均含有率は、微細セルロース繊維の耐熱性低下及びそれに伴う変色を回避する観点から、微細セルロース繊維100質量%に対して、好ましくは、10質量%以下、又は5質量%以下、又は3質量%以下である。上記含有率は、微細セルロース繊維の製造容易性の観点から、0.01質量%以上、又は0.1質量%以上、又は0.2質量%以上、又は0.3質量%以上であってもよい。 In one embodiment, the average content of acid-insoluble components in the fine cellulose fiber is preferably 10% by mass or less, 5% by mass or less, or 3% by mass or less, based on 100% by mass of the fine cellulose fiber, from the viewpoint of avoiding a decrease in the heat resistance of the fine cellulose fiber and the associated discoloration. From the viewpoint of ease of production of the fine cellulose fiber, the above content may be 0.01% by mass or more, 0.1% by mass or more, 0.2% by mass or more, or 0.3% by mass or more.
酸不溶成分平均含有率は、非特許文献(木質科学実験マニュアル、日本木材学会編、92~97頁、2000年)に記載のクラーソン法を用いた酸不溶成分の定量として行う。なおこの方法は当業界においてリグニン量の測定方法として理解されている。硫酸溶液中でサンプルを撹拌してセルロース及びヘミセルロース等を溶解させた後、ガラスファイバーろ紙で濾過し、得られた残渣が酸不溶成分に該当する。この酸不溶成分重量より酸不溶成分含有率を算出し、そして、3サンプルについて算出した酸不溶成分含有率の数平均を酸不溶成分平均含有率とする。 The average acid-insoluble content is determined by quantifying the acid-insoluble content using the Clason method described in the non-patent literature (Wood Science Experiment Manual, edited by the Japan Wood Research Society, pp. 92-97, 2000). This method is understood in the industry as a method for measuring the amount of lignin. The sample is stirred in a sulfuric acid solution to dissolve cellulose, hemicellulose, etc., and then filtered through a glass fiber filter. The resulting residue corresponds to the acid-insoluble components. The acid-insoluble component content is calculated from the weight of this acid-insoluble component, and the number average of the acid-insoluble component contents calculated for the three samples is taken as the average acid-insoluble component content.
微細セルロース繊維は、化学処理(例えば酸化、又は修飾化剤を用いた化学修飾)がされていてもよい。一例として、Cellulose(1998)5,153-164に示されているような2,2,6,6-テトラメチルピペリジン-1-オキシルラジカルによってセルロース繊維を酸化させた後に、洗浄、機械解繊を経ることにより得られる、微細化セルロース繊維を使用してもよい。 The fine cellulose fibers may be chemically treated (e.g., oxidized or chemically modified using a modifying agent). As an example, fine cellulose fibers obtained by oxidizing cellulose fibers with 2,2,6,6-tetramethylpiperidine-1-oxyl radical as shown in Cellulose (1998) 5, 153-164, followed by washing and mechanical fiberization, may be used.
セルロースの修飾化剤としては、セルロースの水酸基と反応する化合物を使用でき、エステル化剤、エーテル化剤、及びシリル化剤が挙げられる。好ましい態様において、化学修飾は、エステル化剤を用いたアシル化である。エステル化剤としては、酸ハロゲン化物、酸無水物、及びカルボン酸ビニルエステル、カルボン酸が好ましい。 As a cellulose modification agent, a compound that reacts with the hydroxyl groups of cellulose can be used, and examples of such agents include esterifying agents, etherifying agents, and silylating agents. In a preferred embodiment, the chemical modification is acylation using an esterifying agent. As an esterifying agent, acid halides, acid anhydrides, vinyl carboxylates, and carboxylic acids are preferred.
酸ハロゲン化物は、下記式(1)で表される化合物からなる群より選択された少なくとも1種であってよい。
R1-C(=O)-X (1)
(式中、R1は炭素数1~24のアルキル基、炭素数2~24のアルケニル基、炭素数3~24のシクロアルキル基、又は炭素数6~24のアリール基を表し、XはCl、Br又はIである。)
酸ハロゲン化物の具体例としては、塩化アセチル、臭化アセチル、ヨウ化アセチル、塩化プロピオニル、臭化プロピオニル、ヨウ化プロピオニル、塩化ブチリル、臭化ブチリル、ヨウ化ブチリル、塩化ベンゾイル、臭化ベンゾイル、ヨウ化ベンゾイル等が挙げられるが、これらに限定されない。中でも、酸塩化物は反応性と取り扱い性の点から好適に採用できる。尚、酸ハロゲン化物の反応においては、触媒として働くと同時に副生物である酸性物質を中和する目的で、アルカリ性化合物を1種又は2種以上添加してもよい。アルカリ性化合物としては、具体的には:トリエチルアミン、トリメチルアミン等の3級アミン化合物;及びピリジン、ジメチルアミノピリジン等の含窒素芳香族化合物;が挙げられるが、これに限定されない。
The acid halide may be at least one selected from the group consisting of compounds represented by the following formula (1):
R 1 -C(=O)-X (1)
(In the formula, R1 represents an alkyl group having 1 to 24 carbon atoms, an alkenyl group having 2 to 24 carbon atoms, a cycloalkyl group having 3 to 24 carbon atoms, or an aryl group having 6 to 24 carbon atoms, and X represents Cl, Br, or I.)
Specific examples of acid halides include, but are not limited to, acetyl chloride, acetyl bromide, acetyl iodide, propionyl chloride, propionyl bromide, propionyl iodide, butyryl chloride, butyryl bromide, butyryl iodide, benzoyl chloride, benzoyl bromide, and benzoyl iodide. Among them, acid chlorides can be preferably used in terms of reactivity and handling. In addition, in the reaction of acid halides, one or more alkaline compounds may be added to act as a catalyst and neutralize the by-product acidic substances. Specific examples of alkaline compounds include, but are not limited to, tertiary amine compounds such as triethylamine and trimethylamine; and nitrogen-containing aromatic compounds such as pyridine and dimethylaminopyridine.
酸無水物としては、任意の適切な酸無水物類を用いることができる。例えば、
酢酸、プロピオン酸、(イソ)酪酸、吉草酸等の飽和脂肪族モノカルボン酸無水物;(メタ)アクリル酸、オレイン酸等の不飽和脂肪族モノカルボン酸無水物;
シクロヘキサンカルボン酸、テトラヒドロ安息香酸等の脂環族モノカルボン酸無水物;
安息香酸、4-メチル安息香酸等の芳香族モノカルボン酸無水物;
二塩基カルボン酸無水物として、例えば、無水コハク酸、アジピン酸等の無水飽和脂肪族ジカルボン酸、無水マレイン酸、無水イタコン酸等の無水不飽和脂肪族ジカルボン酸無水物、無水1-シクロヘキセン-1,2-ジカルボン酸、無水ヘキサヒドロフタル酸、無水メチルテトラヒドロフタル酸等の無水脂環族ジカルボン酸、及び、無水フタル酸、無水ナフタル酸等の無水芳香族ジカルボン酸無水物等;
3塩基以上の多塩基カルボン酸無水物類として、例えば、無水トリメリット酸、無水ピロメリット酸等の(無水)ポリカルボン酸等が挙げられる。
尚、酸無水物の反応においては、触媒として、硫酸、塩酸、燐酸等の酸性化合物、又はルイス酸、(例えば、MYnで表されるルイス酸化合物であって、MはB、As,Ge等の半金属元素、又はAl、Bi、In等の卑金属元素、又はTi、Zn、Cu等の遷移金属元素、又はランタノイド元素を表し、nはMの原子価に相当する整数であり、2又は3を表し、Yはハロゲン原子、OAc、OCOCF3、ClO4、SbF6、PF6又はOSO2CF3(OTf)を表す。)、又はトリエチルアミン、ピリジン等のアルカリ性化合物を1種又は2種以上添加してもよい。
As the acid anhydride, any suitable acid anhydride can be used. For example,
Saturated aliphatic monocarboxylic acid anhydrides, such as acetic acid, propionic acid, (iso)butyric acid, and valeric acid; unsaturated aliphatic monocarboxylic acid anhydrides, such as (meth)acrylic acid and oleic acid;
Alicyclic monocarboxylic acid anhydrides such as cyclohexanecarboxylic acid and tetrahydrobenzoic acid;
Aromatic monocarboxylic acid anhydrides such as benzoic acid and 4-methylbenzoic acid;
Examples of dibasic carboxylic acid anhydrides include saturated aliphatic dicarboxylic acid anhydrides such as succinic anhydride and adipic acid, unsaturated aliphatic dicarboxylic acid anhydrides such as maleic anhydride and itaconic anhydride, alicyclic dicarboxylic acid anhydrides such as 1-cyclohexene-1,2-dicarboxylic acid anhydride, hexahydrophthalic anhydride and methyltetrahydrophthalic anhydride, and aromatic dicarboxylic acid anhydrides such as phthalic anhydride and naphthalic anhydride;
Examples of the polybasic carboxylic acid anhydrides having three or more bases include polycarboxylic acids (anhydrides) such as trimellitic anhydride and pyromellitic anhydride.
In addition, in the reaction of an acid anhydride, one or more types of acidic compounds such as sulfuric acid, hydrochloric acid, phosphoric acid, etc., or Lewis acids (for example, Lewis acid compounds represented by MYn, where M represents a semimetal element such as B, As, Ge, etc., or a base metal element such as Al, Bi, In, etc., or a transition metal element such as Ti, Zn, Cu, etc., or a lanthanoid element, n is an integer corresponding to the atomic valence of M and represents 2 or 3, and Y represents a halogen atom, OAc, OCOCF3 , ClO4 , SbF6 , PF6 , or OSO2CF3 (OTf)), or alkaline compounds such as triethylamine, pyridine, etc. may be added as a catalyst .
カルボン酸ビニルエステルとしては、下記式(1):
R-COO-CH=CH2 …式(1)
{式中、Rは、炭素数1~24のアルキル基、炭素数2~24のアルケニル基、炭素数3~16のシクロアルキル基、又は炭素数6~24のアリール基のいずれかである。}で表されるカルボン酸ビニルエステルが好ましい。カルボン酸ビニルエステルは、酢酸ビニル、プロピオン酸ビニル、酪酸ビニル、カプロン酸ビニル、シクロヘキサンカルボン酸ビニル、カプリル酸ビニル、カプリン酸ビニル、ラウリン酸ビニル、ミリスチン酸ビニル、パルミチン酸ビニル、ステアリン酸ビニル、ピバリン酸ビニル、オクチル酸ビニルアジピン酸ジビニル、メタクリル酸ビニル、クロトン酸ビニル、ピバリン酸ビニル、オクチル酸ビニル、安息香酸ビニル、及び桂皮酸ビニルからなる群より選択された少なくとも1種であることがより好ましい。カルボン酸ビニルエステルによるエステル化反応のとき、触媒として、アルカリ金属水酸化物、アルカリ土類金属水酸化物、アルカリ金属炭酸塩、アルカリ土類金属炭酸塩、アルカリ金属炭酸水素塩、1~3級アミン、4級アンモニウム塩、イミダゾール及びその誘導体、ピリジン及びその誘導体、並びにアルコキシドからなる群より選ばれる1種又は2種以上を添加しても良い。
The vinyl carboxylate may be represented by the following formula (1):
R-COO-CH=CH 2 ...Formula (1)
{wherein R is any one of an alkyl group having 1 to 24 carbon atoms, an alkenyl group having 2 to 24 carbon atoms, a cycloalkyl group having 3 to 16 carbon atoms, and an aryl group having 6 to 24 carbon atoms.} is preferred. The vinyl carboxylate is more preferably at least one selected from the group consisting of vinyl acetate, vinyl propionate, vinyl butyrate, vinyl caproate, vinyl cyclohexanecarboxylate, vinyl caprylate, vinyl caprate, vinyl laurate, vinyl myristate, vinyl palmitate, vinyl stearate, vinyl pivalate, vinyl octylate, divinyl adipate, vinyl methacrylate, vinyl crotonate, vinyl pivalate, vinyl octylate, vinyl benzoate, and vinyl cinnamate. In the esterification reaction with a vinyl carboxylate, one or more catalysts selected from the group consisting of alkali metal hydroxides, alkaline earth metal hydroxides, alkali metal carbonates, alkaline earth metal carbonates, alkali metal hydrogencarbonates, primary to tertiary amines, quaternary ammonium salts, imidazole and derivatives thereof, pyridine and derivatives thereof, and alkoxides may be added.
アルカリ金属水酸化物及びアルカリ土類金属水酸化物としては、水酸化ナトリウム、水酸化カリウム、水酸化リチウム、水酸化カルシウム、水酸化バリウム等が挙げられる。 アルカリ金属炭酸塩、アルカリ土類金属炭酸塩、アルカリ金属炭酸水素塩としては、炭酸リチウム、炭酸ナトリウム、炭酸カリウム、炭酸セシウム、炭酸マグネシウム、炭酸カルシウム、炭酸バリウム、炭酸水素リチウム、炭酸水素ナトリウム、炭酸水素カリウム、炭酸水素セシウム等が挙げられる。 Examples of alkali metal hydroxides and alkaline earth metal hydroxides include sodium hydroxide, potassium hydroxide, lithium hydroxide, calcium hydroxide, barium hydroxide, etc. Examples of alkali metal carbonates, alkaline earth metal carbonates, and alkali metal hydrogen carbonates include lithium carbonate, sodium carbonate, potassium carbonate, cesium carbonate, magnesium carbonate, calcium carbonate, barium carbonate, lithium hydrogen carbonate, sodium hydrogen carbonate, potassium hydrogen carbonate, and cesium hydrogen carbonate, etc.
1~3級アミンとは、1級アミン、2級アミン、及び3級アミンのことであり、具体例としては、エチレンジアミン、ジエチルアミン、プロリン、N,N,N’,N’-テトラメチルエチレンジアミン、N,N,N’,N’-テトラメチル-1,3-プロパンジアミン、N,N,N’,N’-テトラメチル-1,6-ヘキサンジアミン、トリス(3-ジメチルアミノプロピル)アミン、N,N-ジメチルシクロヘキシルアミン、トリエチルアミン等が挙げられる。 Primary, secondary and tertiary amines refer to primary, secondary and tertiary amines, and specific examples include ethylenediamine, diethylamine, proline, N,N,N',N'-tetramethylethylenediamine, N,N,N',N'-tetramethyl-1,3-propanediamine, N,N,N',N'-tetramethyl-1,6-hexanediamine, tris(3-dimethylaminopropyl)amine, N,N-dimethylcyclohexylamine and triethylamine.
イミダゾール及びその誘導体としては、1-メチルイミダゾール、3-アミノプロピルイミダゾール、カルボニルジイミダゾール等が挙げられる。 Imidazole and its derivatives include 1-methylimidazole, 3-aminopropylimidazole, carbonyldiimidazole, etc.
ピリジン及びその誘導体としては、N,N-ジメチル-4-アミノピリジン、ピコリン等が挙げられる。 Examples of pyridine and its derivatives include N,N-dimethyl-4-aminopyridine and picoline.
アルコキシドとしては、ナトリウムメトキシド、ナトリウムエトキシド、カリウム-t-ブトキシド等が挙げられる。 Examples of alkoxides include sodium methoxide, sodium ethoxide, and potassium t-butoxide.
カルボン酸としては、下記式(1)で表される化合物からなる群より選択される少なくとも1種が挙げられる。
R-COOH …(1)
(式中、Rは、炭素数1~16のアルキル基、炭素数2~16のアルケニル基、炭素数3~16のシクロアルキル基、又は炭素数6~16のアリール基を表す。)
The carboxylic acid may be at least one selected from the group consisting of compounds represented by the following formula (1).
R-COOH…(1)
(In the formula, R represents an alkyl group having 1 to 16 carbon atoms, an alkenyl group having 2 to 16 carbon atoms, a cycloalkyl group having 3 to 16 carbon atoms, or an aryl group having 6 to 16 carbon atoms.)
カルボン酸の具体例としては、酢酸、プロピオン酸、酪酸、カプロン酸、シクロヘキサンカルボン酸、カプリル酸、カプリン酸、ラウリン酸、ミリスチン酸、パルミチン酸、ステアリン酸、ピバリン酸、メタクリル酸、クロトン酸、ピバリン酸、オクチル酸、安息香酸、及び桂皮酸からなる群より選択される少なくとも1種が挙げられる。 Specific examples of carboxylic acids include at least one selected from the group consisting of acetic acid, propionic acid, butyric acid, caproic acid, cyclohexane carboxylic acid, caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, pivalic acid, methacrylic acid, crotonic acid, pivalic acid, octylic acid, benzoic acid, and cinnamic acid.
これらカルボン酸の中でも、酢酸、プロピオン酸、及び酪酸からなる群から選択される少なくとも一種、特に酢酸が、反応効率の観点から好ましい。
尚、カルボン酸の反応においては、触媒として、硫酸、塩酸、燐酸等の酸性化合物、又はルイス酸、(例えば、MYnで表されるルイス酸化合物であって、MはB、As,Ge等の半金属元素、又はAl、Bi、In等の卑金属元素、又はTi、Zn、Cu等の遷移金属元素、又はランタノイド元素を表し、nはMの原子価に相当する整数であり、2又は3を表し、Yはハロゲン原子、OAc、OCOCF3、ClO4、SbF6、PF6又はOSO2CF3(OTf)を表す。)、又はトリエチルアミン、ピリジン等のアルカリ性化合物を1種又は2種以上添加してもよい。
Among these carboxylic acids, at least one selected from the group consisting of acetic acid, propionic acid, and butyric acid, and particularly acetic acid, is preferred from the viewpoint of reaction efficiency.
In addition, in the reaction of carboxylic acid, one or more types of acidic compounds such as sulfuric acid, hydrochloric acid, phosphoric acid, etc., or Lewis acids (for example, Lewis acid compounds represented by MYn, where M represents a semimetal element such as B, As, Ge, etc., or a base metal element such as Al, Bi, In, etc., or a transition metal element such as Ti, Zn, Cu, etc., or a lanthanoid element, n is an integer corresponding to the atomic valence of M and represents 2 or 3, and Y represents a halogen atom, OAc, OCOCF3 , ClO4 , SbF6 , PF6 , or OSO2CF3 ( OTf )), or alkaline compounds such as triethylamine, pyridine, etc. may be added as a catalyst.
これらエステル化反応剤の中でも、特に、無水酢酸、無水プロピオン酸、無水酪酸、酢酸ビニル、プロピオン酸ビニル、及び酪酸ビニル、酢酸からなる群から選択された少なくとも一種、中でも無水酢酸及び酢酸ビニルが、反応効率の観点から好ましい。 Among these esterification reactants, at least one selected from the group consisting of acetic anhydride, propionic anhydride, butyric anhydride, vinyl acetate, vinyl propionate, vinyl butyrate, and acetic acid, and among these, acetic anhydride and vinyl acetate are preferred from the viewpoint of reaction efficiency.
本実施形態の化学修飾微細セルロース繊維の修飾度は水酸基の平均置換度(セルロースの基本構成単位であるグルコース当たりの置換された水酸基の平均数、DSともいう)として表される。一態様において、化学修飾微細セルロース繊維のDSは0.01以上2.0以下が好ましい。DSが0.01以上であれば、熱分解開始温度が高い化学修飾微細セルロースを含む樹脂複合体を得ることができる。一方、2.0以下であると、化学修飾微細セルロース中に未修飾のセルロース骨格が残存するため、セルロース由来の高い引張強度及び寸法安定性と化学修飾由来の高い熱分解開始温度を兼ね備えた化学修飾微細セルロースを含む樹脂複合体を得ることができる。DSはより好ましくは0.05以上、さらに好ましくは0.1以上、特に好ましくは0.2以上、最も好ましくは0.3以上であって、より好ましくは1.8以下、さらに好ましくは1.5以下、特に好ましくは1.2以下、最も好ましくは1.0以下である。 The degree of modification of the chemically modified fine cellulose fiber of this embodiment is expressed as the average degree of substitution of hydroxyl groups (the average number of hydroxyl groups substituted per glucose, which is the basic structural unit of cellulose, also referred to as DS). In one aspect, the DS of the chemically modified fine cellulose fiber is preferably 0.01 or more and 2.0 or less. If the DS is 0.01 or more, a resin composite containing chemically modified fine cellulose with a high thermal decomposition onset temperature can be obtained. On the other hand, if the DS is 2.0 or less, an unmodified cellulose skeleton remains in the chemically modified fine cellulose, so that a resin composite containing chemically modified fine cellulose that combines high tensile strength and dimensional stability derived from cellulose and a high thermal decomposition onset temperature derived from chemical modification can be obtained. The DS is more preferably 0.05 or more, even more preferably 0.1 or more, particularly preferably 0.2 or more, and most preferably 0.3 or more, and more preferably 1.8 or less, even more preferably 1.5 or less, particularly preferably 1.2 or less, and most preferably 1.0 or less.
化学修飾微細セルロース繊維の修飾基がアシル基の場合、アシル置換度(DS)は、エステル化微細セルロース繊維の反射型赤外吸収スペクトルから、アシル基由来のピークとセルロース骨格由来のピークとのピーク強度比に基づいて算出することができる。アシル基に基づくC=Oの吸収バンドのピークは1730cm-1に出現し、セルロース骨格鎖に基づくC-Oの吸収バンドのピークは1030cm-1に出現する(図1及び2参照)。エステル化微細セルロース繊維のDSは、後述するエステル化微細セルロース繊維の固体NMR測定から得られるDSと、セルロース骨格鎖C-Oの吸収バンドのピーク強度に対するアシル基に基づくC=Oの吸収バンドのピーク強度の比率で定義される修飾化率(IRインデックス1030)との相関グラフを作製し、相関グラフから算出された検量線
置換度DS = 4.13 × IRインデックス(1030)
を使用することで求めることができる。
When the modifying group of the chemically modified fine cellulose fiber is an acyl group, the degree of acyl substitution (DS) can be calculated based on the peak intensity ratio of the peak derived from the acyl group to the peak derived from the cellulose skeleton from the reflection type infrared absorption spectrum of the esterified fine cellulose fiber. The peak of the absorption band of C=O based on the acyl group appears at 1730 cm -1 , and the peak of the absorption band of C-O based on the cellulose skeleton appears at 1030 cm -1 (see Figures 1 and 2). The DS of the esterified fine cellulose fiber is obtained by preparing a correlation graph between the DS obtained from the solid-state NMR measurement of the esterified fine cellulose fiber described later and the modification rate (IR index 1030) defined as the ratio of the peak intensity of the absorption band of C=O based on the acyl group to the peak intensity of the absorption band of the cellulose skeleton C-O, and calculating the calibration curve substitution degree DS = 4.13 × IR index (1030) from the correlation graph.
It can be found by using
固体NMRによるエステル化微細セルロース繊維のDSの算出方法は、凍結粉砕したエステル化微細セルロース繊維について13C固体NMR測定を行い、50ppmから110ppmの範囲に現れるセルロースのピラノース環由来の炭素C1-C6に帰属されるシグナルの合計面積強度(Inp)に対する修飾基由来の1つの炭素原子に帰属されるシグナルの面積強度(Inf)より下記式で求めることができる。
DS=(Inf)×6/(Inp)
たとえば、修飾基がアセチル基の場合、-CH3に帰属される23ppmのシグナルを用いれば良い。
用いる13C固体NMR測定の条件は例えば以下の通りである。
装置 :Bruker Biospin Avance500WB
周波数 :125.77MHz
測定方法 :DD/MAS法
待ち時間 :75sec
NMR試料管 :4mmφ
積算回数 :640回(約14Hr)
MAS :14,500Hz
化学シフト基準:グリシン(外部基準:176.03ppm)
The DS of the esterified fine cellulose fiber by solid-state NMR can be calculated by carrying out 13C solid-state NMR measurement on the frozen and pulverized esterified fine cellulose fiber, and calculating the DS from the total area intensity (Inp) of the signals assigned to carbons C1-C6 derived from the pyranose ring of cellulose appearing in the range of 50 ppm to 110 ppm relative to the area intensity (Inf) of the signal assigned to one carbon atom derived from the modifying group, using the following formula:
DS=(Inf)×6/(Inp)
For example, when the modifying group is an acetyl group, the signal at 23 ppm assigned to --CH.sub.3 may be used.
The conditions for the 13 C solid state NMR measurement are, for example, as follows:
Equipment: Bruker Biospin Avance500WB
Frequency: 125.77MHz
Measurement method: DD/MAS method Waiting time: 75 sec
NMR sample tube: 4 mm diameter
Accumulation times: 640 times (approximately 14 hours)
MAS: 14,500Hz
Chemical shift reference: glycine (external reference: 176.03 ppm)
≪乾燥体≫
本発明の一態様は、本開示の微細セルロース繊維を含む乾燥体(微細セルロース繊維乾燥体、又は、乾燥体ともいう)を提供する。乾燥体は、微細セルロース繊維のみで構成されてもよいし、微細セルロース繊維と追加の成分とを含んでもよい。追加の成分としては、分散剤、芳香族アラミド等の高耐熱性の有機高分子又は無機高分子からなる微細繊維フィラー成分、相溶化剤、可塑剤、多糖類、天然タンパク質、無機化合物、着色剤、香料、顔料、流動調整剤、レベリング剤、導電剤、帯電防止剤、紫外線吸収剤、紫外線分散剤、消臭剤、防腐剤等が挙げられる。乾燥体は、例えば前述したような微細セルロース繊維の製造方法によって微細セルロース繊維スラリーを得た後、これを撹拌下で加熱乾燥、又は、減圧乾燥することで製造できる。微細セルロース繊維と追加の成分とを含む乾燥体は、微細セルロース繊維スラリーに追加の成分を添加した後、撹拌下で加熱乾燥、又は、減圧乾燥させる方法、又は、微細セルロース繊維の乾燥体と追加の成分とを混合する方法等によって製造できる。
<Dry product>
One aspect of the present invention provides a dried body (also referred to as a fine cellulose fiber dried body or dried body) containing the fine cellulose fibers of the present disclosure. The dried body may be composed of only fine cellulose fibers, or may contain fine cellulose fibers and additional components. Examples of the additional components include dispersants, fine fiber filler components made of highly heat-resistant organic or inorganic polymers such as aromatic aramids, compatibilizers, plasticizers, polysaccharides, natural proteins, inorganic compounds, colorants, fragrances, pigments, flow control agents, leveling agents, conductive agents, antistatic agents, UV absorbers, UV dispersants, deodorants, and preservatives. The dried body can be produced, for example, by obtaining a fine cellulose fiber slurry by the above-mentioned method for producing fine cellulose fibers, and then heating and drying the slurry under stirring or drying under reduced pressure. The dried body containing fine cellulose fibers and additional components can be produced by a method of adding additional components to a fine cellulose fiber slurry, and then heating and drying the slurry under stirring or drying under reduced pressure, or by a method of mixing a dried body of fine cellulose fibers with additional components.
<分散剤>
分散剤は、微細セルロース繊維を安定に分散させる機能を有し、樹脂中での微細セルロース繊維の分散状態を向上又は制御することによって、微細セルロース繊維を用いて製造される樹脂複合体の力学物性を向上させる化合物を意味する。好ましい態様においては、微細セルロース繊維が、分散剤と、該分散剤中に分散された微細セルロース繊維とを含む分散体の形態で樹脂複合体中に分散されている。すなわち樹脂複合体は、好ましくは、分散剤中に微細セルロース繊維が分散されてなる分散体が、樹脂中に分散されているものである。微細セルロース繊維と分散剤との合計100質量%に対する微細セルロース繊維の質量比率は、好ましくは10質量%以上、又は20質量%以上、又は30質量%以上であり、好ましくは99質量%以下、又は95質量%以下、又は90質量%以下である。分散剤は、界面活性剤、沸点160℃以上の有機化合物、及び微細セルロース繊維を高度に分散可能な化学構造を有する樹脂からなる群から選ばれる少なくとも1種であることができ、好ましくは、界面活性剤、及び沸点160℃以上の有機化合物からなる群から選ばれる少なくとも1種である。
<Dispersant>
The dispersant means a compound that has the function of stably dispersing fine cellulose fibers and improves or controls the dispersion state of the fine cellulose fibers in the resin, thereby improving the mechanical properties of the resin composite produced using the fine cellulose fibers. In a preferred embodiment, the fine cellulose fibers are dispersed in the resin composite in the form of a dispersion containing a dispersant and fine cellulose fibers dispersed in the dispersant. That is, the resin composite is preferably a dispersion in which fine cellulose fibers are dispersed in a dispersant, and the dispersion is dispersed in the resin. The mass ratio of the fine cellulose fibers to the total of 100 mass% of the fine cellulose fibers and the dispersant is preferably 10 mass% or more, or 20 mass% or more, or 30 mass% or more, and preferably 99 mass% or less, or 95 mass% or less, or 90 mass% or less. The dispersant can be at least one selected from the group consisting of surfactants, organic compounds having a boiling point of 160 ° C. or more, and resins having a chemical structure capable of highly dispersing fine cellulose fibers, and is preferably at least one selected from the group consisting of surfactants and organic compounds having a boiling point of 160 ° C. or more.
界面活性剤としては、陰イオン系界面活性剤、非イオン系界面活性剤、両性イオン系界面活性剤、及び陽イオン系界面活性剤のいずれも使用することができるが、微細セルロース繊維との親和性の点で、陰イオン系界面活性剤、及び非イオン系界面活性剤が好ましく、非イオン系界面活性剤がより好ましい。 As the surfactant, any of anionic surfactants, nonionic surfactants, amphoteric surfactants, and cationic surfactants can be used, but in terms of affinity with fine cellulose fibers, anionic surfactants and nonionic surfactants are preferred, and nonionic surfactants are more preferred.
界面活性剤の親水基としては、微細セルロース繊維との親和性の点で、ポリオキシエチレン鎖、カルボキシル基、及び水酸基が好ましく、ポリオキシエチレン鎖が特に好ましい。非イオン系のポリオキシエチレン誘導体は特に好ましい。ポリオキシエチレン誘導体のポリオキシエチレン鎖長は、3以上、又は5以上、又は10以上、又は15以上であってよい。鎖長が長いほど微細セルロース繊維との親和性が高まるが、樹脂複合体の所望の特性(例えば機械特性)とのバランスの観点から、ポリオキシエチレン鎖長は、60以下、又は50以下、又は40以下、又は30以下、又は20以下であってよい。 As the hydrophilic group of the surfactant, polyoxyethylene chain, carboxyl group, and hydroxyl group are preferred in terms of affinity with fine cellulose fibers, and polyoxyethylene chain is particularly preferred. Nonionic polyoxyethylene derivatives are particularly preferred. The polyoxyethylene chain length of the polyoxyethylene derivative may be 3 or more, or 5 or more, or 10 or more, or 15 or more. The longer the chain length, the higher the affinity with fine cellulose fibers, but from the viewpoint of balance with the desired properties of the resin composite (e.g., mechanical properties), the polyoxyethylene chain length may be 60 or less, or 50 or less, or 40 or less, or 30 or less, or 20 or less.
界面活性剤の疎水基の構造としては、樹脂との親和性が高い点で、アルキルエーテル型、アルキルフェニルエーテル型、ロジンエステル型、ビスフェノールA型、βナフチル型、スチレン化フェニル型、及び硬化ひまし油型が好ましい。疎水基のアルキル鎖の炭素数(アルキルフェニルの場合はフェニル基を除いた炭素数)は、好ましくは、5以上、又は10以上、又は12以上、又は16以上である。例えば樹脂がポリオレフィン系樹脂の場合、界面活性剤の炭素数が多いほど、樹脂との親和性が高まる。上記炭素数は、例えば30以下、又は25以下であってよい。 As the structure of the hydrophobic group of the surfactant, alkyl ether type, alkyl phenyl ether type, rosin ester type, bisphenol A type, β-naphthyl type, styrenated phenyl type, and hardened castor oil type are preferred in terms of high affinity with resin. The number of carbon atoms in the alkyl chain of the hydrophobic group (the number of carbon atoms excluding the phenyl group in the case of alkyl phenyl) is preferably 5 or more, or 10 or more, or 12 or more, or 16 or more. For example, when the resin is a polyolefin resin, the greater the carbon number of the surfactant, the higher the affinity with the resin. The carbon number may be, for example, 30 or less, or 25 or less.
疎水基としては、環状構造を有するもの、又は嵩高く多官能構造を有するものがより好ましい。環状構造を有する疎水基としては、アルキルフェニルエーテル型、ロジンエステル型、ビスフェノールA型、βナフチル型、及びスチレン化フェニル型の基が好ましく、多官能構造を有するものとしては、硬化ひまし油型(例えば硬化ひまし油エーテル)の基が好ましい。ロジンエステル型、及び硬化ひまし油型は特に好ましい。 As the hydrophobic group, those having a cyclic structure or those having a bulky and multifunctional structure are more preferable. As hydrophobic groups having a cyclic structure, alkylphenyl ether type, rosin ester type, bisphenol A type, β-naphthyl type, and styrenated phenyl type groups are preferable, and as those having a multifunctional structure, hydrogenated castor oil type (e.g. hydrogenated castor oil ether) groups are preferable. Rosin ester type and hydrogenated castor oil type are particularly preferable.
好ましい態様において、界面活性剤は、ポリエチレングリコール(PEG)-ポリプロピレングリコール(PPG)共重合体である。 In a preferred embodiment, the surfactant is a polyethylene glycol (PEG)-polypropylene glycol (PPG) copolymer.
一態様において、微細セルロース繊維を含む乾燥体の含水率は、乾燥体の総量に対し、好ましくは50質量%以下、より好ましくは0.01~40質量%、さらに好ましくは0.1~30質量%、特により好ましくは0.1~20質量%、最も好ましくは0.1~10質量%に制御することができる。 In one embodiment, the moisture content of the dry material containing fine cellulose fibers can be controlled to preferably 50% by mass or less, more preferably 0.01 to 40% by mass, even more preferably 0.1 to 30% by mass, particularly preferably 0.1 to 20% by mass, and most preferably 0.1 to 10% by mass, based on the total amount of the dry material.
一態様において、微細セルロース繊維を含む乾燥体の嵩密度は、樹脂複合体中での微細セルロース繊維の良好な分散を維持しながら貯蔵時及び輸送時のコストを低く抑えることができる点で、好ましくは、0.05g/ml~1g/ml、又は0.1g/ml~0.8g/ml、又は0.2g/ml~0.7g/ml、又は0.3g/ml~0.6g/mlである。上記嵩密度は、パウダテスタ(PT-N型、ホソカワミクロン株式会社製)を用いて測定し、PackDensityの値を嵩密度として測定される値である。 In one embodiment, the bulk density of the dry material containing fine cellulose fibers is preferably 0.05 g/ml to 1 g/ml, or 0.1 g/ml to 0.8 g/ml, or 0.2 g/ml to 0.7 g/ml, or 0.3 g/ml to 0.6 g/ml, in order to keep storage and transportation costs low while maintaining good dispersion of the fine cellulose fibers in the resin complex. The bulk density is measured using a powder tester (PT-N type, manufactured by Hosokawa Micron Corporation), and the value of Pack Density is measured as the bulk density.
一態様において、微細セルロース繊維濃度が0.05質量%である微細セルロース繊維乾燥体を水中で再分散した時のスラリーの沈降高さ比は、樹脂複合体中での微細セルロース繊維の良好な分散を達成する上で、好ましくは、5%~50%、又は5%~40%、又は5%~30%、又は5%~20%、又は10%~20%である。上記沈降高さ比は下記の様に分散液を調製した上で、測定される値である。微細セルロース繊維が0.05質量%になるよう、微細セルロース繊維乾燥体に蒸留水を添加して試験用分散液20gを調製する。試験用分散液20gを30ml容量のガラスバイアルに分取し、ホモジナイザー(回転数10000rpm、5分間)で分散させ、ネジ口試験管(内径12mm)に入れて密封し、手動で振り混ぜて一様な分散液を得ることができる。 In one embodiment, the settling height ratio of the slurry when the dried fine cellulose fiber body having a fine cellulose fiber concentration of 0.05% by mass is redispersed in water is preferably 5% to 50%, or 5% to 40%, or 5% to 30%, or 5% to 20%, or 10% to 20% in order to achieve good dispersion of the fine cellulose fiber in the resin composite. The settling height ratio is a value measured after preparing a dispersion as described below. Distilled water is added to the dried fine cellulose fiber body so that the fine cellulose fiber is 0.05% by mass to prepare 20 g of a test dispersion. 20 g of the test dispersion is dispensed into a 30 ml glass vial, dispersed with a homogenizer (rotation speed 10,000 rpm, 5 minutes), placed in a screw-cap test tube (inner diameter 12 mm), sealed, and manually shaken to obtain a uniform dispersion.
≪樹脂複合体≫
本発明の一態様は、本開示の微細セルロース繊維と、樹脂とを含む樹脂複合体を提供する。一態様においては、微細セルロース繊維が樹脂中で互いに適度な空間を保って良好に分散しているために凝集塊を形成し難い一方で、繊維間の適度な絡み合いによって樹脂に対して優れた物性向上効果を与えるため、少量の微細セルロース繊維の使用であっても樹脂複合体が優れた物性を有することができる。
<Resin composite>
One aspect of the present invention provides a resin composite comprising the fine cellulose fibers of the present disclosure and a resin. In one aspect, the fine cellulose fibers are well dispersed in the resin with an appropriate space between them, so that they are unlikely to form aggregates, while the moderate entanglement between the fibers provides an excellent property-improving effect on the resin, so that even if a small amount of fine cellulose fibers is used, the resin composite can have excellent properties.
<樹脂>
樹脂としては、熱可塑性樹脂、熱硬化性樹脂、及び光硬化性樹脂を用いることができる。樹脂はエラストマーであってもよい。成形性及び生産性の観点から、熱可塑性樹脂がより好ましい。
<Resin>
The resin may be a thermoplastic resin, a thermosetting resin, or a photocurable resin. The resin may be an elastomer. From the viewpoints of moldability and productivity, a thermoplastic resin is more preferable.
(熱可塑性樹脂)
樹脂が熱可塑性樹脂である場合の当該熱可塑性樹脂の融点は、樹脂複合体の用途等に応じて適宜選択してよい。熱可塑性樹脂の融点としては、例えば比較的低融点の樹脂(例えばポリオレフィン系樹脂)について、150℃~190℃、又は160℃~180℃、また例えば比較的高融点の樹脂(例えばポリアミド系樹脂)について、220℃~350℃、又は230℃~320℃、を例示できる。
(Thermoplastic resin)
When the resin is a thermoplastic resin, the melting point of the thermoplastic resin may be appropriately selected depending on the application of the resin composite, etc. The melting point of the thermoplastic resin can be, for example, 150°C to 190°C or 160°C to 180°C for a resin with a relatively low melting point (e.g., a polyolefin resin), or 220°C to 350°C or 230°C to 320°C for a resin with a relatively high melting point (e.g., a polyamide resin).
熱可塑性樹脂は、好ましくは、ポリオレフィン系樹脂、ポリアセテート系樹脂、ポリカーボネート系樹脂、ポリアミド系樹脂、ポリエステル系樹脂、ポリフェニレンエーテル系樹脂、及びアクリル系樹脂からなる群から選ばれる少なくとも1種であることができる。 The thermoplastic resin is preferably at least one selected from the group consisting of polyolefin-based resins, polyacetate-based resins, polycarbonate-based resins, polyamide-based resins, polyester-based resins, polyphenylene ether-based resins, and acrylic-based resins.
熱可塑性樹脂として好ましいポリオレフィン系樹脂は、オレフィン類(例えばα-オレフィン類)及び/又はアルケン類をモノマー単位として重合して得られる高分子である。ポリオレフィン系樹脂の具体例としては、低密度ポリエチレン(例えば線状低密度ポリエチレン)、高密度ポリエチレン、超低密度ポリエチレン、超高分子量ポリエチレン等に例示されるエチレン系(共)重合体、ポリプロピレン、エチレン-プロピレン共重合体、エチレン-プロピレン-ジエン共重合体等に例示されるポリプロピレン系(共)重合体、エチレン-アクリル酸共重合体、エチレン-メタクリル酸メチル共重合体、エチレン-グリシジルメタクリレート共重合体等に代表されるエチレンとα-オレフィンとの共重合体が挙げられる。 Polyolefin-based resins that are preferred as thermoplastic resins are polymers obtained by polymerizing olefins (e.g., α-olefins) and/or alkenes as monomer units. Specific examples of polyolefin-based resins include ethylene-based (co)polymers such as low-density polyethylene (e.g., linear low-density polyethylene), high-density polyethylene, ultra-low-density polyethylene, and ultra-high-molecular-weight polyethylene; polypropylene-based (co)polymers such as polypropylene, ethylene-propylene copolymer, and ethylene-propylene-diene copolymer; and copolymers of ethylene and α-olefins such as ethylene-acrylic acid copolymer, ethylene-methyl methacrylate copolymer, and ethylene-glycidyl methacrylate copolymer.
ここで最も好ましいポリオレフィン系樹脂としては、ポリプロピレンが挙げられる。特に、ISO1133に準拠して230℃、荷重21.2Nで測定されたメルトマスフローレイト(MFR)が、3g/10分以上30g/10分以下であるポリプロピレンが好ましい。MFRの下限値は、より好ましくは5g/10分であり、さらにより好ましくは6g/10分であり、最も好ましくは8g/10分である。また、上限値は、より好ましくは25g/10分であり、さらにより好ましくは20g/10分であり、最も好ましくは18g/10分である。MFRは、樹脂複合体の靱性向上の観点から上記上限値を超えないことが望ましく、樹脂複合体の流動性の観点から上記下限値を超えないことが望ましい。 Here, the most preferred polyolefin resin is polypropylene. In particular, polypropylene having a melt mass flow rate (MFR) of 3 g/10 min or more and 30 g/10 min or less, measured at 230° C. and a load of 21.2 N in accordance with ISO 1133, is preferred. The lower limit of the MFR is more preferably 5 g/10 min, even more preferably 6 g/10 min, and most preferably 8 g/10 min. The upper limit is more preferably 25 g/10 min, even more preferably 20 g/10 min, and most preferably 18 g/10 min. It is desirable that the MFR does not exceed the upper limit from the viewpoint of improving the toughness of the resin composite, and it is desirable that the MFR does not exceed the lower limit from the viewpoint of the fluidity of the resin composite.
また、微細セルロース繊維との親和性を高めるため、酸変性されたポリオレフィン系樹脂も好適に使用可能である。酸変性に用いる酸としては、モノ又はポリカルボン酸を使用でき、例えば、マレイン酸、フマル酸、コハク酸、フタル酸及びこれらの無水物、並びにクエン酸等を例示できる。変性率の高めやすさから、マレイン酸又はその無水物が特に好ましい。変性方法については特に制限はないが、過酸化物の存在下又は非存在下でポリオレフィン系樹脂を融点以上に加熱して溶融混練する方法が一般的である。酸変性するポリオレフィン樹脂としては前出のポリオレフィン系樹脂をすべて使用可能であるが、ポリプロピレンが特に好適である。酸変性されたポリプロピレン系樹脂は、単独で用いても構わないが、樹脂全体としての変性率を調整するため、変性されていないポリプロピレン系樹脂と混合して使用することがより好ましい。この際のすべてのポリプロピレン系樹脂に対する酸変性されたポリプロピレン系樹脂の割合は、好ましくは0.5質量%~50質量%である。より好ましい下限は、1質量%、又は2質量%、又は3質量%、又は4質量%、又は5質量%である。また、より好ましい上限は、45質量%、又は40質量%、又は35質量%、又は30質量%、又は20質量%である。樹脂と微細セルロース繊維との界面強度を維持するためには、下限以上が好ましく、樹脂としての延性を維持するためには、上限以下が好ましい。 In addition, in order to increase the affinity with fine cellulose fibers, acid-modified polyolefin resins can also be suitably used. As the acid used for acid modification, mono- or polycarboxylic acids can be used, and examples thereof include maleic acid, fumaric acid, succinic acid, phthalic acid and their anhydrides, and citric acid. Maleic acid or its anhydride is particularly preferred because it is easy to increase the modification rate. There are no particular restrictions on the modification method, but a method in which a polyolefin resin is heated to above its melting point in the presence or absence of a peroxide and melt-kneaded is common. As the polyolefin resin to be acid-modified, all of the polyolefin resins mentioned above can be used, but polypropylene is particularly suitable. The acid-modified polypropylene resin may be used alone, but it is more preferable to use it in combination with an unmodified polypropylene resin in order to adjust the modification rate of the resin as a whole. In this case, the ratio of the acid-modified polypropylene resin to all polypropylene resins is preferably 0.5% by mass to 50% by mass. A more preferred lower limit is 1% by mass, or 2% by mass, or 3% by mass, or 4% by mass, or 5% by mass. A more preferred upper limit is 45% by mass, or 40% by mass, or 35% by mass, or 30% by mass, or 20% by mass. In order to maintain the interfacial strength between the resin and the fine cellulose fibers, a content equal to or greater than the lower limit is preferred, and in order to maintain the ductility of the resin, a content equal to or less than the upper limit is preferred.
酸変性されたポリプロピレン系樹脂の、ISO1133に準拠して230℃、荷重21.2Nで測定されるメルトマスフローレイト(MFR)は、樹脂と微細セルロース繊維との界面における親和性を高める観点から、好ましくは、50g/10分以上、又は100g/10分以上、又は150g/10分以上、又は200g/10分以上である。上限は特に限定されないが、機械的強度の維持から、好ましくは500g/10分である。 The melt mass flow rate (MFR) of the acid-modified polypropylene resin, measured in accordance with ISO1133 at 230°C and a load of 21.2 N, is preferably 50 g/10 min or more, 100 g/10 min or more, 150 g/10 min or more, or 200 g/10 min or more, from the viewpoint of increasing the affinity at the interface between the resin and the fine cellulose fiber. There is no particular upper limit, but it is preferably 500 g/10 min in order to maintain mechanical strength.
熱可塑性樹脂として好ましいポリアミド系樹脂としては:ラクタム類の重縮合反応により得られるポリアミド(例えばポリアミド6、ポリアミド11、ポリアミド12等);ジアミン類(例えば1,6-ヘキサンジアミン、2-メチル-1,5-ペンタンジアミン、1,7-ヘプタンジアミン、2-メチル-1-6-ヘキサンジアミン、1,8-オクタンジアミン、2-メチル-1,7-ヘプタンジアミン、1,9-ノナンジアミン、2-メチル-1,8-オクタンジアミン、1,10-デカンジアミン、1,11-ウンデカンジアミン、1,12-ドデカンジアミン、m-キシリレンジアミン等)とジカルボン酸類(例えばブタン二酸、ペンタン二酸、ヘキサン二酸、ヘプタン二酸、オクタン二酸、ノナン二酸、デカン二酸、ベンゼン-1,2-ジカルボン酸、ベンゼン-1,3-ジカルボン酸、ベンゼン-1,4ジカルボン酸、シクロヘキサン-1,3-ジカルボン酸、シクロヘキサン-1,4-ジカルボン酸等)との共重合体として得られるポリアミド(例えばポリアミド6,6、ポリアミド6,10、ポリアミド6,11、ポリアミド6,12、ポリアミド6,T、ポリアミド6,I、ポリアミド9,T、ポリアミド10,T、ポリアミド2M5,T、ポリアミドMXD,6、ポリアミド6、C、ポリアミド2M5,C等);及びこれらがそれぞれ共重合された共重合体(例えばポリアミド6,T/6,I等)、が挙げられる。 Preferred polyamide resins as thermoplastic resins include: polyamides obtained by polycondensation reaction of lactams (e.g., polyamide 6, polyamide 11, polyamide 12, etc.); diamines (e.g., 1,6-hexanediamine, 2-methyl-1,5-pentanediamine, 1,7-heptanediamine, 2-methyl-1-6-hexanediamine, 1,8-octanediamine, 2-methyl-1,7-heptanediamine, 1,9-nonanediamine, 2-methyl-1,8-octanediamine, 1,10-decanediamine, 1,11-undecanediamine, 1,12-dodecanediamine, m-xylylenediamine, etc.) and dicarboxylic acids (e.g., butanedioic acid, pentanedioic acid, hexanedioic acid, Polyamides obtained as copolymers with heptanedioic acid, octanedioic acid, nonanedioic acid, decanedioic acid, benzene-1,2-dicarboxylic acid, benzene-1,3-dicarboxylic acid, benzene-1,4-dicarboxylic acid, cyclohexane-1,3-dicarboxylic acid, cyclohexane-1,4-dicarboxylic acid, etc. (e.g. polyamide 6,6, polyamide 6,10, polyamide 6,11, polyamide 6,12, polyamide 6,T, polyamide 6,I, polyamide 9,T, polyamide 10,T, polyamide 2M5,T, polyamide MXD,6, polyamide 6,C, polyamide 2M5,C, etc.); and copolymers in which these are copolymerized (e.g. polyamide 6,T/6,I, etc.).
これらポリアミド系樹脂の中でも、ポリアミド6、ポリアミド11、ポリアミド12、ポリアミド6,6、ポリアミド6,10、ポリアミド6,11、ポリアミド6,12等の脂肪族ポリアミド、及び、ポリアミド6,C、ポリアミド2M5,C等の脂環式ポリアミドがより好ましい。 Among these polyamide resins, aliphatic polyamides such as polyamide 6, polyamide 11, polyamide 12, polyamide 6,6, polyamide 6,10, polyamide 6,11, and polyamide 6,12, and alicyclic polyamides such as polyamide 6,C and polyamide 2M5,C are more preferred.
樹脂複合体の耐熱性を良好にする観点から、ポリアミド系樹脂の融点は、好ましくは220℃以上、又は230℃以上、又は240℃以上、又は245℃以上、又は250℃以上であり、樹脂複合体の製造容易性の観点から、上記融点は、好ましくは、350℃以下、又は320℃以下、又は300℃以下である。 From the viewpoint of improving the heat resistance of the resin composite, the melting point of the polyamide resin is preferably 220°C or higher, or 230°C or higher, or 240°C or higher, or 245°C or higher, or 250°C or higher, and from the viewpoint of ease of manufacturing the resin composite, the melting point is preferably 350°C or lower, or 320°C or lower, or 300°C or lower.
ポリアミド系樹脂の末端カルボキシル基濃度に特に制限はないが、好ましくは、20μモル/g以上、又は30μモル/g以上であり、好ましくは、150μモル/g以下、又は100μモル/g以下、又は80μモル/g以下である。 There is no particular restriction on the terminal carboxyl group concentration of the polyamide resin, but it is preferably 20 μmol/g or more, or 30 μmol/g or more, and preferably 150 μmol/g or less, or 100 μmol/g or less, or 80 μmol/g or less.
ポリアミド系樹脂において、全末端基に対するカルボキシル末端基比率([COOH]/[全末端基])は、微細セルロース繊維の樹脂複合体中での分散性の観点から、好ましくは、0.30以上、又は0.35以上、又は0.40以上、又は0.45以上であり、樹脂複合体の色調の観点から、好ましくは、0.95以下、又は0.90以下、又は0.85以下、又は0.80以下である。 In the polyamide resin, the ratio of carboxyl end groups to all end groups ([COOH]/[total end groups]) is preferably 0.30 or more, or 0.35 or more, or 0.40 or more, or 0.45 or more from the viewpoint of dispersibility of the fine cellulose fibers in the resin composite, and is preferably 0.95 or less, or 0.90 or less, or 0.85 or less, or 0.80 or less from the viewpoint of the color tone of the resin composite.
ポリアミド系樹脂の末端基濃度は、公知の方法で調整できる。調整方法としては、ポリアミドの重合時に、所定の末端基濃度となるように末端基と反応する末端調整剤(例えば、ジアミン化合物、モノアミン化合物、ジカルボン酸化合物、モノカルボン酸化合物、酸無水物、モノイソシアネート、モノ酸ハロゲン化物、モノエステル、モノアルコール等)を重合液に添加する方法が挙げられる。 The end group concentration of polyamide resins can be adjusted by known methods. One adjustment method is to add a terminal regulator (e.g., diamine compound, monoamine compound, dicarboxylic acid compound, monocarboxylic acid compound, acid anhydride, monoisocyanate, monoacid halide, monoester, monoalcohol, etc.) that reacts with the end groups during polymerization of polyamide to the polymerization liquid so as to achieve a predetermined end group concentration.
末端アミノ基と反応する末端調整剤としては、酢酸、プロピオン酸、酪酸、吉草酸、カプロン酸、カプリル酸、ラウリン酸、トリデカン酸、ミリスチン酸、パルミチン酸、ステアリン酸、ピバリン酸、イソ酪酸等の脂肪族モノカルボン酸;シクロヘキサンカルボン酸等の脂環式モノカルボン酸;安息香酸、トルイル酸、α-ナフタレンカルボン酸、β-ナフタレンカルボン酸、メチルナフタレンカルボン酸、フェニル酢酸等の芳香族モノカルボン酸;及びこれらから任意に選ばれる複数の混合物が挙げられる。これらの中でも、反応性、封止末端の安定性、価格等の点から、酢酸、プロピオン酸、酪酸、吉草酸、カプロン酸、カプリル酸、ラウリン酸、トリデカン酸、ミリスチン酸、パルミチン酸、ステアリン酸及び安息香酸からなる群より選ばれる1種以上の末端調整剤が好ましく、酢酸が最も好ましい。 Examples of terminal regulators that react with terminal amino groups include aliphatic monocarboxylic acids such as acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, caprylic acid, lauric acid, tridecanoic acid, myristic acid, palmitic acid, stearic acid, pivalic acid, and isobutyric acid; alicyclic monocarboxylic acids such as cyclohexanecarboxylic acid; aromatic monocarboxylic acids such as benzoic acid, toluic acid, α-naphthalenecarboxylic acid, β-naphthalenecarboxylic acid, methylnaphthalenecarboxylic acid, and phenylacetic acid; and mixtures of a plurality of terminal regulators selected from the above. Among these, in terms of reactivity, stability of the blocked terminal, and price, one or more terminal regulators selected from the group consisting of acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, caprylic acid, lauric acid, tridecanoic acid, myristic acid, palmitic acid, stearic acid, and benzoic acid are preferred, and acetic acid is most preferred.
末端カルボキシル基と反応する末端調整剤としては、メチルアミン、エチルアミン、プロピルアミン、ブチルアミン、ヘキシルアミン、オクチルアミン、デシルアミン、ステアリルアミン、ジメチルアミン、ジエチルアミン、ジプロピルアミン、ジブチルアミン等の脂肪族モノアミン;シクロヘキシルアミン、ジシクロヘキシルアミン等の脂環式モノアミン;アニリン、トルイジン、ジフェニルアミン、ナフチルアミン等の芳香族モノアミン及びこれらの任意の混合物が挙げられる。これらの中でも、反応性、沸点、封止末端の安定性、価格等の点から、ブチルアミン、ヘキシルアミン、オクチルアミン、デシルアミン、ステアリルアミン、シクロヘキシルアミン及びアニリンからなる群より選ばれる1種以上の末端調整剤が好ましい。 Examples of terminal regulators that react with terminal carboxyl groups include aliphatic monoamines such as methylamine, ethylamine, propylamine, butylamine, hexylamine, octylamine, decylamine, stearylamine, dimethylamine, diethylamine, dipropylamine, and dibutylamine; alicyclic monoamines such as cyclohexylamine and dicyclohexylamine; aromatic monoamines such as aniline, toluidine, diphenylamine, and naphthylamine, and any mixtures thereof. Among these, one or more terminal regulators selected from the group consisting of butylamine, hexylamine, octylamine, decylamine, stearylamine, cyclohexylamine, and aniline are preferred in terms of reactivity, boiling point, stability of the blocked terminals, price, and the like.
ポリアミド系樹脂のアミノ末端基及びカルボキシル末端基の濃度は、1H-NMRにより、各末端基に対応する特性シグナルの積分値から求めることができる。この方法は、精度及び簡便さの点で好ましい。より具体的には、特開平7-228775号公報に記載された方法を用い、測定溶媒として重トリフルオロ酢酸を用い、積算回数を300スキャン以上とすることが推奨される。 The concentrations of amino end groups and carboxyl end groups of a polyamide resin can be determined from the integrated values of characteristic signals corresponding to each end group by 1 H-NMR. This method is preferred in terms of accuracy and simplicity. More specifically, it is recommended to use the method described in JP-A-7-228775, deuterated trifluoroacetic acid as the measurement solvent, and to set the number of integration scans to 300 or more.
ポリアミド系樹脂の、濃硫酸中30℃の条件下で測定した固有粘度[η]は、樹脂複合体を例えば射出成形する際に、金型内流動性が良好で成形片の外観が良好であるという観点から、好ましくは、0.6~2.0dL/g、又は0.7~1.4dL/g、又は0.7~1.2dL/g、又は0.7~1.0dL/gである。本開示において、「固有粘度」とは、一般的に極限粘度と呼ばれている粘度と同義である。固有粘度は、96%濃硫酸中、30℃の温度条件下で、濃度の異なるいくつかの測定溶媒のηsp/cを測定し、そのそれぞれのηsp/cと濃度(c)との関係式を導き出し、濃度をゼロに外挿する方法で求められる。このゼロに外挿された値が固有粘度である。上記方法の詳細は、例えば、Polymer Process Engineering(Prentice-Hall,Inc 1994)の291ページ~294ページ等に記載されている。上記の濃度の異なるいくつかの測定溶媒における濃度は、少なくとも4点(例えば、0.05g/dL、0.1g/dL、0.2g/dL、0.4g/dL)とすることが精度の観点から望ましい。 The intrinsic viscosity [η] of a polyamide resin measured in concentrated sulfuric acid at 30°C is preferably 0.6 to 2.0 dL/g, or 0.7 to 1.4 dL/g, or 0.7 to 1.2 dL/g, or 0.7 to 1.0 dL/g, from the viewpoint of good in-mold fluidity and good appearance of the molded piece when the resin composite is, for example, injection molded. In this disclosure, "intrinsic viscosity" is synonymous with the viscosity generally called limiting viscosity. The intrinsic viscosity is determined by measuring the ηsp/c of several measurement solvents with different concentrations in 96% concentrated sulfuric acid at a temperature of 30°C, deriving the relationship between each of the ηsp/c and the concentration (c), and extrapolating the concentration to zero. This value extrapolated to zero is the intrinsic viscosity. Details of the above method are described, for example, in Polymer Process Engineering (Prentice-Hall, Inc. 1994), pages 291 to 294. From the viewpoint of accuracy, it is desirable to set the concentrations in the above-mentioned measurement solvents having different concentrations to at least four points (e.g., 0.05 g/dL, 0.1 g/dL, 0.2 g/dL, 0.4 g/dL).
熱可塑性樹脂として好ましいポリエステル系樹脂としては、ポリエチレンテレフタレート(PET)、ポリエチレンナフタレート(PEN)、ポリブチレンテレフタレート(PBT)、ポリブチレンサクシネート(PBS)、ポリブチレンサクシネートアジペート(PBSA)、ポリブチレンアジペートテレフタレート(PBAT)、ポリヒドロキシアルカン酸(PHA)、ポリ乳酸(PLA)、ポリアリレート(PAR)等から選ばれる1種又は2種以上を用いることができる。中でも、PET、PBS、PBSA、PBT及びPENがより好ましく、PBS、PBSA、及びPBTが特に好ましい。 Preferred polyester resins as thermoplastic resins include one or more selected from polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polybutylene terephthalate (PBT), polybutylene succinate (PBS), polybutylene succinate adipate (PBSA), polybutylene adipate terephthalate (PBAT), polyhydroxyalkanoic acid (PHA), polylactic acid (PLA), polyarylate (PAR), etc. Among these, PET, PBS, PBSA, PBT, and PEN are more preferable, and PBS, PBSA, and PBT are particularly preferable.
ポリエステル系樹脂の末端基は、重合時のモノマー比率、末端安定化剤の添加の有無及び量、等によって任意に変えることができる。ポリエステル系樹脂の全末端基に対するカルボキシル末端基比率([COOH]/[全末端基])は、樹脂複合体中の微細セルロース繊維の分散性の観点から、好ましくは、0.30以上、又は0.35以上、又は0.40であり、又は0.45であり、樹脂複合体の色調の観点から、好ましくは、0.95以下、又は0.90以下、又は0.85以下、又は0.80以下である。 The terminal groups of the polyester resin can be changed as desired by the monomer ratio during polymerization, the presence or absence and amount of addition of a terminal stabilizer, etc. The ratio of carboxyl terminal groups to all terminal groups of the polyester resin ([COOH]/[total terminal groups]) is preferably 0.30 or more, or 0.35 or more, or 0.40, or 0.45 from the viewpoint of dispersibility of the fine cellulose fibers in the resin composite, and is preferably 0.95 or less, or 0.90 or less, or 0.85 or less, or 0.80 or less from the viewpoint of the color tone of the resin composite.
熱可塑性樹脂として好ましいポリアセタール系樹脂としては、ホルムアルデヒドを原料とするホモポリアセタールと、トリオキサンを主モノマーとし、1,3-ジオキソランをコモノマー成分として含むコポリアセタールとが一般的であり、両者とも使用可能であるが、加工時の熱安定性の観点から、コポリアセタールが好ましい。コモノマー成分(例えば1,3-ジオキソラン)由来構造の量は、押出加工及び成形加工時の熱安定性の観点から、好ましくは、0.01モル%以上、又は0.05モル%以上、又は0.1モル%以上、又は0.2モル%以上であり、機械的強度の観点から、好ましくは、4モル%以下、又は3.5モル%以下、又は3.0モル%以下、又は2.5モル%以下、又は2.3モル%以下である。 Preferred polyacetal resins as thermoplastic resins are homopolyacetals made from formaldehyde and copolyacetals containing trioxane as the main monomer and 1,3-dioxolane as a comonomer component. Both can be used, but copolyacetals are preferred from the viewpoint of thermal stability during processing. The amount of the structure derived from the comonomer component (e.g., 1,3-dioxolane) is preferably 0.01 mol% or more, or 0.05 mol% or more, or 0.1 mol% or more, or 0.2 mol% or more from the viewpoint of thermal stability during extrusion and molding, and is preferably 4 mol% or less, or 3.5 mol% or less, or 3.0 mol% or less, or 2.5 mol% or less, or 2.3 mol% or less from the viewpoint of mechanical strength.
(熱硬化性樹脂)
熱硬化性樹脂としては、ビスフェノールA型エポキシ樹脂、ビスフェノールF型エポキシ樹脂、ビスフェノールS型エポキシ樹脂、ビスフェノールE型エポキシ樹脂、ビスフェノールM型エポキシ樹脂、ビスフェノールP型エポキシ樹脂、ビスフェノールZ型エポキシ樹脂等のビスフェノール型エポキシ樹脂、ビスフェノールAノボラック型エポキシ樹脂、フェノールノボラック型エポキシ樹脂、クレゾールノボラックエポキシ樹脂等のノボラック型エポキシ樹脂、ビフェニル型エポキシ樹脂、ビフェニルアラルキル型エポキシ樹脂、アリールアルキレン型エポキシ樹脂、テトラフェニロールエタン型エポキシ樹脂、ナフタレン型エポキシ樹脂、アントラセン型エポキシ樹脂、フェノキシ型エポキシ樹脂、ジシクロペンタジエン型エポキシ樹脂、ノルボルネン型エポキシ樹脂、アダマンタン型エポキシ樹脂、フルオレン型エポキシ樹脂、グリシジルメタアクリレート共重合系エポキシ樹脂、シクロヘキシルマレイミドとグリシジルメタアクリレートとの共重合エポキシ樹脂、エポキシ変性のポリブタジエンゴム誘導体、CTBN変性エポキシ樹脂、トリメチロールプロパンポリグリシジルエーテル、フェニル-1,3-ジグリシジルエーテル、ビフェニル-4,4’-ジグリシジルエーテル、1,6-ヘキサンジオールジグリシジルエーテル、エチレングリコール又はプロピレングリコールのジグリシジルエーテル、ソルビトールポリグリシジルエーテル、トリス(2,3-エポキシプロピル)イソシアヌレート、トリグリシジルトリス(2-ヒドロキシエチル)イソシアヌレート、フェノールノボラック樹脂、クレゾールノボラック樹脂、ビスフェノールAノボラック樹脂等のノボラック型フェノール樹脂、未変性のレゾールフェノール樹脂、桐油、アマニ油、クルミ油等で変性した油変性レゾールフェノール樹脂等のレゾール型フェノール樹脂等のフェノール樹脂、フェノキシ樹脂、尿素(ユリア)樹脂、メラミン樹脂等のトリアジン環含有樹脂、不飽和ポリエステル樹脂、ビスマレイミド樹脂、ジアリルフタレート樹脂、シリコーン樹脂、ベンゾオキサジン環を有する樹脂、ノルボルネン系樹脂、シアネート樹脂、イソシアネート樹脂、ウレタン樹脂、ベンゾシクロブテン樹脂、マレイミド樹脂、ビスマレイミドトリアジン樹脂、ポリアゾメチン樹脂、熱硬化性ポリイミド等が挙げられる。
(Thermosetting resin)
Examples of the thermosetting resin include bisphenol type epoxy resins such as bisphenol A type epoxy resin, bisphenol F type epoxy resin, bisphenol S type epoxy resin, bisphenol E type epoxy resin, bisphenol M type epoxy resin, bisphenol P type epoxy resin, and bisphenol Z type epoxy resin; novolac type epoxy resins such as bisphenol A novolac type epoxy resin, phenol novolac type epoxy resin, and cresol novolac epoxy resin; biphenyl type epoxy resin, biphenyl aralkyl type epoxy resin; aryl alkyl type epoxy resin; epoxy resins modified with cyclohexylmaleimide and glycidyl methacrylate; epoxy resins modified with cyclohexylmaleimide and glycidyl methacrylate; epoxy-modified polybutadiene rubber derivatives; CTBN-modified epoxy resins; trimethylolpropane polyglycidyl ether; Phenyl-1,3-diglycidyl ether, biphenyl-4,4'-diglycidyl ether, 1,6-hexanediol diglycidyl ether, diglycidyl ether of ethylene glycol or propylene glycol, sorbitol polyglycidyl ether, tris(2,3-epoxypropyl)isocyanurate, triglycidyl tris(2-hydroxyethyl)isocyanurate, novolac-type phenolic resins such as phenol novolac resin, cresol novolac resin, and bisphenol A novolac resin, unmodified resol phenolic resin, paulownia wood Examples of the resin include phenolic resins such as resol-type phenolic resins, such as oil-modified resol phenolic resins modified with oil, linseed oil, walnut oil, etc.; triazine ring-containing resins, such as phenoxy resins, urea (urea) resins, and melamine resins; unsaturated polyester resins, bismaleimide resins, diallyl phthalate resins, silicone resins, resins having a benzoxazine ring, norbornene resins, cyanate resins, isocyanate resins, urethane resins, benzocyclobutene resins, maleimide resins, bismaleimide triazine resins, polyazomethine resins, and thermosetting polyimides.
(光硬化性樹脂)
光硬化性樹脂としては、(メタ)アクリレート樹脂、ビニル樹脂、エポキシ樹脂等が挙げられる。これらは、反応機構により、概ね光により発生したラジカルによりモノマーが反応するラジカル反応型と、モノマーがカチオン重合するカチオン反応型とに分類される。ラジカル反応型のモノマーには、(メタ)アクリレート化合物、ビニル化合物(例えばある種のビニルエーテル)等が該当する。カチオン反応型としては、エポキシ化合物、ある種のビニルエーテル等が該当する。なお、例えば、カチオン反応型として用いることができるエポキシ化合物は、熱硬化性樹脂及び光硬化性樹脂の両者のモノマーとなり得る。
(Photocurable resin)
Examples of photocurable resins include (meth)acrylate resins, vinyl resins, and epoxy resins. These are generally classified into radical reaction types in which a monomer reacts with radicals generated by light, and cationic reaction types in which a monomer undergoes cationic polymerization, according to the reaction mechanism. Examples of radical reaction type monomers include (meth)acrylate compounds and vinyl compounds (e.g., certain vinyl ethers). Examples of cationic reaction types include epoxy compounds and certain vinyl ethers. For example, epoxy compounds that can be used as cationic reaction types can be monomers for both thermosetting resins and photocurable resins.
(メタ)アクリレート化合物は、(メタ)アクリレート基を分子内に一つ以上有する化合物である。(メタ)アクリレート化合物としては、単官能(メタ)アクリレート、多官能(メタ)アクリレート、エポキシアクリレート、ポリエステルアクリレート、ウレタンアクリレート等が挙げられる。 A (meth)acrylate compound is a compound that has one or more (meth)acrylate groups in the molecule. Examples of (meth)acrylate compounds include monofunctional (meth)acrylates, polyfunctional (meth)acrylates, epoxy acrylates, polyester acrylates, and urethane acrylates.
ビニル化合物としては、ビニルエーテル、スチレン及びスチレン誘導体等が挙げられる。ビニルエーテルとしては、エチルビニルエーテル、プロピルビニルエーテル、ヒドロキシエチルビニルエーテル、エチレングリコールジビニルエーテル等が挙げられる。スチレン誘導体としては、メチルスチレン、エチルスチレン等が挙げられる。その他のビニル化合物としては、トリアリルイソイシアヌレート、トリメタアリルイソシアヌレート等が挙げられる。 Examples of vinyl compounds include vinyl ether, styrene, and styrene derivatives. Examples of vinyl ethers include ethyl vinyl ether, propyl vinyl ether, hydroxyethyl vinyl ether, and ethylene glycol divinyl ether. Examples of styrene derivatives include methylstyrene and ethylstyrene. Other examples of vinyl compounds include triallyl isocyanurate and trimethallyl isocyanurate.
光硬化性樹脂の原料として、いわゆる反応性オリゴマーを用いてもよい。反応性オリゴマーとしては、(メタ)アクリレート基、エポキシ基、ウレタン結合、及びエステル結合から選ばれる任意の組合せを同一分子内に併せ持つオリゴマー、例えば、(メタ)アクリレート基とウレタン結合とを同一分子内に併せ持つウレタンアクリレート、(メタ)アクリレート基とエステル結合とを同一分子内に併せ持つポリエステルアクリレート、エポキシ樹脂から誘導され、エポキシ基と(メタ)アクリレート基とを同一分子内に併せ持つエポキシアクリレート、等が挙げられる。 As a raw material for the photocurable resin, so-called reactive oligomers may be used. Examples of reactive oligomers include oligomers that have any combination selected from (meth)acrylate groups, epoxy groups, urethane bonds, and ester bonds in the same molecule, such as urethane acrylates that have (meth)acrylate groups and urethane bonds in the same molecule, polyester acrylates that have (meth)acrylate groups and ester bonds in the same molecule, and epoxy acrylates derived from epoxy resins that have epoxy groups and (meth)acrylate groups in the same molecule.
(エラストマー)
エラストマー(すなわちゴム)としては、天然ゴム(NR)、ブタジエンゴム(BR)、スチレン-ブタジエン共重合体ゴム(SBR)、イソプレンゴム(IR)、ブチルゴム(IIR)、アクリロニトリル-ブタジエンゴム(NBR)、アクリロニトリル-スチレン-ブタジエン共重合体ゴム、クロロプレンゴム、スチレン-イソプレン共重合体ゴム、スチレン-イソプレン-ブタジエン共重合体ゴム、イソプレン-ブタジエン共重合体ゴム、クロロスルホン化ポリエチレンゴム、改質天然ゴム(エポキシ化天然ゴム(ENR)、水素化天然ゴム、脱タンパク天然ゴム等)、エチレン-プロピレン共重合体ゴム、アクリルゴム、エピクロルヒドリンゴム、多硫化ゴム、シリコーンゴム、フッ素ゴム、ウレタンゴム等が挙げられる。
(Elastomer)
Examples of elastomers (i.e., rubber) include natural rubber (NR), butadiene rubber (BR), styrene-butadiene copolymer rubber (SBR), isoprene rubber (IR), butyl rubber (IIR), acrylonitrile-butadiene rubber (NBR), acrylonitrile-styrene-butadiene copolymer rubber, chloroprene rubber, styrene-isoprene copolymer rubber, styrene-isoprene-butadiene copolymer rubber, isoprene-butadiene copolymer rubber, chlorosulfonated polyethylene rubber, modified natural rubber (epoxidized natural rubber (ENR), hydrogenated natural rubber, deproteinized natural rubber, etc.), ethylene-propylene copolymer rubber, acrylic rubber, epichlorohydrin rubber, polysulfide rubber, silicone rubber, fluororubber, urethane rubber, and the like.
樹脂(一態様において熱可塑性樹脂)100質量部に対する微細セルロース繊維の量は、好ましくは0.001~100質量部の範囲内である。微細セルロース繊維の量の下限は、より好ましくは0.01質量部、さらに好ましくは0.1質量部、最も好ましくは1質量部である。微細セルロース繊維の量の上限は、より好ましくは80質量部、さらに好ましくは70質量部、最も好ましくは50質量部である。加工性と機械的特性のバランスの観点から、微細セルロース繊維の量を上述の範囲内とすることが望ましい。 The amount of fine cellulose fibers per 100 parts by mass of resin (in one embodiment, thermoplastic resin) is preferably within the range of 0.001 to 100 parts by mass. The lower limit of the amount of fine cellulose fibers is more preferably 0.01 parts by mass, even more preferably 0.1 parts by mass, and most preferably 1 part by mass. The upper limit of the amount of fine cellulose fibers is more preferably 80 parts by mass, even more preferably 70 parts by mass, and most preferably 50 parts by mass. From the viewpoint of the balance between processability and mechanical properties, it is desirable to set the amount of fine cellulose fibers within the above-mentioned range.
≪樹脂複合体の製造方法≫
一態様において、上記の樹脂複合体は、セルロース原料を叩解し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、及び任意に、該微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、を含む方法で微細セルロース繊維を得る微細セルロース繊維製造工程、並びに、上記微細セルロース繊維(すなわち、微細セルロース繊維スラリー又は乾燥体)と樹脂とを混合する混合工程、を含む方法で製造できる。より具体的には、上記の樹脂複合体は、樹脂の種類に応じて例えば以下の方法で製造できる。なお、化学修飾されている微細セルロース繊維を含む樹脂複合体を製造する場合、化学修飾と解繊との順序は問わず、解繊の前、解繊と同時、及び/又は解繊の後にセルロースを化学修飾してよい。
<Method for producing resin composite>
In one embodiment, the resin composite can be produced by a method including a fine cellulose fiber production step of obtaining fine cellulose fibers by a method including beating a cellulose raw material and then defibrating it with a homomixer to obtain a fine cellulose fiber slurry, and optionally drying the fine cellulose fiber slurry under reduced pressure while stirring, and a mixing step of mixing the fine cellulose fibers (i.e., the fine cellulose fiber slurry or dried body) with a resin. More specifically, the resin composite can be produced by, for example, the following method depending on the type of resin. When producing a resin composite containing chemically modified fine cellulose fibers, the order of chemical modification and defibration does not matter, and cellulose may be chemically modified before defibration, simultaneously with defibration, and/or after defibration.
樹脂が熱可塑性樹脂である場合、本開示の微細セルロース繊維を、分散液又は乾燥体(これらは追加の成分を更に含んでもよい)の形態で熱可塑性樹脂と混練して樹脂複合体を製造できる。樹脂複合体のより具体的な製造方法としては、
-樹脂モノマーと微細セルロース繊維とを混合し、重合反応を行い、得られた樹脂組成物をストランド状に押出し、水浴中で冷却固化させ、ペレット状成形体を得る方法、
-単軸又は二軸押出機を用いて、樹脂と微細セルロース繊維との混合物を溶融混練し、ストランド状に押出し、水浴中で冷却固化させ、ペレット状成形体を得る方法、
-単軸又は二軸押出機を用いて、樹脂と微細セルロース繊維との混合物を溶融混練し、棒状又は筒状に押出し冷却して押出成形体を得る方法、
-単軸又は二軸押出機を用いて、樹脂と微細セルロース繊維との混合物を溶融混練し、Tダイより押出しシート、又はフィルム状の成形体を得る方法、
等が挙げられる。好ましい態様においては、単軸又は二軸押出機を用いて、樹脂と微細セルロース繊維との混合物を溶融混練し、ストランド状に押出し、水浴中で冷却固化させ、ペレット状成形体を得る。樹脂と微細セルロース繊維との溶融混練方法の具体例としては、樹脂と、所望の比率で搬送された微細セルロース繊維とを混合した後、溶融混練する方法が挙げられる。
When the resin is a thermoplastic resin, the fine cellulose fibers of the present disclosure can be kneaded with the thermoplastic resin in the form of a dispersion or a dried body (which may further contain additional components) to produce a resin composite.
- A method in which a resin monomer and fine cellulose fibers are mixed, a polymerization reaction is carried out, the resulting resin composition is extruded into a strand shape, and cooled and solidified in a water bath to obtain a pellet-shaped molded product;
A method in which a mixture of resin and fine cellulose fibers is melt-kneaded using a single-screw or twin-screw extruder, extruded into a strand shape, and cooled and solidified in a water bath to obtain a pellet-shaped molded product;
A method in which a mixture of a resin and fine cellulose fibers is melt-kneaded using a single-screw or twin-screw extruder, extruded into a rod-like or tubular shape, and cooled to obtain an extrusion molded product;
A method in which a mixture of resin and fine cellulose fibers is melt-kneaded using a single-screw or twin-screw extruder and extruded through a T-die to obtain a sheet or film-like molded product;
In a preferred embodiment, a mixture of resin and fine cellulose fibers is melt-kneaded using a single-screw or twin-screw extruder, extruded into a strand shape, and cooled and solidified in a water bath to obtain a pellet-shaped molded product. A specific example of a method for melt-kneading a resin and fine cellulose fibers is a method in which a resin and fine cellulose fibers transported in a desired ratio are mixed and then melt-kneaded.
樹脂が熱可塑性樹脂である場合、熱可塑性樹脂供給業者が推奨する最低加工温度は、ナイロン66では255~270℃、ナイロン6では225~240℃、ポリアセタール樹脂では170℃~190℃、ポリプロピレンでは160~180℃である。加熱設定温度は、これらの推奨最低加工温度より20℃高い温度の範囲が好ましい。混合温度をこの温度範囲とすることにより、微細セルロース繊維と樹脂とを均一に混合することができる。 When the resin is a thermoplastic resin, the minimum processing temperatures recommended by thermoplastic resin suppliers are 255-270°C for nylon 66, 225-240°C for nylon 6, 170°C-190°C for polyacetal resin, and 160-180°C for polypropylene. The heating temperature setting is preferably in the range of 20°C higher than these recommended minimum processing temperatures. By setting the mixing temperature within this temperature range, the fine cellulose fibers and the resin can be mixed uniformly.
樹脂として熱可塑性樹脂を含む樹脂複合体は、種々の形状での提供が可能である。具体的には、樹脂ペレット状、シート状、繊維状、板状、棒状等が挙げられるが、樹脂ペレット形状が、後加工の容易性や運搬の容易性からより好ましい。この際の好ましいペレット形状としては、丸型、楕円型、円柱型などが挙げられ、これらは押出加工時のカット方式により異なる。アンダーウォーターカットと呼ばれるカット方法で切断されたペレットは、丸型になることが多く、ホットカットと呼ばれるカット方法で切断されたペレットは丸型又は楕円型になることが多く、ストランドカットと呼ばれるカット方法で切断されたペレットは円柱状になることが多い。丸型ペレットの場合、その好ましい大きさは、ペレット直径として1mm以上、3mm以下である。また、円柱状ペレットの場合の好ましい直径は、1mm以上3mm以下であり、好ましい長さは、2mm以上10mm以下である。上記の直径及び長さは、押出時の運転安定性の観点から、下限以上とすることが望ましく、後加工での成形機への噛み込み性の観点から、上限以下とすることが望ましい。 Resin composites containing thermoplastic resins as resins can be provided in various shapes. Specifically, resin pellets, sheets, fibers, plates, rods, etc. can be mentioned, but the resin pellet shape is more preferable from the viewpoint of ease of post-processing and ease of transportation. Preferred pellet shapes in this case include round, elliptical, and cylindrical shapes, which vary depending on the cutting method used during extrusion processing. Pellets cut by a cutting method called underwater cutting are often round, pellets cut by a cutting method called hot cutting are often round or elliptical, and pellets cut by a cutting method called strand cutting are often cylindrical. In the case of round pellets, the preferred size is a pellet diameter of 1 mm or more and 3 mm or less. In the case of cylindrical pellets, the preferred diameter is 1 mm or more and 3 mm or less, and the preferred length is 2 mm or more and 10 mm or less. It is desirable to set the above diameter and length to the lower limit or more from the viewpoint of operational stability during extrusion, and it is desirable to set them to the upper limit or less from the viewpoint of bite into the molding machine in post-processing.
樹脂として熱可塑性樹脂を含む樹脂複合体は、種々の樹脂成形体として利用が可能である。樹脂成形体の製造方法に関しては特に制限はなく、いずれの製造方法でも構わないが、射出成形法、押出成形法、ブロー成形法、インフレーション成形法、発泡成形法などが使用可能である。これらの中では射出成形法がデザイン性とコストの観点より、最も好ましい。 Resin composites containing a thermoplastic resin as the resin can be used as various resin molded products. There are no particular limitations on the manufacturing method for the resin molded products, and any manufacturing method can be used, including injection molding, extrusion molding, blow molding, inflation molding, and foam molding. Of these, injection molding is the most preferable from the standpoint of design and cost.
樹脂が熱硬化性樹脂又は光硬化性樹脂である場合、例えば、樹脂溶液又は樹脂粉末分散体中に微細セルロース繊維を十分に分散させて乾燥する方法、樹脂モノマー液中に微細セルロース繊維を十分に分散させて熱、UV照射、重合開始剤等によって重合する方法、微細セルロース繊維からなる成形体(例えば、シート、粉末粒子成形体等)に樹脂溶液又は樹脂粉末分散体を十分に含浸させて乾燥する方法、微細セルロース繊維からなる成形体に樹脂モノマー液を十分に含浸させて熱、UV照射、重合開始剤等によって重合する方法等によって、樹脂複合体を製造できる。硬化に際し、種々の重合開始剤、硬化剤、硬化促進剤、重合禁止剤等を配合することができる。 When the resin is a thermosetting resin or a photocurable resin, the resin composite can be produced by, for example, a method of thoroughly dispersing fine cellulose fibers in a resin solution or resin powder dispersion and drying the resulting mixture, a method of thoroughly dispersing fine cellulose fibers in a resin monomer liquid and polymerizing the resulting mixture by heat, UV irradiation, a polymerization initiator, or the like, a method of thoroughly impregnating a molded body (e.g., a sheet, a powder particle molded body, etc.) made of fine cellulose fibers with a resin solution or resin powder dispersion and drying the resulting mixture, or a method of thoroughly impregnating a molded body made of fine cellulose fibers with a resin monomer liquid and polymerizing the resulting mixture by heat, UV irradiation, a polymerization initiator, or the like. When curing, various polymerization initiators, curing agents, curing accelerators, polymerization inhibitors, etc. can be blended.
樹脂が熱硬化性樹脂又は光硬化性樹脂である場合、未硬化又は半硬化のプリプレグと呼ばれるシートを作製した後、プリプレグを単層又は積層にして、加圧及び加熱によって樹脂を硬化及び成形する方法を用いてよい。加圧及び加熱の方法としては、プレス成形法、オートクレーブ成形法、バッギング成形法、ラッピングテープ法、内圧成形法等が挙げられる。 When the resin is a thermosetting resin or a photocurable resin, a method may be used in which an uncured or semi-cured sheet called a prepreg is prepared, the prepreg is then made into a single layer or laminated, and the resin is cured and molded by applying pressure and heat. Examples of the method of applying pressure and heat include press molding, autoclave molding, bagging molding, wrapping tape method, and internal pressure molding.
樹脂が光硬化性樹脂である場合、活性エネルギー線を用いた各種硬化方法を用いて樹脂成形体を製造できる。 When the resin is a photocurable resin, various curing methods using active energy rays can be used to produce a resin molded body.
樹脂がエラストマーである場合、微細セルロース繊維と原料ゴムとを乾式で混練する方法、微細セルロース繊維と原料ゴムとを分散媒中に分散又は溶解させた後、乾燥させて混合する方法等によって、樹脂複合体を製造できる。混合方法としては、高い剪断力と圧力とをかけ、分散を促進できる点で、ホモジナイザーによる混合方法が好ましいが、その他、プロペラ式攪拌装置、ロータリー攪拌装置、電磁攪拌装置、手動による攪拌、等の方法を用いることもできる。エラストマーを含む樹脂複合体を、金型成形、射出成形、押出成形、中空成形、発泡成形等の所望の成形方法を用いて成形し、シート、ペレット、粉末等の所望の形状の未加硫の成形体を得ることができる。未加硫の成形体を、必要に応じて熱処理等で加硫して、樹脂成形体を得ることができる。 When the resin is an elastomer, a resin composite can be produced by a method of dry kneading fine cellulose fibers and raw rubber, or a method of dispersing or dissolving fine cellulose fibers and raw rubber in a dispersion medium, then drying and mixing. As a mixing method, a mixing method using a homogenizer is preferable because it can apply high shear force and pressure and promote dispersion, but other methods such as a propeller-type stirring device, a rotary stirring device, an electromagnetic stirring device, and manual stirring can also be used. A resin composite containing an elastomer can be molded using a desired molding method such as mold molding, injection molding, extrusion molding, hollow molding, and foam molding to obtain an unvulcanized molded product in a desired shape such as a sheet, pellet, or powder. The unvulcanized molded product can be vulcanized by heat treatment or the like as necessary to obtain a resin molded product.
熱可塑性樹脂又はエラストマーを含む樹脂成形体は、その一部(例えば数箇所)を加熱処理して溶融させ、例えば樹脂又は金属の基板に接着して用いても構わない。また、樹脂成形体は、樹脂又は金属の基板に塗布された塗膜であってもよく、基板との積層体を形成してもよい。また、シート状、フィルム状又は繊維状の樹脂成形体には、アニール処理、エッチング処理、コロナ処理、プラズマ処理、シボ転写、切削、表面研磨等の二次加工を行っても構わない。 A resin molded product containing a thermoplastic resin or an elastomer may be partially (e.g., several locations) melted by heat treatment and then bonded to, for example, a resin or metal substrate. The resin molded product may be a coating applied to a resin or metal substrate, or may form a laminate with the substrate. Furthermore, sheet-, film-, or fiber-shaped resin molded products may be subjected to secondary processing such as annealing, etching, corona treatment, plasma treatment, embossing, cutting, and surface polishing.
樹脂複合体において、樹脂100質量部に対する微細セルロース繊維の量は、加工性と機械的特性のバランスの観点から、好ましくは、0.001質量部以上、又は0.01質量部以上、又は0.1質量部以上、又は1質量部以上であってよく、好ましくは、100質量部以下、又は80質量部以下、又は70質量部以下、又は50質量部以下であってよい。 In the resin composite, the amount of fine cellulose fibers per 100 parts by mass of resin may be, from the viewpoint of the balance between processability and mechanical properties, preferably 0.001 parts by mass or more, or 0.01 parts by mass or more, or 0.1 parts by mass or more, or 1 part by mass or more, and may be preferably 100 parts by mass or less, or 80 parts by mass or less, or 70 parts by mass or less, or 50 parts by mass or less.
本実施形態の樹脂複合体は、高耐熱かつ軽量であることから、鋼板の代替、又は炭素繊維強化プラスチック、ガラス繊維強化プラスチック等の繊維強化プラスチック、無機フィラーを含む樹脂コンポジット等の代替ができる。例えば、産業用機械部品(例えば、電磁機器筐体、ロール材、搬送用アーム、医療機器部材等)、一般機械部品、自動車・鉄道・車両等部品(例えば外板、シャーシ、空力部材、座席、トランスミッション内部の摩擦材等)、船舶部材(例えば船体、座席等)、航空関連部品(例えば、胴体、主翼、尾翼、動翼、フェアリング、カウル、ドア、座席、内装材等)、宇宙機、人工衛星部材(モーターケース、主翼、構体、アンテナ等)、電子・電気部品(例えばパーソナルコンピュータ筐体、携帯電話筐体、OA機器、AV機器、電話機、ファクシミリ、家電製品、玩具用品等)、建築・土木材料(例えば、鉄筋代替材料、トラス構造体、つり橋用ケーブル等)、生活用品、スポーツ・レジャー用品(例えば、ゴルフクラブシャフト、釣り竿、テニス又はバトミントンのラケット等)、風力発電用筐体部材等、また容器・包装部材となり得る。 The resin composite of this embodiment is highly heat resistant and lightweight, and can therefore be used as a substitute for steel plates, fiber-reinforced plastics such as carbon fiber reinforced plastics and glass fiber reinforced plastics, and resin composites containing inorganic fillers. For example, industrial machine parts (e.g., electromagnetic device housings, roll materials, transport arms, medical device parts, etc.), general machine parts, automobile, railway, and vehicle parts (e.g., outer panels, chassis, aerodynamic parts, seats, friction materials inside transmissions, etc.), ship parts (e.g., hulls, seats, etc.), aviation-related parts (e.g., fuselages, main wings, tails, movable surfaces, fairings, cowls, doors, seats, interior materials, etc.), spacecraft and artificial satellite parts (motor cases, main wings, structures, antennas, etc.), electronic and electrical parts (e.g., personal computer housings, mobile phone housings, office automation equipment, audio-visual equipment, telephones, facsimiles, home appliances, toys, etc.), construction and civil engineering materials (e.g., rebar replacement materials, truss structures, cables for suspension bridges, etc.), daily necessities, sports and leisure goods (e.g., golf club shafts, fishing rods, tennis or badminton rackets, etc.), wind power generation housing parts, etc., and container and packaging parts.
本発明を実施例に基づいて更に説明するが、本発明はこれら実施例に限定されない。 The present invention will be further explained based on examples, but the present invention is not limited to these examples.
≪微細セルロース繊維の製造≫
[製造例1]
コットンリンターパルプをハンマーミル(ラボネクト社製、HM-500、スクリーンメッシュφ0.7mm)で処理し、粉砕パルプP1を得た。
<Production of fine cellulose fibers>
[Production Example 1]
The cotton linter pulp was treated with a hammer mill (HM-500, manufactured by LabNect, screen mesh φ0.7 mm) to obtain pulverized pulp P1.
[製造例2]
スクリーンメッシュφ0.3mmに代えた以外は製造例1の方法で粉砕パルプP2を得た。
[Production Example 2]
A pulverized pulp P2 was obtained in the same manner as in Production Example 1, except that the screen mesh was changed to φ0.3 mm.
[製造例3]
コットンリンターパルプをアバカパルプに代えた以外は製造例2の方法で、粉砕パルプP3を得た。
[Production Example 3]
Ground pulp P3 was obtained by the method of Production Example 2, except that the cotton linter pulp was replaced with abaca pulp.
[製造例4]
コットンリンターパルプをNBKP代えた以外は製造例1の方法で、粉砕パルプP4を得た。
[Production Example 4]
Ground pulp P4 was obtained in the same manner as in Production Example 1, except that the cotton linter pulp was replaced with NBKP.
[実施例1:解繊のみ、粉砕パルプP1]
KAPPA VITA(登録商標)ホモミキサー(タンクサイズ35L)に、粉砕パルプP1を1質量部、DMSOを19質量部仕込み、ホモミキサー回転数6000rpm(周速度29m/s)で8時間処理し、微細セルロース繊維スラリー(スラリーS1、DMSO溶媒)を得た。スラリーS1を脱水機で濃縮した後、純水30質量部を加えて十分に撹拌し、再度脱水機に入れて濃縮した。この後、純水30質量部の添加、撹拌、濃縮という一連の洗浄操作を合計5回繰り返すことで、DMSOを除去し、固形分率10質量%の微細セルロース繊維ケーキ(ケーキK1、水溶媒)を10質量部得た。
[Example 1: Defibration only, pulverized pulp P1]
A KAPPA VITA (registered trademark) homomixer (tank size 35 L) was charged with 1 part by mass of ground pulp P1 and 19 parts by mass of DMSO, and the mixture was treated for 8 hours at a homomixer rotation speed of 6000 rpm (circumferential speed 29 m/s) to obtain a fine cellulose fiber slurry (slurry S1, DMSO solvent). After concentrating the slurry S1 with a dehydrator, 30 parts by mass of pure water was added, thoroughly stirred, and the mixture was concentrated again in the dehydrator. After this, a series of washing operations, including the addition of 30 parts by mass of pure water, stirring, and concentration, was repeated a total of five times to remove DMSO, and 10 parts by mass of a fine cellulose fiber cake (cake K1, water solvent) with a solid content of 10% by mass was obtained.
[実施例2:逐次法(解繊後にアセチル化)、粉砕パルプP1]
実施例1と同様の方法で得られた微細セルロース繊維スラリー(スラリーS2、DMSO溶媒)に、酢酸ビニルを2.1質量部、炭酸水素ナトリウムを0.32質量部添加し、60℃で4時間アセチル化を実施し、微細セルロース繊維スラリー(スラリーS3、DMSO溶媒)を得た。なお、循環ライン中の滞留を防ぐため、ホモミキサー回転数2000rpm(周速度12m/s)で処理を実施した。スラリーS3に純水30質量部を加えて十分に撹拌することでアセチル化反応を停止させ、脱水機に入れて濃縮した。この後、実施例1と同様の洗浄操作を5回繰り返すことで、DMSO、酢酸ビニル、炭酸水素ナトリウム、副生成物を除去し、固形分率10質量%のアセチル化微細セルロース繊維ケーキ(ケーキK2、水溶媒)を10質量部得た。
[Example 2: Sequential method (acetylation after defibration), pulverized pulp P1]
To the fine cellulose fiber slurry (slurry S2, DMSO solvent) obtained in the same manner as in Example 1, 2.1 parts by mass of vinyl acetate and 0.32 parts by mass of sodium bicarbonate were added, and acetylation was carried out at 60 ° C. for 4 hours to obtain a fine cellulose fiber slurry (slurry S3, DMSO solvent). In order to prevent retention in the circulation line, the treatment was carried out at a homomixer rotation speed of 2000 rpm (circumferential speed 12 m / s). The acetylation reaction was stopped by adding 30 parts by mass of pure water to the slurry S3 and thoroughly stirring it, and the slurry was placed in a dehydrator and concentrated. After this, the same washing operation as in Example 1 was repeated five times to remove DMSO, vinyl acetate, sodium bicarbonate, and by-products, and 10 parts by mass of acetylated fine cellulose fiber cake (cake K2, water solvent) with a solid content of 10% by mass was obtained.
[実施例3:同時法(解繊とアセチル化を同時)、粉砕パルプP2]
KAPPA VITA(登録商標)ホモミキサー(タンクサイズ35L)に、粉砕パルプP2を1質量部、DMSOを19質量部、酢酸ビニルを2.1質量部、炭酸水素ナトリウムを0.32質量部添加し、ホモミキサー回転数6000rpm(周速度29m/s)、処理温度60℃で4時間解繊とアセチル化を同時に行い、微細セルロース繊維スラリー(スラリーS4、DMSO溶媒)を得た。この後の加水による反応停止及び洗浄操作は実施例2と同様に実施し、固形分率10質量%のアセチル化微細セルロース繊維ケーキ(ケーキK3、水溶媒)を10質量部得た。
[Example 3: Simultaneous method (simultaneous defibration and acetylation), pulverized pulp P2]
To a KAPPA VITA (registered trademark) homomixer (tank size 35 L), 1 part by mass of ground pulp P2, 19 parts by mass of DMSO, 2.1 parts by mass of vinyl acetate, and 0.32 parts by mass of sodium bicarbonate were added, and defibration and acetylation were simultaneously performed for 4 hours at a homomixer rotation speed of 6000 rpm (circumferential speed 29 m/s) and a treatment temperature of 60° C. to obtain a fine cellulose fiber slurry (slurry S4, DMSO solvent). The subsequent reaction termination by addition of water and washing operations were performed in the same manner as in Example 2, and 10 parts by mass of an acetylated fine cellulose fiber cake (cake K3, water solvent) with a solid content of 10% by mass was obtained.
[実施例4:逐次法、粉砕パルプP3]
粉砕パルプP1の代わりに粉砕パルプP3を使用した以外は、実施例2と同様の方法で、固形分率10質量%の微細セルロース繊維ケーキ(ケーキK4、水溶媒)を10質量部得た。
[Example 4: Sequential process, ground pulp P3]
A fine cellulose fiber cake (cake K4, water solvent) having a solid content of 10% by mass was obtained in 10 parts by mass in the same manner as in Example 2, except that ground pulp P3 was used instead of ground pulp P1.
[実施例5:解繊のみ、粉砕パルプP1]
ホモミキサー処理時間を24時間にした以外は、実施例1と同様の方法で、固形分率10質量%の微細セルロース繊維ケーキ(ケーキK5、水溶媒)を10質量部得た。
[Example 5: Defibration only, pulverized pulp P1]
The same method as in Example 1 was used to obtain 10 parts by mass of a fine cellulose fiber cake (Cake K5, water solvent) having a solid content of 10% by mass, except that the homomixer treatment time was changed to 24 hours.
[比較例1:同時法、コットンリンターパルプ]
粉砕パルプP1の代わりにコットンリンターパルプを使用した以外は、実施例3と同様の方法で、固形分率10質量%のアセチル化微細セルロース繊維ケーキ(ケーキK6、水溶媒)を10質量部得た。
[Comparative Example 1: Simultaneous method, cotton linter pulp]
10 parts by mass of an acetylated fine cellulose fiber cake (cake K6, water solvent) having a solid content of 10% by mass was obtained in the same manner as in Example 3, except that cotton linter pulp was used instead of ground pulp P1.
[比較例2:逐次法、アバカパルプ]
粉砕パルプP1の代わりにアバカパルプを使用した以外は、実施例2と同様の方法で、固形分率10質量%の微細セルロース繊維ケーキ(ケーキK7、水溶媒)を10質量部得た。
[Comparative Example 2: Sequential process, abaca pulp]
A fine cellulose fiber cake (cake K7, water solvent) having a solid content of 10% by mass was obtained in 10 parts by mass in the same manner as in Example 2, except that abaca pulp was used instead of ground pulp P1.
[比較例3:解繊のみ(水溶媒)、粉砕パルプP4]
粉砕パルプP4を1質量部、一軸撹拌機(アイメックス社製 DKV-1 φ125mmディゾルバー)を用い、水50質量部中で500rpmにて1時間、常温で攪拌した。続いて、ホースポンプでビーズミル(アイメックス社製 NVM-1.5)にフィードし、水のみで180分間循環運転させ、固形分率2.0質量%の微細セルロース繊維スラリー(スラリーS5、水溶媒)を51質量部得た。最終的にスラリーS5は脱水機に入れて濃縮し、固形分率10質量%の微細セルロース繊維ケーキ(K8、水溶媒)を10質量部得た。
循環運転の際、ビーズミルの回転数は2500rpm、周速12m/sとし、用いたビーズはジルコニア製で、φ2.0mm、充填率70%とした(ビーズミルのスリット隙間は0.6mmとした)。また、循環運転の際は、摩擦による発熱を吸収するためにチラーによりスラリー温度を40℃に温度管理した。
[Comparative Example 3: Defibration only (water solvent), pulverized pulp P4]
One part by mass of the ground pulp P4 was stirred at room temperature for 1 hour at 500 rpm in 50 parts by mass of water using a single-shaft agitator (DKV-1 φ125 mm dissolver manufactured by IMEX). The mixture was then fed to a bead mill (NVM-1.5 manufactured by IMEX) using a hose pump, and circulated for 180 minutes using only water to obtain 51 parts by mass of a fine cellulose fiber slurry (slurry S5, water solvent) having a solid content of 2.0% by mass. Finally, the slurry S5 was concentrated in a dehydrator to obtain 10 parts by mass of a fine cellulose fiber cake (K8, water solvent) having a solid content of 10% by mass.
During the circulation operation, the rotation speed of the bead mill was 2500 rpm, the peripheral speed was 12 m/s, the beads used were made of zirconia, had a diameter of 2.0 mm, and a filling rate of 70% (the slit gap of the bead mill was 0.6 mm). During the circulation operation, the slurry temperature was controlled at 40° C. using a chiller to absorb heat generated by friction.
[比較例4:解繊のみ(DMSO溶媒)、粉砕パルプP4]
水50質量部に代わり、DMSO30質量部を用いた以外は比較例3と同様の方法で固形分率3.2質量%の微細セルロース繊維スラリー(スラリーS6、DMSO溶媒)を31質量部得た。スラリーS6は純水30質量部を加えて十分に撹拌した後、脱水機に入れて濃縮した。得られたウェットケーキを再度30質量部の純水に分散、撹拌、濃縮する洗浄操作を合計5回繰り返すことで、DMSOを除去し、固形分率10質量%の微細セルロース繊維ケーキ(K9、水溶媒)を10質量部得た。
セルロース原料及び粉砕パルプ及び微細セルロース繊維の物性を表1に示す。
[Comparative Example 4: Defibration only (DMSO solvent), pulverized pulp P4]
31 parts by mass of a fine cellulose fiber slurry (Slurry S6, DMSO solvent) having a solid content of 3.2% by mass was obtained in the same manner as in Comparative Example 3, except that 30 parts by mass of DMSO was used instead of 50 parts by mass of water. 30 parts by mass of pure water was added to the slurry S6, which was thoroughly stirred and then placed in a dehydrator for concentration. The obtained wet cake was dispersed again in 30 parts by mass of pure water, stirred, and concentrated. The washing operation was repeated a total of five times to remove DMSO, and 10 parts by mass of a fine cellulose fiber cake (K9, water solvent) having a solid content of 10% by mass was obtained.
The physical properties of the cellulose raw material, pulverized pulp, and fine cellulose fibers are shown in Table 1.
粉砕パルプを使用した微細セルロース繊維の沈降高さ比は、粉砕していないセルロース原料を使用した場合と比較して高い値を示した。解繊時の使用溶媒が水であると、DMSOよりも数平均繊維径は小さく、沈降高さ比は高くなった。木材パルプ系のNBKPは粉砕パルプの長さ加重平均繊維長は小さいものの沈降高さ比は小さかった。 The settling height ratio of fine cellulose fibers using pulverized pulp was higher than that of fibers using unpulverized cellulose raw materials. When the solvent used during defibration was water, the number average fiber diameter was smaller than that of DMSO, and the settling height ratio was higher. Although the length-weighted average fiber length of the pulverized pulp of wood pulp-based NBKP was small, the settling height ratio was small.
≪微細セルロース繊維乾燥体の製造方法≫
実施例1~5、比較例1~4で得た微細セルロース繊維ケーキK1~K9に対し硬化ひまし油エーテル(RCW-20、青木油脂製)を3質量%添加し、プラネタリーミキサー(プライミクス株式会社、商品名「ハイビスミックス2P-1」)中で50rpm、10分間、25℃、大気圧で撹拌処理した後、ジャケット温度80℃、-0.1MPaの減圧条件、50rpmで8時間減圧乾燥処理を行い、微細セルロース繊維乾燥体(CNF1~9)を得た。微細セルロース繊維乾燥体において、微細セルロース繊維/硬化ひまし油エーテルの質量比は、10/3であった。
微細セルロース繊維乾燥体及び樹脂複合体の物性を表2に示す。
微細セルロース繊維自体の沈降高さ比が高い場合、微細セルロース繊維乾燥体の沈降高さ比も高い値を示した。これは微細セルロース繊維が良く解繊され、高度なネットワークが水中で形成していると考えられる。そして、それに付随して、樹脂複合体の各種物性も優れている。
<Method for producing dried fine cellulose fiber body>
To the fine cellulose fiber cakes K1 to K9 obtained in Examples 1 to 5 and Comparative Examples 1 to 4, 3% by mass of hydrogenated castor oil ether (RCW-20, manufactured by Aoki Oil & Fat) was added, and the mixture was stirred in a planetary mixer (Primix Corporation, product name "Hibismix 2P-1") at 50 rpm for 10 minutes at 25°C and atmospheric pressure, and then dried under reduced pressure at 50 rpm for 8 hours at a jacket temperature of 80°C and reduced pressure of -0.1 MPa to obtain dried fine cellulose fibers (CNF1 to 9). In the dried fine cellulose fiber, the mass ratio of fine cellulose fiber/hydrogenated castor oil ether was 10/3.
The physical properties of the dried fine cellulose fiber material and the resin composite are shown in Table 2.
When the settling height ratio of the fine cellulose fiber itself was high, the settling height ratio of the dried fine cellulose fiber also showed a high value. This is thought to be because the fine cellulose fiber was well defibrated and an advanced network was formed in water. In addition, various physical properties of the resin composite were also excellent.
≪セルロース原料及び粉砕パルプの評価≫
[長さ加重平均繊維長]
JIS P 8226-2『パルプ-光学的自動分析法による繊維長測定方法 第2部:非偏光法』繊維長測定装置(Kajaani FiberLab V4(Metso Automation社製))を用いて測定した。
<Evaluation of cellulose raw materials and crushed pulp>
[Length-weighted average fiber length]
The fiber length was measured using a fiber length measuring device (Kajaani FiberLab V4 (manufactured by Metso Automation)) in accordance with JIS P 8226-2 "Pulp - Fiber length measurement method by optical automatic analysis method, Part 2: Non-polarized light method."
≪微細セルロース繊維の評価≫
微細セルロース繊維の物性は下記手法で作製された多孔質シートを用いて評価した。
[多孔質シート]
まず、ウェットケーキをtert-ブタノール中に添加し、さらにミキサー等で凝集物が無い状態まで分散処理を行った。微細セルロース繊維固形分重量0.5gに対し、濃度が0.5質量%となるように調整した。得られたtert-ブタノール分散液100gをろ紙上で濾過し、150℃にて乾燥させた後、ろ紙を剥離してシートを得た。このシートの透気抵抗度がシート目付10g/m2あたり100sec/100ml以下のものを多孔質シートとし、測定サンプルとして使用した。
23℃、50%RHの環境で1日静置したサンプルの目付W(g/m2)を測定した後、王研式透気抵抗試験機(旭精工(株)製、型式EG01)を用いて透気抵抗度R(sec/100ml)を測定した。この時、下記式に従い、10g/m2目付あたりの値を算出した。
目付10g/m2あたり透気抵抗度(sec/100ml)=R/W×10
<Evaluation of fine cellulose fibers>
The physical properties of the fine cellulose fibers were evaluated using a porous sheet prepared by the following method.
[Porous sheet]
First, the wet cake was added to tert-butanol, and further dispersed in a mixer or the like until no aggregates were present. The concentration was adjusted to 0.5% by mass per 0.5 g of fine cellulose fiber solid content. 100 g of the obtained tert-butanol dispersion was filtered on filter paper, dried at 150°C, and then the filter paper was peeled off to obtain a sheet. The sheet with an air resistance of 100 sec/100 ml or less per 10 g/ m2 sheet basis weight was used as a porous sheet and as a measurement sample.
After measuring the basis weight W (g/ m2 ) of the sample left to stand for one day in an environment of 23°C and 50% RH, the air resistance R (sec/100 ml) was measured using an Oken type air resistance tester (manufactured by Asahi Seiko Co., Ltd., model EG01). At this time, the value per basis weight of 10 g/ m2 was calculated according to the following formula.
Air resistance per unit area of 10 g/ m2 (sec/100 ml) = R/W x 10
[平均繊維径]
ウェットケーキをtert-ブタノールで0.01質量%まで希釈し、高剪断ホモジナイザー(IKA製、商品名「ウルトラタラックスT18」)を用い、処理条件:回転数25,000rpm×5分間で分散させ、マイカ上にキャストし、風乾したものを、高分解能走査型顕微鏡で測定した。測定は、少なくとも100本のセルロース繊維が観測されるように倍率を調整して行い、無作為に選んだ100本のセルロース繊維の短径(D)を測定し、100本のセルロース繊維の加算平均を算出した。
[Average fiber diameter]
The wet cake was diluted to 0.01% by mass with tert-butanol, dispersed using a high-shear homogenizer (manufactured by IKA, product name "Ultra Turrax T18") under processing conditions of 25,000 rpm for 5 minutes, cast onto mica, air-dried, and measured using a high-resolution scanning microscope. The measurement was performed by adjusting the magnification so that at least 100 cellulose fibers were observed, and the minor diameter (D) of 100 randomly selected cellulose fibers was measured, and the arithmetic average of the 100 cellulose fibers was calculated.
[DS]
多孔質シートの5か所のATR-IR法による赤外分光スペクトルを、フーリエ変換赤外分光光度計(JASCO社製 FT/IR-6200)で測定した。赤外分光スペクトル測定は以下の条件で行った。
積算回数:64回、
波数分解能:4cm-1、
測定波数範囲:4000~600cm-1、
ATR結晶:ダイヤモンド、
入射角度:45°
得られたIRスペクトルよりIRインデックスを、下記式(1):
IRインデックス= H1730/H1030・・・(1)
に従って算出した。式中、H1730及びH1030は1730cm-1、1030cm-1(セルロース骨格鎖C-O伸縮振動の吸収バンド)における吸光度である。ただし、それぞれ1900cm-1と1500cm-1を結ぶ線と800cm-1と1500cm-1を結ぶ線をベースラインとして、このベースラインを吸光度0とした時の吸光度を意味する。
そして、各測定場所の平均置換度をIRインデックスより下記式(2)に従って算出し、その平均値をDSとした。
DS=4.13×IRインデックス・・・(2)
[DS]
The infrared spectrum of the porous sheet at five points was measured by an ATR-IR method using a Fourier transform infrared spectrophotometer (FT/IR-6200 manufactured by JASCO Corp.) The infrared spectrum measurement was performed under the following conditions.
Number of times: 64
Wavenumber resolution: 4cm -1 ,
Measurement wave number range: 4000 to 600 cm -1 ,
ATR crystal: Diamond,
Incident angle: 45°
From the obtained IR spectrum, the IR index was calculated according to the following formula (1):
IR index = H1730/H1030 (1)
In the formula, H1730 and H1030 are the absorbances at 1730 cm -1 and 1030 cm -1 (absorption bands of C-O stretching vibration of the cellulose backbone chain). Note that the lines connecting 1900 cm -1 and 1500 cm -1 and the line connecting 800 cm -1 and 1500 cm -1 are taken as baselines, and the absorbances are calculated based on these baselines as 0 absorbance.
Then, the average degree of substitution at each measurement location was calculated from the IR index according to the following formula (2), and the average value was taken as DS.
DS = 4.13 × IR index (2)
[結晶化度]
多孔質シートのX線回折測定を行い、下記式より結晶化度を算出した。
結晶化度(%)=[I(200)-I(amorphous)]/I(200)×100
I(200):セルロースI型結晶における200面(2θ=22.5°)による回折ピーク強度
I(amorphous):セルロースI型結晶におけるアモルファスによるハローピーク強度であって、200面の回折角度より4.5°低角度側(2θ=18.0°)のピーク強度
[Crystallization degree]
The porous sheet was subjected to X-ray diffraction measurement, and the crystallinity was calculated according to the following formula.
Crystallinity (%) = [I (200) - I (amorphous) ] / I (200) x 100
I (200) : Diffraction peak intensity due to the 200 plane (2θ = 22.5°) in cellulose I type crystals I (amorphous) : Halo peak intensity due to amorphous in cellulose I type crystals, which is the peak intensity at an angle 4.5° lower than the diffraction angle of the 200 plane (2θ = 18.0°)
(X線回折測定条件)
装置 MiniFlex(株式会社リガク製)
操作軸 2θ/θ
線源 CuKα
測定方法 連続式
電圧 40kV
電流 15mA
開始角度 2θ=5°
終了角度 2θ=30°
サンプリング幅 0.020°
スキャン速度 2.0°/min
サンプル:試料ホルダー上に多孔質シートを貼り付け
(X-ray diffraction measurement conditions)
Device: MiniFlex (manufactured by Rigaku Corporation)
Operation axis 2θ/θ
Radiation source: CuKα
Measurement method: Continuous Voltage: 40 kV
Current: 15mA
Starting angle 2θ=5°
End angle 2θ = 30°
Sampling width 0.020°
Scan speed: 2.0°/min
Sample: A porous sheet is attached onto the sample holder.
[重量平均分子量(Mw)、数平均分子量(Mn)及びMw/Mn比]
多孔質シートを0.88g秤量し、ハサミで小片に切り刻んだ後、軽く攪拌したうえで、純水20mLを加え1日放置した。次に遠心分離によって水と固形分を分離した。続いてアセトン20mLを加え、軽く攪拌したうえで1日放置した。次に遠心分離によってアセトンと固形分を分離した。続いてN、N-ジメチルアセトアミド20mLを加え、軽く攪拌したうえで1日放置した。再度、遠心分離によってN、N-ジメチルアセトアミドと固形分を分離したのち、N,N-ジメチルアセトアミド20mLを加え、軽く攪拌したうえで1日放置した。遠心分離によってN,N-ジメチルアセトアミドと固形分を分離し、固形分に塩化リチウムが8質量パーセントになるように調液したN,N-ジメチルアセトアミド溶液を19.2g加え、スターラーで攪拌し、目視で溶解するのを確認した。セルロースを溶解させた溶液を0.45μmフィルターでろ過し、ろ液をゲルパーミエーションクロマトグラフィ用の試料として供した。用いた装置と測定条件は下記である。
装置 :東ソー社 HLC-8120
カラム:TSKgel SuperAWM-H(6.0mmI.D.×15cm)×2本
検出器:RI検出器
溶離液:N、N-ジメチルアセトアミド(塩化リチウム0.2%)
流速:0.6mL/分
検量線:プルラン換算
[Weight average molecular weight (Mw), number average molecular weight (Mn) and Mw/Mn ratio]
0.88 g of the porous sheet was weighed, cut into small pieces with scissors, lightly stirred, and then 20 mL of pure water was added and left for one day. Next, water and solids were separated by centrifugation. Then, 20 mL of acetone was added, lightly stirred, and left for one day. Next, acetone and solids were separated by centrifugation. Then, 20 mL of N,N-dimethylacetamide was added, lightly stirred, and left for one day. N,N-dimethylacetamide and solids were separated again by centrifugation, and then 20 mL of N,N-dimethylacetamide was added, lightly stirred, and left for one day. N,N-dimethylacetamide and solids were separated by centrifugation, and 19.2 g of N,N-dimethylacetamide solution prepared so that lithium chloride was 8 mass percent was added to the solids, and the mixture was stirred with a stirrer, and it was confirmed that it was dissolved by visual observation. The solution in which cellulose was dissolved was filtered through a 0.45 μm filter, and the filtrate was used as a sample for gel permeation chromatography. The apparatus and measurement conditions used are as follows.
Equipment: Tosoh Corporation HLC-8120
Column: TSKgel SuperAWM-H (6.0 mm ID x 15 cm) x 2 Detector: RI detector Eluent: N,N-dimethylacetamide (lithium chloride 0.2%)
Flow rate: 0.6 mL/min. Calibration curve: Pullulan equivalent
[アルカリ可溶多糖類平均含有率]
アルカリ可溶多糖類含有率は微細セルロース繊維について非特許文献(木質科学実験マニュアル、日本木材学会編、92~97頁、2000年)に記載の手法より、ホロセルロース含有率(Wise法)からαセルロース含有率を差し引くことで求めた。1つのサンプルにつき3回アルカリ可溶多糖類含有率を算出し、算出したアルカリ可溶多糖類含有率の数平均を微細セルロース繊維のアルカリ可溶多糖類平均含有率とした。
[Average content of alkali-soluble polysaccharides]
The alkali-soluble polysaccharide content was determined by subtracting the α-cellulose content from the holocellulose content (Wise method) according to the method described in the non-patent literature (Wood Science Experiment Manual, edited by the Japan Wood Research Society, pp. 92-97, 2000) for fine cellulose fibers. The alkali-soluble polysaccharide content was calculated three times for each sample, and the number average of the calculated alkali-soluble polysaccharide contents was taken as the average alkali-soluble polysaccharide content of the fine cellulose fibers.
[沈降高さ比]
微細セルロース繊維ケーキに、微細セルロース繊維が0.05質量%になるよう蒸留水を添加して試験用分散液を調製した。試験用分散液20mlを30ml容量のガラスバイアルに分取した後、ホモジナイザー(回転数10000rpm、1分間)で分散させ、その後素早く試験用分散液8mlをネジ口試験管(内径12mm)に入れて密封し、手動で振り混ぜて分散液を一様にした。このときの分散液の波長850nmにおける透過度(以下、初期透過度ともいう。)を、液中分散安定性評価装置 Turbiscan(三洋貿易(株)製)を用いて測定した。また、この試験管を23℃で24時間静置した後、分散液の底面から分離界面までの高さと、分散液底面から分散液上面までの高さとを、上記液中分散安定性評価装置を用い、上記波長で透過度50%になる高さを分離界面と定義して測定し、下記式(1):
沈降高さ比(%)=([A]/[B])×100・・・(1)
(式(1)中、[A]は分散液底面から分離界面までの高さであり、[B]は分散液底面から分散液上面までの高さである。)
に従って、沈降高さ比を求めた。なお、上記の透過度50%になる高さが画定されない場合には、上記初期透過度よりも透過度が高い領域を上澄部、低い領域を堆積部とし、上澄部と堆積部との境界を分離界面と定義し、上記(1)に従って沈降高さ比を求めた。
沈降高さ比が大きいほど、樹脂複合体中での分散性に優れ、又、微細セルロース繊維のネットワークを形成しやすく、機械特性(特に引張伸度及び引張強度)が良好である。
[Settling height ratio]
A test dispersion was prepared by adding distilled water to the fine cellulose fiber cake so that the fine cellulose fiber was 0.05% by mass. 20 ml of the test dispersion was dispensed into a 30 ml glass vial, and then dispersed with a homogenizer (10,000 rpm, 1 minute). Then, 8 ml of the test dispersion was quickly placed in a screw-top test tube (12 mm inner diameter), sealed, and manually shaken to make the dispersion uniform. The transmittance (hereinafter also referred to as initial transmittance) of the dispersion at a wavelength of 850 nm was measured using a liquid dispersion stability evaluation device Turbiscan (manufactured by Sanyo Trading Co., Ltd.). In addition, after leaving this test tube at 23 ° C. for 24 hours, the height from the bottom of the dispersion to the separation interface and the height from the bottom of the dispersion to the top of the dispersion were measured using the liquid dispersion stability evaluation device, with the height at which the transmittance becomes 50% at the above wavelength being defined as the separation interface, and the following formula (1):
Settling height ratio (%) = ([A] / [B]) × 100 ... (1)
(In formula (1), [A] is the height from the bottom surface of the dispersion to the separation interface, and [B] is the height from the bottom surface of the dispersion to the top surface of the dispersion.)
In addition, when the height at which the permeability becomes 50% was not defined, the region with a higher permeability than the initial permeability was defined as the supernatant portion, and the region with a lower permeability than the initial permeability was defined as the sediment portion, and the boundary between the supernatant portion and the sediment portion was defined as the separation interface, and the sedimentation height ratio was determined according to (1) above.
The larger the sedimentation height ratio, the better the dispersibility in the resin composite, the easier it is to form a network of fine cellulose fibers, and the better the mechanical properties (particularly tensile elongation and tensile strength).
≪微細セルロース繊維乾燥体の評価≫
[沈降高さ比]
微細セルロース繊維が0.5質量%になるよう、微細セルロース繊維乾燥体に蒸留水を添加して試験用分散液20gを調製した。試験用分散液20gを30ml容量のガラスバイアルに分取した後、ホモジナイザー(IKA製、商品名「ウルトラタラックスT18」、回転数10000rpm、5分間)で分散させた。つづいて、ネジ口試験管(内径12mm)に蒸留水7.2ml、試験用分散液0.8mlを入れて密栓し、手動で振り混ぜて分散液を一様にした。得られた分散液を上述の沈降高さ比の測定方法に従い、算出した。
<Evaluation of dried fine cellulose fiber>
[Settling height ratio]
Distilled water was added to the dried fine cellulose fibers so that the fine cellulose fibers were 0.5% by mass to prepare 20 g of a test dispersion. 20 g of the test dispersion was dispensed into a 30 ml glass vial, and then dispersed with a homogenizer (manufactured by IKA, trade name "Ultra Turrax T18", rotation speed 10,000 rpm, 5 minutes). Next, 7.2 ml of distilled water and 0.8 ml of the test dispersion were placed in a screw-cap test tube (inner diameter 12 mm), sealed, and manually shaken to make the dispersion uniform. The obtained dispersion was calculated according to the above-mentioned method for measuring the sedimentation height ratio.
≪樹脂複合体の製造及び評価≫
得られた微細セルロース繊維乾燥体と下記熱可塑性樹脂とを、シリンダーブロック数が13個ある二軸押出機(STEER社製 OMEGA30H、L/D=60)を用いて、熱可塑性樹脂9質量部に対する微細セルロース繊維重量が1質量部となるよう下記条件で溶融混練し、微細セルロース繊維強化熱可塑性樹脂のペレットを得た。
<Production and evaluation of resin composite>
The obtained dried fine cellulose fiber and the following thermoplastic resin were melt-kneaded under the following conditions using a twin-screw extruder (OMEGA30H, L/D = 60) having 13 cylinder blocks so that the weight of the fine cellulose fiber was 1 part by mass per 9 parts by mass of the thermoplastic resin, to obtain pellets of fine cellulose fiber reinforced thermoplastic resin.
(熱可塑性樹脂)
・UBEナイロン 1013B 宇部興産株式会社製
ポリアミド6(以下、PAと称す。)
カルボキシル末端基比率が、([COOH]/[全末端基])=0.59
・プライムポリプロ J105G プライムポリマー製
ポリプロピレン(以下、PPと称す。)
(Thermoplastic resin)
- UBE Nylon 1013B Polyamide 6 (hereinafter referred to as PA) manufactured by Ube Industries, Ltd.
The carboxyl end group ratio ([COOH]/[total end groups]) is 0.59.
- Prime Polypro J105G Made by Prime Polymer Polypropylene (hereinafter referred to as PP)
(溶融混練条件)
回転数:300rpm
シリンダー温度:250℃(PAについて)又は200℃(PPについて)
(Melt-kneading conditions)
Rotation speed: 300 rpm
Cylinder temperature: 250°C (for PA) or 200°C (for PP)
[引張降伏強度、引張降伏伸度(TEy)、引張破断伸度(TEb)]
最大型締圧力75トンの射出成形機を用いて、ISO294-3に準拠した多目的試験片を成形し、JIS K6920-2に準拠した条件でn=10で実施した。なお、ポリアミド樹脂は、吸湿による変化が起きるため、成形直後にアルミ防湿袋に保管し、吸湿を抑制した。
引張降伏伸度(TEy)と引張破断伸度(TEb)との比[TEb/TEy]を算出した。
[Tensile yield strength, tensile yield elongation (TEy), tensile break elongation (TEb)]
Multipurpose test pieces conforming to ISO294-3 were molded using an injection molding machine with a maximum clamping pressure of 75 tons, and tests were performed under conditions conforming to JIS K6920-2 with n=10. Note that since polyamide resin changes due to moisture absorption, it was stored in an aluminum moisture-proof bag immediately after molding to suppress moisture absorption.
The ratio of the tensile elongation at yield (TEy) to the tensile elongation at break (TEb) [TEb/TEy] was calculated.
本開示の微細セルロース繊維は、樹脂複合体に対して良好な物性向上効果を付与できるため、例えば自動車の内装材料及び外装材料用途等の分野で好適に利用できる。 The fine cellulose fibers disclosed herein can impart favorable property-improving effects to resin composites, and can therefore be suitably used in fields such as automotive interior and exterior material applications.
Claims (13)
前記微細セルロース繊維中のアルカリ可溶多糖類平均含有率が、12.5質量%以下であり、
前記微細セルロース繊維を水中に濃度0.05質量%で含有させてなる試験用分散液を23℃、常圧にて24時間静置したときの、試験用分散液の全液高100%に対する微細セルロース繊維の沈降高さの比率が、10%以上50%以下である、微細セルロース繊維。 A fine cellulose fiber,
The average content of alkali-soluble polysaccharides in the fine cellulose fibers is 12.5% by mass or less,
The fine cellulose fibers have a ratio of the settling height of the fine cellulose fibers to the total liquid height of the test dispersion (100%) of 10% or more and 50 % or less when a test dispersion containing the fine cellulose fibers in water at a concentration of 0.05% by mass is allowed to stand at 23°C and normal pressure for 24 hours.
セルロース原料を粉砕し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、及び
任意に、前記微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、
を含む、方法。 A method for producing fine cellulose fibers according to any one of claims 1 to 6,
A cellulose raw material is pulverized and then defibrated with a homomixer to obtain a fine cellulose fiber slurry; and optionally, the fine cellulose fiber slurry is dried under reduced pressure while being stirred.
A method comprising:
セルロース原料を粉砕し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、
前記微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、及び
任意に、前記微細セルロース繊維スラリー及び/又は微細セルロース繊維と追加の成分とを混合すること、
を含む、方法。 A method for producing a dried body according to claim 7 or 8,
A cellulose raw material is pulverized and then defibrated with a homomixer to obtain a fine cellulose fiber slurry;
drying the fine cellulose fiber slurry under reduced pressure while stirring; and optionally mixing the fine cellulose fiber slurry and/or the fine cellulose fibers with additional components.
A method comprising:
セルロース原料を粉砕し、次いでホモミキサーで解繊することによって微細セルロース繊維スラリーを得ること、及び
任意に、前記微細セルロース繊維スラリーを撹拌下で減圧乾燥すること、
を含む方法で微細セルロース繊維を得る微細セルロース繊維製造工程、並びに、
前記微細セルロース繊維と前記樹脂とを混合する混合工程、
を含む、方法。 A method for producing a resin composite according to claim 9,
A cellulose raw material is pulverized and then defibrated with a homomixer to obtain a fine cellulose fiber slurry; and optionally, the fine cellulose fiber slurry is dried under reduced pressure while being stirred.
A process for producing fine cellulose fibers by a method comprising the steps of:
A mixing step of mixing the fine cellulose fibers and the resin;
A method comprising:
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019191926A JP7547040B2 (en) | 2019-10-21 | 2019-10-21 | Fine cellulose fiber and resin composite |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019191926A JP7547040B2 (en) | 2019-10-21 | 2019-10-21 | Fine cellulose fiber and resin composite |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2021066779A JP2021066779A (en) | 2021-04-30 |
| JP2021066779A5 JP2021066779A5 (en) | 2022-10-19 |
| JP7547040B2 true JP7547040B2 (en) | 2024-09-09 |
Family
ID=75636825
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019191926A Active JP7547040B2 (en) | 2019-10-21 | 2019-10-21 | Fine cellulose fiber and resin composite |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP7547040B2 (en) |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012000550A (en) | 2010-06-15 | 2012-01-05 | Asahi Kasei Fibers Corp | Filter medium composed of cellulose fiber |
| JP2012036529A (en) | 2010-08-06 | 2012-02-23 | Asahi Kasei Fibers Corp | Cellulose sheet |
| JP2013185068A (en) | 2012-03-08 | 2013-09-19 | Kyoto City | Modified cellulose nanofiber and resin composition including modified cellulose nanofiber |
| JP2017057243A (en) | 2015-09-14 | 2017-03-23 | 三菱製紙株式会社 | Method for producing finely divided cellulose fiber |
-
2019
- 2019-10-21 JP JP2019191926A patent/JP7547040B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012000550A (en) | 2010-06-15 | 2012-01-05 | Asahi Kasei Fibers Corp | Filter medium composed of cellulose fiber |
| JP2012036529A (en) | 2010-08-06 | 2012-02-23 | Asahi Kasei Fibers Corp | Cellulose sheet |
| JP2013185068A (en) | 2012-03-08 | 2013-09-19 | Kyoto City | Modified cellulose nanofiber and resin composition including modified cellulose nanofiber |
| JP2017057243A (en) | 2015-09-14 | 2017-03-23 | 三菱製紙株式会社 | Method for producing finely divided cellulose fiber |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2021066779A (en) | 2021-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7504650B2 (en) | Dried cellulose fiber body, its manufacturing method, and manufacturing method of resin composite | |
| US20240309208A1 (en) | Highly Heat-Resistant Resin Composite Including Chemically Modified, Fine Cellulose Fibers | |
| JP7488096B2 (en) | Dried cellulose fiber body, its manufacturing method, and manufacturing method of resin composite | |
| JP7527830B2 (en) | Method for producing dried cellulose fiber body and method for producing resin composite | |
| JP7385625B2 (en) | Composite particles and resin compositions | |
| JP7566472B2 (en) | Method for producing cellulose nanofiber dry solid and redispersion of cellulose nanofiber | |
| JP7411378B2 (en) | Cellulose resin composition | |
| JP7444669B2 (en) | Composite particles containing fine cellulose fibers and resin compositions containing composite particles | |
| JP2022001631A (en) | Manufacturing method of dry powder of cellulose fiber | |
| JP2021187885A (en) | Cellulose resin composition and method for producing the same | |
| JP6937817B2 (en) | Cellulose composition | |
| JP7547040B2 (en) | Fine cellulose fiber and resin composite | |
| JP2021070747A (en) | Cellulose nanofiber powder and method for producing the same | |
| JP7417391B2 (en) | Method for producing esterified cellulose fiber | |
| JP6681499B1 (en) | Chemically modified cellulose fine fiber and high heat resistant resin composite containing chemically modified cellulose fine fiber | |
| JP7342142B2 (en) | Cellulose resin composition | |
| JP2021066780A (en) | Fibrous substance fluid dispersion, and fiber-reinforced resin composition | |
| JP2020204112A (en) | Chemically-modified cellulose fiber | |
| JP2024101346A (en) | Method for producing chemical modification fine cellulose fiber | |
| JP2023112485A (en) | Manufacturing method of concentrate of cellulose fine fiber dispersion, and manufacturing method of resin composition | |
| WO2022215756A1 (en) | Polyacetal resin composition and method for manufacturing same | |
| JP2023011541A (en) | Resin composition and method for producing the same | |
| JP2022128430A (en) | Method for producing esterified cellulose nanofiber and resin composition | |
| JP2023177265A (en) | Resin composition and molded body | |
| JP2022171138A (en) | Resin composition and method for manufacturing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20221011 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20221011 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20230822 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20231013 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20231212 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240213 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20240402 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240529 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20240730 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20240828 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7547040 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |