EP0665201B1 - Gas generating mixture - Google Patents
Gas generating mixture Download PDFInfo
- Publication number
- EP0665201B1 EP0665201B1 EP94119382A EP94119382A EP0665201B1 EP 0665201 B1 EP0665201 B1 EP 0665201B1 EP 94119382 A EP94119382 A EP 94119382A EP 94119382 A EP94119382 A EP 94119382A EP 0665201 B1 EP0665201 B1 EP 0665201B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- catalyst
- mixture
- mixture according
- gzt
- oxides
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000203 mixture Substances 0.000 title claims description 28
- 239000003054 catalyst Substances 0.000 claims description 20
- 239000007789 gas Substances 0.000 claims description 9
- 239000000446 fuel Substances 0.000 claims description 7
- 239000007800 oxidant agent Substances 0.000 claims description 7
- IDCPFAYURAQKDZ-UHFFFAOYSA-N 1-nitroguanidine Chemical compound NC(=N)N[N+]([O-])=O IDCPFAYURAQKDZ-UHFFFAOYSA-N 0.000 claims description 5
- 229910044991 metal oxide Inorganic materials 0.000 claims description 5
- 150000004706 metal oxides Chemical group 0.000 claims description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 4
- 239000001301 oxygen Substances 0.000 claims description 4
- 229910052760 oxygen Inorganic materials 0.000 claims description 4
- 230000001590 oxidative effect Effects 0.000 claims description 3
- 239000011541 reaction mixture Substances 0.000 claims description 3
- VAIFDWHLVGCLFM-UHFFFAOYSA-N 3-nitro-4h-triazol-5-one Chemical compound [O-][N+](=O)N1CC(=O)N=N1 VAIFDWHLVGCLFM-UHFFFAOYSA-N 0.000 claims description 2
- 239000002826 coolant Substances 0.000 claims description 2
- UAGLZAPCOXRKPH-UHFFFAOYSA-N nitric acid;1,2,3-triaminoguanidine Chemical compound O[N+]([O-])=O.NNC(NN)=NN UAGLZAPCOXRKPH-UHFFFAOYSA-N 0.000 claims description 2
- 229910052723 transition metal Inorganic materials 0.000 claims description 2
- 150000003624 transition metals Chemical group 0.000 claims description 2
- XTVVROIMIGLXTD-UHFFFAOYSA-N copper(II) nitrate Chemical group [Cu+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O XTVVROIMIGLXTD-UHFFFAOYSA-N 0.000 claims 4
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims 2
- JKQOBWVOAYFWKG-UHFFFAOYSA-N molybdenum trioxide Chemical compound O=[Mo](=O)=O JKQOBWVOAYFWKG-UHFFFAOYSA-N 0.000 claims 2
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 claims 1
- 229910052751 metal Inorganic materials 0.000 claims 1
- 239000002184 metal Substances 0.000 claims 1
- 229910000314 transition metal oxide Inorganic materials 0.000 claims 1
- 238000002485 combustion reaction Methods 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 229910010413 TiO 2 Inorganic materials 0.000 description 1
- 229910000272 alkali metal oxide Inorganic materials 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 230000008094 contradictory effect Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 239000003344 environmental pollutant Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 231100000956 nontoxicity Toxicity 0.000 description 1
- 231100000719 pollutant Toxicity 0.000 description 1
- 239000002893 slag Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C06—EXPLOSIVES; MATCHES
- C06D—MEANS FOR GENERATING SMOKE OR MIST; GAS-ATTACK COMPOSITIONS; GENERATION OF GAS FOR BLASTING OR PROPULSION (CHEMICAL PART)
- C06D5/00—Generation of pressure gas, e.g. for blasting cartridges, starting cartridges, rockets
- C06D5/06—Generation of pressure gas, e.g. for blasting cartridges, starting cartridges, rockets by reaction of two or more solids
-
- C—CHEMISTRY; METALLURGY
- C06—EXPLOSIVES; MATCHES
- C06B—EXPLOSIVES OR THERMIC COMPOSITIONS; MANUFACTURE THEREOF; USE OF SINGLE SUBSTANCES AS EXPLOSIVES
- C06B23/00—Compositions characterised by non-explosive or non-thermic constituents
- C06B23/007—Ballistic modifiers, burning rate catalysts, burning rate depressing agents, e.g. for gas generating
Definitions
- the invention relates to a gas-generating mixture of a fuel, an oxidizer and a catalyst.
- Gas-generating mixtures of the aforementioned type - also called gas generator sets - are characterized in that they enable a high gas yield (> 14 mol / kg) during combustion. They are used for rocket and barrel weapon drives as well as for inflatable restraint (airbag) and rescue systems. In the civil sector in particular, thermal-mechanical insensitivity and non-toxicity of the starting mixtures, but also a lack of toxicity in the resulting gases are required. Many systems in use do not meet these requirements, or meet them only very inadequately.
- the invention has for its object to lower the combustion temperature and increase the rate of combustion in a mixture of the structure mentioned in the introduction.
- the oxidizer provided according to the invention results in cold and rapid combustion.
- the maximum pressure is reached within milliseconds, with the gas temperature remaining below harmful limits.
- Hitherto necessary slag formers, which in known systems for binding pollutants, e.g. Alkali oxides, required, can be omitted in the mixture according to the invention, so that a higher gas yield can be achieved.
- the catalyst which is also used according to the invention is primarily used to reduce harmful gas (CO and NO), the term "catalyst" in the broader sense here denoting an active reaction component which can be implemented itself and has a reaction-guiding and / or reaction-accelerating effect.
- these oxides act as oxygen donors.
- the catalytic effect in the conversion of harmful gases CO + 1/2 O 2nd ⁇ CO 2nd can be determined by the particle size distribution or the average particle size of the oxides below 25 ⁇ m should affect.
- the metal oxide catalyst but also the oxidizer are thermally and mechanically stable and especially not hygroscopic.
- Oxides or mixed oxides of the transition metals are particularly suitable as catalysts, but preferably V 2 O 5 / MoO 3 mixed oxides are used which contain portions of the thermally unstable phase V 2 O 4 , which can be prepared by partial reduction of V 2 O 5 . Further oxides, for example TiO 2 , can be used as promoters.
- N-rich and C-poor fuels include the well-known fuels TAGN (triaminoguanidine nitrate), NIGU (nitroguanidine), NTO (3-nitro-1,2,3-triazol-5-one) and the particularly high nitrogen content of the GZT (diguanidinium 5,5'- azotetrezolate) (DE 4 108 225).
- TAGN, NIGU, NTO, but in particular GZT are preferably used in the mixture according to the invention when used for rescue and restraint systems.
- a preferred mixture consists of GZT and Cu (NO 3 ) 2 * 3Cu (OH) 2 with a balanced oxygen balance and up to 30% by mass of the catalyst.
- the mixture can contain Fe 2 O 3 as the coolant, the oxidative properties of which can additionally be used in the reaction mixture (DE 41 33 655, EP 0 536 525).
- the combustion temperature can be determined very precisely by thermodynamic calculation. It is 2122 K. With the same GZT fuel and a balanced oxygen balance, other oxidizers deliver higher combustion temperatures. For example, they are - 2501 K for KNO 3 , 2850 K for NH 4 NO 3 and 3248 K for K Cl O 3 .
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Combustion & Propulsion (AREA)
- Catalysts (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Description
Die Erfindung betrifft eine gaserzeugende Mischung aus einem Brennstoff, einem Oxidator und einem Katalysator.The invention relates to a gas-generating mixture of a fuel, an oxidizer and a catalyst.
Gaserzeugende Mischungen der vorgenannten Art - auch Gasgeneratorsätze genannt - zeichnen sich dadurch aus, daß sie bei Verbrennung eine hohe Gasausbeute (> 14 mol/kg) ermöglichen. Sie werden für Raketen- und Rohrwaffenantriebe sowie für aufblasbare Rückhalte- (Airbag) und Rettungssysteme verwendet. Besonders im zivilen Bereich werden thermisch-mechanische Unempfindlichkeit und Ungiftigkeit der Ausgangsmischungen, aber auch fehlende Toxizität bei den entstehenden Gasen gefordert. Viele im Einsatz befindlichen Systeme erfüllen diese Forderungen nicht oder nur sehr unzulänglich.Gas-generating mixtures of the aforementioned type - also called gas generator sets - are characterized in that they enable a high gas yield (> 14 mol / kg) during combustion. They are used for rocket and barrel weapon drives as well as for inflatable restraint (airbag) and rescue systems. In the civil sector in particular, thermal-mechanical insensitivity and non-toxicity of the starting mixtures, but also a lack of toxicity in the resulting gases are required. Many systems in use do not meet these requirements, or meet them only very inadequately.
Die Reaktion dieser Brennstoffe mit den bisher eingesetzten Katalysatoren und Oxidatoren zeigen eine unbefriedigende Gaszusammensetzung und/oder ein ungenügendes Abbrandverhalten. Hinzu kommt, daß viele Reaktionsmischungen eine so hohe Verbrennungstemperatur besitzen, daß - bei Airbag-Anwendungen - die thermisch empfindlichen Sackmaterialien geschädigt werden.The reaction of these fuels with the previously used catalysts and oxidizers shows an unsatisfactory gas composition and / or inadequate combustion behavior. Add to that many Reaction mixtures have such a high combustion temperature that - in airbag applications - the thermally sensitive sack materials are damaged.
Der Erfindung liegt die Aufgabe zugrunde, bei einer Mischung des eingangs genannten Aufbaus, die Verbrennungstemperatur abzusenken und die Abbrandgeschwindigkeit zu erhöhen.The invention has for its object to lower the combustion temperature and increase the rate of combustion in a mixture of the structure mentioned in the introduction.
Diese an sich konträren Anforderungen werden erfindungsgemäß dadurch erfüllt, daß der Oxidator Cu(NO3)2 *3Cu(OH)2 und der Katalysator aus einen Metalloxid besteht.According to the invention, these contradictory requirements are met in that the oxidizer Cu (NO 3 ) 2 * 3Cu (OH) 2 and the catalyst consist of a metal oxide.
Durch den erfindungsgemäß vorgesehenen Oxidator ergibt sich eine kalte und schnelle Verbrennung. Der Maximaldruck wird innerhalb Millisekunden erreicht, wobei die Gastemperatur unterhalb schädlicher Grenzen bleibt. Bisher notwendige Schlackenbildner, die bei bekannten Systemen zur Bindung von Schadstoffen, z.B. Alkalioxiden, benötigt werden, können bei der erfindungsgemäßen Mischung entfallen, so daß eine höhere Gasausbeute erzielbar ist.The oxidizer provided according to the invention results in cold and rapid combustion. The maximum pressure is reached within milliseconds, with the gas temperature remaining below harmful limits. Hitherto necessary slag formers, which in known systems for binding pollutants, e.g. Alkali oxides, required, can be omitted in the mixture according to the invention, so that a higher gas yield can be achieved.
Der weiterhin erfindungsgemäß eingesetzte Katalysator dient vornehmlich der Schadgasreduzierung (CO und NO), wobei hier der Begriff "Katalysator" im erweiterten Sinn einen aktiven Reaktionsbestandteil bezeichnet, der selbst umgesetzt werden kann und reaktionslenkend und/oder reaktionsbeschleunigend wirkt. In einer durch die thermische Stabilität der Metalloxide bestimmten Phase der Reaktion wirken diese Oxide als Sauerstoff-Donatoren. Die katalytische Wirkung in der Schadgaskonvertierung
Besonders geeignet als Katalysator sind Oxide oder Mischoxide der Übergangsmetalle, vorzugsweise aber werden V2O5/MoO3-Mischoxide eingesetzt, die Anteile der thermisch instabilen Phase V2O4 enthalten, die durch Teilreduktion von V2O5 darstellbar ist. Weitere Oxide, z.B. TiO2, können als Promotoren eingesetzt werden.Oxides or mixed oxides of the transition metals are particularly suitable as catalysts, but preferably V 2 O 5 / MoO 3 mixed oxides are used which contain portions of the thermally unstable phase V 2 O 4 , which can be prepared by partial reduction of V 2 O 5 . Further oxides, for example TiO 2 , can be used as promoters.
Bei insbesondere zivilen Anwendungen werden ungiftige Ausgangsverbindungen und ungiftige Reaktionsprodukte gefordert. Diese Forderungen werden von N-reichen und C-armen Brennstoffen erfüllt. Hierzu zählen die bekannten Brennstoffe TAGN (triaminoguanidinnitrat), NIGU (Nitroguanidin), NTO (3-Nitro-1,2,3-triazol-5-on) und das sich durch besonders hohen Stickstoffgehalt auszeichnende GZT (Diguanidinium-5,5'-azotetrezolat) (DE 4 108 225). Es werden deshalb im Rahmen der erfindungsgemäßen Mischung bei Verwendung für Rettungs- und Rückhaltsysteme vorzugsweise TAGN, NIGU, NTO, insbesondere aber GZT eingesetzt.In civil applications in particular, non-toxic starting compounds and non-toxic reaction products are required. These requirements are met by N-rich and C-poor fuels. These include the well-known fuels TAGN (triaminoguanidine nitrate), NIGU (nitroguanidine), NTO (3-nitro-1,2,3-triazol-5-one) and the particularly high nitrogen content of the GZT (diguanidinium 5,5'- azotetrezolate) (DE 4 108 225). For this reason, TAGN, NIGU, NTO, but in particular GZT, are preferably used in the mixture according to the invention when used for rescue and restraint systems.
Eine bevorzugte Mischung besteht aus GZT und Cu(NO3)2*3Cu(OH)2 mit ausgeglichener Sauerstoffbilanz und bis zu 30 Mass.-% des Katalysators.A preferred mixture consists of GZT and Cu (NO 3 ) 2 * 3Cu (OH) 2 with a balanced oxygen balance and up to 30% by mass of the catalyst.
Die Mischung kann als Kühlmittel Fe2O3 enthalten, dessen oxidative Eigenschaften in der Reaktionsmischung zusätzlich genutzt werden können (DE 41 33 655, EP 0 536 525).The mixture can contain Fe 2 O 3 as the coolant, the oxidative properties of which can additionally be used in the reaction mixture (DE 41 33 655, EP 0 536 525).
Es wird eine Mischung bestehend aus GZT, einem Mischoxid aus V2O5 und MoO3 mit der Summenformel
V6Mo15O60 als Katalysator und
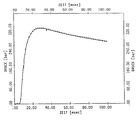
Cu(NO3)2*3Cu(OH)2 als Oxidator im Verhältnis 24,64 : 15,07 : 60,29 Mass.-% hergestellt. Diese Formulierung wird bezüglich ihres Anzünd- und Verbrennungsverhaltens experimentell in der ballistischen Bombe untersucht. Dabei wird ein Druckverlaufsdiagramm gemäß Anlage erhalten. Das Diagramm zeigt, daß die Mischung gute Anzünd- und Verbrennungseigenschaften besitzt. Bei einer Ladedichte von 0,1 g/cm3 liegt der maximale Druck im Bereich von 310 bar (31MPa), der nach etwa 28 ms erreicht wird (t(pmax) = 28 ms). Die Druckanstiegszeit zwischen 30 bis 80 % des Maximaldrucks beträgt t30-80 = 5,52 ms.It is a mixture consisting of GZT, a mixed oxide of V 2 O 5 and MoO 3 with the empirical formula
V 6 Mo 15 O 60 as a catalyst and
Cu (NO 3 ) 2 * 3Cu (OH) 2 as an oxidizer in a ratio of 24.64: 15.07: 60.29% by mass. This formulation is investigated experimentally in the ballistic bomb with regard to its ignition and combustion behavior. A pressure curve diagram according to the system is obtained. The diagram shows that the mixture has good ignition and combustion properties. With a loading density of 0.1 g / cm 3 , the maximum pressure is in the range of 310 bar (31MPa), which is reached after about 28 ms (t (pmax) = 28 ms). The pressure rise time between 30 to 80% of the maximum pressure is t 30-80 = 5.52 ms.
Die Verbrennungstemperatur läßt sich sehr exakt durch thermodynamische Berechnung ermitteln. Sie liegt bei 2122 K. Bei gleichem Brennstoff GZT und ausgeglichener Sauerstoffbilanz liefern andere Oxidatoren höhere Verbrennungs temperaturen. Beispielsweise liegen sie bei KNO3 bei - 2501 K, bei NH4 NO3 bei 2850 K und bei K Cl O3 bei 3248 K.The combustion temperature can be determined very precisely by thermodynamic calculation. It is 2122 K. With the same GZT fuel and a balanced oxygen balance, other oxidizers deliver higher combustion temperatures. For example, they are - 2501 K for KNO 3 , 2850 K for NH 4 NO 3 and 3248 K for K Cl O 3 .
Claims (12)
- A gas generating mixture of a fuel, an oxidant and a catalyst, characterized in that the oxidant is Cu(NO3)2*3Cu(OH)2 and the catalyst is a metal oxide.
- A mixture according to claim 1, characterized in that the catalyst is a metal oxide mixture.
- A mixture according to claim 1, characterized in that the catalyst is a metal mixed oxide.
- A mixture according to claim 1 or 2, characterized in that the catalyst is a mixture of transition metal oxides.
- A mixture according to claim 1 or 3, characterized in that the catalyst is a transition metal mixed oxide.
- A mixture according to claim 5, characterized in that the catalyst consists of V2O5/MoO3 mixed oxides.
- A mixture according to claim 6, characterized in that the catalyst contains proportions of the thermodynamically unstable V2O4 phase.
- A mixture according to any of claims 1 to 7, characterized in that the catalyst also contains TiO2.
- A mixture according to any of claims 1 to 8, characterized in that the catalyst has a mean grain size < 25 µm.
- A mixture according to any of claims 1 to 9, characterized in that TAGN (triaminoguanidine nitrate), NIGU (nitroguanidine), NTO (3-nitro-1,2,3-triazol-5-one) or GZT (diguanidinium-5,5'-azotetrazolate) serves as the fuel.
- A mixture according to any of claims 1 to 10, consisting of a mixture of GZT, Cu(NO3)2*3Cu(OH)2 with equalised oxygen balance and a catalyst content to the reaction mixture up to 30% by mass.
- A mixture according to any of claims 1 to 11, characterized in that it includes Fe2O3 as a coolant.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE4401213A DE4401213C1 (en) | 1994-01-18 | 1994-01-18 | Gas-generating mixture |
| DE4401213 | 1994-01-18 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0665201A1 EP0665201A1 (en) | 1995-08-02 |
| EP0665201B1 true EP0665201B1 (en) | 1996-11-20 |
Family
ID=6508091
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP94119382A Expired - Lifetime EP0665201B1 (en) | 1994-01-18 | 1994-12-08 | Gas generating mixture |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US5542998A (en) |
| EP (1) | EP0665201B1 (en) |
| DE (2) | DE4401213C1 (en) |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR960701817A (en) * | 1994-02-15 | 1996-03-28 | 다이셀 가가꾸 고교 가부시끼가이샤 | GAS GENERATOR COMPOSITION, PROCESS FOR PRODUCING TABLET THEREFROM, AND TRANSPORTATION METHOD |
| DE4442169C1 (en) * | 1994-11-26 | 1995-12-21 | Fraunhofer Ges Forschung | Non-toxic gas-generating mixt. with thermal-mechanical stability |
| DE4442170C1 (en) * | 1994-11-26 | 1995-12-21 | Fraunhofer Ges Forschung | Non-toxic gas-generating mixt. with thermal-mechanical stability |
| DE19531288A1 (en) * | 1995-08-25 | 1997-02-27 | Temic Bayern Chem Airbag Gmbh | Airbag propellant gas generator |
| US5608183A (en) * | 1996-03-15 | 1997-03-04 | Morton International, Inc. | Gas generant compositions containing amine nitrates plus basic copper (II) nitrate and/or cobalt(III) triammine trinitrate |
| US6497774B2 (en) | 1996-07-22 | 2002-12-24 | Daicel Chemical Industries, Ltd. | Gas generant for air bag |
| US6527886B1 (en) | 1996-07-22 | 2003-03-04 | Daicel Chemical Industries, Ltd. | Gas generant for air bag |
| US6306232B1 (en) | 1996-07-29 | 2001-10-23 | Automotive Systems Laboratory, Inc. | Thermally stable nonazide automotive airbag propellants |
| DE19712820A1 (en) * | 1997-03-26 | 1998-10-01 | Basf Ag | Burning moderators for gas-generating mixtures |
| DE29806504U1 (en) | 1998-04-08 | 1998-08-06 | TRW Airbag Systems GmbH & Co. KG, 84544 Aschau | Azide-free, gas generating composition |
| US6132538A (en) * | 1998-07-30 | 2000-10-17 | Autoliv Development Ab | High gas yield generant compositions |
| DE29821544U1 (en) | 1998-12-02 | 1999-02-18 | TRW Airbag Systems GmbH & Co. KG, 84544 Aschau | Azide-free, gas generating composition |
| JP2000319085A (en) * | 1999-04-30 | 2000-11-21 | Daicel Chem Ind Ltd | Gas generating agent composition |
| US6143102A (en) * | 1999-05-06 | 2000-11-07 | Autoliv Asp, Inc. | Burn rate-enhanced basic copper nitrate-containing gas generant compositions and methods |
| CN100465097C (en) * | 1999-09-27 | 2009-03-04 | 大赛璐化学工业株式会社 | Basic metal nitrate, method for producing the same and gas-generating agent composition |
| US6372191B1 (en) | 1999-12-03 | 2002-04-16 | Autoliv Asp, Inc. | Phase stabilized ammonium nitrate and method of making the same |
| US6224697B1 (en) | 1999-12-03 | 2001-05-01 | Autoliv Development Ab | Gas generant manufacture |
| US6436211B1 (en) | 2000-07-18 | 2002-08-20 | Autoliv Asp, Inc. | Gas generant manufacture |
| US6591752B2 (en) | 2001-02-12 | 2003-07-15 | Trw Inc. | Ignition material for an igniter |
| US6589375B2 (en) | 2001-03-02 | 2003-07-08 | Talley Defense Systems, Inc. | Low solids gas generant having a low flame temperature |
| US6875295B2 (en) | 2001-12-27 | 2005-04-05 | Trw Inc. | Cool burning gas generating material for a vehicle occupant protection apparatus |
| US6964716B2 (en) | 2002-09-12 | 2005-11-15 | Daicel Chemical Industries, Ltd. | Gas generating composition |
| US6872265B2 (en) | 2003-01-30 | 2005-03-29 | Autoliv Asp, Inc. | Phase-stabilized ammonium nitrate |
| DE112005000806T5 (en) * | 2004-03-29 | 2007-04-05 | Automotive Systems Laboratory, Inc., Armada | Gas generant and process for its preparation |
| US20130260090A1 (en) * | 2010-12-22 | 2013-10-03 | 3M Innovative Properties Company | Recessed adhesive binding systems and methods of making same |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2604391A (en) * | 1946-11-08 | 1952-07-22 | Ici Ltd | Gas-producing nondetonating composition |
| GB658643A (en) * | 1949-01-12 | 1951-10-10 | Alexander Cantlay Hutchison | Improvements in or relating to solid gas generating charges |
| US3664898A (en) * | 1969-08-04 | 1972-05-23 | Us Navy | Pyrotechnic composition |
| US4336085A (en) * | 1975-09-04 | 1982-06-22 | Walker Franklin E | Explosive composition with group VIII metal nitroso halide getter |
| US4632714A (en) * | 1985-09-19 | 1986-12-30 | Megabar Corporation | Microcellular composite energetic materials and method for making same |
| US4931112A (en) * | 1989-11-20 | 1990-06-05 | Morton International, Inc. | Gas generating compositions containing nitrotriazalone |
| US4994123A (en) * | 1990-05-29 | 1991-02-19 | The United States Of America As Represented By The Secretary Of The Air Force | Polymeric intermolecular emulsion explosive |
| US5145535A (en) * | 1991-02-25 | 1992-09-08 | United States Of America As Represented By The Secretary Of The Air Force | Method for intermolecular explosive with viscosity modifier |
| DE4108225C1 (en) * | 1991-03-14 | 1992-04-09 | Fraunhofer-Gesellschaft Zur Foerderung Der Angewandten Forschung Ev, 8000 Muenchen, De | |
| DE4218531C1 (en) * | 1991-10-11 | 1993-07-15 | Bayern-Chemie Gesellschaft Fuer Flugchemische Antriebe Mbh, 8261 Aschau, De |
-
1994
- 1994-01-18 DE DE4401213A patent/DE4401213C1/en not_active Expired - Fee Related
- 1994-12-08 DE DE59401081T patent/DE59401081D1/en not_active Expired - Lifetime
- 1994-12-08 EP EP94119382A patent/EP0665201B1/en not_active Expired - Lifetime
-
1995
- 1995-01-17 US US08/373,019 patent/US5542998A/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| US5542998A (en) | 1996-08-06 |
| EP0665201A1 (en) | 1995-08-02 |
| DE4401213C1 (en) | 1995-03-02 |
| DE59401081D1 (en) | 1997-01-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0665201B1 (en) | Gas generating mixture | |
| EP0666248B1 (en) | Gas generating mixture | |
| DE3602731C2 (en) | Composition for the production of nitrogen gas | |
| DE4108225C1 (en) | ||
| DE69001893T2 (en) | Composition and method for inflating a safety air bag. | |
| DE69730202T2 (en) | AZIDFREE, GAS-CREATING COMPOSITIONS | |
| DE69609791T2 (en) | CATALYST-CONTAINING, AZID-FREE GAS GENERATING COMPOSITIONS | |
| DE69431991T2 (en) | WATER-FREE GAS-GENERATING TETRAZOLE COMPOSITION AND METHOD FOR THE PRODUCTION THEREOF | |
| EP0716058B1 (en) | Gas generating mixture | |
| DE69609793T2 (en) | Heterogeneous gas-generating propellant charges | |
| EP0691317B1 (en) | Non-azide gas generant formulations | |
| DE69323410T2 (en) | GAS GENERATOR FOR AIRBAGS | |
| EP0519485A1 (en) | Propellant for gas generators | |
| DE2439771A1 (en) | PYROTECHNICAL MIXTURE | |
| DE19742203A1 (en) | Particle-free gas-generating mixture | |
| DE69305793T2 (en) | Pyrotechnic, a non-toxic hot gas generating composition and its use in a protective device for occupants of a motor vehicle | |
| DE4442170C1 (en) | Non-toxic gas-generating mixt. with thermal-mechanical stability | |
| DE10034287C2 (en) | Gas generating composition comprising guanyl urea dinitramide and its use | |
| EP0914306A1 (en) | Pyrotechnic mixture as propellant or a gas charge with carbon monoxide-reduced vapors | |
| DE19840993B4 (en) | Use of a gas-generating mixture as ignition mixture in a gas generator | |
| EP1162183B1 (en) | Ignition mixture for use in gas generators | |
| EP0716060B1 (en) | Gas generating mixture | |
| DE20111410U1 (en) | Nitrocellulose free gas generating composition | |
| DE60012933T2 (en) | COMPOSITE, GAS-PRODUCING MATERIAL FOR GAS-OPERATED VEHICLE SAFETY DEVICES | |
| EP1805123A2 (en) | Substance mixture as a thermally initiatable ignition mixture |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): DE FR GB IT NL SE |
|
| 17P | Request for examination filed |
Effective date: 19950826 |
|
| 17Q | First examination report despatched |
Effective date: 19951016 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): DE FR GB IT NL SE |
|
| ET | Fr: translation filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19961122 |
|
| REF | Corresponds to: |
Ref document number: 59401081 Country of ref document: DE Date of ref document: 19970102 |
|
| ITF | It: translation for a ep patent filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20131217 Year of fee payment: 20 Ref country code: GB Payment date: 20131217 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20131213 Year of fee payment: 20 Ref country code: NL Payment date: 20131216 Year of fee payment: 20 Ref country code: IT Payment date: 20131219 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20131217 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 59401081 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V4 Effective date: 20141208 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20141207 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20141207 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |