JP2010094636A - Moisture absorbing and discharging material, and method of manufacturing the same - Google Patents
Moisture absorbing and discharging material, and method of manufacturing the same Download PDFInfo
- Publication number
- JP2010094636A JP2010094636A JP2008269255A JP2008269255A JP2010094636A JP 2010094636 A JP2010094636 A JP 2010094636A JP 2008269255 A JP2008269255 A JP 2008269255A JP 2008269255 A JP2008269255 A JP 2008269255A JP 2010094636 A JP2010094636 A JP 2010094636A
- Authority
- JP
- Japan
- Prior art keywords
- moisture
- siliceous shale
- sodium chloride
- chloride
- absorbing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Abstract
Description
本発明は、吸放湿材料、及びその製造方法に関する。さらに詳しくは、珪質頁岩を改質することで吸放湿性能を格段に向上させた、調湿建材、デシカント空調装置、厨房排気浄化装置などの幅広い用途に好適に適用できる吸放湿材料、及びその製造方法に関する。 The present invention relates to a moisture absorbing / releasing material and a method for producing the same. More specifically, moisture-absorbing and releasing materials that can be suitably applied to a wide range of applications such as humidity control building materials, desiccant air conditioners, kitchen exhaust purification devices, which have improved moisture absorption and release performance by modifying siliceous shale, And a manufacturing method thereof.
近年、住宅の高気密化・高断熱化にともない、従来の住宅に比べて、室内外の自然換気量が激減している。このような換気不良は、室内に水蒸気がこもって壁や窓に結露を生じさせる原因となり、さらに、それらがカビやダニの発生を誘引する結果となっている。これに対し、水蒸気を吸収及び放出する吸放湿材料を用いることで、自律的に室内の水蒸気量を調整できるようにした調湿建材の提案がされており、実用化されている。また、最近では、冷却・除湿型の空調装置に代わる新しい技術として、吸放湿材料を用いた潜熱・顕熱分離型のデシカント空調装置が使用されるようになり、業務用を中心として実用化が進んでいる。デシカント空調装置は、消費エネルギーや温暖化ガスの排出を抑えることができるなどの特長を有することから、今後、小型化することで家庭用として用いることも期待されている。さらに、燃焼ガスを生じない電気調理器の普及にともない、吸放湿材料などを含む浄化フィルターを備えた室内空気循環型の厨房排気浄化装置が使用されるようになってきている。 In recent years, with the increase in airtightness and heat insulation of houses, the amount of natural ventilation indoors and outdoors has drastically decreased compared to conventional houses. Such poor ventilation causes water vapor to accumulate in the room and cause dew condensation on the walls and windows, which in turn leads to the generation of mold and mites. On the other hand, the use of moisture-absorbing / releasing materials that absorb and release water vapor has been proposed and put into practical use as a humidity control building material that can adjust the amount of water vapor autonomously. Recently, as a new technology to replace the cooling / dehumidification type air conditioner, a latent heat / sensible heat separation type desiccant air conditioner using moisture absorbing / releasing material has been used and put into practical use mainly for commercial use. Is progressing. Since the desiccant air conditioner has features such as the ability to suppress energy consumption and emission of greenhouse gases, it is expected that the desiccant air conditioner will be used for home use by downsizing in the future. Furthermore, along with the widespread use of electric cookers that do not generate combustion gas, indoor air circulation type kitchen exhaust purification devices equipped with purification filters containing moisture absorption / release materials and the like have come to be used.
上記したように、近年、吸放湿材料に対する注目は著しく、その用途は拡大している。吸放湿材料における最も重要な点は、その吸放湿性能にあるが、主に生活空間で使用されることから、健康への影響がないこと、安価であること、繰り返しての使用が容易であること、使い勝手のよいこと、などが要求される。従来より用いられている吸放湿材料としては、例えば、ゼオライト、シリカゲル、活性炭などの多孔質体や、ポリアクリル酸ナトリウムなどの高吸水性ポリマーなどが挙げられる。近年、天然の多孔質体である珪質頁岩に、極めて高い吸放湿性能があることが見出され、その産出量が豊富で、従来の高性能の吸放湿材料に比べて安価であり、健康への影響がなく、使い勝手にも優れることから、吸放湿材料として注目され、壁材などの調湿建材として用いられるようになってきている。 As described above, in recent years, attention has been paid to moisture-absorbing / releasing materials, and their uses are expanding. The most important point in moisture-absorbing / releasing materials is their moisture-absorbing / releasing performance, but since they are mainly used in living spaces, they have no impact on health, are inexpensive, and are easy to use repeatedly. It must be easy to use. Conventionally used moisture absorbing / releasing materials include porous materials such as zeolite, silica gel and activated carbon, and highly water-absorbing polymers such as sodium polyacrylate. In recent years, siliceous shale, which is a natural porous material, has been found to have extremely high moisture absorption and desorption performance, abundant in its production, and cheaper than conventional high performance moisture absorption and desorption materials. It has no influence on health and is excellent in usability, so it has been attracting attention as a moisture absorbing / releasing material and has been used as a humidity control building material such as a wall material.
珪質頁岩は、珪藻プランクトンが堆積して形成された珪藻土が、地圧と熱による地質的変性を受けることによって、一部が結晶化されたものであり、一般に珪藻土と呼ばれている珪藻泥岩とは明確に区別されるものである。そして、珪質頁岩の中でも、極めて微細な細孔径を多数有する珪質頁岩、具体的には、平均細孔直径が3〜12nm、比表面積が80m2/g以上である珪質頁岩は、優れた吸放湿性能を示すことが明らかとなっている(特許文献1参照)。このような特性を有する珪質頁岩としては、例えば、北海道天北地方から産出される稚内層珪質頁岩などが挙げられるが、このような珪質頁岩を調湿機能材料として利用することについて、種々の提案がなされている(特許文献2又は3参照)。
Silica shale is a diatomaceous mudstone generally called diatomaceous earth, which is partly crystallized due to the geological modification of diatomaceous earth formed by the deposition of diatom plankton, which is subjected to geological modification due to earth pressure and heat. Is clearly distinguished. Among siliceous shale, siliceous shale having a large number of extremely fine pore diameters, specifically, siliceous shale having an average pore diameter of 3 to 12 nm and a specific surface area of 80 m 2 / g or more is excellent. It has become clear that the moisture absorption / release performance is shown (see Patent Document 1). As siliceous shale having such characteristics, for example, Wakkanai Formation siliceous shale produced from the Tenboku region of Hokkaido, etc., about using such siliceous shale as a humidity control functional material, Various proposals have been made (see
図9は、稚内層珪質頁岩の原石の水蒸気吸着等温線を示している。図9に示されるとおり、稚内層珪質頁岩は、原石のままでも極めて優れた吸放湿性能を有する。より詳細にみると、低湿域(相対湿度33〜53%)から中湿域(相対湿度53〜75%)付近までは水分吸着量が比較的低い一方で、高湿域(相対湿度75〜93%)では、水蒸気の毛細管凝縮による物理的吸着の進行により、水分吸着量が急激に増大することが示されている。 FIG. 9 shows the water vapor adsorption isotherm of the raw rock of Wakkanai layer siliceous shale. As shown in FIG. 9, the Wakkanai layer siliceous shale has extremely excellent moisture absorption and desorption performance even with the raw stone. More specifically, while the moisture adsorption amount is relatively low from the low humidity range (relative humidity 33 to 53%) to the middle humidity range (relative humidity 53 to 75%), the high humidity range (relative humidity 75 to 93). %) Shows that the amount of moisture adsorption increases rapidly due to the progress of physical adsorption by capillary condensation of water vapor.
しかし、前記した珪質頁岩を調湿建材などの室内用途に適用する場合には、人間が快適に感じるとされる、40〜60%程度の相対湿度を安定的に維持するために、高湿域だけでなく、中湿域でも、ある程度の高い水分吸着能(吸湿性能)を有することが求められる。また、デシカント空調装置の除湿フィルターに用いる場合にも、中湿域から高湿域にかけて高い水分吸着能を有することが必要である。さらに、この場合は、吸着した水分をより速やかに、放湿して、再度、吸湿できる状態に容易にすることができ、繰り返し高い水分吸着能が発揮できるものであることが望まれる。すなわち、珪質頁岩の吸放湿材料としての価値を高め、より幅広い用途への適用を可能とするためには、中湿域においても、原石の高湿域におけると同程度の吸放湿性能を有することが求められる。また、今後、調湿建材や浄化フィルターのさらなる高機能化や、デシカント空調装置の小型化を実現させていくためには、吸放湿材料として使用する珪質頁岩の、中湿域から高湿域にかけての吸放湿性能(最大吸湿量の増大と、蓄湿のない速やかな放湿能など)をより一層向上させることが望まれる。 However, when applying the above siliceous shale for indoor use such as humidity control building materials, in order to stably maintain a relative humidity of about 40 to 60%, which is considered to be comfortable for human beings, It is required to have a certain level of moisture adsorption ability (moisture absorption performance) not only in the region but also in the middle humidity region. Moreover, when using for the dehumidification filter of a desiccant air conditioner, it is necessary to have a high water | moisture-content adsorption capacity from a medium-humidity area to a high-humidity area. Furthermore, in this case, it is desirable that the adsorbed moisture can be released more quickly and can be easily re-absorbed, and can repeatedly exhibit high moisture adsorption ability. In other words, in order to increase the value of siliceous shale as a moisture absorption and desorption material, and to make it applicable to a wider range of applications, the moisture absorption and desorption performance of the same degree as in the high humidity range of the rough ore is also possible in the medium humidity range. It is required to have. Also, in the future, in order to achieve higher functionality of humidity control building materials and purification filters, and downsizing of desiccant air conditioners, siliceous shale used as a moisture absorbing / releasing material from high to low humidity. It is desired to further improve the moisture absorption / release performance (increase in the maximum amount of moisture absorption, quick moisture release capability without moisture accumulation, etc.) over the area.
したがって、本発明の目的は、従来の材料に比べて吸放湿性能に優れる珪質頁岩の、中湿域から高湿域にかけての吸放湿性能を改善し、その性能をより向上させることで、様々な用途において好適に使用できる吸放湿材料を提供することにある。より具体的には、珪質頁岩を改質することで、原石と比べて、中湿域から高湿域にかけての水分吸着量が増大され、また、相対湿度を低下させた場合に、吸湿した水分の多くが放湿され、蓄湿のない速やかな吸放湿を繰り返し行い得る、吸放湿材料を提供することにある。また、本発明の目的は、上記した優れた効果を発揮する改質した吸放湿材料を、簡易かつ経済的に得るための製造方法を提供することにある。 Therefore, the object of the present invention is to improve the moisture absorption and desorption performance of the siliceous shale, which is superior in moisture absorption and desorption performance compared to conventional materials, from the middle to high humidity ranges, and to further improve the performance. Another object of the present invention is to provide a moisture absorbing / releasing material that can be suitably used in various applications. More specifically, by modifying the siliceous shale, the amount of moisture adsorbed from the mid-humidity region to the high-humidity region is increased compared to the rough stone, and when the relative humidity is lowered, the moisture absorption is achieved. An object of the present invention is to provide a moisture-absorbing / releasing material in which a large amount of moisture is released and can be repeatedly absorbed and released quickly without moisture accumulation. Moreover, the objective of this invention is providing the manufacturing method for obtaining the modified moisture absorption / release material which exhibits the above-mentioned outstanding effect simply and economically.
上記目的は以下の本発明によって達成される。すなわち、本発明は、平均細孔直径が3〜18nm、比表面積が80m2/g以上である多数の細孔を有する珪質頁岩の少なくとも細孔内に、塩化ナトリウムが付着されてなることを特徴とする吸放湿材料である。 The above object is achieved by the present invention described below. That is, according to the present invention, sodium chloride is adhered to at least the pores of siliceous shale having an average pore diameter of 3 to 18 nm and a specific surface area of 80 m 2 / g or more. It is a moisture absorbing / releasing material.
また、本発明の吸放湿材料は、塩化ナトリウムの付着量が、10〜150mg/gの範囲内であることが好ましい。また、本発明の吸放湿材料は、前記珪質頁岩の細孔容量が、0.45ml/g以上であることが好ましい。 Moreover, it is preferable that the moisture absorption / release material of this invention has the adhesion amount of sodium chloride in the range of 10-150 mg / g. In the moisture absorbing / releasing material of the present invention, the siliceous shale preferably has a pore volume of 0.45 ml / g or more.
また、本発明の吸放湿材料は、前記珪質頁岩の少なくとも細孔内に、さらに、塩化リチウム、塩化マグネシウム及び/又は塩化カルシウムが付着されていることが好ましい。また、本発明の吸放湿材料は、塩化リチウム、塩化マグネシウム及び/又は塩化カルシウムの付着量が、10〜250mg/gの範囲内であることが好ましい。また、本発明の吸放湿材料は、調湿建材の構成材料として、或いは、デシカント空調装置の除湿フィルターに用いることができる。 In the moisture absorbing / releasing material of the present invention, it is preferable that lithium chloride, magnesium chloride and / or calcium chloride is further adhered to at least the pores of the siliceous shale. Moreover, it is preferable that the moisture absorption / release material of this invention has the adhesion amount of lithium chloride, magnesium chloride, and / or calcium chloride in the range of 10-250 mg / g. In addition, the moisture absorbing / releasing material of the present invention can be used as a constituent material for humidity control building materials or for a dehumidifying filter of a desiccant air conditioner.
本発明の別の実施形態は、平均細孔直径が3〜12nm、比表面積が80m2/g以上である多数の細孔を有する珪質頁岩を1〜20質量%の範囲内の塩化ナトリウム水溶液に浸漬する工程、浸漬した珪質頁岩を乾燥処理する工程を有することを特徴とする吸放湿材料の製造方法である。 Another embodiment of the present invention relates to a sodium chloride aqueous solution containing 1 to 20% by mass of siliceous shale having a number of pores having an average pore diameter of 3 to 12 nm and a specific surface area of 80 m 2 / g or more. It is a manufacturing method of the moisture absorption / release material characterized by having the process immersed in, and the process of drying the immersed siliceous shale.
また、本発明の吸放湿材料の製造方法は、前記珪質頁岩を塩化ナトリウム水溶液に浸漬する工程において、真空引きを行うことが好ましい。本発明の吸放湿材料の製造方法は、前記塩化ナトリウム水溶液に、さらに、塩化リチウム、塩化マグネシウム及び/又は塩化カルシウムを含有させていることが好ましい。また、本発明の吸放湿材料の製造方法は、前記塩化ナトリウム水溶液に浸漬する工程の前に、さらに、珪質頁岩をアルカリエッチング処理して細孔を拡げる工程を有することが好ましく、また、アルカリエッチング処理を、水酸化ナトリウム又は水酸化カリウムの水溶液に浸漬させた後、真空引きすることによって行うことが好ましい。本発明の吸放湿材料の製造方法は、前記塩化ナトリウム水溶液に、さらに、水酸化ナトリウム又は水酸化カリウムを含有させていることが好ましい。 Moreover, it is preferable that the manufacturing method of the moisture absorption / release material of this invention performs evacuation in the process of immersing the said siliceous shale in sodium chloride aqueous solution. In the method for producing a moisture absorbing / releasing material of the present invention, it is preferable that lithium chloride, magnesium chloride and / or calcium chloride is further contained in the sodium chloride aqueous solution. Moreover, the method for producing the moisture absorbing / releasing material of the present invention preferably further includes a step of expanding the pores by performing an alkali etching treatment on the siliceous shale before the step of immersing in the sodium chloride aqueous solution, It is preferable to perform the alkali etching treatment by immersing in an aqueous solution of sodium hydroxide or potassium hydroxide and then evacuating. In the method for producing a moisture absorbing / releasing material of the present invention, it is preferable that sodium chloride or potassium hydroxide is further contained in the sodium chloride aqueous solution.
本発明によれば、従来の材料に比べて吸放湿性能に優れる珪質頁岩における、中湿域から高湿域にかけての吸放湿性能が改善され、また、その性能がより向上された、様々な用途において好適に使用できる吸放湿材料が提供される。すなわち、珪質頁岩を改質することで、原石と比べて、中湿域から高湿域にかけての水分吸着量が増大され、また、相対湿度を低下させた場合に、吸湿した水分の多くが放湿され、蓄湿のない速やかな吸放湿を繰り返し行い得る、吸放湿材料の提供が可能となる。また、本発明によれば、上記した優れた効果を発揮する改質した吸放湿材料を簡易かつ経済的に得ることのできる製造方法が提供される。 According to the present invention, in the siliceous shale excellent in moisture absorption and desorption performance compared with conventional materials, the moisture absorption and desorption performance from the middle to high humidity range has been improved, and the performance has been further improved. A moisture absorbing / releasing material that can be suitably used in various applications is provided. That is, by modifying the siliceous shale, the amount of moisture adsorbed from the mid-humidity to the high-humidity region is increased compared to the rough stone, and when the relative humidity is lowered, much of the moisture absorbed is reduced. It is possible to provide a moisture absorbing / releasing material that can be repeatedly moisture-absorbed and quickly absorbed and released without moisture accumulation. Moreover, according to this invention, the manufacturing method which can obtain the improved moisture absorption / release material which exhibits the outstanding effect mentioned above simply and economically is provided.
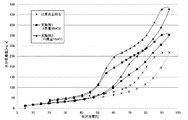
以下に、好ましい実施の形態を挙げて、本発明をさらに詳細に説明する。前記した課題を解決するために、本発明者らは、珪質頁岩の吸放湿性能を向上させることについて、種々の検討を行った。その検討の過程で、前記した特性を有する珪質頁岩の細孔内に、潮解性を有する、塩化リチウムや塩化カルシウムを付着させて、吸放湿性能の変化についての検討を行った。その結果、これらの物質を付着させた場合、原石と比較し、全湿度領域において、吸湿量が飛躍的に増大することがわかった。また、付着させた量に応じてその吸湿量が増大すること、塩化カルシウムを付着させた場合よりも、塩化リチウムを付着させた場合の方が、付着量が少なくても吸湿量が増大することがわかった(図3及び4参照)。 Hereinafter, the present invention will be described in more detail with reference to preferred embodiments. In order to solve the problems described above, the present inventors have made various studies on improving the moisture absorption / release performance of siliceous shale. In the course of the investigation, lithium chloride or calcium chloride having deliquescent properties was deposited in the pores of the siliceous shale having the above-described characteristics, and changes in moisture absorption / release performance were examined. As a result, it was found that when these substances were adhered, the amount of moisture absorption increased dramatically in the entire humidity region as compared with the raw stone. Also, the amount of moisture absorption increases according to the amount of adhesion, and the amount of moisture absorption increases even when the amount of adhesion is small compared to when calcium chloride is adhered. (See FIGS. 3 and 4).
しかし一方で、中湿域から高湿域にかけての水分吸着能(吸湿性能)が特に改善されるわけではなく、全体として向上する傾向にあり、また、原石の場合と同様に、相対湿度を低下させた場合に、吸湿した水分が全て放湿されず、蓄湿が生じ、吸放湿材料としては、さらなる改善の余地があると認識するに至った。そこで、本発明者らが、上記の事実を踏まえて、さらなる検討を重ねた結果、珪質頁岩の少なくとも細孔内に塩化ナトリウムを付着させることで、塩化リチウムや塩化カルシウムを付着させた系からは、到底、予想することができない下記の効果が得られることを見い出し、本発明に到達したものである。すなわち、図1に示されているように、珪質頁岩の細孔内に塩化ナトリウムを付着させた場合には、原石における吸放湿性能と比較して、下記の効果が得られることがわかった。まず、第1に、原石に比較し、吸湿量が飛躍的に増大するが、特に吸放湿材料に期待される、中湿域から高湿域にかけての吸湿量が、1.5〜3倍程度に増大することを見い出した。具体的には、細孔内に付着させた塩化ナトリウムの量にもよるが、特に、原石では、その吸湿量が、中湿域の相対湿度では100mg/g以下と少なかったのに対し、その2〜3倍近くまで大幅に増大する(図1参照)。また、第2に、相対湿度が低湿域になるにつれて水分の放湿が速やかになり、吸湿した水分のほぼ全てを放湿させることができることを見い出した(図1及び2参照)。以下、本発明の吸放湿材料の構成について詳細に説明する。 However, on the other hand, the moisture adsorption capacity (moisture absorption performance) from the middle to high humidity range is not particularly improved and tends to improve as a whole, and the relative humidity is reduced as in the case of the rough stone. In this case, all of the absorbed moisture is not released, and moisture storage occurs, and it has been recognized that there is room for further improvement as a moisture absorption / release material. Therefore, as a result of further studies by the present inventors based on the above fact, by attaching sodium chloride in at least the pores of the siliceous shale, the system in which lithium chloride or calcium chloride is attached is used. The inventors have found that the following effects that cannot be expected are obtained, and have reached the present invention. That is, as shown in FIG. 1, when sodium chloride is deposited in the pores of siliceous shale, it is understood that the following effects can be obtained as compared with the moisture absorption / release performance of the raw stone. It was. First, compared with the rough stone, the amount of moisture absorption increases dramatically, but the amount of moisture absorption from the middle to high humidity range, which is expected especially for moisture-absorbing / releasing materials, is 1.5 to 3 times. Found to increase to the extent. Specifically, although depending on the amount of sodium chloride adhered in the pores, in particular, in the raw stone, the amount of moisture absorption was as low as 100 mg / g or less in the relative humidity of the middle humidity region, whereas It greatly increases up to nearly 2 to 3 times (see FIG. 1). Secondly, it has been found that moisture can be quickly released as the relative humidity becomes a low humidity range, and almost all of the absorbed moisture can be released (see FIGS. 1 and 2). Hereinafter, the structure of the moisture absorption / release material of the present invention will be described in detail.
本発明において、担体として用いる珪質頁岩は、微細な細孔を多数有し、その径の平均細孔直径が3〜12nmであり、かつ、比表面積が80m2/g以上である。なお、後述するが、珪質頁岩原石を改質させて細孔を拡大させたもの(平均細孔直径18nm以下)も本発明において担体として使用することができる。上記した特性の珪質頁岩原石は、先に述べたように、原石においても優れた吸放湿性能を有しており、このような珪質頁岩原石としては、例えば、北海道天北地方で産出される、いわゆる、稚内層珪質頁岩と呼ばれるものが挙げられる。稚内層珪質頁岩は、上記した特性を有する他、細孔容量が0.1〜0.4ml/gの範囲内にあり、最大吸湿率が15質量%以上の、シャープな細孔径分布を有する天然の多孔質体である。本発明では、このような特性の珪質頁岩の細孔に、塩化ナトリウムを付着させることで、吸放湿性能の改質を図る。本発明者らの検討によれば、特に、本発明においては、比表面積が100m2/g以上の珪質頁岩を用いることが好ましい。 In the present invention, the siliceous shale used as a carrier has a large number of fine pores, an average pore diameter of 3 to 12 nm, and a specific surface area of 80 m 2 / g or more. In addition, although mentioned later, what modified the siliceous shale rough | crude and expanded the pore (average pore diameter of 18 nm or less) can also be used as a support | carrier in this invention. The siliceous shale rough with the above characteristics has excellent moisture absorption and desorption performance as described above, and such a siliceous shale rough is produced in, for example, the Tenku region of Hokkaido. What is called Wakkanai Formation siliceous shale is mentioned. The Wakkanai siliceous shale has the above-mentioned characteristics, and has a sharp pore size distribution with a pore volume in the range of 0.1 to 0.4 ml / g and a maximum moisture absorption of 15% by mass or more. It is a natural porous body. In the present invention, the moisture absorption / release performance is improved by attaching sodium chloride to the pores of the siliceous shale having such characteristics. According to the study by the present inventors, it is particularly preferable in the present invention to use siliceous shale having a specific surface area of 100 m 2 / g or more.
なお、本発明において、細孔容量は、BJH(Barrett Joyner Halenda)法で測定した。また、比表面積は、BET(Brunauer Emmett Teller)法で測定した。また、平均細孔直径は、前記の方法で測定した、細孔容量と比表面積とから、細孔が円柱体と仮定して、下記の式から計算により求めた。なお、最大吸湿率は、測定対象物を150℃のオーブンに入れ72時間保持した後に測定した対象物の絶乾質量と、その後、25℃、相対湿度95%の恒温恒湿槽に入れて48時間保持した後に再度測定する対象物の質量との質量増加率により得られる。相対湿度に応じての水分吸着量及び水分放湿量の変化は、水蒸気吸着量測定装置で連続して測定することができる。
(式)平均細孔直径=4×細孔容量(ml/g)÷比表面積(m2/g)
In the present invention, the pore volume was measured by the BJH (Barrett Joyner Halenda) method. The specific surface area was measured by the BET (Brunauer Emmet Teller) method. Further, the average pore diameter was calculated from the following formula based on the pore volume and specific surface area measured by the above method, assuming that the pore was a cylindrical body. The maximum moisture absorption rate is 48 by placing the measurement object in an oven at 150 ° C. and holding it for 72 hours and then measuring the absolute dry mass of the object, and then placing it in a constant temperature and humidity chamber at 25 ° C. and a relative humidity of 95%. It is obtained by the mass increase rate with respect to the mass of the object to be measured again after holding for a time. Changes in the amount of moisture adsorbed and the amount of moisture released according to the relative humidity can be continuously measured with a water vapor adsorption amount measuring apparatus.
(Formula) Average pore diameter = 4 × pore volume (ml / g) ÷ specific surface area (m 2 / g)
前記した特性を有する珪質頁岩の表面及び細孔内に付着される塩化ナトリウムの付着量は、特に制限はないが、10〜150mg/gの範囲内であることが好ましい。10mg/g未満の場合は、吸放湿性能の向上効果が低い場合がある。一方、塩化ナトリウムの付着量を多くするほど吸湿性能をより増大させることができるが、放湿性能や放湿速度を低下させる場合がある。そして、150mg/gを超える場合は、使用する用途によって、通風などで飛まつを生じさせる場合が多くなる。特に好ましい塩化ナトリウムの付着量は、10〜80mg/g、さらには、15〜60mg/gの範囲内である。 The amount of sodium chloride attached to the surface and pores of the siliceous shale having the above-mentioned properties is not particularly limited, but is preferably in the range of 10 to 150 mg / g. In the case of less than 10 mg / g, the improvement effect of moisture absorption / release performance may be low. On the other hand, as the amount of sodium chloride attached increases, the moisture absorption performance can be further increased, but the moisture release performance and moisture release rate may be reduced. And when it exceeds 150 mg / g, depending on the use to be used, there are many cases in which flying is caused by ventilation or the like. Particularly preferable sodium chloride adhesion amount is in the range of 10 to 80 mg / g, more preferably 15 to 60 mg / g.
また、本発明の吸放湿材料は、担体である前記珪質頁岩の少なくとも細孔内に、さらに、塩化リチウム、塩化マグネシウム及び/又は塩化カルシウムが付着されていることが好ましい。これらの物質は、前記したとおり、全湿度領域において、珪質頁岩の吸湿性能を飛躍的に向上させることができるものである。そして、これらの物質を塩化ナトリウムとともに前記珪質頁岩に付着させることにより、より高い吸湿性能向上効果が得られるようになる。本発明においては、上記した中でも、塩化リチウム又は塩化カルシウムを用いることが好ましく、さらには、吸湿性能向上効果が最も得られる塩化リチウムを用いることが特に好ましい。 In the moisture absorbing / releasing material of the present invention, it is preferable that lithium chloride, magnesium chloride and / or calcium chloride is further adhered to at least the pores of the siliceous shale as a carrier. As described above, these substances can drastically improve the moisture absorption performance of siliceous shale in the entire humidity region. And by adhering these substances to the siliceous shale together with sodium chloride, a higher hygroscopic performance improving effect can be obtained. In the present invention, among the above, it is preferable to use lithium chloride or calcium chloride, and it is particularly preferable to use lithium chloride that provides the best effect of improving the hygroscopic performance.
前記した、塩化リチウム、塩化マグネシウム及び/又は塩化カルシウムの珪質頁岩への付着量は、特に制限はないが、10〜250mg/gの範囲内であることが好ましい。10mg/g未満の場合は、吸湿性能向上効果が低い場合があり、250mg/gを超える場合は、水分とともに上記した物質が流出してしまう場合がある。塩化リチウムのさらに好ましい付着量は、20〜120mg/gの範囲内であり、特には、20〜50mg/gの範囲内である。また、塩化カルシウムのさらに好ましい付着量は、20〜180mg/gの範囲内であり、特には、20〜50mg/gの範囲内である。 The amount of lithium chloride, magnesium chloride and / or calcium chloride attached to the siliceous shale is not particularly limited, but is preferably in the range of 10 to 250 mg / g. If it is less than 10 mg / g, the effect of improving the moisture absorption performance may be low, and if it exceeds 250 mg / g, the above substances may flow out together with moisture. The more preferable adhesion amount of lithium chloride is in the range of 20 to 120 mg / g, and particularly in the range of 20 to 50 mg / g. Moreover, the more preferable adhesion amount of calcium chloride is in the range of 20 to 180 mg / g, and particularly in the range of 20 to 50 mg / g.
本発明において、塩化ナトリウム、塩化リチウム、塩化マグネシウム、塩化カルシウムを、前記珪質頁岩の表面及び細孔内に付着させる方法は、特に制限はないが、例えば、前記珪質頁岩を、これらの水溶液に浸漬させた後、乾燥処理する方法が挙げられる。すなわち、本発明の吸放湿材料は、前記した特性を有する珪質頁岩を塩化ナトリウム水溶液に浸漬する工程と、浸漬した珪質頁岩を乾燥処理する工程とを少なくとも有する製造方法により得ることができる。さらに、塩化ナトリウムと、塩化リチウム、塩化マグネシウム及び/又は塩化カルシウムとがともに付着された形態の吸放湿材料は、例えば、塩化ナトリウムを上記の方法で付着させる前後で、塩化リチウムなどを上記の方法で付着させることにより得ることができる。また、付着させる全ての物質を含んだ水溶液に前記珪質頁岩を浸漬させた後、乾燥処理することにより得ることもできる。 In the present invention, the method for attaching sodium chloride, lithium chloride, magnesium chloride, and calcium chloride to the surface and pores of the siliceous shale is not particularly limited. And a method of drying treatment after immersion in the substrate. That is, the moisture-absorbing / releasing material of the present invention can be obtained by a production method having at least a step of immersing siliceous shale having the above-described characteristics in an aqueous sodium chloride solution and a step of drying the immersed siliceous shale. . Furthermore, the moisture absorbing / releasing material in a form in which sodium chloride and lithium chloride, magnesium chloride and / or calcium chloride are attached together is, for example, before and after attaching sodium chloride by the above-described method. It can be obtained by attaching by a method. It can also be obtained by immersing the siliceous shale in an aqueous solution containing all the substances to be adhered, followed by drying treatment.
また、前記した本発明の吸放湿材料を製造する方法においては、前記した最適な付着量を確保するために、水溶液中における濃度を、塩化ナトリウムは1〜20質量%の範囲内、塩化リチウム、塩化マグネシウム、塩化カルシウムは1〜25質量%の範囲内にそれぞれ調製することが好ましい。特には、塩化ナトリウム水溶液の濃度は、1〜15質量%、さらには、1〜8質量%の範囲内に調整することが好ましい。また、塩化リチウム水溶液の濃度は、3〜20質量%、さらには、3〜7質量%の範囲内に調整することが好ましく、塩化カルシウム水溶液の濃度は、3〜20質量%、さらには3〜7質量%の範囲内に調製することが好ましい。 Further, in the method for producing the moisture absorbing / releasing material of the present invention described above, in order to ensure the optimum adhesion amount as described above, the concentration in the aqueous solution is set to sodium chloride within the range of 1 to 20% by mass, lithium chloride. Magnesium chloride and calcium chloride are preferably prepared in the range of 1 to 25% by mass. In particular, the concentration of the aqueous sodium chloride solution is preferably adjusted within the range of 1 to 15% by mass, and more preferably 1 to 8% by mass. Moreover, it is preferable to adjust the density | concentration of lithium chloride aqueous solution in the range of 3-20 mass%, and also 3-7 mass%, and the density | concentration of calcium chloride aqueous solution is 3-20 mass%, Furthermore, 3-3 mass%. It is preferable to prepare within the range of 7% by mass.
前記した、本発明の吸放湿材料を製造する方法においては、前記珪質頁岩を、前記した水溶液に浸漬させた状態で、真空引きを行うことが好ましい。真空引きを行うことにより、珪質頁岩の細孔内部に残存している空気が引き抜かれ、細孔深部まで水溶液を進入させることができるようになる。すなわち、珪質頁岩の細孔内壁と水溶液との接触効率を高めることができるようになり、水溶液中の物質を、より効率的、さらにはより安定的に付着できるようになる。真空引きの条件は、特に制限はないが、例えば、常温で、30〜200分とすることが好ましい。 In the method for producing the moisture-absorbing / releasing material of the present invention, it is preferable to perform evacuation while the siliceous shale is immersed in the aqueous solution. By performing evacuation, the air remaining in the pores of the siliceous shale is extracted, and the aqueous solution can be made to penetrate deep into the pores. That is, the contact efficiency between the pore inner wall of the siliceous shale and the aqueous solution can be increased, and the substance in the aqueous solution can be more efficiently and more stably attached. The vacuuming conditions are not particularly limited, but for example, it is preferably 30 to 200 minutes at room temperature.
真空引きを行う以外にも、珪質頁岩を前記した水溶液に浸漬させた状態で、静置させることで、前記した各物質を付着させてもよい。静置させる場合の条件は、特に制限はないが、例えば、常温で、700〜2,000分とすることが好ましい。 In addition to evacuation, each of the above-mentioned substances may be adhered by allowing the siliceous shale to be immersed in the above-described aqueous solution. The conditions for standing still are not particularly limited, but are preferably 700 to 2,000 minutes at room temperature, for example.
また、乾燥処理は、風乾でもよいし、熱風乾燥やオーブンによる強制乾燥、あるいは減圧乾燥であってもよい。本発明においては、絶乾させることが好ましく、例えば、40〜400℃で乾燥処理することが好ましい。なお、珪質頁岩は、900℃までは、空隙がほとんど変化しないことから、乾燥温度の上限に関しては、特に制限されない。乾燥処理に使用する装置に合わせて効率的な条件に設定すればよい。 The drying process may be air drying, hot air drying, forced drying by an oven, or vacuum drying. In this invention, it is preferable to dry completely, for example, it is preferable to dry-process at 40-400 degreeC. In addition, the siliceous shale is not particularly limited with respect to the upper limit of the drying temperature because the voids hardly change up to 900 ° C. What is necessary is just to set it as efficient conditions according to the apparatus used for a drying process.
また、前記した製造方法の他にも、珪質頁岩をボールミルなどで湿式粉砕する際に、水に変えて塩化ナトリウム水溶液を用いることで、珪質頁岩に塩化ナトリウムを付着させる方法が挙げられる。この方法では、塩化ナトリウムの付着と珪質頁岩の微粉砕化を同時に行うことができるため、非常に効率的であり、安価に行い得る。粉砕条件は、特に制限はなく、所望の粒径の粉末を得るのに適した粉砕時間などを選択することができる。例えば、粒径を20μm以下とする場合は、12時間以上粉砕処理するなど、求める粒径にするための粉砕条件で粉砕すれば、塩化ナトリウムを付着させることができる。なお、この方法における乾燥処理は、前記と同様の方法で行い得る。また、塩化ナトリウム以外の、塩化リチウムなども当該方法で珪質頁岩に付着させることができる。 In addition to the manufacturing method described above, there is a method of attaching sodium chloride to the siliceous shale by using a sodium chloride aqueous solution instead of water when wet grinding the siliceous shale with a ball mill or the like. In this method, the adhesion of sodium chloride and the fine pulverization of the siliceous shale can be performed simultaneously, so that it is very efficient and can be performed at low cost. The pulverization conditions are not particularly limited, and a pulverization time suitable for obtaining a powder having a desired particle diameter can be selected. For example, when the particle size is 20 μm or less, sodium chloride can be adhered by pulverizing under pulverization conditions for obtaining a desired particle size, such as by pulverizing for 12 hours or more. The drying process in this method can be performed by the same method as described above. Moreover, lithium chloride other than sodium chloride can be attached to the siliceous shale by this method.
また、本発明において、担体である珪質頁岩としては、前記した特性を有する珪質頁岩原石をそのまま用いてもよいし、細孔の状態を改質させたものを用いてもよい。珪質頁岩の細孔の状態を改質する方法としては、例えば、アルカリエッチング処理が挙げられる。エッチング処理した場合、珪質頁岩原石よりも、比表面積はやや低下する傾向にあるが、細孔容量を増大させることができ、吸放湿性能も向上させることが可能となる。なお、アルカリエッチング処理した場合、珪質頁岩原石の細孔も拡がる傾向にある。この場合、その平均細孔直径は、18nm以下に調整することが好ましい。すなわち、本発明において、担体として用いる珪質頁岩(原石、改質品)の平均細孔直径は、3〜18nmの範囲内である。さらに、アルカリエッチング処理された珪質頁岩に塩化ナトリウムを付着させた場合には、相乗的に吸放湿性能を向上させることが可能となる。本発明においては、エッチング処理などの方法で、前記珪質頁岩の細孔容量が、0.40ml/g以上、さらには、0.45ml/g以上となるように調整することが好ましい。一方で、細孔容量を大きくし過ぎると、珪質頁岩のもつ優れた吸放湿性能が損なわれるおそれがあるので、細孔容量は、0.65ml/g以下であることが好ましい。特に好ましい細孔容量は、吸放湿性能の向上効果の高い、0.45〜0.50ml/gの範囲内である。 Further, in the present invention, the siliceous shale as the support may be the raw siliceous shale having the above-described characteristics, or may be one having a modified pore state. Examples of the method for modifying the state of the pores of the siliceous shale include alkali etching treatment. When the etching treatment is performed, the specific surface area tends to be slightly lower than that of the siliceous shale rough, but the pore volume can be increased and the moisture absorption / release performance can also be improved. In addition, when alkali etching is performed, the pores of the siliceous shale rough have a tendency to expand. In this case, the average pore diameter is preferably adjusted to 18 nm or less. That is, in the present invention, the average pore diameter of the siliceous shale (raw or modified product) used as the carrier is in the range of 3 to 18 nm. Furthermore, when sodium chloride is adhered to the siliceous shale that has been subjected to the alkali etching treatment, it is possible to synergistically improve the moisture absorption / release performance. In the present invention, it is preferable to adjust the pore volume of the siliceous shale to 0.40 ml / g or more, more preferably 0.45 ml / g or more by a method such as etching treatment. On the other hand, if the pore volume is too large, the excellent moisture absorption / release performance of the siliceous shale may be impaired, so the pore volume is preferably 0.65 ml / g or less. A particularly preferable pore volume is in the range of 0.45 to 0.50 ml / g, which has a high effect of improving moisture absorption / release performance.
アルカリエッチング処理は、水酸化ナトリウムや水酸化カリウムなどのアルカリ性化合物の水溶液に前記珪質頁岩を浸漬させることにより行うことができる。特には、水酸化ナトリウム水溶液を用いることが好ましい。アルカリ性化合物の水溶液の濃度は、例えば、0.5〜3.0mol/lの範囲内にすることができる。また、珪質頁岩をアルカリ性化合物の水溶液に浸漬させた状態で、真空引きを行ってもよいし、静置させたままでもよい。好ましくは、吸放湿性能を向上させる効果がより高い真空引きである。真空引きの条件は、例えば、常温で、45〜300分することが好ましく、また、静置の条件は、常温で、1,000〜2,500分とすることが好ましい。アルカリエッチング処理を行った後は、前記した方法などにより、乾燥処理を行うことが好ましい。なお、アルカリエッチング処理は、前記した塩化ナトリウムの珪質頁岩への付着と同時に行ってもよい。具体的には、塩化ナトリウムと、アルカリ性化合物とをともに含む水溶液に、珪質頁岩を浸漬させて、乾燥処理することで、珪質頁岩への塩化ナトリウム付着とアルカリエッチング処理を同時に行い得る。 The alkali etching treatment can be performed by immersing the siliceous shale in an aqueous solution of an alkaline compound such as sodium hydroxide or potassium hydroxide. In particular, it is preferable to use an aqueous sodium hydroxide solution. The density | concentration of the aqueous solution of an alkaline compound can be in the range of 0.5-3.0 mol / l, for example. Further, evacuation may be performed in a state where the siliceous shale is immersed in an aqueous solution of an alkaline compound, or the siliceous shale may be left still. Preferably, evacuation is more effective in improving the moisture absorption / release performance. For example, the vacuuming condition is preferably 45 to 300 minutes at room temperature, and the standing condition is preferably 1,000 to 2,500 minutes at room temperature. After performing the alkali etching process, it is preferable to perform a drying process by the above-described method or the like. In addition, you may perform an alkali etching process simultaneously with adhesion to the above-mentioned sodium chloride to a siliceous shale. Specifically, the siliceous shale is immersed in an aqueous solution containing both sodium chloride and an alkaline compound, followed by drying treatment, whereby sodium chloride adhesion to the siliceous shale and alkali etching treatment can be performed simultaneously.
本発明の吸放湿材料は、他の機能を付与することなどを目的として、珪質頁岩の表面又は細孔内に、上記した物質以外のものが付着されていてもよい。また、前記した水溶液には、バインダー成分を配合させてもよい。また、本発明の吸放湿材料は、用途などに合わせて、粒径などを適宜調整できる。 In the moisture absorbing / releasing material of the present invention, for the purpose of imparting other functions, materials other than those described above may be adhered to the surface or pores of the siliceous shale. Moreover, you may mix | blend a binder component with above described aqueous solution. In addition, the moisture absorption / release material of the present invention can be appropriately adjusted in particle size and the like according to the application.
以上の構成を有する本発明の吸放湿材料は、中湿域から高湿域にかけて、高い吸湿性能を発揮し、さらに放湿性能にも優れるものである。また、本発明の吸放湿材料は、珪質頁岩と、付着される塩化ナトリウムは、健康への悪影響がないことは明らかであり、さらに、いずれも安価に入手できるものであることから、コスト面でも優れており、様々な用途への使用が期待できるものである。 The moisture-absorbing / releasing material of the present invention having the above configuration exhibits high moisture-absorbing performance from the mid-humidity region to the high-humidity region, and also has excellent moisture-releasing performance. Moreover, it is clear that the moisture absorbing / releasing material of the present invention is siliceous shale and the attached sodium chloride has no adverse health effects and can be obtained at low cost. It is also excellent in terms of use and can be expected to be used for various purposes.
例えば、本発明の吸放湿材料は、調湿建材の構成材料として用いることができる。具体的には、調湿建材、例えば、壁材、天井材、タイルなどを構成する、基材、接着層、表層などに付着又は配合させる材料として用いることができる。この場合、人間が快適に生活できる中湿域の状態を安定的に維持できる調湿建材を得ることが可能となる。 For example, the moisture absorbing / releasing material of the present invention can be used as a constituent material of a humidity control building material. Specifically, it can be used as a material that adheres to or blends with a base material, an adhesive layer, a surface layer, or the like that constitutes a humidity control building material such as a wall material, a ceiling material, or a tile. In this case, it is possible to obtain a humidity control building material that can stably maintain the state of the intermediate humidity region where a human can live comfortably.
また、本発明の吸放湿材料は、デシカント空調装置の除湿フィルターにも好適に用いることもできる。具体的には、除湿フィルターを構成する基材に、付着又は配合させる材料として用いることができる。従来、デシカント空調装置の除湿フィルターに用いられている吸放湿材料は、その再生に80℃以上の高温熱源が必要であった。そのため、装置内には、加熱器や、蒸気コイル、温水コイルなどを組み込まなければならないため、装置が大型化し、その用途の多くは業務用に限定されていた。しかし、本発明の吸放湿材料は、前記したように、中湿域から高湿域にかけて高い吸湿性能を示すと同時に、相対湿度を下げるだけで容易に吸着した水分を放湿できるので、40℃程度の低温で容易に再生できるという、上記の用途に好適な特性を有するものである(図2参照)。このため、本発明の吸放湿材料は、デシカント空調装置の小型化を実現させるものであり、これにより、家庭用としての普及を可能にするものである。 Moreover, the moisture absorption / release material of the present invention can also be suitably used for a dehumidifying filter of a desiccant air conditioner. Specifically, it can be used as a material that adheres to or blends with the base material constituting the dehumidifying filter. Conventionally, moisture absorbing / releasing materials used in a dehumidifying filter of a desiccant air conditioner required a high-temperature heat source of 80 ° C. or higher for regeneration. Therefore, since a heater, a steam coil, a hot water coil, etc. must be incorporated in the apparatus, the apparatus has been increased in size, and many of its applications have been limited to business use. However, as described above, the moisture absorbing / releasing material of the present invention exhibits high moisture absorbing performance from the middle humidity range to the high humidity range, and at the same time, can easily desorb the adsorbed moisture only by lowering the relative humidity. It has a characteristic suitable for the above-mentioned use that it can be easily reproduced at a low temperature of about 0 ° C. (see FIG. 2). For this reason, the moisture-absorbing / releasing material of the present invention realizes downsizing of the desiccant air-conditioning apparatus, and thereby enables widespread use for home use.
また、本発明の吸放湿材料は、厨房排気浄化装置に用いる浄化フィルター用途にも好適である。特に、電気調理器の湯煙から水蒸気を除去するための浄化フィルター用途に好適である。具体的には、浄化フィルターを構成する基材に、付着又は配合させる材料として用いることができる。 Moreover, the moisture absorption / release material of the present invention is also suitable for use as a purification filter used in a kitchen exhaust purification device. In particular, it is suitable for use as a purification filter for removing water vapor from hot smoke in an electric cooker. Specifically, it can be used as a material that adheres to or blends with the substrate constituting the purification filter.
以下、実施例及び比較例を挙げて本発明をさらに具体的に説明するが、本発明は、その要旨を超えない限り、下記実施例により限定されるものではない。なお、本実施例において、比表面積の値はBET法により測定したものであり、細孔容量の値はBJH法により測定したものである。また、平均細孔直径は、比表面積と細孔容量とから、前記した式を用いて算出したものである。 EXAMPLES Hereinafter, although an Example and a comparative example are given and this invention is demonstrated further more concretely, unless the summary is exceeded, this invention is not limited by the following Example. In this example, the value of the specific surface area was measured by the BET method, and the value of the pore volume was measured by the BJH method. The average pore diameter is calculated from the specific surface area and the pore volume using the above formula.
<稚内層珪質頁岩(原石)の利用>
(塩化ナトリウム付着珪質頁岩)
〔試料の作製〕
北海道天北地方から産出した稚内層珪質頁岩を、ハンマークラッシャーにて粉砕し、ふるいをかけて、粒径を1〜2mmに調製した。また、この稚内層珪質頁岩は、比表面積が149m2/g、平均細孔直径が10.2nm、細孔容量が0.38ml/gであった。
<Use of the Wakkanai siliceous shale (rough)>
(Silicon shale with sodium chloride)
[Sample preparation]
The Wakkanai siliceous shale produced from Tenboku, Hokkaido was crushed with a hammer crusher and sieved to adjust the particle size to 1 to 2 mm. The Wakkanai siliceous shale had a specific surface area of 149 m 2 / g, an average pore diameter of 10.2 nm, and a pore volume of 0.38 ml / g.
次に、上記で得られた稚内層珪質頁岩粒子(原石)を、5質量%の塩化ナトリウム水溶液に含浸させた後、デシケータ内で1時間真空引き(常温)を行った。その後、さらに、吸引ろ過し、150℃のオーブンで絶乾することにより、稚内層珪質頁岩の表面及び細孔内部に塩化ナトリウムを付着させた、実施例1の吸放湿材料を得た。なお、実施例1の吸放湿材料における塩化ナトリウムの付着量を、塩化ナトリウムを付着させる前後の珪質頁岩(絶乾)の質量変化により測定したところ、37.2mg/gであった。 Next, after impregnating the 5% by mass sodium chloride aqueous solution with the Wakkanai layer siliceous shale particles (raw ore) obtained above, vacuuming (normal temperature) was performed in a desiccator for 1 hour. Thereafter, the material was further subjected to suction filtration and dried in an oven at 150 ° C. to obtain a moisture absorbing / releasing material of Example 1 in which sodium chloride was adhered to the surface and pores of the Wakkanai layer siliceous shale. In addition, it was 37.2 mg / g when the adhesion amount of sodium chloride in the moisture absorption / release material of Example 1 was measured by the mass change of the siliceous shale (absolutely dry) before and after the sodium chloride was adhered.
また、5質量%の塩化ナトリウム水溶液に代えて、10質量%の塩化ナトリウム水溶液を用い、上記と同様の方法で、実施例2の吸放湿材料(塩化ナトリウムの付着量69.7mg/g)を得た。 Moreover, it replaced with 5 mass% sodium chloride aqueous solution, 10 mass% sodium chloride aqueous solution was used, and the moisture absorption / release material of Example 2 (adhesion amount of sodium chloride 69.7 mg / g) by the method similar to the above. Got.
〔水蒸気吸着等温線の測定〕
稚内層珪質頁岩(原石)、実施例1及び2の吸放湿材料について、水蒸気吸着量測定装置(製品名:HYDROSORB1000,ユアサアイオニクス(株)製)により測定した25℃における水蒸気吸着等温線を図1に示す。図1に示すとおり、塩化ナトリウムを付着させることで、中湿域から高湿域にかけて水分吸着量(吸湿性能)が飛躍的に向上することが確認された。なお、実施例1及び2の吸放湿材料の表面状態を確認したところ、実施例1の吸放湿材料は、珪質頁岩原石と大きな差異はなかったが、実施例2の吸放湿材料は、その表面に再結晶した塩化ナトリウムのかたまりが生じているのを微量ながら見て取れた。
[Measurement of water vapor adsorption isotherm]
Wacoal layer siliceous shale (raw stone), moisture absorption and desorption materials of Examples 1 and 2 were measured with a water vapor adsorption measuring device (product name: HYDROSORB1000, manufactured by Yuasa Ionics Co., Ltd.) at 25 ° C. Is shown in FIG. As shown in FIG. 1, it was confirmed that by adsorbing sodium chloride, the moisture adsorption amount (moisture absorption performance) was dramatically improved from the middle humidity region to the high humidity region. In addition, when the surface condition of the moisture-absorbing / releasing material of Examples 1 and 2 was confirmed, the moisture-absorbing / releasing material of Example 1 was not significantly different from the raw siliceous shale, but the moisture-absorbing / releasing material of Example 2 A small amount of sodium chloride recrystallized on the surface was observed.
〔吸着・再生試験〕
稚内層珪質頁岩(原石)、実施例1の吸放湿材料を、それぞれ、温度40℃、相対湿度30%の条件で24時間養生した後、質量を測定し、基準値とした。次に、これらを、温度30℃、相対湿度70%の条件下で2時間吸湿処理した後、温度40℃、相対湿度30%の条件下で2時間再生処理を行った。さらに、これを1サイクルとして、吸湿・再生を20サイクルまで繰り返し処理した。5サイクルまでの基準値に対する経時の質量変化を図2に示す。また、実施例1の吸放湿材料について、20サイクル終了後に質量を測定したところ、基準値との質量差はほとんどなかった。つまり、図2及び上記事実が示すとおり、本発明の吸放湿材料は、40℃の低温で容易に再生できるとともに、蓄湿がなく、繰り返し使用に耐え得るものであることが確認された。
[Adsorption / regeneration test]
The Wakkanai siliceous shale (raw stone) and the moisture-absorbing / releasing material of Example 1 were cured for 24 hours under the conditions of a temperature of 40 ° C. and a relative humidity of 30%, respectively, and the mass was measured as a reference value. Next, these were subjected to a moisture absorption treatment under conditions of a temperature of 30 ° C. and a relative humidity of 70% for 2 hours, and then subjected to a regeneration treatment for 2 hours under the conditions of a temperature of 40 ° C. and a relative humidity of 30%. Furthermore, this was defined as one cycle, and moisture absorption / regeneration was repeated up to 20 cycles. The mass change over time with respect to the reference value up to 5 cycles is shown in FIG. Moreover, about the moisture absorption / release material of Example 1, when mass was measured after 20 cycles completion, there was almost no mass difference with a reference value. That is, as shown in FIG. 2 and the above fact, it was confirmed that the moisture absorbing / releasing material of the present invention can be easily regenerated at a low temperature of 40 ° C., has no moisture accumulation, and can withstand repeated use.
(塩化リチウム/塩化カルシウム付着珪質頁岩)
〔試料の作製〕
・塩化リチウム
上記で粒径1〜2mmに粉砕した稚内層珪質頁岩(原石)を、5質量%、10質量%、20質量%の塩化リチウム水溶液にそれぞれ含浸させた後、デシケータ内で1時間真空引き(常温)を行った。その後、水分を吸引ろ過し、150℃のオーブンで絶乾することにより、稚内層珪質頁岩の表面及び細孔内部に塩化リチウムを付着させた、参考例1〜3の吸放湿材料を得た。なお、参考例1〜3の吸放湿材料における塩化リチウムの付着量を塩化リチウムを付着させる前後の珪質頁岩(絶乾)の質量変化により測定したところ、それぞれ、33.8mg/g、57.9mg/g、120mg/gであった。
(Silicon shale with lithium chloride / calcium chloride)
[Sample preparation]
・ Lithium chloride After impregnating the 5%, 10%, and 20% by weight lithium chloride aqueous solution with the Wakkanai layer siliceous shale (raw ore) crushed to a particle size of 1 to 2 mm as described above, one hour in a desiccator Vacuuming (room temperature) was performed. Then, moisture is suction filtered and the moisture absorbing / releasing material of Reference Examples 1 to 3 having lithium chloride attached to the surface and pores of the Wakkanai layer siliceous shale is obtained by drying in an oven at 150 ° C. It was. In addition, when the adhesion amount of lithium chloride in the moisture absorption / release materials of Reference Examples 1 to 3 was measured by mass change of siliceous shale (absolutely dry) before and after the lithium chloride was adhered, 33.8 mg / g and 57 respectively. .9 mg / g, 120 mg / g.
・塩化カルシウム
上記で粒径1〜2mmに粉砕した稚内層珪質頁岩(原石)を、5質量%、10質量%、20質量%の塩化カルシウム水溶液にそれぞれ含浸させた後、デシケータ内で1時間真空引き(常温)を行った。その後、水分を吸引ろ過し、150℃のオーブンで絶乾することにより、稚内層珪質頁岩の表面及び細孔内部に塩化カルシウムを付着させた、参考例4〜6の吸放湿材料を得た。なお、参考例4〜6の吸放湿材料における塩化カルシウムの付着量を、塩化カルシウムを付着させる前後の珪質頁岩(絶乾)の質量変化により測定したところ、それぞれ、36.6mg/g、82.8mg/g、179mg/gであった。
-Calcium chloride After impregnating the 5%, 10%, and 20% by weight calcium chloride aqueous solution with the Wakkanai layer siliceous shale (raw stone) crushed to a particle size of 1 to 2 mm as described above, it is one hour in a desiccator. Vacuuming (room temperature) was performed. Thereafter, the moisture absorbing and releasing materials of Reference Examples 4 to 6 in which calcium chloride is adhered to the surface and pores of the Wakkanai layer siliceous shale are obtained by suction filtration of moisture and drying in an oven at 150 ° C. It was. In addition, when the adhesion amount of the calcium chloride in the moisture absorption / release materials of Reference Examples 4 to 6 was measured by the mass change of the siliceous shale (absolutely dry) before and after the calcium chloride was adhered, 36.6 mg / g, They were 82.8 mg / g and 179 mg / g.
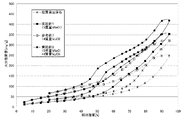
〔水蒸気吸着等温線の測定〕
稚内層珪質頁岩(原石)、参考例1〜6の吸放湿材料について、水蒸気吸着量測定装置(製品名:HYDROSORB1000,ユアサアイオニクス(株)製)により測定した25℃における水蒸気吸着等温線を図3、4に示す。図3、4に示すとおり、塩化リチウム又は塩化カルシウムを付着させることで、全湿度領域において平均的に、水分吸着量が大きく向上することが確認された。また、この効果は、同等の付着量では、塩化リチウムの方がより顕著であることが確認された。
[Measurement of water vapor adsorption isotherm]
Wacoal layer siliceous shale (raw stone), moisture absorption and desorption materials of Reference Examples 1-6, measured with a water vapor adsorption measuring device (product name: HYDROSORB1000, manufactured by Yuasa Ionics Co., Ltd.) at 25 ° C. Are shown in FIGS. As shown in FIGS. 3 and 4, it was confirmed that, by attaching lithium chloride or calcium chloride, the moisture adsorption amount greatly improved on average in the entire humidity region. Moreover, it was confirmed that this effect is more remarkable with lithium chloride at the same amount of adhesion.
(塩化ナトリウム、塩化リチウム複合付着珪質頁岩)
〔試料の作製〕
4質量%の塩化リチウム水溶液を用いる以外は、参考例3と同様の方法で、参考例7の吸放湿材料(塩化リチウムの付着量27.0mg/g)を得た。また、5質量%の塩化ナトリウムと4質量%の塩化リチウムとをともに含む水溶液を用いる以外は、実施例1と同様の方法で、実施例3の吸放湿材料(塩化ナトリウムの付着量35.8mg/g、塩化リチウムの付着量25.8mg/g)を得た。
(Silicon shale with sodium chloride and lithium chloride composite)
[Sample preparation]
Except using 4 mass% lithium chloride aqueous solution, the moisture absorption / release material of Reference Example 7 (attachment amount of lithium chloride 27.0 mg / g) was obtained by the same method as Reference Example 3. In addition, the moisture absorbing / releasing material of Example 3 (adhesive amount of sodium chloride of 35. 5) was obtained in the same manner as in Example 1 except that an aqueous solution containing both 5% by mass of sodium chloride and 4% by mass of lithium chloride was used. 8 mg / g, the adhesion amount of lithium chloride was 25.8 mg / g).
〔水蒸気吸着等温線の測定〕
稚内層珪質頁岩(原石)、参考例7、実施例1、3の吸放湿材料について、水蒸気吸着量測定装置(製品名:HYDROSORB1000,ユアサアイオニクス(株)製)により測定した25℃における水蒸気吸着等温線を図5に示す。図5に示すとおり、珪質頁岩に塩化ナトリウムと塩化リチウムとをともに付着させることで、より高い吸放湿性能が得られることが確認された。
[Measurement of water vapor adsorption isotherm]
Wakkanai siliceous shale (raw stone), moisture absorption / release material of Reference Example 7, Examples 1 and 3 at 25 ° C. measured with a water vapor adsorption measuring device (product name: HYDROSORB1000, manufactured by Yuasa Ionics Co., Ltd.) The water vapor adsorption isotherm is shown in FIG. As shown in FIG. 5, it was confirmed that higher moisture absorption / release performance can be obtained by attaching both sodium chloride and lithium chloride to siliceous shale.
<アルカリエッチング処理された珪質頁岩の利用>
(珪質頁岩のアルカリエッチング処理)
〔真空処理〕
上記で粒径1〜2mmに調製した稚内層珪質頁岩粒子(原石)を、1.0mol/lの水酸化ナトリウム水溶液に含浸させた後、デシケータ内で2時間真空引き(常温)を行った。その後、水分を吸引ろ過し、150℃のオーブンで絶乾することにより、アルカリエッチング処理された珪質頁岩1を得た。
<Use of alkali-etched siliceous shale>
(Alkaline etching treatment of siliceous shale)
[Vacuum treatment]
After impregnating the Wakkanai layer siliceous shale particles (raw ore) prepared above to a particle size of 1 to 2 mm in a 1.0 mol / l sodium hydroxide aqueous solution, vacuuming (normal temperature) was performed for 2 hours in a desiccator. . Thereafter, the moisture was suction filtered and dried in an oven at 150 ° C. to obtain siliceous shale 1 subjected to alkali etching.
〔静置処理〕
上記で粒径1〜2mmに調製した稚内層珪質頁岩粒子(原石)を、1.0mol/lの水酸化ナトリウム水溶液に含浸させた後、デシケータ内で24時間静置(常温)させた。その後、水分を吸引ろ過し、150℃のオーブンで絶乾することにより、アルカリエッチング処理された珪質頁岩2を得た。
[Standing treatment]
The Wakkanai layer siliceous shale particles (raw ore) prepared to have a particle diameter of 1 to 2 mm were impregnated with a 1.0 mol / l sodium hydroxide aqueous solution, and then allowed to stand (normal temperature) for 24 hours in a desiccator. Thereafter, the water was suction filtered and dried in an oven at 150 ° C. to obtain
稚内層珪質頁岩(原石)と、上記で得た珪質頁岩1、2の特性を下記表1に示す。
表1に示すとおり、アルカリエッチング処理を行うことにより、特に真空処理を行った場合は、珪質頁岩の水分吸着量が向上することが確認された。
The properties of the Wakkanai siliceous shale (raw stone) and the
As shown in Table 1, it was confirmed that the moisture adsorption amount of the siliceous shale is improved by performing the alkali etching process, particularly when the vacuum process is performed.
(塩化ナトリウム付着珪質頁岩)
上記で得た珪質頁岩1、2を、それぞれ、5質量%の塩化ナトリウム水溶液に含浸させた後、デシケータ内で1時間真空引き(常温)を行った。その後、さらに、吸引ろ過し、150℃のオーブンで絶乾することにより、珪質頁岩(アルカリ処理)の表面及び細孔内部に塩化ナトリウムを付着させた、実施例4、5の吸放湿材料を得た。なお、実施例4における塩化ナトリウムの付着量は27.9mg/g、実施例5における塩化ナトリウムの付着量は30.4mg/gであった。
(Silicon shale with sodium chloride)
Each of the
〔水蒸気吸着等温線の測定〕
稚内層珪質頁岩(原石)、珪質頁岩1、実施例1、4の吸放湿材料について、水蒸気吸着量測定装置(製品名:HYDROSORB1000,ユアサアイオニクス(株)製)により測定した25℃における水蒸気吸着等温線を図6に示す。図6に示すとおり、アルカリエッチング処理(真空処理)を行った珪質頁岩に、塩化ナトリウムを付着させることで、相乗的に水分吸着量が向上し、単に塩化ナトリウムを付着させた場合よりも、水分吸着量の向上効果が高いことが確認された。
[Measurement of water vapor adsorption isotherm]
Wakkanai layer siliceous shale (rough), siliceous shale 1, and moisture absorption / release materials of Examples 1 and 4 measured at 25 ° C. using a water vapor adsorption measuring device (product name: HYDROSORB1000, manufactured by Yuasa Ionics Co., Ltd.) FIG. 6 shows the water vapor adsorption isotherm. As shown in FIG. 6, by adhering sodium chloride to the siliceous shale that has been subjected to the alkali etching treatment (vacuum treatment), the amount of moisture adsorption is synergistically improved, rather than simply attaching sodium chloride, It was confirmed that the effect of improving the amount of moisture adsorption was high.
稚内層珪質頁岩(原石)、珪質頁岩2、実施例1、5の吸放湿材料について、水蒸気吸着量測定装置(製品名:HYDROSORB1000,ユアサアイオニクス(株)製)により測定した25℃における水蒸気吸着等温線を図7に示す。図7に示すとおり、アルカリエッチング処理(静置処理)を行った珪質頁岩に、塩化ナトリウムを付着させた場合には、最大水分吸着量の変化はほとんどなかったが、高湿域における水分吸着量が相対的に向上していることが確認された。
Wakkanai layer siliceous shale (rough),
(塩化ナトリウム、塩化リチウム複合付着珪質頁岩)
また、5質量%の塩化ナトリウムと4質量%の塩化リチウムとを含む水溶液を用いる以外は、実施例5と同様の方法で、実施例6の吸放湿材料(塩化ナトリウムの付着量35.4mg/g、塩化リチウムの付着量25.2mg/g)を得た。
(Silicon shale with sodium chloride and lithium chloride composite)
Moreover, the moisture absorption / release material of Example 6 (adhesion amount of sodium chloride 35.4 mg) was the same as in Example 5 except that an aqueous solution containing 5% by mass of sodium chloride and 4% by mass of lithium chloride was used. / G, the attached amount of lithium chloride 25.2 mg / g).
〔水蒸気吸着等温線の測定〕
稚内層珪質頁岩(原石)、珪質頁岩2、実施例3、6の吸放湿材料について、水蒸気吸着量測定装置(製品名:HYDROSORB1000,ユアサアイオニクス(株)製)により測定した25℃における水蒸気吸着等温線を図8に示す。図8に示すとおり、アルカリ処理を行った場合には、塩化ナトリウムと塩化リチウムをともに付着させた際の水分吸着量が飛躍的に向上することが確認された。
[Measurement of water vapor adsorption isotherm]
Wakkanai layer siliceous shale (raw stone),
<他の製法(ボールミル)>
粒径2.5mm以下に粉砕処理された珪質頁岩70kgを、100kgボールミル内に投入して、5質量%の塩化ナトリウム水溶液を使用して、20μm以下の粒径となるように粉砕処理した後、乾燥処理し、微粉末状である、実施例7の吸放湿材料を得た。実施例7の吸放湿材料についても、水蒸気吸着等温線を測定した結果、実施例1の吸放湿材料と同程度の吸放湿性能を有していることが確認された。
<Other manufacturing methods (ball mill)>
After 70 kg of siliceous shale pulverized to a particle size of 2.5 mm or less is put into a 100 kg ball mill and pulverized to a particle size of 20 μm or less using a 5 mass% sodium chloride aqueous solution. Then, the moisture-absorbing / releasing material of Example 7 in the form of fine powder was obtained by drying. As a result of measuring the water vapor adsorption isotherm of the moisture absorbing / releasing material of Example 7, it was confirmed that the moisture absorbing / releasing material had the same moisture absorbing / releasing performance as that of Example 1.
本発明によれば、中湿域から高湿域にかけての優れた吸湿性能と、優れた放湿性能とを有し、蓄湿のない速やかな吸放湿を繰り返し行うことのできる吸放湿材料が提供される。したがって、本発明の吸放湿材料は、調湿建材、デシカント空調装置、厨房排気浄化装置などの様々な用途に適用させて有効に活用し得る。 According to the present invention, a moisture absorbing / releasing material that has excellent moisture absorption performance from a medium humidity region to a high humidity region and excellent moisture release performance, and can repeatedly perform moisture absorption / release without moisture accumulation. Is provided. Therefore, the moisture absorbing / releasing material of the present invention can be effectively used by being applied to various applications such as humidity control building materials, desiccant air conditioners, kitchen exhaust purifiers, and the like.
Claims (13)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008269255A JP5382568B2 (en) | 2008-10-19 | 2008-10-19 | Hygroscopic material and method for modifying siliceous shale |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008269255A JP5382568B2 (en) | 2008-10-19 | 2008-10-19 | Hygroscopic material and method for modifying siliceous shale |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010094636A true JP2010094636A (en) | 2010-04-30 |
| JP5382568B2 JP5382568B2 (en) | 2014-01-08 |
Family
ID=42256704
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008269255A Expired - Fee Related JP5382568B2 (en) | 2008-10-19 | 2008-10-19 | Hygroscopic material and method for modifying siliceous shale |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5382568B2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10173168B2 (en) | 2016-03-11 | 2019-01-08 | Kabushiki Kaisha Toshiba | Vapor separator and dehumidifier using the same |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08175814A (en) * | 1994-12-21 | 1996-07-09 | Nittetsu Mining Co Ltd | Method for treating mineral and filtering material using the treated mineral |
| JPH11347341A (en) * | 1998-06-11 | 1999-12-21 | Nippon Steel Chem Co Ltd | Granular moisture absorbing and releasing material and manufacture thereof |
| JP2001137642A (en) * | 1999-10-14 | 2001-05-22 | Air Prod And Chem Inc | Method for removing water from gaseous hydrogen halide and adsorbing agent |
| JP2002212374A (en) * | 2000-11-14 | 2002-07-31 | Nippon Shokubai Co Ltd | Hygroscopic composition, hygroscopic agent and method for producing the same |
| JP2003053128A (en) * | 2001-08-13 | 2003-02-25 | Ohtsu Tire & Rubber Co Ltd :The | Humidity conditioning agent and producing method thereof |
| JP2004223366A (en) * | 2003-01-21 | 2004-08-12 | Panahome Corp | Globular moisture-absorbing/desorbing material and its manufacturing method, moisture-absorbing/desorbing ball, and moisture-absorbing/desorbing device |
| JP2007000701A (en) * | 2005-06-21 | 2007-01-11 | Motoharu Tamai | Enhanced humidifying charcoal |
| JP2008221125A (en) * | 2007-03-13 | 2008-09-25 | Hokkaido Univ | Humidity conditioning and gas adsorption material and manufacturing method of the same |
-
2008
- 2008-10-19 JP JP2008269255A patent/JP5382568B2/en not_active Expired - Fee Related
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH08175814A (en) * | 1994-12-21 | 1996-07-09 | Nittetsu Mining Co Ltd | Method for treating mineral and filtering material using the treated mineral |
| JPH11347341A (en) * | 1998-06-11 | 1999-12-21 | Nippon Steel Chem Co Ltd | Granular moisture absorbing and releasing material and manufacture thereof |
| JP2001137642A (en) * | 1999-10-14 | 2001-05-22 | Air Prod And Chem Inc | Method for removing water from gaseous hydrogen halide and adsorbing agent |
| JP2002212374A (en) * | 2000-11-14 | 2002-07-31 | Nippon Shokubai Co Ltd | Hygroscopic composition, hygroscopic agent and method for producing the same |
| JP2003053128A (en) * | 2001-08-13 | 2003-02-25 | Ohtsu Tire & Rubber Co Ltd :The | Humidity conditioning agent and producing method thereof |
| JP2004223366A (en) * | 2003-01-21 | 2004-08-12 | Panahome Corp | Globular moisture-absorbing/desorbing material and its manufacturing method, moisture-absorbing/desorbing ball, and moisture-absorbing/desorbing device |
| JP2007000701A (en) * | 2005-06-21 | 2007-01-11 | Motoharu Tamai | Enhanced humidifying charcoal |
| JP2008221125A (en) * | 2007-03-13 | 2008-09-25 | Hokkaido Univ | Humidity conditioning and gas adsorption material and manufacturing method of the same |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10173168B2 (en) | 2016-03-11 | 2019-01-08 | Kabushiki Kaisha Toshiba | Vapor separator and dehumidifier using the same |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5382568B2 (en) | 2014-01-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| ES2746198T3 (en) | Adsorbent granule of composite material, process for its production and gas separation process | |
| CN105502385B (en) | A kind of maize straw base porous carbon materials of absorbing carbon dioxide and preparation method thereof | |
| JP5889434B2 (en) | Vacuum insulation including high specific surface area getter material | |
| US11612857B2 (en) | Honeycomb matrix comprising macroporous desiccant, process and use thereof | |
| JP6761999B2 (en) | A water vapor adsorbent in which a hygroscopic salt is supported on an amorphous aluminum silicate granule. | |
| WO2010082456A1 (en) | Regenerative moisture absorbent | |
| JP5382568B2 (en) | Hygroscopic material and method for modifying siliceous shale | |
| EP3366748B1 (en) | A composite material for thermochemical storage and a method for forming a composite material | |
| JP5118864B2 (en) | Humidity conditioning and gas adsorbing material and manufacturing method thereof | |
| WO2023054012A1 (en) | Gas adsorbent and gas adsorption sheet, filter medium, and air filter using same | |
| JP3375927B2 (en) | Humidity control deodorant material using siliceous shale | |
| JP2001259417A (en) | Adsorption material for air conditioner, moisture absorbing element and dehumidifying method | |
| CN102553518A (en) | Preparation process of mineral composite material capable of adjusting moisture | |
| CN108479711A (en) | A kind of preparation method for purifying the modified activated carbon of formaldehyde in air | |
| JP5649024B2 (en) | Dehumidifying filter and desiccant air conditioner using the same | |
| JPWO2019093173A1 (en) | filter | |
| KR102005016B1 (en) | Method manufacturing activated carbon for removal of aldehydes gas | |
| ERDOĞAN | A comparative study of various porous adsorbents for CO2 adsorption | |
| JP2004261702A (en) | Porous material showing steam adsorbing/desorbing behavior and its use | |
| JP3921526B2 (en) | Porous material effective as anti-condensation material and method for producing the same | |
| KR20230086864A (en) | Impregnated activated carbon, filter including this, and method for manufacturing these | |
| JP5794443B1 (en) | Humidity control building materials | |
| JP2011167688A (en) | Humidity controlling and gas adsorbing material, and method for manufacturing the same | |
| JP2021142481A (en) | Gas adsorbent | |
| JP2007216118A (en) | Bamboo charcoal having molecular sieve function, its manufacturing method, and gas separation method and device using the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20111018 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111205 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20121207 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121211 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130208 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130214 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130307 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20130910 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20130920 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5382568 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |