EP0736522A1 - Ceramide compounds, process for their preparation and their use in cosmetics or dermatology - Google Patents
Ceramide compounds, process for their preparation and their use in cosmetics or dermatology Download PDFInfo
- Publication number
- EP0736522A1 EP0736522A1 EP96400546A EP96400546A EP0736522A1 EP 0736522 A1 EP0736522 A1 EP 0736522A1 EP 96400546 A EP96400546 A EP 96400546A EP 96400546 A EP96400546 A EP 96400546A EP 0736522 A1 EP0736522 A1 EP 0736522A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- formula
- radical
- compounds
- compound
- chosen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/68—Sphingolipids, e.g. ceramides, cerebrosides, gangliosides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/16—Emollients or protectives, e.g. against radiation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/02—Preparations for cleaning the hair
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/12—Preparations containing hair conditioners
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/16—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/17—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/18—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom having the carbon atom of the carboxamide group bound to a hydrogen atom or to a carbon atom of an acyclic saturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/16—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/17—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/20—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom having the carbon atom of the carboxamide group bound to a carbon atom of an acyclic unsaturated carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/02—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/04—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton being acyclic and saturated
- C07C235/08—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to acyclic carbon atoms and singly-bound oxygen atoms bound to the same carbon skeleton the carbon skeleton being acyclic and saturated having the nitrogen atom of at least one of the carboxamide groups bound to an acyclic carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/007—Preparations for dry skin
Definitions
- the subject of the present invention is new compounds of the ceramide type, their preparation process and their use, in particular for the treatment and care of the skin, hair, nails and eyelashes in cosmetics or dermatology.
- the skin appears drier, less supple and the skin relief more pronounced.
- the hair which is subjected too frequently to certain hair treatments, loses its shiny appearance and can become coarse and brittle.
- the Applicant has therefore sought compounds which make it possible to prevent or correct these phenomena which result in an apparent dryness and which restore the suppleness to the skin and the hair to their shine and softness.
- ceramides are the predominant constituent elements of the interconéocytaires lipides of the stratum corneum and participate in the maintenance of the integrity of the cutaneous barrier.
- ceramides used in cosmetics are most often natural extracts derived in particular from pigskin, beef brain, egg, blood cells, plants such as wheat, etc. (Japanese patent applications J 86/260008 and J 87/120308). Such ceramides have also been proposed for protecting the hair (EP 0 278 505).
- Each ceramide of natural origin has a precise and unique stereoisomerism, such as those described for sphinganine, phytoshingosine and sphingosine (also called sphingenine) which are respectively ( 2S, 3R ) -2-amino-1,3- octadecanediol, ( 2S , 3S , 4R ) -2-amino-1,3,4-octadecanetriol and ( 2S , 3R , 4E ) -2-amino-4-octadecene-1,3-diol [J. Biochem. 79, 11-21 (1977)].
- these new compounds have very good hydrating power for the skin and / or hair when they are used in cosmetic or dermatological compositions.
- R 1 denotes a saturated and hydroxylated alkyl radical
- the hydroxyl radical is in position ⁇ with respect to the carbon of the group -CHOH-.
- This hydroxyl radical can be in a substituted form.
- This radical can then be represented in particular by a radical of formula -O-CO-CHOH-R 4 , with R4 denoting a linear or branched, saturated or unsaturated, C 1 to C 31 alkyl radical.
- R 1 denotes an unsaturated and hydroxylated alkyl radical
- R 1 has an ethylenic radical in position ⁇ with respect to the carbon of the group -CHOH-. More particularly, at least one, and preferably the hydroxyl radical is in the ⁇ position of the ethylenic radical.
- R 1 denotes a saturated and hydroxylated alkyl radical.
- R 1 denotes a C 12 to C 23 , saturated or unsaturated, hydroxylated alkyl radical.
- R 2 denotes a linear alkyl radical, more particularly of C 2 to C 25 .
- the compounds according to the invention are therefore in the form of mixtures of optical and / or geometric isomers (mixture of enantiomers and / or diastereoisomers) at least on the amino alcohol part, which differentiate them from natural products which are only as a single isomer.
- the compounds according to the invention are preferably in the form of a mixture of at least 4 isomers.
- the compounds of formula (1) are chosen from 2-N-docosanoylaminooctadecane-1,3,4-triol, 2-N- (2-hydroxy-hexadecanoyl) -amino-octadecane-1,3,4 -triol and 2-N-hexadecanoylamino-octadecane-1,3,4-triol.
- the hair treated with these compounds of formula (I) has a shiny appearance, a softer feel and less sensitivity to water, due to the supply of lipid material uniformly distributed over the scales of the hair. Mechanical and nervousness properties are also improved.
- the compounds according to the invention can form with other lipids vesicles.
- the compounds according to the invention can be obtained by acylation of the amine function of the compounds of formula (II) or of reactive derivatives thereof, such as for example the hydrochloride, with an appropriate acylating agent.
- amino alcohol part is meant according to the invention the part of formula (I) coming from the compound of formula (II).
- the process is carried out in the presence of an appropriate solvent.
- solvents which can be used in the process of the present invention, mention may be made of tetrahydrofuran (THF), pyridine, 1,2-dimethoxyethane, dimethylformamide, dichloromethane, tert-butyl methyl ether.
- the OB group is chosen from the following radicals: acetate, benzoate, benzyloxy, -OSi (CH 3 ) 3 , -OSi (CH 3 ) 2 (t-butyl), and -OSi (t-butyl) (- C 6 H 5 ) 2 .
- OB is an acetate group.
- A is a group chosen from the following groups: -Cl, a radical of formula -O-COO-C 2 H 5 , a radical of formula -OCH 3 or -OC 2 H 5 ,
- acylating agents particularly recommended are the succinimide and carbodiimide esters, the imidazole derivatives and the acid chlorides, as defined above.
- salts such as the hydrochlorides of the compounds of formula (II).
- acylating agents of formula (III) are products known to those skilled in the art, they can also be in the form of mixtures of isomers, in particular racemic mixtures.
- the amounts of the compounds of formula (II) and (III) used in the process according to the invention are chosen so that their molar ratio (III) / (II) is greater than or equal to 1.
- the temperature of the process according to the invention can vary to a large extent.
- the temperature of step (a) is generally between 0 and 50 ° C., preferably, it corresponds to ambient temperature.
- stage (b) of the process according to the invention Before stage (b) of the process according to the invention and when X represents the hydroxyl radical, the products of stage (a) can undergo a reaction for protecting the hydroxyl groups, by reaction with an agent chosen from anhydrides d acid, acid halides and chlorosilanes, the reaction being followed, after isolation of the compounds according to step (b), by hydrolysis or hydrogenolysis.
- an agent chosen from anhydrides d acid, acid halides and chlorosilanes the reaction being followed, after isolation of the compounds according to step (b), by hydrolysis or hydrogenolysis.
- the agents used in the process of the present invention are preferably chosen from acetic anhydride, benzoyl chloride, benzyl chloride, benzyl bromide, chlorosilanes of formula ClSi (CH 3 ) 3 , ClSi (CH 3 ) 2 (tBu), ClSi (tBu) (- C 6 H 5 ) 2 .
- the compounds according to the invention can receive various applications, in particular in cosmetic and dermatological compositions. These compounds also have the property of forming vesicles in association with other lipids, when they are dispersed in water.
- the present invention therefore relates to the use of the compounds of formula (I) in emulsions, dispersions or in lotions. It also relates to the use of these compounds, associated with other lipids, for the formation of lipid spherules.
- the present invention also relates to compositions for cosmetic or dermatological use containing the compounds of formula (I).
- Another subject of the invention is a method of cosmetic treatment of the skin, hair, nails or eyelashes consisting in applying to the latter a sufficient amount of at least one compound of formula (I).
- compositions according to the invention can be in the form of emulsions (milk or cream), hydroalcoholic, oily or oleoalcoholic lotions, gels, dispersions or solid sticks, sprays or aerosol foam.
- the compounds of formula (I) represent from 0.005% to 20%, preferably from 0.01 to 10% of the total weight of the composition.
- compositions are, for example, lotions, milks or emollient creams, milks or creams for skin or hair care, creams, lotions or cleansing milks, foundations, foundations, lotions, sunscreen milks or creams, lotions, artificial tanning milks or creams, shaving creams or foams, after shave lotions, shampoos, lipsticks, mascaras or nail polishes.
- compositions can also be in the form of lip sticks intended either to color them or to avoid chapping, or of makeup products for the eyes or eyeshadows and foundations for the face.
- the fatty phase essentially consists of a mixture of compounds of formula (I) with at least one oil, and possibly another fatty substance.
- the fatty phase of the emulsions can constitute from 5 to 60% of the total weight of the emulsion.
- the aqueous phase of said emulsions preferably constitutes from 30 to 85% of the total weight of the emulsion.
- the proportion of the emulsifying agent can be between 1 and 20%, and preferably between 2 and 12% of the total weight of the emulsion.
- compositions according to the invention are in the form of oily, oleoalcoholic or hydroalcoholic lotions, they can constitute, for example, sunscreen lotions containing a filter absorbing UV rays, softening lotions for the skin; oily lotions can also constitute foaming oils containing an oil-soluble surfactant, bath oils, etc.
- fatty substances such as mineral or animal or vegetable oils or waxes, fatty acids, fatty acid esters such as triglycerides of fatty acids having from 6 to 18 carbon atoms, fatty alcohols; emulsifiers such as ethoxyethylenated fatty alcohols or polyglycerol alkyl ethers; solvents such as lower monoalcohols or polyalcohols containing from 1 to 6 carbon atoms or even water.
- fatty substances such as mineral or animal or vegetable oils or waxes, fatty acids, fatty acid esters such as triglycerides of fatty acids having from 6 to 18 carbon atoms, fatty alcohols; emulsifiers such as ethoxyethylenated fatty alcohols or polyglycerol alkyl ethers; solvents such as lower monoalcohols or polyalcohols containing from 1 to 6 carbon atoms or even water.
- the more particularly preferred mono- or poly-alcohols are chosen from ethanol, isopropanol, propylene glycol, glycerol and sorbitol.
- Mention may be made, as fatty substance, of mineral oils, of petrolatum oil; among animal oils, whale, shark, seal, menhaden, halibut, cod, tuna, turtle, ox's foot, horse's foot, sheep's foot, mink's oils , otter, groundhog, etc .; among vegetable oils, almond, wheat germ, olive, corn germ, jojoba, sesame, sunflower, palm, nut, shea, shorea, macadamia, blackcurrant seeds and the like.
- esters of saturated or unsaturated C 12 to C 22 acids and of lower alcohols such as isopropanol or glycerol or of linear C 8 to C 22 fatty alcohols or branched, saturated or unsaturated or alternatively 1,2-C 10 -C 22 alkanediols.
- waxes mention may be made of Sipol wax, lanolin wax, beeswax, Candelila wax, monocrystalline wax, Carnauba wax, spermaceti, cocoa butter, shea butter, silicone waxes, hydrogenated oils concrete at 25 ° C, sucroglycerides, oleates, myristates, linoleates and stearates of calcium, magnesium and aluminum.
- fatty alcohols mention may be made of lauric, cetyl, myristic, stearic, palmitic, oleic alcohols and GUERBET alcohols such as 2-octyldodecanol, 2-decyltetradecanol or 2-hexyldecanol.
- emulsifiers among the polyoxyethylenated fatty alcohols, mention may be made of lauric, cetyl, stearyl and oleic alcohols comprising from 2 to 20 moles of ethylene oxide and among the glycerol alkyl ethers, C 12 -C alcohols 18 comprising from 2 to 10 moles of glycerol.
- thickeners such as cellulose derivatives, polyacrylic acid derivatives, guar or carob gum or xanthan gum.
- composition according to the invention may also contain adjuvants usually used in cosmetics or in dermatology and in particular moisturizers, softeners, products for the treatment of skin conditions, sun filters, germicides, dyes, preservatives, perfumes and propellants.
- compositions according to the invention are dispersions, they may be dispersions of compounds of formula (I) in water in the presence of surfactant or alternatively aqueous dispersions of lipid spherules, consisting of organized molecular layers enclosing an encapsulated aqueous phase, these layers consisting of at least one mixture of isomers of compounds of formula (I) associated with at least one other lipid compound.
- lipid compounds long chain alcohols and diols
- sterols such as cholesterol, phospholipids, cholesteryl sulfate and phosphate, long chain amines and their quaternary ammonium derivatives, dihydroxyalkylamines, polyoxyethylenated fatty amines, esters of long chain amino alcohols, their salts and quaternary ammonium derivatives
- phosphoric esters of fatty alcohols such as acid diketylphosphate or its sodium salt
- alkylsulfates such as cetylsulfate sodium
- lipids comprising a long lipophilic chain containing 12 to 30 carbon atoms, saturated or unsaturated, branched or linear, for example an oleic, lanolic, tetradecylic, hexadecylic, isostearyl, lauric or alkylphenyl chain.
- the hydrophilic group of these lipids can be an ionic or nonionic group.
- nonionic groups mention may be made of groups derived from polyethylene glycol.
- polyglycerol ethers such as those described in French patents Nos. 1,477,048, 2,091,516, 2,465,780 and 2,482,128.
- ionic group it is advantageous to use a group derived from an amphoteric, anionic or cationic compound.
- lipids described in international patent application WO 83/01 571 as being able to be used for the formation of vesicles are the glycolipids such as lactosylceramide, galactocerebroside, gangliosides and trihexosylceramide, as well as phospholipids such as phosphatidylglycerol and phosphatidylinositol.
- the present invention therefore relates to a dispersion of lipid spherules consisting of organized molecular layers of compounds of formula (I) and of lipid defined above containing an aqueous phase to be encapsulated.
- the continuous phase of the dispersion which surrounds the spherules is an aqueous phase.
- the spherules in dispersion generally have a diameter of between 0.05 ⁇ m and 5 ⁇ m.
- the aqueous phase encapsulated in the spherules may be water or an aqueous solution of active substance and in this case is preferably isoosmotic with respect to the continuous phase of the dispersion.
- the spherules can be obtained in particular according to the process described in French patent 2,315,991 of the Applicant, according to which a dispersion of spherules is prepared which consist of organized molecular layers containing an aqueous phase to be encapsulated, by bringing into contact on the one hand mixtures of isomers of compounds of formula (I) associated with one or more lipid (s) defined above and on the other hand the aqueous phase to be encapsulated in the spherules, stirring to ensure mixing and obtaining a lamellar phase, then adding a dispersion liquid in an amount greater than the amount of lamellar phase obtained and vigorously shaking for a period ranging from 15 minutes to 3 hours approximately.
- REV reverse-phase evaporation vesicle
- reverse phase evaporation described in Proc. Natl. Acad. Sci. USA., Vol. 75, n ° 9, pages 4194-4198 (1978), by SZOKA and PAPAHADJOPOULOS.
- the method which comprises the succession of steps consisting in dissolving at least one lipid in at least one organic solvent immiscible with water; adding the organic phase thus obtained to an aqueous phase; forming a dispersion of the two phases with vigorous stirring, the size of the vesicles being adjustable by varying the stirring speed during this phase mixing; conduct the evaporation of the solvent (s) under vigorous stirring; and, if necessary, concentrating the dispersion.
- the active substances can be substances having a pharmaceutical or food interest or substances having a cosmetic activity. When they are water-soluble, they are in the aqueous phase encapsulated inside the vesicles.
- the water-soluble substances having a cosmetic and / or pharmaceutical activity may be products intended for the care or treatment of the skin and the hair, such as for example humectants such as glycerin, sorbitol, pentaerythritol, pyrrolidone carboxylic acid and its salts; artificial browning agents such as dihydroxyacetone, erythrulose, glyceraldehyde, ⁇ -dialdehydes such as tartaric aldehyde, these compounds being optionally combined with dyes; water-soluble sun filters; antiperspirants, deodorants, astringents, refreshing, tonic, healing, keratolytic, depilatory, scented waters; plant tissue extracts, such as polysaccharides; water-soluble dyes; dandruff agents; antiseborrhoeic agents, oxidants such as discoloration such as hydrogen peroxide; reducing agents such as thioglycolic acid and its salts.
- the active substances are liposoluble, they are incorporated into the sheets of the vesicles. They can be chosen from the group formed by fat-soluble sunscreens, substances intended to improve the condition of dry or senile skin, tocopherols, vitamins E, F or A and their esters, retinoic acid, antioxidants, essential fatty acids, glycyrrhetinic acid, keratolytics and carotenoids.
- the dispersions of lipid spherules have the advantage of carrying active substances which are thus masked and protected from the various spoilage agents: oxidants and more generally reactive compounds with respect to the encapsulated active substances.
- the penetration and fixation of the active substances can be modulated by varying the size of the spherules and their electrical charge.
- the action of these active substances can also be postponed (delay effect).
- the subject of the invention is finally the use in cosmetics of an aqueous dispersion of spherules consisting of organized molecular layers of compounds of formula (I) associated with other lipids containing an aqueous phase to be encapsulated, in particular for the treatment of the skin.
- the invention also relates to the use of such a dispersion of lipid spherules in dermatology or in the food industry.
- THF means tetrahydrofuran
- M.A. means active ingredient
- 2-amino-octadecane-1,3,4-triol (2 isomers D, L-ribo, 450mg, 1,4.10 -3 mole) is suspended in 25ml of tetrahydrofuran (THF). Palmitoyl chloride (390 mg, 1.4 ⁇ 10 -3 mole) is added all at once. Triethylamine (145mg, about 1.4.10 -3 mole) is poured slowly. After 4 hours, there is no more amine. By addition of water, a precipitate is obtained in the reaction medium.
- THF tetrahydrofuran
- the D, L-2-hydroxy-hexadecanoic acid (2g - 7.5.10 -3 mole) is suspended in 50ml of ethyl actuate.
- N-hydroxysuccinimide (0.8 g - 7.5.10 -3 mole) and dicyclohexylcarbodiimide (1.5 g - 7.5.10 -3 mole) are rapidly added.
- the mixture is stirred for 2 hours at room temperature.
- the dicyclohexylurea is filtered.
- the solid obtained is resuspended in 20 ml of THF and this solution is poured into a mixture, 2-aminooctadecane-1,3,4-triol (2 isomers D, L-ribo - 2.4g - 7.5.10 -3 mole) and 100ml of THF, the solution is brought to reflux. After 1 hour of reflux, the reaction is stopped and the mixture is allowed to return to ambient temperature. 50 g of silica are added directly to the reaction medium.
- the behenic acid (530 mg - 1.6.10 -3 mole) is dissolved in 10 ml of ethyl acetate. N-hydroxysuccinimide (175mg - 1.6.10 -3 mole) and dicyclohexyl-carbodiimide (325mg - 1.6.10 -3 mole) are quickly added. Stirred at room temperature for 2 hours, filter the dicyclohexylurea and evaporate to dryness.
- the product thus obtained is purified by chromatography on a silica column (the eluent being: 1,2-dichloro-ethane (9) / methanol (1)). 1g of white solid is isolated.
- the mass spectrum of the product obtained corresponds to the expected melting point structure of 138-140 ° C: 2-N- (2-hydroxy-hexadecanoyl) -amino-octadecane-1,3,4-trio (16 isomers)
- This example includes different formulations for the care or treatment of hair with compounds of the previous examples.
- the shampoo thus formulated is of clear appearance Rinse-off conditioner 1-methyl 2-tallow 3-surfamido methosulfate 2 g MA ethylimidazolium / propylene glycol (75/25) sold by Witco as Rewoquat W75PG compound of example 5 0.5 g Cetyl alcohol blend 3 g and oxyethylenated stearyl cetyl Preservative, fragrance Water qs 100g 5.2 spontaneous pH Shampoo Lauryl ether sodium sulfate (28% MA) 60g Cocoylbetaine 9 g Composed of Example 4 0.5 g Preservative, fragrance Water qs 100g HCl qs pH 6
- the shampoo thus formulated is opalescent.
- This example includes different formulations for caring for or treating the skin with compounds from the previous examples
- a comparative test was carried out of the effects on the smoothing of the hair of a solution containing the compound of Example 4 at 1% in tetrahydrofuran (THF), compared to a control consisting of THF, or to a solution containing , instead of the compound according to the invention, either the pure isomer ( D -ribo form) corresponding to the compound of Example 4 (compound A), or the compound of Example 1 of the published European patent application under the number 0 500 437 filed by the Applicant (compound B).
- THF tetrahydrofuran
- This test determines the coefficient of friction of the hair by measuring the force to be applied to a control mass in order to make it slide at constant speed over two hair stretched in parallel. The measurement is carried out by sliding the mass from the root to the tip of the hair (R-> P) and vice versa (P-> R). The results are collated in Table 1 below.
- Example 4 The application of the compound according to the invention (Example 4) allows a marked reduction in the coefficient of friction, thus demonstrating an improvement in the straightening or disentangling of the hair.
- PIE Measuring insensible water loss
- This measurement is carried out using an evaporometer (Servomed) which quantitatively determines the evaporation of water, i.e. a transport of water by diffusion, from a sample of stratum corneum previously defatted sealing a cylindrical capsule containing water, the whole being placed in a chamber at controlled temperature and relative humidity.
- evaporometer Servomed
- Table 2 Compound Composition (concentration) PIE 20 H (%) Compound B 1.5% in THF -8 ⁇ 1
Abstract
Description
La présente invention a pour objet de nouveaux composés de type céramides, leur procédé de préparation ainsi que leur utilisation, notamment pour les traitements et les soins de la peau, des cheveux, des ongles et des cils en cosmétique ou en dermatologie.The subject of the present invention is new compounds of the ceramide type, their preparation process and their use, in particular for the treatment and care of the skin, hair, nails and eyelashes in cosmetics or dermatology.
L'exposition de la peau au froid, au soleil, aux atmosphères à faible humidité relative, les traitements répétés avec des compositions de lavage ou encore le contact avec des solvants organiques, sont des facteurs qui entraînent, à des degrés divers, un déssèchement apparent. La peau apparaît plus sèche, moins souple et le relief cutané plus prononcé. Par ailleurs, les cheveux, qui sont soumis trop fréquemment à certains traitements capillaires, perdent leur aspect brillant et peuvent devenir rèches et cassants.Exposure of the skin to the cold, the sun, atmospheres with low relative humidity, repeated treatments with washing compositions or even contact with organic solvents, are factors which lead, to varying degrees, to an apparent dryness . The skin appears drier, less supple and the skin relief more pronounced. In addition, the hair, which is subjected too frequently to certain hair treatments, loses its shiny appearance and can become coarse and brittle.
La demanderesse a donc recherché des composés qui permettent de prévenir ou de corriger ces phénomènes se traduisant par un déssèchement apparent et qui redonnent à la peau sa souplesse et aux cheveux leur brillance et leur douceur.The Applicant has therefore sought compounds which make it possible to prevent or correct these phenomena which result in an apparent dryness and which restore the suppleness to the skin and the hair to their shine and softness.
Pour résoudre ce problème, on a déjà proposé d'utiliser des céramides. On sait en effet que ces composés sont les éléments constitutifs prépondérants des lipides interconéocytaires du stratum cornéum et participent au maintien de l'intégrité de la barrière cutanée.To solve this problem, it has already been proposed to use ceramides. It is known in fact that these compounds are the predominant constituent elements of the interconéocytaires lipides of the stratum corneum and participate in the maintenance of the integrity of the cutaneous barrier.
Les céramides utilisés en cosmétique sont le plus souvent des extraits naturels issus notamment de la peau de porc, du cerveau de boeuf, de l'oeuf, des cellules de sang, des végétaux comme le blé, etc. (demandes de brevets japonais J 86/260008 et J 87/120308). De tels céramides ont été également proposés pour la protection des cheveux (EP 0 278 505).The ceramides used in cosmetics are most often natural extracts derived in particular from pigskin, beef brain, egg, blood cells, plants such as wheat, etc. (Japanese patent applications J 86/260008 and J 87/120308). Such ceramides have also been proposed for protecting the hair (EP 0 278 505).
Il s'agit donc toujours de mélanges de teneur plus ou moins importante en céramides et dont la composition est difficile à contrôler. De plus, ces mélanges sont sujets à la contamination bactérienne. Leur conservation est donc difficile à maîtriser. Lorsqu'ils sont d'origine animale, il y a en plus un risque de contamination par l'agent responsable de la BSE (encéphalopathie bovine spongiforme).They are therefore always mixtures of more or less significant content of ceramides and the composition of which is difficult to control. In addition, these mixtures are subject to bacterial contamination. Their conservation is therefore difficult to control. When they are of animal origin, there is also a risk of contamination by the agent responsible for BSE (bovine spongiform encephalopathy).
Chaque céramide d'origine naturelle présente une stéréoisomérie précise et unique, telle que celles décrites pour la sphinganine, la phytoshingosine et la sphingosine (encore appelée la sphingénine) qui sont respectivement le (2S,3R)-2-amino-1,3-octadécanediol, le (2S,3S,4R)-2-amino-1,3,4-octadécanetriol et le (2S,3R,4E)-2-amino-4-octadécène-1,3-diol [J. Biochem. 79, 11-21 (1977)].Each ceramide of natural origin has a precise and unique stereoisomerism, such as those described for sphinganine, phytoshingosine and sphingosine (also called sphingenine) which are respectively ( 2S, 3R ) -2-amino-1,3- octadecanediol, ( 2S , 3S , 4R ) -2-amino-1,3,4-octadecanetriol and ( 2S , 3R , 4E ) -2-amino-4-octadecene-1,3-diol [J. Biochem. 79, 11-21 (1977)].
Pour résoudre ces problèmes, nous avons proposé des céramides de synthèse, notamment dans la demande de brevet européen n° 0 500 437. Ces composés, utilisés dans des compositions cosmétiques ou dermatologiques, pour les traitements et les soins de la peau et des cheveux ont un effet hydratant permettant de prévenir ou de corriger certains effets du déssèchement apparent de la peau ou des cheveux.To solve these problems, we have proposed synthetic ceramides, in particular in European patent application No. 0 500 437. These compounds, used in cosmetic or dermatological compositions, for the treatment and care of the skin and hair have a moisturizing effect to prevent or correct certain effects of the apparent drying of the skin or hair.
Toutefois, il serait souhaitable de mettre au point des composés qui, utilisés dans des compositions cosmétiques ou dermatologiques, aient un effet hydratant ou traitant supérieur à celui des composés de cette demande de brevet.However, it would be desirable to develop compounds which, used in cosmetic or dermatological compositions, have a moisturizing or treating effect greater than that of the compounds of this patent application.
Il faut également noter une demande brevet WO 94/10131 décrivant un procédé de fermentation qui conduit à une famille de céramides apparentés aux céramides 1 dans la classification de DOWNING (J.I.D.-84, 410-412,1985), ce procédé est donc malheureusement restreint à cette famille de céramides.Note also a patent application WO 94/10131 describing a fermentation process which leads to a family of ceramides related to ceramides 1 in the classification of DOWNING (JID-84, 410-412,1985), this process is therefore unfortunately restricted to this family of ceramides.
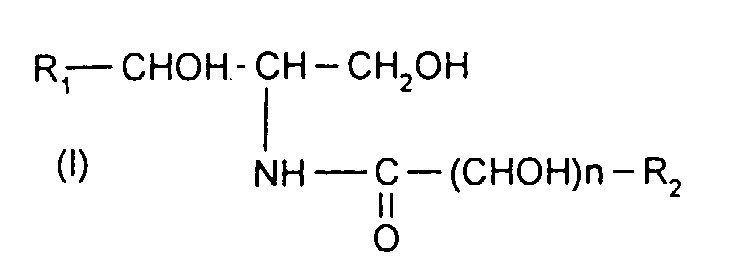
La présente invention a donc pour objet des composés répondant à la formule :
- * R1 désigne un radical alkyle hydroxylé, saturé ou insaturé, en C10 à C25 ;
- * n est égal à 0 ou 1 ;
- * R2 désigne un radical alkyle linéaire ou ramifié, saturé ou insaturé, en C1 à C31,
les composés étant sous forme de mélanges d'isomères au moins sur la partie aminoalcool de ladite formule (I).The present invention therefore relates to compounds corresponding to the formula:
- * R 1 denotes a C 10 to C 25 saturated or unsaturated hydroxylated alkyl radical;
- * n is 0 or 1;
- * R 2 denotes a linear or branched, saturated or unsaturated, C 1 to C 31 alkyl radical,
the compounds being in the form of mixtures of isomers at least on the aminoalcohol part of said formula (I).
Ainsi, ces nouveaux composés présentent un très bon pouvoir hydratant de la peau et/ou des cheveux lorsqu'ils sont utilisés dans des compositions cosmétiques ou dermatologiques.Thus, these new compounds have very good hydrating power for the skin and / or hair when they are used in cosmetic or dermatological compositions.
Lorsque R1 désigne un radical alkyle saturé et hydroxylé, on préfère que le radical hydroxyle se trouve en position α par rapport au carbone du groupement -CHOH-. Ce radical hydroxyle peut se trouver sous une forme subsituée. Ce radical peut alors être représenté notamment par un radical de formule -O-CO-CHOH-R4, avec R4 désignant un radical alkyle linéaire ou ramifié, saturé ou insaturé, en C1 à C31.When R 1 denotes a saturated and hydroxylated alkyl radical, it is preferred that the hydroxyl radical is in position α with respect to the carbon of the group -CHOH-. This hydroxyl radical can be in a substituted form. This radical can then be represented in particular by a radical of formula -O-CO-CHOH-R 4 , with R4 denoting a linear or branched, saturated or unsaturated, C 1 to C 31 alkyl radical.
Lorsque R1 désigne un radical alkyle insaturé et hydroxylé, on préfère que R1 présente un radical éthylénique en position α par rapport au carbone du groupement -CHOH-. Plus particulièrement, au moins un, et de préférence le, radical hydroxyle est en position α du radical éthylénique.When R 1 denotes an unsaturated and hydroxylated alkyl radical, it is preferred that R 1 has an ethylenic radical in position α with respect to the carbon of the group -CHOH-. More particularly, at least one, and preferably the hydroxyl radical is in the α position of the ethylenic radical.
De préférence, R1 désigne un radical alkyle saturé et hydroxylé.Preferably, R 1 denotes a saturated and hydroxylated alkyl radical.
De préférence, R1 désigne un radical alkyle hydroxylé, saturé ou insaturé, en C12 à C23.Preferably, R 1 denotes a C 12 to C 23 , saturated or unsaturated, hydroxylated alkyl radical.
De préférence, R2 désigne un radical alkyle linéaire, plus particulièrement en C2 à C25.Preferably, R 2 denotes a linear alkyl radical, more particularly of C 2 to C 25 .
Les composés selon l'invention sont donc sous forme de mélanges d'isomères optiques et/ou géométriques (mélange d'énantiomères et/ou de diastéréoisomères) au moins sur la partie amino-alcool, ce qui les différencient des produits naturels qui sont uniquement sous forme d'un seul isomère.The compounds according to the invention are therefore in the form of mixtures of optical and / or geometric isomers (mixture of enantiomers and / or diastereoisomers) at least on the amino alcohol part, which differentiate them from natural products which are only as a single isomer.
Ces nouveaux composés ont l'avantage d'améliorer et/ou de rétablir la fonction barrière lorsqu'ils sont appliqués sur la peau.These new compounds have the advantage of improving and / or restoring the barrier function when they are applied to the skin.
Les composés selon l'invention sont de préférence sous forme de mélange d'au moins 4 isomères.The compounds according to the invention are preferably in the form of a mixture of at least 4 isomers.
De préférence, les composés de formule (1) sont choisis parmi le 2-N-docosanoylaminooctadécane-1,3,4-triol, le 2-N-(2-hydroxy-hexadecanoyl)-amino-octadécane-1,3,4-triol et le 2-N-hexadecanoylamino-octadécane-1,3,4-triol.Preferably, the compounds of formula (1) are chosen from 2-N-docosanoylaminooctadecane-1,3,4-triol, 2-N- (2-hydroxy-hexadecanoyl) -amino-octadecane-1,3,4 -triol and 2-N-hexadecanoylamino-octadecane-1,3,4-triol.
Les cheveux traités par ces composés de formule (I) présentent un aspect brillant, un toucher plus doux et une moins grande sensibilité à l'eau, due à l'apport de matière lipidique uniformément répartie sur les écailles du cheveu. Les propriétés mécaniques et de nervosité sont également améliorées.The hair treated with these compounds of formula (I) has a shiny appearance, a softer feel and less sensitivity to water, due to the supply of lipid material uniformly distributed over the scales of the hair. Mechanical and nervousness properties are also improved.
Les composés selon l'invention peuvent former avec d'autres lipides des vésicules.The compounds according to the invention can form with other lipids vesicles.
Les composés selon l'invention peuvent être obtenus par acylation de la fonction amine des composés de formule (II) ou de dérivés réactifs de ceux-ci, tel que par exemple le chlorhydrate, avec un agent acylant approprié.The compounds according to the invention can be obtained by acylation of the amine function of the compounds of formula (II) or of reactive derivatives thereof, such as for example the hydrochloride, with an appropriate acylating agent.
Ainsi, un autre objet de l'invention concerne un procédé de préparation de mélanges d'isomères des composés de formule (I) ci-dessus, caractérisé en ce qu'il comprend :
- (a) l'acylation d'au moins un composé de formule (II) suivante :
formule (II) dans laquelle R1 a la même signification. que donnée ci-dessus, ou d'au moins un dérivé réactif de ces composés de formule (II), à l'aide d'au moins un agent acylant de formule (III) suivante :
R2-(CHX)n-CO-A (III)
formule (III) dans laquelle R2 et n ont les significations données précédemment et X représente un groupe choisi parmi le radical hydroxyle, un atome d'halogène, de préférence Br, Cl ou l, et un radical de formule OB susceptible de former un groupe OH ; A est un groupe choisi parmi un atome d'halogène, un radical de formule -O-COO-R5, un radical de formule OR6, un groupement - (b) l'isolation des composés obtenus, et
- (c) éventuellement, lorsque X est différent du radical hydroxyle, l'hydrolyse ou l'hydrogénolyse des composés obtenus pour transformer le groupe OB en radical hydroxyle, précédé éventuellement, lorsque X est un atome d'halogène, d'une étape de substitution de X par un groupe OB.
- (a) the acylation of at least one compound of formula (II) below:
formula (II) in which R 1 has the same meaning. as given above, or at least one reactive derivative of these compounds of formula (II), using at least one acylating agent of formula (III) below:
R 2 - (CHX) n -CO-A (III)
formula (III) in which R 2 and n have the meanings given above and X represents a group chosen from the hydroxyl radical, a halogen atom, preferably Br, Cl or l, and a radical of formula OB capable of forming a OH group; A is a group chosen from a halogen atom, a radical of formula -O-COO-R 5 , a radical of formula OR 6 , a group - (b) isolating the compounds obtained, and
- (c) optionally, when X is different from the hydroxyl radical, the hydrolysis or hydrogenolysis of the compounds obtained to transform the OB group into a hydroxyl radical, optionally preceded, when X is a halogen atom, by a substitution step of X by an OB group.
Ainsi, par partie amino-alcool, on entend selon l'invention la partie de la formule (I) venant du composé de formule (II).Thus, by amino alcohol part is meant according to the invention the part of formula (I) coming from the compound of formula (II).
De préférence, le procédé est réalisé en présence d'un solvant approprié. Parmi les solvants utilisables dans le procédé de la présente invention, on peut citer le tétrahydrofurane (THF), la pyridine, le 1,2-diméthoxy-éthane, le diméthylformamide, le dichlorométhane, le tertiobutylméthyléther.Preferably, the process is carried out in the presence of an appropriate solvent. Among the solvents which can be used in the process of the present invention, mention may be made of tetrahydrofuran (THF), pyridine, 1,2-dimethoxyethane, dimethylformamide, dichloromethane, tert-butyl methyl ether.
De préférence, le groupement OB est choisi parmi les radicaux suivants : acétate, benzoate, benzyloxy, -OSi(CH3)3, -OSi(CH3)2(t-butyl), et -OSi(t-butyl)(-C6H5)2. De manière encore plus préférentielle, OB est un groupement acétate.Preferably, the OB group is chosen from the following radicals: acetate, benzoate, benzyloxy, -OSi (CH 3 ) 3 , -OSi (CH 3 ) 2 (t-butyl), and -OSi (t-butyl) (- C 6 H 5 ) 2 . Even more preferably, OB is an acetate group.
De préférence, A est un groupe choisi parmi les groupes suivants :
-Cl, un radical de formule -O-COO-C2H5, un radical de formule -OCH3 ou -OC2H5,
-Cl, a radical of formula -O-COO-C 2 H 5 , a radical of formula -OCH 3 or -OC 2 H 5 ,
On recommande tout particulièrement pour A les radicaux
Les agents acylants particulièrement recommandés sont les esters de succinimide et de carbodiimide, les dérivés imidazoles et les chlorures d'acide, tels que définis ci-dessus.The acylating agents particularly recommended are the succinimide and carbodiimide esters, the imidazole derivatives and the acid chlorides, as defined above.
Pour la préparation des composés de formule (I), on peut donc également utiliser des sels, tels que les chlorhydrates des composés de formule (II).For the preparation of the compounds of formula (I), it is therefore also possible to use salts, such as the hydrochlorides of the compounds of formula (II).
Les composés de formule (II) ou leurs dérivés sont connus et ont été notamment décrits par M. Prostenik (Croatia Chemica Acta, 29, 107-113, 1957) et M. Jäger (Angew. Chem. Int., 603-605, 1981). Ces composés sont donc des mélanges d'au moins 2 isomères optiques et/ou géométriques (énantiomères et/ou diastéréoisomères).The compounds of formula (II) or their derivatives are known and have been described in particular by M. Prostenik (Croatia Chemica Acta, 29, 107-113, 1957) and M. Jäger (Angew. Chem. Int., 603-605, nineteen eighty one). These compounds are therefore mixtures of at least 2 optical and / or geometric isomers (enantiomers and / or diastereoisomers).
Les agents acylants de formule (III) sont des produits connus de l'homme du métier, ils peuvent être également sous forme de mélanges d'isomères, notamment de mélanges racémiques.The acylating agents of formula (III) are products known to those skilled in the art, they can also be in the form of mixtures of isomers, in particular racemic mixtures.
Généralement, les quantités des composés de formule (II) et (III) mis en oeuvre dans le procédé selon l'invention sont choisies de telle sorte que leur rapport molaire (III)/(II) est supérieur ou égal à 1.Generally, the amounts of the compounds of formula (II) and (III) used in the process according to the invention are chosen so that their molar ratio (III) / (II) is greater than or equal to 1.
La température du procédé selon l'invention peut varier dans une large mesure. La température de l'étape (a) est généralement comprise entre 0 et 50°C, de préférence, elle correspond à la température ambiante.The temperature of the process according to the invention can vary to a large extent. The temperature of step (a) is generally between 0 and 50 ° C., preferably, it corresponds to ambient temperature.
Avant l'étape (b) du procédé selon l'invention et lorsque X représente le radical hydroxyle, les produits de l'étape (a) peuvent subir une réaction de protection des groupements hydroxyle, par réaction avec un agent choisi parmi les anhydrides d'acide, les halogénures d'acide et les chlorosilanes, la réaction étant suivie, après isolement des composés selon l'étape (b), par une hydrolyse ou une hydrogénolyse.Before stage (b) of the process according to the invention and when X represents the hydroxyl radical, the products of stage (a) can undergo a reaction for protecting the hydroxyl groups, by reaction with an agent chosen from anhydrides d acid, acid halides and chlorosilanes, the reaction being followed, after isolation of the compounds according to step (b), by hydrolysis or hydrogenolysis.
Les agents utilisés dans le procédé de la présente invention sont de préférence choisis parmi l'anhydride acétique, le chlorure de benzoyle, le chlorure de benzyle, le bromure de benzyle, les chlorosilanes de formule ClSi(CH3)3, ClSi(CH3)2(tBu), ClSi(tBu)(-C6H5)2.The agents used in the process of the present invention are preferably chosen from acetic anhydride, benzoyl chloride, benzyl chloride, benzyl bromide, chlorosilanes of formula ClSi (CH 3 ) 3 , ClSi (CH 3 ) 2 (tBu), ClSi (tBu) (- C 6 H 5 ) 2 .
Les composés selon l'invention peuvent recevoir des applications diverses, notamment dans des compositions cosmétiques et dermatologiques. Ces composés possèdent en plus la propriété de former des vésicules en association avec d'autres lipides, lorsqu'ils sont dispersés dans l'eau.The compounds according to the invention can receive various applications, in particular in cosmetic and dermatological compositions. These compounds also have the property of forming vesicles in association with other lipids, when they are dispersed in water.
La présente invention a donc pour objet l'utilisation des composés de formule (I) dans des émulsions, des dispersions ou dans des lotions. Elle a également pour objet l'utilisation de ces composés, associés à d'autres lipides, pour la formation de sphérules lipidiques.The present invention therefore relates to the use of the compounds of formula (I) in emulsions, dispersions or in lotions. It also relates to the use of these compounds, associated with other lipids, for the formation of lipid spherules.
La présente invention a également pour objet des compositions à usage cosmétique ou dermatologique contenant les composés de formule (I).The present invention also relates to compositions for cosmetic or dermatological use containing the compounds of formula (I).
Un autre objet de l'invention est un procédé de traitement cosmétique de la peau, des cheveux, des ongles ou des cils consistant à appliquer sur ces derniers une quantité suffisante d'au moins un composé de formule (I).Another subject of the invention is a method of cosmetic treatment of the skin, hair, nails or eyelashes consisting in applying to the latter a sufficient amount of at least one compound of formula (I).
Les compositions selon l'invention peuvent se présenter sous forme d'émulsions (lait ou crème), de lotions hydroalcooliques, huileuses ou oléoalcooliques, de gels, de dispersions ou de bâtonnets solides, de sprays ou de mousse aérosol.The compositions according to the invention can be in the form of emulsions (milk or cream), hydroalcoholic, oily or oleoalcoholic lotions, gels, dispersions or solid sticks, sprays or aerosol foam.
Selon l'invention, les composés de formule (I) représentent de 0,005% à 20%, de préférence de 0,01 à 10% du poids total de la composition.According to the invention, the compounds of formula (I) represent from 0.005% to 20%, preferably from 0.01 to 10% of the total weight of the composition.
Les compositions sont par exemple des lotions, des laits ou des crèmes émollients, des laits ou des crèmes pour les soins de la peau ou des cheveux, des crèmes, des lotions ou des laits démaquillants, des bases de fond de teint, des lotions, des laits ou des crèmes antisolaires, des lotions, des laits ou des crèmes de bronzage artificiel, des crèmes ou des mousses de rasage, des lotions après rasage, des shampooings, des rouges à lèvres, des mascaras ou des vernis à ongles.The compositions are, for example, lotions, milks or emollient creams, milks or creams for skin or hair care, creams, lotions or cleansing milks, foundations, foundations, lotions, sunscreen milks or creams, lotions, artificial tanning milks or creams, shaving creams or foams, after shave lotions, shampoos, lipsticks, mascaras or nail polishes.
Ces compositions peuvent également se présenter sous la forme de bâtons pour les lèvres destinés soit à les colorer, soit à éviter les gerçures, ou de produits de maquillage pour les yeux ou des fards et fonds de teint pour le visage.These compositions can also be in the form of lip sticks intended either to color them or to avoid chapping, or of makeup products for the eyes or eyeshadows and foundations for the face.
Lorsque les compositions selon l'invention se présentent sous la forme d'émulsions de type eau-dans-l'huile ou huile-dans-l'eau, la phase grasse est essentiellement constituée d'un mélange de composés de formule (I) avec au moins une huile, et éventuellement un autre corps gras.When the compositions according to the invention are in the form of emulsions of water-in-oil or oil-in-water type, the fatty phase essentially consists of a mixture of compounds of formula (I) with at least one oil, and possibly another fatty substance.
La phase grasse des émulsions peut constituer de 5 à 60% du poids total de l'émulsion.The fatty phase of the emulsions can constitute from 5 to 60% of the total weight of the emulsion.
La phase aqueuse desdites émulsions constitue de préférence de 30 à 85% du poids total de l'émulsion.The aqueous phase of said emulsions preferably constitutes from 30 to 85% of the total weight of the emulsion.
La proportion de l'agent émulsionnant peut être comprise entre 1 et 20%, et de préférence entre 2 et 12% du poids total de l'émulsion.The proportion of the emulsifying agent can be between 1 and 20%, and preferably between 2 and 12% of the total weight of the emulsion.
Lorsque les compositions selon l'invention se présentent sous forme de lotions huileuses, oléoalcooliques ou hydroalcooliques, elle peuvent constituer, par exemple, des lotions antisolaires contenant un filtre absorbant les rayons UV, des lotions adoucissantes pour la peau; les lotions huileuses peuvent en outre constituer des huiles moussantes contenant un tensio-actif oléosoluble, des huiles pour le bain, etc.When the compositions according to the invention are in the form of oily, oleoalcoholic or hydroalcoholic lotions, they can constitute, for example, sunscreen lotions containing a filter absorbing UV rays, softening lotions for the skin; oily lotions can also constitute foaming oils containing an oil-soluble surfactant, bath oils, etc.
Parmi les principaux adjuvants pouvant être présents dans les compositions selon l'invention, on peut citer les corps gras tels que les huiles ou les cires minérales, animales ou végétales, les acides gras, les esters d'acide gras tels que les triglycérides d'acides gras ayant de 6 à 18 atomes de carbone, les alcools gras; les émulsionnants comme les alcools gras éthoxyéthylénés ou les alcoyléthers de polyglycérol; les solvants tels que les monoalcools ou polyalcools inférieurs contenant de 1 à 6 atomes de carbone ou encore l'eau.Among the main adjuvants which may be present in the compositions according to the invention, mention may be made of fatty substances such as mineral or animal or vegetable oils or waxes, fatty acids, fatty acid esters such as triglycerides of fatty acids having from 6 to 18 carbon atoms, fatty alcohols; emulsifiers such as ethoxyethylenated fatty alcohols or polyglycerol alkyl ethers; solvents such as lower monoalcohols or polyalcohols containing from 1 to 6 carbon atoms or even water.
Les mono- ou poly-alcools plus particulièrement préférés sont choisis parmi l'éthanol, l'isopropanol, le propylèneglycol, le glycérol et le sorbitol.The more particularly preferred mono- or poly-alcohols are chosen from ethanol, isopropanol, propylene glycol, glycerol and sorbitol.
A titre de corps gras, parmi les huiles minérales, on peut citer l'huile de vaseline; parmi les huiles animales, les huiles de baleine, de requin, de phoque, de menhaden, de foie de flétan, de morue, de thon, de tortue, de pied de boeuf, de pied de cheval, de pied de mouton, de vison, de loutre, de marmotte, etc.; parmi les huiles végétales, les huiles d'amande, de germes de blé, d'olive, de germe de maïs, de jojoba, de sésame, de tournesol, de palme, de noix, de karité, de shoréa, de macadamia, de pépins de cassis et similaires.Mention may be made, as fatty substance, of mineral oils, of petrolatum oil; among animal oils, whale, shark, seal, menhaden, halibut, cod, tuna, turtle, ox's foot, horse's foot, sheep's foot, mink's oils , otter, groundhog, etc .; among vegetable oils, almond, wheat germ, olive, corn germ, jojoba, sesame, sunflower, palm, nut, shea, shorea, macadamia, blackcurrant seeds and the like.
Parmi les esters d'acides gras, on peut utiliser des esters d'acides en C12 à C22 saturés ou insaturés et d'alcools inférieurs comme l'isopropanol ou le glycérol ou d'alcools gras en C8 à C22, linéaires ou ramifiés, saturés ou insaturés ou encore d'alcanediols-1,2 en C10-C22.Among the fatty acid esters, it is possible to use esters of saturated or unsaturated C 12 to C 22 acids and of lower alcohols such as isopropanol or glycerol or of linear C 8 to C 22 fatty alcohols or branched, saturated or unsaturated or alternatively 1,2-C 10 -C 22 alkanediols.
On peut également citer comme corps gras, la vaseline, la paraffine, la lanoline, la lanoline hydrogénée, le suif, la lanoline acétylée, les huiles de silicone.Mention may also be made, as fatty substance, of petroleum jelly, paraffin, lanolin, hydrogenated lanolin, tallow, acetylated lanolin, silicone oils.
Parmi les cires, on peut citer la cire de Sipol, la cire de lanoline, la cire d'abeille, la cire de Candelila, la cire monocristalline, la cire de Carnauba, le spermaceti, le beurre de cacao, le beurre de karité, les cires de silicone, les huiles'hydrogénées concrètes à 25°C, les sucroglycérides, les oléates, myristates, linoléates et stéarates de calcium, magnésium et aluminium.Among the waxes, mention may be made of Sipol wax, lanolin wax, beeswax, Candelila wax, monocrystalline wax, Carnauba wax, spermaceti, cocoa butter, shea butter, silicone waxes, hydrogenated oils concrete at 25 ° C, sucroglycerides, oleates, myristates, linoleates and stearates of calcium, magnesium and aluminum.
Parmi les alcools gras, on peut citer les alcools laurique, cétylique, myristique, stéarique, palmitique, oléique et les alcools de GUERBET comme le 2-octyldodécanol, le 2-décyltétradécanol ou le 2-hexyldécanol.Among the fatty alcohols, mention may be made of lauric, cetyl, myristic, stearic, palmitic, oleic alcohols and GUERBET alcohols such as 2-octyldodecanol, 2-decyltetradecanol or 2-hexyldecanol.
A titre d'émulsionnants, parmi les alcools gras polyoxyéthylénés, on peut citer les alcools laurique, cétylique, stéarylique et oléique comportant de 2 à 20 moles d'oxyde d'éthylène et parmi les alcoyléthers de glycérol, les alcools en C12-C18 comportant de 2 à 10 moles de glycérol.As emulsifiers, among the polyoxyethylenated fatty alcohols, mention may be made of lauric, cetyl, stearyl and oleic alcohols comprising from 2 to 20 moles of ethylene oxide and among the glycerol alkyl ethers, C 12 -C alcohols 18 comprising from 2 to 10 moles of glycerol.
Il peut être aussi utile d'utiliser des épaississants tels que les dérivés de cellulose, les dérivés d'acide polyacrylique, les gommes de guar ou de caroube ou la gomme de xanthane.It may also be useful to use thickeners such as cellulose derivatives, polyacrylic acid derivatives, guar or carob gum or xanthan gum.
La composition selon l'invention peut également contenir des adjuvants habituellement utilisés en cosmétique ou en dermatologie et notamment des produits hydratants, des adoucissants, des produits pour le traitement d'affections cutanées, des filtres solaires, des germicides, des colorants, des conservateurs, des parfums et des propulseurs.The composition according to the invention may also contain adjuvants usually used in cosmetics or in dermatology and in particular moisturizers, softeners, products for the treatment of skin conditions, sun filters, germicides, dyes, preservatives, perfumes and propellants.
Lorsque les compositions selon l'invention sont des dispersions, il peut s'agir de dispersions de composés de formule (I) dans l'eau en présence de tensio-actif ou encore de dispersions aqueuses de sphérules lipidiques, constituées de couches moléculaires organisées enfermant une phase aqueuse encapsulée, ces couches étant constituées d'au moins un mélange d'isomères de composés de formule (I) associé à au moins un autre composé lipidique.When the compositions according to the invention are dispersions, they may be dispersions of compounds of formula (I) in water in the presence of surfactant or alternatively aqueous dispersions of lipid spherules, consisting of organized molecular layers enclosing an encapsulated aqueous phase, these layers consisting of at least one mixture of isomers of compounds of formula (I) associated with at least one other lipid compound.
On peut citer, à cet effet, comme composés lipidiques, les alcools et diols à longue chaîne, les stérols tels que le cholestérol, les phospholipides, les cholestéryl sulfate et phosphate, les amines à longue chaîne et leurs dérivés d'ammonium quaternaire, les dihydroxyalkylamines, les amines grasses polyoxyéthylénées, les esters d'aminoalcools à longue chaîne, leurs sels et dérivés d'ammonium quaternaire, les esters phosphoriques d'alcools gras tels que le dicétylphosphate acide ou son sel de sodium, les alkylsulfates tels que le cétylsulfate de sodium, les acides gras sous forme de sels ou encore les lipides du type de ceux décrits dans les brevets français n° 2 315 991 , 1 477 048 et 2 091 516 ou dans les demandes de brevet international WO 83/01 571 et WO 92/08685.Mention may be made, for this purpose, as lipid compounds, long chain alcohols and diols, sterols such as cholesterol, phospholipids, cholesteryl sulfate and phosphate, long chain amines and their quaternary ammonium derivatives, dihydroxyalkylamines, polyoxyethylenated fatty amines, esters of long chain amino alcohols, their salts and quaternary ammonium derivatives, phosphoric esters of fatty alcohols such as acid diketylphosphate or its sodium salt, alkylsulfates such as cetylsulfate sodium, fatty acids in the form of salts or the lipids of type of those described in French patents 2,315,991, 1,477,048 and 2,091,516 or in international patent applications WO 83/01 571 and WO 92/08685.
On peut par exemple utiliser comme autres lipides, des lipides comportant une chaîne lipophile longue contenant 12 à 30 atomes de carbone, saturée ou insaturée, ramifiée ou linéaire, par exemple une chaîne oléique, lanolique, tétradécylique, hexadécylique, isostéarylique, laurique ou alcoylphénylique. Le groupement hydrophile de ces lipides peut être un groupement ionique ou non-ionique. A titre de groupements non-ioniques, on peut citer des groupements dérivés de polyéthylèneglycol. On peut aussi utiliser avantageusement comme lipides formant la phase lamellaire, des éthers de polyglycérol tels que ceux décrits dans les brevets français n° 1 477 048, 2 091 516, 2 465 780 et 2 482 128.It is possible, for example, to use as other lipids, lipids comprising a long lipophilic chain containing 12 to 30 carbon atoms, saturated or unsaturated, branched or linear, for example an oleic, lanolic, tetradecylic, hexadecylic, isostearyl, lauric or alkylphenyl chain. The hydrophilic group of these lipids can be an ionic or nonionic group. As nonionic groups, mention may be made of groups derived from polyethylene glycol. It is also advantageous to use, as lipids forming the lamellar phase, polyglycerol ethers such as those described in French patents Nos. 1,477,048, 2,091,516, 2,465,780 and 2,482,128.
A titre de groupement ionique, on peut avantageusement utiliser un groupement dérivé d'un composé amphotère, anionique ou cationique.As an ionic group, it is advantageous to use a group derived from an amphoteric, anionic or cationic compound.
D'autres lipides décrits dans la demande de brevet international WO 83/01 571 comme pouvant être utilisés pour la formation de vésicules sont les glycolipides comme le lactosylcéramide, le galactocérébroside, les gangliosides et le trihexosylcéramide, ainsi que les phospholipides tels que le phosphatidylglycérol et le phosphatidylinositol.Other lipids described in international patent application WO 83/01 571 as being able to be used for the formation of vesicles are the glycolipids such as lactosylceramide, galactocerebroside, gangliosides and trihexosylceramide, as well as phospholipids such as phosphatidylglycerol and phosphatidylinositol.
La présente invention a donc pour objet une dispersion de sphérules lipidiques constituées de couches moléculaires organisées de composés de formule (I) et de lipide défini ci-dessus renfermant une phase aqueuse à encapsuler.The present invention therefore relates to a dispersion of lipid spherules consisting of organized molecular layers of compounds of formula (I) and of lipid defined above containing an aqueous phase to be encapsulated.
La phase continue de la dispersion qui entoure les sphérules est une phase aqueuse.The continuous phase of the dispersion which surrounds the spherules is an aqueous phase.
Les sphérules en dispersion ont généralement un diamètre compris entre 0,05 µm et 5 µm.The spherules in dispersion generally have a diameter of between 0.05 µm and 5 µm.
La phase aqueuse encapsulée dans les sphérules peut être de l'eau ou une solution aqueuse de substance active et est dans ce cas de préférence isoosmotique par rapport à la phase continue de la dispersion.The aqueous phase encapsulated in the spherules may be water or an aqueous solution of active substance and in this case is preferably isoosmotic with respect to the continuous phase of the dispersion.
Les sphérules peuvent être obtenues en particulier suivant le procédé décrit dans le brevet français 2 315 991 de la Demanderesse, selon lequel on prépare une dispersion de sphérules constituées de couches moléculaires organisées renfermant une phase aqueuse à encapsuler, en mettant en contact d'une part des mélanges d'isomères de composés de formule (I) associées à un ou plusieurs lipide(s) défini(s) ci-dessus et d'autre part la phase aqueuse à encapsuler dans les sphérules, en agitant pour assurer le mélange et obtenir une phase lamellaire, en ajoutant ensuite un liquide de dispersion en quantité supérieure à la quantité de phase lamellaire obtenue et en secouant énergiquement pendant une durée allant de 15 minutes à 3 heures environ.The spherules can be obtained in particular according to the process described in French patent 2,315,991 of the Applicant, according to which a dispersion of spherules is prepared which consist of organized molecular layers containing an aqueous phase to be encapsulated, by bringing into contact on the one hand mixtures of isomers of compounds of formula (I) associated with one or more lipid (s) defined above and on the other hand the aqueous phase to be encapsulated in the spherules, stirring to ensure mixing and obtaining a lamellar phase, then adding a dispersion liquid in an amount greater than the amount of lamellar phase obtained and vigorously shaking for a period ranging from 15 minutes to 3 hours approximately.
Un autre procédé de préparation peut consister à utiliser le procédé dénommé REV (reverse-phase evaporation vesicle) ou évaporation en phase inverse décrit dans Proc. Natl. Acad. Sci. USA., Vol. 75, n° 9, pages 4194-4198 (1978), par SZOKA et PAPAHADJOPOULOS.Another preparation process can consist in using the process called REV (reverse-phase evaporation vesicle) or reverse phase evaporation described in Proc. Natl. Acad. Sci. USA., Vol. 75, n ° 9, pages 4194-4198 (1978), by SZOKA and PAPAHADJOPOULOS.
On peut également mettre en oeuvre le procédé qui comprend la succession d'étapes consistant à dissoudre au moins un lipide dans au moins un solvant organique non miscible à l'eau ; ajouter la phase organique ainsi obtenue à une phase aqueuse ; former une dispersion des deux phases sous forte agitation, la taille des vésicules pouvant être réglée en faisant varier la vitesse d'agitation au cours de ce mélange de phase ; conduire l'évaporation du (ou des) solvant(s) sous forte agitation ; et, le cas échéant, concentrer la dispersion.It is also possible to implement the method which comprises the succession of steps consisting in dissolving at least one lipid in at least one organic solvent immiscible with water; adding the organic phase thus obtained to an aqueous phase; forming a dispersion of the two phases with vigorous stirring, the size of the vesicles being adjustable by varying the stirring speed during this phase mixing; conduct the evaporation of the solvent (s) under vigorous stirring; and, if necessary, concentrating the dispersion.
Les substances actives peuvent être des substances ayant un intérêt pharmaceutique, alimentaire ou des substances ayant une activité cosmétique. Lorsqu'elles sont hydrosolubles, elles sont dans la phase aqueuse encapsulée à l'intérieur des vésicules.The active substances can be substances having a pharmaceutical or food interest or substances having a cosmetic activity. When they are water-soluble, they are in the aqueous phase encapsulated inside the vesicles.
Les substances hydrosolubles ayant une activité cosmétique et/ou pharmaceutique peuvent être des produits destinés aux soins ou aux traitements de la peau et du cheveu tels que par exemple des humectants comme la glycérine, le sorbitol, le pentaérythritol, l'acide pyrrolidone carboxylique et ses sels; des agents de brunissage artificiel tels que la dihydroxyacétone, l'érythrulose, le glycéraldéhyde, les γ-dialdéhydes tels que l'aldéhyde tartrique, ces composés étant éventuellement associés à des colorants ; des filtres solaires hydrosolubles; des antiperspirants, des déodorants, des astringents, des produits rafraîchissants, toniques, cicatrisants, kératolytiques, dépilatoires, des eaux parfumées ; des extraits de tissus végétaux, tels que les polysaccharides ; des colorants hydrosolubles ; des agents antipelliculaires ; des agents antiséborrhéiques, des oxydants tels que des agents de décoloration comme l'eau oxygénée ; des réducteurs tels que l'acide thioglycolique et ses sels.The water-soluble substances having a cosmetic and / or pharmaceutical activity may be products intended for the care or treatment of the skin and the hair, such as for example humectants such as glycerin, sorbitol, pentaerythritol, pyrrolidone carboxylic acid and its salts; artificial browning agents such as dihydroxyacetone, erythrulose, glyceraldehyde, γ-dialdehydes such as tartaric aldehyde, these compounds being optionally combined with dyes; water-soluble sun filters; antiperspirants, deodorants, astringents, refreshing, tonic, healing, keratolytic, depilatory, scented waters; plant tissue extracts, such as polysaccharides; water-soluble dyes; dandruff agents; antiseborrhoeic agents, oxidants such as discoloration such as hydrogen peroxide; reducing agents such as thioglycolic acid and its salts.
On peut citer également les vitamines, les hormones, les enzymes telles que la superoxyde dismutase, les vaccins, les anti-inflammatoires tels que l'hydrocortisone, les antibiotiques, les bactéricides, les agents cytotoxiques ou anti-tumoraux.Mention may also be made of vitamins, hormones, enzymes such as superoxide dismutase, vaccines, anti-inflammatories such as hydrocortisone, antibiotics, bactericides, cytotoxic or anti-tumor agents.
Lorsque les substances actives sont liposolubles, elles se trouvent incorporées dans les feuillets des vésicules. Elle peuvent être choisies dans le groupe formé par les filtres solaires liposolubles, les substances destinées à améliorer l'état des peaux sèches ou séniles, les tocophérols, les vitamines E, F ou A et leurs esters, l'acide rétinoïque, les antioxydants, les acides gras essentiels, l'acide glycyrrhétinique, les kératolytiques et les caroténoïdes.When the active substances are liposoluble, they are incorporated into the sheets of the vesicles. They can be chosen from the group formed by fat-soluble sunscreens, substances intended to improve the condition of dry or senile skin, tocopherols, vitamins E, F or A and their esters, retinoic acid, antioxidants, essential fatty acids, glycyrrhetinic acid, keratolytics and carotenoids.
Les dispersions de sphérules lipidiques présentent l'intérêt de véhiculer des substances actives qui se trouvent ainsi masquées et protégées vis-à-vis des différents agents d'altération : oxydants et plus généralement composés réactifs vis-à-vis des substances actives encapsulées. La pénétration et la fixation des substances actives peuvent être modulées par la variation de la taille des sphérules et de leur charge électrique. L'action de ces substances actives peut également être ainsi différée (effet retard).The dispersions of lipid spherules have the advantage of carrying active substances which are thus masked and protected from the various spoilage agents: oxidants and more generally reactive compounds with respect to the encapsulated active substances. The penetration and fixation of the active substances can be modulated by varying the size of the spherules and their electrical charge. The action of these active substances can also be postponed (delay effect).
L'invention a enfin pour objet l'utilisation en cosmétique d'une dispersion aqueuse de sphérules constituée de couches moléculaires organisées de composés de formule (I) associés à d'autres lipides renfermant une phase aqueuse à encapsuler, en particulier pour le traitement de la peau.The subject of the invention is finally the use in cosmetics of an aqueous dispersion of spherules consisting of organized molecular layers of compounds of formula (I) associated with other lipids containing an aqueous phase to be encapsulated, in particular for the treatment of the skin.
L'invention a également pour objet l'utilisation d'une telle dispersion de sphérules lipidiques en dermatologie ou dans l'industrie alimentaire.The invention also relates to the use of such a dispersion of lipid spherules in dermatology or in the food industry.
Dans ce qui suit ou ce qui précède, les pourcentages sont donnés en poids, sauf mention contraire.In what follows or what precedes, the percentages are given by weight, unless otherwise stated.
Les exemples qui suivent sont donnés à titre illustratif et non limitatif. THF signifie tétrahydrofurane, M.A. signifie matière active.The examples which follow are given by way of illustration and without limitation. THF means tetrahydrofuran, M.A. means active ingredient.
Le 2-amino-octadécane-1,3,4-triol (2 isomères D,L-ribo, 450mg, 1,4.10-3 mole) est mis en suspension dans 25ml de tétrahydrofurane (THF). On ajoute en une fois le chlorure de palmitoyle (390mg, 1,4.10-3 mole). La triéthylamine (145mg, environ 1,4.10-3 mole) est coulée lentement. Au bout de 4 heures, il ne reste plus d'amine. Par addition d'eau, dans le milieu réactionnel on obtient un précipité.2-amino-octadecane-1,3,4-triol (2 isomers D, L-ribo, 450mg, 1,4.10 -3 mole) is suspended in 25ml of tetrahydrofuran (THF). Palmitoyl chloride (390 mg, 1.4 × 10 -3 mole) is added all at once. Triethylamine (145mg, about 1.4.10 -3 mole) is poured slowly. After 4 hours, there is no more amine. By addition of water, a precipitate is obtained in the reaction medium.
Après filtration, lavage et séchage, on obtient 730mg de solide blanc (rendement : 94%).After filtration, washing and drying, 730 mg of white solid are obtained (yield: 94%).
Le solide est recristallisé dans du méthanol et du THF, on récupère alors 400 mg de cristaux blancs (rendement : 51%). Le spectre RMN 13C, la spectrographie de masse et l'analyse élémentaire du produit obtenu sont conformes au produit attendu de point de fusion de 124-125°C :
le 2-N-hexadecanoylamino-octadécane-1,3,4-triol (2 isomères D,L-ribo)The solid is recrystallized from methanol and THF, 400 mg of white crystals are then recovered (yield: 51%). The 13C NMR spectrum, mass spectrography and elementary analysis of the product obtained are in accordance with the expected product, melting point 124-125 ° C:
2-N-hexadecanoylamino-octadecane-1,3,4-triol (2 isomers D, L-ribo)
L'acide D,L-2-hydroxy-hexadécanoïque (2g - 7,5.10-3 mole) est mis en suspension dans 50ml d'actérate d'éthyle. On ajoute rapidement du N-hydroxysuccinimide (0,8g - 7,5.10-3 mole) et du dicyclohexylcarbodiimide (1,5g - 7,5.10-3 mole). On agite 2 heures à température ambiante. On filtre la dicyclohexylurée. Le solide obtenu est remis en suspension dans 20 ml de THF et cette solution est coulée dans un mélange, 2-aminooctadécane-1,3,4-triol (2 isomères D,L-ribo - 2,4g - 7,5.10-3 mole) et 100ml de THF, la solution est portée à reflux. Après 1 heure de reflux, la réaction est arrêtée et on laisse revenir à température ambiante. On ajoute directement dans le milieu réactionnel 50g de silice.The D, L-2-hydroxy-hexadecanoic acid (2g - 7.5.10 -3 mole) is suspended in 50ml of ethyl actuate. N-hydroxysuccinimide (0.8 g - 7.5.10 -3 mole) and dicyclohexylcarbodiimide (1.5 g - 7.5.10 -3 mole) are rapidly added. The mixture is stirred for 2 hours at room temperature. The dicyclohexylurea is filtered. The solid obtained is resuspended in 20 ml of THF and this solution is poured into a mixture, 2-aminooctadecane-1,3,4-triol (2 isomers D, L-ribo - 2.4g - 7.5.10 -3 mole) and 100ml of THF, the solution is brought to reflux. After 1 hour of reflux, the reaction is stopped and the mixture is allowed to return to ambient temperature. 50 g of silica are added directly to the reaction medium.
Après filtration et évaporation à sec, on obtient 3,8 g de solide blanc (rendement 88%). On recristallise le produit obtenu dans un mélange éthanol/eau (9/1). On obtient alors 2,1 g de cristaux blancs (rendement 49%). Le spectre RMN 13C et l'analyse élémentaire du produit obtenu sont conformes au produit attendu de point de fusion de 134-136°C : le 2-N-(2-hydroxy-hexadecanoyl)-amino-octadécane-1,3,4-triol (4 isomères).After filtration and evaporation to dryness, 3.8 g of white solid are obtained (yield 88%). The product obtained is recrystallized from an ethanol / water mixture (9/1). 2.1 g of white crystals are then obtained (yield 49%). The 13C NMR spectrum and the elemental analysis of the product obtained are in accordance with the expected product with a melting point of 134-136 ° C: 2-N- (2-hydroxy-hexadecanoyl) -amino-octadecane-1,3,4 -triol (4 isomers).
L'acide béhénique (530mg - 1,6.10-3 mole) est mis en solution dans 10ml d'acétate d'éthyle. On ajoute rapidement le N-hydroxysuccinimide (175mg - 1,6.10-3 mole) et le dicyclohexyl-carbodiimide (325mg - 1,6.10-3 mole). On agite à température ambiante pendant 2 heures, on filtre la dicyclohexylurée et on évapore à sec.
Le solide obtenu est remis en solution dans 5ml de THF et coulé rapidement dans une solution de 2-amino-octadécane-1,3,4 - triol (2 isomères D,L-ribo - 500mg - 1,6.10-3 mole - 5ml de THF) au reflux. Ce reflux est maintenu 1 heure.The behenic acid (530 mg - 1.6.10 -3 mole) is dissolved in 10 ml of ethyl acetate. N-hydroxysuccinimide (175mg - 1.6.10 -3 mole) and dicyclohexyl-carbodiimide (325mg - 1.6.10 -3 mole) are quickly added. Stirred at room temperature for 2 hours, filter the dicyclohexylurea and evaporate to dryness.
The solid obtained is redissolved in 5ml of THF and quickly poured into a solution of 2-amino-octadecane-1,3,4 - triol (2 isomers D, L-ribo - 500mg - 1,6.10 -3 mole - 5ml THF) at reflux. This reflux is maintained for 1 hour.
On purifie par chromatographie, le milieu réactionnel étant déposé directement (l'éluant étant un mélange de dichloro1,2-éthane et d'isopropanol (80/20)). On isole ainsi 150 mg de solide blanc. Le spectre RMN 13C et l'analyse élémentaire du produit obtenu sont conformes au produit attendu de point de fusion de 123-124°C :
2-N-docosanoylamino-octadécane-1,3,4-triol (2 isomères D,L-ribo)Purification is carried out by chromatography, the reaction medium being deposited directly (the eluent being a mixture of dichloro1,2-ethane and isopropanol (80/20)). 150 mg of white solid are thus isolated. The 13C NMR spectrum and the elementary analysis of the product obtained are in accordance with the expected product, melting point 123-124 ° C:
2-N-docosanoylamino-octadecane-1,3,4-triol (2 isomers D, L-ribo)
0,5g de chlorhydrate de 2-amino-octadécane-1,3,4-triol (8 isomères - 1,4.10-3 mole) sont mis en solution dans 25 ml de THF. On ajoute rapidement un équivalent de triéthylamine, puis 0,39g de chlorure de palmitoyle (1,4.10-3 mole). On termine par l'addition d'un deuxième équivalent de base.0.5 g of 2-amino-octadecane-1,3,4-triol hydrochloride (8 isomers - 1,4.10 -3 mole) are dissolved in 25 ml of THF. An equivalent of triethylamine is quickly added, then 0.39 g of palmitoyl chloride (1.4.10 -3 mole). We end with the addition of a second basic equivalent.
Après une agitation de 30 minutes à température ambiante et par addition de deux volumes d'eau, on obtient un précipité blanc. Ce précipité est filtré, lavé avec de l'eau puis séché sous vide en présence de P2O5.After stirring for 30 minutes at room temperature and by adding two volumes of water, a white precipitate is obtained. This precipitate is filtered, washed with water and then dried under vacuum in the presence of P 2 O 5 .
Après recristallisation dans de l'acétate d'éthyle, on obtient 0,4g de poudre blanche. (rendemant 78%). Le spectre RMN 13C et l'analyse élémentaire du produit obtenu sont conformes au produit attendu :
2-N-hexadécanoylamino-octadécane-1,3,4-triol (8 isomères)After recrystallization from ethyl acetate, 0.4 g of white powder is obtained. (making 78%). The 13C NMR spectrum and the elementary analysis of the product obtained are in accordance with the expected product:
2-N-hexadecanoylamino-octadecane-1,3,4-triol (8 isomers)
2g d'acide D,L-2-hydroxyhexadécanoïque (7,5.10-3 mole) sont mis en solution dans 50ml d'acétate d'éthyle. On ajoute rapidement 0,8g de N-hydroxysuccinimide (7,5.10-3 mole) et 1,5g de dicyclohexylcarbodiimide (7,5.10-3 mole). On agite à température ambiante pendant 2 heures. On filtre la dicyclohexylurée et on concentre la solution sous vide. On redissout le solide ainsi obtenu dans 20ml de THF. On coule cette solution dans un réacteur contenant 2,4g de 2-amino-octadécane-1,3,4-triol ( 8 isomères - 7,5.10-3 mole) en solution dans 100ml de THF au reflux. Après une heure de reflux, on évapore à sec.2g of D, L-2-hydroxyhexadecanoic acid (7.5.10 -3 mole) are dissolved in 50ml of ethyl acetate. 0.8 g of N-hydroxysuccinimide (7.5.10 -3 mole) and 1.5 g of dicyclohexylcarbodiimide (7.5.10 -3 mole) are rapidly added. The mixture is stirred at ambient temperature for 2 hours. The dicyclohexylurea is filtered and the solution is concentrated in vacuo. The solid thus obtained is redissolved in 20 ml of THF. This solution is poured into a reactor containing 2.4 g of 2-amino-octadecane-1,3,4-triol (8 isomers - 7,5.10 -3 mole) in solution in 100 ml of THF at reflux. After one hour of reflux, evaporated to dryness.
On purifie le produit ainsi obtenu par chromatographie sur colonne de silice (l'éluant étant : dichloro-1,2-éthane (9)/méthanol(1)). 1g de solide blanc est isolé. Le spectre de masse du produit obtenu correspond à la structure attendue de point de fusion de 138-140°C : 2-N-(2-hydroxy-hexadécanoyl)-amino-octadécane-1,3,4-trio (16 isomères)The product thus obtained is purified by chromatography on a silica column (the eluent being: 1,2-dichloro-ethane (9) / methanol (1)). 1g of white solid is isolated. The mass spectrum of the product obtained corresponds to the expected melting point structure of 138-140 ° C: 2-N- (2-hydroxy-hexadecanoyl) -amino-octadecane-1,3,4-trio (16 isomers)
37g de 2-amino-octadécane-1,3,4-triol (8 isomères - 1,16.10-1 mole) sont mis en solution dans 300ml de THF. Quand le milieu est homogène, on refroidit vers 20°C. On ajoute rapidement 41,3g de chlorure d'acide de l'acide D,L-2-bromohexadécanoïque (1,16.10-1 mole) et 16,5ml de triéthylamine (1,16.10-1 mole). On chauffe à 50°C pendant 2 heures. On verse l'ensemble dans 2 litres d'eau glacée sous agitation. On laisse sous agitation pendant 1 heure. On filtre, on lave à l'eau puis on sèche.37g of 2-amino-octadecane-1,3,4-triol (8 isomers - 1,16.10 -1 mole) are dissolved in 300ml of THF. When the medium is homogeneous, it is cooled to around 20 ° C. 41.3 g of acid chloride of D-acid, L-2-bromohexadecanoic acid (1.16.10 -1 mole) and 16.5 ml of triethylamine (1.16.10 -1 mole) are rapidly added. The mixture is heated at 50 ° C for 2 hours. The whole is poured into 2 liters of ice water with stirring. The mixture is left stirring for 1 hour. It is filtered, washed with water and then dried.
On met ensuite en solution 73g de dérivé bromé obtenu (1,15.10-1 mole) dans 250ml de N-méthylpyrrolidone. On ajoute 22,5g d'acétate de potassium (2,3.10-1 mole). On chauffe à 90°C pendant 4 heures. On refroidit et on verse dans 2 litres d'eau glacée. On filtre rapidement, on relave, puis on sèche.73 g of the brominated derivative obtained (1.15 × 10 −1 mole) are then dissolved in 250 ml of N-methylpyrrolidone. 22.5 g of potassium acetate (2.3.10 -1 mole) are added. The mixture is heated at 90 ° C for 4 hours. Cool and pour into 2 liters of ice water. Filter quickly, rewash, then dry.
46g d'acétate obtenu (7,3. 10-2 mole) sont mis en solution dans 350ml de méthanol chaud. On laisse revenir à température ambiante et on ajoute 2,2ml d'une solution de méthylate de sodium à 30% dans le méthanol. Le milieu réactionnel est agité pendant 4 heures. On filtre, on sèche et recristallise dans le minimum d'heptane. On obtient 20g de solide très légèrement beige. L'analyse élémentaire du produit obtenu est conforme au produit attendu de point de fusion de 138-140°C (produit équivalent au produit obtenu par le procédé A) :
2-N-(2-hydroxy-hexadécanoyl)-amino-octadécane-1,3,4-trio (16 isomères)46 g of acetate obtained (7.3 × 10 -2 mole) are dissolved in 350 ml of hot methanol. The mixture is left to return to ambient temperature and 2.2 ml of a 30% sodium methylate solution in methanol are added. The reaction medium is stirred for 4 hours. It is filtered, dried and recrystallized from the minimum of heptane. 20 g of very slightly beige solid are obtained. The elemental analysis of the product obtained is in accordance with the expected product with a melting point of 138-140 ° C (product equivalent to the product obtained by process A):
2-N- (2-hydroxy-hexadecanoyl) -amino-octadecane-1,3,4-trio (16 isomers)
Cette exemple comprend différentes formulations pour le soin ou le traitement des cheveux avec des composés des exemples précédentsThis example includes different formulations for the care or treatment of hair with compounds of the previous examples.
Le shampooing ainsi formulé est d'aspect limpide
Le shampooing ainsi formulé est opalescent.
Cette exemple comprend différentes formulations pour le soin ou le traitement de la peau avec des composés des exemples précédentsThis example includes different formulations for caring for or treating the skin with compounds from the previous examples
Il a été réalisé un test comparatif des effets sur le lissage du cheveu d'une solution contenant le composé de l'exemple 4 à 1 % dans le tétrahydrofurane (THF), par rapport à un témoin constitué de THF, ou à une solution contenant, à la place du composé selon l'invention, soit l'isomère pur (forme D-ribo) correspondant au composé de l'exemple 4 (composé A), soit le composé de l'exemple 1 de la demande de brevet européen publiée sous le n°0 500 437 déposée par la Demanderesse (composé B).A comparative test was carried out of the effects on the smoothing of the hair of a solution containing the compound of Example 4 at 1% in tetrahydrofuran (THF), compared to a control consisting of THF, or to a solution containing , instead of the compound according to the invention, either the pure isomer ( D -ribo form) corresponding to the compound of Example 4 (compound A), or the compound of Example 1 of the published European patent application under the number 0 500 437 filed by the Applicant (compound B).
Ce test détermine le coefficient de frottement des cheveux par mesure de la force à appliquer à une masse témoin pour la faire glisser à vitesse constante sur deux cheveux tendus parallèlement. La mesure est effectuée en faisant glisser la masse de la racine vers la pointe des cheveux (R->P) et inversement (P->R).
Les résultats sont rassemblés dans le tableau 1 suivant.
The results are collated in Table 1 below.
L'application du composé selon l'invention (exemple 4) permet une nette diminution du coefficient de frottement, démontrant ainsi une amélioration du lissage ou du démêlage des cheveux.The application of the compound according to the invention (Example 4) allows a marked reduction in the coefficient of friction, thus demonstrating an improvement in the straightening or disentangling of the hair.
Le composé A, qui correspond à l'isomère pur, se dépose sur le cheveu (pas de possibilité de lissage) et rend ainsi impossible la mesure selon ce test lors d'un traitement non rincé, ce résultat montre donc un des inconvénients des produits sous forme d'un seul isomère.Compound A, which corresponds to the pure isomer, is deposited on the hair (no possibility of straightening) and thus makes measurement impossible according to this test during a leave-in treatment, this result therefore shows one of the drawbacks of the products. as a single isomer.
Cette mesure s'effectue à l'aide d'un évaporimètre (Servomed) qui détermine quantitativement l'évaporation d'eau, c'est-à-dire un transport d'eau par diffusion, à partir d'un échantillon de stratum corneum préalablement délipidé obturant une capsule cylindrique contenant de l'eau, le tout étant placé dans une chambre à température et humidité relatives contrôlées.This measurement is carried out using an evaporometer (Servomed) which quantitatively determines the evaporation of water, i.e. a transport of water by diffusion, from a sample of stratum corneum previously defatted sealing a cylindrical capsule containing water, the whole being placed in a chamber at controlled temperature and relative humidity.
Des capteurs permettent de mesurer la pression partielle de vapeur d'eau en deux points situés à des distances différentes de l'échantillon.Sensors make it possible to measure the partial pressure of water vapor at two points located at different distances from the sample.
On détermine ainsi le gradient de pression partielle de vapeur d'eau entre les deux points, et donc le taux d'évaporation conformément à la loi de Fick.The partial pressure vapor pressure gradient between the two points is thus determined, and therefore the rate of evaporation in accordance with Fick's law.
Il a été réalisé un test comparatif des effets sur la P.l.E. d'une solution contenant le composé de l'exemple 2 ou 4 dans le tétrahydrofuranne (THF), par rapport à une solution contenant, à la place du composé selon l'invention, soit l'isomère pur (forme D-ribo) correspondant au composé de l'exemple 4 (composé A), soit le composé de l'exemple 1 de la demande de brevet européen publiée sous le n°0 500 437 déposée par la Demanderesse (composé B).A comparative test was carried out of the effects on PlE of a solution containing the compound of Example 2 or 4 in tetrahydrofuran (THF), compared to a solution containing, in place of the compound according to the invention, either the pure isomer (form D -ribo) corresponding to the compound of Example 4 (compound A), or the compound of Example 1 of the European patent application published under the number 0 500 437 filed by the Applicant (compound B).
Les résultats sont rassemblés dans le tableau 2 suivant.
On constate donc que l'application des composés selon l'invention permet de réduire de manière significative l'évaporation de l'eau contenue dans le stratum corneum, démontrant ainsi des propriétés améliorées pour les composés selon l'invention de barrière de perméabilité à l'eau de stratum corneumIt can therefore be seen that the application of the compounds according to the invention makes it possible to significantly reduce the evaporation of the water contained in the stratum corneum, thus demonstrating improved properties for the compounds according to the invention of barrier to permeability to water. stratum corneum water
Claims (23)
les composés étant sous forme de mélanges d'isomères au moins sur la partie aminoalcool de ladite formule (I).Compounds corresponding to the formula:
the compounds being in the form of mixtures of isomers at least on the aminoalcohol part of said formula (I).
formule (II) dans laquelle R1 a la signification donnée dans les revendications précédentes, ou d'au moins un dérivé réactif de ces composés de formule (II), à l'aide d'au moins un agent acylant de formule (III) suivante :
R2-(CHX)n-CO-A (III)
formule (III) dans laquelle R2 et n ont les significations données dans les revendications précédentes et X représente un groupe choisi parmi le radical hydroxyle, un atome d'halogène, de préférence Br, Cl ou I, et un radical de formule OB susceptible de former un groupe OH ; A est un groupe choisi parmi un atome d'halogène, un radical de formule -O-COO-R5, un radical de formule OR6, un groupement
formula (II) in which R 1 has the meaning given in the preceding claims, or at least one reactive derivative of these compounds of formula (II), using at least one acylating agent of formula (III) next :
R 2 - (CHX) n -CO-A (III)
formula (III) in which R 2 and n have the meanings given in the preceding claims and X represents a group chosen from the hydroxyl radical, a halogen atom, preferably Br, Cl or I, and a radical of formula OB capable to form an OH group; A is a group chosen from a halogen atom, a radical of formula -O-COO-R 5 , a radical of formula OR 6 , a group
-Cl, un radical de formule -O-COO-C2H5, un radical de formule -OCH3 ou -OC2H5,
-Cl, a radical of formula -O-COO-C 2 H 5 , a radical of formula -OCH 3 or -OC 2 H 5 ,
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR9504050 | 1995-04-05 | ||
| FR9504050A FR2732680B1 (en) | 1995-04-05 | 1995-04-05 | CERAMID-LIKE COMPOUNDS, PROCESS FOR THEIR PREPARATION AND THEIR USE |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0736522A1 true EP0736522A1 (en) | 1996-10-09 |
| EP0736522B1 EP0736522B1 (en) | 1997-12-29 |
Family
ID=9477785
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP96400546A Expired - Lifetime EP0736522B1 (en) | 1995-04-05 | 1996-03-15 | Ceramide compounds, process for their preparation and their use in cosmetics or dermatology |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US5869711A (en) |
| EP (1) | EP0736522B1 (en) |
| JP (2) | JP3053765B2 (en) |
| CN (1) | CN1136555A (en) |
| AR (1) | AR003939A1 (en) |
| AT (1) | ATE161528T1 (en) |
| BR (1) | BR9601509A (en) |
| CA (1) | CA2173543C (en) |
| DE (1) | DE69600130T2 (en) |
| ES (1) | ES2113767T3 (en) |
| FR (1) | FR2732680B1 (en) |
| PL (1) | PL313633A1 (en) |
| RU (1) | RU2125984C1 (en) |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0856510A1 (en) * | 1997-01-31 | 1998-08-05 | Takasago International Corporation | Optically active ceramides and process for producing the same |
| US6251378B1 (en) | 1995-10-20 | 2001-06-26 | L'oreal | Process for decreasing the degradation of the color of oxidation dyed keratin fibers |
| US6312674B1 (en) | 1995-10-20 | 2001-11-06 | L'oreal | Oxidizing composition and novel method for perming or bleaching hair |
| EP1433464A2 (en) * | 2002-12-25 | 2004-06-30 | Kao Corporation | Hair cleansing composition |
| FR2874610A1 (en) * | 2004-09-02 | 2006-03-03 | Oreal | New N-dihydroxyalkyl-hydroxyalkanamide derivatives, useful in cosmetic and pharmaceutical compositions e.g. make-up |
| EP2111842A1 (en) | 2008-03-28 | 2009-10-28 | L'Oréal | Dyeing composition comprising ammonium chloride, method of colouring keratin fibres, and device |
| WO2012131623A2 (en) | 2011-03-31 | 2012-10-04 | L'oreal | Fractional cosmetic treatment process using a laser or microneedles |
| WO2013144871A1 (en) | 2012-03-27 | 2013-10-03 | L'oreal | Cosmetic process for caring for and/or making up keratin materials |
| EP2757090A1 (en) * | 2011-09-13 | 2014-07-23 | Kao Corporation | Method for producing n-acylamino triol |
| WO2014111669A2 (en) | 2013-01-18 | 2014-07-24 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and solid particles, and cosmetic treatment process |
| WO2014111668A2 (en) | 2013-01-18 | 2014-07-24 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and polyols, and cosmetic treatment method |
| WO2015152420A1 (en) | 2014-04-01 | 2015-10-08 | L'oreal | Composition in the form of nano- or micro- emulsion |
| EP2927211A4 (en) * | 2012-11-30 | 2016-07-20 | Amorepacific Corp | Novel pseudoceramide compound and production method for same |
| WO2018097303A1 (en) | 2016-11-28 | 2018-05-31 | L'oreal | Composition in the form of nano- or micro- emulsion |
| WO2019203133A1 (en) | 2018-04-16 | 2019-10-24 | L'oreal | Composition in the form of o/w type |
| US10548832B2 (en) | 2013-01-18 | 2020-02-04 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and polymer conditioning agents, and cosmetic treatment method |
| US11260000B2 (en) | 2014-06-25 | 2022-03-01 | L'oreal | Composition in the form of nano or micro emulsion or with lamellar structure |
| FR3134007A1 (en) | 2022-03-30 | 2023-10-06 | L'oreal | Composition for the care of keratinous materials |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU737402B2 (en) * | 1997-03-05 | 2001-08-16 | Dsm Ip Assets B.V. | Combination of erythrulose and a reducing sugar with self-tanning properties |
| JP2001510487A (en) * | 1997-12-05 | 2001-07-31 | デーエスエム ナムローゼ フェンノートシャップ | Composition comprising a combination of free sphingoid base and ceramide and use thereof |
| FR2776510B1 (en) | 1998-03-31 | 2002-11-29 | Oreal | POLYAMINO-ACID DERIVATIVES AND THEIR USE IN KERATIN FIBER TREATMENT COMPOSITIONS |
| KR100596595B1 (en) | 2000-04-04 | 2006-07-06 | 칼라 액세스, 인크. | Composition for Improving Skin Lipid Barrier Function |
| US6846940B2 (en) * | 2002-01-22 | 2005-01-25 | L'oreal | Ceramides, compositions thereof and methods of use thereof |
| FR2834986B1 (en) * | 2002-01-22 | 2004-02-20 | Oreal | NOVEL CERAMIDES, COMPOSITIONS CONTAINING THEM AND THEIR COSMETIC OR DERMATOLOGICAL USE |
| JP2006257006A (en) * | 2005-03-16 | 2006-09-28 | Tosoh F-Tech Inc | Method for producing allene having fluorine-containing alkyl group |
| CZ298319B6 (en) * | 2006-06-23 | 2007-08-22 | Univerzita Karlova v Praze, Farmaceutická fakultav Hradci Králové | Pseudoceramides and pharmaceutical and/or cosmetic compositions intended for application to skin in which the pseudoceramides are comprised |
| JP2009227593A (en) * | 2008-03-21 | 2009-10-08 | Daicel Chem Ind Ltd | Lamellar liquid-crystal type cosmetic composition |
| CN102058727B (en) * | 2009-11-16 | 2013-03-13 | 桂林莱茵生物科技股份有限公司 | Ceramide-containing tea seed extract and preparation method thereof |
| JP5825672B2 (en) | 2011-12-22 | 2015-12-02 | 高砂香料工業株式会社 | Method for producing high purity ceramides |
| EP4281575A1 (en) | 2021-01-20 | 2023-11-29 | Ajinomoto Co., Inc. | Method for producing phytosphingosine or phytoceramide |
| EP4282399A1 (en) | 2021-01-20 | 2023-11-29 | Ajinomoto Co., Inc. | Cosmetic composition |
| EP4281574A1 (en) | 2021-01-20 | 2023-11-29 | Ajinomoto Co., Inc. | Method for producing phytosphingosine or phytoceramide |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0500437A1 (en) * | 1991-02-21 | 1992-08-26 | L'oreal | Ceramides, method for their preparation and their application in cosmetics and dermopharmacy |
| WO1993020038A1 (en) * | 1992-04-03 | 1993-10-14 | Gist-Brocades N.V. | Selective n-acylation of amino alcohols |
| US5368857A (en) * | 1993-11-15 | 1994-11-29 | Elizabeth Arden Company, Division Of Conopco, Inc. | Ceramide cosmetic compositions |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ATE155771T1 (en) * | 1993-10-04 | 1997-08-15 | Quest Int | METHOD FOR PRODUCING CERAMIDES |
| FR2714829B1 (en) * | 1994-01-10 | 1996-02-02 | Oreal | Cosmetic and / or dermatological composition for the treatment of aging containing ceramides, its use. |
| US5461071A (en) * | 1994-10-21 | 1995-10-24 | Merck & Co., Inc. | Anti-fungal agents |
-
1995
- 1995-04-05 FR FR9504050A patent/FR2732680B1/en not_active Expired - Fee Related
-
1996
- 1996-03-15 ES ES96400546T patent/ES2113767T3/en not_active Expired - Lifetime
- 1996-03-15 DE DE69600130T patent/DE69600130T2/en not_active Expired - Lifetime
- 1996-03-15 AT AT96400546T patent/ATE161528T1/en not_active IP Right Cessation
- 1996-03-15 EP EP96400546A patent/EP0736522B1/en not_active Expired - Lifetime
- 1996-04-03 CN CN96101950A patent/CN1136555A/en active Pending
- 1996-04-03 AR ARP960102039A patent/AR003939A1/en unknown
- 1996-04-04 RU RU96106419A patent/RU2125984C1/en active
- 1996-04-04 BR BR9601509A patent/BR9601509A/en active Search and Examination
- 1996-04-04 CA CA002173543A patent/CA2173543C/en not_active Expired - Fee Related
- 1996-04-04 PL PL96313633A patent/PL313633A1/en unknown
- 1996-04-04 JP JP8082911A patent/JP3053765B2/en not_active Expired - Fee Related
- 1996-04-05 US US08/628,169 patent/US5869711A/en not_active Expired - Fee Related
-
1998
- 1998-11-12 US US09/189,837 patent/US6039963A/en not_active Expired - Fee Related
-
2000
- 2000-02-03 JP JP2000026697A patent/JP3570950B2/en not_active Expired - Lifetime
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0500437A1 (en) * | 1991-02-21 | 1992-08-26 | L'oreal | Ceramides, method for their preparation and their application in cosmetics and dermopharmacy |
| WO1993020038A1 (en) * | 1992-04-03 | 1993-10-14 | Gist-Brocades N.V. | Selective n-acylation of amino alcohols |
| US5368857A (en) * | 1993-11-15 | 1994-11-29 | Elizabeth Arden Company, Division Of Conopco, Inc. | Ceramide cosmetic compositions |
Non-Patent Citations (19)
| Title |
|---|
| ARCH. DERMATOL. RES., vol. 277, no. 4, 1985, pages 284 - 7 * |
| CHEMICAL ABSTRACTS, vol. 103, no. 5, 5 August 1985, Columbus, Ohio, US; abstract no. 35441, S. A. LONG page 337; XP002008993 * |
| CHEMICAL ABSTRACTS, vol. 112, no. 9, 26 February 1990, Columbus, Ohio, US; abstract no. 73760, OHNISHI ET AL. page 474; XP002008992 * |
| CHEMICAL ABSTRACTS, vol. 115, no. 17, 28 October 1991, Columbus, Ohio, US; abstract no. 179361, KOJIMA, MICHIYUKI ET AL. page 537; XP002008991 * |
| CHEMICAL ABSTRACTS, vol. 117, no. 3, 20 July 1992, Columbus, Ohio, US; abstract no. 25005, KOJIMA, MICHIYUKI ET AL. page 565; XP002008990 * |
| CHEMICAL ABSTRACTS, vol. 121, no. 15, 10 October 1994, Columbus, Ohio, US; abstract no. 175530, BERLINCH, ROBERTO GOMES DE SOUZA page 665; XP002008989 * |
| CHEMICAL ABSTRACTS, vol. 122, no. 21, 22 May 1995, Columbus, Ohio, US; abstract no. 261714, ROBSON, KRISTI J. ET AL: "6-Hydroxy-4-sphingenine in human epidermal ceramides" XP002008995 * |
| CHEMICAL ABSTRACTS, vol. 75, no. 13, 27 September 1971, Columbus, Ohio, US; abstract no. 85024, K. L. KARLSSON ET AL. page 78; XP002008994 * |
| J. AGRIC. FOOD CHEM., vol. 39, no. 10, 1991, pages 1709 - 14 * |
| J. LIPID RES. (1994), 35(11), 2060-8 * |
| J. LIPID RES., vol. 12, no. 4, 1971, pages 466 - 472 * |
| K. SISIDO ET AL.: "Syntheses of all of the racemic diastereoisomers of phytosphingosine", JOURNAL OF ORGANIC CHEMISTRY, vol. 35, no. 2, 1970, EASTON US, pages 350 - 353, XP002008985 * |
| K. SISIDO ET AL.: "Synthesis of racemic phytosphingosine and the lyxo isomer", JOURNAL OF ORGANIC CHEMISTRY, vol. 34, no. 11, 1969, EASTON US, pages 3539 - 3544, XP002008986 * |
| KULMACZ, RICHARD J. ET AL: "Sphingolipid base metabolism. Chemical syntheses and properties of N-acetyl derivatives of 4R-, 4S-, 5R-, and 5S-hydroxysphinganine", CHEM. PHYS. LIPIDS (1979), 23(4), 291-319, XP000576829 * |
| MORI, KENJI ET AL: "Synthesis of both 2,3-erythro- and 2,3-threo- isomers of aplidiasphingosine, a bioactive marine terpenoid", TETRAHEDRON LETT. (1981), 22(44), 4429-32, XP002008987 * |
| QUIM. NOVA, vol. 17, no. 2, 1994, pages 167 - 171 * |
| R. KRAUS, G. SPITELLER: "Ceramides from urtica dioica roots", LIEB. ANN. CHEM., 1991, pages 125 - 128, XP002008988 * |
| YUKAGAKU, vol. 38, no. 8, 1989, pages 647 - 53 * |
| YUKAGAKU, vol. 41, no. 3, 1992, pages 252 - 7 * |
Cited By (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6251378B1 (en) | 1995-10-20 | 2001-06-26 | L'oreal | Process for decreasing the degradation of the color of oxidation dyed keratin fibers |
| US6312674B1 (en) | 1995-10-20 | 2001-11-06 | L'oreal | Oxidizing composition and novel method for perming or bleaching hair |
| EP0856510A1 (en) * | 1997-01-31 | 1998-08-05 | Takasago International Corporation | Optically active ceramides and process for producing the same |
| EP1433464A2 (en) * | 2002-12-25 | 2004-06-30 | Kao Corporation | Hair cleansing composition |
| US7612141B2 (en) | 2002-12-25 | 2009-11-03 | Kao Corporation | Hair cleansing composition |
| EP1433464B1 (en) * | 2002-12-25 | 2011-08-24 | Kao Corporation | Hair cleansing composition |
| FR2874610A1 (en) * | 2004-09-02 | 2006-03-03 | Oreal | New N-dihydroxyalkyl-hydroxyalkanamide derivatives, useful in cosmetic and pharmaceutical compositions e.g. make-up |
| EP2111842A1 (en) | 2008-03-28 | 2009-10-28 | L'Oréal | Dyeing composition comprising ammonium chloride, method of colouring keratin fibres, and device |
| WO2012131623A2 (en) | 2011-03-31 | 2012-10-04 | L'oreal | Fractional cosmetic treatment process using a laser or microneedles |
| EP2757090A1 (en) * | 2011-09-13 | 2014-07-23 | Kao Corporation | Method for producing n-acylamino triol |
| EP2757090A4 (en) * | 2011-09-13 | 2015-03-04 | Kao Corp | Method for producing n-acylamino triol |
| WO2013144871A1 (en) | 2012-03-27 | 2013-10-03 | L'oreal | Cosmetic process for caring for and/or making up keratin materials |
| EP2927211A4 (en) * | 2012-11-30 | 2016-07-20 | Amorepacific Corp | Novel pseudoceramide compound and production method for same |
| US10555890B2 (en) | 2013-01-18 | 2020-02-11 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and solid particles, and cosmetic treatment process |
| WO2014111668A2 (en) | 2013-01-18 | 2014-07-24 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and polyols, and cosmetic treatment method |
| US10548832B2 (en) | 2013-01-18 | 2020-02-04 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and polymer conditioning agents, and cosmetic treatment method |
| WO2014111669A2 (en) | 2013-01-18 | 2014-07-24 | L'oreal | Flexible solid cosmetic composition comprising anionic surfactants and solid particles, and cosmetic treatment process |
| WO2015152420A1 (en) | 2014-04-01 | 2015-10-08 | L'oreal | Composition in the form of nano- or micro- emulsion |
| US10561587B2 (en) | 2014-04-01 | 2020-02-18 | L'oreal | Composition in the form of nano- or micro-emulsion |
| US11260000B2 (en) | 2014-06-25 | 2022-03-01 | L'oreal | Composition in the form of nano or micro emulsion or with lamellar structure |
| WO2018097303A1 (en) | 2016-11-28 | 2018-05-31 | L'oreal | Composition in the form of nano- or micro- emulsion |
| WO2019203133A1 (en) | 2018-04-16 | 2019-10-24 | L'oreal | Composition in the form of o/w type |
| FR3134007A1 (en) | 2022-03-30 | 2023-10-06 | L'oreal | Composition for the care of keratinous materials |
Also Published As
| Publication number | Publication date |
|---|---|
| CN1136555A (en) | 1996-11-27 |
| JP3053765B2 (en) | 2000-06-19 |
| RU2125984C1 (en) | 1999-02-10 |
| EP0736522B1 (en) | 1997-12-29 |
| AR003939A1 (en) | 1998-09-30 |
| ATE161528T1 (en) | 1998-01-15 |
| CA2173543C (en) | 2001-07-17 |
| BR9601509A (en) | 1998-03-17 |
| PL313633A1 (en) | 1996-10-14 |
| DE69600130D1 (en) | 1998-02-05 |
| JP2000186013A (en) | 2000-07-04 |
| CA2173543A1 (en) | 1996-10-06 |
| DE69600130T2 (en) | 1998-04-16 |
| JPH08283218A (en) | 1996-10-29 |
| US5869711A (en) | 1999-02-09 |
| FR2732680A1 (en) | 1996-10-11 |
| US6039963A (en) | 2000-03-21 |
| ES2113767T3 (en) | 1998-05-01 |
| JP3570950B2 (en) | 2004-09-29 |
| FR2732680B1 (en) | 1997-05-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0736522B1 (en) | Ceramide compounds, process for their preparation and their use in cosmetics or dermatology | |
| EP0647617B1 (en) | Ceramides, process for their preparation and their cosmetic uses | |
| CA2061692C (en) | Ceramides; process for preparing the same and their use as cosmetic and skin pharmaceutical agents | |
| CA2025790C (en) | New lipidic compositions derivated from spingosins, process for their preparation and uses, especially as cosmetics and dermatologic agents | |
| EP0915081B1 (en) | Ceramide compounds, process for their preparation and use | |
| FR2799650A1 (en) | PROCESS FOR LIMITING THE PENETRATION IN THE SKIN AND / OR KERATINIC FIBERS OF AN ACTIVE COSMETIC AND / OR PHARMACEUTICAL AGENT | |
| EP0942001B1 (en) | Sphingoid Lipid Compounds, a Process for Their Production and Cosmetic and Dermopharmaceutical uses Thereof | |
| EP0720847B1 (en) | Cosmetic or dermatological composition containing at least one ceramide 6 | |
| FR2874610A1 (en) | New N-dihydroxyalkyl-hydroxyalkanamide derivatives, useful in cosmetic and pharmaceutical compositions e.g. make-up |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| 17P | Request for examination filed |
Effective date: 19960812 |
|
| 17Q | First examination report despatched |
Effective date: 19961203 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19971229 Ref country code: FI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19971229 |
|
| REF | Corresponds to: |
Ref document number: 161528 Country of ref document: AT Date of ref document: 19980115 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed |
Owner name: JACOBACCI & PERANI S.P.A. |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19980109 |
|
| REF | Corresponds to: |
Ref document number: 69600130 Country of ref document: DE Date of ref document: 19980205 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19980330 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2113767 Country of ref document: ES Kind code of ref document: T3 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: 78136 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980714 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D Ref document number: 78136 Country of ref document: IE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980930 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20000307 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DK Payment date: 20000313 Year of fee payment: 5 Ref country code: CH Payment date: 20000313 Year of fee payment: 5 Ref country code: AT Payment date: 20000313 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20000330 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20000518 Year of fee payment: 5 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010315 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010315 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010316 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010331 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010331 |
|
| BERE | Be: lapsed |
Owner name: L' OREAL Effective date: 20010331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20011001 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 96400546.6 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EBP |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20011001 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20100317 Year of fee payment: 15 Ref country code: FR Payment date: 20100324 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20100310 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20100408 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20100415 Year of fee payment: 15 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20110315 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20111130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111001 Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110331 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 69600130 Country of ref document: DE Effective date: 20111001 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110315 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110315 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20120424 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20110316 |