JP2016517341A - 腐食条件下で安定しているクロマトグラフィー膜 - Google Patents
腐食条件下で安定しているクロマトグラフィー膜 Download PDFInfo
- Publication number
- JP2016517341A JP2016517341A JP2015562384A JP2015562384A JP2016517341A JP 2016517341 A JP2016517341 A JP 2016517341A JP 2015562384 A JP2015562384 A JP 2015562384A JP 2015562384 A JP2015562384 A JP 2015562384A JP 2016517341 A JP2016517341 A JP 2016517341A
- Authority
- JP
- Japan
- Prior art keywords
- acrylamide
- composite material
- certain embodiments
- membrane
- poly
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000012528 membrane Substances 0.000 title claims abstract description 284
- 238000000034 method Methods 0.000 claims abstract description 162
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims abstract description 141
- 239000002131 composite material Substances 0.000 claims abstract description 130
- 239000000178 monomer Substances 0.000 claims abstract description 115
- 239000011148 porous material Substances 0.000 claims abstract description 58
- 150000002500 ions Chemical class 0.000 claims abstract description 16
- 150000002148 esters Chemical class 0.000 claims abstract description 15
- 239000012530 fluid Substances 0.000 claims description 58
- -1 trimethylammonium halide Chemical class 0.000 claims description 46
- 102000004169 proteins and genes Human genes 0.000 claims description 41
- 108090000623 proteins and genes Proteins 0.000 claims description 41
- 239000003431 cross linking reagent Substances 0.000 claims description 38
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 claims description 37
- KCTMTGOHHMRJHZ-UHFFFAOYSA-N n-(2-methylpropoxymethyl)prop-2-enamide Chemical compound CC(C)COCNC(=O)C=C KCTMTGOHHMRJHZ-UHFFFAOYSA-N 0.000 claims description 34
- 239000000126 substance Substances 0.000 claims description 33
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 claims description 31
- ZIUHHBKFKCYYJD-UHFFFAOYSA-N n,n'-methylenebisacrylamide Chemical group C=CC(=O)NCNC(=O)C=C ZIUHHBKFKCYYJD-UHFFFAOYSA-N 0.000 claims description 31
- 229920000642 polymer Polymers 0.000 claims description 30
- HRPVXLWXLXDGHG-UHFFFAOYSA-N Acrylamide Chemical compound NC(=O)C=C HRPVXLWXLXDGHG-UHFFFAOYSA-N 0.000 claims description 28
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 22
- CNCOEDDPFOAUMB-UHFFFAOYSA-N N-Methylolacrylamide Chemical compound OCNC(=O)C=C CNCOEDDPFOAUMB-UHFFFAOYSA-N 0.000 claims description 21
- 229940088644 n,n-dimethylacrylamide Drugs 0.000 claims description 21
- YLGYACDQVQQZSW-UHFFFAOYSA-N n,n-dimethylprop-2-enamide Chemical compound CN(C)C(=O)C=C YLGYACDQVQQZSW-UHFFFAOYSA-N 0.000 claims description 21
- 239000000872 buffer Substances 0.000 claims description 20
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 claims description 19
- 150000001412 amines Chemical class 0.000 claims description 19
- RWJGITGQDQSWJG-UHFFFAOYSA-N n-(3-methoxypropyl)prop-2-enamide Chemical compound COCCCNC(=O)C=C RWJGITGQDQSWJG-UHFFFAOYSA-N 0.000 claims description 19
- MYRTYDVEIRVNKP-UHFFFAOYSA-N 1,2-Divinylbenzene Chemical compound C=CC1=CC=CC=C1C=C MYRTYDVEIRVNKP-UHFFFAOYSA-N 0.000 claims description 18
- 239000003637 basic solution Substances 0.000 claims description 18
- 239000000463 material Substances 0.000 claims description 18
- 108010014251 Muramidase Proteins 0.000 claims description 17
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 claims description 17
- 239000004325 lysozyme Substances 0.000 claims description 17
- 229960000274 lysozyme Drugs 0.000 claims description 17
- 235000010335 lysozyme Nutrition 0.000 claims description 17
- FQPSGWSUVKBHSU-UHFFFAOYSA-N methacrylamide Chemical compound CC(=C)C(N)=O FQPSGWSUVKBHSU-UHFFFAOYSA-N 0.000 claims description 16
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 claims description 14
- QNILTEGFHQSKFF-UHFFFAOYSA-N n-propan-2-ylprop-2-enamide Chemical compound CC(C)NC(=O)C=C QNILTEGFHQSKFF-UHFFFAOYSA-N 0.000 claims description 12
- 229920001223 polyethylene glycol Polymers 0.000 claims description 12
- FYBFGAFWCBMEDG-UHFFFAOYSA-N 1-[3,5-di(prop-2-enoyl)-1,3,5-triazinan-1-yl]prop-2-en-1-one Chemical compound C=CC(=O)N1CN(C(=O)C=C)CN(C(=O)C=C)C1 FYBFGAFWCBMEDG-UHFFFAOYSA-N 0.000 claims description 11
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 11
- 229920002518 Polyallylamine hydrochloride Polymers 0.000 claims description 11
- 239000007983 Tris buffer Substances 0.000 claims description 11
- 229920001451 polypropylene glycol Polymers 0.000 claims description 11
- 241001465754 Metazoa Species 0.000 claims description 10
- 150000001875 compounds Chemical class 0.000 claims description 10
- 108010038061 Chymotrypsinogen Proteins 0.000 claims description 9
- 229920002873 Polyethylenimine Polymers 0.000 claims description 9
- 241000700605 Viruses Species 0.000 claims description 9
- UUORTJUPDJJXST-UHFFFAOYSA-N n-(2-hydroxyethyl)prop-2-enamide Chemical compound OCCNC(=O)C=C UUORTJUPDJJXST-UHFFFAOYSA-N 0.000 claims description 9
- YBKWKURHPIBUEM-UHFFFAOYSA-N 2-methyl-n-[6-(2-methylprop-2-enoylamino)hexyl]prop-2-enamide Chemical compound CC(=C)C(=O)NCCCCCCNC(=O)C(C)=C YBKWKURHPIBUEM-UHFFFAOYSA-N 0.000 claims description 8
- 108010052832 Cytochromes Proteins 0.000 claims description 8
- 102000018832 Cytochromes Human genes 0.000 claims description 8
- 108060003951 Immunoglobulin Proteins 0.000 claims description 8
- 108010062374 Myoglobin Proteins 0.000 claims description 8
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 claims description 8
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 claims description 8
- 102000018358 immunoglobulin Human genes 0.000 claims description 8
- 229920003213 poly(N-isopropyl acrylamide) Polymers 0.000 claims description 8
- 239000002753 trypsin inhibitor Substances 0.000 claims description 8
- ZJCCRDAZUWHFQH-UHFFFAOYSA-N Trimethylolpropane Chemical compound CCC(CO)(CO)CO ZJCCRDAZUWHFQH-UHFFFAOYSA-N 0.000 claims description 7
- 108010074605 gamma-Globulins Proteins 0.000 claims description 7
- UYJCRMOPNVBSNM-UHFFFAOYSA-N 2-methyl-1-[4-(2-methylprop-2-enoyl)piperazin-1-yl]prop-2-en-1-one Chemical compound CC(=C)C(=O)N1CCN(C(=O)C(C)=C)CC1 UYJCRMOPNVBSNM-UHFFFAOYSA-N 0.000 claims description 6
- AUZRCMMVHXRSGT-UHFFFAOYSA-N 2-methylpropane-1-sulfonic acid;prop-2-enamide Chemical compound NC(=O)C=C.CC(C)CS(O)(=O)=O AUZRCMMVHXRSGT-UHFFFAOYSA-N 0.000 claims description 6
- 102000009027 Albumins Human genes 0.000 claims description 6
- 108010088751 Albumins Proteins 0.000 claims description 6
- 101710162629 Trypsin inhibitor Proteins 0.000 claims description 6
- 229940122618 Trypsin inhibitor Drugs 0.000 claims description 6
- 229920002678 cellulose Polymers 0.000 claims description 6
- 238000004140 cleaning Methods 0.000 claims description 6
- OVHHHVAVHBHXAK-UHFFFAOYSA-N n,n-diethylprop-2-enamide Chemical compound CCN(CC)C(=O)C=C OVHHHVAVHBHXAK-UHFFFAOYSA-N 0.000 claims description 6
- OMNKZBIFPJNNIO-UHFFFAOYSA-N n-(2-methyl-4-oxopentan-2-yl)prop-2-enamide Chemical compound CC(=O)CC(C)(C)NC(=O)C=C OMNKZBIFPJNNIO-UHFFFAOYSA-N 0.000 claims description 6
- MVBJSQCJPSRKSW-UHFFFAOYSA-N n-[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]prop-2-enamide Chemical compound OCC(CO)(CO)NC(=O)C=C MVBJSQCJPSRKSW-UHFFFAOYSA-N 0.000 claims description 6
- ZQXSMRAEXCEDJD-UHFFFAOYSA-N n-ethenylformamide Chemical compound C=CNC=O ZQXSMRAEXCEDJD-UHFFFAOYSA-N 0.000 claims description 6
- 229920000098 polyolefin Polymers 0.000 claims description 6
- 102000004190 Enzymes Human genes 0.000 claims description 5
- 108090000790 Enzymes Proteins 0.000 claims description 5
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 5
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 5
- 108010002350 Interleukin-2 Proteins 0.000 claims description 5
- 102000000588 Interleukin-2 Human genes 0.000 claims description 5
- 102000015696 Interleukins Human genes 0.000 claims description 5
- 108010063738 Interleukins Proteins 0.000 claims description 5
- 108010058846 Ovalbumin Proteins 0.000 claims description 5
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 claims description 5
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 claims description 5
- 102000004142 Trypsin Human genes 0.000 claims description 5
- 108090000631 Trypsin Proteins 0.000 claims description 5
- 239000001913 cellulose Substances 0.000 claims description 5
- 229940088598 enzyme Drugs 0.000 claims description 5
- 239000003112 inhibitor Substances 0.000 claims description 5
- 102000039446 nucleic acids Human genes 0.000 claims description 5
- 108020004707 nucleic acids Proteins 0.000 claims description 5
- 150000007523 nucleic acids Chemical class 0.000 claims description 5
- 229940092253 ovalbumin Drugs 0.000 claims description 5
- 229920001184 polypeptide Polymers 0.000 claims description 5
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 5
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 5
- 239000012588 trypsin Substances 0.000 claims description 5
- YXMISKNUHHOXFT-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) prop-2-enoate Chemical compound C=CC(=O)ON1C(=O)CCC1=O YXMISKNUHHOXFT-UHFFFAOYSA-N 0.000 claims description 4
- KFDVPJUYSDEJTH-UHFFFAOYSA-N 4-ethenylpyridine Chemical compound C=CC1=CC=NC=C1 KFDVPJUYSDEJTH-UHFFFAOYSA-N 0.000 claims description 4
- 108090000672 Annexin A5 Proteins 0.000 claims description 4
- 102000004121 Annexin A5 Human genes 0.000 claims description 4
- 244000068988 Glycine max Species 0.000 claims description 4
- 235000010469 Glycine max Nutrition 0.000 claims description 4
- 108010022181 Phosphopyruvate Hydratase Proteins 0.000 claims description 4
- 102000012288 Phosphopyruvate Hydratase Human genes 0.000 claims description 4
- 108010083644 Ribonucleases Proteins 0.000 claims description 4
- 102000006382 Ribonucleases Human genes 0.000 claims description 4
- 239000002158 endotoxin Substances 0.000 claims description 4
- 102000040430 polynucleotide Human genes 0.000 claims description 4
- 108091033319 polynucleotide Proteins 0.000 claims description 4
- 239000002157 polynucleotide Substances 0.000 claims description 4
- NLVXSWCKKBEXTG-UHFFFAOYSA-N vinylsulfonic acid Chemical compound OS(=O)(=O)C=C NLVXSWCKKBEXTG-UHFFFAOYSA-N 0.000 claims description 4
- 239000011800 void material Substances 0.000 claims description 4
- CGOJOQBYEAVATL-QPJJXVBHSA-N (e)-3-(4-methoxyphenyl)prop-2-enoyl chloride Chemical compound COC1=CC=C(\C=C\C(Cl)=O)C=C1 CGOJOQBYEAVATL-QPJJXVBHSA-N 0.000 claims description 3
- CYIGRWUIQAVBFG-UHFFFAOYSA-N 1,2-bis(2-ethenoxyethoxy)ethane Chemical compound C=COCCOCCOCCOC=C CYIGRWUIQAVBFG-UHFFFAOYSA-N 0.000 claims description 3
- MWZJGRDWJVHRDV-UHFFFAOYSA-N 1,4-bis(ethenoxy)butane Chemical compound C=COCCCCOC=C MWZJGRDWJVHRDV-UHFFFAOYSA-N 0.000 claims description 3
- YERHJBPPDGHCRJ-UHFFFAOYSA-N 1-[4-(1-oxoprop-2-enyl)-1-piperazinyl]-2-propen-1-one Chemical compound C=CC(=O)N1CCN(C(=O)C=C)CC1 YERHJBPPDGHCRJ-UHFFFAOYSA-N 0.000 claims description 3
- DMQYPVOQAARSNF-UHFFFAOYSA-N 3-[2,3-bis(3-prop-2-enoyloxypropoxy)propoxy]propyl prop-2-enoate Chemical compound C=CC(=O)OCCCOCC(OCCCOC(=O)C=C)COCCCOC(=O)C=C DMQYPVOQAARSNF-UHFFFAOYSA-N 0.000 claims description 3
- SFMFACMIOWQIPR-UHFFFAOYSA-N 3-ethoxyprop-2-enoyl chloride Chemical compound CCOC=CC(Cl)=O SFMFACMIOWQIPR-UHFFFAOYSA-N 0.000 claims description 3
- HIBSYUPTCGGRSD-UHFFFAOYSA-N 3-prop-2-enoyl-1,3-oxazolidin-2-one Chemical compound C=CC(=O)N1CCOC1=O HIBSYUPTCGGRSD-UHFFFAOYSA-N 0.000 claims description 3
- XWUNIDGEMNBBAQ-UHFFFAOYSA-N Bisphenol A ethoxylate diacrylate Chemical compound C=1C=C(OCCOC(=O)C=C)C=CC=1C(C)(C)C1=CC=C(OCCOC(=O)C=C)C=C1 XWUNIDGEMNBBAQ-UHFFFAOYSA-N 0.000 claims description 3
- 239000004641 Diallyl-phthalate Substances 0.000 claims description 3
- 239000004695 Polyether sulfone Substances 0.000 claims description 3
- QYKIQEUNHZKYBP-UHFFFAOYSA-N Vinyl ether Chemical compound C=COC=C QYKIQEUNHZKYBP-UHFFFAOYSA-N 0.000 claims description 3
- HFBMWMNUJJDEQZ-UHFFFAOYSA-N acryloyl chloride Chemical compound ClC(=O)C=C HFBMWMNUJJDEQZ-UHFFFAOYSA-N 0.000 claims description 3
- 229960003589 arginine hydrochloride Drugs 0.000 claims description 3
- QUDWYFHPNIMBFC-UHFFFAOYSA-N bis(prop-2-enyl) benzene-1,2-dicarboxylate Chemical compound C=CCOC(=O)C1=CC=CC=C1C(=O)OCC=C QUDWYFHPNIMBFC-UHFFFAOYSA-N 0.000 claims description 3
- QUZSUMLPWDHKCJ-UHFFFAOYSA-N bisphenol A dimethacrylate Chemical compound C1=CC(OC(=O)C(=C)C)=CC=C1C(C)(C)C1=CC=C(OC(=O)C(C)=C)C=C1 QUZSUMLPWDHKCJ-UHFFFAOYSA-N 0.000 claims description 3
- KUQWZSZYIQGTHT-UHFFFAOYSA-N hexa-1,5-diene-3,4-diol Chemical compound C=CC(O)C(O)C=C KUQWZSZYIQGTHT-UHFFFAOYSA-N 0.000 claims description 3
- ZEMHQYNMVKDBFJ-UHFFFAOYSA-N n-(3-hydroxypropyl)prop-2-enamide Chemical compound OCCCNC(=O)C=C ZEMHQYNMVKDBFJ-UHFFFAOYSA-N 0.000 claims description 3
- ZMLXKXHICXTSDM-UHFFFAOYSA-N n-[1,2-dihydroxy-2-(prop-2-enoylamino)ethyl]prop-2-enamide Chemical compound C=CC(=O)NC(O)C(O)NC(=O)C=C ZMLXKXHICXTSDM-UHFFFAOYSA-N 0.000 claims description 3
- QHQMBOKYHCJVKF-UHFFFAOYSA-N n-[12-(prop-2-enoylamino)dodecyl]prop-2-enamide Chemical compound C=CC(=O)NCCCCCCCCCCCCNC(=O)C=C QHQMBOKYHCJVKF-UHFFFAOYSA-N 0.000 claims description 3
- AYGYHGXUJBFUJU-UHFFFAOYSA-N n-[2-(prop-2-enoylamino)ethyl]prop-2-enamide Chemical compound C=CC(=O)NCCNC(=O)C=C AYGYHGXUJBFUJU-UHFFFAOYSA-N 0.000 claims description 3
- YQCFXPARMSSRRK-UHFFFAOYSA-N n-[6-(prop-2-enoylamino)hexyl]prop-2-enamide Chemical compound C=CC(=O)NCCCCCCNC(=O)C=C YQCFXPARMSSRRK-UHFFFAOYSA-N 0.000 claims description 3
- NJLPWLHBSPZUAS-UHFFFAOYSA-N n-[8-(prop-2-enoylamino)octyl]prop-2-enamide Chemical compound C=CC(=O)NCCCCCCCCNC(=O)C=C NJLPWLHBSPZUAS-UHFFFAOYSA-N 0.000 claims description 3
- 229920006393 polyether sulfone Polymers 0.000 claims description 3
- 229960000834 vinyl ether Drugs 0.000 claims description 3
- AGBXYHCHUYARJY-UHFFFAOYSA-N 2-phenylethenesulfonic acid Chemical compound OS(=O)(=O)C=CC1=CC=CC=C1 AGBXYHCHUYARJY-UHFFFAOYSA-N 0.000 claims description 2
- GDFCSMCGLZFNFY-UHFFFAOYSA-N Dimethylaminopropyl Methacrylamide Chemical compound CN(C)CCCNC(=O)C(C)=C GDFCSMCGLZFNFY-UHFFFAOYSA-N 0.000 claims description 2
- 239000005977 Ethylene Substances 0.000 claims description 2
- 239000004721 Polyphenylene oxide Substances 0.000 claims description 2
- 229920002492 poly(sulfone) Polymers 0.000 claims description 2
- 239000004417 polycarbonate Substances 0.000 claims description 2
- 229920000515 polycarbonate Polymers 0.000 claims description 2
- 229920000728 polyester Polymers 0.000 claims description 2
- 229920006380 polyphenylene oxide Polymers 0.000 claims description 2
- 102000016943 Muramidase Human genes 0.000 claims 4
- 102000036675 Myoglobin Human genes 0.000 claims 4
- 229920000724 poly(L-arginine) polymer Polymers 0.000 claims 2
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 claims 1
- 239000002861 polymer material Substances 0.000 claims 1
- 239000004971 Cross linker Substances 0.000 abstract description 78
- 230000004907 flux Effects 0.000 abstract description 65
- 238000000926 separation method Methods 0.000 abstract description 10
- 238000011084 recovery Methods 0.000 abstract description 9
- 238000000746 purification Methods 0.000 abstract description 4
- 238000004587 chromatography analysis Methods 0.000 abstract description 3
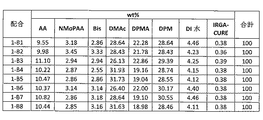
- 239000000203 mixture Substances 0.000 description 104
- 239000000499 gel Substances 0.000 description 102
- 239000002904 solvent Substances 0.000 description 82
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 74
- XHZPRMZZQOIPDS-UHFFFAOYSA-N 2-Methyl-2-[(1-oxo-2-propenyl)amino]-1-propanesulfonic acid Chemical compound OS(=O)(=O)CC(C)(C)NC(=O)C=C XHZPRMZZQOIPDS-UHFFFAOYSA-N 0.000 description 59
- 238000009472 formulation Methods 0.000 description 55
- 239000000243 solution Substances 0.000 description 52
- 238000002156 mixing Methods 0.000 description 49
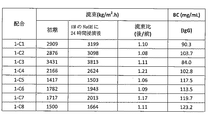
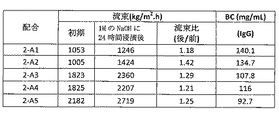
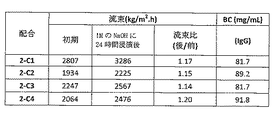
- 230000007797 corrosion Effects 0.000 description 44
- 238000005260 corrosion Methods 0.000 description 44
- 229910001868 water Inorganic materials 0.000 description 40
- WVJDEAVPVAFGLE-UHFFFAOYSA-N 3-(3-methoxypropoxy)propyl acetate Chemical compound COCCCOCCCOC(C)=O WVJDEAVPVAFGLE-UHFFFAOYSA-N 0.000 description 37
- 230000035699 permeability Effects 0.000 description 36
- 235000018102 proteins Nutrition 0.000 description 31
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 27
- 239000008367 deionised water Substances 0.000 description 27
- 229910021641 deionized water Inorganic materials 0.000 description 27
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 24
- KWDCKLXGUZOEGM-UHFFFAOYSA-N 1-methoxy-3-(3-methoxypropoxy)propane Chemical compound COCCCOCCCOC KWDCKLXGUZOEGM-UHFFFAOYSA-N 0.000 description 19
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 18
- 229940113088 dimethylacetamide Drugs 0.000 description 18
- QCAHUFWKIQLBNB-UHFFFAOYSA-N 3-(3-methoxypropoxy)propan-1-ol Chemical group COCCCOCCCO QCAHUFWKIQLBNB-UHFFFAOYSA-N 0.000 description 16
- 239000002585 base Substances 0.000 description 16
- 238000010828 elution Methods 0.000 description 16
- 238000006116 polymerization reaction Methods 0.000 description 16
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 15
- 239000011780 sodium chloride Substances 0.000 description 14
- 102100033468 Lysozyme C Human genes 0.000 description 13
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 12
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 12
- DAKWPKUUDNSNPN-UHFFFAOYSA-N Trimethylolpropane triacrylate Chemical compound C=CC(=O)OCC(CC)(COC(=O)C=C)COC(=O)C=C DAKWPKUUDNSNPN-UHFFFAOYSA-N 0.000 description 12
- 235000017281 sodium acetate Nutrition 0.000 description 12
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 12
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 11
- 238000002474 experimental method Methods 0.000 description 11
- 239000007788 liquid Substances 0.000 description 11
- 238000012545 processing Methods 0.000 description 11
- 230000002035 prolonged effect Effects 0.000 description 11
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 10
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 10
- 239000012148 binding buffer Substances 0.000 description 10
- 150000003839 salts Chemical class 0.000 description 10
- 239000001632 sodium acetate Substances 0.000 description 10
- WEZPLQKRXDBPEP-UHFFFAOYSA-N 1-(1-propoxypropan-2-yloxy)propan-2-ol Chemical compound CCCOCC(C)OCC(C)O WEZPLQKRXDBPEP-UHFFFAOYSA-N 0.000 description 9
- QSSDFXGXUGCLSY-UHFFFAOYSA-N 3-[3-(3-butoxypropoxy)propoxy]propan-1-ol Chemical compound CCCCOCCCOCCCOCCCO QSSDFXGXUGCLSY-UHFFFAOYSA-N 0.000 description 9
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 238000011156 evaluation Methods 0.000 description 9
- CYUZOYPRAQASLN-UHFFFAOYSA-N 3-prop-2-enoyloxypropanoic acid Chemical compound OC(=O)CCOC(=O)C=C CYUZOYPRAQASLN-UHFFFAOYSA-N 0.000 description 8
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 8
- 238000002835 absorbance Methods 0.000 description 8
- 230000007062 hydrolysis Effects 0.000 description 8
- 238000006460 hydrolysis reaction Methods 0.000 description 8
- 239000000758 substrate Substances 0.000 description 8
- 150000007942 carboxylates Chemical group 0.000 description 7
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 7
- 230000015556 catabolic process Effects 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 238000004132 cross linking Methods 0.000 description 7
- 238000006731 degradation reaction Methods 0.000 description 7
- 238000009826 distribution Methods 0.000 description 7
- 239000003999 initiator Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- SXGZJKUKBWWHRA-UHFFFAOYSA-N 2-(N-morpholiniumyl)ethanesulfonate Chemical compound [O-]S(=O)(=O)CC[NH+]1CCOCC1 SXGZJKUKBWWHRA-UHFFFAOYSA-N 0.000 description 6
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- 239000004698 Polyethylene Substances 0.000 description 6
- 229960000583 acetic acid Drugs 0.000 description 6
- 230000008859 change Effects 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- 238000005259 measurement Methods 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 229920000573 polyethylene Polymers 0.000 description 6
- 239000003361 porogen Substances 0.000 description 6
- 239000001509 sodium citrate Substances 0.000 description 6
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 6
- ISAOCJYIOMOJEB-UHFFFAOYSA-N benzoin Chemical compound C=1C=CC=CC=1C(O)C(=O)C1=CC=CC=C1 ISAOCJYIOMOJEB-UHFFFAOYSA-N 0.000 description 5
- 239000000356 contaminant Substances 0.000 description 5
- 239000012149 elution buffer Substances 0.000 description 5
- 239000012527 feed solution Substances 0.000 description 5
- 229920001155 polypropylene Polymers 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 239000012460 protein solution Substances 0.000 description 5
- 230000000717 retained effect Effects 0.000 description 5
- 239000012266 salt solution Substances 0.000 description 5
- 238000004626 scanning electron microscopy Methods 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 4
- WOBHKFSMXKNTIM-UHFFFAOYSA-N Hydroxyethyl methacrylate Chemical compound CC(=C)C(=O)OCCO WOBHKFSMXKNTIM-UHFFFAOYSA-N 0.000 description 4
- 102100030856 Myoglobin Human genes 0.000 description 4
- 239000004743 Polypropylene Substances 0.000 description 4
- 239000008351 acetate buffer Substances 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 239000004615 ingredient Substances 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 4
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 3
- OZAIFHULBGXAKX-UHFFFAOYSA-N 2-(2-cyanopropan-2-yldiazenyl)-2-methylpropanenitrile Chemical compound N#CC(C)(C)N=NC(C)(C)C#N OZAIFHULBGXAKX-UHFFFAOYSA-N 0.000 description 3
- GJKGAPPUXSSCFI-UHFFFAOYSA-N 2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone Chemical group CC(C)(O)C(=O)C1=CC=C(OCCO)C=C1 GJKGAPPUXSSCFI-UHFFFAOYSA-N 0.000 description 3
- KWTQSFXGGICVPE-UHFFFAOYSA-N 2-amino-5-(diaminomethylideneamino)pentanoic acid;hydron;chloride Chemical compound Cl.OC(=O)C(N)CCCN=C(N)N KWTQSFXGGICVPE-UHFFFAOYSA-N 0.000 description 3
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 3
- 244000028419 Styrax benzoin Species 0.000 description 3
- 235000000126 Styrax benzoin Nutrition 0.000 description 3
- 235000008411 Sumatra benzointree Nutrition 0.000 description 3
- 238000005904 alkaline hydrolysis reaction Methods 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 229960002130 benzoin Drugs 0.000 description 3
- 239000007853 buffer solution Substances 0.000 description 3
- 239000012501 chromatography medium Substances 0.000 description 3
- 238000010894 electron beam technology Methods 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 238000005755 formation reaction Methods 0.000 description 3
- 235000019382 gum benzoic Nutrition 0.000 description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 230000000704 physical effect Effects 0.000 description 3
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 description 3
- 235000013772 propylene glycol Nutrition 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 239000007974 sodium acetate buffer Substances 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 230000008961 swelling Effects 0.000 description 3
- 238000002145 thermally induced phase separation Methods 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 2
- FIDRAVVQGKNYQK-UHFFFAOYSA-N 1,2,3,4-tetrahydrotriazine Chemical compound C1NNNC=C1 FIDRAVVQGKNYQK-UHFFFAOYSA-N 0.000 description 2
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 2
- JKEHLQXXZMANPK-UHFFFAOYSA-N 1-[1-(1-propoxypropan-2-yloxy)propan-2-yloxy]propan-2-ol Chemical compound CCCOCC(C)OCC(C)OCC(C)O JKEHLQXXZMANPK-UHFFFAOYSA-N 0.000 description 2
- KWVGIHKZDCUPEU-UHFFFAOYSA-N 2,2-dimethoxy-2-phenylacetophenone Chemical compound C=1C=CC=CC=1C(OC)(OC)C(=O)C1=CC=CC=C1 KWVGIHKZDCUPEU-UHFFFAOYSA-N 0.000 description 2
- JHQVCQDWGSXTFE-UHFFFAOYSA-N 2-(2-prop-2-enoxycarbonyloxyethoxy)ethyl prop-2-enyl carbonate Chemical compound C=CCOC(=O)OCCOCCOC(=O)OCC=C JHQVCQDWGSXTFE-UHFFFAOYSA-N 0.000 description 2
- BDKLKNJTMLIAFE-UHFFFAOYSA-N 2-(3-fluorophenyl)-1,3-oxazole-4-carbaldehyde Chemical compound FC1=CC=CC(C=2OC=C(C=O)N=2)=C1 BDKLKNJTMLIAFE-UHFFFAOYSA-N 0.000 description 2
- IVLXQGJVBGMLRR-UHFFFAOYSA-N 2-aminoacetic acid;hydron;chloride Chemical compound Cl.NCC(O)=O IVLXQGJVBGMLRR-UHFFFAOYSA-N 0.000 description 2
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 2
- TUVYSBJZBYRDHP-UHFFFAOYSA-N acetic acid;methoxymethane Chemical compound COC.CC(O)=O TUVYSBJZBYRDHP-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 2
- ROOXNKNUYICQNP-UHFFFAOYSA-N ammonium persulfate Chemical compound [NH4+].[NH4+].[O-]S(=O)(=O)OOS([O-])(=O)=O ROOXNKNUYICQNP-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000004630 atomic force microscopy Methods 0.000 description 2
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 2
- 239000012965 benzophenone Substances 0.000 description 2
- 229940024874 benzophenone Drugs 0.000 description 2
- 125000002843 carboxylic acid group Chemical group 0.000 description 2
- 238000005341 cation exchange Methods 0.000 description 2
- 239000003518 caustics Substances 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 239000007810 chemical reaction solvent Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 239000011929 di(propylene glycol) methyl ether Substances 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 239000012362 glacial acetic acid Substances 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- 238000007654 immersion Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000005855 radiation Effects 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 238000011012 sanitization Methods 0.000 description 2
- 238000002791 soaking Methods 0.000 description 2
- 229940087562 sodium acetate trihydrate Drugs 0.000 description 2
- 239000011877 solvent mixture Substances 0.000 description 2
- 238000012799 strong cation exchange Methods 0.000 description 2
- 238000007725 thermal activation Methods 0.000 description 2
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 2
- 238000012784 weak cation exchange Methods 0.000 description 2
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 description 1
- AVFZOVWCLRSYKC-UHFFFAOYSA-N 1-methylpyrrolidine Chemical compound CN1CCCC1 AVFZOVWCLRSYKC-UHFFFAOYSA-N 0.000 description 1
- LZHUBCULTHIFNO-UHFFFAOYSA-N 2,4-dihydroxy-1,5-bis[4-(2-hydroxyethoxy)phenyl]-2,4-dimethylpentan-3-one Chemical compound C=1C=C(OCCO)C=CC=1CC(C)(O)C(=O)C(O)(C)CC1=CC=C(OCCO)C=C1 LZHUBCULTHIFNO-UHFFFAOYSA-N 0.000 description 1
- ZIHDWZZSOZGDDK-UHFFFAOYSA-N 2-(2,3-dihydroxy-2-phenylpropanoyl)benzenesulfonic acid Chemical compound OCC(C(C=1C(=CC=CC=1)S(=O)(=O)O)=O)(O)C1=CC=CC=C1 ZIHDWZZSOZGDDK-UHFFFAOYSA-N 0.000 description 1
- KMNCBSZOIQAUFX-UHFFFAOYSA-N 2-ethoxy-1,2-diphenylethanone Chemical compound C=1C=CC=CC=1C(OCC)C(=O)C1=CC=CC=C1 KMNCBSZOIQAUFX-UHFFFAOYSA-N 0.000 description 1
- NLGDWWCZQDIASO-UHFFFAOYSA-N 2-hydroxy-1-(7-oxabicyclo[4.1.0]hepta-1,3,5-trien-2-yl)-2-phenylethanone Chemical compound OC(C(=O)c1cccc2Oc12)c1ccccc1 NLGDWWCZQDIASO-UHFFFAOYSA-N 0.000 description 1
- XMLYCEVDHLAQEL-UHFFFAOYSA-N 2-hydroxy-2-methyl-1-phenylpropan-1-one Chemical compound CC(C)(O)C(=O)C1=CC=CC=C1 XMLYCEVDHLAQEL-UHFFFAOYSA-N 0.000 description 1
- IQAIPUWCYPGRQL-UHFFFAOYSA-N 2-hydroxyacetic acid;hydrate Chemical compound O.OCC(O)=O IQAIPUWCYPGRQL-UHFFFAOYSA-N 0.000 description 1
- BQZJOQXSCSZQPS-UHFFFAOYSA-N 2-methoxy-1,2-diphenylethanone Chemical compound C=1C=CC=CC=1C(OC)C(=O)C1=CC=CC=C1 BQZJOQXSCSZQPS-UHFFFAOYSA-N 0.000 description 1
- 239000004342 Benzoyl peroxide Substances 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 102100030497 Cytochrome c Human genes 0.000 description 1
- 108010075031 Cytochromes c Proteins 0.000 description 1
- 108020005199 Dehydrogenases Proteins 0.000 description 1
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 101000609762 Gallus gallus Ovalbumin Proteins 0.000 description 1
- 102000004407 Lactalbumin Human genes 0.000 description 1
- 108090000942 Lactalbumin Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- AHVYPIQETPWLSZ-UHFFFAOYSA-N N-methyl-pyrrolidine Natural products CN1CC=CC1 AHVYPIQETPWLSZ-UHFFFAOYSA-N 0.000 description 1
- OHLUUHNLEMFGTQ-UHFFFAOYSA-N N-methylacetamide Chemical compound CNC(C)=O OHLUUHNLEMFGTQ-UHFFFAOYSA-N 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 108020002230 Pancreatic Ribonuclease Proteins 0.000 description 1
- 102000005891 Pancreatic ribonuclease Human genes 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 102000045595 Phosphoprotein Phosphatases Human genes 0.000 description 1
- 108700019535 Phosphoprotein Phosphatases Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- KYIKRXIYLAGAKQ-UHFFFAOYSA-N abcn Chemical group C1CCCCC1(C#N)N=NC1(C#N)CCCCC1 KYIKRXIYLAGAKQ-UHFFFAOYSA-N 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- 229910001870 ammonium persulfate Inorganic materials 0.000 description 1
- 239000003011 anion exchange membrane Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 239000008366 buffered solution Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 230000003196 chaotropic effect Effects 0.000 description 1
- 238000002144 chemical decomposition reaction Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000013329 compounding Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000000249 desinfective effect Effects 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000001493 electron microscopy Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000002389 environmental scanning electron microscopy Methods 0.000 description 1
- 229940052303 ethers for general anesthesia Drugs 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000011152 fibreglass Substances 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 239000004745 nonwoven fabric Substances 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 108010040046 poly-beta-hydroxybutyrate depolymerase Proteins 0.000 description 1
- 239000002685 polymerization catalyst Substances 0.000 description 1
- 239000003505 polymerization initiator Substances 0.000 description 1
- 229920000193 polymethacrylate Polymers 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920000131 polyvinylidene Polymers 0.000 description 1
- USHAGKDGDHPEEY-UHFFFAOYSA-L potassium persulfate Chemical compound [K+].[K+].[O-]S(=O)(=O)OOS([O-])(=O)=O USHAGKDGDHPEEY-UHFFFAOYSA-L 0.000 description 1
- KJASTBCNGFYKSR-UHFFFAOYSA-N prop-2-enehydrazide Chemical compound NNC(=O)C=C KJASTBCNGFYKSR-UHFFFAOYSA-N 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 238000002336 sorption--desorption measurement Methods 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000012085 test solution Substances 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
- 235000021241 α-lactalbumin Nutrition 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/281—Sorbents specially adapted for preparative, analytical or investigative chromatography
- B01J20/291—Gel sorbents
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/42—Selective adsorption, e.g. chromatography characterised by the development mode, e.g. by displacement or by elution
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28014—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their form
- B01J20/28033—Membrane, sheet, cloth, pad, lamellar or mat
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28078—Pore diameter
- B01J20/28085—Pore diameter being more than 50 nm, i.e. macropores
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J20/00—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof
- B01J20/28—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties
- B01J20/28054—Solid sorbent compositions or filter aid compositions; Sorbents for chromatography; Processes for preparing, regenerating or reactivating thereof characterised by their form or physical properties characterised by their surface properties or porosity
- B01J20/28095—Shape or type of pores, voids, channels, ducts
- B01J20/28097—Shape or type of pores, voids, channels, ducts being coated, filled or plugged with specific compounds
Landscapes
- Chemical & Material Sciences (AREA)
- Analytical Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Nanotechnology (AREA)
- Separation Using Semi-Permeable Membranes (AREA)
- Treatment Of Liquids With Adsorbents In General (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361781321P | 2013-03-14 | 2013-03-14 | |
| US61/781,321 | 2013-03-14 | ||
| PCT/IB2014/001022 WO2014140860A2 (en) | 2013-03-14 | 2014-03-07 | Chromatography membranes stable under caustic conditions |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2016517341A true JP2016517341A (ja) | 2016-06-16 |
| JP2016517341A5 JP2016517341A5 (enExample) | 2017-04-06 |
Family
ID=51528816
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015562384A Withdrawn JP2016517341A (ja) | 2013-03-14 | 2014-03-07 | 腐食条件下で安定しているクロマトグラフィー膜 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20140273158A1 (enExample) |
| EP (1) | EP2969095A4 (enExample) |
| JP (1) | JP2016517341A (enExample) |
| KR (1) | KR20150131298A (enExample) |
| AU (1) | AU2014229527A1 (enExample) |
| CA (1) | CA2905524A1 (enExample) |
| WO (1) | WO2014140860A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20170120649A (ko) * | 2015-02-20 | 2017-10-31 | 나트릭스 세퍼레이션즈, 인코포레이티드 | 티올-엔 또는 티올-인 클릭 중합 반응에 의해 형성된 크로마토그래피 막 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106006903B (zh) * | 2016-05-27 | 2018-12-18 | 浙江理工大学 | 一种竹浆纤维素&聚n-乙烯基甲酰胺复合絮凝脱色材料的制备方法 |
| KR101952288B1 (ko) * | 2016-12-19 | 2019-05-17 | 예일 유니버시티 | 자가 복원이 가능한 하이드로겔 충진 수처리용 분리막의 제조방법 |
| US12485364B2 (en) | 2018-08-10 | 2025-12-02 | Clemson University Research Foundation | Multi-modal ion-exchange membranes for rapid separations |
| US20220341664A1 (en) * | 2019-09-13 | 2022-10-27 | Merck Millipore Ltd. | Supercritical drying of chromatographic media |
| GB201917711D0 (en) * | 2019-12-04 | 2020-01-15 | Fujifilm Mfg Europe Bv | Affinity membranes, compounds, compositions and processes for their preparation and use |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4198238A (en) * | 1978-06-22 | 1980-04-15 | Hercules Incorporated | Photopolymerizable composition |
| DE602004024190D1 (de) * | 2003-02-19 | 2009-12-31 | Natrix Separations Inc | Geträgerte poröse gele umfassende verbundmaterialien |
| US7452697B2 (en) * | 2003-09-25 | 2008-11-18 | Allergan, Inc. | Chromatographic method and system for purifying a botulinum toxin |
| JP5767584B2 (ja) * | 2008-09-02 | 2015-08-19 | ナトリックス セパレイションズ インコーポレーテッド | クロマトグラフィー膜、それを含む装置及びその使用方法 |
-
2014
- 2014-03-07 US US14/200,615 patent/US20140273158A1/en not_active Abandoned
- 2014-03-07 EP EP14765816.5A patent/EP2969095A4/en not_active Withdrawn
- 2014-03-07 AU AU2014229527A patent/AU2014229527A1/en not_active Abandoned
- 2014-03-07 JP JP2015562384A patent/JP2016517341A/ja not_active Withdrawn
- 2014-03-07 KR KR1020157029525A patent/KR20150131298A/ko not_active Withdrawn
- 2014-03-07 CA CA2905524A patent/CA2905524A1/en not_active Abandoned
- 2014-03-07 WO PCT/IB2014/001022 patent/WO2014140860A2/en not_active Ceased
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20170120649A (ko) * | 2015-02-20 | 2017-10-31 | 나트릭스 세퍼레이션즈, 인코포레이티드 | 티올-엔 또는 티올-인 클릭 중합 반응에 의해 형성된 크로마토그래피 막 |
| JP2018511667A (ja) * | 2015-02-20 | 2018-04-26 | ナトリックス セパレイションズ インコーポレーテッド | チオール−エン又はチオール−インクリック重合反応により形成されたクロマトグラフィー膜 |
| JP2021080456A (ja) * | 2015-02-20 | 2021-05-27 | メルク・ミリポア・リミテッド | チオール−エン又はチオール−インクリック重合反応により形成されたクロマトグラフィー膜 |
| JP7039740B2 (ja) | 2015-02-20 | 2022-03-22 | メルク・ミリポア・リミテッド | チオール-エン又はチオール-インクリック重合反応により形成されたクロマトグラフィー膜 |
| KR102480391B1 (ko) | 2015-02-20 | 2022-12-22 | 머크 밀리포어 리미티드 | 티올-엔 또는 티올-인 클릭 중합 반응에 의해 형성된 크로마토그래피 막 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20140273158A1 (en) | 2014-09-18 |
| KR20150131298A (ko) | 2015-11-24 |
| AU2014229527A1 (en) | 2015-10-29 |
| EP2969095A2 (en) | 2016-01-20 |
| EP2969095A4 (en) | 2017-01-04 |
| WO2014140860A3 (en) | 2014-12-04 |
| WO2014140860A2 (en) | 2014-09-18 |
| CA2905524A1 (en) | 2014-09-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6639236B2 (ja) | 混合モードクロマトグラフィー膜 | |
| JP7039740B2 (ja) | チオール-エン又はチオール-インクリック重合反応により形成されたクロマトグラフィー膜 | |
| Bhattacharjee et al. | Formation of high-capacity protein-adsorbing membranes through simple adsorption of poly (acrylic acid)-containing films at low pH | |
| JP2016517341A (ja) | 腐食条件下で安定しているクロマトグラフィー膜 | |
| JP7614197B2 (ja) | 複合材料に配位子をカップリングするための方法 | |
| CN105636686A (zh) | 色谱介质 | |
| JP2013510918A (ja) | 疎水的相互作用クロマトグラフィー膜、及びその使用方法 | |
| CN103959057A (zh) | 用于蛋白质纯化的基于烯丙胺及其衍生物的新型色谱介质 | |
| JP2021515698A (ja) | バイオセパレーションのための複合材料 | |
| TW201321063A (zh) | 蛋白質之純化方法 | |
| Yusof et al. | Structure variations of the grafted functional polymer brush enhance membrane adsorber performance | |
| Yılmaz Baran et al. | Efficient adsorption of hemoglobin from aqueous solutions by hybrid monolithic cryogel column |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD01 | Notification of change of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7426 Effective date: 20160620 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20160620 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170228 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20170228 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20170601 |