JP7429968B2 - Pest resistance inducer and plant pest control method - Google Patents
Pest resistance inducer and plant pest control method Download PDFInfo
- Publication number
- JP7429968B2 JP7429968B2 JP2020121591A JP2020121591A JP7429968B2 JP 7429968 B2 JP7429968 B2 JP 7429968B2 JP 2020121591 A JP2020121591 A JP 2020121591A JP 2020121591 A JP2020121591 A JP 2020121591A JP 7429968 B2 JP7429968 B2 JP 7429968B2
- Authority
- JP
- Japan
- Prior art keywords
- ionone
- plant
- plants
- pest
- effect
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 241000607479 Yersinia pestis Species 0.000 title claims description 89
- 239000000411 inducer Substances 0.000 title claims description 47
- 238000000034 method Methods 0.000 title claims description 33
- UZFLPKAIBPNNCA-BQYQJAHWSA-N alpha-ionone Chemical compound CC(=O)\C=C\C1C(C)=CCCC1(C)C UZFLPKAIBPNNCA-BQYQJAHWSA-N 0.000 claims description 108
- UZFLPKAIBPNNCA-UHFFFAOYSA-N alpha-ionone Natural products CC(=O)C=CC1C(C)=CCCC1(C)C UZFLPKAIBPNNCA-UHFFFAOYSA-N 0.000 claims description 107
- 241000196324 Embryophyta Species 0.000 claims description 93
- 241000238631 Hexapoda Species 0.000 claims description 19
- 239000004480 active ingredient Substances 0.000 claims description 13
- 241000219193 Brassicaceae Species 0.000 claims description 5
- 241000219104 Cucurbitaceae Species 0.000 claims description 5
- 241000208292 Solanaceae Species 0.000 claims description 5
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 66
- 240000003768 Solanum lycopersicum Species 0.000 description 52
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 50
- 230000000694 effects Effects 0.000 description 48
- PSQYTAPXSHCGMF-BQYQJAHWSA-N β-ionone Chemical compound CC(=O)\C=C\C1=C(C)CCCC1(C)C PSQYTAPXSHCGMF-BQYQJAHWSA-N 0.000 description 35
- 239000000243 solution Substances 0.000 description 26
- 241001414989 Thysanoptera Species 0.000 description 23
- ZNJFBWYDHIGLCU-HWKXXFMVSA-N jasmonic acid Chemical compound CC\C=C/C[C@@H]1[C@@H](CC(O)=O)CCC1=O ZNJFBWYDHIGLCU-HWKXXFMVSA-N 0.000 description 22
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 20
- SFEOKXHPFMOVRM-UHFFFAOYSA-N (+)-(S)-gamma-ionone Natural products CC(=O)C=CC1C(=C)CCCC1(C)C SFEOKXHPFMOVRM-UHFFFAOYSA-N 0.000 description 17
- 230000014509 gene expression Effects 0.000 description 17
- 239000000126 substance Substances 0.000 description 15
- 230000001939 inductive effect Effects 0.000 description 13
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 239000007788 liquid Substances 0.000 description 12
- 108090000623 proteins and genes Proteins 0.000 description 12
- 241000256259 Noctuidae Species 0.000 description 11
- 241000256248 Spodoptera Species 0.000 description 11
- 238000010586 diagram Methods 0.000 description 11
- ZNJFBWYDHIGLCU-UHFFFAOYSA-N jasmonic acid Natural products CCC=CCC1C(CC(O)=O)CCC1=O ZNJFBWYDHIGLCU-UHFFFAOYSA-N 0.000 description 11
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 10
- 229960004889 salicylic acid Drugs 0.000 description 10
- 230000004083 survival effect Effects 0.000 description 10
- 230000006698 induction Effects 0.000 description 9
- 239000000575 pesticide Substances 0.000 description 9
- 240000008067 Cucumis sativus Species 0.000 description 8
- XEVQXKKKAVVSMW-WRWORJQWSA-N loliolide Chemical compound C1[C@@H](O)CC(C)(C)C2=CC(=O)O[C@@]21C XEVQXKKKAVVSMW-WRWORJQWSA-N 0.000 description 8
- 239000002904 solvent Substances 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- 238000005507 spraying Methods 0.000 description 7
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- 208000035240 Disease Resistance Diseases 0.000 description 6
- 241000343234 Scirtothrips citri Species 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 239000000843 powder Substances 0.000 description 6
- 235000009849 Cucumis sativus Nutrition 0.000 description 5
- 238000009825 accumulation Methods 0.000 description 5
- 230000037396 body weight Effects 0.000 description 5
- 230000000052 comparative effect Effects 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- 241000219195 Arabidopsis thaliana Species 0.000 description 4
- XEVQXKKKAVVSMW-UHFFFAOYSA-N D-epiloliolide Natural products C1C(O)CC(C)(C)C2=CC(=O)OC21C XEVQXKKKAVVSMW-UHFFFAOYSA-N 0.000 description 4
- 241000339373 Thrips palmi Species 0.000 description 4
- 235000010724 Wisteria floribunda Nutrition 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- -1 alkali metal salts Chemical class 0.000 description 4
- 238000003556 assay Methods 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 239000000287 crude extract Substances 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 235000013601 eggs Nutrition 0.000 description 4
- 230000000749 insecticidal effect Effects 0.000 description 4
- XWYLNCDCFDBLGT-HTRCEHHLSA-N loliolide Natural products CC1(C)C[C@H](O)C[C@H]2OC(=O)C=C12 XWYLNCDCFDBLGT-HTRCEHHLSA-N 0.000 description 4
- 235000015097 nutrients Nutrition 0.000 description 4
- 239000003960 organic solvent Substances 0.000 description 4
- 244000052769 pathogen Species 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 241000219194 Arabidopsis Species 0.000 description 3
- 235000008534 Capsicum annuum var annuum Nutrition 0.000 description 3
- 241000195493 Cryptophyta Species 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 239000004677 Nylon Substances 0.000 description 3
- 235000006140 Raphanus sativus var sativus Nutrition 0.000 description 3
- 239000000443 aerosol Substances 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 235000021466 carotenoid Nutrition 0.000 description 3
- 150000001747 carotenoids Chemical class 0.000 description 3
- 238000007865 diluting Methods 0.000 description 3
- 239000000839 emulsion Substances 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- HNZUNIKWNYHEJJ-UHFFFAOYSA-N geranyl acetone Natural products CC(C)=CCCC(C)=CCCC(C)=O HNZUNIKWNYHEJJ-UHFFFAOYSA-N 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 229920001778 nylon Polymers 0.000 description 3
- 239000003375 plant hormone Substances 0.000 description 3
- JXJIQCXXJGRKRJ-KOOBJXAQSA-N pseudoionone Chemical compound CC(C)=CCC\C(C)=C\C=C\C(C)=O JXJIQCXXJGRKRJ-KOOBJXAQSA-N 0.000 description 3
- 239000002689 soil Substances 0.000 description 3
- 239000012453 solvate Substances 0.000 description 3
- 235000000832 Ayote Nutrition 0.000 description 2
- 240000007124 Brassica oleracea Species 0.000 description 2
- 235000003899 Brassica oleracea var acephala Nutrition 0.000 description 2
- 235000011299 Brassica oleracea var botrytis Nutrition 0.000 description 2
- 235000011301 Brassica oleracea var capitata Nutrition 0.000 description 2
- 235000001169 Brassica oleracea var oleracea Nutrition 0.000 description 2
- 240000003259 Brassica oleracea var. botrytis Species 0.000 description 2
- 244000221633 Brassica rapa subsp chinensis Species 0.000 description 2
- 235000010149 Brassica rapa subsp chinensis Nutrition 0.000 description 2
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 2
- 241000219122 Cucurbita Species 0.000 description 2
- 235000009854 Cucurbita moschata Nutrition 0.000 description 2
- 235000009804 Cucurbita pepo subsp pepo Nutrition 0.000 description 2
- 241000927584 Frankliniella occidentalis Species 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 101000942959 Punica granatum Acidic endochitinase Pun g 14, amyloplastic Proteins 0.000 description 2
- 244000088415 Raphanus sativus Species 0.000 description 2
- 244000269722 Thea sinensis Species 0.000 description 2
- 241000189579 Thripidae Species 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- ANVAOWXLWRTKGA-XHGAXZNDSA-N all-trans-alpha-carotene Chemical compound CC=1CCCC(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1C(C)=CCCC1(C)C ANVAOWXLWRTKGA-XHGAXZNDSA-N 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 239000002917 insecticide Substances 0.000 description 2
- 150000002611 lead compounds Chemical class 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000037353 metabolic pathway Effects 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000000361 pesticidal effect Effects 0.000 description 2
- 235000015136 pumpkin Nutrition 0.000 description 2
- 238000010898 silica gel chromatography Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 235000013311 vegetables Nutrition 0.000 description 2
- 241001579830 Actinotia Species 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 241001280436 Allium schoenoprasum Species 0.000 description 1
- 235000001270 Allium sibiricum Nutrition 0.000 description 1
- 241000134916 Amanita Species 0.000 description 1
- 241000368734 Apamea Species 0.000 description 1
- 241001605403 Athetis Species 0.000 description 1
- 241000444906 Atrachea Species 0.000 description 1
- 241000774177 Bambusiphila Species 0.000 description 1
- 101710130006 Beta-glucanase Proteins 0.000 description 1
- 235000011293 Brassica napus Nutrition 0.000 description 1
- 235000007294 Brassica nipposinica Nutrition 0.000 description 1
- 244000026811 Brassica nipposinica Species 0.000 description 1
- 235000004221 Brassica oleracea var gemmifera Nutrition 0.000 description 1
- 235000017647 Brassica oleracea var italica Nutrition 0.000 description 1
- 244000308368 Brassica oleracea var. gemmifera Species 0.000 description 1
- 240000008100 Brassica rapa Species 0.000 description 1
- 235000000536 Brassica rapa subsp pekinensis Nutrition 0.000 description 1
- 235000000540 Brassica rapa subsp rapa Nutrition 0.000 description 1
- 235000011960 Brassica ruvo Nutrition 0.000 description 1
- 240000004160 Capsicum annuum Species 0.000 description 1
- 240000008384 Capsicum annuum var. annuum Species 0.000 description 1
- 235000002568 Capsicum frutescens Nutrition 0.000 description 1
- 241000459479 Capsula Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- WTEVQBCEXWBHNA-UHFFFAOYSA-N Citral Natural products CC(C)=CCCC(C)=CC=O WTEVQBCEXWBHNA-UHFFFAOYSA-N 0.000 description 1
- 244000241235 Citrullus lanatus Species 0.000 description 1
- 235000012828 Citrullus lanatus var citroides Nutrition 0.000 description 1
- 241000220362 Cosmia Species 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 241000219112 Cucumis Species 0.000 description 1
- 235000015510 Cucumis melo subsp melo Nutrition 0.000 description 1
- 235000009852 Cucurbita pepo Nutrition 0.000 description 1
- 241000219130 Cucurbita pepo subsp. pepo Species 0.000 description 1
- 235000003954 Cucurbita pepo var melopepo Nutrition 0.000 description 1
- 235000002767 Daucus carota Nutrition 0.000 description 1
- 244000000626 Daucus carota Species 0.000 description 1
- BWLUMTFWVZZZND-UHFFFAOYSA-N Dibenzylamine Chemical class C=1C=CC=CC=1CNCC1=CC=CC=C1 BWLUMTFWVZZZND-UHFFFAOYSA-N 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical class C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- 241000486923 Dryobotodes Species 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical class NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000220370 Eupsilia Species 0.000 description 1
- 241000365767 Frankliniella intonsa Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical class NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical class NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- 102000002704 Leucyl aminopeptidase Human genes 0.000 description 1
- 108010004098 Leucyl aminopeptidase Proteins 0.000 description 1
- 241000566529 Lithophane Species 0.000 description 1
- 244000280244 Luffa acutangula Species 0.000 description 1
- 235000009814 Luffa aegyptiaca Nutrition 0.000 description 1
- UPYKUZBSLRQECL-UKMVMLAPSA-N Lycopene Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1C(=C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=C)CCCC2(C)C UPYKUZBSLRQECL-UKMVMLAPSA-N 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 244000223047 Osmorhiza occidentalis Species 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 241001605379 Phlogophora Species 0.000 description 1
- 241000484385 Polyphaenis Species 0.000 description 1
- 241000206607 Porphyra umbilicalis Species 0.000 description 1
- 244000184734 Pyrus japonica Species 0.000 description 1
- 238000002123 RNA extraction Methods 0.000 description 1
- 235000005733 Raphanus sativus var niger Nutrition 0.000 description 1
- 240000001970 Raphanus sativus var. sativus Species 0.000 description 1
- 241000774167 Sapporia Species 0.000 description 1
- 241000365764 Scirtothrips dorsalis Species 0.000 description 1
- 241001247145 Sebastes goodei Species 0.000 description 1
- 241000931987 Sesamia Species 0.000 description 1
- 235000002597 Solanum melongena Nutrition 0.000 description 1
- 244000061458 Solanum melongena Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 241000256247 Spodoptera exigua Species 0.000 description 1
- 241000256251 Spodoptera frugiperda Species 0.000 description 1
- 241000985245 Spodoptera litura Species 0.000 description 1
- 241000931661 Spodoptera mauritia acronyctoides Species 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 241000339374 Thrips tabaci Species 0.000 description 1
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical class CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 1
- 235000012544 Viola sororia Nutrition 0.000 description 1
- 241001106476 Violaceae Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 241001582722 Xylena Species 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 239000003905 agrochemical Substances 0.000 description 1
- 238000005575 aldol reaction Methods 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 235000003903 alpha-carotene Nutrition 0.000 description 1
- ANVAOWXLWRTKGA-HLLMEWEMSA-N alpha-carotene Natural products C(=C\C=C\C=C(/C=C/C=C(\C=C\C=1C(C)(C)CCCC=1C)/C)\C)(\C=C\C=C(/C=C/[C@H]1C(C)=CCCC1(C)C)\C)/C ANVAOWXLWRTKGA-HLLMEWEMSA-N 0.000 description 1
- 239000011795 alpha-carotene Substances 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229940024606 amino acid Drugs 0.000 description 1
- 235000001014 amino acid Nutrition 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 125000000637 arginyl group Chemical class N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 125000000613 asparagine group Chemical class N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 108010051210 beta-Fructofuranosidase Proteins 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000001511 capsicum annuum Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 150000001746 carotenes Chemical class 0.000 description 1
- 235000005473 carotenes Nutrition 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000001055 chewing effect Effects 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 229940043350 citral Drugs 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 150000001879 copper Chemical class 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical class OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 150000005332 diethylamines Chemical class 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 239000003337 fertilizer Substances 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- WTEVQBCEXWBHNA-JXMROGBWSA-N geranial Chemical compound CC(C)=CCC\C(C)=C\C=O WTEVQBCEXWBHNA-JXMROGBWSA-N 0.000 description 1
- 150000002301 glucosamine derivatives Chemical class 0.000 description 1
- 150000002333 glycines Chemical class 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 239000004009 herbicide Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000001573 invertase Substances 0.000 description 1
- 235000011073 invertase Nutrition 0.000 description 1
- 159000000014 iron salts Chemical class 0.000 description 1
- 229910003002 lithium salt Inorganic materials 0.000 description 1
- 159000000002 lithium salts Chemical class 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 150000002815 nickel Chemical class 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 238000007539 photo-oxidation reaction Methods 0.000 description 1
- 230000029553 photosynthesis Effects 0.000 description 1
- 238000010672 photosynthesis Methods 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 230000008659 phytopathology Effects 0.000 description 1
- 239000005648 plant growth regulator Substances 0.000 description 1
- 230000037039 plant physiology Effects 0.000 description 1
- 159000000001 potassium salts Chemical class 0.000 description 1
- 235000012015 potatoes Nutrition 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 238000010025 steaming Methods 0.000 description 1
- 239000011550 stock solution Substances 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001308 synthesis method Methods 0.000 description 1
- 238000012353 t test Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 235000013616 tea Nutrition 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- QEMXHQIAXOOASZ-UHFFFAOYSA-N tetramethylammonium Chemical class C[N+](C)(C)C QEMXHQIAXOOASZ-UHFFFAOYSA-N 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 238000002723 toxicity assay Methods 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- NCYCYZXNIZJOKI-UHFFFAOYSA-N vitamin A aldehyde Natural products O=CC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-UHFFFAOYSA-N 0.000 description 1
- 239000004563 wettable powder Substances 0.000 description 1
- 150000003751 zinc Chemical class 0.000 description 1
Images
Landscapes
- Catching Or Destruction (AREA)
- Agricultural Chemicals And Associated Chemicals (AREA)
- Cultivation Of Plants (AREA)
Description
特許法第30条第2項適用 (1)ウェブサイトの掲載日:2019年12月19日、ウェブサイトのアドレス:https://www.mdpi.com/1420-3049/25/1/17/htmApplication of Article 30,
本発明は、害虫抵抗性誘導剤及び植物の害虫防除方法に関する。 The present invention relates to a pest resistance inducer and a method for controlling pests in plants.
作物の収量低下を招く病害虫の発生は、農業分野において深刻な問題である。指定有害動植物による被害の半数以上が害虫による被害であり、害虫防除は重要である。作物にかかる害虫の防除方法には様々な技術があるが、主に、殺虫剤等の化学農薬を使用する化学的防除法や、抵抗性品種等を用いる耕種的防除法や天敵を利用する方法等の生物学的防除法が用いられている。中でも、化学農薬は即効性があり、防除効果も高いため、多用されている。しかしながら、化学農薬には、不適切な使用による環境中への残留による生態系への影響や、同一薬剤の連続使用により薬剤抵抗性を持った害虫の出現の問題が懸念されている。また、環境保全や消費者の安全志向の観点からも、化学農薬の使用量の低減が求められている。これらのニーズに呼応して減農薬農業が実施されているが、通常使用量では顕在化しなかった病害虫の発生も問題となっている。 The occurrence of pests and diseases that reduce crop yields is a serious problem in the agricultural field. Pest control is important, as more than half of the damage caused by designated pests is caused by pests. There are various techniques for controlling pests that affect crops, but the main ones are chemical control methods that use chemical pesticides such as insecticides, cultivation control methods that use resistant varieties, and methods that use natural enemies. Biological control methods such as Among them, chemical pesticides are widely used because they are quick-acting and have high pesticidal effects. However, there are concerns about chemical pesticides, such as the impact on the ecosystem due to residue in the environment due to improper use, and the emergence of chemical-resistant pests due to continuous use of the same pesticide. Furthermore, from the viewpoint of environmental conservation and consumer safety, there is a need to reduce the amount of chemical pesticides used. In response to these needs, agriculture with reduced pesticide use has been implemented, but the occurrence of pests and diseases that would not have manifested under normal usage has also become a problem.
生物学的防除法の場合には、化学農薬によるこれらの問題は生じないが、別の問題がある。例えば、通常の品種にかえて抵抗性品種を栽培した場合には、抵抗性を打破する害虫の出現が問題となっている。このため、近年では抵抗性が崩壊することなく安定的に持続する特性を有する品種が求められている。また、天敵を利用する方法では、十分な効果を示す天敵の常時調達が困難である。 Biological control methods do not have these problems with chemical pesticides, but there are other problems. For example, when resistant varieties are grown instead of normal varieties, the emergence of pests that overcome the resistance becomes a problem. For this reason, in recent years there has been a demand for varieties that have the characteristic of stably maintaining resistance without collapse. Furthermore, with the method of using natural enemies, it is difficult to constantly procure natural enemies that are sufficiently effective.
近年、環境保全型病害防除手法として病害抵抗性誘導物質が注目されている。病害抵抗性誘導物質は、化学農薬と異なり、病原体を直接殺すものではなく、植物が本来有する病害抵抗性を誘導及び強化することで病害を防除するものである。広範囲な病原体に効果があり、その効果も長期間持続する特徴がある。植物の病害抵抗性の機構は種を超えて保存されていることから抵抗性誘導物質がその生理活性を示す植物種の範囲は広い。 In recent years, disease resistance inducers have attracted attention as an environmentally friendly disease control method. Unlike chemical pesticides, disease resistance inducers do not directly kill pathogens, but control diseases by inducing and strengthening the plant's inherent disease resistance. It is effective against a wide range of pathogens, and its effects last for a long time. Since the mechanism of plant disease resistance is conserved across species, there is a wide range of plant species in which resistance-inducing substances exhibit physiological activity.
ナス科植物であるトマトやアブラナ科植物であるシロイヌナズナ等を用いた研究により植物の病害抵抗性経路は、生きた植物細胞から栄養を得るウイルス等の活物寄生性病原体に対して有効なサリチル酸経路と、宿主細胞を殺して栄養にする灰色カビ病菌等の殺生性病原体に対して有効なジャスモン酸-エチレン系との2つに大別できることが示されている(例えば、非特許文献1参照)。しかしながら、ジャスモン酸やその類縁体は、広範な害虫に対して極めて強い抵抗性誘導能を示す一方、植物体に処理した場合に、顕著な成長抑制や老化促進を起こす(例えば、非特許文献2参照)。そのため、抵抗性誘導剤のリード化合物としての実用利用は難しい。
Research using tomato, a plant belonging to the Solanaceae family, and Arabidopsis thaliana, a plant belonging to the Brassicaceae family, has revealed that the disease resistance pathway in plants is the salicylic acid pathway, which is effective against living parasitic pathogens such as viruses that obtain nutrients from living plant cells. It has been shown that they can be roughly divided into two types: jasmonic acid-ethylene, which is effective against biocidal pathogens such as gray mold fungus, which kills host cells and provides nutrients (for example, see Non-Patent Document 1). . However, while jasmonic acid and its analogs exhibit extremely strong ability to induce resistance against a wide range of insect pests, when treated with plants, they cause significant growth inhibition and acceleration of aging (for example, Non-Patent
発明者らは、これまで、ロリオライドがミカンキイロアザミウマ等の害虫に対して植物にジャスモン酸非依存的なシグナル伝達経路を介して抵抗性を誘導することを明らかにしている(例えば、特許文献1参照)。
The inventors have previously revealed that loliolide induces resistance in plants to pests such as Occidental thrips through a jasmonic acid-independent signal transduction pathway (for example,
ロリオライドは、市販されておらず入手が困難であり、有機合成のコストも高いという問題がある。 Loliolide has problems in that it is not commercially available and is difficult to obtain, and the cost of organic synthesis is high.
本発明は、上記事情に鑑みてなされたものであって、害虫に対して有効であり、害虫防除剤として有用な新たな抵抗性誘導剤、及び当該抵抗性誘導剤を用いた植物の害虫防除方法を提供する。 The present invention has been made in view of the above circumstances, and provides a new resistance inducer that is effective against pests and useful as a pest control agent, and pest control of plants using the resistance inducer. provide a method.
発明者らは、害虫に対する抵抗性を誘導する物質を見出すべく、天然資源に焦点を絞り、病虫害抵抗性が誘発された植物を用いて探索を試みた結果、カロテノイドの最終代謝産物であるα-ヨノンが、直接的に殺虫活性を有さないにも関わらず、アザミウマ科昆虫やヤガ科昆虫等の害虫に対する優れた抵抗性能を植物に誘導することを見出し、本発明を完成するに至った。 In order to find a substance that induces resistance to pests, the inventors focused on natural resources and tried to search using plants in which pest resistance had been induced. The present inventors have discovered that ionon induces excellent resistance to pests such as Thripsidae and Noctuidae insects in plants, even though it does not have direct insecticidal activity, leading to the completion of the present invention.
すなわち、本発明は、以下の態様を含む。
〔1〕 α-ヨノンを有効成分として含有し、アザミウマ科昆虫に対する抵抗性を植物に誘導する、害虫抵抗性誘導剤。
〔2〕 α-ヨノンを有効成分として含有し、ヤガ科昆虫に対する抵抗性を植物に誘導する、害虫抵抗性誘導剤。
〔3〕 〔1〕又は〔2〕に記載の害虫抵抗性誘導剤を、植物体に接触又は吸収させる、植物の害虫防除方法。
〔4〕 前記植物体が、双子葉植物である、〔3〕に記載の植物の害虫防除方法。
〔5〕 前記植物体が、ナス科植物、アブラナ科植物又はウリ科植物である、〔3〕又は〔4〕に記載の植物の害虫防除方法。
That is, the present invention includes the following aspects.
[1] An insect resistance inducing agent containing α-ionone as an active ingredient and inducing resistance to thripsid insects in plants.
[2] A pest resistance inducer containing α-ionone as an active ingredient and inducing resistance to Noctuaceae insects in plants.
[3] A method for controlling pests in plants, which comprises contacting or absorbing the pest resistance inducer according to [1] or [2] into the plant body.
[4] The method for controlling plant pests according to [3], wherein the plant is a dicotyledonous plant.
[5] The method for controlling plant pests according to [3] or [4], wherein the plant is a Solanaceae plant, a Cruciferae plant, or a Cucurbitaceae plant.
本発明に係る害虫抵抗性誘導剤は、植物体の害虫に対する抵抗性を誘起させて害虫防除効果を示す。このため、当該害虫抵抗性誘導剤及びこれを用いた植物の害虫防除方法は、抵抗性系統の出現のおそれが非常に小さく、環境保護の点からも好ましい害虫防除方法である。 The pest resistance inducer according to the present invention exhibits a pest control effect by inducing resistance to pests in plants. Therefore, the pest resistance inducer and the plant pest control method using the same have very little risk of the appearance of resistant strains, and are preferable pest control methods from the viewpoint of environmental protection.
本発明に係る害虫抵抗性誘導剤は、α-ヨノン(「α-イオノン」、「(E)-4-(2,6,6-トリメチルシクロヘキサ-2-エニル)ブタ-3-エン-2-オン」ともいう)を有効成分として含有し、アザミウマ科昆虫又はヤガ科昆虫に対する抵抗性を植物に誘導する。α-ヨノンは、下記式(1-1)及び(1-2)で表される化合物(以下、「化合物(1-1)」及び「化合物(1-2)」とそれぞれ称する場合がある)である。化合物(1-1)は、α-ヨノンの(R)-(+)体であり、化合物(1-2)はα-ヨノンの(S)-(-)体である。本発明において用いられるα-ヨノンは、(R)-(+)体であってもよく、(S)-(-)体であってもよく、それらの混合物であってもよい。以降、本発明及び本願明細書において、「α-ヨノン」には、(R)-(+)体及び(S)-(-)体が包含される。α-ヨノンのCAS No.は、「127-41-3」である。α-ヨノンは、殺虫活性は示さないが、植物の害虫防除活性を高めることができる。 The pest resistance inducer according to the present invention is α-ionone (“α-ionone”, “(E)-4-(2,6,6-trimethylcyclohex-2-enyl)but-3-ene-2 -on) as an active ingredient, and induces resistance to Thripsidae or Noctuidae insects in plants. α-Yonone is a compound represented by the following formulas (1-1) and (1-2) (hereinafter sometimes referred to as "compound (1-1)" and "compound (1-2)") It is. Compound (1-1) is the (R)-(+) form of α-ionone, and compound (1-2) is the (S)-(-) form of α-ionone. The α-ionone used in the present invention may be in the (R)-(+) form, the (S)-(-) form, or a mixture thereof. Hereinafter, in the present invention and the present specification, "α-ionone" includes the (R)-(+) and (S)-(-) forms. α-Yonone CAS No. is "127-41-3". Although α-ionone does not exhibit insecticidal activity, it can enhance the pest control activity of plants.
本発明において用いられるα-ヨノンは、天然物からの抽出物であってもよい。α-ヨノンは、カロテノイドの代謝産物であり、カロテノイド代謝経路を有する植物や藻類に多く含まれる。 The α-ionone used in the present invention may be an extract from a natural product. α-Yonone is a carotenoid metabolite and is abundantly contained in plants and algae that have a carotenoid metabolic pathway.
α-ヨノンは、カロテノイド代謝経路を有する植物や藻類のうち、特に、ニンジン、カボチャ、タバコ、チャ、アマノリ、テングサ、スミレ等に多く含まれている。これらの植物や藻類からα-ヨノンを抽出することができる。 Among plants and algae that have a carotenoid metabolic pathway, α-ionone is particularly abundant in carrots, pumpkins, tobacco, tea, laver, amanita, violets, and the like. α-ionone can be extracted from these plants and algae.
植物等からのα-ヨノンの抽出は、有機溶剤を用いて常法により行うことができる。具体的には、例えば、α-ヨノンを含む植物の葉や茎等の生鮮物や乾燥物から、メタノール、エタノール、アセトン、ヘキサン等の有機溶媒を用いて抽出する。植物等の乾燥物は、天日乾燥や自然乾燥等の乾燥処理を行ったものであってもよく、液体窒素による急速凍結を行ったものであってもよい。また、有機溶媒を添加する前に、予め細断又は粉砕しておくことも好ましい。得られた抽出物は、減圧蒸留等により濃縮した後、分液ロートを用いて有機溶媒層を分取することにより、α-ヨノンを含む粗抽出物を得ることができる。この粗抽出物は、そのまま、又は必要に応じて濃縮した後、本発明に係る害虫抵抗性誘導剤の有効成分として使用できる。 α-ionone can be extracted from plants and the like by a conventional method using an organic solvent. Specifically, for example, it is extracted from fresh or dried products such as leaves and stems of plants containing α-ionone using an organic solvent such as methanol, ethanol, acetone, or hexane. The dried material such as a plant may be one that has been subjected to a drying process such as sun drying or natural drying, or may be one that has been rapidly frozen using liquid nitrogen. Furthermore, it is also preferable to shred or crush the material in advance before adding the organic solvent. The obtained extract is concentrated by vacuum distillation or the like, and then the organic solvent layer is separated using a separating funnel to obtain a crude extract containing α-ionone. This crude extract can be used as an active ingredient of the pest resistance inducer according to the present invention, either as it is or after being concentrated if necessary.
本発明に係る害虫抵抗性誘導剤の有効成分としては、前記粗抽出物を、クロマトグラフィー法により精製した精製抽出物を用いることも好ましい。例えば、前記粗抽出物を、順相のシリカゲルカラムクロマトグラフィーにかけてα-ヨノンを含む画分を分離してもよい。得られた画分を、そのまま、又は必要に応じて濃縮した後、本発明に係る害虫抵抗性誘導剤の有効成分として使用できる。また、シリカゲルカラムクロマトグラフィーにより得られたα-ヨノンを含む画分を、さらに逆相クロマトグラフィーにかけることにより、α-ヨノンをより高純度に精製することができる。 As the active ingredient of the pest resistance inducer according to the present invention, it is also preferable to use a purified extract obtained by refining the crude extract by chromatography. For example, the crude extract may be subjected to normal phase silica gel column chromatography to separate a fraction containing α-ionone. The obtained fraction can be used as an active ingredient of the pest resistance inducer according to the present invention, either as it is or after being concentrated if necessary. Further, α-ionone can be purified to a higher purity by further subjecting the fraction containing α-ionone obtained by silica gel column chromatography to reversed phase chromatography.
本発明において用いられるα-ヨノンは、合成品であってもよい。α-ヨノンは、公知の合成方法により比較的簡便に合成することができる。α-ヨノンは、例えば、プソイドイオノン(シュードイオノン)から合成することができる。具体的には、例えば、プソイドイオノンに希酸を加えて加熱することで環化し、α-ヨノン及びβ-ヨノンの混合物が得られる。このとき、使用する希酸の種類等の反応条件を適宜調整することで、生成するα体とβ体の比率を変更することができる。例えば、希酸としてリン酸を用いて反応を行なうことで、主としてα-ヨノンが得られる。なお、プソイドイオノンは、シトラールにアセトンを、塩基触媒を用いたアルドール反応によって縮合させることで、プソイドイオノンを生成することができる。その他、α-カロテン等のカロテンから酸化還元酵素や光酸化等により合成することができる。また、本発明において用いられるα-ヨノンは、市販品であってもよい。α-ヨノンの市販品としては、例えば、富士フィルム和光純薬株式会社製のα-ヨノン(製品コード:093-00692)等が挙げられる。 The α-ionone used in the present invention may be a synthetic product. α-ionone can be synthesized relatively easily using known synthesis methods. α-ionone can be synthesized from pseudoionone, for example. Specifically, for example, pseudoionone is cyclized by adding dilute acid and heating to obtain a mixture of α-ionone and β-ionone. At this time, by appropriately adjusting the reaction conditions such as the type of dilute acid used, the ratio of the α-form and the β-form to be produced can be changed. For example, α-ionone is mainly obtained by carrying out the reaction using phosphoric acid as a dilute acid. Note that pseudoionone can be produced by condensing citral with acetone through an aldol reaction using a base catalyst. In addition, it can be synthesized from carotene such as α-carotene by oxidoreductase or photooxidation. Further, α-ionone used in the present invention may be a commercially available product. Commercially available products of α-ionone include, for example, α-ionone (product code: 093-00692) manufactured by Fuji Film Wako Pure Chemical Industries, Ltd.
本発明に係る害虫抵抗性誘導剤の有効成分とするα-ヨノンは、溶媒和物であってもよく、塩として含有されていてもよい。溶媒和物としては、例えば、水、メタノール、エタノール、酢酸エチル等の溶媒和物が挙げられる。また、塩としては、例えば、ナトリウム塩、カリウム塩、リチウム塩等のアルカリ金属塩;カルシウム塩、マグネシウム塩等のアルカリ土類金属塩;アルミニウム塩、鉄塩、亜鉛塩、銅塩、ニッケル塩等の金属塩;酢酸塩、アンモニウム塩等の無機塩;ジベンジルアミン塩、グルコサミン塩、エチレンジアミン塩、ジエチルアミン塩、トリエチルアミン塩、ジシクロヘキシルアミン塩、ジエタノールアミン塩、テトラメチルアンモニア塩等の有機アミン塩;グリシン塩、リジン塩、アルギニン塩、オルニチン塩、アスパラギン塩等のアミノ酸塩等が挙げられる。 α-ionone, which is an active ingredient of the pest resistance inducer according to the present invention, may be a solvate or may be contained as a salt. Examples of the solvate include solvates of water, methanol, ethanol, ethyl acetate, and the like. Examples of salts include alkali metal salts such as sodium salts, potassium salts, and lithium salts; alkaline earth metal salts such as calcium salts and magnesium salts; aluminum salts, iron salts, zinc salts, copper salts, nickel salts, etc. metal salts; inorganic salts such as acetates and ammonium salts; organic amine salts such as dibenzylamine salts, glucosamine salts, ethylenediamine salts, diethylamine salts, triethylamine salts, dicyclohexylamine salts, diethanolamine salts, and tetramethylammonium salts; glycine salts , amino acid salts such as lysine salt, arginine salt, ornithine salt, and asparagine salt.
本発明に係る害虫抵抗性誘導剤におけるα-ヨノンの濃度は、植物体の害虫防除活性を誘起するために充分な濃度であればよく、防除対象である害虫の種類、害虫抵抗性誘導剤が使用される対象植物の種類、一度の散布時における散布量、散布頻度等を考慮して適宜決定される。例えば、害虫防除効果と一度の散布量との兼ね合いから、植物体への施用(散布)時における本発明に係る害虫抵抗性誘導剤中のα-ヨノンの濃度は、10μM(mol/L)以上が好ましく、100μM以上がより好ましく、300μM以上がさらに好ましい。α-ヨノンの濃度が、10μM以上であることで、害虫防除効果をより十分に発揮できる。一方で、α-ヨノンは光合成を行う生物種のほとんど全てにおいて生産される物質であり、多くの食品に含まれていることから、環境や人体に与える影響は小さいと推察される。このため、本発明に係る害虫抵抗性誘導剤中のα-ヨノンの濃度の上限値は、特に規定されないが、費用の点等から2000μMが好ましく、1000μMがより好ましく、700μMがさらに好ましく、500μMが特に好ましい。 The concentration of α-ionone in the pest resistance inducer according to the present invention may be sufficient as long as it induces pest control activity in plants, and the concentration of α-ionone in the pest resistance inducer according to the present invention may be sufficient to induce pest control activity in plants. It is appropriately determined in consideration of the type of target plant to be used, the amount of spraying at one time, the frequency of spraying, etc. For example, in consideration of the pest control effect and the amount of one-time application, the concentration of α-ionone in the pest resistance inducer of the present invention when applied (sprayed) to plants is 10 μM (mol/L) or more. is preferable, 100 μM or more is more preferable, and even more preferably 300 μM or more. When the concentration of α-ionone is 10 μM or more, the pest control effect can be more fully exhibited. On the other hand, α-ionone is a substance produced in almost all species of organisms that perform photosynthesis and is included in many foods, so it is presumed that it has little impact on the environment and the human body. Therefore, the upper limit of the concentration of α-ionone in the pest resistance inducer according to the present invention is not particularly defined, but from the viewpoint of cost etc., it is preferably 2000 μM, more preferably 1000 μM, even more preferably 700 μM, and 500 μM. Particularly preferred.
なお、本発明に係る害虫抵抗性誘導剤は、植物体への施用時に希釈して使用する濃縮物であってもよい。例えば、本発明に係る害虫抵抗性誘導剤のα-ヨノンの濃度を1mM以上1M以下とした場合には、使用時に水やエタノール等の適当な溶媒で100倍以上100000倍以下に希釈して有効成分濃度を所望の濃度(例えば、10μM程度)に調整して使用することができる。 The pest resistance inducer according to the present invention may be a concentrate that is diluted and used when applied to plants. For example, when the concentration of α-ionone in the pest resistance inducer according to the present invention is 1mM or more and 1M or less, it is effective to dilute it 100 times or more and 100,000 times or less with an appropriate solvent such as water or ethanol before use. The component concentration can be adjusted to a desired concentration (for example, about 10 μM) before use.
本発明に係る害虫抵抗性誘導剤の剤型としては、特に限定されるものではなく、例えば、粉剤、粒剤、錠剤、水和剤、乳剤、水溶剤、油剤、エアロゾル剤等が挙げられる。本発明に係る害虫抵抗性誘導剤を農業園芸用として用いる場合には、植物体への散布等が容易であることから、水和剤、乳剤、水溶剤、油剤、エアロゾル剤、粉剤等が好ましく、植物体への吸収性の点から、水和剤、乳剤、水溶剤、エアロゾル剤がより好ましい。これらの製剤は、常法に従って製造することができる。例えば、有効成分(α-ヨノン)を、不活性な液体(溶媒)又は固体の担体で希釈し、必要に応じてその他の添加剤をこれに加えて、各種製剤を製造する。当該添加剤としては、例えば、界面活性剤、賦形剤、結合剤、崩壊剤、滑沢剤、着色料、矯臭剤、pH調整剤等が挙げられる。 The dosage form of the pest resistance inducer according to the present invention is not particularly limited, and examples thereof include powders, granules, tablets, wettable powders, emulsions, aqueous solutions, oils, aerosols, and the like. When using the pest resistance inducer according to the present invention for agricultural and horticultural purposes, wettable powders, emulsions, water-solvents, oils, aerosols, powders, etc. are preferable because they can be easily sprayed onto plants. From the viewpoint of absorbability into plants, wettable powders, emulsions, aqueous solutions, and aerosols are more preferred. These formulations can be manufactured according to conventional methods. For example, various preparations are produced by diluting the active ingredient (α-ionone) with an inert liquid (solvent) or solid carrier, and adding other additives as necessary. Examples of such additives include surfactants, excipients, binders, disintegrants, lubricants, colorants, flavoring agents, pH adjusters, and the like.
本発明に係る害虫抵抗性誘導剤が水溶剤や水和剤等の液状の剤型である場合、α-ヨノンを溶解又は分散させる溶媒としては、α-ヨノンに対して不活性であり、かつ対象となる植物体に悪影響を及ぼしにくい溶媒が好ましい。当該溶媒としては、メタノール、エタノール、及び水等が適宜使用される。水溶剤の場合、例えば、α-ヨノンを、メタノールやエタノールに溶解させ、得られた溶液を水で希釈することにより調製できる。 When the pest resistance inducer according to the present invention is in a liquid form such as an aqueous solution or a wettable powder, the solvent for dissolving or dispersing α-ionone must be inert to α-ionone, and It is preferable to use a solvent that is unlikely to have an adverse effect on the target plant. As the solvent, methanol, ethanol, water, etc. are used as appropriate. In the case of an aqueous solvent, it can be prepared, for example, by dissolving α-ionone in methanol or ethanol and diluting the resulting solution with water.
本発明に係る害虫抵抗性誘導剤は、α-ヨノンによる害虫防除活性誘起効果を阻害しない範囲内で、その他の殺虫剤や殺菌剤、除草剤、植物生長調節剤、肥料等を含有していてもよい。 The pest resistance inducer according to the present invention may contain other insecticides, fungicides, herbicides, plant growth regulators, fertilizers, etc. within the range that does not inhibit the pest control activity-inducing effect of α-ionone. Good too.
α-ヨノンは、植物体の害虫防除活性、特に、アザミウマ科昆虫又はヤガ科昆虫に対する防除活性を誘起し、これらの害虫に対する抵抗性を増強させることができる。このため、本発明に係る害虫抵抗性誘導剤により処理された植物体では、アザミウマ科昆虫による吸汁害又はヤガ科昆虫の幼虫による食害が低減する。また、この害虫抵抗性誘導効果から、α-ヨノンは、アザミウマ科昆虫又はヤガ科昆虫に対する抵抗性誘導剤のリード化合物としての利用も期待できる。 α-ionone can induce pest control activity in plants, particularly against Thripsidae insects or Noctuidae insects, and can enhance resistance to these pests. Therefore, in plants treated with the pest resistance inducer according to the present invention, damage caused by suction by Thripsidae insects or feeding damage by larvae of Noctuidae insects is reduced. Furthermore, due to this insect resistance-inducing effect, α-ionone can be expected to be used as a lead compound for resistance-inducing agents against Thripsidae insects or Noctuidae insects.
本発明に係る害虫抵抗性誘導剤により防除対象となる害虫としては、アザミウマ科(Thripidae)昆虫又はヤガ科(Noctuidae)昆虫に属するものであればよい。
アザミウマ科(Thripidae)昆虫としては、例えば、ミカンキイロアザミウマ(Frankliniella occidentalis)、ミナミキイロアザミウマ(Thrips palmi)、チャノキイロアザミウマ(Scirtothrips dorsalis)、ネギアザミウマ(Thrips tabaci)、ヒラズハナアザミウマ(Frankliniella intonsa)等が挙げられる。中でも、ミカンキイロアザミウマ(Frankliniella occidentalis)又はミナミキイロアザミウマ(Thrips palmi)が好ましい。
ヤガ科(Noctuidae)昆虫としては、多くの属が存在し、例えば、Spodoptera属、Athetis属、Polyphaenis属、Trachea属、Orthogonia属、Actinotia属、Phlogophora属、Apamea属、Sapporia属、Bambusiphila属、Atrachea属、Capsula属、Sesamia属、Dryobotodes属、Xylena属、Lithophane属、Eupsilia属、Rhynchaglaea属、Mesorhynchaglaea属、Sugitania属、Incertobole属、Telorta属、Antivaleria属、Nyctycia属、Cosmia属等が挙げられるが、中でも、Spodoptera属に属するものがより好ましい。Spodoptera属に属するものとしては、例えば、ハスモンヨトウ(Spodoptera litura)、シロイチモジヨトウ(Spodoptera exigua)、シロナヨトウ(Spodoptera mauritia acronyctoides)、ツマジロクサヨトウ(Spodoptera frugiperda)等が挙げられるが、中でも、ハスモンヨトウ(Spodoptera litura)が特に好ましい。
The pests to be controlled by the insect resistance inducer of the present invention may be those belonging to the Thripidae family or the Noctuidae family.
Examples of Thripidae insects include Frankliniella occidentalis, Thrips palmi, Scirtothrips dorsalis, Thrips tabaci, Frankliniella intonsa, etc. It will be done. Among these, preferred is Frankliniella occidentalis or Thrips palmi.
There are many genera of Noctuidae insects, such as Spodoptera, Athetis, Polyphaenis, Trachea, Orthogonia, Actinotia, Phlogophora, Apamea, Sapporia, Bambusiphila, and Atrachea. , Capsula, Sesamia, Dryobotodes, Xylena, Lithophane, Eupsilia, Rhynchaglaea, Mesorhynchaglaea, Sugitania, Incertobole, Telorta, Antivaleria, Nyctycia, Cosmia, etc. Those belonging to the genus Spodoptera are more preferred. Examples of those belonging to the genus Spodoptera include Spodoptera litura, Spodoptera exigua, Spodoptera mauritia acronyctoides, and Spodoptera frugiperda. is particularly preferred.
本発明に係る植物の害虫防除方法は、本発明に係る害虫抵抗性誘導剤を、植物体に接触又は吸収させることを含む。本発明に係る害虫抵抗性誘導剤を植物体に接触又は吸収させることにより、有効成分たるα-ヨノンが植物体に作用し、当該植物体の病害抵抗性が増強され、アザミウマ科昆虫による吸汁害又はヤガ科昆虫の幼虫による食害を低減させることができる。 The plant pest control method according to the present invention includes contacting or absorbing the pest resistance inducer according to the present invention into a plant body. By contacting or absorbing the pest resistance inducer according to the present invention into a plant body, α-ionone as an active ingredient acts on the plant body, and the disease resistance of the plant body is enhanced, resulting in sap-sucking damage caused by Thripsidae insects. Alternatively, feeding damage caused by larvae of insects of the family Noctuidae can be reduced.
本発明に係る植物の害虫防除方法において、本発明に係る害虫抵抗性誘導剤を、植物体に接触又は吸収させる方法は、特に限定されるものではなく、その他の農薬等を散布する方法と同様にして行うことができる。本発明に係る害虫抵抗性誘導剤が液状の剤型である場合には、そのまま、又は適宜水やエタノール等の適当な溶媒で希釈した後に、植物体の葉や茎等の表面に塗布する方法や、噴霧する方法が好ましい。塗布処理や噴霧処理は、通常の葉面散布と同様の方法で行うことができる。その他、液状の害虫抵抗性誘導剤又はその希釈液を、植物体が植えられている土壌にまいてもよい。害虫抵抗性誘導剤を土壌にまいた場合には、有効成分であるα-ヨノンが根や地際部の茎から植物体へ吸収される。その他、液状の害虫抵抗性誘導剤又はその希釈液を、徐放等により蒸気として植物に接触させることができる。その他、定植前等の苗等を、液状の害虫抵抗性誘導剤又はその希釈液に直接浸漬することにより植物に吸収させることもできる。その他、液状の害虫抵抗性誘導剤又はその希釈液を、養液土耕や水耕栽培の養液に混入することにより根や地際部の茎等から吸収させることもできる。本発明に係る害虫抵抗性誘導剤が粉剤の場合には、そのまま、又は適宜水やエタノール等の適当な溶媒に分散させた分散液を、塗布処理や噴霧処理、蒸気処理、浸漬処理、養液混入処理することができる。本発明に係る害虫抵抗性誘導剤が粒剤等の比較的大きな固形の場合には、植物体が植えられている土壌にまくことができる。 In the plant pest control method according to the present invention, the method of bringing the pest resistance inducer according to the present invention into contact with or absorbed into the plant body is not particularly limited, and is the same as the method of spraying other agricultural chemicals. It can be done by When the pest resistance inducer according to the present invention is in liquid form, it is applied to the surfaces of leaves, stems, etc. of plants, either as is or after diluting with an appropriate solvent such as water or ethanol. or spraying is preferred. Application and spraying can be carried out in the same manner as normal foliar spraying. Alternatively, a liquid pest resistance inducer or a diluted solution thereof may be sprinkled on the soil where the plants are planted. When the pest resistance inducer is sprinkled on soil, the active ingredient, α-ionone, is absorbed into the plant body through the roots and stems at the ground level. Alternatively, a liquid pest resistance inducer or a diluted solution thereof can be brought into contact with plants in the form of vapor by slow release or the like. Alternatively, seedlings or the like before planting may be directly immersed in a liquid pest resistance inducer or a diluted solution thereof, so that the plant can absorb the insect resistance inducer. In addition, a liquid pest resistance inducer or its diluted solution can be mixed into a nutrient solution for hydroponic cultivation or hydroponic cultivation, and thereby absorbed through roots, stems, etc. at the ground level. When the pest resistance inducer according to the present invention is a powder, it can be used as it is or as a dispersion in an appropriate solvent such as water or ethanol, by coating, spraying, steaming, dipping, or nutrient solution. Contamination can be treated. When the pest resistance inducing agent according to the present invention is in a relatively large solid form such as granules, it can be sprinkled on the soil where plants are planted.
本発明に係る植物の害虫防除方法において、対象植物体に対する本発明に係る害虫抵抗性誘導剤の施用量は、対象植物体の種類、防除対象である害虫の種類、被害の程度、環境条件、害虫抵抗性誘導剤の有効成分含有量や剤型等を考慮して適宜決定される。本発明においては、充分な害虫防除効果が得られやすいため、植物体への施用時におけるα-ヨノンの濃度が、10μM以上、好ましくは100μM以上、より好ましくは300μM以上となるように本発明に係る害虫抵抗性誘導剤を施用することが好ましい。 In the plant pest control method according to the present invention, the application amount of the pest resistance inducer according to the present invention to the target plant is determined based on the type of target plant, the type of pest to be controlled, the degree of damage, the environmental conditions, It is appropriately determined in consideration of the active ingredient content, dosage form, etc. of the pest resistance inducer. In the present invention, in order to easily obtain a sufficient pest control effect, the concentration of α-ionone at the time of application to plants is 10 μM or more, preferably 100 μM or more, more preferably 300 μM or more. It is preferable to apply such pest resistance inducers.
本発明に係る植物の害虫防除方法において、対象となる植物体としては、アザミウマ科昆虫又はヤガ科昆虫による害が生じ得る植物であれば特に限定されるものではなく、双子葉植物が好ましいが、単子葉植物でもよい。本発明に係る植物の害虫防除方法において害虫防除の対象となる植物体としては、市場で取引される植物であることが好ましく、野菜類、花き類、果樹、チャ等の農作物や観葉植物がより好ましい。中でも、野菜類が好ましく、トマト、ナス、ピーマン、パプリカ、ジャガイモ、トウガラシ等のナス科の植物、キャベツ、ブロッコリー、メキャベツ、カイワレダイコン、カブ、ダイコン、カリフラワー、コマツナ、タアサイ、チンゲンサイ、ナバナ、ハクサイ、ミズナ、ラディッシュ等のアブラナ科の植物、及び、キュウリ、カボチャ、ズッキーニ、ヒョウタン、ヘチマ、トウガン、ツルレイシ、スイカ、メロン等のウリ科の植物がさらに好ましく、トマトが特に好ましい。 In the plant pest control method according to the present invention, the target plant is not particularly limited as long as it is a plant that can be harmed by Thripsidae insects or Noctuidae insects, and dicotyledonous plants are preferable; It can also be a monocotyledonous plant. In the method for controlling pests of plants according to the present invention, the plants to be controlled are preferably plants traded in the market, and more preferably agricultural products such as vegetables, flowers, fruit trees, tea, and ornamental plants. preferable. Among these, vegetables are preferred, such as tomatoes, eggplants, green peppers, paprika, potatoes, plants of the nightshade family such as chili peppers, cabbage, broccoli, Brussels sprouts, Japanese radish, turnips, radish, cauliflower, Komatsuna, Japanese cabbage, Bok choy, Nabana, Chinese cabbage, and Mizuna. More preferred are plants of the Cruciferae family such as , radish, and plants of the Cucurbitaceae family such as cucumbers, pumpkins, zucchini, gourds, loofahs, chives, vines, watermelons, and melons, and tomatoes are particularly preferred.
以下、実施例等により本発明を更に詳細に説明するが、本発明は、その要旨を変更しない限り以下の実施例等に限定されるものではない。 Hereinafter, the present invention will be explained in more detail with reference to examples, but the present invention is not limited to the following examples unless the gist thereof is changed.
[実施例1]
<24時間α-ヨノン処理のトマトにおけるミカンキイロアザミウマ生存数に対する防除効果の検定>
α-ヨノンは市販品(富士フィルム和光純薬株式会社製)を用いた。α-ヨノンはエタノールで所定濃度(1μM、10μM、100μM及び300μM(μmol/L))に希釈した。なお、対照区として、エタノールのみからなる溶液(0μM)も調製した。
[Example 1]
<Examination of the control effect on the survival number of occidental thrips in tomatoes treated with α-ionone for 24 hours>
As α-ionone, a commercially available product (manufactured by Fuji Film Wako Pure Chemical Industries, Ltd.) was used. α-ionone was diluted with ethanol to predetermined concentrations (1 μM, 10 μM, 100 μM, and 300 μM (μmol/L)). As a control, a solution (0 μM) consisting only of ethanol was also prepared.
次いで、ポットで栽培した3週齢以上4週齢以下のトマトを透明樹脂製円柱容器(約1000mL体積;14cm高×10cm直径)に置いた。次いで、100μLの所定濃度のα-ヨノン液及びエタノール(0μM)を予め入れた1.5mLマイクロチューブを当該容器の内壁にテープで固定し、7cm×5cmに切り抜いた箇所にナイロンメッシュを貼付した蓋を載せ、さらに、α-ヨノン蒸気が当該容器から漏れるのを防ぐために食品用ラップフィルムで蓋全体を覆い、24℃±1℃、16時間明期/8時間暗期条件下で制御した恒温室内に置いた。24時間後、α-ヨノン液を入れた1.5mLマイクロチューブを取り出し、ミカンキイロアザミウマの雌成虫をトマト1株あたり20頭放飼し、直ちに蓋をし、上記と同じ条件下で栽培した。14日後に、生存数を計測した。 Next, tomatoes grown in pots that were 3 weeks old or more and 4 weeks old or less were placed in a transparent resin cylindrical container (volume of about 1000 mL; 14 cm height x 10 cm diameter). Next, a 1.5 mL microtube containing 100 μL of α-ionone solution at a predetermined concentration and ethanol (0 μM) was fixed to the inner wall of the container with tape, and a 7 cm x 5 cm cutout was placed in a lid with a nylon mesh pasted on it. To prevent α-ionone vapor from leaking from the container, the entire lid was covered with food-grade cling film, and the container was placed in a thermostatic chamber controlled at 24°C ± 1°C under 16-hour light/8-hour dark conditions. I placed it on. After 24 hours, the 1.5 mL microtube containing the α-ionone solution was taken out, and 20 adult female occidental thrips per tomato plant were released.The tube was immediately covered and cultivated under the same conditions as above. After 14 days, the number of survivors was counted.
図1に、トマトにおけるミカンキイロアザミウマ生存数に対するα-ヨノンの24時間処理の効果を示す。なお、図1において、異なるアルファベットは有意差があることを示す(Tukey Kramer HSD test p<0.05;n=11~15)。α-ヨノン処理区では、対照区と比較して、10μM以上の濃度でミカンキイロアザミウマの生存数の低下が見られた。 Figure 1 shows the effect of 24-hour treatment with α-ionone on the number of Occidentalis thrips survivors in tomatoes. In FIG. 1, different alphabets indicate significant differences (Tukey Kramer HSD test p<0.05; n=11-15). In the α-ionone treated area, a decrease in the number of surviving O. citrus thrips was observed at concentrations of 10 μM or higher compared to the control area.
[比較例1]
<24時間β-ヨノン処理のトマトにおけるミカンキイロアザミウマ生存数に対する防除効果の検定>
α-ヨノンの異性体であるβ-ヨノン処理による防除効果を、実施例1に記載の方法に準じて行った。β-ヨノン(「β-イオノン」、「(E)-4-(2,6,6-トリメチルシクロヘキサ-1-エニル)ブタ-3-エン-2-オン」)は、下記式(2)で表される化合物である。検定においては、最終濃度1μM、10μM、100μM及び300μMのβ-ヨノン(富士フィルム和光純薬株式会社製)を含むエタノール溶液及びエタノールのみからなる溶液(0μM)について、実施例1に記載の方法を用いてトマトを処理した後、当該トマトにミカンキイロアザミウマの雌成虫をトマト1株あたり20頭放飼し、生存数を計測した。なお、エタノールのみからなる溶液(0μM)で処理したトマトを対照とした。
[Comparative example 1]
<Examination of the control effect on the survival number of occidental thrips in tomatoes treated with β-ionone for 24 hours>
The control effect of treatment with β-ionone, which is an isomer of α-ionone, was tested according to the method described in Example 1. β-ionone (“β-ionone”, “(E)-4-(2,6,6-trimethylcyclohex-1-enyl)but-3-en-2-one”) is represented by the following formula (2) This is a compound represented by In the assay, the method described in Example 1 was used for ethanol solutions containing β-ionone (manufactured by Fuji Film Wako Pure Chemical Industries, Ltd.) at final concentrations of 1 μM, 10 μM, 100 μM, and 300 μM, and a solution consisting only of ethanol (0 μM). After treating tomatoes with the tomato, 20 adult female occidental thrips were released per tomato plant, and the number of survivors was counted. Note that tomatoes treated with a solution consisting only of ethanol (0 μM) were used as a control.
図2に、トマトにおけるミカンキイロアザミウマ生存数に対するβ-ヨノンの24時間処理の効果を示す。なお、図2において、「n.s.」はNot Significantの略であり、統計学的有意差がないことを示す。以降、同様である。 Figure 2 shows the effect of 24-hour treatment with β-ionone on the number of Occidentalis thrips survivors in tomatoes. In FIG. 2, "n.s." is an abbreviation for "Not Significant" and indicates that there is no statistically significant difference. The same applies thereafter.
図2に示すとおり、β-ヨノン処理区ではミカンキイロアザミウマの生存数と対照における生存数との間に有意差がなく、生存数の低下は見られなかった。この結果は、α-ヨノンが有する防除効果にとってα-ヨノンの環構造の二重結合の位置が2位と3位の炭素間に存在することが重要であることを示している。 As shown in FIG. 2, there was no significant difference between the number of surviving O. citrus thrips in the β-ionone treatment area and that in the control, and no decrease in the number of survivors was observed. This result shows that it is important for the pesticidal effect of α-ionone that the double bond in the ring structure of α-ionone be located between the 2nd and 3rd carbon positions.
[実施例2]
<ミカンキイロアザミウマに対するα-ヨノンの毒性の検定>
トマト葉を、ガラス板(7.5cm×2.5cm)上に置いた予め水を含ませた脱脂綿の上に載せ、さらにその上にアクリル製マンジャーセル(7.5cm×2.5cm、セル内径:1.5cm)を載せた。ペトリ皿に0.5μLのα-ヨノン(原液、300μM)を滴下し、その上に1頭のミカンキイロアザミウマ2齢幼虫を置き、面相筆で当該幼虫を液上で5秒間転がし、濾紙で余分な液を吸い取った。その後、マンジャーセル内のトマト葉に載せ、24℃±1℃、16時間明期/8時間暗期条件下で制御した恒温室内に置いた。48時間後、生存率(検定開始時の幼虫数に対する検定後の幼虫の生存数の百分率)を調べた。α-ヨノン液に代えて、0.5μLのエタノールで同様に処理したものを、対照区とした。
[Example 2]
<Toxicity assay of α-ionone against occidental thrips>
Place tomato leaves on absorbent cotton pre-soaked with water on a glass plate (7.5 cm x 2.5 cm), and then place an acrylic manger cell (7.5 cm x 2.5 cm, cell (inner diameter: 1.5 cm) was placed. Drop 0.5 μL of α-ionone (stock solution, 300 μM) into a Petri dish, place one 2nd instar larva of Occidental Thrips on top of it, roll the larva on the liquid for 5 seconds with a face brush, and remove excess with filter paper. I sucked up the liquid. Thereafter, it was placed on a tomato leaf in a manger cell and placed in a thermostatic chamber controlled at 24°C ± 1°C under 16-hour light/8-hour dark conditions. After 48 hours, the survival rate (percentage of the number of surviving larvae after the assay to the number of larvae at the start of the assay) was examined. A control group was prepared using 0.5 μL of ethanol instead of the α-ionone solution.
図3に、ミカンキイロアザミウマ生存率に対するα-ヨノン滴下処理の効果を示す。α-ヨノン処理区と対照区でミカンキイロアザミウマの生存率に差は認められなかったことから、α-ヨノンは直接のミカンキイロアザミウマに対する毒性(殺ミカンキイロアザミウマ活性)は有さないことが示された。 FIG. 3 shows the effect of the α-ionone drop treatment on the survival rate of Occidental thrips. There was no difference in the survival rate of O. citrus thrips between the α-ionone treatment area and the control area, indicating that α-ionone does not have direct toxicity (occidental thripsicidal activity) against O. citrus thrips. It was done.
[実施例3]
<トマト遺伝子の発現誘導に対するα-ヨノン処理の効果の検定>
ポットで栽培した3週齢以上4週齢以下のトマトを透明樹脂製円柱容器(約1000mL体積;14cm高×10cm直径)に置いた。次いで、100μLの所定濃度(1μM、10μM、100μM及び300μM)のα-ヨノン液及びエタノール(0μM)を入れた1.5mLマイクロチューブを当該容器の内壁にテープで固定し、7cm×5cmに切り抜いた箇所にナイロンメッシュを貼付した蓋を載せ、さらに、α-ヨノン蒸気が当該容器から漏れるのを防ぐために食品用ラップフィルムで蓋全体を覆い、24℃±1℃、16時間明期/8時間暗期条件下で制御した恒温室内に置いた。24時間後、トマト葉を採取し、直ちに液体窒素で凍結し、全RNA抽出に用いた。トマト葉からの全RNA抽出、RNAからのcDNA合成、cDNAを用いたSYBR法によるリアルタイムPCRは既報(非特許文献3及び4)に従った。サリチル酸応答性遺伝子であるSlGLuB(トマトのβ-glucanase遺伝子)及びSlPR3a(トマトの酸性キチナーゼIII)、ロリオライド応答性遺伝子であるSlLin5(トマトのinvertase)、並びに、ジャスモン酸応答性遺伝子であるSlPR3b(トマトの塩基性キチナーゼIII)、SlLapA1(トマトのleucine aminopeptidase)及びSlPin2(トマトのproteinase inhibitor II)の発現誘導に対するα-ヨノンの効果を調べた。なお、各遺伝子の発現量は、トマトのアクチン遺伝子(Accession number:BT012695)の発現量で補正した。
[Example 3]
<Examination of the effect of α-ionone treatment on tomato gene expression induction>
Tomatoes grown in pots that were 3 weeks old or more and 4 weeks old or less were placed in a transparent resin cylindrical container (volume of about 1000 mL; 14 cm height x 10 cm diameter). Next, a 1.5 mL microtube containing 100 μL of α-ionone solution at a predetermined concentration (1 μM, 10 μM, 100 μM, and 300 μM) and ethanol (0 μM) was fixed to the inner wall of the container with tape and cut into a size of 7 cm x 5 cm. Place a lid with a nylon mesh pasted on the container, cover the entire lid with food-grade cling film to prevent α-ionone vapor from leaking from the container, and store at 24°C ± 1°C, 16 hours light/8 hours dark. The cells were placed in a thermostatic chamber controlled under period conditions. After 24 hours, tomato leaves were collected, immediately frozen in liquid nitrogen, and used for total RNA extraction. Extraction of total RNA from tomato leaves, synthesis of cDNA from RNA, and real-time PCR using the SYBR method using cDNA were conducted in accordance with previous reports (Non-patent Documents 3 and 4). Salicylic acid-responsive genes SlGLuB (tomato β-glucanase gene) and SlPR3a (tomato acid chitinase III), loliolide-responsive gene SlLin5 (tomato invertase), and jasmonic acid-responsive gene SlPR3b (tomato The effect of α-ionone on the induction of expression of basic chitinase III), SlLapA1 (tomato leucine aminopeptidase), and SlPin2 (tomato proteinase inhibitor II) was investigated. Note that the expression level of each gene was corrected by the expression level of the tomato actin gene (Accession number: BT012695).
図4に、トマトの各遺伝子の発現誘導に対するα-ヨノン処理の24時間処理の効果を示す。α-ヨノンは、100μM以上の濃度でSlGLuB及びSlPR3bの発現を誘導した。一方、SlPR3a、SlLapA1、SlPin2及びSlLin5に対するα-ヨノンの発現誘導効果は見られなかった。 FIG. 4 shows the effect of α-ionone treatment for 24 hours on the induction of expression of each gene in tomato. α-ionone induced the expression of SlGLuB and SlPR3b at concentrations of 100 μM or higher. On the other hand, no expression-inducing effect of α-ionone on SlPR3a, SlLapA1, SlPin2, and SlLin5 was observed.
[比較例2]
<トマト遺伝子の発現誘導に対するβ-ヨノン処理の効果の検定>
α-ヨノンの光学異性体であるβ-ヨノン処理によるトマト遺伝子の発現誘導に対する効果を、実施例3に記載の方法に準じて行った。検定においては、最終濃度300μMのβ-ヨノン(富士フィルム和光純薬株式会社製)を含むエタノール溶液及びエタノールのみからなる溶液(0μM)について、実施例3に記載の方法を用いてトマトを24時間処理した後、トマト葉を採取し、トマト葉におけるSlGLuB及びSlPR3bの発現量を調べた。
[Comparative example 2]
<Examination of the effect of β-ionone treatment on tomato gene expression induction>
The effect of treatment with β-ionone, which is an optical isomer of α-ionone, on induction of tomato gene expression was determined according to the method described in Example 3. In the assay, tomatoes were incubated for 24 hours using the method described in Example 3 for an ethanol solution containing β-ionone (manufactured by Fuji Film Wako Pure Chemical Industries, Ltd.) at a final concentration of 300 μM and a solution consisting only of ethanol (0 μM). After the treatment, tomato leaves were collected and the expression levels of SlGLuB and SlPR3b in the tomato leaves were examined.
図5に、トマトの各遺伝子の発現誘導に対するβ-ヨノン処理の24時間処理の効果を示す。300μMのβ-ヨノンは、SlGLuB及びSlPR3bの発現を誘導しなかった。
β-ヨノンは、アザミウマに対する殺虫効果や忌避効果があるといわれている。しかしながら、比較例1及び2の結果から明らかであるように、β-ヨノンはトマトに対してアザミウマ科昆虫に対する抵抗性やSlGLuB及びSlPR3b等のマーカー遺伝子の発現を誘導する効果がないことが示された。
FIG. 5 shows the effect of β-ionone treatment for 24 hours on the induction of expression of each gene in tomato. 300 μM β-ionone did not induce the expression of SlGLuB and SlPR3b.
β-ionone is said to have insecticidal and repellent effects against thrips. However, as is clear from the results of Comparative Examples 1 and 2, β-ionone is not effective in inducing resistance to thripsid insects or the expression of marker genes such as SlGLuB and SlPR3b in tomatoes. Ta.
[実施例4]
<植物ホルモンの蓄積に対するα-ヨノン処理の効果の検定>
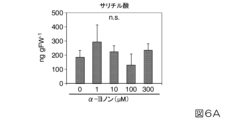
ポットで栽培した3週齢以上4週齢以下のトマトを透明樹脂製円柱容器(約1000mL体積;14cm高×10cm直径)に置いた。次いで、100μLの所定濃度(1μM、10μM、100μM及び300μM)のα-ヨノン液及びエタノール(0μM)を入れた1.5mLマイクロチューブを当該容器の内壁にテープで固定し、7cm×5cmに切り抜いた箇所にナイロンメッシュを貼付した蓋を載せ、さらに、α-ヨノン蒸気が当該容器から漏れるのを防ぐために食品用ラップフィルムで蓋全体を覆い、24℃±1℃、16時間明期/8時間暗期条件下で制御した恒温室内に置いた。24時間後、トマト葉を採取し、直ちに液体窒素で凍結し、植物ホルモン抽出に用いた。植物ホルモンであるサリチル酸及びジャスモン酸の定量は既報(非特許文献3及び4)に従った。なお、エタノールのみからなる溶液(0μM)で処理したトマトを対照とした。
[Example 4]
<Examination of the effect of α-ionone treatment on the accumulation of plant hormones>
Tomatoes grown in pots that were 3 weeks old or more and 4 weeks old or less were placed in a transparent resin cylindrical container (volume of about 1000 mL; 14 cm height x 10 cm diameter). Next, a 1.5 mL microtube containing 100 μL of α-ionone solution at a predetermined concentration (1 μM, 10 μM, 100 μM, and 300 μM) and ethanol (0 μM) was fixed to the inner wall of the container with tape and cut into a size of 7 cm x 5 cm. Place a lid with a nylon mesh pasted on the container, cover the entire lid with food-grade cling film to prevent α-ionone vapor from leaking from the container, and store at 24°C ± 1°C, 16 hours light/8 hours dark. The cells were placed in a thermostatic chamber controlled under period conditions. After 24 hours, tomato leaves were collected, immediately frozen in liquid nitrogen, and used for plant hormone extraction. Quantification of salicylic acid and jasmonic acid, which are plant hormones, was carried out in accordance with previous reports (Non-patent Documents 3 and 4). Note that tomatoes treated with a solution consisting only of ethanol (0 μM) were used as a control.
図6Aにサリチル酸の蓄積に対するα-ヨノン処理の効果を、図6Bにジャスモン酸の蓄積に対するα-ヨノン処理の効果を示す。α-ヨノン処理区と対照区でサリチル酸及びジャスモン酸の内生量に差は認められなかったことから、α-ヨノンはサリチル酸及びジャスモン酸の内生量の増加を起こさないことが示された。 FIG. 6A shows the effect of α-ionone treatment on the accumulation of salicylic acid, and FIG. 6B shows the effect of α-ionone treatment on the accumulation of jasmonic acid. No difference was observed in the endogenous amounts of salicylic acid and jasmonic acid between the α-ionone treated plot and the control plot, indicating that α-ionone did not cause an increase in the endogenous amount of salicylic acid and jasmonic acid.
[実施例5]
<48時間α-ヨノン処理のシロイヌナズナにおけるミカンキイロアザミウマ生存数に対する防除効果の検定>
2ヶ月齢のシロイヌナズナの葉から打ち抜いた葉片(1cm直径)を、予め各ウェルあたり0.8mLの300μMのα-ヨノン液を添加した48穴マイクロタイタープレートに置き、24℃±1℃、16時間明期/8時間暗期条件下で制御した恒温室内に置いた。48時間後、ミカンキイロアザミウマの雌成虫を葉片1株あたり1頭放飼し、直ちに蓋をし、上記と同じ条件下で栽培した。3日後に、産卵数を計測した。なお、エタノールのみからなる溶液(0μM)で処理したトマトを対照(コントロール)とした。
[Example 5]
<Examination of the control effect on the survival number of O. citrus thrips in Arabidopsis thaliana treated with α-ionone for 48 hours>
Leaf pieces (1 cm diameter) punched from 2-month-old Arabidopsis leaves were placed in a 48-well microtiter plate to which 0.8 mL of 300 μM α-ionone solution had been added per well in advance, and incubated at 24°C ± 1°C for 16 hours. It was placed in a thermostatic chamber controlled under light/8 hour dark conditions. After 48 hours, one female adult of Occidental thrips was released per leaf, immediately covered with a lid, and cultivated under the same conditions as above. Three days later, the number of eggs laid was measured. Note that tomatoes treated with a solution consisting only of ethanol (0 μM) were used as a control.
図7に、シロイヌナズナにおけるミカンキイロアザミウマ産卵数に対するα-ヨノンの48時間処理の効果を示す。なお、図7において、異なるアルファベットは有意差があることを示す(p<0.01;n=16~18)。α-ヨノン処理区では、対照区と比較して、ミカンキイロアザミウマの産卵数の低下が見られた。 FIG. 7 shows the effect of 48-hour treatment with α-ionone on O. citrus thrips egg production in Arabidopsis. Note that in FIG. 7, different alphabets indicate significant differences (p<0.01; n=16 to 18). In the α-ionone treated area, a decrease in the number of eggs laid by Occidental thrips was observed compared to the control area.
ミカンキイロアザミウマに対してα-ヨノンは直接的な殺虫効果は示さなかった。このことから、α-ヨノン処理を行ったトマトやシロイヌナズナで見られた生存数の低下は、植物の害虫抵抗性が高まったことによるものであると示唆される。α-ヨノンはSlGLuB及びSlPR3bの発現を高める効果を示したが、他のサリチル酸応答性遺伝子やジャスモン酸応答性遺伝子、及びロリオライド応答性遺伝子に対して、発現誘導効果は示さなかった。また、α-ヨノンはサリチル酸及びジャスモン酸の内生量に対しても増量効果は示さなかった。これらのことから、α-ヨノンによる害虫抵抗性の誘導はサリチル酸やジャスモン酸を介さないものであると推測される。 α-ionone did not show any direct insecticidal effect against occidental thrips. This suggests that the decrease in the number of survivors observed in tomatoes and Arabidopsis thaliana treated with α-ionone is due to increased pest resistance of the plants. Although α-ionone showed an effect of increasing the expression of SlGLuB and SlPR3b, it did not show an expression-inducing effect on other salicylic acid-responsive genes, jasmonic acid-responsive genes, and loliolide-responsive genes. Furthermore, α-ionone did not show any effect on increasing the endogenous amounts of salicylic acid and jasmonic acid. From these facts, it is inferred that the induction of pest resistance by α-ionone is not mediated by salicylic acid or jasmonic acid.
[実施例6]
<48時間α-ヨノン処理のトマトにおけるハスモンヨトウ生存数及び体重に対する防除効果の検定>
3週齢以上4週齢以下のトマトの葉を、予め各濃度のα-ヨノン液を添加したガラス製ペトリ皿(16cm直径)に置き、24℃±1℃、16時間明期/8時間暗期条件下で制御した恒温室内に置いた。48時間後、トマト葉を取り出し、一枚の葉あたり一つの透明樹脂製円柱容器(約1000mL体積;14cm高×10cm直径)内に置き、一葉あたり10頭のハスモンヨトウ幼虫を葉の表面に放飼し、直ちに蓋をし、上記と同じ条件下で栽培した。栽培中に葉が乾燥するのを防ぐために、葉柄を、予め1mLの蒸留水を添加した1.5mLマイクロチューブに突き刺した。5日後に、生存数及び体重を計測した。なお、エタノールのみからなる溶液(0μM)で処理したトマトを対照(コントロール)とした。
[Example 6]
<Examination of the control effect on the survival number and body weight of Spodoptera in tomatoes treated with α-ionone for 48 hours>
Tomato leaves from 3 weeks old to 4 weeks old were placed in a glass Petri dish (16 cm diameter) to which α-ionone solution of each concentration had been added in advance, and incubated at 24°C ± 1°C, 16 hours light/8 hours dark. The cells were placed in a thermostatic chamber controlled under period conditions. After 48 hours, the tomato leaves were removed and each leaf was placed in a transparent resin cylindrical container (approximately 1000 mL volume; 14 cm height x 10 cm diameter), and 10 Spodoptera larvae per leaf were released on the surface of the leaf. The plants were then immediately covered with lids and cultivated under the same conditions as above. To prevent the leaves from drying out during cultivation, the petiole was stuck into a 1.5 mL microtube to which 1 mL of distilled water was previously added. After 5 days, the number of survivors and body weight were measured. Note that tomatoes treated with a solution consisting only of ethanol (0 μM) were used as a control.
図8Aに、トマトにおけるハスモンヨトウ生存数に対するα-ヨノンの48時間処理の効果を示す。図8Bに、トマトにおけるハスモンヨトウ体重に対するα-ヨノンの48時間処理の効果を示す。なお、図8A及び8Bにおいて、異なるアルファベットは有意差があることを示す(p<0.05;n=10)。α-ヨノン処理区では、対照区と比較して、ハスモンヨトウの生存数及び体重の低下が見られた。
以上のことから、α-ヨノンは、アザミウマ科昆虫と異なる摂食様式を示す咀嚼性のハスモンヨトウ(ヤガ科昆虫)の幼虫に対する抵抗性を植物に誘導できることが明らかとなった。
FIG. 8A shows the effect of 48-hour treatment with α-ionone on the number of Spodoptera viables in tomatoes. FIG. 8B shows the effect of 48-hour treatment with α-ionone on Spodoptera spp. body weight in tomatoes. Note that in FIGS. 8A and 8B, different alphabets indicate significant differences (p<0.05; n=10). In the α-ionone treated plot, a decrease in the number of surviving Spodoptera and the body weight was observed compared to the control plot.
From the above, it has been revealed that α-ionone can induce resistance in plants to the chewing larvae of Spodoptera japonica (Noctuidae), which has a feeding style different from that of Thripsidae.
[実施例7]
<24時間α-ヨノン処理のキュウリにおけるミナミキイロアザミウマ生存数に対する防除効果の検定>
ポットで栽培した3週齢のキュウリ株を透明樹脂製円柱容器(約1000mL体積;14cm高×10cm直径)に置き、実施例1に記載の方法に準じて、300μMのα-ヨノン液とエタノール(0μM)を容器内で固定し、各溶液の蒸気にキュウリを24時間曝した。各溶液を入れた1.5mLマイクロチューブを取り出し、ミナミキイロアザミウマの雌成虫をキュウリ1株あたり10頭放飼し、直ちに蓋をし、と同じ条件下で栽培した。14日後に、24℃±1℃、16時間明期/8時間暗期条件下で生存数を計測した。
[Example 7]
<Examination of the control effect on the survival number of southern yellow thrips in cucumbers treated with α-ionone for 24 hours>
A 3-week-old cucumber strain grown in a pot was placed in a transparent resin cylindrical container (approximately 1000 mL volume; 14 cm height x 10 cm diameter), and 300 μM α-ionone solution and ethanol ( (0 μM) was fixed in a container, and cucumbers were exposed to the vapor of each solution for 24 hours. The 1.5 mL microtubes containing each solution were taken out, and 10 female adult southern thrips per cucumber plant were released.The tubes were immediately covered and cultivated under the same conditions. After 14 days, the number of survivors was counted at 24°C±1°C under 16-hour light/8-hour dark conditions.
図9に、キュウリにおけるミナミキイロアザミウマ生存数に対するα-ヨノンの24時間処理の効果を示す。なお、図9において、異なるアルファベットは有意差があることを示す(t-test p<0.05;n=8)。α-ヨノン処理区では、対照区と比較してミナミキイロアザミウマの生存数の低下が見られた。
以上のことから、α-ヨノンは、キュウリ(ウリ科植物)にも、トマト(ナス科植物)及びシロイヌナズナ(アブラナ科植物)と同様に、ミカンキイロアザミウマと同じアザミウマ科昆虫に属するミナミキイロアザミウマに対する抵抗性を誘導できることが明らかとなった。
Figure 9 shows the effect of 24-hour treatment with α-ionone on the number of southern thrips survivors in cucumber. In addition, in FIG. 9, different alphabets indicate that there is a significant difference (t-test p<0.05; n=8). In the α-ionone treated area, a decrease in the number of surviving southern thrips was observed compared to the control area.
From the above, α-ionone is effective against southern yellow thrips, which belongs to the same insect family Thripsidae as occidental thrips, as well as on cucumbers (Cucurbitaceae), as well as tomatoes (Solanaceae) and Arabidopsis (Brassicaceae). It has become clear that resistance can be induced.
本発明に係る害虫抵抗性誘導剤及びこれを用いた植物の害虫防除方法は、アザミウマ科昆虫又はヤガ科昆虫による虫害、特に吸汁害又は食害に対して有効であり、かつ抵抗性系統を出現させるおそれが非常に小さく、また、従来の農薬よりも環境や人体への安全性も高い。このため、本発明に係る害虫抵抗性誘導剤及びこれを用いた植物の害虫防除方法は、特に農作物や観葉植物の栽培、中でも、ナス科植物、アブラナ科植物及びウリ科植物の栽培等の分野において好適に使用できる。 The pest resistance inducer and the method for controlling plant pests using the same according to the present invention are effective against insect damage caused by Thripsidae insects or Noctuidae insects, particularly sap-sucking damage or feeding damage, and cause the emergence of resistant strains. There is very little danger, and it is also safer for the environment and the human body than conventional pesticides. Therefore, the pest resistance inducer according to the present invention and the method for controlling pests of plants using the same are particularly applicable to the cultivation of agricultural crops and ornamental plants, particularly in the cultivation of solanaceous plants, cruciferous plants, and cucurbitaceous plants. It can be suitably used in
Claims (5)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019147367 | 2019-08-09 | ||
| JP2019147367 | 2019-08-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2021028319A JP2021028319A (en) | 2021-02-25 |
| JP7429968B2 true JP7429968B2 (en) | 2024-02-09 |
Family
ID=74667221
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020121591A Active JP7429968B2 (en) | 2019-08-09 | 2020-07-15 | Pest resistance inducer and plant pest control method |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP7429968B2 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116076501A (en) * | 2022-12-01 | 2023-05-09 | 华南农业大学 | Plant-source repellent and application thereof in repelling tropical bed bugs and solenopsis invicta |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008137940A (en) | 2006-12-01 | 2008-06-19 | Taiyo Corp | Thrips controlling agent |
| JP2010132628A (en) | 2007-12-28 | 2010-06-17 | Dainippon Jochugiku Co Ltd | Repellent for grape pest, and method for repelling grape pest |
| JP6108548B2 (en) | 2013-08-09 | 2017-04-05 | 国立研究開発法人農業・食品産業技術総合研究機構 | Pest resistance inducer and plant pest control method |
| CN108353901A (en) | 2018-02-02 | 2018-08-03 | 武汉东昌仓贮技术有限公司 | A kind of attractant and lure for luring control cigarette beetle |
| US20190069543A1 (en) | 2016-03-24 | 2019-03-07 | Evolva Sa | Use of nootkatone to kill sap-sucking insects |
-
2020
- 2020-07-15 JP JP2020121591A patent/JP7429968B2/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008137940A (en) | 2006-12-01 | 2008-06-19 | Taiyo Corp | Thrips controlling agent |
| JP2010132628A (en) | 2007-12-28 | 2010-06-17 | Dainippon Jochugiku Co Ltd | Repellent for grape pest, and method for repelling grape pest |
| JP6108548B2 (en) | 2013-08-09 | 2017-04-05 | 国立研究開発法人農業・食品産業技術総合研究機構 | Pest resistance inducer and plant pest control method |
| US20190069543A1 (en) | 2016-03-24 | 2019-03-07 | Evolva Sa | Use of nootkatone to kill sap-sucking insects |
| CN108353901A (en) | 2018-02-02 | 2018-08-03 | 武汉东昌仓贮技术有限公司 | A kind of attractant and lure for luring control cigarette beetle |
Non-Patent Citations (2)
| Title |
|---|
| α-Ionone, an Apocarotenoid, Induces Plant Resistance to Western Flower Thrips, Frankliniella occidentalis, Independently of Jasmonic Acid,Molecules,2019年12月19日,pp.1-10,doi: 10.3990/molecules25010017 |
| 害虫抵抗性誘導剤の素材として有望な天然物質の発見,環境報告書2020,2020年,17 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2021028319A (en) | 2021-02-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5775539B2 (en) | Use of proline to improve stress tolerance to herbicides | |
| US20080182751A1 (en) | Brassicaceae Plant Materials and Method for Their Use as Biopesticides | |
| KR101904054B1 (en) | Method for improving plant quality | |
| EA023113B1 (en) | Use of agrochemical mixtures for increasing the health of a plant | |
| DK156290B (en) | ALFA-CYANO-3-PHENOXYBENZYL-S - (+) - 2- (4-CHLORPHENYL) ISOVALERATE AND S - (-) - ALFA-CYANO-3-PHENOXYBENZYL-S - (+) - 2- (4-CHLORPHENYL) ISOVALATED, INSECTICID AGENT CONTAINING SUCH A CONNECTION, PROCEDURES FOR REMOVING INSECTS, AND USING SUCH A CONNECTION AS INSECTICID | |
| US11229210B2 (en) | Method for using mustard meal or an extract thereof | |
| JP7429968B2 (en) | Pest resistance inducer and plant pest control method | |
| US11737459B2 (en) | Use of nootkatone to kill sap-sucking insects | |
| US20160366882A1 (en) | Agrochemical compositions for inducing abiotic stress tolerance | |
| CA2535766C (en) | Suppressing plant pathogens and pests with applied or induced auxins | |
| JP2022510614A (en) | Compositions containing choline salts of fatty acids, and their use as fungicides | |
| JP6895433B2 (en) | Application of 7-carboxybenzo [1,2,3] thiadiazoleamide as a plant stimulant | |
| CN100594785C (en) | Controlling plant pathogens and pests with applied or induced auxins | |
| JP5023276B2 (en) | Soil disease control agent and soil disease control method | |
| CA2939323A1 (en) | Extracts of agricultural husks used to modify the metabolism of plants | |
| JPS59118757A (en) | Substituted nitro and cyanoguanidines and use for increasingyield of crops | |
| JP6108548B2 (en) | Pest resistance inducer and plant pest control method | |
| JP5223132B2 (en) | Plant pathogen infection inhibitor and method for suppressing pathogen infection | |
| UA127807C2 (en) | Method for increasing the resistance of a cereal plant | |
| Bruinsma | Chemical control of crop growth and development | |
| EA030020B1 (en) | Binary fungicidal mixtures | |
| KR20180127894A (en) | Composition comprising hinokitiol for controlling plant disease and uses thereof | |
| RU2798880C2 (en) | Method for increasing resistance of grain plant | |
| WO2024144408A2 (en) | Ionic derivatives of aromatic carboxylic acid for use as plant stimulants, method for stimulating plants and use of these derivatives for manufacturing compositions for stimulating plants | |
| CN106962366B (en) | Application of N, N' -methylene bis (2-amino-5-mercapto-1, 3, 4-thiadiazole) in controlling crop pests |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A80 | Written request to apply exceptions to lack of novelty of invention |
Free format text: JAPANESE INTERMEDIATE CODE: A80 Effective date: 20200803 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20230302 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20231222 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20240109 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20240123 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7429968 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |