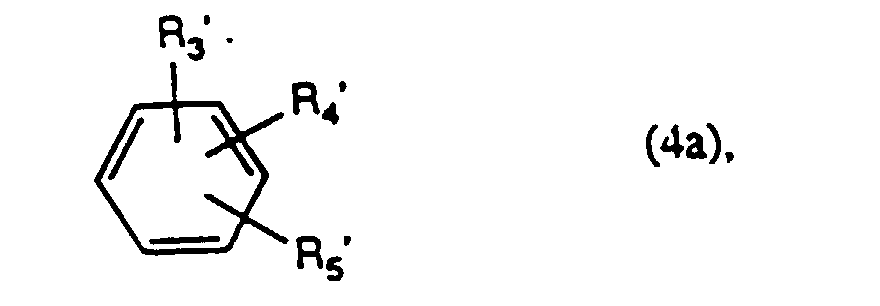EP0572353B2 - Process for dyeing of leather - Google Patents
Process for dyeing of leather Download PDFInfo
- Publication number
- EP0572353B2 EP0572353B2 EP19930810365 EP93810365A EP0572353B2 EP 0572353 B2 EP0572353 B2 EP 0572353B2 EP 19930810365 EP19930810365 EP 19930810365 EP 93810365 A EP93810365 A EP 93810365A EP 0572353 B2 EP0572353 B2 EP 0572353B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- process according
- dye
- weight
- methyl
- radical
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims description 39
- 238000004043 dyeing Methods 0.000 title claims description 29
- 239000010985 leather Substances 0.000 title claims description 22
- 239000000975 dye Substances 0.000 claims description 79
- -1 polyazo Polymers 0.000 claims description 79
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 17
- 229910052739 hydrogen Inorganic materials 0.000 claims description 16
- 239000001257 hydrogen Substances 0.000 claims description 16
- 150000003254 radicals Chemical class 0.000 claims description 13
- 125000002490 anilino group Chemical group [H]N(*)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 claims description 10
- 239000007864 aqueous solution Substances 0.000 claims description 10
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 9
- VOWZNBNDMFLQGM-UHFFFAOYSA-N 2,5-dimethylaniline Chemical compound CC1=CC=C(C)C(N)=C1 VOWZNBNDMFLQGM-UHFFFAOYSA-N 0.000 claims description 8
- JJYPMNFTHPTTDI-UHFFFAOYSA-N 3-methylaniline Chemical compound CC1=CC=CC(N)=C1 JJYPMNFTHPTTDI-UHFFFAOYSA-N 0.000 claims description 8
- PAYRUJLWNCNPSJ-UHFFFAOYSA-N Aniline Chemical compound NC1=CC=CC=C1 PAYRUJLWNCNPSJ-UHFFFAOYSA-N 0.000 claims description 8
- 230000008878 coupling Effects 0.000 claims description 8
- 238000010168 coupling process Methods 0.000 claims description 8
- 238000005859 coupling reaction Methods 0.000 claims description 8
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 claims description 8
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 8
- RNVCVTLRINQCPJ-UHFFFAOYSA-N o-toluidine Chemical compound CC1=CC=CC=C1N RNVCVTLRINQCPJ-UHFFFAOYSA-N 0.000 claims description 8
- 229910052736 halogen Inorganic materials 0.000 claims description 6
- 150000002367 halogens Chemical class 0.000 claims description 6
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 6
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 claims description 6
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 claims description 6
- 125000000129 anionic group Chemical group 0.000 claims description 5
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 claims description 5
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 5
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 claims description 5
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 5
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 claims description 5
- 125000004769 (C1-C4) alkylsulfonyl group Chemical group 0.000 claims description 4
- WZCQRUWWHSTZEM-UHFFFAOYSA-N 1,3-phenylenediamine Chemical compound NC1=CC=CC(N)=C1 WZCQRUWWHSTZEM-UHFFFAOYSA-N 0.000 claims description 4
- CWLKGDAVCFYWJK-UHFFFAOYSA-N 3-aminophenol Chemical compound NC1=CC=CC(O)=C1 CWLKGDAVCFYWJK-UHFFFAOYSA-N 0.000 claims description 4
- 229940018563 3-aminophenol Drugs 0.000 claims description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims description 4
- 239000000872 buffer Substances 0.000 claims description 4
- 125000006125 ethylsulfonyl group Chemical group 0.000 claims description 4
- 239000000203 mixture Substances 0.000 claims description 4
- VMPITZXILSNTON-UHFFFAOYSA-N o-anisidine Chemical compound COC1=CC=CC=C1N VMPITZXILSNTON-UHFFFAOYSA-N 0.000 claims description 4
- IWDCLRJOBJJRNH-UHFFFAOYSA-N p-cresol Chemical compound CC1=CC=C(O)C=C1 IWDCLRJOBJJRNH-UHFFFAOYSA-N 0.000 claims description 4
- RZXMPPFPUUCRFN-UHFFFAOYSA-N p-toluidine Chemical compound CC1=CC=C(N)C=C1 RZXMPPFPUUCRFN-UHFFFAOYSA-N 0.000 claims description 4
- WXWCDTXEKCVRRO-UHFFFAOYSA-N para-Cresidine Chemical compound COC1=CC=C(C)C=C1N WXWCDTXEKCVRRO-UHFFFAOYSA-N 0.000 claims description 4
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 claims description 4
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 claims description 3
- 125000000043 benzamido group Chemical group [H]N([*])C(=O)C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 claims description 3
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 claims description 3
- 239000004094 surface-active agent Substances 0.000 claims description 3
- RUFPHBVGCFYCNW-UHFFFAOYSA-N 1-naphthylamine Chemical compound C1=CC=C2C(N)=CC=CC2=C1 RUFPHBVGCFYCNW-UHFFFAOYSA-N 0.000 claims description 2
- BAHPQISAXRFLCL-UHFFFAOYSA-N 2,4-Diaminoanisole Chemical compound COC1=CC=C(N)C=C1N BAHPQISAXRFLCL-UHFFFAOYSA-N 0.000 claims description 2
- VOZKAJLKRJDJLL-UHFFFAOYSA-N 2,4-diaminotoluene Chemical compound CC1=CC=C(N)C=C1N VOZKAJLKRJDJLL-UHFFFAOYSA-N 0.000 claims description 2
- AVYGCQXNNJPXSS-UHFFFAOYSA-N 2,5-dichloroaniline Chemical compound NC1=CC(Cl)=CC=C1Cl AVYGCQXNNJPXSS-UHFFFAOYSA-N 0.000 claims description 2
- NAZDVUBIEPVUKE-UHFFFAOYSA-N 2,5-dimethoxyaniline Chemical compound COC1=CC=C(OC)C(N)=C1 NAZDVUBIEPVUKE-UHFFFAOYSA-N 0.000 claims description 2
- LOCWBQIWHWIRGN-UHFFFAOYSA-N 2-chloro-4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1Cl LOCWBQIWHWIRGN-UHFFFAOYSA-N 0.000 claims description 2
- AKCRQHGQIJBRMN-UHFFFAOYSA-N 2-chloroaniline Chemical compound NC1=CC=CC=C1Cl AKCRQHGQIJBRMN-UHFFFAOYSA-N 0.000 claims description 2
- XTTIQGSLJBWVIV-UHFFFAOYSA-N 2-methyl-4-nitroaniline Chemical compound CC1=CC([N+]([O-])=O)=CC=C1N XTTIQGSLJBWVIV-UHFFFAOYSA-N 0.000 claims description 2
- JBIJLHTVPXGSAM-UHFFFAOYSA-N 2-naphthylamine Chemical compound C1=CC=CC2=CC(N)=CC=C21 JBIJLHTVPXGSAM-UHFFFAOYSA-N 0.000 claims description 2
- DPJCXCZTLWNFOH-UHFFFAOYSA-N 2-nitroaniline Chemical compound NC1=CC=CC=C1[N+]([O-])=O DPJCXCZTLWNFOH-UHFFFAOYSA-N 0.000 claims description 2
- WAVOOWVINKGEHS-UHFFFAOYSA-N 3-(diethylamino)phenol Chemical compound CCN(CC)C1=CC=CC(O)=C1 WAVOOWVINKGEHS-UHFFFAOYSA-N 0.000 claims description 2
- QNAAQOLWUDNQFY-UHFFFAOYSA-N 3-chloroaniline Chemical compound NC1=CC=CC(Cl)=C1.NC1=CC=CC(Cl)=C1 QNAAQOLWUDNQFY-UHFFFAOYSA-N 0.000 claims description 2
- NDACNGSDAFKTGE-UHFFFAOYSA-N 3-hydroxydiphenylamine Chemical compound OC1=CC=CC(NC=2C=CC=CC=2)=C1 NDACNGSDAFKTGE-UHFFFAOYSA-N 0.000 claims description 2
- XJCVRTZCHMZPBD-UHFFFAOYSA-N 3-nitroaniline Chemical compound NC1=CC=CC([N+]([O-])=O)=C1 XJCVRTZCHMZPBD-UHFFFAOYSA-N 0.000 claims description 2
- ZWUBBMDHSZDNTA-UHFFFAOYSA-N 4-Chloro-meta-phenylenediamine Chemical compound NC1=CC=C(Cl)C(N)=C1 ZWUBBMDHSZDNTA-UHFFFAOYSA-N 0.000 claims description 2
- QSNSCYSYFYORTR-UHFFFAOYSA-N 4-chloroaniline Chemical compound NC1=CC=C(Cl)C=C1 QSNSCYSYFYORTR-UHFFFAOYSA-N 0.000 claims description 2
- TYMLOMAKGOJONV-UHFFFAOYSA-N 4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1 TYMLOMAKGOJONV-UHFFFAOYSA-N 0.000 claims description 2
- 150000004982 aromatic amines Chemical class 0.000 claims description 2
- 229910003002 lithium salt Inorganic materials 0.000 claims description 2
- 159000000002 lithium salts Chemical class 0.000 claims description 2
- NCBZRJODKRCREW-UHFFFAOYSA-N m-anisidine Chemical compound COC1=CC=CC(N)=C1 NCBZRJODKRCREW-UHFFFAOYSA-N 0.000 claims description 2
- BHAAPTBBJKJZER-UHFFFAOYSA-N p-anisidine Chemical compound COC1=CC=C(N)C=C1 BHAAPTBBJKJZER-UHFFFAOYSA-N 0.000 claims description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 claims description 2
- 229960004889 salicylic acid Drugs 0.000 claims description 2
- 150000002431 hydrogen Chemical class 0.000 claims 8
- 229910001508 alkali metal halide Inorganic materials 0.000 claims 4
- 150000008045 alkali metal halides Chemical class 0.000 claims 4
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 claims 2
- 125000000229 (C1-C4)alkoxy group Chemical group 0.000 claims 2
- 230000000844 anti-bacterial effect Effects 0.000 claims 2
- 239000007798 antifreeze agent Substances 0.000 claims 2
- 239000003899 bactericide agent Substances 0.000 claims 2
- 125000001309 chloro group Chemical group Cl* 0.000 claims 2
- 238000009472 formulation Methods 0.000 claims 2
- 239000000417 fungicide Substances 0.000 claims 2
- 150000001412 amines Chemical class 0.000 claims 1
- 238000010016 exhaust dyeing Methods 0.000 claims 1
- 239000003513 alkali Substances 0.000 description 16
- 150000004820 halides Chemical class 0.000 description 16
- 239000000243 solution Substances 0.000 description 11
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 8
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 6
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 6
- 150000001875 compounds Chemical class 0.000 description 5
- 239000000126 substance Substances 0.000 description 4
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- RJSYPKWVIJGNLO-UHFFFAOYSA-N CCOClOC Chemical compound CCOClOC RJSYPKWVIJGNLO-UHFFFAOYSA-N 0.000 description 3
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 229910021529 ammonia Inorganic materials 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 235000019253 formic acid Nutrition 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 150000003839 salts Chemical group 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 125000000738 acetamido group Chemical group [H]C([H])([H])C(=O)N([H])[*] 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000002528 anti-freeze Effects 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000010612 desalination reaction Methods 0.000 description 2
- 125000000664 diazo group Chemical group [N-]=[N+]=[*] 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000011737 fluorine Substances 0.000 description 2
- 125000003253 isopropoxy group Chemical group [H]C([H])([H])C([H])(O*)C([H])([H])[H] 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 125000003506 n-propoxy group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])O* 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- NROKBHXJSPEDAR-UHFFFAOYSA-M potassium fluoride Chemical compound [F-].[K+] NROKBHXJSPEDAR-UHFFFAOYSA-M 0.000 description 2
- 238000001223 reverse osmosis Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical class O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- 239000004280 Sodium formate Substances 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 150000001338 aliphatic hydrocarbons Chemical class 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000004996 alkyl benzenes Chemical class 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 239000002585 base Substances 0.000 description 1
- 235000015278 beef Nutrition 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000007859 condensation product Substances 0.000 description 1
- 238000011033 desalting Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- QGBSISYHAICWAH-UHFFFAOYSA-N dicyandiamide Chemical compound NC(N)=NC#N QGBSISYHAICWAH-UHFFFAOYSA-N 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 125000003754 ethoxycarbonyl group Chemical group C(=O)(OCC)* 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 230000004907 flux Effects 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 125000002510 isobutoxy group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])O* 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 125000006606 n-butoxy group Chemical group 0.000 description 1
- 150000002790 naphthalenes Chemical class 0.000 description 1
- 238000006386 neutralization reaction Methods 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 229920002492 poly(sulfone) Polymers 0.000 description 1
- 235000015277 pork Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 235000003270 potassium fluoride Nutrition 0.000 description 1
- 239000011698 potassium fluoride Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 125000005920 sec-butoxy group Chemical group 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- 235000013024 sodium fluoride Nutrition 0.000 description 1
- 239000011775 sodium fluoride Substances 0.000 description 1
- PUZPDOWCWNUUKD-UHFFFAOYSA-M sodium fluoride Inorganic materials [F-].[Na+] PUZPDOWCWNUUKD-UHFFFAOYSA-M 0.000 description 1
- HLBBKKJFGFRGMU-UHFFFAOYSA-M sodium formate Chemical compound [Na+].[O-]C=O HLBBKKJFGFRGMU-UHFFFAOYSA-M 0.000 description 1
- 235000019254 sodium formate Nutrition 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 125000004213 tert-butoxy group Chemical group [H]C([H])([H])C(O*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P3/00—Special processes of dyeing or printing textiles, or dyeing leather, furs, or solid macromolecular substances in any form, classified according to the material treated
- D06P3/02—Material containing basic nitrogen
- D06P3/04—Material containing basic nitrogen containing amide groups
- D06P3/32—Material containing basic nitrogen containing amide groups leather skins
- D06P3/3206—Material containing basic nitrogen containing amide groups leather skins using acid dyes
- D06P3/3226—Material containing basic nitrogen containing amide groups leather skins using acid dyes dis-polyazo
Definitions
- the present invention relates to a method for dyeing leather with anionic Dyes and leather dyed using the process.
- the invention thus relates to a process for dyeing leather with anionic Dyes, characterized in that an aqueous solution is used for dyeing, containing a polyazo dye with at least 2 sulfo groups and less than 1.5% by weight, based on the weight of the dye, of alkali halide, the Dyes do not exist as lithium salts.
- Suitable substituents on the phenyl or naphthyl radical A are, for example, C 1 -C 4 -alkyl, which here and in general comprises methyl, ethyl, n- or iso-propyl or n-, iso-, sec.- or tert-butyl , C 1 -C 4 alkoxy, which generally means methoxy, ethoxy, n- or iso-propoxy or n-, iso-, sec- or tert-butoxy, halogen, for example fluorine, bromine and especially chlorine; Trifluoromethyl; C 1 -C 4 alkylsulfonyl, especially methyl or ethylsulfonyl; Sulfamoyl; N-mono- or N, N-di-C 1 -C 4 alkylsulfamoyl; Carbamoyl; N-mono- or N, N-di-C 1 -C 4 alkyl
- Preferred diazo components are compounds of the formula wherein R 1 and R 2 are each independently hydrogen, C 1 -C 4 alkyl, C 1 -C 4 alkoxy, halogen, nitro, trifluoromethyl, C 1 -C 4 alkylsulfonyl, acetylamino, hydroxyacetylamino, propionylamino, sulfamoyl, carbamoyl , Cyano, carboxy or phenoxy mean.
- R 1 and R 2 are independently of one another hydrogen, methyl, ethyl, methoxy, ethoxy, chlorine, nitro, trifluoromethyl, methylsulfonyl, ethylsulfonyl, sulfamoyl, carbamoyl, cyano, acetylamino and phenoxy.
- R 1 and R 2 independently of one another particularly preferably represent hydrogen, methyl, methoxy, nitro or chlorine.
- Examples of preferred compounds of the formula (3) are: Aniline, 2-, 3- or 4-methylaniline, 2-, 3- or 4-methoxyaniline, 2-, 3- or 4-chloroaniline, 2-, 3- or 4-nitroaniline, 2-chloro-4-nitroaniline, 2-methyl-4-nitroaniline, 2,5-dichloroaniline, 2,5-dimethylaniline, 2,5-dimethoxyaniline or 2-methoxy-5-methylaniline.
- X is OH and it is also preferred that the group X is not on the same ring as the NH 2 group.
- B denotes the rest of a coupling component, in particular a coupling component from the benzene or naphthalene series.
- B is preferably a radical of the formula wherein R 3 is hydroxy, amino, N-mono- or N, N-di-C 1 -C 4 alkylamino, phenylamino or o-, m- or p-methylphenylamino and R 4 and R 5 each independently of the other meanings R 3 have or represent hydrogen, benzoylamino, C 1 -C 4 alkanoylamino, carboxymethylamino, C 1 -C 4 alkyl, C 1 -C 4 alkoxy, phenoxy, carboxy, halogen, nitro or sulfo.
- R 3 as N-mono- or N, N-di-C 1 -C 4 -alkylamino is, for example, methyl-, ethyl-, n- or iso-propyl- or n-, iso-, sec. - or tert.
- R 3 are hydroxy, amino, N-methyl- and N-ethylamino, N, N-dimethyl- and N, N-diethylamino and phenylamino.
- R 3 particularly preferably represents hydroxy or amino.
- R 4 and / or R 5 as well as the variables below as C 1 -C 4 alkyl are generally methyl, ethyl, n- or iso-propyl or n-, iso-, sec.- or tert-butyl.
- R 4 and / or R 5 or the following variables stand for C 1 -C 4 alkoxy, then methoxy, ethoxy, n- or iso-propoxy or n-, iso-, sec.- or tert.- Butoxy includes.
- R 4 and / or R 5 as halogen is generally, for example, fluorine, bromine and in particular chlorine, as in the following.
- R 4 and / or R 5 with the meaning C 1 -C 4 alkanoylamino are, for example, propionylamino, hydroxyacetylamino and in particular acetylamino.
- Sulfo generally includes the free acid form (-SO 3 H) as well as the salt form, alkali metal salts (Na, K) or ammonium salts being particularly suitable.
- R 4 and R 5 independently of one another preferably represent hydrogen, sulfo, acetylamino, methyl, ethyl, methoxy, ethoxy, chlorine, carboxy, hydroxy, amino, N-methyl- or N-ethylamino, N, N-dimethyl- or N, N-diethylamino or phenylamino and particularly preferably for hydrogen, amino, hydroxy, methyl, methoxy, phenylamino or N, N-dimethyl- or N, N-diethylamino.
- R 3 is hydroxyl, amino, N-methyl- or N-ethylamino, N, N-dimethyl- or N, N-diethylamino or phenylamino
- R 4 and R 5 each independently represent hydrogen, acetylamino, methyl, ethyl, methoxy, ethoxy, chlorine, carboxy, hydroxy, amino, N-methyl- or N-ethylamino, N, N-dimethyl- or N, N-diethylamino or phenylamino.
- a particularly preferred embodiment of the present invention relates to the use of 3-aminophenol, Resorcinol or 1,3-phenylenediamine as a coupling component.
- n is preferably 2 and m is preferably 1.
- the dyes of the formula (1) or (2) prepared in the usual way normally contain about 5 to 30% by weight. % Alkali halide, based on the weight of the dye. This alkali halide is used in the synthesis of the dye formed and / or added to the dye solution obtained to separate the dye. In addition, often the solid or liquid commercial form of the dye by adding alkali halide to the desired color strength set.
- an aqueous solution containing a dye is used with less than 1.5% by weight, based on the weight of the dye, of alkali halide.
- the dyes that are commercially available or produced in the usual way must therefore be used are largely freed from the alkali halide in the dyeing process according to the invention. This happens on itself known way, for example by reverse osmosis, ultrafiltration or dialysis. Such desalination processes are known, e.g. from EP-A 0 059782. Also known are the membranes used in these processes, e.g. from EP-A 0 061 424.
- the process conditions for this desalination are chosen so that dye solutions are obtained which contain less than 1.5% by weight, based on the weight of the dye, of alkali halide.
- Dye salts as well Salts with higher molecular weight cations and / or anions are not or only slightly removed.
- the dye is in the form of an aqueous solution which, if desired, can be concentrated or, e.g. by spray drying, can be evaporated to dryness.
- the dye liquor can contain other additives, e.g. Acids or bases for setting the desired pH value, and auxiliaries, such as. Wetting agents, greasing agents, color-deepening aids, leveling agents, and / or Antifoam.
- additives e.g. Acids or bases for setting the desired pH value
- auxiliaries such as. Wetting agents, greasing agents, color-deepening aids, leveling agents, and / or Antifoam.
- the dyeing is preferably carried out by the exhaust process, e.g. with a liquor ratio of 1: 1.5 to 1: 20, preferably 1: 2 to 1:10, and at temperatures of 20 to 100 ° C, preferably 30 to 60 ° C.
- the leather can be subjected to a pretreatment, for example neutralization or Walke.
- the dyeing time varies depending on the type of leather and the desired depth of color, but is generally between 30 and 180 minutes. After the dyeing, the leather is rinsed and finished as usual.
- Parts mean Parts by weight and percentages by weight.
- the dyes are used in commercially available powder form; the stated amounts of dye refer to the coupe 100% dye commercial form.
- This solution is desalinated in a laboratory reverse osmosis system under the following conditions: polysulfone membrane, approx. 40 ° C, feed: 12 l / h, 25 bar, flux 1289 l / m 2 d (average). After 4.5 hours, 1.9 kg of a solution with a dye content of 18.0% by weight and a sodium chloride content of 0.05% by weight (0.9% by weight, based on dye) are obtained. This desalted and concentrated solution is adjusted to a dye content of 15% after filtration through a 10 ⁇ m sieve with water.
- the leather is made into a liquor made from 1000 parts of water and 2 parts of ammonia 24% and 13.33 parts of the dye solution described above at 50 ° C.
- the mixture After a dyeing time of 60 minutes, the mixture is acidified with 4 parts of 85% formic acid and then 20 Minutes further colored.
- the dyed leather is finished after a treatment in a fresh bath with 1000 parts of water, 2.5 parts of a condensation product of formaldehyde, dicyandiamide, ammonium chloride and ethylenediamine and 0.5 parts of 85% formic acid for 45 minutes at 50 ° C.
- the leather obtained is dyed black is characterized by good fastness properties and good opacity.
- the leather neutralized in this way is then in a liquor of 1000 parts water, 2 parts ammonia 24% and 15 parts of the dye solution described in Example 1 are dyed at 50 ° C. After 30 minutes, the dyeing liquor 3 parts of a synthetic fatliquor (mixture of alkylbenzenes, aliphatic hydrocarbons, Alkanesulfonic acids and surfactants) and after a further 30 minutes 0.5 parts formic acid 85%, diluted with 5 parts Water, added. The treatment is then continued at 50 ° C. for 20 minutes
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Coloring (AREA)
- Treatment And Processing Of Natural Fur Or Leather (AREA)
Description
Die vorliegende Erfindung betrifft ein Verfahren zum Färben von Leder mit anionischen Farbstoffen sowie das nach dem Verfahren gefärbte Leder.The present invention relates to a method for dyeing leather with anionic Dyes and leather dyed using the process.
Verfahren zum Färben von Leder mit anionischen Farbstoffen sind bereits bekannt und werden auch in der Praxis mit grossem Erfolg angewendet. Die Farbstoffe werden dabei üblicherweise in Form fester oder flüssiger Handelsformen eingesetzt, die mehr oder weniger grosse Mengen an Alkalihalogenid enthalten, das als Coupage zur Einstellung einer bestimmten Farbstärke zugesetzt wurde oder aufgrund des Herstellungsverfahrens anwesend ist. Infolgedessen enthalten auch die Färbeflotten Alkalihalogenid.Methods for dyeing leather with anionic dyes are already known and are also used in practice with great success. The dyes are there Usually used in the form of solid or liquid commercial forms, the more or contain less large amounts of alkali halide as a coupling for adjustment a certain color strength was added or due to the manufacturing process is present. As a result, the dyeing liquors also contain alkali halide.
Es wurde nun gefunden, dass überraschenderweise beim Färben von Leder mit speziellen anionischen Farbstoffen das Ausziehvermögen der Farbstoffe erheblich grösser ist, wenn die Färbeflotte wenig oder kein Alkalihalogenid enthält.It has now been found that, surprisingly, when dyeing leather with special anionic dyes the extractability of the dyes is considerably greater if the dye liquor contains little or no alkali halide.
Gegenstand der Erfindung ist somit ein Verfahren zum Ausziehfärben von Leder mit anionischen Farbstoffen, dadurch gekennzeichnet, dass man zum Färben eine wässrige Lösung verwendet, enthaltend einen Polyazofarbstoff mit mindestens 2 Sulfogruppen sowie weniger als 1,5 Gew.%, bezogen auf das Gewicht des Farbstoffes, Alkalihalogenid, wobei die Farbstoffe nicht als Lithiumsalze vorliegen.The invention thus relates to a process for dyeing leather with anionic Dyes, characterized in that an aqueous solution is used for dyeing, containing a polyazo dye with at least 2 sulfo groups and less than 1.5% by weight, based on the weight of the dye, of alkali halide, the Dyes do not exist as lithium salts.
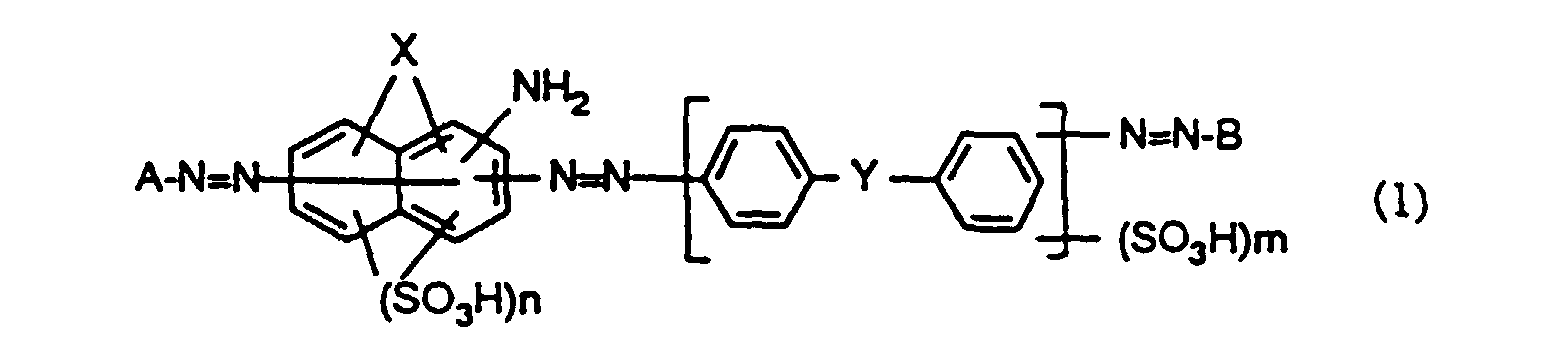
Eine bevorzugte Ausführungsform des erfindungsgemässen Verfahrens besteht darin, dass man zum Färben eine wässrige Lösung verwendet, welche einen Farbstoff der Formel worin
- A
- den Rest einer aromatischen Diazokomponente,
- B
- den Rest einer Kupplungskomponente,
- X
- OH oder NH2,
- Y
- NH, O oder SO2NH,
- n
- 1 oder 2 und
- m
- 0, 1 oder 2 bedeuten, enthält.
- A
- the rest of an aromatic diazo component,
- B
- the rest of a coupling component,
- X
- OH or NH 2 ,
- Y
- NH, O or SO 2 NH,
- n
- 1 or 2 and
- m
- 0, 1 or 2 means contains.
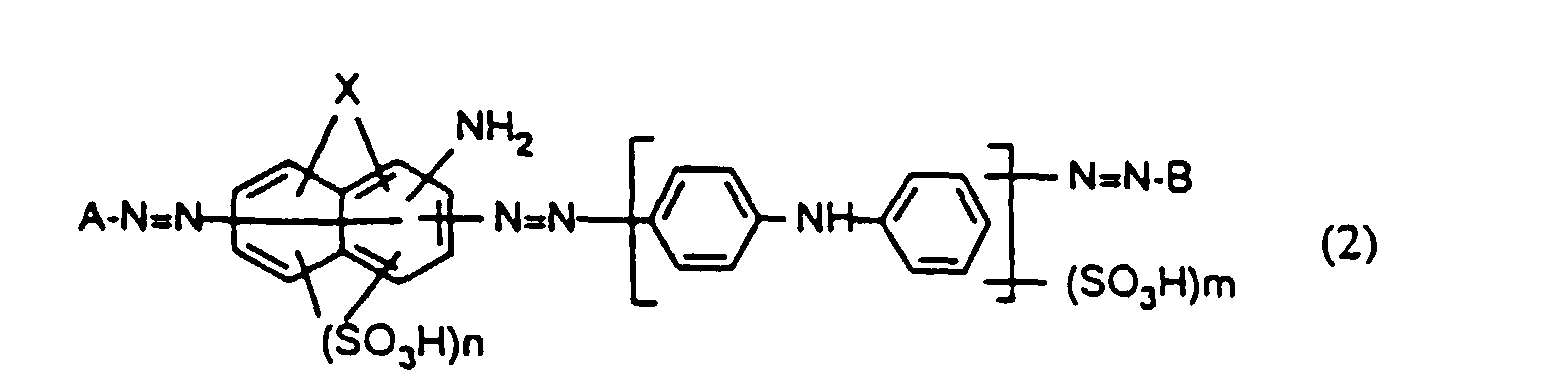
Eine ganz besonders bevorzugte Ausführungsform des erfindungsgemässen Verfahrens besteht darin, dass man zum Färben eine wässrige Lösung verwendet, welche einen Farbstoff der Formel worin
- A
- den Rest einer aromatischen Diazokomponente,
- B
- den Rest einer Kupplungskomponente,
- X
- OH oder NH2,
- n
- 1 oder 2 und
- m
- 0, 1 oder 2 bedeutet, enthält.
- A
- the rest of an aromatic diazo component,
- B
- the rest of a coupling component,
- X
- OH or NH 2 ,
- n
- 1 or 2 and
- m
- 0, 1 or 2 means contains.
Der Diazorest A leitet sich z.B. von einem heterocyclischen oder carbocyclischen aromatischen Amin ab, vorzugsweise von 1- oder 2-Naphthylamin oder Aminobenzol, wobei die genannten aromatischen Amine durch einen oder mehrere gleiche oder verschiedene Reste weitersubstituiert sein können.The diazo residue A leads e.g. from a heterocyclic or carbocyclic aromatic amine, preferably of 1- or 2-naphthylamine or aminobenzene, the aromatic amines mentioned by one or several identical or different radicals can be further substituted.
Geeignete Substituenten am Phenyl- oder Naphthylrest A sind z.B. C1-C4-Alkyl, welches hier und im weiteren generell Methyl, Ethyl, n- oder iso-Propyl oder n-, iso-, sec.- oder tert.-Butyl umfasst, C1-C4-Alkoxy, worunter generell Methoxy, Ethoxy, n- oder iso-Propoxy oder n-, iso-, sec.- oder tert.-Butoxy zu verstehen ist, Halogen, z.B. Fluor, Brom und insbesondere Chlor; Trifluormethyl; C1-C4-Alkylsulfonyl, besonders Methyl- oder Ethylsulfonyl; Sulfamoyl; N-Mono- oder N,N-Di-C1-C4-Alkylsulfamoyl; Carbamoyl; N-Mono- oder N,N-Di-C1-C4-Alkylcarbamoyl, Sulfo; Nitro; Cyano, Carboxy; Phenoxy; C1-C4-Alkanoylamino, besonders Acetylamino oder Propionylamino; Benzoylamino; C1-C4-Alkoxycarbonyl, z.B. Methoxy- oder Ethoxycarbonyl.Suitable substituents on the phenyl or naphthyl radical A are, for example, C 1 -C 4 -alkyl, which here and in general comprises methyl, ethyl, n- or iso-propyl or n-, iso-, sec.- or tert-butyl , C 1 -C 4 alkoxy, which generally means methoxy, ethoxy, n- or iso-propoxy or n-, iso-, sec- or tert-butoxy, halogen, for example fluorine, bromine and especially chlorine; Trifluoromethyl; C 1 -C 4 alkylsulfonyl, especially methyl or ethylsulfonyl; Sulfamoyl; N-mono- or N, N-di-C 1 -C 4 alkylsulfamoyl; Carbamoyl; N-mono- or N, N-di-C 1 -C 4 alkylcarbamoyl, sulfo; Nitro; Cyano, carboxy; Phenoxy; C 1 -C 4 alkanoylamino, especially acetylamino or propionylamino; Benzoylamino; C 1 -C 4 alkoxycarbonyl, for example methoxy or ethoxycarbonyl.
Bevorzugte Diazokomponenten sind Verbindungen der Formel worin R1 und R2 unabhängig voneinander je Wasserstoff, C1-C4-Alkyl, C1-C4-Alkoxy, Halogen, Nitro, Trifluormethyl, C1-C4-Alkylsulfonyl, Acetylamino, Hydroxyacetylamino, Propionylamino, Sulfamoyl, Carbamoyl, Cyano, Carboxy oder Phenoxy bedeuten.Preferred diazo components are compounds of the formula wherein R 1 and R 2 are each independently hydrogen, C 1 -C 4 alkyl, C 1 -C 4 alkoxy, halogen, nitro, trifluoromethyl, C 1 -C 4 alkylsulfonyl, acetylamino, hydroxyacetylamino, propionylamino, sulfamoyl, carbamoyl , Cyano, carboxy or phenoxy mean.
Steht der Rest R1 oder R2 für C1-C4-Alkylsulfonyl, handelt es sich z.B. um Ethylsulfonyl und insbesondere um Methylsulfonyl.If the radical R 1 or R 2 is C 1 -C 4 -alkylsulfonyl, it is, for example, ethylsulfonyl and in particular methylsulfonyl.
Bevorzugte Bedeutungen von R1 und R2 sind unabhängig voneinander Wasserstoff, Methyl, Ethyl, Methoxy, Ethoxy, Chlor, Nitro, Trifluormethyl, Methylsulfonyl, Ethylsulfonyl, Sulfamoyl, Carbamoyl, Cyano, Acetylamino und Phenoxy. R1 und R2 stehen unabhängig voneinander besonders bevorzugt für Wasserstoff, Methyl, Methoxy, Nitro oder Chlor.Preferred meanings of R 1 and R 2 are independently of one another hydrogen, methyl, ethyl, methoxy, ethoxy, chlorine, nitro, trifluoromethyl, methylsulfonyl, ethylsulfonyl, sulfamoyl, carbamoyl, cyano, acetylamino and phenoxy. R 1 and R 2 independently of one another particularly preferably represent hydrogen, methyl, methoxy, nitro or chlorine.
Beispiele für bevorzugte Verbindungen der Formel (3) sind:
Anilin, 2-, 3- oder 4-Methylanilin, 2-, 3- oder 4-Methoxyanilin, 2-, 3- oder 4-Chloranilin, 2-,3- oder 4-Nitroanilin, 2-Chlor-4-nitroanilin,
2-Methyl-4-nitroanilin, 2,5-Dichloranilin, 2,5-Dimethylanilin, 2,5-Dimethoxyanilin oder 2-Methoxy-5-methylanilin.Examples of preferred compounds of the formula (3) are:
Aniline, 2-, 3- or 4-methylaniline, 2-, 3- or 4-methoxyaniline, 2-, 3- or 4-chloroaniline, 2-, 3- or 4-nitroaniline, 2-chloro-4-nitroaniline, 2-methyl-4-nitroaniline, 2,5-dichloroaniline, 2,5-dimethylaniline, 2,5-dimethoxyaniline or 2-methoxy-5-methylaniline.
Die bevorzugte Bedeutung von X ist OH und es ist ausserdem bevorzugt, dass sich die Gruppe X nicht am gleichen Ring befindet wie die NH2-Gruppe.The preferred meaning of X is OH and it is also preferred that the group X is not on the same ring as the NH 2 group.
B bedeutet den Rest einer Kupplungskomponente, insbesondere einer Kupplungskomponente aus der Benzol- oder Naphthalinreihe. Vorzugsweise bedeutet B einen Rest der Formel worin R3 Hydroxy, Amino, N-Mono- oder N,N-Di-C1-C4-Alkylamino, Phenylamino oder o-, m- oder p-Methylphenylamino bedeutet und R4 und R5 unabhängig voneinander je die Bedeutung von R3 haben oder für Wasserstoff, Benzoylamino, C1-C4 -Alkanoylamino, Carboxymethylamino, C1-C4-Alkyl, C1-C4-Alkoxy, Phenoxy, Carboxy, Halogen, Nitro oder Sulfo stehen.B denotes the rest of a coupling component, in particular a coupling component from the benzene or naphthalene series. B is preferably a radical of the formula wherein R 3 is hydroxy, amino, N-mono- or N, N-di-C 1 -C 4 alkylamino, phenylamino or o-, m- or p-methylphenylamino and R 4 and R 5 each independently of the other meanings R 3 have or represent hydrogen, benzoylamino, C 1 -C 4 alkanoylamino, carboxymethylamino, C 1 -C 4 alkyl, C 1 -C 4 alkoxy, phenoxy, carboxy, halogen, nitro or sulfo.
R3 steht als N-Mono- oder N,N-Di-C1-C4 -Alkylamino z.B. für Methyl-, Ethyl-, n- oder iso-Propyl- oder n-, iso-, sec. - oder tert.-Butylamino oder N,N-Dimethyl-, N,N-Diethyl-, N,N-Di-n- oder -iso-Propyl- oder N,N-Di-n-, -iso-, -sec.- oder -tert.-Butylamino und vorzugsweise für N-Methylamino, N-Ethylamino, N,N-Dimethylamino oder N,N-Diethylamino.R 3 as N-mono- or N, N-di-C 1 -C 4 -alkylamino is, for example, methyl-, ethyl-, n- or iso-propyl- or n-, iso-, sec. - or tert. -Butylamino or N, N-dimethyl-, N, N-diethyl-, N, N-Di-n- or -iso-Propyl- or N, N-Di-n-, -iso-, -sec.- or -tert.-butylamino and preferably for N-methylamino, N-ethylamino, N, N-dimethylamino or N, N-diethylamino.
Bevorzugte Bedeutungen von R3 sind Hydroxy, Amino, N-Methyl- und N-Ethylamino, N,N-Dimethyl- und N,N-Diethylamino und Phenylamino.Preferred meanings of R 3 are hydroxy, amino, N-methyl- and N-ethylamino, N, N-dimethyl- and N, N-diethylamino and phenylamino.
R3 steht besonders bevorzugt für Hydroxy oder Amino.R 3 particularly preferably represents hydroxy or amino.
Bei R4 und/oder R5 wie auch bei den weiter unten folgenden Variablen als C1-C4 -Alkyl handelt es sich generell um Methyl, Ethyl, n- oder iso-Propyl oder n-, iso-, sec.- oder tert.-Butyl.R 4 and / or R 5 as well as the variables below as C 1 -C 4 alkyl are generally methyl, ethyl, n- or iso-propyl or n-, iso-, sec.- or tert-butyl.
Stehen R4 und/oder R5 oder weiter unten folgende Variablen für C1-C4-Alkoxy, so ist damit generell Methoxy, Ethoxy, n- oder iso-Propoxy oder n-, iso-, sec.- oder tert.-Butoxy umfasst.If R 4 and / or R 5 or the following variables stand for C 1 -C 4 alkoxy, then methoxy, ethoxy, n- or iso-propoxy or n-, iso-, sec.- or tert.- Butoxy includes.
Bei R4 und/oder R5 als Halogen handelt es sich wie im folgenden generell z.B. um Fluor, Brom und insbesondere um Chlor.R 4 and / or R 5 as halogen is generally, for example, fluorine, bromine and in particular chlorine, as in the following.
Bei R4 und/oder R5 in der Bedeutung C1-C4-Alkanoylamino handelt es sich z.B. um Propionylamino, Hydroxyacetylamino und insbesondere um Acetylamino.R 4 and / or R 5 with the meaning C 1 -C 4 alkanoylamino are, for example, propionylamino, hydroxyacetylamino and in particular acetylamino.
Sulfo umfasst generell die freie Säureform (-SO3H) als auch die Salzform, wobei insbesondere Alkalimetallsalze (Na, K) oder Ammoniumsalze in Frage kommen.Sulfo generally includes the free acid form (-SO 3 H) as well as the salt form, alkali metal salts (Na, K) or ammonium salts being particularly suitable.
R4 und R5 stehen unabhängig voneinander bevorzugt für Wasserstoff, Sulfo, Acetylamino, Methyl, Ethyl, Methoxy, Ethoxy, Chlor, Carboxy, Hydroxy, Amino, N-Methyl- oder N-Ethylamino, N,N-Dimethyl- oder N,N-Diethylamino oder Phenylamino und besonders bevorzugt für Wasserstoff, Amino, Hydroxy, Methyl, Methoxy, Phenylamino oder N,N-Dimethyl- oder N,N-Diethylamino. R 4 and R 5 independently of one another preferably represent hydrogen, sulfo, acetylamino, methyl, ethyl, methoxy, ethoxy, chlorine, carboxy, hydroxy, amino, N-methyl- or N-ethylamino, N, N-dimethyl- or N, N-diethylamino or phenylamino and particularly preferably for hydrogen, amino, hydroxy, methyl, methoxy, phenylamino or N, N-dimethyl- or N, N-diethylamino.
Bevorzugt verwendet man Verbindungen der Formel (4), worin R3 Hydroxy, Amino, N-Methyl- oder N-Ethylamino, N,N-Dimethyl- oder N,N-Diethylamino oder Phenylamino bedeutet und R4 und R5 unabhängig voneinander je für Wasserstoff, Acetylamino, Methyl, Ethyl, Methoxy, Ethoxy, Chlor, Carboxy, Hydroxy, Amino, N-Methyl- oder N-Ethylamino, N,N-Dimethyl- oder N,N-Diethylamino oder Phenylamino stehen.Compounds of the formula (4) are preferably used, in which R 3 is hydroxyl, amino, N-methyl- or N-ethylamino, N, N-dimethyl- or N, N-diethylamino or phenylamino and R 4 and R 5 each independently represent hydrogen, acetylamino, methyl, ethyl, methoxy, ethoxy, chlorine, carboxy, hydroxy, amino, N-methyl- or N-ethylamino, N, N-dimethyl- or N, N-diethylamino or phenylamino.
Beispiele für geeignete Verbindungen der Formel (4) sind: Phenol, 4-Methylphenol, 1,3-Dihydroxybenzol (Resorcin), 3-Aminophenol, 1,3-Phenylendiamin, 3-N,N-Diethylaminophenol, 3-Phenylaminophenol, 2,5-Dimethylanilin, 2-Methoxy-5-methylanilin, Salicylsäure, 2-oder 3-Methylanilin, 2-Methoxyanilin, 1,3-Diamino-4-methylbenzol, 1,3-Diamino-4-chlorbenzol und 1,3-Diamino-4-methoxybenzol.Examples of suitable compounds of the formula (4) are: phenol, 4-methylphenol, 1,3-dihydroxybenzene (resorcinol), 3-aminophenol, 1,3-phenylenediamine, 3-N, N-diethylaminophenol, 3-phenylaminophenol, 2,5-dimethylaniline, 2-methoxy-5-methylaniline, salicylic acid, 2- or 3-methylaniline, 2-methoxyaniline, 1,3-diamino-4-methylbenzene, 1,3-diamino-4-chlorobenzene and 1,3-diamino-4-methoxybenzene.
Besonders bevorzugt verwendet man Verbindungen der Formel worin R3' Amino oder Hydroxy, R4' Amino, Hydroxy, Methyl, Methoxy, Phenylamino oder N,N-Dimethyl- oder N,N-Diethylamino und R5' Wasserstoff, Methyl oder Methoxy sind.Compounds of the formula are particularly preferably used wherein R 3 'amino or hydroxy, R 4 ' amino, hydroxy, methyl, methoxy, phenylamino or N, N-dimethyl- or N, N-diethylamino and R 5 'are hydrogen, methyl or methoxy.
Eine besonders bevorzugte Ausführungsform der vorliegenden Erfindung betrifft die Verwendung von 3-Aminophenol, Resorcin oder 1,3-Phenylendiamin als Kupplungskomponente.A particularly preferred embodiment of the present invention relates to the use of 3-aminophenol, Resorcinol or 1,3-phenylenediamine as a coupling component.
Bei den Farbstoffen der Formel (1) und (2) bedeutet n vorzugsweise 2 und m vorzugsweise 1.In the dyes of the formulas (1) and (2), n is preferably 2 and m is preferably 1.
Die Farbstoffe der Formel (1) und (2) sind bekannt oder können auf bekannte Art und Weise hergestellt werden. Besonders bevorzugt ist die Verwendung solcher Farbstoffe der Formel (1), die in der DE-A-41 24 437 beschrieben sind.The dyes of the formula (1) and (2) are known or can be prepared in a known manner. The use of such dyes of the formula (1), which are described in DE-A-41 24 437, is particularly preferred.
Die auf übliche Weise hergestellten Farbstoffe der Formel (1) oder (2) enthalten normalerweise ca. 5 bis 30 Gew. % Alkalihalogenid, bezogen auf das Gewicht des Farbstoffes. Dieses Alkalihalogenid wird bei der Synthese des Farbstoffes gebildet und/oder der erhaltenen Farbstofflösung zur Abscheidung des Farbstoffes zugesetzt. Zusätzlich wird oft die feste oder flüssige Handelsform des Farbstoffes durch Zugabe von Alkalihalogenid auf die gewünschte Farbstärke eingestellt.The dyes of the formula (1) or (2) prepared in the usual way normally contain about 5 to 30% by weight. % Alkali halide, based on the weight of the dye. This alkali halide is used in the synthesis of the dye formed and / or added to the dye solution obtained to separate the dye. In addition, often the solid or liquid commercial form of the dye by adding alkali halide to the desired color strength set.
Im erfindungsgemässen Färbeverfahren verwendet man dagegen eine wässrige Lösung, enthaltend einen Farbstoff mit weniger als 1,5 Gew.%, bezogen auf das Gewicht des Farbstoffes, Alkalihalogenid.In contrast, in the dyeing process according to the invention, an aqueous solution containing a dye is used with less than 1.5% by weight, based on the weight of the dye, of alkali halide.
Die im Handel erhältlichen oder auf übliche Weise hergestellten Farbstoffe müssen daher vor ihrer Verwendung im erfindungsgemässen Färbeverfahren weitgehend vom Alkalihalogenid befreit werden. Dies geschieht auf an sich bekannte Weise, beispielsweise durch Umkehrosmose, Ultrafiltration oder Dialyse. Solche Entsalzungsverfahren sind bekannt, z.B. aus der EP-A 0 059782. Ebenfalls bekannt sind die in diesen Verfahren verwendeten Membranen, z.B. aus der EP-A 0 061 424.The dyes that are commercially available or produced in the usual way must therefore be used are largely freed from the alkali halide in the dyeing process according to the invention. This happens on itself known way, for example by reverse osmosis, ultrafiltration or dialysis. Such desalination processes are known, e.g. from EP-A 0 059782. Also known are the membranes used in these processes, e.g. from EP-A 0 061 424.
Die Verfahrensbedingungen bei dieser Entsalzung werden so gewählt, dass man Farbstofflösungen erhält, welche weniger als 1,5 Gew.%, bezogen auf das Gewicht des Farbstoffes, Alkalihalogenid enthalten. Farbstoffsalze sowie Salze mit höhermolekularen Kationen und/oder Anionen werden nicht oder nur wenig entfernt.The process conditions for this desalination are chosen so that dye solutions are obtained which contain less than 1.5% by weight, based on the weight of the dye, of alkali halide. Dye salts as well Salts with higher molecular weight cations and / or anions are not or only slightly removed.
Unter Alkalihalogenid sind in dieser Anmeldung Natrium- und Kaliumfluorid, -chlorid, -bromid und -iodid zu verstehen.In this application, alkali halide includes sodium and potassium fluoride, chloride, bromide and iodide to understand.
Nach dem Entsalzen liegt der Farbstoff in Form einer wässrigen Lösung vor, welche gewünschtenfalls noch aufkonzentriert oder, z.B. durch Zerstäubungstrocknung, zur Trockne eingedampft werden kann.After desalting, the dye is in the form of an aqueous solution which, if desired, can be concentrated or, e.g. by spray drying, can be evaporated to dryness.
Eine bevorzugte Ausführungsform des erfindungsgemässen Färbeverfahrens ist dadurch gekennzeichnet, dass man zum Färben eine wässrige Lösung verwendet, enthaltend einen Polyazofarbstoff der Formel (2) sowie weniger als 1,0 Gew.%, bezogen auf das Gewicht des Farbstoffes, Alkalihalogenid.A preferred embodiment of the dyeing process according to the invention is thereby characterized in that an aqueous solution containing one is used for dyeing Polyazo dye of the formula (2) and less than 1.0% by weight, based on the weight of the dye, alkali halide.
Im erfindungsgemässen Färbeverfahren werden die Farbstoffe vorzugsweise als wässrige Farbstoffpräparate eingesetzt. Diese Präparate enthalten vorzugsweise 5 bis 40 Gew.% Farbstoff, 65 bis 95 Gew.% Wasser, 0 bis 20 Gew.% oberflächenaktive Substanzen, Puffersubstanzen, schaumdämpfende Hilfsmittel, Gefrierschutzmittel oder Pilz- und/oder Bakterienwachstum hemmende Stoffe und weniger als 1,5 Gew.%, bezogen auf das Gewicht des Farbstoffes, Alkalihalogenid.In the dyeing process according to the invention, the dyes are preferably aqueous Dye preparations used. These preparations preferably contain 5 to 40% by weight. Dye, 65 to 95% by weight water, 0 to 20% by weight surface-active substances, buffer substances, foam-reducing aids, Antifreezes or substances that inhibit fungal and / or bacterial growth and less than 1.5 % By weight, based on the weight of the dye, alkali halide.
Bevorzugt verwendete Farbstoffpräparate enthalten 10 bis 25 Gew.% Farbstoff, 70 bis 90 Gew.% Wasser, 0 bis 20 Gew.% oberflächenaktive Substanzen, Puffersubstanzen, schaumdämpfende Hilfsmittel, Gefrierschutzmittel sowie Pilz- und/oder Bakterienwachstum hemmende Stoffe und weniger als 1,0 Gew.%, bezogen auf das Gewicht des Farbstoffes, Alkalihalogenid.Dye preparations used with preference contain 10 to 25% by weight of dye, 70 to 90 % By weight of water, 0 to 20% by weight of surface-active substances, Buffer substances, foam-suppressing aids, antifreezes and fungi and / or Bacterial growth-inhibiting substances and less than 1.0% by weight, based on the weight of the dye, alkali halide.
Die Mengen, in denen die Farbstoffe in den Färbebädern eingesetzt werden, können je nach der gewünschten Farbtiefe in weiten Grenzen schwanken. Im allgemeinen sind Farbstoffmengen von 0,1 bis 10, vorzugsweise 0,5 bis 4 Gew.%, bezogen auf das Gewicht des zu färbenden Leders, vorteilhaft.The amounts in which the dyes are used in the dye baths can vary fluctuate within wide limits according to the desired color depth. Generally are Amounts of dye from 0.1 to 10, preferably 0.5 to 4 wt.%, Based on the weight of the leather to be dyed, advantageous.
Neben Wasser und den Farbstoffen kann die Färbeflotte noch weitere Zusätze enthalten, z.B. Säuren oder Basen zur Einstellung des gewünschten pH-Wertes, sowie Hilfsmittel, wie z.B. Netzmittel, Fettungsmittel, farbvertiefende Hilfsmittel, Egalisiermittel, und/oder Antischaumittel.In addition to water and the dyes, the dye liquor can contain other additives, e.g. Acids or bases for setting the desired pH value, and auxiliaries, such as. Wetting agents, greasing agents, color-deepening aids, leveling agents, and / or Antifoam.
Das erfindungsgemässe Verfahren weist den grossen Vorteil auf, dass weniger Farbstoff benötigt wird, um eine bestimmte Farbtiefe zu erzielen, als bei Verwendung von Färbeflotten, welche grössere Mengen Alkalihalogenide enthalten, da das Ausziehvermögen der Farbstoffe grösser ist.The process according to the invention has the great advantage that less dye is needed to achieve a certain depth of color than when using dyeing liquors, which contain larger amounts of alkali halides, because the ability of the Dyes is larger.
Das erfindungsgemässe Verfahren ist nicht nur für einen bestimmten Ledertyp geeignet, sondern auf die verschiedenen Ledertypen anwendbar ist, beispielsweise auf Chromleder, nachgegerbtes Leder oder Velourleder von Ziege, Rind oder Schwein.The method according to the invention is not only suitable for a certain type of leather, but is applicable to the different types of leather, for example chrome leather, retanned leather or suede from goat, Beef or pork.
Das Färben erfolgt vorzugsweise nach dem Ausziehverfahren, z.B. bei einem Flottenverhältnis von 1:1,5 bis 1: 20, vorzugsweise 1:2 bis 1:10, und bei Temperaturen von 20 bis 100° C, vorzugsweise 30 bis 60° C. Falls erwünscht oder erforderlich, kann das Leder einer Vorbehandlung unterworfen werden, beispielsweise einer Neutralisation oder Walke.The dyeing is preferably carried out by the exhaust process, e.g. with a liquor ratio of 1: 1.5 to 1: 20, preferably 1: 2 to 1:10, and at temperatures of 20 to 100 ° C, preferably 30 to 60 ° C. If desired or necessary, the leather can be subjected to a pretreatment, for example neutralization or Walke.
Die Färbedauer schwankt je nach Art des Leders und der gewünschten Farbtiefe, liegt im allgemeinen jedoch zwischen 30 und 180 Minuten. Im Anschluss an die Färbung wird das Leder gespült und wie üblich fertiggestellt.The dyeing time varies depending on the type of leather and the desired depth of color, but is generally between 30 and 180 minutes. After the dyeing, the leather is rinsed and finished as usual.
Man erhält nach dem erfindungsgemässen Verfahren Lederfärbungen in gleichen Farbtönen und Echtheiten wie beim Färben mit alkalihalogenidreicheren Färbeflotten.Leather dyeings are obtained in the same shades and fastnesses as in the process according to the invention when dyeing with dye liquors rich in alkali halide.
Die nachfolgenden Beispiele veranschaulichen die Erfindung, ohne sie darauf zu beschränken. Teile bedeuten Gewichtsteile und Prozente Gewichtsprozente. Die Farbstoffe werden in handelsüblicher Pulverform eingesetzt; die angegebenen Farbstoffmengen beziehen sich auf die coupierte 100%ige Farbstoff-Handelsform.The following examples illustrate the invention without restricting it. Parts mean Parts by weight and percentages by weight. The dyes are used in commercially available powder form; the stated amounts of dye refer to the coupe 100% dye commercial form.
1,25 kg Presskuchen des Rohfarbstoffes der Formel mit einem Trockengehalt von 32 % werden mit 3 kg Wasser (Fabrikwasser) angeschlämmt. Die erhaltene Suspension wird mit 50 %iger Natronlauge auf einen pH von 7 bis 7,5 eingestellt. Man erhält eine dünnflüssige Lösung mit einem Farbstoff-Trockengehalt von 8,0 % und einem Gehalt an Natriumchlorid von 1,3 %.1.25 kg press cake of the raw dye of the formula With a dry content of 32%, 3 kg of water (factory water) are slurried. The suspension obtained is adjusted to a pH of 7 to 7.5 with 50% sodium hydroxide solution. A low-viscosity solution with a dry dye content of 8.0% and a sodium chloride content of 1.3% is obtained.
Diese Lösung wird in einer Labor-Umkehrosmose-Anlage unter folgenden Bedingungen entsalzt: Polysulfon-Membran,
ca. 40°C, Feed: 12 l/h, 25 bar, Flux 1289 l/m2d (Durchschnitt).
Nach 4,5 h erhält man 1,9 kg einer Lösung mit einem Farbstoffgehalt von 18,0 Gew.% und einem Gehalt an Natriumchlorid
von 0,05 Gew.% (0,9 Gew.%, bezogen auf Farbstoff). Diese entsalzte und aufkonzentrierte Lösung wird nach
Filtration über ein 10 µm-Sieb mit Wasser auf einen Farbstoffgehalt von 15 % eingestellt.This solution is desalinated in a laboratory reverse osmosis system under the following conditions: polysulfone membrane, approx. 40 ° C, feed: 12 l / h, 25 bar, flux 1289 l / m 2 d (average).
After 4.5 hours, 1.9 kg of a solution with a dye content of 18.0% by weight and a sodium chloride content of 0.05% by weight (0.9% by weight, based on dye) are obtained. This desalted and concentrated solution is adjusted to a dye content of 15% after filtration through a 10 μm sieve with water.
100 Teile chromgares Bekleidungsveloursleder (Trockengewicht) werden bei 50°C in einer Lösung von 1000 Teilen Wasser und 2 Teilen 24 %igem Ammoniak während 60 Minuten aufgewalkt. Danach folgt ein Waschprozess von 15 Minuten Dauer in 1000 Teilen Wasser von 50°C.100 parts of chrome-colored suede clothing (dry weight) are at 50 ° C in a solution of 1000 parts Water and 2 parts of 24% ammonia rolled on for 60 minutes. This is followed by a washing process of 15 Minutes in 1000 parts of water at 50 ° C.
Nach diesen vorbereitenden Operationen wird das Leder in einer Flotte aus 1000 Teilen Wasser, 2 Teilen Ammoniak 24%ig und 13,33 Teilen der oben beschriebenen Farbstofflösung bei 50°C gefärbt.After these preparatory operations, the leather is made into a liquor made from 1000 parts of water and 2 parts of ammonia 24% and 13.33 parts of the dye solution described above at 50 ° C.
Nach einer Färbedauer von 60 Minuten wird mit 4 Teilen 85 %iger Ameisensäure angesäuert und hierauf noch 20 Minuten weitergefärbt.After a dyeing time of 60 minutes, the mixture is acidified with 4 parts of 85% formic acid and then 20 Minutes further colored.
Die Fertigstellung des gefärbten Leders erfolgt nach einer Behandlung in frischem Bad mit 1000 Teilen Wasser, 2,5 Teilen eines Kondensationsproduktes aus Formaldehyd, Dicyandiamid, Ammoniumchlorid und Ethylendiamin sowie 0,5 Teilen 85 %iger Ameisensäure während 45 Minuten bei 50°C. Das erhaltene Leder ist schwarz gefärbt Es zeichnet sich durch gute Echtheiten und gutes Deckvermögen aus.The dyed leather is finished after a treatment in a fresh bath with 1000 parts of water, 2.5 parts of a condensation product of formaldehyde, dicyandiamide, ammonium chloride and ethylenediamine and 0.5 parts of 85% formic acid for 45 minutes at 50 ° C. The leather obtained is dyed black is characterized by good fastness properties and good opacity.
100 Teile chromgegerbtes Rindleder der Falzstärke 1,4 mm werden in einer aus 200 Teilen Wasser, 1 Teil Natriumbicarbonat sowie 1 Teil Natriumformiat bestehenden Flotte während 45 Minuten bei 30°C gewalkt und dann 2 mal mit 300 Teilen Wasser bei der gleichen Temperatur gewaschen.100 parts of chrome-tanned cowhide with a fold thickness of 1.4 mm are made from 200 parts of water and 1 part of sodium bicarbonate and 1 part of sodium formate existing liquor drummed at 30 ° C for 45 minutes and then 2 times washed with 300 parts of water at the same temperature.
Das so neutralisierte Leder wird anschliessend in einer Flotte aus 1000 Teilen Wasser, 2 Teilen Ammoniak 24%ig und 15 Teilen der im Beispiel 1 beschriebenen Farbstofflösung bei 50°C gefärbt Nach 30 Minuten werden der Färbeflotte 3 Teile eines synthetischen Fettungsmittels (Gemisch aus Alkylbenzolen, aliphatischen Kohlenwasserstoffen, Alkansulfonsäuren und Tensiden) und nach weiteren 30 Minuten 0,5 Teile Ameisensäure 85%ig, verdünnt mit 5 Teilen Wasser, zugesetzt Danach wird noch 20 Minuten bei 50°C weiterbehandelt The leather neutralized in this way is then in a liquor of 1000 parts water, 2 parts ammonia 24% and 15 parts of the dye solution described in Example 1 are dyed at 50 ° C. After 30 minutes, the dyeing liquor 3 parts of a synthetic fatliquor (mixture of alkylbenzenes, aliphatic hydrocarbons, Alkanesulfonic acids and surfactants) and after a further 30 minutes 0.5 parts formic acid 85%, diluted with 5 parts Water, added. The treatment is then continued at 50 ° C. for 20 minutes
Nach gutem Spülen in kaltem Wasser und Fertigstellen in üblicher Weise resultiert ein egal schwarz gefärbtes Leder mit ausgezeichneten Echtheiten, insbesondere einer guten Diffusionsechtheit gegen Weich-PVC. Der Farbton stimmt mit der nach Beispiel 1 erhaltenen Ausfärbung überein.After rinsing well in cold water and finishing in the usual way, the result is a black color Leather with excellent fastness properties, especially good diffusion fastness against soft PVC. The shade agrees with the coloration obtained according to Example 1.
Um die gleiche Farbtiefe mit einer Lösung des gleichen Farbstoffes, welche jedoch einen Gehalt von 1,3 Gew.% (7,2 Gew.%, bezogen auf Farbstoff) Natriumchlorid aufweist, zu erhalten, muss man etwa ein Drittel mehr Farbstoff einsetzen, wenn man unter ansonsten identischen Färbebedingungen färbtTo the same depth of color with a solution of the same dye, which however contains 1.3% by weight (7.2% by weight, based on dye) sodium chloride, to get about a third more dye use if you dye under otherwise identical dyeing conditions
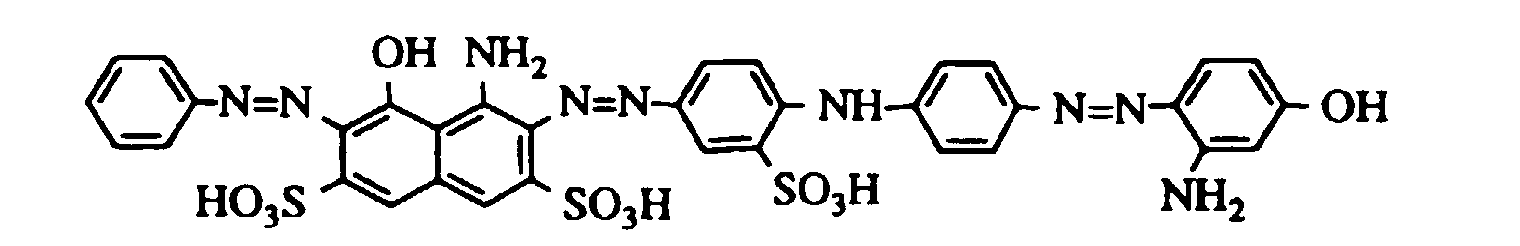
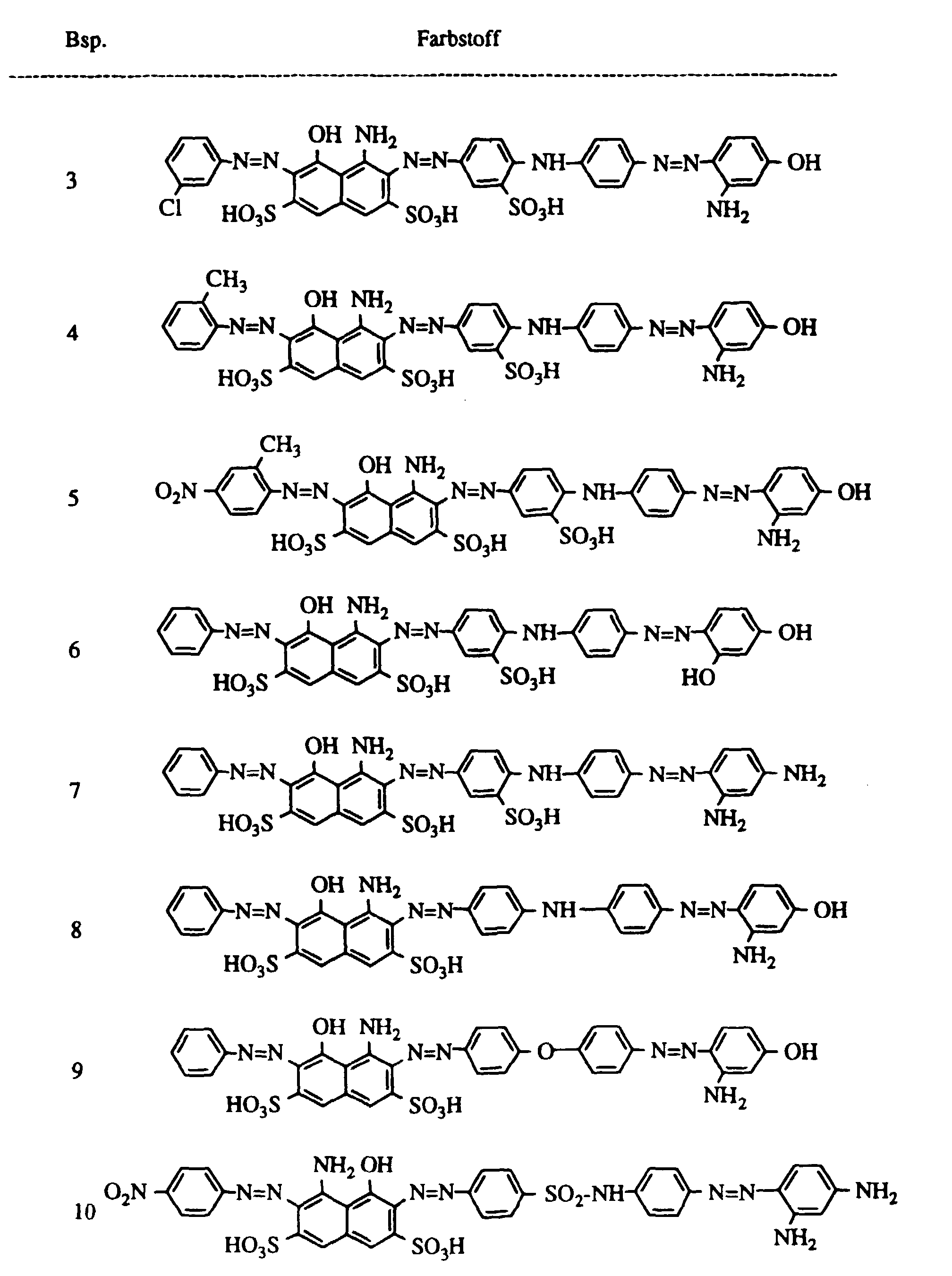
Arbeitet man wie im Beispiel 2 beschrieben, verwendet jedoch anstelle des dort eingesetzten Farbstoffes gleiche Teile der in der folgenden Tabelle aufgeführten Farbstoffe, so erhält man ebenfalls schwarz gefärbtes Leder. If one works as described in Example 2, but instead of the dye used there, the same parts of the dyes listed in the following table are used, black leather is also obtained.
Claims (22)
- A process for exhaust-dyeing leather with anionic dyes, which comprises dyeing with an aqueous solution comprising a polyazo dye which contains at least 2 sulfo groups and less than 1.5 % by weight, based on the weight of the dye, of alkali metal halide, the dyes not being present as lithium salts.
- A process according to any one of claims 1 to 3, which comprises using a dye of formula (1) or (2) wherein the diazo radical A is derived from 1- or 2-naphthylamine or aminobenzene, which aromatic amines may be further substituted by one or more identical or different radicals.
- A process according to any one of claims 1 to 4, which comprises using a dye of formula (1) or (2) wherein the diazo radical A is derived from an amine of formula wherein R1 and R2 are each independently of the other hydrogen, C1-C4alkyl, C1-C4alkoxy, halogen, nitro, trifluoromethyl, C1-C4alkylsulfonyl, acetylamino, hydroxyacetylamino, propionylamino, sulfamoyl, carbamoyl, cyano, carboxy or phenoxy.
- A process according to claim 5, wherein R1 and R2 are each independently of the other hydrogen, methyl, ethyl, methoxy, ethoxy, chloro, nitro, trifluoromethyl, methylsulfonyl, ethylsulfonyl, sulfamoyl, carbamoyl, cyano, acetylamino or phenoxy.
- A process according to claim 6, wherein R1 and R2 are each independently of the other hydrogen, methyl, methoxy, nitro or chloro.
- A process according to claim 7, wherein the diazo radical A is derived from aniline, 2-, 3- or 4-methylaniline, 2-, 3- or 4-methoxyaniline, 2-, 3- or 4-chloroaniline, 2-, 3- or 4-nitroaniline, 2-chloro-4-nitroaniline, 2-methyl-4-nitroaniline, 2,5-dichloroaniline, 2,5-dimethylaniline, 2,5-dimethoxyaniline or 2-methoxy-5-methylaniline.
- A process according to any one of claims 1 to 8, wherein X is OH.
- A process according to any one of claims 1 to 9, wherein X is not on the same ring as the NH2 group.
- A process according to any one of claims 1 to 10, wherein B is a radical of formula wherein R3 is hydroxy, amino, N-mono- or N,N-di-C1-C4alkylamino, phenylamino or o-, m- or p-methylphenylamino, and R4 and R5 each independently of the other have the meaning of R3 or are hydrogen, benzoylamino, C1-C4alkanoylamino, carboxymethylamino, C1-C4alkyl, C1-C4alkoxy, phenoxy, carboxy, halogen, nitro or sulfo.
- A process according to claim 11, wherein R3 is hydroxy, amino, N-methyl-, and N-ethylamino, N,N-dimethyl- and N,N-diethylamino or phenylamino, especially hydroxy or amino.
- A process according to any one of claims 1 to 12, wherein R4 and R5 are each independently of the other hydrogen, sulfo, acetylamino, methyl, ethyl, methoxy, ethoxy, chloro, carboxy, hydroxy, amino, N-methyl- or N-ethylamino, N,N-dimethyl- or N,N-diethylamino or phenylamino, especially hydrogen, amino, hydroxy, methyl, methoxy, phenylamino or N,N-dimethyl- or N,N-diethylamino.
- A process according to any one of claims 1 to 13, wherein B is a radical of formula (4) wherein R3 is hydroxy, amino, N-methyl- or N-ethylamino, N,N-dimethyl- or N,N-diethylamino or phenylamino, and R4 and R5 are each independently of the other hydrogen, acetylamino, methyl, ethyl, methoxy, ethoxy, chloro, carboxy, hydroxy, amino, N-methyl- or N-ethylamino, N,N-dimethyl- or N,N-diethylamino or phenylamino.
- A process according to claim 14, wherein B is the radical of phenol, 4-methylphenol, 1,3-dihydroxybenzene (resorcinol), 3-aminophenol, 1,3-phenylenediamine, 3-N,N-diethylaminophenol, 3-phenylaminophenol, 2,5-dimethylaniline, 2-methoxy-5-methylaniline, salicylic acid, 2- or 3-methylaniline, 2-methoxyaniline, 1,3-diamino-4-methylbenzene, 1,3-diamino-4-chlorobenzene or 1,3-diamino-4-methoxybenzene.
- A process according to any one of claims 1 to 16, wherein B is the radical of 3-aminophenol, resorcinol or 1,3-phenylenediamine.
- A process according to any one of claims 1 to 17, which comprises the use of a dye of formula (1) or (2) wherein n is 2.
- A process according to any one of claims 1 to 18, which comprises the use of a dye of formula (1) or 92) wherein m is 1.
- A process according to any one of claims 1 to 19, which comprises dyeing using an aqueous solution comprising a polyazo dye of formula (1) or (2) and less than 1.0 % by weight, based on the weight of the dye, of alkali metal halide.
- A process according to any one of claims 1 to 20, which comprises dyeing using an aqueous dye formulation containing 5 to 40 % by weight of dye, 65 to 95 % by weight of water, 0 to 20 % by weight of surfactants, buffers, antifoams, antifreeze agents or fungicides and/or bactericides and less than 1.5 % by weight, based on the weight of the dye, of alkali metal halide.
- A process according to any one of claims 2 to 21, which comprises dyeing using an aqueous dye formulation containing 10 to 25 % by weight of dye, 70 to 90 % by weight of water, 0 to 20 % by weight of surfactants, buffers, antifoams, antifreeze agents or fungicides and/or bactericides and less than 1.0 % by weight, based on the weight of the dye, of alkali metal halide.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH168792 | 1992-05-26 | ||
| CH1687/92 | 1992-05-26 | ||
| CH168792A CH685251A5 (en) | 1992-05-26 | 1992-05-26 | A process for dyeing leather. |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0572353A1 EP0572353A1 (en) | 1993-12-01 |
| EP0572353B1 EP0572353B1 (en) | 1996-06-19 |
| EP0572353B2 true EP0572353B2 (en) | 1999-06-09 |
Family
ID=4216257
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19930810365 Expired - Lifetime EP0572353B2 (en) | 1992-05-26 | 1993-05-18 | Process for dyeing of leather |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP0572353B2 (en) |
| JP (1) | JPH0665869A (en) |
| CH (1) | CH685251A5 (en) |
| DE (1) | DE59302989D1 (en) |
| ES (1) | ES2089774T3 (en) |
| MX (1) | MX9303069A (en) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102004027812A1 (en) | 2004-06-08 | 2006-01-05 | Basf Ag | Process for the reactive dyeing of leather |
| EP1882749A1 (en) | 2006-07-25 | 2008-01-30 | Joseph Mellini | Leather-surface repair-composition and the method for surface repair of leather surfaces |
| TWI540186B (en) * | 2015-07-16 | 2016-07-01 | 臺灣永光化學工業股份有限公司 | Black dye composition and use thereof |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2360634A1 (en) * | 1976-08-03 | 1978-03-03 | Ugine Kuhlmann | NEW WATER-SOLUBLE TRISAZOIC DYES |
| DE59004417D1 (en) * | 1989-12-11 | 1994-03-10 | Ciba Geigy | Process for dyeing leather. |
| DE4124437A1 (en) * | 1990-07-26 | 1992-01-30 | Ciba Geigy Ag | Water soluble anionic poly:azo dyestuff cpds. mfr. for dyeing leather - by tetrazotising di:amino:di:phenylamine sulphonic acid, coupling with amino:naphthol and then e.g. phenol etc. |
-
1992
- 1992-05-26 CH CH168792A patent/CH685251A5/en not_active IP Right Cessation
-
1993
- 1993-05-18 EP EP19930810365 patent/EP0572353B2/en not_active Expired - Lifetime
- 1993-05-18 ES ES93810365T patent/ES2089774T3/en not_active Expired - Lifetime
- 1993-05-18 DE DE59302989T patent/DE59302989D1/en not_active Expired - Fee Related
- 1993-05-26 MX MX9303069A patent/MX9303069A/en not_active IP Right Cessation
- 1993-05-26 JP JP5123756A patent/JPH0665869A/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| JPH0665869A (en) | 1994-03-08 |
| ES2089774T3 (en) | 1996-10-01 |
| EP0572353B1 (en) | 1996-06-19 |
| DE59302989D1 (en) | 1996-07-25 |
| EP0572353A1 (en) | 1993-12-01 |
| CH685251A5 (en) | 1995-05-15 |
| MX9303069A (en) | 1994-06-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE2708188B1 (en) | Stabilization of anionic indole dyes | |
| EP0572353B2 (en) | Process for dyeing of leather | |
| DE3034686C2 (en) | Water-soluble 1:2 chromium complexes of disazo compounds, their preparation and use | |
| EP0577556B1 (en) | Process for the trichromatic dyeing of leather with dye mixtures | |
| DE4407802B4 (en) | Azo compounds and intermediates, their preparation and use | |
| EP0061999B1 (en) | Use of 1:2-chrome or cobalt complex dyes for dyeing leather or furs | |
| DE69719345T2 (en) | TETRAKISAZO DYES, THEIR PRODUCTION AND USE | |
| EP0016975B1 (en) | Polyazo dyestuffs and their use in dyeing fibre-materials and leather containing amino- and hydroxyl groups | |
| DE2519657C3 (en) | Mixing dye intermediates and process for making dye | |
| EP0558450A1 (en) | Process for dyeing leather with mixtures of dyes | |
| DE69420102T2 (en) | Stilbene azo dyes | |
| EP0113643B1 (en) | 1:2-cobalt complexes of disazo dyestuffs | |
| DE4319020B4 (en) | Blue chromium complex dyes, their preparation and use | |
| EP0202549B1 (en) | Process for dyeing leather | |
| DE2139148C3 (en) | Telrakisazo dyes, processes for their production and their use for dyeing vegetable and animal fiber materials, as well as leather | |
| EP0468922B1 (en) | Use of polyazodyes for dyeing leather | |
| EP0095441A1 (en) | Asymmetric 1:2 chromium complex dyestuffs | |
| DE4133167A1 (en) | IRON COMPLEX MIXTURES, THEIR PRODUCTION AND USE | |
| DE2017873C3 (en) | Blue disazo dyes | |
| DE899042C (en) | Process for the preparation of new 4,4'-diaminostilbene disulfonic or dicarboxylic acids | |
| DE56456C (en) | Process for the preparation of disazo dyes of azoxyaniline and its homologues | |
| EP0082819B1 (en) | Use of 1:2 cobalt complex dyes for dyeing leather or furs | |
| DE19525496A1 (en) | Azo dyes and intermediates, their manufacture and use | |
| DE639669C (en) | Process for dyeing leather | |
| DE710408C (en) | Process for the preparation of polyazo dyes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE ES FR GB IT LI |
|
| 17P | Request for examination filed |
Effective date: 19940513 |
|
| 17Q | First examination report despatched |
Effective date: 19950206 |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE CH DE ES FR GB IT LI |
|
| ET | Fr: translation filed | ||
| REF | Corresponds to: |
Ref document number: 59302989 Country of ref document: DE Date of ref document: 19960725 |
|
| ITF | It: translation for a ep patent filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19960815 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2089774 Country of ref document: ES Kind code of ref document: T3 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PUE Owner name: CIBA-GEIGY AG TRANSFER- CIBA SC HOLDING AG |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2089774 Country of ref document: ES Kind code of ref document: T3 |
|
| BECN | Be: change of holder's name |
Effective date: 19961129 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: TP |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| 26 | Opposition filed |
Opponent name: BAYER AG Effective date: 19970319 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Free format text: CIBA SC HOLDING AG TRANSFER- CIBA SPECIALTY CHEMICALS HOLDING INC. |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: 732E |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| PLBF | Reply of patent proprietor to notice(s) of opposition |
Free format text: ORIGINAL CODE: EPIDOS OBSO |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19980403 Year of fee payment: 6 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 19980513 Year of fee payment: 6 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: PC2A |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19980616 Year of fee payment: 6 |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| PLAW | Interlocutory decision in opposition |
Free format text: ORIGINAL CODE: EPIDOS IDOP |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19990426 Year of fee payment: 7 |
|
| RAP2 | Party data changed (patent owner data changed or rights of a patent transferred) |
Owner name: CIBA SPECIALTY CHEMICALS HOLDING INC. |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990519 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990531 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990531 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990531 |
|
| 27A | Patent maintained in amended form |
Effective date: 19990609 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): BE CH DE ES FR GB IT LI |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: AEN Free format text: AUFRECHTERHALTUNG DES PATENTES IN GEAENDERTER FORM |
|
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| GBTA | Gb: translation of amended ep patent filed (gb section 77(6)(b)/1977) | ||
| ITF | It: translation for a ep patent filed | ||
| BERE | Be: lapsed |
Owner name: CIBA SPECIALTY CHEMICALS HOLDING INC. Effective date: 19990531 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000518 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20000518 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20010503 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050518 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20070531 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20070313 Year of fee payment: 15 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20090119 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080602 Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20081202 |