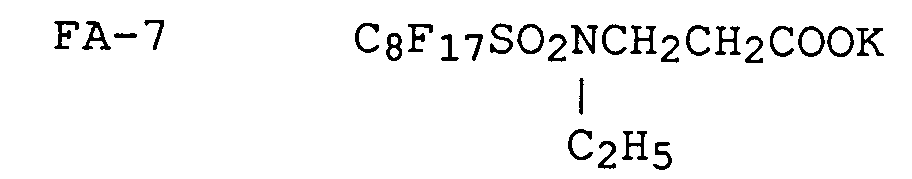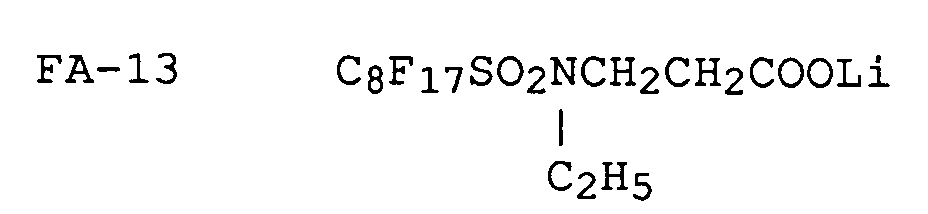Field of the Invention
-
This invention relates to a silver halide photographic light-sensitive material and particularly to the physical surface property improvements, under a highly humid conditions, on a silver halide photographic light-sensitive material having a silver halide emulsion layer containing silver chloride or a silver halide having a silver chloride content of not less than 90 mol%.
Background of the Invention
-
In a silver halide photographic light-sensitive material, a hydrophilic binder principally comprising gelatin is applied to a photographic component layer such as a light-sensitive layer, an intermediate layer and a protective layer. The above-mentioned hydrophilic binder principally comprising gelatin is excellent in dispersibility of silver halide grains and couplers and, at the same time, also excellent in industrial productivity of, for example, a simultaneous multilayer coating.
-
Usually, the above-mentioned hydrophilic binder principally comprising gelatin is cross-linked by a crosslinking agent, so that the layer strength can be increased. However, there has been such a problem that photographic component layers such as a light-sensitive layer, an intermediate layer and a protective layer are liable to be affected by moisture, because the above-mentioned binder is intrinsically hydrophilic, that is to say, for example, such a problem is raised that, when the photographic component layer is put in highly humid conditions, silver halide photographic light-sensitive materials may adhere to each other.
-
As for the measures for improving a physical surface property under highly humid conditions, U.S. Patent No. 4,426,431 and Japanese Patent Publication Open to Public Inspection (hereinafter referred to as JP OPI Publication) Nos.62-264043/1987 and 3-212640/1991 describe respectively that a light-sensitive material is developed and dried, and a protective coated-layer is additionally provided to the top of a protective layer.
-
In the above-mentioned measures, however, not only an additional protective coated-layer has to be provided with the increase of the production load after completing a developing step, but also any problems raised before development cannot be solved.
-
JP OPI Publication Nos. 48-101118/1973, 58-200235/1983, 60-128434/1985 and 2-12145/1990 describe respectively of the attempts for improving a slidability, static chargeability and coatability, by applying a cationic fluorine type surfactant to a silver halide photographic light-sensitive material.
-
When a silver iodobromide emulsion is used as a silver halide emulsion of a light-sensitive material, a physical surface property can be improved under highly humid conditions by making use of a fluorinated cationic surfactant of which the above-given patent publications concretely describe and any particular problem have not been raised. However, when the silver halide emulsion is a silver chloride emulsion or a silver halide emulsion having a high silver chloride content, it was found that the preservation stability of the emulsion was seriously deteriorated when making use of a cationic fluorine type surfactant such as described in the above-given patent publication, although the fluorinated cationic surfactant was not added directly to an emulsion layer, but to a protective layer.
-
When applying the above-mentioned technique to a silver chloride emulsion or a highly silver chloride containing silver halide emulsion with which has widely been applied to a color paper and a film for printing use, there has raised such a serious problem that the preservation stability of the emulsion has seriously been deteriorated.
-
The problem of seriously deteriorating the preservation stability of an emulsion has not been raised when a fluorinated anionic surfactant is used. For example, in the example given in JP OPI Publication No. 2-12145/1990, there is the description of the example in which a silver chloride emulsion and an anionic fluorine type surfactant are used in combination. There does not produce any such a problem as mentioned above in the example. However, the physical properties of light-sensitive material under a high humidity can not be improved by such types of surfactant.
-
Example 1 of JP OPI Publication No. 54-14224/1979 discloses an example in which a silver chlorobromide emulsion and a fluorinated anionic surfactant or a fluorinated betaine surfactant were used in combination. However, there is not such a problem as mentioned above produced in the example.
-
Accordingly, it is presumed that a preservation stability should be deteriorated by a cationic fluorine type surfactant when making use of a silver chloride emulsion or a silver halide emulsion having a high silver chloride content. The inventors have discovered that a physical surface property can be improved under highly humid conditions without deteriorating the preservability of a silver chloride emulsion or a silver halide emulsion having a high silver chloride content, when a fluorinated cationic surfactant having a specific structure is used.
Summary of the Invention
-
An object of the invention to improve the physical surface property of a silver halide photographic light-sensitive material comprising a silver halide emulsion layer having a high silver chloride grain content and to provide a preservation stability excellent to a silver halide emulsion having a high silver chloride grain content.
-
The silver halide photographic light-sensitive material of the invention comprises a support having thereon a photographic layer which comprises a silver halide emulsion layer and optionally a hydrophilic colloid layer in which the silver halide emulsion layer comprises silver halide grains having a silver chloride content of not less than 90 mol % and the outermost layer provided at the position farthest from the surface of the support of said photographic layer contains a fluorinated cationic surfactant represented by formula I;
wherein R₂ is a hydrogen atom or an alkyl group having 1 to 5 carbon atoms; R₃, R₄ and R₅ are each independently a hydrogen atom, an alkyl group having 1 to 5 carbon atoms or a hydroxyalkyl group; X⁻ is Br⁻, Cl⁻, CH₃SO₃⁻, CH₃-C₆H₄-SO₃⁻, CH₃COO⁻, NO₃⁻ or C₆H₅-SO₃⁻; n is an integer of 1 to 10; m is an integer of 1 to 6; ℓ is 0 or 1; and k is an integer of 0 to 5, provided that k is not 0 when ℓ is 1.
-
In a preferable enbodiment of the invention, the outermost layer further contains a fluorinated anionic surfactant rprsented by the following Formula II;
wherein R₁ is a hydrogen atom or an alkyl group having 1 to 5 carbon atoms; A is a methylene group, B is a bivalent linking group; D is -COOM or -SO₃M, in which M is a cation; x is an integer of 3 to 20; and y is an integer of 0 to 10.
Detailed Description of the Invention
-
In a silver halide emulsion layer (hereinafter referred to as a silver halide emulsion layer of the invention) containing silver chloride or a silver halide having a silver chloride content of not less than 90 mol% (hereinafter collectively referred to as a silver halide of the invention), the silver bromide content is preferably not more than 10 mol% and the silver iodide content is preferably not more than 0.5 mol% and silver chlorobromide having a silver bromide content within the range of 0.1 to 2 mol% is more preferable.
-
In a silver halide emulsion layer of the invention, silver halide grains of the invention may be used independently or may also be used upon mixing with silver halide grains of the invention having the different composition therefrom. It is further allowed to use silver halide grains of the invention upon mixing with any silver halide grains other than the silver halide grains of the invention.
-
When a silver halide emulsion layer of the invention contains silver halide- grains other than the silver halide grains of the invention, the silver halide grains of the invention are to occupy, preferably not less than 60% by weight of the whole silver halide grain and, more preferably not less than 80% by weight thereof.
-
Silver halide grains of the invention may be uniform in the silver halide composition all through from the inside of the grains to the outside thereof, or may also be different in the silver halide composition between the inside of the grains and the outside thereof. When the silver halide composition is different between the inside of the grains and the outside thereof, the silver halide composition may be varied continuously or may also be varied discontinuously.
-
There is no special limitation to the grain-sizes of silver halide grains of the invention. However, taking other photographic characteristics such as a rapid processability and a photosensitivity into consideration, the average grain-size thereof is to be within the range of, preferably 0.2 to 1.6µm and, more preferably 0.25 to 1.2µm.
-
Silver halide grains applicable to an emulsion of the invention may be those prepared in any one of the acidic process, neutral process and ammoniacal process. The grains may also be grown up at the same time or may be grown up after seed grains are prepared. The processes of preparing and growing seed grains may be the same with or the different from each other.
-
The systems for reacting a soluble silver salt and a soluble halide salt together may include, for example, an normal precipitation system, a reversal precipitation system, a multiple jet precipitation system and the combination thereof. The reactant obtained in the multiple jet precipitation system is preferred. Further, as one of the multiple jet precipitation systems, it is also allowed to use a pAg-controlled·double jet precipitation system of which detailed, for example, in JP OPI Publication No. 54-48521/1979.
-
If required, it is also allowed to make use of a silver halide solvent such as thioether. It is further allowed to add a mercapto group-containing compound and a nitrogen-containing heterocyclic compound or a compound such as a sensitizing dye either when or after forming silver halide grains.
-
Silver halide grains of the invention may be used in any configuration. One of the preferable examples thereof is a cube having a (100) plane as the crystal surface thereof. In the processes described in U.S. Patent Nos. 4,183,756 and 4,225,666, JP OPI Publication No. 55-26589/1980, JP Examined Publication No. 55-42737/1980, The journal of Photographic Science, 21, 39, (1973), and so forth, a grain having an octahedral, tetradecahedral or dodecahedral configuration is prepared, so that it can be used. In addition to the above, a grain having a twinned crystal surface may also be used. Silver halide grains applicable to the invention may be those having a single configuration or may also be those having a mixture of grains having various configurations.
-
In the invention, a metal ion is added by making use of a cadmium salt; a zinc salt, a lead salt, a thallium salts, an iridium salt including the complexed salts thereof, a rhodium salt including the complexed salts thereof or an iron salt including the complexed salts thereof in the course of forming silver halide grains and/or in the course of growing them, so that the metal ion can be contained inside the grains and/or in the surfaces of the grains. Besides the above, reduction-sensitization nuclei can also be provided to the inside and/or surface of grains by suitably putting them in the reductional atmospheric conditions.
-
From an emulsion containing silver halide grains of the invention, any useless soluble salts may be removed after completing the growth of silver halide grains. Or, such a useless salt as mentioned above may still be contained therein. The useless soluble salts may be removed by following the method described in Research Disclosure No. 17643.
-
In the invention, Silver halide grains applicable to an emulsion of the invention may be those capable of forming a latent image mainly on the surfaces of the grains, or may also be those capable of forming a latent image mainly inside the grains. The silver halide grains applicable to an emulsion of the invention include,-preferably, those capable of forming a latent image mainly on the surfaces thereof.
-
In the invention, an emulsion is to be chemically sensitized in an ordinary method. A chemical sensitization can be performed in, for example, a sulfur sensitization in which a sulfur-containing compound capable of reacting with silver ions or an active gelatin, a selenium sensitization in which a selenium compound is used, a reduction sensitization in which a reducible substance is used, and a noble-metal sensitization in which a gold compound or other noble metal compound is used. The above-given sensitization methods may be used independently or in combination. Among the above-given chemical sensitization methods, it is preferable to carry out a chemical sensitization in a sulfur-sensitization independently or in combination thereof with a noble-metal sensitization.
-
The sulfur sensitizers applicable to a sulfur sensitization include, for example, a thiosulfate, allyl thiocarbazide, thiourea, allyl isothiocyanate, cystine, p-toluene thiosulfinate and rhodanine. Besides the above, it is also allowed to use the sulfur sensitizers given in, for example, U.S. Patent Nos. 1,574,944, 2,410,689, 2,278,947, 2,728,668, 3,501,313 and 3,656,955, West German (OLS) Patent No. 1,422,869, and JP OPI Publication Nos. 56-24937/1981 and 55-45016/1980.
-
As a rough standard, a sulfur sensitizer may be added in an amount within the range of 10⁻⁷ mols to 10⁻¹ mols per mol of silver halide used, provided, however, that the amount thereof added may be varied extending over a wide range according to various conditions such as a pH, a temperature and a silver halide grain size.
-
As for the gold sensitizers, various gold compounds each having a gold oxidation number of +1 valency or +3 valency may be used. The typical gold sensitizers include, for example, chloroaurate, potassium chloroaurate, auric trichloride, potassium auric thiocyanate, potassium iodoaurate, tetracyanoauric acid, ammonium aurothiocyanate and pyridyl trichlorogold.
-
A gold sensitizer may be added in an amount within the range of, preferably 5x10⁻⁷ to 5x10⁻³ mols per mol of silver halide used and, more preferably 2x10⁻⁶ to 1x10⁻⁴ mols, provided, however, that the amounts thereof to be added may be varied according to various conditions.
-
Silver halide grains of the invention may be chemically sensitized even in the presence of elemental sulfur. The term, elemental sulfur, stated herein means the so-called simple sulfur substance that does not form any compound in combination with other elements. However, elemental sulfur used in the invention does not include a sulfur-containing compound which has been well-known as a photographic additive in the field of the art, such as a sulfide, sulfuric acid or the salts thereof, sulfurous acid or the salts thereof, thiosulfuric acid or the salts thereof, sulfonic acid or the salts thereof, a thioether compound, a thiourea compound, a mercapto compound and a sulfur-containing heterocyclic compound.
-
It has been known that elemental sulfur has some allotropes. Any one of the allotropes can also be used in the invention, as elemental sulfur.
-
Among the above-mentioned allotropes, those stable at room temperature include, for example, α-sulfur belonging to a rhombic system. It is preferable in the invention to make use of α-sulfur.
-
When making use of elemental sulfur as a sensitizer, it is allowed to make use of it in the solid state, but it is preferable to make use of it in the.liquid state. It has been known that elemental sulfur is insoluble to water, but is soluble to a solvent such as carbon disulfide, sulfur chloride, benzene, diethyl ether or ethanol. It is preferable to add elemental sulfur to an emulsion upon dissolving it in the above-given solvent. However, taking handling conveniences and photographic influences into consideration, it is particularly preferable to make use of ethanol among the above-given solvents.
-
An amount of elemental sulfur to be added to an emulsion is within the range of 1x10⁻⁵ mg to 10 mg per mol of silver halide used and, preferably 1x10⁻³ mg to 5 mg thereto; provided that a suitable amount thereof may be varied according to the kinds of silver halide emulsions applied and to how much effects to be expected.
-
Elemental sulfur may be added in any step selected from the silver halide photographic light-sensitive material preparation steps consisting of a silver halide grain formation step, a chemical sensitization step or sometimes called a chemical ripening step, a coating solution preparation step and a coating/drying step.
-
When adding elemental sulfur in carrying out a silver halide grain formation step, the "inorganic sulfur" may be added before nucleating a silver halide crystal, or may also be added after completing the nucleation. It is also allowed to add elemental sulfur before growing a crystal and the crystal is then grown up in the presence of elemental sulfur. It is further allowed to add it before or after removing an excess salt upon completion of a crystal growth.
-
When adding elemental sulfur in carrying out a chemical sensitization step, the "inorganic sulfur" may be added at any point of time selected from the series of time consisting of the point of time when starting the chemical sensitization, that is, the point of time when adding a chemical sensitizer, in the course of carrying out the chemical sensitization, and the point of time when completing the chemical sensitization, that is, the point of time when adding a chemical sensitization stopping agent.
-
When adding elemental sulfur in carrying out a coating solution preparation step; provided, in the coating solution preparation step, a coating solution is prepared in the following manner; a coupler dispersing solution and, if required, various kinds of additives such as an aqueous gelatin solution, a surfactant, a thickener, a layer hardener, a dye and a development inhibitor are mixed up together, so that the coating solution can be prepared; elemental sulfur can be added at any point of time from the completion of the chemical sensitization to the coating operation.
-
It is particularly preferable that elemental sulfur is added in the initial stage of a chemical sensitization step and a chemical sensitization and/or a spectral sensitization are carried out in the presence of elemental sulfur, or that elemental sulfur is added at the point of time when completing a chemical sensitization.
-
The above-mentioned chemical sensitization step includes a chemical sensitization starting step and a chemical sensitization stopping step. The chemical sensitization starting step means a step for adding a chemical sensitizer, wherein the point of time when adding a chemical sensitizer is the point of time when starting a chemical sensitization. The chemical sensitization stopping step means a step from the point of time when adding a chemical sensitizer to the point of time when a chemical sensitization is stopped in reaction. When adding elemental sulfur in the course of carrying out a chemical sensitization stopping step, it may be added at any point of time of substantially carrying out a chemical sensitization stopping step. To be more concrete, elemental sulfur may be added at the same time when a chemical sensitizer is added (that is, at the point of time when a chemical sensitization is stopped in reaction), or at any point of time within 10 minutes before or after the chemical sensitization is stopped in reaction and, preferably, at the same time or within 5 minutes before or after the chemical sensitization is stopped in reaction.
-
To an emulsion applicable to the invention, a sensitizing dye may be applied. Particularly preferable sensitizing dyes include, for example, cyanine dye, merocyanine dye and compound merocyanine dye. To these dyes, any nucleus commonly utilized in a cyanine dye may be applied as a basic heterocyclic nucleus, including, for example, nuclei of pyrroline, oxazoline, thiazoline, pyrrole, oxazole, thiazole, selenazole, imidazole, tetrazole, pyridine or a nucleus of an aliphatic or aromatic hydrocarbon ring condensed with each of the above-given nuclei, which include, namely, a nucleus of indolenine, benzindolenine, indole, benzoxozole, naphthoxazole, benzothiazole, naphthothiazole, benzoselenazole, banzimidazole or quinoline. The above-given nuclei may also have each a substituent on the carbon atom thereof.
-
To a merocyanine dye or a compound merocyanine dye, it is allowed to apply a 5- or 6-membered heterocyclic nucleus including, for example, a nucleus of pyrazoline-5-one, thiohydantoin, 2-thioxazolidine-2,4-dione, thiazolidine-2,4-dione, rhodanine or thiobarbituric acid.
-
The above-mentioned sensitizing dyes may be added in any manner having been well-known in the art. For example, such a sensitizing dye as mentioned above is dissolved in a water-soluble solvent such as pyridine, methyl alcohol, ethyl alcohol, methyl cellosolve, acetone or the mixture of such a solvent as given above, dissolved with water in some instance, or dissolved in water in another instance, so that it may be added in the solvent solution. It is also advantageous to make use of a supersonic vibration for dissolving a sensitizing dye. It is further allowed to make use of such a manner; that a sensitizing dye is dissolved in a volatile organic solvent and the resulting solution is dispersed in a hydrophilic colloid, and the resulting dispersion is then added, as described in U.S. Patent No. 3,469,987; and that a sensitizing dye is dispersed in a water-soluble solvent without dissolving a water-insoluble dye and the resulting dispersion is added, as described in JP Examined Publication No. 46-24185/1971. In addition to the above, it is allowed to add to an emulsion a sensitizing dye applicable to the invention in the form of a dispersion prepared in an acidic dissolution/dispersion process. Besides the above, it is also allowed to use the methods described in U.S. Patent Nos. 2,912,345, 3,342,605, 2,996,287 and 3,425,835.
-
Sensitizing dyes which are to be contained in a silver halide emulsion of the invention are each dissolved in the same or the different solvents so as to prepare the sensitizing dye solutions, respectively. In advance of adding the solutions to the silver halide emulsion, the resulting sensitizing dye solutions may be mixedly or separately added to the silver halide emulsion. When adding the sensitizing solutions separately, the adding order, adding time and adding interval may freely be determined so as to meet the purposes. The point of time when adding the sensitizing dyes applicable to the invention may be at any point of time in the course of carrying out an emulsion preparation step. However, they may be added preferably in the course of carrying out or after completing a chemical ripening step and, further preferably in the course of carrying out a chemical ripening step.
-
Together with the above-mentioned sensitizing dyes, it is allowed to use a dye not having any spectral sensitizing function in itself or a substance capable of showing a supersensitizability, that is a substance incapable of substantially absorbing any visible rays of light. The above-mentioned dyes or the substances include, for example, an aromatic organic acid formaldehyde condensate (such as those given in U.S. Patent No. 3,437,510), a cadmium salt, an azaindene compound, and an aminostilbene compound substituted with a nitrogen-containing heterocyclic group (such as those given in U.S. Patent No. 2,933,390). Besides, the combinations described in U.S. Patent Nos. 3,615,613, 3,615,641, 3,617,295 and 3,635,721 are particularly useful.
-
Now, a fluorinated cationic surfactant applicable to the invention will be detailed.
-
A fluorinated cationic surfactant applicable to the invention can be represented by the following formula [I].
wherein R₂ represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms; R₃, R₄ and R₅ represent each a hydrogen atom, an alkyl group having 1 to 5 carbon atoms or a hydroxyalkyl group, provided that R₃, R₄ and R₅ may be the same with or the different from each other; X⁻ represents Br⁻, Cl⁻, CH₃SO₃⁻, CH₃-C₆H₄-SO₃⁻, CH₃COO⁻, NO₃⁻ or C₆H₅-SO₃⁻; n is an integer of 1 to 10, m is an integer of 1 to 6; ℓ is an integer of 0 or 1; and k is an integer of 0 to 5; provided that k is not 0 whenever ℓ is 1.
A lower alkyl group having 1 to 5 carbon atoms, represented by R₂ includes, for example, a methyl, ethyl, propyl, butyl or pentyl group;
A lower alkyl group having 1 to 5 carbon atoms, represented by R₃, R₄ and R₅ includes, for example, a methyl, ethyl, propyl, or butyl group;
A hydroxyalkyl group represented by R₃, R₄, and R₅ includes, for example, a hydroxymethyl, hydroxyethyl, hydroxypropyl or hydroxybutyl group; and
R₃, R₄ and R₅ is independent from each other, and they may also be the same with or the different from each other.
-
In the preferable inbodiment of the invention. X⁻ is Br⁻ or Cl⁻ and R₃, R₄ and R₅ are each an alkyl group.
-
Now, the typical examples of cationic fluorine type surfactants applicable to the invention, represented by Formula [I], will be given below.
-
In the invention, it is preferable to further use an anionic fluorine-containing surfactant together with a cationic fluorine-containing surfactant in combination, because the effects expected to the invention can become remarkable. A particularly preferable fluorinated anionic surfactant is represented by the following formula [II];
wherein R₁ represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms; A represents a methylene group, B represents a divalent bonding group; D represents -COOM or -SO₃M in which M represents a cation; x is an integer of 3 to 20; and y is an integer of 0 to 10.
-
A lower alkyl group having 1 to 5 carbon atoms, represented by R₁, include, for example, a methyl, ethyl, propyl, butyl or pentyl group.
-
A divalent bonding group represented by B include, for example,
-(CH₂)p-, -(CH₂)p-O-(CH₂)r-, -(CH₂)p-(CH₂CH₂O)q-(CH₂)r-, -(CH₂)p-(CHOH)s-(CH₂)r-, and -(CH₂CHOH)t-(CH₂O)u-; in which p,r,t and u are each an integer of 0 to 8 and p and s are each an integer of 1 to 20.
-
A cation represented by M include, for example, an alkali-metal ion and a quaternary ammonium ion.
-
Now, the typical examples of fluorinated anionic surfactants applicable to the invention and represented by formula [II] will be given below.
-
A fluorinated cationic surfactant represented by formula [I] and a fluorinated anionic surfactant represented by formula [II] may be synthesized in any one of the processes described in, for example, U.S. Patent Nos. 2,559,751, 2,567,011, 2,732,398, 2,764,602, 2,806,866, 2,809,998, 2,915,376, 2,915,528, 2,918,501, 2,934,450, 2,937,098, 2,957,031, 3,472,894 and 3,555,089; British Patent Nos. 1,143,927 and 1,130,822; JP Examined Publication No. 45-37304/1970; JP OPI Publication Nos. 47-9613/1972, 49-134614/1974, 50-117705/1975, 50-117727/1975, 50-121243/1975, 52-41182/1977 and 51-12392/1976; Journal of Chemical Society, p. 2789, 1950 and ibid., p. 2574 & 2640, 1957; Journal of American Chemical Society, vol. 79, p. 2549, 1957; Journal of Japan Oil Chemist Society, vol. 12, p. 653; Japan Organic Chemistry, vol. 30, p. 3524, 1965.
-
Among the above-mentioned fluorinated surfactants, some kinds thereof are available on the market under the trade names of, for example, "Megafac F" from Dai-Nippon Ink Industrial Co., "Fluorad FC" from Minnesota Mining and Manufacturing Co., "Monfor" from Imperial Chemical Industries Co., "Zonyls" from E.I. DuPont de Nemours, S.A., and "Licowet VPE" from Farbewerke Hbechst AG., respectively.
-
In the invention, a fluorinated cationic surfactant may be used in an amount within the range of 0.1mg to 1000 mg per sq.meter, more preferably 0.5 mg to 300 mg and further preferably 1.0 mg to 150 mg.
-
In the invention, when a fluorinated cationic surfactant of the invention and a fluorinated anionic surfactant are used in combination, the effects thereof can be displayed even when the amount thereof added is smaller as compared to the case of making independent use of the fluorinated cationic surfactant. The total amount of both cationic and anionic fluorinated surfactants used therein is within the range of, preferably 0.1 mg to 300 mg per sq.meter, more preferably 0.3 mg to 100 mg and, further preferably 0.5 mg to 30 mg. When making combination use thereof, it is allowed to use two or more kinds each of the above-mentioned two types surfactants.
-
Besides the above, it is also allowed to make combination use of a fluorinated nonionic surfactant, a fluorinated betaine surfactant or an anionic, cationic, nonionic or a betaine hydrocarbon surfactant. An adding proportion of a cationic fluorine type surfactant to an anionic fluorine type surfactant is preferably within the range of 1:10 to 10:1 and more preferably 3:7 to 7:3.
-
In the invention, a fluorinated cationic surfactant of Formula I should be existed in an outermost layer provided at the outermost position or the position farthest from the support of a photographic component layer comprising at least one silver halide light-sensitive emulsion layer and optionally a non-light-sensitive hydrophilic colloid layer. Accordingly the outermost layer may be an emulsion layer or a non-light-sensitive hydrophilic colloid layer. The non-light-sensitive hydrophilic colloid layer to be provided at the outermost position of the photographic component layer to protect the emulsion layer from an effect of a physical or mechanical power is usually referred to as a protective layer.
-
A fluorinated cationic surfactant or may be added to any one of the coating solutions for forming a surface protective layer, an intermediate layer, an emulsion layer and so forth of a silver halide photographic light-sensitive material. However, it is necessary to make them present in the uppermost layer after completing the coating and drying and, nevertheless, the above-mentioned surfactant is commonly added to a protective layer coating solution.
-
Gelatin is advantageously used as a binder or a protective colloid for the silver halide emulsion layer and the non-light-sensitive layer of a silver halide photographic light-sensitive material of the invention. It is also allowed to make use of a gelatin derivative, a graft polymer of gelatin and other macromolecule and, besides, a protein, a sugar derivative, a cellulose derivative and such a hydrophilic colloid including a synthetic hydrophilic macromolecular substance such as those of homopolymer or copolymer.
-
As for gelatin, lime processed gelatin and, besides, acid processed gelatin and enzyme processed gelatin such as described in Bulletin of Society of Science and Photography of Japan, No. 16, p. 30, 1966 may also be used. Further, a hydrolyzed matter of gelatin or an enzyme-decomposite thereof may also be used.
-
A gelatin derivative applicable thereto include, for example, those prepared by reacting gelatin with various compounds such as an acid halide, an acid anhydride, an isocyanate, bromacetic acid, an alkane sultone, a vinyl sulfonamide, a maleinimide compound, a polyalkylene oxide or an epoxy compound. The typical examples thereof are given in, for example, U.S. Patent Nos. 2,614,928, 3,132,945, 3,186,846 and 3,312,553; British Patent Nos. 861,414, 1,033,189 and 1,005,784; and JP Examined Publication No. 42-26845/1967.
-
A protein preferably applicable thereto includes, for example, albumin and casein; a cellulose derivative preferably includes, for example, hydroxyethyl cellulose, carboxymethyl cellulose and the sulfuric acid ester of cellulose; and a sugar derivative preferably includes, for example, sodium alginate and a starch derivative, respectively.
-
A graft polymer comprising gelatin and other macromolecule applicable thereto includes, for example, those prepared by grafting gelatin with acrylic acid, methacrylic acid or the esters thereof, a derivative such as those of amide, a homo-polymer or copolymer of a vinyl type monomers, such as acrylonitrile and styrene. It is particularly preferable to use a graft polymer comprising gelatin and a polymer having some extent of compatibility such as acrylic acid, acrylamide, methacrylamide and hydroxyalkyl methacrylate. The typical examples thereof are given in, for example, U.S. Patent Nos. 2,763,625, 2,831,767 and 2,956,884.
-
The typical synthetic hydrophilic macromolecular substances are each a single substance or copolymer of polyvinyl alcohol, a partial acetalized polyvinyl alcohol, poly-N-vinyl pyrrolidone, polyacrylic acid, polymethacrylic acid, polyacrylamide, polyvinyl imidazole or polyvinyl pyrazole. They are given in, for example, West German (OLS) Patent Publication No. 2,312,708; U.S. Patent Nos. 3,620,751 and 3,879,205; and JP Examined Publication No. 43-7561/1968.
-
A binder applicable to the invention is preferable to be cross-linked by a hardener.
-
As for the above-mentioned hardeners, The following layer hardeners may be used independently or in combination. Namely, those of the aldehyde type and the aziridine type such as those given in PB Report 19,921, U.S. Patent Nos. 2,950,197, 2,964,404, 2,983,611 and 3,271,175, JP Examined Publication No. 46-40898/1971 and JP OPI Publication No. 50-91315/1975; the isoxazole type such as those given in U.S. Patent No. 331,609; the epoxy type such as those given in U.S. Patent No. 3,047,394, West German Patent No. 1,085,663, British Patent No. 1,033,518, and JP Examined Publication No. 48-35495/1973; The vinylsulfone type such as those given in PB Report 19,920, West German Patent Nos. 1,100,942, 2,337,412, 2,545,722, 2,635,518, 2,742,308 and 2,749,260, British Patent No. 1,251,091, JP Application Nos. 45-54238/1970 and 48-110996/1973, and U.S. Patent Nos. 3,539,644 and 3,490,911; the acryloyl type such as those given in JP Application No. 48-27949/1973, and U.S. Patent No. 3,640,720; the carbodiimido type such as those given in U.S. Patent Nos. 2,938,892, 4,043,818 and 4,061,499, JP Examined Publication No. 46-38715/1971, and JP Application No. 49-15095/1974; the triazine type such as those given in West German Patent Nos. 2,410,973 and 2,553,915, U.S. Patent No. 3,325,287, and JP OPI Publication No. 52-12722/1977; the macromolecular type such as those given in British Patent No. 822,061, U.S. Patent Nos. 3,623,878, 3,396,029 and 3,226,234, and JP Examined Publication Nos. 47-18578/1972, 47-18579/1972 and 47-48896/1972; and, besides, the layer hardeners of the maleinimido type, acetylene type, methane sulfonate type, N-methylol type.
-
The techniques for combining them include, for example, those described in, for example, West German Patent Nos. 2,447,587, 2,505,746 and 2,514,245, U.S. Patent Nos. 4,047,957, 3,832,181 and 3,840,370, JP OPI Publication Nos. 48-43319/1973, 50-63062/1975 and 52-127329/1977 and JP Examined Publication No. 48-32364/1973.
-
A layer hardener particularly preferable to be applied to the invention include, for example, a vinyl sulfone type layer hardener and an s-triazine type hardener each described in JP OPI Publication No. 5-134367/1993.
-
In the invention, it is also allowed to make use of a coupler capable of developing a color upon reaction with a developing agent.
-
A yellow dye forming coupler preferably applicable to the invention include, for example, a well-known acylacetanilide type coupler. Among the couplers, a benzoyl acetanilide type and pivaloyl acetanilide type compounds are advantageously applicable thereto. The concrete examples of the applicable yellow couplers are given in, for example, British Patent No. 1,077,874, JP Examined Publication No. 45-40757/1970, JP OPI Publication Nos. 47-1031/1972, 47-26133/1972, 48-94432/1973, 50-87650/1975, 51-3631/1976, 52-115219/1977, 54-99433/1979, 54-133329/1979 and 56-30127/1981, U.S. Patent Nos. 2,875,057, 3,253,924, 3,265,506, 3,408,194, 3,551,155, 3,551,156, 3,664,841, 3,725,072, 3,730,722, 3,891,445, 3,900,483, 3,929,484, 3,933,500, 3,973,968, 3,990,896, 4,012,259, 4,022,620, 4,029,508, 4,057,432, 4,106,942, 4,133,958, 4,269,936, 4,286,053, 4,304,845, 4,314,023, 4,336,327, 4,356,258, 4,386,155 and 4,401,752.
-
A magenta dye forming coupler preferably applicable thereto include, for example, a well-known 5-pyrazolone type coupler, pyrazolobenzimidazole type coupler, pyrazolotriazole type coupler and open-chained acylacetonitrile type coupler. The concrete examples of the magenta couplers advantageously applicable thereto are given in, for example, JP Application Nos. 58-164882/1983, 58-167326/1983, 58-206321/1983, 58-214863/1983, 58-217339/1983 and 59-24653/1984, JP Examined Publication Nos. 40-6031/1965, 40-6035/1965, 45-40757/1970, 47-27411/1972 and 49-37854/1974, JP OPI Publication Nos. 50-13041/1975, 51-26541/1976, 51-37646/1976, 51-105820/1976, 52-42121/1977, 53-123129/1978, 53-125835/1878, 53-129035/1978, 54-48540/1979, 56-29236/1981, 56-75648/1981, 57-17950/1982, 57-35858/1982, 57-146251/1982 and 59-99437/1984, British Patent No. 1,252,418, and U.S. Patent Nos. 2,600,788, 3,005,712, 3,062,653, 3,127,269, 3,214,437, 3,253,924, 3,311,476, 3,419,391, 3,519,429, 3,558,319, 3,582,322, 3,615,506, 3,658,544, 3,705,896, 3,725,067, 3,758,309, 3,823,156, 3,834,908, 3,891,445, 3,907,571, 3,926,631, 3,928,044, 3,935,015, 3,960,571, 4,076,533, 4,133,686, 4,237,217, 4,241,168, 4,264,723, 4,301,235 and 4,310,623.
-
A cyan dye forming coupler preferably applicable thereto include, for example, a well-known naphthol type coupler and phenol type coupler are preferably applicable. The concrete examples of the cyan couplers advantageously applicable thereto are given in, for example, British Patent Nos. 1,038,331 and 1,543,040, JP Examined Publication No. 48-36894/1973, JP OPI Publication Nos. 48-59838/1973, 50-137137/1975, 51-146828/1976, 53-105226/1978, 54-115230/1979, 56-29235/1981, 56-104333/1981, 56-126833/1981, 57-133650/1982, 57-155538/1982, 57-204545/1982, 58-118643/1983, 59-31953/1984, 59-31954/1984, 59-59656/1984, 59-124341/1984 and 59-166956/1984, and U.S. Patent Nos. 2,369.929, 2,423,730, 2,434,272, 2,474,293, 2,698,794, 2,772,162, 2,801,171, 2,895,826, 3,253,924, 3,311,476, 3,458,315, 3,476,563, 3,591,383, 3,737,316, 3,758,308, 3,767,411, 3,790,384, 3,880,661, 3,926,634, 4,004,929, 4,009,035, 4,012,258, 4,052,212, 4,124,396, 4,134,766, 4,138,258, 4,146,396, 4,149,886, 4,178,183, 4,205,990, 4,254,212, 4,264,722, 4,288,532, 4,296,199, 4,296,200, 4,299,914, 4,333,999, 4,334,011, 4,386,155, 4,401,752 and 4,427,767.
-
To a silver halide photographic light-sensitive material of the invention, a variety of photographic additives may be added, as well as the above-given compounds.
-
The examples thereof include a UV absorbent such as a benzophenone type compound and a benzotriazole type compound, a development accelerator such as 1-aryl-3-pyrazolidone type compound, a surfactant such as an alkyl naphthalene sulfonate, an alkylsuccinic sulfonate, an itaconate and a polyalkylene oxide compound, a water-soluble anti-irradiation dye such as an azo type compound, a styryl type compound, an oxonol type compound, an anthraquinone type compound and a triphenyl methane type compound, a layer physical property improver such as glycerol, polyalkylene glycol, a polymer latex and solid or liquid paraffin, a color contamination inhibitor such as a diffusion resistant hydroquinone type compound, a dye-image stabilizer such as a hydroquinone derivative, a gallic acid derivative, a phenol type compound, a hydroxy chroman type compound, a polyalkyl piperidine type compound and an aromatic amine type compound, a water-soluble or oil-soluble fluorescent whitening agent and a background tone controller such as an oil-soluble colorant.
-
To a silver halide photographic light-sensitive material of the invention, a variety of photographic additives may be added, as well as the above-given compounds.
-
Among a dye-forming coupler, a colored coupler, a DIR coupler, a DIR compound, an image stabilizer, anti-color foggant, a UV absorbent and a fluorescent whitening agent, each of which is needless to adsorb on the surface of a silver halide crystal, a hydrophobic compound may be added to a silver halide photographic light-sensitive material in various methods such as a solid dispersion method, a latex dispersion method and an oil-in-water type emulsification-dispersion method. A suitable method may be selected out of the above-mentioned methods so as to meet the chemical structure of a subject hydrophobic compound such as a coupler.
-
The above-mentioned oil-in-water type emulsification-dispersion method is so applied as to disperse a hydrophobic additive such as a coupler. This method is usually carried out in the following manner. A hydrophobic additive such as a coupler is dissolved in a high-boiling organic solvent having a boiling point of not lower than 150°C and, if required, by making combination use of an organic solvent having a relatively low boiling point and/or a water-soluble organic solvent. The resulting solution is dispersed in a hydrophilic binder such as an aqueous gelatin solution by making use of a dispersing means such as a stirrer, homogenizer, colloid-mill, flow-jet mixer and supersonic device, and the resulting emulsified dispersion is then added to an objective hydrophilic colloidal layer. When dispersing it, a surfactant may also be used therein. It is also allowed to supplement with a low boiling organic solvent removing step after completing the emulsification and dispersion and, further, to make use of a low boiling organic solvent or a water-soluble organic solvent, in place of the high boiling organic solvent.
-
When making use of a high boiling organic solvent and a low boiling organic solvent in combination, the proportion of the both solvents is within the range of 1:0.1 to 1:50 and, preferably 1:1 to 1:20.
-
The high boiling organic solvents applicable thereto include, for example, those having a boiling point of not lower than 150°C, such as a phenol derivative incapable of reacting with the oxidant of a developing agent, a phthalic acid alkyl ester, a phosphoric acid ester, a citric acid ester, a benzoic acid ester, alkylamide, an aliphatic acid ester and a trimesic acid ester.
-
The high boiling organic solvents applicable thereto include, for example those given in, for example, U.S. Patent Nos. 2,322,027, 2,533,514, 2,835,579, 3,287,134, 2,353,262, 2,852,383, 3,554,755, 3,676,137, 3,676,142, 3,700,454, 3,748,141, 3,779,765 and 3,837,863, British Patent Nos. 958,441 and 1,222,753, West German OLS Patent Publication No. 2,538,889, JP OPI Publication Nos. 47-1031/1972, 49-90523/1974, 50-23823/1975, 51-26037/1976, 51-27921/1976, 51-27922/1976, 51-26035/1976, 51-26036/1976, 50-62632/1975, 53-1520/1978, 53-1521/1978, 53-15127/1978, 54-119921/1979, 54-119922/1979, 55-25057/1980, 55-36869/1980, 56-19049/1981 and 56-81836/1981, and JP Examined Publication No. 48-29060/1973.
-
The low boiling organic solvents or water-soluble organic solvents, which are used together with or in place of a high boiling organic solvent, include, for example, those given in U.S. Patent Nos. 2,801,171 and 2,949,360. The low boiling and substantially water-insoluble organic solvents include, for example, ethyl acetate, propyl acetate, butyl acetate, butanol, chloroform, carbon tetrachloride, nitromethane, nitroethane and benzene. The water-soluble organic solvents include, for example, acetone, methyl isobutyl ketone, β-ethoxyethyl acetate, methoxy glycol acetate, methanol, ethanol, acetonitrile, dioxane, dimethylformamide, dimethylsulfoxide, hexamethylphosphoramide, diethylene glycol, monophenylether and phenoxyethanol.
-
The surfactants preferably applicable thereto as a dispersion aid include, for example, an anionic surfactant such as an alkylbenzenesulfonate, an alkylnaphthalene-sulfonate, an alkylsulfonate, an alkyl sulfate, an alkyl phosphate, a sulfosuccinate and sulfoalkylpolyoxyethylene alkylphenyl ether, a nonionic surfactant such as a steroid type saponin, an alkyleneoxide derivative and a glycidol derivative, an amphoteric surfactant such as an amino acid, an aminoalkylsulfonic acid and an alkylbetaine, and a cationic surfactant such as a quaternary ammonium salt.
-
The concrete examples of the above-given surfactants are given in "A Handbook of Surface Active Agents", Sangyo Tosho Co., 1966 and "The Research·Technical Data on Emulsifying agents and Emulsifying Instruments", Kagaku Hanron Sha Co., 1978.
-
The latex dispersion methods preferably applicable thereto include, for example, those given in U.S. Patent Nos. 4,199,363, 4,214,047, 4,203,716 and 4,247,627, and JP OPI Publication Nos. 49-74538/1974, 51-59942/1976, 51-59943/1976 and 54-32552/1979.
-
When a silver halide photographic light-sensitive material of the invention is a color photographic light-sensitive material, an image can be formed by applying any color development process having been well-known in the art.
-
In the invention, a color developing agent applicable to a color developing process include, for example, well-known developing agents having widely been applied to various color photographic processes. In such a color developing agent as mentioned above contains an aminophenol type or p-phenylene diamine type derivative. These compounds are commonly used in the form of such a salt as a hydrochloride or a sulfate, because these compounds are more stable than in the free state. These compounds are commonly used in a concentration within the range of about 0.1 g to about 30 g per liter of a color developer used and, preferably about 1 g to about 15 g per liter of a color developer used.
-
A color developing agent applicable to a color developer includes, typically, an aromatic primary amine compound and, particularly, those of the p-phenylenediamine type. The preferable examples thereof include, for example, N,N-diethyl-p-phenylenediamine hydrochloride, N-ethyl-p-phenylenediamine hydrochloride, N,N-dimethyl-p-phenylenediamine hydrochloride, 2-amino-5-(N-ethyl-N-dodecylamino)-toluene, N-ethyl-N-(β-methanesulfonamidoethyl)-3-methyl-4-aminoaniline sulfate, N-ethyl-N-β-hydroxyethylaminoaniline, 4-amino-N-(2-methoxyethyl)-N-ethyl-3-methylaniline p-toluene sulfonate, N,N-diethyl-3-ethyl-4-aminoaniline, and N-ethyl-N-(β-hydroxyethyl)-3-methyl-4-aminoaniline.
-
These color developing agents may be used independently or in combination. It is also allowed to use one kind or not less than two kinds of these color developing agents and other black-and-white developing agents such as hydroquinone, 1-phenyl-3-pyrazolidone and an N-methyl-p-aminophenyl in combination.
-
When color developing a silver halide photographic light-sensitive material of the invention, an N-ethyl-N-(β-methane sulfonamidoethyl)-3-methyl-4-aminoaniline sulfate is preferably used among the above-given compounds.
-
If required, a color developer may contains, for example, an alkalizer such as sodium hydroxide, potassium hydroxide, sodium carbonate, tertiary sodium phosphate, potassium carbonate and potassium hydrogen carbonate, a preservative such as N,N-diethylhydroxylamine, N,N-bis(methoxy ethyl)hydroxylamine, triethanolamine, diethanolamine glycol and potassium sulfite, an organic solvent such as methanol, ethanol, butanol, ethylene glycol, diethylene glycol and benzyl alcohol, a development controller such as citrazinic acid and polyethylene glycol, and various photographic additives having been well known in the photographic art, such as a heavy metal ion obliterating agent and a development accelerator.
-
When a color developer contains relatively larger amount of a color formation accelerator such as benzyl alcohol, and if sulfite ions of sodium sulfite or potassium sulfite are added as a color developer preservative in a relatively larger amount for example, in an amount of not less than about 0.01 mols per liter of the color developer, the color developability may not be lowered so much, however, when a color developer contains benzyl alcohol in an amount of the order of 0 to about 5 milliliter per liter of the color developer, the color developability may be lowered. It is therefore preferably to make the concentration of sulfite ions to be not more than about 0.004 mols per liter of the color developer.
-
It is preferable that a silver halide photographic light-sensitive material of the invention does not contain a water-soluble bromide at all, or that a development is to be carried out with a color developer containing a very small amount of a water-soluble bromide. If a color developer contains an excessive amount of a water-soluble bromide, the development speed of a silver halide photographic light-sensitive material is sharply retarded, so that the objects of the invention may not be achieved. In a color developer, a concentration of bromide ions is to be about not more than 0.1 g, in terms of potassium bromide, per liter of the color developer and, preferably not more than 0.05 g.
-
When a silver halide photographic light-sensitive material of the invention is continuously color developed while a color developing replenisher is kept on replenishing, a very small amount of bromide ions are dissolved out of the light-sensitive material, so that the small amount of bromide ions are accumulated in the color developer used. The accumulation of an excess amount of bromide ions is not preferred because bromide ions have a development inhibiting function. It is, therefore, preferable that a replenishment rate of a color developing replenisher is suitably adjusted so that the bromide ion content of the color developer can be within the above-mentioned range.
-
A water-soluble chloride is preferably used as a development modifier in the above-mentioned color developer.
-
The above-mentioned water-soluble chloride is used in an amount within the range of 0.5 g to 5 g in terms of potassium bromide per liter of a color developer used and, preferably 1 g to 3 g.
-
In the above-mentioned color developer, an organic development inhibitor described in JP OPI Publication No. 58-95345/1983 may be used, provided that the effects of the invention shall not be spoiled. For this purpose, adenine or guanine may be preferably used in an amount within the range of 0 to 0.02 g per liter of a color developer.
-
A pH of a developer of the invention is preferably not lower than 9.5 and more preferably not higher than 13. Heretofore, it has been known that a development can be accelerated by raising a pH of a developer. In a silver halide photographic light-sensitive material of the invention, a satisfactory rapid developability can be enjoyed even if the pH thereof is not higher than 11.
-
A temperature of a color developer is to be within the range of 15 to 45°C and preferably 20 to 40°C.
-
After completing a color development, a silver halide photographic light-sensitive material of the invention is bleached and then fixed. The bleaching and fixing treatments may be carried out at the same time. As for a bleaching agent, many kinds of compounds may be used. They include, for example, a polyvalent metal compound such as those of iron (III), cobalt (III) and copper (II). Inter alia, a complex salt comprising the polyvalent metal cation thereof and an organic acid including, for example, an aminopolycarboxylic acid such as ethylenediaminetetracetic acid, nitrilotriacetic acid and N-hydroxyethylethylenediamine diacetic acid, a metal complex salt such as those of malonic acid, tartaric acid, malic acid, diglycolic acid and dithioglycolic acid, or a ferricyanate and a dichromate. They may be used independently or in suitable combination.
-
A fixer applicable thereto include, for example, a soluble complexer capable of dissolving silver halide to be a complex salt. Such a soluble complexer as mentioned above include, for example, sodium thiosulfate, ammonium thiosulfate, potassium thiocyanate, thiourea and thioether.
-
After completing a fixing treatment, a washing treatment is then commonly carried out. For the replacement thereof, a stabilizing treatment may be carried out, or the both treatments thereof may also be carried out. A stabilizer applicable to a stabilizing treatment may also contain a pH controller, a chelating agent, an antimold and so forth. The concrete requirements thereof may be referred to the descriptions in JP OPI Publication No. 58-134636/1983.
Example
Example 1
-
On a paper-made support laminated with polyethylene on one side and with polyethylene containing titanium oxide on the other side, each of the layers having the composition shown below was coated on the polyethylene layer containing titanium oxide, so that a multilayered silver halide photographic light-sensitive material sample No. 1 could be prepared. The coating solutions thereof were each prepared in the following manners.
Coating solution for layer 1
-
Ethyl acetate was added in an amount of 60 ml to 26.7 g of yellow coupler (Y-1), 100 g of dye-image. stabilizer (ST-1) and 6.67 g of dye-image stabilizer (ST-2). The resulting solution was emulsified and dispersed in 220 ml of an aqueous 10% gelatin solution containing 7 ml of 20% surfactant (SU-1) solution by making use of a supersonic homogenizer, so that a yellow coupler dispersion solution could be prepared. The resulting dispersion solution was mixed with a blue-sensitive silver halide emulsion having a silver content of 8.7 g that was prepared under the following conditions, so that a coating solution for layer 1 could be prepared.
-
Coating solutions for layers 2 through 7 were each prepared in the manner similar to that in the above-mentioned coating solution for layer 1. Hardner H-1 were added to layers 2, 4 and 7 in the amounts of 40 mg/m², 50 mg/m² and 60 mg/m². Surfactants SU-1 and SU-2 were each added as a coating aid so that the surface tension thereof could be adjusted. Further, the pH values of each coating solution were adjusted by making use of 0.2M nitric acid so that the pH values of each surface could be 5.8, respectively.
Preparation of the blue-sensitive silver chlorobromide emulsion
-
The following Solution A and Solution B were each added at the same time by taking 30 minutes to 1000 ml of an aqueous 2% gelatin solution being kept at 40°C, while controlling the pAg and pH to be 6.5 and 3.0, respectively. Further, the following Solution C and Solution D were added thereto at the same time by taking 180 minutes, while controlling the pAg and pH to be 7.3 and 5.5, respectively. At this time, the pAg was controlled by following the method described in JP OPI Publication No. 59-45437/1984, and the pH was controlled by making use of an aqueous solution of sulfuric acid or sodium hydroxide.
(Solution A)
| Sodium chloride | 3.42 g |
| Potassium bromide | 0.03 g |
| Add water to make | 200 ml |
(Solution B)
| Silver nitrate | 10 g |
| Add water to make | 200 ml |
(Solution C)
| Sodium chloride | 102.7 g |
| Potassium bromide | 1.0 g |
| Add water to make | 600 ml |
(Solution D)
| Silver nitrate | 300 g |
| Add water to make | 600 ml |
-
After completing the addition, a desalting treatment was carried out by making use of an aqueous 5% solution of Demol N manufactured by Kao-Atlas Corp. and an aqueous 20% solution of magnesium sulfate. The resulting desalted solution was then mixed with an aqueous gelatin solution, so that monodisperse type cubic emulsion EMP-1 having an average grain-size of 0.85µm, a variation coefficient (σ/r) of 0.07 and a silver chloride content of 99.5 mol% could be prepared.
-
The above-mentioned emulsion EMP-1 was chemically ripened at 50°C for 90 minutes by making use of the following compounds, so that blue-sensitive silver chlorobromide emulsion Em-B could be prepared.
| Sodium thiosulfate | 0.8 mg/mol AgX |
| Chloroauric acid | 0.5 mg/mol AgX |
| Stabilizer STAB-1 | 6x10⁻⁴ mols/mol AgX |
| Sensitizing dye BS-1 | 4x10⁻⁴ mols/mol AgX |
| Sensitizing dye BS-2 | 1x10⁻⁴ mols/mol AgX |
Preparing of the green-sensitive silver chlorobromide emulsion
-
A monodisperse type cubic emulsion EMP-2 having an average grain-size of 0.43µm, a variation coefficient (σ/r) of 0.08 and a silver chloride content of 99.5 mol% was prepared in the same manner as in the case of emulsion EMP-1, except that the time for adding Solution A and Solution B and the time for adding Solution C and Solution D were changed.
-
The above-mentioned emulsion EMP-2 was chemically ripened at 55°C for 120 minutes by making use of the following compounds, so that green-sensitive silver chlorobromide emulsion (Em-G) could be prepared.
| Sodium thiosulfate | 1.5 mg/mol AgX |
| Chloroauric acid | 1.0 mg/mol AgX |
| Stabilizer STAB-1 | 6x10⁻⁴ mols/mol AgX |
| Sensitizing dye GS-1 | 4x10⁻⁴ mols/mol AgX |
Preparing of a red-sensitive silver chlorobromide emulsion
-
A monodisperse type cubic emulsion EMP-3 having an average grain-size of 0.50µm, a variation coefficient (σ/r) of 0.08 and a silver chloride content of 99.5 mol% was prepared in the same manner as in the case of emulsion EMP-1, except that the time for adding Solution A and Solution B and the time for adding Solution C and Solution D were changed.
-
The above-mentioned emulsion EMP-3 was chemically ripened at 60°C for 90 minutes by making use of the following compounds, so that red-sensitive silver chlorobromide emulsion (Em-R) could be prepared.
| Sodium thiosulfate | 1.8 mg/mol AgX |
| Chloroauric acid | 2.0 mg/mol AgX |
| Stabilizer STAB-1 | 6x10⁻⁴ mols/mol AgX |
| Sensitizing dye RS-1 | 1x10⁻⁴ mols/mol AgX |
- DBP
- Dibutyl phthalate
- DOP
- Dioctyl phthalate
- DNP
- Dinonyl phthalate
- DIDP
- Diisodecyl phthalate
-
Further, samples No. 2 through No. 20 were each prepared by making use of various fluorinated surfactants in place of the fluorinated surfactant FK-3 used in Layer 7. The amounts and kinds of the fluorinated surfactants used therein are given in Table 3.
-
The resulting samples No. 1 through No. 20 were each evaluated on the aging preservability and adhesion resistance under the highly humid conditions, in the following manner. The results thereof will be shown in Table 3.
<Aging preservability>
-
The samples prepared as above (the samples before aging) and the same samples preserved for 3 weeks under the conditions at 40°C and 40%RH (the same samples after aging) were each exposed to light through an optical wedge and were then processed in the following processing steps. The resulting samples were each measured on the reflection densities of yellow and magenta dye-images by making use of a densitometer, Model PDA-65, so that the minimum density (Dmin) of each sample was obtained. Each of Dmin of the samples before and after aging them was obtained, so that each ΔDmin thereof was calculated out by the following formula.
| [Processing steps] |
| | Temperature (°C) | Time (sec) | Amount replenished |
| Color developing | 38.0±0.3 | 45 | 120 |
| Bleach-fixing | 35.0±0.5 | 45 | 54 |
| Stabilizing | 30 to 40 | 90 | 250 |
| Drying | 50 to 75 | 60 | |
-
The amounts of the processing solutions were each indicated in terms of milliliter per sq.meter of a light-sensitive material used.
-
The composition of each processing solution will be shown below.
| Color developer | For tank | For replenisher |
| Potassium bromide | 20 mg | 8.0 mg |
| Potassium chloride | 2.0 mg | - |
| Potassium sulfited (in an aqueous 50% solution) | 0.6 ml | 1.0 ml |
| N-ethyl-N-(β-methanesulfonamidoethyl)-3-methyl-4-aminoaniline sulfate | 4.5 g | 9.2 g |
| N,N-diethylhydroxylamine | 5.0 g | 9.0 g |
| Triethanolamine | 10.0 g | 15.0 g |
| Potassium carbonate | 27.0 g | 30.0 g |
| Sodium ethylenediaminetetracetate | 1.0 g | 2.0 g |
| Fluorescent whitening agent, (a diaminostilbene disulfonic acid derivative) | 1.0 g | 2.2 g |
-
Add water to make one liter. The pH values of both tank solution and replenisher were adjusted to be 10.10 and 10.60 by making use of sodium hydroxide or sulfuric acid, respectively.
| Bleach-fixer (the tank solution and replenisher were one and the same) |
| Ferric ammonium ethylenediaminetetracetate dihydrate | 20 g |
| Ethylenediaminetetracetic acid | 3 g |
| Ammonium thiosulfate (in an aqueous 70% solution) | 200 ml |
| Ammonium sulfite (in an aqueous 40% solution) | 85 ml |
-
Add water to make one liter and the pH was adjusted to be 5.0 with aqueous ammonia or glacial acetic acid.
| Stabilizer (The tank solution and replenisher were one and the same) |
| 5-chloro-2-methyl-4-isothiazoline-3-one | 0.02 g |
| 2-methyl-4-isothiazoline-3-one | 0.02 g |
| Ethylene glycol | 1.0 g |
| 2-octyl-4-isothiazoline-3-one | 0.01 g |
| 1-hydroxy ethylidene-1,1-diphosphonic acid, (in an aqueous 60% solution) | 3.0 g |
| BiCl₃ (in an aqueous 45% solution) | 0.65 g |
| MgSO₄·7H₂O (in an aqueous 25% solution) | 0.20 g |
| Ammonium hydroxide, (in an aqueous 25% solution) | 2.5 g |
| Trisodium nitrilotriacetate | 1.5 g |
-
Add water to make one liter and the pH was adjusted to be 7.0 with aqueous ammonia or sulfuric acid.
<Adhesion resistance under the highly humid conditions>
-
After subject samples were piled and then fixed up so as to lay one emulsion surface of the sample on top of another, they were allowed to stand for 15 hours under the conditions of 40°C and 80%RH. Thereafter, the samples adhering to each other surfaces were peeled off. At that moment, the sensory resistance against peeling force was obtained and was then evaluated by the following five graded criteria. When a sample was graded, the more the grade is closer to grade 1, the more it was upgraded, and the more the grade is closer to grade 5, the more it was downgraded.
- 1... Sample surfaces did not adhere,
- 2... Intermediate between 1 and 3,
- 3... There was a resistance to such an extent that a noise was produced when peeling off,
- 4... Intermediate between 3 and 5, and
- 5... Sample surfaces adhered to each other so tight that the surfaces could not be separated.
-
As is obvious from the contents of Table 3, It was proved that, among silver halide photographic light-sensitive materials each having a high silver chloride content, those applied with a fluorinated cationic surfactant of the invention have improved in physical surface property under a highly humid condition and excellent in preservability and, in addition, when making combination use of a fluorinated anionic surfactant, the effects of the invention can be remarkable in particular.