EP3349598B1 - Flüssigkeitsformulierung einer elektronischen dampfvorrichtung - Google Patents
Flüssigkeitsformulierung einer elektronischen dampfvorrichtung Download PDFInfo
- Publication number
- EP3349598B1 EP3349598B1 EP16766967.0A EP16766967A EP3349598B1 EP 3349598 B1 EP3349598 B1 EP 3349598B1 EP 16766967 A EP16766967 A EP 16766967A EP 3349598 B1 EP3349598 B1 EP 3349598B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- acid
- liquid formulation
- vapor
- percent
- nicotine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/28—Treatment of tobacco products or tobacco substitutes by chemical substances
- A24B15/287—Treatment of tobacco products or tobacco substitutes by chemical substances by inorganic substances only
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/10—Chemical features of tobacco products or tobacco substitutes
- A24B15/16—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/10—Chemical features of tobacco products or tobacco substitutes
- A24B15/16—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes
- A24B15/167—Chemical features of tobacco products or tobacco substitutes of tobacco substitutes in liquid or vaporisable form, e.g. liquid compositions for electronic cigarettes
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/24—Treatment of tobacco products or tobacco substitutes by extraction; Tobacco extracts
- A24B15/241—Extraction of specific substances
- A24B15/243—Nicotine
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24B—MANUFACTURE OR PREPARATION OF TOBACCO FOR SMOKING OR CHEWING; TOBACCO; SNUFF
- A24B15/00—Chemical features or treatment of tobacco; Tobacco substitutes, e.g. in liquid form
- A24B15/18—Treatment of tobacco products or tobacco substitutes
- A24B15/28—Treatment of tobacco products or tobacco substitutes by chemical substances
- A24B15/42—Treatment of tobacco products or tobacco substitutes by chemical substances by organic and inorganic substances
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F40/00—Electrically operated smoking devices; Component parts thereof; Manufacture thereof; Maintenance or testing thereof; Charging means specially adapted therefor
- A24F40/10—Devices using liquid inhalable precursors
-
- A—HUMAN NECESSITIES
- A24—TOBACCO; CIGARS; CIGARETTES; SIMULATED SMOKING DEVICES; SMOKERS' REQUISITES
- A24F—SMOKERS' REQUISITES; MATCH BOXES; SIMULATED SMOKING DEVICES
- A24F40/00—Electrically operated smoking devices; Component parts thereof; Manufacture thereof; Maintenance or testing thereof; Charging means specially adapted therefor
- A24F40/40—Constructional details, e.g. connection of cartridges and battery parts
- A24F40/48—Fluid transfer means, e.g. pumps
- A24F40/485—Valves; Apertures
Definitions
- the present invention relates to a liquid formulation for e-vaping devices.
- Electronic vaping devices are used to vaporize a liquid material into a vapor in order for a user (an adult electronic vaper or adult vaper) to inhale the vapor.
- E-vaping devices typically include a heater which vaporizes liquid material to produce a vapor.
- An e-vaping device may include several e-vaping elements including a power source, a cartridge or e-vaping tank including the heater and a reservoir capable of holding the liquid material.
- a tobacco-based smoking article typically produces a vapor known to create a familiar sensory experience for adult smokers, including a low to moderate harshness response in the throat and a perceived warmth in the chest.
- the preferred levels of harshness in the throat and perceived warmth in the chest may differ amongst adult smokers.
- Users of e-vaping devices adult vapers typically prefer vaping a device that does not generate too much harshness but that is sufficient to produce a pleasant or familiar experience.
- Liquid formulations for e-vaping devices comprising nicotine and one or more aerosol formers, such as propylene glycol, are widely available.
- CN 101 473 999 A discloses a liquid formulation for an e-vaping device with a health orientation namely preventing and curing decayed teeth.
- the formulation comprises tobacco leaf extract, 40-50% w/v propylene glycol, 10-15% w/v water, tobacco flavor, stabilizer, thickener, xylitol, L-arabinose and sodium fluoride solution.
- CN 101 461 565 discloses a health-care electronic cigarette liquid for medical use, which comprises: tobacco leaf extract, 40-50% w/v propylene glycol, 10-15% w/v pure water, tobacco flavor, stabilizer, thickener and a medicament.
- a liquid formulation for an e-vaping device comprising: a vapor former including propylene glycol, water and substantially no amount of glycerol, wherein a concentration of propylene glycol in the vapor former is 80 percent by weight and a concentration of water in the vapor former is 20 percent by weight; nicotine, wherein a concentration of nicotine in the liquid formulation is equal to or lower than 1.5 percent by weight; and one or more acids, wherein the liquid formulation is configured to form a vapor having a particulate phase and a gas phase when heated in the e-vaping device.
- the liquid formulation is configured to form a vapor having a gas phase upon operation of the e-vaping device.
- the liquid formulation includes one or more acids.
- the acid is operative upon the vapor so as to reduce an amount of nicotine content in the gas phase of the vapor.
- the vapor former including propylene glycol and substantially no glycerol or glycerin provides nicotine delivery, has a higher wicking rate and capillary efficiency in the cartomizer, evaporates easier, and generates a vapor that is less visible than a vapor formed by a vapor former including both propylene glycol and glycerol/glycerin.
- the above advantages may be due, among other reasons, to the fact that propylene glycol is substantially less viscous than glycerol and has a lower boiling point than glycerol.
- less battery power is required to generate a vapor when the vapor former includes propylene glycol and substantially no glycerol/glycerin.
- the performance of the e-vaping device is improved in terms of vapor formation efficiency and battery power usage.
- vapor phase nicotine which is the concentration of nicotine in the vapor phase of the vapor generated during vaping of the e-vaping device, is substantially increased compared to a lower evaporation rate of the vapor.
- a lower nicotine level may be used in the vapor precursor or liquid formulation of the e-vaping device.
- a nicotine level of substantially 1.5 percent, and nicotine levels that are lower than about 1.5 percent, may be used.
- nicotine levels of about 0.5 percent, about 1 percent and about 1.5 percent may be used.
- a vapor precursor or liquid formulation including a vapor former having propylene glycol and substantially no glycerol/glycerin provides the ability for the user to vape without generating a noticeable amount of vapor.
- the concentration of acids may be substantially 3.5 percent.
- the liquid formulation may include an acid having a boiling point of at least about 100 degrees Celsius and configured to volatilize when heated by a heater in the e-vaping device.
- the liquid formulation is configured to form a vapor having a particulate phase and a gas phase when heated by the heater in the e-vaping device, the particulate phase containing protonated nicotine and the gas phase containing unprotonated nicotine, and the vapor has a majority amount of the protonated nicotine and a minority amount of the unprotonated nicotine.
- the acid is operative upon the vapor so as to reduce the amount of perceived throat harshness by a user in comparison to the vapor being formed upon operation of the e-vaping device without the acid.
- the acid is selected to have a liquid to vapor transfer efficiency of about 50 percent or greater and in an amount sufficient to reduce the nicotine gas phase component compared to the nicotine gas phase component of an e-vaping device having a vapor precursor or liquid formulation that does not include the acid.
- the reduction may be of substantially 70 percent or greater.
- the acidic compound that is part of the vapor precursor or liquid formulation may include at least one of pyruvic acid, formic acid, oxalic acid, glycolic acid, acetic acid, isovaleric acid, valeric acid, propionic acid, octanoic acid, lactic acid, sorbic acid, malic acid, tartaric acid, succinic acid, citric acid, benzoic acid, oleic acid, aconitic acid, butyric acid, cinnamic acid, decanoic acid, 3,7-dimethyl-6-octenoic acid, 1-glutamic acid, heptanoic acid, hexanoic acid, 3-hexenoic acid, trans-2-hexenoic acid, isobutyric acid, lauric acid, 2-methylbutyric acid, 2-methylvaleric acid, myristic acid, nonanoic acid, palmitic acid, 4-pentenoic acid, phenylacetic acid, 3-phenyl
- the acidic compound consists of a mixture of pyruvic acid, lactic acid, benzoic acid and acetic acid.
- the liquid formulation includes water.
- Water can be included in an amount ranging from about 5 percent by weight based on the weight of the liquid formulation to about 40 percent by weight based on the weight of the liquid formulation. For example, water may be included at about 20 percent by weight based on the weight of the liquid formulation.
- first, second, third, etc. may be used herein to describe various elements, components, regions, layers or sections, these elements, components, regions, layers, or sections should not be limited by these terms. These terms are only used to distinguish one element, component, region, layer, or section from another region, layer, or section. Thus, a first element, component, region, layer, or section discussed below could be termed a second element, component, region, layer, or section without departing from the teachings of example embodiments.
- spatially relative terms for example, “beneath,” “below,” “lower,” “above,” “upper,” and the like
- the spatially relative terms are intended to encompass different orientations of the device in use or operation in addition to the orientation depicted in the figures. For example, if the device in the figures is turned over, elements described as “below” or “beneath” other elements or features would then be oriented “above” the other elements or features. Thus, the term “below” may encompass both an orientation of above and below.
- the device may be otherwise oriented (rotated 90 degrees or at other orientations) and the spatially relative descriptors used herein interpreted accordingly.
- Example embodiments are described herein with reference to cross-sectional illustrations that are schematic illustrations of idealized embodiments (and intermediate structures) of example embodiments. As such, variations from the shapes of the illustrations as a result, for example, of manufacturing techniques and tolerances, are to be expected. Thus, example embodiments should not be construed as limited to the shapes of regions illustrated herein but are to include deviations in shapes that result, for example, from manufacturing. Thus, the regions illustrated in the figures are schematic in nature and their shapes are not intended to illustrate the actual shape of a region of a device and are not intended to limit the scope of example embodiments.
- an e-vaping device in one embodiment, includes a liquid supply reservoir containing a liquid formulation.
- the liquid formulation is delivered to a heater of the e-vaping device where the liquid formulation is heated and volatilized to form a vapor upon operation of the e-vaping device.
- the liquid formulation includes a mixture of molecular nicotine (unprotonated and uncharged) and an acid, which protonates nearly all of the molecular nicotine in the liquid formulation, so that upon heating of the liquid formulation by a heater in the e-vaping device, a vapor having a majority amount of protonated nicotine and a minority amount of unprotonated nicotine is produced, whereby only a minor portion of all the vaporized nicotine typically remains in the gas phase of the vapor.
- the fraction of nicotine in the gas phase may contribute to perceptions of throat harshness or other perceived off-tastes. Reducing the proportional level of nicotine in the gas phase may improve the perceived subjective deficits associated with nicotine in the gas phase.
- a proportion of nicotine in the gas phase of the vaporized nicotine may be substantially 1.5 percent, substantially 1 percent or less of the total nicotine delivered.
- vapor former describes any suitable known compound or mixture of compounds that, in use, facilitates formation of a vapor and that is substantially resistant to thermal degradation at the operating temperature of the vapor-generating device.
- Suitable vapor-formers consist of various compositions of polyhydric alcohols such as propylene glycol. In one embodiment, the vapor is propylene glycol.
- the liquid formulation may optionally include one or more flavorants in an amount ranging from about 0.01 percent to about 15 percent by weight (for example, about 1 percent to about 12 percent, about 2 percent to about 10 percent, or about 5 percent to about 8 percent).
- the flavorant can be a natural flavorant or an artificial flavorant.
- the flavorant is one of tobacco flavor, menthol, wintergreen, peppermint, herb flavors, fruit flavors, nut flavors, liquor flavors, and combinations thereof.
- a first comparative liquid formulation solution includes about 40 percent propylene glycol (PG), about 60 percent glycerol (Gly), about 15 percent water and about 1.5 percent nicotine by weight (NBW) with substantially no acid.
- a second comparative liquid formulation solution includes about 40 percent propylene glycol, about 60 percent glycerol, about 15 percent water, about 1.5 percent nicotine by weight (NBW) and about 2 percent of menthol as a flavorant, with substantially no acid.
- a first example embodiment of a liquid formulation solution includes about 80 percent propylene glycol, substantially no glycerol, about 20 percent water and about 1.5 percent nicotine by weight (NBW) with substantially no acid.
- a second example embodiment of a liquid formulation solution includes about 80 percent propylene glycol, substantially no glycerol, about 20 percent water and about 1.5 percent nicotine by weight (NBW) with substantially no acid.
- the liquid formulation also includes substantially 2 percent menthol by weight.
- Table 1 describes the reaction of a panel of eight users (adult vapers) who performed a taste-test for the examples described above.
- the users were asked to score the overall enjoyment, or liking, of the e-vaping device, on a scale of 1 to 7.
- the users were asked to rank the "Flavor Liking or Menthol Perception" to provide an evaluation of their liking of the flavorant, and in the case where the flavorant is menthol, of their perception of the menthol in the e-vaping device.
- the users were also asked to rank the impact of the e-vaping device for each one of the comparative examples and the example embodiments, the strength being perceived in the chest of the user.
- the strength of the e-vaping device may be the perception of a strong nicotine taste in the chest of the users.
- the users also ranked the harshness of the e-vaping devices based on the various liquid compositions, the harshness being perceived in one or both of the mouth and the throat of the user.
- the harshness may be the perception of a burning sensation in one or both of the mouth and the throat of the user during use of the e-vaping device, the burning sensation being due to the combination of propylene glycol and glycerol.
- Table 1 Overall Achievement Score of Various E-Vaping Devices Expert Panel (Scale of 1-7) Comparative Example 1 (40/60 PG/Gly, 15 percent Water, 1.5 percent NBW) Comparative Example 2 (40/60 PG/Gly, 15 percent Water, 1.5 percent NBW, 2 percent Menthol) Example Embodiment 1 (80/20 PG/Water, 1.5 percent NBW, flavorant) Example Embodiment 2 (40/20 PG/ Water, 1.5 percent NBW, 2 percent Menthol) Flavor linking or Menthol Perception 3.81 3.88 4.00 4.63 Impact 3.69 3.5 4.00 4.06 Harshness 3.56 3.00 3.25 3.06 Overall Liking 3.5 3.5 3.75 3.88
- Example Embodiments 1 and 2 yield greater average scores of 3.75 and 3.88 on a scale of 1-7 compared to Comparative Examples 1 and 2, which yield average scores of 3.5 each.
- the expert panel of users concluded that e-vaping devices having liquid formulations that include a mixture of propylene glycol, water and nicotine, without including glycerin/glycerol have a more positive perception of the flavor and a better sensation of impact in the chest, harshness in one or both of the mouth and the throat, of the user.
- the following experiments also discuss the taste and perception differences among formulations that include a mixture of propylene glycol and glycerol as well as formulations that do not include glycerol.
- the amount of nicotine in the various formulations is about 1.5 percent, and the amount of water is about 15 percent.
- nicotine measurements per puff of an e-vaping device by a user have been taken with respect to a relative concentration of propylene glycol and glycerol.
- Table 2 Nicotine amount per puff with respect to liquid formulation Percentage PG-Glycerol Puff 1-20 (milligrams of nicotine/puff) Puff 21-40 (milligrams of nicotine/puff) Puff 41-60 (milligrams of nicotine/puff) Puff 61-80 (milligrams of nicotine/puff) 0-100 0.053 0.045 0.040 0.026 20-80 0.060 0.052 0.044 0.023 40-60 0.066 0.057 0.048 0.030 60-40 0.071 0.061 0.055 0.028 80-20 0.075 0.065 0.058 0.038 100-0 0.088 0.077 0.061 0.022
- the vapor mass and nicotine in the vapor generated per puff are different with different propylene glycol to glycerol ratios in the liquid formulation of the e-vaping device. Accordingly, as propylene glycol fraction in the liquid formulation increases, the inhaled vapor produces more strength or impact in the chest of the user, as evidenced by the increasing amount of nicotine per puff. The increasing amount of nicotine per puff is proportional to an increase in the concentration of propylene glycol in the liquid formulation.
- This effect may be due, among other reasons, to the fact that propylene glycol is substantially less viscous than glycerol.
- liquid formulations with increased propylene glycol typically have a higher wicking rate and capillary efficiency.
- Propylene glycol also has a lower boiling point than glycerol.
- generation of the vapor is easier for liquid formulations that have increased propylene glycol.
- less battery power is required to generate a vapor when the vapor former includes propylene glycol and substantially no glycerol because of the easier generation of vapor.
- the performance of the e-vaping device is improved in terms of vapor formation efficiency and battery power usage when more propylene glycol is provided in the liquid formulation.
- a liquid formulation including a vapor former having propylene glycol and substantially no glycerol provides the ability for the user to vape without generating vapor.
- a liquid formulation having substantially 80 percent propylene glycol, substantially 20 percent water and substantially no glycerol may provide the above advantages.
- an acid can be added to the vapor precursor, the acid having the effect of reducing the production of gas phase nicotine with typically minimal sensory and operational impact on the e-vaping device.
- the acid is added within an acceptable sensorial amount according to a sensory impact associated with the acid.
- acetic acid when added at certain levels, may impart a "vinegar" sensorial response. Accordingly, in one embodiment, the acetic acid content may be limited to levels below where such sensory impact arises.
- Other acids can also be used in combination with the acetic (or other) acid in a similar manner so as to establish an acid complex wherein the desired level of acid functionality is achieved (with multiple acids), but with each acid being included at a level below where noticeable or objectionable sensory impact may arise.
- the acid has a boiling point of at least about 100 degrees Celsius, and may be included in the liquid formulation in an amount sufficient to adjust the pH of the liquid formulation in the range of about 3 to about 8.
- the acid is included in an amount sufficient to reduce the amount of nicotine gas phase component by about 30 percent by weight or greater, preferably about 60 percent to about 70 percent by weight, more preferably, about 70 percent by weight or greater, and most preferably about 85 percent by weight or greater, of the level of nicotine gas phase component produced without the acid.
- the acid is operative upon the vapor generated from the liquid formulation upon operation of the e-vaping device so as to reduce the amount of perceived throat harshness in comparison to the vapor formed without the acid.
- the acid included in the liquid formulation includes one or more of pyruvic acid, formic acid, oxalic acid, glycolic acid, acetic acid, isovaleric acid, valeric acid, propionic acid, octanoic acid, lactic acid, levulinic acid, sorbic acid, malic acid, tartaric acid, succinic acid, citric acid, benzoic acid, oleic acid, aconitic acid, butyric acid, cinnamic acid, decanoic acid, 3,7-dimethyl-6-octenoic acid, 1-glutamic acid, heptanoic acid, hexanoic acid, 3-hexenoic acid, trans-2-hexenoic acid, isobutyric acid, lauric acid, 2-methylbutyric acid, 2-methylvaleric acid, myristic acid, nonanoic acid, palmitic acid, 4-pentenoic acid, phenylacetic acid, 3-phenyl
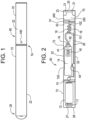
- Fig. 1 is a side view of an e-vaping device, according to a first example.
- the liquid formulation forms a vapor when vaporized in an e-vaping device 60 such as, for example, an e-vaping device, as shown in Fig. 1 .
- the e-vaping device 60 comprises a replaceable cartridge (or first section) 70 and a reusable fixture (or second section) 72, which are coupled together at a threaded joint 74 or by other connecting structure such as one or more of a snug-fit, snap-fit, detent, clamp, clasp, or the like.
- Fig. 3 is a cross-sectional view of another example of an e-vaping device.
- the first section 70 can house a mouth-end insert 20, a capillary vapor generator including a capillary tube 18, a heater 19 to heat at least a portion of the capillary tube 18, a liquid supply reservoir 14, and optionally a valve 40.
- the first section 70 can house a mouth-end insert 20, a heater 319, a flexible, filamentary wick 328 and a liquid supply reservoir 314 as discussed in further detail below.
- the second section 72 can house a power supply 12 (shown in Figs. 2 , 3 and 4 ), a control circuitry 11, and optionally a puff sensor 16 (shown in Figs. 2 and 4 ).
- the threaded portion 74 of the second section 72 can be connected to a battery charger, when not connected to the first section 70, to charge the battery or power supply 12.
- Fig. 2 is a cross-sectional view of an e-vaping device according to another example.
- the e-vaping device 60 can also include a middle section (third section) 73, which can house the liquid supply reservoir 14, the heater 19 and the valve 40.
- the middle section 73 can be configured to be fitted with a threaded joint 74' at an upstream end of the first section 70 and a threaded joint 74 at a downstream end of the second section 72.
- the first section 70 houses the mouth-end insert 20, while the second section 72 houses the power supply 12 and the control circuitry 11.
- the first section 70, the second section 72 and the optional third section 73 include an outer cylindrical housing 22 extending in a longitudinal direction along the length of the e-vaping device 60.
- the middle section 73 is disposable and one or both of the first section 70 and the second section 72 are reusable.
- the sections 70, 72, 73 can be attached by threaded connections or connectors whereby the middle section 73 can be replaced when the liquid supply reservoir 14 is used up.
- the first section 70 can also be replaceable so as to avoid the need for cleaning one or both of the capillary tube 18 and the heater 19.
- first section 70 and the second section 72 may be integrally formed without threaded connections to form a disposable e-vaping device.
- the outer cylindrical housing 22 can include a cutout or depression 102 which allows a user to manually apply pressure to the liquid supply reservoir 14.
- the outer cylindrical housing 22 is one or both of flexible and compressible along the length thereof and fully or partially covers the liquid supply reservoir 14.
- the cutout or depression 102 can extend partially about the circumference of the outer cylindrical housing 22.
- the outer cylindrical housing 22 can be formed of or include a variety of materials including plastics, rubber and combinations thereof.
- the outer cylindrical housing 22 is formed of or includes silicone.
- the outer cylindrical housing 22 can be any suitable color.
- the outer cylindrical housing 22 can include graphics or other indicia printed thereon.
- the liquid supply reservoir 14 is compressible such that when pressure is applied to the liquid supply reservoir, liquid is pumped from the liquid supply reservoir 14 to the capillary tube 18.
- a pressure activated switch 44 can be positioned beneath the liquid supply reservoir 14. When pressure is applied to the liquid supply reservoir 14 to pump liquid, the switch is also pressed and a heater 19 is activated. The heater 19 can be a portion of the capillary tube 18. By applying manual pressure to the pressure switch, the power supply 12 is activated and an electric current heats the liquid in the capillary tube 18 via electrical contacts so as to volatilize the liquid.
- the liquid supply reservoir 14 is a tubular, elongated body formed of or including an elastomeric material so as to be one or both of flexible and compressible when squeezed.
- the elastomeric material can be one of silicone, plastic, rubber, latex, and combinations thereof.
- the compressible liquid supply reservoir 14 has an outlet 17 in fluid communication with a capillary tube 18 so that when squeezed, the liquid supply reservoir 14 can deliver a volume of liquid material to the capillary tube 18.
- the power supply 12 is activated upon the application of the manual pressure on the pressure switch, and the capillary tube 18 is heated to form a heated section wherein the liquid material is volatilized.
- the volatilized material expands, mixes with air and forms a vapor.
- the liquid supply reservoir 14 extends longitudinally within the outer cylindrical housing 22 of the first section 70 (shown in Figs. 3 and 4 ) or the middle section 73 (shown in Fig. 2 ). Moreover, the liquid supply reservoir 14 contains a liquid formulation that is configured to be volatilized when heated and to form a vapor when discharged from the capillary tube 18.
- the capillary tube 18 includes an inlet end 62 in fluid communication with the outlet 17 of the liquid supply reservoir 14, and an outlet end 63 configured to expel volatilized liquid material from the capillary tube 18.
- the liquid supply reservoir 14 may include a valve 40.
- the valve 40 can be a check valve configured to maintain the liquid material within the liquid supply reservoir and to open when the liquid supply reservoir 14 is squeezed and pressure is applied to the reservoir 14.
- the check valve 40 opens when a critical, minimum pressure is reached so as to avoid inadvertent dispensing of liquid material from the liquid supply reservoir 14 or activating the heater 19.
- the critical pressure needed to open the check valve 40 is essentially equal to or slightly less than the pressure required to apply a pressure switch 44 to activate the heater 19.
- the pressure required to press the pressure switch 44 is high enough such that accidental heating is avoided. Such arrangement avoids activation of the heater 19 in the absence of liquid being pumped through the capillary.
- a check valve 40 aids in limiting the amount of liquid that is drawn back from the capillary tube upon release of pressure upon the liquid supply reservoir 14, the switch 44, or both, if manually pumped so as to avoid air uptake into the liquid supply reservoir 14. Presence of air degrades pumping performance of the liquid supply reservoir 14 and can degrade the liquid formulation.
- valve 40 closes.
- the heated capillary tube 18 discharges any liquid remaining downstream of the valve 40.
- a critical flow orifice 41 is located downstream of the check valve 40 to establish a maximum flow rate of liquid to the capillary tube 18.
- the valve 40 can be a two-way valve and the liquid supply reservoir 14 can be pressurized.
- the liquid supply reservoir 14 can be pressurized using a pressurization arrangement 405 configured to apply constant pressure to the liquid supply reservoir 14.
- pressure can be applied to the liquid supply reservoir 14 using an internal or external spring and plate arrangement which constantly applies pressure to the liquid supply reservoir 14.
- the liquid supply reservoir 14 can be compressible and positioned between two plates that are connected by springs or the liquid supply reservoir 14 could be compressible and positioned between the outer housing and a plate that are connected by a spring so that the plate applies pressure to the liquid supply reservoir 14.
- the capillary tube 18 of Figs. 2 and 3 has an internal diameter of about 0.01 millimetres to about 10 millimetres, preferably about 0.05 millimetres to about 1 millimetre, and more preferably about 0.05 millimetres to about 0.4 millimetres.
- Capillary tubes of smaller diameter provide more efficient heat transfer to the fluid because, with the shorter distance to the center of the fluid, less energy and time is required to vaporize the liquid.
- the capillary tube 18 may have a length of about 5 millimetres to about 72 millimetres, more preferably about 10 millimetres to about 60 millimetres or about 20 millimetres to about 50 millimetres. In one example, the capillary tube 18 is substantially straight. In other examples, the capillary tube 18 is coiled or includes one or more bends therein to conserve space, accommodate a long capillary tube, or both.
- the capillary tube 18 is formed of or includes a conductive material, and thus acts as its own heater 19 by passing current through the tube.
- the capillary tube 18 may be any electrically conductive material capable of being resistively heated, while retaining the necessary structural integrity at the operating temperatures experienced by the capillary tube 18, and which is non-reactive with the liquid material.
- Suitable materials for forming the capillary tube 18 are one or more of stainless steel, copper, copper alloys, porous ceramic materials coated with film resistive material, Inconel ® available from Special Metals Corporation, which is a nickel-chromium alloy, nichrome, which is also a nickel-chromium alloy, and combinations thereof.
- the capillary tube 18 is a stainless steel capillary tube 18, which serves as a heater 19 via electrical leads 26 attached thereto for passage of direct or alternating current along a length of the capillary tube 18.
- the stainless steel capillary tube 18 is heated by resistance heating.
- the stainless steel capillary tube 18 may be circular in cross section and may be formed of or include tubing suitable for use as a hypodermic needle of various gauges.
- the capillary tube 18 may comprise a 32 gauge needle having an internal diameter of about 0.11 millimetres and a 26 gauge needle having an internal diameter of about 0.26 millimetres.
- the capillary tube 18 may be a non-metallic tube such as, for example, a glass tube.
- the heater 19 is formed of or includes a conductive material capable of being resistively heated, such as, for example, stainless steel, nichrome or platinum wire, arranged along the glass tube. When the heater arranged along the glass tube is heated, liquid material in the capillary tube 18 is heated to a temperature sufficient to at least partially volatilize liquid material in the capillary tube 18.
- At least two electrical leads 26 are bonded to a metallic capillary tube 18. In an example, the at least two electrical leads 26 are coupled to the capillary tube 18. In one example, one electrical lead 26 is coupled to a first, upstream portion 101 of the capillary tube 18 and a second electrical lead 26 is coupled to a downstream, end portion 102 of the capillary tube 18, as shown in Figs. 2 and 3 .
- the liquid material contained within a heated portion of the capillary tube 18 is volatilized and ejected out of the outlet 63 where the liquid material expands and mixes with air and forms a vapor in a mixing chamber 240.
- the liquid formulation can also be used in an e-vaping device including a heater zone having at least one heater 319 and a filamentary wick 328.
- the first section 70 includes an outer tube (or casing) 22 extending in a longitudinal direction and an inner tube (or chimney) 362 coaxially positioned within the outer tube 22.
- a nose portion 361 of an upstream gasket (or seal) 320 is fitted into an upstream end portion 365 of the inner tube 362, while at the same time, an outer perimeter 367 of the gasket 320 provides a liquid-tight seal with an interior surface 397 of the outer casing 22.
- the upstream gasket 320 also includes a central, longitudinal air passage 315, which opens into an interior of the inner tube 362 that defines a central channel 321.
- a transverse channel 333 at an upstream portion of the gasket 320 intersects and communicates with the central, longitudinal air passage 315 of the gasket 320. This channel 333 assures communication between the central, longitudinal air passage 315 and a space 335 defined between the gasket 320 and a threaded connection 74.
- a nose portion 393 of a downstream gasket 310 is fitted into a downstream end portion 381 of the inner tube 362.
- An outer perimeter 382 of the gasket 310 provides a substantially liquid-tight seal with an interior surface 397 of the outer casing 22.
- the downstream gasket 310 includes a central channel 384 disposed between the central passage 321 of the inner tube 362 and the mouth-end insert 20.
- the liquid supply reservoir 314 is contained in an annulus between an inner tube 362 and an outer casing 22 and between the upstream gasket 320 and the downstream gasket 310.
- the liquid supply reservoir 314 at least partially surrounds the central air passage 321.
- the liquid supply reservoir 314 comprises a liquid material and optionally a liquid storage medium (not shown) configured to store the liquid material therein.
- the inner tube 362 has a central air passage 321 extending therethrough and that houses the heater 319.
- the heater 319 is in contact with the filamentary wick 328, which preferably extends between opposing sections of the liquid supply reservoir 314 so as to deliver the liquid formulation from the liquid supply reservoir to the heater 319.
- the e-vaping device 60 described herein also includes at least one air inlet 440. As shown in Fig. 4 , the at least one air inlet 440 can be located upstream of the heater 319.
- the at least one air inlet 440 is preferably arranged downstream of the capillary tube 18 so as to minimize drawing air along the capillary tube and thereby avoid cooling of the capillary tube 18 during heating cycles.
- the at least one air inlet 440 includes one or two air inlets. Alternatively, there may be three, four, five or more air inlets. Altering the size and number of air inlets 440 can also aid in establishing the resistance to draw of the e-vaping device 60.
- the power supply 12 of the examplescan include a battery or power supply 12 arranged in the e-vaping device 60.
- the power supply 12 is configured to apply voltage across the heater 19 associated with the capillary tube 18, as shown in Figs. 2 and 3 , or the heater 319 associated with the wick 328, as shown in Fig. 4 .
- the heater 19 or 319 volatilizes liquid material according to a power cycle of either a predetermined time period, such as a 2 to 10 second period.
- the electrical contacts or connection between the heater 19, 319 and the electrical leads 26 are substantially conductive and temperature resistant while the heater 19, 319 is substantially resistive so that heat generation occurs primarily along the heater 19 and not at the contacts.
- the battery 12 can be a lithium-ion battery or one of its variants, for example a lithium-ion polymer battery.
- the battery may be a nickel-metal hydride battery, a nickel cadmium battery, a lithium-manganese battery, a lithium-cobalt battery or a fuel cell.
- the e-vaping device 60 is usable by a smoker until the energy in the power supply is depleted.
- the power supply 12 may be rechargeable and include circuitry allowing the battery to be chargeable by an external charging device. In that case, preferably the circuitry, when charged, provides power for a pre-determined number of puffs, after which the circuitry must be re-connected to an external charging device.
- the e-vaping device 60 also includes control circuitry which can be on a printed circuit board 11 (shown in Figs. 2 , 3 and 4 ).
- the control circuitry 11 can also include a heater activation light 27 that is configured to glow when the heater 19, 319 is activated.
- the heater activation light 27 comprises at least one LED and is at an upstream end 28 (shown in Fig. 1 ) of the e-vaping device 60 so that the heater activation light 27 illuminates a cap which takes on the appearance of a burning coal during use.
- the heater activation light 27 can be configured to be visible to the adult vaper.
- the heater activation light 27 can be utilized for smoking article system diagnostics.
- the light 27 can also be configured such that the adult vaper can activate, deactivate, or activate and deactivate the light 27 when desired, such that the light 27 would not activate during vaping if desired.
- the time-period of the electric current supply to the heater 19 may be pre-set depending on the amount of liquid desired to be vaporized.
- the control circuitry 11 can be programmable and can include an application specific integrated circuit (ASIC). In other examples, the control circuitry 11 can include a microprocessor programmed to carry out functions such as heating the capillary tubes, operating the valves, or both.
- the e-vaping device 60 further includes a mouth-end insert 20 having at least two off-axis, preferably diverging outlets 21.
- the mouth-end insert 20 includes at least two diverging outlets 21 (for example, 3, 4, 5, 6 to 8 outlets or more).
- the outlets 21 of the mouth-end insert 20 are located at ends of off-axis passages 23 and are angled outwardly in relation to the longitudinal direction of the e-vaping device 60 (i.e., divergently).
- the term "off-axis" denotes at an angle to the longitudinal direction of the e-vaping device.
- the mouth-end insert (or flow guide) 20 includes outlets uniformly distributed around the mouth-end insert 20 so as to substantially uniformly distribute vapor in a user's mouth during use.
- outlets 21 and off-axis passages 23 are arranged such that droplets of unvaporized liquid material carried in the vapor impact at least one of interior surfaces of the mouth-end insert 20 and interior surfaces of the off-axis passages 23 such that the droplets are removed or broken apart.
- one or more of the outlets 21 may have a diameter of about 0.015 inch to about 0.090 inch (for example, about 0.020 inch to about 0.040 inch or about 0.028 inch to about 0.038 inch).
- the size of the outlets 21 and off-axis passages 23 along with the number of outlets 21 can be selected to adjust the resistance to draw (RTD) of the e-vaping device 60, if desired.
- the e-vaping device 60 is about the same size as a tobacco-based smoking article.
- the e-vaping device 60 can be about 80 millimetres to about 110 millimetres long, preferably about 80 millimetres to about 100 millimetres long and about 7 millimetres to about 10 millimetres in diameter.
- the e-vaping device is about 84 millimetres long and has a diameter of about 7.8 millimetres.
- the outer cylindrical housing 22 of the e-vaping device 60 may be formed of or include any suitable material or combination of materials.
- the outer cylindrical housing 22 is formed at least partially of metal and is part of the electrical circuit.
- the liquid formulation may include one of more acids from pyruvic acid, formic acid, oxalic acid, acetic acid, isovaleric acid, valeric acid, propionic acid, octanoic acid, lactic acid, levulinic acid, sorbic acid, malic acid, tartaric acid, succinic acid, citric acid, benzoic acid, oleic acid, aconitic acid, butyric acid, cinnamic acid, decanoic acid, 3,7-diemthyl-6-octenoic acid, 1-glutamic acid, heptanoic acid, hexanoic acid, 3-hexenoic acid, trans-2-hexenoic acid, isobutyric acid, lauric acid, 2-methylbutyric acid, 2-methylvaleric acid, myristic acid, nonanoic acid, palmitic acid, 4-pentenoic acid, phenylacetic acid, 3-phenylpropionic acid, hydroch
- the acid also may be incorporated into the liquid formulation in the form of a salt.
- the salt form of the acid is selected such that the addition of the acid does not have significant adverse effects on one or both of the vapor transfer efficiency and the reaction of the corresponding free acid form with nicotine.
- the acids included in the liquid formulation can have a boiling point of at least about 100 degrees Celsius.
- the acids may have a boiling point ranging from about 100 degrees Celsius to about 300 degrees Celsius or from about 150 degrees Celsius to about 250 degrees Celsius (for example, about 160 degrees Celsius to about 240 degrees Celsius, about 170 degrees Celsius to about 230 degrees Celsius, about 180 degrees Celsius to about 220 degrees Celsius or about 190 degrees Celsius to about 210 degrees Celsius).
- the acid may volatilize when heated by heater elements of e-vaping devices as previously described. In one example utilizing a heater coil and a wick, the heater coil may reach an operating temperature at or about 300 degrees Celsius.
- the acid is included in the liquid formulation in an amount sufficient to reduce the pH of the liquid formulation in the range of about 3 to about 8. In the examples, the acid is included in the liquid formulation in an amount sufficient to adjust the pH of the liquid formulation in the range of about 3 to about 5. In some other examples, the acid is included in the liquid formulation in an amount sufficient to adjust the pH of the liquid formulation in the range of about 7 to about 8. Moreover, the acid may be condensable at ambient temperature (except for HCl and other acids which are gases at ambient temperature).
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Toxicology (AREA)
- Inorganic Chemistry (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Manufacture Of Tobacco Products (AREA)
Claims (8)
- Flüssige Formulierung für eine E-Dampfvorrichtung, wobei die flüssige Formulierung umfasst:Einen Dampfbildner, der Propylenglykol, Wasser und im Wesentlichen keine Menge an Glycerin umfasst, wobei eine Konzentration an Propylenglykol in dem Dampfbildner 80 Gew.-% und eine Konzentration an Wasser in dem Dampfbildner 20 Gew.-% beträgt;Nikotin, wobei eine Konzentration von Nikotin in der flüssigen Formulierung gleich oder weniger als 1,5 Gew.-% beträgt; und eine oder mehreren Säuren,wobei die flüssige Formulierung so konfiguriert ist, dass diese einen Dampf mit einer teilchenförmigen Phase und einer Gasphase bildet, wenn sie in der e-Dampfvorrichtung erhitzt wird.
- Flüssige Formulierung nach Anspruch 1, wobei eine Konzentration von Nikotin in der flüssigen Formulierung 1,5 Gew.-% beträgt.
- Flüssige Formulierung nach Anspruch 1, wobei eine Konzentration von Nikotin in der flüssigen Formulierung 1 Gew.-% oder 0,5 Gew.-% beträgt.
- Flüssige Formulierung nach einem der vorhergehenden Ansprüche, wobei die von dem Dampfbildner gebildeten Dampfteilchen einen größeren mittleren Durchmesser als die von einem anderen Dampfbildner gebildeten Dampfteilchen aufweisen, der Glycerin enthält.
- Flüssige Formulierung nach Anspruch 4, wobei eine Verdampfungsrate der Dampfteilchen, die von dem Dampfbildner gebildet werden, größer ist als eine Verdampfungsrate der Dampfteilchen, die von dem anderen Dampfbildner einschließlich Glycerin gebildet werden.
- Flüssige Formulierung nach einem beliebigen der vorhergehenden Ansprüche, wobei die flüssige Formulierung in Form einer Lösung vorliegt.
- Die flüssige Formulierung nach einem beliebigen der vorangehenden Ansprüche umfasst ferner eine saure Verbindung, die mindestens eines der folgenden Elemente umfasst: Brenztraubensäure, Ameisensäure, Oxalsäure, Glycolsäure, Ethansäure, Isovaleriansäure, Valeriansäure, Propansäure, Octansäure, Milchsäure, Sorbinsäure, Apfelsäure, Weinsäure, Bernsteinsäure, Zitronensäure, Benzoesäure, Ölsäure, Aconitsäure, Butylsäure, Zimtsäure, Decansäure, 3,7-Dimethyl-6-Octensäure, 1-Glutaminsäure, Heptansäure, Hexansäure, 3-Hexensäure, trans-2-Hexensäure, Isobuttersäure, Laurinsäure, 2-Methylbutylsäure, 2 Methylvaleriansäure, Myristinsäure, Nonansäure, Palmitinsäure, 4-Pentensäure, Phenylessigsäure, 3-Phenylpropionsäure, Chlorwasserstoffsäure, Phosphorsäure und Schwefelsäure.
- Flüssige Formulierung nach einem beliebigen der vorhergehenden Ansprüche, die ferner Nikotinbitartrat umfasst.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/858,638 US20170079322A1 (en) | 2015-09-18 | 2015-09-18 | Liquid formulation of an electronic vapor device |
| PCT/EP2016/072075 WO2017046400A1 (en) | 2015-09-18 | 2016-09-16 | Liquid formulation of an electronic vapor device |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP3349598A1 EP3349598A1 (de) | 2018-07-25 |
| EP3349598C0 EP3349598C0 (de) | 2024-12-18 |
| EP3349598B1 true EP3349598B1 (de) | 2024-12-18 |
Family
ID=56943537
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16766967.0A Active EP3349598B1 (de) | 2015-09-18 | 2016-09-16 | Flüssigkeitsformulierung einer elektronischen dampfvorrichtung |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20170079322A1 (de) |
| EP (1) | EP3349598B1 (de) |
| JP (1) | JP6911011B2 (de) |
| KR (1) | KR102758874B1 (de) |
| CN (1) | CN108024567A (de) |
| CA (1) | CA2998967A1 (de) |
| IL (1) | IL257548A (de) |
| MX (1) | MX2018003035A (de) |
| RU (1) | RU2706839C2 (de) |
| WO (1) | WO2017046400A1 (de) |
Families Citing this family (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20160345631A1 (en) | 2005-07-19 | 2016-12-01 | James Monsees | Portable devices for generating an inhalable vapor |
| US10279934B2 (en) | 2013-03-15 | 2019-05-07 | Juul Labs, Inc. | Fillable vaporizer cartridge and method of filling |
| US10058129B2 (en) | 2013-12-23 | 2018-08-28 | Juul Labs, Inc. | Vaporization device systems and methods |
| USD825102S1 (en) | 2016-07-28 | 2018-08-07 | Juul Labs, Inc. | Vaporizer device with cartridge |
| US20160366947A1 (en) | 2013-12-23 | 2016-12-22 | James Monsees | Vaporizer apparatus |
| US10159282B2 (en) | 2013-12-23 | 2018-12-25 | Juul Labs, Inc. | Cartridge for use with a vaporizer device |
| US10076139B2 (en) | 2013-12-23 | 2018-09-18 | Juul Labs, Inc. | Vaporizer apparatus |
| USD842536S1 (en) | 2016-07-28 | 2019-03-05 | Juul Labs, Inc. | Vaporizer cartridge |
| GB2560652B8 (en) | 2013-12-23 | 2018-12-19 | Juul Labs Uk Holdco Ltd | Vaporization device systems and methods |
| EP3226704B1 (de) | 2014-12-05 | 2021-01-20 | Juul Labs, Inc. | Kalibrierte dosissteuerung |
| WO2016183573A1 (en) | 2015-05-14 | 2016-11-17 | John Cameron | Multi-chambered vaporizer and blend control |
| WO2016187111A1 (en) | 2015-05-15 | 2016-11-24 | John Cameron | Vaporizable material handling for electronic vapor device |
| WO2016187115A1 (en) | 2015-05-15 | 2016-11-24 | John Cameron | Hybrid vapor delivery system utilizing nebulized and non-nebulized elements |
| US10060639B2 (en) | 2015-06-11 | 2018-08-28 | Lunatech, Llc | Air analyzer and treatment apparatus |
| US10088463B2 (en) | 2015-06-11 | 2018-10-02 | Lunatech, Llc | Calibrating electronic vapor device |
| US10215429B2 (en) | 2015-06-15 | 2019-02-26 | Lunatech, Llc | Localized air sensing and treatment |
| US10215430B2 (en) | 2015-06-15 | 2019-02-26 | Lunatech, Llc | Electronic vapor and analysis with HVAC integration |
| US9933790B2 (en) | 2015-06-15 | 2018-04-03 | Lunatech, Llc | Peer-to-peer air analysis and treatment |
| US10042369B2 (en) | 2015-06-16 | 2018-08-07 | Lunatech, Llc | Vapor device for filtering and testing material |
| US10065138B2 (en) | 2015-06-17 | 2018-09-04 | Lunatech, Llc | Remote controllable air treatment apparatus |
| US10405571B2 (en) | 2015-06-26 | 2019-09-10 | Altria Client Services Llc | Compositions and methods for producing tobacco plants and products having altered alkaloid levels |
| US9888725B2 (en) | 2015-07-28 | 2018-02-13 | Lunatech, Llc | Inhalation puff counter gauge and display system |
| US9936737B2 (en) | 2015-10-28 | 2018-04-10 | Lunatech, Llc | Methods and systems for a dual function vapor device |
| EP3419443A4 (de) | 2016-02-11 | 2019-11-20 | Juul Labs, Inc. | Sicheres befestigen von kartuschen für verdampfervorrichtungen |
| EP3413960B1 (de) | 2016-02-11 | 2021-03-31 | Juul Labs, Inc. | Füllbare verdampferkartusche und verfahren zum füllen |
| US10405582B2 (en) | 2016-03-10 | 2019-09-10 | Pax Labs, Inc. | Vaporization device with lip sensing |
| USD849996S1 (en) | 2016-06-16 | 2019-05-28 | Pax Labs, Inc. | Vaporizer cartridge |
| USD851830S1 (en) | 2016-06-23 | 2019-06-18 | Pax Labs, Inc. | Combined vaporizer tamp and pick tool |
| USD836541S1 (en) | 2016-06-23 | 2018-12-25 | Pax Labs, Inc. | Charging device |
| US10701976B2 (en) | 2016-12-12 | 2020-07-07 | VMR Products, LLC | Vaporizer cartridge |
| GB201704999D0 (en) | 2017-03-29 | 2017-05-10 | British American Tobacco Investments Ltd | Aerosol delivery system |
| US12103020B2 (en) | 2017-04-10 | 2024-10-01 | The Procter & Gamble Company | Microfluidic delivery device and method for dispensing a fluid composition upward into the air |
| US20180290158A1 (en) * | 2017-04-10 | 2018-10-11 | The Procter & Gamble Company | Microfluidic delivery device and method of jetting a fluid composition with the same |
| US11305301B2 (en) | 2017-04-10 | 2022-04-19 | The Procter & Gamble Company | Microfluidic delivery device for dispensing and redirecting a fluid composition in the air |
| US11691162B2 (en) | 2017-04-10 | 2023-07-04 | The Procter & Gamble Company | Microfluidic delivery cartridge for use with a microfluidic delivery device |
| GB201713203D0 (en) * | 2017-08-17 | 2017-10-04 | British American Tobacco Investments Ltd | Product |
| USD887632S1 (en) | 2017-09-14 | 2020-06-16 | Pax Labs, Inc. | Vaporizer cartridge |
| US12114688B2 (en) | 2017-10-24 | 2024-10-15 | Rai Strategic Holdings, Inc. | Method for formulating aerosol precursor for aerosol delivery device |
| KR200493437Y1 (ko) * | 2017-12-25 | 2021-03-29 | 상하이 뉴 토바코 프로덕트 리서치 인스티튜트 컴퍼니 리미티드 | 에어로졸 발생 장치 및 전자 담배 |
| US10806816B2 (en) | 2018-05-15 | 2020-10-20 | The Procter & Gamble Company | Microfluidic cartridge and microfluidic delivery device comprising the same |
| US11241044B2 (en) | 2018-07-23 | 2022-02-08 | Juul Labs, Inc. | Airflow management for vaporizer device |
| US20200035118A1 (en) | 2018-07-27 | 2020-01-30 | Joseph Pandolfino | Methods and products to facilitate smokers switching to a tobacco heating product or e-cigarettes |
| US10897925B2 (en) | 2018-07-27 | 2021-01-26 | Joseph Pandolfino | Articles and formulations for smoking products and vaporizers |
| KR102878359B1 (ko) | 2018-07-31 | 2025-10-28 | 쥴 랩스, 인크. | 카트리지-기반 태우지 않고 가열하는 기화기 |
| US12256784B2 (en) | 2018-10-17 | 2025-03-25 | Juul Labs, Inc. | Cartridge for a vaporizer device |
| JP7494170B2 (ja) | 2018-11-05 | 2024-06-03 | ジュール・ラブズ・インコーポレイテッド | 気化器デバイス用のカートリッジ |
| JP7551621B2 (ja) * | 2018-12-28 | 2024-09-17 | フィリップ・モーリス・プロダクツ・ソシエテ・アノニム | 低モル質量金属塩を含む液体ニコチン製剤 |
| BR112021009438A2 (pt) * | 2018-12-28 | 2021-08-17 | Philip Morris Products S.A. | formulação de nicotina compreendendo sal metálico |
| FR3093405B1 (fr) * | 2019-03-05 | 2022-07-29 | V F P France | Compositions de vapotage |
| EP3965601B1 (de) * | 2019-05-07 | 2023-08-16 | Philip Morris Products S.A. | Leckschutzstruktur in einer verdampfervorrichtung |
| CN113710113B (zh) * | 2019-05-16 | 2025-04-18 | 菲利普莫里斯生产公司 | 装置组装方法和根据这种方法制造的装置 |
| WO2020260416A1 (en) * | 2019-06-25 | 2020-12-30 | Philip Morris Products S.A. | Carbonated liquid nicotine formulation |
| EP4140338A4 (de) * | 2020-04-22 | 2024-05-29 | Japan Tobacco Inc. | Tabakprodukt zur erwärmung ohne verbrennung, elektrisch beheiztes tabakprodukt und tabakmaterial zur erwärmung ohne verbrennung |
| CN111961032A (zh) * | 2020-06-20 | 2020-11-20 | 深圳市真味生物科技有限公司 | 一种合成尼古丁盐的制备方法及其组合物 |
| WO2022121449A1 (zh) * | 2020-12-07 | 2022-06-16 | 深圳雾芯科技有限公司 | 电子烟液及包含其的雾化装置 |
| CN113197325B (zh) * | 2021-05-13 | 2022-11-04 | 云南中烟工业有限责任公司 | 一种基于三维网络结构的超分子凝胶 |
| KR102635852B1 (ko) * | 2021-10-28 | 2024-02-13 | 주식회사 케이티앤지 | 복수의 카트리지를 구비한 에어로졸 발생 장치 |
| CN116138510A (zh) * | 2022-12-21 | 2023-05-23 | 惠州市新泓威科技有限公司 | 具有液路与电路同时阻断与导通结构的电子烟 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101461565A (zh) * | 2009-01-08 | 2009-06-24 | 华健 | 药用保健型电子烟烟液 |
| WO2013148810A1 (en) | 2012-03-28 | 2013-10-03 | R. J. Reynolds Tobacco Company | Smoking article incorporating a conductive substrate |
| CN104256888A (zh) | 2014-08-07 | 2015-01-07 | 江苏中烟工业有限责任公司 | 一种热敏性电子烟液及其制备 |
| WO2015167629A1 (en) | 2014-04-30 | 2015-11-05 | Altria Client Services Inc. | Liquid aerosol formulation of an electronic smoking article |
| WO2016071705A1 (en) | 2014-11-07 | 2016-05-12 | Nicoventures Holdings Limited | Solution comprising nicotine in unprotonated from and protonated form |
| EP3145492A1 (de) * | 2014-05-21 | 2017-03-29 | McNeil AB | Flüssige formulierung mit nikotin zur aerosolverabreichung |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0712308D0 (en) * | 2007-06-25 | 2007-08-01 | Kind Group Ltd | An inhalable composition |
| CN101461566B (zh) * | 2009-01-08 | 2012-01-18 | 华健 | 电子香烟雾化液 |
| CN101473999B (zh) * | 2009-01-21 | 2012-01-18 | 华健 | 防治龋齿保健型电子烟烟液 |
| EP2571385B1 (de) * | 2010-05-21 | 2017-01-11 | Hzat Llc. | Verfahren zur herstellung eines tabakextrakts für elektronische rauchvorrichtungen |
| ITMI20112290A1 (it) * | 2011-12-16 | 2013-06-17 | Dks Aromatic Srl | Composizione per sigarette elettroniche |
| RU122000U1 (ru) * | 2012-07-18 | 2012-11-20 | Общество с ограниченной ответственностью "САМАРИН" | Электронная сигарета с изменяемым вкусом |
| PL2925395T3 (pl) * | 2012-11-28 | 2019-08-30 | Fontem Holdings 1 B.V. | Urządzenie wytwarzające aerozol kondensacyjny z ciekłej formulacji |
| RU132954U1 (ru) * | 2013-04-26 | 2013-10-10 | Общество с ограниченной ответственностью "Инфилд" | Одноразовый электронный персональный испаритель с защитным колпачком |
| RU2659887C2 (ru) * | 2013-05-06 | 2018-07-04 | Джуул Лэбз, Инк. | Составы на основе солей никотина для аэрозольных устройств и способы их применения |
| CN105530825A (zh) * | 2013-07-19 | 2016-04-27 | 奥驰亚客户服务有限责任公司 | 电子吸烟器具的液体气溶胶制剂 |
| WO2015042412A1 (en) | 2013-09-20 | 2015-03-26 | E-Nicotine Technology. Inc. | Devices and methods for modifying delivery devices |
| CN104473322A (zh) * | 2014-12-02 | 2015-04-01 | 河南中烟工业有限责任公司 | 一种含烟碱和有机酸的电子烟烟液 |
-
2015
- 2015-09-18 US US14/858,638 patent/US20170079322A1/en not_active Abandoned
-
2016
- 2016-09-16 CN CN201680049548.2A patent/CN108024567A/zh active Pending
- 2016-09-16 EP EP16766967.0A patent/EP3349598B1/de active Active
- 2016-09-16 MX MX2018003035A patent/MX2018003035A/es unknown
- 2016-09-16 KR KR1020187005731A patent/KR102758874B1/ko active Active
- 2016-09-16 JP JP2018510806A patent/JP6911011B2/ja active Active
- 2016-09-16 RU RU2018114060A patent/RU2706839C2/ru active
- 2016-09-16 WO PCT/EP2016/072075 patent/WO2017046400A1/en not_active Ceased
- 2016-09-16 CA CA2998967A patent/CA2998967A1/en not_active Abandoned
-
2018
- 2018-02-15 IL IL257548A patent/IL257548A/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101461565A (zh) * | 2009-01-08 | 2009-06-24 | 华健 | 药用保健型电子烟烟液 |
| WO2013148810A1 (en) | 2012-03-28 | 2013-10-03 | R. J. Reynolds Tobacco Company | Smoking article incorporating a conductive substrate |
| WO2015167629A1 (en) | 2014-04-30 | 2015-11-05 | Altria Client Services Inc. | Liquid aerosol formulation of an electronic smoking article |
| EP3145492A1 (de) * | 2014-05-21 | 2017-03-29 | McNeil AB | Flüssige formulierung mit nikotin zur aerosolverabreichung |
| CN104256888A (zh) | 2014-08-07 | 2015-01-07 | 江苏中烟工业有限责任公司 | 一种热敏性电子烟液及其制备 |
| WO2016071705A1 (en) | 2014-11-07 | 2016-05-12 | Nicoventures Holdings Limited | Solution comprising nicotine in unprotonated from and protonated form |
| EP3214956A1 (de) | 2014-11-07 | 2017-09-13 | Nicoventures Holdings Limited | Lösung mit nikotin im unprotonierter und protonierte form |
Non-Patent Citations (12)
| Title |
|---|
| "The IUPAC Compendium of Chemical Terminology : The Gold Book", 24 February 2014, INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY (IUPAC), article ANONYMOUS: "solution : The Gold Book", XP093318130, DOI: 10.1351/goldbook.S05746 |
| "Tobacco: Production, Chemistry and Technology", 1 January 1999, BLACKWELL SCIENCE, article LEFFINGWELL J C: "Chapter 8 Leaf Chemistry - 8A Basic Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types ", pages: 265 - 284, XP093318126 |
| ANONYMOUS: "Tutorial: Propylene Glycol (PG) vs. Vegetable Glycerin (VG) E-juice", MIST HUB, 19 March 2014 (2014-03-19), XP093318128, Retrieved from the Internet <URL:https://misthub.com/blogs/vape-tutorials/76788613-tutorial-propylene-glycol-pg-vs-vegetable-glycerin-vg-e-juice> |
| BAASSIRI MOHAMAD, TALIH SOHA, SALMAN ROLA, KARAOGHLANIAN NAREG, SALEH RAWAD, EL HAGE RACHEL, SALIBA NAJAT, SHIHADEH ALAN: "Clouds and "throat hit": Effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols", AEROSOL SCIENCE AND TECHNOLOGY., ELSEVIER SCIENCE PUBLISHING, NEW YORK, NY., US, vol. 51, no. 11, 2 November 2017 (2017-11-02), US , pages 1231 - 1239, XP093318127, ISSN: 0278-6826, DOI: 10.1080/02786826.2017.1341040 |
| D18 - Declaration of Alice Lenney |
| JIANG HUANHUAN, GAO XIANG, GAO YONG, LIU YATAO: "Current Knowledge and Challenges of Particle Size Measurements of Mainstream E-Cigarette Aerosols and Their Implication on Respiratory Dosimetry", SCIENTIFIC HORIZONS CONSULTING, IRVINE, CA 92617, USA, vol. 3, no. 1, pages 7 - 28, XP093318120, ISSN: 2673-527X, DOI: 10.3390/jor3010003 |
| MORIE GERALD P.: "RESEARCH NOTE: FRACTION OF PROTONATED AND UNPROTONATED NlCOTINE IN TOBACCO SMOKE", TOBACCO SCIENCE, 1 January 1972 (1972-01-01), pages 167, XP093318124 |
| SON YEONGKWON, MAINELIS GEDIMINAS, DELNEVO CRISTINE, WACKOWSKI OLIVIA A., SCHWANDER STEPHAN, MENG QINGYU: "Investigating E-Cigarette Particle Emissions and Human Airway Depositions under Various E-Cigarette-Use Conditions", CHEMICAL RESEARCH IN TOXICOLOGY, AMERICAN CHEMICAL SOCIETY, WASHINGTON, DC, US, vol. 33, no. 2, 17 February 2020 (2020-02-17), US , pages 343 - 352, XP093318119, ISSN: 0893-228X, DOI: 10.1021/acs.chemrestox.9b00243 |
| TALIH SOHA, BALHAS ZAINAB, EISSENBERG THOMAS, SALMAN ROLA, KARAOGHLANIAN NAREG, EL HELLANI AHMAD, BAALBAKI RIMA, SALIBA NAJAT, SHI: "Effects of User Puff Topography, Device Voltage, and Liquid Nicotine Concentration on Electronic Cigarette Nicotine Yield: Measurements and Model Predictions", NICOTINE & TOBACCO RESEARCH, CARFAX, ABINGDON, GB, vol. 17, no. 2, 1 February 2015 (2015-02-01), GB , pages 150 - 157, XP093318114, ISSN: 1462-2203, DOI: 10.1093/ntr/ntu174 |
| TALIH SOHA, BALHAS ZAINAB, SALMAN ROLA, EL-HAGE RACHEL, KARAOGHLANIAN NAREG, EL-HELLANI AHMAD, BAASSIRI MOHAMAD, JAROUDI EZZAT, EI: "Transport phenomena governing nicotine emissions from electronic cigarettes: Model formulation and experimental investigation", AEROSOL SCIENCE AND TECHNOLOGY., ELSEVIER SCIENCE PUBLISHING, NEW YORK, NY., US, vol. 51, no. 1, 2 January 2017 (2017-01-02), US , pages 1 - 11, XP093318117, ISSN: 0278-6826, DOI: 10.1080/02786826.2016.1257853 |
| VYSHNEVA VIKTORIIA: "Effect of Propylene Glycol and Vegetable Glycerine Ratio in E-Liquid on Aerosol Formation: Overview of Relevant Properties", ADIKTOLOGIE, vol. 22, no. 2, 1 August 2022 (2022-08-01), pages 118 - 125, XP093318121, DOI: 10.35198/01-2022-002-0005 |
| W. H. MARLOW: "Topics in Current Physics: Aerosol Microphysics II - Chemical Physics of Microparticles", 1 June 1982, SPRINGER-VERLAG, ISBN: 3-540-11400-9, article P. E. WAGNER: "5. Aerosol Growth by Condensation", pages: 129 - 178, XP009563941, DOI: 10.1007/978-3-642-81805-9_5 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR102758874B1 (ko) | 2025-01-23 |
| EP3349598C0 (de) | 2024-12-18 |
| MX2018003035A (es) | 2018-05-02 |
| CN108024567A (zh) | 2018-05-11 |
| RU2018114060A (ru) | 2019-10-21 |
| RU2018114060A3 (de) | 2019-10-21 |
| KR20180055807A (ko) | 2018-05-25 |
| IL257548A (en) | 2018-04-30 |
| WO2017046400A1 (en) | 2017-03-23 |
| RU2706839C2 (ru) | 2019-11-21 |
| CA2998967A1 (en) | 2017-03-23 |
| JP6911011B2 (ja) | 2021-07-28 |
| US20170079322A1 (en) | 2017-03-23 |
| JP2018532377A (ja) | 2018-11-08 |
| EP3349598A1 (de) | 2018-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3349598B1 (de) | Flüssigkeitsformulierung einer elektronischen dampfvorrichtung | |
| US20250089758A1 (en) | Liquid aerosol formulation of an electronic smoking article | |
| EP3352591B1 (de) | Vorverdampfungsformulierung zur steuerung des säuregrades in einer elektronische zigarettenvorrichtung | |
| CN106714589B (zh) | 电子吸烟器具的液体气溶胶制剂 | |
| CA3001780C (en) | ELEMENTS FOR IMPROVING RESISTANCE, AND A METHOD FOR OBTAINING IMPROVED RESISTANCE IN AN ELECTRONIC VAPING DEVICE | |
| CN110167364B (zh) | 用于在电子蒸汽烟装置的操作期间形成有机酸的蒸汽前调配物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20171221 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20190910 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 602016090671 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: A24B0015160000 Ipc: A24B0015167000 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A24F 47/00 20060101ALI20240704BHEP Ipc: A24B 15/42 20060101ALI20240704BHEP Ipc: A24B 15/167 20200101AFI20240704BHEP |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20240812 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602016090671 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| U01 | Request for unitary effect filed |
Effective date: 20241218 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT RO SE SI Effective date: 20250102 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241218 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250318 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250319 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250318 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241218 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241218 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241218 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20250418 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241218 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20241218 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: U11 Free format text: ST27 STATUS EVENT CODE: U-0-0-U10-U11 (AS PROVIDED BY THE NATIONAL OFFICE) Effective date: 20251001 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: L10 Free format text: ST27 STATUS EVENT CODE: U-0-0-L10-L00 (AS PROVIDED BY THE NATIONAL OFFICE) Effective date: 20251006 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20250919 Year of fee payment: 10 |
|
| 26 | Opposition filed |
Opponent name: NICOVENTURES TRADING LIMITED Effective date: 20250918 |
|
| U20 | Renewal fee for the european patent with unitary effect paid |
Year of fee payment: 10 Effective date: 20250924 |