EP0202772A2 - Oil upgrading by thermal processing - Google Patents
Oil upgrading by thermal processing Download PDFInfo
- Publication number
- EP0202772A2 EP0202772A2 EP86302916A EP86302916A EP0202772A2 EP 0202772 A2 EP0202772 A2 EP 0202772A2 EP 86302916 A EP86302916 A EP 86302916A EP 86302916 A EP86302916 A EP 86302916A EP 0202772 A2 EP0202772 A2 EP 0202772A2
- Authority
- EP
- European Patent Office
- Prior art keywords
- feed
- phase
- liquid
- product
- hydrogen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G47/00—Cracking of hydrocarbon oils, in the presence of hydrogen or hydrogen- generating compounds, to obtain lower boiling fractions
- C10G47/22—Non-catalytic cracking in the presence of hydrogen
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G9/00—Thermal non-catalytic cracking, in the absence of hydrogen, of hydrocarbon oils
Definitions
- This invention relates to a process for upgrading petroleum residual oils or whole crudes of low quality to make products of higher economic value, especially high quality feedstocks for subsequent catalytic processing or fuels.
- Petroleum residua or resids contain metals, mostly in the form of oil soluble porphyrins, nitrogen, sulfur and other contaminants which interfere with many processing steps or reduce the value of the products obtained from these processing steps. Metals and nitrogen are undesirable if the resid is to be fed to a fluid catalytic cracking (FCC) or hydrocracking unit. The metals and nitrogen deactivate the catalyst. If the residuum is to be coked, the metals end up in the coke and reduce its value. It is desirable to remove the contaminants in a predictable manner.
- FCC fluid catalytic cracking
- Thermal processes used range from mild to severe. Visbreaking is a mild thermal cracking process in which the higher molecular weight components in the residuum are cracked to lighter products. During severe visbreaking, condensation reactions yield carbonaceous materials including toluene insolubles which are incompatible with the remaining products and which therefore tend to form a sludge which separates out of the visbroken effluent.
- coking which is a severe thermal process, the heavy oil feed is heated to cracking temperatures and converted into solid coke and lower molecular weight products which are removed as a vapor.
- coking processes are known, including delayed coking, fluid coking and contact coking and of these, delayed coking is the most common.
- Solvent extraction lacks selectivity for many contaminants, resulting in a requirement for a high solvent/residuum ratio.
- the capital cost and energy consumption to handle the solvent in each step of the process are high.
- the poor selectivity of solvent extraction leads to the production of a large volume of low value product (asphalt).
- the present invention provides a process for thermally upgrading a feed comprising a resid contaminated with sulfur, nitrogen and metal compounds and containing 413°C (775°F) material characterized by heating the feed to 399 to 510°C (750 to 950°F) at a pressure sufficient to maintain a liquid phase long enough to permit liquid/liquid phase separation of thermally upgraded feed into an upper light oil phase of reduced sulfur, nitrogen and metal content containing less than 0.5 wt. % toluene insolubies, and a lower residual oil phase containing from 0.5 to 30 wt. % toluene insolubles, and thereafter recovering the upper light oil phase.
- solvents or lighter boiling crude components are added to augment phase separation after thermal processing.
- thermal processing is carried out in the presence of hydrogen, and the light liquid phase immediately subjected to hydrotreating.
- the hydrotreated liquid is fed to a catalytic cracking process.
- the heavy oil feed comprises a petroleum residuum or a whole or reduced crude.
- the feed will be the bottoms from an atmospheric or vacuum tower, asphalt, various tars, bitumen from heavy oil or tar sands, coal liquids or a heavy shale oil residue.
- Feeds will usually have an initial boiling point, IBP, of at least 350°C and usually, at least 415°C (775°F). Most of the resid will boil above 550°C.
- Whole crudes will have a lower IBP.
- Such feeds usually have relative large amounts of con- radson carbon residue, CCR, materials which are considered uncrackable in a catalytic process such as FCC.
- the feed is thermally cracked optionally in the presence of hydrogen and/or steam.
- This step is carried out under relatively mild conditions so that it may be regarded as a severe visbreaking, or hydrovis- breaking if H 2 is added. It may also be considered a mild coking process, but no solid coke is produced. Substantially all the reactions take place in the liquid phase, even when H 2 is present. Reactions include the thermal cracking of paraffins and naphthenes, dealkylation of aromatics, cracking of resinous constituents to light products and decomposition or cracking of asphaltenes and metal compounds to lighter compounds. Some hydrogenation of various unsaturated and reactive species formed during the cracking occurs if H2 is added.
- the mild thermal cracking forms a denser, residual carbonaceous product which includes a significant content of toluene insoluble, TI, materials and a relatively light liquid product phase with a reduced level of contaminants.
- the contaminants, metal compounds, nitrogen and sulfur-containing heterocompounds in the feed concentrate in the carbonaceous product.
- the light liquid product may be used as a feed for an FCC unit or as a low sulfur fuel. When the feed contains very high contaminate levels, a hydrotreating step may be used to upgrade the light product.
- thermal processing removes 10 to 80, preferably 30 to 40 weight percent of the CCR present in the feed.
- the bulk conversion to light components e.g., to 350°C- materials
- the bulk conversion to light components will be less than 50 percent, typically from 10 to 30 weight percent of the 550°C + material.
- 20 to 70 weight percent of the 550°C + material is converted to 550°C- fractions.
- the 550°C + material in the feed is converted by thermal cracking to distillate material. Conversion of the feed to more volatile materials is not the same as CCR conversion. In general, conversion to lighter materials and CCR conversion increase with increasing severity but it is possible, and preferred, to convert most of the nondistillable portion of the feed to distillable fractions without converting most of the CCR to coke.
- the thermal processing step rejects a toluene insoluble, TI, residue which is removed from a relatively light liquid oil product.
- the conversion of the CCR to solid coke product is relatively low, only the relatively small portion of the most refractory content of the feed is removed, typically 20 to 40 weight percent of the CCR, leaving the rest of the carbon which is more reactive, to be upgraded by downstream processing.
- the carbon removed during this thermal processing is the most troublesome material for downstream processing units to handle, both from the viewpoint of metals contamination and from being refractory to conventional refinery upgrading processes.
- TI content may be measured by washing the total products with hot toluene (100°C), using four volumes of toluene for each volume of product.
- the filter cake on a Whatman No. 2 paper, after washing with hot toluene (100°C) and drying is the TI.
- thermal processing in the present process produces little dry gas, typically from 0.5 to 5 weight percent of the resid feed. This low gas make leads to improved liquid yield and reduced hydrogen loss so that the process as a whole has a markedly higher efficiency in hydrogen use.
- the heavy oil feed is preferably brought to a cracking temperature of 425 to 550°C (800 to 1020°F) with enough pressure to keep most of the liquid feed in the liquid phase typically 1500 to 15000 kPa.
- the heavy oil may be heated to a somewhat lower temperature, at which the degree of carbon deposition in the furnace is acceptable. Additional heat may be supplied by the addition of heated hydrogen or heated diluent (if any).
- the hydrogen may function as a heat transfer medium as well as a reactant both in the thermal cracking step and any downstream hydrotreating step.
- the diluent is preferably a light hydrocarbon such as a distillate, e.g., a light distillate or heavy distillate, or even a fraction boiling in the gasoline boiling range or even heated hydrocarbon gas, e.g. butane, propane.
- the diluent may be lighter portions of the crude oil feed. Use of whole crude as feedstock will save on distillation expenses heretofore incurred prior to visbreaking or coking.
- the diluent preferably acts as an antisol- vent for the residual phase.
- the residual phase is aromatic, containing high molecular weight asphaltenes, so paraffinic diluents are especially effective.
- the hydrogen, and diluent if used as a heating fluid, are heated to a temperature which will bring the heavy oil feed to the desired cracking temperature after mixing.
- the hydrogen and/or diluent will be heated to 450 to 550°C (840 to 1020°F), preferably 450 to 510°C (840 to 950°F) at 1500 to 15000 kPa prior to mixing with the heavy oil feed.
- H 2 and diluent temperature will vary according to the ratio of diluent to feed and their thermal capacities.
- the ratio of diluent to heavy oil feed will generally be 0.05 to 2, preferably 0.1 to 1, by weight.
- the hydrogen circulation preferably is at least 200 n.I.I.-' (200 SCFB) and most preferably 200 to 2000 n.I.I.-' - (1120 to 11235 SCFB).
- Adding H, to the thermal processing step also provides enough hydrogen for downstream hydrotreating. Higher H 2 :oil ratios may be desirable to heat the charge.
- Hydrogen consumption depends on the resid feed and conditions in cracking and hydrotreating. Hydrogen consumption is made up by addition of fresh hydrogen. The cracking temperature, furnace temperature, and so on should be selected for the feed then the H 2 circulation rate set, after which the H 2 addition rate can be determined.
- the diluent has a lower propensity for furnace fouling than the resid.
- the diluent may be heated a lot to heat the resid, thereby reducing the resid heater duty and/or eliminating the necessity for using hot hydrogen. It is expensive to heat hydrogen so use of a heated diluent is preferred.
- the heated diluent, if any, and hydrogen, if any, are mixed with the heated heavy oil feed and charged to a thermal reactor where thermal cracking occurs.
- a phase separator may follow the thermal reactor to separate the light liquid overflow product from residual underflow under optimum conditions.
- the separate phase separator may be hot so that some cracking takes place in it, increasing the total reaction severity. Adequate severity can be obtained in a coil without a separate soaking drum, but with refractory feeds, a drum may be necessary to secure the desired severity.
- a phase separator soaking drum after the furnace provides time at elevated temperature and therefore increases the severity and permits phase separation to take place.
- the thermal cracking is controlled so that the desired phase separation takes place and the desired level of demetalation is achieved.
- the optimum severity varies from feed to feed and may be determined experimentally.
- the objective is to produce a denser, residual carbonaceous phase in which the contaminants are concentrated and a supernatant liquid phase which may be separated by physical means from the residual carbonaceous phase.
- the severity of the thermal cracking step is preferably controlled so the TI content of the dense or carbonaceous phase is 0.5 to 40 wt. % preferably from 5 to 30 wt. %. This ensures that the carbonaceous phase remains thermoplastic and meltable at temperatures below about 200°C and may be handled as a liquid in subsequent processing. Tl is not coke.
- the Tl material is a complex hydrocarbon mixture which is soluble in solvents such as pyridine and tetrahydrofuran but insoluble in toluene. It has a nominal hydrogen content of 6 to 7 wt. %. Pyrolysis of this Tl yields a coke residue which weighs less than about 70 percent of the Tl feed.
- the weight ratio of TI in the total liquid product (upper and lower phases) to the CCR in the residual phase should be 0.5 to 2.0.
- thermal cracking is not sensitive to pressure so pressure is not a critical parameter of the process. A high enough pressure should be present to keep most of the feed in liquid phase.
- the reaction conditions required to achieve the optimum thermal severity vary according to the thermal reactivity of the feed.
- the severity of any thermal processing step may be expressed in terms of "Equivalent Reaction Time” (ERT) measured in seconds at 427°C (800°F). Ten minutes at 427°C (800°F) equals a 600 ERT. This is a well known concept in visbreaking, thermal cracking and coking.
- severity will be controlled to produce a residual phase which can be maintained in a flowable condition for handling.
- the fluidity of the carbonaceous phase may be maintained below certain upper limits to ensure that it remains fluid and readily pumpable to other process units.
- the severity will be from 1500 to 20.000 seconds ERT at 427°C (800°F). The optimum severity will vary from feed to feed and may be determined experimentally by Shaker Bomb or other laboratory test.
- the feed is thermally cracked to produce a residual phase containing the majority of the contaminants, particularly the metals, nitrogen and sulfur heterocompounds, and an upgraded liquid phase which separates from the residual phase.
- the light liquid product is predominantly paraffinic whereas the residual phase, including the TI is predominantly asphaltenic and high in aromatics.
- Phase separation takes place readily in a phase separator, permitting the upgraded liquid product to be removed as an overflow and the thermoplastic, residual phase to be removed as an underflow.
- Hydrogen addition is optional, but preferred. Hydrogen can be used as a heating medium, by superheating the hydrogen and using the superheated hydrogen to heat the feed.
- the hydrogen may also be mixed with the feed, upstream of the thermal processing heater.
- H2 addition may provide hydrogen for subsequent hydrotreating, so that the liquid overflow product can be passed directly to a cascaded hydrotreating step.
- Direct coupling of hydrotreating with cracking enables the light liquid product to be stabilized by hydrogenation of metastable and labile species to prevent recombination to high molecular weight materials. Phase separation between the liquid product and the residual carbonaceous phase is promoted because hydrogen is one of the best antisolvents for the thermoplastic phase. The hydrogen reduces the solubility of the thermoplastic phase in the liquid product.
- Phase separation may also be also improved by the use of solvents, e.g., light hydrocarbons, whether added upstream or downstream of the thermal reactor.
- Light hydrocarbons preferably paraffinic
- the boiling point of the solvent added to the phase separator preferably is chosen so that at the temperature and pressure of the separator, at least 50% of the solvent will be in solution in the liquid product.
- Typical solvents used to enhance phase separation include naphthas and distillates including kerosene, light distillate and heavy distillate.
- Temperatures in the phase separator will generally be from 230 to 450°C, preferably 290 to 425°C. Generally the ratio of any added solvent to the heavy oil feed will be from 0.05:1 to 2:1 by volume.
- the light, supernatant, liquid phase withdrawn as overflow from the phase separator is low in metal, CCR and asphaltenes.
- the contaminants and asphaltenes concentrate in the thermoplastic underflow phase.
- the liquid overflow product contains thermally cracked fragments which are easily hydrogenated to increase their hydrogen content. Hydrogenation improves the storage stability of the product.
- the liquid overflow product is easy to hydrotreat because its molecular weight is lower than the original heavy oil feed. Thermally cracked products are in a metastable or activated state for a significant period of time. Hydrogenation produces stable products with a reduced susceptibility to polymerization condensation and reduces residual heteroatom content (nitrogen, sulfur, possibly oxygen). It is preferred to immediately hydrotreat the liquid overflow product rather than store it, as during storage the activated species may undergo polymerization and condensation to solid, sludge- like products which are hard to hydrotreat.
- the liquid overflow product is hydrogenated with conventional hydrotreating catalyst.
- Suitable catalysts employ a hydrogenation/dehydrogenation component on a substantially neutral or acidic support such as alumina, silica-alumina, carbon, a zeolite or a solid aluminum phosphate.
- the hydrogenation/dehydrogenation component will normally be a transition metal, either a base metal or base metal combination such as cobalt, tung- ston, or a base metal combination such as cobalt- molybdenum, nickel-tungsten, nickel-molybdenum, or a noble metal such as platinum or palladium.
- Hydrotreating conditions are conventional, typically 200 to 450°C, preferably 250 to 400°C.
- Pressures in the hydrotreater are preferably the same as in the thermal processing unit, or hydrovisbreaker if H2 is added during thermal processing, to facilitate direct coupling of the two processes. Therefore, pressure will generally be 1500 to 15000 kPa. Space velocities are normally from 0.1 to 20, preferably 0.5 to 5, hour-'. Liquid flow in the hydrotreater may be up or downflow but downflow is preferred, i.e., a trickle bed reactor is preferred. The severity of the hydrotreating operation will depend on product requirements.
- Hydrotreating is especially preferred when the light liquid product will be FCC feed.
- the sulfur and metals levels are substantially reduced by hydrotreating, and the hydrogen content increased to about that of conventional FCC feed.
- thermoplastic, carbonaceous phase withdrawn as an underflow product from the phase separator can be washed with solvent or stripped with steam to recover more of light liquid oil product.
- the residue from solvent extraction or steam stripping may be made into asphalt or pitch binder, charged to a coker or a gasification unit, or used as refinery fuel.
- Coking treatments include delayed coking, fluid coking or contact coking.
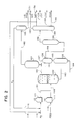
- a resid feed having an initial boiling point of 413°C + (775°F + ) is charged via line 8 to heater 10 and heated to 371 to 538°C.
- the thermal processing can be achieved solely in heater 10, i.e., the time and temperature in the heater can be enough to yield enough thermoplastic carbonaceous material to provide phase separation from the paraffinic light oil phase.
- Reactor 12 is simply a thermal reactor, the elevated temperatures alone initiate the desired reactions. From reactor 12, effluent enters phase separator 14 via line 13.
- Separator 14 is preferably maintained at 204 to 371 °C (400 to 700°F).
- the phenomenon of phase separation is based on the incompatibility of the carbonaceous phase containing toluene insolubles and the liquid paraffinic phase.
- the light or liquid paraffinic phase is withdrawn as overflow via line 16 while the carbonaceous material or sludge is withdrawn as underflow via line 18.
- the underflow may be passed to a heater 20, heated to at least 482°C - (900°F) and charged via line 21 to coking unit 22, e.g., a delayed or fluid coker.
- the underflow via line 18 can be mixed with ligh aromatics from line 5 or steam from line 52 prior to heating.
- the products from coking unit 22 are solid coke removed via line 24 and a vaporized product removed via line 26.
- the vaporized product may be fractionated in fractionator 28 into various components, typically light gaseous components, naphthas, light coker and heavy coker gas oils, removed via lines 31-34, respectively.
- Separation of carbonaceous material from lighter paraffinic liquid in separator 14 may be aided by solvent treatment of the thermal reactor effluent.
- Light products e.g., contained in the effluent from the heater or thermal reactor may first be flashed off in an optional flashing drum. These products will typically be C 2 C. gases. To facilitate flashing, some pressure drop can be taken. The bottom product from the flashing drum may contact solvent upstream of or in the phase separator.
- the important parameters in the solvent treatment are the solvent, solvenflresid ratio, temperature, pressure and number of treatment stages.
- Solvents which may be used include carbon dioxide, light hydrocarbons, C 3 -C 10 paraffins, cycle paraffins, naphthas, gasoline and kerosene, and their mixtures. Propane, butanes, pentane, hexane, and heptane are particularly preferred. The lower the carbon number of the solvent, the higher the quality of the light phase withdrawn but the lower the yield of the treated product.
- the temperature of solvent treatment must be at least 11°C (20°F), preferably 22°C (40°F) higher than the melting point of the carbonaceous bottoms product so that the two phases formed in solvent treatment are both liquids.
- the temperature of solvent treatment must be at least 11°C (20°F), preferably 22°C (40°F) higher than the melting point of the carbonaceous bottoms product so that the two phases formed in solvent treatment are both liquids.
- improved solubility between the solvent, light oil phase and carbonaceous bottoms impairs phase separation.
- a high solvent/resid ratio aids phase separation, but use of large amounts of solvent increases the processing cost.
- cooling feed to the solvent treater and heating product from the solvent treater can be minimized leading to cost savings.
- Higher pressure increases the amount of solvent which is in liquid phase at high temperatures.
- the optimum pressure depends on the solvent used.
- the pressure preferably maintains most of the solvent in the liquid phase. Pressure may equal or exceed the critical pressure of the solvent used. For light hydrocarbons this pressure is about 3,500 Kpa (500 psig).
- solvent/resid ratio As the solvent/resid ratio increases, the quality of the relatively light product increases, but the yield decreases. Solvent/resid ratio should be reduced to minimize the operation cost.
- the optimum solventtresid ratio is the lowest ratio which yields the upgraded product of acceptable quality.
- the sotvent/resid ratio can be 1:10 to 10:1.
- a solvent/resid ratio of 1:2 to 3:1 is preferred.
- the optimum solvent/resid ratio depends substantially upon the severity of thermal treatment. The higher the thermal treating severity, the lower the sotvent/resid ratio requirement.
- a residual oil feed is charged via line 110 to furnace 111 where it is heated.
- Hydrogen gas from recycle line 112, light solvent or diluent from recycle line 114 and fresh hydrogen from inlet 115 are heated in heater 113 and passed via line 151 to mix with the preheated feed in line 116.
- the mixture enters combined thermal reactor and phase separator 120 where thermal cracking and phase separation occurs to form an upper phase of upgraded liquid product and a lower phase of thermoplastic, carbonaceous product.
- the liquid/liquid interface in separator 120 preferably is kept high and the level of inlet line 116 low to improve phase separation and consequently metal removal and carbon residue rejection. As the liquid product passes towards the top of the separator and the fluid carbonaceous product sinks towards the bottom, the toluene insoluble materials formed in the thermal cracking coagulate and agglomerate.
- the light liquid product is removed via line 161 and passed to hydrotreater 121, a conventional hydrotreating process.
- a downflow, trickle bed hydrotreating reactor is preferred.
- Hydrotreating severity depends on the product use. For fuel use, severity is adjusted to meet fuel sulfur content specifications, generally below 0.3 wt % sulfur. If the product will be FCC feed, more sulfur can be tolerated since sulfur rejection may take place during the FCC operation, or in other downstream processing.
- Effluent from hydrotreater 121 passes via line 162 to high pressure separator 122 where a hydrogen rich gas phase is removed from the hydrotreated product.
- the hydrogen may be recycled via line 112 without purification, although an alkaline scrub may be used to remove hydrogenation products, principally hydrogen sulfide, prior to recycle.
- the hydrotreated liquid is removed via line 123.
- phase separator 120 the thermoplastic, carbonaceous phase is withdrawn via line 163 as a liquid or semi-liquid product.
- the residue is passed to a steam stripper 125 where it contacts steam from line 164 which removes residual light products and any added solvent. From the stripper, it passes via line 165 to separator 126 for removal of the light ends which are passed to hydrotreater 121 through line 127.
- the stripped residue passes to a coking unit such as a delayed coker 128.
- the vaporous products of coking are passed via line 129 to fractionator 130 where they are separated into products, coker heavy gas oil (HCGO), coker light gas oil (LCGO), distillate, naphtha and gas, removed via lines 135-131 respectively.
- HCGO coker heavy gas oil
- LCGO coker light gas oil
- Some naphtha is returned as light solvent recycle through line 114 to assist in the heating of the heavy oil feed to the requisite cracking temperature.
- the distillate could be used as light recycle, as could the LCGO and HCGO fractions although these last two are not preferred.
- the gas from fractionator 130 could also be recycled to assist in the heating of the heavy feed, if desired.
- the feed properties were:
- This feed was added to an autoclave, and heated to the desired reaction temperature.
- the autoclave was maintained at the reaction temperature for 1/2 hour and cooled to 316°C (600°F) rapidly with an internal cooling coil.
- 316°C 600°F
- the reaction mixture stood unstirred for phase separation, and then was quenched by ice bath.
- Products collected were the upper phase liquid, extracted lower phase liquid from pentane washing, lower phase tar, cold trap liquid and gas.
- the product distribution is shown in Table 1.
- Table 1 shows as the reaction severity increased from 1800 to 7200 ERT seconds, liquid yield decreased and toluene-insoluble (TI) and gas yields increased.
- TI toluene-insoluble
- the metals, S and N content in the upper phase liquid product all decreased with increasing reaction severity.
- a modest 60 to 70% demetalation was achieved at 1800 ERT sec, equivalent to 30 minutes at 427°C (800°F), with 94% total liquid yield (Table 1).
- the boiling point conversion of this product is shown in Table 3.
- the fractions of 216°C-(420°F-) and 216 to 343°C (420 to 650°F) increased over the feed.
- the conversion of 449°C+ (840°F+) to lighter products was 27 wt %. At a higher severity, e.g., 4,300 ERT, more conversion would be expected.
- the TI in the upper phase liquid was 1.03%, reflecting poor phase separation.
- the TI was very low (0.1 % or less), low enough to feed to .hydrotreaters, FCC units or use as heavy fuel.
- the TI in the upper phase approached 0.09 at 4300 ERT.
- the ratio of TI in the two phases increased from 33 to 570 with severity. This high ratio at high severity reflected the production of more TI materials.
- the optimum treatment severity is about 4300 ERT seconds which yielded large amounts of upgraded liquid product with over 90% of Ni and V removed from the upper liquid phase product.
- a 4300 ERT severity is between visbreaking and coking.
- This example illustrates what can be achieved when mild coking, phase separation and hydrotreating of light liquid product are practiced together.
- a typical low gravity feed is subjected to mild coking in a fluidized bed, liquid phase reactor to reject carbon as a thermoplastic, dense residue which is continuously removed from the reactor.
- Reactor temperature is 470°C (880°F) at 2860 kPa (400 psig) pressure, 3 V/V hr-' space velocity.
- the mildly coked liquid product (MCLP) is removed as a relatively light liquid phase and passed directly to hydrotreating over a conventional hydrotreating catalyst under 7000 kPa (1000 psig) hydrogen pressure at 400°C (750°F), 1 hr' LHSV.
- the hydrotreated product is suitable for use as an FCC feed to produce gas plus distillate, G/D, in a high efficiency process.
- the present process efficiently upgrades residua to low boiling products including gasoline and distillate, with the ability to use existing refinery equipment, thereby minimizing capital cost. It efficiently uses hydrogen, by rejecting the more refractory carbonaceous portions of the resid as a dense phase and using the hydrogen selectively to increase the hydrogen level of the FCC fee. This, in turn, results in an increased yield of low boiling product.
Landscapes
- Chemical & Material Sciences (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Production Of Liquid Hydrocarbon Mixture For Refining Petroleum (AREA)
Abstract
Description
- This invention relates to a process for upgrading petroleum residual oils or whole crudes of low quality to make products of higher economic value, especially high quality feedstocks for subsequent catalytic processing or fuels.
- The high cost of petroleum and the decreasing availability of high quality crudes, with increased demand for gasoline and distillate range materials, led to a greater reliance on processes for upgrading petroleum residua. Petroleum residua or resids contain metals, mostly in the form of oil soluble porphyrins, nitrogen, sulfur and other contaminants which interfere with many processing steps or reduce the value of the products obtained from these processing steps. Metals and nitrogen are undesirable if the resid is to be fed to a fluid catalytic cracking (FCC) or hydrocracking unit. The metals and nitrogen deactivate the catalyst. If the residuum is to be coked, the metals end up in the coke and reduce its value. It is desirable to remove the contaminants in a predictable manner.
- Various methods have been used to upgrade resids. These include hydrogenative processing, solvent extraction and thermal processes.
- Thermal processes used range from mild to severe. Visbreaking is a mild thermal cracking process in which the higher molecular weight components in the residuum are cracked to lighter products. During severe visbreaking, condensation reactions yield carbonaceous materials including toluene insolubles which are incompatible with the remaining products and which therefore tend to form a sludge which separates out of the visbroken effluent. In coking, which is a severe thermal process, the heavy oil feed is heated to cracking temperatures and converted into solid coke and lower molecular weight products which are removed as a vapor. Various coking processes are known, including delayed coking, fluid coking and contact coking and of these, delayed coking is the most common.
- These techniques all have disadvantages. Hydroprocessing requires high pressure hydrogen at high temperatures and this leads to high capital and operating costs.
- Solvent extraction lacks selectivity for many contaminants, resulting in a requirement for a high solvent/residuum ratio. The capital cost and energy consumption to handle the solvent in each step of the process are high. The poor selectivity of solvent extraction leads to the production of a large volume of low value product (asphalt).
- Mild thermal processing, such as visbreaking, requires relatively little equipment and relatively little in the way of process heat. Visbreaking reduces the viscosity of the product, but not the contaminant content, so the "upgrading" is limited to viscosity reduction. More severe thermal processing techniques such as delayed coking are less advantageous. The capital and operating costs of delayed coking are intensive and the value of the coke product may be less than 1/10 that of the feed (for shot coke) or slightly more (for sponge coke) from low quality residua.
- It would be desirable to carry out thermal processing with minimal production of coke. It would be desirable to produce a liquid product with a higher hydrogen content and reduced content of nitrogen, sulfur and metal contaminants. It would be beneficial to have a stable upgraded liquid product which does not undergo any deleterious changes in storage.
- A way has now been discovered to efficiently upgrade heavy oils into lighter products with reduced contaminant contents, without producing large amounts of solid coke.
- Accordingly, the present invention provides a process for thermally upgrading a feed comprising a resid contaminated with sulfur, nitrogen and metal compounds and containing 413°C (775°F) material characterized by heating the feed to 399 to 510°C (750 to 950°F) at a pressure sufficient to maintain a liquid phase long enough to permit liquid/liquid phase separation of thermally upgraded feed into an upper light oil phase of reduced sulfur, nitrogen and metal content containing less than 0.5 wt. % toluene insolubies, and a lower residual oil phase containing from 0.5 to 30 wt. % toluene insolubles, and thereafter recovering the upper light oil phase.
- In one embodiment, solvents or lighter boiling crude components are added to augment phase separation after thermal processing.
- In another embodiment, thermal processing is carried out in the presence of hydrogen, and the light liquid phase immediately subjected to hydrotreating.
- In yet another embodiment, the hydrotreated liquid is fed to a catalytic cracking process.
- Figure 1 is a simplified flow diagram of the present invention wherein a heavy feed is thermally processed to produce an upgraded resid product and a carbonaceous phase.
- Figure 2 is a simplified flow diagram showing the above process with H2 addition to the feed, and hydrotreating of the upgraded product.
- The heavy oil feed comprises a petroleum residuum or a whole or reduced crude. Generally the feed will be the bottoms from an atmospheric or vacuum tower, asphalt, various tars, bitumen from heavy oil or tar sands, coal liquids or a heavy shale oil residue. Feeds will usually have an initial boiling point, IBP, of at least 350°C and usually, at least 415°C (775°F). Most of the resid will boil above 550°C. Whole crudes will have a lower IBP. Such feeds usually have relative large amounts of con- radson carbon residue, CCR, materials which are considered uncrackable in a catalytic process such as FCC.
- In the first stage of the process, the feed is thermally cracked optionally in the presence of hydrogen and/or steam. This step is carried out under relatively mild conditions so that it may be regarded as a severe visbreaking, or hydrovis- breaking if H2 is added. It may also be considered a mild coking process, but no solid coke is produced. Substantially all the reactions take place in the liquid phase, even when H2 is present. Reactions include the thermal cracking of paraffins and naphthenes, dealkylation of aromatics, cracking of resinous constituents to light products and decomposition or cracking of asphaltenes and metal compounds to lighter compounds. Some hydrogenation of various unsaturated and reactive species formed during the cracking occurs if H2 is added.
- The mild thermal cracking forms a denser, residual carbonaceous product which includes a significant content of toluene insoluble, TI, materials and a relatively light liquid product phase with a reduced level of contaminants. The contaminants, metal compounds, nitrogen and sulfur-containing heterocompounds in the feed, concentrate in the carbonaceous product. The light liquid product may be used as a feed for an FCC unit or as a low sulfur fuel. When the feed contains very high contaminate levels, a hydrotreating step may be used to upgrade the light product.
- Preferably thermal processing removes 10 to 80, preferably 30 to 40 weight percent of the CCR present in the feed. Under these conditions, the bulk conversion to light components, e.g., to 350°C- materials, will be less than 50 percent, typically from 10 to 30 weight percent of the 550°C+ material. At the same time, 20 to 70 weight percent of the 550°C+ material is converted to 550°C- fractions. Thus a significant amount of the non-distillables, the 550°C+ material in the feed is converted by thermal cracking to distillate material. Conversion of the feed to more volatile materials is not the same as CCR conversion. In general, conversion to lighter materials and CCR conversion increase with increasing severity but it is possible, and preferred, to convert most of the nondistillable portion of the feed to distillable fractions without converting most of the CCR to coke.
- The thermal processing step rejects a toluene insoluble, TI, residue which is removed from a relatively light liquid oil product. In the thermal processing step the conversion of the CCR to solid coke product is relatively low, only the relatively small portion of the most refractory content of the feed is removed, typically 20 to 40 weight percent of the CCR, leaving the rest of the carbon which is more reactive, to be upgraded by downstream processing. The carbon removed during this thermal processing is the most troublesome material for downstream processing units to handle, both from the viewpoint of metals contamination and from being refractory to conventional refinery upgrading processes.
- The carbon removed is removed as toluene insolubles. TI content may be measured by washing the total products with hot toluene (100°C), using four volumes of toluene for each volume of product. The filter cake on a Whatman No. 2 paper, after washing with hot toluene (100°C) and drying is the TI.
- Another contrast to conventional coking is that thermal processing in the present process produces little dry gas, typically from 0.5 to 5 weight percent of the resid feed. This low gas make leads to improved liquid yield and reduced hydrogen loss so that the process as a whole has a markedly higher efficiency in hydrogen use.
- To initiate the thermal cracking, the heavy oil feed is preferably brought to a cracking temperature of 425 to 550°C (800 to 1020°F) with enough pressure to keep most of the liquid feed in the liquid phase typically 1500 to 15000 kPa. To avoid excessive fouling in the furnace, the heavy oil may be heated to a somewhat lower temperature, at which the degree of carbon deposition in the furnace is acceptable. Additional heat may be supplied by the addition of heated hydrogen or heated diluent (if any). The hydrogen may function as a heat transfer medium as well as a reactant both in the thermal cracking step and any downstream hydrotreating step. The diluent, if present, is preferably a light hydrocarbon such as a distillate, e.g., a light distillate or heavy distillate, or even a fraction boiling in the gasoline boiling range or even heated hydrocarbon gas, e.g. butane, propane. The diluent may be lighter portions of the crude oil feed. Use of whole crude as feedstock will save on distillation expenses heretofore incurred prior to visbreaking or coking. The diluent preferably acts as an antisol- vent for the residual phase. The residual phase is aromatic, containing high molecular weight asphaltenes, so paraffinic diluents are especially effective.
- The hydrogen, and diluent, if used as a heating fluid, are heated to a temperature which will bring the heavy oil feed to the desired cracking temperature after mixing. Generally, the hydrogen and/or diluent will be heated to 450 to 550°C (840 to 1020°F), preferably 450 to 510°C (840 to 950°F) at 1500 to 15000 kPa prior to mixing with the heavy oil feed. H2 and diluent temperature will vary according to the ratio of diluent to feed and their thermal capacities. The ratio of diluent to heavy oil feed will generally be 0.05 to 2, preferably 0.1 to 1, by weight. If hydrogen is added, the hydrogen circulation preferably is at least 200 n.I.I.-' (200 SCFB) and most preferably 200 to 2000 n.I.I.-' - (1120 to 11235 SCFB). Adding H, to the thermal processing step also provides enough hydrogen for downstream hydrotreating. Higher H2:oil ratios may be desirable to heat the charge. Hydrogen consumption depends on the resid feed and conditions in cracking and hydrotreating. Hydrogen consumption is made up by addition of fresh hydrogen. The cracking temperature, furnace temperature, and so on should be selected for the feed then the H2 circulation rate set, after which the H2 addition rate can be determined.
- The diluent has a lower propensity for furnace fouling than the resid. The diluent may be heated a lot to heat the resid, thereby reducing the resid heater duty and/or eliminating the necessity for using hot hydrogen. It is expensive to heat hydrogen so use of a heated diluent is preferred.
- The heated diluent, if any, and hydrogen, if any, are mixed with the heated heavy oil feed and charged to a thermal reactor where thermal cracking occurs. A phase separator may follow the thermal reactor to separate the light liquid overflow product from residual underflow under optimum conditions. The separate phase separator may be hot so that some cracking takes place in it, increasing the total reaction severity. Adequate severity can be obtained in a coil without a separate soaking drum, but with refractory feeds, a drum may be necessary to secure the desired severity. A phase separator soaking drum after the furnace provides time at elevated temperature and therefore increases the severity and permits phase separation to take place.
- The thermal cracking is controlled so that the desired phase separation takes place and the desired level of demetalation is achieved. The optimum severity varies from feed to feed and may be determined experimentally. The objective is to produce a denser, residual carbonaceous phase in which the contaminants are concentrated and a supernatant liquid phase which may be separated by physical means from the residual carbonaceous phase. The severity of the thermal cracking step is preferably controlled so the TI content of the dense or carbonaceous phase is 0.5 to 40 wt. % preferably from 5 to 30 wt. %. This ensures that the carbonaceous phase remains thermoplastic and meltable at temperatures below about 200°C and may be handled as a liquid in subsequent processing. Tl is not coke. The Tl material is a complex hydrocarbon mixture which is soluble in solvents such as pyridine and tetrahydrofuran but insoluble in toluene. It has a nominal hydrogen content of 6 to 7 wt. %. Pyrolysis of this Tl yields a coke residue which weighs less than about 70 percent of the Tl feed. The weight ratio of TI in the total liquid product (upper and lower phases) to the CCR in the residual phase should be 0.5 to 2.0.
- Generally, thermal cracking is not sensitive to pressure so pressure is not a critical parameter of the process. A high enough pressure should be present to keep most of the feed in liquid phase.
- The reaction conditions required to achieve the optimum thermal severity vary according to the thermal reactivity of the feed. The severity of any thermal processing step may be expressed in terms of "Equivalent Reaction Time" (ERT) measured in seconds at 427°C (800°F). Ten minutes at 427°C (800°F) equals a 600 ERT. This is a well known concept in visbreaking, thermal cracking and coking.
- In general, severity will be controlled to produce a residual phase which can be maintained in a flowable condition for handling. The fluidity of the carbonaceous phase may be maintained below certain upper limits to ensure that it remains fluid and readily pumpable to other process units. In general, the severity will be from 1500 to 20.000 seconds ERT at 427°C (800°F). The optimum severity will vary from feed to feed and may be determined experimentally by Shaker Bomb or other laboratory test.
- The feed is thermally cracked to produce a residual phase containing the majority of the contaminants, particularly the metals, nitrogen and sulfur heterocompounds, and an upgraded liquid phase which separates from the residual phase. The light liquid product is predominantly paraffinic whereas the residual phase, including the TI is predominantly asphaltenic and high in aromatics. Phase separation takes place readily in a phase separator, permitting the upgraded liquid product to be removed as an overflow and the thermoplastic, residual phase to be removed as an underflow.
- Hydrogen addition is optional, but preferred. Hydrogen can be used as a heating medium, by superheating the hydrogen and using the superheated hydrogen to heat the feed. The hydrogen may also be mixed with the feed, upstream of the thermal processing heater. H2 addition may provide hydrogen for subsequent hydrotreating, so that the liquid overflow product can be passed directly to a cascaded hydrotreating step. Direct coupling of hydrotreating with cracking enables the light liquid product to be stabilized by hydrogenation of metastable and labile species to prevent recombination to high molecular weight materials. Phase separation between the liquid product and the residual carbonaceous phase is promoted because hydrogen is one of the best antisolvents for the thermoplastic phase. The hydrogen reduces the solubility of the thermoplastic phase in the liquid product.
- Phase separation may also be also improved by the use of solvents, e.g., light hydrocarbons, whether added upstream or downstream of the thermal reactor. Light hydrocarbons, preferably paraffinic, may be added to the phase separator to aid separation. If solvents are added to the phase separator, they are preferably recycled solvents from a subsequent stage of the operation, as described below. The boiling point of the solvent added to the phase separator preferably is chosen so that at the temperature and pressure of the separator, at least 50% of the solvent will be in solution in the liquid product. Typical solvents used to enhance phase separation include naphthas and distillates including kerosene, light distillate and heavy distillate. Temperatures in the phase separator will generally be from 230 to 450°C, preferably 290 to 425°C. Generally the ratio of any added solvent to the heavy oil feed will be from 0.05:1 to 2:1 by volume.
- The light, supernatant, liquid phase withdrawn as overflow from the phase separator is low in metal, CCR and asphaltenes. The contaminants and asphaltenes concentrate in the thermoplastic underflow phase. The liquid overflow product contains thermally cracked fragments which are easily hydrogenated to increase their hydrogen content. Hydrogenation improves the storage stability of the product. The liquid overflow product is easy to hydrotreat because its molecular weight is lower than the original heavy oil feed. Thermally cracked products are in a metastable or activated state for a significant period of time. Hydrogenation produces stable products with a reduced susceptibility to polymerization condensation and reduces residual heteroatom content (nitrogen, sulfur, possibly oxygen). It is preferred to immediately hydrotreat the liquid overflow product rather than store it, as during storage the activated species may undergo polymerization and condensation to solid, sludge- like products which are hard to hydrotreat.
- In the optional, but preferred hydroprocessing step, the liquid overflow product is hydrogenated with conventional hydrotreating catalyst. Suitable catalysts employ a hydrogenation/dehydrogenation component on a substantially neutral or acidic support such as alumina, silica-alumina, carbon, a zeolite or a solid aluminum phosphate. The hydrogenation/dehydrogenation component will normally be a transition metal, either a base metal or base metal combination such as cobalt, tung- ston, or a base metal combination such as cobalt- molybdenum, nickel-tungsten, nickel-molybdenum, or a noble metal such as platinum or palladium. Hydrotreating conditions are conventional, typically 200 to 450°C, preferably 250 to 400°C. Pressures in the hydrotreater are preferably the same as in the thermal processing unit, or hydrovisbreaker if H2 is added during thermal processing, to facilitate direct coupling of the two processes. Therefore, pressure will generally be 1500 to 15000 kPa. Space velocities are normally from 0.1 to 20, preferably 0.5 to 5, hour-'. Liquid flow in the hydrotreater may be up or downflow but downflow is preferred, i.e., a trickle bed reactor is preferred. The severity of the hydrotreating operation will depend on product requirements.
- Hydrotreating is especially preferred when the light liquid product will be FCC feed. The sulfur and metals levels are substantially reduced by hydrotreating, and the hydrogen content increased to about that of conventional FCC feed.
- The thermoplastic, carbonaceous phase withdrawn as an underflow product from the phase separator can be washed with solvent or stripped with steam to recover more of light liquid oil product. The residue from solvent extraction or steam stripping may be made into asphalt or pitch binder, charged to a coker or a gasification unit, or used as refinery fuel. Coking treatments include delayed coking, fluid coking or contact coking.
- The process described above is illustrated in Figure 1. A resid feed having an initial boiling point of 413°C+ (775°F+) is charged via
line 8 toheater 10 and heated to 371 to 538°C. The thermal processing can be achieved solely inheater 10, i.e., the time and temperature in the heater can be enough to yield enough thermoplastic carbonaceous material to provide phase separation from the paraffinic light oil phase. Generally once the resid is heated it is passed via line 11 tothermal reactor 12, operated at 371 to 538°C, and time brings about the thermal reactions which yield the light products due to cracking and the carbonaceous material.Reactor 12 is simply a thermal reactor, the elevated temperatures alone initiate the desired reactions. Fromreactor 12, effluent entersphase separator 14 vialine 13.Separator 14 is preferably maintained at 204 to 371 °C (400 to 700°F). The phenomenon of phase separation is based on the incompatibility of the carbonaceous phase containing toluene insolubles and the liquid paraffinic phase. The light or liquid paraffinic phase is withdrawn as overflow vialine 16 while the carbonaceous material or sludge is withdrawn as underflow vialine 18. The underflow may be passed to aheater 20, heated to at least 482°C - (900°F) and charged via line 21 to coking unit 22, e.g., a delayed or fluid coker. Optionally, the underflow vialine 18 can be mixed with ligh aromatics fromline 5 or steam fromline 52 prior to heating. The products from coking unit 22 are solid coke removed vialine 24 and a vaporized product removed vialine 26. The vaporized product may be fractionated infractionator 28 into various components, typically light gaseous components, naphthas, light coker and heavy coker gas oils, removed via lines 31-34, respectively. - Separation of carbonaceous material from lighter paraffinic liquid in
separator 14 may be aided by solvent treatment of the thermal reactor effluent. - Light products, e.g., contained in the effluent from the heater or thermal reactor may first be flashed off in an optional flashing drum. These products will typically be C2 C. gases. To facilitate flashing, some pressure drop can be taken. The bottom product from the flashing drum may contact solvent upstream of or in the phase separator.
- The important parameters in the solvent treatment are the solvent, solvenflresid ratio, temperature, pressure and number of treatment stages.
- Solvents which may be used include carbon dioxide, light hydrocarbons, C3-C10 paraffins, cycle paraffins, naphthas, gasoline and kerosene, and their mixtures. Propane, butanes, pentane, hexane, and heptane are particularly preferred. The lower the carbon number of the solvent, the higher the quality of the light phase withdrawn but the lower the yield of the treated product.
- The temperature of solvent treatment must be at least 11°C (20°F), preferably 22°C (40°F) higher than the melting point of the carbonaceous bottoms product so that the two phases formed in solvent treatment are both liquids. At high temperatures, above 454°C (850°F), improved solubility between the solvent, light oil phase and carbonaceous bottoms impairs phase separation. A high solvent/resid ratio aids phase separation, but use of large amounts of solvent increases the processing cost. At high temperatures, cooling feed to the solvent treater and heating product from the solvent treater can be minimized leading to cost savings. There is an optimum phase separation temperature depending on the nature of the resid feed and process costs. In general, an operable range is 99 to 427°C (200 to 800°F) and 149 to 371 °C (300 to 700°F) is preferred.
- Higher pressure increases the amount of solvent which is in liquid phase at high temperatures. The optimum pressure depends on the solvent used. The pressure preferably maintains most of the solvent in the liquid phase. Pressure may equal or exceed the critical pressure of the solvent used. For light hydrocarbons this pressure is about 3,500 Kpa (500 psig).
- As the solvent/resid ratio increases, the quality of the relatively light product increases, but the yield decreases. Solvent/resid ratio should be reduced to minimize the operation cost. The optimum solventtresid ratio is the lowest ratio which yields the upgraded product of acceptable quality. The sotvent/resid ratio can be 1:10 to 10:1. A solvent/resid ratio of 1:2 to 3:1 is preferred. In addition to the nature of the resid itself, the optimum solvent/resid ratio depends substantially upon the severity of thermal treatment. The higher the thermal treating severity, the lower the sotvent/resid ratio requirement.
- To minimize the expense of purchased solvent, use of a solvent derived from the products of the process is preferred. Recycle of a naphtha fraction via
line 35 fromfractionation column 28 to phaseseparator 14, in Figure 1 illustrates this concept. - In Figure 2. a residual oil feed is charged via
line 110 to furnace 111 where it is heated. Hydrogen gas fromrecycle line 112, light solvent or diluent fromrecycle line 114 and fresh hydrogen frominlet 115 are heated inheater 113 and passed vialine 151 to mix with the preheated feed inline 116. The mixture enters combined thermal reactor andphase separator 120 where thermal cracking and phase separation occurs to form an upper phase of upgraded liquid product and a lower phase of thermoplastic, carbonaceous product. - The liquid/liquid interface in
separator 120 preferably is kept high and the level ofinlet line 116 low to improve phase separation and consequently metal removal and carbon residue rejection. As the liquid product passes towards the top of the separator and the fluid carbonaceous product sinks towards the bottom, the toluene insoluble materials formed in the thermal cracking coagulate and agglomerate. - The light liquid product is removed via
line 161 and passed to hydrotreater 121, a conventional hydrotreating process. A downflow, trickle bed hydrotreating reactor is preferred. Hydrotreating severity depends on the product use. For fuel use, severity is adjusted to meet fuel sulfur content specifications, generally below 0.3 wt % sulfur. If the product will be FCC feed, more sulfur can be tolerated since sulfur rejection may take place during the FCC operation, or in other downstream processing. - Effluent from
hydrotreater 121 passes vialine 162 tohigh pressure separator 122 where a hydrogen rich gas phase is removed from the hydrotreated product. The hydrogen may be recycled vialine 112 without purification, although an alkaline scrub may be used to remove hydrogenation products, principally hydrogen sulfide, prior to recycle. The hydrotreated liquid is removed vialine 123. - In
phase separator 120 the thermoplastic, carbonaceous phase is withdrawn vialine 163 as a liquid or semi-liquid product. As shown, the residue is passed to asteam stripper 125 where it contacts steam fromline 164 which removes residual light products and any added solvent. From the stripper, it passes vialine 165 toseparator 126 for removal of the light ends which are passed to hydrotreater 121 throughline 127. The stripped residue passes to a coking unit such as a delayedcoker 128. The vaporous products of coking are passed vialine 129 to fractionator 130 where they are separated into products, coker heavy gas oil (HCGO), coker light gas oil (LCGO), distillate, naphtha and gas, removed via lines 135-131 respectively. Some naphtha is returned as light solvent recycle throughline 114 to assist in the heating of the heavy oil feed to the requisite cracking temperature. Alternatively, the distillate could be used as light recycle, as could the LCGO and HCGO fractions although these last two are not preferred. The gas fromfractionator 130 could also be recycled to assist in the heating of the heavy feed, if desired. - This example illustrates what can be achieved by thermal processing. Mild coking causes significant conversion of feed to lighter materials. No hydrogen is added. No diluent or solvent is added, but the use of whole crude as a feedstock, rather than a resid, simulates the addition of some solvent.
-
- This feed was added to an autoclave, and heated to the desired reaction temperature. The autoclave was maintained at the reaction temperature for 1/2 hour and cooled to 316°C (600°F) rapidly with an internal cooling coil. For 1/2 hour at 316°C (600°F), the reaction mixture stood unstirred for phase separation, and then was quenched by ice bath.
- Gas products were collected and measured by a wet test meter. Light liquid products were obtained from the wet gas by collection in a dry ice trap. The distinct liquid phases in the reactor were separated by pipetting. The lower phase contained a considerable amount of light liquid, the separated lower liquid phase was washed with pentane to recover this light liquid.
-
- Table 1 shows as the reaction severity increased from 1800 to 7200 ERT seconds, liquid yield decreased and toluene-insoluble (TI) and gas yields increased. At 7200 ERT seconds delayed coking conditions were approached and 17% TI formed. The TI/CCR ratio was about 2.0 which falls in the range of the coke yield/CCR ratio of delayed coking. The TI/CCR ratio indicates process severity, and increases with increasing severity. At 1800 and 4300 ERT seconds the Ti/CCR ratio 0.44 and 0.71, respectively, and the total liquid yield was greater than 90%.
-
- The metals, S and N content in the upper phase liquid product all decreased with increasing reaction severity. A modest 60 to 70% demetalation was achieved at 1800 ERT sec, equivalent to 30 minutes at 427°C (800°F), with 94% total liquid yield (Table 1). The boiling point conversion of this product is shown in Table 3. The fractions of 216°C-(420°F-) and 216 to 343°C (420 to 650°F) increased over the feed. The conversion of 449°C+ (840°F+) to lighter products was 27 wt %. At a higher severity, e.g., 4,300 ERT, more conversion would be expected.
- As shown in Table 4 below, at 1800 ERT the TI in the upper phase liquid was 1.03%, reflecting poor phase separation. At higher severity, the TI was very low (0.1 % or less), low enough to feed to .hydrotreaters, FCC units or use as heavy fuel. The TI in the upper phase approached 0.09 at 4300 ERT. The ratio of TI in the two phases increased from 33 to 570 with severity. This high ratio at high severity reflected the production of more TI materials. For this feed the optimum treatment severity is about 4300 ERT seconds which yielded large amounts of upgraded liquid product with over 90% of Ni and V removed from the upper liquid phase product. A 4300 ERT severity is between visbreaking and coking.
- This example illustrates what can be achieved when mild coking, phase separation and hydrotreating of light liquid product are practiced together.
- A typical low gravity feed is subjected to mild coking in a fluidized bed, liquid phase reactor to reject carbon as a thermoplastic, dense residue which is continuously removed from the reactor. Reactor temperature is 470°C (880°F) at 2860 kPa (400 psig) pressure, 3 V/V hr-' space velocity. The mildly coked liquid product (MCLP) is removed as a relatively light liquid phase and passed directly to hydrotreating over a conventional hydrotreating catalyst under 7000 kPa (1000 psig) hydrogen pressure at 400°C (750°F), 1 hr' LHSV.
-
- The hydrotreated product is suitable for use as an FCC feed to produce gas plus distillate, G/D, in a high efficiency process.
- The present process efficiently upgrades residua to low boiling products including gasoline and distillate, with the ability to use existing refinery equipment, thereby minimizing capital cost. It efficiently uses hydrogen, by rejecting the more refractory carbonaceous portions of the resid as a dense phase and using the hydrogen selectively to increase the hydrogen level of the FCC fee. This, in turn, results in an increased yield of low boiling product.
Claims (15)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US73298385A | 1985-05-13 | 1985-05-13 | |
| US73298485A | 1985-05-13 | 1985-05-13 | |
| US732984 | 1985-05-13 | ||
| US74643485A | 1985-06-19 | 1985-06-19 | |
| US746434 | 1985-06-19 | ||
| US732983 | 1991-07-19 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0202772A2 true EP0202772A2 (en) | 1986-11-26 |
| EP0202772A3 EP0202772A3 (en) | 1988-07-27 |
Family
ID=27419173
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP86302916A Withdrawn EP0202772A3 (en) | 1985-05-13 | 1986-04-18 | Oil upgrading by thermal processing |
Country Status (3)
| Country | Link |
|---|---|
| EP (1) | EP0202772A3 (en) |
| CA (1) | CA1276125C (en) |
| ES (1) | ES8801358A1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1268711A4 (en) * | 1999-12-22 | 2004-06-09 | Exxonmobil Res & Eng Co | HIGH TEMPERATURE DEPRESSURIZATION FOR THE REMOVAL OF NAPHTA MERCAPTANS |
| EP2031042A1 (en) * | 1999-12-22 | 2009-03-04 | ExxonMobil Research and Engineering Company | High temperature depressurization for naphta mercaptan removal |
| CN102618323A (en) * | 2011-01-27 | 2012-08-01 | 中国石油化工股份有限公司 | Heavy oil raw material processing method for improving liquid product yield |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2847353A (en) * | 1955-12-30 | 1958-08-12 | Texas Co | Treatment of residual asphaltic oils with light hydrocarbons |
| US3796653A (en) * | 1972-07-03 | 1974-03-12 | Universal Oil Prod Co | Solvent deasphalting and non-catalytic hydrogenation |
| US4298457A (en) * | 1978-09-11 | 1981-11-03 | University Of Utah | Hydropyrolysis process for upgrading heavy oils and solids into light liquid products |
| US4379045A (en) * | 1981-05-06 | 1983-04-05 | Mobil Oil Corporation | Co-processing of residual oil and coal |
| JPS6072989A (en) * | 1983-09-30 | 1985-04-25 | Res Assoc Residual Oil Process<Rarop> | Method for thermally cracking heavy oil |
-
1986
- 1986-04-18 EP EP86302916A patent/EP0202772A3/en not_active Withdrawn
- 1986-04-29 CA CA000507806A patent/CA1276125C/en not_active Expired - Lifetime
- 1986-05-12 ES ES554870A patent/ES8801358A1/en not_active Expired
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1268711A4 (en) * | 1999-12-22 | 2004-06-09 | Exxonmobil Res & Eng Co | HIGH TEMPERATURE DEPRESSURIZATION FOR THE REMOVAL OF NAPHTA MERCAPTANS |
| EP2031042A1 (en) * | 1999-12-22 | 2009-03-04 | ExxonMobil Research and Engineering Company | High temperature depressurization for naphta mercaptan removal |
| CN102618323A (en) * | 2011-01-27 | 2012-08-01 | 中国石油化工股份有限公司 | Heavy oil raw material processing method for improving liquid product yield |
| CN102618323B (en) * | 2011-01-27 | 2015-04-29 | 中国石油化工股份有限公司 | Heavy oil raw material processing method for improving liquid product yield |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0202772A3 (en) | 1988-07-27 |
| ES554870A0 (en) | 1987-12-16 |
| CA1276125C (en) | 1990-11-13 |
| ES8801358A1 (en) | 1987-12-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4661241A (en) | Delayed coking process | |
| US5286371A (en) | Process for producing needle coke | |
| EP0121376B1 (en) | Process for upgrading a heavy viscous hydrocarbon | |
| EP0040018B1 (en) | Catalytic hydroconversion of residual stocks | |
| US4615791A (en) | Visbreaking process | |
| US8197668B2 (en) | Process and apparatus for upgrading steam cracker tar using hydrogen donor compounds | |
| CA2908540C (en) | Process for producing distillate fuels and anode grade coke from vacuum resid | |
| CA2326259C (en) | Anode grade coke production | |
| US5124027A (en) | Multi-stage process for deasphalting resid, removing catalyst fines from decanted oil and apparatus therefor | |
| US5124026A (en) | Three-stage process for deasphalting resid, removing fines from decanted oil and apparatus therefor | |
| US5124025A (en) | Process for deasphalting resid, recovering oils, removing fines from decanted oil and apparatus therefor | |
| US4443325A (en) | Conversion of residua to premium products via thermal treatment and coking | |
| US4504377A (en) | Production of stable low viscosity heating oil | |
| JP2926415B2 (en) | Heavy hydrocarbon feedstock conversion method | |
| EP0956324A1 (en) | Method for increasing yield of liquid products in a delayed coking process | |
| US5413702A (en) | High severity visbreaking of residual oil | |
| CA2004882A1 (en) | Process for reducing coke formation during hydroconversion of heavy hydrocarbons | |
| US7033486B2 (en) | Residuum conversion process | |
| CA2011594C (en) | Two catalyst stage hydrocarbon cracking process | |
| US4446004A (en) | Process for upgrading vacuum resids to premium liquid products | |
| EP2880131B1 (en) | Vacuum gas oil conversion process | |
| EP0202772A2 (en) | Oil upgrading by thermal processing | |
| Shen et al. | [17] 2 Thermal Conversion–An Efficient Way for Heavy Residue Processing | |
| US5024752A (en) | Upgrading of resids by liquid phase mild coking | |
| WO2015157379A1 (en) | Process and apparatus for fluid catalytic cracking and hydrocracking hydrocarbons |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AK | Designated contracting states |
Kind code of ref document: A2 Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| PUAL | Search report despatched |
Free format text: ORIGINAL CODE: 0009013 |
|
| AK | Designated contracting states |
Kind code of ref document: A3 Designated state(s): AT BE CH DE FR GB IT LI NL |
|
| 17P | Request for examination filed |
Effective date: 19881216 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN WITHDRAWN |
|
| 18W | Application withdrawn |
Withdrawal date: 19890826 |
|
| R18W | Application withdrawn (corrected) |
Effective date: 19890826 |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: SHU, PAUL Inventor name: YAN, TSOUNG-YUAN |