JP7126830B2 - Method for regenerating fluorinated liquids and regenerating apparatus using same - Google Patents
Method for regenerating fluorinated liquids and regenerating apparatus using same Download PDFInfo
- Publication number
- JP7126830B2 JP7126830B2 JP2018007629A JP2018007629A JP7126830B2 JP 7126830 B2 JP7126830 B2 JP 7126830B2 JP 2018007629 A JP2018007629 A JP 2018007629A JP 2018007629 A JP2018007629 A JP 2018007629A JP 7126830 B2 JP7126830 B2 JP 7126830B2
- Authority
- JP
- Japan
- Prior art keywords
- liquid
- fluorinated
- fluorinated liquid
- water
- lower layer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000007788 liquid Substances 0.000 title claims description 324
- 238000000034 method Methods 0.000 title claims description 65
- 230000001172 regenerating effect Effects 0.000 title claims description 43
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 85
- 239000012459 cleaning agent Substances 0.000 claims description 55
- 238000011069 regeneration method Methods 0.000 claims description 53
- 239000000203 mixture Substances 0.000 claims description 47
- 239000003599 detergent Substances 0.000 claims description 45
- 238000004821 distillation Methods 0.000 claims description 39
- 239000008346 aqueous phase Substances 0.000 claims description 31
- 230000008929 regeneration Effects 0.000 claims description 31
- 239000002798 polar solvent Substances 0.000 claims description 18
- 239000002904 solvent Substances 0.000 claims description 18
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 claims description 16
- 239000002184 metal Substances 0.000 claims description 14
- 229910052751 metal Inorganic materials 0.000 claims description 14
- 238000004519 manufacturing process Methods 0.000 claims description 13
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 12
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 claims description 11
- 239000012071 phase Substances 0.000 claims description 11
- 150000003950 cyclic amides Chemical group 0.000 claims description 6
- 150000001412 amines Chemical class 0.000 claims description 4
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 claims description 4
- 239000012046 mixed solvent Substances 0.000 claims description 4
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 24
- 238000004140 cleaning Methods 0.000 description 17
- 238000000926 separation method Methods 0.000 description 12
- 239000012153 distilled water Substances 0.000 description 11
- 239000011259 mixed solution Substances 0.000 description 8
- 108010081348 HRT1 protein Hairy Proteins 0.000 description 7
- 102100021881 Hairy/enhancer-of-split related with YRPW motif protein 1 Human genes 0.000 description 7
- JNCMHMUGTWEVOZ-UHFFFAOYSA-N F[CH]F Chemical compound F[CH]F JNCMHMUGTWEVOZ-UHFFFAOYSA-N 0.000 description 6
- 230000000052 comparative effect Effects 0.000 description 6
- CWIFAKBLLXGZIC-UHFFFAOYSA-N 1,1,2,2-tetrafluoro-1-(2,2,2-trifluoroethoxy)ethane Chemical compound FC(F)C(F)(F)OCC(F)(F)F CWIFAKBLLXGZIC-UHFFFAOYSA-N 0.000 description 5
- 238000009835 boiling Methods 0.000 description 5
- 125000001309 chloro group Chemical group Cl* 0.000 description 5
- 238000011156 evaluation Methods 0.000 description 4
- VUWZPRWSIVNGKG-UHFFFAOYSA-N fluoromethane Chemical compound F[CH2] VUWZPRWSIVNGKG-UHFFFAOYSA-N 0.000 description 4
- 150000001336 alkenes Chemical class 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000001816 cooling Methods 0.000 description 3
- 125000001153 fluoro group Chemical group F* 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 238000004817 gas chromatography Methods 0.000 description 3
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- OKIYQFLILPKULA-UHFFFAOYSA-N 1,1,1,2,2,3,3,4,4-nonafluoro-4-methoxybutane Chemical compound COC(F)(F)C(F)(F)C(F)(F)C(F)(F)F OKIYQFLILPKULA-UHFFFAOYSA-N 0.000 description 2
- 125000004793 2,2,2-trifluoroethoxy group Chemical group FC(CO*)(F)F 0.000 description 2
- 241000209094 Oryza Species 0.000 description 2
- 235000007164 Oryza sativa Nutrition 0.000 description 2
- 239000012159 carrier gas Substances 0.000 description 2
- 239000003086 colorant Substances 0.000 description 2
- 239000004210 ether based solvent Substances 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000005484 gravity Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 125000006340 pentafluoro ethyl group Chemical group FC(F)(F)C(F)(F)* 0.000 description 2
- -1 polypropylene Polymers 0.000 description 2
- 235000009566 rice Nutrition 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000005292 vacuum distillation Methods 0.000 description 2
- ZHOFTZAKGRZBSL-UHFFFAOYSA-N 1,1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8-heptadecafluoro-8-methoxyoctane Chemical compound COC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F ZHOFTZAKGRZBSL-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 238000011088 calibration curve Methods 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 239000010808 liquid waste Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 239000003586 protic polar solvent Substances 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- ZUHZGEOKBKGPSW-UHFFFAOYSA-N tetraglyme Chemical compound COCCOCCOCCOCCOC ZUHZGEOKBKGPSW-UHFFFAOYSA-N 0.000 description 1
- 238000001771 vacuum deposition Methods 0.000 description 1
- 238000005019 vapor deposition process Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 150000003953 γ-lactams Chemical class 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D11/00—Solvent extraction
- B01D11/04—Solvent extraction of solutions which are liquid
- B01D11/0446—Juxtaposition of mixers-settlers
- B01D11/0457—Juxtaposition of mixers-settlers comprising rotating mechanisms, e.g. mixers, mixing pumps
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D11/00—Solvent extraction
- B01D11/04—Solvent extraction of solutions which are liquid
- B01D11/0492—Applications, solvents used
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23G—CLEANING OR DE-GREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS
- C23G1/00—Cleaning or pickling metallic material with solutions or molten salts
- C23G1/36—Regeneration of waste pickling liquors
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23G—CLEANING OR DE-GREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS
- C23G5/00—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents
- C23G5/02—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents
- C23G5/028—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons
- C23G5/02803—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons containing fluorine
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23G—CLEANING OR DE-GREASING OF METALLIC MATERIAL BY CHEMICAL METHODS OTHER THAN ELECTROLYSIS
- C23G5/00—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents
- C23G5/02—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents
- C23G5/028—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons
- C23G5/02854—Cleaning or de-greasing metallic material by other methods; Apparatus for cleaning or de-greasing metallic material with organic solvents using organic solvents containing halogenated hydrocarbons characterised by the stabilising or corrosion inhibiting additives
- C23G5/02861—Oxygen-containing compounds
- C23G5/02877—Ethers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
- H10K71/10—Deposition of organic active material
- H10K71/16—Deposition of organic active material using physical vapour deposition [PVD], e.g. vacuum deposition or sputtering
- H10K71/166—Deposition of organic active material using physical vapour deposition [PVD], e.g. vacuum deposition or sputtering using selective deposition, e.g. using a mask
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D11/00—Solvent extraction
- B01D11/04—Solvent extraction of solutions which are liquid
- B01D11/0419—Solvent extraction of solutions which are liquid in combination with an electric or magnetic field or with vibrations
- B01D11/0423—Applying ultrasound
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D11/00—Solvent extraction
- B01D11/04—Solvent extraction of solutions which are liquid
- B01D11/0446—Juxtaposition of mixers-settlers
- B01D11/0469—Juxtaposition of mixers-settlers with gas agitation
-
- C11D2111/48—
Description
本開示は、フッ素化液体の再生方法、及び該方法を用いる再生装置に関する。 FIELD OF THE DISCLOSURE The present disclosure relates to a method of regenerating fluorinated liquids and a regenerating apparatus using the method.
例えば、有機ELディスプレイ(以下、「OLED」という場合がある。)の製造方法は、メタルマスクを介してガラス等の基板上にRGB3色の色素を蒸着させて有機発光層を形成する工程を備えている。メタルマスクは高価な部材であるため、N-メチル-2-ピロリドン(以下、「NMP」という場合がある。)溶液で洗浄した後、フッ素化液体によるリンス工程及び乾燥工程を経て、メタルマスクを再利用している。 For example, a manufacturing method of an organic EL display (hereinafter sometimes referred to as "OLED") includes a step of forming an organic light-emitting layer by vapor-depositing pigments of three colors of RGB on a substrate such as glass through a metal mask. ing. Since the metal mask is an expensive member, the metal mask is washed with an N-methyl-2-pyrrolidone (hereinafter sometimes referred to as "NMP") solution, followed by a rinsing process with a fluorinated liquid and a drying process. reused.
特許文献1(特開2006-313753号公報)には、低分子型有機EL素子製造時の真空蒸着工程において使用するメタルマスクを、N-メチル-2-ピロリジノン等の非プロトン性極性溶媒を含む洗浄液組成物を用いて浸漬又はジェット水流により洗浄後、ハイドロフルオロエーテルによってリンスする洗浄方法が記載されている。 Patent Document 1 (Japanese Unexamined Patent Application Publication No. 2006-313753) discloses that a metal mask used in a vacuum vapor deposition process in manufacturing a low-molecular-weight organic EL device contains an aprotic polar solvent such as N-methyl-2-pyrrolidinone. A cleaning method is described in which a cleaning solution composition is used for cleaning by immersion or jet water flow, followed by rinsing with a hydrofluoroether.
特許文献2(特開平07-076787号公報)には、NMPを金属洗浄剤として用いる洗浄装置と、洗浄後のNMP洗浄液から汚染物質を除去して洗浄装置に循環させる再生装置とを備え、再生装置内に設けられる濾材は、少なくともポリプロピレンを含有し、かつ、NMPに対して浮上性を有する粒状の濾材である、金属洗浄剤の再生装置が記載されている。 Patent Document 2 (Japanese Patent Application Laid-Open No. 07-076787) discloses a cleaning device using NMP as a metal cleaning agent, and a regeneration device that removes contaminants from the NMP cleaning liquid after cleaning and circulates it to the cleaning device. A regenerating apparatus for a metal cleaning agent is described in which the filter medium provided in the apparatus is a granular filter medium that contains at least polypropylene and has floating properties with respect to NMP.
特許文献3(特開2008-163400号公報)には、(1a)炭化水素類、(1b)グリコールエーテル類、及び(1c)エステル類から選ばれる1種以上を主成分とする洗浄液を収納し被洗浄物が浸漬される洗浄槽と、(2a)ハイドロフルオロカーボン類、及び(2b)ハイドロフルオロエーテル類から選ばれる1種以上を主成分とするリンス液を収納し被洗浄物が浸漬されるリンス液槽と、リンス液を収納し該リンス液の蒸気を発生させる蒸気槽と、蒸留器を有する再生ユニットを備える、洗浄システムが記載されている。 Patent Document 3 (Japanese Unexamined Patent Application Publication No. 2008-163400) contains a cleaning liquid containing as a main component one or more selected from (1a) hydrocarbons, (1b) glycol ethers, and (1c) esters. A cleaning tank in which the object to be cleaned is immersed, and a rinse containing a rinse liquid mainly composed of one or more selected from (2a) hydrofluorocarbons and (2b) hydrofluoroethers, in which the object to be cleaned is immersed. A cleaning system is described comprising a liquid bath, a steam bath containing a rinse liquid and generating vapor of the rinse liquid, and a regeneration unit having a still.
メタルマスクの洗浄及びリンスの回数が増加するに伴い、洗浄剤のリンス槽への混入比率も増加する。その結果、リンス槽が洗浄剤で汚染されてしまうため、定期的にリンス液を交換する必要があった。しかしながら、リンス液として使用するフッ素化液体も高価な溶剤であるため、一般的には、蒸留手段を使用し、汚染されたリンス液からフッ素化液体を回収して再利用していた。しかしながら、係る蒸留手段では、回収できるフッ素化液体の量が極めて低いため、フッ素化液体の大部分を廃棄処分しているのが現状であった。 As the number of times of cleaning and rinsing the metal mask increases, the mixing ratio of the cleaning agent in the rinsing bath also increases. As a result, the rinsing tank is contaminated with the cleaning agent, and the rinsing liquid needs to be replaced periodically. However, since the fluorinated liquid used as the rinse liquid is also an expensive solvent, generally distillation means are used to recover the fluorinated liquid from the contaminated rinse liquid for reuse. However, since the amount of fluorinated liquid that can be recovered by such distillation means is extremely small, most of the fluorinated liquid is currently disposed of.
本開示は、洗浄剤が混入したフッ素化液体に対する再生効率に優れるフッ素化液体の再生方法、及び該方法を用いる再生装置を提供する。 The present disclosure provides a method for regenerating a fluorinated liquid that is highly efficient in regenerating a fluorinated liquid mixed with a cleaning agent, and a regenerating apparatus using the method.
本開示の一実施態様によれば、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が約80質量%以上にならないように水を接触させる工程と、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する工程と、を備える、フッ素化液体の再生方法であって、洗浄剤が、フッ素化液体に溶解する非プロトン性極性溶媒であり、且つフッ素化液体が、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である、再生方法が提供される。 According to one embodiment of the present disclosure, the step of contacting a fluorinated liquid mixed with a detergent with water such that the concentration of the detergent in an overlying aqueous phase is no more than about 80% by weight; After the mixed liquid is separated into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase, the upper liquid is removed and the lower liquid is collected. A method of regenerating a fluorinated liquid, wherein the cleaning agent is an aprotic polar solvent that dissolves in the fluorinated liquid, and the fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof. A method is provided.
本開示の別の実施態様によれば、上述したフッ素化液体の再生方法を用いて再生されたフッ素化液体を、有機ELディスプレイ製造装置で使用される部材用のリンス液として使用する方法が提供される。 According to another embodiment of the present disclosure, there is provided a method of using the fluorinated liquid regenerated using the method for regenerating the fluorinated liquid described above as a rinse liquid for members used in an organic EL display manufacturing apparatus. be done.
本開示のさらに別の実施態様によれば、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が約80質量%以上にならないように水を接触させる手段と、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する手段と、を備える、フッ素化液体再生装置であって、洗浄剤が、フッ素化液体に溶解する非プロトン性極性溶媒であり、且つフッ素化液体が、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である、フッ素化液体再生装置が提供される。 According to yet another embodiment of the present disclosure, means for contacting the fluorinated liquid mixed with the detergent with water such that the concentration of the detergent in the overlying aqueous phase is no more than about 80% by weight; a means for removing the upper layer liquid and collecting the lower layer liquid after the mixed liquid after contact with water is separated into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase; wherein the cleaning agent is an aprotic polar solvent that dissolves in the fluorinated liquid, and the fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof; A fluorinated liquid regeneration device is provided.
本開示のフッ素化液体の再生方法及び再生装置は、洗浄剤が混入したフッ素化液体の再生効率を向上させることができる。 The method and apparatus for regenerating a fluorinated liquid of the present disclosure can improve the efficiency of regenerating a fluorinated liquid mixed with a cleaning agent.
さらに本開示のいくつかの例では、十分な分離をもたらすことにより追加の蒸留工程や加熱工程を除くことができる。これらの例では常温で完了することができるので、エネルギー効率がより高く、追加の操作が不要となる。 Additionally, in some examples of the present disclosure, additional distillation and heating steps can be eliminated by providing sufficient separation. Since these examples can be completed at ambient temperature, they are more energy efficient and require no additional manipulations.
上述の記載は、本開示の全ての実施態様及び本開示に関する全ての利点を開示したものとみなしてはならない。 The above description should not be considered a disclosure of all implementations of the disclosure and all advantages associated with the disclosure.
本開示の第1の実施形態におけるフッ素化液体の再生方法は、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が約80質量%以上にならないように水を接触させる工程と、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する工程と、を備え、洗浄剤は、フッ素化液体に溶解する非プロトン性極性溶媒であり、フッ素化液体は、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である。本開示の再生方法は、特定の洗浄剤及び特定のフッ素化液体を含む混合系に対し、水を所定量接触させるだけで、純度の高いフッ素化液体を高い産出量(収量)で再生することができる。 In the method for regenerating a fluorinated liquid according to the first embodiment of the present disclosure, water is added to a fluorinated liquid mixed with a cleaning agent so that the concentration of the cleaning agent in the upper aqueous phase does not exceed about 80% by mass. After the step of contacting and contacting with water, the mixed liquid is separated into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase, after which the upper liquid is removed and the lower liquid is collected. wherein the cleaning agent is an aprotic polar solvent soluble in a fluorinated liquid, and the fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof. The regeneration method of the present disclosure regenerates a high-purity fluorinated liquid with a high output (yield) only by bringing a predetermined amount of water into contact with a mixed system containing a specific cleaning agent and a specific fluorinated liquid. can be done.
第1の実施形態におけるフッ素化液体の再生方法における非プロトン性極性溶媒は、環状アミド系溶媒、アミン系溶媒、グリコールエーテル系溶媒、アセトン、ジメチルスルホキシド、ジメチルホルムアミド又はこれらの混合溶媒であってもよい。係る非プロトン性極性溶媒との組み合わせは、フッ素化液体の再生効率をより向上させることができる。中でも、第1の実施形態におけるフッ素化液体の再生方法における非プロトン性極性溶媒が環状アミド系溶媒の場合、フッ素化液体の再生効率をさらに向上させることができる。ここで、再生効率とは、再生したフッ素化液体の純度及び産出量などから求められるものであり、高純度及び高産出量でフッ素化液体を再生できた場合、再生効率が優れるということになる。 The aprotic polar solvent in the method for regenerating a fluorinated liquid in the first embodiment may be a cyclic amide-based solvent, an amine-based solvent, a glycol ether-based solvent, acetone, dimethylsulfoxide, dimethylformamide, or a mixed solvent thereof. good. A combination with such an aprotic polar solvent can further improve the regeneration efficiency of the fluorinated liquid. Above all, when the aprotic polar solvent in the method for regenerating a fluorinated liquid in the first embodiment is a cyclic amide-based solvent, the regeneration efficiency of the fluorinated liquid can be further improved. Here, the regeneration efficiency is determined from the purity and output of the regenerated fluorinated liquid, and when the fluorinated liquid can be regenerated with high purity and high output, the regeneration efficiency is excellent. .
第1の実施形態におけるフッ素化液体の再生方法は、採取された下層の液中のフッ素化液体の純度を約95%以上にすることができる。 The method for regenerating a fluorinated liquid in the first embodiment can achieve a purity of about 95% or higher for the fluorinated liquid in the collected underlying liquid.
第1の実施形態におけるフッ素化液体の再生方法は、下層の液を採取する工程に続いて、下層の液を蒸留する工程を更に備えることができる。蒸留工程をさらに適用することによって、より高純度のフッ素化液体を再生することができる。 The method for regenerating a fluorinated liquid in the first embodiment can further comprise a step of distilling the lower layer liquid following the step of collecting the lower layer liquid. Higher purity fluorinated liquids can be regenerated by further applying a distillation step.
第1の実施形態におけるフッ素化液体の再生方法は、蒸留工程を採用した場合、蒸留によって採取された液中のフッ素化液体の純度を約99.0%以上にすることができる。 In the method for regenerating a fluorinated liquid in the first embodiment, when a distillation process is employed, the purity of the fluorinated liquid in the liquid collected by distillation can be made about 99.0% or higher.
本開示の第2の実施形態における有機ELディスプレイ製造装置で使用される部材用のリンス液として使用する方法は、第1の実施形態におけるフッ素化液体の再生方法を用いて再生されたフッ素化液体を使用することができる。前記部材としては、例えば、メタルマスク又は防着板などを挙げることができる。第1の実施形態におけるフッ素化液体の再生方法は、従来の蒸留のみによる再生方法に比べて廃棄するフッ素化液体の量を大幅に低減し得るため、第1の実施形態の再生方法により得られたフッ素化液体を使用する第2の実施形態における方法は、有機ELディスプレイの製造コストをより削減することができる。 The method of using the rinsing liquid for the members used in the organic EL display manufacturing apparatus according to the second embodiment of the present disclosure is the fluorinated liquid regenerated using the method for regenerating the fluorinated liquid according to the first embodiment. can be used. Examples of the member include a metal mask and an anti-adhesion plate. The method for regenerating a fluorinated liquid in the first embodiment can significantly reduce the amount of fluorinated liquid to be discarded compared to the conventional regeneration method that uses only distillation. The method of the second embodiment using the fluorinated liquid can further reduce the manufacturing cost of the organic EL display.
本開示の第3の実施形態におけるフッ素化液体再生装置は、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が約80質量%以上にならないように水を接触させる手段と、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する手段と、を備え、洗浄剤は、フッ素化液体に溶解する非プロトン性極性溶媒であり、且つフッ素化液体は、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である。本開示の再生装置は、特定の洗浄剤及び特定のフッ素化液体を含む混合系に対し、純度の高いフッ素化液体を高い産出量(収量)で再生することができる。 In the fluorinated liquid regeneration apparatus according to the third embodiment of the present disclosure, the fluorinated liquid mixed with the cleaning agent is brought into contact with water so that the concentration of the cleaning agent in the upper aqueous phase does not exceed about 80% by mass. After the mixed liquid after contact with water is separated into two liquids, an aqueous phase located in the upper layer and a phase containing the fluorinated liquid located in the lower layer, the upper layer liquid is removed and the lower layer liquid is collected. wherein the cleaning agent is an aprotic polar solvent soluble in the fluorinated liquid, and the fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof. The regenerator of the present disclosure is capable of regenerating high purity fluorinated liquids with high output (yield) for mixed systems containing specific cleaning agents and specific fluorinated liquids.
本開示の第3の実施形態におけるフッ素化液体再生装置は、下層の液を採取する手段に続いて、下層の液を蒸留する手段を更に備えることができる。蒸留工程をさらに適用することによって、より高純度のフッ素化液体を再生することができる。 The fluorinated liquid regeneration apparatus in the third embodiment of the present disclosure may further comprise means for distilling the underlying liquid subsequent to the means for collecting the underlying liquid. Higher purity fluorinated liquids can be regenerated by further applying a distillation step.
以下、本開示の代表的な実施態様を例示する目的でより詳細に説明するが、本開示はこれらの実施態様に限定されない。 The present disclosure is described in more detail below for the purpose of illustrating representative embodiments of the present disclosure, but the present disclosure is not limited to these embodiments.
《フッ素化液体の再生方法》
本開示の一実施態様のフッ素化液体の再生方法は、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が約80質量%以上にならないように水を接触させる工程(以下、「水接触工程」という場合がある。)と、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する工程(以下、「分離・採取工程」という場合がある。)と、を備えており、ここで用いる洗浄剤は、フッ素化液体に溶解する非プロトン性極性溶媒であり、且つフッ素化液体は、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である。
《Method for regenerating fluorinated liquid》
A method for regenerating a fluorinated liquid according to an embodiment of the present disclosure includes contacting a fluorinated liquid mixed with a detergent with water such that the concentration of the detergent in the upper aqueous phase does not exceed about 80% by mass. step (hereinafter sometimes referred to as "water contacting step"), and after the mixed liquid after contacting with water is separated into two liquids, an aqueous phase located in the upper layer and a phase containing the fluorinated liquid located in the lower layer, and a step of removing the liquid in the upper layer and collecting the liquid in the lower layer (hereinafter sometimes referred to as a “separation/collection step”). The protic polar solvent and fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or mixtures thereof.
〈洗浄剤〉
本開示のフッ素化液体の再生方法において混入され得る洗浄剤は、各種部材の洗浄時に使用される洗浄剤であり、例えば、有機ELディスプレイ製造装置におけるメタルマスク、防着板等の各種部材の洗浄時に使用される洗浄剤などが挙げられる。係る洗浄剤としては、フッ素化液体に溶解する非プロトン性極性溶媒であればいかなるものであってもよく、次のものに限定されないが、例えば、環状アミド系溶媒、アミン系溶媒、グリコールエーテル系溶媒、アセトン、ジメチルスルホキシド、ジメチルホルムアミド又はこれらの混合溶媒などが挙げられる。メタルマスク又は防着板の洗浄性等の観点から、環状アミド系溶媒を用いることが好ましく、中でも、N-メチル-2-ピロリドン(NMP)、N-ブチル-2-ピロリドン(NBP)などのN-アルキル-ピロリドン類溶媒又はγ-ラクタム類溶媒と称される溶媒などを用いることがより好ましい。非プロトン性極性溶媒は、これらの溶媒を単独で又は2種以上組み合わせて使用することができる。上記の洗浄剤であれば、本開示の再生方法によって、フッ素化液体を効率よく再生することができる。洗浄剤は、フッ素化液体の再生効率を阻害しない範囲で、上記の洗浄剤以外に、他の洗浄剤が含まれていてもよいが、再生効率等の観点から、他の洗浄剤は含まれないことが好ましい。
<Washing soap>
The cleaning agent that can be mixed in the method for regenerating a fluorinated liquid of the present disclosure is a cleaning agent that is used when cleaning various members, for example, cleaning various members such as metal masks and anti-adhesion plates in organic EL display manufacturing equipment. cleaning agents that are sometimes used. Such a cleaning agent may be any aprotic polar solvent that dissolves in the fluorinated liquid, and is not limited to the following, for example, cyclic amide solvents, amine solvents, glycol ether solvents, Examples include solvents such as acetone, dimethylsulfoxide, dimethylformamide, and mixed solvents thereof. From the viewpoint of cleaning the metal mask or the anti-adhesion plate, it is preferable to use a cyclic amide solvent. Solvents called -alkyl-pyrrolidone solvents or γ-lactam solvents are more preferably used. Aprotic polar solvents can be used alone or in combination of two or more. With the cleaning agent described above, the fluorinated liquid can be efficiently regenerated by the regeneration method of the present disclosure. In addition to the above cleaning agents, the cleaning agent may contain other cleaning agents as long as they do not hinder the regeneration efficiency of the fluorinated liquid. preferably not.
以下で説明する蒸留工程の適用等の観点から、洗浄剤の沸点は、約55℃以上、約100℃以上、約150℃以上、約200℃以上、又は約250℃以上であることが好ましい。 From the viewpoint of application of the distillation process described below, etc., the boiling point of the cleaning agent is preferably about 55° C. or higher, about 100° C. or higher, about 150° C. or higher, about 200° C. or higher, or about 250° C. or higher.
〈フッ素化液体〉
本開示のフッ素化液体の再生方法で再生し得るフッ素化液体としては、ハイドロフルオロエーテル(以下、「HFE」と略記する場合がある。)、ハイドロフルオロオレフィン(以下、「HFO」と略記する場合がある。)、又はこれらの混合物を挙げることができる。フッ素化液体は、再生効率を阻害しない範囲で、上記のフッ素化液体以外に、他のフッ素化液体(例えば、ハイドロクロロフルオロカーボン、ハイドロフルオロカーボン等)が含まれていてもよいが、再生効率等の観点から、他のフッ素化液体は含まれないことが好ましい。
<Fluorinated liquid>
Examples of fluorinated liquids that can be regenerated by the method for regenerating fluorinated liquids of the present disclosure include hydrofluoroethers (hereinafter sometimes abbreviated as "HFE") and hydrofluoroolefins (hereinafter abbreviated as "HFO"). ), or a mixture thereof. In addition to the above fluorinated liquids, the fluorinated liquid may contain other fluorinated liquids (for example, hydrochlorofluorocarbons, hydrofluorocarbons, etc.) within a range that does not impair the regeneration efficiency. From a point of view, it is preferred that no other fluorinated liquids are included.
以下で説明する蒸留工程の適用等の観点から、フッ素化液体の沸点は、約30℃以上、約55℃以上、約60℃以上、又は約75℃以上、約150℃以下、約100℃以下、又は約80℃以下であることが好ましい。 From the viewpoint of application of the distillation process described below, etc., the boiling point of the fluorinated liquid is about 30° C. or higher, about 55° C. or higher, about 60° C. or higher, or about 75° C. or higher, about 150° C. or lower, or about 100° C. or lower. , or about 80° C. or less.
(ハイドロフルオロエーテル)
上記のフッ素化液体の中でも、再生効率等の観点から、ハイドロフルオロエーテルの使用が好ましい。ハイドロフルオロエーテルは、ハイドロフルオロカーボンの炭素原子間にエーテル結合性の酸素原子を含む化合物である。ハイドロフルオロエーテル1分子中に含まれるエーテル結合性酸素原子の数は、1であってもよく、2以上であってもよい。溶剤として使いやすい沸点である点、安定性の点などから、1又は2が好ましく、1がより好ましい。ハイドロフルオロエーテルの分子構造は鎖状であればよく、直鎖状でも分岐鎖状でもよいが、再生効率等の観点から、直鎖状が好ましい。ハイドロフルオロエーテルとしては、次のものに限定されないが、C4F9OCH3、C4F9OCH2CH3、C5F11OCH3、C5F11OCH2CH3、C6F13OCH3、C6F13OCH2CH3、C7F15OCH3、C7F15OCH2CH3、C8F17OCH3、C8F17OCH2CH3、C9F19OCH3、C9F19OCH2CH3、C10F21OCH3、C10F21OCH2CH3などのセグリゲート型ハイドロフルオロエーテル;CF3CH2OCF2CF2H、CF3CHFOCH2CF3、CF3CH2OCF2CFHCF3、CHF2CF2CH2OCF2CF2H、C3F7OC3F6OCFHCF3、CF3CF(CF3)CF(OCH3)CF2CF3、CF3CF(CF3)CF(OC2H5)CF2CF3、CF2(OCH2CF3)CF2H、CF2(OCH2CF3)CFHCF3、CF2(OCH2CF2CF2H)CF2H、CF2(OCH2CF2CF2H)CFHCF3等のハイドロフルオロエーテルなどを挙げることができる。中でも、セグリゲート型ハイドロフルオロエーテルの使用は、水と接触させただけで、約97%以上、約98%以上、又は約99%以上の高純度化を達成し得るため、特に好ましいフッ素化液体である。中でも、特に好ましいセグリゲート型ハイドロフルオロエーテルは、C4F9OCH3又はC4F9OCH2CH3である。ここで、「セグリゲート型」とは、エーテル結合を挟んで、一方が完全フッ素化されており、他方が炭素及び水素で構成されている構造を意味する。ハイドロフルオロエーテルは、これらのものを単独で又は2種以上組み合わせて使用することができる。
(hydrofluoroether)
Among the above fluorinated liquids, hydrofluoroether is preferably used from the viewpoint of regeneration efficiency and the like. A hydrofluoroether is a compound containing an etheric oxygen atom between the carbon atoms of a hydrofluorocarbon. The number of etheric oxygen atoms contained in one molecule of the hydrofluoroether may be one, or two or more. 1 or 2 is preferable, and 1 is more preferable, from the viewpoint of the boiling point that is easy to use as a solvent, the stability, and the like. The molecular structure of the hydrofluoroether may be chain-shaped, and may be linear or branched, but linear is preferred from the viewpoint of regeneration efficiency and the like. Hydrofluoroethers include , but are not limited to , C4F9OCH3 , C4F9OCH2CH3 , C5F11OCH3 , C5F11OCH2CH3 , C6F13 OCH3 , C6F13OCH2CH3 , C7F15OCH3 , C7F15OCH2CH3 , C8F17OCH3 , C8F17OCH2CH3 , C9F19OCH3 _ _ _ _ _ _ _ _ _ , C9F19OCH2CH3 , C10F21OCH3 , C10F21OCH2CH3 ; CF3CH2OCF2CF2H , CF3CHFOCH2CF3 , _ _ _ _ _ _ _ CF3CH2OCF2CFHCF3 , CHF2CF2CH2OCF2CF2H , C3F7OC3F6OCFHCF3 , CF3CF ( CF3 ) CF ( OCH3 ) CF2CF3 , CF _ _ 3CF ( CF3 )CF ( OC2H5 ) CF2CF3 , CF2 ( OCH2CF3 ) CF2H , CF2 ( OCH2CF3 ) CFHCF3 , CF2 ( OCH2CF2CF2 H) Hydrofluoroethers such as CF2H , CF2 ( OCH2CF2CF2H ) CFHCF3 , and the like. Among them, the use of a segregated hydrofluoroether is a particularly preferable fluorinated liquid because it can achieve a high purity of about 97% or more, about 98% or more, or about 99% or more just by contacting it with water. be. Among them , a particularly preferred segregated hydrofluoroether is C4F9OCH3 or C4F9OCH2CH3 . Here, the term “seggregate type” means a structure in which one side is fully fluorinated and the other side is composed of carbon and hydrogen with an ether bond interposed therebetween. These hydrofluoroethers can be used alone or in combination of two or more.
(ハイドロフルオロオレフィン)
ハイドロフルオロオレフィンは、オレフィンが有する1個又は2個以上の水素原子がフッ素原子で置換された化合物を意図する。ハイドロフルオロオレフィンが有するフッ素原子の個数は特に限定されるものではないが、1以上又は2以上、10以下又は6以下とすることができる。ハイドロフルオロオレフィンは、E型(トランス型)及びZ型(シス型)のいずれであってもよい。ハイドロフルオロオレフィンは、ハイドロクロロフルオロオレフィン(HCFO)であってもよい。ハイドロクロロフルオロオレフィンは、オレフィンが有する1個又は2個以上の水素原子がフッ素原子で置換されるとともに、該オレフィンが有する1個又は2個以上のその他の水素原子が塩素原子で置換された化合物を意図する。ハイドロクロロフルオロオレフィンが有する塩素原子の個数は特に限定されるものではないが、1以上、5以下又は3以下とすることができる。塩素原子を有しないハイドロフルオロオレフィンとしては、例えば、CF3-CH=CH2、CF3-CF=CH2、CHF2-CH=CHF、CHF2-CF=CH2、CH2F-CH=CF2、CH2F-CF=CHF、CH3-CF=CF2、CF3-CH=CH-CF3、CF3-CH=CF-CH3、CF3-CF=CH-CH3、CF3-CH=CH-CH2F、CHF2-CF=CF-CH3、CHF2-CF=CH-CH2F、CHF2-CH=CF-CH2F、CHF2-CH=CH-CHF2、CH2F-CF=CF-CH2F、CH2F-CH=CH-CF3、CH2F-CF=CH-CHF2、CF3-CH2-CF=CH2、CF3-CHF-CH=CH2、CF3-CH2-CH=CHF、CHF2-CF2-CH=CH2、CHF2-CHF-CF=CH2、CHF2-CHF-CH=CHF、CH2F-CF2-CF=CH2、CH2F-CF2-CH=CHF、CH2F-CHF-CF=CHF、CH2F-CHF-CF=CF2、CH2F-CH2-CF=CF2、CH3-CF2-CF=CHF、CH3-CF2-CH=CF2等が挙げられる。塩素原子を有するハイドロフルオロオレフィン(すなわちハイドロクロロフルオロオレフィン)としては、例えば、CF3-CH=CHCl、CHF2-CF=CHCl、CHF2-CH=CFCl、CHF2-CCl=CHF、CH2F-CCl=CF2、CHFCl-CF=CHF、CH2Cl-CF=CF2、CF3-CCl=CH2等が挙げられる。特に好ましい塩素原子を有するハイドロフルオロオレフィンは、CF3-CH=CHClである。ハイドロフルオロオレフィン(ここには、ハイドロクロロフルオロオレフィンも含まれる。)は、これらのものを単独で又は2種以上組み合わせて使用することができる。
(hydrofluoroolefin)
By hydrofluoroolefin is intended a compound in which one or more hydrogen atoms of an olefin have been replaced with fluorine atoms. The number of fluorine atoms in the hydrofluoroolefin is not particularly limited, but may be 1 or more or 2 or more and 10 or less or 6 or less. Hydrofluoroolefins may be either E-type (trans-type) or Z-type (cis-type). The hydrofluoroolefin may be a hydrochlorofluoroolefin (HCFO). A hydrochlorofluoroolefin is a compound in which one or more hydrogen atoms of an olefin are substituted with fluorine atoms and one or more other hydrogen atoms of the olefin are substituted with chlorine atoms. intended to The number of chlorine atoms in the hydrochlorofluoroolefin is not particularly limited, but can be 1 or more, 5 or less, or 3 or less. Hydrofluoroolefins having no chlorine atom include, for example, CF 3 -CH=CH 2 , CF 3 -CF=CH 2 , CHF 2 -CH=CHF, CHF 2 -CF=CH 2 , CH 2 F-CH= CF2, CH2F-CF = CHF, CH3 - CF=CF2, CF3 - CH=CH- CF3 , CF3 -CH=CF- CH3 , CF3 -CF=CH- CH3 , CF 3 -CH=CH - CH2F, CHF2 - CF=CF- CH3 , CHF2 - CF=CH - CH2F, CHF2 - CH=CF - CH2F, CHF2 - CH=CH-CHF 2 , CH2F-CF=CF-CH2F, CH2F-CH=CH - CF3 , CH2F - CF=CH - CHF2 , CF3 - CH2 - CF= CH2 , CF3- CHF-CH= CH2 , CF3 - CH2 -CH=CHF, CHF2-CF2-CH= CH2 , CHF2 - CHF - CF= CH2 , CHF2 - CHF - CH = CHF, CH2F -CF2 - CF= CH2 , CH2F - CF2 - CH = CHF, CH2F-CHF-CF=CHF, CH2F-CHF-CF=CF2, CH2F - CH2 - CF= CF 2 , CH 3 -CF 2 -CF=CHF, CH 3 -CF 2 -CH=CF 2 and the like. Examples of hydrofluoroolefins having chlorine atoms (that is, hydrochlorofluoroolefins) include CF 3 —CH=CHCl, CHF 2 —CF=CHCl, CHF 2 —CH=CFCl, CHF 2 —CCl=CHF, CH 2 F -CCl=CF 2 , CHFCl-CF=CHF, CH 2 Cl-CF=CF 2 , CF 3 -CCl=CH 2 and the like. A particularly preferred hydrofluoroolefin having chlorine atoms is CF 3 —CH═CHCl. Hydrofluoroolefins (here, hydrochlorofluoroolefins are also included) can be used alone or in combination of two or more.
〈水〉
本開示のフッ素化液体の再生方法における水はいかなるものでもよく、次のものに限定されないが、水道水、蒸留水、イオン交換水などを使用することができる。
<water>
Any water can be used in the method of regenerating a fluorinated liquid of the present disclosure, including, but not limited to, tap water, distilled water, deionized water, and the like.
〈水接触工程〉
本開示のフッ素化液体の再生方法は、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が約80質量%以上にならないように水を接触させる工程(水接触工程)を備える。水接触工程における、上層に位置する水相の洗浄剤の濃度は、再生効率の観点等から、約75質量%以上にならない範囲、又は約70質量%以上にならない範囲とすることができる。係る洗浄剤の濃度の下限値は、特に限定されるものではないが、例えば、約10質量%以下にならない範囲、約15質量%以下にならない範囲、又は約20質量%以下にならない範囲とすることができる。ここで、上層に位置する水相中の洗浄剤濃度の測定は、例えば、上層の混合液から洗浄剤成分を抽出し、ガスクロマトグラフィー及び微量水分測定装置にて分析することにより測定することができる。
<Water contact process>
The method for regenerating a fluorinated liquid of the present disclosure includes a step of contacting a fluorinated liquid mixed with a detergent with water so that the concentration of the detergent in the upper aqueous phase does not exceed about 80% by mass (water contact process). In the water contacting step, the concentration of the cleaning agent in the upper aqueous phase can be set to a range of not more than about 75% by mass, or a range of not more than about 70% by mass, from the viewpoint of regeneration efficiency. The lower limit of the concentration of the cleaning agent is not particularly limited, but may be, for example, a range of about 10% by mass or less, a range of about 15% by mass or less, or a range of about 20% by mass or less. be able to. Here, the concentration of the detergent in the aqueous phase located in the upper layer can be measured, for example, by extracting the detergent component from the mixed liquid in the upper layer and analyzing it with a gas chromatography and a trace moisture analyzer. can.
洗浄剤が混ざったフッ素化液体に水を接触させる方法としては、次のものに限定されないが、例えば、以下の(1)~(7)の方法を、単独で又は二つ以上組み合わせて採用することができ、(1)~(7)の方法における一部を適宜組み合わせて実施することもできる。例えば、(1)又は(2)の方法に対し、(3)、(6)又は(7)に記載される、振動、撹拌子等を用いる物理的撹拌方法、空気を用いる撹拌方法、超音波を用いる撹拌方法などを適用してもよい。 The method of bringing water into contact with a fluorinated liquid mixed with a detergent is not limited to the following, but for example, the following methods (1) to (7) are employed alone or in combination of two or more. It is also possible to combine some of the methods (1) to (7) as appropriate. For example, for the method (1) or (2), physical stirring methods using vibration, stirrers, etc., stirring methods using air, ultrasonic waves, etc. described in (3), (6) or (7) You may apply the stirring method etc. which use.
(1)水が入っている容器に対し、係る容器の上方から、洗浄剤が混入したフッ素化液体を滴下していく方法。
(2)洗浄剤が混入したフッ素化液体が入っている容器に対し、係る容器の下方から、水を添加する方法。
(3)洗浄剤、フッ素化液体及び水の混合液が入っている容器を、振動、又は撹拌子若しくは撹拌羽根などを用いて物理的に撹拌する方法。
(4)洗浄剤、フッ素化液体及び水の混合液が入っている容器において、混合液がすでに二層に分離している状況下、上層及び下層を管等でつなぎ、上層液を重力又はポンプ等で下層へ移動させる方法。
(5)洗浄剤、フッ素化液体及び水の混合液が入っている容器において、混合液がすでに二層に分離している状況下、上層及び下層を管等でつなぎ、下層液を重力又はポンプ等で上層へ移動させる方法。
(6)洗浄剤、フッ素化液体及び水の混合液が入っている容器において、混合液がすでに二層に分離している状況下、該容器中に空気等の気体を吹き込みバブリングして、混合液を混ぜる方法。
(7)洗浄剤、フッ素化液体及び水の混合液が入っている容器において、混合液がすでに二層に分離している状況下、該容器中に超音波を適用して混合液を混ぜる方法。
(1) A method of dripping a fluorinated liquid mixed with a cleaning agent into a container containing water from above the container.
(2) A method of adding water to a container containing a fluorinated liquid mixed with a cleaning agent from below the container.
(3) A method of physically agitating a container containing a mixture of cleaning agent, fluorinated liquid and water, such as by vibrating or using a stirrer or impeller.
(4) In a container containing a mixture of cleaning agent, fluorinated liquid and water, under the condition that the mixture has already separated into two layers, the upper layer and the lower layer are connected with a pipe or the like, and the upper layer is removed by gravity or by a pump. etc. to move to the lower layer.
(5) In a container containing a mixture of cleaning agent, fluorinated liquid and water, under the condition that the mixture has already separated into two layers, the upper layer and the lower layer are connected with a pipe or the like, and the lower layer liquid is removed by gravity or by a pump. etc. to move to the upper layer.
(6) In a container containing a mixture of a cleaning agent, a fluorinated liquid and water, in a state where the mixture has already separated into two layers, a gas such as air is blown into the container to cause mixing. How to mix liquids
(7) A method of mixing a mixture of a cleaning agent, a fluorinated liquid and water by applying ultrasonic waves to the container in which the mixture has already separated into two layers. .
洗浄剤が混ざったフッ素化液体に水を接触させるときの温度及び時間としては、再生するフッ素化液体の純度等の要求性能に応じて変動し得るため、次のものに限定されないが、例えば、温度としては、約20℃以上、約23℃以上又は約25℃以上、約40℃以下、約35℃以下又は約30℃以下の範囲とすることができる。 The temperature and time at which water is brought into contact with the fluorinated liquid mixed with the cleaning agent may vary depending on the required performance such as the purity of the fluorinated liquid to be regenerated, and therefore are not limited to the following, for example: The temperature can range from about 20°C or higher, about 23°C or higher, or about 25°C or higher, to about 40°C or lower, about 35°C or lower, or about 30°C or lower.
〈分離・採取工程〉
本開示のフッ素化液体の再生方法は、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する工程を備える。上層及び下層の二液への分離は、上記の水接触工程を経た後、洗浄剤とフッ素化液体を含む混合液が静置される工程を経ることにより達成することができる。
<Separation/collection process>
In the method for regenerating a fluorinated liquid of the present disclosure, the mixed liquid after contact with water is separated into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase, and then the upper liquid is removed. , and a step of collecting the liquid in the lower layer. Separation into two liquids, the upper layer and the lower layer, can be achieved by passing through the step of allowing the liquid mixture containing the cleaning agent and the fluorinated liquid to stand after the above water contacting step.
下層液の採取は、例えば、混合液を含む容器の下方から管等を介して直接採取してもよく、又は、容器の上方から、上層液を採取し、次いで下層液を採取してもよく、或いは、容器の上方から容器底部付近まで管等を伸ばして吸引して採取してもよい。 The lower layer liquid may be collected, for example, directly from below the container containing the mixed liquid via a pipe or the like, or the upper layer liquid may be collected from above the container, and then the lower layer liquid may be collected. Alternatively, the sample may be collected by extending a tube or the like from the top of the container to near the bottom of the container and sucking.
この段階で採取された下層液の純度は、洗浄剤及びフッ素化液体の組合せなどによって変動し得るが、概ね、水接触工程前における約90%以下のフッ素化液体の純度を、約95%以上、約96%以上、又は約97%以上とすることができる。 The purity of the lower layer liquid collected at this stage may vary depending on the combination of detergent and fluorinated liquid, etc., but generally, the purity of the fluorinated liquid of about 90% or less before the water contacting step is reduced to about 95% or more. , about 96% or more, or about 97% or more.
〈任意の工程〉
本開示のフッ素化液体の再生方法は、任意に、蒸留工程(例えば、沸騰蒸留工程、減圧蒸留工程など)、冷却分離工程などの工程を単独で又は二つ以上組み合わせて適宜適用することができる。
<Optional process>
Optionally, the method for regenerating a fluorinated liquid of the present disclosure can be appropriately applied to a distillation process (e.g., a boiling distillation process, a vacuum distillation process, etc.), a cooling separation process, etc. alone or in combination of two or more. .
任意の工程の中でも、再生したフッ素化液体の純度をより高めたい場合には、下層液を採取した後に、係る下層液に対して蒸留工程を適用することが好ましい。蒸留工程における蒸留温度は、次のものに限定されないが、例えば、約70℃以上、約72℃以上、又は約75℃以上とすることができ、約100℃以下、約95℃以下、又は約90℃以下とすることができる。蒸留によって採取された液中のフッ素化液体の純度としては、洗浄剤及びフッ素化液体の組合せなどによって変動し得るが、概ね、約99.0%以上、約99.2%以上、又は約99.4%以上の純度を達成することができる。 Among the optional steps, when the purity of the regenerated fluorinated liquid is desired to be higher, it is preferable to apply a distillation step to the lower layer liquid after collecting the lower layer liquid. The distillation temperature in the distillation step can be, for example, but not limited to, about 70° C. or higher, about 72° C. or higher, or about 75° C. or higher, and about 100° C. or lower, about 95° C. or lower, or about It can be 90° C. or less. The purity of the fluorinated liquid in the liquid collected by distillation may vary depending on the combination of detergent and fluorinated liquid, but is generally about 99.0% or higher, about 99.2% or higher, or about 99%. Purities of 0.4% and above can be achieved.
《フッ素化液体再生装置》
本開示の一実施態様のフッ素化液体再生装置は、洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が80質量%以上にならないように水を接触させる手段(以下、「水接触手段」という場合がある。)と、水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する手段(以下、「分離・採取手段」という場合がある。)と、を備えており、ここで用いる洗浄剤は、フッ素化液体に溶解する非プロトン性極性溶媒であり、且つフッ素化液体は、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である。係る再生装置における洗浄剤、フッ素化液体及び水については、上述した再生方法におけるものと同一のものを挙げることができる。
《Fluorinated liquid regeneration device》
The fluorinated liquid regenerating apparatus of one embodiment of the present disclosure includes a means ( Hereinafter, it may be referred to as "water contacting means"), and after the mixed liquid after contacting with water is separated into two liquids, an aqueous phase located in the upper layer and a phase containing the fluorinated liquid located in the lower layer, the upper layer means for removing the liquid and collecting the liquid in the lower layer (hereinafter sometimes referred to as "separation/collection means"), and the cleaning agent used here is an aprotic Polar solvents and fluorinated liquids are hydrofluoroethers, hydrofluoroolefins, or mixtures thereof. The cleaning agent, fluorinated liquid and water in the regeneration device may be the same as in the regeneration method described above.
〈水接触手段〉
本開示のフッ素化液体再生装置における水接触手段は、上述したフッ素化液体の再生方法における水接触工程を適用し得る手段であればいかなるものも採用することができ、例えば、洗浄剤、フッ素化液体、及び水を含む混合液を収容する容器(「槽」などと称する場合もある。)の材質、容量、形状、数量、配置箇所などについては、装置の使用用途又は使用環境などに応じて適宜選択することができる。
<Means for contacting water>
As the water contacting means in the fluorinated liquid regenerating apparatus of the present disclosure, any means can be adopted as long as the water contacting step in the method for regenerating the fluorinated liquid described above can be applied. The material, capacity, shape, quantity, location, etc. of the container (sometimes referred to as a “tank”) that holds the liquid and mixed liquid containing water will depend on the intended use or operating environment of the device. It can be selected as appropriate.
〈分離・採取手段〉
本開示のフッ素化液体再生装置における分離・採取手段も、上述したフッ素化液体の再生方法における分離・採取工程を適用し得る手段であればいかなるものも採用することができ、例えば、分離液を収容する容器(「槽」などと称する場合もある。)の材質、容量、形状、数量、配置箇所などについては、装置の使用用途又は使用環境などに応じて適宜選択することができる。
<Separation/collection means>
As the separation/collection means in the fluorinated liquid regeneration apparatus of the present disclosure, any means can be adopted as long as the separation/collection steps in the above-described fluorinated liquid regeneration method can be applied. The material, capacity, shape, quantity, arrangement location, etc. of the container (sometimes referred to as a "tank") can be appropriately selected according to the intended use or use environment of the apparatus.
〈任意の手段〉
本開示のフッ素化液体再生装置は、上述したフッ素化液体の再生方法における、蒸留工程(例えば、沸騰蒸留工程、減圧蒸留工程など)、冷却分離工程などの任意の工程を適用し得る手段であればいかなるものも採用することができ、例えば、蒸留工程で使用される下層液を貯留する容器などの材質、容量、形状、数量、配置箇所などについては、装置の使用用途又は使用環境などに応じて適宜選択することができる。蒸留手段、冷却分離手段等の各種手段は、単独で又は二つ以上組み合わせてフッ素化液体再生装置に対して適用することができる。
<Arbitrary means>
The apparatus for regenerating a fluorinated liquid of the present disclosure is any means that can apply any process such as a distillation process (e.g., a boiling distillation process, a vacuum distillation process, etc.), a cooling separation process, etc. in the above-described fluorinated liquid regeneration method. For example, the material, capacity, shape, quantity, location, etc. of the container for storing the lower layer liquid used in the distillation process will depend on the intended use or operating environment of the device. can be selected as appropriate. Various means, such as distillation means, cooling separation means, etc., can be applied to the fluorinated liquid regenerator either singly or in combination of two or more.
任意の手段の中でも、再生したフッ素化液体の純度をより高めたい場合には、下層液を採取した後に、係る下層液を蒸留する蒸留手段を追加することが好ましい。蒸留手段には、例えば、採取された下層液を貯留し加熱する蒸留釜と、蒸留釜に連通接続され、係る下層液の蒸気を凝縮液化させる冷却器とを備える従来の装置を使用することができる。 Among the optional means, when it is desired to further increase the purity of the regenerated fluorinated liquid, it is preferable to add distillation means for distilling the lower layer liquid after collecting the lower layer liquid. As the distillation means, for example, a conventional apparatus comprising a distillation pot for storing and heating the collected lower layer liquid and a cooler connected to the distillation pot for condensing and liquefying the vapor of the lower layer liquid can be used. can.
《再生したフッ素化液体の使用用途》
本開示のフッ素化液体の再生方法及び再生装置は、例えば、有機ELディスプレイ製造工程などにおいてオンライン又オフラインで使用することができる。オンラインにおいて、本開示のフッ素化液体の再生方法及び再生装置を使用する場合には、これらは、再生したフッ素化液体を洗浄工程に再度投入し得るように適宜構成されていればよい。オフラインでこれらを使用する場合には、再生したフッ素化液体を、有機ELディスプレイ製造工程の洗浄工程で再度使用することができる一方で、係る用途とは別の用途、例えば、プリント配線板のリンス液用として再利用することもできる。
<<Usage of Recycled Fluorinated Liquid>>
The method and apparatus for regenerating a fluorinated liquid according to the present disclosure can be used online or offline, for example, in an organic EL display manufacturing process. When the method and apparatus for regenerating a fluorinated liquid of the present disclosure are used on-line, they need only be appropriately configured so that the regenerated fluorinated liquid can be reintroduced into the cleaning process. When using these offline, the regenerated fluorinated liquid can be reused in the cleaning process of the organic EL display manufacturing process, while other uses such as rinsing printed wiring boards can be used. It can also be reused for liquids.
本開示のフッ素化液体の再生方法及び再生装置から得られた再生したフッ素化液体は、次のものに限定されないが、例えば、有機ELディスプレイ製造装置で使用され、洗浄及びリンス作業に晒されるメタルマスク、防着板などの各種部材用のリンス液の他、各種の電子部品、精密部品、金属部品、プリント配線基板等のリンス液などとして使用することができる。ここで、防着板とは、例えば、有機ELディスプレイの製造時に使用される真空蒸着装置の真空チャンバーの内側に配置される部材であって、蒸発源であるRGB3色の色素から真空チャンバーの汚染を防止するための、取り外して洗浄することが可能な部材である。リンス液としての使用とは、例えば、被洗浄物を浸漬させて付着している洗浄剤等をすすぎ落とす、液体としての直接的な使用に限らず、リンス液を蒸発させて被洗浄物表面に係る蒸発ガスを付着させて洗浄剤等をすすぎ落とす間接的な使用なども包含する。 The regenerated fluorinated liquid obtained from the fluorinated liquid regeneration method and apparatus of the present disclosure includes, but is not limited to, metals used in organic EL display manufacturing equipment and exposed to cleaning and rinsing operations. It can be used as a rinse for various members such as a mask and an anti-adhesion plate, as well as a rinse for various electronic parts, precision parts, metal parts, printed wiring boards, and the like. Here, the anti-adhesion plate is, for example, a member arranged inside the vacuum chamber of a vacuum deposition apparatus used in the manufacture of an organic EL display, and the vacuum chamber is contaminated from the three colors of RGB dyes that are evaporation sources. It is a member that can be removed and washed to prevent The use as a rinsing liquid is not limited to direct use as a liquid, for example, rinsing off the cleaning agent adhering to the object to be washed by immersing it, but evaporating the rinsing liquid to the surface of the object to be washed. It also includes indirect use such as rinsing off the cleaning agent or the like by adhering the evaporative gas.
《実施例1~22及び比較例1~3》
以下の実施例において、本開示の具体的な実施態様を例示するが、本開示はこれに限定されるものではない。
<<Examples 1 to 22 and Comparative Examples 1 to 3>>
The following examples illustrate specific embodiments of the disclosure, but the disclosure is not limited thereto.
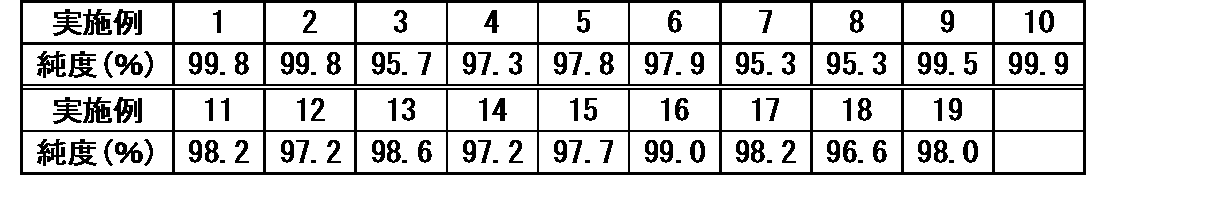
本実施例で使用した商品などを以下の表1に示す。 The products used in this example are shown in Table 1 below.
〈評価方法〉
採取した液体について下記の評価を実施した。
<Evaluation method>
The following evaluations were performed on the sampled liquid.
(純度の評価)
再生したフッ素化液体の純度を、Agilent Technologies社製の7890Aを用い、ガスクロマトグラフィー法により評価した。ガスクロマトグラフィー法の測定条件は以下のとおりである。
カラムの種類:HP-1301
カラムの長さ:60m
カラムの温度:260℃
キャリアガスの種類:ヘリウムガス
キャリアガスの流量:205mL/分
サンプル注入量:1μL
(Purity evaluation)
Purity of the regenerated fluorinated liquid was assessed by gas chromatography using Agilent Technologies 7890A. The measurement conditions for the gas chromatography method are as follows.
Column type: HP-1301
Column length: 60m
Column temperature: 260°C
Carrier gas type: helium gas Carrier gas flow rate: 205 mL/min Sample injection volume: 1 μL
(水分の評価)
水接触工程後に採取した下層液中の水分量を、三菱化学株式会社製の微量水分測定装置を用いて測定した。
(Evaluation of moisture content)
The amount of water in the lower layer liquid sampled after the water contact step was measured using a micro-moisture measuring device manufactured by Mitsubishi Chemical Corporation.
〈試験1:水接触工程後の各種フッ素化液体の純度〉
(実施例1)
サンプル瓶に、100gのNOVEC(商標)7100(フッ素化液体)及び10gのNMP(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を40g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体であるNOVEC(商標)7100の純度を測定した。その結果を表2に示す。なお、非プロトン性極性溶媒の洗浄剤は、フッ素化液体よりも蒸留水側に移行し易いが、蒸留水及び洗浄剤の合計量に対する洗浄剤量の割合(以下、「水中の洗浄剤濃度」という場合がある。)は20質量%であるため、上層に位置する水相の洗浄剤の濃度は20質量%を超えることはない。
<Test 1: Purity of various fluorinated liquids after water contact step>
(Example 1)
100 g of NOVEC™ 7100 (fluorinated liquid) and 10 g of NMP (cleaning agent) were each added to the sample bottle and shaken for 30 minutes. 40 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, and the purity of the fluorinated liquid NOVEC (trademark) 7100 in the lower layer liquid was measured. Table 2 shows the results. The aprotic polar solvent cleaning agent is more likely to migrate to the distilled water side than the fluorinated liquid. ) is 20% by mass, the concentration of the cleaning agent in the upper aqueous phase does not exceed 20% by mass.
(実施例2)
NOVEC(商標)7100に代えて、NOVEC(商標)7200を使用したこと以外は、実施例1と同様にして純度を測定した。
(Example 2)
Purity was measured in the same manner as in Example 1, except that NOVEC™7200 was used instead of NOVEC™7100.
(実施例3)
NOVEC(商標)7100に代えて、1233Zを使用したこと以外は、実施例1と同様にして純度を測定した。
(Example 3)
Purity was determined in the same manner as in Example 1, except that NOVEC™ 7100 was replaced with 1233Z.
(実施例4)
NOVEC(商標)7100に代えて、アサヒクリン(商標)AE3000を使用したこと以外は、実施例1と同様にして純度を測定した。
(Example 4)
Purity was measured in the same manner as in Example 1, except that Asahiklin (trademark) AE3000 was used instead of NOVEC (trademark) 7100.
(実施例5)
サンプル瓶に、100gのNOVEC(商標)7100(フッ素化液体)及び5gのNBP(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を10g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体であるNOVEC(商標)7100の純度を測定した。その結果を表2に示す。なお、本実施例の態様における水中の洗浄剤濃度は33.3質量%であるため、上層に位置する水相の洗浄剤の濃度は33.3質量%を超えることはない。
(Example 5)
100 g of NOVEC™ 7100 (fluorinated liquid) and 5 g of NBP (cleaning agent) were each added to the sample bottle and shaken for 30 minutes. 10 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, and the purity of the fluorinated liquid NOVEC (trademark) 7100 in the lower layer liquid was measured. Table 2 shows the results. In addition, since the concentration of the cleaning agent in water in the aspect of this embodiment is 33.3% by mass, the concentration of the cleaning agent in the aqueous phase positioned in the upper layer does not exceed 33.3% by mass.
(実施例6)
NOVEC(商標)7100に代えて、NOVEC(商標)7200を使用したこと以外は、実施例5と同様にして純度を測定した。
(Example 6)
Purity was measured in the same manner as in Example 5, except that NOVEC™7200 was used instead of NOVEC™7100.
(実施例7)
NOVEC(商標)7100に代えて、1233Zを使用したこと以外は、実施例5と同様にして純度を測定した。
(Example 7)
Purity was determined in the same manner as in Example 5, except that NOVEC™ 7100 was replaced with 1233Z.
(実施例8)
NOVEC(商標)7100に代えて、アサヒクリン(商標)AE3000を使用したこと以外は、実施例5と同様にして純度を測定した。
(Example 8)
Purity was measured in the same manner as in Example 5 except that Asahiklin (trademark) AE3000 was used instead of NOVEC (trademark) 7100.
(実施例9)
サンプル瓶に、100gのNOVEC(商標)7100(フッ素化液体)及び10gのTETRAGLYME(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を80g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体であるNOVEC(商標)7100の純度を測定した。その結果を表2に示す。なお、本実施例の態様における水中の洗浄剤濃度は11.1質量%であるため、上層に位置する水相の洗浄剤の濃度は11.1質量%を超えることはない。
(Example 9)
100 g of NOVEC™ 7100 (fluorinated liquid) and 10 g of TETRAGLYME (cleaner) were each added to the sample bottle and shaken for 30 minutes. 80 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, and the purity of the fluorinated liquid NOVEC (trademark) 7100 in the lower layer liquid was measured. Table 2 shows the results. In addition, since the concentration of the detergent in water in the aspect of this example is 11.1% by mass, the concentration of the detergent in the aqueous phase positioned in the upper layer does not exceed 11.1% by mass.
(実施例10)
NOVEC(商標)7100に代えて、NOVEC(商標)7200を使用したこと以外は、実施例9と同様にして純度を測定した。
(Example 10)
Purity was measured in the same manner as in Example 9, except that NOVEC™7200 was used instead of NOVEC™7100.
(実施例11)
NOVEC(商標)7100に代えて、アサヒクリン(商標)AE3000を使用したこと以外は、実施例9と同様にして純度を測定した。
(Example 11)
Purity was measured in the same manner as in Example 9, except that Asahiklin (trademark) AE3000 was used instead of NOVEC (trademark) 7100.
(実施例12)
サンプル瓶に、100gのNOVEC(商標)7100(フッ素化液体)及び10gのAC(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を80g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体であるNOVEC(商標)7100の純度を測定した。その結果を表2に示す。なお、本実施例の態様における水中の洗浄剤濃度は11.1質量%であるため、上層に位置する水相の洗浄剤の濃度は11.1質量%を超えることはない。
(Example 12)
To the sample bottle, 100 g of NOVEC™ 7100 (fluorinated liquid) and 10 g of AC (cleaner) were each added and shaken for 30 minutes. 80 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, and the purity of the fluorinated liquid NOVEC (trademark) 7100 in the lower layer liquid was measured. Table 2 shows the results. In addition, since the concentration of the detergent in water in the aspect of this example is 11.1% by mass, the concentration of the detergent in the aqueous phase positioned in the upper layer does not exceed 11.1% by mass.
(実施例13)
NOVEC(商標)7100に代えて、NOVEC(商標)7200を使用したこと以外は、実施例12と同様にして純度を測定した。
(Example 13)
Purity was measured in the same manner as in Example 12, except that NOVEC™7200 was used instead of NOVEC™7100.
(実施例14)
サンプル瓶に、100gの1233Z(フッ素化液体)及び10gのDMSO(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を10g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体である1233Zの純度を測定した。その結果を表2に示す。なお、本実施例の態様における水中の洗浄剤濃度は50質量%であるため、上層に位置する水相の洗浄剤の濃度は50質量%を超えることはない。
(Example 14)
100 g of 1233Z (fluorinated liquid) and 10 g of DMSO (detergent) were each added to the sample bottle and shaken for 30 minutes. 10 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was sampled, and the purity of 1233Z, which is a fluorinated liquid, in the lower layer liquid was measured. Table 2 shows the results. In addition, since the concentration of the detergent in water in the aspect of the present embodiment is 50% by mass, the concentration of the detergent in the aqueous phase positioned in the upper layer does not exceed 50% by mass.
(実施例15)
1233Zに代えて、アサヒクリン(商標)AE3000を使用したこと以外は、実施例14と同様にして純度を測定した。
(Example 15)
Purity was measured in the same manner as in Example 14, except that Asahiklin (trademark) AE3000 was used instead of 1233Z.
(実施例16)
サンプル瓶に、100gのNOVEC(商標)7100(フッ素化液体)及び10gのDMF(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を10g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体であるNOVEC(商標)7100の純度を測定した。その結果を表2に示す。なお、本実施例の態様における水中の洗浄剤濃度は50質量%であるため、上層に位置する水相の洗浄剤の濃度は50質量%を超えることはない。
(Example 16)
To the sample bottle, 100 g of NOVEC™ 7100 (fluorinated liquid) and 10 g of DMF (detergent) were each added and shaken for 30 minutes. 10 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, and the purity of the fluorinated liquid NOVEC (trademark) 7100 in the lower layer liquid was measured. Table 2 shows the results. In addition, since the concentration of the detergent in water in the aspect of the present embodiment is 50% by mass, the concentration of the detergent in the aqueous phase positioned in the upper layer does not exceed 50% by mass.
(実施例17)
NOVEC(商標)7100に代えて、NOVEC(商標)7200を使用したこと以外は、実施例16と同様にして純度を測定した。
(Example 17)
Purity was measured in the same manner as in Example 16, except that NOVEC™7200 was used instead of NOVEC™7100.
(実施例18)
NOVEC(商標)7100に代えて、1233Zを使用したこと以外は、実施例16と同様にして純度を測定した。
(Example 18)
Purity was determined in the same manner as in Example 16, except that NOVEC™ 7100 was replaced with 1233Z.
(実施例19)
サンプル瓶に、100gのアサヒクリン(商標)AE3000(フッ素化液体)及び10gのDMF(洗浄剤)を各々添加し、30分間振とうした。この混合液に対して蒸留水を80g添加し、30分間さらに振とうした。次いで、得られた混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、下層液中のフッ素化液体であるアサヒクリン(商標)AE3000の純度を測定した。その結果を表2に示す。なお、本実施例の態様における水中の洗浄剤濃度は11.1質量%であるため、上層に位置する水相の洗浄剤の濃度は11.1質量%を超えることはない。
(Example 19)
100 g of Asahiklin™ AE3000 (fluorinated liquid) and 10 g of DMF (cleaning agent) were each added to the sample bottle and shaken for 30 minutes. 80 g of distilled water was added to this mixed solution, and the mixture was further shaken for 30 minutes. The resulting mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, and the purity of Asahiklin (trademark) AE3000, which is a fluorinated liquid in the lower layer liquid, was measured. Table 2 shows the results. In addition, since the concentration of the detergent in water in the aspect of this example is 11.1% by mass, the concentration of the detergent in the aqueous phase positioned in the upper layer does not exceed 11.1% by mass.
〈結果〉
表2の結果から明らかなように、本開示のフッ素化液体の再生方法を使用すると、単に水と接触させてだけでも、フッ素化液体を約95%以上の純度で再生し得ることが確認できた。
<result>
As can be seen from the results in Table 2, it can be confirmed that the fluorinated liquid can be regenerated to a purity of about 95% or more by simply contacting it with water using the method for regenerating the fluorinated liquid of the present disclosure. rice field.
〈試験2:水接触工程後の各種フッ素化液体の再生具合〉
(実施例20)
蒸留水の添加量を、2.5g(水中の洗浄剤濃度:80.0質量%)、5g(水中の洗浄剤濃度:66.7質量%)、10g(水中の洗浄剤濃度:50.0質量%)、20g(水中の洗浄剤濃度:33.3質量%)、40g(水中の洗浄剤濃度:20.0質量%)、60g(水中の洗浄剤濃度:14.3質量%)、80g(水中の洗浄剤濃度:11.1質量%)とふって水接触工程を各々実施したこと以外は、実施例1と同様にして純度(%)を測定し、さらに採取した下層液の産出量(g)も測定した。測定した純度及び産出量を乗じた値(以下、「再生値」という場合がある。)と、水中の洗浄剤濃度(質量%)とに基づくグラフを図1に示す。ここで、図1に関し、再生値の値が高くなるほど、フッ素化液体の再生具合が良好であることを意図する。
<Test 2: Condition of regeneration of various fluorinated liquids after contact with water>
(Example 20)
The amount of distilled water added was 2.5 g (concentration of detergent in water: 80.0% by mass), 5 g (concentration of detergent in water: 66.7% by mass), 10 g (concentration of detergent in water: 50.0%). % by mass), 20 g (concentration of detergent in water: 33.3% by mass), 40 g (concentration of detergent in water: 20.0% by mass), 60 g (concentration of detergent in water: 14.3% by mass), 80 g Purity (%) was measured in the same manner as in Example 1, except that the water contacting step was performed with (concentration of detergent in water: 11.1% by mass), and the yield of the sampled lower layer liquid. (g) was also measured. FIG. 1 shows a graph based on the value obtained by multiplying the measured purity and production amount (hereinafter sometimes referred to as "regeneration value") and the detergent concentration (% by mass) in water. Now, referring to FIG. 1, it is intended that the higher the regeneration value, the better the regeneration of the fluorinated liquid.
(実施例21)
NOVEC(商標)7100に代えて、NOVEC(商標)7200を使用したこと以外は、実施例20と同様にして純度及び産出量を測定し、それらの結果に基づくグラフを図1に示す。
(Example 21)
Purity and yield were measured in the same manner as in Example 20 except that NOVEC™ 7200 was used instead of NOVEC™ 7100, and a graph based on these results is shown in FIG.
(比較例1)
NOVEC(商標)7100に代えて、VERTREL(商標)XFを使用したこと以外は、実施例20と同様にして純度及び産出量を測定し、それらの結果に基づくグラフを図1に示す。
(Comparative example 1)
Purity and yield were measured in the same manner as in Example 20, except that VERTREL™ XF was used instead of NOVEC™ 7100, and a graph based on these results is shown in FIG.
(比較例2)
NOVEC(商標)7100に代えて、アサヒクリン(商標)AK-225を使用したこと以外は、実施例20と同様にして純度及び産出量を測定し、それらの結果に基づくグラフを図1に示す。
(Comparative example 2)
Purity and yield were measured in the same manner as in Example 20 except that Asahiklin (trademark) AK-225 was used instead of NOVEC (trademark) 7100, and a graph based on these results is shown in FIG. .
〈結果〉
図1の結果から明らかなように、本開示の再生方法に相当する実施例20及び21の態様の方が、ハイドロフルオロカーボン及びハイドロクロロフルオロカーボンを使用する比較例1及び2の態様に比べ、いずれも再生値の値が高くなっていることから、水接触工程による再生は、ハイドロフルオロカーボン及びハイドロクロロフルオロカーボン以外のフッ素化液体に対して有意に作用するということが確認できた。特に、実施例20及び21の態様に関しては、水中の洗浄剤濃度が、約30.0質量%付近から約60.0質量%付近において、再生値がより優れることが確認された。これは、上層に位置する水相の洗浄剤の濃度が、概ね、約30.0質量%以下とならず、かつ、約60.0質量%以上にならないように水を接触させた場合に相当する。
<result>
As is clear from the results in FIG. 1, the embodiments of Examples 20 and 21, which correspond to the regeneration method of the present disclosure, are better than the embodiments of Comparative Examples 1 and 2 using hydrofluorocarbons and hydrochlorofluorocarbons. The higher regeneration value confirms that the regeneration by the water contact step works significantly for fluorinated liquids other than hydrofluorocarbons and hydrochlorofluorocarbons. In particular, with respect to the embodiments of Examples 20 and 21, it was confirmed that the regeneration value was superior when the detergent concentration in water was about 30.0% by mass to about 60.0% by mass. This corresponds to the case where water is brought into contact so that the concentration of the cleaning agent in the aqueous phase located in the upper layer is generally not less than about 30.0% by mass and not more than about 60.0% by mass. do.
〈試験3:水接触工程及び蒸留工程の組合せ〉
(実施例22)
サンプル瓶に、100gのアサヒクリン(商標)AE3000(HFE-347pc-f)及び10gのNMPを各々添加し、30分間振とうした。この混合液に対して蒸留水を40g添加し、30分間さらに振とうし、アサヒクリン(商標)AE3000(HFE-347pc-f)、水及びNMPが、100:40:10の割合で含まれる混合液を作製した。次いで、係る混合液を分液漏斗に移し、混合液が上層及び下層の二層に分離するまで静置した。二層に分離した液の下層液を採取し、実験レベルで使用される一般的な蒸留装置の蒸留フラスコ内に下層液を移し、約80℃の温度で蒸留を開始した。リービッヒ冷却器の入り口付近に設置した温度計の温度が下降した時点を終了のタイミングとし、再生したフッ素化液体と、廃棄する残渣液とに分離した。一連の流れ及びその結果を図2の右側に示す。なお、再生したフッ素化液体の量と、残渣液中に含まれるフッ素化液体の量との合計が100gに満たなかったのは実験誤差であると考える。
<Test 3: Combination of water contact step and distillation step>
(Example 22)
100 g of Asahiklin™ AE3000 (HFE-347pc-f) and 10 g of NMP were each added to the sample bottle and shaken for 30 minutes. Add 40 g of distilled water to this mixed solution, shake further for 30 minutes, and mix Asahiklin (trademark) AE3000 (HFE-347pc-f), water and NMP in a ratio of 100: 40: 10 A liquid was prepared. The mixture was then transferred to a separatory funnel and allowed to stand until the mixture separated into two layers, an upper layer and a lower layer. The lower layer liquid of the liquid separated into two layers was collected, transferred into a distillation flask of a general distillation apparatus used at the experimental level, and distillation was started at a temperature of about 80°C. When the temperature of the thermometer installed near the entrance of the Liebig cooler dropped, the timing was set to end, and the regenerated fluorinated liquid was separated from the residual liquid to be discarded. A series of flows and their results are shown on the right side of FIG. It should be noted that the fact that the sum of the amount of the regenerated fluorinated liquid and the amount of the fluorinated liquid contained in the residual liquid was less than 100 g is considered to be an experimental error.
(比較例3)
サンプル瓶に、80gのアサヒクリン(商標)AE3000(HFE-347pc-f)及び20gのNMPを各々添加し、30分間振とうした。この混合液を実施例22と同一の蒸留装置及び蒸留条件で蒸留を実施し、再生したフッ素化液体と、廃棄する残渣液とに分離した。一連の流れ及びその結果を図2の左側に示す。
(Comparative Example 3)
80 g of Asahiklin™ AE3000 (HFE-347pc-f) and 20 g of NMP were each added to the sample bottle and shaken for 30 minutes. This mixed liquid was distilled using the same distillation apparatus and under the same distillation conditions as in Example 22 to separate the regenerated fluorinated liquid and the residual liquid to be discarded. A series of flows and their results are shown on the left side of FIG.
〈結果〉
フッ素化液体と洗浄剤とを含む混合液を約80℃の温度で蒸留した場合、残渣には、洗浄剤が約30%、フッ素化液体が約70%の割合で含まれることが、検量線等を利用して導き出せる。蒸留のみを使用する従来のフッ素化液体の再生方法である比較例3の場合には、約80℃の温度で蒸留すると、図2の左側に示されるように、33.3gのフッ素化液体(HFE-347pc-f)しか再生できず、残りの46.7gのフッ素化液体は洗浄剤(NMP)と分離できないため廃棄せざるを得なかった。即ち、廃棄しなければならないフッ素化液体の量は、再生前の混合液に含まれるフッ素化液体の58.4%にも及んでいた。一方、蒸留工程を採用する本開示のフッ素化液体の再生方法である実施例22の場合には、水接触工程及び分離・採取工程を経て採取された下層液には、洗浄剤の大部分が既に取り除かれているため、約80℃の温度で蒸留すると、図2の右側に示されるように、92.6gのフッ素化液体を再生することができ、廃棄するフッ素化液体は5.8gと極めて少量に抑えることができた。即ち、廃棄しなければならないフッ素化液体の量(この量は、残渣中のフッ素化液体の量5.8gに加え、誤差分の1.6gも含む。)は、再生前の混合液に含まれるフッ素化液体のわずか7.4%であった。したがって、蒸留工程を採用する本開示のフッ素化液体の再生方法は、従来の蒸留のみを使用する再生方法に比べて、廃棄するフッ素化液体の量を87.3%も削減することが確認できた。
<result>
A calibration curve shows that when a mixture containing a fluorinated liquid and a detergent is distilled at a temperature of about 80° C., the residue contains about 30% detergent and about 70% fluorinated liquid. etc., can be used to derive In the case of Comparative Example 3, which is a conventional method of regenerating a fluorinated liquid using distillation only, distillation at a temperature of about 80° C. yields 33.3 g of fluorinated liquid ( Only HFE-347pc-f) could be regenerated and the remaining 46.7 g of fluorinated liquid had to be discarded as it could not be separated from the cleaning agent (NMP). That is, the amount of fluorinated liquid that had to be discarded was as much as 58.4% of the fluorinated liquid contained in the mixed liquid before regeneration. On the other hand, in the case of Example 22, which is the method for regenerating a fluorinated liquid of the present disclosure that employs a distillation step, the lower layer liquid collected through the water contacting step and the separation/collecting step contains most of the detergent. Since it has already been removed, distillation at a temperature of about 80° C. can recycle 92.6 g of fluorinated liquid, as shown on the right side of FIG. I was able to keep it to a very small amount. That is, the amount of fluorinated liquid that must be discarded (this amount includes the amount of fluorinated liquid in the residue of 5.8 g plus an error of 1.6 g) is included in the mixed liquid before regeneration. was only 7.4% of the fluorinated liquids used. Therefore, it can be confirmed that the disclosed fluorinated liquid regeneration method employing a distillation process reduces the amount of fluorinated liquid to be disposed of by as much as 87.3% compared to the conventional regeneration method using distillation alone. rice field.
本発明の基本的な原理から逸脱することなく、上記の実施態様及び実施例が様々に変更可能であることは当業者に明らかである。また、本発明の様々な改良及び変更が本発明の趣旨及び範囲から逸脱せずに実施できることは当業者には明らかである。
本開示の実施態様の一部を以下の[項目1]-[項目9]に記載する。
[項目1]
洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が80質量%以上にならないように水を接触させる工程と、
水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する工程と、を備える、フッ素化液体の再生方法であって、
前記洗浄剤が、前記フッ素化液体に溶解する非プロトン性極性溶媒であり、且つ前記フッ素化液体が、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である、再生方法。
[項目2]
前記非プロトン性極性溶媒が、環状アミド系溶媒、アミン系溶媒、グリコールエーテル系溶媒、アセトン、ジメチルスルホキシド、ジメチルホルムアミド又はこれらの混合溶媒である、項目1に記載の再生方法。
[項目3]
採取された下層の液中のフッ素化液体の純度が95%以上である、項目1又は2に記載の再生方法。
[項目4]
下層の液を採取する工程に続いて、前記下層の液を蒸留する工程を更に備える、項目1又は2に記載の再生方法。
[項目5]
蒸留によって採取された液中のフッ素化液体の純度が99.0%以上である、項目4に記載の再生方法。
[項目6]
項目1~5のいずれか一項に記載の再生方法を用いて再生されたフッ素化液体を、有機ELディスプレイ製造装置で使用される部材用のリンス液として使用する方法。
[項目7]
前記部材が、メタルマスク又は防着板である、項目6に記載の方法。
[項目8]
洗浄剤が混ざったフッ素化液体に、上層に位置する水相の洗浄剤の濃度が80質量%以上にならないように水を接触させる手段と、
水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する手段と、を備える、フッ素化液体再生装置であって、
前記洗浄剤が、前記フッ素化液体に溶解する非プロトン性極性溶媒であり、且つ前記フッ素化液体が、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である、フッ素化液体再生装置。
[項目9]
下層の液を採取する手段に続いて、前記下層の液を蒸留する手段を更に備える、項目8に記載のフッ素化液体再生装置。
It will be apparent to those skilled in the art that various modifications may be made to the above-described embodiments and examples without departing from the underlying principles of the invention. In addition, it will be apparent to those skilled in the art that various modifications and alterations of this invention can be made without departing from its spirit and scope.
Some of the embodiments of the present disclosure are described in [Item 1]-[Item 9] below.
[Item 1]
a step of contacting the fluorinated liquid mixed with the detergent with water such that the concentration of the detergent in the upper aqueous phase does not exceed 80 mass %;
a step of separating the mixed liquid after contact with water into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase, removing the upper liquid and collecting the lower liquid; A method of regenerating a fluorinated liquid comprising:
A regeneration method, wherein the cleaning agent is an aprotic polar solvent that dissolves in the fluorinated liquid, and the fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof.
[Item 2]
The regeneration method according to item 1, wherein the aprotic polar solvent is a cyclic amide solvent, an amine solvent, a glycol ether solvent, acetone, dimethylsulfoxide, dimethylformamide, or a mixed solvent thereof.
[Item 3]
3. A regeneration method according to item 1 or 2, wherein the purity of the fluorinated liquid in the collected lower layer liquid is 95% or higher.
[Item 4]
3. The regeneration method according to item 1 or 2, further comprising a step of distilling the lower layer liquid following the step of collecting the lower layer liquid.
[Item 5]
5. A regeneration method according to item 4, wherein the purity of the fluorinated liquid in the liquid collected by distillation is 99.0% or higher.
[Item 6]
A method of using the fluorinated liquid regenerated by the regeneration method according to any one of items 1 to 5 as a rinsing liquid for members used in an organic EL display manufacturing apparatus.
[Item 7]
A method according to item 6, wherein the member is a metal mask or an anti-adhesion plate.
[Item 8]
means for bringing water into contact with the fluorinated liquid mixed with the detergent so that the concentration of the detergent in the upper aqueous phase does not exceed 80 mass %;
a means for removing the upper layer liquid and collecting the lower layer liquid after the mixed liquid after contact with water is separated into two liquids, namely, an upper aqueous phase and a lower fluorinated liquid-containing phase; A fluorinated liquid regeneration device comprising:
A fluorinated liquid regenerator, wherein said cleaning agent is an aprotic polar solvent soluble in said fluorinated liquid, and said fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof.
[Item 9]
9. A fluorinated liquid regeneration apparatus according to item 8, further comprising means for distilling said underlayer liquid subsequent to said means for collecting said underlayer liquid.
Claims (9)
水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する工程と、を備える、フッ素化液体の再生方法であって、
前記洗浄剤が、前記フッ素化液体に溶解する非プロトン性極性溶媒であり、且つ前記フッ素化液体が、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である、再生方法。 contacting the fluorinated liquid mixed with the detergent with water such that the concentration of the detergent in the upper aqueous phase is not more than 10% by weight and not more than 80% by weight;
a step of separating the mixed liquid after contact with water into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase, removing the upper liquid and collecting the lower liquid; A method of regenerating a fluorinated liquid comprising:
A regeneration method, wherein the cleaning agent is an aprotic polar solvent that dissolves in the fluorinated liquid, and the fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof.
水接触後の混合液が、上層に位置する水相及び下層に位置するフッ素化液体を含む相の二液に分離した後、上層の液を除去し、下層の液を採取する手段と、を備える、フッ素化液体再生装置であって、
前記洗浄剤が、前記フッ素化液体に溶解する非プロトン性極性溶媒であり、且つ前記フッ素化液体が、ハイドロフルオロエーテル、ハイドロフルオロオレフィン、又はこれらの混合物である、フッ素化液体再生装置。 means for contacting the fluorinated liquid mixed with the cleaning agent with water such that the concentration of the cleaning agent in the upper aqueous phase is not more than 10% by mass and not more than 80% by mass;
a means for removing the upper layer liquid and collecting the lower layer liquid after the mixed liquid after contact with water is separated into two liquids, an upper aqueous phase and a lower fluorinated liquid-containing phase; A fluorinated liquid regeneration device comprising:
A fluorinated liquid regenerator, wherein said cleaning agent is an aprotic polar solvent soluble in said fluorinated liquid, and said fluorinated liquid is a hydrofluoroether, a hydrofluoroolefin, or a mixture thereof.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018007629A JP7126830B2 (en) | 2018-01-19 | 2018-01-19 | Method for regenerating fluorinated liquids and regenerating apparatus using same |
| CN201980008810.2A CN111601873B (en) | 2018-01-19 | 2019-01-16 | Fluorinated liquid regeneration method and regeneration apparatus using such method |
| KR1020207023146A KR20200111717A (en) | 2018-01-19 | 2019-01-16 | Fluorinated liquid regeneration method and regeneration apparatus using such method |
| PCT/IB2019/050343 WO2019142113A1 (en) | 2018-01-19 | 2019-01-16 | Fluorinated liquid regeneration method and regeneration apparatus using such method |
| TW108101984A TW201936998A (en) | 2018-01-19 | 2019-01-18 | Fluorinated liquid regeneration method and regeneration apparatus using such method |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018007629A JP7126830B2 (en) | 2018-01-19 | 2018-01-19 | Method for regenerating fluorinated liquids and regenerating apparatus using same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2019126745A JP2019126745A (en) | 2019-08-01 |
| JP7126830B2 true JP7126830B2 (en) | 2022-08-29 |
Family
ID=67301634
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018007629A Active JP7126830B2 (en) | 2018-01-19 | 2018-01-19 | Method for regenerating fluorinated liquids and regenerating apparatus using same |
Country Status (5)
| Country | Link |
|---|---|
| JP (1) | JP7126830B2 (en) |
| KR (1) | KR20200111717A (en) |
| CN (1) | CN111601873B (en) |
| TW (1) | TW201936998A (en) |
| WO (1) | WO2019142113A1 (en) |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050124524A1 (en) | 2003-12-04 | 2005-06-09 | Kanto Kagaku Kabushiki Kaisha | Cleaning solution and cleaning method for mask used in vacuum vapor deposition step in production of low molecular weight organic EL device |
| JP2006100263A (en) | 2004-09-01 | 2006-04-13 | Sanyo Electric Co Ltd | Cleaning device |
| JP2006313753A (en) | 2006-05-26 | 2006-11-16 | Kanto Chem Co Inc | Mask cleaning liquid composition used at vacuum vapor deposition process of low molecule type organic el element, and cleaning method |
| JP2008163400A (en) | 2006-12-28 | 2008-07-17 | Asahi Glass Co Ltd | Cleaning system and cleaning method |
| JP2008208048A (en) | 2007-02-23 | 2008-09-11 | Three M Innovative Properties Co | Purification process of fluorine-based solvent-containing solution, purification apparatus and cleaning apparatus |
| JP2009248063A (en) | 2008-04-10 | 2009-10-29 | Tosoh Corp | Method for recovering detergent |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0271802A (en) * | 1988-09-06 | 1990-03-12 | Terumo Corp | Method for purifying hydrophobic solvent |
| JPH0731802A (en) * | 1993-07-23 | 1995-02-03 | Minolta Co Ltd | Separation of liquid mixture using distribution liquid |
| JP3290919B2 (en) * | 1997-04-18 | 2002-06-10 | 新オオツカ株式会社 | Cleaning equipment |
| US7513004B2 (en) * | 2003-10-31 | 2009-04-07 | Whirlpool Corporation | Method for fluid recovery in a semi-aqueous wash process |
| CN103739450A (en) * | 2013-12-30 | 2014-04-23 | 山东华夏神舟新材料有限公司 | Preparation method of hydrofluoroether |
| CN206872472U (en) * | 2017-05-26 | 2018-01-12 | 深圳市科伟达超声波设备有限公司 | A kind of cleaning agent reclaiming device |
| CN107243477A (en) * | 2017-05-26 | 2017-10-13 | 深圳市科伟达超声波设备有限公司 | A kind of Cleaning of Parts system and cleaning method |
-
2018
- 2018-01-19 JP JP2018007629A patent/JP7126830B2/en active Active
-
2019
- 2019-01-16 KR KR1020207023146A patent/KR20200111717A/en unknown
- 2019-01-16 WO PCT/IB2019/050343 patent/WO2019142113A1/en active Application Filing
- 2019-01-16 CN CN201980008810.2A patent/CN111601873B/en not_active Expired - Fee Related
- 2019-01-18 TW TW108101984A patent/TW201936998A/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050124524A1 (en) | 2003-12-04 | 2005-06-09 | Kanto Kagaku Kabushiki Kaisha | Cleaning solution and cleaning method for mask used in vacuum vapor deposition step in production of low molecular weight organic EL device |
| JP2006100263A (en) | 2004-09-01 | 2006-04-13 | Sanyo Electric Co Ltd | Cleaning device |
| JP2006313753A (en) | 2006-05-26 | 2006-11-16 | Kanto Chem Co Inc | Mask cleaning liquid composition used at vacuum vapor deposition process of low molecule type organic el element, and cleaning method |
| JP2008163400A (en) | 2006-12-28 | 2008-07-17 | Asahi Glass Co Ltd | Cleaning system and cleaning method |
| JP2008208048A (en) | 2007-02-23 | 2008-09-11 | Three M Innovative Properties Co | Purification process of fluorine-based solvent-containing solution, purification apparatus and cleaning apparatus |
| US20100126934A1 (en) | 2007-02-23 | 2010-05-27 | Daisuke Nakazato | Purification process of fluorine-based solvent-containing solution |
| JP2009248063A (en) | 2008-04-10 | 2009-10-29 | Tosoh Corp | Method for recovering detergent |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019126745A (en) | 2019-08-01 |
| CN111601873A (en) | 2020-08-28 |
| WO2019142113A1 (en) | 2019-07-25 |
| TW201936998A (en) | 2019-09-16 |
| CN111601873B (en) | 2022-04-12 |
| KR20200111717A (en) | 2020-09-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR0168486B1 (en) | Detergent, method and apparatus for cleaning | |
| KR100248175B1 (en) | Solvent cleaning process | |
| JPH02191581A (en) | Method and device for cleaning and drying parts | |
| CA2553080C (en) | Process for removing water and apparatus for removing water | |
| US7473676B2 (en) | Compositions containing fluorinated hydrocarbons and oxygenated solvents | |
| JP7126830B2 (en) | Method for regenerating fluorinated liquids and regenerating apparatus using same | |
| JP4755987B2 (en) | Electronic substrate defraxing composition based on fluorinated hydrocarbon and sec-butanol | |
| JPH08506615A (en) | Multiple solvent cleaning system | |
| JP5494263B2 (en) | Drainer drying method and drainer drying system | |
| JP2021000603A (en) | Method for purifying fluorinated liquid and purification apparatus using same | |
| KR20180021224A (en) | An energy-efficient method for purifying and degreasing volatile compounds | |
| JP4737800B2 (en) | Separation method and apparatus | |
| ITMI960442A1 (en) | SOLVENTS USABLE AS CLEANING AGENTS | |
| JP2011179787A (en) | Draining and drying method and system | |
| JPH05154302A (en) | Water removing composition and removal of water from article | |
| WO2019124239A1 (en) | Method and apparatus for recovering fluorine-based solvent, and method and system for cleaning object to be cleaned | |
| JPH04272194A (en) | Nonaqueous washing method | |
| JP2021041337A (en) | Regeneration method of alcohol-containing-fluorinated liquid and regeneration system using the method | |
| JP5217697B2 (en) | How to drain water | |
| JP2009228048A (en) | Method of draining article | |
| JP2011104528A (en) | Draining drying method and draining drying system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20210106 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20211027 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20211102 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20220114 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220406 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220802 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220817 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7126830 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |