EP0608956A1 - Photographic element and process having improved response to developer variations - Google Patents
Photographic element and process having improved response to developer variations Download PDFInfo
- Publication number
- EP0608956A1 EP0608956A1 EP94200186A EP94200186A EP0608956A1 EP 0608956 A1 EP0608956 A1 EP 0608956A1 EP 94200186 A EP94200186 A EP 94200186A EP 94200186 A EP94200186 A EP 94200186A EP 0608956 A1 EP0608956 A1 EP 0608956A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- substituted
- coupler
- unsubstituted alkyl
- alkoxy
- independently
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims description 14
- 230000008569 process Effects 0.000 title claims description 12
- 230000004044 response Effects 0.000 title description 11
- -1 silver halide Chemical class 0.000 claims abstract description 115
- 229910052709 silver Inorganic materials 0.000 claims abstract description 22
- 239000004332 silver Substances 0.000 claims abstract description 22
- BYHCBLSQLHOONI-UHFFFAOYSA-N 5-anilino-2-phenyl-4h-pyrazol-3-one Chemical compound N=1N(C=2C=CC=CC=2)C(=O)CC=1NC1=CC=CC=C1 BYHCBLSQLHOONI-UHFFFAOYSA-N 0.000 claims abstract description 4
- 125000000217 alkyl group Chemical group 0.000 claims description 33
- 125000001424 substituent group Chemical group 0.000 claims description 26
- 125000003545 alkoxy group Chemical group 0.000 claims description 25
- 229910052739 hydrogen Inorganic materials 0.000 claims description 16
- 239000001257 hydrogen Substances 0.000 claims description 16
- 125000005420 sulfonamido group Chemical group S(=O)(=O)(N*)* 0.000 claims description 16
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 claims description 15
- 125000004104 aryloxy group Chemical group 0.000 claims description 15
- 125000004423 acyloxy group Chemical group 0.000 claims description 13
- 125000004414 alkyl thio group Chemical group 0.000 claims description 12
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 12
- 125000002252 acyl group Chemical group 0.000 claims description 11
- 125000004453 alkoxycarbonyl group Chemical group 0.000 claims description 11
- 229910052736 halogen Inorganic materials 0.000 claims description 11
- 150000002367 halogens Chemical class 0.000 claims description 11
- 125000004390 alkyl sulfonyl group Chemical group 0.000 claims description 10
- 239000003795 chemical substances by application Substances 0.000 claims description 10
- 125000004093 cyano group Chemical group *C#N 0.000 claims description 10
- 125000004391 aryl sulfonyl group Chemical group 0.000 claims description 9
- 125000000623 heterocyclic group Chemical group 0.000 claims description 9
- 150000002431 hydrogen Chemical class 0.000 claims description 9
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 9
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 8
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 7
- 125000003118 aryl group Chemical group 0.000 claims description 7
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 6
- 125000005110 aryl thio group Chemical group 0.000 claims description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 4
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 claims description 4
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical compound C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 claims description 3
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 3
- 229910052717 sulfur Inorganic materials 0.000 claims description 3
- FTJHKZQHQDKPFJ-UHFFFAOYSA-N (carbamoylamino)carbamic acid Chemical compound NC(=O)NNC(O)=O FTJHKZQHQDKPFJ-UHFFFAOYSA-N 0.000 claims description 2
- 125000003277 amino group Chemical group 0.000 claims description 2
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 claims description 2
- 125000004429 atom Chemical group 0.000 claims description 2
- 150000002460 imidazoles Chemical class 0.000 claims description 2
- 125000004434 sulfur atom Chemical group 0.000 claims description 2
- 239000010410 layer Substances 0.000 description 94
- 239000000839 emulsion Substances 0.000 description 50
- 239000000463 material Substances 0.000 description 23
- 230000035945 sensitivity Effects 0.000 description 21
- 239000000975 dye Substances 0.000 description 16
- 108010010803 Gelatin Proteins 0.000 description 14
- 125000004432 carbon atom Chemical group C* 0.000 description 14
- 229920000159 gelatin Polymers 0.000 description 14
- 239000008273 gelatin Substances 0.000 description 14
- 235000019322 gelatine Nutrition 0.000 description 14
- 235000011852 gelatine desserts Nutrition 0.000 description 14
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 14
- ZUNKMNLKJXRCDM-UHFFFAOYSA-N silver bromoiodide Chemical compound [Ag].IBr ZUNKMNLKJXRCDM-UHFFFAOYSA-N 0.000 description 14
- 239000003112 inhibitor Substances 0.000 description 12
- 238000011161 development Methods 0.000 description 11
- 239000000203 mixture Substances 0.000 description 9
- KXDAEFPNCMNJSK-UHFFFAOYSA-N Benzamide Chemical compound NC(=O)C1=CC=CC=C1 KXDAEFPNCMNJSK-UHFFFAOYSA-N 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 6
- 230000000873 masking effect Effects 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 5
- 239000011229 interlayer Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 239000000460 chlorine Substances 0.000 description 4
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 4
- AJDUTMFFZHIJEM-UHFFFAOYSA-N n-(9,10-dioxoanthracen-1-yl)-4-[4-[[4-[4-[(9,10-dioxoanthracen-1-yl)carbamoyl]phenyl]phenyl]diazenyl]phenyl]benzamide Chemical compound O=C1C2=CC=CC=C2C(=O)C2=C1C=CC=C2NC(=O)C(C=C1)=CC=C1C(C=C1)=CC=C1N=NC(C=C1)=CC=C1C(C=C1)=CC=C1C(=O)NC1=CC=CC2=C1C(=O)C1=CC=CC=C1C2=O AJDUTMFFZHIJEM-UHFFFAOYSA-N 0.000 description 4
- 238000001228 spectrum Methods 0.000 description 4
- 125000004149 thio group Chemical group *S* 0.000 description 4
- 239000001043 yellow dye Substances 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- MCSKRVKAXABJLX-UHFFFAOYSA-N pyrazolo[3,4-d]triazole Chemical compound N1=NN=C2N=NC=C21 MCSKRVKAXABJLX-UHFFFAOYSA-N 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- JAAIPIWKKXCNOC-UHFFFAOYSA-N 1h-tetrazol-1-ium-5-thiolate Chemical compound SC1=NN=NN1 JAAIPIWKKXCNOC-UHFFFAOYSA-N 0.000 description 2
- DKIDEFUBRARXTE-UHFFFAOYSA-N 3-mercaptopropanoic acid Chemical compound OC(=O)CCS DKIDEFUBRARXTE-UHFFFAOYSA-N 0.000 description 2
- XVEPKNMOJLPFCN-UHFFFAOYSA-N 4,4-dimethyl-3-oxo-n-phenylpentanamide Chemical class CC(C)(C)C(=O)CC(=O)NC1=CC=CC=C1 XVEPKNMOJLPFCN-UHFFFAOYSA-N 0.000 description 2
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 2
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 2
- 230000001133 acceleration Effects 0.000 description 2
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- IOJUPLGTWVMSFF-UHFFFAOYSA-N benzothiazole Chemical compound C1=CC=C2SC=NC2=C1 IOJUPLGTWVMSFF-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000004061 bleaching Methods 0.000 description 2
- 239000007844 bleaching agent Substances 0.000 description 2
- IAQRGUVFOMOMEM-UHFFFAOYSA-N but-2-ene Chemical compound CC=CC IAQRGUVFOMOMEM-UHFFFAOYSA-N 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 238000012937 correction Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- LNTHITQWFMADLM-UHFFFAOYSA-N gallic acid Chemical compound OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 125000001841 imino group Chemical group [H]N=* 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000002667 nucleating agent Substances 0.000 description 2
- 239000004848 polyfunctional curative Substances 0.000 description 2
- 230000027756 respiratory electron transport chain Effects 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- ILKZXYARHQNMEF-UHFFFAOYSA-N (4-azaniumyl-3-methylphenyl)-ethyl-(2-methoxyethyl)azanium;4-methylbenzenesulfonate Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1.CC1=CC=C(S(O)(=O)=O)C=C1.COCCN(CC)C1=CC=C(N)C(C)=C1 ILKZXYARHQNMEF-UHFFFAOYSA-N 0.000 description 1
- FVRXOULDGSWPPO-UHFFFAOYSA-N 1,2-dihydropyrazole-3-thione Chemical class SC1=CC=NN1 FVRXOULDGSWPPO-UHFFFAOYSA-N 0.000 description 1
- YXIWHUQXZSMYRE-UHFFFAOYSA-N 1,3-benzothiazole-2-thiol Chemical class C1=CC=C2SC(S)=NC2=C1 YXIWHUQXZSMYRE-UHFFFAOYSA-N 0.000 description 1
- YHMYGUUIMTVXNW-UHFFFAOYSA-N 1,3-dihydrobenzimidazole-2-thione Chemical class C1=CC=C2NC(S)=NC2=C1 YHMYGUUIMTVXNW-UHFFFAOYSA-N 0.000 description 1
- 150000005208 1,4-dihydroxybenzenes Chemical class 0.000 description 1
- BAXOFTOLAUCFNW-UHFFFAOYSA-N 1H-indazole Chemical class C1=CC=C2C=NNC2=C1 BAXOFTOLAUCFNW-UHFFFAOYSA-N 0.000 description 1
- KJUGUADJHNHALS-UHFFFAOYSA-N 1H-tetrazole Substances C=1N=NNN=1 KJUGUADJHNHALS-UHFFFAOYSA-N 0.000 description 1
- LLCOQBODWBFTDD-UHFFFAOYSA-N 1h-triazol-1-ium-4-thiolate Chemical class SC1=CNN=N1 LLCOQBODWBFTDD-UHFFFAOYSA-N 0.000 description 1
- CDAWCLOXVUBKRW-UHFFFAOYSA-N 2-aminophenol Chemical class NC1=CC=CC=C1O CDAWCLOXVUBKRW-UHFFFAOYSA-N 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- FLFWJIBUZQARMD-UHFFFAOYSA-N 2-mercapto-1,3-benzoxazole Chemical class C1=CC=C2OC(S)=NC2=C1 FLFWJIBUZQARMD-UHFFFAOYSA-N 0.000 description 1
- BKTNHKCSXCCZBH-UHFFFAOYSA-N 2-methyl-n-[5-oxo-1-(2,4,6-trichlorophenyl)-4h-pyrazol-3-yl]prop-2-enamide Chemical compound O=C1CC(NC(=O)C(=C)C)=NN1C1=C(Cl)C=C(Cl)C=C1Cl BKTNHKCSXCCZBH-UHFFFAOYSA-N 0.000 description 1
- 125000000175 2-thienyl group Chemical group S1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- CLEJZSNZYFJMKD-UHFFFAOYSA-N 3h-1,3-oxazole-2-thione Chemical class SC1=NC=CO1 CLEJZSNZYFJMKD-UHFFFAOYSA-N 0.000 description 1
- OCVLSHAVSIYKLI-UHFFFAOYSA-N 3h-1,3-thiazole-2-thione Chemical class SC1=NC=CS1 OCVLSHAVSIYKLI-UHFFFAOYSA-N 0.000 description 1
- KWIVRAVCZJXOQC-UHFFFAOYSA-N 3h-oxathiazole Chemical class N1SOC=C1 KWIVRAVCZJXOQC-UHFFFAOYSA-N 0.000 description 1
- LUWZTXZFAZCHMX-UHFFFAOYSA-N 3h-oxathiazole-4-thiol Chemical class SC1=COSN1 LUWZTXZFAZCHMX-UHFFFAOYSA-N 0.000 description 1
- 125000004172 4-methoxyphenyl group Chemical group [H]C1=C([H])C(OC([H])([H])[H])=C([H])C([H])=C1* 0.000 description 1
- XTBFKMDOQMQYPP-UHFFFAOYSA-N 4-n,4-n-diethylbenzene-1,4-diamine;hydron;chloride Chemical compound Cl.CCN(CC)C1=CC=C(N)C=C1 XTBFKMDOQMQYPP-UHFFFAOYSA-N 0.000 description 1
- 125000003341 7 membered heterocyclic group Chemical group 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- AJDKZWLPPHJPOJ-UHFFFAOYSA-N C=1C=CC=C(Cl)C=1NN(CC)CC(C=1C=CC=CC=1)NC1=CC=CC=C1 Chemical compound C=1C=CC=C(Cl)C=1NN(CC)CC(C=1C=CC=CC=1)NC1=CC=CC=C1 AJDKZWLPPHJPOJ-UHFFFAOYSA-N 0.000 description 1
- 229920002284 Cellulose triacetate Polymers 0.000 description 1
- KKUKTXOBAWVSHC-UHFFFAOYSA-N Dimethylphosphate Chemical compound COP(O)(=O)OC KKUKTXOBAWVSHC-UHFFFAOYSA-N 0.000 description 1
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- NNLVGZFZQQXQNW-ADJNRHBOSA-N [(2r,3r,4s,5r,6s)-4,5-diacetyloxy-3-[(2s,3r,4s,5r,6r)-3,4,5-triacetyloxy-6-(acetyloxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6s)-4,5,6-triacetyloxy-2-(acetyloxymethyl)oxan-3-yl]oxyoxan-2-yl]methyl acetate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](OC(C)=O)[C@H]1OC(C)=O)O[C@H]1[C@@H]([C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O1)OC(C)=O)COC(=O)C)[C@@H]1[C@@H](COC(C)=O)O[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O NNLVGZFZQQXQNW-ADJNRHBOSA-N 0.000 description 1
- MPLZNPZPPXERDA-UHFFFAOYSA-N [4-(diethylamino)-2-methylphenyl]azanium;chloride Chemical compound [Cl-].CC[NH+](CC)C1=CC=C(N)C(C)=C1 MPLZNPZPPXERDA-UHFFFAOYSA-N 0.000 description 1
- 239000006096 absorbing agent Substances 0.000 description 1
- 229960001413 acetanilide Drugs 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 150000001556 benzimidazoles Chemical class 0.000 description 1
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 1
- 150000001565 benzotriazoles Chemical class 0.000 description 1
- WZTQWXKHLAJTRC-UHFFFAOYSA-N benzyl 2-amino-6,7-dihydro-4h-[1,3]thiazolo[5,4-c]pyridine-5-carboxylate Chemical compound C1C=2SC(N)=NC=2CCN1C(=O)OCC1=CC=CC=C1 WZTQWXKHLAJTRC-UHFFFAOYSA-N 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- CQEYYJKEWSMYFG-UHFFFAOYSA-N butyl acrylate Chemical compound CCCCOC(=O)C=C CQEYYJKEWSMYFG-UHFFFAOYSA-N 0.000 description 1
- 125000004744 butyloxycarbonyl group Chemical group 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-N carbonic acid monoamide Natural products NC(O)=O KXDHJXZQYSOELW-UHFFFAOYSA-N 0.000 description 1
- 125000006297 carbonyl amino group Chemical group [H]N([*:2])C([*:1])=O 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 125000004177 diethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- XNMQEEKYCVKGBD-UHFFFAOYSA-N dimethylacetylene Natural products CC#CC XNMQEEKYCVKGBD-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 1
- XLCGXWPXVZVBTA-UHFFFAOYSA-N dodecyl 3-[[2-(3-benzyl-4-ethoxy-2,5-dioxoimidazolidin-1-yl)-3-(4-methoxyphenyl)-3-oxopropanoyl]amino]-4-chlorobenzoate Chemical compound CCCCCCCCCCCCOC(=O)C1=CC=C(Cl)C(NC(=O)C(N2C(N(CC=3C=CC=CC=3)C(OCC)C2=O)=O)C(=O)C=2C=CC(OC)=CC=2)=C1 XLCGXWPXVZVBTA-UHFFFAOYSA-N 0.000 description 1
- JRBPAEWTRLWTQC-UHFFFAOYSA-N dodecylamine Chemical compound CCCCCCCCCCCCN JRBPAEWTRLWTQC-UHFFFAOYSA-N 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000012992 electron transfer agent Substances 0.000 description 1
- 238000010931 ester hydrolysis Methods 0.000 description 1
- 125000003754 ethoxycarbonyl group Chemical group C(=O)(OCC)* 0.000 description 1
- FPVGTPBMTFTMRT-NSKUCRDLSA-L fast yellow Chemical compound [Na+].[Na+].C1=C(S([O-])(=O)=O)C(N)=CC=C1\N=N\C1=CC=C(S([O-])(=O)=O)C=C1 FPVGTPBMTFTMRT-NSKUCRDLSA-L 0.000 description 1
- 235000019233 fast yellow AB Nutrition 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229940074391 gallic acid Drugs 0.000 description 1
- 235000004515 gallic acid Nutrition 0.000 description 1
- 150000002373 hemiacetals Chemical class 0.000 description 1
- 125000005842 heteroatom Chemical group 0.000 description 1
- 150000002391 heterocyclic compounds Chemical class 0.000 description 1
- 229940042795 hydrazides for tuberculosis treatment Drugs 0.000 description 1
- 125000001165 hydrophobic group Chemical group 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 150000002473 indoazoles Chemical class 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 239000006224 matting agent Substances 0.000 description 1
- 150000004702 methyl esters Chemical class 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 125000006216 methylsulfinyl group Chemical group [H]C([H])([H])S(*)=O 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- QEALYLRSRQDCRA-UHFFFAOYSA-N myristamide Chemical compound CCCCCCCCCCCCCC(N)=O QEALYLRSRQDCRA-UHFFFAOYSA-N 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- DUWWHGPELOTTOE-UHFFFAOYSA-N n-(5-chloro-2,4-dimethoxyphenyl)-3-oxobutanamide Chemical compound COC1=CC(OC)=C(NC(=O)CC(C)=O)C=C1Cl DUWWHGPELOTTOE-UHFFFAOYSA-N 0.000 description 1
- VILFVXYKHXVYAB-UHFFFAOYSA-N naphthalene-2,7-disulfonic acid Chemical compound C1=CC(S(O)(=O)=O)=CC2=CC(S(=O)(=O)O)=CC=C21 VILFVXYKHXVYAB-UHFFFAOYSA-N 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000010534 nucleophilic substitution reaction Methods 0.000 description 1
- KPCHOCIEAXFUHZ-UHFFFAOYSA-N oxadiazole-4-thiol Chemical class SC1=CON=N1 KPCHOCIEAXFUHZ-UHFFFAOYSA-N 0.000 description 1
- 150000004866 oxadiazoles Chemical class 0.000 description 1
- 150000002916 oxazoles Chemical class 0.000 description 1
- 125000001820 oxy group Chemical group [*:1]O[*:2] 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 150000004989 p-phenylenediamines Chemical class 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 125000006678 phenoxycarbonyl group Chemical group 0.000 description 1
- UYWQUFXKFGHYNT-UHFFFAOYSA-N phenylmethyl ester of formic acid Natural products O=COCC1=CC=CC=C1 UYWQUFXKFGHYNT-UHFFFAOYSA-N 0.000 description 1
- 125000003170 phenylsulfonyl group Chemical group C1(=CC=CC=C1)S(=O)(=O)* 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical compound OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 125000001325 propanoyl group Chemical group O=C([*])C([H])([H])C([H])([H])[H] 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 125000006308 propyl amino group Chemical group 0.000 description 1
- 239000011241 protective layer Substances 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 125000004469 siloxy group Chemical group [SiH3]O* 0.000 description 1
- ADZWSOLPGZMUMY-UHFFFAOYSA-M silver bromide Chemical compound [Ag]Br ADZWSOLPGZMUMY-UHFFFAOYSA-M 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 229920006027 ternary co-polymer Polymers 0.000 description 1
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 150000003536 tetrazoles Chemical class 0.000 description 1
- JJJPTTANZGDADF-UHFFFAOYSA-N thiadiazole-4-thiol Chemical class SC1=CSN=N1 JJJPTTANZGDADF-UHFFFAOYSA-N 0.000 description 1
- 150000004867 thiadiazoles Chemical class 0.000 description 1
- YGNGABUJMXJPIJ-UHFFFAOYSA-N thiatriazole Chemical class C1=NN=NS1 YGNGABUJMXJPIJ-UHFFFAOYSA-N 0.000 description 1
- 150000003557 thiazoles Chemical class 0.000 description 1
- KJAMZCVTJDTESW-UHFFFAOYSA-N tiracizine Chemical compound C1CC2=CC=CC=C2N(C(=O)CN(C)C)C2=CC(NC(=O)OCC)=CC=C21 KJAMZCVTJDTESW-UHFFFAOYSA-N 0.000 description 1
- 238000006276 transfer reaction Methods 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- ZMANZCXQSJIPKH-UHFFFAOYSA-O triethylammonium ion Chemical compound CC[NH+](CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-O 0.000 description 1
- 239000006097 ultraviolet radiation absorber Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03C—PHOTOSENSITIVE MATERIALS FOR PHOTOGRAPHIC PURPOSES; PHOTOGRAPHIC PROCESSES, e.g. CINE, X-RAY, COLOUR, STEREO-PHOTOGRAPHIC PROCESSES; AUXILIARY PROCESSES IN PHOTOGRAPHY
- G03C7/00—Multicolour photographic processes or agents therefor; Regeneration of such processing agents; Photosensitive materials for multicolour processes
- G03C7/30—Colour processes using colour-coupling substances; Materials therefor; Preparing or processing such materials
- G03C7/32—Colour coupling substances
- G03C7/3225—Combination of couplers of different kinds, e.g. yellow and magenta couplers in a same layer or in different layers of the photographic material
Definitions
- This invention relates to photographic elements and processes for developing such elements where the elements contain a combination of dye-forming couplers which render the resulting phototgraphic images less susceptible to variations in the developer solution parameters.
- EP 365,282 discloses the use of certain yellow dye-forming couplers (including 2-pivaloylacetanilides) in a single blue light sensitive layer to improve sensitivity to developer pH variations.
- Japanese published applications JO 2027-344-A and J0 2039-047 disclose a broad range of magenta, yellow, and cyan couplers for use to impart stability to elements, and in particular to print images. There is no suggestion of the process sensitivity advantages to be obtained using the combination of the invention.
- Us 4,748,107 discloses the combination of 2-pivaloylacetanilides, 3-anilino-1-phenyl-5-pyrazolones, and the different 2-carbonamidophenols as the yellow, magenta, and cyan dye-forming couplers, respectively, for improved color formation and reproducibility and for image stability in color paper products. None of these proposals provide a solution to the developer sensitivity problem.
- a photographic element which comprises at least one light sensitive silver halide layer sensitized to one or more of green, blue, and red light, and having associated therewith a 3-anilino-1-phenyl-5-pyrazolone magenta dye-forming coupler, a two-equivalent trialkylacetyl-acetanilide yellow dye-forming coupler, and a 2-ureido-5-carbonamidophenol cyan dye-forming coupler.
- the invention also provides a process for forming an image from an exposed element as above described through contact with a color developing agent.
- the invention thus provides photographic images which are more consistent for color density balance and contrast balance regardless of developer parameters which therefore enables the photofinisher the opportunity to print the color negative to a correct color balance.
- This invention also enables photofinishers to maintain the same exposure parameters on automatic printers even as change or variability occurs in the development process due to developer bath variations.
- the magenta dye-forming coupler is represented by formula I
- the yellow dye-forming coupler is represented by formula II
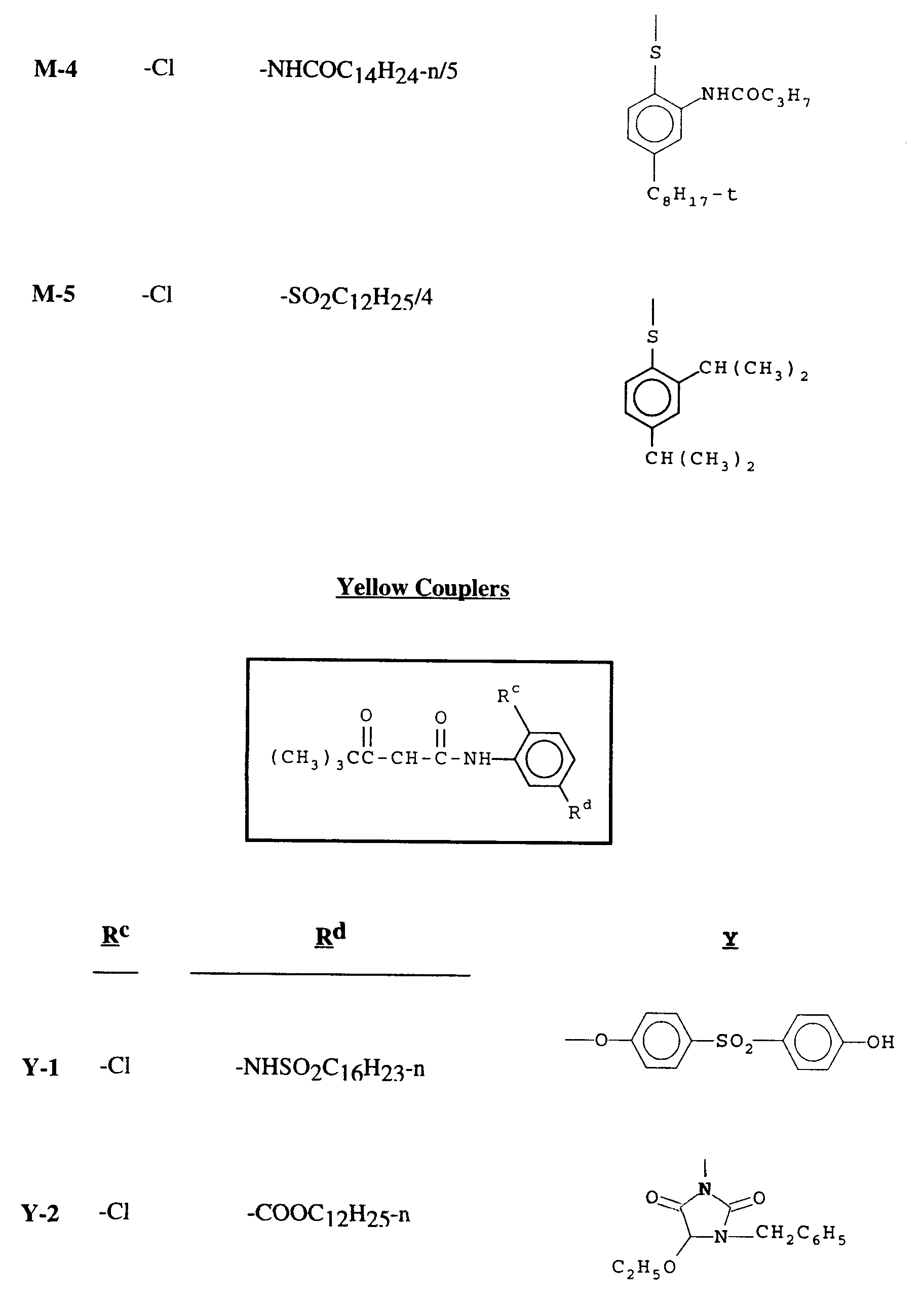
- the cyan dye-forming coupler is represented by formula III: wherein R1 and R2 are independently selected from the group consisting of hydrogen, halogen, trifluoromethyl, cyano, nitro, and substituted or unsubstituted alkyl, alkoxy, aryloxy, alkylthio, carbonamido, carbamoyl, sulfonamido, sulfamoyl, alkylsulfonyl, arylsulfonyl, alkoxycarbonyl, acyloxy, and acyl groups; each R is independently a substituent with r being 0 to 2 and s being 0 to 3 and X is a substituted or unsubstituted arylthio or alkylthio group; wherein R5 and R6 are independently selected from the group consisting of hydrogen, halogen, trifluoromethyl
- substituent unless otherwise specifically stated, has a broad definition.
- the substituent may be, for example, halogen, such as chlorine, bromine or fluorine; nitro; hydroxyl; cyano; and -CO2H and its salts; and groups which may be further substituted, such as alkyl, including straight or branched chain alkyl, such as methyl, trifluoromethyl, ethyl, t -butyl, 3-(2,4-di-t-amylphenoxy) propyl, and tetradecyl; alkenyl, such as ethylene, 2-butene; alkoxy, such as methoxy, ethoxy, propoxy, butoxy, 2-methoxyethoxy, sec -butoxy, hexyloxy, 2-ethylhexyloxy, tetradecyloxy 2-(2,4-di- t -pentylphenoxy)ethoxy, and 2-dodecyl
- the particular substituents used may be selected to attain the desired photographic properties for a specific application and can include, for example, hydrophobic groups, solubilizing groups, blocking groups, etc.
- the above groups and substituents thereof may typically include those having 1 to 30 carbon atoms and usually less than 24 carbon atoms, but greater numbers are possible depending on the particular substituents selected.
- the substituents may themselves be suitably substituted with any of the above groups.
- the R1 group is preferably chlorine or alkoxy of up to 8 carbon atoms.
- R2 is preferably carbonamido, sulfamoyl or sulfonamido.
- the coupling-off group X is an alkylthio or arylthio group.
- the coupling-off group can suitably have the formula: wherein R3 and R4 are individually selected from the group consisting of hydrogen, halogen, carboxyl, and substituted or unsubstituted alkyl, alkoxy, aryloxy, carbonamido, ureido, carbamate, sulfonamido, carbamoyl, sulfamoyl, acyloxy, alkoxycarbonyl, aryloxycarbonyl, and amino groups; q is 0 to 4; and R4 may be para and/or meta to the sulfur atom. It is preferred that R3 have at least one carbon atom and that the total number of carbon atoms in R3 and R4 together be from 4 to 25.
- the yellow dye-forming coupler II also has certain preferred embodiments.
- the R5 substituent is typically chlorine or alkoxy of up to 8 carbon atoms.
- the R6 substituent is preferably carbonamido, sulfamoyl, or sulfonamido.
- Y is suitably an aryloxy coupling-off group.
- R7 is selected from the group consisting of hydrogen, halogen, cyano, nitro, trifluoromethyl and substituted or unsubstituted alkyl, alkoxy, alkylthio, carbonamido, carbamoyl, sulfonamido, sulfamoyl, alkylsulfonyl, arylsulfonyl, alkoxycarbonyl, acyloxy, and acyl groups and each R t is independently a substituent with t being 0 to 4.
- R7 is arylsulfonyl.
- Y may be represented by the formula: wherein W is -O-, -S-, -N(R C )-, or -C(R D )(R E )-; R A is H, or substituted or unsubstituted alkyl, alkoxy, phenyl, or phenoxy; R B and R E are independently H or substituted or unsubstituted alkyl; R C is substituted or unsubstituted alkyl or phenyl; and R D is H or substituted or unsubstituted alkyl or alkoxy.
- W is -NR D ;
- R A is H, or substituted or unsubstituted alkyl or alkoxy;
- R B is H; and
- R C is substituted or unsubstituted alkyl.
- R A is -OC2H5
- R B is H and R C is -CH2-phenyl.
- W is -C(R D )(R E ) with R A , R B , R D , and R E defined as above.
- cyan dye-forming coupler it is preferred that at least one (R) p be present.
- examples are cyano in the 4-position and cyano in the 3-position and Cl in the 4-position.
- R8 is suitably of the formula wherein R9 and R10 are substituted or unsubstituted alkyl of up to 16 carbon atoms and R is a substituent with u being 0 to 3.
- Z is typically hydrogen or aryloxy.
- the materials of the invention can be used in any of the ways and in any of the combinations known in the art.
- the invention materials are incorporated in a silver halide emulsion and the emulsion coated as a layer on a support to form part of a photographic element.
- they can be incorporated at a location adjacent to the silver halide emulsion layer where, during development, they will be in reactive association with development products such as oxidized color developing agent.
- the term "associated" signifies that the compound is in the silver halide emulsion layer or in an adjacent location where, during processing, it is capable of reacting with silver halide development products.
- ballast groups include substituted or unsubstituted alkyl or aryl groups containing 8 to 40 carbon atoms.
- substituents on such groups include alkyl, aryl, alkoxy, aryloxy, alkylthio, hydroxy, halogen, alkoxycarbonyl, aryloxcarbonyl, carboxy, acyl, acyloxy, amino, anilino, carbonamido, carbamoyl, alkylsulfonyl, arysulfonyl, sulfonamido, and sulfamoyl groups wherein the substituents typically contain 1 to 40 carbon atoms. Such substituents can also be further substituted.
- the photographic elements can be single color elements or multicolor elements.
- Multicolor elements contain image dye-forming units sensitive to each of the three primary regions of the spectrum.
- Each unit can comprise a single emulsion layer or multiple emulsion layers sensitive to a given region of the spectrum.
- the layers of the element, including the layers of the image-forming units, can be arranged in various orders as known in the art.
- the emulsions sensitive to each of the three primary regions of the spectrum can be disposed as a single segmented layer.
- a typical multicolor photographic element comprises a support bearing a cyan dye image-forming unit comprised of at least one red-sensitive silver halide emulsion layer having associated therewith at least one cyan dye-forming coupler, a magenta dye image-forming unit comprising at least one green-sensitive silver halide emulsion layer having associated therewith at least one magenta dye-forming coupler, and a yellow dye image-forming unit comprising at least one blue-sensitive silver halide emulsion layer having associated therewith at least one yellow dye-forming coupler.
- the element can contain additional layers, such as filter layers, interlayers, overcoat layers, subbing layers, and the like.
- the photographic element can be used in conjunction with an applied magnetic layer as described in Research Disclosure , November 1992, Item 34390 published by Kenneth Mason Publications, Ltd., Dudley Annex, 12a North Street, Emsworth, Hampshire P010 7DQ, ENGLAND.
- the silver halide emulsions employed in the elements of this invention can be either negative-working or positive-working. Suitable emulsions and their preparation as well as methods of chemical and spectral sensitization are described in Sections I through IV. Color materials and development modifiers are described in Sections V and XXI. Vehicles are described in Section IX, and various additives such as brighteners, antifoggants, stabilizers, light absorbing and scattering materials, hardeners, coating aids, plasticizers, lubricants and matting agents are described , for example, in Sections V, VI, VIII, X, XI, XII, and XVI. Manufacturing methods are described in Sections XIV and XV, other layers and supports in Sections XIII and XVII, processing methods and agents in Sections XIX and XX, and exposure alternatives in Section XVIII.
- Coupling-off groups are well known in the art. Such groups can determine the chemical equivalency of a coupler, i.e., whether it is a 2-equivalent or a 4-equivalent coupler, or modify the reactivity of the coupler. Such groups can advantageously affect the layer in which the coupler is coated, or other layers in the photographic recording material, by performing, after release from the coupler, functions such as dye formation, dye hue adjustment, development acceleration or inhibition, bleach acceleration or inhibition, electron transfer facilitation, color correction and the like.
- the presence of hydrogen at the coupling site provides a 4-equivalent coupler, and the presence of another coupling-off group provides a 2-equivalent coupler.
- Representative classes of such coupling-off groups include, for example, chloro, alkoxy, aryloxy, hetero-oxy, sulfonyloxy, acyloxy, acyl, heterocyclyl, sulfonamido, mercaptotetrazole, benzothiazole, mercaptopropionic acid, phosphonyloxy, arylthio, and arylazo.
- couplers any of which may contain known ballasts or coupling-off groups such as those described in U.S. Patent 4,301,235; U.S. Patent 4,853,319 and U.S. Patent 4,351,897.
- the coupler may also be used in association with "wrong" colored couplers (e.g. to adjust levels of interlayer correction) and, in color negative applications, with masking couplers such as those described in EP 213.490; Japanese Published Application 58-172,647; U.S. Patent 2,983,608; German Application DE 2,706,117C; U.K. Patent 1,530,272; Japanese Application A-113935; U.S. Patents 4,070,191 and 4,273,861; and German Application DE 2,643,965.
- the masking couplers may be shifted or blocked.
- the invention materials may also be used in association with materials that accelerate or otherwise modify the processing steps e.g. of bleaching or fixing to improve the quality of the image.
- Bleach accelerator releasing couplers such as those described in EP 193,389; EP 301,477; U.S. 4,163,669; U.S. 4,865,956; and U.S. 4,923,784, may be useful.
- Also contemplated is use of the compositions in association with nucleating agents, development accelerators or their precursors (UK Patent 2,097,140; U.K. Patent 2,131,188); electron transfer agents (U.S. 4,859,578; U.S.
- antifogging and anti color-mixing agents such as derivatives of hydroquinones, aminophenols, amines, gallic acid; catechol; ascorbic acid; hydrazides; sulfonamidophenols; and non color-forming couplers.
- the invention materials may be substituted in whole or in part in the layers of a color negative photographic element comprising a support bearing the following layers from top to bottom:
- the invention materials may also be used in combination with filter dye layers comprising colloidal silver sol or yellow, cyan, and/or magenta filter dyes, either as oil-in-water dispersions, latex dispersions or as solid particle dispersions. Additionally, they may be used with "smearing" couplers (e.g. as described in U.S. 4,366,237; EP 96,570; U.S. 4,420,556; and U.S. 4,543,323.) Also, the compositions may be blocked or coated in protected form as described, for example, in Japanese Application 61/258,249 or U.S. 5,019,492.
- the invention materials may further be used in combination with image-modifying compounds such as "Developer Inhibitor-Releasing” compounds (DIR's).
- DIR's useful in conjunction with the compositions of the invention are known in the art and examples are described in U.S. Patent Nos.
- DIR Couplers for Color Photography
- C.R. Barr J.R. Thirtle and P.W. Vittum in Photographic Science and Engineering , Vol. 13, p. 174 (1969)
- the developer inhibitor-releasing (DIR) couplers include a coupler moiety and an inhibitor coupling-off moiety (IN).
- the inhibitor-releasing couplers may be of the time-delayed type (DIAR couplers) which also include a timing moiety or chemical switch which produces a delayed release of inhibitor.
- inhibitor moieties are: oxazoles, thiazoles, diazoles, triazoles, oxadiazoles, thiadiazoles, oxathiazoles, thiatriazoles, benzotriazoles, tetrazoles, benzimidazoles, indazoles, isoindazoles, mercaptotetrazoles, selenotetrazoles, mercaptobenzothiazoles, selenobenzothiazoles, mercaptobenzoxazoles, selenobenzoxazoles, mercaptobenzimidazoles, selenobenzimidazoles, benzodiazoles, mercaptooxazoles, mercaptothiadiazoles, mercaptothiazoles, mercaptotriazoles, mercaptooxadiazoles, mercaptodiazoles, mercaptooxathiazoles, telleurotetrazoles or benz
- the inhibitor moiety or group is selected from the following formulas: wherein R I is selected from the group consisting of straight and branched alkyls of from 1 to about 8 carbon atoms, benzyl, phenyl, and alkoxy groups and such groups containing none, one or more than one such substituent; R II is selected from R I and -SR I ; R III is a straight or branched alkyl group of from 1 to about 5 carbon atoms and m is from 1 to 3; and R IV is selected from the group consisting of hydrogen, halogens and alkoxy, phenyl and carbonamido groups, -COOR V and - NHCOOR V wherein R V is selected from substituted and unsubstituted alkyl and aryl groups.
- the coupler moiety included in the developer inhibitor-releasing coupler forms an image dye corresponding to the layer in which it is located, it may also form a different color as one associated with a different film layer. It may also be useful that the coupler moiety included in the developer inhibitor-releasing coupler forms colorless products and/or products that wash out of the photographic material during processing (so-called "universal" couplers).
- the developer inhibitor-releasing coupler may include a timing group which produces the time-delayed release of the inhibitor group such as groups utilizing the cleavage reaction of a hemiacetal (U.S. 4,146,396, Japanese Applications 60-249148; 60-249149); groups using an intramolecular nucleophilic substitution reaction (U.S. 4,248,962); groups utilizing an electron transfer reaction along a conjugated system (U.S. 4,409,323; 4,421,845; Japanese Applications 57-188035; 58-98728; 58-209736; 58-209738) groups utilizing ester hydrolysis (German Patent Application (OLS) No.
- a timing group which produces the time-delayed release of the inhibitor group such as groups utilizing the cleavage reaction of a hemiacetal (U.S. 4,146,396, Japanese Applications 60-249148; 60-249149); groups using an intramolecular nucleophilic substitution reaction (U.S. 4,248,962); groups utilizing an electron
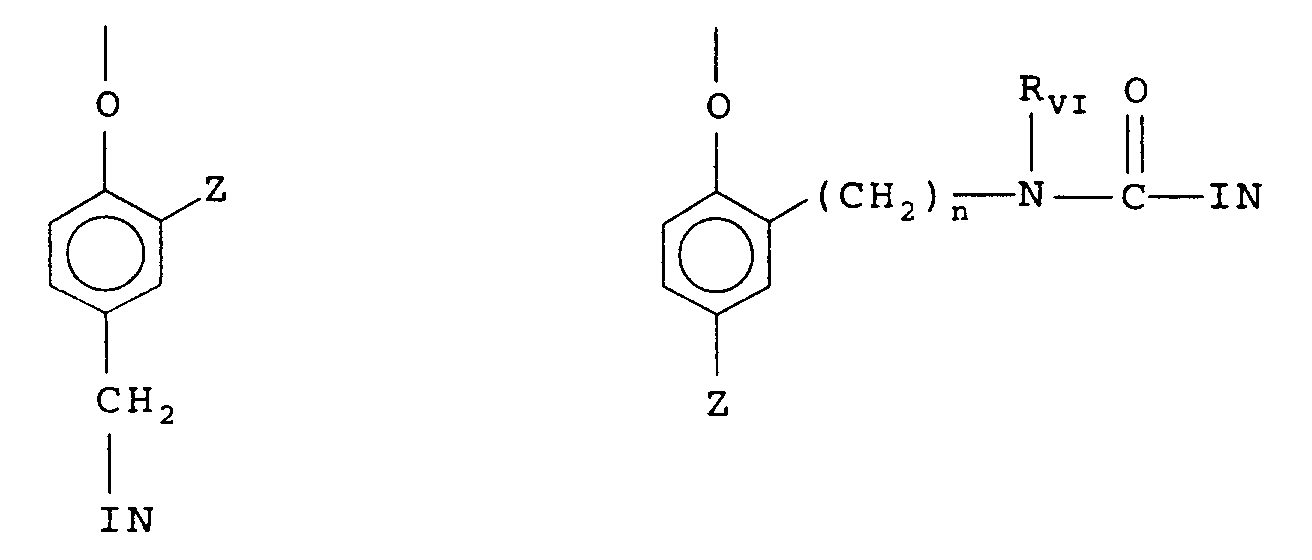
- timing group or moiety is of one of the formulas: wherein IN is the inhibitor moiety, Z is selected from the group consisting of nitro, cyano, alkylsulfonyl; sulfamoyl (-SO2NR2); and sulfonamido (-NRSO2R) groups; n is 0 or 1; and R VI is selected from the group consisting of substituted and unsubstituted alkyl and phenyl groups.
- the oxygen atom of each timing group is bonded to the coupling-off position of the respective coupler moiety of the DIAR.
- T average tabularity
- the average useful ECD of photographic emulsions can range up to about 10 microns, although in practice emulsion ECD's seldom exceed about 4 microns. Since both photographic speed and granularity increase with increasing ECD's, it is generally preferred to employ the smallest tabular grain ECD's compatible with achieving aim speed requirements.
- Emulsion tabularity increases markedly with reductions in tabular grain thickness. It is generally preferred that aim tabular grain projected areas be satisfied by thin (t ⁇ 0.2 micron) tabular grains. To achieve the lowest levels of granularity it is preferred that aim tabular grain projected areas be satisfied with ultrathin (t ⁇ 0.06 micron) tabular grains. Tabular grain thicknesses typically range down to about 0.02 micron. However, still lower tabular grain thicknesses are contemplated. For example, Daubendiek et al U.S. Patent 4,672,027 reports a 3 mole percent iodide tabular grain silver bromoiodide emulsion having a grain thickness of 0.017 micron.
- tabular grains of less than the specified thickness account for at least 50 percent of the total grain projected area of the emulsion.
- tabular grains satisfying the stated thickness criterion account for the highest conveniently attainable percentage of the total grain projected area of the emulsion.
- tabular grains satisfying the stated thickness criteria above account for at least 70 percent of the total grain projected area.
- tabular grains satisfying the thickness criteria above account for at least 90 percent of total grain projected area.
- Suitable tabular grain emulsions can be selected from among a variety of conventional teachings, such as those of the following: Research Disclosure, Item 22534, January 1983, published by Kenneth Mason Publications, Ltd., Emsworth, Hampshire P010 7DD, England; U.S. Patent Nos.
- the emulsions can be surface-sensitive emulsions, i.e., emulsions that form latent images primarily on the surfaces of the silver halide grains, or the emulsions can form internal latent images predominantly in the interior of the silver halide grains.
- the emulsions can be negative-working emulsions, such as surface-sensitive emulsions or unfogged internal latent image-forming emulsions, or direct-positive emulsions of the unfogged, internal latent image-forming type, which are positive-working when development is conducted with uniform light exposure or in the presence of a nucleating agent.
- Photographic elements can be exposed to actinic radiation, typically in the visible region of the spectrum, to form a latent image and can then be processed to form a visible dye image.
- Processing to form a visible dye image includes the step of contacting the element with a color developing agent to reduce developable silver halide and oxidize the color developing agent. Oxidized color developing agent in turn reacts with the coupler to yield a dye.
- the processing step described above provides a negative image.
- the described elements can be processed in the known C-41 color process as described in The British Journal of Photography Annual of 1982, pages 209 - 211 and 1988, pages 191-198.
- the color development step can be preceded by development with a non-chromogenic developing agent to develop exposed silver halide, but not form dye, and followed by uniformly fogging the element to render unexposed silver halide developable.
- a direct positive emulsion can be employed to obtain a positive image.
- Preferred color developing agents are p-phenylenediamines such as: 4-amino-N,N-diethylaniline hydrochloride, 4-amino-3-methyl-N,N-diethylaniline hydrochloride, 4-amino-3-methyl-N-ethyl-N-(b-(methanesulfonamido) ethyl)aniline sesquisulfate hydrate, 4-amino-3-methyl-N-ethyl-N-(b-hydroxyethyl)aniline sulfate, 4-amino-3-b-(methanesulfonamido)ethyl-N,N-diethylaniline hydrochloride and 4-amino-N-ethyl-N-(2-methoxyethyl)-m-toluidine di-p-toluene sulfonic acid.
- Development is usually followed by the conventional steps of bleaching, fixing, or bleach-fixing, to remove silver or silver halide, washing, and drying.
- any reference to a substituent by the identification of a group containing a substitutable hydrogen e.g. alkyl, amine, aryl, alkoxy, heterocyclic, etc.
- a substitutable hydrogen e.g. alkyl, amine, aryl, alkoxy, heterocyclic, etc.
- the substituent will have less than 30 carbon atoms and typically less than 20 carbon atoms.
- a cellulose triacetate film support was coated with the following layers, in sequence (coverages are in grams per meter squared) in order to prepare Photographic Sample 101:
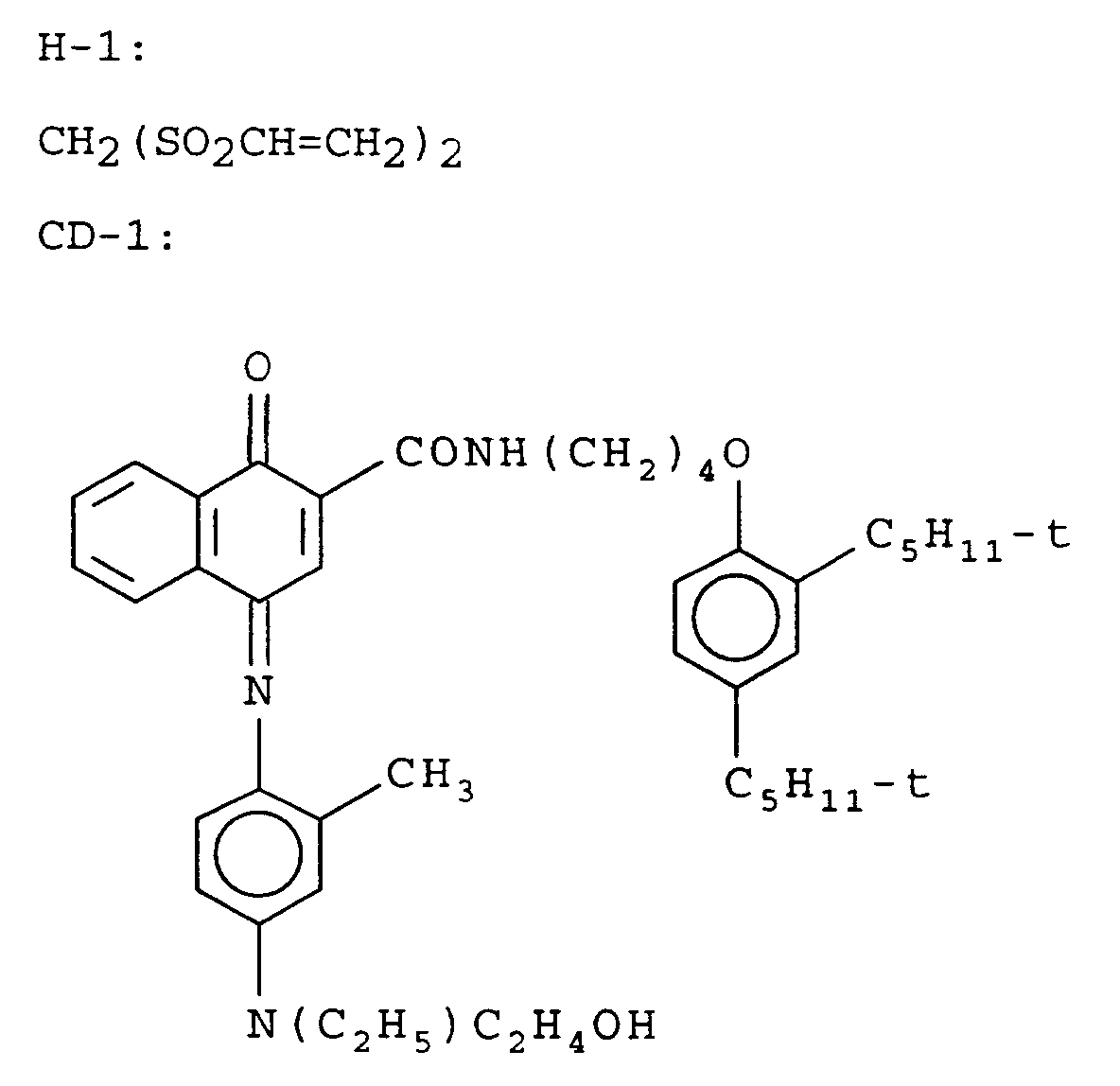
- This film was hardened at coating with 1.75% by weight of total gelatin of hardener H-1.
- Surfactants, coating aids, oxidized developer scavengers, soluble absorber dyes and stabilizers were added to the various layers of this sample as is commonly practiced in the art.
- Photographic Sample 102 was prepared like Photographic Sample 101 except for changes in Layers 6, 7, and 8.
- Magenta dye-forming couplers M-Comp-1 and M-Comp-2 were omitted in all three layers and replaced with magenta dye-forming coupler M-1.
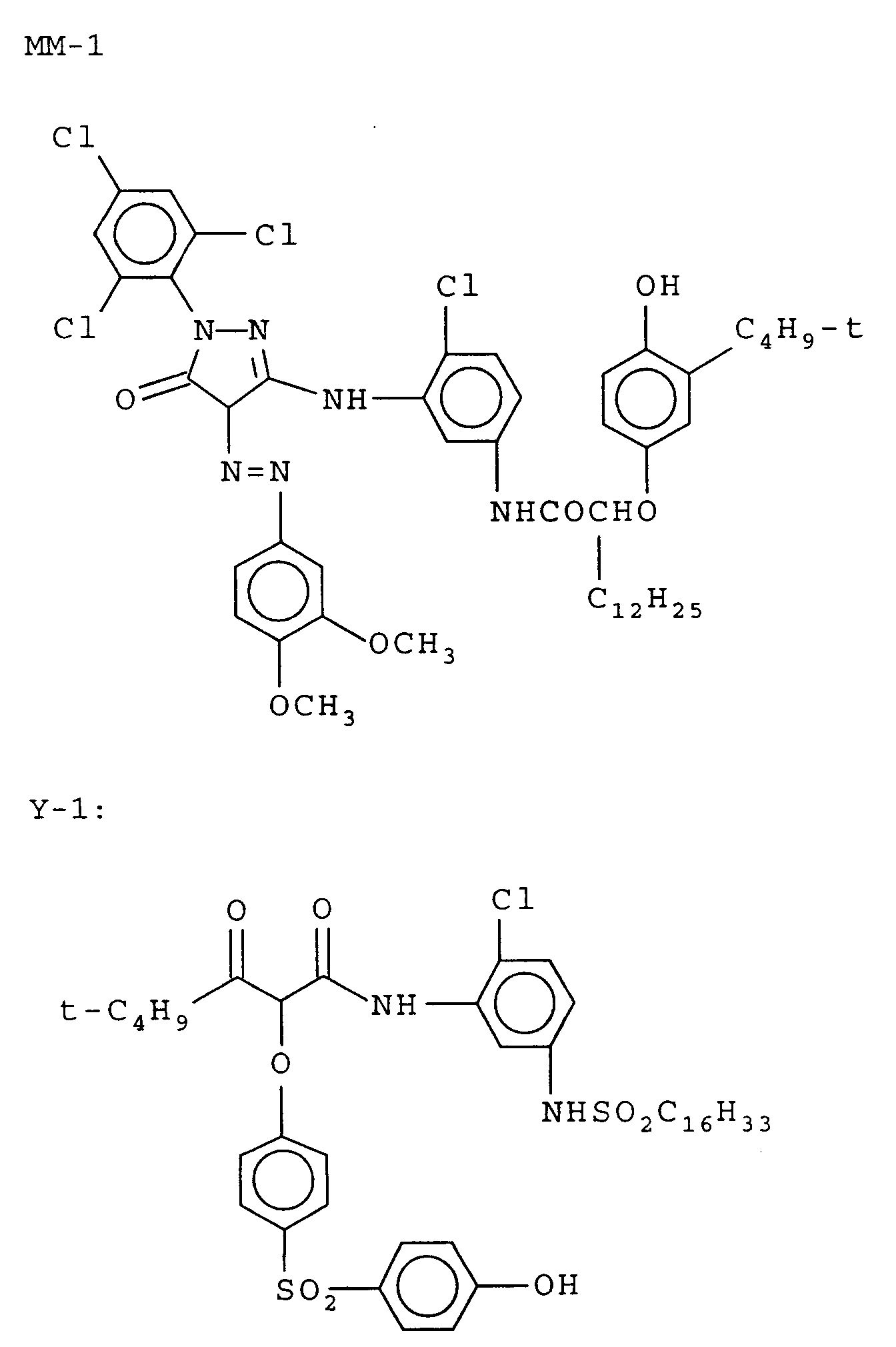
- Photographic Sample 103 was prepared like Photographic Sample 102 except for changes in Layers 6, 7, & 8. In all three layers, the level of M-1 was reduced and magenta dye-forming coupler M-Comp-1 was added.
- the blended couplers provided about 75 mol % of the comparison pyrazolotriazole coupler to 25 mol % of the magenta coupler of the invention.
- the resulting film examples were exposed through a graduated step wedge to a light source at 5500 deg K.
- the exposed elements were processed in a standard C-41 process (as described in the British Journal of Photography Annual, 1988, pages 196-198) for the typical response to the C-41 process and also processed through 8 variations of the C-41 developer to determine sensitivity of the elements to these variations.
- the 8 variations are arrived at by independently varying three separate developer parameters from standard conditions in order to determine the effect of the variation on a selected photographic property.
- the standard condition for the developer was: A. color developer concentration 4.5 g/l B. developer pH 10.05 C. bromide concentration 1.3 g/l
- the above parameters were then varied as follows: Test A B C 1* 4.5 10.05 1.3 2 3.5 9.95 0.8 3 5.5 9.95 0.8 4 3.5 10.15 0.8 5 5.5 10.15 0.8 6 3.5 9.95 1.8 7 5.5 9.95 1.8 8 3.5 10.15 1.8 9 5.5 10.15 1.8 * This is the target or normal value of each of the parameters.
- the sensitivity estimates it is desirable for the sensitivity estimates to approach zero for all developer components and to be as closely matched Red-to-Green-to-Blue as possible.
- the data indicate the invention provides an improvement in this regard for sensitivity to both Br- and pH variations while maintaining a well-balanced response to CD-4.
- This example also demonstrates that when the comparison pyrazolotriazole coupler is used in combination with the coupler of the invention in a mol ratio of 3 : 1, respectively, the desired response is not obtained.
- the improvement is not realized when a pyrazolotriazole coupler is included in an amount exceeding the molar concentration of the invention coupler.
- the element is substantially free of the pyrazozotriazole coupler.
Landscapes
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Silver Salt Photography Or Processing Solution Therefor (AREA)
Abstract
A photographic element comprises at least one light sensitive silver halide layer sensitized to one or more of green, blue, and red light, and having associated therewith a 3-anilino-1-phenyl-5-pyrazolone magenta dye-forming coupler, a two-equivalent trialkylacetylacetanilide yellow dye-forming coupler, and a 2-ureido-5-carbonamidophenol cyan dye-forming coupler.
Description
- This invention relates to photographic elements and processes for developing such elements where the elements contain a combination of dye-forming couplers which render the resulting phototgraphic images less susceptible to variations in the developer solution parameters.
- There are a variety of photofinishers, minilabs, and microlabs that offer the standard C-41 processing for color negative photographic materials. Analyses of these trade processes for C-41 developer components have indicated that various combinations of CD-4 (color developer) concentration, bromide (Br-) concentration, and developer pH value exist in the photofinishing trade. Ideally, color negative photographic materials would be insensitive to these flucuations in developer components, such that density levels and color density balance would be consistent lab-to-lab, day-to-day, and film-to-film. However, currently available color negative photographic materials contain imaging chemistries that display an undesirable sensitivity in this respect. In the absence of totally insensitive materials with which to manufacture color photographic materials, emphasis must be placed on reducing individual chemical sensitivity and matching the sensitometric response of the separate color records so that effects on color contrast balance and color density balance are minimized and overall picture quality is not compromised.
- Commonly used red light sensitive record image chemicals in current color photographic materials display lower sensitivity to C-41 developer perturbations than materials employed in the green and blue light sensitive records. Accordingly, it has been necessary, in order to achieve matched sensitometric responses of the three color records, to reduce the sensitivity of yellow-dye forming and magenta-dye forming couplers. EP 518,101 describes methodology to lower the sensitivity of yellow dye-forming couplers by the addition of a coupler to the blue light sensitive record that releases 3-thiopropionic acid upon coupling with oxidized developer. This has not proven to be the best solution to the problem.
- EP 365,282 discloses the use of certain yellow dye-forming couplers (including 2-pivaloylacetanilides) in a single blue light sensitive layer to improve sensitivity to developer pH variations. Japanese published applications JO 2027-344-A and J0 2039-047 disclose a broad range of magenta, yellow, and cyan couplers for use to impart stability to elements, and in particular to print images. There is no suggestion of the process sensitivity advantages to be obtained using the combination of the invention. Us 4,748,107 discloses the combination of 2-pivaloylacetanilides, 3-anilino-1-phenyl-5-pyrazolones, and the different 2-carbonamidophenols as the yellow, magenta, and cyan dye-forming couplers, respectively, for improved color formation and reproducibility and for image stability in color paper products. None of these proposals provide a solution to the developer sensitivity problem.
- It is thus a problem to be solved to provide a photographic element and process which will provide an improved response in photographic image properties in spite of variations in developer parameters.
- The above problem is solved by providing a photographic element which comprises at least one light sensitive silver halide layer sensitized to one or more of green, blue, and red light, and having associated therewith a 3-anilino-1-phenyl-5-pyrazolone magenta dye-forming coupler, a two-equivalent trialkylacetyl-acetanilide yellow dye-forming coupler, and a 2-ureido-5-carbonamidophenol cyan dye-forming coupler. The invention also provides a process for forming an image from an exposed element as above described through contact with a color developing agent.
- The invention thus provides photographic images which are more consistent for color density balance and contrast balance regardless of developer parameters which therefore enables the photofinisher the opportunity to print the color negative to a correct color balance. This invention also enables photofinishers to maintain the same exposure parameters on automatic printers even as change or variability occurs in the development process due to developer bath variations.
- In a preferred embodiment of the invention, the magenta dye-forming coupler is represented by formula I, the yellow dye-forming coupler is represented by formula II, and the cyan dye-forming coupler is represented by formula III:
wherein R¹ and R² are independently selected from the group consisting of hydrogen, halogen, trifluoromethyl, cyano, nitro, and substituted or unsubstituted alkyl, alkoxy, aryloxy, alkylthio, carbonamido, carbamoyl, sulfonamido, sulfamoyl, alkylsulfonyl, arylsulfonyl, alkoxycarbonyl, acyloxy, and acyl groups; each R is independently a substituent with r being 0 to 2 and s being 0 to 3 and X is a substituted or unsubstituted arylthio or alkylthio group;
wherein R⁵ and R⁶ are independently selected from the group consisting of hydrogen, halogen, cyano, nitro, trifluoromethyl, and substituted or unsubstituted alkyl, alkoxy, aryloxy, alkylthio, carbonamido, carbamoyl, sulfonamido, sulfamoyl, alkylsulfonyl, arylsulfonyl, alkoxycarbonyl, acyloxy, and acyl groups; each R is independently a substituent as hereafter defined with n being 0 to 3; and Y is an aryloxy group or is selected from the group consisting of substituted and unsubstituted imidazole, pyrazole, and heterocyclic compounds represented by the formula:
wherein z represents the atoms necessary to complete a heterocyclic ring; and
wherein each R⁸ is independently selected from the group consisting of substituted and unsubstituted alkyl, alkoxy, aryloxy, alkylthio, carbonamido, aryl, carbamoyl, sulfonamido, sulfamoyl, alkylsulfonyl, arylsulfonyl, acyloxy, acyl, and alkoxycarbonyl groups; each R is independently a substituent with m being 0 to 2 and p being 0 to 5; and Z is hydrogen or a coupling-off group. - As used herein, the term substituent, unless otherwise specifically stated, has a broad definition. The substituent may be, for example, halogen, such as chlorine, bromine or fluorine; nitro; hydroxyl; cyano; and -CO₂H and its salts; and groups which may be further substituted, such as alkyl, including straight or branched chain alkyl, such as methyl, trifluoromethyl, ethyl, t-butyl, 3-(2,4-di-t-amylphenoxy) propyl, and tetradecyl; alkenyl, such as ethylene, 2-butene; alkoxy, such as methoxy, ethoxy, propoxy, butoxy, 2-methoxyethoxy, sec-butoxy, hexyloxy, 2-ethylhexyloxy, tetradecyloxy 2-(2,4-di-t-pentylphenoxy)ethoxy, and 2-dodecyloxyethoxy; aryl such as phenyl, 4-t-butylphenyl, 2,4,6-trimethylphenyl, naphthyl; aryloxy, such as phenoxy, 2-methylphenoxy, alpha- or beta-naphthyloxy, and 4-tolyloxy; carbonamido, such as acetamido, benzamido, butyramido, tetradecanamido, alpha-(2,4-di-t-pentylphenoxy)acetamido, alpha-(2,4-di-t-pentylphenoxy)butyramido, alpha-(3-pentadecylphenoxy)hexanamido, alpha-(4-hydroxy-3-t-butylphenoxy)tetradecanamido, 2-oxo-pyrrolidin-1-yl, 2-oxo-5-tetradecylpyrrolin-1-yl, N-methyltetradecanamido, N-succinimido, N-phthalimido, 2,5-dioxo-1-oxazolidinyl, 3-dodecyl-2,5-dioxo-1-imidazolyl, and N-acetyl-N-dodecylamino, ethoxycarbonylamino, phenoxycarbonylamino, benzyloxycarbonylamino, hexadecyloxycarbonylamino, 2,4-di-t-butylphenoxycarbonylamino, phenylcarbonylamino, 2,5-(di-t-pentylphenyl)carbonylamino, p-dodecylphenylcarbonylamino, p-toluylcarbonylamino, N-methylureido, N,N-dimethylureido, N-methyl-N-dodecylureido, N-hexadecylureido, N,N-dioctadecylureido, N,N-dioctyl-N'-ethylureido, N-phenylureido, N,N-diphenylureido, N-phenyl-N-p-toluylureido, N-(m-hexadecylphenyl)ureido, N,N-(2,5-di-t-pentylphenyl)-N'-ethylureido, and t-butylcarbonamido; sulfonamido, such as methylsulfonamido, benzenesulfonamido, p-toluylsulfonamido, p-dodecylbenzenesulfonamido, N-methyltetradecylsulfonamido, N,N-dipropylsulfamoylamino, and hexadecylsulfonamido; sulfamoyl, such as N-methylsulfamoyl, N-ethylsulfamoyl, N,N-dipropylsulfamoyl, N-hexadecylsulfamoyl, N,N-dimethylsulfamoyl; N-[3-(dodecyloxy)propyl]sulfamoyl, N-[4-(2,4-di-t-pentylphenoxy)butyl]sulfamoyl, N-methyl-N-tetradecylsulfamoyl, and N-dodecylsulfamoyl; carbamoyl, such as N-methylcarbamoyl, N,N-dibutylcarbamoyl, N-octadecylcarbamoyl, N-[4-(2,4-di-t-pentylphenoxy)butyl]carbamoyl, N-methyl-N-tetradecylcarbamoyl, and N,N-dioctylcarbamoyl; acyl, such as acetyl, (2,4-di-t-amylphenoxy)acetyl, phenoxycarbonyl, p-dodecyloxyphenoxycarbonyl methoxycarbonyl, butoxycarbonyl, tetradecyloxycarbonyl, ethoxycarbonyl, benzyloxycarbonyl, 3-pentadecyloxycarbonyl, and dodecyloxycarbonyl; sulfonyl, such as methoxysulfonyl, octyloxysulfonyl, tetradecyloxysulfonyl, 2-ethylhexyloxysulfonyl, phenoxysulfonyl, 2,4-di-t-pentylphenoxysulfonyl, methylsulfonyl, octylsulfonyl, 2-ethylhexylsulfonyl, dodecylsulfonyl, hexadecylsulfonyl, phenylsulfonyl, 4-nonylphenylsulfonyl, and p-toluylsulfonyl; sulfonyloxy, such as dodecylsulfonyloxy, and hexadecylsulfonyloxy; sulfinyl, such as methylsulfinyl, octylsulfinyl, 2-ethylhexylsulfinyl, dodecylsulfinyl, hexadecylsulfinyl, phenylsulfinyl, 4-nonylphenylsulfinyl, and p-toluylsulfinyl; thio, such as ethylthio, octylthio, benzylthio, tetradecylthio, 2-(2,4-di-t-pentylphenoxy)ethylthio, phenylthio, 2-butoxy-5-t-octylphenylthio, and p-tolylthio; acyloxy, such as acetyloxy, benzoyloxy, octadecanoyloxy, p-dodecylamidobenzoyloxy, N-phenylcarbamoyloxy, N-ethylcarbamoyloxy, and cyclohexylcarbonyloxy; amine, such as phenylanilino, 2-chloroanilino, diethylamine, dodecylamine; imino, such as 1 (N-phenylimido)ethyl, N-succinimido or 3-benzylhydantoinyl; phosphate, such as dimethylphosphate and ethylbutylphosphate; phosphite, such as diethyl and dihexylphosphite; azo, such as phenylazo and naphthylazo; a heterocyclic group, a heterocyclic oxy group or a heterocyclic thio group, each of which may be substituted and which contain a 3 to 7 membered heterocyclic ring composed of carbon atoms and at least one hetero atom selected from the group consisting of oxygen, nitrogen and sulfur, such as 2-furyl, 2-thienyl, 2-benzimidazolyloxy or 2-benzothiazolyl; quaternary ammonium, such as triethylammonium; and silyloxy, such as trimethylsilyloxy.
- The particular substituents used may be selected to attain the desired photographic properties for a specific application and can include, for example, hydrophobic groups, solubilizing groups, blocking groups, etc. Generally, the above groups and substituents thereof may typically include those having 1 to 30 carbon atoms and usually less than 24 carbon atoms, but greater numbers are possible depending on the particular substituents selected. Moreover, as indicated, the substituents may themselves be suitably substituted with any of the above groups.
- In the magenta dye-forming coupler there are certain preferred embodiments. The R¹ group is preferably chlorine or alkoxy of up to 8 carbon atoms. R² is preferably carbonamido, sulfamoyl or sulfonamido. Typically, the coupling-off group X is an alkylthio or arylthio group. In the latter case, the coupling-off group can suitably have the formula:
wherein R³ and R⁴ are individually selected from the group consisting of hydrogen, halogen, carboxyl, and substituted or unsubstituted alkyl, alkoxy, aryloxy, carbonamido, ureido, carbamate, sulfonamido, carbamoyl, sulfamoyl, acyloxy, alkoxycarbonyl, aryloxycarbonyl, and amino groups; q is 0 to 4; and R⁴ may be para and/or meta to the sulfur atom. It is preferred that R³ have at least one carbon atom and that the total number of carbon atoms in R³ and R⁴ together be from 4 to 25. - The yellow dye-forming coupler II also has certain preferred embodiments. The R⁵ substituent is typically chlorine or alkoxy of up to 8 carbon atoms. The R⁶ substituent is preferably carbonamido, sulfamoyl, or sulfonamido. Y is suitably an aryloxy coupling-off group. One example would have the formula:
wherein R⁷ is selected from the group consisting of hydrogen, halogen, cyano, nitro, trifluoromethyl and substituted or unsubstituted alkyl, alkoxy, alkylthio, carbonamido, carbamoyl, sulfonamido, sulfamoyl, alkylsulfonyl, arylsulfonyl, alkoxycarbonyl, acyloxy, and acyl groups and each Rt is independently a substituent with t being 0 to 4. Preferably, R⁷ is arylsulfonyl. - In another specific embodiment, Y may be represented by the formula:
wherein W is -O-, -S-, -N(RC)-, or -C(RD)(RE)-; RA is H, or substituted or unsubstituted alkyl, alkoxy, phenyl, or phenoxy; RB and RE are independently H or substituted or unsubstituted alkyl; RC is substituted or unsubstituted alkyl or phenyl; and RD is H or substituted or unsubstituted alkyl or alkoxy. - In one more specific embodiment of the above formula, W is -NRD; RA is H, or substituted or unsubstituted alkyl or alkoxy; RB is H; and RC is substituted or unsubstituted alkyl. In particular, RA is -OC₂H₅, RB is H and RC is -CH₂-phenyl. In another specific embodiment, W is -C(RD)(RE) with RA, RB, RD, and RE defined as above.
- In the cyan dye-forming coupler, it is preferred that at least one (R)p be present. Examples are cyano in the 4-position and cyano in the 3-position and Cl in the 4-position. One R⁸ is suitably of the formula
wherein R⁹ and R¹⁰ are substituted or unsubstituted alkyl of up to 16 carbon atoms and R is a substituent with u being 0 to 3. Z is typically hydrogen or aryloxy. -
- The materials of the invention can be used in any of the ways and in any of the combinations known in the art. Typically, the invention materials are incorporated in a silver halide emulsion and the emulsion coated as a layer on a support to form part of a photographic element. Alternatively, they can be incorporated at a location adjacent to the silver halide emulsion layer where, during development, they will be in reactive association with development products such as oxidized color developing agent. Thus, as used herein, the term "associated" signifies that the compound is in the silver halide emulsion layer or in an adjacent location where, during processing, it is capable of reacting with silver halide development products.
- To control the migration of various components, it may be desirable to include a high molecular weight hydrophobe or "ballast" group in the component molecule. Representative ballast groups include substituted or unsubstituted alkyl or aryl groups containing 8 to 40 carbon atoms. Representative substituents on such groups include alkyl, aryl, alkoxy, aryloxy, alkylthio, hydroxy, halogen, alkoxycarbonyl, aryloxcarbonyl, carboxy, acyl, acyloxy, amino, anilino, carbonamido, carbamoyl, alkylsulfonyl, arysulfonyl, sulfonamido, and sulfamoyl groups wherein the substituents typically contain 1 to 40 carbon atoms. Such substituents can also be further substituted.
- The photographic elements can be single color elements or multicolor elements. Multicolor elements contain image dye-forming units sensitive to each of the three primary regions of the spectrum. Each unit can comprise a single emulsion layer or multiple emulsion layers sensitive to a given region of the spectrum. The layers of the element, including the layers of the image-forming units, can be arranged in various orders as known in the art. In an alternative format, the emulsions sensitive to each of the three primary regions of the spectrum can be disposed as a single segmented layer.
- A typical multicolor photographic element comprises a support bearing a cyan dye image-forming unit comprised of at least one red-sensitive silver halide emulsion layer having associated therewith at least one cyan dye-forming coupler, a magenta dye image-forming unit comprising at least one green-sensitive silver halide emulsion layer having associated therewith at least one magenta dye-forming coupler, and a yellow dye image-forming unit comprising at least one blue-sensitive silver halide emulsion layer having associated therewith at least one yellow dye-forming coupler. The element can contain additional layers, such as filter layers, interlayers, overcoat layers, subbing layers, and the like.
- If desired, the photographic element can be used in conjunction with an applied magnetic layer as described in Research Disclosure, November 1992, Item 34390 published by Kenneth Mason Publications, Ltd., Dudley Annex, 12a North Street, Emsworth, Hampshire P010 7DQ, ENGLAND.
- In the following discussion of suitable materials for use in the emulsions and elements of this invention, reference will be made to Research Disclosure, December 1989, Item 308119, available as described above, which will be identified hereafter by the term "Research Disclosure." The contents of the Research Disclosure, including the patents and publications referenced therein, are incorporated herein by reference, and the Sections hereafter referred to are Sections of the Research Disclosure.
- The silver halide emulsions employed in the elements of this invention can be either negative-working or positive-working. Suitable emulsions and their preparation as well as methods of chemical and spectral sensitization are described in Sections I through IV. Color materials and development modifiers are described in Sections V and XXI. Vehicles are described in Section IX, and various additives such as brighteners, antifoggants, stabilizers, light absorbing and scattering materials, hardeners, coating aids, plasticizers, lubricants and matting agents are described , for example, in Sections V, VI, VIII, X, XI, XII, and XVI. Manufacturing methods are described in Sections XIV and XV, other layers and supports in Sections XIII and XVII, processing methods and agents in Sections XIX and XX, and exposure alternatives in Section XVIII.
- Coupling-off groups are well known in the art. Such groups can determine the chemical equivalency of a coupler, i.e., whether it is a 2-equivalent or a 4-equivalent coupler, or modify the reactivity of the coupler. Such groups can advantageously affect the layer in which the coupler is coated, or other layers in the photographic recording material, by performing, after release from the coupler, functions such as dye formation, dye hue adjustment, development acceleration or inhibition, bleach acceleration or inhibition, electron transfer facilitation, color correction and the like.
- The presence of hydrogen at the coupling site provides a 4-equivalent coupler, and the presence of another coupling-off group provides a 2-equivalent coupler. Representative classes of such coupling-off groups include, for example, chloro, alkoxy, aryloxy, hetero-oxy, sulfonyloxy, acyloxy, acyl, heterocyclyl, sulfonamido, mercaptotetrazole, benzothiazole, mercaptopropionic acid, phosphonyloxy, arylthio, and arylazo. These coupling-off groups are described in the art, for example, in U.S. Pat. Nos. 2,455,169, 3,227,551, 3,432,521, 3,476,563, 3,617,291, 3,880,661, 4,052,212 and 4,134,766; and in U.K. Patents and published application Nos. 1,466,728, 1,531,927, 1,533,039, 2,006,755A and 2,017,704A, the disclosures of which are incorporated herein by reference.
- It may be useful to use a combination of couplers any of which may contain known ballasts or coupling-off groups such as those described in U.S. Patent 4,301,235; U.S. Patent 4,853,319 and U.S. Patent 4,351,897. The coupler may also be used in association with "wrong" colored couplers (e.g. to adjust levels of interlayer correction) and, in color negative applications, with masking couplers such as those described in EP 213.490; Japanese Published Application 58-172,647; U.S. Patent 2,983,608; German Application DE 2,706,117C; U.K. Patent 1,530,272; Japanese Application A-113935; U.S. Patents 4,070,191 and 4,273,861; and German Application DE 2,643,965. The masking couplers may be shifted or blocked.
- The invention materials may also be used in association with materials that accelerate or otherwise modify the processing steps e.g. of bleaching or fixing to improve the quality of the image. Bleach accelerator releasing couplers such as those described in EP 193,389; EP 301,477; U.S. 4,163,669; U.S. 4,865,956; and U.S. 4,923,784, may be useful. Also contemplated is use of the compositions in association with nucleating agents, development accelerators or their precursors (UK Patent 2,097,140; U.K. Patent 2,131,188); electron transfer agents (U.S. 4,859,578; U.S. 4,912,025); antifogging and anti color-mixing agents such as derivatives of hydroquinones, aminophenols, amines, gallic acid; catechol; ascorbic acid; hydrazides; sulfonamidophenols; and non color-forming couplers.
- For example, the invention materials may be substituted in whole or in part in the layers of a color negative photographic element comprising a support bearing the following layers from top to bottom:
- (1) one or more overcoat layers containing ultraviolet absorber(s);
- (2) a two-coat yellow pack with a fast yellow layer containing "Coupler 1": Benzoic acid, 4-chloro-3-((2-(4-ethoxy-2,5-dioxo-3-(phenylmethyl)-1-imidazolidinyl)-3-(4-methoxyphenyl)-1,3-dioxopropyl)amino)-, dodecyl ester and a slow yellow layer containing the same compound together with "Coupler 2": Propanoic acid, 2-[[5-[[4-[2-[[[2,4-bis(1,1-dimethylpropyl)phenoxy]acetyl]amino]-5-[(2,2,3,3,4,4,4-heptafluoro-1-oxobutyl)amino]-4-hydroxyphenoxy]-2,3-dihydroxy-6-[(propylamino)carbonyl ]phenyl]thio]-1,3,4-thiadiazol-2-yl]thio]-, methyl ester and "Coupler 3": 1-((dodecyloxy)carbonyl) ethyl(3-chloro-4-((3-(2-chloro-4-((1-tridecanoylethoxy) carbonyl)anilino)-3-oxo-2-((4)(5)(6)-(phenoxycarbonyl)-1H-benzotriazol-1-yl)propanoyl)amino))benzoate;
- (3) an interlayer containing fine metallic silver;
- (4) a triple-coat magenta pack with a fast magenta layer containing "Coupler 4": Benzamide, 3-((2-(2,4-bis(1,1-dimethylpropyl)phenoxy)-1-oxobutyl)amino)-N-(4,5-dihydro-5-oxo-1-(2,4,6-trichlorophenyl)-1H-pyrazol-3-yl)-,"Coupler 5": Benzamide, 3-((2-(2,4-bis(1,1-dimethylpropyl)phenoxy)-1-oxobutyl)amino)-N-(4',5'-dihydro-5'-oxo-1'-(2,4,6-trichlorophenyl) (1,4'-bi-1H-pyrazol)-3'-yl)-,"Coupler 6": Carbamic acid, (6-(((3-(dodecyloxy)propyl) amino)carbonyl)-5-hydroxy-1-naphthalenyl)-, 2-methylpropyl ester , "Coupler 7": Acetic acid, ((2-((3-(((3-(dodecyloxy)propyl)amino) carbonyl)-4-hydroxy-8-(((2-methylpropoxy)carbonyl) amino)-1-naphthalenyl)oxy )ethyl)thio)-, and "Coupler 8" Benzamide, 3-((2-(2,4-bis(1,1-dimethylpropyl) phenoxy)-1-oxobutyl)amino)-N-(4,5-dihydro-4-((4-methoxyphenyl) azo)-5-oxo-1-(2,4,6-trichlorophenyl)-1H-pyrazol-3-yl)-; a mid-magenta layer and a slow magenta layer each containing "Coupler 9": a ternary copolymer containing by weight in the ratio 1:1:2 2-Propenoic acid butyl ester, styrene, and N-[1-(2,4,6-trichlorophenyl)-4,5-dihydro-5-oxo-1H-pyrazol-3-yl]-2- methyl-2-propenamide; and "Coupler 10": Tetradecanamide, N-(4-chloro-3-((4-((4-((2,2-dimethyl-1-oxopropyl) amino)phenyl)azo)-4,5-dihydro-5-oxo-1-(2,4,6-trichlorophenyl)-1H-pyrazol-3-yl)amino)phenyl)-, in addition to Couplers 3 and 8;
- (5) an interlayer;
- (6) a triple-coat cyan pack with a fast cyan layer containing Couplers 6 and 7; a mid-cyan containing Coupler 6 and "Coupler 11": 2,7-Naphthalenedisulfonic acid, 5-(acetylamino)-3-((4-(2-((3-(((3-(2,4-bis(1,1-dimethylpropyl)phenoxy) propyl)amino)carbonyl)-4-hydroxy-1-naphthalenyl) oxy)ethoxy)phenyl)azo)-4-hydroxy-, disodium salt; and a slow cyan layer containing Couplers 2 and 6;
- (7) an undercoat layer containing Coupler 8; and
- (8) an antihalation layer.
- The invention materials may also be used in combination with filter dye layers comprising colloidal silver sol or yellow, cyan, and/or magenta filter dyes, either as oil-in-water dispersions, latex dispersions or as solid particle dispersions. Additionally, they may be used with "smearing" couplers (e.g. as described in U.S. 4,366,237; EP 96,570; U.S. 4,420,556; and U.S. 4,543,323.) Also, the compositions may be blocked or coated in protected form as described, for example, in Japanese Application 61/258,249 or U.S. 5,019,492.
- The invention materials may further be used in combination with image-modifying compounds such as "Developer Inhibitor-Releasing" compounds (DIR's). DIR's useful in conjunction with the compositions of the invention are known in the art and examples are described in U.S. Patent Nos. 3,137,578; 3,148,022; 3,148,062; 3,227,554; 3,384,657; 3,379,529; 3,615,506; 3,617,291; 3,620,746; 3,701,783; 3,733,201; 4,049,455; 4,095,984; 4,126,459; 4,149,886; 4,150,228; 4,211,562; 4,248,962; 4,259,437; 4,362,878; 4,409,323; 4,477,563; 4,782,012; 4,962,018; 4,500,634; 4,579,816; 4,607,004; 4,618,571; 4,678,739; 4,746,600; 4,746,601; 4,791,049; 4,857,447; 4,865,959; 4,880,342; 4,886,736; 4,937,179; 4,946,767; 4,948,716; 4,952,485; 4,956,269; 4,959,299; 4,966,835; 4,985,336 as well as in patent publications GB 1,560,240; GB 2,007,662; GB 2,032,914; GB 2,099,167; DE 2,842,063, DE 2,937,127; DE 3,636,824; DE 3,644,416 as well as the following European Patent Publications: 272,573; 335,319; 336,411; 346, 899; 362, 870; 365,252; 365,346; 373,382; 376,212; 377,463; 378,236; 384,670; 396,486; 401,612; 401,613.
- Such compounds are also disclosed in "Developer-Inhibitor-Releasing (DIR) Couplers for Color Photography," C.R. Barr, J.R. Thirtle and P.W. Vittum in Photographic Science and Engineering, Vol. 13, p. 174 (1969), incorporated herein by reference. Generally, the developer inhibitor-releasing (DIR) couplers include a coupler moiety and an inhibitor coupling-off moiety (IN). The inhibitor-releasing couplers may be of the time-delayed type (DIAR couplers) which also include a timing moiety or chemical switch which produces a delayed release of inhibitor. Examples of typical inhibitor moieties are: oxazoles, thiazoles, diazoles, triazoles, oxadiazoles, thiadiazoles, oxathiazoles, thiatriazoles, benzotriazoles, tetrazoles, benzimidazoles, indazoles, isoindazoles, mercaptotetrazoles, selenotetrazoles, mercaptobenzothiazoles, selenobenzothiazoles, mercaptobenzoxazoles, selenobenzoxazoles, mercaptobenzimidazoles, selenobenzimidazoles, benzodiazoles, mercaptooxazoles, mercaptothiadiazoles, mercaptothiazoles, mercaptotriazoles, mercaptooxadiazoles, mercaptodiazoles, mercaptooxathiazoles, telleurotetrazoles or benzisodiazoles. In a preferred embodiment, the inhibitor moiety or group is selected from the following formulas:
wherein RI is selected from the group consisting of straight and branched alkyls of from 1 to about 8 carbon atoms, benzyl, phenyl, and alkoxy groups and such groups containing none, one or more than one such substituent; RII is selected from RI and -SRI; RIII is a straight or branched alkyl group of from 1 to about 5 carbon atoms and m is from 1 to 3; and RIV is selected from the group consisting of hydrogen, halogens and alkoxy, phenyl and carbonamido groups, -COORV and - NHCOORV wherein RV is selected from substituted and unsubstituted alkyl and aryl groups. - Although it is typical that the coupler moiety included in the developer inhibitor-releasing coupler forms an image dye corresponding to the layer in which it is located, it may also form a different color as one associated with a different film layer. It may also be useful that the coupler moiety included in the developer inhibitor-releasing coupler forms colorless products and/or products that wash out of the photographic material during processing (so-called "universal" couplers).
- As mentioned, the developer inhibitor-releasing coupler may include a timing group which produces the time-delayed release of the inhibitor group such as groups utilizing the cleavage reaction of a hemiacetal (U.S. 4,146,396, Japanese Applications 60-249148; 60-249149); groups using an intramolecular nucleophilic substitution reaction (U.S. 4,248,962); groups utilizing an electron transfer reaction along a conjugated system (U.S. 4,409,323; 4,421,845; Japanese Applications 57-188035; 58-98728; 58-209736; 58-209738) groups utilizing ester hydrolysis (German Patent Application (OLS) No. 2,626,315; groups utilizing the cleavage of imino ketals (U.S. 4,546,073); groups that function as a coupler or reducing agent after the coupler reaction (U.S. 4,438,193; U.S. 4,618,571) and groups that combine the features describe above. It is typical that the timing group or moiety is of one of the formulas:
wherein IN is the inhibitor moiety, Z is selected from the group consisting of nitro, cyano, alkylsulfonyl; sulfamoyl (-SO₂NR₂); and sulfonamido (-NRSO₂R) groups; n is 0 or 1; and RVI is selected from the group consisting of substituted and unsubstituted alkyl and phenyl groups. The oxygen atom of each timing group is bonded to the coupling-off position of the respective coupler moiety of the DIAR. - Suitable developer inhibitor-releasing couplers for use in the present invention include, but are not limited to, the following:
Especially useful in this invention are tabular grain silver halide emulsions. Specifically contemplated tabular grain emulsions are those in which greater than 50 percent of the total projected area of the emulsion grains are accounted for by tabular grains having a thickness of less than 0.3 micron (0.5 micron for blue sensitive emulsion) and an average tabularity (T) of greater than 25 (preferably greater than 100), where the term "tabularity" is employed in its art recognized usage as
where
ECD is the average equivalent circular diameter of the tabular grains in microns and
t is the average thickness in microns of the tabular grains. - The average useful ECD of photographic emulsions can range up to about 10 microns, although in practice emulsion ECD's seldom exceed about 4 microns. Since both photographic speed and granularity increase with increasing ECD's, it is generally preferred to employ the smallest tabular grain ECD's compatible with achieving aim speed requirements.
- Emulsion tabularity increases markedly with reductions in tabular grain thickness. It is generally preferred that aim tabular grain projected areas be satisfied by thin (t < 0.2 micron) tabular grains. To achieve the lowest levels of granularity it is preferred that aim tabular grain projected areas be satisfied with ultrathin (t < 0.06 micron) tabular grains. Tabular grain thicknesses typically range down to about 0.02 micron. However, still lower tabular grain thicknesses are contemplated. For example, Daubendiek et al U.S. Patent 4,672,027 reports a 3 mole percent iodide tabular grain silver bromoiodide emulsion having a grain thickness of 0.017 micron.
- As noted above tabular grains of less than the specified thickness account for at least 50 percent of the total grain projected area of the emulsion. To maximize the advantages of high tabularity it is generally preferred that tabular grains satisfying the stated thickness criterion account for the highest conveniently attainable percentage of the total grain projected area of the emulsion. For example, in preferred emulsions, tabular grains satisfying the stated thickness criteria above account for at least 70 percent of the total grain projected area. In the highest performance tabular grain emulsions, tabular grains satisfying the thickness criteria above account for at least 90 percent of total grain projected area.
- Suitable tabular grain emulsions can be selected from among a variety of conventional teachings, such as those of the following: Research Disclosure, Item 22534, January 1983, published by Kenneth Mason Publications, Ltd., Emsworth, Hampshire P010 7DD, England; U.S. Patent Nos. 4,439,520; 4,414,310; 4,433,048; 4,643,966; 4,647,528; 4,665,012; 4,672,027; 4,678,745; 4,693,964; 4,713,320; 4,722,886; 4,755,456; 4,775,617; 4,797,354; 4,801,522; 4,806,461; 4,835,095; 4,853,322; 4,914,014; 4,962,015; 4,985,350; 5,061,069 and 5,061,616.
- The emulsions can be surface-sensitive emulsions, i.e., emulsions that form latent images primarily on the surfaces of the silver halide grains, or the emulsions can form internal latent images predominantly in the interior of the silver halide grains. The emulsions can be negative-working emulsions, such as surface-sensitive emulsions or unfogged internal latent image-forming emulsions, or direct-positive emulsions of the unfogged, internal latent image-forming type, which are positive-working when development is conducted with uniform light exposure or in the presence of a nucleating agent.
- Photographic elements can be exposed to actinic radiation, typically in the visible region of the spectrum, to form a latent image and can then be processed to form a visible dye image. Processing to form a visible dye image includes the step of contacting the element with a color developing agent to reduce developable silver halide and oxidize the color developing agent. Oxidized color developing agent in turn reacts with the coupler to yield a dye.
- With negative-working silver halide, the processing step described above provides a negative image. The described elements can be processed in the known C-41 color process as described in The British Journal of Photography Annual of 1982, pages 209 - 211 and 1988, pages 191-198. To provide a positive (or reversal) image, the color development step can be preceded by development with a non-chromogenic developing agent to develop exposed silver halide, but not form dye, and followed by uniformly fogging the element to render unexposed silver halide developable. Alternatively, a direct positive emulsion can be employed to obtain a positive image.
- Preferred color developing agents are p-phenylenediamines such as:
4-amino-N,N-diethylaniline hydrochloride,
4-amino-3-methyl-N,N-diethylaniline hydrochloride,
4-amino-3-methyl-N-ethyl-N-(b-(methanesulfonamido) ethyl)aniline sesquisulfate hydrate,
4-amino-3-methyl-N-ethyl-N-(b-hydroxyethyl)aniline sulfate,
4-amino-3-b-(methanesulfonamido)ethyl-N,N-diethylaniline hydrochloride and
4-amino-N-ethyl-N-(2-methoxyethyl)-m-toluidine di-p-toluene sulfonic acid. - Development is usually followed by the conventional steps of bleaching, fixing, or bleach-fixing, to remove silver or silver halide, washing, and drying.
- It is understood thoroughout this specification and claims that any reference to a substituent by the identification of a group containing a substitutable hydrogen (e.g. alkyl, amine, aryl, alkoxy, heterocyclic, etc.), unless otherwise specifically stated, shall encompass not only the substituent's unsubstituted form, but also its form substituted with any photographically useful substituents. Usually the substituent will have less than 30 carbon atoms and typically less than 20 carbon atoms.
- A cellulose triacetate film support was coated with the following layers, in sequence (coverages are in grams per meter squared) in order to prepare Photographic Sample 101:
- Layer 1 (Antihalation layer): black colloidal silver sol containing 0.215 g of silver, cyan dye material CD-1 (0.032), magenta dye material MD-1 (0.022), yellow dye material YD-1 (0.129) and gelatin (2.44) were contained in this layer.
- Layer 2 (Lowest Sensitivity Red-sensitive layer): This layer comprised a blend of a red-sensitized, tabular grain silver iodobromide emulsion (1.3% iodide, 0.50 microns diameter by 0.08 microns thick) (0.22) and a red-sensitized tabular grain silver iodobromide emulsion (4.1% iodide, 1.00 microns diameter by 0.09 microns thick) (0.32). A cyan dye-forming coupler C-1 (0.54) and a BAR coupler B-1 (0.09) were incorporated in this layer. Gelatin was also included (1.78).
- Layer 3 (Medium Sensitivity Red-sensitive layer): This layer comprised a red-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 1.31 diameter by 0.12 microns thick) (0.54). This layer also comprised a cyan dye-forming coupler C-1 (0.23), a cyan dye-forming masking coupler CM-1 (0.022), DIR coupler D-1 (0.05), and a BAR coupler B-1 (0.003). Gelatin (1.66) was included.
- Layer 4 (Highest Sensitivity Red-sensitive layer): This layer comprised a red-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 2.70 diameter by 0.13 microns thick) (1.08). This layer also comprised a cyan dye-forming coupler C-1 (0.17), a cyan dye-forming masking coupler CM-1 (0.050), DIR coupler D-1 (0.05), and a BAR coupler B-1 (0.002). Gelatin (1.36) was included.
- Layer 5 (Interlayer): This layer comprised gelatin (1.33).
- Layer 6 (Lowest Sensitivity Green-sensitive layer): This layer comprised a blend of a green-sensitized, tabular grain, silver iodobromide emulsion (1.3% iodide, 0.54 microns diameter by 0.08 microns thick) (0.59) and a green-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 1.03 microns diameter by 0.09 microns thick) (0.32). This layer also comprised a blend of magenta dye-forming couplers; M-Comp-1 (0.22) and M-Comp-2 (0.09). The layer also incorporated a BAR coupler B-2 (0.03) and gelatin (1.78).
- Layer 7 (Medium Sensitivity Green-sensitive layer): This layer comprised a green-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 1.22 microns diameter by 0.11 microns thick) (0.97), a blend of magenta dye-forming couplers M-Comp-1 (0.09) and M-Comp-2 (0.03), and a magenta dye-forming masking coupler MM-1 (0.09). This layer also incorporated DIR coupler D-2 (0.02), BAR coupler B-1 (0.003), and gelatin (1.48).
- Layer 8 (Highest Sensitivity Green-sensitive layer): This layer comprised a green-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 2.23 microns diameter by 0.13 microns thick) (0.97), a blend of magenta dye-forming couplers M-Comp-1 (0.09) and M-Comp-2 (0.03), and a magenta dye-forming masking coupler MM-1 (0.05). This layer also incorporated DIR coupler D-2 (0.01), DIR coupler D-3 (0.01), BAR coupler B-1 (0.003), and gelatin (1.33).
- Layer 9 (Yellow filter layer): This layer comprised yellow dye material YD-2 (0.11) and gelatin (1.33).
- Layer 10 (Lowest Sensitivity Blue-sensitive layer): This layer comprised a blend of a blue-sensitized, tabular grain, silver iodobromide emulsion (1.3% iodide, 0.54 microns diameter by 0.08 thick) (0.16), a blue-sensitized, tabular grain silver iodobromide emulsion (4.1% iodide, 1.02 micron diameter by 0.09 micron thick) (0.27), and a blue-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 1.38 microns diameter by 0.11 microns thick) (0.38). This layer incorporated a yellow dye-forming coupler Y-1 (0.91), DIR coupler D-4 (0.05), and BAR coupler B-1 (0.003), and gelatin (2.60).
- Layer 11 (Highest Sensitivity Blue-sensitive layer): This layer comprised a blue-sensitized, conventional 3-D grain, silver iodobromide emulsion (9.0% iodide, 1.0 micron) (0.38) and a blue-sensitized, tabular grain, silver iodobromide emulsion (4.1% iodide, 3.53 microns diameter by 0.14 microns thick) (0.38). This layer also incorporated yellow dye-forming coupler Y-1 (0.27), DIR D-4 (0.04), BAR B-1 (0.005), and gelatin (1.97).
- Layer 12 (UV filtration layer): This layer comprised dye UV-1 (0.11), UV-2 (0.11), and unsensitized silver bromide Lippmann emulsion (0.22). Gelatin was included (1.11).
- Layer 13 (Protective layer): This layer comprised gelatin (0.92) and anti-matte polymethylmethacrylate beads (0.054).
- This film was hardened at coating with 1.75% by weight of total gelatin of hardener H-1. Surfactants, coating aids, oxidized developer scavengers, soluble absorber dyes and stabilizers were added to the various layers of this sample as is commonly practiced in the art.
- Photographic Sample 102 was prepared like Photographic Sample 101 except for changes in Layers 6, 7, and 8. Magenta dye-forming couplers M-Comp-1 and M-Comp-2 were omitted in all three layers and replaced with magenta dye-forming coupler M-1. Layer 6: M-1 (0.22) Layer 7: M-1 (0.12) Layer 8: M-1 (0.12)
Photographic Sample 103 was prepared like Photographic Sample 102 except for changes in Layers 6, 7, & 8. In all three layers, the level of M-1 was reduced and magenta dye-forming coupler M-Comp-1 was added. Layer 6: M-1 (0.11), M-Comp-1 (0.27) Layer 7: M-1 (0.05), M-Comp-1 (0.09) Layer 8: M-1 (0.05), M-Comp-1 (0.09). The blended couplers provided about 75 mol % of the comparison pyrazolotriazole coupler to 25 mol % of the magenta coupler of the invention. - The resulting film examples were exposed through a graduated step wedge to a light source at 5500 deg K. The exposed elements were processed in a standard C-41 process (as described in the British Journal of Photography Annual, 1988, pages 196-198) for the typical response to the C-41 process and also processed through 8 variations of the C-41 developer to determine sensitivity of the elements to these variations.
- The 8 variations are arrived at by independently varying three separate developer parameters from standard conditions in order to determine the effect of the variation on a selected photographic property. The standard condition for the developer was:
A. color developer concentration 4.5 g/l B. developer pH 10.05 C. bromide concentration 1.3 g/l
The above parameters were then varied as follows:Test A B C 1* 4.5 10.05 1.3 2 3.5 9.95 0.8 3 5.5 9.95 0.8 4 3.5 10.15 0.8 5 5.5 10.15 0.8 6 3.5 9.95 1.8 7 5.5 9.95 1.8 8 3.5 10.15 1.8 9 5.5 10.15 1.8 * This is the target or normal value of each of the parameters. - Thus the extremes for each variable were tested with all possible combinations of the extremes for the other two variables. With the nine developer responses, the dependent variables (red, green, blue color densities, color gamma, speed, etc.) were regressed through the nine developer concentrations (CD-4, Br-, pH) which resulted in estimates of sensitivity per color record (sensitivity is expressed as a delta per 2 sigma of the average trade concentration for a given component). For the photographic elements 101 and 102, the results are tabulated below for two photographic parameters: normal gamma (defined as the contrast, by record, throughout the normal (typical) exposure region of the element) and over color (density) balance (defined as density, by record, in the overexposure region (2 stops above normal) of the element. Both parameters are critical for a photofinisher to correctly achieve a balanced print.
- As described above, it is desirable for the sensitivity estimates to approach zero for all developer components and to be as closely matched Red-to-Green-to-Blue as possible. The data indicate the invention provides an improvement in this regard for sensitivity to both Br- and pH variations while maintaining a well-balanced response to CD-4.
- The three photographic elements (#101, #102, #103) were then tested for their sensitometric response in a cross-section of photofinishing trade sites (50 sites evaluated). Exposed film (exposed as in example 1) was taken to the 50 sites and processed in their currently running C-41 process. A ternary plot was generated for the above red, green, and blue sensitometric responses (normal gamma balance and over color balance were plotted.) Statistical information concerning the effect of developer variations in actual field conditions was generated. A 95% confidence interval ellipse was estimated for each of the data sets on the ternary plots. The area of each elipse was measured and the results are recorded in Table III. The larger the area, the larger the variability for the particular parameter.
TABLE III PHOTOFINISHING TRADE RESPONSE Normal Gamma Balance Over Color Balance #101 (comparison) 2701 7913 #102 (invention) 674 2543 #103 (comparison) 2701 8783 - The data indicate a large improvement is realized with the invention (75% reduction in ellipse area for gamma balance and at least a 68% reduction ellipse area for color balance; ideally, the area of the ellipse would be zero). This example also demonstrates that when the comparison pyrazolotriazole coupler is used in combination with the coupler of the invention in a mol ratio of 3 : 1, respectively, the desired response is not obtained. The improvement is not realized when a pyrazolotriazole coupler is included in an amount exceeding the molar concentration of the invention coupler. Preferrably, the element is substantially free of the pyrazozotriazole coupler.
-
Claims (9)
- A photographic element comprising at least one light sensitive silver halide layer sensitized to one or more of green, blue, and red light, and having associated therewith a 3-anilino-1-phenyl-5-pyrazolone magenta dye-forming coupler, a two-equivalent trialkylacetylacetanilide yellow dye-forming coupler, and a 2-ureido-5-carbonamidophenol cyan dye-forming coupler.
- The element of Claim 1 wherein the element contains at least one green sensitive layer containing the magenta coupler, at least one blue sensitive layer containing the yellow coupler, and at least one red sensitive layer containing the cyan coupler.
- The element of Claim 2 wherein said magenta coupler is represented by formula I, said yellow coupler is represented by formula II, and said cyan coupler is represented by formula III:
- The element of Claim 3 wherein Y is represented by the formula:
or by the formula: - The element of Claim 4 wherein Y is a heterocycle having the second formula shown in Claim 5 and in which W is -NRC; RA is H, or substituted or unsubstituted alkyl or alkoxy; RB is H; and RC is substituted or unsubstituted alkyl.
- The element of any of claims 3-5 wherein X is other than hydrogen
- The element of any of claims 3-6 wherein X is represented by the formula:
- A process for forming an image from an exposed element as claim in any of claims 1-8 comprising contacting said element with a color developing agent.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US1092993A | 1993-01-29 | 1993-01-29 | |
| US10929 | 1993-01-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0608956A1 true EP0608956A1 (en) | 1994-08-03 |
Family
ID=21748077
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP94200186A Withdrawn EP0608956A1 (en) | 1993-01-29 | 1994-01-27 | Photographic element and process having improved response to developer variations |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0608956A1 (en) |
| JP (1) | JPH06295041A (en) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2320334A (en) * | 1996-12-12 | 1998-06-17 | Eastman Kodak Co | Photographic materials and process comprising an acylacetanilide yellow dye forming coupler |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0329016A2 (en) * | 1988-02-19 | 1989-08-23 | Agfa-Gevaert AG | Colour-photographic recording material |
| EP0539023A1 (en) * | 1991-09-25 | 1993-04-28 | Konica Corporation | Silver halide color photographic light-sensitive material |
| EP0539024A1 (en) * | 1991-09-25 | 1993-04-28 | Konica Corporation | Silver halide color photographic light-sensitive material |
-
1994
- 1994-01-27 EP EP94200186A patent/EP0608956A1/en not_active Withdrawn
- 1994-01-31 JP JP6009598A patent/JPH06295041A/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0329016A2 (en) * | 1988-02-19 | 1989-08-23 | Agfa-Gevaert AG | Colour-photographic recording material |
| EP0539023A1 (en) * | 1991-09-25 | 1993-04-28 | Konica Corporation | Silver halide color photographic light-sensitive material |
| EP0539024A1 (en) * | 1991-09-25 | 1993-04-28 | Konica Corporation | Silver halide color photographic light-sensitive material |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2320334A (en) * | 1996-12-12 | 1998-06-17 | Eastman Kodak Co | Photographic materials and process comprising an acylacetanilide yellow dye forming coupler |
| GB2320334B (en) * | 1996-12-12 | 2001-09-05 | Eastman Kodak Co | Photographic materials and process comprising a particular acylacetanlide yellow dye-forming coupler |
Also Published As
| Publication number | Publication date |
|---|---|
| JPH06295041A (en) | 1994-10-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5563026A (en) | Color negative element having improved green record printer compatibility | |
| EP0684518A1 (en) | Photographic element containing high dye-yield couplers having improved reactivity | |
| US5925503A (en) | Photographic element having improved magenta dye light stability and process for its use | |
| EP0629913B1 (en) | Photographic elements containing magenta couplers and process for using same | |
| US5972574A (en) | Photographic element containing magenta coupler having improved manufacturability and dye light stability | |
| EP0603964B1 (en) | Azopyrazolone masking couplers | |
| EP0720048B1 (en) | Photographic element containing pyrazolone pug releasing coupler and imaging process employing same | |
| US5723263A (en) | Color negative element having improved blue record printer compatibility | |
| US5451492A (en) | Photographic elements containing certain acylacetanilide couplers in combination with development inhibitor releasing couplers | |
| EP0666502B1 (en) | Photographic element having a blue light sensitive layer containing a particular yellow dye-forming coupler and a magenta image dye-forming coupler | |
| US5677114A (en) | Photographic element containing yellow dye-forming coupler comprising a dye light stability enhancing ballast and process | |
| US5500330A (en) | Photographic material and process comprising a thiol beach assist in the low sensitivity layer of a triple-coat | |
| US5466568A (en) | Photographic element containing an azopyrazolone masking coupler exhibiting reduced fog | |
| EP0628868B1 (en) | Photographic elements containing magenta couplers and process for using same | |
| US5719014A (en) | Color negative films containing yellow methine dyes for filtration and density correction | |
| EP0684515A1 (en) | Photographic element and process incorporating a high dye-yield image coupler providing improved granularity | |
| US5532117A (en) | Photographic element containing certain azoaniline dyes | |
| US5451493A (en) | Photographic element containing a certain sulfonated acylacetanilide coupler in combination with a development inhibitor releasing coupler | |
| US5800971A (en) | Photographic element containing codispersions of yellow methine filter or density correction dyes and reducing agents | |
| EP0672946B1 (en) | Photographic element containing a certain sulfonated acylacetanilide coupler in combination with low- or non-chloride emulsions | |
| EP0608956A1 (en) | Photographic element and process having improved response to developer variations | |
| US5476757A (en) | Photographic element containing a novel cyan dye forming coupler and process for its use | |
| US5482821A (en) | Photographic element containing an azopyrazolone masking coupler exhibiting improved keeping | |
| EP0646840B1 (en) | Photographic element containing an azopyrazolone masking coupler exhibiting improved keeping | |
| EP0718689B1 (en) | Photographic element containing a novel cyan dye forming coupler and process for its use |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE FR GB IT LI NL |
|
| 17P | Request for examination filed |
Effective date: 19941220 |
|
| 17Q | First examination report despatched |
Effective date: 19950727 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19951207 |