EP3598967B1 - Personal cleansing compositions, methods and uses - Google Patents
Personal cleansing compositions, methods and uses Download PDFInfo
- Publication number
- EP3598967B1 EP3598967B1 EP18185894.5A EP18185894A EP3598967B1 EP 3598967 B1 EP3598967 B1 EP 3598967B1 EP 18185894 A EP18185894 A EP 18185894A EP 3598967 B1 EP3598967 B1 EP 3598967B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- surfactant
- composition
- personal cleansing
- sodium
- phase
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 336
- 238000000034 method Methods 0.000 title claims description 37
- 239000004094 surface-active agent Substances 0.000 claims description 156
- -1 fatty acyl isethionate Chemical compound 0.000 claims description 113
- 229920001282 polysaccharide Polymers 0.000 claims description 86
- 239000005017 polysaccharide Substances 0.000 claims description 86
- 239000007788 liquid Substances 0.000 claims description 78
- 229920000642 polymer Polymers 0.000 claims description 68
- 229920001285 xanthan gum Polymers 0.000 claims description 64
- 239000000230 xanthan gum Substances 0.000 claims description 64
- 235000010493 xanthan gum Nutrition 0.000 claims description 64
- 229940082509 xanthan gum Drugs 0.000 claims description 64
- 239000005264 High molar mass liquid crystal Substances 0.000 claims description 46
- 239000004615 ingredient Substances 0.000 claims description 34
- 229910052708 sodium Inorganic materials 0.000 claims description 33
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 32
- 239000003795 chemical substances by application Substances 0.000 claims description 32
- 238000010998 test method Methods 0.000 claims description 32
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 30
- 125000004432 carbon atom Chemical group C* 0.000 claims description 30
- 239000011734 sodium Substances 0.000 claims description 29
- 230000008901 benefit Effects 0.000 claims description 26
- 229920001525 carrageenan Polymers 0.000 claims description 23
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 claims description 22
- 125000000217 alkyl group Chemical group 0.000 claims description 19
- 239000000679 carrageenan Substances 0.000 claims description 18
- 229940113118 carrageenan Drugs 0.000 claims description 18
- 239000003945 anionic surfactant Substances 0.000 claims description 17
- 238000000518 rheometry Methods 0.000 claims description 17
- 239000011780 sodium chloride Substances 0.000 claims description 16
- 239000002888 zwitterionic surfactant Substances 0.000 claims description 16
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 claims description 15
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 15
- 239000003921 oil Substances 0.000 claims description 15
- 235000002639 sodium chloride Nutrition 0.000 claims description 15
- 230000003287 optical effect Effects 0.000 claims description 14
- 239000002245 particle Substances 0.000 claims description 14
- 150000008051 alkyl sulfates Chemical class 0.000 claims description 12
- 239000002280 amphoteric surfactant Substances 0.000 claims description 12
- 239000002736 nonionic surfactant Substances 0.000 claims description 12
- 229940071089 sarcosinate Drugs 0.000 claims description 12
- 229920006395 saturated elastomer Polymers 0.000 claims description 12
- 239000007864 aqueous solution Substances 0.000 claims description 11
- 239000003792 electrolyte Substances 0.000 claims description 11
- 150000004676 glycans Chemical class 0.000 claims description 11
- 229920002907 Guar gum Polymers 0.000 claims description 10
- 229920000161 Locust bean gum Polymers 0.000 claims description 10
- 239000006096 absorbing agent Substances 0.000 claims description 10
- 239000003623 enhancer Substances 0.000 claims description 10
- 239000000665 guar gum Substances 0.000 claims description 10
- 235000010417 guar gum Nutrition 0.000 claims description 10
- 229960002154 guar gum Drugs 0.000 claims description 10
- 235000010420 locust bean gum Nutrition 0.000 claims description 10
- 239000000711 locust bean gum Substances 0.000 claims description 10
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 9
- 125000003342 alkenyl group Chemical group 0.000 claims description 9
- 230000003750 conditioning effect Effects 0.000 claims description 9
- 229910052700 potassium Inorganic materials 0.000 claims description 9
- 229960003975 potassium Drugs 0.000 claims description 9
- 239000011591 potassium Substances 0.000 claims description 9
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 claims description 8
- 125000002252 acyl group Chemical group 0.000 claims description 8
- 239000003086 colorant Substances 0.000 claims description 7
- 238000003801 milling Methods 0.000 claims description 7
- 239000000377 silicon dioxide Substances 0.000 claims description 7
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims description 6
- 238000004140 cleaning Methods 0.000 claims description 6
- 239000003094 microcapsule Substances 0.000 claims description 6
- 239000003605 opacifier Substances 0.000 claims description 6
- 239000000049 pigment Substances 0.000 claims description 6
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 claims description 6
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 claims description 6
- OGIDPMRJRNCKJF-UHFFFAOYSA-N titanium oxide Inorganic materials [Ti]=O OGIDPMRJRNCKJF-UHFFFAOYSA-N 0.000 claims description 6
- ZNOZWUKQPJXOIG-XSBHQQIPSA-L [(2r,3s,4r,5r,6s)-6-[[(1r,3s,4r,5r,8s)-3,4-dihydroxy-2,6-dioxabicyclo[3.2.1]octan-8-yl]oxy]-4-[[(1r,3r,4r,5r,8s)-8-[(2s,3r,4r,5r,6r)-3,4-dihydroxy-6-(hydroxymethyl)-5-sulfonatooxyoxan-2-yl]oxy-4-hydroxy-2,6-dioxabicyclo[3.2.1]octan-3-yl]oxy]-5-hydroxy-2-( Chemical compound O[C@@H]1[C@@H](O)[C@@H](OS([O-])(=O)=O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H]2OC[C@H]1O[C@H](O[C@H]1[C@H]([C@@H](CO)O[C@@H](O[C@@H]3[C@@H]4OC[C@H]3O[C@H](O)[C@@H]4O)[C@@H]1O)OS([O-])(=O)=O)[C@@H]2O ZNOZWUKQPJXOIG-XSBHQQIPSA-L 0.000 claims description 5
- 239000006224 matting agent Substances 0.000 claims description 5
- 239000003607 modifier Substances 0.000 claims description 5
- 239000012748 slip agent Substances 0.000 claims description 5
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 claims description 4
- 229910052783 alkali metal Inorganic materials 0.000 claims description 4
- 150000001340 alkali metals Chemical class 0.000 claims description 4
- 239000001110 calcium chloride Substances 0.000 claims description 4
- 229910001628 calcium chloride Inorganic materials 0.000 claims description 4
- ZCCIPPOKBCJFDN-UHFFFAOYSA-N calcium nitrate Chemical compound [Ca+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O ZCCIPPOKBCJFDN-UHFFFAOYSA-N 0.000 claims description 4
- 239000001103 potassium chloride Substances 0.000 claims description 4
- 235000011164 potassium chloride Nutrition 0.000 claims description 4
- 239000001508 potassium citrate Substances 0.000 claims description 4
- 229960002635 potassium citrate Drugs 0.000 claims description 4
- QEEAPRPFLLJWCF-UHFFFAOYSA-K potassium citrate (anhydrous) Chemical compound [K+].[K+].[K+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O QEEAPRPFLLJWCF-UHFFFAOYSA-K 0.000 claims description 4
- 235000011082 potassium citrates Nutrition 0.000 claims description 4
- JHJLBTNAGRQEKS-UHFFFAOYSA-M sodium bromide Chemical compound [Na+].[Br-] JHJLBTNAGRQEKS-UHFFFAOYSA-M 0.000 claims description 4
- 239000001509 sodium citrate Substances 0.000 claims description 4
- 150000003871 sulfonates Chemical class 0.000 claims description 4
- JIAARYAFYJHUJI-UHFFFAOYSA-L zinc dichloride Chemical compound [Cl-].[Cl-].[Zn+2] JIAARYAFYJHUJI-UHFFFAOYSA-L 0.000 claims description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 claims description 3
- ULUAUXLGCMPNKK-UHFFFAOYSA-N Sulfobutanedioic acid Chemical class OC(=O)CC(C(O)=O)S(O)(=O)=O ULUAUXLGCMPNKK-UHFFFAOYSA-N 0.000 claims description 3
- 235000011148 calcium chloride Nutrition 0.000 claims description 3
- 239000008103 glucose Substances 0.000 claims description 3
- 150000003893 lactate salts Chemical class 0.000 claims description 3
- AGGIJOLULBJGTQ-UHFFFAOYSA-N sulfoacetic acid Chemical class OC(=O)CS(O)(=O)=O AGGIJOLULBJGTQ-UHFFFAOYSA-N 0.000 claims description 3
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 claims description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 claims description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 claims description 2
- SWLVFNYSXGMGBS-UHFFFAOYSA-N ammonium bromide Chemical compound [NH4+].[Br-] SWLVFNYSXGMGBS-UHFFFAOYSA-N 0.000 claims description 2
- WDIHJSXYQDMJHN-UHFFFAOYSA-L barium chloride Chemical compound [Cl-].[Cl-].[Ba+2] WDIHJSXYQDMJHN-UHFFFAOYSA-L 0.000 claims description 2
- 229910001626 barium chloride Inorganic materials 0.000 claims description 2
- 229910001622 calcium bromide Inorganic materials 0.000 claims description 2
- WGEFECGEFUFIQW-UHFFFAOYSA-L calcium dibromide Chemical compound [Ca+2].[Br-].[Br-] WGEFECGEFUFIQW-UHFFFAOYSA-L 0.000 claims description 2
- 150000002190 fatty acyls Chemical group 0.000 claims description 2
- 229910052744 lithium Inorganic materials 0.000 claims description 2
- 229910052749 magnesium Inorganic materials 0.000 claims description 2
- 239000011777 magnesium Substances 0.000 claims description 2
- 235000015424 sodium Nutrition 0.000 claims description 2
- 229910052938 sodium sulfate Inorganic materials 0.000 claims description 2
- 235000011152 sodium sulphate Nutrition 0.000 claims description 2
- 239000011592 zinc chloride Substances 0.000 claims description 2
- 235000005074 zinc chloride Nutrition 0.000 claims description 2
- 239000012071 phase Substances 0.000 description 162
- 150000004804 polysaccharides Chemical class 0.000 description 77
- KWIUHFFTVRNATP-UHFFFAOYSA-N glycine betaine Chemical compound C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 42
- 230000035882 stress Effects 0.000 description 42
- 229960003237 betaine Drugs 0.000 description 26
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 23
- 229940079776 sodium cocoyl isethionate Drugs 0.000 description 19
- 238000002834 transmittance Methods 0.000 description 18
- 108700004121 sarkosyl Proteins 0.000 description 17
- KSAVQLQVUXSOCR-UHFFFAOYSA-M sodium lauroyl sarcosinate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O KSAVQLQVUXSOCR-UHFFFAOYSA-M 0.000 description 17
- 229940045885 sodium lauroyl sarcosinate Drugs 0.000 description 17
- 239000000693 micelle Substances 0.000 description 15
- 239000000047 product Substances 0.000 description 15
- MRUAUOIMASANKQ-UHFFFAOYSA-N cocamidopropyl betaine Chemical compound CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC([O-])=O MRUAUOIMASANKQ-UHFFFAOYSA-N 0.000 description 13
- 235000019198 oils Nutrition 0.000 description 13
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 12
- 229930195712 glutamate Natural products 0.000 description 12
- 238000005259 measurement Methods 0.000 description 10
- 230000015572 biosynthetic process Effects 0.000 description 9
- 229940073507 cocamidopropyl betaine Drugs 0.000 description 9
- 239000003205 fragrance Substances 0.000 description 9
- 239000000499 gel Substances 0.000 description 9
- 239000004973 liquid crystal related substance Substances 0.000 description 9
- 230000003068 static effect Effects 0.000 description 9
- 239000003755 preservative agent Substances 0.000 description 8
- ZUFONQSOSYEWCN-UHFFFAOYSA-M sodium;2-(methylamino)acetate Chemical compound [Na+].CNCC([O-])=O ZUFONQSOSYEWCN-UHFFFAOYSA-M 0.000 description 8
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical compound [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 7
- 230000003098 cholesteric effect Effects 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 230000002335 preservative effect Effects 0.000 description 7
- 108010077895 Sarcosine Proteins 0.000 description 6
- 238000000386 microscopy Methods 0.000 description 6
- 238000002156 mixing Methods 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- 239000006260 foam Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 241000209140 Triticum Species 0.000 description 4
- 235000021307 Triticum Nutrition 0.000 description 4
- 230000032683 aging Effects 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 229940071124 cocoyl glutamate Drugs 0.000 description 4
- 230000000052 comparative effect Effects 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- JOLYVEWZEPKDIJ-UTLKBRERSA-L dipotassium;(2s)-2-(dodecanoylamino)pentanedioate Chemical compound [K+].[K+].CCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O JOLYVEWZEPKDIJ-UTLKBRERSA-L 0.000 description 4
- HWUINYGRRJTXGE-UTLKBRERSA-L disodium;(2s)-2-(dodecanoylamino)pentanedioate Chemical compound [Na+].[Na+].CCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O HWUINYGRRJTXGE-UTLKBRERSA-L 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 229930182478 glucoside Natural products 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 239000002453 shampoo Substances 0.000 description 4
- IKGKWKGYFJBGQJ-UHFFFAOYSA-M sodium;2-(dodecanoylamino)acetate Chemical compound [Na+].CCCCCCCCCCCC(=O)NCC([O-])=O IKGKWKGYFJBGQJ-UHFFFAOYSA-M 0.000 description 4
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 3
- 229940098323 ammonium cocoyl isethionate Drugs 0.000 description 3
- MRUAUOIMASANKQ-UHFFFAOYSA-O carboxymethyl-[3-(dodecanoylamino)propyl]-dimethylazanium Chemical compound CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC(O)=O MRUAUOIMASANKQ-UHFFFAOYSA-O 0.000 description 3
- 235000010418 carrageenan Nutrition 0.000 description 3
- 239000006071 cream Substances 0.000 description 3
- KCIDZIIHRGYJAE-YGFYJFDDSA-L dipotassium;[(2r,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] phosphate Chemical compound [K+].[K+].OC[C@H]1O[C@H](OP([O-])([O-])=O)[C@H](O)[C@@H](O)[C@H]1O KCIDZIIHRGYJAE-YGFYJFDDSA-L 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 238000010316 high energy milling Methods 0.000 description 3
- 238000010348 incorporation Methods 0.000 description 3
- 229940075468 lauramidopropyl betaine Drugs 0.000 description 3
- 229940094506 lauryl betaine Drugs 0.000 description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 3
- DVEKCXOJTLDBFE-UHFFFAOYSA-N n-dodecyl-n,n-dimethylglycinate Chemical compound CCCCCCCCCCCC[N+](C)(C)CC([O-])=O DVEKCXOJTLDBFE-UHFFFAOYSA-N 0.000 description 3
- 238000001907 polarising light microscopy Methods 0.000 description 3
- 229940048106 sodium lauroyl isethionate Drugs 0.000 description 3
- 229940060304 sodium myristoyl sarcosinate Drugs 0.000 description 3
- KHCOJQDJOCNUGV-UHFFFAOYSA-M sodium;2-[methyl(tetradecanoyl)amino]acetate Chemical compound [Na+].CCCCCCCCCCCCCC(=O)N(C)CC([O-])=O KHCOJQDJOCNUGV-UHFFFAOYSA-M 0.000 description 3
- BRMSVEGRHOZCAM-UHFFFAOYSA-M sodium;2-dodecanoyloxyethanesulfonate Chemical compound [Na+].CCCCCCCCCCCC(=O)OCCS([O-])(=O)=O BRMSVEGRHOZCAM-UHFFFAOYSA-M 0.000 description 3
- RTVVXRKGQRRXFJ-UHFFFAOYSA-N sodium;2-sulfobutanedioic acid Chemical compound [Na].OC(=O)CC(C(O)=O)S(O)(=O)=O RTVVXRKGQRRXFJ-UHFFFAOYSA-N 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- DDGPBVIAYDDWDH-UHFFFAOYSA-N 3-[dodecyl(dimethyl)azaniumyl]-2-hydroxypropane-1-sulfonate Chemical compound CCCCCCCCCCCC[N+](C)(C)CC(O)CS([O-])(=O)=O DDGPBVIAYDDWDH-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 241000206572 Rhodophyta Species 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- QNAYBMKLOCPYGJ-UHFFFAOYSA-M alaninate Chemical compound CC(N)C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-M 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 239000008346 aqueous phase Substances 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000013256 coordination polymer Substances 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- PXEDJBXQKAGXNJ-QTNFYWBSSA-L disodium L-glutamate Chemical compound [Na+].[Na+].[O-]C(=O)[C@@H](N)CCC([O-])=O PXEDJBXQKAGXNJ-QTNFYWBSSA-L 0.000 description 2
- 229940079779 disodium cocoyl glutamate Drugs 0.000 description 2
- QKQCPXJIOJLHAL-UHFFFAOYSA-L disodium;2-[2-(carboxylatomethoxy)ethyl-[2-(dodecanoylamino)ethyl]amino]acetate Chemical compound [Na+].[Na+].CCCCCCCCCCCC(=O)NCCN(CC([O-])=O)CCOCC([O-])=O QKQCPXJIOJLHAL-UHFFFAOYSA-L 0.000 description 2
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 150000002191 fatty alcohols Chemical class 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 238000005187 foaming Methods 0.000 description 2
- 235000021588 free fatty acids Nutrition 0.000 description 2
- 238000005227 gel permeation chromatography Methods 0.000 description 2
- 150000008131 glucosides Chemical class 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 229940071145 lauroyl sarcosinate Drugs 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 230000003020 moisturizing effect Effects 0.000 description 2
- LPUQAYUQRXPFSQ-DFWYDOINSA-M monosodium L-glutamate Chemical compound [Na+].[O-]C(=O)[C@@H](N)CCC(O)=O LPUQAYUQRXPFSQ-DFWYDOINSA-M 0.000 description 2
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- 238000000399 optical microscopy Methods 0.000 description 2
- 239000002304 perfume Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- UEZVMMHDMIWARA-UHFFFAOYSA-M phosphonate Chemical compound [O-]P(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-M 0.000 description 2
- 229940082006 potassium cocoyl glutamate Drugs 0.000 description 2
- 229940099874 potassium lauroyl glutamate Drugs 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000013049 sediment Substances 0.000 description 2
- 229940079781 sodium cocoyl glutamate Drugs 0.000 description 2
- 229940065859 sodium cocoyl glycinate Drugs 0.000 description 2
- 229940045944 sodium lauroyl glutamate Drugs 0.000 description 2
- 229940045888 sodium myristoyl isethionate Drugs 0.000 description 2
- NTYZDAJPNNBYED-UHFFFAOYSA-M sodium;2-(2-dodecanoyloxypropanoyloxy)propanoate Chemical compound [Na+].CCCCCCCCCCCC(=O)OC(C)C(=O)OC(C)C([O-])=O NTYZDAJPNNBYED-UHFFFAOYSA-M 0.000 description 2
- MCFLGJDKSROECH-KVVVOXFISA-M sodium;2-[(z)-octadec-9-enoyl]oxyethanesulfonate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC(=O)OCCS([O-])(=O)=O MCFLGJDKSROECH-KVVVOXFISA-M 0.000 description 2
- KWFZLOFTBBTQIE-UHFFFAOYSA-M sodium;2-hexadecanoyloxyethanesulfonate Chemical compound [Na+].CCCCCCCCCCCCCCCC(=O)OCCS([O-])(=O)=O KWFZLOFTBBTQIE-UHFFFAOYSA-M 0.000 description 2
- LEEHDJJMXGSXDH-UHFFFAOYSA-M sodium;2-octadecanoyloxyethanesulfonate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC(=O)OCCS([O-])(=O)=O LEEHDJJMXGSXDH-UHFFFAOYSA-M 0.000 description 2
- WEXQJKLTLYEHLQ-UHFFFAOYSA-M sodium;2-tetradecanoyloxyethanesulfonate Chemical compound [Na+].CCCCCCCCCCCCCC(=O)OCCS([O-])(=O)=O WEXQJKLTLYEHLQ-UHFFFAOYSA-M 0.000 description 2
- 230000007928 solubilization Effects 0.000 description 2
- 238000005063 solubilization Methods 0.000 description 2
- 230000003381 solubilizing effect Effects 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- DIORMHZUUKOISG-UHFFFAOYSA-N sulfoformic acid Chemical compound OC(=O)S(O)(=O)=O DIORMHZUUKOISG-UHFFFAOYSA-N 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 239000003760 tallow Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 150000004043 trisaccharides Chemical class 0.000 description 2
- DCQFFOLNJVGHLW-RDQKPOQOSA-N (1r,3s,4r,5s,8s)-2,6-dioxabicyclo[3.2.1]octane-3,4,8-triol Chemical compound O1[C@H](O)[C@H](O)[C@H]2OC[C@@H]1[C@@H]2O DCQFFOLNJVGHLW-RDQKPOQOSA-N 0.000 description 1
- TUBPSFQENHCYBW-HVDRVSQOSA-N (2s)-2-aminopentanedioic acid;2-[bis(2-hydroxyethyl)amino]ethanol Chemical compound OC(=O)[C@@H](N)CCC(O)=O.OCCN(CCO)CCO TUBPSFQENHCYBW-HVDRVSQOSA-N 0.000 description 1
- FYGDTMLNYKFZSV-WFYNLLPOSA-N (2s,3r,4s,5s,6r)-2-[(2r,4r,5r,6s)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2r,3s,4r,5r,6s)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1[C@@H](CO)O[C@@H](O[C@@H]2[C@H](O[C@H](O)[C@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O FYGDTMLNYKFZSV-WFYNLLPOSA-N 0.000 description 1
- LUEWUZLMQUOBSB-FSKGGBMCSA-N (2s,3s,4s,5s,6r)-2-[(2r,3s,4r,5r,6s)-6-[(2r,3s,4r,5s,6s)-4,5-dihydroxy-2-(hydroxymethyl)-6-[(2r,4r,5s,6r)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol Chemical compound O[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@@H](O[C@@H]2[C@H](O[C@@H](OC3[C@H](O[C@@H](O)[C@@H](O)[C@H]3O)CO)[C@@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O LUEWUZLMQUOBSB-FSKGGBMCSA-N 0.000 description 1
- QURLONWWPWCPIC-UHFFFAOYSA-N 2-(2-aminoethoxy)ethanol;3,6-dichloro-2-methoxybenzoic acid Chemical compound NCCOCCO.COC1=C(Cl)C=CC(Cl)=C1C(O)=O QURLONWWPWCPIC-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- ACCAIGJKLCJFHP-UQKRIMTDSA-N 2-[bis(2-hydroxyethyl)amino]ethanol;(2s)-2-(dodecanoylamino)pentanedioic acid Chemical compound OCCN(CCO)CCO.CCCCCCCCCCCC(=O)N[C@H](C(O)=O)CCC(O)=O ACCAIGJKLCJFHP-UQKRIMTDSA-N 0.000 description 1
- BEABHZRODBBUAU-UHFFFAOYSA-N 2-[bis(2-hydroxyethyl)amino]ethanol;2-(methylamino)acetic acid Chemical compound CNCC(O)=O.OCCN(CCO)CCO BEABHZRODBBUAU-UHFFFAOYSA-N 0.000 description 1
- FUUGOUJDTGRGMR-GMFCBQQYSA-N 2-[bis(2-hydroxyethyl)amino]ethanol;2-[methyl-[(z)-octadec-9-enoyl]amino]acetic acid Chemical compound OCCN(CCO)CCO.CCCCCCCC\C=C/CCCCCCCC(=O)N(C)CC(O)=O FUUGOUJDTGRGMR-GMFCBQQYSA-N 0.000 description 1
- AMRBZKOCOOPYNY-QXMHVHEDSA-N 2-[dimethyl-[(z)-octadec-9-enyl]azaniumyl]acetate Chemical compound CCCCCCCC\C=C/CCCCCCCC[N+](C)(C)CC([O-])=O AMRBZKOCOOPYNY-QXMHVHEDSA-N 0.000 description 1
- HJDITXMCJQRQLU-UHFFFAOYSA-N 2-[dodecanoyl(methyl)amino]acetate;tris(2-hydroxyethyl)azanium Chemical compound OCCN(CCO)CCO.CCCCCCCCCCCC(=O)N(C)CC(O)=O HJDITXMCJQRQLU-UHFFFAOYSA-N 0.000 description 1
- IXOCGRPBILEGOX-UHFFFAOYSA-N 3-[3-(dodecanoylamino)propyl-dimethylazaniumyl]-2-hydroxypropane-1-sulfonate Chemical compound CCCCCCCCCCCC(=O)NCCC[N+](C)(C)CC(O)CS([O-])(=O)=O IXOCGRPBILEGOX-UHFFFAOYSA-N 0.000 description 1
- WQPMYSHJKXVTME-UHFFFAOYSA-N 3-hydroxypropane-1-sulfonic acid Chemical compound OCCCS(O)(=O)=O WQPMYSHJKXVTME-UHFFFAOYSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- 229920000189 Arabinogalactan Polymers 0.000 description 1
- 229920002498 Beta-glucan Polymers 0.000 description 1
- 241000195940 Bryophyta Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 208000001840 Dandruff Diseases 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical class S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 229920000926 Galactomannan Polymers 0.000 description 1
- 229920002581 Glucomannan Polymers 0.000 description 1
- 229920001202 Inulin Polymers 0.000 description 1
- 239000004976 Lyotropic liquid crystal Substances 0.000 description 1
- 229920000057 Mannan Polymers 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- SGAQYTGHTWYTNW-UHFFFAOYSA-N NCCO.CCCCCCCCCCCC(N)=O Chemical compound NCCO.CCCCCCCCCCCC(N)=O SGAQYTGHTWYTNW-UHFFFAOYSA-N 0.000 description 1
- GNMAJAFGCGXYGH-ZOWNYOTGSA-M N[C@@H](CCC(=O)[O-])C(=O)OC(CCCCCCCCC=C)=O.[Na+] Chemical compound N[C@@H](CCC(=O)[O-])C(=O)OC(CCCCCCCCC=C)=O.[Na+] GNMAJAFGCGXYGH-ZOWNYOTGSA-M 0.000 description 1
- ACAXXPXMEBRGLB-IZHYLRJQSA-L N[C@@H](CCC(=O)[O-])C(=O)OC(CCCCCCCCC=C)=O.[Na+].[Na+].C(CCCCCCCCC=C)(=O)OC([C@@H](N)CCC(=O)[O-])=O Chemical compound N[C@@H](CCC(=O)[O-])C(=O)OC(CCCCCCCCC=C)=O.[Na+].[Na+].C(CCCCCCCCC=C)(=O)OC([C@@H](N)CCC(=O)[O-])=O ACAXXPXMEBRGLB-IZHYLRJQSA-L 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 229920001218 Pullulan Polymers 0.000 description 1
- 239000004373 Pullulan Substances 0.000 description 1
- 239000004990 Smectic liquid crystal Substances 0.000 description 1
- 241000589636 Xanthomonas campestris Species 0.000 description 1
- 229920002000 Xyloglucan Polymers 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 150000003973 alkyl amines Chemical class 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000002216 antistatic agent Substances 0.000 description 1
- 235000019312 arabinogalactan Nutrition 0.000 description 1
- 229920000617 arabinoxylan Polymers 0.000 description 1
- 150000004783 arabinoxylans Chemical class 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- VDXDJNXCULYUIU-UHFFFAOYSA-N azane 2-dodecoxy-2-oxoethanesulfonic acid Chemical compound N.CCCCCCCCCCCCOC(=O)CS(O)(=O)=O VDXDJNXCULYUIU-UHFFFAOYSA-N 0.000 description 1
- OCSIXPGPUXCISD-UHFFFAOYSA-N azane;2-[dodecanoyl(methyl)amino]acetic acid Chemical compound N.CCCCCCCCCCCC(=O)N(C)CC(O)=O OCSIXPGPUXCISD-UHFFFAOYSA-N 0.000 description 1
- LLOHIFXFHGMBNO-UHFFFAOYSA-N azane;2-hydroxyethanesulfonic acid Chemical compound [NH4+].OCCS([O-])(=O)=O LLOHIFXFHGMBNO-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-FPRJBGLDSA-N beta-D-galactose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-FPRJBGLDSA-N 0.000 description 1
- 125000001488 beta-D-galactosyl group Chemical group C1([C@H](O)[C@@H](O)[C@@H](O)[C@H](O1)CO)* 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 229920006317 cationic polymer Polymers 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 229940096362 cocoamphoacetate Drugs 0.000 description 1
- 229940047648 cocoamphodiacetate Drugs 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000008406 cosmetic ingredient Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 229940073499 decyl glucoside Drugs 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- SHLKYEAQGUCTIO-UHFFFAOYSA-N diazanium;4-dodecoxy-4-oxo-3-sulfobutanoate Chemical compound [NH4+].[NH4+].CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O.CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O SHLKYEAQGUCTIO-UHFFFAOYSA-N 0.000 description 1
- DRTBPCASGTVPLF-VWLOTQADSA-N didodecanoyl (2s)-2-aminopentanedioate Chemical compound CCCCCCCCCCCC(=O)OC(=O)CC[C@H](N)C(=O)OC(=O)CCCCCCCCCCC DRTBPCASGTVPLF-VWLOTQADSA-N 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- YWGWUEXUIOPNMW-FJSYBICCSA-L dipotassium;(2s)-2-(octadecanoylamino)pentanedioate Chemical compound [K+].[K+].CCCCCCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O YWGWUEXUIOPNMW-FJSYBICCSA-L 0.000 description 1
- TWRWROOHGNQOQC-XRIOVQLTSA-L dipotassium;(2s)-2-(octanoylamino)pentanedioate Chemical compound [K+].[K+].CCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O TWRWROOHGNQOQC-XRIOVQLTSA-L 0.000 description 1
- IKNWNODSTRNMLD-SQKCAUCHSA-L dipotassium;(2s)-2-(tetradecanoylamino)pentanedioate Chemical compound [K+].[K+].CCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O IKNWNODSTRNMLD-SQKCAUCHSA-L 0.000 description 1
- ACNAFCGCINVEDL-GXKRWWSZSA-L dipotassium;(2s)-2-(undec-10-enoylamino)pentanedioate Chemical compound [K+].[K+].[O-]C(=O)CC[C@@H](C([O-])=O)NC(=O)CCCCCCCCC=C ACNAFCGCINVEDL-GXKRWWSZSA-L 0.000 description 1
- 125000000600 disaccharide group Chemical group 0.000 description 1
- 229940079868 disodium laureth sulfosuccinate Drugs 0.000 description 1
- 229940079881 disodium lauroamphodiacetate Drugs 0.000 description 1
- 229940079886 disodium lauryl sulfosuccinate Drugs 0.000 description 1
- 229940047038 disodium myristoyl glutamate Drugs 0.000 description 1
- 229940079784 disodium stearoyl glutamate Drugs 0.000 description 1
- WODOUQLMOIMKAL-FJSYBICCSA-L disodium;(2s)-2-(octadecanoylamino)pentanedioate Chemical compound [Na+].[Na+].CCCCCCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O WODOUQLMOIMKAL-FJSYBICCSA-L 0.000 description 1
- JRHWWJSWHFFZRY-XRIOVQLTSA-L disodium;(2s)-2-(octanoylamino)pentanedioate Chemical compound [Na+].[Na+].CCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O JRHWWJSWHFFZRY-XRIOVQLTSA-L 0.000 description 1
- SXBBFOVRSQCYFE-SQKCAUCHSA-L disodium;(2s)-2-(tetradecanoylamino)pentanedioate Chemical compound [Na+].[Na+].CCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O SXBBFOVRSQCYFE-SQKCAUCHSA-L 0.000 description 1
- GLSRFBDXBWZNLH-UHFFFAOYSA-L disodium;2-chloroacetate;2-(4,5-dihydroimidazol-1-yl)ethanol;hydroxide Chemical compound [OH-].[Na+].[Na+].[O-]C(=O)CCl.OCCN1CCN=C1 GLSRFBDXBWZNLH-UHFFFAOYSA-L 0.000 description 1
- YGAXLGGEEQLLKV-UHFFFAOYSA-L disodium;4-dodecoxy-4-oxo-2-sulfonatobutanoate Chemical compound [Na+].[Na+].CCCCCCCCCCCCOC(=O)CC(C([O-])=O)S([O-])(=O)=O YGAXLGGEEQLLKV-UHFFFAOYSA-L 0.000 description 1
- KHIQYZGEUSTKSB-UHFFFAOYSA-L disodium;4-dodecoxy-4-oxo-3-sulfobutanoate Chemical compound [Na+].[Na+].CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O.CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O KHIQYZGEUSTKSB-UHFFFAOYSA-L 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- GVGUFUZHNYFZLC-UHFFFAOYSA-N dodecyl benzenesulfonate;sodium Chemical compound [Na].CCCCCCCCCCCCOS(=O)(=O)C1=CC=CC=C1 GVGUFUZHNYFZLC-UHFFFAOYSA-N 0.000 description 1
- JRBPAEWTRLWTQC-UHFFFAOYSA-N dodecylamine Chemical compound CCCCCCCCCCCCN JRBPAEWTRLWTQC-UHFFFAOYSA-N 0.000 description 1
- SYELZBGXAIXKHU-UHFFFAOYSA-N dodecyldimethylamine N-oxide Chemical compound CCCCCCCCCCCC[N+](C)(C)[O-] SYELZBGXAIXKHU-UHFFFAOYSA-N 0.000 description 1
- 239000003974 emollient agent Substances 0.000 description 1
- 238000011067 equilibration Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 150000002194 fatty esters Chemical class 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229940046240 glucomannan Drugs 0.000 description 1
- 125000002791 glucosyl group Chemical group C1([C@H](O)[C@@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 1
- 229940049906 glutamate Drugs 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 230000009878 intermolecular interaction Effects 0.000 description 1
- 229940029339 inulin Drugs 0.000 description 1
- JYJIGFIDKWBXDU-MNNPPOADSA-N inulin Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)OC[C@]1(OC[C@]2(OC[C@]3(OC[C@]4(OC[C@]5(OC[C@]6(OC[C@]7(OC[C@]8(OC[C@]9(OC[C@]%10(OC[C@]%11(OC[C@]%12(OC[C@]%13(OC[C@]%14(OC[C@]%15(OC[C@]%16(OC[C@]%17(OC[C@]%18(OC[C@]%19(OC[C@]%20(OC[C@]%21(OC[C@]%22(OC[C@]%23(OC[C@]%24(OC[C@]%25(OC[C@]%26(OC[C@]%27(OC[C@]%28(OC[C@]%29(OC[C@]%30(OC[C@]%31(OC[C@]%32(OC[C@]%33(OC[C@]%34(OC[C@]%35(OC[C@]%36(O[C@@H]%37[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O%37)O)[C@H]([C@H](O)[C@@H](CO)O%36)O)[C@H]([C@H](O)[C@@H](CO)O%35)O)[C@H]([C@H](O)[C@@H](CO)O%34)O)[C@H]([C@H](O)[C@@H](CO)O%33)O)[C@H]([C@H](O)[C@@H](CO)O%32)O)[C@H]([C@H](O)[C@@H](CO)O%31)O)[C@H]([C@H](O)[C@@H](CO)O%30)O)[C@H]([C@H](O)[C@@H](CO)O%29)O)[C@H]([C@H](O)[C@@H](CO)O%28)O)[C@H]([C@H](O)[C@@H](CO)O%27)O)[C@H]([C@H](O)[C@@H](CO)O%26)O)[C@H]([C@H](O)[C@@H](CO)O%25)O)[C@H]([C@H](O)[C@@H](CO)O%24)O)[C@H]([C@H](O)[C@@H](CO)O%23)O)[C@H]([C@H](O)[C@@H](CO)O%22)O)[C@H]([C@H](O)[C@@H](CO)O%21)O)[C@H]([C@H](O)[C@@H](CO)O%20)O)[C@H]([C@H](O)[C@@H](CO)O%19)O)[C@H]([C@H](O)[C@@H](CO)O%18)O)[C@H]([C@H](O)[C@@H](CO)O%17)O)[C@H]([C@H](O)[C@@H](CO)O%16)O)[C@H]([C@H](O)[C@@H](CO)O%15)O)[C@H]([C@H](O)[C@@H](CO)O%14)O)[C@H]([C@H](O)[C@@H](CO)O%13)O)[C@H]([C@H](O)[C@@H](CO)O%12)O)[C@H]([C@H](O)[C@@H](CO)O%11)O)[C@H]([C@H](O)[C@@H](CO)O%10)O)[C@H]([C@H](O)[C@@H](CO)O9)O)[C@H]([C@H](O)[C@@H](CO)O8)O)[C@H]([C@H](O)[C@@H](CO)O7)O)[C@H]([C@H](O)[C@@H](CO)O6)O)[C@H]([C@H](O)[C@@H](CO)O5)O)[C@H]([C@H](O)[C@@H](CO)O4)O)[C@H]([C@H](O)[C@@H](CO)O3)O)[C@H]([C@H](O)[C@@H](CO)O2)O)[C@@H](O)[C@H](O)[C@@H](CO)O1 JYJIGFIDKWBXDU-MNNPPOADSA-N 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 229940045996 isethionic acid Drugs 0.000 description 1
- 229940115478 isopropyl lauroyl sarcosinate Drugs 0.000 description 1
- 229940048866 lauramine oxide Drugs 0.000 description 1
- 229940071188 lauroamphodiacetate Drugs 0.000 description 1
- 125000000400 lauroyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940048848 lauryl glucoside Drugs 0.000 description 1
- IZWSFJTYBVKZNK-UHFFFAOYSA-N lauryl sulfobetaine Chemical compound CCCCCCCCCCCC[N+](C)(C)CCCS([O-])(=O)=O IZWSFJTYBVKZNK-UHFFFAOYSA-N 0.000 description 1
- AIHDCSAXVMAMJH-GFBKWZILSA-N levan Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)OC[C@@H]1[C@@H](O)[C@H](O)[C@](CO)(CO[C@@H]2[C@H]([C@H](O)[C@@](O)(CO)O2)O)O1 AIHDCSAXVMAMJH-GFBKWZILSA-N 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 230000002535 lyotropic effect Effects 0.000 description 1
- LUEWUZLMQUOBSB-GFVSVBBRSA-N mannan Chemical class O[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@H]3[C@H](O[C@@H](O)[C@@H](O)[C@H]3O)CO)[C@@H](O)[C@H]2O)CO)[C@H](O)[C@H]1O LUEWUZLMQUOBSB-GFVSVBBRSA-N 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 235000011929 mousse Nutrition 0.000 description 1
- 125000001419 myristoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- GOQYKNQRPGWPLP-UHFFFAOYSA-N n-heptadecyl alcohol Natural products CCCCCCCCCCCCCCCCCO GOQYKNQRPGWPLP-UHFFFAOYSA-N 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 125000002811 oleoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])/C([H])=C([H])\C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 239000003346 palm kernel oil Substances 0.000 description 1
- 235000019865 palm kernel oil Nutrition 0.000 description 1
- 125000001312 palmitoyl group Chemical group O=C([*])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- XYFCBTPGUUZFHI-UHFFFAOYSA-O phosphonium Chemical compound [PH4+] XYFCBTPGUUZFHI-UHFFFAOYSA-O 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229940079988 potassium cocoyl glycinate Drugs 0.000 description 1
- RJLXQRRDQRGFJR-ZOWNYOTGSA-M potassium;(2s)-5-hydroxy-5-oxo-2-(undec-10-enoylamino)pentanoate Chemical compound [K+].OC(=O)CC[C@@H](C([O-])=O)NC(=O)CCCCCCCCC=C RJLXQRRDQRGFJR-ZOWNYOTGSA-M 0.000 description 1
- KCQOKZAQSWTPIL-BDQAORGHSA-M potassium;(4s)-5-hydroxy-4-(octadecanoylamino)-5-oxopentanoate Chemical compound [H+].[K+].CCCCCCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O KCQOKZAQSWTPIL-BDQAORGHSA-M 0.000 description 1
- WONHSIFWFDNSCE-PPHPATTJSA-M potassium;(4s)-5-hydroxy-4-(octanoylamino)-5-oxopentanoate Chemical compound [H+].[K+].CCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O WONHSIFWFDNSCE-PPHPATTJSA-M 0.000 description 1
- KYLDDUZJZSKJER-NTISSMGPSA-M potassium;(4s)-5-hydroxy-5-oxo-4-(tetradecanoylamino)pentanoate Chemical compound [H+].[K+].CCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O KYLDDUZJZSKJER-NTISSMGPSA-M 0.000 description 1
- WLPORNKIACJZBO-UHFFFAOYSA-M potassium;2-(dodecanoylamino)acetate Chemical compound [K+].CCCCCCCCCCCC(=O)NCC([O-])=O WLPORNKIACJZBO-UHFFFAOYSA-M 0.000 description 1
- JEMLSRUODAIULV-UHFFFAOYSA-M potassium;2-[dodecanoyl(methyl)amino]acetate Chemical compound [K+].CCCCCCCCCCCC(=O)N(C)CC([O-])=O JEMLSRUODAIULV-UHFFFAOYSA-M 0.000 description 1
- XLCIFRJORZNGEV-UHFFFAOYSA-N propan-2-yl 2-[dodecanoyl(methyl)amino]acetate Chemical compound CCCCCCCCCCCC(=O)N(C)CC(=O)OC(C)C XLCIFRJORZNGEV-UHFFFAOYSA-N 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 235000019423 pullulan Nutrition 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000006254 rheological additive Substances 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 229940096501 sodium cocoamphoacetate Drugs 0.000 description 1
- APSBXTVYXVQYAB-UHFFFAOYSA-M sodium docusate Chemical group [Na+].CCCCC(CC)COC(=O)CC(S([O-])(=O)=O)C(=O)OCC(CC)CCCC APSBXTVYXVQYAB-UHFFFAOYSA-M 0.000 description 1
- 229940080264 sodium dodecylbenzenesulfonate Drugs 0.000 description 1
- 229940045998 sodium isethionate Drugs 0.000 description 1
- 239000001540 sodium lactate Substances 0.000 description 1
- 235000011088 sodium lactate Nutrition 0.000 description 1
- 229940005581 sodium lactate Drugs 0.000 description 1
- 229940007636 sodium lauroyl methyl isethionate Drugs 0.000 description 1
- 229940075560 sodium lauryl sulfoacetate Drugs 0.000 description 1
- 229940048109 sodium methyl cocoyl taurate Drugs 0.000 description 1
- 229940077092 sodium myristoyl glutamate Drugs 0.000 description 1
- 229940045898 sodium stearoyl glutamate Drugs 0.000 description 1
- FCBUGCHAVCFTHW-NTISSMGPSA-N sodium;(2s)-2-(tetradecanoylamino)pentanedioic acid Chemical compound [Na].CCCCCCCCCCCCCC(=O)N[C@H](C(O)=O)CCC(O)=O FCBUGCHAVCFTHW-NTISSMGPSA-N 0.000 description 1
- KLIFRVSZGDONER-FERBBOLQSA-M sodium;(4s)-4-(hexadecanoylamino)-5-hydroxy-5-oxopentanoate Chemical compound [H+].[Na+].CCCCCCCCCCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O KLIFRVSZGDONER-FERBBOLQSA-M 0.000 description 1
- KDHFCTLPQJQDQI-BDQAORGHSA-M sodium;(4s)-4-amino-5-octadecanoyloxy-5-oxopentanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC(=O)OC(=O)[C@@H](N)CCC([O-])=O KDHFCTLPQJQDQI-BDQAORGHSA-M 0.000 description 1
- BCGXTKYOBWQPCN-PPHPATTJSA-M sodium;(4s)-5-hydroxy-4-(octanoylamino)-5-oxopentanoate Chemical compound [H+].[Na+].CCCCCCCC(=O)N[C@H](C([O-])=O)CCC([O-])=O BCGXTKYOBWQPCN-PPHPATTJSA-M 0.000 description 1
- SLBXZQMMERXQAL-UHFFFAOYSA-M sodium;1-dodecoxy-4-hydroxy-1,4-dioxobutane-2-sulfonate Chemical compound [Na+].CCCCCCCCCCCCOC(=O)C(S(O)(=O)=O)CC([O-])=O SLBXZQMMERXQAL-UHFFFAOYSA-M 0.000 description 1
- HVFAVOFILADWEZ-UHFFFAOYSA-M sodium;2-[2-(dodecanoylamino)ethyl-(2-hydroxyethyl)amino]acetate Chemical compound [Na+].CCCCCCCCCCCC(=O)NCCN(CCO)CC([O-])=O HVFAVOFILADWEZ-UHFFFAOYSA-M 0.000 description 1
- CAVXVRQDZKMZDB-UHFFFAOYSA-M sodium;2-[dodecanoyl(methyl)amino]ethanesulfonate Chemical compound [Na+].CCCCCCCCCCCC(=O)N(C)CCS([O-])(=O)=O CAVXVRQDZKMZDB-UHFFFAOYSA-M 0.000 description 1
- AUHKUMFBHOJIMU-UHFFFAOYSA-M sodium;2-[hexadecanoyl(methyl)amino]acetate Chemical compound [Na+].CCCCCCCCCCCCCCCC(=O)N(C)CC([O-])=O AUHKUMFBHOJIMU-UHFFFAOYSA-M 0.000 description 1
- VLKIFCBXANYYCK-GMFCBQQYSA-M sodium;2-[methyl-[(z)-octadec-9-enoyl]amino]acetate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC(=O)N(C)CC([O-])=O VLKIFCBXANYYCK-GMFCBQQYSA-M 0.000 description 1
- IZWPGJFSBABFGL-GMFCBQQYSA-M sodium;2-[methyl-[(z)-octadec-9-enoyl]amino]ethanesulfonate Chemical compound [Na+].CCCCCCCC\C=C/CCCCCCCC(=O)N(C)CCS([O-])(=O)=O IZWPGJFSBABFGL-GMFCBQQYSA-M 0.000 description 1
- HYHAWELIVMOSBT-UHFFFAOYSA-M sodium;2-aminopentadecanoate Chemical compound [Na+].CCCCCCCCCCCCCC(N)C([O-])=O HYHAWELIVMOSBT-UHFFFAOYSA-M 0.000 description 1
- NVIZQHFCDBQNPH-UHFFFAOYSA-M sodium;2-dodecanoyloxypropane-1-sulfonate Chemical compound [Na+].CCCCCCCCCCCC(=O)OC(C)CS([O-])(=O)=O NVIZQHFCDBQNPH-UHFFFAOYSA-M 0.000 description 1
- UAJTZZNRJCKXJN-UHFFFAOYSA-M sodium;2-dodecoxy-2-oxoethanesulfonate Chemical compound [Na+].CCCCCCCCCCCCOC(=O)CS([O-])(=O)=O UAJTZZNRJCKXJN-UHFFFAOYSA-M 0.000 description 1
- LADXKQRVAFSPTR-UHFFFAOYSA-M sodium;2-hydroxyethanesulfonate Chemical compound [Na+].OCCS([O-])(=O)=O LADXKQRVAFSPTR-UHFFFAOYSA-M 0.000 description 1
- IWMMSZLFZZPTJY-UHFFFAOYSA-M sodium;3-(dodecylamino)propane-1-sulfonate Chemical compound [Na+].CCCCCCCCCCCCNCCCS([O-])(=O)=O IWMMSZLFZZPTJY-UHFFFAOYSA-M 0.000 description 1
- DUXXGJTXFHUORE-UHFFFAOYSA-M sodium;4-tridecylbenzenesulfonate Chemical compound [Na+].CCCCCCCCCCCCCC1=CC=C(S([O-])(=O)=O)C=C1 DUXXGJTXFHUORE-UHFFFAOYSA-M 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 229960004274 stearic acid Drugs 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229940048912 triethanolamine cocoyl glutamate Drugs 0.000 description 1
- 238000005199 ultracentrifugation Methods 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 1
- 239000004711 α-olefin Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/46—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing sulfur
- A61K8/466—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing sulfur containing sulfonic acid derivatives; Salts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/02—Cosmetics or similar toiletry preparations characterised by special physical form
- A61K8/0295—Liquid crystals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/44—Aminocarboxylic acids or derivatives thereof, e.g. aminocarboxylic acids containing sulfur; Salts; Esters or N-acylated derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
- A61K8/731—Cellulose; Quaternized cellulose derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
- A61K8/736—Chitin; Chitosan; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/10—Washing or bathing preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/02—Preparations for cleaning the hair
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/20—Chemical, physico-chemical or functional or structural properties of the composition as a whole
- A61K2800/30—Characterized by the absence of a particular group of ingredients
Definitions
- the present application generally relates to personal cleansing compositions, their methods of making such compositions, and their uses.
- the personal cleansing compositions have desirable rheologic properties and structure when the personal cleansing composition has a surfactant system comprising a fatty acyl isethionate surfactant and when the personal cleansing composition is free of alkyl sulfate and alkyl ether sulfate type of surfactants.
- Personal cleansing compositions have been traditionally marketed in a variety of forms such as bar soaps, creams, lotions, and gels. Typically, these products must satisfy a number of criteria to be acceptable to consumers. These criteria include cleansing effectiveness, skin feel, mildness to skin, hair, and ocular mucosae, and lather volume. Ideal personal cleansers should gently cleanse the skin or hair, cause little or no irritation, and should not leave the skin or hair overly dry after frequent use.
- Anionic surfactants are widely used in personal cleansing compositions. Many of these anionic surfactants contain elongated micelles and are viscoelastic, which is of great importance, especially in the design of shampoos and body washes. In most personal cleansing compositions, alkyl sulfate and alkyl ether sulfate as the anionic surfactants predominate.
- sulfate-free personal cleansing compositions are difficult to thicken sufficiently to afford the user good usage qualities.
- Two approaches are leveraged to attempt to thicken such formulas.
- One approach for instance is to use high levels of surfactants to benefit from the self-assembling properties of such ingredients. This approach is most common but it is also costly.
- the second approach for instance is to use high levels of rheology modifiers which can adversely impact the properties of the composition such as by decreasing the foam and ease of distribution of the composition.
- WO 2011/120780 A2 is related to super mild surfactant systems, optionally in combination with skin or hair benefit agent.
- WO2008/074617 A1 is related to liquid cleanser compositions which can use fatty acyl isethionates mixtures, regardless of free fatty acid content of isethionates mixture or chain length distribution of isethionates mixture.
- Personal cleansing compositions having a surfactant system comprising a fatty acyl isethionate surfactant and being free of alkyl sulfate and alkyl ether sulfate type of surfactants have been developed.
- Fatty acyl isethionates are mild anionic surfactants highly desirable in personal cleansing products for hair or skin, because fatty acyl isethionates can lather well, are mild to the skin and have good emollient properties.
- fatty acyl isethionates are not readily used in liquid personal cleansing compositions, because of their relatively low solubility in water. This may result in unstable personal cleansing compositions which can exhibit inconsistent rheology profiles.
- a personal cleansing composition comprising a fatty acyl isethionate surfactant and being free of alkyl sulfate and alkyl ether sulfate type of surfactants and having a satisfactory consistent rheology profile.
- Benefit agents in the form of solid particles, liquid droplets are of interest for personal cleansing compositions.
- Benefit agents can be used as pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers.
- a personal cleansing composition comprising a fatty acyl isethionate surfactant imparted with a sufficient structure to hold benefit agents such as solid particles, liquid droplets.
- a personal cleansing composition is provided and comprises:
- Another aspect is related to a method for making the personal cleansing composition according to any of the preceding claims comprising the following steps, preferably in that order:
- a personal cleansing composition is provided and can be made by the method as set out hereinbefore.
- the personal cleansing composition as set out hereinbefore is used for improving the lather of the composition.
- the personal cleansing composition as set out hereinbefore is used for suspending benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- the polymer liquid crystals may form elongated structures and may be nematic or cholesteric.
- QSP or "q.s.” means sufficient quantity for 100% or for 100g. "+/-" indicates the standard deviation. All ranges are inclusive and combinable. The number of significant digits conveys neither a limitation on the indicated amount nor on the accuracy of the measurement.
- Relative humidity refers to the ratio (stated as a percent) of the moisture content of air compared to the saturated moisture level at the same temperature and pressure. Relative humidity can be measured with a hygrometer, in particular with a probe hygrometer from VWR ® International.
- compositions, methods, and uses of the present invention can comprise, consist of, and consist essentially of the elements and limitations of the invention described herein, as well as any of the additional or optional ingredients, components, steps, or limitations described herein.
- Embodiments and aspects described herein may comprise or be combinable with elements, features or components of other embodiments and/or aspects despite not being expressly exemplified in combination, unless an incompatibility is stated.
- composition comprises from 1% to 5% fatty alcohol
- a composition comprising 2% stearyl alcohol and 1% cetyl alcohol and no other fatty alcohol, would fall within this scope.
- the amount of each particular ingredient or mixtures thereof described hereinafter can account for up to 100% (or 100%) of the total amount of the ingredient(s) in the composition.
- mixtures as used herein is meant to include a simple combination of materials and any compounds that may result from their combination.
- molecular weight or "M.Wt.” as used herein refers to the weight average molecular weight unless otherwise stated.
- the weight average molecular weight can be measured by gel permeation chromatography ("GPC").
- personal cleansing composition refers to compositions intended for topical application to the skin or hair for cleansing.
- the personal cleansing composition may be aqueous.
- isotropic refers to a particular phase of the composition wherein the structure is "identical along any three orthogonal directions in space, and is therefore dark or 'nonbirefringent' when viewed between crossed polarized light filters. (One direction is 'orthogonal' to another if the vector component of the first, in the direction of the second, is zero.)" (Laughlin, R. G. (1994). "The Aqueous Phase Behavior of Surfactants," 182, 8.2).
- anisotropic refers a particular phase of the composition wherein the structure exhibits properties with different values when measured in different directions.
- An anisotropic phase is not identical along any three orthogonal directions in space, and is birefringent when viewed between crossed polarized light filters.
- liquid crystals refers to anisotropic fluids or mesophases.
- Liquid crystals as used herein are polymeric liquid crystals.
- the polymeric liquid crystalline phase of the personal cleansing composition is lyotrophic meaning that the polymer liquid crystalline phase contains a solvent, namely water.
- This type of polymer liquid crystals is distinguished in the art from thermotropic, heat, and magnetically induced liquid crystals.
- the liquid crystalline state exists between the boundaries of the solid crystalline phase and the isotropic liquid phase (i.e. an intermediate between the three dimensionally ordered crystalline state and the disordered dissolved state).
- Liquid crystals are also known as anisotropic fluids, a fourth state of matter, polymer association structure or mesophases. Those terms are used interchangeably. Lyotropic means a material is formed through changes in solution behavior (and hence by definition contains a solvent, e.g. water) of the ingredients. The changes involve thermal and salvation energies.
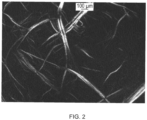
- lyotropic liquid crystal refers to a liquid crystalline phase distinctive by the presence of birefringence under polarized light microscopy.
- birefringence refers the property of the material to capable of transmitting light when viewed with cross polars under static conditions.
- micelle refers structure comprising individual surfactant molecules aggregated to form a hydrophobic core region with externally facing polar head groups in equilibrium with surfactant monomers in a polar phase, having a characteristic dimension that is a single digit multiple of the surfactant length, i.e., generally less than about 10 nm in diameter.
- doctor-off means the intended product usage includes application to skin and/or hair followed by rinsing and/or wiping the product from the skin and/or hair within a few seconds to minutes of the application step.
- the product is generally applied and rinsed in the same usage event, for example, a shower.
- static stability refers to a personal cleansing composition that comprises at least two compositions that maintain at least two "separate" phases with at least two separate benefit concentration zones contained within a single chamber package at ambient conditions for a period of at least about 180 days.
- static stability can be determined by accelerated protocol at elevated temperature.
- One accelerated protocol is based on passing static stability after 10 days at 50° C.
- separatate is meant that there is substantially no mixing of compositions contained in the zones, detected by the benefit analysis method, described hereinafter, prior to dispensing of the composition.
- substantially free of means less than 1%, less than 0.8%, less than 0.5%, less than 0.3%, or less than an immaterial amount of a stated ingredient by total weight of the composition.
- surfactant refers to amphiphilic molecules which can aggregate to form micelles and other surfactant structures, which are soluble in an aqueous phase and contribute to foaming during a cleansing event, i.e., stabilizing an air interface.
- structured means having a rheology that confers stability on the personal cleansing composition.
- the degree of structure is determined by rheologic characteristics such as the yield stress determined by the Herschel-Bulkley Rheology Test Method or the viscosity obtained by the Ultracentrifugation Test Method, all in the Test Method section below.
- the objects of the present invention are to provide personal cleansing compositions and uses of the compositions as described in the Summary or as described hereinbelow for fulfilling the technical effects or goals as set out herein.
- Thickeners are useful for adjusting the viscosity and the rheological behavior of personal cleansing compositions in order to make them easy to pour and dose.
- Structurants thicken, but also provide a suspensive benefit, allowing ingredients such as oils, particulates, and the like, to be stably suspended in the personal cleansing composition.
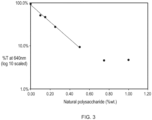
- a natural polysaccharide such as xanthan gum can lead to the formation of a polymer liquid crystalline phase in the personal cleansing composition and even at a relatively low amount from 0.25% to 2% of the natural polysaccharide total weight of the composition.
- the personal cleansing composition comprises a first and second phase.
- the first phase is an isotropic and micellar surfactant phase.
- the second phase is a polymer liquid crystalline phase.
- the first and second phases of the personal cleansing composition may be separated by ultracentrifuge.
- the polymer liquid crystalline phase is characterized by birefringence.
- the polymer liquid crystalline phase and the personal cleansing composition may have a specific rheologic profile.
- the polymer liquid crystalline phase of the composition provides the structure of the personal cleansing composition.
- the polymer liquid crystalline phase comprises polymer liquid crystals.
- the polymer liquid crystals include the natural polysaccharide.
- the polymer liquid crystalline phase can help the personal cleansing composition to suspend a benefit agent selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- a benefit agent selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents,
- the personal cleansing composition may have specific yield stress ⁇ y , as an elastic component of the composition, measured according to the Herschel-Burlley Rheology Test Method.
- the personal cleansing composition may comprise the polymer liquid crystalline phase, and at a sufficient yield stress ⁇ y from 0.005 to 3 Pa measured according to the Herschel-Bulkley Rheology Test Method.
- the personal cleansing composition may also exhibit a flow viscosity from 3 Pa.s to 100 Pa.s at 25°C at a shear rate of 1.5 s-1 according to the Flow Viscosity Test Method.
- a stable personal cleansing composition able to suspend benefits agents and comprising fatty acyl isethionate can be provided when the composition comprises a polymer liquid crystalline phase comprising polymer liquid crystals, wherein the polymer liquid crystals include the natural polysaccharide.
- the personal cleansing composition is free of alkyl sulfate and alkyl ether sulfate type of surfactant.
- the personal cleansing composition does not comprise any alkyl sulfate which comprises C 12 -C 18 alkyl sulfate or any alkyl ether sulfate including alkyl glyceryl ether sulfates.
- the personal cleansing composition may not comprise any alkyl ether sulfates which are those having the formula: RO(CH 2 CH 2 O) n SO 3 M wherein R is an alkyl or alkenyl having 8 to 18 carbons, preferably 12 to 18 carbons, n has an average value of greater than at least 0.5, preferably between 2 and 3; and M is a solubilizing cation such as sodium, potassium, ammonium or substituted ammonium.
- the personal cleansing composition may not comprise any ammonium and sodium lauryl ether sulfates.
- the personal cleansing composition does contain alkyl sulfate and/or alkyl ether sulfate type of surfactant, its content of such a weight proportion of: alkyl sulfates or alkyl ether sulfate type surfactant is less than or equal to the sum of 0.6, more preferably less than or equal to the sum of 0.2, even more preferably equal to 0.
- the personal cleansing composition comprises a surfactant system.
- the surfactant system comprises from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition; and from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15% of a co-surfactant by weight of the composition.
- the fatty acyl isethionate surfactant may be defined as an isethionate according to the general formula (I): wherein R 1 is a saturated or unsaturated, straight or branched, alkyl or alkenyl chain with from 6 to 30 carbon atoms, preferably from 8 to 22 carbon atoms, more preferably from 9 to 18 carbon atoms, R 2 and R 3 are each independently H or (C 1 -C 4 ) alkyl, and M + is an alkali metal, preferably lithium, sodium, potassium; or M + is an alkali-earth metal, preferably magnesium; or M + is an ammonium or a substituted ammonium cation; or
- Suitable fatty acyl isethionate surfactants may include the reaction product of fatty acids esterified with isethionic acid and neutralized with sodium hydroxide. Suitable fatty acids for isethionate surfactants can be derived from coconut oil or palm kernel oil, for instance. Additional examples of suitable isethionic anionic surfactants are described in U.S. Pat. No. 2,486,921 ; U.S. Pat. No. 2,486,922 ; and U.S. Pat. No. 2,396,278 .
- the fatty acyl isethionate surfactant may be selected from the group consisting of sodium lauroyl isethionate, sodium lauroyl methyl isethionate, sodium oleoyl isethionate, sodium oleoyl methyl isethionate, sodium stearoyl isethionate, sodium stearoyl methyl isethionate, sodium myristoyl isethionate, sodium myristoyl methyl isethionate, sodium palmitoyl isethionate, sodium palmitoyl methyl isethionate, sodium cocoyl isethionate, sodium cocoyl methyl isethionate, a blend of stearic acid and sodium cocoyl isethionate, ammonium cocoyl isethionate, ammonium cocoyl methyl isethionate, and mixtures thereof.
- the fatty acyl isethionate surfactant may be preferably selected from the group consisting of sodium lauroyl isethionate, sodium myristoyl isethionate, sodium palmitoyl isethionate, sodium stearoyl isethionate, sodium oleoyl isethionate, sodium cocoyl isethionate, ammonium cocoyl isethionate, and mixtures thereof.
- the fatty acyl isethionate surfactant may be more preferably selected from the group consisting of sodium lauroyl isethionate, sodium cocoyl isethionate, ammonium cocoyl isethionate, and mixtures thereof.
- Corresponding commercial products are available, for example, from the company Innospec under the trade name "Iselux ® " and from Clariant or Uniquema under the trade names "Hostapon ® “ or "Arlatone ® ".
- Examples of other commercial fatty acyl isethionates that may be used can be Hostapon ® surfactants from Clariant such as for sodium cocoyl isethionate: Hostapon ® SCI-85C, Hostapon ® SCI-78C, or a blend of stearic acid with sodium cocoyl isethionate: Hostapon ® SCI-65C.
- Examples of other commercial fatty acyl isethionates that may be used can be "Jordapon ® " surfactants from BASF such as Jordapon ® CI prill or Jordapon ® CI65; and sodium cocoyl isethionate from Yongan Daily Chemical Co. such as YA-SCI-85 ® or YA-SCI-65 ® .
- Fatty acyl isethionates surfactants are typically prepared by the reaction of an isethionate salt such as metal or ammonium isethionate and an a saturated or unsaturated, straight or branched, alkyl or alkenyl chain fatty acid having from 6 to 30 carbon atoms, preferably from 8 to 22 carbon atoms, more preferably from 6 to 18 carbon atoms.
- an isethionate salt such as metal or ammonium isethionate
- an a saturated or unsaturated, straight or branched, alkyl or alkenyl chain fatty acid having from 6 to 30 carbon atoms, preferably from 8 to 22 carbon atoms, more preferably from 6 to 18 carbon atoms.
- the resulting fatty acyl isethionate surfactant can be a mixture of 45 to 95% by weight of fatty acyl isethionates and 40 to 0 wt.% of free fatty acids, in addition to isethionates salts, typically less than 5 wt.%, and trace (less than 2 wt.%) of other impurities, by total weight of the resulting fatty acyl isethionate surfactant.
- a mixture of aliphatic fatty acids may be used for the preparation of commercial fatty acyl isethionates surfactants.
- the personal cleansing composition comprises a surfactant system.
- the surfactant system comprises from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition.
- concentrations mentioned here are total concentration ranges in case more than one fatty acyl isethionate surfactant is present.
- the specified ranges are provided by weight and relate to the total weight of the personal cleansing composition.
- Fatty acyl isethionate surfactants have not typically been used in preparation of personal cleansing compositions because they might readily form solid crystals (when used alone and/or with a co-surfactant) and consequently might make it difficult to form stable liquid personal cleansing compositions.
- the surfactant system comprises from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15% of a co-surfactant by weight of the composition.
- the co-surfactant may be selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant and mixtures thereof.
- the anionic surfactant of the co-surfactant being not an isethionate surfactant may be selected from the group consisting of fatty acyl sarcosinates, sulfosuccinates, sulfonates, sulfoacetates, acyl glycinates, acyl alaninates, acyl glutamates, lactates, lactylates, glucose carboxylates, amphoacetates, taurates, and mixtures thereof.
- alkyl is defined as a saturated or unsaturated, straight or branched alkyl chain with 6 to 30 carbon atoms, preferably with 8 to 22 carbon atoms, more preferably with 9 to 18 carbon atoms.
- acyl is defined as of formula R-C(O)-, wherein R is a saturated or unsaturated, straight or branched alkyl or alkenyl, preferably alkyl chain with 6 to 30 carbon atoms, preferably with 8 to 22 carbon atoms, more preferably with 9 to 18 carbon atoms.
- the fatty acyl sarcosinate may be a sarcosinate according to the general formula (II): wherein R is a saturated or unsaturated, straight or branched alkyl or alkenyl, preferably alkyl chain with 7 to 17 carbon atoms, preferably with 9 to 13 carbon atoms and M + is H, a sodium, potassium or ammonium cation.
- Non-limiting examples of sarcosinates may be selected from the group consisting of sodium lauroyl sarcosinate, sodium cocoyl sarcosinate, sodium myristoyl sarcosinate, TEA-cocoyl sarcosinate, ammonium cocoyl sarcosinate, ammonium lauroyl sarcosinate, dimer dilinoleyl bis-lauroyl glutamate/lauroyl sarcosinate, disodium lauroamphodiacetate, lauroyl sarcosinate, isopropyl lauroyl sarcosinate, potassium cocoyl sarcosinate, potassium lauroyl sarcosinate, sodium oleoyl sarcosinate, sodium palmitoyl sarcosinate, TEA-lauroyl sarcosinate, TEA-oleoyl sarcosinate, TEA-palm kernel
- the fatty acyl sarcosinate may be selected from the group consisting of sodium lauroyl sarcosinate, sodium myristoyl sarcosinate, sodium cocoyl sarcosinate, and mixtures thereof.
- Non-limiting examples of sulfosuccinate surfactants can include disodium N-octadecyl sulfosuccinate, disodium lauryl sulfosuccinate, diammonium lauryl sulfosuccinate, sodium lauryl sulfosuccinate, disodium laureth sulfosuccinate, tetrasodium N-(1,2-dicarboxyethyl)-N-octadecyl sulfosuccinnate, diamyl ester of sodium sulfosuccinic acid, dihexyl ester of sodium sulfosuccinic acid, dioctyl esters of sodium sulfosuccinic acid, and combinations thereof.
- Non-limiting examples of sulfonates can include alpha olefin sulfonates, linear alkylbenzene sulfonates, sodium laurylglucosides hydroxypropylsulfonate, and combinations thereof.
- Non-limiting examples of sulfoacetates can include sodium lauryl sulfoacetate, ammonium lauryl sulfoacetate, and combination thereof.
- Non-limiting examples of acyl glycinates can include sodium cocoyl glycinate, sodium lauroyl glycinate, and combination thereof.
- Non-limiting example of acyl alaninates can include sodium cocoyl alaninate, sodium lauroyl alaninate, sodium N-dodecanoyl-1-alaninate, and combinations thereof.
- Non-limiting examples of acyl glutamates can be selected from the group consisting of sodium cocoyl glutamate, disodium cocoyl glutamate, ammonium cocoyl glutamate, diammonium cocoyl glutamate, sodium lauroyl glutamate, disodium lauroyl glutamate, sodium cocoyl hydrolyzed wheat protein glutamate, disodium cocoyl hydrolyzed wheat protein glutamate, potassium cocoyl glutamate, dipotassium cocoyl glutamate, potassium lauroyl glutamate, dipotassium lauroyl glutamate, potassium cocoyl hydrolyzed wheat protein glutamate, dipotassium cocoyl hydrolyzed wheat protein glutamate, sodium capryloyl glutamate, disodium capryloyl glutamate, potassium capryloyl glutamate, dipotassium capryloyl glutamate, sodium undecylenoyl glutamate, disodium undecylenoyl glutamate, potassium
- Non-limiting example of lactates can include sodium lactate.
- lactylates can include sodium lauroyl lactylate, sodium cocoyl lactylate, and combination thereof.
- glucose carboxylates can include sodium lauryl glucoside carboxylate, sodium cocoyl glucoside carboxylate, and combinations thereof.
- alkylamphoacetates can include sodium cocoyl amphoacetate, sodium lauroyl amphoacetate, and combination thereof.
- Non-limiting examples of acyl taurates can include sodium methyl cocoyl taurate, sodium methyl lauroyl taurate, sodium methyl oleoyl taurate, and combinations thereof.
- the anionic surfactants of the co-surfactant may be selected from the group consisting of sodium tridecyl benzene sulfonate, sodium dodecyl benzene sulfonate, sodium lauroyl sarcosinate, sodium cocoyl sarcosinate, sodium lauroyl glycinate, sodium cocoyl glycinate, potassium lauroyl glycinate, potassium cocoyl glycinate, sodium lauroyl glutamate, potassium lauroyl glutamate, sodium cocoyl glutamate, potassium cocoyl glutamate, disodium lauroyl glutamate, dipotassium lauroyl glutamate, disodium cocoyl glutamate, dipotassium cocoyl glutamate, sodium lauroyl lactylate, and mixtures thereof.
- the surfactant system may comprise from 0.5% to 15%, preferably from 1% to 10%, more preferably from 2% to 5% of an anionic surfactant of the co-surfactant, being not an isethionate surfactant, preferably a fatty acyl sarcosinate, by total weight of the composition.
- the non-ionic surfactant of the co-surfactant may be selected from the group consisting of glucosides, alkyl amines, alcohol ethoxylates, alkyl polyglucosides, alkyl glucosides, acyl glutamide, and mixtures thereof.
- the surfactant system may comprise from 1% to 10% of a non-ionic surfactant, preferably from 3% to 9% of a non-ionic surfactant, more preferably from 5% to 9% of a non-ionic surfactant by total weight of the composition.
- the non-ionic surfactants may be selected from the group consisting of cocoamide monoethanolamine, lauramide monoethanolamine, cocoyl glucoside, lauroyl glucoside, decyl glucoside, and mixtures thereof.
- the co-surfactant of the personal cleansing composition may include an amphoteric surfactant or a zwitterionic surfactant.
- Suitable amphoteric or zwitterionic surfactants can include those described in U.S. Patent No. 5,104,646 and U.S. Patent No. 5,106,609 .
- Amphoteric surfactants can include those that can be broadly described as derivatives of aliphatic secondary and tertiary amines in which an aliphatic radical can be straight or branched chain and wherein an aliphatic substituent can contain from 8 to 18 carbon atoms such that one carbon atom can contain an anionic water solubilizing group, e.g., carboxy, sulfonate, sulfate, phosphate, or phosphonate.
- an anionic water solubilizing group e.g., carboxy, sulfonate, sulfate, phosphate, or phosphonate.
- Examples of compounds falling within this definition can be sodium 3-dodecyl-aminopropionate, sodium 3-dodecylaminopropane sulfonate, N-alkyltaurines such as the one prepared by reacting dodecylamine with sodium isethionate according to the teaching of U.S. Patent No. 2,658,072 , N-higher alkyl aspartic acids such as those produced according to the teaching of U.S. Patent No. 2,438,091 , and products described in U.S. Patent No. 2,528,378 .
- amphoteric surfactant included in the personal cleansing composition described herein may preferably selected from the group consisting of sodium lauroamphoacetate, sodium cocoamphoacetate, disodium lauroamphoacetate, disodium cocodiamphoacetate, and mixtures thereof.
- Zwitterionic surfactants suitable for use in the co-surfactants of the personal cleansing composition described herein may include those that are broadly described as derivatives of aliphatic quaternary ammonium, phosphonium, and sulfonium compounds, in which the aliphatic radicals can be straight or branched chains, and wherein one of the aliphatic substituents can contain from 8 to 18 carbon atoms and one can contain an anionic group, e.g., carboxy, sulfonate, sulfate, phosphate, or phosphonate.
- the zwitterionic surfactant included in the personal cleansing composition described herein may include one or more betaines, including cocoamidopropyl betaine.
- amphoteric or zwitterionic surfactant may be selected from cocamidopropyl betaine, lauramidopropyl betaine, coco-betaine, lauryl betaine, cocoamphoacetate, cocoamphodiacetate, lauroamphoacetate, lauroamphodiacetate, lauramine oxide, and mixtures thereof.
- the surfactant system may further comprise a zwitterionic surfactant selected from the group consisting of: lauryl hydroxysultaine, cocamidopropyl hydroxysultaine, coco-betaine, coco-hydroxysultaine, coco-sultaine, lauryl betaine, lauryl sultaine, and mixtures thereof.
- a zwitterionic surfactant selected from the group consisting of: lauryl hydroxysultaine, cocamidopropyl hydroxysultaine, coco-betaine, coco-hydroxysultaine, coco-sultaine, lauryl betaine, lauryl sultaine, and mixtures thereof.
- betaine zwitterionic surfactants may include coco dimethyl carboxymethyl betaine, cocoamidopropyl betaine (CAPB), coco-betaine, lauryl amidopropyl betaine (LAPB), oleyl betaine, lauryl dimethyl carboxymethyl betaine, lauryl dimethyl alpha-carboxyethyl betaine, cetyl dimethyl carboxymethyl betaine, lauryl bis-(2-hydroxyethyl) carboxymethyl betaine, stearyl bis-(2-hydroxypropyl) carboxymethyl betaine, oleyl dimethyl gamma-carboxypropyl betaine, lauryl bis-(2-hydroxypropyl)alpha-carboxyethyl betaine, and mixtures thereof.
- coco dimethyl carboxymethyl betaine cocoamidopropyl betaine (CAPB), coco-betaine, lauryl amidopropyl betaine (LAPB), oleyl betaine, lauryl dimethyl carboxymethyl betaine, lauryl dimethyl al
- sulfobetaines may include coco dimethyl sulfopropyl betaine, stearyl dimethyl sulfopropyl betaine, lauryl dimethyl sulfoethyl betaine, lauryl bis-(2-hydroxyethyl) sulfopropyl betaine and mixtures thereof.
- the zwitterionic surfactant can comprise or consist of cocamidopropyl betaine (CAPB), lauramidopropyl betaine (LAPB), and combinations thereof.
- CAPB cocamidopropyl betaine
- LAPB lauramidopropyl betaine
- the zwitterionic surfactant of the co-surfacant may be a betaine, preferably a betaine selected from the group consisting of cocamidopropyl betaine, lauramidopropyl betaine, coco betaine, lauryl betaine, coco hydroxysultaine and mixtures thereof; more preferably cocamidopropyl betaine.
- the co-surfactant may comprise at least a combination of a fatty acyl sarcosinate and a betaine; preferably a fatty acyl sarcosinate selected from the group consisting of sodium lauroyl sarcosinate, sodium myristoyl sarcosinate, sodium cocoyl sarcosinate, and mixtures thereof, and a betaine selected from the group consisting of coco hydroxysultaine, lauryl hydroxysultaine, coco betaine, and mixtures thereof; preferably sodium lauroyl sarcosinate and cocamidopropyl betaine.
- a fatty acyl sarcosinate selected from the group consisting of sodium lauroyl sarcosinate, sodium myristoyl sarcosinate, sodium cocoyl sarcosinate, and mixtures thereof
- a betaine selected from the group consisting of coco hydroxysultaine, lauryl hydroxysultaine, coco betaine, and mixtures thereof
- the personal cleansing composition may comprise a total amount from 0.6% to 55% of the surfactant system by total weight of the composition, preferably from 5% to 50% of the surfactant system by total weight of the composition, more preferably from 10% to 45% of the surfactant system by total weight of the composition.
- concentrations mentioned here are total concentration ranges of the surfactant system resulting from at least the addition of the total concentration ranges of the fatty acyl isethionate surfactant and the co-surfactant.
- the specified ranges are provided by weight and relate to the total weight of the personal cleansing composition.
- the personal cleansing composition comprises from 0.25% to 2% of a natural polysaccharide by weight of the composition.
- Carrageenans are structural polysaccharides of marine red algae of the Rhodophyceae class.
- ⁇ -Carrageenans, ⁇ -carrageenans, and furcellarans are linear polysaccharides whose backbone structure is based on a repeating disaccharide sequence of sulphate esters of (1 ⁇ 3) linked ⁇ -D-galactose and (1 ⁇ 4) linked 3,6-anhydro- ⁇ -D-galactose. They differ from each other in the number and position of sulphate groups.
- ⁇ -Carrageenan comprise ⁇ -D-galactopyranosyl residue sulphated at C-2 (instead of C-4 as in ⁇ - and ⁇ -carrageenans) and 2,6-di- O -sulfato- ⁇ -D-galactopyranosyl units (instead of 3,6-anhydro- ⁇ - D-galactopyranosyl residue) (See FIG. 1L ).
- Xanthan gum is an extracellular polysaccharide produced by the bacterium Xanthomonas campestris.
- the primary structure of xanthan gum consists of the cellulose-like backbone of (1 ⁇ 4)-linked ⁇ -DGlc p residues substituted, at O-3 of alternate glucose residues, with a trisaccharide.
- the trisaccharide consists of the ⁇ -D-Man p -(1 ⁇ 4)- ⁇ -D-Glc p A-(1 ⁇ 2)- ⁇ -D-Man p- (1 ⁇ unit ( FIG. 1M ).
- the natural polysaccharide is selected from the group consisting of xanthan gum, ⁇ -carrageenan, t-carrageenan, ⁇ -carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum.
- the natural polysaccharide may be preferably selected from the group consisting of xanthan gum, t-carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum.
- the natural polysaccharide may be a glucomannan such as locust bean gum and guar gum that is combined with xanthan gum and/or carrageenans such as ⁇ -carrageenan, t-carrageenan and ⁇ -carrageenan.
- glucomannan such as locust bean gum and guar gum
- carrageenans such as ⁇ -carrageenan, t-carrageenan and ⁇ -carrageenan.
- Such natural polysaccharide combination can have synergism and provide improved polymer liquid crystals, with an improved yield stress.
- the personal cleansing composition comprises a first and second phase, as identified and isolated by the Ultracentrifuge Test Method.
- the first phase of the personal cleansing composition is an isotropic and micellar surfactant phase.
- the rheologic profile of the first phase has been determined by the Ultracentrifuge Test Method.
- the first phase may exhibit a viscosity greater than 1 Pa.s at 25°C at a shear rate of 1 s -1 according to the Ultracentrifuge Test Method.
- the first phase having a viscosity greater than 1 Pa.s at 25°C at a shear rate of 1 s -1 according to the Ultracentrifuge Test Method can fit the well-known Carreau viscosity profile.
- a Carreau viscosity profile is typical of a micellar phase.
- the micellar phase can find its origin from the surfactant system comprising a fatty acyl isethionate surfactant and a co-surfactant.
- the first phase cannot rotate polarized light and cannot exhibit a birefringent optical morphology. Also, when isolated according to the Ultracentrifuge test, the first phase has a transmittance at 25°C and at 640 nm of 100% according to the Optical Clarity Test Method.
- the personal cleansing composition further comprises from 0.05 to 5%, preferably from 0.1% to 4%, more preferably from 0.5% to 3% of an electrolyte by weight of the composition.
- the addition of an electrolyte can help to elongate the micelles of the surfactant system in the first phase of the personal cleansing composition and to improve the flow viscosity.
- the electrolyte may be selected from the group of sodium or potassium citrate, calcium chloride, calcium bromide, zinc chloride, barium chloride, calcium nitrate, potassium chloride, sodium chloride, potassium iodide, sodium bromide, ammonium bromide, sodium sulfate, and mixtures thereof.
- the electrolyte may be preferably selected from the group of sodium or potassium citrate, calcium chloride, potassium chloride, sodium chloride, and mixtures thereof.
- the second phase of the personal cleansing composition is a polymer liquid crystalline phase.
- the second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase.
- the polymer liquid crystalline phase comprises polymer liquid crystals.
- the polymer liquid crystals include the natural polysaccharide.
- water-soluble polysaccharide are polymers that are used in predominantly aqueous compositions to provide a rheology benefit due to their solubility.
- Water-soluble polysaccharides in solution, absorb substantial amounts of water and increase viscosity of a composition as a result of their size and entanglements. In some cases, the polysaccharides can form gels due to molecular association.
- a relatively poor water-soluble polysaccharide or a polysaccharide that does not form readily a molecular gel in a composition cannot help to improve the performance of the composition. Indeed, a relatively poor water-soluble polysaccharide is generally quite dense and therefore tends to phase separate and become unstable in a composition.
- Polymer liquid crystalline phases are uncommon in personal cleansing compositions, since polymers generally favor disordered structures, especially at low concentration.
- Polymer polydispersity rules out liquid crystalline structures common to small molecules, i.e., smectic liquid crystalline phases such as lamellar and hexagonal.
- Nematic and/or cholesteric polymer liquid crystalline structures are known for some polysaccharides, however only at relatively high polysaccharide concentrations, i.e., at least from 10 wt%, preferably at least from 20 wt% of the polysaccharide by total weight of the composition.
- Inventors have surprisingly found that a natural polysaccharide can lead to the formation of a polymer liquid crystalline phase at a relatively low concentration of the said polysaccharide, and even in the presence of a surfactant system as described hereinbefore.
- a natural polysaccharide can lead to the formation of a polymer liquid crystalline phase, at a level from 0.25% to 2% of a natural polysaccharide by weight of the composition.
- the second phase being a polymer liquid crystalline phase provides a structure for the personal cleansing compositions.
- Such structure is characterized by specific structural features:
- the second phase of the personal cleansing composition is a polymer liquid crystalline phase. Indeed, the second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase.
- the polymer liquid crystalline phase comprises polymer liquid crystals.
- the polymer liquid crystals include the natural polysaccharide.
- the second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase.
- the polymer liquid crystalline phase comprises polymer liquid crystals. Microscopic observations indicate that the polymer liquid crystals form elongated structures, and thus have relatively high degrees of orientational order.
- the polymer liquid crystals may form elongated structures, namely elongated colloidal structures.
- the polymer liquid crystals may be nematic or cholesteric.
- the centers of gravity of the polymer liquid crystals are arranged at random, consequently no positional long-range order exists.
- the axes of all polymer liquid crystals are oriented in a specific direction.
- the arrangement of the polymer liquid crystals are similar to the ones of nematic subclass, however only an orientational order exists in the cholesteric subclass.
- the cholesteric subclass is characterized by the fact that the direction of the long axes of the molecules changes continuously within the sample. This leads to a twist about an axis perpendicular to the long axes of the molecules. If the pitch-of the helical structure agrees with the wavelength of the visible light, selective reflection of monochromatic light can be observed. This effect may lead to the iridescent colors often observed in cholesteric phases.
- the natural polysaccharide may be present in the polymer liquid crystalline phase at a level from 1% to 30%, preferably from 2% to 25%, more preferably from 4% to 10.5% by total weight of the polymer liquid crystalline phase.
- the polymer liquid crystals of the second phase may comprise at least a combination of the natural polysaccharide and one ingredient of the surfactant system.
- the polymer liquid crystals of the second phase may preferably comprise at least a combination of the natural polysaccharide selected from the group consisting of xanthan gum, t-carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum; and the fatty acyl isethionate surfactant.
- the polymer liquid crystals of the second phase may more preferably comprise at least a combination of xanthan gum a fatty acyl isethionate surfactant; and optionally a co-surfactant.
- a natural polysaccharide in an aqueous personal cleansing composition comprising a surfactant system can form a polymer liquid crystalline phase.
- the polymer liquid crystalline phase can form elongated structures which are very efficient at forming an associative network, conferring a structure.
- the elongated structures, forming the associative network can be further characterized by a measurable yield stress, and at surprisingly a relatively low level of the natural polysaccharide.
- the associative network may form a relatively weak gel or a physical gel.
- the polymer liquid crystals of the polymer liquid crystalline phase may be notably soluble during use. Consumer products such as body wash and shampoo are used with water.
- the polymer liquid crystals can dissolve during use with additional water, which increases the viscosity of the dilute aqueous micelles of the personal cleansing composition, resulting in a lather improvement. For example, the increased dilute micelle viscosity can form a lather that is relatively denser and creamier. Those attributes may be desirable for a personal cleansing composition.
- the personal cleansing composition may exhibit a yield stress value ⁇ y from 0.005 Pa to 3 Pa, preferably from 0.02 Pa to 2 Pa, more preferably from 0.03 Pa to 1 Pa, even more preferably from 0.05 Pa to 0.8 Pa according to the Herschel-Bulkley Rheology Test Method as disclosed herein.
- the yield stress of the personal cleansing composition may be a further evidence of the presence of the polymer liquid crystalline phase.
- the yield stress of the personal cleansing composition can be defined as the elastic component characterizing the necessary structure of the composition to be highly effective at suspending any benefit agents, particulates, particles such as silica and titanium oxide, microcapsules, oils, droplets, and mixtures thereof in the personal cleansing composition.
- a polymer liquid crystalline phase and the yield stress value ⁇ y as set out above are the features responsible to provide a personal cleansing composition highly effective at suspending particulates, particles such as silica and titanium oxide, microcapsules, oils, droplets, and mixtures thereof.
- the polymer liquid crystalline phase and optionally the yield stress value ⁇ y as set out above are the features that can help to improve the distribution of benefit agents associated with the personal cleansing composition: skin or hair care ingredients, perfumes, ...
- the personal cleansing composition may also exhibit a flow viscosity from 3 Pa.s to 100 Pa.s at 25°C at a shear rate of 1.5 s -1 according to the Flow Viscosity Test Method as disclosed herein.
- the structure provided by the polymer liquid crystalline phase may also characterized by yield stress and the flow viscosity of the composition, imparting a relatively high stability for the personal cleansing composition.
- the personal cleansing composition may exhibit a transmittance at 25°C and at 640 nm of from 4% to 95%, preferably from 10% to 93%, more preferably from 25% to 90% according to the Optical Clarity Test Method as disclosed herein.
- the personal cleansing composition without a natural polysaccharide forms typically micelles having relatively high transmittance and typical micelle rheology.
- the transmittance of the composition decreases. The decrease of transmittance is due to the formation of a second phase which is a polymer liquid crystalline phase.
- compositions described herein may include a variety of optional components to tailor the properties and characteristics of the composition.
- suitable optional components are well known and can generally include any components which are physically and chemically compatible with the essential components of the compositions described herein.
- Optional components should not otherwise unduly impair product stability, aesthetics, or performance.
- Individual concentrations of optional components can generally range from 0.001% to 10%, by weight of the composition.
- Optional components can be further limited to components which will not impair the clarity of a translucent composition.
- Optional components may include, but are not limited to, conditioning agents (including hydrocarbon oils, fatty esters, silicones), cationic polymers, anti-dandruff actives, and chelating agents.
- Additional suitable optional ingredients include but are not limited to encapsulated and non-encapsulated perfumes or fragrances, colorants, particles, anti-microbials, foam boosters, antistatic agents, moisturizing agents, propellants, self-foaming agents, pH adjusting agents and buffers, preservatives, pearlescent agents, opacifiers, sensates, suspending agents, solvents, diluents, anti-oxidants, vitamins and combinations thereof.
- CTFA Cosmetic Ingredient Handbook, Tenth Edition (published by the Cosmetic, Toiletry, and Fragrance Association, Inc., Washington, D.C.) (2004) (hereinafter "CTFA"), describes a wide variety of nonlimiting materials that can be added to the composition herein.
- the personal cleansing composition may be prepared according to the following method.
- the method for making the personal cleansing composition as set out hereinbefore may be provided and comprises the following steps, preferably in that order:
- the yield stress ⁇ y that may characterize the formation of second polymer liquid crystalline phase cannot be met.
- the step of adding the natural polysaccharide can also impact the orientation of the polymer liquid crystals of the second phase from non-elongated structures that are irregularly shaped with some regularly shaped such as generally spherical domains, to elongated structures.
- the natural polysaccharide needs to be added at the relatively low level from 0.25% to 2% of the natural polysaccharide, by weight of the composition with no milling.
- An increased of the shear when adding the polysaccharide may impede the organization of the second phase into elongated colloidal structures leading to the polymer liquid crystalline phase.
- the elongated colloidal structures forming the polymer liquid crystals may be characterized by the rheological properties.
- the natural polysaccharide may be added to the surfactant system from an aqueous solution comprising from 2% to 30%, preferably from 3% to 20%, more preferably from 4% to 10% of the natural polysaccharide or the chemically modified natural polysaccharide by weight of the aqueous solution.
- the natural polysaccharide may be added to the surfactant system by diluting the natural polysaccharide in water at a dilution level from 2% to 30%, preferably from 3% to 20%, more preferably from 4% to 10%.
- a dilution level of 2% means 2 g of the natural polysaccharide diluted in 100 g of water.
- the personal cleansing composition as set out hereinbefore may be obtained by the method for making the personal cleansing composition comprising the following steps, preferably in that order:
- the personal cleansing composition may be presented in typical personal cleansing formulations. They may be in the form of solutions, dispersion, emulsions, foams, and other delivery mechanisms.
- the personal cleansing composition may be extrudable or dispensable from a single chamber package.
- the personal cleansing compositions can be in the form of liquid, semi-liquid, cream, lotion or gel, or solid compositions intended for topical application to skin or hair.
- Examples of personal cleansing compositions can include but are not limited to shampoo, conditioning shampoo, hair conditioner, body wash, moisturizing body wash, foaming body wash, shower gels, a shower or bath cream, skin cleansers, cleansing milks, hair and body wash, in shower body moisturizer, gel, emulsion, oil, mousse or spray.
- the product forms contemplated for purposes of defining the personal cleansing compositions and methods are rinse-off formulations by which it is meant that the product is applied topically to the skin or hair and then subsequently (i.e., within minutes) rinsed away with water, or otherwise wiped off using a substrate or other suitable removal means.
- the personal cleansing composition as set out hereinabove may be used for improving the lather of the composition.
- the personal cleansing composition as set out hereinabove may be used for suspending benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- the personal cleansing composition can advantageously provide relatively improved ecotoxic or ecologically friendly environmental profile.
- the personal cleansing composition can help to provide good esthetic properties such as good foam, and is thick and creamy in texture, is silky to the touch and affords conditioning.
- the optical clarity of a personal cleansing composition is based on the measurement of the transmittance or light transmission (%T) of the personal cleansing composition at the visible wavelength of light of 640 nm.
- the transmittance of a personal cleansing composition is measured using a spectrophotometer such as a Thermo Fisher model Genesys 10 VIS (Thermo Fisher Inc, USA) and conventional polystyrene cuvettes with 1 cm path length at 25°C.
- the spectrophotometer is set to measure the transmittance (%T) at 640 nm wavelength.
- An identical cuvette filled with distilled water is used as the baseline reference.
- the personal cleansing composition is pipetted into the cuvette and ensured clear of air bubbles by centrifuging to remove air bubbles if present.
- the personal cleansing composition in the cuvette is placed in the holder compartment of the instrument, closing the compartment door, and the transmittance (%T) is recorded.
- the baseline reference will be adjusted accordingly.
- the flow viscosity and the yield stress properties of the personal cleansing composition via the Herschel Bulkley model are measured using a rheometer (available from. TA Instruments of New Castle, Del. AR-G2 series) in a flow mode.
- the measurements are conducted using a cone and plate geometry measuring system, having a diameter of 40 mm and degree cone angle. The measurement commences after about 10 seconds equilibration time at 25°C. If the composition is substantially structured, i.e. having a flow viscosity greater than 30 000 mPa.s or a yield stress greater than 1 Pa, a cross-hatched parallel plate geometry is used.
- the Herschel Bulkley model is described in " Rheometry of Pastes Suspensions and Granular Material” page 163, Philippe Coussot, John Wiley & Sons, Inc., Hoboken, New Jersey (2005 ).
- the flow viscosity and the yield stress value ⁇ y of a personal cleansing composition are measured using a 2-step measurement procedure.
- the rheometer procedure applied herein comprises the step of:
- step 1 the rheometer is set up and operated following standard procedures that include zeroing the gap, bearing friction calibration, and mapping.
- the geometry can be altered as needed to accommodate relatively high or low viscosity personal cleansing compositions, including selecting a parallel plate geometry for compositions containing particles and serrated geometries for compositions that can exhibit wall slip. Viscosity measurements are conducted at 25°C.
- step 2 increase the shear rate from 0.025 s -1 to 250 s -1 over 4 minutes, collecting 15 points per rate decade. Fit the results from the first step to the Herschel-Bulkley rheology model to determine the yield stress of the composition, fitting only the data at and below 0.01 s -1 shear rate. Determine the flow viscosity of the composition using the data from the step 2 at 1.5 s -1 .
- the light microscopy of liquid crystals is described in the Microscopy of Liquid Crystals, Norman Hartshorne, Microscopy publications, Ltd., Chicago, Illinois, U.S.A., 1974 . Methods for microscopic observation and evaluation are discussed in Chapter 1, p. 1-20, and ion Chapter 6, p. 79-90 .
- a preferred method for determining occurrence of polymer liquid crystals is by observing birefringence from relatively thin slices of the personal cleansing composition under a polarizing microscope.
- the characterization of the second phase of the personal cleansing composition in terms of birefringence, type of structures and orientation of the polymer liquid crystals can be thus viewed using an optical microscope such as a Zeiss Zxio Imager or its equivalent.
- An optical microscope such as a Zeiss Zxio Imager or its equivalent.
- a drop of the personal cleansing composition is loaded onto a clean glass slide and thinned using a coverslip.
- Views of the personal cleansing composition using 5x, 10x and 20x objective lenses using bright field can determine the presence of discrete structures of a second phase.
- Under the cross polarized light optical microscope one can rotate into view a polarizer and analyzer in cross-polarized position to determine if the second phase is ordered (transmits light) or isotropic (dark).
- An ordered second phase may characterize a polymer liquid crystalline phase.
- the second phase appears to contain structures that are generally longer than they are wide, including structures that are cylindrical, rod-like, ribbon-like, needle-like or fiber-like in appearance, the second phase is said to contain elongated structures.
- the structure of the second phase of the personal cleansing composition is evaluated with a sufficient number of fields by selecting a composition sample from different parts of the total composition (e.g. different parts of the composition contained in a container). At least 5 drops of the personal cleansing composition are used to prepare 5 microscope slides to obtain an average representation of the personal cleansing composition.
- the weight percentage of the first and second phases of the personal cleansing composition can be determined with the Ultracentrifuge Test using a Beckman Coulter Optima LE-80K ultracentrifuge with swinging bucket rotors (Beckman SW06Ti with 6 buckets, rotor diameter 165 mm, or equivalent).
- the tare weight of an ultracentrifuge tube is determined using a 3-place electronic balance to the closest milligram.
- the ultracentrifuge tube is filled with the personal cleansing composition and the gross weight is measured.
- the personal cleansing composition in the ultracentrifuge tube is placed in the ultracentrifuge bucket, capped, and placed on the ultracentrifuge rotor. The process is continued with other compositions placed on the rotor in counterbalancing positions.
- the rotor is positioned in the ultracentrifuge, the vacuum door closed, and run at a speed of 50,000 rpm, at 25°C for 15 hrs under vacuum. Then, the ultracentrifuge tubes are removed and observed.
- a first phase which is an optically transparent micellar surfactant phase is present in the personal cleansing compositions as a layer which is generally a top layer in the ultracentrifuge tube.
- the micellar surfactant phase has a viscosity which is measured by removing a portion of the first phase using a pipette, transferring it to a rheometer, and measuring the viscosity using the Flow Viscosity Test Method.
- the micelle viscosity as measured in step 2 of the Flow viscosity Test Method is reported as the Micelle Viscosity, in Pa.s.
- the entire micellar surfactant phase is carefully removed using a spatula and absorbent means such as paper towels without disturbing lower phases.
- the second phase which can be a birefringent phase, is a polymer liquid crystal phase.
- the second phase is generally simple to identify based on its structure and appearance.
- compositions (wt.%)
- the polymer liquid crystalline phase comprises polymer liquid crystals.
- the presence of polymer liquid crystals can be characterized by the fact that the personal cleansing composition comprises a yield stress ⁇ y measured according to the Herschel-Bulkley model.
- the personal cleansing composition may exhibit a yield stress ⁇ y from 0.005 Pa to 3 Pa according to the Herschel-Bulkley Rheology Test Method.
- the yield stress ⁇ y and the flow viscosity in some extent both increase.
- the personal cleansing composition is also statically stable.
- the polymer liquids crystals are characterized by their birefringence via optical microscopy. Microscopic observations indicate that the polymer liquid crystals form elongated structures, and thus have relatively high degrees of orientational order.
- FIG. 2 is a cross-polarized image of Example 5 taken with a cross-polarized light microscopy.
- the image of FIG. 2 demonstrates a dispersed second phase which is distributed in the form of elongated structures that are optically birefringent.
- the elongate structures E of the dispersed polymer liquid crystal phase contrast with the first phase being an isotropic and micellar surfactant phase that cannot rotate light and forming the background.
- the presence of the polymer liquid crystalline phase provides the structural feature of the personal cleansing composition that contributes to the relatively high flow viscosity.
- the personal cleansing composition can suspend air bubbles. After 24 hours, the presence of air bubbles within a personal cleansing composition is a further evidence of a structure.
- the personal cleansing composition is provided with a structure characterized by a polymer liquid crystalline phase and optionally a significant yield stress.
- Such structure can suspend one or more benefit agents such that hair care or skin care benefits agents, particulates, particles, oils, liquid droplets.
- the transmittance of the personal cleansing composition is relatively high when no xanthan gum was added to the composition.
- the personal cleansing composition without xanthan gum forms typically micelles having relatively high transmittance and typical micelle rheology.
- the transmittance of the composition decreases. The decrease of transmittance is not only due to the insoluble character of xanthan gum in the first phase being an isotropic and micellar surfactant phase, but also due to the formation of a second phase which is a polymer liquid crystalline phase.
- micellar surfactant phase was isolated by the Ultracentrifuge Test Method. Indeed, two distinct phases were observed and separated for Examples 7-9. A top phase comprising a transparent micellar phase was separated from a lower and denser second phase. The second phase rotates polarized light. The measured micelle viscosities for Examples 7-9 show that the micelles of the micellar surfactant phase can contribute to the flow viscosity of the personal cleansing composition.
- the polymer liquid crystalline phase that only represents for instance 5.3% wt. of the total composition in Example 7 can effectively triple the micellar viscosity to lead to the flow viscosity of the composition.
- the relatively denser second phase was observed to sediment, which is also another characteristic of a liquid crystalline phase enriched with the natural polysaccharide and not a surfactant liquid crystal.
- Surfactant liquid crystals are known to provide creaminess and not to sediment due to their hydrocarbon enrichment.
- compositions (wt.%)
- the personal cleansing compositions useful herein may need a sufficient flow viscosity by adjusting either the amount of the natural polysaccharide, or the total amount of surfactant, optionally with the amount of the electrolyte, e.g. sodium chloride.
- Examples 10 and 11 show that sodium chloride can optionally help to increase the flow viscosity.
- Examples 12, 13 and 14 demonstrate that a respective total amount of 15%wt. or 20%wt. of total surfactant by total weight of the composition with the addition of a natural polysaccharide allows reaching a sufficient flow viscosity without adding an electrolyte such as sodium chloride.
- compositions (wt.%)
- the personal cleansing compositions can be prepared using a mixture of natural polysaccharides such as xanthan gum with locust bean gum or xanthan gum with guar gum; or using a carrageenan like ⁇ -carrageenan.
- xanthan gum was diluted in water as a level of 5-10% by weight (i.e. 5-10 g of xanthan gum in 100 g of water) as a homogeneous aqueous gel with stirring and via a vortex agitation using a Speedmixer.
- the xanthan gum preparation was added and stirred into the composition comprising all the other ingredients as the final step and swirled for 5-10 seconds using a vortexer.
- compositions (wt.%)
- the natural polysaccharide e.g. xanthan gum was added as a dry powder using a Quadro mixer to water, the surfactant system (sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate), followed by the rest of the ingredients of the batch at 70°C, with 6 subsequent theoretical passes following incorporation to provide high energy milling to improve xanthan gum incorporation.
- the surfactant system sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate
- the natural polysaccharide e.g. xanthan gum was added as a dry powder using a pilot plant scale Quadro mixer with no additional milling after incorporation to the batch comprising water, the surfactant system (sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate), followed by the rest of the ingredients.
- the surfactant system sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate
- the natural polysaccharide e.g. xanthan gum was added through a 250 mesh US Standard Sieve onto the moving top surface of the batch comprising the surfactant system (sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate) at 70°C, over several minutes, with no milling or pumping, followed by the rest of the ingredients.
- the surfactant system sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate
- the yield stress ⁇ y that may characterize the formation of second polymer liquid crystalline phase cannot be met.
- the addition method can also impact the orientation of the polymer liquid crystals of the second phase from non-elongated structures that are irregularly shaped with some regularly shaped such as generally spherical domains, to elongated structures.
- Example 21 has a yield stress ⁇ y of 0.242 Pa that enables to suspend silica particles without settling.
- the personal cleansing compositions having a second polymer liquid crystalline phase and a sufficient yield stress ⁇ y can help to suspend benefit agents.
- the polymer liquid crystalline phase can help to provide structuring benefits, in particular the suspension of particles or insoluble liquid droplets throughout the personal cleansing composition without significant settling of such particles or droplets toward the bottom of the container and/or without significant raising or creaming of such particles or droplets toward the top of the container of the composition.
- compositions (wt.%)
- FIGS. 3A and 3B are related to the flow viscosity profile of personal cleansing compositions comprising xanthan gum added dry or added from a 5% aqueous solution to the composition, respectively upon preparation of the compositions or after 3 days aging at ambient temperature.
- Figure 4B demonstrates that even allowing the personal cleansing compositions to age 3 days at ambient temperature, the flow viscosity profile has not been altered. Hence, there is no apparent subsequent hydration of the natural polysaccharide that occurs after the initial processing.
- FIGS. 4C and 4D are related to the yield stress profile of personal cleansing compositions comprising xanthan gum added dry or added from a 5% aqueous solution to the composition, respectively upon preparation of the compositions or after 3 days aging at ambient temperature.
- Figure 4D demonstrates that even allowing the personal cleansing compositions to age 3 days at ambient temperature, the yield stress profile has not been altered. Hence, the structure provided by the polymer liquid crystals forms a network that is stable after its initial formation.
Description
- The present application generally relates to personal cleansing compositions, their methods of making such compositions, and their uses. The personal cleansing compositions have desirable rheologic properties and structure when the personal cleansing composition has a surfactant system comprising a fatty acyl isethionate surfactant and when the personal cleansing composition is free of alkyl sulfate and alkyl ether sulfate type of surfactants.
- Personal cleansing compositions have been traditionally marketed in a variety of forms such as bar soaps, creams, lotions, and gels. Typically, these products must satisfy a number of criteria to be acceptable to consumers. These criteria include cleansing effectiveness, skin feel, mildness to skin, hair, and ocular mucosae, and lather volume. Ideal personal cleansers should gently cleanse the skin or hair, cause little or no irritation, and should not leave the skin or hair overly dry after frequent use.
- Anionic surfactants are widely used in personal cleansing compositions. Many of these anionic surfactants contain elongated micelles and are viscoelastic, which is of great importance, especially in the design of shampoos and body washes. In most personal cleansing compositions, alkyl sulfate and alkyl ether sulfate as the anionic surfactants predominate.
- The formulation of environmentally friendly personal cleansing compositions is becoming a major challenge for satisfying a new expectation of consumers, in particular that of ecologically designed and/or natural products. It becomes necessary to propose personal cleansing compositions free of alkyl sulfate and alkyl ether sulfate, which have good cosmetic qualities, mainly in terms of viscosity and lather.
- Consumers prefer sulfate-free personal cleansing compositions due to perceived mildness and desirable sensorial experience. However, sulfate-free personal cleansing compositions are difficult to thicken sufficiently to afford the user good usage qualities. Two approaches are leveraged to attempt to thicken such formulas. One approach for instance is to use high levels of surfactants to benefit from the self-assembling properties of such ingredients. This approach is most common but it is also costly. The second approach for instance is to use high levels of rheology modifiers which can adversely impact the properties of the composition such as by decreasing the foam and ease of distribution of the composition.
-
WO 2011/120780 A2 is related to super mild surfactant systems, optionally in combination with skin or hair benefit agent. - There is a need today to provide personal cleansing products that comprise alternative mild surfactant systems with relatively improved ecotoxic or ecologically friendly environmental profile.
-
WO2008/074617 A1 is related to liquid cleanser compositions which can use fatty acyl isethionates mixtures, regardless of free fatty acid content of isethionates mixture or chain length distribution of isethionates mixture. - Personal cleansing compositions having a surfactant system comprising a fatty acyl isethionate surfactant and being free of alkyl sulfate and alkyl ether sulfate type of surfactants have been developed. Fatty acyl isethionates are mild anionic surfactants highly desirable in personal cleansing products for hair or skin, because fatty acyl isethionates can lather well, are mild to the skin and have good emollient properties.
- However, fatty acyl isethionates are not readily used in liquid personal cleansing compositions, because of their relatively low solubility in water. This may result in unstable personal cleansing compositions which can exhibit inconsistent rheology profiles.
- Hence, there is still a need to provide a personal cleansing composition comprising a fatty acyl isethionate surfactant and being free of alkyl sulfate and alkyl ether sulfate type of surfactants and having a satisfactory consistent rheology profile.
- Thus, there remains a need for a personal cleansing composition, which is effective at cleaning even while containing lower number of active surfactants than typical cleansing products, but also still possesses good esthetic properties such as good foam, and is thick and creamy in texture, is silky to the touch and affords conditioning.
- Benefit agents in the form of solid particles, liquid droplets are of interest for personal cleansing compositions. Benefit agents can be used as pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers. Thus, there is still a need to provide a personal cleansing composition comprising a fatty acyl isethionate surfactant imparted with a sufficient structure to hold benefit agents such as solid particles, liquid droplets.
- A personal cleansing composition is provided and comprises:
- (a) a surfactant system, wherein the surfactant system comprises:
- from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition;
- from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15% of a co-surfactant by weight of the composition, wherein the co-surfactant is selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant, and mixtures thereof;
- (b) from 0.25% to 2% of a natural polysaccharide by weight of the composition, wherein the natural polysaccharide is selected from the group consisting of xanthan gum, κ-carrageenan gum, t-carrageenan gum, λ-carrageenan gum, xanthan gum/locust bean gum and xanthan gum/guar gum, wherein the total amount of natural polysaccharide ranges from 0.25 to 2% by weight of the composition;
- wherein the personal cleansing composition comprises a first and second phase,
- wherein the first phase is an isotropic and micellar surfactant phase;
- wherein the second phase is a polymer liquid crystalline phase;
- wherein the second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase;
- wherein the polymer liquid crystalline phase comprises polymer liquid crystals, wherein the polymer liquid crystals include the natural polysaccharide;
- wherein the composition is free of from alkyl sulfate and alkyl ether sulfate type of surfactants;
- and wherein the personal cleansing composition comprises from 0.05% to 5% of an electrolyte by weight of the composition.
- Another aspect is related to a method for making the personal cleansing composition according to any of the preceding claims comprising the following steps, preferably in that order:
- (a) providing a surfactant system, wherein the surfactant system comprises:
- from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition;
- from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15%, of a co-surfactant, wherein the co-surfactant is selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant and mixtures thereof, by weight of the composition;
- (b) adding from 0.25% to 2% of a natural polysaccharide, by weight of the composition with no milling.
- A personal cleansing composition is provided and can be made by the method as set out hereinbefore.
- The personal cleansing composition as set out hereinbefore is used for improving the lather of the composition.
- Alternatively, the personal cleansing composition as set out hereinbefore is used for suspending benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- The polymer liquid crystals may form elongated structures and may be nematic or cholesteric.
- While the specification concludes with claims particularly pointing out and distinctly claiming the present invention, it is believed that the same will be better understood from the following description read in conjunction with the accompanying drawings in which:
-
FIG. 1A represents the repeating units of cellulose; -
FIG. 1B is related to the structure of carboxymethylcellulose; -
FIG. 1C is related to the structure of methylcellulose; -
FIG. 1D is related to the structures of mannans and galactomannans; -
FIG. 1E is related to the structure of xyloglucans; -
FIG. 1F is related to the structure of arabinoxylans; -
FIG. 1G is related to the structure of mixed linkage (1→3)(1→4) β-D-glucans; -
FIG. 1H is related to the structure of arabinogalactans; -
FIG. 1I is related to the structure of inulin; -
FIG. 1J is related to the structure of levan; -
FIG. 1K is related to the structural features of alginates; -
FIG. 1L is related to the structures of κ-carrageenan, ι-carrageenan and λ-carrageenan; -
FIG. 1M is related to the structure of xanthan gum; -
FIG. 1N is related to the structure of pullulan; -
FIG. 1O is related to the structures of chitin and chitosan; -
FIG. 2 represents a cross-polarized image of an example according to the present invention taken with a cross-polarized microscopy; -
FIG. 3 represents the optical clarity of the personal cleansing composition by plotting the transmittance at 640 nm versus the weight fraction of the natural polysaccharide; and -
FIGS. 4A and 4B are related to the flow viscosity profile of personal cleansing compositions comprising xanthan gum added dry or added from a 5% aqueous solution to the composition, respectively upon preparation of the compositions or after 3 days aging at ambient temperature; and -
FIGS. 4C and 4D are related to the yield stress profile of personal cleansing compositions comprising xanthan gum added dry or added from a 5% aqueous solution to the composition, respectively upon preparation of the compositions or after 3 days aging at ambient temperature - In this document, including in all embodiments of all aspects of the present invention, the following definitions apply unless specifically stated otherwise.
- All percentages are by weight (w/w) of the respective composition, unless otherwise specified. All ratios or percentages are weight ratios or weight percentages unless specifically stated otherwise. "% wt." means percentage by weight. References to "parts" e.g. a mixture of 1 part X and 3 parts Y, is a ratio by weight. When more than one composition is used during a treatment, the total weight to be considered is the total weight of all the compositions applied on the hair or skin simultaneously (i.e. the weight found "on head"), unless otherwise specified.
- "QSP" or "q.s." means sufficient quantity for 100% or for 100g. "+/-" indicates the standard deviation. All ranges are inclusive and combinable. The number of significant digits conveys neither a limitation on the indicated amount nor on the accuracy of the measurement.
- All measurements are understood to be made at 20°C and at ambient conditions, where "ambient conditions" means at 1 atmosphere (atm) of pressure and at 65% relative humidity, unless otherwise stated. "Relative humidity" refers to the ratio (stated as a percent) of the moisture content of air compared to the saturated moisture level at the same temperature and pressure. Relative humidity can be measured with a hygrometer, in particular with a probe hygrometer from VWR® International.
- Herein "min" means "minute" or "minutes". Herein "mol" means mole. Herein "g" following a number means "gram" or "grams". "Ex." means "example". All amounts as they pertain to listed ingredients are based on the active level ("solids") and do not include carriers or by-products that may be included in commercially available materials.
- Herein "Comp. Ex." means comparative example; and "Ex." means example.
- Herein, "comprising" means that other steps and other ingredients can be included in addition. "Comprising" encompasses the terms "consisting of" and "consisting essentially of". The compositions, methods, and uses of the present invention can comprise, consist of, and consist essentially of the elements and limitations of the invention described herein, as well as any of the additional or optional ingredients, components, steps, or limitations described herein. Embodiments and aspects described herein may comprise or be combinable with elements, features or components of other embodiments and/or aspects despite not being expressly exemplified in combination, unless an incompatibility is stated.
- As used herein, the articles including "a" and "an" when used in a claim, are understood to mean one or more of what is claimed or described.
- The terms "include," "includes," and "including," as used herein are meant to be non-limiting.
- Where amount ranges are given, these are to be understood as being the total amount of said ingredient in the composition, or where more than one species fall within the scope of the ingredient definition, the total amount of all ingredients fitting that definition, in the composition. The concentrations mentioned for a given ingredient are total concentration ranges in case more than one of the given ingredient is present. The specified ranges are provided by weight and relate to the total weight of the personal cleansing composition, unless specifically stated otherwise.
- For example, if the composition comprises from 1% to 5% fatty alcohol, then a composition comprising 2% stearyl alcohol and 1% cetyl alcohol and no other fatty alcohol, would fall within this scope.
- The amount of each particular ingredient or mixtures thereof described hereinafter can account for up to 100% (or 100%) of the total amount of the ingredient(s) in the composition.
- The term "mixtures" as used herein is meant to include a simple combination of materials and any compounds that may result from their combination.
- The term "molecular weight" or "M.Wt." as used herein refers to the weight average molecular weight unless otherwise stated. The weight average molecular weight can be measured by gel permeation chromatography ("GPC").
- The term "personal cleansing composition" as used herein refers to compositions intended for topical application to the skin or hair for cleansing. The personal cleansing composition may be aqueous.
- The term "isotropic" as used herein refers to a particular phase of the composition wherein the structure is "identical along any three orthogonal directions in space, and is therefore dark or 'nonbirefringent' when viewed between crossed polarized light filters. (One direction is 'orthogonal' to another if the vector component of the first, in the direction of the second, is zero.)" (Laughlin, R. G. (1994). "The Aqueous Phase Behavior of Surfactants," 182, 8.2).
- The term "anisotropic" as used herein refers a particular phase of the composition wherein the structure exhibits properties with different values when measured in different directions. An anisotropic phase is not identical along any three orthogonal directions in space, and is birefringent when viewed between crossed polarized light filters.
- The term "liquid crystals" as used herein refers to anisotropic fluids or mesophases. Liquid crystals as used herein are polymeric liquid crystals. The polymeric liquid crystalline phase of the personal cleansing composition is lyotrophic meaning that the polymer liquid crystalline phase contains a solvent, namely water. This type of polymer liquid crystals is distinguished in the art from thermotropic, heat, and magnetically induced liquid crystals. The liquid crystalline state exists between the boundaries of the solid crystalline phase and the isotropic liquid phase (i.e. an intermediate between the three dimensionally ordered crystalline state and the disordered dissolved state). In this state, some of the molecular order characteristics of the solid crystalline phase are retained in the liquid state because of the molecular structure and short range intermolecular interaction. Liquid crystals are also known as anisotropic fluids, a fourth state of matter, polymer association structure or mesophases. Those terms are used interchangeably. Lyotropic means a material is formed through changes in solution behavior (and hence by definition contains a solvent, e.g. water) of the ingredients. The changes involve thermal and salvation energies. The term "lyotropic liquid crystal" as used herein, refers to a liquid crystalline phase distinctive by the presence of birefringence under polarized light microscopy.
- The term "birefringence" as used herein refers the property of the material to capable of transmitting light when viewed with cross polars under static conditions.
- The term "micelle" as used herein refers structure comprising individual surfactant molecules aggregated to form a hydrophobic core region with externally facing polar head groups in equilibrium with surfactant monomers in a polar phase, having a characteristic dimension that is a single digit multiple of the surfactant length, i.e., generally less than about 10 nm in diameter.
- The term "rinse-off" as used herein means the intended product usage includes application to skin and/or hair followed by rinsing and/or wiping the product from the skin and/or hair within a few seconds to minutes of the application step. The product is generally applied and rinsed in the same usage event, for example, a shower.
- The term "statically stable" as used herein, unless otherwise specified, refers to a personal cleansing composition that comprises at least two compositions that maintain at least two "separate" phases with at least two separate benefit concentration zones contained within a single chamber package at ambient conditions for a period of at least about 180 days. Alternatively, static stability can be determined by accelerated protocol at elevated temperature. One accelerated protocol is based on passing static stability after 10 days at 50° C. By "separate" is meant that there is substantially no mixing of compositions contained in the zones, detected by the benefit analysis method, described hereinafter, prior to dispensing of the composition.
- The term "substantially free of" as used herein means less than 1%, less than 0.8%, less than 0.5%, less than 0.3%, or less than an immaterial amount of a stated ingredient by total weight of the composition.
- The term "free of" as used herein refers to no detectable amount of the stated ingredient.
- The term "surfactant" as used herein refers to amphiphilic molecules which can aggregate to form micelles and other surfactant structures, which are soluble in an aqueous phase and contribute to foaming during a cleansing event, i.e., stabilizing an air interface.
- The term "structured" as used herein means having a rheology that confers stability on the personal cleansing composition. The degree of structure is determined by rheologic characteristics such as the yield stress determined by the Herschel-Bulkley Rheology Test Method or the viscosity obtained by the Ultracentrifugation Test Method, all in the Test Method section below.
- The objects of the present invention are to provide personal cleansing compositions and uses of the compositions as described in the Summary or as described hereinbelow for fulfilling the technical effects or goals as set out herein. These objects and other advantages as may be apparent to those skilled in the art can be achieved through the present invention, which is described in the above Summary of the Invention and Detailed Description of the invention and which is defined in the claims which follow.
- Thickeners are useful for adjusting the viscosity and the rheological behavior of personal cleansing compositions in order to make them easy to pour and dose. Structurants thicken, but also provide a suspensive benefit, allowing ingredients such as oils, particulates, and the like, to be stably suspended in the personal cleansing composition.
- The inventors have surprisingly found that instead of thickening the composition, a natural polysaccharide such as xanthan gum can lead to the formation of a polymer liquid crystalline phase in the personal cleansing composition and even at a relatively low amount from 0.25% to 2% of the natural polysaccharide total weight of the composition.
- The personal cleansing composition comprises a first and second phase. The first phase is an isotropic and micellar surfactant phase. The second phase is a polymer liquid crystalline phase. The first and second phases of the personal cleansing composition may be separated by ultracentrifuge. The polymer liquid crystalline phase is characterized by birefringence. The polymer liquid crystalline phase and the personal cleansing composition may have a specific rheologic profile.
- The polymer liquid crystalline phase of the composition provides the structure of the personal cleansing composition. The polymer liquid crystalline phase comprises polymer liquid crystals. The polymer liquid crystals include the natural polysaccharide. The polymer liquid crystalline phase can help the personal cleansing composition to suspend a benefit agent selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- The personal cleansing composition may have specific yield stress τy, as an elastic component of the composition, measured according to the Herschel-Burlley Rheology Test Method.
- In order to provide further structure and suspend further benefit agents, the personal cleansing composition may comprise the polymer liquid crystalline phase, and at a sufficient yield stress τy from 0.005 to 3 Pa measured according to the Herschel-Bulkley Rheology Test Method.
- Furthermore, for providing a stable personal cleansing composition comprising fatty acyl isethionate surfactant, the personal cleansing composition may also exhibit a flow viscosity from 3 Pa.s to 100 Pa.s at 25°C at a shear rate of 1.5 s-1 according to the Flow Viscosity Test Method.
- Hence, it has been found that a stable personal cleansing composition able to suspend benefits agents and comprising fatty acyl isethionate can be provided when the composition comprises a polymer liquid crystalline phase comprising polymer liquid crystals, wherein the polymer liquid crystals include the natural polysaccharide.
- The personal cleansing composition is free of alkyl sulfate and alkyl ether sulfate type of surfactant. Preferably, the personal cleansing composition does not comprise any alkyl sulfate which comprises C12-C18 alkyl sulfate or any alkyl ether sulfate including alkyl glyceryl ether sulfates.
- The personal cleansing composition may not comprise any alkyl ether sulfates which are those having the formula:
RO(CH2CH2O)nSO3M
wherein R is an alkyl or alkenyl having 8 to 18 carbons, preferably 12 to 18 carbons, n has an average value of greater than at least 0.5, preferably between 2 and 3; and M is a solubilizing cation such as sodium, potassium, ammonium or substituted ammonium. - The personal cleansing composition may not comprise any ammonium and sodium lauryl ether sulfates.
- If the personal cleansing composition does contain alkyl sulfate and/or alkyl ether sulfate type of surfactant, its content of such a weight proportion of: alkyl sulfates or alkyl ether sulfate type surfactant is less than or equal to the sum of 0.6, more preferably less than or equal to the sum of 0.2, even more preferably equal to 0.
- The personal cleansing composition comprises a surfactant system. The surfactant system comprises from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition; and from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15% of a co-surfactant by weight of the composition.
- The fatty acyl isethionate surfactant may be defined as an isethionate according to the general formula (I):
- preferably wherein R1 is a saturated or unsaturated, straight or branched alkyl or alkenyl, preferably an alkyl chain with from 6 to 30 carbon atoms, preferably from 8 to 22 carbon atoms, more preferably from 9 to 18 carbon atoms, R2 and R3 are H, and M+ is an alkali metal, preferably sodium, potassium; or M+ is an ammonium cation; or
- more preferably wherein R1 is a saturated or unsaturated, straight or branched alkyl chain with from 9 to 18 carbon atoms, R2 and R3 are H, and M+ is sodium or an ammonium cation.
- Suitable fatty acyl isethionate surfactants may include the reaction product of fatty acids esterified with isethionic acid and neutralized with sodium hydroxide. Suitable fatty acids for isethionate surfactants can be derived from coconut oil or palm kernel oil, for instance. Additional examples of suitable isethionic anionic surfactants are described in
U.S. Pat. No. 2,486,921 ;U.S. Pat. No. 2,486,922 ; andU.S. Pat. No. 2,396,278 . - The fatty acyl isethionate surfactant may be selected from the group consisting of sodium lauroyl isethionate, sodium lauroyl methyl isethionate, sodium oleoyl isethionate, sodium oleoyl methyl isethionate, sodium stearoyl isethionate, sodium stearoyl methyl isethionate, sodium myristoyl isethionate, sodium myristoyl methyl isethionate, sodium palmitoyl isethionate, sodium palmitoyl methyl isethionate, sodium cocoyl isethionate, sodium cocoyl methyl isethionate, a blend of stearic acid and sodium cocoyl isethionate, ammonium cocoyl isethionate, ammonium cocoyl methyl isethionate, and mixtures thereof.
- The fatty acyl isethionate surfactant may be preferably selected from the group consisting of sodium lauroyl isethionate, sodium myristoyl isethionate, sodium palmitoyl isethionate, sodium stearoyl isethionate, sodium oleoyl isethionate, sodium cocoyl isethionate, ammonium cocoyl isethionate, and mixtures thereof.
- The fatty acyl isethionate surfactant may be more preferably selected from the group consisting of sodium lauroyl isethionate, sodium cocoyl isethionate, ammonium cocoyl isethionate, and mixtures thereof.
- Corresponding commercial products are available, for example, from the company Innospec under the trade name "Iselux®" and from Clariant or Uniquema under the trade names "Hostapon®" or "Arlatone®". Examples of other commercial fatty acyl isethionates that may be used can be Hostapon® surfactants from Clariant such as for sodium cocoyl isethionate: Hostapon® SCI-85C, Hostapon® SCI-78C, or a blend of stearic acid with sodium cocoyl isethionate: Hostapon® SCI-65C. Examples of other commercial fatty acyl isethionates that may be used can be "Jordapon®" surfactants from BASF such as Jordapon® CI prill or Jordapon® CI65; and sodium cocoyl isethionate from Yongan Daily Chemical Co. such as YA-SCI-85® or YA-SCI-65®.
- Fatty acyl isethionates surfactants are typically prepared by the reaction of an isethionate salt such as metal or ammonium isethionate and an a saturated or unsaturated, straight or branched, alkyl or alkenyl chain fatty acid having from 6 to 30 carbon atoms, preferably from 8 to 22 carbon atoms, more preferably from 6 to 18 carbon atoms. Depending on the processing conditions used, the resulting fatty acyl isethionate surfactant can be a mixture of 45 to 95% by weight of fatty acyl isethionates and 40 to 0 wt.% of free fatty acids, in addition to isethionates salts, typically less than 5 wt.%, and trace (less than 2 wt.%) of other impurities, by total weight of the resulting fatty acyl isethionate surfactant. A mixture of aliphatic fatty acids may be used for the preparation of commercial fatty acyl isethionates surfactants.
- The personal cleansing composition comprises a surfactant system. The surfactant system comprises from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition. The concentrations mentioned here are total concentration ranges in case more than one fatty acyl isethionate surfactant is present. The specified ranges are provided by weight and relate to the total weight of the personal cleansing composition.
- Fatty acyl isethionate surfactants have not typically been used in preparation of personal cleansing compositions because they might readily form solid crystals (when used alone and/or with a co-surfactant) and consequently might make it difficult to form stable liquid personal cleansing compositions.
- The surfactant system comprises from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15% of a co-surfactant by weight of the composition. The co-surfactant may be selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant and mixtures thereof.
- The anionic surfactant of the co-surfactant, being not an isethionate surfactant may be selected from the group consisting of fatty acyl sarcosinates, sulfosuccinates, sulfonates, sulfoacetates, acyl glycinates, acyl alaninates, acyl glutamates, lactates, lactylates, glucose carboxylates, amphoacetates, taurates, and mixtures thereof.
- In that case, alkyl is defined as a saturated or unsaturated, straight or branched alkyl chain with 6 to 30 carbon atoms, preferably with 8 to 22 carbon atoms, more preferably with 9 to 18 carbon atoms. In that case, acyl is defined as of formula R-C(O)-, wherein R is a saturated or unsaturated, straight or branched alkyl or alkenyl, preferably alkyl chain with 6 to 30 carbon atoms, preferably with 8 to 22 carbon atoms, more preferably with 9 to 18 carbon atoms.
- The fatty acyl sarcosinate may be a sarcosinate according to the general formula (II):
- Non-limiting examples of sarcosinates may be selected from the group consisting of sodium lauroyl sarcosinate, sodium cocoyl sarcosinate, sodium myristoyl sarcosinate, TEA-cocoyl sarcosinate, ammonium cocoyl sarcosinate, ammonium lauroyl sarcosinate, dimer dilinoleyl bis-lauroyl glutamate/lauroyl sarcosinate, disodium lauroamphodiacetate, lauroyl sarcosinate, isopropyl lauroyl sarcosinate, potassium cocoyl sarcosinate, potassium lauroyl sarcosinate, sodium oleoyl sarcosinate, sodium palmitoyl sarcosinate, TEA-lauroyl sarcosinate, TEA-oleoyl sarcosinate, TEA-palm kernel sarcosinate, and mixtures thereof.
- Preferably, the fatty acyl sarcosinate may be selected from the group consisting of sodium lauroyl sarcosinate, sodium myristoyl sarcosinate, sodium cocoyl sarcosinate, and mixtures thereof.
- Non-limiting examples of sulfosuccinate surfactants can include disodium N-octadecyl sulfosuccinate, disodium lauryl sulfosuccinate, diammonium lauryl sulfosuccinate, sodium lauryl sulfosuccinate, disodium laureth sulfosuccinate, tetrasodium N-(1,2-dicarboxyethyl)-N-octadecyl sulfosuccinnate, diamyl ester of sodium sulfosuccinic acid, dihexyl ester of sodium sulfosuccinic acid, dioctyl esters of sodium sulfosuccinic acid, and combinations thereof.
- Non-limiting examples of sulfonates can include alpha olefin sulfonates, linear alkylbenzene sulfonates, sodium laurylglucosides hydroxypropylsulfonate, and combinations thereof.
- Non-limiting examples of sulfoacetates can include sodium lauryl sulfoacetate, ammonium lauryl sulfoacetate, and combination thereof.
- Non-limiting examples of acyl glycinates can include sodium cocoyl glycinate, sodium lauroyl glycinate, and combination thereof.
- Non-limiting example of acyl alaninates can include sodium cocoyl alaninate, sodium lauroyl alaninate, sodium N-dodecanoyl-1-alaninate, and combinations thereof.
- Non-limiting examples of acyl glutamates can be selected from the group consisting of sodium cocoyl glutamate, disodium cocoyl glutamate, ammonium cocoyl glutamate, diammonium cocoyl glutamate, sodium lauroyl glutamate, disodium lauroyl glutamate, sodium cocoyl hydrolyzed wheat protein glutamate, disodium cocoyl hydrolyzed wheat protein glutamate, potassium cocoyl glutamate, dipotassium cocoyl glutamate, potassium lauroyl glutamate, dipotassium lauroyl glutamate, potassium cocoyl hydrolyzed wheat protein glutamate, dipotassium cocoyl hydrolyzed wheat protein glutamate, sodium capryloyl glutamate, disodium capryloyl glutamate, potassium capryloyl glutamate, dipotassium capryloyl glutamate, sodium undecylenoyl glutamate, disodium undecylenoyl glutamate, potassium undecylenoyl glutamate, dipotassium undecylenoyl glutamate, disodium hydrogenated tallow glutamate, sodium stearoyl glutamate, disodium stearoyl glutamate, potassium stearoyl glutamate, dipotassium stearoyl glutamate, sodium myristoyl glutamate, disodium myristoyl glutamate, potassium myristoyl glutamate, dipotassium myristoyl glutamate, sodium cocoyl/hydrogenated tallow glutamate, sodium cocoyl/palmoyl/sunfloweroyl glutamate, sodium hydrogenated tallowoyl glutamate, sodium olivoyl glutamate, disodium olivoyl glutamate, sodium palmoyl glutamate, disodium palmoyl glutamate, TEA-cocoyl glutamate, TEA-hydrogenated tallowoyl glutamate, TEA-lauroyl glutamate, and mixtures thereof.
- Non-limiting example of lactates can include sodium lactate.
- Non-limiting examples of lactylates can include sodium lauroyl lactylate, sodium cocoyl lactylate, and combination thereof.
- Non-limiting examples of glucose carboxylates can include sodium lauryl glucoside carboxylate, sodium cocoyl glucoside carboxylate, and combinations thereof.
- Non-limiting examples of alkylamphoacetates can include sodium cocoyl amphoacetate, sodium lauroyl amphoacetate, and combination thereof.
- Non-limiting examples of acyl taurates can include sodium methyl cocoyl taurate, sodium methyl lauroyl taurate, sodium methyl oleoyl taurate, and combinations thereof.
- Alternatively, the anionic surfactants of the co-surfactant may be selected from the group consisting of sodium tridecyl benzene sulfonate, sodium dodecyl benzene sulfonate, sodium lauroyl sarcosinate, sodium cocoyl sarcosinate, sodium lauroyl glycinate, sodium cocoyl glycinate, potassium lauroyl glycinate, potassium cocoyl glycinate, sodium lauroyl glutamate, potassium lauroyl glutamate, sodium cocoyl glutamate, potassium cocoyl glutamate, disodium lauroyl glutamate, dipotassium lauroyl glutamate, disodium cocoyl glutamate, dipotassium cocoyl glutamate, sodium lauroyl lactylate, and mixtures thereof.
- The surfactant system may comprise from 0.5% to 15%, preferably from 1% to 10%, more preferably from 2% to 5% of an anionic surfactant of the co-surfactant, being not an isethionate surfactant, preferably a fatty acyl sarcosinate, by total weight of the composition.
- The non-ionic surfactant of the co-surfactant may be selected from the group consisting of glucosides, alkyl amines, alcohol ethoxylates, alkyl polyglucosides, alkyl glucosides, acyl glutamide, and mixtures thereof.
- The surfactant system may comprise from 1% to 10% of a non-ionic surfactant, preferably from 3% to 9% of a non-ionic surfactant, more preferably from 5% to 9% of a non-ionic surfactant by total weight of the composition.
- The non-ionic surfactants may be selected from the group consisting of cocoamide monoethanolamine, lauramide monoethanolamine, cocoyl glucoside, lauroyl glucoside, decyl glucoside, and mixtures thereof.
- The co-surfactant of the personal cleansing composition may include an amphoteric surfactant or a zwitterionic surfactant. Suitable amphoteric or zwitterionic surfactants can include those described in
U.S. Patent No. 5,104,646 andU.S. Patent No. 5,106,609 . - Amphoteric surfactants can include those that can be broadly described as derivatives of aliphatic secondary and tertiary amines in which an aliphatic radical can be straight or branched chain and wherein an aliphatic substituent can contain from 8 to 18 carbon atoms such that one carbon atom can contain an anionic water solubilizing group, e.g., carboxy, sulfonate, sulfate, phosphate, or phosphonate. Examples of compounds falling within this definition can be sodium 3-dodecyl-aminopropionate, sodium 3-dodecylaminopropane sulfonate, N-alkyltaurines such as the one prepared by reacting dodecylamine with sodium isethionate according to the teaching of
U.S. Patent No. 2,658,072 , N-higher alkyl aspartic acids such as those produced according to the teaching ofU.S. Patent No. 2,438,091 , and products described inU.S. Patent No. 2,528,378 . - The amphoteric surfactant included in the personal cleansing composition described herein may preferably selected from the group consisting of sodium lauroamphoacetate, sodium cocoamphoacetate, disodium lauroamphoacetate, disodium cocodiamphoacetate, and mixtures thereof.
- Zwitterionic surfactants suitable for use in the co-surfactants of the personal cleansing composition described herein may include those that are broadly described as derivatives of aliphatic quaternary ammonium, phosphonium, and sulfonium compounds, in which the aliphatic radicals can be straight or branched chains, and wherein one of the aliphatic substituents can contain from 8 to 18 carbon atoms and one can contain an anionic group, e.g., carboxy, sulfonate, sulfate, phosphate, or phosphonate.
- The zwitterionic surfactant included in the personal cleansing composition described herein may include one or more betaines, including cocoamidopropyl betaine.
- Alternatively, the amphoteric or zwitterionic surfactant may be selected from cocamidopropyl betaine, lauramidopropyl betaine, coco-betaine, lauryl betaine, cocoamphoacetate, cocoamphodiacetate, lauroamphoacetate, lauroamphodiacetate, lauramine oxide, and mixtures thereof.
- The surfactant system may further comprise a zwitterionic surfactant selected from the group consisting of: lauryl hydroxysultaine, cocamidopropyl hydroxysultaine, coco-betaine, coco-hydroxysultaine, coco-sultaine, lauryl betaine, lauryl sultaine, and mixtures thereof.
- Examples of betaine zwitterionic surfactants may include coco dimethyl carboxymethyl betaine, cocoamidopropyl betaine (CAPB), coco-betaine, lauryl amidopropyl betaine (LAPB), oleyl betaine, lauryl dimethyl carboxymethyl betaine, lauryl dimethyl alpha-carboxyethyl betaine, cetyl dimethyl carboxymethyl betaine, lauryl bis-(2-hydroxyethyl) carboxymethyl betaine, stearyl bis-(2-hydroxypropyl) carboxymethyl betaine, oleyl dimethyl gamma-carboxypropyl betaine, lauryl bis-(2-hydroxypropyl)alpha-carboxyethyl betaine, and mixtures thereof. Examples of sulfobetaines may include coco dimethyl sulfopropyl betaine, stearyl dimethyl sulfopropyl betaine, lauryl dimethyl sulfoethyl betaine, lauryl bis-(2-hydroxyethyl) sulfopropyl betaine and mixtures thereof.
- The zwitterionic surfactant can comprise or consist of cocamidopropyl betaine (CAPB), lauramidopropyl betaine (LAPB), and combinations thereof.
- The zwitterionic surfactant of the co-surfacant may be a betaine, preferably a betaine selected from the group consisting of cocamidopropyl betaine, lauramidopropyl betaine, coco betaine, lauryl betaine, coco hydroxysultaine and mixtures thereof; more preferably cocamidopropyl betaine.
- The co-surfactant may comprise at least a combination of a fatty acyl sarcosinate and a betaine; preferably a fatty acyl sarcosinate selected from the group consisting of sodium lauroyl sarcosinate, sodium myristoyl sarcosinate, sodium cocoyl sarcosinate, and mixtures thereof, and a betaine selected from the group consisting of coco hydroxysultaine, lauryl hydroxysultaine, coco betaine, and mixtures thereof; preferably sodium lauroyl sarcosinate and cocamidopropyl betaine.
- The personal cleansing composition may comprise a total amount from 0.6% to 55% of the surfactant system by total weight of the composition, preferably from 5% to 50% of the surfactant system by total weight of the composition, more preferably from 10% to 45% of the surfactant system by total weight of the composition. The concentrations mentioned here are total concentration ranges of the surfactant system resulting from at least the addition of the total concentration ranges of the fatty acyl isethionate surfactant and the co-surfactant. The specified ranges are provided by weight and relate to the total weight of the personal cleansing composition.
- The personal cleansing composition comprises from 0.25% to 2% of a natural polysaccharide by weight of the composition.
- Carrageenans are structural polysaccharides of marine red algae of the Rhodophyceae class. κ-Carrageenans, ι-carrageenans, and furcellarans are linear polysaccharides whose backbone structure is based on a repeating disaccharide sequence of sulphate esters of (1→3) linked β-D-galactose and (1→4) linked 3,6-anhydro-α-D-galactose. They differ from each other in the number and position of sulphate groups. λ-Carrageenan comprise β-D-galactopyranosyl residue sulphated at C-2 (instead of C-4 as in ι- and κ-carrageenans) and 2,6-di-O-sulfato-α-D-galactopyranosyl units (instead of 3,6-anhydro-α- D-galactopyranosyl residue) (See
FIG. 1L ). - Xanthan gum is an extracellular polysaccharide produced by the bacterium Xanthomonas campestris. The primary structure of xanthan gum consists of the cellulose-like backbone of (1→4)-linked β-DGlcp residues substituted, at O-3 of alternate glucose residues, with a trisaccharide. The trisaccharide consists of the β-D-Manp-(1→4)-β-D-GlcpA-(1→2)-α-D-Manp-(1→ unit (
FIG. 1M ). - The natural polysaccharide is selected from the group consisting of xanthan gum, κ-carrageenan, t-carrageenan, λ-carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum.
- The natural polysaccharide may be preferably selected from the group consisting of xanthan gum, t-carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum.
- Alternatively, the natural polysaccharide may be a glucomannan such as locust bean gum and guar gum that is combined with xanthan gum and/or carrageenans such as κ-carrageenan, t-carrageenan and λ-carrageenan. Such natural polysaccharide combination can have synergism and provide improved polymer liquid crystals, with an improved yield stress.
- The personal cleansing composition comprises a first and second phase, as identified and isolated by the Ultracentrifuge Test Method. The first phase of the personal cleansing composition is an isotropic and micellar surfactant phase. The rheologic profile of the first phase has been determined by the Ultracentrifuge Test Method.
- The first phase may exhibit a viscosity greater than 1 Pa.s at 25°C at a shear rate of 1 s-1 according to the Ultracentrifuge Test Method. The first phase having a viscosity greater than 1 Pa.s at 25°C at a shear rate of 1 s-1 according to the Ultracentrifuge Test Method can fit the well-known Carreau viscosity profile. A Carreau viscosity profile is typical of a micellar phase. The micellar phase can find its origin from the surfactant system comprising a fatty acyl isethionate surfactant and a co-surfactant.
- Furthermore, the first phase cannot rotate polarized light and cannot exhibit a birefringent optical morphology. Also, when isolated according to the Ultracentrifuge test, the first phase has a transmittance at 25°C and at 640 nm of 100% according to the Optical Clarity Test Method.
- The personal cleansing composition further comprises from 0.05 to 5%, preferably from 0.1% to 4%, more preferably from 0.5% to 3% of an electrolyte by weight of the composition. The addition of an electrolyte can help to elongate the micelles of the surfactant system in the first phase of the personal cleansing composition and to improve the flow viscosity.
- The electrolyte may be selected from the group of sodium or potassium citrate, calcium chloride, calcium bromide, zinc chloride, barium chloride, calcium nitrate, potassium chloride, sodium chloride, potassium iodide, sodium bromide, ammonium bromide, sodium sulfate, and mixtures thereof.
- The electrolyte may be preferably selected from the group of sodium or potassium citrate, calcium chloride, potassium chloride, sodium chloride, and mixtures thereof.
- The second phase of the personal cleansing composition is a polymer liquid crystalline phase. The second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase. The polymer liquid crystalline phase comprises polymer liquid crystals. The polymer liquid crystals include the natural polysaccharide.
- As detailed in the example section below, without a natural polysaccharide, e.g. xanthan gum in the personal cleansing composition, no second phase comprising polymer liquid crystals can be observed. In that case, there is no measurable yield stress τy as measured according to the Herschel-Bulkley Rheology Test Method. When adding increasing amount of the natural polysaccharide, a second phase appears. A polymer liquid crystalline phase can be formed. The formation of the second phase being a polymer liquid crystalline phase can be characterized in different ways.
- Generally, water-soluble polysaccharide are polymers that are used in predominantly aqueous compositions to provide a rheology benefit due to their solubility. Water-soluble polysaccharides, in solution, absorb substantial amounts of water and increase viscosity of a composition as a result of their size and entanglements. In some cases, the polysaccharides can form gels due to molecular association. A relatively poor water-soluble polysaccharide or a polysaccharide that does not form readily a molecular gel in a composition cannot help to improve the performance of the composition. Indeed, a relatively poor water-soluble polysaccharide is generally quite dense and therefore tends to phase separate and become unstable in a composition.
- Polymer liquid crystalline phases are uncommon in personal cleansing compositions, since polymers generally favor disordered structures, especially at low concentration. Polymer polydispersity rules out liquid crystalline structures common to small molecules, i.e., smectic liquid crystalline phases such as lamellar and hexagonal. Nematic and/or cholesteric polymer liquid crystalline structures are known for some polysaccharides, however only at relatively high polysaccharide concentrations, i.e., at least from 10 wt%, preferably at least from 20 wt% of the polysaccharide by total weight of the composition.
- Inventors have surprisingly found that a natural polysaccharide can lead to the formation of a polymer liquid crystalline phase at a relatively low concentration of the said polysaccharide, and even in the presence of a surfactant system as described hereinbefore.
- Indeed, a natural polysaccharide can lead to the formation of a polymer liquid crystalline phase, at a level from 0.25% to 2% of a natural polysaccharide by weight of the composition.
- First, the second phase being a polymer liquid crystalline phase provides a structure for the personal cleansing compositions. Such structure is characterized by specific structural features: The second phase of the personal cleansing composition is a polymer liquid crystalline phase. Indeed, the second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase. The polymer liquid crystalline phase comprises polymer liquid crystals. The polymer liquid crystals include the natural polysaccharide.
- The second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase. The polymer liquid crystalline phase comprises polymer liquid crystals. Microscopic observations indicate that the polymer liquid crystals form elongated structures, and thus have relatively high degrees of orientational order. The polymer liquid crystals may form elongated structures, namely elongated colloidal structures. Preferably, the polymer liquid crystals may be nematic or cholesteric.
- When the polymer liquid crystals are of nematic subclass, the centers of gravity of the polymer liquid crystals are arranged at random, consequently no positional long-range order exists. Within volume elements of a macroscopic sample of the polymer liquid crystalline phase, the axes of all polymer liquid crystals are oriented in a specific direction.
- When the polymer liquid crystals are of cholesteric subclass, the arrangement of the polymer liquid crystals are similar to the ones of nematic subclass, however only an orientational order exists in the cholesteric subclass. In contrast to the nematic subclass, the cholesteric subclass is characterized by the fact that the direction of the long axes of the molecules changes continuously within the sample. This leads to a twist about an axis perpendicular to the long axes of the molecules. If the pitch-of the helical structure agrees with the wavelength of the visible light, selective reflection of monochromatic light can be observed. This effect may lead to the iridescent colors often observed in cholesteric phases.
- The natural polysaccharide may be present in the polymer liquid crystalline phase at a level from 1% to 30%, preferably from 2% to 25%, more preferably from 4% to 10.5% by total weight of the polymer liquid crystalline phase.
- The polymer liquid crystals of the second phase may comprise at least a combination of the natural polysaccharide and one ingredient of the surfactant system.
- The polymer liquid crystals of the second phase may preferably comprise at least a combination of the natural polysaccharide selected from the group consisting of xanthan gum, t-carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum; and the fatty acyl isethionate surfactant.
- The polymer liquid crystals of the second phase may more preferably comprise at least a combination of xanthan gum a fatty acyl isethionate surfactant; and optionally a co-surfactant.
- Surprisingly, it has been found that a natural polysaccharide in an aqueous personal cleansing composition comprising a surfactant system can form a polymer liquid crystalline phase. Further, the polymer liquid crystalline phase can form elongated structures which are very efficient at forming an associative network, conferring a structure. The elongated structures, forming the associative network, can be further characterized by a measurable yield stress, and at surprisingly a relatively low level of the natural polysaccharide. The associative network, may form a relatively weak gel or a physical gel.
- The polymer liquid crystals of the polymer liquid crystalline phase may be notably soluble during use. Consumer products such as body wash and shampoo are used with water. The polymer liquid crystals can dissolve during use with additional water, which increases the viscosity of the dilute aqueous micelles of the personal cleansing composition, resulting in a lather improvement. For example, the increased dilute micelle viscosity can form a lather that is relatively denser and creamier. Those attributes may be desirable for a personal cleansing composition.
- Also, the personal cleansing composition may exhibit a yield stress value τy from 0.005 Pa to 3 Pa, preferably from 0.02 Pa to 2 Pa, more preferably from 0.03 Pa to 1 Pa, even more preferably from 0.05 Pa to 0.8 Pa according to the Herschel-Bulkley Rheology Test Method as disclosed herein.
- The yield stress of the personal cleansing composition may be a further evidence of the presence of the polymer liquid crystalline phase. The yield stress of the personal cleansing composition can be defined as the elastic component characterizing the necessary structure of the composition to be highly effective at suspending any benefit agents, particulates, particles such as silica and titanium oxide, microcapsules, oils, droplets, and mixtures thereof in the personal cleansing composition. In other words, a polymer liquid crystalline phase and the yield stress value τy as set out above are the features responsible to provide a personal cleansing composition highly effective at suspending particulates, particles such as silica and titanium oxide, microcapsules, oils, droplets, and mixtures thereof. In other words, the polymer liquid crystalline phase and optionally the yield stress value τy as set out above are the features that can help to improve the distribution of benefit agents associated with the personal cleansing composition: skin or hair care ingredients, perfumes, ...
- The personal cleansing composition may also exhibit a flow viscosity from 3 Pa.s to 100 Pa.s at 25°C at a shear rate of 1.5 s-1 according to the Flow Viscosity Test Method as disclosed herein. The structure provided by the polymer liquid crystalline phase may also characterized by yield stress and the flow viscosity of the composition, imparting a relatively high stability for the personal cleansing composition.
- Another further evidence of the polymer liquid crystalline phase may be the transmittance of the personal cleansing composition. The personal cleansing composition may exhibit a transmittance at 25°C and at 640 nm of from 4% to 95%, preferably from 10% to 93%, more preferably from 25% to 90% according to the Optical Clarity Test Method as disclosed herein. As further detailed below in the example section, the personal cleansing composition without a natural polysaccharide forms typically micelles having relatively high transmittance and typical micelle rheology. However, when adding increasing amount of a natural polysaccharide, the transmittance of the composition decreases. The decrease of transmittance is due to the formation of a second phase which is a polymer liquid crystalline phase.
- As can be appreciated, the compositions described herein may include a variety of optional components to tailor the properties and characteristics of the composition. As can be appreciated, suitable optional components are well known and can generally include any components which are physically and chemically compatible with the essential components of the compositions described herein. Optional components should not otherwise unduly impair product stability, aesthetics, or performance. Individual concentrations of optional components can generally range from 0.001% to 10%, by weight of the composition. Optional components can be further limited to components which will not impair the clarity of a translucent composition.
- Optional components may include, but are not limited to, conditioning agents (including hydrocarbon oils, fatty esters, silicones), cationic polymers, anti-dandruff actives, and chelating agents. Additional suitable optional ingredients include but are not limited to encapsulated and non-encapsulated perfumes or fragrances, colorants, particles, anti-microbials, foam boosters, antistatic agents, moisturizing agents, propellants, self-foaming agents, pH adjusting agents and buffers, preservatives, pearlescent agents, opacifiers, sensates, suspending agents, solvents, diluents, anti-oxidants, vitamins and combinations thereof.
- Such optional ingredients should be physically and chemically compatible with the components of the composition, and should not otherwise unduly impair product stability, aesthetics, or performance. The CTFA Cosmetic Ingredient Handbook, Tenth Edition (published by the Cosmetic, Toiletry, and Fragrance Association, Inc., Washington, D.C.) (2004) (hereinafter "CTFA"), describes a wide variety of nonlimiting materials that can be added to the composition herein.
- The personal cleansing composition may be prepared according to the following method. The method for making the personal cleansing composition as set out hereinbefore may be provided and comprises the following steps, preferably in that order:
- (a) providing a surfactant system, wherein the surfactant system comprises:
- from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition;
- from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15%, of a co-surfactant, wherein the co-surfactant is selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant and mixtures thereof, by weight of the composition;
- (b) adding 0.25% to 2% of a natural polysaccharide, by weight of the composition with no milling.
- As shown in the example section below, when a relatively high energy milling is used for the addition of the natural polysaccharide, the yield stress τy that may characterize the formation of second polymer liquid crystalline phase cannot be met. The step of adding the natural polysaccharide can also impact the orientation of the polymer liquid crystals of the second phase from non-elongated structures that are irregularly shaped with some regularly shaped such as generally spherical domains, to elongated structures.
- The natural polysaccharide needs to be added at the relatively low level from 0.25% to 2% of the natural polysaccharide, by weight of the composition with no milling. An increased of the shear when adding the polysaccharide may impede the organization of the second phase into elongated colloidal structures leading to the polymer liquid crystalline phase. The elongated colloidal structures forming the polymer liquid crystals may be characterized by the rheological properties.
- Preferably, the natural polysaccharide may be added to the surfactant system from an aqueous solution comprising from 2% to 30%, preferably from 3% to 20%, more preferably from 4% to 10% of the natural polysaccharide or the chemically modified natural polysaccharide by weight of the aqueous solution.
- Most preferably, the natural polysaccharide may be added to the surfactant system by diluting the natural polysaccharide in water at a dilution level from 2% to 30%, preferably from 3% to 20%, more preferably from 4% to 10%. A dilution level of 2% means 2 g of the natural polysaccharide diluted in 100 g of water.
- The personal cleansing composition as set out hereinbefore may be obtained by the method for making the personal cleansing composition comprising the following steps, preferably in that order:
- (a) providing a surfactant system, wherein the surfactant system comprises:
- from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition;
- from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15%, of a co-surfactant, wherein the co-surfactant is selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant and mixtures thereof, by weight of the composition;
- (b) adding from 0.25% to 2% of a natural polysaccharide, by weight of the composition with no milling.
- The personal cleansing composition may be presented in typical personal cleansing formulations. They may be in the form of solutions, dispersion, emulsions, foams, and other delivery mechanisms.
- The personal cleansing composition may be extrudable or dispensable from a single chamber package. The personal cleansing compositions can be in the form of liquid, semi-liquid, cream, lotion or gel, or solid compositions intended for topical application to skin or hair.
- Examples of personal cleansing compositions can include but are not limited to shampoo, conditioning shampoo, hair conditioner, body wash, moisturizing body wash, foaming body wash, shower gels, a shower or bath cream, skin cleansers, cleansing milks, hair and body wash, in shower body moisturizer, gel, emulsion, oil, mousse or spray.
- The product forms contemplated for purposes of defining the personal cleansing compositions and methods are rinse-off formulations by which it is meant that the product is applied topically to the skin or hair and then subsequently (i.e., within minutes) rinsed away with water, or otherwise wiped off using a substrate or other suitable removal means.
- The personal cleansing composition as set out hereinabove may be used for improving the lather of the composition.
- The personal cleansing composition as set out hereinabove may be used for suspending benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
- The personal cleansing composition can advantageously provide relatively improved ecotoxic or ecologically friendly environmental profile.
- The personal cleansing composition can help to provide good esthetic properties such as good foam, and is thick and creamy in texture, is silky to the touch and affords conditioning.
- It is understood that the Test Methods that are disclosed in the Test Methods Section of the present application should be used to determine the respective values of the parameters of Applicants' invention as such invention is described and claimed herein.
- The optical clarity of a personal cleansing composition is based on the measurement of the transmittance or light transmission (%T) of the personal cleansing composition at the visible wavelength of light of 640 nm. The transmittance of a personal cleansing composition is measured using a spectrophotometer such as a Thermo
Fisher model Genesys 10 VIS (Thermo Fisher Inc, USA) and conventional polystyrene cuvettes with 1 cm path length at 25°C. The spectrophotometer is set to measure the transmittance (%T) at 640 nm wavelength. An identical cuvette filled with distilled water is used as the baseline reference. The personal cleansing composition is pipetted into the cuvette and ensured clear of air bubbles by centrifuging to remove air bubbles if present. The personal cleansing composition in the cuvette is placed in the holder compartment of the instrument, closing the compartment door, and the transmittance (%T) is recorded. In case the personal cleansing composition optionally comprises a dye, or a colored pigment, the baseline reference will be adjusted accordingly. - The flow viscosity and the yield stress properties of the personal cleansing composition via the Herschel Bulkley model are measured using a rheometer (available from. TA Instruments of New Castle, Del. AR-G2 series) in a flow mode. The measurements are conducted using a cone and plate geometry measuring system, having a diameter of 40 mm and degree cone angle. The measurement commences after about 10 seconds equilibration time at 25°C. If the composition is substantially structured, i.e. having a flow viscosity greater than 30 000 mPa.s or a yield stress greater than 1 Pa, a cross-hatched parallel plate geometry is used.
- The Herschel Bulkley model is described in "Rheometry of Pastes Suspensions and Granular Material" page 163, Philippe Coussot, John Wiley & Sons, Inc., Hoboken, New Jersey (2005). The flow viscosity and the yield stress value τy of a personal cleansing composition are measured using a 2-step measurement procedure. The yield stress can be obtained using a reverse rate curve from 1 s-1 to 10-5 s-1 applied over a 6-minute interval, and applying the Herschel-Bulkley model: τ = τy + Kγ n, using the data analysis software where τ is the shear stress, τy is the yield stress, γ is the shear rate, K is the viscosity and n is a power law viscosity coefficient.
- The rheometer procedure applied herein comprises the step of:
Instep 1, the rheometer is set up and operated following standard procedures that include zeroing the gap, bearing friction calibration, and mapping. The geometry can be altered as needed to accommodate relatively high or low viscosity personal cleansing compositions, including selecting a parallel plate geometry for compositions containing particles and serrated geometries for compositions that can exhibit wall slip. Viscosity measurements are conducted at 25°C. - Load the personal cleansing composition onto the rheometer baseplate, ensuring there are no bubbles or gaps, lower the geometry to 100 µm above measurement position, lock the geometry and trim the sample. Lower the gap to the measurement position and place a protective wind shield around the measurement area. Conduct a first shear rate continuous ramp. Start at 1.0 s-1 and reduce the shear rate logarithmically to 10-5 s-1 over a 6-minute interval collecting 15 points per rate decade change.
- Immediately following, in a step 2, increase the shear rate from 0.025 s-1 to 250 s-1 over 4 minutes, collecting 15 points per rate decade. Fit the results from the first step to the Herschel-Bulkley rheology model to determine the yield stress of the composition, fitting only the data at and below 0.01 s-1 shear rate. Determine the flow viscosity of the composition using the data from the step 2 at 1.5 s-1.
- The light microscopy of liquid crystals is described in the Microscopy of Liquid Crystals, Norman Hartshorne, Microscopy publications, Ltd., Chicago, Illinois, U.S.A., 1974. Methods for microscopic observation and evaluation are discussed in . A preferred method for determining occurrence of polymer liquid crystals is by observing birefringence from relatively thin slices of the personal cleansing composition under a polarizing microscope.
- The characterization of the second phase of the personal cleansing composition in terms of birefringence, type of structures and orientation of the polymer liquid crystals can be thus viewed using an optical microscope such as a Zeiss Zxio Imager or its equivalent. A drop of the personal cleansing composition is loaded onto a clean glass slide and thinned using a coverslip. Views of the personal cleansing composition using 5x, 10x and 20x objective lenses using bright field can determine the presence of discrete structures of a second phase. Under the cross polarized light optical microscope, one can rotate into view a polarizer and analyzer in cross-polarized position to determine if the second phase is ordered (transmits light) or isotropic (dark). An ordered second phase may characterize a polymer liquid crystalline phase.
- If the second phase appears to contain structures that are generally longer than they are wide, including structures that are cylindrical, rod-like, ribbon-like, needle-like or fiber-like in appearance, the second phase is said to contain elongated structures.
- The structure of the second phase of the personal cleansing composition is evaluated with a sufficient number of fields by selecting a composition sample from different parts of the total composition (e.g. different parts of the composition contained in a container). At least 5 drops of the personal cleansing composition are used to prepare 5 microscope slides to obtain an average representation of the personal cleansing composition.
- The weight percentage of the first and second phases of the personal cleansing composition can be determined with the Ultracentrifuge Test using a Beckman Coulter Optima LE-80K ultracentrifuge with swinging bucket rotors (Beckman SW06Ti with 6 buckets, rotor diameter 165 mm, or equivalent).
- The tare weight of an ultracentrifuge tube is determined using a 3-place electronic balance to the closest milligram. The ultracentrifuge tube is filled with the personal cleansing composition and the gross weight is measured. The personal cleansing composition in the ultracentrifuge tube is placed in the ultracentrifuge bucket, capped, and placed on the ultracentrifuge rotor. The process is continued with other compositions placed on the rotor in counterbalancing positions. The rotor is positioned in the ultracentrifuge, the vacuum door closed, and run at a speed of 50,000 rpm, at 25°C for 15 hrs under vacuum. Then, the ultracentrifuge tubes are removed and observed.
- A first phase which is an optically transparent micellar surfactant phase is present in the personal cleansing compositions as a layer which is generally a top layer in the ultracentrifuge tube. The micellar surfactant phase has a viscosity which is measured by removing a portion of the first phase using a pipette, transferring it to a rheometer, and measuring the viscosity using the Flow Viscosity Test Method. The micelle viscosity as measured in step 2 of the Flow viscosity Test Method is reported as the Micelle Viscosity, in Pa.s. The entire micellar surfactant phase is carefully removed using a spatula and absorbent means such as paper towels without disturbing lower phases. Weigh the ultracentrifuge tube with the lower phase, subtracting the ultracentrifuge tube tare weight to obtain the relative amounts of the removed micellar and remaining phases. Continue to remove lower phase individually and weighing the ultracentrifuge tube to obtain the weight of each phase. The second phase which can be a birefringent phase, is a polymer liquid crystal phase. The second phase is generally simple to identify based on its structure and appearance.
- Express the percentage of each phase, including the polymer liquid crystal phase as a percentage by weight of the total composition, excluding the weight of the ultracentrifuge tube.
- The following examples further describe and demonstrate embodiments within the scope of the present invention. Where applicable, ingredients are identified by chemical or CTFA name, or otherwise defined below.
- The following examples were prepared:
Ex. 1-2 are not within the scope of the invention. -
Ingredients Comp. Ex. 1 Ex.1 Ex. 2 Ex. 3 Ex. 4 Ex. 5 Sodium cocoyl isethionate1 4.22 4.22 4.14 4.22 4.22 4.22 Coamidopropyl betaine2 11.03 11.03 10.82 11.03 11.03 11.02 Sodium lauroyl sarcosinate3 4.75 4.75 4.66 4.75 4.75 4.75 Sodium chloride4 4.22 4.22 4.14 4.22 4.22 4.22 Xanthan gum5 - 0.06 0.10 0.25 0.50 1.00 Preservative 0.85 0.85 0.85 0.85 0.85 0.85 Fragrance 0.80 0.80 0.80 0.80 0.80 0.80 water qs qs qs qs qs qs Mixing process vortexer vortexer vortexer vortexer vortexer vortexer Gum addition procedure A A A A A A Total surfactant 20 20 19.6 20 20 20 Flow viscosity (Pa.s) 36.55 36.39 36.04 40.20 47.02 63.80 Yield stress τy (Pa) 0 0.0258 0.0404 0.0198 0.330 0.404 Transmittance (%T at 640 nm) 87% - 64% 38% 26% 7% Hold air bubbles 24 hr No Yes Yes Yes Yes Yes Static stability n.a. stable stable stable stable stable % polymer liquid crystals (Ultracentrifuge Test Method) 0% - - - 4.5% - Birefringence of the 2nd phase No Yes Yes Yes Yes Yes Orientation of the 2nd phase n.a. E E E E E Ingredients Ex. 6 Ex. 7 Ex. 8 Ex. 9 Sodium cocoyl isethionate1 4.22 2.11 2.11 2.11 Coamidopropyl betaine2 11.03 5.51 5.51 5.51 Sodium lauroyl sarcosinate3 4.75 2.37 2.37 2.37 Sodium chloride4 4.22 2.11 2.11 2.11 Xanthan gum5 2.00 0.50 1.00 2.00 Preservative 0.85 0.85 0.85 0.85 Fragrance 0.80 0.80 0.80 0.80 water qs qs qs qs Mixing process vortexer vortexer vortexer vortexer Gum addition procedure A A A A Total surfactant 20 10 10 10 Flow viscosity (Pa.s) 65.69 9.44 17.85 33.99 Yield stress τy (Pa) 0.281 0.793 1.92 0.379 Transmittance (%T at 640 nm) - 10% 4% - Hold air bubbles 24 hr Yes Yes Yes Yes Static stability stable stable stable stable % polymer liquid crystals (Ultracentrifuge Test Method) - 5.3% 6.6% 10.5% Birefringence of the 2nd phase Yes Ye Ye Ye Orientation of the 2nd phase E E E E Micelle viscosity - 1st phase (mPa.s) (Ultracentrifuge Test Method) - 2.95 3.09 2.51 Definitions of Components
* 1 Sodium cocoyl isethionate; Supplier Clariant
*2 Cocamidopropyl Betaine; Supplier BASF
*3 Sodium lauroyl sarcosinate; Supplier Clariant
*4 Sodium chloride; Supplier Morton International
*5 Xanthan gum5; Supplier CP Kelco
qs: sufficient quantity for 100%wt.
n.a. not applicable
E: elongated structures - Without a natural polysaccharide, e.g. xanthan gum in the composition of Comparative Example 1, no second phase comprising polymer liquid crystals has been observed. In that case, there is no yield stress τy measured according to the Herschel-Bulkley Rheology Test Method. When adding increasing amount of the natural polysaccharide, e.g. xanthan gum, a second phase appears. A polymer liquid crystalline phase is thus formed. The polymer liquid crystalline phase may be observed for Examples 1-6 with 20%wt. of total surfactant by total weight of the composition, and for Examples 7-9 with 10%wt. of total surfactant by total weight of the composition.
- The polymer liquid crystalline phase comprises polymer liquid crystals. The presence of polymer liquid crystals can be characterized by the fact that the personal cleansing composition comprises a yield stress τy measured according to the Herschel-Bulkley model. The personal cleansing composition may exhibit a yield stress τy from 0.005 Pa to 3 Pa according to the Herschel-Bulkley Rheology Test Method. When increasing amount of the natural polysaccharide, e.g. xanthan gum, the yield stress τy and the flow viscosity in some extent both increase. The personal cleansing composition is also statically stable.
- The polymer liquids crystals are characterized by their birefringence via optical microscopy. Microscopic observations indicate that the polymer liquid crystals form elongated structures, and thus have relatively high degrees of orientational order.
-
FIG. 2 is a cross-polarized image of Example 5 taken with a cross-polarized light microscopy. The image ofFIG. 2 demonstrates a dispersed second phase which is distributed in the form of elongated structures that are optically birefringent. The elongate structures E of the dispersed polymer liquid crystal phase contrast with the first phase being an isotropic and micellar surfactant phase that cannot rotate light and forming the background. The presence of the polymer liquid crystalline phase provides the structural feature of the personal cleansing composition that contributes to the relatively high flow viscosity. - Due to the presence of a polymer liquid crystalline phase conferring a yield stress, the personal cleansing composition can suspend air bubbles. After 24 hours, the presence of air bubbles within a personal cleansing composition is a further evidence of a structure. In other words, the personal cleansing composition is provided with a structure characterized by a polymer liquid crystalline phase and optionally a significant yield stress. Such structure can suspend one or more benefit agents such that hair care or skin care benefits agents, particulates, particles, oils, liquid droplets.
- It is also observed a reduction of the transmittance when adding xanthan gum, which demonstrates light scattering due to the polymer liquid crystalline phase. The optical clarity upon addition of a natural polysaccharide, e.g. xanthan gum was assessed for the following base composition (wt.%) in
FIG. 3 :Ingredients Base composition Sodium cocoyl isethionate1 2.64 Coamidopropyl betaine2 6.89 Sodium lauroyl sarcosinate3 2.97 Sodium chloride4 3.00 Preservative 0.75 Fragrance 0.80 water qs - As shown in
FIG. 3 , the transmittance of the personal cleansing composition is relatively high when no xanthan gum was added to the composition. The personal cleansing composition without xanthan gum forms typically micelles having relatively high transmittance and typical micelle rheology. However, when adding increasing amount of xanthan gum, the transmittance of the composition decreases. The decrease of transmittance is not only due to the insoluble character of xanthan gum in the first phase being an isotropic and micellar surfactant phase, but also due to the formation of a second phase which is a polymer liquid crystalline phase. - For Examples 7-9, at 10%wt. total surfactant by total weight of the composition, a micellar surfactant phase was isolated by the Ultracentrifuge Test Method. Indeed, two distinct phases were observed and separated for Examples 7-9. A top phase comprising a transparent micellar phase was separated from a lower and denser second phase. The second phase rotates polarized light. The measured micelle viscosities for Examples 7-9 show that the micelles of the micellar surfactant phase can contribute to the flow viscosity of the personal cleansing composition.
- Furthermore, the polymer liquid crystalline phase that only represents for instance 5.3% wt. of the total composition in Example 7 can effectively triple the micellar viscosity to lead to the flow viscosity of the composition. The relatively denser second phase was observed to sediment, which is also another characteristic of a liquid crystalline phase enriched with the natural polysaccharide and not a surfactant liquid crystal. Surfactant liquid crystals are known to provide creaminess and not to sediment due to their hydrocarbon enrichment.
- The following examples were prepared to show that the personal cleansing compositions useful herein needs a sufficient flow viscosity by adjusting either the amount of the natural polysaccharide, or the total surfactant, or the electrolyte.
- Ex. 11-14 are not within the scope of the invention.
-
Ingredients Comp. Ex. 2 Comp. Ex. 3 Comp. Ex. 4 Ex. 10 Ex. 11 Sodium cocoyl isethionate1 2.11 2.11 2.11 2.11 3.17 Coamidopropyl betaine2 5.51 5.51 5.51 5.51 8.27 Sodium lauroyl sarcosinate3 2.37 2.37 2.37 2.37 3.56 Sodium chloride4 2.11 2.11 0 2.11 3.17 Xanthan gum5 - 0.10 0.25 0.25 0.10 Preservative 0.85 0.85 0.75 0.85 0.75 Fragrance 0.80 0.80 0.80 0.80 0.80 water qs qs qs qs qs Mixing process vortexer vortexer vortexer vortexer vortexer Gum addition procedure A A A A A Total surfactant 10 10 10 10 15 Flow viscosity (Pa.s) 1.92 2.59 0.59 3.37 15.33 Yield stress τy (Pa) 0 0.0125 0.0332 0.0531 0.0531 Static stability n.a. separated separated stable stable Birefringence of the 2nd phase No Yes Yes Yes Yes Orientation of the 2nd phase n.a. E E E E Ingredients Ex. 12 Ex. 13 Ex. 14 Sodium cocoyl isethionate1 3.17 3.17 4.22 Coamidopropyl betaine2 8.27 8.27 11.03 Sodium lauroyl sarcosinate3 3.56 3.56 4.75 Sodium chloride 40 0 0 Xanthan gum5 0.25 0.50 0.25 Preservative 0.75 0.75 0.75 Fragrance 0.80 0.80 0.80 water qs qs qs Mixing process vortexer vortexer vortexer Gum addition procedure A A A Total surfactant 15 15 20 Flow viscosity (Pa.s) 8.46 11.16 37.43 Yield stress τy (Pa) 0.0165 0.0282 0.0507 Static stability Stable Stable Stable Birefringence of the 2nd phase Yes Yes Yes Orientation of the 2nd phase E E E - The personal cleansing compositions useful herein may need a sufficient flow viscosity by adjusting either the amount of the natural polysaccharide, or the total amount of surfactant, optionally with the amount of the electrolyte, e.g. sodium chloride.
- Indeed, when the flow viscosity is below 3 Pa.s such as Comparative Example 3 although the yield stress is acceptable, the personal cleansing composition is so unstable that the composition separates in only few days.
- Examples 10 and 11 show that sodium chloride can optionally help to increase the flow viscosity. However, Examples 12, 13 and 14 demonstrate that a respective total amount of 15%wt. or 20%wt. of total surfactant by total weight of the composition with the addition of a natural polysaccharide allows reaching a sufficient flow viscosity without adding an electrolyte such as sodium chloride.
- The following examples were prepared with different combinations of natural polysaccharides or with other natural polysaccharides:
-
Ingredients Ex. 15 Ex. 16 Ex. 17 Ex. 18 Ex. 19 Sodium cocoyl isethionate1 3.17 3.17 3.17 3.17 3.01 Coamidopropyl betaine2 8.27 8.27 8.27 8.27 7.86 Sodium lauroyl sarcosinate3 3.56 3.56 3.56 3.56 3.38 Sodium chloride4 3.16 3.16 3.17 3.17 4.0 Xanthan gum5 0.40 0.25 0.40 0.25 - Locust bean gum6 0.10 0.25 - - - Guar gum7 - - 0.10 0.25 - ι-carrageenan8 - - - - 3.5 water qs qs qs qs qs Mixing process vortexer vortexer vortexer vortexer vortexer addition procedure A A A A A Total surfactant 15 15 15 15 14.25 Flow viscosity (Pa.s) 24.52 23.86 23.16 19.85 39.00 Yield stress τy (Pa) 0.224 0.222 0.156 0.0325 0.59 Hold air bubble 24 hr Yes Yes Yes No Yes Static stability stable stable stable stable stable Birefringence of the 2nd phase Yes Yes Yes Yes Yes Orientation of the 2nd phase E E E E E Definitions of Components
*6 Locust bean gum; Supplier (local food distributor)
*7 guar gum; Supplier Solvay
*8 ι-carrageenan gum; Supplier CP Kelco - The personal cleansing compositions can be prepared using a mixture of natural polysaccharides such as xanthan gum with locust bean gum or xanthan gum with guar gum; or using a carrageenan like ι-carrageenan.
- 24 h prior to making the personal cleansing composition, xanthan gum was diluted in water as a level of 5-10% by weight (i.e. 5-10 g of xanthan gum in 100 g of water) as a homogeneous aqueous gel with stirring and via a vortex agitation using a Speedmixer. The xanthan gum preparation was added and stirred into the composition comprising all the other ingredients as the final step and swirled for 5-10 seconds using a vortexer.
- The following examples were prepared according to different addition procedures of the natural polysaccharide:
-
Ingredients Comp. Ex. 5 Ex. 20 Ex. 21 Ex. 22 Sodium cocoyl isethionate1 3.01 3.01 3.01 3.01 Coamidopropyl betaine2 7.86 7.86 7.86 7.86 Sodium lauroyl sarcosinate3 3.38 3.38 3.38 3.38 Sodium chloride4 3.50 4.0 4.0 4.0 Xanthan gum5 0.25 0.25 0.50 0.50 Preservative 0.85 0.85 0.85 0.85 Fragrance 0.80 0.80 0.80 0.80 Silica (agglomerated) - - - 3.0 water qs qs qs qs addition procedure B C C D Total surfactant 14.25 14.25 14.25 14.25 Total size of the batch 200 kg 100 kg 50 kg 50 kg Flow viscosity (Pa.s) 6.49 10.95 8.67 29.77 Yield stress τy (Pa) 0.0044 0.0353 0.0205 0.242 Hold air bubble 24 hr No No No Yes Static stability stable stable stable stable Birefringence of the 2nd phase Yes Yes Yes Yes Orientation of the 2nd phase X Mostly X with some E Mostly X with some E E X: the second phase of the personal cleansing composition is distributed as non-elongated structures, which are irregularly shaped or regularly shaped such as generally spherical domains, visible by light microscopy with a 5x-10x objective lens;
E: the second phase of the personal cleansing composition is distributed primarily as elongated structures comprising of generally rod-like structures with high aspect ratio observed by light microscopy. In polarized light microscopy with a 5x-10x objective lens the fibers may generally appear optically birefringent. - The natural polysaccharide, e.g. xanthan gum was added as a dry powder using a Quadro mixer to water, the surfactant system (sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate), followed by the rest of the ingredients of the batch at 70°C, with 6 subsequent theoretical passes following incorporation to provide high energy milling to improve xanthan gum incorporation.
- The natural polysaccharide, e.g. xanthan gum was added as a dry powder using a pilot plant scale Quadro mixer with no additional milling after incorporation to the batch comprising water, the surfactant system (sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate), followed by the rest of the ingredients.
- The natural polysaccharide, e.g. xanthan gum was added through a 250 mesh US Standard Sieve onto the moving top surface of the batch comprising the surfactant system (sodium cocoyl isethionate, cocamidopropyl betaine and sodium lauroyl sarcosinate) at 70°C, over several minutes, with no milling or pumping, followed by the rest of the ingredients.
- When a relatively high energy milling is used for the addition of the natural polysaccharide, the yield stress τy that may characterize the formation of second polymer liquid crystalline phase cannot be met. The addition method can also impact the orientation of the polymer liquid crystals of the second phase from non-elongated structures that are irregularly shaped with some regularly shaped such as generally spherical domains, to elongated structures.
- Example 21 has a yield stress τy of 0.242 Pa that enables to suspend silica particles without settling. The personal cleansing compositions having a second polymer liquid crystalline phase and a sufficient yield stress τy can help to suspend benefit agents.
- Indeed, the polymer liquid crystalline phase can help to provide structuring benefits, in particular the suspension of particles or insoluble liquid droplets throughout the personal cleansing composition without significant settling of such particles or droplets toward the bottom of the container and/or without significant raising or creaming of such particles or droplets toward the top of the container of the composition.
- The flow viscosity profiles of different personal cleansing compositions have been generated. For this, the personal cleansing compositions were prepared:
Ex. A and C are not within the scope of the invention. -
Ingredients Comp. Ex. 1A A B C D Sodium cocoyl isethionate1 3.01 3.01 3.01 3.01 3.01 Coamidopropyl betaine2 7.86 7.86 7.86 7.86 7.86 Sodium lauroyl sarcosinate3 3.38 3.38 3.38 3.38 3.38 Sodium chloride4 3.5 3.5 3.5 3.5 3.5 Xanthan gum (dry)5 - 0.10 0.25 - - Xanthan gum (5% aqueous solution)5 - - - 0.10 0.25 Preservative 0.85 0.85 0.85 0.85 0.85 Fragrance 0.80 0.80 0.80 0.80 0.80 water qs qs qs qs qs - FIGS. 3A and 3B are related to the flow viscosity profile of personal cleansing compositions comprising xanthan gum added dry or added from a 5% aqueous solution to the composition, respectively upon preparation of the compositions or after 3 days aging at ambient temperature.
- In
FIG. 4A , relative to the control curve without any xanthan gum (Comparative Example 1A), the addition of xanthan gum increases the flow viscosity across the shear rate spectrum despite the known insolubility of xanthan gum. - In
FIG. 4A , when xanthan gum is added from a 5% aqueous solution (Examples C and D), a significant enhancement of the flow viscosity is obtained than when xanthan gum is added dry (Examples A and B). It is assumed that the pre-solubilization of the natural polysaccharide polymer can improve the flow viscosity profile of the personal cleansing composition. -
Figure 4B demonstrates that even allowing the personal cleansing compositions toage 3 days at ambient temperature, the flow viscosity profile has not been altered. Hence, there is no apparent subsequent hydration of the natural polysaccharide that occurs after the initial processing. -
FIGS. 4C and 4D are related to the yield stress profile of personal cleansing compositions comprising xanthan gum added dry or added from a 5% aqueous solution to the composition, respectively upon preparation of the compositions or after 3 days aging at ambient temperature. - In
FIG. 4C , when xanthan gum is added from a 5% aqueous solution (Examples C and D), a significant enhancement of the yield stress is obtained than when xanthan gum is added dry (Examples A and B). It is assumed that the pre-solubilization of the natural polysaccharide polymer can also improve the yield stress profile of the personal cleansing composition. Improving the yield stress profile of the personal cleansing composition can help to improve the stability of the composition. -
Figure 4D demonstrates that even allowing the personal cleansing compositions toage 3 days at ambient temperature, the yield stress profile has not been altered. Hence, the structure provided by the polymer liquid crystals forms a network that is stable after its initial formation.
Claims (13)
- A personal cleansing composition comprising:(a) a surfactant system, wherein the surfactant system comprises:from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition;from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15% of a co-surfactant by weight of the composition, wherein the co-surfactant is selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant, and mixtures thereof;(b) from 0.25% to 2% of a natural polysaccharide by weight of the composition, wherein the natural polysaccharide is selected from the group consisting of xanthan gum, κ-carrageenan gum, t-carrageenan gum, λ-carrageenan gum, xanthan gum/locust bean gum and xanthan gum/guar gum, wherein the total amount of natural polysaccharide ranges from 0.25 to 2% by weight of the composition;wherein the personal cleansing composition comprises a first and second phase,wherein the first phase is an isotropic and micellar surfactant phase;wherein the second phase is a polymer liquid crystalline phase;wherein the second phase exhibits a birefringent optical morphology indicative of the polymer liquid crystalline phase;wherein the polymer liquid crystalline phase comprises polymer liquid crystals,wherein the polymer liquid crystals include the natural polysaccharide;wherein the composition is free of from alkyl sulfate and alkyl ether sulfate type of surfactants; andwherein the personal cleansing composition comprises from 0.05% to 5% of an electrolyte by weight of the composition.
- The personal cleansing composition of claim 1, wherein the polymer liquid crystals comprise at least a combination of the natural polysaccharide and one ingredient of the surfactant system, preferably at least a combination of the natural polysaccharide selected from the group consisting of xanthan gum, t-carrageenan, xanthan gum/locust bean gum and xanthan gum/guar gum; and the fatty acyl isethionate surfactant, more preferably at least a combination of xanthan gum, the fatty acyl isethionate surfactant and optionally the co-surfactant.
- The personal cleansing composition of any of the preceding claims, wherein the natural polysaccharide is present in the polymer liquid crystalline phase at a level from 1% to 30%, preferably from 2% to 25%, more preferably from 4% to 10.5%, by total weight of the polymer liquid crystalline phase.
- The personal cleansing composition of any of the preceding claims, wherein the fatty acyl isethionate surfactant is an isethionate according to the general Formula (I):
- The personal cleansing composition of any of the preceding claims, wherein the anionic surfactant of the co-surfactant is selected from the group consisting of fatty acyl sarcosinates, sulfosuccinates, sulfonates, sulfoacetates, acyl glycinates, acyl alaninates, acyl glutamates, lactates, lactylates, glucose carboxylates, amphoacetates, taurates, and mixtures thereof; preferably wherein the anionic surfactant of the co-surfactant is a fatty acyl sarcosinate.
- The personal cleansing composition of claim 5, wherein the fatty acyl sarcosinate is a sarcosinate according to the general formula (II):
- The personal composition of any of the preceding claims, wherein the composition exhibits a yield stress value τy from 0.005 Pa to 3 Pa, preferably from 0.02 Pa to 2 Pa according to the Herschel-Bulkley Rheology Test Method as disclosed herein.
- The personal cleansing composition of any of the preceding claims, wherein the composition exhibits a flow viscosity from 3 Pa.s to 100 Pa.s at 25°C at a shear rate of 1.5 s-1 according to the Flow Viscosity Test Method as disclosed herein.
- The personal cleansing composition of any of the preceding claims, wherein the electrolyte is selected from the group of sodium or potassium citrate, calcium chloride, calcium bromide, zinc chloride, barium chloride, calcium nitrate, potassium chloride, sodium chloride, potassium iodide, sodium bromide, ammonium bromide, sodium sulfate, and mixtures thereof, preferably wherein the electrolyte is selected from the group of sodium or potassium citrate, calcium chloride, potassium chloride, sodium chloride, and mixtures thereof.
- A method for making the personal cleansing composition according to any of the preceding claims comprising the following steps, preferably in that order:(a) providing a surfactant system, wherein the surfactant system comprises:from 0.1% to 5%, preferably from 0.2% to 4%, more preferably from 0.5% to 3.5% of a fatty acyl isethionate surfactant by weight of the composition;from 0.5% to 40%, preferably from 1% to 25%, more preferably from 5% to 15%, of a co-surfactant, wherein the co-surfactant is selected from the group consisting of an anionic surfactant being not an isethionate surfactant, a non-ionic surfactant, an amphoteric surfactant, a zwitterionic surfactant and mixtures thereof, by weight of the composition;(b) adding from 0.25% to 2% of a natural polysaccharide, by weight of the composition with no milling.
- The method according to claim 10, wherein the natural polysaccharide is added to the surfactant system from an aqueous solution comprising from 2% to 30%, preferably from 3% to 20%, more preferably from 5% to 10% of the natural polysaccharide by weight of the aqueous solution.
- A personal cleansing composition made by the method according to claim 10 or 11.
- Use of the personal cleansing composition according to any one of the claims 1-9 for suspending benefits agents selected from the group consisting of hair care and skin care benefit agents, particulates, particles, preferably silica and titanium oxide, microcapsules, oils, droplets, pigments or coloring agents, opacifiers, pearlescent agents, feel modifiers, oil absorbers, skin protectants, matting agents, friction enhancers, slip agents, conditioning agents, exfoliants, odor absorbers, or cleaning enhancers, and mixtures thereof.
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP18185894.5A EP3598967B1 (en) | 2018-07-26 | 2018-07-26 | Personal cleansing compositions, methods and uses |
| CN201980048808.8A CN112469387B (en) | 2018-07-26 | 2019-07-02 | Personal cleansing compositions, methods and uses |
| PCT/US2019/040234 WO2020023187A1 (en) | 2018-07-26 | 2019-07-02 | Personal cleansing compositions, methods and uses |
| US16/522,739 US20200030208A1 (en) | 2018-07-26 | 2019-07-26 | Personal cleansing compositions, methods and uses |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP18185894.5A EP3598967B1 (en) | 2018-07-26 | 2018-07-26 | Personal cleansing compositions, methods and uses |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3598967A1 EP3598967A1 (en) | 2020-01-29 |
| EP3598967B1 true EP3598967B1 (en) | 2023-03-15 |
Family
ID=63077824
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18185894.5A Active EP3598967B1 (en) | 2018-07-26 | 2018-07-26 | Personal cleansing compositions, methods and uses |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20200030208A1 (en) |
| EP (1) | EP3598967B1 (en) |
| CN (1) | CN112469387B (en) |
| WO (1) | WO2020023187A1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3598966A1 (en) | 2018-07-26 | 2020-01-29 | The Procter & Gamble Company | Personal cleansing compositions |
| CA3139786A1 (en) | 2019-05-21 | 2020-11-26 | Unilever Global Ip Limited | Lamellar liquid cleansers comprising acyl isethionate and methyl acyl taurate surfactant mixtures |
| US20230050014A1 (en) * | 2020-01-20 | 2023-02-16 | Phase Change Energy Solutions, Inc. | Thermal energy storage compositions and methods of using the same |
| CN115386020A (en) * | 2022-09-30 | 2022-11-25 | 南京智茂新材料科技有限公司 | Preparation method and application of organic silicon modified inulin surfactant |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5246613A (en) | 1990-07-20 | 1993-09-21 | The Procter & Gamble Company | Aqueous isotropic personal liquid cleansing composition with triethanol amine soap, selected electrolyte and synthetic surfacant |
| WO1996031187A2 (en) | 1995-04-04 | 1996-10-10 | Imperial Chemical Industries Plc | Surfactant compositions |
| US5785979A (en) | 1997-01-21 | 1998-07-28 | The Procter & Gamble Company | Personal cleansing compositions |
| WO2008074617A1 (en) | 2006-12-20 | 2008-06-26 | Unilever Plc | Stable liquid cleansing compositions comprising fatty acyl isethionate surfactants |
| US20080153730A1 (en) | 2006-12-20 | 2008-06-26 | Conopco, Inc. D/B/A Unilever | Stable liquid cleansing compositions comprising fatty acyl isethionate surfactant products with high fatty acid content |
| WO2009063250A2 (en) | 2007-11-16 | 2009-05-22 | Innospec Limited | Composition |
| WO2011120780A2 (en) | 2010-03-31 | 2011-10-06 | Unilever Plc | Personal wash cleanser with mild surfactant systems comprising defined alkanoyl compounds and defined fatty acyl isethionate surfactant product and optional skin or hair benefit agent |
| WO2015040488A2 (en) | 2013-08-06 | 2015-03-26 | Rhodia Operations | Sulfate-free structured liquid surfactants |
| US20160095804A1 (en) | 2014-10-01 | 2016-04-07 | L'oreal | Sulfate-free cleansing composition with thickener |
| WO2017189493A1 (en) | 2016-04-25 | 2017-11-02 | L'oreal | Gentle cleansing compositions with makeup removal properties |
| WO2018071353A1 (en) | 2016-10-10 | 2018-04-19 | The Procter & Gamble Company | Personal care compositions substantially free of sulfated surfactants and containing a gel network |
| US20180116937A1 (en) | 2016-10-31 | 2018-05-03 | L'oreal | Cleansing compositions with conditioning properties |
| WO2019193109A1 (en) | 2018-04-05 | 2019-10-10 | L'oreal | Cosmetic composition comprising a polysaccharide, surfactants and fragments of one or more plants |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BE406221A (en) | 1933-11-15 | |||
| US2438091A (en) | 1943-09-06 | 1948-03-16 | American Cyanamid Co | Aspartic acid esters and their preparation |
| BE498391A (en) | 1944-10-16 | |||
| BE498392A (en) | 1945-11-09 | |||
| US2528378A (en) | 1947-09-20 | 1950-10-31 | John J Mccabe Jr | Metal salts of substituted quaternary hydroxy cycloimidinic acid metal alcoholates and process for preparation of same |
| US2658072A (en) | 1951-05-17 | 1953-11-03 | Monsanto Chemicals | Process of preparing amine sulfonates and products obtained thereof |
| US5106609A (en) | 1990-05-01 | 1992-04-21 | The Procter & Gamble Company | Vehicle systems for use in cosmetic compositions |
| US5104646A (en) | 1989-08-07 | 1992-04-14 | The Procter & Gamble Company | Vehicle systems for use in cosmetic compositions |
| JP2010505028A (en) * | 2006-09-26 | 2010-02-18 | ロデイア・インコーポレーテツド | Structured surfactant system |
| US7674759B2 (en) * | 2007-09-05 | 2010-03-09 | Conopco, Inc. | Stable liquid cleansing compositions containing high level of fatty acid isethionate surfactant products having more than 10 wt. % of fatty acid/fatty soap content |
| CN103068445A (en) * | 2010-08-18 | 2013-04-24 | 荷兰联合利华有限公司 | Anti-dandruff shampoo |
| DE102011084888A1 (en) * | 2011-10-20 | 2013-04-25 | Henkel Ag & Co. Kgaa | Mild cosmetic cleaner |
| DE102013226281A1 (en) * | 2013-12-17 | 2015-06-18 | Henkel Ag & Co. Kgaa | Mild cosmetic cleaner |
-
2018
- 2018-07-26 EP EP18185894.5A patent/EP3598967B1/en active Active
-
2019
- 2019-07-02 WO PCT/US2019/040234 patent/WO2020023187A1/en active Application Filing
- 2019-07-02 CN CN201980048808.8A patent/CN112469387B/en active Active
- 2019-07-26 US US16/522,739 patent/US20200030208A1/en active Pending
Patent Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5246613A (en) | 1990-07-20 | 1993-09-21 | The Procter & Gamble Company | Aqueous isotropic personal liquid cleansing composition with triethanol amine soap, selected electrolyte and synthetic surfacant |
| WO1996031187A2 (en) | 1995-04-04 | 1996-10-10 | Imperial Chemical Industries Plc | Surfactant compositions |
| US5785979A (en) | 1997-01-21 | 1998-07-28 | The Procter & Gamble Company | Personal cleansing compositions |
| WO2008074617A1 (en) | 2006-12-20 | 2008-06-26 | Unilever Plc | Stable liquid cleansing compositions comprising fatty acyl isethionate surfactants |
| US20080153730A1 (en) | 2006-12-20 | 2008-06-26 | Conopco, Inc. D/B/A Unilever | Stable liquid cleansing compositions comprising fatty acyl isethionate surfactant products with high fatty acid content |
| WO2009063250A2 (en) | 2007-11-16 | 2009-05-22 | Innospec Limited | Composition |
| WO2011120780A2 (en) | 2010-03-31 | 2011-10-06 | Unilever Plc | Personal wash cleanser with mild surfactant systems comprising defined alkanoyl compounds and defined fatty acyl isethionate surfactant product and optional skin or hair benefit agent |
| WO2015040488A2 (en) | 2013-08-06 | 2015-03-26 | Rhodia Operations | Sulfate-free structured liquid surfactants |
| US20160095804A1 (en) | 2014-10-01 | 2016-04-07 | L'oreal | Sulfate-free cleansing composition with thickener |
| WO2017189493A1 (en) | 2016-04-25 | 2017-11-02 | L'oreal | Gentle cleansing compositions with makeup removal properties |
| WO2018071353A1 (en) | 2016-10-10 | 2018-04-19 | The Procter & Gamble Company | Personal care compositions substantially free of sulfated surfactants and containing a gel network |
| US20180116937A1 (en) | 2016-10-31 | 2018-05-03 | L'oreal | Cleansing compositions with conditioning properties |
| WO2019193109A1 (en) | 2018-04-05 | 2019-10-10 | L'oreal | Cosmetic composition comprising a polysaccharide, surfactants and fragments of one or more plants |
Non-Patent Citations (2)
| Title |
|---|
| CARNALI J. O.: "A dispersed anisotropic phase as the origin of the weak‐gel properties of aqueous xanthan gum", JOURNAL OF APPLIED POLYMER SCIENCE, JOHN WILEY & SONS, INC., US, vol. 43, no. 5, 5 September 1991 (1991-09-05), US , pages 929 - 941, XP093116830, ISSN: 0021-8995, DOI: 10.1002/app.1991.070430511 |
| DATABASE GNPD MINTEL; June 2015 (2015-06-01), "Shampoo", XP093116837, Database accession no. 3197361 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3598967A1 (en) | 2020-01-29 |
| WO2020023187A1 (en) | 2020-01-30 |
| US20200030208A1 (en) | 2020-01-30 |
| CN112469387B (en) | 2023-07-14 |
| CN112469387A (en) | 2021-03-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11638683B2 (en) | Personal cleansing compositions | |
| EP3598967B1 (en) | Personal cleansing compositions, methods and uses | |
| EP2328543B1 (en) | Stablisation of cleansing compositions containing fatty acyl isethionate surfactant products using polyols | |
| JP5698535B2 (en) | Liquid cleaning compositions containing fatty acyl isethionate products stabilized with long and short chain fatty acid / fatty soap mixtures | |
| AU2010365063B2 (en) | Liquid cleaning compositions containing long-chain fatty alcohols | |
| BRPI1013598B1 (en) | formulations for cleaning and caring for human or animal body parts, which contain esters of sorbitancarboxylic acids and use of esters of sorbitancarboxylic acid in cleaning or care formulations | |
| MX2007016588A (en) | Structured surfactant compositions. | |
| JP2010513621A (en) | Stable liquid cleaning composition comprising a fatty acyl isethionate surfactant | |
| KR20050056978A (en) | Biphasic composition induced by polydextrose | |
| US6265368B1 (en) | Aqueous detergent compositions thickened using carrageenan | |
| US9622951B2 (en) | Personal care compositions | |
| KR20150010981A (en) | Use of agglomerated hydroxyethylcellulose in pharmaceutical, personal care and household care applications | |
| EP2320852A1 (en) | Good foaming creamy or paste-like cleansers comprising floor levels of lipids or lipid mimics | |
| BR112015032668B1 (en) | fluid and sparkling composition of personal care and method for preparing a composition | |
| EP3621578B1 (en) | Low viscosity, high polyol self-foaming composition | |
| CA3233022A1 (en) | Effervescent cleansing powder composition | |
| JP2024512093A (en) | cleansing powder composition |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN PUBLISHED |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20200729 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20211126 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20220510 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| INTC | Intention to grant announced (deleted) | ||
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20221006 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602018047180 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1553581 Country of ref document: AT Kind code of ref document: T Effective date: 20230415 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230429 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20230315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230615 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20230608 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1553581 Country of ref document: AT Kind code of ref document: T Effective date: 20230315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230616 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230717 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20230601 Year of fee payment: 6 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230715 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20230531 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602018047180 Country of ref document: DE |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| 26 | Opposition filed |
Opponent name: UNILEVER IP HOLDINGS B.V./ UNILEVER GLOBAL IP LIMITED Effective date: 20231215 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20230315 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230726 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20230726 |