EP0947577A1 - Fuel compositions containing tertiary-alkyl primary amines - Google Patents
Fuel compositions containing tertiary-alkyl primary amines Download PDFInfo
- Publication number
- EP0947577A1 EP0947577A1 EP99302308A EP99302308A EP0947577A1 EP 0947577 A1 EP0947577 A1 EP 0947577A1 EP 99302308 A EP99302308 A EP 99302308A EP 99302308 A EP99302308 A EP 99302308A EP 0947577 A1 EP0947577 A1 EP 0947577A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- fuel
- tertiary alkyl
- primene
- alkyl primary
- cetane
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/222—Organic compounds containing nitrogen containing at least one carbon-to-nitrogen single bond
- C10L1/2222—(cyclo)aliphatic amines; polyamines (no macromolecular substituent 30C); quaternair ammonium compounds; carbamates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/04—Use of additives to fuels or fires for particular purposes for minimising corrosion or incrustation
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L10/00—Use of additives to fuels or fires for particular purposes
- C10L10/12—Use of additives to fuels or fires for particular purposes for improving the cetane number
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/18—Organic compounds containing oxygen
- C10L1/1811—Organic compounds containing oxygen peroxides; ozonides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/23—Organic compounds containing nitrogen containing at least one nitrogen-to-oxygen bond, e.g. nitro-compounds, nitrates, nitrites
- C10L1/231—Organic compounds containing nitrogen containing at least one nitrogen-to-oxygen bond, e.g. nitro-compounds, nitrates, nitrites nitro compounds; nitrates; nitrites
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/22—Organic compounds containing nitrogen
- C10L1/232—Organic compounds containing nitrogen containing nitrogen in a heterocyclic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10L—FUELS NOT OTHERWISE PROVIDED FOR; NATURAL GAS; SYNTHETIC NATURAL GAS OBTAINED BY PROCESSES NOT COVERED BY SUBCLASSES C10G, C10K; LIQUEFIED PETROLEUM GAS; ADDING MATERIALS TO FUELS OR FIRES TO REDUCE SMOKE OR UNDESIRABLE DEPOSITS OR TO FACILITATE SOOT REMOVAL; FIRELIGHTERS
- C10L1/00—Liquid carbonaceous fuels
- C10L1/10—Liquid carbonaceous fuels containing additives
- C10L1/14—Organic compounds
- C10L1/24—Organic compounds containing sulfur, selenium and/or tellurium
- C10L1/2425—Thiocarbonic acids and derivatives thereof, e.g. xanthates; Thiocarbamic acids or derivatives thereof, e.g. dithio-carbamates; Thiurams
Definitions
- This invention relates to improved fuel oil compositions.
- fuel oil compositions containing cetane improvers having improved thermal stability The compositions further enjoy improved stabilization against: 1) sediment formation, and 2) color degradation during storage and distribution as well as improved cetane number, rust inhibition and demulsibility.
- the useful life of a fuel is a function of its quality and of storage conditions.
- middle distillate fuels such as diesel, jet fuels and heating fuels
- Fuel storage stability was a well-understood problem prior to the introduction of low-sulfur diesel fuel. It is well established that diesel fuels can exhibit instability when stored for extended periods of time (storage stability), or when brought into contact with high temperature engine parts (thermal stability). Hydrotreating to meet 1993 regulations reduced storage stability problems for highway fuels. However, low-sulfur fuels resulted in other issues, such as peroxide and thermal stability problems in distillate fuels. However, fuels are often stored for much longer periods because of logistical and economic necessities.
- oxidative degradation products formed under both prolonged storage and thermal stress, continue to be a problem in the utilization of, for example, diesel fuels.
- Fuel-instability reactions are defined in terms of the formation of deleterious products, such as filterable sediment, adherent gums, and peroxides. Sediments and gums which result from the oxidation reactions act to block filters and deposit on surfaces. Both the low-temperature storage and high-temperature thermal degradation, are of concern. Hydrotreating is generally considered the most effective means of improving stability. However, the cost of stability improvement by additives doping can often be less than the hydrotreatment costs.
- Diesel fuel performs multiple functions in a diesel engine and the associated fuel system. In addition to its primary role as an energy source, the fuel also serves as the sole lubricant of critical moving parts and as a heat-transfer fluid. Diesel fuel is increasingly used as a circulating coolant for high pressure fuel injections systems. This causes a problem in that as engine heat passes from injectors into the fuel, it can trigger a process that leads to particle formation, thus clogging filters and injectors. Fuels resistant to such thermal degradation must get a minimum 80% reflectance measurement using a green filter in the updated Octel F21-61 test (180 minutes, 150°C).
- thermal stability may become even more important in the future. Diesel engine manufacturers have indicated that engines under development to meet future exhaust emission standards will expose the fuel to more severe operating environments (stress), e.g., higher pressures and longer contact with high-temperature engine parts. In particular, thermal stability can be troublesome in some heavy-duty applications in some areas with widespread use of cetane improvers. Accordingly, cetane improvers will become increasingly important as engine makers promote and/or require higher cetane fuels, especially in premium diesel products.
- Additives can cause diesel fuel degradation if they are not oxidatively stable. Above 120°C, a cetane improver may oxidize and decompose, leading to particulate and sediment problems that can block filters. It is commonly accepted that 2-ethylhexyl nitrate functions as a diesel ignition improver because it is unstable, i.e., it thermally begins to decompose at about 155°C (311°F), i.e., just above the 300°F stability test temperature (Bacha, John; Lesnini, D. G., Proceedings of the 6 th International Conference on stability and Handling of Liquid Fuels, 1997, Eds., H. N.; US Dept. of Energy, Vol. 2, 671). This result suggests that the stability test temperature and test duration together are just sufficient for 2-ethylhexyl nitrate to contribute to the observed fuel thermal instability in the 300°F test.
- Tertiary alkyl amines are known as diesel fuel additives as antioxidants for storage improvement ( see U.S. Patent 2,945,749 ); in combination with fatty amines to counteract tendency of fatty amines to emulsify (see U.S Patent 3,014,793 ); and as stabilizers in combination with detergent, rust preventors and demulsifier additives (see U.S. Patent 2,793,943 ).
- tertiary alkyl primary amines as thermal stabilizers and cetane improvers especially in the presence of conventional cetane improvers.
- the present inventors have now unexpectedly found that fuels are made thermally stable in the presence of cetane improvers, which are known to make fuels thermally unstable, by the addition of tertiary alkyl primary amines in the C 8 - C 24 range .
- the tertiary alkyl primary amines of the present invention also operate as cetane number improvers and the combination of cetane number improvers with the tertiary alkyl primary amines of the present invention provide a higher cetane number than that provided by the cetane improver alone.
- fuel oil compositions containing these amines are also characterized as having improved dispersability, improved rust inhibition and improved demulsibility.
- a fuel composition including: (A) a major amount of fuel; (B) at least one cetane number improver; and (C) at least one tertiary alkyl primary amine of the formula: wherein: R 1 , R 2 , and R 3 are each independently (C 1 -C 21 ) alkyl, substituted (C 1 -C 21 ) alkyl, (C 1 -C 21 ) alkenyl or substituted (C 1 -C 21 ) alkenyl.
- a fuel composition including: (A) a major amount of diesel fuel; (B) a minor amount of 2-ethylhexylnitrate effective to improve the cetane number of the diesel fuel; and (C) a minor amount of at least one tertiary alkyl primary amine of the formula: effective to provide thermal stability, wherein: R 1 , R 2 , and R 3 are each independently (C 1 -C 21 ) alkyl, substituted (C 1 -C 21 ) alkyl, (C 1 -C 21 ) alkenyl or substituted (C 1 -C 21 ) alkenyl.
- a method of providing thermal stability to fuel containing at least one cetane improver including: introducing into the fuel at least one tertiary alkyl primary amine of the formula: in an amount effective to impart thermal stability to the fuel, wherein: R 1 , R 2 , and R 3 are each independently (C 1 -C 21 ) alkyl, substituted (C 1 -C 21 ) alkyl, (C 1 -C 21 ) alkenyl or substituted (C 1 -C 21 ) alkenyl.
- (C 1 -C 21 ) means a straight chain or branched chain alkyl group having from 1 to 21 carbon atoms per group.
- major amount is understood to mean greater than 50 percent by weight and the term “minor amount” is understood to mean less than 50 percent by weight.
- the fuel of the fuel composition of the present invention is present in a major amount in the fuel composition.
- the fuel oil is present in an amount of at least 60% by weight, preferably at least 75% by weight, more preferably at least 90% by weight of the total fuel composition.
- the fuels useful in the present invention are generally any fuel which may suffer from inadequate cetane number, thermal instability, storage stability problems (sediment and gum formation, color degradation and other deterioration during storage), rusting and emulsion formation.
- the fuels are hydrocarbon fractions having an initial boiling point of at least 200°F and an end point not higher than 750°F, which boil substantially continuously throughout their distillation range.
- Such fuels are generally known as middle distillate fuels.
- middle distillate fuels which may be used in the fuel compositions of the present invention include, but are not limited to, distilled oils, furnace oils, diesel fuels, jet fuels, and residual fuels such as bunker fuels, marine diesel fuels, railroad diesel fuels, etc.
- the fuel is a diesel fuel or a jet fuel.
- the fuel is a diesel fuel.
- the fuel compositions of the present invention also include at least one cetane improver.
- Cetane improvers are compounds that readily decompose to form free radicals and then, in turn, promote the rate of chain initiation. The increased rate of chain initiation improves ignition characteristics for diesel fuel. Accordingly, cetane number (ignition quality) improvers are used to increase the cetane number when the base fuel cetane does not meet required specifications.
- Suitable cetane improvers include, without limitation, alkyl nitrates, such as 2-ethylhexyl nitrate (2-EHN); peroxides, such as di-t-butylperoxide; tetrazoles; thioaldehydes, tertiary alkyl primary amines, and mixtures thereof.
- the at least one cetane improver is an alkyl nitrate.
- the at least one cetane improver is 2-ethylhexyl nitrate (2-EHN).
- the at least one cetane improver is a tertiary alkyl primary amine.
- the at least one cetane improver is a tertiary alkyl primary amine and the fuel composition further includes a second cetane improver selected from alkyl nitrates, peroxides, tetrazoles; thioaldehydes, tertiary alkyl primary amines, and mixtures thereof.
- the at least one cetane improver is a tertiary alkyl primary amine and the fuel composition further includes a second cetane improver which is an alkyl nitrate, such as 2-EHN.
- the cetane improver is present in the fuel composition at a concentration of 50 to 7500, preferably 100 to 5000, more preferably 100 to 2000 ppm.
- the at least one tertiary alkyl primary amine is a tertiary alkyl primary amine according to the formula: wherein: R 1 , R 2 , and R 3 are each independently (C 1 -C 21 ) alkyl, substituted (C 1 -C 21 ) alkyl, (C 1 -C 21 ) alkenyl or substituted (C 1 -C 21 ) alkenyl.
- Suitable examples of (C 1 -C 21 ) alkyl include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, n-pentyl, isopentyl, neopentyl, n-hexyl, 2-ethylhexyl, octyl, decyl, isodecyl, undecyl, dodecyl (also known as lauryl), tridecyl, tetradecyl (also known as myristyl), pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, cosyl, and eicosyl.
- Suitable examples of (C 1 -C 21 ) alkenyl include, but are not limited to, ethenyl, n-propenyl, isopropenyl, 1-butenyl, cis-2-butenyl, isobutylene, trans-2-butenyl, 2-3, dimethyl-2-butenyl, 3-methyl-1-butenyl, 2-methyl-2-butene, 1-pentenyl, cis-2-pentenyl, trans-2-pentenyl, 1-hexenyl, 1-heptenyl, 1-octentl, 1-nonenyl, and 1-decenyl.
- Suitable examples of (C 1 -C 21 ) substituted alkyl and alkenyl include, but are not limited to, the above recited alkyl and alkenyl groups substituted with hydroxy, halide such as fluorine, chlorine or bromine; cyano; alkoxy; haloalkyl; carbalkoxy; carboxy; amino; alkylamino derivatives and the like; or nitro groups.
- the at least one tertiary alkyl primary amine may be a single amine or a mixture of amines, for instance as described following.
- the at least one tertiary alkyl amine is 1,1,3,3-tetramethylbutylamine available from Rohm and Haas Co of Philadelphia, PA as PRIMENE TOA®.
- the at least one tertiary alkyl amine is an isomeric mixture of C 16 to C 22 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE JM-T®.
- the at least one tertiary alkyl amine is an isomeric mixture of C 8 to C 10 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE BC-9® or an isomeric mixture of C 12 to C 14 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE 81-R® or a mixture of PRIMENE BC-9® and PRIMENE 81-R®.
- the at least one tertiary alkyl amine is an isomeric mixture of C 12 to C 14 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE 81-R®.
- the at least one tertiary alkyl primary amine is present in the fuel composition at a concentration of 1 to 1000, preferably 5 to 500, more preferably 10 to 200 ppm, most preferably 10 to 100 ppm. In another embodiment, the at least one tertiary alkyl primary amine is present in the fuel composition at a concentration of 50 to 100, or 1 to 10 ppm.
- the tertiary alkyl primary amines used in the fuel compositions of the present invention are prepared using substrate compounds known as substrates for the Ritter reaction and include, for example, alcohols, alkenes, aldehydes, ketones, and ethers, ( see, generally, L. I. Krimen and D. J. Cota, "The Ritter Reaction", Organic Reactions, Vol. 17, 1969, pp. 213-325 ).
- Processes for preparing tertiary alkyl primary amines are known in the art and are described for instance in U.S Patent 5,527,949 and in co-pending provisional application 60/051,867.
- the fuel compositions of the present invention may also include other additives well known in the art such as, without limitation, anti-oxidants, dispersants, anti-foaming agents and the like.
- Also contemplated is a method of providing thermal stability to fuel containing at least one cetane improver including: introducing into the fuel at least one tertiary alkyl primary amine of the formula: in an amount effective to impart thermal stability to the fuel wherein: - R 1 , R 2 , and R 3 are each independently (C 1 -C 21 ) alkyl, substituted (C 1 -C 21 ) alkyl, (C 1 -C 21 ) alkenyl or substituted (C 1 -C 21 ) alkenyl.
- the fuel, at least one cetane improver, and tertiary alkyl primary amine as well as the levels of usage are as described above.
- Fuel samples #A- # I were fresh test fuels without any additives and were obtained from commercial sources. The fuel samples were analyzed to ensure conformance with specifications and stored under ambient temperature, in dark, and under nitrogen atmosphere. All of the C 8 , C 9 , C 12 , and C 18 tertiary alkyl primary amines samples were commercial products sold under the trademark Primene® by Rohm and Haas Company of Philadelphia, Pa. The results are shown in Table 1.
- Fuel #A was evaluated for thermal stability and cetane number improvement.
- a sample of Fuel #A containing 100 ppm Primene® 81-R and a sample without were prepared. The fuel samples were stored in an air atmosphere at room temperature during the test period.
- the fuel samples were tested for a prolonged period of storage stability. Periodically, as indicated in Table 2, the fuels were sampled and tested for oxidative stability according to ASTM D 2274 Diesel Oxidation Stability test method as follows. A 350 mL sample of fuel was heated at 95°C for 40 hr. while oxygen is bubbled through at the rate of 3 liters per hour. After aging, the sample is cooled to room temperature and filtered to obtain the filterable insoluble quantity. Adherent insolubles are then removed from the associated glassware with trisolvent (TAM). The TAM is then evaporated to obtain the adherent insolubles. The sum of filterable and adherent insolubles, expressed as milligrams per 100 mL, is reported as total insolubles.
- TAM trisolvent
- the Fuel #A samples were also tested for cetane number with and without additives.
- the method used for cetane number determination was ASTM D 613 as well as tested for oxidation stability using ASTM D 2274. The results are shown in Table 3.
- Samples of fuel #C, #D, and #E having a sulfur concentration of 0.047%, 0.035%, and 0.035%, respectively were also evaluated for thermal stability and cetane number improvement.
- the cetane numbers of these fuels are listed in Table 5, and found to be 44.1, 45.5, and 45.4 respectively for the fuel # C, #D, and #E.
- the fuels were tested for oxidation/thermal stability with and without 2-EHN and combined with Primene amines.
- 2-EHN is used as a cetane number improver additive, and the addition of 2-EHN does increase the cetane number.
- it has an adverse effect on the thermal stability of the fuel when subjected to the Octel/DuPont F21-61 test at 150°C for 90 minutes.
- the Octel/DuPont F21-61 test also evaluates the filter pad by comparing with a standard filter color chart provided by Octel/DuPont. A rating of up to 7 is generally considered as a pass, and anything above 7 is considered a fail.
- the data presented in the Table 5 show that the addition of 2-EHN in all three fuel samples increased the filter pad rating to failure, 16, 10, and 9 for the fuels # C, #D, and #E respectively.
- the addition of Primene 81-R in the presence of 2-EHN increased the thermal stability in terms of pad rating and color and increased the cetane number as well.
- Table 8 The results shown in Table 8 were from continued testing of diesel Fuel #F to improve thermal stability in the presence of 2-EHN.
- the results showed that Primene® 81-R, and Primene® BC-9 and a combination of both at 100 ppm concentration with 2000 ppm of 2-EHN is effective in improving the thermal stability of the fuel.
- the combination of 2-ethylhexylnitrate and Primenes® improves the cetane number of the base fuel more than 2-EHN alone.
- Table 7 represents the relationship between the concentration of the additives, cetane number, and the filter pad rating of the DuPont F-21 test.
- Fuel oil samples of 500 mL were stored in 600 mL beakers covered with watch glasses in oven at 40°C. At arbitrary intervals, optical density measurements were made on samples before and after filtering a small portion of vigorously shaken sample through a CORNING 30 F fritted glass crucible. The unused portion was immediately returned to the oven for further aging. The failure time was determined by three methods: 1) the number of days to a stated level of optical density difference ( ⁇ OD) of 0.12 between unfiltered portions, 2) days to reach an OD value of 1.00 for the unfiltered sample, and 3) days to reach a residue level of 2.0 mg/100 mL as determined by filtration.
- ⁇ OD optical density difference
- Oxidative and thermal stability of diesel fuels was studied on fuel samples (a) collected from major regions around the world; (b) containing both high and low levels of sulfur and (c) containing both straight run and cracked components.
- the results of the various stability tests as measured by color, sediments and gum formation show clearly that addition of tertiary alkyl primary amines, at few ppm levels, significantly improves the stability of fuels.
- Results show that the thermal stability of both low and high sulfur fuels can be improved by tertiary alkyl primary amines doping at 8-40 ppm range. Furthermore, thermal stability is achieved without negatively effecting the cetane number. In fact, the cetane number is improved.
Abstract
Description
- This invention relates to improved fuel oil compositions. Particularly, to fuel oil compositions containing cetane improvers having improved thermal stability The compositions further enjoy improved stabilization against: 1) sediment formation, and 2) color degradation during storage and distribution as well as improved cetane number, rust inhibition and demulsibility.
- The useful life of a fuel is a function of its quality and of storage conditions. For instance, depending on the crude source and amount of cracked fraction, middle distillate fuels, such as diesel, jet fuels and heating fuels, can contain very different amounts of gum and color precursors, waxes, aromatics, and other products. Fuel storage stability was a well-understood problem prior to the introduction of low-sulfur diesel fuel. It is well established that diesel fuels can exhibit instability when stored for extended periods of time (storage stability), or when brought into contact with high temperature engine parts (thermal stability). Hydrotreating to meet 1993 regulations reduced storage stability problems for highway fuels. However, low-sulfur fuels resulted in other issues, such as peroxide and thermal stability problems in distillate fuels. However, fuels are often stored for much longer periods because of logistical and economic necessities.
- The oxidative degradation products, formed under both prolonged storage and thermal stress, continue to be a problem in the utilization of, for example, diesel fuels. Fuel-instability reactions are defined in terms of the formation of deleterious products, such as filterable sediment, adherent gums, and peroxides. Sediments and gums which result from the oxidation reactions act to block filters and deposit on surfaces. Both the low-temperature storage and high-temperature thermal degradation, are of concern. Hydrotreating is generally considered the most effective means of improving stability. However, the cost of stability improvement by additives doping can often be less than the hydrotreatment costs.
- The use of cetane improvers and the advent of more complicated diesel fuel utilization compounds problems of stability. Stringent diesel engine emissions regulations are being implemented worldwide. In the United States, the 1990 Clean Air Act mandated lowering NOx emissions to 4.0 grams per horsepower-hour (g/hp-hr) for the 1998 model year. Future proposals by the U.S. Environmental Protection Agency (EPA) call for further reduction of combined NOx and hydrocarbon emissions of heavy-duty trucks and buses to 2.5 g/hp-hr for the 2004 model year. Such reductions will require a combination of new engine technology and economically viable low-emission diesel fuels.
- Numerous studies by the Coordinating Research Council and others have shown that increasing the cetane number by using additives significantly reduces carbon monoxide and NOx emissions; hydrocarbon and particulate matter are slightly reduced. Cetane improvers also enhance the cold startability of diesel cars and trucks. Higher cetane can reduce white smoke, noise, misfire, emissions and improve cold starting in some engines. Diesel fuel with at least a 47 cetane number (measured by ASTM D 613) would qualify for the list.
- Most users are knowledgeable about the fuel's primary role as an energy source. However, few are aware that diesel fuel performs multiple functions in a diesel engine and the associated fuel system. In addition to its primary role as an energy source, the fuel also serves as the sole lubricant of critical moving parts and as a heat-transfer fluid. Diesel fuel is increasingly used as a circulating coolant for high pressure fuel injections systems. This causes a problem in that as engine heat passes from injectors into the fuel, it can trigger a process that leads to particle formation, thus clogging filters and injectors. Fuels resistant to such thermal degradation must get a minimum 80% reflectance measurement using a green filter in the updated Octel F21-61 test (180 minutes, 150°C).
- Adequate thermal stability is a necessary requirement for the effective functioning of diesel fuel as a heat-transfer fluid. In modern heavy-duty diesel engines, only a portion of the fuel that is circulated to the fuel injectors is actually delivered to the combustion cylinders. The remainder is circulated back to the fuel tank carrying heat with it, consequently raising the bulk fuel temperature. Because of the recirculation of fuel through the newer engines, fuel can be exposed momentarily to temperatures as high as 350°C. This process, in some engines and fuel combinations, could accelerate the instability of the diesel fuel. In some cases, this stress can cause the fuel to degrade and form insoluble materials that can restrict fuel flow through filters and injection systems.
- Good thermal stability may become even more important in the future. Diesel engine manufacturers have indicated that engines under development to meet future exhaust emission standards will expose the fuel to more severe operating environments (stress), e.g., higher pressures and longer contact with high-temperature engine parts. In particular, thermal stability can be troublesome in some heavy-duty applications in some areas with widespread use of cetane improvers. Accordingly, cetane improvers will become increasingly important as engine makers promote and/or require higher cetane fuels, especially in premium diesel products.
- Additives can cause diesel fuel degradation if they are not oxidatively stable. Above 120°C, a cetane improver may oxidize and decompose, leading to particulate and sediment problems that can block filters. It is commonly accepted that 2-ethylhexyl nitrate functions as a diesel ignition improver because it is unstable, i.e., it thermally begins to decompose at about 155°C (311°F), i.e., just above the 300°F stability test temperature (Bacha, John; Lesnini, D. G., Proceedings of the 6th International Conference on stability and Handling of Liquid Fuels, 1997, Eds., H. N.; US Dept. of Energy, Vol. 2, 671). This result suggests that the stability test temperature and test duration together are just sufficient for 2-ethylhexyl nitrate to contribute to the observed fuel thermal instability in the 300°F test.
- Accordingly, there is a need for a fuel additive which imparts thermal stability to fuels even in the presence of cetane improver additives. Tertiary alkyl amines are known as diesel fuel additives as antioxidants for storage improvement (see U.S. Patent 2,945,749); in combination with fatty amines to counteract tendency of fatty amines to emulsify (see U.S Patent 3,014,793); and as stabilizers in combination with detergent, rust preventors and demulsifier additives (see U.S. Patent 2,793,943). However, none of these references discusses tertiary alkyl primary amines as thermal stabilizers and cetane improvers especially in the presence of conventional cetane improvers.
- The present inventors, have now unexpectedly found that fuels are made thermally stable in the presence of cetane improvers, which are known to make fuels thermally unstable, by the addition of tertiary alkyl primary amines in the C8- C24 range . Furthermore, the tertiary alkyl primary amines of the present invention also operate as cetane number improvers and the combination of cetane number improvers with the tertiary alkyl primary amines of the present invention provide a higher cetane number than that provided by the cetane improver alone. Also, fuel oil compositions containing these amines are also characterized as having improved dispersability, improved rust inhibition and improved demulsibility.
- In a first aspect of the present invention, there is provided a fuel composition, including: (A) a major amount of fuel; (B) at least one cetane number improver; and (C) at least one tertiary alkyl primary amine of the formula: wherein: R1, R2, and R3 are each independently (C1-C21) alkyl, substituted (C1-C21) alkyl, (C1-C21) alkenyl or substituted (C1-C21) alkenyl.
- In a second aspect of the present invention, there is provided a fuel composition, including: (A) a major amount of diesel fuel; (B) a minor amount of 2-ethylhexylnitrate effective to improve the cetane number of the diesel fuel; and (C) a minor amount of at least one tertiary alkyl primary amine of the formula: effective to provide thermal stability, wherein: R1, R2, and R3 are each independently (C1-C21) alkyl, substituted (C1-C21) alkyl, (C1-C21) alkenyl or substituted (C1-C21) alkenyl.
- In a third aspect of the present invention, there is provided a method of providing thermal stability to fuel containing at least one cetane improver, including: introducing into the fuel at least one tertiary alkyl primary amine of the formula: in an amount effective to impart thermal stability to the fuel, wherein: R1, R2, and R3 are each independently (C1-C21) alkyl, substituted (C1-C21) alkyl, (C1-C21) alkenyl or substituted (C1-C21) alkenyl.
- As used herein the terminology "(C1-C21)" means a straight chain or branched chain alkyl group having from 1 to 21 carbon atoms per group.
- Also, the term "major amount" is understood to mean greater than 50 percent by weight and the term "minor amount" is understood to mean less than 50 percent by weight.
- Throughout this specification and claims, unless otherwise indicated, references to percentages are by weight, all temperatures by degree centigrade and all pressures are atmospheric.
- It is also to be understood that for purposes of this specification and claims that the range and ratio limits, recited herein, are combinable. For example, if ranges of 1-20 and 5-15 are recited for a particular parameter, it is understood that ranges of 1-15 or 5-20 are also contemplated.
- Generally, the fuel of the fuel composition of the present invention is present in a major amount in the fuel composition. In a preferred embodiment, the fuel oil is present in an amount of at least 60% by weight, preferably at least 75% by weight, more preferably at least 90% by weight of the total fuel composition.
- The fuels useful in the present invention are generally any fuel which may suffer from inadequate cetane number, thermal instability, storage stability problems (sediment and gum formation, color degradation and other deterioration during storage), rusting and emulsion formation. In a preferred embodiment, the fuels are hydrocarbon fractions having an initial boiling point of at least 200°F and an end point not higher than 750°F, which boil substantially continuously throughout their distillation range. Such fuels are generally known as middle distillate fuels.
- Examples of middle distillate fuels which may be used in the fuel compositions of the present invention include, but are not limited to, distilled oils, furnace oils, diesel fuels, jet fuels, and residual fuels such as bunker fuels, marine diesel fuels, railroad diesel fuels, etc. In a preferred embodiment, the fuel is a diesel fuel or a jet fuel. In a more preferred embodiment, the fuel is a diesel fuel.
- The fuel compositions of the present invention also include at least one cetane improver. Cetane improvers are compounds that readily decompose to form free radicals and then, in turn, promote the rate of chain initiation. The increased rate of chain initiation improves ignition characteristics for diesel fuel. Accordingly, cetane number (ignition quality) improvers are used to increase the cetane number when the base fuel cetane does not meet required specifications. Suitable cetane improvers include, without limitation, alkyl nitrates, such as 2-ethylhexyl nitrate (2-EHN); peroxides, such as di-t-butylperoxide; tetrazoles; thioaldehydes, tertiary alkyl primary amines, and mixtures thereof. In a preferred embodiment, the at least one cetane improver is an alkyl nitrate. In a more preferred embodiment, the at least one cetane improver is 2-ethylhexyl nitrate (2-EHN).
- In one embodiment, the at least one cetane improver is a tertiary alkyl primary amine. In a preferred embodiment, the at least one cetane improver is a tertiary alkyl primary amine and the fuel composition further includes a second cetane improver selected from alkyl nitrates, peroxides, tetrazoles; thioaldehydes, tertiary alkyl primary amines, and mixtures thereof. In a more preferred embodiment, the at least one cetane improver is a tertiary alkyl primary amine and the fuel composition further includes a second cetane improver which is an alkyl nitrate, such as 2-EHN.
- Generally, the cetane improver is present in the fuel composition at a concentration of 50 to 7500, preferably 100 to 5000, more preferably 100 to 2000 ppm.
-
- Suitable examples of (C1-C21) alkyl include, but are not limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, n-pentyl, isopentyl, neopentyl, n-hexyl, 2-ethylhexyl, octyl, decyl, isodecyl, undecyl, dodecyl (also known as lauryl), tridecyl, tetradecyl (also known as myristyl), pentadecyl, hexadecyl, heptadecyl, octadecyl, nonadecyl, cosyl, and eicosyl.
- Suitable examples of (C1-C21) alkenyl include, but are not limited to, ethenyl, n-propenyl, isopropenyl, 1-butenyl, cis-2-butenyl, isobutylene, trans-2-butenyl, 2-3, dimethyl-2-butenyl, 3-methyl-1-butenyl, 2-methyl-2-butene, 1-pentenyl, cis-2-pentenyl, trans-2-pentenyl, 1-hexenyl, 1-heptenyl, 1-octentl, 1-nonenyl, and 1-decenyl.
- Suitable examples of (C1-C21) substituted alkyl and alkenyl include, but are not limited to, the above recited alkyl and alkenyl groups substituted with hydroxy, halide such as fluorine, chlorine or bromine; cyano; alkoxy; haloalkyl; carbalkoxy; carboxy; amino; alkylamino derivatives and the like; or nitro groups.
- The at least one tertiary alkyl primary amine may be a single amine or a mixture of amines, for instance as described following. In one embodiment, the at least one tertiary alkyl amine is 1,1,3,3-tetramethylbutylamine available from Rohm and Haas Co of Philadelphia, PA as PRIMENE TOA®. In another embodiment, the at least one tertiary alkyl amine is an isomeric mixture of C16 to C22 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE JM-T®. In a preferred embodiment, the at least one tertiary alkyl amine is an isomeric mixture of C8 to C10 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE BC-9® or an isomeric mixture of C12 to C14 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE 81-R® or a mixture of PRIMENE BC-9® and PRIMENE 81-R®. In a more preferred embodiment, the at least one tertiary alkyl amine is an isomeric mixture of C12 to C14 tertiary alkyl primary amines available from Rohm and Haas Co of Philadelphia, PA as PRIMENE 81-R®.
- Generally, the at least one tertiary alkyl primary amine is present in the fuel composition at a concentration of 1 to 1000, preferably 5 to 500, more preferably 10 to 200 ppm, most preferably 10 to 100 ppm. In another embodiment, the at least one tertiary alkyl primary amine is present in the fuel composition at a concentration of 50 to 100, or 1 to 10 ppm.
- The tertiary alkyl primary amines used in the fuel compositions of the present invention are prepared using substrate compounds known as substrates for the Ritter reaction and include, for example, alcohols, alkenes, aldehydes, ketones, and ethers, (see, generally, L. I. Krimen and D. J. Cota, "The Ritter Reaction", Organic Reactions, Vol. 17, 1969, pp. 213-325). Processes for preparing tertiary alkyl primary amines are known in the art and are described for instance in U.S Patent 5,527,949 and in co-pending provisional application 60/051,867.
- The fuel compositions of the present invention may also include other additives well known in the art such as, without limitation, anti-oxidants, dispersants, anti-foaming agents and the like.
- Also contemplated is a method of providing thermal stability to fuel containing at least one cetane improver, including: introducing into the fuel at least one tertiary alkyl primary amine of the formula: in an amount effective to impart thermal stability to the fuel wherein: - R1, R2, and R3 are each independently (C1-C21) alkyl, substituted (C1-C21) alkyl, (C1-C21) alkenyl or substituted (C1-C21) alkenyl. The fuel, at least one cetane improver, and tertiary alkyl primary amine as well as the levels of usage are as described above.
- The following Examples are provided as an illustration of the present invention. Fuel samples #A- # I were fresh test fuels without any additives and were obtained from commercial sources. The fuel samples were analyzed to ensure conformance with specifications and stored under ambient temperature, in dark, and under nitrogen atmosphere. All of the C8, C9, C12, and C18 tertiary alkyl primary amines samples were commercial products sold under the trademark Primene® by Rohm and Haas Company of Philadelphia, Pa. The results are shown in Table 1.
Detailed Analysis of Test Fuel Samples Test Fuel # A Fuel # B Fuel # C Fuel # D Fuel # E Fuel # F Fuel # G Fuel # H Fuel # I Sulfur vol% 0.241 0.4 0.047 0.035 0.038 0.04 1.8 0.1974 0..224 Aromatics vol % 27.5 25 28.1 27.8 27.9 25.5 Olefins vol % 1.7 1.3 1.9 1.8 2.1 Saturates vol % 70.8 73.7 70 70.4 72.4 Cetane Number 49.9 51.5 47 45.4 47 34 40.3 38 - Fuel #A was evaluated for thermal stability and cetane number improvement. A sample of Fuel #A containing 100 ppm Primene® 81-R and a sample without were prepared. The fuel samples were stored in an air atmosphere at room temperature during the test period.
- The fuel samples were tested for a prolonged period of storage stability. Periodically, as indicated in Table 2, the fuels were sampled and tested for oxidative stability according to ASTM D 2274 Diesel Oxidation Stability test method as follows. A 350 mL sample of fuel was heated at 95°C for 40 hr. while oxygen is bubbled through at the rate of 3 liters per hour. After aging, the sample is cooled to room temperature and filtered to obtain the filterable insoluble quantity. Adherent insolubles are then removed from the associated glassware with trisolvent (TAM). The TAM is then evaporated to obtain the adherent insolubles. The sum of filterable and adherent insolubles, expressed as milligrams per 100 mL, is reported as total insolubles. A total sediment of 1 mg/100 mL or less is generally acceptable to pass the test, anything above 1 mg/100 mL sediment results in failing the test. The results are shown in Table 2 following.
Comparison of the Results from Oxidation Stability of diesel Fuel #A @ No. of Weeks by ASTM D 2274 No. of Weeks of Storage Total Sediments (mg/100ml) Diesel Fuel #A Diesel Fuel #A + 100 ppm Primene® 81-R 2 1.0 0.2 5 2.2 0.5 7 2.8 0.6 10 19 1.0 15 27 2.2 - The highest sediment reported in the Table 2 is 27mg for diesel Fuel #A, which is an indication of instability of the fuel at the prolonged period of time. The test results shown in Table 1 exhibit the better stability for fuel #A with 100 ppm 81-R added , at the extended period of time. It is evident that Primene® 81-R is acting as a stabilizer in the diesel fuel, by helping to prevent gum/sediment formation. The most noteworthy point from these results is that Fuel #A containing 100 ppm of Primene® 81-R showed that the fuel is stable even after 10 weeks compared to Fuel #A without Primene® 81-R.
- The Fuel #A samples were also tested for cetane number with and without additives. The method used for cetane number determination was ASTM D 613 as well as tested for oxidation stability using ASTM D 2274. The results are shown in Table 3.
Oxidation Stability Test Results by ASTM D 2274 and Cetane # by ASTM D 613 for Fuel #A ppm Additives Total Insolubles, mg/100mL Cetane Number Diesel #A Diesel #A + 100 ppm Primene® 81-R Diesel #A Diesel #A + 100 ppm Primene ® 81-R None(@ 0 weeks) 1 0.3 49.9 50.6 100 ppm Primene® 81-R 0.2 0.2 1000 ppm 2-EHN# 2.1 --- 53.1 1000 ppm 2-EHN + 100 ppm Primene® 81-R 0.1 53.4 1000 ppm 2-EHN + 100 ppm Primene® 70/30 0.1 < 0.1 53.7 none (@ 5weeks.) 2.2 0.5 51.0 51.5 100 ppm Primene® 81-R 0.3 100 ppm Primene® 70/30 0.7 None (@ 7 weeks.) 2.8 0.6 None (@ 10 weeks.) 19 1.0 None (@ 15 weeks.) 27 2.2 - Addition of 2-ethylhexylnitrate (2-EHN) improves the cetane number, but lowers the oxidative storage stability of the fuel #A as seen in Table 3. Addition of 100 ppm of Primene® 81-R to the fuel #A with 1000 ppm 2-EHN increases the storage stability without sacrificing the effect of 2-EHN as seen in the results in Table 3. Thus Primene® 81-R is not only a stabilizer additive for the diesel fuel by itself, but, also is thermal stabilizer and synergistic cetane improver. Additionally, the tertiary alkyl primary amine (Primene®) enhances the cetane number both with and without the presence of conventional cetane improvers.
- A sample of diesel fuel #B obtained from a Texas Gulf Coast Area refinery was tested for oxidation stability using Primene amines and other primary amines using the ASTM D 2274 test method for 40 hr. The results given in Table 4 show that the Primene 81-R and Primene BC-9 are acting as stabilizers, and help in improving the oxidative storage stability. Other primary amines did not exhibit properties as stabilizers.
Oxidation Stability Results for Diesel Fuels #B (ASTM D 2274 @ 40 hr.) Additives Dosage Fuel #B mg/100mL None --- 2.3 Primene® 81-R 100 <0.1 Primene® JM-T 100 0.1 Primene® TOA 15 0.4 Primene® BC-9 15 <0.1 Primene® 81-R 15 1.2 Primene® JM-T 15 1.4 TAPA C-13-16 blend 15 <0.1 TA PA C16-18 blend 15 1.9 isononylamine 15 2.1 n-nonylamine 15 1.9 2-ethylhexylamine 15 2.2 n-dodecylamine 15 2.2 n-Octadecylamine 15 2.4 - Samples of fuel #C, #D, and #E having a sulfur concentration of 0.047%, 0.035%, and 0.035%, respectively were also evaluated for thermal stability and cetane number improvement. The cetane numbers of these fuels are listed in Table 5, and found to be 44.1, 45.5, and 45.4 respectively for the fuel # C, #D, and #E. The fuels were tested for oxidation/thermal stability with and without 2-EHN and combined with Primene amines. 2-EHN is used as a cetane number improver additive, and the addition of 2-EHN does increase the cetane number. However, it has an adverse effect on the thermal stability of the fuel when subjected to the Octel/DuPont F21-61 test at 150°C for 90 minutes. The Octel/DuPont F21-61 test was done as follows. A 50 mL sample of fuel oil in a test tube is stored in a 300°F bath for 90 minutes (or 180 Min.). After removal from the bath it is allowed to cool to room temperature (about 2 hr.). The aged fuel is then filtered through 4.25 cm Whatman No 1 filter paper. The paper is then washed with heptane and the color of the filter paper is compared to a set of standards (1 = No color, 20 = dark brown).
- Addition of 100 ppm of Primene 81-R helped in increasing the thermal stability while also increasing the cetane number in the presence of 2-EHN as seen in the results in Table 5.
- The Octel/DuPont F21-61 test also evaluates the filter pad by comparing with a standard filter color chart provided by Octel/DuPont. A rating of up to 7 is generally considered as a pass, and anything above 7 is considered a fail. The data presented in the Table 5 show that the addition of 2-EHN in all three fuel samples increased the filter pad rating to failure, 16, 10, and 9 for the fuels # C, #D, and #E respectively. Furthermore, the addition of Primene 81-R in the presence of 2-EHN increased the thermal stability in terms of pad rating and color and increased the cetane number as well.
Octel/Du Pont F21-61 @ 90 min. & Cetane Number Test Results of Diesel fuel # C, #D, & #E Diesel Fuel #C Diesel Fuel #D Diesel Fuel # E Additive(ppm) Cetane No. Thermal Stability Using DuPont F-21 Test Method, (filter pad rating) Cetane No. Thermal Stability Using DuPont F-21 Test Method Cetane No. Thermal Stability Using DuPont F-21 Test Method color before/after Filter pad rating color before/after Filter pad rating Color before/after Filter pad rating None 44.1 1.0/1.5, 2 45.5 1.0/1.0 2 45.4 1.5/2.5 3 70% Primene®81- 44.4 1.0/1.0 1 45.9 1.0/1.0 1 45.4 1.5/2.5 2 R / 30% Plexol®917T (100) 2-EHN (1000) 48.1 1.0/3.0 16 48.5 1.0/3.0 10 48.3 1.5/3.5 9 2-EHN 47.6 1.5/1.5 1 50 1/2 1 48.4 1.5/3 1 (1000)+Primene® 81- R/Plexol®917T (100) 2-EHN (1000) + 47.8 1.0/1.5 1 48.7 1.0/1.5 1 48.8 1.5/3.0 1 Primene®81-R (70) - The fuel was subjected to Octel/DuPont F21-61 test for 180 minutes. The results showed that 2,6-di-t-butyl-4-methyl phenol and N,N'-di-sec-butyl-p-phenylenediamine are not effective anti-oxidants at 20 or 40 ppm concentration under these test conditions. The results shown in Table 6 also suggest that the Primene amines, and dinonyl diphenylamine are effective thermal stabilizers at 40 ppm concentration under these test conditions. However, the combination of Primene 81-R and Primene BC-9 at 1:1 ratio is effective, at 20 ppm concentration, to increase the thermal stability of the test fuel.
Thermal Stability Test Results of Fuel #F (Octel/Du Pont F21-61 @ 180 min.) Additives Octel/Du Pont F 21-61 Stability test @ 180 min. (ppm) Color, before/after Filter pad rating None 1.5/2.0 13 Primene® 81-R 20 1.0/2.0 9 Primene®BC-9 20 0.5/1.5 10 Primene® 81-R + Plexol® 917T 20 0.5/2.0 7 2,6-di-t-butyl-4-methyl phenol 20 0.5/1.5 11 N,N'-di-sec-butyl-p-phenylenediamine 20 0.5/2.5 14 Dinonyl diphenylamine 20 1.0/2.0 9 Primene® JM-T 20 1.0/2.0 8 Primene® 81-R+ Primene® BC-9 (1:1) 20 1.0/2.0 3 Dinonyl diphenylamine 40 3.0/3.0 3 N,N'-di-sec-butyl-p-phenylenediamine 40 0.5/2.5 14 2,6-di-t-butyl-4-methyl phenol 40 0.5/2.0 12 Primene® 81-R 40 1 / 1.5 1 Primene® BC-9 40 1 / 1.5 1 - Testing of fuel # F was extended to evaluate the effect of 2-EHN in the presence and absence of stabilizers. It was learned from Example 3 that the addition of 2-EHN lowers the thermal stability, however the test was run for 90 minutes in Example 3. For fuel #F the cetane number tests showed an increase in cetane number by an average of 7 units with the addition of 2000 ppm of 2-EHN. It was also observed that the addition of 2-EHN decreases the thermal stability, which is reclaimed by the addition of Primene® amines at 100 ppm level. The focus of this experiment was to determine the effect of stabilizers in the presence of 2-EHN, and a comparison was made using Primene® amines and dinonyl diphenylamine only. The results shown in Table 7 suggest that dinonyl diphenylamine failed to stabilize fuel in the presence of 2-EHN. The addition of Primene® amines showed progressively increased thermal stability as the concentration increased. The increased addition of Primene® amine concentration showed a decrease in filter pad rating.
Test Results of Thermal Stability and Cetane Number Improver for Fuel #F Additives (ppm) Octel/DuPont F21-61 Test @ 180 Minutes Cetane Number Color, before/after Filter pad Rating None 1.5 /2.5 13 47 2-EHN (2000) 1 / 7.5 14 54.3 2-EHN (2000) + Primene® 81-R (20) 1 / 7 13 54.3 2-EHN + Dinonyl diphenylamine (20) 1 / 7.5 15 53.4 2-EHN (2000) + Primene® 81-R (40) 1 / 6 13 55.2 2-EHN (2000) + Dinonyl diphenylamine (40) 1 / 7.5 16 54.3 2-EHN (2000) + Primene® BC-9 (40) 1 / 6.5 13 54.3 2-EHN (2000) + Primene® 81-R + Primene® BC-9 (1:1) (40) 1 / 6 10 54.9 - The results shown in Table 8 were from continued testing of diesel Fuel #F to improve thermal stability in the presence of 2-EHN. The results showed that Primene® 81-R, and Primene® BC-9 and a combination of both at 100 ppm concentration with 2000 ppm of 2-EHN is effective in improving the thermal stability of the fuel. The combination of 2-ethylhexylnitrate and Primenes® improves the cetane number of the base fuel more than 2-EHN alone. Table 7 represents the relationship between the concentration of the additives, cetane number, and the filter pad rating of the DuPont F-21 test.
Test Results of Thermal Stability and Cetane Number Improver for Fuel #F Additives (ppm) Octel/DuPont F21-61 Test @ 180 Minutes Color, before/after Filter pad Rating 2-EHN (2000) + Primene® 81-R (80) 1 / 5.5 8 2-EHN (2000) + Primene® 81-R + Primene® BC-9 (1:1) 80 ppm 1 / 5.5 8 2-EHN (2000) + Dinonyl diphenylamine (80) 1 / 8.0 16 2-EHN (2000) + Primene® 81-R (100) 1 / 4 6 2-EHN (2000) + Primene® BC-9 + Primene® 81-R, 1:1 (100) 1 / 4 6 2-EHN (2000) + Primene® BC-9 (100) 1 / 4 6 2-EHN (2000) + Dinonyl diphenylamine (100) 1 / 7 16 - Fuel oil samples of 500 mL were stored in 600 mL beakers covered with watch glasses in oven at 40°C. At arbitrary intervals, optical density measurements were made on samples before and after filtering a small portion of vigorously shaken sample through a CORNING 30 F fritted glass crucible. The unused portion was immediately returned to the oven for further aging. The failure time was determined by three methods: 1) the number of days to a stated level of optical density difference (Δ OD) of 0.12 between unfiltered portions, 2) days to reach an OD value of 1.00 for the unfiltered sample, and 3) days to reach a residue level of 2.0 mg/100 mL as determined by filtration. The results shown in Table 9 shows that addition of Primene® 81-R prolonged the decomposition period for the diesel by detecting the OD = 1 after 120 days compared to the commercially used antioxidants, hence increasing the storage stability with respect to both color and sediment.
Long Term Storage Stability Test Results for Fuel Oil # G Additive Dosage (ppm) Days to failure by Δ OD = 0.12 OD OD= 1.0 2 mg/100 mL None ---- 35 70 59 Primene® 81-R 30 95 120 76 Commercial AO #21 30 40 88 58 Commercial AO #2 30 65 108 75 -
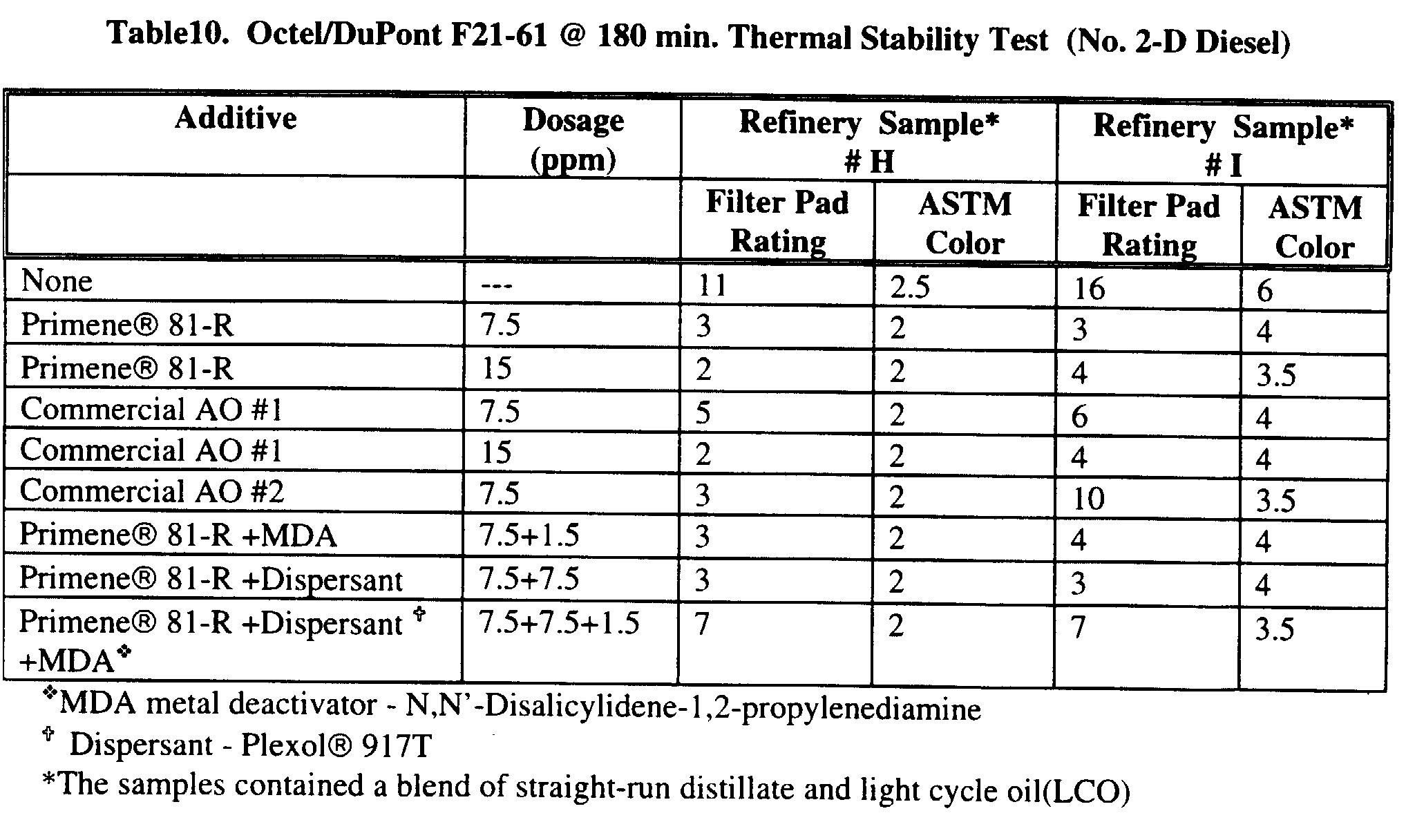
- Presence of water or any other additives reacting water could form an emulsion in the fuel. ASTM test method D-1094 is utilized to test the water reaction of the fuels. Primene® amines show the capability of enhancing the fuel demulsibility property. Table 10 show the results of ASTM D 1094 demonstrating the ability of Primene amines to extract water soluble compounds and keep them soluble in fuel layer.
Test Results of ASTM D 1094 Additive Separation Rating Interface Appearance Rating Free Water separation (mL) Appearance of Fuel layer Blank 2 (small air bubbles in the fuel layer) 2 (2mL of lace) 19 (clear) slightly hazy 9.7 ppm Primene® JM-T 2 (small air bubbles in the fuel layer) 2 (1mL of lace) 18 (oil bubbles in water layer) hazy 9.7 ppm Plexol® 917 T 3 (fuel layer -emulsion) 1b (loose lace at the interface) 20 Fuel layer -cloudy, emulsion 9.7 ppm Primene® JM-T + 9.7 ppm Plexol® 917 T 2 (small air bubbles in the fuel layer) 1b (loose lace at the interface) 20 slight hazy (like blank) 9.7 ppm Triton® CF-32 2 1 20 hazy -Like blank - The results of the various stability tests as measured by color, sediments and gum formation show (See above) clearly shows that the addition of tertiary alkyl primary amines, at a few ppm levels, significantly improves the stability of fuel oils and diesel. It also shows that the stability of diesel containing a catalytically cracked fraction blend can be improved by Primene® 81-R doping at 30 ppm. Several commercial fuel stabilizers at the same dosage level show similar or worse performance.
- Oxidative and thermal stability of diesel fuels was studied on fuel samples (a) collected from major regions around the world; (b) containing both high and low levels of sulfur and (c) containing both straight run and cracked components. The results of the various stability tests as measured by color, sediments and gum formation show clearly that addition of tertiary alkyl primary amines, at few ppm levels, significantly improves the stability of fuels. Results show that the thermal stability of both low and high sulfur fuels can be improved by tertiary alkyl primary amines doping at 8-40 ppm range. Furthermore, thermal stability is achieved without negatively effecting the cetane number. In fact, the cetane number is improved.
- It is noteworthy that both sedimentation and color are improved by tertiary alkyl primary amines. The results of oxidative stability of diesel showing similar tertiary alkyl primary amines benefits were shown earlier. Several commercial fuel stabilizers at the same dosage level show similar or worse performance. The data that tertiary alkyl primary amines are equal or better stabilizers is also seen in comparative experiments with several well-known common fuel stabilizers.
Claims (14)
- A fuel composition, comprising:(A) a major amount of fuel;(B) a minor amount of at least one cetane number improver; and
- The fuel composition of claim 1, wherein the at least one cetane improver is present at a concentration 50 to 7500 ppm.
- The fuel composition of claim 1, wherein the at least one cetane improver is 2-ethylhexylnitrate.
- The fuel composition of claim 1, wherein the at least one tertiary alkyl primary amine is a C9 tertiary alkyl amine.
- The fuel composition of claim 1, wherein the at least one tertiary alkyl primary amine is a C12 tertiary alkyl primary amine.
- The fuel composition of claim 1, wherein the at least one tertiary alkyl primary amine is a C18 tertiary alkyl primary amine.
- The fuel composition of claim 4, wherein the fuel composition further comprises a C12 tertiary alkyl primary amine.
- The fuel composition of claim 1, wherein the at least one tertiary alkyl primary amine is present at a concentration from 1 to 1000 ppm.
- The fuel composition of claim 1, wherein the at least one cetane improver is a tertiary alkyl primary amine.
- The fuel composition of claim 9, further comprising a second cetane improver selected from alkyl nitrates, peroxides, tetrazoles, thioaldehydes, tertiary alkyl primary amines, and mixtures thereof.
- A fuel composition, comprising:(A) a major amount of diesel fuel;(B) a minor amount of 2-ethylhexylnitrate effective to improve the cetane number of the diesel fuel; and
- The composition of claim 11, wherein the at least one tertiary alkyl primary amine is present in a concentration from 1 to 1000 ppm.
- A method of providing thermal stability to fuel containing cetane improvers, comprising: introducing into the fuel at least one tertiary alkyl primary amine of the formula: in an amount effective to impart thermal stability to the fuel, wherein: R1, R2, and R3 are each independently (C1-C21) alkyl, substituted (C1-C21) alkyl, (C1-C21) alkenyl or substituted (C1-C21) alkenyl.
- The method of claim 13, wherein the tertiary alkyl primary amine is introduced in an amount from 1 to 1000 ppm.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE69922566T DE69922566T2 (en) | 1999-03-25 | 1999-03-25 | Use of tertiary alkyl primary amines in fuel compositions used as heat transfer fluid |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US7725098P | 1998-03-09 | 1998-03-09 | |
| US77250P | 1998-04-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0947577A1 true EP0947577A1 (en) | 1999-10-06 |
| EP0947577B1 EP0947577B1 (en) | 2004-12-15 |
Family
ID=22136966
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP99302308A Expired - Lifetime EP0947577B1 (en) | 1998-03-09 | 1999-03-25 | Use of tertiary-alkyl primary amines in fuel compositions used as heat-transfer fluid |
Country Status (1)
| Country | Link |
|---|---|
| EP (1) | EP0947577B1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1054051A1 (en) * | 1999-05-19 | 2000-11-22 | Rohm And Haas Company | Diesel fuel compositions containing tertiary alkyl primary amines |
| EP1531174A2 (en) * | 2003-11-04 | 2005-05-18 | Afton Chemical Corporation | Composition and method to reduce peroxides in middle distillate fuels containing oxygenates |
| WO2013160294A1 (en) * | 2012-04-24 | 2013-10-31 | Basf Se | Use of additives with detergent action for further increasing the cetane number of fuel oils |
| CN102093917B (en) * | 2009-12-09 | 2013-11-13 | 济南开发区星火科学技术研究院 | Blended fuel oil capable of substituting petrochemical diesel and preparation method thereof |
| US9056268B2 (en) | 2010-02-12 | 2015-06-16 | Donaldson Company, Inc. | Liquid filtration media, filter elements and methods |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2456569A (en) * | 1944-12-18 | 1948-12-14 | Union Oil Co | Motor fuel |
| US2945749A (en) * | 1956-04-18 | 1960-07-19 | Socony Mobil Oil Co Inc | Stabilized fuel oil containing tertiary alkyl primary amines |
| US3014793A (en) * | 1956-02-28 | 1961-12-26 | Exxon Research Engineering Co | Distillate fuel oil compositions |
| US4482355A (en) * | 1983-12-30 | 1984-11-13 | Ethyl Corporation | Diesel fuel compositions |
| EP0890570A2 (en) * | 1997-07-07 | 1999-01-13 | Rohm And Haas Company | Tertiary alkyl primary amines and process for preparing the same |
-
1999
- 1999-03-25 EP EP99302308A patent/EP0947577B1/en not_active Expired - Lifetime
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2456569A (en) * | 1944-12-18 | 1948-12-14 | Union Oil Co | Motor fuel |
| US3014793A (en) * | 1956-02-28 | 1961-12-26 | Exxon Research Engineering Co | Distillate fuel oil compositions |
| US2945749A (en) * | 1956-04-18 | 1960-07-19 | Socony Mobil Oil Co Inc | Stabilized fuel oil containing tertiary alkyl primary amines |
| US4482355A (en) * | 1983-12-30 | 1984-11-13 | Ethyl Corporation | Diesel fuel compositions |
| EP0890570A2 (en) * | 1997-07-07 | 1999-01-13 | Rohm And Haas Company | Tertiary alkyl primary amines and process for preparing the same |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1054051A1 (en) * | 1999-05-19 | 2000-11-22 | Rohm And Haas Company | Diesel fuel compositions containing tertiary alkyl primary amines |
| EP1531174A2 (en) * | 2003-11-04 | 2005-05-18 | Afton Chemical Corporation | Composition and method to reduce peroxides in middle distillate fuels containing oxygenates |
| EP1531174A3 (en) * | 2003-11-04 | 2005-08-24 | Afton Chemical Corporation | Composition and method to reduce peroxides in middle distillate fuels containing oxygenates |
| US7615085B2 (en) | 2003-11-04 | 2009-11-10 | Afton Chemical Corporation | Composition and method to reduce peroxides in middle distillate fuels containing oxygenates |
| CN102093917B (en) * | 2009-12-09 | 2013-11-13 | 济南开发区星火科学技术研究院 | Blended fuel oil capable of substituting petrochemical diesel and preparation method thereof |
| US9056268B2 (en) | 2010-02-12 | 2015-06-16 | Donaldson Company, Inc. | Liquid filtration media, filter elements and methods |
| US10226723B2 (en) | 2010-02-12 | 2019-03-12 | Donaldson Company, Inc. | Liquid filtration media, filter elements and methods |
| US11565206B2 (en) | 2010-02-12 | 2023-01-31 | Donaldson Company, Inc. | Liquid filtration media, filter elements and methods |
| WO2013160294A1 (en) * | 2012-04-24 | 2013-10-31 | Basf Se | Use of additives with detergent action for further increasing the cetane number of fuel oils |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0947577B1 (en) | 2004-12-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0247706B1 (en) | Fuel composition and additive concentrates, and their use in inhibiting engine coking | |
| US5669938A (en) | Emulsion diesel fuel composition with reduced emissions | |
| EP0902824B1 (en) | Fuel additives | |
| KR20050083779A (en) | Fuel compositions | |
| EP0467628B1 (en) | Fuel compositions with enhanced combustion characteristics | |
| JP3663429B2 (en) | Method and composition for reducing pollutant deposit formation in jet engines | |
| EP0034968B1 (en) | N-substituted succinimides, their preparation and their use as additives for fuels | |
| CA2343296C (en) | Fuel additive composition and method for the treatment of fuels | |
| US20080256846A1 (en) | Fuel composition for diesel engines | |
| EP0457589B1 (en) | Fuel compositions with enhanced combustion characteristics | |
| EP0947577B1 (en) | Use of tertiary-alkyl primary amines in fuel compositions used as heat-transfer fluid | |
| US4482355A (en) | Diesel fuel compositions | |
| JP3812853B2 (en) | Vegetable oil-containing diesel fuel | |
| KR100307417B1 (en) | How to increase the economics of fuels and fuel compositions for them | |
| CA2263938A1 (en) | Fuel compositions containing tertiary-alkyl primary amines | |
| US4482353A (en) | Compression ignition fuel compositions | |
| US6379530B1 (en) | Polyisobutene substituted succinimides | |
| CN111465676B (en) | Method for reducing oxidation | |
| CN112004916B (en) | Diesel fuel with improved ignition properties | |
| US7396450B2 (en) | Method of reducing amount of peroxides, reducing fuel sediments and enhancing fuel system elastomer durability, fuel stability and fuel color durability | |
| WO2000017293A1 (en) | Diesel fuel additive composition and method for the treatment of diesel fuels | |
| EP1854867A1 (en) | New stabilized fuel composition | |
| JPH08283754A (en) | Fuel oil composition | |
| RU2788009C2 (en) | Diesel fuel with improved ignition characteristics |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19990412 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR GB IT |
|
| AX | Request for extension of the european patent |
Free format text: AL;LT;LV;MK;RO;SI |
|
| AKX | Designation fees paid |
Free format text: DE FR GB IT |
|
| 17Q | First examination report despatched |
Effective date: 20020903 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| RTI1 | Title (correction) |
Free format text: USE OF TERTIARY-ALKYL PRIMARY AMINES IN FUEL COMPOSITIONS USED AS HEAT-TRANSFER FLUID |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB IT |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REF | Corresponds to: |
Ref document number: 69922566 Country of ref document: DE Date of ref document: 20050120 Kind code of ref document: P |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| ET | Fr: translation filed | ||
| 26N | No opposition filed |
Effective date: 20050916 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20060317 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20060331 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20060502 Year of fee payment: 8 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20070325 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20071130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20071002 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070402 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20060329 Year of fee payment: 8 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20070325 |