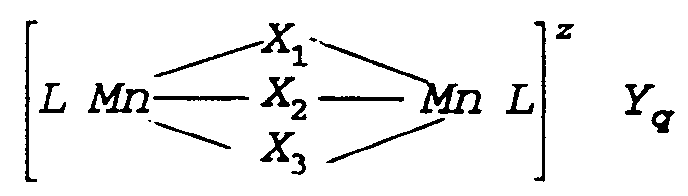EP0544490A1 - Detergent bleach compositions - Google Patents
Detergent bleach compositions Download PDFInfo
- Publication number
- EP0544490A1 EP0544490A1 EP92310719A EP92310719A EP0544490A1 EP 0544490 A1 EP0544490 A1 EP 0544490A1 EP 92310719 A EP92310719 A EP 92310719A EP 92310719 A EP92310719 A EP 92310719A EP 0544490 A1 EP0544490 A1 EP 0544490A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- weight
- composition according
- alkyl
- bleach
- aryl
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/39—Organic or inorganic per-compounds
- C11D3/3902—Organic or inorganic per-compounds combined with specific additives
- C11D3/3905—Bleach activators or bleach catalysts
- C11D3/3932—Inorganic compounds or complexes
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/395—Bleaching agents
Definitions
- This invention relates to detergent bleach compositions. More particularly, it relates to improved detergent bleach compositions, especially but not exclusively adapted for washing and cleaning of fabrics, containing, a surfactant material, a peroxide bleaching agent and a manganese complex as bleach catalyst.
- the manganese complex used in the present invention is a dinuclear manganese complex of the formula: wherein Mn is manganese which can individually be in the III or IV oxidation state; X1, X2 and X3 each independently represent a coordinating or bridging species selected from the group consisting of H2O, O22 ⁇ , O2 ⁇ , OH ⁇ , HO2 ⁇ , SH ⁇ , S2 ⁇ , >SO, Cl ⁇ , N3 ⁇ , SCN ⁇ , RCOO ⁇ , RSO ⁇ 3, NH2 ⁇ and NR3, with R being H, alkyl, aryl, both optionally substituted, or R'COO ⁇ where R' is alkyl, aryl, both optionally substituted; L is a ligand which is an organic molecule containing at least three nitrogen atoms which coordinates via all or some of the nitrogen atoms to the manganese centres; z denotes the charge of the complex and is an integer which can be positive or negative; Y is a monovalent or multivalent counter-i
- Preferred manganese-complexes are those wherein X1, X2 and X3 are either CH3COO ⁇ or O2 ⁇ or mixtures thereof and, most preferably, wherein the manganese is in the IV oxidation state and X1, X2 and X3 are O2 ⁇ .
- ligand is of formula wherein t is an integer from 2 to 3; s is an integer from 3 to 4; u is zero or one; R1, R2 and R3 are each independently selected from H, alkyl, aryl, both optionally substituted.
- the type of counter-ion Y for charge neutrality is not critical for the activity of the complex and can be selected from for example any of the following counter-ions : chloride; sulphate; nitrate; methylsulphate; surfactant-anions, such as the long-chain alkylsulphates, alkylsulphonates, alkylbenzenesulphonates, tosylate; trifluormethylsulphonate; perchlorate (C104 ⁇ ), BPh4 ⁇ and PF6-, though some counter-ions are more preferred than others for reasons of product property and safety.
- manganese complexes as used in the present invention are: (I) [ (Me3TACN)Mn IV ( ⁇ -O)3Mn IV (Me3TACN) ]2+(PF6 ⁇ )2 (II) [ (Me4TACN)Mn IV ( ⁇ -O)3Mn IV (Me4TACN) ]2+ (PF6 ⁇ )2 (III) [ (Me3TACN)Mn III ( ⁇ -O)( ⁇ -OAc)2Mn III (Me3TACN) ]2+ (PF6 ⁇ )2 (IV) [ (Me4TACN)Mn III ( ⁇ -O)( ⁇ -OAc)2Mn III (Me4TACN) ]2+ (PF6 ⁇ )2 (V) [ (Et-bridged(Me2TACN)2)Mn III Mn IV ( ⁇ -O)2( ⁇ -OAc) ]2+ (ClO4 ⁇ )2 which are hereinafter also abbreviated as: (I) [
- the invention now relates to detergent bleaching compositions comprising a surface-active material, a peroxide bleaching agent and the above-described dinuclear manganese-complex as bleach catalyst.
- the peroxide bleaching agents are normally compounds which are capable of yielding hydrogen peroxide in aqueous solution.
- Hydrogen peroxide sources are well known in the art. They,include the alkali metal peroxides, organic peroxides such as urea peroxide, and inorganic persalts, such as the alkali metal perborates, percarbonates, perphosphates, persilicates and persulphates. Mixtures of two or more such compounds may also be suitable.
- Particularly preferred are sodium perborate tetrahydrate and, especially, sodium perborate monohydrate.
- Sodium perborate monohydrate is preferred because of its high active oxygen content.
- Sodium percarbonate may also be preferred for environmental reasons.
- the amount thereof in the composition of the invention usually will be within the range of about 5-35% by weight, preferably from 10-25% by weight.
- Alkylhydroxy peroxides are another class of peroxide bleaching agents. Examples of these materials include cumene hydroperoxide and t-butyl hydroperoxide.
- Organic peroxyacids may also be suitable as the peroxide bleaching agent.
- Such materials normally have the general formula: wherein R is an alkylene or substituted alkylene group containing from 1 to about 20 carbon atoms, optionally having an internal amide linkage; or a phenylene or substituted phenylene group; and Y is hydrogen, halogen, alkyl, aryl, an imido-aromatic or non-aromatic group, a COOH or group or a quaternary ammonium group.
- Typical monoperoxy acids useful herein include, for example:
- Typical diperoxyacids useful herein include, for example:
- inorganic peroxyacid compounds are suitable, such as for example potassium monopersulphate (MPS). If organic or inorganic peroxyacids are used as the peroxygen compound, the amount thereof will normally be within the range of about 2-10% by weight, preferably from 4-8% by weight.
- MPS potassium monopersulphate
- All these peroxide compounds may be utilized alone or in conjunction with a peroxyacid bleach precursor and/or an organic bleach catalyst.
- Peroxyacid bleach precursors are known and amply described in literature, such as in the British Patents 836,988; 864,798; 907,356; 1,003,310 and 1,519,351; German Patent 3,337,921; EP-A-0185522; EP-A-0174132; EP-A-0120591; and US Patents 1,246,339; 3,332,882; 4,128,494; 4,412,934 and 4,675,393.
- peroxyacid bleach precursors are that of the cationic i.e. quaternary ammonium substituted peroxyacid precursors as disclosed in US Patents 4,751,015 and 4,397,757, in EP-A-284292 and EP-A-331,229.
- peroxyacid bleach precursors of this class are: 2-(N,N,N-trimethyl ammonium) ethyl sodium-4-sulphonphenyl carbonate chloride - (SPCC); N-octyl,N,N-dimethyl-N10-carbophenoxy decyl ammonium chloride - (ODC); 3-(N,N,N-trimethyl ammonium) propyl sodium-4-sulphophenyl carboxylate; and N,N,N-trimethyl ammonium toluyloxy benzene sulphonate.
- SPCC 2-(N,N,N-trimethyl ammonium) ethyl sodium-4-sulphonphenyl carbonate chloride -
- ODC N-octyl,N,N-dimethyl-N10-carbophenoxy decyl ammonium chloride -
- a further special class of cationic peroxyacid bleach precursors is formed by the cationic nitriles as disclosed in EP-A-0303520 and in European Patent Specification No.'s 458396 and 464880.
- any one of these peroxyacid bleach precursors can be used in the present invention, though some may be more preferred than others.
- the preferred classes are the esters, including acyl phenol sulphonates and acyl alkyl phenol sulphonates; the acyl-amides; and the quaternary ammonium substituted peroxyacid precursors including the cationic nitriles.
- Examples of said preferred peroxyacid bleach precursors or activators are sodium-4-benzoloxy benzene sulphonate (SBOBS); N,N,N',N'-tetraacetyl ethylene diamine (TAED); sodium-1-methyl-2-benzoyloxy benzene-4-sulphonate; sodium-4-methyl-3-benzoloxy benzoate; SPCC; trimethyl ammonium toluyloxy-benzene sulphonate; sodium nonanoyloxybenzene sulphonate (SNOBS); sodium 3,5,5-trimethyl hexanoyloxybenzene sulphonate (STHOBS); and the substituted cationic nitriles.
- SBOBS sodium-4-benzoloxy benzene sulphonate
- TAED N,N,N',N'-tetraacetyl ethylene diamine
- TAED sodium-1-methyl-2-benzoyloxy benzene-4-sulphon
- the precursors may be used in an amount of about 1-8%, preferably from 2-5% by weight, of the composition.
- Organic bleach catalyst most suitable for being utilized here are the so-called suphonimides as disclosed in EP-A-0,453,003 and EP-A-0,446,982.
- the surface-active material normally used in detergent bleach compositions may be naturally derived, such as soap, or a synthetic material selected from anionic, nonionic, amphoteric, zwiterionic, cationic actives and mixtures thereof.
- suitable actives are commercially available and are fully described in the literature, for example in "Surface Active Agents and Detergents", Volumes I and II, by Schwartz, Perry and Berch.
- Typical synthetic anionic surface-actives are usually water-soluble alkali metal salts of organic sulphates and sulphonates having alkyl radicals containing from about 8 to about 22 carbon atoms, the term alkyl being used to include the alkyl portion of higher aryl radicals.
- suitable synthetic anionic detergent compounds are sodium and ammonium alkyl sulphates, especially those obtained by sulphating higher (C8-C18) alcohols produced, for example, from tallow or coconut oil; sodium and ammonium alkyl (C9-C10) benzene sulphonates, particularly sodium linear secondary alkyl (C10-C15) benzene sulphonates; sodium alkyl glyceryl ether sulphates, especially those esters of the higher alcohols derived from tallow or coconut oil and synthetic alcohols derived from petroleum; sodium coconut oil fatty acid monoglyceride sulphates and sulphonates; sodium and ammonium salts of sulphuric acid esters of higher (C9-C18) fatty alcohol alkylene oxide, particularly ethylene oxide, reaction products; the reaction products of fatty acids such as coconut fatty acids esterified with isethionic acid and neutralised with sodium hydroxide; sodium and ammonium salts of fatty acid amides of methyl
- nonionic surface-active compounds which may be used, preferably together with the anionic surface-active compounds, include, in particular, the reaction products of alkylene oxides, usually ethylene oxide, with alkyl (C6-C22) phenols, generally 5-25 EO, i.e 5-25 units of ethylene oxides per molecule; the condensation products of aliphatic (C8-C18) primary or secondary linear or branched alcohols with ethylene oxide, generally 2-30 EO, and products may be condensation of ethylene oxide with the reaction products of propylene oxide and ethylene diamine.
- alkylene oxides usually ethylene oxide
- alkyl (C6-C22) phenols generally 5-25 EO, i.e 5-25 units of ethylene oxides per molecule
- condensation products of aliphatic (C8-C18) primary or secondary linear or branched alcohols with ethylene oxide generally 2-30 EO
- products may be condensation of ethylene oxide with the reaction products of propylene oxide and ethylene diamine.
- nonionic surface-actives include alkyl polyglycosides, sugar esters, long-chain tertiary amine oxides, long-chain tertiary phosphine oxides and dialkyl sulphoxides.
- Amount of amphoteric or zwitterionic surface-active compounds can also be used in the compositions-of the invention but this is not normally desired owing to their relatively high cost. If any amphoteric or zwitterionic detergent compounds are used, it is generally in small amounts in compositions based on the much more commonly used synthetic anionic and nonionic actives.
- It is therefore an object of the present invention to provide a detergent bleach composition comprising a surface-active material, a peroxide bleaching agent and a dinuclear manganese complex as hereinbefore described having optimal bleaching action.
- nonionic provided it is present in sufficient amounts, compensates the strong negative effect of anionic surfactants by resolubilization of the catalyst.
- the invention provides a detergent bleach composition
- a detergent bleach composition comprising a surface-active material, a peroxide bleaching agent and a dinuclear manganese complex as the bleach catalyst, which composition is characterized in that it comprises from 0-25% by weight of anionic surfactant and from 7.5-55% by weight of nonionic surfactant, the weight ratio of nonionic surfactant to anionic surfactant being at least 0.75 if the composition contains 0-7.5% by weight of a carbonate builder, expressed as sodium carbonate, and at least 2.2 if the composition contains more than 7.5% by weight of carbonate builder.
- composition will comprise from 0-15% by weight of anionic surfactant and from 10-40% by weight of nonionic surfactant.
- the detergent active system is free from C16-C22 fatty acid soaps.
- the detergent bleach composition is an all-nonionic based formulation exempt from any anionic surfactant.
- composition of the invention normally and preferably also contains a detergency builder in an amount of from about 5-80% by weight, preferably from about 10-60% by weight.
- Builder materials may be selected from 1) calcium sequestrant materials, 2) precipitating materials, 3) calcium ion-exchange materials and 4) mixture thereof.
- Examples of calcium sequestrant builder materials include alkali metal polyphosphates, such as sodium tripolyphosphate; nitrilotriacetic acid and its water-soluble salts; the alkali metal salts of carboxymethyloxy succinic acid, ethylene diamine tetraacetic acid, oxydisuccinic acid, mellitic acid, benzene polycarboxylic acids, citric acid; and polyacetal carboxylates as disclosed in US patents 4,144,226 and 4,146,495.
- alkali metal polyphosphates such as sodium tripolyphosphate
- the alkali metal salts of carboxymethyloxy succinic acid, ethylene diamine tetraacetic acid, oxydisuccinic acid, mellitic acid, benzene polycarboxylic acids, citric acid and polyacetal carboxylates as disclosed in US patents 4,144,226 and 4,146,495.
- precipitating builder materials examples include sodium orthophosphate and sodium carbonate.
- Examples of calcium ion-exchange builder materials include the various types of water-insoluble crystalline or amorphous aluminosilicates, of which zeolites are the best known representatives, e.g. zeolite A, zeolite B (also known as Zeolite P), zeolite C, zeolite X, zeolite Y and also the zeolite P type as described in EP-A-0,384,070.
- zeolites are the best known representatives, e.g. zeolite A, zeolite B (also known as Zeolite P), zeolite C, zeolite X, zeolite Y and also the zeolite P type as described in EP-A-0,384,070.
- compositions of the invention may contain any one of the organic or inorganic builder materials, though, for environmental reasons, phosphate builders are preferably omitted or only used in very small amounts.
- Typical builders usable in the present invention are, for example, sodium carbonate, calcite/carbonate, the sodium salt of nitrilotriacetic acid, sodium citrate, carboxymethyloxy malonate, carboxymethyloxy succinate and the water-insoluble crystalline or amorphous aluminosilicate builder material, each of which can be used as the main builder, either alone or in admixture with other builders or polymers as co-builder.
- alkalimetal carbonates and phosphates especially sodium carbonate, sodium bicarbonate and sodium triphosphate, also have a negative influence on the catalytic bleach performance of hydroperoxide bleaches, but surprisingly, only to a much lesser extent on the catalytic bleach performance of peroxyacid bleaches.
- the carbonate effect is however pH dependent in that upon increasing the pH to above 10, particularly to 10.5 and above, the negative effect is largely removed.
- the composition contains not more than 5% by weight of a carbonate builder, expressed as sodium carbonate, more preferable not more than 2.5% by weight to substantially nil, if the composition pH lies in the lower alkaline region of up to 10.
- the detergent compositions of the invention can contain any of the conventional additives in amounts of which such materials are normally employed in fabric washing detergent compositions.
- these additives include buffers such as carbonates, lather boosters, such as alkanolamides, particularly the monoethanol amides derived from palmkernel fatty acids and coconut fatty acids; lather depressants, such as alkyl phosphates and silicones; anti-redeposition agents, such as sodium carboxymethyl cellulose and alkyl or substituted alkyl cellulose ethers; stabilizers, such as ethylene diamine tetraacetic acid (EDTA) and the phosphonic acid derivatives (i.e.
- buffers such as carbonates
- lather boosters such as alkanolamides, particularly the monoethanol amides derived from palmkernel fatty acids and coconut fatty acids
- lather depressants such as alkyl phosphates and silicones
- anti-redeposition agents such as sodium carboxymethyl cellulose and alkyl or
- Dequest ® types fabric softening agents; inorganic salts and alkaline buffering agents, such as sodium sulphate, sodium silicate etc.; and usually in very small amounts, fluorescent agents; perfumes; enzymes, such as proteases, cellulases, lipases, amylases and oxidases; germicides and colourants.
- the composition contains not more than 5% by weight of a carbonate buffer, expressed as sodium carbonate, more preferable not more than 2.5% by weight to substantially nil, if the composition pH lies in the lower alkaline region of up to 10.
- transition metal sequestrants such as EDTA and the phosphonic acid derivatives, e.g. ethylene diamine tetra- (methylene phosphonate) -EDTMP- are of special importance, as not only do they improve the stability of the catalyst/H2O2 system and sensitive ingredients, such as enzymes, fluorescent agents, perfumes and the like, but also improve the bleach performance, especially at the higher pH region of above 10, particularly at pH 10.5 and above.
- detergent bleach compositions comprising a surface-active material, a peroxide bleaching agent, the manganese complex bleach catalyst, a carbonate builder and a transition metal sequestrant, having pH in solution of above 10, especially of 10.5 and above, are within the purview of the present invention.
- detergent bleach compositions comprising a surface-active material, a peroxyacid bleach, such as DPDA, PAP and MPS, the bleach catalyst, and a phosphate builder, preferably sodium triphosphate.
- Another optional but highly desirable additive ingredient with multi-functional characteristics in detergent compositions is from 0.1% to about 3% by weight of a polymeric material having a molecular weight of from 1,000 to 2,000,000 and which can be a homo- or co-polymer of acrylic acid, maleic acid, or salt or anhydride thereof, vinyl pyrrolidone, methyl- or ethylvinyl ethers, and other polymerizable vinyl monomers.
- polyacrylic acid or polyacrylate are polyacrylic acid or polyacrylate; polymaleic acid/acrylic acid copolymer; 70:30 acrylic acid/hydroxyethyl maleate copolymer; 1:1 styrene/maleic acid copolymer; isobutylene/maleic acid and diisobutylene/maleic acid copolymers; methyl- and ethylvinylether/maleic acid copolymers; ethylene/maleic acid copolymer; polyvinyl pyrrolidone; and vinyl pyrrolidone/maleic acid copolymer.
- Detergent bleach compositions of the invention when formulated as free-flowing particles, e.g. in powdered or granulated form, can be produced by any of the conventional techniques employed in the manufacture of detergent compositions, for instance by slurry-making, followed by spray-drying to form a detergent base powder to which the heat-sensitive ingredients including the peroxide compound bleach and optionally some other ingredients as desired, and the bleach catalyst, can be added as dry substances.
- the detergent base powder compositions, to which the bleach catalyst is added can itself be made in a variety of other ways, such as the so-called part-part processing, non-tower route processing, dry-mixing, agglomeration, granulation, extrusion, compacting and densifying processes etc., such ways being well known to those skilled in the art and not forming the essential part of the present invention.
- the bleach system in the wash solution was added at a concentration of 2.5 ⁇ mol/1 catalyst and 8.6 mmol/1 PBM, corresponding to 0.03% by weight of catalyst and 14.3% by weight of PBM if a detergent bleach formulation is dosed at 6 g/1.
- Test cloths were immersed for 30 minutes in each of the compositions of the examples. After rinsing with tap water, the cloths were dried in a tumble drier. The reflectance (R 460* .) was measured on a Zeiss Elrephometer before and after treatment. The difference ( ⁇ R 460* .) in the value gives a measure of the effectiveness of the treatment.
- Example 2 shows the effect of nonionic addition on the bleach performance of a formulation containing 9% C18-soap.
- the experiments were carried out under exactly the same conditions as used in Example 1.
- Example 3 shows the effect of nonionic addition on the bleach performance of a formulation containing 9% ABS.
- the experiments were carried out under exactly the same conditions as used in Example II and the results are shown in Table 3.
- Table 3 present - 9% ABS addition - - NI 2) NI 2) wt.% - - 4 8 ⁇ R460* 25.0 16.6 23.9 26.7
- Example 2 shows the effect of increasing amounts of C12-soap on the catalytic bleach performance of a catalyst/PBM bleach system on tea-stained test cloths.
- the experiments were carried out under the same conditions as used in Example I. The results are given in Table 4.
- Table C12-soap(% by weight) 0 9 21 33 74 mmol/1 0 2.4 5.8 9.0 20 ⁇ R460* 25.0 28.6 25.1 23.4 20.2
- This Example shows the effect of Na2CO3 on the bleach performance of a detergent bleach composition of the following nominal formulation: Formulation % by Weight Alkyl benzene sulphonate (ABS) 3.0 C18-soap 1.7 Nonionic (Synperonic A3/A7) 9.8 Zeolite 24.0 Polymer (Sokalan CP5 ex BASF) 4.0 Sodium carboxymethylcellulose 0.6 Sodium perborate monohydrate 14.3 Mn-complex (I) catalyst 0.03
- Example I was repeated except the bleach catalyst used was Mn III Mn IV ( ⁇ -O)2( ⁇ -OAc)(Et-bridged(Me2TACN)2) # and millipore water (ie. demineralised water which was further purified by treatment through a Milli-Q plus water purification system ex Millipore Corporation).
- # - prepared according to a method described by Prof Wieghardt at the 1991 ICBIC Conference in Oxford (UK)
- the level of catalyst corresponds to 0.037% by weight and of PBM to 14.3% by weight if a detergent bleach formulation is dosed at 6g/1.
Abstract
Detergent bleach compositions are disclosed comprising a surface active material, a peroxide bleaching agent and a dinuclear manganese complex as the bleach catalyst, characterised in that the composition comprises from 0-25% by weight of anionic surfactant and from 7.5-55% by weight of nonionic surfactant, the weight ratio of nonionic surfactant to anionic surfactant being at least 0.75 if the composition contains not more than 7.5% by weight of a carbonate, expressed as sodium carbonate, and at least 2.2. if the composition contains more than 7.5% by weight of a carbonate.
Description
- This invention relates to detergent bleach compositions. More particularly, it relates to improved detergent bleach compositions, especially but not exclusively adapted for washing and cleaning of fabrics, containing, a surfactant material, a peroxide bleaching agent and a manganese complex as bleach catalyst.
- The manganese complex used in the present invention is a dinuclear manganese complex of the formula:
wherein Mn is manganese which can individually be in the III or IV oxidation state; X₁, X₂ and X₃ each independently represent a coordinating or bridging species selected from the group consisting of H₂O, O₂²⁻, O²⁻, OH⁻, HO₂⁻, SH⁻, S²⁻, >SO, Cl⁻, N³⁻, SCN⁻, RCOO⁻, RSO⁻₃, NH₂⁻ and NR₃, with R being H, alkyl, aryl, both optionally substituted, or R'COO⁻ where R' is alkyl, aryl, both optionally substituted; L is a ligand which is an organic molecule containing at least three nitrogen atoms which coordinates via all or some of the nitrogen atoms to the manganese centres; z denotes the charge of the complex and is an integer which can be positive or negative; Y is a monovalent or multivalent counter-ion, leading to charge neutrality, which is dependent upon the charge z of the complex; and q = z/[charge Y]. - Preferred manganese-complexes are those wherein X₁, X₂ and X₃ are either CH₃COO⁻ or O²⁻ or mixtures thereof and, most preferably, wherein the manganese is in the IV oxidation state and X₁, X₂ and X₃ are O²⁻.
-
- Other preferred complexes are those which comprise two ligands of formula
wherein t is an integer from 2 to 3;
s is an integer from 3 to 4;
u is zero or one;
R¹ and R² are each independently selected from H, alkyl, aryl, both optionally substituted; and
R³ is independently selected from hydrogen, alkyl, aryl, both optionally substituted, with the proviso that a bridging unit R⁴ is formed by one R³ unit from each ligand where
R⁴ is the group CnR⁵R⁶-(D)p-CmR⁵R⁶ where p is zero or one;
D is selected from a heteroatom such as oxygen and NR⁷ or is part of an aromatic or saturated homonuclear or heteronuclear ring,
n is an integer from 1 to 4;
m is an integer from 1 to 4;
with the proviso that n + m <= 4 if p is zero or p is one and D is part of an aromatic or saturated homonuclear or heteronuclear ring; and that n + m <=3 if p is one and D is a heteroatom such as oxygen or NR⁷;
R⁵ and R⁶ are each independently selected from H, NR⁸ and OR⁹, alkyl, aryl, optionally substituted and R⁷, R⁸, R⁹ are each independently selected from H, alkyl, aryl, both optionally substituted. - Examples of suitable ligands in their simplest forms are:
- (i)
- 1,4,7-triazacyclononane;
1,4,7-triazacylclodecane;
1,4,8-triazacycloundecane;
1,5,9-triazacyclododecane.
1,4,7-trimethyl-1,4,7-triazacyclononane
1,4,7-trimethyl-1,4,7-triazacyclodecane;
1,4,8-trimethyl-1,4,8-triazacycloundecane;
1,5,9-trimethyl-1,5,9-triazacyclododecane.
1,2-bis-(4,7-dimethyl-1,4,7-triaza-1,-cyclononyl)ethane. - (ii)
- Tris(pyridin-2-yl)methane;
Tris(pyrazol-1-yl)methane;
Tris(imidazol-2-yl)methane;
Tris(triazol-1-yl)methane. - (iii)
- Tris(pyridin-2-yl)borate;
Tris(triazol-1-yl)borate;
Tris(pyrazol-1-yl)borate;
Tris(imidazol-2-yl)phosphine;
Tris(imidazol-2-yl)borate. - (iv)
- 1,3,5-trisamino-cyclohexane;
1,1,1-tris(methylamino)ethane. - (v)
- Bis(pyridin-2-yl-methyl)amine;
Bis(pyrazol-1-yl-methyl)amine;
Bis(triazol-1-yl-methyl)amine;
Bis(imidazol-2-yl-methyl)amine, - Of these the ligands of group (i) and their carbon-substituted derivatives are especially preferred, particularly:
- (1)
- 1,4,7-trimethyl-1,4,7-triazacyclononane (Me₃TACN), and
- (2)
- 1,2,4,7-tetramethyl-1,4,7-triazacyclononane (Me₄TACN)
- (3)
- 1,2,2,4,7-pentamethyl-1,4,7-triazacyclononane (Me₅TACN)
- (4)
- 2-benzyl-1,4,7-trimethyl-1,4,7-triazacyclononane
- (5)
- 1,2-bis(4,7-dimethyl-1,4,7-triaza-1-cyclonoyl)ethane(Et-bridged(Me₂TACN)₂)
- The type of counter-ion Y for charge neutrality is not critical for the activity of the complex and can be selected from for example any of the following counter-ions : chloride; sulphate; nitrate; methylsulphate; surfactant-anions, such as the long-chain alkylsulphates, alkylsulphonates, alkylbenzenesulphonates, tosylate; trifluormethylsulphonate; perchlorate (C10₄⁻), BPh₄⁻ and PF₆-, though some counter-ions are more preferred than others for reasons of product property and safety.
- However the most preferred manganese complexes as used in the present invention are:
(I) [ (Me₃TACN)MnIV(µ-O)₃MnIV(Me₃TACN) ]²⁺(PF₆⁻)₂
(II) [ (Me₄TACN)MnIV(µ-O)₃MnIV(Me₄TACN) ]²⁺ (PF₆⁻)₂
(III) [ (Me₃TACN)MnIII(µ-O)(µ-OAc)₂MnIII(Me₃TACN) ]²⁺ (PF₆⁻)₂
(IV) [ (Me₄TACN)MnIII(µ-O)(µ-OAc)₂MnIII(Me₄TACN) ]²⁺ (PF₆⁻)₂
(V) [ (Et-bridged(Me₂TACN)₂)MnIIIMnIV(µ-O)₂(µ-OAc) ]²⁺ (ClO₄⁻)₂
which are hereinafter also abbreviated as:
(I) [MnIV₂(µ-O)₃(Me₃TACN)₂] (PF₆)₂
(II) [ MnIV₂(µ-O)₃(Me₄TACN)₂] (PF₆)₂
(III) [MnIII₂(µ-O)(µ-OAc)₂(Me₃TACN)₂] (PF₆)₂
(IV) [MnIII₂(µ-O)(µ-OAc)₂(Me₄TACN)₂] (PF₆)₂
-
- These manganese complexes are reported in Applicant's copending European Patent Specification No's 458 397 and 458 398 as unusually effective bleach and oxidation catalysts. In the further description of the invention they will also be referred to as the "bleach catalyst" or simply "catalyst".
- The invention now relates to detergent bleaching compositions comprising a surface-active material, a peroxide bleaching agent and the above-described dinuclear manganese-complex as bleach catalyst.
- The peroxide bleaching agents are normally compounds which are capable of yielding hydrogen peroxide in aqueous solution. Hydrogen peroxide sources are well known in the art. They,include the alkali metal peroxides, organic peroxides such as urea peroxide, and inorganic persalts, such as the alkali metal perborates, percarbonates, perphosphates, persilicates and persulphates. Mixtures of two or more such compounds may also be suitable. Particularly preferred are sodium perborate tetrahydrate and, especially, sodium perborate monohydrate. Sodium perborate monohydrate is preferred because of its high active oxygen content. Sodium percarbonate may also be preferred for environmental reasons. The amount thereof in the composition of the invention usually will be within the range of about 5-35% by weight, preferably from 10-25% by weight.
- Alkylhydroxy peroxides are another class of peroxide bleaching agents. Examples of these materials include cumene hydroperoxide and t-butyl hydroperoxide.
- Organic peroxyacids may also be suitable as the peroxide bleaching agent. Such materials normally have the general formula:
wherein R is an alkylene or substituted alkylene group containing from 1 to about 20 carbon atoms, optionally having an internal amide linkage; or a phenylene or substituted phenylene group; and Y is hydrogen, halogen, alkyl, aryl, an imido-aromatic or non-aromatic group, a COOH or
group or a quaternary ammonium group. - Typical monoperoxy acids useful herein include, for example:
- (i)
- peroxybenzoic acid and ring-substituted peroxybenzoic acids, e.g. peroxy-α-naphthoic acid;
- (ii)
- aliphatic, substituted aliphatic and arylalkyl monoperoxyacids, e.g. peroxylauric acid, peroxystearic acid and N,N-phthaloylaminoperoxy caproic acid (PAP); and
- (iii)
- 6-octylamino-6-oxo-peroxyhexanoic acid.
- Typical diperoxyacids useful herein include, for example:
- (iv)
- 1,12-diperoxydodecanedioic acid (DPDA);
- (v)
- 1,9-diperoxyazelaic acid;
- (vi)
- diperoxybrassilic acid; diperoxysebasic acid and diperoxyisophthalic acid;
- (vii)
- 2-decyldiperoxybutane-1,4-dioic acid; and
- (viii)
- 4,4'-sulphonylbisperoxybenzoic acid.
- Also inorganic peroxyacid compounds are suitable, such as for example potassium monopersulphate (MPS). If organic or inorganic peroxyacids are used as the peroxygen compound, the amount thereof will normally be within the range of about 2-10% by weight, preferably from 4-8% by weight.
- All these peroxide compounds may be utilized alone or in conjunction with a peroxyacid bleach precursor and/or an organic bleach catalyst.
- Peroxyacid bleach precursors are known and amply described in literature, such as in the British Patents 836,988; 864,798; 907,356; 1,003,310 and 1,519,351; German Patent 3,337,921; EP-A-0185522; EP-A-0174132; EP-A-0120591; and US Patents 1,246,339; 3,332,882; 4,128,494; 4,412,934 and 4,675,393.
- Another useful class of peroxyacid bleach precursors is that of the cationic i.e. quaternary ammonium substituted peroxyacid precursors as disclosed in US Patents 4,751,015 and 4,397,757, in EP-A-284292 and EP-A-331,229. Examples of peroxyacid bleach precursors of this class are:
2-(N,N,N-trimethyl ammonium) ethyl sodium-4-sulphonphenyl carbonate chloride - (SPCC);
N-octyl,N,N-dimethyl-N₁₀-carbophenoxy decyl ammonium chloride - (ODC);
3-(N,N,N-trimethyl ammonium) propyl sodium-4-sulphophenyl carboxylate; and
N,N,N-trimethyl ammonium toluyloxy benzene sulphonate. - A further special class of cationic peroxyacid bleach precursors is formed by the cationic nitriles as disclosed in EP-A-0303520 and in European Patent Specification No.'s 458396 and 464880.
- Any one of these peroxyacid bleach precursors can be used in the present invention, though some may be more preferred than others.
- Of the above classes of bleach precursors, the preferred classes are the esters, including acyl phenol sulphonates and acyl alkyl phenol sulphonates; the acyl-amides; and the quaternary ammonium substituted peroxyacid precursors including the cationic nitriles.
- Examples of said preferred peroxyacid bleach precursors or activators are sodium-4-benzoloxy benzene sulphonate (SBOBS); N,N,N',N'-tetraacetyl ethylene diamine (TAED); sodium-1-methyl-2-benzoyloxy benzene-4-sulphonate; sodium-4-methyl-3-benzoloxy benzoate; SPCC; trimethyl ammonium toluyloxy-benzene sulphonate; sodium nonanoyloxybenzene sulphonate (SNOBS); sodium 3,5,5-trimethyl hexanoyloxybenzene sulphonate (STHOBS); and the substituted cationic nitriles.
- The precursors may be used in an amount of about 1-8%, preferably from 2-5% by weight, of the composition. Organic bleach catalyst most suitable for being utilized here are the so-called suphonimides as disclosed in EP-A-0,453,003 and EP-A-0,446,982.
- The surface-active material normally used in detergent bleach compositions may be naturally derived, such as soap, or a synthetic material selected from anionic, nonionic, amphoteric, zwiterionic, cationic actives and mixtures thereof. Many suitable actives are commercially available and are fully described in the literature, for example in "Surface Active Agents and Detergents", Volumes I and II, by Schwartz, Perry and Berch.
- Typical synthetic anionic surface-actives are usually water-soluble alkali metal salts of organic sulphates and sulphonates having alkyl radicals containing from about 8 to about 22 carbon atoms, the term alkyl being used to include the alkyl portion of higher aryl radicals. Examples of suitable synthetic anionic detergent compounds are sodium and ammonium alkyl sulphates, especially those obtained by sulphating higher (C₈-C₁₈) alcohols produced, for example, from tallow or coconut oil; sodium and ammonium alkyl (C₉-C₁₀) benzene sulphonates, particularly sodium linear secondary alkyl (C₁₀-C₁₅) benzene sulphonates; sodium alkyl glyceryl ether sulphates, especially those esters of the higher alcohols derived from tallow or coconut oil and synthetic alcohols derived from petroleum; sodium coconut oil fatty acid monoglyceride sulphates and sulphonates; sodium and ammonium salts of sulphuric acid esters of higher (C₉-C₁₈) fatty alcohol alkylene oxide, particularly ethylene oxide, reaction products; the reaction products of fatty acids such as coconut fatty acids esterified with isethionic acid and neutralised with sodium hydroxide; sodium and ammonium salts of fatty acid amides of methyl taurine; alkane monosulphonates such as those derived by reacting alpha-olefins (C₈-C₂₀) with sodium bisulphite and those derived by reaction paraffins with SO₂ and C₁₂ and then hydrolysing with a base to produce a random sulphonate; sodium and ammonium C₇-C₁₂ dialkyl sulphosuccinates; and olefin sulphonates, which term is used to describe material made by reacting olefins, particularly C₁₀-C₂₀ alpha-olefins, with SO₃ and then neutralising and hydrolysing the reaction product. The preferred anionic detergent compounds are sodium (C₁₀-C₁₅) alkylbenzene sulphonates, sodium (C₁₆-C₁₈) alkyl ether sulphates.
- Examples of suitable nonionic surface-active compounds which may be used, preferably together with the anionic surface-active compounds, include, in particular, the reaction products of alkylene oxides, usually ethylene oxide, with alkyl (C₆-C₂₂) phenols, generally 5-25 EO, i.e 5-25 units of ethylene oxides per molecule; the condensation products of aliphatic (C₈-C₁₈) primary or secondary linear or branched alcohols with ethylene oxide, generally 2-30 EO, and products may be condensation of ethylene oxide with the reaction products of propylene oxide and ethylene diamine. Other so-called nonionic surface-actives include alkyl polyglycosides, sugar esters, long-chain tertiary amine oxides, long-chain tertiary phosphine oxides and dialkyl sulphoxides.
- Amount of amphoteric or zwitterionic surface-active compounds can also be used in the compositions-of the invention but this is not normally desired owing to their relatively high cost. If any amphoteric or zwitterionic detergent compounds are used, it is generally in small amounts in compositions based on the much more commonly used synthetic anionic and nonionic actives.
- It has been found however, that if the catalysts hereinbefore described are used in detergent bleach compositions their performance is highly dependent upon the active detergent system and the builder system of the composition. Experiments have shown that anionic surfactants in general and long chain C₁₆-C₂₂ fatty acid soaps in particular have a strong negative effect on the catalyst activity, thereby decreasing the performance dramatically, whereas nonionic detergent surfactants do not interact with the catalyst. Short chain C₁₂-C₁₄ fatty acid soaps at lower levels up to about 10% by weight have a positive effect on the catalyst activity, but at high concentrations, e.g. >20% by weight they also become detrimental.
- The mechanism causing these effects is not fully understood, though it can be hypothesized that by some mechanism the catalyst is believed to be (partly) withdrawn from the solution in the presence of anionic surfactants. This may for instance happen by precipitation of the negatively charged actives with the positively charged catalyst molecule or only by ion pair formation which causes catalytic inactivity. Another possibility may be that the catalyst is enclosed in the micelles of the anion-active surfactant.
- Whatever the reason may be, it is clear that the bleach performance of detergent bleach compositions comprising a dinuclear manganese complex catalysed peroxide bleach system, is not optimal if the active detergent system comprises an anionic surface-active material.
- It is therefore an object of the present invention to provide a detergent bleach composition comprising a surface-active material, a peroxide bleaching agent and a dinuclear manganese complex as hereinbefore described having optimal bleaching action.
- It has now surprisingly been found that by including a nonionic surfactant in sufficient amounts it is possible to enhance the bleaching action back to optimal.
- Without wishing to be bound to any theory, it is believed that the nonionic, provided it is present in sufficient amounts, compensates the strong negative effect of anionic surfactants by resolubilization of the catalyst.
- Accordingly the invention provides a detergent bleach composition comprising a surface-active material, a peroxide bleaching agent and a dinuclear manganese complex as the bleach catalyst, which composition is characterized in that it comprises from 0-25% by weight of anionic surfactant and from 7.5-55% by weight of nonionic surfactant, the weight ratio of nonionic surfactant to anionic surfactant being at least 0.75 if the composition contains 0-7.5% by weight of a carbonate builder, expressed as sodium carbonate, and at least 2.2 if the composition contains more than 7.5% by weight of carbonate builder.
- Preferably the composition will comprise from 0-15% by weight of anionic surfactant and from 10-40% by weight of nonionic surfactant.
- In a further preferred embodiment the detergent active system is free from C₁₆-C₂₂ fatty acid soaps. In another preferred embodiment the detergent bleach composition is an all-nonionic based formulation exempt from any anionic surfactant.
- The composition of the invention normally and preferably also contains a detergency builder in an amount of from about 5-80% by weight, preferably from about 10-60% by weight.
- Builder materials may be selected from 1) calcium sequestrant materials, 2) precipitating materials, 3) calcium ion-exchange materials and 4) mixture thereof.
- Examples of calcium sequestrant builder materials include alkali metal polyphosphates, such as sodium tripolyphosphate; nitrilotriacetic acid and its water-soluble salts; the alkali metal salts of carboxymethyloxy succinic acid, ethylene diamine tetraacetic acid, oxydisuccinic acid, mellitic acid, benzene polycarboxylic acids, citric acid; and polyacetal carboxylates as disclosed in US patents 4,144,226 and 4,146,495.
- Examples of precipitating builder materials include sodium orthophosphate and sodium carbonate.
- Examples of calcium ion-exchange builder materials include the various types of water-insoluble crystalline or amorphous aluminosilicates, of which zeolites are the best known representatives, e.g. zeolite A, zeolite B (also known as Zeolite P), zeolite C, zeolite X, zeolite Y and also the zeolite P type as described in EP-A-0,384,070.
- In particular, the compositions of the invention may contain any one of the organic or inorganic builder materials, though, for environmental reasons, phosphate builders are preferably omitted or only used in very small amounts.
- Typical builders usable in the present invention are, for example, sodium carbonate, calcite/carbonate, the sodium salt of nitrilotriacetic acid, sodium citrate, carboxymethyloxy malonate, carboxymethyloxy succinate and the water-insoluble crystalline or amorphous aluminosilicate builder material, each of which can be used as the main builder, either alone or in admixture with other builders or polymers as co-builder.
- As to the builder system, it has been furthermore observed that alkalimetal carbonates and phosphates, especially sodium carbonate, sodium bicarbonate and sodium triphosphate, also have a negative influence on the catalytic bleach performance of hydroperoxide bleaches, but surprisingly, only to a much lesser extent on the catalytic bleach performance of peroxyacid bleaches. The carbonate effect is however pH dependent in that upon increasing the pH to above 10, particularly to 10.5 and above, the negative effect is largely removed.
- Accordingly when using a hydroperoxide, such as sodium perborate or sodium percarbonate, as the bleaching agent, it is preferred that the composition contains not more than 5% by weight of a carbonate builder, expressed as sodium carbonate, more preferable not more than 2.5% by weight to substantially nil, if the composition pH lies in the lower alkaline region of up to 10.
- Apart from the components already mentioned, the detergent compositions of the invention can contain any of the conventional additives in amounts of which such materials are normally employed in fabric washing detergent compositions. Examples of these additives include buffers such as carbonates, lather boosters, such as alkanolamides, particularly the monoethanol amides derived from palmkernel fatty acids and coconut fatty acids; lather depressants, such as alkyl phosphates and silicones; anti-redeposition agents, such as sodium carboxymethyl cellulose and alkyl or substituted alkyl cellulose ethers; stabilizers, such as ethylene diamine tetraacetic acid (EDTA) and the phosphonic acid derivatives (i.e. Dequest ® types); fabric softening agents; inorganic salts and alkaline buffering agents, such as sodium sulphate, sodium silicate etc.; and usually in very small amounts, fluorescent agents; perfumes; enzymes, such as proteases, cellulases, lipases, amylases and oxidases; germicides and colourants.
- When using a hydroperoxide, such as sodium perborate or sodium percarbonate, as the bleaching agent, it is preferred that the composition contains not more than 5% by weight of a carbonate buffer, expressed as sodium carbonate, more preferable not more than 2.5% by weight to substantially nil, if the composition pH lies in the lower alkaline region of up to 10.
- Of the additives, transition metal sequestrants, such as EDTA and the phosphonic acid derivatives, e.g. ethylene diamine tetra- (methylene phosphonate) -EDTMP- are of special importance, as not only do they improve the stability of the catalyst/H₂O₂ system and sensitive ingredients, such as enzymes, fluorescent agents, perfumes and the like, but also improve the bleach performance, especially at the higher pH region of above 10, particularly at pH 10.5 and above.
- Accordingly detergent bleach compositions comprising a surface-active material, a peroxide bleaching agent, the manganese complex bleach catalyst, a carbonate builder and a transition metal sequestrant, having pH in solution of above 10, especially of 10.5 and above, are within the purview of the present invention.
- Also within the purview of the present invention are detergent bleach compositions comprising a surface-active material, a peroxyacid bleach, such as DPDA, PAP and MPS, the bleach catalyst, and a phosphate builder, preferably sodium triphosphate.
- Another optional but highly desirable additive ingredient with multi-functional characteristics in detergent compositions is from 0.1% to about 3% by weight of a polymeric material having a molecular weight of from 1,000 to 2,000,000 and which can be a homo- or co-polymer of acrylic acid, maleic acid, or salt or anhydride thereof, vinyl pyrrolidone, methyl- or ethylvinyl ethers, and other polymerizable vinyl monomers. Preferred examples of such polymeric materials are polyacrylic acid or polyacrylate; polymaleic acid/acrylic acid copolymer; 70:30 acrylic acid/hydroxyethyl maleate copolymer; 1:1 styrene/maleic acid copolymer; isobutylene/maleic acid and diisobutylene/maleic acid copolymers; methyl- and ethylvinylether/maleic acid copolymers; ethylene/maleic acid copolymer; polyvinyl pyrrolidone; and vinyl pyrrolidone/maleic acid copolymer.
- Detergent bleach compositions of the invention, when formulated as free-flowing particles, e.g. in powdered or granulated form, can be produced by any of the conventional techniques employed in the manufacture of detergent compositions, for instance by slurry-making, followed by spray-drying to form a detergent base powder to which the heat-sensitive ingredients including the peroxide compound bleach and optionally some other ingredients as desired, and the bleach catalyst, can be added as dry substances.
- It will be appreciated, however, that the detergent base powder compositions, to which the bleach catalyst is added, can itself be made in a variety of other ways, such as the so-called part-part processing, non-tower route processing, dry-mixing, agglomeration, granulation, extrusion, compacting and densifying processes etc., such ways being well known to those skilled in the art and not forming the essential part of the present invention.
- The invention will now be illustrated by way of the following non-limiting Examples.
- The following examples were carried out in glass vessels, equipped with a temperature controlled heating spiral in quartz, magnetic stirrer, thermo-couple and pH electrode.
- At 40°C isothermal, experiments in demineralised water at pH 10, were carried out to determine the effect of soaps, primary alcohol sulphate (PAS), alkyl benzene sulphonate (ABS), and nonionic surfactant (NI) on the bleach performance of sodium perborate monohydrate (PBM) and [ MnIV₂(µ-O)₃(Me-TACN)₂ ] (PF₆)₂, i.e. complex (I), as the bleach catalyst, on standard tea-stained test cloths.
- The bleach system in the wash solution was added at a concentration of 2.5 µmol/1 catalyst and 8.6 mmol/1 PBM, corresponding to 0.03% by weight of catalyst and 14.3% by weight of PBM if a detergent bleach formulation is dosed at 6 g/1.
- Test cloths were immersed for 30 minutes in each of the compositions of the examples. After rinsing with tap water, the cloths were dried in a tumble drier. The reflectance (R460*.) was measured on a Zeiss Elrephometer before and after treatment. The difference (ΔR460*.) in the value gives a measure of the effectiveness of the treatment.
-
- The results show that when a C₁₈-soap is present in a very low quantity eg. 1:6% by weight there is a detectable decrease in the performance of the catalyst. At 9% by weight C₁₈-soap decreases the performance dramatically from 25 Δ R units to about 9 units. Surprisingly, C₁₂-soap at 9% enhances the catalytic bleach performance up to 29 units. Both synthetic anionic surfactants PAS (at 9%) and ABS (at 9%) also have a strong negative effect on the catalyst activity, decreasing the catalytic bleach performance from 25 units to about 16 units.
- On the other hand 4% of NI hardly influences the catalyst activity.
- This Example shows the effect of nonionic addition on the bleach performance of a formulation containing 9% C₁₈-soap. The experiments were carried out under exactly the same conditions as used in Example 1.
- The results are shown in Table 2
Table 2 present - 9% C₁₈-soap addition - - NI2) NI2) wt.% - - 4 16 Δ R₄₆₀* 25.0 8.7 21.0 25.7 - These results clearly show that addition of 4% NI is not sufficient to adequately improve the bleach performance, whereas an addition of 16% (NI: Anionic ratio of 1:8), enhances the catalytic bleach performance back to optimal approximately 25 ΔR units.
- This Example shows the effect of nonionic addition on the bleach performance of a formulation containing 9% ABS. The experiments were carried out under exactly the same conditions as used in Example II and the results are shown in Table 3.
Table 3 present - 9% ABS addition - - NI2) NI2) wt.% - - 4 8 Δ R₄₆₀* 25.0 16.6 23.9 26.7 - These results clearly show that addition of 4% NI is not sufficient to adequately improve the bleach performance, whereas an addition of 8% (NI:anionic ratio 0.9) enhances the catalytic bleach performance and gives a better result than the control experiment without active.
- This Example shows the effect of increasing amounts of C₁₂-soap on the catalytic bleach performance of a catalyst/PBM bleach system on tea-stained test cloths. The experiments were carried out under the same conditions as used in Example I. The results are given in Table 4.
Table C₁₂-soap(% by weight) 0 9 21 33 74 mmol/1 0 2.4 5.8 9.0 20 Δ R₄₆₀* 25.0 28.6 25.1 23.4 20.2 - This Example shows the effect of Na₂CO₃ on the bleach performance of a detergent bleach composition of the following nominal formulation:
Formulation % by Weight Alkyl benzene sulphonate (ABS) 3.0 C₁₈-soap 1.7 Nonionic (Synperonic A3/A7) 9.8 Zeolite 24.0 Polymer (Sokalan CP5 ex BASF) 4.0 Sodium carboxymethylcellulose 0.6 Sodium perborate monohydrate 14.3 Mn-complex (I) catalyst 0.03 - The washing experiments were carried out at 40°C isothermal, using tap water of 16°FH at pH 10 and pH 10.5. Tea-stained test cloths were immersed in the wash solution, containing the bleach composition dosed at 6g/1, for 30 minutes
- The results expressed as reflectance difference Δ R₄₆₀* are shown in the following Tables.
Table 5 (at pH 10) Na₂CO₃ added (% by weight) - 2.5 5.0 10.0 20.0 Δ R₄₆₀* 25.6 21.6 21.7 16.8 15.7
The slightly negative effect of sodium carbonate at levels up to 5.0% and the significance decrease of catalytic bleach performance by the addition of higher levels, i.e. 10% and 20%, of sodium carbonate are clearly shown.Table 6 (at pH 10.5) Na₂CO₃ added (% by weight) - 10.0 20.0 Δ R₄₆₀* (-EDTA) 24.9 22.0 18.1 Δ R₄₆₀* (+ 0.3% EDTA) 28.0 26.1 - - The positive effect of increasing pH and adding EDTA are shown by the results in Table 6.
- Example I was repeated except the bleach catalyst used was MnIII MnIV (µ-O)₂(µ-OAc)(Et-bridged(Me₂TACN)₂)# and millipore water (ie. demineralised water which was further purified by treatment through a Milli-Q plus water purification system ex Millipore Corporation).
(# - prepared according to a method described by Prof Wieghardt at the 1991 ICBIC Conference in Oxford (UK)) - The level of catalyst corresponds to 0.037% by weight and of PBM to 14.3% by weight if a detergent bleach formulation is dosed at 6g/1.
-
- The results show a bridged material related to Mn₂(IV)(µ-0)₃(Me₃TACN)₂ catalyses perborate bleaching. The bleach catalysis is significantly reduced by the use of ABS but not if nonionic is used. However, if ABS as well as nonionic are present, a larger amount of nonionic is required to compensate for the poor effect of ABS than in the case when a non-bridged complex is used as bleach catalyst.
all optionally substituted on amine N-atom and/or CH₂ carbon atom and/or aromatic ring.
Claims (12)
- A detergent bleach composition comprising a surface active material, a peroxide bleaching agent and a dinuclear manganese complex, as the bleach catalyst, having the formula:
- A composition according to claim 1, characterised in that it comprises from 0-15% by weight of anionic surfactant and from 10-40% by weight of nonionic surfactant.
- A composition according to claim 1 or 2, characterised in that the detergent active system is free from C₁₆-C₂₂ fatty acid soaps.
- A composition according to claim 1, 2 or 3, characterised in that the surface active agent is a nonionic surface active agent.
- A composition according to any of the above claims 1-4, characterised in that the bleaching agent is a hydroperoxide compound.
- A composition according to claim 5, characterised in that it has a pH of up to 10 and contains not more than 5% by weight of a carbonate, expressed as sodium carbonate.
- A composition according to claim 6, characterised in that it is substantially free of carbonate.
- A composition according to any one of the claims 1 to 4 characterised in that the bleaching agent is a peroxyacid compound.
- A composition according to claim 1 wherein the bleach catalyst comprises two ligands of formula
s is an integer from 3 to 4;
u is zero or one;
R¹ and R² are each independently selected from H, alkyl, aryl, both optionally substituted; and
R³ is independently selected from hydrogen, alkyl, aryl, both optionally substituted, with the proviso that a bridging unit R⁴ is formed by one R³ unit from each ligand where R⁴ is the group CnR⁵R⁶-(D)p-CmR⁵R⁶ where p is zero or one;
D is selected from a heteroatom such as oxygen and NH⁷ or is part of an aromatic or saturated homonuclear or heteronuclear ring,
n is an integer from 1 to 4;
m is an integer from 1 to 4;
with the proviso that n + m <= 4 if p is zero or p is one and D is part of an aromatic or saturated homonuclear or heteronuclear ring; and that n + m <=3 if p is one and D is a heteroatom such as oxygen or NR⁷;
R⁵ and R⁶ are each independently selected from H, NR⁸ and OR⁹, alkyl, aryl, optionally substituted and R⁷,
R⁸, R⁹ are each independently selected from H, alkyl, aryl, both optionally substituted. - A composition according to claims 1-8, characterised in that the bleach catalyst is selected from the complexes:
(I) [MnIV₂(µ-O)₃(Me₃TACN)₂] (PF₆)₂
(II) [MnIV₂(µ-O)₃(Me₄TACN)₂] (PF₆)₂
(III) [MnIII₂(µ-O) (µ-OAc)₂(Me₃TACN)₂] (PF₆)₂
(IV) [MnIII₂(µ-O) (µ-OAc)₂(Me₄TACN)₂] (PF₆)₂
(V) [(Et-bridged(Me₂TACN)₂)MnIIIMnIV(µ-O)₂(µ-OAc)]²⁺ (ClO₄⁻)₂
- A composition according to claim 11, characterised in that said bleach catalyst is complex (I).
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP91203078 | 1991-11-26 | ||
| EP91203078 | 1991-11-26 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0544490A1 true EP0544490A1 (en) | 1993-06-02 |
Family
ID=8208033
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP92310719A Withdrawn EP0544490A1 (en) | 1991-11-26 | 1992-11-24 | Detergent bleach compositions |
Country Status (11)
| Country | Link |
|---|---|
| EP (1) | EP0544490A1 (en) |
| JP (1) | JPH0768543B2 (en) |
| KR (1) | KR960000205B1 (en) |
| CN (1) | CN1031647C (en) |
| AU (1) | AU661522B2 (en) |
| BR (1) | BR9204549A (en) |
| CA (1) | CA2083661A1 (en) |
| MX (1) | MX9206779A (en) |
| TR (1) | TR27075A (en) |
| TW (1) | TW234144B (en) |
| ZA (1) | ZA928188B (en) |
Cited By (165)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994019445A1 (en) * | 1993-02-22 | 1994-09-01 | Unilever N.V. | Machine dishwashing composition |
| WO1994021775A1 (en) * | 1993-03-18 | 1994-09-29 | Unilever Plc | Detergent compositions |
| EP0693550A2 (en) | 1994-07-21 | 1996-01-24 | Ciba-Geigy Ag | Fabric bleaching composition |
| WO1996006157A1 (en) * | 1994-08-19 | 1996-02-29 | Unilever N.V. | Detergent bleach composition |
| WO1996006154A1 (en) * | 1994-08-19 | 1996-02-29 | Unilever N.V. | Detergent bleach composition |
| EP0699745A2 (en) | 1994-08-31 | 1996-03-06 | The Procter & Gamble Company | Automatic dishwashing compositions comprising quaternary ammonium compounds bleach activators and quaternary ammonium |
| WO1996025478A1 (en) | 1995-02-15 | 1996-08-22 | The Procter & Gamble Company | Detergent composition comprising an amylase enzyme and a nonionic polysaccharide ether |
| US5560748A (en) * | 1994-06-10 | 1996-10-01 | The Procter & Gamble Company | Detergent compositions comprising large pore size redox catalysts |
| US5580485A (en) * | 1994-06-13 | 1996-12-03 | Lever Brothers Company, Division Of Conopco, Inc. | Bleach activation |
| EP0751142A2 (en) * | 1995-06-30 | 1997-01-02 | Hoechst Aktiengesellschaft | Chiral manganese-triazanonane complexes and process for their preparation |
| US5622646A (en) * | 1994-04-07 | 1997-04-22 | The Procter & Gamble Company | Bleach compositions comprising metal-containing bleach catalysts and antioxidants |
| GB2307250A (en) * | 1995-11-18 | 1997-05-21 | Ciba Geigy Ag | Fabric bleaching composition |
| WO1997034985A1 (en) * | 1996-03-16 | 1997-09-25 | The Procter & Gamble Company | Bleaching composition comprising metal catalyst, cellulase enzyme, and oxygen bleach |
| US5686014A (en) * | 1994-04-07 | 1997-11-11 | The Procter & Gamble Company | Bleach compositions comprising manganese-containing bleach catalysts |
| WO1997042282A1 (en) | 1996-05-03 | 1997-11-13 | The Procter & Gamble Company | Detergent compositions comprising polyamine polymers with improved soil dispersancy |
| WO1997044520A1 (en) * | 1996-05-20 | 1997-11-27 | Rudolf Patt | A method for the delignification of fibrous material and use of a catalyst |
| WO1997048787A1 (en) * | 1996-06-19 | 1997-12-24 | Unilever N.V. | Bleach activation |
| US5703030A (en) * | 1995-06-16 | 1997-12-30 | The Procter & Gamble Company | Bleach compositions comprising cobalt catalysts |
| US5703034A (en) * | 1995-10-30 | 1997-12-30 | The Procter & Gamble Company | Bleach catalyst particles |
| US5705464A (en) * | 1995-06-16 | 1998-01-06 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt catalysts |
| US5798326A (en) * | 1995-02-02 | 1998-08-25 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt III catalysts |
| US5850086A (en) * | 1996-06-21 | 1998-12-15 | Regents Of The University Of Minnesota | Iron complexes for bleach activation and stereospecific oxidation |
| WO1999020726A1 (en) | 1997-10-23 | 1999-04-29 | The Procter & Gamble Company | Bleaching compositions comprising multiply-substituted protease variants |
| US5939373A (en) * | 1995-12-20 | 1999-08-17 | The Procter & Gamble Company | Phosphate-built automatic dishwashing composition comprising catalysts |
| US5969171A (en) * | 1997-07-01 | 1999-10-19 | Clariant Gmbh | Metal complexes as bleach activators |
| US5998645A (en) * | 1997-05-07 | 1999-12-07 | Clariant Gmbh | Bleaching-active metal complexes |
| US6020294A (en) * | 1995-02-02 | 2000-02-01 | Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt chelated catalysts |
| US6139769A (en) * | 1997-04-05 | 2000-10-31 | Clariant Gmbh | Bleaching-active metal complexes |
| US6153576A (en) * | 1996-02-16 | 2000-11-28 | Henkel Kommanditgesellschaft Auf Aktien | Transition-metal complexes used as activators for peroxy compounds |
| US6187739B1 (en) | 1995-09-21 | 2001-02-13 | Henkel Kommanditgesellschaft Auf Aktien | Paste-form washing and cleaning agents |
| US6200946B1 (en) | 1996-04-01 | 2001-03-13 | Henkel Kommanditgesellschaft Auf Aktien | Transition metal ammine complexes as activators for peroxide compounds |
| EP1083173A2 (en) * | 1999-09-10 | 2001-03-14 | Clariant GmbH | Metal complexes with bleaching activity |
| US6221824B1 (en) | 1999-02-25 | 2001-04-24 | Henkel Kommanditgesellschaft Auf Aktien | Process for the production of compounded acetonitrile derivatives |
| US6221820B1 (en) | 1997-12-31 | 2001-04-24 | Henkel Kommanditgesellschaft Auf Aktien | Granular component containing alkylaminotriazole for use in machine dishwashing detergents |
| US6225274B1 (en) | 1996-11-29 | 2001-05-01 | Henkel Kommandigesellschaft Auf Aktien | Acetonitrile derivatives as bleaching activators in detergents |
| US6235695B1 (en) * | 1996-04-01 | 2001-05-22 | Henkel Kommanditgesellschaft Auf Aktien | Cleaning agent with oligoammine activator complexes for peroxide compounds |
| US6248708B1 (en) | 1996-09-05 | 2001-06-19 | Henkel-Ecolab Gmbh & Co. Ohg | Paste-form detergent containing a mixture of ethoxylated alcohols |
| US6329333B1 (en) | 1997-01-30 | 2001-12-11 | Henkel-Ecolab Gmbh & Co. Ohg | Pastelike detergent and cleaning agent |
| US6391838B1 (en) | 1999-03-31 | 2002-05-21 | Henkel Kommanditgesellschaft Auf Aktien | Detergents containing enzymes and bleach activators |
| US6410500B1 (en) | 1997-12-30 | 2002-06-25 | Henkel Kommanditgesellschaft Auf Aktien | Moulded body dishwasher detergents with soil release polymers |
| US6409770B1 (en) | 1995-12-08 | 2002-06-25 | Henkel Kommanditgesellschaft Auf Aktien | Bleaching and washing agents with enzyme bleaching system |
| US6417152B1 (en) | 1997-07-30 | 2002-07-09 | Henkel Kommanditgesellshaft Auf Aktien | Detergent containing glucanase |
| US6462006B1 (en) | 1998-04-30 | 2002-10-08 | Henkel Kommanditgesellschaft Auf Aktien | Solid machine dishwashing detergent with phosphate and crystalline lamellar silicates |
| US6541233B1 (en) | 1997-07-30 | 2003-04-01 | Henkel Kommanditgesellschaft Auf Aktien | β-glucanase from a bacillus |
| US6610752B1 (en) | 1999-10-09 | 2003-08-26 | Cognis Deutschland Gmbh | Defoamer granules and processes for producing the same |
| US6616705B2 (en) | 2000-09-08 | 2003-09-09 | Cognis Deutschland Gmbh & Co. Kg | Laundry detergent compositions |
| US6620209B2 (en) | 2000-09-08 | 2003-09-16 | Cognis Deutschland Gmbh & Co. Kg | Laundry detergent compositions |
| US6649085B2 (en) | 2000-11-25 | 2003-11-18 | Clariant Gmbh | Cyclic sugar ketones as catalysts for peroxygen compounds |
| US6686327B1 (en) | 1999-10-09 | 2004-02-03 | Cognis Deutschland Gmbh & Co. Kg | Shaped bodies with improved solubility in water |
| US6696401B1 (en) | 1999-11-09 | 2004-02-24 | The Procter & Gamble Company | Laundry detergent compositions comprising zwitterionic polyamines |
| US6703357B1 (en) | 1997-07-30 | 2004-03-09 | Henkel Kommanditgesellschaft Auf Aktien | Cleaning agent for hard surfaces, containing glucanase |
| US6723135B2 (en) | 2000-09-19 | 2004-04-20 | Cognis Deutschland Gmbh & Co. Kg | Laundry detergents and cleaning products based on alkyl and/or alkenyl oligoglycosides and fatty alcohols |
| US6746996B2 (en) | 2001-01-19 | 2004-06-08 | Clariant Gmbh | Use of transition metal complexes having oxime ligands as bleach catalysts |
| US6756351B2 (en) | 2000-04-18 | 2004-06-29 | Cognis Deutschland Gmbh & Co. Kg | Detergents and cleaning agents |
| WO2004069979A2 (en) | 2003-02-03 | 2004-08-19 | Unilever Plc | Laundry cleansing and conditioning compositions |
| US6812198B2 (en) | 1999-11-09 | 2004-11-02 | The Procter & Gamble Company | Laundry detergent compositions comprising hydrophobically modified polyamines |
| US6841614B1 (en) | 1998-10-29 | 2005-01-11 | Henkel Kommanditgesellschaft Auf Aktien | Polymer granules produced by fluidized bed granulation |
| US6846791B1 (en) | 1999-11-09 | 2005-01-25 | The Procter & Gamble Company | Laundry detergent compositions comprising hydrophobically modified polyamines |
| US6875734B2 (en) | 2003-02-03 | 2005-04-05 | Clariant Gmbh | Use of transition metal complexes as bleach catalysts |
| US6881359B2 (en) | 2000-01-26 | 2005-04-19 | Cognis Deutschland Gmbh & Co. Kg | Processes for the preparation of low dust, limited particle size distribution, surfactant granules |
| US6897193B2 (en) | 2001-12-22 | 2005-05-24 | Cognis Deutschland Gmbh & Co., Kg | Hydroxy mixed ethers and polymers in the form of solid preparations as a starting compound for laundry detergents, dishwashing detergents and cleaning compositions |
| US6936581B2 (en) | 2000-04-19 | 2005-08-30 | Cognis Deutschland Gmbh & Co. Kg | Processes for preparing anhydrous detergent granules |
| US6951838B1 (en) | 1999-09-15 | 2005-10-04 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US6977239B1 (en) | 1999-11-25 | 2005-12-20 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US6992056B1 (en) | 1997-12-30 | 2006-01-31 | Henkel Kgaa | Process for preparing detergent tablets having two or more regions |
| US7087570B2 (en) | 1999-12-24 | 2006-08-08 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US7091168B2 (en) | 2000-06-29 | 2006-08-15 | Cognis Deutschland Gmbh & Co. Kg | Liquid detergents |
| US7186678B2 (en) | 1999-12-24 | 2007-03-06 | Cognis Deutschland Gmbh & Co. Kg | Tenside granules with improved disintegration rate |
| US7199096B1 (en) | 1999-11-09 | 2007-04-03 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US7335629B2 (en) | 2001-12-21 | 2008-02-26 | Henkel Kommanditgesellschaft Auf Aktien | Support-fixed bleaching catalyst complex compounds suitable as catalysts for peroxygen compounds |
| DE102007003885A1 (en) | 2007-01-19 | 2008-07-24 | Lanxess Deutschland Gmbh | Use of a builder system comprising alkali metal tripolyphosphate and iminodisuccinic acid to produce automatic dishwasher formulations |
| EP1978081A2 (en) | 2000-10-27 | 2008-10-08 | The Procter and Gamble Company | Stabilized liquid compositions |
| DE102008000029A1 (en) | 2008-01-10 | 2009-07-16 | Lanxess Deutschland Gmbh | Use of phosphate reduced building system comprising alkali tripolyphosphate and imino disuccinic acid, for manufacturing formulations e.g. for the automatic or mechanical dish cleaning and crockery cleaning machines on ships |
| DE102008024800A1 (en) | 2008-05-23 | 2009-11-26 | Henkel Ag & Co. Kgaa | Method for washing textiles in the presence of a peroxygenated bleaching agent and a bleach boosting transition metal complex |
| WO2010010334A1 (en) | 2008-07-23 | 2010-01-28 | Reckitt Benckiser N.V. | Container |
| DE102008045297A1 (en) | 2008-09-02 | 2010-03-04 | Friedrich-Alexander-Universität Erlangen-Nürnberg | Method for washing textiles in the presence of a peroxygenated bleaching agent and a bleach boosting transition metal complex |
| WO2011005910A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | Method of laundering fabric using a compacted laundry detergent composition |
| WO2011005730A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | A catalytic laundry detergent composition comprising relatively low levels of water-soluble electrolyte |
| WO2011005623A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | Laundry detergent composition comprising low level of bleach |
| WO2011005913A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | A catalytic laundry detergent composition comprising relatively low levels of water-soluble electrolyte |
| WO2011005804A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | Method of laundering fabric using a liquid laundry detergent composition |
| WO2011025615A2 (en) | 2009-08-13 | 2011-03-03 | The Procter & Gamble Company | Method of laundering fabrics at low temperature |
| US7956027B2 (en) | 2006-07-27 | 2011-06-07 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US7972386B2 (en) | 2005-10-12 | 2011-07-05 | Conopco, Inc. | Bleaching of substrates |
| US7976582B2 (en) | 2007-01-16 | 2011-07-12 | Conopco, Inc. | Bleaching of substrates |
| WO2011088089A1 (en) | 2010-01-12 | 2011-07-21 | The Procter & Gamble Company | Intermediates and surfactants useful in household cleaning and personal care compositions, and methods of making the same |
| WO2011146602A2 (en) | 2010-05-18 | 2011-11-24 | Milliken & Company | Optical brighteners and compositions comprising the same |
| WO2011146604A2 (en) | 2010-05-18 | 2011-11-24 | Milliken & Company | Optical brighteners and compositions comprising the same |
| WO2011149871A1 (en) | 2010-05-28 | 2011-12-01 | Milliken & Company | Colored speckles having delayed release properties |
| WO2012003360A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Detergent product and method for making same |
| WO2012003319A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Filaments comprising an active agent nonwoven webs and methods for making same |
| WO2012003300A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Filaments comprising a non-perfume active agent nonwoven webs and methods for making same |
| WO2012003367A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Method for delivering an active agent |
| WO2012003316A1 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Process for making films from nonwoven webs |
| WO2012009525A2 (en) | 2010-07-15 | 2012-01-19 | The Procter & Gamble Company | Compositions comprising a near terminal-branched compound and methods of making the same |
| WO2012009660A2 (en) | 2010-07-15 | 2012-01-19 | The Procter & Gamble Company | Detergent compositions comprising microbially produced fatty alcohols and derivatives thereof |
| US8153576B2 (en) | 2006-07-27 | 2012-04-10 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| WO2012112828A1 (en) | 2011-02-17 | 2012-08-23 | The Procter & Gamble Company | Bio-based linear alkylphenyl sulfonates |
| WO2012116014A1 (en) | 2011-02-25 | 2012-08-30 | Milliken & Company | Capsules and compositions comprising the same |
| US8262804B2 (en) | 2007-10-12 | 2012-09-11 | Basf Se | Dishwasher detergent formulations comprising a mixture of hydrophobically modified polycarboxylates and hydrophilically modified polycarboxylates |
| WO2012138423A1 (en) | 2011-02-17 | 2012-10-11 | The Procter & Gamble Company | Compositions comprising mixtures of c10-c13 alkylphenyl sulfonates |
| WO2013002786A1 (en) | 2011-06-29 | 2013-01-03 | Solae | Baked food compositions comprising soy whey proteins that have been isolated from processing streams |
| WO2013043852A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Easy-rinse detergent compositions comprising isoprenoid-based surfactants |
| WO2013043857A1 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Detergent compositions comprising sustainable surfactant systems comprising isoprenoid-derived surfactants |
| WO2013043803A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Detergent compositions comprising specific blend ratios of isoprenoid-based surfactants |
| WO2013043855A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | High suds detergent compositions comprising isoprenoid-based surfactants |
| WO2013043805A1 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Detergent compositions comprising primary surfactant systems comprising highly branched surfactants especially isoprenoid - based surfactants |
| US8455423B2 (en) | 2005-05-27 | 2013-06-04 | Conopco, Inc. | Process of bleaching |
| FR2985273A1 (en) | 2012-01-04 | 2013-07-05 | Procter & Gamble | FIBROUS STRUCTURES CONTAINING ASSETS AND HAVING MULTIPLE REGIONS |
| WO2014018309A1 (en) | 2012-07-26 | 2014-01-30 | The Procter & Gamble Company | Low ph liquid cleaning compositions with enzymes |
| US8658590B2 (en) | 2006-07-27 | 2014-02-25 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| WO2014160820A1 (en) | 2013-03-28 | 2014-10-02 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| US8945671B2 (en) | 2007-12-19 | 2015-02-03 | Evonik Treibacher Gmbh | Method for producing encapsulated sodium percarbonate particles |
| US8993504B2 (en) | 2007-12-19 | 2015-03-31 | Lion Corporation | Oxidation catalyst for bleaching, and bleaching composition using the same |
| FR3014456A1 (en) | 2013-12-09 | 2015-06-12 | Procter & Gamble | |
| WO2015148361A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| WO2015148360A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| WO2015187757A1 (en) | 2014-06-06 | 2015-12-10 | The Procter & Gamble Company | Detergent composition comprising polyalkyleneimine polymers |
| EP2966161A1 (en) | 2014-07-08 | 2016-01-13 | Dalli-Werke GmbH & Co. KG | Enzyme-bleach catalyst cogranulate suitable for detergent compositions |
| EP3075832A1 (en) | 2015-03-30 | 2016-10-05 | Dalli-Werke GmbH & Co. KG | Manganese-amino acid compounds in cleaning compositions |
| WO2016161253A1 (en) | 2015-04-03 | 2016-10-06 | Ecolab Usa Inc. | Enhanced peroxygen stability using anionic surfactant in taed-containing peroxygen solid |
| WO2016161249A1 (en) | 2015-04-03 | 2016-10-06 | Ecolab Usa Inc. | Enhanced peroxygen stability in multi-dispense taed-containing peroxygen solid |
| US9469666B2 (en) | 2010-03-03 | 2016-10-18 | Catexel Limited | Preparation of bleaching catalysts |
| US9624119B2 (en) | 2014-06-13 | 2017-04-18 | Ecolab Usa Inc. | Enhanced catalyst stability in activated peroxygen and/or alkaline detergent formulations |
| WO2017066337A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017066334A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017065979A1 (en) | 2015-10-13 | 2017-04-20 | The Procter & Gamble Company | Laundry care compositions comprising whitening agents for cellulosic substrates |
| WO2017066343A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017065978A1 (en) | 2015-10-13 | 2017-04-20 | The Procter & Gamble Company | Laundry care compositions comprising whitening agents for cellulosic substrates |
| WO2017065977A1 (en) | 2015-10-13 | 2017-04-20 | The Procter & Gamble Company | Laundry care compositions comprising whitening agents for cellulosic substrates |
| WO2017112016A1 (en) | 2015-12-22 | 2017-06-29 | Milliken & Company | Occult particles for use in granular laundry care compositions |
| EP3190168A1 (en) | 2016-01-06 | 2017-07-12 | Dalli-Werke GmbH & Co. KG. | Coated bleach catalyst |
| US9856439B2 (en) | 2010-11-12 | 2018-01-02 | The Procter & Gamble Company | Thiophene azo dyes and laundry care compositions containing the same |
| WO2018140454A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles and product-shipping assemblies for containing the same |
| WO2018140432A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| WO2018140431A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| WO2018140472A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| EP3369845A1 (en) | 2012-01-04 | 2018-09-05 | The Procter & Gamble Company | Active containing fibrous structures with multiple regions having differing densities |
| US10144005B2 (en) | 2011-09-08 | 2018-12-04 | Richard William Kemp | Catalysts |
| US10196592B2 (en) | 2014-06-13 | 2019-02-05 | Ecolab Usa Inc. | Enhanced catalyst stability for alkaline detergent formulations |
| US10260025B2 (en) | 2008-02-11 | 2019-04-16 | Ecolab Usa Inc. | Use of activator complexes to enhance lower temperature cleaning in alkaline peroxide cleaning systems |
| WO2019182856A1 (en) | 2018-03-19 | 2019-09-26 | Ecolab Usa Inc. | Liquid detergent compositions containing bleach catalyst |
| WO2019241629A1 (en) | 2018-06-15 | 2019-12-19 | Ecolab Usa Inc. | Enhanced peroxygen stability using fatty acid in bleach activating agent containing peroxygen solid |
| US10526566B2 (en) | 2007-01-19 | 2020-01-07 | The Procter & Gamble Company | Whitening agents for cellulosic substrates |
| WO2020081297A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081293A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081296A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Laundry care compositions comprising polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081300A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Process for controlling odor on a textile substrate and polyethyleneimine compounds containing n-halamine |
| WO2020081301A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081294A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081299A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Articles comprising a textile substrate and polyethyleneimine compounds containing n-halamine |
| WO2020123889A1 (en) | 2018-12-14 | 2020-06-18 | The Procter & Gamble Company | Foaming fibrous structures comprising particles and methods for making same |
| EP3719192A1 (en) | 2012-01-04 | 2020-10-07 | The Procter & Gamble Company | Fibrous structures comprising particles and methods for making same |
| WO2021026556A1 (en) | 2019-08-02 | 2021-02-11 | The Procter & Gamble Company | Foaming compositions for producing a stable foam and methods for making same |
| WO2021097004A1 (en) | 2019-11-15 | 2021-05-20 | The Procter & Gamble Company | Graphic-containing soluble articles and methods for making same |
| US11021681B2 (en) | 2015-05-07 | 2021-06-01 | Novozymes A/S | Manganese bleach catalyst granules for use in dishwash detergents |
| WO2021178098A1 (en) | 2020-03-02 | 2021-09-10 | Milliken & Company | Composition comprising hueing agent |
| WO2021178100A1 (en) | 2020-03-02 | 2021-09-10 | Milliken & Company | Composition comprising hueing agent |
| WO2021178099A1 (en) | 2020-03-02 | 2021-09-10 | Milliken & Company | Composition comprising hueing agent |
| WO2022056204A1 (en) | 2020-09-14 | 2022-03-17 | Milliken & Company | Oxidative hair cream composition containing thiophene azo colorant |
| WO2022056205A1 (en) | 2020-09-14 | 2022-03-17 | Milliken & Company | Hair care composition containing polymeric colorant |
| WO2022056203A1 (en) | 2020-09-14 | 2022-03-17 | Milliken & Company | Oxidative hair cream composition containing polymeric colorant |
| WO2022197295A1 (en) | 2021-03-17 | 2022-09-22 | Milliken & Company | Polymeric colorants with reduced staining |
| WO2022251838A1 (en) | 2021-05-28 | 2022-12-01 | The Procter & Gamble Company | Natural polymer-based fibrous elements comprising a surfactant and methods for making same |
| US11970821B2 (en) | 2023-07-26 | 2024-04-30 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR100555040B1 (en) * | 1998-12-29 | 2006-05-16 | 주식회사 엘지생활건강 | Tris (2-pyridylmethyl) amine manganese complex, bleach and bleach composition containing the same |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0443651A2 (en) * | 1990-02-19 | 1991-08-28 | Unilever N.V. | Bleach activation |
| EP0458398B1 (en) * | 1990-05-21 | 1997-03-26 | Unilever N.V. | Bleach activation |
-
1992
- 1992-11-24 EP EP92310719A patent/EP0544490A1/en not_active Withdrawn
- 1992-11-24 CA CA002083661A patent/CA2083661A1/en not_active Abandoned
- 1992-11-25 CN CN92111815A patent/CN1031647C/en not_active Expired - Fee Related
- 1992-11-25 BR BR9204549A patent/BR9204549A/en not_active Application Discontinuation
- 1992-11-25 MX MX9206779A patent/MX9206779A/en not_active Application Discontinuation
- 1992-11-25 AU AU29620/92A patent/AU661522B2/en not_active Ceased
- 1992-11-26 TR TR01152/92A patent/TR27075A/en unknown
- 1992-11-26 JP JP4317228A patent/JPH0768543B2/en not_active Expired - Lifetime
- 1992-11-26 KR KR1019920022431A patent/KR960000205B1/en not_active IP Right Cessation
- 1992-11-26 ZA ZA928188A patent/ZA928188B/en unknown
- 1992-12-04 TW TW081109741A patent/TW234144B/zh active
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0443651A2 (en) * | 1990-02-19 | 1991-08-28 | Unilever N.V. | Bleach activation |
| EP0458398B1 (en) * | 1990-05-21 | 1997-03-26 | Unilever N.V. | Bleach activation |
| EP0458397B1 (en) * | 1990-05-21 | 1997-03-26 | Unilever N.V. | Bleach activation |
Cited By (210)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994019445A1 (en) * | 1993-02-22 | 1994-09-01 | Unilever N.V. | Machine dishwashing composition |
| WO1994021775A1 (en) * | 1993-03-18 | 1994-09-29 | Unilever Plc | Detergent compositions |
| US5409627A (en) * | 1993-03-18 | 1995-04-25 | Lever Brothers Company, Division Of Conopco, Inc. | Particulate bleaching detergent compositions containing zeolite map and a stable bleach catalyst |
| US5622646A (en) * | 1994-04-07 | 1997-04-22 | The Procter & Gamble Company | Bleach compositions comprising metal-containing bleach catalysts and antioxidants |
| US5686014A (en) * | 1994-04-07 | 1997-11-11 | The Procter & Gamble Company | Bleach compositions comprising manganese-containing bleach catalysts |
| US5560748A (en) * | 1994-06-10 | 1996-10-01 | The Procter & Gamble Company | Detergent compositions comprising large pore size redox catalysts |
| US5580485A (en) * | 1994-06-13 | 1996-12-03 | Lever Brothers Company, Division Of Conopco, Inc. | Bleach activation |
| EP0693550A2 (en) | 1994-07-21 | 1996-01-24 | Ciba-Geigy Ag | Fabric bleaching composition |
| GB2291440A (en) * | 1994-07-21 | 1996-01-24 | Ciba Geigy Ag | Activated fabric bleaching composition |
| GB2291440B (en) * | 1994-07-21 | 1999-02-24 | Ciba Geigy Ag | Fabric bleaching composition |
| US5965506A (en) * | 1994-07-21 | 1999-10-12 | Ciba Specialty Chemicals Corporation | Fabric bleaching composition |
| WO1996006154A1 (en) * | 1994-08-19 | 1996-02-29 | Unilever N.V. | Detergent bleach composition |
| WO1996006157A1 (en) * | 1994-08-19 | 1996-02-29 | Unilever N.V. | Detergent bleach composition |
| EP0699745A2 (en) | 1994-08-31 | 1996-03-06 | The Procter & Gamble Company | Automatic dishwashing compositions comprising quaternary ammonium compounds bleach activators and quaternary ammonium |
| US5798326A (en) * | 1995-02-02 | 1998-08-25 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt III catalysts |
| US5968881A (en) * | 1995-02-02 | 1999-10-19 | The Procter & Gamble Company | Phosphate built automatic dishwashing compositions comprising catalysts |
| US6020294A (en) * | 1995-02-02 | 2000-02-01 | Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt chelated catalysts |
| US6119705A (en) * | 1995-02-02 | 2000-09-19 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt chelated catalysts |
| WO1996025478A1 (en) | 1995-02-15 | 1996-08-22 | The Procter & Gamble Company | Detergent composition comprising an amylase enzyme and a nonionic polysaccharide ether |
| US5705464A (en) * | 1995-06-16 | 1998-01-06 | The Procter & Gamble Company | Automatic dishwashing compositions comprising cobalt catalysts |
| US5703030A (en) * | 1995-06-16 | 1997-12-30 | The Procter & Gamble Company | Bleach compositions comprising cobalt catalysts |
| EP0751142A2 (en) * | 1995-06-30 | 1997-01-02 | Hoechst Aktiengesellschaft | Chiral manganese-triazanonane complexes and process for their preparation |
| EP0751142A3 (en) * | 1995-06-30 | 1998-08-19 | Hoechst Aktiengesellschaft | Chiral manganese-triazanonane complexes and process for their preparation |
| US6187739B1 (en) | 1995-09-21 | 2001-02-13 | Henkel Kommanditgesellschaft Auf Aktien | Paste-form washing and cleaning agents |
| US5703034A (en) * | 1995-10-30 | 1997-12-30 | The Procter & Gamble Company | Bleach catalyst particles |
| US6528469B2 (en) | 1995-11-18 | 2003-03-04 | Ciba Specialty Chemicals Corporation | Fabric bleaching composition |
| GB2307250A (en) * | 1995-11-18 | 1997-05-21 | Ciba Geigy Ag | Fabric bleaching composition |
| US6409770B1 (en) | 1995-12-08 | 2002-06-25 | Henkel Kommanditgesellschaft Auf Aktien | Bleaching and washing agents with enzyme bleaching system |
| US5939373A (en) * | 1995-12-20 | 1999-08-17 | The Procter & Gamble Company | Phosphate-built automatic dishwashing composition comprising catalysts |
| US6153576A (en) * | 1996-02-16 | 2000-11-28 | Henkel Kommanditgesellschaft Auf Aktien | Transition-metal complexes used as activators for peroxy compounds |
| WO1997034985A1 (en) * | 1996-03-16 | 1997-09-25 | The Procter & Gamble Company | Bleaching composition comprising metal catalyst, cellulase enzyme, and oxygen bleach |
| US6235695B1 (en) * | 1996-04-01 | 2001-05-22 | Henkel Kommanditgesellschaft Auf Aktien | Cleaning agent with oligoammine activator complexes for peroxide compounds |
| US6200946B1 (en) | 1996-04-01 | 2001-03-13 | Henkel Kommanditgesellschaft Auf Aktien | Transition metal ammine complexes as activators for peroxide compounds |
| WO1997042282A1 (en) | 1996-05-03 | 1997-11-13 | The Procter & Gamble Company | Detergent compositions comprising polyamine polymers with improved soil dispersancy |
| WO1997044520A1 (en) * | 1996-05-20 | 1997-11-27 | Rudolf Patt | A method for the delignification of fibrous material and use of a catalyst |
| WO1997048787A1 (en) * | 1996-06-19 | 1997-12-24 | Unilever N.V. | Bleach activation |
| US6022490A (en) * | 1996-06-19 | 2000-02-08 | Lever Brothers Company | Bleach activation |
| US6107528A (en) * | 1996-06-21 | 2000-08-22 | Regents Of The University Of Minnesota | Iron complexes for bleach activation and stereospecific oxidation |
| US5850086A (en) * | 1996-06-21 | 1998-12-15 | Regents Of The University Of Minnesota | Iron complexes for bleach activation and stereospecific oxidation |
| US6248708B1 (en) | 1996-09-05 | 2001-06-19 | Henkel-Ecolab Gmbh & Co. Ohg | Paste-form detergent containing a mixture of ethoxylated alcohols |
| US6225274B1 (en) | 1996-11-29 | 2001-05-01 | Henkel Kommandigesellschaft Auf Aktien | Acetonitrile derivatives as bleaching activators in detergents |
| US6329333B1 (en) | 1997-01-30 | 2001-12-11 | Henkel-Ecolab Gmbh & Co. Ohg | Pastelike detergent and cleaning agent |
| US6602441B1 (en) | 1997-04-05 | 2003-08-05 | Clariant Gmbh | Bleaching-active metal complexes |
| US6139769A (en) * | 1997-04-05 | 2000-10-31 | Clariant Gmbh | Bleaching-active metal complexes |
| US5998645A (en) * | 1997-05-07 | 1999-12-07 | Clariant Gmbh | Bleaching-active metal complexes |
| US5969171A (en) * | 1997-07-01 | 1999-10-19 | Clariant Gmbh | Metal complexes as bleach activators |
| US6541233B1 (en) | 1997-07-30 | 2003-04-01 | Henkel Kommanditgesellschaft Auf Aktien | β-glucanase from a bacillus |
| US6703357B1 (en) | 1997-07-30 | 2004-03-09 | Henkel Kommanditgesellschaft Auf Aktien | Cleaning agent for hard surfaces, containing glucanase |
| US6417152B1 (en) | 1997-07-30 | 2002-07-09 | Henkel Kommanditgesellshaft Auf Aktien | Detergent containing glucanase |
| WO1999020726A1 (en) | 1997-10-23 | 1999-04-29 | The Procter & Gamble Company | Bleaching compositions comprising multiply-substituted protease variants |
| US6410500B1 (en) | 1997-12-30 | 2002-06-25 | Henkel Kommanditgesellschaft Auf Aktien | Moulded body dishwasher detergents with soil release polymers |
| US6992056B1 (en) | 1997-12-30 | 2006-01-31 | Henkel Kgaa | Process for preparing detergent tablets having two or more regions |
| US6221820B1 (en) | 1997-12-31 | 2001-04-24 | Henkel Kommanditgesellschaft Auf Aktien | Granular component containing alkylaminotriazole for use in machine dishwashing detergents |
| US6462006B1 (en) | 1998-04-30 | 2002-10-08 | Henkel Kommanditgesellschaft Auf Aktien | Solid machine dishwashing detergent with phosphate and crystalline lamellar silicates |
| US6841614B1 (en) | 1998-10-29 | 2005-01-11 | Henkel Kommanditgesellschaft Auf Aktien | Polymer granules produced by fluidized bed granulation |
| US6221824B1 (en) | 1999-02-25 | 2001-04-24 | Henkel Kommanditgesellschaft Auf Aktien | Process for the production of compounded acetonitrile derivatives |
| US6391838B1 (en) | 1999-03-31 | 2002-05-21 | Henkel Kommanditgesellschaft Auf Aktien | Detergents containing enzymes and bleach activators |
| EP1083173A3 (en) * | 1999-09-10 | 2003-03-19 | Clariant GmbH | Metal complexes with bleaching activity |
| EP1083173A2 (en) * | 1999-09-10 | 2001-03-14 | Clariant GmbH | Metal complexes with bleaching activity |
| US6951838B1 (en) | 1999-09-15 | 2005-10-04 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US6686327B1 (en) | 1999-10-09 | 2004-02-03 | Cognis Deutschland Gmbh & Co. Kg | Shaped bodies with improved solubility in water |
| US6610752B1 (en) | 1999-10-09 | 2003-08-26 | Cognis Deutschland Gmbh | Defoamer granules and processes for producing the same |
| US6696401B1 (en) | 1999-11-09 | 2004-02-24 | The Procter & Gamble Company | Laundry detergent compositions comprising zwitterionic polyamines |
| US7199096B1 (en) | 1999-11-09 | 2007-04-03 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US6812198B2 (en) | 1999-11-09 | 2004-11-02 | The Procter & Gamble Company | Laundry detergent compositions comprising hydrophobically modified polyamines |
| US6846791B1 (en) | 1999-11-09 | 2005-01-25 | The Procter & Gamble Company | Laundry detergent compositions comprising hydrophobically modified polyamines |
| US6977239B1 (en) | 1999-11-25 | 2005-12-20 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US7186678B2 (en) | 1999-12-24 | 2007-03-06 | Cognis Deutschland Gmbh & Co. Kg | Tenside granules with improved disintegration rate |
| US7087570B2 (en) | 1999-12-24 | 2006-08-08 | Cognis Deutschland Gmbh & Co. Kg | Detergent tablets |
| US6881359B2 (en) | 2000-01-26 | 2005-04-19 | Cognis Deutschland Gmbh & Co. Kg | Processes for the preparation of low dust, limited particle size distribution, surfactant granules |
| US6756351B2 (en) | 2000-04-18 | 2004-06-29 | Cognis Deutschland Gmbh & Co. Kg | Detergents and cleaning agents |
| US6936581B2 (en) | 2000-04-19 | 2005-08-30 | Cognis Deutschland Gmbh & Co. Kg | Processes for preparing anhydrous detergent granules |
| US7091168B2 (en) | 2000-06-29 | 2006-08-15 | Cognis Deutschland Gmbh & Co. Kg | Liquid detergents |
| US6620209B2 (en) | 2000-09-08 | 2003-09-16 | Cognis Deutschland Gmbh & Co. Kg | Laundry detergent compositions |
| US6616705B2 (en) | 2000-09-08 | 2003-09-09 | Cognis Deutschland Gmbh & Co. Kg | Laundry detergent compositions |
| US6723135B2 (en) | 2000-09-19 | 2004-04-20 | Cognis Deutschland Gmbh & Co. Kg | Laundry detergents and cleaning products based on alkyl and/or alkenyl oligoglycosides and fatty alcohols |
| EP1978081A2 (en) | 2000-10-27 | 2008-10-08 | The Procter and Gamble Company | Stabilized liquid compositions |
| US6649085B2 (en) | 2000-11-25 | 2003-11-18 | Clariant Gmbh | Cyclic sugar ketones as catalysts for peroxygen compounds |
| US6746996B2 (en) | 2001-01-19 | 2004-06-08 | Clariant Gmbh | Use of transition metal complexes having oxime ligands as bleach catalysts |
| US7335629B2 (en) | 2001-12-21 | 2008-02-26 | Henkel Kommanditgesellschaft Auf Aktien | Support-fixed bleaching catalyst complex compounds suitable as catalysts for peroxygen compounds |
| US6897193B2 (en) | 2001-12-22 | 2005-05-24 | Cognis Deutschland Gmbh & Co., Kg | Hydroxy mixed ethers and polymers in the form of solid preparations as a starting compound for laundry detergents, dishwashing detergents and cleaning compositions |
| US6875734B2 (en) | 2003-02-03 | 2005-04-05 | Clariant Gmbh | Use of transition metal complexes as bleach catalysts |
| WO2004069979A2 (en) | 2003-02-03 | 2004-08-19 | Unilever Plc | Laundry cleansing and conditioning compositions |
| US8722608B2 (en) | 2005-05-27 | 2014-05-13 | Conopco, Inc. | Process of bleaching |
| US8455423B2 (en) | 2005-05-27 | 2013-06-04 | Conopco, Inc. | Process of bleaching |
| US7972386B2 (en) | 2005-10-12 | 2011-07-05 | Conopco, Inc. | Bleaching of substrates |
| US7956027B2 (en) | 2006-07-27 | 2011-06-07 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US8658590B2 (en) | 2006-07-27 | 2014-02-25 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US8153576B2 (en) | 2006-07-27 | 2012-04-10 | Evonik Degussa Gmbh | Coated sodium percarbonate particles |
| US7976582B2 (en) | 2007-01-16 | 2011-07-12 | Conopco, Inc. | Bleaching of substrates |
| US11198838B2 (en) | 2007-01-19 | 2021-12-14 | The Procter & Gamble Company | Whitening agents for cellulosic substrates |
| US10526566B2 (en) | 2007-01-19 | 2020-01-07 | The Procter & Gamble Company | Whitening agents for cellulosic substrates |
| US11946025B2 (en) | 2007-01-19 | 2024-04-02 | The Procter & Gamble Company | Whitening agents for cellulosic substrates |
| DE102007003885A1 (en) | 2007-01-19 | 2008-07-24 | Lanxess Deutschland Gmbh | Use of a builder system comprising alkali metal tripolyphosphate and iminodisuccinic acid to produce automatic dishwasher formulations |
| US8262804B2 (en) | 2007-10-12 | 2012-09-11 | Basf Se | Dishwasher detergent formulations comprising a mixture of hydrophobically modified polycarboxylates and hydrophilically modified polycarboxylates |
| US8993504B2 (en) | 2007-12-19 | 2015-03-31 | Lion Corporation | Oxidation catalyst for bleaching, and bleaching composition using the same |
| US8945671B2 (en) | 2007-12-19 | 2015-02-03 | Evonik Treibacher Gmbh | Method for producing encapsulated sodium percarbonate particles |
| DE102008000029A1 (en) | 2008-01-10 | 2009-07-16 | Lanxess Deutschland Gmbh | Use of phosphate reduced building system comprising alkali tripolyphosphate and imino disuccinic acid, for manufacturing formulations e.g. for the automatic or mechanical dish cleaning and crockery cleaning machines on ships |
| US10260025B2 (en) | 2008-02-11 | 2019-04-16 | Ecolab Usa Inc. | Use of activator complexes to enhance lower temperature cleaning in alkaline peroxide cleaning systems |
| DE102008024800A1 (en) | 2008-05-23 | 2009-11-26 | Henkel Ag & Co. Kgaa | Method for washing textiles in the presence of a peroxygenated bleaching agent and a bleach boosting transition metal complex |
| WO2010010334A1 (en) | 2008-07-23 | 2010-01-28 | Reckitt Benckiser N.V. | Container |
| DE102008045297A1 (en) | 2008-09-02 | 2010-03-04 | Friedrich-Alexander-Universität Erlangen-Nürnberg | Method for washing textiles in the presence of a peroxygenated bleaching agent and a bleach boosting transition metal complex |
| WO2011005730A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | A catalytic laundry detergent composition comprising relatively low levels of water-soluble electrolyte |
| WO2011005623A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | Laundry detergent composition comprising low level of bleach |
| WO2011005804A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | Method of laundering fabric using a liquid laundry detergent composition |
| WO2011005913A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | A catalytic laundry detergent composition comprising relatively low levels of water-soluble electrolyte |
| WO2011005910A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | Method of laundering fabric using a compacted laundry detergent composition |
| EP2292725A1 (en) | 2009-08-13 | 2011-03-09 | The Procter & Gamble Company | Method of laundering fabrics at low temperature |
| WO2011025615A2 (en) | 2009-08-13 | 2011-03-03 | The Procter & Gamble Company | Method of laundering fabrics at low temperature |
| WO2011088089A1 (en) | 2010-01-12 | 2011-07-21 | The Procter & Gamble Company | Intermediates and surfactants useful in household cleaning and personal care compositions, and methods of making the same |
| US8933131B2 (en) | 2010-01-12 | 2015-01-13 | The Procter & Gamble Company | Intermediates and surfactants useful in household cleaning and personal care compositions, and methods of making the same |
| US9469666B2 (en) | 2010-03-03 | 2016-10-18 | Catexel Limited | Preparation of bleaching catalysts |
| EP3020768A1 (en) | 2010-05-18 | 2016-05-18 | Milliken & Company | Optical brighteners and compositions comprising the same |
| WO2011146604A2 (en) | 2010-05-18 | 2011-11-24 | Milliken & Company | Optical brighteners and compositions comprising the same |
| WO2011146602A2 (en) | 2010-05-18 | 2011-11-24 | Milliken & Company | Optical brighteners and compositions comprising the same |
| WO2011149871A1 (en) | 2010-05-28 | 2011-12-01 | Milliken & Company | Colored speckles having delayed release properties |
| WO2012003351A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Web material and method for making same |
| WO2012003316A1 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Process for making films from nonwoven webs |
| WO2012003319A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Filaments comprising an active agent nonwoven webs and methods for making same |
| WO2012003300A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Filaments comprising a non-perfume active agent nonwoven webs and methods for making same |
| WO2012003360A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Detergent product and method for making same |
| WO2012003367A2 (en) | 2010-07-02 | 2012-01-05 | The Procter & Gamble Company | Method for delivering an active agent |
| EP3533908A1 (en) | 2010-07-02 | 2019-09-04 | The Procter & Gamble Company | Nonwoven web comprising one or more active agents |
| WO2012009525A2 (en) | 2010-07-15 | 2012-01-19 | The Procter & Gamble Company | Compositions comprising a near terminal-branched compound and methods of making the same |
| WO2012009660A2 (en) | 2010-07-15 | 2012-01-19 | The Procter & Gamble Company | Detergent compositions comprising microbially produced fatty alcohols and derivatives thereof |
| US10435651B2 (en) | 2010-11-12 | 2019-10-08 | The Procter & Gamble Company | Thiophene azo dyes and laundry care compositions containing the same |
| US10655091B2 (en) | 2010-11-12 | 2020-05-19 | The Procter & Gamble Company | Thiophene azo dyes and laundry care compositions containing the same |
| US9856439B2 (en) | 2010-11-12 | 2018-01-02 | The Procter & Gamble Company | Thiophene azo dyes and laundry care compositions containing the same |
| WO2012138423A1 (en) | 2011-02-17 | 2012-10-11 | The Procter & Gamble Company | Compositions comprising mixtures of c10-c13 alkylphenyl sulfonates |
| WO2012112828A1 (en) | 2011-02-17 | 2012-08-23 | The Procter & Gamble Company | Bio-based linear alkylphenyl sulfonates |
| US9193937B2 (en) | 2011-02-17 | 2015-11-24 | The Procter & Gamble Company | Mixtures of C10-C13 alkylphenyl sulfonates |
| WO2012116021A1 (en) | 2011-02-25 | 2012-08-30 | Milliken & Company | Capsules and compositions comprising the same |
| WO2012116014A1 (en) | 2011-02-25 | 2012-08-30 | Milliken & Company | Capsules and compositions comprising the same |
| WO2012116023A1 (en) | 2011-02-25 | 2012-08-30 | Milliken & Company | Capsules and compositions comprising the same |
| WO2013002786A1 (en) | 2011-06-29 | 2013-01-03 | Solae | Baked food compositions comprising soy whey proteins that have been isolated from processing streams |
| US10144005B2 (en) | 2011-09-08 | 2018-12-04 | Richard William Kemp | Catalysts |
| WO2013043805A1 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Detergent compositions comprising primary surfactant systems comprising highly branched surfactants especially isoprenoid - based surfactants |
| WO2013043855A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | High suds detergent compositions comprising isoprenoid-based surfactants |
| WO2013043803A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Detergent compositions comprising specific blend ratios of isoprenoid-based surfactants |
| WO2013043857A1 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Detergent compositions comprising sustainable surfactant systems comprising isoprenoid-derived surfactants |
| WO2013043852A2 (en) | 2011-09-20 | 2013-03-28 | The Procter & Gamble Company | Easy-rinse detergent compositions comprising isoprenoid-based surfactants |
| EP3719192A1 (en) | 2012-01-04 | 2020-10-07 | The Procter & Gamble Company | Fibrous structures comprising particles and methods for making same |
| EP3369845A1 (en) | 2012-01-04 | 2018-09-05 | The Procter & Gamble Company | Active containing fibrous structures with multiple regions having differing densities |
| FR2985273A1 (en) | 2012-01-04 | 2013-07-05 | Procter & Gamble | FIBROUS STRUCTURES CONTAINING ASSETS AND HAVING MULTIPLE REGIONS |
| WO2014018309A1 (en) | 2012-07-26 | 2014-01-30 | The Procter & Gamble Company | Low ph liquid cleaning compositions with enzymes |
| WO2014160821A1 (en) | 2013-03-28 | 2014-10-02 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine, a soil release polymer, and a carboxymethylcellulose |
| WO2014160820A1 (en) | 2013-03-28 | 2014-10-02 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| EP3572572A1 (en) | 2013-12-09 | 2019-11-27 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| US11795622B2 (en) | 2013-12-09 | 2023-10-24 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| EP3805350A1 (en) | 2013-12-09 | 2021-04-14 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| US11624156B2 (en) | 2013-12-09 | 2023-04-11 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| WO2015088826A1 (en) | 2013-12-09 | 2015-06-18 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| FR3014456A1 (en) | 2013-12-09 | 2015-06-12 | Procter & Gamble | |
| US10494767B2 (en) | 2013-12-09 | 2019-12-03 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| DE112014005598B4 (en) | 2013-12-09 | 2022-06-09 | The Procter & Gamble Company | Fibrous structures including an active substance and with graphics printed on it |
| US11293144B2 (en) | 2013-12-09 | 2022-04-05 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
| WO2015148360A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| WO2015148361A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| WO2015187757A1 (en) | 2014-06-06 | 2015-12-10 | The Procter & Gamble Company | Detergent composition comprising polyalkyleneimine polymers |
| US9624119B2 (en) | 2014-06-13 | 2017-04-18 | Ecolab Usa Inc. | Enhanced catalyst stability in activated peroxygen and/or alkaline detergent formulations |
| US10196592B2 (en) | 2014-06-13 | 2019-02-05 | Ecolab Usa Inc. | Enhanced catalyst stability for alkaline detergent formulations |
| EP2966161A1 (en) | 2014-07-08 | 2016-01-13 | Dalli-Werke GmbH & Co. KG | Enzyme-bleach catalyst cogranulate suitable for detergent compositions |
| EP3075832A1 (en) | 2015-03-30 | 2016-10-05 | Dalli-Werke GmbH & Co. KG | Manganese-amino acid compounds in cleaning compositions |
| WO2016161253A1 (en) | 2015-04-03 | 2016-10-06 | Ecolab Usa Inc. | Enhanced peroxygen stability using anionic surfactant in taed-containing peroxygen solid |
| WO2016161249A1 (en) | 2015-04-03 | 2016-10-06 | Ecolab Usa Inc. | Enhanced peroxygen stability in multi-dispense taed-containing peroxygen solid |
| US11021681B2 (en) | 2015-05-07 | 2021-06-01 | Novozymes A/S | Manganese bleach catalyst granules for use in dishwash detergents |
| WO2017066343A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017065978A1 (en) | 2015-10-13 | 2017-04-20 | The Procter & Gamble Company | Laundry care compositions comprising whitening agents for cellulosic substrates |
| WO2017065979A1 (en) | 2015-10-13 | 2017-04-20 | The Procter & Gamble Company | Laundry care compositions comprising whitening agents for cellulosic substrates |
| WO2017066334A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017066413A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017066337A1 (en) | 2015-10-13 | 2017-04-20 | Milliken & Company | Novel whitening agents for cellulosic substrates |
| WO2017065977A1 (en) | 2015-10-13 | 2017-04-20 | The Procter & Gamble Company | Laundry care compositions comprising whitening agents for cellulosic substrates |
| WO2017112016A1 (en) | 2015-12-22 | 2017-06-29 | Milliken & Company | Occult particles for use in granular laundry care compositions |
| EP3190168A1 (en) | 2016-01-06 | 2017-07-12 | Dalli-Werke GmbH & Co. KG. | Coated bleach catalyst |
| EP3991962A1 (en) | 2017-01-27 | 2022-05-04 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| WO2018140431A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| DE112018000558T5 (en) | 2017-01-27 | 2019-10-10 | The Procter & Gamble Company | Active substance-containing articles which have acceptable consumer properties acceptable to the consumer |
| WO2018140454A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles and product-shipping assemblies for containing the same |
| WO2018140432A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| EP4197598A1 (en) | 2017-01-27 | 2023-06-21 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| EP3915643A1 (en) | 2017-01-27 | 2021-12-01 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| WO2018140472A1 (en) | 2017-01-27 | 2018-08-02 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| DE112018000568T5 (en) | 2017-01-27 | 2019-10-17 | The Procter & Gamble Company | Active substance-containing articles and product shipping arrangements for enclosing the same |
| DE112018000563T5 (en) | 2017-01-27 | 2019-10-24 | The Procter & Gamble Company | Active substance-containing articles which have acceptable consumer properties acceptable to the consumer |
| EP3881900A1 (en) | 2017-01-27 | 2021-09-22 | The Procter & Gamble Company | Active agent-containing articles that exhibit consumer acceptable article in-use properties |
| DE112018000565T5 (en) | 2017-01-27 | 2019-10-24 | The Procter & Gamble Company | Active substance-containing articles which have acceptable consumer properties acceptable to the consumer |
| US11225631B2 (en) | 2018-03-19 | 2022-01-18 | Ecolab Usa Inc. | Acidic liquid detergent compositions containing bleach catalyst and free of anionic surfactant |
| WO2019182856A1 (en) | 2018-03-19 | 2019-09-26 | Ecolab Usa Inc. | Liquid detergent compositions containing bleach catalyst |
| EP4349951A2 (en) | 2018-06-15 | 2024-04-10 | Ecolab USA Inc. | Enhanced peroxygen stability using fatty acid in bleach activating agent containing peroxygen solid |
| WO2019241629A1 (en) | 2018-06-15 | 2019-12-19 | Ecolab Usa Inc. | Enhanced peroxygen stability using fatty acid in bleach activating agent containing peroxygen solid |
| WO2020081296A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Laundry care compositions comprising polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081293A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081297A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081301A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081300A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Process for controlling odor on a textile substrate and polyethyleneimine compounds containing n-halamine |
| WO2020081294A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Polyethyleneimine compounds containing n-halamine and derivatives thereof |
| WO2020081299A1 (en) | 2018-10-18 | 2020-04-23 | Milliken & Company | Articles comprising a textile substrate and polyethyleneimine compounds containing n-halamine |
| WO2020123889A1 (en) | 2018-12-14 | 2020-06-18 | The Procter & Gamble Company | Foaming fibrous structures comprising particles and methods for making same |
| WO2021026556A1 (en) | 2019-08-02 | 2021-02-11 | The Procter & Gamble Company | Foaming compositions for producing a stable foam and methods for making same |
| WO2021097004A1 (en) | 2019-11-15 | 2021-05-20 | The Procter & Gamble Company | Graphic-containing soluble articles and methods for making same |
| WO2021178099A1 (en) | 2020-03-02 | 2021-09-10 | Milliken & Company | Composition comprising hueing agent |
| WO2021178098A1 (en) | 2020-03-02 | 2021-09-10 | Milliken & Company | Composition comprising hueing agent |
| WO2021178100A1 (en) | 2020-03-02 | 2021-09-10 | Milliken & Company | Composition comprising hueing agent |
| WO2022056203A1 (en) | 2020-09-14 | 2022-03-17 | Milliken & Company | Oxidative hair cream composition containing polymeric colorant |
| WO2022056205A1 (en) | 2020-09-14 | 2022-03-17 | Milliken & Company | Hair care composition containing polymeric colorant |
| WO2022056204A1 (en) | 2020-09-14 | 2022-03-17 | Milliken & Company | Oxidative hair cream composition containing thiophene azo colorant |
| WO2022197295A1 (en) | 2021-03-17 | 2022-09-22 | Milliken & Company | Polymeric colorants with reduced staining |
| WO2022251838A1 (en) | 2021-05-28 | 2022-12-01 | The Procter & Gamble Company | Natural polymer-based fibrous elements comprising a surfactant and methods for making same |
| US11970821B2 (en) | 2023-07-26 | 2024-04-30 | The Procter & Gamble Company | Fibrous structures including an active agent and having a graphic printed thereon |
Also Published As
| Publication number | Publication date |
|---|---|
| MX9206779A (en) | 1993-05-01 |
| TW234144B (en) | 1994-11-11 |
| AU661522B2 (en) | 1995-07-27 |
| ZA928188B (en) | 1994-05-26 |
| JPH0641586A (en) | 1994-02-15 |
| BR9204549A (en) | 1993-06-01 |
| AU2962092A (en) | 1993-06-17 |
| TR27075A (en) | 1994-10-18 |
| JPH0768543B2 (en) | 1995-07-26 |
| CN1073714A (en) | 1993-06-30 |
| KR960000205B1 (en) | 1996-01-03 |
| KR930010171A (en) | 1993-06-22 |
| CN1031647C (en) | 1996-04-24 |
| CA2083661A1 (en) | 1993-05-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU661522B2 (en) | Detergent bleach compositions | |
| EP0549271B1 (en) | Bleach activation using a manganese compound and an organic ligand | |
| EP0392592B1 (en) | Bleach activation | |
| CA2083658C (en) | Manganese catalyst | |
| EP0458398B1 (en) | Bleach activation | |
| EP0549272A1 (en) | Bleach activation | |
| EP0408131B1 (en) | Bleach activation | |
| US6022490A (en) | Bleach activation | |
| EP0765381B1 (en) | Bleach activation | |
| WO1996006154A1 (en) | Detergent bleach composition | |
| ZA200103295B (en) | Bleach and oxidation catalyst | |
| EP0909809A2 (en) | Bleach activation | |
| WO1996006157A1 (en) | Detergent bleach composition | |
| US6432901B2 (en) | Bleach catalysts | |
| AU733805B2 (en) | Bleach activation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): CH DE ES FR GB IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19930529 |
|
| 17Q | First examination report despatched |
Effective date: 19960809 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION IS DEEMED TO BE WITHDRAWN |
|
| 18D | Application deemed to be withdrawn |
Effective date: 19961220 |