EP1325151B1 - Improved binding interactions in dipstick assays - Google Patents
Improved binding interactions in dipstick assays Download PDFInfo
- Publication number
- EP1325151B1 EP1325151B1 EP01947639A EP01947639A EP1325151B1 EP 1325151 B1 EP1325151 B1 EP 1325151B1 EP 01947639 A EP01947639 A EP 01947639A EP 01947639 A EP01947639 A EP 01947639A EP 1325151 B1 EP1325151 B1 EP 1325151B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- probe
- detection
- nucleic acid
- capture
- target nucleic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6888—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms
- C12Q1/689—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for detection or identification of organisms for bacteria
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6813—Hybridisation assays
- C12Q1/6832—Enhancement of hybridisation reaction
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6813—Hybridisation assays
- C12Q1/6834—Enzymatic or biochemical coupling of nucleic acids to a solid phase
Definitions
- the present invention relates to enhanced nucleic acid detection by dipsticks.
- Dipsticks of the invention are used to detect the presence of a target nucleic acid in a sample solution, for example to identify whether a patient is infected with a disease causing microorganism such as Chlamydia trachomatis.
- PCR polymerase chain reaction
- dipsticks detect unamplified target nucleic acid without the requirement for any specialist equipment and the results can be obtained much more rapidly than PCR-based methods. Patients can then be treated in the same visit. This is particularly advantageous where the patient is unlikely to, or cannot, return at a later date.
- a single stranded DNA capture probe is immobilised on a nitrocellulose filter at a capture zone remote from one end of the filter (the contact end). Part of the sequence of the capture probe is complementary to the sequence of a first region of the target nucleic acid to be detected.
- a labelled single stranded DNA detection probe is immobilised on the nitrocellulose filter at a probe zone located between the capture zone and the contact end of the filter. The detection probe has sequence complementary to the sequence of a second region (distinct from the first region) of the target nucleic acid.
- the contact end of the nitrocellulose filter is contacted with the sample solution.
- the sample solution wicks up the filter by capillary action and passes the probe zone and the capture zone. As the sample solution passes the probe zone, it mobilises the detection probe and causes it to rise with the sample solution towards the capture zone. Mobilised detection probe can then hybridise to the second region of any target DNA present in the sample solution.
- the hybridised detection probe and target DNA arrive at the capture zone, the first region of the target DNA can hybridise to the immobilised capture probe.
- a ternary complex is thereby formed between the target nucleic acid, the capture probe and the labelled detection probe. Presence of label at the capture zone, therefore, indicates that target DNA is present in the sample solution.
- the labelled DNA detection probe is not immobilised on the nitrocellulose filter. Instead the detection probe is added to the sample solution under conditions allowing hybridisation of the detection probe to any target nucleic acid in the sample solution. The contact end of the nitrocellulose filter is then contacted with the sample solution and as the sample solution wicks up the dipstick, target nucleic acid which is hybridised to the detection probe is captured at the capture zone by the capture probe.
- the sensitivity of nucleic acid detection by conventional dipsticks can be low. If the target nucleic acid is double stranded, the sensitivity of dipstick detection can be particularly low. Consequently, the presence of target nucleic acid in a sample solution can sometimes be undetected. It is desired, therefore, to improve the sensitivity of target nucleic acid detection by dipsticks.
- a dipstick for testing for the presence of target nucleic acid in a sample solution which comprises:
- 'chromatographic strip' is used herein to mean any porous strip of material capable of transporting a solution by capillarity.
- the capture probe spacer may comprise any component which spaces the capture probe from the capture zone without preventing the capture probe from being able to hybridise to the target nucleic acid or hook capture probe.
- the capture probe spacer comprises a biopolymer.
- the capture probe spacer may comprise a protein.
- the term 'protein' is used herein to mean any compound comprising one or more amino acid residues.
- preferred proteins are naturally occurring proteins, preferably bovine serum albumin (BSA), thyroglobulin, or derivatives thereof. Derivatives include proteins which differ from BSA or thyroglobulin by amino acid substitution, addition, or deletion, or by post-translational modification.
- BSA bovine serum albumin
- thyroglobulin or derivatives thereof.
- Derivatives include proteins which differ from BSA or thyroglobulin by amino acid substitution, addition, or deletion, or by post-translational modification.
- a modifier comprising a first reactive group capable of reacting with the capture probe and a second reactive group capable of reacting with the protein.
- a suitable modifier comprises a phosphoramidite group and a primary amino group (or a protected primary amino group which is deprotected before use).
- the phosphoramidite group is capable of reacting with a hydroxyl group (usually the 5'-OH or the 3'-OH of the capture probe when the capture probe is a nucleic acid) and the primary amino group is capable of reacting with a carboxyl group of the protein.
- C6-TFA 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
- the protein is adsorbed directly to the capture zone.
- the detection probe or capture probe is immobilised to the dipstick by covalent attachment, adsorption, use of heat or by UV cross-linking.
- proteins may be more readily and efficiently immobilised to the dipstick than the capture probe. Consequently, direct adsorption of the protein to the capture zone is a more convenient and more efficient means of immobilising the capture probe to the dipstick than conventional immobilisation.
- the capture probe spacer may comprise a non protein.
- the capture probe spacer comprises a protein and a non protein, the capture probe being coupled to the non protein and the non protein being coupled to the protein thereby spacing the capture probe from the protein.
- a modifier comprising a first reactive group capable of reacting with the non protein and a second reactive group capable of reacting with the protein.
- a suitable modifier for use with non proteins which comprise a hydroxyl group is 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA).
- C6-TFA 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
- non protein spacer components examples include 1',2'-Dideoxyribose phosphate (dS), 3-Hydroxypropyl phosphate (S C3 ), and Hexaethyleneglycol phosphate (S).
- dS 1',2'-Dideoxyribose phosphate
- S C3 3-Hydroxypropyl phosphate
- S Hexaethyleneglycol phosphate
- the non protein comprises a nucleobase capable of forming a stacking interaction with a base pair formed when the capture probe has hybridised to the target nucleic acid or hook capture probe. More preferably the non protein comprises a nucleotide. Most preferably the non protein consists only of one or more nucleotides.
- the non protein is at least the length of three nucleotide monomers.
- the length of one nucleotide monomer (N) is approximately equal to the length of one 1', 2'-Dideoxyribose phosphate (dS) or one 3-Hydroxypropyl phosphate (S C3 ).
- Three nucleotide monomers are approximately equal to the length of one Hexaethyleneglycol phosphate (S).
- the capture probe spacer may be linked to any part of the capture probe which does not prevent the capture probe from hybridising to the target nucleic acid or the hook capture probe.
- the hook capture probe may comprise a first region capable of hybridising to the target nucleic acid and a second region capable of hybridising to the capture probe thereby enabling indirect binding of the capture probe to the target nucleic acid.
- the hook capture probe may comprise at least one nucleic acid or nucleic acid analogue.
- Dipsticks of the first aspect of the invention may be used in methods for testing for the presence of target nucleic acid in a sample solution in which a detection probe capable of hybridising to the target nucleic acid is incubated with the sample solution under conditions for hybridisation of the detection probe to target nucleic acid.
- the contact end of the dipstick is contacted with the sample solution allowing sample solution to move up the dipstick by capillary action.
- Target nucleic acid hybridised to the detection probe in the sample solution can then be captured by the capture probe at the capture zone.
- the presence of target nucleic acid in the sample solution is then indicated by the presence of the detection probe at the capture zone.
- the invention also provides a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- the detection probe may be releasably immobilised to the dipstick, for example at a probe zone between the contact end and the capture zone of the chromatographic strip.
- the contact end of the dipstick can be contacted with the sample solution so that sample solution wicks up the dipstick by capillary action.
- the detection probe can hybridise to target nucleic acid in the sample solution and move with the target nucleic acid to the capture zone.
- Target nucleic acid hybridised to the detection probe is captured by the capture probe as the sample solution passes the capture zone. The presence of target nucleic acid in the sample solution is then indicated by the presence of the detection probe at the capture zone.
- the detection probe may be coupled to a label thereby allowing direct detection of target nucleic acid at the capture zone.
- the detection probe may be coupled to a detection ligand allowing indirect detection of target nucleic acid using a detection ligand binding moiety.
- the label or the detection ligand may be linked to a detection probe spacer which is linked to the detection probe, thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe.
- a dipstick for testing for the presence of target nucleic acid in a sample solution which comprises:
- the detection probe spacer may comprise any component which spaces the detection probe from the label or ligand without preventing the detection probe from being able to hybridise to the target nucleic acid.
- the detection probe spacer comprises a biopolymer.
- the detection probe spacer may comprise a protein.
- the term 'protein' is used herein to mean any compound comprising one or more amino acid residues.
- preferred proteins are naturally occurring proteins such as bovine serum albumin (BSA), thyroglobulin, or derivatives thereof.
- BSA bovine serum albumin
- thyroglobulin or derivatives thereof.
- Derivatives include proteins which differ from BSA or thyroglobulin by amino acid substitution, addition, or deletion, or by post-translational modification.
- a modifier comprising a first reactive group capable of reacting with the detection probe and a second reactive group capable of reacting with the protein.
- C6-TFA 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
- the label or detection ligand may be part of or attached to a particle such as a bead.
- the protein is adsorbed directly to the label or the detection ligand.
- the detection probe spacer may comprise a non protein.
- the detection probe spacer comprises a protein and a non protein, the detection probe being coupled to the non protein and the non protein being coupled to the protein thereby spacing the detection probe from the protein.
- a modifier comprising a first reactive group capable of reacting with the non protein and a second reactive group capable of reacting with the protein.
- a suitable modifier for use with non proteins which comprise a hydroxyl group is 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA).
- C6-TFA 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
- the detection probe spacer comprises a non protein only. With such arrangements it will generally be necessary to functionalise the non protein in order to link the non protein to the label or detection ligand. This can be done by the use of a modifier comprising a first reactive group capable of reacting with the non protein and a second reactive group capable of reacting with the label or detection ligand.
- a suitable modifier comprises a phosphoramidite group and a primary amino group (or protected primary amino group which is deprotected before use). 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA) is a suitable example.
- C6-TFA 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
- non protein detection probe spacer components examples include 1',2'-Dideoxyribose phosphate (dS), 3-Hydroxypropyl phosphate (S C3 ), and Hexaethyleneglycol phosphate (S).
- dS 1',2'-Dideoxyribose phosphate
- S C3 3-Hydroxypropyl phosphate
- S Hexaethyleneglycol phosphate
- the non protein comprises a base capable of forming a stacking interaction with a base pair formed when the detection probe has hybridised to the target nucleic acid. More preferably the non protein comprises a nucleotide. Most preferably the non protein consists only of one or more nucleotides.
- the non protein is at least the length of three nucleotides.
- the detection probe spacer may be linked to any part of the detection probe which does not prevent the detection probe from hybridising to the target nucleic acid.
- the capture moiety may be capable of binding directly or indirectly to the target nucleic acid by base pairing or non base pairing interaction.
- the capture moiety may comprise a capture probe capable of hybridising directly to the target nucleic acid.
- the capture moiety may comprise a capture probe capable of hybridising to a hook capture probe bound to the target nucleic acid.
- the capture moiety may be capable of binding to a capture ligand coupled to a capture probe bound to the target nucleic acid, thereby allowing indirect binding of the capture moiety to the target nucleic acid.
- the capture moiety may be an antibody or antibody fragment. If the capture probe is coupled to a capture ligand, the capture probe may be linked to a capture probe spacer which is linked to the capture ligand to space the capture ligand from the capture probe.
- the hook capture probe can be added to the sample solution so that it can bind to target nucleic acid in the sample solution and be captured by the capture probe as the sample solution wicks up the dipstick by capillary action.
- the capture probe, the hook capture probe and the detection probe may each comprise at least one nucleic acid or nucleic acid analogue. Where a probe comprises more than one nucleic acid or nucleic acid analogue, they are preferably hybridised together.
- the invention also provides a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- suitable labels include textile dyes, metal sol such as colloidal gold, and coloured particles such as coloured latex particles. Such labels can be coupled directly to the detection probe or, if the detection probe is coupled to a detection ligand, to the detection ligand binding moiety.
- Suitable capture or detection ligands include biotin (captured or detected for example by an anti-biotin antibody, avidin, streptavidin or a derivative thereof),fluorescein (captured or detected for example by an anti-fluorescein antibody) and 2,4-dinitrophenol (DNP) (captured or detected for example by an anti-DNP antibody).
- the detection probe may comprise a universal detection probe which is capable of hybridising to a hook detection probe bound to the target nucleic acid.
- the universal detection probe may be linked to a label or a detection ligand thereby allowing detection of the detection probe.
- kits and dipsticks of the invention may further comprise any reagent required for the kit to be used to detect target nucleic acid in a sample solution.
- kits of the invention which comprise a detection probe coupled to a detection ligand may further comprise a detection ligand binding moiety. This may be separate to the dipstick or releasably immobilised to the dipstick between the contact end and the capture zone.
- the detection ligand binding moiety may comprise an antibody or antibody fragment, or a non antibody.
- the detection ligand comprises biotin
- the detection ligand binding moiety may comprise an anti-biotin antibody, streptavidin, avidin or a derivative thereof which retains biotin binding activity.
- the detection ligand binding moiety is labelled thereby allowing indirect detection of target nucleic acid utilising the detection probe and the detection ligand binding moiety.
- the invention relates to use of detection probes and/or capture probes linked to a spacer and that the detection probe or capture probe may be immobilised to the dipstick or incubated with the sample solution depending on the method of detection of target nucleic acid.
- kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- a dipstick assay probe for detecting or capturing a target nucleic acid in a dipstick assay as herein defined, the probe comprising a nucleic acid or nucleic acid analogue capable of hybridising to the target nucleic acid, and a label allowing direct detection of the target nucleic acid utilising the probe, or a ligand allowing capture or indirect detection of the target nucleic acid utilising the probe, wherein the label or the ligand is linked to a spacer and the spacer is linked to the nucleic acid or nucleic acid analogue thereby spacing the label or the ligand from the nucleic acid or nucleic acid analogue, characterised in that:
- a method for immobilising a probe to a solid phase which comprises providing a probe coupled to a protein and adsorbing the protein to the solid phase.
- Methods for immobilising a probe to a solid phase may further comprise coupling the probe to the protein.
- the probe is preferably coupled to a linker and the linker coupled to the protein.
- the probe is preferably coupled to the linker before the linker is coupled to the protein.
- the probe may comprise a nucleic acid or nucleic acid analogue.
- the linker is preferably coupled to a non nucleobase part of the probe.
- the linker is preferably coupled to a sugar or phosphate group of the probe.
- the probe comprises a the nucleic acid analogue PNA (protein nucleic acid)
- the linker is preferably coupled to an amino group of the Probe. This is so that the linker does not interfere with base pairing of the nucleobase.
- the linker may be coupled to the 5' or 3' end of the part of the probe which is capable of hybridising to the binding partner of the probe (for example target nucleic acid) to avoid interference of the linker with the base pairing interactions of the prob and its binding partner.
- the probe is preferably coupled to the linker by reaction of a phosphoramidite group attached to the linker with a hydroxyl group of the probe or by reaction of a hydroxyl group of the linker with a phosphoramidite group attached to the probe.
- the linker is preferably coupled to the protein by reaction of a primary amino group attached to the linker with a carboxyl group of the protein.
- the solid phase may comprise a membrane, preferably a nitrocellulose membrane.
- the solid phase may comprise a particle such as a bead.
- probe immobilised to a solid phase by adsorption to the solid phase of a protein coupled to the probe immobilised to a probe immobilised to a solid phase by adsorption to the solid phase of a protein coupled to the probe.
- dipstick, kit, or probe of the invention in a dipstick assay to test for the presence of target nucleic acid in a sample solution.
- dipsticks of the invention having a capture probe spacer is improved because the capture probe linked to the capture probe spacer is more accessible for hybridisation to the target nucleic acid.
- the sensitivity of dipsticks or kits of the invention in which the capture probe is coupled to a capture ligand by a capture probe spacer may be improved because the capture probe is thereby more accessible for hybridisation to the target nucleic acid and the capture ligand is more accessible for binding by the capture moiety.
- the sensitivity of dipsticks or kits of the invention having a detection probe spacer coupling the detection probe to a label is improved because the detection probe spacer makes the detection probe more accessible for hybridisation to the target nucleic acid. If the detection probe is coupled to a detection ligand by a detection probe spacer, the detection probe is thought to be more accessible for hybridisation to the target nucleic acid and the detection probe ligand may also be more accessible to the detection ligand binding moiety.
- spacers comprising nucleobases in accordance with the invention is thought to be particularly effective because the nucleotide or nucleobase is able to form stacking interactions with the base pairs formed between the capture probe or the detection probe and the target nucleic acid. Formation of the stacking interactions is thought to enhance the hybridisation of the capture probe or the detection probe to the target nucleic acid thereby improving the efficiency of capture or detection of the target nucleic acid at the capture zone of the dipstick.
- dipsticks and kits of the invention may be used in the following types of dipstick assay to test for the presence of a target nucleic acid in a sample solution:
- the capture probe instead of being mixed with the sample solution, may be releasably immobilised to the dipstick between the contact end and the capture zone.
- the capture probe When the contact end of the dipstick is contacted with the sample solution causing the sample solution to move by capillary action to the capture zone, the capture probe is released into the sample solution so that released capture probe is released into the sample solution so that released capture probe can hybridise to target nucleic acid in the sample solution as it moves to the capture zone.
- Target nucleic acid may be detected for using a detection probe which may be contacted with the sample solution, releasably immobilised to the dipstick between the contact end and the capture zone, or contacted separately with the capture zone.
- the capture probe may be contacted with the capture zone before, (or exceptionally, at the same time as) the sample solution reaches the capture zone by capillary action. This will allow the capture probe to be bound by the capture moiety at the capture zone so that target nucleic acid may be captured.
- the capture probe may be in a separate capture probe solution which is contacted separately with the capture zone by directly applying it to the capture zone, or by contacting the capture probe solution with the contact end of the dipstick to cause the capture probe to move by capillary action to the capture zone. Subsequent contact of the contact end of the dipstick with the sample solution will allow target nucleic acid reaching the capture zone by capillary action to be captured there.
- target nucleic acid may be detected for using a detection probe which may be contacted with the sample solution, releasably immobilised to the dipstick between the contact end and the capture zone, or contacted separately with the capture zone.
- the target nucleic acid may be labelled directly in the sample solution, for example by covalent attachment of a label to the target nucleic acid. This may be achieved by contact of a precursor label with the sample solution and incubation of the sample solution and precursor label under conditions for covalent attachment of the label to target nucleic acid.
- the capture moiety of assay (2) may be a universal capture probe capable of hybridising to the capture probe, or the capture moiety may be capable of binding by non base pairing interaction to the capture probe.
- the capture probe comprises one or more capture ligands
- the capture moiety is a capture ligand binding moiety.
- the dipstick assay uses more than one probe capable of hybridising to the target nucleic acid it is preferred that all the probes are added to the sample solution and that hybridisation is carried out in a single step. This simplifies the assay, making it easier and quicker to perform. It has been found that the sensitivity of detection of target nucleic acid using a one step hybridisation assay is about equal to the sensitivity of detection when hybridisation is carried out in multiple steps. Multiple step hybridisation may be carried out by sequential hybridisation of the different probes to the target nucleic acid in the sample solution, or by contacting the dipstick with different solutions each containing a different probe. Usually, the latter method of multiple step hybridisation will involve washing the dipstick between each contact with a different probe solution. Whilst there may be circumstances in which multiple step hybridisation is preferred, it will be appreciated that the simpler and quicker format of one step hybridisation will usually be preferred.
- the sample solution is of suitable composition to allow the hybridisation reactions to take place in a single hybridisation step and also to allow non base pairing interactions to take place (for example between a detection ligand and a detection ligand binding moiety and between a capture ligand and a capture ligand binding moiety) and transport a complex comprising target nucleic acid and one or more hybridised probes and (optionally) ligand binding moieties by capillary action up the dipstick.
- any ligand-ligand binding moiety interactions can take place, before the sample solution is contacted directly with the contact end of the dipstick (without the need to first dilute or alter the sample solution).
- Ligand-ligand binding moiety interactions can additionally or alternatively take place on the dipstick if desired as the sample solution travels to the capture zone. Simple and rapid dipstick detection of target nucleic acid is thereby facilitated.
- sample solutions comprising a standard hybridisation buffer (such as SSPE buffer or Tris buffer) with salt, detergent and a blocking protein such as BSA or powdered milk.
- a standard hybridisation buffer such as SSPE buffer or Tris buffer
- salt such as SSPE buffer or Tris buffer
- detergent such as BSA or powdered milk.
- blocking protein such as BSA or powdered milk.
- CT Chlamydia trachomatis
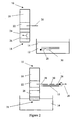
- a dipstick 10 is used to try to detect single or double stranded CT target nucleic acid 12 in a sample solution 14 (see Figure 2 ).
- the dipstick 10 comprises a strip of nitrocellulose 16 having a contact end 18 for contacting the sample solution 14 and a capture probe 20 immobilised at a capture zone 22 of the nitrocellulose strip 16 remote from the contact end 18.
- An anti-biotin antibody-dye conjugate 24 (or an anti-fluorescein antibody-dye conjugate in example 5) is releasably immobilised at a conjugate zone 26 of the nitrocellulose strip located between the contact end 18 and the capture zone 22.
- the capture probe 20 is capable of hybridising to a first region of one strand (the first strand) of the target nucleic acid 12.
- the sample solution 14 is prepared by spiking 1ml urine with Chlamydia trachomatis bacteria then spinning the urine at 15K rpm for 30 minutes. The pellets are resuspended in 100ml standard hybridisation buffer (including a blocking agent such as casein or BSA). A detection probe 28 capable of hybridising to the target nucleic acid is then added (together with a helper probe capable of hybridising to the target nucleic acid adjacent the region recognised by the detection probe and/or the capture probe if used). The detection probe 28, is coupled to biotin (or to fluorescein in example 5) (using methods well known to those of skill in the art). The sample solution 14 is then heated to 100°C for 7 minutes and cooled.
- the contact end 18 of the dipstick 10 is then contacted with the sample solution 14.
- the sample solution 14 and any target nucleic acid 12 hybridised to the detection probe 28 moves up the dipstick 10 by capillary action.
- Complex formed between the anti-biotin antibody-dye conjugate 24, the detection probe 28 and the target nucleic acid 12 then moves up the dipstick 10 to the capture zone 22 where the target nucleic acid of the complex can hybridise to the immobilised capture probe 20.
- the capture probe 20 is immobilised at the capture zone 22 in such a way that it cannot be mobilised by the sample solution 14 as it moves past the capture zone 22. Consequently, the complex bound to the capture probe remains in the capture zone and can be detected by the presence of the dye of the anti-biotin antibody-dye conjugate at the capture zone.
- the detection probe 28 cannot be captured at the capture zone 22 and so no dye is visible at the capture zone. If there is CT target nucleic acid in the sample solution, but insufficient amounts of the target nucleic acid can be captured at the capture zone the presence of the target nucleic acid in the sample solution will not be detected.
- the sensitivity of detection of target nucleic acid can be reduced if the distance between the region of the target nucleic acid to which the capture probe hybridises and the region to which the detection probe hybridises is less than 26 nucleotides.
- the distance between these regions is at least 26 nucleotides and preferably at least 200 nucleotides.
- the capture of target nucleic acid described above is referred to as direct probe capture in the examples below.
- the strength of the detection of the target nucleic acid in the examples is recorded on a scale of 0 to 5, with 5 representing the strongest detection, and 0 no detection.
- sequences of the probes used in the following examples are:
- Capture format direct probe capture (cp) Seq ID No 14 immobilised on the dipstick; Detection probe (dp) : Seq ID No 13 or Seq ID No 15 with biotin coupled directly to the 5'-end, or by a 3 nucleotide (N 3 ), 6 nucleotide (N 6 ), S, or SS spacer, or SEQ ID No 13 with biotin coupled directly to the 3'-end. 10 12 copies of each. Detection format: anti-biotin antibody-dye conjugate; Target DNA: 73 or 76 nucleotide single stranded DNA fragments at 5x10 11 - 10 10 copies.
- the sensitivity of target nucleic acid detection using detection probes with biotin coupled to the 5'-end by a N 6 , N 3 , SS or S spacer was higher than the sensitivity using a detection probe with biotin coupled directly to the 5'-end.
- the N 3 and N 6 spacers were better than the S and SS spacers.
- the biotin (or other detection ligand) is coupled to one end of the detection probe.
- the biotin (or other detection ligand) can be at the end of the detection probe proximal to the region of the target nucleic acid which hybridises to the capture probe (internally orientated) or, preferably, at the end of the detection probe distal to the region of the target nucleic acid which hybridises to the capture probe (externally orientated). If no spacer is used to couple the detection ligand to the detection probe, then the sensitivity of detection of target nucleic acid is generally higher if the detection ligand is externally orientated. Consequently, the detection probe is usually chosen so that the detection ligand is externally orientated.
- the sensitivity of detection using a detection probe with externally orientated biotin coupled directly to the 3'-end of the detection probe was as good as the sensitivity using a detection probe with internally orientated biotin coupled to the 5'-end of the detection probe by a six nucleotide spacer.
- the detection probe does not have to be chosen so that the detection ligand is externally orientated in the complex captured at the capture zone.
- Capture format direct probe capture (cp) Seq ID No 14 immobilised on dipstick membrane; Detection probe: detection probe (dp) Seq ID No 13, Seq ID No 15 and Seq ID No 16 with biotin coupled to the 5'-end by a 3 nucleotide (N 3 ), 6 nucleotide (N 6 ), S or SS spacer. 10 12 copies of each. Detection format: anti-biotin antibody-dye conjugate; Target DNA: 214 bp double stranded DNA fragments at 10 11 - 10 10 copies.
- the sensitivity of detection of double stranded target nucleic acid was improved more than five-fold using N 3 , N 6 , S or SS spacers.
- the sensitivity of detection was higher with the N 6 and SS spacers than with the N 3 and S spacers, indicating that the length of the spacer is important for improved sensitivity of detection.
- the sensitivity of detection was higher with the N 6 spacer than the SS spacer, despite the fact that these spacers are of equivalent length, indicating that the physicochemical properties of the spacer are important for improved sensitivity of detection.
- the sensitivity of detection using only two detection probes each coupled at the 5'-end to biotin by an N 6 spacer was greater than the sensitivity of detection using three detection probes each coupled at the 5'-end directly to biotin (dp13-B + dp15-B + dp16-B) in which the biotin of one detection probe (dpl3-B) is externally orientated in the complex captured by the capture probe.
- the sensitivity of target nucleic acid detection is increased by using a spacer to couple the detection ligand to the detection probe.
- spacers of equivalent length but with different physicochemical properties had different effects on the sensitivity of detection.
- spacers in which the non protein component consists only of nucleotides are better than spacers which include non nucleotide components. Possible explanations for this are:
- Capture format direct probe capture (cp) Seq ID No 10 immobilised on the dipstick; Detection probe: detection probe (dp) Seq ID No 13 coupled to biotin at the 5'-end either directly or by a N 6 , SS, (dS) 6 , (SC 3 ) 6 or SN 3 SN 3 S spacer. 10 12 copies of each.
- Detection format anti-biotin antibody-dye conjugate; Helper probes: SEQ ID No 5 and SEQ ID No 6 adjacent to SEQ ID No 10; SEQ ID No 1 and SEQ ID No 2 adjacent to SEQ ID No 13 at 10 12 copies; Target DNA: 872 bp double stranded DNA fragment at 2x10 11 - 5x10 10 copies.
- the SS, (dS) 6 , and (SC 3 ) 6 spacers are of equivalent length and had a similar effect on enhancing the sensitivity of detection of target nucleic acid despite their structural differences and properties.
- the N 6 spacer is of equivalent length to the SS, (dS) 6 , and (SC 3 ) 6 spacers.
- the N 6 spacer had the greatest effect on improving the sensitivity of detection of target nucleic acid.
- the sensitivity of target nucleic acid detection was greater using the N 6 spacer than the SN 3 SN 3 S spacer (the longest spacer tested).
- a possible explanation for this could be that the S monomer reduces or eliminates stacking interaction between the nucleobases of the N 3 components of the spacer and the base pairs formed between the detection probe and the target nucleic acid. This data supports the conclusion that stacking interactions between unpaired nucleobases of the spacer and the duplex formed between the target nucleic acid and the detection probe are important in enhancing the sensitivity of detection of target nucleic acid.
- the membrane was then incubated with an anti-biotin antibody coupled to alkaline phosphatase (capable of converting a Nitro Blue Tetrazolium/5-Bromo-4-Chloro-3-Indolyl Phosphate (NBT/BCIP) chromogenic substrate), washed, and incubated with the NBT/BCIP chromogenic substrate, and the membrane was observed to see if any colour formed at the capture zone.
- NBT/BCIP Nitro Blue Tetrazolium/5-Bromo-4-Chloro-3-Indolyl Phosphate
- Capture format direct probe capture (cp) Seq ID No 14 immobilised on the dipstick; Detection probe: detection probe (dp) Seq ID No 13 coupled to biotin at the 5'-end either directly or by a nucleotide or non-nucleotide spacer. 10 12 copies of each. Detection format: anti-biotin antibody-dye conjugate; Helper probes: SEQ ID No 2 and SEQ ID No 3 adjacent to SEQ ID No 14 at 10 12 copies; Target DNA: 416 bp double stranded DNA fragment at 5x10 10 - 5x10 9 copies.
- Detection probes detection probe Seq ID No 13 coupled to biotin at the 5'-end, either directly or by a nucleotide or non-nucleotide spacer.
- Detection format anti-biotin antibody coupled to alkaline phosphatase, detection by NBT/BSIP chromogenic substrate.
- the sensitivity of detection with a six nucleotide spacer is marginally better than 3-5 nucleotide spacers.
- the sensitivity of detection with spacers consisting only of nucleotides was better than with spacers which include non-nucleotides.
- spacers which include non-nucleotides were better than with spacers which include non-nucleotides.
- the N 3 spacer was better than the longest spacer SN 3 SN 3 S, equivalent to 15 nucleotides in length.
- the (dS) 6 spacer is slightly better than the SS spacer.
- the sensitivity of detection was highest with the longest spacers (SSSS and SN 3 SN 3 S).
- the dS component has a similar structure to a nucleotide. Both have a ribose residue, expected to provide rigidity. However, the nucleotide has a nucleobase which is not present in the dS component.
- the different effect of the (dS) 6 spacer on the sensitivity of target nucleic acid detection compared to the N 6 spacer suggests that the greater effect on the improvement of sensitivity of detection using a nucleotide spacer is explained principally by the presence of the nucleobases which do not base pair with the target nucleic acid.
- composition of the spacer appears to be more important than its length (compare the results for dp-N 3 -B 5' and dp-SN 3 SN 3 S-B 5' in the dipstick test of example 4).
- the sensitivity of detection using a (dS) 6 spacer was greater than with an SS spacer.
- These spacers are of equivalent length. This suggests that the physicochemical properties of the spacer, such as rigidity or polarity, may also have an effect on the improvement of sensitivity of detection by the spacer.
- the dot blot analysis of example 4 shows that the length of the spacer is important for the availability of the biotin to the anti-biotin antibody.
- the sensitivity of biotin recognition by the anti-biotin antibody was similar whether the biotin was coupled to the immobilised probe by an N 6 , (dS) 6 or SS spacer.
- the effect of these spacers on the sensitivity of detection in the dipstick test of example 4 was different. This suggests that the composition of the spacer is more important in the hybridisation of the detection probe to the target nucleic acid than in the recognition of the biotin by the anti-biotin antibody.
- the length of the spacer appears to be more important than the composition of the spacer for recognition of the biotin (or other detection ligand) by the anti-biotin antibody (or other detection ligand binding moiety).
- Capture format direct probe capture (cp) Seq ID No 14 coupled to BSA immobilised to the dipstick.
- the capture probe is coupled to the BSA either directly or by a six nucleotide spacer;
- Detection probe detection probe (dp) Seq ID No 13 coupled to fluorescein. 10 12 copies.
- Detection format anti-fluorescein antibody-dye conjugate;
- Target DNA 73 nt or 76 nt single stranded DNA fragments at 10 11 copies.
- Capture format direct probe capture (cp) Seq ID No 14 coupled at the 5'-end to BSA immobilised on the dipstick.
- the capture probe is coupled to the BSA by an N 6 or SN 3 SN 3 S spacer;
- Detection probes detection probes (dp) Seq ID No 7, 8, 9, 10, 11, 12, 13, 15, 16 and 17 coupled to biotin. 10 12 copies of each;
- Detection format anti-biotin antibody-dye conjugate; Target : 872 bp double stranded DNA at 10 11 - 2.5x10 9 copies.
- Capture format direct probe capture (cp) Seq ID No 15 coupled to BSA immobilised on the dipstick.
- the capture probe is coupled to the BSA by an N 6 spacer; Helper probes: SEQ ID No 3 and SEQ ID No 4 adjacent to SEQ ID No 15;
- Detection probe In this example, the detection probe comprises a hook probe and a universal probe.
- the hook probe has sequence corresponding to Seq ID No 17 (capable of hybridising to the target nucleic acid) and sequence complementary to the sequence of a universal probe.
- the universal probe is coupled to a textile dye by an N 6 or SN 3 SN 6 spacer. There are 10 12 copies of the hook probe.
- Target 872 bp double stranded DNA at 10 11 and 10 10 copies.
- spacers comprising a protein immobilised to the dipstick and a non protein to couple the capture probe to the immobilised protein (examples 5 and 6), or use of non protein spacers to couple the label to the detection probe (example 7) improve the sensitivity of detection of target nucleic acid.
- the sensitivity of nucleic acid detection was highest when the non protein component of the spacer consisted entirely of nucleotides.
- Capture probe:BSA ratios of 2.7:1, 3.2:1, 4.1:1, 5.2:1, 6.6:1, 10.9:1 were studied. 1, 1.5, 2, 4, 5 and 20mM concentrations of EDAC were studied. Concentrations higher than 5 mM caused the BSA-capture probe to precipitate out of the solution.
- Capture format direct probe capture by Seq ID No 14 immobilised directly on the dipstick membrane or by a BSA protein spacer.
- the capture probe and the capture probe-BSA were applied to the dipstick and immobilised by treating the dipstick for 1h at 80°C.
- Detection probe biotin labelled detection probe (dp) Seq ID No 13 at 10 12 copies.
- Detection format anti-biotin antibody-dye conjugate; Target DNA: 73 nucleotide single stranded DNA fragments at 1x10 11 copies.
- Capture probe Signal capture probe-BSA 4.0 Capture probe 0.0
- the target nucleic acid was undetected using a capture probe directly immobilised to the dipstick. However, a strong detection signal was obtained if the capture probe was coupled to BSA and the BSA was immobilised to the dipstick.
- the following examples relate to detection of target nucleic acid using a probe coupled to a coloured particle by a protein spacer.
- Coloured dye particles were coated with probe coupled to BSA.
- the protein is adsorbed to the dye particles thereby coupling the probe to the dye particles.
- the procedure used was as follows:
- Capture format Anti-biotin antibody/biotin coupled to capture probes Seq ID No 13, No 14, No 15, No 16 and No 17 at 10 12 copies per test.
- Direct probe capture format (Seq ID No 14 or Seq ID No 15) were used for comparison ; Direct detection probe Seq ID No 10 coupled to dye particle by BSA; Helper probes: SEQ ID No 5 and SEQ ID No 6 adjacent to SEQ ID No 10; Target: 872 bp ds DNA at 10 11 to 10 8 copies.
- Capture format direct probe capture (cp) Seq ID No 15 coupled to BSA immobilised to the dipstick.
- Helper probes SEQ ID No 3 and SEQ ID No 4 adjacent to SEQ ID No 15;
- a "hook probe" with sequence complimentary to target DNA in this region and to the universal probe sequence was used at 10 12 copies per test;
- Detection format Textile dye coupled to the universal probe by BSA; Target: 872 bp ds DNA at 10 11 and 10 10 copies.
- Capture format direct probe capture Seq ID No 15 coupled to BSA immobilised to the dipstick.
- Helper probes SEQ ID No 3 and SEQ ID No 4 adjacent to SEQ ID No 15; Detection probe region Seq ID No 17.
- a "hook" detection probe 1 with sequence complementary to target DNA in this region and to the universal probe sequence 2, was used at 10 12 copies per test;
- Detection format a hook detection probe comprises sequence complementary to sequence of the target nucleic acid and to the sequence of a first universal detection probe.
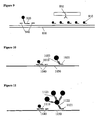
- the first universal detection probe is coupled to BSA and the BSA is adsorbed to a first coloured particle thereby coupling the first universal detection probe to the first coloured particle.
- Several second universal detection probes are also each coupled to BSA adsorbed to the first coloured particle.
- the second universal detection probes comprise sequence complementary to sequence of a third universal detection probe.
- the third universal detection probe is coupled to BSA adsorbed to a second coloured particle.
- target nucleic acid is detected using the hook detection probe, the first, second and third universal detection probes and the first and second coloured particles, a single first coloured particle forms a first layer on the target nucleic acid and several second coloured particles form a second layer on the target nucleic acid as in Figure 11 . Because several coloured particles can be attached to each target nucleic acid in this way, it is thought that the sensitivity of detection of target nucleic acid is improved compared to use of a hook detection probe and a single universal detection probe coupled to a coloured particle.
- Example 12 One-step Nucleic Acid Dipstick Assay Detection of Chlamydia trachomatis
- the detection probe, helper probe and 5x10 6 - 5x10 3 copies of EB diluted in hybridisation buffer made up to 80 ⁇ l and heated at 100°C for 7 minutes. The mixure was then centrifuged briefly to collect all the liquid and mixed with 20 ⁇ l anti-biotin Ab colloidal gold. The whole 100 ⁇ l mixture were wicked up on dipstick and let to develop a signal.
- a sandwich hybridisation assay for detection of Ct disclosed in PCT WO 93/1322 for example is a complex multi-component microtitre plate format assay, which coul dnot be accomplished for less than 5 hours.
- This assay is a multistep assay, which requires a gradual addition of its components in a defined order with incubations and washing steps after the addition of every new component.
- the nucleic acid dipstick assay subject of this invention could be done in one step with no need of different steps for addition of components and washings.
- This sandwich hybridisation assay does not require more than one solution conditions in order to render them advantageous for hybridisation and other affinity pair formations. The same solution conditions could serve a free migration of the components through the dipstick membrane as well.
- the sensitivity of detection of single and double stranded target nucleic acid by dipsticks has been found to be significantly improved by the use of spacers in accordance with the invention.
- the sensitivity of detection of double stranded circular target nucleic acid can also be increased by use of spacers in accordance with the invention.
- Single stranded target nucleic acid is known to form secondary structure by means of intramolecular base pairing interactions.
- Such secondary structure can inhibit binding of the capture probe and the detection probe to the target nucleic acid. Consequently, the region of the target nucleic acid to which the detection probe and capture probe bind is often chosen as a region predicted to be substantially free of secondary structure.
- the improved sensitivity of detection of double stranded target nucleic acid achieved by use of spacers in accordance with the invention means that the sensitivity of detection of single stranded target nucleic acid using a capture probe and/or a detection probe which recognises a region of the target nucleic acid involved in secondary structure will also be improved.
- An advantage of this is that the capture probe and/or detection probe may not have to be chosen based on predictions of the secondary structure formed by the target nucleic acid, thus simplifying the choice of capture and detection probe.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Health & Medical Sciences (AREA)
- Zoology (AREA)
- Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Measurement Of Length, Angles, Or The Like Using Electric Or Magnetic Means (AREA)
Abstract
Description
- The present invention relates to enhanced nucleic acid detection by dipsticks. Dipsticks of the invention are used to detect the presence of a target nucleic acid in a sample solution, for example to identify whether a patient is infected with a disease causing microorganism such as Chlamydia trachomatis.
- Some conventional tests for detecting the presence of a target nucleic acid in a sample solution rely on amplification of the target nucleic acid using the polymerase chain reaction (PCR). Whilst this reaction allows detection of small quantities of target nucleic acid, it can take several hours before a result is obtained. This can be a significant disadvantage because it is often desired to obtain the result as quickly as possible, for example, to keep patient waiting times to a minimum. Further disadvantages of such methods are the requirement for expensive specialist equipment to perform the reaction and the relatively high cost of the reagents.
- In contrast, dipsticks detect unamplified target nucleic acid without the requirement for any specialist equipment and the results can be obtained much more rapidly than PCR-based methods. Patients can then be treated in the same visit. This is particularly advantageous where the patient is unlikely to, or cannot, return at a later date.
- In a typical conventional dipstick described in
US 5,310,650 , a single stranded DNA capture probe is immobilised on a nitrocellulose filter at a capture zone remote from one end of the filter (the contact end). Part of the sequence of the capture probe is complementary to the sequence of a first region of the target nucleic acid to be detected. A labelled single stranded DNA detection probe is immobilised on the nitrocellulose filter at a probe zone located between the capture zone and the contact end of the filter. The detection probe has sequence complementary to the sequence of a second region (distinct from the first region) of the target nucleic acid. - To detect target DNA in a sample solution thought to contain target DNA, the contact end of the nitrocellulose filter is contacted with the sample solution. The sample solution wicks up the filter by capillary action and passes the probe zone and the capture zone. As the sample solution passes the probe zone, it mobilises the detection probe and causes it to rise with the sample solution towards the capture zone. Mobilised detection probe can then hybridise to the second region of any target DNA present in the sample solution.
- When the hybridised detection probe and target DNA arrive at the capture zone, the first region of the target DNA can hybridise to the immobilised capture probe. A ternary complex is thereby formed between the target nucleic acid, the capture probe and the labelled detection probe. Presence of label at the capture zone, therefore, indicates that target DNA is present in the sample solution.
- With a second type of conventional dipstick, the labelled DNA detection probe is not immobilised on the nitrocellulose filter. Instead the detection probe is added to the sample solution under conditions allowing hybridisation of the detection probe to any target nucleic acid in the sample solution. The contact end of the nitrocellulose filter is then contacted with the sample solution and as the sample solution wicks up the dipstick, target nucleic acid which is hybridised to the detection probe is captured at the capture zone by the capture probe.
- It has been found, however, that the sensitivity of nucleic acid detection by conventional dipsticks can be low. If the target nucleic acid is double stranded, the sensitivity of dipstick detection can be particularly low. Consequently, the presence of target nucleic acid in a sample solution can sometimes be undetected. It is desired, therefore, to improve the sensitivity of target nucleic acid detection by dipsticks.
- According to a first aspect of the invention there is provided a dipstick for testing for the presence of target nucleic acid in a sample solution which comprises:
- a chromatographic strip having a contact end for contacting the sample solution; and
- a capture probe immobilised at a capture zone of the chromatographic strip remote from the contact end, the capture probe being capable of hybridising to the target nucleic acid or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is linked to a capture probe spacer and the capture probe spacer is linked to the capture zone, thereby immobilising the capture probe at the capture zone and spacing the capture probe from the capture zone, characterized in that:
- i) the capture probe spacer is linked to one end of the capture probe, and the end of the capture probe not linked to the capture probe spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the capture probe has hybridised to the target nucleic acid or to the hook capture probe when the capture probe has hybridised to the hook capture probe;
- ii) the capture probe spacer is linked to a part of the capture probe between the ends of the capture probe, and one or both ends of the capture probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the capture probe has hybridised to the target nucleic acid or to the hook capture probe when the capture probe has hybridised to the hook capture probe;
- iii) the capture probe spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the capture probe has hybridised to the target nucleic acid or hook capture probe; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; or
- iv) the capture probe spacer comprises a protein directly adsorbed to the capture zone, and covalently linked to the capture probe by a linker.
- The term 'chromatographic strip' is used herein to mean any porous strip of material capable of transporting a solution by capillarity.
- The capture probe spacer may comprise any component which spaces the capture probe from the capture zone without preventing the capture probe from being able to hybridise to the target nucleic acid or hook capture probe. Preferably the capture probe spacer comprises a biopolymer.
- The capture probe spacer may comprise a protein. The term 'protein' is used herein to mean any compound comprising one or more amino acid residues. Examples of preferred proteins are naturally occurring proteins, preferably bovine serum albumin (BSA), thyroglobulin, or derivatives thereof. Derivatives include proteins which differ from BSA or thyroglobulin by amino acid substitution, addition, or deletion, or by post-translational modification.
- In order to link the capture probe to the protein spacer it will generally be necessary to functionalise the capture probe. This can be done by the use of a modifier comprising a first reactive group capable of reacting with the capture probe and a second reactive group capable of reacting with the protein. A suitable modifier comprises a phosphoramidite group and a primary amino group (or a protected primary amino group which is deprotected before use). The phosphoramidite group is capable of reacting with a hydroxyl group (usually the 5'-OH or the 3'-OH of the capture probe when the capture probe is a nucleic acid) and the primary amino group is capable of reacting with a carboxyl group of the protein. An example of a suitable modifier is 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA). Other suitable modifiers will be known to those of ordinary skill in the art.
- Once the modifier has reacted with the capture probe and the protein to link the capture probe to the protein the reacted modifier is termed herein a 'linker'.
- Preferably the protein is adsorbed directly to the capture zone.
- In conventional dipsticks, the detection probe or capture probe is immobilised to the dipstick by covalent attachment, adsorption, use of heat or by UV cross-linking. However, it has been found that proteins may be more readily and efficiently immobilised to the dipstick than the capture probe. Consequently, direct adsorption of the protein to the capture zone is a more convenient and more efficient means of immobilising the capture probe to the dipstick than conventional immobilisation.
- The capture probe spacer may comprise a non protein. In a preferred arrangement, the capture probe spacer comprises a protein and a non protein, the capture probe being coupled to the non protein and the non protein being coupled to the protein thereby spacing the capture probe from the protein.
- In order to link the non protein to the protein it will generally be necessary to use a modifier comprising a first reactive group capable of reacting with the non protein and a second reactive group capable of reacting with the protein.
- A suitable modifier for use with non proteins which comprise a hydroxyl group is 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA). Other suitable modifiers will be known to those of ordinary skill in the art.
- Once the modifier has reacted with the non protein and the protein to link the non protein to the protein the reacted modifier is termed herein a 'linker'.
- Examples of suitable non protein spacer components include 1',2'-Dideoxyribose phosphate (dS), 3-Hydroxypropyl phosphate (SC3), and Hexaethyleneglycol phosphate (S). The chemical structures of these compounds are shown in
Figure 1 . - Preferably, however, the non protein comprises a nucleobase capable of forming a stacking interaction with a base pair formed when the capture probe has hybridised to the target nucleic acid or hook capture probe. More preferably the non protein comprises a nucleotide. Most preferably the non protein consists only of one or more nucleotides.
- Preferably the non protein is at least the length of three nucleotide monomers. The length of one nucleotide monomer (N) is approximately equal to the length of one 1', 2'-Dideoxyribose phosphate (dS) or one 3-Hydroxypropyl phosphate (SC3). Three nucleotide monomers are approximately equal to the length of one Hexaethyleneglycol phosphate (S).
- The capture probe spacer may be linked to any part of the capture probe which does not prevent the capture probe from hybridising to the target nucleic acid or the hook capture probe.
- The hook capture probe may comprise a first region capable of hybridising to the target nucleic acid and a second region capable of hybridising to the capture probe thereby enabling indirect binding of the capture probe to the target nucleic acid. The hook capture probe may comprise at least one nucleic acid or nucleic acid analogue.
- Dipsticks of the first aspect of the invention may be used in methods for testing for the presence of target nucleic acid in a sample solution in which a detection probe capable of hybridising to the target nucleic acid is incubated with the sample solution under conditions for hybridisation of the detection probe to target nucleic acid. The contact end of the dipstick is contacted with the sample solution allowing sample solution to move up the dipstick by capillary action. Target nucleic acid hybridised to the detection probe in the sample solution can then be captured by the capture probe at the capture zone. The presence of target nucleic acid in the sample solution is then indicated by the presence of the detection probe at the capture zone.
- Accordingly the invention also provides a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- a dipstick of the first aspect of the invention; and
- a detection probe capable of hybridising to the target nucleic acid thereby allowing detection of target nucleic utilising the detection probe.
- Instead of incubating the detection probe with the sample solution, the detection probe may be releasably immobilised to the dipstick, for example at a probe zone between the contact end and the capture zone of the chromatographic strip. To test for the presence of target nucleic acid in a sample solution, the contact end of the dipstick can be contacted with the sample solution so that sample solution wicks up the dipstick by capillary action. As the sample solution passes the probe zone of the dipstick it mobilises the detection probe so that the detection probe can hybridise to target nucleic acid in the sample solution and move with the target nucleic acid to the capture zone. Target nucleic acid hybridised to the detection probe is captured by the capture probe as the sample solution passes the capture zone. The presence of target nucleic acid in the sample solution is then indicated by the presence of the detection probe at the capture zone.
- The detection probe may be coupled to a label thereby allowing direct detection of target nucleic acid at the capture zone. Alternatively the detection probe may be coupled to a detection ligand allowing indirect detection of target nucleic acid using a detection ligand binding moiety. The label or the detection ligand may be linked to a detection probe spacer which is linked to the detection probe, thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe.
- According to a second aspect of the invention there is provided a dipstick for testing for the presence of target nucleic acid in a sample solution which comprises:
- a chromatographic strip having a contact end for contacting the sample solution;
- a capture moiety, immobilised at a capture zone remote from the contact end, the capture moiety being capable of binding directly or indirectly to the target nucleic acid; and a detection probe, releasably immobilised at a probe zone located between the contact end and the capture zone, the detection probe being capable of hybridising to the target nucleic acid and the detection probe being coupled to a label allowing direct detection of the detection probe, or
- the detection probe being coupled to a detection ligand allowing indirect detection of the detection probe;
- i) the detection probe spacer is linked to one end of the detection probe, and the end of the detection probe not linked to the detection probe spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;
- ii) the detection probe spacer is linked to a part of the detection probe between the ends of the detection probe, and one or both ends of the detection probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;
- iii) the detection probe spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the detection probe has hybridised to the target nucleic acid; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; or iv) the detection probe spacer comprises a protein.
- The detection probe spacer may comprise any component which spaces the detection probe from the label or ligand without preventing the detection probe from being able to hybridise to the target nucleic acid. Preferably the detection probe spacer comprises a biopolymer.
- The detection probe spacer may comprise a protein. The term 'protein' is used herein to mean any compound comprising one or more amino acid residues. Examples of preferred proteins are naturally occurring proteins such as bovine serum albumin (BSA), thyroglobulin, or derivatives thereof. Derivatives include proteins which differ from BSA or thyroglobulin by amino acid substitution, addition, or deletion, or by post-translational modification.
- In order to link the detection probe to the protein spacer it will generally be necessary to functionalise the detection probe. This can be done by the use of a modifier comprising a first reactive group capable of reacting with the detection probe and a second reactive group capable of reacting with the protein.
- An example of a suitable modifier is 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA). Other suitable modifiers will be known to those of ordinary skill in the art.
- Once the modifier has reacted with the detection probe and the protein to link the detection probe to the protein the reacted modifier is termed herein a 'linker'.
- The label or detection ligand may be part of or attached to a particle such as a bead. In such cases, preferably the protein is adsorbed directly to the label or the detection ligand.
- The detection probe spacer may comprise a non protein. In a preferred arrangement, the detection probe spacer comprises a protein and a non protein, the detection probe being coupled to the non protein and the non protein being coupled to the protein thereby spacing the detection probe from the protein.
- In order to link the non protein to the protein it will generally be necessary to use a modifier comprising a first reactive group capable of reacting with the non protein and a second reactive group capable of reacting with the protein. An example of a suitable modifier for use with non proteins which comprise a hydroxyl group is 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA). Other suitable modifiers will be known to those of ordinary skill in the art.
- Once the modifier has reacted with the non protein and the protein to link the non protein to the protein the reacted modifier is termed herein a 'linker'.
- In other arrangements the detection probe spacer comprises a non protein only. With such arrangements it will generally be necessary to functionalise the non protein in order to link the non protein to the label or detection ligand. This can be done by the use of a modifier comprising a first reactive group capable of reacting with the non protein and a second reactive group capable of reacting with the label or detection ligand.
- If the non protein comprises a hydroxyl group and the label or detection ligand comprises a carboxyl group, a suitable modifier comprises a phosphoramidite group and a primary amino group (or protected primary amino group which is deprotected before use). 6-(Trifluoroacetylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite (C6-TFA) is a suitable example. Other suitable modifiers will be known to those of ordinary skill in the art.
- Examples of suitable non protein detection probe spacer components include 1',2'-Dideoxyribose phosphate (dS), 3-Hydroxypropyl phosphate (SC3), and Hexaethyleneglycol phosphate (S).
- Preferably, however, the non protein comprises a base capable of forming a stacking interaction with a base pair formed when the detection probe has hybridised to the target nucleic acid. More preferably the non protein comprises a nucleotide. Most preferably the non protein consists only of one or more nucleotides.
- Preferably the non protein is at least the length of three nucleotides.
- The detection probe spacer may be linked to any part of the detection probe which does not prevent the detection probe from hybridising to the target nucleic acid.
- The capture moiety may be capable of binding directly or indirectly to the target nucleic acid by base pairing or non base pairing interaction.
- For example, the capture moiety may comprise a capture probe capable of hybridising directly to the target nucleic acid. Alternatively, the capture moiety may comprise a capture probe capable of hybridising to a hook capture probe bound to the target nucleic acid.
- The capture moiety may be capable of binding to a capture ligand coupled to a capture probe bound to the target nucleic acid, thereby allowing indirect binding of the capture moiety to the target nucleic acid. For example the capture moiety may be an antibody or antibody fragment. If the capture probe is coupled to a capture ligand, the capture probe may be linked to a capture probe spacer which is linked to the capture ligand to space the capture ligand from the capture probe.
- The hook capture probe can be added to the sample solution so that it can bind to target nucleic acid in the sample solution and be captured by the capture probe as the sample solution wicks up the dipstick by capillary action.
- The capture probe, the hook capture probe and the detection probe may each comprise at least one nucleic acid or nucleic acid analogue. Where a probe comprises more than one nucleic acid or nucleic acid analogue, they are preferably hybridised together.
- The invention also provides a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- a dipstick according to the second aspect of the invention in which the capture moiety is capable of binding to a capture ligand coupled to a capture probe bound to the target nucleic acid; and
- a capture probe capable of hybridising to the target nucleic acid or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is coupled to a capture ligand which can be bound by the capture moiety.
- Examples of suitable labels include textile dyes, metal sol such as colloidal gold, and coloured particles such as coloured latex particles. Such labels can be coupled directly to the detection probe or, if the detection probe is coupled to a detection ligand, to the detection ligand binding moiety.
- Examples of suitable capture or detection ligands include biotin (captured or detected for example by an anti-biotin antibody, avidin, streptavidin or a derivative thereof),fluorescein (captured or detected for example by an anti-fluorescein antibody) and 2,4-dinitrophenol (DNP) (captured or detected for example by an anti-DNP antibody).
- The detection probe may comprise a universal detection probe which is capable of hybridising to a hook detection probe bound to the target nucleic acid. The universal detection probe may be linked to a label or a detection ligand thereby allowing detection of the detection probe.
- It will be appreciated that kits and dipsticks of the invention may further comprise any reagent required for the kit to be used to detect target nucleic acid in a sample solution. For example, kits of the invention which comprise a detection probe coupled to a detection ligand may further comprise a detection ligand binding moiety. This may be separate to the dipstick or releasably immobilised to the dipstick between the contact end and the capture zone.
- The detection ligand binding moiety may comprise an antibody or antibody fragment, or a non antibody. For example, if the detection ligand comprises biotin the detection ligand binding moiety may comprise an anti-biotin antibody, streptavidin, avidin or a derivative thereof which retains biotin binding activity. Preferably the detection ligand binding moiety is labelled thereby allowing indirect detection of target nucleic acid utilising the detection probe and the detection ligand binding moiety.
- It will be understood that the invention relates to use of detection probes and/or capture probes linked to a spacer and that the detection probe or capture probe may be immobilised to the dipstick or incubated with the sample solution depending on the method of detection of target nucleic acid.
- There is also provided according to the invention a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- i) a dipstick comprising a chromatographic strip having a contact end for contacting the sample solution and a capture probe capable of hybridising to the target nucleic acid, or to a hook capture probe bound to the target nucleic acid, the capture probe being immobilised at a capture zone of the chromatographic strip remote from the contact end; and
- ii) a detection probe capable of hybridising to the target nucleic acid, the detection probe being coupled to a label allowing direct detection of the target nucleic acid utilising the detection probe, or the detection probe being coupled to a ligand allowing indirect detection of the target nucleic acid utilising the detection probe, wherein the label or the detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe and wherein:
- i) the detection probe spacer is linked to one end of the detection probe, and the end of the detection probe not linked to the detection probe spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;
- ii) the detection probe spacer is linked to a part of the detection probe between the ends of the detection probe, and one or both ends of the detection probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;
- iii) the detection probe spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the detection probe has hybridised to the target nucleic acid; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; or iv) the detection probe spacer comprises a protein.
- There is also provided according to the invention a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- i) a dipstick comprising a chromatographic strip having a contact end for contacting the sample solution and a capture moiety immobilised at a capture zone of the chromatographic strip remote from the contact end, the capture moiety being capable of binding directly or indirectly to the target nucleic acid;
- ii) a capture probe capable of hybridising to the target nucleic acid, or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is coupled to a capture ligand which can be bound by the capture moiety; and iii) a detection probe capable of hybridising to the target nucleic acid, the detection probe being coupled to a label allowing direct detection of the target nucleic acid utilising the detection probe, or the detection probe being coupled to a detection ligand allowing indirect detection of the target nucleic acid utilising the detection probe, wherein the label or the detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe wherein:
- i) the spacer is linked to one end of the probe, and the end of the probe not linked to the spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid or the hook capture probe;
- ii) the spacer is linked to a part of the probe between the ends of the probe, and one or both ends of the probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid or the hook capture probe;
- iii) the spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the probe has hybridised to the target nucleic acid or the hook capture probe; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; or iv) the spacer comprises a protein.
- There is also provided according to the invention a kit for testing for the presence of target nucleic acid in a sample solution which comprises:
- i) a dipstick comprising a chromatographic strip having a contact end for contacting the sample solution and a capture moiety immobilised at a capture zone of the chromatographic strip remote from the contact end;
- ii) a capture probe capable of hybridising to the target nucleic acid, or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is linked to a capture probe spacer and the capture probe spacer is linked to a capture ligand thereby coupling the capture ligand to the capture probe and spacing the capture ligand from the capture probe; and
- iii) a detection probe capable of hybridising to the target nucleic acid, the detection probe being coupled to a label allowing direct detection of the target nucleic acid utilising the detection probe, or the detection probe being coupled to a detection ligand allowing indirect detection of the target nucleic acid utilising the detection probe,
- i) the spacer is linked to one end of the probe, and the end of the probe not linked to the spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid or the hook capture probe;
- ii) the spacer is linked to a part of the probe between the ends of the probe, and one or both ends of the probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid or the hook capture probe;
- iii) the spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the probe has hybridised to the target nucleic acid or the hook capture probe; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; or
- iv) the spacer comprises a protein.
- According to the invention there is also provided a dipstick assay probe for detecting or capturing a target nucleic acid in a dipstick assay as herein defined, the probe comprising a nucleic acid or nucleic acid analogue capable of hybridising to the target nucleic acid, and a label allowing direct detection of the target nucleic acid utilising the probe, or a ligand allowing capture or indirect detection of the target nucleic acid utilising the probe, wherein the label or the ligand is linked to a spacer and the spacer is linked to the nucleic acid or nucleic acid analogue thereby spacing the label or the ligand from the nucleic acid or nucleic acid analogue, characterised in that:
- i) the spacer is linked to one end of the probe, and the end of the probe not linked to the spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid;
- ii) the spacer is linked to a part of the probe between the ends of the probe, and one or both ends of the probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid;
- iii) the spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the probe has hybridised to the target nucleic acid; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; or
- iv) the spacer comprises a protein.
- According to a further aspect there is provided a method for immobilising a probe to a solid phase which comprises providing a probe coupled to a protein and adsorbing the protein to the solid phase.
- Methods for immobilising a probe to a solid phase may further comprise coupling the probe to the protein.
- The probe is preferably coupled to a linker and the linker coupled to the protein. The probe is preferably coupled to the linker before the linker is coupled to the protein.
- The probe may comprise a nucleic acid or nucleic acid analogue.
- The linker is preferably coupled to a non nucleobase part of the probe. When the probe comprises nucleic acid, the linker is preferably coupled to a sugar or phosphate group of the probe. When the probe comprises a the nucleic acid analogue PNA (protein nucleic acid), the linker is preferably coupled to an amino group of the Probe. This is so that the linker does not interfere with base pairing of the nucleobase. Alternatively, the linker may be coupled to the 5' or 3' end of the part of the probe which is capable of hybridising to the binding partner of the probe (for example target nucleic acid) to avoid interference of the linker with the base pairing interactions of the prob and its binding partner.
- The probe is preferably coupled to the linker by reaction of a phosphoramidite group attached to the linker with a hydroxyl group of the probe or by reaction of a hydroxyl group of the linker with a phosphoramidite group attached to the probe.
- The linker is preferably coupled to the protein by reaction of a primary amino group attached to the linker with a carboxyl group of the protein.
- The solid phase may comprise a membrane, preferably a nitrocellulose membrane. Alternatively, the solid phase may comprise a particle such as a bead.
- There is also provided a probe immobilised to a solid phase by adsorption to the solid phase of a protein coupled to the probe.
- There is also provided according to the invention use of a dipstick, kit, or probe of the invention in a dipstick assay to test for the presence of target nucleic acid in a sample solution.
- It is thought that the sensitivity of dipsticks of the invention having a capture probe spacer is improved because the capture probe linked to the capture probe spacer is more accessible for hybridisation to the target nucleic acid. The sensitivity of dipsticks or kits of the invention in which the capture probe is coupled to a capture ligand by a capture probe spacer may be improved because the capture probe is thereby more accessible for hybridisation to the target nucleic acid and the capture ligand is more accessible for binding by the capture moiety.
- Similarly, it is thought that the sensitivity of dipsticks or kits of the invention having a detection probe spacer coupling the detection probe to a label is improved because the detection probe spacer makes the detection probe more accessible for hybridisation to the target nucleic acid. If the detection probe is coupled to a detection ligand by a detection probe spacer, the detection probe is thought to be more accessible for hybridisation to the target nucleic acid and the detection probe ligand may also be more accessible to the detection ligand binding moiety.
- Use of spacers comprising nucleobases in accordance with the invention is thought to be particularly effective because the nucleotide or nucleobase is able to form stacking interactions with the base pairs formed between the capture probe or the detection probe and the target nucleic acid. Formation of the stacking interactions is thought to enhance the hybridisation of the capture probe or the detection probe to the target nucleic acid thereby improving the efficiency of capture or detection of the target nucleic acid at the capture zone of the dipstick.
- Where appropriate, dipsticks and kits of the invention may be used in the following types of dipstick assay to test for the presence of a target nucleic acid in a sample solution:
- 1) A dipstick is provided which comprises a chromatographic strip having a contact end and a capture probe immobilised at a capture zone remote from the contact end, the capture probe being capable of hybridising to the target nucleic acid. A detection probe is contacted with the sample solution under conditions for hybridisation of the detection probe to the target nucleic acid. The sample solution is contacted with the contact end of the dipstick to cause sample solution to move by capillary action to the capture zone, thereby allowing target nucleic acid and the detection probe to move with the sample solution to the capture zone, and target nucleic acid to be captured at the capture zone. Detection probe can then be detected for at the capture zone. The presence of detection probe at the capture zone indicates that target nucleic acid was present in the sample solution.
In a variation of this assay, the detection probe may be releasably immobilised to the dipstick between the contact end and the capture zone instead of being separate from the dipstick. When the contact end of the dipstick is contacted with the sample solution causing the sample solution to move by capillary action to the capture zone, the detection probe is released into the sample solution so that released detection probe can hybridise to target nucleic acid in the sample solution as it moves to the capture zone.
In further variations of this assay, the detection probe may be separate from the sample solution and contacted with the capture zone of the dipstick. This will usually be done after the contact end of the dipstick has been contacted with the sample solution. The detection probe may be contacted directly with the capture zone, or the detection probe may be in a separate probe solution which is contacted with the contact end of the dipstick to cause the probe solution to move by capillary action to the capture zone. - 2) A dipstick is provided which comprises a chromotographic strip having a contact end and a capture moiety immobilised at a capture zone remote from the contact end, the capture moiety being capable of binding a capture probe hybridised to the target nucleic acid. The capture probe is contacted with the sample solution under conditions for hybridisation of the capture probe to the target nucleic acid. The sample solution is contacted with the contact end of the dipstick to cause sample solution to move by capillary action to the capture zone, thereby allowing target nucleic acid and the capture probe to move with the sample solution to the capture zone, and target nucleic acid to be captured at the capture zone by binding of the capture moiety to the capture probe. Target nucleic acid can then be detected for at the capture zone. Target nucleic acid may be detected using a detection probe as described for assay (1). The detection probe may be added to the sample solution with the capture probe or separately from the capture probe (in any order). Alternatively the detection probe may be releasably immobilised to the dipstick between the contact end and the capture zone, or may be contacted separately with the capture zone as described for assay (1).
- In a variation of assay (2), the capture probe instead of being mixed with the sample solution, may be releasably immobilised to the dipstick between the contact end and the capture zone. When the contact end of the dipstick is contacted with the sample solution causing the sample solution to move by capillary action to the capture zone, the capture probe is released into the sample solution so that released capture probe is released into the sample solution so that released capture probe can hybridise to target nucleic acid in the sample solution as it moves to the capture zone. Target nucleic acid may be detected for using a detection probe which may be contacted with the sample solution, releasably immobilised to the dipstick between the contact end and the capture zone, or contacted separately with the capture zone.
- In a further variation of assay (2), the capture probe may be contacted with the capture zone before, (or exceptionally, at the same time as) the sample solution reaches the capture zone by capillary action. This will allow the capture probe to be bound by the capture moiety at the capture zone so that target nucleic acid may be captured. The capture probe may be in a separate capture probe solution which is contacted separately with the capture zone by directly applying it to the capture zone, or by contacting the capture probe solution with the contact end of the dipstick to cause the capture probe to move by capillary action to the capture zone. Subsequent contact of the contact end of the dipstick with the sample solution will allow target nucleic acid reaching the capture zone by capillary action to be captured there. Again, target nucleic acid may be detected for using a detection probe which may be contacted with the sample solution, releasably immobilised to the dipstick between the contact end and the capture zone, or contacted separately with the capture zone. As an alternative to use of a detection probe in assay (2), the target nucleic acid may be labelled directly in the sample solution, for example by covalent attachment of a label to the target nucleic acid. This may be achieved by contact of a precursor label with the sample solution and incubation of the sample solution and precursor label under conditions for covalent attachment of the label to target nucleic acid.
- The capture moiety of assay (2) may be a universal capture probe capable of hybridising to the capture probe, or the capture moiety may be capable of binding by non base pairing interaction to the capture probe. For example, when the capture probe comprises one or more capture ligands, the capture moiety is a capture ligand binding moiety.
- Where the dipstick assay uses more than one probe capable of hybridising to the target nucleic acid it is preferred that all the probes are added to the sample solution and that hybridisation is carried out in a single step. This simplifies the assay, making it easier and quicker to perform. It has been found that the sensitivity of detection of target nucleic acid using a one step hybridisation assay is about equal to the sensitivity of detection when hybridisation is carried out in multiple steps. Multiple step hybridisation may be carried out by sequential hybridisation of the different probes to the target nucleic acid in the sample solution, or by contacting the dipstick with different solutions each containing a different probe. Usually, the latter method of multiple step hybridisation will involve washing the dipstick between each contact with a different probe solution. Whilst there may be circumstances in which multiple step hybridisation is preferred, it will be appreciated that the simpler and quicker format of one step hybridisation will usually be preferred.
- It is most preferred that the sample solution is of suitable composition to allow the hybridisation reactions to take place in a single hybridisation step and also to allow non base pairing interactions to take place (for example between a detection ligand and a detection ligand binding moiety and between a capture ligand and a capture ligand binding moiety) and transport a complex comprising target nucleic acid and one or more hybridised probes and (optionally) ligand binding moieties by capillary action up the dipstick. Using such a sample solution, it will be appreciated that the hybridisation reactions can then be carried out in a single step, and any ligand-ligand binding moiety interactions can take place, before the sample solution is contacted directly with the contact end of the dipstick (without the need to first dilute or alter the sample solution). Ligand-ligand binding moiety interactions can additionally or alternatively take place on the dipstick if desired as the sample solution travels to the capture zone. Simple and rapid dipstick detection of target nucleic acid is thereby facilitated.
- We have found that such results are achieved with sample solutions comprising a standard hybridisation buffer (such as SSPE buffer or Tris buffer) with salt, detergent and a blocking protein such as BSA or powdered milk. The sensitivity of detection of target nucleic acid using such assays has been found to be about equal to or better than that of other dipstick assays.
- Embodiments of the invention are now described by way of example with reference to the accompanying drawings in which:
-
Figure 1 shows the chemical structure of non protein components suitable as components of the capture probe spacer or the detection probe spacer of the invention; -
Figure 2 shows detection of Chlamydia trachomatis target nucleic acid using an embodiment of the invention; -
Figure 3 shows the experimental setup for Example 1; -
Figure 4 shows the experimental setup for Example 2; -
Figure 5 shows the experimental setup for Example 3; -
Figure 6 shows the experimental setup for Example 4; -
Figure 7 shows the experimental setup for Example 6; -
Figure 8 shows the experimental setup for Example 7; -
Figure 9 shows the experimental setup for Example 9; -
Figure 10 shows the experimental setup for Example 10; -
Figure 11 shows the experimental setup for Example 11; and -
Figure 12 shows schematically the structures of different detection probes coupled to biotin detection ligand used in the examples. - The examples relate to detection of a DNA fragment of Chlamydia trachomatis (CT) cryptic plasmid DNA. CT is one of the most common causes of sexually transmitted disease. CT infections can cause infertility and, during pregnancy, can result in spontaneous abortion, still birth or postpartum endometritis. In neonates, CT infection can cause blindness and chronic respiratory disease. Approximately 10% of infected men and upto 70% of infected women do not show symptoms of CT infection. Consequently, accurate diagnosis of CT infection is important so that early treatment of the disease can be initiated.
- In the examples, a
dipstick 10 is used to try to detect single or double stranded CT targetnucleic acid 12 in a sample solution 14 (seeFigure 2 ). Thedipstick 10 comprises a strip ofnitrocellulose 16 having acontact end 18 for contacting thesample solution 14 and acapture probe 20 immobilised at acapture zone 22 of thenitrocellulose strip 16 remote from thecontact end 18. An anti-biotin antibody-dye conjugate 24 (or an anti-fluorescein antibody-dye conjugate in example 5) is releasably immobilised at aconjugate zone 26 of the nitrocellulose strip located between thecontact end 18 and thecapture zone 22. Thecapture probe 20 is capable of hybridising to a first region of one strand (the first strand) of the targetnucleic acid 12. - The
sample solution 14 is prepared by spiking 1ml urine with Chlamydia trachomatis bacteria then spinning the urine at 15K rpm for 30 minutes. The pellets are resuspended in 100ml standard hybridisation buffer (including a blocking agent such as casein or BSA). Adetection probe 28 capable of hybridising to the target nucleic acid is then added (together with a helper probe capable of hybridising to the target nucleic acid adjacent the region recognised by the detection probe and/or the capture probe if used). Thedetection probe 28, is coupled to biotin (or to fluorescein in example 5) (using methods well known to those of skill in the art). Thesample solution 14 is then heated to 100°C for 7 minutes and cooled. - The
contact end 18 of thedipstick 10 is then contacted with thesample solution 14. Thesample solution 14 and any targetnucleic acid 12 hybridised to thedetection probe 28 moves up thedipstick 10 by capillary action. As thesample solution 14 passes theconjugate zone 26, it mobilises the anti-biotin antibody-dye conjugate 24. Released anti-biotin antibody-dye conjugate 24 can then bind to the biotin coupled to thedetection probe 28 hybridised to the targetnucleic acid 12. - Complex formed between the anti-biotin antibody-
dye conjugate 24, thedetection probe 28 and the targetnucleic acid 12 then moves up thedipstick 10 to thecapture zone 22 where the target nucleic acid of the complex can hybridise to the immobilisedcapture probe 20. Thecapture probe 20 is immobilised at thecapture zone 22 in such a way that it cannot be mobilised by thesample solution 14 as it moves past thecapture zone 22. Consequently, the complex bound to the capture probe remains in the capture zone and can be detected by the presence of the dye of the anti-biotin antibody-dye conjugate at the capture zone. - If there is no CT target nucleic acid in the sample solution, the
detection probe 28 cannot be captured at thecapture zone 22 and so no dye is visible at the capture zone. If there is CT target nucleic acid in the sample solution, but insufficient amounts of the target nucleic acid can be captured at the capture zone the presence of the target nucleic acid in the sample solution will not be detected. - It has been found that the sensitivity of detection of target nucleic acid can be reduced if the distance between the region of the target nucleic acid to which the capture probe hybridises and the region to which the detection probe hybridises is less than 26 nucleotides. Thus, it is preferred that the distance between these regions is at least 26 nucleotides and preferably at least 200 nucleotides.
- The capture of target nucleic acid described above is referred to as direct probe capture in the examples below. The strength of the detection of the target nucleic acid in the examples is recorded on a scale of 0 to 5, with 5 representing the strongest detection, and 0 no detection.
- The sequences of the probes used in the following examples are:
- SEQ ID No 1: 5' TGC AAC TCT TGG TGG TAG ACT TTG C
- SEQ ID No 2: 5' GCG CAC AGA CGA TCT ATT TTT TGC A
- SEQ ID No 3: 5' CGG GCG ATT TGC CTT AAC CCC ACC A
- SEQ ID No 4: 5' CCA AGC TTA AGA CTT CAG AGG AGC G
- SEQ ID No 5: 5' CAT GCG TTT CCA ATA GGA TTC TTG G
- SEQ ID No 6: 5' CAC AGT CAG AAA TTG GAG TGC TGG C
- SEQ ID No 7: 5' CTT GCT GCT CGA ACT TGT TTA GTA C
- SEQ ID No 8: 5' AGA AGT CTT GGC AGA GGA AAC TTT T
- SEQ ID No 9: 5' CTA GAA TTA GAT TAT GAT TTA AAA GGG
- SEQ ID No 10: 5' TTC ATA TCC AAG GAC AAT AGA CCA A
- SEQ ID No 11: 5' TGA TCT ACA AGT ATG TTT GTT GAG T
- SEQ ID No 12: 5' TGC ATA ATA ACT TCG AAT AAG GAG AAG
- SEQ ID No 13: 5' TCC CTC GTG ATA TAA CCT ATC CG
- SEQ ID No 14: 5' CAG GTT GTT AAC AGG ATA GCA CGC
- SEQ ID No 15: 5' CTC GTT CCG AAA TAG AAA ATC GCA
- SEQ ID No 16: 5' GGT AAA GCT CTG ATA TTT GAA GAC
- SEQ ID No 17: 5' CTG AGG CAG CTT GCT AAT TAT GAG T
- The structures of the detection probes in the examples described below are shown schematically in
Figure 12 . - Capture format: direct probe capture (cp)
Seq ID No 14 immobilised on the dipstick;
Detection probe (dp) : Seq ID No 13 or Seq ID No 15 with biotin coupled directly to the 5'-end, or by a 3 nucleotide (N3), 6 nucleotide (N6), S, or SS spacer, or SEQ ID No 13 with biotin coupled directly to the 3'-end. 1012 copies of each.
Detection format: anti-biotin antibody-dye conjugate; Target DNA: 73 or 76 nucleotide single stranded DNA fragments at 5x1011 - 1010 copies. -
dp Seq ID No 13 Copies of target: 5x10 111011 5x10 101010 Detection probe Signal strength dp-B 5' 3.5 3.0 2.0 1.0 dp-N3-B 5' 4.5 3.5 3.0 1.5 dp-N6-B 5' 5.0 4.0 3.0 2.0 dp-S-B 5' 4.0 3.0 2.0 1.0 dp-SS-B 5' 4.5 3.5 2.5 1.0 3' B-dp 5.0 3.5 3.0 2.0 dp Seq ID No 15 Copies of target: 5x10 111011 5x1010 Detection probe Signal strength dp-B 5' 3.5 2.0 1.0 dp-N3-B 5' 4.0 3.0 2.0 dp-N6-B 5' 4.5 3.0 2.0 dp-S-B 5' 4.0 2.5 1.5 dp-SS-B 5' 4.0 2.5 1.5 - The sensitivity of target nucleic acid detection using detection probes with biotin coupled to the 5'-end by a N6, N3, SS or S spacer was higher than the sensitivity using a detection probe with biotin coupled directly to the 5'-end.
- The N3 and N6 spacers were better than the S and SS spacers.
- It is preferred that the biotin (or other detection ligand) is coupled to one end of the detection probe. In the complex formed when the capture probe and the detection probe are hybridised to the target nucleic acid at the capture zone then the biotin (or other detection ligand) can be at the end of the detection probe proximal to the region of the target nucleic acid which hybridises to the capture probe (internally orientated) or, preferably, at the end of the detection probe distal to the region of the target nucleic acid which hybridises to the capture probe (externally orientated). If no spacer is used to couple the detection ligand to the detection probe, then the sensitivity of detection of target nucleic acid is generally higher if the detection ligand is externally orientated. Consequently, the detection probe is usually chosen so that the detection ligand is externally orientated.
- However, in this example, the sensitivity of detection using a detection probe with externally orientated biotin coupled directly to the 3'-end of the detection probe was as good as the sensitivity using a detection probe with internally orientated biotin coupled to the 5'-end of the detection probe by a six nucleotide spacer. Thus, when spacers are used in accordance with the invention, the detection probe does not have to be chosen so that the detection ligand is externally orientated in the complex captured at the capture zone.
- Capture format: direct probe capture (cp)
Seq ID No 14 immobilised on dipstick membrane;
Detection probe: detection probe (dp) Seq ID No 13, Seq ID No 15 andSeq ID No 16 with biotin coupled to the 5'-end by a 3 nucleotide (N3), 6 nucleotide (N6), S or SS spacer. 1012 copies of each.
Detection format: anti-biotin antibody-dye conjugate; Target DNA: 214 bp double stranded DNA fragments at 1011 - 1010 copies. -
Copies of target: 1011 5x10 101010 Detection probe Signal Strength dp13-B + dp15-B + dp16-B 1.5 1.0 0.0 dp13-N3-B + dp15-N3-B + dp16-N3-B 3.0 2.0 0.5 dp13-N6-B + dp15-N6-B + dp16-N6-B 4.5 3.0 1.0 dp13-S-B + dp15-S-B + dp16-S-B 3.0 2.0 <0.5 dp13-SS-B + dp15-SS-B + dp16-SS-B 3.5 3.0 0.5 dp13 non-lab + dp15-N6-B + dp16-N6-B 2.5 1.5 0.5 - The sensitivity of detection of double stranded target nucleic acid was improved more than five-fold using N3, N6, S or SS spacers.
- The sensitivity of detection was higher with the N6 and SS spacers than with the N3 and S spacers, indicating that the length of the spacer is important for improved sensitivity of detection.
- The sensitivity of detection was higher with the N6 spacer than the SS spacer, despite the fact that these spacers are of equivalent length, indicating that the physicochemical properties of the spacer are important for improved sensitivity of detection.
- The sensitivity of detection using only two detection probes each coupled at the 5'-end to biotin by an N6 spacer (dp13 non-lab + dp15-N6-B + dp16-N6-B), in which the biotin is internally orientated in the complex captured by the capture probe, was greater than the sensitivity of detection using three detection probes each coupled at the 5'-end directly to biotin (dp13-B + dp15-B + dp16-B) in which the biotin of one detection probe (dpl3-B) is externally orientated in the complex captured by the capture probe.
- The sensitivity of target nucleic acid detection is increased by using a spacer to couple the detection ligand to the detection probe.
- Longer spacers are better than shorter spacers.
- Spacers of equivalent length but with different physicochemical properties had different effects on the sensitivity of detection. In particular, spacers in which the non protein component consists only of nucleotides are better than spacers which include non nucleotide components. Possible explanations for this are:
- 1. Nucleotide spacers may improve the sensitivity of detection by enhancing hybridisation of the detection probe to the target nucleic acid. The nucleotides of these spacers are not expected to base pair to nucleotides of the target nucleic acid when the detection probe hybridises to the target nucleic acid. The nucleobases of these nucleotides may form stacking interactions with the base pairs formed when the detection probe hybridises to the target nucleic acid. These stacking interactions may enhance the stability of the hybrid formed between the target nucleic acid and the detection probe, thereby enhancing the sensitivity of detection of target nucleic acid.
- 2. Nucleotide spacers may be more rigid than the S and SS spacers. The ribose rings of the nucleotide spacers are expected to provide much more rigidity than the polyethylene glycol groups of the S and SS spacers. This greater rigidity might increase the availability of the detection ligand coupled to the nucleotide spacer for interaction with the detection ligand binding moiety.
- 3. Polarity differences between the nucleotide and non nucleotide spacers may cause differences in the sensitivity of detection.
- Capture format: direct probe capture (cp)
Seq ID No 10 immobilised on the dipstick;
Detection probe: detection probe (dp) Seq ID No 13 coupled to biotin at the 5'-end either directly or by a N6, SS, (dS)6, (SC3)6 or SN3SN3S spacer. 1012 copies of each. Detection format: anti-biotin antibody-dye conjugate; Helper probes:SEQ ID No 5 andSEQ ID No 6 adjacent toSEQ ID No 10;SEQ ID No 1 andSEQ ID No 2 adjacent to SEQ ID No 13 at 1012 copies;
Target DNA: 872 bp double stranded DNA fragment at 2x1011 - 5x1010 copies. -
Copies of target: 2x1011 5x1010 Detection probe Signal strength dp-B <1.0 0.0 dp -N6-B 2.0 0.5 dp -SS-B 1.0 0.0 dp - (dS)6-B 1.0 0.0 dp - (SC3)6-B 1.0 0.0 dp -SN3SN3S-B 1.5 0.0 - The SS, (dS)6, and (SC3)6 spacers are of equivalent length and had a similar effect on enhancing the sensitivity of detection of target nucleic acid despite their structural differences and properties.
- The N6 spacer is of equivalent length to the SS, (dS)6, and (SC3)6 spacers. However, the N6 spacer had the greatest effect on improving the sensitivity of detection of target nucleic acid.
- The sensitivity of target nucleic acid detection was greater using the N6 spacer than the SN3SN3S spacer (the longest spacer tested). A possible explanation for this could be that the S monomer reduces or eliminates stacking interaction between the nucleobases of the N3 components of the spacer and the base pairs formed between the detection probe and the target nucleic acid. This data supports the conclusion that stacking interactions between unpaired nucleobases of the spacer and the duplex formed between the target nucleic acid and the detection probe are important in enhancing the sensitivity of detection of target nucleic acid.
- Spacers with different physicochemical properties and lengths were evaluated by the dipstick test and by dot blot analysis. Dot blot analysis enables the efficiency of the interaction of the anti-biotin antibody with the biotin coupled to the detection probe to be analysed in the absence of hybridisation of the detection probe to the target nucleic acid. 5x108-5x1011 copies of detection probes coupled to biotin by different spacers were spotted at different places on a positively charged nylon membrane and UV crosslinked to the membrane. The membrane was then incubated with an anti-biotin antibody coupled to alkaline phosphatase (capable of converting a Nitro Blue Tetrazolium/5-Bromo-4-Chloro-3-Indolyl Phosphate (NBT/BCIP) chromogenic substrate), washed, and incubated with the NBT/BCIP chromogenic substrate, and the membrane was observed to see if any colour formed at the capture zone.
- Capture format: direct probe capture (cp)
Seq ID No 14 immobilised on the dipstick;
Detection probe: detection probe (dp) Seq ID No 13 coupled to biotin at the 5'-end either directly or by a nucleotide or non-nucleotide spacer. 1012 copies of each.
Detection format: anti-biotin antibody-dye conjugate; Helper probes:SEQ ID No 2 andSEQ ID No 3 adjacent toSEQ ID No 14 at 1012 copies;
Target DNA: 416 bp double stranded DNA fragment at 5x1010 - 5x109 copies. -
copies of target: 5x1010 5x109 Detection probe Signal strength dp-B5' 3.5 0.5 dp-N3-B5' 4.5 1.5 dp-N4-B5' 4.5 1.0 dp-N5-B5' 4.5 1.0 dp-N6-B5' 5.0 1.5 dp-(dS)6-B5' 4.0 0.5 dp-S-B5' 3.5 0.0 dp-SS-B5' 4.0 0.0 dp-SSS-B5' 4.5 0.0 dp-SSSS-B5' 4.0 0.0 dp-S N3 S N3 S-B5' 4.5 0.5 - Detection probes: detection probe Seq ID No 13 coupled to biotin at the 5'-end, either directly or by a nucleotide or non-nucleotide spacer.
Detection format: anti-biotin antibody coupled to alkaline phosphatase, detection by NBT/BSIP chromogenic substrate. -
Spacer Detection limit without 5.0 x E11 N3 5.0 x E10 N4 5.0 x E10 N5 5.0 x E10 N6 2.5 x E10 (dS)6 2.5 x E10 SN3SN3S 2.5 x E9 SSSS 2.5 x E9 SSS 5.0 x E9 SS 2.5 x E10 S 5.0 x E11 - There is no significant difference in the sensitivity of target nucleic acid detection using detection probes with three, four or five nucleotide spacers.
- The sensitivity of detection with a six nucleotide spacer is marginally better than 3-5 nucleotide spacers.
- The sensitivity of detection with spacers consisting only of nucleotides was better than with spacers which include non-nucleotides. For example the N3 spacer was better than the longest spacer SN3SN3S, equivalent to 15 nucleotides in length.
- The (dS)6 spacer is slightly better than the SS spacer.
- The sensitivity of detection was highest with the longest spacers (SSSS and SN3SN3S).
- The sensitivity of detection using equivalent length spacers (N6, (dS)6 and SS) with different physicochemical properties was similar.
- The dS component has a similar structure to a nucleotide. Both have a ribose residue, expected to provide rigidity. However, the nucleotide has a nucleobase which is not present in the dS component. The different effect of the (dS)6 spacer on the sensitivity of target nucleic acid detection compared to the N6 spacer suggests that the greater effect on the improvement of sensitivity of detection using a nucleotide spacer is explained principally by the presence of the nucleobases which do not base pair with the target nucleic acid.
- The composition of the spacer appears to be more important than its length (compare the results for dp-N3-B5' and dp-SN3SN3S-B5' in the dipstick test of example 4).
- The sensitivity of detection using a (dS)6 spacer was greater than with an SS spacer. These spacers are of equivalent length. This suggests that the physicochemical properties of the spacer, such as rigidity or polarity, may also have an effect on the improvement of sensitivity of detection by the spacer.
- The dot blot analysis of example 4 shows that the length of the spacer is important for the availability of the biotin to the anti-biotin antibody. The sensitivity of biotin recognition by the anti-biotin antibody was similar whether the biotin was coupled to the immobilised probe by an N6, (dS)6 or SS spacer. However, the effect of these spacers on the sensitivity of detection in the dipstick test of example 4 was different. This suggests that the composition of the spacer is more important in the hybridisation of the detection probe to the target nucleic acid than in the recognition of the biotin by the anti-biotin antibody. The length of the spacer appears to be more important than the composition of the spacer for recognition of the biotin (or other detection ligand) by the anti-biotin antibody (or other detection ligand binding moiety).
- Capture format: direct probe capture (cp)
Seq ID No 14 coupled to BSA immobilised to the dipstick. The capture probe is coupled to the BSA either directly or by a six nucleotide spacer;
Detection probe: detection probe (dp) Seq ID No 13 coupled to fluorescein. 1012 copies.
Detection format: anti-fluorescein antibody-dye conjugate; Target DNA: 73 nt or 76 nt single stranded DNA fragments at 1011 copies. -
Copies of target: 1011 capture probe signal strength cp-BSA-dipstick 4.0 cp-N6-BSA-dipstick 5.0 - Capture format: direct probe capture (cp)
Seq ID No 14 coupled at the 5'-end to BSA immobilised on the dipstick. The capture probe is coupled to the BSA by an N6 or SN3SN3S spacer;
Detection probes: detection probes (dp)Seq ID No
Detection format: anti-biotin antibody-dye conjugate; Target : 872 bp double stranded DNA at 1011 - 2.5x109 copies. -
Copies of target: 1011 2.5 x10 101010 5x109 2.5x109 Capture probe signal strength cp-SN3SN3S-BSA-dipstick 4.0 3.5 2.5 1.0 0.0 cp-N6-BSA-dipstick 4.5 4.0 3.0 1.5 0.0 - Capture format: direct probe capture (cp) Seq ID No 15 coupled to BSA immobilised on the dipstick. The capture probe is coupled to the BSA by an N6 spacer;
Helper probes:SEQ ID No 3 andSEQ ID No 4 adjacent to SEQ ID No 15;
Detection probe: In this example, the detection probe comprises a hook probe and a universal probe. The hook probe has sequence corresponding to Seq ID No 17 (capable of hybridising to the target nucleic acid) and sequence complementary to the sequence of a universal probe. The universal probe is coupled to a textile dye by an N6 or SN3SN6 spacer. There are 1012 copies of the hook probe.
Target: 872 bp double stranded DNA at 1011 and 1010 copies. -
Copies of target: 1011 1010 Capture probe signal strength Universal probe-SN3SN6-Dye 2.0 0.0 Universal probe-N6 -Dye 2.0 0.5 - Use of spacers comprising a protein immobilised to the dipstick and a non protein to couple the capture probe to the immobilised protein (examples 5 and 6), or use of non protein spacers to couple the label to the detection probe (example 7) improve the sensitivity of detection of target nucleic acid.
- The sensitivity of nucleic acid detection was highest when the non protein component of the spacer consisted entirely of nucleotides.
- The sensitivity of target nucleic acid detection using a capture probe immobilised on the dipstick membrane by a protein carrier or by passive adsorption are compared in this example.
- 10 nmoles of capture probe functionalised at the 5'- or 3'-end with a primary amino group was mixed with 1µl 10% BSA and 25µl of 50 mM MES buffer (pH 6.1) and made up to 49µl with water in a 500µl microfuge tube. 1µl of coupling reagent (freshly prepared 300mM 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) in water) was then added and mixed in well. The mixture was left at room temperature overnight to couple the BSA to the capture probe, and then stored at 2-8°C. The BSA-capture probe was then applied to the capture zone of the dipstick and the dipstick was heated at 80°C for 1 hour.
- The molar ratio of capture probe to BSA and the concentration of the EDAC coupling reagent was optimised. Capture probe:BSA ratios of 2.7:1, 3.2:1, 4.1:1, 5.2:1, 6.6:1, 10.9:1 were studied. 1, 1.5, 2, 4, 5 and 20mM concentrations of EDAC were studied. Concentrations higher than 5 mM caused the BSA-capture probe to precipitate out of the solution.
- Capture format: direct probe capture by
Seq ID No 14 immobilised directly on the dipstick membrane or by a BSA protein spacer. The capture probe and the capture probe-BSA were applied to the dipstick and immobilised by treating the dipstick for 1h at 80°C.
Detection probe: biotin labelled detection probe (dp) Seq ID No 13 at 1012 copies.
Detection format: anti-biotin antibody-dye conjugate; Target DNA: 73 nucleotide single stranded DNA fragments at 1x1011 copies. -
Capture probe: Signal capture probe-BSA 4.0 Capture probe 0.0 - The target nucleic acid was undetected using a capture probe directly immobilised to the dipstick. However, a strong detection signal was obtained if the capture probe was coupled to BSA and the BSA was immobilised to the dipstick.
- The following examples relate to detection of target nucleic acid using a probe coupled to a coloured particle by a protein spacer. Coloured dye particles were coated with probe coupled to BSA. The protein is adsorbed to the dye particles thereby coupling the probe to the dye particles. The procedure used was as follows:
- Dilute 11 µl of washed dye (A575 =900) in 434µl of 10mM phosphate buffer containing 10mM NaCl, 1 mM EDTA, pH 7.5.
- Add 5 µl of probe-BSA (2 mg/ml), mix well and rotate at room temperature for 1 hour;
- Add 50 µl of 20% alkaline treated casein. Mix well and rotate at room temperature for 1 hour;
- Centrifuge at 4000rpm in microfuge at room temperature for 15 min;
- Remove supernatant and resuspend the pellet in 500 µl of conjugation buffer containing 5
% sucrose 2% alkaline treated casein, 0.02% Na azide. - Capture format: Anti-biotin antibody/biotin coupled to capture probes Seq ID No 13, No 14, No 15, No 16 and No 17 at 1012 copies per test.
- Alternatively direct probe capture format (
Seq ID No 14 or Seq ID No 15) were used for comparison ;
Direct detection probeSeq ID No 10 coupled to dye particle by BSA;
Helper probes:SEQ ID No 5 andSEQ ID No 6 adjacent toSEQ ID No 10;
Target: 872 bp ds DNA at 1011 to 108 copies. -
Capture probe(s) Seq ID No 13 Seq ID No 14Seq ID No 15 Seq ID No 16Seq ID No 17 All 5 Signal 1 0 1 1 1 5 Strength 1011 copies target -
Target copies 1x1011 1x1010 5x109 1x109 5x108 1x108 Antibody 5 4.5 4 2.5 2 0.5 Capture (5 probes) Direct Probe 4.5 3 2.5 1.5 0 0 Capture (Seq ID No 15) Direct Probe 3 2 0.5 0 0 0 Capture (Seq ID No 14) - These results show that direct detection probe - dye conjugate works with both antibody capture format and direct probe capture format.
- Capture format: direct probe capture (cp) Seq ID No 15 coupled to BSA immobilised to the dipstick.
Helper probes:SEQ ID No 3 andSEQ ID No 4 adjacent to SEQ ID No 15;
Detection probe region Seq ID No 17. A "hook probe" with sequence complimentary to target DNA in this region and to the universal probe sequence was used at 1012 copies per test;
Detection format: Textile dye coupled to the universal probe by BSA;
Target: 872 bp ds DNA at 1011 and 1010 copies. -
Copies of target 1011 1010 signal 2.0 0.0 - Capture format: direct probe capture Seq ID No 15 coupled to BSA immobilised to the dipstick.
Helper probes:SEQ ID No 3 andSEQ ID No 4 adjacent to SEQ ID No 15;
Detection probe region Seq ID No 17. A "hook"detection probe 1, with sequence complementary to target DNA in this region and to theuniversal probe sequence 2, was used at 1012 copies per test;
Detection format: a hook detection probe comprises sequence complementary to sequence of the target nucleic acid and to the sequence of a first universal detection probe. The first universal detection probe is coupled to BSA and the BSA is adsorbed to a first coloured particle thereby coupling the first universal detection probe to the first coloured particle. Several second universal detection probes are also each coupled to BSA adsorbed to the first coloured particle. The second universal detection probes comprise sequence complementary to sequence of a third universal detection probe. The third universal detection probe is coupled to BSA adsorbed to a second coloured particle. When target nucleic acid is detected using the hook detection probe, the first, second and third universal detection probes and the first and second coloured particles, a single first coloured particle forms a first layer on the target nucleic acid and several second coloured particles form a second layer on the target nucleic acid as inFigure 11 . Because several coloured particles can be attached to each target nucleic acid in this way, it is thought that the sensitivity of detection of target nucleic acid is improved compared to use of a hook detection probe and a single universal detection probe coupled to a coloured particle.
Target: 872 bp ds DNA at 1011 and 1010 copies. -
Copies of target 1011 1010 signal 2.0 0.5 -
- Capture format: oligonucleotide probe capture immobilised on dipstick membrane via BSA carrier;
- Detection format: multiple biotin labelled detector probe; anti-biotin antibody - colloidal gold conjugate;
- Sample preparation: Chlamydia trachomatis (Ct) elementary bodies (EB) celles were prepared in ceoncentrations from 106 copies/µl to 103 copies/µl in PBS buffer and heated at 100°C for 20 minutes;
- Hybridisation/dipstick running buffer: Standard hybridisation buffer comprising salt, detergent and a blocking protein such as BSA or powdered milk.
- The detection probe, helper probe and 5x106 - 5x103 copies of EB diluted in hybridisation buffer made up to 80 µl and heated at 100°C for 7 minutes. The mixure was then centrifuged briefly to collect all the liquid and mixed with 20 µl anti-biotin Ab colloidal gold. The whole 100µl mixture were wicked up on dipstick and let to develop a signal.
- The results presented in the Table and
Figure 13 showed that about 104 copies of Ct EB could be detected with one step nucleic acid dipstick assay in less than an hour including the sample preparation step. - Although the so presented dipstick detection assay has a sensitivity of detection about equal to other sandwich hybridisation assays it has the major advantages of speed and simplicity.
A sandwich hybridisation assay for detection of Ct disclosed inPCT WO 93/1322
The nucleic acid dipstick assay subject of this invention could be done in one step with no need of different steps for addition of components and washings. This sandwich hybridisation assay does not require more than one solution conditions in order to render them advantageous for hybridisation and other affinity pair formations. The same solution conditions could serve a free migration of the components through the dipstick membrane as well. - The sensitivity of detection of single and double stranded target nucleic acid by dipsticks has been found to be significantly improved by the use of spacers in accordance with the invention. The sensitivity of detection of double stranded circular target nucleic acid can also be increased by use of spacers in accordance with the invention.
- Single stranded target nucleic acid is known to form secondary structure by means of intramolecular base pairing interactions. Such secondary structure can inhibit binding of the capture probe and the detection probe to the target nucleic acid. Consequently, the region of the target nucleic acid to which the detection probe and capture probe bind is often chosen as a region predicted to be substantially free of secondary structure.
- The improved sensitivity of detection of double stranded target nucleic acid achieved by use of spacers in accordance with the invention means that the sensitivity of detection of single stranded target nucleic acid using a capture probe and/or a detection probe which recognises a region of the target nucleic acid involved in secondary structure will also be improved. An advantage of this is that the capture probe and/or detection probe may not have to be chosen based on predictions of the secondary structure formed by the target nucleic acid, thus simplifying the choice of capture and detection probe.
- Conventional methods of dipstick detection are thought to be very poor at detecting circular double stranded target nucleic acid. Consequently, such target nucleic acid is usually treated with an enzyme that linearises the double stranded target before the target is detected. Improved detection of circular double stranded target nucleic acid using spacers in accordance with the invention means that linearisation of the target nucleic acid may not be required, thus simplifying the detection methods.
-
-
Figure 1 - Design of Spacer- B=biotin coupled to a linker
-
Figure 3 - 310 - cp
- 320 - dp
- 330 - target
-
Figure 4 - 450 - dp Seq ID No 13
- 460 - cp
Seq ID No 14 - 470 - dp Seq ID No 15
- 480 - dp
Seq ID No 16
-
Figure 5 - 510 - cp
- 520 - dp
- 540 - helper probes dp
-
Figure 6 - 610 - cp
- 620 - dp
- 640 - hp
-
Figure 7 - 710 - cp-N6-BSA or cp-SN3-SN3-S-BSA
-
Figure 8 - 810 - cp
- 821 - detection hook
- 822 - Universal probe-N6-Dye or Universal probe-SN3-SN6- Dye
- 840 - helper probes
-
Figure 9 - 910 - capture probe coupled to ligand
- 920 - detection probe coupled to dye particle by BSA
- 930 - 872bp dsDNA Target
- 940 - helper probes
- 950 - membrane bound anti-biotin antibody
-
Figure 10 - 1010 - cp
- 1021 - detection hook
- 1022 - Universal probe- Dye
- 1040 - helper probes
-
Figure 11 - 1110 - direct probe capture
- 1121 - hook probe
- 1122 - first layer dye conjugate
- 1123 - second layer dye conjugate
- 1130 - 872bp dsDNA target
- 1140 - helper probes
-
Figure 12 - -------
- represents nucleic acid of the detection probe
- B
- represents biotin coupled to a linker
- x
- represents the number of nucleotides
- y
- represents the number of hexaethyleneglycol phosphate monomers
-
Figure 13
One-step nucleic acid dipstick assay detection of Chlamydia trachomatis
The numbers indicate the number of elementary bodies of Chlamydia trachomatis.
*NC: negative control -
Figure 14 - Table: one-step nucleic acid dipstick assay detection of Chlamydia trachomatis
wherein:
Claims (28)
- A dipstick for testing for the presence of target nucleic acid in a sample solution which comprises:a chromatographic strip having a contact end for contacting the sample solution; and a capture probe immobilised at a capture zone of the chromatographic strip remote from the contact end, the capture probe being capable of hybridising to the target nucleic acid or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is linked to a capture probe spacer and the capture probe spacer is linked to the capture zone, thereby immobilising the capture probe at the capture zone and spacing the capture probe from the capture zone, characterised in that:i) the capture probe spacer is linked to one end of the capture probe, and the end of the capture probe not linked to the capture probe spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the capture probe has hybridised to the target nucleic acid or to the hook capture probe when the capture probe has hybridised to the hook capture probe;ii) the capture probe spacer is linked to a part of the capture probe between the ends of the capture probe, and one or both ends of the capture probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the capture probe has hybridised to the target nucleic acid or to the hook capture probe when the capture probe has hybridised to the hook capture probe;iii) the capture probe spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the capture probe has hybridised to the target nucleic acid or hook capture probe; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; oriv) the capture probe spacer comprises a protein directly adsorbed to the capture zone, and covalently linked to the capture probe by a linker.
- A dipstick according to claim 1(iii) in which the capture probe spacer further comprises a protein, the capture probe being coupled to the nucleotide and the nucleotide being coupled to the protein thereby spacing the capture probe from the protein.
- A dipstick according to claim 2 in which the nucleotide is coupled to the protein by a linker.
- A dipstick according to any preceding claim in which the protein is BSA, thyroglobulin, or a derivative thereof.
- A dipstick according to any preceding claim which further comprises a detection probe, releasably immobilised at a probe zone located between the contact end and the capture zone of the chromatographic strip, the detection probe being capable of hybridising to the target nucleic acid to allow detection of the target nucleic acid.
- A dipstick according to claim 5 in which the detection probe is coupled to a label which allows direct detection of the target nucleic acid when the detection probe has hybridised to the target nucleic acid.
- A dipstick according to claim 5 in which the detection probe is coupled to a detection ligand which can be bound by a detection ligand binding moiety to allow indirect detection of the target nucleic acid when the detection probe has hybridised to the target nucleic acid.
- A dipstick according to claim 6 or 7 in which the label or detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe, thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe.
- A dipstick for testing for the presence of target nucleic acid in a sample solution which comprises:a chromatographic strip having a contact end for contacting the sample solution;a capture moiety, immobilised at a capture zone remote from the contact end, the capture moiety being capable of binding directly or indirectly to the target nucleic acid; anda detection probe, releasably immobilised at a probe zone located between the contact end and the capture zone, the detection probe being capable of hybridising to the target nucleic acid and the detection probe being coupled to a label allowing direct detection of the target nucleic acid utilising the detection probe, or the detection probe being coupled to a detection ligand allowing indirect detection of the target nucleic acid utilising the detection probe,wherein the label or the detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe, thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe, and
wherein:i) the detection probe spacer is linked to one end of the detection probe, and the end of the detection probe not linked to the detection probe spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;ii) the detection probe spacer is linked to a part of the detection probe between the ends of the detection probe, and one or both ends of the detection probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;iii) the detection probe spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the detection probe has hybridised to the target nucleic acid; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; oriv) the detection probe spacer comprises a protein. - A dipstick according to claim 9(iv) in which the protein is BSA, thyroglobulin, or a derivative thereof.
- A dipstick according to claim 9(iv) or 10 in which the label or the detection ligand is attached to or part of a bead and the protein is adsorbed directly to the bead.
- A dipstick according to any of claims 9(iv), 10, or 11 in which the protein is linked to the detection probe by a linker.
- A dipstick according to any of claims 9(iv), or 10 to 12 in which the detection probe spacer further comprises a non protein.
- A dipstick according to claim 13 in which the detection probe is coupled to the non protein and the non protein is coupled to the protein thereby spacing the detection probe from the protein.
- A dipstick according to claim 14 in which the non protein is coupled to the protein by a linker.
- A dipstick according to any of claims 13 to 15 in which the non protein is at least the length of three nucleotides.
- A dipstick according to any of claims 13 to 16 in which the non protein comprises a nucleotide.
- A dipstick according to claim 17 in which the non protein consists only of one or more nucleotides.
- A dipstick according to any of claims 9 to 18 in which the capture moiety comprises a capture probe capable of hybridising to the target nucleic acid or to a hook capture probe bound to the target nucleic acid.
- A dipstick according to any of claims 9 to 18 in which the capture moiety is capable of binding to a capture ligand coupled to a capture probe bound to the target nucleic acid.
- A kit for testing for the presence of target nucleic acid in a sample solution which comprises:a dipstick according to any of claims 1 to 4; anda detection probe capable of hybridising to the target nucleic acid thereby allowing detection of target nucleic utilising the detection probe.
- A kit according to claim 21 in which the detection probe is coupled to a label allowing direct detection of the detection probe hybridised to the target nucleic acid, or a detection ligand allowing indirect detection of the detection probe hybridised to the target nucleic acid by a detection ligand binding moiety, wherein the label or the detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe.
- A kit for testing for the presence of target nucleic acid in a sample solution which comprises:a dipstick according to claim 20; anda capture probe capable of hybridising to the target nucleic acid, or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is coupled to a capture ligand which can be bound by the capture moiety.
- A kit according to claim 23 in which the capture ligand is linked to a capture probe spacer and the capture probe spacer is linked to the capture probe thereby coupling the capture ligand to the capture probe and spacing the capture ligand from the capture probe.
- A kit for testing for the presence of target nucleic acid in a sample solution which comprises:a dipstick comprising a chromatographic strip having a contact end for contacting the sample solution and a capture probe capable of hybridising to the target nucleic acid, or to a hook capture probe bound to the target nucleic acid, the capture probe being immobilised at a capture zone of the chromatographic strip remote from the contact end; anda detection probe capable of hybridising to the target nucleic acid, the detection probe being coupled to a label allowing direct detection of the target nucleic acid utilising the detection probe, or the detection probe being coupled to a ligand allowing indirect detection of the target nucleic acid utilising the detection probe, wherein the label or the detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe, and wherein:i) the detection probe spacer is linked to one end of the detection probe, and the end of the detection probe not linked to the detection probe spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;ii) the detection probe spacer is linked to a part of the detection probe between the ends of the detection probe, and one or both ends of the detection probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the detection probe has hybridised to the target nucleic acid;iii) the detection probe spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the detection probe has hybridised to the target nucleic acid; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; oriv) the detection probe spacer comprises a protein.
- A kit for testing for the presence of target nucleic acid in a sample solution which comprises:a dipstick comprising a chromatographic strip having a contact end for contacting the sample solution and a capture moiety immobilised at a capture zone of the chromatographic strip remote from the contact end, the capture moiety being capable of binding directly or indirectly to the target nucleic acid;a capture probe capable of hybridising to the target nucleic acid, or to a hook capture probe bound to the target nucleic acid, wherein the capture probe is coupled to a capture ligand which can be bound by the capture moiety; anda detection probe capable of hybridising to the target nucleic acid, the detection probe being coupled to a label allowing direct detection of the target nucleic acid utilising the detection probe, or the detection probe being coupled to a detection ligand allowing indirect detection of the target nucleic acid utilising the detection probe;wherein the capture probe is linked to a capture probe spacer and the capture probe spacer is linked to the capture ligand thereby coupling the capture ligand to the capture probe and spacing the capture ligand from the capture probe, or the label or the detection ligand is linked to a detection probe spacer and the detection probe spacer is linked to the detection probe thereby coupling the label or the detection ligand to the detection probe and spacing the label or the detection ligand from the detection probe; and wherein:i) the spacer is linked to one end of the probe, and the end of the probe not linked to the spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid or the hook capture probe;ii) the spacer is linked to a part of the probe between the ends of the probe, and one or both ends of the probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid or the hook capture probe;iii) the spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the probe has hybridised to the target nucleic acid or the hook capture probe; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; oriv) the spacer comprises a protein.
- A dipstick assay probe for detecting or capturing a target nucleic acid in a dipstick assay as herein defined, the probe comprising a nucleic acid or nucleic acid analogue capable of hybridising to the target nucleic acid, and a label allowing direct detection of the target nucleic acid utilising the probe, or a ligand allowing capture or indirect detection of the target nucleic acid utilising the probe, wherein the label or the ligand is linked to a spacer and the spacer is linked to the nucleic acid or nucleic acid analogue thereby spacing the label or the ligand from the nucleic acid or nucleic acid analogue, characterised in that:i) the spacer is linked to one end of the probe, and the end of the probe not linked to the spacer is coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid;ii) the spacer is linked to a part of the probe between the ends of the probe, and one or both ends of the probe are coupled to one or more nucleotides which do not hybridise to the target nucleic acid when the probe has hybridised to the target nucleic acid;iii) the spacer comprises: a nucleotide comprising a nucleobase capable of forming a stacking interaction with a base pair formed when the probe has hybridised to the target nucleic acid; 3-Hydroxypropyl phosphate; or Hexaethylene glycol phosphate; oriv) the spacer comprises a protein.
- Use of a dipstick according to any of claims 1 to 20, a kit according to any of claims 21 to 26, or a probe according to claim 27, to test for the presence of target nucleic acid in a sample solution.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB0016836.9A GB0016836D0 (en) | 2000-07-07 | 2000-07-07 | Improved dipstick assays (1) |
| GB0016836 | 2000-07-07 | ||
| PCT/GB2001/003039 WO2002004671A2 (en) | 2000-07-07 | 2001-07-06 | Improved binding interactions in dipstick assays |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1325151A2 EP1325151A2 (en) | 2003-07-09 |
| EP1325151B1 true EP1325151B1 (en) | 2011-08-24 |
Family
ID=9895306
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01947639A Expired - Lifetime EP1325151B1 (en) | 2000-07-07 | 2001-07-06 | Improved binding interactions in dipstick assays |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US8431336B2 (en) |
| EP (1) | EP1325151B1 (en) |
| JP (2) | JP5341291B2 (en) |
| CN (2) | CN100355905C (en) |
| AT (1) | ATE521714T1 (en) |
| AU (2) | AU2001269285B2 (en) |
| ES (1) | ES2372477T3 (en) |
| GB (1) | GB0016836D0 (en) |
| WO (1) | WO2002004671A2 (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2986359A4 (en) * | 2014-03-11 | 2017-02-15 | Bio-rad Laboratories, Inc. | Dip-stick western blot |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB0016813D0 (en) * | 2000-07-07 | 2000-08-30 | Lee Helen | Improved dipstick assays (4) |
| GB0016836D0 (en) | 2000-07-07 | 2000-08-30 | Lee Helen | Improved dipstick assays (1) |
| GB0025245D0 (en) | 2000-10-14 | 2000-11-29 | Lee Helen | Multiple target detection |
| DE10154291B4 (en) * | 2001-11-05 | 2005-05-19 | Hain Lifescience Gmbh | Method for the detection of nucleic acids in the form of a dry rapid test |
| US8669052B2 (en) | 2008-06-10 | 2014-03-11 | Rapid Pathogen Screening, Inc. | Lateral flow nucleic acid detector |
| GB0701253D0 (en) | 2007-01-23 | 2007-02-28 | Diagnostics For The Real World | Nucleic acid amplification and testing |
| US20110086359A1 (en) | 2008-06-10 | 2011-04-14 | Rapid Pathogen Screening, Inc. | Lateral flow assays |
| WO2010009206A2 (en) * | 2008-07-15 | 2010-01-21 | Rapid Pathogen Screening, Inc. | In situ lysis of cells in lateral flow immunoassays |
| WO2010139010A1 (en) * | 2009-06-02 | 2010-12-09 | Monoquant Pty Ltd | A method of nucleic acid amplification |
| EP2644699B1 (en) | 2010-11-24 | 2018-02-21 | Kaneka Corporation | Amplified nucleic acid detection method and detection device |
| JP2013198482A (en) * | 2012-02-22 | 2013-10-03 | Nippon Meat Packers Inc | Detection method of nucleic acid |
| EP2871244A4 (en) * | 2012-07-05 | 2016-03-23 | Ngk Insulators Ltd | Nucleic acid chromatography method, composition for nucleic acid chromatography, and kit containing same |
| JP6734011B2 (en) * | 2014-06-06 | 2020-08-05 | 古河電気工業株式会社 | Biomolecule detection test kit, biomolecule detection method using the same, biomolecule detection test piece and biomolecule detection labeling reagent used therein |
| JP2018518180A (en) | 2015-06-19 | 2018-07-12 | ケンブリッジ エンタープライズ リミテッド | Diagnosis and treatment of infectious diseases |
| WO2018180987A1 (en) * | 2017-03-29 | 2018-10-04 | 東洋紡株式会社 | Nucleic acid detection method |
| CN114040983A (en) * | 2019-06-26 | 2022-02-11 | 南京金斯瑞生物科技有限公司 | Oligonucleotide containing blocker |
| MX2020006374A (en) * | 2020-07-13 | 2022-01-14 | Univ Mexico Nac Autonoma | Hybridization method for detecting nucleic acids using functionalized magnetic particles. |
Family Cites Families (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA1223222A (en) * | 1984-02-22 | 1987-06-23 | Nanibhushan Dattagupta | Immobilized nucleic acid-containing probes |
| CA1253777A (en) | 1984-06-01 | 1989-05-09 | Robert J. Carrico | Nucleic acid hybridization assay employing immobilized rna probes |
| ATE40572T1 (en) | 1985-01-10 | 1989-02-15 | Molecular Diagnostics Inc | PHOTOCHEMICAL METHOD FOR LABELING NUCLEIC ACIDS FOR DETECTION IN HYBRIDISATION SAMPLES. |
| GB8509367D0 (en) * | 1985-04-12 | 1985-05-15 | Amersham Int Plc | Nucleic acid hybridisation |
| US4751177A (en) * | 1985-06-13 | 1988-06-14 | Amgen | Methods and kits for performing nucleic acid hybridization assays |
| US4868105A (en) | 1985-12-11 | 1989-09-19 | Chiron Corporation | Solution phase nucleic acid sandwich assay |
| US4822731A (en) | 1986-01-09 | 1989-04-18 | Cetus Corporation | Process for labeling single-stranded nucleic acids and hybridizaiton probes |
| US5317098A (en) | 1986-03-17 | 1994-05-31 | Hiroaki Shizuya | Non-radioisotope tagging of fragments |
| US5310650A (en) * | 1986-09-29 | 1994-05-10 | Abbott Laboratoires | Method and device for improved reaction kinetics in nucleic acid hybridizations |
| US5283174A (en) * | 1987-09-21 | 1994-02-01 | Gen-Probe, Incorporated | Homogenous protection assay |
| WO1989004375A1 (en) | 1987-10-23 | 1989-05-18 | Siska Diagnostics, Inc. | Lanthanide chelate-tagged nucleic acid probes |
| WO1990001564A1 (en) | 1988-08-09 | 1990-02-22 | Microprobe Corporation | Methods for multiple target analyses through nucleic acid hybridization |
| GB8822228D0 (en) | 1988-09-21 | 1988-10-26 | Southern E M | Support-bound oligonucleotides |
| DE3842700A1 (en) * | 1988-12-19 | 1990-06-21 | Boehringer Mannheim Gmbh | METHOD FOR PROTEIN IMMOBILIZATION ON A SOLID PHASE, PROTEIN-CARRYING SOLID PHASE PRODUCED THEREOF AND THE USE THEREOF |
| EP0387696B1 (en) | 1989-03-17 | 1997-08-27 | Abbott Laboratories | Method and device for improved reaction kinetics in nucleic acid hybridizations |
| DE69032847T2 (en) * | 1989-06-30 | 1999-05-12 | Chiron Corp., Emeryville, Calif. | Hydrophobic nucleic acid probe |
| US5232829A (en) | 1989-09-29 | 1993-08-03 | Hoffmann-La Roche Inc. | Detection of chlamydia trachomatis by polymerase chain reaction using biotin labelled lina primers and capture probes |
| ES2103795T3 (en) | 1989-12-04 | 1997-10-01 | Microprobe Corp | IMPROVEMENT OF THE CAPTURE OF A NUCLEIC ACID DIANA THROUGH THE USE OF UNITED OLIGONUCLEOTIDES COVALENTLY TO POLYMERS. |
| CA2031659A1 (en) * | 1990-01-26 | 1991-07-27 | John B. Findlay | Water-insoluble reagent, nucleic acid probe, test kit and diagnostic and purification methods |
| FR2663040B1 (en) * | 1990-06-11 | 1995-09-15 | Bio Merieux | METHOD OF DETECTING A NUCLEOTIDE SEQUENCE ACCORDING TO THE SANDWICH HYBRIDIZATION TECHNIQUE. |
| US5695926A (en) * | 1990-06-11 | 1997-12-09 | Bio Merieux | Sandwich hybridization assays using very short capture probes noncovalently bound to a hydrophobic support |
| US5210015A (en) | 1990-08-06 | 1993-05-11 | Hoffman-La Roche Inc. | Homogeneous assay system using the nuclease activity of a nucleic acid polymerase |
| US5663318A (en) * | 1991-03-01 | 1997-09-02 | Pegg; Randall Kevin | Assay preparation containing capture and detection polynucleotides covalently bound to substrates with a heterobifunctional crosslinking agent |
| FR2679252B1 (en) * | 1991-07-17 | 1993-12-10 | Bio Merieux | PROBE SYSTEM FOR HLA DR TYPING, AND TYPING METHOD USING THE SAME. |
| FR2679255B1 (en) | 1991-07-17 | 1993-10-22 | Bio Merieux | METHOD OF IMMOBILIZING A NUCLEIC FRAGMENT BY PASSIVE FIXING ON A SOLID SUPPORT, SOLID SUPPORT THUS OBTAINED AND ITS USE. |
| US5976789A (en) | 1991-07-17 | 1999-11-02 | Bio Merieux | System of probes enabling HLA-DR typing to be performed, and typing method using said probes |
| US5747244A (en) * | 1991-12-23 | 1998-05-05 | Chiron Corporation | Nucleic acid probes immobilized on polystyrene surfaces |
| WO1994000600A1 (en) | 1992-06-25 | 1994-01-06 | Microprobe Corporation | Solid supports for nucleic acid hybridization assays |
| WO1994002634A1 (en) * | 1992-07-24 | 1994-02-03 | University Of South Australia | Amplification and detection process |
| US5438131A (en) | 1992-09-16 | 1995-08-01 | Bergstrom; Donald E. | 3-nitropyrrole nucleoside |
| WO1994006940A1 (en) | 1992-09-18 | 1994-03-31 | Abbott Laboratories | Multiple assay test strip devices |
| EP0637342B1 (en) * | 1992-11-27 | 1999-04-28 | N.V. Innogenetics S.A. | Process for typing of hcv isolates |
| WO1994023299A1 (en) | 1993-03-31 | 1994-10-13 | Quidel Corporation | Multiple assay device |
| US5496934A (en) * | 1993-04-14 | 1996-03-05 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Nucleic acids encoding a cellulose binding domain |
| JPH08511621A (en) | 1993-06-09 | 1996-12-03 | クイデル コーポレイション | Antigen-specific one-step assay |
| US5635602A (en) * | 1993-08-13 | 1997-06-03 | The Regents Of The University Of California | Design and synthesis of bispecific DNA-antibody conjugates |
| CA2145750A1 (en) | 1993-08-18 | 1995-02-23 | Michael J. Conrad | Applications of fluorescent n-nucleosides and fluorescent structural analogs of n-nucleosides |
| CA2170264A1 (en) * | 1993-09-10 | 1995-03-16 | Michael W. Konrad | Optical detection of position of oligonucleotides on large dna molecules |
| US5925517A (en) * | 1993-11-12 | 1999-07-20 | The Public Health Research Institute Of The City Of New York, Inc. | Detectably labeled dual conformation oligonucleotide probes, assays and kits |
| US5681697A (en) | 1993-12-08 | 1997-10-28 | Chiron Corporation | Solution phase nucleic acid sandwich assays having reduced background noise and kits therefor |
| DE4406524A1 (en) * | 1994-02-28 | 1995-08-31 | Boehringer Mannheim Gmbh | 3 'RNA tag with terminal transferase |
| WO1995027081A1 (en) * | 1994-03-31 | 1995-10-12 | E.I. Du Pont De Nemours And Company | A method for detection of nucleic acid fragments |
| US5747248A (en) | 1994-12-05 | 1998-05-05 | Chiron Corporation | Discontinuous probe design using hybritope mapping |
| EP1019420B1 (en) | 1995-05-09 | 2003-08-06 | Kreatech Biotechnology B.V. | Methods for the production of platinum-based linkers between labels and bio-organic molecules, for labelling bio-organic molecules, for detecting biological substances of interest |
| DE19537952A1 (en) * | 1995-10-12 | 1997-04-17 | Boehringer Mannheim Gmbh | Method for the detection of an analyte |
| US5874216A (en) * | 1996-02-23 | 1999-02-23 | Ensys Environmental Products, Inc. | Indirect label assay device for detecting small molecules and method of use thereof |
| ATE221894T1 (en) | 1996-10-08 | 2002-08-15 | Kreatech Biotech Bv | METHOD FOR LABELING NUCLEOTIDES, LABELED NUCLEOTIDES AND USEFUL INTERMEDIATE PRODUCTS |
| JP2002510505A (en) * | 1998-04-03 | 2002-04-09 | フィロス インク. | Localizable protein arrays |
| US20020012913A1 (en) | 1998-09-15 | 2002-01-31 | Kevin L. Gunderson | Nucleic acid analysis using complete n-mer arrays |
| AU1681000A (en) | 1998-12-10 | 2000-06-26 | Takara Shuzo Co., Ltd. | Method for immobilizing oligonucleotide on a carrier |
| DE19901761A1 (en) * | 1999-01-18 | 1999-07-01 | Gerhard Dr Hartwich | Oligonucleotides tagged with photoinducible redox-active unit |
| DE19910761A1 (en) * | 1999-03-11 | 2000-09-14 | Tellmann Wilfried | Magnetic motor has intermediate magnets arranged diagonally on both sides of rotor such that attractive and repulsive forces between rotor and intermediate magnets generate rotary motion |
| WO2000061806A2 (en) | 1999-04-09 | 2000-10-19 | Arcturus Engineering, Inc. | GENERIC cDNA OR PROTEIN ARRAY FOR CUSTOMIZED ASSAYS |
| GB0016833D0 (en) | 2000-07-07 | 2000-08-30 | Lee Helen | Improved dipstick assays (2) |
| GB0016836D0 (en) | 2000-07-07 | 2000-08-30 | Lee Helen | Improved dipstick assays (1) |
| GB0016814D0 (en) | 2000-07-07 | 2000-08-30 | Lee Helen | Improved dipstick assays (3) |
| GB0025245D0 (en) | 2000-10-14 | 2000-11-29 | Lee Helen | Multiple target detection |
| GB0029154D0 (en) | 2000-11-30 | 2001-01-17 | Lee Helen | Signal enhancement with multiple labelled-antibodies |
| GB0130947D0 (en) | 2001-12-24 | 2002-02-13 | Lee Helen | Improved sample preparation for the detection of infectious agents |
| GB0217390D0 (en) | 2002-07-26 | 2002-09-04 | Lee Helen | Liquid sampler and method |
-
2000
- 2000-07-07 GB GBGB0016836.9A patent/GB0016836D0/en not_active Ceased
-
2001
- 2001-07-06 ES ES01947639T patent/ES2372477T3/en not_active Expired - Lifetime
- 2001-07-06 CN CNB018153097A patent/CN100355905C/en not_active Expired - Lifetime
- 2001-07-06 WO PCT/GB2001/003039 patent/WO2002004671A2/en active Application Filing
- 2001-07-06 CN CNA2007101849913A patent/CN101230393A/en active Pending
- 2001-07-06 AU AU2001269285A patent/AU2001269285B2/en not_active Expired
- 2001-07-06 AU AU6928501A patent/AU6928501A/en active Pending
- 2001-07-06 US US10/332,142 patent/US8431336B2/en not_active Expired - Lifetime
- 2001-07-06 JP JP2002509524A patent/JP5341291B2/en not_active Expired - Lifetime
- 2001-07-06 AT AT01947639T patent/ATE521714T1/en not_active IP Right Cessation
- 2001-07-06 EP EP01947639A patent/EP1325151B1/en not_active Expired - Lifetime
-
2011
- 2011-09-29 JP JP2011215672A patent/JP2012019797A/en not_active Withdrawn
-
2013
- 2013-04-19 US US13/866,891 patent/US20140106347A1/en not_active Abandoned
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2986359A4 (en) * | 2014-03-11 | 2017-02-15 | Bio-rad Laboratories, Inc. | Dip-stick western blot |
| US10107780B2 (en) | 2014-03-11 | 2018-10-23 | Bio-Rad Laboratories, Inc. | Dip-stick western blot |
| US10852273B2 (en) | 2014-03-11 | 2020-12-01 | Bio-Rad Laboratories, Inc. | Dip-stick western blot |
Also Published As
| Publication number | Publication date |
|---|---|
| ATE521714T1 (en) | 2011-09-15 |
| JP2012019797A (en) | 2012-02-02 |
| CN101230393A (en) | 2008-07-30 |
| GB0016836D0 (en) | 2000-08-30 |
| CN100355905C (en) | 2007-12-19 |
| US20140106347A1 (en) | 2014-04-17 |
| US20040072176A1 (en) | 2004-04-15 |
| JP2004512499A (en) | 2004-04-22 |
| WO2002004671A3 (en) | 2003-05-01 |
| ES2372477T3 (en) | 2012-01-20 |
| CN1452664A (en) | 2003-10-29 |
| EP1325151A2 (en) | 2003-07-09 |
| JP5341291B2 (en) | 2013-11-13 |
| WO2002004671A2 (en) | 2002-01-17 |
| AU6928501A (en) | 2002-01-21 |
| AU2001269285B2 (en) | 2007-08-16 |
| US8431336B2 (en) | 2013-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20140106347A1 (en) | Binding interactions in dipstick assays | |
| US7867706B2 (en) | Capture and detection of target nucleic acid in dipstick assays | |
| US20150099265A1 (en) | Detection signal and capture in dipstick assays | |
| EP0248896B1 (en) | Amplified hybridization assay | |
| AU2001269285A1 (en) | Improved binding interactions in dipstick assays | |
| US20080160516A1 (en) | Capture and detection format versatility for dipstick assays | |
| WO2007114519A1 (en) | Probe, probe set, probe-immobilized carrier, and genetic testing method | |
| JPH08501689A (en) | Improved Strand Displacement Assay and Complexes Useful Therefor | |
| AU2001267752A1 (en) | Improved capture and detection of target nucleic acid in dipstick assays | |
| AU2001267750A1 (en) | Improved detection signal and capture in dipstick assays | |
| AU2001269279A1 (en) | Improved capture and detection format versatility for dipstick assays | |
| JPH11502124A (en) | All-in-one nucleic acid amplification assay | |
| JPH08501220A (en) | Isolated nucleotide sequence for identification of Neisseria gonorrhoeae | |
| EP0622464A2 (en) | Nucleic acid assay procedure | |
| WO2002004122A2 (en) | Improved stability of hybridisation interactions in dipstick assays | |
| JPH0588A (en) | New nucleic acid fragment and detection of mycoplasma using the same | |
| JPH0698797A (en) | Method for detecting nucleic acid | |
| AU2007234492A1 (en) | Improved detection signal and capture in dipstick assays | |
| WO2007114517A1 (en) | Probe, probe set, probe-immobilized carrier, and genetic testing method |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20030206 |
|
| AK | Designated contracting states |
Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK RO SI |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: DIAGNOSTICS FOR THE REAL WORLD, LTD. |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: DIAGNOSTICS FOR THE REAL WORLD, LTD |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: LEE, HELEN Inventor name: DINEVA, MAGDA, ANASTASSOVA Inventor name: HU, HSIANG, YUN |
|
| 17Q | First examination report despatched |
Effective date: 20050301 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: DIAGNOSTICS FOR THE REAL WORLD, LTD |
|
| 17Q | First examination report despatched |
Effective date: 20050301 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 60145209 Country of ref document: DE Effective date: 20111027 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: VDEP Effective date: 20110824 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2372477 Country of ref document: ES Kind code of ref document: T3 Effective date: 20120120 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111226 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK05 Ref document number: 521714 Country of ref document: AT Kind code of ref document: T Effective date: 20110824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20111125 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 Ref country code: AT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20120525 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60145209 Country of ref document: DE Effective date: 20120525 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120731 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120731 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120731 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120706 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20110824 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120706 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 18 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20200612 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20200709 Year of fee payment: 20 Ref country code: ES Payment date: 20200813 Year of fee payment: 20 Ref country code: DE Payment date: 20200708 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20200710 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60145209 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20210705 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20211026 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210705 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210707 |