EP2711045B1 - Inflatable structure with braided layer - Google Patents
Inflatable structure with braided layer Download PDFInfo
- Publication number
- EP2711045B1 EP2711045B1 EP13198920.4A EP13198920A EP2711045B1 EP 2711045 B1 EP2711045 B1 EP 2711045B1 EP 13198920 A EP13198920 A EP 13198920A EP 2711045 B1 EP2711045 B1 EP 2711045B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- balloon
- portions
- braid
- yarns
- braiding
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000002787 reinforcement Effects 0.000 claims description 39
- 239000000835 fiber Substances 0.000 claims description 35
- 239000000463 material Substances 0.000 claims description 34
- 238000009954 braiding Methods 0.000 description 54
- 239000011159 matrix material Substances 0.000 description 27
- 239000002131 composite material Substances 0.000 description 26
- 238000000034 method Methods 0.000 description 23
- 230000003115 biocidal effect Effects 0.000 description 14
- 230000006835 compression Effects 0.000 description 12
- 238000007906 compression Methods 0.000 description 12
- 230000005684 electric field Effects 0.000 description 12
- 239000003139 biocide Substances 0.000 description 11
- 239000004744 fabric Substances 0.000 description 10
- 239000000203 mixture Substances 0.000 description 10
- 238000000576 coating method Methods 0.000 description 8
- 229920000642 polymer Polymers 0.000 description 8
- 238000009941 weaving Methods 0.000 description 8
- 238000005452 bending Methods 0.000 description 7
- 239000011248 coating agent Substances 0.000 description 7
- 239000000853 adhesive Substances 0.000 description 6
- 230000001070 adhesive effect Effects 0.000 description 6
- 239000004020 conductor Substances 0.000 description 6
- 238000010586 diagram Methods 0.000 description 6
- 229910003460 diamond Inorganic materials 0.000 description 6
- 239000010432 diamond Substances 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 125000006850 spacer group Chemical group 0.000 description 6
- 238000000465 moulding Methods 0.000 description 5
- YLQBMQCUIZJEEH-UHFFFAOYSA-N Furan Chemical compound C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 4
- -1 aminoglycosides Chemical class 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 239000005020 polyethylene terephthalate Substances 0.000 description 4
- 239000004642 Polyimide Substances 0.000 description 3
- 239000003242 anti bacterial agent Substances 0.000 description 3
- 238000005520 cutting process Methods 0.000 description 3
- 235000004879 dioscorea Nutrition 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000002844 melting Methods 0.000 description 3
- 230000008018 melting Effects 0.000 description 3
- 229920000139 polyethylene terephthalate Polymers 0.000 description 3
- 229920001721 polyimide Polymers 0.000 description 3
- 229920005989 resin Polymers 0.000 description 3
- 239000011347 resin Substances 0.000 description 3
- 229920001169 thermoplastic Polymers 0.000 description 3
- 239000004416 thermosoftening plastic Substances 0.000 description 3
- 238000011282 treatment Methods 0.000 description 3
- CPKVUHPKYQGHMW-UHFFFAOYSA-N 1-ethenylpyrrolidin-2-one;molecular iodine Chemical compound II.C=CN1CCCC1=O CPKVUHPKYQGHMW-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 229920000153 Povidone-iodine Polymers 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 125000002091 cationic group Chemical group 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000009413 insulation Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 229920000570 polyether Polymers 0.000 description 2
- 229960001621 povidone-iodine Drugs 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000001954 sterilising effect Effects 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 239000004753 textile Substances 0.000 description 2
- 229920000785 ultra high molecular weight polyethylene Polymers 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- OSDLLIBGSJNGJE-UHFFFAOYSA-N 4-chloro-3,5-dimethylphenol Chemical compound CC1=CC(O)=CC(C)=C1Cl OSDLLIBGSJNGJE-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229920000049 Carbon (fiber) Polymers 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- 229920004934 Dacron® Polymers 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 229920000271 Kevlar® Polymers 0.000 description 1
- 229920000106 Liquid crystal polymer Polymers 0.000 description 1
- 239000004977 Liquid-crystal polymers (LCPs) Substances 0.000 description 1
- 229920002292 Nylon 6 Polymers 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- XEFQLINVKFYRCS-UHFFFAOYSA-N Triclosan Chemical compound OC1=CC(Cl)=CC=C1OC1=CC=C(Cl)C=C1Cl XEFQLINVKFYRCS-UHFFFAOYSA-N 0.000 description 1
- 239000004699 Ultra-high molecular weight polyethylene Substances 0.000 description 1
- 229920000508 Vectran Polymers 0.000 description 1
- 239000004979 Vectran Substances 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 239000004676 acrylonitrile butadiene styrene Substances 0.000 description 1
- 239000011157 advanced composite material Substances 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000004917 carbon fiber Substances 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 239000000645 desinfectant Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 239000011152 fibreglass Substances 0.000 description 1
- 239000002657 fibrous material Substances 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 210000003709 heart valve Anatomy 0.000 description 1
- ACGUYXCXAPNIKK-UHFFFAOYSA-N hexachlorophene Chemical compound OC1=C(Cl)C=C(Cl)C(Cl)=C1CC1=C(O)C(Cl)=CC(Cl)=C1Cl ACGUYXCXAPNIKK-UHFFFAOYSA-N 0.000 description 1
- 229960004068 hexachlorophene Drugs 0.000 description 1
- 235000010299 hexamethylene tetramine Nutrition 0.000 description 1
- VKYKSIONXSXAKP-UHFFFAOYSA-N hexamethylenetetramine Chemical compound C1N(C2)CN3CN1CN2C3 VKYKSIONXSXAKP-UHFFFAOYSA-N 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- PNDPGZBMCMUPRI-UHFFFAOYSA-N iodine Chemical compound II PNDPGZBMCMUPRI-UHFFFAOYSA-N 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229920000554 ionomer Polymers 0.000 description 1
- 239000004761 kevlar Substances 0.000 description 1
- 238000009940 knitting Methods 0.000 description 1
- 238000003475 lamination Methods 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 1
- 229960004011 methenamine Drugs 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- IAIWVQXQOWNYOU-FPYGCLRLSA-N nitrofural Chemical compound NC(=O)N\N=C\C1=CC=C([N+]([O-])=O)O1 IAIWVQXQOWNYOU-FPYGCLRLSA-N 0.000 description 1
- 229960000564 nitrofurantoin Drugs 0.000 description 1
- NXFQHRVNIOXGAQ-YCRREMRBSA-N nitrofurantoin Chemical compound O1C([N+](=O)[O-])=CC=C1\C=N\N1C(=O)NC(=O)C1 NXFQHRVNIOXGAQ-YCRREMRBSA-N 0.000 description 1
- 229960001907 nitrofurazone Drugs 0.000 description 1
- 239000003973 paint Substances 0.000 description 1
- 150000002960 penicillins Chemical class 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920002577 polybenzoxazole Polymers 0.000 description 1
- 239000011160 polymer matrix composite Substances 0.000 description 1
- 229920013657 polymer matrix composite Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 210000003492 pulmonary vein Anatomy 0.000 description 1
- 150000007660 quinolones Chemical class 0.000 description 1
- 229920003252 rigid-rod polymer Polymers 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 239000012209 synthetic fiber Substances 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229940040944 tetracyclines Drugs 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 229960003500 triclosan Drugs 0.000 description 1
- 210000003708 urethra Anatomy 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1002—Balloon catheters characterised by balloon shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1027—Making of balloon catheters
- A61M25/1029—Production methods of the balloon members, e.g. blow-moulding, extruding, deposition or by wrapping a plurality of layers of balloon material around a mandril
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1027—Making of balloon catheters
- A61M25/1038—Wrapping or folding devices for use with balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/104—Balloon catheters used for angioplasty
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M29/00—Dilators with or without means for introducing media, e.g. remedies
- A61M29/02—Dilators made of swellable material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- D—TEXTILES; PAPER
- D04—BRAIDING; LACE-MAKING; KNITTING; TRIMMINGS; NON-WOVEN FABRICS
- D04C—BRAIDING OR MANUFACTURE OF LACE, INCLUDING BOBBIN-NET OR CARBONISED LACE; BRAIDING MACHINES; BRAID; LACE
- D04C1/00—Braid or lace, e.g. pillow-lace; Processes for the manufacture thereof
- D04C1/02—Braid or lace, e.g. pillow-lace; Processes for the manufacture thereof made from particular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0018—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in elasticity, stiffness or compressibility
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0039—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in diameter
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1002—Balloon catheters characterised by balloon shape
- A61M2025/1004—Balloons with folds, e.g. folded or multifolded
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1027—Making of balloon catheters
- A61M25/1029—Production methods of the balloon members, e.g. blow-moulding, extruding, deposition or by wrapping a plurality of layers of balloon material around a mandril
- A61M2025/1031—Surface processing of balloon members, e.g. coating or deposition; Mounting additional parts onto the balloon member's surface
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1075—Balloon catheters with special features or adapted for special applications having a balloon composed of several layers, e.g. by coating or embedding
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1079—Balloon catheters with special features or adapted for special applications having radio-opaque markers in the region of the balloon
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1084—Balloon catheters with special features or adapted for special applications having features for increasing the shape stability, the reproducibility or for limiting expansion, e.g. containments, wrapped around fibres, yarns or strands
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1088—Balloon catheters with special features or adapted for special applications having special surface characteristics depending on material properties or added substances, e.g. for reducing friction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1011—Multiple balloon catheters
-
- D—TEXTILES; PAPER
- D10—INDEXING SCHEME ASSOCIATED WITH SUBLASSES OF SECTION D, RELATING TO TEXTILES
- D10B—INDEXING SCHEME ASSOCIATED WITH SUBLASSES OF SECTION D, RELATING TO TEXTILES
- D10B2403/00—Details of fabric structure established in the fabric forming process
- D10B2403/02—Cross-sectional features
- D10B2403/024—Fabric incorporating additional compounds
- D10B2403/0241—Fabric incorporating additional compounds enhancing mechanical properties
- D10B2403/02411—Fabric incorporating additional compounds enhancing mechanical properties with a single array of unbent yarn, e.g. unidirectional reinforcement fabrics
-
- D—TEXTILES; PAPER
- D10—INDEXING SCHEME ASSOCIATED WITH SUBLASSES OF SECTION D, RELATING TO TEXTILES
- D10B—INDEXING SCHEME ASSOCIATED WITH SUBLASSES OF SECTION D, RELATING TO TEXTILES
- D10B2509/00—Medical; Hygiene
- D10B2509/06—Vascular grafts; stents
Definitions
- the invention relates to composite structures for medical balloons and in particular, such structures that promote predictable folding.
- Woven and braided fabrics have been used to reinforce various devices. Compared to weaving, braiding may impart greater strength for a unit of weight. The strength of a braid comes from the fact that multiple yarns can be intertwined without any being twisted around another. Generally these are continuously braided at an angle and there is no need for any yarn to suffer a sharp bend. As a result, loads may be distributed evenly and efficiently throughout the braid.
- Braids can also be formed without any underlying support (freestanding) or over a mandrel or a part to be reinforced, such as the mast of a sailboat. Braiding can also be done over a three-dimensional part, such as a tool.
- a single braid can incorporate multiple yarn materials to form a hybrid weave. This is often done to make patterns in the resulting product.
- Yarns can be of metal, carbon fiber, glass fiber, mono or multifilament threads, etc. Braiding can be done with very delicate materials.
- Braid has been used as a reinforcement for some surgical devices such as endoscopes and catheters and for implantable devices such as splints and stents.
- Non-woven fiber reinforcements are also known, for example, randomly arrayed fibers such as in fiberglass and hand-laid fibers arrayed over and within a matrix are known strategies. Both have been described in connection with the reinforcement of medical balloons.
- the company, 3TEX provides information about state of the art three-dimensional automated braiding at [online] ]Retrieved on 2005-6-21] Retrieved from the Internet ⁇ http://www.3tex.com/3braid.cfm>.
- the page shows photographs and an animation of a large Cartesian braiding machine.
- One of the points made is that with computer control, it is possible to shift the braiding pattern at any time without changing the number or continuity of the yarns.
- NTC National Textile Center
- WO 03/020334 A3 is concerned with a catheter.
- US 2001/0056299 A1 is concerned with a braided covered stent.
- US 6,012,457 is concerned with a method for forming a circumferential conduction block in a pulmonary vein.
- US 2002/010489 A1 is concerned with a balloon catheter.
- a balloon for medical treatments such as percutaneous transluminal coronary angioplasty (PTCA), delivery of a vascular stents or stent grafts, employs reinforcement materials that are patterned so as to promote consistent, predictable, or tighter, folding of the balloon.
- PTCA percutaneous transluminal coronary angioplasty
- reinforcement materials that are patterned so as to promote consistent, predictable, or tighter, folding of the balloon.
- Claim 2 defines a preferred embodiment. Examples that do not fall under the scope of the claims are included for information purposes.
- An example provides a medical balloon whose walls have relatively stiff and relatively flexible regions to promote folding along the flexible regions.
- the variation in stiffness is achieved, according to the different embodiments, by variably arranging composite elements on, or within, the wall of the balloon; by adding stiffening members to the wall at selected portions; by varying the properties of a fabric or braid or other filamentous structure to define variable stiffness, and by other means.
- the example is a foldable composite balloon with a wall.
- the wall has first and second filaments and first and second wall portions.
- the wall has compression elements separating the first and second filaments in the first wall portions so that they define opposing tension elements running in a wind direction.
- the opposing tension elements have a component in a specified direction and are separated by the at least one compression element resulting in the first portion being stiffer than the second portion, at least in the specified direction, the first and second portions being arranged such that when the balloon is folded, the first portions resist bending more than the second portions.
- This may be so the folds are generally aligned with the second portions and it may help to ensure a neat and predictable folding behavior when the balloon is collapsed. This in turn can help to ensure a compact configuration in tight areas.
- the first and second filaments may be portions of elongate members that run continuously through the first portions and the second portions.
- the first and second filaments may be braided to define at least a portion of a braid.
- the braid may include a triaxial portion having third filaments running as a 0° braid yarn in the first and second portions, the third filaments in the first portions being thicker than the third filaments in the second portion and the third filaments forming at least part of the at compression element.
- the 0° yarn refers to yarns running in a longitudinal direction, which is the direction along which the braid extends (or gets longer) as the braid is woven.
- the first and second filaments may define at least a portion of a braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions.
- the first and second filaments may define at least a portion of a biaxial braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions.
- the wall may be elongated such as to have a longitudinal axis and the second portion may be aligned with the axis or follow a helical path around the longitudinal axis.
- the wall may include a matrix, such as a polymer matrix, and members embedded therein with the first and second filaments being embedded in the matrix and the members forming at least portions of the compression elements.
- the wall may include a matrix and flat members embedded therein, the first and second filaments being embedded in the matrix and the members forming at least portions of the compression element.
- a foldable composite balloon with a wall of polymer matrix with first and second filaments attached to it.
- the wall may have first and second portions, the first and second filaments spaced apart by a portion of the polymer matrix in the first wall portions, such that they define opposing tension elements running in a wind direction having a component in a specified direction and separated by the matrix portion.
- the spacing of the tension elements on opposite sides of the matrix portion is such that the matrix portion acts as a compression element and the result is that the first portion is stiffer than the second portion, at least in the specified direction.
- the first and second portions may be arranged such that when the balloon is folded, the first portions resist bending more than the second portions. Or the second portions may be aligned with folding lines of the balloon so the structure helps to promote folding or creates a natural folding behavior.
- first and second filaments being portions of elongate members running continuously through the first portions and the second portions.
- the first and second filaments may be braided to form a braid.
- the first and second filaments may define a braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions.
- the second portions may define folding contours and the alternations are staggered in the first region such that no consecutive trains of side alternations occur that are parallel to the folding contours.
- a foldable composite balloon with a body that has a polymer matrix and a filamentous structure attached thereto.
- the body may have first and second portions and folding lines with the filamentous structure defining first and second portions, the folding lines lying within the second portions and the first portions lying between the second portions.
- the filamentous structure may be configured to promote folding along the folding lines either by being configured to cause the body to be stiffer in the first regions, at least in a direction perpendicular to the folding line, than the second portions.
- the filamentous structure may be configured to generate a mechanical bias that favors folding along the folding lines as a result of being formed over a form with edges on it.
- the filamentous structure may be configured to cause the body to be stiffer in the first regions, at least in a direction perpendicular to the folding lines, than the second portions.
- the filamentous structure may include a braid.
- the filamentous structure may have first and second filaments and a compression element, the first portions being stiffer, at least in a direction perpendicular to the folding lines, at least in part as a result of the first and second filaments of the first portions being arranged with the compression element between then, thereby defining opposing tension elements separated by the compression element.
- the braid may have layers with more layers in the first portions than in the second portions such that the first portions are stiffer than the second portions.
- the body may have a longitudinal axis and the folding lines are parallel to the longitudinal axis.
- the body may have a longitudinal axis and the folding lines may wind helically around a longitudinal axis.
- the braid may be triaxial or biaxial.
- a foldable composite balloon which has a wall with a polymer matrix included elements attached to, or within, the polymer matrix.
- the included elements are arranged to define first portions, and second portions of the wall such that the wall folds more readily in the first portions than the second portions.
- a foldable composite balloon is provided with a wall having elongate reinforcement members, first portions, and second portions, the stiffness of the first portions being lower than the stiffness of the second portions.
- An arrangement of the elongate reinforcement members causes the wall to be stiffer in the second portions than in the first portions, whereby the balloon tends to fold along contours coinciding with the low stiffness portions.
- a method for the treatment of an infected area within a body includes applying an electrically conductive biocide composition to an infected area within the body that has been exposed during surgery, and applying an electric field to the biocide composition by contacting a surface with the biocide composition with an inflatable member having conductive surface of alternate polarity to generate an electric field.
- the electric field strength and duration of application are sufficient to produce killing of microorganisms in the infected area.
- the infected area is composed of a biofilm that is composed predominately of bacteria, yeast or fungus.
- the biocide is an antibiotic selected from the family of antibiotics consisting of penicillins, cephalosporins, aminoglycosides, tetracyclines, sulfonamides, macrolide antibiotics and quinolones.
- the electrically conductive biocide composition is a buffered saline composition.
- the biocide composition includes a thickener.
- the electric field is substantially constant.
- the electrical field is a pulsed or alternating electric field.
- the electric field strength is generated by currents having a value in the range from about 1 to about 200 milliamps.
- said electric field is applied to the electrically conductive biocide composition for a period of time of between about 1 minute to about 48 hours.
- the biocide is present in the composition, in an amount which would be ineffective to completely kill the infected area if used in the absence of the electric field.
- the method is performed during the course of heart valve replacement surgery.
- the biocide is an antibiotic, an anti-fungal agent, a disinfectant, a sterilant, other antiseptic agents, hexachlorophene, cationic bisiguanides, iodine, iodophores, para-chloro-meta-xylenol, triclosan, furan preparations, methenamine, aldehydes, or alcohols.
- the cationic bisiguanides include chlorhexidene or cyclohexidene.
- iodine include povidone-iodine.
- iodophores include povidone-iodine.
- furan preparations include nitrofurantoin or nitrofurazone.
- aldehydes is in glute form.
- the example is a medical balloon, comprising: a balloon body having an array of reinforcement fibers exposed on an external surface thereon; at least some of the reinforcement fibers being electrically conductive subsets of which are connectable to a source of voltage such that an electric field can be continuously generated on the surface of the body.
- the fibers form a braided pattern.
- the at least some of the reinforcement fibers are of metal.
- the at least some of the reinforcement fibers are zero-angle fibers of a triaxial braid.
- a foldable composite balloon with a wall of polymer matrix with first and second filaments attached to it.
- the wall may have first and second portions, the first and second filaments spaced apart by one or more radio-opaque elements in the first wall portions, such that the yarns overlying them define opposing tension elements running in a wind direction having a component in a specified direction and separated by the radio-opaque portions.
- This allows the radio-opaqu included elements to be relatively stiff without impeding (in fact promoting) the folding of the balloon.
- the spacing of the tension elements on opposite sides of the matrix portion is such that the included radio-opaque elements act as compression elements and the result is that the first portion is stiffer than the second portion, at least in the specified direction.
- the first and second portions may be arranged such that when the balloon is folded, the first portions resist bending more than the second portions. Or the second portions may be aligned with folding lines of the balloon so the structure helps to promote folding or creates a natural folding behavior.
- first and second filaments being portions of elongate members running continuously through the first portions and the second portions.
- the first and second filaments may be braided to form a braid.
- the first and second filaments may define a braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions.
- the second portions may define folding contours and the alternations are staggered in the first region such that no consecutive trains of side alternations occur that are parallel to the folding contours.
- a foldable composite balloon has a braided reinforcement structure defining a wall.
- the braided reinforcement pattern is such that the wall is stiffer at the first wall portions than at the second wall portions.
- the first and second wall portions are arranged such that when the balloon is folded, the first portions resist bending more than the second portions.
- at least the first wall portions have a radio-opaque coating thereon.
- only the first wall portions have a radio-opaque coating thereon.
- a radio-opaque material is integrated in the fist portions only.
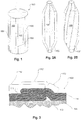
- Fig. 1 shows a reinforcement structure, such as a tube braid, that may be used in a composite balloon, the reinforcement structure having relatively flexible portions 106 or facets and relatively stiff portions 102 or facets.
- a tubular braid 103 has stiff portions 102 that are relatively stiff, or at least relatively stiff in the circumferential direction (i.e., the direction about the balloon axis).
- the tubular braid 103 also has flexible portions 106 that are flexible relative to the stiff portions 102, also, at least in the circumferential direction.
- the tube braid 103 may be of strong filaments (not shown separately) of any type, but in the present examples of folding medical balloons, they include relatively inelastic high strength synthetic fibers.
- the filaments may be a mix of different materials and cross-sectional shapes and different materials may be combined in various ways as discussed below.
- the braiding may be done using a variety of mechanisms which are known in the art employing braid patterns and other structures described herein.
- the braiding of the tube braid 103 may be performed using a programmable tube braider (not shown).
- the tube braid 103 may be a portion of a non-cylindrical (three-dimensional) braid as illustrated in Fig. 2A with progressively tapering ends.
- the filaments may be braided over a three-dimensional form (for example, as at 220 in Fig. 5B , described below) to create a desired balloon shape.
- the tube braid 103 may be embedded in, impregnated with, or otherwise combined with a flexible material that can hold pressure and ensure against leakage to form a medical balloon.
- the tube braid may be glued over a base liner that has the shape of the desired kind of balloon.
- a variety of known processes for forming composite structures are suitable so the subject will not be expansively discussed here.
- Figs. 2A and 2B are figurative illustrations of medical balloon fiber preforms with longitudinal folding patterns.
- balloon preform 105 has straight folding contours and in Fig. 2B , balloon preform 107 has helical folding contours.
- the preforms 105 and 107 as described below with reference to Figs. 5A and 5B , may be braided over a three-dimensional form to achieve the illustrated shape.
- the different portions with variegated flexibility, as identified above, are indicated collectively at 108 and 109. Note that a variety of other shapes may be used with the present inventive features and the shapes shown are merely for purposes of describing various folding features and structures.
- Fig. 3 is a planar development of a portion of folded balloon wall lying adjacent a catheter surface according to an example. As will be understood by those of skill in the art, a very low profile can be achieved in a medical balloon by configuring it to fold upon deflation.
- the folded shape shown in Fig. 3 shows a portion of the wall of a folded balloon 109.
- the illustration is a planar development and it is to be understood that the surface indicated at 104 would wrap around the axis of the balloon which passes through the plane of the drawing, as would the overlying balloon wall 114 and folds.
- the structure 111 may be a catheter with a circular cross-section. Fig.
- FIG. 3 shows how the relatively stiff portions 112 lie relatively flat while the folds coincide with the relatively flexible portions 113.
- a composite fiber reinforcement such as the braid discussed above, is preferably incorporated within the wall 114 of the balloon 109.
- balloons may not be completely folded, in the sense that the wall is bent 180° and completely overlaps and contacts an adjacent portion, in order for the balloon to achieve a compact shape. That is, the wall may simply be wrap or bend without making a fully 180° turn and/or adjacent portions may not lap once in the folded configuration.
- the portions of the wall that are stiffer will resist bending more than other portions.
- portion 112 resists bending more than portion 113. Note that since Fig. 3 shows a planar development, portion 112 is generally wrapped around the axis of the balloon, even though it is represented as a flat portion.
- a balloon may have a relaxed condition in the folded state (in fact this may be preferred in some examples) in which case some parts of the balloon may actually provide a negative resistance to bending to form the folded configuration.
- resistance here is used in a general sense that covers zero, negative, and positive resistance.
- the portions of the balloon that are bent the most, such as in folding may achieve their most relaxed state in, for example, a folded configuration, in which case, the balloon would not generate a positive resistance to folding because it tends to fold. So in that case, the resistance at certain portions, for example fold lines, would be negative.
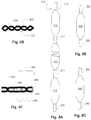
- Fig. 4A illustrates a braiding pattern that provides relatively stiff and relatively flexible portions.
- Fig. 4A shows a triaxial braid fabric 100 that may form part of a reinforcement structure for the wall of a medical balloon (not shown in the present drawing).
- the braiding pattern is a so-called diamond braid pattern with the yarns alternating sides each time they cross a yarn.
- longitudinal longitudinal being defined as the direction of the long axis of the page which is also the long axis of the balloon
- “seams" are formed where all the yarns cross sides along the same longitudinal line. This makes the diamond pattern easier to bend because the seams are thin offer less resistance.
- yarns generally cross more than one 0° yarn before they cross sides.
- a yarn crosses three other 0° yarns before crossing sides.
- the yarns cross three other 0° yarns before crossing. The pattern is preferably such that the crossing points lined up in the longitudinal direction lines.
- Figs. 4B and 4C illustrate how the braiding patterns of Fig. 4A of the flexible 110 and stiff 115 regions differ and how the differences contribute to relative stiffness.
- Fig. 4B the relatively flexible diamond pattern is shown in a figurative cross-section.
- the yarns 250 shown in section, represent longitudinal fibers of the axial braid. Note that the figure is a figurative cross-section because, although the yams 253 cross at a diagonal and, at any straight cross-section, continuous runs of crossing yarns could not be seen in a real cross-section, the illustration is functionally similar, as may be confirmed by inspection.
- the seams such as indicated at 252, where yarns 253 cross, are arranged in successive ranks along the longitudinal direction because yams alternate at every crossing.
- a stiffer structure is formed with crossing yarns 280 and 284 form tension elements that, in combination with the embedded matrix (which resist compression) define a stiff segment 288.
- the positions of the crossing points of other crossing yarns such as those shown crossing at 286, do not coincide in the lateral direction (i.e., the crossing lines are not aligned along longitudinal lines) so there no seam arises where the braid would be easier to bend.
- a stiff segment 290 (shown with broken lines) which is longitudinally adjacent the foreground stiff segment 288, is offset in the lateral direction. In this way, the stiffness is extended laterally beyond the range of the stiff segment 288.
- This kind of stiff arrangement is illustrated in Fig. 4A at 115.
- Fig. 5A illustrates a multi-balloon form 215, or mandrel, that may be used for braiding (or weaving) multiple reinforcement structures for a balloon in a single braiding operation.
- a braid may begin at a top extension portion 216 and widen into a balloon portion 210 and then neck down to a connecting section 217A.
- the braiding operation may continue over balloon sections 211 and connecting section 217 (arbitrary number of them), and then over a final balloon section 212, and extension section 218, to form a structure (not shown) that may be cut into multiple balloon preforms.
- Fig. 5B illustrates a single-balloon form 220 with extension sections 222 that may be used for braiding (or weaving) a single reinforcement structure for a balloon.
- the form may be made of a disintegrating, or otherwise collapsible structure to permit it to be removed from the finished form 215, 220 to leave one or more preforms.
- the cutting operation to divide the segments of example 215 may be done before or after the collapsing of the form 215.
- Fig. 5C illustrates a base balloon over which a reinforcement structure may be braided.
- the base balloon 240 with extensions 242 may act as a liner portion of a finished balloon, functioning as a braiding form for fabrication of the braided structure and, optionally, then remaining as part of the finished balloon.
- the base balloon 240 is fabricated of an inelastic material to facilitate its use as a form and base for braiding.
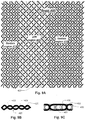
- Fig. 6A is a flow diagram of an example of a method for making a reinforced medical balloon using a multi-balloon form.
- step S10 the multiple part form 215 is fabricated and in step S15, a braiding device is used to braid over the form 215 ( Fig. 5A ).
- step S20 the resulting form and braided preform are then cut into segments to define the individual preforms to be used in the separate balloons.
- the underlying form is collapsed, for example by dissolving in acid or water, melting, reconfiguring, or performing some other step or steps depending on the structure of the form.
- step S30 a liner is inserted in each balloon preform and inflated.
- the liner may be formed such that it assumes the desired shape and size when inflated.
- the liner is inflated with a pressurized fluid and the braided preform coated with a matrix which is cured in step S40 and which serves to adhere the preform, the liner balloon, and the matrix together, forming an integral structure.
- Fig. 6B is a flow diagram of a method for making a reinforced medical balloon using a single-balloon form.
- step S110 the form 215 is fabricated and in step S115, a braiding device is used to braid over the form 225 ( Fig. 5B ).
- step S120 the underlying form is collapsed, for example by dissolving in acid or water, melting, reconfiguring, or performing some other step or steps depending on the structure of the form.
- a liner is inserted in the balloon preform and inflated.
- the liner may be of a form and shape that requires molding before adopting its final shape.
- a cylindrical mandrel may be inserted in step S135 to help seal and fill the balloon.
- step S140 in this example, the liner is inflated with a pressurized fluid and then the braided preform coated with a matrix that is cured in step S145 and which serves to adhere the preform, the liner balloon, and the matrix together, forming an integral structure.
- Fig. 6C is a flow diagram of a method for making a reinforced medical balloon using a liner balloon as a form.
- step S210 the liner balloon 220 ( Fig. 5C ) is fabricated and in step S215, a braiding device is used to braid over the liner balloon 220 ( Fig. 5C ).
- step S235 a cylindrical mandrel may be inserted in the liner to help seal and fill the balloon and the subassembly inserted in a mold.
- step S240 in this example, the liner is inflated with a pressurized fluid and the subassembly is heated until the braid is melted into the liner balloon, which thereby forms a matrix that seals the braided structure. The matrix is then cooled in step S245 to form an integral structure.
- the method steps were deliberately varied to illustrate that there are multiple ways to form the balloon with an integrated fiber braid reinforcement.
- a curable coating is placed on a preform while in Fig. 6C , a liner of thermoplastic is partially melted and cooled to form the balloon.
- the various methods and features can be altered and varied to form balloons and that no particular method is required to realized the benefits of the reinforcement structure described in the instant application.
- the fibers that are braided may be coated, or the preform impregnated with finely divided thermoplastic or adhesive with the liner balloon being of a high melting temperature than the molding temperature. Then in the method of Fig. 6C , the coating or impregnated material would then adhere the braid to the liner to form the balloon during thermal molding.
- An alternative method of making a balloon without employing a mold is to braid over a liner balloon using yarns that contain resin that flows at a lower temperature than the base material of the yarns of the base balloon.
- high melting-temperature yarns may be coated with low melting-temperature thermoplastic.
- the braid and base balloon may be heated to a temperature that causes the low melting-temperature resin to flow sealing any openings between the yarns.
- the base balloon material and thickness may be chosen such that it may either be removed or left in place depending on the properties of the material of the base balloon.
- Figs. 7 and Figs. 8A, 8B, and 8C illustrate the manufacture of a composite balloon according to an example.
- a mandrel 302 with openings 304 is inserted in a tube 314 of Polyethylene Terephthalate (PET), Nylon, or other suitable material, that will be molded to form a balloon liner.
- PET Polyethylene Terephthalate
- the mandrel 302 and tube 314 are inserted in a braided preform 308 and the substructure 332 then placed in a mold 310, 312, here illustrated as a two part mold. See Fig. 8A .
- Clamps 320 are placed over the ends of the mandrel 306 to seal the tube 314 and preform 308 against the mandrel 306.
- the mold is then assembled (as indicated by broken lines 322 or arrows 355) and compressed over the preform 308 (See Fig. 8B ) and heated while air or other fluid is injected in the mandrel 302 at an end opening 306.
- the tube 314 balloon is softened and expands under pressure. See Fig. 8C .
- the mold is cooled and the composite balloon 314 is removed.
- Figs. 9A - 9C illustrate a braiding pattern and yarn set that provides relatively stiff and relatively pliable portions.
- the present example is similar to that of Figs. 4A - 4C except that the 0° yarns 420 are larger in cross-section than the other yarns within the stiff regions 415. In the flexible regions 410 the yarns may be identical.
- the 0° yarns 420 in the present example have the effect of separating the tension portions 420 further apart than the example of Figs. 4A - 4C thereby creating even greater stiffness.
- Fig. 9B the relatively flexible diamond pattern is shown in a figurative cross-section with the 0° yarns 408, shown in section.
- the seams, such as indicated at 423, where yarns 425 cross are longitudinally arranged because yarns 425 alternate at every crossing.
- Fig. 9C where yarns 425 do not alternate at every crossing, a less flexible structure is formed with crossing yarns 420 forming tension elements as described above with reference to Fig. 4C .
- the braid pattern is such that stiff segments are offset and staggered in a direction perpendicular to the longitudinal direction to provide a cooperative continuous extension of the stiffness between stiff segments.
- Such a staggered arrangement is illustrated in Fig. 9C by observing the positions of the alternation points of other crossing yarns 435, which do not coincide in the lateral direction.
- a braid may that employs a biaxial braid structure may be created, which uses the same principle.
- the elements 420 could be, for example, PET or Nylon filaments.
- the 0° elements may be of another material that helps to provide the resistance to compression along with the material that forms the matrix.
- Fig. 10 illustrates a balloon example in which reinforcement portions are added in stages and sections.
- the braiding structures described herein may be braided as a cylindrical tube 515.
- the tube 515 may be slid over a balloon 505 and bonded or molded to it.
- the end portions may be formed by teasing the braid, folding it over (lapping it), cutting notches in it, or simply terminating it before completely covering the ends.
- braid may be formed over the ends as indicated at 510.
- the tube 515 may be teased and the fibers laid over the end (which is conical in the example) and a helical wind may be wrapped over the conical ends. See US Patent No.
- 6,746,425 for Medical Balloon which describes a structure and method for wrapping a balloon with a helical wind.
- the method which may involve the use of adhesive according to an example in the reference, may be followed after the tube braid 515 is laid over a liner balloon and inflated to form the desired shape.
- 0° yarns may be extended beyond the end of the tube where the conical end portion is reached.
- These free longitudinal fibers may be adhesively bonded in place as described in US Patent No. 6,746,425 and a helical wind added over the top in the manner described in this patent.
- the free ends of the 0° yarns may be obtained by the braiding device or by cutting the or unraveling.
- the diagonal yarns may be included in the longitudinal reinforcements over the conical portions.
- Fig. 11 illustrates a reinforced balloon having a non-cylindrical shape.
- Fig. 11 is included to illustrate that the inventive reinforcement structures are not limited to cylindrical balloons.
- a balloon 524 that collapses using accordion folds 530 and 535 and inflates to an expanded shape such as a cylinder may be formed.
- Fig. 12 shows a cylindrical form 550 with a non-circular cross-section. It may be beneficial to braid over such a non-cylindrical form, in some cases, to further promote folding, depending on the compatibility with yarn angles and other considerations. By braiding over such a form, the lengths of yarns are biased to favor the folded configuration, which is a property that is in addition to the variegated stiff and flexible regions property discussed above.
- the cylinder 550 (note that technically, non-circular column shapes are still called "cylinders”) may have a helical wind to it if the fold lines are not longitudinal. In some cases, it may be beneficial to use a stiffer braid throughout the entire braided structure (for example as described with reference to Figs.
- Fig. 13 illustrates a molding apparatus for forming reinforcement structures or combinations of balloons and reinforcement structures.
- the mold includes a central portion 582 and a wing portions 584.
- a balloon can have a folded shape bias molded into the matrix as well as the properties of additional flexibility in the folded portions, and/or folded bias, in the composite substructure (e.g., the braid).
- Fig. 14 illustrates another braiding pattern and structure that provides relatively stiff and relatively pliable portions.
- a stiff region 570 has a spacer 576 over which the braid is woven.
- the spacer 576 may be held as a 0° yarn and braiding may be performed around it.
- the layers of the flexible regions 572 are adjacent to allow them to be more flexible.
- the separated layers of the stiff region 570 contribute stiffness in a manner similar to that described with reference to Figs. 4B - 4C and Figs. 9B - 9C . That is the upper layer and lower layers act as tension elements over an incompressible core in the form of the spacer 576.
- the layers in the flexible regions 574 may be woven into a single fabric using three-dimensional braiding equipment. See for example, three dimensional braiding as described in US Patent Nos. 5,357,839 ; 5,772,848 ; and 6,090,137 .
- Fig. 15 illustrates yet another braiding pattern that provides relatively stiff and relatively pliable portions.
- the stiff portions 592 are formed by overbraiding (braiding on top of a braid to form an additional layer) with an overbraided layer 586 in only the regions 592 that are to be stiffened. This may be done using less expensive two-dimensional braiding equipment.
- the braid pattern may include yarns 590 that connect the overbraided layers 586.
- the thin region is defined mostly by the lower layer 588.
- a spacer such as 576 in Fig. 14 is included between the layers in the stiff regions. Note in this example, a spacer may be included by an intermediate lamination or coating step in which the spacer is placed on a balloon rather than being positioned and included as part of a braid preform.
- the stiff portions 592 can be made of a radio-opaque material to enhance visualization of the balloon in situ.
- the stiff portions 592 have a radio-opaque material coated on them to make them radio-opaque. This may allow the use of radio-opaque materials which might be too inflexible or otherwise difficult to integrate in a medical balloon.
- the balloon is coated with a radio-opaque material.
- the radio-opaque material are integrated into the balloon, such as by the braiding process as discussed in the instant specification.
- Such radio-opaque materials can be restricted the relatively flat (non-folding) portions of the balloon wall which may permit the use of materials that cannot tolerate as high a degree of strain if used in portions that are folded tightly.
- Examples of materials that may be used for the matrix and or liner of the above examples include polycaprolactam, polyesters, polyethers, polyamides, polyurethanes, polyimides, ABS copolymers, polyester/polyether block copolymers, ionomer resins, liquid crystal polymers, and rigid rod polymers.
- vascular dilatation vascular dilatation
- stent delivery drug delivery
- delivery and operation of sensors and surgical devices such as blades, and the like.
- Exemplary design parameters of balloons include balloons with burst pressures of 6,895 bar (100 psi) or more.
- the words “yarn” and “fiber” are used interchangeably.
- the term “yarn” is commonly used in the field of braiding. The term is not intended to limit the material, composition, or structure of the fiber material that is used in any of the above-described examples.
- the structures disclosed may be created in various ways including mechanisms that do not include braiding. Thus, even where the term “yarn” is used and/or where braiding is described as a preferred means of forming a structure, the uses are not necessarily intended to limit the structures described to ones formed by braiding.
- fibers/yams A variety of materials can be used for the fibers/yams. Examples include, but are not limited to, high strength inelastic fibers such as Kevlar, Vectran, Spectra, Dacron, Dyneema, Terlon (PBT), Zylon (PBO), Polyimide (PIM), ultra high molecular weight polyethylene, and the like.
- fibers/yarns may have non-circular cross-sections. For example, flat fibers/yarns may provide superior amenability to folding.
- the balloon or the braid pre-form can be coated with suitable materials (paint) to render the resulting medical balloon radio-opaque.
- suitable materials are known, for example, as discussed in US Patent 6,599,448 .
- some or all of the yarns or fibers employed may be radio-opaque to enhance the radio-opacity of the resulting balloon. This can be performed, for example, by applying a coating to the balloon, or fibers using vapor deposition or electro-energy deposition, for example, a metal coating such as tantalum or other materials such as barium sulfate.
- a metallic layer can be used in treatment such as to provide a means for creating an electric field inside the body for sterilizing a site. Examples of such applications and biocides are described in US Patent 6,258,249 (Charles Lee Simpson for "Sterilization of surgical sites").
- a medical balloon 601 may be of any suitable structure including monolithic polymer, composite with fibers, including braided fibers, laminates, or any other suitable structure.
- the medical balloon 601 has conductive surfaces (not shown in this view) on its external surfaces to allow the generation of an electric field for performing the biocidal function described in US Patent No. 6,258,249 .
- the balloon 601 is inserted between two surfaces 606 and 608 of a body cavity, a surgical or traumatic wound in a host 604.
- the balloon 601 may be coated with a biocide as described in US Patent No. 6,258,249 patent and the conductive surfaces connected to a source of voltage (alternating or direct) to destroy biofilm or other susceptible infectious material.
- the balloon 601 may be inserted and moved around, with an internal pressure that allows the balloon 6021 to conform to the surfaces 606 and 608.
- a cylindrical balloon 614 may be inserted in a lumen structure 616 of the host 604 to perform the same function.
- the lumen could be a blood vessel, a urethra, a duct, or any other cylindrical cavity or conduit.
- the internal space 602, 610 of either balloon 601, 614 can be filled with any suitable material such as, for example, saline.
- Figs. 17A, 17B Further details of the balloons of Fig. 16 are shown in Figs. 17A, 17B , as well as Figs. 18 through 20 , according to various alternative examples.
- the surface of balloons 630 and 660 are striped with first and second conductive strips 634 and 636 for balloon 630 and 664 and 666 for balloon 660.
- the strips are labeled 1 and 2 to indicate which of two poles of a voltage source (not shown) they are connected to.
- the strips 634 and 638 or 664 and 666 can be painted, sputtered, laminated, or otherwise deposited on the balloon in any suitable manner.
- the strips 634 and 638 or 664 and 666 may be realized by employing conductive yarns in a braid pattern whose surface is exposed on the balloon 630, 660.
- the surface may be exposed by creating a braided preform in which parallel sets of conductive yarns are used.
- a base balloon with an adhesive for example, a thermally activated adhesive or thermopolymer layer, may be inflated within the pre-form.
- the adhesive may not be required depending on the requirements of the application, but the result is preferably one in which the conductive yarns are exposed on the surface of the balloon to create the patterns shown in Figs. 17A and 17B .
- the conductive surfaces of the balloon 630 and 660 may be carried to terminals 638, 640 or 668, 670, respectively, for connection to the voltage source.
- Fig. 18 shows an example of parallel conductive yarns, in this case, the longitudinal yarns 704 are held in position and maintained parallel by the triaxial braiding pattern of the fabric 706.
- the conductive yarns 704 may be interspersed with non-conductive yarns.
- some or all of the diagonal yarns 703 may be made conductive.
- the polarity may alternate as every N yarns in any desired manner to achieve a desired spacing of the oppositely polarized yarns.
- the conductive yarns may be of metal, carbon-impregnated fiber, carbon composite, or any suitable material.
- a braid is not required to employ conductive fibers in this manner. For example yarns can be laid into a mold and a balloon molded into it as described in US Patent No. 6,746,425 . Any adhesive or matrix remaining on the surface can be polished off to expose the conductive yarns.
- the conductive yarns can be provided with insulation in parts to provide that the conductors are exposed only at desired locations on a balloon.
- conductors 752 and 754 (which indicate sets of alternating polarity), in the array 706, depicted in Fig. 19 , are exposed only in a region 756 (or 786 in the Fig. 19 example).
- a region 757 exposes the conductors 752 and 754 at a terminal region 757 for connection to a source.
- the conductors 752 of one polarity may be exposed by lack of insulation in a different axial position than the conductors 754 of the other polarity to facilitate connection to voltage sources as illustrated in Fig. 20 .
- region 782 is a region in which the yarns of one polarity are exposed and the yarns of the other polarity are insulated.
- region 780 is a region in which the yarns of the other polarity are exposed and the yarns of the one polarity are insulated.
- conductive take-offs 781 and 783 can be soldered or otherwise electrically connected to the conductive yarns without causing shorting.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Child & Adolescent Psychology (AREA)
- Vascular Medicine (AREA)
- Manufacturing & Machinery (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Cardiology (AREA)
- Textile Engineering (AREA)
- General Chemical & Material Sciences (AREA)
- Communicable Diseases (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Chemical & Material Sciences (AREA)
- Oncology (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Materials For Medical Uses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Woven Fabrics (AREA)
- Electrotherapy Devices (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
- The present application claims priority to
US Provisional Application Serial Number 60/829,231, filed Oct 12, 2006 - The invention relates to composite structures for medical balloons and in particular, such structures that promote predictable folding.
- Woven and braided fabrics have been used to reinforce various devices. Compared to weaving, braiding may impart greater strength for a unit of weight. The strength of a braid comes from the fact that multiple yarns can be intertwined without any being twisted around another. Generally these are continuously braided at an angle and there is no need for any yarn to suffer a sharp bend. As a result, loads may be distributed evenly and efficiently throughout the braid.
- Automated fabrication of braids generally results in tubular or flat configurations. Braids can also be formed without any underlying support (freestanding) or over a mandrel or a part to be reinforced, such as the mast of a sailboat. Braiding can also be done over a three-dimensional part, such as a tool.
- A single braid can incorporate multiple yarn materials to form a hybrid weave. This is often done to make patterns in the resulting product. Yarns can be of metal, carbon fiber, glass fiber, mono or multifilament threads, etc. Braiding can be done with very delicate materials.
- Braid has been used as a reinforcement for some surgical devices such as endoscopes and catheters and for implantable devices such as splints and stents.
- Non-woven fiber reinforcements are also known, for example, randomly arrayed fibers such as in fiberglass and hand-laid fibers arrayed over and within a matrix are known strategies. Both have been described in connection with the reinforcement of medical balloons.
- Many composite balloon structures are reinforced by inelastic filaments, which is a good match for folding to minimize the collapsed balloon's volume. However, the fiber can be an impediment to folding, an issue that is addressed by at least some of the inventive embodiments disclosed below.
- The following are some references to background in the field of braiding technology. A brief overview and comparison of 2D and 3D braiding machines and the kinds of structures they can create is provided by an article, "Braiding," 2005 Advanced Composite Materials & Textile Research Laboratory, University of Massachusetts-Lowell. [online] August 2007 [Retrieved on 2006-6-21]. Retrieved from the Internet. <http://mechanical.uml.edu/acmtrl/research-Braiding.htm>.
- The company, 3TEX, provides information about state of the art three-dimensional automated braiding at [online] ]Retrieved on 2005-6-21] Retrieved from the Internet <http://www.3tex.com/3braid.cfm>. The page shows photographs and an animation of a large Cartesian braiding machine. One of the points made is that with computer control, it is possible to shift the braiding pattern at any time without changing the number or continuity of the yarns.
- A report by the National Textile Center (NTC) in Springhouse, PA discusses different kinds of braiding patterns such as diamond, regular, and Hercules braids and discusses behavior of braids under tensile load, the effect of yarn angle with respect to load and jamming condition, and other issues. "Engineered Non-Linear Elastic Blended Fabrics," NTC Project F00-PH05 2005 [Retrived on 2006-6-21]. Retrieved from the Internet. <http://www.ntcresearch.org/pdf-rpts/AnRp02/F00-PH05-A2.pdf>
- The following articles discuss braids with different mechanical properties, including mixing materials. "Analysis of three-dimensional textile preforms for multidirectional reinforcement of composites;" Guang-Wu Du, Tsu-Wei Chou and P. Popper; J. Mater. Sci. 26 (1991) 3438-3448. Dunn, Matthew; Armstrong-Carroll, Eileen; Gowayed, Yasser; "Engineered Non-linear Elastic Bland Fabrics" [Retrived on 2006-6-21]. Retrieved from the Internet. <http://www.ntcresearch.org/pdf-rpts/Bref0601/F00-P05.pdf>.
- The following article discusses the effect of braids on the mechanical properties of braided fabrics. There is considerable background on hybrid braids and their performance. Seneviratne, Waruna P. and Tomblin, John S.; "Design Of A Braided Composite Structure With A Tapered Cross-Section;" National Institute for Aviation Research Wichita State University Wichita, KS 67260-0093 The Department Of Defense Handbook Composite Materials Handbook
Volume 2, "Polymer Matrix Composites Materials Properties," discusses braids in the context of composite materials. [Retrived on 2006-6-21]. Retrieved from the Internet. <http://www.lib.ucdavis.edu/dept/pse/resources/fulltext/HDBK17-2F.pdf> -
WO 03/020334 A3 US 2001/0056299 A1 is concerned with a braided covered stent.US 6,012,457 is concerned with a method for forming a circumferential conduction block in a pulmonary vein. -
US 2002/010489 A1 is concerned with a balloon catheter. - A balloon for medical treatments such as percutaneous transluminal coronary angioplasty (PTCA), delivery of a vascular stents or stent grafts, employs reinforcement materials that are patterned so as to promote consistent, predictable, or tighter, folding of the balloon.
- The present invention is defined in
claim 1.Claim 2 defines a preferred embodiment. Examples that do not fall under the scope of the claims are included for information purposes. - An example provides a medical balloon whose walls have relatively stiff and relatively flexible regions to promote folding along the flexible regions. The variation in stiffness is achieved, according to the different embodiments, by variably arranging composite elements on, or within, the wall of the balloon; by adding stiffening members to the wall at selected portions; by varying the properties of a fabric or braid or other filamentous structure to define variable stiffness, and by other means.
- According to an example, the example is a foldable composite balloon with a wall. The wall has first and second filaments and first and second wall portions. The wall has compression elements separating the first and second filaments in the first wall portions so that they define opposing tension elements running in a wind direction. The opposing tension elements have a component in a specified direction and are separated by the at least one compression element resulting in the first portion being stiffer than the second portion, at least in the specified direction, the first and second portions being arranged such that when the balloon is
folded, the first portions resist bending more than the second portions. This may be so the folds are generally aligned with the second portions and it may help to ensure a neat and predictable folding behavior when the balloon is collapsed. This in turn can help to ensure a compact configuration in tight areas. - Variations of this example and others are possible. For example, the first and second filaments may be portions of elongate members that run continuously through the first portions and the second portions. The first and second filaments may be braided to define at least a portion of a braid. The braid may include a triaxial portion having third filaments running as a 0° braid yarn in the first and second portions, the third filaments in the first portions being thicker than the third filaments in the second portion and the third filaments forming at least part of the at compression element. Note, the 0° yarn refers to yarns running in a longitudinal direction, which is the direction along which the braid extends (or gets longer) as the braid is woven.
- The first and second filaments may define at least a portion of a braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions. The first and second filaments may define at least a portion of a biaxial braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions.
- The wall may be elongated such as to have a longitudinal axis and the second portion may be aligned with the axis or follow a helical path around the longitudinal axis. The wall may include a matrix, such as a polymer matrix, and members embedded therein with the first and second filaments being embedded in the matrix and the members forming at least portions of the compression elements.
- The wall may include a matrix and flat members embedded therein, the first and second filaments being embedded in the matrix and the members forming at least portions of the compression element.
- According to an example, also provided is a foldable composite balloon with a wall of polymer matrix with first and second filaments attached to it. The wall may have first and second portions, the first and second filaments spaced apart by a portion of the polymer matrix in the first wall portions, such that they define opposing tension elements running in a wind direction having a component in a specified direction and separated by the matrix portion. The spacing of the tension elements on opposite sides of the matrix portion is such that the matrix portion acts as a compression element and the result is that the first portion is stiffer than the second portion, at least in the specified direction. The first and second portions may be arranged such that when the balloon is folded, the first portions resist bending more than the second portions. Or the second portions may be aligned with folding lines of the balloon so the structure helps to promote folding or creates a natural folding behavior.
- This example has variations as well, such as may include the first and second filaments being portions of elongate members running continuously through the first portions and the second portions. The first and second filaments may be braided to form a braid. The first and second filaments may define a braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions. The second portions may define folding contours and the alternations are staggered in the first region such that no consecutive trains of side alternations occur that are parallel to the folding contours.
- According to yet another example, described is a foldable composite balloon with a body that has a polymer matrix and a filamentous structure attached thereto. The body may have first and second portions and folding lines with the filamentous structure defining first and second portions, the folding lines lying within the second portions and the first portions lying between the second portions. In one embodiment, the filamentous structure may be configured to promote folding along the folding lines either by being configured to cause the body to be stiffer in the first regions, at least in a direction perpendicular to the folding line, than the second portions. In another embodiment, the filamentous structure may be configured to generate a mechanical bias that favors folding along the folding lines as a result of being formed over a form with edges on it.
- The filamentous structure may be configured to cause the body to be stiffer in the first regions, at least in a direction perpendicular to the folding lines, than the second portions. The filamentous structure may include a braid. The filamentous structure may have first and second filaments and a compression element, the first portions being stiffer, at least in a direction perpendicular to the folding lines, at least in part as a result of the first and second filaments of the first portions being arranged with the compression element between then, thereby defining opposing tension elements separated by the compression element. The braid may have layers with more layers in the first portions than in the second portions such that the first portions are stiffer than the second portions.
- The body may have a longitudinal axis and the folding lines are parallel to the longitudinal axis. The body may have a longitudinal axis and the folding lines may wind helically around a longitudinal axis. The braid may be triaxial or biaxial.
- According to yet another example, a foldable composite balloon is provided which has a wall with a polymer matrix included elements attached to, or within, the polymer matrix. The included elements are arranged to define first portions, and second portions of the wall such that the wall folds more readily in the first portions than the second portions.
- According to yet another example, a foldable composite balloon is provided with a wall having elongate reinforcement members, first portions, and second portions, the stiffness of the first portions being lower than the stiffness of the second portions. An arrangement of the elongate reinforcement members causes the wall to be stiffer in the second portions than in the first portions, whereby the balloon tends to fold along contours coinciding with the low stiffness portions.
- According to an example, described is a method for the treatment of an infected area within a body. The method includes applying an electrically conductive biocide composition to an infected area within the body that has been exposed during surgery, and applying an electric field to the biocide composition by contacting a surface with the biocide composition with an inflatable member having conductive surface of alternate polarity to generate an electric field. The electric field strength and duration of application are sufficient to produce killing of microorganisms in the infected area. Preferably, the infected area is composed of a biofilm that is composed predominately of bacteria, yeast or fungus. Preferably, the biocide is an antibiotic selected from the family of antibiotics consisting of penicillins, cephalosporins, aminoglycosides, tetracyclines, sulfonamides, macrolide antibiotics and quinolones. Preferably, the electrically conductive biocide composition is a buffered saline composition. Preferably, the biocide composition includes a thickener. Preferably, the electric field is substantially constant. Preferably, the electrical field is a pulsed or alternating electric field. Preferably, the electric field strength is generated by currents having a value in the range from about 1 to about 200 milliamps. Preferably, said electric field is applied to the electrically conductive biocide composition for a period of time of between about 1 minute to about 48 hours. Preferably, the biocide is present in the composition, in an amount which would be ineffective to completely kill the infected area if used in the absence of the electric field.
- In a particular variation of the above method examples, the method is performed during the course of heart valve replacement surgery. Preferably, the biocide is an antibiotic, an anti-fungal agent, a disinfectant, a sterilant, other antiseptic agents, hexachlorophene, cationic bisiguanides, iodine, iodophores, para-chloro-meta-xylenol, triclosan, furan preparations, methenamine, aldehydes, or alcohols. Preferably, the cationic bisiguanides include chlorhexidene or cyclohexidene. Preferably, iodine include povidone-iodine. Preferably, iodophores include povidone-iodine. Preferably, furan preparations include nitrofurantoin or nitrofurazone. Preferably, aldehydes is in glute form.
- According to another example, the example is a medical balloon, comprising: a balloon body having an array of reinforcement fibers exposed on an external surface thereon; at least some of the reinforcement fibers being electrically conductive subsets of which are connectable to a source of voltage such that an electric field can be continuously generated on the surface of the body. Preferably, the fibers form a braided pattern. Preferably, the at least some of the reinforcement fibers are of metal. Preferably, the at least some of the reinforcement fibers are zero-angle fibers of a triaxial braid.
- According to an example, also provided may be a foldable composite balloon with a wall of polymer matrix with first and second filaments attached to it. The wall may have first and second portions, the first and second filaments spaced apart by one or more radio-opaque elements in the first wall portions, such that the yarns overlying them define opposing tension elements running in a wind direction having a component in a specified direction and separated by the radio-opaque portions. This allows the radio-opaqu included elements to be relatively stiff without impeding (in fact promoting) the folding of the balloon. The spacing of the tension elements on opposite sides of the matrix portion is such that the included radio-opaque elements act as compression elements and the result is that the first portion is stiffer than the second portion, at least in the specified direction. The first and second portions may be arranged such that when the balloon is folded, the first portions resist bending more than the second portions. Or the second portions may be aligned with folding lines of the balloon so the structure helps to promote folding or creates a natural folding behavior.
- This example has variations as well, such as may include the first and second filaments being portions of elongate members running continuously through the first portions and the second portions. The first and second filaments may be braided to form a braid. The first and second filaments may define a braid having, within the second portions, a greater number of crossings between layer alternations than within the first portions. The second portions may define folding contours and the alternations are staggered in the first region such that no consecutive trains of side alternations occur that are parallel to the folding contours.
- In another example, a foldable composite balloon has a braided reinforcement structure defining a wall. The braided reinforcement pattern is such that the wall is stiffer at the first wall portions than at the second wall portions. The first and second wall portions are arranged such that when the balloon is folded, the first portions resist bending more than the second portions. Preferably, at least the first wall portions have a radio-opaque coating thereon. Alternatively, only the first wall portions have a radio-opaque coating thereon. In another preferred embodiment, a radio-opaque material is integrated in the fist portions only.
- The accompanying drawings, which are incorporated herein and constitute part of this specification, illustrate exemplary embodiments of the invention, and, together with the general description given above and the detailed description given below, serve to explain the features of the invention.
-
Fig. 1 shows a reinforcement structure, such as a tube braid, that may be used in a composite balloon, the reinforcement structure having relatively flexible portions or facets and relatively stiff portions or facets. -
Figs. 2A and 2B are figurative illustrations of medical balloon fiber preforms with longitudinal folding patterns, the first being straight and the second being helical. -
Fig. 3 is a planar development of a portion of folded balloon wall lying adjacent a catheter surface. -
Fig. 4A illustrates a braiding pattern that provides relatively stiff and relatively pliable portions. -
Figs. 4B and 4C are illustrations for helping to explain a feature of the braid pattern ofFig. 4A . -
Fig. 5A illustrates a multi-balloon mandrel that may be used for braiding or weaving multiple reinforcement structures for a balloon. -
Fig. 5B illustrates a single-balloon mandrel that may be used for braiding or weaving a reinforcement structure for a balloon. -
Fig. 5C illustrates a base balloon over which a reinforcement structure may be braided. -
Fig. 6A is a flow diagram of a method for making a reinforced medical balloon using a multi-balloon form. -
Fig. 6B is a flow diagram of a method for making a reinforced medical balloon using a single-balloon form. -
Fig. 6C is a flow diagram of a method for making a reinforced medical balloon using a liner balloon as a form. -
Fig. 7 illustrates elements used in manufacturing a composite balloon. -
Figs. 8A - 8C illustrate steps in the manufacture of a composite balloon according to the example ofFig. 7 . -
Figs. 9A - 9C illustrate a braiding pattern and yarn set that provides relatively stiff and relatively pliable portions. -
Fig. 10 illustrates a balloon embodiment in which reinforcement portions are added in stages and sections. -
Fig. 11 illustrates a reinforced balloon having a non-cylindrical shape. -
Fig. 12 illustrates a cylindrical form with a non-circular cross-section. -
Fig. 13 illustrates a molding apparatus for forming reinforcement structures or combinations of balloons and reinforcement structures. -
Fig. 14 illustrates another braiding pattern and structure that provides relatively stiff and relatively pliable portions. -
Fig. 15 illustrates yet another braiding pattern that provides relatively stiff and relatively pliable portions. -
Fig. 16 illustrates two alternative types of balloons inserted in an opening of a host. -
Figs. 17A and 17B illustrate embodiments of a medical balloon embodiments with conducting surfaces for use in biocidal procedures or other procedures where balloons having conductors are used. -
Fig. 18 illustrates a fabric with conductive yarns. -
Fig. 19 illustrates partly insulated conductive yarns for use with embodiments of the invention. -
Fig. 20 illustrates a balloon with partly insulated conductive yarns. - The various embodiments will be described in detail with reference to the accompanying drawings. Wherever possible, the same reference numbers will be used throughout the drawings to refer to the same or like parts.
-
Fig. 1 shows a reinforcement structure, such as a tube braid, that may be used in a composite balloon, the reinforcement structure having relativelyflexible portions 106 or facets and relativelystiff portions 102 or facets. Atubular braid 103 hasstiff portions 102 that are relatively stiff, or at least relatively stiff in the circumferential direction (i.e., the direction about the balloon axis). Thetubular braid 103 also hasflexible portions 106 that are flexible relative to thestiff portions 102, also, at least in the circumferential direction. - The
tube braid 103 may be of strong filaments (not shown separately) of any type, but in the present examples of folding medical balloons, they include relatively inelastic high strength synthetic fibers. The filaments may be a mix of different materials and cross-sectional shapes and different materials may be combined in various ways as discussed below. The braiding may be done using a variety of mechanisms which are known in the art employing braid patterns and other structures described herein. For example, the braiding of thetube braid 103 may be performed using a programmable tube braider (not shown). Alternatively, thetube braid 103 may be a portion of a non-cylindrical (three-dimensional) braid as illustrated inFig. 2A with progressively tapering ends. In such a case, the filaments may be braided over a three-dimensional form (for example, as at 220 inFig. 5B , described below) to create a desired balloon shape. - The
tube braid 103 may be embedded in, impregnated with, or otherwise combined with a flexible material that can hold pressure and ensure against leakage to form a medical balloon. For example, the tube braid may be glued over a base liner that has the shape of the desired kind of balloon. A variety of known processes for forming composite structures are suitable so the subject will not be expansively discussed here. -
Figs. 2A and 2B are figurative illustrations of medical balloon fiber preforms with longitudinal folding patterns. InFig. 2A ,balloon preform 105 has straight folding contours and inFig. 2B ,balloon preform 107 has helical folding contours. Thepreforms Figs. 5A and 5B , may be braided over a three-dimensional form to achieve the illustrated shape. The different portions with variegated flexibility, as identified above, are indicated collectively at 108 and 109. Note that a variety of other shapes may be used with the present inventive features and the shapes shown are merely for purposes of describing various folding features and structures. -
Fig. 3 is a planar development of a portion of folded balloon wall lying adjacent a catheter surface according to an example. As will be understood by those of skill in the art, a very low profile can be achieved in a medical balloon by configuring it to fold upon deflation. The folded shape shown inFig. 3 shows a portion of the wall of a foldedballoon 109. As indicated, the illustration is a planar development and it is to be understood that the surface indicated at 104 would wrap around the axis of the balloon which passes through the plane of the drawing, as would the overlyingballoon wall 114 and folds. For example, the structure 111 may be a catheter with a circular cross-section.Fig. 3 shows how the relativelystiff portions 112 lie relatively flat while the folds coincide with the relativelyflexible portions 113. As a result, when theballoon 109 is deflated, the fold pattern may be more readily assumed by theballoon 109. Though not illustrated, a composite fiber reinforcement, such as the braid discussed above, is preferably incorporated within thewall 114 of theballoon 109. - The examples described above and below may be modified in such a way that balloons may not be completely folded, in the sense that the wall is bent 180° and completely overlaps and contacts an adjacent portion, in order for the balloon to achieve a compact shape. That is, the wall may simply be wrap or bend without making a fully 180° turn and/or adjacent portions may not lap once in the folded configuration. In such examples, the portions of the wall that are stiffer will resist bending more than other portions. For example, in
Fig. 3 ,portion 112 resists bending more thanportion 113. Note that sinceFig. 3 shows a planar development,portion 112 is generally wrapped around the axis of the balloon, even though it is represented as a flat portion. A balloon may have a relaxed condition in the folded state (in fact this may be preferred in some examples) in which case some parts of the balloon may actually provide a negative resistance to bending to form the folded configuration. So the term "resistance" here is used in a general sense that covers zero, negative, and positive resistance. In other words, it is understood that the portions of the balloon that are bent the most, such as in folding, may achieve their most relaxed state in, for example, a folded configuration, in which case, the balloon would not generate a positive resistance to folding because it tends to fold. So in that case, the resistance at certain portions, for example fold lines, would be negative. -
Fig. 4A illustrates a braiding pattern that provides relatively stiff and relatively flexible portions.Fig. 4A shows atriaxial braid fabric 100 that may form part of a reinforcement structure for the wall of a medical balloon (not shown in the present drawing). In aflexible region regions 113 inFig. 3 , the braiding pattern is a so-called diamond braid pattern with the yarns alternating sides each time they cross a yarn. With the diamond braid pattern, longitudinal (longitudinal being defined as the direction of the long axis of the page which is also the long axis of the balloon) "seams" are formed where all the yarns cross sides along the same longitudinal line. This makes the diamond pattern easier to bend because the seams are thin offer less resistance. In the stiff regions 115 (only one shown, but there would ordinarily be more) yarns generally cross more than one 0° yarn before they cross sides. For example, in a Hercules braid, a yarn crosses three other 0° yarns before crossing sides. In the illustrated braiding pattern for thestiff region 115, the yarns cross three other 0° yarns before crossing. The pattern is preferably such that the crossing points lined up in the longitudinal direction lines. - Refer now to
Figs. 4B and 4C , which illustrate how the braiding patterns ofFig. 4A of the flexible 110 and stiff 115 regions differ and how the differences contribute to relative stiffness. InFig. 4B , the relatively flexible diamond pattern is shown in a figurative cross-section. Theyarns 250, shown in section, represent longitudinal fibers of the axial braid. Note that the figure is a figurative cross-section because, although theyams 253 cross at a diagonal and, at any straight cross-section, continuous runs of crossing yarns could not be seen in a real cross-section, the illustration is functionally similar, as may be confirmed by inspection. The seams, such as indicated at 252, whereyarns 253 cross, are arranged in successive ranks along the longitudinal direction because yams alternate at every crossing. In contrast, as shown inFig. 4C , where yarns do not alternate at every crossing, a stiffer structure is formed with crossingyarns stiff segment 288. The positions of the crossing points of other crossing yarns such as those shown crossing at 286, do not coincide in the lateral direction (i.e., the crossing lines are not aligned along longitudinal lines) so there no seam arises where the braid would be easier to bend. Thus, for example, a stiff segment 290 (shown with broken lines) which is longitudinally adjacent the foregroundstiff segment 288, is offset in the lateral direction. In this way, the stiffness is extended laterally beyond the range of thestiff segment 288. This kind of stiff arrangement is illustrated inFig. 4A at 115. -
Fig. 5A illustrates amulti-balloon form 215, or mandrel, that may be used for braiding (or weaving) multiple reinforcement structures for a balloon in a single braiding operation. A braid may begin at atop extension portion 216 and widen into aballoon portion 210 and then neck down to a connecting section 217A. The braiding operation may continue overballoon sections 211 and connecting section 217 (arbitrary number of them), and then over afinal balloon section 212, andextension section 218, to form a structure (not shown) that may be cut into multiple balloon preforms. -
Fig. 5B illustrates a single-balloon form 220 withextension sections 222 that may be used for braiding (or weaving) a single reinforcement structure for a balloon. In both the 215 and 220 examples, the form may be made of a disintegrating, or otherwise collapsible structure to permit it to be removed from thefinished form form 215.Fig. 5C illustrates a base balloon over which a reinforcement structure may be braided. Thebase balloon 240 withextensions 242 may act as a liner portion of a finished balloon, functioning as a braiding form for fabrication of the braided structure and, optionally, then remaining as part of the finished balloon. Preferably, thebase balloon 240 is fabricated of an inelastic material to facilitate its use as a form and base for braiding. -
Fig. 6A is a flow diagram of an example of a method for making a reinforced medical balloon using a multi-balloon form. In step S10, themultiple part form 215 is fabricated and in step S15, a braiding device is used to braid over the form 215 (Fig. 5A ). In step S20, the resulting form and braided preform are then cut into segments to define the individual preforms to be used in the separate balloons. In step S25, next, the underlying form is collapsed, for example by dissolving in acid or water, melting, reconfiguring, or performing some other step or steps depending on the structure of the form. In step S30, a liner is inserted in each balloon preform and inflated. The liner may be formed such that it assumes the desired shape and size when inflated. In step S35, in an example, the liner is inflated with a pressurized fluid and the braided preform coated with a matrix which is cured in step S40 and which serves to adhere the preform, the liner balloon, and the matrix together, forming an integral structure. -
Fig. 6B is a flow diagram of a method for making a reinforced medical balloon using a single-balloon form. In step S110, theform 215 is fabricated and in step S115, a braiding device is used to braid over the form 225 (Fig. 5B ). In step S120, the underlying form is collapsed, for example by dissolving in acid or water, melting, reconfiguring, or performing some other step or steps depending on the structure of the form. In step S130, a liner is inserted in the balloon preform and inflated. The liner may be of a form and shape that requires molding before adopting its final shape. A cylindrical mandrel may be inserted in step S135 to help seal and fill the balloon. In step S140, in this example, the liner is inflated with a pressurized fluid and then the braided preform coated with a matrix that is cured in step S145 and which serves to adhere the preform, the liner balloon, and the matrix together, forming an integral structure. -
Fig. 6C is a flow diagram of a method for making a reinforced medical balloon using a liner balloon as a form. In step S210, the liner balloon 220 (Fig. 5C ) is fabricated and in step S215, a braiding device is used to braid over the liner balloon 220 (Fig. 5C ). In step S235, a cylindrical mandrel may be inserted in the liner to help seal and fill the balloon and the subassembly inserted in a mold. In step S240, in this example, the liner is inflated with a pressurized fluid and the subassembly is heated until the braid is melted into the liner balloon, which thereby forms a matrix that seals the braided structure. The matrix is then cooled in step S245 to form an integral structure. - In the foregoing examples, the method steps were deliberately varied to illustrate that there are multiple ways to form the balloon with an integrated fiber braid reinforcement. For example in the methods of
Figs. 6A and 6B , a curable coating is placed on a preform while inFig. 6C , a liner of thermoplastic is partially melted and cooled to form the balloon. It will be recognized that the various methods and features can be altered and varied to form balloons and that no particular method is required to realized the benefits of the reinforcement structure described in the instant application. For example, the fibers that are braided may be coated, or the preform impregnated with finely divided thermoplastic or adhesive with the liner balloon being of a high melting temperature than the molding temperature. Then in the method ofFig. 6C , the coating or impregnated material would then adhere the braid to the liner to form the balloon during thermal molding. - An alternative method of making a balloon without employing a mold is to braid over a liner balloon using yarns that contain resin that flows at a lower temperature than the base material of the yarns of the base balloon. For example high melting-temperature yarns may be coated with low melting-temperature thermoplastic. After braiding over the base balloon with the two-part yarns, the braid and base balloon may be heated to a temperature that causes the low melting-temperature resin to flow sealing any openings between the yarns. The base balloon material and thickness may be chosen such that it may either be removed or left in place depending on the properties of the material of the base balloon.
-
Figs. 7 andFigs. 8A, 8B, and 8C illustrate the manufacture of a composite balloon according to an example. Amandrel 302 withopenings 304 is inserted in atube 314 of Polyethylene Terephthalate (PET), Nylon, or other suitable material, that will be molded to form a balloon liner. Themandrel 302 andtube 314 are inserted in abraided preform 308 and thesubstructure 332 then placed in amold Fig. 8A .Clamps 320 are placed over the ends of themandrel 306 to seal thetube 314 and preform 308 against themandrel 306. The mold is then assembled (as indicated bybroken lines 322 or arrows 355) and compressed over the preform 308 (SeeFig. 8B ) and heated while air or other fluid is injected in themandrel 302 at anend opening 306. As a result thetube 314 balloon is softened and expands under pressure. SeeFig. 8C . After thetube 314, now aballoon 314A, has formed acomposite balloon 314, the mold is cooled and thecomposite balloon 314 is removed. -
Figs. 9A - 9C illustrate a braiding pattern and yarn set that provides relatively stiff and relatively pliable portions. The present example is similar to that ofFigs. 4A - 4C except that the 0°yarns 420 are larger in cross-section than the other yarns within thestiff regions 415. In theflexible regions 410 the yarns may be identical. The 0°yarns 420 in the present example have the effect of separating thetension portions 420 further apart than the example ofFigs. 4A - 4C thereby creating even greater stiffness. - In
Fig. 9B , the relatively flexible diamond pattern is shown in a figurative cross-section with the 0°yarns 408, shown in section. Again, the seams, such as indicated at 423, whereyarns 425 cross, are longitudinally arranged becauseyarns 425 alternate at every crossing. InFig. 9C , whereyarns 425 do not alternate at every crossing, a less flexible structure is formed with crossingyarns 420 forming tension elements as described above with reference toFig. 4C . Again, preferably, the braid pattern is such that stiff segments are offset and staggered in a direction perpendicular to the longitudinal direction to provide a cooperative continuous extension of the stiffness between stiff segments. Such a staggered arrangement is illustrated inFig. 9C by observing the positions of the alternation points ofother crossing yarns 435, which do not coincide in the lateral direction. - Note that another example of a braid may that employs a biaxial braid structure may be created, which uses the same principle. In such an example, no 0° yarns exist in the
flexible regions 410 but 0° elements - not necessarily yarns - serve to separate the biaxial layers of biaxial yarns in stiff regions. In such an embodiment, theelements 420 could be, for example, PET or Nylon filaments. In this case, the 0° elements may be of another material that helps to provide the resistance to compression along with the material that forms the matrix. -
Fig. 10 illustrates a balloon example in which reinforcement portions are added in stages and sections. Instead of braiding over a form to create a three-dimensional preform, the braiding structures described herein may be braided as acylindrical tube 515. Thetube 515 may be slid over aballoon 505 and bonded or molded to it. In that case, the end portions may be formed by teasing the braid, folding it over (lapping it), cutting notches in it, or simply terminating it before completely covering the ends. In addition, braid may be formed over the ends as indicated at 510. For example, thetube 515 may be teased and the fibers laid over the end (which is conical in the example) and a helical wind may be wrapped over the conical ends. SeeUS Patent No. 6,746,425 for Medical Balloon; which describes a structure and method for wrapping a balloon with a helical wind. For example, the method which may involve the use of adhesive according to an example in the reference, may be followed after thetube braid 515 is laid over a liner balloon and inflated to form the desired shape. - In variation on the example of
fig. 10 , 0° yarns may be extended beyond the end of the tube where the conical end portion is reached. These free longitudinal fibers may be adhesively bonded in place as described inUS Patent No. 6,746,425 and a helical wind added over the top in the manner described in this patent. The free ends of the 0° yarns may be obtained by the braiding device or by cutting the or unraveling. Alternatively, the diagonal yarns may be included in the longitudinal reinforcements over the conical portions. -
Fig. 11 illustrates a reinforced balloon having a non-cylindrical shape.Fig. 11 is included to illustrate that the inventive reinforcement structures are not limited to cylindrical balloons. For example, aballoon 524 that collapses using accordion folds 530 and 535 and inflates to an expanded shape such as a cylinder may be formed. -
Fig. 12 shows acylindrical form 550 with a non-circular cross-section. It may be beneficial to braid over such a non-cylindrical form, in some cases, to further promote folding, depending on the compatibility with yarn angles and other considerations. By braiding over such a form, the lengths of yarns are biased to favor the folded configuration, which is a property that is in addition to the variegated stiff and flexible regions property discussed above. The cylinder 550 (note that technically, non-circular column shapes are still called "cylinders") may have a helical wind to it if the fold lines are not longitudinal. In some cases, it may be beneficial to use a stiffer braid throughout the entire braided structure (for example as described with reference toFigs. 4A- 4C andFigs. 9A - 9C ), which would result inshorter tension members Fig. 13 illustrates a molding apparatus for forming reinforcement structures or combinations of balloons and reinforcement structures. The mold includes acentral portion 582 and a wing portions 584. Using such a structure, a balloon can have a folded shape bias molded into the matrix as well as the properties of additional flexibility in the folded portions, and/or folded bias, in the composite substructure (e.g., the braid). -
Fig. 14 illustrates another braiding pattern and structure that provides relatively stiff and relatively pliable portions. Here, astiff region 570 has aspacer 576 over which the braid is woven. Thespacer 576 may be held as a 0° yarn and braiding may be performed around it. The layers of theflexible regions 572 are adjacent to allow them to be more flexible. The separated layers of thestiff region 570 contribute stiffness in a manner similar to that described with reference toFigs. 4B - 4C andFigs. 9B - 9C . That is the upper layer and lower layers act as tension elements over an incompressible core in the form of thespacer 576. Note that the layers in theflexible regions 574 may be woven into a single fabric using three-dimensional braiding equipment. See for example, three dimensional braiding as described inUS Patent Nos. 5,357,839 ;5,772,848 ; and6,090,137 . -
Fig. 15 illustrates yet another braiding pattern that provides relatively stiff and relatively pliable portions. Thestiff portions 592 are formed by overbraiding (braiding on top of a braid to form an additional layer) with anoverbraided layer 586 in only theregions 592 that are to be stiffened. This may be done using less expensive two-dimensional braiding equipment. The braid pattern may includeyarns 590 that connect the overbraided layers 586. The thin region is defined mostly by thelower layer 588. In another example, a spacer such as 576 inFig. 14 is included between the layers in the stiff regions. Note in this example, a spacer may be included by an intermediate lamination or coating step in which the spacer is placed on a balloon rather than being positioned and included as part of a braid preform. - In an example, the
stiff portions 592 can be made of a radio-opaque material to enhance visualization of the balloon in situ. In an embodiment, thestiff portions 592 have a radio-opaque material coated on them to make them radio-opaque. This may allow the use of radio-opaque materials which might be too inflexible or otherwise difficult to integrate in a medical balloon. - In any of the examples described herein, the balloon is coated with a radio-opaque material. The radio-opaque material are integrated into the balloon, such as by the braiding process as discussed in the instant specification. Such radio-opaque materials can be restricted the relatively flat (non-folding) portions of the balloon wall which may permit the use of materials that cannot tolerate as high a degree of strain if used in portions that are folded tightly.
- Examples of materials that may be used for the matrix and or liner of the above examples include polycaprolactam, polyesters, polyethers, polyamides, polyurethanes, polyimides, ABS copolymers, polyester/polyether block copolymers, ionomer resins, liquid crystal polymers, and rigid rod polymers.
- Applications of the medical balloon examples include vascular dilatation, stent delivery, drug delivery, delivery and operation of sensors and surgical devices such as blades, and the like. Exemplary design parameters of balloons include balloons with burst pressures of 6,895 bar (100 psi) or more.
- Note that although many of the examples discussed and illustrated above were based on triaxial braid structures, biaxial the benefits of the examples may be applied to other braid patterns. Such patterns include multilayer and so-called thick braids or three-dimensional braids.
- Also note that there are types of braiding technology that allow a high degree of flexibility and control for forming braids. Suitable technology and techniques as may be combined with the teachings of this disclosure may be found in:
US Patent Nos. 5,085,252 ;5,465,760 ;6,129,122 ;6,315,007 6,439,096 - Also note that although the examples are described in terms of braids as a base technology, it possible to achieve the same benefits using a weaving or combination weaving and braiding technology. In such cases the stiffening properties may be derive from warp and/or weft yarns in a fabric weave. Moreover, current technologies for knitting, weaving, and braiding have blurred the boundaries of these categories so the terms should not be taken as limiting.
- In the instant disclosure, the words "yarn" and "fiber" are used interchangeably. The term "yarn" is commonly used in the field of braiding. The term is not intended to limit the material, composition, or structure of the fiber material that is used in any of the above-described examples. In addition, the structures disclosed may be created in various ways including mechanisms that do not include braiding. Thus, even where the term "yarn" is used and/or where braiding is described as a preferred means of forming a structure, the uses are not necessarily intended to limit the structures described to ones formed by braiding.
- A variety of materials can be used for the fibers/yams. Examples include, but are not limited to, high strength inelastic fibers such as Kevlar, Vectran, Spectra, Dacron, Dyneema, Terlon (PBT), Zylon (PBO), Polyimide (PIM), ultra high molecular weight polyethylene, and the like. In addition, fibers/yarns may have non-circular cross-sections. For example, flat fibers/yarns may provide superior amenability to folding.
- In any of the above examples, the balloon or the braid pre-form can be coated with suitable materials (paint) to render the resulting medical balloon radio-opaque. Suitable coatings are known, for example, as discussed in
US Patent 6,599,448 . In addition, some or all of the yarns or fibers employed may be radio-opaque to enhance the radio-opacity of the resulting balloon. This can be performed, for example, by applying a coating to the balloon, or fibers using vapor deposition or electro-energy deposition, for example, a metal coating such as tantalum or other materials such as barium sulfate. Also, a metallic layer can be used in treatment such as to provide a means for creating an electric field inside the body for sterilizing a site. Examples of such applications and biocides are described inUS Patent 6,258,249 (Charles Lee Simpson for "Sterilization of surgical sites"). - Referring now to
Fig. 16 , a medical balloon 601 may be of any suitable structure including monolithic polymer, composite with fibers, including braided fibers, laminates, or any other suitable structure. The medical balloon 601 has conductive surfaces (not shown in this view) on its external surfaces to allow the generation of an electric field for performing the biocidal function described inUS Patent No. 6,258,249 . In the example shown, the balloon 601 is inserted between twosurfaces host 604. The balloon 601 may be coated with a biocide as described inUS Patent No. 6,258,249 patent and the conductive surfaces connected to a source of voltage (alternating or direct) to destroy biofilm or other susceptible infectious material. The balloon 601 may be inserted and moved around, with an internal pressure that allows the balloon 6021 to conform to thesurfaces cylindrical balloon 614 may be inserted in a lumen structure 616 of thehost 604 to perform the same function. For example, the lumen could be a blood vessel, a urethra, a duct, or any other cylindrical cavity or conduit. Theinternal space balloon 601, 614 can be filled with any suitable material such as, for example, saline. - Further details of the balloons of
Fig. 16 are shown inFigs. 17A, 17B , as well asFigs. 18 through 20 , according to various alternative examples. Referring now toFigs. 17A and 17B , the surface ofballoons conductive strips balloon balloon 660. The strips are labeled 1 and 2 to indicate which of two poles of a voltage source (not shown) they are connected to. Thestrips - In an alternative example, the
strips balloon Figs. 17A and 17B . The conductive surfaces of theballoon terminals -
Fig. 18 shows an example of parallel conductive yarns, in this case, thelongitudinal yarns 704 are held in position and maintained parallel by the triaxial braiding pattern of thefabric 706. Theconductive yarns 704 may be interspersed with non-conductive yarns. In alternative examples, some or all of the diagonal yarns 703 may be made conductive. The polarity may alternate as every N yarns in any desired manner to achieve a desired spacing of the oppositely polarized yarns. Note that the conductive yarns may be of metal, carbon-impregnated fiber, carbon composite, or any suitable material. Note also that a braid is not required to employ conductive fibers in this manner. For example yarns can be laid into a mold and a balloon molded into it as described inUS Patent No. 6,746,425 . Any adhesive or matrix remaining on the surface can be polished off to expose the conductive yarns. - Referring to
Figs. 19 and 20 , the conductive yarns can be provided with insulation in parts to provide that the conductors are exposed only at desired locations on a balloon. For example,conductors 752 and 754 (which indicate sets of alternating polarity), in thearray 706, depicted inFig. 19 , are exposed only in a region 756 (or 786 in theFig. 19 example). A region 757 exposes theconductors conductors 752 of one polarity may be exposed by lack of insulation in a different axial position than theconductors 754 of the other polarity to facilitate connection to voltage sources as illustrated inFig. 20 . Here,region 782 is a region in which the yarns of one polarity are exposed and the yarns of the other polarity are insulated.Region 780 is a region in which the yarns of the other polarity are exposed and the yarns of the one polarity are insulated. - In this way conductive take-offs 781 and 783, can be soldered or otherwise electrically connected to the conductive yarns without causing shorting.
Claims (2)
- A medical balloon, comprising:a balloon body (215) having an array of reinforcement fibers exposed on an external surface thereon;at least some of the reinforcement fibers incorporating a radio-opaque material,characterised in that: the at least some of the reinforcement fibers are coated with a radio-opaque material, and in that: the at least some of the reinforcement fibers are zero-angle fibers of a triaxial braid (100).
- A balloon as in claim 1, wherein the fibers form a braided pattern (100).
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP18172890.8A EP3384953B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US82923106P | 2006-10-12 | 2006-10-12 | |
| EP07871161A EP2073885B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
| EP12158569.9A EP2462975B1 (en) | 2006-10-12 | 2007-10-12 | Inflatables structure with braided layer |
Related Parent Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP07871161A Division EP2073885B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
| EP12158569.9A Division EP2462975B1 (en) | 2006-10-12 | 2007-10-12 | Inflatables structure with braided layer |
| EP12158569.9A Division-Into EP2462975B1 (en) | 2006-10-12 | 2007-10-12 | Inflatables structure with braided layer |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18172890.8A Division EP3384953B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2711045A2 EP2711045A2 (en) | 2014-03-26 |
| EP2711045A3 EP2711045A3 (en) | 2014-06-18 |
| EP2711045B1 true EP2711045B1 (en) | 2018-06-13 |
Family
ID=39430419
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18172890.8A Active EP3384953B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
| EP07871161A Active EP2073885B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
| EP12158569.9A Active EP2462975B1 (en) | 2006-10-12 | 2007-10-12 | Inflatables structure with braided layer |
| EP13198920.4A Active EP2711045B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
Family Applications Before (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP18172890.8A Active EP3384953B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
| EP07871161A Active EP2073885B1 (en) | 2006-10-12 | 2007-10-12 | Inflatable structure with braided layer |
| EP12158569.9A Active EP2462975B1 (en) | 2006-10-12 | 2007-10-12 | Inflatables structure with braided layer |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US9440055B2 (en) |
| EP (4) | EP3384953B1 (en) |
| JP (3) | JP5337037B2 (en) |
| CA (1) | CA2663961C (en) |
| ES (1) | ES2764436T3 (en) |
| WO (1) | WO2008063782A2 (en) |
Families Citing this family (51)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8845672B2 (en) | 2002-05-09 | 2014-09-30 | Reshape Medical, Inc. | Balloon system and methods for treating obesity |
| EP1846077A4 (en) * | 2005-02-09 | 2009-11-25 | Angiodynamics Inc | Reinforced balloon for a catheter |
| US20070100368A1 (en) * | 2005-10-31 | 2007-05-03 | Quijano Rodolfo C | Intragastric space filler |
| ES2623654T3 (en) | 2006-12-18 | 2017-07-11 | C.R. Bard, Inc. | Balloon with dividing fabric layers and method for braiding on three-dimensional shapes |
| WO2008095046A2 (en) * | 2007-01-30 | 2008-08-07 | Loma Vista Medical, Inc., | Biological navigation device |
| US8226602B2 (en) * | 2007-03-30 | 2012-07-24 | Reshape Medical, Inc. | Intragastric balloon system and therapeutic processes and products |
| US8142469B2 (en) * | 2007-06-25 | 2012-03-27 | Reshape Medical, Inc. | Gastric space filler device, delivery system, and related methods |
| EP2008598A1 (en) * | 2007-06-29 | 2008-12-31 | Edward A. Loeser | Composite fiber electrosurgical instrument |
| JP5304006B2 (en) * | 2008-04-17 | 2013-10-02 | 株式会社カネカ | Compound balloon for catheter and method for producing the same |
| JP5304005B2 (en) * | 2008-04-17 | 2013-10-02 | 株式会社カネカ | Compound balloon for catheter and method for producing the same |
| WO2009149108A1 (en) * | 2008-06-02 | 2009-12-10 | Loma Vista Medical, Inc. | Inflatable medical devices |
| JP5428209B2 (en) * | 2008-06-11 | 2014-02-26 | 株式会社カネカ | Medical balloon catheter |
| BRPI0919958A2 (en) * | 2008-10-30 | 2015-12-08 | R4 Vascular Inc | Deformable and rupture-resistant radiopaque catheter balloon and methods for its use in an intravascular surgical procedure |
| US9174031B2 (en) * | 2009-03-13 | 2015-11-03 | Reshape Medical, Inc. | Device and method for deflation and removal of implantable and inflatable devices |
| EP2414025A4 (en) * | 2009-04-03 | 2017-08-02 | ReShape Medical, Inc. | Improved intragastric space fillers and methods of manufacturing including in vitro testing |
| US9358143B2 (en) | 2009-07-22 | 2016-06-07 | Reshape Medical, Inc. | Retrieval mechanisms for implantable medical devices |
| WO2011011741A2 (en) | 2009-07-23 | 2011-01-27 | Reshape Medical, Inc. | Inflation and deflation mechanisms for inflatable medical devices |
| EP2456505B1 (en) | 2009-07-23 | 2017-05-24 | ReShape Medical, Inc. | Deflation and removal of implantable medical devices |
| EP2480279A4 (en) | 2009-09-24 | 2017-11-15 | Reshape Medical, Inc. | Normalization and stabilization of balloon surfaces for deflation |
| US8992553B2 (en) | 2009-10-01 | 2015-03-31 | Cardioniti | Cutting balloon assembly and method of manufacturing thereof |
| EP2533846B1 (en) | 2010-02-08 | 2018-08-22 | ReShape Medical LLC | Materials and methods for improved intragastric balloon devices |
| US9622896B2 (en) | 2010-02-08 | 2017-04-18 | Reshape Medical, Inc. | Enhanced aspiration processes and mechanisms for instragastric devices |
| WO2011106637A1 (en) | 2010-02-25 | 2011-09-01 | Reshape Medical, Inc. | Improved and enhanced explant processes and mechanisms for intragastric devices |
| US9629740B2 (en) | 2010-04-06 | 2017-04-25 | Reshape Medical, Inc. | Inflation devices for intragastric devices with improved attachment and detachment and associated systems and methods |
| US9227041B2 (en) * | 2010-04-09 | 2016-01-05 | Boston Scientific Scimed, Inc. | Balloon catheters with fibers for delivery of therapeutic agent and methods of making the same |
| EP2593171B1 (en) | 2010-07-13 | 2019-08-28 | Loma Vista Medical, Inc. | Inflatable medical devices |
| US20130261654A1 (en) * | 2010-10-07 | 2013-10-03 | Reshape Medical, Inc. | Materials and methods for improved intragastric balloon devices |
| US10188436B2 (en) | 2010-11-09 | 2019-01-29 | Loma Vista Medical, Inc. | Inflatable medical devices |
| GB2501064B (en) * | 2012-03-27 | 2014-06-18 | Cook Medical Technologies Llc | Medical balloon with incorporated fibres |
| US20160135939A1 (en) * | 2012-12-17 | 2016-05-19 | Atex Technologies, Inc. | Medical textile and methods of making the same |
| WO2014123983A2 (en) * | 2013-02-05 | 2014-08-14 | Loma Vista Medical, Inc. | Inflatable medical devices |
| US10729570B2 (en) | 2013-09-17 | 2020-08-04 | West Coast Catheter, Inc. | Medical balloon with varied compliance |
| US9782571B2 (en) | 2014-01-30 | 2017-10-10 | Chuter A. M. Timothy | Flexible high-pressure angioplasty balloons |
| US10201685B2 (en) | 2013-11-13 | 2019-02-12 | West Coast Catheter, Inc. | High-pressure balloons |
| US9149612B2 (en) | 2013-11-13 | 2015-10-06 | West Coast Catheter, Inc. | Flexible high-pressure balloons |
| US10568686B2 (en) * | 2013-11-21 | 2020-02-25 | Biosense Webster (Israel) Ltd. | Multi-electrode balloon catheter with circumferential and point electrodes |
| RU2540755C1 (en) * | 2013-12-19 | 2015-02-10 | Российская Федерация от имени которой выступает Министерство промышленности и торговли Российской Федерации (Минпромторг России) | Braided preform for manufacture of composite articles of complex shape |
| US10280951B2 (en) | 2014-03-02 | 2019-05-07 | Drexel University | Articulating devices |
| US20150328027A1 (en) * | 2014-05-16 | 2015-11-19 | Terumo Kabushiki Kaisha | Method and apparatus for treating urethral stricture |
| WO2017044982A1 (en) * | 2015-09-10 | 2017-03-16 | Ikonano Venture Partners, Llc | Polymeric electrospun embolization device and methods of use |
| WO2017062753A1 (en) * | 2015-10-07 | 2017-04-13 | Mayo Foundation For Medical Education And Research | Electroporation for obesity or diabetes treatment |
| US10208410B2 (en) * | 2015-11-13 | 2019-02-19 | Federal-Mogul Powertrain Llc | Braided textile sleeve with axially collapsible, anti-kinking feature and method of construction thereof |
| DE102017210821A1 (en) * | 2017-06-27 | 2018-12-27 | Adidas Ag | Specially-designed braided hose |
| US20190257326A1 (en) * | 2018-02-19 | 2019-08-22 | The Regents Of The University Of Michigan | Method For Mass-Customization And Multi-Axial Motion With A Knit-Constrained Actuator |
| US11944331B2 (en) | 2021-02-26 | 2024-04-02 | Fastwave Medical Inc. | Intravascular lithotripsy |
| US11911056B2 (en) | 2021-02-26 | 2024-02-27 | Fastwave Medical Inc. | Intravascular lithotripsy |
| US11484327B2 (en) | 2021-02-26 | 2022-11-01 | Fastwave Medical Inc. | Intravascular lithotripsy |
| WO2023235665A1 (en) | 2022-06-01 | 2023-12-07 | Fastwave Medical Inc. | Intravascular lithotripsy |
| EP4311527A1 (en) * | 2022-07-29 | 2024-01-31 | Intressa Vascular S.A. | Implantable 3d braided endovascular prosthesis for remodeling of dissected aorta |
| CN115350383B (en) * | 2022-08-30 | 2023-05-23 | 铂珑生物科技(苏州)有限公司 | Balloon catheter system |
| CN117482365B (en) * | 2023-12-27 | 2024-04-09 | 杭州德晋医疗科技有限公司 | Balloon dilation catheter |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020010489A1 (en) * | 2000-07-24 | 2002-01-24 | Jeffrey Grayzel | Stiffened balloon catheter for dilatation and stenting |
Family Cites Families (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3646247A (en) * | 1971-01-11 | 1972-02-29 | Electroweave Inc | Foldable woven multistrand electrical cable |
| CA1266412A (en) * | 1984-10-24 | 1990-03-06 | J. Richard Spears | Method and apparatus for angioplasty |
| US4816028A (en) * | 1987-07-01 | 1989-03-28 | Indu Kapadia | Woven vascular graft |
| EP0414350B1 (en) | 1989-08-25 | 1994-08-24 | C.R. Bard, Inc. | Pleated balloon dilatation catheter and method of manufacture |
| US5357839A (en) | 1990-07-12 | 1994-10-25 | Albany International Corp. | Solid braid structure |
| US5085252A (en) | 1990-08-29 | 1992-02-04 | North Carolina State University | Method of forming variable cross-sectional shaped three-dimensional fabrics |
| US5409467A (en) | 1992-10-02 | 1995-04-25 | Board Of Regents, The University Of Texas System | Antimicrobial catheter |
| US5913894A (en) | 1994-12-05 | 1999-06-22 | Meadox Medicals, Inc. | Solid woven tubular prosthesis |
| US5746745A (en) * | 1993-08-23 | 1998-05-05 | Boston Scientific Corporation | Balloon catheter |
| US5465760A (en) | 1993-10-25 | 1995-11-14 | North Carolina State University | Multi-layer three-dimensional fabric and method for producing |
| US5458572A (en) * | 1994-07-01 | 1995-10-17 | Boston Scientific Corp. | Catheter with balloon folding into predetermined configurations and method of manufacture |
| NL9500468A (en) * | 1995-03-08 | 1996-10-01 | Cordis Europ | Balloon catheter and method of making it. |
| US5853389A (en) * | 1996-03-07 | 1998-12-29 | Cordis Corporation | Balloon catheter and method for manufacturing |
| US6592617B2 (en) * | 1996-04-30 | 2003-07-15 | Boston Scientific Scimed, Inc. | Three-dimensional braided covered stent |
| US6746425B1 (en) | 1996-06-14 | 2004-06-08 | Futuremed Interventional | Medical balloon |
| US5772848A (en) | 1996-12-03 | 1998-06-30 | Albany International Corp. | Braided base fabrics for shoe press belts |
| US6012457A (en) | 1997-07-08 | 2000-01-11 | The Regents Of The University Of California | Device and method for forming a circumferential conduction block in a pulmonary vein |
| WO1999039648A1 (en) * | 1998-02-10 | 1999-08-12 | Dubrul William R | Entrapping apparatus and method for use |
| US6129122A (en) | 1999-06-16 | 2000-10-10 | 3Tex, Inc. | Multiaxial three-dimensional (3-D) circular woven fabric |
| US6575966B2 (en) | 1999-08-23 | 2003-06-10 | Cryocath Technologies Inc. | Endovascular cryotreatment catheter |
| US6482309B1 (en) * | 1999-10-20 | 2002-11-19 | Oxibio, Inc. | Electrolytic generation of nascent iodine as a method of treatment and for the prevention of infections associated with medical implant devices |
| US6258249B1 (en) | 1999-11-10 | 2001-07-10 | Sulzer Carbomedics Inc. | Sterilization of surgical sites |
| JP3651336B2 (en) | 1999-11-24 | 2005-05-25 | 東レ・デュポン株式会社 | Reinforcing fiber sheet |
| US6599448B1 (en) | 2000-05-10 | 2003-07-29 | Hydromer, Inc. | Radio-opaque polymeric compositions |
| JP2003534064A (en) * | 2000-05-26 | 2003-11-18 | ヴァルステン・メディカル・エス・アー | Balloon catheter |
| AU2002211639A1 (en) * | 2000-10-09 | 2002-04-22 | Tricardia, L.L.C. | Material useable for medical balloons and catheters |
| US6439096B1 (en) | 2000-11-28 | 2002-08-27 | 3Tex, Inc. | Automated 3-D braiding machine and method |
| US20020161388A1 (en) * | 2001-02-27 | 2002-10-31 | Samuels Sam L. | Elastomeric balloon support fabric |
| US6315007B1 (en) | 2001-03-23 | 2001-11-13 | 3Tex, Inc. | High speed three-dimensional weaving method and machine |
| US6638245B2 (en) | 2001-06-26 | 2003-10-28 | Concentric Medical, Inc. | Balloon catheter |
| JP2003310762A (en) * | 2002-02-19 | 2003-11-05 | Kawasumi Lab Inc | Balloon adaptor and balloon catheter with the same |
| US7985234B2 (en) * | 2002-02-27 | 2011-07-26 | Boston Scientific Scimed, Inc. | Medical device |
| US20050123702A1 (en) * | 2003-12-03 | 2005-06-09 | Jim Beckham | Non-compliant medical balloon having a longitudinal fiber layer |
| CA2552198A1 (en) | 2003-12-31 | 2005-07-21 | Biosense Webster, Inc. | Circumferential ablation device assembly with an expandable member |
| WO2006034396A2 (en) | 2004-09-21 | 2006-03-30 | Stout Medical Group, L.P. | Balloon and methods of making and using |
| US7309324B2 (en) * | 2004-10-15 | 2007-12-18 | Futuremed Interventional, Inc. | Non-compliant medical balloon having an integral woven fabric layer |
| EP1846077A4 (en) | 2005-02-09 | 2009-11-25 | Angiodynamics Inc | Reinforced balloon for a catheter |
| US7691082B2 (en) * | 2005-07-01 | 2010-04-06 | Boston Scientific Scimed, Inc. | Medical devices |
-
2007
- 2007-10-12 ES ES18172890T patent/ES2764436T3/en active Active
- 2007-10-12 WO PCT/US2007/081264 patent/WO2008063782A2/en active Application Filing
- 2007-10-12 CA CA2663961A patent/CA2663961C/en active Active
- 2007-10-12 US US12/444,796 patent/US9440055B2/en active Active
- 2007-10-12 EP EP18172890.8A patent/EP3384953B1/en active Active
- 2007-10-12 EP EP07871161A patent/EP2073885B1/en active Active
- 2007-10-12 JP JP2009532609A patent/JP5337037B2/en active Active
- 2007-10-12 EP EP12158569.9A patent/EP2462975B1/en active Active
- 2007-10-12 EP EP13198920.4A patent/EP2711045B1/en active Active
-
2013
- 2013-05-22 JP JP2013107880A patent/JP5820431B2/en active Active
-
2015
- 2015-10-01 JP JP2015195880A patent/JP6247266B2/en active Active
-
2016
- 2016-09-12 US US15/262,767 patent/US10143577B2/en active Active
-
2018
- 2018-11-13 US US16/189,324 patent/US11033414B2/en active Active
-
2021
- 2021-06-11 US US17/345,035 patent/US11766345B2/en active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20020010489A1 (en) * | 2000-07-24 | 2002-01-24 | Jeffrey Grayzel | Stiffened balloon catheter for dilatation and stenting |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2711045A2 (en) | 2014-03-26 |
| US20190231570A1 (en) | 2019-08-01 |
| JP2013236935A (en) | 2013-11-28 |
| US10143577B2 (en) | 2018-12-04 |
| JP6247266B2 (en) | 2017-12-13 |
| WO2008063782A2 (en) | 2008-05-29 |
| EP2711045A3 (en) | 2014-06-18 |
| US20100023047A1 (en) | 2010-01-28 |
| WO2008063782A3 (en) | 2009-05-14 |
| EP2462975B1 (en) | 2015-09-09 |
| US11766345B2 (en) | 2023-09-26 |
| JP2016041254A (en) | 2016-03-31 |
| ES2764436T3 (en) | 2020-06-03 |
| EP2073885B1 (en) | 2012-06-20 |
| JP5337037B2 (en) | 2013-11-06 |
| EP2462975A1 (en) | 2012-06-13 |
| JP5820431B2 (en) | 2015-11-24 |
| US9440055B2 (en) | 2016-09-13 |
| EP2073885A4 (en) | 2010-08-04 |
| JP2010514462A (en) | 2010-05-06 |
| EP3384953B1 (en) | 2019-11-27 |
| US20160374841A1 (en) | 2016-12-29 |
| US11033414B2 (en) | 2021-06-15 |
| EP2073885A2 (en) | 2009-07-01 |
| US20210298932A1 (en) | 2021-09-30 |
| EP3384953A1 (en) | 2018-10-10 |
| CA2663961A1 (en) | 2008-05-29 |
| CA2663961C (en) | 2015-07-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11766345B2 (en) | Inflatable structure with braided layer | |
| US11173288B2 (en) | Non-compliant medical balloon having braided or knitted reinforcement | |
| US9802027B2 (en) | Semi-compliant medical balloon | |
| JP4009324B2 (en) | Reinforced catheter with a moldable distal tip | |
| JP5054539B2 (en) | Method for manufacturing a medical device formed using a sacrificial structure | |
| JP2008534032A (en) | Reinforcing balloon for catheter | |
| US20070110935A1 (en) | Silk reinforcement of expandable medical balloons | |
| JP2012507372A (en) | Fracture resistant compliant radiopaque catheter balloon and method for using it in endovascular surgical procedures | |
| JP5304006B2 (en) | Compound balloon for catheter and method for producing the same | |
| JP7321254B2 (en) | Inflatable medical balloon containing sigmoidal fibers | |
| JP2012065804A (en) | Catheter balloon and balloon manufacturing method | |
| WO2017106358A1 (en) | Rupture-resistant compliant radiopaque catheter balloon and methods for use of same in an intravascular surgical procedure |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2073885 Country of ref document: EP Kind code of ref document: P Ref document number: 2462975 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61M 25/10 20130101AFI20140513BHEP |
|
| 17P | Request for examination filed |
Effective date: 20141017 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| 17Q | First examination report despatched |
Effective date: 20170406 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R079 Ref document number: 602007055136 Country of ref document: DE Free format text: PREVIOUS MAIN CLASS: A61M0025100000 Ipc: A61M0029020000 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: A61M 29/02 20060101AFI20171218BHEP Ipc: D04C 1/02 20060101ALI20171218BHEP Ipc: A61M 25/10 20130101ALI20171218BHEP Ipc: A61F 2/958 20130101ALI20171218BHEP |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20180209 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 2073885 Country of ref document: EP Kind code of ref document: P Ref document number: 2462975 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP Ref country code: AT Ref legal event code: REF Ref document number: 1007837 Country of ref document: AT Kind code of ref document: T Effective date: 20180615 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602007055136 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 12 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180913 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180914 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 1007837 Country of ref document: AT Kind code of ref document: T Effective date: 20180613 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20181013 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602007055136 Country of ref document: DE |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20190314 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181012 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181031 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181031 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181012 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181012 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180613 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20071012 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20230920 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20230920 Year of fee payment: 17 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20240919 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20240919 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20240919 Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20240919 Year of fee payment: 18 |