ES2703220A1 - Method for the preparation of a new ERI molecular sieve (Machine-translation by Google Translate, not legally binding) - Google Patents
Method for the preparation of a new ERI molecular sieve (Machine-translation by Google Translate, not legally binding) Download PDFInfo
- Publication number
- ES2703220A1 ES2703220A1 ES201731089A ES201731089A ES2703220A1 ES 2703220 A1 ES2703220 A1 ES 2703220A1 ES 201731089 A ES201731089 A ES 201731089A ES 201731089 A ES201731089 A ES 201731089A ES 2703220 A1 ES2703220 A1 ES 2703220A1
- Authority
- ES
- Spain
- Prior art keywords
- molecular sieve
- bis
- eri
- cyclohexane
- silica
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 title claims abstract description 121
- 239000002808 molecular sieve Substances 0.000 title claims abstract description 119
- 238000000034 method Methods 0.000 title claims abstract description 34
- 238000002360 preparation method Methods 0.000 title claims abstract description 15
- 125000005208 trialkylammonium group Chemical group 0.000 claims abstract description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 62
- 239000000203 mixture Substances 0.000 claims description 33
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 claims description 31
- 239000000377 silicon dioxide Substances 0.000 claims description 30
- 230000015572 biosynthetic process Effects 0.000 claims description 23
- 238000003786 synthesis reaction Methods 0.000 claims description 22
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 15
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 14
- 229910052751 metal Inorganic materials 0.000 claims description 13
- 239000002184 metal Substances 0.000 claims description 13
- 239000010949 copper Substances 0.000 claims description 11
- 150000002739 metals Chemical class 0.000 claims description 9
- 229910052742 iron Inorganic materials 0.000 claims description 8
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 7
- 229910052802 copper Inorganic materials 0.000 claims description 7
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 claims description 6
- 229910052783 alkali metal Inorganic materials 0.000 claims description 5
- 150000001340 alkali metals Chemical class 0.000 claims description 5
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 5
- 150000001342 alkaline earth metals Chemical class 0.000 claims description 5
- 229910052782 aluminium Inorganic materials 0.000 claims description 5
- 229910052719 titanium Inorganic materials 0.000 claims description 5
- 239000010936 titanium Substances 0.000 claims description 5
- 238000002441 X-ray diffraction Methods 0.000 claims description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 4
- 150000001768 cations Chemical group 0.000 claims description 4
- 230000005855 radiation Effects 0.000 claims description 4
- 229910052718 tin Inorganic materials 0.000 claims description 4
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 claims description 3
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 claims description 3
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 3
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 3
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 claims description 3
- 229910052796 boron Inorganic materials 0.000 claims description 3
- 238000001354 calcination Methods 0.000 claims description 3
- 239000000428 dust Substances 0.000 claims description 3
- 229910052733 gallium Inorganic materials 0.000 claims description 3
- 229910052732 germanium Inorganic materials 0.000 claims description 3
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 claims description 3
- 229910052735 hafnium Inorganic materials 0.000 claims description 3
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 claims description 3
- 229910052738 indium Inorganic materials 0.000 claims description 3
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 claims description 3
- 229910052710 silicon Inorganic materials 0.000 claims description 3
- 229910052726 zirconium Inorganic materials 0.000 claims description 3
- DAZXVJBJRMWXJP-UHFFFAOYSA-N n,n-dimethylethylamine Chemical compound CCN(C)C DAZXVJBJRMWXJP-UHFFFAOYSA-N 0.000 claims description 2
- GNVRJGIVDSQCOP-UHFFFAOYSA-N n-ethyl-n-methylethanamine Chemical compound CCN(C)CC GNVRJGIVDSQCOP-UHFFFAOYSA-N 0.000 claims description 2
- 239000010703 silicon Substances 0.000 claims description 2
- ZMANZCXQSJIPKH-UHFFFAOYSA-O triethylammonium ion Chemical compound CC[NH+](CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-O 0.000 claims description 2
- 239000003795 chemical substances by application Substances 0.000 abstract description 5
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical group N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 45
- 229910052675 erionite Inorganic materials 0.000 description 45
- 239000003054 catalyst Substances 0.000 description 31
- MWUXSHHQAYIFBG-UHFFFAOYSA-N nitrogen oxide Inorganic materials O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 23
- 229910021529 ammonia Inorganic materials 0.000 description 21
- 239000007789 gas Substances 0.000 description 21
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 18
- 239000000047 product Substances 0.000 description 18
- 239000000463 material Substances 0.000 description 17
- 230000003647 oxidation Effects 0.000 description 16
- 238000007254 oxidation reaction Methods 0.000 description 16
- 230000003197 catalytic effect Effects 0.000 description 15
- 235000012239 silicon dioxide Nutrition 0.000 description 14
- 229910052681 coesite Inorganic materials 0.000 description 13
- 229910052593 corundum Inorganic materials 0.000 description 13
- 229910052906 cristobalite Inorganic materials 0.000 description 13
- 229910052682 stishovite Inorganic materials 0.000 description 13
- 239000000758 substrate Substances 0.000 description 13
- 229910052905 tridymite Inorganic materials 0.000 description 13
- 229910001845 yogo sapphire Inorganic materials 0.000 description 13
- 239000007864 aqueous solution Substances 0.000 description 11
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 10
- GQPLMRYTRLFLPF-UHFFFAOYSA-N Nitrous Oxide Chemical compound [O-][N+]#N GQPLMRYTRLFLPF-UHFFFAOYSA-N 0.000 description 9
- 239000010457 zeolite Substances 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 8
- 239000013078 crystal Substances 0.000 description 8
- 229930195733 hydrocarbon Natural products 0.000 description 8
- 150000002430 hydrocarbons Chemical class 0.000 description 8
- 238000000634 powder X-ray diffraction Methods 0.000 description 8
- HNPSIPDUKPIQMN-UHFFFAOYSA-N dioxosilane;oxo(oxoalumanyloxy)alumane Chemical compound O=[Si]=O.O=[Al]O[Al]=O HNPSIPDUKPIQMN-UHFFFAOYSA-N 0.000 description 7
- 229910021536 Zeolite Inorganic materials 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 239000011230 binding agent Substances 0.000 description 6
- 238000002425 crystallisation Methods 0.000 description 6
- 230000008025 crystallization Effects 0.000 description 6
- 239000012071 phase Substances 0.000 description 6
- 239000012265 solid product Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000011282 treatment Methods 0.000 description 6
- -1 N, N-dimethylpiperidinium cations Chemical class 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 229910021645 metal ion Inorganic materials 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- BJAARRARQJZURR-UHFFFAOYSA-N trimethylazanium;hydroxide Chemical compound O.CN(C)C BJAARRARQJZURR-UHFFFAOYSA-N 0.000 description 5
- 238000005406 washing Methods 0.000 description 5
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 4
- LCGLNKUTAGEVQW-UHFFFAOYSA-N Dimethyl ether Chemical compound COC LCGLNKUTAGEVQW-UHFFFAOYSA-N 0.000 description 4
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 4
- 239000004809 Teflon Substances 0.000 description 4
- 229920006362 Teflon® Polymers 0.000 description 4
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 4
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 4
- 239000003638 chemical reducing agent Substances 0.000 description 4
- 239000008367 deionised water Substances 0.000 description 4
- 229910021641 deionized water Inorganic materials 0.000 description 4
- 239000012153 distilled water Substances 0.000 description 4
- 238000005342 ion exchange Methods 0.000 description 4
- 239000001272 nitrous oxide Substances 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- 150000001298 alcohols Chemical class 0.000 description 3
- 229910000323 aluminium silicate Inorganic materials 0.000 description 3
- 239000011959 amorphous silica alumina Substances 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 229910052763 palladium Inorganic materials 0.000 description 3
- 229910052697 platinum Inorganic materials 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 239000010970 precious metal Substances 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 238000006722 reduction reaction Methods 0.000 description 3
- 238000004626 scanning electron microscopy Methods 0.000 description 3
- 229910052723 transition metal Inorganic materials 0.000 description 3
- 150000003624 transition metals Chemical class 0.000 description 3
- IMNIMPAHZVJRPE-UHFFFAOYSA-N triethylenediamine Chemical compound C1CN2CCN1CC2 IMNIMPAHZVJRPE-UHFFFAOYSA-N 0.000 description 3
- 238000011144 upstream manufacturing Methods 0.000 description 3
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000001336 alkenes Chemical class 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 238000010531 catalytic reduction reaction Methods 0.000 description 2
- CETPSERCERDGAM-UHFFFAOYSA-N ceric oxide Chemical compound O=[Ce]=O CETPSERCERDGAM-UHFFFAOYSA-N 0.000 description 2
- 229910000422 cerium(IV) oxide Inorganic materials 0.000 description 2
- 238000002485 combustion reaction Methods 0.000 description 2
- 239000012973 diazabicyclooctane Substances 0.000 description 2
- 150000002170 ethers Chemical class 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000002354 inductively-coupled plasma atomic emission spectroscopy Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 229910000069 nitrogen hydride Inorganic materials 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 239000002243 precursor Substances 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000001878 scanning electron micrograph Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 229910001428 transition metal ion Inorganic materials 0.000 description 2
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- XEZNGIUYQVAUSS-UHFFFAOYSA-N 18-crown-6 Chemical compound C1COCCOCCOCCOCCOCCO1 XEZNGIUYQVAUSS-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- 229910004298 SiO 2 Inorganic materials 0.000 description 1
- 229910010413 TiO 2 Inorganic materials 0.000 description 1
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 150000004645 aluminates Chemical class 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 150000001491 aromatic compounds Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- YOUGRGFIHBUKRS-UHFFFAOYSA-N benzyl(trimethyl)azanium Chemical compound C[N+](C)(C)CC1=CC=CC=C1 YOUGRGFIHBUKRS-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 229910001593 boehmite Inorganic materials 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 150000001728 carbonyl compounds Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000004517 catalytic hydrocracking Methods 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 239000003518 caustics Substances 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000007324 demetalation reaction Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000001493 electron microscopy Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 229910021485 fumed silica Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 238000005216 hydrothermal crystallization Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-M hydroxide Chemical compound [OH-] XLYOFNOQVPJJNP-UHFFFAOYSA-M 0.000 description 1
- FAHBNUUHRFUEAI-UHFFFAOYSA-M hydroxidooxidoaluminium Chemical compound O[Al]=O FAHBNUUHRFUEAI-UHFFFAOYSA-M 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- INQOMBQAUSQDDS-UHFFFAOYSA-N iodomethane Chemical compound IC INQOMBQAUSQDDS-UHFFFAOYSA-N 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 239000013335 mesoporous material Substances 0.000 description 1
- 229910044991 metal oxide Inorganic materials 0.000 description 1
- 150000004706 metal oxides Chemical class 0.000 description 1
- 239000012229 microporous material Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000002707 nanocrystalline material Substances 0.000 description 1
- 239000003345 natural gas Substances 0.000 description 1
- 229910052605 nesosilicate Inorganic materials 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 239000006259 organic additive Substances 0.000 description 1
- 239000011368 organic material Substances 0.000 description 1
- 150000004762 orthosilicates Chemical class 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 238000006303 photolysis reaction Methods 0.000 description 1
- 230000015843 photosynthesis, light reaction Effects 0.000 description 1
- 238000004375 physisorption Methods 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 239000010948 rhodium Substances 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910052596 spinel Inorganic materials 0.000 description 1
- 239000011029 spinel Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 229910000314 transition metal oxide Inorganic materials 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B39/00—Compounds having molecular sieve and base-exchange properties, e.g. crystalline zeolites; Their preparation; After-treatment, e.g. ion-exchange or dealumination
- C01B39/02—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof; Direct preparation thereof; Preparation thereof starting from a reaction mixture containing a crystalline zeolite of another type, or from preformed reactants; After-treatment thereof
- C01B39/30—Erionite or offretite type, e.g. zeolite T
- C01B39/305—Erionite or offretite type, e.g. zeolite T using at least one organic template directing agent
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B39/00—Compounds having molecular sieve and base-exchange properties, e.g. crystalline zeolites; Their preparation; After-treatment, e.g. ion-exchange or dealumination
- C01B39/02—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof; Direct preparation thereof; Preparation thereof starting from a reaction mixture containing a crystalline zeolite of another type, or from preformed reactants; After-treatment thereof
- C01B39/30—Erionite or offretite type, e.g. zeolite T
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/50—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the erionite or offretite type, e.g. zeolite T, as exemplified by patent document US2950952
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J29/00—Catalysts comprising molecular sieves
- B01J29/04—Catalysts comprising molecular sieves having base-exchange properties, e.g. crystalline zeolites
- B01J29/06—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof
- B01J29/50—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the erionite or offretite type, e.g. zeolite T, as exemplified by patent document US2950952
- B01J29/52—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof of the erionite or offretite type, e.g. zeolite T, as exemplified by patent document US2950952 containing iron group metals, noble metals or copper
- B01J29/56—Iron group metals or copper
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J35/00—Catalysts, in general, characterised by their form or physical properties
- B01J35/30—Catalysts, in general, characterised by their form or physical properties characterised by their physical properties
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B39/00—Compounds having molecular sieve and base-exchange properties, e.g. crystalline zeolites; Their preparation; After-treatment, e.g. ion-exchange or dealumination
- C01B39/02—Crystalline aluminosilicate zeolites; Isomorphous compounds thereof; Direct preparation thereof; Preparation thereof starting from a reaction mixture containing a crystalline zeolite of another type, or from preformed reactants; After-treatment thereof
- C01B39/026—After-treatment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C29/00—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring
- C07C29/48—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by oxidation reactions with formation of hydroxy groups
- C07C29/50—Preparation of compounds having hydroxy or O-metal groups bound to a carbon atom not belonging to a six-membered aromatic ring by oxidation reactions with formation of hydroxy groups with molecular oxygen only
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G11/00—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
- C10G11/02—Catalytic cracking, in the absence of hydrogen, of hydrocarbon oils characterised by the catalyst used
- C10G11/04—Oxides
- C10G11/05—Crystalline alumino-silicates, e.g. molecular sieves
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G3/00—Production of liquid hydrocarbon mixtures from oxygen-containing organic materials, e.g. fatty oils, fatty acids
- C10G3/42—Catalytic treatment
- C10G3/44—Catalytic treatment characterised by the catalyst used
- C10G3/48—Catalytic treatment characterised by the catalyst used further characterised by the catalyst support
- C10G3/49—Catalytic treatment characterised by the catalyst used further characterised by the catalyst support containing crystalline aluminosilicates, e.g. molecular sieves
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G45/00—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds
- C10G45/58—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to change the structural skeleton of some of the hydrocarbon content without cracking the other hydrocarbons present, e.g. lowering pour point; Selective hydrocracking of normal paraffins
- C10G45/60—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to change the structural skeleton of some of the hydrocarbon content without cracking the other hydrocarbons present, e.g. lowering pour point; Selective hydrocracking of normal paraffins characterised by the catalyst used
- C10G45/64—Refining of hydrocarbon oils using hydrogen or hydrogen-generating compounds to change the structural skeleton of some of the hydrocarbon content without cracking the other hydrocarbons present, e.g. lowering pour point; Selective hydrocracking of normal paraffins characterised by the catalyst used containing crystalline alumino-silicates, e.g. molecular sieves
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G47/00—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions
- C10G47/02—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions characterised by the catalyst used
- C10G47/10—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions characterised by the catalyst used with catalysts deposited on a carrier
- C10G47/12—Inorganic carriers
- C10G47/16—Crystalline alumino-silicate carriers
- C10G47/20—Crystalline alumino-silicate carriers the catalyst containing other metals or compounds thereof
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D2255/00—Catalysts
- B01D2255/50—Zeolites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D53/00—Separation of gases or vapours; Recovering vapours of volatile solvents from gases; Chemical or biological purification of waste gases, e.g. engine exhaust gases, smoke, fumes, flue gases, aerosols
- B01D53/34—Chemical or biological purification of waste gases
- B01D53/92—Chemical or biological purification of waste gases of engine exhaust gases
- B01D53/94—Chemical or biological purification of waste gases of engine exhaust gases by catalytic processes
- B01D53/9404—Removing only nitrogen compounds
- B01D53/9409—Nitrogen oxides
- B01D53/9413—Processes characterised by a specific catalyst
- B01D53/9418—Processes characterised by a specific catalyst for removing nitrogen oxides by selective catalytic reduction [SCR] using a reducing agent in a lean exhaust gas
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2229/00—Aspects of molecular sieve catalysts not covered by B01J29/00
- B01J2229/10—After treatment, characterised by the effect to be obtained
- B01J2229/18—After treatment, characterised by the effect to be obtained to introduce other elements into or onto the molecular sieve itself
- B01J2229/186—After treatment, characterised by the effect to be obtained to introduce other elements into or onto the molecular sieve itself not in framework positions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2229/00—Aspects of molecular sieve catalysts not covered by B01J29/00
- B01J2229/30—After treatment, characterised by the means used
- B01J2229/42—Addition of matrix or binder particles
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2002/00—Crystal-structural characteristics
- C01P2002/70—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data
- C01P2002/72—Crystal-structural characteristics defined by measured X-ray, neutron or electron diffraction data by d-values or two theta-values, e.g. as X-ray diagram
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/30—Particle morphology extending in three dimensions
- C01P2004/40—Particle morphology extending in three dimensions prism-like
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01P—INDEXING SCHEME RELATING TO STRUCTURAL AND PHYSICAL ASPECTS OF SOLID INORGANIC COMPOUNDS
- C01P2004/00—Particle morphology
- C01P2004/90—Other morphology not specified above
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2400/00—Products obtained by processes covered by groups C10G9/00 - C10G69/14
- C10G2400/22—Higher olefins
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G2400/00—Products obtained by processes covered by groups C10G9/00 - C10G69/14
- C10G2400/30—Aromatics
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02C—CAPTURE, STORAGE, SEQUESTRATION OR DISPOSAL OF GREENHOUSE GASES [GHG]
- Y02C20/00—Capture or disposal of greenhouse gases
- Y02C20/10—Capture or disposal of greenhouse gases of nitrous oxide (N2O)
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/52—Improvements relating to the production of bulk chemicals using catalysts, e.g. selective catalysts
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P30/00—Technologies relating to oil refining and petrochemical industry
- Y02P30/20—Technologies relating to oil refining and petrochemical industry using bio-feedstock
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Crystallography & Structural Chemistry (AREA)
- Materials Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Geology (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Combustion & Propulsion (AREA)
- Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Environmental & Geological Engineering (AREA)
- Analytical Chemistry (AREA)
- Catalysts (AREA)
- Silicates, Zeolites, And Molecular Sieves (AREA)
Abstract
Description
DESCRIPCIÓNDESCRIPTION
Método para la preparación de un nuevo tamiz molecular de ERIMethod for the preparation of a new ERI molecular sieve
La presente invención se refiere a un método para la preparación de un nuevo tamiz molecular con el tipo de estructura de ERI.The present invention relates to a method for the preparation of a new molecular sieve with the ERI structure type.
En particular, la invención es un método para la preparación de un material de tamiz molecular cristalino que pertenece a la familia de estructura de ERI esencialmente sin intercrecimiento de OFF, con una relación elevada de sílice a alúmina y una morfología cristalina tabular a prismática.In particular, the invention is a method for the preparation of a crystalline molecular sieve material that belongs to the ERI structure family essentially without OFF intergrowth, with a high ratio of silica to alumina and a tabular to prismatic crystal morphology.
Las zeolitas son materiales microporosos cristalinos formados al compartir por las esquinas tetraedros de TO4 (T = Si, Al, P, Ge, B, Ti, Sn, etc.), interconectados mediante átomos de oxígeno para formar poros y cavidades de tamaño uniforme y forma definida precisamente mediante su estructura cristalina. Las zeolitas también se designan "tamices moleculares” debido a que los poros y cavidades son de tamaño similar a moléculas pequeñas. Esta clase de materiales tiene aplicaciones comerciales importantes como absorbentes, intercambiadores de iones y catalizadores.Zeolites are crystalline microporous materials formed by sharing tetrahedral TO4 corners (T = Si, Al, P, Ge, B, Ti, Sn, etc.), interconnected by oxygen atoms to form pores and cavities of uniform size and precisely defined by its crystal structure. Zeolites are also referred to as "molecular sieves" because the pores and cavities are similar in size to small molecules.This class of materials has important commercial applications as absorbers, ion exchangers and catalysts.
Los tamices moleculares zeolíticos se clasifican por la International Zeolite Association (IZA) según las reglas de la Comisión de la IUPAC sobre Nomenclatura de Tamices Moleculares. Una vez que se establece la topología de una nueva estructura, se asigna un código de tres letras. Este código define la estructura atómica de la estructura, a partir de la cual se puede describir un patrón de difracción de rayos X nítido.Zeolitic molecular sieves are classified by the International Zeolite Association (IZA) according to the rules of the IUPAC Commission on Molecular Sieve Nomenclature. Once the topology of a new structure is established, a three-letter code is assigned. This code defines the atomic structure of the structure, from which a clear X-ray diffraction pattern can be described.
La expresión tipo de estructura o topología de estructura como se usa aquí, se refiere a la estructura atómica única de un tamiz molecular específico, nombrado por un código de tres letras ideado por la International Zeolite Association [Atlas of Zeolite Framework Types, 6a edición revisada, 2007, Ch. Baerlocher, L.B. McCusker y D.H. Olson, ISBN: 978-0-444 53064-6].The term structure type or structure topology as used herein, refers to the unique atomic structure of a specific molecular sieve, named by a three-letter code devised by the International Zeolite Association [Atlas of Zeolite Framework Types, 6th revised edition , 2007, Ch. Baerlocher, LB McCusker and D.H. Olson, ISBN: 978-0-444 53064-6].
La erionita (ERI) es una zeolita de aluminosilicato de origen natural [Staples, L.W. y Gard, J.A., Mineral. Mag., 32, 261-281 (1959)] con una relación de Si/Al de alrededor de 3. Se encuentra a menudo como un intercrecimiento con OFF [Schlenker, J.L., Pluth, J.J. y Smith, J.V., Acta Crystallogr., B33, 3265-3268 (1977)].Erionite (ERI) is an aluminosilicate zeolite of natural origin [Staples, LW and Gard, JA, Mineral. Mag., 32 , 261-281 (1959)] with a Si / Al ratio of around 3. It is often found as an intergrowth with OFF [Schlenker, JL, Pluth, JJ and Smith, JV, Acta Crystallogr., B33 , 3265-3268 (1977)].
Se han descrito varias maneras para preparar ERI mediante métodos sintéticos.Several ways have been described for preparing ERI by synthetic methods.
La patente US 2.950.952 describe la preparación de tamiz molecular tipo T, que se ha mostrado que es un intercrecimiento de ERI y OFF [J.M. Bennet et al., Nature, 1967, 214, 1005-1006. La patente US 3.699.139 describe la síntesis de ERI/OFF usando trimetilbencilamonio. La patente US 4.086.186 describe la síntesis de ZSM-34, que también es un intercrecimiento de ERI y OFF. La patente US 4.503.023 describe la síntesis de LZ-220, que es una forma ligeramente más silícea de tamiz molecular tipo T, y también es un intercrecimiento. También se ha dado a conocer que el uso de DABCO(I) y DABCO(II) da intercrecimientos de ERI y OFF [M. L. Ocelli et al., Zeolites, 1987, 7, 265-271].US Patent 2,950,952 describes the preparation of type T molecular sieve, which has been shown to be an intergrowth of ERI and OFF [J.M. Bennet et al., Nature, 1967, 214, 1005-1006. US Patent 3,699,139 describes the synthesis of ERI / OFF using trimethylbenzylammonium. US Patent 4,086,186 describes the synthesis of ZSM-34, which is also an intergrowth of ERI and OFF. US Patent 4,503,023 describes the synthesis of LZ-220, which is a slightly more siliceous form of T-type molecular sieve, and is also an intergrowth. It has also been reported that the use of DABCO (I) and DABCO (II) gives inter-growths of ERI and OFF [M. L. Ocelli et al., Zeolites, 1987, 7, 265-271].
Como se ilustra mediante las referencias anteriores, la preparación de ERI conduce típicamente a intercrecimientos con OFF. Estos intercrecimientos no se pueden considerar topologías de ERI pura y conducen a diferentes sistemas de canales y distribución de jaulas dentro de los materiales zeolíticos en comparación con ERI pura, lo que en conjunto influirá en las propiedades de esta clase de materiales.As illustrated by the above references, the preparation of ERI typically leads to intergrowths with OFF. These intergrowths can not be considered pure ERI topologies and lead to different channel systems and cage distribution within the zeolitic materials compared to pure ERI, which together will influence the properties of this class of materials.
Solamente unas pocas publicaciones se refieren a la síntesis de ERI esencialmente libre de intercrecimientos de OFF. La patente US 7.344.694 da a conocer la preparación de UZM-12, que se sugiere que tiene una relación de Si/Al por encima de 5,5 (= SiO2/Al2O3 > 11). Prácticamente, en los ejemplos no se dio ningún caso en el que se llevase a cabo la invención para lograr relaciones de sílice a alúmina (SiO2/Al2O3) mayores que 12,6. Además, UZM-12 se prepara usando un enfoque de disparidad de densidades, en el que se puede obtener material nanocristalino con cristalitos de 15 a 50 nm con morfologías cristalinas esferoidales a "de grano de arroz”. Especialmente, los nanocristalitos son difíciles de separar del licor de cristalización.Only a few publications refer to the synthesis of ERI essentially free of OFF inter-growth. US Patent 7,344,694 discloses the preparation of UZM-12, which is suggested to have a Si / Al ratio above 5.5 (= SiO2 / Al2O3> 11). Practically, in the examples there was no case in which the invention was carried out to achieve silica to alumina ratios (SiO2 / Al2O3) greater than 12.6. In addition, UZM-12 is prepared using a density disparity approach, in which nanocrystalline material can be obtained with crystallites of 15 to 50 nm with spherical crystalline morphologies to "rice grain." Especially, nanocrystallites are difficult to separate of the crystallization liquor.
Recientemente, otro tamiz molecular de ERI, designado SSZ-98, se dio a conocer en las patentes US 9.409.786, 9.416.017 y en la solicitud de patente US 2016/0001273. Este material también está esencialmente libre de intercrecimiento de OFF.Recently, another molecular sieve of ERI, designated SSZ-98, was disclosed in US Patents 9,409,786, 9,416,017 and in US Patent Application 2016/0001273. This material is also essentially free of OFF intergrowth.
Se reivindica que SSZ-98 tiene una relación de SiO2/Al2O3 entre 15 y 50, con una morfología cristalina similar a varillas o de lámina, y se prepara usando el dicatión de N,N’-dimetil-1,4diazobicido[2.2.2]octano como un agente director de la estructura.It is claimed that SSZ-98 has a SiO2 / Al2O3 ratio between 15 and 50, with a crystal-like morphology similar to rods or sheet, and is prepared using the dication of N, N'-dimethyl-1,4-diazobicido [2.2.2] octane as a directing agent of the structure.
Solicitudes de patentes posteriores también reivindican cationes de N,N-dimetilpiperidinio, cationes de 1,3-diciclohexilimidazalio, y su combinación, en las solicitudes de patentes US 2017/0088432, 2017/0073240 y 2016/0375428, respectivamente.Subsequent patent applications also claim N, N-dimethylpiperidinium cations, 1,3-dicyclohexylimidazole cations, and their combination, in patent applications US 2017/0088432, 2017/0073240 and 2016/0375428, respectively.
Es normalmente conocido en la técnica que la estabilidad hidrotérmica de tamices moleculares de aluminosilicatos se hace más elevada cuando se incrementa la relación molar de SiO2/Al2O3. En consecuencia, existe la necesidad de incrementar las relaciones molares de SiO2/Al2O3 de los materiales de tamices moleculares de ERI conocidos, en particular para aplicaciones en las que la estabilidad hidrotérmica es un problema. Además, también se conoce normalmente en la técnica que la morfología cristalina tiene un gran impacto sobre el comportamiento del tamiz molecular en aplicaciones catalíticas. En [S. Teketel, L. F. Lundegaard, W. Skistad, S. M. Chavan, U. Olsbye, K. P. Lillerud, P. Beato, S. Svelle, J. Catal. 2015, 327, 22-32] se puede encontrar una descripción del comportamiento de las diferentes morfologías cristalinas en la catálisis zeolítica. De este modo, también existe la necesidad de preparar materiales con morfologías específicas para aplicaciones catalíticas específicas.It is commonly known in the art that the hydrothermal stability of molecular sieves of aluminosilicates becomes higher when the molar ratio of SiO2 / Al2O3 is increased. Accordingly, there is a need to increase the molar ratios of SiO2 / Al2O3 of the known ERI molecular sieve materials, in particular for applications in which hydrothermal stability is a problem. In addition, it is also commonly known in the art that crystalline morphology has a great impact on the behavior of the molecular sieve in catalytic applications. In [S. Teketel, LF Lundegaard, W. Skistad, SM Chavan, U. Olsbye, KP Lillerud, P. Beato, S. Svelle, J. Catal. 2015 , 327, 22-32] a description of the behavior of the different crystalline morphologies in zeolitic catalysis can be found. Thus, there is also a need to prepare materials with specific morphologies for specific catalytic applications.
Para distinguir diferentes morfologías cristalinas, se define un parámetro (rc/ra), que describe la relación entre las diferentes dimensiones a lo largo (rc) y ortogonal (ra) al eje c único de los cristalitos preparados, por ejemplo determinadas mediante métodos de microscopía electrónica (para cristales hexagonales, el eje c único es paralelo al eje de simetría de seis veces). Las morfologías del cristalito se describirán usando las palabras lámina, tabular, prismática, aguja y similar a varilla. La relación entre estas descripciones y los valores de rc/ra se define en la Tabla a continuaciónTo distinguish different crystalline morphologies, a parameter (rc / ra) is defined , which describes the relationship between the different dimensions along (rc) and orthogonal (ra) to the single c-axis of the prepared crystallites, for example determined by methods of electron microscopy (for hexagonal crystals, the single c-axis is parallel to the six-fold axis of symmetry). The morphologies of the crystallite will be described using the words sheet, tabular, prismatic, needle and rod-like. The relationship between these descriptions and the rc / ra values is defined in the Table below
De este modo, un objeto general de esta invención es proporcionar un tamiz molecular cristalino de ERI esencialmente libre de intercrecimientos de OFF, con relaciones molares de SiO2/Al2O3 elevadas y morfologías cristalinas diferentes a lo que ya se conoce. Thus, a general object of this invention is to provide an ERI crystalline molecular sieve essentially free of OFF inter-flows, with high molar ratios of SiO2 / Al2O3 and crystal morphologies different from what is already known.
Hemos encontrado que el uso de un dicatión de cidohexano-1,4-bis(trialquilamonio) como un agente director de la estructura orgánica (OSDA) da como resultado la consecución exitosa de ERI pura con relaciones elevadas de sílice a alúmina de hasta 100, y con morfologías cristalinas diferentes a aquella de SSZ-98.We have found that the use of a dication of cidohexane-1,4-bis (trialkylammonium) as a directing agent of the organic structure (OSDA) results in the successful achievement of pure ERI with high silica to alumina ratios of up to 100, and with crystalline morphologies different from that of SSZ-98.
De acuerdo con el hallazgo anterior la presente invención proporciona un método para la preparación de un producto de tamiz molecular con el tipo de estructura de ERI, que comprende las etapas deAccording to the above finding, the present invention provides a method for the preparation of a molecular sieve product with the ERI structure type, comprising the steps of
i) preparar una mezcla de síntesis que comprende al menos una fuente de sílice y al menos una fuente de alúmina, o una fuente combinada tanto de sílice como de alúmina, una fuente de metal alcalino o metal alcalino-térreo (A), al menos un OSDA que es un dicatión de ciclohexano-1,4-bis(trialquilamonio), y agua en relaciones molares de:i) preparing a synthesis mixture comprising at least one source of silica and at least one source of alumina, or a combined source of both silica and alumina, a source of alkali metal or alkaline earth metal (A), at least an OSDA that is a dication of cyclohexane-1,4-bis (trialkylammonium), and water in molar ratios of:
ii) someter la mezcla a condiciones capaces de hacer cristalizar el tamiz molecular; yii) subjecting the mixture to conditions capable of crystallizing the molecular sieve; Y
iii) separar el producto del tamiz molecular para obtener el tamiz molecular según se sintetiza.iii) separating the product from the molecular sieve to obtain the molecular sieve as it is synthesized.
La fuente de sílice puede comprender sílice, sílice pirolizada, ácido silícico, silicatos amorfos o cristalinos, sílice coloidal, ortosilicatos de tetraalquilo, y mezclas de los mismos.The silica source may comprise silica, fumed silica, silicic acid, amorphous or crystalline silicates, colloidal silica, tetraalkyl orthosilicates, and mixtures thereof.
La fuente de alúmina puede comprender alúmina, boehmita, aluminatos, y mezclas de los mismos.The alumina source may comprise alumina, boehmite, aluminates, and mixtures thereof.
Una fuente combinada de sílice y alúmina puede ser sílice-alúmina amorfa coprecipitada, caolín, materiales mesoporosos, aluminosilicatos microporosos cristalinos, y mezclas de los mismos. A combined source of silica and alumina can be amorphous amorphous silica-alumina, kaolin, mesoporous materials, crystalline microporous aluminosilicates, and mixtures thereof.
En una realización de la invención, el producto del tamiz molecular tiene, en el estado según se sintetiza y anhidro, una composición con las relaciones molares dadas en la tabla:In one embodiment of the invention, the product of the molecular sieve has, in the state as it is synthesized and anhydrous, a composition with the molar ratios given in the table:
El OSDA es un dicatión de ciclohexano-1,4-bis(trialquilamonio) que tiene las estructuras (R = grupo alquilo) como se muestran a continuación.OSDA is a dication of cyclohexane-1,4-bis (trialkylammonium) having the structures (R = alkyl group) as shown below.
Preferiblemente, el OSDA se selecciona del grupo que consiste en ciclohexano-1,4-bis(trimetilamonio), ciclohexano-1,4-bis(trietilamonio), ciclohexano-1,4-bis(etildimetilamonio), ciclohexano-1,4-bis(dietilmetilamonio).Preferably, the OSDA is selected from the group consisting of cyclohexane-1,4-bis (trimethylammonium), cyclohexane-1,4-bis (triethylammonium), cyclohexane-1,4-bis (ethyldimethylammonium), cyclohexane-1,4- bis (diethylmethylammonium).
Actualmente, el OSDA más preferido es ciclohexano-1,4-bis(trimetilamonio).Currently, the most preferred OSDA is cyclohexane-1,4-bis (trimethylammonium).
El catión del OSDA está asociado con aniones, que pueden ser típicamente hidróxido, cloruro, bromuro, yoduro, etc., en tanto que no sean perjudiciales para la formación del tamiz molecular.The cation of OSDA is associated with anions, which may typically be hydroxide, chloride, bromide, iodide, etc., as long as they are not detrimental to the formation of the molecular sieve.
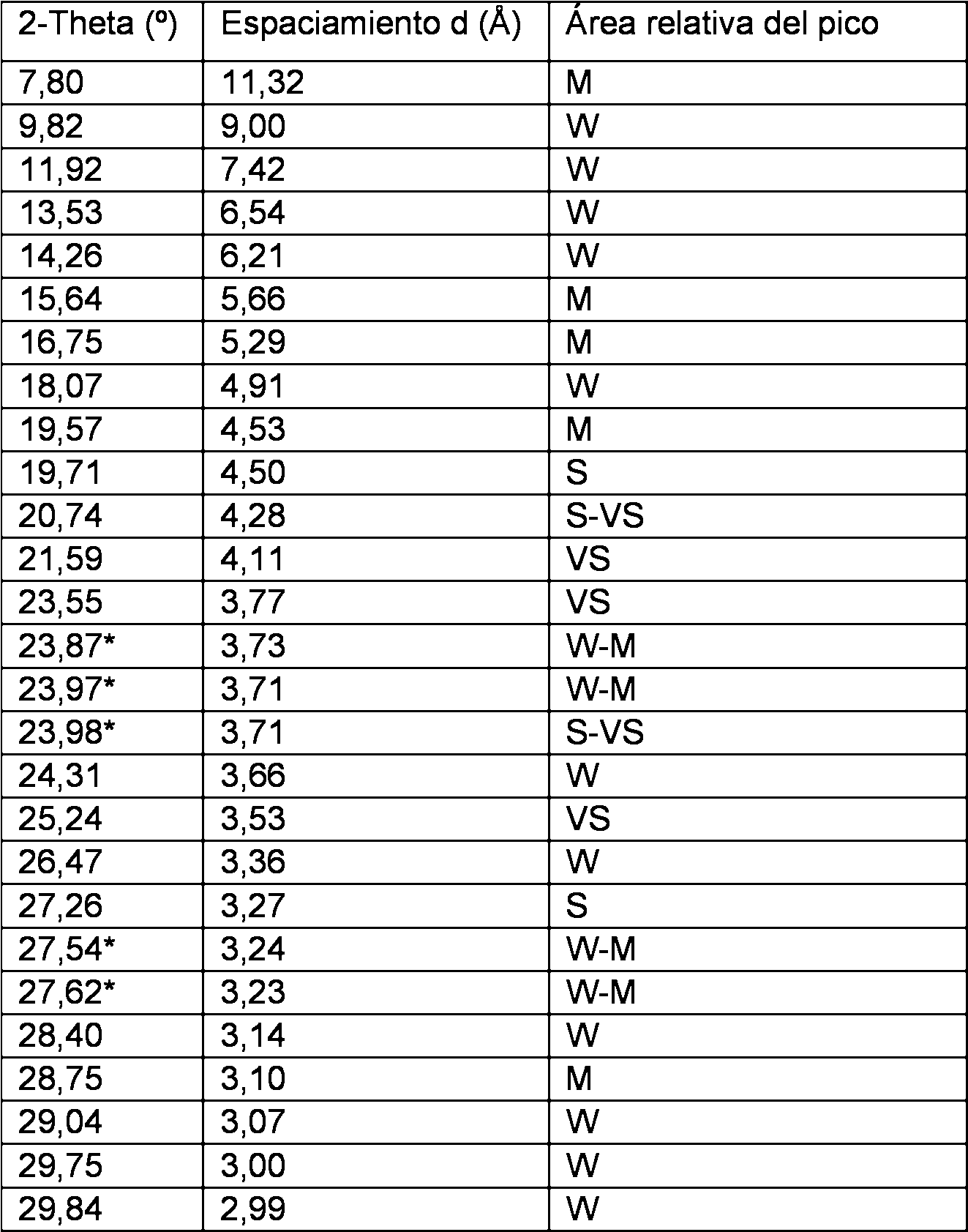
En una realización, la forma según se sintetiza del tamiz molecular tiene un patrón de difracción de rayos X de polvo, recogido en geometría de Bragg-Brentano con una ranura de divergencia variable usando radiación de Cu K-alfa, esencialmente como se muestra en la siguiente Tabla:In one embodiment, the shape as synthesized from the molecular sieve has a powder X-ray diffraction pattern, collected in Bragg-Brentano geometry with a variable divergence groove using Cu K-alpha radiation, essentially as shown in FIG. Next Table:
* Las intensidades de los picos y la asignación de las letras es incierta debido al solapamiento significativo de los picos.* The intensities of the peaks and the assignment of the letters is uncertain due to the significant overlap of the peaks.
en la que las áreas relativas de los picos observados en el intervalo 2-Theta se muestran según: W = débil: 0-20%; M = medio: 20-40%; S = fuerte: 40-60% y VS = muy fuerte: 60-100%. Los valores de 2-Theta son ± 0,20°.in which the relative areas of the peaks observed in the 2-Theta interval are shown according to: W = weak: 0-20%; M = medium: 20-40%; S = strong: 40-60% and VS = very strong: 60-100%. The values of 2-Theta are ± 0.20 °.
El catión del OSDA orgánico todavía retenido en el tamiz molecular según se sintetiza es eliminado en la mayoría de los casos, excepto que se use en la forma según se sintetiza, mediante tratamiento térmico en presencia de oxígeno. La temperatura del tratamiento térmico debería de ser suficiente para eliminar las moléculas orgánicas ya sea mediante evaporación, descomposición, combustión, o una combinación de las mismas. Típicamente, se aplica una temperatura entre 150 y 750°C durante un período de tiempo suficiente para eliminar la molécula o moléculas orgánicas. Una persona experta en la técnica será capaz fácilmente de determinar una temperatura y tiempo mínimos para este tratamiento térmico. Otros métodos para eliminar el material o materiales orgánicos retenidos en el tamiz molecular según se sintetiza incluyen extracción, calcinación a vacío, fotolisis, o tratamiento con ozono.The cation of the organic OSDA still retained in the molecular sieve as it is synthesized is eliminated in most cases, except that it is used in the form as it is synthesized, by heat treatment in the presence of oxygen. The temperature of the heat treatment should be sufficient to remove the organic molecules either by evaporation, decomposition, combustion, or a combination thereof. Typically, a temperature between 150 and 750 ° C is applied for a period of time sufficient to remove the organic molecule or molecules. A person skilled in the art will readily be able to determine a minimum temperature and time for this heat treatment. Other methods for removing the material or organic materials retained in the molecular sieve as synthesized include extraction, vacuum calcination, photolysis, or treatment with ozone.
En una realización, la forma calcinada del producto del tamiz molecular tiene un patrón de difracción de rayos X de polvo, recogido en geometría de Bragg-Brentano con una ranura de divergencia variable usando radiación de Cu K-alfa, esencialmente como se muestra en la siguiente Tabla:In one embodiment, the calcined form of the molecular sieve product has a dust X-ray diffraction pattern, collected in Bragg-Brentano geometry with a variable divergence groove using Cu K-alpha radiation, essentially as shown in FIG. Next Table:
* Las intensidades de los picos y la asignación de las letras es incierta debido al solapamiento significativo de los picos.* The intensities of the peaks and the assignment of the letters is uncertain due to the significant overlap of the peaks.
en la que las áreas relativas de los picos observados en el intervalo 2-Theta se muestran según: W = débil: 0-20%; M = medio: 20-40%; S = fuerte: 40-60% y VS = muy fuerte: 60-100%. Los valores de 2-Theta son ± 0,20°. in which the relative areas of the peaks observed in the 2-Theta interval are shown according to: W = weak: 0-20%; M = medium: 20-40%; S = strong: 40-60% and VS = very strong: 60-100%. The values of 2-Theta are ± 0.20 °.
El nuevo tamiz molecular con el tipo de estructura de ERI tiene una relación en moles de sílice a alúmina de alrededor de 8 a alrededor de 100 y una morfología cristalina, definida por la relación entre las dimensiones rc a lo largo de y ra ortogonal al eje c único, entre 0,5 y 2,0.The new molecular sieve structure type ERI has a mole ratio of silica to alumina of about 8 to about 100 and a crystal morphology, defined by the relationship between rc dimensions along orthogonal ra axis c only, between 0.5 and 2.0.
La morfología cristalina del nuevo tamiz molecular de ERI con una relación rc/ra de entre 0,5 y 2 tiene una morfología cristalina prismática a tabular, como se muestra en las Figuras 2 y 4 en los ejemplos más abajo, que es diferente a una morfología cristalina similar a varillas o de lámina del tamiz molecular de ERI conocido SSZ-98.The crystalline morphology of the new molecular sieve of ERI with a rc / ra ratio of between 0.5 and 2 has a prismatic to tabular crystalline morphology, as shown in Figures 2 and 4 in the examples below, which is different from a Crystal-like morphology similar to rods or molecular sieve sheet from ERI known SSZ-98.
En una realización adicional, la relación en moles de sílice a alúmina del nuevo tamiz molecular de ERI está entre 8 y 100, preferiblemente entre 10 y 60.In a further embodiment, the molar ratio of silica to alumina of the new molecular sieve of ERI is between 8 and 100, preferably between 10 and 60.
También se pueden introducir en la mezcla de síntesis otros elementos tetravalentes. Tales elementos incluyen estaño, circonio, titanio, hafnio, germanio, y combinaciones de los mismos. También se pueden incluir en la mezcla de síntesis elementos trivalentes, ya sea junto con aluminio o sin la presencia de aluminio. Tales elementos trivalentes incluyen boro, hierro, indio, galio, y combinaciones de los mismos. Tanto los elementos tetravalentes como trivalentes se pueden añadir en forma de metales, sales, óxidos, sulfuros, y combinaciones de los mismos.Other tetravalent elements can also be introduced into the synthesis mixture. Such elements include tin, zirconium, titanium, hafnium, germanium, and combinations thereof. Trivalent elements can also be included in the synthesis mixture, either together with aluminum or without the presence of aluminum. Such trivalent elements include boron, iron, indium, gallium, and combinations thereof. Both tetravalent and trivalent elements can be added in the form of metals, salts, oxides, sulfides, and combinations thereof.
De este modo, en una realización adicional, al menos una parte del aluminio en la fuente de alúmina y/o del silicio en la fuente de sílice en la mezcla de síntesis se sustituye por uno o más elementos seleccionados de estaño, circonio, titanio, hafnio, germanio, boro, hierro, indio y galio.Thus, in a further embodiment, at least a portion of the aluminum in the source of alumina and / or the silicon in the silica source in the synthesis mixture is replaced by one or more elements selected from tin, zirconium, titanium, hafnium, germanium, boron, iron, indium and gallium.
En la mezcla de síntesis se pueden incluir metales de transición, ya sea como sales simples o como complejos que protegen al metal de transición de la precipitación en las condiciones cáusticas dictadas por la mezcla de síntesis. Especialmente, los complejos de poliaminas son útiles para proteger iones de metales de transición de cobre y hierro durante la preparación, y también pueden actuar para dirigir la síntesis hacia tamices moleculares específicos (véase, por ejemplo, el uso de poliaminas en combinación con iones de cobre en la solicitud de patente US 2016/271596). De tal manera, los iones de metales de transición se pueden introducir en el interior del tamiz molecular fácilmente durante la cristalización. Transition metals may be included in the synthesis mixture, either as simple salts or as complexes that protect the transition metal from precipitation under the caustic conditions dictated by the synthesis mixture. Especially, the polyamine complexes are useful for protecting transition metal ions of copper and iron during the preparation, and can also act to direct the synthesis towards specific molecular sieves (see, for example, the use of polyamines in combination with ions of copper in the patent application US 2016/271596). In such a manner, the transition metal ions can be introduced into the interior of the molecular sieve easily during crystallization.
La mezcla de síntesis también puede contener agentes de llenado de poros baratos, que pueden ayudar en la preparación de productos más silíceos. Tales agentes de llenado de poros pueden ser éteres corona (por ejemplo, 18-corona-6), aminas simples (por ejemplo trimetil- y trietilamina), y otras moléculas no cargadas.The synthesis mixture can also contain inexpensive pore filling agents, which can aid in the preparation of more siliceous products. Such pore-filling agents may be crown (eg, 18-crown-6) ethers, simple amines (eg trimethyl- and triethylamine), and other uncharged molecules.
La cristalización de la mezcla de síntesis para formar el nuevo tamiz molecular se lleva a cabo a temperaturas elevadas hasta que se forma el tamiz molecular. Habitualmente se realiza la cristalización hidrotérmica de una manera para generar una presión autógena a temperaturas de 100-200°C en un autoclave y durante períodos de tiempo entre dos horas y 20 días. La mezcla de síntesis se puede someter a agitación durante la cristalización.The crystallization of the synthesis mixture to form the new molecular sieve is carried out at elevated temperatures until the molecular sieve is formed. Hydrothermal crystallization is usually carried out in a manner to generate an autogenous pressure at temperatures of 100-200 ° C in an autoclave and for periods of time between two hours and 20 days. The synthesis mixture can be subjected to stirring during crystallization.
Una vez que la cristalización se ha terminado, el producto del tamiz molecular sólido resultante se separa de la mezcla de síntesis líquida que queda mediante técnicas de separación convencionales tales como decantación, filtración (a vacío) o centrifugación. Los sólidos recuperados se enjuagan entonces típicamente con agua y se secan usando métodos convencionales (por ejemplo, calentando hasta 75-150°C a presión atmosférica, secado a vacío o liofilización, etc.), para obtener el tamiz molecular "según se sintetiza”. El producto "según se sintetiza” se refiere aquí al tamiz molecular tras la cristalización y antes de la eliminación del agente o agentes directores de la estructura u otros aditivos orgánicos.Once the crystallization is complete, the resulting solid molecular sieve product is separated from the remaining liquid synthesis mixture by conventional separation techniques such as decantation, filtration (in vacuo) or centrifugation. The recovered solids are then typically rinsed with water and dried using conventional methods (e.g., heating to 75-150 ° C at atmospheric pressure, vacuum drying or lyophilization, etc.), to obtain the "as synthesized" molecular sieve. The product "as synthesized" refers here to the molecular sieve after crystallization and before the elimination of the agent or agents directing the structure or other organic additives.
Habitualmente, es deseable eliminar los iones alcalinos o alcalino-térreos restantes (por ejemplo Na+) del tamiz molecular esencialmente libre de moléculas orgánicas ocluidas mediante intercambio iónico u otros métodos conocidos. El intercambio iónico con amonio y/o hidrógeno son métodos bien reconocidos para obtener la forma de NH4 o la forma de H del tamiz molecular. También se pueden incluir en el procedimiento de intercambio iónico iones metálicos deseados, o se pueden llevar a cabo separadamente. La forma de NH4 del material también se puede convertir en la forma de H mediante tratamiento térmico simple, de una manera similar a como se describe anteriormente.Usually, it is desirable to remove the remaining alkali or alkaline earth (for example Na +) ions from the molecular sieve essentially free of occluded organic molecules by ion exchange or other known methods. Ion exchange with ammonium and / or hydrogen are well-recognized methods for obtaining the NH4 form or the H form of the molecular sieve. Also, desired metal ions can be included in the ion exchange process, or they can be carried out separately. The NH 4 form of the material can also be converted to the H form by simple heat treatment, in a manner similar to that described above.
En ciertos casos, también puede ser deseable alterar la composición química del tamiz molecular obtenido, tal como alterando la relación molar de sílice a alúmina. Sin estar atados por cualquier orden de los tratamientos post-sintéticos, en este caso pueden ser útiles la lixiviación de ácidos (se pueden usar inorgánicos y orgánicos usando agentes complejantes tales como EDTA, etc.), el tratamiento con vapor, la desilicación, y combinaciones de los mismos, u otros métodos de desmetalación. In certain cases, it may also be desirable to alter the chemical composition of the molecular sieve obtained, such as by altering the molar ratio of silica to alumina. Without being bound by any order of the post-synthetic treatments, in this case the leaching of acids can be useful (they can be used inorganic and organic using complexing agents such as EDTA, etc.), steam treatment, desilication, and combinations thereof, or other methods of demetalation.
Para promover aplicaciones catalíticas específicas, se pueden introducir ciertos metales en el nuevo tamiz molecular para obtener un tamiz molecular sustituido con metal, impregnado con metal o intercambiado con metal. Los iones metálicos se pueden introducir mediante intercambio iónico, impregnación, procedimientos en estado sólido, y otras técnicas conocidas. Los metales se pueden introducir para producir esencialmente iones metálicos atómicamente dispersados, o se pueden introducir para producir pequeños agrupamientos o nanopartículas con carácter iónico o metálico. Como alternativa, los metales se pueden precipitar simplemente sobre la superficie y en los poros del tamiz molecular. En el caso en el que se prefieran nanopartículas, puede ser útil el tratamiento consecutivo en, por ejemplo, una atmósfera reductora. En otros casos, también puede ser deseable calcinar el material tras la introducción de metales o iones metálicos.To promote specific catalytic applications, certain metals can be introduced into the new molecular sieve to obtain a molecular sieve substituted with metal, impregnated with metal or exchanged with metal. Metal ions can be introduced by ion exchange, impregnation, solid state processes, and other known techniques. The metals can be introduced to produce essentially atomically dispersed metal ions, or they can be introduced to produce small groupings or nanoparticles with ionic or metallic character. Alternatively, the metals can be simply precipitated on the surface and in the pores of the molecular sieve. In the case where nanoparticles are preferred, consecutive treatment in, for example, a reducing atmosphere may be useful. In other cases, it may also be desirable to calcine the material after the introduction of metals or metal ions.
De este modo, en otra realización, el método según la invención comprende la etapa adicional de introducir cobre y/o hierro sobre o en el producto del tamiz molecular.Thus, in another embodiment, the method according to the invention comprises the additional step of introducing copper and / or iron onto or in the product of the molecular sieve.
El tamiz molecular según la invención es particularmente útil en reacciones de conversión catalíticas heterogéneas, tal como cuando el tamiz molecular cataliza la reacción de moléculas en fase gaseosa o fase líquida. También se puede formular para otras aplicaciones no catalíticas comercialmente importantes, tal como la separación de gases. El tamiz molecular proporcionado por la invención y procedente de cualquiera de las etapas de preparación descritas anteriormente se puede conformar en una variedad de formas físicas útiles para aplicaciones específicas. Por ejemplo, el tamiz molecular se puede usar en forma de polvo, o se le puede dar la forma de peletes, extrusados o formas monolíticas moldeadas, por ejemplo como un sustrato de cuerpo totalmente corrugado que contiene el tamiz molecular.The molecular sieve according to the invention is particularly useful in heterogeneous catalytic conversion reactions, such as when the molecular sieve catalyses the reaction of molecules in gas phase or liquid phase. It can also be formulated for other commercially important non-catalytic applications, such as gas separation. The molecular sieve provided by the invention and from any of the preparation steps described above can be formed into a variety of physical forms useful for specific applications. For example, the molecular sieve may be used in the form of a powder, or it may be in the form of pellets, extruded or molded monolithic forms, for example as a fully corrugated body substrate containing the molecular sieve.
A la hora de dar forma al tamiz molecular, típicamente será útil aplicar componentes orgánicos o inorgánicos adicionales. Para aplicaciones catalíticas, es particularmente útil aplicar una combinación con alúmina, sílice, titania, ceria, circonia, diversas estructuras de espinela, u otros óxidos o combinaciones de las mismas. También se puede formular con otros compuestos activos tales como metales activos u otros tamices moleculares, etc.When shaping the molecular sieve, it will typically be useful to apply additional organic or inorganic components. For catalytic applications, it is particularly useful to apply a combination with alumina, silica, titania, ceria, zirconia, various spinel structures, or other oxides or combinations thereof. It can also be formulated with other active compounds such as active metals or other molecular sieves, etc.
El tamiz molecular también se puede emplear revestido sobre o introducido en un sustrato que mejora el área de contacto, la difusión, las características del fluido y la fluidez de la corriente gaseosa. El sustrato puede ser un sustrato metálico, un sustrato extruido o un sustrato corrugado, estando formado este último de papel cerámico. El sustrato se puede diseñar como un diseño de flujo continuo o un diseño de flujo a través de pared. En el último caso, el gas fluye a través de las paredes del sustrato, y de esta manera también puede contribuir con un efecto filtrante adicional.The molecular sieve can also be used coated on or introduced into a substrate that improves the contact area, diffusion, fluid characteristics and fluidity of the gaseous current. The substrate can be a metal substrate, an extruded substrate or a corrugated substrate, the latter being formed of ceramic paper. The substrate can be designed as a continuous flow design or a flow through wall design. In the latter case, the gas flows through the walls of the substrate, and in this way can also contribute an additional filtering effect.
El tamiz molecular está presente típicamente sobre o en el sustrato en cantidades entre 10 y 600 g/l, preferiblemente 100 y 300 g/l, según se calcula mediante el peso del tamiz molecular por volumen del artículo catalítico total.The molecular sieve is typically present on or in the substrate in amounts between 10 and 600 g / l, preferably 100 and 300 g / l, as calculated by the weight of the molecular sieve per volume of the total catalytic article.
El tamiz molecular se reviste sobre o en el interior del sustrato usando técnicas conocidas de revestimiento por lavado. En este enfoque, el polvo del tamiz molecular se suspende en un medio líquido junto con aglutinante o aglutinantes y estabilizante o estabilizantes. El revestimiento de lavado se puede aplicar entonces sobre las superficies y paredes del sustrato. El revestimiento de lavado también contiene opcionalmente aglutinantes a base de TiO2, SiO2, Al2O3, ZrO2, CeO2, y combinaciones de los mismos.The molecular sieve is coated on or in the interior of the substrate using known techniques of coating by washing. In this approach, the molecular sieve powder is suspended in a liquid medium together with binder or binders and stabilizer or stabilizers. The washing coating can then be applied on the surfaces and walls of the substrate. The wash coating also optionally contains binders based on TiO2, SiO2, Al2O3, ZrO2, CeO2, and combinations thereof.
El tamiz molecular también se puede aplicar como una o más capas sobre el sustrato en combinación con otras funcionalidades catalíticas u otros catalizadores zeolíticos. Una combinación específica es una capa con un catalizador de oxidación que contiene, por ejemplo, platino o paladio, o combinaciones de los mismos. El tamiz molecular se puede aplicar adicionalmente en zonas limitadas a lo largo de la dirección del caudal del gas del sustrato.The molecular sieve can also be applied as one or more layers on the substrate in combination with other catalytic functionalities or other zeolitic catalysts. A specific combination is a layer with an oxidation catalyst containing, for example, platinum or palladium, or combinations thereof. The molecular sieve can be additionally applied in limited areas along the direction of the gas flow of the substrate.
El tamiz molecular según la invención se puede usar en la conversión catalítica de óxidos de nitrógeno, típicamente en presencia de oxígeno. En particular, el tamiz molecular se puede usar en la reducción catalítica selectiva (SCR) de óxidos de nitrógeno con un agente reductor tal como amoníaco y precursores del mismo, incluyendo urea o hidrocarburos. Para este tipo de aplicación, el tamiz molecular se cargará típicamente con un metal de transición, tal como cobre o hierro, o combinaciones de los mismos, usando cualquiera de los procedimientos descritos anteriormente, en una cantidad suficiente para catalizar la reacción específica.The molecular sieve according to the invention can be used in the catalytic conversion of nitrogen oxides, typically in the presence of oxygen. In particular, the molecular sieve can be used in the selective catalytic reduction (SCR) of nitrogen oxides with a reducing agent such as ammonia and precursors thereof, including urea or hydrocarbons. For this type of application, the molecular sieve will typically be loaded with a transition metal, such as copper or iron, or combinations thereof, using any of the methods described above, in an amount sufficient to catalyze the specific reaction.
En ciertos aspectos de la invención, puede ser beneficiosa una cierta cantidad de metal alcalino o metal alcalino-térreo. Véase, por ejemplo, una descripción de los efectos de metales alcalinos y metales alcalino-térreos sobre CHA promovida por cobre en [F. Gao, Y. Wang, N. M. Washton, M. Kollár, J. Szanyi, C. H. F. Peden, ACS Catal. 2015, 5, 6780 6791]. En otros aspectos, se puede preferir usar el tamiz molecular esencialmente libre de metal alcalino o metal alcalino-térreo.In certain aspects of the invention, a certain amount of alkali metal or alkaline earth metal may be beneficial. See, for example, a description of the effects of alkali metals and alkaline-earth metals on CHA promoted by copper in [F. Gao, Y. Wang, NM Washton, M. Kollár, J. Szanyi, CHF Peden, ACS Catal. 2015 , 5, 6780 6791]. In other aspects, it may be preferred to use the molecular sieve essentially free of alkali metal or alkaline earth metal.
El tamiz molecular de ERI según la invención se puede usar ventajosamente como catalizador en la reducción de óxidos de nitrógeno en el escape procedente de un motor de combustión interna vehicular (es decir, móvil). En esta aplicación, el sistema de escape puede comprender uno o más de los siguientes componentes: un catalizador de oxidación de diésel (DOC), un filtro de partículas de diésel (DPF), un catalizador de reducción catalítica selectiva (SCR), y/o un catalizador antifuga de amoníaco (ASC). Tal sistema también contiene típicamente medios para medir el agente reductor, así como la posibilidad para medir hidrocarburos en el sistema de escape aguas arriba del SCR y del DOC, respectivamente.The ERI molecular sieve according to the invention can be advantageously used as a catalyst in the reduction of nitrogen oxides in the exhaust from a vehicular (ie mobile) internal combustion engine. In this application, the exhaust system may comprise one or more of the following components: a diesel oxidation catalyst (DOC), a diesel particulate filter (DPF), a selective catalytic reduction catalyst (SCR), and / or an ammonia leakage catalyst (ASC). Such a system also typically contains means for measuring the reducing agent, as well as the possibility to measure hydrocarbons in the exhaust system upstream of the SCR and the DOC, respectively.
Preferiblemente, el catalizador de SCR comprende el tamiz molecular de ERI de la invención. El catalizador de SCR también puede contener otros componentes activos, tales como otros tamices moleculares. Cuando el catalizador de SCR está situado en tal sistema de escape, está expuesto a temperaturas elevadas procedentes del motor o durante la regeneración térmica de uno o más de los componentes en el sistema.Preferably, the SCR catalyst comprises the ERI molecular sieve of the invention. The SCR catalyst may also contain other active components, such as other molecular sieves. When the SCR catalyst is located in such an exhaust system, it is exposed to elevated temperatures from the engine or during thermal regeneration of one or more of the components in the system.
En el sistema de escape como se describe anteriormente, el catalizador de SCR, que comprende el tamiz molecular de ERI, puede estar situado entre los componentes del DPF y del ASC. Otra posibilidad es disponer el catalizador de SCR aguas arriba del DOC, donde se requiere cierta tolerancia a hidrocarburos sin quemar. La funcionalidad de SCR también se puede incluir en el DPF, o se puede combinar con el ASC en un único componente con una función dual.In the exhaust system as described above, the SCR catalyst, comprising the ERI molecular sieve, can be located between the components of the DPF and the ASC. Another possibility is to arrange the SCR catalyst upstream of the DOC, where a certain tolerance to unburned hydrocarbons is required. The SCR functionality can also be included in the DPF, or it can be combined with the ASC in a single component with a dual function.
El tamiz molecular de ERI según la invención también puede ser parte de un catalizador antifuga de amoníaco (ASC). El catalizador de ASC se usa en combinación con el artículo de SCR, y su función es eliminar la cantidad en exceso de amoníaco, o un precursor del mismo, que es necesario en la etapa de SCR para eliminar cantidades elevadas de óxidos de nitrógeno del gas de escape.The ERI molecular sieve according to the invention can also be part of an ammonia leakage catalyst (ASC). The ASC catalyst is used in combination with the SCR article, and its function is to remove the excess amount of ammonia, or a precursor thereof, which is necessary in the SCR stage to remove high quantities of nitrogen oxides from the gas escape
Los catalizadores de tipo ASC son catalizadores bifuncionales. La primera función es oxidar amoníaco con oxígeno, que produce NOx, y la segunda función es la SCR del NH3, en la que NOx y cantidades residuales de amoníaco reaccionan hasta nitrógeno.ASC type catalysts are bifunctional catalysts. The first function is to oxidize ammonia with oxygen, which produces NOx, and the second function is the SCR of NH3, in which NOx and residual amounts of ammonia react to nitrogen.
Por tanto, los catalizadores de ASC consisten en una combinación de un componente activo para la oxidación de amoníaco mediante oxígeno y un componente activo para la SCR del NH3.Therefore, the ASC catalysts consist of a combination of an active component for the oxidation of ammonia by oxygen and an active component for the SCR of NH3.
Los componentes aplicados más habitualmente para la oxidación de amoníaco por oxígeno se basan en metales como Pt, Pd, Rh, Ir, Ru, pero para este fin también se pueden usar óxidos de metales de transición o una combinación de óxidos metálicos, por ejemplo óxidos de Ce, Ti, V, Cr, Mn, Fe, Co, Nb, Mo, Ta, W. Cuando tales materiales se combinan con la forma cargada con metal del tamiz molecular de la invención que tiene actividad de SCR, se obtiene un catalizador antifuga de amoníaco.The most commonly applied components for the oxidation of ammonia by oxygen are based on metals such as Pt, Pd, Rh, Ir, Ru, but for this purpose you can also use transition metal oxides or a combination of metal oxides, for example oxides of Ce, Ti, V, Cr, Mn, Fe, Co, Nb, Mo, Ta, W. When such materials are combined with the metal-charged form of the molecular sieve of the invention having SCR activity, a catalyst is obtained anti-ammonia.
Los catalizadores antifuga de amoníaco basados en el tamiz molecular de la invención también pueden contener materiales auxiliares, por ejemplo, y sin limitarse a aglutinantes, materiales de soporte para los componentes de metales nobles, tales como Al2O3, TiO2, SiO2. Tales combinaciones pueden tener diferentes formas, tal como una mezcla del componente de oxidación de amoníaco con la forma activa de SCR del tamiz molecular de la invención, reactores o artículos catalíticos en serie (véanse los ejemplos de la patente US 4.188.364).The ammonia leakage catalysts based on the molecular sieve of the invention can also contain auxiliary materials, for example, and without being limited to binders, support materials for noble metal components, such as Al 2 O 3, TiO 2, SiO 2. Such combinations may have different forms, such as a mixture of the ammonia oxidation component with the active form of SCR of the molecular sieve of the invention, reactors or serial catalytic articles (see examples of US Pat. No. 4,188,364).
En particular, el catalizador antifuga de amoníaco puede ser una capa revestida por lavado de una mezcla del componente de oxidación de amoníaco con la forma activa de SCR del tamiz molecular de ERI de la invención en un monolito, o una disposición de múltiples capas revestida por lavado sobre un monolito, en la que las diferentes capas contienen diferentes cantidades del componente de oxidación de amoníaco, o de la forma activa de SCR del tamiz molecular de la invención, o de cualquier combinación del componente de oxidación de amoníaco y la forma activa de SCR del tamiz molecular de la invención (documentos JP3436567, EP1992409).In particular, the ammonia leakage catalyst may be a layer coated by washing a mixture of the ammonia oxidation component with the active form of SCR of the ERI molecular sieve of the invention in a monolith, or a multilayer array coated by washing over a monolith, in which the different layers contain different amounts of the ammonia oxidation component, or of the active form of SCR of the molecular sieve of the invention, or of any combination of the ammonia oxidation component and the active form of SCR of the molecular sieve of the invention (JP3436567, EP1992409).
En otra configuración, el componente de oxidación del amoníaco o la forma activa de SCR del tamiz molecular de ERI de la invención, o cualquier combinación del componente de oxidación del amoníaco y la forma activa de SCR del tamiz molecular de la invención, está presente en las paredes de un monolito. Esta configuración se puede combinar además con diferentes combinaciones de capas revestidas por lavado.In another configuration, the oxidation component of the ammonia or the active form of SCR of the ERI molecular sieve of the invention, or any combination of the oxidation component of the ammonia and the active form of SCR of the molecular sieve of the invention, is present in the walls of a monolith. This configuration can also be combined with different combinations of layers coated by washing.
Otra configuración del catalizador de ASC es un artículo catalítico con un extremo de entrada de gas y un extremo de salida de gas, en el que el extremo de salida contiene un componente de oxidación del amoníaco y la forma activa de SCR del tamiz molecular de la invención. El extremo de entrada del artículo catalítico puede contener entonces otras funcionalidades.Another configuration of the ASC catalyst is a catalytic article with a gas inlet end and a gas outlet end, wherein the outlet end contains an oxidation component of the ammonia and the active form of SCR of the molecular sieve of the gas. invention. The input end of the catalytic article can then contain other functionalities.
El tamiz molecular de ERI de la invención es útil como catalizador en la reducción de óxidos de nitrógeno en el gas de escape procedente de una turbina de gas que usa amoníaco como agente reductor. En esta aplicación, el catalizador se puede disponer directamente aguas abajo de la turbina de gas. También se puede exponer a grandes fluctuaciones de temperatura durante los procedimientos de puesta en marcha y apagado de la turbina de gas.The ERI molecular sieve of the invention is useful as a catalyst in the reduction of nitrogen oxides in the exhaust gas from a gas turbine using ammonia as a reducing agent. In this application, the catalyst can be arranged directly downstream of the gas turbine. It can also be exposed to large temperature fluctuations during the start-up and shut-down procedures of the gas turbine.
En ciertas aplicaciones, el catalizador del tamiz molecular se usa en un sistema de turbina de gas con un modo operacional de un único ciclo, sin ningún sistema de recuperación de calor aguas abajo de la turbina. Cuando se coloca directamente después de la turbina de gas, el tamiz molecular es capaz de soportar temperaturas del gas de escape hasta 650°C con una composición del gas que contiene agua.In certain applications, the molecular sieve catalyst is used in a gas turbine system with a single-cycle operational mode, without any heat recovery system downstream of the turbine. When placed directly after the gas turbine, the molecular sieve is able to withstand exhaust gas temperatures up to 650 ° C with a composition of the gas containing water.
Otras aplicaciones del tamiz molecular de la invención son en un sistema de tratamiento del escape de una turbina de gas, en combinación con un sistema de recuperación de calor tal como un Generador del Sistema de Recuperación de Calor (HRSG). En tal diseño del procedimiento, el catalizador del tamiz molecular está dispuesto entre la turbina de gas y el HRSG. El tamiz molecular también se puede disponer en varias localizaciones dentro del HRSG.Other applications of the molecular sieve of the invention are in a gas turbine exhaust treatment system, in combination with a heat recovery system such as a Heat Recovery System Generator (HRSG). In such process design, the molecular sieve catalyst is disposed between the gas turbine and the HRSG. The molecular sieve can also be arranged in several locations within the HRSG.
Todavía una aplicación del tamiz molecular de ERI según la invención es el empleo como catalizador en combinación con un catalizador de oxidación para la disminución de hidrocarburos y monóxido de carbono en el gas de escape.Still an application of the ERI molecular sieve according to the invention is the use as catalyst in combination with an oxidation catalyst for the reduction of hydrocarbons and carbon monoxide in the exhaust gas.
El catalizador de oxidación, típicamente compuesto de metales preciosos tales como Pt y Pd, se puede disponer por ejemplo aguas arriba o aguas abajo del tamiz molecular, y tanto dentro como fuera del HRSG. La funcionalidad de oxidación también se puede combinar con el catalizador del tamiz molecular en una única unidad catalítica.The oxidation catalyst, typically composed of precious metals such as Pt and Pd, can be arranged for example upstream or downstream of the molecular sieve, and both inside and outside the HRSG. Oxidation functionality can also be combined with the molecular sieve catalyst in a single catalytic unit.
La funcionalidad de oxidación se puede combinar directamente con el tamiz molecular usando el tamiz molecular como soporte para los metales preciosos. Los metales preciosos también se pueden soportar sobre otro material soporte y se pueden mezclar físicamente con el tamiz molecular.The oxidation functionality can be combined directly with the molecular sieve using the molecular sieve as support for the precious metals. The precious metals can also be supported on another support material and can be physically mixed with the molecular sieve.
El tamiz molecular de la invención es capaz de eliminar óxido nitroso. Por ejemplo, se puede disponer en combinación con un bucle de producción de ácido nítrico en un montaje de disminución primario, secundario o terciario. En tal procedimiento de disminución, el tamiz molecular se puede usar para eliminar óxido nitroso, así como óxidos de nitrógeno, como artículos catalíticos independientes o combinado en un único artículo catalítico. El óxido de nitrógeno se puede usar para facilitar la eliminación del óxido nitroso. También se pueden añadir como agente reductor amoníaco o hidrocarburos inferiores, incluyendo metano, para reducir adicionalmente óxidos de nitrógeno y/u óxido nitroso.The molecular sieve of the invention is capable of eliminating nitrous oxide. For example, it can be arranged in combination with a nitric acid production loop in a primary, secondary or tertiary decrease assembly. In such a decreasing process, the molecular sieve can be used to remove nitrous oxide, as well as nitrogen oxides, as independent catalytic articles or combined in a single catalytic article. Nitrogen oxide can be used to facilitate the removal of nitrous oxide. Ammonia or lower hydrocarbons, including methane, can also be added as a reducing agent to further reduce nitrogen oxides and / or nitrous oxide.
El tamiz molecular de ERI de la invención también se puede usar en la conversión de sustancias oxigenadas en diversos hidrocarburos. La materia prima de las sustancias oxigenadas es típicamente alcoholes inferiores y éteres que contienen uno a cuatro átomos de carbono, y/o combinaciones de los mismos. Las sustancias oxigenadas también pueden ser compuestos carbonílicos tales como aldehído, cetonas y ácidos carboxílicos. Los compuestos oxigenados particularmente adecuados son metanol, éter dimetílico, y mezclas de los mismos. Tales sustancias oxigenadas se pueden convertir en hidrocarburos en presencia del tamiz molecular. En tal procedimiento, la materia prima de la sustancia oxigenada se diluye típicamente, y la temperatura y la velocidad espacial se controlan para obtener el intervalo de producto deseado.The ERI molecular sieve of the invention can also be used in the conversion of oxygenated substances into various hydrocarbons. The raw material of the oxygenated substances is typically lower alcohols and ethers containing one to four carbon atoms, and / or combinations thereof. Oxygenated substances can also be carbonyl compounds such as aldehyde, ketones and carboxylic acids. Particularly suitable oxygenates are methanol, dimethyl ether, and mixtures thereof. Such oxygenated substances can be converted into hydrocarbons in the presence of the molecular sieve. In such a process, the raw material of the oxygenated substance is typically diluted, and the temperature and the space velocity are controlled to obtain the desired product range.
Un uso adicional del tamiz molecular de la invención es como catalizador en la producción de olefinas inferiores, en particular olefinas adecuadas para uso en gasolina, o como catalizador en la producción de compuestos aromáticos.A further use of the molecular sieve of the invention is as a catalyst in the production of lower olefins, in particular olefins suitable for use in gasoline, or as a catalyst in the production of aromatic compounds.
En las aplicaciones anteriores, el tamiz molecular de ERI se usa típicamente en forma ácida, y se extruirá con materiales aglutinantes o se conformará en peletes junto con una matriz adecuada y materiales aglutinantes como se describió anteriormente. In the above applications, the ERI molecular sieve is typically used in acid form, and will be extruded with binder materials or be formed into pellets together with a suitable matrix and binder materials as described above.
También se pueden incluir otros compuestos activos adecuados, tales como metales e iones metálicos, para cambiar la selectividad por el intervalo de producto deseado.Other suitable active compounds, such as metals and metal ions, may also be included to change the selectivity for the desired product range.
El tamiz molecular de ERI según la invención se puede usar además en la oxidación parcial de metano a metanol u otros compuestos oxigenados tales como éter dimetílico.The ERI molecular sieve according to the invention can be further used in the partial oxidation of methane to methanol or other oxygenated compounds such as dimethyl ether.
En el documento WO11046621A1 se proporciona un ejemplo de un procedimiento para la conversión directa de metano en metanol a temperaturas por debajo de 300°C en fase gaseosa. En tal procedimiento, el tamiz molecular de la invención se carga con una cantidad de cobre suficiente para llevar a cabo la conversión. Típicamente, el tamiz molecular se tratará en una atmósfera oxidante en la que después el metano se hace pasar subsiguientemente sobre el tamiz molecular activado para formar directamente metanol. Subsiguientemente, el metanol se puede extraer mediante métodos adecuados, y los sitios activos se pueden regenerar mediante otro tratamiento oxidativo.In WO11046621A1 an example of a method for the direct conversion of methane to methanol at temperatures below 300 ° C in the gas phase is provided. In such a procedure, the molecular sieve of the invention is charged with a sufficient amount of copper to carry out the conversion. Typically, the molecular sieve will be treated in an oxidizing atmosphere in which then the methane is subsequently passed over the activated molecular sieve to directly form methanol. Subsequently, the methanol can be extracted by suitable methods, and the active sites can be regenerated by another oxidative treatment.
En [K. Narsimhan, K. lyoki, K. Dinh, Y. Román-Leshkov, ACS Cent. Sci. 2016, 2, 424-429] se describe otro ejemplo en el que se logra un incremento o una producción continua de metanol mediante adición de agua a la corriente de agentes reaccionantes para extraer continuamente metanol sin tener que alterar las condiciones entre tratamientos oxidativos y la formación de metanol.In [K. Narsimhan, K. lyoki, K. Dinh, Y. Roman-Leshkov, ACS Cent. Sci. 2016 , 2, 424-429] describes another example in which an increase or continuous production of methanol is achieved by adding water to the stream of reactants to continuously extract methanol without having to alter the conditions between oxidative treatments and the formation of methanol.
El tamiz molecular de ERI de la invención se puede usar para separar diversos gases. Los ejemplos incluyen la separación de dióxido de carbono de gas natural, y alcoholes inferiores de alcoholes superiores. Típicamente, la aplicación práctica del tamiz molecular será como parte de una membrana para este tipo de separación.The ERI molecular sieve of the invention can be used to separate various gases. Examples include the separation of carbon dioxide from natural gas, and lower alcohols of higher alcohols. Typically, the practical application of the molecular sieve will be as part of a membrane for this type of separation.
El tamiz molecular de ERI de la invención se puede usar además en reacciones de isomerización, craqueo, hidrocraqueo, y otras reacciones, para mejorar el petróleo.The ERI molecular sieve of the invention can be further used in isomerization, cracking, hydrocracking, and other reactions to improve the oil.
El tamiz molecular de ERI de la invención también se puede usar como una trampa de hidrocarburos, por ejemplo procedentes de emisiones de inicio en frío de diversos motores.The ERI molecular sieve of the invention can also be used as a hydrocarbon trap, for example from cold start emissions of various engines.
Además, el tamiz molecular se puede usar para la preparación de aminas pequeñas, tales como metilamina y dimetilamina, mediante reacción de amoníaco con metanol. In addition, the molecular sieve can be used for the preparation of small amines, such as methylamine and dimethylamine, by reaction of ammonia with methanol.
EJEMPLOSEXAMPLES
Ejemplo 1: Síntesis de OSDA cidohexano-1,4-bis(hidróxido de trimetilamonio)Example 1: Synthesis of OSDA cidohexane-1,4-bis (trimethylammonium hydroxide)
Se puso a reflujo una mezcla de 30 ml de ácido fórmico (disolución acuosa al 89,5% en peso), 6,1 g de NaHCO3, 5 g de trans-1,4-diaminocidohexano (polvo de pureza de 98%) y 14 ml de formaldehído (disolución acuosa al 37% en peso) hasta que no se observó ninguna evolución visible de CO2. La mezcla de síntesis se destiló a vacío después de que se añadieron 50 ml de HCl (disolución acuosa 2 moles/l), seguido de la adición de un exceso de NaOH y extracción 3 veces con cloroformo. Las porciones de cloroformo se combinaron, y se añadieron 8 ml de yoduro de metilo (99% en peso), seguido del mezclamiento durante toda la noche. El sólido obtenido se disolvió en agua y se intercambió iónicamente a la forma de hidróxido, usando una resina de intercambio iónico.A mixture of 30 ml of formic acid (89.5% by weight aqueous solution), 6.1 g of NaHCO3, 5 g of trans-1,4-diamino-monohexane (powder of 98% purity) was refluxed. 14 ml of formaldehyde (37% by weight aqueous solution) until no visible evolution of CO2 was observed. The synthesis mixture was distilled in vacuo after 50 ml of HCl (aqueous solution 2 mol / l) was added, followed by the addition of an excess of NaOH and extraction 3 times with chloroform. The chloroform portions were combined, and 8 ml of methyl iodide (99% by weight) was added, followed by mixing overnight. The solid obtained was dissolved in water and ionically exchanged to the hydroxide form, using an ion exchange resin.
Ejemplo 2: Síntesis de ERIExample 2: Synthesis of ERI
Se preparó una mezcla de 1,87 g de ciclohexano-1,4-bis(hidróxido de trimetilamonio) (disolución acuosa al 12,7% en peso), 1,7 g de KOH (disolución acuosa al 10% en peso), 0,48 g de agua destilada y 0,94 g de sílice-alúmina amorfa coprecipitada (SiO2/Al2O3 = 12). La mezcla se calentó en un autoclave cerrado forrado con Teflon a 135°C durante 7 días, y el producto sólido se separó por filtración y se lavó con agua desionizada. Mediante análisis de difracción de polvo de rayos X, se puede ver que el producto según se sintetiza es ERI de fase pura.A mixture of 1.87 g of cyclohexane-1,4-bis (trimethylammonium hydroxide) (12.7% by weight aqueous solution), 1.7 g of KOH (10% by weight aqueous solution) was prepared, 0.48 g of distilled water and 0.94 g of amorphous silica-alumina co-precipitated (SiO2 / Al2O3 = 12). The mixture was heated in a closed autoclave lined with Teflon at 135 ° C for 7 days, and the solid product was filtered off and washed with deionized water. By X-ray powder diffraction analysis, it can be seen that the product as synthesized is pure phase ERI.
Ejemplo 3: Síntesis de ERIExample 3: ERI synthesis
Se preparó una mezcla de 1,97 g de ciclohexano-1,4-bis(hidróxido de trimetilamonio) (disolución acuosa al 12,7% en peso), 1,79 g de KOH (disolución acuosa al 10% en peso), 0,46 g de agua destilada y 0,79 g de zeolita FAU (SiO2/Al2O3 = 12). La mezcla se calentó en un autoclave cerrado forrado con Teflon a 135°C durante 7 días, y el producto sólido se separó por filtración y se lavó con agua desionizada.A mixture of 1.97 g of cyclohexane-1,4-bis (trimethylammonium hydroxide) (12.7% by weight aqueous solution), 1.79 g of KOH (10% by weight aqueous solution) was prepared, 0.46 g of distilled water and 0.79 g of zeolite FAU (SiO2 / Al2O3 = 12). The mixture was heated in a closed autoclave lined with Teflon at 135 ° C for 7 days, and the solid product was filtered off and washed with deionized water.
El producto sólido seco tuvo una relación de SiO2/Al2O3 de 9,8 determinada mediante análisis de ICP-AES. Mediante análisis de difracción de polvo de rayos X, se observa que el producto según se sintetiza es ERI de fase pura. El análisis de SEM revela además una morfología cristalina tabular a prismática.The dry solid product had an SiO2 / Al2O3 ratio of 9.8 determined by ICP-AES analysis. By X-ray powder diffraction analysis, it is observed that the product as synthesized is pure phase ERI. The SEM analysis also reveals a Tabular to prismatic crystal morphology.
Ejemplo 4: Síntesis de ERIExample 4: Synthesis of ERI
Se preparó una mezcla de 1,95 g de cidohexano-1,4-bis(hidróxido de trimetilamonio) (disolución acuosa al 12,7% en peso), 1,77 g de KOH (disolución acuosa al 10% en peso), 0,5 g de agua destilada y 0,79 g de sílice-alúmina amorfa coprecipitada (SiO2/Al2O3 = 30). La mezcla se calentó en un autoclave cerrado forrado con Teflon a 135°C durante 7 días, y el producto sólido se separó por filtración y se lavó con agua desionizada.A mixture of 1.95 g of cidohexane-1,4-bis (trimethylammonium hydroxide) (12.7% by weight aqueous solution), 1.77 g of KOH (10% by weight aqueous solution) was prepared, 0.5 g of distilled water and 0.79 g of amorphous silica-alumina coprecipitated (SiO2 / Al2O3 = 30). The mixture was heated in a closed autoclave lined with Teflon at 135 ° C for 7 days, and the solid product was filtered off and washed with deionized water.
Mediante análisis de difracción de polvo de rayos X, se observa que el producto según se sintetiza es ERI de fase pura. En la Figura 1 se muestra el difractograma medido para el producto según se sintetiza. El análisis de SEM revela además una morfología cristalina tabular (véase la Figura 2).By X-ray powder diffraction analysis, it is observed that the product as synthesized is pure phase ERI. Figure 1 shows the diffractogram measured for the product as it is synthesized. The SEM analysis also reveals a tabular crystal morphology (see Figure 2).
La Figura 1 representa XRPD del tamiz molecular preparado según se prepara en el Ejemplo 4.Figure 1 represents XRPD of the molecular sieve prepared as prepared in Example 4.
La Figura 2 representa una micrografía de SEM del tamiz molecular preparado según se prepara en el Ejemplo 4.Figure 2 represents a SEM micrograph of the molecular sieve prepared as prepared in Example 4.
Ejemplo 5: Síntesis de ERIExample 5: Synthesis of ERI
Se preparó una mezcla de 1,99 g de ciclohexano-1,4-bis(hidróxido de trimetilamonio) (disolución acuosa al 12,7% en peso), 1,81 g de KOH (disolución acuosa al 10% en peso), 0,45 g de agua destilada y 0,74 g de zeolita FAU (SiO2/Al2O3 = 30). La mezcla se calentó en un autoclave cerrado forrado con Teflon a 135°C durante 7 días, y el producto sólido se separó por filtración y se lavó con agua desionizada.A mixture of 1.99 g of cyclohexane-1,4-bis (trimethylammonium hydroxide) (12.7% by weight aqueous solution), 1.81 g of KOH (10% by weight aqueous solution) was prepared, 0.45 g of distilled water and 0.74 g of zeolite FAU (SiO2 / Al2O3 = 30). The mixture was heated in a closed autoclave lined with Teflon at 135 ° C for 7 days, and the solid product was filtered off and washed with deionized water.
El producto sólido seco tuvo una relación de SiO2/Al2O3 de 22,0, determinada mediante análisis de ICP-AES. Mediante análisis de difracción de polvo de rayos X, se observa que el producto según se sintetiza es ERI de fase pura. El difractograma medido para el producto según se sintetiza se muestra en la Figura 3. El análisis de SEM revela además una morfología cristalina prismática (véase la Figura 4). The dry solid product had an SiO2 / Al2O3 ratio of 22.0, determined by ICP-AES analysis. By X-ray powder diffraction analysis, it is observed that the product as synthesized is pure phase ERI. The diffractogram measured for the product as synthesized is shown in Figure 3. The SEM analysis also reveals a prismatic crystal morphology (see Figure 4).
La Figura 3 XRPD del tamiz molecular preparado según se prepara en el Ejemplo 5.Figure 3 XRPD of the molecular sieve prepared as prepared in Example 5.
La Figura 4 representa una micrografía de SEM del tamiz molecular preparado según se prepara en el Ejemplo 5.Figure 4 represents a SEM micrograph of the molecular sieve prepared as prepared in Example 5.
La calcinación del tamiz molecular según se prepara seco, se llevó a cabo a 550°C durante 3 h. Después, el producto calcinado se intercambió iónicamente con Na4+. En la Figura 5 se muestra el difractograma de rayos X medido para el producto calcinado. Además, la fisisorción de N2 reveló una superficie específica de BET de múltiples puntos de 559 m2/g y un volumen de microporo de 0,19 cm3/g, indicando claramente la naturaleza microporosa del material preparado.Calcination of the molecular sieve as prepared dry was carried out at 550 ° C for 3 h. Then, the calcined product was ionically exchanged with Na 4 +. In Figure 5 the X-ray diffractogram measured for the calcined product is shown. In addition, the physisorption of N2 revealed a BET specific surface of multiple points of 559 m2 / g and a micropore volume of 0.19 cm 3 / g, clearly indicating the microporous nature of the prepared material.
La Figura 5 representa XRPD del tamiz molecular calcinado preparado en el Ejemplo 5. Figure 5 represents XRPD of the calcined molecular sieve prepared in Example 5.
Claims (10)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES201731089A ES2703220A1 (en) | 2017-09-07 | 2017-09-07 | Method for the preparation of a new ERI molecular sieve (Machine-translation by Google Translate, not legally binding) |
| PCT/EP2018/073599 WO2019048373A1 (en) | 2017-09-07 | 2018-09-03 | Method for preparation of a novel eri-molecular sieve |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES201731089A ES2703220A1 (en) | 2017-09-07 | 2017-09-07 | Method for the preparation of a new ERI molecular sieve (Machine-translation by Google Translate, not legally binding) |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| ES2703220A1 true ES2703220A1 (en) | 2019-03-07 |
Family
ID=63528730
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| ES201731089A Withdrawn ES2703220A1 (en) | 2017-09-07 | 2017-09-07 | Method for the preparation of a new ERI molecular sieve (Machine-translation by Google Translate, not legally binding) |
Country Status (2)
| Country | Link |
|---|---|
| ES (1) | ES2703220A1 (en) |
| WO (1) | WO2019048373A1 (en) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113636572B (en) * | 2021-08-27 | 2022-11-29 | 昆明贵研催化剂有限责任公司 | High-yield Me-SSZ-98 type molecular sieve material, catalyst and application |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20170088432A1 (en) * | 2015-09-25 | 2017-03-30 | Chevron U.S.A. Inc. | Method for preparing zeolite ssz-98 |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NL238953A (en) | 1958-05-08 | |||
| US3699139A (en) | 1969-10-16 | 1972-10-17 | Mobil Oil Corp | Synthetic crystalline aluminosilicate |
| US4086186A (en) | 1976-11-04 | 1978-04-25 | Mobil Oil Corporation | Crystalline zeolite ZSM-34 and method of preparing the same |
| GB1586530A (en) | 1977-05-31 | 1981-03-18 | Caterpillar Tractor Co | Two-stage catalysts of engine exhaust |
| US4503023A (en) | 1979-08-14 | 1985-03-05 | Union Carbide Corporation | Silicon substituted zeolite compositions and process for preparing same |
| JP3436567B2 (en) | 1993-06-23 | 2003-08-11 | バブコック日立株式会社 | Exhaust gas purification catalyst and method for producing the same |
| US7344694B2 (en) * | 2004-10-06 | 2008-03-18 | Uop Llc | UZM-12 and UZM-12HS: crystalline aluminosilicate zeolitic compositions and processes for preparing and using the compositions |
| JP5110954B2 (en) | 2007-05-09 | 2012-12-26 | エヌ・イーケムキャット株式会社 | Exhaust gas purification catalyst apparatus using selective reduction catalyst and exhaust gas purification method |
| GB201007623D0 (en) | 2009-10-14 | 2010-06-23 | Univ Leuven Kath | Low temperture direct selective methane to methanol conversion |
| PL2931406T3 (en) | 2012-12-12 | 2020-03-31 | Umicore Ag & Co. Kg | One-pot method for the synthesis of cu-ssz-13 |
| US9416017B2 (en) | 2014-07-03 | 2016-08-16 | Chevron U.S.A. Inc. | Method for making molecular sieve SSZ-98 |
| US9409786B2 (en) | 2014-07-03 | 2016-08-09 | Chevron U.S.A. Inc. | Molecular sieve SSZ-98 |
| EP3164362B1 (en) | 2014-07-03 | 2019-10-16 | Chevron U.S.A. Inc. | Processes using molecular sieve ssz-98 |
| WO2017003627A1 (en) | 2015-06-29 | 2017-01-05 | Chevron U.S.A. Inc. | Synthesis of aluminosilicate zeolite ssz-98 |
| EP3347308B1 (en) | 2015-09-11 | 2019-07-31 | Chevron U.S.A. Inc. | Method for preparing zeolite ssz-98 |
-
2017
- 2017-09-07 ES ES201731089A patent/ES2703220A1/en not_active Withdrawn
-
2018
- 2018-09-03 WO PCT/EP2018/073599 patent/WO2019048373A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20170088432A1 (en) * | 2015-09-25 | 2017-03-30 | Chevron U.S.A. Inc. | Method for preparing zeolite ssz-98 |
Non-Patent Citations (2)
| Title |
|---|
| MARTÍN, NURIA, ET AL. Cage-based small-pore catalysts for NH 3-SCR prepared by combining bulky organic structure directing agents with modified zeolites as reagents.. Applied Catalysis B: Environmental, 29/05/2017, Vol. 217, Páginas 125-136 [en línea][recuperado el 01/12/2017]. (DOI: 10.1016/j.apcatb.2017.05.082) apartado 1. * |
| ZHU, JIE, ET AL. . Ultrafast Synthesis of High-Silica Erionite Zeolite with Improved Hydrothermal Stability. . Chemical Communications,, 24/05/2017, Vol. 53, Páginas 6796-6799 [en línea][recuperado el 04/12/2017]. (DOI: 10.1039/C7CC03166A) todo el documento. * |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2019048373A1 (en) | 2019-03-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10919773B2 (en) | Method for the preparation of a molecular sieve belonging to the ABC-6 framework family with disorder in the ABC stacking sequence | |
| ES2465004T3 (en) | Method of direct synthesis of zeolites containing Cu that have a CHA structure | |
| ES2589059B1 (en) | DIRECT SYNTHESIS OF Cu-CHA THROUGH THE COMBINATION OF A CU AND TETRAETHYLAMM COMPLEX, AND APPLICATIONS IN CATALYSIS | |
| US10865117B2 (en) | Disordered ABC-6 molecular sieve | |
| JP6951343B2 (en) | Methods for Direct Synthesis of Iron-Containing AEI Zeolite Catalysts | |
| Liu et al. | Morphology-oriented ZrO2-supported vanadium oxide for the NH3-SCR process: importance of structural and textural properties | |
| EP3388392A1 (en) | Copper-containing zeolites having a low alkali metal content, method of making thereof, and their use as scr catalysts | |
| WO2021114208A1 (en) | Denitration catalyst and denitration method using the catalyst | |
| JP6943861B2 (en) | Hydrothermal stability Iron-containing AEI zeolite SCR catalyst | |
| JP2019512376A (en) | Process for removing nitrous oxide from off-gas in the presence of a catalyst comprising Fe-AEI zeolitic material essentially free of alkali metals | |
| US11667536B2 (en) | Method for the preparation of a molecular sieve of the CHA-type | |
| US20240075466A1 (en) | One-Pot Synthesis of Transition Metal-Promoted Chabazites | |
| JP6987766B2 (en) | A method for removing nitrogen oxides from exhaust gas by selective catalytic reduction in the presence of an SCR catalyst containing an FE-AEI zeolite material that is essentially free of alkali metals. | |
| US10933408B2 (en) | Catalyst comprising a molecular sieve belonging to the ABC-6 framework family with disorder in the ABC stacking sequence and use of the catalyst | |
| JP7251471B2 (en) | Transition metal-supported zeolite, method for producing the same, catalyst for purifying nitrogen oxides, and method for using the same | |
| ES2703220A1 (en) | Method for the preparation of a new ERI molecular sieve (Machine-translation by Google Translate, not legally binding) | |
| US11879374B2 (en) | STA-30, a new member of the SWY family of molecular sieves, methods of preparation and use | |
| ES2703221A1 (en) | New ERI molecular sieve and a method for its preparation (Machine-translation by Google Translate, not legally binding) | |
| ES2703222A1 (en) | Catalyst comprising a new molecular sieve that belongs to the ERI family and use of the catalyst (Machine-translation by Google Translate, not legally binding) | |
| Guo et al. | Rational design on hierarchically porous Cu/ZSM-5 zeolite for promoting low-temperature NH3-SCR performance based on protectively alkali-etching strategy | |
| WO2022185038A1 (en) | Jmz-12, a disordered aei/cha family of zeolites, its synthesis and use | |
| EP3887313A1 (en) | Jmz-1, a cha-containing zeolite and methods of preparation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| BA2A | Patent application published |
Ref document number: 2703220 Country of ref document: ES Kind code of ref document: A1 Effective date: 20190307 |
|
| FA2A | Application withdrawn |
Effective date: 20190702 |