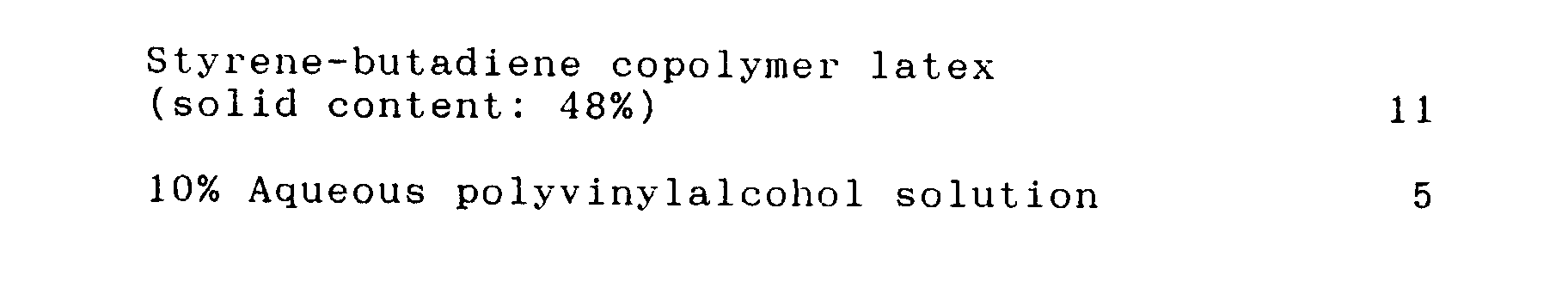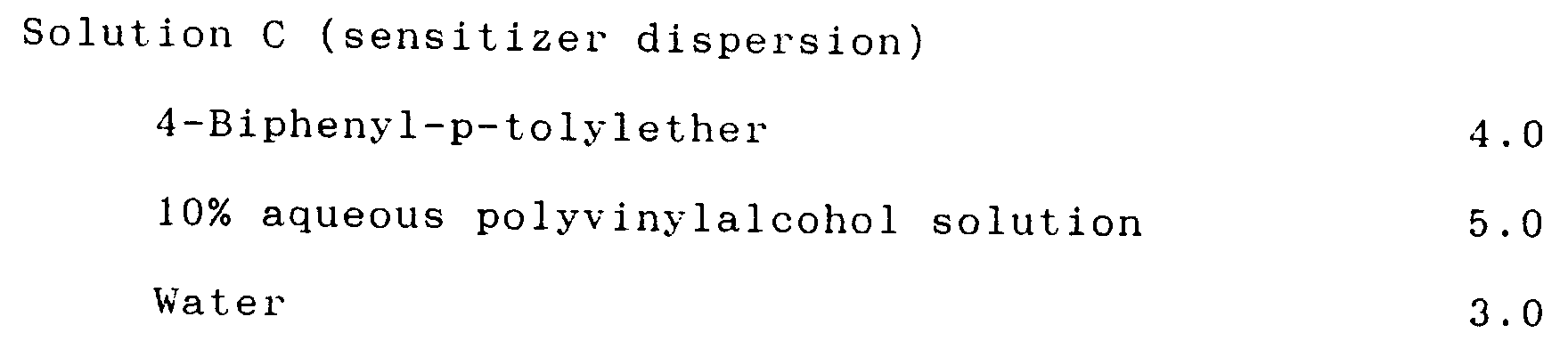EP0570186A1 - Thermal recording sheet - Google Patents
Thermal recording sheet Download PDFInfo
- Publication number
- EP0570186A1 EP0570186A1 EP93303616A EP93303616A EP0570186A1 EP 0570186 A1 EP0570186 A1 EP 0570186A1 EP 93303616 A EP93303616 A EP 93303616A EP 93303616 A EP93303616 A EP 93303616A EP 0570186 A1 EP0570186 A1 EP 0570186A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- intermediate layer
- cross
- thermal recording
- thermal
- methyl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/26—Thermography ; Marking by high energetic means, e.g. laser otherwise than by burning, and characterised by the material used
- B41M5/40—Thermography ; Marking by high energetic means, e.g. laser otherwise than by burning, and characterised by the material used characterised by the base backcoat, intermediate, or covering layers, e.g. for thermal transfer dye-donor or dye-receiver sheets; Heat, radiation filtering or absorbing means or layers; combined with other image registration layers or compositions; Special originals for reproduction by thermography
- B41M5/42—Intermediate, backcoat, or covering layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M2205/00—Printing methods or features related to printing methods; Location or type of the layers
- B41M2205/04—Direct thermal recording [DTR]
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M2205/00—Printing methods or features related to printing methods; Location or type of the layers
- B41M2205/38—Intermediate layers; Layers between substrate and imaging layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/26—Thermography ; Marking by high energetic means, e.g. laser otherwise than by burning, and characterised by the material used
- B41M5/40—Thermography ; Marking by high energetic means, e.g. laser otherwise than by burning, and characterised by the material used characterised by the base backcoat, intermediate, or covering layers, e.g. for thermal transfer dye-donor or dye-receiver sheets; Heat, radiation filtering or absorbing means or layers; combined with other image registration layers or compositions; Special originals for reproduction by thermography
- B41M5/42—Intermediate, backcoat, or covering layers
- B41M5/426—Intermediate, backcoat, or covering layers characterised by inorganic compounds, e.g. metals, metal salts, metal complexes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B41—PRINTING; LINING MACHINES; TYPEWRITERS; STAMPS
- B41M—PRINTING, DUPLICATING, MARKING, OR COPYING PROCESSES; COLOUR PRINTING
- B41M5/00—Duplicating or marking methods; Sheet materials for use therein
- B41M5/26—Thermography ; Marking by high energetic means, e.g. laser otherwise than by burning, and characterised by the material used
- B41M5/40—Thermography ; Marking by high energetic means, e.g. laser otherwise than by burning, and characterised by the material used characterised by the base backcoat, intermediate, or covering layers, e.g. for thermal transfer dye-donor or dye-receiver sheets; Heat, radiation filtering or absorbing means or layers; combined with other image registration layers or compositions; Special originals for reproduction by thermography
- B41M5/42—Intermediate, backcoat, or covering layers
- B41M5/44—Intermediate, backcoat, or covering layers characterised by the macromolecular compounds
Definitions
- This invention relates to a thermal recording sheet which is superior in dynamic sensitivity, dot reproducibility, and image quality, without sticking and adherence of residue.
- thermal recording sheets In general, in thermal recording sheets, a normally colorless or pale colored basic chromogenic dye and an organic color developer such as a phenolic substance are individually dispersed into fine particles, mixed, and a binder, a filler, a sensitivity improver, a slip agent, and other additives are added to obtain a coating color, which is coated on a substrate such as paper, synthetic paper, plastic films, cloths, and the like.
- the thermal recording sheet enables color recording by a momentary chemical reaction caused by heating with a thermal pen, a thermal head, a hot stamp, laser light, or the like.
- thermal recording sheets are applied in a variety of areas such as measurement recorders, computer terminal printers, facsimiles, automatic ticket vendors, and bar-code labels, however, with recent diversification and improvement of these recording devices, requirements to the thermal recording sheets have become stricter. For example, with increasing recording speed, it is required to obtain a high-concentration, sharp color image even with a small heat energy and, in addition, to have improved storage stability in terms of light resistance, weather resistance, and oil resistance.
- thermal recording sheet which is superior in dot reproducibility and image quality over the energy regions from low energy to high energy.
- polystyrene is a thermoplastic resin, it tends to increase adherence of residue to the thermal head and sticking, and is thus less practical.
- Japanese Patent Laid-open Publications 62-117787, 63-21180 in which, in order to apply heat from the thermal head to the thermal color developing layer, a heat insulating intermediate layer containing thermoplastic hollow fine particles is introduced to obtain a high density and sharp image.
- thermoplastic hollow fine particles themselves melt by heat, resulting in increased adherence of residue to the thermal head and sticking.
- thermo recording sheet which is superior in dot reproducibility and image quality with reduced adherence of residue and sticking.
- a thermal recording sheet having a thermal color developing layer containing a chromogenic agent and a color developer as main ingredients provided on a substrate, characterized in that an intermediate layer containing polymeric fine particles having a cross-linking structure with a particle diameter of 0.2 to 5.0 microns and an inorganic pigment having an oil absorption (according to JIS K5101) of less than 170 ml/100g in a ratio of 1:1 to 1:9.
- polymeric fine particles having such a cross-linking structure Various substances are known as polymeric fine particles having such a cross-linking structure.
- cross-linkable polymeric fine particles which have a particle diameter of 5 microns or less, more preferably 2 microns or less, and most preferably 0.2 to 1.0 micron.
- those which have a particle diameter of 0.2 microns or less When the particle diameter is 0.2 microns or less, spaces between particles in the intermediate layer become small, penetration into the substrate is increased, and it is impossible to obtain a thermal recording sheet which is the object of the present invention, resulting in considerable deterioration in quality such as sticking and adherence of residue.
- the particle diameter is 5.0 microns or more, voids between particles in the intermediate layer are large, which allow the ingredients of the thermal recording layer to penetrate into the intermediate layer, resulting in lowering the color developing sensitivity, sticking, and adherence of residue.
- the polymeric fine particles having such a cross-linking structure used in the intermediate layer of the present invention are obtained by emulsion polymerization of a monomer mixture containing a cross-linkable monomer.
- the cross-linkable monomer includes such monomers having at least two polymerizable unsaturated double bonds in one molecule such as trimethylolpropane-trimethacrylate, divinylbenzene, ethyleneglycoldiacrylate, and the like.
- the cross-linkable monomer is used in an amount of 0.5 to 1% by weight to a vinyl monomer mixture polymer which is described below.
- an aromatic vinyl compound such as (meth)acrylic esters, vinylacetate, vinyl esters, vinylcyano compounds, halogenated vinyl compounds, styrene, ⁇ -methylstyrene, or vinyltoluene can be used as a vinyl monomer, and it is preferable to use styrene or methylmethacrylate as a main ingredient in view of heat resistance.
- the polymeric fine particles having such a specific cross-linking structure are dispersed in a binder to obtain a solution, as will be described later.
- a latex-based binder gives a good coating color stability and endows the intermediate layer with an elasticity, and allows even contact with the thermal head, thereby remarkably improving the dot reproducibility.
- the pigment used in combination with the polymeric fine particles having the specific cross-linking structure in the intermediate layer is an inorganic pigment having an oil absorption (according to JIS K5101) of 170 ml/100 g.
- This type of pigment includes alumina, magnesium hydroxide, calcium hydroxide, magnesium carbonate, zinc oxide, barium sulfate, silica, calcium carbonate, kaolin, calcined kaolin, diatomaceous earth, talc, titanium oxide, aluminum hydroxide, and the like.
- the polymeric fine particles having the specific cross-linking structure and the inorganic pigment having an oil absorption of less than 170 ml/100 g are used in a ratio of 1:1 to 1:9 (weight ratio).
- a greater amount of the polymeric fine particles having the specific cross-linking structure than 1:1 tends to result in deterioration in dot reproducibility and image quality, sticking, and adherence of residue.
- a greater amount of inorganic pigment having an oil absorption of 170 ml/100 g than 1:9 tends to result in the dynamic sensitivity.
- the amounts of the polymeric fine particles having the cross-linking structure and the inorganic pigment having an oil absorption of less than 170 ml/100 g used in the intermediate layer are not specifically limited, but it is desirable to use in amounts of typically 60 to 95 % by weight based on the total solids, preferably 70 to 90 % by weight.
- the coating amount is not specifically limited, but it is coated typically in an amount of 2 to 20 g/m2, preferably 4 to 15 g/m2.
- the intermediate layer containing the fine particles having the cross-linking structure with a particle diameter of 0.2 to 5.0 microns and an inorganic pigment having an oil absorption (according to JIS K5101) of 170 ml/100 g in a ratio of 1:1 to 1:9 is coated on a substrate, and the thermal color developing layer is coated on top, followed by drying, to produce the thermal recording sheet of the present invention.
- the combination of the chromogenic agent and the color developer is not specifically limited, but can be applied to various types of thermal recording sheets such as a leuco dye type color developing material comprising a basic colorless dye and an acid substance, a chelate type color developing material comprising iron salt of a higher fatty acid and stearyl gallate, a pigment type color developing material comprising an imino compound and an isocyanate compound, and the like, and the present invention includes these types of recording sheets.
- the specific intermediate layer of the present invention provided on the substrate provides the best effect when the leuco dye type color developing material comprising a basic colorless dye and an acid substance is used.
- examples of the color developer include bisphenols A, 4-hydroxybenzoic esters, 4-hydroxyphthalic diesters, phthalic monoesters, bis(hydroxyphenyl) sulfides, 4-hydroxyphenylarylsulfones, 4-hydroxyphenylarylsulfonates, 1,3-di[2-(hydroxyphenyl)-2-propyl]-benzenes, 4-hydroxybenzoyloxybenzoic esters, and bisphenolsulfones. These examples are shown below:
- the basic colorless dye used in the present invention is not specifically limited, however, it is preferable to use triphenylmethane-type dyes, fluorane-type dyes, fluorene-type dyes, divinyl-type dyes, or the like, and practical examples of these dyes are shown below.
- These dyes can be used alone or as mixtures of two or more.
- the binder used in the intermediate and the thermal recording layer can be completely-hydrolyzed polyvinylalcohol with a polymerization degree of 200 to 1,900, partially-hydrolyzed polyvinylalcohol, carboxy-modified polyvinylalcohol, amide-modified polyvinylalcohol, sulfonic acid-modified polyvinylalcohol, butyral-modified polyvinylalcohol, and other modified polyvinylalcohols, hydroxyethylcellulose, methylcellulose, carboxymethylcellulose, styrene-maleic anhydride copolymer, styrene-butadiene copolymer, styrene-acrylate copolymer, acrylonitrile-butadiene copolymer; cellulose derivatives such as ethylcellulose and acetylcellulose; polyvinylchloride, polyvinylacetate, polyacrylamide, polyacrylic esters, polyvinylalco
- the pigment used in the present invention can be inorganic fillers such as alumina, magnesium hydroxide, calcium hydroxide, magnesium carbonate, zinc oxide, barium sulfate, silica, calcium carbonate, kaolin, calcined kaolin, diatomaceous earth, talc, titanium oxide, aluminum hydroxide, or the like, and organic pigments such as urea-formaldehyde resins, styrene-methacrylic acid copolymer, polystyrene resins, and amino resin fillers.
- inorganic fillers such as alumina, magnesium hydroxide, calcium hydroxide, magnesium carbonate, zinc oxide, barium sulfate, silica, calcium carbonate, kaolin, calcined kaolin, diatomaceous earth, talc, titanium oxide, aluminum hydroxide, or the like
- organic pigments such as urea-formaldehyde resins, styrene-methacrylic acid copolymer,
- releasing agents such as fatty acid metal salts, slip agents such as wax, benzophenone- or triazole-based ultraviolet absorbers, water resistant agents such as glyoxal, dispersants, defoamers, and the like.
- the amounts of the organic color developer and the basic colorless dye used in the present invention and the types and amounts of other constituents are determined according to the required properties and recording adaptability, and are not specifically limited, but it is usually preferable to use 1 to 8 parts of the organic color developer and 1 to 20 parts of the filler to 1 part of the basic colorless dye, and the binder is used in an amount of 10 to 25% of the total solid.
- the coating color of the above composition can be coated on any type of substrate such as paper, synthetic paper, plastic films, non-woven fabrics, or the like to obtain the objective thermal recording sheet.
- the sheet can be provided on the thermal color developing layer with an overcoating layer comprising a polymeric substance containing a pigment, or on the substrate with a back coating layer comprising a polymeric substance, to improve the storage stability.
- the organic color developer, the basic colorless dye, and the materials which are added as needed are dispersed by a dispersing machine such as a ball mill, an attriter, a sand grinder, or the like, or by an appropriate emulsifying apparatus to a particle diameter of several microns or less, and mixed with the binder and various additives according to the purpose to obtain a coating color.
- a dispersing machine such as a ball mill, an attriter, a sand grinder, or the like, or by an appropriate emulsifying apparatus to a particle diameter of several microns or less, and mixed with the binder and various additives according to the purpose to obtain a coating color.
- the formation method of the intermediate layer and the recording layer is not specifically limited, but these layers can be formed by a conventional method known in the art, and off-machine coaters or on-machine coaters provided with an air knife coater, a rod blade coater, a bill blade coater, a roll coater, or the like can be appropriately selected.
- the individual layer can be smoothed as needed by a super-calender or the like.
- the intermediate layer containing polymeric fine particles having the specific cross-linking structure and the inorganic pigment having an oil absorption of 170 ml/100 g is provided between the substrate and the thermal color developing layer.
- the pigment mixture mutually functions, and the intermediate layer containing the pigment fills and smooths microscopic irregularities on the surface of the base paper to suppress penetration of the coating color of thermal recording layer which is subsequently coated, thereby obtaining a heat insulating layer having a high void ratio and enabling uniform coating of the thermal recording layer.
- the polymeric fine particles having the specific cross-linking structure of the present invention are low in thermal conductivity, the intermediate layer itself containing the particles is also low in the conductivity, the heat energy supplied from the thermal head can be effectively used for color developing. Moreover, the cross-linking-structured polymeric fine particles are also superior in heat resistance, sticking and adherence of residue is prevented. Thus, improved dynamic sensitivity, dot reproducibility, and image quality, and prevention of sticking and adherence of residue are achieved.
- part means part by weight.
- the above thermal color developing layer coating color was coated on top of the intermediate layer obtained above to a dry coating amount of 6.0 g/m2 and dried.
- the resulting sheet was super-calendered to a smoothness of 700-800 seconds to obtain a thermal recording sheet.
- Example 2 In the formation of the intermediate layer, the same procedure as Example 1 was used, except that the intermediate layer of the following composition was provided, to obtain a thermal recording sheet.
- Example 2 In the formation of the intermediate layer, the same procedure as Example 1 was used, except that the intermediate layer of the following composition was provided, to obtain a thermal recording sheet.
- Part Styrene-based polymeric fine particles having cross-linking structure (tradename: GLOSSDERU OPP-100, Mitsui Toatsu)
- X Calcined kaolin (tradename: ANSILEX, ENGEL HARD, oil absorption: 90ml/100 g)
- Y Styrene-butadiene copolymer latex solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5
- Example 2 In the formation of the intermediate layer, the same procedure as Example 1 was used, except that the intermediate layer of the following composition was provided, to obtain a thermal recording sheet.
- the above compositions were blended to obtain an intermediate layer coating color.
- the coating color was coated on fine paper with a substance of 50 g/m2 to a dry coating amount of 8 g/m2 and dried.
- the above thermal color developing layer coating color was coated on top of the intermediate layer obtained above to a dry coating amount of 6.0 g/m2 and dried.
- the resulting sheet was super-calendered to a smoothness of 700-800 seconds to obtain a thermal recording sheet.
- the intermediate layer In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m2 to a dry amount of 8 g/m2 and dried, to obtain a thermal recording sheet.
- Part Styrene-based polymeric fine particles having cross-linking structure (tradename: GLOSSDERU OPP-100, Mitsui Toatsu) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5
- the intermediate layer In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m2 to a dry amount of 8 g/m2 and dried, to obtain a thermal recording sheet.
- Part Calcined kaolin (tradename: ANSILEX, ENGEL HARD, oil absorption: 90ml/100 g) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5
- the intermediate layer In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m2 to a dry amount of 8 g/m2 and dried, to obtain a thermal recording sheet.
- Part Silicon dioxide (tradename: NIPSIL E-743, Nippon Silica) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5
- the intermediate layer In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m2 to a dry amount of 8 g/m2 and dried, to obtain a thermal recording sheet.
- Part Styrene-acrylic copolymer hollow fine particles (tradename: LOPAQUE OP-62, Rohm & Haas) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5
- the intermediate layer In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m2 to a dry amount of 8 g/m2 and dried, to obtain a thermal recording sheet.
- Part Polystyrene-fine particles (tradename: L8801, Asahi Kasei) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5
Landscapes
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Heat Sensitive Colour Forming Recording (AREA)
Abstract
Description
- This invention relates to a thermal recording sheet which is superior in dynamic sensitivity, dot reproducibility, and image quality, without sticking and adherence of residue.
- In general, in thermal recording sheets, a normally colorless or pale colored basic chromogenic dye and an organic color developer such as a phenolic substance are individually dispersed into fine particles, mixed, and a binder, a filler, a sensitivity improver, a slip agent, and other additives are added to obtain a coating color, which is coated on a substrate such as paper, synthetic paper, plastic films, cloths, and the like. The thermal recording sheet enables color recording by a momentary chemical reaction caused by heating with a thermal pen, a thermal head, a hot stamp, laser light, or the like.
- These thermal recording sheets are applied in a variety of areas such as measurement recorders, computer terminal printers, facsimiles, automatic ticket vendors, and bar-code labels, however, with recent diversification and improvement of these recording devices, requirements to the thermal recording sheets have become stricter. For example, with increasing recording speed, it is required to obtain a high-concentration, sharp color image even with a small heat energy and, in addition, to have improved storage stability in terms of light resistance, weather resistance, and oil resistance.
- Furthermore, with the diversification of market needs, demand is increasing for a thermal recording sheet which is superior in dot reproducibility and image quality over the energy regions from low energy to high energy.
- On the other hand, a method is disclosed (Japanese Patent Laid-open Publication 2-307784) in which an intermediate layer containing polystyrene and a pigment having an oil absorption of less than 100 ml/100 g is introduced between a substrate and a thermal color developing layer to obtain a thermal recording paper with high density and reduced adherence of residue.
- However, in this method, since polystyrene is a thermoplastic resin, it tends to increase adherence of residue to the thermal head and sticking, and is thus less practical.
- Furthermore, there is disclosed a method (Japanese Patent Laid-open Publications 62-117787, 63-21180) in which, in order to apply heat from the thermal head to the thermal color developing layer, a heat insulating intermediate layer containing thermoplastic hollow fine particles is introduced to obtain a high density and sharp image.
- Furthermore, there is disclosed a method (Japanese Patent Laid-open Publication 62-5886) in which a heat insulating intermediate layer containing thermoplastic hollow fine particles and an intermediate layer containing an inorganic or organic pigment are laminated between the substrate and the thermal color developing layer. However, even these methods are not practical because they involve problems in that the thermoplastic hollow fine particles themselves melt by heat, resulting in increased adherence of residue to the thermal head and sticking.
- Therefore, it is a primary object of the present invention to provide a thermal recording sheet which is superior in dot reproducibility and image quality with reduced adherence of residue and sticking.
- In accordance with the present invention which solves all of the above problems, there is provided a thermal recording sheet having a thermal color developing layer containing a chromogenic agent and a color developer as main ingredients provided on a substrate, characterized in that an intermediate layer containing polymeric fine particles having a cross-linking structure with a particle diameter of 0.2 to 5.0 microns and an inorganic pigment having an oil absorption (according to JIS K5101) of less than 170 ml/100g in a ratio of 1:1 to 1:9.
- Various substances are known as polymeric fine particles having such a cross-linking structure. In the present invention, it is desirable to use cross-linkable polymeric fine particles which have a particle diameter of 5 microns or less, more preferably 2 microns or less, and most preferably 0.2 to 1.0 micron. When those which have a particle diameter of 0.2 microns or less. When the particle diameter is 0.2 microns or less, spaces between particles in the intermediate layer become small, penetration into the substrate is increased, and it is impossible to obtain a thermal recording sheet which is the object of the present invention, resulting in considerable deterioration in quality such as sticking and adherence of residue.
- On the other hand, when the particle diameter is 5.0 microns or more, voids between particles in the intermediate layer are large, which allow the ingredients of the thermal recording layer to penetrate into the intermediate layer, resulting in lowering the color developing sensitivity, sticking, and adherence of residue.
- The polymeric fine particles having such a cross-linking structure used in the intermediate layer of the present invention are obtained by emulsion polymerization of a monomer mixture containing a cross-linkable monomer.
- The cross-linkable monomer includes such monomers having at least two polymerizable unsaturated double bonds in one molecule such as trimethylolpropane-trimethacrylate, divinylbenzene, ethyleneglycoldiacrylate, and the like. The cross-linkable monomer is used in an amount of 0.5 to 1% by weight to a vinyl monomer mixture polymer which is described below.
- In the present invention, an aromatic vinyl compound such as (meth)acrylic esters, vinylacetate, vinyl esters, vinylcyano compounds, halogenated vinyl compounds, styrene, α-methylstyrene, or vinyltoluene can be used as a vinyl monomer, and it is preferable to use styrene or methylmethacrylate as a main ingredient in view of heat resistance.
- The polymeric fine particles having such a specific cross-linking structure are dispersed in a binder to obtain a solution, as will be described later. In this case, a latex-based binder gives a good coating color stability and endows the intermediate layer with an elasticity, and allows even contact with the thermal head, thereby remarkably improving the dot reproducibility.
- The pigment used in combination with the polymeric fine particles having the specific cross-linking structure in the intermediate layer is an inorganic pigment having an oil absorption (according to JIS K5101) of 170 ml/100 g. This type of pigment includes alumina, magnesium hydroxide, calcium hydroxide, magnesium carbonate, zinc oxide, barium sulfate, silica, calcium carbonate, kaolin, calcined kaolin, diatomaceous earth, talc, titanium oxide, aluminum hydroxide, and the like. In this case, when the oil absorption is greater than 170 ml/100 g, the residue absorbing ability is improved, but the ingredients of the thermal recording layer tend to be absorbed into the intermediate layer in coating the thermal recording layer in coating the thermal recording layer, resulting in a deterioration in printing density and dot reproducibility.
- The polymeric fine particles having the specific cross-linking structure and the inorganic pigment having an oil absorption of less than 170 ml/100 g are used in a ratio of 1:1 to 1:9 (weight ratio). A greater amount of the polymeric fine particles having the specific cross-linking structure than 1:1 tends to result in deterioration in dot reproducibility and image quality, sticking, and adherence of residue. A greater amount of inorganic pigment having an oil absorption of 170 ml/100 g than 1:9 tends to result in the dynamic sensitivity.
- The amounts of the polymeric fine particles having the cross-linking structure and the inorganic pigment having an oil absorption of less than 170 ml/100 g used in the intermediate layer are not specifically limited, but it is desirable to use in amounts of typically 60 to 95 % by weight based on the total solids, preferably 70 to 90 % by weight. The coating amount is not specifically limited, but it is coated typically in an amount of 2 to 20 g/m², preferably 4 to 15 g/m².
- In the present invention, the intermediate layer containing the fine particles having the cross-linking structure with a particle diameter of 0.2 to 5.0 microns and an inorganic pigment having an oil absorption (according to JIS K5101) of 170 ml/100 g in a ratio of 1:1 to 1:9 is coated on a substrate, and the thermal color developing layer is coated on top, followed by drying, to produce the thermal recording sheet of the present invention.
- In the present invention, the combination of the chromogenic agent and the color developer is not specifically limited, but can be applied to various types of thermal recording sheets such as a leuco dye type color developing material comprising a basic colorless dye and an acid substance, a chelate type color developing material comprising iron salt of a higher fatty acid and stearyl gallate, a pigment type color developing material comprising an imino compound and an isocyanate compound, and the like, and the present invention includes these types of recording sheets.
- However, the specific intermediate layer of the present invention provided on the substrate provides the best effect when the leuco dye type color developing material comprising a basic colorless dye and an acid substance is used.
- In the present invention, examples of the color developer include bisphenols A, 4-hydroxybenzoic esters, 4-hydroxyphthalic diesters, phthalic monoesters, bis(hydroxyphenyl) sulfides, 4-hydroxyphenylarylsulfones, 4-hydroxyphenylarylsulfonates, 1,3-di[2-(hydroxyphenyl)-2-propyl]-benzenes, 4-hydroxybenzoyloxybenzoic esters, and bisphenolsulfones. These examples are shown below:
- 4,4'-Isopropylidene-diphenol (Bisphenol A)
4,4'-Cyclohexylidene-diphenol
p,p'-(1-Methyl-n-hexylidene)-diphenol
1,7-di(4-hydroxyphenylthio)-3,5-dioxaheptane - Benzyl-4-hydroxybenzoate
Ethyl-4-hydroxybenzoate
Propyl-4-hydroxybenzoate
Isopropyl-4-hydroxybenzoate
Butyl-4-hydroxybenzoate
Isobutyl-4-hydroxybenzoate
Methylbenzyl-4-hydroxybenzoate - Dimethyl-4-hydroxyphthalate
Diisopropyl-4-hydroxyphthalate
Dibenzyl-4-hydroxyphthalate
Dihexyl-4-hydroxyphthalate - Monobenzylphthalate
Monocyclohexylphthalate
Monophenylphthalate
Monomethylphenylphthalate
Monoethylphenylphthalate
Monopropylbenzylphthalate
Monohalogenhenzylphthalate
Monoethoxybenzylphthalate - Bis-(4-hydroxy-3-tert-butyl-6-methylphenyl)-sulfide
Bis-(4-hydroxy-2,5-dimethylphenyl)-sulfide
Bis-(4-hydroxy-2-methyl-5-ethylphenyl)-sulfide
Bis-(4-hydroxy-2-methyl-5-isopropylphenyl)-sulfide
Bis-(4-hydroxy-2,3-dimethylphenyl)-sulfide
Bis-(4-hydroxy-2,5-dimethylphenyl)-sulfide
Bis-(4-hydroxy-2,5-diisopropylphenyl)-sulfide
Bis-(4-hydroxy-2,3,6-trimethylphenyl)-sulfide
Bis-(2,4,5-trihydroxyphenyl)-sulfide
Bis-(4-hydroxy-2-cyclohexyl-5-methylphenyl)-sulfide
Bis-(2,3,4-trihydroxyphenyl)-sulfide
Bis-(4,5-dihydroxy-2-tert-butylphenyl)-sulfide
Bis-(4-hydroxy-2,5-diphenylphenyl)-sulfide
Bis-(4-hydroxy-2-tert-octyl-5-methylphenyl)-sulfide - 4-Hydroxy-4'-isopropoxydiphenylsulfone
4-Hydroxy-4'-propoxydiphenylsulfone
4-Hydroxy-4'-n-butyloxydiphenylsulfone
4-Hydroxy-4'-n-propoxydiphenylsulfone - 4-Hydroxyphenylbenzenesulfonate
4-Hydroxyphenyl-p-toluylsulfonate
4-Hydroxyphenylmethylenesulfonate
4-Hydroxyphenyl-p-chlorobenzenesulfonate
4-Hydroxyphenyl-p-tert-butylbenzenesulfonate
4-Hydroxypllenyl-p-isopropoxybenzenesulfonate
4-Hydroxyphenyl-1'-naphthalenesulfonate
4-Hydroxyphenyl-2'-naphthalenesulfonate - 1,3-Di[2-(4-hydroxyphenyl)-2-propyl]-benzene
1,3-Di[2-(4-hydroxy-3-alkylphenyl-2-propyl]-benzene
1,3-Di[2-(2,4-dihydroxyphenyl)-2-propyl]-benzene
1,3-Di[2-(4-hydroxy-5-methylphenyl)-2-propyl]-benzene - 1,3-Dihydroxy-6(α,α-dimethylbenzyl)-benzene
- Benzyl-4-hydroxybenzoyloxybenzoate
Methyl-4-hydroxybenzoyloxybenzoate
Ethyl-4-hydroxybenzoyloxybenzoate
Propyl-4-hydroxybenzoyloxybenzoate
Butyl-4-hydroxybenzoyloxybenzoate
Isopropyl-4-hydroxybenzoyloxybenzoate
tert-Butyl-4-hydroxybenzoyloxybenzoate
Hexyl-4-hydroxybenzoyloxybenzoate
Octyl-4-hydroxybenzoyloxybenzoate
Nonyl-4-hydroxybenzoyloxybenzoate
Cyclohexyl-4-hydroxybenzoyloxybenzoate
β-Phenethyl-4-hydroxybenzoyloxybenzoate
Phenyl-4-hydroxybenzoyloxybenzoate
α-Naphthyl-4-hydroxybenzoyloxybenzoate
β-Naphthyl-4-hydroxybenzoyloxybenzoate
sec-Butyl-4-hydroxybenzoyloxybenzoate - Bis-(3-1-butyl-4-hydroxy-6-methylphenyl)-sulfone
Bis-(3-ethyl-4-hydroxyphenyl)-sulfone
Bis-(3-propyl-4-hydroxyphenyl)-sulfone
Bis-(3-methyl-4-hydroxyphenyl)-sulfone
Bis-(3-isopropyl-4-hydroxyphenyl)-sulfone
Bis-(2-ethyl-4-hydroxyphenyl)-sulfone
Bis(3-chloro-4-hydroxyphenyl)-sulfone
Bis-(2,3-dimethyl-4-hydroxyphenyl)-sulfone
Bis-(2,5-dimethyl-4-hydroxyphenyl)-sulfone
Bis-(3-methoxy-4-hydroxyphenyl)-sulfone
4-Hydroxyphenyl-2'-ethyl-4'-hydroxyphenylsulfone
4-Hydroxyphenyl-2'-isopropyl-4'-hydroxyphenylsulfone
4-Hydroxyphenyl-3'-isopropyl-4'-hydroxyphenylsulfone
4-Hydroxyphenyl-3'-sec-butyl-4'-hydroxyphenylsulfone
3-Chloro-4-hyydroxyphenyl-3'-isopropyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-aminophenyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-isopropylphenyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-octylphenyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-chloro-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-methyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-isopropyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-chloro-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-methyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-isopropyl-4'-hydroxyphenylsulfone
2-Hydroxy-5-t-butylphenyl-3'-methyl-4'-hydroxyphenylsulfone - 4,4'-Sulfonyldiphenol
2,4'-Sulfonyldiphenol
3,3'-Dichloro-4,4'-sulfonyldiphenol
3,3, -Dibromo-4 ,4, -sulfonyldiphenol
3,3',5,5'-Tetrabromo-4,4'-sulfonyldiphenol
3,3'-Diamino-4,4'-sulfonyldiphenol
- p-tert-Butylphenol
2,4-Dihydroxybenzophenone
Novolac type phenolic resin
4-Hydroxyacetophenone
p-Phenylphenol
Benzyl-4-hydroxyphenylacetate
p-Benzylphenol - The basic colorless dye used in the present invention is not specifically limited, however, it is preferable to use triphenylmethane-type dyes, fluorane-type dyes, fluorene-type dyes, divinyl-type dyes, or the like, and practical examples of these dyes are shown below.
- 3,3-bis(p-dimethylaminophenyl)-6-dimethylaminophthalide [Crystal Violet Lactone]
- 3-Diethylamino-6-methyl-7-anilinofluorane
3-(N-ethyl-p-toluidino)-6-methyl-7-anilinofluorane
3-(N-ethyl-N-isoamylamino)-6-methyl-7-anilinofluorane
3-Diethylamino-6-methyl-7-(o,p-dimethylanilino)fluorane
3-Pyrrolidino-6-methyl-7-anilinofluorane
3-Piperidino-6-methyl-7-anilinofluorane
3-(N-cyclohexyl-N-methylamino)-6-methyl-7-anilinofluorane
3-Diethylamino-7-(m-trifluoromethylanilino)fluorane
3-N-n-Dibutylamino-6-methyl-7-anilinofluorane
3-N-n-Dibutylamino-7-(o-chloroanilino)fluorane
3-(N-ethyl-N-tetrahdrofurfurylamino)6-methyl-7-anilinofluorane
3-Dibutylamino-6-methyl-7-(o,p-dimethylanilino)fluorane
3-(N-methyl-N-propylamino)-6-methyl-7-anilinofluorane
3-diethylamino-6-chloro-7-anilinofluorane
3-Dibutylamino-7-(o-chloroanilino)fluorane
3-Diethylamino-7-(o-chloroanilino)fluorane.
3-Diethylamino-6-methyl-7-(m-methylanilino)fluorane
3-n-Dibutylamino-6-methyl-7-(m-methylanilino)fluorane
3-Diethylamino-6-methyl-chlorofluorane
3-Diethylamino-6-methyl-fluorane
3-Cyclohexylamino-6-chlorofluorane
3-Diethylamino-benzo[a]-fluorane
3-n-Dipentylamino-6-methyl-7-anilinofluorane
2-(4-Oxo-hexyl)-3-dimethylamino-6-methyl-7-anilinofluorane
2-(4-Oxo-hexyl)-3-diethylamino-6-methyl-7-anilinofluorane
2-(4-Oxo-hexyl)-3-dipropylamino-6-methyl-7-anilinofluorane - 3,6,6'-tris(dimethylamino)spiro[fluorene-9,3'-phthalide
3,6,6'-tris(diethylamino)spiro[fluorene-9,3'-phthalide - 2-Methyl-6-p-(p-dimetylaminophenyl)aminoanilinofluorane
2-Methoxy-6-p-(p-dimetylaminophenyl)aminoanilinofluorane
2-Chloro-3-methyl-6-p-(p-dimetylaminophenyl)aminoanilinofluorane
2-Chloro-6-p-(p-dimetylaminophenyl)aminoanilinofluorane
2-Nitro-6-p-(p-diethylaminophenyl)aminoanilinofluorane
2-Amino-6-p-(p-diethylaminophenyl)aminoanilinofluorane
2-Diethylamino-6-p-(p-diethylaminophenyl)aminoanilinofluorane
2-Phenyl-6-methyl-6-p-(p-phenylaminophenyl)aminoanilinofluorane
2-Benzyl-6-p-(p-phenylaminophenyl)aminoanilinofluorane
2-Hydroxy-6-p-(p-phenylaminophenyl)aminoanilinofluorane
3-Methyl-6-p-(p-dimethylaminophenyl)aminoanilinofluorane
3-Diethyamino-6-p-(p-diethylaminophenyl)aminoanilinofluorane
3-Diethyamino-6-p-(p-dibutylaminophenyl)aminoanilino-fluorane - 3,3-Bis-[2-(p-dimethylaminophenyl)-2-(p-methoxyphenyl)-ethenyl]-4,5,6,7-tetrabromophthalide
3,3-Bis-[2-(p-dimethylaminophenyl)-2-(p-methoxyphenyl)-ethenyl]-4,5,6,7-tetrachlorophthalide
3,3-Bis-[1,1-bis(4-pyrrolidinophenyl)ethylene-2-yl]-4,5,6,7-tetrabromophthalide
3,3-Bis-[1-(4-methoxyphenyl)-1-(4-pyrrolidinophenyl)-ethylen-2-yl]-4,5,6,7-tetrachlorophthalide - 1,1-Bis-[2',2',2'',2''-tetrakis-(p-dimethylaminophenyl)-ethenyl]-2,2-dinitrileethane
1,1-Bis-[2',2',2'',2''-tetrakis-(p-dimethylaminophenyl)-ethenyl]-2-β-naphthoylethane
1,1-Bis-[2',2',2'',2''-tetrakis-(p-dimethylaminophenyl)-ethenyl]-2,2-diacetylethane
Dimethyl-bis-[2',2',2'',2''-tetrakis-(p-dimethylaminophenyl)-ethenyl]-methylmalonate - These dyes can be used alone or as mixtures of two or more.
- Furthermore, as a sensitizer, fatty acid amides such as stearamide, palmitamide, or the like; ethylene-bisamide, montan wax, polyethylene wax, dibenzyl terephthalate, benzyl p-benzyloxybenzoate, di-p-tolylcarbonate, p-benzylbiphenyl, phenyl-α-naphthylcarbonate, 1,4-diethoxynaphthalene, phenyl-1-hydroxy-2-naphthoate, 1,2-di-(3-methylphenoxy) ethane, di(p-methylbenzyl)oxalate, β-benzyloxynaphthalene, 4-biphenyl-p-tolylether, o-xylylene-bis-(phenylether), 4-(m-methylphenoxymethyl)biphenyl, or the like can be added.
- In the present invention, the binder used in the intermediate and the thermal recording layer can be completely-hydrolyzed polyvinylalcohol with a polymerization degree of 200 to 1,900, partially-hydrolyzed polyvinylalcohol, carboxy-modified polyvinylalcohol, amide-modified polyvinylalcohol, sulfonic acid-modified polyvinylalcohol, butyral-modified polyvinylalcohol, and other modified polyvinylalcohols, hydroxyethylcellulose, methylcellulose, carboxymethylcellulose, styrene-maleic anhydride copolymer, styrene-butadiene copolymer, styrene-acrylate copolymer, acrylonitrile-butadiene copolymer; cellulose derivatives such as ethylcellulose and acetylcellulose; polyvinylchloride, polyvinylacetate, polyacrylamide, polyacrylic esters, polyvinylbutyral, polystyrene and its copolymers, polyamide resins, silicone resins, petroleum resins, terpene resins, ketone resins, coumarone resins, starch, starch derivatives, and casein. These polymeric substances are used in the state emulsified in water or other solvents, or can be used in combination according to the quality requirements.
- In the present invention, it is also possible to add known stabilizers based on metal salts (Ca, Zn) of p-nitrobenzoic acid or metal salts (Ca, Zn) of monobenzylphthalate as much as the effect of the present invention is not impaired.
- The pigment used in the present invention can be inorganic fillers such as alumina, magnesium hydroxide, calcium hydroxide, magnesium carbonate, zinc oxide, barium sulfate, silica, calcium carbonate, kaolin, calcined kaolin, diatomaceous earth, talc, titanium oxide, aluminum hydroxide, or the like, and organic pigments such as urea-formaldehyde resins, styrene-methacrylic acid copolymer, polystyrene resins, and amino resin fillers.
- In addition to the above, it is possible to use releasing agents such as fatty acid metal salts, slip agents such as wax, benzophenone- or triazole-based ultraviolet absorbers, water resistant agents such as glyoxal, dispersants, defoamers, and the like.
- The amounts of the organic color developer and the basic colorless dye used in the present invention and the types and amounts of other constituents are determined according to the required properties and recording adaptability, and are not specifically limited, but it is usually preferable to use 1 to 8 parts of the organic color developer and 1 to 20 parts of the filler to 1 part of the basic colorless dye, and the binder is used in an amount of 10 to 25% of the total solid.
- The coating color of the above composition can be coated on any type of substrate such as paper, synthetic paper, plastic films, non-woven fabrics, or the like to obtain the objective thermal recording sheet.
- Furthermore, the sheet can be provided on the thermal color developing layer with an overcoating layer comprising a polymeric substance containing a pigment, or on the substrate with a back coating layer comprising a polymeric substance, to improve the storage stability.
- The organic color developer, the basic colorless dye, and the materials which are added as needed are dispersed by a dispersing machine such as a ball mill, an attriter, a sand grinder, or the like, or by an appropriate emulsifying apparatus to a particle diameter of several microns or less, and mixed with the binder and various additives according to the purpose to obtain a coating color.
- In the thermal recording sheet of the present invention, the formation method of the intermediate layer and the recording layer is not specifically limited, but these layers can be formed by a conventional method known in the art, and off-machine coaters or on-machine coaters provided with an air knife coater, a rod blade coater, a bill blade coater, a roll coater, or the like can be appropriately selected.
- Furthermore, after the intermediate layer and the recording layer are coated and dried, the individual layer can be smoothed as needed by a super-calender or the like.
- In the present invention, the reason why the effect of the present invention is obtained by providing the intermediate layer is considered as follows:
- In the present invention, the intermediate layer containing polymeric fine particles having the specific cross-linking structure and the inorganic pigment having an oil absorption of 170 ml/100 g is provided between the substrate and the thermal color developing layer. With this arrangement, the pigment mixture mutually functions, and the intermediate layer containing the pigment fills and smooths microscopic irregularities on the surface of the base paper to suppress penetration of the coating color of thermal recording layer which is subsequently coated, thereby obtaining a heat insulating layer having a high void ratio and enabling uniform coating of the thermal recording layer.
- Furthermore, since the polymeric fine particles having the specific cross-linking structure of the present invention are low in thermal conductivity, the intermediate layer itself containing the particles is also low in the conductivity, the heat energy supplied from the thermal head can be effectively used for color developing. Moreover, the cross-linking-structured polymeric fine particles are also superior in heat resistance, sticking and adherence of residue is prevented. Thus, improved dynamic sensitivity, dot reproducibility, and image quality, and prevention of sticking and adherence of residue are achieved.
- The present invention will now be described with reference to the examples. In the description, part means part by weight.
-
(Formation of the intermediate layer) Part Styrene-based polymeric fine particles having cross-linking structure (tradename: GLOSSDERU 201-S, Mitsui Toatsu) X Calcined kaolin (tradename: ANSILEX, ENGEL HARD, oil absorption: 90ml/100 g) Y Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 - The above compositions were blended to obtain various coating colors for the intermediate layer. These coating colors were coated on fine paper with a substance of 50 g/m² to a dry coating amount of 8 g/m² and dried.
(Formation of the thermal color developing layer) Solution A (color developer dispersion) Part 4,4'-Isopropylidene-diphenol 6.0 10% Aqueous polyvinylalcohol solution 18.8 Water 11.2 Solution B (dye dispersion) 3-N-n-dibutylamino-6-methyl-7-anilinofluorane 2.0 10% aqueous polyvinylalcohol solution 4.6 Water 2.6 Solution C (sensitizer dispersion) 4-Biphenyl-p-tolylether 4.0 10% aqueous polyvinylalcohol solution 5.0 Water 3.0 - The above dispersions were individually ground by a sand grinder to an average particle diameter of 1 micron. Then, the dispersions were mixed in the following ratio to obtain a coating color.
Solution A 36.0 parts Solution B 9.2 Solution C 12.0 Kaolin clay (50% dispersion) 12.0 - The above thermal color developing layer coating color was coated on top of the intermediate layer obtained above to a dry coating amount of 6.0 g/m² and dried. The resulting sheet was super-calendered to a smoothness of 700-800 seconds to obtain a thermal recording sheet.
-
- In the formation of the intermediate layer, the same procedure as Example 1 was used, except that the intermediate layer of the following composition was provided, to obtain a thermal recording sheet.
(Formation of the intermediate layer) Part Styrene-based polymeric fine particles having cross-linking structure (tradename: GLOSSDERU OPP-100, Mitsui Toatsu) X Calcined kaolin (tradename: ANSILEX, ENGEL HARD, oil absorption: 90ml/100 g) Y Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 -
-
(Formation of the intermediate layer) Part Styrene-based polymeric fine particles having cross-linking structure (tradename: GLOSSDERU 201-S, Mitsui Toatsu) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 -
- The above dispersions were individually ground by a sand grinder to an average particle diameter of 1 micron. Then, the dispersions were mixed in the following ratio to obtain a coating color.
Solution A 36.0 parts Solution B 9.2 Solution C 12.0 Kaolin clay (50% dispersion) 12.0 - The above thermal color developing layer coating color was coated on top of the intermediate layer obtained above to a dry coating amount of 6.0 g/m² and dried. The resulting sheet was super-calendered to a smoothness of 700-800 seconds to obtain a thermal recording sheet.
- In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m² to a dry amount of 8 g/m² and dried, to obtain a thermal recording sheet.
(Formation of the intermediate layer) Part Styrene-based polymeric fine particles having cross-linking structure (tradename: GLOSSDERU OPP-100, Mitsui Toatsu) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 - In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m² to a dry amount of 8 g/m² and dried, to obtain a thermal recording sheet.
(Formation of the intermediate layer) Part Calcined kaolin (tradename: ANSILEX, ENGEL HARD, oil absorption: 90ml/100 g) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 - In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m² to a dry amount of 8 g/m² and dried, to obtain a thermal recording sheet.
(Formation of the intermediate layer) Part Silicon dioxide (tradename: NIPSIL E-743, Nippon Silica) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 - In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m² to a dry amount of 8 g/m² and dried, to obtain a thermal recording sheet.
(Formation of the intermediate layer) Part Styrene-acrylic copolymer hollow fine particles (tradename: LOPAQUE OP-62, Rohm & Haas) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 - In the formation of the intermediate layer, the same procedure as Comparative Example 1 was used, except that the intermediate layer of the following composition was formed on fine paper with a substance of 50 g/m² to a dry amount of 8 g/m² and dried, to obtain a thermal recording sheet.
(Formation of the intermediate layer) Part Polystyrene-fine particles (tradename: L8801, Asahi Kasei) 100 Styrene-butadiene copolymer latex (solid content: 48%) 11 10% Aqueous polyvinylalcohol solution 5 - The thermal recording sheets obtained in the above Examples and Comparative Examples were tested for the properties. The test results are summarized in Table 1 and Table 2. As can be seen from Examples 1-4 in Table 1 and Table 2, the best effects are obtained when the polymeric fine particles having the cross-linking structure and the inorganic pigment having an oil absorption of less than 170 ml/100 g are used in a ratio of 1:1 to 1:9 (weight ratio). When the content of the polymeric fine particles having the cross-linking structure is greater than the ratio of 1:1, deterioration in dot reproducibility and image quality, sticking, and adherence of grounds result, and when the content of the inorganic pigment having an oil absorption of less than 170 ml/100 g is greater than the ratio of 1:9, a considerable reduction occurs in the dynamic sensitivity.
- The effects of the present invention are as follows:
- (1) With superior heat response, a sharp, high-density image can be obtained even in high-speed, high-density recording (high sensitivity).
- (2) Superior dot reproducibility and image quality.
- (3) No sticking or residue adherence occurs during thermal printing.
Claims (6)
- A thermal recording sheet comprising, in order, a substrate, an intermediate layer and a thermal color developing layer containing a chromogenic agent and a color developer as main ingredients, characterized in that the intermediate layer contains particles of a cross-linked polymer having a particle diameter of 0.2 to 5.0 µm and an inorganic pigment having an oil absorption according to JIS K5101 of less than 170 ml/lOOg in a weight ratio of 1:1 to 1:9.
- A sheet according to claim 1 wherein the polymer is a styrene-based resin.
- A sheet according to claim 1 wherein the polymer is a copolymer of a cross-linkable monomer and a vinyl monomer.
- A sheet according to claim 3 wherein the polymer particles are obtainable by the emulsion polymerization of a monomer mixture containing the cross-linking monomer.
- A sheet according to claim 3 or 4 wherein said cross-linkable monomer has two polymerizable unsaturated bonds in a molecule.
- A sheet according to claim 5 wherein the cross-linkable monomer is selected from trimethylolpropanemethacrylate, divinylbenzene and ethyleneglycol-diacrylate.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP4123644A JPH05318927A (en) | 1992-05-15 | 1992-05-15 | Thermal recording sheet |
| JP123644/92 | 1992-05-15 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0570186A1 true EP0570186A1 (en) | 1993-11-18 |
| EP0570186B1 EP0570186B1 (en) | 1997-08-13 |
Family
ID=14865700
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19930303616 Expired - Lifetime EP0570186B1 (en) | 1992-05-15 | 1993-05-11 | Thermal recording sheet |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0570186B1 (en) |
| JP (1) | JPH05318927A (en) |
| CA (1) | CA2095965A1 (en) |
| DE (1) | DE69313009T2 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1391317A1 (en) * | 2002-08-21 | 2004-02-25 | CTP Papierhilfsmittel GmbH & Co. KG | Thermosensitive recording material and its' use |
| WO2004045862A1 (en) | 2002-11-19 | 2004-06-03 | Mitsubishi Hitec Paper Flensburg Gmbh | Heat-sensitive printing material and use thereof |
| EP2070714A1 (en) | 2007-12-11 | 2009-06-17 | Mitsubishi HiTec Paper Bielfeld GmbH | Heat sensitive recording material |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0399785A1 (en) * | 1989-05-23 | 1990-11-28 | Oji Paper Company Limited | Heat-sensitive recording material |
-
1992
- 1992-05-15 JP JP4123644A patent/JPH05318927A/en active Pending
-
1993
- 1993-05-11 CA CA 2095965 patent/CA2095965A1/en not_active Abandoned
- 1993-05-11 DE DE1993613009 patent/DE69313009T2/en not_active Expired - Fee Related
- 1993-05-11 EP EP19930303616 patent/EP0570186B1/en not_active Expired - Lifetime
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0399785A1 (en) * | 1989-05-23 | 1990-11-28 | Oji Paper Company Limited | Heat-sensitive recording material |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1391317A1 (en) * | 2002-08-21 | 2004-02-25 | CTP Papierhilfsmittel GmbH & Co. KG | Thermosensitive recording material and its' use |
| WO2004045862A1 (en) | 2002-11-19 | 2004-06-03 | Mitsubishi Hitec Paper Flensburg Gmbh | Heat-sensitive printing material and use thereof |
| EP2070714A1 (en) | 2007-12-11 | 2009-06-17 | Mitsubishi HiTec Paper Bielfeld GmbH | Heat sensitive recording material |
Also Published As
| Publication number | Publication date |
|---|---|
| CA2095965A1 (en) | 1993-11-16 |
| JPH05318927A (en) | 1993-12-03 |
| DE69313009D1 (en) | 1997-09-18 |
| DE69313009T2 (en) | 1997-12-18 |
| EP0570186B1 (en) | 1997-08-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2184175A1 (en) | Thermal recording medium | |
| EP1079356B1 (en) | Adhesive label for thermosensitive recording | |
| EP3103649B2 (en) | Thermosensitive recording medium | |
| US6410479B1 (en) | Thermally sensitive recording medium | |
| JP2008044227A (en) | Thermal recording medium | |
| EP0570186B1 (en) | Thermal recording sheet | |
| EP0585127B1 (en) | Thermal Recording sheet | |
| JP7354483B1 (en) | heat sensitive recording material | |
| JP2624952B2 (en) | Thermal recording sheet | |
| HK1005614B (en) | Thermal recording sheet | |
| US5747414A (en) | Thermal recording sheet | |
| JP2012076228A (en) | Thermosensitive recording body | |
| EP0399785A1 (en) | Heat-sensitive recording material | |
| JP2681905B2 (en) | Thermal recording sheet | |
| JP2727885B2 (en) | Thermal recording sheet | |
| WO2023190314A1 (en) | Heat-sensitive recording body | |
| JPH10272848A (en) | Thermal recording medium | |
| JP2006175636A (en) | Method for manufacturing thermal recording medium | |
| JP3642248B2 (en) | Thermal recording material | |
| JP2967709B2 (en) | Thermal recording medium | |
| JP2668858B2 (en) | Thermal recording sheet | |
| JP2012076230A (en) | Thermosensitive recording body | |
| JPH10272842A (en) | Thermal recording medium | |
| JP2005096323A (en) | Thermal recording body | |
| JPH07329422A (en) | Thermal recording |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE DE FR GB IT SE |
|
| 17P | Request for examination filed |
Effective date: 19931206 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| 17Q | First examination report despatched |
Effective date: 19961106 |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): BE DE FR GB IT SE |
|
| ET | Fr: translation filed | ||
| REF | Corresponds to: |
Ref document number: 69313009 Country of ref document: DE Date of ref document: 19970918 |
|
| ITF | It: translation for a ep patent filed | ||
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 19990728 Year of fee payment: 7 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20000531 |
|
| BERE | Be: lapsed |
Owner name: NIPPON PAPER INDUSTRIES CO. LTD Effective date: 20000531 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050511 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20070508 Year of fee payment: 15 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20080515 Year of fee payment: 16 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20080514 Year of fee payment: 16 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20090511 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20100129 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090602 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20080514 Year of fee payment: 16 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20090511 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20091201 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20080512 |