EP0477772A1 - Photographic silver halide materials - Google Patents
Photographic silver halide materials Download PDFInfo
- Publication number
- EP0477772A1 EP0477772A1 EP91115913A EP91115913A EP0477772A1 EP 0477772 A1 EP0477772 A1 EP 0477772A1 EP 91115913 A EP91115913 A EP 91115913A EP 91115913 A EP91115913 A EP 91115913A EP 0477772 A1 EP0477772 A1 EP 0477772A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- emulsion
- iodide
- silver halide
- twinned
- core
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 229910052709 silver Inorganic materials 0.000 title claims abstract description 44
- 239000004332 silver Substances 0.000 title claims abstract description 44
- 239000000463 material Substances 0.000 title claims abstract description 40
- -1 silver halide Chemical class 0.000 title claims abstract description 33
- 239000000839 emulsion Substances 0.000 claims abstract description 94
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 claims abstract description 27
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 claims abstract description 13
- 239000000203 mixture Substances 0.000 claims abstract description 13
- 239000013078 crystal Substances 0.000 claims description 44
- ZUNKMNLKJXRCDM-UHFFFAOYSA-N silver bromoiodide Chemical compound [Ag].IBr ZUNKMNLKJXRCDM-UHFFFAOYSA-N 0.000 claims description 8
- 239000000243 solution Substances 0.000 description 28
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 18
- 238000000034 method Methods 0.000 description 14
- JHJLBTNAGRQEKS-UHFFFAOYSA-M sodium bromide Chemical compound [Na+].[Br-] JHJLBTNAGRQEKS-UHFFFAOYSA-M 0.000 description 14
- LSNNMFCWUKXFEE-UHFFFAOYSA-L sulfite Chemical compound [O-]S([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-L 0.000 description 10
- 108010010803 Gelatin Proteins 0.000 description 9
- 239000008273 gelatin Substances 0.000 description 9
- 229920000159 gelatin Polymers 0.000 description 9
- 235000019322 gelatine Nutrition 0.000 description 9
- 235000011852 gelatine desserts Nutrition 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 229910001961 silver nitrate Inorganic materials 0.000 description 9
- 150000004694 iodide salts Chemical group 0.000 description 8
- 239000002585 base Substances 0.000 description 7
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 6
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 5
- 238000000576 coating method Methods 0.000 description 5
- 239000000084 colloidal system Substances 0.000 description 5
- 150000004820 halides Chemical class 0.000 description 5
- 229920002284 Cellulose triacetate Polymers 0.000 description 4
- 206010070834 Sensitisation Diseases 0.000 description 4
- NNLVGZFZQQXQNW-ADJNRHBOSA-N [(2r,3r,4s,5r,6s)-4,5-diacetyloxy-3-[(2s,3r,4s,5r,6r)-3,4,5-triacetyloxy-6-(acetyloxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6s)-4,5,6-triacetyloxy-2-(acetyloxymethyl)oxan-3-yl]oxyoxan-2-yl]methyl acetate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](OC(C)=O)[C@H]1OC(C)=O)O[C@H]1[C@@H]([C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O1)OC(C)=O)COC(=O)C)[C@@H]1[C@@H](COC(C)=O)O[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O NNLVGZFZQQXQNW-ADJNRHBOSA-N 0.000 description 4
- SWLVFNYSXGMGBS-UHFFFAOYSA-N ammonium bromide Chemical compound [NH4+].[Br-] SWLVFNYSXGMGBS-UHFFFAOYSA-N 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- JKFYKCYQEWQPTM-UHFFFAOYSA-N 2-azaniumyl-2-(4-fluorophenyl)acetate Chemical compound OC(=O)C(N)C1=CC=C(F)C=C1 JKFYKCYQEWQPTM-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 229910021612 Silver iodide Inorganic materials 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 239000004816 latex Substances 0.000 description 3
- 229920000126 latex Polymers 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 230000005070 ripening Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 230000001235 sensitizing effect Effects 0.000 description 3
- 229940045105 silver iodide Drugs 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- 238000001016 Ostwald ripening Methods 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 239000004133 Sodium thiosulphate Substances 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- 239000005864 Sulphur Substances 0.000 description 2
- 238000013019 agitation Methods 0.000 description 2
- 229910052783 alkali metal Inorganic materials 0.000 description 2
- 150000001340 alkali metals Chemical class 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- XYXNTHIYBIDHGM-UHFFFAOYSA-N ammonium thiosulfate Chemical compound [NH4+].[NH4+].[O-]S([O-])(=O)=S XYXNTHIYBIDHGM-UHFFFAOYSA-N 0.000 description 2
- 239000002518 antifoaming agent Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- HIOZQPNKVSTJOQ-UHFFFAOYSA-N bis(2-hydroxyethyl)azanium;hydrogen sulfite Chemical compound OS(O)=O.OCCNCCO HIOZQPNKVSTJOQ-UHFFFAOYSA-N 0.000 description 2
- 239000000298 carbocyanine Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- CBEQRNSPHCCXSH-UHFFFAOYSA-N iodine monobromide Chemical compound IBr CBEQRNSPHCCXSH-UHFFFAOYSA-N 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- QWYZFXLSWMXLDM-UHFFFAOYSA-M pinacyanol iodide Chemical compound [I-].C1=CC2=CC=CC=C2N(CC)C1=CC=CC1=CC=C(C=CC=C2)C2=[N+]1CC QWYZFXLSWMXLDM-UHFFFAOYSA-M 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- ADZWSOLPGZMUMY-UHFFFAOYSA-M silver bromide Chemical compound [Ag]Br ADZWSOLPGZMUMY-UHFFFAOYSA-M 0.000 description 2
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 2
- 235000019345 sodium thiosulphate Nutrition 0.000 description 2
- GGCZERPQGJTIQP-UHFFFAOYSA-N sodium;9,10-dioxoanthracene-2-sulfonic acid Chemical compound [Na+].C1=CC=C2C(=O)C3=CC(S(=O)(=O)O)=CC=C3C(=O)C2=C1 GGCZERPQGJTIQP-UHFFFAOYSA-N 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- STCOOQWBFONSKY-UHFFFAOYSA-N tributyl phosphate Chemical compound CCCCOP(=O)(OCCCC)OCCCC STCOOQWBFONSKY-UHFFFAOYSA-N 0.000 description 2
- 229930024421 Adenine Natural products 0.000 description 1
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 229960000643 adenine Drugs 0.000 description 1
- 235000011114 ammonium hydroxide Nutrition 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 230000000712 assembly Effects 0.000 description 1
- 238000000429 assembly Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 238000004182 chemical digestion Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 238000001246 colloidal dispersion Methods 0.000 description 1
- 239000011258 core-shell material Substances 0.000 description 1
- 238000002109 crystal growth method Methods 0.000 description 1
- LNGNZSMIUVQZOX-UHFFFAOYSA-L disodium;dioxido(sulfanylidene)-$l^{4}-sulfane Chemical compound [Na+].[Na+].[O-]S([O-])=S LNGNZSMIUVQZOX-UHFFFAOYSA-L 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- BLCTWBJQROOONQ-UHFFFAOYSA-N ethenyl prop-2-enoate Chemical compound C=COC(=O)C=C BLCTWBJQROOONQ-UHFFFAOYSA-N 0.000 description 1
- 230000003311 flocculating effect Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- DZVCFNFOPIZQKX-LTHRDKTGSA-M merocyanine Chemical compound [Na+].O=C1N(CCCC)C(=O)N(CCCC)C(=O)C1=C\C=C\C=C/1N(CCCS([O-])(=O)=O)C2=CC=CC=C2O\1 DZVCFNFOPIZQKX-LTHRDKTGSA-M 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910000069 nitrogen hydride Inorganic materials 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 239000004014 plasticizer Substances 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000233 poly(alkylene oxides) Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 239000010948 rhodium Substances 0.000 description 1
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 229940065287 selenium compound Drugs 0.000 description 1
- 150000003343 selenium compounds Chemical class 0.000 description 1
- 231100000489 sensitizer Toxicity 0.000 description 1
- 239000003352 sequestering agent Substances 0.000 description 1
- 229940080262 sodium tetrachloroaurate Drugs 0.000 description 1
- SDKPSXWGRWWLKR-UHFFFAOYSA-M sodium;9,10-dioxoanthracene-1-sulfonate Chemical compound [Na+].O=C1C2=CC=CC=C2C(=O)C2=C1C=CC=C2S(=O)(=O)[O-] SDKPSXWGRWWLKR-UHFFFAOYSA-M 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 239000001117 sulphuric acid Substances 0.000 description 1
- 235000011149 sulphuric acid Nutrition 0.000 description 1
- 150000003567 thiocyanates Chemical class 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- G—PHYSICS
- G03—PHOTOGRAPHY; CINEMATOGRAPHY; ANALOGOUS TECHNIQUES USING WAVES OTHER THAN OPTICAL WAVES; ELECTROGRAPHY; HOLOGRAPHY
- G03C—PHOTOSENSITIVE MATERIALS FOR PHOTOGRAPHIC PURPOSES; PHOTOGRAPHIC PROCESSES, e.g. CINE, X-RAY, COLOUR, STEREO-PHOTOGRAPHIC PROCESSES; AUXILIARY PROCESSES IN PHOTOGRAPHY
- G03C1/00—Photosensitive materials
- G03C1/005—Silver halide emulsions; Preparation thereof; Physical treatment thereof; Incorporation of additives therein
- G03C1/035—Silver halide emulsions; Preparation thereof; Physical treatment thereof; Incorporation of additives therein characterised by the crystal form or composition, e.g. mixed grain
Definitions
- This invention relates to photographic silver halide material.
- One method of producing high speed emulsions is to employ silver halide grains of the core/shell type which have a relatively high iodide core. That is to say a core which can comprise up to 40 mole iodide. But it has proved difficult to incorporate iodide into the silver halide crystals in a controlled manner.
- step b) which comprises mixing in a dispersion medium which contains the seed crystals an aqueous solution of a silver salt and an aqueous solution of an alkali metal or ammonium bromide or chloride or mixtures thereof so forming twinned silver halide crystals containing the halide being added and iodide from the seed crystals and continuing the addition of the silver halide until dissolution of the seed crystals is substantially complete, then c) causing the twinned crystals formed in step b) to increase in size by adding to the colloidal dispersion further alkali metal or ammonium halide and aqueous silver salt solution and then d) removing the water-soluble salts and chemically sensitising the silver halide crystals.
- a controlled high iodide containing core can be formed in step b) and a shell added in step c).
- Photographic silver halide emulsion prepared by the above method are hereinafter referred to as twinned high iodide core emulsions.
- Twinned high iodide core emulsions of this type have been used in the preparation of photographic material and they yield high speed photographic material. Moreover, the granularity of the processed negative is satisfactory. However, variable results are obtained when this material after exposure is developed in different types of developing solutions. Whilst the manufacturers of photographic materials often state in the instruction leaflet or on the film box what is the preferred developing solution it is not possible for the manufacturer to control this. The use by the film user of other developing solutions can lead to inferior results thus it is desirable that photographic materials should be able to produce optimum results in all the main classes of developing solutions.
- emulsion is present in an assembly which comprises the twinned high iodide core emulsion in order to extend the useable exposure range of the material and to improve its tone scale.
- drawbacks have arisen when the two emulsions are coated as a bipack with the high iodide core-shell above the other emulsion with regard to granularity of the developed image on development of such material in low-sulphite developers.
- photographic silver halide material which comprises coated on a subbed film base a silver halide emulsion which is a blend of a silver halide emulsion of the twinned high-iodide core type as just described which comprises from 10 to 20 mole % iodide and of a silver iodobromide emulsion which comprises less than 10 mole % iodide and which has a smaller crystal size and a photographic speed of from 0.2 to 1.0 log E units less than the twinned high-iodide core emulsion, the amounts of the emulsions in the blend being from 20 to 70% by weight of the total silver present being derived from the emulsion of the twinned high-iodide core type and the remainder from the smaller crystal size silver iodobromide emulsion.
- the blend Preferably in the blend 30-50% by weight of the total silver present is derived from the twinned high-iodide core emulsion and most preferably 35 - 45% by weight.
- high iodide core is meant a core which comprises from 20 to 40 mole% iodide.
- the amount of iodide in the core is from 35 to 40 mole % iodide.
- the volume of the core can be from 10% of the total volume of the crystal up to 50% of the total volume of the crystal.
- a useful volume of the core is from 25 to 45% of the total volume of the crystal.
- the core is at least one silver bromide shell which can comprise up to 10 mole % iodide overall. Often a plurality of shells are present. In one embodiment there is present a so-called development control layer which comprises up to 15 mole % iodide.
- the silver iodobromide emulsion which is of smaller crystal size is of the type used in a number of commercially available black and white films.
- the Theory of the Photographic Process 4th Edition by J H James, published in 1977, chapter 3, pages 88-104 describes the preparation of such emulsions.
- the iodobromide emulsion of smaller crystal size is of the ammonia ripening process type. In another embodiment the iodobromide emulsion of the smaller crystal size has been prepared by a controlled crystal growth method.
- the range of crystal size for the silver halide of the twinned high iodide core type is from 0.6 to 1.2 /1 .m.
- the range of crystal size for the silver iodobromide emulsion which comprises less than 10 mole % iodide is from 0.4 to 0.9tim. There must be a difference of at least 0.1 ⁇ m in the crystal size of the two emulsions in the blend.
- high sulphite developers often supplied as solid ingredients and which comprise when made up from 0.6 to 0.8 moles of sulphite per litre and so-called low sulphite developers which are often supplied as concentrated liquids and which comprise from 0.01 to 0.1 moles of sulphite per litre.
- low sulphite developers which are often supplied as concentrated liquids and which comprise from 0.01 to 0.1 moles of sulphite per litre.
- the photographic material as shown in the examples which follow exhibits a high speed and low granularity in both types of developing solutions.
- the water-soluble salts formed in the emulsification processes may be removed by any of the well-known methods. Such methods often involve flocculating the silver halide and colloid dispersing agent, removing this flocculate from the then aqueous medium, washing it and redispersing it in water.
- One other common method is ultrafiltration, in which the emulsion is passed over a membrane under pressure. The pore size of the membrane is such that the silver halide crystals and most of the colloid dispersing medium is retained, whilst water and solutes permeate through.
- Most of the well-known methods allow the emulsion to be concentrated as well as washed. This is important when weak reagent solutions are employed, particularly those with concentrations below 3M.
- the silver halide crystals in the emulsion are preferably chemically sensitised on the surface by any of the well known means, for example by use of sulphur or selenium compounds and/or salts of the noble metals such as gold, iridium, rhodium, osmium, palladium or platinum.
- Chemical sensitisation is preferably carried out in the presence of sulphur-containing ripening agents such as thioethers or thiocyanate compounds.
- the fully grown crystals are sensitised in this manner, so that the products of chemical sensitisation are formed on the surface of the crystal so that such sensitised crystals become developable in a surface developer after exposure to light.
- the silver halide crystals in the emulsion of the present invention are preferably spectrally sensitised by the addition of spectral sensitisers for example carbocyanine and merocyanine dyes to the emulsions.
- the emulsions may contain any of the additives commonly used in photographic emulsions for example wetting agents, such as polyalkene oxides, stabilising agents, such as tetraazaindenes, metal sequestering agents, growth or crystal habit modifying agents commonly used for silver halide such as adenine and plasticisers such as glycerol to reduce the effect of mechanical stress.
- wetting agents such as polyalkene oxides
- stabilising agents such as tetraazaindenes, metal sequestering agents
- growth or crystal habit modifying agents commonly used for silver halide such as adenine
- plasticisers such as glycerol
- the colloid dispersing medium is gelatin or a mixture of gelatin and a water-soluble latex for example or latex vinyl acrylate-containing polymer. Most preferably if such a latex is present in the final emulsion it is added after all crystal growth has occurred.
- a water-soluble latex for example casein, polyvinyl pyrrolidone or polyvinyl alcohol may be used alone or together with gelatin.
- the silver halide emulsion assemblies of the present invention exhibit high speed and low granularity, thus they are of particular use in high speed camera film material.
- the invention includes coated photographic materials containing the defined emulsion blends in at least one layer.
- Step A Preparation of monosized silver iodide emulsion
- the silver iodide seed emulsion was grown as described in Example 1 of British Patent Specification No. 1596602 except that the crystals had an average size of 0.65tim.
- step C 48% of the emulsion prepared in step C was taken and the crystal volume was increased by double jetting 1.5 Molar solultions of silver nitrate and sodium bromide maintaining the potential at -33mV at 65C. A total of 1070 cm3 of each solution was added at 20 cm3/minute.
- the emulsion was composed of AglBr in which 15 mole % of the halide was iodide and the median crystal size was 1.0 um (based on equivalent circular diameter).
- the emulsion was then adjusted at 40 °C to pH 6 and pAg 8.2. It was then digested at 52 °C with sodium thiosulphate and sodium tetrachloroaurate to chemically sensitise it. After optimised chemical sensitisation a tetrazindene stabiliser was added.
- the mole % iodide in the core was 35% and the volume of the core was 43% of the whole crystal.
- the average mean size of the crystals was 1.0 ⁇ m.
- a mixed halide solution which comprised 90.8% ammonium bromide and 9.2% potassium iodide 266 litres with a molar concentration of 1.75 was then jetted in to the gelatin solution.
- the emulsion as thus prepared was then desalinated and then chemically digested using sodium thiosulphite and a gold salt as with emulsion A. After chemical digestion was complete a tetraazindene stabiliser was added to the emulsion. An optimum quantity of a carbocyanine sensitising dye was added to both emulsions A and B.
- Both emulsions A and B were coated on to a film base, exposed and developed and their photographic speeds were compared at full development.
- Emulsion A had speed of 5.92 log E units and Emulsion B had a speed of 5.42 log E units.
- a blended emulsion was then prepared by mixing emulsions A and B so that 40% by weight of the silver was derived from emulsion A and 60% by weight of the silver was derived from emulsion B.
- Samples of material 1 and 2 were then light exposed through a step-wedge of 1/30 second by a light source of 250 lux.
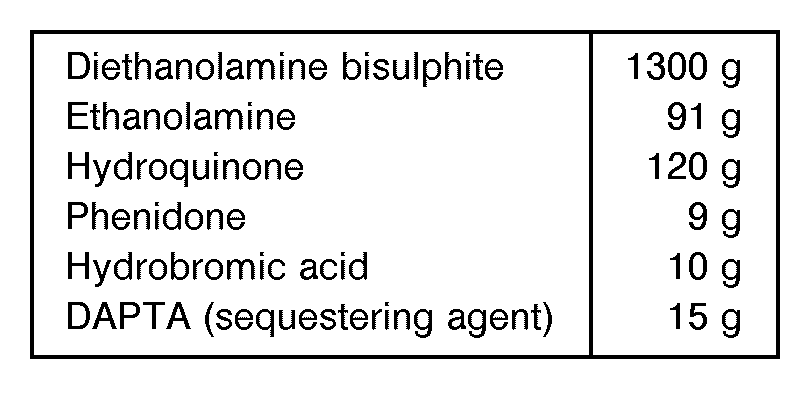
- Developer 1 was a high sulphite developer of the following formula.
- Developer 2 was a low sulphite developer in which the sulphite was present as diethanolamine bisulphite.
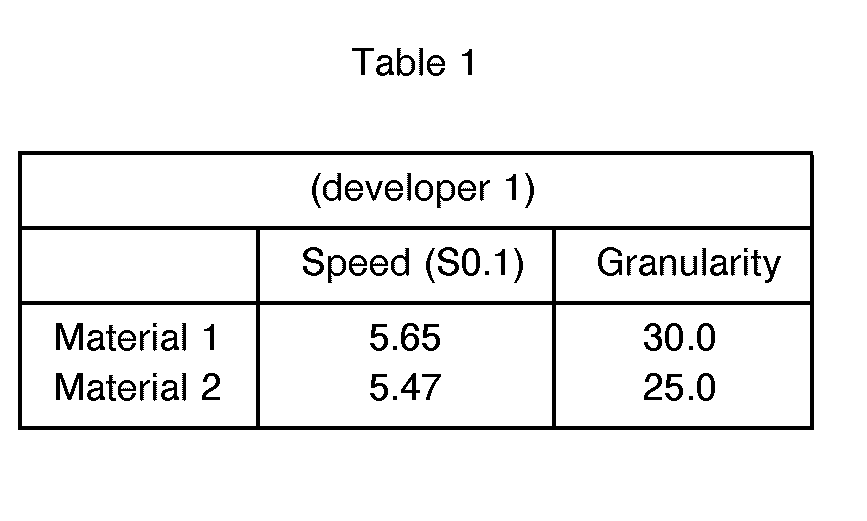
- Both materials 1 and 2 were found to exhibit a photographic speed of approximately 400 I.S.O.. Both materials exhibited low fog.
- the granularity of material 1 was acceptable but the image in the high lights was noticeably grainy. However in material 2 the granularity was low and even in the high lights very little if any grain was visible.
- the silver iodide seed emulsion was grown as described in Example 1 of British Patent Specification No. 1596602 except that the crystals had an average size of 0.65 ⁇ m.
- Step D Formation of development control layer and final growth
- step C 48% of the emulsion prepared in step C was taken and the crystal volume was increased by double jetting 1.5 Molar solutions of silver nitrate and a 95 : 5 mole percent mixture of sodium bromide and potassium iodide maintaining the potential at -33mV at 65C. A total of 713 cm3 of each solution was added at 20cm3/minute. Finally, 357 cm3 of solutions of 1.5 molar silver bromide and 1.5 molar sodium bromide were added maintaining the same temperature and potential at 20cm 3 /minute.
- the emulsion was composed of AglBr in which 16.3 mole % of the halide was iodide and the median crystal was 1.0 um (based on equivalent circle diameter).
- the emulsion was desalinated and redispersed with a solution of limed ossein gelatin.
- the emulsion was then chemically digested using sodium thiosulphate and gold. After chemical sensitisation was complete a tetraazindine stabiliser was added to the emulsion.
- a sample of emulsion B as described in Example 1 was also prepared.
- Emulsion C was coated on a film base, exposed and fully developed. It was found to have a speed of 5.90 log E units. This is to be compared with the speed of 5.42 log E units for Emulsions B as tested in Example 1.
- Material 1 Sufficient of emulsion B was then coated on a cellulose triacetate base to yield a coating weight of 2.8g Ag/m 2 . Over this layer was coated sufficient of emulsion C to yield a coating weight of 4.2 Ag/ m2.
- a blended emulsion was then prepared by mixing emulsions C and B so that 40% by weight of the silver was derived from emulsion C and 60% by weight of the silver was derived from emulsion B.
- Samples of material 1 and 2 were then light exposed through a step-wedge of 1/30 second by a light source of 250 lux.
- Developer 1 was a high sulphite developer of the following formula.
- Developer 2 was a low sulphite developer in which the sulphite was present as diethanolamine bisulphite. Made up to 1 litre and then diluted 1 + 31 for use.
- the granularity measured was the root mean square granularity which was measured at a density of 1.0 above Dmin using a 21 ⁇ square aperture.
- the density of the scale was specular.
Landscapes
- Chemical & Material Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Silver Salt Photography Or Processing Solution Therefor (AREA)
Abstract
Photographic silver halide material which comprises coated on a subbed film base a silver halide emulsion which is a blend of a silver halide emulsion of the twinned high-iodide core type as just described which comprises from 10 to 20 mole % iodide and of a silver iodiobromide emulsion.
Description
- This invention relates to photographic silver halide material.
- It is a continuing objective of manufacturers of photographic silver halide material to produce high speed film material which has a low granularity on development. One method of producing high speed emulsions is to employ silver halide grains of the core/shell type which have a relatively high iodide core. That is to say a core which can comprise up to 40 mole iodide. But it has proved difficult to incorporate iodide into the silver halide crystals in a controlled manner. However in British patent specifications 1520976; 1570581 and 1596602 a method is described for preparing twinned monosize crystal which have a desirable high iodide core and which exhibit a high photographic speed and wherein the iodide is incorporated into the core in a controlled manner.
- The method described in these patent specifications can be summed up by the following steps:- a) forming in a colloid dispensing medium silver halide crystals being predominantly of the Agl hexagonal lattice structure, these are then used as seed crystals for step b) which comprises mixing in a dispersion medium which contains the seed crystals an aqueous solution of a silver salt and an aqueous solution of an alkali metal or ammonium bromide or chloride or mixtures thereof so forming twinned silver halide crystals containing the halide being added and iodide from the seed crystals and continuing the addition of the silver halide until dissolution of the seed crystals is substantially complete, then c) causing the twinned crystals formed in step b) to increase in size by adding to the colloidal dispersion further alkali metal or ammonium halide and aqueous silver salt solution and then d) removing the water-soluble salts and chemically sensitising the silver halide crystals.
- Thus in this process a controlled high iodide containing core can be formed in step b) and a shell added in step c).
- Photographic silver halide emulsion prepared by the above method are hereinafter referred to as twinned high iodide core emulsions.
- Twinned high iodide core emulsions of this type have been used in the preparation of photographic material and they yield high speed photographic material. Moreover, the granularity of the processed negative is satisfactory. However, variable results are obtained when this material after exposure is developed in different types of developing solutions. Whilst the manufacturers of photographic materials often state in the instruction leaflet or on the film box what is the preferred developing solution it is not possible for the manufacturer to control this. The use by the film user of other developing solutions can lead to inferior results thus it is desirable that photographic materials should be able to produce optimum results in all the main classes of developing solutions.
- Moreover it may be desirable that another emulsion is present in an assembly which comprises the twinned high iodide core emulsion in order to extend the useable exposure range of the material and to improve its tone scale. However drawbacks have arisen when the two emulsions are coated as a bipack with the high iodide core-shell above the other emulsion with regard to granularity of the developed image on development of such material in low-sulphite developers.
- Therefore according to the present invention there is provided photographic silver halide material which comprises coated on a subbed film base a silver halide emulsion which is a blend of a silver halide emulsion of the twinned high-iodide core type as just described which comprises from 10 to 20 mole % iodide and of a silver iodobromide emulsion which comprises less than 10 mole % iodide and which has a smaller crystal size and a photographic speed of from 0.2 to 1.0 log E units less than the twinned high-iodide core emulsion, the amounts of the emulsions in the blend being from 20 to 70% by weight of the total silver present being derived from the emulsion of the twinned high-iodide core type and the remainder from the smaller crystal size silver iodobromide emulsion.
- Preferably in the blend 30-50% by weight of the total silver present is derived from the twinned high-iodide core emulsion and most preferably 35 - 45% by weight.
- By high iodide core is meant a core which comprises from 20 to 40 mole% iodide. Preferably the amount of iodide in the core is from 35 to 40 mole % iodide.
- The volume of the core can be from 10% of the total volume of the crystal up to 50% of the total volume of the crystal. A useful volume of the core is from 25 to 45% of the total volume of the crystal.
- Present on the core is at least one silver bromide shell which can comprise up to 10 mole % iodide overall. Often a plurality of shells are present. In one embodiment there is present a so-called development control layer which comprises up to 15 mole % iodide.
- The silver iodobromide emulsion which is of smaller crystal size is of the type used in a number of commercially available black and white films. The Theory of the Photographic Process 4th Edition by J H James, published in 1977, chapter 3, pages 88-104 describes the preparation of such emulsions.
- In one embodiment the iodobromide emulsion of smaller crystal size is of the ammonia ripening process type. In another embodiment the iodobromide emulsion of the smaller crystal size has been prepared by a controlled crystal growth method.
- The range of crystal size for the silver halide of the twinned high iodide core type is from 0.6 to 1.2/1.m.
- The range of crystal size for the silver iodobromide emulsion which comprises less than 10 mole % iodide is from 0.4 to 0.9tim. There must be a difference of at least 0.1µm in the crystal size of the two emulsions in the blend.
- Two types of developer which are used to develop exposed black and white films are so-called high sulphite developers often supplied as solid ingredients and which comprise when made up from 0.6 to 0.8 moles of sulphite per litre and so-called low sulphite developers which are often supplied as concentrated liquids and which comprise from 0.01 to 0.1 moles of sulphite per litre. Examples of both types of developing solutions are set forth in the example which follows.
- The photographic material as shown in the examples which follow exhibits a high speed and low granularity in both types of developing solutions.
- The water-soluble salts formed in the emulsification processes may be removed by any of the well-known methods. Such methods often involve flocculating the silver halide and colloid dispersing agent, removing this flocculate from the then aqueous medium, washing it and redispersing it in water. One other common method is ultrafiltration, in which the emulsion is passed over a membrane under pressure. The pore size of the membrane is such that the silver halide crystals and most of the colloid dispersing medium is retained, whilst water and solutes permeate through. Most of the well-known methods allow the emulsion to be concentrated as well as washed. This is important when weak reagent solutions are employed, particularly those with concentrations below 3M.
- The silver halide crystals in the emulsion are preferably chemically sensitised on the surface by any of the well known means, for example by use of sulphur or selenium compounds and/or salts of the noble metals such as gold, iridium, rhodium, osmium, palladium or platinum. Chemical sensitisation is preferably carried out in the presence of sulphur-containing ripening agents such as thioethers or thiocyanate compounds. The fully grown crystals are sensitised in this manner, so that the products of chemical sensitisation are formed on the surface of the crystal so that such sensitised crystals become developable in a surface developer after exposure to light.
- The silver halide crystals in the emulsion of the present invention are preferably spectrally sensitised by the addition of spectral sensitisers for example carbocyanine and merocyanine dyes to the emulsions.
- The emulsions may contain any of the additives commonly used in photographic emulsions for example wetting agents, such as polyalkene oxides, stabilising agents, such as tetraazaindenes, metal sequestering agents, growth or crystal habit modifying agents commonly used for silver halide such as adenine and plasticisers such as glycerol to reduce the effect of mechanical stress.
- Preferably the colloid dispersing medium is gelatin or a mixture of gelatin and a water-soluble latex for example or latex vinyl acrylate-containing polymer. Most preferably if such a latex is present in the final emulsion it is added after all crystal growth has occurred. However other water-soluble colloids for example casein, polyvinyl pyrrolidone or polyvinyl alcohol may be used alone or together with gelatin.
- The silver halide emulsion assemblies of the present invention exhibit high speed and low granularity, thus they are of particular use in high speed camera film material.
- The invention includes coated photographic materials containing the defined emulsion blends in at least one layer.
- The following Examples will serve to illustrate the invention.
- Preparation of silver iodobromide emulsion which is of the twinned high-iodide core type. This is emulsion A.
- The silver iodide seed emulsion was grown as described in Example 1 of British Patent Specification No. 1596602 except that the crystals had an average size of 0.65tim.
- 16.9 litres of 4.1 % gel solution was added to a quantity of the emulsion prepared in step A containing 35 moles of silver at a temperature of 70 ° C and with constant stirring. 1.5m solutions of silver nitrate and sodium bromide were added by double jetting until 35 litres of silver nitrate had been added. The initial jetting rate was 199 cm3/minute. The potential was maintained at -58mV at 70 C.
- 3.6% of the emulsion produced in step B was taken, and .35 litres of a 29% w/v inert gel solution added with thorough agitation at 65 °C. 0.2 cm3 of tri-n-butyl phosphate was added as an antifoam. The volume of each crystal was then increased by double jetting 1.5 m solutions of silver nitrate and sodium bromide while maintaining the potential at -33 mV at 65 °C. The first 355 cm3 was added as a jetting rate of 10 cm3 minute, a second 355 cm3 was added at a jetting rate of 15 cm3/minute and then 533 cm3 was added at 20 cm3/minute.
- 48% of the emulsion prepared in step C was taken and the crystal volume was increased by double jetting 1.5 Molar solultions of silver nitrate and sodium bromide maintaining the potential at -33mV at 65C. A total of 1070 cm3 of each solution was added at 20 cm3/minute.
- At this stage the emulsion was composed of AglBr in which 15 mole % of the halide was iodide and the median crystal size was 1.0 um (based on equivalent circular diameter).
- The emulsion was then adjusted at 40 °C to pH 6 and pAg 8.2. It was then digested at 52 °C with sodium thiosulphate and sodium tetrachloroaurate to chemically sensitise it. After optimised chemical sensitisation a tetrazindene stabiliser was added.
- The mole % iodide in the core was 35% and the volume of the core was 43% of the whole crystal. The average mean size of the crystals was 1.0µm.
- Preparation of silver iodobromide emulsion which is prepared by a double-jetting and ammonia ripening process. This is emulsion B.
- 5.26 kg of gelatin was dissolved in 262 litres of distilled water.
- A mixed halide solution which comprised 90.8% ammonium bromide and 9.2% potassium iodide 266 litres with a molar concentration of 1.75 was then jetted in to the gelatin solution.
- Shortly after the commencement of the jetting of the mixed halide solution into the gelatin solution 228 litres of a 2.31 M silver nitrate solution was jetted slowly into the gelatin solution.
- Then after a short period 34 litres of a mixed ammonium bromide/ammonia solution was added which comprised 20% NH3 and 2.73 mole % ammonium bromide.
- Then after a short period 52 litres of a 20% ammonium solution is added to cause Ostwald ripening.
- Then after another short period 61 litres of a 7.2% N sulphuric acid is added to the gelatin solution to stop the Ostwald ripening process. The average mean size of the crystals thus prepared was 0.6µm.
- The emulsion as thus prepared was then desalinated and then chemically digested using sodium thiosulphite and a gold salt as with emulsion A. After chemical digestion was complete a tetraazindene stabiliser was added to the emulsion. An optimum quantity of a carbocyanine sensitising dye was added to both emulsions A and B.
- Both emulsions A and B were coated on to a film base, exposed and developed and their photographic speeds were compared at full development.
- Emulsion A had speed of 5.92 log E units and Emulsion B had a speed of 5.42 log E units.
- Preparation of photographic materials.
- Material 1. Sufficient of emulsion A was then coated on a cellulose triacetate base to yield a coating weight of 7.0g Ag/m 2.
- Material 2. A blended emulsion was then prepared by mixing emulsions A and B so that 40% by weight of the silver was derived from emulsion A and 60% by weight of the silver was derived from emulsion B.
- Then sufficient of this blended emulsion was coated on a cellulose triacetate base to yield a coating weight of 7g Ag/m 2.
- Samples of material 1 and 2 were then light exposed through a step-wedge of 1/30 second by a light source of 250 lux.
- Samples of materials 1 and 2 were then developed to the same contrast both in developer 1 and developer 2.
-
-
- Made up to 1 litre and then diluted 1 + 31 for use.
- Both sets of material were then fixed in an ammonium thiosulphate fixing bath, washed and then sensitometric results were measured.
- Both materials 1 and 2 were found to exhibit a photographic speed of approximately 400 I.S.O.. Both materials exhibited low fog. The granularity of material 1 was acceptable but the image in the high lights was noticeably grainy. However in material 2 the granularity was low and even in the high lights very little if any grain was visible.
- Preparation of silver iodobromide emulsion which is of the twinned high iodide core type and which has a development control layer. This is emulsion C.
- The silver iodide seed emulsion was grown as described in Example 1 of British Patent Specification No. 1596602 except that the crystals had an average size of 0.65µm.
- 16.9 litres of 4.1% gel solution was added to a quantity of the emulsion prepared in step A containing 35 moles of silver at a temperature of 70 °C and with constant stirring. 1.5m solutions of silver nitrate and sodium bromide were added by double jetting until 35 litres of silver nitrate had been added. The initial jetting rate was 199 cm3/minute at the end of addition. The potential was maintained at -58mV at 70 C. The mole % iodide in the core was 35% and the volume of the core was 43% of the whole crystal.
- 3.6% of the emulsion produced in step B was taken, and 0.35 litres of a 29% w/v inert gel solution added with thorough agitation at 65 °C. 0.2 cm3 of tri-n-butyl phosphate was added as an antifoam. The volume of each crystal was then increased by double jetting 1.5m solutions of silver nitrate and sodium bromide while maintaining the potential at -33 mV at 65 ° C. The first 355 cm3 was added as a jetting rate of 10cm3 minute, a second 355cm3 was added at jetting rate of 15cm3/minute and then 553 cm3 was added at 20cm3/minute.
- 48% of the emulsion prepared in step C was taken and the crystal volume was increased by double jetting 1.5 Molar solutions of silver nitrate and a 95 : 5 mole percent mixture of sodium bromide and potassium iodide maintaining the potential at -33mV at 65C. A total of 713 cm3 of each solution was added at 20cm3/minute. Finally, 357 cm3 of solutions of 1.5 molar silver bromide and 1.5 molar sodium bromide were added maintaining the same temperature and potential at 20cm3/minute.
- At this stage the emulsion was composed of AglBr in which 16.3 mole % of the halide was iodide and the median crystal was 1.0 um (based on equivalent circle diameter).
- After the completion of crystal growth, the emulsion was desalinated and redispersed with a solution of limed ossein gelatin.
- The emulsion was then chemically digested using sodium thiosulphate and gold. After chemical sensitisation was complete a tetraazindine stabiliser was added to the emulsion.
- A sample of emulsion B as described in Example 1 was also prepared.
- An optimum quantity of a carbcyamine optical sensitising dye was added to both emulsion B and C.
- A sample of Emulsion C was coated on a film base, exposed and fully developed. It was found to have a speed of 5.90 log E units. This is to be compared with the speed of 5.42 log E units for Emulsions B as tested in Example 1.
- Preparation of photographic materials.
- Material 1. Sufficient of emulsion B was then coated on a cellulose triacetate base to yield a coating weight of 2.8g Ag/m2. Over this layer was coated sufficient of emulsion C to yield a coating weight of 4.2 Ag/m2.
- Material 2. A blended emulsion was then prepared by mixing emulsions C and B so that 40% by weight of the silver was derived from emulsion C and 60% by weight of the silver was derived from emulsion B.
- Then sufficient of this blended emulsion was coated on a cellulose triacetate base to yield a coating weight of 7g Ag/m 2.
- Samples of material 1 and 2 were then light exposed through a step-wedge of 1/30 second by a light source of 250 lux.
- These samples of materials 1 and 2 were then developed in developer 1 and developer 2 to equal contrast.
-
-
- Both sets of material were then fixed in an ammonium thiosulphate fixing bath, washed and then sensitometric results were measured.
- In developer 1 both materials were developed to a contrast of G1.5 = 0.62.
-
- The granularity measured was the root mean square granularity which was measured at a density of 1.0 above Dmin using a 21µ square aperture. The density of the scale was specular.
Claims (6)
1. Photographic silver halide material which comprises coated on a subbed film base a silver halide emulsion which is characterised in that it is a blend of a silver halide emulsion of the twinned high-iodide core type as just described which comprises from 10 to 20 mole % iodide and of a silver iodiobromide emulsion which comprises less than 10 mole % iodide and which has a smaller crystal size and a photographic speed of from 0.2 to 1.0 log E units less than the twinned high-iodide core emulsion, the amounts of the emulsions in the blend being from 20 to 70% by weight of the total silver present being derived from the emulsion of the twinned high-iodide core type and the remainder from the smaller crystal size silver iodobromide emulsion.
2. Photographic silver halide material according to claim 1 characterised in that the blend comprises 30-50% by weight of the total silver present derived from the twinned high-iodide core emulsion.
3. Photographic silver halide material according to claim 1 characterised in that the blend comprises 35-45% by weight of the total silver present derived from the twinned high-iodide core emulsion.
4. Photographic silver halide material according to claim 1 characterised in that the twinned high-iodide core emulsion the core comprises from 20 to 40 mole % iodide.
5. Photographic silver halide material according to claim 1 characterised in that in the twinned high iodide core emulsion the core comprises from 35 to 40 mole % iodide.
6. Photographic silver halide material according to claim 1 characterised in that the twinned high iodide core emulsion comprises a development control layer which comprises up to 15 mole % iodide.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB9020947 | 1990-09-26 | ||
| GB909020947A GB9020947D0 (en) | 1990-09-26 | 1990-09-26 | Photographic material |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP0477772A1 true EP0477772A1 (en) | 1992-04-01 |
Family
ID=10682779
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP91115913A Ceased EP0477772A1 (en) | 1990-09-26 | 1991-09-19 | Photographic silver halide materials |
Country Status (2)
| Country | Link |
|---|---|
| EP (1) | EP0477772A1 (en) |
| GB (1) | GB9020947D0 (en) |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4184877A (en) * | 1976-06-10 | 1980-01-22 | Ciba-Geigy Ag | Process for the manufacture of photographic silver halide emulsions containing silver halide crystals of the twinned type |
| EP0359507A2 (en) * | 1988-09-13 | 1990-03-21 | ILFORD Limited | Silver halide emulsions |
| EP0359506A2 (en) * | 1988-09-13 | 1990-03-21 | ILFORD Limited | Silver halide emulsions |
-
1990
- 1990-09-26 GB GB909020947A patent/GB9020947D0/en active Pending
-
1991
- 1991-09-19 EP EP91115913A patent/EP0477772A1/en not_active Ceased
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4184877A (en) * | 1976-06-10 | 1980-01-22 | Ciba-Geigy Ag | Process for the manufacture of photographic silver halide emulsions containing silver halide crystals of the twinned type |
| EP0359507A2 (en) * | 1988-09-13 | 1990-03-21 | ILFORD Limited | Silver halide emulsions |
| EP0359506A2 (en) * | 1988-09-13 | 1990-03-21 | ILFORD Limited | Silver halide emulsions |
Also Published As
| Publication number | Publication date |
|---|---|
| GB9020947D0 (en) | 1990-11-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4150994A (en) | Process for the manufacture of photographic silver halide emulsions containing silver halide crystals of the twinned type | |
| DE3875517T2 (en) | METHOD FOR PRODUCING EMULSIONS WITH TABLED SILVER CHLORIDE GRAINS. | |
| DE3241646C2 (en) | Photosensitive silver halide emulsion and process for its preparation | |
| DE3402873A1 (en) | PHOTOTHERMOGRAPHIC RECORDING MATERIAL | |
| US5017469A (en) | Twinned emulsions made from silver iodide seed crystals having an aspect ratio of at least 2:1 | |
| JPS6158027B2 (en) | ||
| DE2951670A1 (en) | SILVER HALOGENID HYBRID CRYSTALS AND THEIR PRODUCTION | |
| JPS59177535A (en) | Silver halide photographic emulsion and its production | |
| EP0391560B1 (en) | Process for the preparation of photographic silver halide emulsions having tabular grains | |
| US4350758A (en) | Photographic emulsion containing copper halide host crystals | |
| US5164292A (en) | Selenium and iridium doped emulsions with improved properties | |
| JPH0534851A (en) | Silver halide emulsion and its production | |
| JP2000321696A (en) | Preparation of emulsion | |
| US5009991A (en) | Silver halide emulsions containing twinned silver halide crystals | |
| EP0477772A1 (en) | Photographic silver halide materials | |
| EP0445444A1 (en) | Photographic emulsions | |
| RU2085989C1 (en) | Silver halide photographic material | |
| JP2731715B2 (en) | An improved method for the manufacture of photographic elements | |
| RU2085990C1 (en) | Method of preparing silver bromide/iodide photographic emulsions | |
| EP0428334A1 (en) | Process for the spectral sensitisation of photographic silver halide emulsions and products thereof | |
| JP3012093B2 (en) | Silver halide photographic emulsion and silver halide photographic material using the same | |
| JP3245760B2 (en) | Silver halide color photographic light-sensitive material | |
| DE3878995T2 (en) | AUTOMATICALLY TREATABLE PHOTOGRAPHIC ELEMENT. | |
| JP3388926B2 (en) | Method for producing silver halide photographic emulsion | |
| EP0462528A1 (en) | Method for preparing a silver halide emulsion |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): BE CH DE FR GB LI |
|
| 17P | Request for examination filed |
Effective date: 19920430 |
|
| 17Q | First examination report despatched |
Effective date: 19931216 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN REFUSED |
|
| 18R | Application refused |
Effective date: 19941225 |