EP0349534B1 - Hydraulic fluids - Google Patents
Hydraulic fluids Download PDFInfo
- Publication number
- EP0349534B1 EP0349534B1 EP88901051A EP88901051A EP0349534B1 EP 0349534 B1 EP0349534 B1 EP 0349534B1 EP 88901051 A EP88901051 A EP 88901051A EP 88901051 A EP88901051 A EP 88901051A EP 0349534 B1 EP0349534 B1 EP 0349534B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- oxidant
- hydraulic fluids
- composition
- hydraulic
- base composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000012530 fluid Substances 0.000 title claims abstract description 50
- 239000000203 mixture Substances 0.000 claims abstract description 25
- 239000003963 antioxidant agent Substances 0.000 claims abstract description 19
- 150000003626 triacylglycerols Chemical class 0.000 claims abstract description 19
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 claims abstract description 18
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims abstract description 12
- 235000006708 antioxidants Nutrition 0.000 claims abstract description 12
- 235000014113 dietary fatty acids Nutrition 0.000 claims abstract description 9
- 229930195729 fatty acid Natural products 0.000 claims abstract description 9
- 239000000194 fatty acid Substances 0.000 claims abstract description 9
- 229910052751 metal Inorganic materials 0.000 claims abstract description 9
- 239000002184 metal Substances 0.000 claims abstract description 9
- 230000003078 antioxidant effect Effects 0.000 claims abstract description 8
- -1 C22 fatty acid Chemical class 0.000 claims abstract description 7
- 150000004982 aromatic amines Chemical class 0.000 claims abstract description 7
- 150000001875 compounds Chemical class 0.000 claims abstract description 5
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 claims abstract description 4
- 150000002148 esters Chemical class 0.000 claims abstract description 4
- 239000011630 iodine Substances 0.000 claims abstract description 4
- 229910052740 iodine Inorganic materials 0.000 claims abstract description 4
- 235000013824 polyphenols Nutrition 0.000 claims abstract description 4
- 150000001408 amides Chemical class 0.000 claims abstract description 3
- 229940042795 hydrazides for tuberculosis treatment Drugs 0.000 claims abstract description 3
- AQSJGOWTSHOLKH-UHFFFAOYSA-N phosphite(3-) Chemical class [O-]P([O-])[O-] AQSJGOWTSHOLKH-UHFFFAOYSA-N 0.000 claims abstract description 3
- 150000003839 salts Chemical class 0.000 claims abstract description 3
- 150000003568 thioethers Chemical class 0.000 claims abstract description 3
- 235000019484 Rapeseed oil Nutrition 0.000 claims description 16
- 239000000654 additive Substances 0.000 claims description 8
- 150000004665 fatty acids Chemical class 0.000 claims description 8
- 238000005461 lubrication Methods 0.000 claims description 4
- 230000007797 corrosion Effects 0.000 claims description 2
- 238000005260 corrosion Methods 0.000 claims description 2
- 239000006260 foam Substances 0.000 claims description 2
- 239000003112 inhibitor Substances 0.000 claims description 2
- 150000004671 saturated fatty acids Chemical class 0.000 claims description 2
- 235000003441 saturated fatty acids Nutrition 0.000 claims description 2
- 229960004232 linoleic acid Drugs 0.000 claims 1
- 230000000063 preceeding effect Effects 0.000 claims 1
- 239000003921 oil Substances 0.000 description 37
- 235000019198 oils Nutrition 0.000 description 37
- 238000012360 testing method Methods 0.000 description 26
- 229930195733 hydrocarbon Natural products 0.000 description 13
- 150000002430 hydrocarbons Chemical class 0.000 description 13
- 239000004215 Carbon black (E152) Substances 0.000 description 10
- 239000002480 mineral oil Substances 0.000 description 8
- 230000003647 oxidation Effects 0.000 description 8
- 238000007254 oxidation reaction Methods 0.000 description 8
- 238000000034 method Methods 0.000 description 6
- PDEDQSAFHNADLV-UHFFFAOYSA-M potassium;disodium;dinitrate;nitrite Chemical compound [Na+].[Na+].[K+].[O-]N=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O PDEDQSAFHNADLV-UHFFFAOYSA-M 0.000 description 6
- 239000003925 fat Substances 0.000 description 5
- 235000019197 fats Nutrition 0.000 description 5
- 229940042472 mineral oil Drugs 0.000 description 5
- 235000010446 mineral oil Nutrition 0.000 description 5
- 239000002253 acid Substances 0.000 description 4
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- DPUOLQHDNGRHBS-KTKRTIGZSA-N erucic acid Chemical compound CCCCCCCC\C=C/CCCCCCCCCCCC(O)=O DPUOLQHDNGRHBS-KTKRTIGZSA-N 0.000 description 3
- 239000010720 hydraulic oil Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 239000003208 petroleum Substances 0.000 description 3
- 238000010998 test method Methods 0.000 description 3
- 235000015112 vegetable and seed oil Nutrition 0.000 description 3
- 239000008158 vegetable oil Substances 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 2
- WJQOZHYUIDYNHM-UHFFFAOYSA-N 2-tert-Butylphenol Chemical class CC(C)(C)C1=CC=CC=C1O WJQOZHYUIDYNHM-UHFFFAOYSA-N 0.000 description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 2
- DPUOLQHDNGRHBS-UHFFFAOYSA-N Brassidinsaeure Natural products CCCCCCCCC=CCCCCCCCCCCCC(O)=O DPUOLQHDNGRHBS-UHFFFAOYSA-N 0.000 description 2
- GHKOFFNLGXMVNJ-UHFFFAOYSA-N Didodecyl thiobispropanoate Chemical compound CCCCCCCCCCCCOC(=O)CCSCCC(=O)OCCCCCCCCCCCC GHKOFFNLGXMVNJ-UHFFFAOYSA-N 0.000 description 2
- 235000013830 Eruca Nutrition 0.000 description 2
- 241000801434 Eruca Species 0.000 description 2
- URXZXNYJPAJJOQ-UHFFFAOYSA-N Erucic acid Natural products CCCCCCC=CCCCCCCCCCCCC(O)=O URXZXNYJPAJJOQ-UHFFFAOYSA-N 0.000 description 2
- DMBHHRLKUKUOEG-UHFFFAOYSA-N N-phenyl aniline Natural products C=1C=CC=CC=1NC1=CC=CC=C1 DMBHHRLKUKUOEG-UHFFFAOYSA-N 0.000 description 2
- JZTPOMIFAFKKSK-UHFFFAOYSA-N O-phosphonohydroxylamine Chemical class NOP(O)(O)=O JZTPOMIFAFKKSK-UHFFFAOYSA-N 0.000 description 2
- 239000005642 Oleic acid Substances 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 239000003570 air Substances 0.000 description 2
- XITRBUPOXXBIJN-UHFFFAOYSA-N bis(2,2,6,6-tetramethylpiperidin-4-yl) decanedioate Chemical compound C1C(C)(C)NC(C)(C)CC1OC(=O)CCCCCCCCC(=O)OC1CC(C)(C)NC(C)(C)C1 XITRBUPOXXBIJN-UHFFFAOYSA-N 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 2
- 230000001050 lubricating effect Effects 0.000 description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920000193 polymethacrylate Polymers 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 125000001273 sulfonato group Chemical class [O-]S(*)(=O)=O 0.000 description 2
- 229910052725 zinc Inorganic materials 0.000 description 2
- 239000011701 zinc Substances 0.000 description 2
- OYHQOLUKZRVURQ-NTGFUMLPSA-N (9Z,12Z)-9,10,12,13-tetratritiooctadeca-9,12-dienoic acid Chemical compound C(CCCCCCC\C(=C(/C\C(=C(/CCCCC)\[3H])\[3H])\[3H])\[3H])(=O)O OYHQOLUKZRVURQ-NTGFUMLPSA-N 0.000 description 1
- ONRGCKHNIPGJGT-BFIYWSAOSA-N (9z,12z)-octadeca-9,12-dienoic acid;(z)-octadec-9-enoic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O.CCCCC\C=C/C\C=C/CCCCCCCC(O)=O ONRGCKHNIPGJGT-BFIYWSAOSA-N 0.000 description 1
- UPYPTOCXMIWHSG-UHFFFAOYSA-N 1-dodecylsulfanyldodecane Chemical group CCCCCCCCCCCCSCCCCCCCCCCCC UPYPTOCXMIWHSG-UHFFFAOYSA-N 0.000 description 1
- FOKDITTZHHDEHD-PFONDFGASA-N 2-ethylhexyl (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(CC)CCCC FOKDITTZHHDEHD-PFONDFGASA-N 0.000 description 1
- DTBDAFLSBDGPEA-UHFFFAOYSA-N 3-Methylquinoline Natural products C1=CC=CC2=CC(C)=CN=C21 DTBDAFLSBDGPEA-UHFFFAOYSA-N 0.000 description 1
- OYHQOLUKZRVURQ-UHFFFAOYSA-N 9,12-Octadecadienoic Acid Chemical compound CCCCCC=CCC=CCCCCCCCC(O)=O OYHQOLUKZRVURQ-UHFFFAOYSA-N 0.000 description 1
- 235000005637 Brassica campestris Nutrition 0.000 description 1
- 241001301148 Brassica rapa subsp. oleifera Species 0.000 description 1
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 1
- BSMUHMONAIFTDF-UHFFFAOYSA-N C(C=1C(O)=CC=CC1)=NC=C(C)N=CC=1C(O)=CC=CC1 Chemical compound C(C=1C(O)=CC=CC1)=NC=C(C)N=CC=1C(O)=CC=CC1 BSMUHMONAIFTDF-UHFFFAOYSA-N 0.000 description 1
- 102100039496 Choline transporter-like protein 4 Human genes 0.000 description 1
- 239000003508 Dilauryl thiodipropionate Substances 0.000 description 1
- 101000889282 Homo sapiens Choline transporter-like protein 4 Proteins 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 229920002367 Polyisobutene Polymers 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- ZOJBYZNEUISWFT-UHFFFAOYSA-N allyl isothiocyanate Chemical compound C=CCN=C=S ZOJBYZNEUISWFT-UHFFFAOYSA-N 0.000 description 1
- 239000012080 ambient air Substances 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 235000019304 dilauryl thiodipropionate Nutrition 0.000 description 1
- 229940035422 diphenylamine Drugs 0.000 description 1
- 239000012990 dithiocarbamate Substances 0.000 description 1
- 150000004659 dithiocarbamates Chemical class 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- ZQPPMHVWECSIRJ-MDZDMXLPSA-N elaidic acid Chemical compound CCCCCCCC\C=C\CCCCCCCC(O)=O ZQPPMHVWECSIRJ-MDZDMXLPSA-N 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 239000003517 fume Substances 0.000 description 1
- 150000002314 glycerols Chemical class 0.000 description 1
- 239000003673 groundwater Substances 0.000 description 1
- 230000008642 heat stress Effects 0.000 description 1
- 229910001385 heavy metal Inorganic materials 0.000 description 1
- OYHQOLUKZRVURQ-IXWMQOLASA-N linoleic acid Natural products CCCCC\C=C/C\C=C\CCCCCCCC(O)=O OYHQOLUKZRVURQ-IXWMQOLASA-N 0.000 description 1
- 239000000944 linseed oil Substances 0.000 description 1
- 235000021388 linseed oil Nutrition 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 238000005065 mining Methods 0.000 description 1
- 239000008164 mustard oil Substances 0.000 description 1
- FSWDLYNGJBGFJH-UHFFFAOYSA-N n,n'-di-2-butyl-1,4-phenylenediamine Chemical compound CCC(C)NC1=CC=C(NC(C)CC)C=C1 FSWDLYNGJBGFJH-UHFFFAOYSA-N 0.000 description 1
- KCZLICCXIBLZPN-UHFFFAOYSA-N n,n'-diethyl-n,n'-diphenyloxamide Chemical compound C=1C=CC=CC=1N(CC)C(=O)C(=O)N(CC)C1=CC=CC=C1 KCZLICCXIBLZPN-UHFFFAOYSA-N 0.000 description 1
- YCWSUKQGVSGXJO-NTUHNPAUSA-N nifuroxazide Chemical group C1=CC(O)=CC=C1C(=O)N\N=C\C1=CC=C([N+]([O-])=O)O1 YCWSUKQGVSGXJO-NTUHNPAUSA-N 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 238000010525 oxidative degradation reaction Methods 0.000 description 1
- 150000002989 phenols Chemical class 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- OJMIONKXNSYLSR-UHFFFAOYSA-N phosphorous acid Chemical compound OP(O)O OJMIONKXNSYLSR-UHFFFAOYSA-N 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 239000000344 soap Substances 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 238000007655 standard test method Methods 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 235000011044 succinic acid Nutrition 0.000 description 1
- VLYWMPOKSSWJAL-UHFFFAOYSA-N sulfamethoxypyridazine Chemical compound N1=NC(OC)=CC=C1NS(=O)(=O)C1=CC=C(N)C=C1 VLYWMPOKSSWJAL-UHFFFAOYSA-N 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 150000003852 triazoles Chemical class 0.000 description 1
- IKXFIBBKEARMLL-UHFFFAOYSA-N triphenoxy(sulfanylidene)-$l^{5}-phosphane Chemical compound C=1C=CC=CC=1OP(OC=1C=CC=CC=1)(=S)OC1=CC=CC=C1 IKXFIBBKEARMLL-UHFFFAOYSA-N 0.000 description 1
- WGKLOLBTFWFKOD-UHFFFAOYSA-N tris(2-nonylphenyl) phosphite Chemical compound CCCCCCCCCC1=CC=CC=C1OP(OC=1C(=CC=CC=1)CCCCCCCCC)OC1=CC=CC=C1CCCCCCCCC WGKLOLBTFWFKOD-UHFFFAOYSA-N 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
- JGSUMMPGKPITGK-UHFFFAOYSA-L zinc;n,n-dipentylcarbamodithioate Chemical compound [Zn+2].CCCCCN(C([S-])=S)CCCCC.CCCCCN(C([S-])=S)CCCCC JGSUMMPGKPITGK-UHFFFAOYSA-L 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M169/00—Lubricating compositions characterised by containing as components a mixture of at least two types of ingredient selected from base-materials, thickeners or additives, covered by the preceding groups, each of these compounds being essential
- C10M169/04—Mixtures of base-materials and additives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M101/00—Lubricating compositions characterised by the base-material being a mineral or fatty oil
- C10M101/04—Fatty oil fractions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/04—Hydroxy compounds
- C10M129/10—Hydroxy compounds having hydroxy groups bound to a carbon atom of a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/16—Ethers
- C10M129/18—Epoxides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/68—Esters
- C10M129/74—Esters of polyhydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/04—Amines, e.g. polyalkylene polyamines; Quaternary amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/04—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M133/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/04—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M133/12—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to a carbon atom of a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/16—Amides; Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/38—Heterocyclic nitrogen compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M137/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus
- C10M137/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus having no phosphorus-to-carbon bond
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M137/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus
- C10M137/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus having no phosphorus-to-carbon bond
- C10M137/04—Phosphate esters
- C10M137/08—Ammonium or amine salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M137/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus
- C10M137/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus having no phosphorus-to-carbon bond
- C10M137/04—Phosphate esters
- C10M137/10—Thio derivatives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/024—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings having at least two phenol groups but no condensed ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/026—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings with tertiary alkyl groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/027—Neutral salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/04—Ethers; Acetals; Ortho-esters; Ortho-carbonates
- C10M2207/042—Epoxides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/281—Esters of (cyclo)aliphatic monocarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/282—Esters of (cyclo)aliphatic oolycarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/283—Esters of polyhydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/286—Esters of polymerised unsaturated acids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/287—Partial esters
- C10M2207/289—Partial esters containing free hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/30—Complex esters, i.e. compounds containing at leasst three esterified carboxyl groups and derived from the combination of at least three different types of the following five types of compounds: monohydroxyl compounds, polyhydroxy xompounds, monocarboxylic acids, polycarboxylic acids or hydroxy carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/40—Fatty vegetable or animal oils
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/40—Fatty vegetable or animal oils
- C10M2207/401—Fatty vegetable or animal oils used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/40—Fatty vegetable or animal oils
- C10M2207/404—Fatty vegetable or animal oils obtained from genetically modified species
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/40—Fatty vegetable or animal oils
- C10M2207/404—Fatty vegetable or animal oils obtained from genetically modified species
- C10M2207/4045—Fatty vegetable or animal oils obtained from genetically modified species used as base material
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/04—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/062—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings containing hydroxy groups bound to the aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/064—Di- and triaryl amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/064—Di- and triaryl amines
- C10M2215/065—Phenyl-Naphthyl amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/066—Arylene diamines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/067—Polyaryl amine alkanes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/068—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings having amino groups bound to polycyclic aromatic ring systems, i.e. systems with three or more condensed rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/08—Amides [having hydrocarbon substituents containing less than thirty carbon atoms]
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/08—Amides [having hydrocarbon substituents containing less than thirty carbon atoms]
- C10M2215/082—Amides [having hydrocarbon substituents containing less than thirty carbon atoms] containing hydroxyl groups; Alkoxylated derivatives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/086—Imides [having hydrocarbon substituents containing less than thirty carbon atoms]
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/12—Partial amides of polycarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/12—Partial amides of polycarboxylic acids
- C10M2215/122—Phtalamic acid
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/22—Heterocyclic nitrogen compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/22—Heterocyclic nitrogen compounds
- C10M2215/221—Six-membered rings containing nitrogen and carbon only
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/22—Heterocyclic nitrogen compounds
- C10M2215/225—Heterocyclic nitrogen compounds the rings containing both nitrogen and oxygen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/22—Heterocyclic nitrogen compounds
- C10M2215/225—Heterocyclic nitrogen compounds the rings containing both nitrogen and oxygen
- C10M2215/226—Morpholines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/24—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions having hydrocarbon substituents containing thirty or more carbon atoms, e.g. nitrogen derivatives of substituted succinic acid
- C10M2215/28—Amides; Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions
- C10M2215/24—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant Compositions having hydrocarbon substituents containing thirty or more carbon atoms, e.g. nitrogen derivatives of substituted succinic acid
- C10M2215/30—Heterocyclic compounds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/06—Thio-acids; Thiocyanates; Derivatives thereof
- C10M2219/062—Thio-acids; Thiocyanates; Derivatives thereof having carbon-to-sulfur double bonds
- C10M2219/066—Thiocarbamic type compounds
- C10M2219/068—Thiocarbamate metal salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/08—Thiols; Sulfides; Polysulfides; Mercaptals
- C10M2219/082—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms
- C10M2219/085—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms containing carboxyl groups; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/04—Phosphate esters
- C10M2223/041—Triaryl phosphates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/04—Phosphate esters
- C10M2223/043—Ammonium or amine salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/04—Phosphate esters
- C10M2223/045—Metal containing thio derivatives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/049—Phosphite
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/10—Phosphatides, e.g. lecithin, cephalin
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/04—Groups 2 or 12
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/08—Hydraulic fluids, e.g. brake-fluids
Definitions
- the present invention is concerned with hydraulic fluids based on oily triglycerides of fatty acids.
- the hydraulic fluids commonly used are petroleum-based, chemically saturated or unsaturated, straight-chained, branched or ring-type hydrocarbons.
- Hydrocarbons may constitute a cancer risk when in prolonged contact with the skin, as well as a risk of damage to the lungs when inhaled with the air.
- oil allowed to escape into the environment causes spoiling of the soil and the ground water, even in small quantities. They are also toxic to the aquatic life in rivers, lakes, etc.
- hydrocarbon oils as such have in fact a rather limited applicability for hydraulic purposes, wherefor the hydraulic fluids based on such oils contain a variety of additives in considerable amounts. Petroleum is also a non-renewable, and consequently limited, natural resource.
- the triglycerides described in the said specification GB 2 134 923 are glycerol esters of fatty acids, and the chemical structure of the said esters can be defined by means of the following formula: wherein R1, R2 and R3 can be the same or different and are selected from the group consisting of saturated and unsaturated straight-chained alkyl, alkenyl, and alkadienyl chains of ordinarily 9 to 22 carbon atoms.
- the triglyceride may also, according to the teaching of the specification GB 2 134 923, contain a small quantity of an alkatrienylic acid residue, but a larger quantity is detrimental, because it promotes oxidation of the triglyceride oil.
- Certain triglyceride oils, so-called drying oils contain considerable quantities of alkatrienyl and alkadienyl groups, and they form solid films, under the effect of the oxygen in the air.
- Such oils the iodine number of which is usually higher than 130 and which are used i.a. as components of special coatings, cannot be considered for use in the hydraulic fluids.
- any other oily triglyceride with an iodine number of at least 50 and no more than 128 is suitable for the purpose.
- Particularly suitable are the triglycerides of the oleic acid-linoleic acid type which contain no more than 20 per cent by weight of esterified saturated fatty acids calculated on the quantity of esterified fatty acids.
- These oils are liquids at 15 to 20°C, and their most important fatty acid residues are derived from the following unsaturated acids: oleic acid, 9-octadecenoic acid, linoleic acid, 9,12-octa-decadienoic acid.
- the fume point of triglycerides is above 200°C and the flash point above 300°C (both determinations as per AOCS Ce 9a-48 or ASTM D 1310).
- the flash points of hydrocarbon basic oils are, as a rule, clearly lower.
- the triglyceride oils differ from the non-polar hydrocarbons completely in the respect that they are of a polar nature. This accounts for the superb ability of triglycerides to be adsorbed on metal faces as very thin adhering films.
- Rape seed oil has been considered as an example of the monomeric triglyceride oils used in the hydraulic fluids in accordance with the specification GB 2 134 923, which rape seed oil is also obtained from the sub-species Brassica campestris and which oil, in its present-day commercial form, contains little or no erucic acid, 13-docosenoic acid.
- applicable triglyceride oils differ from rape seed oil only in respect of the composition of the fatty acids esterified with glycerol, which difference comes out as different pour points and viscosities of the oils.
- oils obtained from different sub-species of rape and from their related sub-species display differences in pour points and viscosities, owing to differences in the compositions of fatty acids, as appears from Table 3.
- the first one (eruca) has been obtained from a sub-species that has a high content of erucic acid (C 22:1).
- the characterizing data of rape seed oil are compared in Table 4 with certain commercial basic mineral oils.
- Table 4 Characteristic data of rape seed oil and certain basic mineral oils Rape seed oil Gulf 300 paramid Gulf 300 Texas oil Nynäs S 100 Nynäs H 22 Density g/cm3 1) 15°C 0.9205 0.878 0.914 0.910 0.926 Viscosity mm2/s -20°C 660 40°C 34.2 60.7 57.9 99 26 100°C 8 8.1 6.6 8.6 3.9
- Method ASTM D 1298 Method ASTM D 93 3) Method ASTM D 974
- the viscosity index (VI) of triglycerides is superior.
- the viscosity index of the triclyceride oils is apparently also more stable against mechanical and heat stresses existing in the hydraulic systems than the viscosity index of the hydraulic fluids based on formulated mineral oils and containing polymeric viscosity index improves.
- the ability of the polar triglyceride molecule to adhere onto metallic surfaces improves the lubricating properties of these triglycerides.
- the oxidation has many negative effects to the properties of a natural triglyceride based hydraulic fluid, wherefore the fluid has to be replaced by fresh fluid more frequently than fluids based on hydrocarbon oils.
- the viscosity of the natural triglyceride hydraulic fluid is increased due to the oxidation.
- the oxidation causes also foaming of the fluid, the filtration properties of the fluid are decreased, and the higher water solubility causes problems in the hydraulic system.
- the oxidation products are also corrosive. In order to avoid these problems caused by oxidation the working temperature of the hydraulic system is to be kept lower than when hydrocarbon based oils are used.
- the stability of the hydraulic fluids against oxidative degradation was tested.
- the fluids were tested with an apparatus according to the test method ASTM D 525 by introducing into a pressure vessel 100 ml of the fluid to be tested. The vessel was closed and placed into boiling water. During the test the oxygen pressure in the vessel was determined.
- the additives used were: Irgalube 349 , amino phosphate derivative, Ciba-Geigy; Irganox L 130 , mixture of tertiary-butyl phenol derivatives, Ciba-Geigy; Reomet 39 , triazole derivative, Ciba-Geigy; Anglamol 75 , zinc dialkyldithio-phosphate, Lubrizol; EN 1235 , kortacid T derivative, Akzo Chemie; Hitec 4735 , mixture of tertiary-butyl phenol derivatives, Ethyl Pertoleum Additives Ltd; Irganox PS 800 , dilauryl thio di propionate, Ciba-Geigy; Irganox L 180 , triaryl phosphite, Ciba-Geigy; Irganox L 57 , mixed alkyl diphenyl amine, Ciba-Geigy
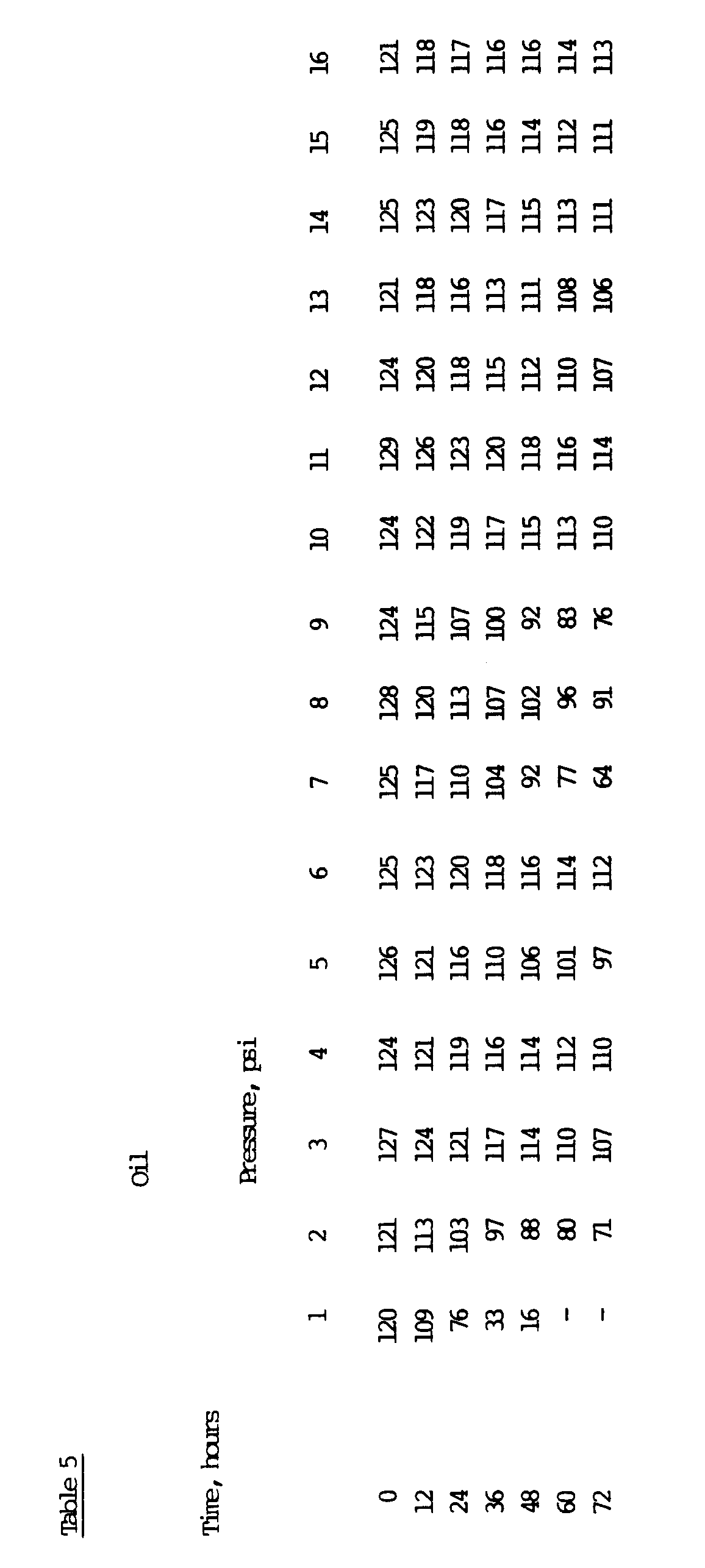
- compositions 3, 4, 5, 6, 8, 10, 11, 12, 13 and 14 are clearly comparable with the common mineral-oil based hydraulic oils 15 and 16 used for comparison in this example.
- the compositions 2 and 9 contain the anti-oxidant additives selected according to the invention, but the amounts used have not been sufficient. From the data in Table 5 it can be derived that a triglyceride complying with the definitions presented at the beginning of this description and containing a certain amount of carefully selected anti-oxidant additives can form a base for a fluid composition usable for hydraulic purposes.
- the anti-oxidant fraction in the composition forms 2.0 to 4.5 percent by weight of the composition
- the anti-oxidants are selected so that at least one compound is from the group (1) of hindered phenolics and aromatic amines, and the remaining compound(s) forming the balance in the composition, is from the group (2) of metal salts of dithioacids, phosphites and sulphides, or from the group (3) of amides, non-aromatic amines, hydrazides and triazols.
- a Cameron Plint tester High Frequency Friction Machine TE. 77
- the friction between a moving and a stationary element is determined at increasing temperatures.
- the moving element a steel ball having a diameter of 6 mm
- the stationary element consists of a steel plate.
- the lubricant to be tested is spread on the plate, and it is exposed to the ambient air oxygen during the tests.
- the ball was pressed towards the plate by a force of 40 N during its reciprocating movement having an amplitude of 5 mm and a frequency of 20 Hz.
- the temperature at the beginning of each test was adjusted to 40 °C, whereafter it was increased by 2 °C per minute.
- the temperature in which the friction began to increase sharply was registered, and it was used as an indication of the failure of the lubricative film between the ball and the plate.
- the film failure temperature is a measure of the oxidation resistance of the oil.
- a vegetable oil based hydraulic fluid was tested using as a reference a commercial mineral oil based hydraulic fluid.
- two new identical hydraulic driven mining loaders were used.
- the pressures in the hydraulic circuits varied from 0 to 165 bar and the hydraulic fluid temperature from 60 to 80°C. Hydraulic pressure was generated by gear pumps and the power was taken out by means of cylinder-piston devices.
- the hydraulic fluids tested were: The following Table 7 gives the viscosity of the oils after a prolonged time in operation. Table 7 Time, hours Viscosity, mm2/s / 40 °C Fluid 1 2 3 0 34 33.2 44.6 300 36.8 33.2 38.1 600 39.5 33.5 35.2 900 44.3 33.9 34.3 1200 51.8 34.1 34.2 1500 55.6 34.3 34.2
- the efficiency tests were conducted using a fluid pressure of 165 bar, and a temperature of 65°C.
- test results of Table 8 indicate that the efficiency of the system containing the vegetable oil based fluid decreased slower than that of the mineral oil based fluid.
- hydraulic fluid according to the invention may also comprise other constituents such as:
- From the base composition according to the invention can be made hydraulic fluids for different purposes by adjusting its viscosity.
- Table 12 gives one example of adjusting possibilities.
- Table 12 From a base composition according to the invention was made hydraulic fluids for different viscosity classes (ASTM D 2422) Oil comp. % by weight visc. mm2/s class 1.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Organic Chemistry (AREA)
- Lubricants (AREA)
- Fluid-Pressure Circuits (AREA)
Abstract
Description
- The present invention is concerned with hydraulic fluids based on oily triglycerides of fatty acids.
- The hydraulic fluids commonly used are petroleum-based, chemically saturated or unsaturated, straight-chained, branched or ring-type hydrocarbons.
- The petroleum-based hydraulic fluids involve, however, a number of environmental and health risks. Hydrocarbons may constitute a cancer risk when in prolonged contact with the skin, as well as a risk of damage to the lungs when inhaled with the air. Moreover, oil allowed to escape into the environment causes spoiling of the soil and the ground water, even in small quantities. They are also toxic to the aquatic life in rivers, lakes, etc.
- In addition to the above, hydrocarbon oils as such have in fact a rather limited applicability for hydraulic purposes, wherefor the hydraulic fluids based on such oils contain a variety of additives in considerable amounts. Petroleum is also a non-renewable, and consequently limited, natural resource.
- Thus there is an obvious need for fluids for hydraulic purposes which are based on renewable natural resources, and which are, at the same time, environmentally acceptable. One such a natural base component for hydraulic fluids is the oily triglycerides, as suggested in the patent specification GB 2 134 923.
- The triglycerides described in the said specification GB 2 134 923 are glycerol esters of fatty acids, and the chemical structure of the said esters can be defined by means of the following formula:
wherein R₁, R₂ and R₃ can be the same or different and are selected from the group consisting of saturated and unsaturated straight-chained alkyl, alkenyl, and alkadienyl chains of ordinarily 9 to 22 carbon atoms. - The triglyceride may also, according to the teaching of the specification GB 2 134 923, contain a small quantity of an alkatrienylic acid residue, but a larger quantity is detrimental, because it promotes oxidation of the triglyceride oil. Certain triglyceride oils, so-called drying oils, contain considerable quantities of alkatrienyl and alkadienyl groups, and they form solid films, under the effect of the oxygen in the air. Such oils, the iodine number of which is usually higher than 130 and which are used i.a. as components of special coatings, cannot be considered for use in the hydraulic fluids.
- As is stated in the specification GB 2 134 923, any other oily triglyceride with an iodine number of at least 50 and no more than 128 is suitable for the purpose. Particularly suitable are the triglycerides of the oleic acid-linoleic acid type which contain no more than 20 per cent by weight of esterified saturated fatty acids calculated on the quantity of esterified fatty acids. These oils are liquids at 15 to 20°C, and their most important fatty acid residues are derived from the following unsaturated acids: oleic acid, 9-octadecenoic acid, linoleic acid, 9,12-octa-decadienoic acid. The most preferred among these triglycerides of vegetable origin, under normal temperatures of use, are described to be those that contain esterified oleic acid in a quantity in excess of 50 per cent by weight of the total quantity of fatty acids (Table 1).
It is characteristic of all of these oily triglycerides that their viscosities change on change in temperature to a lesser extent than the viscosities of hydrocarbon basic oils. The viscosity-to-temperature ratio characteristic of each oil can be characterized by means of the empiric viscosity index (VI), the numerical value of which is the higher the less the viscosity of the oil concerned changes with a change in temperature. The viscosity indexes of triglycerides are clearly higher than those of hydrocarbon oils with no additives, so that triglycerides are to their nature so-called multi-grade oils. This is of considerable importance under conditions in which the operating temperature may vary within rather wide limits. The viscosities and viscosity indexes of certain triglycerides are given in Table 2.Table 2 Viscosity properties of oils Viscosity mm²/s Viscosity index 38°C 99°C 1) 2) Olive oil 46.68 9.09 194 Rape seed oil (eruca) 50.64 10.32 210 Rape seed oil 36.04 8.03 217 Mustard oil 45.13 9.46 215 Cottonseed oil 35.88 8.39 214 Soybean oil 28.49 7.60 271 Linseed oil 29.60 7.33 242 Sunflower oil 33.31 7.68 227 Hydrocarbon-based basic oils 0 - 120 1) Method ASTM D 445 2) Method ASTM D 2270 - The fume point of triglycerides is above 200°C and the flash point above 300°C (both determinations as per AOCS Ce 9a-48 or ASTM D 1310). The flash points of hydrocarbon basic oils are, as a rule, clearly lower.
- The triglyceride oils differ from the non-polar hydrocarbons completely in the respect that they are of a polar nature. This accounts for the superb ability of triglycerides to be adsorbed on metal faces as very thin adhering films. A study of the operation of glide faces placed in close relationship to each other, and considering pressure and temperature to be the fundamental factors affecting lubrication, shows that the film-formation properties of triglycerides are particularly advantageous in hydraulic systems.
- In addition, water cannot force a triglyceride oil film off a metal face as easily as a hydrocarbon film.
- Rape seed oil has been considered as an example of the monomeric triglyceride oils used in the hydraulic fluids in accordance with the specification GB 2 134 923, which rape seed oil is also obtained from the sub-species Brassica campestris and which oil, in its present-day commercial form, contains little or no erucic acid, 13-docosenoic acid. However, it is to be kept in mind that applicable triglyceride oils differ from rape seed oil only in respect of the composition of the fatty acids esterified with glycerol, which difference comes out as different pour points and viscosities of the oils. Even oils obtained from different sub-species of rape and from their related sub-species display differences in pour points and viscosities, owing to differences in the compositions of fatty acids, as appears from Table 3. Of the rape seed oils mentioned in the table, the first one (eruca) has been obtained from a sub-species that has a high content of erucic acid (C 22:1).
- The characterizing data of rape seed oil are compared in Table 4 with certain commercial basic mineral oils.
Table 4 Characteristic data of rape seed oil and certain basic mineral oils Rape seed oil Gulf 300 paramid Gulf 300 Texas oil Nynäs S 100 Nynäs H 22 Density g/cm³ 1) 15°C 0.9205 0.878 0.914 0.910 0.926 Viscosity mm²/s -20°C 660 40°C 34.2 60.7 57.9 99 26 100°C 8 8.1 6.6 8.6 3.9 Viscosity index 217 101 26 31 - Pour point °C -27 -12 -34 -18 -33 Flash point °C 2) > 300 238 188 215 180 Acid value mg KOH/g 3) 0.06 0.04 0.09 0.01 0.01 1) Method ASTM D 1298 2) Method ASTM D 93 3) Method ASTM D 974 - The above data indicate that the said triglycerides have many properties which are of advantage especially in hydraulic fluids. As mentioned already before, the viscosity index (VI) of triglycerides, as compared with mineral oil products, is superior. The viscosity index of the triclyceride oils is apparently also more stable against mechanical and heat stresses existing in the hydraulic systems than the viscosity index of the hydraulic fluids based on formulated mineral oils and containing polymeric viscosity index improves. In addition it can be expected that the ability of the polar triglyceride molecule to adhere onto metallic surfaces improves the lubricating properties of these triglycerides.
- The only property of the natural triglycerides which has shown to impede their intended use for hydraulic purposes is their tendency to be easily oxidized.
- The oxidation has many negative effects to the properties of a natural triglyceride based hydraulic fluid, wherefore the fluid has to be replaced by fresh fluid more frequently than fluids based on hydrocarbon oils.
- For instance the viscosity of the natural triglyceride hydraulic fluid is increased due to the oxidation. The oxidation causes also foaming of the fluid, the filtration properties of the fluid are decreased, and the higher water solubility causes problems in the hydraulic system. The oxidation products are also corrosive. In order to avoid these problems caused by oxidation the working temperature of the hydraulic system is to be kept lower than when hydrocarbon based oils are used.
- It has, however, been noted that the tendency of the said natural triglycerides to be oxidized can be decreased essentially to the same level as that of the common hydrocarbon based hydraulic oils, by using additives in very moderate amounts, which additives have been selected according to the invention. This fact is evident from the results of the following example 1.
- In this example the stability of the hydraulic fluids against oxidative degradation was tested. The fluids were tested with an apparatus according to the test method ASTM D 525 by introducing into a pressure vessel 100 ml of the fluid to be tested. The vessel was closed and placed into boiling water. During the test the oxygen pressure in the vessel was determined.
- The additives used were: Irgalube 349, amino phosphate derivative, Ciba-Geigy; Irganox L 130, mixture of tertiary-butyl phenol derivatives, Ciba-Geigy; Reomet 39, triazole derivative, Ciba-Geigy; Anglamol 75, zinc dialkyldithio-phosphate, Lubrizol; EN 1235, kortacid T derivative, Akzo Chemie; Hitec 4735, mixture of tertiary-butyl phenol derivatives, Ethyl Pertoleum Additives Ltd; Irganox PS 800, dilauryl thio di propionate, Ciba-Geigy; Irganox L 180, triaryl phosphite, Ciba-Geigy; Irganox L 57, mixed alkyl diphenyl amine, Ciba-Geigy; Irganlube TPPT, triphenylphosphorothionate, Ciba-Geigy; Tinuvin 770, bis (2,2,6,6-tetrametyl-4-piperidyl) sebacate, Ciba-Geigy; Vanlube AZ, zinc diamyldithiocarbamate, R.T. Vanderbilt; Additin 10, 2,6-di-tertiary-butyl-4-mathylphenol, Rhein-Chemie.
-
- As can bee seen from the results of Table 5, the compositions 3, 4, 5, 6, 8, 10, 11, 12, 13 and 14 are clearly comparable with the common mineral-oil based hydraulic oils 15 and 16 used for comparison in this example. The compositions 2 and 9 contain the anti-oxidant additives selected according to the invention, but the amounts used have not been sufficient. From the data in Table 5 it can be derived that a triglyceride complying with the definitions presented at the beginning of this description and containing a certain amount of carefully selected anti-oxidant additives can form a base for a fluid composition usable for hydraulic purposes.
- According to the invention the anti-oxidant fraction in the composition forms 2.0 to 4.5 percent by weight of the composition, and the anti-oxidants are selected so that at least one compound is from the group (1) of hindered phenolics and aromatic amines, and the remaining compound(s) forming the balance in the composition, is from the group (2) of metal salts of dithioacids, phosphites and sulphides, or from the group (3) of amides, non-aromatic amines, hydrazides and triazols.
- Examples of compounds which belong to the abovementioned groups can be named as follows:
- 1) 2,6-di-tert-butyl-4-methyl phenol; 2'2-methylenebis-(4-methyl-6-tert-butylphenol); N,N'-disecbutyl-p-phenylene-diamine; alkylated diphenyl amine; alkylated phenyl-alfa-naphtyl amine
- 2) zinc dialkyldithiophosphates; tris (nonylphenyl) phosphite; dilauryl thiodipropionate
- 3) N,N'-diethyl-N,N'-diphenyloxamide;
N,N'-disalicylidene-1,2-propenylenediamine;
N,N'-bis (beta-3,5-ditertbutyl-4-hydroxyphenylpropiono) hydrazide - In the tests a Cameron Plint tester (High Frequency Friction Machine TE. 77) was used. In this tester the friction between a moving and a stationary element is determined at increasing temperatures. As the moving element is used a steel ball having a diameter of 6 mm, whereas the stationary element consists of a steel plate. The lubricant to be tested is spread on the plate, and it is exposed to the ambient air oxygen during the tests. In the tests conducted the ball was pressed towards the plate by a force of 40 N during its reciprocating movement having an amplitude of 5 mm and a frequency of 20 Hz. The temperature at the beginning of each test was adjusted to 40 °C, whereafter it was increased by 2 °C per minute. The temperature in which the friction began to increase sharply was registered, and it was used as an indication of the failure of the lubricative film between the ball and the plate. The film failure temperature is a measure of the oxidation resistance of the oil.
-
- From the results of table 6 (tests 2 and 3) it can be seen, that if the oil contains an anti-oxidant which can be classified to hindered phenolics (Hitec 4735) or to aromatic amines (Irganox L 57) the film failure temperature is higher than that of pure rape seed oil. The test 3, however, shows that the percentage of the anti-oxidant has not been high enough. The result is clearly better if the oil contains also a small amount of other anti-oxidant (Irgalube 349, amino phosphate derivative, test 5). Higher percentages of the anti-oxidants give results which are superior to the results of commercial hydrocarbon based hydraulic oils.
- The ability of the hydraulic fluids according to the invention was also tested in a full scale test, which is described in the following example 3.
- A vegetable oil based hydraulic fluid was tested using as a reference a commercial mineral oil based hydraulic fluid. In the test two new identical hydraulic driven mining loaders were used. During the test the pressures in the hydraulic circuits varied from 0 to 165 bar and the hydraulic fluid temperature from 60 to 80°C. Hydraulic pressure was generated by gear pumps and the power was taken out by means of cylinder-piston devices.
-
- In the same test also the volumetric efficiency of the hydraulic systems 2 and 3 was recorded during the test period and the results are given in the following table 8.
Table 8 Time, hours µ v / µ ref Fluid 2 3 0 1 1 300 0.960 0.94 600 0.945 0.88 900 0.940 0.84 1200 0.935 0.79 1500 0.93 0.76 µ v means efficiency recorded
µ ref means efficiency at the beginning of the test - The efficiency tests were conducted using a fluid pressure of 165 bar, and a temperature of 65°C.
- The test results of Table 7 indicate that the durability against shear stress of the vegetable oil based fluid was better than that of the mineral oil based fluid.
- The test results of Table 8 indicate that the efficiency of the system containing the vegetable oil based fluid decreased slower than that of the mineral oil based fluid.
- The lubricative properties of a hydraulic fluid based on the triglyceride composition of the invention were tested by using the testing method described in the following example 4.
- The suitability of rape seed oil as a hydraulic fluid was tested in a four ball tester according to the test method IP 239, in which the test period is one hour and the load 1 kg, as well as according to the standard Test Method STD No 791/6503,1, in which the load is increased stepwise during the test period of 10 seconds. The oils tested are given in the Table 9.
- All the oils tested belong to the viscosity cathegory ISO VG 32 according to the test method ASTM D 2422.
- The results of the said tests are given in the Table 10.
Table 10 IP 239, 1 h / 50 kg wear, mm STD No 791/6503,1 load to welding of the balls 1. 0.46 over 300 2. 0.71 200 3. 1,52 140 4. 1.49 200 5. 0.81 260 6. 0.57 200 - The lubricating properties were compared also by using a gear system, which test is described in the following Example 5.
- The protective action of three hydraulic fluids on gear systems against wear was tested by using the FZG-method according to the standard DIN 51354 E (FZG gear rig test machine).
-
- The results of this test are given in the following table 11.
Table 11 Oil Load degree to damage Specific wear, mg/horsepower/hour 1 above 12 0.05 2 above 12 0.033 3 11 0.10 - In addition to the basic composition the hydraulic fluid according to the invention may also comprise other constituents such as:
- Boundary lubrication additives, such as
metal dialkyl dithiophosphates;
metal diaryl dithiophosphates;
metal dialkyl dithiocarbamates;
alkyl phosphates;
phosphorized fats and olefins; sulphurized fats and fat derivatives; chlorinated fats and fat derivatives - Corrosion inhibitors, such as metal sulfonates;
acid phosphate esters; amines; alkyl succinic acids - VI (Viscosity Index) improvers, such as polymethacrylates; styrene butadiene copolymers; polyisobutylenes
- Pour point depressants, such as chlorinated polymers; alkylated phenol polymers; polymethacrylates
- Foam decomposers, such as polysiloxanes; polyacrylates
- Demulsifiers, such as heavy metal soaps; Ca and Mg sulphonates
- From the base composition according to the invention can be made hydraulic fluids for different purposes by adjusting its viscosity. The following table 12 gives one example of adjusting possibilities.
Table 12 From a base composition according to the invention was made hydraulic fluids for different viscosity classes (ASTM D 2422) Oil comp. % by weight visc. mm²/s class 1. Refined rape seed oil 62.5 20.6 ISO VG 22 Rilanit EHO 35 Hitec 4735 2.0 Anglamol 75 1.5 2. Refined rape seed oil 96.5 34.3 ISO VG 32 Hitec 4735 2.0 Anglamol 75 1.5 3. Refined rape seed oil 73.5 45.2 ISO VG 46 Priolube 3987 23 Hitec 4735 2.0 Anglamol 75 1.5 4. Refined rape seed oil 76.5 73.0 ISO VG 68 Priolube 3987 14 Priolube 3986 6 Hitec 4735 2.0 Anglamol 75 1.5 Rilanit EHO, 2-ethylhexyl oleate, Henkel
Priolube 3987, pentaerythritol ester, Unichema
Priolube 3986, complex ester, Unichema
Claims (6)
- A base composition for hydraulic fluids consisting of:- one or several natural triglycerides which are esters of a straight-chain C₁₀ to C₂₂ fatty acid and glycerol, which triglyceride has an iodine number of at least 50 and not more than 128, and- one or several anti-oxidant additives,- characterized in- that the anti-oxidant additive fraction forms at least 1.5 percent by weight of the composition,- that the anti-oxidant additive fraction contains one or several compounds selected from the group:- (I) hindered phenolics and aromatic amines,- and in that at least in anti-oxidant percentages of 1.5 to 2.0 the anti-oxidant fraction contains one or several additional anti-oxidants selected from one or both of the following groups:- (II) metal salts of dithioacids, phosphites and sulfides.- (III) amides, non aromatic amines, hydrazides and triazols.
- A base composition for hydraulic fluids according to the claim 1, characterized in that the anti-oxidant fraction forms 1.5 to 4.5 percentage by weight of the composition.
- A base composition for hydraulic fluids according to the claims 1 or 2, characterized in that the anti-oxidants selected from the group I form 1.0 to 3.0 percentage by weight of the composition.
- A base composition for hydraulic fluids according to the claims 1 to 3, characterized in that the triglyceride is of oleic-acid-linoleic-acid type and contains saturated fatty acids of not more than 20 percent by weight calculated on the quantity of fatty acids esterified with glycerol.
- A base composition for hydraulic fluids according to the claim 4, characterized in that the triglyceride consists of rape seed oil.
- A hydraulic fluid made of a base composition according to any of the preceeding claims 1 to 5, characterized in that the fluid in addition contains at least one component selected from: boundary lubrication additives, corrosion inhibitors, VI-improvers, pour point depressants, foam decomposers, demulsifiers.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT88901051T ATE83003T1 (en) | 1987-01-28 | 1988-01-27 | HYDRAULIC FLUIDS. |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US7627 | 1987-01-28 | ||
| US07/007,627 US4783274A (en) | 1983-02-11 | 1987-01-28 | Hydraulic fluids |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0349534A1 EP0349534A1 (en) | 1990-01-10 |
| EP0349534B1 true EP0349534B1 (en) | 1992-12-02 |
Family
ID=21727264
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP88901051A Expired - Lifetime EP0349534B1 (en) | 1987-01-28 | 1988-01-27 | Hydraulic fluids |
Country Status (7)
| Country | Link |
|---|---|
| EP (1) | EP0349534B1 (en) |
| AT (1) | ATE83003T1 (en) |
| AU (1) | AU1227388A (en) |
| DE (1) | DE3876432T2 (en) |
| DK (1) | DK536188A (en) |
| NO (1) | NO174060B (en) |
| WO (1) | WO1988005808A1 (en) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU662595B2 (en) * | 1991-08-09 | 1995-09-07 | Lubrizol Corporation, The | Functional fluid with triglycerides, detergent-inhibitor additives and viscosity modifying additives |
| DE4202034A1 (en) * | 1992-01-25 | 1993-07-29 | Rwe Dea Ag | hydraulic fluids |
| FI94278C (en) * | 1992-04-22 | 1995-08-10 | Risto Wisakanto | Use of a pressure medium in drilling |
| ES2133363T3 (en) * | 1992-09-02 | 1999-09-16 | Lubrizol Corp | ANTIOXIDANTS FOR HIGHLY MONO-UNSATURATED VEGETABLE OILS. |
| GB9221846D0 (en) * | 1992-10-17 | 1992-12-02 | Castrol Ltd | Lubricants |
| GB9221841D0 (en) * | 1992-10-17 | 1992-12-02 | Castrol Ltd | Industrial oils |
| US5358652A (en) * | 1992-10-26 | 1994-10-25 | Ethyl Petroleum Additives, Limited | Inhibiting hydrolytic degradation of hydrolyzable oleaginous fluids |
| DE4323771A1 (en) * | 1993-07-15 | 1995-01-19 | Henkel Kgaa | Triglyceride-based base oil for hydraulic oils |
| US5338471A (en) * | 1993-10-15 | 1994-08-16 | The Lubrizol Corporation | Pour point depressants for industrial lubricants containing mixtures of fatty acid esters and vegetable oils |
| US6156228A (en) * | 1994-11-16 | 2000-12-05 | Houghton International, Inc. | Trialkoxyalkylphosphate-based fire resistant fluid containing triglyceride |
| DE4444137A1 (en) * | 1994-12-12 | 1996-06-13 | Henkel Kgaa | Synthetic esters from alcohols and fatty acid mixtures from oleic acid-rich, low stearic acid vegetable oils |
| US5580482A (en) * | 1995-01-13 | 1996-12-03 | Ciba-Geigy Corporation | Stabilized lubricant compositions |
| JP2001214187A (en) * | 2000-02-04 | 2001-08-07 | Nippon Mitsubishi Oil Corp | Hydraulic fluid composition |
| DE102004018732A1 (en) * | 2004-04-17 | 2005-11-03 | Gunnar Berle | oil separator |
| EP2228425A1 (en) * | 2009-02-27 | 2010-09-15 | Dako Ag | Lubricant |
| DE102012222747A1 (en) | 2012-12-11 | 2014-06-12 | Gunnar Berle | Compressor i.e. rotary screw compressor, for use in compressed air-powered machine or tool to produce pressurized air, has heat exchanger through which compressed air is dewatered and cooled in cooling device |
| CN116814312B (en) * | 2023-06-26 | 2025-08-05 | 上海奇克氟硅材料有限公司 | A high temperature resistant antioxidant composition and preparation method thereof |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1789927A (en) * | 1927-09-23 | 1931-01-20 | Vanderbilt Co R T | Oil composition |
| DK117012B (en) * | 1966-04-19 | 1970-03-09 | Sun Oil Co | Hydraulic oil that is resistant to oxidation. |
| US4278554A (en) * | 1978-12-04 | 1981-07-14 | Ethyl Corporation | Antioxidant |
| US4532059A (en) * | 1982-11-25 | 1985-07-30 | Ciba-Geigy Corporation | Benzylated phenols |
| FI66899C (en) * | 1983-02-11 | 1984-12-10 | Kasvisoeljy Vaextolje Ab Oy | SMOERJMEDEL MED TRIGLYCERIDER SOM HUVUDKONPONENT |
| JPS59227986A (en) * | 1983-06-10 | 1984-12-21 | Kao Corp | Metal working oil composition |
| GB2152073B (en) * | 1983-12-23 | 1986-10-22 | Ciba Geigy | Lubricant stabilizer additives |
| CA1248516A (en) * | 1985-07-15 | 1989-01-10 | Stephen C. Cohen | Lubricating oil compositions containing novel combination of stabilizers |
-
1988
- 1988-01-27 AU AU12273/88A patent/AU1227388A/en not_active Abandoned
- 1988-01-27 EP EP88901051A patent/EP0349534B1/en not_active Expired - Lifetime
- 1988-01-27 WO PCT/FI1988/000011 patent/WO1988005808A1/en not_active Ceased
- 1988-01-27 AT AT88901051T patent/ATE83003T1/en not_active IP Right Cessation
- 1988-01-27 DE DE8888901051T patent/DE3876432T2/en not_active Revoked
- 1988-09-23 NO NO884237A patent/NO174060B/en unknown
- 1988-09-27 DK DK536188A patent/DK536188A/en not_active Application Discontinuation
Also Published As
| Publication number | Publication date |
|---|---|
| DE3876432T2 (en) | 1993-04-08 |
| DK536188D0 (en) | 1988-09-27 |
| DE3876432D1 (en) | 1993-01-14 |
| EP0349534A1 (en) | 1990-01-10 |
| NO884237D0 (en) | 1988-09-23 |
| DK536188A (en) | 1988-09-27 |
| ATE83003T1 (en) | 1992-12-15 |
| WO1988005808A1 (en) | 1988-08-11 |
| AU1227388A (en) | 1988-08-24 |
| NO174060B (en) | 1993-11-29 |
| NO884237L (en) | 1988-09-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4783274A (en) | Hydraulic fluids | |
| EP0349534B1 (en) | Hydraulic fluids | |
| KR100855112B1 (en) | Vegetable oil-based lubricants, including pre-hydrogenated synthetic oils | |
| CA2498816C (en) | Biodegradable penetrating lubricant | |
| US5102567A (en) | High performance food-grade lubricating oil | |
| CN103205301B (en) | Gear oil composition and preparation method thereof | |
| US5094763A (en) | Hydraulic fluid composition for power steering containing a phosphorous compound and a thiadiazole derivative | |
| EP0667389B1 (en) | Metal free hydraulic fluid with amine salt | |
| JPH08231976A (en) | Stabilized lubricant composition | |
| CN1166180A (en) | Hydraulic operating oil composition | |
| EP1026225B1 (en) | Electrical and transformer oil composition | |
| US5990055A (en) | Biodegradable lubricant composition from triglycerides and oil soluble antimony | |
| KR100575423B1 (en) | Lubricating composition | |
| EP0239088A2 (en) | A lubricant and process for its production | |
| WO2006116775A1 (en) | Vegetable oil lubricant comprising fischer tropsch synthetic oils | |
| US20060211585A1 (en) | Vegetable oil lubricant comprising Fischer Tropsch synthetic oils | |
| US5198129A (en) | Lubricating oil composition containing zinc dithiophosphate | |
| DE112011103822T5 (en) | Lubricant for percussion equipment | |
| EP0741736B1 (en) | Anti-wear additives and their use | |
| EP0025774B1 (en) | Dithiophosphates, their preparation and lubricants containing them | |
| JP5496502B2 (en) | Lubricating oil composition | |
| EP0079302A2 (en) | Lubricants containing thio ethers of beta-dicarbonyl or beta-cyano-carbonyl compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19890714 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| 17Q | First examination report despatched |
Effective date: 19910227 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE FR GB IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 83003 Country of ref document: AT Date of ref document: 19921215 Kind code of ref document: T |
|
| REF | Corresponds to: |
Ref document number: 3876432 Country of ref document: DE Date of ref document: 19930114 |
|
| ITF | It: translation for a ep patent filed | ||
| ET | Fr: translation filed | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: CIBA-GEIGY AG PATENTABTEILUNG Effective date: 19930830 |
|
| 26 | Opposition filed |
Opponent name: DEN NORSKE STATS OJESELSKAP A.S. Effective date: 19930902 Opponent name: THE LUBRIZOL CORPORATION Effective date: 19930902 Opponent name: MOBIL OIL CORPORATION Effective date: 19930902 Opponent name: KARLSHAMNS AB Effective date: 19930829 Opponent name: CIBA-GEIGY AG PATENTABTEILUNG Effective date: 19930830 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: DEN NORSKE STATS OJESELSKAP A.S. Opponent name: LUBRIZOL CORPORATION Opponent name: MOBIL OIL CORPORATION Opponent name: KARLSHAMNS AB Opponent name: CIBA-GEIGY AG PATENTABTEILUNG |
|
| EPTA | Lu: last paid annual fee | ||
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |
|
| R26 | Opposition filed (corrected) |
Opponent name: CIBA-GEIGY AG PATENTABTEILUNG * 930829 KARLSHAMNS Effective date: 19930830 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: LU Payment date: 19950101 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 19950104 Year of fee payment: 8 Ref country code: GB Payment date: 19950104 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 19950117 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19950124 Year of fee payment: 8 |
|
| EAL | Se: european patent in force in sweden |
Ref document number: 88901051.8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 19950131 Year of fee payment: 8 Ref country code: BE Payment date: 19950131 Year of fee payment: 8 Ref country code: AT Payment date: 19950131 Year of fee payment: 8 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 19950202 Year of fee payment: 8 |
|
| RDAG | Patent revoked |
Free format text: ORIGINAL CODE: 0009271 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT REVOKED |
|
| 27W | Patent revoked |
Effective date: 19950702 |
|
| GBPR | Gb: patent revoked under art. 102 of the ep convention designating the uk as contracting state |
Free format text: 950702 |
|
| NLR2 | Nl: decision of opposition | ||
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |