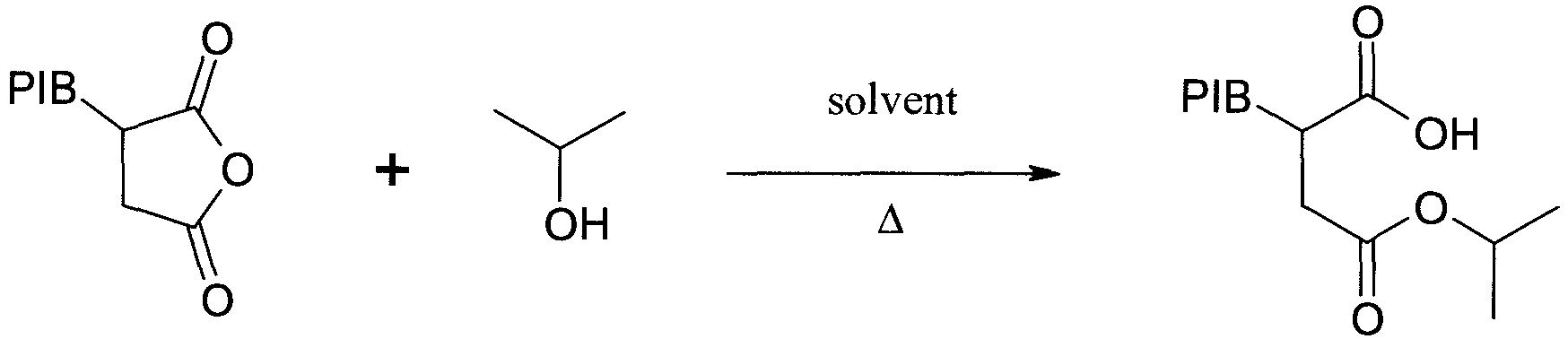EP1512736B1 - Stabilised diesel fuel additive compositions - Google Patents
Stabilised diesel fuel additive compositions Download PDFInfo
- Publication number
- EP1512736B1 EP1512736B1 EP04255073.1A EP04255073A EP1512736B1 EP 1512736 B1 EP1512736 B1 EP 1512736B1 EP 04255073 A EP04255073 A EP 04255073A EP 1512736 B1 EP1512736 B1 EP 1512736B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fuel
- ppm
- oil
- diesel fuel
- additive
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 *C(NC(CCC(O)=O)C(O)=O)=O Chemical compound *C(NC(CCC(O)=O)C(O)=O)=O 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/188—Carboxylic acids; metal salts thereof
- C10L1/1881—Carboxylic acids; metal salts thereof carboxylic group attached to an aliphatic carbon atom
- C10L1/1883—Carboxylic acids; metal salts thereof carboxylic group attached to an aliphatic carbon atom polycarboxylic acid
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/19—Esters ester radical containing compounds; ester ethers; carbonic acid esters
- C10L1/1905—Esters ester radical containing compounds; ester ethers; carbonic acid esters of di- or polycarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/19—Esters ester radical containing compounds; ester ethers; carbonic acid esters
- C10L1/191—Esters ester radical containing compounds; ester ethers; carbonic acid esters of di- or polyhydroxyalcohols
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/195—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
- C10L1/196—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derived from monomers containing a carbon-to-carbon unsaturated bond and a carboxyl group or salts, anhydrides or esters thereof homo- or copolymers of compounds having one or more unsaturated aliphatic radicals each having one carbon bond to carbon double bond, and at least one being terminated by a carboxyl radical or of salts, anhydrides or esters thereof
- C10L1/1966—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derived from monomers containing a carbon-to-carbon unsaturated bond and a carboxyl group or salts, anhydrides or esters thereof homo- or copolymers of compounds having one or more unsaturated aliphatic radicals each having one carbon bond to carbon double bond, and at least one being terminated by a carboxyl radical or of salts, anhydrides or esters thereof poly-carboxylic
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/198—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid
- C10L1/1985—Macromolecular compounds obtained otherwise than by reactions involving only carbon-to-carbon unsaturated bonds homo- or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon to carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid polyethers, e.g. di- polygylcols and derivatives; ethers - esters
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/224—Amides; Imides carboxylic acid amides, imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/234—Macromolecular compounds
- C10L1/236—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof
- C10L1/2364—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derivatives thereof homo- or copolymers derived from unsaturated compounds containing amide and/or imide groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/06—Use of additives to fuels or fires for particular purposes for facilitating soot removal
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/08—Use of additives to fuels or fires for particular purposes for improving lubricity; for reducing wear
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/14—Use of additives to fuels or fires for particular purposes for improving low temperature properties
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/18—Use of additives to fuels or fires for particular purposes use of detergents or dispersants for purposes not provided for in groups C10L10/02 - C10L10/16
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/12—Inorganic compounds
- C10L1/1233—Inorganic compounds oxygen containing compounds, e.g. oxides, hydroxides, acids and salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G OR C10K; LIQUIFIED PETROLEUM GAS; USE OF ADDITIVES TO FUELS OR FIRES; FIRE-LIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/192—Macromolecular compounds
- C10L1/195—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds
- C10L1/196—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derived from monomers containing a carbon-to-carbon unsaturated bond and a carboxyl group or salts, anhydrides or esters thereof homo- or copolymers of compounds having one or more unsaturated aliphatic radicals each having one carbon bond to carbon double bond, and at least one being terminated by a carboxyl radical or of salts, anhydrides or esters thereof
- C10L1/1963—Macromolecular compounds obtained by reactions involving only carbon-to-carbon unsaturated bonds derived from monomers containing a carbon-to-carbon unsaturated bond and a carboxyl group or salts, anhydrides or esters thereof homo- or copolymers of compounds having one or more unsaturated aliphatic radicals each having one carbon bond to carbon double bond, and at least one being terminated by a carboxyl radical or of salts, anhydrides or esters thereof mono-carboxylic
Definitions
- This invention relates to novel fuel additive compositions. More particularly, this invention relates to fuel compositions containing metallic additives which are stabilised against phase separation. Metallic additives are added to fuels since they are especially effective in improving the performance of particulate traps which are used in the exhaust systems of diesel engines, amongst other uses.
- Diesel engines equipped with particulate traps, mounted in the exhaust stream, to "trap” or collect particulates in the exhaust to prevent their emission to the atmosphere are expected to be in greater use in the next few years.
- HC hydrocarbons
- CO carbon monoxide
- NO x nitrogen oxides
- the problems of controlling these pollutants are compounded because there is a trade-off between particulates and nitrogen oxides: when the combustion conditions are modified to favor low nitrogen oxides emissions, particulates are increased.
- Particulate traps are employed to reduce the severity of the particulate emissions.
- the use of diesel traps and the need to improve them has resulted in a great deal of research and a great number of patents and technical publications.
- the traps are typically constructed of metal or ceramic and are capable of collecting the particulates from the exhaust and withstanding the heat produced by oxidation of carbonaceous deposits which must be burned off at regular intervals.
- organometallic salts and complexes to improve the operation of diesel engine particulate traps is disclosed, for example, in U.S. Patent No. 5,344,467 issued September 6, 1994 , which teaches the use of a combination of an organometallic complex and an antioxidant.
- the organometallic complex is soluble or dispersible in the diesel fuel and is derived from an organic compound containing at least two functional groups attached to a hydrocarbon linkage.
- WO 99/36488 published July 22, 1999 discloses fuel additive compositions which contain at least one iron-containing fuel-soluble or fuel-dispersible species in synergistic combination with at least one alkaline earth group metal-containing fuel-soluble or fuel-dispersible species. This combination of metallic additives is said to improve the operation of the diesel particulate filter traps.
- WO 94/11467 published May 26, 1994 teaches a method to improve the operation of diesel traps through the use of a fuel additive comprising fuel-soluble compositions of a platinum group metal in effective amounts to lower the emissions of unburned hydrocarbons and carbon monoxide from the trap.
- the platinum group metals comprise platinum, palladium, rhodium or iridium.
- Co-pending European application EP-A-1 344 809 describes a diesel fuel composition which improves the diesel engine particulate traps, comprising a metal salt-ashless detergent additive combination effective in improving the performance of the particulate trap.
- a metal salt-ashless detergent additive combination effective in improving the performance of the particulate trap.
- US-A-3,438,756 describes barium compounds provided to fuels to reduce smoking and comprising the reaction product of alkylphenol with a relatively high molecular weight alkenyl succinic acid and barium carbonate, prepared by carbonation of barium oxide dispersed in the presence of the alkyl phenol and alkenyl succinic acid.
- EP 671205 , EP 599717 and EP 575189 disclose the use of various cerium compounds in fuels.
- the present invention is based upon a discovery that such fuels may be stabilised against such phase separation or haze formation by the addition of very small amounts of an oil-dispersible or oil-soluble compound having two or more contiguous polar head groups.
- diesel fuel compositions stabilised against phase separation comprising a diesel fuel, a colloidally dispersed or solubilised metal catalyst compound for diesel particulate trap regeneration and an oil soluble or oil dispersible organic compound having a lipophilic hydrocarbyl chain having attached directly thereto at least two contiguous polar head functional groups, the organic compound being present in an amount effective to stabilise the metal catalyst compound against phase separation, wherein the metal catalyst compound consists of cerium or iron oxides or mixtures thereof, and wherein the composition of the invention is further particularised in claim 1.
- the organic compound is generally present in an amount of 10 to 1,000 ppm, preferably 10 to 200 ppm, most preferably 10 to 50 ppm (weight) of compound per weight of diesel fuel composition in order to effectively stabilise the metal catalyst compound.
- 'contiguous polar head functional groups' is used to represent polar (functional chemical) groups which are separated by no more than three, preferably no more than two carbon atoms within the molecule.
- the invention is particularly applicable to diesel fuel compositions which contain as catalysts for diesel particulate trap regeneration effective amounts of metallic compounds, typically sufficient to provide 1 to 200, 1 to 100, 1 to 20, 1 to 10, or 1 to 5 ppm metal (by weight) in the fuel, in the form of colloidally dispersed or solubilised compounds.
- composition's metal catalyst compound consists of cerium or iron oxides or mixtures thereof.
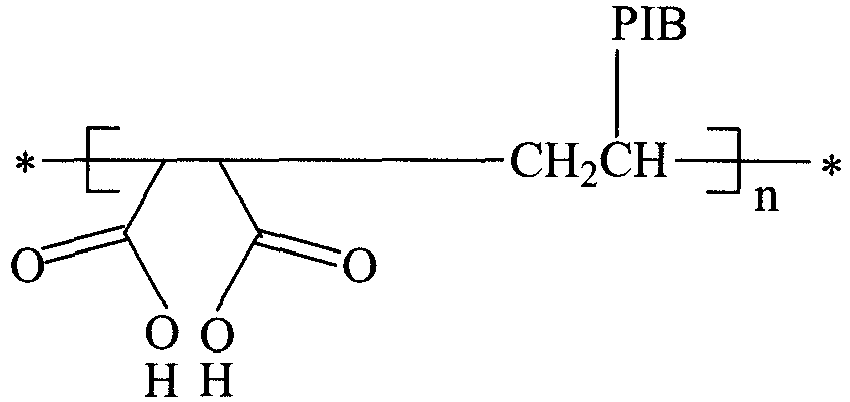
- the stabiliser compound of the present invention is represented by the generalised formula A-C-B, where C represents a hydrocarbyl chain of Mn (number average molecular weight) 200 - 4,000, preferably 200 - 1,300, more preferably 200 - 1,000 such as 400 - 1,000, 700-1000 or 450 - 700.
- C represents a hydrocarbyl chain of Mn (number average molecular weight) 200 - 4,000, preferably 200 - 1,300, more preferably 200 - 1,000 such as 400 - 1,000, 700-1000 or 450 - 700.
- the stabilisers may also be described in the following pictorial representation:
- n may be 1-20, but is preferably 1-10, more preferably 1-5.
- the stabiliser includes compounds of the following formulas, where "PIB” is polyisobutenyl, "PIBSA” is polyisobutenyl succinic anhydride and R is the lipophilic hydrocarbyl group: Hydrolysed poly(PIBSA) Hydrolysed alternating copolymers of (alpha-olefin- alt -maleic anhydride)
- This hydrocarbyl chain may be straight or branched, but branched hydrocarbyl chains are preferred because of their increased degree of solubility and preferably the hydrocarbyl chain is a polyisobutenyl group in the molecular weight ranges given above.
- the head grouping can be made to be tridentate, tetradentate and polydentate in surface binding ability.
- Particularly preferred stabiliser additives for use in the composition of the present invention are polyisobutenyl succinic acid wherein the polyisobutenyl group has a Mn of 1,000, and polyisobutenyl succinic acid wherein the polyisobutenyl group has a molecular weight of 450.
- Further embodiments of this invention comprise fuel compositions comprising the stabiliser compound, metal catalyst compound and one or more other fuel additive compounds, such as a lubricity enhancing additive, diesel detergent additive or a cold flow additive.
- Still further embodiments comprise additive concentrate compositions containing 3 to 75 % by weight of the stabiliser of this invention, in combination with the particulate trap metal catalyst compound, optionally in further combination with one or more other fuel additive compounds such as a lubricity enhancing additive, diesel detergent additive or a cold flow additive as described herein below.
- a concentrate comprising the additive dispersed in carrier liquid is convenient as a means of incorporating the additive.
- the concentrates of the present invention are convenient as a means for incorporating the additive into bulk oil such as distillate fuel, which incorporation may be done by methods known in the art.
- the concentrates may also contain other additives as required and preferably contain from 3 to 75 wt %, more preferably 3 to 60 wt %, and most preferably 10 to 50 wt % of the additive or additives preferably in solution in oil.
- carrier liquid are organic solvents including hydrocarbon solvents, for example petroleum fractions such as naphtha, kerosene, diesel and heater oil; aromatic hydrocarbons such as aromatic fractions, e.g.
- alkylphenols such as nonylphenol and 2,4-di-t-butylphenol either alone or in combination with any of the above have also been found to be particularly useful as carrier solvents.
- the carrier liquid must, of course, be selected having regard to its compatibility with the additive and with the fuel.
- the fuel oil may be a petroleum-based fuel oil, suitably a middle distillate fuel oil, i.e. a fuel oil obtained in refining crude oil as the fraction between the lighter kerosene and jet fuels fraction and the heavy fuel oil fraction.
- a middle distillate fuel oil i.e. a fuel oil obtained in refining crude oil as the fraction between the lighter kerosene and jet fuels fraction and the heavy fuel oil fraction.
- distillate fuel oils generally boil above about 100°C.
- the fuel oil can comprise atmospheric distillate or vacuum distillate, or cracked gas oil or a blend in any proportion of straight run and thermally and/or catalytically cracked and/or hydroprocessed distillates.
- the most common petroleum-based fuel oils are kerosene, jet fuels and preferably diesel fuel oils.
- the sulphur content of the fuel oil may be 2000 or less, preferably 500 or less, more preferably 50 or less, most preferably 10 or less, ppm by mass based on the mass of the fuel oil.
- the art describes methods for reducing the sulphur content of hydrocarbon middle distillate fuels, such methods including solvent extraction, sulphuric acid treatment, and hydrodesulphurisation.
- Preferred fuel oils have a cetane number of at least 40, preferably above 45 and more preferably above 50.
- the fuel oil may have such cetane numbers prior to the addition of any cetane improver or the cetane number of the fuel may be raised by the addition of a cetane improver.
- the fuel oils are those that have low solvency properties caused by low aromatic concentrations (e.g. below 30, below 20, below 15, below 10, or below 5, mass per cent), and/or those that are required to operate at low temperatures such as at -5, -10, -15, or -20, °C or lower.
- low aromatic concentrations e.g. below 30, below 20, below 15, below 10, or below 5, mass per cent
- fuel oils include jet-fuels; Fischer-Tropsch fuels; biofuels such as fuels made from vegetable matter such as rape seed methyl ester; and diesel/alcohol or diesel/water emulsions or solutions.
- Fischer-Tropsch fuels also known as FT fuels, include those that are described as gas-to-liquid fuels and coal conversion fuels. To make such fuels, syngas (CO + H 2 ) is first generated and then converted to normal paraffins by a Fischer-Tropsch process.
- the normal paraffins may then be modified by processes such as catalytic cracking/reforming or isomerisation, hydrocracking and hydroisomerisation to yield a variety of hydrocarbons such as iso-paraffins, cyclo-paraffins and aromatic compounds.
- the resulting FT fuel can be used as such or in combination with other fuel components and fuel types such as those mentioned in this specification.
- fuels emulsified with water and alcohols which contain suitable surfactants, and residual fuel oil used in marine diesel engines.
- WO-A- 0104239 ; WO-A- 0015740 ; WO-A-0151593 ; WO-A- 9734969 ; and WO-155282 describe examples of diesel/water emulsions.
- WO-A- 0031216 ; WO-A- 9817745 ; and WO-A- 024 8294 describe examples of diesel-ethanol emulsions/mixtures.
- Preferred vegetable-based fuel oils are triglycerides of monocarboxylic acids, and these typically have the general formula shown below where R is an aliphatic radical of 10-25 carbon atoms which may be saturated or unsaturated.
- oils contain glycerides of a number of acids, the number and kind varying with the source vegetable of the oil.
- Suitable fuel oils also include mixtures of 1-100% by weight of vegetable oils or methylesters of fatty acid, with petroleum based diesel fuel oils.
- oils and methyl ester derived fuel are tall oil, rapeseed oil, coriander oil, soyabean oil, cottonseed oil, sunflower oil, castor oil, olive oil, peanut oil, maize oil, almond oil, palm kernel oil, coconut oil, mustard seed oil, beef tallow and fish oils.
- Rapeseed oil which is a mixture of fatty acids esterified with glycerol, is preferred as it is available in large quantities and can be obtained in a simple way by pressing from rapeseed.
- vegetable-based fuel oils are alkyl esters, such as methyl esters, of fatty acids of the vegetable or animal oils. Such esters can be made by transesterification.
- lower alkyl esters of fatty acids consideration may be given to the following, for example as commercial mixtures containing, for example: the ethyl, propyl, butyl and especially methyl esters of fatty acids with 12 to 22 carbon atoms, for example, mixtures of lauric acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, elaidic acid, petroselic acid, ricinoleic acid, elaeostearic acid, linoleic acid, linolenic acid, eicosanoic acid, gadoleic acid, docosanoic acid or erucic acid, and rosin acid and isomers which have an iodine number from 50 to 180, especially 90 to 180.
- the ethyl, propyl, butyl and especially methyl esters of fatty acids with 12 to 22 carbon atoms for example, mixtures of lauric acid, myr
- Mixtures with particularly advantageous properties are those which contain mainly, i.e. to at least 50 wt % methyl esters of fatty acids with 16 to 22 carbon atoms and 1, 2 or 3 double bonds.
- the preferred lower alkyl esters of fatty acids are the methyl esters of oleic acid, linoleic acid, linolenic acid and erucic acid.
- rapeseed methyl ester Most preferred as a vegetable-based fuel oil is rapeseed methyl ester.
- the fuel comprises the above-defined biofuels (either alone, or in combination with other fuels from other sources, such as petroleum-based fuels)
- the amount of the stabiliser compound should typically exceed 20 ppm weight (per weight of fuel), more preferably 25 to 200 ppm of stabiliser compound. This effect is particularly prevalent with lower alkyl esters of fatty acids, such as rapeseed and other vegetable oil methyl esters.
- the additive compositions and/or the fuel compositions of the invention may additionally comprise one or more other fuel additives, or co-additives, as indicated above.
- fuel additives include other lubricity-enhancing compounds; cold flow improvers such as ethylene-unsaturated ester copolymers, hydrocarbon polymers, polar nitrogen compounds, alkylated aromatics, linear polymer compounds and comb polymers; detergents; corrosion inhibitors (anti-rust additives); dehazers; demulsifiers; metal deactivators; antifoaming agents; combustion improvers such as cetane improvers; cosolvents; package compatibilisers; reodorants; and metallic-based additives such as metallic combustion improvers.
- cold flow improvers such as ethylene-unsaturated ester copolymers, hydrocarbon polymers, polar nitrogen compounds, alkylated aromatics, linear polymer compounds and comb polymers
- detergents corrosion inhibitors (anti-rust additives); dehazers; demulsifiers; metal deactivators;
- inventive diesel fuel compositions can contain other fuel additives which are well known to those of skill in the art. These include dyes, cetane improvers, rust inhibitors such as alkylated succinic acids and anhydrides, bacteriostatic agents, gum inhibitors, metal deactivators, demulsifiers, upper cylinder lubricants and anti-icing agents and antioxidants.
- Stabilised compositions of this invention will preferably contain one or more of the various lubricity additives which are now commonly used in low sulphur fuels, i.e., fuels having less than 0.2 wt % sulphur, preferably less than 0.1 wt % such as 0.005 or 0.001 wt % sulphur or less.
- Such lubricity additives include monohydric or polyhydric alcohol esters of C 2 -C 50 carboxylic acids such as glycerol monooleate, esters of polybasic acids with C 1 -C 5 monohydric alcohols, esters of dimerized carboxylic acids, reaction products of polycarboxylic acids and epoxides such as 1,2-epoxyethane and 1,2-epoxypropane and lubricity additives derived from fatty acids such as vegetable oil fatty acid methyl esters, as well as fatty acid amides of monoethanolamine and diethanolamine.
- carboxylic acids such as glycerol monooleate
- esters of polybasic acids with C 1 -C 5 monohydric alcohols esters of dimerized carboxylic acids
- reaction products of polycarboxylic acids and epoxides such as 1,2-epoxyethane and 1,2-epoxypropane
- lubricity additives derived from fatty acids such as vegetable oil
- lubricity additives prepared by combining the aforesaid esters of C 2 -C 50 carboxylic acids with an ashless dispersant comprising an acylated nitrogen compound having a hydrocarbyl substituent of at least 10 carbon atoms made by reacting an acylating agent with an amino compound, such as the reaction products of polyisobutenyl (C 80 -C5 00 ) succinic anhydride with ethylene polyamines having 3 to 7 amino nitrogen atoms.
- lubricity additives are combinations of the aforesaid esters with ethylene-unsaturated ester copolymers having, in addition to units derived from ethylene, units of the formula -CR 1 R 2 -CHR 3 - wherein R 1 represents hydrogen or methyl; R 2 represents COOR 4 , wherein R 4 represents an alkyl group having from 1 to 9 carbon atoms which is straight chain or, if it contains 2 or more carbon atoms, branched, or R 2 represents OOCR 5 , wherein R 5 represents R 4 or H; and R 3 represents H or COOR 4 .
- Examples are ethylene-vinyl acetate and ethylene-vinyl propionate and other copolymers where there is present 5-40% of the vinyl ester.
- the lubricity additive may comprise one or more carboxylic acids of the types disclosed in relation to the ester lubricity additives.
- Such acids may be mono- or polycarboxylic, saturated or unsaturated, straight or branched chain and may be generalised by the formula R 1 (COOH) x where x is 1-4 and R 1 is a C 2 to C 50 hydrocarbyl.
- Examples are capric, lauric, myristic, palmitic, oleic, elaidic, palmitoleic, petaoselic, ricinoleic, linoleic, linolenic, eicosanic, tall oil fatty and dehydrated castor oil fatty acids, and rosin acids and isomers and mixtures thereof.
- the polycarboxylic acid may be a dimer acid such as that formed by dimerization of unsaturated fatty acids such as linoleic or oleic acid.
- R 1 is an alkenyl radical having one or more double bonds or an alkyl radical and containing from 4 to 50 carbon atoms, or a radical of the formula where each of R 2 , R 3 , R 4 , R 5 , R 6 and R 7 is independently hydrogen or a lower alkyl radical
- R 8 is an alkenyl radical having one or more double bonds or an alkyl radical and containing from 4 to 50 carbon atoms
- R 9 is an alkylene radical containing from 2 to 35, e.g. 2 to 6, carbon atoms
- each of p, q and v is an integer between 1 and 4
- each of a, b and c may be 0, providing that at least one of a, b or c is an integer between 1 and 75.
- lubricity additives are ester, amine and amine salt derivatives of salicylic acid and alkylated salicylic acids.
- the additives of the invention may also be used in combination with diesel performance additives such as silicon-containing anti-foam agents such as siloxane block copolymers or cetane improvers such as 2-ethyl hexyl nitrate.
- diesel performance additives such as silicon-containing anti-foam agents such as siloxane block copolymers or cetane improvers such as 2-ethyl hexyl nitrate.
- the additives of the present invention may also be used in combination with an appropriate carrier liquid or organic solvent.
- carrier liquid are organic solvents including hydrocarbon solvents, for example petroleum fractions such as naphtha, kerosene, diesel and heater oil; aromatic hydrocarbons such as aromatic fractions, e.g. those sold under the 'SOLVESSO' tradename; paraffinic hydrocarbons such as hexane and pentane and isoparaffins, e.g., those sold under the 'ISOPAR' tradename; and oxygenated solvents such as alcohols.
- the carrier liquid must, of course, be selected having regard to its compatibility with the additive and with the fuel.
- the stabiliser composition can be any suitable stabiliser composition.
- additive compositions of the invention may be incorporated into bulk fuel oil by methods such as those known in the art. If co-additives are required, they may be incorporated into the bulk fuel oil at the same time as the additives of the invention or at a different time.
- PIB 1000 SA succinic anhydride (10 g, 9.5 mmol), toluene (50 ml) and deionised water (15 ml, excess) were heated with stirring under reflux ( ⁇ 85°C) for 6 hours.
- PIB 1000 SA (10 g, 9.5 mmol), toluene (50 ml) and isopropanol (50 ml, excess) were heated with stirring under reflux ( ⁇ 85°C) for 6 hours.
- Diesel Fuel A German Low Sulphur CFPP (°C) -10 Cloud Point (°C) -9 Density @15 deg.C (kg/l) 0.827 Sulphur ⁇ 10 ppm D86 Distillation (°C) IBP 179 10% 198 50% 248 95% 340 FBP 353 Diesel Fuel B: German Low Sulphur CFPP (°C) -9 Cloud Point (°C) -8 Density @15 deg.C (kg/l) 0.831 Sulphur ⁇ 10 ppm D86 Distillation (°C) IBP 174 10% 204 50% 264 95% 347 FBP 359 Diesel Fuel C: Spanish 300 ppm Sulphur KV@40C (cSt) 3.133 Sulphur content (ppm) 300 Density @15C Kg/litre 833.9 Cloud Point (°C) +1 IBP (°C) 183.7 10% Recovery 224.3 50% Recovery 281 95% Recovery 353.5 FBP 368.9 Diesel Fuel D: Swedish Class 1 Test Result Units Cloud point (Auto
- Example 4 Investigation of Stability of Colloidal Cerium DPF System in German Low Sulphur ( ⁇ 10 ppm) Diesel Fuels A and B at 80°C
- Tables 1 and 2 detail stability results for the colloidal cerium-based DPF additive Eolys®, a cerium containing oil fuel additive marketed by "Rhodia Electronics and Catalysis", a Rhodia Group subsidiary, in low sulphur diesel fuels in the presence of various lubricity improver additive chemistries and in the presence of percentage levels of biodiesel (rapeseed oil methyl ester - RME) respectively.
- the stability test involved the separate addition of the additive(s) to the respective fuel using normal laboratory blending practices, and thereafter visually observing the blended fuel composition for phase separation and general appearance whilst being stored at 80°C.
- phase sep. phase sep. Fuel B 25 400 phase sep. phase sep. phase sep. Fuel B 25 200 phase sep. phase sep. phase sep. Fuel B 25 400 phase sep. phase sep. phase sep. Fuel B 25 5% phase sep. phase sep. phase sep. Fuel B 25 30% hazy phase sep. phase sep.
- Example 5 Investigation of Stability of Colloidal Cerium DPF Additive in the Presence of Commercial Lubricity Additives in Diesel Fuel C (300 ppm sulphur) and Fuel D (Swedish Class 1, ⁇ 10 ppm sulphur) at 80°C
- Example 6 Improved Stability of Colloidal Cerium in the Presence of Lubricity Additives when using Stabiliser A of the Present Invention (Part I)
- Stabiliser B (Comparative) is able to stabilise the Ce-based composition up to 7 days.
- Stabiliser A at 50-100 ppm is able to stabilise the similar composition for up to 12 days versus the control samples which exhibit the onset of phase separation from initial sample blending.
- Both A and B are capable of stabilizing the compositions to such an extent that clear compositions are formed, in some cases for extended periods (and particularly with the stabiliser A).
- low levels of PIBSA-PAM (10-100 ppm) a polyisobutenyl succinimide diesel detergent, which contains one imide polar head group per lipophilic chain, does not provide sufficient stabilization to form clear compositions.
- Example 7 Improved Stability of Colloidal Cerium in the Presence of Commercial Lubricity Additives When Using Stabilisers of the Present Invention (Part II)
- Table 5 gives further stability data for the stabilised Ce colloid, Eolys® in the presence of various lubricity additives.
- 35-50 ppm of the stabiliser C is able to stabilise Eolys® for up to 8 days static storage at 80°C in the presence of large amounts (200 ppm) of lubricity additives I and IV, compared to control samples.
- the same stabiliser molecule at 50 ppm is able to control the stability of the Ce colloid in the presence of lubricity improver II for up to 5 days.
- Example 8 Improved Stability of Colloidal Metal Oxide DPF Additives in Fuel D When Using Stabilisers of the Present Invention (Part III)
- lubricity improver ('LI') chemistry types V and VI* offer no stabilising effect in this system.
- Table 7 indicates the excellent stability that may be achieved by adding Stabiliser A from Example 1 directly into a stock solution of Eolys® 176, prior to doping the mixture into either Class 1 diesel fuel E or a fuel composition comprising fuel E and the biofuel fatty acid methyl ester ('FAME').
- results indicate the markedly improved stability that may be achieved from adding the stabiliser moiety directly into the colloidal metal catalyst concentrate prior to doping the mixtures into the fuel.
- results may also be compared to those obtained from adding the stabiliser and colloidal DPF additive separately into a fuel composition comprising petroleum-derived diesel fuel and the biofuel fatty acid methyl ester ('FAME') (previous Example 8 above). It is believed that the pre-addition of the stabiliser to the metal additive concentrate causes the re-organisation of the colloidal metal complex in such a way that its oil soluble or oil dispersible character is substantially improved, leading to better performance when subsequently blended into the fuel.
- Tables 8 and 9 give analytical results for Ce and Fe from samples obtained from the fuel tank of a Peugeot 307 car operated on (i) the mixed cerium and iron additive described in the previous two examples, and (ii) the cerium-only additive described in earlier examples, when used in Fuel F.
- This Ford car was equipped (as standard factory fit) with a separate on-board tank for storage of a diesel particulate trap additive concentrate, this additive being introduced into the fuel contained in the tank of the car under the control of the on-board engine management system, to achieve the regeneration of the particulate trap.
- the factory-filled additive was replaced by untreated test fuel (ie. containing no additive) to avoid subsequent interference in the test, and the fuel tank filled with test fuel already comprising the mixed cerium and iron additive and stabiliser A previously described, these additives having been added separately to the fuel.
- the stability of the fuel - additive mixture in the car fuel tank was thereafter assessed by analysis at periodic intervals during the running of the vehicle. At each assessment, sampling from both the top and bottom of the tank fuel gave an indication of the degree of phase separation and/or settlement of the metal additive from the fuel during the normal running of the vehicle.
- test fuel contained 25 ppm (weight) of stabiliser A and additionally contained a constant level of lubricity additive LI-I.
- Table 8 - mixed cerium and iron additive Test sample Ce (ppm) Fe (ppm) Initial sample 0.1 0.5 One hour in test, top tank 6.1 2.7 One hour in test, bottom tank 6 2.7 Halfway through test, top tank 6.1 2.8 Halfway through test, bottom tank 5.8 2.7 End of test + 1 hour 5.2 2.4 After fuel drained 5.3 2.5
- Table 8 demonstrate the maintenance of equivalent levels of metals throughout the fuel tank during the test, consistent with effective stabilization of the metal additive within the fuel.
- Table 9 cerium-only additive Test sample Ce (ppm) with Stabiliser A Initial sample 21 One hour in test, top tank 22 One hour in test, bottom tank 22 Halfway through test, top tank 21 Halfway through test, bottom tank 21 End of test + 1 hour 16 After fuel drained 22 Test sample Ce (ppm) without Stabiliser A Initial sample 0.5 One hour in test, top tank 0.5 One hour in test, bottom tank 0.5 Halfway through test, top tank 0.5 Halfway through test, bottom tank 0.5 End of test + 1 hour 0.5 After fuel drained 0.5
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Emergency Medicine (AREA)
- Combustion & Propulsion (AREA)
- Liquid Carbonaceous Fuels (AREA)
Description
- This invention relates to novel fuel additive compositions. More particularly, this invention relates to fuel compositions containing metallic additives which are stabilised against phase separation. Metallic additives are added to fuels since they are especially effective in improving the performance of particulate traps which are used in the exhaust systems of diesel engines, amongst other uses.
- Diesel engines equipped with particulate traps, mounted in the exhaust stream, to "trap" or collect particulates in the exhaust to prevent their emission to the atmosphere are expected to be in greater use in the next few years.
- Diesel engines running without particulate traps emit unburned hydrocarbons (HC), carbon monoxide (CO), nitrogen oxides (NOx), and particulates, all of which are subject to current or proposed regulation. The problems of controlling these pollutants are compounded because there is a trade-off between particulates and nitrogen oxides: when the combustion conditions are modified to favor low nitrogen oxides emissions, particulates are increased. Particulate traps are employed to reduce the severity of the particulate emissions.
- Diesel particulates, their effect and control, are at the center of much concern and controversy. Their chemistry and environmental impact present complex issues. Generally, the diesel particulate matter is principally solid particles of carbon and metal compounds with adsorbed hydrocarbons, sulphates and aqueous species. Among the adsorbed species are aldehydes and polycyclic aromatic hydrocarbons. Some of these organics have been reported to be potential carcinogens or mutagens. Unburned hydrocarbons are related to the characteristic diesel odour and include aldehydes such as formaldehyde and acrolein. The need to control nanoparticles is likely to lead to mandates requiring traps.
- The use of diesel traps and the need to improve them has resulted in a great deal of research and a great number of patents and technical publications. The traps are typically constructed of metal or ceramic and are capable of collecting the particulates from the exhaust and withstanding the heat produced by oxidation of carbonaceous deposits which must be burned off at regular intervals.
- This burning off, or regeneration, could occur by itself if the operating temperature of the trap were sufficiently high. However, in the typical situation, the exhaust temperature is not constantly high enough, and secondary measures such as electrically heating to raise the trap temperature or using a catalyst on the washcoat to reduce the combustion temperature of particulates, have not been fully successful.
- The use of organometallic salts and complexes to improve the operation of diesel engine particulate traps is disclosed, for example, in
U.S. Patent No. 5,344,467 issued September 6, 1994 , which teaches the use of a combination of an organometallic complex and an antioxidant. The organometallic complex is soluble or dispersible in the diesel fuel and is derived from an organic compound containing at least two functional groups attached to a hydrocarbon linkage. -
WO 99/36488 published July 22, 1999 -
WO 94/11467 published May 26, 1994 - Co-pending European application
EP-A-1 344 809 describes a diesel fuel composition which improves the diesel engine particulate traps, comprising a metal salt-ashless detergent additive combination effective in improving the performance of the particulate trap. A wide variety of metals are disclosed, in the context of organo-metallic salts. -
US-A-3,438,756 describes barium compounds provided to fuels to reduce smoking and comprising the reaction product of alkylphenol with a relatively high molecular weight alkenyl succinic acid and barium carbonate, prepared by carbonation of barium oxide dispersed in the presence of the alkyl phenol and alkenyl succinic acid. -
EP 671205 EP 599717 EP 575189 - A problem observed in connection with the formulation of diesel fuels having solubilised or colloidally dispersed metals such as metal oxides is the tendency to undergo phase separation upon storage, as evidenced by formation of haze or actual separation of layers. This problem is even more pronounced in connection with low sulphur diesel fuels which also contain a variety of additives.
- The present invention is based upon a discovery that such fuels may be stabilised against such phase separation or haze formation by the addition of very small amounts of an oil-dispersible or oil-soluble compound having two or more contiguous polar head groups.
- In accordance with this invention there have been discovered diesel fuel compositions stabilised against phase separation comprising a diesel fuel, a colloidally dispersed or solubilised metal catalyst compound for diesel particulate trap regeneration and an oil soluble or oil dispersible organic compound having a lipophilic hydrocarbyl chain having attached directly thereto at least two contiguous polar head functional groups, the organic compound being present in an amount effective to stabilise the metal catalyst compound against phase separation, wherein the metal catalyst compound consists of cerium or iron oxides or mixtures thereof, and wherein the composition of the invention is further particularised in claim 1.
- The organic compound is generally present in an amount of 10 to 1,000 ppm, preferably 10 to 200 ppm, most preferably 10 to 50 ppm (weight) of compound per weight of diesel fuel composition in order to effectively stabilise the metal catalyst compound.
- In this specification, the term 'contiguous polar head functional groups' is used to represent polar (functional chemical) groups which are separated by no more than three, preferably no more than two carbon atoms within the molecule.
- The invention is particularly applicable to diesel fuel compositions which contain as catalysts for diesel particulate trap regeneration effective amounts of metallic compounds, typically sufficient to provide 1 to 200, 1 to 100, 1 to 20, 1 to 10, or 1 to 5 ppm metal (by weight) in the fuel, in the form of colloidally dispersed or solubilised compounds.
- The composition's metal catalyst compound consists of cerium or iron oxides or mixtures thereof.
- The stabiliser compound of the present invention is represented by the generalised formula A-C-B, where C represents a hydrocarbyl chain of Mn (number average molecular weight) 200 - 4,000, preferably 200 - 1,300, more preferably 200 - 1,000 such as 400 - 1,000, 700-1000 or 450 - 700.
-
- In the formula above, n may be 1-20, but is preferably 1-10, more preferably 1-5. When n is greater than 1, the stabiliser includes compounds of the following formulas, where "PIB" is polyisobutenyl, "PIBSA" is polyisobutenyl succinic anhydride and R is the lipophilic hydrocarbyl group:
- This hydrocarbyl chain may be straight or branched, but branched hydrocarbyl chains are preferred because of their increased degree of solubility and preferably the hydrocarbyl chain is a polyisobutenyl group in the molecular weight ranges given above. In the aforesaid formula A and B represent at least two contiguous carboxylate residues attached directly to one end of the lipophilic C chain, , being either groups of the formula -COOH or ionized as -(COO-)nMn+ where M may be a uni- or dipositively charged metal cation (ie, where n = 1 or 2) or a quaternary ammonium cation. Typical examples of suitable quaternary ammonium cations are the ammonium ion itself, NH4 +, and the following quaternary ammonium cations R4N+, R3NH+, R2NH2 +, RNH3 + derived from tertiary, secondary and primary amines respectively where R = H or a straight or branched alkyl chain or aromatic moiety containing from 1 to 22 carbon atoms.
- Compounds with two contiguous groups that are capable of binding or otherwise coordinating to a metal or metal oxide moiety are known in the art as bidentate. By varying the nature of the carboxylate derivative used, the head grouping can be made to be tridentate, tetradentate and polydentate in surface binding ability.
- Particularly preferred stabiliser additives for use in the composition of the present invention are polyisobutenyl succinic acid wherein the polyisobutenyl group has a Mn of 1,000, and polyisobutenyl succinic acid wherein the polyisobutenyl group has a molecular weight of 450.
- Further embodiments of this invention comprise fuel compositions comprising the stabiliser compound, metal catalyst compound and one or more other fuel additive compounds, such as a lubricity enhancing additive, diesel detergent additive or a cold flow additive.
- Still further embodiments comprise additive concentrate compositions containing 3 to 75 % by weight of the stabiliser of this invention, in combination with the particulate trap metal catalyst compound, optionally in further combination with one or more other fuel additive compounds such as a lubricity enhancing additive, diesel detergent additive or a cold flow additive as described herein below.
- A concentrate comprising the additive dispersed in carrier liquid (e.g. in solution) is convenient as a means of incorporating the additive. The concentrates of the present invention are convenient as a means for incorporating the additive into bulk oil such as distillate fuel, which incorporation may be done by methods known in the art. The concentrates may also contain other additives as required and preferably contain from 3 to 75 wt %, more preferably 3 to 60 wt %, and most preferably 10 to 50 wt % of the additive or additives preferably in solution in oil. Examples of carrier liquid are organic solvents including hydrocarbon solvents, for example petroleum fractions such as naphtha, kerosene, diesel and heater oil; aromatic hydrocarbons such as aromatic fractions, e.g. those sold under the 'SOLVESSO' trade name; alcohols and/or esters; and paraffinic hydrocarbons such as hexane and pentane and isoparaffins. Higher boiling paraffinic liquids are preferred. Alkylphenols, such as nonylphenol and 2,4-di-t-butylphenol either alone or in combination with any of the above have also been found to be particularly useful as carrier solvents. The carrier liquid must, of course, be selected having regard to its compatibility with the additive and with the fuel.
- Further embodiments of the invention include :
- the use, in a diesel fuel composition comprising a diesel fuel and the colloidally dispersed or solubilised metal catalyst compound as defined above, of the oil soluble or oil dispersible organic compound defined above to reduce the tendency of the diesel fuel and the colloidally dispersed or solubilised metal catalyst compound to form separate phases within the diesel fuel composition over time;
- the use of the oil soluble or oil dispersible organic compound defined above, in an additive concentrate comprising the metal catalyst compound defined above, to improve the colloidal dispersability or solubility of the metal catalyst compound in diesel fuel; and
- a process for enhancing the oil dispersibility or solubility of the metal catalyst compound defined above, comprising the addition thereto of the oil soluble or oil dispersible organic compound defined above :
- (a) either to a diesel fuel composition comprising a diesel fuel and the metal catalyst compound,
- (b) or, preferably, to an additive concentrate containing the metal catalyst compound,
- (c) or to both.
- Examples of other stabiliser compounds are as follows wherein R is hydrocarbyl:
- the acylated aspartic and glutamic acid moieties
- The fuel oil may be a petroleum-based fuel oil, suitably a middle distillate fuel oil, i.e. a fuel oil obtained in refining crude oil as the fraction between the lighter kerosene and jet fuels fraction and the heavy fuel oil fraction. Such distillate fuel oils generally boil above about 100°C. The fuel oil can comprise atmospheric distillate or vacuum distillate, or cracked gas oil or a blend in any proportion of straight run and thermally and/or catalytically cracked and/or hydroprocessed distillates. The most common petroleum-based fuel oils are kerosene, jet fuels and preferably diesel fuel oils.
- The sulphur content of the fuel oil may be 2000 or less, preferably 500 or less, more preferably 50 or less, most preferably 10 or less, ppm by mass based on the mass of the fuel oil. The art describes methods for reducing the sulphur content of hydrocarbon middle distillate fuels, such methods including solvent extraction, sulphuric acid treatment, and hydrodesulphurisation.
- Preferred fuel oils have a cetane number of at least 40, preferably above 45 and more preferably above 50. The fuel oil may have such cetane numbers prior to the addition of any cetane improver or the cetane number of the fuel may be raised by the addition of a cetane improver.
- Advantageously, the fuel oils are those that have low solvency properties caused by low aromatic concentrations (e.g. below 30, below 20, below 15, below 10, or below 5, mass per cent), and/or those that are required to operate at low temperatures such as at -5, -10, -15, or -20, °C or lower.
- Other examples of fuel oils include jet-fuels; Fischer-Tropsch fuels; biofuels such as fuels made from vegetable matter such as rape seed methyl ester; and diesel/alcohol or diesel/water emulsions or solutions. Fischer-Tropsch fuels, also known as FT fuels, include those that are described as gas-to-liquid fuels and coal conversion fuels. To make such fuels, syngas (CO + H2) is first generated and then converted to normal paraffins by a Fischer-Tropsch process. The normal paraffins may then be modified by processes such as catalytic cracking/reforming or isomerisation, hydrocracking and hydroisomerisation to yield a variety of hydrocarbons such as iso-paraffins, cyclo-paraffins and aromatic compounds. The resulting FT fuel can be used as such or in combination with other fuel components and fuel types such as those mentioned in this specification. Also suitable are fuels emulsified with water and alcohols, which contain suitable surfactants, and residual fuel oil used in marine diesel engines.
WO-A- 0104239 WO-A- 0015740 WO-A-0151593 WO-A- 9734969 WO-155282 WO-A- 0031216 WO-A- 9817745 WO-A- 024 8294 -
- Generally, such oils contain glycerides of a number of acids, the number and kind varying with the source vegetable of the oil. Suitable fuel oils also include mixtures of 1-100% by weight of vegetable oils or methylesters of fatty acid, with petroleum based diesel fuel oils.
- Examples of oils and methyl ester derived fuel are tall oil, rapeseed oil, coriander oil, soyabean oil, cottonseed oil, sunflower oil, castor oil, olive oil, peanut oil, maize oil, almond oil, palm kernel oil, coconut oil, mustard seed oil, beef tallow and fish oils. Rapeseed oil, which is a mixture of fatty acids esterified with glycerol, is preferred as it is available in large quantities and can be obtained in a simple way by pressing from rapeseed.
- Further preferred examples of vegetable-based fuel oils are alkyl esters, such as methyl esters, of fatty acids of the vegetable or animal oils. Such esters can be made by transesterification.
- As lower alkyl esters of fatty acids, consideration may be given to the following, for example as commercial mixtures containing, for example: the ethyl, propyl, butyl and especially methyl esters of fatty acids with 12 to 22 carbon atoms, for example, mixtures of lauric acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, elaidic acid, petroselic acid, ricinoleic acid, elaeostearic acid, linoleic acid, linolenic acid, eicosanoic acid, gadoleic acid, docosanoic acid or erucic acid, and rosin acid and isomers which have an iodine number from 50 to 180, especially 90 to 180. Mixtures with particularly advantageous properties are those which contain mainly, i.e. to at least 50 wt % methyl esters of fatty acids with 16 to 22 carbon atoms and 1, 2 or 3 double bonds. The preferred lower alkyl esters of fatty acids are the methyl esters of oleic acid, linoleic acid, linolenic acid and erucic acid.
- Commercial mixtures of the stated kind are obtained for example by cleavage and esterification of natural fats and oils by their transesterification with lower aliphatic alcohols. For production of lower alkyl esters of fatty acids it is advantageous to start from fats and oils with high iodine number, such as, for example, sunflower oil, rapeseed oil, coriander oil, castor oil, soyabean oil, cottonseed oil, peanut oil or beef tallow. Lower alkyl esters of fatty acids based on a new variety of rapeseed oil, the fatty acid component of which is derived to more than 80 wt % from unsaturated fatty acids with 18 carbon atoms, are preferred.
- Most preferred as a vegetable-based fuel oil is rapeseed methyl ester.
- Where the fuel comprises the above-defined biofuels (either alone, or in combination with other fuels from other sources, such as petroleum-based fuels), it has been found that higher proportions of the stabiliser compound can be required to impart effective stability against phase separation. Thus, in such embodiments, the amount of the stabiliser compound should typically exceed 20 ppm weight (per weight of fuel), more preferably 25 to 200 ppm of stabiliser compound. This effect is particularly prevalent with lower alkyl esters of fatty acids, such as rapeseed and other vegetable oil methyl esters.
- The additive compositions and/or the fuel compositions of the invention may additionally comprise one or more other fuel additives, or co-additives, as indicated above. Examples include other lubricity-enhancing compounds; cold flow improvers such as ethylene-unsaturated ester copolymers, hydrocarbon polymers, polar nitrogen compounds, alkylated aromatics, linear polymer compounds and comb polymers; detergents; corrosion inhibitors (anti-rust additives); dehazers; demulsifiers; metal deactivators; antifoaming agents; combustion improvers such as cetane improvers; cosolvents; package compatibilisers; reodorants; and metallic-based additives such as metallic combustion improvers.
- The inventive diesel fuel compositions can contain other fuel additives which are well known to those of skill in the art. These include dyes, cetane improvers, rust inhibitors such as alkylated succinic acids and anhydrides, bacteriostatic agents, gum inhibitors, metal deactivators, demulsifiers, upper cylinder lubricants and anti-icing agents and antioxidants.
- Stabilised compositions of this invention will preferably contain one or more of the various lubricity additives which are now commonly used in low sulphur fuels, i.e., fuels having less than 0.2 wt % sulphur, preferably less than 0.1 wt % such as 0.005 or 0.001 wt % sulphur or less. Such lubricity additives include monohydric or polyhydric alcohol esters of C2-C50 carboxylic acids such as glycerol monooleate, esters of polybasic acids with C1-C5 monohydric alcohols, esters of dimerized carboxylic acids, reaction products of polycarboxylic acids and epoxides such as 1,2-epoxyethane and 1,2-epoxypropane and lubricity additives derived from fatty acids such as vegetable oil fatty acid methyl esters, as well as fatty acid amides of monoethanolamine and diethanolamine.
- Further examples are lubricity additives prepared by combining the aforesaid esters of C2-C50 carboxylic acids with an ashless dispersant comprising an acylated nitrogen compound having a hydrocarbyl substituent of at least 10 carbon atoms made by reacting an acylating agent with an amino compound, such as the reaction products of polyisobutenyl (C80-C500) succinic anhydride with ethylene polyamines having 3 to 7 amino nitrogen atoms.
-
- Other lubricity additives are combinations of the aforesaid esters with ethylene-unsaturated ester copolymers having, in addition to units derived from ethylene, units of the formula
-CR1R2-CHR3-
wherein R1 represents hydrogen or methyl; R2 represents COOR4, wherein R4 represents an alkyl group having from 1 to 9 carbon atoms which is straight chain or, if it contains 2 or more carbon atoms, branched, or R2 represents OOCR5, wherein R5 represents R4 or H; and R3 represents H or COOR4. Examples are ethylene-vinyl acetate and ethylene-vinyl propionate and other copolymers where there is present 5-40% of the vinyl ester. - As an alternative to the above described esters, or in combination therewith, the lubricity additive may comprise one or more carboxylic acids of the types disclosed in relation to the ester lubricity additives. Such acids may be mono- or polycarboxylic, saturated or unsaturated, straight or branched chain and may be generalised by the formula R1(COOH)x where x is 1-4 and R1 is a C2 to C50 hydrocarbyl. Examples are capric, lauric, myristic, palmitic, oleic, elaidic, palmitoleic, petaoselic, ricinoleic, linoleic, linolenic, eicosanic, tall oil fatty and dehydrated castor oil fatty acids, and rosin acids and isomers and mixtures thereof. The polycarboxylic acid may be a dimer acid such as that formed by dimerization of unsaturated fatty acids such as linoleic or oleic acid.
- Other lubricity additives are hydroxy amines of the formula
- Other lubricity additives are ester, amine and amine salt derivatives of salicylic acid and alkylated salicylic acids.
- The additives of the invention may also be used in combination with diesel performance additives such as silicon-containing anti-foam agents such as siloxane block copolymers or cetane improvers such as 2-ethyl hexyl nitrate.
- The additives of the present invention may also be used in combination with an appropriate carrier liquid or organic solvent. Examples of carrier liquid are organic solvents including hydrocarbon solvents, for example petroleum fractions such as naphtha, kerosene, diesel and heater oil; aromatic hydrocarbons such as aromatic fractions, e.g. those sold under the 'SOLVESSO' tradename; paraffinic hydrocarbons such as hexane and pentane and isoparaffins, e.g., those sold under the 'ISOPAR' tradename; and oxygenated solvents such as alcohols. The carrier liquid must, of course, be selected having regard to its compatibility with the additive and with the fuel.
- The stabiliser composition can be
- 1. Preferably added to the DPF (diesel particulate filter) additive composition prior to doping the mixture into the fuel.
- 2. Added to the fuel separately before or after addition of the DPF additive.
- 3. Added to any typical fuel additive composition, e.g., lubricity improver, detergent, cold flow improver, corrosion inhibitor, antistatic or mixtures thereof prior to doping the mixture into the fuel.
- 4. Added to the fuel separately before or after addition of any typical fuel additive composition.
- The additive compositions of the invention, with or without diluent or solvent, may be incorporated into bulk fuel oil by methods such as those known in the art. If co-additives are required, they may be incorporated into the bulk fuel oil at the same time as the additives of the invention or at a different time.
- The invention is further illustrated by the following examples.
-
- PIB1000SA (succinic anhydride) (10 g, 9.5 mmol), toluene (50 ml) and deionised water (15 ml, excess) were heated with stirring under reflux (∼85°C) for 6 hours.
- After cooling, the organic phase was separated and dried over anhydrous MgSO4. After filtration, the solvent was removed in vacuo at low temperature, giving a product which was used directly.
- Infrared: vCO 1714 (free acid) cm-1.
- %C, H, N: C, 80.8; H, 12.5; N, <0.1%.
- TAV = 1.96 Meq/g; TAN = 110 mg KOH/g.
-
- PIB1000SA (10 g, 9.5 mmol), toluene (50 ml) and isopropanol (50 ml, excess) were heated with stirring under reflux (∼85°C) for 6 hours.
- After cooling, the solvent mixture was removed in vacuo at low temperature, giving a partially esterified product which was used directly.
- Infrared: vCO 1713 (free acid); 1734 (ester) cm-1.
- %C, H, N: C, 81.0; H, 12.5; N, 0.2%.
- TAV = 1.37 Meq/g; TAN = 76 mg KOH/g.
- PIB450SA (50 g), Isopar L, a paraffinic solvent, (150 ml) and water (4 ml, excess) were heated with stirring under a reflux (∼120°C) for 12 hours.
- After cooling, the organic phase was separated and dried over anhydrous MgSO4. After filtration, the solvent mixture was removed in vacuo at low temperature, giving a product which was used directly.
- The characteristics of the fuels tested are given below:
-
Diesel Fuel A: German Low SulphurCFPP (°C) -10 Cloud Point (°C) -9 Density @15 deg.C (kg/l) 0.827 Sulphur <10 ppm D86 Distillation (°C) IBP 179 10% 198 50% 248 95% 340 FBP 353
Diesel Fuel B: German Low SulphurCFPP (°C) -9 Cloud Point (°C) -8 Density @15 deg.C (kg/l) 0.831 Sulphur <10 ppm D86 Distillation (°C) IBP 174 10% 204 50% 264 95% 347 FBP 359
Diesel Fuel C: Spanish 300 ppm SulphurKV@40C (cSt) 3.133 Sulphur content (ppm) 300 Density @15C Kg/litre 833.9 Cloud Point (°C) +1 IBP (°C) 183.7 10% Recovery 224.3 50% Recovery 281 95% Recovery 353.5 FBP 368.9
Diesel Fuel D: Swedish Class 1Test Result Units Cloud point (Auto) -40 °C CFPP -40 °C kV @ 40°C (Auto) 1.574 cSt Density @ 15°C 817.6 Kg/m3 Sulphur 9 mg/kg Distillation D86 IBP 190.9 °C 10% 208.8 °C 50% 233.3 °C 95% 278.8 °C FBP 290.1 °C
Diesel Fuel E : Shell Class 1 diesel fuel.
Diesel Fuel F : a further Class 1 diesel fuel. - Tables 1 and 2 detail stability results for the colloidal cerium-based DPF additive Eolys®, a cerium containing oil fuel additive marketed by "Rhodia Electronics and Catalysis", a Rhodia Group subsidiary, in low sulphur diesel fuels in the presence of various lubricity improver additive chemistries and in the presence of percentage levels of biodiesel (rapeseed oil methyl ester - RME) respectively. The stability test involved the separate addition of the additive(s) to the respective fuel using normal laboratory blending practices, and thereafter visually observing the blended fuel composition for phase separation and general appearance whilst being stored at 80°C.
- The results demonstrate that the cerium-based colloid is fundamentally unstable in low sulphur diesel fuel and evidence of gross phase separation of insoluble precipitates is seen within 1 day during storage at 80°C, even in the absence of the lubricity additive or biodiesel.
- The results also show that the RME and the various lubricity improver additives are unlikely to improve the stability of the metal catalyst compound to levels required to ensure safe field operation.
Table 1. Stability of Colloidal Cerium DPF Additive in German Low Sulphur Diesel Fuel A in the Presence of Representative Types of Lubricity Improver Chemistry Fuel Eolys® (ppm Ce) Lubricity Improver Chemistry I (ppm) Lubricity Improver Chemistry II (ppm) Lubricity Improver Chemistry III (ppm) Lubricity Improver Chemistry IV (ppm) RME (%) day 1 day 2 day 4 (end) Fuel A 25 phase sep. phase sep. phase sep. Fuel A 25 200 phase sep. phase sep. phase sep. Fuel A 25 400 phase sep. phase sep. phase sep. Fuel A 25 200 phase sep. phase sep. phase sep. Fuel A 25 400 phase sep. phase sep. phase sep. Fuel A 25 200 phase sep. phase sep. phase sep. Fuel A 25 400 phase sep. phase sep. phase sep. Fuel A 25 200 phase sep. phase sep. phase sep. Fuel A 25 400 phase sep. phase sep. phase sep. Fuel A 25 5% phase sep. phase sep. phase sep. Fuel A 25 30% hazy phase sep. phase sep. RME = Rapeseed Methyl Ester (biodiesel) Table 2. Stability of Colloidal Cerium DPF Additive in German Low Sulphur Diesel Fuel B in the Presence of Representative Types of Lubricity Improver Chemistry Fuel Eolys® (ppm Ce) Lubricity Improver Chemistry I (ppm) Lubricity Improver Chemistry II (ppm) Lubricity Improver Chemistry III (ppm) Lubricity Improver Chemistry IV (ppm) RME (%) day 1 day 2 day 4 (end) Fuel B 25 phase sep. phase sep. phase, sep. Fuel B 25 200 phase sep. phase sep. phase sep. Fuel B 25 400 phase sep. phase sep. phase sep. Fuel B 25 200 phase sep. phase sep. phase sep. Fuel B 25 400 phase sep. phase sep. phase sep. Fuel B 25 200 phase sep. phase sep. phase sep. Fuel B 25 400 phase sep. phase sep. phase sep. Fuel B 25 200 phase sep. phase sep. phase sep. Fuel B 25 400 phase sep. phase sep. phase sep. Fuel B 25 5% phase sep. phase sep. phase sep. Fuel B 25 30% hazy phase sep. phase sep. - It may be observed from Table 3 that the cerium-based DPF additive (Eolys®) is fundamentally unstable with respect to precipitation/phase separation in the presence of various lubricity additive chemistries in these two different diesel fuels. The same test protocol of separate addition of the additives to the fuel was followed.
- This instability of the cerium additive at two concentrations (25 ppm and 250 ppm respectively) is only mitigated in the presence of very high levels (200-1000 ppm) of PIBSA-PAM, a polyisobutenyl succinimide diesel detergent, which contains one imide polar head group per lipophilic chain (ie. not an example according to the invention.)
Table 3. Stability of Cerium-based DPF Additives at 80°C in the Presence of Some Lubricity Additives in Diesel Fuels C and D Diesel Eolys® (ppm Ce) Lubricity Improver Chemistry 1 (ppm) Lubricity Improver Chemistry II (ppm) Lubricity Improver Chemistry IV (ppm) PIBSA-PAM (ppm) 1 hr. day 1 day 4 day 5 day 7 (end) Fuel C 250 200 clear hazy phase sep. phase sep. phase sep. Fuel C 250 200 200 clear clear clear clear clear Fuel C 250 600 600 clear clear hazy hazy hazy Fuel C 250 600 800 clear clear clear clear slightly hazy Fuel C 250 600 1000 clear clear clear clear clear Fuel C 25 clear clear clear hazy phase sep. Fuel C 25 200 clear hazy hazy hazy phase sep. Fuel C 25 200 I 50 clear hazy hazy hazy slightly hazy Fuel C 25 200 200 clear clear clear clear clear Fuel C 25 200 400 clear clear clear clear clear Fuel C 25 200 hazy phase sep. phase sep. phase sep. phase sep. Fuel D 25 200 clear clear/hazy? clear sl phase sep. phase sep. Fuel D 25 200 clear clear phase sep. phase sep. phase sep. Fuel D 25 200 clear clear/hazy? phase sep. phase sep. phase sep. - Results given in Table 4 indicate the significantly improved stability of the colloidal DPF system in static storage at 80°C in diesel fuel C in the presence of a lubricity improver when using low levels of the stabiliser molecules of the present invention (Stabiliser A from Example 1).
- Between 50-100 ppm of Stabiliser B (Comparative) is able to stabilise the Ce-based composition up to 7 days. Stabiliser A at 50-100 ppm is able to stabilise the similar composition for up to 12 days versus the control samples which exhibit the onset of phase separation from initial sample blending. Both A and B are capable of stabilizing the compositions to such an extent that clear compositions are formed, in some cases for extended periods (and particularly with the stabiliser A). In comparison, low levels of PIBSA-PAM (10-100 ppm), a polyisobutenyl succinimide diesel detergent, which contains one imide polar head group per lipophilic chain, does not provide sufficient stabilization to form clear compositions.
- Furthermore, the significantly-improved stability performance of the stabiliser molecule A versus the unreacted starting material, PIBSA, at similar concentration indicates the effectiveness of the present invention in stabilising the cerium colloid in the presence of the lubricity improver additive I.
Table 4. Improved Stability of Cerium-based DPF Additives at 80°C in the Presence of Lubricity Improver in Diesel Fuel C When Using Stabilising Compositions Eolys® (ppm Ce) Lubricity Improver Chemistry I (ppm) Stabiliser A (ppm) Stabiliser B (ppm) PIBSA - PAM (ppm) PIBSA (ppm) Day 0 Day 3 Day 4 Day 5 Day 6 Day 7 Day 10 Day 11 Day 12 Day 13 Day 14 25 200 hazy PS PS PS PS PS PS PS PS PS PS 25 200 10 clear clear clear vs haze s haze hazy v hazy v hazy v hazy sPS s PS 25 200 50 clear clear clear clear clear clear clear clear clear hazy PS 25 200 100 clear clear clear clear clear clear clear clear clear PS PS 25 200 10 hazy PS PS PS PS PS PS PS PS PS PS 25 200 50 clear clear clear clear vs haze hazy v hazy PS PS PS PS 25 200 100 clear clear clear clear clear clear PS PS PS PS PS 25 200 10 v hazy PS PS PS PS PS PS PS PS PS PS 25 200 50 hazy v hazy v hazy v hazy v hazy v hazy s PS s PS s PS s PS s PS 25 200 100 s haze hazy hazy hazy hazy hazy v hazy v hazy v hazy v hazy v hazy 25 200 10 hazy v hazy PS PS PS PS PS PS PS PS PS 25 200 50 clear clear clear s haze hazy s PS PS PS PS PS PS 25 200 100 clear clear clear clear clear clear PS PS PS PS PS PS = phase separation, v = very, s = slight - Table 5 gives further stability data for the stabilised Ce colloid, Eolys® in the presence of various lubricity additives.
- It may be seen that 35-50 ppm of the stabiliser C (from Example 3) is able to stabilise Eolys® for up to 8 days static storage at 80°C in the presence of large amounts (200 ppm) of lubricity additives I and IV, compared to control samples. The same stabiliser molecule at 50 ppm is able to control the stability of the Ce colloid in the presence of lubricity improver II for up to 5 days.
- The various sample controls of Ce colloid and lubricity improver (without stabiliser present) exhibit haze formation and/or phase separation within 1 day. Likewise, the unreacted starting material, PIBSA, shows lesser ability to stabilise the composition against phase separation.
Table 5. Improved Stability of Cerium-based DPF Additives at 80°C in the Presence of Some Commercial Lubricity Additives When Using Stabilising Compositions Fuel Eolys® (ppm Ce) Lubricity Improver Chemistry I (ppm) Lubricity Improver Chemistry II (ppm) Lubricity Improver Chemistry IV (ppm) Stabiliser C (ppm) PIBSA (ppm) Day 0 Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day 8 Fuel C 25 clear PS - - PS PS PS PS PS Fuel C 25 200 v s haze hazy - - PS PS PS PS PS Fuel C 25 200 10 clear clear - - v hazy s PS s PS PS PS Fuel C 25 200 20 clear clear - - clear clear hazy PS PS Fuel C 25 200 35 clear clear - - clear clear clear clear clear Fuel C 25 200 50 clear clear - - clear clear clear clear clear Fuel C 25 200 10 v s haze hazy - - PS PS PS PS PS Fuel C 25 200 20 clear v s haze - - PS PS PS PS PS Fuel C 25 200 35 clear clear - - clear v hazy PS PS PS Fuel C 25 200 50 clear clear - - clear hazy PS PS PS Fuel C 25 200 v hazy PS - - PS PS PS PS PS Fuel C 25 200 10 s hazy hazy - - PS PS PS PS PS Fuel C 25 200 20 v s haze v s haze - - PS PS PS PS PS Fuel C 25 200 35 clear clear - - v hazy PS PS PS PS Fuel C 25 200 50 clear clear - - clear clear hazy PS PS Fuel C 25 200 10 v hazy PS - - PS PS PS PS PS Fuel C 25 200 20 v hazy PS - - PS PS PS PS PS Fuel C 25 200 35 hazy PS - - PS PS PS PS PS Fuel C 25 200 50 hazy sPS - - PS PS PS PS PS Fuel C 25 200 clear PS - - PS PS PS PS PS Fuel C 25 200 10 clear clear - - PS PS PS PS PS Fuel C 25 200 20 clear clear - - clear clear hazy PS PS Fuel C 25 200 35 clear clear - - clear clear clear clear clear Fuel C 25 200 50 clear clear - - clear clear clear clear clear Fuel C 25 200 20 clear clear - - PS PS PS PS PS Fuel C 25 200 35 clear clear - - PS PS PS PS PS Fuel C 25 200 50 clear clear - - clear PS PS PS PS - The results given in the Table 6 indicate the stabilising effect of Stabiliser A from Example 1 on a colloidal mixed cerium oxide and iron oxides additive, 'Eolys® 176', in fuel compositions comprising petroleum-derived diesel fuel D and the biofuel fatty acid methyl ester ('FAME'). In Table 6, the treat rates shown for the CeFe additive are ppm (weight) of total metal in the fuel.
Table 6 Fuel Blend Additives Stability (days) 5% FAME in fuel D composition 10 ppm CeFe 1 10 ppm CeFe + 10 ppm stabiliser A 1 10 ppm CeFe + 25 ppm stabiliser A 2 10 ppm CeFe + 50 ppm stabiliser A 4 10 ppm CeFe + 75 ppm stabiliser A 5 10 ppm CeFe + 100 ppm stabiliser A 5 10 ppm CeFe + 200 ppm LI-V 1 10 ppm CeFe + 200 ppm LI-VI 1 2% FAME in fuel D composition 10 ppm CeFe 1 10 ppm CeFe + 10 ppm stabiliser A 1 10 ppm CeFe + 25 ppm stabiliser A 2 10 ppm CeFe + 50 ppm stabiliser A 5 10 ppm CeFe + 75 ppm stabiliser A 12 10 ppm CeFe + 100 ppm stabiliser A 5 10 ppm CeFe + 200 ppm LI-V 1 10 ppm CeFe + 200 ppm LI-VI 1 - It may be observed that addition of moderate amounts (25-75 ppm) of stabiliser A can markedly improve the stability of the colloidal metallic additive towards phase separation in the presence of the biofuel FAME, compared to the control situation with no stabiliser present.
- Moreover, it may be seen that lubricity improver ('LI') chemistry types V and VI* offer no stabilising effect in this system.
- Table 7 indicates the excellent stability that may be achieved by adding Stabiliser A from Example 1 directly into a stock solution of Eolys® 176, prior to doping the mixture into either Class 1 diesel fuel E or a fuel composition comprising fuel E and the biofuel fatty acid methyl ester ('FAME').
- The results indicate the markedly improved stability that may be achieved from adding the stabiliser moiety directly into the colloidal metal catalyst concentrate prior to doping the mixtures into the fuel. The results may also be compared to those obtained from adding the stabiliser and colloidal DPF additive separately into a fuel composition comprising petroleum-derived diesel fuel and the biofuel fatty acid methyl ester ('FAME') (previous Example 8 above). It is believed that the pre-addition of the stabiliser to the metal additive concentrate causes the re-organisation of the colloidal metal complex in such a way that its oil soluble or oil dispersible character is substantially improved, leading to better performance when subsequently blended into the fuel.
Table 7 Fuel Blend Additives Stability (days) Class I diesel fuel Separate blending into fuel 25 ppm Ce 1 10 ppm CeFe 2 25 ppm Ce + 25 ppm Stabiliser A 4 25 ppm Ce + 50 ppm Stabiliser A 5 10 ppm CeFe + 25 ppm Stabiliser A 5 10 ppm CeFe + 50 ppm Stabiliser A 15 Pre-blend of stabiliser into concentrate 25 ppm Ce + 25 ppm Stabiliser A 5 25 ppm Ce + 50 ppm Stabiliser A 15 10 ppm CeFe + 25 ppm Stabiliser A 10 10 ppm CeFe + 50 ppm Stabiliser A 15 2% FAME in fuel composition 10 ppm CeFe 1 Pre-blend of stabiliser into concentrate 10 ppm CeFe +25 ppm Stabiliser A 16 10 ppm CeFe +50 ppm Stabiliser A 5 10 ppm CeFe +75 ppm Stabiliser A 5 10 ppm CeFe +100 ppm Stabiliser A 15 5% FAME in fuel composition 10 ppm CeFe 1 Pre-blend of stabiliser into concentrate 10 ppm CeFe +25 ppm Stabiliser A 10 10 ppm CeFe +50 ppm Stabiliser A 12 10 ppm CeFe +75 ppm Stabiliser A 16 10 ppm CeFe +100 ppm Stabiliser A 9 - The following Tables 8 and 9 give analytical results for Ce and Fe from samples obtained from the fuel tank of a Peugeot 307 car operated on (i) the mixed cerium and iron additive described in the previous two examples, and (ii) the cerium-only additive described in earlier examples, when used in Fuel F.
- This Peugeot car was equipped (as standard factory fit) with a separate on-board tank for storage of a diesel particulate trap additive concentrate, this additive being introduced into the fuel contained in the tank of the car under the control of the on-board engine management system, to achieve the regeneration of the particulate trap. In these tests, the factory-filled additive was replaced by untreated test fuel (ie. containing no additive) to avoid subsequent interference in the test, and the fuel tank filled with test fuel already comprising the mixed cerium and iron additive and stabiliser A previously described, these additives having been added separately to the fuel. The stability of the fuel - additive mixture in the car fuel tank was thereafter assessed by analysis at periodic intervals during the running of the vehicle. At each assessment, sampling from both the top and bottom of the tank fuel gave an indication of the degree of phase separation and/or settlement of the metal additive from the fuel during the normal running of the vehicle.
- In each table, the test fuel contained 25 ppm (weight) of stabiliser A and additionally contained a constant level of lubricity additive LI-I.
Table 8 - mixed cerium and iron additive Test sample Ce (ppm) Fe (ppm) Initial sample 0.1 0.5 One hour in test, top tank 6.1 2.7 One hour in test, bottom tank 6 2.7 Halfway through test, top tank 6.1 2.8 Halfway through test, bottom tank 5.8 2.7 End of test + 1 hour 5.2 2.4 After fuel drained 5.3 2.5 - The results in Table 8 demonstrate the maintenance of equivalent levels of metals throughout the fuel tank during the test, consistent with effective stabilization of the metal additive within the fuel.
Table 9 - cerium-only additive Test sample Ce (ppm) with Stabiliser A Initial sample 21 One hour in test, top tank 22 One hour in test, bottom tank 22 Halfway through test, top tank 21 Halfway through test, bottom tank 21 End of test + 1 hour 16 After fuel drained 22 Test sample Ce (ppm) without Stabiliser A Initial sample 0.5 One hour in test, top tank 0.5 One hour in test, bottom tank 0.5 Halfway through test, top tank 0.5 Halfway through test, bottom tank 0.5 End of test + 1 hour 0.5 After fuel drained 0.5 - In Table 9, the presence of stabiliser A again leads to maintenance of metal distribution within the tank, in contrast to the control experiment lacking stabiliser A.
Claims (11)
- A diesel fuel composition comprising a diesel fuel, a colloidally dispersed or solubilised metal catalyst compound for diesel particulate trap regeneration and 10 to 1,000 ppm of an oil soluble or oil dispersible organic compound having a lipophilic hydrocarbyl chain having attached directly thereto at least two contiguous polar head functional groups, wherein the metal catalyst compound consists of cerium or iron oxides or mixtures thereof, and wherein the organic compound is represented by the generalised formula A-C-B, where C represents a hydrocarbyl chain of Mn (number average molecular weight) of 200 - 4,000, and A and B represent at least two contiguous carboxylate residues attached directly to one end of the lipophilic C chain, being either groups of the formula -COOH or ionized as -(COO-)nMn+ where M is a uni- or dipositively charged metal cation (i.e. where n = 1 or 2) or a quaternary ammonium cation.
- The composition of claim 1 wherein the hydrocarbyl chain is a polyisobutenyl group of Mn 200 - 4,000.
- The composition of claim 2 wherein the organic compound is polyisobutenyl succinic acid wherein the polyisobutenyl group has a Mn of 1,000 or 450.
- The composition of any preceding claim further comprising a lubricity additive.
- The composition of any preceding claim further comprising a cold flow additive.
- The composition of any preceding claim further comprising a diesel detergent additive.
- The composition of any preceding claim wherein the diesel fuel contains 2000 ppm or less of sulphur.
- A diesel fuel additive concentrate composition comprising a carrier fluid, the colloidally dispersed or solubilised metal catalyst compound defined in claim 1, and 3 to 75 wt. % of a stabiliser additive comprising the oil soluble or oil dispersible organic compound defined in any of claims 1 to 3.
- The use, in a diesel fuel composition comprising a diesel fuel and the colloidally dispersed or solubilised metal catalyst compound defined in claim 1, of the oil soluble or oil dispersible organic compound defined in any of claims 1 to 3, to reduce the tendency of the diesel fuel and the colloidally dispersed or solubilised metal catalyst compound to form separate phases within the diesel fuel composition over time.
- The use of the oil soluble or oil dispersible organic compound defined in any of claims 1 to 3 in an additive concentrate comprising the metal catalyst compound defined in claim 1 to improve the colloidal dispersability or solubility of the metal catalyst compound in diesel fuel.
- A process for enhancing the oil dispersibility or solubility of the metal catalyst compound defined in claim 1, comprising the addition thereto of the oil soluble or oil dispersible organic compound defined in any of claims 1 to 3:(a) either to a diesel fuel composition comprising a diesel fuel and the metal catalyst compound,(b) or, preferably, to an additive concentrate containing the metal catalyst compound,(c) or to both.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP04255073.1A EP1512736B2 (en) | 2003-09-05 | 2004-08-24 | Stabilised diesel fuel additive compositions |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP03255551 | 2003-09-05 | ||
| EP03255551 | 2003-09-05 | ||
| EP04255073.1A EP1512736B2 (en) | 2003-09-05 | 2004-08-24 | Stabilised diesel fuel additive compositions |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP1512736A1 EP1512736A1 (en) | 2005-03-09 |
| EP1512736B1 true EP1512736B1 (en) | 2018-05-02 |
| EP1512736B2 EP1512736B2 (en) | 2025-12-17 |
Family
ID=34137600
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP04255073.1A Expired - Lifetime EP1512736B2 (en) | 2003-09-05 | 2004-08-24 | Stabilised diesel fuel additive compositions |
Country Status (1)
| Country | Link |
|---|---|
| EP (1) | EP1512736B2 (en) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1731590A3 (en) * | 2005-06-06 | 2007-09-05 | Peugeot Citroën Automobiles S.A. | Additive for a device for automatic supply of means forming an additive into a motor vehicle fuel tank. |
| FR2886647B1 (en) * | 2005-06-06 | 2010-06-25 | Peugeot Citroen Automobiles Sa | ADDITIVE FOR A DEVICE FOR AUTOMATICALLY INTRODUCING MEANS FORMING ADDITIVE IN A FUEL TANK OF A MOTOR VEHICLE |
| FR2886649B1 (en) * | 2005-06-06 | 2010-06-11 | Peugeot Citroen Automobiles Sa | ADDITIVE FOR A DEVICE FOR AUTOMATICALLY INTRODUCING MEANS FORMING ADDITIVE IN A FUEL TANK OF A MOTOR VEHICLE |
| FR2886648B1 (en) * | 2005-06-06 | 2007-09-21 | Peugeot Citroen Automobiles Sa | ADDITIVE FOR A DEVICE FOR AUTOMATICALLY INTRODUCING MEANS FORMING ADDITIVE IN A FUEL TANK OF A MOTOR VEHICLE |
| US7959693B2 (en) | 2005-11-18 | 2011-06-14 | Ferox, LLC | Combustion catalyst carriers and methods of using the same |
| GB0705922D0 (en) * | 2007-03-28 | 2007-05-09 | Infineum Int Ltd | Process for the manufacture of a colloid of iron oxide |
| GB0705920D0 (en) * | 2007-03-28 | 2007-05-09 | Infineum Int Ltd | Method of supplying iron to the particulate trap of a diesel engine exhaust |
| GB2447922C (en) * | 2007-03-28 | 2011-03-09 | Infineum Int Ltd | Iron-containing polymer suitable for regenerating diesel exhaust particulate traps. |
| US7901472B2 (en) | 2007-08-29 | 2011-03-08 | Conseal International Incorporated | Combustion modifier and method for improving fuel combustion |
| JP5551244B2 (en) | 2009-06-23 | 2014-07-16 | ロディア オペレーションズ | Combination of synergistic detergent and active metal compound |
| FR2969655B1 (en) * | 2010-12-22 | 2014-01-10 | Rhodia Operations | FUEL ADDITIVE COMPOSITION BASED ON AN IRON PARTICLE DISPERSION AND A POLYESTER QUATERNARY AMMONIUM SALT DETERGENT |
| CN106753617A (en) * | 2017-01-22 | 2017-05-31 | 甘肃华新能源科技有限公司 | Automobile nutrient |
| EP3839014A3 (en) * | 2019-12-20 | 2021-09-15 | Infineum International Limited | Process for commissioning an exhaust particulate filter |
| DE102023112356A1 (en) | 2023-05-10 | 2024-11-14 | Volkswagen Aktiengesellschaft | Device and method for producing a solubility-stable fuel mixture based on renewable fuels |
Citations (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3346354A (en) | 1963-07-02 | 1967-10-10 | Chvron Res Company | Long-chain alkenyl succinic acids, esters, and anhydrides as fuel detergents |
| US3438756A (en) | 1966-06-27 | 1969-04-15 | Chevron Res | Antismoke barium phenate compositions |
| US3447918A (en) | 1967-10-26 | 1969-06-03 | Standard Oil Co | Rust inhibitors |
| FR2097879A5 (en) | 1970-06-15 | 1972-03-03 | Avco Corp | Oil slick treatment - by ferrofluids |
| US4522631A (en) | 1983-11-18 | 1985-06-11 | Texaco Inc. | Diesel fuel containing rare earth metal and oxygenated compounds |
| WO1987005924A1 (en) | 1986-04-04 | 1987-10-08 | Revertex Limited | Dispersions of solids in organic liquids |
| US4775389A (en) | 1986-12-29 | 1988-10-04 | Texaco Inc. | Exhaust particulate reducing and color stabilizing additives for diesel fuels |
| US4778480A (en) | 1986-10-03 | 1988-10-18 | Texaco Inc. | Color stabilization additives for diesel fuel containing rare earth metals and oxygenated compounds |
| GB2248068A (en) | 1990-09-21 | 1992-03-25 | Exxon Chemical Patents Inc | Oil compositions and novel additives |
| EP0671205A2 (en) | 1994-02-18 | 1995-09-13 | Rhone-Poulenc Chimie | Organic sole of or tetravalent metal oxid and their use as additive in hydrocarbon compositions |
| US5501714A (en) | 1988-12-28 | 1996-03-26 | Platinum Plus, Inc. | Operation of diesel engines with reduced particulate emission by utilization of platinum group metal fuel additive and pass-through catalytic oxidizer |
| WO1996034075A1 (en) | 1995-04-24 | 1996-10-31 | The Associated Octel Company Ltd. | Synergistic process for improving combustion |
| WO1996034074A1 (en) | 1995-04-24 | 1996-10-31 | The Associated Octel Company Ltd. | Improved combustion |
| WO1997044414A1 (en) | 1996-05-20 | 1997-11-27 | Bp Chemicals (Additives) Limited | Marine diesel process and fuel therefor |
| US5919276A (en) | 1997-02-07 | 1999-07-06 | Ethyl Petroleum Additives Limited | Use of mixed alkaline earth-alkali metal systems as emissions reducing agents in compression ignition engines |
| WO1999036488A1 (en) | 1998-01-15 | 1999-07-22 | The Associated Octel Company Limited | Fuel additives |
| US6003303A (en) | 1993-01-11 | 1999-12-21 | Clean Diesel Technologies, Inc. | Methods for reducing harmful emissions from a diesel engine |
| US6023928A (en) | 1997-04-17 | 2000-02-15 | Clean Diesel Technologies, Inc. | Method for reducing emissions from a diesel engine |
| WO2000011119A1 (en) | 1998-08-21 | 2000-03-02 | The Associated Octel Company Limited | Fuel additives |
| WO2001010545A1 (en) | 1999-08-04 | 2001-02-15 | Rhodia Terres Rares | Organic colloidal dispersion of a rare earth compound monocrystalline particles |
| WO2002000812A2 (en) | 2000-06-29 | 2002-01-03 | Neuftec Limited | A fuel additive |
| WO2002092703A1 (en) | 2001-05-16 | 2002-11-21 | Oxonica Limited | Comminution of coated metal oxides and hydroxides |
| WO2003040270A2 (en) | 2001-11-06 | 2003-05-15 | Oxonica Limited | Cerium oxide nanoparticles |
| WO2003053560A1 (en) | 2001-12-21 | 2003-07-03 | Rhodia Electronics And Catalysis | ORGANIC COLLOIDAL DISPERSION OF IRON PARTICLES, PROCESS FOR PREPARING THE SAME, AND ITS USE AS A FUEL ADJUVANT FOR INTERNAL COMBUSTION ENGINES |
| WO2004065529A1 (en) | 2003-01-23 | 2004-08-05 | Oxonica Limited | Cerium oxide nanoparticles as fuel additives |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3272746A (en) * | 1965-11-22 | 1966-09-13 | Lubrizol Corp | Lubricating composition containing an acylated nitrogen compound |
| US5344467A (en) | 1991-05-13 | 1994-09-06 | The Lubrizol Corporation | Organometallic complex-antioxidant combinations, and concentrates and diesel fuels containing same |
| DE69308651T2 (en) | 1992-06-17 | 1997-07-24 | Rhone Poulenc Chemicals | Organic cerium IV compounds and their production and use |
| DK0668899T3 (en) | 1992-11-10 | 2001-01-08 | Clean Diesel Tech Inc | Process for reducing harmful emissions from a diesel engine equipped with a particle trap |
| FR2698346B1 (en) | 1992-11-25 | 1995-01-27 | Rhone Poulenc Chimie | Ceric oxide crystallite aggregate, process for obtaining it and its use for reducing combustion residues. |
| FR2746106B1 (en) | 1996-03-15 | 1998-08-28 | EMULSIFIED FUEL AND ONE OF ITS PROCESSES | |
| DK0902824T3 (en) | 1996-05-31 | 2005-01-10 | Ass Octel | fuel additives |
| GB9621753D0 (en) | 1996-10-18 | 1996-12-11 | Williamson Ian V | Fuel composition |
| FR2768155B1 (en) † | 1997-09-11 | 2000-03-31 | Rhodia Chimie Sa | COMPOSITION BASED ON AN ORGANIC SOL OF TETRAVALENT OXIDE, AND OF AN ORGANIC COMPOUND OF ALKALINE OR ALKALINE EARTH, ITS USE AS ADDITIVE OF HYDROCARBON COMPOUNDS |
| US6368366B1 (en) | 1999-07-07 | 2002-04-09 | The Lubrizol Corporation | Process and apparatus for making aqueous hydrocarbon fuel compositions, and aqueous hydrocarbon fuel composition |
| US6648929B1 (en) | 1998-09-14 | 2003-11-18 | The Lubrizol Corporation | Emulsified water-blended fuel compositions |
| JP2002530515A (en) | 1998-11-23 | 2002-09-17 | ピュア エナジー コーポレイション | Diesel fuel composition |
| FR2789601B1 (en) * | 1999-02-17 | 2001-05-11 | Rhodia Chimie Sa | ORGANIC SOL AND SOLID COMPOUND BASED ON CERIUM OXIDE AND AN AMPHIPHILIC COMPOUND AND METHODS OF PREPARATION |
| WO2001051593A1 (en) | 2000-01-12 | 2001-07-19 | Cam Tecnologie S.P.A. | Fuel comprising an emulsion between water and a liquid hydrocarbon |
| AR028780A1 (en) | 2000-07-03 | 2003-05-21 | Ass Octel | A METHOD FOR INCREASING THE LUBRICITY OF A LIQUID HYDROCARBON FUEL; THE ADDITIVE COMPOSITION AND THE COMPOUND USED |
| SE523228C2 (en) | 2000-12-15 | 2004-04-06 | Akzo Nobel Nv | Fuel composition containing a hydrocarbon fraction, ethanol and an additive with water solubilizing capacity |
| EP1344812A1 (en) * | 2002-03-13 | 2003-09-17 | Infineum International Limited | Overbased metallic salt diesel fuel additive compositions for improvement of particulate traps |
| JP2015017888A (en) | 2013-07-11 | 2015-01-29 | Ntn株式会社 | Wheel bearing device with sensor |
-
2004
- 2004-08-24 EP EP04255073.1A patent/EP1512736B2/en not_active Expired - Lifetime
Patent Citations (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3346354A (en) | 1963-07-02 | 1967-10-10 | Chvron Res Company | Long-chain alkenyl succinic acids, esters, and anhydrides as fuel detergents |
| US3438756A (en) | 1966-06-27 | 1969-04-15 | Chevron Res | Antismoke barium phenate compositions |
| US3447918A (en) | 1967-10-26 | 1969-06-03 | Standard Oil Co | Rust inhibitors |
| FR2097879A5 (en) | 1970-06-15 | 1972-03-03 | Avco Corp | Oil slick treatment - by ferrofluids |
| CA970553A (en) | 1970-06-15 | 1975-07-08 | Robert Kaiser | Process and composition for cleaning up oil spills |
| US4522631A (en) | 1983-11-18 | 1985-06-11 | Texaco Inc. | Diesel fuel containing rare earth metal and oxygenated compounds |
| WO1987005924A1 (en) | 1986-04-04 | 1987-10-08 | Revertex Limited | Dispersions of solids in organic liquids |
| US4778480A (en) | 1986-10-03 | 1988-10-18 | Texaco Inc. | Color stabilization additives for diesel fuel containing rare earth metals and oxygenated compounds |
| US4775389A (en) | 1986-12-29 | 1988-10-04 | Texaco Inc. | Exhaust particulate reducing and color stabilizing additives for diesel fuels |
| US5501714A (en) | 1988-12-28 | 1996-03-26 | Platinum Plus, Inc. | Operation of diesel engines with reduced particulate emission by utilization of platinum group metal fuel additive and pass-through catalytic oxidizer |
| GB2248068A (en) | 1990-09-21 | 1992-03-25 | Exxon Chemical Patents Inc | Oil compositions and novel additives |
| US6003303A (en) | 1993-01-11 | 1999-12-21 | Clean Diesel Technologies, Inc. | Methods for reducing harmful emissions from a diesel engine |
| EP0671205A2 (en) | 1994-02-18 | 1995-09-13 | Rhone-Poulenc Chimie | Organic sole of or tetravalent metal oxid and their use as additive in hydrocarbon compositions |
| WO1996034075A1 (en) | 1995-04-24 | 1996-10-31 | The Associated Octel Company Ltd. | Synergistic process for improving combustion |
| WO1996034074A1 (en) | 1995-04-24 | 1996-10-31 | The Associated Octel Company Ltd. | Improved combustion |
| WO1997044414A1 (en) | 1996-05-20 | 1997-11-27 | Bp Chemicals (Additives) Limited | Marine diesel process and fuel therefor |
| US5919276A (en) | 1997-02-07 | 1999-07-06 | Ethyl Petroleum Additives Limited | Use of mixed alkaline earth-alkali metal systems as emissions reducing agents in compression ignition engines |
| US6023928A (en) | 1997-04-17 | 2000-02-15 | Clean Diesel Technologies, Inc. | Method for reducing emissions from a diesel engine |
| WO1999036488A1 (en) | 1998-01-15 | 1999-07-22 | The Associated Octel Company Limited | Fuel additives |
| WO2000011119A1 (en) | 1998-08-21 | 2000-03-02 | The Associated Octel Company Limited | Fuel additives |
| WO2001010545A1 (en) | 1999-08-04 | 2001-02-15 | Rhodia Terres Rares | Organic colloidal dispersion of a rare earth compound monocrystalline particles |
| WO2002000812A2 (en) | 2000-06-29 | 2002-01-03 | Neuftec Limited | A fuel additive |
| WO2002092703A1 (en) | 2001-05-16 | 2002-11-21 | Oxonica Limited | Comminution of coated metal oxides and hydroxides |
| WO2003040270A2 (en) | 2001-11-06 | 2003-05-15 | Oxonica Limited | Cerium oxide nanoparticles |
| WO2003053560A1 (en) | 2001-12-21 | 2003-07-03 | Rhodia Electronics And Catalysis | ORGANIC COLLOIDAL DISPERSION OF IRON PARTICLES, PROCESS FOR PREPARING THE SAME, AND ITS USE AS A FUEL ADJUVANT FOR INTERNAL COMBUSTION ENGINES |
| WO2004065529A1 (en) | 2003-01-23 | 2004-08-05 | Oxonica Limited | Cerium oxide nanoparticles as fuel additives |
| EP1587898A1 (en) | 2003-01-23 | 2005-10-26 | Oxonica Limited | Cerium oxide nanoparticles as fuel additives |
Non-Patent Citations (4)
| Title |
|---|
| ANONYMOUS: "Using paraffin oil as a fuel", THE COMMERCIAL MOTOR ARCHIVE, 19 July 1921 (1921-07-19), XP055558586, Retrieved from the Internet <URL:http://archive.commercialmotor.com/article/19th-july-1921/16/-using-paraffin-oil-as-a-fuel> |
| BARBELLA R; CIAJOLO A; D'ANNA A; BERTOLI C: "Effect of fuel aromaticity on diesel emissions", COMBUSTION AND FLAME, vol. 77, 1989, pages 267 - 277, XP023609843 |
| DOINA BICA: "PREPARATION OF MAGNETIC FLUIDS FOR VARIOUS APPLICATIONS", ROMANIAN REPORTS IN PHYSICS, vol. 47, no. 3-5, 1995, pages 265 - 272, XP055558574 |
| JEAN-MARC DI MEGLIO: "Colloides et nanosciences", TECHNIQUES DE L'LNGENIEUR RÉF.: J2130 V1, 10 September 2007 (2007-09-10), pages 1 - 12, XP055558628 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1512736A1 (en) | 2005-03-09 |
| EP1512736B2 (en) | 2025-12-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8147568B2 (en) | Stabilised diesel fuel additive compositions | |
| AU2004205788B2 (en) | Cerium oxide nanoparticles as fuel additives | |
| EP1512736B1 (en) | Stabilised diesel fuel additive compositions | |
| US7585336B2 (en) | Fuel additive compositions for diesel engine equipped with a particulate trap | |
| EP1435386B1 (en) | Use of a fuel additive composition for improving acceleration of a gasoline engine | |
| EP1612256B1 (en) | Fuel additives comprising a colloidal metal compound. | |
| CZ20021730A3 (en) | Use of salts of alkoxylated oligoamines with fatty acids as compositions improving lubrication power for crude oil products | |
| EP2129751B1 (en) | Iron-containing polymer suitable for regenerating diesel exhaust particulate traps | |
| JPH06509124A (en) | Additives for fuel oil | |
| EP1274820B1 (en) | Fuel oil compositions | |
| EP1721955B1 (en) | Fuel compositions | |
| JP2004530739A (en) | Combustion enhancers for normally liquid fuels |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20040824 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL HR LT LV MK |
|
| AKX | Designation fees paid |
Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PL PT RO SE SI SK TR |
|
| 17Q | First examination report despatched |
Effective date: 20100201 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: EXAMINATION IS IN PROGRESS |
|
| RIC1 | Information provided on ipc code assigned before grant |
Ipc: C10L 10/06 20060101ALI20170829BHEP Ipc: C10L 10/18 20060101ALI20170829BHEP Ipc: C10L 1/12 20060101ALI20170829BHEP Ipc: C10L 1/198 20060101ALI20170829BHEP Ipc: C10L 1/10 20060101AFI20170829BHEP Ipc: C10L 1/18 20060101ALI20170829BHEP |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20171012 |
|
| INTG | Intention to grant announced |
Effective date: 20171019 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 995247 Country of ref document: AT Kind code of ref document: T Effective date: 20180515 Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: PT Ref legal event code: SC4A Ref document number: 1512736 Country of ref document: PT Date of ref document: 20180529 Kind code of ref document: T Free format text: AVAILABILITY OF NATIONAL TRANSLATION Effective date: 20180523 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602004052654 Country of ref document: DE Ref country code: IE Ref legal event code: FG4D Ref country code: ES Ref legal event code: FG2A Ref document number: 2670344 Country of ref document: ES Kind code of ref document: T3 Effective date: 20180530 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: FP |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180802 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: T3 Ref document number: E 27862 Country of ref document: SK |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180803 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 995247 Country of ref document: AT Kind code of ref document: T Effective date: 20180502 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R026 Ref document number: 602004052654 Country of ref document: DE |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLAN | Information deleted related to communication of a notice of opposition and request to file observations + time limit |
Free format text: ORIGINAL CODE: EPIDOSDOBS2 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| PLAX | Notice of opposition and request to file observation + time limit sent |
Free format text: ORIGINAL CODE: EPIDOSNOBS2 |
|
| 26 | Opposition filed |
Opponent name: THE LUBRIZOL CORPORATION Effective date: 20190204 Opponent name: RHODIA OPERATIONS Effective date: 20190202 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180824 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180831 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180831 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 |
|
| PLAF | Information modified related to communication of a notice of opposition and request to file observations + time limit |
Free format text: ORIGINAL CODE: EPIDOSCOBS2 |
|
| PLBB | Reply of patent proprietor to notice(s) of opposition received |
Free format text: ORIGINAL CODE: EPIDOSNOBS3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20040824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20180502 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180824 |
|
| RIC2 | Information provided on ipc code assigned after grant |
Ipc: C10L 10/18 20060101ALI20210319BHEP Ipc: C10L 10/14 20060101ALI20210319BHEP Ipc: C10L 10/08 20060101ALI20210319BHEP Ipc: C10L 10/06 20060101ALI20210319BHEP Ipc: C10L 1/236 20060101ALI20210319BHEP Ipc: C10L 1/224 20060101ALI20210319BHEP Ipc: C10L 1/198 20060101ALI20210319BHEP Ipc: C10L 1/196 20060101ALI20210319BHEP Ipc: C10L 1/19 20060101ALI20210319BHEP Ipc: C10L 1/188 20060101ALI20210319BHEP Ipc: C10L 1/18 20060101ALI20210319BHEP Ipc: C10L 1/12 20060101ALI20210319BHEP Ipc: C10L 1/10 20060101AFI20210319BHEP |
|
| APAH | Appeal reference modified |
Free format text: ORIGINAL CODE: EPIDOSCREFNO |
|
| APBM | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOSNREFNO |
|
| APBP | Date of receipt of notice of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA2O |
|
| APBM | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOSNREFNO |
|
| APBP | Date of receipt of notice of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA2O |
|
| APBM | Appeal reference recorded |
Free format text: ORIGINAL CODE: EPIDOSNREFNO |
|
| APBP | Date of receipt of notice of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA2O |
|
| APBQ | Date of receipt of statement of grounds of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA3O |
|
| APBQ | Date of receipt of statement of grounds of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA3O |
|
| APBQ | Date of receipt of statement of grounds of appeal recorded |
Free format text: ORIGINAL CODE: EPIDOSNNOA3O |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: RHODIA OPERATIONS Effective date: 20190202 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: PT Payment date: 20230628 Year of fee payment: 20 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20230720 Year of fee payment: 20 |
|
| R26 | Opposition filed (corrected) |
Opponent name: RHODIA OPERATIONS Effective date: 20190202 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20230810 Year of fee payment: 20 Ref country code: GB Payment date: 20230712 Year of fee payment: 20 Ref country code: ES Payment date: 20230906 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SK Payment date: 20230711 Year of fee payment: 20 Ref country code: FR Payment date: 20230710 Year of fee payment: 20 Ref country code: DE Payment date: 20230711 Year of fee payment: 20 Ref country code: BE Payment date: 20230712 Year of fee payment: 20 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| APBU | Appeal procedure closed |
Free format text: ORIGINAL CODE: EPIDOSNNOA9O |
|
| R26 | Opposition filed (corrected) |
Opponent name: RHODIA OPERATIONS Effective date: 20190202 |
|
| PLAY | Examination report in opposition despatched + time limit |
Free format text: ORIGINAL CODE: EPIDOSNORE2 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 602004052654 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MK Effective date: 20240823 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20240830 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MK Effective date: 20240824 |
|
| REG | Reference to a national code |
Ref country code: SK Ref legal event code: MK4A Ref document number: E 27862 Country of ref document: SK Expiry date: 20240824 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20240823 |
|
| PLBC | Reply to examination report in opposition received |
Free format text: ORIGINAL CODE: EPIDOSNORE3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240904 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240823 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240825 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240824 Ref country code: PT Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240904 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240823 Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20240825 |
|
| PLAY | Examination report in opposition despatched + time limit |
Free format text: ORIGINAL CODE: EPIDOSNORE2 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: M12 Free format text: ST27 STATUS EVENT CODE: U-0-0-M10-M12 (AS PROVIDED BY THE NATIONAL OFFICE) Effective date: 20251119 |
|
| 27A | Patent maintained in amended form |
Effective date: 20251217 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HU IE IT LI LU MC NL PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R102 Ref document number: 602004052654 Country of ref document: DE |