EP0328128B2 - Process concerning the adhesion between metallic materials and galvanic aluminium layers and the non-aqueous electrolyte used therein - Google Patents
Process concerning the adhesion between metallic materials and galvanic aluminium layers and the non-aqueous electrolyte used therein Download PDFInfo
- Publication number
- EP0328128B2 EP0328128B2 EP89102319A EP89102319A EP0328128B2 EP 0328128 B2 EP0328128 B2 EP 0328128B2 EP 89102319 A EP89102319 A EP 89102319A EP 89102319 A EP89102319 A EP 89102319A EP 0328128 B2 EP0328128 B2 EP 0328128B2
- Authority
- EP
- European Patent Office
- Prior art keywords
- metal
- anhydrous
- nickel
- compounds
- process according
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000000034 method Methods 0.000 title claims abstract description 22
- 239000007769 metal material Substances 0.000 title claims abstract description 10
- 229910052782 aluminium Inorganic materials 0.000 title claims abstract description 9
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 title claims abstract description 9
- 239000011255 nonaqueous electrolyte Substances 0.000 title claims abstract description 6
- 239000004411 aluminium Substances 0.000 title claims abstract 4
- 229910052751 metal Inorganic materials 0.000 claims abstract description 46
- 239000002184 metal Substances 0.000 claims abstract description 46
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims abstract description 41
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims abstract description 31
- 229910045601 alloy Inorganic materials 0.000 claims abstract description 23
- 239000000956 alloy Substances 0.000 claims abstract description 23
- 229910052759 nickel Inorganic materials 0.000 claims abstract description 23
- 229910052742 iron Inorganic materials 0.000 claims abstract description 18
- 239000010949 copper Substances 0.000 claims abstract description 15
- 150000002739 metals Chemical class 0.000 claims abstract description 13
- 229910052802 copper Inorganic materials 0.000 claims abstract description 12
- 229910000831 Steel Inorganic materials 0.000 claims abstract description 8
- 239000010959 steel Substances 0.000 claims abstract description 8
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims abstract description 7
- 239000010941 cobalt Substances 0.000 claims abstract description 7
- 229910017052 cobalt Inorganic materials 0.000 claims abstract description 7
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 claims abstract description 7
- 229910000990 Ni alloy Inorganic materials 0.000 claims abstract description 3
- 150000003839 salts Chemical class 0.000 claims description 17
- 239000003792 electrolyte Substances 0.000 claims description 15
- AMXOYNBUYSYVKV-UHFFFAOYSA-M lithium bromide Chemical compound [Li+].[Br-] AMXOYNBUYSYVKV-UHFFFAOYSA-M 0.000 claims description 12
- 150000001875 compounds Chemical class 0.000 claims description 10
- -1 alkylene glycol Chemical compound 0.000 claims description 9
- 239000002904 solvent Substances 0.000 claims description 9
- 239000011261 inert gas Substances 0.000 claims description 8
- LYCAIKOWRPUZTN-UHFFFAOYSA-N ethylene glycol Natural products OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 7
- 150000002170 ethers Chemical class 0.000 claims description 6
- 239000000203 mixture Substances 0.000 claims description 6
- 238000005868 electrolysis reaction Methods 0.000 claims description 5
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 5
- 150000001298 alcohols Chemical class 0.000 claims description 4
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 claims description 4
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M lithium chloride Chemical compound [Li+].[Cl-] KWGKDLIKAYFUFQ-UHFFFAOYSA-M 0.000 claims description 4
- 229910052718 tin Chemical class 0.000 claims description 4
- 125000000217 alkyl group Chemical group 0.000 claims description 3
- 239000012298 atmosphere Substances 0.000 claims description 3
- 150000004820 halides Chemical class 0.000 claims description 3
- 150000002736 metal compounds Chemical class 0.000 claims description 3
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 3
- 238000007747 plating Methods 0.000 claims description 3
- 239000011135 tin Chemical class 0.000 claims description 3
- DNIAPMSPPWPWGF-GSVOUGTGSA-N (R)-(-)-Propylene glycol Chemical compound C[C@@H](O)CO DNIAPMSPPWPWGF-GSVOUGTGSA-N 0.000 claims description 2
- 238000004070 electrodeposition Methods 0.000 claims description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims description 2
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 2
- CLDVQCMGOSGNIW-UHFFFAOYSA-N nickel tin Chemical compound [Ni].[Sn] CLDVQCMGOSGNIW-UHFFFAOYSA-N 0.000 claims description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 2
- 235000013772 propylene glycol Nutrition 0.000 claims description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical class [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims 2
- 239000003115 supporting electrolyte Substances 0.000 claims 2
- 150000003842 bromide salts Chemical class 0.000 claims 1
- 150000001768 cations Chemical class 0.000 claims 1
- 150000003841 chloride salts Chemical class 0.000 claims 1
- 125000004051 hexyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 claims 1
- 229910001509 metal bromide Inorganic materials 0.000 claims 1
- 229910001510 metal chloride Inorganic materials 0.000 claims 1
- 239000011248 coating agent Substances 0.000 abstract description 7
- 238000000576 coating method Methods 0.000 abstract description 7
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 abstract 1
- 229910001297 Zn alloy Inorganic materials 0.000 abstract 1
- 239000011701 zinc Substances 0.000 abstract 1
- 239000010410 layer Substances 0.000 description 23
- 239000001257 hydrogen Substances 0.000 description 10
- 229910052739 hydrogen Inorganic materials 0.000 description 10
- 239000000243 solution Substances 0.000 description 10
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 9
- 238000000151 deposition Methods 0.000 description 7
- 230000008021 deposition Effects 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 6
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- 229910021586 Nickel(II) chloride Inorganic materials 0.000 description 4
- 239000000853 adhesive Substances 0.000 description 4
- 230000001070 adhesive effect Effects 0.000 description 4
- 238000005269 aluminizing Methods 0.000 description 4
- QMMRZOWCJAIUJA-UHFFFAOYSA-L nickel dichloride Chemical compound Cl[Ni]Cl QMMRZOWCJAIUJA-UHFFFAOYSA-L 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 3
- 229910001914 chlorine tetroxide Inorganic materials 0.000 description 3
- 238000009713 electroplating Methods 0.000 description 3
- XLYOFNOQVPJJNP-ZSJDYOACSA-N heavy water Substances [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 description 3
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Chemical compound [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 229910021591 Copper(I) chloride Inorganic materials 0.000 description 2
- GQPLMRYTRLFLPF-UHFFFAOYSA-N Nitrous Oxide Chemical compound [O-][N+]#N GQPLMRYTRLFLPF-UHFFFAOYSA-N 0.000 description 2
- 229910052786 argon Inorganic materials 0.000 description 2
- 239000004327 boric acid Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- OXBLHERUFWYNTN-UHFFFAOYSA-M copper(I) chloride Chemical compound [Cu]Cl OXBLHERUFWYNTN-UHFFFAOYSA-M 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 239000008151 electrolyte solution Substances 0.000 description 2
- 229940021013 electrolyte solution Drugs 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- UUFQTNFCRMXOAE-UHFFFAOYSA-N 1-methylmethylene Chemical compound C[CH] UUFQTNFCRMXOAE-UHFFFAOYSA-N 0.000 description 1
- XNWFRZJHXBZDAG-UHFFFAOYSA-N 2-METHOXYETHANOL Chemical compound COCCO XNWFRZJHXBZDAG-UHFFFAOYSA-N 0.000 description 1
- ZNQVEEAIQZEUHB-UHFFFAOYSA-N 2-ethoxyethanol Chemical compound CCOCCO ZNQVEEAIQZEUHB-UHFFFAOYSA-N 0.000 description 1
- 229940093475 2-ethoxyethanol Drugs 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 229910000531 Co alloy Inorganic materials 0.000 description 1
- 229910021589 Copper(I) bromide Inorganic materials 0.000 description 1
- XTHFKEDIFFGKHM-UHFFFAOYSA-N Dimethoxyethane Chemical compound COCCOC XTHFKEDIFFGKHM-UHFFFAOYSA-N 0.000 description 1
- 229910021577 Iron(II) chloride Inorganic materials 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000002318 adhesion promoter Substances 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000005619 boric acid group Chemical group 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 238000012733 comparative method Methods 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- CAPAZTWTGPAFQE-UHFFFAOYSA-N ethane-1,2-diol Chemical compound OCCO.OCCO CAPAZTWTGPAFQE-UHFFFAOYSA-N 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- NMCUIPGRVMDVDB-UHFFFAOYSA-L iron dichloride Chemical compound Cl[Fe]Cl NMCUIPGRVMDVDB-UHFFFAOYSA-L 0.000 description 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 229910001507 metal halide Inorganic materials 0.000 description 1
- 150000005309 metal halides Chemical class 0.000 description 1
- 238000001465 metallisation Methods 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- LGQLOGILCSXPEA-UHFFFAOYSA-L nickel sulfate Chemical compound [Ni+2].[O-]S([O-])(=O)=O LGQLOGILCSXPEA-UHFFFAOYSA-L 0.000 description 1
- 229910000363 nickel(II) sulfate Inorganic materials 0.000 description 1
- 239000001272 nitrous oxide Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D5/00—Electroplating characterised by the process; Pretreatment or after-treatment of workpieces
- C25D5/10—Electroplating with more than one layer of the same or of different metals
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D3/00—Electroplating: Baths therefor
- C25D3/02—Electroplating: Baths therefor from solutions
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D5/00—Electroplating characterised by the process; Pretreatment or after-treatment of workpieces
- C25D5/627—Electroplating characterised by the visual appearance of the layers, e.g. colour, brightness or mat appearance
Definitions
- the invention relates to methods for the metal coating of metal materials, in particular of low-alloy, high-strength steels.
- the invention also relates to the non-aqueous electrolytes used in the above process.
- Certain metals such as copper, can be directly electroplated with aluminum after appropriate mechanical and / or chemical pretreatments to remove fat and / or oxide layers from the surface of the workpieces.
- metals such as, for example, iron materials and in particular special steels, generally no adhesive layers of galvanoaluminium can be obtained in the same way.
- DE-PS 22 60 191 (Siemens AG, priority: 08.12.1972) describes such a method, which is characterized in that at least the last process step, which is used to shape the workpieces, is carried out under an aprotic, water- and oxygen-free protective medium.
- milling, sawing or sanding are mentioned as the last process step used for shaping.
- a decisive disadvantage of the two last-mentioned procedures is that during the electrodeposition of the metals serving as adhesion promoters, co-separation of hydrogen cannot be avoided.
- Low-alloy high-strength steels for example as shown in Table 1, are very sensitive to embrittlement to hydrogen. Aqueous electrolyte solutions are therefore unsuitable for coating such steels.
- DE-A-2012846 discloses non-aqueous, DMSO-containing solutions of metal salts for electroplating without hydrogen fragility.
- the invention consequently relates to methods for the metal coating of metal materials, in particular low-alloy high-strength steels, galvanic bonding layers being formed from non-aqueous electrolytes of anhydrous metal compounds Iron, iron and nickel, nickel, cobalt, copper or alloys of the above-mentioned metals or tin-nickel alloys are deposited on these metal materials and then subsequently galvanically aluminum is deposited in a manner known per se.
- a layer thickness of 1 to 4 ⁇ m is generally sufficient to ensure adhesion between the material, intermediate layer and galvanoaluminium layer.
- electrolytes are solutions of anhydrous metal salts of Fe, Co, Ni, Cu or Sn, in particular their anhydrous halides and / or the complex compounds of these metal halides with ethers, such as for example tetrahydrofuran, or with alcohols, such as ethanol, in anhydrous alkyl half ethers of a C2-C3 alkylene glycol of the formula in the R for C1 to C6 and phenyl, and R1 represents H or methyl, or mixtures of these solutions with the addition of anhydrous conductive salts, in particular lithium chloride, lithium bromide or corresponding tetraorganylammonium halides.
- ethers such as for example tetrahydrofuran
- alcohols such as ethanol
- Such soluble anodes are expediently those from the concerned metal or in the deposition of alloys used from the metals to be deposited or corresponding alloy anodes.
- the metal (II) compounds are expediently used; in the deposition of Cu, Cu (I) compounds are generally used.
- the anhydrous metal salts are preferably the anhydrous metal dichlorides or dibromides in the case of Fe, Co and Ni or copper (I) chloride or bromide or their addition compounds with alcohols, such as, for example, methanol or ethanol, or with ethers, such as, for example, diethyl ether, THF or dimethoxyethane , used.
- Alkyl half ethers of an alkylene glycol such as 1-alkoxy-2-hydroxyethane or 1-alkoxy-2-hydroxypropane, in particular the easily and inexpensively accessible half ethers of 1,2-ethanediol ROCH2CH2OH, preferably those with R methyl, ethyl, propyl or isopropyl, are used as solvents or those of 1,2-propanediol, in particular CH3CH (OH) CH2OCH3, used.
- concentrations of conductive salt, especially lithium bromide, should be of approximately the same to twice the order of magnitude.
- the electrolysis temperatures are between room temperature and approximately 120 ° C. Temperatures between 50 and 80 ° C. are preferred. Good, uniform and shiny metal layers made of Fe, Co, Ni or Cu can be achieved with current densities between 0.2 and 1.5 A / dm2, preferably 0.5 to 1.0 A / dm2, see Table 1.
- alloy deposits mixtures of solutions of the metal salts of the alloy components are generally used according to the invention.
- the anodes can then be either those made of appropriate alloys or a plurality of electrodes made of the metals of the individual alloy components. If there is a large electrolyte supply, it is possible to work only with the anode made from one of the alloy components. The concentration of the other alloy component must then be refreshed from time to time in the solution by adding the appropriate salt. If the deposition tendencies of the individual metals are very different, electrolytes containing only the salt of the more difficult to deposit metal can also be used when using alloy anodes.
- the electrolyses are carried out in closed vessels in an inert gas atmosphere of, for example, argon and / or nitrous oxide and / or nitrogen.
- the workpieces are first washed with the solvent of the electrolyte.
- the workpieces are washed with dry toluene and then transferred to the aluminizing bath via an inert gas lock.
- a particular advantage of such a procedure is that no new oxide or water layer can be formed on the metal surface. Furthermore, there is no need for subsequent, complex drying processes before entering the aluminizing bath, such as treatment with fluorocarbons containing wetting agents.
- a cylindrical glass vessel with an edge ground flat at the top serves as the electrolysis cell and can be firmly closed with a lid made of insulating material.
- a cathode made of the material to be coated for example WL-1.6359, is suspended from the lid between two anode plates made of the electrochemically dissolvable metal, for example nickel.
- the attachments of the electrodes also serve as a power supply.
- the dry cell is filled with inert gas, such as argon or nitrogen.
- To coat the cathode with nickel a solution of 0.05 mol NiCl2 ⁇ 0.63 THF and 0.05 mol LiBr in 1 liter CH3OCH2CH2OH is used as the electrolyte. Electrolysis is carried out at 60 ° C.
- the anodic and cathodic current yields are quantitative in relation to the amount of electricity.
- the workpieces are washed with the solvent of the electrolyte and dried in a stream of inert gas.
- the workpieces are then washed with dry toluene and transferred to the aluminizing bath via an inert gas lock.
- the "tape test” is a quantitative comparative method that enables liability assessments to be carried out in a simple manner. For this purpose, an adhesive strip is first pressed firmly onto the galvanic layer and then quickly torn off. In the event of poor or moderate adhesion, the galvanic layer and the adhesive strip come off the material base. If the adhesion is good, only small areas of the electroplating layer will be removed and if the adhesion is very good, the bonding of the electroplating layer to the base will remain intact.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Electroplating And Plating Baths Therefor (AREA)
- Electroplating Methods And Accessories (AREA)
- Application Of Or Painting With Fluid Materials (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Paints Or Removers (AREA)
- Laminated Bodies (AREA)
- Electrolytic Production Of Metals (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
- Prevention Of Electric Corrosion (AREA)
- Secondary Cells (AREA)
- Sealing Battery Cases Or Jackets (AREA)
Abstract
Description
Die Erfindung betrifft Verfahren zur Metallbeschichtung von Metallwerkstoffen, insbesondere von niedriglegierten, hochfesten Stählen.The invention relates to methods for the metal coating of metal materials, in particular of low-alloy, high-strength steels.
Die Erfindung betrifft außerdem die im Rahmen des obigen Verfahrens eingesetzten nichtwäßrigen Elektrolyte.The invention also relates to the non-aqueous electrolytes used in the above process.
Bestimmte Metalle, wie beispielsweise Kupfer, können nach entsprechenden mechanischen und/oder chemischen Vorbehandlungen zur Entfernung von Fett- und/oder Oxidschichten von der Oberfläche der Werkstücke direkt mit Aluminium haftfest galvanisch beschichtet werden. Auf anderen Metallen, wie beispielsweie Eisenwerkstoffen und insbesondere Spezialstählen, können im allgemeinen auf gleiche Weise keine haftfesten Schichten von Galvanoaluminium erhalten werden.Certain metals, such as copper, can be directly electroplated with aluminum after appropriate mechanical and / or chemical pretreatments to remove fat and / or oxide layers from the surface of the workpieces. On other metals, such as, for example, iron materials and in particular special steels, generally no adhesive layers of galvanoaluminium can be obtained in the same way.
Daher wurden verschiedene Verfahren zur Vorbereitung von Werkstücken aus elektrisch leitfähigen Materialien, inbesondere Metallen für eine haftfeste galvanische Beschichtung mit Aluminium, vorgeschlagen.Therefore, various methods for the preparation of workpieces made of electrically conductive materials, in particular metals for an adhesive galvanic coating with aluminum, have been proposed.
Die DE-PS 22 60 191 (Siemens AG, Priorität: 08.12.1972) beschreibt ein solches Verfahren, das dadurch gekennzeichnet ist, daß zumindest der letzte, der Formgebung der Werkstücke dienende Verfahrensschritt unter aprotischem wasser- und sauerstoff-freiem Schutzmedium durchgeführt wird. In den Beispielen wird als letzter der Formgebung dienender Verfahrensschritt Fräsen, Sägen oder Schmirgeln genannt.DE-PS 22 60 191 (Siemens AG, priority: 08.12.1972) describes such a method, which is characterized in that at least the last process step, which is used to shape the workpieces, is carried out under an aprotic, water- and oxygen-free protective medium. In the examples, milling, sawing or sanding are mentioned as the last process step used for shaping.
In der DE-OS 31 12 919 (Siemens AG, Priorität: 31.03.1981) wird vorgeschlagen, zur Haftvermittlung auf Eisenwerkstücken galvanisch aus wäßrigen Lösungen dünne Schichten aus Kobalt oder Kobaltlegierungen aufzubringen. Zur Haftvermittlung mit dem anschließend galvanisch aufzubringenden Aluminium sollen Schichtdicken von maximal 1 µm ausreichen.In DE-OS 31 12 919 (Siemens AG, priority: March 31, 1981) it is proposed to apply thin layers of cobalt or cobalt alloys galvanically from aqueous solutions to impart adhesion to iron workpieces. Layer thicknesses of a maximum of 1 µm should be sufficient to impart adhesion with the aluminum that is subsequently to be electroplated.
Bereits früher (H. Lehmkuhl, Dissertation, Technische Hochschule Aachen, 1954) wurde empfohlen, galvanisch aus wäßrigen Elektrolyten abgeschiedene Kupferschichten zur Haftvermittlung zwischen Eisenwerkstücken und galvanisch erzeugten Aluminiumschichten zu verwenden.Previously (H. Lehmkuhl, dissertation, Technical University of Aachen, 1954), it was recommended to use copper layers electroplated from aqueous electrolytes to promote adhesion between iron workpieces and electroplated aluminum layers.
Entscheidender Nachteil der beiden zuletzt genannten Verfahrensweisen ist, daß bei der galvanischen Abscheidung der als Haftvermittler dienenden Metalle aus wäßrigen Lösungen eine Mitabscheidung von Wasserstoff nicht zu vermeiden ist. Niedriglegierte hochfeste Stähle, wie beispielsweise in Tabelle 1 angegeben, sind jedoch sehr versprödungempfindlich gegenüber Wasserstoff. Wäßrige Elektrolytlösungen sind daher zur Beschichtung solcher Stähle ungeeignet.A decisive disadvantage of the two last-mentioned procedures is that during the electrodeposition of the metals serving as adhesion promoters, co-separation of hydrogen cannot be avoided. Low-alloy high-strength steels, for example as shown in Table 1, are very sensitive to embrittlement to hydrogen. Aqueous electrolyte solutions are therefore unsuitable for coating such steels.
Aus der DE-A-2012846 sind nicht wäßrige, DMSO enthaltende Lösungen von Metallsalzen zum Elektroplattieren ohne Wasserstoffbrüchigkeit bekannt.DE-A-2012846 discloses non-aqueous, DMSO-containing solutions of metal salts for electroplating without hydrogen fragility.
Die Erfindung betrifft folglich Verfahren zur Metallbeschichtung von Metallwerkstoffen, insbesondere niedriglegierten hochfesten Stählen, wobei aus nichtwäßrigen Elektrolyten wasserfreier Metallverbindungen galvanisch Haftvermittlungsschichten aus Eisen, Eisen und Nickel, Nickel, Cobalt, Kupfer oder Legierungen der vorstehend genannten Metalle oder Zinn-Nickel-Legierungen auf diese Metallwerkstoffe abgeschieden und darauf dann anschließend in an sich bekannter Weise galvanisch Aluminium abgeschieden wird.The invention consequently relates to methods for the metal coating of metal materials, in particular low-alloy high-strength steels, galvanic bonding layers being formed from non-aqueous electrolytes of anhydrous metal compounds Iron, iron and nickel, nickel, cobalt, copper or alloys of the above-mentioned metals or tin-nickel alloys are deposited on these metal materials and then subsequently galvanically aluminum is deposited in a manner known per se.
Bei Verwendung dieser Metalle als Zwischenschichten für eine anschließende Aluminierung auf galvanischem Weg ist im allgemeinen eine Schichtdicke von 1 bis 4 µm ausreichend, um Haftung zwischen Werkstoff, Zwischenschicht und Galvanoaluminiumschicht zu gewährleisten.When using these metals as intermediate layers for a subsequent aluminizing by galvanic means, a layer thickness of 1 to 4 µm is generally sufficient to ensure adhesion between the material, intermediate layer and galvanoaluminium layer.
Als Elektrolyte werden, um die Abscheidung von Wasserstoff und die damit verbundene Gefahr der Versprödung der Werkstoffe zu vermeiden, Lösungen wasserfreier Metallsalze des Fe, Co, Ni, Cu oder Sn, insbesondere deren wasserfreie Halogenide und/oder die Komplexverbindungen dieser Metallhalogenide mit Ethern, wie beispielsweise Tetrahydrofuran, oder mit Alkoholen, wie beispielsweise Ethanol, in wasserfreien Alkylhalbethern eines C₂-C₃-Alkylenglykols der Formel
in der
R für C₁ bis C₆ und Phenyl, und
R¹ für H oder Methyl steht,
oder Mischungen dieser Lösungen unter Zusatz wasserfreier Leitsalze, insbesondere Lithiumchlorid, Lithiumbromid oder entsprechende Tetraorganylammoniumhalogenide verwendet.In order to avoid the deposition of hydrogen and the associated risk of embrittlement of the materials, electrolytes are solutions of anhydrous metal salts of Fe, Co, Ni, Cu or Sn, in particular their anhydrous halides and / or the complex compounds of these metal halides with ethers, such as for example tetrahydrofuran, or with alcohols, such as ethanol, in anhydrous alkyl half ethers of a C₂-C₃ alkylene glycol of the formula
in the
R for C₁ to C₆ and phenyl, and
R¹ represents H or methyl,
or mixtures of these solutions with the addition of anhydrous conductive salts, in particular lithium chloride, lithium bromide or corresponding tetraorganylammonium halides.
Weiter werden als lösliche Anoden zweckmäßig solche aus dem betreffenden Metall oder bei der Abscheidung von Legierungen solche aus den abzuscheidenden Metallen oder entsprechende Legierungsanoden eingesetzt.Furthermore, such soluble anodes are expediently those from the concerned metal or in the deposition of alloys used from the metals to be deposited or corresponding alloy anodes.
Bei Fe-, Co-, Ni- und Sn-Verbindungen werden zweckmäßig die Metall(II)-Verbindungen eingesetzt, bei der Abscheidung von Cu geht man im allgemeinen von Cu(I)-Verbindungen aus.In the case of Fe, Co, Ni and Sn compounds, the metal (II) compounds are expediently used; in the deposition of Cu, Cu (I) compounds are generally used.
Die Verwendung von 2-Ethoxyethanol als Lösungsmittel von Elektrolyten zur Abscheidung von Cu, Ni, Co ist von A.L. Chaney, C.A. Mann, J. Phys. Chem 35 (1931) 2289 beschrieben worden. Im Gegensatz zum erfindungsgemäßen Verfahren wurden jedoch nur die Wasser enthaltenen Verbindungen (Cu(ClO₄ )₂·2H₂O, Ni(ClO₄)₂ ·2H₂O und Co(ClO₄)₂ ·2H₂O beschrieben. Die Art der Metallabscheidung wird von den Autoren beim Cu als gut, beim Ni als weniger gut, weil spröde, und beim Co auch als weniger gut, weil schwarz, schwammig beschrieben. Ob diese Schichten als Haftvermittlungsschichten für Galvanoaluminium geeignet sind, ist nicht bekannt. Dies muß jedoch, insbesondere wegen der Eigenschaften der Ni- oder Co-Schichten, wie Sprödigkeit oder schwammiger Charakter, bezweifelt werden. Auf jeden Fall bleibt bei den durch die Metallsalze eingebrachten Wasseranteilen die Mitabscheidung von Wasserstoff unvermeidbar und damit verbunden die Gefahr der Versprödung der Werkstoffe durch Wasserstoff erhalten.The use of 2-ethoxyethanol as a solvent of electrolytes for the deposition of Cu, Ni, Co has been described by AL Chaney, CA Mann, J. Phys. Chem 35 (1931) 2289. In contrast to the process according to the invention, however, only the water-containing compounds (Cu (ClO₄) ₂ · 2H₂O, Ni (ClO₄) ₂ · 2H₂O and Co (ClO₄) ₂ · 2H₂O have been described. The authors describe the type of metal deposition for Cu as good , Ni is less good because it is brittle, and Co less good because it is black, spongy, and it is not known whether these layers are suitable as adhesion-promoting layers for galvanoaluminium, but this must be the case, particularly because of the properties of the Ni or Co-layers such as brittleness or spongy character are doubted In any case, with the water components brought in by the metal salts, the co-separation of hydrogen remains unavoidable and the associated risk of embrittlement of the materials by hydrogen is maintained.
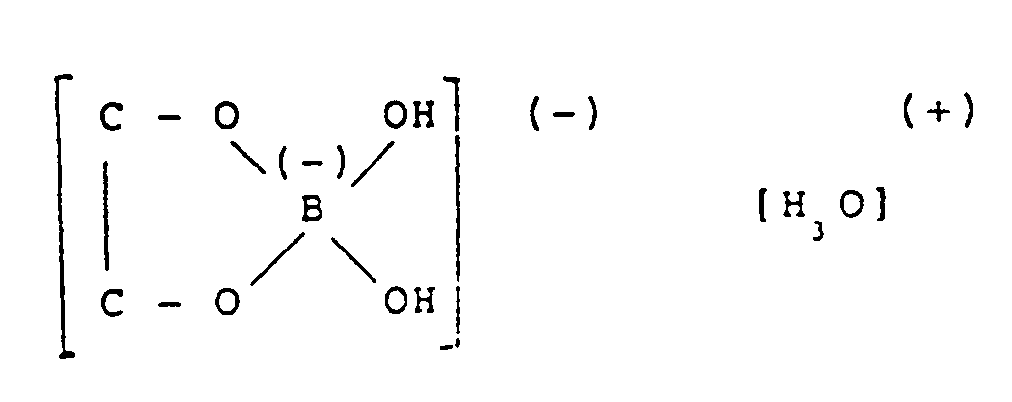
Die von A.J. Dill (Plating 1972, 59 (11), 1048-1052, Galvano-Organo 1974, 43, 151-156) beschriebene Abscheidung von Nickel aus Lösungen von NiCl₂·6H₂O in Ethylenglykol (1,2-Ethandiol) geht ebenfalls von einem wasserhaltigen Metallsalz aus. Die Mitabscheidung von Wasserstoff kann daher nicht vermieden werden. Ähnliches gilt für die von A.A. Sarabi, V.B. Singh, Indian J. of Technology 25 (1987) 119 untersuchte Nickelabscheidung aus 0.2 M Lösungen von NiCl₂ nicht definierten Wassergehaltes oder NiSO₄·7 H₂O in 1,2-Ethandiol oder 2-Methoxyethanol unter Zusatz von Borsäure (0.2 M). In 2-Methoxyethanol-NiCl₂-H₃BO₃-x H₂O-Elektrolyten sind die Nickelabscheidungen gleichmäßig, grauglänzend und haftend bei Stromdichten von 0.1-0.3 A/dm², bei höheren Stromdichten zeigen sie eine Tendenz sich abzuschälen. Da die kathodischen Stromausbeuten nur 90-98 % betragen, muß angenommen werden, daß Wasserstoff mit abgeschieden wird. Hierzu ist allgemein bekannt (F.A. Cotton, G. Wilkinson, Anorganische Chemie, Verlag Chemie, Weinheim 1967, S. 245), daß Borsäure mit Alkoholen sehr leicht unter Abspaltung von Wasser Borsäureester bildet. Mit 1,2-Alkandiolen, wie 1,2-Ethandiol, entstehen stark saure Chelatkomplexe vom Typ
Beide Effekte verstärken die Gefahr der Wasserstoffabspaltung.The deposition of nickel from solutions of NiCl₂ · 6H₂O in ethylene glycol (1,2-ethanediol) also described by AJ Dill (Plating 1972 , 59 (11), 1048-1052, Galvano-Organo 1974 , 43, 151-156) a water-containing metal salt. The co-separation of hydrogen cannot therefore be avoided. The same applies to the nickel deposition from 0.2 M solutions by AA Sarabi, VB Singh, Indian J. of Technology 25 (1987) 119 NiCl₂ undefined water content or NiSO₄ · 7 H₂O in 1,2-ethanediol or 2-methoxyethanol with the addition of boric acid (0.2 M). In 2-methoxyethanol-NiCl₂-H₃BO₃-x H₂O electrolytes, the nickel deposits are uniform, shiny gray and sticky at current densities of 0.1-0.3 A / dm², at higher current densities they show a tendency to peel off. Since the cathodic current yields are only 90-98%, it must be assumed that hydrogen is also deposited. It is generally known (FA Cotton, G. Wilkinson, Inorganic Chemistry, Verlag Chemie, Weinheim 1967, p. 245) that boric acid forms very easily boric acid esters with the elimination of water with alcohols. With 1,2-alkanediols, such as 1,2-ethanediol, strongly acidic chelate complexes of the type are formed
Both effects increase the risk of hydrogen elimination.
Diese Gefahr besteht beim erfindungsgemäßen Verfahren nicht, da hier die wasserfreien Salze eingesetzt werden und das Lösungsmittel ebenfalls wasserfrei und ohne Zusatz von Säuren, insbesondere Borsäure, verwendet wird. Die anodischen und kathodischen Stromausbeuten sind bezogen auf aufgelöstes bzw. abgeschiedenes Metall quantitativ. Wasserstoff wird nicht abgeschieden. 1 Faraday, d.s. 26.8 Amperestunden, lösen anodisch 55.85/2 g Eisen, 58.94/2 g Kobalt oder 58.71/2 g Nickel entsprechend dem elektrolytischen Vorgang
Als Lösungsmittel werden Alkylhalbether eines Alkylenglykols wie 1-Alkoxy-2-hydroxyethan oder 1-Alkoxy-2-hydroxypropan, insbesondere die leicht und preiswert zugänglichen Halbether des 1,2-Ethandiols ROCH₂CH₂OH, bevorzugt solche mit R = Methyl, Ethyl, Propyl oder Isopropyl oder solche des 1,2-Propandiols, insbesondere CH₃CH(OH)CH₂OCH₃, eingesetzt.Alkyl half ethers of an alkylene glycol such as 1-alkoxy-2-hydroxyethane or 1-alkoxy-2-hydroxypropane, in particular the easily and inexpensively accessible half ethers of 1,2-ethanediol ROCH₂CH₂OH, preferably those with R = methyl, ethyl, propyl or isopropyl, are used as solvents or those of 1,2-propanediol, in particular CH₃CH (OH) CH₂OCH₃, used.
Als Metallsalzkonzentration in diesen Lösungsmitteln werden 0,02 bis 0,1 M, bevorzugt 0,044 bis 0,05 M Lösungen empfohlen. Die Konzentrationen an Leitsalz, insbesondere Lithiumbromid, sollten von etwa gleicher bis doppelter Größenordnung sein.0.02 to 0.1 M, preferably 0.044 to 0.05 M solutions are recommended as the metal salt concentration in these solvents. The concentrations of conductive salt, especially lithium bromide, should be of approximately the same to twice the order of magnitude.
Die Elektrolysetemperaturen liegen zwischen Raumtemperatur und ca. 120°C bevorzugt werden Temperaturen zwischen 50 und 80 °C. Gute, gleichmäßige und glänzende Metallschichten aus Fe, Co, Ni oder Cu lassen sich mit Stromdichten zwischen 0,2 und 1,5 A/dm² erreichen, bevorzugt werden 0,5 bis 1,0 A/dm², siehe Tabelle 1.The electrolysis temperatures are between room temperature and approximately 120 ° C. Temperatures between 50 and 80 ° C. are preferred. Good, uniform and shiny metal layers made of Fe, Co, Ni or Cu can be achieved with current densities between 0.2 and 1.5 A / dm², preferably 0.5 to 1.0 A / dm², see Table 1.
Bei Legierungsabscheidungen werden erfindungsgemäß im allgemeinen Mischungen aus Lösungen der Metallsalze der Legierungsbestandteile eingesetzt. Als Anoden können dann entweder solche aus entsprechenden Legierungen oder mehrere Elektroden aus den Metallen der einzelnen Legierungsbestandteile verwendet werden. Bei Vorhandensein eines größeren Elektrolytvorrats ist es möglich, nur mit der Anode aus einem der Legierungsbestandteile zu arbeiten. Die Konzentration des anderen Legierungsbestandteils muß dann in der Lösung durch Zugabe des entsprechenden Salzes von Zeit zu Zeit aufgefrischt werden. Wenn die Abscheidungstendenzen der Einzelmetalle sehr verschieden sind, lassen sich bei Verwendung von Legierungsanoden auch Elektrolyte verwenden, die nur das Salz des sich schwerer abscheidenden Metalls enthalten.In the case of alloy deposits, mixtures of solutions of the metal salts of the alloy components are generally used according to the invention. The anodes can then be either those made of appropriate alloys or a plurality of electrodes made of the metals of the individual alloy components. If there is a large electrolyte supply, it is possible to work only with the anode made from one of the alloy components. The concentration of the other alloy component must then be refreshed from time to time in the solution by adding the appropriate salt. If the deposition tendencies of the individual metals are very different, electrolytes containing only the salt of the more difficult to deposit metal can also be used when using alloy anodes.
Die Zusammensetzung der abzuscheidenden Legierung kann in weiten Bereichen variiert werden (siehe Tabelle 2) und zwar
- 1. durch Veränderung des Verhältnisses der Metallsalze im Elektrolyten zueinander und/oder
- 2. durch Verwendung von mehreren Anoden unterschiedlicher wirksamer Fläche aus den Metallen der einzelnen Legierungsbestandteile und/oder
- 3. bei Einsatz mehrerer Anoden aus den Metallen der Legierungsbestandteile durch unterschiedliche Stromkreise zwischen der Kathode und den einzelnen Anoden.
- 1. by changing the ratio of the metal salts in the electrolyte to one another and / or
- 2. by using several anodes of different effective area from the metals of the individual alloy components and / or
- 3. when using multiple anodes made of the metals of the alloy components through different circuits between the cathode and the individual anodes.
Um eine Luftoxidation der Metallsalzlösungen und/oder der galvanisch abgeschiedenen Metallschichten zu vermeiden, werden die Elektrolysen in geschlossenen Gefäßen in einer Inertgasatmosphäre von z.B. Argon und/oder Lachgas und/oder Stickstoff ausgeführt. Nach Beendigung der Zwischenbeschichtung werden die Werkstücke zunächst mit dem Lösungsmittel des Elektrolyten gewaschen. Nach Abtropfen des Lösungsmittels und Trocknen im Inertgasstrom oder im Vakuum werden die Werkstücke mit trockenem Toluol gewaschen und dann über eine Inertgasschleuse in das Aluminierbad überführt. Besonderer Vorteil einer solchen Verfahrensweise ist, daß auf der Metalloberfläche keine neue Oxid- oder Wasserschicht gebildet werden kann. Weiter entfallen nachträgliche aufwendige Trocknungsverfahren vor Eintritt in das Aluminierbad, wie beispielsweise die Behandlung mit Netzmittel enthaltenden Fluorkohlenwasserstoffen.In order to avoid air oxidation of the metal salt solutions and / or the galvanically deposited metal layers, the electrolyses are carried out in closed vessels in an inert gas atmosphere of, for example, argon and / or nitrous oxide and / or nitrogen. After finishing the intermediate coating, the workpieces are first washed with the solvent of the electrolyte. After draining the Solvents and drying in an inert gas stream or in a vacuum, the workpieces are washed with dry toluene and then transferred to the aluminizing bath via an inert gas lock. A particular advantage of such a procedure is that no new oxide or water layer can be formed on the metal surface. Furthermore, there is no need for subsequent, complex drying processes before entering the aluminizing bath, such as treatment with fluorocarbons containing wetting agents.
Die Erfindung wird anhand der in den folgenden beiden Tabellen beschriebenen Ausführungsbeispiele erläutert.The invention is explained using the exemplary embodiments described in the following two tables.
Als Elektrolysezelle dient ein zylindrisches Glasgefäß mit oben plan geschliffenem Rand, das mit einem Deckel aus isolierendem Material fest verschlossen werden kann. Am Deckel ist zwischen zwei Anodenplatten aus dem elektrochemisch aufzulösenden Metall, z.B. Nickel, eine Kathode aus dem zu beschichtenden Werkstoff, z.B. WL-1.6359 aufgehängt. Die Befestigungen der Elektroden dienen gleichzeitig als Stromzuführung. Die trockene Zelle wird mit Inertgas gefüllt, z.B. Argon oder Stickstoff. Zur Beschichtung der Kathode mit Nickel wird als Elektrolyt eine Lösung von 0.05 mol NiCl₂·0.63 THF und 0.05 mol LiBr in 1 Liter CH₃OCH₂CH₂OH eingesetzt. Es wird bei 60 °C mit einer Kathodenstromdichte von 0.5 A/dm² bei ca. 3-4 Volt und guter Duchmischung des Elektrolyten solange elektrolysiert, bis sich eine 1 µ dicke Nickelschicht auf der Kathode abgeschieden hat. Bezogen auf die Strommenge sind die anodischen und kathodischen Stromausbeuten quantitativ.A cylindrical glass vessel with an edge ground flat at the top serves as the electrolysis cell and can be firmly closed with a lid made of insulating material. A cathode made of the material to be coated, for example WL-1.6359, is suspended from the lid between two anode plates made of the electrochemically dissolvable metal, for example nickel. The attachments of the electrodes also serve as a power supply. The dry cell is filled with inert gas, such as argon or nitrogen. To coat the cathode with nickel, a solution of 0.05 mol NiCl₂ · 0.63 THF and 0.05 mol LiBr in 1 liter CH₃OCH₂CH₂OH is used as the electrolyte. Electrolysis is carried out at 60 ° C. with a cathode current density of 0.5 A / dm² at about 3-4 volts and thorough mixing of the electrolyte until a 1 μm thick nickel layer has deposited on the cathode. The anodic and cathodic current yields are quantitative in relation to the amount of electricity.
In analoger Weise wurden andere, in Tabelle 1 aufgeführte Metallabscheidungen mit den in Tabelle 1 angegebenen Elektrolyten ausgeführt.
Dieses Beispiel beschreibt den in Tabelle 2 aufgeführten Versuch 1 detailliert. Eine 0.05 molare (M) Lösung von LiBr in CH₃OCH₂CH₂OH, die außerdem noch 0.029 M an NiCl₂ und 0.015 M an FeCl₂ ist, wird bei 65 °C in einer Inertgasatmosphäre mit einer Stromdichte von 0.5 A/dm² elektrolysiert. Als Anoden werden Nickel- und Eisenblech verwendet; das Flächenverhältnis beider Metallanoden ist 1.0 : 0.5. Als Kathode wird ein Werkstück aus WL-1.7176 eingesetzt. Man elektrolysiert solange, bis sich auf der Kathode eine ca. 3 µ dicke Legierungsschicht abgeschieden hat. Die Legierung besteht zu 75 % aus Fe und zu 25 % aus Ni. Die Versuche 2-9 wurden analog mit den in Tabelle 2 angegebenen Elektrolyten und Anoden durchgeführt.This example describes Experiment 1 listed in Table 2 in detail. A 0.05 molar (M) solution of LiBr in CH₃OCH₂CH₂OH, which is also 0.029 M in NiCl₂ and 0.015 M in FeCl₂, is electrolyzed at 65 ° C in an inert gas atmosphere with a current density of 0.5 A / dm². Nickel and iron sheets are used as anodes; the area ratio of both metal anodes is 1.0: 0.5. A workpiece made of WL-1.7176 is used as the cathode. Electrolysis is carried out until an approximately 3 μ thick alloy layer has been deposited on the cathode. The alloy consists of 75% Fe and 25% Ni. Experiments 2-9 were carried out analogously with the electrolytes and anodes specified in Table 2.
Nach der Zwischenbeschichtung entsprechend den Beispielen 1 und 2 und den in Tabelle 1 und 2 zusaammengefaßten Versuchen werden die Werkstücke mit dem Lösungsmittel des Elektrolyten gewaschen und im Inertgasstrom getrocknet. Dann wäscht man die Werkstücke mit trockenem Toluol und überführt sie über eine Inertgasschleuse in das Aluminierbad.After the intermediate coating according to Examples 1 and 2 and the tests summarized in Tables 1 and 2, the workpieces are washed with the solvent of the electrolyte and dried in a stream of inert gas. The workpieces are then washed with dry toluene and transferred to the aluminizing bath via an inert gas lock.
Um ein quantitatives Maß für die Haftfestigkeit galvanisch erzeugter Schichten auf Werkstoffen zu erhalten, bedient man sich Verfahren zur Messung der Kraft, die benötigt wird, um den Niederschlag von der Unterlage abzureißen. Der "Tape-Test" ist eine quantitativ vergleichende Methode, die Bewertungen der Haftung auf einfache Weise ermöglicht. Dazu wird zunächst ein Klebestreifen fest auf die Galvanoschicht gepreßt und dann rasch abgerissen. Bei schlechter oder mäßiger Haftung löst sich dabei die Galvanoschicht zusammen mit dem Klebestreifen von der Werkstoffunterlage. Bei guter Haftung werden nur kleine Bezirke der Galvanoschicht abgelöst und bei sehr guter Haftung bleibt die Bindung der Galvanoschicht an die Unterlage vollständig erhalten.
Claims (8)
- A process for metal-plating of metallic materials, and more specifically low-alloy high-strength steels, wherein adhesion-bonding layers of iron, iron and nickel, nickel, cobalt, copper or alloys of said metals or tin-nickel alloys are electrodeposited on said metallic materials from non-aqueous electrolytes of anhydrous metal compounds, and then aluminium is electrodeposited thereon in a per se known manner, wherein, as the anhydrous compounds, there are employed metal salts of iron, cobalt, nickel, copper or tin, and more specifically of the anhydrous chlorides thereof and/or anhydrous bromides thereof and/or the complex compounds of the metal chlorides and/or metal bromides with ethers or with alcohols in water-free alkyl semi-ethers of a C₂- to C₃-alkylene glycol of the formula
- The process according to claim 1, characterized in that as the anhydrous metal compounds there are employed metal(II) compounds of iron, cobalt, nickel or tin or metal(I) compounds of copper.
- The process according to claims 1 or 2, characterized in that as the anode a metal anode is used which has the same alloy composition as the metal cations of the metal salts of the electrolyte.
- The process according to claims 1 to 3, characterized in that as the anhydrous solvent there is used a C₁- to C₄-alkyl semi-ether, and preferably a C₁- to C₃-alkyl semi-ether, of an alkylene glycol, namely of 1,2-ethylene diol or of 1,2-propanediol.
- The process according to claims 1 to 4, characterized in that in the solvent of the water-free electrolyte the metal salts are present in concentrations of from 0.02 to 0.1M, and preferably of from 0.044 to 0.05M, and the supporting electrolyte, more particularly the lithium bromide, is present in an equimolar to twice the equimolar concentration relative to said metal salts.
- The process according to claims 1 to 5, characterized in that the bonding layers are electrodeposited at current densities at from 0.2 to 1.5 A/dm², and preferably at from 0.5 to 1.0 A/dm², at electrolysis temperatures between room temperature (20 °C) and about 120 °C, and preferably between 50 °C and 80 °C.
- The process according to claims 1 to 6, characterized in that the electrodeposition is effected in an inert gas atmosphere.
- Non-aqueous electrolytes having the compositions as set forth in claims 1, 2, 4 and 5 for metal-plating of metallic materials, and more specifically low-alloy high-strength steels, and for adhesion-bonding between said metallic materials and aluminium layers electrodeposited thereon.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE3804303 | 1988-02-12 | ||
| DE3804303A DE3804303A1 (en) | 1988-02-12 | 1988-02-12 | METHOD FOR ADMINISTERING BETWEEN METAL MATERIALS AND GLAVAN ALUMINUM LAYERS AND NON-AQUE ELECTROLYTE USED THEREOF |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP0328128A1 EP0328128A1 (en) | 1989-08-16 |
| EP0328128B1 EP0328128B1 (en) | 1992-04-08 |
| EP0328128B2 true EP0328128B2 (en) | 1995-09-20 |
Family
ID=6347224
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP89102319A Expired - Lifetime EP0328128B2 (en) | 1988-02-12 | 1989-02-10 | Process concerning the adhesion between metallic materials and galvanic aluminium layers and the non-aqueous electrolyte used therein |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US4925536A (en) |
| EP (1) | EP0328128B2 (en) |
| JP (1) | JP2824267B2 (en) |
| AT (1) | ATE74630T1 (en) |
| CA (1) | CA1337690C (en) |
| DE (2) | DE3804303A1 (en) |
| DK (1) | DK64789A (en) |
| ES (1) | ES2032341T5 (en) |
| IE (1) | IE61700B1 (en) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0499638B1 (en) * | 1989-04-20 | 1998-12-02 | Tokin Corporation | Method for Plating a Permanent Magnet of a R2T14B Intermetallic Compound |
| US8225481B2 (en) * | 2003-05-19 | 2012-07-24 | Pratt & Whitney Rocketdyne, Inc. | Diffusion bonded composite material and method therefor |
| EP1524336A1 (en) * | 2003-10-18 | 2005-04-20 | Aluminal Oberflächtentechnik GmbH & Co. KG | Workpieces coated with an aluminum magnesium alloy |
| DE102008048109B4 (en) * | 2008-04-17 | 2015-01-29 | Ks Aluminium-Technologie Gmbh | Method for producing a metallic component and use of a cylinder part as basic body for carrying out the method |
| DE102017201559A1 (en) | 2017-01-31 | 2018-08-02 | Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. | Atmospheric pressure plasma process for the production of plasma polymer coatings |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3616280A (en) * | 1969-03-24 | 1971-10-26 | Atomic Energy Commission | Nonaqueous electroplating solutions and processing |
| JPS5334711B2 (en) * | 1971-09-16 | 1978-09-21 | ||
| JPS5137082B2 (en) * | 1972-03-31 | 1976-10-13 | ||
| DE3112834A1 (en) * | 1981-03-31 | 1982-10-14 | Siemens AG, 1000 Berlin und 8000 München | Metal-coated ferrous materials |
| WO1986001840A1 (en) * | 1984-09-17 | 1986-03-27 | Eltech Systems Corporation | Protective coating |
-
1988
- 1988-02-12 DE DE3804303A patent/DE3804303A1/en not_active Withdrawn
-
1989
- 1989-02-02 US US07/305,838 patent/US4925536A/en not_active Expired - Fee Related
- 1989-02-10 IE IE42489A patent/IE61700B1/en not_active IP Right Cessation
- 1989-02-10 DK DK064789A patent/DK64789A/en not_active Application Discontinuation
- 1989-02-10 AT AT89102319T patent/ATE74630T1/en not_active IP Right Cessation
- 1989-02-10 ES ES89102319T patent/ES2032341T5/en not_active Expired - Lifetime
- 1989-02-10 EP EP89102319A patent/EP0328128B2/en not_active Expired - Lifetime
- 1989-02-10 DE DE8989102319T patent/DE58901105D1/en not_active Expired - Lifetime
- 1989-02-10 CA CA000590782A patent/CA1337690C/en not_active Expired - Fee Related
- 1989-02-10 JP JP1032540A patent/JP2824267B2/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| JP2824267B2 (en) | 1998-11-11 |
| EP0328128A1 (en) | 1989-08-16 |
| CA1337690C (en) | 1995-12-05 |
| DK64789A (en) | 1989-08-13 |
| DK64789D0 (en) | 1989-02-10 |
| ES2032341T5 (en) | 1995-11-16 |
| US4925536A (en) | 1990-05-15 |
| ES2032341T3 (en) | 1993-02-01 |
| DE58901105D1 (en) | 1992-05-14 |
| ATE74630T1 (en) | 1992-04-15 |
| EP0328128B1 (en) | 1992-04-08 |
| IE890424L (en) | 1989-08-12 |
| JPH01247593A (en) | 1989-10-03 |
| DE3804303A1 (en) | 1989-08-24 |
| IE61700B1 (en) | 1994-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE60109182T2 (en) | IONIC LIQUIDS AND ITS USE | |
| DE3428345C2 (en) | ||
| DE2947821C2 (en) | ||
| DE3048083C2 (en) | Process for the chemical removal of oxide layers from objects made of titanium or titanium alloys | |
| DE19523307A1 (en) | Chrome plating process using trivalent chromium | |
| Rashwan et al. | Electrodeposition and characterization of thin layers of Zn–Co alloys obtained from glycinate baths | |
| Crousier et al. | Influence of substrate on the electrodeposition of nickel-molybdenum alloys | |
| DE3710368A1 (en) | AQUEOUS ACID BATH AND METHOD FOR GALVANIC DEPOSITION OF A ZINC-NICKEL ALLOY | |
| CA1129805A (en) | Electrodeposition of ruthenium-iridium alloy | |
| EP0328128B2 (en) | Process concerning the adhesion between metallic materials and galvanic aluminium layers and the non-aqueous electrolyte used therein | |
| CA1256057A (en) | Process for electrolytic treatment of metal by liquid power feeding | |
| DE2114119A1 (en) | Process for the electrolytic deposition of ruthenium and electrolysis bath to carry out this process | |
| EP2635724B1 (en) | Process for electroplating hard chromium from a cr(vi) free electrolyte | |
| DE68905429T2 (en) | METHOD FOR TIN ELECTROPLATING METALLIC MATERIAL. | |
| DE2917019C2 (en) | Process for the metallization of composite material and bath composition suitable for this | |
| DE3347593C2 (en) | ||
| Levason et al. | Studies of platinum electroplating baths: Part V: Solutions derived from Pt (NO2) 42− in aqueous acid | |
| EP2989236B1 (en) | Electrically conducting liquids based on metal-diphosphonate complexes | |
| DE2818780A1 (en) | GALVANIC BATH FOR ELECTROPLATING CHROME OR CHROME ALLOYS | |
| DE3108202A1 (en) | METHOD FOR ELECTROLYTICALLY DEPOSITING LAYERS OF NICKEL ALLOYS WITH ALLOY ELEMENTS | |
| EP0360863A1 (en) | Method and nickel-oxide electrode for applying a composite nickel-oxide coating to a metal carrier | |
| Gardam | Polarisation in the electrodeposition of metals | |
| Rudnik et al. | Effect of organic additives on electrodeposition of tin from acid sulfate solution | |
| DE2606418C2 (en) | Process for the production of magnetic recording media with a wear-resistant surface | |
| DE2743847A1 (en) | METHOD FOR GALVANIC DEPOSITION OF NICKEL AND COBALT ALONE OR AS BINARY OR TERNAIRE ALLOYS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE ES FR GB IT LI LU NL SE |
|
| 17P | Request for examination filed |
Effective date: 19890920 |
|
| 17Q | First examination report despatched |
Effective date: 19910717 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE ES FR GB IT LI LU NL SE |
|
| REF | Corresponds to: |
Ref document number: 74630 Country of ref document: AT Date of ref document: 19920415 Kind code of ref document: T |
|
| ITF | It: translation for a ep patent filed | ||
| REF | Corresponds to: |
Ref document number: 58901105 Country of ref document: DE Date of ref document: 19920514 |
|
| ET | Fr: translation filed | ||
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) | ||
| PLBI | Opposition filed |
Free format text: ORIGINAL CODE: 0009260 |
|
| 26 | Opposition filed |
Opponent name: SIEMENS AG GR PA 8 Effective date: 19930108 |
|
| NLR1 | Nl: opposition has been filed with the epo |
Opponent name: SIEMENS AG |
|
| EPTA | Lu: last paid annual fee | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 89102319.4 |
|
| PUAH | Patent maintained in amended form |
Free format text: ORIGINAL CODE: 0009272 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: PATENT MAINTAINED AS AMENDED |
|
| 27A | Patent maintained in amended form |
Effective date: 19950920 |
|
| AK | Designated contracting states |
Kind code of ref document: B2 Designated state(s): AT BE CH DE ES FR GB IT LI LU NL SE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: AEN |
|
| ET3 | Fr: translation filed ** decision concerning opposition | ||
| NLR2 | Nl: decision of opposition | ||
| GBTA | Gb: translation of amended ep patent filed (gb section 77(6)(b)/1977) |
Effective date: 19951018 |
|
| ITF | It: translation for a ep patent filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: DC2A Kind code of ref document: T5 Effective date: 19951116 |
|
| NLR3 | Nl: receipt of modified translations in the netherlands language after an opposition procedure | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 19990218 Year of fee payment: 11 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20000114 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 20000117 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20000122 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20000124 Year of fee payment: 12 Ref country code: LU Payment date: 20000124 Year of fee payment: 12 Ref country code: FR Payment date: 20000124 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20000125 Year of fee payment: 12 Ref country code: AT Payment date: 20000125 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20000208 Year of fee payment: 12 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010210 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010210 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010210 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010211 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010212 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010228 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010228 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010228 |
|
| BERE | Be: lapsed |
Owner name: STUDIEN G.- KOHLE M.B.H. Effective date: 20010228 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20010901 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20010210 |
|
| EUG | Se: european patent has lapsed |
Ref document number: 89102319.4 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20011031 |
|
| NLV4 | Nl: lapsed or anulled due to non-payment of the annual fee |
Effective date: 20010901 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20011201 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20020916 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20050210 |
|
| PLAB | Opposition data, opponent's data or that of the opponent's representative modified |
Free format text: ORIGINAL CODE: 0009299OPPO |