EP2673265B1 - Formation of n-protected bis-3,6-(4-aminoalkyl) -2,5,diketopiperazine - Google Patents
Formation of n-protected bis-3,6-(4-aminoalkyl) -2,5,diketopiperazine Download PDFInfo
- Publication number
- EP2673265B1 EP2673265B1 EP12744320.8A EP12744320A EP2673265B1 EP 2673265 B1 EP2673265 B1 EP 2673265B1 EP 12744320 A EP12744320 A EP 12744320A EP 2673265 B1 EP2673265 B1 EP 2673265B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- diketopiperazine
- water
- mixture
- trifluoroacetyl
- lysine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 230000015572 biosynthetic process Effects 0.000 title claims description 20
- BXRNXXXXHLBUKK-UHFFFAOYSA-N piperazine-2,5-dione Chemical compound O=C1CNC(=O)CN1 BXRNXXXXHLBUKK-UHFFFAOYSA-N 0.000 title description 11
- DLYUQMMRRRQYAE-UHFFFAOYSA-N tetraphosphorus decaoxide Chemical group O1P(O2)(=O)OP3(=O)OP1(=O)OP2(=O)O3 DLYUQMMRRRQYAE-UHFFFAOYSA-N 0.000 claims description 36
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 34
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 claims description 29
- 239000000203 mixture Substances 0.000 claims description 25
- 239000003054 catalyst Substances 0.000 claims description 23
- 238000000034 method Methods 0.000 claims description 23
- 150000001413 amino acids Chemical class 0.000 claims description 22
- 238000003786 synthesis reaction Methods 0.000 claims description 19
- -1 proglyme Chemical compound 0.000 claims description 18
- 239000004472 Lysine Substances 0.000 claims description 15
- 239000002904 solvent Substances 0.000 claims description 13
- 238000010438 heat treatment Methods 0.000 claims description 8
- NGXZRXLIUBALRQ-AKGZTFGVSA-N (2s)-2,6-diamino-8,8,8-trifluoro-7-oxooctanoic acid Chemical compound OC(=O)[C@@H](N)CCCC(N)C(=O)C(F)(F)F NGXZRXLIUBALRQ-AKGZTFGVSA-N 0.000 claims description 7
- SBZXBUIDTXKZTM-UHFFFAOYSA-N diglyme Chemical compound COCCOCCOC SBZXBUIDTXKZTM-UHFFFAOYSA-N 0.000 claims description 7
- 238000010791 quenching Methods 0.000 claims description 7
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 claims description 6
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 5
- 230000000171 quenching effect Effects 0.000 claims description 5
- LZDKZFUFMNSQCJ-UHFFFAOYSA-N 1,2-diethoxyethane Chemical compound CCOCCOCC LZDKZFUFMNSQCJ-UHFFFAOYSA-N 0.000 claims description 3
- RRQYJINTUHWNHW-UHFFFAOYSA-N 1-ethoxy-2-(2-ethoxyethoxy)ethane Chemical compound CCOCCOCCOCC RRQYJINTUHWNHW-UHFFFAOYSA-N 0.000 claims description 3
- 239000003960 organic solvent Substances 0.000 claims description 3
- 125000004044 trifluoroacetyl group Chemical group FC(C(=O)*)(F)F 0.000 claims description 3
- RDHOLFGDARNAPR-BKLSDQPFSA-N (2s)-2-amino-4-(aminomethyl)-6,6,6-trifluoro-5-oxohexanoic acid Chemical compound FC(F)(F)C(=O)C(CN)C[C@H](N)C(O)=O RDHOLFGDARNAPR-BKLSDQPFSA-N 0.000 claims description 2
- 125000004103 aminoalkyl group Chemical group 0.000 claims description 2
- 235000001014 amino acid Nutrition 0.000 description 20
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 13
- 239000013543 active substance Substances 0.000 description 13
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 12
- 238000001914 filtration Methods 0.000 description 12
- 125000006239 protecting group Chemical group 0.000 description 12
- 238000007363 ring formation reaction Methods 0.000 description 12
- 239000007787 solid Substances 0.000 description 11
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 239000011541 reaction mixture Substances 0.000 description 9
- 230000004888 barrier function Effects 0.000 description 8
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 8
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 229910052757 nitrogen Inorganic materials 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 4
- QWVGKYWNOKOFNN-UHFFFAOYSA-N o-cresol Chemical compound CC1=CC=CC=C1O QWVGKYWNOKOFNN-UHFFFAOYSA-N 0.000 description 4
- IWDCLRJOBJJRNH-UHFFFAOYSA-N p-cresol Chemical compound CC1=CC=C(O)C=C1 IWDCLRJOBJJRNH-UHFFFAOYSA-N 0.000 description 4
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 4
- BHCRIIUZDBIUKV-DTIOYNMSSA-N (2S)-2-amino-4-(aminomethyl)-5-oxo-5-phenylmethoxypentanoic acid Chemical compound OC(=O)[C@@H](N)CC(CN)C(=O)OCC1=CC=CC=C1 BHCRIIUZDBIUKV-DTIOYNMSSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 3
- 150000001408 amides Chemical class 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 230000035484 reaction time Effects 0.000 description 3
- OJTJKAUNOLVMDX-LBPRGKRZSA-N (2s)-6-amino-2-(phenylmethoxycarbonylamino)hexanoic acid Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)OCC1=CC=CC=C1 OJTJKAUNOLVMDX-LBPRGKRZSA-N 0.000 description 2
- KXDHJXZQYSOELW-UHFFFAOYSA-N Carbamic acid Chemical group NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 description 2
- IAZDPXIOMUYVGZ-WFGJKAKNSA-N Dimethyl sulfoxide Chemical compound [2H]C([2H])([2H])S(=O)C([2H])([2H])[2H] IAZDPXIOMUYVGZ-WFGJKAKNSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- QLZHNIAADXEJJP-UHFFFAOYSA-N Phenylphosphonic acid Chemical compound OP(O)(=O)C1=CC=CC=C1 QLZHNIAADXEJJP-UHFFFAOYSA-N 0.000 description 2
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000004821 distillation Methods 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 230000002496 gastric effect Effects 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229960003104 ornithine Drugs 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- NSETWVJZUWGCKE-UHFFFAOYSA-N propylphosphonic acid Chemical compound CCCP(O)(O)=O NSETWVJZUWGCKE-UHFFFAOYSA-N 0.000 description 2
- 238000010189 synthetic method Methods 0.000 description 2
- STCOOQWBFONSKY-UHFFFAOYSA-N tributyl phosphate Chemical compound CCCCOP(=O)(OCCCC)OCCCC STCOOQWBFONSKY-UHFFFAOYSA-N 0.000 description 2
- 239000008096 xylene Substances 0.000 description 2
- 150000003738 xylenes Chemical class 0.000 description 2
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 description 1
- 125000006648 (C1-C8) haloalkyl group Chemical group 0.000 description 1
- 238000005160 1H NMR spectroscopy Methods 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- 125000004648 C2-C8 alkenyl group Chemical group 0.000 description 1
- 102000055006 Calcitonin Human genes 0.000 description 1
- 108060001064 Calcitonin Proteins 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 229920001499 Heparinoid Polymers 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- PZZHRSVBHRVIMI-UHFFFAOYSA-N NC(CCCCNC(C(F)(F)F)=O)C(O)=O Chemical compound NC(CCCCNC(C(F)(F)F)=O)C(O)=O PZZHRSVBHRVIMI-UHFFFAOYSA-N 0.000 description 1
- YKAUOWFSSCHPMG-UHFFFAOYSA-N O=C(C(F)(F)F)NCCCCC(C(NC1CCCCNC(C(F)(F)F)=O)=O)NC1=O Chemical compound O=C(C(F)(F)F)NCCCCC(C(NC1CCCCNC(C(F)(F)F)=O)=O)NC1=O YKAUOWFSSCHPMG-UHFFFAOYSA-N 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 238000005903 acid hydrolysis reaction Methods 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 150000001370 alpha-amino acid derivatives Chemical class 0.000 description 1
- 235000008206 alpha-amino acids Nutrition 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- BBBFJLBPOGFECG-VJVYQDLKSA-N calcitonin Chemical compound N([C@H](C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(N)=O)C(C)C)C(=O)[C@@H]1CSSC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 BBBFJLBPOGFECG-VJVYQDLKSA-N 0.000 description 1
- 229960004015 calcitonin Drugs 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 102000038379 digestive enzymes Human genes 0.000 description 1
- 108091007734 digestive enzymes Proteins 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000000921 elemental analysis Methods 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- GATNOFPXSDHULC-UHFFFAOYSA-N ethylphosphonic acid Chemical compound CCP(O)(O)=O GATNOFPXSDHULC-UHFFFAOYSA-N 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 239000002554 heparinoid Substances 0.000 description 1
- 229940025770 heparinoids Drugs 0.000 description 1
- 125000003187 heptyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 150000002829 nitrogen Chemical class 0.000 description 1
- 125000002347 octyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 125000001147 pentyl group Chemical group C(CCCC)* 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 239000002831 pharmacologic agent Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/02—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D241/00—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings
- C07D241/02—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings
- C07D241/06—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having one or two double bonds between ring members or between ring members and non-ring members
- C07D241/08—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having one or two double bonds between ring members or between ring members and non-ring members with oxygen atoms directly attached to ring carbon atoms
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J27/00—Catalysts comprising the elements or compounds of halogens, sulfur, selenium, tellurium, phosphorus or nitrogen; Catalysts comprising carbon compounds
- B01J27/14—Phosphorus; Compounds thereof
- B01J27/16—Phosphorus; Compounds thereof containing oxygen, i.e. acids, anhydrides and their derivates with N, S, B or halogens without carriers or on carriers based on C, Si, Al or Zr; also salts of Si, Al and Zr
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/02—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides
- B01J31/0215—Sulfur-containing compounds
- B01J31/0225—Sulfur-containing compounds comprising sulfonic acid groups or the corresponding salts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/02—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides
- B01J31/0234—Nitrogen-, phosphorus-, arsenic- or antimony-containing compounds
- B01J31/0255—Phosphorus containing compounds
- B01J31/0257—Phosphorus acids or phosphorus acid esters
- B01J31/0258—Phosphoric acid mono-, di- or triesters ((RO)(R'O)2P=O), i.e. R= C, R'= C, H
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J31/00—Catalysts comprising hydrides, coordination complexes or organic compounds
- B01J31/02—Catalysts comprising hydrides, coordination complexes or organic compounds containing organic compounds or metal hydrides
- B01J31/0234—Nitrogen-, phosphorus-, arsenic- or antimony-containing compounds
- B01J31/0255—Phosphorus containing compounds
- B01J31/0257—Phosphorus acids or phosphorus acid esters
- B01J31/0259—Phosphorus acids or phosphorus acid esters comprising phosphorous acid (-ester) groups ((RO)P(OR')2) or the isomeric phosphonic acid (-ester) groups (R(R'O)2P=O), i.e. R= C, R'= C, H
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2231/00—Catalytic reactions performed with catalysts classified in B01J31/00
- B01J2231/40—Substitution reactions at carbon centres, e.g. C-C or C-X, i.e. carbon-hetero atom, cross-coupling, C-H activation or ring-opening reactions
Definitions
- the present invention relates to compositions for delivering active agents, and particularly biologically active agents.
- Disclosed embodiments are in the field of chemical synthesis and more particularly are related to improved synthetic methods for the preparation and purification of 3,6-di-substituted-2,5-diketopiperazines.
- Drug delivery is a persistent problem in the administration of active agents to patients.
- Conventional means for delivering active agents are often severely limited by biological, chemical, and physical barriers. Typically, these barriers are imposed by the environment through which delivery occurs, the environment of the target for delivery, or the target itself.
- Biologically active agents are particularly vulnerable to such barriers.
- barriers are imposed by the body. Examples of physical barriers are the skin and various organ membranes that must be traversed before reaching a target.
- Chemical barriers include, but are not limited to, pH variations, lipid bi-layers, and degrading enzymes.
- Oral delivery of many biologically active agents would be the route of choice for administration to animals if not for biological, chemical, and physical barriers such as varying pH in the gastrointestinal (Gl) tract, powerful digestive enzymes, and active agent impermeable gastrointestinal membranes.
- Gl gastrointestinal
- agents which are not typically amenable to oral administration are biologically active peptides, such as calcitonin and insulin; polysaccharides, and in particular mucopolysaccharides including, but not limited to, heparin; heparinoids; antibiotics; and other organic substances. These agents are rapidly rendered ineffective or are destroyed in the gastrointestinal tract by acid hydrolysis, enzymes, or the like.
- DKP diketopiperazines
- 3,6-bis-substituted-2,5-diketopiperazines have been shown to effectively deliver biologically active agents across the lining of the lung.
- diketopiperazines Conventional synthesis of diketopiperazines proceeds via a cyclocondensation of two amino acid molecules or a dipeptide.
- One exemplary process for the synthesis of diketopiperazines entails heating an amino acid (Cbz-L-lysine for example) in m-cresol for between 17 and 22 hours at 160-170oC, and recrystallizing the diketopiperazine from acetic acid for a yield of about 48%.
- N-substituted 3,6-aminoalkyl-2,5-diketopiperazines as pharmaceutical excipients has shown considerable promise. As noted above, these compounds are often synthesized via cyclocondensation of amino acids. If the amino acid has a free nitrogen on its side-chain (as in, for example, lysine or ornithine) it is often necessary to have this nitrogen blocked prior to the cyclization reaction. Because of the potential for disparate synthetic processes after cyclization, compatibility with a variety of protecting groups is desired. Thus a synthetic method that can accommodate a number of diverse N-protecting groups and produce good yield of N-protected diketopiperazine is desired.
- Some useful protecting groups include trifluoroacteyl, acetyl and other amide forming protecting groups; carbamate protecting groups including benzyloxycarbonyl (Cbz) and t-butoxycarbonyl (BOC).
- 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine is formed by heating ⁇ -trifluoroacetyl-L-lysine in a water miscible solvent such as N-methyl-2-pyrrolidone (NMP) in the presence of a catalyst chosen from the group comprising phosphoric acid, sulfuric acid and phosphorous pentoxide to a temperature of about 150-175oC.
- NMP N-methyl-2-pyrrolidone
- the diketopiperazine is isolated by quenching with water and filtering the resulting solid.
- Disclosed embodiments describe methods for the synthesis of 3,6-bis-[N-protected aminoalkyl]-2,5-diketopiperazine comprising heating a mixture of an amino acid of general formula I in the presence of a catalyst in an organic solvent; wherein the catalyst is selected from the group comprising sulfuric acid, phosphoric acid, p-toluenesulfonic acid, 1-propylphosphonic acid cyclic anhydride, tributyl phosphate, phenyl phosphonic acid and phosphorous pentoxide among others; and wherein the solvent is selected from the group comprising: dimethylacetamide, N-methyl-2-pyrrolidone, diglyme, ethyl glyme, proglyme, ethyldiglyme, m-cresol, p-cresol, o-cresol, xylenes, ethylene glycol and phenol among others.
- the catalyst is selected from the group comprising sulfuric acid, phosphoric acid, p
- n is equal to 3, wherein n is equal to 2, wherein PG is an amide forming protecting group, wherein the protecting group is trifluoroacetyl, wherein PG is a carbamate forming protecting group, wherein the protecting group is Cbz, wherein the solvent is substantially water miscible, wherein the solvent is N-methyl-2-pyrrolidone, wherein the amino acid is ⁇ -trifluoroacetyl-L-lysine, wherein the amino acid is ⁇ -Cbz-L-lysine, wherein the amino acid is ⁇ -trifluoroacetyl-ornithine, wherein the amino acid is ⁇ -Cbz-ornithine, wherein the catalyst is phosphorous pentoxide, wherein the concentration of phosphorous pentoxide is from 10% to about 50% that of the amino acid, and embodiments further comprising the step of quenching the mixture with water.
- Disclosed embodiments describe methods for the synthesis of 3,6-bis-[N-protected aminobutyl]-2,5-diketopiperazine comprising: heating a mixture of a N-protected lysine in the presence of a catalyst in an organic solvent, to a temperature of between 110o and 175oC for between 0.25 and 5 hours; wherein the catalyst is selected from the group comprising sulfuric acid, phosphoric acid and phosphorous pentoxide, the concentration of catalyst from about 5% to about 50% that of the lysine; and the solvent is selected from the group comprising: dimethylacetamide, N-methyl-2-pyrrolidone, diglyme, ethyl glyme, proglyme, ethyldiglyme, m-cresol, p-cresol, o-cresol, xylenes, ethylene glycol and phenol.
- Disclosed embodiments describe methods for the synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine comprising: heating a mixture of ⁇ -trifluoroacetyl-L-lysine in the presence of phosphorous pentoxide in N-methyl-2-pyrrolidone, to a temperature of between 150o and 175oC for between 0.25 and 5 hours, the concentration of phosphorous pentoxide is from about 10% to about 40% that of the lysine; and quenching the mixture with a second solvent, or alternatively, wherein the concentration of phosphorous pentoxide to lysine is between 20% and 35% and the mixture is quenched with water.
- methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, hexyl, heptyl or octyl and all bond isomers are to be considered as alkyl.
- These can be mono- or poly-substituted with (C1-C8)-alkoxy, (C1 - C8)-haloalkyl, OH, halogen, NH 2 , NO 2 , SH, S-(C1 -C8) alkyl.
- C2-C8)-alkenyl is understood to mean a (C1-C8)-alkyl group as illustrated above having at least one double bond.
- a side-chain group of an ⁇ -amino acid is understood to mean the changeable group on the ⁇ -C atom of glycine as the basic amino acid.
- Natural amino acids are given for example in Bayer-Walter, Lehrbuch der organischen Chemie, S. Hirzel Verlag, Stuttgart, 22nd edition, page 822ff .
- Preferred synthetic amino acids and protected amino acids are available from the Sigma -Aldrich Company.
- the side chain groups can be derived from those referred to there.
- the stated chemical structures relate to all possible stereoisomers that can be obtained by varying the configuration of the individual chiral centers, axes or surfaces, in other words all possible diastereomers as well as all optical isomers (enantiomers) falling within this group.
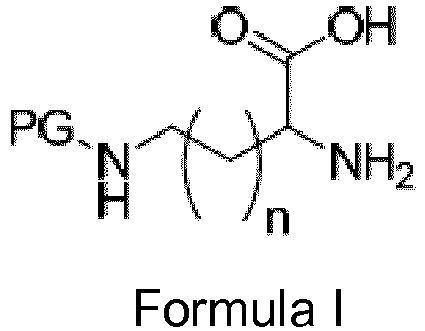
- FIGURE 1 shows a general scheme for the synthesis of a disubstituted diketopiperazine.
- This scheme shows an N-protected amino acid undergoing a cyclocondensation with a second amino acid molecule.
- PG represents a protecting group for the nitrogen and n may be from 0 to 7. It is evident from the scheme that it is necessary when forming a diketopiperazine with an amine on a side chain that the nitrogen(s) must be blocked prior to the cyclization reaction or yields will be affected by unwanted side condensations.
- a variety of protecting groups are desired, and thus a method that accommodates many groups is preferred.
- Some useful protecting groups include trifluoroacteyl, acetyl and other amide forming protecting groups; carbamate protecting groups including benzyloxycarbonyl (Cbz) and t-butoxycarbonyl (BOC).
- Known methods of cyclocondensation of amino acids to form DKP employed solvents such as n-butanol (water miscibility of about 7-8%), whereas solvents such as NMP are more miscible with water allowing a simple water quench/wash to remove reaction solvent and, if the catalyst has significant water solubility, the catalyst, all at once.
- the catalyst for the amino acid cyclocondensation is water soluble allowing a water quench and subsequent removal by filtration.
- Figure 2 illustrates an embodiment wherein PG is trifluoroacetyl and n is equal to 3.
- the starting amino acid is ⁇ -trifluoroacetyl lysine and the product is 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine.
- An example of a method for the synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine follows:
- N-methyl-2-pyrollidone 200 L
- stirring was started.
- TFA-lysine 100 kg, 413 mol
- phosphorous pentoxide 15.2 kg, 107 mol
- the mixture was then heated to 160oC for 1 h. After 1 h at 160oC the mixture was cooled to 100oC and water (500 L) was added. The resulting mixture was cooled to 25oC and held there for 90 minutes.
- the resulting solids were washed twice with water (265 L each) and isolated by filtration to give 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine in 50% yield.
- TFA-lysine (50 g), N-methyl pyrrolidone (125 mL), and P 2 O 5 (8.8 g, 0.3 eq.) were charged to a round bottom flask. The mixture was heated to a reaction temperature for a reaction time. The reaction mixture was cooled, poured into water, and then cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. Table 4 . Reaction times and temperatures for TFA-lysine diketopiperazine synthesis. Reaction temp (°C) Reaction time Reaction yield 110 0.25 19% 110 5 54% 170 0.25 59% 170 5 42%
- TFA-lysine (10.0 g), m-cresol (22 mL), and P 2 O 5 were charged to a 250 mL round bottom flask. The mixture was heated to 160 - 165 °C for 1 hour. The reaction mixture was cooled to 65 °C, poured into a solution of 5% aqueous NaOH and methanol, and then cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. Product yield was 12%.
- TFA-lysine (50.0 g) and ethylene glycol (150 mL) were charged to a 500 mL round bottom flask. The mixture was heated to 160 - 170 °C for 2 hours. The reaction mixture was poured into water and cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. Product yield was 2%.
- Figure 3 shows a general scheme for the cyclocondensation of ⁇ -Cbz-ornithine.
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Low-Molecular Organic Synthesis Reactions Using Catalysts (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Description
- The present invention relates to compositions for delivering active agents, and particularly biologically active agents. Disclosed embodiments are in the field of chemical synthesis and more particularly are related to improved synthetic methods for the preparation and purification of 3,6-di-substituted-2,5-diketopiperazines.
- Drug delivery is a persistent problem in the administration of active agents to patients. Conventional means for delivering active agents are often severely limited by biological, chemical, and physical barriers. Typically, these barriers are imposed by the environment through which delivery occurs, the environment of the target for delivery, or the target itself.
- Biologically active agents are particularly vulnerable to such barriers. For example, in the delivery of pharmacological and therapeutic agents to humans, barriers are imposed by the body. Examples of physical barriers are the skin and various organ membranes that must be traversed before reaching a target. Chemical barriers include, but are not limited to, pH variations, lipid bi-layers, and degrading enzymes.
- These barriers are of particular significance in the design of oral delivery systems. Oral delivery of many biologically active agents would be the route of choice for administration to animals if not for biological, chemical, and physical barriers such as varying pH in the gastrointestinal (Gl) tract, powerful digestive enzymes, and active agent impermeable gastrointestinal membranes. Among the numerous agents which are not typically amenable to oral administration are biologically active peptides, such as calcitonin and insulin; polysaccharides, and in particular mucopolysaccharides including, but not limited to, heparin; heparinoids; antibiotics; and other organic substances. These agents are rapidly rendered ineffective or are destroyed in the gastrointestinal tract by acid hydrolysis, enzymes, or the like.
- However, broad spectrum use of drug delivery systems is often precluded because: (1) the systems require toxic amounts of adjuvants or inhibitors; (2) suitable low molecular weight active agents are not available; (3) the systems exhibit poor stability and inadequate shelf life; (4) the systems are difficult to manufacture; (5) the systems fail to protect the active agent; (6) the systems adversely alter the active agent; or (7) the systems fail to allow or promote absorption of the active agent.
- There is still a need in the art for simple, inexpensive delivery systems which are easily prepared and which can deliver a broad range of active agents. One class of delivery system that has shown promise as excipients is diketopiperazines (DKP). In particular, 3,6-bis-substituted-2,5-diketopiperazines have been shown to effectively deliver biologically active agents across the lining of the lung.
- Conventional synthesis of diketopiperazines proceeds via a cyclocondensation of two amino acid molecules or a dipeptide. One exemplary process for the synthesis of diketopiperazines, entails heating an amino acid (Cbz-L-lysine for example) in m-cresol for between 17 and 22 hours at 160-170ºC, and recrystallizing the diketopiperazine from acetic acid for a yield of about 48%.
-
US Patent No. 7,709,639 to Stevenson et. al. details methods for the synthesis of bis-Cbz-N-protected diketopiperazines, the disclosure of which is hereby incorporated by reference in its entirety as if recited fully herein. - Others have generated diketopiperazines from isolated dipetides by heating in an appropriate solvent while removing water by distillation. While these provide the desired diketopiperazines, the methods provide suboptimal yields and may require prolonged purification. Thus, there is a need for an improved method for the synthesis of disubstituted 2,5-diketopiperazines that provides the N-protected diketopiperazines in good yield while preserving the protecting groups and requiring minimal purification.
- This and other unmet needs of the prior art are met by compounds and methods as described in more detail below. The use of N-substituted 3,6-aminoalkyl-2,5-diketopiperazines as pharmaceutical excipients has shown considerable promise. As noted above, these compounds are often synthesized via cyclocondensation of amino acids. If the amino acid has a free nitrogen on its side-chain (as in, for example, lysine or ornithine) it is often necessary to have this nitrogen blocked prior to the cyclization reaction. Because of the potential for disparate synthetic processes after cyclization, compatibility with a variety of protecting groups is desired. Thus a synthetic method that can accommodate a number of diverse N-protecting groups and produce good yield of N-protected diketopiperazine is desired.
- Some useful protecting groups include trifluoroacteyl, acetyl and other amide forming protecting groups; carbamate protecting groups including benzyloxycarbonyl (Cbz) and t-butoxycarbonyl (BOC).
- In an embodiment, 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine is formed by heating ε-trifluoroacetyl-L-lysine in a water miscible solvent such as N-methyl-2-pyrrolidone (NMP) in the presence of a catalyst chosen from the group comprising phosphoric acid, sulfuric acid and phosphorous pentoxide to a temperature of about 150-175ºC. The diketopiperazine is isolated by quenching with water and filtering the resulting solid.
- Disclosed embodiments describe methods for the synthesis of 3,6-bis-[N-protected aminoalkyl]-2,5-diketopiperazine comprising heating a mixture of an amino acid of general formula I in the presence of a catalyst in an organic solvent; wherein the catalyst is selected from the group comprising sulfuric acid, phosphoric acid, p-toluenesulfonic acid, 1-propylphosphonic acid cyclic anhydride, tributyl phosphate, phenyl phosphonic acid and phosphorous pentoxide among others; and wherein the solvent is selected from the group comprising: dimethylacetamide, N-methyl-2-pyrrolidone, diglyme, ethyl glyme, proglyme, ethyldiglyme, m-cresol, p-cresol, o-cresol, xylenes, ethylene glycol and phenol among others.
- The disclosed embodiments also describe methods wherein n is equal to 3, wherein n is equal to 2, wherein PG is an amide forming protecting group, wherein the protecting group is trifluoroacetyl, wherein PG is a carbamate forming protecting group, wherein the protecting group is Cbz, wherein the solvent is substantially water miscible, wherein the solvent is N-methyl-2-pyrrolidone, wherein the amino acid is ε-trifluoroacetyl-L-lysine, wherein the amino acid is ε-Cbz-L-lysine, wherein the amino acid is γ-trifluoroacetyl-ornithine, wherein the amino acid is γ-Cbz-ornithine, wherein the catalyst is phosphorous pentoxide, wherein the concentration of phosphorous pentoxide is from 10% to about 50% that of the amino acid, and embodiments further comprising the step of quenching the mixture with water.
- Disclosed embodiments describe methods for the synthesis of 3,6-bis-[N-protected aminobutyl]-2,5-diketopiperazine comprising: heating a mixture of a N-protected lysine in the presence of a catalyst in an organic solvent, to a temperature of between 110º and 175ºC for between 0.25 and 5 hours; wherein the catalyst is selected from the group comprising sulfuric acid, phosphoric acid and phosphorous pentoxide, the concentration of catalyst from about 5% to about 50% that of the lysine; and the solvent is selected from the group comprising: dimethylacetamide, N-methyl-2-pyrrolidone, diglyme, ethyl glyme, proglyme, ethyldiglyme, m-cresol, p-cresol, o-cresol, xylenes, ethylene glycol and phenol.
- Disclosed embodiments describe methods for the synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine comprising: heating a mixture of ε-trifluoroacetyl-L-lysine in the presence of phosphorous pentoxide in N-methyl-2-pyrrolidone, to a temperature of between 150º and 175ºC for between 0.25 and 5 hours, the concentration of phosphorous pentoxide is from about 10% to about 40% that of the lysine; and quenching the mixture with a second solvent, or alternatively, wherein the concentration of phosphorous pentoxide to lysine is between 20% and 35% and the mixture is quenched with water.
- Any combination of the above described elements in all possible variations thereof is encompassed by the disclosed embodiments unless otherwise indicated herein or otherwise clearly contradicted by context.
- A better understanding of the exemplary embodiments of the invention will be had when reference is made to the accompanying drawings, wherein identical parts are identified with identical reference numerals, and wherein:
-
FIGURE 1 is a scheme showing the cyclocondensation of an N-protected amino acid into a diketopiperazine. -
FIGURE 2 is a scheme showing the cyclocondensation of ε-trifluoroacetyl lysine. -
FIGURE 3 is a scheme showing the cyclocoondensation of γ-Cbz-ornithine. - As used herein, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, hexyl, heptyl or octyl and all bond isomers are to be considered as alkyl. These can be mono- or poly-substituted with (C1-C8)-alkoxy, (C1 - C8)-haloalkyl, OH, halogen, NH2, NO2, SH, S-(C1 -C8) alkyl. (C2-C8)-alkenyl, with the exception of methyl, is understood to mean a (C1-C8)-alkyl group as illustrated above having at least one double bond.
- A side-chain group of an α-amino acid is understood to mean the changeable group on the α-C atom of glycine as the basic amino acid. Natural amino acids are given for example in Bayer-Walter, Lehrbuch der organischen Chemie, S. Hirzel Verlag, Stuttgart, 22nd edition, page 822ff. Preferred synthetic amino acids and protected amino acids are available from the Sigma -Aldrich Company. The side chain groups can be derived from those referred to there.
- The stated chemical structures relate to all possible stereoisomers that can be obtained by varying the configuration of the individual chiral centers, axes or surfaces, in other words all possible diastereomers as well as all optical isomers (enantiomers) falling within this group.
- Turning to the drawings for a better understanding,
FIGURE 1 shows a general scheme for the synthesis of a disubstituted diketopiperazine. This scheme shows an N-protected amino acid undergoing a cyclocondensation with a second amino acid molecule. In this embodiment, PG represents a protecting group for the nitrogen and n may be from 0 to 7. It is evident from the scheme that it is necessary when forming a diketopiperazine with an amine on a side chain that the nitrogen(s) must be blocked prior to the cyclization reaction or yields will be affected by unwanted side condensations. Depending on the chemistry that will be performed after ring formation, a variety of protecting groups are desired, and thus a method that accommodates many groups is preferred. Some useful protecting groups include trifluoroacteyl, acetyl and other amide forming protecting groups; carbamate protecting groups including benzyloxycarbonyl (Cbz) and t-butoxycarbonyl (BOC). - Known methods of cyclocondensation of amino acids to form DKP employed solvents such as n-butanol (water miscibility of about 7-8%), whereas solvents such as NMP are more miscible with water allowing a simple water quench/wash to remove reaction solvent and, if the catalyst has significant water solubility, the catalyst, all at once. In an embodiment, the catalyst for the amino acid cyclocondensation is water soluble allowing a water quench and subsequent removal by filtration.
-
Figure 2 illustrates an embodiment wherein PG is trifluoroacetyl and n is equal to 3. Thus, the starting amino acid is ε-trifluoroacetyl lysine and the product is 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine. An example of a method for the synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine follows: - Example 1 and 2
-
- To a 1 L, 3-neck round bottom flask equipped with a nitrogen purge, a distillation apparatus, a mechanical stirrer and a thermocouple with a temperature display, was added: NMP (256 mL), TFA-Lys (125 g, 0.52 mol) and P2O5 (22 g, 0.15 mol). The reaction mixture was heated to 160ºC and held there for 1.5 h. The mixture was then cooled to 100ºC and poured into DI water. The mixture was then cooled below 25ºC. and the solids were isolated via filtration, washed with DI water and dried in vacuo at 50ºC. to yield 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine (65.28g, 56.4%). 1H-NMR (DMSO-d6): 1.3 (m, 4H), 1.5 (m, 4H), 1.7 (m, 4H), 3.2 (q, 4H), 3.8 (m, 2H), 8.1 (s, 2H), 9.4 (s, 2H). Elemental analysis, calc'd C: 42.86; H: 4.95; N 12.50; F: 25.42. Found: C: 42.95; H: 4.91; N: 12.53; F: 24.99.
- To a 100 gallon glass-lined reactor was added N-methyl-2-pyrollidone (200 L) and stirring was started. To the solvent was added TFA-lysine (100 kg, 413 mol) at ambient temperature. To the resulting slurry was added phosphorous pentoxide (15.2 kg, 107 mol). The mixture was then heated to 160ºC for 1 h. After 1 h at 160ºC the mixture was cooled to 100ºC and water (500 L) was added. The resulting mixture was cooled to 25ºC and held there for 90 minutes. The resulting solids were washed twice with water (265 L each) and isolated by filtration to give 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine in 50% yield.
- A variety of catalysts were examined for the formation of bis-substituted diketopiperazines. The results of the catalyst survey are shown in Table 1. A general scheme and example for this survey follows:
-
- Cbz-lysine (10.0 g), diethylene glycol dimethyl ether (diglyme; 50 mL), and a catalyst were charged to a 250 mL round bottom flask. The mixture was heated to 160 - 165 °C for 2.5 hours. The reaction mixture was poured into water and cooled to ambient temperature overnight. The precipitated solid was isolated by filtration, washed with water, and dried in vacuo at 50 °C.
Table 1. Catalysts for diketopiperazine synthesis. Catalyst Amount Reaction yield P2O5 0.76 g (0.15 eq.) 55% P2O5 1.76 g (0.30 eq.) 45% H2SO4 1.27 mL (0.35 eq.) 55% H3PO4 0.73 mL (0.30 eq.) 65% p-toluene sulfonic acid 3.39 g (0.50 eq.) 52% 1-propylphosphonic acid cyclic anhydride 4.54 g (0.20 eq.) 79% tributyl phosphate 2.44 g (0.30 eq.) 89% ethyl phosphonic acid 1.18 g (0.30 eq.) 0% phenyl phosphonic acid 1.13 g (0.20 eq.) 78% - Sulfuric acid and phosphorous pentoxide (at two concentrations) were surveyed further for synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine in diglyme. The results are shown in Table 2.
-
- TFA-lysine (10.0 g), diethylene glycol dimethyl ether (50 mL), and a catalyst were charged to a 250 mL round bottom flask. The mixture was heated to 160 - 165 °C for 2.5 hours. The reaction mixture was poured into water and cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C.
Table 2. Catalysts for TFA-lysine diketopiperazine synthesis. Catalyst Amount Reaction yield P2O5 0.88 g (0.15 eq.) 41% P2O5 1.76 g (0.30 eq.) 55% H2SO4 0.8 mL (0.35 eq.) 40% - Sulfuric acid and phosphorous pentoxide (at two concentrations) were surveyed further for synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine in dimethylacetamide (DMAc). The results are shown in Table 3.
-
- TFA-lysine (25.0 g), dimethylacetamide (125 mL), and a catalyst were charged to a 250 mL round bottom flask. The mixture was heated to 160 - 165 °C for 2.5 hours. The reaction mixture was cooled to 100 °C, poured into water, and then cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. The results are shown in Table 3.
Table 3. Catalysts for TFA-lysine diketopiperazine synthesis. Catalyst Amount Reaction yield P2O5 2.2 g (0.15 eq.) 35% P2O5 5.13 g (0.35 eq.) 50% H2SO4 4.19 g (0.40 eq.) 16% - The use of phosphorous pentoxide was examined for the synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine in N-methyl-2-pyrrolidone (NMP) at different times and temperatures. The results are shown in Table 4.
-
- TFA-lysine (50 g), N-methyl pyrrolidone (125 mL), and P2O5 (8.8 g, 0.3 eq.) were charged to a round bottom flask. The mixture was heated to a reaction temperature for a reaction time. The reaction mixture was cooled, poured into water, and then cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C.
Table 4. Reaction times and temperatures for TFA-lysine diketopiperazine synthesis. Reaction temp (°C) Reaction time Reaction yield 110 0.25 19% 110 5 54% 170 0.25 59% 170 5 42% -
- TFA-lysine (10.0 g), m-cresol (22 mL), and P2O5 were charged to a 250 mL round bottom flask. The mixture was heated to 160 - 165 °C for 1 hour. The reaction mixture was cooled to 65 °C, poured into a solution of 5% aqueous NaOH and methanol, and then cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. Product yield was 12%.
-
- TFA-lysine (50.0 g) and ethylene glycol (150 mL) were charged to a 500 mL round bottom flask. The mixture was heated to 160 - 170 °C for 2 hours. The reaction mixture was poured into water and cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. Product yield was 2%.
-
- Cbz-lysine (100.0 g) and ethylene glycol (300 mL) were charged to a 1000 mL round bottom flask. The mixture was heated to 160 - 170 °C for 6 hours. The reaction mixture was poured into a mixture of water and methanol and cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with water and dried in vacuo at 50 °C. Product yield was 64%.
-
Figure 3 shows a general scheme for the cyclocondensation of γ-Cbz-ornithine. -
- CBz-ornithine (100 g), N-methyl pyrrolidone (194 mL), and P2O5 (8 g) were charged to a 1000 mL round bottom flask. The mixture was heated to 160 - 165 °C for 2 hours. The reaction mixture was poured into water and cooled to ambient temperature. The precipitated solid was isolated by filtration, washed with methanol and water, and dried in vacuo at 50 °C. The product yield was 51 %.
- Unless otherwise indicated, all numbers expressing quantities of ingredients, properties such as molecular weight, reaction conditions, and so forth used in the specification and claims are to be understood as being modified in all instances by the term "about". Accordingly, unless indicated to the contrary, the numerical parameters set forth in the following specification and attached claims are approximations that may vary depending upon the desired properties sought to be obtained by the disclosed embodiments. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques. Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the disclosed embodiments are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements.
- The terms "a" and "an" and "the" and similar references used in the context of describing the disclosed embodiments (especially in the context of the following claims) are to be construed to cover both the singular and the plural, unless otherwise indicated herein or clearly contradicted by context.
- Recitation of ranges of values herein is merely intended to serve as a shorthand method of referring individually to each separate value falling within the range. Unless otherwise indicated herein, each individual value is incorporated into the specification as if it were individually recited herein. All methods described herein can be performed in any suitable order unless otherwise indicated herein or otherwise clearly contradicted by context. The use of any and all examples, or exemplary language (e.g. "such as") provided herein is intended merely to better illuminate the disclosed embodiments and does not pose a limitation on the scope of the disclosed embodiments unless otherwise claimed. No language in the specification should be construed as indicating any non-claimed element essential to the practice of the disclosed embodiments or any variants thereof.
- Groupings of alternative elements or embodiments disclosed herein are not to be construed as limitations. Each group member may be referred to and claimed individually or in any combination with other members of the group or other elements found herein. It is anticipated that one or more members of a group may be included in, or deleted from, a group for reasons of convenience and/or patentability. When any such inclusion or deletion occurs, the specification is herein deemed to contain the group as modified thus fulfilling the written description of any and all Markush groups used in the appended claims.
- Preferred embodiments of this invention are described herein, including the best mode known to the inventors for carrying out the invention(s). Of course, variations on the disclosed embodiments will become apparent to those of ordinary skill in the art upon reading the foregoing description. The inventors expect skilled artisans to employ such variations as appropriate, and the inventors intend for the invention(s) to be practiced otherwise than specifically described herein. Accordingly, this disclosure includes all modifications and equivalents of the subject matter recited in the claims appended hereto as permitted by applicable law. Moreover, any combination of the above described elements in all possible variations thereof is encompassed by the disclosed embodiments unless otherwise indicated herein or otherwise clearly contradicted by context.
- Furthermore, references have been made to patents and printed publications throughout this specification.
- Having shown and described an embodiment of the invention, those skilled in the art will realize that many variations and modifications may be made to affect the described invention and still be within the scope of the claimed invention. Additionally, many of the elements indicated above may be altered or replaced by different elements which will provide the same result.
- It is the intention, therefore, to limit the invention only as indicated by the scope of the claims.
Claims (7)
- A method for the synthesis of 3,6-bis-[N-protected aminoalkyl]-2,5-diketopiperazine comprising:heating a mixture of an amino acid of general formula I in the presence of a catalyst in an organic solvent;wherein the catalyst is phosphorous pentoxide, wherein the concentration of phosphorous pentoxide to the amino acid is 20 mol % to 50 mol %;wherein n is 2 or 3;wherein the PG is trifluoroacetyl; andwherein the solvent is selected from the group consisting of:dimethylacetamide, N-methyl-2-pyrrolidone, diglyme, ethyl glyme, proglyme, ethyldiglyme.
- The method of claim 1 wherein the solvent is water miscible.
- The method of claim 1 wherein the solvent is N-methyl-2-pyrrolidone.
- The method of claim 1 wherein the amino acid is ε-trifluoroacetyl-L-lysine.
- The method of claim 1 wherein the amino acid is γ-trifluoroacetyl-ornithine.
- The method of claim 1 further comprising the step of quenching the mixture with water.
- A method according to claim 1 for the synthesis of 3,6-bis-4-(N-trifluoroacetyl)aminobutyl-2,5-diketopiperazine comprising:heating a mixture of ε-trifluoroacetyl-L-lysine in the presence of phosphorous pentoxide in N-methyl-2-pyrrolidone, to a temperature of between 150° and 175°C for between 0.25 and 5 hours, the concentration of phosphorous pentoxide is 20 mol % to 35 mol % that of the lysine; andquenching the mixture with water.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161441525P | 2011-02-10 | 2011-02-10 | |
| PCT/US2012/024160 WO2012109256A2 (en) | 2011-02-10 | 2012-02-07 | Formation of n-protected bis-3,6-(4-aminoalkyl) -2,5,diketopiperazine |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP2673265A2 EP2673265A2 (en) | 2013-12-18 |
| EP2673265A4 EP2673265A4 (en) | 2014-08-06 |
| EP2673265B1 true EP2673265B1 (en) | 2016-10-19 |
Family
ID=46639161
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP12744320.8A Active EP2673265B1 (en) | 2011-02-10 | 2012-02-07 | Formation of n-protected bis-3,6-(4-aminoalkyl) -2,5,diketopiperazine |
Country Status (13)
| Country | Link |
|---|---|
| US (6) | US8912328B2 (en) |
| EP (1) | EP2673265B1 (en) |
| JP (1) | JP6018586B2 (en) |
| KR (1) | KR20140027937A (en) |
| CN (2) | CN105884700A (en) |
| AU (2) | AU2012214592B2 (en) |
| BR (1) | BR112013020514B1 (en) |
| CA (1) | CA2826973C (en) |
| IL (1) | IL227904A (en) |
| MX (1) | MX346331B (en) |
| RU (1) | RU2606624C2 (en) |
| SG (3) | SG10201600967VA (en) |
| WO (1) | WO2012109256A2 (en) |
Families Citing this family (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| CA2479751C (en) | 2002-03-20 | 2008-06-03 | Trent Poole | Inhalation apparatus |
| KR101273120B1 (en) * | 2004-08-20 | 2013-06-13 | 맨카인드 코포레이션 | Catalysis of diketopiperazine synthesis |
| KR101644250B1 (en) | 2004-08-23 | 2016-07-29 | 맨카인드 코포레이션 | Diketopiperazine salts, diketomorpholine salts or diketodioxane salts for drug delivery |
| KR101486397B1 (en) | 2005-09-14 | 2015-01-28 | 맨카인드 코포레이션 | Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents |
| BRPI0707991B8 (en) | 2006-02-22 | 2021-05-25 | Mannkind Corp | methods of preparing a dry powder medicine with an improved pharmaceutical property, said dry powder and using an effective amount of the dry powder |
| CN104491962B (en) | 2008-06-13 | 2018-10-23 | 曼金德公司 | Diskus and the system conveyed for drug |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| ES2421385T3 (en) | 2008-06-20 | 2013-09-02 | Mannkind Corp | Interactive device and procedure to establish the profile, in real time, of inhalation efforts |
| TWI494123B (en) | 2008-08-11 | 2015-08-01 | Mannkind Corp | Use of ultrarapid acting insulin |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| CA2754595C (en) | 2009-03-11 | 2017-06-27 | Mannkind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| US8734845B2 (en) | 2009-06-12 | 2014-05-27 | Mannkind Corporation | Diketopiperazine microparticles with defined specific surface areas |
| US9016147B2 (en) | 2009-11-03 | 2015-04-28 | Mannkind Corporation | Apparatus and method for simulating inhalation efforts |
| EP2582421A1 (en) | 2010-06-21 | 2013-04-24 | MannKind Corporation | Dry powder drug delivery system and methods |
| CN105884700A (en) * | 2011-02-10 | 2016-08-24 | 麦康公司 | Formation of N-Protected bis-3,6-(4-aminoalkyl)-2,5,diketopiperazine |
| SG10201606220QA (en) | 2011-04-01 | 2016-09-29 | Mannkind Corp | Blister package for pharmaceutical cartridges |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| KR20140095483A (en) | 2011-10-24 | 2014-08-01 | 맨카인드 코포레이션 | Methods and compositions for treating pain |
| WO2013142969A1 (en) * | 2012-03-28 | 2013-10-03 | Jian Ping Gao | Urethanes and ureas and processes |
| KR20150023315A (en) | 2012-04-27 | 2015-03-05 | 맨카인드 코포레이션 | Methods for the synthesis of ethylfumarates and their use as intermediates |
| JP6312262B2 (en) | 2012-07-12 | 2018-04-18 | マンカインド コーポレイション | Dry powder drug delivery system |
| WO2014066856A1 (en) | 2012-10-26 | 2014-05-01 | Mannkind Corporation | Inhalable influenza vaccine compositions and methods |
| MX2015012603A (en) | 2013-03-15 | 2016-06-10 | Mannkind Corp | Formation of n-protected bis-3,6-(4-aminobutyl)-2,5-diketopiperaz ine through a cyclic alpha-n-protected amino ester. |
| SG11201507564PA (en) | 2013-03-15 | 2015-10-29 | Mannkind Corp | Microcrystalline diketopiperazine compositions and methods |
| AU2014290438B2 (en) | 2013-07-18 | 2019-11-07 | Mannkind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| US11446127B2 (en) | 2013-08-05 | 2022-09-20 | Mannkind Corporation | Insufflation apparatus and methods |
| CN105254576A (en) * | 2014-02-26 | 2016-01-20 | 中国科学院长春应用化学研究所 | Method for preparing glutamic piperazinedione |
| WO2015148905A1 (en) | 2014-03-28 | 2015-10-01 | Mannkind Corporation | Use of ultrarapid acting insulin |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| WO2017025031A1 (en) * | 2015-08-10 | 2017-02-16 | 于跃 | Diazaoxa heterocyclic spiro-dione piperazine alkaloid derivative having antiviral activity and preparation method thereof |
| AR108812A1 (en) | 2016-06-20 | 2018-09-26 | Shionogi & Co | PROCESS TO PREPARE A SUBSTITUTED AND CRYSTAL POLYCHYCLIC PIRIDONA DERIVATIVE |
| CN108997168B (en) * | 2018-07-14 | 2021-09-28 | 上海应用技术大学 | General synthesis method of fluorine-containing non-natural lysine derivative |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5352461A (en) * | 1992-03-11 | 1994-10-04 | Pharmaceutical Discovery Corporation | Self assembling diketopiperazine drug delivery system |
| GB9217331D0 (en) * | 1992-08-14 | 1992-09-30 | Xenova Ltd | Pharmaceutical compounds |
| JPH06321916A (en) * | 1993-05-14 | 1994-11-22 | Mitsui Toatsu Chem Inc | Production of 3,6-bis((4-hydroxyphenyl)methyl)-2,5-diketopiperazine |
| JP2001511810A (en) * | 1997-02-13 | 2001-08-14 | モンサント カンパニー | Method for producing aminocarboxylic acid |
| US6337678B1 (en) * | 1999-07-21 | 2002-01-08 | Tactiva Incorporated | Force feedback computer input and output device with coordinated haptic elements |
| US6590993B2 (en) * | 1999-09-06 | 2003-07-08 | Koninklijke Philips Electronics N.V. | Panel-shaped loudspeaker |
| DE10019879A1 (en) * | 2000-04-20 | 2001-10-25 | Degussa | Production of known and new 2,5-diketopiperazine derivatives useful for the synthesis of bioactive compounds, e.g. cyclo(Lys-Lys) |
| KR101273120B1 (en) * | 2004-08-20 | 2013-06-13 | 맨카인드 코포레이션 | Catalysis of diketopiperazine synthesis |
| JP4968848B2 (en) * | 2008-01-30 | 2012-07-04 | 株式会社Adeka | Polyolefin resin composition |
| CN101851213A (en) * | 2010-06-21 | 2010-10-06 | 于清 | Synthetic methods of 3,6-bis(4-bisfumaroyl aminobutyl)-2,5-diketopiperazine and salt substitute thereof |
| CN101914032B (en) * | 2010-07-15 | 2013-06-05 | 启东市沪东化工有限公司 | Synthetic method of (s)-N-trifluoroacetyl-2-(4-Methoxyphenyl)ethylamine |
| CN105884700A (en) * | 2011-02-10 | 2016-08-24 | 麦康公司 | Formation of N-Protected bis-3,6-(4-aminoalkyl)-2,5,diketopiperazine |
-
2012
- 2012-02-07 CN CN201610169104.4A patent/CN105884700A/en active Pending
- 2012-02-07 MX MX2013009260A patent/MX346331B/en active IP Right Grant
- 2012-02-07 SG SG10201600967VA patent/SG10201600967VA/en unknown
- 2012-02-07 CN CN201280008649.7A patent/CN103534242B/en active Active
- 2012-02-07 SG SG10201802008TA patent/SG10201802008TA/en unknown
- 2012-02-07 SG SG2013060793A patent/SG192708A1/en unknown
- 2012-02-07 US US13/368,172 patent/US8912328B2/en active Active
- 2012-02-07 JP JP2013553494A patent/JP6018586B2/en active Active
- 2012-02-07 AU AU2012214592A patent/AU2012214592B2/en active Active
- 2012-02-07 BR BR112013020514-8A patent/BR112013020514B1/en active IP Right Grant
- 2012-02-07 KR KR1020137023801A patent/KR20140027937A/en not_active Application Discontinuation
- 2012-02-07 WO PCT/US2012/024160 patent/WO2012109256A2/en active Application Filing
- 2012-02-07 CA CA2826973A patent/CA2826973C/en active Active
- 2012-02-07 RU RU2013138609A patent/RU2606624C2/en active
- 2012-02-07 EP EP12744320.8A patent/EP2673265B1/en active Active
-
2013
- 2013-08-11 IL IL227904A patent/IL227904A/en not_active IP Right Cessation
-
2014
- 2014-11-17 US US14/543,464 patent/US9416113B2/en active Active
-
2016
- 2016-08-15 US US15/237,427 patent/US10196366B2/en active Active
-
2017
- 2017-06-07 AU AU2017203860A patent/AU2017203860B2/en active Active
-
2019
- 2019-02-04 US US16/266,683 patent/US10640471B2/en active Active
-
2020
- 2020-05-04 US US16/866,056 patent/US11440891B2/en active Active
-
2022
- 2022-09-12 US US17/942,576 patent/US20230034201A1/en active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11440891B2 (en) | Formation of N-protected 3,6-bis-(4-aminoalkyl)-2,5,diketopiperazine | |
| US20200190041A1 (en) | Catalysis of Diketopiperazine Synthesis | |
| KR20010043416A (en) | Process for producing carboxylic acid derivative and condensing agent comprising quaternary ammonium salt | |
| US10870628B2 (en) | Formation of N-protected 3,6-bis-(4-aminobutyl)-2,5-diketopiperazine through a cyclic α-N-protected amino ester |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20130903 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| DAX | Request for extension of the european patent (deleted) | ||
| A4 | Supplementary search report drawn up and despatched |
Effective date: 20140707 |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C07C 229/00 20060101ALI20140701BHEP Ipc: B01J 31/02 20060101ALI20140701BHEP Ipc: C07D 241/08 20060101AFI20140701BHEP |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 1192883 Country of ref document: HK |
|
| 17Q | First examination report despatched |
Effective date: 20151009 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: MANNKIND CORPORATION |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20160502 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 838195 Country of ref document: AT Kind code of ref document: T Effective date: 20161115 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602012024339 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20161019 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 838195 Country of ref document: AT Kind code of ref document: T Effective date: 20161019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170120 Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170119 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170219 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170220 Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 602012024339 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170119 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 602012024339 Country of ref document: DE |
|
| 26N | No opposition filed |
Effective date: 20170720 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20170207 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20171031 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 7 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R073 Ref document number: 602012024339 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170207 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: S28 Free format text: APPLICATION FILED |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R074 Ref document number: 602012024339 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170228 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: RN Effective date: 20171226 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: S28 Free format text: RESTORATION ALLOWED Effective date: 20180123 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: FC Effective date: 20180116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170207 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170207 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170228 |
|
| PGRI | Patent reinstated in contracting state [announced from national office to epo] |
Ref country code: FR Effective date: 20180116 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170207 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20120207 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20161019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20161019 |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: WD Ref document number: 1192883 Country of ref document: HK |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230613 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20240228 Year of fee payment: 13 Ref country code: CH Payment date: 20240301 Year of fee payment: 13 Ref country code: GB Payment date: 20240227 Year of fee payment: 13 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20240226 Year of fee payment: 13 |