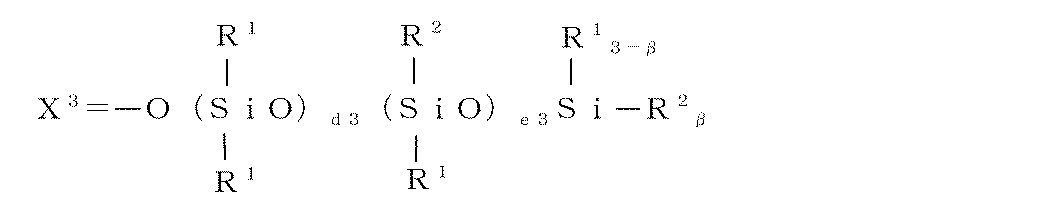JP4530255B2 - Water-dispersed water / oil repellent and water / oil repellent paper and sheet using the same - Google Patents
Water-dispersed water / oil repellent and water / oil repellent paper and sheet using the same Download PDFInfo
- Publication number
- JP4530255B2 JP4530255B2 JP2003357041A JP2003357041A JP4530255B2 JP 4530255 B2 JP4530255 B2 JP 4530255B2 JP 2003357041 A JP2003357041 A JP 2003357041A JP 2003357041 A JP2003357041 A JP 2003357041A JP 4530255 B2 JP4530255 B2 JP 4530255B2
- Authority
- JP
- Japan
- Prior art keywords
- water
- group
- mass
- parts
- component
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Landscapes
- Materials Applied To Surfaces To Minimize Adherence Of Mist Or Water (AREA)
- Paper (AREA)
Description
本発明は、フッ素化合物及び溶剤を使用することなく、各種紙基材に対し撥水撥油性を付与できる処理剤組成物を提供する。 The present invention provides a treating agent composition capable of imparting water and oil repellency to various paper base materials without using a fluorine compound and a solvent.
食品用の包装紙や包装容器、クッキングペーパなどに用いられる紙材料は、食品の油分や水分が浸透して周囲を汚さないように、また食品が粘着あるいは接着して取り出す際に変形や破損することのないように、撥水撥油性や非粘着性を付与されている。 Paper materials used for food wrapping paper, packaging containers, cooking paper, etc. are deformed or damaged when taking out the oil and moisture of the food to prevent the surroundings from getting dirty, and when the food is sticky or adhered and taken out. Water and oil repellency and non-tackiness are imparted to prevent this.
従来からこの目的には、パーフルオロアルキル基を有する各種の化合物が好適に利用され、様々な改良がなされながら今日に到っている。その一つには、パーフルオロアルキル基を有する重合性単量体の重合単位を有する重合体を利用する方法が知られている。多くは水に分散された形態で、抄紙する際にこれらの処理剤を添加する内添法に、または、抄紙した紙を処理液に浸漬させる外添法に、広く用いられてきた。 Conventionally, various compounds having a perfluoroalkyl group have been suitably used for this purpose, and various improvements have been made so far. As one of the methods, a method using a polymer having a polymerization unit of a polymerizable monomer having a perfluoroalkyl group is known. Many of them are dispersed in water, and have been widely used in an internal addition method in which these processing agents are added during papermaking or in an external addition method in which the papermaking paper is immersed in a treatment solution.
例えば、参考文献1には溶解性の改良、参考文献2には処理法による撥油性低下の防止、参考文献3には二次加工性の改良、参考文献4は密着性の改良について、この方法を利用した提案が見られる。 For example, Reference 1 is a method for improving solubility, Reference 2 is a method for preventing a decrease in oil repellency by treatment, Reference 3 is for improving secondary processability, and Reference 4 is for improving adhesion. Proposals using can be seen.
もう一つには、パーフルオロアルキル基を有するリン酸エステルのアミン塩を用いる方法が知られており、上述の重合体を利用するものと同様に広く用いられてきた。例えば、参考文献5や参考文献6には分散安定性の改善、参考文献7や参考文献8には貯蔵安定性の改良の提案が、この方法について成されている。 Another method is known that uses an amine salt of a phosphoric ester having a perfluoroalkyl group, and has been widely used in the same manner as that using the above-mentioned polymer. For example, Reference 5 and Reference 6 propose improvements in dispersion stability, and Reference 7 and Reference 8 propose improvements in storage stability for this method.
しかし、深刻化する環境問題の一因として、以前からフルオロ脂肪族炭化水素がオゾン層の破壊物質または地球温暖化物質とされ、その使用が規制されている。そのため、類似構造を有するフッ素化合物においても、近い将来に環境問題に関連して何らかの規制がなされる可能性は否めない。また、食品用途においては、電子レンジ等による調理の際に僅かではあるがフッ素を含有した有害性物質が生成する可能性を指摘されている。類似の問題は廃棄処分のために焼却された場合にも指摘されており、フッ酸などのフッ素を含有する有害性物質を環境に排出することにもなる。 However, as one of the causes of a serious environmental problem, fluoroaliphatic hydrocarbons have been regarded as ozone layer depleting substances or global warming substances and their use has been regulated. Therefore, even in the case of a fluorine compound having a similar structure, there is no denying the possibility that some kind of regulation will be made in the near future in relation to environmental problems. In addition, in food applications, it has been pointed out that there is a possibility that a harmful substance containing fluorine is generated even when cooking with a microwave oven or the like. A similar problem has been pointed out when incinerated for disposal, and it also releases harmful substances containing fluorine such as hydrofluoric acid to the environment.
これらの状況から、最近ではパーフルオロアルキル基を有する化合物を利用することなく、紙材料に撥水撥油性や非粘着性を付与する方法が求められるようになっている。PVA樹脂は古くから目止めなどに紙基材処理に利用されてきたが、その高い親水性は撥油性を与える効果も持っており、パーフルオロアルキル基を有する化合物の代替えとして期待されている。しかし、その親水性ゆえに撥水性や非粘着性が悪く、実際の使用に際しバランスのとれた性能を得ることが難しい。 Under these circumstances, recently, a method for imparting water / oil repellency and non-tackiness to paper materials without using a compound having a perfluoroalkyl group has been demanded. PVA resin has long been used for paper substrate processing for sealing and the like, but its high hydrophilicity also has an effect of imparting oil repellency, and is expected as a substitute for a compound having a perfluoroalkyl group. However, due to its hydrophilicity, water repellency and non-adhesiveness are poor, and it is difficult to obtain a balanced performance in actual use.
一方、シリコーンは撥水性や非粘着性を付与する加工に利用されているが、撥油性の点では代替えに十分な性能を持っているものが見当たらない。シリコーンは疎水性の、PVA樹脂は親水性の材料であり、この両者を単に混合しても均一に混ざり合うことはなく、撥水と撥油性を両立させること難しい。 Silicone, on the other hand, is used for processing to impart water repellency and non-adhesiveness, but there are no substitutes that have sufficient performance in terms of oil repellency. Silicone is a hydrophobic material, and PVA resin is a hydrophilic material. Even if these are simply mixed, they are not mixed uniformly, and it is difficult to achieve both water and oil repellency.
本発明は、上記事情に鑑みなされたもので、エマルジョン型シリコーン系剥離剤とポリビニルアルコール(以下PVAと略す)系樹脂とから成る処理剤を用いて、クラフト紙、上質紙、ダンボールなどの紙基材に撥油性と撥水性を付与することを目的とする。 The present invention has been made in view of the above circumstances, and uses a processing agent comprising an emulsion type silicone release agent and a polyvinyl alcohol (hereinafter abbreviated as PVA) resin, to make a paper base such as kraft paper, fine paper, and cardboard. The object is to impart oil and water repellency to the material.
本発明者は、上記目的を達成するために鋭意努力を行った結果、
(A)官能性基含有オルガノポリシロキサン 100質量部
(B)架橋剤 0.1〜30質量部
(C)PVA系樹脂 50〜1000質量部
(D)触媒 有効成分として0〜5質量部
(E)水 100〜100000質量部
(F)加水分解性基含有シラン及び又はそれらの部分加水分解縮合物
1〜250質量部
これらからなる水分散型撥水撥油剤で処理することにより、優れた撥水撥油性を紙基材に付与することができることを知見し、本発明をなすに至った。
As a result of diligent efforts to achieve the above object,
(A) Functional group-containing organopolysiloxane 100 parts by mass (B) 0.1-30 parts by mass of cross-linking agent (C) PVA-based resin 50-1000 parts by mass (D) catalyst 0-5 parts by mass (E ) 100-100,000 parts by weight of water (F) Hydrolyzable group-containing silanes and / or their partially hydrolyzed condensates
1 to 250 parts by mass It has been found that excellent water and oil repellency can be imparted to a paper substrate by treatment with a water-dispersed water and oil repellent comprising these, and the present invention has been made.
撥油性に優れたPVA樹脂と、撥水性や非粘着性の良好なシリコーンを組み合わせて、両方の材料の利点を両立させるべく検討した結果、特定の構造を有する材料を特定の条件で組み合わせ、加水分解性基含有シラン及びその部分加水分解縮合物を配合することで、撥油性、撥水性、非粘着性を合わせ持つ処理用組成物を見出し本発明に到った。環境に対する安全性が高く無害な材料の組み合わせた組成物であり、パーフルオロアルキル基を有する化合物の代替えとして好適に利用できる。有機溶剤を含有しない水分散型として利用できるため、溶剤使用による環境問題や危険性などからの不利益を回避できる。この組成物で処理された紙基材はリサイクルが容易で、環境負荷の小さい製品となる。フッ素化合物に由来する有害性や環境問題を解決できる。溶剤を含まず、使用する際にも不要なため管理やコストの面で有利。リサイクルが容易で、環境負荷が軽減される。 As a result of examining the combination of PVA resin with excellent oil repellency and silicone with good water repellency and non-adhesiveness to achieve the advantages of both materials, a material having a specific structure is combined under specific conditions. By blending a decomposable group-containing silane and a partially hydrolyzed condensate thereof, a treatment composition having both oil repellency, water repellency and non-tackiness was found and the present invention was reached. The composition is a combination of materials that are safe and harmless to the environment, and can be suitably used as a substitute for a compound having a perfluoroalkyl group. Since it can be used as a water-dispersed type that does not contain an organic solvent, disadvantages from environmental problems and dangers due to the use of the solvent can be avoided. The paper base treated with this composition is easy to recycle and becomes a product with low environmental impact. It can solve harmful and environmental problems derived from fluorine compounds. It does not contain a solvent and is unnecessary for use, so it is advantageous in terms of management and cost. Recycling is easy and environmental impact is reduced.
本発明に使用される紙基材としては一般に市販されている、クラフト紙、上質紙、ライナー、ダンボールなどが使用可能で、例えば、マニラ麻、こうぞ、みつまたなどの天然繊維、テトロン、ビニロン、アクリルなどの合成繊維を主原料としたものが利用できる。 Kraft paper, fine paper, liner, cardboard, etc., which are generally commercially available, can be used as the paper base material used in the present invention. For example, natural fibers such as Manila hemp, ridges, honey bees, etc., Tetron, vinylon, acrylic Those made from synthetic fibers such as these can be used.
本発明に使用される(A)成分としての官能性基含有オルガノポリシロキサンは、主骨格構造が分岐鎖構造を含んでいてもよい直鎖で主にジメチルシロキサンから構成され、25℃での粘度が0.05〜500Pa・sのものである。官能基として水酸基又はアルケニル基を持ち、後述する(B)成分の架橋剤と(D)成分の触媒とを組み合わせて、硬化反応しうるものが撥水性や非粘着性の点から好ましく利用される。
一つの好ましい(A)成分、(B)成分、(D)成分の組み合わせは、下記(A2)成分の官能性基含有オルガノポリシロキサンと(B2)成分の架橋剤が(D2)成分の触媒により縮合反応するものである。この(A2)成分は、平均組成式(1)で示される構造を有し、1分子中に少なくとも2個の水酸基を持つものである。
The functional group-containing organopolysiloxane used as the component (A) for use in the present invention is composed of a straight chain which may contain a branched chain structure, mainly dimethylsiloxane, and has a viscosity at 25 ° C. Is 0.05 to 500 Pa · s. Those having a hydroxyl group or an alkenyl group as a functional group and capable of undergoing a curing reaction by combining a crosslinking agent of component (B) and a catalyst of component (D) described later are preferably used from the viewpoint of water repellency and non-adhesiveness. .
One preferred combination of component (A), component (B), and component (D) is that the functional group-containing organopolysiloxane of component (A2) below and the crosslinking agent of component (B2) depend on the catalyst of component (D2). It undergoes a condensation reaction. This component (A2) has a structure represented by the average composition formula (1) and has at least two hydroxyl groups in one molecule.
[式中、R1は一価炭化水素基で、R3は水酸基を示し、X2は以下の式で示される基である。
[Wherein R 1 represents a monovalent hydrocarbon group, R 3 represents a hydroxyl group, and X 2 represents a group represented by the following formula:
(a2、b2、c2、d2はオルガノポリシロキサンの25℃での粘度が0.05〜500Pa・sを満たす正数から選ばれ、b2、c2、d2は0であってもよい。)]
(A2, b2, c2, d2 the viscosity at 25 ° C. of the organopolysiloxane is chosen from a positive number to fully plus the 0.05~500Pa · s, b2, c2, d2 may be zero.) ]
ここでのR2は炭素数1〜20の一価の炭化水素基を示し、例えば、それぞれメチル基、エチル基、プロピル基、ブチル基などのアルキル基、シクロヘキシル基などのシクロアルキル基、ビニル基、プロペニル基、ブテニル基などのアルケニル基、フェニル基、トリル基などのアリール基、あるいはこれらの基の炭素原子に結合した水素原子の一部または全部をハロゲン原子、シアノ基などで置換したクロロメチル基、シアノエチル基などのような非置換または置換1価炭化水素基などから選択される基である。(A2)成分の官能性基含有オルガノポリシロキサン全体に含まれるR2はその少なくとも80%がメチル基であることが製造上及び特性上好ましい。 Wherein R 2 in represents a monovalent hydrocarbon group having 1 to 20 carbon atoms, for example, each a methyl group, an ethyl group, a propyl group, an alkyl group such as butyl group, cycloalkyl groups such as a cyclohexyl group, a vinyl group Chloromethyl in which some or all of the hydrogen atoms bonded to carbon atoms of alkenyl groups such as propenyl and butenyl groups, phenyl and tolyl groups, or carbon atoms of these groups are substituted with halogen atoms, cyano groups, etc. And a group selected from an unsubstituted or substituted monovalent hydrocarbon group such as a cyanoethyl group and the like. From the viewpoint of production and characteristics, it is preferable that at least 80% of R 2 contained in the entire functional group-containing organopolysiloxane of the component (A2) is a methyl group.
(A2)成分の官能性基含有オルガノポリシロキサンの1分子が持つ水酸基は縮合反応で硬化するための官能基として2個以上が必要であり、後述する架橋剤(B2)成分及び必要により触媒の(D2)成分と組み合わされて硬化型として利用される。水酸基が2個未満では硬化後も未架橋分子が残る可能性が高く、撥水性が経時で低下する傾向が大きくなるため望ましくない。 The hydroxyl group possessed by one molecule of the functional group-containing organopolysiloxane of component (A2) requires at least two functional groups for curing by a condensation reaction, and the crosslinking agent (B2) component described below and, if necessary, the catalyst Combined with the component (D2), it is used as a curing type. If there are less than two hydroxyl groups, there is a high possibility that uncrosslinked molecules remain even after curing, and the water repellency tends to decrease with time, which is not desirable.
望ましくは官能性基含有オルガノポリシロキサン100gあたりの含有量としては0.0001モルから0.1モルであり、0.0001モル未満では撥水性が経時で低下し、0.1モルを越えるとポットライフが短くなり取り扱いが難しくなる。相当する式(2)及び置換基X2のa2、b2、c2,d2としては、1分子が持つ水酸基の数(b2+c2+2)が2〜150の範囲になるように選ばれる。 Desirably, the content per 100 g of the functional group-containing organopolysiloxane is 0.0001 mol to 0.1 mol, and if it is less than 0.0001 mol, the water repellency decreases with time. Life is shortened and handling becomes difficult. The corresponding compound of formula (2) and the substituent X 2 a2, b2, c2, d2, the number of hydroxyl groups possessed by one molecule (b2 + c2 + 2) is selected to be in the range of 2 to 150.
(A2)成分と組み合わせて縮合反応する好ましい架橋剤としての(B2)成分は、オルガノハイドロジェンポリシロキサンまたはオルガノポリシロキサンであって1分子中にSiHまたは加水分解性基を少なくとも3個有するものが利用できる。含有されるSiHまたは加水分解性基のモル数が、(A2)成分に含まれる水酸基のモル数の5〜200倍に相当する量が用いられるが、一般的なオルガノポリシロキサンでの配合質量部としては、(A2)成分の官能性基含有オルガノポリシロキサン100質量部に対して0.1〜30質量部の範囲である。 Component (B2) as a preferred crosslinking agent that undergoes a condensation reaction in combination with component (A2) is an organohydrogenpolysiloxane or organopolysiloxane having at least three SiH or hydrolyzable groups in one molecule. Available. The amount of SiH or hydrolyzable group contained is equivalent to 5 to 200 times the number of moles of the hydroxyl group contained in the component (A2). As, it is the range of 0.1-30 mass parts with respect to 100 mass parts of functional group containing organopolysiloxane of (A2) component.
(B2)成分の配合量に含有されるSiHまたは加水分解性基のモル数が(A2)成分に含まれる水酸基のモル数の5倍未満では、水酸基とSiHまたは加水分解性基の化学反応による橋掻け結合が十分ではなく撥水性や非粘着性が低下する一方、200倍を超えて配合しても効果の顕著な増加は見られず、かえって経時変化の原因となるうえ、経済的にも不利となる。 If the number of moles of SiH or hydrolyzable group contained in the blending amount of component (B2) is less than 5 times the number of moles of hydroxyl group contained in component (A2), it depends on the chemical reaction between the hydroxyl group and SiH or hydrolyzable group. While the bridging bond is not sufficient and the water repellency and non-adhesiveness are reduced, the effect does not increase remarkably even if it is added more than 200 times. Is also disadvantageous.
(B2)成分に使用されるオルガノハイドロジエンポリシロキサンは、組成式R2 fHgSiO(4−f−g)/2(式中、R2は上述の平均組成式(1)のR2と同様の意味を示し、fは0≦f≦3、gは0<g≦3、f+gは1≦f+g<3の実数である。)で示され、1分子中にSiHを少なくとも3個有することが必要である他は特に限定されず、分子構造は直鎖状、分岐鎖状もしくは環状のいずれであってもよい。粘度も数mPa・s〜数万mPa・sの範囲であれば良い。 (B2) organohydrogenpolysiloxane used in component composition formula R 2 f H g SiO (4 -f-g) / 2 R 2 of (wherein, R 2 is above average composition formula (1) F is 0 ≦ f ≦ 3, g is 0 <g ≦ 3, and f + g is a real number of 1 ≦ f + g <3), and has at least three SiH in one molecule The molecular structure may be linear, branched or cyclic. The viscosity may be in the range of several mPa · s to tens of thousands mPa · s.
オルガノハイドロジエンポリシロキサンの具体例として下記のオルガノポリシロキサンを挙げることができる。 The following organopolysiloxanes can be mentioned as specific examples of the organohydropolysiloxane.
但し、上記構造式及び組成式において、YとZは以下の構造式で示される基であり、かつ、hからwは次に示される範囲の整数である。h,l,nは3〜500、m,p,sは1〜500、i,j,k,o,q,r,t,u,v,wは0〜500。 However, in the above structural formula and composition formula, Y and Z are groups represented by the following structural formula, and h to w are integers in the following range. h, l and n are 3 to 500, m, p and s are 1 to 500, i, j, k, o, q, r, t, u, v and w are 0 to 500.
(B2)成分に使用されるオルガノポリシロキサンは、組成式R2 fWgSiO
(4−f−g)/2(式中、R2は上述の平均組成式(1)のR2と同様の意味を、Wは加水分解性基を示し、fは0≦f≦3、gは0<g≦3、f+gは1≦f+g<3の実数である。)で示され、1分子中に珪素原子に結合した加水分解性基を少なくとも3個有することが必要である他は特に限定されず、分子構造は直鎖状、分岐鎖状もしくは環状のいずれであってもよい。粘度も数mPa・s〜数万mPa・sの範囲であれば良い。
The organopolysiloxane used for the component (B2) has a composition formula R 2 f W g SiO
In (4-f-g) / 2 ( wherein, R 2 is the same meaning as the R 2 in the average composition formula described above (1), W represents a hydrolyzable group, f is 0 ≦ f ≦ 3, g is a real number of 0 <g ≦ 3 and f + g is 1 ≦ f + g <3), and it is necessary to have at least three hydrolyzable groups bonded to a silicon atom in one molecule. There is no particular limitation, and the molecular structure may be linear, branched or cyclic. The viscosity may be in the range of several mPa · s to tens of thousands mPa · s.
加水分解性基としては、珪素に直接結合したメトキシ基、エトキシ基、プロポキシ基、ブトキシ基、メトキシエトキシ基、イソプロペノキシ基などのアルコキシ基、アセトキシ基などのアシルオキシ基、エチルアミノ基などのアミノ基、アミド基、エチルメチルブタノキシム基などのオキシム基、塩素、臭素などのハロゲン原子を有するものが挙げられる。 Hydrolyzable groups include alkoxy groups such as methoxy, ethoxy, propoxy, butoxy, methoxyethoxy, and isopropenoxy, which are directly bonded to silicon, acyloxy groups such as acetoxy, amino groups such as ethylamino, Examples thereof include those having an oxime group such as an amide group and an ethylmethylbutanoxime group, and a halogen atom such as chlorine and bromine.
具体的には以下のポリオルガノシロキサンが使用できる。 Specifically, the following polyorganosiloxane can be used.
ここでのWはCH3COO−,CH3(C2H5)C=NO−,(C2H5)2N−CH3CO(C2H5)N−,CH2=(CH3)CO−などの加水分解性基を示し、x、y、zは0〜500の範囲の整数である。
Here, W is CH 3 COO—, CH 3 (C 2 H 5 ) C═NO—, (C 2 H 5 ) 2 N—CH 3 CO (C 2 H 5 ) N—, CH 2 = (CH 3 ) Represents a hydrolyzable group such as CO-, and x, y and z are integers in the range of 0 to 500.
(A2)と(B2)成分の組み合わせに対して好ましい触媒の(D2)成分は、縮合反応を促進して架橋させ、撥水性と非粘着性を付与し持続性を高めるために用いられる。かかる縮合触媒としては、塩酸、リン酸、メタンスルホン酸、パラトルエンスルホン酸、マレイン酸、トリフロロ酢酸などの酸類、水酸化ナトリウム、水酸化カリウム、ナトリウムエトキシド、テトラエチルアンモニウムヒドロキシドなどのアルカリ類、塩化アンモニウム、酢酸アンモニウム、フッ化アンモニウム、炭酸ナトリウムなどの塩類、マグネシウム、アルミニウム、、亜鉛、鉄、ジルコニウム、セリウム、チタン等の金属の有機酸塩、アルコキシド、キレート化合物などの有機金属化合物が挙げられる。例えば、亜鉛ジオクテート、チタンテトライソプロポキシド、アルミニウムトリブトキシド、ジルコニウムテトラアセチルアセトネート等が挙げられる。 The (D2) component of the preferred catalyst for the combination of the (A2) and (B2) components is used to accelerate the condensation reaction to crosslink, impart water repellency and non-tackiness, and increase durability. Examples of the condensation catalyst include acids such as hydrochloric acid, phosphoric acid, methanesulfonic acid, paratoluenesulfonic acid, maleic acid, and trifluoroacetic acid, alkalis such as sodium hydroxide, potassium hydroxide, sodium ethoxide, and tetraethylammonium hydroxide, Examples include salts such as ammonium chloride, ammonium acetate, ammonium fluoride, and sodium carbonate, organic metal salts of metals such as magnesium, aluminum, zinc, iron, zirconium, cerium, and titanium, alkoxides, and chelate compounds. . For example, zinc dioctate, titanium tetraisopropoxide, aluminum tributoxide, zirconium tetraacetylacetonate and the like can be mentioned.
上記縮合触媒は通常添加する必要はないが、(A2)成分と(B2)成分が反応するのに十分な加熱乾燥条件を確保できず、本来の性能が得られ難い場合などに添加される。(A2)成分と(B2)成分の合計質量に対して有効成分として0.1〜5%(質量比)配合することが、性能を付与持続する上で好ましいが、前記成分の反応性又は所望の硬化速度に応じて適宜増減させることができる。 Although the above condensation catalyst does not normally need to be added, it is added when it is difficult to obtain the original performance because sufficient heating and drying conditions for the reaction between the component (A2) and the component (B2) cannot be ensured. It is preferable to add 0.1 to 5% (mass ratio) as an active ingredient with respect to the total mass of the component (A2) and the component (B2) in order to impart and maintain performance, but the reactivity or desired of the components The amount can be appropriately increased or decreased depending on the curing rate.
もう一つの好ましい(A)成分、(B)成分、(D)成分の組み合わせは、下記(A3)成分の官能性基含有オルガノポリシロキサンと(B3)成分の架橋剤が(D3)成分の触媒により付加反応するものである。この(A3)成分の官能性基含有オルガノポリシロキサンは、平均組成式(2)で示される構造を有し、1分子中に少なくとも2個のアルケニル基を持つものである。 Another preferred combination of the component (A), the component (B), and the component (D) is a functional group-containing organopolysiloxane of the following component (A3) and a crosslinking agent of the component (B3) (D3) It is an addition reaction. The functional group-containing organopolysiloxane of component (A3) has a structure represented by the average composition formula (2) and has at least two alkenyl groups in one molecule.
ここでのR1は上記したと同じ炭素数1〜20の一価の炭化水素基から選択される基である。(A3)成分の官能性基含有オルガノポリシロキサン全体に含まれるR1はその少なくとも80%がメチル基であることが、R2はビニル基であることが製造上及び特性上好ましい。 R 1 here is a group selected from the same monovalent hydrocarbon group having 1 to 20 carbon atoms as described above. From the viewpoint of production and characteristics, it is preferable that at least 80% of R 1 contained in the entire functional group-containing organopolysiloxane of component (A3) is a methyl group, and R 2 is a vinyl group.
(A3)成分の官能性基含有オルガノポリシロキサンの1分子が持つアルケニル基は付加反応で硬化するための官能基として2個以上が必要であり、後述する架橋剤(B3)成分及び触媒(D3)成分と組み合わされて硬化型として利用される。アルケニル基が、2個未満では硬化後も未架橋分子が残る可能性が高く、(A2)の場合と同じように撥水性が経時で低下する傾向が大きくなるため望ましくない。 The alkenyl group of one molecule of the functional group-containing organopolysiloxane of the component (A3) requires two or more functional groups for curing by addition reaction, and the crosslinking agent (B3) component and catalyst (D3 described later) ) In combination with ingredients, it is used as a curable mold. If the number of alkenyl groups is less than 2, there is a high possibility that uncrosslinked molecules remain after curing, and the water repellency tends to decrease with time as in the case of (A2), which is not desirable.
望ましくは官能性基含有オルガノポリシロキサン100gあたりの含有量としては0.001モルから0.1モルであり、0.001モル未満では撥水性が経時で低下し、0.1モルを越えるとポットライフが短くなり取り扱いが難しくなる。相当する式(2)及び置換基X3のa3、b3、c3、d3、e3としては、1分子が持つアルケニル基の数{b3×(e3+α)+c3+2×α}が2〜150の範囲になるように選ばれる。 Desirably, the content per 100 g of the functional group-containing organopolysiloxane is 0.001 mol to 0.1 mol, and if it is less than 0.001 mol, the water repellency decreases with time. Life is shortened and handling becomes difficult. The a3, b3, c3, d3, e3 of the corresponding formula (2) and substituents X 3, number {b3 × (e3 + α) + c3 + 2 × α} alkenyl group having 1 molecule is in the range of 2 to 150 So chosen.
(A3)成分と付加反応する好ましい架橋剤としての(B3)成分は、1分子中にSiHを少なくとも3個有するオルガノハイドロジェンポリシロキサンであって、上述(B2)成分のうちのオルガノハイドロジェンポリシロキサンと同じものが使用できる。含有されるSiHのモル数が、(A3)成分に含まれるアルケニル基のモル数の1〜5倍に相当する量が用いられ、一般的なオルガノハイドロジエンポリシロキサンでの配合質量部としては、(A3)成分の官能性基含有オルガノポリシロキサン100質量部に対して0.1〜20質量部の範囲である。 The component (B3) as a preferred crosslinking agent that undergoes addition reaction with the component (A3) is an organohydrogenpolysiloxane having at least three SiHs in one molecule, and the organohydrogenpolysiloxane among the components (B2) described above. The same as siloxane can be used. The amount of SiH contained is an amount corresponding to 1 to 5 times the number of moles of the alkenyl group contained in the component (A3), and as a blending mass part in a general organohydropolyenepolysiloxane, (A3) It is the range of 0.1-20 mass parts with respect to 100 mass parts of functional group containing organopolysiloxane of a component.
(B3)成分の配合量に含有されるSiHのモル数が(A3)成分に含まれるアルケニル基のモル数の1倍未満では、アルケニル基とSiHの付加反応による橋架け結合が十分ではなく撥水性や非粘着性が低下する一方、5倍を超えて配合しても効果の顕著な増加は見られず、かえって経時変化の原因となるうえ、経済的にも不利となる。 When the number of moles of SiH contained in the blending amount of component (B3) is less than one times the number of moles of alkenyl groups contained in component (A3), the bridging bond due to the addition reaction between the alkenyl group and SiH is not sufficient. On the other hand, the aqueous and non-adhesive properties are reduced, but even if blended in excess of 5 times, a significant increase in the effect is not observed, which causes a change with time and is disadvantageous economically.
(A3)と(B3)成分の組み合わせに対して好ましい触媒(D3)成分は、付加反応を促進して架橋させ、撥水性と非粘着性を付与し持続性を高めるために用いられる。かかる付加反応触媒としては、例えば、白金黒、塩化白金酸、塩化白金酸−オレフィンコンプレックス、塩化白金酸−アルコール配位化合物、ロジウム、ロジウム−オレフィンコンプレックス等が挙げられる。上記付加反応触媒は、(A3)成分と(B3)成分の合計質量部に対し、白金の量又はロジウムの量として5〜1000ppm(質量比)配合することが、性能を付与持続する上で好ましいが、前記成分の反応性又は所望の硬化速度に応じて適宜増減させることができる。 A preferred catalyst (D3) component for the combination of the components (A3) and (B3) is used to promote the addition reaction to crosslink, impart water repellency and non-tackiness, and increase durability. Examples of such an addition reaction catalyst include platinum black, chloroplatinic acid, chloroplatinic acid-olefin complex, chloroplatinic acid-alcohol coordination compound, rhodium, rhodium-olefin complex, and the like. The addition reaction catalyst is preferably blended in an amount of 5 to 1000 ppm (mass ratio) as the amount of platinum or the amount of rhodium with respect to the total mass part of the component (A3) and the component (B3) in order to provide and maintain performance. However, it can be appropriately increased or decreased depending on the reactivity of the components or the desired curing rate.
(A2)、(A3)成分の官能性基含有オルガノポリシロキサンは、25℃における粘度の範囲が0.05〜500Pa・sであり、粘度が0.05Pa・s未満では非粘着性が得られ難く、500Pa・sを超えると(C)成分との分散性が低下する。望ましくは粘度が0.1〜100Pa・sである。相当する式(2)〜(3)及び置換基X2〜X3のa、b、c、d、eとしては、重合度{a+d+b×(c+e+1)+2}が30〜2,000の範囲になるように選ばれる。 The functional group-containing organopolysiloxanes (A2) and (A3) have a viscosity range of 0.05 to 500 Pa · s at 25 ° C., and non-adhesiveness is obtained when the viscosity is less than 0.05 Pa · s. It is difficult, and if it exceeds 500 Pa · s, the dispersibility with the component (C) decreases. Desirably, the viscosity is 0.1 to 100 Pa · s. As a, b, c, d, e of the corresponding formulas (2) to (3) and substituents X 2 to X 3 , the polymerization degree {a + d + b × (c + e + 1) +2} is in the range of 30 to 2,000. Chosen to be.
(A2)、(A3)成分の官能性基含有オルガノポリシロキサンの主骨格構造は直鎖であるが、bが0でない場合で示されるように分岐鎖構造を含むものも使用できる。これらのオルガノポリシロキサンは、それぞれ有機系樹脂で変性されたオルガノポリシロキサンを用いてもよい。有機系樹脂としては、例えば、PVA樹脂、アクリル樹脂、ポリエステル樹脂、アルキッド樹脂など極性基や親水性の構造を有するものが挙げられる。 The main skeleton structure of the functional group-containing organopolysiloxane of the components (A2) and (A3) is a straight chain, but those containing a branched chain structure as shown in the case where b is not 0 can also be used. As these organopolysiloxanes, organopolysiloxanes modified with organic resins may be used. Examples of the organic resin include those having a polar group and a hydrophilic structure such as PVA resin, acrylic resin, polyester resin, and alkyd resin.
また、(A2)、(A3)成分の官能性基含有オルガノポリシロキサンに対して、その特性を損なわない範囲において、下記平均組成式(3)で示される官能性基を含有しないオルガノポリシロキサン(A1)を併用することができる。 Moreover, in the range which does not impair the characteristic with respect to the functional group containing organopolysiloxane of (A2) and (A3) component, the organopolysiloxane which does not contain the functional group shown by the following average composition formula (3) ( A1) can be used in combination.
式中、R1は上記したと同様の一価炭化水素基を示す。X1は以下の式で示される基である。
In the formula, R 1 represents the same monovalent hydrocarbon group as described above. X 1 is a group represented by the following formula.
a1、b1、d1はオルガノポリシロキサンの25℃での粘度が0.05〜500Pa・sを満を満たす正数から選ばれ、28≦a1+b1×(d1+1)≦2,000、b1、d1は0であってもよい。
a1, b1, and d1 are selected from positive numbers satisfying the viscosity of the organopolysiloxane at 25 ° C. of 0.05 to 500 Pa · s, and 28 ≦ a1 + b1 × (d1 + 1) ≦ 2,000, b1, and d1 are 0. It may be.
ここでR1は上記したと同様の一価炭化水素基を示し、例えば、それぞれメチル基、エチル基、プロピル基、ブチル基などのアルキル基、シクロヘキシル基などのシクロアルキル基、フェニル基、トリル基などのアリール基、あるいはこれらの基の炭素原子に結合した水素原子の一部または全部をハロゲン原子、シアノ基などで置換したクロロメチル基、シアノエチル基などのような非置換または置換1価炭化水素基などから選択される基である。このオルガノポリシロキサン全体に含まれるR1はその少なくとも80%がメチル基であることが製造上及び特性上好ましい。 Here, R 1 represents the same monovalent hydrocarbon group as described above, for example, an alkyl group such as a methyl group, an ethyl group, a propyl group, or a butyl group, a cycloalkyl group such as a cyclohexyl group, a phenyl group, or a tolyl group. An unsubstituted or substituted monovalent hydrocarbon such as a chloromethyl group or a cyanoethyl group in which a part or all of the hydrogen atoms bonded to carbon atoms of these groups are substituted with a halogen atom, a cyano group, or the like A group selected from groups and the like. From the viewpoint of production and characteristics, it is preferable that at least 80% of R 1 contained in the whole organopolysiloxane is a methyl group.
(A1)のオルガノポリシロキサンは、上記した(A2)や(A3)成分とは異なり、(B)及び(D)とは非反応性のシリコーン系エマルジョンとして利用される。(A1)成分は、皮膜と化学的に結合されていないため皮膜から除去されやすく、そのため撥水性は経時でより低下し易い傾向にある。これを抑制する方法として、R1として極性基や親水性の基を、撥水性が損なわれない程度に導入してもよいし、後述するように極性基や構造を多く持った樹脂で変性されたものを選択あるいは併用してもよい。(A)成分のPVA系樹脂との相互作用がより大きくなり皮膜からの流出を抑える効果が期待できる。また、組み合わせる(A)成分のPVA系樹脂を、上述の公知の重合性ビニル系モノマーを共重合したものを用いることもできる。 The organopolysiloxane (A1) is used as a silicone-based emulsion that is non-reactive with (B) and (D), unlike the components (A2) and (A3) described above. Since the component (A1) is not chemically bonded to the film, it is easily removed from the film, so that the water repellency tends to be lowered with time. As a method of suppressing this, a polar group or a hydrophilic group may be introduced as R 1 to such an extent that the water repellency is not impaired, or it is modified with a resin having a large number of polar groups or structures as described later. May be selected or used together. The interaction with the PVA resin of the component (A) becomes larger, and an effect of suppressing the outflow from the film can be expected. Moreover, what combined the above-mentioned well-known polymerizable vinyl-type monomer can also be used for the PVA-type resin of (A) component to combine.
(A1)のオルガノポリシロキサンでの配合質量部としては、(A2)成分の官能性基含有オルガノポリシロキサン100質量部に対して0〜30質量部の範囲である。また、(A3)成分の官能性基含有オルガノポリシロキサン100質量部に対して0〜30質量部の範囲である。 The blending part by mass of the organopolysiloxane (A1) is in the range of 0 to 30 parts by mass with respect to 100 parts by mass of the functional group-containing organopolysiloxane of the component (A2). Moreover, it is the range of 0-30 mass parts with respect to 100 mass parts of functional group containing organopolysiloxane of (A3) component.
オルガノポリシロキサンは、変性されることで(C)成分のPVA系樹脂との相互作用がより強くなって分散性が向上し、また皮膜内により強固に保持されて撥水性や非粘着性の経時低下が軽減される。変性に用いる有機系樹脂の量は、本発明が目的とする撥水性や非粘着性を損なわない程度、オルガノポリシロキサンに対して5質量%以下を目安とするが、有機系樹脂の種類や構造により適宜調整されるものである。 By modifying the organopolysiloxane, the interaction with the PVA resin of the component (C) becomes stronger and the dispersibility is improved. Further, the organopolysiloxane is more strongly retained in the film and has a water repellency and non-adhesive property over time. Reduction is reduced. The amount of the organic resin used for modification is 5 mass% or less based on the organopolysiloxane to the extent that the water repellency and non-adhesiveness intended by the present invention are not impaired. Is adjusted as appropriate.
本発明に使用される(C)成分のPVA系樹脂としては、一般に市販されているPVA系樹脂を利用することができるが、以下に述べるような特定のPVA系樹脂を選択した方がより有利に本発明の目的を達成できる。 As the PVA resin of the component (C) used in the present invention, a commercially available PVA resin can be used, but it is more advantageous to select a specific PVA resin as described below. The object of the present invention can be achieved.
PVA系樹脂の大まかな特性は重合度とケン化度で規定されるが、重合度は4%水溶液の20℃での粘度として2〜80mPa・s、ケン化度は80〜99.5モル%を満たすPVA系樹脂を一種類又は複数種類選択して使用することが好ましい。4%水溶液の20℃での粘度が2mPa・s未満では造膜性が不足してしまい、80mPa・sを超えると塗工性が悪くなってしまう。ケン化度80モル%未満では撥油性が十分得られない場合があり、99.5モル%を超えると撥水性が低下してしまうことがある。 Rough characteristics of the PVA resin are defined by the degree of polymerization and the degree of saponification, but the degree of polymerization is 2 to 80 mPa · s as a viscosity of a 4% aqueous solution at 20 ° C., and the degree of saponification is 80 to 99.5 mol%. It is preferable to use one or more types of PVA-based resins that satisfy the requirements. When the viscosity at 20 ° C. of the 4% aqueous solution is less than 2 mPa · s, the film forming property is insufficient, and when it exceeds 80 mPa · s, the coating property is deteriorated. If the degree of saponification is less than 80 mol%, sufficient oil repellency may not be obtained, and if it exceeds 99.5 mol%, the water repellency may decrease.
特に好ましくは、4%水溶液の20℃での粘度が10〜50mPa・s、かつケン化度が85〜95モル%を満たすPVA系樹脂を選択する。4%水溶液の20℃での粘度が10mPa・s未満でも50mPa・sを超えても(B)成分のシリコーン系エマルジョンとの良好な分散状態が得られ難くなり、またケン化度が85〜95モル%の範囲から外れると得られる撥油性の水準が処理条件などに左右され易くなってくる。相当する概略の重合度としては200〜3000、さらに好ましくは500〜2500のものである。 Particularly preferably, a PVA resin satisfying a viscosity of 10 to 50 mPa · s at 20 ° C. of a 4% aqueous solution and a saponification degree of 85 to 95 mol% is selected. Even if the viscosity at 20 ° C. of the 4% aqueous solution is less than 10 mPa · s or more than 50 mPa · s, it is difficult to obtain a good dispersion state with the silicone emulsion of the component (B), and the saponification degree is 85 to 95. If it is out of the mol% range, the level of oil repellency obtained tends to depend on the processing conditions. The corresponding approximate degree of polymerization is 200 to 3000, more preferably 500 to 2500.
本発明の撥水撥油紙を使用する用途にもよるが、熱可塑性を示す温度の高いPVA系樹脂が好ましく利用でき、150℃以下では明らかな可塑性を示さない方が処理時の加熱による悪影響が少ない。 Although depending on the use of the water- and oil-repellent paper of the present invention, a PVA-based resin having a high temperature exhibiting thermoplasticity can be preferably used. Few.
本発明に使用するPVA系樹脂には、公知の重合性ビニル系モノマーを5モル%以下を目安に、その撥油性効果を損なわない範囲で、共重合したものも用いることができる。重合性ビニル系モノマーとしては、例えば、メチルメタアクリレート、プロピルメタアクリレート、アリルメタアクリレート等のメタアクリル酸エステル、メチルアクリレート、ブチルアクリレート等のアクリル酸エステル、ブチルビニルエーテル、スチレン、ブテン、ブタジエン、アクリロニトリル、アクリルアミド、無水マレイン酸、塩化ビニル等があげられる。 As the PVA resin used in the present invention, a copolymerized copolymer of a known polymerizable vinyl monomer within a range that does not impair the oil-repellent effect with 5 mol% or less as a guide. Examples of the polymerizable vinyl monomer include methacrylic acid esters such as methyl methacrylate, propyl methacrylate, and allyl methacrylate, acrylic acid esters such as methyl acrylate and butyl acrylate, butyl vinyl ether, styrene, butene, butadiene, acrylonitrile, Examples include acrylamide, maleic anhydride, vinyl chloride and the like.
また同じ様に、側鎖基の5モル%以下を目安に炭素数1〜20の炭化水素基、例えばアルキル基、アリール基、及びそれらの水素原子がケイ素含有基で置換したものも用いることができる。 Similarly, a hydrocarbon group having 1 to 20 carbon atoms such as an alkyl group, an aryl group, and a hydrogen atom thereof substituted with a silicon-containing group can be used with 5 mol% or less of the side chain group as a guide. it can.
本発明に用いるPVA系樹脂には、その効果を損なわない範囲で各種添加剤を加えてもよい。例えば、シランカップリング剤をPVA系樹脂に対して0.5〜10質量%の添加すれば密着性の向上が期待できる。適当なシランカップリング剤としては3−グリシドキシプロピルトリメトキシシラン、3−グリシドキシプロピルメチルジメトキシシランシ、3−メタクリロキシプロピルトリメトキシシラン、3−メタクリロキシプロピルメチルジメトキシシラン等で良好な結果が得られる。 Various additives may be added to the PVA resin used in the present invention as long as the effect is not impaired. For example, if a silane coupling agent is added in an amount of 0.5 to 10% by mass with respect to the PVA resin, an improvement in adhesion can be expected. Suitable silane coupling agents include 3-glycidoxypropyltrimethoxysilane, 3-glycidoxypropylmethyldimethoxysilane, 3-methacryloxypropyltrimethoxysilane, 3-methacryloxypropylmethyldimethoxysilane and the like. Results are obtained.
(C)成分のPVA系樹脂の配合量は(A)成分のオルガノポリシロキサンの100質量部に対し50〜1000質量部であり、50質量部未満では撥油性が低下し、1000質量部を超えると撥水性が不足してしまう。 (C) The compounding quantity of the PVA-type resin of a component is 50-1000 mass parts with respect to 100 mass parts of the organopolysiloxane of (A) component, and if it is less than 50 mass parts, oil repellency falls and exceeds 1000 mass parts. And water repellency will be insufficient.
(E)成分の水は、本発明の組成物においてPVA系樹脂の溶媒及び官能性基含有オルガノポリシロキサンなど疎水性成分の分散媒として使用される。水道水程度の不純物濃度であれば十分であるが、強酸、強アルカリ、多量のアルコール、塩類などの混入した水は分散性を低下させるため使用には適さない。 (E) Water of a component is used as a dispersion medium of hydrophobic components, such as a solvent of PVA-type resin, and functional group containing organopolysiloxane in the composition of this invention. An impurity concentration of about the same level as tap water is sufficient, but water mixed with strong acid, strong alkali, a large amount of alcohol, salt, etc. is not suitable for use because it lowers dispersibility.
水の量は、実際に使用する塗工装置に適した粘度と、目標とする紙材料への塗工量を本発明の組成物が満たすように調整されるもので、特に限定されるものではないが、一般的には濃度1〜20%である。水の配合量としては(A)成分の100質量部に対して100〜100000質量部が好ましい。100質量部未満では分散が難しくなり、100000質量部を超えると分散状態の経時での低下が大きくなる。 The amount of water is adjusted so that the composition of the present invention satisfies the viscosity suitable for the coating apparatus actually used and the coating amount on the target paper material, and is not particularly limited. In general, the concentration is 1 to 20%. As a compounding quantity of water, 100-100000 mass parts is preferable with respect to 100 mass parts of (A) component. When the amount is less than 100 parts by mass, dispersion becomes difficult, and when the amount exceeds 100000 parts by mass, the degradation of the dispersed state with time increases.
本発明に使用される(F)成分としての加水分解性基含有シランは、1分子あたり1個以上の加水分解性基を持つもので、好ましくは2個以上持つもの、より望ましくは3個以上持つものから選ばれる。1分子あたりの加水分解性基が多いほど撥油性を向上させる効果がより得られ易い。 The hydrolyzable group-containing silane as the component (F) used in the present invention has one or more hydrolyzable groups per molecule, preferably two or more, more preferably three or more. Choose from what you have. The more hydrolyzable groups per molecule, the more easily the effect of improving oil repellency.
加水分解性基としては、珪素に直接結合したメトキシ基、エトキシ基、プロポキシ基、ブトキシ基、メトキシエトキシ基、イソプロペノキシ基などのアルコキシ基、アセトキシ基などのアシルオキシ基、エチルアミノ基などのアミノ基、アミド基、エチルメチルブタノキシム基などのオキシム基、が挙げられる。 Hydrolyzable groups include alkoxy groups such as methoxy, ethoxy, propoxy, butoxy, methoxyethoxy, and isopropenoxy, which are directly bonded to silicon, acyloxy groups such as acetoxy, amino groups such as ethylamino, And oxime groups such as an amide group and an ethylmethylbutanoxime group.
加水分解性基以外の基は、基炭素数1〜20の一価の炭化水素基から選ばれる。例えばメチル基、エチル基、プロピル基、ブチル基などのアルキル基、シクロヘキシル基などのシクロアルキル基、フェニル基、トリル基などのアリール基、ビニル基、プロペニル基などのアルケニル基などの1価炭化水素基が挙げられる。あるいはこれらの基は以下の置換基を持ったものでもよく、例えば水素原子の一部または全部をハロゲン原子などで置換したクロロメチル基、シアノ基で置換したシアノエチル基、エポキシ基で置換したグリシドキシプロピル基、アミノ基で置換したアミノプロピル基、アクリル及びメタアクリル基で置換したメタアクリロキシプロピル基、チオール基で置換したメルカプトプロピル基などのような置換1価炭化水素基などが挙げられる。 The group other than the hydrolyzable group is selected from monovalent hydrocarbon groups having 1 to 20 carbon atoms. For example, monovalent hydrocarbons such as alkyl groups such as methyl group, ethyl group, propyl group and butyl group, cycloalkyl groups such as cyclohexyl group, aryl groups such as phenyl group and tolyl group, alkenyl groups such as vinyl group and propenyl group Groups. Alternatively, these groups may have the following substituents, for example, a chloromethyl group in which some or all of the hydrogen atoms are substituted with halogen atoms, a cyanoethyl group substituted with a cyano group, or a glycidide substituted with an epoxy group. Examples thereof include a substituted monovalent hydrocarbon group such as a xylpropyl group, an aminopropyl group substituted with an amino group, a methacryloxypropyl group substituted with an acryl and methacryl group, and a mercaptopropyl group substituted with a thiol group.
具体的には、テトラメチルシリケート、テトラエチルシリケート、テトラプロピルシリケート、メチルトリメトキシシラン、メチルトリエトキシシラン、メチルトリプロポキシシラン、ジメチルジメトキシシラン、ジメチルジエトキシシラン、ビニルトリメトキシシラン、γ−グリシドキシプロピルトリメトキシシラン、N−β(アミノエチル)γ−アミノプロピルトリメトキシシラン、γ−メルカプトプロピルトリメトキシシラン、γ−メタクリロキシプロピルトリメトキシシランなどである。 Specifically, tetramethyl silicate, tetraethyl silicate, tetrapropyl silicate, methyltrimethoxysilane, methyltriethoxysilane, methyltripropoxysilane, dimethyldimethoxysilane, dimethyldiethoxysilane, vinyltrimethoxysilane, γ-glycidoxy Examples thereof include propyltrimethoxysilane, N-β (aminoethyl) γ-aminopropyltrimethoxysilane, γ-mercaptopropyltrimethoxysilane, and γ-methacryloxypropyltrimethoxysilane.
これら(F)成分としての加水分解性基含有シランは、部分加水分解縮合させたオリゴシロキサンとして使用することもできる。部分加水分解縮合物を用いる利点は、加水分解副生物を減らしてそれによる影響を小さくすることにあるが、オリゴシロキサンの重合度が高くなりすぎると組成物中への分散性が悪くなり、撥油性を向上させる効果自体が低下する傾向が見られる。選択したシランの構造により最適な重合度は異なるが、オリゴシロキサンの平均重合度を50以下、望ましくは10以下とすることで多くの場合に好ましい結果が期待できる。また、部分加水分解縮合させたオリゴシロキサンとシランモノマーとの併用も可能である。 These hydrolyzable group-containing silanes as the component (F) can also be used as partially hydrolyzed and condensed oligosiloxanes. The advantage of using a partially hydrolyzed condensate is that it reduces the amount of hydrolysis by-products and reduces the effect thereof. However, if the degree of polymerization of the oligosiloxane becomes too high, the dispersibility in the composition becomes poor and the repellent properties are reduced. There is a tendency that the effect of improving the oiliness itself decreases. Although the optimum degree of polymerization varies depending on the structure of the selected silane, favorable results can be expected in many cases by setting the average degree of polymerization of oligosiloxane to 50 or less, preferably 10 or less. Moreover, the combined use of the partially hydrolyzed and condensed oligosiloxane and the silane monomer is also possible.
(F)成分の配合量は(A)成分の官能性基含有オルガノポリシロキサン100質量部に対して1〜250質量部であり、1質量部未満では目立った撥水性の向上が見られず、250質量部を超えて配合すると経時で分散性の低下が見られる場合がある。 The blending amount of the component (F) is 1 to 250 parts by mass with respect to 100 parts by mass of the functional group-containing organopolysiloxane of the component (A), and a marked improvement in water repellency is not seen at less than 1 part by mass. When the amount exceeds 250 parts by mass, a decrease in dispersibility may be observed over time.
組成物の調製は、(A)成分の官能性基含有オルガノポリシロキサン100質量部に対して、(B)成分の架橋剤を0.1〜30質量部、(C)PVA系樹脂の50〜1000質量部、(F)成分の加水分解性基含有シラン及びその部分加水分解縮合物を1〜250質量部配合し、後述する塗工方法や塗工量に合わせて粘度及び濃度を調整するため(E)成分の希釈水100〜100000質量部を適宜加えて、公知の方法を用いて均一に分散して処理剤組成物とする。(D)成分の触媒を使用する場合は塗工する直前に必要量を配合して均一に分散させる。 Preparation of the composition is 0.1 to 30 parts by mass of the crosslinking agent of component (B) and 50 to 50 of PVA-based resin for 100 parts by mass of the functional group-containing organopolysiloxane of component (A). 1000 parts by mass, 1 to 250 parts by mass of the hydrolyzable group-containing silane of component (F) and its partial hydrolysis condensate are blended, and the viscosity and concentration are adjusted in accordance with the coating method and coating amount described below. (E) 100-100000 mass parts of dilution water of a component is added suitably, and it disperse | distributes uniformly using a well-known method, and is set as a processing agent composition. (D) When using the catalyst of a component, it mix | blends a required amount just before coating and disperse | distributes it uniformly.
(A)成分の官能性基含有オルガノポリシロキサンと(B)成分の架橋剤は疎水性であるため、水を含む組成物への分散性をより向上させる目的で、予めエマルジョンにしてから配合してもよい。エマルジョンの製造は、公知の方法を用いて上記成分を均一に分散すればよく、例えば(A)成分、(E)成分の一部、(B)成分を、プラネタリーミキサー、コンビミキサーなどの高剪断可能な撹拌装置を用いて混合し、転相法により乳化し、(E)成分の残分を加えて希釈してエマルジョンにする方法が挙げられる。各成分は単一で使用しても2種類以上を併用しても良い。 Since the functional group-containing organopolysiloxane of component (A) and the cross-linking agent of component (B) are hydrophobic, they are pre-emulsified for the purpose of further improving dispersibility in water-containing compositions. May be. The emulsion may be produced by uniformly dispersing the above components using a known method. For example, the component (A), a part of the component (E), and the component (B) are mixed with a high amount such as a planetary mixer or a combination mixer. Examples of the method include mixing using a shearable stirrer, emulsifying by a phase inversion method, and adding the residue of component (E) to dilute to form an emulsion. Each component may be used alone or in combination of two or more.
エマルジョンの製造をより容易にし、その安定性を向上させるために界面活性剤を利用することもできる。好ましい界面活性剤としては、ノニオン系、例えば、ポリオキシエチレンラウリルエーテル、ポリオキシエチレントリデシルエーテル等のアルキルエーテル型のもの、ポリオキシエチレンオレート、ポリオキシエチレンラウレート等のアルキルエステル型のものが挙げられる。これらのノニオン系乳化剤は1種単独又は2種以上を組み合わせて使用することができる。安定なシリコーンエマルジョンを得るには、これらノニオン系乳化剤の単独あるいは混合後のHLBが10〜15であることが望ましい。 Surfactants can also be utilized to make the emulsion easier to manufacture and improve its stability. Preferred surfactants are nonionic, for example, alkyl ether types such as polyoxyethylene lauryl ether and polyoxyethylene tridecyl ether, and alkyl ester types such as polyoxyethylene oleate and polyoxyethylene laurate. Can be mentioned. These nonionic emulsifiers can be used singly or in combination of two or more. In order to obtain a stable silicone emulsion, it is desirable that these nonionic emulsifiers have an HLB of 10 to 15 either alone or after mixing.
また、アニオン型界面活性剤やカチオン型界面活性剤も使用できるが、ノニオン系界面活性剤と併用することが、分散性の点から望ましい。 Anionic surfactants and cationic surfactants can also be used, but it is desirable to use them together with nonionic surfactants from the viewpoint of dispersibility.
界面活性剤の配合量は、良好な分散状態とその持続性が十分得られる最少の量とすることが望ましい。具体的には(A)成分の官能性基含有オルガノシロキサンの100質量部に対し0.1〜10質量部、好ましくは0.2〜6質量部である。0.1質量部未満では乳化を促進する効果が得られず、10質量部を超えると、(A3)成分と(B3)成分の付加反応を阻害して撥水性や非粘着性が低下する場合がある。 The blending amount of the surfactant is desirably a minimum amount that can sufficiently obtain a good dispersion state and its sustainability. Specifically, it is 0.1 to 10 parts by mass, preferably 0.2 to 6 parts by mass with respect to 100 parts by mass of the functional group-containing organosiloxane of component (A). When the amount is less than 0.1 parts by mass, the effect of promoting emulsification cannot be obtained, and when the amount exceeds 10 parts by mass, the addition reaction between the component (A3) and the component (B3) is inhibited, resulting in a decrease in water repellency and non-adhesiveness. There is.
エマルジョン化する際の水(E)成分は、(A)成分の100質量部に対して100〜400質量部が好ましい。100質量部未満では分散が難しくなり、400質量部を超えると分散状態の経時での低下が大きくなる。 As for the water (E) component at the time of emulsification, 100-400 mass parts is preferable with respect to 100 mass parts of (A) component. If the amount is less than 100 parts by mass, the dispersion becomes difficult, and if the amount exceeds 400 parts by mass, the deterioration of the dispersed state over time increases.
エマルジョン化に代わる方法として、市販されている汎用品のシリコーン系エマルジョンのなかから、水分散型のもので、かつPVA系樹脂と混合して良好な分散状態を形成できるものを選択して使用することもできる。骨格構造が主にジメチルシロキサンから構成されているシリコーンを主成分とするシリコーン系エマルジョンが撥水性や非粘着性の点から好ましく、例えば撥水処理用、離形処理用、剥離紙用などのシリコーン系エマルジョンで硬化型のものが利用に適している。 As an alternative to emulsification, use a commercially available silicone emulsion that is water-dispersed and that can be mixed with a PVA resin to form a good dispersion. You can also. A silicone-based emulsion mainly composed of dimethylsiloxane and having a skeleton structure mainly composed of silicone is preferable from the viewpoint of water repellency and non-adhesiveness. For example, silicone for water repellent treatment, release treatment, release paper, etc. A curable emulsion is suitable for use.
縮合触媒(D2)及び付加反応触媒(D3)成分は上記(A)(B)成分と同時に乳化せず、使用する直前に添加することが望ましい。より好ましくは、触媒は添加に先立ち水分散可能なものとするのが好ましく、例えば、界面活性剤と予め混合しておく方法や、上述の方法でエマルジョンにしておく方法などが有効である。 It is desirable that the condensation catalyst (D2) and the addition reaction catalyst (D3) component are not emulsified at the same time as the components (A) and (B) and are added immediately before use. More preferably, the catalyst is preferably water-dispersible prior to addition. For example, a method in which the catalyst is mixed in advance with a surfactant or a method in which an emulsion is formed by the above-described method is effective.
(F)成分につては、選択する加水分解性基含有シラン及びその部分加水分解縮合物の構造により組成物への分散性が左右されるため、上述の組成物製造方法では十分な分散性が得られない場合には、(F)成分についても分散性を向上させる処置を採用することが好ましい。そのためには公知の方法を使用できるが、例えば以下の方法を採ることができる。方法の一つは、(F)成分と乳化剤及び水を加えて予め自己乳化型としたものや、溶液又はエマルジョン化しておいたものを配合する方法である。用いられる乳化剤や乳化方法については、公知のもの及び方法を採用できるが、上述した(A)(B)成分のエマルジョン化に用いられる乳化剤や方法を利用してもよいし、場合によっては(A)(B)成分をエマルジョン化する際に(F)成分も配合して、まとめてエマルジョン化することも可能である。他の方法としては、予め(F)成分と水を混合撹拌して、加水分解性官能基の一部あるいは全部を加水分解させてシラノール基に変えることで分散性を高め、均一な水溶液となったものを配合する方法である。均一化し難い場合は温度を上げたり、微量の酸を用いて水相のpHを2〜4に調整すると加水分解を促すことができるが、pHを下げ過ぎると縮合も進み逆効果となる。 As for the component (F), the dispersibility in the composition depends on the structure of the hydrolyzable group-containing silane to be selected and the partially hydrolyzed condensate thereof. When it cannot be obtained, it is preferable to adopt a treatment for improving the dispersibility of the component (F). For this purpose, a known method can be used. For example, the following method can be adopted. One of the methods is a method of adding a self-emulsifying type by adding the component (F), an emulsifier and water, or a solution or emulsion. As the emulsifier and the emulsification method used, known ones and methods can be adopted. However, the emulsifier and method used for emulsifying the components (A) and (B) described above may be used, and in some cases (A ) When the component (B) is emulsified, the component (F) can also be blended and emulsified together. As another method, the (F) component and water are mixed and stirred in advance, and part or all of the hydrolyzable functional groups are hydrolyzed to change to silanol groups, thereby increasing dispersibility and obtaining a uniform aqueous solution. It is a method of blending the food. If it is difficult to homogenize, hydrolysis can be promoted by raising the temperature or adjusting the pH of the aqueous phase to 2 to 4 using a small amount of acid. However, if the pH is lowered too much, condensation proceeds and the reverse effect is obtained.
使用できる水は、水道水程度の不純物濃度であれば十分であるが、強酸、強アルカリ、多量のアルコール、塩類などの混入した水は分散性を低下させるため使用には適さない。 Water that can be used is sufficient if the impurity concentration is about the same as tap water, but water mixed with strong acid, strong alkali, a large amount of alcohol, salts, etc. is not suitable for use because it lowers dispersibility.
これらの成分以外に、他の任意成分、例えば白金族金属系触媒の触媒活性を抑制する目的で、各種有機窒素化合物、有機リン化合物、アセチレン誘導体、オキシム化合物、有機ハロゲン化物などの触媒活性抑制剤、非粘着性を制御する目的でシリコーンレジン、シリカ、又はケイ素原子に結合した水素原子やアルケニル基を有さないオルガノポリシロキサン、界面活性剤などのレベリング剤、水溶性高分子、例えばメチルセルロースなどのセルロース誘導体、デンプン誘導体、などの増粘剤、造膜性を高める目的でスチレン・無水マレイン酸共重合体等などの公知の改良剤を必要に応じて添加することができる。なお、任意成分の添加量は、本発明の効果を妨げない範囲で通常量とすることができる。 In addition to these components, catalyst activity inhibitors such as various organic nitrogen compounds, organic phosphorus compounds, acetylene derivatives, oxime compounds, and organic halides are used for the purpose of suppressing the catalytic activity of other optional components such as platinum group metal catalysts. For the purpose of controlling non-stickiness, silicone resin, silica, organopolysiloxane having no hydrogen atom or alkenyl group bonded to silicon atom, leveling agent such as surfactant, water-soluble polymer such as methyl cellulose Thickeners such as cellulose derivatives and starch derivatives, and known improvers such as styrene / maleic anhydride copolymers may be added as necessary for the purpose of improving film-forming properties. In addition, the addition amount of an arbitrary component can be made into a normal amount in the range which does not inhibit the effect of this invention.
PVA系樹脂の100質量部に対し、シリコーン系エマルジョンを20〜300質量部を含有し、好ましくはエマルジョン中のシリコーン成分として5〜100質量部の範囲になるように配合し、後述する塗工方法や塗工量に合わせて粘度及び濃度を調整するため(C)成分の希釈水を適宜加えて、公知の方法を用いて均一に分散して処理剤組成物とする。使用できる(C)成分の水は、水道水程度の不純物濃度であれば十分であるが、強酸、強アルカリ、多量のアルコール、塩類などの混入した水は分散性を低下させるため使用には適さない。 A coating method which contains 20 to 300 parts by mass of the silicone emulsion and preferably 5 to 100 parts by mass as a silicone component in the emulsion with respect to 100 parts by mass of the PVA resin, and which will be described later. In order to adjust the viscosity and concentration according to the coating amount, the (C) component dilution water is added as appropriate, and uniformly dispersed using a known method to obtain a treating agent composition. The water of component (C) that can be used is sufficient if it has an impurity concentration equivalent to tap water, but water mixed with strong acid, strong alkali, a large amount of alcohol, salts, etc. is suitable for use because it reduces dispersibility. Absent.
水の量は、実際に使用する塗工装置に適した粘度と、目標とする紙材料への塗工量を満たすように調整されるもので、特に限定されるものではないが、一般的には濃度1〜20%である。水の配合量としては(A)成分の100質量部に対して100から10000質量部が好ましい。100質量部未満では分散が難しくなり、10000質量部を超えると分散状態の経時での低下が大きくなる。 The amount of water is not particularly limited as it is adjusted so as to satisfy the viscosity suitable for the coating apparatus actually used and the coating amount to the target paper material. The concentration is 1 to 20%. As a compounding quantity of water, 100 to 10000 mass parts is preferable with respect to 100 mass parts of (A) component. If the amount is less than 100 parts by mass, the dispersion is difficult, and if it exceeds 10,000 parts by mass, the deterioration of the dispersed state over time increases.
これらの成分以外に、他の任意成分、例えば非粘着性を制御する目的でシリコーンレジン、シリカ、又はケイ素原子に結合した水素原子やアルケニル基を有さないオルガノポリシロキサン、界面活性剤などのレベリング剤、水溶性高分子、例えばメチルセルロースなどのセルロース誘導体、デンプン誘導体、などの増粘剤、造膜性を高める目的でスチレン・無水マレイン酸共重合体等などの公知の改良剤を必要に応じて添加することができる。なお、任意成分の添加量は、本発明の効果を妨げない範囲で通常量とすることができる。 In addition to these components, other optional components such as silicone resin, silica, or organopolysiloxane having no hydrogen atom or alkenyl group bonded to a silicon atom for the purpose of controlling non-stickiness, a surfactant, etc. Agents, water-soluble polymers, for example, cellulose derivatives such as methylcellulose, thickeners such as starch derivatives, and known improving agents such as styrene / maleic anhydride copolymer for the purpose of improving film-forming properties. Can be added. In addition, the addition amount of an arbitrary component can be made into a normal amount in the range which does not inhibit the effect of this invention.
紙基材上に処理剤組成物を塗工する方法は、塗工液の粘度、塗工速度等を考慮した通常行われている塗工方法、カレンダー塗工、グラビアコーター、エアーナイフコーター、ロールコーター、ワイヤーバーなどの各種コーターを用いた塗工、スプレー塗工等を利用することができる。処理剤組成物の塗工量は固形分として0.1g/m2以上、好ましくは1〜5g/m2の範囲でありる。0.1g/m2未満では良好な撥油性を維持することが難しく、5g/m2を越えても性能向上は小さくコスト上不利である。 The method of coating the treating agent composition on the paper substrate is usually performed in consideration of the viscosity of the coating liquid, coating speed, etc., calendar coating, gravure coater, air knife coater, roll Coating and spray coating using various coaters such as a coater and a wire bar can be used. The coating amount of the treatment composition is 0.1 g / m 2 or more as a solid content, allyl preferably in the range of 1 to 5 g / m 2. It is less than 0.1 g / m 2 it is difficult to maintain good oil repellency, even performance improvement beyond 5 g / m 2 is cost-disadvantageous small.
塗工後、乾燥機を通過させて加熱乾燥させて撥水撥油紙を得る。加熱乾燥の条件は、例えば140℃以上の温度で10秒以上の条件が一般的である。 After coating, a water- and oil-repellent paper is obtained by passing through a dryer and drying by heating. The heat drying conditions are typically, for example, a temperature of 140 ° C. or higher and a time of 10 seconds or longer.
以下、実施例及び比較例を示し、本発明を具体的に説明するが、本発明は下記の実施例に制限されるものではない。 EXAMPLES Hereinafter, although an Example and a comparative example are shown and this invention is demonstrated concretely, this invention is not restrict | limited to the following Example.
A.原料の調製
調製例1
容器内全体を撹拌できる錨型撹拌装置と、周縁に小さな歯型突起が上下に交互に設けられている回転可能な円板とを有する5リットルの複合乳化装置に、(A2)成分として以下の式で示されるポリオルガノシロキサンを100質量部
A. Preparation of raw materials
Preparation Example 1
To a 5 liter composite emulsifying device having a vertical stirring device capable of stirring the entire interior of the container and a rotatable disc having small tooth-shaped projections alternately provided on the periphery, the following as component (A2) 100 parts by mass of the polyorganosiloxane represented by the formula
(式中のMeはメチル基を示し、25℃での粘度が2Pa・s、シラノール基含有量=0.01モル/100g)、
(B2)成分として以下の式で示されるメチルハイドロジエンポリシロキサンを3質量部
(Me in the formula represents a methyl group, the viscosity at 25 ° C. is 2 Pa · s, the silanol group content = 0.01 mol / 100 g),
(B2) 3 parts by mass of methylhydrogenpolysiloxane represented by the following formula as a component
(式中のMeはメチル基を示し、粘度が25mPa・s、H含有量=1.5モル/100g)、界面活性剤としてポリオキシエチレンラウリルエーテルのHLBが13.6のもの1質量部、4%水溶液の20℃での粘度30mPa・s、ケン化度90モル%のPVA樹脂5質量部(予め10%水溶液に調整したもの50質量部として使用)を仕込み、均一に撹拌混合した。
(Me in the formula represents a methyl group, viscosity is 25 mPa · s, H content = 1.5 mol / 100 g), 1 part by mass of polyoxyethylene lauryl ether having an HLB of 13.6 as a surfactant, 5 parts by mass of a PVA resin having a viscosity of 30 mPa · s at 20 ° C. and a saponification degree of 90 mol% (used as 50 parts by mass in advance of a 10% aqueous solution) was charged and mixed uniformly.
この混合物に水10質量部を添加して転相させ、引続き30分間撹拌した。追加の水836質量部を加えて希釈して撹拌し、固形分10%のO/W型エマルジョンを得た。これを(A2)と(B2)成分のシリコーンエマルジョンとして用いた。 To this mixture, 10 parts by mass of water was added for phase inversion, followed by stirring for 30 minutes. An additional 836 parts by mass of water was added, diluted and stirred to obtain an O / W emulsion having a solid content of 10%. This was used as a silicone emulsion of components (A2) and (B2).
調製例2
容器内全体を撹拌できる錨型撹拌装置と、周縁に小さな歯型突起が上下に交互に設けられている回転可能な円板とを有する5リットルの複合乳化装置に、
(A3)成分として以下の式で示されるポリオルガノシロキサンを100質量部
Preparation Example 2
In a 5 liter composite emulsifying device having a vertical stirring device capable of stirring the entire inside of the container and a rotatable disk in which small tooth-shaped projections are alternately provided on the periphery on the periphery,
(A3) 100 parts by mass of a polyorganosiloxane represented by the following formula as a component
(式中のMeはメチル基を示し、Viはビニル基を示し、25℃での粘度が0.4Pa・s、ビニル基含有量は0.03モル/100g)、(B3)成分として以下の式で示されるメチルハイドロジエンポリシロキサンを6質量部
(Me in the formula represents a methyl group, Vi represents a vinyl group, the viscosity at 25 ° C. is 0.4 Pa · s, and the vinyl group content is 0.03 mol / 100 g). 6 parts by mass of methylhydrogenpolysiloxane represented by the formula
(式中のMeはメチル基を示し、粘度が25mPa・s、H含有量=1.5モル/100g)、界面活性剤としてポリオキシエチレンラウリルエーテルのHLBが13.6のもの1質量部、4%水溶液の20℃での粘度30mPa・s、ケン化度90モル%のPVA樹脂5質量部(予め10%水溶液に調整したもの50質量部として使用)、触媒活性抑制剤としてエチニルシクロヘキサノール0.4質量部を仕込み、均一に撹拌混合した。
(Me in the formula represents a methyl group, viscosity is 25 mPa · s, H content = 1.5 mol / 100 g), 1 part by mass of polyoxyethylene lauryl ether having an HLB of 13.6 as a surfactant, 5 parts by mass of a PVA resin having a viscosity of 30 mPa · s at 20 ° C. in a 4% aqueous solution and a saponification degree of 90 mol% (used as 50 parts by mass previously adjusted to a 10% aqueous solution), ethynylcyclohexanol 0 as a catalyst activity inhibitor .4 parts by mass were charged and stirred and mixed uniformly.
この混合物に水10質量部を添加して転相させ、引続き30分間撹拌した。追加の水833質量部を加えて希釈して撹拌し、固形分10%のO/W型エマルジョンを得た。これを(A3)と(B3)成分のシリコーンエマルジョンとして用いた。 To this mixture, 10 parts by mass of water was added for phase inversion, followed by stirring for 30 minutes. An additional 833 parts by weight of water was added, diluted and stirred to obtain an O / W emulsion having a solid content of 10%. This was used as a silicone emulsion of components (A3) and (B3).
調製例3
4%水溶液の20℃での粘度30mPa・s、ケン化度90モル%のPVA樹脂100質量部と、水900質量部を混合し、均一な溶液になるまで撹拌して10%水溶液を調整した。これを(C)成分のPVA系樹脂として用いた。
Preparation Example 3
A 10% aqueous solution was prepared by mixing 100 parts by mass of a PVA resin having a viscosity of 30 mPa · s at 20 ° C. and a saponification degree of 90 mol% with 900 parts by mass of water and stirring until a uniform solution was obtained. . This was used as a PVA-based resin as component (C).
調製例4
4%水溶液の20℃での粘度5mPa・s、ケン化度80モル%のPVA樹脂100質量部と、水900質量部を混合し、均一な溶液になるまで撹拌して10%水溶液を調整した。これを(C)成分のPVA系樹脂として用いた。
Preparation Example 4
A 10% aqueous solution was prepared by mixing 100 parts by mass of a 4% aqueous solution with a viscosity of 5 mPa · s at 20 ° C. and 100 parts by mass of PVA resin having a saponification degree of 80 mol% and 900 parts by mass of water and stirring until a uniform solution was obtained. . This was used as a PVA-based resin as component (C).
調製例5
4%水溶液の20℃での粘度60mPa・s、ケン化度98モル%のPVA樹脂100質量部と、水900質量部を混合し、均一な溶液になるまで撹拌して10%水溶液を調整した。これを(C)成分のPVA系樹脂として用いた。
Preparation Example 5
A 4% aqueous solution was mixed with 100 parts by mass of PVA resin having a viscosity of 60 mPa · s at 20 ° C. and a saponification degree of 98 mol% and 900 parts by mass of water, and stirred until a uniform solution was obtained to prepare a 10% aqueous solution. . This was used as a PVA-based resin as component (C).
B.処理剤組成物の調製
実施例1
調製例1のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を200質量部、テトラエトキシシラン3質量部を配合し良く混合したものを処理剤組成物とした。
B. Preparation of treatment composition
Example 1
100 parts by mass of the silicone emulsion of Preparation Example 1, 200 parts by mass of the PVA-based resin aqueous solution of Preparation Example 3 and 3 parts by mass of tetraethoxysilane were mixed well and used as a treating agent composition.
実施例2
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を200質量部、メチルトリエトキシシランを3質量部、信越化学工業(株)製の白金触媒エマルジョンCAT−PM−10Aを1質量部(シリコーン分に対する白金質量100ppm)配合し良く混合したものを処理剤組成物とした。
Example 2
100 parts by mass of the silicone emulsion of Preparation Example 2, 200 parts by mass of the PVA resin aqueous solution of Preparation Example 3, 3 parts by mass of methyltriethoxysilane, and platinum catalyst emulsion CAT-PM-10A manufactured by Shin-Etsu Chemical Co., Ltd. A treatment composition was prepared by mixing 1 part by mass (platinum mass 100 ppm with respect to silicone) and mixing well.
実施例3
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を200質量部、テトラエトキシシランの平均2量化物を3質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Example 3
100 parts by mass of the silicone emulsion of Preparation Example 2, 200 parts by mass of the PVA resin aqueous solution of Preparation Example 3, 3 parts by mass of an average dimerized tetraethoxysilane, and 1 part by mass of CAT-PM-10A are mixed well. This was used as a treating agent composition.
実施例4
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を1000質量部、テトラエトキシシランを25質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Example 4
A treating agent prepared by mixing 100 parts by mass of the silicone emulsion of Preparation Example 2, 1000 parts by mass of the PVA resin aqueous solution of Preparation Example 3, 25 parts by mass of tetraethoxysilane, and 1 part by mass of CAT-PM-10A and mixing them well. It was set as the composition.
実施例5
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を50質量部、テトラエトキシシランを0.1質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Example 5
100 parts by mass of the silicone emulsion of Preparation Example 2, 50 parts by mass of the PVA resin aqueous solution of Preparation Example 3, 0.1 part by mass of tetraethoxysilane, and 1 part by mass of CAT-PM-10A were mixed well. A treating agent composition was obtained.
実施例6
調製例2のシリコーンエマルジョンを100質量部、調製例4のPVA系樹脂水溶液を200質量部、テトラエトキシシランを3質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Example 6
Treatment agent prepared by mixing 100 parts by mass of the silicone emulsion of Preparation Example 2, 200 parts by mass of the PVA resin aqueous solution of Preparation Example 4, 3 parts by mass of tetraethoxysilane, and 1 part by mass of CAT-PM-10A. It was set as the composition.
実施例7
調製例2のシリコーンエマルジョンを100質量部、調製例5のPVA系樹脂水溶液を200質量部、テトラエトキシシランを3質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Example 7
100 parts by mass of the silicone emulsion of Preparation Example 2, 200 parts by mass of the PVA resin aqueous solution of Preparation Example 5, 3 parts by mass of tetraethoxysilane, and 1 part by mass of CAT-PM-10A were mixed well and treated. It was set as the composition.
比較例1
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を200質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Comparative Example 1
100 parts by mass of the silicone emulsion of Preparation Example 2, 200 parts by mass of the PVA-based resin aqueous solution of Preparation Example 3, and 1 part by mass of CAT-PM-10A were mixed well to obtain a treating agent composition.
比較例2
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を200質量部、テトラエトキシシランを0.05質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Comparative Example 2
100 parts by mass of the silicone emulsion of Preparation Example 2, 200 parts by mass of the PVA resin aqueous solution of Preparation Example 3, 0.05 parts by mass of tetraethoxysilane, and 1 part by mass of CAT-PM-10A were mixed well. A treating agent composition was obtained.
比較例3
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を200質量部、テトラエトキシシランを27質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Comparative Example 3
100 parts by weight of the silicone emulsion of Preparation Example 2, 200 parts by weight of the aqueous PVA resin solution of Preparation Example 3, 27 parts by weight of tetraethoxysilane, and 1 part by weight of CAT-PM-10A are mixed well and treated. It was set as the composition.
比較例4
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を1200質量部、テトラエトキシシランを3質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした。
Comparative Example 4
100 parts by weight of the silicone emulsion of Preparation Example 2, 1200 parts by weight of the PVA aqueous resin solution of Preparation Example 3, 3 parts by weight of tetraethoxysilane, and 1 part by weight of CAT-PM-10A were mixed well and treated. It was set as the composition.
比較例5
調製例2のシリコーンエマルジョンを100質量部、調製例3のPVA系樹脂水溶液を30質量部、テトラエトキシシランを3質量部、CAT−PM−10Aを1質量部配合し良く混合したものを処理剤組成物とした
Comparative Example 5
100 parts by mass of the silicone emulsion of Preparation Example 2, 30 parts by mass of the PVA resin aqueous solution of Preparation Example 3, 3 parts by mass of tetraethoxysilane, and 1 part by mass of CAT-PM-10A were mixed well and treated. As a composition
C.撥水撥油紙の作成
調製例で調製した処理剤組成物を坪量50g/m2の市販クラフト紙に、固形分としての塗工量が2g/m2になるようにバーコーターを用いて塗工し、乾燥機で140℃×30秒の条件で加熱して撥水撥油紙を作成した。
C. Preparation of water- and oil-repellent paper Apply the treatment composition prepared in the Preparation Example to a commercial kraft paper having a basis weight of 50 g / m 2 using a bar coater so that the coating amount as a solid content is 2 g / m 2. And heated with a drier at 140 ° C. for 30 seconds to prepare a water / oil repellent paper.
D.評価方法
分散状態
室温で1週間放置した後の外観を目視で観察し、分離が見られず良好ないものを○、僅かに分離傾向が見られるものを△、分離しているものを×とした。
D. Evaluation methods
The appearance after standing at room temperature for 1 week in a dispersed state was visually observed. The case where separation was not observed was unsatisfactory.
撥油性
3Mキットテスト(TAPPI−RC−338)により測定した。3Mキットテスト法は、ヒマシ油、トルエン、ヘプタンが配合された試験油を撥水撥油紙表面におき、浸透を受けるか否かを測定する試験である。浸透を受けなかった最大の試験油のキット番号を評価結果とし、数値が大きいほど撥油性に優れることを示す。キット番号が12以上を○、キット番号が8〜11を△、キット番号が7以下を×として示した。
It was measured by an oil repellency 3M kit test (TAPPI-RC-338). The 3M kit test method is a test in which a test oil containing castor oil, toluene, and heptane is placed on the surface of a water- and oil-repellent paper to determine whether or not it is permeated. The kit number of the largest test oil that did not permeate was taken as the evaluation result, and the larger the value, the better the oil repellency. A kit number of 12 or more was shown as ◯, a kit number of 8 to 11 as Δ, and a kit number of 7 or less as x.
撥水性
撥水撥油紙表面の水に対する接触角で測定した。接触角が大きいほど撥水性が良好であることを示す。接触角が100°を超えるものを○、100°未満90°以上のものを△、90°未満のものを×として示した。
The water- repellent water-repellent and oil-repellent paper surface was measured by the contact angle with water. A larger contact angle indicates better water repellency. Those having a contact angle exceeding 100 ° were shown as ◯, those having a contact angle of less than 100 ° and 90 ° or more were shown as Δ, and those having a contact angle of less than 90 ° were shown as ×.
非粘着性
撥水撥油紙表面にニットー31Bテープ(巾50mm)を貼り、20g荷重で70℃の条件で20時間エージング後、テープを180°方向で剥がす際に必要な力をオートグラフで測定した。剥離力が1N以下のものを○、1Nを超えるものを×として示した。
A Nitto 31B tape (width 50 mm) was applied to the surface of non-adhesive water and oil repellent paper, and after aging for 20 hours under the condition of 70 ° C. under a load of 20 g, the force required to peel the tape in the 180 ° direction was measured with an autograph. . Those having a peeling force of 1 N or less are indicated by ◯, and those having a peeling force exceeding 1 N are indicated by ×.
溶出試験
塗工面10cm2分の撥水撥油紙を20mlの蒸留水に浸し60℃で30分間放置後、ろ過して溶出液を得た。三角フラスコに溶出液10ml、硫酸0.5ml、0.002モル/l過マンガン酸カリウム溶液1mlを採り5分間煮沸した。加熱後、0.01モル/lシュウ酸ナトリウム溶液1mlを加えて0.002モル/l過マンガン酸カリウム溶液で微紅色になるまで滴定した。別に、溶出液を蒸留水に替えて滴定しブランクを求め、撥水撥油紙塗工面からの溶出物量を以下の式を用いて過マンガン酸カリウム消費量としてを算出した。
過マンガン酸カリウム消費量(ppm)={溶出液の滴定量(ml)−ブランクの滴定量(ml)}×31.6
この値が小さいほど撥水性及び耐水性が良好であることを示す。
Elution test A water- and oil-repellent paper having a coating surface of 10 cm 2 was immersed in 20 ml of distilled water, allowed to stand at 60 ° C. for 30 minutes, and then filtered to obtain an eluate. In an Erlenmeyer flask, 10 ml of eluate, 0.5 ml of sulfuric acid, and 1 ml of 0.002 mol / l potassium permanganate solution were taken and boiled for 5 minutes. After heating, 1 ml of a 0.01 mol / l sodium oxalate solution was added and titrated with a 0.002 mol / l potassium permanganate solution until the color turned slightly red. Separately, the eluate was titrated with distilled water to obtain a blank, and the amount of eluate from the water- and oil-repellent paper coated surface was calculated as the potassium permanganate consumption amount using the following formula.
Potassium permanganate consumption (ppm) = {eluent titration (ml) -blank titration (ml)} × 31.6
A smaller value indicates better water repellency and water resistance.
E.評価結果
以下の表1に結果をまとめた。
E. Evaluation results The results are summarized in Table 1 below.
Claims (8)
(A)官能性基含有オルガノポリシロキサン 100質量部
(B)架橋剤 0.1〜30質量部
(C)ポリビニルアルコール(以下PVAと略す)系樹脂50〜1000質量部
(D)触媒 有効成分として0〜5質量部
(E)水 100〜100000質量部
(F)加水分解性基含有シラン及び又はその部分加水分解縮合物
1〜250質量部 Ing from the following ingredients water dispersion type water and oil repellent composition.
(A) Functional group-containing organopolysiloxane 100 parts by mass (B) Cross-linking agent 0.1-30 parts by mass (C) Polyvinyl alcohol (hereinafter abbreviated as PVA) resin 50-1000 parts by mass (D) Catalyst As an active ingredient 0 to 5 parts by mass (E) Water 100 to 100000 parts by mass (F) Hydrolyzable group-containing silane and / or its partial hydrolysis condensate
1 to 250 parts by mass
(A2)下記平均組成式(1)で示される構造を有し、1分子中に少なくとも2個の水酸基を持つオルガノポリシロキサン
[式中、R1は一価炭化水素基であり、R3は水酸基を示し、X2は以下の式で示される基である。
(a2、b2、c2、d2はオルガノポリシロキサンの25℃での粘度が0.05〜500Pa・sを満たす正数から選ばれ、b2、c2、d2は0であってもよい。)]
(B2)1分子中にケイ素原子に結合した水素原子(以下SiHと略す)または加水分解性基を少なくとも3個有するオルガノハイドロジェンポリシロキサンまたはオルガノポリシロキサンであって、含有されるSiHまたは加水分解性基のモル数が、(A2)成分に含まれる水酸基のモル数の5〜200倍に相当する質量部
(D2)触媒量の縮合触媒 The water-dispersed water / oil repellent according to claim 1 , wherein the components ( A) , (B) and (D) are the following components (A2) , (B2) and (D2), respectively.
(A2) Organopolysiloxane having a structure represented by the following average composition formula (1) and having at least two hydroxyl groups in one molecule
[Wherein, R 1 represents a monovalent hydrocarbon group, R 3 represents a hydroxyl group, and X 2 represents a group represented by the following formula:
(A2, b2, c2, d2 the viscosity at 25 ° C. of the organopolysiloxane is chosen from a positive number to fully plus the 0.05~500Pa · s, b2, c2, d2 may be zero.) ]
(B2) Organohydrogenpolysiloxane or organopolysiloxane having at least three hydrogen atoms bonded to silicon atoms (hereinafter abbreviated as SiH) or hydrolyzable groups in one molecule, and contained SiH or hydrolysis moles sex groups, (A2) weight portion corresponding to 5 to 200 times the number of moles of hydroxyl groups contained in the component (D2) catalytic amount of a condensation catalyst
(A3)下記平均組成式(2)で示される構造を有し、1分子中に少なくとも2個のアルケニル基を持つオルガノポリシロキサン
(B3)1分子中にSiHを少なくとも3個有するオルガノハイドロジェンポリシロキサンであって、含有されるSiHのモル数が、(A3)成分に含まれるアルケニル基のモル数の1〜5倍に相当する質量部
(D3)触媒量の白金族金属系触媒 The water-dispersed water / oil repellent according to claim 1 , wherein the components ( A) , (B) and (D) are the following components (A3) , (B3) and (D3), respectively.
(A3) Organopolysiloxane having a structure represented by the following average composition formula (2) and having at least two alkenyl groups in one molecule
(B3) An organohydrogenpolysiloxane having at least three SiHs in one molecule, wherein the number of moles of SiH contained corresponds to 1 to 5 times the number of moles of alkenyl groups contained in component (A3) Part (D3) catalyst amount of a platinum group metal catalyst
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003357041A JP4530255B2 (en) | 2003-10-16 | 2003-10-16 | Water-dispersed water / oil repellent and water / oil repellent paper and sheet using the same |
| TW93100990A TWI282349B (en) | 2003-01-24 | 2004-01-15 | Silicone composition and paper treatment agent including the same |
| KR1020040004282A KR100996306B1 (en) | 2003-01-24 | 2004-01-20 | Silicone composition and a paper treatment agent comprising the same |
| EP20040075217 EP1441009B1 (en) | 2003-01-24 | 2004-01-23 | Silicone composition and a paper treatment agent comprising the same |
| DE200460023342 DE602004023342D1 (en) | 2003-01-24 | 2004-01-23 | Silicone composition and paper treating agent containing the same |
| EP20080156836 EP1970413B1 (en) | 2003-01-24 | 2004-01-23 | Silicone composition and a paper treatment agent comprising the same |
| US10/762,462 US7459213B2 (en) | 2003-01-24 | 2004-01-23 | Silicone composition and a paper treatment agent comprising the same |
| EP20090171540 EP2135899A1 (en) | 2003-01-24 | 2004-01-23 | Silicone composition and a paper treatment agent comprising the same |
| CNB2004100390827A CN100360612C (en) | 2003-01-24 | 2004-01-29 | Polysiloxane compsn. and paper treatment agent contg. same |
| US11/542,169 US20070032596A1 (en) | 2003-01-24 | 2006-10-04 | Silicone composition and a paper treatment agent comprising the same |
| US11/927,193 US20080064814A1 (en) | 2003-01-24 | 2007-10-29 | Silicone composition and a paper treatment agent comprising the same |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2003357041A JP4530255B2 (en) | 2003-10-16 | 2003-10-16 | Water-dispersed water / oil repellent and water / oil repellent paper and sheet using the same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005120236A JP2005120236A (en) | 2005-05-12 |
| JP4530255B2 true JP4530255B2 (en) | 2010-08-25 |
Family
ID=34614043
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003357041A Expired - Fee Related JP4530255B2 (en) | 2003-01-24 | 2003-10-16 | Water-dispersed water / oil repellent and water / oil repellent paper and sheet using the same |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4530255B2 (en) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4829513B2 (en) * | 2005-03-15 | 2011-12-07 | 信越化学工業株式会社 | Water / oil repellent composition and paper treating agent containing the composition |
| JP2006330277A (en) * | 2005-05-25 | 2006-12-07 | Fuji Xerox Co Ltd | Carrier for electrostatic latent image developer and method for manufacturing the same, electrostatic latent image developer, and image forming apparatus |
| EP1911801B1 (en) * | 2006-10-13 | 2011-05-18 | Shin-Etsu Chemical Co., Ltd. | Coating emulsion composition, and water/oil-repellent paper and making method |
| JP2008095251A (en) * | 2006-10-13 | 2008-04-24 | Shin Etsu Chem Co Ltd | Water- and oil-repellent paper and method for producing the same |
| JP5136952B2 (en) * | 2008-04-18 | 2013-02-06 | 信越化学工業株式会社 | Water / oil repellent composition, water / oil repellent treatment agent for paper-like sheet substrate comprising the composition, and water / oil repellent paper-like sheet treated with the composition |
| US20130052356A1 (en) * | 2011-08-31 | 2013-02-28 | Wacker Chemical Corporation | Paper Coating Compositions For Grease and Water Resistance |
| JP6346456B2 (en) * | 2013-02-22 | 2018-06-20 | 国立研究開発法人産業技術総合研究所 | Water / oil repellent coating and method for producing the same |
| DE102013107329A1 (en) * | 2013-07-10 | 2015-01-15 | Kuraray Europe Gmbh | Impregnating materials for release papers |
| KR102553617B1 (en) | 2014-10-31 | 2023-07-07 | 스미또모 가가꾸 가부시키가이샤 | Transparent coating film |
| CN107109124B (en) | 2014-10-31 | 2021-07-09 | 住友化学株式会社 | Transparent coating film |
| JP6715530B2 (en) | 2014-10-31 | 2020-07-01 | 住友化学株式会社 | Water- and oil-repellent coating mixed composition |
| TWI723964B (en) | 2014-11-12 | 2021-04-11 | 日商住友化學股份有限公司 | Water and oil repellent coating composition and transparent film |
| CN111254712A (en) * | 2020-01-17 | 2020-06-09 | 江西赛欧特科新材料有限公司 | Fluorine-free waterproof breathable fabric and preparation method thereof |
| JP7505551B2 (en) * | 2020-05-22 | 2024-06-25 | 信越化学工業株式会社 | Water repellent composition and fiber treatment agent |
| JP2023057598A (en) * | 2021-10-12 | 2023-04-24 | 信越化学工業株式会社 | Organoalkoxysilane-containing composition and production method of the same, and water absorption-preventive agent |
| CN114481010A (en) * | 2021-12-30 | 2022-05-13 | 西安九洲生物材料有限公司 | Method for improving surface hardness and mechanical property of metal dental medical instrument |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6151057A (en) * | 1984-06-12 | 1986-03-13 | ロ−ヌ−プ−ラン・スペシアリテ・シミ−ク | Aqueous emulsion composition for cellulose material adhesion-repellent and water-repellent treatment |
| JP2000119642A (en) * | 1998-10-12 | 2000-04-25 | Dow Corning Asia Ltd | Water-repellent film-forming composition |
| JP2001139358A (en) * | 1999-09-10 | 2001-05-22 | Dow Corning Corp | Water-repellent composition and production process of water-repellent base material |
-
2003
- 2003-10-16 JP JP2003357041A patent/JP4530255B2/en not_active Expired - Fee Related
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6151057A (en) * | 1984-06-12 | 1986-03-13 | ロ−ヌ−プ−ラン・スペシアリテ・シミ−ク | Aqueous emulsion composition for cellulose material adhesion-repellent and water-repellent treatment |
| JP2000119642A (en) * | 1998-10-12 | 2000-04-25 | Dow Corning Asia Ltd | Water-repellent film-forming composition |
| JP2001139358A (en) * | 1999-09-10 | 2001-05-22 | Dow Corning Corp | Water-repellent composition and production process of water-repellent base material |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2005120236A (en) | 2005-05-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR100996306B1 (en) | Silicone composition and a paper treatment agent comprising the same | |
| JP4743757B2 (en) | Silicone paper treatment agent | |
| JP4530255B2 (en) | Water-dispersed water / oil repellent and water / oil repellent paper and sheet using the same | |
| JP3933700B2 (en) | Epoxysilane emulsion additive compositions in water-based reactive polymer dispersions and methods for their preparation | |
| US6071987A (en) | Silicone emulsion composition and process for producing silicone powder therefrom | |
| US6794444B2 (en) | Silicone emulsion composition adherent to plastic film substrates and release film | |
| WO2008019953A1 (en) | Silicone release coating compositions | |
| JPH10195387A (en) | Curable silicone release coating composition | |
| JP4229850B2 (en) | Water-dispersed water / oil repellent composition and water / oil repellent paper treated with the same | |
| JP4829513B2 (en) | Water / oil repellent composition and paper treating agent containing the composition | |
| JP5296412B2 (en) | Release silicone emulsion composition and releasable substrate using the same | |
| EP0117607A1 (en) | Siloxane-PVA coating compositions | |
| JPH05287217A (en) | Production of diorganopolysiloxane/acrylic ester copolymer emulsion | |
| JP3521775B2 (en) | Silicone resin-containing emulsion composition and article having cured coating of the composition | |
| JP5136952B2 (en) | Water / oil repellent composition, water / oil repellent treatment agent for paper-like sheet substrate comprising the composition, and water / oil repellent paper-like sheet treated with the composition | |
| JP6666827B2 (en) | Silicone resin-containing emulsion composition and cured film-forming substrate | |
| JPH11130962A (en) | Silicone-resin-containing emulsion composition, article with cured film formed therefrom, and production thereof | |
| JP3951135B2 (en) | Emulsion composition for building material and building material | |
| JP3481683B2 (en) | Surface treatment agent for plastic film | |
| JP6767766B2 (en) | Two-component curable paint composition and coating film | |
| JP2005036187A (en) | Emulsion composition used as building material | |
| JPH1149955A (en) | Water base silicone composition | |
| JPH07310051A (en) | Rubber article surface treating agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20051012 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20070912 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20071016 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20071214 |
|
| RD02 | Notification of acceptance of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7422 Effective date: 20071214 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080820 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090430 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20100602 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20100603 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 4530255 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130618 Year of fee payment: 3 |
|
| LAPS | Cancellation because of no payment of annual fees |