EP0273688B1 - Process for the manufacture of spray-dried detergent powder - Google Patents
Process for the manufacture of spray-dried detergent powder Download PDFInfo
- Publication number
- EP0273688B1 EP0273688B1 EP87311311A EP87311311A EP0273688B1 EP 0273688 B1 EP0273688 B1 EP 0273688B1 EP 87311311 A EP87311311 A EP 87311311A EP 87311311 A EP87311311 A EP 87311311A EP 0273688 B1 EP0273688 B1 EP 0273688B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- slurry
- detergent
- active compound
- wholly
- acid form
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000000843 powder Substances 0.000 title claims description 44
- 238000000034 method Methods 0.000 title claims description 36
- 239000003599 detergent Substances 0.000 title claims description 25
- 238000004519 manufacturing process Methods 0.000 title claims description 8
- 239000002002 slurry Substances 0.000 claims description 78
- BDHFUVZGWQCTTF-UHFFFAOYSA-N sulfonic acid Chemical compound OS(=O)=O BDHFUVZGWQCTTF-UHFFFAOYSA-N 0.000 claims description 55
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 38
- 239000000463 material Substances 0.000 claims description 27
- 150000001875 compounds Chemical class 0.000 claims description 25
- 239000002253 acid Substances 0.000 claims description 23
- 125000000129 anionic group Chemical group 0.000 claims description 21
- 235000019832 sodium triphosphate Nutrition 0.000 claims description 18
- 238000001694 spray drying Methods 0.000 claims description 15
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 14
- 150000004996 alkyl benzenes Chemical class 0.000 claims description 13
- 239000004115 Sodium Silicate Substances 0.000 claims description 12
- NTHWMYGWWRZVTN-UHFFFAOYSA-N sodium silicate Chemical compound [Na+].[Na+].[O-][Si]([O-])=O NTHWMYGWWRZVTN-UHFFFAOYSA-N 0.000 claims description 12
- 229910052911 sodium silicate Inorganic materials 0.000 claims description 12
- 150000003839 salts Chemical class 0.000 claims description 11
- 238000010348 incorporation Methods 0.000 claims description 10
- 239000000203 mixture Substances 0.000 claims description 7
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 7
- 239000011149 active material Substances 0.000 claims description 4
- 230000002378 acidificating effect Effects 0.000 claims description 2
- 229910017053 inorganic salt Inorganic materials 0.000 claims description 2
- 239000007788 liquid Substances 0.000 claims description 2
- 239000004615 ingredient Substances 0.000 description 14
- 239000003513 alkali Substances 0.000 description 11
- -1 for example Substances 0.000 description 11
- 238000006386 neutralization reaction Methods 0.000 description 9
- 239000002736 nonionic surfactant Substances 0.000 description 8
- 230000008901 benefit Effects 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 150000007513 acids Chemical class 0.000 description 6
- 238000009826 distribution Methods 0.000 description 5
- 239000000344 soap Substances 0.000 description 5
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 235000014113 dietary fatty acids Nutrition 0.000 description 4
- 239000000194 fatty acid Substances 0.000 description 4
- 229930195729 fatty acid Natural products 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 230000003472 neutralizing effect Effects 0.000 description 4
- 239000007921 spray Substances 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- AKEJUJNQAAGONA-UHFFFAOYSA-N sulfur trioxide Chemical compound O=S(=O)=O AKEJUJNQAAGONA-UHFFFAOYSA-N 0.000 description 4
- 235000011149 sulphuric acid Nutrition 0.000 description 4
- BPQQTUXANYXVAA-UHFFFAOYSA-N Orthosilicate Chemical compound [O-][Si]([O-])([O-])[O-] BPQQTUXANYXVAA-UHFFFAOYSA-N 0.000 description 3
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 3
- 239000003945 anionic surfactant Substances 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 230000009286 beneficial effect Effects 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- 230000036571 hydration Effects 0.000 description 3
- 238000006703 hydration reaction Methods 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 159000000000 sodium salts Chemical class 0.000 description 3
- 239000001117 sulphuric acid Substances 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 238000005273 aeration Methods 0.000 description 2
- 159000000011 group IA salts Chemical class 0.000 description 2
- 239000002304 perfume Substances 0.000 description 2
- 150000003138 primary alcohols Chemical class 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 238000005086 pumping Methods 0.000 description 2
- 150000003333 secondary alcohols Chemical class 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 206010067484 Adverse reaction Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 244000020551 Helianthus annuus Species 0.000 description 1
- 235000003222 Helianthus annuus Nutrition 0.000 description 1
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 description 1
- 229910000503 Na-aluminosilicate Inorganic materials 0.000 description 1
- 206010067482 No adverse event Diseases 0.000 description 1
- 235000019484 Rapeseed oil Nutrition 0.000 description 1
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical compound OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 description 1
- 229920006243 acrylic copolymer Polymers 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 125000002877 alkyl aryl group Chemical group 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 235000015278 beef Nutrition 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000007844 bleaching agent Substances 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000007922 dissolution test Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000011552 falling film Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000005187 foaming Methods 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 150000004687 hexahydrates Chemical class 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 229910001425 magnesium ion Inorganic materials 0.000 description 1
- 159000000003 magnesium salts Chemical class 0.000 description 1
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Chemical group CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229920005646 polycarboxylate Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000000429 sodium aluminium silicate Substances 0.000 description 1
- 235000012217 sodium aluminium silicate Nutrition 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- NNMHYFLPFNGQFZ-UHFFFAOYSA-M sodium polyacrylate Polymers [Na+].[O-]C(=O)C=C NNMHYFLPFNGQFZ-UHFFFAOYSA-M 0.000 description 1
- 229910052938 sodium sulfate Inorganic materials 0.000 description 1
- 235000011152 sodium sulphate Nutrition 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 230000003019 stabilising effect Effects 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 125000001273 sulfonato group Chemical group [O-]S(*)(=O)=O 0.000 description 1
- 239000003760 tallow Substances 0.000 description 1
- UNXRWKVEANCORM-UHFFFAOYSA-I triphosphate(5-) Chemical compound [O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O UNXRWKVEANCORM-UHFFFAOYSA-I 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D11/00—Special methods for preparing compositions containing mixtures of detergents
- C11D11/02—Preparation in the form of powder by spray drying
Definitions
- the present invention relates to a process for the production of detergent powders containing anionic detergent-active compounds, by slurry-making and spray-drying.
- Spray-dried detergent powders are generally produced by preparing an aqueous slurry of detergent-active compounds, builder and other salts, sodium silicate, fluorescers and other non-heat-sensitive ingredients, and then spray-drying the slurry to form a free-flowing powder. Ingredients unsuitable for spray-drying, such as bleaches, perfumes and enzymes, may be postdosed subsequently to the spray-dried base powder.
- Anionic detergent-active compounds such as alkylbensene sulphonates are incorporated in spray-dried detergent powders via the slurry.
- Alkylbenzene sulphonates are manufactured by sulphonation of the corresponding alkylbensene to give a sulphonic acid which can then be neutralised with a suitable base, for example, sodium or potassium hydroxide, sodium carbonate, ammonia or triethanolamine, to give the salt of the desired cation.
- a suitable base for example, sodium or potassium hydroxide, sodium carbonate, ammonia or triethanolamine, to give the salt of the desired cation.
- the sodium salt which is the most commonly used salt in detergent powders, is generally prepared by neutralisation with aqueous sodium hydroxide solution to yield a paste containing 50% or more water, and that material can be incorporated directly in the slurry.
- EP-A-242138 published 21.10.87, forms part of the state of the art by reason of Article 54(3) EPC. This document discloses a procedure in which alkylbenzene sulphonic acid is added to slurry containing sodium carbonate.
- a significant characteristic of the invention is the incorporation of an anionic detergent-active compound wholly or predominantly in its acid form at a relatively late stage in the slurry-making process, when a large reservoir of alkaline material is already present in the slurry.
- the invention is applicable to the incorporation of any anionic detergent-active compound having a stable acid form. It is of especial interest in relation to sulphonic acids, especially alkylaryl sulphonic acids, more especially C8-C15 alkylbenzene sulphonic acids, both linear and branched. It may be advantageous for the sulphonic acid to be partially neutralised, for example, up to 10% by weight neutralised, before introduction into the slurry-making vessel: a neutralisation level of about 5% appears to be especially beneficial. This can be helpful in stabilising the sulphonic acid and preventing it from darkening in colour during storage. When the sodium salt of the sulphonic acid is desired, such preneutralisation is conveniently carried out with aqueous sodium hydroxide solution. For convenience, acid or partially neutralised acid added to the slurry will be referred to hereinafter simply as "sulphonic acid".
- alkaline material As is required fully to neutralise the sulphonic acid.
- This will generally be constituted in part by a base such as sodium hydroxide which is especially intended for the purpose, in part by alkaline sodium salts present as builders, and possibly in part by alkaline sodium silicate.
- the detergent composition prepared in accordance with the invention is to include alkaline sodium silicate
- the silicate is preferably incorporated in the slurry before the sulphonic acid, and thus contributes to the alkaline material, mentioned previously, that must be present in at least a twofold amount relative to the sulphonic acid at the time when the latter component is added.
- alkaline builder salts present in the slurry when the sulphonic acid is added are sodium tripolyphosphate, sodium carbonate and mixtures of these. These materials are commonly used in detergent powders as builders which sequester or precipitate calcium and magnesium salts in the wash liquor. They generally comprise at least 25% by weight of the detergent powder so that if they are incorporated in the slurry before addition of the sulphonic acid they can serve as a large alkaline buffer to prevent or minimise any adverse interaction between sodium silicate and sulphonic acid. Efficient stirring in the slurry-making vessel also helps to eliminate such adverse interaction.
- any alkali added to the slurry specifically for neutralisation of the sulphonic acid makes an additional contribution to the reservoir of alkaline material in the slurry.
- the amount of sodium hydroxide required to neutralise the sulphonic acid completely generally ranges from 10 to 20g per 100g sulphonic acid. This relatively large variation occurs because the amount of sulphuric acid present as a by-product in the sulphonic acid can vary quite widely: it is likely to be higher in sulphonic acids prepared by oleum sulphonation than in sulphonic acids prepared by sulphur trioxide sulphonation.
- Sodium hydroxide solution of specific gravity 1.5 is conveniently used as the neutralising alkali.
- a neutralising alkali When a neutralising alkali is used, it need not be introduced into the slurry before the sulphonic acid, provided that sufficient alkaline material from other sources is present when the sulphonic acid is added. It may be preferable to add the alkali and sulphonic acid simultaneously.
- the process of the invention gives several worthwhile advantages.
- Sodium silicate is a desirable ingredient in detergent compositions because it improves powder structure, prevents corrosion of washing machine parts in use, and provides building capacity (towards magnesium ions).
- the process of the invention is, however, also beneficial for the production of silicate-free powders and gives other advantages applicable both to silicate-containing and to silicate-free powders.
- a less aerated slurry is also obtained by the process of the invention, especially when other, high-foaming anionic, surfactants such as alkyl sulphates or alkyl ether sulphates are absent.
- a less aerated slurry gives a spray-dried powder of higher particle density, and allows high bulk density powders to be prepared without the need for a separate deaeration step.
- a less aerated slurry is also less bulky so that larger batches of slurry can be processed in a given slurry-making vessel: for powders containing high levels of alkylbenzene sulphonate (20% or more) an increase in batch size of up to 20% has been achieved, leading to a corresponding increase in the output of the slurry making plant. Pumping of a less aerated slurry is also easier because its viscosity is lower and cavitation problems are less likely to occur.
- sodium tripolyphosphate is a major building and structuring component of the powder: in a preferred embodiment of the invention, all sodium tripolyphosphate is added before the addition of the sulphonic acid.
- the temperature of the slurry at the time at which the sodium tripolyphosphate is added is then lower than in the prior art process in which the sulphonic acid neutralisation step has already occurred and raised the slurry temperature.
- the sodium tripolyphosphate can dissolve and hydrate more quickly at a lower temperature: this ensures that less degradation of the tripolyphosphate occurs during spray-drying, and also favours the growth of very small sodium tripolyphosphate hexahydrate crystals in the slurry, thus giving spray-dried powders of improved structure and absorptivity.
- a period of at least 2 minutes may be allowed to lapse between addition of the sodium tripolyphosphate and addition of the sulphonic acid to the slurry, in order to maximise hydration of the sodium tripolyphosphate before any temperature increase due to the neutralisation reaction occurs.
- the finished slurry is pumped, first via low-pressure pipes and then via high-pressure pipes, to a distribution manifold for the atomising nozzles of a spray-drying tower.
- the sulphonic acid in the slurry-making vessel itself, it may be injected either into the low-pressure pump or pipes, or into the high-pressure pump or pipes. It has been found especially beneficial to inject the sulphonic acid into the high-pressure pump or pipes, very shortly before the slurry arrives at the spray-nozzle distribution manifold.
- Alkylbenzene sulphonic acids are fairly viscous liquids at ambient temperatures but can be pumped without too much difficulty using positive displacement pumps. It has been found that sulphonic acid can be used at ambient temperature if it is to be added to the slurry-making vessel. When the alternative procedure of injection into a slurry transfer pump or pipe is to be used, however, the sulphonic acid is preferably heated to a temperature of about 50 to 60°C to lower its viscosity to a value at which accurate metering with a pump of the piston type is possible.
- An alternative method of reducing the viscosity of the sulphonic acid is to premix it with a viscosity-lowering ingredient, especially a nonionic surfactant, but this method is suitable only for preparing powders in which the viscosity-lowering ingredient is required.
- the process of the invention may be used with advantage to prepare any spray-dried detergent powder containing an anionic detergent-active compound having a stable acid form.
- an anionic detergent-active compound having a stable acid form This will normally be a sulphonate-type anionic detergent, especially an alkylaryl sulphonate and more especially a C8-C15 linear alkylbenzene sulphonate.
- Other anionic detergent-active compounds may also be present, for example, primary and secondary alkyl sulphates, olefin sulphonates, alkane sulphonates, alkyl ether sulphates, and fatty acid ester sulphonates.
- Nonionic surfactants may also be used in detergent powders prepared according to the invention. These include the primary and secondary alcohol ethoxylates, especially the C12-C15 primary and secondary alcohols ethoxylated with an average of from 3 to 20 moles of ethylene oxide per mole of alcohol.
- soaps of fatty acids are preferably sodium soap derived from naturally occurring fatty acids, for example, the fatty acids from coconut oil, beef tallow, sunflower or hardened rape seed oil.
- the total amount of detergent-active material (surfactant), excluding soap, in detergent powders prepared according to the invention is generally within the range of from 5 to 40% by weight.
- the preferred range is from 5 to 20% by weight, with a weight ratio of anionic surfactant to nonionic surfactant not exceeding 10:1, and preferably not exceeding 6:1.
- the present invention is especially applicable to the manufacture of medium- and high-sudsing powders containing higher levels of anionic surfactant and/or higher ratios of anionic surfactant to nonionic surfactant.
- Detergent powders prepared in accordance with the invention will also comprise one or more detergency builders, suitably in an amount of from 10 to 60% by weight.
- Detergency builders are very well known to those skilled in the art and include sodium tripolyphosphate, orthophosphate and pyrophosphate; crystalline and amorphous sodium aluminosilicate; sodium carbonate; and monomeric and polymeric polycarboxylates, for example, sodium citrate, nitrilotriacetate and polyacrylate, and acrylic copolymers.
- at least some of the builders incorporated in the powder must be alkaline salts.
- inorganic salts without a detergency building function for example, sodium sulphate, may also be included in detergent powders prepared according to the invention.
- Detergent powders prepared according to the invention will also generally contain various additives to enhance the efficiency of the product, notably bleach systems, antiredeposition agents, fluorescers, lather suppressors, enzymes and perfumes. These may be added to the slurry or postdosed into the spray-dried powder in accordance with their known suitability for undergoing spray-drying processes.
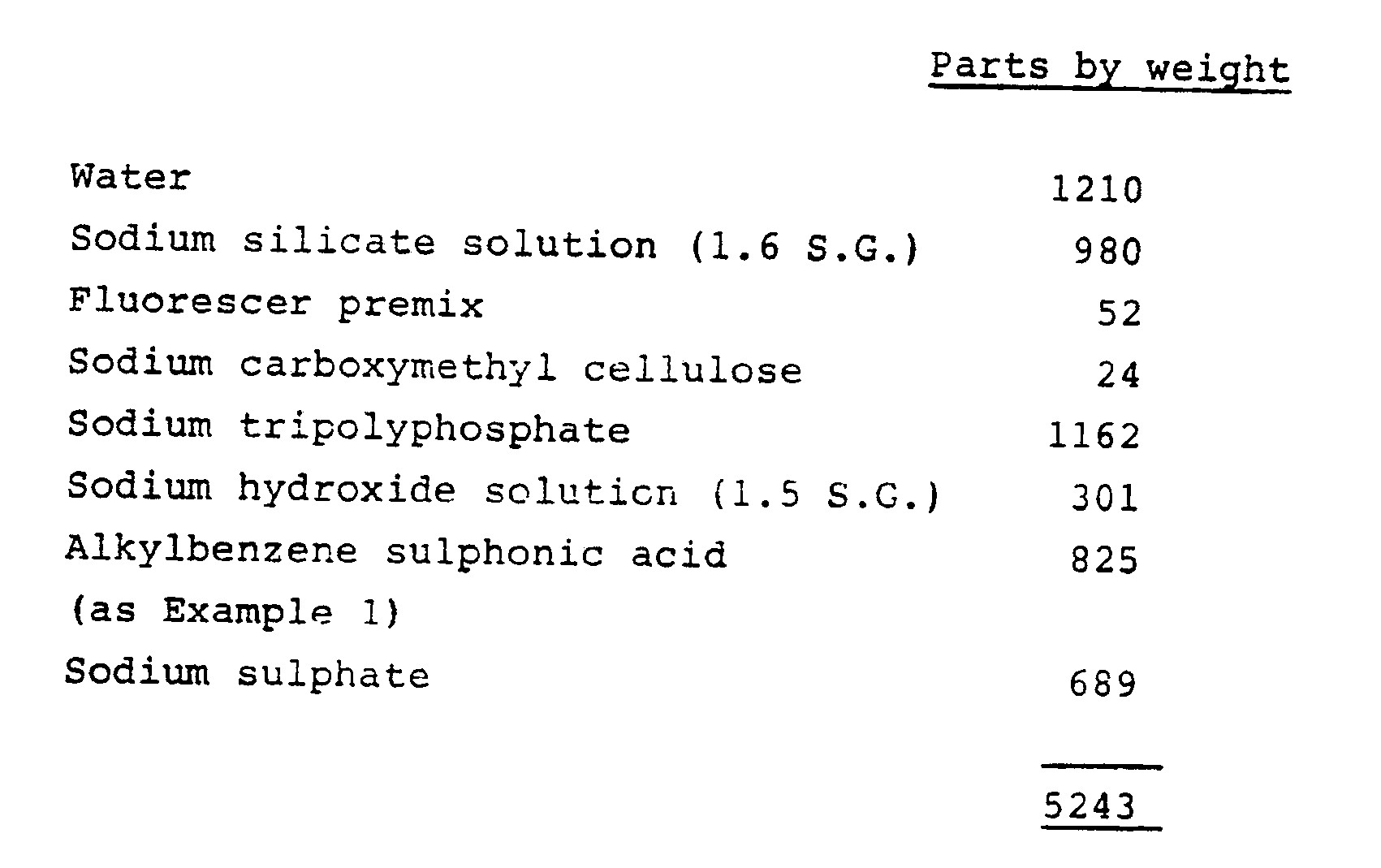
- a slurry was prepared in a batch slurry-making apparatus from the following ingredients, added in the order listed:
- the contents of the slurry-making vessel were stirred continuously during the preparation of the slurry.
- the temperature of the slurry rose from about 80°C to about 85°. It was noticeable that much less air than usual was entrained in the slurry during its preparation.
- the slurry was transferred via low and high-pressure transfer pipes to a spray-drying tower, 162 parts of a nonionic surfactant being injected into the high-pressure slurry transfer pipe just before it entered the spray jet distribution manifold.
- the solubility of the powder was assessed by stirring 4g into 400 ml of water at a temperature of 40°C for 2 minutes and then filtering the undissolved material.
- the weight of undissolved material under these test conditions should be less than 0.16g for a detergent powder, equivalent to 4% undissolved powder: the weight of undissolved material is greatly affected by the amount of insoluble siliceous material present.
- Example 1 Under the above testing conditions, the level of undissolved material in the detergent powder of Example 1 was very satisfactory at 1.1%.
- the sulphonic acid used in Example 1 had been prepared by sulphonating an alkylbenzene with sulphur trioxide and air on a falling film reactor. It was of very good quality and contained only 0.9% by weight of free sulphuric acid.
- This Example describes the preparation of a high-sudsing detergent powder containing 20% by weight of alkylbenzene sulphonate.
- the alkylbenzene sulphonic acid used had been prepared by reacting the corresponding alkylbenzene directly with 20% oleum and then allowing the resulting mixture of sulphonic and sulphuric acids to separate into two layers; its sulphuric acid content was 6.9% by weight.
- a slurry was prepared in a batch slurry-making vessel by adding the following ingredients with mixing, in the order stated:
- the slurry temperature before addition of the sodium tripolyphosphate was 56°C and this rose to 72°C after a 2-minute period to allow the sodium tripolyphosphate to hydrate: this temperature rise indicated that 93% hydration has been achieved.
- the temperature rose by a further 22°C to a final value of 94°C; at no time during the slurry-making process was heat from an external source applied to the vessel.
- the degree of aeration of the slurry after all the slurry ingredients had been added was significantly less than normal.
- the slurry was easy to pump.
- the slurry was transferred to a spray-drying tower and spray-dried to give a free-flowing powder having a bulk density of 413 g/litre.
- Example 1 The standard dissolution test described in Example 1 established that the dissolving properties of the detergent powder were very good. The amount of undissolved material remaining after 2 minutes' stirring was only 0.1%. This result confirmed that no adverse reaction between the sulphonic acid and the sodium silicate had occurred in the slurry.
- Example 2 During addition of the sulphonic acid the temperature in the slurry-making vessel rose by 42°C. The extent of hydration of the sodium tripolyphosphate was 83%. The slurry was considerably more aerated than that of Example 2, so that a smaller batch size (about 80% of that of Example 2) had to be used. The slurry was spray-dried to form a powder having a bulk density of 390 g/litre.
- This Example illustrates an alternative procedure according to the invention, in which alkylbenzene sulphonic acid was injected continuously into the slurry immediately before the slurry reached the distribution manifold for the spray nozzles of the spray-drying tower.
- the slurry was pumped through low and high-pressure pipes to the distribution manifold.
- the alkylbenzene sulphonic acid used in Example 1 (689 parts) was injected continuously at a pressure of 50 bar into the slurry just before it entered the manifold: the slurry pressure at this point was 45 bar.
- a simple static in-line mixer installed after the injection point ensured that the sulphonic acid mixed well with the slurry. Further and more intense local mixing also took place in the high shear swirl type of spray jets in the spray-drying tower.
- the spray dried detergent powder had very good physical properties and a bulk density of 490 g/litre.
- the level of undissolved material remaining after 2 minutes' stirring in water at 40°C was 0.1%. This very low result confirmed that no unusual adverse reaction had occurred between the injected sulphonic acid and the silicate in the slurry leading to the formation of high levels of insoluble siliceous material.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Detergent Compositions (AREA)
Description
- The present invention relates to a process for the production of detergent powders containing anionic detergent-active compounds, by slurry-making and spray-drying.
- Spray-dried detergent powders are generally produced by preparing an aqueous slurry of detergent-active compounds, builder and other salts, sodium silicate, fluorescers and other non-heat-sensitive ingredients, and then spray-drying the slurry to form a free-flowing powder. Ingredients unsuitable for spray-drying, such as bleaches, perfumes and enzymes, may be postdosed subsequently to the spray-dried base powder.
- Anionic detergent-active compounds such as alkylbensene sulphonates are incorporated in spray-dried detergent powders via the slurry. Alkylbenzene sulphonates are manufactured by sulphonation of the corresponding alkylbensene to give a sulphonic acid which can then be neutralised with a suitable base, for example, sodium or potassium hydroxide, sodium carbonate, ammonia or triethanolamine, to give the salt of the desired cation. The sodium salt, which is the most commonly used salt in detergent powders, is generally prepared by neutralisation with aqueous sodium hydroxide solution to yield a paste containing 50% or more water, and that material can be incorporated directly in the slurry.
- It has already been proposed to omit a separate neutralisation step by dosing the alkylbenzene sulphonic acid directly into the slurry-making vessel and neutralising it there with sodium hydroxide solution: this procedure is disclosed, for example, in GB-A-1 151 767 (Colgate-Palmolive) and GB-A-1 355 187 (Unilever). The heat liberated in the neutralisation reaction is useful for heating the slurry to the desired temperature which is generally about 80°C. The alkylbensene sulphonic acid is conventionally one of the first ingredients introduced into the slurry-making vessel, and is neutralised with sodium hydroxide solution before other ingredients are added. It has hitherto been believed that it is essential to neutralise the sulphonic acid before any sodium silicate is added, in order to prevent those components from interacting to form relatively insoluble siliceous compounds which could seriously affect the solubility of the finished detergent powder and could also lead to jet blocking during the spray-drying operation.
- It has now been found that it is possible to delay the addition of sulphonic acid until a later stage in the slurry-making process without encountering this disadvantage. The new procedure gives both product and process benefits, especially in the manufacture of powders containing a relatively high level of anionic detergent-active material.
- EP-A-242138, published 21.10.87, forms part of the state of the art by reason of Article 54(3) EPC. This document discloses a procedure in which alkylbenzene sulphonic acid is added to slurry containing sodium carbonate.
- The present invention provides a process for the manufacture of a particulate detergent composition, which comprises the steps of:
- (i) preparing an aqueous slurry comprising one or more anionic non-soap detergent-active compounds, and one or more alkaline inorganic salts, and
- (ii) spray-drying the slurry to form a powder;
- A significant characteristic of the invention is the incorporation of an anionic detergent-active compound wholly or predominantly in its acid form at a relatively late stage in the slurry-making process, when a large reservoir of alkaline material is already present in the slurry.
- The invention is applicable to the incorporation of any anionic detergent-active compound having a stable acid form. It is of especial interest in relation to sulphonic acids, especially alkylaryl sulphonic acids, more especially C₈-C₁₅ alkylbenzene sulphonic acids, both linear and branched. It may be advantageous for the sulphonic acid to be partially neutralised, for example, up to 10% by weight neutralised, before introduction into the slurry-making vessel: a neutralisation level of about 5% appears to be especially beneficial. This can be helpful in stabilising the sulphonic acid and preventing it from darkening in colour during storage. When the sodium salt of the sulphonic acid is desired, such preneutralisation is conveniently carried out with aqueous sodium hydroxide solution. For convenience, acid or partially neutralised acid added to the slurry will be referred to hereinafter simply as "sulphonic acid".
- At the time at which the sulphonic acid is introduced into the slurry-making vessel, there must already be present at least twice as much alkaline material as is required fully to neutralise the sulphonic acid. This will generally be constituted in part by a base such as sodium hydroxide which is especially intended for the purpose, in part by alkaline sodium salts present as builders, and possibly in part by alkaline sodium silicate. It is normally desirable to include in the slurry sufficient sodium hydroxide solution or other alkali to neutralise the sulphonic acid fully but in the manufacture of low-pH detergent powders intended for washing delicate fabrics it may be preferable to use a less than stoichiometric quantity of alkali and allow the neutralisation reaction to be completed by the alkaline builder salts present.
It is even possible for neutralising alkali to be omitted completely and the neutralisation reaction to be effected entirely by means of alkaline salts. - If the detergent composition prepared in accordance with the invention is to include alkaline sodium silicate, the silicate is preferably incorporated in the slurry before the sulphonic acid, and thus contributes to the alkaline material, mentioned previously, that must be present in at least a twofold amount relative to the sulphonic acid at the time when the latter component is added.
- Preferred examples of alkaline builder salts present in the slurry when the sulphonic acid is added are sodium tripolyphosphate, sodium carbonate and mixtures of these. These materials are commonly used in detergent powders as builders which sequester or precipitate calcium and magnesium salts in the wash liquor. They generally comprise at least 25% by weight of the detergent powder so that if they are incorporated in the slurry before addition of the sulphonic acid they can serve as a large alkaline buffer to prevent or minimise any adverse interaction between sodium silicate and sulphonic acid. Efficient stirring in the slurry-making vessel also helps to eliminate such adverse interaction.
- Any alkali added to the slurry specifically for neutralisation of the sulphonic acid of course makes an additional contribution to the reservoir of alkaline material in the slurry. In the case of alkylbenzenesulphonic acid, the amount of sodium hydroxide required to neutralise the sulphonic acid completely generally ranges from 10 to 20g per 100g sulphonic acid. This relatively large variation occurs because the amount of sulphuric acid present as a by-product in the sulphonic acid can vary quite widely: it is likely to be higher in sulphonic acids prepared by oleum sulphonation than in sulphonic acids prepared by sulphur trioxide sulphonation. Sodium hydroxide solution of specific gravity 1.5 is conveniently used as the neutralising alkali.
- When a neutralising alkali is used, it need not be introduced into the slurry before the sulphonic acid, provided that sufficient alkaline material from other sources is present when the sulphonic acid is added. It may be preferable to add the alkali and sulphonic acid simultaneously.
- The process of the invention gives several worthwhile advantages. One has already been mentioned: if sodium silicate is present, interaction between sodium silicate and sulphonic acid to form insoluble species can be minimised. Sodium silicate is a desirable ingredient in detergent compositions because it improves powder structure, prevents corrosion of washing machine parts in use, and provides building capacity (towards magnesium ions). The process of the invention is, however, also beneficial for the production of silicate-free powders and gives other advantages applicable both to silicate-containing and to silicate-free powders.
- Better control of the slurry temperature during the in-situ sulphonation step can be achieved than in the prior art process when the sulphonic acid is neutralised before addition of the builder salts and other components, because of the larger mass of material present, and available to act as a heat sink, at the time of the addition of the sulphonic acid. This benefit is especially noticeable when spray-dried powders containing 20% by weight or more of alkylaryl sulphonate are prepared: using the prior art procedure, so much heat may be generated under those circumstances that the slurry can boil over.
- A less aerated slurry is also obtained by the process of the invention, especially when other, high-foaming anionic, surfactants such as alkyl sulphates or alkyl ether sulphates are absent. A less aerated slurry gives a spray-dried powder of higher particle density, and allows high bulk density powders to be prepared without the need for a separate deaeration step. A less aerated slurry is also less bulky so that larger batches of slurry can be processed in a given slurry-making vessel: for powders containing high levels of alkylbenzene sulphonate (20% or more) an increase in batch size of up to 20% has been achieved, leading to a corresponding increase in the output of the slurry making plant. Pumping of a less aerated slurry is also easier because its viscosity is lower and cavitation problems are less likely to occur.
- Another benefit of the process of the invention is seen when sodium tripolyphosphate is a major building and structuring component of the powder: in a preferred embodiment of the invention, all sodium tripolyphosphate is added before the addition of the sulphonic acid. The temperature of the slurry at the time at which the sodium tripolyphosphate is added is then lower than in the prior art process in which the sulphonic acid neutralisation step has already occurred and raised the slurry temperature. The sodium tripolyphosphate can dissolve and hydrate more quickly at a lower temperature: this ensures that less degradation of the tripolyphosphate occurs during spray-drying, and also favours the growth of very small sodium tripolyphosphate hexahydrate crystals in the slurry, thus giving spray-dried powders of improved structure and absorptivity. Advantageously, a period of at least 2 minutes may be allowed to lapse between addition of the sodium tripolyphosphate and addition of the sulphonic acid to the slurry, in order to maximise hydration of the sodium tripolyphosphate before any temperature increase due to the neutralisation reaction occurs.
- In a typical slurry-making plant, the finished slurry is pumped, first via low-pressure pipes and then via high-pressure pipes, to a distribution manifold for the atomising nozzles of a spray-drying tower. As an alternative to incorporating the sulphonic acid in the slurry-making vessel itself, it may be injected either into the low-pressure pump or pipes, or into the high-pressure pump or pipes. It has been found especially beneficial to inject the sulphonic acid into the high-pressure pump or pipes, very shortly before the slurry arrives at the spray-nozzle distribution manifold.
- In this procedure slurry aeration and interaction with other materials in the slurry, particularly other detergent-active materials, are minimised: this is of especial advantage if nonionic surfactants are present, since alkylbenzene sulphonates can form viscous phases with nonionic surfactants in slurries, especially when the weight ratio of alkylbenzene sulphonate to nonionic surfactant is within the range of from 1:5 to 5:1. Furthermore, the slurry viscosity is lower before the addition of the sulphonic acid, and this lower viscosity can be exploited to the full, in easier pumping operations, if the sulphonic acid addition is delayed as long as possible.
- Alkylbenzene sulphonic acids are fairly viscous liquids at ambient temperatures but can be pumped without too much difficulty using positive displacement pumps. It has been found that sulphonic acid can be used at ambient temperature if it is to be added to the slurry-making vessel. When the alternative procedure of injection into a slurry transfer pump or pipe is to be used, however, the sulphonic acid is preferably heated to a temperature of about 50 to 60°C to lower its viscosity to a value at which accurate metering with a pump of the piston type is possible. An alternative method of reducing the viscosity of the sulphonic acid is to premix it with a viscosity-lowering ingredient, especially a nonionic surfactant, but this method is suitable only for preparing powders in which the viscosity-lowering ingredient is required.
- The process of the invention may be used with advantage to prepare any spray-dried detergent powder containing an anionic detergent-active compound having a stable acid form. This will normally be a sulphonate-type anionic detergent, especially an alkylaryl sulphonate and more especially a C₈-C₁₅ linear alkylbenzene sulphonate. Other anionic detergent-active compounds may also be present, for example, primary and secondary alkyl sulphates, olefin sulphonates, alkane sulphonates, alkyl ether sulphates, and fatty acid ester sulphonates.
- Nonionic surfactants may also be used in detergent powders prepared according to the invention. These include the primary and secondary alcohol ethoxylates, especially the C₁₂-C₁₅ primary and secondary alcohols ethoxylated with an average of from 3 to 20 moles of ethylene oxide per mole of alcohol.
- It may also be desirable to include one or more soaps of fatty acids. The soaps which can be used are preferably sodium soap derived from naturally occurring fatty acids, for example, the fatty acids from coconut oil, beef tallow, sunflower or hardened rape seed oil.
- The total amount of detergent-active material (surfactant), excluding soap, in detergent powders prepared according to the invention is generally within the range of from 5 to 40% by weight. For low-sudsing powders intended for use in European front-loading automatic washing machines the preferred range is from 5 to 20% by weight, with a weight ratio of anionic surfactant to nonionic surfactant not exceeding 10:1, and preferably not exceeding 6:1. As indicated above, however, the present invention is especially applicable to the manufacture of medium- and high-sudsing powders containing higher levels of anionic surfactant and/or higher ratios of anionic surfactant to nonionic surfactant.
- Detergent powders prepared in accordance with the invention will also comprise one or more detergency builders, suitably in an amount of from 10 to 60% by weight. Detergency builders are very well known to those skilled in the art and include sodium tripolyphosphate, orthophosphate and pyrophosphate; crystalline and amorphous sodium aluminosilicate; sodium carbonate; and monomeric and polymeric polycarboxylates, for example, sodium citrate, nitrilotriacetate and polyacrylate, and acrylic copolymers. As mentioned above, at least some of the builders incorporated in the powder must be alkaline salts.
- Other inorganic salts without a detergency building function, for example, sodium sulphate, may also be included in detergent powders prepared according to the invention.
- Detergent powders prepared according to the invention will also generally contain various additives to enhance the efficiency of the product, notably bleach systems, antiredeposition agents, fluorescers, lather suppressors, enzymes and perfumes. These may be added to the slurry or postdosed into the spray-dried powder in accordance with their known suitability for undergoing spray-drying processes.
- The invention will now be illustrated in further detail by the following non-limiting Examples.
-
- The contents of the slurry-making vessel were stirred continuously during the preparation of the slurry. During addition of the sulphonic acid, which was at ambient temperature, the temperature of the slurry rose from about 80°C to about 85°. It was noticeable that much less air than usual was entrained in the slurry during its preparation.
- It will be noted that at the time of addition of the alkylbenzene sulphonic acid (746 equivalents) to the slurry, 16782 equivalents of alkaline material (16033 equivalents from the sodium tripolyphosphate and 749 equivalents from the sodium hydroxide) were available in the slurry-making vessel, that is to say, the equivalent ratio of alkali to sulphonic acid was 22:1 without counting the sodium silicate and minor ingredients.
- The slurry was transferred via low and high-pressure transfer pipes to a spray-drying tower, 162 parts of a nonionic surfactant being injected into the high-pressure slurry transfer pipe just before it entered the spray jet distribution manifold.
- The solubility of the powder was assessed by stirring 4g into 400 ml of water at a temperature of 40°C for 2 minutes and then filtering the undissolved material. The weight of undissolved material under these test conditions should be less than 0.16g for a detergent powder, equivalent to 4% undissolved powder: the weight of undissolved material is greatly affected by the amount of insoluble siliceous material present.
- Under the above testing conditions, the level of undissolved material in the detergent powder of Example 1 was very satisfactory at 1.1%. The sulphonic acid used in Example 1 had been prepared by sulphonating an alkylbenzene with sulphur trioxide and air on a falling film reactor. It was of very good quality and contained only 0.9% by weight of free sulphuric acid.
- This Example describes the preparation of a high-sudsing detergent powder containing 20% by weight of alkylbenzene sulphonate. The alkylbenzene sulphonic acid used had been prepared by reacting the corresponding alkylbenzene directly with 20% oleum and then allowing the resulting mixture of sulphonic and sulphuric acids to separate into two layers; its sulphuric acid content was 6.9% by weight.
-
- It will be noted that at the time of addition of the alkylbenzene sulphonic acid (3525 equivalents) to the slurry, 16155 equivalents of alkaline material from the sodium tripolyphosphate and the sodium hydroxide were available, that is to say, the equivalent ratio of alkali to sulphonic acid was 4.6:1.
- The slurry temperature before addition of the sodium tripolyphosphate was 56°C and this rose to 72°C after a 2-minute period to allow the sodium tripolyphosphate to hydrate: this temperature rise indicated that 93% hydration has been achieved. During addition of the alkylbenzene sulphonic acid the temperature rose by a further 22°C to a final value of 94°C; at no time during the slurry-making process was heat from an external source applied to the vessel.
- The degree of aeration of the slurry after all the slurry ingredients had been added was significantly less than normal. The slurry was easy to pump.
- Without deaeration the slurry was transferred to a spray-drying tower and spray-dried to give a free-flowing powder having a bulk density of 413 g/litre.
- The standard dissolution test described in Example 1 established that the dissolving properties of the detergent powder were very good. The amount of undissolved material remaining after 2 minutes' stirring was only 0.1%. This result confirmed that no adverse reaction between the sulphonic acid and the sodium silicate had occurred in the slurry.
-
- During addition of the sulphonic acid the temperature in the slurry-making vessel rose by 42°C. The extent of hydration of the sodium tripolyphosphate was 83%. The slurry was considerably more aerated than that of Example 2, so that a smaller batch size (about 80% of that of Example 2) had to be used. The slurry was spray-dried to form a powder having a bulk density of 390 g/litre.
- This Example illustrates an alternative procedure according to the invention, in which alkylbenzene sulphonic acid was injected continuously into the slurry immediately before the slurry reached the distribution manifold for the spray nozzles of the spray-drying tower.
-
- The slurry was pumped through low and high-pressure pipes to the distribution manifold. The alkylbenzene sulphonic acid used in Example 1 (689 parts) was injected continuously at a pressure of 50 bar into the slurry just before it entered the manifold: the slurry pressure at this point was 45 bar. A simple static in-line mixer installed after the injection point ensured that the sulphonic acid mixed well with the slurry. Further and more intense local mixing also took place in the high shear swirl type of spray jets in the spray-drying tower.
- At the point of injection of the sulphonic acid (2110 equivalents) to the slurry, 20457 equivalents of alkaline material (18315 equivalents from the sodium tripolyphosphate and 2142 equivalents from the sodium hydroxide) were present in the slurry, that is to say, the equivalent ratio of alkali to sulphonic acid was 9.7:1, without counting the alkali present in the sodium silicate solution and minor ingredients.
- The spray dried detergent powder had very good physical properties and a bulk density of 490 g/litre.
The level of undissolved material remaining after 2 minutes' stirring in water at 40°C was 0.1%. This very low result confirmed that no unusual adverse reaction had occurred between the injected sulphonic acid and the silicate in the slurry leading to the formation of high levels of insoluble siliceous material.
wherein in step (i) an anionic detergent-active compound is incorporated in wholly or predominantly acid form into the slurry after the incorporation of alkaline material in an amount such that a total of at least two equivalents of alkaline material per equivalent of the said acidic detergent-active compound are present, with the proviso, that sodium carbonate is not used as the only alkaline inorganic salt.
Claims (12)
- A process for the manufacture of a particulate detergent composition, which comprises the steps of:(i) preparing an aqueous slurry comprising one or more anionic detergent-active compounds, and one or more alkaline inorganic salts, and(ii) spray-drying the slurry to form a powder;wherein in step (i) an anionic detergent-active compound is incorporated in wholly or predominantly acid form into the slurry after the incorporation of alkaline material in an amount such that a total of at least two equivalents of alkaline material per equivalent of the said acidic detergent-active compounds are present, with the proviso, that sodium carbonate is not used as the only alkaline inorganic salt.
- A process as claimed in claim 1, wherein the anionic detergent-active compound incorporated into the slurry in wholly or predominantly acid form is an C₈-C₁₅ alkylbenzene sulphonic acid.
- A process as claimed in claim 1 or claim 2, wherein the alkaline material incorporated in the slurry before the incorporation of the anionic detergent-active compound in wholly or predominantly acid form includes sodium hydroxide.
- A process as claimed in any one of claims 1 to 3, wherein the alkaline material incorporated in the slurry before the incorporation of the anionic detergent-active compound in wholly or predominantly acid form includes one or more alkaline builder salts.
- A process as claimed in claim 4, wherein the alkaline builder salts comprise sodium tripolyphosphate, sodium carbonate or a mixture thereof.
- A process as claimed in claim 5, wherein the detergent-active compound in wholly or predominantly acid form is incorporated in the slurry after the incorporation of any sodium tripolyphosphate.
- A process as claimed in any preceding claim, wherein the alkaline material incorporated in the slurry before the incorporation of the anionic detergent-active compound in wholly or predominantly acid form includes alkaline sodium silicate.
- A process as claimed in any preceding claim, wherein the anionic detergent-active compound in wholly or predominantly acid form is incorporated in the slurry in an amount corresponding to 20% by weight or more of the neutralised detergent-active material in the spray-dried powder.
- A process as claimed in any preceding claim, wherein the anionic detergent-active compound is incorporated in the slurry in 0 to 10% by weight neutralised form.
- A process as claimed in any preceding claim, wherein the slurry is prepared in a slurry-making vessel and then pumped through pipes to a spray-drying tower, characterised in that the anionic detergent-active compound in wholly or predominantly acid form is injected into a pipe or pump between the slurry-making vessel and the spray-drying tower.
- A process as claimed in claim 10, wherein the anionic detergent-active compound in wholly or predominantly acid form is premixed with a liquid nonionic detergent-active compound before incorporation in the slurry.
- A process as claimed in claim 10, wherein the anionic detergent-active compound in wholly or predominantly acid form is heated to a temperature within the range of from 50 to 60°C before incorporation in the slurry.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB868630726A GB8630726D0 (en) | 1986-12-23 | 1986-12-23 | Manufacture of spray-dried detergent powder |
| GB8630726 | 1986-12-23 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0273688A1 EP0273688A1 (en) | 1988-07-06 |
| EP0273688B1 true EP0273688B1 (en) | 1991-09-04 |
Family
ID=10609493
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP87311311A Expired - Lifetime EP0273688B1 (en) | 1986-12-23 | 1987-12-22 | Process for the manufacture of spray-dried detergent powder |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP0273688B1 (en) |
| DE (1) | DE3772739D1 (en) |
| ES (1) | ES2025672T3 (en) |
| GB (1) | GB8630726D0 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7811980B1 (en) | 2009-06-09 | 2010-10-12 | The Procter & Gamble Company | Spray-drying process |
| US7842657B2 (en) | 2008-06-25 | 2010-11-30 | The Procter & Gamble Company | Spray-drying process |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE69707480T2 (en) * | 1996-08-26 | 2002-08-14 | THE PROCTER & GAMBLE COMPANY, CINCINNATI | SPRAY DRYING METHOD FOR PRODUCING DETERGENT COMPOSITIONS WITH PRE-MIXING OF A MODIFIED POLYAMINE |
| DE19936613B4 (en) * | 1999-08-04 | 2010-09-02 | Henkel Ag & Co. Kgaa | Process for the preparation of a detergent with a soluble builder system |
| CN103002878B (en) | 2010-04-09 | 2015-07-01 | 帕西拉制药有限公司 | Method for formulating large diameter synthetic membrane vesicles |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0242138A2 (en) * | 1986-04-14 | 1987-10-21 | Unilever Plc | Process for the preparation of detergent powders |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1355187A (en) * | 1970-07-10 | 1974-06-05 | Unilever Ltd | Production of detergent compositions |
| GB1371101A (en) * | 1971-02-03 | 1974-10-23 | Unilever Ltd | Production of detergent compositions |
| PH10800A (en) * | 1972-10-31 | 1977-09-07 | Procter & Gamble | Detergent composition |
| GB8502032D0 (en) * | 1985-01-28 | 1985-02-27 | Unilever Plc | Detergent powder |
-
1986
- 1986-12-23 GB GB868630726A patent/GB8630726D0/en active Pending
-
1987
- 1987-12-22 DE DE8787311311T patent/DE3772739D1/en not_active Expired - Lifetime
- 1987-12-22 EP EP87311311A patent/EP0273688B1/en not_active Expired - Lifetime
- 1987-12-22 ES ES87311311T patent/ES2025672T3/en not_active Expired - Lifetime
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0242138A2 (en) * | 1986-04-14 | 1987-10-21 | Unilever Plc | Process for the preparation of detergent powders |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7842657B2 (en) | 2008-06-25 | 2010-11-30 | The Procter & Gamble Company | Spray-drying process |
| US7811980B1 (en) | 2009-06-09 | 2010-10-12 | The Procter & Gamble Company | Spray-drying process |
Also Published As
| Publication number | Publication date |
|---|---|
| ES2025672T3 (en) | 1992-04-01 |
| GB8630726D0 (en) | 1987-02-04 |
| EP0273688A1 (en) | 1988-07-06 |
| DE3772739D1 (en) | 1991-10-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0265203B1 (en) | Detergent compositions | |
| CA2555244C (en) | A granular laundry detergent composition comprising a ternary detersive surfactant system and low levels of, or no, zeolite builders and phosphate builders | |
| US5665694A (en) | Block detergent containing nitrilotriacetic acid | |
| EP2138567A1 (en) | Spray-drying process | |
| US4298493A (en) | Method for retarding gelation of bicarbonate-carbonate-silicate crutcher slurries | |
| EP2138564A1 (en) | A process for preparing a detergent powder | |
| US3576748A (en) | Free-flowing granular detergent compositions containing nta and soap | |
| US4294718A (en) | Non-gelling inorganic salt crutcher slurries | |
| US5447651A (en) | Process for producing concentrated laundry detergent by manufacture of low moisture content detergent slurries utilizing liquid active surfactant blend technology | |
| US4032465A (en) | Production of detergent compositions | |
| US5419850A (en) | Block detergent containing nitrilotriacetic acid | |
| JPH07506610A (en) | Method for producing detergent containing anionic surfactant | |
| EP0273688B1 (en) | Process for the manufacture of spray-dried detergent powder | |
| US5453215A (en) | Process for producing concentrated laundry detergent by manufacture of low moisture content detergent slurries | |
| US5490949A (en) | Block detergent containing nitrilotriacetic acid | |
| US20050187127A1 (en) | Laundry detergent composition comprising an anionic detersive surfactant, sulphamic acid and/or water soluble salts thereof, and a sulphate salt | |
| US3355390A (en) | Method for preparing homogeneous detergent slurry | |
| EP2832843B1 (en) | Method of making granular detergent compositions comprising polymers | |
| US3953379A (en) | Manufacture of improved aqueous alkali metal silicate-alkali metal hydroxyalkyl iminodiacetate compositions | |
| US5219495A (en) | Detergent compositions containing mobile liquid active systems | |
| NO179077B (en) | Process for the preparation of a concentrated liquid detergent composition containing magnesium alkylbenzenesulfonate and alkanolamide | |
| EP0328190B1 (en) | Particulate laundry detergent composition | |
| US5425895A (en) | Block detergent containing nitrilotriacetic acid | |
| AU688277B2 (en) | Detergent composition and process for its production | |
| WO2015112342A1 (en) | Method of making detergent compositions comprising polymers |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): CH DE ES FR GB IT LI NL SE |
|
| 17P | Request for examination filed |
Effective date: 19880723 |
|
| 17Q | First examination report despatched |
Effective date: 19890630 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): CH DE ES FR GB IT LI NL SE |
|
| REF | Corresponds to: |
Ref document number: 3772739 Country of ref document: DE Date of ref document: 19911010 |
|
| ET | Fr: translation filed | ||
| ITF | It: translation for a ep patent filed | ||
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2025672 Country of ref document: ES Kind code of ref document: T3 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| EAL | Se: european patent in force in sweden |
Ref document number: 87311311.2 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: CH Payment date: 19961203 Year of fee payment: 10 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19971231 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19971231 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: SE Payment date: 20031219 Year of fee payment: 17 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20041223 |
|
| EUG | Se: european patent has lapsed | ||
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20061220 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20061221 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20061222 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20061226 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20061231 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20070131 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20071222 |
|
| NLV7 | Nl: ceased due to reaching the maximum lifetime of a patent |
Effective date: 20071222 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20071224 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20071224 Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20071221 |