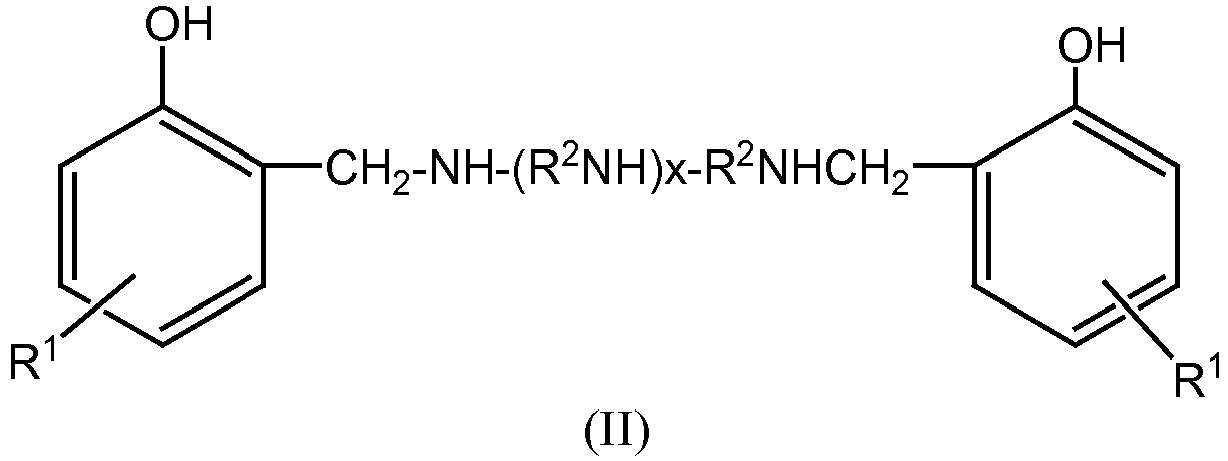EP3134496B1 - Multigrade lubricating compositions - Google Patents
Multigrade lubricating compositions Download PDFInfo
- Publication number
- EP3134496B1 EP3134496B1 EP15719586.8A EP15719586A EP3134496B1 EP 3134496 B1 EP3134496 B1 EP 3134496B1 EP 15719586 A EP15719586 A EP 15719586A EP 3134496 B1 EP3134496 B1 EP 3134496B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- lubricating
- composition
- oil
- lubricating composition
- viscosity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000000203 mixture Substances 0.000 title claims description 142
- 230000001050 lubricating effect Effects 0.000 title claims description 104
- 239000003599 detergent Substances 0.000 claims description 68
- 239000002270 dispersing agent Substances 0.000 claims description 52
- 229910052751 metal Inorganic materials 0.000 claims description 40
- 239000002184 metal Substances 0.000 claims description 40
- 239000000314 lubricant Substances 0.000 claims description 29
- 238000002485 combustion reaction Methods 0.000 claims description 19
- 239000000446 fuel Substances 0.000 claims description 16
- 238000000034 method Methods 0.000 claims description 15
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims description 14
- 239000011575 calcium Substances 0.000 claims description 14
- 229910052791 calcium Inorganic materials 0.000 claims description 14
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 claims description 13
- 239000011777 magnesium Substances 0.000 claims description 13
- 229910052749 magnesium Inorganic materials 0.000 claims description 13
- BDHFUVZGWQCTTF-UHFFFAOYSA-M sulfonate Chemical compound [O-]S(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-M 0.000 claims description 12
- ZMRQTIAUOLVKOX-UHFFFAOYSA-L calcium;diphenoxide Chemical compound [Ca+2].[O-]C1=CC=CC=C1.[O-]C1=CC=CC=C1 ZMRQTIAUOLVKOX-UHFFFAOYSA-L 0.000 claims description 3
- 239000003921 oil Substances 0.000 description 71
- 235000019198 oils Nutrition 0.000 description 68
- -1 C12 monocarboxylic acids Chemical class 0.000 description 39
- 239000000654 additive Substances 0.000 description 28
- 239000002199 base oil Substances 0.000 description 24
- 239000003795 chemical substances by application Substances 0.000 description 20
- 125000001183 hydrocarbyl group Chemical group 0.000 description 20
- 239000003963 antioxidant agent Substances 0.000 description 19
- 125000004432 carbon atom Chemical group C* 0.000 description 18
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Natural products OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 17
- 229920000768 polyamine Polymers 0.000 description 17
- KZNICNPSHKQLFF-UHFFFAOYSA-N succinimide Chemical compound O=C1CCC(=O)N1 KZNICNPSHKQLFF-UHFFFAOYSA-N 0.000 description 17
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 13
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical group [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 13
- 239000000463 material Substances 0.000 description 13
- 229910052717 sulfur Inorganic materials 0.000 description 13
- 239000011593 sulfur Substances 0.000 description 13
- 239000011701 zinc Substances 0.000 description 13
- 229910052725 zinc Inorganic materials 0.000 description 13
- 125000000217 alkyl group Chemical group 0.000 description 12
- 150000002148 esters Chemical class 0.000 description 12
- 229920000570 polyether Polymers 0.000 description 12
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 11
- 239000004721 Polyphenylene oxide Substances 0.000 description 11
- 150000001412 amines Chemical class 0.000 description 11
- DMBHHRLKUKUOEG-UHFFFAOYSA-N diphenylamine Chemical class C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 11
- 229910052698 phosphorus Inorganic materials 0.000 description 11
- 239000011574 phosphorus Substances 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- 239000003607 modifier Substances 0.000 description 10
- 125000001931 aliphatic group Chemical group 0.000 description 9
- 230000003078 antioxidant effect Effects 0.000 description 9
- 229920001577 copolymer Polymers 0.000 description 9
- 238000005260 corrosion Methods 0.000 description 9
- 230000007797 corrosion Effects 0.000 description 9
- 239000003112 inhibitor Substances 0.000 description 9
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 9
- 229960001860 salicylate Drugs 0.000 description 9
- 239000001993 wax Substances 0.000 description 9
- 239000002253 acid Substances 0.000 description 8
- 125000002947 alkylene group Chemical group 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 150000002989 phenols Chemical class 0.000 description 8
- 150000003242 quaternary ammonium salts Chemical class 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- 239000000344 soap Substances 0.000 description 8
- 239000004071 soot Substances 0.000 description 8
- 229960002317 succinimide Drugs 0.000 description 8
- 230000000996 additive effect Effects 0.000 description 7
- 150000004982 aromatic amines Chemical class 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N nitrogen Substances N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 7
- 125000001424 substituent group Chemical group 0.000 description 7
- 239000004034 viscosity adjusting agent Substances 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 229920002367 Polyisobutene Polymers 0.000 description 6
- 125000003118 aryl group Chemical group 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 125000005266 diarylamine group Chemical group 0.000 description 6
- 229930195733 hydrocarbon Natural products 0.000 description 6
- 150000002430 hydrocarbons Chemical class 0.000 description 6
- 239000005078 molybdenum compound Substances 0.000 description 6
- 150000002752 molybdenum compounds Chemical class 0.000 description 6
- 239000010705 motor oil Substances 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 150000003873 salicylate salts Chemical class 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 239000010936 titanium Substances 0.000 description 6
- 229910052719 titanium Inorganic materials 0.000 description 6
- 150000003609 titanium compounds Chemical class 0.000 description 6
- 239000004215 Carbon black (E152) Substances 0.000 description 5
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 5
- 150000007513 acids Chemical class 0.000 description 5
- 229910052782 aluminium Inorganic materials 0.000 description 5
- 239000002585 base Substances 0.000 description 5
- FPYJFEHAWHCUMM-UHFFFAOYSA-N maleic anhydride Chemical compound O=C1OC(=O)C=C1 FPYJFEHAWHCUMM-UHFFFAOYSA-N 0.000 description 5
- 229910052750 molybdenum Inorganic materials 0.000 description 5
- 239000011733 molybdenum Substances 0.000 description 5
- 229920005862 polyol Polymers 0.000 description 5
- 150000003871 sulfonates Chemical class 0.000 description 5
- 230000002378 acidificating effect Effects 0.000 description 4
- 150000001336 alkenes Chemical class 0.000 description 4
- 230000029936 alkylation Effects 0.000 description 4
- 238000005804 alkylation reaction Methods 0.000 description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 4
- 150000001408 amides Chemical class 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 125000002573 ethenylidene group Chemical group [*]=C=C([H])[H] 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000010687 lubricating oil Substances 0.000 description 4
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 4
- 150000002924 oxiranes Chemical class 0.000 description 4
- 229920013639 polyalphaolefin Polymers 0.000 description 4
- 229920000098 polyolefin Polymers 0.000 description 4
- 150000003077 polyols Chemical class 0.000 description 4
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 4
- DKCPKDPYUFEZCP-UHFFFAOYSA-N 2,6-di-tert-butylphenol Chemical compound CC(C)(C)C1=CC=CC(C(C)(C)C)=C1O DKCPKDPYUFEZCP-UHFFFAOYSA-N 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- 229920000089 Cyclic olefin copolymer Polymers 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 3
- 239000005977 Ethylene Substances 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 3
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical class C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 3
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 150000001735 carboxylic acids Chemical class 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 235000014113 dietary fatty acids Nutrition 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 229930195729 fatty acid Natural products 0.000 description 3
- 239000000194 fatty acid Substances 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 150000002194 fatty esters Chemical class 0.000 description 3
- 239000006260 foam Substances 0.000 description 3
- WOLATMHLPFJRGC-UHFFFAOYSA-N furan-2,5-dione;styrene Chemical compound O=C1OC(=O)C=C1.C=CC1=CC=CC=C1 WOLATMHLPFJRGC-UHFFFAOYSA-N 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 150000003949 imides Chemical group 0.000 description 3
- 150000002646 long chain fatty acid esters Chemical class 0.000 description 3
- 239000002480 mineral oil Substances 0.000 description 3
- LVZUNTGFCXNQAF-UHFFFAOYSA-N n-nonyl-n-phenylaniline Chemical compound C=1C=CC=CC=1N(CCCCCCCCC)C1=CC=CC=C1 LVZUNTGFCXNQAF-UHFFFAOYSA-N 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 239000002530 phenolic antioxidant Substances 0.000 description 3
- 229920000193 polymethacrylate Polymers 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 239000010689 synthetic lubricating oil Substances 0.000 description 3
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- VOJUXHHACRXLTD-UHFFFAOYSA-N 1,4-dihydroxy-2-naphthoic acid Chemical compound C1=CC=CC2=C(O)C(C(=O)O)=CC(O)=C21 VOJUXHHACRXLTD-UHFFFAOYSA-N 0.000 description 2
- QVXGKJYMVLJYCL-UHFFFAOYSA-N 2,3-di(nonyl)-N-phenylaniline Chemical compound C(CCCCCCCC)C=1C(=C(C=CC1)NC1=CC=CC=C1)CCCCCCCCC QVXGKJYMVLJYCL-UHFFFAOYSA-N 0.000 description 2
- WXTMDXOMEHJXQO-UHFFFAOYSA-N 2,5-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC=C1O WXTMDXOMEHJXQO-UHFFFAOYSA-N 0.000 description 2
- AKEUNCKRJATALU-UHFFFAOYSA-N 2,6-dihydroxybenzoic acid Chemical compound OC(=O)C1=C(O)C=CC=C1O AKEUNCKRJATALU-UHFFFAOYSA-N 0.000 description 2
- GOXQRTZXKQZDDN-UHFFFAOYSA-N 2-Ethylhexyl acrylate Chemical compound CCCCC(CC)COC(=O)C=C GOXQRTZXKQZDDN-UHFFFAOYSA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- 229910000838 Al alloy Inorganic materials 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 2
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- XQVWYOYUZDUNRW-UHFFFAOYSA-N N-Phenyl-1-naphthylamine Chemical compound C=1C=CC2=CC=CC=C2C=1NC1=CC=CC=C1 XQVWYOYUZDUNRW-UHFFFAOYSA-N 0.000 description 2
- 239000005642 Oleic acid Substances 0.000 description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 2
- GLOYGJPNNKTDIG-UHFFFAOYSA-N SC=1N=NSC=1S Chemical class SC=1N=NSC=1S GLOYGJPNNKTDIG-UHFFFAOYSA-N 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 2
- 125000003342 alkenyl group Chemical group 0.000 description 2
- 150000004703 alkoxides Chemical class 0.000 description 2
- 229940053200 antiepileptics fatty acid derivative Drugs 0.000 description 2
- 150000001491 aromatic compounds Chemical class 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 125000002837 carbocyclic group Chemical group 0.000 description 2
- 230000006315 carbonylation Effects 0.000 description 2
- 238000005810 carbonylation reaction Methods 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000012612 commercial material Substances 0.000 description 2
- 239000012141 concentrate Substances 0.000 description 2
- 239000007859 condensation product Substances 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 239000013020 final formulation Substances 0.000 description 2
- LNTHITQWFMADLM-UHFFFAOYSA-N gallic acid Chemical compound OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 description 2
- 229910052736 halogen Inorganic materials 0.000 description 2
- 150000002367 halogens Chemical class 0.000 description 2
- 150000002462 imidazolines Chemical class 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 150000004668 long chain fatty acids Chemical class 0.000 description 2
- 238000005461 lubrication Methods 0.000 description 2
- 239000006078 metal deactivator Substances 0.000 description 2
- 239000010688 mineral lubricating oil Substances 0.000 description 2
- 235000010446 mineral oil Nutrition 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- KHYKFSXXGRUKRE-UHFFFAOYSA-J molybdenum(4+) tetracarbamodithioate Chemical class C(N)([S-])=S.[Mo+4].C(N)([S-])=S.C(N)([S-])=S.C(N)([S-])=S KHYKFSXXGRUKRE-UHFFFAOYSA-J 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- RQVGZVZFVNMBGS-UHFFFAOYSA-N n-octyl-n-phenylaniline Chemical compound C=1C=CC=CC=1N(CCCCCCCC)C1=CC=CC=C1 RQVGZVZFVNMBGS-UHFFFAOYSA-N 0.000 description 2
- VBEGHXKAFSLLGE-UHFFFAOYSA-N n-phenylnitramide Chemical compound [O-][N+](=O)NC1=CC=CC=C1 VBEGHXKAFSLLGE-UHFFFAOYSA-N 0.000 description 2
- 125000004433 nitrogen atom Chemical group N* 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 125000005702 oxyalkylene group Chemical group 0.000 description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 2
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 2
- 239000003208 petroleum Substances 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 229960004889 salicylic acid Drugs 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 125000001302 tertiary amino group Chemical group 0.000 description 2
- UMGDCJDMYOKAJW-UHFFFAOYSA-N thiourea Chemical compound NC(N)=S UMGDCJDMYOKAJW-UHFFFAOYSA-N 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- WBYWAXJHAXSJNI-VOTSOKGWSA-M .beta-Phenylacrylic acid Natural products [O-]C(=O)\C=C\C1=CC=CC=C1 WBYWAXJHAXSJNI-VOTSOKGWSA-M 0.000 description 1
- 150000000178 1,2,4-triazoles Chemical class 0.000 description 1
- 150000000180 1,2-diols Chemical group 0.000 description 1
- KGRVJHAUYBGFFP-UHFFFAOYSA-N 2,2'-Methylenebis(4-methyl-6-tert-butylphenol) Chemical compound CC(C)(C)C1=CC(C)=CC(CC=2C(=C(C=C(C)C=2)C(C)(C)C)O)=C1O KGRVJHAUYBGFFP-UHFFFAOYSA-N 0.000 description 1
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 description 1
- BVUXDWXKPROUDO-UHFFFAOYSA-N 2,6-di-tert-butyl-4-ethylphenol Chemical compound CCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 BVUXDWXKPROUDO-UHFFFAOYSA-N 0.000 description 1
- SZATXRHXOOLEFV-UHFFFAOYSA-N 2,6-ditert-butyl-4-dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 SZATXRHXOOLEFV-UHFFFAOYSA-N 0.000 description 1
- STHGHFNAPPFPQV-UHFFFAOYSA-N 2,6-ditert-butyl-4-propylphenol Chemical compound CCCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 STHGHFNAPPFPQV-UHFFFAOYSA-N 0.000 description 1
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 1
- JAHNSTQSQJOJLO-UHFFFAOYSA-N 2-(3-fluorophenyl)-1h-imidazole Chemical compound FC1=CC=CC(C=2NC=CN=2)=C1 JAHNSTQSQJOJLO-UHFFFAOYSA-N 0.000 description 1
- QDCPNGVVOWVKJG-VAWYXSNFSA-N 2-[(e)-dodec-1-enyl]butanedioic acid Chemical compound CCCCCCCCCC\C=C\C(C(O)=O)CC(O)=O QDCPNGVVOWVKJG-VAWYXSNFSA-N 0.000 description 1
- KTXWGMUMDPYXNN-UHFFFAOYSA-N 2-ethylhexan-1-olate;titanium(4+) Chemical compound [Ti+4].CCCCC(CC)C[O-].CCCCC(CC)C[O-].CCCCC(CC)C[O-].CCCCC(CC)C[O-] KTXWGMUMDPYXNN-UHFFFAOYSA-N 0.000 description 1
- ROGIWVXWXZRRMZ-UHFFFAOYSA-N 2-methylbuta-1,3-diene;styrene Chemical class CC(=C)C=C.C=CC1=CC=CC=C1 ROGIWVXWXZRRMZ-UHFFFAOYSA-N 0.000 description 1
- NFCPRRWCTNLGSN-UHFFFAOYSA-N 2-n-phenylbenzene-1,2-diamine Chemical compound NC1=CC=CC=C1NC1=CC=CC=C1 NFCPRRWCTNLGSN-UHFFFAOYSA-N 0.000 description 1
- GPNYZBKIGXGYNU-UHFFFAOYSA-N 2-tert-butyl-6-[(3-tert-butyl-5-ethyl-2-hydroxyphenyl)methyl]-4-ethylphenol Chemical compound CC(C)(C)C1=CC(CC)=CC(CC=2C(=C(C=C(CC)C=2)C(C)(C)C)O)=C1O GPNYZBKIGXGYNU-UHFFFAOYSA-N 0.000 description 1
- QTLJDONMABRFLR-UHFFFAOYSA-N 3,5-dihydroxynaphthalene-1-carboxylic acid Chemical compound C1=CC=C2C(C(=O)O)=CC(O)=CC2=C1O QTLJDONMABRFLR-UHFFFAOYSA-N 0.000 description 1
- YWQIVUUGLJFRHG-UHFFFAOYSA-N 3,7-dihydroxynaphthalene-1-carboxylic acid Chemical compound C1=C(O)C=C2C(C(=O)O)=CC(O)=CC2=C1 YWQIVUUGLJFRHG-UHFFFAOYSA-N 0.000 description 1
- WVRNUXJQQFPNMN-VAWYXSNFSA-N 3-[(e)-dodec-1-enyl]oxolane-2,5-dione Chemical compound CCCCCCCCCC\C=C\C1CC(=O)OC1=O WVRNUXJQQFPNMN-VAWYXSNFSA-N 0.000 description 1
- OFNISBHGPNMTMS-UHFFFAOYSA-N 3-methylideneoxolane-2,5-dione Chemical compound C=C1CC(=O)OC1=O OFNISBHGPNMTMS-UHFFFAOYSA-N 0.000 description 1
- MDWVSAYEQPLWMX-UHFFFAOYSA-N 4,4'-Methylenebis(2,6-di-tert-butylphenol) Chemical compound CC(C)(C)C1=C(O)C(C(C)(C)C)=CC(CC=2C=C(C(O)=C(C=2)C(C)(C)C)C(C)(C)C)=C1 MDWVSAYEQPLWMX-UHFFFAOYSA-N 0.000 description 1
- UNBOSJFEZZJZLR-UHFFFAOYSA-N 4-(4-nitrophenylazo)aniline Chemical compound C1=CC(N)=CC=C1N=NC1=CC=C([N+]([O-])=O)C=C1 UNBOSJFEZZJZLR-UHFFFAOYSA-N 0.000 description 1
- KJWMCPYEODZESQ-UHFFFAOYSA-N 4-Dodecylphenol Chemical compound CCCCCCCCCCCCC1=CC=C(O)C=C1 KJWMCPYEODZESQ-UHFFFAOYSA-N 0.000 description 1
- WTWGHNZAQVTLSQ-UHFFFAOYSA-N 4-butyl-2,6-ditert-butylphenol Chemical compound CCCCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 WTWGHNZAQVTLSQ-UHFFFAOYSA-N 0.000 description 1
- CMGDVUCDZOBDNL-UHFFFAOYSA-N 4-methyl-2h-benzotriazole Chemical compound CC1=CC=CC2=NNN=C12 CMGDVUCDZOBDNL-UHFFFAOYSA-N 0.000 description 1
- DQKVMDNCLZIACU-UHFFFAOYSA-J 7,7-dimethyloctanoate;titanium(4+) Chemical compound [Ti+4].CC(C)(C)CCCCCC([O-])=O.CC(C)(C)CCCCCC([O-])=O.CC(C)(C)CCCCCC([O-])=O.CC(C)(C)CCCCCC([O-])=O DQKVMDNCLZIACU-UHFFFAOYSA-J 0.000 description 1
- RREANTFLPGEWEN-MBLPBCRHSA-N 7-[4-[[(3z)-3-[4-amino-5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidin-2-yl]imino-5-fluoro-2-oxoindol-1-yl]methyl]piperazin-1-yl]-1-cyclopropyl-6-fluoro-4-oxoquinoline-3-carboxylic acid Chemical group COC1=C(OC)C(OC)=CC(CC=2C(=NC(\N=C/3C4=CC(F)=CC=C4N(CN4CCN(CC4)C=4C(=CC=5C(=O)C(C(O)=O)=CN(C=5C=4)C4CC4)F)C\3=O)=NC=2)N)=C1 RREANTFLPGEWEN-MBLPBCRHSA-N 0.000 description 1
- YJKJAYFKPIUBAW-UHFFFAOYSA-N 9h-carbazol-1-amine Chemical compound N1C2=CC=CC=C2C2=C1C(N)=CC=C2 YJKJAYFKPIUBAW-UHFFFAOYSA-N 0.000 description 1
- PNEYBMLMFCGWSK-UHFFFAOYSA-N Alumina Chemical class [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- UUNBFTCKFYBASS-UHFFFAOYSA-N C(CCCCCCC)C=1C(=C(C=CC1)NC1=CC=CC=C1)CCCCCCCC Chemical compound C(CCCCCCC)C=1C(=C(C=CC1)NC1=CC=CC=C1)CCCCCCCC UUNBFTCKFYBASS-UHFFFAOYSA-N 0.000 description 1
- WBYWAXJHAXSJNI-SREVYHEPSA-N Cinnamic acid Chemical compound OC(=O)\C=C/C1=CC=CC=C1 WBYWAXJHAXSJNI-SREVYHEPSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 229910001366 Hypereutectic aluminum Inorganic materials 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 1
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 150000007933 aliphatic carboxylic acids Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- 125000005037 alkyl phenyl group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 229920005603 alternating copolymer Polymers 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 150000005005 aminopyrimidines Chemical class 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000007866 anti-wear additive Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 150000003819 basic metal compounds Chemical class 0.000 description 1
- 150000001556 benzimidazoles Chemical class 0.000 description 1
- 150000001565 benzotriazoles Chemical class 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical class C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 150000001639 boron compounds Chemical class 0.000 description 1
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical class C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 description 1
- SNCZNSNPXMPCGN-UHFFFAOYSA-N butanediamide Chemical compound NC(=O)CCC(N)=O SNCZNSNPXMPCGN-UHFFFAOYSA-N 0.000 description 1
- DFMYXZSEXKBYDI-UHFFFAOYSA-N butyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate Chemical compound CCCCOC(=O)CCC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 DFMYXZSEXKBYDI-UHFFFAOYSA-N 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001721 carbon Chemical group 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 230000021523 carboxylation Effects 0.000 description 1
- 238000006473 carboxylation reaction Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 238000010531 catalytic reduction reaction Methods 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 229930016911 cinnamic acid Natural products 0.000 description 1
- 235000013985 cinnamic acid Nutrition 0.000 description 1
- HNEGQIOMVPPMNR-IHWYPQMZSA-N citraconic acid Chemical compound OC(=O)C(/C)=C\C(O)=O HNEGQIOMVPPMNR-IHWYPQMZSA-N 0.000 description 1
- 229940018557 citraconic acid Drugs 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 239000003245 coal Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000000994 depressogenic effect Effects 0.000 description 1
- 150000001991 dicarboxylic acids Chemical class 0.000 description 1
- 239000010710 diesel engine oil Substances 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- 150000002009 diols Chemical group 0.000 description 1
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical class C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 description 1
- LTYMSROWYAPPGB-UHFFFAOYSA-N diphenyl sulfide Chemical class C=1C=CC=CC=1SC1=CC=CC=C1 LTYMSROWYAPPGB-UHFFFAOYSA-N 0.000 description 1
- 230000032050 esterification Effects 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- HQQADJVZYDDRJT-UHFFFAOYSA-N ethene;prop-1-ene Chemical group C=C.CC=C HQQADJVZYDDRJT-UHFFFAOYSA-N 0.000 description 1
- 125000001033 ether group Chemical group 0.000 description 1
- 238000006266 etherification reaction Methods 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 125000000816 ethylene group Chemical group [H]C([H])([*:1])C([H])([H])[*:2] 0.000 description 1
- 230000005496 eutectics Effects 0.000 description 1
- 125000001153 fluoro group Chemical group F* 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 125000002541 furyl group Chemical group 0.000 description 1
- 229940074391 gallic acid Drugs 0.000 description 1
- 235000004515 gallic acid Nutrition 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 125000004356 hydroxy functional group Chemical group O* 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 125000002883 imidazolyl group Chemical group 0.000 description 1
- 230000003116 impacting effect Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- WBYWAXJHAXSJNI-UHFFFAOYSA-N methyl p-hydroxycinnamate Natural products OC(=O)C=CC1=CC=CC=C1 WBYWAXJHAXSJNI-UHFFFAOYSA-N 0.000 description 1
- LVHBHZANLOWSRM-UHFFFAOYSA-N methylenebutanedioic acid Natural products OC(=O)CC(=C)C(O)=O LVHBHZANLOWSRM-UHFFFAOYSA-N 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- LSHROXHEILXKHM-UHFFFAOYSA-N n'-[2-[2-[2-(2-aminoethylamino)ethylamino]ethylamino]ethyl]ethane-1,2-diamine Chemical compound NCCNCCNCCNCCNCCN LSHROXHEILXKHM-UHFFFAOYSA-N 0.000 description 1
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 1
- CDYHCLPQXKUDMV-UHFFFAOYSA-N n-decyl-n-phenylaniline Chemical compound C=1C=CC=CC=1N(CCCCCCCCCC)C1=CC=CC=C1 CDYHCLPQXKUDMV-UHFFFAOYSA-N 0.000 description 1
- RRTKAPLFMTUHDH-UHFFFAOYSA-N n-octyloctanamide Chemical compound CCCCCCCCNC(=O)CCCCCCC RRTKAPLFMTUHDH-UHFFFAOYSA-N 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 150000002825 nitriles Chemical class 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 125000000018 nitroso group Chemical group N(=O)* 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- ATGUVEKSASEFFO-UHFFFAOYSA-N p-aminodiphenylamine Chemical compound C1=CC(N)=CC=C1NC1=CC=CC=C1 ATGUVEKSASEFFO-UHFFFAOYSA-N 0.000 description 1
- WXZMFSXDPGVJKK-UHFFFAOYSA-N pentaerythritol Chemical compound OCC(CO)(CO)CO WXZMFSXDPGVJKK-UHFFFAOYSA-N 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- 150000003018 phosphorus compounds Chemical class 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000151 polyglycol Polymers 0.000 description 1
- 239000010695 polyglycol Substances 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920006389 polyphenyl polymer Polymers 0.000 description 1
- 229920005606 polypropylene copolymer Polymers 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 125000004076 pyridyl group Chemical group 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- CQRYARSYNCAZFO-UHFFFAOYSA-N salicyl alcohol Chemical compound OCC1=CC=CC=C1O CQRYARSYNCAZFO-UHFFFAOYSA-N 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- RINCXYDBBGOEEQ-UHFFFAOYSA-N succinic anhydride Chemical class O=C1CCC(=O)O1 RINCXYDBBGOEEQ-UHFFFAOYSA-N 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical class [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- FAGUFWYHJQFNRV-UHFFFAOYSA-N tetraethylenepentamine Chemical compound NCCNCCNCCNCCN FAGUFWYHJQFNRV-UHFFFAOYSA-N 0.000 description 1
- 125000001544 thienyl group Chemical group 0.000 description 1
- VXUYXOFXAQZZMF-UHFFFAOYSA-N titanium(IV) isopropoxide Chemical compound CC(C)O[Ti](OC(C)C)(OC(C)C)OC(C)C VXUYXOFXAQZZMF-UHFFFAOYSA-N 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M169/00—Lubricating compositions characterised by containing as components a mixture of at least two types of ingredient selected from base-materials, thickeners or additives, covered by the preceding groups, each of these compounds being essential
- C10M169/04—Mixtures of base-materials and additives
- C10M169/045—Mixtures of base-materials and additives the additives being a mixture of compounds of unknown or incompletely defined constitution and non-macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M169/00—Lubricating compositions characterised by containing as components a mixture of at least two types of ingredient selected from base-materials, thickeners or additives, covered by the preceding groups, each of these compounds being essential
- C10M169/04—Mixtures of base-materials and additives
- C10M169/048—Mixtures of base-materials and additives the additives being a mixture of compounds of unknown or incompletely defined constitution, non-macromolecular and macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2203/00—Organic non-macromolecular hydrocarbon compounds and hydrocarbon fractions as ingredients in lubricant compositions
- C10M2203/10—Petroleum or coal fractions, e.g. tars, solvents, bitumen
- C10M2203/102—Aliphatic fractions
- C10M2203/1025—Aliphatic fractions used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
- C10M2205/022—Ethene
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2205/00—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions
- C10M2205/02—Organic macromolecular hydrocarbon compounds or fractions, whether or not modified by oxidation as ingredients in lubricant compositions containing acyclic monomers
- C10M2205/024—Propene
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/028—Overbased salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/26—Overbased carboxylic acid salts
- C10M2207/262—Overbased carboxylic acid salts derived from hydroxy substituted aromatic acids, e.g. salicylates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/28—Amides; Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/06—Macromolecular compounds obtained by functionalisation op polymers with a nitrogen containing compound
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/04—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions containing sulfur-to-oxygen bonds, i.e. sulfones, sulfoxides
- C10M2219/046—Overbasedsulfonic acid salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/04—Groups 2 or 12
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/02—Viscosity; Viscosity index
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2020/00—Specified physical or chemical properties or characteristics, i.e. function, of component of lubricating compositions
- C10N2020/01—Physico-chemical properties
- C10N2020/04—Molecular weight; Molecular weight distribution
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/02—Pour-point; Viscosity index
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/04—Detergent property or dispersant property
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/06—Oiliness; Film-strength; Anti-wear; Resistance to extreme pressure
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/52—Base number [TBN]
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2030/00—Specified physical or chemical properties which is improved by the additive characterising the lubricating composition, e.g. multifunctional additives
- C10N2030/54—Fuel economy
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
- C10N2040/252—Diesel engines
Definitions
- the disclosed technology relates to a lubricating composition for a heavy duty diesel engine that provides for improved fuel economy without reducing durability (i.e. anti-wear) performance of the engine. This may be achieved through the use of high viscosity index base oils in combination with ashless dispersants and overbased metal-containing detergents.
- Lubricating oil compositions are used for the smooth operation of internal combustion engines.
- the engine oils for internal combustion engines in particular serve to (i) lubricate various sliding interfaces between the piston ring and cylinder liner, in bearings of the crank shaft and the connecting rod, and in the valve driving mechanism including cams and valve lifters, (ii) cool the engine, (iii) clean and disperse the combustion products and (iv) prevent corrosion and consequent rust formation.
- the stringent requirements for high performance engines in recent years has meant greater demand from lubricants used in such engines.
- Polymeric viscosity index improvers are typically added to lubricating base oils, including highly refined mineral oils, to improve the viscosity-temperature characteristics and/or low-temperature viscosity characteristics of the lubricating compositions.
- the viscosity index is commonly evaluated as the viscosity-temperature characteristic of lubricating base oils and lubricating compositions, while the properties evaluated for the low-temperature viscosity characteristics are generally the pour point, cloud point and freezing point.
- Polymeric viscosity index improvers have also been implicated in deposit formation.
- EP 2 610 333 A1 discloses fuel economical lubricating oil compositions for internal combustion engines.
- the objectives of the invention described herein include providing improved fuel economy while also providing at least one of (i) reduced wear (such as cam wear or lifter wear), (ii) decreased deposit formation, (iii) improved soot handling, (iv) reduced lead or copper corrosion, (v) increased oxidation resistance, and/or (vi) improved seal compatibility in an internal combustion engine.
- the objectives of the present invention includes providing improved fuel economy while also providing at least one of (i) reduced wear or (ii) decreased deposit formation, especially in compression ignition (i.e. diesel) engines.
- the invention described herein provides a multigrade lubricating , comprising: (a) 80 wt % to 95 wt % of an oil of lubricating viscosity having a viscosity index of at least 130; (b) an ashless dispersant; and (c) an overbased metal detergent; wherein the lubricating composition contains less than 0.01 weight percent of a polymeric viscosity index improver; and the lubricant composition has a SAE viscosity grade of XW-Y, wherein X may be 0, 5, or 10; and Y may be 16, 20, 26, 30, or 40.
- the invention further provides a lubricating composition as described herein wherein the multigrade crankcase lubricant is a SAE 0W-16, 0W-20, 0W-26, 5W-16, 5W-20, 5W-26, 5W-30, 10W-16, 10W-30, or 10W-40 lubricant.
- the multigrade crankcase lubricant is a SAE 0W-16, 0W-20, 0W-26, 5W-16, 5W-20, 5W-26, 5W-30, 10W-16, 10W-30, or 10W-40 lubricant.
- the invention further provides a lubricating composition as described herein in which the ashless dispersant is derived from a polyolefin having a number average molecular weight of 500 to 5000.
- the invention further provides a lubricating composition as described herein in which the metal-containing overbased detergent includes one or more of a calcium sulfonate, a calcium phenate, a magnesium sulfonate or a magnesium phenate.
- the invention further provides a lubricating composition as described herein in which the metal-containing overbased detergent is present in an amount to deliver at least 4 TBN to the composition.
- the invention further provides a lubricating composition as described herein in which the lubricating composition has a viscosity index of at least 130 or at least 135.
- the lubricating composition has a viscosity index of 130 to 230, 135 to 195, or 140 to 175.
- the invention further provides a lubricating composition as described herein in which the oil of lubricating viscosity includes from 0.1 weight percent to 20 weight percent of an oil derived from hydroisomerization of a high wax-containing feed stream.
- the invention further provides a lubricating composition as described herein in which the oil of lubricating viscosity includes from 0.5 weight percent to 5 weight percent of an oil derived from hydroisomerization of a high wax-containing feed stream.
- the invention further provides a use of a lubricant composition for lubricating a compression-ignition internal combustion engine including supplying the engine a lubricant composition as described herein.
- the invention further provides a use of a lubricant composition for improving the fuel economy of a compression-ignition internal combustion engine including supplying to the engine the lubricant composition as described herein, wherein the lubricant is a SAE 0W-16, 0W-20, 0W-26, 5W-16, 5W-20, 5W-30, 10W-16, 10W-30 or 10W-40 lubricant .
- the oil of lubricating viscosity of the invention may be defined as an API Group II+ base oil.
- API Group II+ base oils are known and described for example in SAE publication entitled “ Design Practice: Passenger Car Automatic Transmissions", fourth Edition, AE-29, published 2012, page 12-9 .
- US 8,216,448 also defines a API Group II+ as a "Group II plus base oil” having a viscosity index greater than or equal to 110 and less than 120.
- the oil of lubricating viscosity of the invention has a viscosity index (VI) of at least 130, or at least 135.
- Examples of an oil of lubricating viscosity of the present invention include base oils sold under the registered trade names of Ultra-S, Nexbase®, Yubase®, Petrocanada, and Chevron neutral oil 110RLV.
- the oil of lubricating viscosity of the invention is present at 80 wt % to 95 wt % of the lubricating composition.
- the oil of lubricating viscosity of the invention may also be blended with a conventional oil of lubricating viscosity (i.e., an oil of lubricating viscosity other than that defined by the present invention) with the proviso that the base oil mixture continues to exhibit a viscosity index as described above.
- a conventional oil of lubricating viscosity i.e., an oil of lubricating viscosity other than that defined by the present invention
- the conventional oil of lubricating viscosity may be defined as specified in the American Petroleum Institute (API) Base Oil Interchangeability Guidelines.
- the five base oil groups are as follows: Group I (sulfur content >0.03 wt %, and/or ⁇ 90 wt % saturates, viscosity index 80-120); Group II (sulfur content ⁇ 0.03 wt %, and ⁇ 90 wt % saturates, viscosity index 80-120); Group III (sulfur content ⁇ 0.03 wt %, and ⁇ 90 wt % saturates, viscosity index ⁇ 120); Group IV (all polyalphaolefins (PAOs)); and Group V (all others not included in Groups I, II, III, or IV).
- the oil of lubricating viscosity comprises an API Group I, Group II (other than the oil of lubricating viscosity defined by the present invention), Group III, Group IV, Group V oil or mixtures thereof.
- the conventional oil of lubricating viscosity is an API Group I, Group II (other than the oil of lubricating viscosity defined by the present invention), Group III, Group IV oil or mixtures thereof.
- the conventional oil of lubricating viscosity is often an API Group II (other than the oil of lubricating viscosity defined by the present invention), Group III or Group IV oil or mixtures thereof.
- Groups I, II and III are mineral oil base stocks.
- the oil of lubricating viscosity can include natural or synthetic oils and mixtures thereof. Mixture of mineral oil and synthetic oils, e.g., polyalphaolefin oils and/or polyester oils, may be used.
- Base oils of API Group II and Group III are subjected to hydrotreating to reduce/remove aromatics and raise viscosity index.
- Group III base oils are subjected to severe hydrotreating process conditions in order to produce oils with viscosity index of at least 120.
- the lubricating composition of the invention comprises a severely hydrotreated base oil with a viscosity index of at least 110, at least 120, at least 130, or even at least 140.
- the lubricating composition comprises a Group III base oil.
- Some high viscosity index Group III base oils are produced in part by inclusion of high wax containing feed streams that are subjected to hydroisomerization to produce high viscosity index isoparaffinic base oils.
- Natural oils include animal oils and vegetable oils (e.g. vegetable acid esters) as well as mineral lubricating oils such as liquid petroleum oils and solvent-treated or acid treated mineral lubricating oils of the paraffinic, naphthenic, or mixed paraffinic-naphthenic types. Hydrotreated or hydrocracked oils are also useful oils of lubricating viscosity. Oils of lubricating viscosity derived from coal or shale are also useful.
- Synthetic oils include hydrocarbon oils and halosubstituted hydrocarbon oils such as polymerized and interpolymerized olefins and mixtures thereof, alkylbenzenes, polyphenyl, alkylated diphenyl ethers, and alkylated diphenyl sulfides and their derivatives, analogs and homologues thereof.
- Alkylene oxide polymers and interpolymers and derivatives thereof, and those where terminal hydroxyl groups have been modified by, e.g., esterification or etherification, are other classes of synthetic lubricating oils.
- suitable synthetic lubricating oils comprise esters of dicarboxylic acids and those made from C5 to C12 monocarboxylic acids and polyols or polyol ethers.
- Other synthetic lubricating oils include liquid esters of phosphorus-containing acids, polymeric tetrahydrofurans, silicon-based oils such as poly-alkyl-, polyaryl-, polyalkoxy-, or polyaryloxy-siloxane oils, and silicate oils.
- oils include those produced by Fischer-Tropsch reactions, typically hydroisomerized Fischer-Tropsch hydrocarbons or waxes.
- oils may be prepared by a Fischer-Tropsch gas-to-liquid synthetic procedure as well as other gas-to-liquid oils.
- the base oil of lubricating viscosity contains 0.1 weight percent to 20 weight percent of a base oil fraction derived from hydroisomerization of a high wax-containing feed stream and/or base oil derived from hydroisomerization of a Fischer-Tropsch wax feed stream. In one embodiment, the oil of lubricating viscosity contains 0.5 weight percent to 5 weight percent of a base oil fraction derived from hydroisomerization of a high wax-containing feed stream and/or base oil derived from hydroisomerization of a Fischer-Tropsch wax feed stream. In one embodiment, the lubricating composition is free of (or substantially free of) a hydroisomerized wax-derived base oil.
- Unrefined, refined, and rerefined oils either natural or synthetic (as well as mixtures thereof) of the types disclosed hereinabove can used.
- Unrefined oils are those obtained directly from a natural or synthetic source without further purification treatment.
- Refined oils are similar to the unrefined oils except they have been further treated in one or more purification steps to improve one or more properties.
- Rerefined oils are obtained by processes similar to those used to obtain refined oils applied to refined oils which have been already used in service. Rerefined oils often are additionally processed to remove spent additives and oil breakdown products.
- the amount of the oil of lubricating viscosity present is typically the balance remaining after subtracting from 100 wt % the sum of the amount of performance additives of the present invention.
- the lubricating composition may be in the form of a concentrate and/or a fully formulated lubricant. If the performance additives of this invention are in the form of a concentrate (which may be combined with additional oil to form, in whole or in part, a finished lubricant), the ratio of the performance additives to the oil of lubricating viscosity and/or to diluent oil include the ranges of 1:99 to 99:1 by weight, or 80:20 to 10:90 by weight.
- Dispersants generally, are well known in the field of lubricants and include primarily what is known as ashless dispersants and polymeric dispersants. Ashless dispersants are so-called because, as supplied, they do not contain metal and thus do not normally contribute to sulfated ash when added to a lubricant. However, they may interact with ambient metals once they are added to a lubricant which includes a metal-containing species. Ashless dispersants are characterized by a polar group attached to a relatively high molecular weight hydrocarbon chain.
- Typical ashless dispersants include N-substituted long chain alkenyl succinimides, having a variety of chemical structures, including those represented by Formula (I): where each R 1 is independently an alkyl group, frequently a polyisobutylene group with a molecular weight (M n ) of 500-5000 based on the polyisobutylene precursor, and R 2 are alkylene groups, commonly ethylene (C 2 H 4 ) groups.
- Such molecules are commonly derived from reaction of an alkenyl acylating agent with a polyamine, and a wide variety of linkages between the two moieties is possible beside the simple imide structure shown above, including a variety of amides and quaternary ammonium salts.
- the amine portion is shown as an alkylene polyamine, although other aliphatic and aromatic mono- and polyamines may also be used.
- a variety of modes of linkage of the R 1 groups onto the imide structure are possible, including various cyclic linkages.
- the ratio of the carbonyl groups of the acylating agent to the nitrogen atoms of the amine may be 1:0.5 to 1:3, and in other instances 1:1 to 1:2.75 or 1:1.5 to 1:2.5.
- Succinimide dispersants are more fully described in U.S. Patents 4,234,435 and 3,172,892 and in EP 0355895 .
- the dispersant is prepared by a process that involves the presence of small amounts of chlorine or other halogen, as described in U.S. Patent 7,615,521 (see, e.g., col. 4, lines 18-60 and preparative example A). Such dispersants typically have some carbocyclic structures in the attachment of the hydrocarbyl substituent to the acidic or amidic "head” group.
- the dispersant is prepared by a thermal process involving an "ene” reaction, without the use of any chlorine or other halogen, as described in U.S. Patent 7,615,521 ; dispersants made in this manner are often derived from high vinylidene (i.e. greater than 50% terminal vinylidene) polyisobutylene(See col.
- dispersants typically do not contain the above-described carbocyclic structures at the point of attachment.
- the dispersant is prepared by free radical catalyzed polymerization of high-vinylidene polyisobutylene with an ethylenically unsaturated acylating agent, as described in United States Patent 8,067,347 .
- Dispersants may be derived from, as the polyolefin, high vinylidene polyisobutylene, that is, having greater than 50, 70, or 75% terminal vinylidene groups ( ⁇ and ⁇ isomers).
- the succinimide dispersant may be prepared by the direct alkylation route. In other embodiments it may comprise a mixture of direct alkylation and chlorine-route dispersants.
- Suitable dispersants for use in the compositions of the present invention include succinimide dispersants.
- the dispersant may be present as a single dispersant.
- the dispersant may be present as a mixture of two or three different dispersants, wherein at least one may be a succinimide dispersant.
- the succinimide dispersant may be a derivative of an aliphatic polyamine, or mixtures thereof.
- the aliphatic polyamine may be aliphatic polyamine such as an ethylenepolyamine, a propylenepolyamine, a butylenepolyamine, or mixtures thereof.
- the aliphatic polyamine may be ethylenepolyamine.
- the aliphatic polyamine may be selected from the group consisting of ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, pentaethylenehexamine, polyamine still bottoms, and mixtures thereof.
- the succinimide dispersant may be a derivative of an aromatic amine, an aromatic polyamine, or mixtures thereof.
- the aromatic amine may be 4-aminodiphenylamine (ADPA) (also known as N -phenylphenylenediamine), derivatives of ADPA (as described in United States Patent Publications 2011/0306528 and 2010/0298185 ), a nitroaniline, an aminocarbazole, an amino-indazolinone, an aminopyrimidine, 4-(4-nitrophenylazo)aniline, or combinations thereof.
- the dispersant is derivative of an aromatic amine wherein the aromatic amine has at least three non-continuous aromatic rings.
- the succinimide dispersant may be a derivative of a polyether amine or polyether polyamine.
- Typical polyether amine compounds contain at least one ether unit and will be chain terminated with at least one amine moiety.
- the polyether polyamines can be based on polymers derived from C 2 -C 6 epoxides such as ethylene oxide, propylene oxide, and butylene oxide. Examples of polyether polyamines are sold under the Jeffamine® brand and are commercially available from Hunstman Corporation located in Houston, Texas.
- esters Another class of ashless dispersant is high molecular weight esters. These materials are similar to the above-described succinimides except that they may be seen as having been prepared by reaction of a hydrocarbyl acylating agent and a polyhydric aliphatic alcohol such as glycerol, pentaerythritol, or sorbitol. Such materials are described in more detail in U.S. Patent 3,381,022 . Aromatic succinate esters may also be prepared as described in United States Patent Publication 2010/0286414 .
- a succinic-based dispersant may be formed by reacting maleic anhydride or a reactive equivalent thereof, such as an acid or ester, with a hydrocarbon chain by any method such as those disclosed above (e.g., chlorine-based process or thermal process).
- Other acids or equivalents thereof may be used in place of the maleic anhydride. These include fumaric acid, itaconic acid, itaconic anhydride, citraconic acid, citaconic anhydride, and cinnamic acid as well as other ethylenically unsaturated acids such as acrylic or methacrylic acid; and their reactive equivalents.
- Mannich bases Another class of ashless dispersant is Mannich bases. These are materials which are formed by the condensation of a higher molecular weight, alkyl substituted phenol, an alkylene polyamine, and an aldehyde such as formaldehyde. Such materials may have the general structure as represented by Formula (II) (including a variety of isomers and the like) and are described in more detail in U.S. Patent 3,634,515 .
- ashless dispersants include dispersants comprising a quaternary ammonium salt.
- Quaternary ammonium salts include the reaction product of: (i) a compound comprising at least one tertiary amino group; and (ii) a quaternizing agent suitable for converting the tertiary amino group of compound (i) to a quaternary nitrogen.
- quaternary ammonium salts examples include (i) imide quaternary ammonium salts, (ii) Mannich quaternary ammonium salts, (iii) polyalkene substituted amine quaternary ammonium salts, (iv) amide quaternary ammonium salts, (v) ester quaternary ammonium salts, (vi) polyester quaternary ammonium salts, or (vii) any combination thereof.
- quaternary ammonium salts may be prepared in any number of ways but generally are prepared by reacting a non-quaternized nitrogen-containing compound with a quaternizing agent.
- Each of the different types of quaternary ammonium salts described uses a different non-quaternized nitrogen-containing compound in its preparation, but generally the non-quaternized nitrogen-containing compound contains a tertiary nitrogen capable of being quaternized (or a primary or secondary nitrogen atom that can be alkylated to a tertiary nitrogen that can then be quaternized) and a hydrocarbyl substituent group.
- the preparation and use of quaternized ammonium dispersants is described in detail in United States Patent 7,951,211 and United States Patent 7,906,470 .
- the dispersant may also be post-treated by conventional methods by a reaction with any of a variety of agents.
- agents include boron compounds, urea, thiourea, dimercaptothiadiazoles, carbon disulfide, aldehydes, ketones, carboxylic acids, hydrocarbon-substituted succinic anhydrides, maleic anhydride, nitriles, epoxides, and phosphorus compounds.
- the dispersant may also exhibit basicity, as measured by Total Base Number (TBN).
- TBN may be determined by ASTM D2896. This will particularly be the case if the dispersant is prepared with an amine, such as a polyamine, and the amine contains one or more amino groups that have not reacted with acidic groups of the dispersant.
- the TBN of the dispersant may be 1 to 110, or 5 to 50, or 10 to 40 or 30 to 70.
- the dispersant may not exhibit basicity (that is, have a TBN of 0 or nearly 0).
- the dispersant has a TBN of zero as measured by D2896. Such could be the case if no basic nitrogen is present on the dispersant.
- the dispersant may be present at 0.01 wt % to 20 wt %, or 0.1 wt % to 15 wt %, or 0.1 wt % to 10 wt %, or 0.5 wt % to 8 wt % , or 1.0 wt % to 6.5 wt % of the lubricating composition.
- Metal overbased detergents otherwise referred to as overbased detergents, metal-containing overbased detergents or superbased salts, are characterized by a metal content in excess of that which would be necessary for neutralization according to the stoichiometry of the metal and the particular acidic organic compound, i.e. the substrate, reacted with the metal.
- the overbased detergent may comprise one or more of non-sulfur containing phenates, sulfur containing phenates, sulfonates, salicylates, and mixtures thereof.
- the amount of excess metal is commonly expressed in terms of substrate to metal ratio.
- the terminology "metal ratio" is used in the prior art and herein to define the ratio of the total chemical equivalents of the metal in the overbased salt to the chemical equivalents of the metal in the salt which would be expected to result from the reaction between the hydrocarbyl substituted organic acid; the hydrocarbyl-substituted phenol or mixtures thereof to be overbased, and the basic metal compound according to the known chemical reactivity and the stoichiometry of the two reactants.
- a normal or neutral salt i.e. soap
- the metal ratio is one and, in an overbased salt, the metal ratio is greater than one, especially greater than 1.3.
- the overbased detergent of the invention may have a metal ratio of 5 to 30, or a metal ratio of 7 to 22, or a metal ratio of at least 11.
- the metal-containing detergent may also include "hybrid" detergents formed with mixed surfactant systems including phenate and/or sulfonate components, e.g. phenate/salicylates, sulfonate/phenates, sulfonate/salicylates, sulfonates/phenates/salicylates, as described, for example, in US Patents 6,429,178 ; 6,429,179 ; 6,153,565 ; and 6,281,179 .
- phenate/salicylates e.g. phenate/salicylates, sulfonate/phenates, sulfonate/salicylates, sulfonates/phenates/salicylates, as described, for example, in US Patents 6,429,178 ; 6,429,179 ; 6,153,565 ; and 6,281,179 .
- hybrid detergent would be considered equivalent to amounts of distinct phenate and sulfonate detergents introducing like amounts of phenate and sulfonate soaps, respectively.
- Overbased phenates and salicylates typically have a total base number of 180 to 450 TBN.
- Overbased sulfonates typically have a total base number of 250 to 600, or 300 to 500.
- Overbased detergents are known in the art.
- Alkylphenols are often used as constituents in and/or building blocks for overbased detergents.
- Alkylphenols may be used to prepare phenate, salicylate, salixarate, or saligenin detergents or mixtures thereof.
- Suitable alkylphenols may include para-substituted hydrocarbyl phenols.
- the hydrocarbyl group may be linear or branched aliphatic groups of 1 to 60 carbon atoms, 8 to 40 carbon atoms, 10 to 24 carbon atoms, 12 to 20 carbon atoms, or 16 to 24 carbon atoms.
- the alkylphenol overbased detergent is prepared from an alkylphenol or mixture thereof that is free of or substantially free of (i.e.
- the lubricating composition of the invention contains less than 0.1 weight percent) p-dodecylphenol. In one embodiment, the lubricating composition of the invention contains less than 0.3 weight percent of alkylphenol, less than 0.1 weight percent of alkylphenol, or less than 0.05 weight percent of alkylphenol.
- the overbased metal-containing detergent may be alkali metal or alkaline earth metal salts.
- the overbased detergent may be sodium salts, calcium salts, magnesium salts, or mixtures thereof of the phenates, sulfur-containing phenates, sulfonates, salixarates and salicylates.
- the overbased detergent is a calcium detergent, a magnesium detergent or mixtures thereof.
- the overbased calcium detergent may be present in an amount to deliver at least 500 ppm calcium by weight and no more than 3000 ppm calcium by weight, or at least 1000 ppm calcium by weight, or at least 2000 ppm calcium by weight, or no more than 2500 ppm calcium by weight to the lubricating composition.
- the overbased detergent may be present in an amount to deliver no more than 500 ppm by weight of magnesium to the lubricating composition, or no more than 330 ppm by weight, or no more than 125 ppm by weight, or no more than 45 ppm by weight.
- the lubricating composition is essentially free of (i.e. contains less than 10 ppm) magnesium resulting from the overbased detergent.
- the overbased detergent may be present in an amount to deliver at least 200 ppm by weight of magnesium, or at least 450 ppm by weight magnesium, or at least 700 ppm by weight magnesium to the lubricating composition.
- both calcium and magnesium containing detergents may be present in the lubricating composition. Calcium and magnesium detergents may be present such that the weight ratio of calcium to magnesium is 10:1 to 1:10, or 8:3 to 4:5, or 1:1 to 1:3.
- the overbased detergent is free of or substantially free of sodium.
- the sulfonate detergent may be predominantly a linear alkylbenzene sulfonate detergent having a metal ratio of at least 8 as is described in paragraphs [0026] to [0037] of US Patent Publication 2005/065045 (and granted as US 7,407,919 ).
- the linear alkylbenzene sulfonate detergent may be particularly useful for assisting in improving fuel economy.
- the linear alkyl group may be attached to the benzene ring anywhere along the linear chain of the alkyl group, but often in the 2, 3 or 4 position of the linear chain, and in some instances, predominantly in the 2 position, resulting in the linear alkylbenzene sulfonate detergent.
- Salicylate detergents and overbased salicylate detergents may be prepared in at least two different manners. Carbonylation (also referred to as carboxylation) of a p-alkylphenol is described in many references including US Patent 8,399,388 . Carbonylation may be followed by overbasing to form overbased salicylate detergent. Suitable p-alkylphenols include those with linear and/or branched hydrocarbyl groups of 1 to 60 carbon atoms. Salicylate detergents may also be prepared by alkylation of salicylic acid, followed by overbasing, as described in US Patent 7,009,072 .
- Salicylate detergents prepared in this manner may be prepared from linear and/or branched alkylating agents (usually 1-olefins) containing 6 to 50 carbon atoms, 10 to 30 carbon atoms, or 14 to 24 carbon atoms.
- the overbased detergent of the invention is a salicylate detergent.
- the salicylate detergent of the invention is free of unreacted p-alkylphenol (i.e. contains less than 0.1 weight percent).
- the salicylate detergent of the invention is prepared by alkylation of salicylic acid.
- the overbased detergent may be present at 0.2 wt % to 15 wt %, or 0.3 wt % to 10 wt %, or 0.3 wt % to 8 wt %, or 0.4 wt % to 3 wt %.
- the detergent may be present at 2 wt % to 3 wt % of the lubricating composition.
- the detergent may be present at 0.2 wt % to 1 wt % of the lubricating composition.
- Metal-containing overbased detergents often provide TBN to the lubricating composition.
- the overbased detergent is present in an amount to deliver at least 4 mg KOH/g of TBN to the lubricating composition, or 4 to 15 mg KOH/g, or 5 to 9 mg KOH/g of TBN to the lubricating composition.
- Metal-containing detergents contribute sulfated ash to a lubricating composition.
- Sulfated ash may be determined by ASTM D874.
- the lubricating composition of the invention comprises a metal-containing detergent in an amount to deliver at least 0.4 weight percent sulfated ash to the total composition.
- the metal-containing detergent is present in an amount to deliver at least 0.6 weight percent sulfated ash, or at least 0.75 weight percent sulfated ash, or even at least 0.9 weight percent sulfated ash to the lubricating composition.
- overbased detergents contribute detergent soap, also referred to as neutral detergent salt, to the lubricating composition.
- Soap being a metal salt of the substrate, may act as a surfactant in the lubricating composition.
- the lubricating composition comprises 0.05 weight percent to 1.5 weight percent detergent soap, or 0.1 weight percent to 0.9 weight percent detergent soap.
- the lubricating composition contains no more than 0.5 weight percent detergent soap.
- the overbased detergent may have a weight ratio of ash:soap of 5:1 to 1:2.3, or 3.5:1 to 1:2, or 2.9:1 to 1:1:7.
- compositions of the invention may optionally comprise one or more additional performance additives.
- additional performance additives may include one or more metal deactivators, detergents, friction modifiers, antiwear agents, corrosion inhibitors, soot-dispersing additives, extreme pressure agents, antioxidants, foam inhibitors, demulsifiers, pour point depressants, seal swelling agents, and any combination or mixture thereof.
- fully-formulated lubricating oil will contain one or more of these performance additives, and often a package of multiple performance additives.
- the invention provides a lubricating composition further comprising an antiwear agent, a dispersant viscosity modifier, a friction modifier, a viscosity modifier, an antioxidant, an overbased detergent, a dispersant (different from that of the invention), or a combination thereof, where each of the additives listed may be a mixture of two or more of that type of additive.
- the invention provides a lubricating composition further comprising an antiwear agent, a dispersant viscosity modifier, a friction modifier, a viscosity modifier (typically an olefin copolymer such as an ethylene-propylene copolymer), an antioxidant (including phenolic and aminic antioxidants), an overbased detergent (including overbased sulfonates and phenates), or a combination thereof, where each of the additives listed may be a mixture of two or more of that type of additive.
- an antiwear agent typically an olefin copolymer such as an ethylene-propylene copolymer
- an antioxidant including phenolic and aminic antioxidants
- an overbased detergent including overbased sulfonates and phenates
- antiwear agent Another additive is an antiwear agent.
- anti-wear agents include phosphorus-containing antiwear/extreme pressure agents such as metal thiophosphates, phosphoric acid esters and salts thereof, phosphorus-containing carboxylic acids, esters, ethers, and amides, and phosphites.
- a phosphorus antiwear agent may be present in an amount to deliver 0.01 to 0.2 or 0.015 to 0.15 or 0.02 to 0.1 or 0.025 to 0.08 percent phosphorus.
- the antiwear agent is a zinc dialkyldithiophosphate (ZDP).
- Zinc dialkyldithiophosphates may be described as primary zinc dialkyldithiophosphates or as secondary zinc dialkyldithiophosphates, depending on the structure of the alcohol used in its preparation.
- the compositions of the invention include primary zinc dialkyldithiophosphates.
- the compositions of the invention include secondary zinc dialkyldithiophosphates.
- the compositions of the invention include a mixture of primary and secondary zinc dialkyldithiophosphates.
- component (b) is a mixture of primary and secondary zinc dialkyldithiophosphates where the ratio of primary zinc dialkyldithiophosphates to secondary zinc dialkyldithiophosphates (one a weight basis) is at least 1:1, or even at least 1:1.2, or even at least 1:1.5 or 1:2, or 1:10. In some embodiments component (b) is a mixture of primary and secondary zinc dialkyldithiophosphates that is at least 50 percent by weight primary, or even at least 60, 70, 80, or even 90 percent by weight primary. In some embodiments component (b) is free of primary zinc dialkyldithiophosphates.
- the phosphorus antiwear agent may be present at 0 wt % to 3 wt %, or 0.1 wt % to 1.5 wt %, or 0.5 wt % to 0.9 wt % of the lubricating composition.
- oil-soluble titanium compounds as disclosed in U.S. Pat. No. 7,727,943 and US20060014651 .
- the oil-soluble titanium compounds may function as antiwear agents, friction modifiers, antioxidants, deposit control additives, or more than one of these functions.
- the oil soluble titanium compound may be a titanium (IV) alkoxide.
- the titanium alkoxide may be formed from a monohydric alcohol, a polyol or mixtures thereof.
- the monohydric alkoxides may have 2 to 16, or 3 to 10 carbon atoms.
- the titanium alkoxide may be titanium (IV) isopropoxide.
- the titanium alkoxide may be titanium (IV) 2-ethylhexoxide.
- the titanium compound comprises the alkoxide of a vicinal 1,2-diol or polyol.
- the 1,2-vicinal diol comprises a fatty acid mono-ester of glycerol, often the fatty acid may be oleic acid.
- the oil soluble titanium compound may be a titanium carboxylate.
- the titanium (IV) carboxylate may be titanium neodecanoate.
- the oil soluble titanium compound may be present in the lubricating composition in an amount necessary to provide for 10 ppm to 1500 ppm titanium by weight or 25 ppm to 150 ppm titanium by weight.
- the invention provides a lubricating composition which further comprises ashless antioxidant.
- Ashless antioxidants may comprise one or more of arylamines, diarylamines, alkylated arylamines, alkylated diaryl amines, phenols, hindered phenols, sulfurized olefins, or mixtures thereof.
- the lubricating composition includes an antioxidant, or mixtures thereof.
- the antioxidant may be present at 0 wt % to 15 wt %, or 0.1 wt % to 10 wt %, or 0.5 wt % to 5 wt %, or 0.5 wt % to 3 wt %, or 0.3 wt % to 1.5 wt % of the lubricating composition.
- the diarylamine or alkylated diarylamine may be a phenyl- ⁇ -naphthylamine (PANA), an alkylated diphenylamine, or an alkylated phenylnapthylamine, or mixtures thereof.
- the alkylated diphenylamine may include di-nonylated diphenylamine, nonyl diphenylamine, octyl diphenylamine, di-octylated diphenylamine, di-decylated diphenylamine, decyl diphenylamine and mixtures thereof.
- the diphenylamine may include nonyl diphenylamine, dinonyl diphenylamine, octyl diphenylamine, dioctyl diphenylamine, or mixtures thereof.
- the alkylated diphenylamine may include nonyl diphenylamine, or dinonyl diphenylamine.
- the alkylated diarylamine may include octyl, di-octyl, nonyl, di-nonyl, decyl or di-decyl phenylnapthylamines.
- the diarylamine antioxidant of the invention may be present on a weight basis of the lubrication composition at 0.1% to 10%, 0.35% to 5%, or even 0.5% to 2%.
- the phenolic antioxidant may be a simple alkyl phenol, a hindered phenol, or coupled phenolic compounds.
- the hindered phenol antioxidant often contains a secondary butyl and/or a tertiary butyl group as a sterically hindering group.
- the phenol group may be further substituted with a hydrocarbyl group (typically linear or branched alkyl) and/or a bridging group linking to a second aromatic group.
- hindered phenol antioxidants examples include 2,6-di-tert-butylphenol, 4-methyl-2,6-di-tert-butylphenol, 4-ethyl-2,6-di-tert-butylphenol, 4-propyl-2,6-di-tert-butylphenol or 4-butyl-2,6-di-tert-butylphenol, 4-dodecyl-2,6-di-tert-butylphenol, or butyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate.
- the hindered phenol antioxidant may be an ester and may include, e.g., IrganoxTM L-135 from Ciba.
- Coupled phenols often contain two alkylphenols coupled with alkylene groups to form bisphenol compounds.
- suitable coupled phenol compounds include 4,4'- methylene bis-(2,6-di-tert-butyl phenol), 4-methyl-2,6-di-tert-butylphenol, 2,2'-bis-(6-t-butyl-4-heptylphenol); 4,4'-bis(2,6-di-t-butyl phenol), 2,2'-methylenebis(4-methyl-6-t-butylphenol), and 2,2'-methylene bis(4-ethyl-6-t-butylphenol).
- Phenols of the invention also include polyhydric aromatic compounds and their derivatives.
- suitable polyhydric aromatic compounds include esters and amides of gallic acid, 2,5-dihydroxybenzoic acid, 2,6-dihydroxybenzoic acid, 1,4-dihydroxy-2-naphthoic acid, 3,5-dihydroxynaphthoic acid, 3,7-dihydroxy naphthoic acid, and mixtures thereof.
- the phenolic antioxidant comprises a hindered phenol.
- the hindered phenol is derived from 2,6-ditertbutyl phenol.
- the lubricating composition of the invention comprises a phenolic antioxidant in a range of 0.01 wt % to 5 wt %, or 0.1 wt % to 4 wt %, or 0.2 wt % to 3 wt %, or 0.5 wt % to 2 wt % of the lubricating composition.
- Sulfurized olefins are well known commercial materials, and those which are substantially nitrogen-free, that is, not containing nitrogen functionality, are readily available.
- the olefinic compounds which may be sulfurized are diverse in nature. They contain at least one olefinic double bond, which is defined as a nonaromatic double bond; that is, one connecting two aliphatic carbon atoms. These materials generally have sulfide linkages having 1 to 10 sulfur atoms, for instance, 1 to 4, or 1 or 2.
- Ashless antioxidants may be used separately or in combination.
- two or more different antioxidants are used in combination, such that there is at least 0.1 weight percent of each of the at least two antioxidants and wherein the combined amount of the ashless antioxidants is 0.5 to 5 weight percent. In one embodiment, there may be at least 0.25 to 3 weight percent of each ashless antioxidant.
- the invention provides a lubricating composition further comprising a molybdenum compound.
- the molybdenum compound may be selected from the group consisting of molybdenum dialkyldithiophosphates, molybdenum dithiocarbamates, amine salts of molybdenum compounds, molybdenum-containing dispersants, and mixtures thereof. Examples of commercially available molybdenum compounds include Sakura-lubeTM 525 and Sakura-lubeTM 710, both available from Adeka Corporation; and Molyvan® 855 available from Vanderbilt Chemicals, LLC.
- the molybdenum compound may provide the lubricating composition with 0 to 1000 ppm, or 5 to 1000 ppm, or 10 to 750 ppm, or 5 ppm to 300 ppm, or 20 ppm to 250 ppm of molybdenum.
- the molybdenum compound is a molybdenum dithiocarbamate compound present in an amount to provide 300 ppm to 750 ppm molybdenum to the lubricating composition.
- the lubricating composition of the invention further comprises a soot dispersing additive.
- the soot dispersing additive may be present at 0 wt % to 5 wt %, or 0 wt % to 4 wt %, or 0.05 wt % to 2 wt % of the lubricating composition.
- Suitable soot dispersing additives include functionalized low molecular weight polyolefins, for example, ethylene-propylene copolymers with a number average molecular weight (Mn) of less than 20,000 that have been functionalized with an acylating agent such as maleic anhydride and an amine, preferably an aromatic amine.
- Other soot dispersing additives may be prepared from acylated polyisobutylene that has been functionalized with aromatic (poly)amines. More detailed description of soot-dispersing additives are disclosed in U.S. Patents 4,863,623 ; 5,182,041 ; 7,790,661 ; 8,557,753 ; and 8,637,437 .
- the soot dispersing additive may include those described in U.S. Patent 7,790,661 or in U.S. Patent 8,557,753 .
- the invention provides a lubricating composition further comprising a friction modifier.
- friction modifiers include long chain fatty acid derivatives of amines, fatty esters, or epoxides; fatty imidazolines such as condensation products of carboxylic acids and polyalkylene-polyamines; amine salts of alkylphosphoric acids; fatty esters, amides, and/or imides of ⁇ -hydroxy-carbonyl compounds such as tartaric acid, malic acid, citric acid, glycolic acid, lactic acid and mandelic acid.
- fatty as used herein, can mean having a C8-22 linear alkyl group.
- Friction modifiers may also encompass materials such as sulfurized fatty compounds and olefins, molybdenum dialkyldithiophosphates, molybdenum dithiocarbamates, sunflower oil or monoester of a polyol and an aliphatic carboxylic acid.
- the friction modifier may be selected from the group consisting of long chain fatty acid derivatives of amines, long chain fatty esters, or long chain fatty epoxides; fatty imidazolines; amine salts of alkylphosphoric acids; fatty alkyl tartrates; fatty alkyl tartrimides; and fatty alkyl tartramides.
- the friction modifier may be present at 0 wt % to 6 wt %, or 0.05 wt % to 4 wt %, or 0.1 wt % to 2 wt % of the lubricating composition.
- the friction modifier may be a long chain fatty acid ester.
- the long chain fatty acid ester may be a mono-ester or a diester or a mixture thereof, and in another embodiment the long chain fatty acid ester may be a triglyceride.
- corrosion inhibitors include those described in paragraphs 5 to 8 of US Application US05/038319 , published as WO2006/047486 , octyl octanamide, condensation products of dodecenyl succinic acid or anhydride and a fatty acid such as oleic acid with a polyamine.
- the corrosion inhibitors include the Synalox® (a registered trademark of The Dow Chemical Company) corrosion inhibitor.
- the Synalox® corrosion inhibitor may be a homopolymer or copolymer of propylene oxide.
- the Synalox® corrosion inhibitor is described in more detail in a product brochure with Form No. 118-01453-0702 AMS, published by The Dow Chemical Company.
- the product brochure is entitled "SYNALOX Lubricants, High-Performance Polyglycols for Demanding Applications.”
- the lubricating composition may further include metal deactivators, including derivatives of benzotriazoles (typically tolyltriazole), dimercaptothiadiazole derivatives, 1,2,4-triazoles, benzimidazoles, 2-alkyldithiobenzimidazoles, or 2-alkyldithiobenzothiazoles; foam inhibitors, including copolymers of ethyl acrylate and 2-ethylhexylacrylate and copolymers of ethyl acrylate and 2-ethylhexylacrylate and vinyl acetate; demulsifiers including trialkyl phosphates, polyethylene glycols, polyethylene oxides, polypropylene oxides and (ethylene oxide-propylene oxide) polymers; and pour point depressants, including esters of maleic anhydride-styrene, polymethacrylates, polyacrylates or polyacrylamides.
- metal deactivators including derivatives of benzotriazoles (typically tolyltri
- the lubricating composition may further comprise a polyether compound.
- the polyether compound may be a polyether, a polyetheramine, a (poly)alkoxylated amine, an ethoxylated alcohol, or mixtures thereof.
- the polyether can be represented by the formula RO[CH 2 CH(R 1 )O] x H where R is a hydrocarbyl group; R 1 is selected from the group consisting of hydrogen, alkyl groups of 1 to 14 carbon atoms, and mixtures thereof; and x is a number from 2 to 50.
- the hydrocarbyl group R is a univalent hydrocarbon group, has one or more carbon atoms, and includes alkyl and alkyl-phenyl groups having 7 to 30 total carbon atoms, such as 9 to 25 total carbon atoms, or 11 to 20 total carbon atoms.
- the repeating oxyalkylene units may be derived from ethylene oxide, propylene oxide, or butylene oxide.
- the number of oxyalkylene units x may be 10 to 35, or 18 to 27.
- the polyether of the present invention can be prepared by various well-known methods including condensing one mole of an alcohol or alkylphenol with two or more moles of an alkylene oxide, mixture of alkylene oxides, or with several alkylene oxides in sequential fashion, usually in the presence of a base catalyst.
- U.S. Pat. No. 5,094,667 provides reaction conditions for preparing a polyether.
- Suitable polyethers are commercially available from Dow Chemicals, Huntsman, ICI and include the Actaclear® series from Bayer.
- Suitable ethoxylates include Surfonic® ethoxylates, for example Surfonic L24-5, available from Huntsman International LLC.
- Polymeric viscosity modifiers may be present with the proviso that they are present in an amount less than 0.01 weight percent of the lubricating composition.
- Suitable viscosity modifiers include ethylene-olefin co-polymers, especially ethylene-propylene; maleic anhydride-styrene alternating copolymers and esters thereof, polymethacrylates (including random, block and star architectures), hydrogenated styrene-butadiene block copolymers, hydrogenated styrene-isoprene radial and/or block copolymers, or mixtures thereof.
- the polymeric viscosity modifier includes an ethylene-olefin based copolymer.
- the ethylene makes up from 50 weight percent to 90 weight percent or from 65 weight percent to 85 weight percent, on a molar basis of the monomer used to prepare the copolymer of the ethylene-olefin-based copolymer. In another embodiment, the ethylene makes up at least 70 weight percent of the monomer used to prepare the copolymer. In one embodiment, the ethylene-olefin copolymer has a molecular weight from 5,000 to 40,000 Mn.
- Pour point depressants that may be useful in the compositions of the invention further include polyalphaolefins, esters of maleic anhydride-styrene, poly(meth)acrylates, polyacrylates or polyacrylamides.
- the lubricant is added to the lubricating system of the internal combustion engine, which then delivers the lubricating composition to the critical parts of the engine, during its operation, that require lubrication.
- the lubricating compositions described above may be utilized in an internal combustion engine.
- the engine components may have a surface of steel or aluminum (typically a surface of steel), and may also be coated for example with a diamond-like carbon (DLC) coating.
- DLC diamond-like carbon
- An aluminum surface may be comprised of an aluminum alloy that may be a eutectic or hyper-eutectic aluminum alloy (such as those derived from aluminum silicates, aluminum oxides, or other ceramic materials).
- the aluminum surface may be present on a cylinder bore, cylinder block, or piston ring having an aluminum alloy, or aluminum composite.
- the internal combustion engine may be fitted with an emission control system, an oil mist separator or a turbocharger.
- emission control system examples include diesel particulate filters (DPF), or systems employing selective catalytic reduction (SCR).
- the internal combustion engine is distinct from a gas turbine.
- individual combustion events translate from a linear reciprocating force into a rotational torque through the rod and crankshaft.
- a gas turbine which may also be referred to as a j et engine
- a continuous combustion process generates a rotational torque continuously without translation, and can also develop thrust at the exhaust outlet.
- the lubricant composition for an internal combustion engine may be suitable for any engine lubricant irrespective of the sulfur, phosphorus or sulfated ash (ASTM D-874) content.
- the sulfur content of the engine oil lubricant may be 1 wt % or less, or 0.8 wt % or less, or 0.5 wt % or less, or 0.3 wt % or less. In one embodiment, the sulfur content may be in the range of 0.001 wt % to 0.5 wt %, or 0.01 wt % to 0.3 wt %.
- the phosphorus content may be 0.2 wt % or less, or 0.12 wt % or less, or 0.1 wt % or less, or 0.085 wt % or less, or 0.08 wt % or less, or even 0.06 wt % or less, 0.055 wt % or less, or 0.05 wt % or less.
- the phosphorus content may be 100 ppm to 1000 ppm, or 200 ppm to 600 ppm.
- the total sulfated ash content may be 2 wt % or less, or 1.5 wt % or less, or 1.1 wt % or less, or 1 wt % or less, or 0.8 wt % or less, or 0.5 wt % or less, or 0.4 wt % or less.
- the sulfated ash content may be 0.05 wt % to 0.9 wt %, or 0.1 wt % to 0.2 wt % or to 0.45 wt %.
- the lubricating composition may be an engine oil, wherein the lubricating composition may be characterized as having at least one of (i) a sulfur content of 0.5 wt % or less, (ii) a phosphorus content of 0.1 wt % or less, (iii) a sulfated ash content of 1.5 wt % or less, or combinations thereof.
- a series of 10W-30 diesel lubricating compositions are prepared according to Table 1 below. Compositions are prepared by blending components as shown in Table 1 into a lubricant. The amounts in Table 1 are on an oil-free basis. TABLE 1 - Lubricating Compositions Embodiments (wt %) Inv.
- the lubricating compositions are evaluated for wear protection in GM 6.5L Roller Follower Wear Test (RFWT) (ASTM D5966). This test measures wear on camshaft roller follower pins to determine the ability of an engine oil to control wear in the presence of soot-laden oil. The results obtained are summarized in Table 2, below.
- the lubricating compositions were also evaluated in the Volvo D12D fuel economy engine test; this test estimates the fuel economy benefits of the candidate oil versus a baseline oil (HVES 540).
- the baseline lubricant was a commercial SAE 15W-40 API CJ-4 Heavy-Duty Diesel Engine Oil. The results obtained are summarized in Table 2, below. TABLE 2 - Wear and Fuel Economy Test Results Inv.
- each chemical component described is presented exclusive of any solvent or diluent oil, which may be customarily present in the commercial material, that is, on an active chemical basis, unless otherwise indicated.
- each chemical or composition referred to herein should be interpreted as being a commercial grade material which may contain the isomers, byproducts, derivatives, and other such materials which are normally understood to be present in the commercial grade.
- hydrocarbyl substituent or “hydrocarbyl group” is used in its ordinary sense, which is well-known to those skilled in the art. Specifically, it refers to a group having a carbon atom directly attached to the remainder of the molecule and having predominantly hydrocarbon character.
- hydrocarbyl groups include:
- hydrocarbon substituents that is, aliphatic (e.g., alkyl or alkenyl), alicyclic (e.g., cycloalkyl, cycloalkenyl) substituents, and aromatic-, aliphatic-, and alicyclic-substituted aromatic substituents, as well as cyclic substituents wherein the ring is completed through another portion of the molecule (e.g., two substituents together form a ring);
- aliphatic e.g., alkyl or alkenyl
- alicyclic e.g., cycloalkyl, cycloalkenyl
- aromatic-, aliphatic-, and alicyclic-substituted aromatic substituents as well as cyclic substituents wherein the ring is completed through another portion of the molecule (e.g., two substituents together form a ring);
- substituted hydrocarbon substituents that is, substituents containing non-hydrocarbon groups which, in the context of this invention, do not alter the predominantly hydrocarbon nature of the substituent (e.g., halo (especially chloro and fluoro), hydroxy, alkoxy, mercapto, alkylmercapto, nitro, nitroso, and sulfoxy);
- hetero substituents that is, substituents which, while having a predominantly hydrocarbon character, in the context of this invention, contain other than carbon in a ring or chain otherwise composed of carbon atoms and encompass substituents as pyridyl, furyl, thienyl and imidazolyl.
- Heteroatoms include sulfur, oxygen, and nitrogen.
- no more than two, or no more than one, non-hydrocarbon substituent will be present for every ten carbon atoms in the hydrocarbyl group; alternatively, there may be no non-hydrocarbon substituents in the hydrocarbyl group.
- the molecular weight of the materials described above have been determined using known methods, such as GPC analysis using polystyrene standards.
- Methods for determining molecular weights of polymers are well known. The methods are described for instance: (i) P.J. Flory, “Principles of star polymer Chemistry", Cornell University Press 91953), Chapter VII, pp 266-315 ; or (ii) " Macromolecules, an Introduction to star polymer Science", F. A. Bovey and F. H. Winslow, Editors, Academic Press (1979), pp 296-312 .
- weight average and number weight average molecular weights of the materials described are obtained by integrating the area under the peak corresponding to the material of interest, excluding peaks associated with diluents, impurities, uncoupled star polymer chains and other additives.
- the transitional term "comprising,” which is synonymous with “including,” “containing,” or “characterized by,” is inclusive or open-ended and does not exclude additional, un-recited elements or method steps.
- the term also encompass, as alternative embodiments, the phrases “consisting essentially of' and “consisting of,” where “consisting of' excludes any element or step not specified and “consisting essentially of' permits the inclusion of additional un-recited elements or steps that do not materially affect the essential or basic and novel characteristics of the composition or method under consideration.
Description
- The disclosed technology relates to a lubricating composition for a heavy duty diesel engine that provides for improved fuel economy without reducing durability (i.e. anti-wear) performance of the engine. This may be achieved through the use of high viscosity index base oils in combination with ashless dispersants and overbased metal-containing detergents.
- Lubricating oil compositions are used for the smooth operation of internal combustion engines. The engine oils for internal combustion engines in particular serve to (i) lubricate various sliding interfaces between the piston ring and cylinder liner, in bearings of the crank shaft and the connecting rod, and in the valve driving mechanism including cams and valve lifters, (ii) cool the engine, (iii) clean and disperse the combustion products and (iv) prevent corrosion and consequent rust formation. The stringent requirements for high performance engines in recent years has meant greater demand from lubricants used in such engines.
- There is increasing interest in improving the fuel efficiency of internal combustion engines. Vehicle manufacturers have improved fuel economy through engine design, improvements which take advantage of advances in lubricating oils which provide better oxidative stability, wear protection, and reduced friction. Operators of heavy duty diesel vehicles have been reluctant to adopt low viscosity grade engine oils to improve fuel economy; durability, i.e. the ability to maintain vehicles on the road for extended periods of time and mileage, has been and remains the primary concern. Thus, the most widely used viscosity grades for on-highway heavy duty diesel vehicles has been SAE 15W-40. In recent years, there is a growing push to improve the fuel efficiency of heavy duty diesel vehicles. Consequently, there is a need to improve the fuel economy of diesel engines without compromising the durability of the engines or otherwise negatively impacting the lubricant performance, including deposit and soot control and oxidation and corrosion resistance. This is particularly relevant for viscosity grades lighter than that of SAE 15W-40, for example 10W-30.
- Polymeric viscosity index improvers are typically added to lubricating base oils, including highly refined mineral oils, to improve the viscosity-temperature characteristics and/or low-temperature viscosity characteristics of the lubricating compositions. The viscosity index is commonly evaluated as the viscosity-temperature characteristic of lubricating base oils and lubricating compositions, while the properties evaluated for the low-temperature viscosity characteristics are generally the pour point, cloud point and freezing point. Polymeric viscosity index improvers have also been implicated in deposit formation.
-
EP 2 610 333 A1 discloses fuel economical lubricating oil compositions for internal combustion engines. - The objectives of the invention described herein include providing improved fuel economy while also providing at least one of (i) reduced wear (such as cam wear or lifter wear), (ii) decreased deposit formation, (iii) improved soot handling, (iv) reduced lead or copper corrosion, (v) increased oxidation resistance, and/or (vi) improved seal compatibility in an internal combustion engine. For example, the objectives of the present invention includes providing improved fuel economy while also providing at least one of (i) reduced wear or (ii) decreased deposit formation, especially in compression ignition (i.e. diesel) engines.
- The invention described herein provides a multigrade lubricating , comprising: (a) 80 wt % to 95 wt % of an oil of lubricating viscosity having a viscosity index of at least 130; (b) an ashless dispersant; and (c) an overbased metal detergent; wherein the lubricating composition contains less than 0.01 weight percent of a polymeric viscosity index improver; and the lubricant composition has a SAE viscosity grade of XW-Y, wherein X may be 0, 5, or 10; and Y may be 16, 20, 26, 30, or 40.
- The invention further provides a lubricating composition as described herein wherein the multigrade crankcase lubricant is a SAE 0W-16, 0W-20, 0W-26, 5W-16, 5W-20, 5W-26, 5W-30, 10W-16, 10W-30, or 10W-40 lubricant.
- The invention further provides a lubricating composition as described herein in which the ashless dispersant is derived from a polyolefin having a number average molecular weight of 500 to 5000.
- The invention further provides a lubricating composition as described herein in which the metal-containing overbased detergent includes one or more of a calcium sulfonate, a calcium phenate, a magnesium sulfonate or a magnesium phenate.
- The invention further provides a lubricating composition as described herein in which the metal-containing overbased detergent is present in an amount to deliver at least 4 TBN to the composition.
- The invention further provides a lubricating composition as described herein in which the lubricating composition has a viscosity index of at least 130 or at least 135. In one embodiment, the lubricating composition has a viscosity index of 130 to 230, 135 to 195, or 140 to 175.
- The invention further provides a lubricating composition as described herein in which the oil of lubricating viscosity includes from 0.1 weight percent to 20 weight percent of an oil derived from hydroisomerization of a high wax-containing feed stream.
- The invention further provides a lubricating composition as described herein in which the oil of lubricating viscosity includes from 0.5 weight percent to 5 weight percent of an oil derived from hydroisomerization of a high wax-containing feed stream.
- The invention further provides a use of a lubricant composition for lubricating a compression-ignition internal combustion engine including supplying the engine a lubricant composition as described herein.
- The invention further provides a use of a lubricant composition for improving the fuel economy of a compression-ignition internal combustion engine including supplying to the engine the lubricant composition as described herein, wherein the lubricant is a SAE 0W-16, 0W-20, 0W-26, 5W-16, 5W-20, 5W-30, 10W-16, 10W-30 or 10W-40 lubricant .
- Various preferred features and embodiments will be described below by way of non-limiting illustration. As used herein, reference to the amounts of additives present in the lubricating composition disclosed are quoted on an oil free basis, i.e., amount of actives, unless otherwise indicated.
- The oil of lubricating viscosity of the invention may be defined as an API Group II+ base oil. API Group II+ base oils are known and described for example in SAE publication entitled "Design Practice: Passenger Car Automatic Transmissions", fourth Edition, AE-29, published 2012, page 12-9.
US 8,216,448 also defines a API Group II+ as a "Group II plus base oil" having a viscosity index greater than or equal to 110 and less than 120. - The oil of lubricating viscosity of the invention has a viscosity index (VI) of at least 130, or at least 135.
- Examples of an oil of lubricating viscosity of the present invention include base oils sold under the registered trade names of Ultra-S, Nexbase®, Yubase®, Petrocanada, and Chevron neutral oil 110RLV.
- The oil of lubricating viscosity of the invention is present at 80 wt % to 95 wt % of the lubricating composition.
- The oil of lubricating viscosity of the invention may also be blended with a conventional oil of lubricating viscosity (i.e., an oil of lubricating viscosity other than that defined by the present invention) with the proviso that the base oil mixture continues to exhibit a viscosity index as described above. The conventional oil of lubricating viscosity may be defined as specified in the American Petroleum Institute (API) Base Oil Interchangeability Guidelines. The five base oil groups are as follows: Group I (sulfur content >0.03 wt %, and/or <90 wt % saturates, viscosity index 80-120); Group II (sulfur content ≤0.03 wt %, and ≥90 wt % saturates, viscosity index 80-120); Group III (sulfur content ≤0.03 wt %, and ≥90 wt % saturates, viscosity index ≥120); Group IV (all polyalphaolefins (PAOs)); and Group V (all others not included in Groups I, II, III, or IV). The oil of lubricating viscosity comprises an API Group I, Group II (other than the oil of lubricating viscosity defined by the present invention), Group III, Group IV, Group V oil or mixtures thereof.
- Often the conventional oil of lubricating viscosity is an API Group I, Group II (other than the oil of lubricating viscosity defined by the present invention), Group III, Group IV oil or mixtures thereof. Alternatively the conventional oil of lubricating viscosity is often an API Group II (other than the oil of lubricating viscosity defined by the present invention), Group III or Group IV oil or mixtures thereof. Groups I, II and III are mineral oil base stocks. The oil of lubricating viscosity can include natural or synthetic oils and mixtures thereof. Mixture of mineral oil and synthetic oils, e.g., polyalphaolefin oils and/or polyester oils, may be used.
- Base oils of API Group II and Group III are subjected to hydrotreating to reduce/remove aromatics and raise viscosity index. Group III base oils are subjected to severe hydrotreating process conditions in order to produce oils with viscosity index of at least 120. In one embodiment, the lubricating composition of the invention comprises a severely hydrotreated base oil with a viscosity index of at least 110, at least 120, at least 130, or even at least 140. In one embodiment the lubricating composition comprises a Group III base oil. Some high viscosity index Group III base oils are produced in part by inclusion of high wax containing feed streams that are subjected to hydroisomerization to produce high viscosity index isoparaffinic base oils.
- Natural oils include animal oils and vegetable oils (e.g. vegetable acid esters) as well as mineral lubricating oils such as liquid petroleum oils and solvent-treated or acid treated mineral lubricating oils of the paraffinic, naphthenic, or mixed paraffinic-naphthenic types. Hydrotreated or hydrocracked oils are also useful oils of lubricating viscosity. Oils of lubricating viscosity derived from coal or shale are also useful.
- Synthetic oils include hydrocarbon oils and halosubstituted hydrocarbon oils such as polymerized and interpolymerized olefins and mixtures thereof, alkylbenzenes, polyphenyl, alkylated diphenyl ethers, and alkylated diphenyl sulfides and their derivatives, analogs and homologues thereof. Alkylene oxide polymers and interpolymers and derivatives thereof, and those where terminal hydroxyl groups have been modified by, e.g., esterification or etherification, are other classes of synthetic lubricating oils. Other suitable synthetic lubricating oils comprise esters of dicarboxylic acids and those made from C5 to C12 monocarboxylic acids and polyols or polyol ethers. Other synthetic lubricating oils include liquid esters of phosphorus-containing acids, polymeric tetrahydrofurans, silicon-based oils such as poly-alkyl-, polyaryl-, polyalkoxy-, or polyaryloxy-siloxane oils, and silicate oils.
- Other synthetic oils include those produced by Fischer-Tropsch reactions, typically hydroisomerized Fischer-Tropsch hydrocarbons or waxes. In one embodiment oils may be prepared by a Fischer-Tropsch gas-to-liquid synthetic procedure as well as other gas-to-liquid oils.
- In one embodiment, the base oil of lubricating viscosity contains 0.1 weight percent to 20 weight percent of a base oil fraction derived from hydroisomerization of a high wax-containing feed stream and/or base oil derived from hydroisomerization of a Fischer-Tropsch wax feed stream. In one embodiment, the oil of lubricating viscosity contains 0.5 weight percent to 5 weight percent of a base oil fraction derived from hydroisomerization of a high wax-containing feed stream and/or base oil derived from hydroisomerization of a Fischer-Tropsch wax feed stream. In one embodiment, the lubricating composition is free of (or substantially free of) a hydroisomerized wax-derived base oil.
- Unrefined, refined, and rerefined oils, either natural or synthetic (as well as mixtures thereof) of the types disclosed hereinabove can used. Unrefined oils are those obtained directly from a natural or synthetic source without further purification treatment. Refined oils are similar to the unrefined oils except they have been further treated in one or more purification steps to improve one or more properties. Rerefined oils are obtained by processes similar to those used to obtain refined oils applied to refined oils which have been already used in service. Rerefined oils often are additionally processed to remove spent additives and oil breakdown products.
- The amount of the oil of lubricating viscosity present is typically the balance remaining after subtracting from 100 wt % the sum of the amount of performance additives of the present invention.
- The lubricating composition may be in the form of a concentrate and/or a fully formulated lubricant. If the performance additives of this invention are in the form of a concentrate (which may be combined with additional oil to form, in whole or in part, a finished lubricant), the ratio of the performance additives to the oil of lubricating viscosity and/or to diluent oil include the ranges of 1:99 to 99:1 by weight, or 80:20 to 10:90 by weight.
- Dispersants, generally, are well known in the field of lubricants and include primarily what is known as ashless dispersants and polymeric dispersants. Ashless dispersants are so-called because, as supplied, they do not contain metal and thus do not normally contribute to sulfated ash when added to a lubricant. However, they may interact with ambient metals once they are added to a lubricant which includes a metal-containing species. Ashless dispersants are characterized by a polar group attached to a relatively high molecular weight hydrocarbon chain. Typical ashless dispersants include N-substituted long chain alkenyl succinimides, having a variety of chemical structures, including those represented by Formula (I):
- Such molecules are commonly derived from reaction of an alkenyl acylating agent with a polyamine, and a wide variety of linkages between the two moieties is possible beside the simple imide structure shown above, including a variety of amides and quaternary ammonium salts. In the above Formula (I), the amine portion is shown as an alkylene polyamine, although other aliphatic and aromatic mono- and polyamines may also be used. Also, a variety of modes of linkage of the R1 groups onto the imide structure are possible, including various cyclic linkages. The ratio of the carbonyl groups of the acylating agent to the nitrogen atoms of the amine may be 1:0.5 to 1:3, and in other instances 1:1 to 1:2.75 or 1:1.5 to 1:2.5. Succinimide dispersants are more fully described in
U.S. Patents 4,234,435 and3,172,892 and inEP 0355895 . - In certain embodiments, the dispersant is prepared by a process that involves the presence of small amounts of chlorine or other halogen, as described in
U.S. Patent 7,615,521 (see, e.g., col. 4, lines 18-60 and preparative example A). Such dispersants typically have some carbocyclic structures in the attachment of the hydrocarbyl substituent to the acidic or amidic "head" group. In other embodiments, the dispersant is prepared by a thermal process involving an "ene" reaction, without the use of any chlorine or other halogen, as described inU.S. Patent 7,615,521 ; dispersants made in this manner are often derived from high vinylidene (i.e. greater than 50% terminal vinylidene) polyisobutylene(See col. 4, line 61 to col. 5, line 30 and preparative example B). Such dispersants typically do not contain the above-described carbocyclic structures at the point of attachment. In certain embodiments, the dispersant is prepared by free radical catalyzed polymerization of high-vinylidene polyisobutylene with an ethylenically unsaturated acylating agent, as described in United States Patent8,067,347 . - Dispersants may be derived from, as the polyolefin, high vinylidene polyisobutylene, that is, having greater than 50, 70, or 75% terminal vinylidene groups (α and β isomers). In certain embodiments, the succinimide dispersant may be prepared by the direct alkylation route. In other embodiments it may comprise a mixture of direct alkylation and chlorine-route dispersants.
- Suitable dispersants for use in the compositions of the present invention include succinimide dispersants. In one embodiment, the dispersant may be present as a single dispersant. In one embodiment, the dispersant may be present as a mixture of two or three different dispersants, wherein at least one may be a succinimide dispersant.
- The succinimide dispersant may be a derivative of an aliphatic polyamine, or mixtures thereof. The aliphatic polyamine may be aliphatic polyamine such as an ethylenepolyamine, a propylenepolyamine, a butylenepolyamine, or mixtures thereof. In one embodiment, the aliphatic polyamine may be ethylenepolyamine. In one embodiment the aliphatic polyamine may be selected from the group consisting of ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine, pentaethylenehexamine, polyamine still bottoms, and mixtures thereof.
- The succinimide dispersant may be a derivative of an aromatic amine, an aromatic polyamine, or mixtures thereof. The aromatic amine may be 4-aminodiphenylamine (ADPA) (also known as N-phenylphenylenediamine), derivatives of ADPA (as described in United States Patent Publications
2011/0306528 and2010/0298185 ), a nitroaniline, an aminocarbazole, an amino-indazolinone, an aminopyrimidine, 4-(4-nitrophenylazo)aniline, or combinations thereof. In one embodiment, the dispersant is derivative of an aromatic amine wherein the aromatic amine has at least three non-continuous aromatic rings. - The succinimide dispersant may be a derivative of a polyether amine or polyether polyamine. Typical polyether amine compounds contain at least one ether unit and will be chain terminated with at least one amine moiety. The polyether polyamines can be based on polymers derived from C2-C6 epoxides such as ethylene oxide, propylene oxide, and butylene oxide. Examples of polyether polyamines are sold under the Jeffamine® brand and are commercially available from Hunstman Corporation located in Houston, Texas.
- Another class of ashless dispersant is high molecular weight esters. These materials are similar to the above-described succinimides except that they may be seen as having been prepared by reaction of a hydrocarbyl acylating agent and a polyhydric aliphatic alcohol such as glycerol, pentaerythritol, or sorbitol. Such materials are described in more detail in
U.S. Patent 3,381,022 . Aromatic succinate esters may also be prepared as described in United States Patent Publication2010/0286414 . - A succinic-based dispersant (succinimide, succinamide, succinic ester, and mixtures thereof) may be formed by reacting maleic anhydride or a reactive equivalent thereof, such as an acid or ester, with a hydrocarbon chain by any method such as those disclosed above (e.g., chlorine-based process or thermal process). Other acids or equivalents thereof may be used in place of the maleic anhydride. These include fumaric acid, itaconic acid, itaconic anhydride, citraconic acid, citaconic anhydride, and cinnamic acid as well as other ethylenically unsaturated acids such as acrylic or methacrylic acid; and their reactive equivalents.
- Another class of ashless dispersant is Mannich bases. These are materials which are formed by the condensation of a higher molecular weight, alkyl substituted phenol, an alkylene polyamine, and an aldehyde such as formaldehyde. Such materials may have the general structure as represented by Formula (II)
U.S. Patent 3,634,515 . - Another class of ashless dispersants include dispersants comprising a quaternary ammonium salt. Quaternary ammonium salts include the reaction product of: (i) a compound comprising at least one tertiary amino group; and (ii) a quaternizing agent suitable for converting the tertiary amino group of compound (i) to a quaternary nitrogen. Examples of suitable quaternary ammonium salts include (i) imide quaternary ammonium salts, (ii) Mannich quaternary ammonium salts, (iii) polyalkene substituted amine quaternary ammonium salts, (iv) amide quaternary ammonium salts, (v) ester quaternary ammonium salts, (vi) polyester quaternary ammonium salts, or (vii) any combination thereof.
- These various types of quaternary ammonium salts may be prepared in any number of ways but generally are prepared by reacting a non-quaternized nitrogen-containing compound with a quaternizing agent. Each of the different types of quaternary ammonium salts described uses a different non-quaternized nitrogen-containing compound in its preparation, but generally the non-quaternized nitrogen-containing compound contains a tertiary nitrogen capable of being quaternized (or a primary or secondary nitrogen atom that can be alkylated to a tertiary nitrogen that can then be quaternized) and a hydrocarbyl substituent group. The preparation and use of quaternized ammonium dispersants is described in detail in United States Patent
7,951,211 and United States Patent7,906,470 . - The dispersant may also be post-treated by conventional methods by a reaction with any of a variety of agents. Among these are boron compounds, urea, thiourea, dimercaptothiadiazoles, carbon disulfide, aldehydes, ketones, carboxylic acids, hydrocarbon-substituted succinic anhydrides, maleic anhydride, nitriles, epoxides, and phosphorus compounds.
- The dispersant may also exhibit basicity, as measured by Total Base Number (TBN). TBN may be determined by ASTM D2896. This will particularly be the case if the dispersant is prepared with an amine, such as a polyamine, and the amine contains one or more amino groups that have not reacted with acidic groups of the dispersant. In some embodiments, the TBN of the dispersant may be 1 to 110, or 5 to 50, or 10 to 40 or 30 to 70. In some embodiments, however, the dispersant may not exhibit basicity (that is, have a TBN of 0 or nearly 0). In one embodiment the dispersant has a TBN of zero as measured by D2896. Such could be the case if no basic nitrogen is present on the dispersant.
- The dispersant may be present at 0.01 wt % to 20 wt %, or 0.1 wt % to 15 wt %, or 0.1 wt % to 10 wt %, or 0.5 wt % to 8 wt % , or 1.0 wt % to 6.5 wt % of the lubricating composition.
- Metal overbased detergents, otherwise referred to as overbased detergents, metal-containing overbased detergents or superbased salts, are characterized by a metal content in excess of that which would be necessary for neutralization according to the stoichiometry of the metal and the particular acidic organic compound, i.e. the substrate, reacted with the metal. The overbased detergent may comprise one or more of non-sulfur containing phenates, sulfur containing phenates, sulfonates, salicylates, and mixtures thereof.
- The amount of excess metal is commonly expressed in terms of substrate to metal ratio. The terminology "metal ratio" is used in the prior art and herein to define the ratio of the total chemical equivalents of the metal in the overbased salt to the chemical equivalents of the metal in the salt which would be expected to result from the reaction between the hydrocarbyl substituted organic acid; the hydrocarbyl-substituted phenol or mixtures thereof to be overbased, and the basic metal compound according to the known chemical reactivity and the stoichiometry of the two reactants. Thus, in a normal or neutral salt (i.e. soap) the metal ratio is one and, in an overbased salt, the metal ratio is greater than one, especially greater than 1.3. The overbased detergent of the invention may have a metal ratio of 5 to 30, or a metal ratio of 7 to 22, or a metal ratio of at least 11.
- The metal-containing detergent may also include "hybrid" detergents formed with mixed surfactant systems including phenate and/or sulfonate components, e.g. phenate/salicylates, sulfonate/phenates, sulfonate/salicylates, sulfonates/phenates/salicylates, as described, for example, in
US Patents 6,429,178 ;6,429,179 ;6,153,565 ; and6,281,179 . Where, for example, a hybrid sulfonate/phenate detergent is employed, the hybrid detergent would be considered equivalent to amounts of distinct phenate and sulfonate detergents introducing like amounts of phenate and sulfonate soaps, respectively. Overbased phenates and salicylates typically have a total base number of 180 to 450 TBN. Overbased sulfonates typically have a total base number of 250 to 600, or 300 to 500. Overbased detergents are known in the art. - Alkylphenols are often used as constituents in and/or building blocks for overbased detergents. Alkylphenols may be used to prepare phenate, salicylate, salixarate, or saligenin detergents or mixtures thereof. Suitable alkylphenols may include para-substituted hydrocarbyl phenols. The hydrocarbyl group may be linear or branched aliphatic groups of 1 to 60 carbon atoms, 8 to 40 carbon atoms, 10 to 24 carbon atoms, 12 to 20 carbon atoms, or 16 to 24 carbon atoms. In one embodiment, the alkylphenol overbased detergent is prepared from an alkylphenol or mixture thereof that is free of or substantially free of (i.e. contains less than 0.1 weight percent) p-dodecylphenol. In one embodiment, the lubricating composition of the invention contains less than 0.3 weight percent of alkylphenol, less than 0.1 weight percent of alkylphenol, or less than 0.05 weight percent of alkylphenol.
- The overbased metal-containing detergent may be alkali metal or alkaline earth metal salts. In one embodiment, the overbased detergent may be sodium salts, calcium salts, magnesium salts, or mixtures thereof of the phenates, sulfur-containing phenates, sulfonates, salixarates and salicylates. In one embodiment, the overbased detergent is a calcium detergent, a magnesium detergent or mixtures thereof. In one embodiment, the overbased calcium detergent may be present in an amount to deliver at least 500 ppm calcium by weight and no more than 3000 ppm calcium by weight, or at least 1000 ppm calcium by weight, or at least 2000 ppm calcium by weight, or no more than 2500 ppm calcium by weight to the lubricating composition. In one embodiment, the overbased detergent may be present in an amount to deliver no more than 500 ppm by weight of magnesium to the lubricating composition, or no more than 330 ppm by weight, or no more than 125 ppm by weight, or no more than 45 ppm by weight. In one embodiment, the lubricating composition is essentially free of (i.e. contains less than 10 ppm) magnesium resulting from the overbased detergent. In one embodiment, the overbased detergent may be present in an amount to deliver at least 200 ppm by weight of magnesium, or at least 450 ppm by weight magnesium, or at least 700 ppm by weight magnesium to the lubricating composition. In one embodiment, both calcium and magnesium containing detergents may be present in the lubricating composition. Calcium and magnesium detergents may be present such that the weight ratio of calcium to magnesium is 10:1 to 1:10, or 8:3 to 4:5, or 1:1 to 1:3. In one embodiment, the overbased detergent is free of or substantially free of sodium.
- In one embodiment, the sulfonate detergent may be predominantly a linear alkylbenzene sulfonate detergent having a metal ratio of at least 8 as is described in paragraphs [0026] to [0037] of
US Patent Publication 2005/065045 (and granted asUS 7,407,919 ). The linear alkylbenzene sulfonate detergent may be particularly useful for assisting in improving fuel economy. The linear alkyl group may be attached to the benzene ring anywhere along the linear chain of the alkyl group, but often in the 2, 3 or 4 position of the linear chain, and in some instances, predominantly in the 2 position, resulting in the linear alkylbenzene sulfonate detergent. - Salicylate detergents and overbased salicylate detergents may be prepared in at least two different manners. Carbonylation (also referred to as carboxylation) of a p-alkylphenol is described in many references including
US Patent 8,399,388 . Carbonylation may be followed by overbasing to form overbased salicylate detergent. Suitable p-alkylphenols include those with linear and/or branched hydrocarbyl groups of 1 to 60 carbon atoms. Salicylate detergents may also be prepared by alkylation of salicylic acid, followed by overbasing, as described inUS Patent 7,009,072 . Salicylate detergents prepared in this manner, may be prepared from linear and/or branched alkylating agents (usually 1-olefins) containing 6 to 50 carbon atoms, 10 to 30 carbon atoms, or 14 to 24 carbon atoms. In one embodiment, the overbased detergent of the invention is a salicylate detergent. In one embodiment, the salicylate detergent of the invention is free of unreacted p-alkylphenol (i.e. contains less than 0.1 weight percent). In one embodiment, the salicylate detergent of the invention is prepared by alkylation of salicylic acid. - The overbased detergent may be present at 0.2 wt % to 15 wt %, or 0.3 wt % to 10 wt %, or 0.3 wt % to 8 wt %, or 0.4 wt % to 3 wt %. For example, in a heavy duty diesel engine, the detergent may be present at 2 wt % to 3 wt % of the lubricating composition. For a passenger car engine, the detergent may be present at 0.2 wt % to 1 wt % of the lubricating composition.
- Metal-containing overbased detergents often provide TBN to the lubricating composition. In one embodiment, the overbased detergent is present in an amount to deliver at least 4 mg KOH/g of TBN to the lubricating composition, or 4 to 15 mg KOH/g, or 5 to 9 mg KOH/g of TBN to the lubricating composition.
- Metal-containing detergents contribute sulfated ash to a lubricating composition. Sulfated ash may be determined by ASTM D874. In one embodiment, the lubricating composition of the invention comprises a metal-containing detergent in an amount to deliver at least 0.4 weight percent sulfated ash to the total composition. In another embodiment, the metal-containing detergent is present in an amount to deliver at least 0.6 weight percent sulfated ash, or at least 0.75 weight percent sulfated ash, or even at least 0.9 weight percent sulfated ash to the lubricating composition.
- In addition to ash and TBN, overbased detergents contribute detergent soap, also referred to as neutral detergent salt, to the lubricating composition. Soap, being a metal salt of the substrate, may act as a surfactant in the lubricating composition. In one embodiment, the lubricating composition comprises 0.05 weight percent to 1.5 weight percent detergent soap, or 0.1 weight percent to 0.9 weight percent detergent soap. In one embodiment, the lubricating composition contains no more than 0.5 weight percent detergent soap. The overbased detergent may have a weight ratio of ash:soap of 5:1 to 1:2.3, or 3.5:1 to 1:2, or 2.9:1 to 1:1:7.
- The compositions of the invention may optionally comprise one or more additional performance additives. These additional performance additives may include one or more metal deactivators, detergents, friction modifiers, antiwear agents, corrosion inhibitors, soot-dispersing additives, extreme pressure agents, antioxidants, foam inhibitors, demulsifiers, pour point depressants, seal swelling agents, and any combination or mixture thereof. Typically, fully-formulated lubricating oil will contain one or more of these performance additives, and often a package of multiple performance additives.
- In one embodiment, the invention provides a lubricating composition further comprising an antiwear agent, a dispersant viscosity modifier, a friction modifier, a viscosity modifier, an antioxidant, an overbased detergent, a dispersant (different from that of the invention), or a combination thereof, where each of the additives listed may be a mixture of two or more of that type of additive. In one embodiment, the invention provides a lubricating composition further comprising an antiwear agent, a dispersant viscosity modifier, a friction modifier, a viscosity modifier (typically an olefin copolymer such as an ethylene-propylene copolymer), an antioxidant (including phenolic and aminic antioxidants), an overbased detergent (including overbased sulfonates and phenates), or a combination thereof, where each of the additives listed may be a mixture of two or more of that type of additive.
- Another additive is an antiwear agent. Examples of anti-wear agents include phosphorus-containing antiwear/extreme pressure agents such as metal thiophosphates, phosphoric acid esters and salts thereof, phosphorus-containing carboxylic acids, esters, ethers, and amides, and phosphites. In certain embodiments a phosphorus antiwear agent may be present in an amount to deliver 0.01 to 0.2 or 0.015 to 0.15 or 0.02 to 0.1 or 0.025 to 0.08 percent phosphorus. Often the antiwear agent is a zinc dialkyldithiophosphate (ZDP).
- Zinc dialkyldithiophosphates may be described as primary zinc dialkyldithiophosphates or as secondary zinc dialkyldithiophosphates, depending on the structure of the alcohol used in its preparation. In some embodiments the compositions of the invention include primary zinc dialkyldithiophosphates. In some embodiments the compositions of the invention include secondary zinc dialkyldithiophosphates. In some embodiments the compositions of the invention include a mixture of primary and secondary zinc dialkyldithiophosphates. In some embodiments component (b) is a mixture of primary and secondary zinc dialkyldithiophosphates where the ratio of primary zinc dialkyldithiophosphates to secondary zinc dialkyldithiophosphates (one a weight basis) is at least 1:1, or even at least 1:1.2, or even at least 1:1.5 or 1:2, or 1:10. In some embodiments component (b) is a mixture of primary and secondary zinc dialkyldithiophosphates that is at least 50 percent by weight primary, or even at least 60, 70, 80, or even 90 percent by weight primary. In some embodiments component (b) is free of primary zinc dialkyldithiophosphates.
- The phosphorus antiwear agent may be present at 0 wt % to 3 wt %, or 0.1 wt % to 1.5 wt %, or 0.5 wt % to 0.9 wt % of the lubricating composition.
- Another class of anti-wear additives includes oil-soluble titanium compounds as disclosed in
U.S. Pat. No. 7,727,943 andUS20060014651 . The oil-soluble titanium compounds may function as antiwear agents, friction modifiers, antioxidants, deposit control additives, or more than one of these functions. In one embodiment the oil soluble titanium compound may be a titanium (IV) alkoxide. The titanium alkoxide may be formed from a monohydric alcohol, a polyol or mixtures thereof. The monohydric alkoxides may have 2 to 16, or 3 to 10 carbon atoms. In one embodiment, the titanium alkoxide may be titanium (IV) isopropoxide. In one embodiment, the titanium alkoxide may be titanium (IV) 2-ethylhexoxide. In one embodiment, the titanium compound comprises the alkoxide of a vicinal 1,2-diol or polyol. In one embodiment, the 1,2-vicinal diol comprises a fatty acid mono-ester of glycerol, often the fatty acid may be oleic acid. In one embodiment, the oil soluble titanium compound may be a titanium carboxylate. In one embodiment the titanium (IV) carboxylate may be titanium neodecanoate. In one embodiment the oil soluble titanium compound may be present in the lubricating composition in an amount necessary to provide for 10 ppm to 1500 ppm titanium by weight or 25 ppm to 150 ppm titanium by weight. - In one embodiment, the invention provides a lubricating composition which further comprises ashless antioxidant. Ashless antioxidants may comprise one or more of arylamines, diarylamines, alkylated arylamines, alkylated diaryl amines, phenols, hindered phenols, sulfurized olefins, or mixtures thereof. In one embodiment the lubricating composition includes an antioxidant, or mixtures thereof. The antioxidant may be present at 0 wt % to 15 wt %, or 0.1 wt % to 10 wt %, or 0.5 wt % to 5 wt %, or 0.5 wt % to 3 wt %, or 0.3 wt % to 1.5 wt % of the lubricating composition.
- The diarylamine or alkylated diarylamine may be a phenyl-α-naphthylamine (PANA), an alkylated diphenylamine, or an alkylated phenylnapthylamine, or mixtures thereof. The alkylated diphenylamine may include di-nonylated diphenylamine, nonyl diphenylamine, octyl diphenylamine, di-octylated diphenylamine, di-decylated diphenylamine, decyl diphenylamine and mixtures thereof. In one embodiment, the diphenylamine may include nonyl diphenylamine, dinonyl diphenylamine, octyl diphenylamine, dioctyl diphenylamine, or mixtures thereof. In one embodiment the alkylated diphenylamine may include nonyl diphenylamine, or dinonyl diphenylamine. The alkylated diarylamine may include octyl, di-octyl, nonyl, di-nonyl, decyl or di-decyl phenylnapthylamines.
- The diarylamine antioxidant of the invention may be present on a weight basis of the lubrication composition at 0.1% to 10%, 0.35% to 5%, or even 0.5% to 2%.
- The phenolic antioxidant may be a simple alkyl phenol, a hindered phenol, or coupled phenolic compounds.
- The hindered phenol antioxidant often contains a secondary butyl and/or a tertiary butyl group as a sterically hindering group. The phenol group may be further substituted with a hydrocarbyl group (typically linear or branched alkyl) and/or a bridging group linking to a second aromatic group. Examples of suitable hindered phenol antioxidants include 2,6-di-tert-butylphenol, 4-methyl-2,6-di-tert-butylphenol, 4-ethyl-2,6-di-tert-butylphenol, 4-propyl-2,6-di-tert-butylphenol or 4-butyl-2,6-di-tert-butylphenol, 4-dodecyl-2,6-di-tert-butylphenol, or butyl 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate. In one embodiment, the hindered phenol antioxidant may be an ester and may include, e.g., Irganox™ L-135 from Ciba.
- Coupled phenols often contain two alkylphenols coupled with alkylene groups to form bisphenol compounds. Examples of suitable coupled phenol compounds include 4,4'- methylene bis-(2,6-di-tert-butyl phenol), 4-methyl-2,6-di-tert-butylphenol, 2,2'-bis-(6-t-butyl-4-heptylphenol); 4,4'-bis(2,6-di-t-butyl phenol), 2,2'-methylenebis(4-methyl-6-t-butylphenol), and 2,2'-methylene bis(4-ethyl-6-t-butylphenol).
- Phenols of the invention also include polyhydric aromatic compounds and their derivatives. Examples of suitable polyhydric aromatic compounds include esters and amides of gallic acid, 2,5-dihydroxybenzoic acid, 2,6-dihydroxybenzoic acid, 1,4-dihydroxy-2-naphthoic acid, 3,5-dihydroxynaphthoic acid, 3,7-dihydroxy naphthoic acid, and mixtures thereof.
- In one embodiment, the phenolic antioxidant comprises a hindered phenol. In another embodiment the hindered phenol is derived from 2,6-ditertbutyl phenol.
- In one embodiment the lubricating composition of the invention comprises a phenolic antioxidant in a range of 0.01 wt % to 5 wt %, or 0.1 wt % to 4 wt %, or 0.2 wt % to 3 wt %, or 0.5 wt % to 2 wt % of the lubricating composition.
- Sulfurized olefins are well known commercial materials, and those which are substantially nitrogen-free, that is, not containing nitrogen functionality, are readily available. The olefinic compounds which may be sulfurized are diverse in nature. They contain at least one olefinic double bond, which is defined as a nonaromatic double bond; that is, one connecting two aliphatic carbon atoms. These materials generally have sulfide linkages having 1 to 10 sulfur atoms, for instance, 1 to 4, or 1 or 2.
- Ashless antioxidants may be used separately or in combination. In one embodiment of the invention, two or more different antioxidants are used in combination, such that there is at least 0.1 weight percent of each of the at least two antioxidants and wherein the combined amount of the ashless antioxidants is 0.5 to 5 weight percent. In one embodiment, there may be at least 0.25 to 3 weight percent of each ashless antioxidant.
- In one embodiment, the invention provides a lubricating composition further comprising a molybdenum compound. The molybdenum compound may be selected from the group consisting of molybdenum dialkyldithiophosphates, molybdenum dithiocarbamates, amine salts of molybdenum compounds, molybdenum-containing dispersants, and mixtures thereof. Examples of commercially available molybdenum compounds include Sakura-lube™ 525 and Sakura-lube™ 710, both available from Adeka Corporation; and Molyvan® 855 available from Vanderbilt Chemicals, LLC. The molybdenum compound may provide the lubricating composition with 0 to 1000 ppm, or 5 to 1000 ppm, or 10 to 750 ppm, or 5 ppm to 300 ppm, or 20 ppm to 250 ppm of molybdenum. In one embodiment, the molybdenum compound is a molybdenum dithiocarbamate compound present in an amount to provide 300 ppm to 750 ppm molybdenum to the lubricating composition.
- In one embodiment, the lubricating composition of the invention further comprises a soot dispersing additive. The soot dispersing additive may be present at 0 wt % to 5 wt %, or 0 wt % to 4 wt %, or 0.05 wt % to 2 wt % of the lubricating composition.
- Suitable soot dispersing additives include functionalized low molecular weight polyolefins, for example, ethylene-propylene copolymers with a number average molecular weight (Mn) of less than 20,000 that have been functionalized with an acylating agent such as maleic anhydride and an amine, preferably an aromatic amine. Other soot dispersing additives may be prepared from acylated polyisobutylene that has been functionalized with aromatic (poly)amines. More detailed description of soot-dispersing additives are disclosed in
U.S. Patents 4,863,623 ;5,182,041 ;7,790,661 ;8,557,753 ; and8,637,437 . In one embodiment, the soot dispersing additive may include those described inU.S. Patent 7,790,661 or inU.S. Patent 8,557,753 . - In one embodiment, the invention provides a lubricating composition further comprising a friction modifier. Examples of friction modifiers include long chain fatty acid derivatives of amines, fatty esters, or epoxides; fatty imidazolines such as condensation products of carboxylic acids and polyalkylene-polyamines; amine salts of alkylphosphoric acids; fatty esters, amides, and/or imides of α-hydroxy-carbonyl compounds such as tartaric acid, malic acid, citric acid, glycolic acid, lactic acid and mandelic acid. The term fatty, as used herein, can mean having a C8-22 linear alkyl group.
- Friction modifiers may also encompass materials such as sulfurized fatty compounds and olefins, molybdenum dialkyldithiophosphates, molybdenum dithiocarbamates, sunflower oil or monoester of a polyol and an aliphatic carboxylic acid.
- In one embodiment the friction modifier may be selected from the group consisting of long chain fatty acid derivatives of amines, long chain fatty esters, or long chain fatty epoxides; fatty imidazolines; amine salts of alkylphosphoric acids; fatty alkyl tartrates; fatty alkyl tartrimides; and fatty alkyl tartramides. The friction modifier may be present at 0 wt % to 6 wt %, or 0.05 wt % to 4 wt %, or 0.1 wt % to 2 wt % of the lubricating composition.
- In one embodiment, the friction modifier may be a long chain fatty acid ester. In another embodiment the long chain fatty acid ester may be a mono-ester or a diester or a mixture thereof, and in another embodiment the long chain fatty acid ester may be a triglyceride.
- Other performance additives such as corrosion inhibitors include those described in paragraphs 5 to 8 of US Application
US05/038319 , published asWO2006/047486 , octyl octanamide, condensation products of dodecenyl succinic acid or anhydride and a fatty acid such as oleic acid with a polyamine. In one embodiment, the corrosion inhibitors include the Synalox® (a registered trademark of The Dow Chemical Company) corrosion inhibitor. The Synalox® corrosion inhibitor may be a homopolymer or copolymer of propylene oxide. The Synalox® corrosion inhibitor is described in more detail in a product brochure with Form No. 118-01453-0702 AMS, published by The Dow Chemical Company. The product brochure is entitled "SYNALOX Lubricants, High-Performance Polyglycols for Demanding Applications." - The lubricating composition may further include metal deactivators, including derivatives of benzotriazoles (typically tolyltriazole), dimercaptothiadiazole derivatives, 1,2,4-triazoles, benzimidazoles, 2-alkyldithiobenzimidazoles, or 2-alkyldithiobenzothiazoles; foam inhibitors, including copolymers of ethyl acrylate and 2-ethylhexylacrylate and copolymers of ethyl acrylate and 2-ethylhexylacrylate and vinyl acetate; demulsifiers including trialkyl phosphates, polyethylene glycols, polyethylene oxides, polypropylene oxides and (ethylene oxide-propylene oxide) polymers; and pour point depressants, including esters of maleic anhydride-styrene, polymethacrylates, polyacrylates or polyacrylamides.
- The lubricating composition may further comprise a polyether compound. The polyether compound may be a polyether, a polyetheramine, a (poly)alkoxylated amine, an ethoxylated alcohol, or mixtures thereof. The polyether can be represented by the formula RO[CH2CH(R1)O]xH where R is a hydrocarbyl group; R1 is selected from the group consisting of hydrogen, alkyl groups of 1 to 14 carbon atoms, and mixtures thereof; and x is a number from 2 to 50. The hydrocarbyl group R is a univalent hydrocarbon group, has one or more carbon atoms, and includes alkyl and alkyl-phenyl groups having 7 to 30 total carbon atoms, such as 9 to 25 total carbon atoms, or 11 to 20 total carbon atoms. The repeating oxyalkylene units may be derived from ethylene oxide, propylene oxide, or butylene oxide. The number of oxyalkylene units x may be 10 to 35, or 18 to 27. The polyether of the present invention can be prepared by various well-known methods including condensing one mole of an alcohol or alkylphenol with two or more moles of an alkylene oxide, mixture of alkylene oxides, or with several alkylene oxides in sequential fashion, usually in the presence of a base catalyst.
U.S. Pat. No. 5,094,667 provides reaction conditions for preparing a polyether. Suitable polyethers are commercially available from Dow Chemicals, Huntsman, ICI and include the Actaclear® series from Bayer. Suitable ethoxylates include Surfonic® ethoxylates, for example Surfonic L24-5, available from Huntsman International LLC. - Polymeric viscosity modifiers may be present with the proviso that they are present in an amount less than 0.01 weight percent of the lubricating composition. Suitable viscosity modifiers include ethylene-olefin co-polymers, especially ethylene-propylene; maleic anhydride-styrene alternating copolymers and esters thereof, polymethacrylates (including random, block and star architectures), hydrogenated styrene-butadiene block copolymers, hydrogenated styrene-isoprene radial and/or block copolymers, or mixtures thereof. In one embodiment, the polymeric viscosity modifier includes an ethylene-olefin based copolymer. In some embodiments, the ethylene makes up from 50 weight percent to 90 weight percent or from 65 weight percent to 85 weight percent, on a molar basis of the monomer used to prepare the copolymer of the ethylene-olefin-based copolymer. In another embodiment, the ethylene makes up at least 70 weight percent of the monomer used to prepare the copolymer. In one embodiment, the ethylene-olefin copolymer has a molecular weight from 5,000 to 40,000 Mn.
- Pour point depressants that may be useful in the compositions of the invention further include polyalphaolefins, esters of maleic anhydride-styrene, poly(meth)acrylates, polyacrylates or polyacrylamides.
- Generally, the lubricant is added to the lubricating system of the internal combustion engine, which then delivers the lubricating composition to the critical parts of the engine, during its operation, that require lubrication.
- The lubricating compositions described above may be utilized in an internal combustion engine. The engine components may have a surface of steel or aluminum (typically a surface of steel), and may also be coated for example with a diamond-like carbon (DLC) coating.
- An aluminum surface may be comprised of an aluminum alloy that may be a eutectic or hyper-eutectic aluminum alloy (such as those derived from aluminum silicates, aluminum oxides, or other ceramic materials). The aluminum surface may be present on a cylinder bore, cylinder block, or piston ring having an aluminum alloy, or aluminum composite.
- The internal combustion engine may be fitted with an emission control system, an oil mist separator or a turbocharger. Examples of the emission control system include diesel particulate filters (DPF), or systems employing selective catalytic reduction (SCR).
- The internal combustion engine is distinct from a gas turbine. In an internal combustion engine, individual combustion events translate from a linear reciprocating force into a rotational torque through the rod and crankshaft. In contrast, in a gas turbine (which may also be referred to as a j et engine) a continuous combustion process generates a rotational torque continuously without translation, and can also develop thrust at the exhaust outlet. These differences in operation conditions of a gas turbine and internal combustion engine result in different operating environments and stresses.
- The lubricant composition for an internal combustion engine may be suitable for any engine lubricant irrespective of the sulfur, phosphorus or sulfated ash (ASTM D-874) content. The sulfur content of the engine oil lubricant may be 1 wt % or less, or 0.8 wt % or less, or 0.5 wt % or less, or 0.3 wt % or less. In one embodiment, the sulfur content may be in the range of 0.001 wt % to 0.5 wt %, or 0.01 wt % to 0.3 wt %. The phosphorus content may be 0.2 wt % or less, or 0.12 wt % or less, or 0.1 wt % or less, or 0.085 wt % or less, or 0.08 wt % or less, or even 0.06 wt % or less, 0.055 wt % or less, or 0.05 wt % or less. In one embodiment the phosphorus content may be 100 ppm to 1000 ppm, or 200 ppm to 600 ppm. The total sulfated ash content may be 2 wt % or less, or 1.5 wt % or less, or 1.1 wt % or less, or 1 wt % or less, or 0.8 wt % or less, or 0.5 wt % or less, or 0.4 wt % or less. In one embodiment, the sulfated ash content may be 0.05 wt % to 0.9 wt %, or 0.1 wt % to 0.2 wt % or to 0.45 wt %.
- In one embodiment, the lubricating composition may be an engine oil, wherein the lubricating composition may be characterized as having at least one of (i) a sulfur content of 0.5 wt % or less, (ii) a phosphorus content of 0.1 wt % or less, (iii) a sulfated ash content of 1.5 wt % or less, or combinations thereof.
- The following examples provide illustrations of the invention. These examples are non-exhaustive and are not intended to limit the scope of the invention. Examples:
- A series of 10W-30 diesel lubricating compositions are prepared according to Table 1 below. Compositions are prepared by blending components as shown in Table 1 into a lubricant. The amounts in Table 1 are on an oil-free basis.
TABLE 1 - Lubricating Compositions Embodiments (wt %) Inv. Ex1 Comp Ex 1 Group III Base Oil 84.81 76.82 PIBsuccinimide Dispersant3 3.1 3.1 Calcium Sulfonate Detergent4 1.2 1.2 Calcium Phenate Detergent5 0.8 0.8 Soot Dispersing Additive6 0.67 0.67 Zinc dialkyldithiophosphate 1.0 1.0 Other Performance Additives7 1.44 1.44 E-P Viscosity Index Improver 0 0.7 Pour Point Depressant 0.2 0.2 Dil Oil (from components) Balance to 100% 1. Ultra-S (S-Oil) VHVI-8cSt; VI = 131
2. Ultra-S (S-Oil) VHVI-4cSt (29.64%) (VI = 125)/Ultra-S (S-Oil) VHV-8cSt(46.36%) VI = 131
3. PIBsuccinimide
4. Combination of low metal ratio (<3) and high metal ratio (>10) alkylbenzene sulfonate
5. Combination of low metal ratio (<2) and higher metal ratio (>5) sulfur-coupled phenates
6. Ethylene-propylene copolymer (Mn 7000) acylated with maleic anhydride and functionalized with nitroaniline
7. Other additives include metal passivators, surfactant, ashless antioxidants, and foam inhibitor - The lubricating compositions are evaluated for wear protection in GM 6.5L Roller Follower Wear Test (RFWT) (ASTM D5966). This test measures wear on camshaft roller follower pins to determine the ability of an engine oil to control wear in the presence of soot-laden oil. The results obtained are summarized in Table 2, below.
- In addition, the lubricating compositions were also evaluated in the Volvo D12D fuel economy engine test; this test estimates the fuel economy benefits of the candidate oil versus a baseline oil (HVES 540). The baseline lubricant was a commercial SAE 15W-40 API CJ-4 Heavy-Duty Diesel Engine Oil. The results obtained are summarized in Table 2, below.
TABLE 2 - Wear and Fuel Economy Test Results Inv. Ex1 Comp Ex 1 Base Oil Viscosity @100C 5.80 7.11 KV100 10.5 11.5 VI (D2270) 137 159 Calcium (wt %) 0.29 0.29 Phosphorus (wt %) 0.11 0.11 Sulfur (wt %) 0.38 0.38 HTHS (cP) (D4683) 3.3 3.3 RFWT - GM 6.5L Average Wear (mils) 0.10 0.24 D12D FE Screen Test Ave. FE improvement (%) 0.71 0.47 - The results obtained demonstrate that the lubricating composition disclosed herein provides improved fuel economy performance without sacrificing wear protection. This has been accomplished in a composition free of polymeric viscosity index improver.
- The amount of each chemical component described is presented exclusive of any solvent or diluent oil, which may be customarily present in the commercial material, that is, on an active chemical basis, unless otherwise indicated. However, unless otherwise indicated, each chemical or composition referred to herein should be interpreted as being a commercial grade material which may contain the isomers, byproducts, derivatives, and other such materials which are normally understood to be present in the commercial grade.
- As used herein, the term "hydrocarbyl substituent" or "hydrocarbyl group" is used in its ordinary sense, which is well-known to those skilled in the art. Specifically, it refers to a group having a carbon atom directly attached to the remainder of the molecule and having predominantly hydrocarbon character. Examples of hydrocarbyl groups include:
- hydrocarbon substituents, that is, aliphatic (e.g., alkyl or alkenyl), alicyclic (e.g., cycloalkyl, cycloalkenyl) substituents, and aromatic-, aliphatic-, and alicyclic-substituted aromatic substituents, as well as cyclic substituents wherein the ring is completed through another portion of the molecule (e.g., two substituents together form a ring);
- substituted hydrocarbon substituents, that is, substituents containing non-hydrocarbon groups which, in the context of this invention, do not alter the predominantly hydrocarbon nature of the substituent (e.g., halo (especially chloro and fluoro), hydroxy, alkoxy, mercapto, alkylmercapto, nitro, nitroso, and sulfoxy);
- hetero substituents, that is, substituents which, while having a predominantly hydrocarbon character, in the context of this invention, contain other than carbon in a ring or chain otherwise composed of carbon atoms and encompass substituents as pyridyl, furyl, thienyl and imidazolyl. Heteroatoms include sulfur, oxygen, and nitrogen. In general, no more than two, or no more than one, non-hydrocarbon substituent will be present for every ten carbon atoms in the hydrocarbyl group; alternatively, there may be no non-hydrocarbon substituents in the hydrocarbyl group.
- It is known that some of the materials described above may interact in the final formulation, so that the components of the final formulation may be different from those that are initially added. For instance, metal ions (of, e.g., a detergent) can migrate to other acidic or anionic sites of other molecules. The products formed thereby, including the products formed upon employing the composition of the present invention in its intended use, may not be susceptible of easy description. Nevertheless, all such modifications and reaction products are included within the scope of the present invention; the present invention encompasses the composition prepared by admixing the components described above.
- As described hereinafter the molecular weight of the materials described above have been determined using known methods, such as GPC analysis using polystyrene standards. Methods for determining molecular weights of polymers are well known. The methods are described for instance: (i) P.J. Flory, "Principles of star polymer Chemistry", Cornell University Press 91953), Chapter VII, pp 266-315; or (ii) "Macromolecules, an Introduction to star polymer Science", F. A. Bovey and F. H. Winslow, Editors, Academic Press (1979), pp 296-312. As used herein the weight average and number weight average molecular weights of the materials described are obtained by integrating the area under the peak corresponding to the material of interest, excluding peaks associated with diluents, impurities, uncoupled star polymer chains and other additives.
- The mention of any document is not an admission that such document qualifies as prior art or constitutes the general knowledge of the skilled person in any jurisdiction. Except in the Examples, or where otherwise explicitly indicated, all numerical quantities in this description specifying amounts of materials, reaction conditions, molecular weights, number of carbon atoms, and the like, are to be understood as modified by the word "about." It is to be understood that the upper and lower amount, range, and ratio limits set forth herein may be independently combined. Similarly, the ranges and amounts for each element of the invention can be used together with ranges or amounts for any of the other elements.
- As used herein, the transitional term "comprising," which is synonymous with "including," "containing," or "characterized by," is inclusive or open-ended and does not exclude additional, un-recited elements or method steps. However, in each recitation of "comprising" herein, it is intended that the term also encompass, as alternative embodiments, the phrases "consisting essentially of' and "consisting of," where "consisting of' excludes any element or step not specified and "consisting essentially of' permits the inclusion of additional un-recited elements or steps that do not materially affect the essential or basic and novel characteristics of the composition or method under consideration.
- While certain representative embodiments and details have been shown for the purpose of illustrating the subject invention, it will be apparent to those skilled in this art that various changes and modifications can be made therein without departing from the scope of the subject invention. In this regard, the scope of the invention is to be limited only by the following claims.
Claims (7)
- A multigrade lubricant composition, comprising:(a) 80 wt % to 95 wt % of an oil of lubricating viscosity having a viscosity index of at least 130;(b) an ashless dispersant; and(c) an overbased metal detergent;wherein the lubricating composition contains less than 0.01 weight percent of a polymeric viscosity index improver; and
the lubricant composition has a SAE viscosity grade of XW-Y, wherein X may be 0, 5, or 10; and Y may be 16, 20, 26, 30, or 40. - The lubricant composition of claim 1, wherein the multigrade crank-case lubricant is a SAE OW-16, 0W-20, 0W-26, 5W-16, 5W-20, 5W-26, 5W-30, 10W-16, 10W-30, or 10W-40 lubricant.
- The lubricating composition of claim 1, wherein the metal-containing overbased detergent comprises one or more of a calcium sulfonate, a calcium phenate, a magnesium sulfonate or a magnesium phenate.
- The lubricating composition of claim 3, where the metal-containing overbased detergent is present in amount to deliver at least 4 TBN to the composition, wherein the TBN is measured using the method in ASTM D2896
- The lubricating composition of claim 1, wherein the lubricating composition has a viscosity index of at least 135.
- Use of a lubricant composition according to claim 1 for lubricating a compression-ignition internal combustion engine, wherein the use comprises supplying the composition to said engine.
- Use of a lubricant composition according to claim 2 for improving the fuel economy of a compression-ignition internal combustion engine.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201461984232P | 2014-04-25 | 2014-04-25 | |
| PCT/US2015/027416 WO2015164682A1 (en) | 2014-04-25 | 2015-04-24 | Multigrade lubricating compositions |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP3134496A1 EP3134496A1 (en) | 2017-03-01 |
| EP3134496B1 true EP3134496B1 (en) | 2021-03-10 |
Family
ID=53016803
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP15719586.8A Active EP3134496B1 (en) | 2014-04-25 | 2015-04-24 | Multigrade lubricating compositions |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20170044460A1 (en) |
| EP (1) | EP3134496B1 (en) |
| CN (2) | CN106459818A (en) |
| CA (1) | CA2946865C (en) |
| WO (1) | WO2015164682A1 (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20180037841A1 (en) * | 2016-08-03 | 2018-02-08 | Exxonmobil Research And Engineering Company | Lubricating engine oil for improved wear protection and fuel efficiency |
| US10513668B2 (en) * | 2017-10-25 | 2019-12-24 | Afton Chemical Corporation | Dispersant viscosity index improvers to enhance wear protection in engine oils |
| US20210002577A1 (en) * | 2017-11-28 | 2021-01-07 | The Lubrizol Corporation | Lubricant compositions for high efficiency engines |
| CA3193463A1 (en) * | 2020-09-22 | 2022-03-31 | The Lubrizol Corporation | Diesel engine lubricating compositions and methods of use thereof |
Citations (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3915843A (en) | 1972-12-08 | 1975-10-28 | Inst Francais Du Petrole | Hydrocracking process and catalyst for producing multigrade oil of improved quality |
| US4956122A (en) | 1982-03-10 | 1990-09-11 | Uniroyal Chemical Company, Inc. | Lubricating composition |
| US4992183A (en) | 1987-04-01 | 1991-02-12 | Ethyl Corporation | Multigrade hydrogenated decene-1 oligomer engine oils |
| US5208403A (en) | 1992-01-09 | 1993-05-04 | Mobil Oil Corporation | High VI lubricant blends from slack wax |
| WO1995034615A1 (en) | 1994-06-16 | 1995-12-21 | Exxon Chemical Limited | Multigrade lubricating compositions containing no viscosity modifier |
| US5612295A (en) | 1994-05-18 | 1997-03-18 | Ethyl Corporation | Lubricant additive compositions |
| US6071863A (en) | 1995-11-14 | 2000-06-06 | Bp Amoco Corporation | Biodegradable polyalphaolefin fluids and formulations containing the fluids |
| JP2003155492A (en) | 2001-11-22 | 2003-05-30 | Nippon Oil Corp | Lubricating oil composition for internal combustion engine |
| US20030171223A1 (en) | 2002-01-31 | 2003-09-11 | Winemiller Mark D. | Lubricating oil compositions with improved friction properties |
| US20040242433A1 (en) | 2002-10-31 | 2004-12-02 | Chevron Oronite Company Llc | Low-phosphorus lubricating oil composition for extended drain intervals |
| US20050133407A1 (en) | 2003-12-23 | 2005-06-23 | Chevron U.S.A. Inc. | Finished lubricating comprising lubricating base oil with high monocycloparaffins and low multicycloparaffins |
| US20060009366A1 (en) | 2004-07-08 | 2006-01-12 | Peter Sant | Lubricating oil composition |
| US20060025316A1 (en) | 2004-07-30 | 2006-02-02 | The Lubrizol Corporation | Dispersant viscosity modifiers containing aromatic amines |
| US20060052252A1 (en) | 2002-06-26 | 2006-03-09 | Wedlock David J | Lubricant composition |
| US7067049B1 (en) | 2000-02-04 | 2006-06-27 | Exxonmobil Oil Corporation | Formulated lubricant oils containing high-performance base oils derived from highly paraffinic hydrocarbons |
| US20060196807A1 (en) | 2005-03-03 | 2006-09-07 | Chevron U.S.A. Inc. | Polyalphaolefin & Fischer-Tropsch derived lubricant base oil lubricant blends |
| US20060223718A1 (en) | 2005-04-01 | 2006-10-05 | Bastien Paul F | Engine oils for racing applications and method of making same |
| US20070129266A1 (en) | 2005-11-18 | 2007-06-07 | Peter Busse | Lubricating Oil Composition |
| EP1903093A1 (en) | 2006-09-19 | 2008-03-26 | Infineum International Limited | A lubricating oil composition |
| US20090163393A1 (en) | 2007-12-21 | 2009-06-25 | Boffa Alexander B | Lubricating oil compositions for internal combustion engines |
| US20090203563A1 (en) | 2005-12-20 | 2009-08-13 | The Lubrizol Corporation | Method of Preparing an Overbased or Neutral Detergent |
| US20120129741A1 (en) | 2009-06-24 | 2012-05-24 | Simon William Dunning | Lubricating composition |
| WO2014209712A1 (en) | 2013-06-28 | 2014-12-31 | Dow Global Technologies Llc | Process for the preparation of branched polyolefins for lubricant applications |
Family Cites Families (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE1248643B (en) | 1959-03-30 | 1967-08-31 | The Lubrizol Corporation, Cleveland, Ohio (V. St. A.) | Process for the preparation of oil-soluble aylated amines |
| US3381022A (en) | 1963-04-23 | 1968-04-30 | Lubrizol Corp | Polymerized olefin substituted succinic acid esters |
| US3634515A (en) | 1968-11-08 | 1972-01-11 | Standard Oil Co | Alkylene polyamide formaldehyde |
| US4234435A (en) | 1979-02-23 | 1980-11-18 | The Lubrizol Corporation | Novel carboxylic acid acylating agents, derivatives thereof, concentrate and lubricant compositions containing the same, and processes for their preparation |
| US4464277A (en) * | 1982-10-25 | 1984-08-07 | Standard Oil Company (Indiana) | Synthetic lubricant composition |
| US4863623A (en) | 1988-03-24 | 1989-09-05 | Texaco Inc. | Novel VI improver, dispersant, and anti-oxidant additive and lubricating oil composition containing same |
| GB8818711D0 (en) | 1988-08-05 | 1988-09-07 | Shell Int Research | Lubricating oil dispersants |
| US5182041A (en) | 1989-05-01 | 1993-01-26 | Texaco Inc. | Dispersant - anti-oxidant additive and lubricating oil composition containing same |
| US5094667A (en) | 1990-03-20 | 1992-03-10 | Exxon Research And Engineering Company | Guerbet alkyl ether mono amines |
| GB9611316D0 (en) | 1996-05-31 | 1996-08-07 | Exxon Chemical Patents Inc | Overbased metal-containing detergents |
| GB9611424D0 (en) | 1996-05-31 | 1996-08-07 | Exxon Chemical Patents Inc | Overbased metal-containing detergents |
| GB9611318D0 (en) | 1996-05-31 | 1996-08-07 | Exxon Chemical Patents Inc | Overbased metal-containing detergents |
| GB9611428D0 (en) | 1996-05-31 | 1996-08-07 | Exxon Chemical Patents Inc | Overbased metal-containing detergents |
| EP1442105B1 (en) | 2001-11-05 | 2005-04-06 | The Lubrizol Corporation | Lubricating composition with improved fuel economy |
| ES2311715T3 (en) * | 2002-06-10 | 2009-02-16 | The Lubrizol Corporation | LUBRICATION PROCEDURE OF AN INTERNAL COMBUSTION ENGINE AND THE EFFICIENCY OF THE ENGINE EMISSION CONTROL SYSTEM. |
| US7009072B2 (en) | 2002-10-31 | 2006-03-07 | Crompton Corporation | Method for producing lubricant detergents |
| US6790813B2 (en) * | 2002-11-21 | 2004-09-14 | Chevron Oronite Company Llc | Oil compositions for improved fuel economy |
| CA2535107A1 (en) | 2003-08-01 | 2005-02-10 | The Lubrizol Corporation | Mixed dispersants for lubricants |
| US7053254B2 (en) | 2003-11-07 | 2006-05-30 | Chevron U.S.A, Inc. | Process for improving the lubricating properties of base oils using a Fischer-Tropsch derived bottoms |
| US7615519B2 (en) | 2004-07-19 | 2009-11-10 | Afton Chemical Corporation | Additives and lubricant formulations for improved antiwear properties |
| US7807611B2 (en) * | 2004-10-12 | 2010-10-05 | The Lubrizol Corporation | Tartaric acid derivatives as fuel economy improvers and antiwear agents in crankcase oils and preparation thereof |
| JP2008518059A (en) | 2004-10-25 | 2008-05-29 | ザ ルブリゾル コーポレイション | Corrosion prevention |
| EP2290044B1 (en) | 2005-03-28 | 2016-11-09 | The Lubrizol Corporation | Method using titanium compounds and complexes as additives in lubricants |
| ES2561424T3 (en) | 2005-06-16 | 2016-02-26 | The Lubrizol Corporation | Quaternary ammonium salt detergents for use in fuels |
| CN101479367A (en) * | 2006-04-24 | 2009-07-08 | 卢布里佐尔公司 | Star polymer lubricating composition |
| US7906470B2 (en) | 2006-09-01 | 2011-03-15 | The Lubrizol Corporation | Quaternary ammonium salt of a Mannich compound |
| US8067347B2 (en) | 2006-10-27 | 2011-11-29 | Chevron Oronite Company Llc | Lubricating oil additive composition and method of making the same |
| WO2008058108A2 (en) * | 2006-11-08 | 2008-05-15 | The Lubrizol Corporation | Crosslinked polymer |
| US7786057B2 (en) | 2007-02-08 | 2010-08-31 | Infineum International Limited | Soot dispersants and lubricating oil compositions containing same |
| RU2494140C2 (en) * | 2007-08-13 | 2013-09-27 | Шелл Интернэшнл Рисерч Маатсхаппий Б.В. | Lubricating oil composition and method for production thereof |
| CN101970622A (en) | 2007-11-13 | 2011-02-09 | 卢布里佐尔公司 | Lubricating composition containing a polymer |
| EP2231840B1 (en) * | 2007-12-27 | 2013-04-10 | The Lubrizol Corporation | Lubricating composition containing detergent |
| US20090186784A1 (en) * | 2008-01-22 | 2009-07-23 | Diggs Nancy Z | Lubricating Oil Composition |
| SG171382A1 (en) | 2008-11-26 | 2011-07-28 | Lubrizol Corp | Lubricating composition containing a polymer functionalised with a carboxylic acid and an aromatic polyamine |
| SG175117A1 (en) * | 2009-04-07 | 2011-11-28 | Infineum Int Ltd | Marine engine lubrication |
| US8399388B2 (en) | 2009-07-01 | 2013-03-19 | Chevron Oronite Company Llc | Low temperature performance lubricating oil detergents and method of making the same |
| US20120196778A1 (en) * | 2009-08-18 | 2012-08-02 | The Lubrizol Corporation | Lubricating Composition Containing an Antiwear Agent |
| US8349776B2 (en) * | 2009-09-29 | 2013-01-08 | Chevron Oronite Company Llc | Trunk piston engine lubricating oil compositions |
| CA2826107A1 (en) * | 2011-01-31 | 2012-08-09 | The Lubrizol Corporation | Lubricant composition comprising anti-foam agents |
| BR112013030491A2 (en) * | 2011-05-31 | 2017-08-08 | Lubrizol Corp | lubricant composition with improved nbt retention |
| US20130005622A1 (en) * | 2011-06-29 | 2013-01-03 | Exxonmobil Research And Engineering Company | Low viscosity engine oil with superior engine wear protection |
| JP5773365B2 (en) * | 2011-12-27 | 2015-09-02 | シェブロンジャパン株式会社 | Fuel-saving lubricating oil composition for internal combustion engines |
| WO2013159570A1 (en) * | 2012-04-26 | 2013-10-31 | 中国石油化工股份有限公司 | Mannich base, preparation method for same, and application thereof |
| CN104471041A (en) * | 2012-06-06 | 2015-03-25 | 范德比尔特化学品有限责任公司 | Fuel efficient lubricating oils |
-
2015
- 2015-04-24 WO PCT/US2015/027416 patent/WO2015164682A1/en active Application Filing
- 2015-04-24 EP EP15719586.8A patent/EP3134496B1/en active Active
- 2015-04-24 US US15/306,157 patent/US20170044460A1/en not_active Abandoned
- 2015-04-24 CN CN201580033464.5A patent/CN106459818A/en active Pending
- 2015-04-24 CA CA2946865A patent/CA2946865C/en active Active
- 2015-04-24 CN CN202210757995.0A patent/CN115093893A/en active Pending
Patent Citations (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3915843A (en) | 1972-12-08 | 1975-10-28 | Inst Francais Du Petrole | Hydrocracking process and catalyst for producing multigrade oil of improved quality |
| US4956122A (en) | 1982-03-10 | 1990-09-11 | Uniroyal Chemical Company, Inc. | Lubricating composition |
| US4992183A (en) | 1987-04-01 | 1991-02-12 | Ethyl Corporation | Multigrade hydrogenated decene-1 oligomer engine oils |
| US5208403A (en) | 1992-01-09 | 1993-05-04 | Mobil Oil Corporation | High VI lubricant blends from slack wax |
| US5612295A (en) | 1994-05-18 | 1997-03-18 | Ethyl Corporation | Lubricant additive compositions |
| WO1995034615A1 (en) | 1994-06-16 | 1995-12-21 | Exxon Chemical Limited | Multigrade lubricating compositions containing no viscosity modifier |
| US6071863A (en) | 1995-11-14 | 2000-06-06 | Bp Amoco Corporation | Biodegradable polyalphaolefin fluids and formulations containing the fluids |
| US7067049B1 (en) | 2000-02-04 | 2006-06-27 | Exxonmobil Oil Corporation | Formulated lubricant oils containing high-performance base oils derived from highly paraffinic hydrocarbons |
| JP2003155492A (en) | 2001-11-22 | 2003-05-30 | Nippon Oil Corp | Lubricating oil composition for internal combustion engine |
| US20030171223A1 (en) | 2002-01-31 | 2003-09-11 | Winemiller Mark D. | Lubricating oil compositions with improved friction properties |
| US20060052252A1 (en) | 2002-06-26 | 2006-03-09 | Wedlock David J | Lubricant composition |
| US20040242433A1 (en) | 2002-10-31 | 2004-12-02 | Chevron Oronite Company Llc | Low-phosphorus lubricating oil composition for extended drain intervals |
| US20050133407A1 (en) | 2003-12-23 | 2005-06-23 | Chevron U.S.A. Inc. | Finished lubricating comprising lubricating base oil with high monocycloparaffins and low multicycloparaffins |
| US20060009366A1 (en) | 2004-07-08 | 2006-01-12 | Peter Sant | Lubricating oil composition |
| US20060025316A1 (en) | 2004-07-30 | 2006-02-02 | The Lubrizol Corporation | Dispersant viscosity modifiers containing aromatic amines |
| US20060196807A1 (en) | 2005-03-03 | 2006-09-07 | Chevron U.S.A. Inc. | Polyalphaolefin & Fischer-Tropsch derived lubricant base oil lubricant blends |
| US20060223718A1 (en) | 2005-04-01 | 2006-10-05 | Bastien Paul F | Engine oils for racing applications and method of making same |
| US20070129266A1 (en) | 2005-11-18 | 2007-06-07 | Peter Busse | Lubricating Oil Composition |
| US20090203563A1 (en) | 2005-12-20 | 2009-08-13 | The Lubrizol Corporation | Method of Preparing an Overbased or Neutral Detergent |
| EP1903093A1 (en) | 2006-09-19 | 2008-03-26 | Infineum International Limited | A lubricating oil composition |
| US20090163393A1 (en) | 2007-12-21 | 2009-06-25 | Boffa Alexander B | Lubricating oil compositions for internal combustion engines |
| US20120129741A1 (en) | 2009-06-24 | 2012-05-24 | Simon William Dunning | Lubricating composition |
| WO2014209712A1 (en) | 2013-06-28 | 2014-12-31 | Dow Global Technologies Llc | Process for the preparation of branched polyolefins for lubricant applications |
Non-Patent Citations (21)
| Title |
|---|
| "Automotive Lubricants Reference Book. 2nd ed.", 1 January 2004, PROFESSIONAL ENGINEERING PUBLISHING , article HAYCOCK R.F., ET AL: "Diesel Engine oils", pages: 175 - 190, XP055500772 |
| "Chemistry and Technology of Lubricants", 1 January 2010, article MORTIER: "Viscosity Index Improvers and Thickeners", pages: 181,498, XP055874141 |
| "Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing", 1 June 2003, article TOTTEN GEORGE E, ET AL: "Performance Testing", pages: 235 - 236, XP055874142 |
| "Lubricant Additives, Chemistry and Applications", 1 January 2009, article RIZVI: "Detergents", pages: 1 - 8, XP055874133 |
| "Lubricants and Lubrication", 1 January 2007, article MANG THEO, ET AL: "Rheology of lubricants", pages: 1 - 5, XP055874137 |
| "Process Chemistry of Lubricant Base Stocks", 4 October 2013, article LYNCH THOMAS R: "Viscosity Index Distributions in Base Stocks: Use of Thermal Diffusion", pages: 63 - 63, XP055874143 |
| "The IUPAC Compendium of Chemical Terminology : The Gold Book", 24 February 2014, INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY (IUPAC), Research Triangle Park, NC, article ANONYMOUS: "Paraffin ", XP093112317, DOI: 10.1351/goldbook.P04401 |
| ANONYMOUS: "Base Oil SN-150 Specification", DYM RESOURCES GMBH, 1 July 2020 (2020-07-01), XP093112324, Retrieved from the Internet <URL:https://dymresources.com/wp-content/uploads/2020/07/SN150.pdf> [retrieved on 20231214] |
| ANONYMOUS: "LUBRICANT ADDITIVES and THE ENVIRONMENT Document 49 (revision 1) ", ATC, pages 1 - 35, XP093013399, Retrieved from the Internet <URL:https://www.atc-europe.org/public/doc49rev1.pdf> [retrieved on 20230112] |
| ANONYMOUS: "Viscosity The single most important physical characteristic of any oil", OELCHECKER, 1 January 2012 (2012-01-01), pages 5 - 7, XP055874150 |
| ANONYMOUS: "WHICH OIL IS RIGHT FOR YOU?", API, 1 January 2013 (2013-01-01), pages 1 - 4, XP055874149, Retrieved from the Internet <URL:https://www.api.org/~/media/files/certification/engine-oil-diesel/publications/mom_guide_english_2013.pdf> [retrieved on 20211217] |
| CARDEN PHIL, ET AL: "The Effect of Low Viscosity Oil on the Wear, Friction and Fuel Consumption of a Heavy Duty Truck Engine ", SAE INTERNATIONAL, vol. 6, no. 2, 1 June 2013 (2013-06-01), pages 311 - 319, XP055874144 |
| FAIRLEY PETER: "GTL Transforming - The Future of Lubricant Production", TRIBOLOGY & LUBRICATION TECHNOLOGY, 1 October 2003 (2003-10-01), XP093112314, Retrieved from the Internet <URL:https://www.stle.org/images/pdf/STLE_ORG/BOK/LS/Base%20Oils/GTL_Transforming%20the%20Future%20of%20Lubricant%20Production_tlt%20article_Oct03.pdf> [retrieved on 20231214] |
| FRANK MARKUS: "Is the Era of the Camless Valvetrain Finally Upon us?-Technologue", 27 January 2018 (2018-01-27), XP093125541, Retrieved from the Internet <URL:https://www.motortrend.com/features/is-the-era-of-the-camless-valvetrain-finally-upon-us-technologue/> [retrieved on 20240130] |
| MANG ET AL.: "Lubricants and Lubrication", 1 January 2007, ISBN: 978-3-527-31497-3, article THEO MANG: "6.2 Viscosity Modifiers", pages: 94 - 97, XP009550875, DOI: 10.1002/9783527610341.ch6 |
| MORTIER ET AL., 1 December 2009, SPRINGER VERLAG, ISBN: 978-1-4020-8661-8, article D. ATKINSON, A. J. BROWN, D. JILBERT, G. LAMB: "Formulation of Automotive Lubricants", pages: 293 - 324, XP009550876, DOI: 10.1023/b105569_9 |
| REPKO COLIN: "The ins and outs of high-temp, high-shear oils", FLEET EQUIPEMENT, 26 February 2018 (2018-02-26), pages 1 - 6, XP055874147, Retrieved from the Internet <URL:https://www.fleetequipmentmag.com/ins-outs-high-temp-high-shear-oils/> [retrieved on 20211217] |
| RUDNICK ET AL., 1 January 2009, CRC PRESS INC , ISBN: 978-1-4200-5964-9, article LESLIE R. RUDNICK: "Passages; Lubricant additives: chemistry and applications", pages: 463 - 488, XP009550874 |
| STUNNENBERG F., DE VRIES FEYENS A.W.L., OTTERHOLM B., BUDD A.: "Truck Field Test vs. Bench Engine - A Comparison", SAE 2010 COMMERCIAL VEHICLE ENGINEERING CONGRESS SAE TECHNICAL PAPERS, SAE INTERNATIONAL, US, vol. 1, 8 June 2004 (2004-06-08), US , pages 1338 - 1342, XP093110657, ISSN: 0148-7191, DOI: 10.4271/2004-01-2020 |
| THOMAS R. LYNCH: "Process of Chemistry of Lubricant Base Stocks", 1 January 2008, CRC PRESS, US, ISBN: 0-8493-3849-2, article THOMAS R. LYNCH: "Passages; Process of Chemistry of Lubricant Base Stocks", pages: 205, 355, XP009550877 |
| VAN DAM W., MILLER T., PARSONS G. M., TAKEUCHI Y.: "The Impact of Lubricant Viscosity and Additive Chemistry on Fuel Economy in Heavy Duty Diesel Engines", SAE INTERNATIONAL JOURNAL OF FUELS AND LUBRICANTS, S A E INC., US, vol. 5, no. 1, 30 January 2012 (2012-01-30), US , pages 459 - 469, XP093071129, ISSN: 1946-3960, DOI: 10.4271/2011-01-2124 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2946865C (en) | 2023-03-28 |
| CN106459818A (en) | 2017-02-22 |
| CN115093893A (en) | 2022-09-23 |
| CA2946865A1 (en) | 2015-10-29 |
| WO2015164682A1 (en) | 2015-10-29 |
| US20170044460A1 (en) | 2017-02-16 |
| EP3134496A1 (en) | 2017-03-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20220145204A1 (en) | Method Of Lubricating An Internal Combustion Engine | |
| EP3134496B1 (en) | Multigrade lubricating compositions | |
| US9663744B2 (en) | Dispersant viscosity modifiers | |
| KR20210092785A (en) | low viscosity lubricant composition | |
| EP2938715B1 (en) | Lubricating composition containing an acylated polyalkylene oxide | |
| EP3851508B1 (en) | Method of lubricating an internal combustion engine | |
| JP7315482B2 (en) | Lubricating engine oil composition containing detergent compound | |
| EP2609179A1 (en) | Lubricants containing aromatic dispersants and titanium | |
| US20190352577A1 (en) | Engine lubricant containing polyether compounds | |
| US10513668B2 (en) | Dispersant viscosity index improvers to enhance wear protection in engine oils | |
| US11608477B1 (en) | Engine oil formulations for low timing chain stretch | |
| CA2920023A1 (en) | Reduced engine deposits from dispersant treated with cobalt | |
| WO2016018462A1 (en) | Sae 15w-30 lubricating oil composition having improved oxidative stability | |
| CA2920022A1 (en) | Reduced engine deposits from dispersant treated with copper | |
| EP4077605A1 (en) | Lubricating oil compositions comprising a polyalphaolefin | |
| CA3193463A1 (en) | Diesel engine lubricating compositions and methods of use thereof | |
| WO2024019952A1 (en) | Deposit control compounds for lubricating compositions | |
| CA3216888A1 (en) | Lubricating oil compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE INTERNATIONAL PUBLICATION HAS BEEN MADE |
|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20161125 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| AX | Request for extension of the european patent |
Extension state: BA ME |
|
| DAV | Request for validation of the european patent (deleted) | ||
| DAX | Request for extension of the european patent (deleted) | ||
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| 17Q | First examination report despatched |
Effective date: 20190219 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10M 169/04 20060101AFI20200814BHEP Ipc: C10N 30/04 20060101ALI20200814BHEP Ipc: C10N 30/06 20060101ALI20200814BHEP Ipc: C10N 30/00 20060101ALN20200814BHEP Ipc: C10N 30/02 20060101ALI20200814BHEP Ipc: C10N 40/25 20060101ALI20200814BHEP Ipc: C10N 20/04 20060101ALN20200814BHEP Ipc: C10N 30/12 20060101ALI20200814BHEP Ipc: C10N 20/02 20060101ALN20200814BHEP |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10N 30/02 20060101ALI20200904BHEP Ipc: C10N 20/04 20060101ALN20200904BHEP Ipc: C10N 40/25 20060101ALI20200904BHEP Ipc: C10N 20/02 20060101ALN20200904BHEP Ipc: C10N 30/12 20060101ALI20200904BHEP Ipc: C10N 30/00 20060101ALN20200904BHEP Ipc: C10M 169/04 20060101AFI20200904BHEP Ipc: C10N 30/04 20060101ALI20200904BHEP Ipc: C10N 30/06 20060101ALI20200904BHEP |
|
| INTG | Intention to grant announced |
Effective date: 20200928 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 1369812 Country of ref document: AT Kind code of ref document: T Effective date: 20210315 Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602015066621 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG9D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210610 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210610 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210611 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1369812 Country of ref document: AT Kind code of ref document: T Effective date: 20210310 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20210310 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210710 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210712 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602015066621 Country of ref document: DE |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210424 |
|
| 26 | Opposition filed |
Opponent name: AFTON CHEMICAL CORPORATION Effective date: 20211203 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20210430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210430 Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210430 Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210424 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210710 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20210430 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20150424 |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230516 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20210310 |
|
| P02 | Opt-out of the competence of the unified patent court (upc) changed |
Effective date: 20230527 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20230425 Year of fee payment: 9 Ref country code: DE Payment date: 20230427 Year of fee payment: 9 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20230427 Year of fee payment: 9 |
|
| RDAF | Communication despatched that patent is revoked |
Free format text: ORIGINAL CODE: EPIDOSNREV1 |
|
| APBM | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOSNREFNO |
|
| APBP | Date of receipt of notice of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA2O |
|
| APAH | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNO |
|
| APBQ | Date of receipt of statement of grounds of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA3O |