EP0512946B1 - Process for improving the fastness to light of leather - Google Patents
Process for improving the fastness to light of leather Download PDFInfo
- Publication number
- EP0512946B1 EP0512946B1 EP19920810292 EP92810292A EP0512946B1 EP 0512946 B1 EP0512946 B1 EP 0512946B1 EP 19920810292 EP19920810292 EP 19920810292 EP 92810292 A EP92810292 A EP 92810292A EP 0512946 B1 EP0512946 B1 EP 0512946B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- hydroxy
- hydrogen
- sulfo
- leather
- benzotriazole
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 *c1cc2n[n](-c(cc(*)c(*)c3*)c3O)nc2cc1* Chemical compound *c1cc2n[n](-c(cc(*)c(*)c3*)c3O)nc2cc1* 0.000 description 3
Classifications
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/44—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders
- D06P1/64—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders using compositions containing low-molecular-weight organic compounds without sulfate or sulfonate groups
- D06P1/642—Compounds containing nitrogen
- D06P1/6426—Heterocyclic compounds
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06P—DYEING OR PRINTING TEXTILES; DYEING LEATHER, FURS OR SOLID MACROMOLECULAR SUBSTANCES IN ANY FORM
- D06P1/00—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed
- D06P1/44—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders
- D06P1/64—General processes of dyeing or printing textiles, or general processes of dyeing leather, furs, or solid macromolecular substances in any form, classified according to the dyes, pigments, or auxiliary substances employed using insoluble pigments or auxiliary substances, e.g. binders using compositions containing low-molecular-weight organic compounds without sulfate or sulfonate groups
- D06P1/651—Compounds without nitrogen
- D06P1/65106—Oxygen-containing compounds
- D06P1/65112—Compounds containing aldehyde or ketone groups
Definitions
- the present invention relates to a method for improving the light fastness of colored and uncolored leather.
- leather dyeings generally have a lower light fastness than comparable dyeings on textile fiber materials, e.g. from B. Magerkurth, The Leather Manufacturer, January 1987, page 10-26: Identical dyes gave a lightfastness rating of 6-7 when dyed nylon, a lightfastness rating lowered by 1 grade on wool and a maximum of 4 on leather, each according to ISO -Blue scale measured.
- the carboxy and sulfor radicals can generally be used as a free acid or as a salt, e.g. as an alkali metal, alkaline earth metal, ammonium or amine salt.
- R2 or any other substituents are C1-C4alkoxy, they represent e.g. Methoxy, ethoxy, n- or iso-propoxy, or n-, iso-, sec.- or tert.-butoxy.
- R1 is C1-C12-alkoxy, in addition to the above-mentioned C1-C4-alkoxy groups e.g. straight or branched pentyloxy, hexyloxy, heptyloxy, octyloxy, nonyloxy, decyloxy, undecyloxy or dodecyloxy in question.
- C1-C4-alkoxy groups e.g. straight or branched pentyloxy, hexyloxy, heptyloxy, octyloxy, nonyloxy, decyloxy, undecyloxy or dodecyloxy in question.
- UV absorbers of the formula (1) are 2-hydroxy-4-methoxy-5-sulfobenzophenone and 2,2'-dihydroxy-4,4'-dimethoxy-5-sulfobenzophenone.
- R4 or any variables other than halogen are e.g. fluorine, bromine or preferably chlorine.
- R4 or any other variables for C1-C4-alkyl here are the meanings methyl, ethyl, n- or iso-propyl or n-, iso-, sec- or tert-butyl in question.
- R4 or R8 as C1-C8 alkoxycarbonyl is preferably methoxycarbonyl or ethoxycarbonyl.
- R4 preferably represents hydrogen, chlorine, methyl, methoxy, methoxycarbonyl, Ethoxycarbonyl, carboxy or sulfo and particularly preferably for hydrogen or sulfo.
- R5 is preferably hydrogen.
- R6 or any other substituents are C1-C12-alkyl, in addition to the aforementioned C1-C4-alkyl radicals e.g. a linear or branched pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl or dodecyl radical in question.
- C1-C4-alkyl radicals e.g. a linear or branched pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl or dodecyl radical in question.
- R6 or any variables other than C5-C6 cycloalkyl are e.g. cyclopentyl or preferably cyclohexyl.
- C7-C9-alalkyl R6 or any other variables are preferably phenylethyl or in particular benzyl.
- R6 are hydrogen, chlorine, C1-C6-alkyl, benzyl, phenylethyl or sulfo.
- R6 particularly preferably represents methyl, ethyl, n- or isopropyl, n-, iso-, sec- or tert-butyl or tert-amyl.
- R7 is preferably hydrogen, hydroxy or methoxy and particularly preferably hydrogen.
- R8 preferably represents hydrogen, chlorine, C1-C8-alkyl or sulfo, particularly preferably C1-C4-alkyl or sulfo and particularly preferably sulfo.
- Preferred UV absorbers correspond to the formula wherein R'4 is hydrogen, chlorine, methyl, methoxy, methoxycarbonyl, ethoxycarbonyl, carboxy or sulfo, R'6 is hydrogen, chlorine, C1-C6-alkyl, benzyl, phenylethyl or sulfo, R'7 is hydrogen, hydroxy or methoxy is and R'8 is hydrogen, chlorine, C1-C8-alkyl or sulfo and at least one of the radicals R'4 or R'8 must be sulfo.
- UV absorbers of the formula (2a) given above are particularly preferred, in which R'4 is hydrogen or sulfo, R'6 is methyl, ethyl, n- or iso-propyl, n-, iso-, sec- or tert-butyl ( or tert-amyl, R'7 is hydrogen and R'8 is C1-C4alkyl or sulfo.
- UV absorbers of the formula (2) or (2a) are: 2- (2'-hydroxy-3'-sulfo-5'-tert-octylphenyl) benzotriazole, 2- (2'-hydroxy-3'-sulfo-5'-tert-butylphenyl) benzotriazole, 2- (2'-hydroxy-3'-sulfo-5'-methylphenyl) benzotriazole, 2- (2 ', 4'dihydroxy-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-4'-methoxy-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-5'-sulfophenyl) -5-chlorobenzotriazole, 2- (2'-hydroxy-3'-methyl-5'-sulfophenyl) -5-chlorobenzotriazole, 2- (2'-hydroxy-5'-methylphenyl) -5-sulfobenzotriazole, 2- (2'-Hydr
- UV absorbers of the formula (2) or (2a) are: 2- (2'-hydroxy-3'-tert-amyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-tert-butyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-sec-butyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-n-butyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-isobutyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-isopropyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-n-propyl-5'-sulfophenyl) benzotriazole, 2- (2'-hydroxy-3'-ethyl-5'-sulfophenyl) benzotriazole,
- improving lightfastness means preventing or at least reducing yellowing.
- the process according to the invention is advantageously carried out in such a way that the leather initially subjected to pretreatment, for example retanning, neutralization and / or rolling.
- the leather pretreated in this way is then, if desired, dyed by means of a known pull-out process using one or more dyes; for example, the leather is dyed in an aqueous solution at a liquor ratio of 1: 1.5 to 1:20, preferably 1: 2 to 1:10 and a temperature of e.g. 20 to 100 ° C, preferably 40 to 60 ° C.
- a temperature e.g. 20 to 100 ° C, preferably 40 to 60 ° C.
- the dyeing time also depends on the type of leather to be dyed, but is generally e.g. 20 to 180 minutes.
- the UV absorber can be added during the pretreatment, which is particularly useful for leather that should not be subsequently dyed. However, it can also be added to the dye bath before, during or after the dyeing process, i.e. the addition can take place during the pretreatment, during the actual coloring or only after the treatment.
- the UV absorber is preferably added after the dyeing process. Instead of a UV absorber, a mixture of different UV absorbers can also be used.
- the UV absorbers are used in an amount of e.g. 0.25 to 7.5, preferably 0.5 to 4.0 and particularly 0.5 to 2.5% by weight, based on the weight of the leather, is used.
- the dye bath can be admixed with other commonly used additives, e.g. Wetting agents, leveling agents, color deepening agents and / or fatty agents are added.
- Wetting agents e.g. Acidified with formic acid to improve bath exhaustion and let it run for some time.
- the dyed leather is finished in a manner known per se.
- customary anionic dyes such as are known, for example, from the Color Index, are used for dyeing the leather.
- Anionic dyes that can be used are all dyes commonly used in leather dyeing; acid dyes and direct dyes, such as, in particular, sulfo-containing monoazo, disazo and polyazo dyes, and 1: 1 or 1: 2 metal complex dyes are preferred.
- anionic dyes can also be used.
- dye mixtures of 2, 3, 4 or more components can be used.
- a preferred embodiment of the present invention relates to a process for improving the light fastness of leather dyeings, characterized in that the leather is treated with 0.5 to 4.0% by weight, based on the weight of the leather, of a UV absorber of the formula wherein R'4 is hydrogen, chlorine, methyl, methoxy, methoxycarbonyl, ethoxycarbonyl, carboxy or sulfo, R'6 is hydrogen, chlorine, C1-C6-alkyl, benzyl, phenylethyl or sulfo, R'7 is hydrogen, hydroxy or methoxy is and R'8 is hydrogen, chlorine, C1-C8 alkyl or sulfo and at least one of the radicals R'4 or R'8 must be sulfo treated.
- a particularly preferred embodiment of the present invention relates to a method for improving the lightfastness of leather dyeings, characterized in that after the dyeing process with 0.5 to 2.5% by weight, based on the weight of the leather, of a UV absorber Formula (2a) given above, in which R'4 is hydrogen or sulfo, R'6 is methyl, ethyl, n- or isopropyl, n-, iso-, sec- or tert-butyl or tert-amyl, R ' 7 is hydrogen and R'8 C1-C4 alkyl or sulfo treated.
- a UV absorber Formula (2a) given above, in which R'4 is hydrogen or sulfo, R'6 is methyl, ethyl, n- or isopropyl, n-, iso-, sec- or tert-butyl or tert-amyl, R ' 7 is hydrogen and R'8 C1-C4 alkyl or
- the method according to the invention is suitable for all types of grain, suede and suede, for example chrome leather, retanned leather or suede from goat, sheep, beef and pork.
- level, deep, well-covering dyeings with good general fastness properties are obtained, for example water, washing, welding, dry cleaning, acid, Alkali, solvent and diffusion fastness to soft PVC.
- the lightfastness of the leather obtainable according to the invention which is greatly improved compared to a comparable method without UV absorber, deserves special mention.
- Example 1 100 parts of chrome-tanned cowhide (fold weight) are washed for 15 minutes in 300 parts of water at 30 ° C. and then for 60 minutes in one of 300 parts of water, 2 parts of neutralizing agent (Na salts of aromatic sulfonic acids and aliphatic dicarboxylic acids) and 0. 5 parts of sodium bicarbonate existing liquor neutralized at 30 ° C. This is followed by a 15-minute washing operation in 300 parts of water at room temperature.
- neutralizing agent Na salts of aromatic sulfonic acids and aliphatic dicarboxylic acids
- the leather treated in this way is now in a from 300 parts of water, 1 part of dye of the formula and 2 parts of UV absorber of the formula existing fresh liquor dyed at 50 ° C for 30 minutes. Then 3 parts of a fatliquor (preparation based on sulfonated chlorinated paraffin) are added to the dye liquor, and after a further 30 minutes 0.5 part of 85% formic acid is added. Dyeing continues for a further 30 minutes at unchanged temperature and the dyed leather is made after Rinse done as usual. A level red color is obtained with a light fastness that is greatly improved compared to a color depth of the same color without a UV absorber.
- Example 2 100 parts of chrome-tanned cowhide (fold weight) are washed for 15 minutes in 300 parts of water at 30 ° C. and then for 60 minutes in one of 300 parts of water, 2 parts of neutralizing agent (Na salts of aromatic sulfonic acids and aliphatic dicarboxylic acids) and 0. 5 parts of sodium bicarbonate existing liquor neutralized at 30 ° C.
- neutralizing agent Na salts of aromatic sulfonic acids and aliphatic dicarboxylic acids
- the neutralizing liquor is supplemented with 2 parts of the UV absorber of the formula and let it run for 60 minutes. This is followed by a 15-minute washing operation in 300 parts of water at room temperature.
- the leather treated in this way is now in a from 300 parts of water and 2 parts of dye of the formula existing fresh liquor dyed at 50 ° C for 30 minutes. Then 3 parts of a fatliquor (preparation based on sulfonated chlorinated paraffin) are added to the dyeing liquor and, after a further 30 minutes, 0.5 part of 85% formic acid is added. The dyeing is continued for 30 minutes at unchanged temperature and the dyed leather is finished as usual after rinsing . The result is a level red color with a light fastness that is greatly improved compared to a color depth of the same color without UV absorber.
- Example 3 100 parts of chrome cowhide (shaved weight) are neutralized as described in Example 1 and then at 30 ° C. in the same liquor with 7 parts of a liquid synthetic retanning agent (condensation products of polyphenolsulfonic acids with formaldehyde and urea) and 2 parts of that used in Example 1 UV absorber treated for another 60 minutes. This is followed by a 15-minute washing process in 300 parts of water at room temperature. This retanned chrome cowhide is then at 50 ° C in a liquor consisting of 300 parts of water and 2.01 parts of the dye mixture consisting of 0.67 parts of the yellow dye of the formula 0.67 part of the red dye used in Example 1 and 0.67 part of the blue dye of the formula colored.
- a liquid synthetic retanning agent condensation products of polyphenolsulfonic acids with formaldehyde and urea
- UV absorber treated for another 60 minutes. This is followed by a 15-minute washing process in 300 parts of water at room temperature.
- Example 4 100 parts of chrome cowhide (shaved weight) are neutralized and retanned as described in Example 3 (without the UV absorber mentioned in Example 3) and then dyed in a liquor containing 300 parts of water and 1.5 parts of the dye mixture used in Example 3 at 50 ° C. for 30 minutes. After 30 minutes, 3 parts of the synthetic fatliquor from Example 1 are added, and after a further 30 minutes, 1 part of 85% formic acid is added. After acidification, the treatment is continued for 30 minutes at 50 ° C. The leather is then aftertreated in a fresh bath with 1 part of a color-deepening auxiliary (polyquaternary amine-ethylene oxide adduct) in 300 parts of water.
- a color-deepening auxiliary polyquaternary amine-ethylene oxide adduct
- Example 5 100 parts of chrome cowhide (shaved weight) are neutralized as described in Example 1 and then at 30 ° C. in the same liquor with 7 parts of a liquid synthetic retanning agent (condensation products of naphthalenesulfonic acids with formaldehyde and urea) and 2 parts of that used in Example 1 UV absorber treated for a further 60 minutes. This is followed by a washing process of 15 minutes in 300 parts of water at room temperature. This retanned chrome cowhide is then dyed in a liquor containing 300 parts of water and 1.5 parts of the dye mixture used in Example 3 at 50 ° C. for 30 minutes.
- a liquid synthetic retanning agent condensation products of naphthalenesulfonic acids with formaldehyde and urea
- Example 6 100 parts of chrome leather (shaved weight) are neutralized and retanned as described in Example 3 (without the UV absorber mentioned in Example 3) and dyed with 2.0 or 4.0 parts of the red dye listed in Example 1.
- This finished crust leather is aftertreated by a fuel application with a solution containing 25 g of the UV absorber listed in Example 1, 100 g of methoxyisopropanol and 875 g of water. With this milky solution, the leather is sprayed 2 times crosswise and air-dried.
- the leather aftertreated with UV absorber shows very much improved light fastness compared to leather that has not been aftertreated.
- Example 7 100 parts of chrome-tanned cowhide (fold weight) are neutralized and dyed as described in Example 1, but 1 part of 2-hydroxy-4-methoxy-5-sulfobenzophenone is used instead of the UV absorber used there.
- the red color is characterized by a significantly improved light fastness.
- Example 8 100 parts of chrome cowhide (shaved weight) are neutralized as described in Example 1 and then at 30 ° C. in the same liquor with 4 parts of a synthetic retanning agent (formaldehyde condensation product of polyphenolsulfone and polynaphthalenesulfonic acid) and 2 parts of the UV listed in Example 1 -Absorbers treated another 60 minutes. This is followed by a washing process of 15 minutes in 300 parts of water at room temperature. The leather is then treated in a float of 300 parts of water and 3 parts of the synthetic fatliquor from Example 1 for 30 minutes at 50 ° C. After 30 minutes, 0.5 part of 85% formic acid is added and the treatment is continued for 30 minutes at 50 ° C. It is then rinsed in cold water and finished. The leather treated with UV absorber is markedly improved over leather treated in the same way but without UV absorber Lightfastness off, ie the leather with the UV absorber shows significantly less yellowing under the influence of light.
Landscapes
- Engineering & Computer Science (AREA)
- Textile Engineering (AREA)
- Treatment And Processing Of Natural Fur Or Leather (AREA)
- Coloring (AREA)
Description
Gegenstand der vorliegenden Erfindung ist ein Verfahren zur Verbesserung der Lichtechtheit von gefärbtem und ungefärbtem Leder.The present invention relates to a method for improving the light fastness of colored and uncolored leather.
Die vermehrte Verwendung von Ledern z.B. für die Herstellung von Autositzen, Möbeln oder Bekleidungsartikeln hat dazu geführt, dass man heute an die Lichtechtheit von Leder immer höhere Anforderungen stellt.The increased use of leather e.g. for the manufacture of car seats, furniture or articles of clothing has led to increasing demands being placed on the lightfastness of leather.
Lederfärbungen weisen jedoch grundsätzlich eine geringere Lichtechtheit auf als vergleichbare Färbungen auf textilen Fasermaterialien, wie z.B. aus B. Magerkurth, The Leather Manufacturer, January 1987, Seite 10-26 hervorgeht: Identische Farbstoffe ergaben auf Nylon ausgefärbt eine Lichtechtheitsnote 6-7, auf Wolle eine um 1 Note erniedrigte Lichtechtheitsnote und auf Leder maximal nur die Note 4, jeweils gemäss ISO-Blaumassstab gemessen.However, leather dyeings generally have a lower light fastness than comparable dyeings on textile fiber materials, e.g. from B. Magerkurth, The Leather Manufacturer, January 1987, page 10-26: Identical dyes gave a lightfastness rating of 6-7 when dyed nylon, a lightfastness rating lowered by 1 grade on wool and a maximum of 4 on leather, each according to ISO -Blue scale measured.
Bei ungefärbtem Leder tritt unter der Einwirkung von Licht eine mehr oder weniger starke Vergilbung auf, die meist unerwünscht ist.In the case of undyed leather, more or less yellowing occurs under the influence of light, which is usually undesirable.
Es besteht daher ein Bedarf, gefärbtes und ungefärbtes Leder in seiner Lichtechtheit zu verbessern.There is therefore a need to improve dyed and undyed leather in its light fastness.
In der US-A-3 444 164 und in der EP-A-0 165 608 sind Triazinderivate als UV-Absorber beschrieben und die EP-A-0 245 204 lehrt, dass man Polyamidfasermaterial mit Mischungen aus UV-Absorbern und organischen Kupferkomplexen stabilisieren kann.In US-A-3 444 164 and in EP-A-0 165 608 triazine derivatives are described as UV absorbers and EP-A-0 245 204 teaches that polyamide fiber material is stabilized with mixtures of UV absorbers and organic copper complexes can.
Es hat sich gezeigt, dass man überraschenderweise Leder mit verbesserter Lichtechtheit erhält, wenn man das Leder mit spezifischen UV-Absorbern behandelt.It has been shown that, surprisingly, leather with improved light fastness can be obtained if the leather is treated with specific UV absorbers.
Gegenstand der vorliegenden Anmeldung ist daher ein Verfahren zur Verbesserung der Lichtechtheit von Leder, welches dadurch gekennzeichnet ist, dass man dass Leder in Gegenwart eines UV-Absorbers, ausgewählt aus der Gruppe der
- a) 2-Hydroxybenzophenone der Formel (1)
R₁ Wasserstoff, Hydroxy, C₁-C₁₄-Alkoxy oder Phenoxy,
R₂ Wasserstoff, Hydroxy oder C₁-C₄-Alkoxy und
R₃ Wasserstoff, Hydroxy oder Carboxy
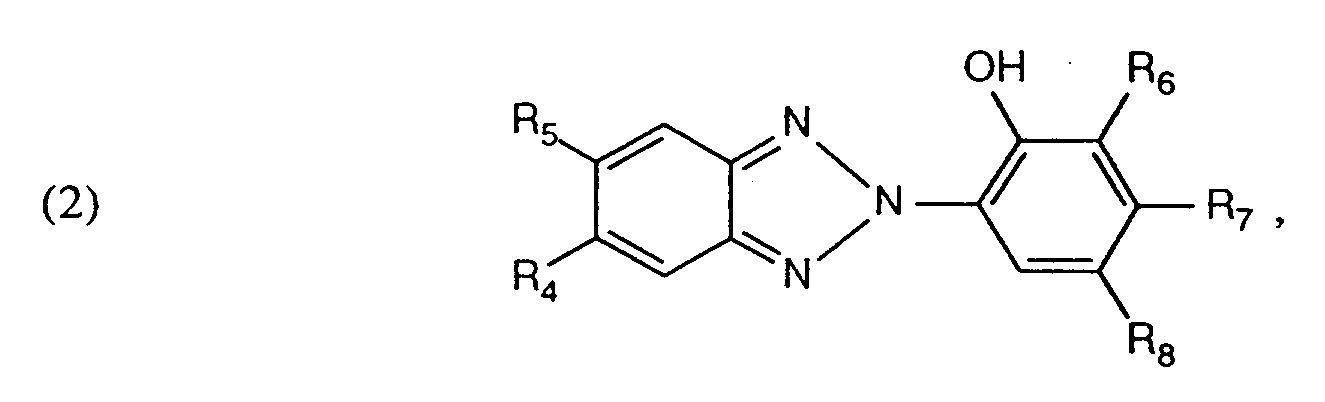
bedeuten, und - b) 2-(2'-Hydroxyphenyl)-benzotriazole der Formel (2)
R₄ Wasserstoff, Halogen, C₁-C₄-Alkyl, C₁-C₄-Alkoxy, C₁-C₈-Alkoxycarbonyl, Carboxy oder Sulfo,
R₅ Wasserstoff oder Halogen,
R₆ Wasserstoff, Halogen, C₁-C₁₂-Alkyl, C₅-C₆-Cycloalkyl, C₇-C₉-Arylalkyl oder Sulfo,
R₇ Wasserstoff, Halogen, C₁-C₄-Alkyl, C₁-C₄-Alkoxy, Hydroxy oder Sulfo, und
R₈ C₁-C₁₂-Alkyl, Halogen, C₅-C₆-Cycloalkyl, C₁-C₄-Alkoxy, Phenyl, (C₁-C₈-Alkyl)-phenyl, C₁-C₈-Alkoxycarbonyl, Carboxyethyl, C₇-C₉-Aralkyl oder Sulfo bedeuten und mindestens einer der Reste R₄ oder R₈ Sulfo sein muss, behandelt.
- a) 2-hydroxybenzophenones of the formula (1)
R₁ is hydrogen, hydroxy, C₁-C₁₄ alkoxy or phenoxy,
R₂ is hydrogen, hydroxy or C₁-C₄ alkoxy and
R₃ is hydrogen, hydroxy or carboxy
mean and - b) 2- (2'-hydroxyphenyl) benzotriazoles of the formula (2)
R₄ is hydrogen, halogen, C₁-C₄-alkyl, C₁-C₄-alkoxy, C₁-C₈-alkoxycarbonyl, carboxy or sulfo,
R₅ is hydrogen or halogen,
R₆ is hydrogen, halogen, C₁-C₁₂-alkyl, C₅-C₆-cycloalkyl, C₇-C₉-arylalkyl or sulfo,
R₇ is hydrogen, halogen, C₁-C₄-alkyl, C₁-C₄-alkoxy, hydroxy or sulfo, and
R₈ is C₁-C₁₂-alkyl, halogen, C₅-C₆-cycloalkyl, C₁-C₄-alkoxy, phenyl, (C₁-C₈-alkyl) -phenyl, C₁-C₈-alkoxycarbonyl, carboxyethyl, C₇-C₉-aralkyl or sulfo and at least one of the radicals R₄ or R₈ must be sulfo treated.
Die Carboxy- und Sulforeste können generell als freie Säure oder auch als Salz, z.B. als Alkalimetall-, Erdalkalimetall-, Ammonium- oder Aminsalz, vorliegen.The carboxy and sulfor radicals can generally be used as a free acid or as a salt, e.g. as an alkali metal, alkaline earth metal, ammonium or amine salt.
Bedeuten R₂ oder etwaige andere Substituenten C₁-C₄-Alkoxy, so stellen sie z.B. Methoxy, Ethoxy, n- oder iso-Propoxy, oder n-, iso-, sec.- oder tert.-Butoxy dar.If R₂ or any other substituents are C₁-C₄alkoxy, they represent e.g. Methoxy, ethoxy, n- or iso-propoxy, or n-, iso-, sec.- or tert.-butoxy.
Steht R₁ für C₁-C₁₂-Alkoxy, so kommen ausser den oben genannten C₁-C₄-Alkoxy-Gruppen z.B. geradliniges oder verzweigtes Pentyloxy, Hexyloxy, Heptyloxy, Octyloxy, Nonyloxy, Decyloxy, Undecyloxy oder Dodecyloxy in Frage.If R₁ is C₁-C₁₂-alkoxy, in addition to the above-mentioned C₁-C₄-alkoxy groups e.g. straight or branched pentyloxy, hexyloxy, heptyloxy, octyloxy, nonyloxy, decyloxy, undecyloxy or dodecyloxy in question.
Beispiele für geeignete UV-Absorber der Formel (1) sind 2-Hydroxy-4-methoxy-5-sulfobenzophenon und 2,2'-Dihydroxy-4,4'-dimethoxy-5-sulfobenzophenon.Examples of suitable UV absorbers of the formula (1) are 2-hydroxy-4-methoxy-5-sulfobenzophenone and 2,2'-dihydroxy-4,4'-dimethoxy-5-sulfobenzophenone.
Bei R₄ oder etwaigen anderen Variablen als Halogen handelt es sich z.B. um Fluor, Brom oder vorzugsweise um Chlor.R₄ or any variables other than halogen are e.g. fluorine, bromine or preferably chlorine.
Stehen R₄ oder etwaige andere Variablen für C₁-C₄-Alkyl, so kommen hierbei die Bedeutungen Methyl, Ethyl, n- oder iso-Propyl oder n-, iso-, sec.- oder tert.-Butyl in Frage.R₄ or any other variables for C₁-C₄-alkyl, here are the meanings methyl, ethyl, n- or iso-propyl or n-, iso-, sec- or tert-butyl in question.
Bei R₄ oder R₈ als C₁-C₈-Alkoxycarbonyl handelt es sich vorzugsweise um Methoxycarbonyl oder Ethoxycarbonyl.R₄ or R₈ as C₁-C₈ alkoxycarbonyl is preferably methoxycarbonyl or ethoxycarbonyl.
R₄ steht vorzugsweise für Wasserstoff, Chlor, Methyl, Methoxy, Methoxycarbonyl, Ethoxycarbonyl, Carboxy oder Sulfo und besonders bevorzugt für Wasserstoff oder Sulfo.R₄ preferably represents hydrogen, chlorine, methyl, methoxy, methoxycarbonyl, Ethoxycarbonyl, carboxy or sulfo and particularly preferably for hydrogen or sulfo.
R₅ hat vorzugsweise die Bedeutung Wasserstoff.R₅ is preferably hydrogen.
Stehen R₆ oder etwaige andere Substituenten für C₁-C₁₂-Alkyl, so kommen ausser den zuvor genannten C₁-C₄-Alkylresten z.B. ein geradliniger oder verzweigter Pentyl-, Hexyl-, Heptyl-, Octyl-, Nonyl-, Decyl-, Undecyl- oder Dodecylrest in Frage.If R₆ or any other substituents are C₁-C₁₂-alkyl, in addition to the aforementioned C₁-C₄-alkyl radicals e.g. a linear or branched pentyl, hexyl, heptyl, octyl, nonyl, decyl, undecyl or dodecyl radical in question.
Bei R₆ oder etwaigen anderen Variablen als C₅-C₆-Cycloalkyl handelt es sich z.B. um Cyclopentyl oder vorzugsweise um Cyclohexyl.R₆ or any variables other than C₅-C₆ cycloalkyl are e.g. cyclopentyl or preferably cyclohexyl.
Als C₇-C₉-Alalkyl stehen R₆ oder etwaige andere Variablen bevorzugt für Phenylethyl oder insbesondere für Benzyl.As C₇-C₉-alalkyl R₆ or any other variables are preferably phenylethyl or in particular benzyl.
Bevorzugte Bedeutungen von R₆ sind Wasserstoff, Chlor, C₁-C₆-Alkyl, Benzyl, Phenylethyl oder Sulfo. R₆ steht besonders bevorzugt für Methyl, Ethyl, n- oder iso-Propyl, n-, iso-, sec.- oder tert.-Butyl oder tert.-Amyl.Preferred meanings of R₆ are hydrogen, chlorine, C₁-C₆-alkyl, benzyl, phenylethyl or sulfo. R₆ particularly preferably represents methyl, ethyl, n- or isopropyl, n-, iso-, sec- or tert-butyl or tert-amyl.
R₇ ist bevorzugt Wasserstoff, Hydroxy oder Methoxy und besonders bevorzugt Wasserstoff.R₇ is preferably hydrogen, hydroxy or methoxy and particularly preferably hydrogen.
R₈ steht bevorzugt für Wasserstoff, Chlor, C₁-C₈-Alkyl oder Sulfo, besonders bevorzugt für C₁-C₄-Alkyl oder Sulfo und insbesondere bevorzugt für Sulfo.R₈ preferably represents hydrogen, chlorine, C₁-C₈-alkyl or sulfo, particularly preferably C₁-C₄-alkyl or sulfo and particularly preferably sulfo.
Bevorzugte UV-Absorber entsprechen der Formel
worin R'₄ Wasserstoff, Chlor, Methyl, Methoxy, Methoxycarbonyl, Ethoxycarbonyl, Carboxy oder Sulfo bedeutet, R'₆ für Wasserstoff, Chlor, C₁-C₆-Alkyl, Benzyl, Phenylethyl oder Sulfo steht, R'₇ Wasserstoff, Hydroxy oder Methoxy ist und R'₈ Wasserstoff, Chlor, C₁-C₈-Alkyl oder Sulfo bedeutet und mindestens einer der Reste R'₄ oder R'₈ Sulfo sein muss.Preferred UV absorbers correspond to the formula
wherein R'₄ is hydrogen, chlorine, methyl, methoxy, methoxycarbonyl, ethoxycarbonyl, carboxy or sulfo, R'₆ is hydrogen, chlorine, C₁-C₆-alkyl, benzyl, phenylethyl or sulfo, R'₇ is hydrogen, hydroxy or methoxy is and R'₈ is hydrogen, chlorine, C₁-C₈-alkyl or sulfo and at least one of the radicals R'₄ or R'₈ must be sulfo.
Besonders bevorzugt sind UV-Absorber der oben angegebenen Formel (2a), worin R'₄ Wasserstoff oder Sulfo, R'₆ Methyl, Ethyl, n- oder iso-Propyl, n-, iso-, sec- oder tert.-Butyl (oder tert.-Amyl, R'₇ Wasserstoff und R'₈ C₁-C₄-Alkyl oder Sulfo bedeuten.UV absorbers of the formula (2a) given above are particularly preferred, in which R'₄ is hydrogen or sulfo, R'₆ is methyl, ethyl, n- or iso-propyl, n-, iso-, sec- or tert-butyl ( or tert-amyl, R'₇ is hydrogen and R'₈ is C₁-C₄alkyl or sulfo.
Beispiele für geeignete UV-Absorber der Formel (2) bzw. (2a) sind:
2-(2'-Hydroxy-3'-sulfo-5'-tert.-octylphenyl)-benzotriazol,
2-(2'-Hydroxy-3'-sulfo-5'-tert.-butylphenyl)-benzotriazol,
2-(2'-Hydroxy-3'-sulfo-5'-methylphenyl)-benzotriazol,
2-(2',4'dihydroxy-5'-sulfophenyl)-benzotriazol,
2-(2'-hydroxy-4'-methoxy-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-5'-sulfophenyl)-5-chlorobenzotriazol,
2-(2'-Hydroxy-3'-methyl-5'-sulfophenyl)-5-chlorobenzotriazol,
2-(2'-Hydroxy-5'-methylphenyl)-5-sulfobenzotriazol,
2-(2'-Hydroxy-3'-benzyl-5'-sulfophenyl)-benzotriazol.Examples of suitable UV absorbers of the formula (2) or (2a) are:
2- (2'-hydroxy-3'-sulfo-5'-tert-octylphenyl) benzotriazole,
2- (2'-hydroxy-3'-sulfo-5'-tert-butylphenyl) benzotriazole,
2- (2'-hydroxy-3'-sulfo-5'-methylphenyl) benzotriazole,
2- (2 ', 4'dihydroxy-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-4'-methoxy-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-5'-sulfophenyl) -5-chlorobenzotriazole,
2- (2'-hydroxy-3'-methyl-5'-sulfophenyl) -5-chlorobenzotriazole,
2- (2'-hydroxy-5'-methylphenyl) -5-sulfobenzotriazole,
2- (2'-Hydroxy-3'-benzyl-5'-sulfophenyl) benzotriazole.
Beispiele für insbesondere bevorzugte UV-Absorber der Formel (2) bzw. (2a) sind:
2-(2'-Hydroxy-3'-tert.-amyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-tert.-butyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-sec.-butyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-n-butyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-iso-butyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-isopropyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-n-propyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-ethyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-methyl-5'-sulfophenyl)-benzotriazol,
2-(2'-Hydroxy-3'-tert.-butyl-5'-methylphenyl)-5-sulfobenzotriazol,
2-(2'-Hydroxy-3',5'-dimethylphenyl)-5-sulfobenzotriazol,
2-(2'-Hydroxy-3'-isopropyl-5'-tert.-butylphenyl)-5-sulfobenzotriazol,
2-(2'-Hydroxy-3'-tert.-amyl-5'-methylphenyl)-5-sulfobenzotriazol.Examples of particularly preferred UV absorbers of the formula (2) or (2a) are:
2- (2'-hydroxy-3'-tert-amyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-tert-butyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-sec-butyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-n-butyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-isobutyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-isopropyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-n-propyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-ethyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-methyl-5'-sulfophenyl) benzotriazole,
2- (2'-hydroxy-3'-tert-butyl-5'-methylphenyl) -5-sulfobenzotriazole,
2- (2'-hydroxy-3 ', 5'-dimethylphenyl) -5-sulfobenzotriazole,
2- (2'-hydroxy-3'-isopropyl-5'-tert-butylphenyl) -5-sulfobenzotriazole,
2- (2'-Hydroxy-3'-tert-amyl-5'-methylphenyl) -5-sulfobenzotriazole.
Bei ungefärbtem Leder ist unter Verbesserung der Lichtechtheit eine Verhinderung oder zumindest Verminderung des Vergilbens zu verstehen.In the case of uncoloured leather, improving lightfastness means preventing or at least reducing yellowing.
Das erfindungsgemässe Verfahren wird vorteilhaft so ausgeführt, dass man das Leder zunächst einer Vorbehandlung, z.B. einer Nachgerbung, Neutralisation und/oder Walke unterwirft.The process according to the invention is advantageously carried out in such a way that the leather initially subjected to pretreatment, for example retanning, neutralization and / or rolling.
Das solchermassen vorbehandelte Leder wird dann, falls gewünscht, mittels eines an sich bekannten Ausziehverfahrens unter Verwendung eines oder mehrerer Farbstoffe gefärbt; beispielsweise färbt man das Leder in einer wässrigen Lösung bei einem Flottenverhältnis von 1:1,5 bis 1:20, vorzugsweise 1:2 bis 1:10 und einer Temperatur von z.B. 20 bis 100°C, vorzugsweise 40 bis 60°C. Man verwendet je nach Art des zu färbenden Leders z.B. 0,25 bis 15 Gew.-%, vorzugsweise 1,0 bis 10 Gew.-%, bezogen auf das Gewicht des Leders, Farbstoff. Auch die Färbedauer hängt von der Art des zu färbenden Leders ab, beträgt aber im allgemeinen z.B. 20 bis 180 Minuten.The leather pretreated in this way is then, if desired, dyed by means of a known pull-out process using one or more dyes; for example, the leather is dyed in an aqueous solution at a liquor ratio of 1: 1.5 to 1:20, preferably 1: 2 to 1:10 and a temperature of e.g. 20 to 100 ° C, preferably 40 to 60 ° C. Depending on the type of leather to be dyed, e.g. 0.25 to 15 wt .-%, preferably 1.0 to 10 wt .-%, based on the weight of the leather, dye. The dyeing time also depends on the type of leather to be dyed, but is generally e.g. 20 to 180 minutes.
Der UV-Absorber kann bereits während der Vorbehandlung zugesetzt werden, was vor allem bei Leder zweckmässig ist, welches nicht anschliesend gefärbt werden soll. Er kann aber auch dem Färbebad vor, während oder nach dem Färbevorgang zugegeben werden, d.h. die Zugabe kann bereits während der Vorbehandlung, während der eigentlichen Färbung oder aber erst bei der Nachbehandlung erfolgen. Die Zugabe des UV-Absorbers erfolgt vorzugsweise nach dem Färbevorgang. Anstelle eines UV-Absorbers kann auch ein Gemisch verschiedener UV-Absorber eingesetzt werden.The UV absorber can be added during the pretreatment, which is particularly useful for leather that should not be subsequently dyed. However, it can also be added to the dye bath before, during or after the dyeing process, i.e. the addition can take place during the pretreatment, during the actual coloring or only after the treatment. The UV absorber is preferably added after the dyeing process. Instead of a UV absorber, a mixture of different UV absorbers can also be used.
Die UV-Absorber werden in einer Menge von z.B. 0,25 bis 7,5, vorzugsweise 0,5 bis 4,0 und besonders 0,5 bis 2,5 Gew.-%, bezogen auf das Gewicht des Leders, eingesetzt.The UV absorbers are used in an amount of e.g. 0.25 to 7.5, preferably 0.5 to 4.0 and particularly 0.5 to 2.5% by weight, based on the weight of the leather, is used.
Dem Färbebad können gegebenenfalls vor, während oder nach der Färbung weitere allgemein übliche Zusätze, z.B. Netzmittel, Egalisiermittel, Farbvertiefungsmittel und/oder Fettungsmittel zugegeben werden. Am Ende des Färbevorgangs wird vorteilhaft z.B. mit Ameisensäure zur besseren Baderschöpfung angesäuert und noch einige Zeit weiterlaufen gelassen. Die Fertigstellung des gefärbten Leders erfolgt in an sich bekannter Weise.If necessary, the dye bath can be admixed with other commonly used additives, e.g. Wetting agents, leveling agents, color deepening agents and / or fatty agents are added. At the end of the dyeing process, e.g. Acidified with formic acid to improve bath exhaustion and let it run for some time. The dyed leather is finished in a manner known per se.
Für das erfindungsgemässe Verfahren werden zum Färben des Leders übliche anionische Farbstoffe, wie sie z.B. aus dem Colour Index bekannt sind, verwendet. Als anionische Farbstoffe kommen alle üblicherweise in der Lederfärberei verwendeten Farbstoffe in Frage; bevorzugt sind hierbei Säurefarbstoffe und Direktfarbstoffe, wie insbesondere sulfogruppenhaltige Monoazo-, Disazo- und Polyazofarbstoffe, sowie 1:1- oder 1:2-Metallkomplexfarbstoffe.For the process according to the invention, customary anionic dyes, such as are known, for example, from the Color Index, are used for dyeing the leather. Anionic dyes that can be used are all dyes commonly used in leather dyeing; acid dyes and direct dyes, such as, in particular, sulfo-containing monoazo, disazo and polyazo dyes, and 1: 1 or 1: 2 metal complex dyes are preferred.
Es können auch Mischungen der anionischen Farbstoffe eingesetzt werden. Beispielsweise können Farbstoffmischungen aus 2, 3, 4 oder noch mehreren Komponenten verwendet werden.Mixtures of the anionic dyes can also be used. For example, dye mixtures of 2, 3, 4 or more components can be used.
Eine bevorzugte Ausführungsform der vorliegenden Erfindung betrifft ein Verfahren zur Verbesserung der Lichtechtheit von Lederfärbungen, dadurch gekennzeichnet, dass man das Leder mit 0,5 bis 4,0 Gew.-%, bezogen auf das Gewicht des Leders, eines UV-Absorbers der Formel
worin R'₄ Wasserstoff, Chlor, Methyl, Methoxy, Methoxycarbonyl, Ethoxycarbonyl, Carboxy oder Sulfo bedeutet, R'₆ für Wasserstoff, Chlor, C₁-C₆-Alkyl, Benzyl, Phenylethyl oder Sulfo steht, R'₇ Wasserstoff, Hydroxy oder Methoxy ist und R'₈ Wasserstoff, Chlor, C₁-C₈-Alkyl oder Sulfo bedeutet und mindestens einer der Reste R'₄ oder R'₈ Sulfo sein muss, behandelt.A preferred embodiment of the present invention relates to a process for improving the light fastness of leather dyeings, characterized in that the leather is treated with 0.5 to 4.0% by weight, based on the weight of the leather, of a UV absorber of the formula
wherein R'₄ is hydrogen, chlorine, methyl, methoxy, methoxycarbonyl, ethoxycarbonyl, carboxy or sulfo, R'₆ is hydrogen, chlorine, C₁-C₆-alkyl, benzyl, phenylethyl or sulfo, R'₇ is hydrogen, hydroxy or methoxy is and R'₈ is hydrogen, chlorine, C₁-C₈ alkyl or sulfo and at least one of the radicals R'₄ or R'₈ must be sulfo treated.
Eine besonders bevorzugte Ausführungsform der vorliegenden Erfindung betrifft ein Verfahren zur Verbesserung der Lichtechtheit von Lederfärbungen, dadurch gekennzeichnet, dass man nach dem Färbevorgang mit 0,5 bis 2,5 Gew.-%, bezogen auf das Gewicht des Leders, eines UV-Absorbers der zuvor angegebenen Formel (2a), worin R'₄ Wasserstoff oder Sulfo, R'₆ Methyl, Ethyl, n- oder iso-Propyl, n-, iso-, sec- oder tert.-Butyl oder tert.-Amyl, R'₇ Wasserstoff und R'₈ C₁-C₄-Alkyl oder Sulfo bedeuten, behandelt.A particularly preferred embodiment of the present invention relates to a method for improving the lightfastness of leather dyeings, characterized in that after the dyeing process with 0.5 to 2.5% by weight, based on the weight of the leather, of a UV absorber Formula (2a) given above, in which R'₄ is hydrogen or sulfo, R'₆ is methyl, ethyl, n- or isopropyl, n-, iso-, sec- or tert-butyl or tert-amyl, R ' ₇ is hydrogen and R'₈ C₁-C₄ alkyl or sulfo treated.
Das erfindungsgemässe Verfahren ist für alle Arten von Narben-, Rauh- und Veloursledern, z.B. Chromleder, nachgegerbtes Leder oder Veloursleder von Ziege, Schaf, Rind und Schwein geeignet.
Bei Anwendung des erfindungsgemässen Verfahrens in Verbindung mit einem Färbeverfahren erhält man egale, tiefe, gut deckende Färbungen mit guten Allgemeinechtheiten, z.B. Wasser-, Wasch-, Schweiss-, Trockenreinigungs-, Säure-, Alkali-, Lösungsmittel- und Diffusionsechtheit gegenüber Weich-PVC.
Besondere Erwähnung verdient die gegenüber einem vergleichbaren Verfahren ohne UV-Absorber stark verbesserte Lichtechtheit der erfindungsgemäss erhältlichen Leder.The method according to the invention is suitable for all types of grain, suede and suede, for example chrome leather, retanned leather or suede from goat, sheep, beef and pork.
When the process according to the invention is used in conjunction with a dyeing process, level, deep, well-covering dyeings with good general fastness properties are obtained, for example water, washing, welding, dry cleaning, acid, Alkali, solvent and diffusion fastness to soft PVC.
The lightfastness of the leather obtainable according to the invention, which is greatly improved compared to a comparable method without UV absorber, deserves special mention.
Die folgenden Beispiele veranschaulichen die Erfindung. Teile bedeuten Gewichtsteile, Prozente Gewichtsprozente. Die Temperaturen sind in °C angegeben.The following examples illustrate the invention. Parts mean parts by weight, percentages by weight. The temperatures are given in ° C.
Beispiel 1: 100 Teile chromgegerbtes Rindleder (Falzgewicht) werden 15 Minuten lang in 300 Teilen Wasser bei 30°C gewaschen und anschliessend 60 Minuten in einer aus 300 Teilen Wasser, 2 Teilen Neutraiisationsmittel (Na-Salze aromatischer Sulfosäuren und aliphatischer Dicarbonsäuren) und 0,5 Teilen Natriumbicarbonat bestehenden Flotte bei 30°C neutralisiert. Anschliessend folgt eine 15 Minuten dauernde Waschoperation in 300 Teilen Wasser bei Raumtemperatur. Example 1: 100 parts of chrome-tanned cowhide (fold weight) are washed for 15 minutes in 300 parts of water at 30 ° C. and then for 60 minutes in one of 300 parts of water, 2 parts of neutralizing agent (Na salts of aromatic sulfonic acids and aliphatic dicarboxylic acids) and 0. 5 parts of sodium bicarbonate existing liquor neutralized at 30 ° C. This is followed by a 15-minute washing operation in 300 parts of water at room temperature.
Das derart behandelte Leder wird nun in einer aus 300 Teilen Wasser, 1 Teil Farbstoff der Formel
und 2 Teilen UV-Absorber der Formel
bestehenden frischen Flotte 30 Minuten bei 50°C gefärbt. Dann werden der Färbeflotte 3 Teile eines Fettungsmittels (Zubereitung auf Basis von sulfoniertem Chlorparaffin) und nach weiteren 30 Minuten 0,5 Teile 85 %ige Ameisensäure zugesetzt. Man färbt noch 30 Minuten bei unveränderter Temperatur weiter und stellt das gefärbte Leder nach dem Spülen wie üblich fertig. Man erhält eine egale Rotfärbung mit einer gegenüber einer Färbung gleicher Farbtiefe ohne UV-Absorber stark verbesserten Lichtechtheit.The leather treated in this way is now in a from 300 parts of water, 1 part of dye of the formula
and 2 parts of UV absorber of the formula
existing fresh liquor dyed at 50 ° C for 30 minutes. Then 3 parts of a fatliquor (preparation based on sulfonated chlorinated paraffin) are added to the dye liquor, and after a further 30 minutes 0.5 part of 85% formic acid is added. Dyeing continues for a further 30 minutes at unchanged temperature and the dyed leather is made after Rinse done as usual. A level red color is obtained with a light fastness that is greatly improved compared to a color depth of the same color without a UV absorber.
Beispiel 2: 100 Teile chromgegerbtes Rindleder (Falzgewicht) werden 15 Minuten lang in 300 Teilen Wasser bei 30°C gewaschen und anschliessend 60 Minuten in einer aus 300 Teilen Wasser, 2 Teilen Neutralisationsmittel (Na-Salze aromatischer Sulfosäuren und aliphatischer Dicarbonsäuren) und 0,5 Teilen Natriumbicarbonat bestehenden Flotte bei 30°C neutralisiert. Example 2: 100 parts of chrome-tanned cowhide (fold weight) are washed for 15 minutes in 300 parts of water at 30 ° C. and then for 60 minutes in one of 300 parts of water, 2 parts of neutralizing agent (Na salts of aromatic sulfonic acids and aliphatic dicarboxylic acids) and 0. 5 parts of sodium bicarbonate existing liquor neutralized at 30 ° C.
Man ergänzt die Neutralisierungsflotte mit 2 Teilen UV-Absorber der Formel
und lässt 60 Minuten weiterlaufen. Anschliessend folgt wiederum eine 15 Minuten dauernde Waschoperation in 300 Teilen Wasser bei Raumtemperatur.The neutralizing liquor is supplemented with 2 parts of the UV absorber of the formula
and let it run for 60 minutes. This is followed by a 15-minute washing operation in 300 parts of water at room temperature.
Das derart behandelte Leder wird nun in einer aus 300 Teilen Wasser und 2 Teilen Farbstoff der Formel
bestehenden frischen Flotte 30 Minuten bei 50°C gefärbt. Dann werden der Färbeflotte 3 Teile eines Fettungsmittels (Zubereitung auf Basis von sulfoniertem Chlorparaffin) und nach weiteren 30 Minuten 0,5 Teile 85 %ige Ameisensäure zugesetzt Man färbt noch 30 Minuten bei unveränderter Temperatur weiter und stellt das gefärbte Leder nach dem Spülen wie üblich fertig. Es ergibt sich eine egale Rotfärbung mit einer gegenüber einer Färbung gleicher Farbtiefe ohne UV-Absorber stark verbesserten Lichtechtheit.The leather treated in this way is now in a from 300 parts of water and 2 parts of dye of the formula
existing fresh liquor dyed at 50 ° C for 30 minutes. Then 3 parts of a fatliquor (preparation based on sulfonated chlorinated paraffin) are added to the dyeing liquor and, after a further 30 minutes, 0.5 part of 85% formic acid is added. The dyeing is continued for 30 minutes at unchanged temperature and the dyed leather is finished as usual after rinsing . The result is a level red color with a light fastness that is greatly improved compared to a color depth of the same color without UV absorber.
Beispiel 3: 100 Teile Chromrindleder (Falzgewicht) werden nach den Angaben im Beispiel 1 neutralisiert und dann bei 30° C in gleicher Flotte mit 7 Teilen eines flüssigen synthetischen Nachgerbstoffes (Kondensationsprodukte von Polyphenolsulfosäuren mit Formaldehyd und Harnstoff) und 2 Teilen des im Beispiel 1 verwendeten UV-Absorbers weitere 60 Minuten behandelt Danach folgt ein Waschprozess von 15 Minuten Dauer in 300 Teilen Wasser bei Raumtemperatur. Dieses nachgegerbte Chromrindleder wird anschliessend bei 50° C in einer Flotte, bestehend aus 300 Teilen Wasser sowie 2,01 Teilen der Farbstoffmischung, bestehend aus 0,67 Teilen des gelben Farbstoffes der Formel
0,67 Teilen des im Beispiel 1 verwendeten roten Farbstoffes und 0,67 Teilen des blauen Farbstoffes der Formel
gefärbt. Nach einer Färbedauer von 30 Minuten erfolgt ein Zusatz von 3 Teilen des synthetischen Fettungsmittels aus Beispiel 1 und nach weiteren 30 Minuten eine Zugabe von 1 Teil 85 %iger Ameisensäure. Nach dem Ansäuern wird die Behandlung noch 30 Minuten bei 50°C fortgesetzt. Anschliessend wird in kaltem Wasser gespült und fertiggestellt. Die erhaltene egale braune Färbung zeichnet sich gegenüber der Färbung gleicher Farbtiefe ohne UV-Absorber durch eine deutlich verbesserte Lichtechtheit aus. Example 3: 100 parts of chrome cowhide (shaved weight) are neutralized as described in Example 1 and then at 30 ° C. in the same liquor with 7 parts of a liquid synthetic retanning agent (condensation products of polyphenolsulfonic acids with formaldehyde and urea) and 2 parts of that used in Example 1 UV absorber treated for another 60 minutes. This is followed by a 15-minute washing process in 300 parts of water at room temperature. This retanned chrome cowhide is then at 50 ° C in a liquor consisting of 300 parts of water and 2.01 parts of the dye mixture consisting of 0.67 parts of the yellow dye of the formula
0.67 part of the red dye used in Example 1 and 0.67 part of the blue dye of the formula
colored. After a dyeing time of 30 minutes, 3 parts of the synthetic fatliquor from Example 1 are added, and after a further 30 minutes, 1 part of 85% formic acid is added. After acidification, the treatment is continued for 30 minutes at 50 ° C. It is then rinsed in cold water and finished. The level brown dyeing obtained is distinguished from the dyeing of the same color depth without UV absorber by a significantly improved light fastness.
Beispiel 4: 100 Teile Chromrindleder (Falzgewicht) werden nach den Angaben im Beispiel 3 neutralisiert und nachgegerbt (ohne den in Beispiel 3 genannten UV-Absorber) und danach in einer Flotte, enthaltend 300 Teile Wasser und 1,5 Teile der im Beispiel 3 eingesetzten Farbstoffmischung, während 30 Minuten bei 50°C gefärbt. Nach 30 Minuten erfolgt ein Zusatz von 3 Teilen des synthetischen Fettungsmittels aus Beispiel 1 und nach weiteren 30 Minuten eine Zugabe von 1 Teil 85 %iger Ameisensäure. Nach dem Ansäuern wird die Behandlung noch 30 Minuten bei 50°C fortgesetzt. Anschliessend wird in einem frischen Bad noch mit 1 Teil eines farbvertiefenden Hilfsmittels (Polyquaternäres Amin-Ethylenoxid-Addukt) in 300 Teilen Wasser das Leder nachbehandelt. Nach einer Behandlungsdauer von 15 Minuten wird dem Färbebad ein Drittel der obengenannten Farbstoffmischung und 2 Teile des im Beispiel 1 verwendeten UV-Absorbers zugesetzt und danach 15 Minuten bei 50°C weitergefärbt. Dann erfolgt ein Zusatz von 0,25 Teilen 85 %iger Ameisensäure. Nach weiteren 20 Minuten ist die Färbung beendet. Es wird dann in kaltem Wasser gespült. Die wie üblich fertiggestellte Färbung zeichnet sich durch sehr gute Lichtechtheit aus im Vergleich zu einer Färbung gleicher Farbtiefe ohne UV-Absorber. Example 4: 100 parts of chrome cowhide (shaved weight) are neutralized and retanned as described in Example 3 (without the UV absorber mentioned in Example 3) and then dyed in a liquor containing 300 parts of water and 1.5 parts of the dye mixture used in Example 3 at 50 ° C. for 30 minutes. After 30 minutes, 3 parts of the synthetic fatliquor from Example 1 are added, and after a further 30 minutes, 1 part of 85% formic acid is added. After acidification, the treatment is continued for 30 minutes at 50 ° C. The leather is then aftertreated in a fresh bath with 1 part of a color-deepening auxiliary (polyquaternary amine-ethylene oxide adduct) in 300 parts of water. After a treatment period of 15 minutes, a third of the above-mentioned dye mixture and 2 parts of the UV absorber used in Example 1 are added to the dyebath and then further dyeing at 50 ° C. for 15 minutes. Then 0.25 part of 85% formic acid is added. The coloring is complete after a further 20 minutes. It is then rinsed in cold water. The dyeing, which is finished as usual, is characterized by very good lightfastness in comparison to a dyeing of the same color depth without UV absorber.
Beispiel 5: 100 Teile Chromrindleder (Falzgewicht) werden nach den Angaben im Beispiel 1 neutralisiert und dann bei 30° C in gleicher Flotte mit 7 Teilen eines flüssigen synthetischen Nachgerbstoffes (Kondensationsprodukte von Naphthalinsulfosäuren mit Formaldehyd und Harnstoff) und 2 Teilen des im Beispiel 1 verwendeten UV-Absorbers weitere 60 Minuten behandelt. Danach folgt ein Waschprozess von 15 Minuten Dauer in 300 Teilen Wasser bei Raumtemperatur. Dieses nachgegerbte Chromrindleder wird anschliessend in einer Flotte, enthaltend 300 Teile Wasser und 1,5 Teile der im Beispiel 3 eingesetzten Farbstoffmischung, während 30 Minuten bei 50°C gefärbt. Nach 30 Minuten erfolgt ein Zusatz von 3 Teilen des synthetischen Fettungsmittels aus Beispiel 1 und nach weiteren 30 Minuten eine Zugabe von 1 Teil 85 %iger Ameisensäure. Nach dem Ansäuern wird die Behandlung noch 30 Minuten bei 50°C fortgesetzt. Anschliessend wird in einem frischen Bad noch mit 1 Teil eines farbvertiefenden Hilfsmittels (Polyquaternäres Amin-Ethylenoxid-Addukt) in 300 Teilen Wasser das Leder während 15 Minuten nachbehandelt. Nach einer Behandlungsdauer von 15 Minuten wird dem Färbebad ein Drittel der obengenannten Farbstoffmischung zugesetzt und danach 15 Minuten bei 50°C weitergefärbt. Dann erfolgt ein Zusatz von 0,25 Teilen 85 %iger Ameisensäure. Nach weiteren 20 Minuten ist die Färbung beendet. Es wird dann in kaltem Wasser gespült. Es ergibt sich eine volle Braunfärbung mit einer gegenüber der Färbung gleicher Farbtiefe ohne UV-Absorber stark verbesserten Lichtechtheit. Diese Färbung zeigt einen geringeren Aufhellungseffekt im Vergleich zu dem nach Beispiel 4 behandelten Leder. Example 5: 100 parts of chrome cowhide (shaved weight) are neutralized as described in Example 1 and then at 30 ° C. in the same liquor with 7 parts of a liquid synthetic retanning agent (condensation products of naphthalenesulfonic acids with formaldehyde and urea) and 2 parts of that used in Example 1 UV absorber treated for a further 60 minutes. This is followed by a washing process of 15 minutes in 300 parts of water at room temperature. This retanned chrome cowhide is then dyed in a liquor containing 300 parts of water and 1.5 parts of the dye mixture used in Example 3 at 50 ° C. for 30 minutes. After 30 minutes, 3 parts of the synthetic fatliquor from Example 1 are added, and after a further 30 minutes, 1 part of 85% formic acid is added. After acidification, the treatment is continued for 30 minutes at 50 ° C. The leather is then aftertreated in a fresh bath with 1 part of a color-deepening auxiliary (polyquaternary amine-ethylene oxide adduct) in 300 parts of water for 15 minutes. After a treatment period of 15 minutes, a third of the dye mixture mentioned above is added to the dyebath and then further dyeing at 50 ° C. for 15 minutes. Then 0.25 part of 85% formic acid is added. The coloring is complete after a further 20 minutes. It is then rinsed in cold water. The result is a full brown color with a light fastness that is greatly improved compared to the color depth of the same color without UV absorber. This coloration shows a lower lightening effect compared to the leather treated according to Example 4.
Beispiel 6: 100 Teile Chromleder (Falzgewicht) werden nach den Angaben im Beispiel 3 neutralisiert und nachgegerbt (ohne den im Beispiel 3 genannten UV-Absorber) und mit 2,0 bzw. 4,0 Teilen des im Beispiel 1 aufgeführten roten Farbstoffes gefärbt. Dieses fertiggestellte Crust-Leder wird nachbehandelt durch eine Sprit-Applikation mit einer Lösung, enthaltend 25g des im Beispiel 1 aufgeführten UV-Absorbers, 100g Methoxyisopropanol und 875g Wasser. Mit dieser milchigen Lösung wird das Leder 2 mal kreuzweise sattgespritzt und luftgetrocknet. Die mit UV-Absorber nachbehandelten Leder zeigen sehr stark verbesserte Lichtechtheit gegenüber nicht nachbehandeltem Leder. Example 6: 100 parts of chrome leather (shaved weight) are neutralized and retanned as described in Example 3 (without the UV absorber mentioned in Example 3) and dyed with 2.0 or 4.0 parts of the red dye listed in Example 1. This finished crust leather is aftertreated by a fuel application with a solution containing 25 g of the UV absorber listed in Example 1, 100 g of methoxyisopropanol and 875 g of water. With this milky solution, the leather is sprayed 2 times crosswise and air-dried. The leather aftertreated with UV absorber shows very much improved light fastness compared to leather that has not been aftertreated.
Beispiel 7: 100 Teile chromgegerbtes Rindleder (Falzgewicht) werden nach den Angaben im Beispiel 1 neutralisiert und gefärbt, man verwendet jedoch anstelle des dort eingesetzten UV-Absorbers 1 Teil 2-Hydroxy-4-methoxy-5-sulfobenzophenon. Example 7: 100 parts of chrome-tanned cowhide (fold weight) are neutralized and dyed as described in Example 1, but 1 part of 2-hydroxy-4-methoxy-5-sulfobenzophenone is used instead of the UV absorber used there.
Die Rotfärbung zeichnet sich gegenüber einer Färbung gleicher Farbtiefe ohne UV-Absorber durch eine deutlich verbesserte Lichtechtheit aus.Compared to dyeing of the same color depth without UV absorber, the red color is characterized by a significantly improved light fastness.
Beispiel 8: 100 Teile Chromrindleder (Falzgewicht) werden nach den Angaben im Beispiel 1 neutralisiert und dann bei 30° C in gleicher Flotte mit 4 Teilen eines synthetischen Nachgerbstoffes (Formaldehyd-Kondensationsprodukt aus Polyphenolsulfon und Polynaphthalinsulfosäure) und 2 Teilen des im Beispiel 1 aufgeführten UV-Absorbers weitere 60 Minuten behandelt. Danach folgt ein Waschprozess von 15 Minuten Dauer in 300 Teilen Wasser bei Raumtemperatur. Danach wird das Leder in einer Flotte aus 300 Teilen Wasser und 3 Teilen des synthetischen Fettungsmittels aus Beispiel 1 während 30 Minuten bei 50°C behandelt. Nach 30 Minuten erfolgt ein Zusatz von 0,5 Teilen 85 %iger Ameisensäure, und die Behandlung wird noch 30 Minuten bei 50°C fortgesetzt. Anschliessend wird in kaltem Wasser gespült und fertiggestellt. Das mit UV-Absorber behandelte Leder zeichnet sich gegenüber einem in gleicher Weise, aber ohne UV-Absorber, behandeltem Leder durch eine stark verbesserte Lichtechtheit aus, d.h. das Leder mit dem UV-Absorber zeigt deutlich geringere Vergilbung unter dem Einfluss von Licht. Example 8: 100 parts of chrome cowhide (shaved weight) are neutralized as described in Example 1 and then at 30 ° C. in the same liquor with 4 parts of a synthetic retanning agent (formaldehyde condensation product of polyphenolsulfone and polynaphthalenesulfonic acid) and 2 parts of the UV listed in Example 1 -Absorbers treated another 60 minutes. This is followed by a washing process of 15 minutes in 300 parts of water at room temperature. The leather is then treated in a float of 300 parts of water and 3 parts of the synthetic fatliquor from Example 1 for 30 minutes at 50 ° C. After 30 minutes, 0.5 part of 85% formic acid is added and the treatment is continued for 30 minutes at 50 ° C. It is then rinsed in cold water and finished. The leather treated with UV absorber is markedly improved over leather treated in the same way but without UV absorber Lightfastness off, ie the leather with the UV absorber shows significantly less yellowing under the influence of light.
Beispiel 9: Arbeitet man wie im Beispiel 7 beschrieben, verwendet jedoch als UV-Absorber 1 Teil 2,2'-Dihydroxy-4,4'-dimethoxy-5-sulfobenzophenon, so erhält man eine Rotfärbung, welche sich gegenüber einer Färbung gleicher Farbtiefe ohne UV-Absorber durch eine deutlich verbesserte Lichtechtheit auszeichnet. EXAMPLE 9 If the procedure is as described in Example 7, but 1 part of 2,2'-dihydroxy-4,4'-dimethoxy-5-sulfobenzophenone is used as the UV absorber, a red coloration is obtained which differs from a coloration of the same color depth characterized by a significantly improved light fastness without UV absorber.
Claims (11)
- A process for improving the light fastness of leather, which comprises treating the leather with a UV absorber chosen from the group comprising2) 2-hydroxybenzophenones of the formula (1)
C₁-C₈alkoxycarbonyl, carboxyl or sulfo, R₅ is hydrogen or halogen, R₆ is hydrogen, halogen, C₁-C₁₂alkyl, C₅-C₆cycloalkyl, C₇-C₉arylalkyl or sulfo, R₇ is hydrogen, halogen, C₁-C₄alkyl, C₁-C₄alkoxy, hydroxy or sulfo, and R₈ is C₁-C₁₂alkyl, halogen, C₅-C₆cycloalkyl, C₁-C₄alkoxy, phenyl,
(C₁-C₈alkyl)phenyl, C₁-C₈alkoxycarbonyl, carboxyethyl, C₇-C₉aralkyl or sulfo, and at least one of the radicals R₄ or R₈ must be sulfo. - A process according to claim 1, wherein a UV absorber of the formula (2) is used.
- A process according to claim 1 or 2, wherein a UV absorber of the formula
- A process according to claim 3, wherein a UV absorber of the formula (2a) in which R'₄ is hydrogen or sulfo, R'₆ is methyl, ethyl, n- or iso-propyl, n-, iso-, sec- or tert-butyl or tert-amyl, R'₇ is hydrogen and R'₈ is C₁-C₄alkyl or sulfo is used.
- A process according to one of claims 1 to 4, wherein
2-(2'-hydroxy-3'-tert-amyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-tert-butyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-sec-butyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-n-butyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-iso-butyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-isopropyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-n-propyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-ethyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-methyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-tert-butyl-5'-methylphenyl)-5-sulfobenzotriazole,
2-(2'-hydroxy-3',5'-dimethylphenyl)-5-sulfobenzotriazole,
2-(2'-hydroxy-3'-isopropyl-5'-tert-butylphenyl)-5-sulfobenzotriazole or
2-(2'-hydroxy-3'-tert-amyl-5'-methylphenyl)-5-sulfobenzotriazole
is used as the UV absorber. - A process according to one of claims 1 to 5, wherein
2-(2'-hydroxy-3'-tert-butyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-sec-butyl-5'-sulfophenyl)-benzotriazole,
2-(2'-hydroxy-3'-n-butyl-5'-sulfophenyl)-benzotriazole or
2-(2'-hydroxy-3'-iso-butyl-5'-sulfophenyl)-benzotriazole
is used as the UV absorber. - A process according to one of claims 1 to 6, wherein the treatment with the UV absorber is carried out after a dyeing operation.
- A process according to one of claims 1 to 7, wherein the UV absorber is employed in an amount of 0.25 to 7.5, preferably 0.5 to 4.0 and in particular 0.5 to 2.5% by weight, based on the weight of the leather.
- A process for improving the light-fastness of a leather dyeing, which comprises treating the leather, after dyeing, with 0.5 to 4.0% by weight, based on the weight of the leather, of a UV absorber of the formula
- A process according to claim 9, wherein 0.5 to 2.5% by weight, based on the weight of the leather, of a UV absorber of the formula (2a) defined in claim 9, in which R'₄ is hydrogen or sulfo, R'₆ is methyl, ethyl, n- or iso-propyl, n-, iso-, sec- or tert-butyl or tert-amyl, R'₇ is hydrogen and R'₈ is C₁-C₄alkyl or sulfo, is used.
- The leather treated according to claims 1 to 10.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CH1321/91 | 1991-05-02 | ||
| CH132191 | 1991-05-02 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0512946A1 EP0512946A1 (en) | 1992-11-11 |
| EP0512946B1 true EP0512946B1 (en) | 1995-09-13 |
Family
ID=4207712
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP19920810292 Expired - Lifetime EP0512946B1 (en) | 1991-05-02 | 1992-04-22 | Process for improving the fastness to light of leather |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP0512946B1 (en) |
| JP (1) | JPH05272076A (en) |
| DE (1) | DE59203628D1 (en) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5556973A (en) * | 1994-07-27 | 1996-09-17 | Ciba-Geigy Corporation | Red-shifted tris-aryl-s-triazines and compositions stabilized therewith |
| TW440633B (en) * | 1996-09-27 | 2001-06-16 | Kuraray Co | Suede-like artificial leather and its preparation |
| GB0118156D0 (en) * | 2001-07-25 | 2001-09-19 | Pittards Plc | Leather production |
| JP5308039B2 (en) | 2007-02-20 | 2013-10-09 | 富士フイルム株式会社 | Polymer materials containing UV absorbers |
| JP5276876B2 (en) | 2007-03-30 | 2013-08-28 | 富士フイルム株式会社 | UV absorber composition |
| WO2009022736A1 (en) | 2007-08-16 | 2009-02-19 | Fujifilm Corporation | Heterocyclic compound, ultraviolet ray absorbent, and composition comprising the ultraviolet ray absorbent |
| JP5250289B2 (en) | 2008-03-31 | 2013-07-31 | 富士フイルム株式会社 | UV absorber composition |
| JP5244437B2 (en) | 2008-03-31 | 2013-07-24 | 富士フイルム株式会社 | UV absorber composition |
| JP2009270062A (en) | 2008-05-09 | 2009-11-19 | Fujifilm Corp | Ultraviolet absorbent composition |
| ES2463674T3 (en) | 2009-01-19 | 2014-05-28 | Basf Se | Organic black pigments and their preparation |
| JP2011202144A (en) * | 2010-03-01 | 2011-10-13 | Fujifilm Corp | Adhesive composition, adhesive tape or film using the same, surface protective film, laminated glass and solar cell module |
| ES2930671T3 (en) | 2014-08-04 | 2022-12-21 | Citizen Watch Co Ltd | Leather or leather article and method for producing the same, hexavalent chromium treatment agent, method for treating hexavalent chromium in raw leather or raw leather article |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH478589A (en) * | 1965-09-24 | 1969-09-30 | Ciba Geigy | Use of aryl-1,3,5-triazines as stabilizers against ultraviolet radiation and the effects of heat outside the textile industry |
| DE3581002D1 (en) * | 1984-06-22 | 1991-02-07 | Ilford Ag | HYDROXYPHENYLTRIAZINE, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE AS A UV ABSORBER. |
| US4775386A (en) * | 1986-05-05 | 1988-10-04 | Ciba-Geigy Corporation | Process for photochemical stabilization of undyed and dyed polyamide fibre material and blends thereof with other fibres: copper complex and light stabilizer treatment |
-
1992
- 1992-04-22 DE DE59203628T patent/DE59203628D1/en not_active Expired - Fee Related
- 1992-04-22 EP EP19920810292 patent/EP0512946B1/en not_active Expired - Lifetime
- 1992-05-01 JP JP4112671A patent/JPH05272076A/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| EP0512946A1 (en) | 1992-11-11 |
| JPH05272076A (en) | 1993-10-19 |
| DE59203628D1 (en) | 1995-10-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0512946B1 (en) | Process for improving the fastness to light of leather | |
| EP0459950B1 (en) | Stabilisation of dyes on polyamide fibres | |
| EP0475905A1 (en) | Process for the photochemical stabilisation of wool | |
| DE863982C (en) | Process for refining untanned collagenous material | |
| EP0350803B1 (en) | Process for the pigmentation of leather in a bath | |
| CH671052A5 (en) | ||
| DE2255256A1 (en) | PROCESS TO INCREASE THE AFFINITY FOR ANIONIC DYES OF HIGH MOLECULAR ORGANIC COMPOUNDS CONTAINING ALKYLABLE GROUPS | |
| DE2835035B2 (en) | Process for dyeing cellulose fibers with reactive dyes using the exhaust method | |
| CH673465A5 (en) | ||
| EP0558450B1 (en) | Process for dyeing leather with mixtures of dyes | |
| EP0577556B1 (en) | Process for the trichromatic dyeing of leather with dye mixtures | |
| DE10044642A1 (en) | Dyed leather and dyeing process for tanned leather | |
| EP0564404B1 (en) | Process for leather dyeing with mixtures of dyes | |
| EP0024014B1 (en) | Method of retanning mineral tanned leather with aromatic sulfonic acids | |
| DE1619349A1 (en) | Dye mixture for browning fur | |
| DE4330283B4 (en) | Stabilization of dyeings on polyamide fibers | |
| DE69527064T2 (en) | Leather tanning process and tanning agents | |
| EP0060433A1 (en) | Process for dyeing hydrophobic fibrous material | |
| EP0423561A1 (en) | Process for dyeing leather | |
| DE4404904A1 (en) | Retanning process | |
| DE4319020B4 (en) | Blue chromium complex dyes, their preparation and use | |
| DE2056009C (en) | Process for uniform and streak-free dyeing of synthetic polyamide fiber material | |
| DE933023C (en) | Process for the production of water-insoluble azo dyes on the fiber | |
| DE3151594C2 (en) | ||
| DE1057061B (en) | Process for the production of water-insoluble azo dyes on vegetable fibers |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): CH DE FR GB IT LI |
|
| 17P | Request for examination filed |
Effective date: 19930510 |
|
| 17Q | First examination report despatched |
Effective date: 19940221 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): CH DE FR GB IT LI |
|
| ET | Fr: translation filed | ||
| REF | Corresponds to: |
Ref document number: 59203628 Country of ref document: DE Date of ref document: 19951019 |
|
| ITF | It: translation for a ep patent filed |
Owner name: SOCIETA' ITALIANA BREVETTI S.P.A. |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19951020 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19960306 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 19960319 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19960320 Year of fee payment: 5 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19960620 Year of fee payment: 5 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Effective date: 19970422 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970430 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19970430 |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 19970422 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19971231 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980101 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20050422 |