JP6412629B1 - 放射性フッ素含有ヒドロキサム酸型造影剤、その製造方法及びその応用 - Google Patents
放射性フッ素含有ヒドロキサム酸型造影剤、その製造方法及びその応用 Download PDFInfo
- Publication number
- JP6412629B1 JP6412629B1 JP2017208279A JP2017208279A JP6412629B1 JP 6412629 B1 JP6412629 B1 JP 6412629B1 JP 2017208279 A JP2017208279 A JP 2017208279A JP 2017208279 A JP2017208279 A JP 2017208279A JP 6412629 B1 JP6412629 B1 JP 6412629B1
- Authority
- JP
- Japan
- Prior art keywords
- hydroxamic acid
- fluorine
- contrast agent
- acid type
- radioactive fluorine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C259/00—Compounds containing carboxyl groups, an oxygen atom of a carboxyl group being replaced by a nitrogen atom, this nitrogen atom being further bound to an oxygen atom and not being part of nitro or nitroso groups
- C07C259/04—Compounds containing carboxyl groups, an oxygen atom of a carboxyl group being replaced by a nitrogen atom, this nitrogen atom being further bound to an oxygen atom and not being part of nitro or nitroso groups without replacement of the other oxygen atom of the carboxyl group, e.g. hydroxamic acids
- C07C259/06—Compounds containing carboxyl groups, an oxygen atom of a carboxyl group being replaced by a nitrogen atom, this nitrogen atom being further bound to an oxygen atom and not being part of nitro or nitroso groups without replacement of the other oxygen atom of the carboxyl group, e.g. hydroxamic acids having carbon atoms of hydroxamic groups bound to hydrogen atoms or to acyclic carbon atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/0402—Organic compounds carboxylic acid carriers, fatty acids
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B59/00—Introduction of isotopes of elements into organic compounds ; Labelled organic compounds per se
- C07B59/001—Acyclic or carbocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C205/00—Compounds containing nitro groups bound to a carbon skeleton
- C07C205/27—Compounds containing nitro groups bound to a carbon skeleton the carbon skeleton being further substituted by etherified hydroxy groups
- C07C205/35—Compounds containing nitro groups bound to a carbon skeleton the carbon skeleton being further substituted by etherified hydroxy groups having nitro groups and etherified hydroxy groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton
- C07C205/36—Compounds containing nitro groups bound to a carbon skeleton the carbon skeleton being further substituted by etherified hydroxy groups having nitro groups and etherified hydroxy groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton to carbon atoms of the same non-condensed six-membered aromatic ring or to carbon atoms of six-membered aromatic rings being part of the same condensed ring system
- C07C205/37—Compounds containing nitro groups bound to a carbon skeleton the carbon skeleton being further substituted by etherified hydroxy groups having nitro groups and etherified hydroxy groups bound to carbon atoms of six-membered aromatic rings of the carbon skeleton to carbon atoms of the same non-condensed six-membered aromatic ring or to carbon atoms of six-membered aromatic rings being part of the same condensed ring system the oxygen atom of at least one of the etherified hydroxy groups being further bound to an acyclic carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07B—GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR
- C07B2200/00—Indexing scheme relating to specific properties of organic compounds
- C07B2200/05—Isotopically modified compounds, e.g. labelled
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Physics & Mathematics (AREA)
- Medicinal Chemistry (AREA)
- Optics & Photonics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Abstract
Description
一般式(III)
一般式(I)
一般式(III)
一般式(I)
−実施形態−
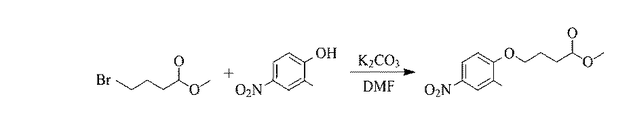
図1は本発明の一般式(I)で表されるフッ素18-ヒドロキサム酸類化合物の前駆体の合成反応フローチャートである。4-ニトロ-2-メキノール(1.53g、8.2mmol、1.0eq)、4-ブロモ酪酸メチル(1.98g、11.0mmol、1.3eq)、及び炭酸カリウム(2.76g、20.0mmol、2.4eq)がN,N-ジメチルホルムアミド(25mL)中に溶かされ、80℃で一晩撹拌反応させる。酢酸エチル(100mL)が添加され、重炭酸ナトリウム飽和水溶液(100mL)、1Nの塩酸水溶液(100mL)、及び飽和食塩水(100mL)により抽出が行われ、有機層が収集される。硫酸マグネシウムにより除水が行われた後、減圧濃縮、濾過が実行され、最後にシリコーンカラムクロマトグラフィーによる純化が行われ、フッ素18-ヒドロキサム酸類化合物の前駆体(2.26 g)となる黄色の固体が得られる。その生成率は96%に達する。酢酸エチル/n-ヘキサン(30:70)は移動相となる。また、1H NMR (300 MHz, D2O, δ): 8.10-8.03 (m, 2H)、6.86-6.83 (d, J= 8.7 Hz, 1H)、4.15-4.11 (t, J= 6.3 Hz, 2H)、3.70 (s, 3H)、2.59-2.55 (t, J= 7.2 Hz, 2H)、2.27 (s, 3H)、2.22-2.17 (m, 2H)である。
一般式(III)で表される構造の化合物は一般式(I)で表される構造の化合物が弗素化及びヒドロキサム酸化の2つのステップを経た後に得られる。図2は本発明の一般式(I)で表されるフッ素18-ヒドロキサム酸類化合物の前駆体が弗素化及びヒドロキサム酸化を経る反応フローチャートである。
一般式(II)
フッ素18-ヒドロキサム酸類化合物がヒストン脱アセチル化酵素の阻害剤となりうるか否かを更に確かめるため、フッ素19-ヒドロキサム酸類化合物を選択してヒストン脱アセチル化酵素の阻害性の分析が行われた。フッ素18-ヒドロキサム酸類化合物及びフッ素19-ヒドロキサム酸類化合物の化性は同じであり、差異はフッ素18-ヒドロキサム酸類化合物が放射性を有し、フッ素19-ヒドロキサム酸類化合物が放射性を有しない点のみである。
小脳萎縮症の動物モデルのマウス(SCA17)をガス麻酔させた後、定位固定装置により0.75MBq(20μCi)のフッ素18-ヒドロキサム酸類化合物(放射性薬物)を2mLマウスの側脳室に注射し、高解像度の小動物の陽電子放出断層撮影(PET/CT)を利用してマウスの放射性薬物の分布のスキャンを行った。撮影時間は1時間であり、脳内における薬物の分布表現が観察された。CT及びPET画像の再編及び融合(fusion)が行われた後、PmodのソフトウェアでROIを選択し、結果は薬物が小脳萎縮症のマウスの小脳部位に蓄積したことを示し、好ましいターゲット特性を有することを証明した(図6に示す)。但し、MRIの結果は正常なマウス及び疾病を患ったマウスの小脳の画像には明確な差異が無いことを示す(図7に示す)。TGは疾病を患ったマウスを示し、WTは正常なマウスを示す。
Claims (10)
- 診断用造影剤とすることを特徴とする請求項1に記載の放射性フッ素含有ヒドロキサム酸型造影剤。
- 前記診断用造影剤は小脳萎縮症の診断用造影剤であることを特徴とする請求項2に記載の放射性フッ素含有ヒドロキサム酸型造影剤。
- 4-ニトロ-2-メキノール、4-ブロモ酪酸メチル、及び炭酸カリウムがN,N-ジメチルホルムアミド中に溶かされ、60〜90℃で12〜36時間反応させ、次いで酢酸エチルが添加され、且つ重炭酸ナトリウム飽和水溶液、塩酸水溶液、及び飽和食塩水により抽出が行われ、有機層が収集されて除水、減圧濃縮、及び濾過のステップが順に実行され、最後にシリコーンカラムクロマトグラフィーによる純化が行われ、請求項4に記載の前駆体となる黄色の固体が得られる前記前駆体の製造のステップ1と、
製造された前記前駆体が弗化反応を経て中間生成物が形成されるステップ2と、
次いで、前記中間生成物がヒドロキサム酸化反応を経て最終生成物が形成されるステップ3とを含むことを特徴とする放射性フッ素含有ヒドロキサム酸型造影剤の製造方法。 - 前記4-ニトロ-2-メキノール、前記4-ブロモ酪酸メチル、及び前記炭酸カリウムのモル濃度比は8.2:11:20であることを特徴とする請求項5に記載の放射性フッ素含有ヒドロキサム酸型造影剤の製造方法。
- 前記弗化反応はフッ素同位体18またはフッ素同位体19の化学的インジケーターであることを特徴とする請求項5に記載の放射性フッ素含有ヒドロキサム酸型造影剤の製造方法。
- 前記弗化反応は、無水フッ素18及び前記前駆体を摂氏100〜120℃で10〜30分間反応させることを特徴とする請求項5に記載の放射性フッ素含有ヒドロキサム酸型造影剤の製造方法。
- 前記ヒドロキサム酸化反応は、前記中間生成物、ヒドロキシルアミン、及び水酸化ナトリウムを摂氏30〜50℃で5〜20分間反応させることを特徴とする請求項5に記載の放射性フッ素含有ヒドロキサム酸型造影剤の製造方法。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TW106134788A TWI650139B (zh) | 2017-10-11 | 2017-10-11 | 含有放射性同位素氟的異羥肟酸類造影劑、其製備方法及其用途 |
| TW106134788 | 2017-10-11 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP6412629B1 true JP6412629B1 (ja) | 2018-10-24 |
| JP2019073492A JP2019073492A (ja) | 2019-05-16 |
Family
ID=60262724
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017208279A Active JP6412629B1 (ja) | 2017-10-11 | 2017-10-27 | 放射性フッ素含有ヒドロキサム酸型造影剤、その製造方法及びその応用 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US10538486B2 (ja) |
| EP (1) | EP3470090B1 (ja) |
| JP (1) | JP6412629B1 (ja) |
| TW (1) | TWI650139B (ja) |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10233412A1 (de) * | 2002-07-23 | 2004-02-12 | 4Sc Ag | Neue Verbindungen als Histondeacetylase-Inhibitoren |
| US7705017B2 (en) * | 2004-05-03 | 2010-04-27 | En Vivo Pharmaceuticals, Inc. | Compounds for treatment of neurodegenerative diseases |
| GB0509223D0 (en) * | 2005-05-05 | 2005-06-15 | Chroma Therapeutics Ltd | Enzyme inhibitors |
| NZ592686A (en) | 2008-10-15 | 2012-12-21 | Generics Uk Ltd | Process for the preparation of vorinostat from aniline, hydroxylamine and suberic acid starting materials |
| CN102786448B (zh) | 2012-08-09 | 2014-03-12 | 深圳万乐药业有限公司 | 一种合成belinostat的方法 |
| US10188756B2 (en) * | 2013-10-18 | 2019-01-29 | The General Hospital Corporation | Imaging histone deacetylases with a radiotracer using positron emission tomography |
| CN106045923A (zh) * | 2016-03-17 | 2016-10-26 | 广东众生药业股份有限公司 | 一种组蛋白去乙酰化酶抑制剂及其制备方法和用途 |
-
2017
- 2017-10-11 TW TW106134788A patent/TWI650139B/zh active
- 2017-10-27 JP JP2017208279A patent/JP6412629B1/ja active Active
- 2017-10-27 US US15/795,600 patent/US10538486B2/en active Active
- 2017-10-27 EP EP17198831.4A patent/EP3470090B1/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| EP3470090B1 (en) | 2020-10-07 |
| TW201914618A (zh) | 2019-04-16 |
| JP2019073492A (ja) | 2019-05-16 |
| EP3470090A1 (en) | 2019-04-17 |
| US20190106381A1 (en) | 2019-04-11 |
| US10538486B2 (en) | 2020-01-21 |
| TWI650139B (zh) | 2019-02-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20250177578A1 (en) | Psma-binding agents and uses thereof | |
| US12491270B2 (en) | Imaging histone deacetylases with a radiotracer using positron emission tomography | |
| Hu et al. | Synthesis and biological evaluation of N-(2-[18F] Fluoropropionyl)-L-methionine for tumor imaging | |
| Petersen et al. | 18F-Labelling of electron rich iodonium ylides: application to the radiosynthesis of potential 5-HT2A receptor PET ligands | |
| US20140336503A1 (en) | Radiolabeled biomarkers for osteoclast activation and related methods thereof | |
| JP6412629B1 (ja) | 放射性フッ素含有ヒドロキサム酸型造影剤、その製造方法及びその応用 | |
| CA2911307C (en) | Use of fluorinated derivatives of 4-aminopyridine in therapeutics and medical imaging | |
| Li et al. | Synthesis and evaluation of 18F-INER-1577-3 as a central nervous system (CNS) histone deacetylase imaging agent | |
| HK40062845A (zh) | Psma-結合劑及其用途 | |
| WO2026006561A1 (en) | L-(2-(6-[18f]fluoropyridin-3-yl)-4-(trifluoromethyl)phenyl)-n-(pyrimidin-2-yl)-2,3-dihydro-lh-indene-5-sulfonamide and uses in pet imaging | |
| HK1161248B (en) | Psma-binding agents and uses thereof | |
| Fantoni | Development and Evaluation of a Library of Novel 18F-labelled PET Tracers Targeting the P2X7 Receptor | |
| HK1161248A (en) | Psma-binding agents and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20171027 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20180904 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20180928 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6412629 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |