JP3842829B2 - Adhesive sheets - Google Patents
Adhesive sheets Download PDFInfo
- Publication number
- JP3842829B2 JP3842829B2 JP15685995A JP15685995A JP3842829B2 JP 3842829 B2 JP3842829 B2 JP 3842829B2 JP 15685995 A JP15685995 A JP 15685995A JP 15685995 A JP15685995 A JP 15685995A JP 3842829 B2 JP3842829 B2 JP 3842829B2
- Authority
- JP
- Japan
- Prior art keywords
- component
- weight
- sensitive adhesive
- adhesive
- monomer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Description
【0001】
【産業上の利用分野】
本発明は、アクリル系共重合体を主成分とした再剥離型感圧接着剤と、これをシ―ト状やテ―プ状などの形態とした接着シ―ト類に関する。
【0002】
【従来の技術】
再剥離型感圧接着剤は、表面保護フイルム、塗装用マスキングテ―プ、粘着メモなどに広く用いられている。たとえば、表面保護フイルムは、金属製品やプラスチツク製品の運搬、貯蔵、加工時に、傷つき、汚染、腐食を防止するために、一時的に表面を保護し、上記目的を達したのちは剥離される。
【0003】
このため、表面保護フイルムは、金属製品やプラスチツク製品の運搬、貯蔵、加工時には被着体に接着して剥離することがなく、上記目的を達したのちは容易に剥離できることが要求される。しかし、通常の感圧接着剤を用いたものでは、貼り付け後に経時変化により接着力の上昇が起こりやすい。
【0004】
その結果、運搬、貯蔵、加工などの目的を達したのちに、表面保護フイルムを被着体から剥離するのが難しく、剥離作業に時間がかかつたり、感圧接着剤の糊残りを生じたりする。とくに、塗装鋼板のような被着体に対しては、その表面粗さのために、感圧接着剤のぬれの進行により接着力の上昇が起こりやすく、上記のような問題がより顕著となる。
【0005】
【発明が解決しようとする課題】
本発明は、上記従来の事情に照らし、塗装鋼板などの被着体に対する接着力の経時上昇性が小さく、容易に剥離可能であり、また糊残りによる被着体の汚染といつた問題のない再剥離型感圧接着剤と、これをシ―ト状やテ―プ状などの形態とした接着シ―ト類を提供することを目的としている。
【0006】
【課題を解決するための手段】
本発明者らは、上記の目的を達成するため、鋭意検討した結果、高鎖長でかつ分岐したアルキル基を有する特定の(メタ)アクリル酸アルキルエステルを共重合成分としたアクリル系共重合体を用いることにより、接着力の経時上昇性が小さく、糊残りによる被着体の汚染の問題のない再剥離型感圧接着剤が得られることを見い出し、本発明を完成するに至つた。
【0007】
すなわち、本発明は、支持体の片面または両面に、下記のA〜C成分;
A)アルキル基の炭素数が14〜22でかつ分岐を有する(メタ)アクリル酸アルキル エステルからなるアクリレ―ト系単量体
B)一般式:CH2 =CR1 COOR2 (R1 は水素またはメチル基、R2 は炭素数4 〜8の炭化水素基または置換炭化水素基である)で表されるアクリレ―ト系単量体 C)上記A,B成分と共重合可能なビニル系単量体
のうち、A成分が30〜90重量%、B成分+C成分が70〜10重量%で、B成分が60〜0重量%、C成分が40〜0重量%である単量体混合物の共重合体を含有する再剥離型感圧接着剤からなる層を有し、この層の弾性率が1〜40 kg / cm 2 であることを特徴とするシ―ト状やテ―プ状などの形態とした接着シ―ト類に係るものである。
【0008】
【発明の構成・作用】
本発明に用いるA成分のアクリレ―ト系単量体は、アルキル基の炭素数が14〜22でかつ分岐を有する(メタ)アクリル酸アルキルエステルであつて、具体的には、イソミリスチル(メタ)アクリレ―ト、イソステアリル(メタ)アクリレ―ト、イソセチル(メタ)アクリレ―トなどが用いられる。このA成分のアクリレ―ト系単量体は、これとB,C成分からなる単量体混合物中、30〜90重量%、好ましくは40〜85重量%の割合で用いられる。
【0009】
A成分のアクリレ―ト系単量体が30重量%未満となると、被着体に対する、とくに被着体表面の塗料に対する親和性が高くなり、接着力の経時上昇性が高くなり、90重量%を超えると、初期接着力の低下を招きやすい。また、この単量体のアルキル基の炭素数が14未満となると、接着力の経時上昇性が小さくなり、さらに、上記アルキル基の炭素数が22を超えたり、分岐を有しないアルキル基を有するものでは、初期接着力を損ないやすい。
【0010】
本発明に用いるB成分のアクリレ―ト系単量体は、前記の一般式:CH2 =CR1 COOR2 (R1 は水素またはメチル基、R2 は炭素数4〜8の炭化水素基または置換炭化水素基である)で表されるものであつて、主として初期接着力の調整に用いられるものである。具体的には、前記の一般式中のR2 がブチル基、イソブチル基、イソアミル基、ヘキシル基、ヘプチル基、2−エチルヘキシル基、イソオクチル基、4−ヒドロキシブチル基、4−メトキシブチル基などからなる(メタ)アクリル酸エステルが挙げられる。
【0011】
本発明に用いるC成分の単量体は、上記A,B成分の単量体と共重合可能なものであつて、初期接着力や凝集力などの調整のために用いられるものである。とくに好ましい単量体は、アクリル酸、メタクリル酸、イタコン酸、マレイン酸、クロトン酸などのカルボキシル基含有単量体である。その他の単量体として、酢酸ビニル、スチレン、(メタ)アクリル酸グリシジル、(メタ)アクリル酸2−ヒドロキシエチル、(メタ)アクリル酸2−ヒドロキシプロピル、(メタ)アクリル酸メチル、(メタ)アクリル酸エチル、(メタ)アクリル酸プロピル、N−(メタ)アクリロイルモルホリン、N,N−ジメチル(メタ)アクリルアミド、N−ビニル−2−ピロリドン、シクロペンチル(メタ)アクリレ―ト、イソボルニル(メタ)アクリレ―トなどが挙げられる。
【0012】
本発明において、上記のB,C成分の単量体は、これとA成分とからなる単量体混合物中、70〜10重量%、好ましくは60〜15重量%の割合で用いられる。また、このうち、B成分は60〜0重量%、好ましくは50〜10重量%、C成分は40〜0重量%、好ましくは30〜0重量%であり、C成分の1種としてカルボキシル基含有単量体を用いるときは、このカルボキシル基含有単量体が5〜0重量%となるようにするのがよい。
【0013】
B成分の使用量が60重量%を超えたり、C成分の使用量が40重量%を超えたりすると、被着体に対する、とくに被着体表面の塗料に対する親和性が高くなり、接着力の経時上昇性が大きくなりやすい。また、同様に、C成分のうち、カルボキシル基含有単量体の使用量が、単量体全体の5重量%より多くなると、やはり接着力の経時上昇性が大きくなり、好ましくない。
【0014】
本発明においては、上記のA〜C成分からなる単量体混合物を、溶液重合、塊状重合、乳化重合、懸濁重合などの公知の重合法にて共重合させることにより、アクリル系共重合体を合成する。このアクリル系共重合体は、これをそのまま再剥離型感圧接着剤としてもよいし、必要に応じて通常使用される架橋剤や、無機粉末や金属粉末などの充填剤、顔料、着色剤などの添加剤を配合してもよい。これら任意成分の配合量は、通常用いられている使用量でよい。
【0015】
本発明の再剥離型感圧接着剤は、アクリル系共重合体の単量体組成や分子量、架橋剤、充填剤の種類や量などを適宜選択することにより、弾性率が1〜40Kg/cm2 、とくに1〜20Kg/cm2 となるようにするのが望ましい。弾性率が1Kg/cm2 より小さいと、接着力の経時上昇性が大きくなり、再剥離性の低下や被着体の汚染を起こし、また40Kg/cm2 より大きいと、初期接着力に劣り、いずれも、再剥離型感圧接着剤として望まれるすぐれた性能が得られにくい。
【0016】
本発明の接着シ―ト類は、このような再剥離型感圧接着剤をポリエステルフイルムなどの合成フイルムやその他公知の各種材質からなる支持体の片面または両面に設けて、シ―ト状やテ―プ状などの形態としたものである。その際、再剥離型感圧接着剤からなる層の厚さは、個々の用途目的に応じて適宜決定されるが、一般には2〜20μm程度の厚さとすればよい。
【0017】
【発明の効果】
本発明は、高鎖長でかつ分岐したアルキル基を有する特定のアクリレ―ト系単量体を共重合成分としたアクリル系共重合体を用いたことにより、塗装鋼板などの被着体に対する接着力の経時上昇性が小さく、容易に剥離可能であり、また糊残りによる被着体の汚染といつた問題のない再剥離型感圧接着剤と、そのシ―ト状やテ―プ状などの形態とした接着シ―ト類を提供できる。
【0018】
【実施例】
つぎに、本発明の実施例を記載して、より具体的に説明する。なお、以下において、部とあるのは重量部を意味するものとする。
【0019】
実施例1
冷却管、窒素導入管、温度計、攪拌装置を備えた反応容器に、イソステアリルアクリレ―ト70部、アクリル酸n−ブチル29部、アクリル酸1部、重合開始剤として2,2´−アゾビス(2−アミジノプロパン)ジヒドロクロライド0.1部、乳化剤としてポリオキシエチレンノニルフエニルエ―テル0.3部、ポリオキシエチレンノニルフエニルエ―テル硫酸アンモニウム0.3部、水100部を入れ、70℃で4時間乳化重合し、10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。
【0020】
このポリマ―エマルシヨンに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この接着剤を、厚さが60μmの低密度ポリエチレンフイルムの片面に、乾燥後の厚さが10μmとなるように塗布し、80℃で5分間乾燥して、接着テ―プを作製した。また、これとは別に、剥離処理したポリエステルフイルムにも同様に塗布、乾燥し、弾性率測定用のサンプルを作製した。
【0021】
実施例2
冷却管、窒素導入管、温度計、攪拌装置を備えた反応容器に、イソステアリルアクリレ―ト40部、アクリル酸n−ブチル40部、メタクリル酸イソブチル19.5部、アクリル酸0.5部、重合開始剤として過硫酸カリウム0.2部、乳化剤としてポリオキシエチレンノニルフエニルエ―テル0.5部、ドデシルベンゼンスルホン酸ナトリウム0.3部を入れ、70℃で4時間乳化重合し、10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。
【0022】
このポリマ―エマルシヨンに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この再剥離型感圧接着剤を用い、以下実施例1と同様にして、接着テ―プおよび弾性率測定用のサンプルを作製した。
【0023】
実施例3
冷却管、窒素導入管、温度計、攪拌装置を備えた反応容器に、イソミリスチルアクリレ―ト69.5部、メタクリル酸イソブチル30部、アクリル酸0.5部、重合開始剤として2,2´−アゾビス(2−アミジノプロパン)ジヒドロクロライド0.2部、乳化剤としてポリオキシエチレンノニルフエニルエ―テル硫酸アンモニウム1部、水100部を入れ、70℃で4時間乳化重合し、10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。
【0024】
このポリマ―エマルシヨンに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この再剥離型感圧接着剤を用い、以下実施例1と同様にして、接着テ―プおよび弾性率測定用のサンプルを作製した。
【0025】
実施例4
冷却管、窒素導入管、温度計、攪拌装置を備えた反応容器に、イソミリスチルアクリレ―ト40部、アクリル酸n−ブチル30部、メタクリル酸イソブチル29.5部、アクリル酸0.5部、重合開始剤として2,2´−アゾビス(2−アミジノプロパン)ジヒドロクロライド0.2部、乳化剤としてポリオキシエチレンノニルフエニルエ―テル硫酸アンモニウム1部、水100部を入れ、70℃で4時間乳化重合し、10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。
【0026】
このポリマ―エマルシヨンに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この再剥離型感圧接着剤を用い、実施例1と同様にして、接着テ―プおよび弾性率測定用のサンプルを作製した。
【0027】
比較例1
モノマ―として、ラウリルアクリレ―ト40部、アクリル酸n−ブチル40部、メタクリル酸イソブチル19.5部、アクリル酸0.5部を用いた以外は、実施例1と同様に乳化重合し、その後10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。これに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この再剥離型感圧接着剤を用い、実施例1と同様にして、接着テ―プおよび弾性率測定用のサンプルを作製した。
【0028】
比較例2
モノマ―として、ステアリルアクリレ―ト80部、アクリル酸n−ブチル19.5部、アクリル酸0.5部を用いた以外は、実施例1と同様に乳化重合し、その後10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。このポリマ―エマルシヨンに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この再剥離型感圧接着剤を用い、実施例1と同様にして、接着テ―プおよび弾性率測定用のサンプルを作製した。
【0029】
比較例3
モノマ―として、イソステアリルアクリレ―ト99.5部、アクリル酸0.5部を用いた以外は、実施例1と同様に乳化重合し、その後10重量%アンモニア水でpH8に調整して、ポリマ―エマルシヨンを得た。これに、エマルシヨンの固形分100部あたりヘキサメチロ―ルメラミン1部を混合して、水分散型の再剥離型感圧接着剤を調製した。この再剥離型感圧接着剤を用い、実施例1と同様にして、接着テ―プおよび弾性率測定用のサンプルを作製した。
他は実施例1と同様にして評価サンプルを得た。
【0030】
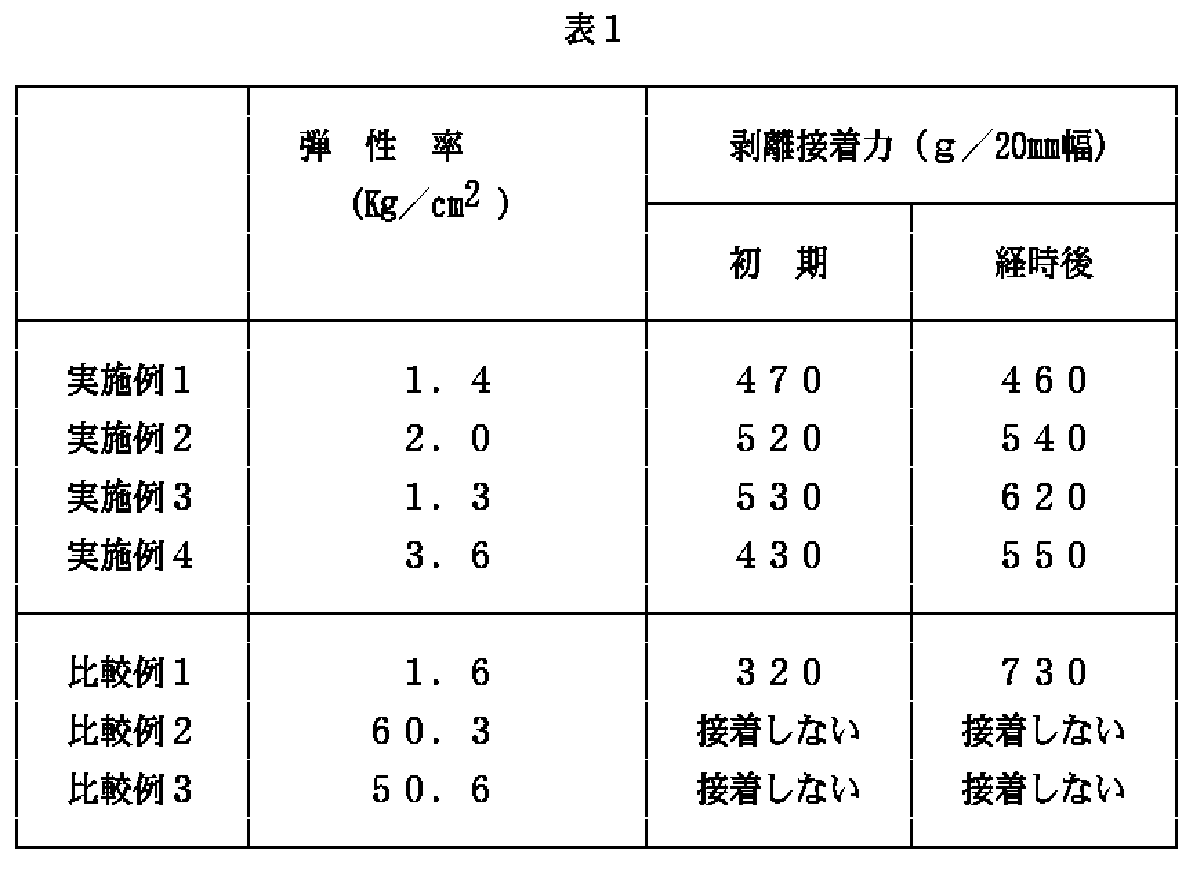
上記の実施例1〜4および比較例1〜3の各再剥離型感圧接着剤について、以下の要領にて、弾性率を測定した。また、各接着テ―プについて、以下の要領にて、初期(23℃,20分放置)および経時後(70℃で48時間放置)の剥離接着力を調べた。結果は、後記の表1に示されるとおりであつた。
【0031】
<弾性率>
再剥離型感圧接着剤を40mm×40mmの大きさに切り出し、幅方向に丸める。これを用いて、万能引張り試験機により、23℃、65%RHの条件下、チヤツク間距離10mm、引張り速度100mm/分で初期の傾きを測定した。
【0032】
<剥離接着力>
接着テ―プを幅20mm×長さ100mmの大きさに裁断して、試験片を作製し、この試験片を、被着体としてのメラミン塗装鋼板に2kgのロ―ラ―を一往復させる方法で圧着し、23℃、65%RHの条件下、初期(23℃,20分放置)および経時後(70℃で48時間放置)に、引張り速度300mm/分の条件にて180度剥離に要する力を測定した。
【0033】
【表1】
【0034】
上記の表1から明らかなように、本発明の再剥離型感圧接着剤を用いた接着テ―プは、適度な初期接着力を有するとともに、接着力の経時上昇性が小さく、このため、経時後(70℃で48時間放置)に容易に剥離可能で、糊残りによる被着体汚染の問題もないことが確認された。[0001]
[Industrial application fields]
The present invention relates to a re-peelable pressure-sensitive adhesive mainly composed of an acrylic copolymer, and an adhesive sheet in the form of a sheet or tape.
[0002]
[Prior art]
Re-peeling pressure-sensitive adhesives are widely used for surface protection films, coating masking tapes, adhesive memos, and the like. For example, the surface protective film temporarily protects the surface in order to prevent damage, contamination, and corrosion during transportation, storage, and processing of metal products and plastic products, and is peeled off after reaching the above purpose.
[0003]
For this reason, it is required that the surface protective film does not adhere to and peel off from the adherend during transportation, storage or processing of metal products or plastic products, and can be easily peeled off after achieving the above purpose. However, in the case of using a normal pressure-sensitive adhesive, the adhesive force tends to increase due to a change with time after pasting.
[0004]
As a result, it is difficult to peel off the surface protection film from the adherend after the purpose of transportation, storage, processing, etc., and it takes time for the peeling work, and the pressure sensitive adhesive remains untouched. To do. In particular, for adherends such as coated steel sheets, due to the surface roughness, the adhesive force tends to increase due to the progress of wetting of the pressure-sensitive adhesive, and the above problems become more prominent. .
[0005]
[Problems to be solved by the invention]
In light of the above-described conventional circumstances, the present invention has a small increase in adhesive strength with time on an adherend such as a coated steel plate, can be easily peeled off, and is free from contamination with adherend residue. It is an object of the present invention to provide a re-peelable pressure-sensitive adhesive and adhesive sheets in the form of a sheet or tape.
[0006]
[Means for Solving the Problems]
As a result of intensive investigations to achieve the above object, the present inventors have found that an acrylic copolymer having a specific (meth) acrylic acid alkyl ester having a high chain length and a branched alkyl group as a copolymerization component. As a result, it has been found that a re-peelable pressure-sensitive adhesive can be obtained which has a small increase in adhesive strength over time and does not have a problem of contamination of the adherend due to adhesive residue, thereby completing the present invention.
[0007]
That is, the present invention provides the following AC components on one side or both sides of the support :
A) An acrylate monomer comprising an alkyl group having 14 to 22 carbon atoms and a branched (meth) acrylic acid alkyl ester B) General formula: CH 2 = CR 1 COOR 2 (R 1 is hydrogen or An acrylate monomer represented by a methyl group and R 2 is a hydrocarbon group having 4 to 8 carbon atoms or a substituted hydrocarbon group) C) a vinyl monomer that is copolymerizable with the above A and B components In the body, a monomer mixture comprising 30 to 90% by weight of the A component, 70 to 10% by weight of the B component + C component, 60 to 0% by weight of the B component, and 40 to 0% by weight of the C component. such as looped - a layer consisting of removable pressure-sensitive adhesive containing the polymer, the modulus of elasticity of the layer is 1 to 40 kg /, characterized in that cm is 2 - DOO shape and Te It relates to adhesive sheets in the form.
[0008]
[Configuration and operation of the invention]
The acrylate monomer of the component A used in the present invention is a (meth) acrylic acid alkyl ester having an alkyl group having 14 to 22 carbon atoms and having a branch. ) Acrylate, isostearyl (meth) acrylate, isocetyl (meth) acrylate, etc. are used. The acrylate monomer of the component A is used in a proportion of 30 to 90% by weight, preferably 40 to 85% by weight, in the monomer mixture comprising this and the B and C components.
[0009]
When the amount of the acrylate monomer of component A is less than 30% by weight, the affinity for the adherend, particularly the paint on the surface of the adherend, is increased, and the aging property of the adhesive force is increased. Exceeding the value tends to cause a decrease in initial adhesive strength. Further, when the carbon number of the alkyl group of this monomer is less than 14, the aging property of the adhesive force is reduced, and further, the alkyl group has a carbon number exceeding 22 or having an alkyl group having no branch. In a thing, it is easy to impair initial adhesive force.
[0010]
The acrylate monomer of the component B used in the present invention has the general formula: CH 2 = CR 1 COOR 2 (where R 1 is hydrogen or a methyl group, R 2 is a hydrocarbon group having 4 to 8 carbon atoms, or It is a substituted hydrocarbon group) and is mainly used for adjusting the initial adhesive strength. Specifically, R 2 in the above general formula is from butyl group, isobutyl group, isoamyl group, hexyl group, heptyl group, 2-ethylhexyl group, isooctyl group, 4-hydroxybutyl group, 4-methoxybutyl group and the like. (Meth) acrylic acid ester is mentioned.
[0011]
The monomer of component C used in the present invention is copolymerizable with the monomers of components A and B, and is used for adjusting the initial adhesive force and cohesive force. Particularly preferred monomers are carboxyl group-containing monomers such as acrylic acid, methacrylic acid, itaconic acid, maleic acid and crotonic acid. Other monomers include vinyl acetate, styrene, glycidyl (meth) acrylate, 2-hydroxyethyl (meth) acrylate, 2-hydroxypropyl (meth) acrylate, methyl (meth) acrylate, (meth) acrylic Ethyl acetate, propyl (meth) acrylate, N- (meth) acryloylmorpholine, N, N-dimethyl (meth) acrylamide, N-vinyl-2-pyrrolidone, cyclopentyl (meth) acrylate, isobornyl (meth) acrylate And so on.
[0012]
In the present invention, the monomers of the above B and C components are used in a proportion of 70 to 10% by weight, preferably 60 to 15% by weight, in the monomer mixture comprising this and the A component. Of these, the B component is 60 to 0% by weight, preferably 50 to 10% by weight, the C component is 40 to 0% by weight, preferably 30 to 0% by weight, and contains a carboxyl group as one of the C components. When using a monomer, the carboxyl group-containing monomer is preferably 5 to 0% by weight.
[0013]
When the usage amount of the B component exceeds 60% by weight or the usage amount of the C component exceeds 40% by weight, the affinity for the adherend, particularly the paint on the surface of the adherend, is increased, and the adhesive strength is deteriorated over time. Elevation tends to increase. Similarly, if the amount of the carboxyl group-containing monomer used in the component C is more than 5% by weight of the total amount of the monomer, the adhesive strength increases with time, which is not preferable.
[0014]
In the present invention, the acrylic copolymer is prepared by copolymerizing the monomer mixture composed of the above-described components A to C by a known polymerization method such as solution polymerization, bulk polymerization, emulsion polymerization, suspension polymerization, or the like. Is synthesized. This acrylic copolymer may be used as a re-peelable pressure-sensitive adhesive as it is, or a crosslinking agent that is usually used as necessary, a filler such as inorganic powder or metal powder, a pigment, a colorant, etc. These additives may be blended. The amount of these optional components may be a commonly used amount.
[0015]
The re-peelable pressure-sensitive adhesive of the present invention has an elastic modulus of 1 to 40 kg / cm by appropriately selecting the monomer composition and molecular weight of the acrylic copolymer, the crosslinking agent, the type and amount of the filler, and the like. 2 , particularly 1 to 20 kg / cm 2 is desirable. If the elastic modulus is less than 1 kg / cm 2 , the adhesive strength increases with time, resulting in a decrease in removability and contamination of the adherend. If it is greater than 40 kg / cm 2 , the initial adhesive strength is poor. In either case, the excellent performance desired as a re-peelable pressure-sensitive adhesive is difficult to obtain.
[0016]
In the adhesive sheet of the present invention, such a re-peelable pressure-sensitive adhesive is provided on one or both sides of a support made of a synthetic film such as a polyester film or other known materials, and a sheet-like or It is in the form of a tape or the like. At that time, the thickness of the layer made of the re-peelable pressure-sensitive adhesive is appropriately determined according to the purpose of each application, but generally may be about 2 to 20 μm.
[0017]
【The invention's effect】
The present invention uses an acrylic copolymer having a specific acrylate monomer having a high chain length and a branched alkyl group as a copolymerization component, thereby allowing adhesion to an adherend such as a coated steel sheet. Removable pressure-sensitive adhesive that does not cause problems due to contamination of the adherend due to adhesive residue, and its sheet shape and tape shape, etc. Adhesive sheets in the form of can be provided.
[0018]
【Example】
Next, examples of the present invention will be described in more detail. In the following, “parts” means parts by weight.
[0019]
Example 1
In a reaction vessel equipped with a cooling tube, a nitrogen introduction tube, a thermometer, and a stirrer, 70 parts of isostearyl acrylate, 29 parts of n-butyl acrylate, 1 part of acrylic acid, and 2,2′- as a polymerization initiator Add 0.1 part of azobis (2-amidinopropane) dihydrochloride, 0.3 part of polyoxyethylene nonyl phenyl ether, 0.3 part of polyoxyethylene nonyl phenyl ammonium sulfate, and 100 parts of water as emulsifier. The emulsion was polymerized at 70 ° C. for 4 hours and adjusted to pH 8 with 10 wt% aqueous ammonia to obtain a polymer emulsion.
[0020]
This polymer emulsion was mixed with 1 part of hexamethylol melamine per 100 parts of the solid content of emulsion to prepare a water dispersion type re-peelable pressure sensitive adhesive. This adhesive was applied to one side of a low-density polyethylene film having a thickness of 60 μm so that the thickness after drying was 10 μm, and dried at 80 ° C. for 5 minutes to prepare an adhesive tape. Separately from this, it was similarly applied to a peeled polyester film and dried to prepare a sample for measuring elastic modulus.
[0021]
Example 2
In a reaction vessel equipped with a cooling tube, a nitrogen introduction tube, a thermometer, and a stirring device, 40 parts of isostearyl acrylate, 40 parts of n-butyl acrylate, 19.5 parts of isobutyl methacrylate, 0.5 parts of acrylic acid , 0.2 parts of potassium persulfate as a polymerization initiator, 0.5 parts of polyoxyethylene nonylphenyl ether and 0.3 parts of sodium dodecylbenzenesulfonate as an emulsifier, and emulsion polymerization at 70 ° C. for 4 hours, A polymer emulsion was obtained by adjusting the pH to 8 with 10% by weight aqueous ammonia.
[0022]
This polymer emulsion was mixed with 1 part of hexamethylol melamine per 100 parts of the solid content of emulsion to prepare a water dispersion type re-peelable pressure sensitive adhesive. Using this re-peelable pressure sensitive adhesive, a sample for measuring an adhesive tape and elastic modulus was prepared in the same manner as in Example 1 below.
[0023]
Example 3
In a reaction vessel equipped with a cooling tube, a nitrogen introduction tube, a thermometer, and a stirrer, 69.5 parts of isomyristyl acrylate, 30 parts of isobutyl methacrylate, 0.5 part of acrylic acid, 2,2 as a polymerization initiator 2 parts of ′ -azobis (2-amidinopropane) dihydrochloride, 1 part of polyoxyethylene nonylphenyl ether sulfate as an emulsifier and 100 parts of water were added, and emulsion polymerization was carried out at 70 ° C. for 4 hours. The pH was adjusted to 8 with water to obtain a polymer emulsion.
[0024]
This polymer emulsion was mixed with 1 part of hexamethylol melamine per 100 parts of the solid content of emulsion to prepare a water dispersion type re-peelable pressure sensitive adhesive. Using this re-peelable pressure sensitive adhesive, a sample for measuring an adhesive tape and elastic modulus was prepared in the same manner as in Example 1 below.
[0025]
Example 4
In a reaction vessel equipped with a cooling tube, a nitrogen introduction tube, a thermometer, and a stirrer, 40 parts of isomyristyl acrylate, 30 parts of n-butyl acrylate, 29.5 parts of isobutyl methacrylate, 0.5 part of acrylic acid , 0.22 part of 2,2′-azobis (2-amidinopropane) dihydrochloride as a polymerization initiator, 1 part of polyoxyethylene nonylphenyl ether ammonium sulfate and 100 parts of water as an emulsifier, and 4 hours at 70 ° C. Emulsion polymerization was performed, and the pH was adjusted to 8 with 10% by weight aqueous ammonia to obtain a polymer emulsion.
[0026]
This polymer emulsion was mixed with 1 part of hexamethylol melamine per 100 parts of the solid content of emulsion to prepare a water dispersion type re-peelable pressure sensitive adhesive. Using this re-peelable pressure-sensitive adhesive, a sample for measuring an adhesive tape and elastic modulus was prepared in the same manner as in Example 1.
[0027]
Comparative Example 1
As a monomer, emulsion polymerization was performed in the same manner as in Example 1 except that 40 parts of lauryl acrylate, 40 parts of n-butyl acrylate, 19.5 parts of isobutyl methacrylate, and 0.5 part of acrylic acid were used. Thereafter, the pH was adjusted to 8 with 10% by weight aqueous ammonia to obtain a polymer emulsion. To this, 1 part of hexamethylol melamine was mixed with 100 parts of solid content of emulsion to prepare a water-dispersible re-peelable pressure-sensitive adhesive. Using this re-peelable pressure-sensitive adhesive, a sample for measuring an adhesive tape and elastic modulus was prepared in the same manner as in Example 1.
[0028]
Comparative Example 2
Emulsion polymerization was carried out in the same manner as in Example 1 except that 80 parts of stearyl acrylate, 19.5 parts of n-butyl acrylate, and 0.5 part of acrylic acid were used as monomers, and then 10% by weight aqueous ammonia. To adjust the pH to 8 to obtain a polymer emulsion. This polymer emulsion was mixed with 1 part of hexamethylol melamine per 100 parts of the solid content of emulsion to prepare a water dispersion type re-peelable pressure sensitive adhesive. Using this re-peelable pressure-sensitive adhesive, a sample for measuring an adhesive tape and elastic modulus was prepared in the same manner as in Example 1.
[0029]
Comparative Example 3
Emulsion polymerization was carried out in the same manner as in Example 1 except that 99.5 parts of isostearyl acrylate and 0.5 part of acrylic acid were used as monomers, and then adjusted to pH 8 with 10 wt% aqueous ammonia, Obtained polymer emulsion. To this, 1 part of hexamethylol melamine was mixed with 100 parts of solid content of emulsion to prepare a water-dispersible re-peelable pressure-sensitive adhesive. Using this re-peelable pressure sensitive adhesive, a sample for measuring an adhesive tape and elastic modulus was prepared in the same manner as in Example 1.
Other than that, an evaluation sample was obtained in the same manner as in Example 1.
[0030]
About each re-peeling type pressure sensitive adhesive of said Examples 1-4 and Comparative Examples 1-3, the elasticity modulus was measured in the following ways. For each adhesive tape, the peel adhesive strength at the initial stage (23 ° C., left for 20 minutes) and after aging (left at 70 ° C. for 48 hours) was examined in the following manner. The results were as shown in Table 1 below.
[0031]
<Elastic modulus>
The peelable pressure sensitive adhesive is cut into a size of 40 mm × 40 mm and rounded in the width direction. Using this, the initial inclination was measured with a universal tensile tester under the conditions of 23 ° C. and 65% RH at a distance between the chucks of 10 mm and a pulling speed of 100 mm / min.
[0032]
<Peel adhesion>
A method in which an adhesive tape is cut into a size of 20 mm wide × 100 mm long to prepare a test piece, and this test piece is reciprocated by a 2 kg roller on a melamine-coated steel sheet as an adherend. It is necessary to peel 180 degrees under the conditions of 23 ° C. and 65% RH, initially (23 ° C., left for 20 minutes) and after time (left at 70 ° C. for 48 hours) at a pulling speed of 300 mm / min. The force was measured.
[0033]
[Table 1]
[0034]
As apparent from Table 1 above, the adhesive tape using the re-peelable pressure-sensitive adhesive of the present invention has an appropriate initial adhesive force and a small increase in the adhesive force with time. It was confirmed that the film could be easily peeled after a lapse of time (left at 70 ° C. for 48 hours) and there was no problem of adherend contamination due to adhesive residue.
Claims (2)
A)アルキル基の炭素数が14〜22でかつ分岐を有する(メタ)アクリル酸アルキル エステルからなるアクリレ―ト系単量体
B)一般式:CH2 =CR1 COOR2 (R1 は水素またはメチル基、R2 は炭素数4 〜8の炭化水素基または置換炭化水素基である)で表されるアクリレ―ト系単量体 C)上記A,B成分と共重合可能なビニル系単量体
のうち、A成分が30〜90重量%、B成分+C成分が70〜10重量%で、B成分が60〜0重量%、C成分が40〜0重量%である単量体混合物の共重合体を含有する再剥離型感圧接着剤からなる層を有し、この層の弾性率が1〜40 Kg / cm 2 であることを特徴とする接着シ―ト類。The following A to C components on one or both sides of the support :
A) An acrylate monomer comprising an alkyl group having 14 to 22 carbon atoms and a branched (meth) acrylic acid alkyl ester B) General formula: CH 2 = CR 1 COOR 2 (R 1 is hydrogen or An acrylate monomer represented by a methyl group and R 2 is a hydrocarbon group having 4 to 8 carbon atoms or a substituted hydrocarbon group) C) a vinyl monomer that is copolymerizable with the above A and B components In the body, a monomer mixture comprising 30 to 90% by weight of the A component, 70 to 10% by weight of the B component + C component, 60 to 0% by weight of the B component, and 40 to 0% by weight of the C component. DOO acids - adhesive sheet, characterized in that a layer consisting of removable pressure-sensitive adhesive containing the polymer, the modulus of elasticity of this layer is 1~40 Kg / cm 2.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP15685995A JP3842829B2 (en) | 1995-05-30 | 1995-05-30 | Adhesive sheets |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP15685995A JP3842829B2 (en) | 1995-05-30 | 1995-05-30 | Adhesive sheets |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JPH08325544A JPH08325544A (en) | 1996-12-10 |
| JP3842829B2 true JP3842829B2 (en) | 2006-11-08 |
Family
ID=15636949
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP15685995A Expired - Lifetime JP3842829B2 (en) | 1995-05-30 | 1995-05-30 | Adhesive sheets |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP3842829B2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8137807B2 (en) | 2010-03-26 | 2012-03-20 | 3M Innovative Properties Company | Pressure-sensitive adhesives derived from 2-alkyl alkanols |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4824154B2 (en) * | 2000-05-16 | 2011-11-30 | 日東電工株式会社 | Adhesive and surface protective film for optical member using the adhesive |
| JP2005314513A (en) * | 2004-04-28 | 2005-11-10 | Nitto Denko Corp | Adhesive composition, and surface-protective film obtained by using the same |
| JP4854092B2 (en) * | 2008-05-16 | 2012-01-11 | 日東電工株式会社 | Re-peelable pressure-sensitive adhesive, pressure-sensitive adhesive layer, and pressure-sensitive adhesive member using these |
| JP5616005B2 (en) | 2008-06-02 | 2014-10-29 | スリーエム イノベイティブ プロパティズ カンパニー | Adhesive composition and adhesive tape |
| JP5469194B2 (en) * | 2011-05-02 | 2014-04-09 | 日東電工株式会社 | Adhesive, adhesive layer, and adhesive sheet |
| KR20140074958A (en) * | 2011-09-26 | 2014-06-18 | 쓰리엠 이노베이티브 프로퍼티즈 컴파니 | Pressure-sensitive adhesives with a (meth)acrylic-based elastomeric material |
| WO2013101827A1 (en) | 2011-12-29 | 2013-07-04 | 3M Innovative Properties Company | Low temperature vibration damping pressure sensitive adhesives and constructions |
| JP5612754B2 (en) * | 2013-12-04 | 2014-10-22 | スリーエム イノベイティブ プロパティズ カンパニー | Adhesive composition and adhesive tape |
| WO2017078026A1 (en) * | 2015-11-06 | 2017-05-11 | リンテック株式会社 | Release agent composition, release sheet, and adhesive body |
| JP7370716B2 (en) * | 2019-03-19 | 2023-10-30 | 日東電工株式会社 | Surface protection film and optical components with protection film |
| JP7370714B2 (en) * | 2019-03-19 | 2023-10-30 | 日東電工株式会社 | Optical components with protective film |
| JP7370715B2 (en) * | 2019-03-19 | 2023-10-30 | 日東電工株式会社 | Surface protection film and optical components with protection film |
| WO2020188950A1 (en) * | 2019-03-19 | 2020-09-24 | 日東電工株式会社 | Surface protection film and protection film-attached optical member |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63202682A (en) * | 1987-02-18 | 1988-08-22 | Nitto Electric Ind Co Ltd | Pressure-sensitive adhesive |
| JPH059449A (en) * | 1991-06-28 | 1993-01-19 | Toyo Ink Mfg Co Ltd | Rereleasable self-adhesive composition |
| TW221061B (en) * | 1991-12-31 | 1994-02-11 | Minnesota Mining & Mfg | |
| JPH06150815A (en) * | 1992-11-02 | 1994-05-31 | Nitto Denko Corp | Removing method for resist film pattern and adhesive or adhesive sheet used for it in picture pattern process of color television shadow mask |
| JPH06151671A (en) * | 1992-11-02 | 1994-05-31 | Nitto Denko Corp | Removal of resist film image in lead frame image formation process, and adhesive or sheet used therefor |
| JP3262607B2 (en) * | 1992-11-09 | 2002-03-04 | 日本カーバイド工業株式会社 | Active energy ray-curable adhesive composition and tape |
| JP3411065B2 (en) * | 1993-07-13 | 2003-05-26 | 積水化学工業株式会社 | Acrylic pressure-sensitive adhesive composition and double-sided tape |
-
1995
- 1995-05-30 JP JP15685995A patent/JP3842829B2/en not_active Expired - Lifetime
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8137807B2 (en) | 2010-03-26 | 2012-03-20 | 3M Innovative Properties Company | Pressure-sensitive adhesives derived from 2-alkyl alkanols |
Also Published As
| Publication number | Publication date |
|---|---|
| JPH08325544A (en) | 1996-12-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4572007B2 (en) | Water-dispersible pressure-sensitive adhesive for re-peeling and its adhesive sheets | |
| JP3810490B2 (en) | Re-peelable pressure-sensitive adhesive and its adhesive sheets | |
| US5118750A (en) | Pressure-sensitive adhesive comprising solid tacky microspheres and macromonomer-containing binder copolymer | |
| JP3842829B2 (en) | Adhesive sheets | |
| US5502108A (en) | Pressure-sensitive adhesive comprising solid tacky microspheres and macromonomer-containing binder copolymer | |
| JPH06166857A (en) | Pressure-sensitive adhesive and adhesive sheet or the like containing the same | |
| EP1054932A1 (en) | Hot-melt adhesive composition | |
| JP3571460B2 (en) | Removable pressure-sensitive adhesive sheets | |
| JP2003027026A (en) | Re-releasable water-dispersed pressure-sensitive adhesive | |
| JPH07138544A (en) | Pressure-sensitive acrylic adhesive composition | |
| JPH08253750A (en) | Re-releasable pressure-sensitive adhesive and adhesive sheet coated therewith | |
| JPH08143842A (en) | Repeelable pressure-sensitive adhesive and its adhesive sheet | |
| JP2002105420A (en) | Re-releasable, water-dispersible, pressure-sensitive adhesive | |
| JPH10158617A (en) | Repeatedly peelable pressure-sensitive adhesive composition of water dispersion type and repeatedly peelable pressure-sensitive adhesive sheet and the like using the same | |
| JP3775811B2 (en) | Method for producing re-peelable adhesive sheets | |
| JPH0819392B2 (en) | Pressure-sensitive adhesives and their adhesive sheets | |
| JP3154739B2 (en) | Photopolymerizable adhesive composition, pressure-sensitive adhesive using the same, and adhesive sheets thereof | |
| KR102642819B1 (en) | Water-based acrylic pressure sensitive adhesive compositno, method for preparing the same, and pressure sensitive adhesive sheet comprising the composition | |
| JP2014070122A (en) | Adhesive for label, and adhesive tape | |
| JPS6191277A (en) | Releasable self-adhesive | |
| JPH07188630A (en) | Releasable pressure-sensitive adhesive | |
| JP3105695B2 (en) | paint remover | |
| JP4380823B2 (en) | Re-peelable pressure-sensitive adhesive and its adhesive sheets | |
| JP2600360B2 (en) | Adhesive for surface protection film | |
| JPH05320604A (en) | Pressure-sensitive acrylic adhesive composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20040907 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20041104 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20060811 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120818 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120818 Year of fee payment: 6 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150818 Year of fee payment: 9 |
|
| EXPY | Cancellation because of completion of term |